Abstract
Background
We assessed the safety profile of lasmiditan, a selective 5-HT1F receptor agonist without vasoconstrictive activity being developed as an acute therapy for migraine.
Methods
SAMURAI and SPARTAN were Phase 3 double-blind studies of patients with migraine, randomized to oral lasmiditan 50 mg (SPARTAN only), 100 mg, 200 mg, or placebo to be taken within 4 hours of onset of migraine pain. Safety data from the studies were integrated. Treatment-emergent adverse events (occurring within 48 hours of first dose) were considered in the analyses.
Results
The safety population comprised 1262 patients assigned placebo, and 654, 1265, and 1258 assigned lasmiditan 50 mg, 100 mg, and 200 mg, respectively. There were no deaths; serious adverse events were reported for seven patients (placebo, n = 2 [0.2%]; lasmiditan 50 mg, n = 1 [0.2%]; lasmiditan 100 mg, n = 1 [0.2%]; lasmiditan 200 mg, n = 3 [0.2%]). Patients reporting ≥ 1 treatment-emergent adverse events were: Placebo, n = 174 (13.5%); lasmiditan 50 mg, n = 166 (25.4%); lasmiditan 100 mg, n = 458 (36.2%); and lasmiditan 200 mg, n = 510 (40.6%). Treatment-emergent adverse events were generally mild or moderate in severity. The most common treatment-emergent adverse events with lasmiditan were dizziness, paresthesia, somnolence, fatigue, nausea, muscular weakness and hypoesthesia. There were no ischemic events.
Conclusions
As a centrally-penetrant drug, lasmiditan use was associated with neurologic treatment-emergent adverse events; most were mild or moderate in severity and self-limiting.
Trial registration at clinicaltrials.gov
SAMURAI (NCT02439320) and SPARTAN (NCT02605174).
Keywords: Lasmiditan, safety, phase 3
Introduction
Lasmiditan is a centrally-penetrant, highly selective, 5-HT1F receptor agonist being developed as an acute therapy for migraine (1).
Two Phase 3 single migraine attack studies of lasmiditan, SAMURAI and SPARTAN, have been completed (2,3). In these studies, treatment with oral lasmiditan at all doses resulted in a significant increase in the proportion of patients who were headache pain-free (primary endpoint) and most bothersome symptom (MBS)-free (key secondary endpoint) at 2 hours. In both studies, the overall incidence of adverse events was higher with lasmiditan versus placebo and increased with increasing lasmiditan dose. Adverse events (AEs) were generally mild or moderate in severity. The most frequently reported adverse events with lasmiditan were dizziness, fatigue, lethargy, nausea, paresthesia, and somnolence; the incidence of cardiovascular-related treatment-emergent adverse events (TEAEs) was low.
SAMURAI and SPARTAN were nearly identical in design and provide data from 4439 patients with migraine. The purpose of this paper is to describe the safety profile of lasmiditan using pooled data from SAMURAI and SPARTAN.
Methods
Study designs
SAMURAI (2) and SPARTAN (3) were randomized, double-blind, placebo-controlled Phase 3 trials for acute treatment of migraine, conducted in the United States, and, in the case of SPARTAN, the UK and Germany also. The studies were almost identical in design; SAMURAI, but not SPARTAN, excluded individuals with known coronary artery disease, clinically significant arrhythmia, and uncontrolled hypertension. Key inclusion criteria were a Migraine Disability Assessment score ≥11 (moderate or severe disability), a history of migraine ≥1 year, 3–8 migraine attacks/month, and migraine onset at <50 years of age. There was no upper age limit for study inclusion.
Patients were randomized equally to receive the first dose of lasmiditan 50 mg (SPARTAN only), 100 mg, 200 mg, or placebo. Patients were instructed to take the study drug on an outpatient basis within 4 hours of onset of a migraine pain of at least moderate severity and not improving. If needed for rescue or recurrence, a second dose was taken 2 to 24 hours after first dose. For the second dose, patients initially assigned lasmiditan were randomized to the same active dose or placebo (2:1 ratio), and patients originally assigned placebo received placebo.
Since somnolence, dizziness and fatigue have been documented with lasmiditan, study participants were advised not to drive or operate machinery for 12 hours after treatment.
The primary and key secondary endpoints in SAMURAI and SPARTAN were, respectively, the proportion of patients who were headache pain-free and the proportion who were MBS-free at 2 hours post-first dose.
The studies were conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, and local regulatory requirements. The study protocols were approved by an independent ethics committee or institutional review board at each study site. All patients provided written informed consent for study participation prior to the start of the study. This paper conforms to the extended CONSORT guidelines.
Safety data collection and adverse event coding
At 0.5, 1, 1.5, 2, 3, 4, 24, and 48 hours after the first dose (and again, if taken, at the same intervals after a second dose of the study drug), an electronic diary asked: “Do you feel anything unusual since taking the study medication that you have not felt with a migraine before?” If the patient answered “yes”, they were to receive a call from the site within 48 hours to determine if an AE had been experienced. AEs that occurred or worsened within 48 hours of dosing were considered treatment-emergent. Severity of an AE was recorded by the site, based on patient reporting, with the following severity definitions provided to guide the reporting: Mild – usually transient, does not interfere with the subject's daily activities; Moderate – low level of inconvenience or concern to the subject and may interfere with daily activities; Severe – interrupts the subject's daily activities; Life-threatening.
The Columbia Suicide Severity Rating Scale (C-SSRS), a suicidal ideation and behavior rating scale, was administered at baseline and endpoint. Vital sign assessment, clinical laboratory tests, and 12-lead ECG assessment were also performed at baseline and at the end of the study. The end of study visit was scheduled by the patient for one week after a migraine attack had been treated.
Because lasmiditan is an agonist at the serotonin 1F receptor, medical review was performed to assess for possible cases of serotonin syndrome in patients assigned lasmiditan. Potential cases of serotonin syndrome were identified through a search for TEAEs within the Standardised MedDRA Queries (SMQ) of Neuroleptic Malignant Syndrome (SMQ 20000044; v21.0) and the additional terms of orthostatic hypotension, urinary incontinence, urinary retention, oesophageal dysmotility, gastroparesis, diarrhoea, faecal incontinence, constipation, muscle twitching, muscle stiffness, and muscle spasm. Cases for which one or more of these events were reported were medically reviewed by three physicians at Eli Lilly and Company to determine whether they met Hunter Criteria, considering associated AEs, timing of AEs, severity, seriousness, concomitant medications, and medical history.
Outcome measures
At baseline, 0.5, 1, 2, 3, 4, 24, and 48 hours after dosing, patients were asked to rate their headache pain as none, mild, moderate, or severe. Additionally, they were asked whether they were experiencing nausea, phonophobia, photophobia, or vomiting. If they had nausea, phonophobia, or photophobia, at baseline they were asked to identify which of these was their most bothersome migraine associated symptom. Patients were asked the following questions: a) At all time points, “How much is your migraine interfering with your usual activities?” (responded using a four-point numeric rating); and b) at 2 hours, the Patient Global Impression of Change (PGIC) question (4), “How do you feel after taking study medication?” (responded using a seven-point Likert scale from “very much better” to “very much worse”). The PGIC is an integrated measure of drug tolerability and efficacy.
Statistical analysis
The safety population was defined as all patients who were randomized and received at least one dose of study medication. A TEAE was defined as an AE that first occurred or worsened in severity from baseline within 48 hours of the first dose of study drug, regardless of whether a second dose was taken.
The number and percentage of patients who reported TEAEs, coded using MedDRA® Preferred Term, were summarized by randomized lasmiditan treatment groups (lasmiditan 50 mg, 100 mg, 200 mg, placebo), for all lasmiditan dose groups combined (lasmiditan pooled dose group), and for the placebo group. Statistical comparisons were made between the all lasmiditan doses combined and placebo. The Cochran-Mantel-Haenszel test stratified by study was used for treatment comparisons of percentages.
The time to onset and duration of individual common TEAEs ( ≥ 2% incidence in any lasmiditan dose group) were summarized. Only events for which the relevant information was available (start and stop times/dates, and dosing start times/dates) were considered in these analyses. For patients with multiple events falling under the same Preferred Term, the time to onset and duration for all treatment-emergent occurrences of that term were averaged.
To determine whether there was a relationship between reporting of individual common TEAEs and outcomes, the percentages of those assigned lasmiditan who reported a) being pain free; b) being MBS free; c) no interference with normal activities or d) feeling “very much better” and “much better” on PGIC at 2 hours after dosing were calculated for those who reported the common TEAE and those who did not.
Results
Patient disposition, baseline demographics and study drug exposure
The integrated safety database included a total of 4439 patients who received at least one dose of study drug (placebo, n = 1262; lasmiditan, n = 3177). Of those who received a study dose, 97% assigned either placebo or to a lasmiditan dose group completed the study (i.e. all required follow-ups).
Baseline demographics are summarized in Table 1. Medical history or pre-existing conditions occurring in ≥ 10% in at least one treatment group were seasonal allergy, depression, drug hypersensitivity, headache, hypertension, anxiety, gastroesophageal reflux disease, hysterectomy, female sterilization, asthma, back pain, insomnia, obesity, and postmenopause.
Table 1.
Baseline demographics.
| Placebo (n = 1262) | Lasmiditan pooled doses (n = 3177) | |
|---|---|---|
| Age, years, mean (SD) | 42.4 (12.5) | 42.2 (12.3) |
| Female (%) | 85 | 84 |
| BMI ≥ 30 kg/m2 (%) | 46 | 43 |
| Race (%) | ||
| Black/African-American | 18 | 18 |
| Caucasian | 79 | 78 |
| Hispanic or Latino ethnicity | 17 | 18 |
| Region (%) | ||
| Europe | 8 | 10 |
| North America | 92 | 90 |
| Duration migraine history, years, mean (SD) | 18.7 (12.7) | 18.9 (13.0) |
| Migraine attacks/ month, mean (SD) | 5.2 (2.2) | 5.1 (2.0) |
| Pre-existing CVDa (%) | 21 | 20 |
| Baseline CVD risk factor(s)b (%) | 80 | 79 |
Presence of one or more of the following: Cardiac arrhythmia, cardiac failure, cardiomyopathy, CNS vascular disorder, embolic or thrombotic event, hypertension, ischemic heart disease, pulmonary hypertension, Torsade de pointes/QT prolongation.
Identified based on the American College of Cardiology and American Heart Association Task Force on Practice Guidelines. Risk factors considered were age >40 years, current smoker, systolic blood pressure ≥140 mm Hg and/or a medical history of hypertension at baseline, total cholesterol ≥240 mg/dL, HDL cholesterol <40 mg/dL for men and < 50 mg/dL for women, and diabetes mellitus (5,6).
BMI: body mass index; CVD: cardiovascular disease; CNS: central nervous system; HDL: high-density lipoprotein; SD: standard deviation.
A summary of drug exposure (one or two doses) is shown in Table 2. A higher percentage of patients assigned placebo took a second dose compared to those in any of the lasmiditan treatment groups. The majority of second doses (about 94%) were taken for rescue.
Table 2.
Exposure to study drug (received doses).
| Placebo (n = 1262) | Lasmiditan 50 mg (n = 654) | Lasmiditan 100 mg (n = 1265) | Lasmiditan 200 mg (n = 1258) | Lasmiditan pooled doses (n = 3177) | |
|---|---|---|---|---|---|
| One dose only, n, (%) | 500 (40) | 352 (54) | 716 (57) | 802 (64) | 1870 (59) |
| Two dosesa, n, (%) | 762 (60) | 302 (46) | 549 (43) | 456 (36) | 1307 (41) |
| Placebo | 762 | 96 | 169 | 153 | |
| Active | – | 206 | 380 | 303 |
Second dose taken for rescue (patient did not achieve headache pain-free status at 2 hours, completed the 2-hour assessments, and took a second dose of study drug between 2 and 24 hours post-first dose) or recurrence (patient achieved headache pain-free status at 2 hours but then experienced recurrence of mild, moderate, or severe migraine pain and took a second dose of study drug up to 24 hours after the first dose).
Adverse event summary
Adverse event findings are summarized by dose group in Table 3. There were no deaths reported and treatment-emergent serious adverse events were reported for two (0.2%) patients assigned placebo and five (0.2%) assigned to one of the lasmiditan dose groups. Treatment-emergent SAEs were as follows: Placebo – cholelithiasis, non-cardiac chest pain; Lasmiditan – hypertension, hypotension, pituitary tumor benign, presyncope, surgery. Additional SAEs that were not treatment-emergent were as follows: Placebo – intestinal obstruction; Lasmiditan – asthma, deep vein thrombosis, somatic symptom disorder.
Table 3.
Overview of AE findings.
| Number of patients experiencing: | Placebo (n = 1262) | Lasmiditan 50 mg (n = 654) | Lasmiditan 100 mg (n = 1265) | Lasmiditan 200 mg (n = 1258) | Lasmiditan pooled doses (n = 3177) |
|---|---|---|---|---|---|
| Death | 0 | 0 | 0 | 0 | 0 |
| ≥1 treatment emergent SAE | 2 (0.2) | 1 (0.2) | 1 (0.1) | 3 (0.2) | 5 (0.2) |
| ≥1 SAE | 3 (0.2) | 1 (0.2) | 2 (0.2) | 5 (0.4) | 8 (0.3) |
| ≥1 TEAE | 174 (13.8) | 166 (25.4) | 458 (36.2) | 510 (40.5) | 1134 (35.9)* |
| Discontinuation due to TEAE | 0 | 0 | 0 | 1 (0.1)a | 1 (0) |
Number of patients (%) reported.
p < 0.001 vs. placebo. Formal comparisons for pooled lasmiditan doses (not individual doses) vs placebo only.
Patient discontinued due to fatigue and dizziness (both of mild severity).
SAE: serious adverse event; TEAE: treatment-emergent adverse event; vs: versus.
TEAEs overall were reported more frequently in patients assigned lasmiditan versus placebo, and, in general, the frequency of TEAEs increased with increasing lasmiditan dose. The difference in TEAE frequency between the lasmiditan and placebo groups was primarily driven by nervous system TEAEs occurring at a higher incidence in all lasmiditan dose groups compared to placebo; nervous system TEAEs included dizziness, paresthesia, somnolence, hypoesthesia, lethargy, balance disorder, tremor, and sedation. Of the TEAEs reported, most TEAEs were mild (placebo, 70%; lasmiditan pooled 57%) or moderate (placebo, 24%; lasmiditan pooled 36%) in severity.
The most frequently reported severe TEAEs in patients assigned lasmiditan were dizziness, somnolence, fatigue and nausea (see Common TEAEs section). The only SAE with incidence >1% in any group was dizziness in the lasmiditan 200 mg group. There were no life-threatening events.
The incidence of TEAEs reported was numerically higher in those who took one versus two doses of study drug (Table 4). Among patients who took lasmiditan for their first dose and then took a second dose of study drug, the incidence of TEAEs reported after the second dose was similar regardless of whether the second dose was lasmiditan or placebo (Table 4).
Table 4.
Incidence of TEAEs by dosing regimen.
| First dose: | Placebo | Lasmiditan 50 mg | Lasmiditan 100 mg | Lasmiditan 200 mg | |||
|---|---|---|---|---|---|---|---|
| ≥1 TEAE after first dose, n (%) | |||||||
| Took only one dosea, n (%) | 76 (15) | 93 (26) | 287 (40) | 354 (44) | |||
| Took two dosesb, n (%) | 59 (7.7) | 56 (19) | 127 (23) | 123 (27) | |||
| Second dose: |
Placebo (n = 762) |
Placebo (n = 96) |
Lasmiditan 50 mg (n = 206) |
Placebo (n = 169) |
Lasmiditan 100 mg (n = 380) |
Placebo (n = 153) |
Lasmiditan 200 mg (n = 303) |
| ≥1 TEAE after second dose, n (%) | 64 (8.4) | 13 (14) | 35 (17) | 31 (18) | 65 (17) | 33 (22) | 55 (18) |
Denominator for % is number of patients who took only one dose.
TEAEs occurring from time of first dose to time of second dose. Denominator for % is number of patients who took two doses.
Common TEAEs
Common TEAEs are summarized in Table 5. Dizziness was the most common TEAE reported with lasmiditan (14.7%). Most events were mild or moderate in severity (Table 6).
Table 5.
Summary of TEAEs that occurred in 2% or more of patients in any lasmiditan dose group and more frequently with lasmiditan than placebo (p < 0.05).
| Placebo (n = 1262) | Lasmiditan 50 mg (n = 654) | Lasmiditan 100 mg (n = 1265) | Lasmiditan 200 mg (n = 1258) | Lasmiditan pooled doses (n = 3177) | |
|---|---|---|---|---|---|
| Dizziness | 37 (2.9) | 56 (8.6)a | 194 (15.3)a,b | 216 (17.2)a,b | 466 (14.7)a |
| OR vs. placebo | 3.7 | 6.0 | 6.9 | 5.7 | |
| Paresthesia | 19 (1.5) | 16 (2.4)a | 73 (5.8)a,b | 91 (7.2)a,b | 180 (5.7)a |
| OR vs. placebo | 2.7 | 4.0 | 5.1 | 4.1 | |
| Somnolence | 27 (2.1) | 35 (5.4)a | 65 (5.1)a | 75 (6.0)a | 175 (5.5)a |
| OR vs. placebo | 2.7 | 2.5 | 2.9 | 2.7 | |
| Fatigue | 8 (0.6) | 18 (2.8)a | 52 (4.1)a | 50 (4.0)a | 120 (3.8)a |
| OR vs. placebo | 3.0 | 6.7 | 6.5 | 6.0 | |
| Nausea | 20 (1.6) | 18 (2.8) | 52 (4.1)a | 50 (4.0)a | 107 (3.4)a |
| OR vs. placebo | 2.3 (ns) | 2.0 | 2.5 | 2.3 | |
| Muscular weakness | 0 | 7 (1.1)a | 16 (1.3)a | 19 (1.5)a | 42 (1.3)a |
| OR vs. placebo | na | na | na | na | |
| Hypoesthesia | 3 (0.2) | 2 (0.3) | 17 (1.3)a,b | 20 (1.6)a | 39 (1.2)a |
| OR vs. placebo | 1.0 (ns) | 5.7 | 6.8 | 5.3 |
Number of patients (%) reported. OR: odds ratio point estimate; vs: versus; na: not applicable.
Significant difference vs. placebo (p < 0.05).
Significant difference vs. lasmiditan 50 mg (p < 0.05).
No significant differences between lasmiditan 100 mg vs. 200 mg.
Table 6.
Summary of TEAEs by severity.
| Placebo (n = 1262) | Lasmiditan 50 mg (n = 654) | Lasmiditan 100 mg (n = 1265) | Lasmiditan 200 mg (n = 1258) | Lasmiditan pooled doses (n = 3177) | |
|---|---|---|---|---|---|
| Dizziness | |||||
| Mild | 29 (2.3)a | 40 (6.1) | 118 (9.3) | 121 (9.6) | 279 (8.8) |
| Moderate | 7 (0.6) | 14 (2.1) | 66 (5.2) | 78 (6.2) | 158 (5.0) |
| Severe | 1 (0.1) | 2 (0.3) | 10 (0.8) | 17 (1.4) | 29 (0.9) |
| Paresthesia | |||||
| Mild | 18 (1.4) | 11 (1.7) | 47 (3.7) | 60 (4.8) | 118 (3.7) |
| Moderate | 1 (0.1) | 5 (0.8) | 26 (2.1) | 31 (2.5) | 62 (2.0) |
| Severe | 1 (0.1) | 1 (0.2) | 4 (0.3) | 3 (0.2) | 8 (0.3) |
| Somnolence | |||||
| Mild | 20 (1.6) | 24 (3.7) | 38 (3.0) | 48 (3.8) | 110 (3.5) |
| Moderate | 6 (0.5) | 10 (1.5) | 23 (1.8) | 24 (1.9) | 57 (1.8) |
| Severe | 1 (0.1) | 1 (0.2) | 4 (0.3) | 3 (0.2) | 8 (0.3) |
| Fatigue | |||||
| Mild | 3 (0.2) | 8 (1.2) | 22 (1.7) | 25 (2.0) | 55 (1.7) |
| Moderate | 4 (0.3) | 8 (1.2) | 26 (2.1) | 22 (1.7) | 56 (1.8) |
| Severe | 1 (0.1) | 2 (0.3) | 4 (0.3) | 3 (0.2) | 9 (0.3) |
| Nausea | |||||
| Mild | 13 (1.0) | 10 (1.5) | 22 (1.7) | 34 (2.7) | 66 (2.1) |
| Moderate | 6 (0.5) | 7 (1.1) | 16 (1.3) | 10 (0.8) | 33 (1.0) |
| Severe | 1 (0.1) | 1 (0.2) | 2 (0.2) | 5 (0.4) | 8 (0.3) |
| Muscular weakness | |||||
| Mild | 0 | 2 (0.3) | 11 (0.9) | 11 (0.9) | 24 (0.8) |
| Moderate | 0 | 5 (0.8) | 5 (0.4) | 8 (0.6) | 18 (0.6) |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Hypoesthesia | |||||
| Mild | 3 (0.2) | 1 (0.2) | 10 (0.8) | 8 (0.6) | 19 (0.6) |
| Moderate | 0 | 1 (0.2) | 6 (0.5) | 10 (0.8) | 17 (0.5) |
| Severe | 0 | 0 | 1 (0.1) | 2 (0.2) | 3 (0.1) |
Number of participants experiencing an event (%).
Generally, the onset of common TEAEs with lasmiditan occurred approximately 30–50 minutes after dosing. The median durations (interquartile range) of common TEAEs with lasmiditan were, in hours, 2.0 (0.8–4) for dizziness, 1.0 (0.5–3) for paresthesia, 3.9 (2–8.2) for somnolence, 4.8 (2–10.5) for fatigue, 3.0 (1.5–6.3) for nausea, 1.3 (0.8–4.0) for muscular weakness, and 1.5 (0.6–4.8) for hypoesthesia.
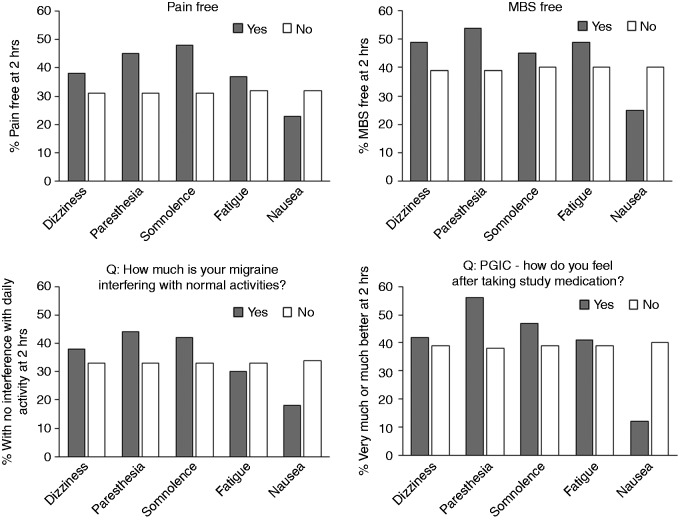
The presence of dizziness, paresthesia, somnolence, or fatigue did not appear to have a negative influence on freedom from pain or MBS, daily activity or PGIC at 2 hours post dosing (Figure 1). The presence of nausea did appear to have a potentially negative influence on these parameters. No formal comparisons were made due to the small sample sizes.
Figure 1.
Association between the presence/absence of common TEAEs and outcomes at 2 hours in patients who took lasmiditan. For five most common TEAEs (common TEAES with >100 events in total).
Note: Yes/No on Figure refers to presence/absence of the common TEAE.
MBS: most bothersome symptom; PGIC: Patient Global Impression of Change.
Evaluation of other potential harms
Suicide ideation and behavior
The C-SSRS reporting of affirmative response(s) was low and similar across treatment groups. Of patients who had no suicidal ideation or behavior at baseline, one of 1188 patients assigned placebo and one of 2976 assigned lasmiditan (pooled doses) had a post-baseline affirmative response. There were no instances of suicidal behavior in either group. One TEAE of suicidal ideation (“wanting to die feeling,”) was reported in a lasmiditan group; this event was not captured on the C-SSRS.
Injury and accident
Neurologic TEAEs were the most frequently observed events with lasmiditan. An analysis was performed to assess for potential relationship between these TEAEs and injuries or accidents. The numbers of patients who had both at least one neurologic TEAE and at least one injury and/or accident AE (irrespective of temporal association) were similar between placebo (n = 1, 0.1%) and pooled lasmiditan (n = 9, 0.3%) groups (p = 0.17). Medical review of each AE of any injury, poisoning, and procedural complication was also undertaken to determine whether neurologic TEAEs preceded any of these events. In the lasmiditan treatment groups, there were no cases of an injury or accident being temporally associated with a neurologic TEAE.
Cardiovascular safety
TEAEs: No ischemic cardiovascular events potentially attributable to vasoconstriction such as myocardial infarction, transient ischemic attack, ischemic stroke, or angina were reported in any treatment group. TEAEs determined by medical review to be cardiovascular in nature were reported for five (0.4%) in the placebo group and 30 (0.9%) in the pooled lasmiditan group (p = 0.057). This difference was driven by three cardiovascular events – palpitations (placebo, n = 1 [0.1%]; lasmiditan, n = 12 [0.4%]); tachycardia (placebo, n = 0; lasmiditan, n = 6 [0.2%]); and increased heart rate (placebo, n = 1 [0.1%]; lasmiditan, n = 5 [0.2%]).
Vital signs: Systolic and diastolic blood pressure and pulse were similar across treatment groups, with no statistically or clinically significant differences between groups at baseline and post-baseline. Mean change from baseline was small in both treatment groups. Overall, the number of patients with low, high or potentially clinically significant elevations in blood pressure (either systolic or diastolic) either at baseline or post-baseline was low and similar across treatment groups.
ECG findings: Mean changes in quantitative ECG parameters from baseline to last post-baseline assessment were minimal. There were no statistically significant differences in the least square mean changes for any parameter (heart rate, PR interval, QRS interval, QT interval, corrected QT interval by Fridericia [QTcF]). A similar proportion of the placebo and lasmiditan pooled treatment groups had categorical (low or high) ECG changes in heart rate, PR interval, QRS interval, QT interval uncorrected, and QTcF, with no statistically significant differences between the two groups for any categorical change. One patient assigned lasmiditan had an ECG change reported as a likely cardiovascular TEAE. This patient had an event of abnormal ECG reported (right bundle branch block and left axis deviation) on study completion. No patient experienced a QTcF value >500 msec in either treatment group.
Hepatic safety
There were no significant differences between lasmiditan and placebo groups in the frequency of abnormal hepatic laboratory results (alanine transaminase or aspartate transaminase ≥3×, 5×, 10× the upper limit of normal (ULN); ALP ≥ 2 × ULN; total bilirubin ≥ 2 × ULN) during the treatment period.
Serotonin syndrome
Since lasmiditan is an agonist at the serotonin 1F receptor, medical review was performed to assess for possible cases of serotonin syndrome in patients assigned lasmiditan. Five cases were identified via the search strategy described. Medical review of these cases revealed that none of the TEAEs were severe or serious, and no cases met Hunter Criteria (7).
Hypersensitivity
Medical review of cases revealed seven patients with a total of nine suspected events of immediate hypersensitivity, including rashes, all in patients who received lasmiditan. The events were all mild or moderate in severity and none were serious. There were no cases of suspected non-immediate hypersensitivity or anaphylaxis.
Discussion
We present the pooled safety findings from two Phase 3 studies of lasmiditan for acute treatment of migraine. There were no deaths, and SAEs were reported for 0.2% of patients in the placebo and pooled lasmiditan groups.
TEAEs were more frequently reported with lasmiditan (placebo 13.5% versus lasmiditan pooled groups 35.9%) and there was a numerical increase in the incidence of TEAEs with increasing lasmiditan dose. For those who received lasmiditan as first dose and then took a second dose, the incidence of TEAEs was similar whether the second dose was placebo or lasmiditan, supporting the idea that a second dose of lasmiditan for migraine pain does not confer an additional risk of TEAEs.
The most frequent TEAEs associated with lasmiditan use were generally neurologic events, most frequently dizziness, which included patient reports of dizziness, lightheadedness, lightheaded, dizzy, woozy, lightheaded feeling, giddiness, feeling drunk and feeling of faintness. Dizziness with lasmiditan use has been previously explored.8 Other common TEAEs were paresthesia, somnolence, fatigue, nausea, muscular weakness, and hypoesthesia. The odds ratios for these events for the pooled lasmiditan versus placebo group ranged from 2.3 to 6.0. Most events were mild or moderate in severity. The onset of common TEAEs with lasmiditan was rapid, generally less than one hour after dosing, and generally lasted 1.0 to 4.8 hours (median values), depending on event; this time course generally coincides with the maximum plasma concentration of lasmiditan (9). Since the most frequent TEAEs were neurologic, we assessed whether there was any increased risk of injury with lasmiditan use. We found no increased risk of injury associated with neurologic TEAEs and there were no treatment-emergent road traffic accidents, impaired ability to use machinery, or accidents reported.
The common TEAEs reported in SAMURAI and SPARTAN could, theoretically, be expected to be associated with tolerability concerns; for example, interference with daily activity and PGIC. Although nausea was associated with a numerically greater interference with daily activity and worsened PGIC, the proportion of patients assigned lasmiditan with no interference with daily activity, and very much/much better on PGIC at 2 hours appeared, in general, to be numerically higher in those reporting dizziness, paresthesia, somnolence or fatigue versus not, though no formal analysis was performed.
The finding that the reporting of dizziness, paresthesia, somnolence and fatigue, but not nausea, appeared to be associated with numerically greater freedom from pain and MBS raises the possibility that these TEAEs could be mediated through a similar mechanism of action as the efficacy.
While triptans have affinity for 5-HT1B, located on vascular smooth muscle receptors and mediating vasoconstrictor effect, lasmiditan has a high affinity for the 5-HT1F receptor and is not a vasoconstrictor (10–12). Due to the increased risk of cardiovascular disease in patients with migraine (13,14), the vasoconstrictive effects of triptans as well as ergotamines (12,15–17), and warnings concerning rare serious cardiovascular events with triptans, ergotamines, and NSAIDS (17–19), we evaluated the cardiovascular safety of lasmiditan. No TEAEs related to vasoconstriction, including angina pectoris, cerebral infarction, hypertensive crisis, and ischemic stroke, were reported in either SAMURAI or SPARTAN. In addition, available information suggests that lasmiditan use is not associated with increased risks of serious gastrointestinal events (as reported with NSAIDs (19)), hepatotoxicity (as reported with high dose acetaminophen), or respiratory depression (as reported with opiates). Lasmiditan may be a new option for the acute treatment of migraine, including for patients in whom currently available options are not desirable because of safety concerns.
Comparisons of the TEAE findings observed with lasmiditan versus those observed in trials of other migraine agents have limitations due to differences in AE collection methodologies. Importantly, the lasmiditan Phase 3 trials used an electronic diary and included a question to solicit AE information at 0.5, 1, 1.5, 2, 3, 4, 24, and 48 hours after the first dose (and again, if taken, at the same intervals after a second dose of study drug); a positive response to this question triggered phone contact from the site. This questioning likely resulted in relatively high ascertainment of TEAEs. Notwithstanding this difference, we compared the common TEAEs (those reaching 2% in a group and higher with active drug than placebo) with lasmiditan to those reported with a triptan with relatively low (naratriptan (20)), medium (sumatriptan (18)), and high (rizatriptan (21)) efficacy, based on findings from a meta-analysis (22). The number of individual TEAEs meeting this criterion of “common” were six for naratriptan, eight for sumatriptan, 11 for rizatriptan, and seven for lasmiditan. Consistent with high specificity for 5-HT1F and central penetrance, lasmiditan appears to have a distinct adverse event profile from that of triptans, with common TEAEs being CNS-related and without the TEAEs related to chest/throat/neck/jaw pain, tightness, pressure, or heaviness that have been reported with triptan use (18). The most common TEAE reported with naratriptan was nausea (4–5% of patients); with sumatriptan, paresthesia (3–5%); with rizatriptan, dizziness (4–9%); and with lasmiditan, dizziness (9–17%).
Based on findings from these Phase 3 studies, there is no indication that lasmiditan is associated with an increase in suicidal ideation or behavior. In addition, there was no significant effect of lasmiditan on hepatic safety, ECG findings or vital signs, though these parameters were not assessed in temporal proximity to dosing. Additionally, a medical review did not reveal any probable cases of serotonin syndrome. There were cases of immediate hypersensitivity ( < 1%), including rashes, with lasmiditan; all were mild or moderate in severity.
There are limitations to this analysis. These placebo-controlled studies included assessment of lasmiditan during a single attack and data pertaining to long-term adherence and compliance are not available; additional safety data from a long-term safety study of lasmiditan for the acute treatment of migraine are needed and studies are under way. C-SSRS, laboratory measures, ECG findings and vital signs were collected during scheduled visits and not necessarily collected in close temporal proximity to dosing since, per protocol, the endpoint visit could have occurred up to 7 days after dosing. Apart from the PGIC, the outcomes evaluated for their association with presence or absence of common TEAEs are migraine specific and do not reflect the overall patient status. In addition, we evaluated TEAEs occurring at or prior to 2 hours in relation to outcomes occurring at 2 hours and did not consider a more complex temporal association between TEAE and outcome that may have existed.
In conclusion, TEAEs were reported for 36% of those assigned lasmiditan; they were mostly neurologic, of mild or moderate severity, with early onset and self-limiting with short duration. There were no deaths, and the incidence of SAEs, suicidal ideation or injuries and accidents were similar in the lasmiditan and placebo groups. The most common TEAEs were not associated with worse outcomes relating to pain, most bothersome migraine symptoms, daily activity, and overall treatment experience (measured using PGIC). There were no ischemic events suggesting vasoconstriction. Lasmiditan may represent a useful acute treatment option for patients with migraine, including those in whom vasoconstriction is not desirable.
Key findings
Lasmiditan, a centrally-penetrant selective 5-HT1F receptor agonist, was associated with neurologic treatment-emergent adverse events, which were mostly mild or moderate in severity and self-limiting, and of short duration.
The most common adverse events with lasmiditan were dizziness, paresthesia, somnolence, fatigue, nausea, muscular weakness and hypoesthesia.
There were no ischemic events reported.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JHK, EL, EGD, SAD, JW and ASB are full-time employees and minor stockholders at Eli Lilly and Company. PBR serves on advisory boards for Biohaven Pharmaceuticals and Xoc Pharmaceuticals.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The SPARTAN and SAMURAI studies were sponsored by CoLucid Pharmaceuticals, a wholly owned subsidiary of Eli Lilly and Company.
References
- 1.Vila-Pueyo M. Targeted 5-HT1F therapies for migraine. Neurotherapeutics 2018; 15: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuca B, Silberstein SD, Wietecha L, et al. Lasmiditan is an effective acute treatment for migraine: A phase 3 randomized study. Neurology 2018; 91: e2222–e2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goadsby PJ LL, Dennehy EB, Kuca B, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. Epub ahead of print 27 May 2019. DOI: 10.1093/brain/awz134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guy W. Clinical global impression. In: ECDEU assessment manual for psychopharmacology – revised. Rockville, MD: US Department of Health, Education and Welfare, pp.218–222. https://archive.org/details/ecdeuassessmentm1933guyw (1976, accessed 28 May 2019).
- 5.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: S49–S73. [DOI] [PubMed] [Google Scholar]
- 6.American Heart Association resource page. Understanding blood pressure readings: Know your numbers, https://www.heart.org/HEARTORG/Conditions/HighBloodPressure/KnowYourNumbers/Und%20erstanding-Blood-Pressure-Readings_UCM_301764_Article.jsp (2017, accessed 1 May 2019).
- 7.Dunkley E, Isbister G, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: Simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003; 96: 635–642. [DOI] [PubMed] [Google Scholar]
- 8.Tepper SK, Krege J, Lombard L, et al. Characterization of dizziness after lasmiditan: Findings from the SAMURAI and SPARTAN acute migraine treatment randomized trials. Headache. (in press). [DOI] [PubMed] [Google Scholar]
- 9.Pilgrim A, Dussault B, Rupniak NM, et al. col-144, an orally bioavailable selective 5-HT1F receptor agonist for acute migraine therapy: Po34. Cephalalgia 2009; 29: 24–25. [Google Scholar]
- 10.Nilsson T, Longmore J, Shaw D, et al. Contractile 5-HT1B receptors in human cerebral arteries: Pharmacological characterization and localization with immunocytochemistry. Brit J Pharmacol 1999; 128: 1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neeb L, Meents J, Reuter U. 5-HT 1F receptor agonists: A new treatment option for migraine attacks? Neurotherapeutics 2010; 7: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubio-Beltrán E, Labastida-Ramírez A, Villalon CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther 2018; 186: 88–97. [DOI] [PubMed] [Google Scholar]
- 13.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017; 357: j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmoud AN, Mentias A, Elgendy AY, et al. Migraine and the risk of cardiovascular and cerebrovascular events: A meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open 2018; 8: e020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphrey P, Feniuk W, Perren MJ, et al. Serotonin and migraine. Ann NY Acad Sci 1990; 600: 587–598. [DOI] [PubMed] [Google Scholar]
- 16.Mitsikostas D, Tfelt-Hansen P. Targeting to 5-HT1F receptor subtype for migraine treatment: Lessons from the past, implications for the future. Cent Nerv Syst Agents Med Chem 2012; 12: 241–249. [DOI] [PubMed] [Google Scholar]
- 17.Valeant Pharmaceuticals North America. D.H.E. 45® (dihydroergotamine mesylate) injection, USP, https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/005929s044lbl.pdf (2008, accessed 1 May 2019).
- 18.GlaxoSmithKline. IMITREX (sumatriptan succinate) tablets, for oral use. Highlights of prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020132s029lbl.pdf (2017, accessed 1 May 2019).
- 19.Depomed Inc. CAMBIA® (diclofenac potassium), for oral solution. Highlights of prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022165s012lbl.pdf (2017, accessed 1 May 2019).
- 20.GlaxoSmithKline. AMERGE® (naratriptan hydrochloride) tablets, for oral use, https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020763s011lbl.pdf (2016, accessed 1 May 2019).
- 21.Merck & Co, Inc. MAXALT (rizatriptan benzoate) tablets, for oral use, https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020864s011s016s017s018s019,020865s012s016s018s020s021lbl.pdf (2011, accessed 1 May 2019).
- 22.Ferrari MD, Roon KI, Lipton RB, et al. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: A meta-analysis of 53 trials. Lancet 2001; 358: 1668–1675. [DOI] [PubMed] [Google Scholar]