Keywords: nerve regeneration, growth arrest-specific 5, PC12 cell, neuron, proliferation, cell cycle, choline acetyltransferase, acetylcholine, Alzheimer's disease, neural regeneration
Abstract
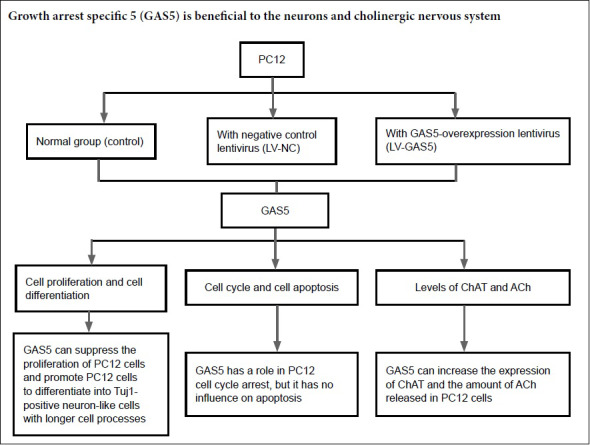
Growth arrest-specific 5 (GAS5) is an anti-oncogene that has been extensively studied in tumors. However, research on GAS5 in the context of nervous system disease is rare at present. This study aimed to investigate the role of the long non-coding RNA GAS5 in rat pheochromocytoma cells (PC12 cells). GAS5-overexpressing lentivirus was transfected into PC12 cells, and expression levels of GAS5 and C-myc were detected by real-time PCR. Ratios of cells in S phase were detected by 5-ethynyl-2′-deoxyuridine. Immunohistochemical staining was used to detect the immunoreactivity of neuron microtubule markers Tuj1, doublecortin, and microtubule-associated protein 2. Apoptosis was detected by flow cytometry, while expression of acetylcholine in cells was detected by western blot assay. We found that GAS5 can promote PC12 cells to differentiate into Tuj1-positive neuron-like cells with longer processes. In addition, cell proliferation and cell cycle were significantly suppressed by GAS5, whereas it had no effect on apoptosis of PC12 cells. Our results indicate that GAS5 could increase the expression of choline acetyltransferase and acetylcholine release. Thus, we speculate that GAS5 is beneficial to the recovery of neurons and the cholinergic nervous system.
Chinese Library Classification No. R446; Q421; Q78
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by progressive impairment of memory and the loss of acquired knowledge until a total loss of activity in daily life occurs, thus imposing a heavy burden on society and families. As such, AD has become one of the most important diseases threatening the health of the older adult. Although there are many studies about AD, its exact mechanism remains unclear. During the course of this disease, cholinergic system injury takes place in AD patient brains, where it plays an important role in AD-related memory deficits (Ferreira-Vieira et al., 2016; Volpato and Holzgrabe, 2018).
Lhx8 (L3/Lhx7) is a member of the LIM-homeobox gene family, whose structures are highly conserved across many organisms. Lhx8 is closely related to development of the cholinergic nervous system and a critical factor for cholinergic cell fates (Zhao et al., 2003; Mori et al., 2004; Manabe et al., 2005). Cholinergic neurons in the basal forebrain were decreased in Lhx8-null mice, and cholinergic phenotypes were specifically lost in Lhx8-knockout embryonic stem cells (Manabe et al., 2007, 2008; Li et al., 2014). Lhx8 can also promote choline acetyltransferase (ChAT) expression and acetylcholine (ACh) release, and regulate cholinergic differentiation of murine embryonic stem cells, Neuro-2a cells, hippocampal progenitor cells, and hippocampal newborn neurons (Manabe et al., 2005, 2007; Shi et al., 2012; Zhu et al., 2013).
Rat pheochromocytoma cells (PC12 cells) can express ChAT and release Ach (Pongrac and Rylett, 1998; Roghani and Carroll, 2002). As such, they are regularly used to investigate the cholinergic system in AD studies (Roghani and Carroll, 2002; Jin et al., 2015; Zhang et al., 2016). In our previous study, we found that Lhx8 inhibited both cell proliferation and cell cycle in PC12 cells (Li et al., 2015), although the exact mechanism was unclear.
Long non-coding RNAs (lncRNAs), a major component of the human transcriptome, play major roles in biological processes such as gene transcription, cell growth, and tumorigenesis (Mattick, 2004; Wapinski and Chang, 2011; Derrien et al., 2012; Kung et al., 2013; Pan et al., 2017); although, many have yet to be functionally annotated (Kung et al., 2013; Morris and Mattick, 2014). Growth arrest-specific 5 (GAS5), which belongs to the GAS family, was originally isolated from mouse NIH 3T3 fibroblasts (Schneider et al., 1988). GAS5, an anti-oncogene, has been extensively studied in tumors (Mourtada-Maarabouni et al., 2008, 2009; Kino et al., 2010; García-Claver et al., 2013; Liu et al., 2013; Lu et al., 2013; Tu et al., 2014; Sun et al., 2017). However, research investigating the role of GAS5 in nervous system diseases such as AD is rare at present. In this report, we focused on analyzing the effect of GAS5 on cell proliferation, neuronal differentiation, cell cycle, cell apoptosis, ChAT expression, and ACh release in vitro using the neuronal cell line PC12.
Materials and Methods
Cell culture
PC12 cells were bought from Shanghai Zhong Qiao Xin Zhou Biotechnology (Shanghai, China) and cultured in Dulbecco’s Modified Eagle’s Medium/Ham’s F12 (DMEM/F12; Gibco, Invitrogen, CA, USA) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) in a CO2 incubator. For differentiation experiments, PC12 cells were maintained in DMEM/F12 containing 1% FBS.
Cell transfection
Both lncRNA GAS5-overexpressing lentivirus (LV-GAS5) and negative control lentivirus (LV-NC) were synthesized by Genechem (Shanghai, China). First, the multiplicity of infection (MOI) was determined through a pre-experiment; we selected MOI = 50 for subsequent formal experiments. All experiments were carried out according to the manufacturer’s lentivirus operation manual (Genechem). After transfection, cells were examined for green fluorescent protein (GFP) fluorescence using an EVOS FL Auto (Life Technologies, Carlsbad, CA, USA). Transfection efficiency was calculated by comparing GFP-positive cells to total cells. First, the number of cells in the bright field was counted, and then the number of cells emitting GFP in the same visual field was counted. The EVOS FL Auto has an automatic counting function.
Quantitative real-time PCR
Total RNA of each group was extracted using TRIzol reagent (Invitrogen) under strict quality control. Quantification and quality checks were performed using a Nanodrop 2000 (Thermo Scientific, Waltham, MA, USA). LncRNAs were reverse transcribed using a RevertAid™ First Strand cDNA Synthesis Kit (Thermo Scientific). Detection of GAS5 was performed using a SYBR Green Master Mix (Roche, Basel, Switzerland). Quantitative real-time PCR (qRT-PCR) was performed with a Corbett RG-6000 PCR system (Qiagen, Hilden, Germany), with sense and antisense primers as follows: glyceraldehyde 3-phosphate dehydrogenase (GAPDH): 5′-GCA AGT TCA ACG GCA CAG-3′, 5′-GCC AGT AGA CTC CAC GAC AT-3′; GAS5: 5′-ATG GGA TGG TGG AGT TTG AAT C-3′, 5′-GTC AGA GGA GCC CTT GAA ATT C-3′; and C-myc: 5′-AGT CAG GGT CAT CCC CAT CA-3′, 5′-TGG AGC ATT TGC GGT TGT TG-3′. Fold changes in mRNA expression were determined using the 2–ΔΔCt method (Pfaffl, 2001).
5-Ethynyl-2′-deoxyuridine assay
We used an 5-ethynyl-2′-deoxyuridine (EdU) assay kit (Ribobio, Guangzhou, China) to detect S-phase cells. Detailed steps were consistent with our previously published report (Li et al., 2015). EdU-labeled cells, which were red, were viewed under the EVOS FL Auto.
Immunocytochemical assay of neuronal markers
Cells in different groups were examined according to previously reported experimental steps (Zhao et al., 2011). Briefly, cells were incubated with primary antibodies including mouse anti-Tuj1 (1:500; Abcam, Cambridge, UK), guinea pig anti-doublecortin (DCX; 1:1000; Millipore, Billerica, MA, USA), and rabbit anti-microtubule-associated protein 2 (MAP2; 1:400; Abcam) overnight at 4ºC. Cells were incubated with corresponding secondary antibodies (Alexa Fluor568-conjugated goat anti-mouse, goat anti-guinea pig, or goat anti-rabbit IgG, 1:1000; Invitrogen) at room temperature for 4 hours. Nuclei were labeled with Hoechst 33342 (1:1000; Sigma, St. Louis, MO, USA) at 37ºC for 30 minutes. All cells were examined under the EVOS FL Auto.
Fluorescence-activated cell sorting (FACS) assay
BD Cycletest™ Plus DNA Reagent Kit (BD Biosciences, Franklin Lakes, NJ, USA) and an Annexin V-PE Apoptosis Detection Kit (Beyotime, Shanghai, China) were used for FACS sorting on a Calibur instrument (BD Biosciences). Detailed steps were consistent with our previously published report (Li et al., 2015).
Enzyme-linked immunosorbent assay (ELISA)
A total of 200 µL of culture medium from each group was collected at 24, 48, 72, and 96 hours. ACh release in medium was detected with an ACh ELISA Kit (Yanyu, Shanghai, China) using a Synergy 2 microplate reader (BioTek, Winooski, VT, USA).
Western blot analysis
Total protein of PC12 cells in each group was extracted with a protein extraction kit (Beyotime). Protein concentrations were detected with a bicinchoninic assay kit (Thermo Scientific). Forty micrograms of protein were loaded and then separated on sodium dodecyl sulfate–polyacrylamide gels (10%). Next, target proteins were electronically transferred to polyvinylidene difluoride membranes using semi-dry transfer at 25 V for 7 minutes. Subsequently, membranes were blocked with 5% skim milk powder at room temperature for 2 hours, followed by primary antibodies [rabbit polyclonal anti-ChAT (1:800; Abcam) and mouse monoclonal anti-β-actin (1:2000; Beyotime)] at 4°C overnight. Secondary horseradish peroxidase-conjugated anti-rabbit IgG (1:1000; Bioss, Woburn, MA, USA) and anti-mouse IgG (1:1000; Bioss) were used to detect related proteins for 2 hours at room temperature. Finally, Enhanced Chemiluminescence-Plus reagent (Bio-Rad, Hercules, CA, USA) and Software Quantity One (Bio-Rad) were used to visualize immunoblots, and relative protein expression was calculated with β-actin as the internal reference.
Statistical analysis
All experimental data were collected from at least three independent experiments. SPSS 22.0 software (IBM, Armonk, NY, USA) was used to analyze experimental data by one-way analysis of variance followed by independent sample t-test. Statistical data are expressed as the mean ± standard error of the mean (SEM). P < 0.05 was considered statistically significant.
Results
Efficiency of transfection and GAS5 expression level detection
We first analyzed differences between normal PC12 cells and PC12 cells overexpressing Lhx8 by DE-ribosomal RNA sequence and qRT-PCR. The results revealed increased expression of the lncRNA GAS5 in PC12 cells after overexpression of Lhx8 compared with the normal group (Figure 1A & B). To study the function of GAS5 in PC12 cells, both GAS5-overexpressing lentivirus and negative control lentivirus were transfected into PC12 cells. After lentivirus transfection, GFP was detected in both LV-GAS5 and LV-NC groups (Figure 1C). First, we confirmed that the trans-fection efficiency was approximately 95% (Figure 1D). Next, relative expression of GAS5 in each group was detected using qRT-PCR. The results displayed low GAS5 expression in normal PC12 cells, and no difference in GAS5 expression between control and LV-NC groups (P > 0.05). However, mRNA levels of GAS5 were obviously increased after GAS5 lentivirus transfection (P < 0.001; Figure 1E).
Figure 1.
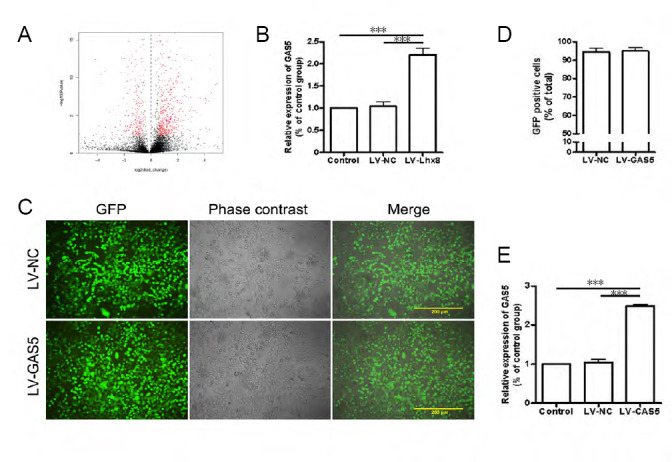
Level of growth arrest-specific 5 (GAS5) expression.
(A, B) We used a deribosomal RNA sequence (A) and real-time polymerase chain reaction (B) to detect differences in GAS5 expression between Lhx8-overexpressing and normal PC12 cells. Results showed that the expression of long non-coding RNA (lncRNA) GAS5 increased after overexpression of Lhx8 compared with negative control lentivirus (LV-NC) and control groups. (C) GAS5 lentivirus and negative lentivirus were transfected into PC12 cells. Green fluorescent protein (GFP) was found in both lncRNA GAS5-overexpressing lentivirus (LV-GAS5) and LV-NC groups. Bars: 200 μm. (D) Transfection efficiencies were approximately 95% in the two groups. (E) mRNA levels of GAS5 were obviously increased after GAS5 transfection. Data are expressed as the mean ± SEM. ***P < 0.001 (one-way analysis of variance followed by independent sample t-test). The experiment was repeated three times.
Effect of GAS5 on cell proliferation and neuronal differentiation
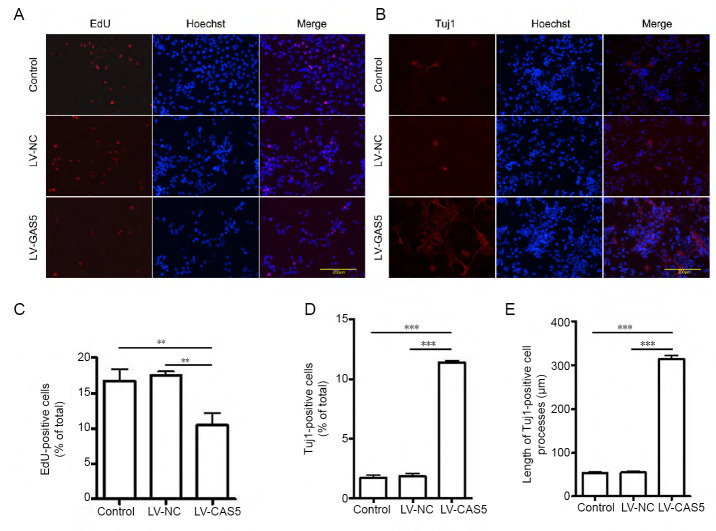
We examined the proliferative ability of cells in different groups by EdU (Figure 2A). EdU-positive cell ratios in the LV-GAS5 group were apparently lower than in the other two groups (P < 0.01; Figure 2C). Next, we examined expression of three neuronal markers (Tuj1, DCX, and MAP2) to detect the differentiation of PC12 cells; cells were only positive for Tuj1 (Figure 2B), while the other two markers were immunofluorescent negative. Percentages of Tuj1-positive cells in the LV-GAS5 group were higher than in control and LV-NC groups (P < 0.001; Figure 2D). Moreover, average lengths of Tuj1-positive cell processes in the LV-GAS5 group were markedly increased compared with either LV-NC or control groups (P < 0.001; Figure 2E). Differences in percentages of Tuj1-positive cells and average lengths of Tuj1-positive cell processes between control and LV-NC groups were not statistically significant (P > 0.05). Thus, we concluded that GAS5 can suppress the proliferation of PC12 cells and promote their differentiation into Tuj1-positive neuron-like cells with longer cell processes.
Figure 2.
Effect of growth arrest-specific 5 (GAS5) on cell proliferation and differentiation.
(A, C) 5-Ethynyl-2′-deoxyuridine (EdU) immunofluorescence labeling was used to detect cell proliferation. The lncRNA GAS5-overexpressing lentivirus (LV-GAS5) group had a lower ratio of EdU-positive cells compared with the other two groups. (B) Tuj1 immunopositivity (red, stained by Alexa Fluor568) in different groups. Bars: 200 μm. (D) The ratio of Tuj1-positive cells in the LV-GAS5 group was higher than in control and negative control lentivirus (LV-NC) groups. (E) Average lengths of Tuj1-positive cell processes in the LV-GAS5 group were markedly longer than observed in either LV-NC or control groups. Data are expressed as the mean ± SEM. **P < 0.01, ***P < 0.001 (one-way analysis of variance followed by independent sample t-test). The experiment was repeated three times.
Effect of GAS5 on cell cycle and apoptosis
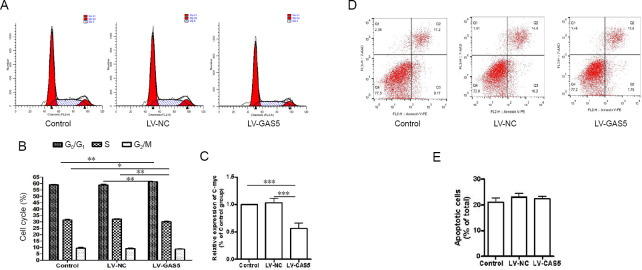
We used flow cytometry to assess the effects of GAS5 on cell cycle and cell apoptosis. A FACS assay revealed that more cells remained in G0/G1 phase following the increase of GAS5. Percentages of cells in G0/G1 phase in the LV-GAS5 group were obviously higher than in LV-NC or control groups (P < 0.01). Ratios of cells in S phase in the LV-GAS5 group were clearly less than in LV-NC or control groups (P < 0.01 or P < 0.05). For G2/M phase, notably, although the ratio of cells in the LV-GAS5 group was less than observed in the other two groups, there were no differences among the three groups (P > 0.05; Figure 3A & B). We further examined the C-myc gene, which can promote cell division (Li et al. 2015). Compared with the other two groups, relative expression of C-myc in the LV-GAS5 group was significantly reduced (P < 0.001; Figure 3C).
Figure 3.
Effect of growth arrest-specific 5 (GAS5) on cell cycle and apoptosis.
Detection of cell cycle progression and cell apoptosis using flow cytometry. (A, B) In the cell cycle assay, DNA histograms show propidium iodide fluorescence in different groups. (A) Proportion of cells at G0/G1 phase in the lncRNA GAS5-overexpressing lentivirus (LV-GAS5) group was obviously increased compared with the other two groups. In contrast, the ratio of cells in S phase in the LV-GAS5 group was clearly reduced compared with negative control lentivirus (LV-NC) and control groups. For G2/M phase, there were no differences among the three groups (B). (C) The C-myc gene can promote cell division; real-time polymerase chain reaction assay showed that relative levels of C-myc in the LV-GAS5 group were decreased compared with the other two groups. (D) Cell apoptosis was detected by flow cytometry analysis. (E) Apoptotic cell ratio (Annexin V single-positive cells vs. Annexin V/PI double-positive cells); there were no differences among groups. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way analysis of variance followed by independent sample t-test). The experiment was repeated three times.
Annexin V-positive cells were detected to assess apoptosis by flow cytometry (Figure 3D). Interestingly, there were no differences in ratios of Annexin V-positive cells among the three groups (P > 0.05; Figure 3E). These results showed that GAS5 had a role in PC12 cell cycle arrest, but had no influence on apoptosis.
Levels of ChAT and ACh in cells after GAS5 transfection
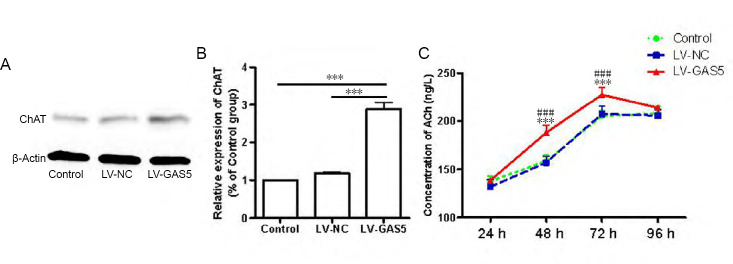
To explore whether upregulation of GAS5 could affect the expression of ChAT and ACh release levels in PC12 cells, western blot and ELISA were performed. The results indicated ChAT protein expression levels in the LV-GAS5 group were 2.88 ± 0.31 times and 2.45 ± 0.35 times that of control and LV-NC groups (P < 0.001), whereas there were no differences between control and LV-NC groups (P > 0.05; Figure 4A & B). ACh levels in the LV-GAS5 group were higher than the other two groups at 48 hours (P < 0.001) and 72 hours (P < 0.01). Notably, there were no differences in ACh levels among the three groups at 24 or 96 hours (P > 0.05; Figure 4C).
Figure 4.
Effect of growth arrest-specific 5 (GAS5) on levels of choline acetyltransferase (ChAT) and acetylcholine (ACh) in cells.
(A, B) ChAT protein content of the lncRNA GAS5-overexpressing lentivirus (LV-GAS5) group was significantly increased compared with other groups. (C) The LV-GAS5 group had increased ACh levels at 48 and 72 hours (h) compared with other groups, whereas, there were no differences among groups at 24 and 96 hours. Data are expressed as the mean ± SEM. ***P < 0.001, vs. control group; ###P < 0.001, vs. negative control lentivirus (LV-NC) group (one-way analysis of variance followed by independent sample t-test). The experiment was repeated three times.
Discussion
GAS5, which consists of 12 exons, only contains a short open reading frame. It is localized at 1q25.1 and seems to play different roles in different studies, which may be related to disease types, cell types, and culture environments. For example, many studies have shown that malignant tumor cell lines are often accompanied by reduced GAS5 expression, especially in advanced disease models. In addition, high expression of GAS5 inhibits proliferation and promotes apoptosis (Mourtada-Maarabouni et al., 2009; Romanuik et al., 2010; Gee et al., 2011; Liu et al., 2013; Lu et al., 2013; Qiao et al., 2013; Zhang et al., 2013a, b; Cao et al., 2014; Isin et al., 2014; Renganathan et al., 2014; Tu et al., 2014; Dong et al., 2015; Glover et al., 2015); therefore, GAS5 is an anti-oncogene. However, in the case of multiple sclerosis, GAS5 seems to play a negative role, as microglia M2 polarization was inhibited and demyelination was worsened as a result of increased GAS5 expression (Sun et al., 2014). Interestingly, in our study, GAS5 inhibited the proliferation but not apoptosis of PC12 cells, which may be PC12 specific. At the same time, cells in the LV-GAS5 group showed increased proportions of cells in G0/G1 phase and lower proportions of S or G2/M phase cells. Therefore, it is likely that inhibition of PC12 cell proliferation by GAS5 was related to cell cycle arrest, but not apoptosis in vitro. C-myc is a regulatory gene related to cell proliferation and cell cycle; the decline of C-myc expression leads to delayed transition from G1 to S (Hölscher, 2014; Liu et al., 2014). In this study, C-myc levels were significantly decreased following GAS5 overexpression. As it was previously reported that GAS5 and the eukaryotic translation initiation factor 4E coordinated control C-myc translation (Hu et al., 2014), we speculated that GAS5 induced the growth arrest of PC12 cells potentially through the reverse regulation of C-myc.
Cell cycle arrest and neurogenesis are highly coordinated and interactive processes. Rozental et al. (2000) studied the temporal expression of neuronal connexins during hippocampal ontogeny, and found that expression of GAS5 was involved in the regulation of neuronal differentiation. Moreover, GAS5 could make mouse embryonic hippocampal neuronal progenitor (MK31) cells quit the proliferation cycle and differentiate into neurons. Hence, we used Tuj1, DCX, and MAP2 to detect the differentiation of PC12 cells. Immunofluorescence results only revealed positivity for Tuj1, and the number of Tuj1-positive neuron-like cells was significantly increased after overexpression of GAS5. GAS7, another member of the GAS family, is expressed in neurons of the hippocampus, cerebellum, and cerebral cortex, where it seems to actively regulate neurite outgrowth (Ju et al., 1998; Rozental et al., 2000). In our study, we also found that the average lengths of Tuj1-positive cell processes in the GAS5 overexpression group were significantly increased compared with the other two groups. Therefore, we believe that GAS5 can promote the differentiation of PC12 cells into Tuj1-positive neuron-like cells, while promoting the growth of cell processes. It must be noted that Tuj1 positivity does not necessarily mean those neuron-like cells are functional neurons. As such, we next need to measure their electrophysiological properties as a direct characterization of whether or not a cell is a neuron.
It is known that cell cycle arrest can stimulate the transition of cells to a so-called senescent state; moreover, aging is another risk factor for AD (Wei et al., 2016). Senescenceassociated secretory phenotypes, which arise from senescence-associated growth arrest, have different effects on adaptive mechanisms of the brain at different stages of disease progression (Nekrasov and Vorobyov, 2018). A primary limitation of this study is that it is only a simple in vitro cell study, which cannot fully recapitulate the complex body environment. Indeed, astrocytes, microglia, cytokines interleukin-1α and interleukin-1β, and the senescence-associated marker p16 can all affect neurons. How to develop these factors in a better direction is worth further exploring.
As Lhx8 has a critical and specific effect on the development and sustenance of cholinergic neurons (Shibaguchi et al., 2003; Zhao et al., 2003; Mori et al., 2004; Manabe et al., 2007, 2008), we further compared the expression of ChAT in PC12 cells and the amount of ACh released. We found that the expression level of ChAT protein in LV-GAS5 group was significantly increased compared with control and LV-NC groups. In addition, ACh release levels in the LV-GAS5 group were increased compared with other groups at 48 and 72 hours, whereas there were no differences at 24 and 96 hours between the three groups. These results indicate that GAS5 can promote cholinergic systems similar to Lhx8.
GAS5 is a 5′-terminal oligopyrimidine tract RNA (Smith and Steitz, 1998) whose expression is negatively regulated by the mechanistic target of rapamycin (mTOR) pathway (Meyuhas, 2000; Mourtada-Maarabouni et al., 2010; Williams et al., 2011). That is, when the mTOR pathway is activated, GAS5 is low; but when the mTOR pathway is inhibited, GAS5 is high. In an animal model of AD, rapamycin, an mTOR pathway inhibitor, improved learning and memory, and reduced amyloid β and Tau pathology (Caccamo et al., 2010). Vellai et al. (2003) found that decreasing the activity of the mTOR protein complex mTORC1 could significantly prolong the longevity and function of Caenorhabditis elegans. In mouse hematopoietic stem cells, mTORC1 activity was increased in an age-dependent manner and when mTORC1 was activated, expression of p16 was increased. However, use of rapamycin can maintain hematopoietic stem cells at a level similar to that of young animals (Chen et al., 2009). Based on the above experimental results and previous literature, we speculate that the mTOR signaling pathway may play a crucial role in regulation of GAS5 by Lhx8 in PC12 cells, and GAS5 may be beneficial for AD patients by eliciting an anti-aging effect; although, further studies are necessary to verify these claims.
In summary, we found that increasing GAS5 promoted PC12 cells to differentiate into Tuj1-positive neuron-like cells with longer processes. In addition, cell proliferation and cell cycle were significantly suppressed by GAS5, whereas it had no effect on apoptosis of PC12 cells. In addition, GAS5 could also promote the expression of ChAT and ACh release, similar to Lhx8. Thus, we speculate that GAS5 may be beneficial to the recovery of neurons and the cholinergic nervous system in AD patients.
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81501133 (to HML); Postgraduate Research & Practice Innovation Program of Jiangsu Province of China, No. KYCX17-1931 (to HYZ); Undergraduate Innovation and Entrepreneurship Training Project of Nantong University of China, No. 2018150 (to STZ); Pre-research Project of Natural Science Foundation of Nantong University of China, No. 17ZY19 (to HH); Scientific Research Fund Project of Nantong University Xinglin College of China, No. 2018K131 (to HYZ); and Nantong Science and Technology Project of China, No. JC2018064 (to HYZ). The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81501133 (to HML); Postgraduate Research & Practice Innovation Program of Jiangsu Province of China, No. KYCX17-1931 (to HYZ); Undergraduate Innovation and Entrepreneurship Training Project of Nantong University of China, No. 2018150 (to STZ); Pre-research Project of Natural Science Foundation of Nantong University of China, No. 17ZY19 (to HH); Scientific Research Fund Project of Nantong University Xinglin College of China, No. 2018K131 (to HYZ); and Nantong Science and Technology Project of China, No. JC2018064 (to HYZ).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Van Deusen A, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao S, Liu W, Li F, Zhao W, Qin C. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int J Clin Exp Pathol. 2014;7:6776–6783. [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong S, Qu X, Li W, Zhong X, Li P, Yang S, Chen X, Shao M, Zhang L. The long non-coding RNA, GAS5, enhances ge-fitinib-induced cell death in innate EGFR tyrosine kinase inhibitor-resistant lung adenocarcinoma cells with wide-type EGFR via downregulation of the IGF-1R expression. J Hematol Oncol. 2015;8:43. doi: 10.1186/s13045-015-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: targeting the cholinergic system. Curr Neuro-pharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Claver A, Lorente M, Mur P, Campos-Martín Y, Mollejo M, Velasco G, Meléndez B. Gene expression changes associated with erlotinib response in glioma cell lines. Eur J Cancer. 2013;49:1641–1653. doi: 10.1016/j.ejca.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Gee HE, Buffa FM, Camps C, Ramachandran A, Leek R, Taylor M, Patil M, Sheldon H, Betts G, Homer J, West C, Ragoussis J, Harris AL. The small-nucleolar RNAs commonly used for microRNA normalisation correlate with tumour pathology and prognosis. Br J Cancer. 2011;104:1168–1177. doi: 10.1038/sj.bjc.6606076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glover AR, Zhao JT, Ip JC, Lee JC, Robinson BG, Gill AJ, Soon PS, Sidhu SB. Long noncoding RNA profiles of adrenocortical cancer can be used to predict recurrence. Endocr Relat Cancer. 2015;22:99–109. doi: 10.1530/ERC-14-0457. [DOI] [PubMed] [Google Scholar]
- 10.Hölscher C. Drugs developed for treatment of diabetes show protective effects in Alzheimer’s and Parkinson’s diseases. Sheng Li Xue Bao. 2014;66:497–510. [PubMed] [Google Scholar]
- 11.Hu G, Lou Z, Gupta M. The long non-coding RNA GAS5 cooperates with the eukaryotic translation initiation factor 4E to regulate c-Myc translation. PLoS One. 2014;9:e107016. doi: 10.1371/journal.pone.0107016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isin M, Ozgur E, Cetin G, Erten N, Aktan M, Gezer U, Dalay N. Investigation of circulating lncRNAs in B-cell neoplasms. Clin Chim Acta. 2014;431:255–259. doi: 10.1016/j.cca.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Jin Y, Tsuchiya A, Kanno T, Nishizaki T. Amyloid-beta peptide increases cell surface localization of alpha7 ACh receptor to protect neurons from amyloid beta-induced damage. Biochem Biophys Res Commun. 2015;468:157–160. doi: 10.1016/j.bbrc.2015.10.141. [DOI] [PubMed] [Google Scholar]
- 14.Ju YT, Chang AC, She BR, Tsaur ML, Hwang HM, Chao CC, Cohen SN, Lin-Chao S. gas7: A gene expressed preferentially in growth-arrested fibroblasts and terminally differentiated Purkinje neurons affects neurite formation. Proc Natl Acad Sci U S A. 1998;95:11423–11428. doi: 10.1073/pnas.95.19.11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193:651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Qin J, Jin G, Zou L, Shi J, Han X, Cheng X, Zhang X. Overexpression of Lhx8 inhibits cell proliferation and induces cell cycle arrest in PC12 cell line. In Vitro Cell Dev Biol Anim. 2015;51:329–335. doi: 10.1007/s11626-014-9838-y. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Jin G, Zhu P, Zou L, Shi J, Yi X, Zhang X, Tian M, Qin J. Upregulation of Lhx8 increase VAChT expression and ACh release in neuronal cell line SHSY5Y. Neurosci Lett. 2014;559:184–188. doi: 10.1016/j.neulet.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Liu N, Zang S, Liu H, Wang P, Ji C, Sun X. Tumor suppressor DYRK1A effects on proliferation and chemoresistance of AML cells by downregulating c-Myc. PLoS One. 2014;9:e98853. doi: 10.1371/journal.pone.0098853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Wang W, Jiang J, Bao E, Xu D, Zeng Y, Tao L, Qiu J. Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PLoS One. 2013;8:e73991. doi: 10.1371/journal.pone.0073991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu X, Fang Y, Wang Z, Xie J, Zhan Q, Deng X, Chen H, Jin J, Peng C, Li H, Shen B. Downregulation of gas5 increases pancreatic cancer cell proliferation by regulating CDK6. Cell Tissue Res. 2013;354:891–896. doi: 10.1007/s00441-013-1711-x. [DOI] [PubMed] [Google Scholar]
- 22.Manabe T, Tatsumi K, Inoue M, Matsuyoshi H, Makinodan M, Yokoyama S, Wanaka A. L3/Lhx8 is involved in the determination of cholinergic or GABAergic cell fate. J Neurochem. 2005;94:723–730. doi: 10.1111/j.1471-4159.2005.03261.x. [DOI] [PubMed] [Google Scholar]
- 23.Manabe T, Tatsumi K, Inoue M, Makinodan M, Yamauchi T, Makinodan E, Yokoyama S, Sakumura R, Wanaka A. L3/Lhx8 is a pivotal factor for cholinergic differentiation of murine embryonic stem cells. Cell Death Differ. 2007;14:1080–1085. doi: 10.1038/sj.cdd.4402106. [DOI] [PubMed] [Google Scholar]
- 24.Manabe T, Tatsumi K, Inoue M, Matsuyoshi H, Makinodan M, Yamauchi T, Makinodan E, Yokoyama S, Sakumura R, Okuda H, Wanaka A. Knockdown of the L3/Lhx8 gene suppresses cholinergic differentiation of murine embryonic stem cell-derived spheres. Int J Dev Neurosci. 2008;26:249–252. doi: 10.1016/j.ijdevneu.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Mattick JS. RNA regulation: a new genetics? Nat Rev Genet. 2004;5:316–323. doi: 10.1038/nrg1321. [DOI] [PubMed] [Google Scholar]
- 26.Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem. 2000;267:6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- 27.Mori T, Yuxing Z, Takaki H, Takeuchi M, Iseki K, Hagino S, Kitanaka J, Takemura M, Misawa H, Ikawa M, Okabe M, Wanaka A. The LIM homeobox gene, L3/Lhx8, is necessary for proper development of basal forebrain cholinergic neurons. Eur J Neurosci. 2004;19:3129–3141. doi: 10.1111/j.0953-816X.2004.03415.x. [DOI] [PubMed] [Google Scholar]
- 28.Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet. 2014;15:423–437. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mourtada-Maarabouni M, Hasan AM, Farzaneh F, Williams GT. Inhibition of human T-cell proliferation by mammalian target of rapamycin (mTOR) antagonists requires noncoding RNA growth-arrest-specific transcript 5 (GAS5) Mol Pharmacol. 2010;78:19–28. doi: 10.1124/mol.110.064055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mourtada-Maarabouni M, Hedge VL, Kirkham L, Farzaneh F, Williams GT. Growth arrest in human T-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (GAS5) J Cell Sci. 2008;121:939–946. doi: 10.1242/jcs.024646. [DOI] [PubMed] [Google Scholar]
- 31.Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 32.Nekrasov PV, Vorobyov VV. Dopaminergic mediation in the brain aging and neurodegenerative diseases: a role of senescent cells. Neural Regen Res. 2018;13:649–650. doi: 10.4103/1673-5374.230290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan B, Shi ZJ, Yan JY, Li JH, Feng SQ. Long non-coding RNA NONMMUG014387 promotes Schwann cell proliferation after peripheral nerve injury. Neural Regen Res. 2017;12:2084–2091. doi: 10.4103/1673-5374.221168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pongrac JL, Rylett RJ. NGF-induction of the expression of ChAT mRNA in PC12 cells and primary cultures of embryonic rat basal forebrain. Brain Res Mol Brain Res. 1998;62:25–34. doi: 10.1016/s0169-328x(98)00215-0. [DOI] [PubMed] [Google Scholar]
- 36.Qiao HP, Gao WS, Huo JX, Yang ZS. Long non-coding RNA GAS5 functions as a tumor suppressor in renal cell carcinoma. Asian Pac J Cancer Prev. 2013;14:1077–1082. doi: 10.7314/apjcp.2013.14.2.1077. [DOI] [PubMed] [Google Scholar]
- 37.Renganathan A, Kresoja-Rakic J, Echeverry N, Ziltener G, Vrugt B, Opitz I, Stahel RA, Felley-Bosco E. GAS5 long non-coding RNA in malignant pleural mesothelioma. Mol Cancer. 2014;13:119. doi: 10.1186/1476-4598-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roghani A, Carroll PT. Analysis of uptake and release of newly synthesized acetylcholine in PC12 cells overexpressing the rat vesicular acetylcholine transporter (VAChT) Brain Res Mol Brain Res. 2002;100:21–30. doi: 10.1016/s0169-328x(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 39.Romanuik TL, Wang G, Morozova O, Delaney A, Marra MA, Sadar MD. LNCaP Atlas: gene expression associated with in vivo progression to castration-recurrent prostate cancer. BMC Med Genomics. 2010;3:43. doi: 10.1186/1755-8794-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rozental R, Srinivas M, Gokhan S, Urban M, Dermietzel R, Kessler JA, Spray DC, Mehler MF. Temporal expression of neuronal connexins during hippocampal ontogeny. Brain Res Brain Res Rev. 2000;32:57–71. doi: 10.1016/s0165-0173(99)00096-x. [DOI] [PubMed] [Google Scholar]
- 41.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 42.Shi J, Li H, Jin G, Zhu P, Tian M, Qin J, Tan X, Zhao S, Wang F, Hua Y, Xiao Y. Lhx8 promote differentiation of hippocampal neural stem/progenitor cells into cholinergic neurons in vitro. In Vitro Cell Dev Biol Anim. 2012;48:603–609. doi: 10.1007/s11626-012-9562-4. [DOI] [PubMed] [Google Scholar]
- 43.Shibaguchi T, Kato J, Abe M, Tamamura Y, Tabata MJ, Liu JG, Iwamoto M, Wakisaka S, Wanaka A, Kurisu K. Expression and role of Lhx8 in murine tooth development. Arch Histol Cytol. 2003;66:95–108. doi: 10.1679/aohc.66.95. [DOI] [PubMed] [Google Scholar]
- 44.Smith CM, Steitz JA. Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5′-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Mol Cell Biol. 1998;18:6897–6909. doi: 10.1128/mcb.18.12.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun D, Yu Z, Fang X, Liu M, Pu Y, Shao Q, Wang D, Zhao X, Huang A, Xiang Z, Zhao C, Franklin RJ, Cao L, He C. LncRNA GAS5 inhibits microglial M2 polarization and exacerbates demyelination. EMBO Rep. 2017;18:1801–1816. doi: 10.15252/embr.201643668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun M, Jin FY, Xia R, Kong R, Li JH, Xu TP, Liu YW, Zhang EB, Liu XH, De W. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. doi: 10.1186/1471-2407-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tu ZQ, Li RJ, Mei JZ, Li XH. Down-regulation of long non-coding RNA GAS5 is associated with the prognosis of hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:4303–4309. [PMC free article] [PubMed] [Google Scholar]
- 48.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 49.Volpato D, Holzgrabe U. Designing hybrids targeting the cholinergic system by modulating the muscarinic and nicotinic receptors: a concept to treat Alzheimer’s disease. Molecules. 2018;23:E3230. doi: 10.3390/molecules23123230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Wei Z, Chen XC, Song Y, Pan XD, Dai XM, Zhang J, Cui XL, Wu XL, Zhu YG. Amyloid beta protein aggravates neuronal senescence and cognitive deficits in 5XFAD mouse model of Alzheimer’s disease. Chin Med J (Engl) 2016;129:1835–1844. doi: 10.4103/0366-6999.186646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams GT, Mourtada-Maarabouni M, Farzaneh F. A critical role for non-coding RNA GAS5 in growth arrest and rapamycin inhibition in human T-lymphocytes. Biochem Soc Trans. 2011;39:482–486. doi: 10.1042/BST0390482. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, An S, Hu W, Teng M, Wang X, Qu Y, Liu Y, Yuan Y, Wang D. The neuroprotective properties of hericium erinaceus in glutamate-damaged differentiated PC12 cells and an Alzheimer’s disease mouse model. Int J Mol Sci. 2016;17:E1810. doi: 10.3390/ijms17111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang XQ, Sun S, Lam KF, Kiang KM, Pu JK, Ho AS, Lui WM, Fung CF, Wong TS, Leung GK. A long non-coding RNA signa-ture in glioblastoma multiforme predicts survival. Neurobiol Dis. 2013a;58:123–131. doi: 10.1016/j.nbd.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z, Zhu Z, Watabe K, Zhang X, Bai C, Xu M, Wu F, Mo YY. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013b;20:1558–1568. doi: 10.1038/cdd.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao H, Jin G, Tian M, Li H, Zhang X. Extract of deafferented hippocampus promotes in vitro radial glial cell differentiation into neurons. Neurosci Lett. 2011;498:93–98. doi: 10.1016/j.neulet.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y, Marín O, Hermesz E, Powell A, Flames N, Palkovits M, Rubenstein JL, Westphal H. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci U S A. 2003;100:9005–9010. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu P, Li H, Jin G, Tian M, Tan X, Shi J, Zou L, Qin J. LIM-homeobox gene Lhx8 promote the differentiation of hippocampal newborn neurons into cholinergic neurons in vitro. In Vitro Cell Dev Biol Anim. 2013;49:103–107. doi: 10.1007/s11626-013-9582-8. [DOI] [PubMed] [Google Scholar]