Abstract
Given that cardiovascular (CV) safety concerns remain the leading cause of drug attrition at the preclinical drug development stage, the National Center for Toxicological Research (NCTR) of the US Food and Drug Administration (FDA) hosted a workshop to discuss current gaps and challenges in translating preclinical CV safety data to humans. This white paper summarizes the topics presented by speakers from academia, industry, and government intended to address the theme of improving cardiotoxicity assessment in drug development. The main conclusion is that in order to reduce CV safety liabilities of new therapeutic agents, there is an urgent need to integrate human-relevant platforms/approaches into drug development. Potential regulatory applications of human-derived cardiomyocytes and future directions in employing human-relevant platforms to fill the gaps and overcome barriers and challenges in preclinical CV safety assessment were discussed. This paper is intended to serve as an initial step in a public-private collaborative development program for human-relevant cardiotoxicity tools, particularly for cardiotoxicities characterized by contractile dysfunction or structural injury.
Keywords: Cardiotoxicity, cardiomyocyte, risk assessment, human, stem cell, human platforms, drug development
Subject Terms: Basic Science Research, Stem Cells, Translational Studies
Executive Summary
CV safety concerns continue to be the most common reason for drug termination during preclinical drug development. These liabilities are most often identified in animal studies and represent putative risks of significant or irreversible CV harm. However, such preclinical risks are often not confirmed in human patients because many of these drugs are terminated prior to clinical testing. Poor translational specificity of preclinical animal models and our recognized shortcomings in understanding comparative pathophysiology suggest that we may be terminating beneficial medicines for liabilities in animals that would not be liabilities in patients. Additionally, animal studies have low throughput and are conducted late in preclinical development, which eliminates the opportunity to leverage the power of molecular design to avoid liabilities that are truly human relevant.
There is a clear need to change our approach on preclinical CV safety assessment so that we can increase the specificity of assessments performed at higher throughput to optimize our ability to design safer and more effective drugs. Human-relevant platforms, including human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) and adult human primary cardiomyocytes (CMs), are increasingly used in pre-clinical in vitro cardiac toxicity screening. The use of these new in vitro preclinical models has been accepted for investigational new drug (IND) applications and has great potential to reveal mechanisms of drug-drug interactions (DDIs) and personalize cardiac safety predictions. We believe that current pharmaceutical CV safety assessment would benefit from an approach that may prove to be more efficient in cost and time, mechanistically informative, and translatable to human patients. Such an approach may enable the earlier recognition of development-limiting safety liabilities, reduction of false positives that lead to premature and unnecessary development termination, discovery of more relevant biomarkers, and a significant decrease of late-stage attrition.
Introduction
A working group of cardiovascular scientists from academia, industry, and government assembled at the FDA/NCTR in Jefferson, Arkansas to identify gaps and challenges in translating current approaches of preclinical CV safety assessment to human patients. Technical applications and opportunities in using human-relevant platforms to improve cardiotoxicity detection and characterization were presented and the path forward was discussed. It should be emphasized that the goal of the workshop was not to seek a consensus on new approaches or platforms, but to help inform the planning process for a consortium effort to shift the CV safety pharmacology and toxicity test battery toward human-relevant platforms that are more mechanism-based and hypothesis-driven. The goal of the workshop was aligned with the mission of FDA and the NCTR strategic plan.
Background
Drug development is a long and expensive process with far more failures than successes. The aim of the process is to advance novel drug candidates with maximum benefits and minimal or manageable safety risks. The safety assessment component of the process tends to be relatively risk-averse to protect patients from unintended harm. According to the Pharmaceutical Research and Manufacturers of America (PhRMA) 2016 Biopharmaceutical Research Industry Profile,1 the average time to develop a drug is 10–15 years with an average cost of $2.6 billion in 2000s to early 2010s. Fewer than 12% of drugs that entered clinical trials have ultimately been approved. In analyses of reasons for attrition during drug discovery and development, clinical safety concerns and non-clinical toxicity are major contributors.2 The current paradigm for preclinical safety assessment and clinical safety prediction relies heavily on animal studies founded on the belief that animals most closely approximate the biological complexity of human patients. However, species differences are increasingly being recognized as potentially leading to false positive and false negative predictions of patient responses and risk liabilities. A recent analysis conducted by a cross-pharma working group concluded that preclinical animal in vivo studies generally have a high negative predictive value (NPV)3 (i.e., if organ-specific toxicity did not occur in the animal studies, it would likely not occur in human clinical trials), which is supportive of an approach built on conservative risk/liability aversion. The potential for low specificity in animal safety studies and high rates of false positives3 (i.e., low positive predictive value (PPV)), particularly for CV liabilities, has been less explored and may be adding to the crippling rate of safety-related attrition. Human-based in vitro methods (e.g., human cells, stem cells, and organ-on-chips) and computational models have been proposed to replace animal testing, at least partially, in preclinical drug development.4
Gaps and Challenges to Translating Current Preclinical CV Safety Data Approaches to Humans
Gaps:
Drugs can intentionally or unintentionally target many of the cellular components of the CV system as well as signaling receptors, ion channels, and fundamental cellular processes to produce cardiotoxic liabilities. These toxicities can manifest variously as functional and/or structural perturbations of the heart muscle, valves, or systemic vasculature. The integrated CV system is difficult to model effectively in reductionist systems, so animal studies are crucial to the current preclinical CV safety assessment. Traditional approaches to drug safety assessment include acute (single dose) and chronic (multiple-dose) studies of increasing durations as preclinical development progresses. At least 2 animal species are used (one rodent and one non-rodent) to assess both functional and structural liabilities. Single-dose, short-duration studies are performed using chronically instrumented animals in a Latin square design can assess drug-induced changes in CV function (e.g., arterial blood pressure (BP), heart rate (HR), electrophysiology (ECG), and cardiac contractility). Repeat-dose general toxicity studies are more enriched for morphologic, hematologic, and biochemical endpoints to evaluate a full spectrum of potential target organs. The primary purpose of preclinical safety studies is to identify potential human hazards and establish a safe starting dose for human clinical trials. The aim to identify potential hazards dictates that doses used in the animal preclinical studies generally far exceed those intended in the clinic.
Despite their general relevance to humans, preclinical animal studies have a number of potential drawbacks. Laboratory animals do not faithfully represent general human biology in every respect, to say nothing of individual variability in disease susceptibilities, co-morbidities, and polypharmacy. Additional potential issues include:
-
▪
Studies are unblinded
-
▪
Results are observational
-
▪
There is no hypothesis testing and no type-I error control
-
▪
Large doses cause clinically irrelevant effects
-
▪
Phenotypic toxicology studies provide limited mechanistic insights.
Consequently, it is not surprising that the manifestation of some toxicities in patients differ significantly from those demonstrated in traditional animal studies. A typical example of the difference in sensitivity to CV toxicity is the dog’s predilection for vascular injury induced by vasodilators and positive inotropic agents that are not always recapitulated in humans.5 Another example is rat-specific cardiomyopathy.6 Animals are not good model systems for safety assessment for drug classes that have high specificity for human-specific targets, such as recombinant proteins, monoclonal antibodies, oligonucleotides, and siRNAs.
False-positive findings in animals may be more common than we understand. Unfortunately, there is no database on the rate of false positives and their impact on drug development. Recently, the IQ Consortium analyzed a blinded database of 182 molecules and animal toxicology data coupled with clinical observations from phase I human studies, finding that the PPVs (the proportion of positive nonclinical findings that had positive clinical findings) of CV toxicity in rodents, dogs, and non-human primates were only 17%, 50%, and 20%, respectively.7 Another cross-company database for the concordance between conscious dog telemetry data and phase I clinical CV toxicity assessment found that within the 10–30x exposure range, the unadjusted PPV ranges were only 10–13% for HR and 6–8% for diastolic BP, although the NPV was high.3 The loss of opportunities in testing these false-positive drugs in human patients may present an even bigger problem not including drug candidates that were dropped from development prior to being tested in humans in the two databases.
Challenges:
Clearly, there are significant limitations for preclinical animal studies to be used in CV safety assessment. A better preclinical assay would ideally include the following attributes: 1) higher throughput; 2) sample size adequate to make reliable inferences; 3) blinded/unbiased assessment of objective endpoints; 4) formal hypothesis testing to assess false-positive/negative findings; 5) mechanistic insight leveraging toxicopathological failure modes; and 6) human-relevant biology so that findings are more likely to translate to the clinic. However, the creation of a new paradigm in shifting our testing from phenotypic to mechanistic would face challenges including the following:
Biology − Transition from normal to abnormal is generally non-binary. Thresholds of biological perturbation that represent “toxicity” are difficult to define precisely and not generally well understood mechanistically. Contextualizing those perturbations in a myriad of possible individual susceptibilities is even more difficult.
Math − Traditional approaches to preclinical safety assessment identify putative risks, characterize their adversity, and determine dose/exposure margins from predicted clinical exposures. Quantitatively determining the window between no-observed-adverse-effect level (NOAEL) and clinical peak serum concentration (Cmax) is challenging.
Complex pathogeneses – Pathogenesis of toxicity can be complex. Multiple injuries can happen in the CV system coincidentally, and we need to determine whether there is a pathogenic connection among these changes, whether these changes can be replicated in humans, and whether the toxicities are manageable in patients.
Human element – There is a process of building credibility and confidence for novel assays and models that needs to be accepted by regulatory decision-makers.
Opportunities and Technology Applications of Human-Relevant Platforms
Given the limitations of preclinical animal studies in translating preclinical safety assessment in the clinical setting, human-based models have been introduced in drug discovery, screening, and toxicity testing. The Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative is an example of how human-relevant platforms are being used for drug-induced proarrhythmia detection.4, 8
The CiPA initiative:
QTc interval prolongation and Torsade de Pointes (TdPs) were the single most common cause of withdrawal or restriction on marketed drugs in the 1990s, which led to the international regulatory guidance of ICH S7B and ICH E14 calling for careful assessment of drug effects on human ether-a-go-go-related (hERG) or IKr channel and the QTc-corrected interval in preclinical and clinical studies. This approach was taken in response to the known links between drug-induced reductions in the hERG-related current and increases in the QTc interval and the development of TdPs. Instead of focusing on the actual proarrhythmic risk, ICH S7B and E14 focus on surrogates, which can identify drugs with the potential to induce TdPs but whose PPV is low. A number of drugs inhibit hERG or prolong the QTc interval (e.g., verapamil, ranolazine, and phenobarbital) but are not associated with TdPs, because they inhibit other cardiac currents that are necessary for TdPs development (i.e., the late inward sodium current and the L-type calcium current). Consequences of developing a compound with a hERG or QTc effect include significantly increased development burden, delays in filing and/or regulatory approval, label warnings (that may not be necessary) with competitive implications, and licensing/partnering challenges. Consequently, many drugs are terminated during discovery because of preclinical findings of a hERG or QTc effect, despite their potential to have a net favorable benefit. It has been estimated that the discontinuation rate of preclinical programs due to these issues is ~60%.9 In fact, the finding of a QTc signal during clinical development often leads to the discontinuation of a drug’s development altogether.
In the last 14 years since ICH S7B and E14 were ratified, a deeper understanding of the mechanisms responsible for TdPs and the role of early afterdepolarizations in the genesis of the arrhythmia has evolved. This has led to a new paradigm – CiPA.4 This approach focuses on assessing a drug’s mechanistic proclivity to be proarrhythmic and cause TdPs. In this approach, drugs that inhibit hERG or result in QTc prolongation due to drug-induced ion channel effects, but are otherwise safe from a proarrhythmic perspective (due to inhibiting the late inward sodium current or the L-type calcium current), will be identified and labeled accordingly. Briefly, CiPA has 4 major components:
Drug effects on multiple individual human cardiac ion channels in heterologous expression systems are assessed with commercial high-throughput voltage clamp systems. This effort focuses on creating uniform ion channel testing protocols that best represent the actual ion channel properties in humans.
The data obtained from ion channel studies are integrated into an in silico human ventricular myocyte computational model to assess proarrhythmic liabilities; this is the primary determinant of proarrhythmic potential under CiPA.
The ECGs in a Phase 1 study, including T-wave analysis, are assessed to determine if there are unexpected electrocardiographic effects based on the preclinical data in humans (e.g., due to a human specific metabolite or different pharmacokinetics).
Human stem cell-derived cardiomyocytes will be used for unanticipated nonclinical effects, or if human ECG data are insufficient.
CiPA is a new in vitro paradigm for cardiac safety evaluation of drugs that is expected to provide a more accurate and comprehensive mechanistic-based assessment of proarrhythmic potential. This should improve candidate selection decisions, enhance efficiency of drug development, and permit drugs to be efficiently developed that are safe despite hERG and/or QTc effects, ultimately providing product labeling that more accurately reflects the actual risks.
Moving Beyond CiPA
As part of the commitment to Replacing, Reducing, and/or Refining animal studies (the “3Rs”), CiPA has established a good example of using human-based technologies to enable more robust nonclinical proarrhythmia screening of new drug candidates and reduce the use of animals for torsadogenic risk assessment. Besides proarrhythmia assessment, other aspects of cardiac safety may also be addressed through human materials in the early drug screening process, including the uncoupling in excitation-contraction (E-C), interference with force generation, interference with mitochondrial energetics, and direct cytotoxicity (Figure 1). A few examples of how the human-relevant materials and platforms may improve our understanding of mechanisms of direct cardiotoxicity and drug-induced cardiotoxicity detection (Figure 1) were presented and discussed at the workshop.
Figure 1.
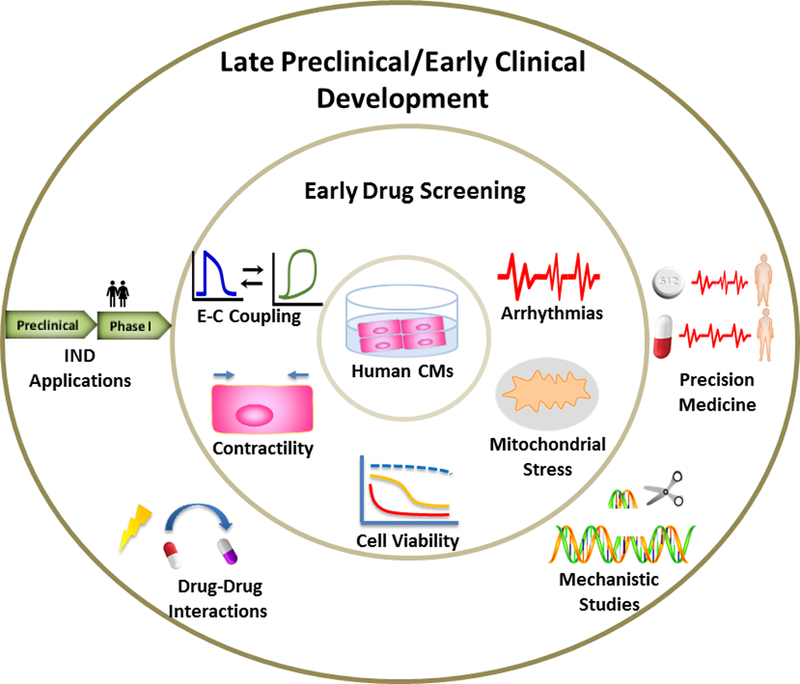
Phenotypic changes that can be assessed with human cardiomyocytes in early drug screening and applications of human-relevant platforms in late preclinical and early clinical drug development process.
hiPSC-CMs Recapitulate Side Effects Not Revealed in Preclinical Animal Studies
Activators of Sphingosine 1‑Phosphate (S1P) receptor have been used to treat multiple sclerosis. One of the major side effects of Gilenya® (fingolimod), an S1P1 and S1P3 agonist,10, 11 is bradycardia. As the study with S1P3 knock-out mice suggested that S1P3 is a major contributor to the HR effect of Gilenya,12 BAF-312 and CS-0777, the selective S1P1 agonists, were developed. In preclinical species, the selective S1P1 agonists showed no significant HR effects. However, clinically significant bradycardia was still observed in humans, suggesting that the distributions of S1P receptors in preclinical species and humans are different. S1P3 is dominant in hearts of preclinical species, while S1P1 plays a major role in modulating HR in humans.13, 14 To develop a S1P1-selective agonist with minimal HR effects in humans, hiPSC-CMs and multielectrode array (MEA) technologies were applied to assess the beat rate (BR) effects of S1P agonists.15, 16 S1P1 and S1P3 exhibited a similar RNA expression in hiPSC-CMs as in adult human heart tissues in QPCR assay. The hiPSC-CM MEA assay recapitulated the slow HR effect of Gilenya, BAF-312, and CS-0777 at clinically relevant concentrations, and had the same rank order in BR reduction potencies found in different agents as in human bradycardia. A mechanistic study using pharmacological tool agents of the G protein-gated inward-rectifying potassium channel (IKACh) inhibitor, Gi blocker, S1P1, and muscarinic receptor antagonists in hiPSC-CMs indicated that the slow HR effect of S1P1 is caused by activation of IKACh through the S1P1-coupled Gi protein. The slow BR effects of selective S1P1 agonists were more substantial in hiPSC-CMs than in rat embryonic CMs, consistent with more significant bradycardia seen in humans. These data were used for internal risk assessment and IND filling.
The Use of hiPSC-CMs In vitro Assays to Reveal Unexpected Cardiac Drug-Drug Interaction in Clinics
Assessment of any potential ECG and dysrhythmic effects is an important part of the preclinical development process for all new pharmaceutical agents. While tachyarrhythmic ECG events including supraventricular proarrhythmias (i.e., atrial fibrillation) and ventricular proarrhythmias (i.e., TdPs) have always been major concerns, recent significant bradyarrhythmias resulting from drug-drug interactions (DDIs) pose a difficult safety problem.17, 18 In 2015, the European Medicines Agency (EMA) and U.S. FDA issued warning letters in response to 9 reported, post-marketing clinical cases of severe and life-threatening symptomatic bradycardias/bradyarrhythmias in Hepatitis C-Virus (HCV)-infected patients treated with a HCV-NS5B pronucleotide inhibitor (HCV-NS5B-NI) antiviral agent, sofosbuvir (SOF), in combination with other direct-acting antivirals (DAAs) and/or the class-III antiarrhythmic drug: amiodarone (AMIO). 19, 20 Investigations were initially conducted in anesthetized guinea-pigs and conscious, chair-restrained telemetered nonhuman primate models, in which the serious, clinical SOF+AMIO cardiac DDIs were replicated convincingly to provide hypotheses for potential mechanism(s) of action.21 However, since AMIO presents a very prolonged half-life in animals as well as in patients (average t1/2=58 days) and an uncertain elimination (t1/2=36 days for its active metabolite – desethylamiodarone), the selective cardiac electrophysiological effects were examined in a hiPSC-CM functional assay capable of providing a rapid assessment of potential cellular/molecular mechanism(s) and identification of cell types, receptors, and/or ion channels responsible for the clinical DDIs observed with SOF+AMIO. In the preliminary in vitro experiments, the impact of six HCV-NS5B-NI prodrug phosphoramidate diastereochemistry (D-/L-alanine, Rp-/Sp-phosphoryl) molecules (SOF, MNI-1, MNI-2, MNI-3, MNI-4 and MK-3682 or uprifosbuvir) was evaluated in hiPSC-CMs, as well as their effects when co-applied with AMIO. Interestingly, L-ala, Sp prodrugs (SOF, MNI-1, MNI-3) increased the BR or field potential (FP) rate and decreased the beat amplitude or impedance (IMP) in hiPSC-CMs, but D-ala, Rp prodrugs, including MNI-2, MNI-4 and MK-3682 (uprifosbuvir), did not have the same effects. Furthermore, as shown on Figure 2, this stereochemical selectivity on emerging bradycardia was confirmed in vivo.22 Because the analogous effect was not observed with MK-3682, a different prodrug NS5B nucleotide polymerase inhibitor, this suggests that it is not a class effect. In addition, the combination of SOF+AMIO in hiPSC-CMs mirrored the phenotypic effects of Ca2+ channel blockers (CCBs) in this assay, but surprisingly, replacing AMIO with CCBs did not induce the cardiac DDI. Moreover, dronedarone (an AMIO analog) did not substitute for AMIO, but rather competed with AMIO in the cardiac DDI. Ryanodine and thapsigargin (depleting the intracellular Ca2+ stores), and SEA-400 (a Na+/Ca2+ exchanger NCX1 inhibitor) partially alienated or diminished the DDI effects. Comparing with the respective individual effects, other FP rate-affecting compounds either showed no effects or only exerted additive or subtractive effects. The data in hiPSC-CMs indicated that the DDI between AMIO and HCV-NS5B NI agents such as SOF and MN1 is attributed to their potential interaction(s) with key intracellular Ca2+-handling mechanisms. Finally, to identify whether the cardiac DDI phenotype/signature between HCV-NS5B pronucleotide inhibitors and AMIO in hiPSC-CMs was caused exclusively by AMIO’s chemical structure features (Structure Activity Relationship: SAR) or from interaction with other chemical entities, 20 AMIO’s chemical analogs and 72 chemical entities (marketed drugs or reference pharmacological agents) were synthesized, and their effects on FP and IMP in hiPSC-CMs were evaluated. In vitro SAR studies demonstrated the required “high specificity” of AMIO chemical structure features to induce this type of cardiac DDI when combined with HCV-NS5B NI agents. These data support the utility of hiPSC-CMs as a potential comprehensive yet scalable tool, which can be used to identify and examine the unexpected, cardioactive pharmacodynamic DDIs for new chemical entities.
Figure 2.
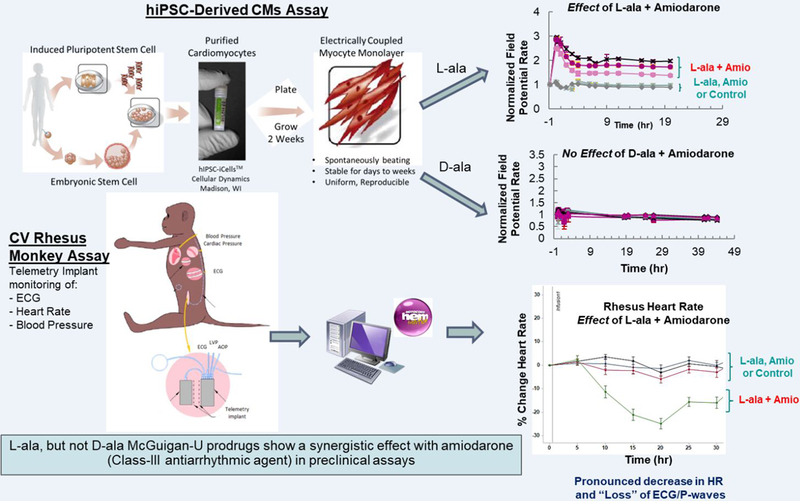
hiPSC-CMs assay can replicate the HCV-NS5B NI/Amiodarone cardiac DDIs observed in clinic and preclinical models, provide a competitive advantage for early screening/de-risking of new HCV-NI candidates, and rapidly assess the putative mechanism(s) responsible of DDIs.
hiPSC-CMs in Discovery Toxicology: Balancing Promise and Translational Value
The technological co-evolution of hiPSC-CMs and platforms to address their functional output has recently enabled in vitro CV safety testing in phenotypically-relevant cellular models. Concomitant technical advances such as incorporation of the underlying measurement technology (e.g., MEA and impedance electrodes) into standard format multi-well plates, and automated processing thereof, have increased the throughput of these assays to a level that is compatible with discovery-phase chemistry counter screening. The pilot and validation phases of CiPA myocyte studies demonstrate that occurrence of in vitro arrhythmias and prolongation of field potential duration (FPD) corrected for BR can have high translational value for clinical TdPs.23, 24 The relatively large size of the CiPA compound sets has also provided a convenient means for confirming low well-to-well, plate-to-plate, and site-to-site variability for these translational cardiac beat metrics.
A noteworthy difference exists, however, in the origin of human cardiomyocyte beating in vitro vs. in vivo. In the intact human heart, spontaneous beating is maintained by pacing at anatomical nodes, and transmitted through the cardiac conduction system. Drug or endocrine action at these nodes is thus a primary driver of clinical chronotropic effects. In contrast, growing evidence suggests spontaneous beating in syncytial hiPSC-CMs is mainly determined by chronological ectopic expression of HCN4, a hyperpolarization-activated cyclic nucleotide-gated ion channel representing the molecular correlate of the pacemaker (If) current.25 This may lead to a complicated mechanistic interpretation of drug effects. For example, drug-induced cessation of spontaneous beating has historically been scored as an arrhythmia in hiPSC-CM assays such as those used in the Japan iPS Cardiac Safety Assessment study26, despite the ectopic cell-intrinsic role of If in maintenance of beating. Similarly it is not uncommon for changes in BR to be used as a primary safety metric, without exclusion of If modulation as a confounding factor.27–30 While If modulation would most directly confound drug effects on BR, a corollary concern is that FPD values in hiPSC-CM assays are almost universally “corrected” for the rate of this ectopic beating using clinically derived formulas.
In summary, while hiPSC-CMs have generated justifiable excitement as an in vitro CV safety screening tool, the following concerns should be considered when experimental outcomes are decisional: 1) cessation of spontaneous beating during drug application has a particularly dubious translational value, as it is unknown whether cardiac beating would continue when driven by an anatomical node; 2) for chronotropic effects, potentially confounding pharmacological targets (e.g. HCN4 and M2 muscarinic G-protein-coupled receptors) should be addressed, if not ruled out, when results are decisional; and 3) whenever possible, spontaneous BR should be obviated using platforms capable of electrical or optogenetic pacing.23, 31, 32 Such extrinsic pacing methods may also recover data for other metrics (e.g., FPD and presence of arrhythmias) at drug concentrations in which spontaneous beating is completely eliminated.
Stem Cells and Genomics for Precision Cardiovascular Medicine
Emerging big data technologies and advances in genetics and genomics are providing an unprecedented opportunity for precision medicine to treat patients using a more tailored approach based on their individual genetic backgrounds. However, there remains a significant gap between patients’ genotypes and phenotypes (e.g., disease and drug response). Patient-specific hiPSC-CMs hold great potential for studying genotype-phenotype associations in patients with various CV diseases, if these models can sufficiently recapitulate the requisite biological function.
The concept of in vitro disease modeling with hiPSC-CMs is well established for inherited cardiomyopathies (e.g., hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM)) and inherited cardiac channelopathies (e.g., long QT syndrome (LQTS) and Brugada syndrome) through many studies showing their ability to recapitulate human disease phenotypes in a dish (e.g., abnormal contractile properties and arrhythmia).33 Moreover, hiPSC-CMs can serve as a novel platform for in vitro cardiotoxicity testing. For example, inter-individual variation in susceptibility to doxorubicin-induced cardiotoxicity was reproduced in vitro using patient-specific hiPSC-CMs.34 In another study, cardiac toxicities of 21 FDA-approved tyrosine kinase inhibitors were screened and tested systematically in a high-throughput fashion using hiPSC-CMs.35 hiPSC-CMs were also employed to investigate mechanisms of off-target cardiotoxic effects induced by trastuzumab, by targeting the altered pathways, to evaluate it as a potential therapeutic approach in protecting CMs from the off-target toxic effects.36 In addition, advances in genome editing tools now make it possible to precisely link genetic variations to human diseases. Recently, the pathogenicity of variants of uncertain significance in heart disease-related genes (HCM37 and LQTS38) has been determined for the first time by combining genome-editing and hiPSC technologies.
Furthermore, the aforementioned findings support the emerging “clinical trial in a dish” concept, in which hiPSC-CMs from a large patient cohort can serve as a new robust platform for preclinical drug testing. Further development of this concept may enable us to identify druggable targets, predict drug response, and facilitate individualized therapeutic interventions. Ultimately, the precise information generated through hiPSC-CMs platform is expected to lead to more efficient and effective clinical trials and thereby improve patient outcomes.
Metabolic Maturation of hiPSC-CMs
hiPSC-CMs are gaining recognition for in vitro cardiac toxicity screening of new drug candidates and hold great promise for precision medicine. However, the immaturity and embryonic-/fetal-like nature of hiPSC-CMs can be a potential problem for drug-induced cardiotoxicity prediction that truly reflects the toxicity in adult human hearts. To maximize the benefits of hiPSC-CMs in disease modeling and drug discovery, various techniques have been employed to improve hiPSC-CM maturation both functionally and structurally. Long-term culture,39 enhanced expression of KCNJ2/IK1,40 chronic electrical stimulation,41 stretch or mechanical loading,42 developmental and epigenetic cues,43 micropatterning,44 and 3D cultures of hiPSC-CMs as engineered heart tissues45 are among the advances that have been reported to improve hiPSC-CM maturation in terms of electrophysiological property, metabolic signature, contractile function, and structure modeling.33
One of the most difficult challenges in maturing hiPSC-CMs is to promote energy metabolism shift from anaerobic glycolysis to fatty acid beta oxidation.46 This is because hiPSC-CMs produced in current protocols show a relatively low mitochondrial density and are predominantly glycolytic, whereas adult human CMs typically depend on fatty acids, rather than glucose, as the main energy substrate. It is well known that cells that mainly rely on mitochondrial oxidative phosphorylation (OXPHOS) for energy production are more susceptible to mitochondrial insults than glycolysis-dependent cells.47, 48 To overcome these limitations, hiPSC-CMs were exposed to a fatty acid-based maturation medium to mimic the usage of metabolic substrates in adult human CMs. The preliminary data indicate that compared to cells grown in normal maintenance media, hiPSC-CMs cultured in maturation media had increased mitochondrial membrane potential, mitochondrial mass, and oxygen consumption as they are more reliant on fatty acids for energy generation. As expected, mitochondrial function assay and cytotoxicity assay demonstrated that maturation medium-cultured hiPSC-CMs were more sensitive to mitotoxicants than cells maintained in normal maintenance media. CMs have a high energy demand for contraction and thus can be particularly susceptible to agents that impair mitochondrial function. The fatty acid-based maturation media’s ability to improve hiPSC-CM maturation may be a better approach for modeling the responses of adult human CMs to cardiotoxicants with mitochondrial liabilities.49
Adult Human Primary Cardiomyocyte Model for the Simultaneous Prediction of Drug-Induced Inotropic and Pro-Arrhythmia Risk
Although some academic laboratories have used adult human primary CMs to conduct cardiac research,50–53 the production of CMs has never been scaled for drug discovery purposes. Methods enabling standardized procurement protocols and utilization of high-quality viable human heart samples from organ donors have recently been established, and cardiac safety studies aiming at validating a pro-arrhythmia model based on action potential recordings in ventricular human trabeculae have been published.54, 55 While this approach provides data highly predictive of clinical cardiac risk, the limited throughput of tissue-based assays prevents their use in early stages of preclinical development. This prompted the development and validation of an adult human primary cardiomyocyte model, amenable to high-throughput screening and reliant on non-invasive measurements of contraction using bright-field imaging.56 By selecting endpoints measured from the contractility transients, both pro-arrhythmia as well as inotropic risks can be identified. Validation results involving 33 clinically well-characterized positive and negative controls, including 23 torsadogenic and 10 non-torsadogenic drugs, were presented at the workshop. When drug effects were evaluated at 10-fold of the free effective therapeutic plasma concentration (fETPC), the detection of drug-induced aftercontractions led to risk assessment with excellent sensitivity and specificity of 96 and 100%, respectively. This high predictivity supports the translational safety potential of the adult human primary cardiomyocyte preparation and the predictivity of the selected endpoint. Additionally, by monitoring the amplitude of sarcomere shortening, both multi-ion channel blockers associated with negative inotropic risk56 as well as positive inotropes can be readily identified. Furthermore, a detailed analysis of the parameters describing the contractility transient allows for the identification of the different mechanisms of action in drugs with inotropic activity.57 In addition to the study of normal adult myocytes, adult primary CMs are now available from diseased donors, and it is now possible to assess how cardiac toxicity risk may be affected by common comorbidities. These data provide evidence that adult human primary CMs can be a valuable model for the reliable assessment of human cardiac safety.
Future Directions
Integrated Cardiotoxicity Prediction with Animal Studies and Human-Relevant Platforms
CiPA represents a paradigm shift for drug safety evaluation, by shifting the focus from a single ion channel activity to an integrated risk assessment of proarrhythmia based on multiple ion channels; it is also innovative by reducing the reliance on animal models and focusing on human-relevant cellular electrophysiology. The studies presented at this workshop and other emerging evidence strongly suggest that the integration of human cardiomyocyte-based in vitro assays in the drug development process may fill the gaps in current CV safety assessment; this may be particularly relevant for improving cardiotoxicity prediction, especially for arrhythmias (including non-TdP arrhythmia58) and for contractile and structural cardiotoxicity assessment.59 However, the current human-relevant cellular platforms have limitations related to the intrinsic inability to model and predict adverse hemodynamic effects and other off-target effects in non-cardiac tissue. Therefore, the new technologies are not expected to completely eliminate the reliance on animal models for CV safety and toxicity assessment. Moreover, animal studies can provide valuable information on pharmacokinetics of therapeutics. The development of human-based organ-on-chips and microphysiological systems may offer additional opportunities in assessing drug-induced off-target effects in the vasculature60 and other non-cardiac tissues,61 and in advancing modeling-informed quantitative clinical pharmacology evaluation.62
hiPSC-CMs have been widely used in drug-induced cardiotoxicity screening and offer several practical advantages: 1) iPSC culture is scalable and the sustainable production of large numbers CMs enables drug screening campaigns for industry applications;33 2) hiPSC-CMs carry patient-specific genetic information and their production requires minimally invasive procedures, which allow clinicians to link patients’ clinical/genetic information with in vitro pharmacological responses and guide the selection of a personalized therapeutic regime;63 3) disease-specific hiPSC-CMs models have the potential to provide platforms for investigating disease mechanisms and evaluating novel therapeutics; 33, 36 4) hiPSC-CMs derived from a representative pool of the general population may enable patient stratification for clinical testing34, 64 and may be employed to further assess the risk of cardiotoxicities if adverse events are reported in post-marketing surveillance; and 5) hiPSC technology may be utilized in human organ-on-chips and microphysiological systems to evaluate DDIs and off-target effects.
As highlighted at the workshop, important limitations related to the biology of hiPSC-CMs cannot be overlooked and, undoubtedly, additional work needs to be done to fully understand the predictive value of this system in the context of preclinical cardiac safety risk assessment. The issues related to metabolic, structural, and functional maturation remain incompletely resolved, and any one of these factors could result in inconsistent predictive value across diverse classes of drug candidates.
Isolated adult human primary CMs provide a fully mature system that is physiologically and pharmacologically relevant to the assessment of cardiac safety risk. Several platforms originally developed for the study of hiPSC-CMs can also be used to measure drug effects in adult human primary CMs, thereby enabling sufficient throughput to employ the adult primary CMs in early- to mid-stage discovery programs.65, 66 The use of optical-based methods to measure contractility, intracellular calcium dynamics, membrane potential fluctuations, and mitochondrial function offers great opportunities to also employ adult human primary CMs for mechanistic investigations in a system that has all the native physiological components, avoiding the potential pitfalls of artificially engineered cells. While major improvements in scaling adult primary CM isolation have been accomplished in recent years,56, 67–69 additional work needs to be performed to further expand the availability of these cells. It will also be interesting to merge efforts in large-scale primary CM isolation with advances in biomaterials and substrate technologies, so that better platforms can be developed to support the long-term culture of primary CMs under continuous electrical stimulation.70 These methods could greatly facilitate the study of chronic cardiac toxicities.
Understanding the advantages and limitations of adult human CMs and hiPSC-CMs (Table 1) and combining the appropriate in vitro assays with animal studies will lead to a better prediction of CV liabilities of new therapeutics.
Table 1:
Comparison of Adult Human CMs and hiPSC-CMs for Drug-Induced Cardiotoxicity In Vitro Assay
| Adult Human CMs | hiPSC-CMs | |
|---|---|---|
| Resemble of human biology | ||
| Structure | Rod-shaped; Sarcomere highly organized; Immature: Round or polygonal; Sarcomere disorganized; T-tubules well developed33 | Immature: Round or polygonal; Sarcomere disorganized; No T-tubes33 |
| Electrophysiology | Adult and electrical-pacing-induced contraction; Physiological ion channel density; Matured Ca2+ handling | Immature: Spontaneous beating; Some ion channels are under- or over-expressed; Immature Ca2+ handling33 |
| Contractility | Significant force; 33 Positive force-frequency relationship71 | Immature: reduced force;33 Positive force-frequency relationship can be generated in some 2D/3D cultures72, 73 |
| Mitochondrial | Occupy 20-40% of cell volume; Throughout cell; Mainly rely on fatty acids33 | Immature: Low numbers; Perinuclear; Primarily employ glycolysis33 |
| CM Quality & Consistency | Single cell with known chamber information; | Single cell assay or in 2D/3D cultures; A mix population of CMs, possible for chamber-specific categorization;33, 74 3D cultures may enable evaluate the propagation of pacemaker signals75 |
| Reproducibility can be achieved by implementation of standardized procurement protocols and novel isolation methods54, 56, 67 | Ensure reproducibility is critical as many issues in reprogramming, differentiation, and maintenance are not certain33 | |
| Scalability | Possible to scale up with the improvement in preservation of primary CMs56 and development of immortalized CMs68 | Easy scale-up iPSC culture, CMs can be generated in large scales33 |
| Duration of exposure | Short (up to 30 minutes)56 and long (up to few days)70 for both acute and prolonged exposure to drugs | Long-term culture (months) is feasible,39, 74 for both acute and chronic treatment |
| Ease of access to technology | Medium-throughput industrial platforms are available65, 66, 76 | Multiple industrial platforms are commercially available for medium- and high-throughput assays33, 76 |
| Autologous to “single patient” testing | No | Yes, minimally invasive |
| Disease Model | Hearts can be procured from patients with end- stage heart diseases; CMs can be isolated to study cardiac cellular pathophysiology and develop potential new treatments67 | Many monogenetic disease models have been generated with patient-specific hiPSC-CMs and genome editing technology33; It is possible to develop disease models of polygenetic disease74 |
| Population-based testing | Possible56, 77 | Yes64,78 |
| Development of MPS & human Organ-on-chips | In development79 | Yes72, 79 |
From a regulatory standpoint, in vitro CMs assays may be used at multiple stages of drug development59, including: 1) lead candidate selection and optimization at the early drug discovery phase; 2) assessment of cardiotoxic potential before the initiation of clinical trial, especially for first-in-human (FIH) compounds when they are of a drug class with known safety concern; 3) mechanistic assessment (e.g., mitochondrial stress, lipid metabolism, cell viability, gene expression changes, cellular biomarker release, and other cellular morphological measurements) when post-marketing monitoring indicates safety concerns or DDIs; 4) waiver of human echocardiogram monitoring if a negative profile for cardiac liability was demonstrated with qualified tests; and 5) use in discovery of noninvasive biomarkers to provide earlier detection of cardiotoxic risk before irreversible injury has occurred. Integrated cardiotoxicity prediction with animal studies and human-relevant platforms may represent a new paradigm of drug development to fill the gaps and unmet needs of the current strategy and to improve drug-induced CV liability prediction.
Summary
The take home messages from this workshop are: (i) human-relevant models can assess both structural and functional abnormalities of CMs and may avoid false-positives due to animal-specific findings; (ii) hiPSC-CMs show the potential to identify delayed responses, reveal patient-specific susceptibilities, and detect/predict some off-target effects; (iii) adult human primary CMs are a physiological system with the full complement of ion channels, receptors and contractile machinery that are critical for proper cardiac function and could be considered the gold standard for cell-based cardiac toxicity studies; and (iv) to reduce CV safety liabilities of new therapeutic agents, there is an urgent need to integrate human-relevant platforms/approaches into drug development.
For a new nonclinical method to be accepted for use in CV safety assessment, there must be a systematic evaluation of the ability of the new technology to predict adverse clinical outcomes. This will involve a continuous dialogue and feedback among all relevant stakeholders, and careful qualification studies must be conducted to test the assays’ precision, selectivity, sensitivity, reliability, reproducibility, and applicability. To this end, NCTR sponsored the workshop as an initiative step in building a consortium to emphasize the importance and feasibility of integrating human-relevant platforms into the drug development process. Further collaborations among participants from academia, government, and industry are discussed and will be organized by HESI Cardiac Research Safety Committee (CSRC).
Acknowledgements
The authors would like to thank Dr. Andre Ghetti and Mr. Blake Wu for reviewing and editing the manuscript.
Nonstandard Abbreviations and Acronyms:
- CMs
cardiomyocytes
- IND
investigational new drug
- DDIs
drug-drug interactions
- PhRMA
Pharmaceutical Research and Manufacturers of America
- NPV
negative predictive value
- PPV
positive predictive value
- TdPs
Torsade de Pointes
- hERG
human ether-a-go-go-related gene
- fETPC
free effective therapeutic plasma concentration
- FIH
first-in-human
Footnotes
Disclaimer & Disclosures
The views expressed in this paper are those of the authors and do not necessarily reflect the opinions of their companies/institutions or the official policy of the FDA. No official support or endorsement by the FDA is intended or should be inferred.
Philip T. Sager has a stock ownership in LQT Therapeutics (which holds patents to treat the Long QT syndrome) and AnaBios. He is or has been a consultant to Charles River, AnaBios, MedPace, ERT, BioTelemetry, AbbVie, and is a founder of LQT Therapeutics. Hong Shi is an employee of BMS, Frederick Sannajust was an employee of MERCK & Co., Mathew Brock was an employee of Genentech Inc., and Najah Abi-Gerges is an employee of AnaBios Corporation. Joseph C. Wu is a cofounder of Khloris Biosciences but has no competing interests, as the work presented here is completely independent.
Contributor Information
Li Pang, Division of Systems Biology, National Center for Toxicological Research, U.S. Food and Drug Administration..
Philip T Sager, Stanford University School of Medicine..
Xi Yang, Division of Cardiovascular and Renal Products, Center for Drug Evaluation and Research, U.S. Food and Drug Administration..
Hong Shi, Discovery Toxicology, Bristol-Myers Squibb (BMS) Company..
Frederick Sannajust, Safety & Exploratory Pharmacology Department, SALAR Division, Merck & Co..
Mathew Brock, Investigative Toxicology, Genentech Inc. Current address: MyoKardia, Incorporation.
Joseph C. Wu, Stanford University School of Medicine, Stanford Cardiovascular Institute
Najah Abi-Gerges, AnaBios Corporation..
Beverly Lyn-Cook, Division of Biochemical Toxicology, National Center for Toxicological Research, U.S. Food and Drug Administration..
Brian R. Berridge, Division of the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health
Norman Stockbridge, Division of Cardiovascular and Renal Products, Center for Drug Evaluation and Research, U.S. Food and Drug Administration..
Reference:
- 1.PhRMA. Phrma 2016 biopharmaceutical research industry profile. 2016 [Google Scholar]
- 2.Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, Pairaudeau G, Pennie WD, Pickett SD, Wang J, Wallace O, Weir A An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nature reviews. Drug discovery. 2015;14:475–486. [DOI] [PubMed] [Google Scholar]
- 3.Ewart L, Aylott M, Deurinck M, et al. The concordance between nonclinical and phase i clinical cardiovascular assessment from a cross-company data sharing initiative. Toxicol Sci. 2014;142:427–435. [DOI] [PubMed] [Google Scholar]
- 4.Sager PT, Gintant G, Turner JR, Pettit S, Stockbridge N Rechanneling the cardiac proarrhythmia safety paradigm: A meeting report from the cardiac safety research consortium. Am Heart J. 2014;167:292–300. [DOI] [PubMed] [Google Scholar]
- 5.Dogterom P, Zbinden G, Reznik GK Cardiotoxicity of vasodilators and positive inotropic/vasodilating drugs in dogs: An overview. Crit Rev Toxicol. 1992;22:203–241. [DOI] [PubMed] [Google Scholar]
- 6.Faqi AS A comprehensive guide to toxicology in nonclinical drug development Elsevier Inc, 2 edition; 2016. [Google Scholar]
- 7.Monticello TM, Jones TW, Dambach DM, Potter DM, Bolt MW, Liu M, Keller DA, Hart TK, Kadambi VJ Current nonclinical testing paradigm enables safe entry to first-in-human clinical trials: The iq consortium nonclinical to clinical translational database. Toxicol Appl Pharmacol. 2017;334:100–109. [DOI] [PubMed] [Google Scholar]
- 8.Colatsky T, Fermini B, Gintant G, Pierson JB, Sager P, Sekino Y, Strauss DG, Stockbridge N The comprehensive in vitro proarrhythmia assay (CiPA) initiative - update on progress. J Pharmacol Toxicol Methods. 2016;81:15–20. [DOI] [PubMed] [Google Scholar]
- 9.De Ponti F Pharmacological and regulatory aspects of QT prolongation In: Vaz RJ, Klabunde T, eds Antitargets: prediction and prevention of drug side effects. Weinheim, Germany: Wiley-VCH; 2008:55–88. [Google Scholar]
- 10.FDA drug safety communication: Safety review of a reported death after the first dose of multiple sclerosis drug Gilenya (fingolimod). 2011 [Google Scholar]
- 11.FDA drug safety communication: Revised recommendations for cardiovascular monitoring and use of multiple sclerosis drug Gilenya (fingolimod), May 14, 2012. [Google Scholar]
- 12.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H Sphingosine 1-phosphate (s1p) receptor subtypes s1p1 and s1p3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. [DOI] [PubMed] [Google Scholar]
- 13.Moberly JB, Ford DM, Zahir H, Chen S, Mochizuki T, Truitt KE, Vollmer TL Pharmacological effects of cs-0777, a selective sphingosine 1-phosphate receptor-1 modulator: Results from a 12-week, open-label pilot study in multiple sclerosis patients. J Neuroimmunol. 2012;246:100–107. [DOI] [PubMed] [Google Scholar]
- 14.Gergely P, Nuesslein-Hildesheim B, Guerini D, et al. The selective sphingosine 1-phosphate receptor modulator baf312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol. 2012;167:1035–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcoux D, Xiao HY, Murali Dhar TG, et al. Identification of potent tricyclic prodrug s1p1 receptor modulators. Medchemcomm. 2017;8:725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhar TG, Xiao HY, Xie J, et al. Identification and preclinical pharmacology of bms-986104: A differentiated s1p1 receptor modulator in clinical trials. ACS Med Chem Lett. 2016;7:283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Back DJ, Burger DM Interaction between amiodarone and sofosbuvir-based treatment for hepatitis C virus infection: Potential mechanisms and lessons to be learned. Gastroenterology. 2015;149:1315–1317. [DOI] [PubMed] [Google Scholar]
- 18.Renet S, Chaumais MC, Antonini T, Zhao A, Thomas L, Savoure A, Samuel D, Duclos-Vallee JC, Algalarrondo V Extreme bradycardia after first doses of sofosbuvir and daclatasvir in patients receiving amiodarone: 2 cases including a rechallenge. Gastroenterology. 2015;149:1378–1380 e1371. [DOI] [PubMed] [Google Scholar]
- 19.European medicines agency (EMA): EMA recommends avoidance of certain hepatitis C medicines and amiodarone together. 2015:4–24. [Google Scholar]
- 20.Gilead sciences, inc.: Gilead – Solvadi warning letter. 8–20-0015 [Google Scholar]
- 21.Regan CP, Morissette P, Regan HK, Travis JJ, Gerenser P, Wen J, Fitzgerald K, Gruver S, DeGeorge JJ, Sannajust FJ Assessment of the clinical cardiac drug-drug interaction associated with the combination of hepatitis C virus nucleotide inhibitors and amiodarone in guinea pigs and rhesus monkeys. Hepatology. 2016;64:1430–1441. [DOI] [PubMed] [Google Scholar]
- 22.Lagrutta A, Zeng H, Imredy J, Balasubramanian B, Dech S, Lis E, Wang J, Zhai J, DeGeorge J, Sannajust F Interaction between amiodarone and hepatitis-c virus nucleotide inhibitors in human induced pluripotent stem cell-derived cardiomyocytes and HEK-293 cav1.2 over-expressing cells. Toxicol Appl Pharmacol. 2016;308:66–76. [DOI] [PubMed] [Google Scholar]
- 23.Millard D, Dang Q, Shi H, et al. Cross-site reliability of human induced pluripotent stem cell-derived cardiomyocyte based safety assays using microelectrode arrays: Results from a blinded CiPA pilot study. Toxicol Sci. 2018;164:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blinova K, Dang Q, Millard D, et al. International multisite study of human-induced pluripotent stem cell-derived cardiomyocytes for drug proarrhythmic potential assessment. Cell Rep. 2018;24:3582–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huo J, Kamalakar A, Yang X, Word B, Stockbridge N, Lyn-Cook B, Pang L Evaluation of batch variations in induced pluripotent stem cell-derived human cardiomyocytes from 2 major suppliers. Toxicol Sci. 2017;156:25–38. [DOI] [PubMed] [Google Scholar]
- 26.Ando H, Yoshinaga T, Yamamoto W, et al. A new paradigm for drug-induced torsadogenic risk assessment using human iPS cell-derived cardiomyocytes. J Pharmacol Toxicol Methods. 2017;84:111–127. [DOI] [PubMed] [Google Scholar]
- 27.Cohen JD, Babiarz JE, Abrams RM, Guo L, Kameoka S, Chiao E, Taunton J, Kolaja KL Use of human stem cell derived cardiomyocytes to examine sunitinib mediated cardiotoxicity and electrophysiological alterations. Toxicol Appl Pharmacol. 2011;257:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirenko O, Cromwell EF, Crittenden C, Wignall JA, Wright FA, Rusyn I Assessment of beating parameters in human induced pluripotent stem cells enables quantitative in vitro screening for cardiotoxicity. Toxicol Appl Pharmacol. 2013;273:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamore SD, Ahlberg E, Boyer S, Lamb ML, Hortigon-Vinagre MP, Rodriguez V, Smith GL, Sagemark J, Carlsson L, Bates SM, Choy AL, Stalring J, Scott CW, Peters MF Deconvoluting kinase inhibitor induced cardiotoxicity. Toxicol Sci. 2017;158:213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koci B, Luerman G, Duenbostell A, Kettenhofen R, Bohlen H, Coyle L, Knight B, Ku W, Volberg W, Woska JR Jr., Brown MP An impedance-based approach using human iPSC-derived cardiomyocytes significantly improves in vitro prediction of in vivo cardiotox liabilities. Toxicol Appl Pharmacol. 2017;329:121–127. [DOI] [PubMed] [Google Scholar]
- 31.Rehnelt S, Malan D, Juhasz K, Wolters B, Doerr L, Beckler M, Kettenhofen R, Bohlen H, Bruegmann T, Sasse P Frequency-dependent multi-well cardiotoxicity screening enabled by optogenetic stimulation. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18122634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng H, Balasubramanian B, Lagrutta A, Sannajust F Response of human induced pluripotent stem cell-derived cardiomyocytes to several pharmacological agents when intrinsic syncytial pacing is overcome by acute external stimulation. J Pharmacol Toxicol Methods. 2018;91:18–26. [DOI] [PubMed] [Google Scholar]
- 33.Denning C, Borgdorff V, Crutchley J, et al. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim Biophys Acta. 2016;1863:1728–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burridge PW, Li YF, Matsa E, et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med. 2016;22:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma A, Burridge PW, McKeithan WL, et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Science translational medicine. 2017;9 doi: 10.1126/scitranslmed.aaf2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitani T, Ong SG, Lam CK, Rhee JW, Zhang JZ, Oikonomopoulos A, Ma N, Tian L, Lee J, Telli ML, Witteles RM, Sharma A, Sayed N, Wu JC Human-induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in patients with breast cancer. Circulation. 2019;139:2451–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, Yang H, Rhee JW, Zhang JZ, Lam CK, Sallam K, Chang ACY, Ma N, Lee J, Zhang H, Blau HM, Bers DM, Wu JC Modelling diastolic dysfunction in induced pluripotent stem cell-derived cardiomyocytes from hypertrophic cardiomyopathy patients. Eur Heart J. 2019. doi: 10.1093/eurheartj/ehz326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garg P, Oikonomopoulos A, Chen H, Li Y, Lam CK, Sallam K, Perez M, Lux RL, Sanguinetti MC, Wu JC Genome editing of induced pluripotent stem cells to decipher cardiac channelopathy variant. Journal of the American College of Cardiology. 2018;72:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundy SD, Zhu WZ, Regnier M, Laflamme MA Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaidyanathan R, Markandeya YS, Kamp TJ, Makielski JC, January CT, Eckhardt LL Ik1-enhanced human-induced pluripotent stem cell-derived cardiomyocytes: An improved cardiomyocyte model to investigate inherited arrhythmia syndromes. Am J Physiol Heart Circ Physiol. 2016;310:H1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieu DK, Fu JD, Chiamvimonvat N, Tung KC, McNerney GP, Huser T, Keller G, Kong CW, Li RA Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Arrhythm Electrophysiol. 2013;6:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu R, Blazeski A, Poon E, Costa KD, Tung L, Boheler KR Physical developmental cues for the maturation of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther. 2014;5:117. doi: 10.1186/scrt507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keung W, Boheler KR, Li RA Developmental cues for the maturation of metabolic, electrophysiological and calcium handling properties of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther. 2014;5:17. doi: 10.1186/scrt406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao C, Prodromakis T, Kolker L, Chaudhry UA, Trantidou T, Sridhar A, Weekes C, Camelliti P, Harding SE, Darzi A, Yacoub MH, Athanasiou T, Terracciano CM The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials. 2013;34:2399–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen A, Eder A, Bonstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwoerer AP, Uebeler J, Eschenhagen T Development of a drug screening platform based on engineered heart tissue. Circulation research. 2010;107:35–44. [DOI] [PubMed] [Google Scholar]
- 46.Magdy T, Schuldt AJT, Wu JC, Bernstein D, Burridge PW Human induced pluripotent stem cell (hipsc)-derived cells to assess drug cardiotoxicity: Opportunities and problems. Annu Rev Pharmacol Toxicol. 2018;58:83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rana P, Anson B, Engle S, Will Y Characterization of human-induced pluripotent stem cell-derived cardiomyocytes: Bioenergetics and utilization in safety screening. Toxicol Sci. 2012;130:117–131. [DOI] [PubMed] [Google Scholar]
- 48.Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y Circumventing the crabtree effect: Replacing media glucose with galactose increases susceptibility of hepg2 cells to mitochondrial toxicants. Toxicol Sci. 2007;97:539–547. [DOI] [PubMed] [Google Scholar]
- 49.Cai CZ WF, Shi Q, Yang X, Strauss DG, Stockbridge N, and Pang L Fatty acid-based medium promoted metabolic maturation of human induced pluripotent stem cell-derived cardiomyocytes. Circulation. 2018;138 (Abstract #16287). [Google Scholar]
- 50.Bustamante JO, Watanabe T, Murphy DA, McDonald TF Isolation of single atrial and ventricular cells from the human heart. Can Med Assoc J. 1982;126:791–793. [PMC free article] [PubMed] [Google Scholar]
- 51.Beuckelmann DJ, Nabauer M, Erdmann E Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. [DOI] [PubMed] [Google Scholar]
- 52.Iost N, Virag L, Opincariu M, Szecsi J, Varro A, Papp JG Delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc Res. 1998;40:508–515. [DOI] [PubMed] [Google Scholar]
- 53.Coppini R, Ferrantini C, Aiazzi A, Mazzoni L, Sartiani L, Mugelli A, Poggesi C, Cerbai E Isolation and functional characterization of human ventricular cardiomyocytes from fresh surgical samples. J Vis Exp. 2014. doi: 10.3791/51116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Page G, Ratchada P, Miron Y, Steiner G, Ghetti A, Miller PE, Reynolds JA, Wang K, Greiter-Wilke A, Polonchuk L, Traebert M, Gintant GA, Abi-Gerges N Human ex-vivo action potential model for pro-arrhythmia risk assessment. J Pharmacol Toxicol Methods. 2016;81:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qu Y, Page G, Abi-Gerges N, Miller PE, Ghetti A, Vargas HM Action potential recording and pro-arrhythmia risk analysis in human ventricular trabeculae. Front Physiol. 2017;8:1109. doi: 10.3389/fphys.2017.01109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen N, Nguyen W, Nguyenton B, Ratchada P, Page G, Miller PE, Ghetti A, Abi-Gerges N Adult human primary cardiomyocyte-based model for the simultaneous prediction of drug-induced inotropic and pro-arrhythmia risk. Front Physiol. 2017;8:1073. doi: 10.3389/fphys.2017.01073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abi-Gerges N IT, Nguyen W, Page G, Nguyen N, MIller P and Ghetti A A human cardiomyocyte-based platform for the profiling of positive inotropes with potential to treat heart failure. Mol Biol Cell. 2018;28:3727 (Abstract # P3492). [Google Scholar]
- 58.Guo L, Abrams RM, Babiarz JE, Cohen JD, Kameoka S, Sanders MJ, Chiao E, Kolaja KL Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes. Toxicol Sci. 2011;123:281–289. [DOI] [PubMed] [Google Scholar]
- 59.Yang X, Papoian T Moving beyond the comprehensive in vitro proarrhythmia assay: Use of human-induced pluripotent stem cell-derived cardiomyocytes to assess contractile effects associated with drug-induced structural cardiotoxicity. J Appl Toxicol. 2018;38:1166–1176. [DOI] [PubMed] [Google Scholar]
- 60.Cochrane A, Albers HJ, Passier R, Mummery CL, van den Berg A, Orlova VV, van der Meer AD Advanced in vitro models of vascular biology: Human induced pluripotent stem cells and organ-on-chip technology. Adv Drug Deliv Rev. 2018. doi: 10.1016/j.addr.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 61.Vernetti L, Gough A, Baetz N, et al. Functional coupling of human microphysiology systems: Intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal muscle. Sci Rep. 2017;7:42296. doi: 10.1038/srep42296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Isoherranen N, Madabushi R, Huang SM Emerging role of organ-on-a-chip technologies in quantitative clinical pharmacology evaluation. Clin Transl Sci. 2019;12:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terrenoire C, Wang K, Tung KW, Chung WK, Pass RH, Lu JT, Jean JC, Omari A, Sampson KJ, Kotton DN, Keller G, Kass RS Induced pluripotent stem cells used to reveal drug actions in a long qt syndrome family with complex genetics. J Gen Physiol. 2013;141:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stillitano F, Hansen J, Kong CW, et al. Modeling susceptibility to drug-induced long qt with a panel of subject-specific induced pluripotent stem cells. Elife. 2017;6 doi: 10.7554/eLife.19406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sala L, van Meer BJ, Tertoolen LGJ, et al. Musclemotion: A versatile open software tool to quantify cardiomyocyte and cardiac muscle contraction in vitro and in vivo. Circulation research. 2018;122:e5–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDonough PM BR, Ingermanson R, Azimi B, Abi-Gerges N, Miller P, and Price J High-throughput analysis of contractile function in adult canine cardiomyocytes via kinetic image cytometry. The Toxicologist, Supplement to Toxicological Sciences. 2019;168:158 (Abstract #1668). [Google Scholar]
- 67.Guo GR, Chen L, Rao M, Chen K, Song JP, Hu SS A modified method for isolation of human cardiomyocytes to model cardiac diseases. J Transl Med. 2018;16:288. doi: 10.1186/s12967-018-1649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J, Volkers L, Jangsangthong W, Bart CI, Engels MC, Zhou G, Schalij MJ, Ypey DL, Pijnappels DA, de Vries AAF Generation and primary characterization of iAM-1, a versatile new line of conditionally immortalized atrial myocytes with preserved cardiomyogenic differentiation capacity. Cardiovasc Res. 2018;114:1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abi-Gerges N, Pointon A, Pullen GF, Morton MJ, Oldman KL, Armstrong D, Valentin JP, Pollard CE Preservation of cardiomyocytes from the adult heart. J Mol Cell Cardiol. 2013;64:108–119. [DOI] [PubMed] [Google Scholar]
- 70.Ou Q, Jacobson Z, Abouleisa R, et al. A physiological biomimetic culture system for pig and human heart slices. Circulation research. 2019. doi: 10.1161/CIRCRESAHA.119.314996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR Altered myocardial force-frequency relation in human heart failure. Circulation. 1992;85:1743–1750. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Y, Rafatian N, Wang EY, Feric NT, Lai BFL, Knee-Walden EJ, Backx PH, Radisic M Engineering microenvironment for human cardiac tissue assembly in heart-on-a-chip platform. Matrix Biol. 2019. doi: 10.1016/j.matbio.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X YM, Oikonomopoulos A, Willits C, Green K, and Abassi YA Assessment of cardiac contractile force modulator using functionally mature human ipsc-derived cardiomyocytes. The Toxicologist, Supplement to Toxicological Sciences. 2019;168:158 (Abstract #1669). [Google Scholar]
- 74.Zhao Y, Rafatian N, Feric NT, et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell. 2019;176:913–927 e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schulze ML, Lemoine MD, Fischer AW, Scherschel K, David R, Riecken K, Hansen A, Eschenhagen T, Ulmer BM Dissecting hipsc-cm pacemaker function in a cardiac organoid model. Biomaterials. 2019;206:133–145. [DOI] [PubMed] [Google Scholar]
- 76.Feaster TK, Cadar AG, Wang L, Williams CH, Chun YW, Hempel JE, Bloodworth N, Merryman WD, Lim CC, Wu JC, Knollmann BC, Hong CC Matrigel mattress: A method for the generation of single contracting human-induced pluripotent stem cell-derived cardiomyocytes. Circulation research. 2015;117:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Britton OJ, Abi-Gerges N, Page G, Ghetti A, Miller PE, Rodriguez B Quantitative comparison of effects of dofetilide, sotalol, quinidine, and verapamil between human ex vivo trabeculae and in silico ventricular models incorporating inter-individual action potential variability. Front Physiol. 2017;8:597. doi: 10.3389/fphys.2017.00597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blanchette AD, Grimm FA, Dalaijamts C, Hsieh NH, Ferguson K, Luo YS, Rusyn I, Chiu WA Thorough qt/qtc in a dish: An in vitro human model that accurately predicts clinical concentration-qtc relationships. Clin Pharmacol Ther. 2019;105:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Polonchuk L, Chabria M, Badi L, Hoflack JC, Figtree G, Davies MJ, Gentile C Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci Rep. 2017;7:7005. [DOI] [PMC free article] [PubMed] [Google Scholar]


