Abstract
Aspartate-glutamate carrier 1 (AGC1) is one of two exchangers within the malate-aspartate shuttle. AGC1 is encoded by the SLC25A12 gene. Three patients with pathogenic variants in SLC25A12 have been reported in the literature. These patients were clinically characterized by neurodevelopmental delay, epilepsy, hypotonia, cerebral atrophy, and hypomyelination; however, there has been discussion in the literature as to whether this hypomyelination is primary or secondary to a neuronal defect. Here we report a 12-year-old patient with variants in SLC25A12 and magnetic resonance imaging (MRI) at multiple ages. Novel compound heterozygous, recessive variants in SLC25A12 were identified: c.1295C>T (p.A432V) and c.1447–2_1447–1delAG. Clinical presentation is characterized by severe intellectual disability, non-ambulatory, non-verbal status, hypotonia, epilepsy, spastic quadriplegia, and a happy disposition. The serial neuroimaging findings are notable for cerebral atrophy with white matter involvement, namely, early hypomyelination yet subsequent progression of myelination. The longitudinal MRI findings are most consistent with a leukodystrophy of the leuko-axonopathy category, i.e., white matter abnormalities that are most suggestive of mechanisms that result from primary neuronal defects. We present here the first case of a patient with compound heterozygous variants in SLC25A12, including brain MRI findings, in the oldest individual reported to date with this neurogenetic condition.
Keywords: AGC1, SLC25A12, Genetics, Intellectual disability, MRI
1. INTRODUCTION
The aspartate-glutamate carrier (AGC) proteins transport intramitochondrial aspartate to the cytoplasm in exchange for glutamate and a proton (Amoedo et al., 2016), a process that is regulated by calcium (Palmieri et al., 2001). AGC1 is primarily expressed in the heart, skeletal muscle, and central nervous system whereas AGC2, a paralog of AGC1, is primarily expressed in epithelial cells and the liver (Amoedo et al., 2016). As one of two primary exchangers within the malate-aspartate shuttle (the other being 2-oxoglutarate carrier), AGC1 is involved in the transfer of nicotinamide adenine dinucleotide (NADH)-reducing equivalents from the cytosol to the mitochondria, a transfer essential to connect the glycolytic and oxidative phases of glucose catabolism (Amoedo et al., 2016; Greenhouse & Lehninger, 1976; Ramos et al., 2003; Taylor, 2017). Aspartate produced in the mitochondria and exported to the cytosol is crucial for cell growth, proliferation, and synaptic transmission (Alkan et al., 2018; Birsoy et al., 2015; Sullivan et al., 2015), and in the brain, neuronal aspartate is continuously acetylated to form N-acetylaspartate (NAA). NAA is transported to glial cells, particularly oligodendrocytes, where it is likely used for lipid and myelin synthesis (Burri, Steffen, & Herschkowitz, 1991; Chakraborty, Mekala, Yahya, Wu, & Ledeen, 2001; Francis et al., 2016; Moffett, Ross, Arun, Madhavarao, & Namboodiri, 2007), although an absence of NAA does not preclude myelin formation (Martin, Capone, Schneider, Hennig, & Thiel, 2001). NAA is also potentially involved in osmoregulation (Baslow, 2003), nitrogen balance (Moffett et al., 2007), and the inhibition of protein aggregation (Dolle et al., 2018).
AGC1 is encoded by the SLC25A12 gene (chromosome 2q31.1) and has been studied in various model organisms (Amoedo et al., 2016). Mouse models of SLC25A12 loss-of-function variants have identified a common phenotype, which is characterized by postnatal global neurodevelopmental delay, motor abnormalities (i.e., tremor and motor coordination impairment), and slowed body growth (Jalil et al., 2005; Sakurai et al., 2010). Noted neural abnormalities include abnormal brain growth, hypomyelination, and abnormal neurofilamentous accumulations in neurons (Sakurai et al., 2010). Metabolic alterations include reduced aspartate and NAA in the brain, and decreased baseline and maximal uncoupled respiration in neuronal cultures (Llorente-Folch et al., 2013; Sakurai et al., 2010). Intriguingly, administration of pyruvate rescued myelination deficits in cerebellar slice cultures and respiration in cortical neurons (Llorente-Folch et al., 2013; Sakurai et al., 2010). As utilization of pyruvate obviates the accumulation of NADH that occurs during glucose catabolism, the ability of pyruvate to rescue these deficits is suggestive of the importance of AGC1’s role in NAD+/NADH cycling.
To date, three patients with pathogenic variants in SLC25A12 have been described in the literature, with a clinical phenotype consisting of arrested psychomotor development, profound developmental delay, hypotonia, and epilepsy (Falk et al., 2014; Wibom et al., 2009). In terms of the neural abnormalities, cerebral atrophy, abnormal myelination, fluctuating basal ganglia changes, and decreased NAA levels, as indicated by magnetic resonance spectroscopy (MRS), were reported in two siblings (Falk et al., 2014). In another patient (Wibom et al., 2009), global cerebral hypomyelination was noted under magnetic resonance imaging (MRI), in the absence of gray matter, basal ganglia, brainstem, or spectroscopy-measured abnormalities. However, the argument made by Wibom et al. for a primary hypomyelinating disorder in this patient was subsequently questioned in the literature. Namely, Wolf and van der Knaap suggested that the MRI demonstrated evidence of primary severe cerebral atrophy, with hypomyelinating disease most likely secondary to defects in neurons as opposed to intrinsic myelin defects (Wolf & van der Knaap, 2009). AGC1 deficiency (in the absence of genetic results to confirm the SLC25A12 variant) was reported in a fourth patient, with the clinical implementation of the ketogenic diet resulting in notable clinical and radiological improvements, including improved psychomotor functioning and resumed myelination (Dahlin et al., 2015). Here we present the oldest patient to date with SLC25A12-related disease. Notably, the patient has compound heterozygous pathogenic variants in SLC25A12, with each single variant present in one parent. MRI results from multiple ages are most consistent with a leukodystrophy of the leuko-axonopathy category (i.e., mechanisms stemming from neuronal and/or axonal defects), as proposed by van der Knaap and Bugiani (van der Knaap & Bugiani, 2017), and not a primary hypomyelinating disorder.
2. METHODS
Editorial Policies and Ethical Considerations
Institutional Review Boards at Brown University and Lifespan Healthcare approved this study, and legal guardians of the patient provided written informed consent. Clinical information was collected by direct parental interview and by physical examination of the patient. Clinical information was also extracted from available medical records.
DNA Sequence Analysis
Parent and patient variants were verified by Sanger DNA sequencing. References for variant coordinates are based on GenBank transcript ID NM_003705, Ensembl transcript ID ENST00000422440, and UniProt peptide O75746.
In Silico Structural Modeling
Modeling of the human SLC25A12 crystal structure was performed using the Rosetta software suite, available at https://www.rosettacommons.org/, following the RosettaCM package (Song et al., 2013). To find homologous proteins with known crystal structures, a blastp search of the transmembrane region (amino acids 312–678) was performed against proteins available in the Protein Data Bank (PDB) database, and the following proteins were selected: the Bos taurus ADP-ATP carrier (SLC25A4/ANT1), PDB accession 2C3E (30% homology); the Saccharomyces cerevisiae ADP-ATP carrier (PET9/ANT2), PDB accession 4C9J (28% homology); and the Mus musculus mitochondrial uncoupling protein 2 (SLC25A8/UCP2), PDB accession 2LCK (27% homology). Sequence alignments were generated using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/), and transmembrane alignments were generated and verified using Octopus (http://octopus.cbr.su.se/) (Viklund & Elofsson, 2008). Ten models of the transmembrane domain were generated, and the model with the lowest calculated thermodynamic score was selected to serve as a reference model. To generate the models containing the SLC25A12 variant, the generated PDB file and the variant information were submitted to RosettaBackrub (https://kortemmeweb.ucsf.edu/backrub/) (Davis, Arendall, Richardson, & Richardson, 2006; Smith & Kortemme, 2008). Twenty models of the variant-containing protein were generated, and the model with the lowest thermodynamic score was selected for comparison. Protein visualization was performed using PyMOL (Schrodinger, 2015).
3. CLINICAL REPORT AND RESULTS
The patient is a male of 11 years of age at the time of evaluation. He was born at term at healthy weight without complications. Vigorous movement in utero was noted. He was admitted to the neonatal intensive care unit post-partum for delayed transition, during which time he received oxygen for approximately 8 days.
Global delays were noted early in development, although some eye contact and smiling were reported prior to a regression around seizure onset. The patient experienced his first seizures starting at 3 months of life, manifesting as gaze deviation to the left and left head turning. The electroencephalogram (EEG) at that time was noted to be abnormal due to epileptiform discharges within the right hemisphere with a wide distribution. Two seizures were captured on EEG, both of which originated within the right hemisphere, one with focal to bilateral spread and another that remained focal. He was placed on oxcarbazepine and levetiracetam at that time. During the course of treatment, topiramate and phenobarbital have also been prescribed, and he required up to three antiepileptic medications for control. His parents reported that he experienced hundreds of seizures prior to medical control with combination treatment (including phenobarbital and levetiracetam) at 3 years of age, at which time seizures stopped. He remained seizure free for many years and lived without seizure medications; yet at age 12 years, seizures appeared to recommence, which required medication (levetiracetam) to control. Seizure episodes were characterized as staring spells observed in school and nocturnal episodes characterized as staring with repetitive mouth movements and “clucking”-like sounds. EEG was notable for diffuse slowing and epileptiform discharges seen independently in the left and right frontal, central, and parietal regions, suggesting epileptic foci in these regions. Throughout the patient’s life, there is no report of prior epileptic spasms or features consistent with Lennox-Gastaut syndrome.
The patient has experienced profound developmental delays and is unable to engage in purposeful movements or actions. His medical history includes gastroesophageal reflux, constipation, chronic ear infections, seasonal allergies, blepharitis, osteopenia, scoliosis, and bilateral dislocated hips. His active neurological diagnoses include optic neuropathy, cortical visual impairment, exotropia, spastic quadriplegic cerebral palsy, metabolic encephalopathy, and epilepsy without status epilepticus.
On most recent examination at age 11 years, the patient had a limited functional status. He liked sitting in a swing. He responded to familial interaction and stimulation by his siblings. He was non-verbal. Some teachers believed that he understood some language but was non-verbal. He was non-ambulatory and wheelchair bound. He appeared to feel pain distally in feet. Head circumference at time of exam was 50.5 cm (2nd percentile), weight was 65 lbs (10th percentile), and length was 127 cm (<5th percentile). He was unable to follow directions, either at the midline or laterally. He did vocalize and smile. He had no recognizable words. He did not grimace to light. On cranial nerve exam, his left eye deviated out and down, and he was very slow to fix and follow. He localized to sound correctly. He exhibited right lower face weakness. His tongue was midline without evident rooting reflex. On motor exam, he had spastic quadriplegia with axial rigidity. His right and left wrists were flexed, and his right hand was flexed except for his index and middle fingers, which were mostly extended. His right ankle was everted and dorsiflexed. His left ankle had comparatively decreased tone. He had no response to light touch. His right biceps reflexed, and right brachioradialis showed spreading but no clonus. His toe was upgoing on the right.
Adaptive functioning was assessed with the Vineland parental interview, with his overall scores falling in the severe impairment range (standard scores ranging from 30–33 and age-equivalent scores ranging from <1 month to 1 year 10 months). At time of this report, his feeding is done through a gastrostomy tube. He receives special education services through his school district. He receives outpatient occupational therapy, physical therapy, and speech/language therapy.
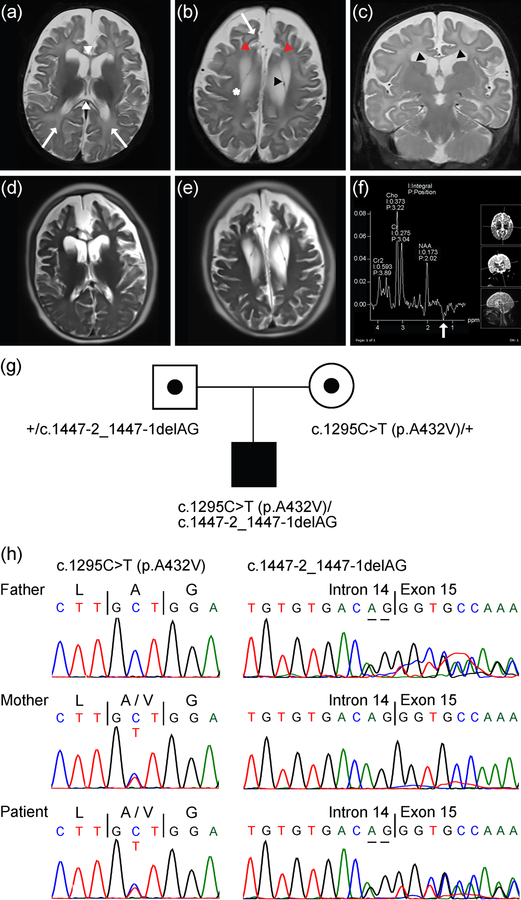
The patient underwent brain MRI at three ages: 4.5 months (MRI), 7 months (MRI/MRS), and 10 years 5 months (MRI) (Figure 1a–f and Supporting Information Figure S1). MRI at 4.5 months (data not shown) and 7 months of age (Figure 1a–c) showed diffuse white matter volume loss associated with mild to moderate ventriculomegaly and an abnormally thin corpus callosum (Figure 1a, white arrowheads). Panlobar gyral contraction that results in reciprocal sulcal prominence and prominence of the subarachnoid spaces was also noted (Figure 1b, red arrowheads). Fine paired linear signal in the anterior horns and bodies of both lateral ventricles may represent cysts or membranes (Figure 1b,c, black arrowheads). The deep bilateral frontal and parietal white matter showed confluent abnormally intermediate signal on the T1-weighted images, where high signal is expected, and abnormally elevated signal on the T2-weighted images. Although some intermediate to high signal on T2-weighted images in the deep white matter reflecting unmyelinated white matter is an expected MRI finding at 7 months of age, the patient’s MRI results at 7 months of age show an exaggerated signal. Such findings may initially raise concern for a permanent or primary hypomyelinating disorder; however, follow-up MRI at 10 years 5 months of age demonstrates progression of myelination (Figure 1d,e). MRS performed using intermediate echo time (TE 135 ms) revealed an inverted double peak at approximately 1.3 parts per million (ppm), which is consistent with the presence of lactate that may reflect the ongoing seizures at this time (i.e., 7 months) and is suggestive of a primary neuronal defect at this early stage of development (Figure 1f). Further, MRI depicting cerebellum in this patient suggests mild cerebellar atrophy at age 10 years 5 months but not at prior ages examined (i.e., 4.5 months and 7 months) (Supporting Information Figure S1).
Figure 1.
MRI and MRS findings in male patient with compound heterozygous SLC25A12 pathogenic variants. (a-c) T2-weighted images at 7 months of age, with axial images shown in (a) and (b) and a coronal image in (c). Images reveal diffusely accentuated signal in the bilateral frontal and parietal deep white matter (a,b, arrows) that contrasts with myelinating periventricular and corticospinal tract white matter (b, *). The corpus callosum (a, white arrowheads) is abnormally thin for age, which is also reflective of white matter volume loss. Images also reveal diffuse panlobar sulcal prominence and white matter volume loss that results in contraction of the gyri, most conspicuously in the bilateral superior frontal gyri (b, red arrowheads). Thin membranes in the bilateral lateral ventricles that may outline intraventricular cysts (b,c, black arrowheads) can also be seen. (d,e) Axial HASTE images at 10 years 5 months of age suggest further contraction of the gyri and white matter, yet also reveal interval myelination on the T2-weighted images. (f) Results of MRS performed over the left basal ganglia at 7 months of age. The spectrum acquired at intermediate echo time (TE 135 ms) shows an inverted lactate peak at 1.3 ppm (white arrow), which may reflect sequela of seizures. (g) Pedigree showing heterozygous parents, each of which is a carrier of a distinct SLC25A12 variant, and the affected male patient with compound heterozygous variants in SLC25A12. Note, information regarding siblings of the patient is not shown to aid in protecting the anonymity and privacy of the family. (h) Sanger DNA sequencing chromatograms showing SLC25A12 variants in the father, mother, and patient, as indicated in (g). References for variant coordinates are based on GenBank transcript ID NM_003705 and Ensembl transcript ID ENST00000422440.
Initially, the child’s metabolic and genetic testing results were nondiagnostic, including normal mitochondrial genome sequencing and microarray analysis. Subsequent whole-exome sequencing, however, revealed compound heterozygous pathogenic variants in SLC25A12: c.1295C>T (p.A432V) and c.1447–2_1447–1delAG (GenBank transcript ID: NM_003705, UniProt peptide: O75746). These variants were confirmed by Sanger DNA sequencing and, additionally, appear to have been inherited from the parents (Figure 1g,h). These results are consistent with a diagnosis of AGC1 deficiency.
Analysis of the SLC25A12 A432V variant by PROVEAN (Choi & Chan, 2015), SIFT (Sim et al., 2012), and PolyPhen (Adzhubei et al., 2010) predicted that this variant is likely to be deleterious. Further, this variant has not been observed in large population cohorts (Lek et al., 2016). The A432V variant is located in transmembrane helix 3 in the transport domain, and based on alignment to homologous structures, it is in a highly conserved region at the interface between two helices. To model the effects of the A432V variant on the AGC1 protein, a comparative homology analysis was first performed to approximate the structure of the SLC25A12 transporter domain (amino acids 312–678), after which this structure was used to assess the impact of the A432V variant on the folding of the structure (see Materials and Methods). The AGC1 transmembrane model (Figure 2a) was generated by referencing the known structures of the bovine ADP-ATP carrier (ANT1) (Figure 2b), the yeast ADP-ATP carrier (PET9) (Figure 2c), and the mouse mitochondrial uncoupling protein 2 (UCP2) (Figure 2d), which also exports 4-carbon metabolites, including aspartate (Vozza et al., 2014). The A432V variant was then introduced into the structure, and the variant-containing structure was modeled (see Materials and Methods). As predicted, the A432V variant appears to alter secondary structure of the transmembrane helices and packing of the protein (Figure 2e,f). The solute-carrying transmembrane helix containing residue A432V appears to be less efficiently packed, and the variant appears to de-structure a portion of the alpha helix conformation of both transmembrane helices 2 and 3 (Figure 2g). Moreover, the variant substantially alters the position of several residues in the adjacent transmembrane helix 2 (Figure 2h).
Figure 2.
In silico structural modeling of SLC25A12 and of the A432V pathogenic variant. (a) The in silico model of the transmembrane region of SLC25A12. N- and C-termini have been excluded to facilitate modeling and for clarity. (b) The model of SLC25A12 (gray) overlaid on the structure of bovine SLC25A4 (aqua). (c) The model of SLC25A12 (gray) overlaid on the structure of yeast SLC25A4 (green). (d) The model of SLC25A12 (gray) overlaid on the structure of mouse SLC25A8 (magenta). (e) A view of transmembrane helices 2 (bottom) and 3 (top) of the consensus sequence model. A432 is highlighted in green. (f) The same region of the variant-containing sequence model. V432 is highlighted in red. (g) The consensus sequence (gray) and variant sequence (yellow) model overlaid. (h) An overlay of A432V, with the position shifts of residues 414–417 in the consensus sequence and variant-containing sequence highlighted.
The c.1447–2_1447–1delAG pathogenic variant is an intronic deletion, located immediately before exon 15. Analysis of the c.1447–2_1447–1delAG variant using MutationTaster (Schwarz, Cooper, Schuelke, & Seelow, 2014) determined that this deletion removes a splice acceptor site, which may result in the loss of exon 15 and subsequent exons, which encode for three of the transmembrane helices of the AGC1 transporter domain. Namely, if exon 15 is skipped, splicing to the subsequent exon (i.e., exon 16) creates a frame shift and premature stop codon after amino acid 489. Consequently, this variant is likely to be a complete loss of function.
4. DISCUSSION
Here we report on novel pathogenic variants in the SLC25A12 gene, specifically a compound heterozygous inheritance of c.1295C>T (p.A432V) and c.1447–2_1447–1delAG in a 12-year-old male. To date, this is the oldest individual reported with this genetic syndrome. The clinical phenotype of this child is highly consistent with the three other children reported in the literature (Falk et al., 2014; Wibom et al., 2009). Together, the SLC25A12 syndrome phenotype is uniformly characterized by intellectual disability (severe-profound), non-ambulatory, non-verbal status, hypotonia, epilepsy, and a happy disposition. Small stature, microcephaly, dysmorphic features, and prior developmental regression have also been noted in two patients (Falk et al., 2014).
SLC25A12-related disease may be associated with successful medical seizure control. Seizures were successfully treated or reduced in frequency with medication in three previously reported patients (Falk et al., 2014; Wibom et al., 2009), while the currently described patient was seizure free without medication for several years until seizure recurrence at approximately 12 years of age. The ketogenic diet has also been shown to be effective: one patient in the literature described as having AGC1 deficiency, although without genetic confirmation, and treatment-resistant seizures exhibited motor improvement and increased myelination after clinical treatment with the ketogenic diet (Dahlin et al., 2015).
With regard to the classification of the epilepsy in this patient, as per the 2017 International League Against Epilepsy (ILAE) classification of seizures (Brodie, Zuberi, Scheffer, & Fisher, 2018), the seizure onset at 3 months of age appears to be focal on the right side with motor symptoms, and additionally, on EEG there is evidence of focal to bilateral spreading. Classification of seizures at age 12 years is more difficult. The later EEG shows evidence of multi-focal pathology without capturing seizure onset; however, the automatisms still suggest focal seizures, even though the possibility of this patient having mixed focal and generalized onset events cannot be entirely ruled out. Further, the diffusely slow background demonstrates the development of an encephalopathy, which is likely metabolic, but may also have an epileptic component.
The mouse model of SLC25A12 deficiency is described as showing postnatal global neurodevelopmental delay, motor abnormalities, and slowed body growth (Jalil et al., 2005; Sakurai et al., 2010). The mouse model has further identified abnormalities in overall brain growth, hypomyelination, and neurofilamentous accumulations in neurons (Sakurai et al., 2010). Although the mouse model may support hypomyelination and hypomyelination has been used to describe the neuroimaging findings of a previously described patient (Wibom et al., 2009), whether the presentation is truly reflective of primary hypomyelinating disease has been questioned (Wolf & van der Knaap, 2009). Similarly, the serial MRI findings in our case are less characteristic of a primary or permanent hypomyelinating disorder (i.e., resulting from gene-related mechanisms autonomous to myelin) but rather are much more suggestive of a leukodystrophy of the leuko-axonopathy category (i.e., mechanisms stemming from neuronal and/or axonal defects with secondary white matter involvement) (Schiffman & van der Knaap, 2009; van der Knaap & Bugiani, 2017). It could be possible that there is a requirement for AGC1 in both neurons and oligodendrocytes, such that there are intrinsic, cell autonomous defects in both tissue types. However, the strict imaging definition of a permanent hypomyelinating disorder requires no evidence of myelination between two MRI studies separated by at least a 6 month interval in a child older than 1 year (Schiffman & van der Knaap, 2009). In our case, although MRI at 4.5 months and 7 months of age suggested a derangement in myelination, the marked gray matter loss and the evidence of some interval myelination on the MRI performed at age 10 years 5 months, in the setting of a seizure disorder, are much more suggestive of a leukodystrophy, leuko-axonopathy type. Overall, we suggest that SLC25A12-related disease be classified as a leukodystrophy of the leuko-axonopathy category, a genetic condition with white matter involvement that arises likely due to central disease mechanisms involving neurons and axons (van der Knaap & Bugiani, 2017).
In summary, current and prior reports indicate that SLC25A12 syndrome or AGC1 deficiency is one identified form of autosomal recessive neurodevelopmental delay characterized by cerebral atrophy, hypotonia, seizures, severe-profound intellectual disability, non-ambulatory, non-verbal status, and happy disposition. Our support for classification of SLC25A12-related disease as a leukodystrophy of the leuko-axonopathy category is based in examination of a trajectory of MRI findings in the oldest patient reported to date.
Supplementary Material
Figure S1 MRI findings in male patient with compound heterozygous SLC25A12 pathogenic variants. (a-c) MRI scans taken at 4 months 15 days of age. Images shown are a T1-weighted sagittal image (a), a T2-weighted axial image (b), and a T2-weighted coronal image (c). There is no evidence of cerebellar volume loss on these images. The dentate nuclei hila appear subtly hyperintense. (d-f) MRI scans taken at 7 months 6 days of age. Images shown are a T2-weighted sagittal image (d), a T2-weighted axial image (e), and a T2-weighted coronal image (f). There is no evidence of cerebellar volume loss on these images. The dentate nuclei hila again appear slightly hyperintense for age. (g-i) MRI scans taken at 10 years 5 months of age. Images shown are a T1-weighted axial image (g), a T2-weighted axial image through the superior cerebellum (h), and a T2-weighted axial image at a slightly lower position through the cerebellum (i). There is mild cerebellar fissural prominence seen on these images, consistent with mild atrophy.
ACKNOWLEDGEMENTS
The authors would like to thank the family for its participation in the current study.
Grant numbers: R01MH102418, R01MH105442, and T32MH019927
FUNDING
Research reported in this publication was supported in part by the following: National Institute of Mental Health grant numbers R01MH102418 and R01MH105442, and a gift through the Hassenfeld Child Health Innovation Institute at Brown University (EMM); a Carney Institute for Brain Science and Suna Kıraç Fellowship Graduate Award in Brain Science (OB); and National Institute of Mental Health grant number T32MH019927 (EBW).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
DATA AVAILABILITY
All data generated or analyzed as part of this study are included herein or are available from the corresponding author on reasonable request.
REFERENCES
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, … Sunyaev SR (2010). A method and server for predicting damaging missense mutations. Nat Methods, 7(4), 248–249. doi: 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkan HF, Walter KE, Luengo A, Madreiter-Sokolowski CT, Stryeck S, Lau AN, … Bogner-Strauss JG (2018). Cytosolic Aspartate Availability Determines Cell Survival When Glutamine Is Limiting. Cell Metab, 28(5), 706–720 10.1016/j.cmet.2018.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoedo ND, Punzi G, Obre E, Lacombe D, De Grassi A, Pierri CL, & Rossignol R (2016). AGC1/2, the mitochondrial aspartate-glutamate carriers. Biochim Biophys Acta, 1863(10), 2394–2412. doi: 10.1016/j.bbamcr.2016.04.011 [DOI] [PubMed] [Google Scholar]
- Baslow MH (2003). N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res, 28(6), 941–953. [DOI] [PubMed] [Google Scholar]
- Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, & Sabatini DM (2015). An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell, 162(3), 540–551. doi: 10.1016/j.cell.2015.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MJ, Zuberi SM, Scheffer IE, & Fisher RS (2018). The 2017 ILAE classification of seizure types and the epilepsies: what do people with epilepsy and their caregivers need to know? Epileptic Disord, 20(2), 77–87. doi: 10.1684/epd.2018.0957 [DOI] [PubMed] [Google Scholar]
- Burri R, Steffen C, & Herschkowitz N (1991). N-acetyl-L-aspartate is a major source of acetyl groups for lipid synthesis during rat brain development. Dev Neurosci, 13(6), 403–411. doi: 10.1159/000112191 [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Mekala P, Yahya D, Wu G, & Ledeen RW (2001). Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. J Neurochem, 78(4), 736–745. [DOI] [PubMed] [Google Scholar]
- Choi Y, & Chan AP (2015). PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics, 31(16), 2745–2747. doi: 10.1093/bioinformatics/btv195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin M, Martin DA, Hedlund Z, Jonsson M, von Dobeln U, & Wedell A (2015). The ketogenic diet compensates for AGC1 deficiency and improves myelination. Epilepsia, 56(11), e176–181. doi: 10.1111/epi.13193 [DOI] [PubMed] [Google Scholar]
- Davis IW, Arendall WB 3rd, Richardson DC, & Richardson JS (2006). The backrub motion: how protein backbone shrugs when a sidechain dances. Structure, 14(2), 265–274. doi: 10.1016/j.str.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Dolle JP, Rodgers JM, Browne KD, Troxler T, Gai F, & Smith DH (2018). Newfound effect of N-acetylaspartate in preventing and reversing aggregation of amyloid-beta in vitro. Neurobiol Dis, 117, 161–169. doi: 10.1016/j.nbd.2018.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MJ, Li D, Gai X, McCormick E, Place E, Lasorsa FM, … Hakonarson H (2014). AGC1 Deficiency Causes Infantile Epilepsy, Abnormal Myelination, and Reduced N-Acetylaspartate. JIMD Rep, 14, 77–85. doi: 10.1007/8904_2013_287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis JS, Wojtas I, Markov V, Gray SJ, McCown TJ, Samulski RJ, … Leone P (2016). N-acetylaspartate supports the energetic demands of developmental myelination via oligodendroglial aspartoacylase. Neurobiol Dis, 96, 323–334. doi: 10.1016/j.nbd.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse WV, & Lehninger AL (1976). Occurrence of the malate-aspartate shuttle in various tumor types. Cancer Res, 36(4), 1392–1396. [PubMed] [Google Scholar]
- Jalil MA, Begum L, Contreras L, Pardo B, Iijima M, Li MX, … Saheki T (2005). Reduced N-acetylaspartate levels in mice lacking aralar, a brain- and muscle-type mitochondrial aspartate-glutamate carrier. J Biol Chem, 280(35), 31333–31339. doi: 10.1074/jbc.M505286200 [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, … Exome Aggregation, C. (2016). Analysis of protein-coding genetic variation in 60,706 humans. Nature, 536(7616), 285–291. doi: 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Folch I, Rueda CB, Amigo I, del Arco A, Saheki T, Pardo B, & Satrustegui J (2013). Calcium-regulation of mitochondrial respiration maintains ATP homeostasis and requires ARALAR/AGC1-malate aspartate shuttle in intact cortical neurons. J Neurosci, 33(35), 13957–13971, 10.1523/JNEUROSCI.0929-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Capone A, Schneider J, Hennig J, & Thiel T (2001). Absence of N-acetylaspartate in the human brain: impact on neurospectroscopy? Ann Neurol, 49(4), 518–521. [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, & Namboodiri AM (2007). N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol, 81(2), 89–131. doi: 10.1016/j.pneurobio.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri L, Pardo B, Lasorsa FM, del Arco A, Kobayashi K, Iijima M, … Palmieri F (2001). Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J, 20(18), 5060–5069. doi: 10.1093/emboj/20.18.5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M, del Arco A, Pardo B, Martinez-Serrano A, Martinez-Morales JR, Kobayashi K, … Satrustegui J (2003). Developmental changes in the Ca2+-regulated mitochondrial aspartate-glutamate carrier aralar1 in brain and prominent expression in the spinal cord. Brain Res Dev Brain Res, 143(1), 33–46. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Ramoz N, Barreto M, Gazdoiu M, Takahashi N, Gertner M, … Buxbaum JD (2010). Slc25a12 disruption alters myelination and neurofilaments: a model for a hypomyelination syndrome and childhood neurodevelopmental disorders. Biol Psychiatry, 67(9), 887–894. doi: 10.1016/j.biopsych.2009.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman R, & van der Knaap MS (2009). Invited article: An MRI-based approach to the diagnosis of white matter disorders. Neurology, 72, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrodinger L (2015). The PyMOL Molecular Graphics System, Version 1.8
- Schwarz JM, Cooper DN, Schuelke M, & Seelow D (2014). MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods, 11(4), 361–362. doi: 10.1038/nmeth.2890 [DOI] [PubMed] [Google Scholar]
- Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, & Ng PC (2012). SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res, 40 (Web Server issue), W452–457. doi: 10.1093/nar/gks539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, & Kortemme T (2008). Backrub-like backbone simulation recapitulates natural protein conformational variability and improves mutant side-chain prediction. J Mol Biol, 380(4), 742–756. doi: 10.1016/j.jmb.2008.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, DiMaio F, Wang RY, Kim D, Miles C, Brunette T, … Baker D (2013). High-resolution comparative modeling with RosettaCM. Structure, 21(10), 1735–1742. doi: 10.1016/j.str.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, & Vander Heiden MG (2015). Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell, 162(3), 552–563. doi: 10.1016/j.cell.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor EB (2017). Functional Properties of the Mitochondrial Carrier System. Trends Cell Biol, 27(9), 633–644. doi: 10.1016/j.tcb.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, & Bugiani M (2017). Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol, 134(3), 351–382. doi: 10.1007/s00401-017-1739-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viklund H, & Elofsson A (2008). OCTOPUS: improving topology prediction by two-track ANN-based preference scores and an extended topological grammar. Bioinformatics, 24(15), 1662–1668. doi: 10.1093/bioinformatics/btn221 [DOI] [PubMed] [Google Scholar]
- Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, … Fiermonte G (2014). UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci U S A, 111(3), 960–965. doi: 10.1073/pnas.1317400111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibom R, Lasorsa FM, Tohonen V, Barbaro M, Sterky FH, Kucinski T, … Wedell A (2009). AGC1 deficiency associated with global cerebral hypomyelination. N Engl J Med, 361(5), 489–495. doi: 10.1056/NEJMoa0900591 [DOI] [PubMed] [Google Scholar]
- Wolf NI, & van der Knaap MS (2009). AGC1 deficiency and cerebral hypomyelination. N Engl J Med, 361(20), 1997–1998; 10.1056/NEJMc091723 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 MRI findings in male patient with compound heterozygous SLC25A12 pathogenic variants. (a-c) MRI scans taken at 4 months 15 days of age. Images shown are a T1-weighted sagittal image (a), a T2-weighted axial image (b), and a T2-weighted coronal image (c). There is no evidence of cerebellar volume loss on these images. The dentate nuclei hila appear subtly hyperintense. (d-f) MRI scans taken at 7 months 6 days of age. Images shown are a T2-weighted sagittal image (d), a T2-weighted axial image (e), and a T2-weighted coronal image (f). There is no evidence of cerebellar volume loss on these images. The dentate nuclei hila again appear slightly hyperintense for age. (g-i) MRI scans taken at 10 years 5 months of age. Images shown are a T1-weighted axial image (g), a T2-weighted axial image through the superior cerebellum (h), and a T2-weighted axial image at a slightly lower position through the cerebellum (i). There is mild cerebellar fissural prominence seen on these images, consistent with mild atrophy.