Abstract
Background:
Despite the two-fold higher prevalence of major depressive disorder and posttraumatic stress disorder in females compared to males, most clinical and preclinical studies focus on male subjects. We introduce an ethological murine model to study several cardinal symptoms of affective disorders in the female targets of female aggression.
Methods:
Intact Swiss Webster (CFW) female mice were housed with castrated males and tested for aggression toward female intruders. For 10 days, aggressive CFW females defeated C57BL/6J (B6) females during 5-min encounters. Measures of corticosterone, c-Fos activation in hypothalamic and limbic structures, and species-typical behaviors were collected from defeated and non-defeated females. Ketamine (20 mg/kg) was tested for its potential to reverse stress-induced social deficits.
Results:
Housed with a castrated male, most intact CFW females readily attacked unfamiliar B6 females, inflicting >40 bites in a 5-min encounter. Compared to controls, defeated B6 females exhibited elevated plasma corticosterone and increased c-Fos activation in the medial amygdala, ventral lateral septum, ventromedial hypothalamus, and hypothalamic paraventricular nucleus. Chronically defeated females also showed vigilance-like behavior and deficits in social interactions, novel object investigation, and nesting. The duration of social interactions increased 24 hrs after chronically defeated females received a systemic dose of ketamine.
Conclusions:
These findings demonstrate that CFW females living with male conspecifics can be used as aggressive residents in an ethological model of female social defeat stress. These novel behavioral methods will encourage further studies of sex-specific neural, physiological, and behavioral adaptations to chronic stress and on the biological bases for interfemale aggression.
Keywords: Aggression, Female, Social defeat stress, Affective disorders, Social interaction, Ketamine
Introduction
The prevalence of affective disorders including major depressive disorder (MDD) and posttraumatic stress disorder (PTSD) is two-fold higher in women than in men, and sex can shape the trajectory of these disorders in terms of their onset, duration, and rate of recurrence (1–4). Despite substantial evidence for sex differences in the development, course and biological underpinnings of affective disorders (5, 6), most clinical and preclinical studies focus on males. In preclinical research, the murine chronic social defeat stress protocol employs species-typical male aggression toward a submissive conspecific to induce several cardinal symptoms of stress-related affective disorders in a subset of defeated male mice (7–14). This protocol is highly reproducible between and within laboratories, suggesting that the translational effects of chronic social stress are both robust and reliable. In addition, chronic treatment with tricyclic antidepressants or serotonin reuptake inhibitors or acute ketamine administration can normalize defeat-associated social deficits and stress-induced molecular adaptations in male mice (9, 13, 15–18), making this protocol a valuable tool for investigating novel drugs and mechanisms.
In developing a female model of social defeat stress that parallels the existing male protocol, substantial efforts have been made to foster female-directed, male aggression by using male odorants transferred onto females (19) and through chemogenetic stimulation of the male ventromedial hypothalamus (20). A vicarious social defeat stress model in which females witness intermale aggression (21) has also been employed. To provide an alternative method that closely mirrors the male chronic social defeat protocol and eliminates the need to generate atypical patterns of male aggression, we identify specific conditions that promote aggression in outbred female mice toward female opponents.
While male mice are aggressive under diverse experimental conditions (22), female aggression is most often studied either during pregnancy (23–27; but 28) or during the first postpartum week while neonatal pups are suckling (29–31). As an adaptive defensive behavior, dams will engage in maternal aggression to prevent postpartum fertilization and to protect their offspring against unfamiliar male or female intruders (29, 31–33). Outside of these brief gestational and postpartum windows, female mice are minimally aggressive when subjected to isolation housing (34), a technique often used to induce territorial aggression in outbred male mice (35, 36), in female California mice (Peromyscus californicus; 37), and in female Syrian golden hamsters (Mesocricetus auratus; 38, 39). In contrast, we report that most intact female mice (Mus musculus) housed with an intact or castrated male engage in intense aggression when confronted by a female opponent; although isolation-induced territorial competition may not promote interfemale agonistic behavior, a significant subset of females will readily fight a rival female, possibly in competition for an available mate.
Upon identifying specific conditions to engender interfemale aggression, studies were conducted to: 1.) generate an ethological model of female chronic social defeat stress, 2.) examine the effects of social defeat stress on plasma corticosterone concentrations, 3.) determine if social defeat stress increases c-Fos activation in brain areas including the medial amygdala, lateral septum, ventromedial hypothalamus, and paraventricular nucleus, 4.) characterize defeat-associated deficits in species-typical social and non-social behaviors, and to 5.) increase the duration of social interactions initiated by chronically defeated females with acutely administered ketamine.
Methods and Materials
See Supplement for additional details.
Animals
Twelve-week-old intact (n=74) or ovariectomized (OVX; n=27) Swiss Webster (CFW) female mice (Charles River Laboratories, Wilmington, MA, USA) were housed in resident pairs with age-matched intact (n=47) or castrated (n=61) CFW males in clear polycarbonate cages (18.9×29.7×12.8 cm) lined with pine shavings. Twelve-week-old intact intruder C57BL/6J (B6; n=190; Jackson Laboratories, Bar Harbor, ME, USA) or CFW females (n=40) were group-housed in cages (25.7×48.3×15.2 cm; n=10/cage) with corn cob bedding. Experimental twelve-week-old B6 females were housed individually and assigned to control (n=23), acute (n=13) or chronic social defeat (n=28) conditions. Animals were cared for according to the National Research Council’s Guide for the Care and Use of Laboratory Animals and procedures were approved by the Tufts University Institutional Animal Care and Use Committee.
Aggression in outbred OVX females
OVX CFW females, housed in resident pairs with intact CFW males, were evaluated for aggression in modified resident-intruder confrontations every other day starting two weeks after pairhousing (36). To test the effect of intruder strain and familiarity, males were removed and intruder females were introduced to resident home cages for 2-min confrontations (Fig. S2). The attack latency and attack bite frequency were recorded. Following confrontations, female intruders were removed and males were returned to their resident home cages.
Aggression in intact outbred female mice
Intact CFW females, housed with intact CFW males, were evaluated for aggression toward unfamiliar B6 females. CFW litters were culled on postnatal day one (PND1) at which time CFW females were housed singly. After the first postpartum week, there was a substantial decrease in interfemale aggression. To address whether this reduction was related to isolation housing, CFW females were housed with castrated males and three days later, aggressive confrontations continued. After five days of living with castrated males, most resident females attacked unfamiliar B6 females. Here, male cohabitation-induced interfemale aggression is referred to as rival aggression to distinguish it from maternal or gestational aggression. In subsequent groups, intact nulliparous CFW females were housed exclusively with castrated males in resident pairs. A significant subset of these females were highly aggressive toward unfamiliar B6 intruders (n=39/61; >15 bites/2-min); these residents were used as aggressors during the chronic social defeat protocol.
Sex-specific patterns of aggression
Ten 5-min resident-intruder confrontations between intact CFW female residents and unfamiliar B6 female intruders were videotaped in the home cage for detailed behavioral analyses (Video S1). Similar archival videos of intermale confrontations were analyzed to compare the behavioral composition of aggressive encounters in males versus females (Video S2).
Testing the aggressive potential of females that do not display rival aggression
To test whether pregnancy-induced aggression was distinct from rival aggression, consistently non-aggressive females that were pair-housed with castrated males (n=7) for at least two months were assessed for gestational aggression. These females were housed with intact CFW males for three days, then returned to their original castrated male partners. Aggression was evaluated every two or four days during 2-min resident-intruder confrontations. Pups were culled on PND1 and resident females were tested for aggression four and seven days postpartum.
Ten-day female chronic social defeat stress
Highly aggressive CFW females were used as resident stimulus animals for the chronic social defeat stress protocol. Two days prior to the initial defeat episode, resident CFW pairs comprised of intact females and castrated males were transferred to large polycarbonate cages (25.7×48.3×15.2 cm) divided in half by perforated, clear polycarbonate partitions (cf., 8, 14). One day before defeats, females were tested for aggression to ensure behavioral reliability under the new housing conditions.
Daily 5-min defeat episodes occurred in the large divided cages (Video S3); males were temporarily removed and intruder experimental B6 females were exposed to unfamiliar aggressive CFW females. Following defeats, B6 females were housed opposite the CFW females that defeated them and males were returned to be pair-housed with CFW females. During this 24-hr threat period, cage dividers permitted sensory contact between CFW resident pairs and B6 females but protected experimental mice from attack. For 10 consecutive days, B6 females were defeated by and rehoused adjacent to unfamiliar, aggressive CFW females. Non-defeated, control B6 females were housed opposite unfamiliar resident CFW pairs daily, but were never physically attacked. Acutely defeated B6 females were treated as controls until the tenth day when they were defeated once. B6 females were weighed every other day and singly housed after defeats concluded on the tenth day. During the ten day protocol, resident CFW pairs received ~15 g of fresh pine shavings every other day and cages were cleaned on the fifth day following the defeat. Reliably aggressive CFW females (i.e., >40 bites/5-min or >15 bites/2-min) were used in chronic social defeat stress experiments for 6–12 months.
Estrous cycling
Vaginal cytology was monitored in experimental B6 females during the 10-day social defeat stress protocol using the lavage technique (40). Cyclicity was also evaluated in a subset of highly aggressive nulliparous resident females (n=23) housed with castrated males to determine if aggression varied according to estrous cycle phase (41–43; but 44, 45).
Corticosterone measurements
At two time points (Fig. S1A), blood was collected from the submandibular vein of B6 females (n=5 control; n=5 acute defeat; n=10 chronic defeat) using sterile 4 mm lancets (Goldenrod Animal Lancet, Medipoint, Inc., Mineola, NY, USA), centrifuged at 4°C at 3000 rpm for 10 min. Fifteen microliters of plasma were collected and stored at −80°C for corticosterone enzyme immunoassay (Arbor Assays, Ann Arbor, MI, USA); standards (7.8125–1,000 ng/mL) and samples were run in duplicate.
Tissue collection and c-Fos immunohistochemistry
On the tenth day of chronic social defeat stress, brains were collected from B6 females after no defeat or a 5-min social defeat stress episode followed by an hour-long threat period (Fig. S1B). Fifty micron brain slices containing the anteromedial bed nucleus of the stria terminalis (amBNST), ventral lateral septum (LSv), hypothalamic paraventricular nucleus (hPVN), periventricular nucleus (PeN), ventromedial hypothalamus (VMH), medial amygdala (MeA), and dentate gyrus (DG) were selected for c-Fos immunohistochemistry.
Open field social interaction
After a 2.5-min habituation period in the social interaction apparatus (84×29×36 cm) containing an empty wire mesh stimulus cage (11 cm height, 10.5 cm diameter; Fig. 5C), control and defeated females were briefly removed while an unfamiliar, aggressive CFW female was placed in the stimulus cage. Experimental mice were returned to the apparatus and evaluated for social interactions during a 2.5-min test. Social interaction time was defined as the duration spent within a social interaction zone extending 2.25 cm past the radius of the stimulus cage (Ethovision XT v. 14). Social interaction videos were scored manually for vigilance-like behavior, defined here as time spent oriented toward but not interacting with the stimulus animal (cf., 46). The open field and stimulus cage were cleaned and dried between mice.
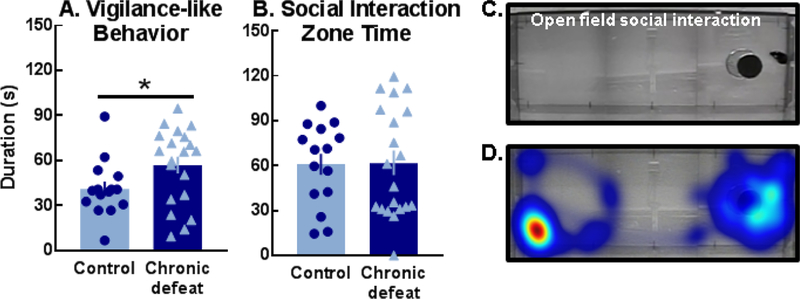
Figure 5.
Chronically defeated females expressed more (A) vigilance-like behavior than controls (t(33)=2.05, *p=0.048) despite both groups spending (B) similar durations within the social interaction zone. (C) Representative image of vigilance-like behavior displayed by a chronically defeated female and (D) the corresponding heat map of activity during the open field social interaction test.
Novel object investigation
Experimental females were briefly moved to clean holding cages while four rubber stoppers (14-135G/14-130G; Fisher Scientific, Agawam, MA) were placed in the home cage (Fig. 7A). Females were returned to the home cage for a 5-min test. Rectal temperatures were collected from experimental females immediately prior to and following tests using a thermo-probe (2100 Tele-thermometer, YSI, Inc., Yellow Springs, OH, USA) lubricated with mineral oil.
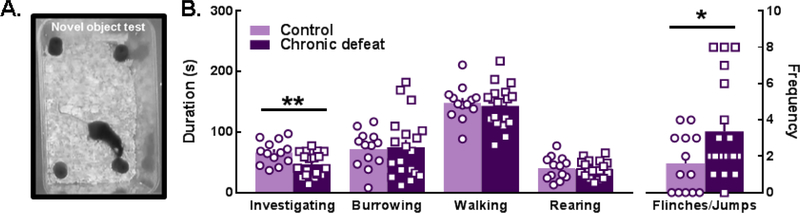
Figure 7.
(A, B) Chronically defeated females spent less time investigating novel objects (t(30)=2.849, p=0.0078) and exhibited more defensive flinches and jumps (t(30)=2.126, p=0.0419) compared to nondefeated females. All data are shown as the Mean ± SEM; *p<0.05, **p<0.01, control vs. chronic defeat.
Home cage social interactions and ketamine administration
Experimental female-initiated social contact with a non-aggressive, group-housed B6 stimulus female was evaluated during 1.5-min tests in the experimental female’s home cage. Alternatively, home cage social interaction tests were conducted using anesthetized stimulus females (Fig. S1A). Rectal temperatures were collected from experimental females immediately prior to and following testing.
Chronically defeated and control females received intraperitoneal injections of 0.9% NaCl or the N-methyl-D-aspartate (NMDA) receptor antagonist, ketamine hydrochloride diluted in 0.9% NaCl (20 mg/kg; VedCo Inc., Saint Joseph, MO, USA;17, 21, 47, 48). Social interactions were evaluated 30 min, 24 hrs, and 5 days post-injection.
Nesting
Females received two grams of nesting material (Nestlets, Ancare Corp., Bellmore, NY, USA) at 1330hr in the home cage. Five days later, nests were scored on a scale of 1–5 (49), nest heights and diameters were recorded, and nest images were evaluated for shape (i.e., circularity) in ImageJ.
Results
Interfemale rival aggression
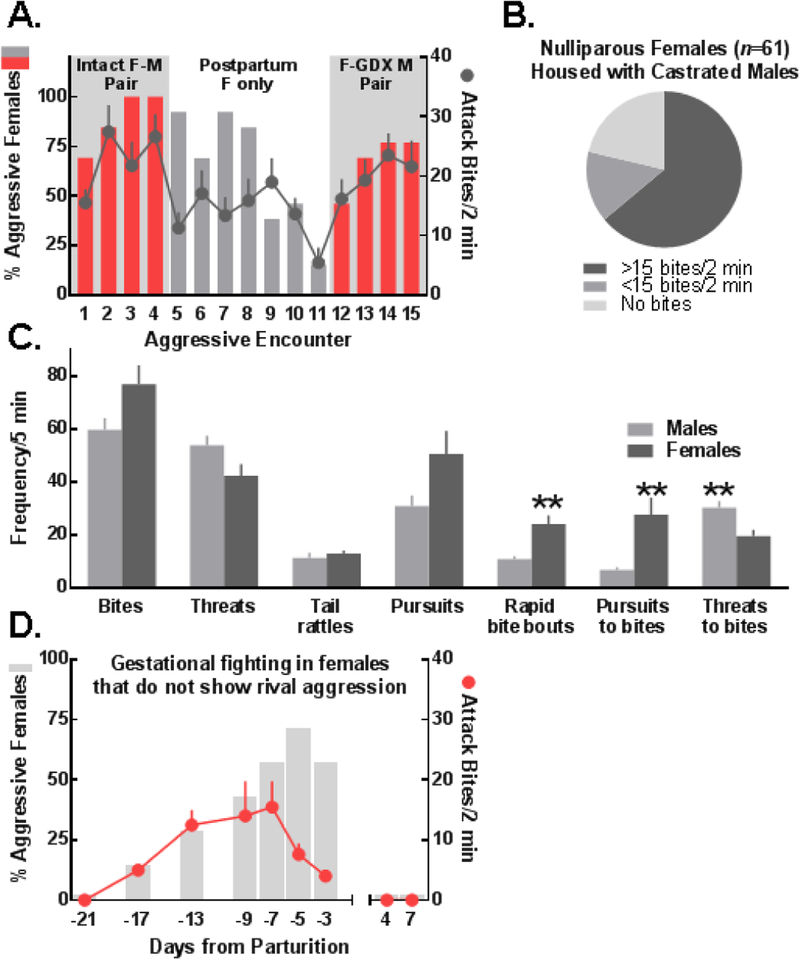
Most intact resident CFW females were aggressive when housed with a male, but not when housed in isolation (Fig. 1A) or following ovariectomy (Fig. S2). By the third aggressive confrontation, >90% of resident CFW females that were housed with an intact male expressed gestational aggression (attack bites: M±SEM=26.62±3.83). However, by the seventh day after litters were culled and intact males were removed, <20% of females were aggressive and females that did express aggression showed substantially reduced attack bite frequencies (M±SEM=5.5±2.5). Five days after being rehoused with castrated males, most resident females fought (75%; attack bites: M±SEM=21.6±4.34). Similarly, most intact nulliparous CFW females housed exclusively with castrated males fought and a significant subset (65%) emerged as highly aggressive toward unfamiliar B6 intruders (Fig. 1B).
Figure 1.
(A) Most multiparous and (B) nulliparous outbred females living with intact or gonadectomized (GDX; i.e., castrated) males developed aggression toward unfamiliar C57BL/6J female intruders. (C) Male and female attack bite frequencies were comparable, but females (n=10) displayed more sequential bites (rapid bite bouts; t(18)=3.85) and their bites were more frequently preceded by pursuits (pursuits to bites; t(18)=3.20) whereas males (n=10) exhibited more bites preceded by sideways threats (threats to bites; t(18)=3.14); data shown as Mean ± SEM; **p<0.01 male vs. female. (D) Most non-aggressive nulliparous females displayed pregnancy-induced aggression that was time-locked with the gestational period. (A, D) Left axes denote the percentage of animals that were aggressive and right axes depict attack bite frequencies as Mean ± SEM, calculated from females that fought.
Attack bite frequencies were similar between resident female and male aggressors (Fig. 1C). During confrontations with an unfamiliar intruder, resident females exhibited more rearing behavior (Fig. S4; 50) and rapid bouts of consecutive bites which were often preceded by pursuits (Fig. 1C; Video S1). In contrast, male attacks were often preceded by sideways threats (Video S2). Estrous cycle phase was determined in highly aggressive females (n=23). While an effect of phase on aggression was not apparent (Fig. S3), this could be explained by a ceiling effect and low variability in attack bite frequencies among aggressive females.
A subset of nulliparous resident females housed with castrated males were consistently nonaggressive toward intruder females (n=13/61). Half of these mice (n=7) were tested for their sensitivity to the pro-aggressive effects of pregnancy. By late-pregnancy, 75% of formerly non-aggressive females attacked an unfamiliar female intruder; however, after pups were culled on PND1, these residents returned to their non-aggressive, pre-pregnancy baselines (Fig. 1D).
Neural and physiological effects of chronic female social defeat stress
Acute or ten-day social defeat stress (Fig. 2A; Video S3) followed by a threat period increased circulating corticosterone more than the threat period alone (Fig. 2B). Similar corticosterone concentrations in acutely and chronically defeated mice suggest that females do not habituate to social defeat stress, much like acutely and repeatedly defeated outbred males (51). However, neither estrous cycle nor body weight was significantly affected by chronic social defeat (Fig. S5A–E).
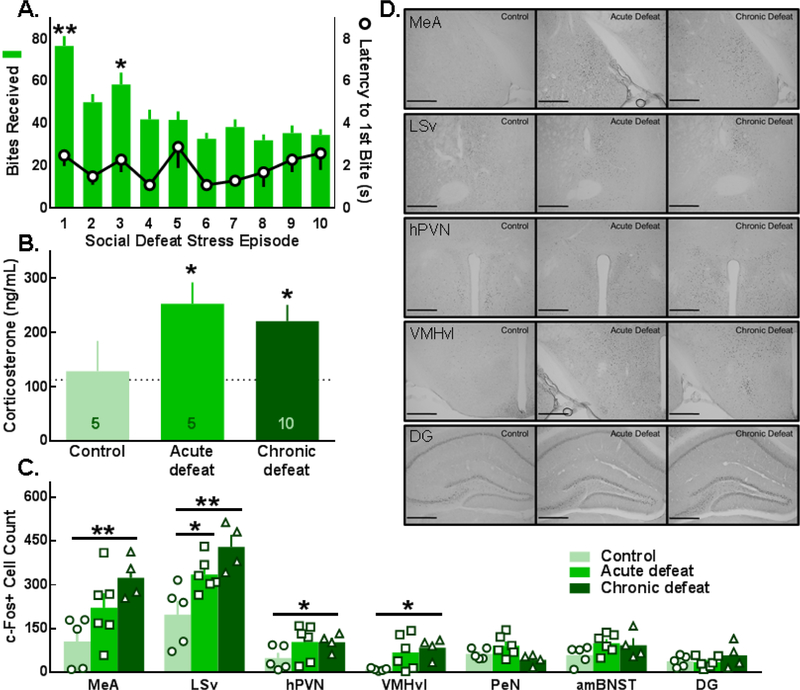
Figure 2.
Female C57BL/6J mice were defeated by aggressive resident CFW females for 10 consecutive days. (A; right axis). Attack latencies were <5 sec and (A; left axis) the greatest number of attacks were delivered early in the defeat protocol (left axis; F(9,190)=13.03, p<0.0001; *p<0.05, **p<0.0001 compared to day 5). (B) Elevated concentrations of plasma corticosterone were detected after acute or chronic social defeat stress (time: F(1,17)=9.6, p=0.007; defeat: F(2,17)=4.2, p=0.033; *p<0.05 compared to control). (C) Social defeat stress increased c-Fos activation in the medial amygdala (MeA; F(2,12)=6.43, p=0.013), the ventral lateral septum (LSv; F(2,12)=9.28, p=0.004), the hypothalamic paraventricular nucleus (hPVN; F(2,12)=4.48, p=0.035), and the ventrolateral division of the ventromedial hypothalamus (VMHvl; F(2,12)=4.17, p=0.042); *p<0.05, **p<0.01, compared to control. (D) Representative images of c-Fos in the MeA, LSv, hPVN, VMHvl, and dentate gyrus (DG); PeN, periventricular nucleus; amBNST, anteromedial bed nucleus of the stria terminalis; scale bars are 200 μm. Data are shown as Mean ± SEM.
Compared to the control condition, acute or chronic social defeat stress significantly increased c-Fos activation in the LSv whereas only chronic social defeat significantly increased the number of c-Fos+ cells in the MeA, hPVN, and VMHvl (Fig. 2C, D). Exploratory correlational analyses revealed patterns of interregional c-Fos activation. Specifically, there was a positive relationship between c-Fos+ cell counts in the MeA and amBNST in control whereas an inverse correlation was observed in defeated mice (Table S1). Females subjected to chronic social defeat also exhibited a unique pattern of inverse correlations in c-Fos in the PeN and amBNST or DG.
Behavioral effects of chronic social defeat stress in female mice
During social interactions in the home cage (Fig. 3E) with a non-aggressive B6 stimulus female, chronically defeated individuals displayed a greater number of defensive kicks and flinches compared to controls (Fig. 3A) along with deficits in both anogenital/flank and total social contact (Fig. 3C, D). When an anesthetized social stimulus mouse was placed into their home cage, chronically defeated females actually engaged in more nasal contact compared to controls (Fig. 3B). Social investigation in the home cage also produced a substantial hyperthermic response which was greater in chronically defeated females compared to controls (Fig. 4A); in contrast, there was no group difference in hyperthermia induced by novel object investigation (Fig. 4B).
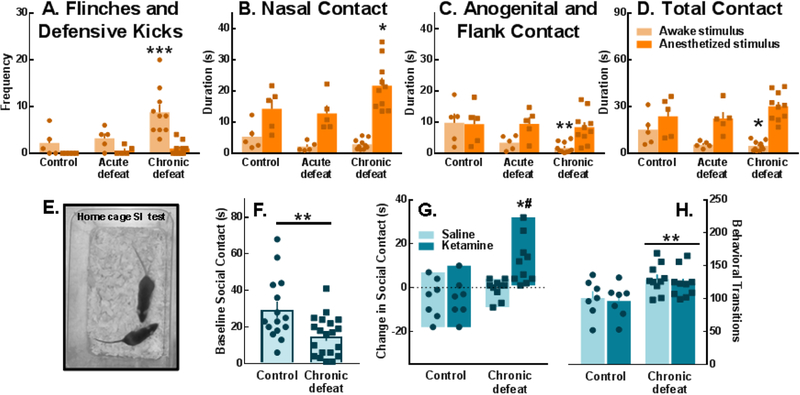
Figure 3.
Chronically defeated females exhibited substantial social contact deficits which were improved with ketamine, 24 hrs post-injection. (A) Defensive flinches and kicks were observed in chronically defeated females during social interactions with an awake conspecific (light bars; stimulus × defeat interaction: F(2,17)=3.67, p=0.047; stimulus: F(1,17)=15.3, p=0.001; defeat: F(2,17)=7.73, p=0.004). (B-D) Though chronically defeated females engaged in more nasal contact with anesthetized stimulus animals (dark bars; stimulus × defeat interaction: F(2,17)=3.8, p=0.043; stimulus: F(1,17)=54.53, p<0.0001), they displayed significantly less anogenital/flank contact (stimulus × defeat interaction: F(2,17)=3.87, p=0.041; stimulus: F(1,17)=13.67, p=0.002) and total contact (stimulus × defeat interaction: F(2,17)=6.18, p=0.0096; stimulus: F(1,17)=65.11, p<0.0001) with awake stimulus mice as compared to non-defeated controls. (E, F) Baseline total social contact with an awake stimulus female in the home cage was suppressed in chronically defeated mice (t(33)=3.056, p=0.0044). (G) Twenty-four hours post-injection, ketamine (20 mg/kg) significantly increased social contact in chronically defeated females (drug × defeat interaction: F(1,30)=4.26, p=0.0478; drug: F(1,30)= 4.26, p=0.0478; defeat: F(1,30)= 12.52, p=0.0013), (H) but did not reverse the high rate of behavioral transitions during social interaction tests (defeat: F(1,30)=11.00, p=0.0024). (A-D, F, H) Data are portrayed as the Mean ± SEM. (G) Bars depict the max and min values. The dotted line marks no change in social contact between baseline and post-injection tests; values above the dotted line are increases from baseline while values below are decreases from baseline social contact time. Circles and squares represent individuals. *p<0.05, **p<0.01, ***p<0.001 (A, C, D) compared to controls interacting with an awake social stimulus female, (B) compared to controls interacting with an anesthetized social stimulus female, (F, H) compared to controls, or (G) compared to ketamine-treated controls; #p<0.05, compared to saline-treated chronically defeated females.
Figure 4.
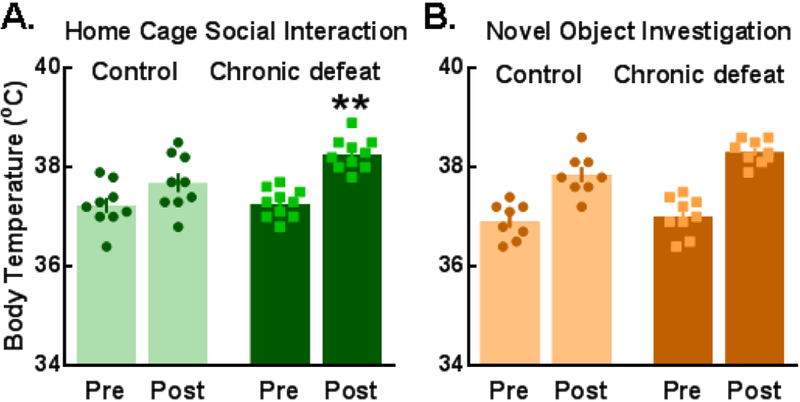
Hyperthermia was observed in response to 1.5-min social interaction and 5-min novel object investigation tests. Temperatures were measured immediately prior to (pre) and following (post) testing. (A) Chronically defeated females experienced a greater elevation in body temperature after social interactions compared to non-defeated controls (defeat × time interaction: F(1,17)=5.91, p=0.027; time: F(1,17)=42.21, p<0.0001). (B) All mice showed a similar degree of hyperthermia in response to novel object investigation (time: F(1,15)=113.6, p<0.0001). Data are depicted as the Mean ± SEM; **p<0.05, compared to control post-test temperature.
The total duration of experimental female-initiated social contact (i.e., non-aggressive social interaction) during baseline home cage testing (Fig. 3E) in mice that later received ketamine or saline was significantly lower among defeated animals (Fig. 3F). Twenty-four hours after receiving a dose of ketamine (20 mg/kg), defeated females exhibited a significant increase from pre-treatment social interactions compared to ketamine-treated controls and compared to defeated mice that received saline (Fig. 3G; Videos S4–7). This effect of ketamine was not evident 30 min or 5 days post-injection (Fig. S6). Importantly, although ketamine increased social contact duration in chronically defeated females, a defeat-associated increase in behavioral transitions during social interactions persisted (Fig. 3H).
Control and defeated females were also examined in an open field social interaction test (Fig. 5C) which is often employed to identify depressive-like phenotypes in chronically defeated male mice (8–10). Chronically defeated females exhibited significantly more vigilance-like behavior compared to controls (Fig. 5A, C, D; Video S8) though the duration of time spent in a predefined social interaction zone was comparable between groups (Fig. 5B). Correlational analyses of chronically defeated individuals revealed a significant inverse relationship between time investigating a social partner in the open field and vigilance-like behavior (Table S2).
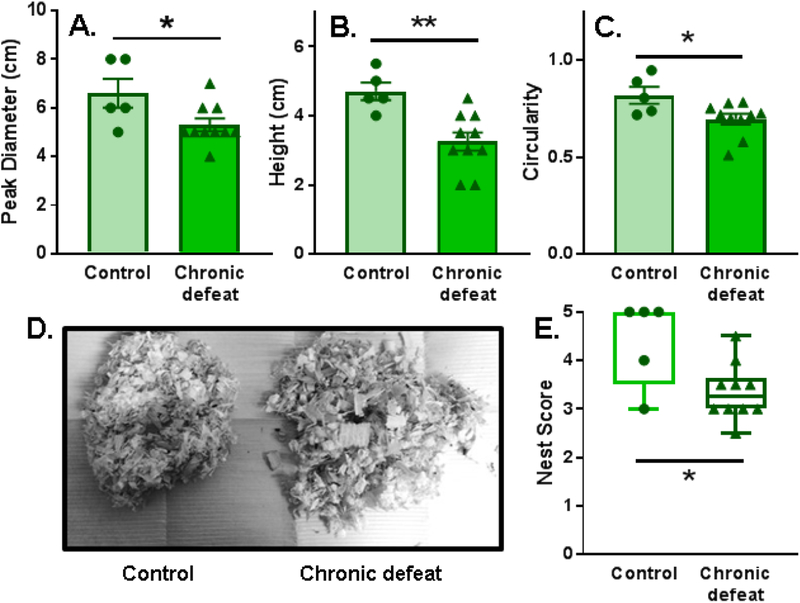
In terms of non-social behaviors, chronically defeated females constructed nests that were significantly less developed than controls as illustrated by measures of nest peak diameter, height, circularity, and overall nest score (Fig. 6A–E). Importantly, there was no group difference in baseline body temperature (Fig. 4), suggesting that nest-building deficits in defeated females were probably not due to stress effects on thermoregulation. A defeat phenotype was also evident when novel objects were placed in the home cage. Defeated females spent significantly less time investigating and exhibited a greater number of defensive startle-like behaviors (i.e. flinching and jumping) compared to controls (Fig. 7B). In contrast, measures of general anxiety-like behavior collected during light-dark box testing were similar between control and chronically defeated mice (Fig. S8). Interestingly, a greater number of attack bites received during the 10-day chronic social defeat stress protocol predicted reduced home cage social interactions and time spent in the light chamber during light dark box testing (Fig. S9).
Figure 6.
Nests constructed by chronically defeated females were underdeveloped compared to nests built by non-defeated animals, as measured by (A) nest peak diameter (t(13)=2.347), (B) nest maximal height (t(13)=3.503), (C, D) circularity (measured on a scale of 0–1, 1=perfect circle; t(13)=2.515), and (E) nest score (Mann-Whitney U=8.5, p=0.039). (A-C) Data are portrayed as the Mean ± SEM (D) or as the max and min surrounding the median; *p<0.05, **p<0.01 compared to control.
Discussion
We designed a novel and ethologically relevant model of chronic female social defeat stress that produces a distinct profile of neural and physiological effects along with pronounced depressive- and anxiety-like behaviors in defeated female mice (Table 1). After ten days of continuous social stress, females exhibited elevated levels of the stress hormone, corticosterone, and increased c-Fos activation in the MeA, LSv, VMHvl, and hPVN. In the days to weeks following social defeat, females engaged in atypical behaviors during novel object investigation, nest-building, and social interactions. Active investigation of a non-aggressive social partner during social interactions increased in defeated ketamine-treated females, indicating that our model of female chronic social defeat stress produces a phenotype that is sensitive to some antidepressant compounds (52, 53). Though ketamine increased social contact, defeated females that received drug treatment continued to exhibit atypically high rates of behavioral transitioning. Reduced behavioral stability in the presence of a non-aggressive individual may reflect a sensitized social threat response. The behavioral selectivity of ketamine raises the possibility that distinct mechanisms underlie chronic stress-induced deficits in social contact vs. vigilance-related impairments. These observations should be considered and extended in future preclinical studies focusing on stress-related psychopathologies that occur at higher rates in women than in men.
Table 1.
Effects of social defeat stress in female mice
| Neural activation (c-Fos) | Acute defeat | Chronic defeat |
|---|---|---|
| MeA | ↔ | ↑ |
| LSv | ↑ | ↑ |
| hPVN | ↔ | ↑ |
| VMHvl | ↔ | ↑ |
| PeN | ↔ | ↔ |
| amBNST | ↔ | ↔ |
| DG | ↔ | ↔ |
| Plasma corticosterone | ↑ | ↑ |
| Social interactions (HCSI) | ↔ | ↓ |
| Conspecific-induced defense (HCSI) | ↔ | ↑ |
| Social hyperthermia (HCSI) | N/A | ↑ |
| Social vigilance (OFSI) | N/A | ↑ |
| Social interaction zone time (OFSI) | N/A | ↔ |
| Novel object investigation (HCNO) | N/A | ↓ |
| Novelty-induced defense (HCNO) | N/A | ↑ |
| Novelty-induced hyperthermia (HCNO) | N/A | ↔ |
| Nest quality | N/A | ↓ |
| Anxiety-like behavior (LDB) | N/A | ↔ |
Consequences of acute or chronic social defeat stress in female C57BL/6J mice compared to non-defeated controls (p <0.05); no difference from controls, ↔; significantly greater than controls, ↑; significantly less than controls, ↓; not available, N/A; medial amygdala, MeA; ventral lateral septum, LSv; hypothalamic paraventricular nucleus, hPVN; ventrolateral division of the ventromedial hypothalamus, VMHvl; periventricular nucleus, PeN; anteromedial bed nucleus of the stria terminalis, amBNST; dentate gyrus, DG; home cage social interaction test, HCSI; open field social interaction test, OFSI; home cage novel object test, HCNO; light dark box test, LDB
Behavioral effects of chronic female social defeat stress
The pattern of aggressive behaviors recorded during interfemale agonistic encounters differed significantly from attack sequences during intermale fights. Considering the sophisticated exchange of multimodal sensory information between animals during ethological agonistic interactions, chronic social defeat stress procedures that rely on species-typical aggression may increase the translational potential of experimental findings. Like the pattern of aggression, significant features of the defeated female phenotype are distinct from males, and in female mice, the severity of persistent stress-induced behavioral deficits may depend on the severity of the stress experience. These observations should encourage a sex-specific approach to evaluating the consequences of defeat in male and female mice and the development of strategies tailored to treat specific symptoms (54).
We observed a hypervigilant-like phenotype in females subjected to chronic stress (46, 55), which may reflect an inability to distinguish threatening from non-threatening stimuli. Defeated females showed exaggerated defensive behaviors such as whole-body flinches, backwards jumps and defensive kicks toward non-aggressive social partners. These tests occurred within a familiar, nonthreatening environment, further illustrating impairments in threat assessment. Importantly, social deficits were not readily detected when social interaction zone time, a putative indicator of depressive-like behavior in males (9), was used as the dependent measure. Some defeat-induced behavioral deficits manifest in a sex-specific fashion, highlighting the importance of evaluating novel potential pharmacotherapies with probes that can detect sexually dimorphic adaptations to chronic stress.
Nesting behavior was also impaired in chronically defeated females. Measures of nest construction can serve as an overall indicator of rodent health (56–58) and can be inhibited in males exposed to social stressors (59, 60). As a goal-directed behavior, nesting requires a sequence of intricate actions to ultimately construct a protected, concave nest site (49, 61). Among other possibilities, poor nesting may result from decreased concentration on task completion or impaired motivation to engage in potentially rewarding species-typical behaviors (nest material as a reinforcer: 61–64). Defeated mice constructed incomplete nests, suggesting indecision or issues with concentration, both of which are cardinal symptoms of PTSD and MDD (65). Preferential allocation of attentional resources for threat assessment may impede nest completion in animals that exhibit a hypervigilant-like phenotype. Future studies that evaluate action sequence planning as well as the anticipatory, motivational, and learning processes that drive nesting behaviors could reveal unique circuit-level mechanisms that contribute to the defeat phenotype observed in female mice (61, 66).
Estrous cycling was similar between stressed and non-stressed females during chronic social defeat. Additional investigations need to determine if cycle phase and circulating hormone concentrations influence specific behavioral endpoints in females defeated by aggressive conspecifics. Further work is also required to fully examine which stress-induced behavioral impairments are sensitive to acute vs. repeated ketamine in female mice subjected to ethological stress conditions (67). We also did not observe a distinct bimodal distribution of “susceptible” and “resilient” chronically defeated females (Fig. S7A). Large-scale studies paralleling those conducted by Krishnan, Han et al. (10) in defeated males are necessary to definitively address the possibility of subgroups within the defeated female population. Such work may clarify the mechanisms that render some individuals more likely to develop affective psychopathologies compared to others and may guide the development of personalized treatment options.
Chronic social defeat stress and increased c-Fos activation in sexually dimorphic brain regions
Chronically defeated females exhibited c-Fos activation within several sexually dimorphic brain regions that comprise overlapping social (68–70) and defensive behavioral and threat-processing networks (46, 55, 71–74) including the MeA, LSv, VMHvl, and hPVN. While these areas are also activated in male rodents (75–80) exposed to repeated social defeat stress, regional sexual dimorphism can contribute to significant sex-dependent behavioral outcomes in response to cues (81–83) and experiences (84–86), illustrating the potential for sex-specific social defeat phenotypes despite similar patterns of c-Fos activation in chronically defeated male and female mice.
Interestingly, estrogen receptor alpha-expressing cells in the anterior portion of the VMHvl may control some aspects of active defense during acute social defeat (74). Our findings point to a similar cluster of cells that may be relevant to atypical social behaviors observed in chronically defeated females. Persistent stress-induced changes in estrogen signaling (5, 87, 88) within this cell population could contribute to exaggerated active defense and hypervigilance in defeated females.
Future directions: Female stress and aggression
Aggression is most often studied in the context of the male behavioral repertoire; yet, male and female rodents, non-human primates, and humans will readily engage in aggressive acts under certain conditions (22, 89–93). Here, we show that consistent and intense interfemale aggression can be generated in intact, but not ovariectomized, female mice living with a male conspecific. In addition, distinct aggressive and non-aggressive female subtypes are present within the aggressive subpopulation; some females display exclusively gestational aggression while others engage in gestational and rival aggression (Fig. S10). Comparable studies in rats have shown that intact, nulliparous females housed with sterile males become highly and persistently aggressive toward unfamiliar females (94), suggesting that the present murine model could be extended to study female rats under analogous defeat conditions. Additional similarities between female mice and rats (94–96) raise the possibility that there may be some adaptive and potentially conserved elements of interfemale rival aggression in rodents, and perhaps in other mammalian species.
Chronic social defeat stress produced a pattern of functional activation in brain areas also activated during mating or aggression in female mice including the MeA (82, 97) and VMHvl (98–101). It remains unclear whether these cell populations are functionally, molecularly, and spatially discrete or overlapping (86, 102). Interactions between these networks could allow one social experience to modify later behaviors; for example, stress-associated activation of cells in aggression-related brain regions could affect aggressive performance or motivation to engage in future agonistic behaviors. To address this, further behavioral and molecular studies are necessary to test the potential for rival aggression to motivate operant responding in control and socially defeated females (cf., 103–108).
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Antibody | Rabbit polyclonal anti-c-Fos | Proteintech Group, Inc. | Cat# 26192–1-AP | |
| Antibody | Biotinylated goat anti-rabbit | Vector Laboratories | Cat# BA-1000 | |
| Chemical Compound or Drug | Ketamine HCl | VedCo Inc. | Cat# VINV-KETA-0VED | |
| Commercial Assay or Kit | Vectastain avidin-biotin complex (ABC) kit | Vector Laboratories | Cat#PK-4000 | |
| Commercial Assay or Kit | 3,3'-diaminobenzidine (DAB) kit | Sigma-Aldrich | Cat# D4418 | |
| Organism/Strain | Mouse (Mus musculus): CFW, male and female | Charles River Laboratories | RRID: IMSR_CRL:24 | |
| Organism/Strain | Mouse (Mus musculus): C57BL/6J, female | The Jackson Laboratory | RRID: IMSR_JAX:000664 | |
| Software; Algorithm | Ethovision XT | Noldus Information Technology | RRID: SCR_000441 | |
| Software; Algorithm | The Observer XT | Noldus Information Technology | RRID: SCR_004074 | |
| Software; Algorithm | ImageJ | National Institutes of Health | RRID: SCR_003070 | |
| Software; Algorithm | Prism | GraphPad | RRID: SCR_002798 |
Acknowledgements and disclosures
Research reported in this publication was supported by the National Institutes of Health under award numbers F31AA025827 (E.L.N.), R01MH108665 (K.J.R.), R01AA013983 (K.A.M.), and R01DA031734 (K.A.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Mr. Thomas J. Sopko for his technical expertise and the Tufts University undergraduate research assistants Avni Rajpal and Susannah LaPointe.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB (1993): Sex and depression in the national comorbidity survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 29:85–96. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995): Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 52:1048–1060. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC (1997): The effects of stressful life events on depression. Annu Rev Psychol. 48:191–214. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC (2003): Epidemiology of women and depression. J Affect Disord. 74:5–13. [DOI] [PubMed] [Google Scholar]
- 5.Ramikie TS, Ressler KJ (2018): Mechanisms of sex differences in fear and posttraumatic stress disorder. Biol Psychiatry. 83:876–885. [DOI] [PubMed] [Google Scholar]
- 6.Rubinow DR, Schmidt PJ (2019): Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology. 44:111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miczek KA, Thompson ML, Shuster L (1982): Opioid-like analgesia in defeated mice. Science. 215:1520–1522. [DOI] [PubMed] [Google Scholar]
- 8.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA (1991): Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 38:315–320. [DOI] [PubMed] [Google Scholar]
- 9.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. (2006): Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 311:864–868. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. (2007): Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 131:391–404. [DOI] [PubMed] [Google Scholar]
- 11.Nestler EJ, Hyman SE (2010): Animal models of neuropsychiatric disorders. Nat Neurosci. 13:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covington HE, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. (2010): Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 30:16082–16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vialou V, Robison AJ, LaPlant QC, Covington HE, Dietz DM, Ohnishi YN, et al. (2010): DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 13:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden SA, Covington HE, Berton O, Russo SJ (2011): A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 6:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ (2006): Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 9:519–525. [DOI] [PubMed] [Google Scholar]
- 16.Covington HE, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. (2009): Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 29:11451–11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA (2014): Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 76:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, et al. (2017): Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol Psychiatry. 81:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, et al. (2018): A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology. 43:1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi A, Chung JR, Zhang S, Zhang HX, Grossman Y, Aleyasin H, et al. (2017): Establishment of a repeated social defeat stress model in female mice. Scientific Reports. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iniguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, et al. (2018): Vicarious social defeat stress induces depression-related outcomes in female mice. Biol Psychiatry. 83:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miczek KA, Maxson SC, Fish EW, Faccidomo S (2001): Aggressive behavioral phenotypes in mice. Behav Brain Res. 125:167–181. [DOI] [PubMed] [Google Scholar]
- 23.Noirot E (1969): Interactions between reproductive and territorial behavior in female mice. Int Ment Health Res Newsl. 11:10–11. [Google Scholar]
- 24.Noirot E, Goyens J, Buhot MC (1975): Aggressive behavior of pregnant mice toward males. Horm Behav. 6:9–17. [DOI] [PubMed] [Google Scholar]
- 25.Mann MA, Svare B (1982): Factors influencing pregnancy-induced aggression in mice. Behav Neural Biol. 36:242–258. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa S, Makino J (1984): Aggressive behavior in inbred strains of mice during pregnancy. Behav Neural Biol. 40:195–204. [DOI] [PubMed] [Google Scholar]
- 27.Hedricks C, Daniels CE (1981): Agonistic behavior between pregnant mice and male intruders. Behav Neural Biol. 31:236–241. [DOI] [PubMed] [Google Scholar]
- 28.DeBold JF, Miczek KA (1984): Aggression persists after ovariectomy in female rats. Horm Behav. 18:177–190. [DOI] [PubMed] [Google Scholar]
- 29.Svare B, Gandelman R (1973): Postpartum aggression in mice: experiential and environmental factors. Horm Behav. 4:323–334. [Google Scholar]
- 30.Svare B, Gandelman R (1976): Suckling stimulation induces aggression in virgin female mice. Nature. 260:606–608. [DOI] [PubMed] [Google Scholar]
- 31.Haney M, DeBold JF, Miczek KA (1989): Maternal aggression in mice and rats towards male and female conspecifics. Aggress Behav. 15:443–453. [Google Scholar]
- 32.Parmigiani S, Brain PF, Mainardi D, Brunoni V (1988): Different patterns of biting attack employed by lactating female mice (Mus domesticus) in encounters with male and female conspecific intruders. J Comp Psychol. 102:287–293. [DOI] [PubMed] [Google Scholar]
- 33.Rosenson LM, Asheroff AK (1975): Maternal aggression in CD-1 mice: influence of the hormonal condition of the intruder. Behav Biol. 15:219–224. [DOI] [PubMed] [Google Scholar]
- 34.Clipperton-Allen AE, Cragg CL, Wood AJ, Pfaff DW, Choleris E (2010): Agonistic behavior in males and females: effects of an estrogen receptor beta agonist in gonadectomized and gonadally intact mice. Psychoneuroendocrinology. 35:1008–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valzelli L (1973): The “isolation syndrome” in mice. Psychopharmacology. 31:305–320. [DOI] [PubMed] [Google Scholar]
- 36.Miczek KA, O’Donnell JM (1978): Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology. 57:47–55. [DOI] [PubMed] [Google Scholar]
- 37.Davis ES, Marler CA (2003): The progesterone challenge: steroid hormone changes following a simulated territorial intrusion in female Peromyscus californicus. Horm Behav. 44:185–198. [DOI] [PubMed] [Google Scholar]
- 38.Brain PF (1972): Effects of isolation/grouping on endocrine function and fighting behavior in male and female golden hamsters. (Mesocricetus auratus Waterhouse). Behav Biol. 7:349–357. [DOI] [PubMed] [Google Scholar]
- 39.Grelk DF, Papson BA, Cole JE, Rowe FA (1974): The influence of caging conditions and hormone treatments on fighting in male and female hamsters. Horm Behav. 5:355–366. [DOI] [PubMed] [Google Scholar]
- 40.McLean AC, Valenzuela N, Fai S, Bennett SA (2012): Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Been LE, Gibbons AB, Meisel RL (2018): Towards a neurobiology of female aggression. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floody OR, Pfaff DW (1977): Aggressive behavior in female hamsters: the hormonal basis for fluctuations in female aggressiveness correlated with estrous state. J Comp Physiol Psychol. 91:443–464. [DOI] [PubMed] [Google Scholar]
- 43.Hyde J, Sawyer TF (1977): Estrous cycle fluctuations in aggressiveness of house mice. Horm Behav. 9:290–295. [DOI] [PubMed] [Google Scholar]
- 44.de Jong TR, Beiderbeck DI, Neumann ID (2014): Measuring virgin female aggression in the female intruder test (FIT): effects of oxytocin, estrous cycle, and anxiety. PLoS One. 9:e91701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.More L (2008): Intra-female aggression in the mouse (Mus musculus domesticus) is linked to the estrous cycle regularity but not to ovulation. Aggress Beh. 34:46–50. [DOI] [PubMed] [Google Scholar]
- 46.Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, et al. (2018): Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress induced social avoidance in female California mice. Biol Psychiatry. 83:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. (2016): NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hultman R, Ulrich K, Sachs BD, Blount C, Carlson DE, Ndubuizu N, et al. (2018): Brain-wide electrical spatiotemporal dynamics encode depression vulnerability. Cell. 173:166–180.e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deacon RM (2006): Assessing nest building in mice. Nat Protoc. 1:1117–1119. [DOI] [PubMed] [Google Scholar]
- 50.Lisciotto CA, DeBold JF, Miczek KA (1990): Sexual differentiation and the effects of alcohol on aggressive behavior in mice. Pharmacol Biochem Behav. 35:357–362. [DOI] [PubMed] [Google Scholar]
- 51.Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, et al. (2015): Social stress and escalated drug self-administration in mice I. alcohol and corticosterone. Psychopharmacology. 232:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. (2000): Antidepressant effects of ketamine in depressed patients. Biological Psychiatry. 47:351–354. [DOI] [PubMed] [Google Scholar]
- 53.Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. (2006): A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression Arch Gen Psychiatry. 63:856–864. [DOI] [PubMed] [Google Scholar]
- 54.Dzirasa K, Covington HE (2012): Increasing the validity of experimental models for depression. Ann N Y Acad Sci. 1265:36–45. [DOI] [PubMed] [Google Scholar]
- 55.Blanchard DC, Griebel G, Pobbe R, Blanchard RJ (2011): Risk assessment as an evolved threat detection and analysis process. Neurosci Biobehav Rev. 35:991–998. [DOI] [PubMed] [Google Scholar]
- 56.Van de Weerd HA, Van Loo PL, Van Zutphen LF, Koolhaas JM, Baumans V (1997): Preferences for nesting material as environmental enrichment for laboratory mice. Lab Anim. 31:133–143. [DOI] [PubMed] [Google Scholar]
- 57.Jirkof P (2014): Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods. 234:139–146. [DOI] [PubMed] [Google Scholar]
- 58.Gaskill BN, Karas AZ, Garner JP, Pritchett-Corning KR (2013): Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp.51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Otabi H, Goto T, Okayama T, Kohari D, Toyoda A (2016): Subchronic and mild social defeat stress alter mouse nest building behavior. Behav Processes. 122:21–25. [DOI] [PubMed] [Google Scholar]
- 60.Rettich A, Käsermann HP, Pelczar P, Bürki K, Arras M (2006): The physiological and behavioral impact of sensory contact among unfamiliar adult mice in the laboratory. J Appl Anim Welf Sci. 9:277–288. [DOI] [PubMed] [Google Scholar]
- 61.Roper TJ (1976): Self-sustaining activities and reinforcement in the nest building behaviour of mice. Behaviour. 59:40–58. [Google Scholar]
- 62.Roper TJ (1973): Nesting material as a reinforcer for female mice. Anim Behav. 21:733–740. [Google Scholar]
- 63.Jansen PE, Goodman ED, Jowaisas D, Bunnell BN (1969): Paper as a positive reinforcer for acquisition of bar press response by the golden hamster. Psychon Sci. 16:113–114. [Google Scholar]
- 64.Oley NN, Slotnick BM (1970): Nesting material as a reinforcement for operant behavior in the rat. Psychon Sci. 21:41–43. [Google Scholar]
- 65.American Psychiatric Association (2013): Diagnostic and statistical manual of mental disorders. 5th ed Washington, DC. [Google Scholar]
- 66.Der-Avakian A, Markou A (2012): The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 35:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strong CE, Kabbaj M (2018): On the safety of repeated ketamine infusions for the treatment of depression: effects of sex and developmental periods. Neurobiol Stress. 9:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Newman SW (1999): The medial extended amygdala in male reproductive behavior: a node in the mammalian social behavior network. Ann N Y Acad Sci. 877:242–257. [DOI] [PubMed] [Google Scholar]
- 69.O’Connell LA, Hofmann HA (2011): The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 519:3599–3639. [DOI] [PubMed] [Google Scholar]
- 70.Kim Y, Venkataraju KU, Pradhan K, Mende C, Taranda J, Turaga SC, et al. (2015): Mapping social behavior-induced brain activation at cellular resolution in the mouse. Cell Rep. 10:292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canteras NS (2002): The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 71:481–491. [DOI] [PubMed] [Google Scholar]
- 72.Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, et al. (2016): Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol Psychiatry. 80:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang L, Chen IZ, Lin D (2015): Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron. 85:1344–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L, Talwar V, Osakada T, Kuang A, Guo Z, Yamaguchi T, et al. (2019): Hypothalamic control of conspecific self-defense. Cell Rep. 26:1747–1758.e1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M (1996): Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci Res. 26:157–170. [PubMed] [Google Scholar]
- 76.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. (2011): Beta-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 31:6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez M, Phillips PJ, Herbert J (1998): Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 10:20–33. [DOI] [PubMed] [Google Scholar]
- 78.Nikulina EM, Covington HE, Ganschow L, Hammer RP, Miczek KA (2004): Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: fos in the ventral tegmental area and amygdala. Neuroscience. 123:857–865. [DOI] [PubMed] [Google Scholar]
- 79.Kollack-Walker S, Don C, Watson SJ, Akil H (1999): Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J Neuroendocrinol. 11:547–559. [DOI] [PubMed] [Google Scholar]
- 80.Martinez M, Calvo-Torrent A, Herbert J (2002): Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 5:3–13. [DOI] [PubMed] [Google Scholar]
- 81.Xu PS, Lee D, Holy TE (2016): Experience-dependent plasticity drives individual differences in pheromone-sensing neurons. Neuron. 91:878–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishii KK, Osakada T, Mori H, Miyasaka N, Yoshihara Y, Miyamichi K, et al. (2017): A labeled-line neural circuit for pheromone-mediated sexual behaviors in mice. Neuron. 95:123–137.e128. [DOI] [PubMed] [Google Scholar]
- 83.Lischinsky JE, Sokolowski K, Li P, Esumi S, Kamal Y, Goodrich M, et al. (2017): Embryonic transcription factor expression in mice predicts medial amygdala neuronal identity and sex-specific responses to innate behavioral cues. Elife. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, Schnitzer MJ, et al. (2017): Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell. 171:1176–1190.e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Remedios R, Kennedy A, Zelikowsky M, Grewe BF, Schnitzer MJ, Anderson DJ (2017): Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature. 550:388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, et al. (2018): Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science. 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. (2011): Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 470:492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mercer KB, Dias B, Shafer D, Maddox SA, Mulle JG, Hu P, et al. (2016): Functional evaluation of a PTSD-associated genetic variant: estradiol regulation and ADCYAP1R1. Transl Psychiatry. 6:e978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haug M (1978): Attack by female mice on “strangers”. Aggress Behav. 4:133–139. [Google Scholar]
- 90.Stockley P, Campbell A (2013): Female competition and aggression: interdisciplinary perspectives. Philos Trans R Soc Lond B Biol Sci. 368:20130073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reinhardt V, Reinhardt A, Reinhardt C (1987): Evaluating sex differences in aggressiveness in cattle, bison and rhesus monkeys. Behaviour. 102:58–66. [Google Scholar]
- 92.Duque-Wilckens N, Trainor BC (2017): Behavioral neuroendocrinology of female aggression. Oxford Res Encycl Neurosc. DOI: 10.1093/acrefore/9780190264086.013.11 [DOI] [Google Scholar]
- 93.Denson TF, O’Dean SM, Blake KR, Beames JR (2018): Aggression in women: behavior, brain and hormones. Front Behav Neurosci. 12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Albert DJ, Dyson EM, Petrovic DM, Walsh ML (1988): Activation of aggression in female rats by normal males and by castrated males with testosterone implants. Physiol Behav. 44:9–13. [DOI] [PubMed] [Google Scholar]
- 95.Albert DJ, Petrovic DM, Walsh ML (1989): Ovariectomy attenuates aggression by female rats cohabiting with sexually active sterile males. Physiol Behav. 45:225–228. [DOI] [PubMed] [Google Scholar]
- 96.Albert DJ, Jonik RH, Watson NV, Moe IV, Walsh ML (1991): Aggression by a female rat cohabitating with a sterile male: termination of pseudopregnancy does not abolish aggression. Physiol Behav. 50:519–523. [DOI] [PubMed] [Google Scholar]
- 97.Unger EK, Burke KJ, Yang CF, Bender KJ, Fuller PM, Shah NM (2015): Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 10:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, et al. (2013): Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 153:896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, et al. (2017): Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat Neurosci. 20:1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen P, Hong W (2018): Neural circuit mechanisms of social behavior. Neuron. 98:16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hashikawa K, Hashikawa Y, Lischinsky J, Lin D (2018): The neural mechanisms of sexually dimorphic aggressive behaviors. Trends Genet. 34:755–776. [DOI] [PubMed] [Google Scholar]
- 102.Sakurai K, Zhao S, Takatoh J, Rodriguez E, Lu J, Leavitt AD, et al. (2016): Capturing and manipulating activated neuronal ensembles with CANE delineates a hypothalamic social-fear circuit. Neuron. 92:739–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Covington HE, Newman EL, Tran S, Walton L, Hayek W, Leonard MZ, et al. (2018): The urge to fight: persistent escalation by alcohol and role of NMDA receptors in mice. Front Behav Neurosci. 12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Golden SA, Heins C, Venniro M, Caprioli D, Zhang M, Epstein DH, et al. (2017): Compulsive addiction-like aggressive behavior in mice. Biol Psychiatry. 82:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, Lin D (2016): Hypothalamic control of male aggression-seeking behavior. Nat Neurosci. 19:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fish EW, De Bold JF, Miczek KA (2002): Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology. 163:459–466. [DOI] [PubMed] [Google Scholar]
- 107.Fish EW, McKenzie-Quirk SD, Bannai M, Miczek KA (2008): 5-HT(1B) receptor inhibition of alcohol-heightened aggression in mice: comparison to drinking and running. Psychopharmacology. 197:145–156. [DOI] [PubMed] [Google Scholar]
- 108.Couppis MH, Kennedy CH (2008): The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology. 197:449–456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.