Abstract
Plants encounter a variety of adverse environmental conditions, such as high salinity, drought, extreme heat/cold and heavy metals contamination (abiotic stress) or attack of various pathogens (biotic stress). These detrimental environmental factors enhanced the ROS production such as singlet oxygen (1O2), superoxide (O•−2), hydrogen peroxide (H2O2) and hydroxyl radicals (OH•). ROS are highly reactive and directly target several cellular molecules and metabolites, which lead to severe cellular dysfunction. Plants respond to oxidative damages by activating antioxidant machinery to trigger signalling cascades for stress tolerance. H2O2 signalling balances the plant metabolism through cross-talk with other signals and plant hormones during growth, development and stress responses. H2O2 facilitates the regulation of different stress-responsive transcription factors (TFs) including NAC, Zinc finger, WRKY, ERF, MYB, DREB and bZIP as both upstream and downstream events during stress signalling. The present review focuses on the biological synthesis of the H2O2 and its effect on the upregulation of kinase genes and stress related TFs for imparting stress tolerance.
Keywords: Reactive oxygen species, Hydrogen peroxide, Oxidative stress, Transcription factor, Stress signalling, Phytohormone
Introduction
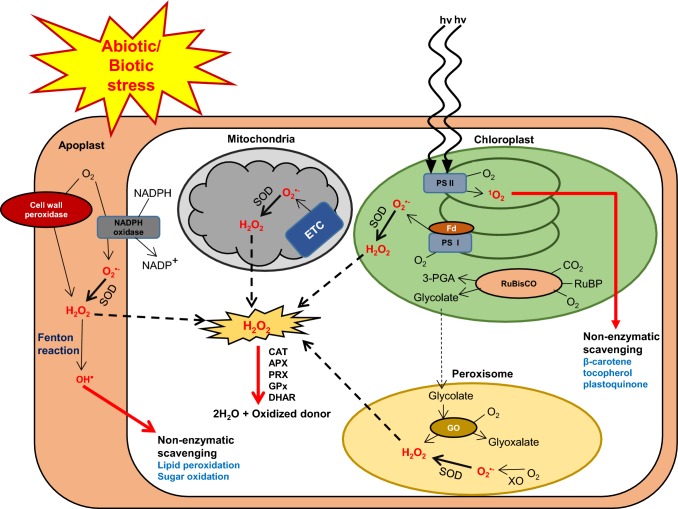
During stress-free conditions, plants’ aerobic metabolic processes such as respiration and photosynthesis produce several reactive oxygen species (ROS; O•−2, H2O2, OH• and 1O2) continuously as byproducts. The ROS are distributed in plant cellular organelles such as chloroplast, peroxisomes and mitochondria. The electron transport chain (ETC) during the aerobic metabolism and enzymatic catalysis by xanthine oxidase and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase produce O•−2 by directly reducing the O2. Spontaneous or catalytic breakdown of O•−2 leads to H2O2 production mainly by superoxide dismutase (SOD), an important antioxidative enzyme. SODs are categorized in three groups: copper/zinc SOD (Cu/Zn–SOD); iron SOD (Fe–SOD) and manganese SOD (Mn–SOD) based on the metal cofactors. Cu/Zn-SOD is predominantly localized in cytosol and chloroplast; while Fe–SOD and Mn–SOD are confined to chloroplast and mitochondria, respectively (McKersie et al. 2000; Xing et al. 2013, 2015). H2O2 is known for its cytotoxic effects and is controlled by non-enzymatic and enzymatic regulation. Major non-enzymatic antioxidants are ascorbate (vitamin C), glutathione (GSH), tocopherol (vitamin E), flavonoids, alkaloids, and carotenoids in plants. While enzymatic mechanisms include SOD, catalase (CAT), ascorbate peroxidase (APX), peroxidase (POX), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), guaiacol peroxidase (GOPX), and glutathione-S- transferase (GST) (Apel and Hirt 2004). However, H2O2 is potentially quite reactive to Fe2+ and consequently the Fe2+-containing cofactors found in some proteins are oxidized, by means of the Fenton reaction (Becana et al. 1998). The homolysis of H2O2 to 2 (OH•) is the “unpleasant” result of this reaction and ultimately oxidize the polyunsaturated fatty acids (PUFA) of lipid membrane and release water molecule (Fig. 1).
Fig. 1.
Schematic representation of ROS generation in different organelles of plant cell during abiotic and biotic stress, its scavenging through enzymatic and non-enzymatic pathways to pursue oxidative stress tolerance. ROS generation is inevitable by-products of aerobic cell metabolism and dramatically enhance in adverse environmental conditions. Apoplastic ROS is generated through cell membrane-bound NADPH oxidase, cell wall-associated peroxidases and fenton reaction. Singlet oxygen (1O2) and hydrogen peroxide (H2O2) are produced in high light intensity as well as during photosynthesis and photorespiration in chloroplast. Mitochondrial ETC generates superoxide anion (O•−2) in response to stress, which is ultimately converted to H2O2 by superoxide dismutase (SOD). In peroxisomes, H2O2 is accumulated during glyoxalate synthesis and SOD-mediated dismutation of O•−2, produced by Xanthine oxidase (XO). O•−2 is dismutated into the H2O2 through SOD enzyme. H2O2 is catalysed by various enzymes such as catalase (CAT), peroxiredoxins (PRX), ascorbate peroxidase (APx), glutathione peroxidase (GPx) and dehydroascorbate reductase (DHAR). Hydroxyl radicle (OH•) is reduced non-enzymatically through lipid peroxidation and sugar oxidation. The 1O2 is also non-enzymatically processed by donating electron to β-carotene, tocopherol and plastoquinone and confer oxidative stress tolerance. Black arrow (→) indicates ROS generation; Red arrow (→) indicates ROS scavenging
Adverse stress conditions such as salinity, drought, temperature, high light intensity and wounding disturb the equilibrium between production and scavenging of ROS. Gill and Tuteja (2010) reported the enhanced production of ROS in chloroplasts during abiotic stresses such as salt, drought and excess light conditions. Salinity stress damages photosystem and causes the production of O•−2 via Mehler reaction by overloading of electron flow in the ETC (Apel and Hirt 2004). During salinity and drought, plants face reduction in water availability and stomatal closure and thus decrease in CO2 to O2 ratio in mesophyll cells which finally enhance the photorespiration and production of glycolate in chloroplasts (Miller et al. 2008). Glycolate oxidase-mediated oxidation of glycolate in peroxisomes accounts for the majority of H2O2 production during photorespiration (Noctor et al. 2002; Karpinski et al. 2003). In peroxisomes, other major processes such as β-oxidation of fatty acid, the flavin oxidase pathway and the dismutation of O•−2 radicals also involved in H2O2 production (Corpas et al. 2001; Del Río 2011). Peroxisomes are also source of O•−2 production by matrix localised enzyme, xanthine oxidase and NADPH-dependent ETC (Karuppanapandian et al. 2011; Del Río 2011). Under environmental stress, especially in drought and salinity, ROS production has been shown to upsurge in mitochondria (Alscher et al. 1997; Bartoli et al. 2004; Pastore et al. 2007). Over-reduction of the ubiquinone after disruption in mitochondrial ETC function, results in increased ROS production (Rhoads et al. 2006). Abiotic stress also mediate apoplastic ROS production through any of the known four different mechanisms such as NADPH oxidase, peroxidases (O’Brien et al. 2012), oxalate oxidase (Voothuluru and Sharp 2012) and xanthine dehydrogenase (Ma et al. 2016). Plasma membrane located NADPH oxidase-Respiratory burst oxidase homologs (RBOH) protein is the most studied mechanism, which is associated with calcium and ROS signalling during stress and produce superoxide in the apoplast (Gilroy et al. 2016).
ROS mediated cellular damages
Lipid peroxidation
At cellular level, the most damaging consequence of ROS generation and accumulation is lipid peroxidation on both cell and organelle membranes. Beyond the utmost level of ROS accumulation, lipid peroxidation ultimately cause the oxidative stress through production of lipid-derived radicals (Montillet et al. 2005). The membrane PUFAs [linoleic acid (18:2) and linolenic acid (18:3)] are the main targets of O•−2 and OH• and enhance the formation of the polar lipid hydroperoxide complex mixtures, resulting in enhanced membrane fluidity, cytosolic solutes efflux and membrane associated protein functional loss (Moller et al. 2007). As a result of PUFA peroxidation, some aldehydes such as malondialdehyde (MDA) and 4-hydroxy-2-nonenal are created which attack amino acid side chains in proteins and also cause fragmentation of DNA (Gill and Tuteja 2010).
Protein oxidation
The important ROS-mediated post-translational modifications are sulfonylation, carbonylation, glutathionylation and s-nitrosylation. Sulfonylation is the oxidation of sulfhydryl groups, one of the main mechanisms that control the activity of many enzymes and transcription factors (TFs) in plants. H2O2 generating sulfenic acid (R-SOH) mainly induces the oxidation that can cause disulfide (S–S) bond formation between cysteine residues and resulted in conformational changes that alter protein/enzyme activity. The oxidation of cysteine residues to sulfenic acid is the first step in the ROS-dependent redox signalling pathway. The process can be relapsed mainly via thioredoxins (Trxs), peroxiredoxins (PRXs) and glutathione (GSH) system, which respond to stress and regulate redox homeostasis.
S-Glutathionylation is the further modification of the sulfenic acid-containing side-chains of proteins through covalent bond formation with low molecular weight thiols (e.g. GSH) and can regulate redox-driven signal transduction cascades and metabolic pathways (Fratelli et al. 2004). Glutathionylation can be reversed through the thiol-disulfide oxidoreductases Grxs activity (also known as thioltransferases; Gallogly and Mieyal 2007).
Another form of protein oxidation is the carbonylation which occurs at arginine, histidine, lysine, proline and threonine residues and considered to be irreversible (Shacter 2000). Indirect reactions of lipoperoxidation products with Cys and His residues can also lead to carbonylation of proteins (Madian and Regnier 2010).
During stress, some proteins get modified by the S-nitrosylation, the covalent binding of nitric oxide (NO) to thiol groups of Cys, modulates the signalling cascades. The different enzymes involved in respiration, antioxidation and photorespiration have been modified via S-nitrosylation during salinity stress (Camejo et al. 2013; Htet Hlaing and Clement 2014). It has been reported that s-nitrosylation also affect DNA binding activity of some TFs as well as inactivate RBOH (Yun et al. 2011). For example, AtMYB30 TF from Arabidopsis thaliana, an essential stress regulatory protein, is negatively regulated by S-nitrosylation (Tavares et al. 2014). H2O2 lead to alteration in conformational changes, nucleocytoplasmic shuttling, proteolysis, or interaction with other regulators of some H2O2-regulated TFs with redox-sensitive Cys residue (Dietz 2014).
ROS cross-talk between biotic and abiotic stresses
ROS and biotic stress
ROS play pivotal role in biotic stress during plant pathogen defence and wounding. ROS are generated by plant cells through the plasma-membrane bound NADPH-oxidases, cell wall-bound peroxidases, oxalate oxidase and xanthine dehydrogenase in the apoplast (Grant and Loake 2000; O’Brien et al. 2012; Voothuluru and Sharp 2012; Ma et al. 2016). Plant PCD is induced by the incompatible plant–pathogen interaction at the infection sites and response known as hypersensitive response (HR; Greenberg and Yao 2004). HR and associated ROS work against hemi-biotrophic and biotrophic pathogens. To avoid the death of the entire plant, HR response may further lead to activation of systemic acquired resistance (SAR). During plant–pathogen interaction, the SA plays an important role for onset of PCD. The coordinated production of ROS (over a certain threshold) and down-regulation of ROS scavenging mechanisms are the absolute requirement for PCD (Delledonne et al. 2001; Mittler et al. 1999). During plant pathogen reactions, a virulent pathogens are recognized by plants via R proteins and generates biphasic ROS (H2O2) production; a low-amplitude and transient first phase (ROS burst) followed by an unremitting phase of extreme magnitude (threshold level). Biphasic increases of H2O2 also produces during ozone or wounding stress (Lamb and Dixon 1997; Grant and Loake 2000; Joo et al. 2005). Alvarez et al. (1998) reported the oxidative burst in infected leaves of Arabidopsis leading to secondary systemic burst in distal parts and express the defence-related genes to provide plant immunity. Furthermore, AtRbohD also facilitates a long-distance signal for triggering the defence response at distal sites, in response to Pseudomonas syringae pathovar tomato (Pst) DC3000 (Dubiella et al. 2013). In an another study, pathogens Phytophthora cryptogea elevates the ROS generation in chloroplasts, which plays an important role in executing HR-like cell death (Ren et al. 2006).
ROS and abiotic stress
Abiotic stress such as salinity, drought, temperature, flooding generates ROS, which is further scavenged by the enzymatic and non-enzymatic anti-oxidant system. PCD occurs not only after the oxidative burst during pathogen challenge, but also by the exposure of abiotic stress. For example, the ozone-induced oxidative burst causes cell death process that shares similarities with the HR. It is probably due to the initial step of ozone signalling in the regulation of RBOH-dependent ROS production. It was reported that, exposure of acute ozone on A. thaliana induced biphasic ROS generation that is associated with the pathogen response (Joo et al. 2005). The ROS mostly performs opposite role during abiotic stress than it plays during pathogen defence. During abiotic stresses, ROS scavenging mechanisms are induced for the reduction in lethal concentration of intracellular ROS levels. These diversities in the function of ROS between biotic and abiotic stresses could evolve from the cross-talk between different signalling pathways and hormonal actions or from the spatial ROS production and/or accumulation during different stresses.
Stress hormones associated with ROS
Recent studies have shown that abiotic stress also generates a cross-talk among the plant hormones and ROS. Tognetti et al. (2012) have reported mutual interactions between ROS and auxin during abiotic stress causing auxin imbalance. Alteration in the auxin gradient have also been shown by stress-induced ROS production (Xia et al. 2015). Change in auxin equilibrium and perturbed signalling are associated with ROS-mediated auxin degradation (Kawano 2003); auxin conjugation (Tognetti et al. 2010); and auxin bypass through changes in the gene expression encoding auxin transporters (Grunewald and Friml 2010). Zhou et al. (2018) reported that exogenously applied H2O2 caused asymmetrical distribution of indole 3-acetic acid (IAA) in the primary root and influenced the gravitropism. During abiotic stress plants synthesisze brassinosteroids (BRs) hormone which helps in acclimation via physiological and biochemical processes (Xia et al. 2009). Nie et al. (2013) have showed correlation of BR synthesis and abiotic stress tolerance in tomato. RBOH transcription and enhanced NADPH oxidase activity were induced by BRs with the concurrent increase in apoplastic H2O2. Similarly the effect of abscisic acid (ABA) on RBOH expression and the generation of apoplastic H2O2 have been reported (Xia et al. 2015). In a recent study, the H2O2-dependent BRASSINAZOLE-RESISTANT1 (BZR1) TF showed regulation of auxin-signalling (AUXIN RESPONSE FACTOR6; ARF6) and light-signalling (PHYTOCHROME INTERACTING FACTOR4; PIF4) pathways during shoot cell elongation. Such significant overlaps of ROS with BR synthesis and auxin-light responses regulate shoot cell elongation through a central module of BZR1 interaction with the PIF4 and ARF6 factors (Tian et al. 2018). During abiotic stress conditions, gibberellins (GAs) promote cell division, cell elongation and plant growth (Colebrook et al. 2014). GAs execute the function under the regulation of the DELLA proteins, which are negative regulators of GA signalling (Achard et al. 2006). Cellular redox steady-state is also controlled via GA signalling and imparts stress tolerance. During drought condition, reduction in leaf GA contents lead to enhanced DELLA activity that resulted in increased ROS quenching capacity in maize and improved survival (Wang et al. 2008). Overexpression of the SUB1A (SUBMERGENCE 1A) gene in rice plants limits the ROS accumulation and oxidative damage during submergence stress by increased expression of the DELLA protein SLR1 and the SLR-like 1 (Fukao et al. 2006, 2011). Salicylic acid (SA) and nitrous oxide (NO) supress the activity of the ROS detoxifying enzymes APX and CAT, thus produce more ROS and activate program cell death (PCD) during the plant pathogen interaction (Klessig et al. 2000). Phytohormone ethylene is also having important role in stress signalling by modulating ethylene response factor (ERF) family TF. H2O2 activateds the phosphorylation of ERF6 via MPK6 and in turn bind to ROS-responsive cis-acting element 7 (ROSE7)/GCC box of the promoters of genes up-regulated by ROS. ROS-responsive expression of ERF6 is dependent on the NADPH oxidase (RbohD) and calcium signalling. The erf6 mutant study revealed its possible role in regulation of photooxidative damage as well as in anthocyanin synthesis (Wang et al. 2013; Sewelam et al. 2013). Collectively, all above findings propose the inter-linked role of ROS and phytohormones which regulate growth, development and stress responses in plants.
H2O2 as a member of ROS family
Even though H2O2 is known for its cytotoxic properties, it has also been recognized to play role as an important regulator of signal transduction. The H2O2 has very stringent regulatory mechanisms from production to degradation or to maintain ROS homeostasis. The small molecular structure and life longevity (1 ms) of H2O2 amongst other ROS make it precise messenger for signalling functions through crossing cellular membranes (Bienert et al. 2006) and acts in both autocrine and paracrine level in plants. H2O2 functions in concentration-dependent manner. At a low concentration, H2O2 acts as a signalling molecule and at high concentrations provokes cellular death (Gechev and Hille 2005).
Biological sources of H2O2
The H2O2 production in plant cells occurs during enhanced ETC or redox reactions in chloroplasts or mitochondria, fatty acid oxidation and photorespiration. Oxidation of glycolate by glycolate-oxidase is most significant in the peroxisome during the photosynthetic carbon oxidation cycle for H2O2 production (Noctor et al. 2002; Karpinski et al. 2003). The biphasic ROS production or oxidative burst, a part of HR during plant pathogen interaction, is also the cause of rapid increase in H2O2 concentration (Miller et al. 2010). The membrane-bound NADPH-dependent oxidases, similar to the RBOH, mostly cooperate in oxidative burst during stress signalling along with other inter-related cell wall-associated enzymes. The spontaneous or catalytic breakdown of superoxide anions, which are produced from oxygen reduction process during aerobic respiration and/or various environmental stresses, is also the predominant source of H2O2 generation.
H2O2 signalling in plants
Plants developed the mechanism to utilize H2O2 as a signalling molecule with remarkable impact on plant growth and behaviour during the stress treatments (Fig. 2). Apoplastic redox status elicits intra- and intercellular signalling. Due to high stability, H2O2 can be moved through the plasma membrane by diffusion or by aquaporin channels (Bienert and Chaumont 2014; Tian et al. 2016). H2O2 performs as a signalling molecule at both cellular and organ level as an autocrine (includes intracellular) and paracrine (intercellular) signals (Bienert et al. 2006).
Autocrine signalling
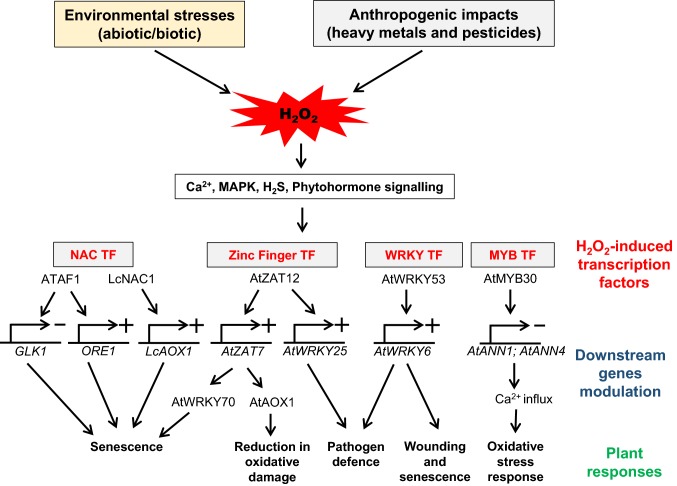
Fig. 2.
Schematic representation of H2O2 -mediated transcriptional cascading. Environmental stresses and anthropogenic activities produce stress conditions and lead to accumulation of H2O2, which serves as a second messenger and transmits the signal through sensors, including Ca2+, MAPK, H2S and phytohormone. Downstream cascading activates the H2O2–responsive transcription factors (TFs), which relays the signal to expression of downstream genes and regulates positively and negatively at transcriptional level for various oxidative stress responses
Autocrine signalling occurs in the same cell through chemical messengers. H2O2-mediated stomatal closure in guard cells is an example of autocrine signalling (Pei et al. 2000; Desikan et al. 2004). During water deficit condition, plants produce ABA as a downstream signalling molecule and cause the stomatal closure. NADPH oxidase acts as an important component for this signalling by elevation of apoplastic H2O2 levels. H2O2 can function directly as an effector molecule as well as a second messenger in signal amplification. It is postulated that redox-sensitive TFs could sense enhanced H2O2 levels and regulate downstream cascades. Class A heat shock factors (Hsfs) are the suitable candidates for such TFs, which are revealed as an oxidative stress-responsive both in animals and plants (Miller and Mittler 2006; Kotak et al. 2007). Mitogen-activated protein kinase (MAPK) cascades the external stimuli to cytoplasmic and nuclear responses and involve during osmotic stress (Hirt 1997), ozone (Samuel et al. 2000) and plant–pathogen interactions (Somssich 1997), which are linked to H2O2 through the redox network (Kovtun et al. 2000; Matern et al. 2015).
Paracrine signalling
Transport of H2O2 signals between the cells (intercellular), which need to be pass across membrane is called as paracrine signalling. Mittler et al. (2011) have reported the “ROS wave” cascade signal from cell-to-cell after the initial oxidative burst that transmits throughout different tissues and transports the signal over long distances. The “ROS wave model” linked with calcium signalling and other systemic signals such as ABA, hydraulic waves, and electric signals to imparts acclimation during abiotic stresses. Perfect example of paracrine signalling pathway is the H2O2-influenced xylem differentiation and lignification. During xylem lignification H2O2 lead to the synthesis of lignin biosynthesis enzymes such as phenylalanine ammonia lyase and help in polymerisation of coumaryl, coniferyl and sinapyl alcohols to lignin. In this manner, H2O2 instigates the secondary cell wall formation and finally the PCD in differentiating xylem cells (Fukuda 1996; Barcelo 2005).
H2O2 and mitogen-activated protein kinases (MAPKs) cascade
In plants, MAPKs are involved in diverse functions such as growth, development and acclimation during environmental changes. The MAPK signalling pathway is composed of three kinases, MAPK (MPK), MAPK kinase (MKK), and MAPK kinase kinase (MAPKKK or MEKK). It is suggested that exogenously applied H2O2, its accumulation or H2O2-induced oxidative burst induce the activation of MAPK pathway and relays the intracellular H2O2 signal. The MAPK cascades the upstream or downstream application of H2O2, and reveals its complexity and multi directionality during the stress signalling. H2O2 activates the ANP1 (MAPKKK) protein which subsequently induces AtMPK3 and AtMPK6 phosphorylation (Kovtun et al. 2000). While some MAPK pathways such as MEK2 perform upstream of Rboh genes and ultimately produce H2O2 (Yoshioka et al. 2003). In Arabidopsis, one of the ROS-mediated MAPK pathway includes the MEKK1–MKK1/2–MPK4 cascading, where MAPK kinase kinase MEKK1 is controlled by H2O2 and various stresses in a proteasome-dependent manner (Pitzschke et al. 2009) and activates the downstream kinases MPK3, MPK4 and MPK6. Remarkably, a unique MAPK pathway has been reported (Miao et al. 2007), where MEKK1 directly interacts and phosphorylates the TF WRKY53. Activated WRKY53 subsequently induces stress and defence-related target genes through DNA-binding activity. MAPKs also get activated in a H2O2-dependent manner during plant pathogen interaction and disease signals. In Arabidopsis, MPK3 and MPK6 (Nakagami et al. 2005) are associated with H2O2, whereas similar regulatory mechanism have been shown by respective homologes SIPK and WIPK kinases in tobacco (Asai and Yoshioka 2008). BRs-mediated apoplastic H2O2 accumulation switches on the MPKs and its cascading to impart stress tolerance in plants. In cucumber, BR-induced process upregulate the Rboh, MPK1 and MAPK3 genes (Xia et al. 2009) while in maize, BR-stimulated ZmMPK5 which drives NADPH oxidase-mediated activities of antioxidant enzymes (Zhang et al. 2010). Recently, the calcium-dependent protein kinase CPK27 had shown cross-talk between H2O2, NO, and MPK1/2 towards cold acclimation-induced ABA biosynthesis and cold tolerance in tomato (Lv et al. 2018). Similarly, Wang et al. (2017) reported that the H2O2 treatment enhanced the expressions of tomato SlMAPK1/2/3 and SlCBF1 genes and imparted the chilling tolerance by modulating the phytohormones concentration. The tolerance towards other stresses such as drought and salinity is also reported by the application of H2O2 through MAP kinase pathways (Wang et al. 2010). The cotton MKK1 (GhMKK1) is upregulated by H2O2 and showed enhanced stress tolerance in Nicotiana benthamiana transgenics (Lu et al. 2013). The H2O2 is also known to activate the rice salt-responsive ERF1 (SERF1) TF, which showed binding to the MAPK kinase kinase6 (MAP3K6) and MAPK5 and involved in initial phase of salt stress and resulted further in salt tolerance (Schmidt et al. 2013). The MAP cascade genes also reported to reduce the H2O2 content in the transgenic plants during abiotic stress condition and provides the tolerance. The Raf-like MAPKKK gene, GhRaf19 from the cotton showed chilling tolerance by alleviating the ROS via increased activity of ROS-related enzymes in N. benthamiana (Jia et al. 2016). A poplar PtMKK4 gene showed strong tolerance to drought stress, the transgenics showed significantly reduced level of H2O2 and higher antioxidant enzyme activity (Wang et al. 2014). Similarly, the cotton MAPK (GhMPK17) transgenics showed reduced level of H2O2 and tolerance to salt and drought tolerance (Zhang et al. 2014).
H2O2-mediated transcription factors
H2O2 is reported to induce the signalling cascade by modulating downstream genes expression by switch on or off the transcript expression machinery (Fig. 2). Transcription factor families such as NAC, Zinc finger (ZINC FINGER OF ARABIDOPSIS THALIANA; ZAT), WRKY, ERF, MYB, DEHYDRATION RESPONSIVE ELEMENT BINDING FACTOR (DREB), and BASIC LEUCINE ZIPPER (bZIP) are linked with H2O2-induced signalling. Induction of various members of plant TF family and their response in acclimatization of plants under oxidative stress condition after H2O2 treatment are mentioned in Table 1.
Table 1.
H2O2-induced transcription factors and their downstream cascading in regulation of oxidative stress after H2O2 treatment
| Sr. no. | Plant | H2O2-induced transcription factor | Regulation of oxidative stress | References |
|---|---|---|---|---|
| 1 | Arabidopsis thaliana | ZINC FINGER of ARABIDOPSIS THALIANA 6 (ZAT6) | Activation of anthocyanin biosynthesis | Shi et al. (2018) |
| 2 | Arabidopsis thaliana | BRASSINAZOLE-RESISTANT1 (BZR1) | Regulation of cell elongation | Tian et al. (2018) |
| 3 | Arabidopsis thaliana | REDOX RESPONSIVE TRANSCRIPTION FACTOR 1 (RRTF1)/ERF109 | Generation of an oxidative burst | Heyman et al. (2018) |
| 4 | Arabidopsis thaliana | MYB30 | Regulation of root elongation and influences plant immunity; Annexin-mediated oxidative stress response through elevation of calcium influx | Mabuchi et al. (2018), Liao et al. (2017) |
| 5 | Arabidopsis thaliana | WRKY75 | Positive regulator of leaf senescence through repressing the CATALASE2 (CAT2) expression | Guo et al. (2017) |
| 6 | Arabidopsis thaliana | Cytokinin Response Factor 6 (CRF6) | Repression of cytokinin-related genes to control the cytokinin-mediated ROS generation and improved oxidative stress | Zwack et al. (2016) |
| 7 | Arabidopsis thaliana | Heat shock factor class A (HsfA3) | Increased levels of galactinol and raffinose; Upregulation of APX2 (Ascorbate peroxidase 2) gene | Song et al. (2016), Hwang et al. (2012) |
| 8 | Arabidopsis thaliana | ZAT12 | Negatively regulate Iron (Fe) uptake; role in oxidative stress signalling | Le et al. (2016), Davletova et al. (2005) |
| 9 | Arabidopsis thaliana | Arabidopsis thaliana ACTIVATING FACTOR1 (ATAF1) | Progression of senescence | Garapati et al. (2015) |
| 10 | Arabidopsis thaliana | ANAC017 | Induction of AOX1a and function in mitochondrial retrograde signalling | Ng et al. (2013) |
| 11 | Arabidopsis thaliana | ANAC013 | Regulation of Mitochondrial retrograde signalling during oxidative stress | De Clercq et al. (2013) |
| 12 | Arabidopsis thaliana | Ethylene response factor6 (ERF6) | Role in the transcriptional regulation of ROS-responsive genes | Wang et al. (2013) |
| 13 | Arabidopsis thaliana | JUNGBRUNNEN1 (JUB1) | Delays senescence, reduces intracellular H2O2 levels and enhances abiotic tolerance | Wu et al. (2012) |
| 14 | Arabidopsis thaliana | ORESARA1 SISTER1 (ORS1) | Triggers expression of senescence-associated genes and accelerates senescence | Balazadeh et al. (2011) |
| 15 | Aeluropus lagopoides | AlNAC4 | ROS homeostasis and oxidative stress tolerance in transgenic tobacco | Khedia et al. (2018) |
| 16 | Litchi chinensis | LcNAC1 | Induction of LcAOX1 gene and regulate ROS production and energy metabolism | Jiang et al. (2017) |
| 17 | Lycopersicon esculentum | TOMATO ERF1 (TERF1) | Reduction of ROS accumulation and alleviate H2O2 stress in tobacco transgenics | Zhang et al. (2016) |
| 18 | Malus domestica | MdNAC1 | Enhances antioxidative enzymes activity (POD, CAT and SOD) as well as their gene expression | Jia et al. (2019) |
| 19 | Oriza sativa | Salt-responsive ERF1 (SERF1) | Confer salinity tolerance through MAPK pathway | Schmidt et al. (2013) |
| 20 | Poncirus trifoliata | basic/helix-loop-helix (bHLH) | Activation of POD-mediated H2O2 scavenging in transgenic tobacco | Huang et al. (2013) |
| 21 | Populus euphratica | PeSTZ1 | ROS scavenging through regulation of PeAPX2 and confers cold tolerance | He et al. (2019) |
| 22 | Solanum lycopersicum | CBF1 (C-repeat/dehydration-responsive factor) | Enhance chilling stress with phytohormone modulation | Wang et al. (2017) |
In Arabidopsis, many H2O2-regulated leaf senescence-associated NAC TFs have been identified, regulating senescence both positively (ATAF1, ANAC092, ANAC059, ANAC029 and ANAC016) and negatively (ANAC042, ANAC083). The H2O2 and ABA is reported to activate the Arabidopsis thaliana Activating Factor1 (ATAF1), an upstream regulator of senescence. The ATAF1 induced senescence by activating ANAC092 and repressing Golden 2-like1 (GLK1) genes by shifting the physiological balance (Garapati et al. 2015). Balazadeh et al. (2010a) have reported that H2O2-upregulated 15 senescence-associated NAC TFs, among which ANAC032 and ANAC042 TFs showed significantly higher induction. ANAC042 has already been reported in H2O2-induced relay cascades via OXI1/MPK3/6 activation (Gechev and Hille 2005). ANAC092/ORE1 have also been proved to play role in the regulation of salt-induced senescence (Balazadeh et al. 2010b) along with the developmental senescence in Arabidopsis (Kim et al. 2009). Some of the NAC TFs are reported to delay the senescence and help in providing the stress tolerance during abiotic stress. JUNGBRUNNEN1 (JUB1) gene, one of the NAC TF family member, showed delayed senescence and conferred abiotic stress tolerance by reducing intracellular H2O2 in transgenic Arabidopsis (Wu et al. 2012). The possible mechanism of JUB1 is by activating the DREB2A TF which in turn upregulate several ROS-responsive genes such as glutathione S-transferase. In Litchi chinensis, the enhanced expression of LcNAC1 was observed by ABA and H2O2 treatments. LcNAC1 positively activates the LcAOX1a gene and reduces the senescence time by regulating the ROS related genes and energy metabolism, whereas, LcWRKY1 regulates the LcAOX1a antagonistically (Jiang et al. 2017). Recently, it has been reported that overexpression of MusaSNAC1 increased H2O2 content in guard cells augmented stomatal closure for facilitating drought tolerance (Negi et al. 2018). Chilling tolerance was found to be enhanced by overexpressing tomato SlNAM1 TF in tobacco, wherein increased H2O2 content reduced by antioxidative enzymes (Li et al. 2016). The Aeluropus AlNAC4 TF showed significantly higher expression with H2O2 and dehydration treatments. The overexpression of the AlNAC4 TF in the tobacco confers oxidative stress tolerance by reducing H2O2 content through ROS homeostasis and regulation of stress-related downstream genes (Khedia et al. 2018). Borgohain et al. (2019) reported the role of tomato SlNAC2 TF in increased abiotic stress tolerance with expression of the important glutathione biosynthetic genes, which showed improved antioxidative response and reduced ROS accumulation (H2O2 and O•−2) in transgenic plants. Similarly, the apple MdNAC1 exhibited drought tolerance through enhanced activity of antioxidant enzymes such as peroxidase (POD), catalase (CAT) and superoxide dismutase (SOD) and reduced level of H2O2 content (Jia et al. 2019).
H2O2 also strongly induces the zinc finger TF family, especially various members of the ZINC FINGER OF ARABIDOPSIS THALIANA (ZAT) family at the transcript level (Gadjev et al. 2006). ZAT12 TF is one of the important component of the oxidative stress which transduce the signal to ZAT7 and WRKY25 TFs and express the cytosolic APX1 during elevation of H2O2 in Arabidopsis (Rizhsky et al. 2004). The Zat12 mutant plants showed the reduced expression of Zat7 by H2O2 treatment, which confirmed that ZAT12 regulate the activity of ZAT7. Miller et al. (2008) have shown the role of AtZAT proteins (AtZAT7, AtZAT10, and AtZAT12) in the ROS signalling and plant stress responses. Shi et al. (2018) showed pivotal role of the AtZAT6 in H2O2-mediated secondary metabolite synthesis such as anthocyanin through direct binding to promoters of anthocyanin synthesis genes. Studies have supported the antioxidant role of anthocyanin through reduction in photooxidative damage under high irradiance (Zeng et al. 2010; Zhang et al. 2012; Maruta et al. 2014) and wounding (Gould et al. 2002). Populus euphratica, PeSTZ1, a C2H2-type Zn finger TF showed freezing tolerance through direct regulation of PeAPX2 and modulate ROS scavenging (He et al. 2019).
WRKY TFs also play important role in biotic, abiotic and oxidative stresses (Eulgem and Somssich 2007). Improved expression of several WRKYs in Arabidopsis have been reported by H2O2 treatment (Chen et al. 2010) that regulate the senescence and defence-related gene expression (Besseau et al. 2012). WRKY52 and WRKY70 are widely reported H2O2-inducible TFs, from which WRKY52 is a senescence-associated and regulates functions of various stress and defence-responsive genes (Miao et al. 2004). Guo et al. (2017) showed involvement of WRKY75 TF in positive regulation of leaf senescence in the presence of SA and ROS in Arabidopsis. Similarly, WRKY53 TF positively accelerated leaf senescence through SA and JA equilibrium, and negative regulation through interaction with JA-inducible protein EPITHIOSPECIFYING SENESCENCE REGULATOR (ESR/ESP) (Miao and Zentgraf 2007). The jatropha, JcWRKY showed lower accumulation of ROS (H2O2 content) in transgenic tobacco compared to wild-type plants during Macrophomina infestation as well as by SA treatment (Agarwal et al. 2018).
Oryza sativa SALT-RESPONSIVE ERF1 (SERF1) a root-specific TF induces during salinity and H2O2 treatments. It promotes the expression of the H2O2-responsive genes, such as MAPK KINASE KINASE6 (MAP3K6), MAPK KINASE5 (MAPK5), DREB2A, and ZFP179 (Schmidt et al. 2013). Ectopic expression of tomato TERF1 in Nicotiana tabacum showed enhanced H2O2 stress tolerance in seedlings as well as activation of downstream genes involved in oxidative responses, including carbonic anhydrase (in hypersensitive defence), catalase, glutathione peroxidase (in antioxidant response) and GDP-d-mannose pyrophosphorylase (in ascorbic acid biosynthesis) (Zhang et al. 2016).
Arabidopsis myb30 mutant showed Ca2+ mediated signalling in response to H2O2 and heat stress (Liao et al. 2017) and similarly Mabuchi et al. (2018) reported strong response of ROS-induced AtMYB30 in the inhibition of root cell elongation. ZmMYB31 has been reported to play a role in chilling and peroxide stress alleviation in transgenic Arabidopsis through declined photoinhibition and ROS accumulation (Li et al. 2019). SbMYB15 TF from Salicornia brachiata conferred heavy metal (Cd2+ and Ni2+) tolerance in transgenic tobacco with reduced level of H2O2 content and increased ROS scavenging antioxidative enzyme activities (Sapara et al. 2019). SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) TF showed enhanced resistance against P. syringae with higher H2O2 accumulation and SA signalling pathway. Foliar H2O2 application confirmed the SA-mediated defence response with reduction of miR156 expression, which negatively regulates SPL9 TF (Yin et al. 2019). DREB2A TF is also included in the H2O2-induced TFs family (Rizhsky et al. 2003). DREB2A showed salinity and drought tolerance in transgenic tobacco plants by lowering the H2O2 content (Gupta et al. 2014). Li et al. (2017) have reported the involvement of bZIP TFs in H2O2-regulated family through conferring disease resistance against cassava bacterial blight.
Conclusion and perspectives
Plants being sessile have an innate ability to adapt itself to the various natural and manmade variations through complex stress signalling and leading to rapid alterations in physiology and metabolism pathways. The generation of ROS serves as a key molecular marker for adverse external stimuli and participates both positively and negatively for transcriptional signalling. Understanding the molecular mechanism towards oxidative stress responses of plants is very essential, as it acts as a central dogma towards coordinating the multiple stress responses. The synthesis of ROS-associated phytohormones such as auxin, brassinosteroid, gibberellin, salicylic acid and ethylene cross-talk during stress conditions and facilitates plant acclimation through various physiological and biochemical processes. H2O2 has the higher longevity amongst the different ROS family members and function as a secondary messenger during various stress responses. The oxidative burst regulates the ROS scavenging mechanism to maintain the cellular redox status that facilitates the proper enzymatic activation and metabolism. The research on the interaction of ROS production and scavenging for maintain redox status is gaining momentum. The NAC, Zinc finger, WRKY, ERF, MYB, DREB and bZIP are the major H2O2-induced TF families involved in oxidative stress responses. It is needed to understand the downstream events leading to specific responses. The dose- age specific ROS responses also is an important aspect of ROS signalling. The integration of the ROS is the central component of the signalling network, however, still the gene network, threshold concentration in different cellular compartments, ROS scavenging and interaction with other signalling components need to be elucidated to gain insights into the biological role of ROS in alleviating stress and also promoting growth and development.
Acknowledgements
CSIR-CSMCRI Communication No.-PRIS014. The financial assistance from the CSIR-SRF, DST-WOS-A and CSIR, New Delhi, India is duly acknowledged.
Compliance with ethical standards
Conflict of interest
No conflict of interests exist.
References
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311(5757):91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- Agarwal P, Patel K, Agarwal PK. Ectopic expression of JcWRKY confers enhanced resistance in transgenic tobacco against Macrophomina phaseolina. DNA Cell Biol. 2018;37(4):298–307. doi: 10.1089/dna.2017.4057. [DOI] [PubMed] [Google Scholar]
- Alscher RG, Donahue JL, Cramer CL. Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant. 1997;100:224–233. [Google Scholar]
- Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92(6):773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Asai S, Yoshioka H. The role of radical burst via MAPK signaling in plant immunity. Plant Signal Behav. 2008;3(11):920–922. doi: 10.4161/psb.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazadeh S, Siddiqui H, Allu AD, Matallana-Ramirez LP, Caldana C, Mehrnia M, Zanor MI, Köhler B, Mueller-Roeber B. A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J. 2010;62(2):250–264. doi: 10.1111/j.1365-313X.2010.04151.x. [DOI] [PubMed] [Google Scholar]
- Balazadeh S, Wu A, Mueller-Roeber B. Salt-triggered expression of the ANAC092-dependent senescence regulon in Arabidopsis thaliana. Plant Signal Behav. 2010;5(6):733–735. doi: 10.4161/psb.5.6.11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazadeh S, Kwasniewski M, Caldana C, Mehrnia M, Zanor MI, Xue GP, Mueller-Roeber B. ORS1, an H2O2-responsive NAC transcription factor, controls senescence in Arabidopsis thaliana. Mol Plant. 2011;4(2):346–360. doi: 10.1093/mp/ssq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo AR. Xylem parenchyma cells deliver the H2O2 necessary for lignification in differentiating xylem vessels. Planta. 2005;220(5):747–756. doi: 10.1007/s00425-004-1394-3. [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Gómez F, Martínez DE, Guiamet JJ. Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.) J Exp Bot. 2004;55:1663–1669. doi: 10.1093/jxb/erh199. [DOI] [PubMed] [Google Scholar]
- Becana M, Morán JF, Iturbe-Ormaetxe I. Iron-dependent oxygen free radical generation in plants subjected to environmental stress: toxicity and antioxidant protection. Plant Soil. 1998;201(1):137–147. [Google Scholar]
- Besseau S, Li J, Palva ET. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J Exp Bot. 2012;63(7):2667–2679. doi: 10.1093/jxb/err450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienert GP, Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. BBA Biomembr. 2014;5:1596–1604. doi: 10.1016/j.bbagen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. BBA Biomembr. 2006;1758(8):994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Borgohain P, Saha B, Agrahari R, Chowardhara B, Sahoo S, van der Vyver C, Panda SK. SlNAC2 overexpression in Arabidopsis results in enhanced abiotic stress tolerance with alteration in glutathione metabolism. Protoplasma. 2019;27:1–13. doi: 10.1007/s00709-019-01368-0. [DOI] [PubMed] [Google Scholar]
- Camejo D, del Carmen Romero-Puertas M, Rodríguez-Serrano M, Sandalio LM, Lázaro JJ, Jiménez A, Sevilla F. Salinity-induced changes in S-nitrosylation of pea mitochondrial proteins. J Proteomics. 2013;79:87–99. doi: 10.1016/j.jprot.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang L, Yu D. Wounding-induced WRKY8 is involved in basal defense in Arabidopsis. Mol Plant Microbe Interact. 2010;23(5):558–565. doi: 10.1094/MPMI-23-5-0558. [DOI] [PubMed] [Google Scholar]
- Colebrook EH, Thomas SG, Phillips AL, Hedden P. The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol. 2014;217(1):67–75. doi: 10.1242/jeb.089938. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB, Luis A. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 2001;6:145–150. doi: 10.1016/s1360-1385(01)01898-2. [DOI] [PubMed] [Google Scholar]
- Davletova S, Schlauch K, Coutu J, Mittler R. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005;139(2):847–856. doi: 10.1104/pp.105.068254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq I, Vermeirssen V, Van Aken O, Vandepoele K, Murcha MW, Law SR, Inzé A, Ng S, Ivanova A, Rombaut D, Van De Cotte B. The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell. 2013;25:3472–3490. doi: 10.1105/tpc.113.117168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Río LA. Peroxisomes as a cellular source of reactive nitrogen species signal molecules. Arch Biochem Biophys. 2011;506:1–11. doi: 10.1016/j.abb.2010.10.022. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci. 2001;98(23):13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot. 2004;55(395):205–212. doi: 10.1093/jxb/erh033. [DOI] [PubMed] [Google Scholar]
- Dietz KJ. Redox regulation of transcription factors in plant stress acclimation and development. Antioxid Redox Signal. 2014;21(9):1356–1372. doi: 10.1089/ars.2013.5672. [DOI] [PubMed] [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci. 2013;110(21):8744–8749. doi: 10.1073/pnas.1221294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Somssich IE. Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol. 2007;10(4):366–371. doi: 10.1016/j.pbi.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Fratelli M, Gianazza E, Ghezzi P. Redox proteomics: identification and functional role of glutathionylated proteins. Expert Rev Proteomics. 2004;1(3):365–376. doi: 10.1586/14789450.1.3.365. [DOI] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006;18(8):2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J. The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell. 2011;23:412–427. doi: 10.1105/tpc.110.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H. Xylogenesis: initiation, progression, and cell death. Annu Rev Plant Biol. 1996;47(1):299–325. doi: 10.1146/annurev.arplant.47.1.299. [DOI] [PubMed] [Google Scholar]
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inzé D, Mittler R, Van Breusegem F. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 2006;141(2):436–445. doi: 10.1104/pp.106.078717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallogly MM, Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7(4):381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Garapati P, Xue GP, Munné-Bosch S, Balazadeh S. Transcription factor ATAF1 in Arabidopsis promotes senescence by direct regulation of key chloroplast maintenance and senescence transcriptional cascades. Plant Physiol. 2015;168(3):1122–1139. doi: 10.1104/pp.15.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev TS, Hille J. Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol. 2005;168(1):17–20. doi: 10.1083/jcb.200409170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, Mittler R. ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol. 2016;171(3):1606–1615. doi: 10.1104/pp.16.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KS, McKelvie J, Markham KR. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell Environ. 2002;25(10):1261–1269. [Google Scholar]
- Grant JJ, Loake GJ. Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol. 2000;124(1):21–30. doi: 10.1104/pp.124.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JT, Yao N. The role and regulation of programmed cell death in plant–pathogen interactions. Cell Microbiol. 2004;6(3):201–211. doi: 10.1111/j.1462-5822.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- Grunewald W, Friml J. The March of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J. 2010;29(16):2700–2714. doi: 10.1038/emboj.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Li Z, Huang P, Li B, Fang S, Chu J, Guo H. A tripartite amplification loop involving the transcription factor WRKY75, salicylic acid, and reactive oxygen species accelerates leaf senescence. Plant Cell. 2017;29:2854–2870. doi: 10.1105/tpc.17.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Jha B, Agarwal PK. A dehydration-responsive element binding (DREB) transcription factor from the succulent halophyte Salicornia brachiata enhances abiotic stress tolerance in transgenic tobacco. Mar Biotechnol. 2014;16(6):657–673. doi: 10.1007/s10126-014-9582-z. [DOI] [PubMed] [Google Scholar]
- He F, Li HG, Wang JJ, Su Y, Wang HL, Feng CH, Yang Y, Niu MX, Liu C, Yin W, Xia X. PeSTZ1, a C2H2‐type zinc finger transcription factor from Populus euphratica, enhances freezing tolerance through modulation of ROS scavenging by directly regulating PeAPX2. Plant Biotechnol J. 2019 doi: 10.1111/pbi.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman J, Canher B, Bisht A, Christiaens F, De Veylder L. Emerging role of the plant ERF transcription factors in coordinating wound defense responses and repair. J Cell Sci. 2018;131(2):208215. doi: 10.1242/jcs.208215. [DOI] [PubMed] [Google Scholar]
- Hirt H. Multiple roles of MAP kinases in plant signal transduction. Trends Plant Sci. 1997;2(1):11–15. [Google Scholar]
- Htet Hlaing K, Clement MV. Formation of protein S-nitrosylation by reactive oxygen species. Free Radical Res. 2014;48(9):996–1010. doi: 10.3109/10715762.2014.942842. [DOI] [PubMed] [Google Scholar]
- Huang X, Wang W, Zhang Q, Liu JH. A basic helix-loop-helix transcription factor PtrbHLH of Poncirus trifoliata confers cold tolerance and modulates POD-mediated scavenging of H2O2. Plant Physiol. 2013;162:1178–1194. doi: 10.1104/pp.112.210740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JE, Lim CJ, Chen H, Je J, Song C, Lim CO. Overexpression of Arabidopsis dehydration-responsive element-binding protein 2C confers tolerance to oxidative stress. Mol Cell. 2012;33(2):135–140. doi: 10.1007/s10059-012-2188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Hao L, Guo X, Liu S, Yan Y, Guo X. A Raf-like MAPKKK gene, GhRaf19, negatively regulates tolerance to drought and salt and positively regulates resistance to cold stress by modulating reactive oxygen species in cotton. Plant Sci. 2016;252:267–281. doi: 10.1016/j.plantsci.2016.07.014. [DOI] [PubMed] [Google Scholar]
- Jia D, Jiang Q, van Nocker S, Gong X, Ma F. An apple (Malus domestica) NAC transcription factor enhances drought tolerance in transgenic apple plants. Plant Physiol Biochem. 2019;139:504–512. doi: 10.1016/j.plaphy.2019.04.011. [DOI] [PubMed] [Google Scholar]
- Jiang G, Yan H, Wu F, Zhang D, Zeng W, Qu H, Chen F, Tan L, Duan X, Jiang Y. Litchi fruit LcNAC1 is a target of LcMYC2 and regulator of fruit senescence through its interaction with LcWRKY1. Plant Cell Physiol. 2017;58(6):1075–1089. doi: 10.1093/pcp/pcx054. [DOI] [PubMed] [Google Scholar]
- Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV. Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell. 2005;17(3):957–970. doi: 10.1105/tpc.104.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Gabrys H, Mateo A, Karpinska B, Mullineaux PM. Light perception in plant disease defence signalling. Curr Opin Plant Biol. 2003;6:390–396. doi: 10.1016/s1369-5266(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Karuppanapandian T, Moon J, Kim C, Manoharan K. Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci. 2011;5:709–725. [Google Scholar]
- Kawano T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003;21(9):829–837. doi: 10.1007/s00299-003-0591-z. [DOI] [PubMed] [Google Scholar]
- Khedia J, Agarwal P, Agarwal PK. AlNAC4 transcription factor from halophyte Aeluropus lagopoides mitigates oxidative stress by maintaining ROS homeostasis in transgenic tobacco. Front Plant Sci. 2018;9:1522. doi: 10.3389/fpls.2018.01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Woo HR, Kim J, Lim PO, Lee IC, Choi SH, Hwang D, Nam HG. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science. 2009;323(5917):1053–1057. doi: 10.1126/science.1166386. [DOI] [PubMed] [Google Scholar]
- Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, Zhou JM, Shah J, Zhang S, Kachroo P, Trifa Y. Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci. 2000;97(16):8849–8855. doi: 10.1073/pnas.97.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf KD. Complexity of the heat stress response in plants. Curr Opin Plant Biol. 2007;10(3):310–316. doi: 10.1016/j.pbi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci. 2000;97(6):2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Biol. 1997;48(1):251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Le CTT, Brumbarova T, Ivanov R, Stoof C, Weber E, Mohrbacher J, Fink-Straube C, Bauer P. Zinc finger of Arabidopsis thaliana12 (ZAT12) interacts with FER-like iron deficiency-induced transcription factor (FIT) linking iron deficiency and oxidative stress responses. Plant Physiol. 2016;170(1):540–557. doi: 10.1104/pp.15.01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XD, Zhuang KY, Liu ZM, Yang DY, Ma NN, Meng QW. Overexpression of a novel NAC-type tomato transcription factor, SlNAM1, enhances the chilling stress tolerance of transgenic tobacco. J Plant Physiol. 2016;204:54–65. doi: 10.1016/j.jplph.2016.06.024. [DOI] [PubMed] [Google Scholar]
- Li SW, Leng Y, Shi RF. Transcriptomic profiling provides molecular insights into hydrogen peroxide-induced adventitious rooting in mung bean seedlings. BMC Genom. 2017;18(1):188. doi: 10.1186/s12864-017-3576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Lin L, Zhang Y, Sui N. ZmMYB31, a R2R3-MYB transcription factor in maize, positively regulates the expression of CBF genes and enhances resistance to chilling and oxidative stress. Mol Biol Rep. 2019;46:3937–3944. doi: 10.1007/s11033-019-04840-5. [DOI] [PubMed] [Google Scholar]
- Liao C, Zheng Y, Guo Y. MYB30 transcription factor regulates oxidative and heat stress responses through ANNEXIN-mediated cytosolic calcium signalling in Arabidopsis. New Phytol. 2017;216(1):163–177. doi: 10.1111/nph.14679. [DOI] [PubMed] [Google Scholar]
- Lu W, Chu X, Li Y, Wang C, Guo X. Cotton GhMKK1 induces the tolerance of salt and drought stress, and mediates defence responses to pathogen infection in transgenic Nicotiana benthamiana. PLoS One. 2013;8(7):68503. doi: 10.1371/journal.pone.0068503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X, Li H, Chen X, Xiang X, Guo Z, Yu J, Zhou Y. The role of calcium-dependent protein kinase in hydrogen peroxide, nitric oxide and ABA-dependent cold acclimation. J Exp Bot. 2018;69:4127–4139. doi: 10.1093/jxb/ery212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Wang WM, Bittner F, Schmidt N, Berkey R, Zhang L, King H, Zhang Y, Feng J, Wen Y, Tan L. Dual and opposing roles of xanthine dehydrogenase in defense-associated reactive oxygen species metabolism in Arabidopsis. Plant Cell. 2016;28:1108–1126. doi: 10.1105/tpc.15.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi K, Maki H, Itaya T, Suzuki T, Nomoto M, Sakaoka S, Morikami A, Higashiyama T, Tada Y, Busch W, Tsukagoshi H. MYB30 links ROS signaling, root cell elongation, and plant immune responses. Proc Natl Acad Sci. 2018;115(20):E4710–E4719. doi: 10.1073/pnas.1804233115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madian AG, Regnier FE. Proteomic identification of carbonylated proteins and their oxidation sites. J Proteome Res. 2010;9(8):3766–3780. doi: 10.1021/pr1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta T, Noshi M, Nakamura M, Matsuda S, Tamoi M, Ishikawa T, Shigeoka S. Ferulic acid 5-hydroxylase 1 is essential for expression of anthocyanin biosynthesis-associated genes and anthocyanin accumulation under photooxidative stress in Arabidopsis. Plant Sci. 2014;219:61–68. doi: 10.1016/j.plantsci.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Matern S, Peskan-Berghoefer T, Gromes R, Kiesel RV, Rausch T. Imposed glutathione-mediated redox switch modulates the tobacco wound-induced protein kinase and salicylic acid-induced protein kinase activation state and impacts on defence against Pseudomonas syringae. J Exp Bot. 2015;66(7):1935–1950. doi: 10.1093/jxb/eru546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie BD, Murnaghan J, Jones KS, Bowley SR. Iron-superoxide dismutase expression in transgenic alfalfa increases winter survival without a detectable increase in photosynthetic oxidative stress tolerance. Plant Physiol. 2000;122(4):1427–1438. doi: 10.1104/pp.122.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Zentgraf U. The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell. 2007;19(3):819–830. doi: 10.1105/tpc.106.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Laun T, Zimmermann P, Zentgraf U. Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol Biol. 2004;55(6):853–867. doi: 10.1007/s11103-004-2142-6. [DOI] [PubMed] [Google Scholar]
- Miao Y, Laun TM, Smykowski A, Zentgraf U. Arabidopsis MEKK1 can take a short cut: it can directly interact with senescence-related WRKY53 transcription factor on the protein level and can bind to its promoter. Plant Mol Biol. 2007;65(1–2):63–76. doi: 10.1007/s11103-007-9198-z. [DOI] [PubMed] [Google Scholar]
- Miller GAD, Mittler RON. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann Bot. 2006;98(2):279–288. doi: 10.1093/aob/mcl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Shulaev V, Mittler R. Reactive oxygen signaling and abiotic stress. Physiol Plant. 2008;133:481–489. doi: 10.1111/j.1399-3054.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Mittler R, Herr EH, Orvar BL, Van Camp W, Willekens H, Inzé D, Ellis BE. Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc Natl Acad Sci. 1999;96(24):14165–14170. doi: 10.1073/pnas.96.24.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller GAD, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci. 2011;16(6):300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Moller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annu Rev Plant Biol. 2007;58:459–481. doi: 10.1146/annurev.arplant.58.032806.103946. [DOI] [PubMed] [Google Scholar]
- Montillet JL, Chamnongpol S, Rustérucci C, Dat J, Van De Cotte B, Agnel JP, Battesti C, Inzé D, Van Breusegem F, Triantaphylides C. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 2005;138(3):1516–1526. doi: 10.1104/pp.105.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 2005;10(7):339–346. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Negi S, Tak H, Ganapathi TR. A banana NAC transcription factor (MusaSNAC1) impart drought tolerance by modulating stomatal closure and H2O2 content. Plant Mol Biol. 2018;96(4–5):457–471. doi: 10.1007/s11103-018-0710-4. [DOI] [PubMed] [Google Scholar]
- Ng S, Ivanova A, Duncan O, Law SR, Van Aken O, De Clercq I, Wang Y, Carrie C, Xu L, Kmiec B, Walker H. A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell. 2013;25:3450–3471. doi: 10.1105/tpc.113.113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie WF, Wang MM, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ. Silencing of tomato RBOH1 and MPK2 abolishes brassinosteroid-induced H2O2 generation and stress tolerance. Plant Cell Environ. 2013;36(4):789–803. doi: 10.1111/pce.12014. [DOI] [PubMed] [Google Scholar]
- Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer CH. Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot. 2002;89:841–850. doi: 10.1093/aob/mcf096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JA, Daudi A, Finch P, Butt VS, Whitelegge JP, Souda P, Ausubel FM, Bolwell GP. A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defence. Plant Physiol. 2012;158:2013–2027. doi: 10.1104/pp.111.190140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore D, Trono D, Laus MN, Di Fonzo N, Flagella Z. Possible plant mitochondria involvement in cell adaptation to drought stress—a case study: durum wheat mitochondria. J Exp Bot. 2007;58(2):195–210. doi: 10.1093/jxb/erl273. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406(6797):731. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Djamei A, Bitton F, Hirt H. A major role of the MEKK1–MKK1/2–MPK4 pathway in ROS signalling. Mol Plant. 2009;2(1):120–137. doi: 10.1093/mp/ssn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Yang KY, Li GJ, Liu Y, Zhang S. Activation of Ntf4, a tobacco mitogen-activated protein kinase, during plant defense response and its involvement in hypersensitive response-like cell death. Plant Physiol. 2006;141(4):1482–1493. doi: 10.1104/pp.106.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006;141(2):357–366. doi: 10.1104/pp.106.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Mittler R. The water-water cycle is essential for chloroplast protection in the absence of stress. J Biol Chem. 2003;278:38921–38925. doi: 10.1074/jbc.M304987200. [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Davletova S, Liang H, Mittler R. The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J Biol Chem. 2004;279(12):11736–11743. doi: 10.1074/jbc.M313350200. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Miles GP, Ellis BE. Ozone treatment rapidly activates MAP kinase signalling in plants. Plant J. 2000;22(4):367–376. doi: 10.1046/j.1365-313x.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- Sapara K, Khedia J, Agarwal P, Gangapur D, Agarwal PK. SbMYB15 transcription factor mitigates cadmium and nickel stress in transgenic tobacco by limiting uptake and modulating antioxidative defence system. Func Plant Biol. 2019 doi: 10.1071/FP18234. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Mieulet D, Hubberten HM, Obata T, Hoefgen R, Fernie AR, Fisahn J, San Segundo B, Guiderdoni E, Schippers JH, Mueller-Roeber B. SALT-RESPONSIVE ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell. 2013;25:2115–2131. doi: 10.1105/tpc.113.113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewelam N, Kazan K, Thomas-Hall SR, Kidd BN, Manners JM, Schenk PM. Ethylene response factor 6 is a regulator of reactive oxygen species signaling in Arabidopsis. PLoS One. 2013;8(8):e70289. doi: 10.1371/journal.pone.0070289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacter E. Protein oxidative damage. Methods Enzymol. 2000;319:428–436. doi: 10.1016/s0076-6879(00)19040-8. [DOI] [PubMed] [Google Scholar]
- Shi H, Liu G, Wei Y, Chan Z. The zinc-finger transcription factor ZAT6 is essential for hydrogen peroxide induction of anthocyanin synthesis in Arabidopsis. Plant Mol Biol. 2018;97(1–2):165–176. doi: 10.1007/s11103-018-0730-0. [DOI] [PubMed] [Google Scholar]
- Somssich IE. MAP kinases and plant defense. Trends Plant Sci. 1997;2(11):406–408. [Google Scholar]
- Song C, Chung WS, Lim CO. Overexpression of heat shock factor gene HsfA3 increases galactinol levels and oxidative stress tolerance in Arabidopsis. Mol Cells. 2016;39(6):477. doi: 10.14348/molcells.2016.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares CP, Vernal J, Delena RA, Lamattina L, Cassia R, Terenzi H. S-nitrosylation influences the structure and DNA binding activity of AtMYB30 transcription factor from Arabidopsis thaliana. BBA Proteins Proteomics. 2014;4:810–817. doi: 10.1016/j.bbapap.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Tian S, Wang X, Li P, Wang H, Ji H, Xie J, Qiu Q, Shen D, Dong H. Plant aquaporin AtPIP1; 4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol. 2016;171:1635–1650. doi: 10.1104/pp.15.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Fan M, Qin Z, Lv H, Wang M, Zhang Z, Zhou W, Zhao N, Li X, Han C, Ding Z. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat Commun. 2018;9(1):1063. doi: 10.1038/s41467-018-03463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti VB, Van Aken O, Morreel K, Vandenbroucke K, Van De Cotte B, De Clercq I, Chiwocha S, Fenske R, Prinsen E, Boerjan W, Genty B. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell. 2010;22:2660–2679. doi: 10.1105/tpc.109.071316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti VB, Mühlenbock PER, Van Breusegem F. Stress homeostasis–the redox and auxin perspective. Plant Cell Environ. 2012;35(2):321–333. doi: 10.1111/j.1365-3040.2011.02324.x. [DOI] [PubMed] [Google Scholar]
- Voothuluru P, Sharp RE. Apoplastic hydrogen peroxide in the growth zone of the maize primary root under water stress. I. Increased levels are specific to the apical region of growth maintenance. J Exp Bot. 2012;64(5):1223–1233. doi: 10.1093/jxb/ers277. [DOI] [PubMed] [Google Scholar]
- Wang C, Yang A, Yin H, Zhang J. Influence of water stress on endogenous hormone contents and cell damage of maize seedlings. J Integr Plant Biol. 2008;50(4):427–434. doi: 10.1111/j.1774-7909.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding H, Zhang A, Ma F, Cao J, Jiang M. A novel mitogen-activated protein kinase gene in maize (Zea mays), ZmMPK3, is involved in response to diverse environmental cues. J Integr Plant Biol. 2010;52(5):442–452. doi: 10.1111/j.1744-7909.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- Wang P, Du Y, Zhao X, Miao Y, Song CP. The MPK6-ERF6-ROSE7/GCC-box complex modulates oxidative gene transcription and the oxidative response in Arabidopsis thaliana. Plant Physiol. 2013;161:1392–1408. doi: 10.1104/pp.112.210724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Su H, Han L, Wang C, Sun Y, Liu F. Differential expression profiles of poplar MAP kinase kinases in response to abiotic stresses and plant hormones, and overexpression of PtMKK4 improves the drought tolerance of poplar. Gene. 2014;545(1):141–148. doi: 10.1016/j.gene.2014.04.058. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhao R, Zheng Y, Chen L, Li R, Ma J, Hong X, Ma P, Sheng J, Shen L. SlMAPK1/2/3 and antioxidant enzymes are associated with H2O2-induced chilling tolerance in tomato plants. J Agric Food Chem. 2017;65(32):6812–6820. doi: 10.1021/acs.jafc.7b01685. [DOI] [PubMed] [Google Scholar]
- Wu A, Allu AD, Garapati P, Siddiqui H, Dortay H, Zanor MI, Asensi-Fabado MA, Munné-Bosch S, Antonio C, Tohge T, Fernie AR. JUNGBRUNNEN1, a reactive oxygen species–responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell. 2012;24:482–506. doi: 10.1105/tpc.111.090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009;150(2):801–814. doi: 10.1104/pp.109.138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XJ, Zhou YH, Shi K, Zhou J, Foyer CH, Yu JQ. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot. 2015;66(10):2839–2856. doi: 10.1093/jxb/erv089. [DOI] [PubMed] [Google Scholar]
- Xing Y, Cao Q, Zhang Q, Qin L, Jia W, Zhang J. MKK5 regulates high light-induced gene expression of Cu/Zn superoxide dismutase 1 and 2 in Arabidopsis. Plant Cell Physiol. 2013;54(7):1217–1227. doi: 10.1093/pcp/pct072. [DOI] [PubMed] [Google Scholar]
- Xing Y, Chen WH, Jia W, Zhang J. Mitogen-activated protein kinase kinase 5 (MKK5)-mediated signalling cascade regulates expression of iron superoxide dismutase gene in Arabidopsis under salinity stress. J Exp Bot. 2015;66(19):5971–5981. doi: 10.1093/jxb/erv305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Hong G, Li L, Zhang X, Kong Y, Sun Z, Li J, Chen J, He Y. miR156/SPL9 regulates reactive oxygen species accumulation and immune response in Arabidopsis thaliana. Phytopathology. 2019;109(4):632–642. doi: 10.1094/PHYTO-08-18-0306-R. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JD, Doke N. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell. 2003;15(3):706–718. doi: 10.1105/tpc.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun BW, Feechan A, Yin M, Saidi NB, Le Bihan T, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH, Pallas JA. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature. 2011;478(7368):264. doi: 10.1038/nature10427. [DOI] [PubMed] [Google Scholar]
- Zeng XQ, Chow WS, Su LJ, Peng XX, Peng CL. Protective effect of supplemental anthocyanins on Arabidopsis leaves under high light. Physiol Plant. 2010;138(2):215–225. doi: 10.1111/j.1399-3054.2009.01316.x. [DOI] [PubMed] [Google Scholar]
- Zhang A, Zhang J, Ye N, Cao J, Tan M, Zhang J, Jiang M. ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defense in leaves of maize. J Exp Bot. 2010;61(15):4399–4411. doi: 10.1093/jxb/erq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Su LJ, Chen JW, Zeng XQ, Sun BY, Peng CL. The antioxidative role of anthocyanins in Arabidopsis under high-irradiance. Biol Plantarum. 2012;56(1):97–104. [Google Scholar]
- Zhang J, Zou D, Li Y, Sun X, Wang NN, Gong SY, Zheng Y, Li XB. GhMPK17, a cotton mitogen-activated protein kinase, is involved in plant response to high salinity and osmotic stresses and ABA signaling. PLoS One. 2014;9(4):95642. doi: 10.1371/journal.pone.0095642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li A, Zhang Z, Huang Z, Lu P, Zhang D, Liu X, Zhang ZF, Huang R. Ethylene response factor TERF1, regulated by ETHYLENE-INSENSITIVE3-like factors, functions in reactive oxygen species (ROS) scavenging in tobacco (Nicotiana tabacum L.) Sci rep. 2016;6:29948. doi: 10.1038/srep29948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Hou H, Yang T, Lian Y, Sun Y, Bian Z, Wang C. Exogenous hydrogen peroxide inhibits primary root gravitropism by regulating auxin distribution during Arabidopsis seed germination. Plant Physiol Biochem. 2018;128:126–133. doi: 10.1016/j.plaphy.2018.05.014. [DOI] [PubMed] [Google Scholar]
- Zwack PJ, De Clercq I, Howton TC, Hallmark HT, Hurny A, Keshishian EA, Parish AM, Benkova E, Mukhtar MS, Van Breusegem F, Rashotte AM. Cytokinin response factor 6 represses cytokinin-associated genes during oxidative stress. Plant Physiol. 2016;172:1249–1258. doi: 10.1104/pp.16.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]