Abstract
Parkinson’s disease (PD) is an age-related neurodegenerative disease caused by the progressive loss of dopaminergic (DA) neurons in the substantia nigra. As DA neurons degenerate, PD patients gradually lose their ability of movement. To date no effective therapies are available for the treatment of PD and its pathogenesis remains unknown. Experimental models that appropriately mimic the development of PD are certainly needed for gaining mechanistic insights into PD pathogenesis and identifying new therapeutic targets. Human induced pluripotent stem cells (iPSCs) could provide a promising model for fundamental research and drug screening. In this review, we summarize various iPSCs-based PD models either derived from PD patients through reprogramming technology or established by gene-editing technology, and the promising application of iPSC-based PD models for mechanistic studies and drug testing.
Keywords: Parkinson’s disease, Dopaminergic neurons, Induced pluripotent stem cells, Somatic cell reprogramming, Aging
Core tip: Human induced pluripotent stem cell (iPSC)-derived dopaminergic neurons hold great promise for studying disease mechanisms underlying Parkinson’s disease (PD) and testing drug effects. A number of reviews have previously summarized the potential use of patient iPSCs for modeling PD. However, few of them comprehensively discuss the establishment of gene-editing-based iPSCs for PD and their application in research. Our objective is to consolidate the current literature on various iPSC-based PD models either derived from PD patients through reprogramming technology or established by gene-editing technology, and provide new insights into the application of iPSC PD models.
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disease caused by the death of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) and a decrease in dopamine level, which lead to hypokinetic motor symptoms such as shaking, rigidity, slowness of movement, and difficulty in walking[1,2]. Although the standard treatments of PD that focus on boosting dopamine or dopamine receptor signaling can reduce symptoms at an early stage of the disease, none is effective at slowing or preventing progression of PD[3].
The onset of PD typically occurs at age > 60 years and its incidence is a global health concern with the increase in the aged population[4,5]. Aging is considered to be the most important risk factor for PD[6,7], but multiple genetic and environmental factors are also widely recognized to play critical roles in its development[8,9]. PD is generally classified into two forms: sporadic and familial PD. Sporadic PD is usually late onset and accounts for the majority of PD cases[10]. Some genetic backgrounds have been reported to increase the incidence of sporadic PD such as cytochrome P450 2D6 and glutathione S-transferase pi 1. However, these are not defined as genetic diseases because they have not been demonstrated to be associated with the development of PD[11,12]. In addition, long-term exposure to industrial chemicals and pollutants such as pesticides[13], metals[14] and solvents[15] is considered to contribute to development of sporadic PD. Familial PD is caused by gene mutations, which accounts for about 10% of PD cases[16], and shares some clinical features with sporadic PD. Currently, 16 familial PD-related genes have been identified. Their classification depends on chromosomal locus, which is named PARK and numbered in chronological order of their identification[17,18]. Among these mutations, PARK1/4, PARK3, PARK5, PARK8 and PARK11 are identified as autosomal dominant mutations. Except for PARK1/4, the other autosomal dominant mutations are late-onset PD. Autosomal recessive mutations in PARK2, PARK6, PARK7, PARK9, PARK14 and PARK15 are reported in early-onset PD. Other mutations in vacuolar protein sorting 35 and eukaryotic translation initiation factor 4G1 are identified in large families with dominant late-onset PD but not yet assigned with a PARK locus[19].
Previous studies on the pathology of PD have indicated that the deterioration is caused by formation of α-synuclein immunoreactive inclusion bodies that develop into globular Lewy bodies or Lewy neurites[20,21]. α-synuclein aggregation is recognized as not only a key event in familial PD but also the most consistent marker to define Lewy body pathology in sporadic PD[22]. These findings hint that both familial and sporadic PD have similar etiology[23]. In addition to abnormal aggregation of α-synuclein, other pathogenic factors involved in progress of PD have been reported, including mitochondrial dysfunction, oxidative and nitrative stress, neuroinflammation, and impaired autophagy[24-28]. However, up to now, the exact etiology and pathogenesis of PD are still unknown. The major barrier to study PD is the lack of brain tissue samples from patients, and current understanding of PD neuronal dysfunction has been largely derived from postmortem and pathological specimens[29]. Experimentally modeling PD is conventionally based on biochemical abnormalities in the brains of PD patients, such as oxidative stress and mitochondrial dysfunction. Toxins such as 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), paraquat and rotenone are conventionally used in PD modeling[30]. Commonly, 6-OHDA is stereotactically injected into animal brains and the other toxins are injected subcutaneously or intraperitoneally to induce PD models[31-33]. These neurotoxins can be taken up by DA neurons via dopamine transporters and cause neuronal damage[34]. Their toxicities are possibly due to the inhibition of complex I of the mitochondrial electron transport chain, which leads to depletion of ATP and an increase in reactive oxygen species (ROS), and eventually results in neuronal death[35]. Although these toxins can destroy DA neurons in the SNpc, 6-OHDA and MPTP treatment does not yield aggregation of α-synuclein (Lewy bodies), which is a major pathological marker of PD[36]. Typical PD is a type of chronic neurodegeneration. However, 6-OHDA or MPTP causes acute damage, which is not an appropriate model to mimic the pathogenic factors of PD. Chronic exposure to rotenone in rats leads to aggregation of α-synuclein, DA neurodegeneration and behavior defects. A major concern about rotenone-induced models is that, in addition to degeneration of nigral DA neurons, rotenone causes pronounced degeneration in basal ganglia and brainstem nuclei[37], and leads to high systemic toxicity[38].
Transgenic mice are alternative models for exploring pathogenic mechanisms of PD-linked genetic factors. Currently, duplication or triplication of the α-synuclein locus could cause PD symptoms[39], indicating that increasing expression of PD-related genes could be applied to establishment of PD models[40,41]. Some PD characteristics can be observed in transgenic mice generated by overexpressing wild-type PD genes or PD gene mutation at age ≤ 2 years[42]. However, in mammalian models, knockin or knockout mutation of PD-linked genes can only cause a moderate decrease in striatal DA levels accompanied by low locomotor activity in an age-dependent manner, without evident loss of dopaminergic neurons in SNpc[43]. Transgenic mouse models may illuminate some pathogenic processes of PD, but cannot fully replicate the phenotypes of human PD because they are insufficient to cause significant nigral degeneration within the animal lifespan[40]. The genetic mouse models may not properly represent interaction of genetic factors, aging process, and environmental insults in human PD[19,44]. Thus, establishing suitable models to fully represent the characteristics of PD is urgently needed.
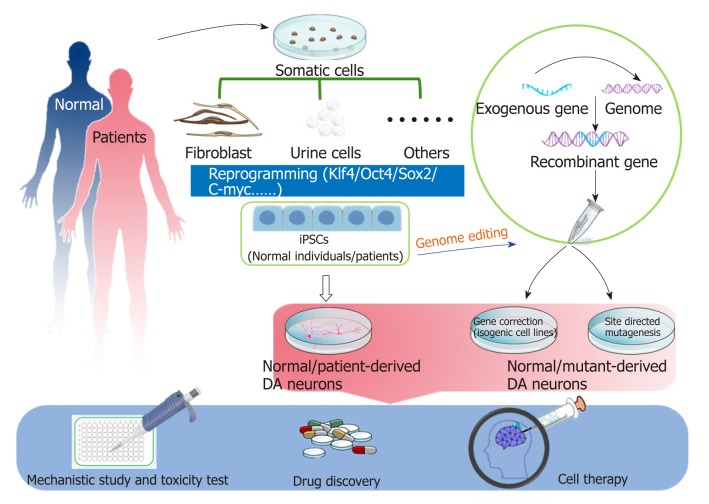
Human induced pluripotent stem cells (iPSCs) are generated from somatic cells by reprogramming. The somatic cells reprogramming technology was pioneered by Shinya Yamanaka in 2006 and showed that introduction of four transcription factors (OCT4, SOX2, KLF4 and c-MYC) could convert somatic cells into PSCs[45]. The ability of iPSCs to differentiate into DA neurons overcomes the challenges and shortcomings associated with PD modeling. Two types of iPSC-based PD models are widely used: patient-specific and gene-modifying models. Patient-derived specific disease models have been used in discovering novel biomarkers for diagnosis or candidate drug therapy[46]. Genome editing can offset the variation of genetic background among individuals[47,48]. In this review, we summarize various iPSC-based PD models either derived from PD patients through reprogramming technology or established by gene-editing technology, and the promising application of iPSC-based PD models for mechanistic studies and drug testing (Figure 1).
Figure 1.
Potential applications of induced pluripotent stem cells for study of Parkinson’s disease. Somatic cells are extracted from either normal individuals or patients and then reprogrammed to induced pluripotent stem cells (iPSCs). Gene editing technology enables one to generate knockin mutant Parkinson’s disease (PD) iPSCs and isogenic control cell lines. Dopamine (DA) neurons can be successfully differentiated from iPSCs. Both patient-derived and gene-editing iPSCs could be powerful tools for modeling PD for mechanistic studies and drug discovery. Healthy or corrected iPSCs could serve as normal controls and cell sources for cell therapy. iPSCs: Induced pluripotent stem cells; DA: Dopaminergic.
PATIENT-SPECIFIC iPSCs
It is widely accepted that PD results from a complex interaction of environmental and genetic factors[49]. In the hope of copying traits of PD, DA neurons derived from PD-patient-specific iPSCs display the cellular characteristics of PD in vitro. Investigators have established iPSC banks of neurodegenerative disease over the past decade. To date, patient-specific iPSCs have been used in a variety of fields including drug discovery, basic research and cytotoxicity testing.
iPSC models for familial PD
It is well known that sporadic PD occupies > 90% of total PD cases, but genetic factors still play an important role in understanding PD etiology. The use of genetic PD iPSCs offers the promise of addressing the contribution of individual genetic factors and functional relevance of underlying molecular pathways in the development of PD. Currently, 16 familial PD-related genes have been identified. Among them, SNCA, LRRK2, Parkin, PINK1 and GBA mutations have mostly been studied in genetic PD iPSCs[50] (Table 1). Researchers have made efforts to illustrate the pathological features of DA neurons or other neuronal cells derived from these genetic PD iPSCs. It is generally considered that the fate of these mutated genes is often loss of function that induces aberrant accumulation of inactive proteins[51].
Table 1.
Patient-derived induced pluripotent stem cell-based modeling of Parkinson’s disease
| Gene mutation | Inheritance type | Differentiated cell types | In vitro phenotypes (normalized to normal control / non-isogenic control) | Ref. |
| A53T SNCA | Familial | DA neurons | Not demonstrated | [52] |
| Triplication SNCA | Familial | DA neurons | Elevated levels of SNCA mRNA Increased cellular and secreted α-synuclein protein | [40] |
| Triplication SNCA | Familial | DA neurons | Elevated α-synuclein protein expression Increased expression of oxidative stress-related genes Increased susceptibility to oxidative stress | [53] |
| SNCA (A53T)/triplication SNCA | Familial | Forebrain cortical neurons | Nitrosative stress Accumulation of ERAD substrates ER stress | [58] |
| Triplication SNCA | Familial | Neural precursor cells | High vulnerability to stress Increased ROS production | [59] |
| Triplication SNCA | Familial | Neural precursor cells/DA neurons | Impaired neuronal differentiation and maturation pSer129-aSyn accumulation Increased susceptibility to oxidative stress | [56] |
| LRRK2 (G2019S) | Familial | |||
| SNCA (A53T) | Familial | DA, GABAergic,and glutamatergic neurons | Protein aggregation (thioflavin S and pSer129-aSyn) Axonal neuropathology Altered expression of synaptic transcripts | [57] |
| LRRK2 (G2019S) | Familial | DA neurons | Reduced neurite outgrowth Dysregulated autophagy system Increased cell death in response to neurotoxins Elevated αsynuclein protein level Dysregulation of genes related to DA neurodegeneration | [61] |
| LRRK2 (G2019S) | Familial | Neural stem cells | Increased sensitivity to stress Progressive impairment in nuclear envelope organization Defective self-renewal and neuronal differentiation | [62] |
| PINK1 (Q456X) | Familial | DA and nonDA neurons, and immature cells | Increased vulnerability to stress Dysfunction of mitochondria | [63] |
| LRRK2 (G2019S) | Familial | [63] | ||
| PINK1 (Q456X or R275W) | Familial | DA neurons | Increased neuronal death Degenerated dendrites Impaired AKT signaling | [72] |
| PARK2 (V324A) | Familial | [72] | ||
| LRRK2 (G2019S) and Sporadic | Familial and Sporadic | DA neurons | Increased apoptosis Reduced neurite numbers and complexity Increased autophagic vacuoles | [81] |
| SCNA (A53T) | Familial | DA neurons | Elevated αsynuclein aggregation and Lewy-body-like deposition Induced nitrosative and oxidative stress Increased vulnerability to mitochondrial toxin-induced cell death | [55] |
| SCNA (A53T) | Familial | DA neurons | Decreased αsynuclein tetramers Increased neurotoxicity | [56] |
| PARK2 (exon 2–4 deletion or exon 6, 7 deletion) | Familial | DA neurons | Increased oxidative stress, activated NRF2 pathway Abnormal mitochondrial morphology and turnover. Elevated αsynuclein accumulation | [70] |
| PARK2 (exon 3, 5 deletion or exon 3 deletion) | Familial | DA neurons | Increased oxidative stress Reduced dopamine uptake Enhanced spontaneous dopamine release | [71] |
| PINK1 (c.1366C>T; p.Q456X or c.509T>G; p.V170G) | Familial | DA neurons | Impaired recruitment of Parkin to mitochondria Increased mitochondria copy number PGC1α upregulation | [74] |
| PARK2 (exon 3, 5 deletion or exon 3 deletion) | Familial | DA neurons | Reduced neurite complexity Diminished microtubule stability | [73] |
| PARK2 (R42P, exon 3 deletion, exon 3, 4 deletion, 255A deletion, R275W or R42P) | Familial | DA neurons | Reduced capacity to differentiate into DA neurons Altered mitochondrial volume fraction | [75] |
| LRRK2 (G2019S) and sporadic type | Familial/sporadic | DA neurons | Elevated oxidative stress response Increased sensitivity to stress-induced cell death | [64] |
| LRRK2 (G2019S) and Sporadic PD | Familial/sporadic | DA neurons | Hypermethylation in gene regulatory regions Reduced expression of transcription factors related to disease | [65] |
| GBA1 (RecNcil/+, L444P/+ or N370S/+) and sporadic type | Familial/sporadic | DA neurons | Reduced dopamine storage and uptake Elevated α-synuclein and glucosylceramide levels Defective autophagic and lysosomal machinery Increased basal and induced calcium levels Enhanced vulnerability to ER stress | [77] |
| GBA (N370S/+) and sporadic type | Familial/ sporadic | DA neurons | Elevated αsynuclein levels Reduced dopamine levels Induced MAOB expression Disrupted network activity | [78] |
| GBA1 and sporadic | Familial/ sporadic | DA neurons | Decreased dopamine storage and uptake Elevated αsynuclein levels | [79] |
| SCNA SNP | Sporadic | Neurons | Disease-associated risk variant that regulates SCNA expression | [49] |
| SCNA (A53T) | Familial | DA neurons | Elevated nitrosative stress SNCAA53T or mitochondrial toxins induce S-nitrosylated (SNO)-MEF2C in DA neurons S-nitrosylation of MEF2C reduces PGC1α expression and impairs mitochondrial function | [54] |
ERAD: Endoplasmic-reticulum-associated degradation; MAOB: Monoamine oxidase B; NRF2: Nuclear factor erythroid 2related factor 2; SNP: Single-nucleotide polymorphism; TH: Tyrosine hydroxylase.
Accumulation of α-synuclein is a major feature of PD, which is encoded by SNCA gene. SNCA A53T mutant and triplication SNCA are familial PD SNCA mutants. In these patients, α-synuclein level in the midbrain region is three times that in somatic cells, revealing that it is easy to cause accumulation of α-synuclein in DA neurons. After Soldner et al[52] successfully generated the first DA neurons from SNCA mutant iPSCs, a number of SNCA A53T mutant or triplication SNCA mutant iPSC lines were generated, and elevated α-synuclein levels were found in these iPSC-derived DA neurons[40,53-57]. Other cellular types such as forebrain cortical neurons, neural precursor cells, and GABAergic neurons were further induced from SNCA mutant iPSCs, and endoplasmic reticulum (ER) stress, oxidative stress, or maturation inability but no significant alteration of α-synuclein protein or mRNA level were detected in these iPSC-derived cells[56-59]. These studies show that SNCA gene mutations may have great influence on a variety of neuronal subtypes and not just DA neurons.
N1437H, R1441C and G2019S mutations of LRRK2 are known to cause PD. Among them, G2019S is the most common familial PD mutation in LRRK2[60]. Many studies have pointed out the crucial role of the loss-of-function of LRRK2 mutant in reducing neurite outgrowth and numbers, and process complexity[61-65]. Oxidative stress, mitophagic dysfunction, and DNA damage have been observed in LRRK2 G2019S iPSC-derived DA neurons. In addition, self-renewal and neuronal differentiation of LRRK2 G2019 mutant neural stem cells are reported to be impaired, providing evidence on the crucial role of LRRK2 in neural development[62].
Synergistic effect of PINK1 and Parkin are important to maintain cellular homeostasis such as mitochondrial quality in DA neurons[66,67]. It is suggested that overexpression of Parkin can largely rescue the defects in PINK1 mutants through mitochondrial translocation[66,68]. Parkin improves the uptake of dopamine through enhancing the expression of DAT as well[69]. PINK1 or Parkin mutant iPSC-derived DA neurons display abnormal phenotypes such as mitophagy and autophagy impairment, vulnerability to various stresses, and accumulation of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α), which are consistent with previous studies[63,70-75]. These findings support the synergistic effect of PINK1 and Parkin, providing an inspiration for developing therapeutic strategies for PD.
GBA1 gene mutations are reported to be associated with an increased risk of sporadic PD[76]. A number of studies on patient-specific iPSC-derived DA neurons harboring GBA1 mutations have indicated that β-glucocerebrosidase (GBA1) has high correlation with elevated α-synuclein levels as well as autophagic and lysosomal defects[77-79]. Furthermore, calcium homeostasis imbalance, and reduced dopamine storage and uptake are also found in GBA1 mutant DA neurons[77-79]. However, how these above-mentioned phenotypes are triggered by GBA1 mutant remains to be explored.
iPSC models for sporadic PD
Sporadic PD-derived iPSCs lack known PD mutations. The first case of generation of sporadic PD patient iPSCs was reported by Soldner et al[80]. They generated iPSCs from skin biopsies obtained from sporadic PD patients by application of modified lentiviruses carrying loxP sites flanking the integrated provirus for improving the efficiency of reprogramming. The advantage of this method was the use of inducible excisable lentivirus, rendering the iPSCs free of reprogramming transgenes. However, the phenotypic analysis of these iPSCs was not performed in that study. Sanchez-Danes et al[71] generated healthy and sporadic PD iPSCs via retroviral delivery of OCT4, KLF4 and SOX2, and then differentiated them into DA neurons. By comparing with the healthy control group, they found that DA neurons differentiated from sporadic PD-patient-derived iPSCs after long-term culture displayed increased expression of cleaved caspase 3, shortened neurite length, and defective autophagosome clearance[81]. Fernández-Santiago et al[65] reported genome-wide DNA methylation of DA neurons derived from LRRK2-mutant and sporadic PD patients to explore the relationship between DNA methylation and alteration of gene expression and enhancer elements. They found that alterations of epigenetic signature significantly affected DNA methylation in sporadic PD patients. This study provides evidence that it is a common phenomenon that aberrant protein turnover and altered morphology and methylation patterns occur in sporadic PD-patient-derived DA neurons. Piwi-interacting RNA (piRNA) seems to have relevance to aberrant protein turnover and altered morphology and methylation patterns in sporadic PD-patient-derived DA neurons. piRNA is a complex of piwi protein and RNA, and is a large class of small noncoding RNA molecules expressed in animal cells that are involved in the epigenetic and post-transcriptional silencing of transposons. Schulze et al[82] have shown that the specific alteration of sin- and line-derived piRNA in DA neurons could be a new mechanism for the causation of PD[82-84].
iPSCs-derived astrocytes in PD modeling
Astrocytes are the major group of cells in the central nervous system, with a range of functions that provide both structural and metabolic support for neurons. Accumulating evidence suggests that astrocyte dysfunction leads to the pathogenesis of PD, especially familial PD[85-88]. As summarized by Booth et al[85], mutations in DJ-1, SNCA, PLA2G6, LRRK2 and GBA lead to abnormal glutamate uptake, mitochondrial dysfunction, inflammatory response, water transport defect, and autophagy impairment. Gunhanlar et al[86] found that in a coculture system of astrocytes and neurons at a consistent ratio (60:40), neuron maturation was distinctly upregulated according to electrophysiological maturity. Accordingly, astrocytes are widely used in implementing cellular modeling approaches to the study of neurodegenerative disorders[87]. Similarly, astrocytes could help DA neurons defend against the neurotoxins and attenuate the mitochondrial dysfunction, as observed by Feng et al[88]. Remarkably, the astrocytes and neuron co-culture system improved the outgrowth of neuron markers, and stabilized the mitochondrial function through downregulation of ROS and increased mitochondrial function[88]. On the contrary, astrocytes were also used in inducing PD degeneration phenotypes by Santos et al[89] and di Domenico et al[90]. In Santos et al’s research, astrocytes were activated and became inflammatory, and were co-cultured with DA neurons[89], while Domenico et al[90] co-cultured PD-patient-derived astrocytes with normal DA neurons. Normal DA neurons were induced to display apoptosis and multiple system dysfunction after co-culture with dysfunctional astrocytes[89,90]. These compelling findings emphasized that astrocytes may substantially participate in PD pathogenesis (Table 2).
Table 2.
Induced pluripotent stem cell-derived astrocytes in Parkinson’s disease modeling
| Cell lines | Differentiated types | Phenotype demonstrated | Ref. |
| Bone marrow 2-3(BM2-3) hiPSCs | Astrocytes/DA neurons coculture system | Elevated DA neuron identities Stablization of mitochondrial function Downregulation of mitoROS Increased mitochondrial length (normalized to non-co-culture DA neurons) | [88] |
| iPSCs and ESCs | Astrocytes/DA neurons coculture system | Non-activated astrocytes co-culture system improved DA neurons survival Non-activated astrocytes co-culture system increased DA neurons neurite lengths (normalized to inflammatory-activated astrocytes coculture system) | [89] |
| LRRK2 mutant and normal iPSCs | PD Astrocytes/normal DA neurons coculture system | Non-cell-autonomous damage is triggered by impaired autophagy in PD astrocytes Dysfunctional PD astrocytes accumulate and transfer α-synuclein to healthy DA neurons CMA activator drug prevents α-synuclein accumulation and neurodegeneration (normalized to the single culture system) | [90] |
iPSCs: Induced pluripotent stem cells; ESCs: Embryonic stem cells; hiPSCs: Human induced pluripotent stem cells; PD: Parkinson’s disease; DA: Dopaminergic.
GENOME-EDITING-BASED iPSCs and ISOGENIC CONTROLS
Since Yamanaka opened the door for the generation of iPSCs[45], iPSCs differentiation into DA neurons is commonly used for studying PD pathophysiology. Appropriate healthy iPSC-derived cells with higher quality and availability are often used as controls to study the phenotypes of a patient’s iPSC-derived cells. However, differences in genetic background are of concern: healthy siblings used as controls share only about 50% of the genome of patients[91]. The differences in genetic background lead to higher variations in phenotypic presentation. This critical issue should only be interpreted via comparison with isogenic control cells[92]. A locus mutation or target gene correction is introduced by editing a specific site in the human genome of iPSCs, which has become a routine procedure in many studies of PD iPSCs. Many genome-editing tools, including zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and CRISPR/Cas9 system are commonly used in editing iPSCs[93]. Among them, CRISPR/Cas9 may be the best one in gene editing because of the prospect of flexibility in site selection and affordability, although there are still some concerns about the efficacy and off-target risk[92]. The bottleneck in investigating connections between gene function and disease mechanisms is likely to be overcome by these genome-editing technologies.
Many iPSC PD cell lines have been established with genome editing (Table 3). Soldner et al[52] performed genetic correction in A53T (G209A) α-synuclein mutation in PD patient-derived iPSCs via ZFN-based gene editing. The G2019S mutation in LRRK2 is the most common cause of familial PD. Reinhardt et al[94] performed genetic correction of an LRRK2 mutation in PD-patient iPSCs via ZFN-based gene editing and Cre/LoxP systems, and linked parkinsonian neurodegeneration to extracellular signal-regulated kinase (ERK)-dependent changes in gene expression. They further demonstrated that ERK inhibitor reduces multiple PD-associated phenotypes, including lower neurite outgrowth, autophagy defects, synaptic defects, increased apoptosis, accumulation of tau and α-synuclein in LRRK2 G2019S mature DA neurons. Sanders et al reported that LRRK2 G2019S iPSC-derived DA neurons displayed greater levels of mitochondrial DNA damage, whereas this abnormal mitochondrial damage was no longer detected in corrected iPSC-derived neurons by ZFN-mediated genomic correction[95]. The mutations and genomic multiplications of the SNCA gene (α-synuclein) account for up to 15% of cases of familial PD[41,96,97]. Soldner et al[52] used CRISPR/Cas9-mediated insertion and exchange of risk-associated enhancer sequences. They identified a common PD-associated risk variant in a noncoding distal enhancer element that regulates expression of SNCA. Arias-Fuenzalida et al[98] induced heterozygous missense A30P and A53T mutations in the SNCA gene in healthy iPSCs via combined use of CRISPR/Cas9 and fluorescence-activated cell sorting analysis. These edited SNCA mutant iPSC-derived neuroepithelial stem cells displayed a significant decrease in maximal respiration, proton leak, basal respiration, ATP production, and nonmitochondrial respiration for the extracellular energy flux. Qing et al[48] used CRISPR/Cas9 and piggyBac system to establish heterozygous LRRK2 G2019S mutation in healthy iPSCs. They observed that the number of tyrosine-hydroxylase-positive neurons and their neurite complexity were significantly decreased in LRRK2 G2019S DA neurons.
Table 3.
Pluripotent stem cell-based genome-editing Parkinson’s disease models
| Gene mutation | Editing system | Cell line | Phenotype demonstrated | Application | Ref. |
| SNCA A30P | CRISPR/CAS 9 | hiPSC | Not demonstrated | Locus mutation | [48] |
| LRRK2 G2019S | ZFN | hiPSC | Not demonstrated | Gene correction | [95] |
| LRRK2 G2019S | CRISPR/CAS 9 | hiPSC | Synaptic defect, fraction of TH+/S129P-αS+ neurons was significantly reduced | Locus mutation | [48] |
| SNCA E46K | ZFN | hESC | Not demonstrated | Locus mutation | [53] |
| SNCA A53T | ZFN | hiPSC | Not demonstrated | Gene correction | [52] |
| SNCA A30P/A53T | CRISPR/CAS 9 | hiPSC | Not demonstrated | Locus mutation | [98] |
| SNCA (rs356165 A/G) | CRISPR/CAS 9 | hiPSC | Not demonstrated | Locus mutation | [49] |
| LRRK2 G2019S | ZFN | hiPSC | Basic phenotypes: autophagy defects, synaptic defects, increased apoptosis, accumulation of τ and α-synuclein. Phenotypes were alleviated after genetic correction | Gene correction | [94] |
hiPSC: Human induced pluripotent stem cell.
CHALLENGES IN USING iPSCs TO MODEL PD
Efficient generation of DA neurons from human iPSCs
DA neurons modulate several brain functions such as motor control, reward behavior, and cognition[99]. Recapitulation of the in vivo developmental profile of a specific cell type provides a powerful strategy for manipulating cell-fate choice during the process of human iPSC differentiation. In vitro generation of functional DA neurons is critical in pluripotent cell biology for both experimental and clinical applications. The neural stem/progenitor cell (NSPC) strategy and the floor-plate cell strategy are two useful protocols for generating DA neurons. The NSPC strategy is widely used in neuronal differentiation, in which NSPCs are isolated from rosettes[100,101] or induced by defined medium with many supplements[102]. The floor-plate strategy was proposed by Hynes et al and is based on the fact that the floor plate is a critical signaling center during neural development located along the ventral midline of the embryo[103]. Lorens et al[100] derived the floor-plate cells from human embryonic stem cells (hESCs) using a modified protocol by dual Smad inhibition. The floor-plate cells are predisposed to differentiate into mature ventral midbrain DA neurons with a higher efficiency than rosette-based neurons[101]. The floor-plate-derived midbrain DA neurons are able to control dopamine release and selective dopamine reuptake, as well as other features such as synaptic transmission. Importantly, PD patient iPSC-derived DA neurons show cellular PD phenotypes such as increased accumulation of mitochondrial ROS and cytoplasmic α-synuclein, mitochondrial DNA damage, shortened neurites, and impaired autophagy[80,104,105].
Disease phenotypes in modeling late-onset PD
Aging is a crucial risk factor for all late-onset neurodegenerative diseases. One important challenge found in iPSC-based PD models is to appropriately reproduce the late-onset characteristics. Reprogramming somatic cells to iPSCs resets their identity back to an embryonic state. The ability of iPSCs to undergo unlimited division while maintaining genomic integrity provides a way to overcome the senescence barrier. Even if these iPSCs differentiate into mature DA neurons, it still needs a lot of time to culture for mimicking aging DA neurons, presenting a significant hurdle for modeling PD. Thus, how to induce aging in iPSC-derived DA neurons is an important issue. Justine et al[72] compared young and aged human fibroblasts; they found a predominant difference in progerin level between adolescent and aged cells. They further demonstrated that overexpression of progerin in PD iPSC-derived DA neurons in vitro or in vivo promoted cell aging for modeling late-onset PD features such as pronounced dendrite degeneration, progressive loss of tyrosine hydroxylase expression, and enlarged mitochondria or Lewy body inclusions[72]. Thus, progerin expression could accelerate aging in iPSC-derived DA neurons for inducing PD pathogenic phenotypes, and introducing progerin expression could be a useful strategy to manifest disease phenotypes in iPSC-based late-onset PD models. However, it should be noted that neurons are fragile for undertaking the delivery of exogenous genes. The low efficiency of transfection in neurons remains a big challenge for widespread and convenient application of progerin expression to model iPSC-based late-onset neurodegenerative diseases.
Previous studies have indicated that chronic treatment with anticancer drug hydroxyurea (HU) could induce cellular senescence in human fibroblasts[106] and mouse neural stem cells[107] via induction of genes related to DNA damage and repair, mitochondrial dysfunction, and ROS increase. In our recent study[108], we found that HU induced disease phenotypes of sporadic PD-patient-specific iPSC-derived DA neurons. HU treatment significantly reduced neurite outgrowth, expression of p-Akt and its downstream targets (p-4EBP1 and p-ULK1), as well as increased the level of cleaved caspase 3 in iPSC-derived DA neurons from sporadic PD patients. Transcriptome analysis and Western blotting indicate that HU alters the expression of genes and proteins related to the ER stress pathway in healthy iPSC-derived DA neurons. It reveals that ER stress might contribute to HU-induced aging in iPSC-derived DA neurons from sporadic PD patients. Our study also found that the midbrain characteristics decline after iPSC-derived DA neurons are treated with HU, which is similar to the characterization of PD. Thus, increasing chemically induced ER stress could be an alternative approach to accelerating aging of iPSC-derived DA neurons from PD patients for manifestation of PD cellular phenotypes.
POTENTIAL APPLICATION OF iPSCs IN PD
iPSCs-based mechanistic studies for exploring clinical therapeutic strategies
Due to the ability of human iPSCs to differentiate into human DA neurons and astrocytes, human iPSCs are a promising model for studying the pathogenesis of PD. Compared with neurotoxin-induced injuries, human iPSC-derived DA neurons from sporadic or familiar PD patients could give help us to understand the progressive changes of PD neuronal phenotypes as culture time increases[50,109]. Through this PD iPSCs model, we can verify the possible mechanisms of pathogenesis suggested in previous studies in other cell or animal models. Most importantly, studying these PD-iPSC-derived DA neurons could explain how the clinical degenerative features of human DA neurons occur[110,111]. At least the changes in some human familial- PD-iPSC-derived neurons can represent the middle or final stage of PD because this iPSC-derived DA neuronal death occurs with α-synuclein accumulation, which is consistent with the observation in PD patients. Compared with familial PD, the etiology of sporadic PD is still a major challenge because of the multifactorial etiopathogenesis of sporadic PD. Since sporadic PD is complicated, the changes in neuronal phenotype cannot reflect the pathogenic alteration in the whole brain or other systems of sporadic PD patients[112,113].
iPSCs as a powerful and convincing drug discovery tool in PD
Current PD therapies help patients relieve motor symptoms, but do not effectively prevent, slow or halt the progression of PD, particularly in the loss of DA neurons[114]. Neurotoxin-based neurons or animal models are commonly used for anti-PD drug screening. Many drugs based on these artificial models have been developed but do not significantly prevent PD progression[115]. One reasonable explanation is that these neurotoxins that usually cause strong injuries in DA neurons cannot mimic the progressive death of human DA neurons in PD. In addition, DA neurons from animals are distinct from human DA neurons. In PD-iPSC-derived DA neurons, the typical PD features such as accumulation of α-synuclein, progressive degeneration, and death could be observed. These robust and reproducible PD phenotypes are amenable to screening potential compounds. Thus, PD-iPSC-derived DA neurons are more suitable for screening anti-PD drugs than artificial models are.
iPSC-based research enhanced development of cell therapy
The inspiring success in PD treatment was achieved through allograft of human fetal midbrain cell suspensions in 1980[116]. In addition, in MPTP-treated monkey brains, monkey ESC-derived neural progenitor cells differentiated into DA neurons and cells integrated well in the striatum, thereby PD-related motor symptoms improved[117]. Compared with ESCs, iPSCs have more potential in cell replacement therapies for PD because they can be generated from patients’ own cells and differentiate into DA neural progenitor cells that specifically develop into DA neurons[118]. iPSCs have the advantage of eliminating immune rejection concerns as they are obtained from the host. The generation of iPSCs from a patient’s own somatic cells would potentially allow for a plentiful source of cells for autotransplantation. In addition, using iPSCs rather than ESCs means that this treatment would be potentially available in some countries that ban the application of ESCs, including Italy, Ireland, and most African and South American countries[119,120]. For familial PD patients, the corrected iPSCs are also a reasonable source for the transplantation of normal DA neurons to reduce motor symptoms. Recently, scientists from Japan have started a clinical trial[121] (ClinicalTrials.gov NCT02452723) to treat PD with human iPSCs. All these suggest that the transplant of human iPSCs-derived DA neurons will be a promising therapeutic strategy and customized treatment is practical due to individual differences.
CONCLUSION
In this review, we have summarized iPSC-based PD models from patient-specific as well as genome-editing-based iPSCs. Patient-specific iPSCs that harbor the disease-associated genotype have provided extensive insights into pathogenic mechanisms of PD. However, given inherent genetic heterogeneity between individuals, it is understandable that disease phenotypes could be confounded by use of patient-specific iPSCs with different genetic backgrounds. The use of gene-targeting strategies based on ZFNs, TALENs or CRISPR/Cas9 to induce or correct a particular genetic mutation, has become indispensable in developing isogenic lines with and without a disease genotype. However, although great advances have been made in gene editing, high off-target risk and low efficacy still make it difficult and time-consuming in generating genome-editing-based iPSCs. Thus, improving the efficiency and precision of gene editing is important for generating more isogenic PD-specific iPSCs and control cell lines. How to reliably establish iPSC-based models for late-onset PD remains to be resolved. Inducing an aged state by long-term culture, overexpression of an aging protein, or small molecules is encouraged in most iPSC-based age-related PD models. Finally, iPSCs cannot mimic motor symptoms and some nonmotor symptoms such as depression, agrypnia, hyposmia and impairment of cognition. Thus, how to bridge the gap between animal and cell studies should be addressed in the future.
Footnotes
Conflict-of-interest statement: The authors have declared no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: February 27, 2019
First decision: April 11, 2019
Article in press: August 20, 2019
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kujawska M, Li SC, Tarnawski AS S-Editor: Ma RY L-Editor: A E-Editor: Xing YX
Contributor Information
Minjing Ke, State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao 999078, China.
Cheong-Meng Chong, State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao 999078, China.
Huanxing Su, State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao 999078, China. huanxingsu@umac.mo.
References
- 1.Kalia LV, Lang AE. Parkinson disease in 2015: Evolving basic, pathological and clinical concepts in PD. Nat Rev Neurol. 2016;12:65–66. doi: 10.1038/nrneurol.2015.249. [DOI] [PubMed] [Google Scholar]
- 2.Alota Ignacio Pereira V, Augusto Barbieri F, Moura Zagatto A, Cezar Rocha Dos Santos P, Simieli L, Augusto Barbieri R, Pivetta Carpes F, Teresa Bucken Gobbi L. Muscle Fatigue Does Not Change the Effects on Lower Limbs Strength Caused by Aging and Parkinson's Disease. Aging Dis. 2018;9:988–998. doi: 10.14336/AD.2018.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong CM, Shen M, Zhou ZY, Pan P, Hoi PM, Li S, Liang W, Ai N, Zhang LQ, Li CW, Yu H, Hou T, Lee SM. Discovery of a benzofuran derivative (MBPTA) as a novel ROCK inhibitor that protects against MPP⁺-induced oxidative stress and cell death in SH-SY5Y cells. Free Radic Biol Med. 2014;74:283–293. doi: 10.1016/j.freeradbiomed.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Benito-León J, Bermejo-Pareja F, Morales-González JM, Porta-Etessam J, Trincado R, Vega S, Louis ED Neurological Disorders in Central Spain (NEDICES) Study Group. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology. 2004;62:734–741. doi: 10.1212/01.wnl.0000113727.73153.68. [DOI] [PubMed] [Google Scholar]
- 5.de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63:1240–1244. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- 6.Shetty AK, Kodali M, Upadhya R, Madhu LN. Emerging Anti-Aging Strategies - Scientific Basis and Efficacy. Aging Dis. 2018;9:1165–1184. doi: 10.14336/AD.2018.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao YF, Qiong-Zhang, Zhang JF, Lou ZY, Zu HB, Wang ZG, Zeng WC, Kai-Yao, Xiao BG. The Synergy of Aging and LPS Exposure in a Mouse Model of Parkinson's Disease. Aging Dis. 2018;9:785–797. doi: 10.14336/AD.2017.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defazio G, Abbruzzese G, Aniello MS, Bloise M, Crisci C, Eleopra R, Fabbrini G, Girlanda P, Liguori R, Macerollo A, Marinelli L, Martino D, Morgante F, Santoro L, Tinazzi M, Berardelli A. Environmental risk factors and clinical phenotype in familial and sporadic primary blepharospasm. Neurology. 2011;77:631–637. doi: 10.1212/WNL.0b013e3182299e13. [DOI] [PubMed] [Google Scholar]
- 9.Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 10.Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso-Navarro H, Jimenez-Jimenez FJ, Garcia-Martin E, Agundez JA. Genomic and pharmacogenomic biomarkers of Parkinson's disease. Curr Drug Metab. 2014;15:129–181. doi: 10.2174/138920021502140327175404. [DOI] [PubMed] [Google Scholar]
- 12.Singh NK, Banerjee BD, Bala K, Chhillar M, Chhillar N. Gene-gene and gene-environment interaction on the risk of Parkinson's disease. Curr Aging Sci. 2014;7:101–109. doi: 10.2174/1874609807666140805123621. [DOI] [PubMed] [Google Scholar]
- 13.Pezzoli G, Cereda E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology. 2013;80:2035–2041. doi: 10.1212/WNL.0b013e318294b3c8. [DOI] [PubMed] [Google Scholar]
- 14.Vlaar T, Kab S, Schwaab Y, Fréry N, Elbaz A, Moisan F. Association of Parkinson's disease with industry sectors: a French nationwide incidence study. Eur J Epidemiol. 2018;33:1101–1111. doi: 10.1007/s10654-018-0399-3. [DOI] [PubMed] [Google Scholar]
- 15.Goldman SM. Environmental toxins and Parkinson's disease. Annu Rev Pharmacol Toxicol. 2014;54:141–164. doi: 10.1146/annurev-pharmtox-011613-135937. [DOI] [PubMed] [Google Scholar]
- 16.Lin MK, Farrer MJ. Genetics and genomics of Parkinson's disease. Genome Med. 2014;6:48. doi: 10.1186/gm566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson's disease pathology and genetics. Nature. 2016;539:207–216. doi: 10.1038/nature20414. [DOI] [PubMed] [Google Scholar]
- 18.Klein C, Westenberger A. Genetics of Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood-Kaczmar A, Gandhi S, Wood NW. Understanding the molecular causes of Parkinson's disease. Trends Mol Med. 2006;12:521–528. doi: 10.1016/j.molmed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Roca L, Adame-Castillo C, Campdelacreu J, Ispierto L, Vilas D, Rene R, Alvarez R, Gascon-Bayarri J, Serrano-Munoz MA, Ariza A, Beyer K. Glucocerebrosidase mRNA is Diminished in Brain of Lewy Body Diseases and Changes with Disease Progression in Blood. Aging Dis. 2018;9:208–219. doi: 10.14336/AD.2017.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiesling C, Kieper N, Seidel K, Krüger R. Review: Familial Parkinson's disease--genetics, clinical phenotype and neuropathology in relation to the common sporadic form of the disease. Neuropathol Appl Neurobiol. 2008;34:255–271. doi: 10.1111/j.1365-2990.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 23.Carr J, de la Fuente-Fernández R, Schulzer M, Mak E, Calne SM, Calne DB. Familial and sporadic Parkinson's disease usually display the same clinical features. Parkinsonism Relat Disord. 2003;9:201–204. doi: 10.1016/s1353-8020(02)00048-2. [DOI] [PubMed] [Google Scholar]
- 24.Di Maio R, Barrett PJ, Hoffman EK, Barrett CW, Zharikov A, Borah A, Hu X, McCoy J, Chu CT, Burton EA, Hastings TG, Greenamyre JT. α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson's disease. Sci Transl Med. 2016;8:342ra78. doi: 10.1126/scitranslmed.aaf3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson's disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147:93–104. doi: 10.1196/annals.1427.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, Deng YN, Zhang JY, Liu J, Li YB, Su H, Qu QM. SIRT3 Protects Rotenone-induced Injury in SH-SY5Y Cells by Promoting Autophagy through the LKB1-AMPK-mTOR Pathway. Aging Dis. 2018;9:273–286. doi: 10.14336/AD.2017.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Hao J, Zheng Y, Su R, Liao Y, Gong X, Liu L, Wang X. Fucoidan Protects Dopaminergic Neurons by Enhancing the Mitochondrial Function in a Rotenone-induced Rat Model of Parkinson's Disease. Aging Dis. 2018;9:590–604. doi: 10.14336/AD.2017.0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morroni F, Sita G, Graziosi A, Turrini E, Fimognari C, Tarozzi A, Hrelia P. Neuroprotective Effect of Caffeic Acid Phenethyl Ester in A Mouse Model of Alzheimer's Disease Involves Nrf2/HO-1 Pathway. Aging Dis. 2018;9:605–622. doi: 10.14336/AD.2017.0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. 2017;18:573–584. doi: 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potashkin JA, Blume SR, Runkle NK. Limitations of animal models of Parkinson's disease. Parkinsons Dis. 2010;2011:658083. doi: 10.4061/2011/658083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su R, Sun M, Wang W, Zhang J, Zhang L, Zhen J, Qian Y, Zheng Y, Wang X. A Novel Immunosuppressor, (5R)-5-Hydroxytriptolide, Alleviates Movement Disorder and Neuroinflammation in a 6-OHDA Hemiparkinsonian Rat Model. Aging Dis. 2017;8:31–43. doi: 10.14336/AD.2016.0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hipkiss AR. On the Relationship between Energy Metabolism, Proteostasis, Aging and Parkinson's Disease: Possible Causative Role of Methylglyoxal and Alleviative Potential of Carnosine. Aging Dis. 2017;8:334–345. doi: 10.14336/AD.2016.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lv D, Li J, Li H, Fu Y, Wang W. Imaging and Quantitative Analysis of the Interstitial Space in the Caudate Nucleus in a Rotenone-Induced Rat Model of Parkinson's Disease Using Tracer-based MRI. Aging Dis. 2017;8:1–6. doi: 10.14336/AD.2016.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiba K, Trevor A, Castagnoli N., Jr Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun. 1984;120:574–578. doi: 10.1016/0006-291x(84)91293-2. [DOI] [PubMed] [Google Scholar]
- 35.Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson's disease. Bioessays. 2002;24:308–318. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt WJ, Alam M. Controversies on new animal models of Parkinson's disease pro and con: the rotenone model of Parkinson's disease (PD) J Neural Transm Suppl. 2006:273–276. [PubMed] [Google Scholar]
- 37.Höglinger GU, Féger J, Prigent A, Michel PP, Parain K, Champy P, Ruberg M, Oertel WH, Hirsch EC. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J Neurochem. 2003;84:491–502. doi: 10.1046/j.1471-4159.2003.01533.x. [DOI] [PubMed] [Google Scholar]
- 38.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 39.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 40.Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M, Drummond NJ, Taanman JW, Schapira AH, Gwinn K, Hardy J, Lewis PA, Kunath T. Parkinson's disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 42.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron. 2010;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blesa J, Przedborski S. Parkinson's disease: animal models and dopaminergic cell vulnerability. Front Neuroanat. 2014;8:155. doi: 10.3389/fnana.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krüger R, Eberhardt O, Riess O, Schulz JB. Parkinson's disease: one biochemical pathway to fit all genes? Trends Mol Med. 2002;8:236–240. doi: 10.1016/s1471-4914(02)02333-x. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 46.Sterneckert JL, Reinhardt P, Schöler HR. Investigating human disease using stem cell models. Nat Rev Genet. 2014;15:625–639. doi: 10.1038/nrg3764. [DOI] [PubMed] [Google Scholar]
- 47.Khurana V, Tardiff DF, Chung CY, Lindquist S. Toward stem cell-based phenotypic screens for neurodegenerative diseases. Nat Rev Neurol. 2015;11:339–350. doi: 10.1038/nrneurol.2015.79. [DOI] [PubMed] [Google Scholar]
- 48.Qing X, Walter J, Jarazo J, Arias-Fuenzalida J, Hillje AL, Schwamborn JC. CRISPR/Cas9 and piggyBac-mediated footprint-free LRRK2-G2019S knock-in reveals neuronal complexity phenotypes and α-Synuclein modulation in dopaminergic neurons. Stem Cell Res. 2017;24:44–50. doi: 10.1016/j.scr.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Soldner F, Stelzer Y, Shivalila CS, Abraham BJ, Latourelle JC, Barrasa MI, Goldmann J, Myers RH, Young RA, Jaenisch R. Parkinson-associated risk variant in distal enhancer of α-synuclein modulates target gene expression. Nature. 2016;533:95–99. doi: 10.1038/nature17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang JF, Tang BS, Guo JF. The Progress of Induced Pluripotent Stem Cells as Models of Parkinson's Disease. Stem Cells Int. 2016;2016:4126214. doi: 10.1155/2016/4126214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schulte C, Gasser T. Genetic basis of Parkinson's disease: inheritance, penetrance, and expression. Appl Clin Genet. 2011;4:67–80. doi: 10.2147/TACG.S11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soldner F, Laganière J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, Khurana V, Golbe LI, Myers RH, Lindquist S, Zhang L, Guschin D, Fong LK, Vu BJ, Meng X, Urnov FD, Rebar EJ, Gregory PD, Zhang HS, Jaenisch R. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146:318–331. doi: 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byers B, Cord B, Nguyen HN, Schüle B, Fenno L, Lee PC, Deisseroth K, Langston JW, Pera RR, Palmer TD. SNCA triplication Parkinson's patient's iPSC-derived DA neurons accumulate α-synuclein and are susceptible to oxidative stress. PLoS One. 2011;6:e26159. doi: 10.1371/journal.pone.0026159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan SD, Dolatabadi N, Chan SF, Zhang X, Akhtar MW, Parker J, Soldner F, Sunico CR, Nagar S, Talantova M, Lee B, Lopez K, Nutter A, Shan B, Molokanova E, Zhang Y, Han X, Nakamura T, Masliah E, Yates JR, 3rd, Nakanishi N, Andreyev AY, Okamoto S, Jaenisch R, Ambasudhan R, Lipton SA. Isogenic human iPSC Parkinson's model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription. Cell. 2013;155:1351–1364. doi: 10.1016/j.cell.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dettmer U, Newman AJ, Soldner F, Luth ES, Kim NC, von Saucken VE, Sanderson JB, Jaenisch R, Bartels T, Selkoe D. Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun. 2015;6:7314. doi: 10.1038/ncomms8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin L, Göke J, Cukuroglu E, Dranias MR, VanDongen AM, Stanton LW. Molecular Features Underlying Neurodegeneration Identified through In Vitro Modeling of Genetically Diverse Parkinson's Disease Patients. Cell Rep. 2016;15:2411–2426. doi: 10.1016/j.celrep.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 57.Kouroupi G, Taoufik E, Vlachos IS, Tsioras K, Antoniou N, Papastefanaki F, Chroni-Tzartou D, Wrasidlo W, Bohl D, Stellas D, Politis PK, Vekrellis K, Papadimitriou D, Stefanis L, Bregestovski P, Hatzigeorgiou AG, Masliah E, Matsas R. Defective synaptic connectivity and axonal neuropathology in a human iPSC-based model of familial Parkinson's disease. Proc Natl Acad Sci U S A. 2017;114:E3679–E3688. doi: 10.1073/pnas.1617259114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung CY, Khurana V, Auluck PK, Tardiff DF, Mazzulli JR, Soldner F, Baru V, Lou Y, Freyzon Y, Cho S, Mungenast AE, Muffat J, Mitalipova M, Pluth MD, Jui NT, Schüle B, Lippard SJ, Tsai LH, Krainc D, Buchwald SL, Jaenisch R, Lindquist S. Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons. Science. 2013;342:983–987. doi: 10.1126/science.1245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flierl A, Oliveira LM, Falomir-Lockhart LJ, Mak SK, Hesley J, Soldner F, Arndt-Jovin DJ, Jaenisch R, Langston JW, Jovin TM, Schüle B. Higher vulnerability and stress sensitivity of neuronal precursor cells carrying an alpha-synuclein gene triplication. PLoS One. 2014;9:e112413. doi: 10.1371/journal.pone.0112413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouhouche A, Tibar H, Ben El Haj R, El Bayad K, Razine R, Tazrout S, Skalli A, Bouslam N, Elouardi L, Benomar A, Yahyaoui M, Regragui W. LRRK2 G2019S Mutation: Prevalence and Clinical Features in Moroccans with Parkinson's Disease. Parkinsons Dis. 2017;2017:2412486. doi: 10.1155/2017/2412486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young JE, Boulanger-Weill J, Williams DA, Woodruff G, Buen F, Revilla AC, Herrera C, Israel MA, Yuan SH, Edland SD, Goldstein LS. Elucidating molecular phenotypes caused by the SORL1 Alzheimer's disease genetic risk factor using human induced pluripotent stem cells. Cell Stem Cell. 2015;16:373–385. doi: 10.1016/j.stem.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu GH, Qu J, Suzuki K, Nivet E, Li M, Montserrat N, Yi F, Xu X, Ruiz S, Zhang W, Wagner U, Kim A, Ren B, Li Y, Goebl A, Kim J, Soligalla RD, Dubova I, Thompson J, Yates J, 3rd, Esteban CR, Sancho-Martinez I, Izpisua Belmonte JC. Progressive degeneration of human neural stem cells caused by pathogenic LRRK2. Nature. 2012;491:603–607. doi: 10.1038/nature11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cooper O, Seo H, Andrabi S, Guardia-Laguarta C, Graziotto J, Sundberg M, McLean JR, Carrillo-Reid L, Xie Z, Osborn T, Hargus G, Deleidi M, Lawson T, Bogetofte H, Perez-Torres E, Clark L, Moskowitz C, Mazzulli J, Chen L, Volpicelli-Daley L, Romero N, Jiang H, Uitti RJ, Huang Z, Opala G, Scarffe LA, Dawson VL, Klein C, Feng J, Ross OA, Trojanowski JQ, Lee VM, Marder K, Surmeier DJ, Wszolek ZK, Przedborski S, Krainc D, Dawson TM, Isacson O. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson's disease. Sci Transl Med. 2012;4:141ra90. doi: 10.1126/scitranslmed.3003985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schüle B, Dolmetsch RE, Langston W, Palmer TD, Pera RR. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernández-Santiago R, Carballo-Carbajal I, Castellano G, Torrent R, Richaud Y, Sánchez-Danés A, Vilarrasa-Blasi R, Sánchez-Pla A, Mosquera JL, Soriano J, López-Barneo J, Canals JM, Alberch J, Raya Á, Vila M, Consiglio A, Martín-Subero JI, Ezquerra M, Tolosa E. Aberrant epigenome in iPSC-derived dopaminergic neurons from Parkinson's disease patients. EMBO Mol Med. 2015;7:1529–1546. doi: 10.15252/emmm.201505439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang C, Han H, Yan M, Zhu S, Liu J, Liu Z, He L, Tan J, Liu Y, Liu H, Sun L, Duan S, Peng Y, Liu F, Yin XM, Zhang Z, Dong Z. PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy. 2018;14:880–897. doi: 10.1080/15548627.2017.1405880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scarffe LA, Stevens DA, Dawson VL, Dawson TM. Parkin and PINK1: much more than mitophagy. Trends Neurosci. 2014;37:315–324. doi: 10.1016/j.tins.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang H, Jiang Q, Feng J. Parkin increases dopamine uptake by enhancing the cell surface expression of dopamine transporter. J Biol Chem. 2004;279:54380–54386. doi: 10.1074/jbc.M409282200. [DOI] [PubMed] [Google Scholar]
- 70.Imaizumi Y, Okada Y, Akamatsu W, Koike M, Kuzumaki N, Hayakawa H, Nihira T, Kobayashi T, Ohyama M, Sato S, Takanashi M, Funayama M, Hirayama A, Soga T, Hishiki T, Suematsu M, Yagi T, Ito D, Kosakai A, Hayashi K, Shouji M, Nakanishi A, Suzuki N, Mizuno Y, Mizushima N, Amagai M, Uchiyama Y, Mochizuki H, Hattori N, Okano H. Mitochondrial dysfunction associated with increased oxidative stress and α-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol Brain. 2012;5:35. doi: 10.1186/1756-6606-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, Azabdaftari G, Nakaso K, Yan Z, Feng J. Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun. 2012;3:668. doi: 10.1038/ncomms1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller JD, Ganat YM, Kishinevsky S, Bowman RL, Liu B, Tu EY, Mandal PK, Vera E, Shim JW, Kriks S, Taldone T, Fusaki N, Tomishima MJ, Krainc D, Milner TA, Rossi DJ, Studer L. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell. 2013;13:691–705. doi: 10.1016/j.stem.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ren Y, Jiang H, Hu Z, Fan K, Wang J, Janoschka S, Wang X, Ge S, Feng J. Parkin mutations reduce the complexity of neuronal processes in iPSC-derived human neurons. Stem Cells. 2015;33:68–78. doi: 10.1002/stem.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D. Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci. 2011;31:5970–5976. doi: 10.1523/JNEUROSCI.4441-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shaltouki A, Sivapatham R, Pei Y, Gerencser AA, Momčilović O, Rao MS, Zeng X. Mitochondrial alterations by PARKIN in dopaminergic neurons using PARK2 patient-specific and PARK2 knockout isogenic iPSC lines. Stem Cell Reports. doi: 10.1016/j.stemcr.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ortega RA, Torres PA, Swan M, Nichols W, Boschung S, Raymond D, Barrett MJ, Johannes BA, Severt L, Shanker V, Hunt AL, Bressman S, Pastores GM, Saunders-Pullman R. Glucocerebrosidase enzyme activity in GBA mutation Parkinson's disease. J Clin Neurosci. 2016;28:185–186. doi: 10.1016/j.jocn.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aflaki E, Borger DK, Moaven N, Stubblefield BK, Rogers SA, Patnaik S, Schoenen FJ, Westbroek W, Zheng W, Sullivan P, Fujiwara H, Sidhu R, Khaliq ZM, Lopez GJ, Goldstein DS, Ory DS, Marugan J, Sidransky E. A New Glucocerebrosidase Chaperone Reduces α-Synuclein and Glycolipid Levels in iPSC-Derived Dopaminergic Neurons from Patients with Gaucher Disease and Parkinsonism. J Neurosci. 2016;36:7441–7452. doi: 10.1523/JNEUROSCI.0636-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woodard CM, Campos BA, Kuo SH, Nirenberg MJ, Nestor MW, Zimmer M, Mosharov EV, Sulzer D, Zhou H, Paull D, Clark L, Schadt EE, Sardi SP, Rubin L, Eggan K, Brock M, Lipnick S, Rao M, Chang S, Li A, Noggle SA. iPSC-derived dopamine neurons reveal differences between monozygotic twins discordant for Parkinson's disease. Cell Rep. 2014;9:1173–1182. doi: 10.1016/j.celrep.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schöndorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B, Sardi SP, Valsecchi M, Hoffmann S, Schwarz LK, Hedrich U, Berg D, Shihabuddin LS, Hu J, Pruszak J, Gygi SP, Sonnino S, Gasser T, Deleidi M. iPSC-derived neurons from GBA1-associated Parkinson's disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun. 2014;5:4028. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- 80.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O, Jaenisch R. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136 doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sánchez-Danés A, Richaud-Patin Y, Carballo-Carbajal I, Jiménez-Delgado S, Caig C, Mora S, Di Guglielmo C, Ezquerra M, Patel B, Giralt A, Canals JM, Memo M, Alberch J, López-Barneo J, Vila M, Cuervo AM, Tolosa E, Consiglio A, Raya A. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson's disease. EMBO Mol Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schulze M, Sommer A, Plötz S, Farrell M, Winner B, Grosch J, Winkler J, Riemenschneider MJ. Sporadic Parkinson's disease derived neuronal cells show disease-specific mRNA and small RNA signatures with abundant deregulation of piRNAs. Acta Neuropathol Commun. 2018;6:58. doi: 10.1186/s40478-018-0561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seto AG, Kingston RE, Lau NC. The coming of age for Piwi proteins. Mol Cell. 2007;26:603–609. doi: 10.1016/j.molcel.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 84.Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol. 2011;12:246–258. doi: 10.1038/nrm3089. [DOI] [PubMed] [Google Scholar]
- 85.Booth HDE, Hirst WD, Wade-Martins R. The Role of Astrocyte Dysfunction in Parkinson's Disease Pathogenesis. Trends Neurosci. 2017;40:358–370. doi: 10.1016/j.tins.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gunhanlar N, Shpak G, van der Kroeg M, Gouty-Colomer LA, Munshi ST, Lendemeijer B, Ghazvini M, Dupont C, Hoogendijk WJG, Gribnau J, de Vrij FMS, Kushner SA. A simplified protocol for differentiation of electrophysiologically mature neuronal networks from human induced pluripotent stem cells. Mol Psychiatry. 2018;23:1336–1344. doi: 10.1038/mp.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li H, Jiang H, Zhang B, Feng J. Modeling Parkinson's Disease Using Patient-specific Induced Pluripotent Stem Cells. J Parkinsons Dis. 2018;8:479–493. doi: 10.3233/JPD-181353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du F, Yu Q, Chen A, Chen D, Yan SS. Astrocytes Attenuate Mitochondrial Dysfunctions in Human Dopaminergic Neurons Derived from iPSC. Stem Cell Reports. 2018;10:366–374. doi: 10.1016/j.stemcr.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santos R, Vadodaria KC, Jaeger BN, Mei A, Lefcochilos-Fogelquist S, Mendes APD, Erikson G, Shokhirev M, Randolph-Moore L, Fredlender C, Dave S, Oefner R, Fitzpatrick C, Pena M, Barron JJ, Ku M, Denli AM, Kerman BE, Charnay P, Kelsoe JR, Marchetto MC, Gage FH. Differentiation of Inflammation-Responsive Astrocytes from Glial Progenitors Generated from Human Induced Pluripotent Stem Cells. Stem Cell Reports. 2017;8:1757–1769. doi: 10.1016/j.stemcr.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.di Domenico A, Carola G, Calatayud C, Pons-Espinal M, Muñoz JP, Richaud-Patin Y, Fernandez-Carasa I, Gut M, Faella A, Parameswaran J, Soriano J, Ferrer I, Tolosa E, Zorzano A, Cuervo AM, Raya A, Consiglio A. Patient-Specific iPSC-Derived Astrocytes Contribute to Non-Cell-Autonomous Neurodegeneration in Parkinson's Disease. Stem Cell Reports. 2019;12:213–229. doi: 10.1016/j.stemcr.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin J, Musunuru K. Genome engineering tools for building cellular models of disease. FEBS J. 2016;283:3222–3231. doi: 10.1111/febs.13763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Musunuru K. Genome editing of human pluripotent stem cells to generate human cellular disease models. Dis Model Mech. 2013;6:896–904. doi: 10.1242/dmm.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heidenreich M, Zhang F. Applications of CRISPR-Cas systems in neuroscience. Nat Rev Neurosci. 2016;17:36–44. doi: 10.1038/nrn.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reinhardt P, Schmid B, Burbulla LF, Schöndorf DC, Wagner L, Glatza M, Höing S, Hargus G, Heck SA, Dhingra A, Wu G, Müller S, Brockmann K, Kluba T, Maisel M, Krüger R, Berg D, Tsytsyura Y, Thiel CS, Psathaki OE, Klingauf J, Kuhlmann T, Klewin M, Müller H, Gasser T, Schöler HR, Sterneckert J. Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell. 2013;12:354–367. doi: 10.1016/j.stem.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 95.Sanders LH, Laganière J, Cooper O, Mak SK, Vu BJ, Huang YA, Paschon DE, Vangipuram M, Sundararajan R, Urnov FD, Langston JW, Gregory PD, Zhang HS, Greenamyre JT, Isacson O, Schüle B. LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson's disease patients: reversal by gene correction. Neurobiol Dis. 2014;62:381–386. doi: 10.1016/j.nbd.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bozi M, Papadimitriou D, Antonellou R, Moraitou M, Maniati M, Vassilatis DK, Papageorgiou SG, Leonardos A, Tagaris G, Malamis G, Theofilopoulos D, Kamakari S, Stamboulis E, Hadjigeorgiou GM, Athanassiadou A, Michelakakis H, Papadimitriou A, Gasser T, Stefanis L. Genetic assessment of familial and early-onset Parkinson's disease in a Greek population. Eur J Neurol. 2014;21:963–968. doi: 10.1111/ene.12315. [DOI] [PubMed] [Google Scholar]
- 97.Kim HJ, Jeon BS, Yoon MY, Park SS, Lee KW. Increased expression of α-synuclein by SNCA duplication is associated with resistance to toxic stimuli. J Mol Neurosci. 2012;47:249–255. doi: 10.1007/s12031-012-9732-6. [DOI] [PubMed] [Google Scholar]
- 98.Arias-Fuenzalida J, Jarazo J, Qing X, Walter J, Gomez-Giro G, Nickels SL, Zaehres H, Schöler HR, Schwamborn JC. FACS-Assisted CRISPR-Cas9 Genome Editing Facilitates Parkinson's Disease Modeling. Stem Cell Reports. 2017;9:1423–1431. doi: 10.1016/j.stemcr.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chinta SJ, Andersen JK. Dopaminergic neurons. Int J Biochem Cell Biol. 2005;37:942–946. doi: 10.1016/j.biocel.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 100.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, Zeng X. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells. 2010;28:1893–1904. doi: 10.1002/stem.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hynes M, Poulsen K, Tessier-Lavigne M, Rosenthal A. Control of neuronal diversity by the floor plate: contact-mediated induction of midbrain dopaminergic neurons. Cell. 1995;80:95–101. doi: 10.1016/0092-8674(95)90454-9. [DOI] [PubMed] [Google Scholar]
- 104.Giordano S, Darley-Usmar V, Zhang J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2013;2:82–90. doi: 10.1016/j.redox.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cooper O, Hargus G, Deleidi M, Blak A, Osborn T, Marlow E, Lee K, Levy A, Perez-Torres E, Yow A, Isacson O. Differentiation of human ES and Parkinson's disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010;45:258–266. doi: 10.1016/j.mcn.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yeo EJ, Hwang YC, Kang CM, Kim IH, Kim DI, Parka JS, Choy HE, Park WY, Park SC. Senescence-like changes induced by hydroxyurea in human diploid fibroblasts. Exp Gerontol. 2000;35:553–571. doi: 10.1016/s0531-5565(00)00108-x. [DOI] [PubMed] [Google Scholar]
- 107.Dong CM, Wang XL, Wang GM, Zhang WJ, Zhu L, Gao S, Yang DJ, Qin Y, Liang QJ, Chen YL, Deng HT, Ning K, Liang AB, Gao ZL, Xu J. A stress-induced cellular aging model with postnatal neural stem cells. Cell Death Dis. 2014;5:e1116. doi: 10.1038/cddis.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan Y, Ke M, Huang Z, Chong C, Cen X, Lu JH, Yao X, Qin D, Su H. Hydroxyurea facilitates manifestation of disease relevant phenotypes in patients-derived iPSCs-based modeling of late-onset Parkinson's disease disease. Aging Dis. 2019 doi: 10.14336/AD.2018.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tong G, Izquierdo P, Raashid RA. Human Induced Pluripotent Stem Cells and the Modelling of Alzheimer's Disease: The Human Brain Outside the Dish. Open Neurol J. 2017;11:27–38. doi: 10.2174/1874205X01711010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deng W. Induced pluripotent stem cells: paths to new medicines. A catalyst for disease modelling, drug discovery and regenerative therapy. EMBO Rep. 2010;11:161–165. doi: 10.1038/embor.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bahmad H, Hadadeh O, Chamaa F, Cheaito K, Darwish B, Makkawi AK, Abou-Kheir W. Modeling Human Neurological and Neurodegenerative Diseases: From Induced Pluripotent Stem Cells to Neuronal Differentiation and Its Applications in Neurotrauma. Front Mol Neurosci. 2017;10:50. doi: 10.3389/fnmol.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson's disease. Biochim Biophys Acta. 2010;1802:29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 113.Perfeito R, Cunha-Oliveira T, Rego AC. Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse. Free Radic Biol Med. 2013;62:186–201. doi: 10.1016/j.freeradbiomed.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 114.Jankovic J, Aguilar LG. Current approaches to the treatment of Parkinson's disease. Neuropsychiatr Dis Treat. 2008;4:743–757. doi: 10.2147/ndt.s2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bové J, Perier C. Neurotoxin-based models of Parkinson's disease. Neuroscience. 2012;211:51–76. doi: 10.1016/j.neuroscience.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 116.Morizane A, Takahashi J. Cell Therapy for Parkinson's Disease. Neurol Med Chir (Tokyo) 2016;56:102–109. doi: 10.2176/nmc.ra.2015-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115:102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM, Sundberg M, Moore MA, Perez-Torres E, Brownell AL, Schumacher JM, Spealman RD, Isacson O. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson's disease. Cell Stem Cell. 2015;16:269–274. doi: 10.1016/j.stem.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yasuhara T, Kameda M, Sasaki T, Tajiri N, Date I. Cell Therapy for Parkinson's Disease. Cell Transplant. 2017;26:1551–1559. doi: 10.1177/0963689717735411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liras A. Future research and therapeutic applications of human stem cells: general, regulatory, and bioethical aspects. J Transl Med. 2010;8:131. doi: 10.1186/1479-5876-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garitaonandia I, Gonzalez R, Sherman G, Semechkin A, Evans A, Kern R. Novel Approach to Stem Cell Therapy in Parkinson's Disease. Stem Cells Dev. 2018;27:951–957. doi: 10.1089/scd.2018.0001. [DOI] [PubMed] [Google Scholar]