Abstract
Strongyloides spp. are parasitic nematodes that are transmitted through the environment and are capable of causing disease. These nematodes affect an estimated 3–300 million humans worldwide. Identifying the environmental reservoirs of Strongyloides spp. is essential for the development of appropriate control strategies. This systematic literature review examined all published studies that identified Strongyloides stercoralis, Strongyloides fuelleborni, Strongyloides fuelleborni kellyi, and Strongyloides spp. from an environmental source. Most studies detected the nematode from dog and primate fecal samples. Other environmental sources identified were ruminants, cats, rodents, insects, water, soil, as well as fruit and vegetables. Most studies used microscopy-based identification techniques; however, several employed molecular-based techniques, which have become increasingly popular for the detection of Strongyloides spp. A limitation identified was a lack of studies that comprehensively screened all potential environmental samples in a region. Future research should undertake this holistic screening process to identify which environmental reservoirs pose the greatest significance to human health. Potential controls can be identified through the identification of environmental sources. Understanding where Strongyloides spp. is commonly found within the environment of endemic areas will inform environmental control strategies to reduce this neglected disease.
Keywords: Strongyloides spp., Strongyloides stercoralis, Strongyloides fuelleborni, strongyloidiasis, environmental reservoirs
1. Introduction
Strongyloidiasis is a disease caused by parasitic nematodes of the genus Strongyloides. Within this genus, three species, Strongyloides stercoralis, Strongyloides fuelleborni, and Strongyloides fuelleborni kellyi are known to parasitize humans [1,2].
S. stercoralis, S. fuelleborni, and S. fuelleborni kellyi are capable of autoinfecting the host. This occurs after adult female parthenogenic nematodes within the infected human shed eggs. These eggs develop to larvae that are passed within the stool. A certain number burrow through the wall of the large intestine, thereby reinfecting the body. Infected individuals can have a low-level undetected infection for many years [3]. When this auto-infective life cycle becomes uncontrolled in immunocompromised, young, and elderly patients, a disseminated infection can develop. Disseminated infection occurs when the parasite travels throughout the body. This can result in sepsis, bacterial meningitis, or gastrointestinal hemorrhage [4]. The mortality rate from a disseminated infection and its comorbidities is estimated to be 80% [3]. The larvae that are passed within the stool are then capable of completing a free-living cycle, in which they molt twice to develop into filariform larvae. These infective filariform larvae are capable of then reinfecting humans, where they can be involved with the autoinfection cycle again [5].
Both S. stercoralis and S. fuelleborni are able to complete their life cycle within animals such as canids, primates, and insects. Animal species-specific strains of S. stercoralis unable to infect humans have been identified [6]. This ability for the nematode to reproduce within other animals indicates that all infected animals’ feces may pose an infection threat to humans.
After excretion in the stool, larvae can survive and reproduce within the environment, and environmental sources contaminated with larvae can cause reinfection. Although Strongyloides spp. are classified as soil-transmitted helminths, locations that harbor Strongyloides spp. within the environment, with the exception of soil, have not been investigated holistically [7,8]. By reviewing and collating all reported environmental sources of S. stercoralis, S. fuelleborni, and S. fuelleborni kellyi, environmental interventions can be implemented.
We need a better understanding of the environmental sources of Strongyloides spp.; resistance to the current anthelminthic drugs has been observed in other Strongyloides spp. [9]. Both environmental and clinical control of Strongyloides spp. is essential [10]. The aim of this review is to identify all research reporting S. stercoralis, S. fuelleborni, S. fuelleborni kellyi, and Strongyloides spp. within environmental sources worldwide.
2. Results
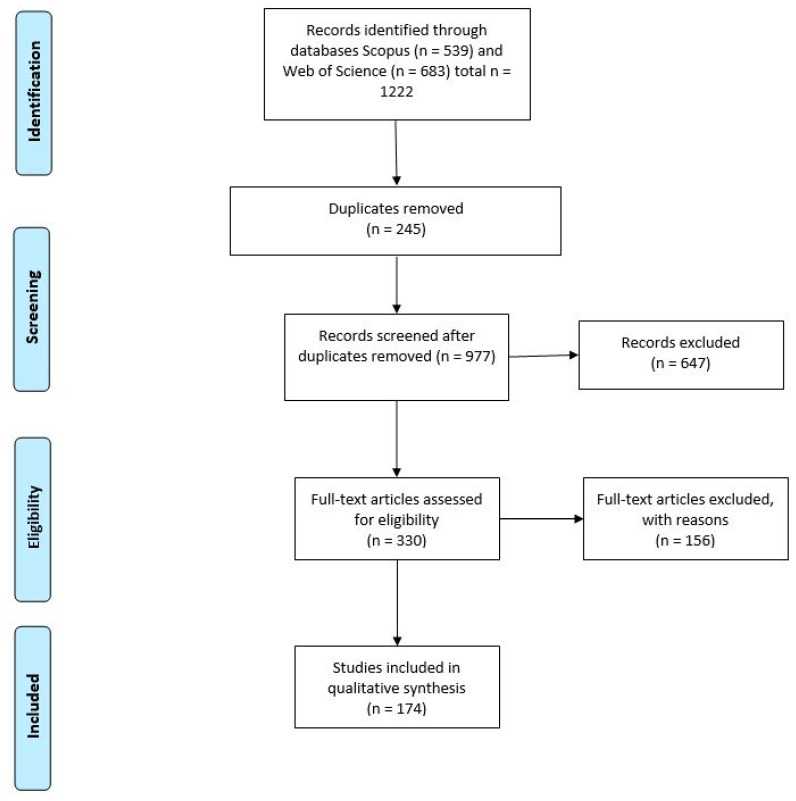
One thousand two hundred and twenty-two papers were retrieved from SCOPUS and Web of Science using the search terms identified as suitable, as seen in Table 1, with 174 articles identified as eligible for inclusion.
Table 1.
Complete search strategy and all key words used to identify relevant literature.
| Search Terms Employed to Identify Relevant Literature |
|---|
| Strongyloides OR Strongyloidiasis OR “Strongyloides stercoralis” OR “S. stercoralis” OR “Strongyloides fuelleborni” OR “S. fulleborni” OR “Strongyloides fulleborni kellyi” OR “S. fulleborni kellyi” |
| AND |
| “Tap Water” OR “Potable water” OR Water OR Soil OR Dirt OR sediment OR synanthropic OR “synanthropic insect” OR Insect OR “Musca domestica” OR flies OR “Musca vetustissima” OR Sarcophagidae OR “Chrysomya megacephala” OR “Musca sorbens” OR “Lucilia cuprina” OR “Calliphora vicina” OR “Blattella germanica” OR “Periplaneta Americana” OR Cockroach OR dog OR “Canis lupis” OR zoonotic OR Monkey OR “septic tank” OR waste OR wastewater OR rubbish OR trash OR environment |
S. stercoralis was identified in 35% of all studies and S. fuelleborni in 10% of all studies; both S. stercoralis and S. fuelleborni were identified in 1% of all studies. S. fuelleborni and Strongyloides spp. were identified in 0.5% of studies, and genus-level identification was identififed in 55% of all studies, as seen in Table A1. S. fuelleborni kellyi was not identified within any papers.
The most commonly identified reports of Strongyloides spp. were within primates (26% of all published works), and dogs (14% of all published works), as seen in Table A1. Other animals identified as environmental sources included cats, ruminants, rodents, and insects. Water, soil, as well as fruit and vegetables were all also identified as containing Strongyloides spp.
Fifty percent of all studies identifying Strongyloides spp. within primate populations identified the larvae to genus level only. S. fuelleborni was the next most frequently identified species at 40%. Parasitic infections were identified more frequently in terrestrial primates than arboreal primates [11,12]. Most studies (80%) employed microscopy, as seen in Table A1. Proximity to human populations and increased interaction with human populations was also frequently reported in infected populations [13,14]. Captive primates treated with anthelmintic drugs were also reported as carriers of Strongyloides spp. [15]. Sample size ranged from 7 to 3349, and prevalence within primate studies ranged from <1% to 100%, as seen in Table A1.
Domestic and stray dogs were the second most commonly identified source. Fourteen percent of all studies identifying Strongyloides spp. were within dogs, with sample sizes ranging from 35 to 879 and prevalence ranged from <1% to 45%, as seen in Table A1.
All studies reporting incidences of Strongyloides spp. within ruminant farming animals only identified Strongyloides to the genus level, as seen in Table A1.
Studies identifying rodents as a source of Strongyloides spp. accounted for 5% of the published works. Studies identified Rattus rattus, Rattus norvegicus, Mus musculus, Dasyprocta, and Hydrochoerus hydrochaeris as carriers of Strongyloides spp. [16,17,18,19,20,21,22]. Sample sizes for rodent-based studies ranged from 10 to 502. The prevalence ranged from 10% to 97%, as seen in Table A1.
Studies identifying insects within the order Diptera as a source of Strongyloides spp. accounted for 2% of the published works, as seen in Table A1. Identified insects within this order included flies of the genus Musca spp. and Lucilia spp. All studies identifying Strongyloides spp. within Diptera identified it from sites within the continent of Africa [23,24,25]. The sample sizes ranged from 5000 to 9950, and prevalence was between <1% and 2%.
Insects within the order Blattodea were identified in 2% of all studies, as seen in Table A1. Identified insects within this order include cockroaches from the genus Periplaneta spp. and Blattella spp. Four of the five identified studies reported Strongyloides spp. within populations of Blattodea in Africa. The remaining study identified Strongyloides spp. in Blattodea in Thailand. All studies identified infected insects within housing and food preparation areas [26,27,28,29]. The sample sizes of studies identifying insects within the order Blattodea ranged from 70 to 920, with the prevalence ranging from 1% to 81%.
Half (50%) of published works identifying parasitic contamination of vegetables and fruits found S. stercoralis upon leafy, rough-surfaced vegetables such as lettuces, cabbage, celery, spinach, and carrot [30,31,32,33,34,35,36,37]. The sample sizes for fruit and vegetable-based studies ranged from 36 to 1130, with prevalence ranging from <1% to 46%.
Countries where Strongyloides spp. was identified in soils in public areas included Spain, Iran, Malaysia, Nigeria, Brazil, the Czech Republic, Slovakia, and Romania, as seen in Figure 2 [38,39,40,41,42,43,44,45,46,47]. Geophagy, the purposeful consumption of soils, was also commonly identified as a factor in infection from soil-based sources.
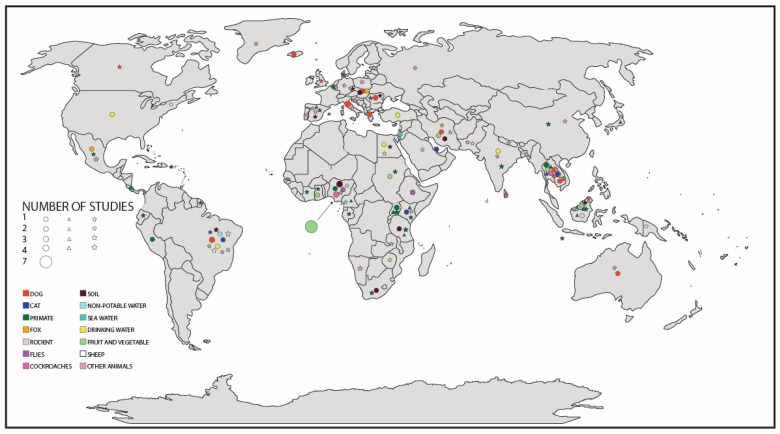
Studies identifying environmental sources of S. stercoralis, S. fuelleborni, and Strongyloides spp. are distributed across the world. Areas with a large amount of research included Europe, Africa, and South East Asia, as seen in Figure 1. Areas lacking research include Oceania, and the Americas, as seen in Figure 1. Many published studies identified Strongyloides spp. within temperate regions as opposed to tropical regions, as seen in Figure 1.
Figure 1.
Map displaying the global distribution of all reported environmental cases of Strongyloides stercoralis, Strongyloides fuelleborni, and Strongyloides spp. Where circles are representative of Strongyloides stercoralis, diamonds are representative of Strongyloides fuelleborni, and stars are representative of Strongyloides spp. The size of each shape is mapped to the number of studies published in that country. Location of shapes does not represent exact location of study, but country in which the study was completed. Colored fill of shapes was assigned to a single source and is consistent across all helminth species.
Microscopy was the most commonly used identification technique (90%). However, molecular detection was more common in recent publications. For example, in 2018, 6 of the 14 papers identified employed molecular-based techniques; however, in 2011, 1 of 11 papers published used molecular-based techniques, as seen in Table A1.
3. Discussion
3.1. Animals
Primates and domesticated or feral dogs (canids) adapt well to association with human settlements and cohabitation with humans, indicating the potential for transmission to humans. Contamination with feces from domesticated or synanthropic primates and dogs may lead to other environmental sources, such as water and soil, becoming reservoirs of Strongyloides spp. capable of causing infection. Most studies found in this review were based on primate and dog investigation, suggesting that these animals preferentially live closely with and benefit from humans. This habitual closeness presents a chance for environmental transmission of Strongyloides spp.
3.1.1. Canids
Studies that report parasites found in canid feces frequently investigate multiple parasites such as Ancylostoma spp., Giardia spp., and Strongyloides spp. These studies have often found low levels of S. stercoralis within otherwise highly parasitically infected populations [40,48,49,50,51,52,53,54,55,56]. Infection occurs more frequently in canids when they are living stray. This might be a result of exposure to infective Strongyloides spp. larvae occurring more frequently to these dogs than dogs living within homes [57]. Mass drug administration (MDA) to stray dogs has been implemented successfully for the control of rabies; accordingly, it may be an option for the control of Strongyloides spp. [10]. Isolated or infrequent anthelmintic treatment increases infection rates and so considered treatment must be implemented [10,58]. Studies identifying canid feces as containing Strongyloides spp. commonly also screened the samples for other parasites. Sample sizes ranged from 35 to 3465. The highest prevalence was reported by Beknazarova et al. [59] who screened 35 canine fecal samples from Australia, of which 49% were positive for Strongyloides spp. This low sample size with a high positivity rate in comparison with other studies is representative of the inconsistent fecal shedding of Strongyloides spp. as well as the endemic location of the study. The lowest prevalence was reported by Ardelean et al. [60] with 1% of 3465 samples positive from dogs within Romania. Strongyloidiasis was observed most commonly in dogs three to six months of age in this study. This variance based on age and study location may be further impacted by the detection method. Ardelean et al. [60] reported high levels of Ancylostomidae spp. which is morphologically similar to Strongyloides spp., therefore making reliable identification with microscopy alone difficult.
3.1.2. Primates
Areas sparsely populated by humans increase roaming in primates due to the attractive food sources but offer a low threat from the decreased human numbers. More frequent entry to communities in search of food potentially increases the numbers of Strongyloides spp.-infected primate feces within these sparsely populated communities [13,14]. Terrestrial Papio primates were likely to excrete Strongyloides spp. larvae; however, arboreal Cercopithecus neglectus were less likely [11,12]. This may be due to less frequent contact with soil containing Strongyloides spp. larvae. The impact of human populations upon forests has led to an increased chance of interaction between humans and potentially infected primates. Hasegawa et al. [61] observed that degraded forest increased the chance of roaming and transfer of parasites.
Captive primates present an infection risk to handlers because anthelmintic treatment has been observed to not eliminate Strongyloides spp. larvae shedding within feces [15]. This may be due to the introduction of new individuals to groups, a phenomenon also observed within wild individuals [15]. This indicates the value in introducing physical environmental controls beyond anthelmintic drugs, especially in communities exposed to roaming wild primates.
Tourist sanctuaries provide an ideal environment for contact between primates and humans. Environmental controls such as fecal contamination removal can decrease helminthic infection in both primates and humans without interfering with natural behaviors [62]. Strongyloides spp. is unable to transfer either from animal to human or from human to human directly [63]. This further supports the importance of clearing feces because contact with the animals does not cause infection; however, contact with fecal matter can cause infection. Larger groups, such as those within tourist sanctuaries, are generally associated with higher parasitic species richness. Some variation of infection can be expected based on food availability and stress levels [64].
Studies of primates had sample sizes ranging from 7 to 3349, with prevalence also ranging from <1% to 100%. Prevalence within primate populations was reported to be higher than in canine populations. Hasegawa et al. [61] reported 100% prevalence within seven gorilla and chimpanzees from Uganda; whereas Li et al. [65] screened 3349 fecal samples and identified a prevalence rate of 6%. This variation in prevalence may be due to the inconsistent shedding of Strongyloides spp. larvae. Li et al. [65] employed microscopy whereas Hasegawa et al. [61] employed molecular techniques, which may account for differentiation in prevalence.
3.1.3. Ruminants
Strongyloides spp. has also been found within the feces of ruminants used in western farming settings including pigs, sheep, and cattle. All studies identifying Strongyloides spp. within farm-associated ruminants only identified the parasite to the species level [66,67]. These may have been genus specific, such as the pork threadworm, Strongyloides ransomi, or the more general Strongyloides papillosis. All studies used microscopy, a technique that can have low success in identifying Strongyloides spp. to species level. These recorded observations indicate the potential for infected ruminant feces to provide an environmental source of Strongyloides spp.
3.1.4. Rodents
Rodents are known to carry a range of communicable diseases. Strongyloides spp. has been found in several rodent species, including common house rats, and non-synanthropic rodents such as Hydrochoerus hydrochaeris. S. stercoralis has been identified in house rat feces in East Java, Indonesia, using microscopy [20]. The area in which S. stercoralis was identified in house rat feces is an area with poor sanitation and hygiene. People reported a large house rat population within these areas [20]. In such cases, where a zoonotic pathogen is identified, control of the offending carrier can be employed. The sample sizes of rodents were low in comparison with other sources, with the highest sample size being 502 [19]. The highest prevalence was within a population of Rattus norvegicus within a Brazilian slum [17]. The rodents sampled within this study had particularly high levels of infection with helminths; in all except five, helminths were present within their feces [17]. This prevalence of 97% from a sample size of 299 is representative of animals living within an area highly contaminated by human waste.
3.1.5. Insects
Increasing urbanization has allowed for synanthropic dependence to increase within insect populations. Densely urbanizing areas lead to an increase in available food for insects, and areas with poor sanitation and hygiene practices attract disease-carrying insects such as those within the order Blattodea (cockroaches) and Diptera (flies). Filth flies present a source of helminth transmission. Their preference for consuming wet, rotting substances indicates a high probability for the consumption and carriage of Strongyloides spp. Carriage of Strongyloides spp. has been observed on the external body of flies despite frequent preening and cleaning [68]. Fetene and Worku [23] identified S. stercoralis within Chrysomya rufifacies, Musca sorbens, and Lucilia cuprina. C. rufifacies were identified largely within butcheries and defecating grounds; M. sorbens was found more frequently within the market collection sites. Furthermore, Musca domestica, a species always found in association with humans, has been observed to carry Strongyloides spp. [24]. The presence of these flies within human food areas presents a potential transmission route for Strongyloides spp. larvae. Prevalence was higher in the internal structures of flies than on the external surface of flies [25]. This observation is further supported by the preference of these flies for consumption of wet substances. Insects within the order Blattodea, commonly known as cockroaches, also present a transmission source. Parasite prevalence has been found to be associated with housing type. Low-cost housing with pit latrines as well as housing in close proximity to dumpsites was reported to contain higher levels of carrier cockroaches [26,28,29]. Through the introduction of environmental controls such as fly screens or nets, movement of carrier insects can be decreased [27]. Sample sizes for both orders were high. Studies found low prevalence with the exception of Morenikeji et al. [28] who reported 81% in 70 cockroaches.
3.2. Water
Contamination of water is also a potential source of helminth transferal. Pollution of water sources with human and agricultural waste can render water sources unsuitable for use as drinking and irrigation water. In areas where water access is limited, contaminated water may be employed for these uses [69]. Waste stabilization ponds, chlorination, or activated sludge treatment systems may be suitable approaches for reducing helminth levels; however, many studies monitoring wastewater treatment methods have provided contradictory results [70,71,72,73]. Some studies identified standard treatment techniques as adequate for removing larvae; however, others did not. Frequent monitoring of treated waste water is important because treated water has been identified as containing higher than acceptable levels of helminths including Strongyloides spp. [74]. To date, studies have focused on the helminth burden of treated water instead of comparing treated with untreated levels. Through focusing on untreated and treated waters from the same area, reduction in burden levels of treated water may be better understood.
Untreated water used for drinking can contain Strongyloides spp., particularly when water is sourced from storm water or collected rainwater [75]. According to one study, when water runoff moves into drinking water sources such as rivers, it can carry Strongyloides spp. larvae with it [75]. Bore and ground-water contamination can also occur and has been identified [76,77]. Jonnalagadda and Bhat [77] found that improper washing of vessels used to collect and store water can lead to helminth contamination. Implementation of appropriate washing and sanitation education in areas with high contamination risk may decrease incidences of infection.
Prevalence of Strongyloides spp. within non-potable water was higher than in potable water; this was expected because most sources of non-potable water were collected from wastewater treatment facilities [71]. The prevalence of Strongyloides spp. within non-treated wastewater was between 40–100%. Treated waste water intended for use on crops for human consumption had a much lower prevalence (2%); however, it was still observed to be present, supporting the importance of monitoring water intended for reuse [70].
3.3. Fruit and Vegetables
The rough nature of green, leafy vegetables surfaces means that adhesion of parasitic larvae and eggs occurs easily when these vegetables are either washed with contaminated water or come into contact with contaminated human fecal-based fertilizers (i.e., night soil) [30]. Studies identified S. stercoralis contamination most frequently within leafy, rough-surfaced vegetables such as lettuces, cabbage, celery, spinach, and carrot [30,31,32,33,34,35,36,37]. This correlation may be due to these vegetables growing close to or in the ground, which may lead to increased contamination from fertilizers [78,79]. Market vendors commonly wash vegetables prior to purchase, and consumption of raw vegetables such as salad leaves is frequently noted [31,79,80]. There is an increasing focus on the study of vegetables, washing water, and farm soil to determine where in the food chain parasites are being introduced [33].
Prevalence of Strongyloides spp. within fruit and vegetable samples was generally low, ranging from <1% to 46%. Ogbolu et al. [81] found S. stercoralis in 46% of fresh vegetables sold at open markets in Nigeria. The application of night soils and untreated wastewater is common within low-income nations may have led to the high level of prevalence [81]. Lower prevalence was also reported within Nigeria, between <1% and 19%. This variation may be due to differences in handling of samples, treatment during farming, and cross contamination [30,31].
3.4. Soil
Increasing urbanization has led to an ever-increasing amount of waste. Modern waste includes not only fecal waste but waste produced in the form of rubbish. Dumpsites and landfills are commonly employed to deal with this large amount of waste; Strongyloides spp. contamination can occur throughout the nearby environments. Dumps and landfills pose a transmission risk due to the ability of Strongyloides spp. larvae to survive effectively in the soil [82].
Contamination of soils with animal feces within public recreation areas also presents a transmission source. High levels of soil-transmitted helminths were reported in public area soils such as parks in Spain, Iran, Malaysia, Nigeria, Brazil, the Czech Republic, Slovakia, and Romania [38,39,40,41,42,43,44,45,46,47].
The texture and chemistry of soil also plays a role in the prevalence of Strongyloides spp. larvae. Moisture levels in soils increases the incidence of rhabditiform larvae developing into filariform larvae [83]. This requirement for moisture is supported by the findings of a study of helminth larvae during the wet season, which found that no larvae were located during the dry season despite contamination [39]. High sand and silt content soils favor the survival of Strongyloides spp. and other helminth larvae. This is due to the high porosity of these soils, which allows larvae to move effectively through the soils towards sources of nutrition and moisture [84].
Strongyloides spp. is transmitted from soil-based sources to humans through skin-to-soil contact; however, the behavior of purposefully ingesting soil known as geophagy can also lead to soil-based infections. Geophagy is culturally accepted and common in sub-Saharan Africa. This behavior is common in pregnant women; S. stercoralis infections have been observed, along with other soil-transmitted helminth infections, in these women [85,86]. Geophagy may be undertaken as a method for diet supplementation in low-income areas. Notably, these areas are more likely to have helminth-contaminated soil, which leads to an increased chance of infection.
Prevalence of Strongyloides spp. within soil was between 1% and 20%. Ivoke et al. [85] screened 797 pregnant women for parasitic infections related to geophagy. The prevalence of infection was 1% within this cohort. This low prevalence is likely due to picking soils specifically for consumption. Higher prevalence was observed in soils sampled in soil directly from areas densely populated with poor health infrastructure [41].
3.5. PCR and Microscopy
Identification of Strongyloides spp. nematodes can be undertaken using several methods. These fall into either techniques involving the identification of Strongyloides spp. larvae using a microscope or molecular-based techniques. Microscopy presents several problems but it accounts for most larval identification-based techniques (90%) as presented in this literature review. This study did not exclude papers based on year; accordingly, this overrepresentation of microscopy is likely a result of the recent introduction of polymerase chain reaction (PCR) techniques. Recent papers have increasingly employed PCR as accessibility to the equipment increases. In 2018, 5 of the 14 papers published employed molecular-based techniques; in contrast, in 2011, only 1 of 11 studies published employed molecular-based techniques, as seen in Table A1. This increasing use of molecular-based techniques is expected, and its use will allow for more accurate identification of species of Strongyloides spp. The strengths of microscopy include that it can be employed within the field or where resources are limited; however, microscopy alone cannot reliably differentiate S. stercoralis from S. fuelleborni. The reliance on microscopy-based techniques is hazardous because both species are morphologically similar [34]. Molecular techniques allow for the accurate identification of Strongyloides spp. to the species level; however, set up of molecular protocols can be expensive. Each technique has strengths and weaknesses; when looking at all published works, a consideration of the identification techniques allows for more accurate assessment of reports.
3.6. Global Distribution
Globally, published research has mainly focused in countries within Africa, Europe, and South East Asia. Commonly, Strongyloides spp. is reported as a tropical disease; however, Strongyloides spp. was often also reported in temperate regions such as Europe, as seen in Figure 1 [83,87]. This may be due to a lack of resources within low-income nations, leading to an overrepresentation of the generally higher income countries within Europe. Australia and the Americas both lacked studies looking at the environmental sources of Strongyloides spp. (Figure 1). This indicates a need for more research into the environmental transmission of S. stercoralis, S. fuelleborni, and Strongyloides spp. within these areas.
4. Materials and Methods
This systematic literature review is based on an adapted version of the PRISMA statement. This tool allows for the transparent and reliable reporting of evidence. A systematic search of the databases Scopus and Web of Science was undertaken, and all articles published prior to 2019 were included. Key words used in searches included Strongyloides spp., strongyloidiasis, tap water, soil, insect, zoonotic, and waste, as seen in Table 1. A search strategy was developed to ensure a transparent and complete literature review of all identified environmental sources of Strongyloides spp. was completed. This strategy is as follows; All non-English documents were excluded from the search.
To be included, published data must have reported Strongyloides spp. in one of the three spp. capable of human infection or to the genus level because these studies cannot be excluded as identifying disease-causing Strongyloides spp. The document must have reported this presence within an environmental source. Documents were excluded if they were reviews, reports of humans with Strongyloides spp. infection with no mention of contributing environmental source, or lab-based studies, as seen in Figure 2.
Figure 2.
Flow diagram representing the search strategies used (based on the PRISMA statement reporting guidelines for systematic literature reviews) showing an overview of the retrieved articles and the total articles identified as eligible.
First, all titles and abstracts of all papers were manually reviewed to ensure the papers met inclusion criteria. If it was unclear from titles and abstracts if papers met the criteria, they were included for full text review. Papers that were unclear were included. Papers were then read as full text and compared against inclusion and exclusion criteria. Articles that met these criteria were included in the study. All papers included in the study had key points extracted and recorded including the environmental source reported, species of Strongyloides spp. observed, detection method used, and the country from which the sample was taken.
5. Conclusions
Although Strongyloides spp. is considered a soil-transmitted helminth, there are several environmental sources that can potentially provide a route of transmission of the disease. Understanding the potential sources, combined with the adoption of environmental controls for Strongyloides spp. is likely to decrease transmission and therefore infections. Animals such as dogs, primates, and insects, as well as soil, water, and fruit and vegetables have all been reported to contain Strongyloides spp. larvae, capable of perpetuating infection within humans who have come into contact. Future research is needed to undertake a holistic screening of all environmental sources within endemic areas to identify those which pose the greatest significance to human health. By understanding the established and recorded environmental reservoirs of S. stercoralis, S. fuelleborni, and S. fuelleborni kellyi, better environmental controls can be implemented.
Acknowledgments
Thanks to Natasha White for producing the map using Adobe Illustrator.
Appendix A
Table A1.
Summary of all reports, and studies identifying S. stercoralis, S. fuelleborni, S. fuelleborni kellyi, and Strongyloides spp. within environmental sources worldwide.
| Species Parasite | Prevalence | Sample Size | Detection Method | Country | Reference | Source |
|---|---|---|---|---|---|---|
| Strongyloides stercoralis | 1% | 3465 | Microscopy | Romania | (Ardelean et al., 2005) [60] | Dog |
| Strongyloides stercoralis | 49% | 35 | Molecular | Australia | (Beknazarova et al., 2017) [59] | Dog |
| Strongyloides stercoralis | <1% | 3208 | Microscopy | Iceland | (Eydal and Skirnisson 2016) [88] | Dog |
| Strongyloides stercoralis | <1% | 215 | Microscopy | Brazil | (Ferreira et al., 2006) [89] | Dog |
| Strongyloides spp. | <1% | 457 | Microscopy | Canada | (Gaunt and Carr 2011) [90] | Dog |
| Strongyloides stercoralis | <1% | 181 | Microscopy | Brazil | (Goncalves et al., 2007) [91] | Dog |
| Strongyloides stercoralis | 87% | 88 | Molecular | Cambodia | (Jaleta et al., 2017) [6] | Dog |
| Strongyloides stercoralis | <1% | 879 | Microscopy | Greece | (Kostopoulou et al., 2017) [48] | Dog |
| Strongyloides stercoralis | <1% | 189 | Microscopy | Thailand | (Leelayoova et al., 2009) [49] | Dog |
| Strongyloides stercoralis | 45% | 171 | Microscopy | Brazil | (Martins et al., 2012) [58] | Dog |
| Strongyloides stercoralis | 4% | 52 | Microscopy | Romania | (Mircean et al., 2012) [92] | Dog |
| Strongyloides spp. | 5% | 175 | Microscopy | Malaysia | (Noor Azian et al., 2008) [40] | Dog |
| Strongyloides stercoralis | 2% | 281 | Microscopy | Greece | (Papazahariadouet al., 2007) [50] | Dog |
| Strongyloides stercoralis | 2% | 272 | Microscopy | Italy | (Paradies et al., 2017) [93] | Dog |
| Strongyloides spp. | 11% | 90 | Microscopy | Sri Lanka | (Perera et al., 2013) [57] | Dog |
| Strongyloides stercoralis | 6% | 174 | Microscopy | Iran | (Razmi et al., 2009) [52] | Dog |
| Strongyloides stercoralis | <1% | 239 | Microscopy | Italy | (Riggio et al., 2013) [53] | Dog |
| Strongyloides stercoralis | <1% | 639 | Microscopy | Italy | (Sauda et al., 2018) [55] | Dog |
| Strongyloides spp. | 15% | 94 | Microscopy | Cambodia | (Schär et al.,2014) [87] | Dog |
| Strongyloides stercoralis | 10% | 60 | Microscopy | Slovakia | (Štrkolcová et al., 2017) [45] | Dog |
| Strongyloides spp. | 2% | 171 | Microscopy | England | (Wright et al., 2016) [56] | Dog |
| Strongyloides stercoralis | <1% | 463 | Microscopy | Italy | (Zanzani et al., 2014) [94] | Dog |
| Strongyloides spp. | 2% | 197 | Microscopy | Thailand | (Pumidonming et al., 2016) [51] | Dog |
| Strongyloides stercoralis | 18% | 824 | Microscopy | Qatar | (Abu-Madi et al., 2007) [95] | Cat |
| Strongyloides spp. | 47% | 28 | Microscopy | Christmas Island | (Adams et al., 2008) [96] | Cat |
| Strongyloides spp. | 54% | 37 | Microscopy | Brazil | (Lima et al., 2017) [97] | Cat |
| Strongyloides spp. | 3% | 414 | Microscopy | Romania | (Mircean et al., 2010) [98] | Cat |
| Strongyloides stercoralis | 14% | 173 | Microscopy | Brazil | (Monteiro et al., 2016) [99] | Cat |
| Strongyloides stercoralis | 44% | 103 | Microscopy | Kenya | (Njuguna et al., 2017) [100] | Cat |
| Strongyloides spp. | <1% | 300 | Microscopy | Thailand | (Rojekittikhun et al., 2014) [101] | Cat |
| Strongyloides stercoralis | 21% | 38 | Microscopy | Thailand | (Sedionoto and Anamnart 2018) [102] | Cat |
| Strongyloides spp. | 99 1.0% | 99 | Microscopy | Denmark | (Takeuchi-Storm et al., 2015) [103] | Cat |
| Strongyloides fuelleborni | UNK | UNK | Molecular and Microscopy | Japan | (Arizono et al., 2012) [13] | Primate |
| Strongyloides spp. | 41 44% | 41 | Microscopy | Uganda | (Bezjian et al., 2008) [11] | Primate |
| Strongyloides spp. | 37% | 24 | Microscopy | French Guiana | (De Thoisy et al., 2001) [14] | Primate |
| Strongyloides spp. | 21% | 125 | Microscopy | India | (Ekanayake et al., 2006) [104] | Primate |
| Strongyloides fuelleborni | 28% | 293 | Microscopy | Uganda | (Gillespie et al., 2004) [105] | Primate |
| Strongyloides fuelleborni and Strongyloides stercoralis | <1% S. stercoralis, 4% S. fuelleborni | 2103 | Microscopy | Uganda | (Gillespie et al., 2005) [106] | Primate |
| Strongyloides fuelleborni | 84% | 153 | Microscopy | Tanzania | (Gillespie et al., 2010) [107] | Primate |
| Strongyloides fuelleborni and Strongyloides spp. | 11% S. fulleborni, 15% S. spp | 27 | Microscopy | Spain | (Gomez et al., 1996) [15] | Primate |
| Strongyloides fuelleborni | 23% | 401 | Microscopy | Japan | (Gotoh 2000) [108] | Primate |
| Strongyloides fuelleborni and Strongyloides stercoralis | 100% | 7 | Molecular | Uganda | (Hasegawa et al., 2016) [61] | Primate |
| Strongyloides spp. | 88% | 96 | Microscopy | Ecuador | (Helenbrook et al., 2015) [64] | Primate |
| Strongyloides spp. | 4% | 238 | Microscopy | Uganda | (Hodder and Chapman 2012) [109] | Primate |
| Strongyloides spp. | 7% | 40 | Microscopy | Kenya | (Karere and Munene 2002) [12] | Primate |
| Strongyloides spp. | 41% | 624 | Microscopy | Borneo | (Klaus et al., 2018) [62] | Primate |
| Strongyloides fuelleborni | 32% | 652 | Microscopy | Borneo | (Klaus et al., 2017) [110] | Primate |
| Strongyloides fuelleborni | 57% | 141 | Microscopy | Puerto Rico | (Knezevich et al., 1998) [111] | Primate |
| Strongyloides spp. | 43% | 686 | Microscopy | Tanzania | (Kooriyama et al., 2012) [112] | Primate |
| Strongyloides spp. | 74% | 3142 | Microscopy | Côte d’Ivoire | (Kouassi et al., 2015 [113] | Primate |
| Strongyloides spp. | 13% | 366 | Microscopy | India | (Kumar et al., 2018) [114] | Primate |
| Strongyloides fuelleborni | 44% S. fuelleborni, 4% S. spp. | 25 | Microscopy | Malaysia | (Kuze et al., 2010) [115] | Primate |
| Strongyloides fuelleborni | 95% | 20 | Molecular and Microscopy | Indonesia | (Labes et al., 2011) [116] | Primate |
| Strongyloides spp. | 37% | 59 | Microscopy | Ethiopia | (Legesse and Erko 2004) [117] | Primate |
| Strongyloides spp. | 5% | 222 | Microscopy | Belgium | (Levecke et al., 2007) [118] | Primate |
| Strongyloides spp. | 6% | 3349 | microscopy | China | (Li et al., 2017) [65] | Primate |
| Strongyloides stercoralis | 6% | 46 | Microscopy | Nigeria | (Mafuyai et al., 2013) [119] | Primate |
| Strongyloides spp. | 50% | 134 | Microscopy | Costa Rica | (Maldonado-Lopez et al., 2014) [120] | Primate |
| Strongyloides spp. | 77% | 78 | Microscopy | Ecuador | (Martin-Solano et al., 2017) [121] | Primate |
| Strongyloides spp. | 17% | 53 | Microscopy | Uganda | (Matsubayashi et al., 1992) [122] | Primate |
| Strongyloides fuelleborni | 58% | 432 | Molecular | Uganda | (McLennan et al., 2017) [123] | Primate |
| Strongyloides spp. | 84% | 121 | Microscopy | Uganda | (Muehlenbein et al., 2005) [124] | Primate |
| Strongyloides fuelleborni | 45% | 315 | Microscopy | Kenya | (Munene et al., 1998) [125] | Primate |
| Strongyloides fuelleborni | 21% | 297 | Microscopy | Kenya | (Muriuki et al., 1998) [126] | Primate |
| Strongyloides spp. | 76% | 83 | Microscopy | Costa Rica | (Parr et al., 2013) [127] | Primate |
| Strongyloides spp. | 13% | 366 | Microscopy | Tanzania | (Petrasova et al., 2010) [128] | Primate |
| Strongyloides spp. | 44% | 130 | Microscopy | Tanzania | (Petrzelkova et al., 2010) [129] | Primate |
| Strongyloides stercoralis | 15% | 86 | Microscopy | Peru | (Phillips et al., 2004) [130] | Primate |
| Strongyloides spp. | 43% | 47 | Microscopy | Gabon | (Pouillevet et al., 2017) [131] | Primate |
| Strongyloides fuelleborni | 6% | 125 | Microscopy | Cameroon | (Pourrut et al., 2011) [132] | Primate |
| Strongyloides spp. | 53% | 55 | Microscopy | Ghana | (Ryan et al., 2012) [133] | Primate |
| Strongyloides spp. | 8% | 420 | Molecular | Mexico | (Solorzano-Garcia and de Leon 2017) [134] | Primate |
| Strongyloides fuelleborni | 39% | 243 | Molecular | Thailand and Laos | (Thanchomnang et al., 2018) [135] | Primate |
| Strongyloides spp. | 35% | 283 | Microscopy | India | (Tiwari et al., 2017) [136] | Primate |
| Strongyloides stercoralis | 31% | 135 | Microscopy | Thailand | (Wenz-Mucke et al., 2013) [137] | Primate |
| Strongyloides spp. | 24% | 272 | Microscopy | South Africa | (Wren et al., 2015) [138] | Primate |
| Strongyloides spp. | 24% | 332 | Microscopy | South Africa | (Wren et al., 2016) [139] | Primate |
| Strongyloides fuelleborni and Strongyloides spp. | UNK | 14 | Molecular | Malaysian Borneo | (Frias et al., 2018) [140] | Primate |
| Strongyloides spp. | 29% | 64 | Microscopy | Brazil | (De Souza et al., 2012) [66] | Sheep |
| Strongyloides spp. | 8% | 165 | Microscopy | Papua New Guinea | (Koinari et al., 2013) [141] | Sheep |
| Strongyloides spp. | <1% | 27 | Microscopy | New England | (MacGlaflin et al., 2011) [142] | Sheep |
| Strongyloides spp. | UNK | 1798 | Microscopy | Brazil | (McManus et al., 2009) [143] | Sheep |
| Strongyloides spp. | 2% | 275 | Microscopy | Greenland | (Andreassen et al., 2017) [144] | Fox |
| Strongyloides spp. | 4% | 22 | Microscopy | Iran | (Dalimi et al., 2006) [145] | Fox |
| Strongyloides stercoralis | 16% | 249 | Microscopy | Mexico | (Hernandez-Camacho et al., 2011) [146] | Fox |
| Strongyloides stercoralis | 2% | 1198 | Microscopy | Slovakia | (Miterpakova et al., 2009) [147] | Fox |
| Strongyloides spp. | 65% | 60 | Microscopy | Pakistan | (Afshan et al., 2013) [16] | Rat |
| Strongyloides spp. | 97% | 299 | Microscopy | Brazil | (Carvalho-Pereira et al., 2018) [17] | Rat |
| Strongyloides spp. | 40% | 25 | Microscopy | Brazil | (Lima et al., 2017) [97] | Rat |
| Strongyloides spp. | 13% | 76 | Microscopy | Bangladesh | (Fuehrer et al., 2012) [18] | Rat |
| Strongyloides spp. | 10% | 502 | Microscopy | Nigeria | (Isaac et al., 2018) [19] | Mouse and rat |
| Strongyloides stercoralis | 53% | 98 | Microscopy | Indonesia | (Prasetyo et al., 2016) [20] | House rat |
| Strongyloides spp. | 10% | 10 | Microscopy | Brazil | (Souza et al., 2015) [21] | Capybaras |
| Strongyloides spp. | 10% | 31 | Microscopy | Brazil | (Gioia-Di Chiacchio et al., 2014) [22] | Capybaras |
| Strongyloides stercoralis | 2% | 6530 | Microscopy | Ethiopia | (Fetene and Worku 2009) [23] | Flies |
| Strongyloides stercoralis | <1% | 9950 | Microscopy | Ethiopia | (Getachew et al., 2007) [24] | Flies |
| Strongyloides stercoralis | 2% | 5000 | Microscopy | Nigeria | (Umeche 1989b) [25] | Flies |
| Strongyloides stercoralis | 12% | 749 | Microscopy | Nigeria | (Adenusi et al., 2018) [26] | Cockroaches |
| Strongyloides stercoralis | 1% | 920 | Microscopy | Thailand | (Chamavit et al., 2010) [27] | Cockroaches |
| Strongyloides stercoralis | 81% | 70 | Microscopy | Nigeria | (Morenikeji et al., 2016) [28] | Cockroaches |
| Strongyloides stercoralis | UNK | 234 | Microscopy | Nigeria | (Tatfeng et al., 2005) [29] | Cockroaches |
| Strongyloides stercoralis | 2% | 125 | Microscopy | Nigeria | (Adesewa and Morenikeji, 2017) [82] | Soil |
| Strongyloides spp. | 3% | 625 | Microscopy | Spain | (Dado et al., 2012) [38] | Soil |
| Strongyloides spp. | 8% | 120 | Microscopy | Egypt | (Etewa et al., 2016) [83] | Soil |
| Strongyloides stercoralis | 1% | 797 | Microscopy | Nigeria | (Ivoke et al., 2017) [85] | Geophagy |
| Strongyloides stercoralis | 2% | 1078 | Microscopy | Tanzania | (Kawai et al., 2009) [86] | Geophagy |
| Strongyloides stercoralis | 3% | 112 | Microscopy | Iran | (Motazedian et al., 2006) [39] | Soil |
| Strongyloides spp. | 7% | 182 | Microscopy | Malaysia | (Noor Azian et al., 2008) [40] | Soil |
| Strongyloides stercoralis | 20% | 102 | Microscopy | Nigeria | (Ogbolu et al., 2011) [41] | Soil |
| Strongyloides spp. | 5% | 2520 | Microscopy | Brazil | (Rocha et al., 2011) [42] | Soil |
| Strongyloides spp. | 2% | 500 | Microscopy | Czech Republic | (Valkounova 1982) [43] | Soil |
| Strongyloides spp. | 3% | 125 | Microscopy | Brazil | (Mandarino-Pereira et al., 2010) [44] | Soil |
| Strongyloides stercoralis | 14% | 14 | Microscopy | Slovakia | (Strkolcova et al., 2017) [45] | Soil |
| Strongyloides stercoralis | 12% | 17 | Microscopy | South Africa | (Sumbele et al., 2014) [84] | Soil |
| Strongyloides spp. | 4% | 45 | Microscopy | Romania | (Tudor 2015) [46] | Soil |
| Strongyloides stercoralis | 6% | 150 | Microscopy | Nigeria | (Umeche 1989a) [47] | Soil |
| Strongyloides spp. | 6% | 16 | Microscopy | Brazil | (da Silva et al., 2014) [148] | Soil |
| Strongyloides spp. | UNK | 8 | Microscopy | Cameroon | (Aghaindum and Landry, 2019) [149] | Non-potable water |
| Strongyloides spp. | 40% - 100% | 100 | Microscopy | Saudi Arabia | (Bolbol 1992) [69] | Non-potable water |
| Strongyloides stercoralis | 2%% | UNK | Microscopy | Brazil | (Bastos et al., 2008) [70] | Non-potable water |
| Strongyloides spp. | 100% | 3 | Microscopy | Brazil | (Cutolo et al., 2006) [71] | Non-potable water |
| Strongyloides stercoralis | 19% | 52 | Microscopy | Palestine | (Hilles et al., 2014) [150] | Seawater |
| Strongyloides stercoralis | 1% | 85 | Microscopy | Turkey | (Bakir et al., 2003) [151] | Drinking water |
| Strongyloides fuelleborni and Strongyloides spp. | 11% S. fuelleborni, 15% S. spp. | 9950 | Microscopy | Zimbabwe | (Dalu et al., 2011) [76] | Drinking water |
| Strongyloides spp. | UNK | UNK | Microscopy | Egypt | (El Shazly et al., 2003) [75] | Drinking water |
| Strongyloides stercoralis | 7% | 80 | Microscopy | Egypt | (El-Badry et al., 2018) [152] | Drinking water |
| Strongyloides stercoralis | 81% | 16 | Microscopy | Brazil | (Freitas et al., 2017) [153] | Drinking water |
| Strongyloides stercoralis | 51% | 232 | Microscopy | India | (Jonnalagadda and Bhat 1995) [77] | Drinking water |
| Strongyloides stercoralis | 100% | UNK | Microscopy | USA | (Klotz et al., 1992) [154] | Drinking water |
| Strongyloides stercoralis | UNK | UNK | Molecular | Malaysia | (Zeehaida et al., 2011) [155] | Fruit & vegetables |
| Strongyloides stercoralis | <1% | 1130 | Microscopy | Nigeria | (Adamu et al., 2012) [30] | Fruit & vegetables |
| Strongyloides stercoralis | <1% | 960 | Microscopy | Nigeria | (Adenusi et al., 2015) [31] | Fruit & vegetables |
| Strongyloides stercoralis | 10% | 150 | Microscopy | Nigeria | (Amaechi et al., 2016) [78] | Fruit & vegetables |
| Strongyloides stercoralis | 7% | 190 | Microscopy | Nigeria | (Amuta et al., 2017) [32] | Fruit & vegetables |
| Strongyloides stercoralis | 7% | 240 | Microscopy | Nigeria | (Dada et al., 2015) [33] | Fruit & vegetables |
| Strongyloides spp. | 1% | 453 | Microscopy | Iran | (Fallah et al., 2016) [34] | Fruit & vegetables |
| Strongyloides stercoralis | 36% | 360 | Microscopy | Ghana | (Kudah et al., 2018) [35] | Fruit & vegetables |
| Strongyloides spp. | 13% | 108 | Microscopy | Brazil | (Luz et al., 2017) [156] | Fruit & vegetables |
| Strongyloides spp. | 19% | 199 | Microscopy | Nigeria | (Maikai et al., 2012) [157] | Fruit & vegetables |
| Strongyloides spp. | 11% | 36 | Microscopy | Malaysia | (Matyusof et al., 2017) [80] | Fruit & vegetables |
| Strongyloides stercoralis | 1% | 260 | Microscopy | Sudan | (Mohamed et al., 2016) [36] | Fruit & vegetables |
| Strongyloides stercoralis | 10% | 265 | Microscopy | Thailand | (Punsawad et al., 2019) [37] | Fruit & vegetables |
| Strongyloides stercoralis | 46% | 120 | Microscopy | Nigeria | (Ogbolu et al., 2009) [81] | Fruit & vegetables |
| Strongyloides stercoralis | 14% | 140 | Microscopy | Iran | (Madadi 2010) [158] | Fruit & vegetables |
| Strongyloides stercoralis | 19% | 80 | Microscopy | Nigeria | (Ohaeri and Unogu 2011) [79] | Fruit & vegetables |
| Strongyloides spp. | 7% | 15 | Microscopy | Zambia | (Berentsen et al., 2012) [159] | Other animals |
| Strongyloides spp. | 5% | 272 | Microscopy | Nepal | (Bista et al., 2017) [160] | Other animals |
| Strongyloides spp. | 100% | 1 | Microscopy | Brazil | (Cardia et al., 2016) [161] | Other animals |
| Strongyloides spp. | 4% | 432 | Microscopy | Spain | (Cordon et al., 2008) [162] | Other animals |
| Strongyloides spp. | 40% | 52 | Microscopy | Russia | (González et al., 2007) [163] | Other animals |
| Strongyloides spp. | 2% | 956 | Microscopy | India | (Gupta et al., 2018) [164] | Other animals |
| Strongyloides spp. | <1% | 1005 | Microscopy | Germany | (Hallinger et al., 2018) [165] | Other animals |
| Strongyloides spp. | 31% | 42 | Microscopy | Japan | (Hasegawa et al., 2017) [166] | Other animals |
| Strongyloides spp. | <1% | 400 | Microscopy | Croatia | (Hermosilla et al., 2017) [167] | Other animals |
| Strongyloides spp. | 64% - 99% | 990 | Microscopy | Mexico | (Hu et al., 2018) [168] | Other animals |
| Strongyloides spp. | 4% | 821 | Microscopy | China | (Huang et al., 2014) [169] | Other animals |
| Strongyloides spp. | 15% | 2280 | Microscopy | Pakistan | (Khan et al., 2010) [67] | Other animals |
| Strongyloides spp. | UNK | 6 | Microscopy | Namibia | (Kumba et al., 2003) [170] | Other animals |
| Strongyloides spp. | 36% | 58 | Microscopy | Poland | (Mizgajska-Wiktor et al., 2010) [171] | Other animals |
| Strongyloides spp. | 67% | 12 | Microscopy | Mexico | (Mukul-Yerves et al., 2014) [172] | Other animals |
| Strongyloides spp. | 57% | 201 | Microscopy | Estonia | (Oja et al., 2017) [173] | Other animals |
| Strongyloides spp. | 47% | 383 | Microscopy | Mexico | (Ojeda-Robertos et al., 2017) [174] | Other animals |
| Strongyloides spp. | 7% | 6 | Molecular | Iberian Peninsula | (Perera et al., 2013) [175] | Other animals |
| Strongyloides spp. | 3% | 468 | Microscopy | Poland | (Pilarczyk et al., 2015) [176] | Other animals |
| Strongyloides spp. | 17% | 86 | Microscopy | Bangladesh | (Rahman et al., 2018) [177] | Other animals |
| Strongyloides spp. | 3% | 1883 | Microscopy | Italy | (Rinaldi et al., 2009) [178] | Other animals |
| Strongyloides spp. | 44% | 163 | Microscopy | Portugal | (Rosalino et al., 2006) [179] | Other animals |
| Strongyloides spp. | 45% | 82 | Microscopy | Australia | (Turni and Smales 2001) [180] | Other animals |
| Strongyloides spp. | UNK | UNK | Microscopy | Namibia | (Turner et al., 2010) [181] | Other animals |
| Strongyloides spp. | UNK | UNK | Microscopy | Namibia | (Turner et al., 2012) [182] | Other animals |
| Strongyloides spp. | <1% | 213 | Microscopy | Kenya | (VanderWaal et al., 2014) [183] | Other animals |
| Strongyloides spp. | 74% | 243 | Microscopy | Philippines | (Ybanez et al., 2018) [184] | Other animals |
Author Contributions
M.A.F.W., H.W., and K.E.R. designed and participated in review design. M.A.F.W. drafted and edited the manuscript, H.W. corrected and contributed to the manuscript, K.E.R. corrected and contributed to the manuscript. All authors approved of the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Olsen A., Van Lieshout L., Marti H., Polderman T., Polman K., Steinmann P., Stothard R., Thybo S., Verweij J.J., Magnussen P. Strongyloidiasis—The most neglected of the neglected tropical diseases? Trans. R. Soc. Trop. Med. Hyg. 2009;103:967–972. doi: 10.1016/j.trstmh.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Ashford R.W., Barnish G., Viney M.E. Strongyloides fuelleborni kellyi: Infection and disease in Papua New Guinea. Parasitol. Today. 1992;8:314–318. doi: 10.1016/0169-4758(92)90106-C. [DOI] [PubMed] [Google Scholar]
- 3.Hauber H.P., Galle J., Chiodini P.L., Rupp J., Birke R., Vollmer E., Zabel P., Lange C. Fatal outcome of a hyperinfection syndrome despite successful eradication of Strongyloides with subcutaneous ivermectin. Infect. 2005;33:383–386. doi: 10.1007/s15010-005-5060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearns T.M., Currie B.J., Cheng A.C., McCarthy J., Carapetis J.R., Holt D.C., Page W., Shield J., Gundjirryirr R., Mulholland E., et al. Strongyloides seroprevalence before and after an ivermectin mass drug administration in a remote Australian Aboriginal community. PLoS Negl. Trop. Dis. 2017;11:e0005607. doi: 10.1371/journal.pntd.0005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vadlamudi R.S., Chi D.S., Krishnaswamy G. Intestinal strongyloidiasis and hyperinfection syndrome. Clin. Mol. Allergy. 2006;4:8. doi: 10.1186/1476-7961-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaleta T.G., Zhou S., Bemm F.M., Schär F., Khieu V., Muth S., Odermatt P., Lok J.B., Streit A. Different but overlapping populations of Strongyloides stercoralis in dogs and humans—Dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisoffi Z., Buonfrate D., Montresor A., Requena-Méndez A., Muñoz J., Krolewiecki A.J., Gotuzzo E., Mena M.A., Chiodini P.L., Anselmi M., et al. Strongyloides stercoralis: A plea for action. PLoS Negl. Trop. Dis. 2013;7:e2214. doi: 10.1371/journal.pntd.0002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jourdan P.M., Lamberton P.H.L., Fenwick A., Addiss D.G. Soil-transmitted helminth infections. Lancet. 2018;391:252–265. doi: 10.1016/S0140-6736(17)31930-X. [DOI] [PubMed] [Google Scholar]
- 9.Maroto R., Jiménez A.E., Romero J.J., Alvarez V., De Oliveira J.B., Hernández J. First report of anthelmintic resistance in gastrointestinal nematodes of sheep from Costa Rica. Vet. Med. Int. 2011;2011:145312. doi: 10.4061/2011/145312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beknazarova M., Whiley H., Ross K. Mass drug administration for the prevention human strongyloidiasis should consider concomitant treatment of dogs. PLoS Negl. Trop. Dis. 2017;11:e0005735. doi: 10.1371/journal.pntd.0005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bezjian M., Gillespie T.R., Chapman C.A., Greiner E.C. Coprologic evidence of gastrointestinal helminths of forst baboons, Papio anubis, in Kibale national park, Uganda. J. Wildl. Dis. 2008;44:878–887. doi: 10.7589/0090-3558-44.4.878. [DOI] [PubMed] [Google Scholar]
- 12.Karere G.M., Munene E. Some gastro-intestinal tract parasites in wild De Brazza’s monkeys (Cercopithecus neglectus) in Kenya. Vet. Parasitol. 2002;110:153–157. doi: 10.1016/S0304-4017(02)00348-5. [DOI] [PubMed] [Google Scholar]
- 13.Arizono N., Yamada M., Tegoshi T., Onishi K. Molecular identification of oesophagostomum and trichuris eggs isolated from wild Japanese macaques. Korean J. Parasitol. 2012;50:253–257. doi: 10.3347/kjp.2012.50.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Thoisy B., Vogel I., Reynes J.M., Pouliquen J.F., Carme B., Kazanji M., Vié J.C. Health evaluation of translocated free-ranging primates in French Guiana. Am. J. Primatol. 2001;54:1–16. doi: 10.1002/ajp.1008. [DOI] [PubMed] [Google Scholar]
- 15.Gomez M.S., Gracenea M., Montoliu I., Feliu C., Monleon A., Fernandez J., Ensenat C. Intestinal parasitism—Protozoa and helminths—In primates at the Barcelona zoo. J. Med. Primatol. 1996;25:419–423. doi: 10.1111/j.1600-0684.1996.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 16.Afshan K., Beg M.A., Rizvi S.S.R., Qayyum M. Helminths and nematode infection in Norway rats (Rattus norvegicus) captured from Northern Punjab, Pakistan. Pak. J. Zool. 2013;45:1456–1459. [Google Scholar]
- 17.Carvalho-Pereira T., Souza F.N., Santos L.R.N., Walker R., Pertile A.C., de Oliveira D.S., Pedra G.G., Minter A., Rodrigues M.G., Bahiense T.C., et al. The helminth community of a population of Rattus norvegicus from an urban Brazilian slum and the threat of zoonotic diseases. Parasitol. 2018;145:797–806. doi: 10.1017/S0031182017001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuehrer H.P., Baumann T.A., Riedl J., Treiber M., Igel P., Swoboda P., Joachim A., Noedl H. Endoparasites of rodents from the Chittagong hill tracts in Southeastern Bangladesh. Wien. Klin. Wochenschr. 2012;124:27–30. doi: 10.1007/s00508-012-0237-7. [DOI] [PubMed] [Google Scholar]
- 19.Isaac C., Igbinosa B.I., Ohiolei J.A., Osimen C.E. Endoparasites of small mammals in Edo State, Nigeria: Public health implications. Korean J. Parasitol. 2018;56:93–100. doi: 10.3347/kjp.2018.56.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasetyo R.H. Survey of house rat intestinal parasites from Surabaya District, East Java, Indonesia that can cause opportunistic infections in humans. Southeast Asian J. Trop. Med. Public Health. 2016;47:194–198. [PubMed] [Google Scholar]
- 21.Souza G.T.R., Ribeiro T.S., Antonucci A.M., Ueda B.H., Carniel M.K., Karling L.C., Eiras J.C., Takemoto R.M., Pavanelli G.C. Endoparasite fauna of wild capybaras (Hydrochoerus hydrochaeris) (linnaeus, 1766) from the Upper Parana River floodplain, Brazil. Aquat. Mamm. 2015;41:213–221. doi: 10.1578/AM.41.2.2015.213. [DOI] [Google Scholar]
- 22.Gioia-Di Chiacchio R., Prioste F.E.S., Vanstreels R.E.T., Knobl T., Kolber M., Miyashiro S.I., Matushima E.R. Health evaluation and survey of zoonotic pathogens in free-ranging capybaras (Hydrochoerus hydrochaeris) J. Wildl. Dis. 2014;50:496–504. doi: 10.7589/2013-05-109. [DOI] [PubMed] [Google Scholar]
- 23.Fetene T., Worku N. Public health importance of non-biting cyclorrhaphan flies. Trans. R. Soc. Trop. Med. Hyg. 2009;103:187–191. doi: 10.1016/j.trstmh.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Getachew S., Gebre-Michael T., Erko B., Balkew M., Medhin G. Non-biting cyclorrhaphan flies (diptera) as carriers of intestinal human parasites in slum areas of Addis Ababa, Ethiopia. Acta Trop. 2007;103:186–194. doi: 10.1016/j.actatropica.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Umeche N., Mandah L.E. Musca domestica as a carrier of intestinal helminths in Calabar, Nigeria. East. Afr. Med. J. 1989;66:349–352. [PubMed] [Google Scholar]
- 26.Adenusi A.A., Akinyemi M.I., Akinsanya D. Domiciliary cockroaches as carriers of human intestinal parasites in Lagos metropolis, Southwest Nigeria: Implications for public health. J. Arthropod-Borne Dis. 2018;12:141–151. doi: 10.18502/jad.v12i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamavit P., Sahaisook P., Niamnuy N. The majority of cockroaches from the Samutprakarn province of Thailand are carriers of parasitic organisms. EXCLI J. 2011;10:218–222. [PMC free article] [PubMed] [Google Scholar]
- 28.Morenikeji O.A., Adebiyi A., Oluwayiose O.A. Parasites in cockroaches recovered from residential houses around awotan dumpsite in Ido local government area of Oyo State, Nigeria. Annu. Res. Rev. Biol. 2016;9 doi: 10.9734/ARRB/2016/19881. [DOI] [Google Scholar]
- 29.Tatfeng Y.M., Usuanlele M.U., Orukpe A., Digban A.K., Okodua M., Oviasogie F., Turay A.A. Mechanical transmission of pathogenic organisms: The role of cockroaches. J. Vector Borne Dis. 2005;42:129–134. [PubMed] [Google Scholar]
- 30.Adamu N.B., Adamu J.Y., Mohammed D. Prevalence of helminth parasites found on vegetables sold in Maiduguri, Northeastern Nigeria. Food Control. 2012;25:23–26. doi: 10.1016/j.foodcont.2011.10.016. [DOI] [Google Scholar]
- 31.Adenusi A.A., Abimbola W.A., Adewoga T.O.S. Human intestinal helminth contamination in pre-washed, fresh vegetables for sale in major markets in Ogun State, Southwest Nigeria. Food Control. 2015;50:843–849. doi: 10.1016/j.foodcont.2014.10.033. [DOI] [Google Scholar]
- 32.Amuta E.U., Obisike V.U., Acham N.I. Prevalence of helminth eggs on raw vegetables and fruits sold in selected markets in Makurdi, Benue State, Nigeria. Annu. Res. Rev. Biol. 2017;19 doi: 10.9734/ARRB/2017/35806. [DOI] [Google Scholar]
- 33.Dada A.J., Wartu J.R., Auta T., Diya A.W. Public health significance of helminthes eggs isolated from raw vegetables obtained from farms and those sold within Kaduna Metropolis, Nigeria. Asian J. Microbiol. Biotechnol. Environ. Sci. 2015;17:527–532. [Google Scholar]
- 34.Fallah A.A., Makhtumi Y., Pirali-Kheirabadi K. Seasonal study of parasitic contamination in fresh salad vegetables marketed in Shahrekord, Iran. Food Control. 2016;60:538–542. doi: 10.1016/j.foodcont.2015.08.042. [DOI] [Google Scholar]
- 35.Kudah C., Sovoe S., Baiden F. Parasitic contamination of commonly consumed vegetables in two markets in Ghana. Ghana Med. J. 2018;52:88–93. doi: 10.4314/gmj.v52i2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohamed M.A., Siddig E.E., Elaagip A.H., Edris A.M.M., Nasr A.A. Parasitic contamination of fresh vegetables sold at central markets in Khartoum State, Sudan. Ann. Clin. Microb. Antimicrob. 2016;15 doi: 10.1186/s12941-016-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Punsawad C., Phasuk N., Thongtup K., Nagavirochana S., Viriyavejakul P. Prevalence of parasitic contamination of raw vegetables in Nakhon Si Thammarat province, Southern Thailand. BMC Public Health. 2019;19 doi: 10.1186/s12889-018-6358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dado D., Izquierdo F., Vera O., Montoya A., Mateo M., Fenoy S., Galvan A.L., Garcia S., Garcia A., Aranguez E., et al. Detection of zoonotic intestinal parasites in public parks of Spain. Potential Epidemiological Role of Microsporidia. Zoonoese Public Health. 2012;59:23–28. doi: 10.1111/j.1863-2378.2011.01411.x. [DOI] [PubMed] [Google Scholar]
- 39.Motazedian H., Mehrabani D., Tabatabaee S.H.R., Pakniat A., Tavalali M. Prevalence of helminth ova in soil samples from public places in Shiraz. East. Med. Health J. 2006;12:562–565. [PubMed] [Google Scholar]
- 40.Noor Azian M.Y., Sakhone L., Hakim S.L., Yusri M.Y., Nurulsyamzawaty Y., Zuhaizam A.H., Rodi I.M., Maslawaty M.N. Detection of helminth infections in dogs and soil contamination in rural and urban areas. Southeast Asian J. Trop. Med. Public Health. 2008;39:205–212. [PubMed] [Google Scholar]
- 41.Ogbolu D.O., Alli O.A., Amoo A.O., Olaosun I.I., Ilozavbie G.W., Olusoga-Ogbolu F.F. High-level parasitic contamination of soil sampled in Ibadan Metropolis. Afr. J. Med. Med. Sci. 2011;40:321–325. [PubMed] [Google Scholar]
- 42.Rocha S., Pinto R.M.F., Floriano A.P., Teixeira L.H., Bassili B., Martinez A., da Costa S.O.P., Caseiro M.M. Environmental analyses of the parasitic profile found in the sandy soil from the Santos municipality beaches, sp, Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2011;53:277–281. doi: 10.1590/S0036-46652011000500007. [DOI] [PubMed] [Google Scholar]
- 43.Valkounová J. Parasitological investigation of children’s sandboxes and dog faeces from public areas of housing development in Prague. Folia Parasitol. 1982;29:133–138. [PubMed] [Google Scholar]
- 44.Mandarino-Pereira A., de Souza F.S., Lopes C.W.G., Pereira M.J.S. Prevalence of parasites in soil and dog feces according to diagnostic tests. Vet. Parasitol. 2010;170:176–181. doi: 10.1016/j.vetpar.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Štrkolcová G., Goldová M., Bocková E., Mojžišová J. The roundworm Strongyloides stercoralis in children, dogs, and soil inside and outside a segregated settlement in Eastern Slovakia: Frequent but hardly detectable parasite. Parasitol. Res. 2017;116:891–900. doi: 10.1007/s00436-016-5362-1. [DOI] [PubMed] [Google Scholar]
- 46.Tudor P. Soil Contamination with canine intestinal parasites eggs in the parks and shelter dogs from Bucharest Area. Agric. Agric. Sci. Procedia. 2015;6:387–391. doi: 10.1016/j.aaspro.2015.08.103. [DOI] [Google Scholar]
- 47.Umeche N. Helminth ova in soil from children’s playgrounds in Calabar, Nigeria. Central Afr. J. Med. 1989;35:432–434. [PubMed] [Google Scholar]
- 48.Kostopoulou D., Claerebout E., Arvanitis D., Ligda P., Voutzourakis N., Casaert S., Sotiraki S. Abundance, zoonotic potential and risk factors of intestinal parasitism amongst dog and cat populations: The scenario of Crete, Greece. Parasit. Vectors. 2017;10 doi: 10.1186/s13071-017-1989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leelayoova S., Siripattanapipong S., Naaglor T., Taamasri P., Mungthin M. Prevalence of intestinal parasitic infections in military personnel and military dogs, Thailand. J. Med. Assoc. Thail. Chotmaihet Thangphaet. 2009;92(Suppl. 1):S53–S59. [PubMed] [Google Scholar]
- 50.Papazahariadou A., Founta A., Papadopoulos E., Chliounakis S., Antoniadou-Sotiriadou K., Theodorides Y. Gastrointestinal parasites of shepherd and hunting dogs in the Serres Prefecture, Northern Greece. Vet. Parasitol. 2007;148:170–173. doi: 10.1016/j.vetpar.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Pumidonming W., Salman D., Gronsang D., Abdelbaset A.E., Sangkaeo K., Kawazu S., Igarashi M. Prevalence of gastrointestinal helminth parasites of zoonotic significance in dogs and cats in lower Northern Thailand. J.Vet. Med. Sci. 2016;78:1779–1784. doi: 10.1292/jvms.16-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Razmi G.R. Survey of dogs’ parasites in Khorasan Razavi province, Iran. Iran. J. Parasitol. 2009;4:48–54. [Google Scholar]
- 53.Riggio F., Mannella R., Ariti G., Perrucci S. Intestinal and lung parasites in owned dogs and cats from central Italy. Vet. Parasitol. 2013;193:78–84. doi: 10.1016/j.vetpar.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 54.Santos J.L.C., Magalhanes N.B., dos Santos H.A., Ribeiro R.R., Guimaraes M.P. Parasites of domestic and wild canids in the region of Serra Do Cipo national park, Brazil. Rev. Bras. De Parasitol. Vet. 2012;21:270–277. doi: 10.1590/S1984-29612012000300016. [DOI] [PubMed] [Google Scholar]
- 55.Sauda F., Malandrucco L., Macri G., Scarpulla M., De Liberato C., Terracciano G., Fichi G., Berrilli F., Perrucci S. Leishmania infantum, dirofilaria spp. And other endoparasite infections in kennel dogs in central Italy. Parasite. 2018;25 doi: 10.1051/parasite/2018001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright I., Stafford K., Coles G. The prevalence of intestinal nematodes in cats and dogs from Lancashire, North-West England. J. Small Anim. Pract. 2016;57:393–395. doi: 10.1111/jsap.12478. [DOI] [PubMed] [Google Scholar]
- 57.Perera P.K., Rajapakse R., Rajakaruna R.S. Gastrointestinal parasites of dogs in Hantana area in the Kandy district. J. National Sci. Found. Sri Lanka. 2013;41:81–91. doi: 10.4038/jnsfsr.v41i2.5703. [DOI] [Google Scholar]
- 58.Martins C.M., de Barros C.D., Bier D., Marinho A.P., Figueiredo J.M.G., Hoffmann J.L., Molento M.B., Biondo A.W. Dog parasite incidence and risk factors, from sampling after one-year interval, in Pinhais, Brazil. Rev. Bras. De Parasitol. Vet. 2012;21:101–106. doi: 10.1590/S1984-29612012000200006. [DOI] [PubMed] [Google Scholar]
- 59.Beknazarova M., Millsteed S., Robertson G., Whiley H., Ross K. Validation of dess as a DNA preservation method for the detection of Strongyloides spp. In canine feces. Int. J. Environmen. Res. Public Health. 2017;14:624. doi: 10.3390/ijerph14060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ardelean A.I., Suteu E., Cozma V. Epidemiology of digestive helminthosis from urban area of Clujnapoca, Romania. Bull. Univ. Agric. Sci. Vet. Med. Vet. Med. 2005;62:322–329. [Google Scholar]
- 61.Hasegawa H., Kalousova B., McLennan M.R., Modry D., Profousova-Psenkova I., Shutt-Phillips K.A., Todd A., Huffman M.A., Petrzelkova K.J. Strongyloides infections of humans and great apes in Dzangasangha protected areas, Central African republic and in degraded forest fragments in Bulindi, Uganda. Parasitol. Int. 2016;65:367–370. doi: 10.1016/j.parint.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Klaus A., Strube C., Roper K.M., Radespiel U., Schaarschmidt F., Nathan S., Goossens B., Zimmermann E. Fecal parasite risk in the endangered proboscis monkey is higher in an anthropogenically managed forest environment compared to a Riparian rain forest in Sabah, Borneo. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0195584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grove D.I. Strongyloidiasis: Is it transmitted from husband to wife? Br. J. Vener. Dis. 1982;58:271–272. doi: 10.1136/sti.58.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Helenbrook W.D., Wade S.E., Shields W.M., Stehman S.V., Whipps C.M. Gastrointestinal parasites of Ecuadorian mantled howler monkeys (Alouatta palliata aequatorialis) based on fecal analysis. J. Parasitol. 2015;101:341–350. doi: 10.1645/13-356.1. [DOI] [PubMed] [Google Scholar]
- 65.Li J.Q., Dong H.J., Wang R.J., Yu F.C., Wu Y.Y., Chang Y.K., Wang C.R., Qi M., Zhang L.X. An investigation of parasitic infections and review of molecular characterization of the intestinal protozoa in nonhuman primates in China from 2009 to 2015. Int. J. Parasitol. Parasit. Wildl. 2017;6:8–15. doi: 10.1016/j.ijppaw.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Souza M.D., Pimentel-Neto M., da Silva R.M., Farias A.C.B., Guimaraes M.P. Gastrointestinal parasites of sheep, municipality of Lajes, Rio Grande Do Norte, Brazil. Rev. Bras. De Parasitol. Vet. 2012;21:71–73. doi: 10.1590/S1984-29612012000100015. [DOI] [PubMed] [Google Scholar]
- 67.Khan M.N., Sajid M.S., Khan M.K., Iqbal Z., Hussain A. Gastrointestinal helminthiasis: Prevalence and associated determinants in domestic ruminants of district Toba Tek Singh, Punjab, Pakistan. Parasitol. Res. 2010;107:787–794. doi: 10.1007/s00436-010-1931-x. [DOI] [PubMed] [Google Scholar]
- 68.Förster M., Klimpel S., Sievert K. The house fly (Musca domestica) as a potential vector of metazoan parasites caught in a pig-pen in Germany. Vet. Parasitol. 2009;160:163–167. doi: 10.1016/j.vetpar.2008.10.087. [DOI] [PubMed] [Google Scholar]
- 69.Bolbol A.S. Risk of contamination of human and agricultural environment with parasites through reuse of treated municipal wastewater in Riyadh, Saudi Arabia. J. Hyg. Epidemiol. Microbiol. Immunol. 1992;36:330–337. [PubMed] [Google Scholar]
- 70.Bastos R.K.X., Bevilacqua P.D., Silva C.A.B., Silva C.V. Wastewater irrigation of salad crops: Further evidence for the evaluation of the WHO guidelines. Water Sci. Technol. 2008;57:1213–1219. doi: 10.2166/wst.2008.244. [DOI] [PubMed] [Google Scholar]
- 71.Cutolo S.A., Matté M.H., Rocha A.A. Monitoring of parasitological contamination in treated wastewater from activated sludge system. Manag. Environ. Qual. Int. J. 2006;17:43–56. doi: 10.1108/14777830610639431. [DOI] [Google Scholar]
- 72.Saqer A.S., Seham H., Ragaa G., Yehia A.E.W., Wafaa S. Optimum methods of inactivation of Strongyloides stercoralis larvae from reclaimed wastewater. Environ. Monit. Assess. 2007;130:341–346. doi: 10.1007/s10661-006-9401-8. [DOI] [PubMed] [Google Scholar]
- 73.Tonani K.A.A., Julião F.C., Trevilato T.M.B., Takayanagui A.M.M., Bocio A., Domingo J.L., Segura-Muñoz S.I. Behavior of metals, pathogen parasites, and indicator bacteria in sewage effluents during biological treatment by activated sludge. Biol. Trace Elem. Res. 2011;143:1193–1201. doi: 10.1007/s12011-010-8906-8. [DOI] [PubMed] [Google Scholar]
- 74.Hatam-Nahavandi K., Mahvi A.H., Mohebali M., Keshavarz H., Mobedi I., Rezaeian M. Detection of parasitic particles in domestic and urban wastewaters and assessment of removal efficiency of treatment plants in Tehran, Iran. J. Environ. Health Sci. Eng. 2015;13 doi: 10.1186/s40201-015-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El Shazly A.M., Soliman M., Nemr H.I., El Moafyo N., Abel Gawad A.G., El Bendary M. Nematodes and water pollution in eastern part of Nile Delta. J. Egy. Soc. Parasitol. 2003;33:631–636. [PubMed] [Google Scholar]
- 76.Dalu T., Barson M., Nhiwatiwa T. Impact of intestinal microorganisms and protozoan parasites on drinking water quality in Harare, Zimbabwe. J. Water Sanit. Hyg. Dev. 2011;1:153–163. doi: 10.2166/washdev.2011.049. [DOI] [Google Scholar]
- 77.Jonnalagadda P.R., Bhat R.V. Parasitic contamination of stored water used for drinking/cooking in Hyderabad. Southeast Asian J. Trop. Med. Public Health. 1995;26:789–794. [PubMed] [Google Scholar]
- 78.Amaechi E.C., Ohaeri C.C., Ukpai O.M., Adegbite R.A. Prevalence of parasitic contamination of salad vegetables in Ilorin, North Central, Nigeria. Momona Ethiop. J. Sci. 2016;8:136–145. doi: 10.4314/mejs.v8i2.3. [DOI] [Google Scholar]
- 79.Ohaeri C.C., Unogu L.O. Soil transmitted helminths of some common fruits and vegetables in Umuahia, Abia State Nigeria. Niger. J. Parasitol. 2011;32:305–308. [Google Scholar]
- 80.Matyusof A., Mohammad M., Abshir Abdullahi M., Mohamed Z., Zakaria R., Abdul Wahab R. Occurrence of intestinal parasitic contamination in select consumed local raw vegetables and fruits in Kuantan, Pahang. Trop. Life Sci. Res. 2017;28:23–32. doi: 10.21315/tlsr2017.28.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ogbolu D.O., Alli O.A., Ogunleye V.F., Olusoga-Ogbolu F.F., Olaosun I. The presence of intestinal parasites in selected vegetables from open markets in South Western Nigeria. Afr. J. Med. Med. Sci. 2009;38:319–324. [PubMed] [Google Scholar]
- 82.Adesewa A., Morenikeji O. Helminths and heavy metals in soils from a dumpsite in Ibadan City, Nigeria. J. Prev. Med. Hyg. 2017;58:E328–E333. doi: 10.15167/2421-4248/jpmh2017.58.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Etewa S.E., Abdel-Rahman S.A., Abd El-Aal N.F., Fathy G.M., El-Shafey M.A., Ewis A.M.G. Geohelminths distribution as affected by soil properties, physicochemical factors and climate in Sharkyia Governorate Egypt. J. Parasit. Dis. 2016;40:496–504. doi: 10.1007/s12639-014-0532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sumbele I.U., Ngole V.M., Ekosse G.I.E. Influence of physico-chemistry and mineralogy on the occurrence of geohelminths in geophagic soils from selected communities in the Eastern cape, South Africa, and their possible implication on human health. Int. J. Environ. Health Res. 2014;24:18–30. doi: 10.1080/09603123.2013.782600. [DOI] [PubMed] [Google Scholar]
- 85.Ivoke N., Ikpor N., Ivoke O., Ekeh F., Ezenwaji N., Odo G., Iyaji F., Onoja U., Eyo J. Geophagy as risk behaviour for gastrointestinal nematode infections among pregnant women attending antenatal clinics in a humid tropical zone of Nigeria. Afr. Health Sci. 2017;17:24–31. doi: 10.4314/ahs.v17i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kawai K., Saathoff E., Antelman G., Msamanga G., Fawzi W.W. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV-infected pregnant women in Tanzania. Am. J. Trop. Med. Hyg. 2009;80:36–43. doi: 10.4269/ajtmh.2009.80.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schär F., Trostdorf U., Giardina F., Khieu V., Muth S., Marti H., Vounatsou P., Odermatt P. Strongyloides stercoralis: Global distribution and risk factors. PLoS Negl. Trop. Dis. 2013;7:e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eydal M., Skirnisson K. Strongyloides stercoralis found in imported dogs, household dogs and kennel dogs in Iceland. Icel. Agric. Sci. 2016;29:39–51. doi: 10.16886/IAS.2016.04. [DOI] [Google Scholar]
- 89.Ferreira A., Goncalves-Pires M.R.F., Silva D.A.O., Goncalves A.L.R., Costa-Cruz J.M. Parasitological and serological diagnosis of Strongyloides stercoralis in domesticated dogs from Southeastern Brazil. Vet. Parasitol. 2006;136:137–145. doi: 10.1016/j.vetpar.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 90.Gaunt M.C., Carr A.P. A survey of intestinal parasites in dogs from Saskatoon, Saskatchewan. Can. Vet. J. Revue Vet. Can. 2011;52:497–500. [PMC free article] [PubMed] [Google Scholar]
- 91.Goncalves A.L.R., Machado G.A., Goncalves-Pires M.R.F., Ferreira-Junior A., Silva D.A.O., Costa-Cruz J.M. Evaluation of strongyloidiasis in kennel dogs and keepers by parasitological and serological assays. Vet. Parasitol. 2007;147:132–139. doi: 10.1016/j.vetpar.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 92.Mircean V., Gyorke A., Cozma V. Prevalence and risk factors of Giardia duodenalis in dogs from Romania. Vet. Parasitol. 2012;184:325–329. doi: 10.1016/j.vetpar.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 93.Paradies P., Iarussi F., Sasanelli M., Capogna A., Lia R.P., Zucca D., Greco B., Cantacessi C., Otranto D. Occurrence of strongyloidiasis in privately owned and sheltered dogs: Clinical presentation and treatment outcome. Parasit. Vectors. 2017;10 doi: 10.1186/s13071-017-2275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zanzani S.A., Di Cerbo A.R., Gazzonis A.L., Genchi M., Rinaldi L., Musella V., Cringoli G., Manfredi M.T. Canine fecal contamination in a metropolitan area (Milan, North-Western Italy): Prevalence of intestinal parasites and evaluation of health risks. Sci. World J. 2014;2014 doi: 10.1155/2014/132361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abu-Madi M.A., Al-Ahbabi D.A., Al-Mashhadani M.M., Al-Ibrahim R., Pal P., Lewis J.W. Patterns of parasitic infections in faecal samples from stray cat populations in Qatar. J. Helminthol. 2007;81:281–286. doi: 10.1017/S0022149X07818505. [DOI] [PubMed] [Google Scholar]
- 96.Adams P.J., Elliot A.D., Algar D., Brazell R.I. Gastrointestinal parasites of feral cats from Christmas Island. Aust. Vet. J. 2008;86:60–63. doi: 10.1111/j.1751-0813.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 97.Lima V.F.S., Ramos R.A.N., Lepold R., Borges J.C.G., Ferreira C.D., Rinaldi L., Cringoli G., Alves L.C. Gastrointestinal parasites in feral cats and rodents from the Fernando De Noronha Archipelago, Brazil. Rev. Bras. Parasit. Vet. 2017;26:521–524. doi: 10.1590/s1984-29612017066. [DOI] [PubMed] [Google Scholar]
- 98.Mircean V., Titilincu A., Vasile C. Prevalence of endoparasites in household cat (felis catus) populations from Transylvania (Romania) and association with risk factors. Vet. Parasitol. 2010;171:163–166. doi: 10.1016/j.vetpar.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 99.Monteiro M.F.M., Ramos R.A.N., Calado A.M.C., Lima V.F.S., Ramos I.C.D., Tenorio R.F.L., Faustino M.A.D., Alves L.C. Gastrointestinal parasites of cats in Brazil: Frequency and zoonotic risk. Rev. Bras. Parasitol. Vet. 2016;25:254–257. doi: 10.1590/S1984-29612016019. [DOI] [PubMed] [Google Scholar]
- 100.Njuguna A.N., Kagira J.M., Karanja S.M., Ngotho M., Mutharia L., Maina N.W. Prevalence of Toxoplasma gondii and other gastrointestinal parasites in domestic cats from households in Thika region, Kenya. Biomed Res. Int. 2017 doi: 10.1155/2017/7615810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rojekittikhun W., Chaisiri K., Mahittikorn A., Pubampen S., Sa-nguankiat S., Kusolsuk T., Maipanich W., Udonsom R., Mori H. Gastrointestinal parasites of dogs and cats in a refuge in Nakhon Nayok, Thailand. Southeast Asian J. Trop. Med. Public Health. 2014;45:31–39. [PubMed] [Google Scholar]
- 102.Sedionoto B., Anamnart W. Prevalence of hookworm infection and strongyloidiasis in cats and potential risk factor of human diseases. E3S Web of Conf. 2018;31:06002. doi: 10.1051/e3sconf/20183106002. [DOI] [Google Scholar]
- 103.Takeuchi-Storm N., Mejer H., Al-Sabi M.N.S., Olsen C.S., Thamsborg S.M., Enemark H.L. Gastrointestinal parasites of cats in Denmark assessed by necropsy and concentration mcmaster technique. Vet. Parasitol. 2015;214:327–332. doi: 10.1016/j.vetpar.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 104.Ekanayake D.K., Arulkanthan A., Horadagoda N.U., Sanjeevani G.K.M., Kieft R., Gunatilake S., Dittus W.P.J. Prevalence of cryptosporidium and other enteric parasites among wild non-human primates in Polonnaruwa, Sri Lanka. Am. J. Trop. Med. Hyg. 2006;74:322–329. doi: 10.4269/ajtmh.2006.74.322. [DOI] [PubMed] [Google Scholar]
- 105.Gillespie T.R., Greiner E.C., Chapman C.A. Gastrointestinal parasites of the Guenons of Western Uganda. J. Parasitol. 2004;90:1356–1360. doi: 10.1645/GE-311R. [DOI] [PubMed] [Google Scholar]
- 106.Gillespie T.R., Greiner E.C., Chapman C.A. Gastrointestinal parasites of the Colobus monkeys of Uganda. J. Parasitol. 2005;91:569–573. doi: 10.1645/GE-434R. [DOI] [PubMed] [Google Scholar]
- 107.Gillespie T.R., Lonsdorf E.V., Canfield E.P., Meyer D.J., Nadler Y., Raphael J., Pusey A.E., Pond J., Pauley J., Mlengeya T., et al. Demographic and ecological effects on patterns of parasitism in Eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe national park, Tanzania. Am. J. Phys. Anthropol. 2010;143:534–544. doi: 10.1002/ajpa.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gotoh S. Regional differences in the infection of wild Japanese macaques by gastrointestinal helminth parasites. Primates. 2000;41:291–298. doi: 10.1007/BF02557598. [DOI] [PubMed] [Google Scholar]
- 109.Hodder S.A.M., Chapman C.A. Do nematode infections of red colobus (Procolobus rufomitratus) and black-and-white colobus (Colobus guereza) on humanized forest edges differ from those on nonhumanized forest edges? Int. J. Primatol. 2012;33:845–859. doi: 10.1007/s10764-012-9619-y. [DOI] [Google Scholar]
- 110.Klaus A., Zimmermann E., Roper K.M., Radespiel U., Nathan S., Goossens B., Strube C. Co-infection patterns of intestinal parasites in arboreal primates (Proboscis monkeys, Nasalis larvatus) in Borneo. Int. J. Parasitol. Parasit. Wildl. 2017;6:320–329. doi: 10.1016/j.ijppaw.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Knezevich M. Geophagy as a therapeutic mediator of endoparasitism in a free-ranging group of Rhesus macaques (Macaca mulatta) Am. J. Primatol. 1998;44:71–82. doi: 10.1002/(SICI)1098-2345(1998)44:1<71::AID-AJP6>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 112.Kooriyama T., Hasegawa H., Shimozuru M., Tsubota T., Nishida T., Iwaki T. Parasitology of five primates in Mahale mountains national park, Tanzania. Primates. 2012;53:365–375. doi: 10.1007/s10329-012-0311-9. [DOI] [PubMed] [Google Scholar]
- 113.Kouassi R.Y.W., McGraw S.W., Yao P.K., Abou-Bacar A., Brunet J., Pesson B., Bonfoh B., N’Goran E.K., Candolfi E. Diversity and prevalence of gastrointestinal parasites in seven non-human primates of the Tai national park, Cote D’ivoire. Parasite. 2015;22 doi: 10.1051/parasite/2015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kumar S., Sundararaj P., Kumara H.N., Pal A., Santhosh K., Vinoth S. Prevalence of gastrointestinal parasites in bonnet macaque and possible consequences of their unmanaged relocations. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0207495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuze N., Kanamori T., Malim T.P., Bernard H., Zamma K., Kooriyama T., Morimoto A., Hasegawa H. Parasites found from the feces of Bornean orangutans in Danum Valley, Sabah, Malaysia, with a redescription of Pongobius hugoti and the description of a new species of Pongobius (nematoda: Oxyuridae) J. Parasitol. 2010;96:954–960. doi: 10.1645/GE-2379.1. [DOI] [PubMed] [Google Scholar]
- 116.Labes E.M., Wijayanti N., Deplazes P., Mathis A. Genetic characterization of Strongyloides spp. From captive, semi-captive and wild Bornean orangutans (Pongo pygmaeus) in central and east Kalimantan, Borneo, Indonesia. Parasitology. 2011;138:1417–1422. doi: 10.1017/S0031182011001284. [DOI] [PubMed] [Google Scholar]
- 117.Legesse M., Erko B. Zoonotic intestinal parasites in Papio anubis (Baboon) and Cercopithecus aethiops (Vervet) from four localities in Ethiopia. Acta Trop. 2004;90:231–236. doi: 10.1016/j.actatropica.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 118.Levecke B., Dorny P., Geurden T., Vercammen F., Vercruysse J. Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet. Parasitol. 2007;148:236–246. doi: 10.1016/j.vetpar.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 119.Mafuyai H.B., Barshep Y., Audu B.S., Kumbak D., Ojobe T.O. Baboons as potential reservoirs of zoonotic gastrointestinal parasite infections at Yankari National Park, Nigeria. Afr. Health Sci. 2013;13:252–254. doi: 10.4314/ahs.v13i2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maldonado-Lopez S., Maldonado-Lopez Y., Ch A.G.T., Cuevas-Reyes P., Stoner K.E. Patterns of infection by intestinal parasites in sympatric howler monkey (Alouatta palliata) and spider monkey (Ateles geoffroyi) populations in a tropical dry forest in Costa Rica. Primates. 2014;55:383–392. doi: 10.1007/s10329-014-0413-7. [DOI] [PubMed] [Google Scholar]
- 121.Martin-Solano S., Carrillo-Bilbao G.A., Ramirez W., Celi-Erazo M., Huynen M.C., Levecke B., Benitez-Ortiz W., Losson B. Gastrointestinal parasites in captive and free-ranging Cebus albifrons in the Western Amazon, Ecuador. Int. J. Parasitol. Parasit. Wil. 2017;6:209–218. doi: 10.1016/j.ijppaw.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsubayashi K., Gotoh S., Kawamoto Y., Watanabe T., Nozawa K., Takasaka M., Narita T., Griffiths O., Stanley M.A. Clinical examinations on crab-eating macaques in Mauritius. Primates. 1992;33:281–288. doi: 10.1007/BF02382759. [DOI] [Google Scholar]
- 123.McLennan M.R., Hasegawa H., Bardi M., Huffman M.A. Gastrointestinal parasite infections and self-medication in wild chimpanzees surviving in degraded forest fragments within an agricultural landscape mosaic in Uganda. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0180431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muehlenbein M.P. Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale national park, Uganda. Am. J. Primatol. 2005;65:167–179. doi: 10.1002/ajp.20106. [DOI] [PubMed] [Google Scholar]
- 125.Munene E., Otsyula M., Mbaabu D.A.N., Mutahi W.T., Muriuki S.M.K., Muchemi G.M. Helminth and protozoan gastrointestinal tract parasites in captive and wild-trapped African non-human primates. Vet. Parasitol. 1998;78:195–201. doi: 10.1016/S0304-4017(98)00143-5. [DOI] [PubMed] [Google Scholar]
- 126.Muriuki S.M.K., Murugu R.K., Munene E., Karere G.M., Chai D.C. Some gastro-intestinal parasites of zoonotic (public health) importance commonly observed in old world non-human primates in Kenya. Acta Trop. 1998;71:73–82. doi: 10.1016/S0001-706X(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 127.Parr N.A., Fedigan L.M., Kutz S.J. A coprological survey of parasites in white-faced Capuchins (Cebus capucinus) from sector Santa Rosa, acg, Costa Rica. Folia Primatol. 2013;84:102–114. doi: 10.1159/000348287. [DOI] [PubMed] [Google Scholar]
- 128.Petrasova J., Modry D., Huffman M.A., Mapua M.I., Bobakova L., Mazoch V., Singh J., Kaur T., Petrzelkova K.J. Gastrointestinal parasites of Indigenous and introduced primate species of Rubondo island national park, Tanzania. Int. J. Primatol. 2010;31:920–936. doi: 10.1007/s10764-010-9439-x. [DOI] [Google Scholar]
- 129.Petrzelkova K.J., Hasegawa H., Appleton C.C., Huffman M.A., Archer C.E., Moscovice L.R., Mapua M.I., Singh J., Kaur T. Gastrointestinal parasites of the chimpanzee population introduced onto Rubondo island national park, Tanzania. Am. J. Primatol. 2010;72:307–316. doi: 10.1002/ajp.20783. [DOI] [PubMed] [Google Scholar]
- 130.Phillips K.A., Haas M.E., Grafton B.W., Yrivarren M. Survey of the gastrointestinal parasites of the primate community at Tambopata national reserve, Peru. J. Zool. 2004;264:149–151. doi: 10.1017/S0952836904005680. [DOI] [Google Scholar]
- 131.Pouillevet H., Dibakou S.E., Ngoubangoye B., Poirotte C., Charpentier M.J.E. A comparative study of four methods for the detection of nematode eggs and large protozoan cysts in mandrill faecal material. Folia Primatol. 2017;88:344–357. doi: 10.1159/000480233. [DOI] [PubMed] [Google Scholar]
- 132.Pourrut X., Diffo J.L.D., Somo R.M., Bilong C.F.B., Delaportee E., LeBreton M., Gonzalez J.P. Prevalence of gastrointestinal parasites in primate bushmeat and pets in Cameroon. Vet. Parasitol. 2011;175:187–191. doi: 10.1016/j.vetpar.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 133.Ryan S.J., Brashares J.S., Walsh C., Milbers K., Kilroy C., Chapman C.A. A survey of gastrointestinal parasites of olive baboons (Papio anubis) in human settlement areas of Mole national park, Ghana. J. Parasitol. 2012;98:885–888. doi: 10.1645/GE-2976.1. [DOI] [PubMed] [Google Scholar]
- 134.Solorzano-Garcia B., de Leon G.P.P. Helminth parasites of howler and spider monkeys in Mexico: Insights into molecular diagnostic methods and their importance for zoonotic diseases and host conservation. Int. J. Parasitol. Parasit. Wil. 2017;6:76–84. doi: 10.1016/j.ijppaw.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thanchomnang T., Intapan P.M., Sanpool O., Rodpai R., Sadaow L., Phosuk I., Somboonpatarakun C., Laymanivong S., Tourtip S., Maleewong W. First molecular identification of Strongyloides fuelleborni in long-tailed macaques in Thailand and Lao People’s Democratic Republic reveals considerable genetic diversity. J. Helminthol. 2018:1–8. doi: 10.1017/S0022149X18000512. [DOI] [PubMed] [Google Scholar]
- 136.Tiwari S., Reddy D.M., Pradheeps M., Sreenivasamurthy G.S., Umapathy G. Prevalence and co-occurrence of gastrointestinal parasites in Nilgiri Langur (Trachypithecus johnii) of fragmented landscape in Anamalai hills, Western Ghats, India. Curr. Sci. 2017;113:2194–2200. doi: 10.18520/cs/v113/i11/2194-2200. [DOI] [Google Scholar]
- 137.Wenz A., Heymann E.W., Petney T.N., Taraschewski H.F. The influence of human settlements on the parasite community in two species of Peruvian Tamarin. Parasitology. 2010;137:675–684. doi: 10.1017/S0031182009991570. [DOI] [PubMed] [Google Scholar]
- 138.Wren B.T., Gillespie T.R., Camp J.W., Remis M.J. Helminths of vervet monkeys, Chlorocebus aethiops, from Loskop Dam nature reserve, South Africa. Comp. Parasitol. 2015;82:101–108. doi: 10.1654/4712RR.1. [DOI] [Google Scholar]
- 139.Wren B.T., Remis M.J., Camp J.W., Gillespie T.R. Number of grooming partners is associated with hookworm infection in wild vervet monkeys (Chlorocebus aethiops) Folia Primatol. 2016;87:168–179. doi: 10.1159/000448709. [DOI] [PubMed] [Google Scholar]
- 140.Frias L., Stark D.J., Lynn M.S., Nathan S.K., Goossens B., Okamoto M., MacIntosh A.J.J. Lurking in the dark: Cryptic strongyloides in a Bornean slow loris. Int. J. Parasitol. Parasites Wil. 2018;7:141–146. doi: 10.1016/j.ijppaw.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koinari M., Karl S., Ryan U., Lymbery A.J. Infection levels of gastrointestinal parasites in sheep and goats in Papua New Guinea. J. Helminthol. 2013;87:409–415. doi: 10.1017/S0022149X12000594. [DOI] [PubMed] [Google Scholar]
- 142.MacGlaflin C.E., Zajac A.M., Rego K.A., Petersson K.H. Effect of vitamin E supplementation on naturally acquired parasitic infection in lambs. Vet. Parasitol. 2011;175:300–305. doi: 10.1016/j.vetpar.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 143.McManus C., Louvandini H., Paiva S.R., de Oliveira A.A., Azevedo H.C., de Melo C.B. Genetic factors of sheep affecting gastrointestinal parasite infections in the Distrito Federal, Brazil. Vet. Parasitol. 2009;166:308–313. doi: 10.1016/j.vetpar.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 144.Andreassen P.N.S., Schmidt N.M., Kapel C.M.O., Christensen M.U., Sittler B., Gilg O., Enemark H.L., Al-Sabi M.N.S. Gastrointestinal parasites of two populations of arctic foxes (Vulpes lagopus) from North-East Greenland. Polar Res. 2017;36 doi: 10.1080/17518369.2017.1308667. [DOI] [Google Scholar]
- 145.Dalimi A., Sattari A., Motamedi G. A study on intestinal helminthes of dogs, foxes and jackals in the western part of Iran. Vet. Parasitol. 2006;142:129–133. doi: 10.1016/j.vetpar.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 146.Hernandez-Camacho N., Pineda-Lopez R., Lopez-Gonzalez C.A., Jones R.W. Nematodes parasites of the gray fox (Urocyon cinereoargenteus schreber, 1775) in the seasonally dry tropical highlands of central Mexico. Parasitol. Res. 2011;108:1425–1429. doi: 10.1007/s00436-010-2191-5. [DOI] [PubMed] [Google Scholar]
- 147.Miterpakova M., Hurnikova Z., Antolova D., Dubinsky P. Endoparasites of red fox (Vulpes vulpes) in the Slovak Republic with the emphasis on zoonotic species Echinococcus multilocularis and Trichinella spp. Helminthologia. 2009;46:73–79. doi: 10.2478/s11687-009-0015-x. [DOI] [Google Scholar]
- 148.Da Silva S.R.M., Maldonade I.R., Ginani V.C., Lima S.A., Mendes V.S., Azevedo M.L.X., Gurgel-Gonçalves R., Machado E.R. Detection of intestinal parasites on field-grown strawberries in the federal district of Brazil. Rev. Soc. Bras. Med. Trop. 2014;47:801–805. doi: 10.1590/0037-8682-0044-2014. [DOI] [PubMed] [Google Scholar]
- 149.Aghaindum A.G., Landry F.K.A. Dissemination of the resistant forms of intestinal worms in the marshy areas of the city of Yaounde (Cameroon): Importance of some abiotic factors of the medium. Appl. Water Sci. 2019;9 doi: 10.1007/s13201-019-0895-y. [DOI] [Google Scholar]
- 150.Hilles A.H., Al Hindi A.I., Abu Safieh Y.A. Assessment of parasitic pollution in the coastal seawater of Gaza city. J. Environ. Health Sci. Eng. 2014;12 doi: 10.1186/2052-336X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bakir B., Tanyuksel M., Saylam F., Tanriverdi S., Araz R.E., Hacim A.K., Hasde M. Investigation of waterborne parasites in drinking water sources of Ankara, Turkey. J. Microbiol. 2003;41:148–151. [Google Scholar]
- 152.El-Badry A.A., Hamdy D.A., El Wahab W.M.A. Strongyloides stercoralis larvae found for the first time in tap water using a novel culture method. J. Parasitol. Res. 2018;117:3775–3780. doi: 10.1007/s00436-018-6078-1. [DOI] [PubMed] [Google Scholar]
- 153.Freitas D.A., Cabral J.J.S.P., Rocha F.J.S., Paiva A.L.R., Sens M.L., Veras T.B. Cryptosporidium spp. and Giardia spp. removal by bank filtration at Beberibe River, Brazil. J. River Res. App. 2017;33:1079–1087. doi: 10.1002/rra.3151. [DOI] [Google Scholar]
- 154.Klotz S.A., Normand R.E., Kalinsky R.G. “Through a drinking glass and what was found there”: Pseudocontamination of a hospital's drinking water. J. Infect. Control Hosp. Epidemiol. 1992;8:477–481. doi: 10.1086/646576. [DOI] [PubMed] [Google Scholar]
- 155.Zeehaida M., Zairi N.Z., Rahmah N., Maimunah A., Madihah B. Strongyloides stercoralis in common vegetables and herbs in Kota Bharu, Keletan, Malaysia. J. Trop. Biomed. 2011;28:188–193. [PubMed] [Google Scholar]
- 156.Luz J.G.G., Barbosa M.V., de Carvalho A.G., Resende S.D., Dias J.V.L., Martins H.R. Contamination by intestinal parasites in vegetables marketed in an area of Jequitinhonha Valley, Minas Gerais, Brazil. Rev. Nutricao-Braz. J. Nutr. 2017;30:127–136. doi: 10.1590/1678-98652017000100012. [DOI] [Google Scholar]
- 157.Maikai B.V., Elisha I.A., Baba-Onoja E.B.T. Contamination of vegetables sold in markets with helminth eggs in Zaria metropolis, Kaduna State, Nigeria. Food Control. 2012;28:345–348. doi: 10.1016/j.foodcont.2012.05.035. [DOI] [Google Scholar]
- 158.Madadi M. Parasitic contamination in the table vegetables planted in Shiraz plain, Iran. Pak. J. Sci. Ind. Res. 2010;53:42–45. [Google Scholar]
- 159.Berentsen A.R., Becker M.S., Stockdale-Walden H., Matandiko W., McRobb R., Dunbar M.R. Survey of gastrointestinal parasite infection in African lion (Panthera leo), African wild dog (Lycaon pictus) and spotted hyaena (Crocuta crocuta) in the Luangwa valley, Zambia. Afr. Zool. 2012;47:363–368. doi: 10.1080/15627020.2012.11407561. [DOI] [Google Scholar]
- 160.Bista D., Shrestha S., Kunwar A.J., Acharya S., Jnawali S.R., Acharya K.P. Status of gastrointestinal parasites in red panda of Nepal. PeerJ. 2017;2017 doi: 10.7717/peerj.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cardia D.F.F., Camossi L.G., Fornazari F., Babboni S.D., Teixeira C.R., Bresciani K.D.S. First report of Strongyloides sp. (nematoda, Strongyloididae) in Lutreolina crassicaudata (didelphimorphia: Didelphidae) Braz. J. Biol. 2016;76:884–887. doi: 10.1590/1519-6984.03315. [DOI] [PubMed] [Google Scholar]
- 162.Cordon G.P., Prados A.H., Romero D., Moreno M.S., Pontes A., Osuna A., Rosales M.J. Intestinal parasitism in the animals of the zoological garden “Pena Escrita” (Almunecar, Spain) Vet. Parasitol. 2008;156:302–309. doi: 10.1016/j.vetpar.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 163.González P., Carbonell E., Urios V., Rozhnov V.V. Coprology of Panthera tigris altaica and Felis bengalensis euptilurus from the Russian far East. J. Parasitol. 2007;93:948–950. doi: 10.1645/GE-3519RN.1. [DOI] [PubMed] [Google Scholar]
- 164.Gupta A., Singh N.K., Singh H., Rath S.S. Assessment of risk factors associated with prevalence of gastrointestinal helminths in buffaloes from Punjab State, India. Buffalo Bull. 2018;37:279–290. [Google Scholar]
- 165.Hallinger M.J., Taubert A., Hermosilla C., Mutschmann F. Occurrence of health-compromising protozoan and helminth infections in tortoises kept as pet animals in Germany. Parasites Vectors. 2018;11 doi: 10.1186/s13071-018-2936-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hasegawa H., Ota H. Parasitic Helminths Found from Polypedates leucomystax (Amphibia: Rhacophoridae) on Miyakojima Island, Ryukyu Archipelago, Japan. Curr. Herpetol. 2017;36:1–10. doi: 10.5358/hsj.36.1. [DOI] [Google Scholar]
- 167.Hermosilla C., Kleinertz S., Silva L.M.R., Hirzmann J., Huber D., Kusak J., Taubert A. Protozoan and helminth parasite fauna of free-living Croatian wild wolves (Canis lupus) analyzed by scat collection. Vet. Parasitol. 2017;233:14–19. doi: 10.1016/j.vetpar.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 168.Hu X.L., Liu G., Wei Y.T., Wang Y.H., Zhang T.X., Yang S., Hu D.F., Liu S.Q. Regional and seasonal effects on the gastrointestinal parasitism of captive forest musk deer. Acta Tropica. 2018;177:1–8. doi: 10.1016/j.actatropica.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 169.Huang W., Zhou L.Z., Zhao N.N. Temporal-spatial patterns of intestinal parasites of the Hooded Crane (Grus monacha) wintering in lakes of the middle and lower Yangtze River floodplain. Avian Res. 2014;5 doi: 10.1186/s40657-014-0006-6. [DOI] [Google Scholar]
- 170.Kumba F.F., Katjivena H., Kauta G., Lutaaya E. Seasonal evolution of faecal egg output by gastrointestinal worms in goats on communal farms in eastern Namibia. Onderstepoort J. Vet. Res. 2003;70:265–271. doi: 10.4102/ojvr.v70i4.291. [DOI] [PubMed] [Google Scholar]
- 171.Mizgajska-Wiktor H., Jarosz W. Potential risk of zoonotic infections in recreational areas visited by Sus scrofa and Vulpes vulpes. Case study--Wolin Island, Poland. Wiad. Parazytol. 2010;56:243–251. [PubMed] [Google Scholar]
- 172.Mukul-Yerves J.M., Zapata-Escobedo M.D., Montes-Perez R.C., Rodriguez-Vivas R.I., Torres-Acosta J.F. Gastrointestinal and ectoparasites in wildlife-ungulates under captive and free-living conditions in the Mexican tropic. Rev. Mex. Cienc. Pecu. 2014;5:459–469. [Google Scholar]
- 173.Oja R., Velstrom K., Moks E., Jokelainen P., Lassen B. How does supplementary feeding affect endoparasite infection in wild boar? Parasitol. Res. 2017;116:2131–2137. doi: 10.1007/s00436-017-5512-0. [DOI] [PubMed] [Google Scholar]
- 174.Ojeda-Robertos N., Torres-Chable O.M., Peralta-Torres J., Luna-Palomera C., Aguilar-Cabrales A., Chay-Canul A., Gonzalez-Garduno R., Machain-Williams C., Camara-Sarmiento R. Study of gastrointestinal parasites in water buffalo (Bubalus bubalis) reared under Mexican humid tropical conditions. Trop. Anim. Health Prod. 2017;49:613–618. doi: 10.1007/s11250-017-1237-4. [DOI] [PubMed] [Google Scholar]
- 175.Perera A., Maia J., Jorge F., Harris D.J. Molecular screening of nematodes in lacertid lizards from the Iberian Peninsula and Balearic Islands using 18s rRNA sequences. J. Helminthol. 2013;87:189–194. doi: 10.1017/S0022149X12000181. [DOI] [PubMed] [Google Scholar]
- 176.Pilarczyk B., Tomza-Marciniak A., Udala J., Kuba J. The prevalence and control of gastrointestinal nematodes in farmed fallow deer (Dama dama l.) Vet. Arh. 2015;85:415–423. [Google Scholar]
- 177.Rahman M., Islam S., Masuduzzaman M., Alam M., Chawdhury M.N.U., Ferdous J., Islam M.N., Hassan M.M., Hossain M.A., Islam A. Prevalence and diversity of gastrointestinal helminths in free-ranging Asian house shrew (Suncus murinus) in Bangladesh. Vet. World. 2018;11:549–556. doi: 10.14202/vetworld.2018.549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rinaldi L., Musella V., Veneziano V., Condoleo R.U., Cringoli G. Helmintic infections in water buffaloes on Italian farms: A spatial analysis. Geospat. Health. 2009;3:233–239. doi: 10.4081/gh.2009.223. [DOI] [PubMed] [Google Scholar]
- 179.Rosalino L.M., Torres J., Santos-Reis M. A survey of helminth infection in Eurasian badgers (Meles meles) in relation to their foraging behaviour in a mediterranean environment in Southwest Portugal. Eur. J. Wildl. Res. 2006;52:202–206. doi: 10.1007/s10344-006-0033-7. [DOI] [Google Scholar]
- 180.Turni C., Smales L.R. Parasites of the bridled nailtail wallaby (Onychogalea fraenata) (marsupialia : Macropodidae) Wildl. Res. 2001;28:403–411. doi: 10.1071/WR99108. [DOI] [Google Scholar]
- 181.Turner W.C., Getz W.M. Seasonal and demographic factors influencing gastrointestinal parasitism in ungulates of Etosha national park. J. Wildl. Dis. 2010;46:1108–1119. doi: 10.7589/0090-3558-46.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Turner W.C., Cizauskas C.A., Getz W.M. Variation in faecal water content may confound estimates of gastro-intestinal parasite intensity in wild African herbivores. J. Helminthol. 2010;84:99–105. doi: 10.1017/S0022149X09990320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.VanderWaal K., Omondi G.P., Obanda V. Mixed-host aggregations and helminth parasite sharing in an East African wildlife-livestock system. Vet. Parasitol. 2014;205:224–232. doi: 10.1016/j.vetpar.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 184.Ybañez R.H.D., Resuelo K.J.G., Kintanar A.P.M., Ybañez A.P. Detection of gastrointestinal parasites in small-scale poultry layer farms in Leyte, Philippines. Vet. World. 2018;11:1587–1591. doi: 10.14202/vetworld.2018.1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]