Significance
Caspase is the enzyme involved in cell death, and its activation via the apoptosome is thought to represent irreversible cellular destruction. Furthermore, accumulating evidence suggests increasingly diverse functions of caspase beyond apoptosis. Here, using Drosophila wing as a model, we reveal that the specific executioner caspases, Dcp-1 and Decay, promote, rather than suppress by inducing apoptosis, tissue growth. These executioner caspases act independently of initiator caspase Dronc and apoptosis. We further show that the caspase-mediated cleavage of Acinus is important for sustaining tissue growth. Our research highlights the importance of executioner caspase-mediated basal proteolytic cleavage of substrates during tissue growth, and the findings hint at the original function of caspase—not apoptosis, but basal proteolytic cleavages for cell vigor.
Keywords: caspase-dependent nonlethal cellular processes, executioner caspase, tissue-size regulation, fluctuating asymmetry, TurboID
Abstract
Caspase is best known as an enzyme involved in programmed cell death, which is conserved among multicellular organisms. In addition to its role in cell death, caspase is emerging as an indispensable enzyme in a wide range of cellular functions, which have recently been termed caspase-dependent nonlethal cellular processes (CDPs). In this study, we examined the involvement of cell death signaling in tissue-size determination using Drosophila wing as a model. We found that the Drosophila executioner caspases Dcp-1 and Decay, but not Drice, promoted wing growth independently of apoptosis. Most of the reports on CDPs argue the importance of the spatiotemporal regulation of the initiator caspase, Dronc; however, this sublethal caspase function was independent of Dronc, suggesting a more diverse array of CDP regulatory mechanisms. Tagging of TurboID, an improved promiscuous biotin ligase that biotinylates neighboring proteins, to the C terminus of caspases revealed the differences among the neighbors of executioner caspases. Furthermore, we found that the cleavage of Acinus, a substrate of the executioner caspase, was important in promoting wing growth. These results demonstrate the importance of executioner caspase-mediated basal proteolytic cleavage of substrates in sustaining tissue growth. Given the existence of caspase-like DEVDase activity in a unicellular alga, our results likely highlight the original function of caspase—not cell death, but basal proteolytic cleavages for cell vigor.
Caspase is best known as an enzyme involved in programmed cell death, which is conserved among multicellular organisms (1). The conserved cell death platform in multicellular organisms is an Apaf-1–Caspase-9 complex called apoptosome. A rise in the local concentration of procaspase-9 leads to its autoactivation and subsequent activation of downstream executioner caspases (2). In Drosophila melanogaster, the genetic architecture of apoptosis and its regulatory mechanisms are well characterized. Various apoptotic stimuli are transduced for the transcriptional up-regulation of proapoptotic genes rpr, hid, and grim (RHG), which antagonize DIAP-1 (mammalian inhibitors of apoptosis protein). This leads to formation of the apoptosome by complexation of Dronc (mammalian Caspase-9) with Dark (mammalian Apaf-1), thereby initiating a proteolytic cascade for activation of executioner caspases, Drice and Dcp-1 (mammalian Caspase-3/6/7), and finally leading to apoptosis (3) (Fig. 1A). Although both Drice and Dcp-1 preferentially target primary amino acid sequences of DEVD, Drice is more effective in inducing apoptosis (4). In addition to its role in cell death, caspase is emerging as an indispensable enzyme for a wide range of cellular functions, including partial cell destruction, cell fate determination, and cell migration (3, 5), which have recently been termed caspase-dependent nonlethal cellular processes (CDPs) (6). To date, studies in Drosophila caspase have demonstrated the importance of the spatiotemporal regulation of Dronc in various processes, including dendrite pruning (7), cell size expansion (8), apoptosis-induced proliferation (9), and sperm individualization (10, 11). However, events downstream of caspase activation and the mechanisms through which cells avoid death remain largely unexplored.
Fig. 1.
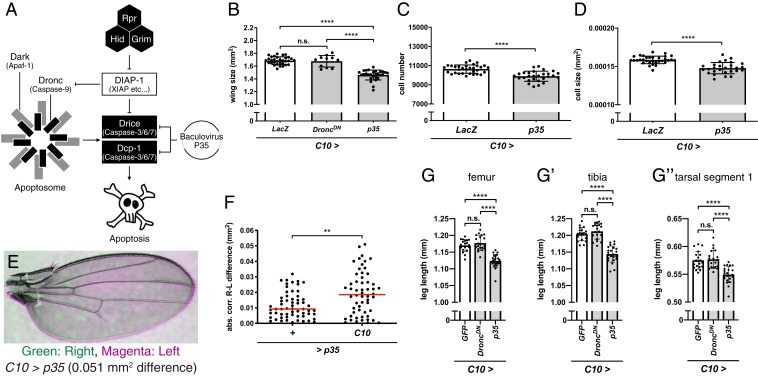
Cell death signaling inhibition reduces Drosophila imaginal tissue size. (A) Schematic diagram of Drosophila apoptosis signaling. (B) Wing sizes under control (C10 > LacZ; n = 32) and caspase-inhibited conditions (C10 > DroncDN, n = 11; C10 > p35, n = 28). (C and D) Cell sizes (C) and cell numbers (D) of B. (E) Representative image of a bilaterally asymmetric Drosophila wing pair of C10 > p35. Green, right wing; magenta, left wing. (F) Wing size differences between right and left wings within the same flies in control (+ > p35, n = 60) and caspase-inhibited conditions (C10 > p35, n = 56). Red bars indicate the median of each group. (G) Femur, (G′) tibia, and (G″) tarsal segment 1 lengths in the control (C10 > GFP, n = 19) and caspase-inhibited conditions (C10 > DroncDN, n = 20; C10 > p35, n = 23). For all graphs except F, data are mean ± SD. Statistical analyses were performed with Tukey’s multiple comparison test after 1-way ANOVA (B, G, G′, and G″), unpaired Student’s t test (C and D), or the Mann–Whitney U test (F). n.s., P > 0.05; **P < 0.01; ****P < 0.0001. n.s., not significant.
Tissue-size regulation is one of the most fundamental questions in the field of developmental biology. The size of a tissue is the integrated outcome of cell growth, division, and death. Drosophila wing imaginal disc (WD) is a well-established model system for studying tissue-size regulation (12). The WD emerges as a sac of 50 epithelial cells and grows to approximately 50,000 cells by the end of the third instar larval stage, accompanying cell death (13). Mutant flies lacking the irradiation-responsive enhancer region (IRER) of proapoptotic genes show increased wing size. Thus, developmental cell death, or at least myc overexpression-induced apoptosis, most likely negatively regulates tissue-size determination (14). However, there is an opposing observation that inhibition of caspase activity by overexpressing p35 results in wing size reduction (15). Perturbation of cell death machinery has even led to a disturbance of the bilateral symmetry of wing blade sizes (16). Overall, it is likely that the cell death machinery plays a major role in tissue-size tuning and homeostasis; however, the underlying mechanisms remain unknown.
In this study, we examined the involvement of various caspases in wing size determination. We found that the relatively minor Drosophila executioner caspases Dcp-1 and Decay, but not Drice, promoted wing growth independently of apoptosis. In addition, we found that the basal cleavage of one of the executioner caspase substrates, Acinus (Acn), was important in promoting wing growth. Collectively, our findings highlight the importance of executioner caspase-mediated basal proteolytic cleavage of substrates in sustaining tissue growth.
Results
Executioner Caspase Activity Inhibition Leads to Reduction in Wing and Leg Size.
The WD experiences caspase activation, which induces both apoptosis and CDPs, during development (13, 17). To examine the contribution of caspase activation to wing growth, we first inhibited caspase activity by overexpressing either p35, a baculovirus-derived caspase inhibitor, or Dronc Dominant Negative form (DroncDN) (Drosophila apoptosis signaling in Fig. 1A) in the entire WD using a C10-Gal4 driver (SI Appendix, Fig. S1) and examined their effects on wing size (SI Appendix, Fig. S2A). We found that wing size decreased significantly under p35 overexpression but did not change under DroncDN overexpression (Fig. 1B). We also checked the cell number and cell size (SI Appendix, Fig. S2B) and found that both the cell size and number decreased under p35 overexpression (Fig. 1 C and D). Interestingly, comparison of the bilateral symmetry of wing size in C10 > p35 caspase-inhibited flies revealed increased bilateral asymmetry (Fig. 1 E and F). On the other hand, we found no reduction in overall body size under p35 overexpression (SI Appendix, Fig. S3 A and B). In addition, there was no difference in the developmental duration of larval stage in p35-overexpressing flies (SI Appendix, Fig. S3C). Taken together, these results suggest that caspase mainly regulates the growth rate of the WD in a tissue-autonomous manner, without affecting the duration of larval development or entire body size.
We also inhibited caspase activity by knocking down the RHG genes (Fig. 1A), using UAS-RHG microRNA (UAS-miRHG). Wing size decreased on knockdown of RHG genes (SI Appendix, Fig. S4A). In this condition, cell numbers decreased, whereas cell size increased slightly (SI Appendix, Fig. S4 B and C). Thus, these results, together with those of p35 overexpression, suggest that caspase promotes wing growth mainly by regulating cell number. We further tested the effect of caspase inhibition on the length of hind legs (SI Appendix, Fig. S2C). We found that leg length decreased under p35 overexpression but not under DroncDN overexpression (Fig. 1 G–G″). We also found that leg length decreased on knockdown of RHG genes (SI Appendix, Fig. S4 D–D″). These results support the notion that the growth-promoting effect of caspase is rather general, at least in imaginal tissues. Furthermore, we examined the effect of caspase inhibition on WD size. Using apterous-Gal4 (ap-Gal4), which is expressed in the dorsal part of wing pouch, we found that p35 overexpression significantly reduced the volume of the dorsal part of the WD (SI Appendix, Fig. S5 A–C). Thus, we concluded that caspase activity promotes WD growth.
Increased Caspase Activity in diap1 Heterozygous Mutant Results in Increased Wing Size.
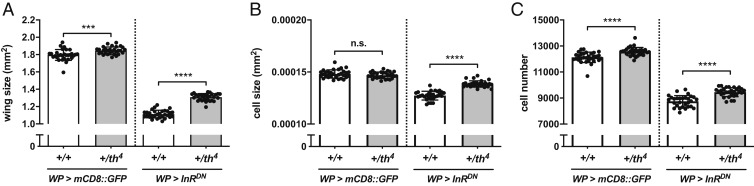
To further validate that caspase activity promotes wing growth, we attempted to increase caspase activity without inducing massive apoptosis. We used a diap1 heterozygous mutant of the th4 allele, which is known to exhibit increased caspase activity for both apoptosis and CDPs in WD (18). As we expected, the diap1 mutant showed increased wing size (Fig. 2A). Using WP-Gal4, which is expressed in the wing pouch of the WD (19), we overexpressed Insulin Receptor Dominant Negative form (InRDN), which leads to a significant reduction in wing size, to sensitize the effect of caspase on tissue growth (Fig. 2A). The growth-promoting effect of caspase was more evident in the sensitized condition (Fig. 2A). Cell size increased only in the sensitized condition (Fig. 2B), while cell number increased in the diap1 heterozygous mutant in both normal and sensitized conditions (Fig. 2C). This result further supports the idea that caspase activity promotes the growth of imaginal tissue by increasing cell number and, in part, cell size.
Fig. 2.
Enhanced cell death signaling increases Drosophila wing size. (A) Wing size in control (WP > mCD8::GFP, n = 30; th4, WP > mCD8::GFP, n = 30) and wing size-reduced conditions (WP > InRDN, n = 30; th4, WP > InRDN, n = 29). (B and C) Cell sizes (B) and cell numbers (C) of A. For all graphs, data are mean ± SD. Statistical analyses were performed with unpaired Student’s t test. n.s., P > 0.05; ***P < 0.001; ****P < 0.0001. n.s., not significant.
Executioner Caspases Dcp-1 and Decay Are Responsible for Wing Growth.
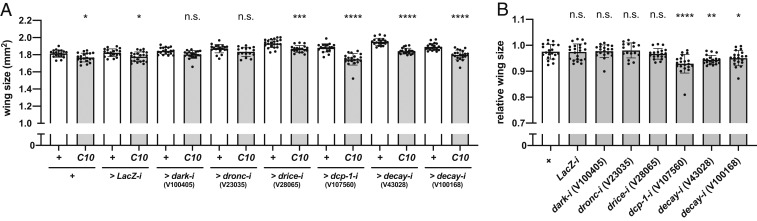
To gain mechanical insights into the observed phenomena, we screened the responsible caspase(s), as p35 overexpression and RHG knockdown simultaneously inhibit the activation of multiple caspases (20) (Fig. 1A). D. melanogaster is known to have 7 caspases (1). We used the curly-up wing phenotype and the opaque wing phenotype as indicators of growth inhibition and apoptosis inhibition, respectively (more details in SI Appendix, Fig. S6 A–F). From the RNA interference (RNAi) screening, we identified Dcp-1 and Decay, but not Dronc or Drice, as the most prominent candidates of growth-promoting caspases (more details in SI Appendix, Figs. S6 G–L and S7). Of note, while dark, dronc, and drice RNAi showed an opaque wing phenotype without the curly wing phenotype, dcp-1 and decay RNAi showed the curly wing phenotype without the opaque wing phenotype, suggesting the Dronc- and apoptosis-independent nature of the wing growth-promoting effect. To evaluate the screening results, we directly examined the growth-promoting effects of Dark, Dronc, Drice, Dcp-1, and Decay on wing sizes using C10-Gal4. Introduction of C10-Gal4 resulted in 2.48% (against w1118) and 2.52% (against LacZ RNAi) reductions in wing size compared with No-Gal4 control (Fig. 3 A and B). Similar extents of reduction were observed with dark, dronc, and drice RNAi (2.28%, 1.98%, and 3.31% respectively; Fig. 3 A and B). In contrast, dcp-1 and decay RNAi led to large reductions in wing size (7.07%, 5.64%, and 4.92%, respectively; Fig. 3 A and B). Importantly, Dcp-1 is relatively minor caspase for apoptosis compared with Drice (4). In addition, Decay is not required for apoptosis in WD (21). Overall, our screening results support the idea that the nonapoptotic caspase activity of Drosophila cell death signaling, especially Dcp-1 and Decay, but not Drice or Dronc, is required for promoting wing growth.
Fig. 3.
Dcp-1 and Decay, but not Dronc or Drice, are required for promoting wing growth. (A) Wing size in No-Gal4 controls (+ > +, n = 20; + > LacZ-i, n = 19; + > dark-iV100405, n = 19; + >dronc-iV23035, n = 20; + > drice-iV28065, n = 22; + > dcp-1-iV107560, n = 20; + > decay-iV43028, n = 20; + > decay-iV100168, n = 20) and RNAi groups (C10 > +, n = 20; C10 > LacZ-i, n = 22; C10 > dark-iV100405, n = 20; C10 >dronc-iV23035, n = 16; C10 > drice-iV28065, n = 19; C10 > dcp-1-iV107560, n = 20; C10 > decay-iV43028, n = 20; C10 > decay-iV100168, n = 20) for screening results validation. (B) Relative wing sizes of A normalized to the mean of corresponding No-Gal4 control. For graphs A and B, data are mean ± SD. Statistical analyses were performed with unpaired Student’s t test with Bonferroni correction for A and Dunnett’s multiple comparison test against control (C10 > +) after 1-way ANOVA for B. n.s., P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. n.s., not significant.
Growth-Promoting Effect of Executioner Caspase Activity Is Independent of Apoptosis.
Next, we performed terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay to examine the involvement of apoptosis for promoting wing growth. Compared with LacZ RNAi (SI Appendix, Fig. S8 A and G), p35 overexpression and RHG genes RNAi showed reduced TUNEL signals (SI Appendix, Fig. S8 B, C, and G), while dcp-1 RNAi and decay RNAi showed no significant reduction (SI Appendix, Fig. S8 D–G). We also used the genetically encoded mCD8::PARP::VENUS probe (7) to detect strong DEVDase activity in the WD (4, 18). Expression of the probe in the entire WD using C10-Gal4 yielded similar results to those of the TUNEL experiment; compared with LacZ RNAi (SI Appendix, Fig. S8 H and N), the signals were sharply reduced following p35 overexpression and RHG RNAi (SI Appendix, Fig. S8 I, J, and N) and showed no reduction with dcp-1 and decay RNAi (SI Appendix, Fig. S8 K–N). These results showed no correlation between the amount of apoptosis and wing size reduction, suggesting that the growth-promoting effect of executioner caspase activity is independent of its function in cell death.
Executioner Caspase Activity Exists Independently of Dronc during Larval Development.
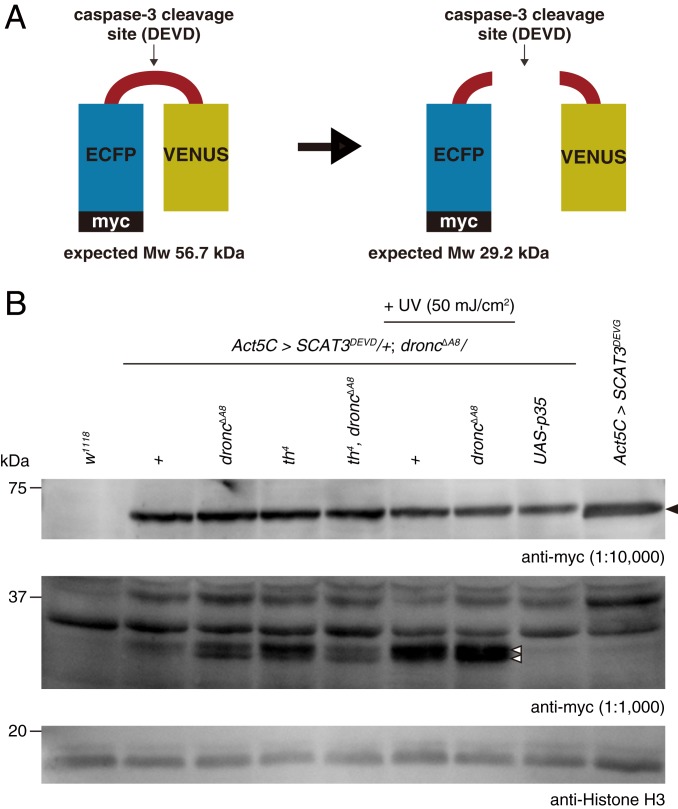
It is widely accepted that executioner caspase activation requires activation of the initiator caspase, Dronc. However, our results suggest that Dronc is not involved in the wing growth phenotype. This raises the possibility that the executioner caspases, Dcp-1 and Decay, might be activated independently of Dronc. To test this hypothesis, we took advantage of caspase activity probe SCAT3 (22) (Fig. 4A) and examined whether the cleaved SCAT3 bands were present in the droncΔA8 null mutant. We first confirmed that weak cleaved SCAT3 probe bands were detected on UV irradiation (SI Appendix, Fig. S9). Using a 10-fold higher concentration of the anti-myc antibody, we found faint cleaved SCAT3 bands even in the absence of Dronc (Fig. 4B). The detected bands got stronger on UV irradiation, were weaker (or even absent) by expressing p35, and were not detected in SCAT3DEVG-negative control probe, all supporting the idea that the detected bands were produced by the proteolytic cleavage by DEVDases (Fig. 4B). These results show that executioner caspase can be basally activated in the absence of Dronc.
Fig. 4.
Dronc-independent executioner caspase activity in Drosophila. (A) Schematic diagram of SCAT3 probe. (B) Western blotting against full-length SCAT3 probe (anti-myc antibody, dilution 1:10,000) and cleaved SCAT3 probe (anti-myc antibody, dilution 1:1,000) in the absence of Dronc. Genotypes are described in the figure. Black arrowhead indicates full-length SCAT3; white arrowheads, cleaved SCAT3.
Dcp-1, Drice, and Dronc Are Ubiquitously Expressed throughout the WD with Different Neighboring Proteins.
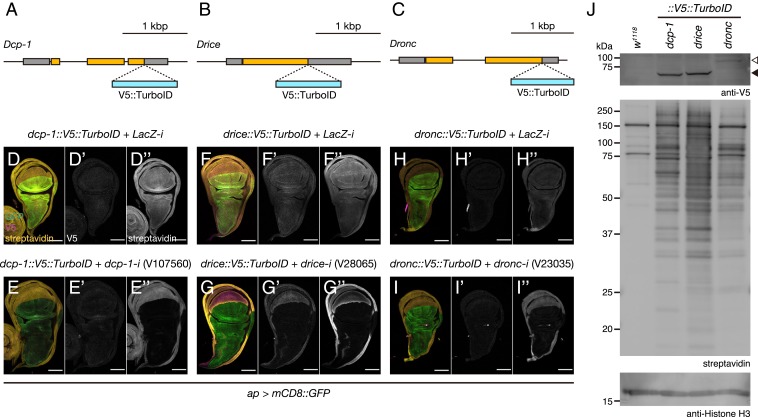
Our results so far showed the potential differential activity of caspases on cellular phenomena. While Dronc and Drice efficiently induce apoptosis, Dcp-1 and Decay promote wing growth. To elucidate the cause of functional differences among caspases, we established V5::TurboID (23) knockin fly lines against Dcp-1, Drice, and Dronc at their C termini (Fig. 5 A–C). TurboID is an improved promiscuous biotin ligase applicable to Drosophila that biotinylates the proximal proteins (23). We first examined the spatial expression patterns of the 3 caspases during wing development. Staining with an anti-V5 antibody weakly detected the expression of Drice, Dcp-1, and Dronc in the WDs and pupal wings (Fig. 5 D–I and SI Appendix, Fig. S10 A–H). Importantly, staining with streptavidin produced highly improved signals for all 3 caspases, which were depleted on RNAi (Fig. 5 D–I and SI Appendix, Fig. S10 A–H). Western blot analysis confirmed high Drice and Dcp-1 expression and lower Dronc expression in the WDs (Fig. 5J). Importantly, streptavidin blotting to visualize the biotinylated proteins revealed the nonidentical patterns among the caspases (Fig. 5J). Of note, some bands were detected only in Dcp-1 but not in Drice and vice versa (Fig. 5J). These results suggest that functional differences among executioner caspases might originate from the differences in their neighboring proteins.
Fig. 5.
The expression patterns of caspases during wing development. (A–C) Schematic diagrams of dcp-1 (A), drice (B), and dronc (C) gene loci. TurboID with V5-tag was knocked in right before the stop codon. (D–I) Expression patterns of Dcp-1 (D and E), Drice (F and G), and Dronc (H and I) in WDs. (Left) Merged image of GFP (green), V5 (magenta), and streptavidin (yellow) staining. (Middle) V5 (gray). (Right) Streptavidin (gray). (Scale bar: 100 µm.) dcp-1::V5::TurboID, ap > mCD8::GFP, LacZ-i (D); dcp-1::V5::TurboID, ap > mCD8::GFP, dcp-1-iV107560 (E); drice::V5::TurboID, ap > mCD8::GFP, LacZ-i (F), drice::V5::TurboID, ap > mCD8::GFP, drice-iV28065 (G); dronc::V5::TurboID, ap > mCD8::GFP, LacZ-i (H); dronc::V5::TurboID, ap > mCD8::GFP, dronc-iV23035 (I). (J) Western blotting against V5-tagged caspases and their potential neighboring (biotinylated) proteins. The white arrowhead indicates Dronc::V5::TurboID; black arrowhead, Dcp-1::V5::TurboID and Drice::V5::TurboID.
Caspase-Dependent Cleavage of Acn Promotes Wing Growth.
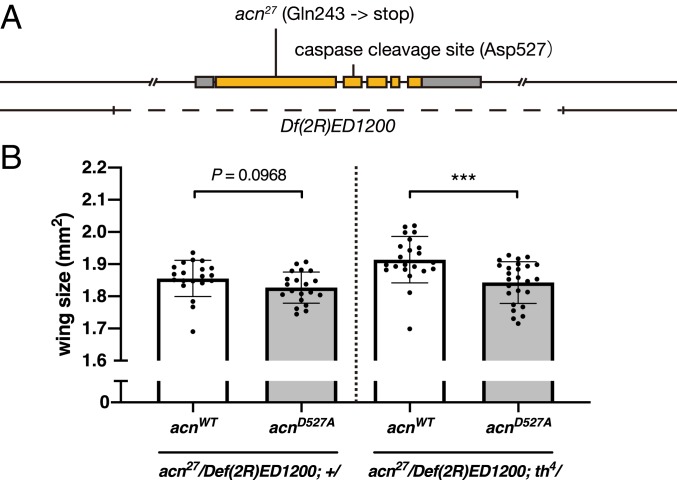
To further characterize the molecular mechanisms downstream of caspase activation, we first determined the caspase inhibition-related changes in the overall transcriptome. However, we could not identify the change in the major signaling pathway for promoting tissue growth (more details in SI Appendix, Fig. S11). Thus, we looked at the caspase substrates. Acn, a nuclear protein that regulates alternative splicing (24) and basal autophagy (25), is reported to be a substrate for executioner caspase in Drosophila (26). A previous genetic analysis suggested that Acn is regulated mainly by Dcp-1, but not by Drice and Dronc (26). We examined the effect of Acn cleavage on wing size (Fig. 6A). Wing size measurements revealed a slight reduction in wing size in the caspase-cleavage resistant acnD527A-carrying flies compared with control flies (Fig. 6B). We further tested the genetic interaction between acn and caspase activity. Introduction of diap-1 heterozygous mutation significantly increased the difference in wing size between in acnWT and acnD527A flies (Fig. 6B). These results support the idea that the basal caspase activity of Dcp-1, and possibly that of Decay, restricts the basal proteolytic cleavage of Acn to sustain basal tissue growth.
Fig. 6.
Caspase-mediated cleavage of Acn increases wing size. (A) Schematic diagram of the acn gene locus. (B) Wing size in acnWT (acn27/Df(2R)ED1200; acnWT/+, n = 20; acn27/Df(2R)ED1200; acnWT/th4, n = 22), and acnD527A (acn27/Df(2R)ED1200; acnD527A/+, n = 20; acn27/Df(2R)ED1200; acnD527A/th4, n = 25) flies. Data are mean ± SD. Statistical analyses were performed using an unpaired Student’s t test. ***P < 0.001.
As Acn is known to induce basal autophagy, we further tested whether the curly-up wing phenotype caused by dcp-1 and decay RNAi can be rescued by the simultaneous knockdown of genes involved in autophagy. We performed RNAi of several autophagy-related genes, including atg3, 7, 9, 13, and 18a. We observed a partial rescue of the dcp-1 and decay RNAi curly-up wing phenotype by some of the RNAi lines, including atg18a RNAi (SI Appendix, Fig. S12 A–E), suggesting the partial involvement of autophagy in Dcp-1- and Decay-mediated growth. Overall, our results suggest the importance of caspase-mediated basal proteolytic cleavage of their substrates, including Acn, to sustain basal tissue growth (SI Appendix, Fig. S13).
Discussion
The role of executioner caspases in promoting tissue growth is highly conserved among higher multicellular organisms. Mammalian studies showed that intrinsic cell death pathway-mediated caspase activation is essential for cardiomyocyte hypertrophy (27) and that the myocyte number is reduced in caspase-3 and caspase-7 double-knockout mice (28). Most recently, it has been shown in mouse sebocytes that caspase-3 mediates cell proliferation via the activation of YAP through the cleavage of α-catenin (29). Here we report an alternative mechanism in which the Dronc-independent basal caspase activity promotes tissue growth, partly via the cleavage of Acn. We also confirmed that cell death signaling is related to wing bilateral asymmetry. Although cell death is currently thought to be important in adjusting bilateral asymmetry (16), our findings suggest the possibility that the growth-promoting effect of caspase might be important for achieving robust wing size. Our inference is supported by a report that dysregulation of growth regulating signals also resulted in bilaterally asymmetric body appendages (30).
The observation that executioner caspase could exert nondestructive proteolytic activity in the absence of the apoptosome component Dronc is noteworthy, as most of the observed caspase activation in both apoptosis and CDPs in Drosophila is acquired via Dronc. Given that the Dronc-independent basal caspase activity was not high enough to induce apoptosis, we believe that this is an alternative mechanism for cells to escape from cell death. Identifying how basal executioner caspase activity is regulated, especially from the perspective of posttranslational modification, is important for future research (SI Appendix, Discussion).
In this paper, we report the physiological function of Dronc-independent basal caspase activity in vivo using one of the caspase substrates, Acn, as an example. The mechanism through which stabilized Acn reduces wing size could not be determined in this study (SI Appendix, Discussion). Our findings also highlight the importance of precise analysis of caspase substrates in vivo. While more than 400 caspase proteolytic targets have been identified by degradomes in mammals (31, 32), there could still be many uncharacterized substrates with nonapoptotic caspase functions. In addition, we showed that executioner caspases have functional specificity for CDPs, possibly because of the difference in substrate specificity. A previous study has also shown that human executioner caspases differ in their substrate specificity (33). Such substrate specificity seems to be partly acquired via caspases’ neighboring proteins; for example, CRINKLED contributes to the substrate specificity of Dronc (34). Furthermore, the neighboring proteins of caspases also regulate their localization; for example, Myo1D is needed for Dronc translocation to the plasma membrane (9). In this study, we generated TurboID knockin Drosophila lines for the major apoptotic caspases Dcp-1, Drice, and Dronc; the established fly lines facilitated examination of the molecular mechanisms of cell death and CDPs from the perspective of the innate neighboring proteins.
Our findings highlight some important aspects of caspase function from the standpoint of evolution and emergence of apoptosis. In metazoans, apoptosis requires a regulated, rapid, and strong activation of executioner caspase in the whole cytosolic fraction. In this case, the apoptosome—caspase activation and recruitment domain (CARD)-mediated Apaf-1/caspase-9 complex—plays a pivotal role in initiating the caspase-activating cascade. Nonmetazoans, including choanoflagellates, the closest living relatives of the metazoans, lack both the CARD domain and caspase (35); however, biochemical analysis shows the existence of caspase-like DEVDase activity in the unicellular alga Dunaliella viridis even in the absence of the CARD domain (36). These lines of evidence suggest that the original roles of caspase, or DEVDase, could be independent of the apoptosome and apoptosis. Thus, our present findings hint at the original function of executioner caspase in basal proteolytic cleavages for cell vigor, which in turn sheds light on the function of the apoptosome as an efficient cell death inducer acquired during the evolution of unicellular organisms into multicellular organisms.
In conclusion, we found that executioner caspase, originally identified as a cell death enzyme, promotes wing growth independently of cell death. Our data show that the basal caspase activity could be Dronc-independent; this result is in sharp contrast with the apoptotic function as well as with the nonapoptotic functions of caspase that have been revealed so far. Because the basal cleavage of Acn was in part responsible for promoting wing growth, our research highlights the importance of executioner caspase-mediated basal proteolytic cleavages of substrates in promoting tissue growth.
Materials and Methods
Detailed information on fly strains and rearing conditions (including detailed genotypes of the flies used in the figures); generation of caspase::V5::TurboID knockin alleles using CRISPR/Cas9 (including the sequence of pBac[3xP3-DsRed_polyA_Scarless_TK]); Gal4 expression check; wing size, cell size, cell number, and leg length measurements; pupal size measurement; fluctuating asymmetry measurement; pupation time measurement; curly up and opaque (cell remaining) fly wing scoring and screening; immunohistochemistry; quantification of wing imaginal disc volume; TUNEL assay; quantification of the TUNEL and cPARP ratio; Western blot analysis; microarray sample preparation and analysis; and statistical analysis are provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank E. Hafen, C. Goodman, H. Richardson, B. Hay, C.-H. Cheng, Y. Hiromi, H. Krämer, G. Juhasz, the Kyoto Stock Center, the Vienna Drosophila Resource Center, and the Bloomington Drosophila Stock Center for providing the fly strains; N. Perrimon for providing the plasmid; and S. Cohen for providing the antibody. We thank K. H. Takahashi for his technical advice and discussions on fluctuating asymmetry analysis. We thank members of the M.M. laboratory for their technical assistance and discussions; in particular, K. Takenaga for preparation of the fly food, R. Takamoto for support during the wing size measurement experiments, S. Haraguchi for the assistance with the microarray analysis, T. Katsuyama for the generation of pBac[3xP3-DsRed_polyA_Scarless_TK] plasmid and assistance with the generation of caspase::V5::TurboID knockin alleles by CRISPR/Cas9, and F. Obata for the support in the DNA staining of fly wings. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [KAKENHI Grants 19K16137 (to N.S.), 18H05369 (to T.C.), and 16H06385 (to M.M.)]. Support was also provided by the Frontier Development Program for Genome Editing, Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers, a grant from the Toray Science Foundation (to T.C.), and Japan Agency for Medical Research and Development Grants JP17gm0610004 and JP19gm5010001 (to M.M.). N.S. was a research fellow of the Japan Society for the Promotion of Science (KAKENHI Grant 17J10971).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data have been deposited in the NCBI Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE136170).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904647116/-/DCSupplemental.
References
- 1.Kumar S., Caspase function in programmed cell death. Cell Death Differ. 14, 32–43 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Yuan S., Akey C. W., Apoptosome structure, assembly, and procaspase activation. Structure 21, 501–515 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakajima Y. I., Kuranaga E., Caspase-dependent non-apoptotic processes in development. Cell Death Differ. 24, 1422–1430 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florentin A., Arama E., Caspase levels and execution efficiencies determine the apoptotic potential of the cell. J. Cell Biol. 196, 513–527 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell R. A. V., Megeney L. A., Evolution of caspase-mediated cell death and differentiation: Twins separated at birth. Cell Death Differ. 24, 1359–1368 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aram L., Yacobi-Sharon K., Arama E., CDPs: Caspase-dependent non-lethal cellular processes. Cell Death Differ. 24, 1307–1310 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams D. W., Kondo S., Krzyzanowska A., Hiromi Y., Truman J. W., Local caspase activity directs engulfment of dendrites during pruning. Nat. Neurosci. 9, 1234–1236 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Kang Y., Neuman S. D., Bashirullah A., Tango7 regulates cortical activity of caspases during reaper-triggered changes in tissue elasticity. Nat. Commun. 8, 603 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amcheslavsky A., et al. , Plasma membrane localization of apoptotic caspases for non-apoptotic functions. Dev. Cell 45, 450–464.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arama E., Agapite J., Steller H., Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev. Cell 4, 687–697 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Huh J. R., et al. , Multiple apoptotic caspase cascades are required in nonapoptotic roles for Drosophila spermatid individualization. PLoS Biol. 2, E15 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hariharan I. K., Organ size control: Lessons from Drosophila. Dev. Cell 34, 255–265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milán M., Campuzano S., García-Bellido A., Developmental parameters of cell death in the wing disc of Drosophila. Proc. Natl. Acad. Sci. U.S.A. 94, 5691–5696 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C., et al. , An intergenic regulatory region mediates Drosophila Myc-induced apoptosis and blocks tissue hyperplasia. Oncogene 34, 2385–2397 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding A. X., et al. , CasExpress reveals widespread and diverse patterns of cell survival of caspase-3 activation during development in vivo. eLife 5, e10936 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neto-Silva R. M., Wells B. S., Johnston L. A., Mechanisms of growth and homeostasis in the Drosophila wing. Annu. Rev. Cell Dev. Biol. 25, 197–220 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanuka H., et al. , Drosophila caspase transduces Shaggy/GSK-3β kinase activity in neural precursor development. EMBO J. 24, 3793–3806 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinoda N., Obata F., Zhang L., Miura M., Drosophila SETDB1 and caspase cooperatively fine-tune cell fate determination of sensory organ precursor. Genes Cells 21, 378–386 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Obata F., et al. , Necrosis-driven systemic immune response alters SAM metabolism through the FOXO-GNMT axis. Cell Rep. 7, 821–833 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Kim C. H., Paik D., Rus F., Silverman N., The caspase-8 homolog Dredd cleaves Imd and Relish but is not inhibited by p35. J. Biol. Chem. 289, 20092–20101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo S., Senoo-Matsuda N., Hiromi Y., Miura M., DRONC coordinates cell death and compensatory proliferation. Mol. Cell. Biol. 26, 7258–7268 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takemoto K., Nagai T., Miyawaki A., Miura M., Spatio-temporal activation of caspase revealed by indicator that is insensitive to environmental effects. J. Cell Biol. 160, 235–243 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branon T. C., et al. , Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi R., Handler D., Ish-Horowicz D., Brennecke J., The exon junction complex is required for definition and excision of neighboring introns in Drosophila. Genes Dev. 28, 1772–1785 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haberman A. S., Akbar M. A., Ray S., Krämer H., Drosophila acinus encodes a novel regulator of endocytic and autophagic trafficking. Development 137, 2157–2166 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nandi N., Tyra L. K., Stenesen D., Krämer H., Acinus integrates AKT1 and subapoptotic caspase activities to regulate basal autophagy. J. Cell Biol. 207, 253–268 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Putinski C., et al. , Intrinsic-mediated caspase activation is essential for cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 110, E4079–E4087 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardona M., et al. , Executioner caspase-3 and 7 deficiency reduces myocyte number in the developing mouse heart. PLoS One 10, e0131411 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yosefzon Y., et al. , Caspase-3 regulates YAP-dependent cell proliferation and organ size. Mol. Cell 70, 573–587.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Trotta V., et al. , Developmental instability of the Drosophila wing as an index of genomic perturbation and altered cell proliferation. Evol. Dev. 7, 234–243 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Lüthi A. U., Martin S. J., The CASBAH: A searchable database of caspase substrates. Cell Death Differ. 14, 641–650 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Julien O., Wells J. A., Caspases and their substrates. Cell Death Differ. 24, 1380–1389 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julien O., et al. , Quantitative MS-based enzymology of caspases reveals distinct protein substrate specificities, hierarchies, and cellular roles. Proc. Natl. Acad. Sci. U.S.A. 113, E2001–E2010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orme M. H., et al. , The unconventional myosin CRINKLED and its mammalian orthologue MYO7A regulate caspases in their signalling roles. Nat. Commun. 7, 10972 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richter D. J., Fozouni P., Eisen M. B., King N., Gene family innovation, conservation and loss on the animal stem lineage. eLife 7, e34226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiménez C., et al. , Different ways to die: Cell death modes of the unicellular chlorophyte Dunaliella viridis exposed to various environmental stresses are mediated by the caspase-like activity DEVDase. J. Exp. Bot. 60, 815–828 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.