Abstract
Lungless salamanders (Family Plethodontidae) form a highly speciose group that has undergone spectacular adaptive radiation to colonize a multitude of habitats. Substantial morphological variation in the otic region coupled with great ecological diversity within this clade make plethodontids an excellent model for exploring the ecomorphology of the amphibian ear. We examined the influence of habitat, development, and vision on inner ear morphology in 53 plethodontid species. We collected traditional and 3D geometric morphometric measurements to characterize variation in size and shape of the otic endocast and peripheral structures of the salamander ear. Phylogenetic comparative analyses demonstrate structural convergence in the inner ear across ecologically similar species. Species that dwell in spatially-complex microhabitats exhibit robust, highly curved semicircular canals suggesting enhanced vestibular sense, whereas species with reduced visual systems demonstrate reduced canal curvature indicative of relaxed selection on the vestibulo-ocular reflex. Cave specialists show parallel enlargement of auditory-associated structures. The morphological correlates of ecology among diverse species reveal underlying evidence of habitat specialization in the inner ear and suggest that there exists physiological variation in the function of the salamander ear even in the apparent absence of selective pressures on the auditory system to support acoustic behavior.
Keywords: amphibian, auditory, vestibular, geometric morphometrics, ecomorphology
Introduction
A primary goal of evolutionary biology is to identify the factors driving morphological diversification. One tool used to address this goal is ecomorphology, or the study of the relationship between the ecology of organisms and the morphology of phenotypic traits to infer potential adaptive qualities of structural differences (Wainwright and Reilly 1994). Variation in morphological traits can mediate shifts in behavior and life history that permit colonization of new ecological niches; conversely, biotic and abiotic conditions within habitats can shape adaptive modifications to functional morphology to support an organism’s survival within their niche (Wainwright and Reilly 1994; Schluter 2000; Losos 2011). These expectations predict that ecological niche-specific selective pressures drive phenotypic variation such that phylogenetically independent lineages within similar niches will share behavioral and morphological traits.
Salamanders of the family Plethodontidae present an excellent model system for investigating the role of ecology in the generation of morphological variation. Plethodontids are a speciose and widespread group of salamanders that have undergone remarkable adaptive radiation to a wide range of habitats from arboreal bromeliads in the tropics to perpetually dark caves in temperate regions. Although plethodontid salamanders demonstrate strong phylogenetic conservatism in body morphology across diverse microhabitats (Blankers et al. 2012), there are notable examples of parallel morphological evolution in the elongate, worm-like body forms of fossorial taxa (Wake 1966; Parra-Olea and Wake 2001), and in the extensive convergent modification to foot size and inter-digit webbing in climbing species (Adams and Nistri 2010). Adaptation to a multitude of habitat types has also resulted in a suite of life history strategies to support survival including modifications to the timing of developmental events (heterochrony) leading to permanently aquatic paedomorphic species, facultatively metamorphic species, as well as highly terrestrial species that bypass the aquatic larval stage via direct development within the egg (Wilbur and Collins 1973; Whiteman 1994; Wake and Hanken 1996; Chippindale et al. 2004; Denoël et al. 2005; Bonett et al. 2014). In addition to locomotor and developmental adaptations, variable challenges presented by diverse habitat types result in changes to sensory systems to cope with different levels of environmentally-imposed sensory constraint. These are especially apparent among cave-dwelling plethodontid species which display varying degrees of reduction to the visual system in low light environments. Morphological diversity is also apparent in an unexpected plethodontid structure: the inner ear. The salamander inner ear has been observed to present a surprising amount of structural diversity with inter-specific variation in the size and shape of the membranous labyrinth, the fluid-filled sac that contains the auditory and vestibular end organs of the ear (Lombard 1977). Few groups present such a unique complement of ecological, developmental, and sensory diversity as is observed in the family Plethodontidae. Here, we use plethodontid salamanders as a model system for a comparative study of the influence of habitat, development, and sensory ecology on the functional morphology of the inner ear.
The interplay of environmental heterogeneity and functional morphology can be particularly salient in sensory system evolution. Sensory traits integrate ontogeny (e.g., metamorphic changes to the amphibian visual (Hoskins 1990), mechanosensory lateral line (Fritzsch 1988; Roth et al. 1992), and olfactory (Thewissen et al. 2012) systems), physiological demands, and ecological pressures experienced by organisms; therefore variability of sensory structures among closely related species may reveal ecological specialization. Further, structures related to sensory perception of environmental stimuli demonstrate a strong form-function relationship and thus are useful for investigating ecomorphological variation (Bock and von Wahlert 1965). Although the inner ear is a functionally constrained structure (i.e., its features are phylogenetically conservative and gross morphological similarities exist at high organizational levels), structural variation of the components of the ear correlates with functional differences and sensory specializations among ecologically diverse species. For example, fossorial (burrowing) mammals (Burda et al. 1992; Mason 2004), lizards (Toerien 1963), snakes (Yi and Norell 2015), and amphibians (Maddin and Sherratt 2014) exhibit repeated, parallel evolution of hypertrophic auditory structures believed to contribute to enhanced seismic sensitivity to ground vibrations. Additionally, the size and shape of the semicircular canals are correlated with locomotor behavior in a broad range of vertebrate taxa that perform agile and/or spatially complex behaviors, including arboreal Anolis lizard ecomorphs (Dickson et al. 2017), fossorial caecilian amphibians (Maddin and Sherratt 2014), gliding snakes (Boistel et al. 2011), and fossorial, aquatic, and arboreal mammals (Lindenlaub et al. 1995; Spoor et al. 2002; Spoor 2003; Pfaff et al. 2015).
Compared to most terrestrial vertebrates, salamanders demonstrate a highly reduced auditory system lacking a tympanic membrane and middle ear cavity, and retain only the membranous inner ear enclosed within the bony labyrinth and one to two middle ear ossicles (reviewed by Capshaw and Soares 2016). The salamander inner ear may be functionally subdivided into the pars superior which includes the three semicircular canals of the vestibular system and the pars inferior which contains the sensory epithelia that detect acoustic stimuli. Salamanders are not known to rely on acoustic cues to mediate behavior, and therefore the salamander ear is not expected to be under selection for increased auditory sensitivity. Despite this, there is great morphological variation in the salamander inner ear (Lombard 1977). What processes mediate the high degree of variation observed in the ears of non-vocal, atympanic salamanders? The trends underlying this structural variation have not yet been explored; however, the extraordinary diversity of habitats occupied by plethodontid salamanders result in a range of navigational challenges, developmental strategies, and sensory constraints that may have influenced the morphology of the inner ear.
Here, we investigate the effects of three traits (habitat, developmental strategy, and vision) on the functional morphology of the inner ear with an ecologically diverse sampling of plethodontid species. First, we test the hypothesis that variation in the salamander bony labyrinth reflects the morphological trends observed in other species that occupy similar ecological niches. For example, species occupying habitats that necessitate spatially complex behaviors, such as climbing in arboreal habitats or swimming in aquatic habitats, may benefit from enhanced vestibular sensitivity and are expected to possess hypertrophied semicircular canals as are observed in taxa that inhabit similar ecological niches. Second, we hypothesize that heterochronic shifts in developmental sequence among salamander species influence structural variation in the otic region. The general ossification pattern of the salamander skull anticipates late ossification of the endochondral bones of the otic capsule and stapes (Rose 1994). We therefore expect paedomorphic species that retain aquatic larval traits into maturity to possess poorly ossified otic structures demonstrated by larger foramina compared to direct developing and metamorphic species. Finally, because the diversity of habitats occupied by plethodontid salamanders presents a range of visual constraints that may influence reliance on acoustic and vestibular cues, we test the hypothesis that variation in the bony labyrinth reflects diversity in visual systems. Species that occupy visually-restricted habitats, such as caves and subterranean burrows, may display hypertrophic semicircular canals indicative of enhanced vestibular sense. Species with reduced visual systems may also rely on substrate vibration as an information channel, and so might possess large middle ear bones to magnify inertial bone conduction of sound to the ear, and large saccules to support hypertrophied sensory epithelia within the inner ear. We use both traditional and 3D geometric morphometrics to evaluate differences in the size and shape of the structures of the salamander inner ear, and use phylogenetically informed statistics to analyze these data for ecological trends among diverse plethodontid species.
Methods
Study species
We sampled 172 adult specimens representing 52 ecologically diverse species of plethodontid salamanders, and 4 Rhyacotriton variegatus specimens representing an outgroup to the family Plethodontidae (supplementary table S1). Preliminary analyses were obtained incorporating Amphiuma pholeter and Amphiuma means as outgroup species; however, measurements taken from these species fell out of the upper quartile for our sample and were therefore excluded from our analyses as outliers. For each species of interest, specimens were randomly selected and included both male and female individuals of comparable developmental stage and head width in order to reduce the effects of intra-specific size variation and sexual dimorphism that might confound the species means used in our comparative morphometric analysis.
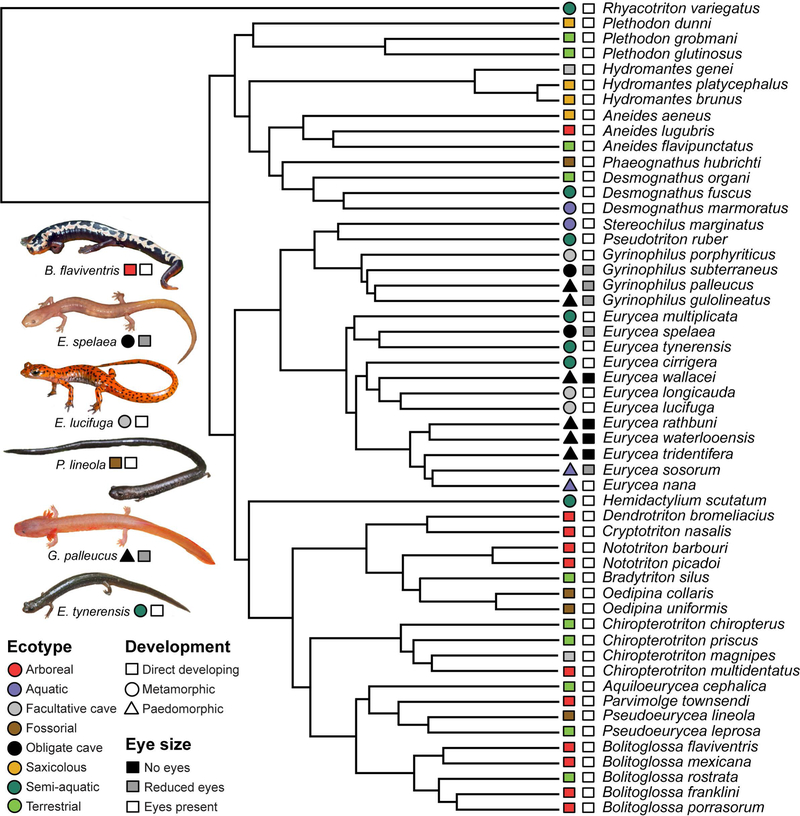
To test the relationship between otic morphology and habitat type, we classified species into ecotype categories using data from the literature (supplementary table S1). We defined the following ecotype classifications: obligate cave, facultative cave, aquatic, arboreal, fossorial, saxicolous, and terrestrial. Obligate cave species demonstrate troglomorphic adaptations to the cave environment, including reduced or absent eyes and loss of pigmentation. Facultative cave species are those that commonly inhabit caves and cave twilight zones, but do not demonstrate cave-adapted morphology and may also be found in epigean (surface) microhabitats. We also used an alternative ecotype classification scheme which distributed facultative cave-dwelling species among surface ecotypes that closely match their microhabitat (e.g., Chiropterotriton magnipes was alternatively coded as saxicolous). Surface-dwelling species that have strong association with an aquatic lifestyle were categorized as aquatic. The arboreal ecotype was used to characterize species that climb vegetation higher than 1 meter above the ground, and includes those that dwell in arboreal microhabitats such as bromeliads. Burrowing species were categorized into the fossorial ecotype. Saxicolous species are those that live primarily on rocky substrates. Semi-aquatic species are those that live at the aquatic/terrestrial interface, and commonly transition between aquatic and terrestrial microhabitats throughout their lives. The terrestrial ecotype encompasses ground-dwelling species with a reduced dependence on water, including those that are commonly found under rocks, logs, or other vegetation.
Often, salamander species grouped within the aquatic and the obligate cave ecotype have a paedomorphic life cycle and therefore possess similar traits (e.g., the retention of external gills into adulthood) that may confound morphological comparisons. In order to reduce the effect of these morphological correlations among the aquatic and obligate cave ecotypes, we incorporated paedomorphic and metamorphic species in both categories. Additionally, we included developmental strategy as a separate factor in our analyses to distinguish between paedomorphic (here defined as species with mature, gilled, fully aquatic adult body forms) and non-paedomorphic (i.e., metamorphic and direct developing) species. Finally, we incorporated eye size in order to evaluate the influence of the visual system on auditory structures. We drew eye size information from the literature see (supplementary table S1); because measurements of visual traits vary across studies, we summarized eye size in broad terms to incorporate presence, absence, and reduction of the eyes as a categorical factor in our analyses.
In sum, our comparative analysis included 8 independently evolved obligate cave-adapted species, 5 facultative cave-dwelling species, and 40 surface species encompassing a diverse array of habitat types (4 aquatic species, 11 arboreal species, 4 fossorial species, 4 saxicolous species, 7 semi-aquatic species, and 10 terrestrial species, see figure 1). Of these, 31 species are direct developing, 14 species are metamorphic, and 8 species are paedomorphic. Our sample included 4 species with no external eyes (i.e., vestigial eyes covered by skin), 5 species with eyes that are reduced in size but retain functional lenses, and 44 species with well-developed visual systems.
Figure 1.
Maximum likelihood phylogeny of all species sampled. Tip labels: first column indicates ecotype (color) and developmental strategy (shape); second column colors indicate eye size. Inset photos have been edited from the originals (B. flaviventris, E. tynerensis, and P. lineola from S. M. Rovito (CC BY-NC 3.0), E. lucifuga from T. Pierson (CC BY-NC 3.0), E. spelaea and G. palleucus from M. Niemiller).
Data acquisition
Specimens were scanned using a variety of micro-computed tomography (μCT) imaging systems and settings (see supplementary table S1) and were digitally reconstructed using Mimics software (Materialise NV). μCT data were supplemented with serially sectioned histological specimens that were reconstructed using Neurolucida software (MBF Bioscience). We created 3D surface models of osteological structures of the otic region of the skull including the brain case, the otic capsules, and the fenestral element of the oval window, hereafter referred to as the stapes although the homology of this structure is unresolved (Hetherington, 1988). Additionally, we generated surface models of the endocast of the otic capsule, which represent a close approximation of the soft tissue morphology of the inner ear.
Traditional Morphometrics
We measured the total volume of the otic endocast, the relative volume of the saccule (calculated as a proportion relative to the total endocast volume), the area of the oval window and of three foramina in the medial wall of the otic capsule: the periotic foramen, representing the putative pressure relief window for the salamander ear, and the foramina for the anterior and posterior branches of the eighth cranial nerve that innervate the vestibular and auditory organs of the inner ear, respectively. Additionally, we measured the total volume of the stapes, and the medial surface area of the stapes footplate. Because several histological specimens presented incomplete data for whole head measurements such as skull length, we calculated head size using a proxy measurement of the distance separating the two otic endocasts at the medial margin of the pars superior and pars inferior, following Maddin and Sherratt (2014). A regression analysis of the measured inter-endocast distance against skull length for specimens in which these data were available validated the accuracy of this proxy measurement to predict head size for all specimens in our study (R2 = 0.79).
Measurements of otic structures were calculated on 3D surface models using Mimics and Neurolucida. All structures were measured three times and the average measurement used for subsequent analyses. We assessed the precision of our measurements by calculating the percent error of each measurement relative to the mean measurement from an individual specimen; measurements with a percent error greater than 5% were re-measured or were excluded. Data were averaged to obtain a species mean for each trait, and all measurements were log-transformed to meet assumptions of normality for statistical testing (species mean data are provided in supplementary table S2).
Geometric morphometrics
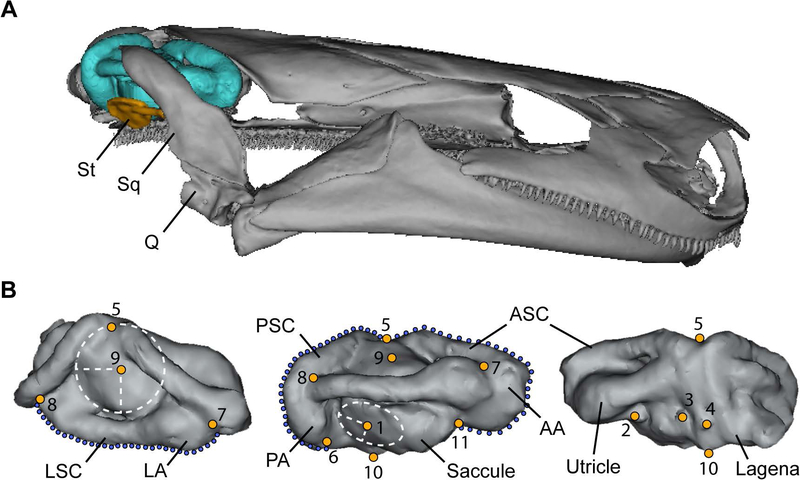
Morphometric analyses of otic endocast shape variation were based on landmark configurations placed on the 3D surface models using Landmark Editor software (v 3.6, Institute for Data Analysis and Visualization, University of California, Davis). We defined 11 landmarks representing clearly distinguishable, homologous anatomical locations on the otic endocasts that could be reliably placed across all specimens in this study (see figure 2 and supplementary table S3 for a description of landmark placement). Additionally, the three semicircular canals were each defined by 30 sliding semilandmarks that were evenly distributed along the line of maximum curvature. These semilandmarks were anchored by fixed landmarks at each end of the semicircular canals.
Figure 2.
3D rendering of the skull of Eurycea spelaea (A) with bony otic capsule removed to show the otic endocast. (B) Placement of landmarks 1–11 and sliding semilandmarks on the right otic capsule endocast of the inner ear in dorsal (left), lateral (middle) and medial (right) views. Placement of landmarks 1 and 5 are indicated at the center of a circumscribed ellipse. Abbreviations: AA anterior ampulla, ASC anterior semicircular canal, LA lateral ampulla, LSC lateral semicircular canal, PA posterior ampulla, PSC posterior semicircular canal, Q quadrate, Sq squamosal, St stapes.
Landmark coordinate data characterizing the shape of the otic endocasts were aligned with a Generalized Procrustes analysis using the R package geomorph (v 3.1.2; Adams et al. 2014). The generalized Procrustes analysis is a least-squares method that superimposes, rotates, and scales landmarks to an average configuration, resulting in shape information for each specimen that is independent of size, position, and orientation of the original structure (Rohlf and Slice 1990). The sliding semilandmarks were aligned to minimize bending energy between each specimen and the Procrustes average of all specimens. The resulting Procrustes coordinates were used as allometry-free shape variables in subsequent analyses.
Phylogenetic analyses
We used maximum likelihood method of estimation to construct a phylogeny based on 9 nuclear genes and 4 mitochondrial genes obtained from GenBank see (supplementary table S1). We created a concatenated sequence alignment in Geneious v 11.1 using the MAFFT plug-in (Katoh et al. 2002). We used PartitionFinder2 (Lanfear et al. 2016) to identify optimal substitution and site heterogeneity models based on the greedy algorithm and AICc model selection. We used RAxML v 8.2.12 (Stamatakis 2014) on the CIPRES Science Gateway (Miller et al. 2010) for maximum likelihood inference with a GTR + Γ model and 1,000 rapid bootstrapping replicates. The resulting maximum likelihood tree was used in all phylogenetic comparative analyses.
Statistical analyses
We performed phylogenetic generalized least squares (PGLS) analyses for each measurement regressed against head size using the R package caper (v 0.5.2; Orme et al., 2013). The PGLS regression method incorporates the variance-covariance matrix derived from the phylogeny into the error structure of the model to account for the evolutionary relationships among species under a Brownian motion model of trait evolution (Grafen 1989; Martins and Hansen 1997; Rohlf 2001). We extracted size-corrected phylogenetic residuals from PGLS models for each measurement, and assessed the phylogenetic dependence of these residuals by calculating Pagel’s lambda estimated by maximum likelihood in caper. Pagel’s lambda ranges from 0 to 1, where 0 indicates no phylogenetic signal (i.e., a given trait varies among species independent of phylogeny) and 1 indicates that trait evolution corresponds to a Brownian motion model (i.e., trait variation among species reflect phylogenetic relatedness) (Pagel 1999). Size-corrected PGLS residuals were used as response variables in subsequent analyses.
To test for variation in size measurements of otic structures among species, we performed a phylogenetic multivariate analysis of variance (MANOVA) with ecotype, developmental strategy, and eye size incorporated as predictor variables using the procD.pgls function in the geomorph package in R. This test uses a randomized residual permutation procedure (1,000 iterations) to test for correlation between a multivariate response variable and the predictor variables while incorporating the phylogeny to account for species relatedness (see Adams and Collyer 2018). The phylogenetic MANOVA was performed using type II sums of squares and cross-products which conforms to the principle of marginality and tests the main effects of each factor after all other factors have been accounted for, independent of the order in which they are incorporated into the model. Significant results were further investigated using univariate PGLS regression analyses in caper to test for the relationships among otic traits, ecotype, development, and eye size among species, followed by model selection using the Akaike information criterion corrected for small sample sizes (AICc). Models with ΔAICc within two points of the best model were considered equally supported (Burnham and Anderson 2002). We performed phylogenetic analysis of variance (ANOVA) analyses on the residuals from PGLS models that best fit the data, followed by post-hoc pairwise testing corrected for multiple comparisons using the Bonferroni method with 10,000 replicates using the package phytools in R (Revell 2012).
We explored variation in otic endocast shape among species using principal components analysis of the Procrustes coordinates of all specimens, and visualized shape change using thin plate spline deformation grids in geomorph. We assessed the phylogenetic signal in the shape data using the multivariate version of Blomberg’s K-statistic in geomorph. A strong phylogenetic signal is indicated by K values greater than 1. We tested for the effects of ecotype, developmental strategy, and eye size on otic endocast shape using phylogenetic MANOVA with randomized residual permutation procedure and type II sums of squares and cross-products in geomorph. We then used the pairwise function in the R package RRPP (v 0.4.2, Collyer and Adams 2018) to test the significance of Euclidean distances (d) between least-squares means of groups defined by ecotype, developmental strategy, and eye size. For each factor of interest, test statistics and p-values for pairwise comparisons were obtained using 1,000 permutations under a reduced null model (see Freedman and Lane 1983) and a 95% confidence interval. All statistical analyses were performed using R v 3.3.3 (R Core Team 2017).
Results
Body size correlations and phylogenetic conservativism
In general, the size of otic structures scale allometrically with head size (supplementary table S4); however, the volume of the saccule and the areas of the anterior and posterior foramina of the eighth cranial nerve were weakly correlated with head size (R2 = 0.20, 0.33, 0.44 respectively). Volume of the stapes was most strongly influenced by head size. Univariate testing found no significant effects of ecotype, developmental strategy, or eye size on size-corrected stapes volume residuals, indicating that variability in the size of this structure is dominated by allometry in our sampled species.
All measured otic traits were significantly influenced by phylogenetic relatedness among species under a Brownian motion model of evolutionary change (table 1). Variation in the shape of the otic endocast was found to be weakly influenced by phylogenetic relatedness among species under a Brownian motion process (Kmult = 0.17); however phylogenetic conservatism in the Procrustes coordinates was still greater than expected by chance (p = 0.001; 1,000 permutations).
Table 1.
Phylogenetic signal of size-corrected phylogenetic residuals for otic measurements
| Measurement | λ | Log-likelihood | Log-likelihood for λ=0 | P-value of likelihood ratio test |
|---|---|---|---|---|
| Ant. 8th cranial nerve foramen area | 0.38 | 1.66 | −1.36 | 0.01 |
| Post. 8th cranial nerve foramen area | 0.81 | 7.44 | −11.74 | 5.8e-10 |
| Endocast volume | 0.84 | 29.27 | 9.03 | 2.0e-10 |
| Oval window area | 0.91 | 34.76 | 4.95 | 1.2e-14 |
| Periotic foramen area | 0.83 | 20.62 | 3.55 | 5.1e-9 |
| Relative saccule volume | 0.67 | 46.39 | 38.65 | 8.3e-5 |
| Stapes footplate area | 0.81 | 24.57 | 15.48 | 2.0e-5 |
| Stapes volume | 0.83 | 6.43 | −5.51 | 1.0e-6 |
Significance of Pagel’s lambda (λ) was evaluated using 10,000 permutations.
Ecological correlates of structural variation in the salamander ear
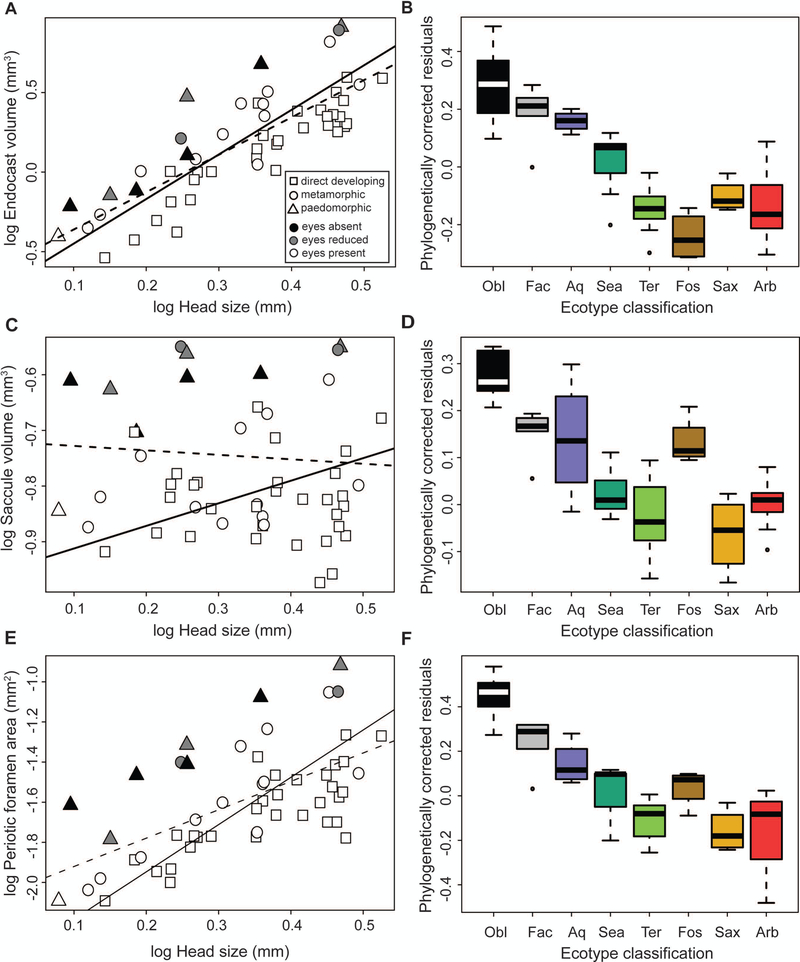
Phylogenetic MANOVA and permutation testing of otic measurements indicated a significant effect of ecotype classification (table 2). Univariate analyses found that ecotype classification was significantly correlated with variation in size-corrected residuals for total endocast volume (F7, 45 = 21.62; p = 0.002), saccule volume (F7, 45 = 19.64; p < 0.001), and periotic foramen area (F7, 45 = 22.39; p <0.001). Pairwise post-hoc comparisons revealed that obligate cave, facultative cave, and aquatic species had larger otic endocasts compared to all other surface-dwelling ecotypes (p<0.05 for all pairwise comparisons; see figure 3a–b). Residual endocast volume of obligate cave species differed significantly from arboreal (p = 0.03), fossorial (p = 0.03), saxicolous (p = 0.031), and terrestrial species (p = 0.003). Endocast volume in facultative cave species was significantly different from that of arboreal (p = 0.011), fossorial (p = 0.003), and terrestrial species (p = 0.006). Endocast volume of aquatic species differed significantly from fossorial (p = 0.008) and terrestrial (p = 0.014) species. A separate multivariate analysis incorporating the alternative ecotype classification scheme that distributed facultative cave species among surface ecotypes did not recover any significant effects of ecotype on otic measurements (supplementary table S5); however, the alternative ecotype classification scheme was not favored in subsequent model selection or univariate analyses of otic measurements (supplementary table S4).
Table 2.
Results of phylogenetic MANOVA on otic measurements
| Effect | Df | SS | MS | R2 | F | Z | P |
|---|---|---|---|---|---|---|---|
| Development | 2 | 5.02 | 2.51 | 0.05 | 1.84 | 1.15 | 0.129 |
| Ecotype | 7 | 18.78 | 2.68 | 0.17 | 1.97 | 2.20 | 0.021 |
| Eye | 2 | 26.84 | 13.42 | 0.25 | 9.84 | 3.24 | 0.002 |
| Residuals | 41 | 108.60 | 1.36 | 0.52 |
Type II Phylogenetic MANOVA with randomized residual permutation procedure (1,000 permutations) implemented using procD.pgls in R. Significant p-values are in bold.
Figure 3.
Linear regressions of (A) otic endocast volume, (C) relative saccule volume (calculated as a proportion of total endocast volume), and (E) periotic foramen area against head size; results of both GLS (dotted line) and PGLS (solid line) models are shown. Symbol shape indicates developmental strategy and color indicates external eye size. Boxplots represent the phylogenetic residuals of (B) endocast volume and (D) relative saccule volume, and (F) periotic foramen area grouped by ecotype classification.
Variation in relative saccule volume correlated significantly with both ecotype and eye size. Species within the obligate cave ecotype possessed the largest saccules relative to total endocast volume, and significantly differed from all other ecotypes (p < 0.05) except for the fossorial ecotype. Although relative saccule volume appears large within the fossorial ecotype relative to other non-aquatic, surface-dwelling ecotypes, pairwise comparisons did not support significant differences between species of this ecotype and any other ecotype. The area of the periotic foramen demonstrated similar trends: variation in this structure is strongly correlated with habitat type, with the largest periotic foramina found in obligate cave-adapted species (p < 0.05 for all pairwise comparisons).
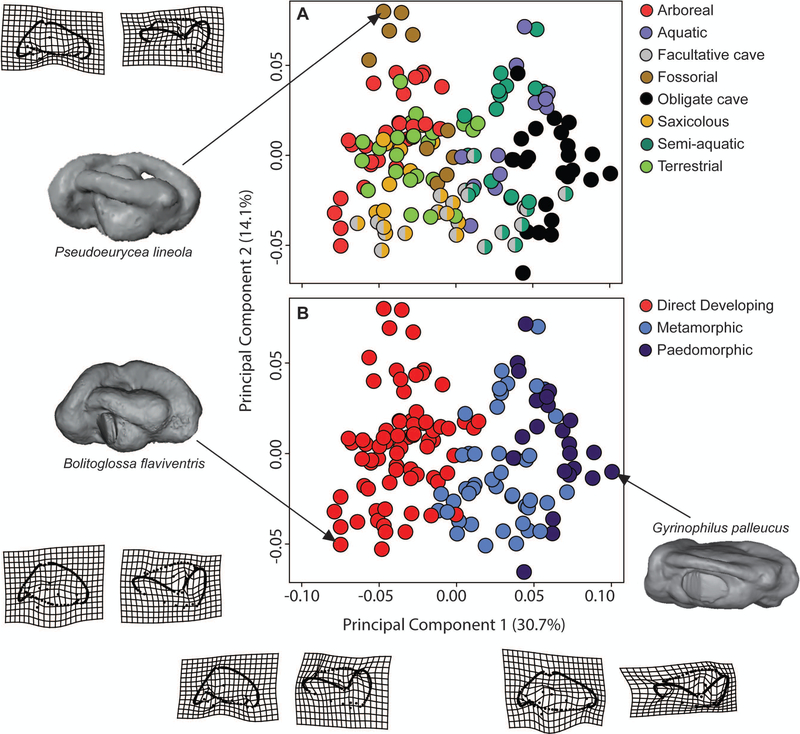
The PCA of the multivariate endocast shape data produced 132 PCs, the first three of which account for approximately 53% of the total variance in shape. Ecotype classification correlates well with PC1 (figure 4a). Moving from PC1 negative to positive, the pars inferior becomes more elongated and narrowed anteriorly, and the anterior and posterior semicircular canals exhibit a smaller degree of curvature and thickness. All obligate cave species are grouped at the PC1 maximum, indicating that the inner ear of this ecotype is generally similar across these independently derived lineages. The inner ears of arboreal, fossorial, and saxicolous species are generally constrained to the PC1 minimum, where the anterior and posterior semicircular canals are thicker, more strongly curved, and more bulbous at the anterior end.
Figure 4.
Principal component (PC) analysis of inner ear shape. Individual points represent specimens; colors indicate ecotype classification (A) and developmental strategy (B). The facultative cave ecotype in (A) is split-colored to indicate the alternative ecotype classification scheme. Thin-plate spline deformation grids illustrating deviation from the Procrustes mean shape along each PC axis are shown.
Shape changes along PC2 are primarily associated with changes to the lateral semicircular canal, which becomes more curved and projects further from the labyrinth in the negative direction along the axis, and is more adpressed to the labyrinth in the positive direction. Additionally, at the PC2 minimum, the overall curvature of all three semicircular canals is more extreme, and the angle of the crus communis (where the anterior and posterior semicircular canals meet) is more acute than that observed in specimens at the PC2 maximum. Saxicolous and facultative cave-dwelling species are constrained to more negative values of PC2, however, species within all ecotypes exist in a continuum along the PC2 axis.
A MANOVA of the Procrustes coordinates found that endocast shape was significantly correlated with ecotype, regardless of the classification scheme (table 3). Post-hoc pairwise comparisons of the Euclidean distance separating least squares means for ecotypes recovered significant differences only between the obligate cave ecotype relative to the terrestrial (p = 0.037) and arboreal ecotype (p = 0.014), with marginal differences separating the obligate cave ecotype from the fossorial (p = 0.065) and saxicolous ecotype (p = 0.073) – see supplementary table S6. The significant effects of ecotype on inner ear shape in plethodontid salamanders appear to be largely driven by the disparate shape of the obligate cave-adapted species, indicated by the PCA (figure 4a). In contrast, the inner ear shape of the facultative cave ecotype did not differ significantly from any other ecotype. These results, coupled with broad distribution of facultative cave-dwelling species across the PC1 axis, may indicate that disparate microhabitat usage by these species within caves and surface habitats influences inner ear shape to a larger degree than their troglophilic habit.
Table 3.
Results of phylogenetic MANOVAs on otic endocast shape coordinates incorporating both ecotype classification schemes
| Effect | Df | SS | MS | R2 | F | Z | P |
|---|---|---|---|---|---|---|---|
| Development | 2 | 0.149 | 0.074 | 0.032 | 1.850 | 2.198 | 0.012 |
| Ecotype | 7 | 1.259 | 0.180 | 0.275 | 4.477 | 5.703 | 0.001 |
| Eye | 2 | 0.818 | 0.409 | 0.179 | 10.177 | 5.119 | 0.001 |
| Residuals | 41 | 1.647 | 0.040 | ||||
| Development | 2 | 0.108 | 0.054 | 0.024 | 1.013 | 0.215 | 0.349 |
| Alt. Ecotype | 2 | 0.675 | 0.112 | 0.147 | 2.115 | 2.703 | 0.020 |
| Eye | 6 | 0.705 | 0.352 | 0.154 | 6.629 | 3.974 | 0.001 |
| Residuals | 42 | 2.232 | 0.053 |
Type II Phylogenetic MANOVA with randomized residual permutation procedure (1,000 permutations) implemented using procD.pgls in R. Significant p-values are in bold.
Heterochrony and the ossification of the otic region
Multivariate analysis of the effect of developmental strategy on otic measurements found no significant differences among groups (table 2). However, we found significant effects of development on the univariate traits of oval window area (F2, 50 = 64.24; p < 0.001) and foramen area for the posterior branch of the eighth cranial nerve (F2, 50 = 23.00; p=0.029). Direct developing species have proportionally smaller foramina relative to both paedomorphic and metamorphic species; however, there is much variation in the size of these structures within groups (supplemental figures S1–2). Although model selection favored the interaction of head size and developmental strategy on the foramen area for the anterior branch of the eighth cranial nerve and stapes footplate area, the phylogenetic ANOVA of size-corrected residuals found no significant effects of development, likely due to the high variability of these traits within groups (supplemental figure S4).
The PCA of otic endocast shape reveals a correlation of developmental strategy with shape changes along the first PC axis: paedomorphic and metamorphic species cluster nearer to the positive end of PC1 with narrow, less curved semicircular canals, and direct developing species are constrained to the negative end with more robust semicircular canals (figure 4b). Although the phylogenetic MANOVA recovered a significant effect of developmental strategy on otic endocast shape (table 3), post-hoc pairwise comparisons of the least-squares means recovered no significant differences separating any group (supplementary table S7). The shape of the paedomorphic otic endocast is only marginally disparate relative to the direct developing otic endocast (p = 0.057).
The influence of visual system reduction on otic structures
A significant relationship between external eye size and otic measurements is supported by phylogenetic MANOVA (table 2; F2, 41 = 9.84; p =0.002) and subsequent univariate testing. We found that saccule volume (F2, 50 = 31.75; p < 0.001) and periotic foramen area (F2, 50 = 30.52; p < 0.001) are highly correlated with eye size. Saccule volume exists in a broad continuum among species with eyes, but is larger in general among species with reduced (p < 0.001) and absent (p < 0.001) eyes compared to species with well-developed eyes (supplemental figure S3). Similarly, larger periotic foramina are associated with species that have reduced (p = 0.003) or absent (p < 0.001) eyes relative to species with well-developed eyes (figure 3e–f). A high lambda value for the best fit model explaining variance in periotic foramen area (λ=0.67; supplementary table S4) indicates a strong phylogenetic signal in this trait, which may be due to the fact that many species with reduced or absent eyes are cave-adapted species of the plethodontid tribe Spelerpinae. In contrast, the best fit model explaining variance in saccule volume has negligible phylogenetic signal (λ=0.00; supplementary table S4), indicating possible convergence in this trait among species with reduced visual systems.
Univariate testing of the stapes footplate area detected significant effects of both developmental strategy and eye size on the size of this structure; however, subsequent post-hoc testing revealed no significant correlations among any groups. Examination of the size-corrected residuals for footplate area revealed large amounts of variation within groups, particularly among direct developing species and species with well-developed eyes relative to those with reduced or absent eyes (supplementary figure S4).
Additionally, the phylogenetic MANOVA confirmed a significant association of eye size and inner ear shape (table 3).Pairwise comparisons of Euclidean distance between least-squares means of each group revealed significant differences in otic endocast shape among species with no external eyes and species with well-developed eyes (p = 0.003, supplementary table S8). There was no significant difference separating endocast shape of species with reduced eyes and species with well-developed external eyes. These results suggest that morphological disparity in inner ear shape among diverse salamander species is significantly influenced by the presence or absence of eyes.
Discussion
The vertebrate inner ear is a crucial sensory structure that serves dual functionality for detection of biotic and abiotic acoustic stimuli and encoding linear and rotational acceleration of the head for maintenance of balance. The vestibular pars superior is comprised of the three semicircular canals and the utricle, which act as inertial sensors of head movement and are generally conserved structurally among all vertebrates to preserve function (Carey and Amin 2006). In contrast, the auditory pars inferior is known to demonstrate inter-specific morphological variation indicating adaptation for auditory specialization, exemplified by variation in the elongation and coiling of the mammalian cochlea to support extended frequency ranges of acoustic sensitivity. Salamanders have an unspecialized, atympanic ear and are non-vocal in general (but see Gehlbach and Walker 1970; Thurow and Gould 1977; Brodie 1978; Crovo et al. 2016); therefore, the salamander otic region is not expected to experience strong directional selection for auditory function. Under relaxed selection, the structures of the salamander ear may show somewhat random phenotypic variation, but are primarily expected to retain an unspecialized form. Here we find compelling evidence that not only does the vestibular component of the salamander ear show ecomorphological trends among diverse species, but also the auditory structures of the ear demonstrate convergent hypertrophy in species that inhabit visually-restrictive environments. The results of our analyses reveal a relationship between the functional morphology of the salamander inner ear with habitat (and associated locomotor behaviors), developmental strategy, and visual system, and imply that the structures of the ear are malleable to selective pressures even in species not known to display acoustic behaviors.
Ecological signal in salamander inner ear morphology indicates variation in vestibular function
We found significant variation in the size and shape of inner ear structures among sampled salamander species such that those inhabiting similar ecological niches cluster together within the morphospace. The semicircular canals of the vestibular system, which detect angular accelerations and support gaze stabilization during head movement via the vestibulo-ocular reflex, are highly variable across salamander ecotypes. Sensitivity of the semicircular canals is dependent on their physical parameters. The internal radius of a cross-sectional slice of the canal and radius of curvature along the length of the canal determine the flow dynamics of the enclosed endolymphatic fluid and subsequent stimulation of the sensory hair cells in response to head movement (Jones and Spells 1963). There is a generally positive relationship between semicircular canal curvature and locomotor behavior in which more agile species tend to have relatively large, strongly curvilinear canals (Spoor et al. 2007; Cox and Jeffery 2008, 2010). The form-function relationship of semicircular canal morphology and afferent sensitivity has been confirmed across a wide sample of mammals (Yang and Hullar 2007). Although salamanders have a sprawling gait with a relatively simple locomotor module and are not typically considered agile compared to most other vertebrates, our study indicates that variation in otic endocast shape among salamander ecotypes correlates with variability in locomotor behavior and differential reliance on the vestibulo-ocular reflex.
Semicircular canal curvature is greatest among salamander species that are known to perform complex locomotor behaviors and navigate cluttered and complex microhabitats. Salamanders that inhabit arboreal, fossorial, and saxicolous microhabitats have relatively small otic endocasts compared to aquatic and cave-dwelling species; however, the inner ear of these species is characterized by robust semicircular canals with greater curvature compared to that observed in cave-adapted and aquatic species. In particular, species with the most extreme degree of curvature to the semicircular canals are arboreal species associated with epiphytic microhabitats such as bromeliads and have specialized foot morphology adapted for climbing (e.g., Bolitoglossa porrasorum, B. mexicana).
The facultative cave ecotype exhibits the largest amount of shape variation of the inner ear among sampled species. This may be attributable to the diversity in locomotor modes and microhabitat usage of facultative cave species that range from the semi-aquatic Gyrinophilus porphyriticus to the scansorial Chiropterotriton magnipes, which possesses morphological adaptations to the appendicular skeleton to support climbing behavior (Darda and Wake 2015).
The semicircular canals of cave-adapted species with reduced or absent external eyes exhibit the smallest degree of curvature among sampled salamander species, which may result from a limited reliance on the vestibulo-ocular reflex due to environmental constraints to vision. Trends towards reduced curvature of the semicircular canals are also observed in fossorial caecilians with reduced visual systems (Maddin and Sherratt 2014). It is notable that our results do not support a similar reduction in canal curvature among fossorial salamander species; however, this may be explained by a difference in the reliance on visual information to guide behavior among caecilians and salamanders. Caecilians demonstrate strong morphological adaptations to support a burrowing lifestyle, including hyperossification of the cranial bones, degenerated eyes covered by skin or bone, and reduced visual innervation (Wake 1985). Fossorial salamanders, in contrast, often retain well developed eyes. The fossorial species incorporated in this study are visual predators that hunt using protrusible or freely projectile tongues (Lombard and Wake 1977; Herrel et al. 2019); therefore retaining a keen vestibulo-ocular reflex, demonstrated by robustly curved semicircular canals, may be beneficial to coordinate head movement and gaze stabilization in these species.
Shape, but not size, of the structures of the inner ear is influenced by heterochrony
Contrary to our expectations, there were no clear patterns in otic measurements that indicated heterochronic effects on variable ossification of the salamander ear. Although the oval window and medial foramina of the otic capsule were generally smaller in direct-developing species relative to paedomorphic and metamorphic species, all groups displayed large amounts of variation suggesting that development as it is characterized in this study is not the strongest influence on the size of otic structures. In this study, we limit our definition of paedomorphism to encompass species that retain a permanently aquatic larval body form at maturity; however, differential ontogenetic patterning among direct developing species leads to variably paedomorphic cranial features, including the loss or reduction of elements of the adult skull among Bolitoglossines (Wake 1966; Alberch 1983; Rose 1994). We did not address this form of osteological paedomorphism in plethodontid salamanders; however, further exploration of the influence of developmental patterning of the skull could refine our understanding of morphological variation in the otic region of diverse plethodontid species.
Although principal components analyses appear to support variability of otic endocast shape among developmental groups, it appears that these morphological differences are more strongly linked to ecological and sensory specialization in plethodontid salamanders. Direct developing species are more likely to encounter complex microhabitats (e. g., arboreal bromeliads) and adaptation to these habitats may be facilitated by changes to vestibular sensitivity to coordinate locomotor behaviors, signified here by robust, highly curved semicircular canals. In contrast, the majority of metamorphic and paedomorphic salamander species that we sampled possess narrower, weakly curved semicircular canals. A trend toward reduction of the vestibular system in permanently aquatic paedomorphic species is shared by cave-adapted and surface-dwelling species, indicating that enhanced vestibular sensitivity is not necessary to coordinate aquatic locomotor behavior in paedomorphic salamanders, regardless of the degree of reduction in the visual system.
Feeding mode is a notable trait distinguishing the sensory ecology of surface-dwelling direct developers and permanently aquatic paedomorphic species: the ballistic tongue projection mechanism observed in many direct developing plethodontids requires a robust vestibular capacity to coordinate vision and head posture, which likely plays a less significant role in aquatic suction feeding among paedomorphic species (Roth 1987; Wake and Deban 2000). Additionally, paedomorphic cave and surface species tend to possess dorso-ventrally flattened skulls which may represent morphological homoplasies to support the biomechanical requirements of suction feeding in an aquatic habitat (Heiss et al. 2013, 2018; Herrel et al. 2019). Although the relationship of cranial depth with inner ear morphology has not been explored in this study, the shape and depth of the salamander skull may further influence the shape of the inner ear and constrain the degree of curvature of the semicircular canals. Further study of developmental ecomorphology of the plethodontid skull and its influence on elements of the otic region are an important next step.
Hypertrophic auditory structures correlate with cave habitat and visual reduction
The size of the inner ear varies widely across salamander ecotypes, independent of the effects of evolutionary allometry, and a substantial trend appears linked to cave colonization. Independently-evolved cave lineages have larger otic endocasts relative to head size, with proportionally larger saccules that may support hypertrophic sensory organs. This trend towards a hypertrophic inner ear is observed in obligate cave species that display morphological indicators of cave adaptation (i.e., loss or reduction of the visual system, depigmentation, etc.), and in facultative cave-dwelling species that otherwise possess no troglomorphic features. Even with a comparable hair cell density across species, a proportionally larger epithelial surface area within the saccule may increase the total number of sensory receptors in the ear and could contribute to enhanced sensitivity to acoustic stimuli in these species. A quantitative analysis of the saccular macula (the acoustic end organ of the saccule) in the aquatic cave-adapted species Proteus anguinus demonstrated a positive correlation between body size, diameter of the sensory epithelium of the saccule, and the total number of hair cells (Bulog 1989). Additionally, burrowing caecilians have similarly expanded saccules which are believed to indicate greater sensitivity to substrate vibration (Maddin and Sherratt 2014). Although histology is necessary to confirm any correlation between the observed variation in ear size and size of the sensory epithelia enclosed within, it is generally accepted that sensory structures present strong morpho-functional correlations and that enlargement of sensory organs signifies increased sensitivity. Our findings indicate that cave adaptation in plethodontid salamanders extends to structural changes to the inner ear that may support increased sensitivity to acoustic energy, particularly seismic vibrations.
Cave species with reduced or completely absent external eyes also have proportionally larger periotic foramina compared to species dwelling in epigean habitats. The salamander auditory system lacks a round window, the pressure relief window found in the otic capsules of most tympanic vertebrates, and in the absence of this pressure relief window the fluid-filled inner ear may be considered incompressible by the movement of the stapes within the oval window. The salamander auditory system is therefore theorized to rely upon the periotic foramen as a fluid pressure relief pathway (Wever 1978), and experimental evidence from salamander cranial preparations have demonstrated that the periotic foramen presents the path of least resistance for release of mechanical energy within the otic capsule (Smith 1968). Size variation of the periotic foramen therefore has great potential significance for auditory function in salamanders. A larger portal for pressure relief could increase the overall compliance of the fluid-filled membranous inner ear, leading to less resistance to fluid displacement across the hair cells of the sensory epithelia in response to movements of the stapes in the oval window. Although the observed enlargement of the periotic foramen in the otic capsule of these cave species may be attributable to ontogenetic changes in skull ossification related to paedomorphism and/or eye degeneration, the functional consequences of this structural change could still result in enhanced compressibility of the fluid-filled inner ear as a byproduct.
Loss of vision is often accompanied by concomitant hypertrophy of non-visual sensory organs to support behavioral guidance. Independent cavefish lineages demonstrate expansions to the mechanosensory lateral line (Poulson 1963; Teyke 1990; Montgomery et al. 2001; Yoshizawa et al. 2010) and to gustatory and chemosensory receptors (Varatharasan et al. 2009; Yamamoto et al. 2009) to support greater sensitivity to non-visual stimuli. Although similar enhancements to hearing are not observed in the cavefish species in which it has been studied (Popper 1970; Schulz-Mirbach et al. 2010; Niemiller et al. 2013), deep sea fish that inhabit light-restricted marine habitats possess hypertrophic auditory structures including enlarged otoliths, saccules, and brain regions involved in audition (Buran et al. 2005; Deng et al. 2013). Our results reveal that the inner ear of cave-adapted salamanders demonstrate a mosaic of regressive and progressive structural changes related to reduced visual input, including narrow, less curved semicircular canals indicative of a reduction in the role of the vestibulo-ocular reflex, and hypertrophy of the inner ear as a whole and expansion of the acoustically-sensitive saccule. These results reinforce the potential significance of acoustic vibration as an information source to guide behavior in visually-constrained species, and highlight the need for further physiological and behavioral investigation of the salamander auditory system within a comparative framework.
Concluding remarks
Our study shows that the morphology of the salamander inner ear reflects the evolutionary diversity found within the Family Plethodontidae, and reveals potential adaptation of the auditory and vestibular system to the variety of sensory and locomotor challenges encountered across different habitat types. Morphological trends observed in the vestibular systems of other taxa hold true for salamanders: species that perform complex locomotor behaviors (i.e., climbing and burrowing) demonstrate structural changes to the semicircular canals of the inner ear that are associated with increased vestibular sensitivity. Species with reduced visual input demonstrate narrower semicircular canals with less pronounced curvature indicating a reduced sensitivity of the vestibular system, possibly connected to a reduced need for maintaining sensory input for the vestibulo-ocular reflex. Convergent enlargement of the inner ear and saccule in independently-evolved cave species indicates potentially overlooked selective pressures on the sensory capacity of the inner ear in the absence of visual input, and highlights the need for additional investigation of auditory function in cave-dwelling species. Size variation of the periotic foramen, a key feature for fluid pressure relief within the salamander inner ear, may reveal variation in the biophysics of energy flow in the ear with concomitant changes to acoustic sensitivity among salamander species.
The convergent patterns observed in the salamander inner ear do not necessarily indicate adaptation under a selective regime: physiological experimentation is required to determine the functional significance of the observed morphological variation in the salamander ear. However, phenotypic similarities in the bony labyrinth among salamanders and more distantly-related taxa that encounter similar environmental contexts provides evidence that predictions for parallel trait evolution of inner ear morphology can be extended to species in which the auditory system is not expected to be under strong selection. Our findings provide additional support and validation for the use of the otic endocast as a tool for predicting locomotor behavior, habitat type, and the associated sensory requirements of the auditory and vestibular system across a wide range of taxa.
Supplementary Material
Acknowledgements:
We gratefully acknowledge the support of Rayna Bell, curator of Amphibians and Reptiles at the National Museum of Natural History (NMNH), and Addison Wynn, NMNH collection manager. We thank members of the Bell lab for their helpful comments on this manuscript. We also thank Jennifer J. Hill, Scott Whittaker, and Jessica Maisano for their invaluable assistance with CT data acquisition, and Jessica Goodheart and Daniel Escobar-Camacho for their help with phylogenetic analyses.
Funding: This work was supported by National Institute on Deafness and Other Communications Disorders (NIDCD) training grant NIDCD/NIH: T32 DC-000046 to the University of Maryland’s Center for Comparative and Evolutionary Biology of Hearing, and by DC-000436 (C.E.C.), a Smithsonian Institution graduate student fellowship (G.C.), and grants in aid of research from the Society for Integrative and Comparative Biology and the Cosmos Club Foundation (G.C.).
Footnotes
Data Accessibility: Raw data will be uploaded to MorphoSource digital repository (http://morphosource.org).
Conflict of Interests: The authors declare no conflict of interests.
References
- Adams DC, and Collyer ML. 2018. Phylogenetic ANOVA: Group-clade aggregation, biological challenges, and a refined permutation procedure. Evolution (N. Y). 72:1204–1215. [DOI] [PubMed] [Google Scholar]
- Adams DC, Collyer ML, Otarola-Castillo E, and Sherratt E. 2014. Geomorph: Software for geometric morphometric analyses. R package version 3.0.5 https://cran.r-project.org/package=geomorph.
- Adams DC, and Nistri A. 2010. Ontogenetic convergence and evolution of foot morphology in European cave salamanders (Family: Plethodontidae). BMC Evol. Biol 10:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberch P 1983. Morphological Variation in the Neotropical Salamander Genus Bolitoglossa. Evolution (N. Y). 37:906–919. [DOI] [PubMed] [Google Scholar]
- Blankers T, Adams DC, and Wiens JJ. 2012. Ecological radiation with limited morphological diversification in salamanders. J. Evol. Biol 25:634–646. [DOI] [PubMed] [Google Scholar]
- Bock WJ, and von Wahlert G. 1965. Adaptation and the Form-Function Complex. Evolution (N. Y). 19:269–299. [Google Scholar]
- Boistel R, Herrel A, Lebrun R, Daghfous G, Tafforeau P, Losos JB, and Vanhooydonck B. 2011. Shake Rattle and Roll: The Bony Labyrinth and Aerial Descent in Squamates. Integr. Comp. Biol 51:957–968. [DOI] [PubMed] [Google Scholar]
- Bonett RM, Steffen MA, Lambert SM, Wiens JJ, and Chippindale PT. 2014. Evolution of paedomorphosis in plethodontid salamanders: Ecological correlates and re-evolution of metamorphosis. Evolution (N. Y). 68:466–482. [DOI] [PubMed] [Google Scholar]
- Brodie ED 1978. Biting and Vocalization as Antipredator Mechanisms in Terrestrial Salamanders. Copeia 1978:127–129. [Google Scholar]
- Bulog B 1989. Differentiation of the inner ear sensory epithelia of Proteus anguinus (Urodela, Amphibia). J. Morphol 202:325–338. [DOI] [PubMed] [Google Scholar]
- Buran BN, Deng X, and Popper AN. 2005. Structural variation in the inner ears of four deep-sea elopomorph fishes. J. Morphol 265:215–225. [DOI] [PubMed] [Google Scholar]
- Burda H, Bruns V, and Hickman GC. 1992. The ear in subterranean Insectivora and Rodentia in comparison with ground-dwelling representatives. I. Sound conducting system of the middle ear. J. Morphol 214:49–61. [DOI] [PubMed] [Google Scholar]
- Burnham KP, and Anderson DR. 2002. Model Selection and Multimodal Inference. Second. Springer, New York. [Google Scholar]
- Capshaw G, and Soares D. 2016. Hearing in Plethodontid Salamanders: A Review. Copeia 104:157–164. [Google Scholar]
- Carey J, and Amin N. 2006. Evolutionary changes in the cochlea and labyrinth: Solving the problem of sound transmission to the balance organs of the inner ear. Anat. Rec. - Part A Discov. Mol. Cell. Evol. Biol 288A:482–489. [DOI] [PubMed] [Google Scholar]
- Chippindale PT, Bonett RM, Baldwin AS, and Wiens JJ. 2004. Phylogenetic Evidence for a Major Reversal of Life-History Evolution in Plethodontid Salamanders. Evolution (N. Y). 58:2809–2822. [DOI] [PubMed] [Google Scholar]
- Collyer ML, and Adams DC. 2018. RRPP: An r package for fitting linear models to high-dimensional data using residual randomization. Methods Ecol. Evol 9:1772–1779. [Google Scholar]
- Cox PG, and Jeffery N. 2008. Geometry of the semicircular canals and extraocular muscles in rodents, lagomorphs, felids and modern humans. J. Anat 213:583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox PG, and Jeffery N. 2010. Semicircular canals and agility: The influence of size and shape measures. J. Anat 216:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crovo JA, Zeyl JN, and Johnston CE. 2016. Hearing and Sound Production in the Aquatic Salamander, Amphiuma means. Herpetologica 72:167–173. [Google Scholar]
- Darda DM, and Wake DB. 2015. Osteological variation among extreme morphological forms in the Mexican salamander genus Chiropterotriton (Amphibia: Plethodontidae): Morphological evolution and homoplasy. PLoS One 10:e0127248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Wagner HJ, and Popper AN. 2013. Interspecific variations of inner ear structure in the deep-sea fish family melamphaidae. Anat. Rec 296:1064–1082. [DOI] [PubMed] [Google Scholar]
- Denoël M, Joly P, and Whiteman HH. 2005. Evolutionary ecology of facultative paedomorphosis in newts and salamanders. Biol. Rev. Camb. Philos. Soc 80:663–671. [DOI] [PubMed] [Google Scholar]
- Dickson BV, Sherratt E, Losos JB, and Pierce SE. 2017. Semicircular canals in Anolis lizards: Ecomorphological convergence and ecomorph affinities of fossil species. R. Soc. Open Sci. 4:170058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D, and Lane D. 1983. A nonstochastic interpretation of reported significance levels. J. Bus. Econ. Stat 1:292–298. [Google Scholar]
- Fritzsch B 1988. The lateral-line and inner-ear afferents in larval and adult urodeles. Brain Behav. Evol 31:325–348. [DOI] [PubMed] [Google Scholar]
- Gehlbach FR, and Walker B. 1970. Acoustic Behavior of the Aquatic Salamander Siren intermedia. Bioscience 20:1107–1108. [Google Scholar]
- Grafen A 1989. The phylogenetic regression. Philos. Trans. R. Soc. Lond. B. Biol. Sci 326:119–157. [DOI] [PubMed] [Google Scholar]
- Heiss E, Aerts P, and Van Wassenbergh S. 2018. Aquatic–terrestrial transitions of feeding systems in vertebrates: a mechanical perspective. J. Exp. Biol 221:jeb154427. [DOI] [PubMed] [Google Scholar]
- Heiss E, Natchev N, Gumpenberger M, Weissenbacher A, and Van Wassenbergh S. 2013. Biomechanics and hydrodynamics of prey capture in the Chinese giant salamander reveal a high-performance jaw-powered suction feeding mechanism. J. R. Soc. Interface 10:20121028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrel A, O’Reilly JC, Fabre A-C, Bardua C, Lowie A, Boistel R, and Gorb SN. 2019. Feeding in amphibians: Evolutionary transformations and phenotypic diversity as drivers of feeding system diversity Pp. 431–467 in Bels V and Whishaw IQ, eds. Feeding in Vertebrates. Springer Nature; Switzerland. [Google Scholar]
- Hetherington TE 1988. Metamorphic changes in the middle ear Pp. 339–357 in Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, and Walkowiak W, eds. The Evolution of the Amphibian Auditory System. John Wiley & Sons, Inc, New York. [Google Scholar]
- Hoskins SG 1990. Metamorphosis of the amphibian eye. J. Neurobiol 21:970–989. [DOI] [PubMed] [Google Scholar]
- Jones GM, and Spells KE. 1963. A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc. R. Soc. London. Ser. B. Biol. Sci 157:403–419. [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, and Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30:3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, and Calcott B. 2016. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol, doi: dx.doi.org/10.1093/molbev/msw260. [DOI] [PubMed] [Google Scholar]
- Lindenlaub T, Burda H, and Nevo E. 1995. Convergent evolution of the vestibular organ in the subterranean mole‐rats, Cryptomys and Spalax, as compared with the aboveground rat, Rattus. J. Morphol 224:303–311. [DOI] [PubMed] [Google Scholar]
- Lombard RE 1977. Comparative Morphology of the Inner Ear in Salamanders (Caudata: Amphibia). Karger, New York. [Google Scholar]
- Lombard RE, and Wake DB. 1977. Tongue evolution in the lungless salamanders, family plethodontidae. II. Function and evolutionary diversity. J. Morphol 153:39–80. [DOI] [PubMed] [Google Scholar]
- Losos JB 2011. Convergence, adaptation, and constraint. Evolution (N. Y). 65:1827–1840. [DOI] [PubMed] [Google Scholar]
- Maddin HC, and Sherratt E. 2014. Influence of fossoriality on inner ear morphology: Insights from caecilian amphibians. J. Anat 225:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins EP, and Hansen TF. 1997. Phylogenies and the comparative method: A general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat 149:646–667. [Google Scholar]
- Mason MJ 2004. Functional morphology of the middle ear in Chlorotalpa golden moles (Mammalia, Chrysochloridae): Predictions from three models. J. Morphol 261:162–174. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, and Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees Pp. 1–8 in Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov 2010. New Orleans, LA. [Google Scholar]
- Montgomery JC, Coombs S, and Baker CF. 2001. The mechanosensory lateral line system of the hypogean form of Astyanax fasciatus. Environ. Biol. Fishes 62:87–96. [Google Scholar]
- Niemiller ML, Higgs DM, and Soares D. 2013. Evidence for hearing loss in amblyopsid cavefishes. Biol. Lett 9:20130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme D, Freckleton RP, Thomas G, Petzoldt T, Fritz S, Isaac N, and Pearse W. 2013. The caper package : Comparative Analyses of Phlogenetics and Evolution in R. R Dev. Core Team, 2011, doi: 1. [Google Scholar]
- Pagel M 1999. Inferring the historical patterns of biological evolution. Nature 401:877–884. [DOI] [PubMed] [Google Scholar]
- Parra-Olea G, and Wake DB. 2001. Extreme morphological and ecological homoplasy in tropical salamanders. Proc. Natl. Acad. Sci 98:7888–7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff C, Martin T, and Ruf I. 2015. Bony labyrinth morphometry indicates locomotor adaptations in the squirrel-related clade (Rodentia, Mammalia). Proc. R. Soc. B Biol. Sci 282:20150744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper AN 1970. Auditory capacities of the Mexican blind cave fish (Astyanax jordani) and its eyed ancestor (Astyanax mexicanus). Anim. Behav 18:552–562. [Google Scholar]
- Poulson TL 1963. Cave adaptation in Amblyopsid fishes. Am. Midl. Nat 70:257–290. [Google Scholar]
- R Core Team. 2017. R: A Language and Environment for Statistical Computing. Vienna, Austria. [Google Scholar]
- Reiss JO, and Eisthen HL. 2008. Comparative Anatomy and Physiology of Chemical Senses in Amphibians Pp. 43–64 in Thewissen JGM and Nummela S, eds. Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates. University of California Press. [Google Scholar]
- Revell LJ 2012. phytools: An R package for phylogenetic comparative biology (and other things ). Methods Ecol. Evol 3:217–223. [Google Scholar]
- Rohlf FJ 2001. Comparative methods for the analysis of continuous variables: Geometric interpretations. Evolution (N. Y). 55:2143–2160. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ, and Slice D. 1990. Extensions of the Procrustes Method for the Optimal Superimposition of Landmarks. Syst. Zool 39:40–59. [Google Scholar]
- Rose CS 1994. The developmental morphology of salamander skulls Pp. 1684–1781 in Heatwole H and Davies M, eds. Amphibian Biology, Vol. 5 Osteology; Australia. [Google Scholar]
- Roth G 1987. Visual Behavior in Salamanders. Springer-Verlag, Heidelberg. [Google Scholar]
- Roth G, Dicke U, and Nishikawa KC. 1992. How do ontogeny, morphology, and physiology of sensory systems constrain and direct the evolution of amphibians. Am. Nat 139:S105–S124. [Google Scholar]
- Schluter D 2000. The Ecology of Adaptive Radiation. Oxford University Press, New York. [Google Scholar]
- Schulz-Mirbach T, Ladich F, Riesch R, and Plath M. 2010. Otolith morphology and hearing abilities in cave- and surface-dwelling ecotypes of the Atlantic molly, Poecilia mexicana (Teleostei: Poeciliidae). Hear. Res 267:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJB 1968. Hearing in terrestrial urodeles: a vibration-sensitive mechanism in the ear. J. Exp. Biol 48:191–205. [DOI] [PubMed] [Google Scholar]
- Spoor F 2003. The semicircular canal system and locomotor behaviour, with special reference to hominin evolution. Cour. Forsch.-Inst. Senckenb 243:93–104. [Google Scholar]
- Spoor F, Bajpai S, Kumar K, Hussain ST, and Thewissen JGM. 2002. Vestibular evidence for the evolution of aquatic behaviour in early cetaceans. Nature 417:163–166. [DOI] [PubMed] [Google Scholar]
- Spoor F, Garland T, Krovitz G, Ryan TM, Silcox MT, and Walker A. 2007. The primate semicircular canal system and locomotion. Proc. Natl. Acad. Sci 104:10808–10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A 2014. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyke T 1990. Morphological differences in neuromasts of the blind cave fish Astyanax hubbsi and the sighted river fish Astyanax mexicanus. Brain. Behav. Evol 35:23–30. [DOI] [PubMed] [Google Scholar]
- Thurow GA, and Gould HJ. 1977. Sound Production in a Caecilian. Herpetologica 33:234–237. [Google Scholar]
- Toerien MJ 1963. The sound conducting systems of lizards without tympanic membranes. Evolution (N. Y) 17:540–547. [Google Scholar]
- Varatharasan N, Croll RP, and Franz-Odendaal TA. 2009. Taste bud development and patterning in sighted and blind morphs of Astyanax mexicanus. Dev. Dyn 238:3056–3064. [DOI] [PubMed] [Google Scholar]
- Wainwright PC, and Reilly SM (eds). 1994. Ecological Morphology: Integrative Organismal Biology. University of Chicago Press, Chicago, IL. [Google Scholar]
- Wake DB 1966. Comparative Osteology and Evolution of the Lungless Salamanders, Family Plethodontidae. Mem. South. Calif. Acad. Sci 4:130 pp. [Google Scholar]
- Wake DB, and Deban SM. 2000. Terrestrial Feeding in Salamanders Pp. 95–116 in Schwenk K, ed. Feeding: Form, function and evolution in tetrapod vertebrates. Academic Press, San Diego CA. [Google Scholar]
- Wake DB, and Hanken J. 1996. Direct development in the lungless salamanders: what are the consequences for developmental biology, evolution and phylogenesis? Int. J. Dev. Biol 40:859–869. [PubMed] [Google Scholar]
- Wake MH 1985. The comparative morphology and evolution of the eyes of caecilians (Amphibia, Gymnophiona). Zoomorphology 105:277–295. [Google Scholar]
- Wever EG 1978. Sound transmission in the salamander ear. Proc. Natl. Acad. Sci. U. S. A 75:529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman HH 1994. Evolution of facultative paedomorphosis in salamanders. Q. Rev. Biol 69:205–221. [Google Scholar]
- Wilbur HM, and Collins JP. 1973. Ecological aspects of amphibian metamorphosis. Science (80-. ). 182:1305–1314. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Byerly MS, Jackman WR, and Jeffery WR. 2009. Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Dev. Biol 330:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, and Hullar TE. 2007. Relationship of semicircular canal size to vestibular-nerve afferent sensitivity in mammals. J. Neurophysiol 98:3197–3205. [DOI] [PubMed] [Google Scholar]
- Yi H, and Norell MA. 2015. The burrowing origin of modern snakes. Sci. Adv 1:e1500743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Gorički Š, Soares D, and Jeffery WR. 2010. Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr. Biol 20:1631–16366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.