Abstract
The kynurenine pathway (KP) plays a critical role in generating cellular energy in the form of nicotinamide adenine dinucleotide (NAD+). Because energy requirements are substantially increased during an immune response, the KP is a key regulator of the immune system. Perhaps more importantly in the context of psychiatry, many kynurenines are neuroactive, modulating neuroplasticity and/or exerting neurotoxic effects in part through their effects on NMDA receptor signaling and glutamatergic neurotransmission. As such, it is not surprising that the kynurenines have been implicated in psychiatric illness in the context of inflammation. However, because of their neuromodulatory properties, the kynurenines are not just additional members of a list of inflammatory mediators linked with psychiatric illness, but in preclinical studies have been shown to be necessary components of the behavioral analogues of depression and schizophrenia-like cognitive deficits. Further, as the title suggests, the KP is regulated by, and in turn regulates multiple other physiological systems that are commonly disrupted in psychiatric disorders, including endocrine, metabolic, and hormonal systems. This review provides a broad overview of the mechanistic pathways through which the kynurenines interact with these systems, thus impacting emotion, cognition, pain, metabolic function, and aging, and in so doing potentially increasing the risk of developing psychiatric disorders. Novel therapeutic approaches targeting the KP are discussed. Moreover, electroconvulsive therapy, ketamine, physical exercise, and certain non-steroidal anti-inflammatories have been shown to alter kynurenine metabolism, raising the possibility that kynurenine metabolites may have utility as treatment response or therapeutic monitoring biomarkers.
1. OVERVIEW
The kynurenine pathway (KP) is best known for its link with inflammatory disease. However, many of its metabolites, collectively termed “kynurenines”, are physiologically active and not only play a key immunoregulatory role, but affect diverse physiological systems. Unlike other reviews which have generally focused on one particular sphere of influence or disorder, this review provides an overview of the impact of the kynurenines on several physiological pathways and symptom domains. In so doing, it highlights the importance of the KP to the field of psychiatry, not only in terms of enhancing our mechanistic understanding of pathophysiology, but with respect to current and future therapeutic interventions. While the focus on psychiatric illness is intended to be wide-ranging, by necessity, the depression and schizophrenia literature is emphasized since minimal research has been performed on other psychiatric disorders.
2. BASIC Biochemistry and neurophysiology
Tryptophan (TRP) is converted into several bioactive molecules, the best known of which is serotonin. However, only a small percentage of TRP is metabolized into serotonin. More than 95% of TRP is converted into kynurenine (KYN) and its breakdown products, culminating in the generation of nicotinamide adenine dinucleotide (NAD+), an important cellular energy source1 (figure 1). The kynurenines are produced in many different tissues, notably in the liver by the enzyme, tryptophan dioxygenase (TDO)2, and cells of the immune system and brain, where indoleamine 2,3-dioxygenase (IDO) catalyzes the conversion of TRP to KYN.
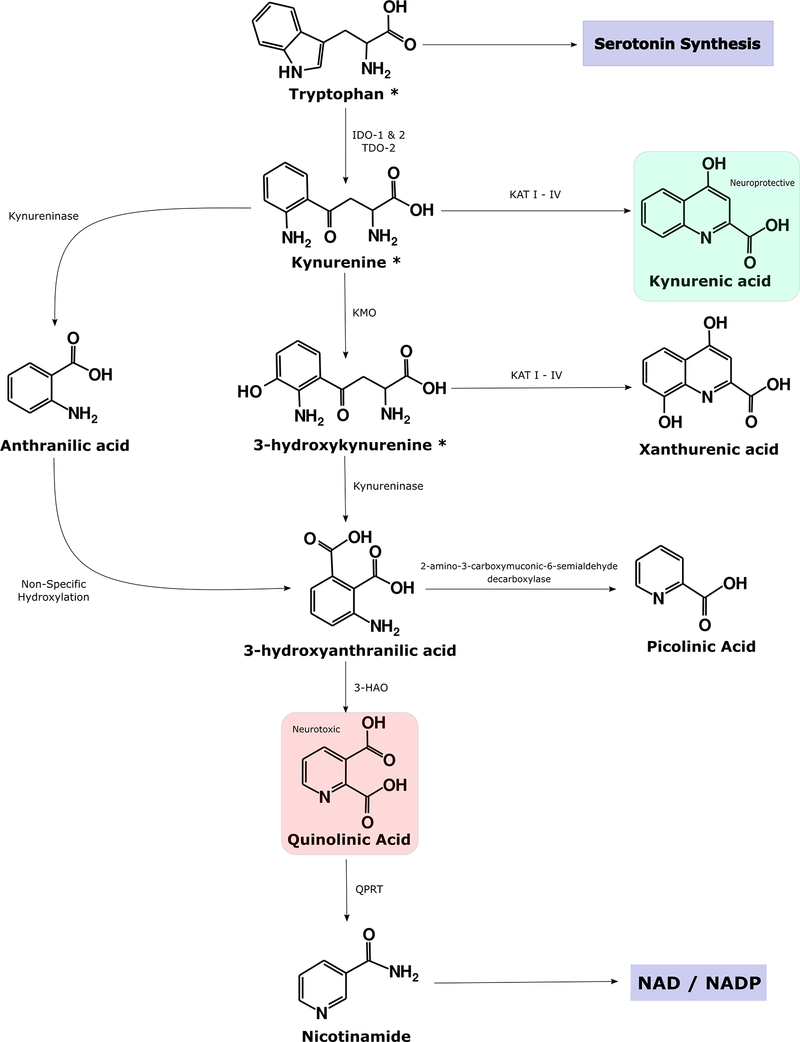
Figure 1: Simplified Illustration of the Kynurenine Pathway.
Tryptophan (TRP) is predominantly converted into kynurenine (KYN) by the indoleamine 2,3-dioxygenase (IDO) isozymes and tryptophan dioxygenase (TDO). IDO-1 is expressed in various immune cells throughout the body, notably dendritic cells, monocytes, and macrophages. Less is known about the more recently discovered IDO-2 enzyme although it is more selectively expressed in dendritic cells, liver, kidney, and the brain180 and it does not appear to have a significant effect on peripheral kynurenine concentration181. This review focuses on IDO-1 (hereafter IDO). TDO-2 is an alternative nomenclature for TDO. KYN can be metabolized into kynurenic acid (KYNA), which is usually considered to be neuroprotective, by the KAT isozymes. Alternatively, it may be converted into anthranilic acid by kynureninase or 3-hydroxykynurenine (3HK) by kynurenine mono-oxygenase (KMO). Metabolism down the latter pathway increases under inflammatory conditions25, 30. 3HK is a free radical generator while quinolinic acid (QA) is a known neurotoxin and gliotoxin. Thus, metabolites in this pathway are usually considered to be neurotoxic. QA is the endogenous source of nicotinamide and nicotinamide adenine dinucleotide (NAD+).
The KP has two main branches. Under physiological conditions, KYN is preferentially converted into 3-hydroxykynurenine (3HK) and then 3-hydroxyanthranilic acid (3HAA), quinolinic acid (QA), and ultimately NAD+3. The remaining balance of KYN is converted into kynurenic acid (KynA) by the kynurenine aminotransferase (KAT) enzymes. This review will focus primarily on two metabolites that have received the most attention in the literature, KynA and QA.
KynA, which is generally considered to be neuroprotective4, competitively inhibits ionotropic glutamate receptors at high concentrations but preferentially attenuates activity at the glycine co-agonist site of the NMDA receptor5. The administration of even low concentrations of KynA (nanomolar range) into the brain is capable of decreasing glutamate levels by 30–40%6. KynA is also generally believed to act as a negative allosteric modulator at the α7-nicotinic receptor7. More recently, KynA has also been shown to act as an agonist at an orphan G-protein-coupled receptor (GPR35)8, modulating cAMP production and inhibiting the N-type Ca2+ channels of sympathetic neurons and astrocytes9, ultimately leading to a suppression of several inflammatory pathways10. KynA also regulates the immune response through its agonistic effects on the aryl hydrocarbon receptor (AhR), a transcription factor involved in the metabolism of xenobiotics11. AhR signaling appears to play an important role in terminating cytokine release in several cell types including macrophages11.
QA is an NMDA receptor agonist that can additionally inhibit reuptake of glutamate by astrocytes, leading to excitotoxicity12. QA exerts neurotoxic effects via at least nine different mechanisms (reviewed in detail elsewhere13) including the generation of reactive oxygen species, disruption of the blood brain barrier, destabilization of the cellular cytoskeleton, promotion of tau phosphorylation, and disruption of autophagy. QA also potentiates the inflammatory response by inducing the production of proinflammatory mediators in astrocytes14. Theoretically, QA may also activate microglia through their NMDA receptors, a pathway that has been previously shown to trigger neuronal cell death15 – although see reference16.
TRP, KYN, and 3HK can be transported across the blood brain barrier. In fact, 60%−80% of KYN in the brain is thought to be of exogenous origin under physiological conditions, being actively transported into the brain by the large neutral amino acid transporter17, 18. These figures may approach 100% following systemic immune activation, although if inflammation is limited to the brain, KYN can be produced centrally from TRP18. In contrast, the classical view is that KynA and QA poorly cross the blood brain barrier17, and thus brain KynA and QA are thought to be derived from brain KYN. Nevertheless, the issue of whether molecules pass through the blood brain barrier is complex, and the traditional view that peripheral QA and KynA do not enter the brain may be too simplistic. For instance, in gerbils, a significant correlation between serum and QA was measured in several brain regions (r≥0.53) and ∼50%−70% of subcutaneously-infused radiolabeled QA was eventually detected in the brain and CSF, respectively19. Potentially as a result of inflammatory episodes20, blood brain barrier integrity is putatively decreased in several psychiatric disorders21. Thus, the penetrance of circulating QA (and potentially other metabolites) into the CNS may depend on the degree of underlying inflammation. In this regard, the significant correlations between circulating and CSF levels of QA in HIV patients22 and Hepatitis C patients treated with interferon α23 (r=0.65 and r=0.72, respectively), is potentially noteworthy.
3. INFLAMMATION
Proinflammatory cytokines shunt the metabolism of TRP towards KYN by upregulating IDO expression. IDO is activated primarily via the interferon gamma receptor (IFN-γR)24, but also through IFN-γR-independent pathways, notably the toll-like receptor 4 (TLR4)25 and the synergistic activation of TLR4, the interleukin 1 beta receptor (IL-1R)26, 27, and the tumor necrosis factor alpha receptor (TNFR)27, 28. CNS concentrations of KYN also appear to increase via an IDO-independent mechanism, i.e. an increase in the transport of KYN into the brain during systemic inflammation18. From an evolutionary perspective, activated immune cells need large amounts of energy to fight off an infection and hence QA is needed to produce adequate amounts of NAD+29. Thus, one would expect increased metabolism down the QA branch of the KP under inflammatory conditions, and this is indeed borne out in the preclinical literature. KMO but not KAT-II expression is increased in rat brain after systemic LPS administration25, 30, and in human hippocampal progenitor cells, IL-1β was shown to increase KMO transcripts26.
3.1. Relevance to Psychiatry
3.1.1. Mood Disorders
There is increasingly persuasive evidence that inflammation plays a pathophysiological role in some cases of depression, with reports of: (a) depression-associated elevations of circulating pro-inflammatory cytokines31, (b) differential expression of inflammation-related genes in monocytes or peripheral blood mononuclear cells (PBMCs) of subjects with mood disorders32, 33, (c) depressive episodes occurring in ∼30% of patients receiving immune-stimulating treatments34, (d) the development of depressive symptoms in some healthy participants given low-dose endotoxin35, (e) prospective studies demonstrating a positive association between the concentrations of inflammatory mediators at baseline and the development of de novo cases of major depressive disorder (MDD)36, (f) an epidemiological association between depression and diseases with an autoimmune or inflammatory component37, 38, (g) higher numbers and/or activation of microglial cells measured in vivo with positron emission tomography39, and (h) an increased number and/or activation of microglia/macrophages in depressed suicides at postmortem40.
The effect of inflammatory mediators on neural function – particularly dopaminergic signaling – has been well characterized41. What then does the KP contribute to our understanding of the pathophysiology of mood disorders? The short answer is that the kynurenines exert pathological and behavioral effects that extend beyond those of inflammatory mediators. Firstly, preclinical work has shown that the pro-depressive effects of lipopolysaccharide (LPS) can be blocked by the genetic deletion or pharmacological inhibition of IDO without affecting cytokine levels, indicating that IDO activity within the brain is necessary for the manifestation of depression-like behavior in mice42. Similarly, KMO knockout mice are protected from the pro-depressive effects of LPS, demonstrating that – at least in mice - neurotoxic kynurenine metabolites are key mediators of inflammation-induced depression-like behaviors43.
Clearly, similar kinds of experimental manipulations are not possible to perform in humans. Nevertheless, ∼30% of patients receiving immune-activating treatments (e.g. IFNα) for hepatitis C or cancer develop depressive episodes that co-occur with KP activation44. IFNα treatment-induced depression also coincides with increase in the ratio of KYN to KynA, indicating a role for activation of the QA pathway in the genesis of depression45. More recently, depressive symptoms at 6–9 months post initiation of IFNα-treatment were found to be associated with higher plasma concentrations of QA at these timepoints46.
There is also a growing literature showing cross-sectional reductions in peripheral concentrations of KynA or the ratio of KynA to QA in patients with MDD versus comparison controls47–57. Moreover, my colleagues and I have demonstrated that the balance between the KynA and QA pathway metabolism is associated with brain structure and function, particularly the hippocampus. This relative anatomical specificity is consistent with the high density of NMDA receptors in the hippocampus, and preclinical data demonstrating a stronger shift towards the neurotoxic KP in the hippocampus relative to other brain regions58. Specifically, we found that lower “neuroprotective indices”, i.e. the ratios of KynA/3HK and KynA/QA, were associated with reduced hippocampal volumes in individuals with MDD48, 59, concussed athletes with symptoms of depression60, and individuals with bipolar disorder (BD)61. Moreover, a follow-up study showed an inverse relationship between KynA/3HK and left hippocampal activity during the recall of autobiographical memories in depressed participants, indicating a link between kynurenine metabolism and hippocampal function62.
While most research has focused on the balance between the production of KynA and QA, it is also possible that neurotoxicity may result from a failure to adequately metabolize QA once it is formed. In human neurons, quinolinate phosphoribosyltransferase (QPRT), the enzyme that metabolizes QA into nicotinic acid mononucleotide and ultimately NAD+, becomes saturated in the presence of high extracellular concentrations (300–500 nM) of QA13, 63. Interestingly, this is a similar threshold above which QA has been shown to become neurotoxic in vitro13. Conceivably, when QA is produced at a faster rate than its conversion to NAD+, QA accumulates at toxic concentrations and it is thus possible that increasing the expression or activity of QPRT may have therapeutic benefits in the context of inflammatory illness64. Other factors may also be at play. Mitochondrial dysfunction has been implicated in several psychiatric disorders65, 66 (see section 6) and when cellular bioenergetics are compromised, KMO activity is likely increased to compensate for this deficit by producing more NAD+ from QA67. This process may become counter-productive during an inflammatory response. Activated immune cells have been reported to shift their metabolism away from NAD-dependent oxidative-phosphorylation to glycolysis and lactic acid production in order to rapidly generate energy, including in hypoxic tissue microenvironments associated with infections68, 69. Theoretically, this metabolic shift may also result in an accumulation of QA.
How do KynA and QA impinge on neural function and behavior? As discussed above, KynA is an NMDA receptor antagonist while QA is an NMDA receptor agonist. While QA has a similar potency to glutamate at the NMDA receptor, it remains in the synaptic cleft for a longer period of time due to less-efficient reuptake, and therefore its excitotoxic effects are stronger70. Thus, high enough concentrations of QA could contribute to excitotoxicity although QA (and KynA) are perhaps more likely to alter neuroplasticity through the NMDA receptor71–73 (figure 2). Consequently, the possibility has been raised that the competing actions of KynA and QA at the NMDA receptor may unify the inflammation and glutamate models of depression74. This hypothesis receives support from preclinical studies. Notably, Dantzer and colleagues demonstrated that LPS increases QA in the brain but that the pro-depressive effects of LPS could be blocked by low-dose ketamine without altering sickness behavior, inflammatory processes, or IDO activity75. Rather, the anti-depressant effects of ketamine were shown to be mediated by ketamine’s antagonistic effects at the NMDA receptor and the promotion of AMPA receptor-mediated glutamatergic neurotransmission. This result suggests that inflammation-induced activation of the NMDA receptor by QA is a key mechanistic pathway through which inflammation exerts its depressogenic effects75.
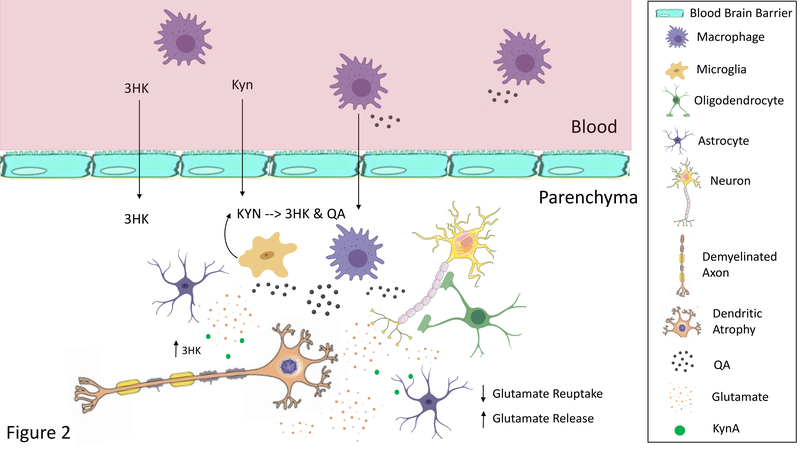
Figure 2: Heuristic Model of the Pathological Effects of Neurotoxic Kynurenines.
Under inflammatory conditions circulating macrophages produce more kynurenine (KYN), 3-hydroxykynurenine (3HK), and quinolinic acid (QA). 3HK can cross the blood brain barrier damaging neuronal cells through the production of free radicals. KYN also crosses the blood brain barrier where it is preferentially metabolized into 3HK and QA by microglia. Further, macrophages can infiltrate the brain parenchyma, where they are estimated to produce 32 times more QA than resident microglia182. Thus, although QA is usually found in low nanomolar concentrations in the human brain and CSF, a significant increase in QA levels to micromolar concentrations likely occurs in patients with neuroinflammation183. QA may contribute to the excitotoxic processes caused by the deficient glutamate reuptake (and paradoxical release) by dysfunctional astrocytes. Dendritic atrophy and remodeling also likely occurs altering functional connectivity. Further, 3HK and QA may damage oligodendrocytes leading to white matter abnormalities. Oligodendrocytes are highly sensitive to inflammation and reductions in their numbers or density are one of the most prominent findings in mood disorders at postmortem40. Note that other inflammatory mediators also play an important role in these neuropathological processes. Only the kynurenines are shown in the figure for clarity.
3.1.2. Suicide
Suicidal behavior cuts across diagnostic boundaries and has been strongly linked with inflammation40, 76. As in the case of depression, activation of the KP has been reported in suicide attempters77, 78 but arguably the most salient finding is the relative increase in QA in suicide attempters versus controls. Increased concentrations of QA and decreased concentrations of KynA were found in both the CSF and plasma of suicide attempters79 and these abnormalities were sustained for two years after the suicide attempt51. Consistent with these data, increased numbers of microglia immunoreactive for QA were reported within sub-regions of the anterior cingulate cortex in depressed suicides at postmortem80. Brundin and colleagues81 additionally showed that suicide attempters had reduced levels of the neuroprotective metabolite, picolinic acid (PIC)82, and a decreased PIC/QA ratio in both the CSF and plasma. 2-amino-3-carboxymuconic-6-semialdehyde (ACMS) is spontaneously degraded to form QA but can also be converted to PIC by the amino-β-carboxymuconate-semialdehyde-decarboxylase (ACMSD) enzyme (figure 1). Thus, the aforementioned decrease in PIC raises the possibility of a deficiency in ACMSD activity in the context of suicidal behavior81. More generally, the collective data suggest that aberrant QA-mediated glutamate signaling driven by altered enzymatic activity at several different points in the KP may contribute to suicidality.
3.1.3. Psychosis and Schizophrenia
The classic dopamine model viewed psychosis and schizophrenia as disorders of excess striatal dopaminergic neurotransmission resulting from increased dopamine synthesis and presynaptic dopamine release83. This model was modified over time to take the psychotomimetic effects of non-competitive NMDA receptor antagonists like phencyclidine into account. That is, the upstream cause of the striatal hyperdopaminergia was postulated to be NMDA receptor hypofunction on GABAergic interneurons that in turn disinhibited excitatory projections onto midbrain dopamine neurons84. Since KynA is the only known endogenous NMDA receptor antagonist, psychosis and schizophrenia were postulated to be caused by the effect of elevated KynA on glutamatergic and ultimately, dopaminergic neurotransmission85–87 (figure 3). Consistent with this model, experimentally increasing central levels of KynA has been demonstrated to increase the burst-firing of midbrain dopaminergic neurons in animal models88, perhaps explaining why increased KynA has been reported in the postmortem brain89, and the CSF90, 91 of patients with schizophrenia. Similar results have been reported in BD – but the elevation in KynA was reported to be limited to patients with a history of psychosis86, 87.
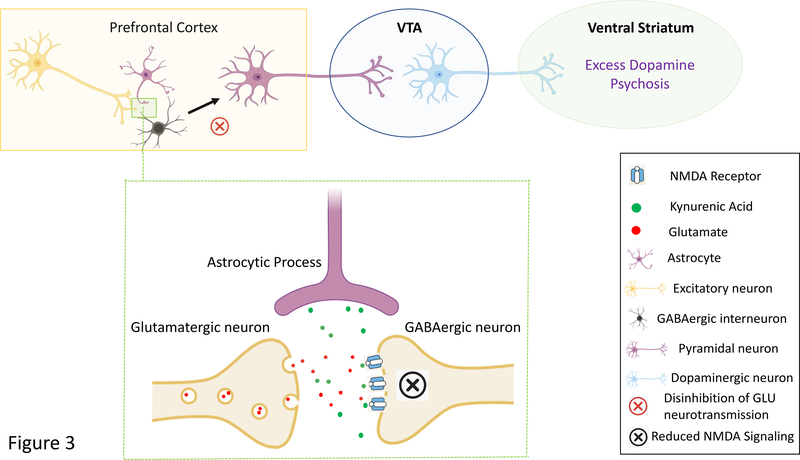
Figure 3: Simplified Model of the Putative Psychosis-Inducing Effects of Kynurenic Acid.
Cortical GABAergic interneurons normally exert an inhibitory tone on glutamatergic pyramidal neurons that project to the ventral tegmental area (VTA) and modulate dopaminergic neurotransmission. Excess kynurenic acid production by astrocytes may cause NMDA receptor hypofunction on cortical GABA interneurons leading to reductions in GABAergic neurotransmission and the disinhibition of cortical glutamate projections. Theoretically, this abnormally increased glutamatergic activity causes overactivation of the mesolimbic DA pathway and the excessive release of dopamine in the ventral striatum, ultimately leading to the development of psychosis.
The reported increase in KynA in schizophrenia and psychosis may originate from inflammation-driven increases in KYN92. As in the case of mood disorders, there is evidence that inflammation may play a mechanistic role in schizophrenia93 and PET studies have reported increased microglial activation in patients with schizophrenia94. The problem with this model is that inflammation should theoretically cause an increase in the production of QA and NAD+ at the expense of KynA as is seen in depression. In fact, my colleagues and I did indeed find a decrease in serum KynA in patients with schizoaffective disorder, psychotic BD, and MDD relative to healthy controls50. Further, it was the patients with affective psychosis who showed the largest decrease in KynA. Similarly, Myint et al. had previously reported a reduction in KynA together with an increase in 3HK in the plasma of the patients with schizophrenia compared with healthy controls, and this decrease in the KynA/3HK ratio significantly increased relative to baseline after 6 weeks of treatment95. Further research is needed to test whether schizophrenia and psychosis-associated reductions in KynA are limited to the periphery, and if so, why peripheral and central KynA concentrations appear to be in opposite directions to each other (as opposed to simply uncorrelated).
Conceivably, some as yet uncharacterized brain-specific immune process is elevating central but not peripheral concentrations of KynA. Another possibility is that psychosis-related increases in KynA could theoretically be related factors other than inflammation. Cortisol is known to activate TDO96 and adrenergic activity may increase IDO activity through IFNγ97. Hong and colleagues showed that salivary KynA concentrations increased immediately after a stress-inducing cognitive task in schizophrenia although there was no difference in KynA concentrations between participants with schizophrenia and healthy controls over time97. However, stress has also been reported to increase the productions of neurotoxic kynurenines: self-reported stressful life events were positively correlated with 3HK, but not KynA concentrations in elderly subjects at risk of dementia98. Genetic variants may also influence KP metabolism. For instance, a functional SNP in the KMO gene that decreases KMO activity was associated with elevated KynA concentrations in the CSF that were specific to BD patients with psychosis87. Conceivably, biotypes of psychotic disorders that differ in genetics, the degree of underlying inflammation or other etiological factors such as stress-induced HPA axis dysfunction may also differ in KynA. Differential changes in KynA in the acute versus the recovery phase of illness may also affect findings50. Larger sample sizes are required to disentangle these putative effects.
3.1.4. Cognition
As in the case of schizophrenia, there are two competing narratives as to the role of the KP in cognition. On the one hand, neurotoxic metabolites such as QA have been shown to damage the brain and lead to cognitive deficits. For instance, Heisler and O’Connor demonstrated that mice deficient in IDO or KMO were protected from endotoxin-induced deficits in novel object recognition, a task dependent on normal functioning of the hippocampus99. This result is potentially consistent with our report of an association between lower serum KynA to 3HK concentrations and greater hippocampal activity (indicating more effortful recall) during an autobiographical memory task in MDD subjects62. The link between “cold” cognitive deficits and KP metabolism has not been widely studied in patients with mood disorders although there is some equivocal evidence to suggest that lower neuroprotective ratios (e.g. KynA/3HK) are associated with greater cognitive impairment in BD100.
On the other hand, elevated KynA has been postulated to underlie the cognitive deficits associated with schizophrenia. In preclinical studies, elevation of brain KynA through the genetic or pharmacological knockdown of KMO, has been shown to induce cognitive abnormalities that bear some resemblance to those observed in schizophrenia, including prepulse inhibition of the acoustic startle reflex101, learning and memory102, and cognitive flexibility103. Conversely, reducing brain KynA through pharmacological inhibition or genetic knockdown of KAT-II, improves cognitive function73, 102. Consistent with these preclinical data, subjects with schizophrenia (and to a lesser extent healthy controls) who were homozygous for a single nucleotide polymorphism (SNP) in the KMO gene that reduces KMO activity, had lower scores than their counterparts on a range of neuropsychological tests104.
3.1.5. Pain
While pro-inflammatory cytokines may alone be pain-inducing, preclinical work has demonstrated that IDO activation also contributes to inflammation-related pain and associated depression-like behavior. With respect to behavior, the hippocampus appears to play a central role: Kim et al. demonstrated that the induction of chronic pain in rats with Freund’s adjuvant induced depressive-like behavior together with IDO upregulation in the hippocampus via IL-6 signaling105. Both the nociceptive and depression-like behavior was attenuated in IDO gene knockouts or by pharmacological inhibition of IDO105. Consistent with these data, virus-induced IDO expression in the lungs and lymphoid tissue was reported to induce pain hypersensitivity. This effect was absent in IDO knockouts but could be recapitulated by intravenously-administered KYN106.
Because glutamate signaling through the NMDA receptor can produce hypersensitivity of the spinal sensory neurons thereby increasing pain sensation, the NMDA receptor is a key therapeutic target in pain disorders107. An example is ketamine which exerts analgesic effects by blocking NMDA receptors at sub-anesthetic doses107. Thus, it is not surprising that neurotoxic KP metabolites have been implicated in pain-related behavior. In a preclinical study, spared nerve injury was shown to increase KMO expression and QA concentration (but decrease KynA) in the contralateral hippocampus via an IL-1-dependent mechanism108. Pharmacological inhibition of KMO eliminated the depression-like behavior but not the mechanical allodynia likely because inhibition of NMDA receptors in the spinal cord is required for analgesia108.
The increase in metabolism down the neurotoxic branch of the KP in both mood disorders and pain conditions has led to the hypothesis that the activation of NMDA receptors by QA may be a common pathophysiological mechanism that explains their high level of comorbidity109. Chronic pain is a significant risk factor for depression: 30–60% of individuals with clinical pain have comorbid depression110. Conversely, idiopathic pain is common in MDD. For instance, 75% of subjects in the Sequenced Treatment Alternative to Relieve Depression (STAR*D) study reported pain111.
4. Infectious Disease, Immune Tolerance and Immunosuppression
Activation of the KP is an important negative feedback loop that short-circuits the inflammatory response112, 113. This process initially was hypothesized to be predominantly mediated through T-cells which are sensitive to reductions in TRP, an important energy source for the T-cells. However, the reduction in TRP is now generally viewed through the lens of increased substrate utilization to provide the required energy (via the conversion of QA to NAD+) for the immune response114. Rather, increases in KYN, 3HK, and 3‐HAA suppress and induce the apoptosis of Th1 and natural killer cells (NKC) as well as down-regulate the expression of CD8+ receptors, impairing their cytotoxic activity112 (figure 4). In addition, TGFβ production and consequently regulatory T-cell (Treg) numbers are increased, leading to a tolerogenic environment112.
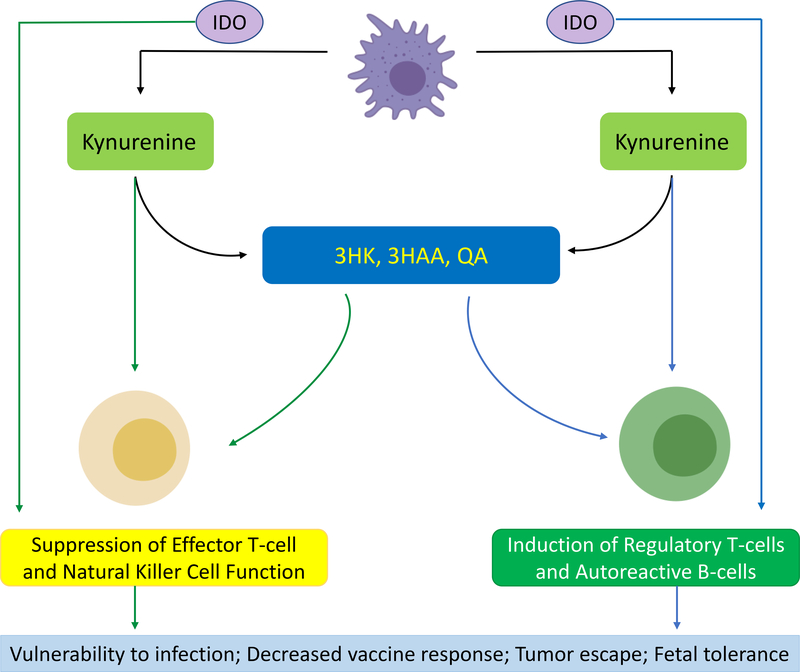
Figure 4: The Immune-Modulatory Effects of Kynurenine Pathway Activation.
The kynurenine pathway may explain the counter-intuitive phenomenon of co-occurring inflammation and immunosuppression in depression. Under inflammatory conditions, IDO is upregulated, catalyzing the conversion of tryptophan (TRP) to kynurenine (KYN). KYN is preferentially metabolized down the quinolinic acid (QA) pathway into 3-hydroxykynurenine (3HK), 3-hydroxyanthranilic acid (3HAA), and QA. IDO, KYN and its metabolites exert a variety of immunosuppressive effects, including downregulation of NKC receptors and induction of NKC death184; induction of lymphocyte cell-cycle arrest and apoptosis, and downregulation of the T-cell receptor (TCR)185 (readers left, green arrows). They also promote a tolerogenic environment by facilitating the differentiation of naïve T-cells into regulatory T-cells (Treg)186 and the development of autoreactive B-cells187. This suppression of adaptive immune function has clinical consequences, including increased vulnerability to infectious disease, deficient vaccine-induced immunogenicity, and tumor escape120–122 (reader’s right, blue arrows).
Immune suppression is not only needed to resolve the inflammatory response but also in pregnancy where the allogenic fetus has to be protected from the mother’s immune response. Munn and Mellor initially demonstrated that pregnant mice given an IDO inhibitor rejected semiallogenic but not syngenic fetuses, suggesting that the maternal T-cells response against paternal MHC antigens may occur through an IDO-dependent mechanism115. Nevertheless, the applicability of Munn and Mellor’s model to humans remains controversial since blocking IDO does not completely inhibit T-cell function in vitro116 and TRP is required by the fetus for normal development116, 117. Some researchers have concluded that the putative depletion of TRP is either highly localized or that increased utilization of TRP has a more modest effect on immunotolerance in the context of pregnancy via the production of immunosuppressive KP metabolites114, 116.
4.1. Relevance to Psychiatry
If chronic activation of the KP leads to a state of immune tolerance that increases susceptibility to infections and malignancies then one would expect to observe this phenomenon in a subgroup of individuals with psychiatric disorders. At least in the case of mood disorders, impairments of adaptive immunity are well-characterized. These impairments take the form of reduced numbers, gene expression and mitogen-stimulated proliferation of lymphocytes33, reduced NKC numbers or cytotoxicity118, 119, and increased numbers of Treg cells118. The public health consequences of compromised cellular immunity are significant. Depressed and stressed individuals are more vulnerable to infection120, show reduced immunogenic responses to vaccines, acutely121, and may even lose humoral immunity more rapidly in cases where the onset of depression occurs many years after vaccination122. In a similar vein, depression also predicts greater mortality in cancer patients123.
Given its putative role in fetal tolerance, it is plausible that the kynurenines play a role in the development of postpartum depression. Conceivably, increases in KYN, 3HK, and 3‐HAA, which suppress Th1 and NKC112 may protect against fetal rejection at the expense of increasing the risk of depression. In women without depressive symptoms, TRP levels were reported to increase within two days of birth whereas this increase was absent in women with mild increases in depressive symptoms (“postpartum blues”)124. Partly consistent with this result, an increase in KYN as well as amino acids that compete with TRP for transport across the blood brain barrier was found in the first three days after birth in a general population sample125. This finding was postulated to reflect a decrease in the brain availability of TRP, which was in turn hypothesized to result in “postpartum blues”125. Bergink and colleagues observed a shift towards the QA pathway in the first two months postpartum but this phenomenon occurred in both women with mood disorders and healthy controls126. However, a recent study reported increased serum concentrations of 3HK and 3HK/KYN (putatively reflecting increased KMO activity) in women with postpartum depression versus those without postpartum depression after cesarean section127.
5. METABOLISM AND METABOLIC DISEASE
Immune cells are important regulators of energy metabolism in adipose tissue modulating lipid storage, glucose homeostasis, and the expenditure of energy128. Macrophages in particular accumulate and shift to a pro-inflammatory state as fat levels increase, theoretically leading to the preferential production of neurotoxic KP metabolites. Emerging preclinical evidence suggests that the kynurenines could play a mechanistic role in the evolution of metabolic dysfunction. A high-fat diet increased IDO expression in the plasma, adipose tissue, and muscle of mice, however, IDO knockout animals were protected from the obesogenic, inflammatory, and insulin resistance-inducing effects of the diet129. This effect appeared to be dependent on the maintenance of a healthy microbiome through the production of sufficient indole derivatives from TRP129. Another mechanistic pathway conceivably relates to the balance between 3HK/QA and KynA production. An increase in 3HK and its metabolite xanthurenic acid (XA) is thought to interfere with insulin production and activity, and therefore with glucose homeostasis130. Ruas and colleagues also point out that the sustained activation of NMDA receptors on pancreatic β-cells may impair the function of these cells, raising the possibility that excess QA is a driver of metabolic disease128. KynA in contrast, serves as an endogenous measure of nutrient availability in Caenorhabditis elegans with KynA levels increasing during fasting, thus forming a homeostatic feedback loop that allows feeding behavior to be altered131. In a recent study, KynA was also reported to increase energy expenditure and promote an anti-inflammatory environment in adipose tissue thereby ameliorating the negative effects of a high-fat diet on weight and adiposity in a mouse-model of obesity132. Genetic deletion of GPR35 caused a progressive increase in obesity and loss of glucose tolerance suggesting that the effect of KynA was mediated by activation of GPR35 and downstream crosstalk with a variety of cellular pathways involved in thermogenesis, lipid metabolism and immunoregulation132.
5.1. Relevance to Psychiatry
MDD is highly correlated with obesity and the two disorders have an overlapping biology133, 134. Similarly, there is a bidirectional relationship between metabolic diseases such as type II diabetes (T2D) and depression. Individuals with T2D are more likely to become depressed while a history of depression increases the risk of T2D135. One component of the shared biology is chronic low-grade inflammation, with the effect of pro-inflammatory cytokines the major focus of attention. However, given the preclinical studies linking kynurenines with metabolic function and the robust evidence for decreased KynA in depression47–57, it is conceivable that dysregulation of the KP is part of this shared biology and may be a fruitful avenue for future research. Extant evidence suggests that patients with T2D display the same pattern of reduced KynA versus QA-pathway metabolism as in depression with reports of reduced expression of KAT1 and KAT2 in skeletal muscle136 and increases in QA over the course of a longitudinal study increasing the risk of T2D137. Similarly, pro-inflammatory macrophages in the adipose tissue of obese women were shifted towards the neurotoxic branch of the KP and KMO expression was positively correlated with hemoglobin A1c levels138.
6. AGING AND NEURODEGENERATIVE DISEASE
The biological mechanisms underlying aging are not yet well understood. One factor that consistently has been shown to correlate with age is mitochondrial activity. The age-associated reprograming of mitochondrial gene expression is thought to be a leading cause of cellular senescence, which is characterized by a state of permanent cell-cycle arrest and the acquisition of a proinflammatory phenotype named the senescence-associated secretory phenotype (SASP)139. Mitochondrial dysfunction is underpinned by many factors but at least two relate to the KP. Neurotoxic KP metabolites may directly compromise mitochondrial function - a mammalian cell-line overexpressing human KMO showed increased production of 3HK and reactive oxygen species, leading to impaired mitochondrial capacity67. Another pathway may involve an age-associated decrease in PGC-1 activity140. PGC-1 is a cofactor for the peroxisome proliferator-activated receptor (PPAR) family of transcription factors that regulate mitochondrial biogenesis and function. At least in muscle, activation of the PGC-1-PPARα/δ pathway increases circulating concentrations of the neuroprotective metabolite, KYNA141. PGC-1 expression is decreased in several neurodegenerative disorders140 and consistent with this putative decrease in PPAR signaling, there is evidence for increased metabolism down the neurotoxic branch of the KP in Alzheimer’s Disease (AD) and Parkinson’s Disease (PD)142, 143. For instance, plasma concentrations of KynA are decreased in AD144 and postmortem studies have reported decreases in KynA in the frontal cortex, putamen, and substantia nigra of PD patients145. Further, genetic variants of ACMSD, which converts 3HAA (a precursor of QA) to PIC, are replicated risk factors for PD, providing additional evidence that excess QA may cause PD-associated excitotoxicity146.
Consistent with these clinical data, preclinical studies have demonstrated that KMO inhibition ameliorates degeneration in models of neurodegenerative diseases such as AD147, PD148, and Huntington’s disease149. Further, knockdown of kynureninase (the enzyme that converts 3HK to 3HAA) not only increased the lifespan of C. elegans, but also delayed age-dependent paralysis in worms expressing amyloid β150 (a model of neurodegenerative disease).
6.1. Relevance to Psychiatry
Markers of cellular senescence including increased mitochondrial DNA copy number and SASP gene expression signatures have been reported in a range of psychiatric disorders151. Similarly, imaging studies are suggestive of reduced ATP availability in the brain in mood and psychotic disorders during high energy demand, potentially coinciding with a shift in energy production away from oxidative phosphorylation due to mitochondrial dysfunction65, 66. Consistent with these data, psychiatric disorders are associated with an increased risk of developing diseases of aging, including neurodegenerative disorders such as AD152 and PD153. The underlying mechanisms linking accelerated cellular aging and neurodegeneration with psychiatric illness are still unclear but conceivably may partly involve dysregulation of the balance between neurotoxic and neuroprotective kynurenine metabolites in the context of mitochondrial dysfunction and inflammation.
7. SEX HORMONES
Males and females differ significantly in immune function. Females for example, respond more strongly to infections and vaccines, and are significantly more likely than males to suffer from inflammatory and autoimmune diseases154. These sex differences likely relate to the close interaction between hormones and the immune system. For instance, estrogen, androgen, and progesterone receptors are expressed on most immune cells, and many genes of the innate immune system contain estrogen response elements155, 156. Interestingly, estrogen was reported almost 60 years ago to exert an inhibitory effect on the KAT enzymes, leading to a decrease in KynA157. This finding was recently extended by an in vitro study which reported that estradiol disulfate is a strong inhibitor of the KAT-I and KAT-II enzyme isoforms158. However, the effects of estrogen are likely dose and context-dependent since treatment of rhesus monkeys with estradiol also resulted in a significant reduction in the expression of KMO in the raphe159. Progesterone is generally considered to have neuroprotective properties although as in the case of estrogen, its effects on the immune system may be complex. In vitro treatment of human macrophages with a supra-physiological dose of progesterone was shown to attenuate IFNγ-induced activation of the KP, decrease QA, and increase KynA concentrations160.
7.1. Relevance to Psychiatry
The lifetime prevalence of MDD in women is twice that of men161. This statistic has traditionally been attributed to psychosocial or hormonal factors. However, given evidence of sex differences in immune function154 and the effects of estrogen on the KAT and KMO enzymes, the higher rate of depression in women versus men may also be related to alterations in KP metabolism that increase vulnerability to depression in females162. Consistent with these in vitro and preclinical studies, my colleagues and I reported that in both depressed subjects and healthy controls, women had reduced serum concentrations of KynA relative to males, and further that women taking oral contraceptives (OC) had lower levels of KynA relative to women not taking OC162. In contrast to depression, the prevalence of schizophrenia in males is ∼2.5 times greater than in females163. If, as has been hypothesized, elevated brain KynA is a pathophysiological mechanism underlying schizophrenia, then estrogen-associated decreases in KynA may be protective, conceivably partly explaining why females are at lower risk for schizophrenia-related disorders.
8. THERAPEUTIC IMPLICATIONS
Dysregulation of the KP is a candidate pathogenic mechanism for psychiatric disorders. Preclinical work has demonstrated that KP metabolites play a causal role in the manifestation of the behavioral analogues of depression and schizophrenia-like cognitive deficits. Cross-sectional studies of clinical populations are generally consistent with the animal literature although cannot demonstrate causality. Thus, in theory, modulation of KP metabolism holds therapeutic potential. Enzymes in the KP are druggable. IDO inhibitors are in various stages of development for the treatment of cancer164 and clinical trials are underway with drugs that target various points in the metabolic pathway (see below). Further, because the KP is a nexus for diverse physiological processes – immunological, endocrine, metabolic, hormonal – that are dysregulated across multiple psychiatric disorders, unlike anti-inflammatory medications, modulation of the KP could theoretically benefit multiple biotypes of mood and psychotic disorders.
8.1. Novel Medications
Given evidence for increased production of neurotoxic metabolites at the expense of KynA in mood disorders, inhibiting KMO or augmenting activity of the KAT enzymes could conceivably have anti-depressant and neuroprotective effects. KMO inhibitors have shown some efficacy in preclinical models of neuropathic pain165, HD, and AD147. However, because of the central regulatory role of the KP, an excess reduction in QA together with an abnormal elevation in KynA may be an undesirable outcome of therapeutic approaches involving inhibition of KMO. Further, because KMO inhibition may drive-up circulating KYN concentrations which can then be converted into QA in the CNS (see below), sufficient brain penetrance is likely needed for therapeutic efficacy. As a result of these challenges, additional approaches are in development.
First-in-human clinical trials for MDD are currently underway with the KynA analogue, 4-chlorokynurenine or AV-101 (NCT02484456 and NCT03078322). Like KynA, AV-101 is a selective antagonist at the glycine-binding site of the NMDA receptor. Manufactured by VistaGen, AV-101 recently received Fast Track Designation by the FDA for the treatment of MDD166. Another potential intervention is predicated on the mechanics of KYN transport across the blood brain barrier. KYN is transported across the blood brain barrier by the large amino acid transporter (LAT1) where, under inflammatory conditions, it is preferentially metabolized into neurotoxic metabolites. Therefore, reducing the access of circulating KYN to the brain will in theory have therapeutic effects. Dantzer and colleagues have recently demonstrated that leucine treatment is a feasible method of competitively blocking the LAT1 to prevent exogenous KYN from entering into the brain167. Moreover, they reported that leucine blocked LPS-induced depression-like behavior in mice without affecting levels of inflammation or sickness behavior167. A phase 2 clinical trial to test the anti-depressant effects of leucine in individuals with MDD is in progress (NCT03079297).
8.2. Extant Treatments
While compounds targeting the KP hold promise as novel treatments, the literature suggests that several current treatments for depression alter KP metabolism. A recent study showed that prior to treatment with electroconvulsive therapy (ECT), depressed patients had significantly lower levels of serum KynA/QA than controls which increased significantly after three ECT treatments performed over two weeks56. Similarly, in another study, increases in KynA and KynA/3HK were observed in depressed patients receiving twice-weekly ECT for an average of three weeks168.
In mice, Dantzer and colleagues demonstrated that ketamine was able to abrogate LPS-induced depressive behavior likely by blocking QA-mediated activation of NMDA receptors75. The link between ketamine and the KP has also been investigated in clinical populations. Ketamine treatment was shown to acutely decrease circulating KYN and the KYN/TRP ratio with a greater magnitude of reduction in treatment responders versus non-responders169. Partially consistent with this result, a non-significant reduction in KYN and KYN/TRP also was reported in ketamine responders versus non-responders at two hours and 24 hours post initial infusion53. While these two studies did not report significant ketamine-induced changes in downstream KP metabolites, in another study, ketamine was shown to increase KynA concentrations from 24 hours after the first infusion until at least two weeks post initiation of treatment170. Further, the elevation in KynA was greater in treatment responders versus non-responders. Metabolites in the QA pathway were not measured but the increase in the ratio of KynA to KYN suggested that ketamine may shunt metabolism of kynurenine towards KynA170.
Physical exercise protects against the future onset of depression171 and can be an efficacious treatment for MDD172. In a landmark study, skeletal muscle exercise was shown to protect against stress-induced depression in mice by increasing expression of the KAT enzymes in muscle, thereby reducing KYN concentrations in the periphery and by implication, KYN levels and neurotoxic metabolites in the brain141. Further, human volunteers displayed increased KAT gene expression in muscle after a three-week endurance exercise program141. In a follow-up study, endurance exercise was found to increased plasma concentrations of KynA as well as KynA/QA and well-conditioned subjects appeared to show adaptive increases in KAT expression in the muscle tissue173. Nevertheless, discrepant effects have been reported in several other studies174 likely because of the diversity of the trial designs, types of exercise and the length of the regimens employed. At least three ongoing clinical trials of exercise propose alterations in the KP as secondary outcome measures (NCT03539835; NCT02765568, and NCT03324152).
Given a pathoetiological role for inflammatory processes in mood disorders, there is emerging interest in employing anti-inflammatory medications to treat a subgroup of depressed patients with systemic inflammation. Among the medications that have shown therapeutic potential are cyclooxygenase (COX) inhibitors – particularly COX-1 inhibitors175. We recently completed the first clinical trial of aspirin for the treatment of bipolar depression. Patients receiving low-dose aspirin, which preferentially inhibits COX-1, were significantly more likely to respond to treatment than placebo-treated subjects, suggesting that aspirin may be an efficacious adjunctive treatment for bipolar depression176. Interestingly, the COX-1 inhibitors, indometacin and diclofenac increased brain levels of KynA in the rat177, 178 and KynA levels in both the plasma and the hippocampus were shown to be significantly increased one hour after a single dose of ibuprofen179. Whether the impact of COX inhibition on KynA is independent of its general anti-inflammatory effects remains to be determined but either way these data raise the possibility that changes in KP metabolism may contribute to the putative anti-depressant effects of COX inhibitors.
9. SUMMARY AND FUTURE DIRECTIONS
Given its central role in immune function and energy metabolism, any dysregulation of KP metabolism is likely to “reverberate through the system”, ultimately impacting neural function and leading to neuropsychiatric disorders and associated medical comorbidity. While preclinical studies have demonstrated that the KP plays a causal role in psychiatric illness that is independent of inflammatory processes per se, one challenge for the field is to extend this work to humans by developing methods to experimentally manipulate KP activity with the necessary degree of specificity to draw causal conclusions. Beyond the immune system, the KP also interacts with other physiological systems and clarifying how inflammation, stress, pain, metabolic, and hormonal dysfunction overlap or differ from each other in their downstream effects on the KP may help to advance the field. Third, the role of genetic or epigenetic factors in influencing the activity of other key KP enzymes such as ACMSD, QPRT, and the KATs, is ripe for exploration. Finally, in addition to clinical trials of novel therapeutic interventions, an assessment of whether the kynurenines possess clinical utility as biomarkers of response to existing treatments is indicated. Since dysregulation of the KP may weaken the capacity for neuroplasticity, the kynurenines may also hold promise as prognostic biomarkers for mood disorders and schizophrenia.
ACKNOWLEDGMENTS
Support was received from the National Institute of General Medical Sciences (P20GM121312), the National Institute of Mental Health (R21MH113871), and the Laureate Institute for Brain Research (LIBR).
Footnotes
DECLARATION OF INTEREST
No conflicts of interest are declared.
REFERENCES
- 1.Bender DA. Effects of a dietary excess of leucine on the metabolism of tryptophan in the rat: a mechanism for the pellagragenic action of leucine. Br J Nutr 1983; 50(1): 25–32. [DOI] [PubMed] [Google Scholar]
- 2.Kanai M, Funakoshi H, Takahashi H, Hayakawa T, Mizuno S, Matsumoto K et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain 2009; 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017; 357(6349). [DOI] [PubMed] [Google Scholar]
- 4.Foster AC, Vezzani A, French ED, Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett 1984; 48(3): 273–278. [DOI] [PubMed] [Google Scholar]
- 5.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem 1989; 52(4): 1319–1328. [DOI] [PubMed] [Google Scholar]
- 6.Carpenedo R, Pittaluga A, Cozzi A, Attucci S, Galli A, Raiteri M et al. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur J Neurosci 2001; 13(11): 2141–2147. [DOI] [PubMed] [Google Scholar]
- 7.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. The Journal of neuroscience : the official journal of the Society for Neuroscience 2001; 21(19): 7463–7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem 2006; 281(31): 22021–22028. [DOI] [PubMed] [Google Scholar]
- 9.Guo J, Williams DJ, Puhl HL 3rd, Ikeda SR. Inhibition of N-type calcium channels by activation of GPR35, an orphan receptor, heterologously expressed in rat sympathetic neurons. J Pharmacol Exp Ther 2008; 324(1): 342–351. [DOI] [PubMed] [Google Scholar]
- 10.Wirthgen E, Hoeflich A, Rebl A, Gunther J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front Immunol 2017; 8: 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011; 478(7368): 197–203. [DOI] [PubMed] [Google Scholar]
- 12.Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol 1981; 72(4): 411–412. [DOI] [PubMed] [Google Scholar]
- 13.Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. Febs J 2012; 279(8): 1356–1365. [DOI] [PubMed] [Google Scholar]
- 14.Guillemin GJ, Croitoru-Lamoury J, Dormont D, Armati PJ, Brew BJ. Quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia 2003; 41(4): 371–381. [DOI] [PubMed] [Google Scholar]
- 15.Kaindl AM, Degos V, Peineau S, Gouadon E, Chhor V, Loron G et al. Activation of microglial N-methyl-D-aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol 2012; 72(4): 536–549. [DOI] [PubMed] [Google Scholar]
- 16.Garrison AM, Parrott JM, Tunon A, Delgado J, Redus L, O’Connor JC. Kynurenine pathway metabolic balance influences microglia activity: Targeting kynurenine monooxygenase to dampen neuroinflammation. Psychoneuroendocrinology 2018; 94: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. Journal of neurochemistry 1991; 56(6): 2007–2017. [DOI] [PubMed] [Google Scholar]
- 18.Kita T, Morrison PF, Heyes MP, Markey SP. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. J Neurochem 2002; 82(2): 258–268. [DOI] [PubMed] [Google Scholar]
- 19.Heyes MP, Morrison PF. Quantification of local de novo synthesis versus blood contributions to quinolinic acid concentrations in brain and systemic tissues. J Neurochem 1997; 68(1): 280–288. [DOI] [PubMed] [Google Scholar]
- 20.Skaper SD. Impact of Inflammation on the Blood-Neural Barrier and Blood-Nerve Interface: From Review to Therapeutic Preview. Int Rev Neurobiol 2017; 137: 29–45. [DOI] [PubMed] [Google Scholar]
- 21.Pollak TA, Drndarski S, Stone JM, David AS, McGuire P, Abbott NJ. The blood-brain barrier in psychosis. Lancet Psychiatry 2018; 5(1): 79–92. [DOI] [PubMed] [Google Scholar]
- 22.Heyes MP, Brew BJ, Saito K, Quearry BJ, Price RW, Lee K et al. Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and beta 2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. J Neuroimmunol 1992; 40(1): 71–80. [DOI] [PubMed] [Google Scholar]
- 23.Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 2010; 15(4): 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida R, Imanishi J, Oku T, Kishida T, Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci U S A 1981; 78(1): 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connor TJ, Starr N, O’Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma? Neurosci Lett 2008; 441(1): 29–34. [DOI] [PubMed] [Google Scholar]
- 26.Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM et al. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2012; 37(4): 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babcock TA, Carlin JM. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine 2000; 12(6): 588–594. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci 2009; 29(13): 4200–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol 2003; 81(4): 247–265. [DOI] [PubMed] [Google Scholar]
- 30.Molteni R, Macchi F, Zecchillo C, Dell’agli M, Colombo E, Calabrese F et al. Modulation of the inflammatory response in rats chronically treated with the antidepressant agomelatine. Eur Neuropsychopharmacol 2013; 23(11): 1645–1655. [DOI] [PubMed] [Google Scholar]
- 31.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71(2): 171–186. [DOI] [PubMed] [Google Scholar]
- 32.Savitz J, Frank MB, Victor T, Bebak M, Marino JH, Bellgowan PS et al. Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain, behavior, and immunity 2013; 31: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leday GGR, Vertes PE, Richardson S, Greene JR, Regan T, Khan S et al. Replicable and Coupled Changes in Innate and Adaptive Immune Gene Expression in Two Case-Control Studies of Blood Microarrays in Major Depressive Disorder. Biol Psychiatry 2018; 83(1): 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capuron L, Hauser P, Hinze-Selch D, Miller AH, Neveu PJ. Treatment of cytokine-induced depression. Brain, behavior, and immunity 2002; 16(5): 575–580. [DOI] [PubMed] [Google Scholar]
- 35.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun 2010; 24(4): 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasco JA, Nicholson GC, Williams LJ, Jacka FN, Henry MJ, Kotowicz MA et al. Association of high-sensitivity C-reactive protein with de novo major depression. The British journal of psychiatry : the journal of mental science 2010; 197(5): 372–377. [DOI] [PubMed] [Google Scholar]
- 37.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 2007; 22(7): 613–626. [DOI] [PubMed] [Google Scholar]
- 38.Dalton EJ, Heinrichs RW. Depression in multiple sclerosis: a quantitative review of the evidence. Neuropsychology 2005; 19(2): 152–158. [DOI] [PubMed] [Google Scholar]
- 39.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015; 72(3): 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry 2016; 6(11): e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016; 16(1): 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawson MA, Parrott JM, McCusker RH, Dantzer R, Kelley KW, O’Connor JC. Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2,3-dioxygenase-dependent depression-like behaviors. J Neuroinflammation 2013; 10: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry 2016; 6(10): e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Molecular psychiatry 2002; 7(5): 468–473. [DOI] [PubMed] [Google Scholar]
- 45.Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry 2005; 10(6): 538–544. [DOI] [PubMed] [Google Scholar]
- 46.Baranyi A, Meinitzer A, Breitenecker RJ, Amouzadeh-Ghadikolai O, Stauber R, Rothenhausler HB. Quinolinic Acid Responses during Interferon-alpha-Induced Depressive Symptomatology in Patients with Chronic Hepatitis C Infection - A Novel Aspect for Depression and Inflammatory Hypothesis. PLoS One 2015; 10(9): e0137022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 2007; 98(1–2): 143–151. [DOI] [PubMed] [Google Scholar]
- 48.Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS et al. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 2015; 40(2): 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PS, Victor TA et al. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun 2015; 46: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wurfel BE, Drevets WC, Bliss SA, McMillin JR, Suzuki H, Ford BN et al. Serum kynurenic acid is reduced in affective psychosis. Transl Psychiatry 2017; 7(5): e1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-d-aspartate receptor in depression and suicidality. Brain Behav Immun 2015; 43: 110–117. [DOI] [PubMed] [Google Scholar]
- 52.Doolin K, Allers KA, Pleiner S, Liesener A, Farrell C, Tozzi L et al. Altered tryptophan catabolite concentrations in major depressive disorder and associated changes in hippocampal subfield volumes. Psychoneuroendocrinology 2018; 95: 8–17. [DOI] [PubMed] [Google Scholar]
- 53.Allen AP, Naughton M, Dowling J, Walsh A, O’Shea R, Shorten G et al. Kynurenine pathway metabolism and the neurobiology of treatment-resistant depression: Comparison of multiple ketamine infusions and electroconvulsive therapy. J Psychiatr Res 2018; 100: 24–32. [DOI] [PubMed] [Google Scholar]
- 54.Birner A, Platzer M, Bengesser SA, Dalkner N, Fellendorf FT, Queissner R et al. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS One 2017; 12(2): e0172699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poletti S, Myint AM, Schuetze G, Bollettini I, Mazza E, Grillitsch D et al. Kynurenine pathway and white matter microstructure in bipolar disorder. Eur Arch Psychiatry Clin Neurosci 2018; 268(2): 157–168. [DOI] [PubMed] [Google Scholar]
- 56.Schwieler L, Samuelsson M, Frye MA, Bhat M, Schuppe-Koistinen I, Jungholm O et al. Electroconvulsive therapy suppresses the neurotoxic branch of the kynurenine pathway in treatment-resistant depressed patients. J Neuroinflammation 2016; 13(1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maes M, Galecki P, Verkerk R, Rief W. Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2,3-dioxygenase and lowered kynurenine aminotransferase activity. Neuro endocrinology letters 2011; 32(3): 264–273. [PubMed] [Google Scholar]
- 58.Parrott JM, Redus L, O’Connor JC. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J Neuroinflammation 2016; 13(1): 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA et al. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun 2016; 53: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meier TB, Savitz J, Singh R, Teague TK, Bellgowan PS. Smaller Dentate Gyrus and CA2 and CA3 Volumes Are Associated with Kynurenine Metabolites in Collegiate Football Athletes. J Neurotrauma 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Savitz J, Dantzer R, Wurfel BE, Victor TA, Ford BN, Bodurka J et al. Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology 2015; 52: 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young KD, Drevets WC, Dantzer R, Teague TK, Bodurka J, Savitz J. Kynurenine pathway metabolites are associated with hippocampal activity during autobiographical memory recall in patients with depression. Brain Behav Immun 2016; 56: 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One 2009; 4(7): e6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones SP, Franco NF, Varney B, Sundaram G, Brown DA, de Bie J et al. Expression of the Kynurenine Pathway in Human Peripheral Blood Mononuclear Cells: Implications for Inflammatory and Neurodegenerative Disease. PLoS One 2015; 10(6): e0131389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry 2004; 61(5): 450–458. [DOI] [PubMed] [Google Scholar]
- 66.Du F, Yuksel C, Chouinard VA, Huynh P, Ryan K, Cohen BM et al. Abnormalities in High-Energy Phosphate Metabolism in First-Episode Bipolar Disorder Measured Using (31)P-Magnetic Resonance Spectroscopy. Biol Psychiatry 2018; 84(11): 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castellano-Gonzalez G, Jacobs KR, Don E, Cole NJ, Adams S, Lim CK et al. Kynurenine 3-Monooxygenase Activity in Human Primary Neurons and Effect on Cellular Bioenergetics Identifies New Neurotoxic Mechanisms. Neurotox Res 2019. [DOI] [PubMed] [Google Scholar]
- 68.Saha S, Shalova IN, Biswas SK. Metabolic regulation of macrophage phenotype and function. Immunol Rev 2017; 280(1): 102–111. [DOI] [PubMed] [Google Scholar]
- 69.Palsson-McDermott EM, O’Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays 2013; 35(11): 965–973. [DOI] [PubMed] [Google Scholar]
- 70.Foster AC, Miller LP, Oldendorf WH, Schwarcz R. Studies on the disposition of quinolinic acid after intracerebral or systemic administration in the rat. Exp Neurol 1984; 84(2): 428–440. [DOI] [PubMed] [Google Scholar]
- 71.Latif-Hernandez A, Shah D, Ahmed T, Lo AC, Callaerts-Vegh Z, Van der Linden A et al. Quinolinic acid injection in mouse medial prefrontal cortex affects reversal learning abilities, cortical connectivity and hippocampal synaptic plasticity. Sci Rep 2016; 6: 36489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forrest CM, McNair K, Pisar M, Khalil OS, Darlington LG, Stone TW. Altered hippocampal plasticity by prenatal kynurenine administration, kynurenine-3-monoxygenase (KMO) deletion or galantamine. Neuroscience 2015; 310: 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu HQ et al. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2010; 35(8): 1734–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller AH. Conceptual confluence: the kynurenine pathway as a common target for ketamine and the convergence of the inflammation and glutamate hypotheses of depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2013; 38(9): 1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2013; 38(9): 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brundin L, Bryleva EY, Thirtamara Rajamani K. Role of Inflammation in Suicide: From Mechanisms to Treatment. Neuropsychopharmacology 2017; 42(1): 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bradley KA, Case JA, Khan O, Ricart T, Hanna A, Alonso CM et al. The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res 2015; 227(2–3): 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okusaga O, Duncan E, Langenberg P, Brundin L, Fuchs D, Groer MW et al. Combined Toxoplasma gondii seropositivity and high blood kynurenine--Linked with nonfatal suicidal self-directed violence in patients with schizophrenia. J Psychiatr Res 2016; 72: 74–81. [DOI] [PubMed] [Google Scholar]
- 79.Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 2013; 38(5): 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? Journal of neuroinflammation 2011; 8: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brundin L, Sellgren CM, Lim CK, Grit J, Palsson E, Landen M et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl Psychiatry 2016; 6(8): e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grant RS, Coggan SE, Smythe GA. The physiological action of picolinic Acid in the human brain. Int J Tryptophan Res 2009; 2: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol 2015; 29(2): 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995; 52(12): 998–1007. [DOI] [PubMed] [Google Scholar]
- 85.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nature reviews Neuroscience 2012; 13(7): 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Olsson SK, Sellgren C, Engberg G, Landen M, Erhardt S. Cerebrospinal fluid kynurenic acid is associated with manic and psychotic features in patients with bipolar I disorder. Bipolar disorders 2012; 14(7): 719–726. [DOI] [PubMed] [Google Scholar]
- 87.Lavebratt C, Olsson S, Backlund L, Frisen L, Sellgren C, Priebe L et al. The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Molecular psychiatry 2014; 19(3): 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nilsson LK, Linderholm KR, Erhardt S. Subchronic treatment with kynurenine and probenecid: effects on prepulse inhibition and firing of midbrain dopamine neurons. J Neural Transm (Vienna) 2006; 113(5): 557–571. [DOI] [PubMed] [Google Scholar]
- 89.Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 2001; 50(7): 521–530. [DOI] [PubMed] [Google Scholar]
- 90.Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 2001; 313(1–2): 96–98. [DOI] [PubMed] [Google Scholar]
- 91.Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull 2012; 38(3): 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plitman E, Iwata Y, Caravaggio F, Nakajima S, Chung JK, Gerretsen P et al. Kynurenic Acid in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr Bull 2017; 43(4): 764–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull 2013; 39(6): 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR et al. Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [(11)C]PBR28 PET Brain Imaging Study. Am J Psychiatry 2016; 173(1): 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Myint AM, Schwarz MJ, Verkerk R, Mueller HH, Zach J, Scharpe S et al. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naive and medication-free schizophrenic patients. Brain, behavior, and immunity 2011; 25(8): 1576–1581. [DOI] [PubMed] [Google Scholar]
- 96.Salter M, Pogson CI. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects of glucocorticoids and experimental diabetes. Biochem J 1985; 229(2): 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiappelli J, Rowland LM, Notarangelo FM, Wijtenburg SA, Thomas MAR, Pocivavsek A et al. Salivary kynurenic acid response to psychological stress: inverse relationship to cortical glutamate in schizophrenia. Neuropsychopharmacology 2018; 43(8): 1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuster OC, Laptinskaya D, Fissler P, Schnack C, Zugel M, Nold V et al. Novel Blood-Based Biomarkers of Cognition, Stress, and Physical or Cognitive Training in Older Adults at Risk of Dementia: Preliminary Evidence for a Role of BDNF, Irisin, and the Kynurenine Pathway. J Alzheimers Dis 2017; 59(3): 1097–1111. [DOI] [PubMed] [Google Scholar]
- 99.Heisler JM, O’Connor JC. Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav Immun 2015; 50: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Platzer M, Dalkner N, Fellendorf FT, Birner A, Bengesser SA, Queissner R et al. Tryptophan breakdown and cognition in bipolar disorder. Psychoneuroendocrinology 2017; 81: 144–150. [DOI] [PubMed] [Google Scholar]
- 101.Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry 2004; 56(4): 255–260. [DOI] [PubMed] [Google Scholar]
- 102.Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2011; 36(11): 2357–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alexander KS, Wu HQ, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) 2012; 220(3): 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wonodi I, McMahon RP, Krishna N, Mitchell BD, Liu J, Glassman M et al. Influence of kynurenine 3-monooxygenase (KMO) gene polymorphism on cognitive function in schizophrenia. Schizophr Res 2014; 160(1–3): 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim H, Chen L, Lim G, Sung B, Wang S, McCabe MF et al. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. The Journal of clinical investigation 2012; 122(8): 2940–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang L, Ou R, Rabelo de Souza G, Cunha TM, Lemos H, Mohamed E et al. Virus Infections Incite Pain Hypersensitivity by Inducing Indoleamine 2,3 Dioxygenase. PLoS Pathog 2016; 12(5): e1005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bannister K, Kucharczyk M, Dickenson AH. Hopes for the Future of Pain Control. Pain Ther 2017; 6(2): 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laumet G, Zhou W, Dantzer R, Edralin JD, Huo X, Budac DP et al. Upregulation of neuronal kynurenine 3-monooxygenase mediates depression-like behavior in a mouse model of neuropathic pain. Brain Behav Immun 2017; 66: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev 2014; 66(1): 80–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arnow BA, Blasey CM, Lee J, Fireman B, Hunkeler EM, Dea R et al. Relationships among depression, chronic pain, chronic disabling pain, and medical costs. Psychiatr Serv 2009; 60(3): 344–350. [DOI] [PubMed] [Google Scholar]
- 111.Husain MM, Rush AJ, Trivedi MH, McClintock SM, Wisniewski SR, Davis L et al. Pain in depression: STAR*D study findings. J Psychosom Res 2007; 63(2): 113–122. [DOI] [PubMed] [Google Scholar]
- 112.Vecsei L, Szalardy L, Fulop F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov 2013; 12(1): 64–82. [DOI] [PubMed] [Google Scholar]
- 113.Badawy AA. Hypothesis kynurenic and quinolinic acids: The main players of the kynurenine pathway and opponents in inflammatory disease. Med Hypotheses 2018; 118: 129–138. [DOI] [PubMed] [Google Scholar]
- 114.Badawy AA, Namboodiri AM, Moffett JR. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin Sci (Lond) 2016; 130(15): 1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281(5380): 1191–1193. [DOI] [PubMed] [Google Scholar]
- 116.Durr S, Kindler V. Implication of indolamine 2,3 dioxygenase in the tolerance toward fetuses, tumors, and allografts. J Leukoc Biol 2013; 93(5): 681–687. [DOI] [PubMed] [Google Scholar]
- 117.Badawy AA. Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology 2017; 112(Pt B): 248–263. [DOI] [PubMed] [Google Scholar]
- 118.Suzuki H, Savitz J, Kent Teague T, Gandhapudi SK, Tan C, Misaki M et al. Altered populations of natural killer cells, cytotoxic T lymphocytes, and regulatory T cells in major depressive disorder: Association with sleep disturbance. Brain Behav Immun 2017; 66: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Irwin M, Lacher U, Caldwell C. Depression and reduced natural killer cytotoxicity: a longitudinal study of depressed patients and control subjects. Psychological medicine 1992; 22(4): 1045–1050. [DOI] [PubMed] [Google Scholar]
- 120.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med 1991; 325(9): 606–612. [DOI] [PubMed] [Google Scholar]
- 121.Irwin MR, Levin MJ, Laudenslager ML, Olmstead R, Lucko A, Lang N et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2013; 56(8): 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ford BN, Yolken RH, Dickerson FB, Teague TK, Irwin MR, Paulus MP et al. Reduced immunity to measles in adults with major depressive disorder. Psychol Med 2018: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer 2009; 115(22): 5349–5361. [DOI] [PubMed] [Google Scholar]
- 124.Kohl C, Walch T, Huber R, Kemmler G, Neurauter G, Fuchs D et al. Measurement of tryptophan, kynurenine and neopterin in women with and without postpartum blues. J Affect Disord 2005; 86(2–3): 135–142. [DOI] [PubMed] [Google Scholar]
- 125.Bailara KM, Henry C, Lestage J, Launay JM, Parrot F, Swendsen J et al. Decreased brain tryptophan availability as a partial determinant of post-partum blues. Psychoneuroendocrinology 2006; 31(3): 407–413. [DOI] [PubMed] [Google Scholar]
- 126.Veen C, Myint AM, Burgerhout KM, Schwarz MJ, Schutze G, Kushner SA et al. Tryptophan pathway alterations in the postpartum period and in acute postpartum psychosis and depression. J Affect Disord 2016; 189: 298–305. [DOI] [PubMed] [Google Scholar]
- 127.Wang SY, Duan KM, Tan XF, Yin JY, Mao XY, Zheng W et al. Genetic variants of the kynurenine-3-monooxygenase and postpartum depressive symptoms after cesarean section in Chinese women. J Affect Disord 2017; 215: 94–101. [DOI] [PubMed] [Google Scholar]
- 128.Dadvar S, Ferreira DMS, Cervenka I, Ruas JL. The weight of nutrients: kynurenine metabolites in obesity and exercise. J Intern Med 2018; 284(5): 519–533. [DOI] [PubMed] [Google Scholar]
- 129.Laurans L, Venteclef N, Haddad Y, Chajadine M, Alzaid F, Metghalchi S et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med 2018. [DOI] [PubMed] [Google Scholar]
- 130.Oxenkrug G Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol Neurobiol 2013; 48(2): 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lemieux GA, Cunningham KA, Lin L, Mayer F, Werb Z, Ashrafi K. Kynurenic acid is a nutritional cue that enables behavioral plasticity. Cell 2015; 160(1–2): 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Agudelo LZ, Ferreira DMS, Cervenka I, Bryzgalova G, Dadvar S, Jannig PR et al. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell metabolism 2018; 27(2): 378–392 e375. [DOI] [PubMed] [Google Scholar]
- 133.Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry 2018. [DOI] [PubMed] [Google Scholar]
- 134.Capuron L, Lasselin J, Castanon N. Role of Adiposity-Driven Inflammation in Depressive Morbidity. Neuropsychopharmacology 2017; 42(1): 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tabak AG, Akbaraly TN, Batty GD, Kivimaki M. Depression and type 2 diabetes: a causal association? Lancet Diabetes Endocrinol 2014; 2(3): 236–245. [DOI] [PubMed] [Google Scholar]
- 136.Mudry JM, Alm PS, Erhardt S, Goiny M, Fritz T, Caidahl K et al. Direct effects of exercise on kynurenine metabolism in people with normal glucose tolerance or type 2 diabetes. Diabetes Metab Res Rev 2016; 32(7): 754–761. [DOI] [PubMed] [Google Scholar]
- 137.Yu E, Papandreou C, Ruiz-Canela M, Guasch-Ferre M, Clish CB, Dennis C et al. Association of Tryptophan Metabolites with Incident Type 2 Diabetes in the PREDIMED Trial: A Case-Cohort Study. Clin Chem 2018; 64(8): 1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Favennec M, Hennart B, Caiazzo R, Leloire A, Yengo L, Verbanck M et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity (Silver Spring) 2015; 23(10): 2066–2074. [DOI] [PubMed] [Google Scholar]
- 139.Sun N, Youle RJ, Finkel T. The Mitochondrial Basis of Aging. Mol Cell 2016; 61(5): 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. Febs J 2015; 282(4): 647–672. [DOI] [PubMed] [Google Scholar]
- 141.Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014; 159(1): 33–45. [DOI] [PubMed] [Google Scholar]
- 142.Maddison DC, Giorgini F. The kynurenine pathway and neurodegenerative disease. Semin Cell Dev Biol 2015; 40: 134–141. [DOI] [PubMed] [Google Scholar]
- 143.Lim CK, Fernandez-Gomez FJ, Braidy N, Estrada C, Costa C, Costa S et al. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog Neurobiol 2017; 155: 76–95. [DOI] [PubMed] [Google Scholar]
- 144.Hartai Z, Juhasz A, Rimanoczy A, Janaky T, Donko T, Dux L et al. Decreased serum and red blood cell kynurenic acid levels in Alzheimer’s disease. Neurochem Int 2007; 50(2): 308–313. [DOI] [PubMed] [Google Scholar]
- 145.Ogawa T, Matson WR, Beal MF, Myers RH, Bird ED, Milbury P et al. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology 1992; 42(9): 1702–1706. [DOI] [PubMed] [Google Scholar]
- 146.Thirtamara-Rajamani K, Li P, Escobar Galvis ML, Labrie V, Brundin P, Brundin L. Is the Enzyme ACMSD a Novel Therapeutic Target in Parkinson’s Disease? J Parkinsons Dis 2017; 7(4): 577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 2011; 145(6): 863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gregoire L, Rassoulpour A, Guidetti P, Samadi P, Bedard PJ, Izzo E et al. Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behav Brain Res 2008; 186(2): 161–167. [DOI] [PubMed] [Google Scholar]
- 149.Campesan S, Green EW, Breda C, Sathyasaikumar KV, Muchowski PJ, Schwarcz R et al. The kynurenine pathway modulates neurodegeneration in a Drosophila model of Huntington’s disease. Curr Biol 2011; 21(11): 961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sutphin GL, Backer G, Sheehan S, Bean S, Corban C, Liu T et al. Caenorhabditis elegans orthologs of human genes differentially expressed with age are enriched for determinants of longevity. Aging Cell 2017; 16(4): 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cai N, Chang S, Li Y, Li Q, Hu J, Liang J et al. Molecular signatures of major depression. Curr Biol 2015; 25(9): 1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Herbert J, Lucassen PJ. Depression as a risk factor for Alzheimer’s disease: Genes, steroids, cytokines and neurogenesis - What do we need to know? Front Neuroendocrinol 2016; 41: 153–171. [DOI] [PubMed] [Google Scholar]
- 153.Gustafsson H, Nordstrom A, Nordstrom P. Depression and subsequent risk of Parkinson disease: A nationwide cohort study. Neurology 2015; 84(24): 2422–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16(10): 626–638. [DOI] [PubMed] [Google Scholar]
- 155.Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun 2009; 10(5): 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kadel S, Kovats S. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front Immunol 2018; 9: 1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mason M, Gullekson EH. Estrogen-enzyme interactions: Inhibition and protection of kynurenine transaminase by the sulfate esters of diethylstilbestrol, estradiol, and estrone. J Biol Chem 1960; 235: 1312–1316. [PubMed] [Google Scholar]
- 158.Jayawickrama GS, Nematollahi A, Sun G, Gorrell MD, Church WB. Inhibition of human kynurenine aminotransferase isozymes by estrogen and its derivatives. Sci Rep 2017; 7(1): 17559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reddy AP, Bethea CL. Preliminary array analysis reveals novel genes regulated by ovarian steroids in the monkey raphe region. Psychopharmacology (Berl) 2005; 180(1): 125–140. [DOI] [PubMed] [Google Scholar]
- 160.de Bie J, Lim CK, Guillemin GJ. Progesterone Alters Kynurenine Pathway Activation in IFN-gamma-Activated Macrophages - Relevance for Neuroinflammatory Diseases. Int J Tryptophan Res 2016; 9: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kessler RC. Epidemiology of women and depression. J Affect Disord 2003; 74(1): 5–13. [DOI] [PubMed] [Google Scholar]
- 162.Meier TB, Drevets WC, Teague TK, Wurfel BE, Mueller SC, Bodurka J et al. Kynurenic acid is reduced in females and oral contraceptive users: Implications for depression. Brain Behav Immun 2018; 67: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bao AM, Swaab DF. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist 2010; 16(5): 550–565. [DOI] [PubMed] [Google Scholar]
- 164.Moon YW, Hajjar J, Hwu P, Naing A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. Journal for immunotherapy of cancer 2015; 3: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rojewska E, Piotrowska A, Makuch W, Przewlocka B, Mika J. Pharmacological kynurenine 3-monooxygenase enzyme inhibition significantly reduces neuropathic pain in a rat model. Neuropharmacology 2016; 102: 80–91. [DOI] [PubMed] [Google Scholar]
- 166.Wilkinson ST, Sanacora G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Walker AK, Wing EE, Banks WA, Dantzer R. Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol Psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guloksuz S, Arts B, Walter S, Drukker M, Rodriguez L, Myint AM et al. The impact of electroconvulsive therapy on the tryptophan-kynurenine metabolic pathway. Brain Behav Immun 2015; 48: 48–52. [DOI] [PubMed] [Google Scholar]
- 169.Moaddel R, Shardell M, Khadeer M, Lovett J, Kadriu B, Ravichandran S et al. Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology (Berl) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H et al. Antidepressant effect of repeated ketamine administration on kynurenine pathway metabolites in patients with unipolar and bipolar depression. Brain Behav Immun 2018. [DOI] [PubMed] [Google Scholar]
- 171.Harvey SB, Overland S, Hatch SL, Wessely S, Mykletun A, Hotopf M. Exercise and the Prevention of Depression: Results of the HUNT Cohort Study. Am J Psychiatry 2018; 175(1): 28–36. [DOI] [PubMed] [Google Scholar]
- 172.Mura G, Moro MF, Patten SB, Carta MG. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr 2014; 19(6): 496–508. [DOI] [PubMed] [Google Scholar]
- 173.Schlittler M, Goiny M, Agudelo LZ, Venckunas T, Brazaitis M, Skurvydas A et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol 2016; 310(10): C836–840. [DOI] [PubMed] [Google Scholar]
- 174.Metcalfe AJ, Koliamitra C, Javelle F, Bloch W, Zimmer P. Acute and chronic effects of exercise on the kynurenine pathway in humans - A brief review and future perspectives. Physiol Behav 2018; 194: 583–587. [DOI] [PubMed] [Google Scholar]
- 175.Savitz J, Preskorn S, Teague TK, Drevets D, Yates W, Drevets W. Minocycline and aspirin in the treatment of bipolar depression: a protocol for a proof-of-concept, randomised, double-blind, placebo-controlled, 2×2 clinical trial. BMJ Open 2012; 2(1): e000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M et al. Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2×2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry 2018; 8(1): 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Edwards SR, Mather LE, Lin Y, Power I, Cousins MJ. Glutamate and kynurenate in the rat central nervous system following treatments with tail ischaemia or diclofenac. J Pharm Pharmacol 2000; 52(1): 59–66. [DOI] [PubMed] [Google Scholar]
- 178.Schwieler L, Erhardt S, Erhardt C, Engberg G. Prostaglandin-mediated control of rat brain kynurenic acid synthesis--opposite actions by COX-1 and COX-2 isoforms. J Neural Transm (Vienna) 2005; 112(7): 863–872. [DOI] [PubMed] [Google Scholar]
- 179.Maciejak P, Szyndler J, Turzynska D, Sobolewska A, Kolosowska K, Lehner M et al. The kynurenine pathway: a missing piece in the puzzle of valproate action? Neuroscience 2013; 234: 135–145. [DOI] [PubMed] [Google Scholar]
- 180.Fukunaga M, Yamamoto Y, Kawasoe M, Arioka Y, Murakami Y, Hoshi M et al. Studies on tissue and cellular distribution of indoleamine 2,3-dioxygenase 2: the absence of IDO1 upregulates IDO2 expression in the epididymis. J Histochem Cytochem 2012; 60(11): 854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Metz R, Smith C, DuHadaway JB, Chandler P, Baban B, Merlo LM et al. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int Immunol 2014; 26(7): 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Espey MG, Chernyshev ON, Reinhard JF Jr., Namboodiri MA, Colton CA. Activated human microglia produce the excitotoxin quinolinic acid. Neuroreport 1997; 8(2): 431–434. [DOI] [PubMed] [Google Scholar]
- 183.Stone TW. Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Progress in neurobiology 2001; 64(2): 185–218. [DOI] [PubMed] [Google Scholar]
- 184.Della Chiesa M, Carlomagno S, Frumento G, Balsamo M, Cantoni C, Conte R et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 2006; 108(13): 4118–4125. [DOI] [PubMed] [Google Scholar]
- 185.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 2005; 22(5): 633–642. [DOI] [PubMed] [Google Scholar]
- 186.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol 2009; 183(4): 2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Scott GN, DuHadaway J, Pigott E, Ridge N, Prendergast GC, Muller AJ et al. The immunoregulatory enzyme IDO paradoxically drives B cell-mediated autoimmunity. J Immunol 2009; 182(12): 7509–7517. [DOI] [PMC free article] [PubMed] [Google Scholar]