Abstract
With the mechanistic understanding of immune checkpoints and success in checkpoint blockade using antibodies for the treatment of certain cancers, immunotherapy has become one of the hottest areas in cancer research, with promise of long‐lasting therapeutic effect. Currently, however, only a proportion of cancers have a good response to checkpoint inhibition immunotherapy. Better understanding of the cancer response and resistance mechanisms is essential to fully explore the potential of immunotherapy to cure the majority of cancers. Bladder cancer, one of the most common and aggressive malignant diseases, has been successfully treated both at early and advanced stages by different immunotherapeutic approaches, bacillus Calmette–Guérin (BCG) intravesical instillation and anti‐PD‐1/PD‐L1 immune checkpoint blockade, respectively. Therefore, it provides a good model to investigate cancer immune response mechanisms and to improve the efficiency of immunotherapy. Here, we review bladder cancer immunotherapy with equal weight on BCG and anti‐PD‐1/PD‐L1 therapies and demonstrate why and how bladder cancer can be used as a model to study the predictors and mechanisms of cancer immune response and shine light on further development of immunotherapy approaches and response predictive biomarkers to improve immunotherapy of bladder cancer and other malignancies. We review the success of BCG and anti‐PD‐1/PD‐L1 treatment of bladder cancer, the underlying mechanisms and the therapeutic response predictors, including the limits to our knowledge. We then highlight briefly the adaptation of immunotherapy approaches and predictors developed in other cancers for bladder cancer therapy. Finally, we explore the potential of using bladder cancer as a model to investigate cancer immune response mechanisms and new therapeutic approaches, which may be translated into immunotherapy of other human cancers. © 2019 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: bladder cancer, immune response, immunotherapy, bacillus Calmette–Guérin, intravesical instillation, immune checkpoint blockade, PD‐1/PD‐L1 inhibitors, biomarkers, model system
Introduction
In 1891, Coley treated sarcoma patients with streptococcal organisms to prevent tumour progression 1. The concept of cancer immunotherapy began. To avoid lethal infection, Coley later implemented heat‐killed microorganisms, which subsequently became known as Coley's toxins 2. Based on this concept of infection induced immune response to enhance immune recognition of tumour‐associated antigens, a live, attenuated strain of Mycobacterium bovis, bacillus Calmette–Guérin (BCG), used for vaccination against tuberculosis, was tested as cancer therapy 3. In 1976, the clinical benefit of BCG intravesical instillation for bladder cancer was reported 4, which encouraged further clinical trials and established BCG intravesical instillation as the gold‐standard adjuvant treatment for non‐muscle invasive bladder cancer (NMIBC) 5.
Towards the end of last century, other immunotherapeutic approaches were also developed for cancer treatment, but with high toxicity and low specificity 6. In the 1990s, the immune checkpoint key proteins, CTLA4, PD‐1 and PD‐L1, were identified, which led to the success of cancer immunotherapy by immune checkpoint blockade (ICB). Together with the development of the chimeric antigen receptor T‐cell (CAR‐T) technology 7, in the last 10 years, immunotherapies demonstrate many breakthrough achievements, making immunotherapy a promising approach to cure certain cancers. Figure 1 summarises the history of immunotherapy development with milestones. However, many cancers are still not responsive to immunotherapy. A better understanding of the cancer response and inhibition mechanisms is essential to fully explore the potential of immunotherapy to cure cancers. Bladder cancer, which can be successfully treated by immunotherapy both for early‐ and later‐stage disease, provides a good model to investigate cancer immune response and improve efficacy.
Figure 1.
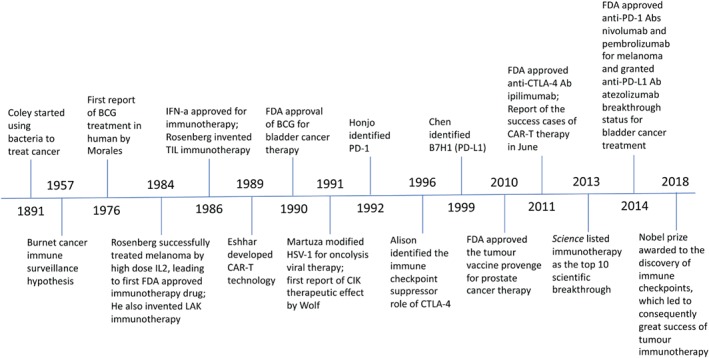
Milestones in cancer immunotherapy development. LAK, lymphokine‐activated killer cells; CIK, cytokine‐induced killer cells.
Bladder cancer is the sixth most common cancer with 70% of cases presenting as non‐muscle invasive lesions (NMIBC) 8. Around 25–75% high‐risk NMIBCs progress to muscle invasive cancer (MIBC) and further metastatic cancer, with poor prognosis. Due to the unique function of the bladder, urine storage, intravesical instillation of drugs is used to treat NMIBC, with BCG intravesical instillation the most successful approach, and the current standard clinical treatment. The mechanisms of BCG‐induced tumour‐specific immunity have been extensively investigated, although many unclear issues remain 5. Only 25% advanced/metastatic bladder cancers respond to anti‐PD‐1/PD‐L1 ICB 9, thus further improvement is required. As previous reviews have focused on either BCG or anti‐PD‐1/PD‐L1 immunotherapies, rarely both, here we review bladder cancer BCG and anti‐PD1/PD‐L1 immunotherapies together to explore our knowledge and the potential to improve immunotherapy. We demonstrate that bladder cancer is a good model to study cancer immune response mechanisms and predictors, which may help to improve immunotherapy of other cancers.
The success of BCG intravesical instillations for early‐stage NMIBC
Based on the observation that people with active tuberculosis develop cancer less frequently than the general population 10, the potential therapeutic effect of BCG against cancer was tested initially in 1936 for stomach cancer 11. Because of the unique function of the bladder, BCG intravesical instillation was applied in treating NMIBC patients in 1976 resulting in efficacy 4. Later several larger scale clinical trials with BCG instillation were reported at the beginning of 1980s, comparing outcomes with surgery alone 12, 13, 14 and with intravesical chemotherapy 12, 15.
Lamm et al reported in 1980 that BCG intravesical therapy following transurethral resection of bladder tumours (TURBT) better prevents tumour recurrence compared to patients receiving TURBT only (3/18 versus 8/19) 13. Similar findings were reported in later publications 12, 14. In addition to preventing recurrence after TURBT, the therapeutic efficacy of BCG instillations also achieved 70% complete remission rate in bladder cancer patients, who were not suitable for cystectomy or with incompletely resected tumour lesions 14, 16. In 1982, Brosman demonstrated that BCG treatment was more effective in preventing recurrence compared to thiotepa intravesical chemotherapy (0% versus 40% recurrence rate, respectively with 2‐year follow‐up) 15. The efficacy of BCG over chemotherapy was further supported by a study evaluating 176 patients randomised into intravesical BCG and chemotherapy groups, showing BCG treatment with 13% (9/67), doxorubicin treatment 43% (23/53) and thiotepa 36% (20/56) recurrence rates 17. Another 5‐year follow‐up study of 262 patients, also demonstrated that intravesical BCG significantly prevented recurrence compared to doxorubicin (63% versus 87% recurrence rate) 18. In 1990, the FDA approved intravesical instillation of BCG for NMIBC treatment, which was considered as a breakthrough cancer therapy 5, 18, 19.
Following FDA approval, many additional clinical trials have been conducted to investigate the long‐term clinical benefits of BCG intravesical instillation. A significant difference in time to first recurrence between BCG and chemotherapy was confirmed after follow‐up periods of greater than 8 years 20, 21. However, there were no significant differences in disease progression and long‐term end points 3, 18, 19, 20, 21, 22, although marginal differences for distant metastases (p = 0.046), overall survival (OS; p = 0.023), and disease‐specific survival (p = 0.026) comparing BCG and epirubicin chemotherapy were reported in a randomised phase III clinical trial with 837 patients 21. These studies also helped to optimise the dosage and schemes of BCG instillations 23, 24, 25.
Mechanisms of the response of NMIBC to BCG immunotherapy
While BCG has been FDA approved for nearly three decades and many mechanistic studies have been carried out, the mechanisms of BCG‐induced immunotherapeutic effect are still not fully understood due to the multiple biological aspects involved, including the innate and adaptive immune systems. There are review articles focusing on bladder cancer BCG therapeutic mechanisms 5, 26, 27. Here we summarise key immune response factors that are involved.
Innate immune response
The innate immune cells, including dendritic cells (DCs), neutrophils, monocytes, macrophages, NKs and other innate lymphocytes, are the front line of host defence and recruit immune cells through the production of cytokines and chemokines. BCG induces infiltration of neutrophils and mononuclear cells into the bladder wall 28. NKs, T and B cells are also recruited 5, 27. The in vitro interaction of bladder cancer cells and BCG or its cell wall skeleton stimulates the maturation of DCs, the major antigen‐presenting cells (APCs) 29, 30. The addition of BCG‐infected DCs into co‐cultured bladder cancer and white blood cells facilitates the immune inhibition of cancer cells 31. In a study with limited patients, low levels of post‐BCG treatment urine DCs was associated with recurrence 32. However, pre‐treatment tumour infiltration DCs were not significantly (p = 0.117) associated with recurrence and were inversely associated (p = 0.002) with BCG maintenance efficacy 33. Further studies are required.
In a mouse model study, depletion of neutrophils abolished the therapeutic effect of BCG by diminishing monocyte and CD4+ T cell infiltration in the bladder 34. However, it is yet to be verified if neutrophils are essential in BCG‐induced immunity in human bladder cancer 5. In a mouse model, depletion of NK cells also reduced efficacy of BCG immunotherapy 35. The role of NK cells in BCG‐induced cytotoxicity is supported by additional reports 36, 37, 38. However, in some in vitro and in vivo BCG studies, bladder cancer cell cytolysis or treatment efficacy in mice were not significantly affected by modulating NK cell activity 39, 40.
Although macrophages are detected in the bladder wall and urine of patients after BCG instillation 27, 41, 42, the role of macrophages in BCG immunotherapy is not clear. While BCG stimulates macrophages to produce cytotoxicity against certain bladder cancer cell lines, in patients, high pre‐BCG treatment tumour infiltrating macrophages are associated with cancer recurrence, potentially through the macrophage‐induced immunosuppression 27, 33, 43.
Adaptive immune response
The human adaptive/acquired immune response system consists of two types of responses: the cell‐mediated immune response, which is carried out by T lymphocytes, and the humoral immune response, which depends on B lymphocytes and B cell‐generated antibodies. Both preclinical and clinical studies suggest that BCG induces a strong adaptive host immune response to maximally inhibit cancer cell growth 5, 44. Essentially, BCG may work as a vaccine to stimulate host immune defences against tumour‐associated antigens.
BCG antigens are presented by DCs and urothelial cells via MHC class II 45, 46, leading to a TH1 cell immune response with the production of IL‐2, IL‐12, IFN‐γ, TNF, and TNF‐β, which is associated with successful BCG immunotherapy 47, 48. If a TH2 cell response is induced instead of TH1, patient response to BCG treatment is generally poor 47. The TH1 cell cytokine environment, in particular IFN‐γ 49 facilitates cytotoxic CD8+ T lymphocyte activation through MHC class I antigen presentation, and consequently anti‐tumour activity. The necessity of T cells for BCG immunotherapy is supported by mouse models with T cell depletion or in T cell absent athymic nude mice 50. In human bladder cancer, tumour‐infiltrating CD4+ T cells are increased in tumour samples in patients successfully treated with BCG 51. Mice vaccinated with BCG prior to BCG instillation have increased local acute inflammatory responses and infiltrating T cell recruitment after the first BCG instillation compared to unvaccinated mice 52 and the inflammatory response was significantly reduced by T cell depletion, suggesting the presence of BCG‐specific T cells enhances the BCG induced inflammatory response 52. However, the antigen(s) that activate T cells have not been identified, although tumour‐specific immunity has been induced by BCG in mice 53.
BCG intravesical instillation can also stimulate systemic immune responses. Following BCG therapy, lymphoproliferation and mycobacteria‐specific humoral responses and serum levels of cytokines and chemokines, such as IFN‐γ, IL‐1, IL‐2, IL‐8, TNF, CCL2 and CCL5 increased 5, 54. The purified protein derivative (PPD) skin test, indicative of a previous exposure to BCG or tuberculosis, frequently changes from negative to positive after BCG intravesical instillation 5, 54, 55. Although it is still debatable, a positive PPD skin test both prior and post‐BCG induction has been associated with better outcome compared with those with negative PPD skin test in some studies 55, 56, 57 and PPD test prior to BCG instillation has recently been reported to improve the therapeutic outcomes 58.
The success of immunotherapy with PD‐1/PDL‐1 inhibitors of advanced‐stage bladder cancer
Currently, there are five anti‐PD‐1/PD‐L1 ICB immunotherapeutic drugs approved by the FDA for the treatment of bladder and other urothelial carcinomas (UCs), including three anti‐PD‐L1 and two anti‐PD‐1 antibodies 59 (Table 1).
Table 1.
FDA approvals of anti‐PD‐1/PD‐L1 immunotherapeutic drugs in bladder and other cancers
| Atezolizumab | Durvalumab | Avelumab | Nivolumab | Pembrolizumab |
|---|---|---|---|---|
| May 2016, pre‐treated AMUC (bladder cancer) | May 2017, pre‐treated advanced/metastatic (bladder cancer) | Mar 2017, metastatic Merkel cell carcinoma | December 2014, advanced melanoma | September 2014, advanced melanoma |
| October 2016, metastatic NSCLC cancer | February 2018, unresectable Stage III NSCLC cancer | May 2017, AMUC (bladder cancer) | May 2015, lung cancer | October 2015, advanced/metastatic NSCLC cancer |
| April 2017, first line treatment advanced/metastatic (bladder cancer) | November 2015, metastatic renal cell carcinoma | August 2016, recurrent/metastatic head and neck squamous carcinoma | ||
| May 2016, Hodgkin lymphoma | October 2016, first line treatment of metastatic NSCLC | |||
| November 2016, head and neck cancer | March 2017, classical Hodgkin lymphoma | |||
| February 2017, pre‐treated AMUC (bladder cancer) | May 2017, AMUC (bladder cancer) | |||
| August 2017, metastatic colorectal cancer with MSI or MMR deficiency | May 2017, any solid cancer with MSI or MMR deficiency | |||
| September 2017, pre‐treated hepatocellular carcinoma | September 2017, pre‐treated advanced/metastatic gastric, gastroesophageal cancer | |||
| August 2018, pre‐treated SCLC | June 2018, pre‐treated advanced/metastatic cervical cancer | |||
| June 2018, pre‐treated PMBCL |
Information obtained through https://www.drugs.com/history/ [Accessed 20 November 2018]. Only the first FDA approval for a non‐bladder cancer was included in the table.
AMUC, advanced/metastatic urothelial carcinoma; MMR, mismatch repair; MSI, microsatellite instability; NSCLC, non‐small cell lung cancer; PMBCL, primary mediastinal large B‐cell lymphoma.
Anti‐PD‐L1 immunotherapies
The first anti‐PD‐1/PD‐L1 antibody drug tested for bladder/urothelial cancer immunotherapy is atezolizumab, which was reported in 2014 by Powles 60. Atezolizumab showed in post‐chemotherapy metastatic cancer good efficacy, which was associated with tumour infiltration immune cell (TIC) PD‐L1 expression (p = 0.026) but not tumour cell PD‐L1 expression (p = 0.93) and favourable toxicity profile. Consequently, a multicentre phase II trial of atezolizumab (IMvigor 210) demonstrated a better (15% overall objective response rate [ORR]) than an historical chemotherapy control (10% ORR) 61. Patients with continued atezolizumab beyond radiographic progression also benefited from the treatment 62. IMvigor 210 also demonstrated that cisplatin‐ineligible patients benefited from first‐line atezolizumab treatment (see supplementary material, Table S1). ORR and OS were significantly associated with tumour mutation load (TML) but not much with PD‐L1 expression 63. Consequently, the FDA approved atezolizumab as both a second‐line and first‐line (cisplatin‐ineligible patients) treatments of locally advanced/metastatic UC. In 2018, a phase III trial showed that atezolizumab has a longer response duration and less side‐effects than chemotherapy 64(see supplementary material, Table S1).
Following the success of atezolizumab, Powles and colleagues tested another anti‐PD‐L1 drug, durvalumab, in advanced bladder cancer. Two publications 65, 66 reported the results from the phase I/II multicentre trial, which led to FDA accelerated approval of durvalumab as second‐line therapy for locally advanced/metastatic UC 66(see supplementary material, Table S1). The third anti‐PD‐L1 antibody approved by the FDA for locally advanced/metastatic bladder cancer (second‐line therapy) is avelumab. The safety and dosage of avelumab was initially investigated in a single‐centre phase Ia solid tumour trial not limited to UC 67. The therapeutic efficacy was later demonstrated in large cohorts of post‐platinum chemotherapy locally advanced/metastatic UCs 68, 69 (see supplementary material, Table S1).
Anti‐PD‐1 immunotherapies
Results from two multicentre clinical trials of nivolumab, phase I/II CheckMate 032 and phase II CheckMate 275, on locally advanced/metastatic UC have been reported for the efficacy and safety 70, 71 (see supplementary material, Table S1). While CheckMate 032 did not show significant association of PD‐L1 expression with therapeutic response 70, CheckMate 275 did 71. In February 2017, the FDA granted nivolumab accelerated approval for second‐line therapy of post‐platinum locally advanced/metastatic UC.
The efficacy and safety profile of pembrolizumab in treating post‐platinum locally advanced/metastatic UC was firstly investigated in a phase Ib clinical trial KEYNOTE‐012 72. The efficacy of pembrolizumab for advanced UC was then demonstrated in a phase III trial KEYNOTE‐045, with significantly higher ORR than chemotherapy both in the overall patient population (p = 0.001) and in combined PD‐L1 positive score ≥10% patients (p = 0.0034). The OS of the pembrolizumab‐treated group was also significantly longer than the chemotherapy group, overall (p = 0.0004) or in the high PD‐L1 expression population (p = 0.005) 73. The benefit of pembrolizumab as first‐line treatment for cisplatin‐ineligible locally advanced /metastatic UC has also been demonstrated in a phase II study, where PD‐L1 expression was correlated with response 74 (see supplementary material, Table S1). The FDA has approved pembrolizumab separately as second‐line therapy for post‐platinum and first‐line treatment for cisplatin ineligible patients with locally advanced/metastatic UC. Recently, the benefit of using pembrolizumab for prior radical cystectomy neoadjuvant therapy has been reported 75 (see supplementary material, Table S1).
Mechanisms of the response to anti‐PD1/PD‐L1 immunotherapy
The mechanisms of the immunotherapeutic effects of anti‐PD‐1/PD‐L1 immune checkpoint inhibitors are much simpler and clearer than BCG immunotherapy. PD‐1 (CD279) is a transmembrane protein expressed on activated T cells, which is necessary for the termination of immune response. It interacts with its ligand PD‐L1 (B7‐H1/CD274), which is constitutively expressed at low levels on APCs and a wide variety of non‐hematopoietic cells 76. Cells use the PD‐L1/PD‐1 interaction to suppress T‐cell receptor (TCR)‐mediated cytotoxic function and inhibit proliferation of CD8+ T cells, to avoid autoimmunity and resolve inflammation 76. Tumour cells also use this immune suppression mechanism to escape immune surveillance by upregulating PD‐L1 expression or stimulating PD‐L1 expression in tumour microenvironment (TME) cells 76. For details please read the review article by Boussiotis 76. The anti‐PD‐1 and anti‐PD‐L1 inhibitors are antibodies which specifically bind to PD‐1 on T cell and PD‐L1 on cancer or TME cells respectively to prevent the interaction of PD‐1 and PD‐L1, consequently reactivate the anti‐tumour immune response of cytotoxic T‐cells 9 (Figure 2).
Figure 2.
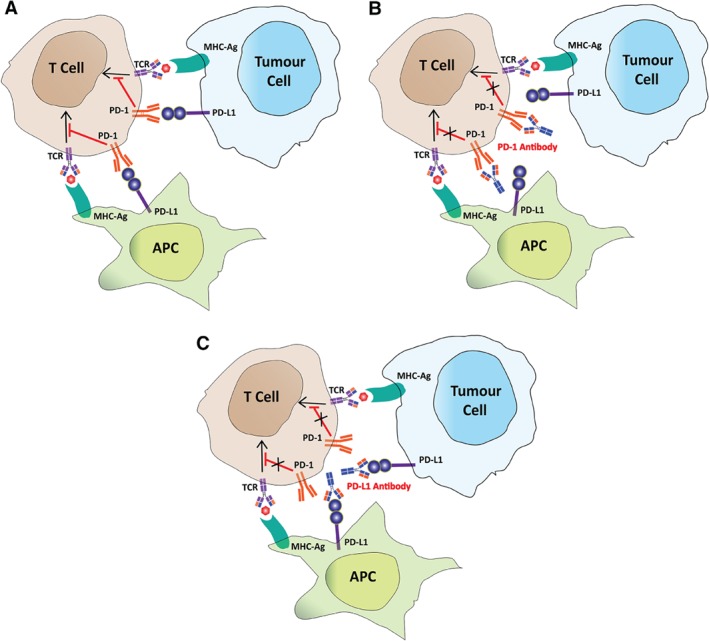
The schematic presentation of the mechanisms underlying how anti‐PD1/PDL‐1 antibodies work for immunotherapy. (A) The PD‐1–PD‐L1 interaction inhibits T cell activation. (B) PD‐1 antibody blocks PD‐1 on the T cell, which allows the cytotoxic T cell to remain activate and to infiltrate tumours to kill cancerous cells. (C) Anti‐PD‐L1 blocks the PD‐L1 immune checkpoint protein on immune cells, such as APCs and on tumour cells, preventing the inactivation of cytotoxic T cells.
Based on the above mechanism, the outcome of anti‐PD‐1/PD‐L1 immunotherapy should be predicted by tumour neoantigen level, the level of tumour infiltrating lymphocytes (TILs) and cancer/TME PD‐L1 expression. While there are positive correlations of these factors to anti‐PD‐1/PD‐L1 immunotherapy sensitivity in many studies, the associations are not always strong or significant 9, 77, 78, 79. The response of PD1/PD‐L1 negative tumours to checkpoint inhibitors was unexpected. Dynamic expression of PD‐L1 and multifactorial determination of immunotherapy responses are the potential explanations 9, 80, 81, 82, 83, 84. Some of the known resistance mechanisms are summarised in the biomarker section below and further mechanistic investigations are required.
Biomarkers to predict the response of immune therapies
The response of immunotherapies, even targeting specific immune response molecules such as in the case of anti‐PD‐1/PD‐L1 ICB, is determined by multiple factors, including cancer cell immunogenicity, TME and the strength of local/systematic immune activity 80, 81, 82, 83, 84, 85. Therefore, we review the relevant literature of immunotherapy response predictors in consideration of these aspects. One of the unique features of bladder cancer is the relative convenience of sampling for biomarker analysis, particularly the urine sample for cancer and TME materials. Primary tumour growth can also be easily monitored by cystoscopy, hence cystoscopy and cytological assessment are currently used to determine BCG treatment response.
BCG immunotherapy response prediction biomarkers
Although intravesical BCG immunotherapy has been used for bladder cancer treatment for over 40 years, biomarkers to predict the therapeutic response has not been extensively investigated as in ICD response. Certain cancer clinicopathological features were convincingly established as predictive factors, including recurrent tumours, multiplicity BCG treatment recurrence and high tumour grade and stage 47, 85, 86, 87, 88. However, these are general factors for poor cancer prognosis instead of predictive biomarkers. Urine cell genomic alterations detected by fluorescence in situ hybridisation during BCG treatment, although with low sensitivity 89, increased several fold cancer recurrence and progression risk 85, 89, 90, 91, 92. Next generation sequencing (NGS) is infrequently applied for BCG response prediction studies. We only found three tissue‐based and one urine cell‐based genomic NGS studies, each with limited cases, for BCG response predictors, with only one recurrence‐associated mutation in ARID1A identified 93, 94, 95, 96. DNA damage repair gene alterations and TML were not correlated with BCG immunotherapy response 95. No cancer transcriptome study has been reported and the correlation of DNA methylation of myopodin (SYNPO2), PAX6, MSH6, RB1, THBS1, PYCARD, TP73, ESR1, GATA5, PMF‐1, CDKN2B and MUS81a (MUS81) with cancer recurrence, progression and/or survival under BCG treatment were detected through candidate gene analysis 97, 98, 99, 100. Further NGS studies of tumour immunogenicity are required. It is not surprising that cancers expressing high levels of antigen‐presenting molecules and chemokines respond better to BCG immunotherapy 101, 102 and the increase of PD‐L1 positivity in cancer cells and TICs after BCG induction has been associated with poor outcome 103, 104, 105, explaining the high BCG treatment recurrence/resistance risk of NMIBC with carcinoma in situ 85, which has the high frequency (45%) of PD‐L1 expression 104.
TME and anti‐tumour immunity predictors for BCG treatment response have been much better investigated than the cancer cells. Increased TILs and CD4+/CD8+ ratio on BCG induction, have been linked to good therapeutic response 28, 51 and pre‐BCG treatment tumour infiltrating macrophages are associated with poor treatment response 33, 43. Changes in DCs and NK cells 85 and urine leukocytes 106 on BCG induction have also been suggested as potential predictors of BCG therapeutic response. The induction of a TH1 immune response after BCG instillation, particular from a TH2 baseline, is strongly associated with a good response 47, 48, 107, 108. Several studies have suggested the BCG response prediction value of urinary cytokines, such as IL‐2, IL‐6, IL‐8, IL‐10, IL‐12, IL‐18, IFN‐γ, TNF‐α and TRAIL 47, 85, 109, 110 and a nine‐cytokine urinary nomogram (CyPRIT) has been shown with 85.5% accuracy in predicting recurrence 111. PD‐L1 expression in TICs was also increased after BCG induction 103, 105, a potential treatment failure predictive biomarker. A positive PPD skin test both prior and post BCG instillation has been associated with BCG immunotherapy outcome, but requires further investigation 55, 56, 57. The systemic inflammatory response markers neutrophil‐to‐lymphocyte ratio and circulating C‐reactive protein have also been correlated to cancer recurrence and progression 112. Importantly, the current data suggest that pre‐treatment features have limited predictive accuracy for BCG immunotherapy sensitivity/resistance and change of cancer cell and immune response status after BCG induction may be more accurate predictors of BCG response, a principle may be applicable to predict anti‐PD‐1/PD‐L1 immunotherapy response.
Anti‐PD‐1/PD‐L1 immunotherapy response prediction biomarkers
Predicting which patient will benefit from the expensive ICB immunotherapy is critical. Although the mechanism of anti‐PD‐1/PD‐L1 immunotherapy is simple, patient response is determined by multiple factors 81, 82, 84. Extensive research has been carried out with dozens of review articles on anti‐PD‐1/PD‐L1 immunotherapy biomarkers in the last 2 years. The current most reliable positive predictor is the FDA approved anti‐PD‐1/PD‐L1 immunotherapy sensitivity predictor, microsatellite instability, caused by mismatch repair gene deficiency 9. Additionally, high TML and neo‐antigen 113, 114, 115, high clonality of tumour neoantigen 116, high patient HLA‐1 genotype heterozygosity and HLA‐B44 supertype 117, high immuno‐predictive score (IMPRES) 118, high levels of TICs and immune score 115, 119, 120, PD‐L1 expression in cancer and TME cells 120, 121, 122, 123, clonal TCRs 119, 124 and T cell expansion and activation 114, 119, 124, elevated IFN‐γ and tumour expression of IFN‐γ induced genes 114, high relative blood eosinophil and lymphocyte counts and low lactate dehydrogenase (LDH) levels 125, increase in circulating classical monocyte 126, early ctDNA reduction 127, 128, high diversity of gut microbiota and certain species, such as Ruminococcaceae, Bifidobacteria, Dorea formicogenerans, Collinsella aerofaciens and Enterococcus faecium 129, 130, 131, 132, 133, have all been correlated to good therapeutic response, although some of them, in particular those associated with tumour burden and stage are mainly prognostic but not predictive markers 81, 82, 84. High tumour burden/stage 125, 134, 135, 136, evasion to immune recognition due to absence of tumour neoantigens and loss‐of‐function mutations in the IFN response pathway and antigen presentation machinery (including loss of putative tumour neoantigens, loss of HLA haplotypes, somatic mutations in HLA or JAK1/JAK2 and B2M genes) 114, 137, 138, 139, 140, upregulation of alternative immune checkpoints, such as CTLA4, IDO, LAG3, TIM‐3, TIGIT and VISA 9, 121, 141, 142, 143, the innate anti‐PD‐1 resistance gene signatures (IPRES) 114, low‐IMPRES 118, activation of PI3K signalling (by PTEN loss) 144, low heterozygosity of HLA‐1 genotype and presence of HLA‐B62 supertype 117, tumour immune dysfunction and exclusion 145, the presence of tumour‐associated macrophages 146, immune suppressive cytokines released by cancer or TME cells, such as TGF‐b and CD73 115, 120, 147, high blood Angiopoietin‐2 level 148 and gut microbiota such as Bacteriodales 131 have all been associated with therapeutic resistance, while many more resistance mechanisms have been proposed in pre‐clinical studies 80, 81, 82, 84. These potential anti‐PD‐1/PD‐L1 immunotherapy responsive indicators in cancer, TME and systematic anti‐tumour immunity categories are listed in supplementary material, Table S2. However, none of these factors can reliably predict individual ICB response and immunograms have been proposed recently to consider multiple cancer, TME and immune activity factors to predict anti‐PD‐1/PD‐L1 immunotherapy outcome 83, 149, 150, which theoretically should increase prediction accuracy, however clinical feasibility is yet to be tested.
For bladder cancer, although the Ventana PD‐L1 assays have been FDA approved as biomarkers for atezolizumab (https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm501878.htm) and durvalumab (https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm555930) treatments, there is some degree of ambiguity in data from clinical studies for the correlation of PD‐L1 expression to therapeutic response and it has limited negative predictive value 60, 61, 63, 64, 65, 66, 69, 70, 71, 73, 74, 75. TML, which is generally high in bladder cancer 151, 152, has been correlated to bladder cancer anti‐PD‐1/PD‐L1 immunotherapy responses 61, 73, 75, although with an exception of a study with limited samples 79. TCGA subtype has been correlated to therapeutic response, but with conflicting messages from different studies 61, 71, 153. High levels of IFN‐ɣ‐induced gene expression and high density of infiltrating CD8+ T cells have also been associated with good ICB therapeutic outcome 61, 71, 115, 154; and a TGFβ signalling signature in fibroblasts, epithelial–mesenchymal transition and stroma related gene expression predicted therapeutic resistance 115, 155. Peripheral blood factors have been explored and high TCR clonality was associated with poor outcome 79 and early ctDNA reduction predicted good survival 127 (see supplementary material, Table S2). Recently, a multifactorial model including pre‐treatment clinical, tumour, and circulating features, has been developed, which increased accuracy in predicting anti‐PD‐L1 therapy response 124. We expect that including dynamic changes in this prediction model would further increase its efficiency. Although PD‐L1 expression on CTCs has been reported 156, its potential as a therapeutic response predictor has not been established. Urine sample analysis, an advantage of bladder cancer for dynamic immune response evaluation, has not been explored for response predictive biomarkers.
Previous research on ICB response predictive biomarkers are mainly focused on pre‐treatment conditions, which can only predict the likelihood of therapeutic response and outcome with limited accuracy 80, 81, 82, 84. The immune response is determined by the dynamic interaction of cancer cells, TME and anti‐tumour immunity 137, 157, 158, 159, 160. Recent studies in ICB response predictive biomarkers by analysing on‐treatment changes have shown good therapeutic response predictive values, frequently better than the static pre‐treatment status 80, 81, 115, 117, 127, 158, 159, 160, 161, 162. However, analysing the dynamic changes of cancer cells, TME and anti‐tumour immunity requires frequent tissue sampling, which is difficult for many cancers and suffers from intratumour heterogeneity 163. Liquid biopsy 164, 165, 166, 167, 168, using cancer (circulating and urine tumour cells, cancer cell extracellular vesicles, cell free nucleic acid and proteins) and immune system (immune cells and cytokines/chemokines) factors in body fluid, such as blood and urine samples, have great potential to be developed into efficient biomarkers for frequent analysis to predict/monitor immunotherapy efficacy 159, 160. Many circulating factors have been reported as potential predictors for anti‐PD‐1/PD‐L1 immunotherapy response 79, 125, 126, 127, 128, 148, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188 (see supplementary material, Table S2). We expect that further exploration in liquid biopsy ICB response predictive/monitoring biomarkers, including urine where bladder cancer has the unique opportunity, will greatly enhance the practice of precision immunotherapy.
Adaptation of immunotherapy approaches developed in other cancers for bladder cancer therapy and potential future development
Both BCG and ICB therapies were initially developed as therapies for other cancers but were later adapted to treat bladder cancers. Indeed, initial BCG cancer treatment was carried out in other tumour types, including stomach cancer, acute lymphoblastic leukaemia and melanoma 11, 189, 190, 191. However, BCG local immunotherapy is currently only used for NMIBC as standard of care. Formulations emulating Coley's toxin and other bacterial products with an immune stimulation role may also be used for bladder cancer immunotherapy. In China, as BCG has only been approved for bladder cancer therapy recently, a strain of Pseudomonas aeruginosa, P. aeruginosa‐mannose‐sensitive hemagglutinin has been developed and commonly used for intravesical instillation immunotherapy of NMIBCs 192. Only two recurrences have been recorded in our preliminary data from 27 patients treated with this intravesical instillation after TURBT each with minimum 1 year follow‐up (unpublished data).
The concept of ICB as immunotherapy was also adapted to bladder cancer from studies in other cancers 193, 194. However, of the five anti‐PD‐1/PD‐L1 antibody drugs approved for cancer immunotherapy, two of them, atezolizumab and durvalumab, were first approved for the treatment of bladder cancer 59 (Table 1). Many ICB therapeutic response prediction biomarkers have also been developed in other cancers and subsequently applied to bladder cancer anti‐PD‐1/PD‐L1 therapy as mentioned early (see supplementary material, Table S2), such as TML, initially identified in melanoma, lung, and colon cancer studies 61, 195, 196, 197, 198.
Based on the above observations, bladder cancer is immunogenic from early development to any stage during cancer progression that it can be treated at any clinical stage by some form of immunotherapy. In addition to BCG and ICB immunotherapies, other immunotherapy methods successfully used now or in the future in other cancers would be effective and should be assessed for bladder cancer treatment. For example, the FDA approved virus cancer vaccine, lmlygic (talimogene laherparepvec/T‐VEC) for melanoma treatment 199 may have better therapeutic efficacy than BCG instillation for NMIBC immunotherapy, as it has both oncolytic and immune stimulation effects. Another example is the FDA approved sipuleucel‐T for prostate cancer immunotherapy, where the design approach was used to develop the lapuleucel‐T vaccine for bladder cancer immunotherapy, using HER2 instead of PSMA for specific targeting 200.
Using bladder cancer as a model to investigate cancer immune response mechanisms and to translate them into immunotherapy of other human cancers
The successes of immunotherapy in a proportion of bladder cancer cases at each disease stage makes bladder cancer a good model to investigate the mechanisms of cancer immune genesis/response and develop novel immunotherapies and associated prediction markers. Bladder cancer has many features suitable for cancer immunotherapy development, including the genetic and molecular nature of disease development; and the bladder's unique function and anatomy, enabling well controlled local therapy application and cellular and molecular therapeutic response monitoring. All these help to test novel forms of cancer immunotherapies, which will benefit the development and application of immunotherapy in other cancers.
Cancer immune surveillance is activated by cancer neoantigens, reflected by TML, and other immunogenic factors. Bladder cancer has a high mutation rate 151, 152 and large cohorts of bladder cancer cases from early stage disease NMIBC to advanced stage MIBC have been sequenced for cancer TML 95, 201, 202, 203, 204, 205, 206. A considerable proportion of bladder cancer cases also host mismatch repair and DNA damage response deficiencies 95, 207. A proportion of bladder cancers are positive for human papillomavirus, a strong immune response stimulator 208. All these features facilitate the investigation of neoantigen generation mechanisms in ICB immunotherapy response. The extensive mechanistic studies and long‐period clinical response data of BCG immunotherapy are also rich resources to understand cancer immunity and develop immunotherapies.
Given the unique anatomy and function of the bladder, new cancer immune boosting therapeutic approaches may be easier to test in bladder cancer than other malignant diseases by intravesical instillation to evaluate and monitor efficacy, as well as mechanistic investigation to fully understand response and resistance mechanisms. Cancer and immune cells, cytokines, and other molecular factors in the urine can be easily collected and analysed to facilitate the development of accurate predictive biomarkers, not only for bladder cancer, but also to translate into other cancers.
There are two strategies for cancer immunotherapy, boosting the general immune response and removing the immune suppressor effect, such as by ICB. Both of them, in the forms of BCG and anti‐PD‐1/PD‐L1 immunotherapies, are standard therapeutic methods benefiting a proportion of bladder cancer patients. This makes bladder cancer a good model to test new immunotherapy approaches aiming to either boost general anti‐tumour immunity or block cancer immune evasion, as well as the combination. Most importantly, bladder cancer provides a good model to test the combined therapy in early stage disease as BCG is already the gold standard treatment of early stage NMIBC. There are clinical trials of combined immunotherapies in bladder cancer 209, 210 and we expect promising results to come and the associated opportunity for immunotherapy mechanism studies. These successes will undoubtedly shine light on immunotherapies of other human cancers.
Due to regulations, currently clinical trials are conducted on untreatable advanced cancers to evaluate firstly the safety (phase I) and then efficacy (phase II and III) of a new therapy. Certain immunotherapies, in particular immune boosting therapies, as demonstrated by BCG intravesical instillation in NMIBC, may be more effective in early rather than late stage cancer, when multiple immunotherapeutic resistant mechanisms have been developed. The high PD‐L1 positive rate in early stage melanoma 211 and lung cancer 212, 213, 214, 215 suggests that these cancers may have high neo‐antigens at early disease stage. The combined immune boosting and ICB immunotherapies may be more effective for these cancers when applied at an early cancer stage.
In conclusion, both BCG immune stimulation at early disease stage and anti‐PD‐1/PD‐L1 ICB at late disease stage are effective immunotherapies for bladder cancer, due to its molecular/biological characteristics and unique anatomical structure and location. These specific features of bladder cancer make it easy for local and systematic immunotherapy applications and easy to sample, in particular urine, for mechanistic studies and biomarker development. Therefore bladder cancer is a unique model for immunotherapy research to develop/test new immunotherapeutic approaches and predictive biomarkers. Using bladder cancer as a model, we expect to accelerate immunotherapy in cancer treatment, not only at late, but also early stage diseases.
Author contributions statement
DS and YJL designed the review outline. All authors contributed to the writing and critical review of the manuscript.
SUPPLEMENTARY MATERIAL ONLINE.
Table S1. Anti‐PD‐1/PD‐L1 immunotherapy clinical trials in bladder/urothelial cancer published in peer‐reviewed journals
Table S2. Potential predictive biomarkers for anti‐PD‐1/PD‐L1 immunotherapy response
Supporting information
Table S1. Anti‐PD‐1/PD‐L1 immunotherapy clinical trials in bladder/urothelial cancer published in peer‐reviewed journals
Table S2. Potential predictive biomarkers for anti‐PD‐1/PD‐L1 immunotherapy response
Acknowledgements
We thank Jianjian Yin for her assistance in the production of Figure 2.
Conflict of interest statement: TP has acted as consultant/advisor for Genentech/Roche, Bristol‐Myers Squibb, Merck, Novartis, GlaxoSmithKline and AstraZeneca and received research funding from Genentech/Roche and AstraZeneca/MedImmune. All other authors declared no conflict of interest.
References
- 1. Coley WB. II. Contribution to the knowledge of sarcoma. Ann Surg 1891; 14: 199–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft‐tissue sarcomas. Iowa Orthop J 2006; 26: 154–158. [PMC free article] [PubMed] [Google Scholar]
- 3. Zheng YQ, Naguib YW, Dong Y, et al Applications of bacillus Calmette–Guerin and recombinant bacillus Calmette–Guerin in vaccine development and tumor immunotherapy. Expert Rev Vaccines 2015; 14: 1255–1275. [PMC free article] [PubMed] [Google Scholar]
- 4. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette–Guerin in the treatment of superficial bladder tumors. J Urol 1976; 116: 180–183. [DOI] [PubMed] [Google Scholar]
- 5. Pettenati C, Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol 2018; 15: 615–625. [DOI] [PubMed] [Google Scholar]
- 6. Jiang J, Wu C, Lu B. Cytokine‐induced killer cells promote antitumor immunity. J Transl Med 2013; 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013; 3: 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 9. Gopalakrishnan D, Koshkin VS, Ornstein MC, et al Immune checkpoint inhibitors in urothelial cancer: recent updates and future outlook. Ther Clin Risk Manag 2018; 14: 1019–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pearl R. On the pathological relations between cancer and tuberculosis. Exp Biol Med 1928; 26: 73–75. [Google Scholar]
- 11. Holmgren I. Employment of B. C. G. especially in Intravenous Injection. Acta Med Scand 1936; 90: 350–361. [Google Scholar]
- 12. Adolphs HD, Bastian HP. Chemoimmune prophylaxis of superficial bladder cancer. J Urol 1983; 129: 29–32. [DOI] [PubMed] [Google Scholar]
- 13. Lamm DL, Thor DE, Harris SC, et al Bacillus Calmette–Guerin immunotherapy of superficial bladder cancer. J Urol 1980; 124: 38–40. [DOI] [PubMed] [Google Scholar]
- 14. Lamm DL, Thor DE, Stogdill VD, et al Bladder cancer immunotherapy. J Urol 1982; 128: 931–935. [DOI] [PubMed] [Google Scholar]
- 15. Brosman SA. Experience with bacillus Calmette–Guerin in patients with superficial bladder carcinoma. J Urol 1982; 128: 27–30. [DOI] [PubMed] [Google Scholar]
- 16. Schellhammer PF, Ladaga LE, Fillion MB. Bacillus Calmette–Guerin for superficial transitional cell carcinoma of the bladder. J Urol 1986; 135: 261–264. [DOI] [PubMed] [Google Scholar]
- 17. Martinez‐Pineiro JA, Jimenez Leon J, Martinez‐Pineiro L Jr, et al Bacillus Calmette–Guerin versus doxorubicin versus thiotepa: a randomized prospective study in 202 patients with superficial bladder cancer. J Urol 1990; 143: 502–506. [DOI] [PubMed] [Google Scholar]
- 18. Lamm DL, Blumenstein BA, Crawford ED, et al A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette–Guerin for transitional‐cell carcinoma of the bladder. N Engl J Med 1991; 325: 1205–1209. [DOI] [PubMed] [Google Scholar]
- 19. Malmstrom PU, Sylvester RJ, Crawford DE, et al An individual patient data meta‐analysis of the long‐term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette–Guerin for non‐muscle‐invasive bladder cancer. Eur Urol 2009; 56: 247–256. [DOI] [PubMed] [Google Scholar]
- 20. Jarvinen R, Kaasinen E, Sankila A, et al Long‐term efficacy of maintenance bacillus Calmette–Guerin versus maintenance mitomycin C instillation therapy in frequently recurrent TaT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised FinnBladder I study with a 20‐year follow‐up. Eur Urol 2009; 56: 260–265. [DOI] [PubMed] [Google Scholar]
- 21. Sylvester RJ, Brausi MA, Kirkels WJ, et al Long‐term efficacy results of EORTC genito‐urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette–Guerin, and bacillus Calmette–Guerin plus isoniazid in patients with intermediate‐ and high‐risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol 2010; 57: 766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shang PF, Kwong J, Wang ZP, et al Intravesical bacillus Calmette–Guerin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database Syst Rev 2011: CD006885. [DOI] [PubMed] [Google Scholar]
- 23. Lamm DL, Blumenstein BA, Crissman JD, et al Maintenance bacillus Calmette–Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000; 163: 1124–1129. [PubMed] [Google Scholar]
- 24. Oddens J, Brausi M, Sylvester R, et al Final results of an EORTC‐GU cancers group randomized study of maintenance bacillus Calmette–Guerin in intermediate‐ and high‐risk Ta, T1 papillary carcinoma of the urinary bladder: one‐third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 2013; 63: 462–472. [DOI] [PubMed] [Google Scholar]
- 25. Ehdaie B, Sylvester R, Herr HW. Maintenance bacillus Calmette–Guerin treatment of non‐muscle‐invasive bladder cancer: a critical evaluation of the evidence. Eur Urol 2013; 64: 579–585. [DOI] [PubMed] [Google Scholar]
- 26. Ingersoll MA, Albert ML. From infection to immunotherapy: host immune responses to bacteria at the bladder mucosa. Mucosal Immunol 2013; 6: 1041–1053. [DOI] [PubMed] [Google Scholar]
- 27. Redelman‐Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer – a current perspective. Nat Rev Urol 2014; 11: 153–162. [DOI] [PubMed] [Google Scholar]
- 28. Bohle A, Gerdes J, Ulmer AJ, et al Effects of local bacillus Calmette–Guerin therapy in patients with bladder carcinoma on immunocompetent cells of the bladder wall. J Urol 1990; 144: 53–58. [DOI] [PubMed] [Google Scholar]
- 29. Tsuji S, Matsumoto M, Takeuchi O, et al Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette–Guerin: involvement of toll‐like receptors. Infect Immun 2000; 68: 6883–6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim KD, Lee HG, Kim JK, et al Enhanced antigen‐presenting activity and tumour necrosis factor‐alpha‐independent activation of dendritic cells following treatment with Mycobacterium bovis bacillus Calmette–Guerin. Immunology 1999; 97: 626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higuchi T, Shimizu M, Owaki A, et al A possible mechanism of intravesical BCG therapy for human bladder carcinoma: involvement of innate effector cells for the inhibition of tumor growth. Cancer Immunol Immunother 2009; 58: 1245–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beatty JD, Islam S, North ME, et al Urine dendritic cells: a noninvasive probe for immune activity in bladder cancer? BJU Int 2004; 94: 1377–1383. [DOI] [PubMed] [Google Scholar]
- 33. Ayari C, LaRue H, Hovington H, et al Bladder tumor infiltrating mature dendritic cells and macrophages as predictors of response to bacillus Calmette–Guerin immunotherapy. Eur Urol 2009; 55: 1386–1396. [DOI] [PubMed] [Google Scholar]
- 34. Suttmann H, Riemensberger J, Bentien G, et al Neutrophil granulocytes are required for effective bacillus Calmette–Guerin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res 2006; 66: 8250–8257. [DOI] [PubMed] [Google Scholar]
- 35. Brandau S, Riemensberger J, Jacobsen M, et al NK cells are essential for effective BCG immunotherapy. Int J Cancer 2001; 92: 697–702. [DOI] [PubMed] [Google Scholar]
- 36. Garcia‐Cuesta EM, Lopez‐Cobo S, Alvarez‐Maestro M, et al NKG2D is a key receptor for recognition of bladder cancer cells by IL‐2‐activated NK cells and BCG promotes NK cell activation. Front Immunol 2015; 6: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sonoda T, Sugimura K, Ikemoto S, et al Significance of target cell infection and natural killer cells in the anti‐tumor effects of bacillus Calmette–Guerin in murine bladder cancer. Oncol Rep 2007; 17: 1469–1474. [PubMed] [Google Scholar]
- 38. Suttmann H, Jacobsen M, Reiss K, et al Mechanisms of bacillus Calmette–Guerin mediated natural killer cell activation. J Urol 2004; 172: 1490–1495. [DOI] [PubMed] [Google Scholar]
- 39. Ratliff TL, Shapiro A, Catalona WJ. Inhibition of murine bladder tumor growth by bacille Calmette–Guerin: lack of a role of natural killer cells. Clin Immunol Immunopathol 1986; 41: 108–115. [DOI] [PubMed] [Google Scholar]
- 40. Wang MH, Flad HD, Bohle A, et al Cellular cytotoxicity of human natural killer cells and lymphokine‐activated killer cells against bladder carcinoma cell lines. Immunol Lett 1991; 27: 191–197. [DOI] [PubMed] [Google Scholar]
- 41. Prescott S, James K, Hargreave TB, et al Intravesical Evans Strain BCG therapy – quantitative immunohistochemical analysis of the immune‐response within the bladder wall. J Urol 1992; 147: 1636–1642. [DOI] [PubMed] [Google Scholar]
- 42. Saint F, Patard JJ, Groux Muscatelli B, et al Evaluation of cellular tumour rejection mechanisms in the peritumoral bladder wall after bacillus Calmette–Guerin treatment. BJU Int 2001; 88: 602–610. [DOI] [PubMed] [Google Scholar]
- 43. Takayama H, Nishimura K, Tsujimura A, et al Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette–Guerin instillation. J Urol 2009; 181: 1894–1900. [DOI] [PubMed] [Google Scholar]
- 44. Liakou CI, Narayanan S, Ng Tang D, et al Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human bladder cancer. Cancer Immun 2007; 7: 10. [PMC free article] [PubMed] [Google Scholar]
- 45. Prescott S, James K, Busuttil A, et al HLA‐DR expression by high grade superficial bladder cancer treated with BCG. Br J Urol 1989; 63: 264–269. [DOI] [PubMed] [Google Scholar]
- 46. Stefanini GF, Bercovich E, Mazzeo V, et al Class I and class II HLA antigen expression by transitional cell carcinoma of the bladder: correlation with T‐cell infiltration and BCG treatment. J Urol 1989; 141: 1449–1453. [DOI] [PubMed] [Google Scholar]
- 47. Zuiverloon TC, Nieuweboer AJ, Vekony H, et al Markers predicting response to bacillus Calmette–Guerin immunotherapy in high‐risk bladder cancer patients: a systematic review. Eur Urol 2012; 61: 128–145. [DOI] [PubMed] [Google Scholar]
- 48. Saint F, Patard JJ, Maille P, et al Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette–Guerin treatment for superficial bladder cancer. J Urol 2002; 167: 364–367. [PubMed] [Google Scholar]
- 49. Luo Y, Chen X, O'Donnell MA. Role of Th1 and Th2 cytokines in BCG‐induced IFN‐gamma production: cytokine promotion and simulation of BCG effect. Cytokine 2003; 21: 17–26. [DOI] [PubMed] [Google Scholar]
- 50. Ratliff TL, Ritchey JK, Yuan JJ, et al T‐cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol 1993; 150: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 51. Pichler R, Fritz J, Zavadil C, et al Tumor‐infiltrating immune cell subpopulations influence the oncologic outcome after intravesical bacillus Calmette–Guerin therapy in bladder cancer. Oncotarget 2016; 7: 39916–39930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Biot C, Rentsch CA, Gsponer JR, et al Preexisting BCG‐specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med 2012; 4: 137ra172. [DOI] [PubMed] [Google Scholar]
- 53. Vandeveer AJ, Fallon JK, Tighe R, et al Systemic immunotherapy of non‐muscle invasive mouse bladder cancer with avelumab, an anti‐PD‐L1 immune checkpoint inhibitor. Cancer Immunol Res 2016; 4: 452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Taniguchi K, Koga S, Nishikido M, et al Systemic immune response after intravesical instillation of bacille Calmette–Guerin (BCG) for superficial bladder cancer. Clin Exp Immunol 1999; 115: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kelley DR, Haaff EO, Becich M, et al Prognostic value of purified protein derivative skin test and granuloma formation in patients treated with intravesical bacillus Calmette–Guerin. J Urol 1986; 135: 268–271. [DOI] [PubMed] [Google Scholar]
- 56. Krajewski W, Zdrojowy R, Kolodziej A. Purified protein derivative skin test‐one swallow does not make a summer. Re: purified protein derivative skin test reactions are associated with clinical outcomes of patients with nonmuscle invasive bladder cancer treated with induction bacillus Calmette–Guerin therapy. Urol Oncol 2019; 37: 346–347. [DOI] [PubMed] [Google Scholar]
- 57. Niwa N, Kikuchi E, Matsumoto K, et al Purified protein derivative skin test reactions are associated with clinical outcomes of patients with nonmuscle invasive bladder cancer treated with induction bacillus Calmette–Guerin therapy. Urol Oncol 2018; 36: 77 e15–77 e21. [DOI] [PubMed] [Google Scholar]
- 58. Niwa N, Kikuchi E, Matsumoto K, et al Purified protein derivative skin test prior to bacillus Calmette–Guerin therapy may have therapeutic impact in patients with nonmuscle invasive bladder cancer. J Urol 2018; 199: 1446–1451. [DOI] [PubMed] [Google Scholar]
- 59. Katz H, Wassie E, Alsharedi M. Checkpoint inhibitors: the new treatment paradigm for urothelial bladder cancer. Med Oncol 2017; 34: 170. [DOI] [PubMed] [Google Scholar]
- 60. Powles T, Eder JP, Fine GD, et al MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–562. [DOI] [PubMed] [Google Scholar]
- 61. Rosenberg JE, Hoffman‐Censits J, Powles T, et al Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: a single‐arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Necchi A, Joseph RW, Loriot Y, et al Atezolizumab in platinum‐treated locally advanced or metastatic urothelial carcinoma: post‐progression outcomes from the phase II IMvigor210 study. Ann Oncol 2017; 28: 3044–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Balar AV, Galsky MD, Rosenberg JE, et al Atezolizumab as first‐line treatment in cisplatin‐ineligible patients with locally advanced and metastatic urothelial carcinoma: a single‐arm, multicentre, phase 2 trial. Lancet 2017; 389: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Powles T, Duran I, van der Heijden MS, et al Atezolizumab versus chemotherapy in patients with platinum‐treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open‐label, phase 3 randomised controlled trial. Lancet 2018; 391: 748–757. [DOI] [PubMed] [Google Scholar]
- 65. Massard C, Gordon MS, Sharma S, et al Safety and efficacy of durvalumab (MEDI4736), an anti‐programmed cell death ligand‐1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 2016; 34: 3119–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Powles T, O'Donnell PH, Massard C, et al Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open‐label study. JAMA Oncol 2017; 3: e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heery CR, O'Sullivan‐Coyne G, Madan RA, et al Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose‐escalation trial. Lancet Oncol 2017; 18: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Patel MR, Ellerton J, Infante JR, et al Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open‐label, phase 1 trial. Lancet Oncol 2018; 19: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Apolo AB, Infante JR, Balmanoukian A, et al Avelumab, an anti‐programmed death‐ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol 2017; 35: 2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sharma P, Callahan MK, Bono P, et al Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open‐label, two‐stage, multi‐arm, phase 1/2 trial. Lancet Oncol 2016; 17: 1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sharma P, Retz M, Siefker‐Radtke A, et al Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single‐arm, phase 2 trial. Lancet Oncol 2017; 18: 312–322. [DOI] [PubMed] [Google Scholar]
- 72. Plimack ER, Bellmunt J, Gupta S, et al Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE‐012): a non‐randomised, open‐label, phase 1b study. Lancet Oncol 2017; 18: 212–220. [DOI] [PubMed] [Google Scholar]
- 73. Bellmunt J, de Wit R, Vaughn DJ, et al Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Balar AV, Castellano D, O'Donnell PH, et al First‐line pembrolizumab in cisplatin‐ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (keynote‐052): a multicentre, single‐arm, phase 2 study. Lancet Oncol 2017; 18: 1483–1492. [DOI] [PubMed] [Google Scholar]
- 75. Necchi A, Anichini A, Raggi D, et al Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle‐invasive urothelial bladder carcinoma (PURE‐01): an open‐label, single‐arm, Phase II study. J Clin Oncol 2018; 36: 3353–3360. [DOI] [PubMed] [Google Scholar]
- 76. Boussiotis VA. Molecular and biochemical aspects of the PD‐1 checkpoint pathway. N Engl J Med 2016; 375: 1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mehta K, Patel K, Parikh RA. Immunotherapy in genitourinary malignancies. J Hematol Oncol 2017; 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chabanon RM, Pedrero M, Lefebvre C, et al Mutational landscape and sensitivity to immune checkpoint blockers. Clin Cancer Res 2016; 22: 4309–4321. [DOI] [PubMed] [Google Scholar]
- 79. Snyder A, Nathanson T, Funt SA, et al Contribution of systemic and somatic factors to clinical response and resistance to PD‐L1 blockade in urothelial cancer: an exploratory multi‐omic analysis. PLoS Med 2017; 14: e1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sharma P, Hu‐Lieskovan S, Wargo JA, et al Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017; 168: 707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zappasodi R, Wolchok JD, Merghoub T. Strategies for predicting response to checkpoint inhibitors. Curr Hematol Malig Rep 2018; 13: 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Park YJ, Kuen DS, Chung Y. Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance. Exp Mol Med 2018; 50: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. van Dijk N, Funt SA, Blank CU, et al The cancer Immunogram as a framework for personalized immunotherapy in urothelial cancer. Eur Urol 2019; 75: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Weber JS. Biomarkers for checkpoint inhibition. Am Soc Clin Oncol Educ Book 2017; 37: 205–209. [DOI] [PubMed] [Google Scholar]
- 85. Kamat AM, Li R, O'Donnell MA, et al Predicting response to intravesical bacillus Calmette–Guerin Immunotherapy: are we there yet? a systematic review. Eur Urol 2018; 73: 738–748. [DOI] [PubMed] [Google Scholar]
- 86. Cambier S, Sylvester RJ, Collette L, et al EORTC nomograms and risk groups for predicting recurrence, progression, and disease‐specific and overall survival in non‐muscle‐invasive stage Ta‐T1 urothelial bladder cancer patients treated with 1‐3 years of maintenance bacillus Calmette–Guerin. Eur Urol 2016; 69: 60–69. [DOI] [PubMed] [Google Scholar]
- 87. Fernandez‐Gomez J, Madero R, Solsona E, et al Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette–Guerin: the CUETO scoring model. J Urol 2009; 182: 2195–2203. [DOI] [PubMed] [Google Scholar]
- 88. Malmstrom PU, Hemdan T, Segersten U. Validation of the ezrin, CK20, and Ki‐67 as potential predictive markers for BCG instillation therapy of non‐muscle‐invasive bladder cancer. Urol Oncol 2017; 35: 532 e531–532 e536. [DOI] [PubMed] [Google Scholar]
- 89. Bao Y, Tu X, Chang T, et al The role of fluorescence in situ hybridization to predict patient response to intravesical Bacillus Calmette–Guerin therapy for bladder cancer: a diagnostic meta‐analysis and systematic review. Medicine (Baltimore) 2018; 97: e12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kamat AM, Dickstein RJ, Messetti F, et al Use of fluorescence in situ hybridization to predict response to bacillus Calmette–Guerin therapy for bladder cancer: results of a prospective trial. J Urol 2012; 187: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kamat AM, Willis DL, Dickstein RJ, et al Novel fluorescence in situ hybridization‐based definition of bacille Calmette–Guerin (BCG) failure for use in enhancing recruitment into clinical trials of intravesical therapies. BJU Int 2016; 117: 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liem EIML, Baard J, Cauberg ECC, et al Fluorescence in situ hybridization as prognostic predictor of tumor recurrence during treatment with bacillus Calmette–Guerin therapy for intermediate‐ and high‐risk non‐muscleinvasive bladder cancer. Medical Oncology 2017; 34: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jungels C, Martinez Chanza N, Albisinni S, et al Interest of next‐generation sequencing in BCG‐treated high‐risk bladder cancer. Prog Urol 2018; 28: 344–350. [DOI] [PubMed] [Google Scholar]
- 94. Meeks JJ, Carneiro BA, Pai SG, et al Genomic characterization of high‐risk non‐muscle invasive bladder cancer. Oncotarget 2016; 7: 75176–75184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pietzak EJ, Bagrodia A, Cha EK, et al Next‐generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur Urol 2017; 72: 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Scott SN, Ostrovnaya I, Lin CM, et al Next‐generation sequencing of urine specimens: a novel platform for genomic analysis in patients with non‐muscle‐invasive urothelial carcinoma treated with bacille Calmette–Guerin. Cancer Cytopathol 2017; 125: 416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Agundez M, Grau L, Palou J, et al Evaluation of the methylation status of tumour suppressor genes for predicting bacillus Calmette–Guerin response in patients with T1G3 high‐risk bladder tumours. Eur Urol 2011; 60: 131–140. [DOI] [PubMed] [Google Scholar]
- 98. Alvarez‐Mugica M, Cebrian V, Fernandez‐Gomez JM, et al Myopodin methylation is associated with clinical outcome in patients with T1G3 bladder cancer. J Urol 2010; 184: 1507–1513. [DOI] [PubMed] [Google Scholar]
- 99. Alvarez‐Mugica M, Fernandez‐Gomez JM, Cebrian V, et al Polyamine‐modulated factor‐1 methylation predicts bacillus Calmette–Guerin response in patients with high‐grade non‐muscle‐invasive bladder carcinoma. Eur Urol 2013; 63: 364–370. [DOI] [PubMed] [Google Scholar]
- 100. Husek P, Pacovsky J, Chmelarova M, et al Methylation status as a predictor of intravesical bacillus Calmette–Guerin (BCG) immunotherapy response of high grade non‐muscle invasive bladder tumor. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017; 161: 210–216. [DOI] [PubMed] [Google Scholar]
- 101. Kitamura H, Torigoe T, Honma I, et al Effect of human leukocyte antigen class I expression of tumor cells on outcome of intravesical instillation of bacillus Calmette–Guerin immunotherapy for bladder cancer. Clin Cancer Res 2006; 12: 4641–4644. [DOI] [PubMed] [Google Scholar]
- 102. Videira PA, Calais FM, Correia M, et al Efficacy of bacille Calmette–Guerin immunotherapy predicted by expression of antigen‐presenting molecules and chemokines. Urology 2009; 74: 944–950. [DOI] [PubMed] [Google Scholar]
- 103. Hashizume A, Umemoto S, Yokose T, et al Enhanced expression of PD‐L1 in non‐muscle‐invasive bladder cancer after treatment with bacillus Calmette–Guerin. Oncotarget 2018; 9: 34066–34078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Inman BA, Sebo TJ, Frigola X, et al PD‐L1 (B7‐H1) expression by urothelial carcinoma of the bladder and BCG‐induced granulomata: associations with localized stage progression. Cancer 2007; 109: 1499–1505. [DOI] [PubMed] [Google Scholar]
- 105. Chevalier MF, Schneider AK, Cesson V, et al Conventional and PD‐L1‐expressing regulatory T cells are enriched during BCG therapy and may limit its efficacy. Eur Urol 2018; 74: 540–544. [DOI] [PubMed] [Google Scholar]
- 106. Saint F, Patard JJ, Irani J, et al Leukocyturia as a predictor of tolerance and efficacy of intravesical BCG maintenance therapy for superficial bladder cancer. Urology 2001; 57: 617–621 discussion 621–612. [DOI] [PubMed] [Google Scholar]
- 107. Nunez‐Nateras R, Castle EP, Protheroe CA, et al Predicting response to bacillus Calmette–Guerin (BCG) in patients with carcinoma in situ of the bladder. Urol Oncol 2014; 32: 45 e23–45 e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pichler R, Gruenbacher G, Culig Z, et al Intratumoral Th2 predisposition combines with an increased Th1 functional phenotype in clinical response to intravesical BCG in bladder cancer. Cancer Immunol Immunother 2017; 66: 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Qu K, Gu J, Ye Y, et al High baseline levels of interleukin‐8 in leukocytes and urine predict tumor recurrence in non‐muscle invasive bladder cancer patients receiving bacillus Calmette–Guerin therapy: a long‐term survival analysis. Oncoimmunology 2017; 6: e1265719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Salmasi A, Elashoff DA, Guo R, et al Urinary cytokine profile to predict response to intravesical BCG with or without HS‐410 therapy in patients with non‐muscle invasive bladder cancer. Cancer Epidemiol Biomarkers Prev 2019; 28: 1036–1044. [DOI] [PubMed] [Google Scholar]
- 111. Kamat AM, Briggman J, Urbauer DL, et al Cytokine panel for response to intravesical therapy (CyPRIT): nomogram of changes in urinary cytokine levels predicts patient response to bacillus Calmette–Guerin. Eur Urol 2016; 69: 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mbeutcha A, Shariat SF, Rieken M, et al Prognostic significance of markers of systemic inflammatory response in patients with non‐muscle‐invasive bladder cancer. Urol Oncol 2016; 34: 483 e417–483 e424. [DOI] [PubMed] [Google Scholar]
- 113. Samstein RM, Lee CH, Shoushtari AN, et al Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019; 51: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hugo W, Zaretsky JM, Sun L, et al Genomic and transcriptomic features of response to anti‐PD‐1 therapy in metastatic melanoma. Cell 2016; 165: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mariathasan S, Turley SJ, Nickles D, et al TGFbeta attenuates tumour response to PD‐L1 blockade by contributing to exclusion of T cells. Nature 2018; 554: 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. McGranahan N, Furness AJ, Rosenthal R, et al Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016; 351: 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chowell D, Morris LGT, Grigg CM, et al Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 2018; 359: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Auslander N, Zhang G, Lee JS, et al Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat Med 2018; 24: 1545–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tumeh PC, Harview CL, Yearley JH, et al PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Taube JM, Klein A, Brahmer JR, et al Association of PD‐1, PD‐1 ligands, and other features of the tumor immune microenvironment with response to anti‐PD‐1 therapy. Clin Cancer Res 2014; 20: 5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Herbst RS, Soria JC, Kowanetz M, et al Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Larkin J, Chiarion‐Sileni V, Gonzalez R, et al Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Garon EB, Rizvi NA, Hui R, et al Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 124. Leiserson MDM, Syrgkanis V, Gilson A, et al A multifactorial model of T cell expansion and durable clinical benefit in response to a PD‐L1 inhibitor. PLoS One 2018; 13: e0208422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Weide B, Martens A, Hassel JC, et al Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 2016; 22: 5487–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Krieg C, Nowicka M, Guglietta S, et al High‐dimensional single‐cell analysis predicts response to anti‐PD‐1 immunotherapy. Nat Med 2018; 24: 144–153. [DOI] [PubMed] [Google Scholar]
- 127. Raja R, Kuziora M, Brohawn PZ, et al Early reduction in ctDNA predicts survival in patients with lung and bladder cancer treated with durvalumab. Clin Cancer Res 2018; 24: 6212–6222. [DOI] [PubMed] [Google Scholar]
- 128. Cabel L, Riva F, Servois V, et al Circulating tumor DNA changes for early monitoring of anti‐PD1 immunotherapy: a proof‐of‐concept study. Ann Oncol 2017; 28: 1996–2001. [DOI] [PubMed] [Google Scholar]
- 129. Frankel AE, Coughlin LA, Kim J, et al Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia 2017; 19: 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gopalakrishnan V, Helmink BA, Spencer CN, et al The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 2018; 33: 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gopalakrishnan V, Spencer CN, Nezi L, et al Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science 2018; 359: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Matson V, Fessler J, Bao R, et al The commensal microbiome is associated with anti‐PD‐1 efficacy in metastatic melanoma patients. Science 2018; 359: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Routy B, Le Chatelier E, Derosa L, et al Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science 2018; 359: 91–97. [DOI] [PubMed] [Google Scholar]
- 134. Nosrati A, Tsai KK, Goldinger SM, et al Evaluation of clinicopathological factors in PD‐1 response: derivation and validation of a prediction scale for response to PD‐1 monotherapy. Br J Cancer 2017; 116: 1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tumeh PC, Hellmann MD, Hamid O, et al Liver metastasis and treatment outcome with anti‐PD‐1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017; 5: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Joseph RW, Elassaiss‐Schaap J, Kefford R, et al Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res 2018; 24: 4960–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Anagnostou V, Smith KN, Forde PM, et al Evolution of neoantigen landscape during immune checkpoint blockade in non‐small cell lung cancer. Cancer Discov 2017; 7: 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zaretsky JM, Garcia‐Diaz A, Shin DS, et al Mutations associated with acquired resistance to PD‐1 blockade in melanoma. N Engl J Med 2016; 375: 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Shukla SA, Rooney MS, Rajasagi M, et al Comprehensive analysis of cancer‐associated somatic mutations in class I HLA genes. Nat Biotechnol 2015; 33: 1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sade‐Feldman M, Jiao YJ, Chen JH, et al Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun 2017; 8: 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Koyama S, Akbay EA, Li YY, et al Adaptive resistance to therapeutic PD‐1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016; 7: 10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Llosa NJ, Cruise M, Tam A, et al The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter‐inhibitory checkpoints. Cancer Discov 2015; 5: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sweis RF, Spranger S, Bao R, et al Molecular drivers of the non‐T‐cell‐Inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunol Res 2016; 4: 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Peng W, Chen JQ, Liu C, et al Loss of PTEN promotes resistance to T cell‐mediated immunotherapy. Cancer Discov 2016; 6: 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Jiang P, Gu S, Pan D, et al Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med 2018; 24: 1550–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Peranzoni E, Lemoine J, Vimeux L, et al Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti‐PD‐1 treatment. Proc Natl Acad Sci U S A 2018; 115: E4041–E4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Beavis PA, Slaney CY, Milenkovski N, et al CD73: a potential biomarker for anti‐PD‐1 therapy. Oncoimmunology 2015; 4: e1046675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wu X, Giobbie‐Hurder A, Liao X, et al Angiopoietin‐2 as a biomarker and target for immune checkpoint therapy. Cancer Immunol Res 2017; 5: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Karasaki T, Nagayama K, Kuwano H, et al An Immunogram for the cancer‐immunity cycle: towards personalized immunotherapy of lung cancer. J Thorac Oncol 2017; 12: 791–803. [DOI] [PubMed] [Google Scholar]
- 150. Blank CU, Haanen JB, Ribas A, et al CANCER IMMUNOLOGY. The ‘cancer immunogram’. Science 2016; 352: 658–660. [DOI] [PubMed] [Google Scholar]
- 151. Alexandrov LB, Nik‐Zainal S, Wedge DC, et al Signatures of mutational processes in human cancer. Nature 2013; 500: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lawrence MS, Stojanov P, Polak P, et al Mutational heterogeneity in cancer and the search for new cancer‐associated genes. Nature 2013; 499: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kim J, Kwiatkowski D, McConkey DJ, et al The Cancer Genome Atlas expression subtypes stratify response to checkpoint inhibition in advanced urothelial cancer and identify a subset of patients with high survival probability. Eur Urol 2019; 75: 961–964. [DOI] [PubMed] [Google Scholar]
- 154. Higgs BW, Morehouse CA, Streicher K, et al Interferon gamma messenger RNA signature in tumor biopsies predicts outcomes in patients with non‐small cell lung carcinoma or urothelial cancer treated with durvalumab. Clin Cancer Res 2018; 24: 3857–3866. [DOI] [PubMed] [Google Scholar]
- 155. Wang L, Saci A, Szabo PM, et al EMT‐ and stroma‐related gene expression and resistance to PD‐1 blockade in urothelial cancer. Nat Commun 2018; 9: 3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Anantharaman A, Friedlander T, Lu D, et al Programmed death‐ligand 1 (PD‐L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer 2016; 16: 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Verdegaal EM, de Miranda NF, Visser M, et al Neoantigen landscape dynamics during human melanoma‐T cell interactions. Nature 2016; 536: 91–95. [DOI] [PubMed] [Google Scholar]
- 158. Riaz N, Havel JJ, Makarov V, et al Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 2017; 171: 934–949 e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Huang AC, Postow MA, Orlowski RJ, et al T‐cell invigoration to tumour burden ratio associated with anti‐PD‐1 response. Nature 2017; 545: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zappasodi R, Budhu S, Hellmann MD, et al Non‐conventional inhibitory CD4(+)Foxp3(‐)PD‐1(hi) T cells as a biomarker of immune checkpoint blockade activity. Cancer Cell 2018; 33: 1017–1032 e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Huang AC, Orlowski RJ, Xu X, et al A single dose of neoadjuvant PD‐1 blockade predicts clinical outcomes in resectable melanoma. Nat Med 2019; 25: 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chen PL, Roh W, Reuben A, et al Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 2016; 6: 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Marusyk A, Almendro V, Polyak K. Intra‐tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 2012; 12: 323–334. [DOI] [PubMed] [Google Scholar]
- 164. Schwarzenbach H, Hoon DS, Pantel K. Cell‐free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011; 11: 426–437. [DOI] [PubMed] [Google Scholar]
- 165. Bardelli A, Pantel K. Liquid biopsies, what we do not know (yet). Cancer Cell 2017; 31: 172–179. [DOI] [PubMed] [Google Scholar]
- 166. Siravegna G, Marsoni S, Siena S, et al Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017; 14: 531–548. [DOI] [PubMed] [Google Scholar]
- 167. Buder‐Bakhaya K, Hassel JC. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment‐a review from the melanoma perspective and beyond. Front Immunol 2018; 9: 1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kitano S, Nakayama T, Yamashita M. Biomarkers for immune checkpoint inhibitors in melanoma. Front Oncol 2018; 8: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Hong X, Sullivan RJ, Kalinich M, et al Molecular signatures of circulating melanoma cells for monitoring early response to immune checkpoint therapy. Proc Natl Acad Sci U S A 2018; 115: 2467–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bao H, Bai T, Takata K, et al High expression of carcinoembryonic antigen and telomerase reverse transcriptase in circulating tumor cells is associated with poor clinical response to the immune checkpoint inhibitor nivolumab. Oncol Lett 2018; 15: 3061–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Khagi Y, Goodman AM, Daniels GA, et al Hypermutated circulating tumor DNA: correlation with response to checkpoint inhibitor‐based immunotherapy. Clin Cancer Res 2017; 23: 5729–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Chasseuil E, Saint‐Jean M, Chasseuil H, et al Blood predictive biomarkers for nivolumab in advanced melanoma. Acta Derm Venereol 2018; 98: 406–410. [DOI] [PubMed] [Google Scholar]
- 173. Fujisawa Y, Yoshino K, Otsuka A, et al Baseline neutrophil to lymphocyte ratio combined with serum lactate dehydrogenase level associated with outcome of nivolumab immunotherapy in a Japanese advanced melanoma population. Br J Dermatol 2018; 179: 213–215. [DOI] [PubMed] [Google Scholar]
- 174. Diem S, Schmid S, Krapf M, et al Neutrophil‐to‐Lymphocyte ratio (NLR) and platelet‐to‐Lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017; 111: 176–181. [DOI] [PubMed] [Google Scholar]
- 175. Jeyakumar G, Kim S, Bumma N, et al Neutrophil lymphocyte ratio and duration of prior anti‐angiogenic therapy as biomarkers in metastatic RCC receiving immune checkpoint inhibitor therapy. J Immunother Cancer 2017; 5: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Bagley SJ, Kothari S, Aggarwal C, et al Pretreatment neutrophil‐to‐lymphocyte ratio as a marker of outcomes in nivolumab‐treated patients with advanced non‐small‐cell lung cancer. Lung Cancer 2017; 106: 1–7. [DOI] [PubMed] [Google Scholar]
- 177. Kamphorst AO, Pillai RN, Yang S, et al Proliferation of PD‐1+ CD8 T cells in peripheral blood after PD‐1‐targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017; 114: 4993–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Takeuchi Y, Tanemura A, Tada Y, et al Clinical response to PD‐1 blockade correlates with a sub‐fraction of peripheral central memory CD4+ T cells in patients with malignant melanoma. Int Immunol 2018; 30: 13–22. [DOI] [PubMed] [Google Scholar]
- 179. Nonomura Y, Otsuka A, Nakashima C, et al Peripheral blood Th9 cells are a possible pharmacodynamic biomarker of nivolumab treatment efficacy in metastatic melanoma patients. Oncoimmunology 2016; 5: e1248327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Subrahmanyam PB, Dong Z, Gusenleitner D, et al Distinct predictive biomarker candidates for response to anti‐CTLA‐4 and anti‐PD‐1 immunotherapy in melanoma patients. J Immunother Cancer 2018; 6: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Zhou J, Mahoney KM, Giobbie‐Hurder A, et al Soluble PD‐L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res 2017; 5: 480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Morello S, Capone M, Sorrentino C, et al Soluble CD73 as biomarker in patients with metastatic melanoma patients treated with nivolumab. J Transl Med 2017; 15: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Oya Y, Yoshida T, Kuroda H, et al Predictive clinical parameters for the response of nivolumab in pretreated advanced non‐small‐cell lung cancer. Oncotarget 2017; 8: 103117–103128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Maccalli C, Giannarelli D, Chiarucci C, et al Soluble NKG2D ligands are biomarkers associated with the clinical outcome to immune checkpoint blockade therapy of metastatic melanoma patients. Oncoimmunology 2017; 6: e1323618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Weber JS, Sznol M, Sullivan RJ, et al A serum protein signature associated with outcome after anti‐PD‐1 therapy in metastatic melanoma. Cancer Immunol Res 2018; 6: 79–86. [DOI] [PubMed] [Google Scholar]
- 186. Sanmamed MF, Perez‐Gracia JL, Schalper KA, et al Changes in serum interleukin‐8 (IL‐8) levels reflect and predict response to anti‐PD‐1 treatment in melanoma and non‐small‐cell lung cancer patients. Ann Oncol 2017; 28: 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Yamazaki N, Kiyohara Y, Uhara H, et al Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci 2017; 108: 1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Bigot F, Castanon E, Baldini C, et al Prospective validation of a prognostic score for patients in immunotherapy phase I trials: the Gustave Roussy Immune Score (GRIm‐Score). Eur J Cancer 2017; 84: 212–218. [DOI] [PubMed] [Google Scholar]
- 189. Mathe G, Amiel JL, Schwarzenberg L, et al Active immunotherapy for acute lymphoblastic leukaemia. Lancet 1969; 1: 697–699. [DOI] [PubMed] [Google Scholar]
- 190. Morton DL, Eilber FR, Holmes EC, et al BCG immunotherapy of malignant melanoma: summary of a seven‐year experience. Ann Surg 1974; 180: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Morton DL, Eilber FR, Malmgren RA, et al Immunological factors which influence response to immunotherapy in malignant melanoma. Surgery 1970; 68: 158–163. [PubMed] [Google Scholar]
- 192. Liu JJ, Duan XY. PA‐MSHA induces apoptosis and suppresses metastasis by tumor associated macrophages in bladder cancer cells. Cancer Cell Int 2017; 17: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Eggermont AM, Maio M, Robert C. Immune checkpoint inhibitors in melanoma provide the cornerstones for curative therapies. Semin Oncol 2015; 42: 429–435. [DOI] [PubMed] [Google Scholar]
- 194. O'Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T‐lymphocyte antigen‐4 (CTLA‐4): a novel strategy for the treatment of melanoma and other malignancies. Cancer 2007; 110: 2614–2627. [DOI] [PubMed] [Google Scholar]
- 195. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 196. Le DT, Uram JN, Wang H, et al PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Rizvi NA, Hellmann MD, Snyder A, et al Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Snyder A, Makarov V, Merghoub T, et al Genetic basis for clinical response to CTLA‐4 blockade in melanoma. N Engl J Med 2014; 371: 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Greig SL. Talimogene laherparepvec: first global approval. Drugs 2016; 76: 147–154. [DOI] [PubMed] [Google Scholar]
- 200. Brancato SJ, Lewi K, Agarwal PK. Evolving immunotherapy strategies in urothelial cancer. Am Soc Clin Oncol Educ Book 2015; e284–e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Audenet F, Isharwal S, Cha EK, et al Clonal relatedness and mutational differences between upper tract and bladder urothelial carcinoma. Clin Cancer Res 2018; 25: 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Christensen E, Nordentoft I, Vang S, et al Optimized targeted sequencing of cell‐free plasma DNA from bladder cancer patients. Sci Rep 2018; 8: 1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Hovelson DH, Udager AM, McDaniel AS, et al Targeted DNA and RNA sequencing of paired urothelial and squamous bladder cancers reveals discordant genomic and transcriptomic events and unique therapeutic implications. Eur Urol 2018; 74: 741–753. [DOI] [PubMed] [Google Scholar]
- 204. Hurst CD, Alder O, Platt FM, et al Genomic subtypes of non‐invasive bladder cancer with distinct metabolic profile and female gender bias in KDM6A mutation frequency. Cancer Cell 2017; 32: 701–715 e707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Robertson AG, Kim J, Al‐Ahmadie H, et al Comprehensive molecular characterization of muscle‐invasive bladder cancer. Cell 2017; 171: 540–556 e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Roy S, Pradhan D, Ernst WL, et al Next‐generation sequencing‐based molecular characterization of primary urinary bladder adenocarcinoma. Mod Pathol 2017; 30: 1133–1143. [DOI] [PubMed] [Google Scholar]
- 207. Abbosh PH, Plimack ER. Molecular and clinical insights into the role and significance of mutated dna repair genes in bladder cancer. Bladder Cancer 2018; 4: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Li N, Yang L, Zhang Y, et al Human papillomavirus infection and bladder cancer risk: a meta‐analysis. J Infect Dis 2011; 204: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. US National Library of Medicine Internet . http://ClinicalTrials.gov. Record No. 02808143. [Accessed 14 November 2018]. Available from: https://clinicaltrials.gov/show/NCT02808143
- 210. US National Library of Medicine Internet . http://CrlinicalTrials.gov. Record No. 02324582. [Accessed 14 November 2018]. Available from: https://clinicaltrials.gov/show/NCT02324582
- 211. Iacono D, Cinausero M, Gerratana L, et al Tumour‐infiltrating lymphocytes, programmed death ligand 1 and cyclooxygenase‐2 expression in skin melanoma of elderly patients: clinicopathological correlations. Melanoma Res 2018; 28: 547–554. [DOI] [PubMed] [Google Scholar]
- 212. Conde E, Caminoa A, Dominguez C, et al Aligning digital CD8(+) scoring and targeted next‐generation sequencing with programmed death ligand 1 expression: a pragmatic approach in early‐stage squamous cell lung carcinoma. Histopathology 2018; 72: 270–284. [DOI] [PubMed] [Google Scholar]
- 213. Cooper WA, Tran T, Vilain RE, et al PD‐L1 expression is a favorable prognostic factor in early stage non‐small cell carcinoma. Lung Cancer 2015; 89: 181–188. [DOI] [PubMed] [Google Scholar]
- 214. Hirai A, Yoneda K, Shimajiri S, et al Prognostic impact of programmed death‐ligand 1 expression in correlation with human leukocyte antigen class I expression status in stage I adenocarcinoma of the lung. J Thorac Cardiovasc Surg 2018; 155: 382–392 e381. [DOI] [PubMed] [Google Scholar]
- 215. Yu H, Chen Z, Ballman KV, et al Correlation of PD‐L1 expression with tumor mutation burden and gene signatures for prognosis in early‐stage squamous cell lung carcinoma. J Thorac Oncol 2018; 14: 25–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Anti‐PD‐1/PD‐L1 immunotherapy clinical trials in bladder/urothelial cancer published in peer‐reviewed journals
Table S2. Potential predictive biomarkers for anti‐PD‐1/PD‐L1 immunotherapy response


