Abstract
Background
A video and medical data recorder in the operating theatre is possible, but concerns over privacy, data use and litigation have limited widespread implementation. The literature on legal considerations and challenges to overcome, and guidelines related to use of data recording in the surgical environment, are presented in this narrative review.
Methods
A review of PubMed and Embase databases and Cochrane Library was undertaken. International jurisprudence on the topic was searched. Practice recommendations and legal perspectives were acquired based on experience with implementation and use of a video and medical data recorder in the operating theatre.
Results
After removing duplicates, 116 citations were retrieved and abstracts screened; 31 articles were assessed for eligibility and 20 papers were finally included. According to the European General Data Protection Regulation and US Health Insurance Portability and Accountability Act, researchers are required to make sure that personal data collected from patients and healthcare professionals are used fairly and lawfully, for limited and specifically stated purposes, in an adequate and relevant manner, kept safe and secure, and stored for no longer than is absolutely necessary. Data collected for the sole purpose of healthcare quality improvement are not required to be added to the patient's medical record.
Conclusion
Transparency on the use and purpose of recorded data should be ensured to both staff and patients. The recorded video data do not need to be used as evidence in court if patient medical records are well maintained. Clear legislation on data responsibility is needed to use the medical recorder optimally for quality improvement initiatives.
A video and medical data recorder in the operating theatre is possible, but concerns over privacy, data use and litigation have limited widespread implementation. Transparency on the use and purpose of the recorded data should be ensured to both staff and patients. Clear legislation on data responsibility is needed to use the medical recorder optimally for quality improvement initiatives.

Coming to a theatre near you
Antecedentes
Es posible instalar un sistema de video y grabación de datos médicos en el quirófano, pero su implementación se ha visto limitada por las dudas relativas a la privacidad, uso de datos y aspectos de litigio. Estas dudas deberían superarse, motivo por el que en este trabajo se proponen unas guías sobre el uso de sistemas de registro en el ambiente quirúrgico.
Métodos
Se realizó una revisión en las bases de datos Pubmed y Embase y de la Biblioteca Cochrane. Se buscó la jurisprudencia internacional sobre el tema. Se establecieron unas recomendaciones prácticas y de las perspectivas legales adquiridas a través de la experiencia de la implementación y el uso de sistemas de video y registro de datos médicos en el quirófano.
Resultados
Se obtuvieron 116 referencias, de las que una vez eliminadas las duplicadas (n = 5) y revisados los resumenes, 31 artículos cumplían los criterios de eligibilidad. En el estudio final se incluyeron 20 artículos. De acuerdo con la Ley Orgánica de Protección de Datos (General Data Protection Regulation , GDRP) y la Ley de Transferencia y Responsabilidad de Seguro Médico (Health Insurance Portability and Accountability Act, HIPAA), los investigadores deben asegurar que los datos personales recopilados pertenecientes a los pacientes y profesionales de la salud se utilicen de manera justa y legal, con fines definidos y bien establecidos, de manera adecuada y relevante, y mantenidos a resguardo y almacenados no más tiempo del estrictamente necesario. No es necesario que los datos recopilados con el único propósito de mejorar la calidad de la atención médica se agreguen a la historia clínica del paciente.
Conclusión
Se debe asegurar por parte del personal sanitario como del paciente, la transparencia tanto en la utilización como en el objetivo de los datos almacenados. Los datos registrados en video no es necesario que sean usados como evidencia en procesos judiciales si la historia clínica de los pacientes cumple los estandares establecidos. Se precisa una legislación clara sobre la responsabilidad de los datos para la utilización óptima de los registros médicos en las iniciativas de mejora de la calidad.
Introduction
The number of healthcare professionals using an audio, video or complete data recorder in the surgical environment, sometimes referred to as a medical data recorder (MDR) or ‘black box’, is increasing1, 2, 3. A MDR is able to record operational data (for example from overview cameras, laparoscopic cameras, anaesthetic and environmental equipment), enabling analysis of technical and non‐technical elements4. It provides theatre staff the opportunity to learn from their performance or suboptimal situations to enhance team performance5, 6, 7, 8, 9, 10, 11. Surgical procedures may be recorded for purposes of education, research and quality improvement3, 12. Although this has been associated with a reduction in errors, there are concerns about the adequacy of implementation related to privacy, ownership of data and medical negligence4, 8, 10, 13, 14. Understandably, medical practitioners fear that a MDR could be misused for punitive or controlling purposes, a situation that inevitably leads to scepticism, user resistance and loss of autonomy7, 13, 15. These very real medicolegal concerns are hindering the optimal use of the MDR3, 5.
Other high‐risk industries such as aviation (flight data recorder), offshore oil platforms and maritime transport (voyage data recorder) have used black boxes to analyse suboptimal situations and errors for quite some time16. In these industries, they have been embedded in legal and operational frameworks that are sorely lacking in the surgical environment7, 17. This study reviewed the privacy law concerns, medicolegal considerations and universal legal requirements regarding MDR use.
Methods
A comprehensive search for peer‐reviewed literature published in the past 12 years (January 2007 to December 2018) was conducted using the PubMed and Embase databases and the Cochrane Library. The following search terms were included: video recording, operating room, theatre, endoscopic, medicolegal, legislation, ethics and law. Non‐English and non‐Dutch publications were excluded. The exact search algorithms can be found in Appendix S1 (supporting information). The articles reviewed comprised a broad range of methods, including mainly descriptive, opinion or narrative reviews. For this reason, no attempt was made to grade the levels of evidence systematically or to undertake a statistical analysis18.
In addition, jurisprudence on the topic from North American and European jurisdictions was searched to find examples of medicolegal cases in which video recordings were used as evidence19, 20. A professor of health law at the University of Amsterdam collaborated in this study, to ensure correct interpretation of the legal literature.
Results
The literature search yielded 95 citations from the PubMed database, no review citations from the Cochrane Library and 26 from Embase. After removing duplicates, unrelated fields, abstracts without full text and non‐relevant papers, 20 manuscripts were included in the review (Fig. 1).
Figure 1.
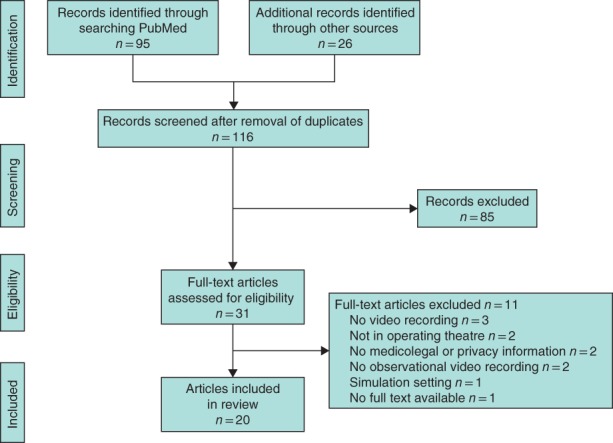
Flow chart showing selection of articles for review
In 2016, one MDR was installed in an ENDOALPHA operating suite (Olympus Europa, Hamburg, Germany) in the Amsterdam University Medical Centre4, 21. It has since been used to record selected laparoscopic abdominal procedures. This recorder is able to capture a multitude of data streams (overview cameras, laparoscopic camera, microphones, anaesthesia monitor). Procedures were recorded between the time‐out and sign‐out time stamp of the surgical procedure22, 23. These recordings were analysed by a specialized trained team in Toronto, Canada4. The performance report generated was used as a tool for structured postoperative team debriefing24, 25.
Aviation safety system perspective
The safety initiatives of the aviation industry have been compared with those of healthcare15, 26, 27.
Following a series of high‐profile crashes that threatened the sustainability of the passenger jet industry, the National Aeronautics and Space Administration (NASA) research community and regulatory industries led investigations in the 1970s28. Since then, as part of joint NASA and Federal Aviation Administration (FAA) initiatives, behavioural science researchers have scrutinized tens of thousands of simulator and live flights. These recognized human performance as factors in aviation safety29, 30, 31. NASA now operates an Aviation Safety Reporting System (ASRS) that offers the incentives of anonymity and immunity to pilots who report an unsafe situation within 10 days of its occurrence26. All identifying information in the report is then removed before the incident is investigated and any lessons are publicized. Later, if the FAA attempts to take punitive action against those involved, the ASRS reference number provides evidence of a constructive safety attitude, such that penalties are not imposed (provided that the mistakes were inadvertent and did not constitute a criminal offence)26.
Safety management system requirements have also been introduced into European Union (EU) law. The European aviation safety system is based on a comprehensive set of common safety rules, which are overseen by the European Commission, the European Aviation Safety Agency and the National Aviation Authorities. These rules are directly applicable to all EU member states17. In addition, the EU has regulated the reporting, analysis and follow‐up of aviation safety threats32. The current legislation sets out how relevant safety information relating to civil aviation is reported, collected, stored, protected, exchanged, disseminated, analysed and acted upon17, 33.
The aviation industry holds Six Sigma (nearly perfect) safety records, because it uses the system approach, deals with errors non‐punitively yet proactively, and reduces the consequences of error before escalation28, 34, 35, 36. This way of reporting and managing error results in a ‘just culture’, where aviation professionals feel confident to report events (even their own mistakes), by promoting balanced accountability for individuals and organizations responsible17. This is a critical ingredient to the creation of a safety culture37. Other high‐risk industries have adopted this philosophy, accepting that human error is both inevitable and ubiquitous36. The medical profession has incorporated some of these safety lessons30, 31, 38.
In the past few years, the number of patients harmed by medical error has gained public attention. Some of these mishaps have reached unsatisfactory conclusions for all involved parties31, 39. The medical profession traditionally employs the personal approach, which acts as a disincentive to voluntary reporting, and inhibits the search for systemic conditions or triggers that lead to error40, 41. These conclusions have resulted in several national and international guidelines and regulations, aimed at the broad implementation of safety systems that address human factors, such as teamwork and communication37, 41, 42.
Privacy perspective
The use of a MDR should conform to certain rules and requirements relating to the privacy of both the healthcare professional and the patient2, 43. Throughout Western legislation, privacy laws relating to personal data, medical records and professional confidentiality apply to MDRs44, 45, 46, 47. The new European General Data Protection Regulation (GDPR) took effect in May 2018. It was designed to harmonize all the data privacy laws across the EU48, 49. It has a processing obligation that requires all individuals involved to be strictly and clearly informed about what happens to their personal data44, 48, 49. Researchers are respectively required to make sure that personal data collected from patients and healthcare professionals are used fairly and lawfully, for limited and specifically stated purposes, in an adequate, relevant and sober manner, and kept safe and secure and stored for no longer than is absolutely necessary47, 50, 51, 52.
The privacy‐by‐design principle is of great importance, regardless of the country in which a project collecting medical data using a MDR is carried out48, 53. According to this principle, the privacy of the users has to be taken into account from the very beginning of engineering the system, mainly by making optimal use of privacy‐enhancing technical solutions54, 55. Thus, video, audio and medical data related to healthcare staff should be anonymized as early as possible. This entails deidentifying the data (for example by voice alteration and image blurring), so that it cannot be linked back to the person56. The Health Insurance Portability and Accountability Act (HIPAA) in the USA, the Personal Information Protection and Electronic Documents Act in Canada and the GDPR in the EU require data protection with confidentiality and integrity57. Furthermore, they require that identifiable personal health information in any form, either electronic, written or oral, should be made available to patients3. As Henken and colleagues43 state in their review, the distinction between information that must be included in a patient record and information that can be excluded is not as clear in the USA as in the EU. However, as in the GDPR, the HIPAA allows for the use of limited data sets (deidentified) for the purposes of research and quality improvement initiatives57.
In laparoscopic surgery, the patient's consent to the making of an intra‐abdominal video could be included in the informed consent for the complete treatment, as it is used to perform the surgical procedure58, 59, 60, 61, 62. Consequently, only the laparoscopically generated video stream, but not the operating room overview video stream in which the theatre staff is visible, becomes part of the patient's medical record63. The GDPR data retention rule of thumb is ‘as long as necessary, as short as possible’48. Data included in the patient's medical record must be accessible to the patient and stored for at least 5 years, depending on the country and state the patient is treated in64, 65.
Medicolegal perspective
Data collected by a MDR for the sole purpose of quality improvement and training of the operating team is not intended to be used for patient diagnosis, evaluation or treatment. The patient's medical record should only include information relevant to the patient's health and healthcare7, 51, 66. Thus, such data should not be added to the patient's medical record nor handed over to the patient or their legal representatives3, 7. This does not preclude the healthcare professional from reporting a calamity or a ‘near miss’ just as in an unrecorded surgical procedure. In the face of such an event, it is common for hospital protocols in North America and most European countries to require that the patient is informed of the situation as early as possible, and the incident clearly noted in the patient's medical record37, 40, 61, 67.
In the case of a serious adverse event (a critical unexpected incident with the outcome severe injury or death) resulting in a lawsuit, a judge may decide to breach the legal protection of the healthcare professional by asking the institute for the video MDR data. However, reported cases indicate that in most jurisdictions judges are aware of the importance of protecting information that is collected for the sole purpose of quality improvement, and will breach this protection only if vital information is lacking in the medical record and cannot be retrieved in any other way7, 44, 68, 69. However, even if video data have to be provided, various court cases have demonstrated that these recordings actually predominantly lend legal support specifically to the healthcare professional or surgeon43, 70, 71, 72, 73, 74, 75, 76. An American medical malpractice claim showed that a surgeon could indeed prove, with the help of reviewing the videotape of the laparoscopic cholecystectomy in court, that the standard of care was not breached72. In a similar case, a Dutch urologist proved that he did not act negligently during the nephrectomy by showing the video recording of the procedure71.
Hoschtitzky and colleagues (London, UK)70 demonstrated in their care report that the video recording provided supportive evidence of good practice and an open attitude to patient safety. With the help of the video recording, they were able to document all the surgical steps accurately and it allowed them to state confidently that no missing equipment was inadvertently left behind in the patient. On the other hand, in January 2016, a Dutch surgeon had a medical malpractice suit filed against him after a complicated cholecystectomy. He was unable to prove that he obtained the critical view of safety because he could not show the judge the video recordings. The surgeon was hence found guilty73. Besides that, when privileged information is used in court without justification, both American and European laws contain provisions that have consequences in favour of the unjustly accused37.
Discussion
As is often the case with relatively new technology, legal guidelines on the use of MDRs are currently lacking. However, the general privacy principles are clear on how to design such a system and how to optimize conditions for use. Lessons are learned from the aviation industry, and the main issues that should be addressed are related to the privacy and legislation perspectives.
Patients may rely on professional ethics and best judgement in deciding which of the permissive uses of the MDR and disclosures the healthcare professional has to make57. Regardless of the national differences in legislation, the importance of the general privacy principles, to ensure clear consensus and openness between participants and researchers about the methods and purpose of the MDR, is to be highlighted2, 56, 77. Any possible information that might identify the patient or healthcare provider should either be blurred, scrambled or, whenever possible, removed as early as possible and not be reflected in the reporting output. Most importantly, as the patient is not the object of the study itself, patient identifiers should be removed. This means that written informed consent does not necessarily have to be obtained from the patient57, 78, 79. According to the general privacy rules, an opt‐out option is sufficient and should be provided to the patient in a timely manner, with their decision clearly noted in the medical record3, 5, 80.
As far as the operating theatre staff is concerned, authors recommend that theatre staff, including medical students, are asked formally, upon embarking on such a quality initiative, to volunteer to work with the innovation62. An official informed consent stating the purpose of the data recordings, where the data recordings are analysed, what the expected benefits for the participants are, and how the data are stored securely may help in gaining support and momentum for the MDR initiative48, 81, 82. It should be emphasized that their safety and personal privacy is protected, ensuring full transparency of the methods used58, 80, 83, 84, 85. Based on this review and the authors' experience, an overview of the recommended practice and legal guidelines is presented in Table 1.
Table 1.
Key dimension, recommendations and legal guidelines on the use of a medical data recorder in the operating theatre
| Key dimension | Practice recommendations | Legal implications |
|---|---|---|
| What is the purpose? | Quality improvement, such as structured team debriefing or enhanced morbidity and mortality meetings. The purpose of data collection is for theatre staff to learn from what went well and what can be done better | It is important that the goal is clearly specified. When a MDR is used in the authors' centre, the patient is not the main focus of the initiative. The purpose is quality improvement of operating teams and workflow, or support of hospital quality safeguarding systems. Hence, the data are not required to be added to the patient's medical file. Only the laparoscopic camera footage is added and accessible to patient, in accordance with standard protocol |
| Who and what do the data cover? | The theatre staff is being recorded using audio and video during the surgical procedure; patient parameters on the anaesthesia monitor and the laparoscopic camera views are recorded. Other data sources considered to be of relevance may be added to the data set collected (door movements, room temperature, etc.) | Given that the purpose is quality improvement, patient consent may be assumed. The patient needs to be informed about planning of the operation by the surgeon and has the possibility to opt out without negative consequences (no delay in planning). The MDR is used as a quality improvement tool and so, if adequate safeguards are put in place, the hospital may state that the theatre staff is expected to participate |
| What about privacy and the privacy‐by‐design principle? | Recordings may initially collect, but not process, the patient's personal identifiers. The patient's personal identifiers need to be stripped from the file as soon as possible (deidentification). Faces of theatre staff need to be scrambled and voices altered. To protect the patient's privacy maximally, it is advised that their face and genitals are not recorded by cameras when this serves no purpose |
General privacy principles must be respected Data are kept safe and secure, and stored for no longer than is absolutely necessary. The privacy of staff and patients needs to be taken into account from the very beginning by making use of privacy‐enhancing technical solutions |
| Who is responsible for the data? | The hospital needs to assign a responsible person for the MDR. In trial settings, project coordinators and principal investigators are responsible for collecting the data and secure storage of the outcome report. In this case, the original data set (including video recordings) is sent immediately to the data analysis centre and, after it has been analysed, the pseudoanonymous outcome report is sent back. The original data are deleted, as the purpose of the original data has been fulfilled and the original data are no longer needed | An official agreement on confidentiality signed by the hospital directorate assures that the original and outcome data cannot be requested and used for any purpose other than that stated in the agreement Clear legislation is needed to make sure the inspectorate and other external parties cannot request the data |
| Which format should the data be in? | The original recordings are used for systematic analysis of the theatre team's performance. A performance report is created. Only the performance report, enhanced with video clips, is presented to the team. As soon as the performance report has been created (in this case within 48 h), the original data are deleted |
The general privacy‐by‐design principles Data are used fairly, and for limited and specifically stated purposes in an adequate and relevant manner. The performance report is stripped of any identifiable information. To enhance privacy, the faces of theatre staff are blurred and voices altered |
MDR, medical data recorder.
Informing patients about having a MDR that is used solely for the purposes of team debriefing may significantly contribute to the patient's trust, as most of them value this quality improvement measure. Regardless of this, healthcare professionals should not ignore the fact that, in time, society may shift towards favouring the idea that MDR‐generated video and data recordings should be accessible to patients, next to the information that is already accessible via their medical record70, 86, 87, 88. In the future, society may decide to choose transparency over the medicolegal concerns of medical employees and demand full legal access for the patient to the information generated by MDRs4, 85. In the USA, the state of Wisconsin89 has already drafted legislation to allow patients to access video recordings of their surgical procedures. If future legislation were to support the position that the MDR should become part of standard care, and if the output should become part of the patient's medical record, healthcare professionals would be bound to work in a continuously monitored environment, where all results are accessible to patients. This may be an argument for organizations to start exploring optimal use of MDRs, which may secure optimal conditions for both patient and providers, as soon as possible.
In the authors' opinion, the fear that a MDR bears an increased risk of medical negligence litigation, limited performance or loss of professional status is unjustified, as long as good professional standards of patient medical record keeping and reporting of adverse events are maintained31, 39, 40, 70, 75, 90. To help dissipate any remaining fear, resistance or doubt, the principal investigator of the MDR project can instigate an official agreement on confidentiality signed and supported by the hospital directorate. The researchers and the institute are, in accordance with the official agreement, bound to refuse the disclosure of any output obtained by the MDR77.
It is important to emphasize that, if a severe adverse event occurs, video recordings usually help rather than harm the healthcare professionals involved. The chain of (re)actions and decisions resulting in the unwanted event are better understood with the objective help of the MDR. MDR data may help in augmenting the analysis of a calamity or near miss when constructing a public calamity report. The data source itself is protected by law. Besides, if he or she has provided reasonable quality standard of care, no punitive measures can be imposed91, 92, 93. Nevertheless, several hospitals in the USA ceased video recording after receiving legal advice to do so, as a result of their medicolegal concerns and the introduction of the HIPAA in 19963. Hospital administrators, especially in the USA, are often extra cautious, owing to an increasingly hostile medicolegal environment12. Plenty of court cases have demonstrated that video recordings actually lend legal support to the healthcare professional or surgeon70, 71, 72, 73, 74, 75, 76.
Healthcare professionals who are not well informed may also respond reluctantly to the use of a MDR, because they are afraid they will have to behave differently: ‘Can I still play music, make jokes or use bad language?’. It is important to take this viewpoint into account as well. Differences in staff perceptions of good behaviour may exist among team members working in a high‐risk environment for behaviour that unsettles the team94, 95, 96, 97, 98, 99. Disturbing behaviour or even bullying in the operating theatre, such as inappropriate joking or degrading comments, usually goes unreported and is considered part of the job99, 100, 101. Team members may feel powerless to address certain behaviour while it is occurring96, 102. The ultimate impact of these issues is poor teamwork and an increased risk of adverse perioperative events94, 99, 103, 104, 105. Being able to look back on shared performance in a safe, neutral and moderated setting may help all team members get a clearer perspective on the situation. Indeed, it may help healthcare institutions in the further development of a framework for dealing with disruptive behaviour. This would ensure a productive, healthy and safe working environment, which is focused on education and rehabilitation rather than punishment106. Systematic postoperative team debriefing using a MDR, led by an independent facilitator, may help in objective assessment of issues that have traditionally been ignored, creating a unique opportunity to discuss appropriate solutions with the entire operating team safely and respectfully.
Supporting information
Appendix S1. Search details
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Disclosure: The authors declare no conflict of interest.
References
- 1. Gambadauro P, Magos A. Digital video recordings for training, assessment, and revalidation of surgical skills. Surg Technol Int 2010; 20: 36–39. [PubMed] [Google Scholar]
- 2. Prigoff JG, Sherwin M, Divino CM. Ethical recommendations for video recording in the operating room. Ann Surg 2016; 264: 34–35. [DOI] [PubMed] [Google Scholar]
- 3. Taylor K, Mayell A, Vandenberg S, Blanchard N, Parshuram CS. Prevalence and indications for video recording in the health care setting in North American and British paediatric hospitals. Paediatr Child Health 2011; 16: e57–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg 2017; 152: 972–973. [DOI] [PubMed] [Google Scholar]
- 5. Makary MA. The power of video recording: taking quality to the next level. JAMA 2013; 309: 1591–1592. [DOI] [PubMed] [Google Scholar]
- 6. Grenda TR, Pradarelli JC, Dimick JB. Using surgical video to improve technique and skill. Ann Surg 2016; 264: 32–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henken KR, Jansen FW, Klein J, Stassen LP, Dankelman J, van den Dobbelsteen JJ. Implications of the law on video recording in clinical practice. Surg Endosc 2012; 26: 2909–2916. [DOI] [PubMed] [Google Scholar]
- 8. Oremakinde AA, Bernstein M. A reduction in errors is associated with prospectively recording them. J Neurosurg 2014; 121: 297–304. [DOI] [PubMed] [Google Scholar]
- 9. Hu YY, Parker SH, Lipsitz SR, Arriaga AF, Peyre SE, Corso KA et al. Surgeons' leadership styles and team behavior in the operating room. J Am Coll Surg 2016; 222: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Mahoney PR, Yeo HL, Lange MM, Milsom JW. Driving surgical quality using operative video. Surg Innov 2016; 23: 337–340. [DOI] [PubMed] [Google Scholar]
- 11. Hu YY, Greenberg CC. Patient safety in surgical oncology: perspective from the operating room. Surg Oncol Clin N Am 2012; 21: 467–478 ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campbell S, Sosa JA, Rabinovici R, Frankel H. Do not roll the videotape: effects of the health insurance portability and accountability act and the law on trauma videotaping practices. Am J Surg 2006; 191: 183–190. [DOI] [PubMed] [Google Scholar]
- 13. Silas MR, Grassia P, Langerman A. Video recording of the operating room – is anonymity possible? J Surg Res 2015; 197: 272–276. [DOI] [PubMed] [Google Scholar]
- 14. Birkmeyer JD, Finks JF, O'Reilly A, Oerline M, Carlin AM, Nunn AR et al.; Michigan Bariatric Surgery Collaborative. Surgical skill and complication rates after bariatric surgery. N Engl J Med 2013; 369: 1434–1442. [DOI] [PubMed] [Google Scholar]
- 15. Xiao Y, Schimpff S, Mackenzie C, Merrell R, Entin E, Voigt R et al. Video technology to advance safety in the operating room and perioperative environment. Surg Innov 2007; 14: 52–61. [DOI] [PubMed] [Google Scholar]
- 16. Kontogiannis T, Malakis S. A proactive approach to human error detection and identification in aviation and air traffic control. Saf Sci 2009; 47: 693–706. [Google Scholar]
- 17. European Commission . Aviation Safety Policy in Europe; 2018. https://ec.europa.eu/transport/modes/air/safety_en [accessed 19 March 2018].
- 18. Sawyer T, Eppich W, Brett‐Fleegler M, Grant V, Cheng A. More than one way to debrief: a critical review of healthcare simulation debriefing methods. Simul Healthc 2016; 11: 209–217. [DOI] [PubMed] [Google Scholar]
- 19.Leagle. The Leagle Lawyer Directory. http://www.leagle.com/ [accessed 16 August 2018].
- 20.de Rechtspraak. Uitspraken. https://www.rechtspraak.nl/Uitspraken-en-nieuws/Uitspraken [accessed 12 June 2018].
- 21.Olympus. ENDOALPHA Olympus Europe (Operating Room Integration) https://www.olympus-europa.com/medical/en/Products-and-Solutions/Medical-Solutions/OR-Integration/2018 [accessed 13 February 2018].
- 22. de Vries E, Smorenburg S, Gouma D, Boermeester M. Een checklist voor het gehele chirurgische traject. Ned Tijdschr Evid Based Pract 2008; 6: 17–19. [Google Scholar]
- 23. Rydenfält C, Johansson G, Odenrick P, Åkerman K, Larsson PA. Compliance with the WHO Surgical Safety Checklist: deviations and possible improvements. Int J Qual Health Care 2013; 25: 182–187. [DOI] [PubMed] [Google Scholar]
- 24. van Dalen ASHM, Jansen M, van Haperen M, van Dieren S, Buskens CJ, Nieveen van Dijkum EJM et al. Implementing structured team debriefing with the use of an operating room black box to improve surgical safety; results of a pilot study. Surg Endosc 2018; 32(Suppl 2): S434. [Google Scholar]
- 25. van Dalen ASHM, van Haperen M, Swinkels JA, Grantcharov TP, Schijven MP. Debriefing using medical data recorders in the operating room: this is how we do it. Surg Endosc 2017; 31(Suppl 2): S187. [Google Scholar]
- 26. Lewis GH, Vaithianathan R, Hockey PM, Hirst G, Bagian JP. Counterheroism, common knowledge, and ergonomics: concepts from aviation that could improve patient safety. Milbank Q 2011; 89: 4–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guerlain S, Adams RB, Turrentine FB, Shin T, Guo H, Collins SR et al. Assessing team performance in the operating room: development and use of a ‘black‐box’ recorder and other tools for the intraoperative environment. J Am Coll Surg 2005; 200: 29–37. [DOI] [PubMed] [Google Scholar]
- 28. Sexton JB, Thomas EJ, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ 2000; 320: 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Helmreich RL. On error management: lessons from aviation. BMJ 2000; 320: 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reason J. Human error: models and management. BMJ 2000; 320: 768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Connor T, Papanikolaou V, Keogh I. Safe surgery, the human factors approach. Surgeon 2010; 8: 93–95. [DOI] [PubMed] [Google Scholar]
- 32. European Commission . European Aviation Safety Management https://ec.europa.eu/transport/modes/air/safety/safety_management_en [accessed 19 March 2018].
- 33. European Parliament and of the Council . Regulation (EU) No. 376/2014; 3 April 2014 http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1446648394542&uri=CELEX:32014R0376 [accessed 19 March 2018].
- 34. Boet S, Bould MD, Sharma B, Revees S, Naik V, Triby E et al. Within‐team debriefing versus instructor‐led debriefing for simulation‐based education: a randomized controlled trial. Ann Surg 2013; 258: 53–58. [DOI] [PubMed] [Google Scholar]
- 35. Moorman DW. On the quest for Six Sigma. Am J Surg 2005; 189: 253–258. [DOI] [PubMed] [Google Scholar]
- 36. Hickey EJ, Nosikova Y, Pham‐Hung E, Gritti M, Schwartz S, Caldarone CA et al. National Aeronautics and Space Administration ‘threat and error’ model applied to pediatric cardiac surgery: error cycles precede ∼85% of patient deaths. J Thorac Cardiovasc Surg 2015; 149: 496–507.e4. [DOI] [PubMed] [Google Scholar]
- 37. Legemaate J. Blame free reporting: international developments In Patient Safety, Law, Policy and Practice, Tingle J, Bark P. (eds). Routledge: London, 2011; 85–96. [Google Scholar]
- 38. WHO . World Alliance for Patient Safety: WHO Draft Guidelines for Adverse Event Reporting and Learning Systems: From Information to Action Report no. WHO/EIP/SPO/QPS/05.3. WHO: Geneva, 2005.
- 39. Groopman J. How Doctors Think. Houghton Mifflin: New York, 2007. [Google Scholar]
- 40. Legemaate J. ‘Just culture’: legal potential and limits. Ned Tijdschr Geneeskd 2017; 161: D1725. [PubMed] [Google Scholar]
- 41. Rodziewicz TL, Hipskind JE. Medical Error Prevention. StatPearls Publishing: Treasure Island, 2018. [PubMed] [Google Scholar]
- 42. Gawande A. Complications: a Surgeon's Notes on an Imperfect Science. Profile Books: London, 2003. [Google Scholar]
- 43. Henken KR, Jansen FW, Klein J, Stassen LP, Dankelman J, van den Dobbelsteen JJ. Implications of the law on video recording in clinical practice. Surg Endosc 2012; 26: 2909–2916. [DOI] [PubMed] [Google Scholar]
- 44. Thorarensen B. The processing of health information – protecting the individual right to privacy through effective legal remedies. Health Technol 2017; 7: 401–413. [Google Scholar]
- 45. Christiansen EK, Skipenes E, Hausken MF, Skeie S, Østbye T, Iversen MM. Shared electronic health record systems: key legal and security challenges. J Diabetes Sci Technol 2017; 11: 1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang DS. Re: ethical recommendations for video recording in the operating room. J Urol 2017; 197: 789. [DOI] [PubMed] [Google Scholar]
- 47. Turnbull AM, Emsley ES. Video recording of ophthalmic surgery – ethical and legal considerations. Surv Ophthalmol 2014; 59: 553–558. [DOI] [PubMed] [Google Scholar]
- 48. European Union . General Data Protection Regulation; 2016. https://autoriteitpersoonsgegevens.nl/sites/default/files/atoms/files/gdpr.pdf [accessed 09 March 2018].
- 49. Allen & Overy . The EU General Data Protection Regulation; 2016. http://www.allenovery.com/SiteCollectionDocuments/Radical%20changes%20to%20European%20data%20protection%20legislation.pdf [accessed 25 August 2018].
- 50. Xiao Y, Dexter F, Hu P, Dutton RP. The use of distributed displays of operating room video when real‐time occupancy status was available. Anesth Analg 2008; 106: 554–560. [DOI] [PubMed] [Google Scholar]
- 51. Ploem C, Gevers S. Introduction of a national electronic patient record in the Netherlands: some legal issues. Eur J Health Law 2011; 18: 191–204. [DOI] [PubMed] [Google Scholar]
- 52. Dougherty M, Washington L. Still seeking the legal EHR. The push for electronic records increases, the record management questions remain. J AHIMA 2010; 81: 42–45. [PubMed] [Google Scholar]
- 53. Hustinx P. Privacy by design: delivering the promises. Identity in the Information Society 2010; 3: 253–255. [Google Scholar]
- 54. Cavoukian A. Privacy by Design, Take the Challenge. Information and Privacy Commissioner of Ontario: Toronto, 2009. [Google Scholar]
- 55. Pagallo U. On the principle of privacy by design and its limits: technology, ethics and the rule of law In European Data Protection: In Good Health? Gutwirth S, Leenes R, De Hert P, Poullet Y. (eds). Springer Netherlands: Dordrecht, 2012; 331–346. [Google Scholar]
- 56. Yamamoto R. Large‐scale health information database and privacy protection. Japan Med Assoc J 2016; 59: 91–109. [PMC free article] [PubMed] [Google Scholar]
- 57. HIPAA Compliance Assistance . Summary of the HIPAA Privacy Rule. Office for Civil Rights: Washington DC, 2003. [Google Scholar]
- 58. Blaauw CB, van den Dobbelsteen JJ, Hubben JH. [Legal aspects of video registration during operations – the digital operating room assistant: opportunity or threat?] Ned Tijdschr Geneeskd 2011; 155: A3487. [PubMed] [Google Scholar]
- 59. Willner N, Peled‐Raz M, Shteinberg D, Shteinberg M, Keren D, Rainis T. Digital recording and documentation of endoscopic procedures: do patients and doctors think alike? Can J Gastroenterol Hepatol 2016; 2016: 2493470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Matsuda K, Tanaka K, Fujishiro M, Saito Y, Ohtsuka K, Oda I et al. Design paper: Japan Endoscopy Database (JED): a prospective, large database project related to gastroenterological endoscopy in Japan. Dig Endosc 2018; 30: 5–19. [DOI] [PubMed] [Google Scholar]
- 61. Cottrill E, Becker SS, Delaurentis D. Pearls and pitfalls: medico‐legal considerations for sinus surgery. Curr Opin Otolaryngol Head Neck Surg 2014; 22: 75–79. [DOI] [PubMed] [Google Scholar]
- 62. van de Graaf FW, Menon AG, Lange MM. [An image says more than a thousand words; standardising video registration in the operating theatre.] Ned Tijdschr Geneeskd 2017; 161: D498. [PubMed] [Google Scholar]
- 63. Schijven MP, Legemate DA, Legemaate J. [Video recording and data collection in the operating room: the way to a ‘just culture'in the OR.] Ned Tijdschr Geneeskd 2017; 161: D1655. [PubMed] [Google Scholar]
- 64. Kierkegaard P. Electronic health record: wiring Europe's healthcare. Comput Law Secur Rev 2011; 27: 503–515. [Google Scholar]
- 65. Tovino SA. The HIPAA Privacy Rule and the EU GDPR: illustrative comparisons. Seton Hall Law Rev 2017; 47: 973–993. [PubMed] [Google Scholar]
- 66. Wynia M, Dunn K. Dreams and nightmares: practical and ethical issues for patients and physicians using personal health records. J Law Med Ethics 2010; 38: 64–73. [DOI] [PubMed] [Google Scholar]
- 67. Hassan M. Informed consent and the law – an English legal perspective. Dig Dis 2008; 26: 23–27. [DOI] [PubMed] [Google Scholar]
- 68. Legemaate J, de Roode R. Veilig melden van incidenten in de gezondheidszorg: voorbeelden van (buitenlandse) wetgeving. Tijdschr Veiligheid 2009; 1: 20–34. [Google Scholar]
- 69. Kerbage Y, Collinet P, Rubod C, Merlot B, Cosson M. Litigation in gynecological surgery: a retrospective study in the French university hospital of Lille between 1997 and 2015. Gynecol Obstet Fertil 2016; 44: 196–199. [DOI] [PubMed] [Google Scholar]
- 70. Hoschtitzky JA, Trivedi DB, Elliott MJ. Saved by the video: added value of recording surgical procedures on video. Ann Thorac Surg 2009; 87: 940–941. [DOI] [PubMed] [Google Scholar]
- 71.Overheid.nl. Gezondheidszorg RTvd. 's Gravenhage; 2012. http://tuchtrecht.overheid.nl/YG2045 [accessed 16 August 2018].
- 72.Court of Appeal of Louisiana FC. Lois Gisclair, Wife of/and Dan GISCLAIR v. Monte M. BONNEVAL, M.D. Louisiana [No.2004 CA 474]; 2005. http://www.leagle.com/decision/2005967928So2d39_1960/GISCLAIR%20v.%20BONNEVAL [accessed 16 August 2018].
- 73. Rechtbank Gelderland . C/05/291197 / HZ RK 15‐86 http://deeplink.rechtspraak.nl/uitspraak?id=ECLI:NL:RBGEL:2016:420 [accessed 16 August 2018].
- 74. Desai N, Gaddam S, Gupta N, Singh M, Balasubramanian G, Kanakadandi V et al. Developing a database of high definition endoscopic videos and images in your institution. Endoscopy 2013; 45: 370–376. [DOI] [PubMed] [Google Scholar]
- 75. Koninckx PR, Stepanian A, Adamyan L, Ussia A, Donnez J, Wattiez A. The digital operating room and the surgeon. Gynecol Surg 2013; 10: 57–62. [Google Scholar]
- 76. Sullivan TP. The evolution of law enforcement attitudes to recording custodial interviews. J Psychiatry Law 2010; 38: 137–175. [Google Scholar]
- 77. Asan O, Montague E. Using video‐based observation research methods in primary care health encounters to evaluate complex interactions. Inform Prim Care 2014; 21: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jacobs B, Duncan JR, Street M, Murray D. Audio and video recording system for routine documentation of fluoroscopic procedures. J Vasc Interv Radiol 2010; 21: 725–729. [DOI] [PubMed] [Google Scholar]
- 79. Burkle CM, Anderson KA, Xiong Y, Guerra AE, Tschida‐Reuter DA. Assessment of the efficiency of language interpreter services in a busy surgical and procedural practice. BMC Health Serv Res 2017; 17: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Birgand G, Azevedo C, Toupet G, Pissard‐Gibollet R, Grandbastien B, Fleury E et al. Attitudes, risk of infection and behaviours in the operating room (the ARIBO Project): a prospective, cross‐sectional study. BMJ Open 2014; 4: e004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Reid AM, Brown JM, Smith JM, Cope AC, Jamieson S. Ethical dilemmas and reflexivity in qualitative research. Perspect Med Educ 2018; 7: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stevens J, Schmied V, Burns E, Dahlen HG. Video ethnography during and after caesarean sections: methodological challenges. J Clin Nurs 2017; 26: 2083–2092. [DOI] [PubMed] [Google Scholar]
- 83. Blikkendaal MD, Driessen SR, Rodrigues SP, Rhemrev JP, Smeets MJ, Dankelman J et al. Surgical flow disturbances in dedicated minimally invasive surgery suites: an observational study to assess its supposed superiority over conventional suites. Surg Endosc 2017; 31: 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hu YY, Mazer LM, Yule SJ, Arriaga AF, Greenberg CC, Lipsitz SR et al. Complementing operating room teaching with video‐based coaching. JAMA Surg 2017; 152: 318–325. [DOI] [PubMed] [Google Scholar]
- 85. Taylor K, Vandenberg S, le Huquet A, Blanchard N, Parshuram CS. Parental attitudes to digital recording: a paediatric hospital survey. J Paediatr Child Health 2011; 47: 335–339. [DOI] [PubMed] [Google Scholar]
- 86. Delbanco T, Walker J, Bell SK, Darer JD, Elmore JG, Farag N et al. Inviting patients to read their doctors' notes: a quasi‐experimental study and a look ahead. Ann Intern Med 2012; 157: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tierney WM, Alpert SA, Byrket A, Caine K, Leventhal JC, Meslin EM et al. Provider responses to patients controlling access to their electronic health records: a prospective cohort study in primary care. J Gen Intern Med 2015; 30: S31–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ozair FF, Jamshed N, Sharma A, Aggarwal P. Ethical issues in electronic health records: a general overview. Perspect Clin Res 2015; 6: 73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. State of Wisconsin . 2015. Assembly Bill 255. http://docs.legis.wisconsin.gov/2015/related/proposals/ab255.pdf [accessed 18 October 2018].
- 90. Paradis E, Sutkin G. Beyond a good story: from Hawthorne effect to reactivity in health professions education research. Med Educ 2017; 51: 31–39. [DOI] [PubMed] [Google Scholar]
- 91. Attri JP, Momin S, Kaur G, Sandhu KS, Bala N, Channa SS. Anaesthesia provider's perception of law: focus on preventive measures. Indian J Anaesth 2015; 59: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mujovic‐Zornic H. Legislation and patient's right: some necessary remarks. Med Law 2007; 26: 709–719. [PubMed] [Google Scholar]
- 93. Zwaanswijk M, Ploem MC, Wiesman FJ, Verheij RA, Friele RD, Gevers JK. Understanding health care providers' reluctance to adopt a national electronic patient record: an empirical and legal analysis. Med Law 2013; 32: 13–31. [PubMed] [Google Scholar]
- 94. Cochran A, Elder WB. A model of disruptive surgeon behavior in the perioperative environment. J Am Coll Surg 2014; 219: 390–398. [DOI] [PubMed] [Google Scholar]
- 95. Bigony L, Lipke TG, Lundberg A, McGraw CA, Pagac GL, Rogers A. Lateral violence in the perioperative setting. AORN J 2009; 89: 688–696. [DOI] [PubMed] [Google Scholar]
- 96. Coe R, Gould D. Disagreement and aggression in the operating theatre. J Adv Nurs 2008; 61: 609–618. [DOI] [PubMed] [Google Scholar]
- 97. Makary MA, Sexton JB, Freischlag JA, Holzmueller CG, Millman EA, Rowen L et al. Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder. J Am Coll Surg 2006; 202: 746–752. [DOI] [PubMed] [Google Scholar]
- 98. Sexton JB, Makary MA, Tersigni AR, Pryor D, Hendrich A, Thomas EJ et al. Teamwork in the operating room: frontline perspectives among hospitals and operating room personnel. Anesthesiology 2006; 105: 877–884. [DOI] [PubMed] [Google Scholar]
- 99. Strauss S. Bullying in health care: a hazard for caregivers and patients In Bullies in the Workplace: Seeing and Stopping Adults Who Abuse Their Co‐Workers and Employees, Paludi M. (ed). Santa Barbara: Praeger, 2015; 109–148. [Google Scholar]
- 100. Felblinger DM. Bullying, incivility, and disruptive behaviors in the healthcare setting: identification, impact, and intervention. Front Health Serv Manage 2009; 25: 13–23. [PubMed] [Google Scholar]
- 101. Halim UA, Riding DM. Systematic review of the prevalence, impact and mitigating strategies for bullying, undermining behaviour and harassment in the surgical workplace. Br J Surg 2018; 105: 1390–1397. [DOI] [PubMed] [Google Scholar]
- 102. Mardell A, Rees C. The theatre nurse: getting the image right. Nurs Stand 1998; 13: 46–47. [DOI] [PubMed] [Google Scholar]
- 103. Rosenstein AH, O'Daniel M. A survey of the impact of disruptive behaviors and communication defects on patient safety. Jt Comm J Qual Patient Saf 2008; 34: 464–471. [DOI] [PubMed] [Google Scholar]
- 104. Healey T, Peterson TC, Healey J, El‐Othmani MM, Saleh KJ. Improving operating room efficiency, part 2: intraoperative and postoperative strategies. JBJS Rev 2015; 3. [DOI] [PubMed] [Google Scholar]
- 105. Frasier LL, Pavuluri Quamme SR, Becker A, Booth S, Gutt A, Wiegmann D et al. Investigating teamwork in the operating room: engaging stakeholders and setting the agenda. JAMA Surg 2017; 152: 109–111. [DOI] [PubMed] [Google Scholar]
- 106. Jacobs GB, Wille RL. Consequences and potential problems of operating room outbursts and temper tantrums by surgeons. Surg Neurol Int 2012; 3(Suppl 3): S167–S173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search details


