Abstract
Catatonia is a psychomotor syndrome associated with several psychiatric and medical conditions. Psychomotor signs range from stupor to agitation and include pathognomonic features such as verbigeration and waxy flexibility. Disturbances of volition led to the classification as a subtype of schizophrenia, but recent changes in nosology now recognize high prevalence in mood disorders, overlap with delirium, and comorbidity with medical conditions. Initial psychometric studies have revealed three behavioral factors, but the structure of catatonia is still unknown.
Recent evidence from patients with psychotic disorders indicates increased neural activity in premotor areas in hypokinetic catatonia patients. It is unclear whether this localized hyperactivity is due to cortico-cortical inhibition or excess activity of inhibitory cortico-basal ganglia loops. Current treatment of catatonia relies on benzodiazepines and electroconvulsive therapy, both effective yet unspecific in their modes of action.
Longitudinal and treatment studies, using neuroimaging and brain stimulation techniques, are needed to advance our understanding of catatonia.
Keywords: catatonia syndrome, ECT, motor network, motor inhibition
Introduction
“… the scrivener was the victim of innate and incurable disorder. I might give alms to his body; but his body did not pain him; it was his soul that suffered, and his soul I could not reach.”
Herman Melville, Bartleby, the Srivener. A story of Wall-street. 1853
Herman Melville’s Bartleby is a story about a New York City law clerk, who retreats from the world around him, becomes mute and stuporous, wastes away and dies1. The tale is the skillful description of a hitherto unknown psychiatric condition. Two decades later, Karl Kahlbaum publishes the monograph Die Katatonie oder das Spannungsirresein2, in which he describes peculiar abnormalities of apparently volitional motor acts. Catatonia is now known as a psychomotor syndrome, with symptoms ranging from inhibition to agitation, with prominent disturbances of volition and, in severe cases, autonomic nervous system dysregulation3–6.
Kahlbaum and others have speculated about the pathology of catatonia, but the disease mechanisms remain elusive7–10. Mortality of catatonia was very high before the introduction of medication and electroconvulsive therapy11,12. Today it still remains high in malignant forms of catatonia13–16. There is an abundance of case reports and conceptual reviews of catatonia, but well-powered research studies are rare17. This paucity of catatonia research is one reason that current treatment approaches remain non-specific and without evidence-based criteria.
In order to improve treatment and prediction of course and outcome, it is critical to unravel the disease mechanisms of catatonia. Specifically, neuroimaging studies of catatonia may inform the development of novel brain stimulation techniques18,19. This review will summarize the current state of knowledge on catatonia focusing on clinical and neuroimaging findings. In the same issue, Rogers and colleagues will report on neuroinflammation associated with catatonia.
Catatonia is a psychomotor syndrome
Catatonia, a well-known syndrome in the early part of the 20th century, almost disappeared from psychiatric discourse, leading some to ask: “Where have all the catatonics gone?”20 Exact prevalence and incidence estimates of catatonia depend on the proper detection and description of the signs and symptoms of catatonia21. But clinicians are often confused by catatonia, reframing the signs and symptoms as something else or missing them altogether.
Much of the confusion stems from the poorly defined term psychomotor22–24. It refers to the volitional aspects and affective modulation of spontaneous or cued motor behavior. On the one hand, it includes the will to act, the planning and the execution of a motor act. But it also includes the modulation of motor behavior by sensorium and affect, leading to the remarkable complexity regarding the voluntariness of motor acts. Just consider the many ways to speak a sentence, sing a song or play an instrument.
Carl Wernicke suggested that psychomotor behavior is abnormal if there is too little (hypofunction/akinesis) or too much (hyperfunction/hyperkinesis) motor activity or if the act itself is qualitatively abnormal (parakinesis)25. This framework is still helpful today. Panel 1a separates the signs of catatonia into increased, abnormal and decreased psychomotor behavior, as categorized by observation, interview and physical exam. Many signs of catatonia are triggered as a response to the examiner. Even observed signs may change in response to the examiner’s physical presence, for example, the patient’s mutism or hypokinesis may be pronounced in the presence of the examiner, but decreases when the examiner leaves. The mercurial nature of catatonia may mislead the examiner to interpret the signs simply as volitional or behavioral, when they are better characterized as read-outs of dysfunctional motor circuits, similar to functional movement disorders in neurological patients26.
Panel 1A.
Catatonia checklist
| Observation | Interview | Physical exam | ||
|---|---|---|---|---|
| Psychomotor activity | Increased | Agitation/Excitement | ||
| Impulsivity | ||||
| Combativeness | ||||
| Abnormal | Grimacing | Echolalia * | Waxy Flexibility | |
| Stereotypy | Echopraxia * | Catalepsy * | ||
| Mannerism | Verbigeration | Rigidity | ||
| Posturing * | Automatic obedience | Gegenhalten | ||
| Perseveration | Mitgehen | |||
| Grasp reflex | ||||
| Decreased | Stupor | Negativism | ||
| Ambitendency | Mutism | |||
| Staring | Withdrawal | |||
| Autonomic abnormality |
The checklist recognizes all 23 items of the Bush-Francis Catatonia Rating Scale (BFCRS)40.
Red indicates the 12 DSM-5 criteria for catatonia3
Asterisk (*): indicates items that are combined in the BFCRS (item #5: posturing/catalepsy, item #7: echolalia/echopraxia), but are separate items in the DSM-5.
The patients in Kahlbaum’s monograph can be captured well with this checklist (Supplemental Table 1). One classical feature of catatonia, autonomic abnormality (i.e., tachycardia, hypertension, fever), which does not occur in every patient, is not a psychomotor sign and should be listed separately.
Psychomotor abnormalities are found in various clinical settings. Three psychiatric disorders include psychomotor abnormalities as DSM-5 diagnostic criteria or specifiers: mood disorders, schizophrenia spectrum disorders and delirium. Additionally, the DSM-5 diagnostic criteria for autism spectrum disorders describes psychomotor signs of catatonia as core diagnostic criterion. Not surprisingly, catatonia can be a prominent feature in patients diagnosed with these psychiatric disorders.
Psychomotor retardation has long been recognized as a cardinal feature of depression, especially melancholia27,28. Signs include, but are not limited to, slowed reaction time, discrete body movements (especially of the face) and abnormalities of speech (such as speed and modulation). Psychomotor retardation/agitation is recognized in DSM-5 as one of the nine diagnostic criteria for major depressive disorder. Whether psychomotor retardation/agitation in depression resembles a minor form of catatonia remains to be determined.
Schizophrenia has been associated with catatonia since Kraepelin29 defined dementia praecox as a natural disease unit of three previously separate clinical syndromes: dementia paranoides, hebephrenia and catatonia30. Critics have observed that most patients who present with catatonic features do not have a diagnosis of schizophrenia31. But the DSM continued Kraepelin’s tradition of linking catatonia with schizophrenia until 2013, when DSM-5 removed all schizophrenia subtypes, allowed for the recognition of catatonic symptoms in many psychiatric and medical conditions via a specifier and introduced «Unspecified Catatonia», for cases with insufficient information or where the underlying condition is unclear.
Delirium is defined by disturbances of attention, awareness and cognition, frequently with perceptual disturbances and delusional thought content that develops over a short period of time and tends to fluctuate in course. For some, delirium can result in a dementia-like illness at long-term follow-up32,33. Delirious patients can be further characterized as hyperactive, hypoactive or mixed forms, based on abnormalities of psychomotor behavior34. Catatonia is now recognized to frequently co-occur with delirium in the context of general medical and critical illnesses35–38, however the clinical relevance of this co-occurrence remains unknown.
It is not clear to what extent the clinical presentation and course of psychomotor abnormalities differ between schizophrenia, affective disorders, delirium, and other conditions. Studying this issue will foster diagnosis and treatment of catatonia.
Structure of catatonia
Several rating scales are available to measure catatonia39. Panel 1b summarizes the definition of items of the Bush Francis Catatonia Rating Scale (BFCRS)40 and the DSM-5 criteria3. Is there an underlying structure to the clinical features of catatonia? Few psychometric studies of catatonia have been published and most of them have focused on psychiatric cohorts, primarily patients with psychotic disorders41–46.
Panel 1B.
Definitions of catatonia signs
| 1 | 9 | Agitation/Excitement- extreme hyperactivity with nonpurposeful movements and uncontrollable, extreme emotional reactions |
| 19 | Ambitendency- appearance of being “stuck” in indecisive or hesitant movement | |
| 16 | Automatic Obedience- mechanical and reproducible compliance with examiner’s request, even if dangerous | |
| 23 | Autonomic Abnormality- diaphoresis, palpitations, or abnormal temperature, pulse, blood pressure, pulse or respiratory rate | |
| 5* | 2 | Catalepsy- passive induction of a posture held against gravity |
| 22 | Combativeness- striking out against others, with or without potential for injury | |
| 7 | 11 | Echolalia- mimicking another’s speech |
| 7* | 12 | Echopraxia- mimicking another’s movements |
| 18 | Gegenhalten- resistance to positioning by examiner which increases proportionally to applied force | |
| 20 | Grasp reflex | |
| 6 | 10 | Grimacing- odd and inappropriate facial expressions, irrespective of situation |
| 15 | Impulsivity- patient suddenly engages in inappropriate behavior without provocation; afterwards can give no, or only a facile explanation | |
| 9 | 7 | Mannerism- odd, circumstantial caricature of normal actions |
| 17 | Mitgehen- exaggerated movements in response to light pressure | |
| 3 | 4 | Mutism- no, or very little, verbal response (exclude if known aphasia) |
| 12 | 5 | Negativism- opposing or not responding to instructions or external stimuli |
| 21 | Perseveration- whole or partial non-goal-directed repetition of actions or verbal content | |
| 5 | 6 | Posturing- spontaneous and active maintenance of a posture against gravity |
| 11 | Rigidity- resistance by way of increased muscle tone | |
| 4 | Staring/Blinking- fixed gaze, decreased blinking, and widely opened eyes. Or increased blinking | |
| 8 | 8 | Stereotypy- repetitive, abnormally frequent, non-goal-directed movements |
| 2 | 1 | Stupor- no or markedly reduced psychomotor activity; no active relation to environment |
| 10 | Verbigeration- continuous, directionless, repetition of words, phrases, or sentences | |
| 13 | 3 | Waxy Flexibility- slight, even resistance to positioning by examiner |
| 14 | Withdrawal- turning away from examiner, no eye contact and/or refusal to take food or drink when offered. Or social isolation |
Catatonia signs are not fully operationalized and definitions vary slightly between authors.
In a mixed medical and psychiatric population, Wilson et al. studied 339 acutely ill patients who were evaluated for catatonia using the Bush Francis Catatonia Rating Scale at a single academic medical center41. Of the 339 patients screened for catatonia, 232 met their definition of validated catatonia (≥2 catatonia items present on exam and a positive response to treatment with a benzodiazepine or electroconvulsive therapy). Of the “validated catatonia” cases, 211 (91%) met DSM-IV catatonia criteria, however only 170 (73%) met DSM-5 criteria. Principal component analysis identified three catatonia components: “Increased”, “Decreased” and “Abnormal” psychomotor behaviors. Item response theory analysis showed that excitement, immobility/stupor and waxy flexibility were the best indicators of each component. Only 37% of the variance of the condition was explained, leading the authors to conclude that the underlying structure of catatonia remains unknown.
The scarce psychometric evidence for a model of catatonia has not kept authors from speculating how best to conceptualize this condition. Here we contrast catatonia as a motor syndrome, paralysis of will, a fear syndrome, or a result of immune dysregulation, with the emphasis of this review on the motor syndrome.
Motor syndrome
While volitional disturbances and fear might drive abnormal psychomotor behaviors, most researchers have focused on motor acts as the central feature of catatonia. Karl Kleist developed an elaborate neural circuity model for psychomotor abnormalities in various psychiatric conditions7,47. More recently, Georg Northoff integrated current neuroscience data into a top-down-modulation model of catatonia10. Many studies in schizophrenia reported striking covariance between catatonia and other motor abnormalities or symptom dimensions (e.g. parkinsonism, negative symptoms, dyskinesia, neurological soft signs or disorganization). This co-occurrence is due to both conceptual overlap between rating scales and to truly common pathways in pathobiology5,48–50. Depending on which scale is used, catatonia symptoms are readily missed or confused. Rogers’ termed this problem the “conflict of paradigms”, which particularly hampers research of psychomotor symptoms in severe psychiatric illness48. More research is needed to clarify whether the underlying symptom structure differs between psychiatric conditions associated with catatonia. Objective assessment and comparison between disorders is most likely to be achieved for pure motor symptoms, which can be approached with instrumentation51,52.
Paralysis of will
Kraepelin identified 11 disturbances of volition and action53. Most are now represented in DSM-5 as criterion A.4. of schizophrenia (grossly disorganized and/or catatonic behavior), but some are scored separately as criterion A.5. (avolition). After separating Kraepelin’s “paralysis of will” into two distinct domains, much of the clinical and research focus shifted to negative symptoms of schizophrenia54.
Bleuler recognized loosening of associations as the primary feature of schizophrenia, leading to it’s recognition as a thought disorder55,56. But he also considered negativism and ambivalence as fundamental57. Ambivalence captures the waxing and waning of willful behavior seen in catatonia very well.
Fear syndrome
Similarities between catatonia and tonic immobility, an animal defense strategy in response to fear, led to the hypothesis that catatonia is a primitive fear syndrome58,59. The affective symptoms and neuroendocrine abnormalities seen in melancholic patients with catatonic symptoms and the therapeutic effects of benzodiazepines have been cited in support of catatonia as a fear syndrome60. Imaging studies of the amygdala and the ventromedial / dorsolateral prefrontal cortices can explore this hypothesis61.
Immune dysregulation
The course and presentation of acute catatonia mimics encephalitis in some cases, which led to the hypothesis that catatonia results from immune dysregulation62. This evolving research field is covered by the article of Rogers and colleagues in this issue.
Nosology and epidemiology of catatonia
The nosology of catatonia has changed recently. With DSM-5, the syndrome is now recognized in many psychiatric and medical conditions, but likely remains under-diagnosed21. Most estimates of catatonia prevalence are derived from studies of acutely ill psychiatric inpatients, and range from 9 to 18%40,60,63–65. The prevalence critically depends on the assessment method, rating scales such as the BFCRS provide higher numbers than DSM-5 criteria21,65. In a meta-analysis of 74 studies of catatonia, the mean catatonia prevalence was 9% but rose to 23.9% in patients receiving ECT17. In a population based cohort study, Kleinhaus et al, found that 7.6% of patients with schizophrenia had the catatonic subtype66. Work by Abrams and Taylor reported an even higher prevalence in patients with affective conditions with up to 20% of patients with bipolar disorder exhibiting catatonic signs67–70. Catatonia is a frequent manifestation of many general medical conditions64,71–73. Studies have suggested that perhaps as many as one third of patients with delirium secondary to a general medical condition will develop catatonia during their acute hospitalization35,36. Catatonia incidence ranged from 1·6% to 8·9% on medical services assessed by a psychiatry liaison service, with prevalence varying across age groups and associated medical conditions74,75.
Catatonia is a medical condition
Max Fink has advocated that catatonia should be given a home of its own in psychiatric nosology76. While there are many etiologies, illness course and response to treatment are considered strong validators. From this perspective, various conditions may be related as catatonia-spectrum illnesses, including malignant catatonia, neuroleptic malignant syndrome (NMS), serotonin syndrome, lethal catatonia77, and the growing number of autoantibody-related encephalitis cases with catatonia78. These life-threatening conditions are characterized by autonomic abnormalities and encephalopathy, both of which are rarely studied in catatonia. Future studies should focus on the characterization and course of symptoms such as tachycardia and hyperthermia in catatonia.
Disease Mechanisms
Neuroimaging findings
Catatonia is associated with alterations of cerebral motor circuits5,79,80. It is important to understand the mechanism of catatonic motor dysfunction in order to design specific treatments. Similar to Parkinson disease, catatonia comprises hypo- and hyperkinetic motor abnormalities, suggesting dysfunction of more than one motor circuit.
The Research Domain Criteria (RDoC) initiative considers including a motor domain with three main circuits. One is thought to serve inhibition and excitation of movements and comprises a circuit from primary motor cortex (M1) to putamen, internal and external pallidum, thalamus, and back to M1; another circuit focuses on motor dynamics and timing including M1, thalamus, cerebellum and pontine nuclei; and the final cortico-cortical circuit is thought to control motor organization and speed including M1, supplementary motor area (SMA), posterior parietal cortex, and medial prefrontal cortex24. Thus, according to this model, catatonia symptoms affecting motor inhibition/excitation as well as motor organization would be associated with at least two of the three circuits. Some authors, however, argue that “horizontal abnormalities” within the cortico-cortical loop are most relevant to catatonia24,79.
Initial neuroimaging studies of patients with a history of catatonia indicated poor functional activation of SMA, primary and secondary motor cortices, inferior parietal cortex and basal ganglia during self-initiated movements81–83, lower cerebral blood flow (CBF) in right prefrontal and parietal cortex84, and reduced GABA-A-receptor density in the left sensorimotor cortex85. Furthermore, functional MRI studies reported that orbitofrontal cortex dysfunction would contribute to poor connectivity in medial prefrontal cortices in catatonia, which is partly reversible by lorazepam administration86,87. A number of case reports also point to reduced neural activity in frontal and parietal cortices in catatonia5,6.
New studies using arterial spin labeling revealed that schizophrenia patients in a catatonic episode have increased cerebral blood flow (CBF) in the left primary motor cortex M1 and supplementary motor area (SMA) compared to schizophrenia patients without catatonia history19. This study is supported by another recent investigation in patients with chronic periodic catatonia, indicating increased perfusion of the SMA and left M1 in catatonic patients compared to non-catatonic patients and controls88. In addition, case reports demonstrated increased neural activity in SMA and M1 in acute catatonia89,90. Increased resting state neural activity in the SMA could also explain the lack of activation in functional MRI tasks in catatonia82,83.
Transcranial magnetic stimulation (TMS) indicated a hyper-imitative state in a case of acute catatonia: a patient was found to exhibit increased motor evoked potentials (MEP) in response to TMS while this patient was observing motor acts91. But this effect was no longer seen after catatonia remitted, suggesting disinhibition within the motor and premotor cortex during catatonia. Likewise, an EEG study in catatonia reported delayed late readiness potentials and movement potentials over fronto-parietal midline structures92. This finding suggests ineffective coupling of premotor and motor cortices in catatonia. Interestingly, lorazepam administration further delayed readiness potentials arguing for compromised inhibitory control within the motor network in catatonia.
We found aberrant hyperconnectivity between thalamus and bilateral M1 and SMA in schizophrenia18, similar to previous studies93,94. This resting state thalamocortical hyperconnectivity (to M1) was linked to ratings on the Bush Francis Catatonia Rating Scale, i.e. increased connectivity in patients with more severe catatonia symptoms18.
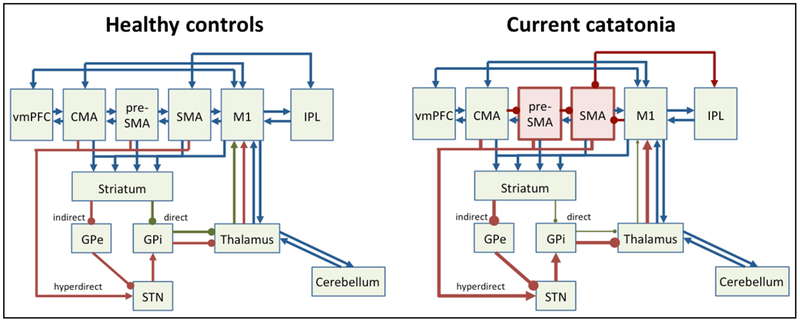
Together, these findings implicate the motor system in catatonia (see figure 1). They point to abnormal hyperactivity within the SMA and pre-SMA, tightly interconnected brain structures that are critically involved in motor control, movement selection, initiation, timing, and inhibition95–97. Particularly, the concentration of the inhibitory neurotransmitter GABA determines individual variation in responding to motor cues98. In addition, SMA is thought to initiate the inhibitory processes in downstream brain areas such as basal ganglia98. SMA hyperactivity could result from increased feed-forward stimulation of STN, from activation of other cortical areas exerting inhibitory control or from an attempt to overcome inhibitory processes, such as basal ganglia output on the (pre)motor cortex (see figure 1). Connecting white matter could also be involved, even though direct evidence in catatonia patients is lacking99.
Figure 1. Motor network pathology in catatonia.
Left panel depicts motor system in healthy controls, right panel in current hypokinetic catatonia. Cortical (pre)motor areas are: ventromedial prefrontal cortex (vmPFC), cingulate motor area (CMA), pre-supplementary motor area (pre-SMA), supplementary motor area (SMA), primary motor cortex (M1), and inferior parietal lobe (IPL). GPe = external globus pallidus, GPi = internal globus pallidus, STN = subthalamic nucleus. Green arrows indicate net facilitatory pathways, such as the direct pathway. Red arrows indicate net inhibitory pathways, such as indirect or hyperdirect pathways. Blue arrows indicate neutral or modulatory connections.
Note: in catatonia preSMA and SMA have increased neural activity concurrently with motor behavioral inhibition, which may either result from cortico-cortical inhibition or from dominant indirect/hyperdirect pathway activity.
Genetics
First-degree relatives of patients with catatonia are more likely to suffer from affective disorders100,101. A study of multiplex families reported substantial heritability estimates for catatonia symptoms, symptom endorsement frequency and syndrome severity102. Interestingly, the most frequent signs such as psychomotor retardation and excitement had moderate levels of familiality estimates, whereas classical signs as mutism or rigidity had very high familiality estimates. Karl Leonhard reported that periodic catatonia runs in families, while stable (systematic) catatonias or reactive motility psychoses show little familial aggregation103,104. In fact, lifetime morbidity risk of catatonia for first-degree relatives of patients with periodic catatonia is 27%, with an autosomal dominant linkage to chromosome 15q15 and heterogeneous linkage to chromosome 22q13105,106. Likewise, in patients with intellectual disability who present with catatonia, researchers detected rare copy number variants107,108.
Mice heterozygous for oligodendroglial myelin basic protein (mbp+/−) or cyclic nucleotid phosphodiesterase (CNP+/−), both critical for oligodendroglial function and myelination109,110, developed spontaneous catalepsy, apathy and social withdrawal during aging. Likewise, schizophrenia patients with the same genotype display a similar phenotype in terms of behavior and corpus callosum white matter after age 40110. Interestingly, the CNP+/− genotype is more prevalent among schizophrenia patients with catatonic symptoms62. Finally, in the mutant mice, catatonia was alleviated by pharmacological microglia inactivation62. White matter alterations in frontal motor areas have been reported in patients with schizophrenia and major depression, who demonstrated reduced spontaneous motor behavior, potentially a minor form of catatonia111,112.
Treatment
Current state
Catatonia is a potentially life-threatening condition, often requiring inpatient medical or psychiatric treatment. Medically, catatonia can lead to deep vein thrombosis and pulmonary embolus, decubiti, muscle contracture, and rhabdomyolysis leading to renal failure113–115. General preventive recommendations for patients with catatonia include (1) pharmacologic prophylaxis of venous thromboembolism, (2) skin assessments and frequent repositioning to prevent pressure ulcer, (3) daily stretching to prevent muscle contractions, and (4) tube feeding and intravenous fluids when necessary to avoid dehydration and malnutrition116. A summary of clinical recommendations is given in Panel 2.
Panel 2:
Clinical Recommendations
|
Few prospective investigations have examined the effectiveness of available treatments for catatonia (see Supplemental Table 2). In fact, a systematic review failed to identify trial-derived data meeting inclusion criteria117. Benzodiazepines60,118,119 and electroconvulsive therapy (ECT)120,121 are safe and effective standard treatments, particularly in acute catatonia. Lorazepam is the preferred benzodiazepine for catatonia. It is administered sublingually, intravenous or intramuscularly and effective at both low (1–3mg/day)60 or high doses (6–16 mg/day)118,120. Although lorazepam had no effect on chronic catatonia symptoms in one randomized controlled trial (RCT)122, acute catatonia responds well to benzodiazepines in approximately 70% of the cases according to prospective investigations (see Supplemental Table 2). Still, response to benzodiazepines is not satisfactory in a considerable number of patients121, with 27% of patients experiencing only partial symptom reduction63,118. Predictive factors for response to benzodiazepines include chronicity, symptomatology, age and aetiology (i.e. affective, psychotic or organic)122 with evidence for poorer response with increasing age, increased duration of illness, chronicity and in psychotic patients. If effective for ameliorating catatonia, the effects of repeated benzodiazepine administration should be observed within a few days121.
The treatment of patients who present with signs of catatonia and delirium poses challenges (i.e., antipsychotic drugs are currently standard treatment for delirium but may worsen catatonia; benzodiazepines are first-line treatment for catatonia but may worsen delirium). One of us (JEW) is studying the overlap of delirium and catatonia35, but further research must be done before clear treatment recommendations can be made.
When patients fail to respond to benzodiazepines, they should be referred to ECT121. ECT can also be applied as first line treatment in life-threatening conditions (e.g., severe autonomic instability or withdrawal leading to insufficient oral intake). One meta-analysis on the effect of ECT for the treatment of catatonia documented symptom improvement with ECT (SMD = −3·1, 95% CI [−3·95; −2·34]). However, according to the authors the studies (three prospective RCTs, five prospective observational and one retrospective chart review) failed to demonstrate efficacy and effectiveness of ECT due to low quality (missing rigorous quality RCT) and heterogeneity (different ECT protocols, different scales assessed treatment response)123. The combined administration of ECT with benzodiazepines has been associated with reduced efficacy and efficiency, yet some synergism has been discussed124. Bifrontal ECT results in superior clinical and cognitive outcomes than bitemporal ECT in schizophrenia patients with catatonia125. Importantly, the suggested reduced risk of cognitive side effects with unilateral stimulation was not confirmed in a small case series126.
Prospective investigations of treatments for catatonia are rare. There is some evidence that patients with psychotic disorders who present with catatonic signs respond better to clozapine and to antipsychotics with low affinity for dopamine receptors127,128. A recent systematic review of alternative treatment strategies for catatonia concluded that intravenous lorazepam and ECT remain first-line treatments129. The authors proposed a treatment algorithm with glutamate antagonists and anti-epileptic drugs (carbamazepine and valproic acid) as second-line treatments if benzodiazepines and a full course of ECT are not sufficient.
New developments:
Novel brain stimulation techniques hold promise in targeting the motor network directly with local and distant effects within the network130, ultimately allowing for individualized treatment of catatonia. Repetitive transcranial magnetic stimulation (rTMS) is being studied in two randomized controlled trials, informed by recent neuroimaging results19,88 (see figure 1). One trial (, www.clinicaltrials.gov) is a four-arm prospective parallel trial, testing add-on rTMS in patients with schizophrenia spectrum disorders and major depressive disorder. One arm tests inhibitory rTMS and one arm facilitatory rTMS of the SMA. The other trial aims to treat catatonia by rTMS based on individual brain perfusion abnormalities (, www.clinicaltrials.gov). Both trials target motor inhibition in catatonia. Without conclusive evidence regarding pathophysiology, the field is struggling to develop novel treatment approaches. Currently, noninvasive brain stimulation is a promising new development that allows for careful hypothesis testing of disease mechanism and treatment efficacy.
Future perspectives
Adoption of Kraepelin’s nosology impeded progress in catatonia research for many years. With the greater recognition of catatonia as not just a subtype of schizophrenia, we are now ready to explore clinical presentation, symptom structure, and course of catatonia in various conditions. Dimensional assessments of psychomotor behavior in various psychiatric and medical conditions may prove especially fruitful.
Neuroimaging is likely to advance our understanding of catatonia. Resting-state functional, perfusion, and structural MRI scans are feasible methods to study catatonic patients who are unable to engage in functional MRI tasks. We will need well-powered studies, longitudinal studies, as well as studies in patients with distinct catatonia symptom profiles. Motor network properties are likely different between patients with volitional issues such as negativism and patients with pure motor inhibition. Furthermore, these neuroimaging approaches should be applied to all types of catatonia. Finally, vegetative signs remain poorly understood in catatonia. We need characterization of vegetative dysregulation in cross-sectional and longitudinal studies.
Negativism, avolition, and mutism have made it difficult to obtain informed consent from catatonic patients. Consenting a surrogate decision maker and prospective recruitment of patients during catatonia-free periods are ethical solutions to this obstacle. Given the prevalence of catatonia, larger medical centers should be able to conduct interventional studies, ideally with multi-center designs. In addition to testing established treatments (benzodiazepines, ECT), we need to explore the use of non-invasive brain stimulation techniques such as rTMS; and finally even considerdeep brain stimulation for severe and chronic cases. For the development of invasive brain stimulation, we need animal models of catatonia.
Finally, establishing individualized treatment regimens is a high priority. Treatment effects will depend on the underlying condition and the specific symptom clusters. Indeed, current studies with broad inclusion criteria may fail because of heterogeneous responses to treatment.
We have summarized next steps in catatonia research in panel 3. Clinicians may aid this process by applying screening instruments such as the BFCRS to recognize catatonia patients.
Panel 3:
Next steps in catatonia research
|
Conclusions
Catatonia remains poorly understood. The syndrome of abnormal volition and motor behavior occurs in several psychiatric and medical conditions. We conceptualize it as a disorder of cerebral motor network dysfunction. Future research should clarify the various presentations, the course and the disease mechanisms. Neuroimaging stands to aid the development of novel brain-network focused treatment options.
Search strategy
We searched PubMed for relevant publications using the terms: “catatonia” AND “prevalence” OR “neuroimaging”, “MRI”, “ECT”, “treatment”, “clinical trial”, “meta-analysis”, “transcranial magnetic stimulation”, “benzodiazepines”, “lorazepam”, “antipsychotic”, “symptom structure”. In addition, we carefully checked books, reference lists of articles, and the clinicaltrials.gov database for further studies, reports or reviews on catatonia. The retrieved publications in English or German were further selected for their relevance to the topics of this review, i.e. prevalence, presentation, neuroimaging, treatment. Finally, we also searched specifically for some topics using Google Scholar. We applied no date restrictions to our search; the last search was carried out June 27, 2018.
Supplementary Material
Supplemental Table 1. Catatonia symptom distribution in Kahlbaum’s case series
Supplemental Table 2. Prospective studies of catatonia treatment with blinded assessments: benzodiazepines, electroconvulsive therapy (ECT), and antipsychotics
Role of the funding source
The manuscript was prepared without funding.
Footnotes
Conflicts of interest
Dr. Walther reports personal fees from Eli Lilly, personal fees from Janssen-Cilag, personal fees from Lundbeck, outside the submitted work. Dr. Wilson reports grants from Vanderbilt Clinical and Translational Science Award (KL2). Dr. Heckers reports grants from NIMH and personal fees from JAMA Psychiatry and from lectures. Dr. Stegmayer has nothing to disclose.
References
- 1.Schneck JM. Karl Kahlbaum’s Catatonia and Herman Melville’s Bartleby the Scrivener. Arch Gen Psychiatry 1972; 27(1): 48–51. [DOI] [PubMed] [Google Scholar]
- 2.Kahlbaum K. Die Katatonie oder das Spannungsirresein. Eine klinische Form psychischer Krankheit. Berlin: A. Hirschwald; 1874. [Google Scholar]
- 3.Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res 2013; 150(1): 26–30. [DOI] [PubMed] [Google Scholar]
- 4.Fink M, Taylor MA. Catatonia: A Clinician’s Guide to Diagnosis and Treatment: Cambridge University Press; 2006. [Google Scholar]
- 5.Walther S, Strik W. Motor Symptoms and Schizophrenia. Neuropsychobiology 2012; 66(2): 77–92. [DOI] [PubMed] [Google Scholar]
- 6.Walther S, Strik W. Catatonia. Cns Spectrums 2016; 21(4): 341–8. [DOI] [PubMed] [Google Scholar]
- 7.Kleist K. Untersuchungen zur Kenntnis der psychomotorischen Bewegungsstörungen bei Geisteskranken. Leipzig: Verlag von Dr. Werner Klinkhardt; 1908. [Google Scholar]
- 8.Danziger L, Elmergreen GL. Mathematical Theory of Periodic Relapsing Catatonia. Bulletin of Mathematical Biophysics 1954; 16: 15–21. [Google Scholar]
- 9.Gjessing LR. The switch mechanism in periodic catatonia and manic-depressive disorder. Chronobiologia 1975; 2(4): 307–16. [PubMed] [Google Scholar]
- 10.Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci 2002; 25(5): 555–77; discussion 78–604. [DOI] [PubMed] [Google Scholar]
- 11.Niswander GD, Haslerud GM, Mitchell GD. Effect of Catatonia on Schizophrenic Mortality. Arch Gen Psychiatry 1963; 9: 548–51. [DOI] [PubMed] [Google Scholar]
- 12.Healy D, Le Noury J, Harris M, et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875–1924 and 1994–2010. BMJ Open 2012; 2(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical care and treatment. Gen Hosp Psychiatry 2010; 32(6): 631–5. [DOI] [PubMed] [Google Scholar]
- 14.Cornic F, Consoli A, Tanguy ML, et al. Association of adolescent catatonia with increased mortality and morbidity: evidence from a prospective follow-up study. Schizophr Res 2009; 113(2–3): 233–40. [DOI] [PubMed] [Google Scholar]
- 15.Shenai N, White CD, Azzam PN, Gopalan P, Solai LK. Practical and Legal Challenges to Electroconvulsive Therapy in Malignant Catatonia. Harv Rev Psychiatry 2016; 24(3): 238–41. [DOI] [PubMed] [Google Scholar]
- 16.Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl 2013; (441): 1–47. [DOI] [PubMed] [Google Scholar]
- 17.Solmi M, Pigato GG, Roiter B, et al. Prevalence of Catatonia and Its Moderators in Clinical Samples: Results from a Meta-analysis and Meta-regression Analysis. Schizophr Bull 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant Hyperconnectivity in the Motor System at Rest Is Linked to Motor Abnormalities in Schizophrenia Spectrum Disorders. Schizophr Bull 2017; 43(5): 982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walther S, Schappi L, Federspiel A, et al. Resting-State Hyperperfusion of the Supplementary Motor Area in Catatonia. Schizophr Bull 2017; 43(5): 972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahendra B. Where have all the catatonics gone? Psychol Med 1981; 11(4): 669–71. [DOI] [PubMed] [Google Scholar]
- 21.van der Heijden FM, Tuinier S, Arts NJ, Hoogendoorn ML, Kahn RS, Verhoeven WM. Catatonia: disappeared or under-diagnosed? Psychopathology 2005; 38(1): 3–8. [DOI] [PubMed] [Google Scholar]
- 22.Schrijvers D, Hulstijn W, Sabbe BG. Psychomotor symptoms in depression: a diagnostic, pathophysiological and therapeutic tool. J Affect Disord 2008; 109(1–2): 1–20. [DOI] [PubMed] [Google Scholar]
- 23.Morrens M, Hulstijn W, Sabbe B. Psychomotor slowing in schizophrenia. Schizophr Bull 2007;33(4): 1038–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittal VA, Bernard JA, Northoff G. What Can Different Motor Circuits Tell Us About Psychosis? An RDoC Perspective. Schizophr Bull 2017; 43(5): 949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wernicke C. Grundriss der Psychiatrie: Verlag von Georg Thieme; 1900. [Google Scholar]
- 26.Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol 2012; 11(3): 250–60. [DOI] [PubMed] [Google Scholar]
- 27.Sobin C, Sackeim HA. Psychomotor symptoms of depression. Am J Psychiatry 1997; 154(1): 4–17. [DOI] [PubMed] [Google Scholar]
- 28.Taylor MA, Fink M. Melancholia: The Diagnosis, Pathophysiology and Treatment of Depressive Illness: Cambridge University Press; 2010. [Google Scholar]
- 29.Kraepelin E. Psychiatrie Ein Lehrbuch für Studirende und Aerzte. 6 ed. Leipzig: Verlag von Johann Ambrosius Barth; 1899. [Google Scholar]
- 30.Heckers S. Making progress in schizophrenia research. Schizophr Bull 2008; 34(4): 591–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fink M, Shorter E, Taylor MA. Catatonia is not schizophrenia: Kraepelin’s error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull 2010; 36(2): 314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14): 1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012; 367(1): 30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meagher D, Moran M, Raju B, et al. A new data-based motor subtype schema for delirium. J Neuropsychiatry Clin Neurosci 2008; 20(2): 185–93. [DOI] [PubMed] [Google Scholar]
- 35.Wilson JE, Carlson R, Duggan MC, et al. Delirium and Catatonia in Critically Ill Patients: The Delirium and Catatonia Prospective Cohort Investigation. Crit Care Med 2017; 45(11): 1837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grover S, Ghosh A, Ghormode D. Do patients of delirium have catatonic features? An exploratory study. Psychiatry Clin Neurosci 2014; 68(8): 644–51. [DOI] [PubMed] [Google Scholar]
- 37.Meyen R, Acevedo-Diaz EE, Reddy SS. Challenges of managing delirium and catatonia in a medically ill patient. Schizophr Res 2018. [DOI] [PubMed] [Google Scholar]
- 38.Oldham MA, Lee HB. Catatonia vis-a-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry 2015; 37(6): 554–9. [DOI] [PubMed] [Google Scholar]
- 39.Sienaert P, Rooseleer J, De Fruyt J. Measuring catatonia: a systematic review of rating scales. J Affect Disord 2011; 135(1–3): 1–9. [DOI] [PubMed] [Google Scholar]
- 40.Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand 1996; 93(2): 129–36. [DOI] [PubMed] [Google Scholar]
- 41.Wilson JE, Niu K, Nicolson SE, Levine SZ, Heckers S. The diagnostic criteria and structure of catatonia. Schizophr Res 2015; 164(1–3): 256–62. [DOI] [PubMed] [Google Scholar]
- 42.Wong E, Ungvari GS, Leung SK, Tang WK. Rating catatonia in patients with chronic schizophrenia: Rasch analysis of the Bush-Francis Catatonia Rating Scale. Int J Methods Psychiatr Res 2007; 16(3): 161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogt BA. Regions and subregions of the cingulate cortex In: Vogt BA, ed. Cingulate Neurobiology And Disease. New York: Oxford University Press, Inc.; 2009. [Google Scholar]
- 44.Peralta V, Campos MS, de Jalon EG, Cuesta MJ. DSM-IV catatonia signs and criteria in first-episode, drug-naive, psychotic patients: psychometric validity and response to antipsychotic medication. Schizophr Res 2010; 118(1–3): 168–75. [DOI] [PubMed] [Google Scholar]
- 45.Ungvari GS, Goggins W, Leung SK, Gerevich J. Schizophrenia with prominent catatonic features (‘catatonic schizophrenia’). II. Factor analysis of the catatonic syndrome. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31(2): 462–8. [DOI] [PubMed] [Google Scholar]
- 46.Kruger S, Bagby RM, Hoffler J, Braunig P. Factor analysis of the catatonia rating scale and catatonic symptom distribution across four diagnostic groups. Compr Psychiatry 2003; 44(6): 472–82. [DOI] [PubMed] [Google Scholar]
- 47.Kleist K. Weitere Untersuchungen an Geisteskranken mit psychomotorischen Störungen. Leipzig: Verlag von Dr. Werner Klinkhardt; 1909. [Google Scholar]
- 48.Rogers D. The motor disorders of severe psychiatric illness: a conflict of paradigms. Br J Psychiatry 1985; 147: 221–32. [DOI] [PubMed] [Google Scholar]
- 49.McKenna PJ, Lund CE, Mortimer AM, Biggins CA. Motor, volitional and behavioural disorders in schizophrenia. 2: The ‘conflict of paradigms’ hypothesis. Br J Psychiatry 1991; 158: 328–36. [DOI] [PubMed] [Google Scholar]
- 50.Strik W, Stegmayer K, Walther S, Dierks T. Systems Neuroscience of Psychosis: Mapping Schizophrenia Symptoms onto Brain Systems. Neuropsychobiology 2017; 75(3): 100–16. [DOI] [PubMed] [Google Scholar]
- 51.van Harten PN, Walther S, Kent JS, Sponheim SR, Mittal VA. The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neurosci Biobehav Rev 2017; 80: 476–87. [DOI] [PubMed] [Google Scholar]
- 52.Walther S, Horn H, Razavi N, Koschorke P, Muller TJ, Strik W. Quantitative motor activity differentiates schizophrenia subtypes. Neuropsychobiology 2009; 60(2): 80–6. [DOI] [PubMed] [Google Scholar]
- 53.Kraepelin E. General Symptomatology: Section D: Disturbances of volition and action In: Kraepelin E, ed. Clinical Psychiatry: Macmillian; 1907: 77–96. [Google Scholar]
- 54.Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry 2018. [DOI] [PubMed] [Google Scholar]
- 55.Peralta V, Cuesta MJ. Eugen Bleuler and the schizophrenias: 100 years after. Schizophr Bull 2011; 37(6): 1118–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moskowitz A, Heim G. Eugen Bleuler’s Dementia praecox or the group of schizophrenias (1911): a centenary appreciation and reconsideration. Schizophr Bull 2011; 37(3): 471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bleuler E. The theory of schizophrenic negativism. The Journal of Nervous and Mental Disease 1912; 39(3): 195–202. [Google Scholar]
- 58.Moskowitz AK. “Scared stiff”: catatonia as an evolutionary-based fear response. Psychol Rev 2004; 111(4): 984–1002. [DOI] [PubMed] [Google Scholar]
- 59.Shorter E, Fink M. Madness of Fear: A History of Catatonia: Oxford University Press; 2018. [Google Scholar]
- 60.Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990; 51(9): 357–62. [PubMed] [Google Scholar]
- 61.Ellul P, Choucha W. Neurobiological Approach of Catatonia and Treatment Perspectives. Front Psychiatry 2015; 6: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janova H, Arinrad S, Balmuth E, et al. Microglia ablation alleviates myelin-associated catatonic signs in mice. J Clin Invest 2018; 128(2): 734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JW, Schwartz DL, Hallmayer J. Catatonia in a psychiatric intensive care facility: incidence and response to benzodiazepines. Ann Clin Psychiatry 2000; 12(2): 89–96. [DOI] [PubMed] [Google Scholar]
- 64.Braunig P, Kruger S. [Catatonia]. Psychiatr Prax 2005; 32 Suppl 1: S7–24. [DOI] [PubMed] [Google Scholar]
- 65.Stuivenga M, Morrens M. Prevalence of the catatonic syndrome in an acute inpatient sample. Front Psychiatry 2014; 5: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kleinhaus K, Harlap S, Perrin MC, et al. Catatonic Schizophrenia: A Cohort Prospective Study. Schizophr Bull 2012; 38(2): 331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abrams R, Taylor MA. Catatonia. A prospective clinical study. Arch Gen Psychiatry 1976; 33(5): 579–81. [DOI] [PubMed] [Google Scholar]
- 68.Abrams R, Taylor MA. Catatonia: prediction of response to somatic treatments. Am J Psychiatry 1977; 134(1): 78–80. [DOI] [PubMed] [Google Scholar]
- 69.Abrams R, Taylor MA, Coleman Stolurow KA. Catatonia and mania: patterns of cerebral dysfunction. Biol Psychiatry 1979; 14(1): 111–7. [PubMed] [Google Scholar]
- 70.Taylor MA, Abrams R. The phenomenology of mania. A new look at some old patients. Arch Gen Psychiatry 1973; 29(4): 520–2. [DOI] [PubMed] [Google Scholar]
- 71.Fink M. Catatonia: A Clinician’s Guide to Diagnosis and Treatment: Cambridge University Press; 2003. [Google Scholar]
- 72.Gelenberg AJ. The catatonic syndrome. Lancet 1976; 1(7973): 1339–41. [DOI] [PubMed] [Google Scholar]
- 73.Morrison JR. Catatonia: diagnosis and management. Hosp Community Psychiatry 1975; 26(2): 91–4. [DOI] [PubMed] [Google Scholar]
- 74.Carroll BT, Spetie L. Catatonia on the consultation-liaison service: a replication study. Int J Psychiatry Med 1994; 24(4): 329–37. [DOI] [PubMed] [Google Scholar]
- 75.Jaimes-Albornoz W, Serra-Mestres J. Prevalence and clinical correlations of catatonia in older adults referred to a liaison psychiatry service in a general hospital. Gen Hosp Psychiatry 2013. [DOI] [PubMed] [Google Scholar]
- 76.Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003; 160(7): 1233–41. [DOI] [PubMed] [Google Scholar]
- 77.Mann SC, Caroff SN, Bleier HR, Welz WK, Kling MA, Hayashida M. Lethal catatonia. Am J Psychiatry 1986; 143(11): 1374–81. [DOI] [PubMed] [Google Scholar]
- 78.Kayser MS, Kohler CG, Dalmau J. Psychiatric manifestations of paraneoplastic disorders. Am J Psychiatry 2010; 167(9): 1039–50. [DOI] [PubMed] [Google Scholar]
- 79.Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC. Motor dysfunction within the schizophrenia-spectrum: A dimensional step towards an underappreciated domain. Schizophr Res 2015; 169(1–3): 217–33. [DOI] [PubMed] [Google Scholar]
- 80.Walther S. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res 2015; 233(3): 293–8. [DOI] [PubMed] [Google Scholar]
- 81.Northoff G, Braus DF, Sartorius A, et al. Reduced activation and altered laterality in two neuroleptic-naive catatonic patients during a motor task in functional MRI. Psychol Med 1999; 29(4): 997–1002. [DOI] [PubMed] [Google Scholar]
- 82.Scheuerecker J, Ufer S, Kapernick M, et al. Cerebral network deficits in post-acute catatonic schizophrenic patients measured by fMRI. J Psychiatr Res 2009; 43(6): 607–14. [DOI] [PubMed] [Google Scholar]
- 83.Payoux P, Boulanouar K, Sarramon C, et al. Cortical motor activation in akinetic schizophrenic patients: a pilot functional MRI study. Mov Disord 2004; 19(1): 83–90. [DOI] [PubMed] [Google Scholar]
- 84.Northoff G, Steinke R, Nagel DC, et al. Right lower prefronto-parietal cortical dysfunction in akinetic catatonia: a combined study of neuropsychology and regional cerebral blood flow. Psychol Med 2000; 30(3): 583–96. [DOI] [PubMed] [Google Scholar]
- 85.Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry 1999; 67(4): 445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Northoff G, Kotter R, Baumgart F, et al. Orbitofrontal cortical dysfunction in akinetic catatonia: a functional magnetic resonance imaging study during negative emotional stimulation. Schizophr Bull 2004; 30(2): 405–27. [DOI] [PubMed] [Google Scholar]
- 87.Richter A, Grimm S, Northoff G. Lorazepam modulates orbitofrontal signal changes during emotional processing in catatonia. Hum Psychopharmacol 2010; 25(1): 55–62. [DOI] [PubMed] [Google Scholar]
- 88.Foucher JR, Zhang YF, Roser M, et al. A double dissociation between two psychotic phenotypes: Periodic catatonia and cataphasia. Prog Neuropsychopharmacol Biol Psychiatry 2018; 86: 363–9. [DOI] [PubMed] [Google Scholar]
- 89.De Tiege X, Bier JC, Massat I, et al. Regional cerebral glucose metabolism in akinetic catatonia and after remission. J Neurol Neurosurg Psychiatry 2003; 74(7): 1003–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iseki K, Ikeda A, Kihara T, et al. Impairment of the cortical GABAergic inhibitory system in catatonic stupor: a case report with neuroimaging. Epileptic Disord 2009; 11(2): 126–31. [DOI] [PubMed] [Google Scholar]
- 91.Mehta UM, Basavaraju R, Thirthalli J. Mirror neuron disinhibition may be linked with catatonic echo-phenomena: a single case TMS study. Brain Stimul 2013; 6(4): 705–7. [DOI] [PubMed] [Google Scholar]
- 92.Northoff G, Pfennig A, Krug M, et al. Delayed onset of late movement-related cortical potentials and abnormal response to lorazepam in catatonia. Schizophr Res 2000; 44(3): 193–211. [DOI] [PubMed] [Google Scholar]
- 93.Woodward ND, Heckers S. Mapping Thalamocortical Functional Connectivity in Chronic and Early Stages of Psychotic Disorders. Biol Psychiatry 2016; 79(12): 1016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martino M, Magioncalda P, Yu H, et al. Abnormal Resting-State Connectivity in a Substantia Nigra-Related Striato-Thalamo-Cortical Network in a Large Sample of First-Episode Drug-Naive Patients With Schizophrenia. Schizophr Bull 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB. The “what” and “when” of self-initiated movements. Cereb Cortex 2013; 23(3): 520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol 2005; 15(2): 122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen X, Scangos KW, Stuphorn V. Supplementary motor area exerts proactive and reactive control of arm movements. J Neurosci 2010; 30(44): 14657–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol 2010; 20(19): 1779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bracht T, Schnell S, Federspiel A, et al. Altered cortico-basal ganglia motor pathways reflect reduced volitional motor activity in schizophrenia. Schizophr Res 2013; 143(2–3): 269–76. [DOI] [PubMed] [Google Scholar]
- 100.Van Os J, Marcelis M, Sham P, Jones P, Gilvarry K, Murray R. Psychopathological syndromes and familial morbid risk of psychosis. Br J Psychiatry 1997; 170: 241–6. [DOI] [PubMed] [Google Scholar]
- 101.Peralta V, Cuesta MJ. The relationship between syndromes of the psychotic illness and familial liability to schizophrenia and major mood disorders. Schizophr Res 2007; 91(1–3): 200–9. [DOI] [PubMed] [Google Scholar]
- 102.Peralta V, Fananas L, Martin-Reyes M, Cuesta MJ. Dissecting the catatonia phenotype in psychotic and mood disorders on the basis of familial-genetic factors. Schizophr Res 2017. [DOI] [PubMed] [Google Scholar]
- 103.Beckmann H, Franzek E, Stober G. Genetic heterogeneity in catatonic schizophrenia: a family study. Am J Med Genet 1996; 67(3): 289–300. [DOI] [PubMed] [Google Scholar]
- 104.Leonhard K. Different causative factors in different forms of schizophrenia. Br J Psychiatry 1986; 149: 1–6. [DOI] [PubMed] [Google Scholar]
- 105.Selch S, Strobel A, Haderlein J, et al. MLC1 polymorphisms are specifically associated with periodic catatonia, a subgroup of chronic schizophrenia. Biol Psychiatry 2007; 61(10): 1211–4. [DOI] [PubMed] [Google Scholar]
- 106.Stober G, Saar K, Ruschendorf F, et al. Splitting schizophrenia: periodic catatonia-susceptibility locus on chromosome 15q15. Am J Hum Genet 2000; 67(5): 1201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Breckpot J, Vercruyssen M, Weyts E, et al. Copy number variation analysis in adults with catatonia confirms haploinsufficiency of SHANK3 as a predisposing factor. Eur J Med Genet 2016; 59(9): 436–43. [DOI] [PubMed] [Google Scholar]
- 108.Butcher NJ, Boot E, Lang AE, et al. Neuropsychiatric expression and catatonia in 22q11.2 deletion syndrome: An overview and case series. Am J Med Genet A 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poggi G, Boretius S, Mobius W, et al. Cortical network dysfunction caused by a subtle defect of myelination. Glia 2016; 64(11): 2025–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hagemeyer N, Goebbels S, Papiol S, et al. A myelin gene causative of a catatonia-depression syndrome upon aging. EMBO Mol Med 2012; 4(6): 528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walther S, Hugli S, Hofle O, et al. Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol Dis 2012; 47(1): 13–9. [DOI] [PubMed] [Google Scholar]
- 112.Walther S, Federspiel A, Horn H, et al. Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiol Dis 2011; 42(3): 276–83. [DOI] [PubMed] [Google Scholar]
- 113.Carroll BT. Complications of catatonia. J Clin Psychiatry 1996; 57(2): 95. [PubMed] [Google Scholar]
- 114.Gross AF, Smith FA, Stern TA. Dread complications of catatonia: a case discussion and review of the literature. Prim Care Companion J Clin Psychiatry 2008; 10(2): 153–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fricchione GL, Huffman JC, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonine syndrome In: Stern TA, Fricchione GL, Cassem NH, eds. Massachusetss General Hospital Handbook of General Hospital Psychiatry. Philadelphia: Mosby; 2004. [Google Scholar]
- 116.Clinebell K, Azzam PN, Gopalan P, Haskett R. Guidelines for preventing common medical complications of catatonia: case report and literature review. J Clin Psychiatry 2014; 75(6): 644–51. [DOI] [PubMed] [Google Scholar]
- 117.Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Db Syst Rev 2008; (4). [DOI] [PubMed] [Google Scholar]
- 118.Seethalakshmi R, Dhavale S, Suggu K, Dewan M. Catatonic syndrome: importance of detection and treatment with lorazepam. Ann Clin Psychiatry 2008; 20(1): 5–8. [DOI] [PubMed] [Google Scholar]
- 119.Northoff G, Wenke J, Demisch L, Eckert J, Gille B, Pflug B. Catatonia: short-term response to lorazepam and dopaminergic metabolism. Psychopharmacology (Berl) 1995; 122(2): 182–6. [DOI] [PubMed] [Google Scholar]
- 120.Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand 1996; 93(2): 137–43. [DOI] [PubMed] [Google Scholar]
- 121.Girish K, Gill NS. Electroconvulsive therapy in Lorazepam non-responsive catatonia. Indian J Psychiatry 2003; 45(1): 21–5. [PMC free article] [PubMed] [Google Scholar]
- 122.Ungvari GS, Chiu HF, Chow LY, Lau BS, Tang WK. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999; 142(4): 393–8. [DOI] [PubMed] [Google Scholar]
- 123.Leroy A, Naudet F, Vaiva G, Francis A, Thomas P, Amad A. Is electroconvulsive therapy an evidence-based treatment for catatonia? A systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2017. [DOI] [PubMed] [Google Scholar]
- 124.Petrides G, Divadeenam KM, Bush G, Francis A. Synergism of lorazepam and electroconvulsive therapy in the treatment of catatonia. Biol Psychiatry 1997; 42(5): 375–81. [DOI] [PubMed] [Google Scholar]
- 125.Phutane VH, Thirthalli J, Muralidharan K, Naveen Kumar C, Keshav Kumar J, Gangadhar BN. Double-blind randomized controlled study showing symptomatic and cognitive superiority of bifrontal over bitemporal electrode placement during electroconvulsive therapy for schizophrenia. Brain Stimul 2013; 6(2): 210–7. [DOI] [PubMed] [Google Scholar]
- 126.Cristancho P, Jewkes D, Mon T, Conway C. Successful use of right unilateral ECT for catatonia: a case series. J ECT 2014; 30(1): 69–72. [DOI] [PubMed] [Google Scholar]
- 127.Martenyi F, Metcalfe S, Schausberger B, Dossenbach MR. An efficacy analysis of olanzapine treatment data in schizophrenia patients with catatonic signs and symptoms. J Clin Psychiatry 2001; 62 Suppl 2: 25–7. [PubMed] [Google Scholar]
- 128.Madigand J, Lebain P, Callery G, Dollfus S. Catatonic syndrome: From detection to therapy. Encephale 2016; 42(4): 340–5. [DOI] [PubMed] [Google Scholar]
- 129.Beach SR, Gomez-Bernal F, Huffman JC, Fricchione GL. Alternative treatment strategies for catatonia: A systematic review. Gen Hosp Psychiatry 2017; 48: 1–19. [DOI] [PubMed] [Google Scholar]
- 130.Ji GJ, Yu F, Liao W, Wang K. Dynamic aftereffects in supplementary motor network following inhibitory transcranial magnetic stimulation protocols. Neuroimage 2017; 149: 285–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Catatonia symptom distribution in Kahlbaum’s case series
Supplemental Table 2. Prospective studies of catatonia treatment with blinded assessments: benzodiazepines, electroconvulsive therapy (ECT), and antipsychotics