Abstract
Objective:
Breast cancer survivors experience problems with cognition that interfere with daily life and can last for years. In the general population, obesity and diabetes are risk factors for cognitive decline, and weight loss can improve cognition; however, the impact of intentional weight loss on cancer survivors’ cognition has not been tested. We investigated the impact of weight loss and metformin on changes in cognitive function in a sample of breast cancer survivors.
Methods:
Overweight/obese postmenopausal breast cancer survivors (n = 333) were randomized to a weight loss intervention versus control and metformin versus placebo in a 2 × 2 factorial design. Outcomes were changes in five cognitive domains from baseline to 6 months measured by objective neurocognitive tests.
Results:
There were no statistically significant intervention effects for the metformin or weight loss interventions in five neurocognitive domains. Baseline body mass index (BMI) was a significant effect modifier of the changes in verbal functioning for the weight loss (P = 0.009) and metformin interventions (P = 0.0125). These effect modifications were independent of percent weight loss achieved during the 6-month study period.
Conclusions:
This randomized controlled trial of weight loss and metformin interventions that examined changes to cognition among breast cancer survivors suggests that these interventions may not improve cognitive functioning among breast cancer survivors in general. However, weight loss may improve verbal functioning among individuals with a higher BMI.
Keywords: cancer survivors, cognition, metformin, obesity, weight loss
1. BACKGROUND
Up to 75% of the 3.5 million breast cancer survivors experience cognitive impairment,1 which is a troubling, disruptive, and persistent symptom that can last for years after systemic therapy.2–6 Cognitive impairment in breast cancer survivors have negative effects on quality of life, daily functioning, and the ability to return to work—all of which can create substantial hardship.7–12 There are no established treatments for cognitive impairment in breast cancer survivors. Therefore, there is an urgent need to identify effective intervention strategies that clinicians and their patients can use to improve cognitive difficulties for women in this large population subgroup.
Obesity and diabetes have been identified as risk factors for age-related cognitive decline in the general population,13,14 and emerging evidence suggests they also contribute to the cognitive challenges experienced by breast cancer survivors.15–18 Recent prospective studies have identified obesity to be a risk factor for adverse cognitive changes in breast cancer survivors,15 as well as for the persistence of cognitive complaints.16 Given the evidence linking obesity and diabetes to cognition and their potential modifiability, it is of considerable public health importance to examine whether interventions that target these risk factors can improve cognitive function in breast cancer survivors.
Studies testing whether weight loss can improve cognition in populations of breast cancer survivors are lacking; however, evidence from the general population suggests a benefit.19,20 Some of the most robust evidence to date comes from a 2017 systematic review that used a random-effects meta-analysis including data from seven randomized trials, to evaluate the impact of intentional weight loss on cognitive function among overweight or obese adults. The studies included men and women with samples size ranging from 37 to 119 participants, with mean ages ranging from 36 to 70 years old. Mean baseline body mass index (BMI) varied greatly across the seven studies ranging from 27 to 37 kg/m2. Lengths of the studies were between 8 and 48 weeks long, with five of the seven being less than 6 months long. Participants were generally healthy adults with one study targeting men and women with high blood pressure, and the interventions varied greatly and included both dietary only and exercise plus diet interventions. The authors of this study concluded that weight loss may have a beneficial effect on several cognitive domains, including attention, memory, executive function, and language.19 In contrast to this meta-analysis, the Look AHEAD trial, with 3802 over-weight or obese adults with type 2 diabetes, which evaluated a 10-year weight loss intervention, did not reduce the risk of cognitive impairment or dementia.21 The discrepant findings between the generally healthy populations in the meta-analysis and a sample with type 2 diabetes highlight the need to examine this in cancer survivors separately.
Metformin, a medication primarily used to treat diabetes, may decrease breast cancer risk.22 The potential therapeutic effect has increased the interest in using metformin in breast cancer survivors. Although diabetes is generally identified as a risk factor for cognitive impairment and dementia, it remains unclear whether interventions that prevent or control diabetes, such as metformin, will benefit the brain. For example, there is well-documented evidence from animal and prospective cohort studies suggesting that metformin use may reduce the risk of cognitive decline and dementia.23–25 However, other reports indicate that metformin may increase the risk of cognitive problems.26–28 In a study by Moore and colleagues involving 126 patients with diabetes, individuals using metformin had worse cognitive performance compared with individuals that managed their diabetes using other approaches.26 While metformin is not Food and Drug Administration (FDA) approved for use as a weight loss agent, it may result in modest weight loss, as seen with the 2.1-kg weight loss in the Diabetes Prevention Program,29 which could potentially support cognitive health. These inconsistences make it difficult to draw definitive conclusions about the effects of metformin on cognitive function.
In the current study, we investigate the impact of a 6-month duration weight loss and metformin use on cognitive function among breast cancer survivors. Specifically, we test the impact of weight loss and metformin on changes in cognitive functioning in a sample of 333 overweight/obese early-stage breast cancer survivors who were enrolled in the Reach for Health randomized trial.30,31 We also explore potential modifiers of the intervention effects. To our knowledge, this is the first study to test the impact of weight loss or metformin on cognitive functioning among cancer survivors in the setting of a randomized controlled trial (RCT).
2. METHODS
The Reach for Health trial () was conducted as part of the University of California (UC) San Diego Transdisciplinary Research in Energetics and Cancer Center initiative to examine the role of insulin resistance and inflammation in breast cancer risk (1U54CA155435; PI, Patterson). The study used a 2 × 2 factorial design to randomly allocate 333 participants in (roughly) equal numbers to one of four arms: placebo + control, weight loss intervention alone, metformin alone, and weight loss + metformin. The a priori primary analysis tested the main effects of metformin versus placebo and a weight loss intervention versus control. Measurements were collected during in-person clinic visits conducted at baseline and 6-month follow-up. Details of the study design, recruitment, and interventions are described elsewhere.30,31 All participants provided signed informed consent, and the study was approved by the Human Research Protections Program at UC San Diego (IRB#101977).
2.1. Participants and recruitment
Breast cancer survivors were recruited between August 2011 and May 2015 from San Diego and surrounding communities using cancer registry mailings, physician referrals, community outreach, and mass media approaches (Figure S1).31 Eligible participants were post-menopausal breast cancer survivors with a BMI ≥ 25.0 kg/m2, diagnosed with primary operable stage IA-IIIC breast cancer within 10 years of enrollment, and had completed planned surgery, and active treatments. Participants who were taking adjuvant endocrine or biological therapy for breast cancer had to be willing and able to continue throughout the 6-month intervention period. Women were excluded if they were taking diabetes medication, had elevated fasting blood glucose test at screening, were using hormone replacement therapy, or had serious medical conditions such as renal insufficiency or congestive heart failure.
2.2. Intervention groups
2.2.1. Metformin versus placebo
Participants were randomly assigned to receive metformin or placebo pills (we received an FDA waiver to provide metformin to these non-diabetic women). Participants and study staff were blinded to medication group assignment. To enhance drug tolerance, participants gradually increased from a single 500-mg metformin pill to three pills (1500 mg total) at 1 month.30,31
2.2.2. Weight loss intervention versus control
Participants were randomly assigned to a telephone-based weight loss intervention or control (ie, usual care). The weight loss intervention was delivered by trained lifestyle coaches through 12 phone calls over the 6-month intervention period. Intervention weight loss strategies included encouraging portion control, healthy eating, and counting calories to reduce daily energy intake by 500 to 1000 calories. Women randomized to the control group were provided with the US Dietary Guidelines for Americans, 2010.30,31
2.3. Measures
Cognitive functioning was assessed at the baseline and 6-month clinic visits using a 45-minute computerized testing battery designed to sample a range of cognitive domains (NeuroTrax).32 The computerized tests are adaptive, which reduces the time needed to complete the tests, increases sensitivity, and minimizes ceiling effects. Cognitive domain index scores were computed from individual test scores standardized for age and educational level and fit to a scale with mean of 100 and standard deviation (SD) of 15. Higher domain scores represent better cognitive functioning. This measure has been used in a variety of patient populations including breast cancer survivors.17
Demographic data were obtained via self-report questionnaires at baseline (prior to randomization). Height and weight were measured using standard procedures. Medical charts were abstracted to obtain information regarding the original cancer diagnosis and treatment, including stage, grade, and hormone receptor status. Details of measurement procedures are published.31
2.4. Statistical considerations and analysis
Participants were assigned to the study arms using a random permuted–block design that included strata for stage at diagnosis (stage I versus stages II and III) and BMI (<30.0 versus ≥30.0 kg/m2). All analyses were performed using an intent-to-treat principle with missing data assumed missing at random and accounted for in the longitudinal random-effects models by using a likelihood-based estimation method, which uses all available data and does not omit subjects with partially missing data. Sample size estimates were based on main effects comparisons of metformin versus nonmetformin and weight loss versus control. Assuming a two-sided test with alpha = 0.05 and a sample size of 320, there was 80% power to detect a main effect of 0.32 for a standardized group mean difference in change (ie, effect size) for a cognition outcome.
Baseline distributions (mean/SD and n/percent) were calculated, and differences in baseline characteristics were assessed using t tests for continuous and chi-squared tests for categorical variables.
To align with the original study design, intervention effects were assessed separately for the metformin versus placebo and for the weight loss intervention versus control arms. As a sensitivity analysis, we also assessed the four-group comparison (weight loss plus metformin, weight loss plus placebo, metformin only, and placebo only). Main effects of the intervention on neurocognitive domains (attention, executive function, memory, verbal function, and visual spatial) were assessed using mixed effects regression models with a subject-level random intercept and an unstructured covariance structure, as determined by model Akaike information criterion. These models included a fixed effect term for time, group, and the time by group interaction. Contrasts were used to calculate an estimate of the group difference in neurocognitive change based on the regression model.
We examined chemotherapy, tamoxifen, or aromatase inhibitor use, time since diagnosis, and baseline BMI as potential effect modifiers of the interventions on cognitive outcomes by adding the potential effect modifier and a three-way interaction (time, group, and effect modifier) to the mixed effect regression models. Interpretation of significant effect modifiers was done by calculating estimated group differences at various values of the effect modifier using the regression model that included the time, group, and effect modifier three-way interaction plus all lower order terms. In models assessing baseline BMI as an effect modifier, we evaluated percent weight loss as a potential confounder, with a greater than 10% change in the effect size used as determination for confounding. Lastly, we also examined baseline associations of the potential effect modifiers with each of the five neurocognitive measures using separate simple linear regression models.
3. RESULTS
Participants were 333 breast cancer survivors who were a mean 63 years of age (SD = 6.9; range 49–83 years old), predominantly white (84%), and were generally obese (mean BMI of 31.1 kg/m2, SD = 5.0; BMI range 25–53 kg/m2); over 90% had some college education. Time since breast cancer diagnosis was an average of 2.7 years (SD 2.0), with approximately half of participants diagnosed with stage I cancer. Mean age- and education-adjusted, standardized, neurocognitive scores were consistent with normal cognitive function in all five domains ranging from a mean of 101 (SD = 17.2) for verbal function to a mean of 110 (SD = 13.5) for visual spatial performance (see Table 1). There were no significant differences between the arms in baseline characteristics (P > 0.05). At baseline, having had chemotherapy or hormone therapy and time since diagnosis were not significantly associated with any of the five neurocognitive tests. Baseline BMI was associated with the verbal functioning score only (b = −0.38, P = 0.047). As previously reported, the weight loss intervention group lost a significantly greater percent of initial weight than those in the control group (5.5% vs 2.7%; P < 0.001); participants prescribed metformin lost significantly more weight than those prescribed placebo (5.3% vs 2.9%; P < 0.001); and 65.9% of participants took 80% or more of the prescribed metformin.30
TABLE 1.
Baseline characteristics of breast cancer survivors in a 2 × 2 factorial randomized controlled trial of metformin and weight loss (n = 333)a
| Age, mean years (SD) | 62.6 (6.9) |
| Education, N (%) | |
| College graduate or graduate school | 171 (51.3%) |
| Some college | 132 (39.6%) |
| High school or less | 30 (9.0%) |
| Time since diagnosis, mean years (SD) | 2.7 (2.0) |
| BMI, kg/m2 (SD) | 31.1 (5.0) |
| Race, N (%) | |
| White | 278 (83.5%) |
| Black or African-American | 12 (3.6%) |
| sian | 6 (1.8%) |
| Mixed or other race | 37 (11.1%) |
| Ethnicity, N (%) | |
| Non-Hispanic | 295 (88.6%) |
| Hispanic | 38 (11.4%) |
| Cancer stage, N (%) | |
| Stage I | 161 (48.4%) |
| Stage II | 116 (34.8%) |
| Stage III | 56 (16.8%) |
| Receptor status, N (%) | |
| ER+ or PR+ HER2- | 240 (72.1%) |
| HER2+ | 51 (15.3%) |
| Triple negative (ER-, PR-, and HER2-) | 30 (9.0%) |
| Missing data | 12 (3.6%) |
| Received chemotherapy, N (%) | 177 (53.2%) |
| Received hormone therapy, N (%) | 229 (68.8%) |
| Neurocognitive score, mean (SD) | |
| Attention | 102.9 (8.84) |
| Executive function | 102.9 (10.96) |
| Memory | 105.1 (8.97) |
| Verbal function | 101.1 (17.20) |
| Visual spatial | 110.3 (13.54) |
Abbreviations: BMI, body mass index; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; SD, standard deviation.
There were no significant differences across the study arms.
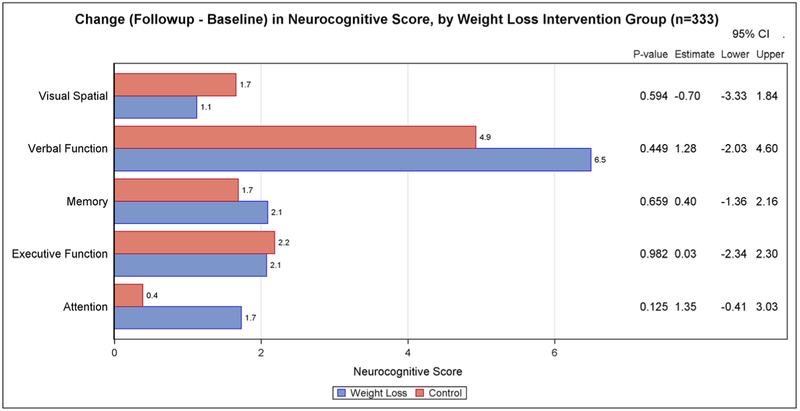
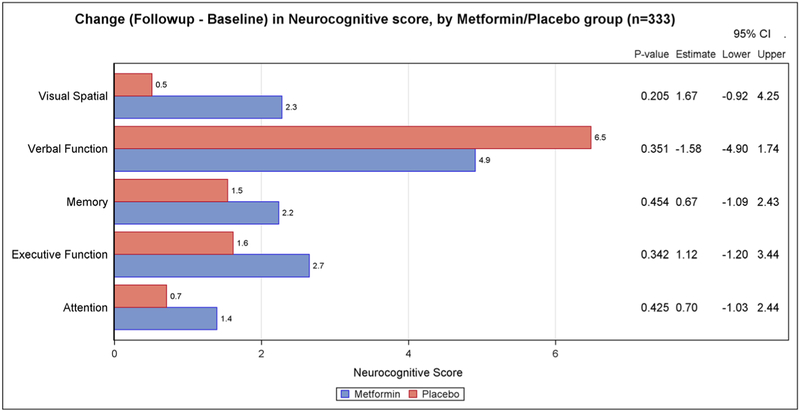
Changes in neurocognitive testing from baseline to 6 months for the main intervention effects are presented in Figure 1 (weight loss versus control) and Figure 2 (metformin versus placebo). There was no statistically significant difference in the two-arm comparisons between the intervention and control/placebo groups for any of the neurocognitive domain scores. We also found no significant intervention effects when the four arms were compared (weight loss plus metformin, weight loss plus placebo, metformin only, and placebo only).
FIGURE 1.
Change (follow-up–baseline) in neurocognitive score, by weight loss intervention group (n = 333)
FIGURE 2.
Change (follow-up–baseline) in neurocognitive score, by metformin/placebo group (n = 333)
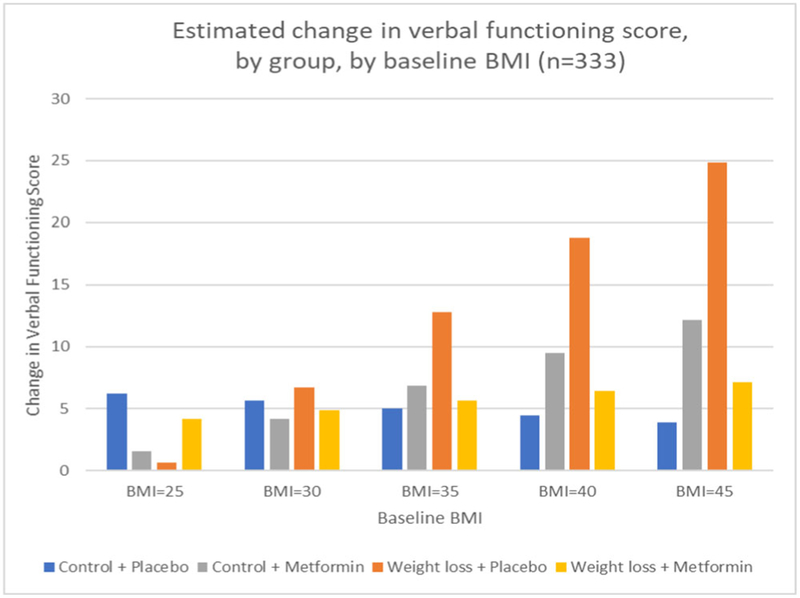
BMI, having had chemotherapy, hormonal therapy, and time since diagnosis were tested as potential effect modifiers of the interventions. Only BMI was found to be a significant effect modifier. Baseline BMI was associated with a differential change in verbal functioning for both the weight loss intervention effect (three-way interaction P = 0.009) and the metformin effect (three-way interaction P = 0.0125). These effect modifications were independent of percent weight loss. To interpret the direction of the interactions, we calculated estimated group differences of change in cognition, based on the resulting regression model, at different levels of baseline BMI. This indicated that participants in the weight loss intervention with a greater baseline BMI increased their verbal function score to a greater degree than those in the control group, compared to participants with a lower baseline BMI. However, the effect modification of BMI on metformin appeared to be in the opposite direction. To further disentangle these effects, we explored effect modification in the four arms (weight loss plus metformin, weight loss plus placebo, metformin only, and placebo only). Baseline BMI remained a significant effect modifier (three-way interaction P = 0.0014). Estimated change in verbal functioning scores, by group, at different levels of baseline BMI showed that the weight loss plus placebo arm had the greatest improvement in verbal functioning with increasing baseline BMI (see Figure 3).
FIGURE 3.
Estimated change in verbal functioning score, by group, by baseline body mass index (BMI; n = 333)
4. CONCLUSIONS
This is the first study within the setting of an RCT among breast cancer survivors to test the impact of weight loss or metformin on cognitive functioning. Neither the weight loss intervention nor metformin significantly improved cognitive functioning over the 6-month period. The lack of intervention effects, particularly for the weight loss intervention, is inconsistent with a recent meta-analysis of seven RCTs in noncancer survivors.19 The meta-analysis showed benefits of intentional weight loss across multiple cognitive domains; however, most of the studies had small sample sizes (total seven studies N = 468), enrolled healthy participants, and had a lower mean age (54 years old) than the current study, and five out of the seven studies had a shorter intervention duration. Many of the RCTs in the meta-analysis also achieved much greater weight loss than the current study, and some had more intensive exercise components in their interventions, which may also independently influence cognition.33 These differences in populations, amount of weight loss achieved, and differences in exercise prescriptions across interventions are all possible reasons why findings from the current study may contrast with previous reports. However, our results were consistent with the Look AHEAD trial of over 3000 type 2 diabetics where a weight loss intervention was not shown to improve cognition.21 Weight loss or metformin may not be effective strategies to improve cognition for many breast cancer survivors.
In exploratory analyses, BMI was a significant moderator of both the weight loss and the metformin intervention effects on verbal functioning, but the direction of the interaction appeared to be in opposite directions for each main effect. To try to understand this moderator effect, we looked at the study four arms separately and found that the effect modification was driven mainly by the weight loss plus placebo group, with all other groups behaving similarly to the control/placebo group. The observed benefit of the weight loss intervention for verbal functioning in our exploratory analyses is somewhat consistent with a meta-analysis of 12 weight loss studies, which found greater effects of weight loss on cognition among obese participants than overweight participants, highlighting the plausibility that this may be a true association.20 If it is a true association, one putative mechanism through which weight loss may improve verbal functioning among at least some subsets of breast cancer survivors is by altering brain structure. Breast cancer survivors have been shown in neuroimaging studies to have structural alterations in both gray and white matter,34–37 and weight loss has been shown to be increase brain volume. Specifically, weight loss has been shown to increase gray matter volume in the left inferior frontal gyrus, the part of the brain that is important for verbal processing.38 Future research with obese cancer survivors is needed to replicate this finding and explore potential mechanisms through by weight loss may be able to improve verbal functioning among breast cancer survivors with high BMI.
The weight loss plus metformin group did not have a similar improvement in verbal functioning with increasing baseline BMI in our exploratory analyses. Although this finding was unexpected, there is some evidence that metformin may be associated with impaired cognition. One study showed that diabetics who take metformin have a higher risk of cognitive impairment than diabetics who do not take metformin.24 There is also evidence that metformin, but not other diabetes medications, is associated with increased risk of developing Alzheimer disease.28 One possible mechanism is that metformin suppresses AMP-activated protein kinase (AMPK) in the hypothalamus, where in contrast, weight loss strategies, such as exercise and dietary changes, have been shown to activate AMPK in the hypothalamus.39 This difference in AMPK activation between metformin and weight loss could be responsible for the difference in outcomes between the four study arms. As metformin continues to be explored as a potential prevention strategy for breast and other cancers, more research is needed to understand its broader impact on cognition.
4.1. Study limitations
Several limitations of this study should be noted. The intervention was 6 months long achieving five percent weight loss, which may not have been sufficient to demonstrate changes on the neurocognitive tests. Overall, participants were cognitively intact at baseline and may have had little ability to improve their cognitive functioning, or there may not have been enough power to detect small changes. In addition, although our results were intended to be hypothesis generating, it should be noted that multiple statistical tests were performed on the data without correction for multiple comparisons. Therefore, we must acknowledge the possibility that the exploratory BMI findings are chance findings. Future studies focusing on survivors with cognitive deficits, which are longer in duration and can potentially achieve greater weight loss, are needed to replicate these findings and fully elucidate the impact of weight loss on cognitive changes in breast cancer survivors.
4.2. Clinical implications
Findings provide preliminary support that weight loss or metformin may not be effective strategies to improve cognitive functioning among breast cancer survivors in general. This study provides a suggestion that breast cancer survivors with very high BMIs may improve their verbal functioning with weight loss. Given the increasing interest in repurposing metformin as an intervention strategy for cancer prevention and control, the impact of metformin on cognition should be carefully examined in larger and longer-term trials with more diverse samples of breast cancer survivors so that physicians and their patients can be fully informed of its benefits and potential side effects.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by funding from the National Cancer Institute (U54 CA155435–01). Dr Hartman was supported by the National Cancer Institute under award number K07CA181323. Dr Marinac was supported by the National Cancer Institute under award numbers F32CA220859. The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
National Cancer Institute, Grant/Award Numbers: F32CA220859, K07CA181323 and U54 CA155435
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy restrictions.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26(1):102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(29): 3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips K-A. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta-analysis of the current literature. Brain Cogn. 2005;59(1):60–70. [DOI] [PubMed] [Google Scholar]
- 4.Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Intern Neuropsychol Soc: JINS. 2003;9(7):967–982. [DOI] [PubMed] [Google Scholar]
- 5.Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104(10): 2222–2233. [DOI] [PubMed] [Google Scholar]
- 6.Ono M, Ogilvie JM, Wilson JS, et al. A meta-analysis of cognitive impairment and decline associated with adjuvant chemotherapy in women with breast cancer. Front Oncol. 2015;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid-Arndt SA, Yee A, Perry MC, Hsieh C. Cognitive and psychological factors associated with early posttreatment functional outcomes in breast cancer survivors. J Psychosoc Oncol. 2009;27(4):415–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100(11):2292–2299. [DOI] [PubMed] [Google Scholar]
- 9.Duijts SF, van Egmond MP, Spelten E, van Muijen P, Anema JR, van der Beek AJ. Physical and psychosocial problems in cancer survivors beyond return to work: a systematic review. Psycho-Oncology. 2014;23(5): 481–492. [DOI] [PubMed] [Google Scholar]
- 10.Jagsi R, Hawley ST, Abrahamse P, et al. Impact of adjuvant chemotherapy on long-term employment of survivors of early-stage breast cancer. Cancer. 2014;120(12):1854–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauzier S, Maunsell E, Drolet M, et al. Wage losses in the year after breast cancer: extent and determinants among Canadian women. J Natl Cancer Inst. 2008;100(5):321–332. [DOI] [PubMed] [Google Scholar]
- 12.Steiner JF, Cavender TA, Main DS, Bradley CJ. Assessing the impact of cancer on work outcomes: what are the research needs? Cancer. 2004; 101(8):1703–1711. [DOI] [PubMed] [Google Scholar]
- 13.Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015;9(2):93–113. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z, Zheng Y, Bao P, et al. Aging, obesity, and post-therapy cognitive recovery in breast cancer survivors. Oncotarget. 2017;8(7): 12364–12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klemp JR, Myers JS, Fabian CJ, et al. Cognitive functioning and quality of life following chemotherapy in pre- and peri-menopausal women with breast cancer. Support Care Cancer. 2018;26(2):575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartman SJ, Marinac CR, Natarajan L, Patterson RE. Lifestyle factors associated with cognitive functioning in breast cancer survivors. Psycho-Oncology. 2015;24(6):669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandelblatt JS, Stern RA, Luta G, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol. 2014;32(18): 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veronese N, Facchini S, Stubbs B, et al. Weight loss is associated with improvements in cognitive function among overweight and obese people: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;72:87–94. [DOI] [PubMed] [Google Scholar]
- 20.Siervo M, Arnold R, Wells JC, et al. Intentional weight loss in over-weight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev. 2011;12(11):968–983. [DOI] [PubMed] [Google Scholar]
- 21.Espeland MA, Luchsinger JA, Baker LD, et al. Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology. 2017;88(21):2026–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Col NF, Ochs L, Springmann V, Aragaki AK, Chlebowski RT. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat. 2012;135(3):639–646. [DOI] [PubMed] [Google Scholar]
- 23.Hsu CC, Wahlqvist ML, Lee MS, Tsai HN. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis. 2011;24(3):485–493. [DOI] [PubMed] [Google Scholar]
- 24.Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis. 2014;41(1):61–68. [DOI] [PubMed] [Google Scholar]
- 25.Hervas D, Fornes-Ferrer V, Gomez-Escribano AP, et al. Metformin intake associates with better cognitive function in patients with Huntington’s disease. PLoS ONE. 2017;12(6):e0179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore EM, Mander AG, Ames D, et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36(10):2981–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wennberg AMV, Hagen CE, Edwards K, et al. Association of antidiabetic medication use, cognitive decline, and risk of cognitive impairment in older people with type 2 diabetes: results from the population-based Mayo Clinic Study of Aging. Int J Geriatr Psychiatry. 2018;33(8):1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imfeld P, Bodmer M, Jick SS, Meier CR. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc. 2012;60(5):916–921. [DOI] [PubMed] [Google Scholar]
- 29.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson RE, Marinac CR, Sears DD, et al. The effects of metformin and weight loss on biomarkers associated with breast cancer outcomes. J Natl Cancer Inst. 2018;110(11):1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson RE, Marinac CR, Natarajan L, et al. Recruitment strategies, design, and participant characteristics in a trial of weight-loss and metformin in breast cancer survivors. Contemp Clin Trials. 2016;47:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dwolatzky T, Whitehead V, Doniger GM, et al. Validity of the mindstreams computerized cognitive battery for mild cognitive impairment. J Mol Neurosci: MN. 2004;24(1):33–44. [DOI] [PubMed] [Google Scholar]
- 33.Hartman SJ, Nelson SH, Myers E, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory & motion study. Cancer. 2018;124(1):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koppelmans V, de Ruiter MB, van der Lijn F, et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132(3):1099–1106. [DOI] [PubMed] [Google Scholar]
- 35.de Ruiter MB, Reneman L, Boogerd W, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;33(12):2971–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deprez S, Amant F, Yigit R, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011; 32(3):480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lepage C, Smith AM, Moreau J, et al. A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus. 2014;3(1):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prehn K, Jumpertz von Schwartzenberg R, Mai K, et al. Caloric restriction in older adults-differential effects of weight loss and reduced weight on brain structure and function. Cereb Cortex. 2017;27(3): 1765–1778. [DOI] [PubMed] [Google Scholar]
- 39.Nakano M, Inui A. Metformin and incretin-based therapies up-regulate central and peripheral adenosine monophosphate-activated protein affecting appetite and metabolism. Indian J Endocrinol Metab. 2012; 16(Suppl 3):S529–S531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.