Abstract
Background:
Long-term ozone () exposure is associated with cardiovascular mortality, but little is known about the associations between and subclinical arterial disease.
Objectives:
We studied the longitudinal association of exposure to and progression of key subclinical arterial markers in adults: intima-media thickness of common carotid artery (), carotid plaque (CP) burden, and coronary artery calcification (CAC).
Methods:
CAC was measured one to four times at baseline and at follow-up exams (1999–2012) by computed tomography (CT) in 6,619 healthy adults, recruited at age 45–84 y without cardiovascular disease (CVD), over a mean of 6.5 y (standard deviation: 3.5 y). and CP burden were quantified in 3,392 participants using carotid artery ultrasound imaging acquired over a mean of 9 y (1.7 y). Over 91% and 89% participants had at least one follow-up and CAC measurement, respectively. Residence-specific concentrations were estimated by a validated spatiotemporal model spanning from 1999 to 2012. This model relied on comprehensive monitoring data and geographical variables to predict individualized long-term average concentrations since baseline. Linear mixed models and logistic regression model were used to evaluate relationships of long-term average exposure to with longitudinal change in , CAC, and CP formation, respectively.
Results:
Mean progression rates of and CAC were and . CP formation was identified in 55% of the subjects. A increase in long-term average exposure was associated with a [95% confidence interval (CI): 1.4, 9.7] greater increase in over 10 y. A increase in was also associated with new CP formation [odds ratio (OR): 1.2 (95% CI: 1.1, 1.4)] but not CAC progression [ (95% CI: , 2)]. Associations were robust in the analysis with extended covariate adjustment, including copollutants, i.e., nitrogen oxides () and particulate matter with diameter ().
Conclusion:
Over almost a decade of follow-up, outdoor concentrations were associated with increased rate of carotid wall thickness progression and risk of new plaque formation, suggesting arterial injury in this cohort. https://doi.org/10.1289/EHP3325
Introduction
Ground-level ozone () is a powerful oxidizing agent and is one of the most harmful air pollutants currently addressed by air quality standards in the European Union and United States. Over recent decades, has not shown a discernible trend of decline in Europe and the United States, and it will likely remain an important environmental health issue, especially given projected increases in temperature related to climate change (EEA 2015), since ground-level ozone is formed when a complex set of chemical reactions is triggered by heat and sunlight (U.S. EPA 2013). Strong and consistent evidence for health effects of exposure has been demonstrated for the short-term exposure effects on the respiratory system (EEA 2011; U.S. EPA 2013). The literature on cardiovascular effects of long-term exposure to have been considered less consistent (U.S. EPA 2013; Jerrett et al. 2009) and focused primarily on mortality effects. (Carey et al. 2013; Di et al. 2017; Jerrett et al. 2009; Turner et al. 2016; Zanobetti and Schwartz 2011). There has been little attempt to investigate the underlying association between long-term exposure to and subclinical vascular disease (Breton et al. 2012).
Intima-media thickness of common carotid artery () and coronary artery calcification (CAC) are noninvasive markers of subclinical arterial disease that predict risk of coronary heart disease and stroke in people without cardiovascular diseases (CVDs) (Stein et al. 2008). Compared to , carotid plaque (CP) represents a later stage of arterial injury where diffuse thickening of the intima media complex accelerates focally, leading to atherosclerotic plaque formation (Stein et al. 2008). CP presence and burden are associated with increased risk of CVD events, and this measure improves the predictive accuracy of (Gepner et al. 2015).
To date, the association of long-term exposure to and has only been reported in a cross-sectional study of schoolchildren (Breton et al. 2012). Findings on the effects of exposure on progression of , CAC, and CP have not been reported previously.
The Multi-Ethnic Study of Atherosclerosis (MESA, https://www.mesa-nhlbi.org/) is a population-based prospective cohort study of adults free of CVD at baseline with repeated measurements of , CAC, and CP for up to 10 y. This provides a unique opportunity to assess the longitudinal relationship between long-term exposure to and progression of , CAC, and CP in a well-characterized cohort of adults.
Methods
Study Population
Study objectives and design have been previously published (Kaufman et al. 2012). MESA enrolled 6,814 participants aged 45–84 y without a clinical history of CVD in six U.S. city regions (Baltimore, Maryland; Chicago, Illinois; Los Angeles County, California; New York City, New York; St. Paul, Minnesota; and Winston-Salem, North Carolina). Recruitment of the MESA study started in 2000, and participants were followed for approximately 10 y. Participants had to meet age and race/ethnicity eligibility criteria and be free of prevalent CVD to optimize the study of subclinical CVD progression and its association with predictors of clinical CVDs. Between 2005 and 2007, the MESA Air Pollution Study (MESA Air) recruited an additional 257 participants from the Los Angeles Basin and the New York region to capitalize on exposure heterogeneity in the vicinity of two existing MESA population communities and to increase the size of the cohort for follow-up of clinical events. CP, , and CAC, together with standard cardiovascular risk factors, sociodemographic factors, lifestyle habits, and psychosocial factors, were collected for individuals at baseline and in the follow-up examinations (Table 1; Table S1). Participants had an average of two measurements (minimum to maximum: one to three times) for and CP, and three measurements (minimum to maximum: one to four) for CAC. We developed air pollution estimates for all MESA participants. There are approximately 21% participants who changed residential location within 5 y of their examination. The current analysis includes participants in the MESA Air study for whom we had developed air pollutant exposure estimates. This study met the Declaration of Helsinki guidelines. Written informed consent was obtained from all participants. The study was approved by the institutional review boards of all of the field and reading centers.
Table 1.
Participant characteristics with intima-media thickness of common carotid artery () and carotid plaque (CP) burden at baseline and during follow-up by study regions. Values provided are for continuous variables or number (%) for categorical variables.
| Site | Winston-Salem | New York City | Baltimore | St. Paul | Chicago | Los Angeles |
|---|---|---|---|---|---|---|
| concentrations (ppb), 2000–2012 | ||||||
| Number of participants | ||||||
| Baseline | 568 | 624 | 435 | 529 | 668 | 568 |
| Follow-up | 534 | 570 | 383 | 489 | 587 | 481 |
| Follow-up time (years) | ||||||
| Outcomes | ||||||
| Mean at baseline () | ||||||
| Increase in during follow-up () | ||||||
| Baseline: CP prevalence, (%) | 293 (53) | 275 (44) | 187 (44) | 280 (54) | 308 (46) | 237 (43) |
| Follow-up: CP formation, (%) | 294 (54) | 299 (51) | 238 (57) | 298 (60) | 357 (56) | 281 (53) |
| Baseline demographics | ||||||
| Age | ||||||
| Male, (%) | 261 (46) | 256 (41) | 200 (46) | 270 (51) | 314 (47) | 284 (50) |
| Race/ethnicity, (%) | ||||||
| White | 284 (50) | 131 (21) | 248 (57) | 312 (59) | 307 (46) | 62 (11) |
| Chinese | — | — | — | — | 207 (31) | 216 (38) |
| Black | 284 (50) | 193 (31) | 187 (43) | — | 154 (23) | 74 (13) |
| Hispanic | — | 300 (48) | — | 217 (41) | — | 216 (38) |
| Education, (%) | ||||||
| 142 (25) | 256 (41) | 105 (24) | 185 (35) | 94 (14) | 267 (47) | |
| Some college/technical | 170 (30) | 181 (29) | 126 (29) | 196 (37) | 154 (23) | 159 (28) |
| College or graduate | 256 (45) | 187 (30) | 204 (47) | 148 (28) | 420 (63) | 142 (25) |
| Employment, (%) | 392 (69) | 394 (63) | 302 (69) | 409 (77) | 478 (72) | 306 (54) |
| Neighborhood SES index | ||||||
| Income () | ||||||
| Baseline risk factors | ||||||
| Body mass index () | ||||||
| Smoking status, (%) | ||||||
| Never | 244 (43) | 331 (53) | 187 (43) | 217 (41) | 314 (47) | 364 (64) |
| Former | 233 (41) | 206 (33) | 196 (45) | 217 (41) | 274 (41) | 148 (26) |
| Current | 91 (16) | 87 (14) | 52 (12) | 95 (18) | 80 (12) | 56 (10) |
| Pack-years smoking | ||||||
| Secondhand smoking, (%) | 351 (62) | 281 (45) | 198 (45) | 331 (63) | 354 (53) | 178 (31) |
| Physical activity, (%) | ||||||
| Q1 | 128 (23) | 146 (23) | 89 (21) | 106 (20) | 121 (18) | 178 (31) |
| Q2 | 165 (29) | 135 (22) | 105 (24) | 140 (27) | 180 (27) | 174 (31) |
| Q3 | 133 (23) | 155 (25) | 110 (25) | 125 (23) | 185 (28) | 126 (22) |
| Q4 | 142 (25) | 188 (30) | 131 (30) | 158 (30) | 182 (27) | 90 (16) |
| Systolic blood pressure (mm Hg) | ||||||
| Diastolic blood pressure (mm Hg) | ||||||
| Hypertension, (%)a | 284 (50) | 268 (43) | 191 (44) | 175 (33) | 227 (34) | 227 (40) |
| High-density lipid ()b | ||||||
| Total cholesterol ()b | ||||||
| Statin use, (%)c | 74 (13) | 100 (16) | 96 (22) | 69 (13) | 94 (14) | 74 (13) |
| Antihypertensive medication, (%)c | 228 (40) | 242 (39) | 168 (39) | 151 (29) | 203 (30) | 172 (30) |
| Diabetes, (%)d | ||||||
| Normal | 453 (80) | 487 (78) | 338 (78) | 420 (79) | 547 (82) | 383 (67) |
| Impaired fasting glucose | 65 (12) | 68 (11) | 61 (14) | 62 (12) | 77 (12) | 107 (19) |
| Diabetic | 49 (9) | 69 (11) | 37 (8) | 47 (9) | 44 (7) | 79 (14) |
| Family history of premature CVD, (%)e | 168 (30) | 154 (25) | 138 (32) | 170 (32) | 166 (25) | 113 (20) |
| Fibrinogen () | ||||||
| C-reactive protein () | ||||||
| Creatinine () | ||||||
Outcome, demographic covariates, and risk factors were available for all the participants. Note: —, no data; CVD, cardiovascular disease; SES, socioeconomic status.
Hypertension was defined as systolic blood pressure , diastolic blood pressure , or reported use of antihypertensive medication.
Plasma lipid measurements for high-density lipid and total cholesterol.
Medication use was defined as any positive report of a statin and/or antihypertensive medication use on the medication inventory for the participants at each of the five clinical exams.
Diabetes mellitus was defined as fasting glucose or the use of hypoglycemic medications. Among those not reporting use of hypoglycemic medications, we defined impaired fasting glucose between 100 and and normal fasting glucose as fasting blood glucose .
Family history of premature cardiovascular disease was defined as myocardial infarction/heart attack, stroke/brain attack, or cardiovascular procedure (coronary bypass or balloon angioplasty) in a female primary relative (parent, sibling, or child) aged or a male primary relative aged .
Carotid Artery Measurements
Ultrasound images were acquired from the baseline examination (Exam 1, 2000–2002) and follow-up examinations between 2005–2007 (Exam 4) and/or between 2010–2012 (Exam 5).
Details of the measurements of the common carotid artery and CP have been described previously (Gepner et al. 2015; Tattersall et al. 2014). In brief, high-resolution B-mode ultrasound images of the right and left common, bifurcation, and internal carotid artery segments were recorded at all exams with the same ultrasound model (Logiq 700, GE Medical Systems) using the M12L transducer (GE Medical Systems) with a standardized imaging protocol. Trained and certified sonographers from all six MESA sites acquired images for all three examinations. All ultrasound images for this analysis were centrally read and interpreted by the University of Wisconsin Atherosclerosis Imaging Research Program’s MESA Carotid Ultrasound Reading Center.
CCA was assessed in the distal of the vessel. was defined as the IMT measured as the mean of the left and right mean far wall distal CCA wall thickness. CP burden was defined as the number of CPs (0–12) in the internal, bifurcation, and common segments of both carotid arteries (Tattersall et al. 2014). CP was defined as a discrete, focal wall thickening or focal thickening greater than the surrounding IMT (Stein et al. 2008). We defined CP formation as an increase in CP burden from baseline to the final follow-up exam (Tattersall et al. 2014).
Inter- and intrareader variability measures were derived from measurements of 24 scans from Exam 1 and Exam 5, each read blindly by all four sonographers twice. Scan–rescan reproducibility variability measures were based on 44 scans from Exam 5 repeated by three sonographers and read blindly by as single reader (Tattersall et al. 2014).
Coronary Artery Calcium Assessment
Details of the measurement of the CAC have been published (Kaufman et al. 2016). Briefly, the CAC score was obtained using either a cardiac-gated electron beam computed tomography (CT) scanner or a multidetector CT system at all sites in all examinations. A four-density calibration phantom was scanned with each participant to enhance comparability of images between scanners and was used to scale the voxels in each composite scan before the calculation of CAC scores. Images were interpreted using the Agatston method at the MESA CT reading center for all examinations from Exam 1 to 5 (Table S1).
Exposure Assessment
A detailed description of the methodology for estimating long-term outdoor, residence-specific concentrations has been published elsewhere (Wang et al. 2015). In brief, region-specific spatiotemporal exposure models developed for were based upon continuous daily measurements (1999–2013) from the Air Quality System of the U.S. Environmental Protection Agency (U.S. EPA), and the spatially dense supplementary monitoring data specific to the MESA Air Study to capture time-varying trends and spatial gradients of included monitoring at a sample of participant homes. The models incorporated a large number of geographical variables covering a wide diversity of geographic features, such as traffic, industrial emissions, population density, and land use. The performance of city-specific models ranged from good to excellent as assessed by the overall cross-validation (range: 0.61–0.90) at participants’ homes (Wang et al. 2015).
Exposures were estimated longitudinally and cross-sectionally. Longitudinal exposures, i.e., long-term average, were time-varying mean predictions between the baseline exam and each follow-up exam. To avoid possible seasonal differences of exposure due to different starting and ending dates among the participants, we averaged the time- and location-specific 2-wk predictions, rounding the time period to the nearest whole year between recruitment and each examination. Cross-sectional exposures were assigned the concentrations for the year preceding the baseline exam.
In the sensitivity analysis, we explore the different exposure time windows, including the 1-y average exposure prior to each exam and exposures averaged only in the warm season (April to September) between baseline and follow-up exams.
Statistical Analysis
Our primary interest is to assess the longitudinal relationship between long-term exposure and progression of mean and CAC over time. This association was estimated using linear mixed-effect models with random slopes and intercepts for each participant and for each Census tract. The model analyzed longitudinal relationships of the outcome ( or CAC) with long-term average air pollution exposure and other risk factors (Kaufman et al. 2016). We controlled for cross-sectional effects in this analysis because the cross-sectional relationships of outcomes with air pollution and other risk factors can produce biased results in a progression analysis (Yanez et al. 2002). This model has three components: a) the cross-sectional relationship between the baseline outcome and values of covariates at baseline, b) the longitudinal relationship for rate of change, and c) measurement error associated with outcome, as follows:
where is the outcome measurement ( or CAC) for subject i at vth follow-up exam, and are the time-invariant covariates at baseline for subject i, including cross-sectional confounders, risk factors, and mean exposure during the baseline year. are the time-varying longitudinal covariates at exam v for subject i, including possible confounders, other risk factors, and mean exposure during the time period between baseline () and vth follow-up exam, rounded to the nearest whole year or annual average prior to each exam. is the time in years from baseline () to the vth follow-up exam for subject i. is the average outcome progression (annual rate of change) when . are the coefficients for the interaction between longitudinal covariates and time; this includes the exposure by time interaction, which is interpreted as a rate (association between air pollution and annual progression). is the average outcome at baseline when . are the coefficients for cross-sectional associations between baseline outcome and covariates (including baseline exposure). is the subject-specific random slope and intercept. is the error associated with .
We developed models in stages. The base model (Model 1) included age, sex, race/ethnicity, and study region. For CAC, the base model also adjusted for CT scanner types. The moderate adjustment model (Model 2) added various risk factors [body mass index, smoking (status and pack-years), secondhand smoke exposure, alcohol consumption, physical activity (metabolic equivalents: minutes/week by quartiles from low to vigorous activity at baseline), employment status, high-density lipoprotein cholesterol, total cholesterol, and statin use]. Pack-years smoking provided information on smoking duration in addition to smoking status. The primary model (Model 3) for reporting results further adjusted for neighborhood (2000 U.S. Census tract level) socioeconomic status (SES) index [continuous variable constructed by factor analysis of six indicators of neighborhood-level SES, i.e., wealth, income, education, employment, and occupation, with higher value indicating more socioeconomic disadvantage (Diez Roux et al. 2001)], individual baseline social economic factors, e.g., income (continuous) and education categories (less than high school, high school, some college/technical college, or graduate school), blood pressure, hypertension (defined as systolic blood pressure , diastolic blood pressure , or reported use of antihypertensive medication), antihypertensive medications, and diabetic categories (normal, impaired fasting glucose, diabetic). Medication use was defined as any positive report of a statin and/or antihypertensive medication use on the medication inventory for the participants at each of the five clinical exams. These covariates were selected a priori according to the findings from former studies that may potentially influence the exposure–outcome association (Gassett et al. 2015). In secondary analysis (Model 4), we extended our regression models by adjusting for CVD risk biomarkers and family history of premature CVD. Family history of premature CVD was defined as myocardial infarction/heart attack, stroke/brain attack, or cardiovascular procedure (coronary bypass or balloon angioplasty) in a female primary relative (parent, sibling, or child) aged or a male primary relative aged .
For CP, logistic regression models were used to estimate an odds ratio (OR) for association between long-term exposure (between the last exam and baseline) and CP formation (any increase in CP burden from baseline to the last measured visit) in the same cohort for all individuals with repeat measurements. Furthermore, CP incidence among the population with no CP at baseline (i.e., baseline CP burden is zero) was also examined.
We evaluated potential effect modification by age category ( or ), gender, race (white vs. black, Hispanic, and Chinese), smoking status (never vs. former and current), diabetes, hypertension, statin therapy, and presence of CP at baseline using three-way interaction terms between study time (), the effect modifier, and exposure concentration.
To assess the concentration–response relationship of with the subclinical outcomes, we refitted the model using a natural spline with 4 degrees of freedom for long-term average concentrations. In sensitivity analyses, we explored health effects using the 1-y average exposure prior to each exam and exposures in the warm season (April to September) for each participant as a proxy for long-term exposure. Additional sensitivity analyses included addition of copollutants, i.e., nitrogen oxides () and particulate matter with diameter (), in the models. Interquartile range (IQR) increases in () for each study region were used to express the model parameter estimates. All analyses were performed using SAS (version 9.4; SAS Institute).
Results
Study Participants
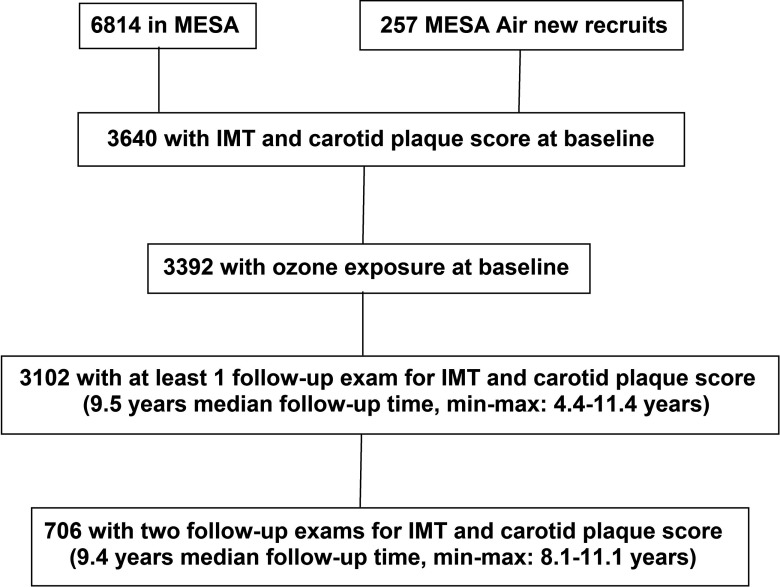
Of the 3,640 participants with and any CP measurements, 3,392 had estimated outdoor residential concentrations for the year of their baseline exam and also during the follow-up period (Figure 1), and 91% of the participants had at least one follow-up ultrasound measurement. Table 1 shows the population characteristics for all the participants by study regions. Mean follow-up duration ranged from 8.4 (Los Angeles) to 9.2 y (St. Paul and Chicago), and participants were, on average, 60 y old at baseline. Approximately equal numbers of men and women were included. Almost half were lifelong nonsmokers (49%), one-third were non-Hispanic white (39%), and two-thirds had at least a college education (70%). Moreover, 41% of participants had hypertension, 10% had diabetes, and nearly 47% had CP at baseline. For CAC, 6,619 participants had estimated concentrations, and 89% had at least one follow-up CAC measurement, with mean follow-up duration of 6.5 y (Figure S1; Table S2). The small subgroup with only baseline data had similar characteristics compared with the majority with longitudinal data.
Figure 1.
Description of study design and data characteristics for intima-media thickness of common carotid artery () and carotid plaque (CP). Note: MESA, Multi-Ethnic Study of Atherosclerosis.
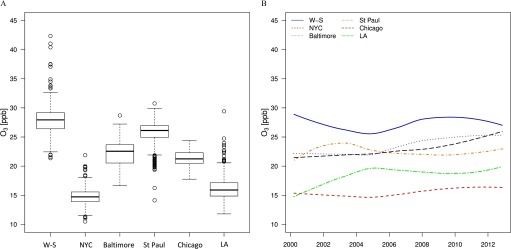
Long-term average concentrations at baseline varied substantially within and across the study regions (Figure 2A; Table S3), with the highest mean value and variation in Winston-Salem and the lowest in New York (Table 1). Mean concentrations at the participants’ homes remained constant over time in each study region (Figure 2B). Correlations of predictions of with and were negative and relatively low within each study region (Table S4). Unadjusted mean and CAC at baseline were and 194 Agatston units, respectively. Mean () progression of and CAC were and (Table 1; Table S2). Among all participants, 1,767 (53%) had CP progression, of which 1,036 (31%) without CP at baseline developed new CP during follow-up.
Figure 2.
Ambient ozone () exposure estimated at the participants’ residential locations. (A) Distribution of concentrations at baseline by study regions, and (B) mean estimated concentrations at all participants’ homes from 1999 to 2013. Boxes cover the 25–75th percentile (interquartile range; IQR) with a center line for the median concentration. Whiskers extend to the highest observation within 3 IQRs of the box, with more extreme observations shown as points. Note: W-S, Winston-Salem; NYC, New York; LA, Los Angeles.
and .
In the longitudinal analysis, an IQR increment in long-term average exposure () was associated with a (95%CI: 1.4, 9.7) faster increase in over 10 y (Table 2). The association was not sensitive to additional adjustment for family history of CVD and relevant biomarkers. In sensitivity analyses, the measure of association became slightly larger with long-term average exposures that were based only on the warm season (; 95% CI: 2.6, 10.3) but was much smaller using the 1-y average exposure prior to each exam (; 95% CI: 0.1, 7.1). Addition of or as covariates did not weaken the associations with exposure to . There is little evidence of a nonlinear relationship between concentration and change in (Figure S2). We found suggestive evidence of effect modification by CP, with larger effect estimates in those without CP at baseline (Table 3).
Table 2.
Main and sensitivity analyses for estimates of the effect of a increase of ozone () exposure on progression and CP formation over 10 y from models with increasing amounts of covariate adjustment.
| Change in over 10 y | CP formation over 10 y | |
|---|---|---|
| (, 95% CI) | Odds ratio (95% CI) | |
| Main analysesa | ||
| Baseb | 5.6 (1.6, 9.6) | 1.3 (1.2, 1.4) |
| Moderatec | 5.5 (1.4, 9.6) | 1.2 (1.1, 1.4) |
| Fulld | 5.6 (1.4, 9.7) | 1.2 (1.1, 1.4) |
| Extendede | 5.7 (1.5, 9.9) | 1.3 (1.1, 1.4) |
| Sensitivity analysesf | ||
| , warm seasons | 6.5 (2.6, 10.3) | 1.0 (0.9, 1.1) |
| , 1-y mean | 3.6 (0.1, 7.1) | 1.0 (0.9, 1.2) |
| Adjustment for | 15.2 (7.2, 23.2) | 1.3 (1.1, 1.4) |
| Adjustment for | 7.8 (3.1, 12.6) | 1.3 (1.1, 1.5) |
| Full (no site adjustment) | 5.3 (1.1, 9.4) | 1.0 (1.0, 1.1) |
Note: CI, confidence interval; CP, carotid plaque; , intima-media thickness of common carotid artery; , nitrogen oxides; , particulate matter with diameter .
Main analyses [within-city interquartile range (IQR) of ].
Base model includes age, sex, race/ethnicity, and study region.
, smoking status, pack-years, secondhand smoke exposure, alcohol consumption, physical activity, employment status, high-density lipoprotein cholesterol, total cholesterol, statin use.
, income, education, systolic and diastolic blood pressure, hypertension, antihypertensive medication, diabetes; full model is the primary model for report in the main text and discussion.
, fibrinogen, C-reactive protein, creatinine.
Sensitivity analyses: covariates in the sensitivity analyses are the same as those in the full model of the main analyses.
Table 3.
Estimated effects of a increase in ozone () exposure on progression and CP formation over 10 y, according to baseline participant characteristics.
| Change of () | Odds ratio of CP formation | ||||
|---|---|---|---|---|---|
| 95% CI | Interaction p-valuea | 95% CI | Interaction p-valuea | ||
| Sex | — | — | 0.99 | — | 0.69 |
| Female | 1,800 | 5.6 (0.3, 11.0) | — | 1.3 (1.1, 1.5) | — |
| Male | 1,592 | 5.6 (, 11.5) | — | 1.2 (1.0, 1.4) | — |
| Race | — | — | 0.99 | — | 0.09 |
| White | 1,334 | 5.9 (0.8, 11.0) | — | 1.4 (1.2, 1.6) | — |
| Nonwhite | 2,058 | 5.9 (, 12.4) | — | 1.1 (0.9, 1.3) | — |
| Age at baseline | — | — | 0.25 | — | 0.58 |
| 1,658 | 3.5 (, 9.0) | — | 1.3 (1.1, 1.5) | — | |
| 1,734 | 7.8 (2.2, 13.4) | — | 1.4 (1.2, 1.6) | — | |
| Smoking | — | — | 0.36 | — | 0.09 |
| Never | 1,658 | 3.9 (, 9.5) | — | 1.4 (1.2, 1.6) | — |
| Former and current | 1,729 | 7.4 (1.6, 13.1) | — | 1.1 (1.0, 1.3) | — |
| Diabetes | — | — | 0.05 | — | 0.11 |
| No | 3,067 | 6.5 (2.1, 10.8) | — | 1.2 (1.1, 1.3) | — |
| Yes | 325 | (, 12.8) | — | 1.6 (1.2, 2.3) | — |
| Hypertension | — | — | 0.91 | — | 0.30 |
| No | 1,996 | 5.4 (0.1, 10.7) | — | 1.1 (1.0, 1.3) | — |
| Yes | 1,396 | 5.9 (0.0, 11.7) | — | 1.4 (1.2, 1.6) | — |
| Statin use | — | — | 0.87 | — | 0.19 |
| No | 2,880 | 5.9 (0.3, 11.4) | — | 1.2 (1.1, 1.4) | — |
| Yes | 511 | 5.3 (0.0, 10.6) | — | 1.3 (1.1, 1.5) | — |
| Presence of carotid plaqueb | — | — | 0.04 | — | 0.79 |
| No | 1,792 | 7.9 (2.1, 13.7) | — | 1.2 (1.1, 1.4) | — |
| Yes | 1,600 | 0.5 (, 6.3) | — | 1.3 (1.1, 1.5) | — |
Models included age, sex, race/ethnicity, study region, body mass index, smoking status, pack-years, secondhand smoke exposure, alcohol consumption, physical activity, employment status, high-density lipoprotein cholesterol, total cholesterol, statin use, neighborhood socioeconomic status (SES) index, income, education, systolic and diastolic blood pressure, hypertension, antihypertensive medication, and diabetes. Interquartile range (IQR) for within-city . Note: —, no data; CI, confidence interval; CP, carotid plaque; , intima-media thickness of common carotid artery.
p-Value by the F test of the three-way interaction between , study time, and stratification variable.
Presence of carotid plaque at baseline. Subset with no presence of carotid plaque indicate incidence of carotid plaque during follow-up.
and carotid plaque.
The increment in long-term exposure was also associated with CP formation (OR: 1.2; 95% CI: 1.1, 1.4) (Table 2), with little evidence of nonlinearity observed (Figure S2). These ORs were stable with little change for the different covariate adjustments. Effect estimates were smaller when different exposure metrics were used, but were robust to adjustment for or . exposure was also associated with CP incidence (OR: 1.2: 95% CI: 1.1, 1.4; full model) among those without CP at baseline (Table S5).
and coronary artery calcification.
We did not observe association between exposure and CAC change in any analyses. The estimate for the effect of a higher long-term exposure to in CAC was (95% CI: , 2) over 10 y (Table S6).
Discussion
In this well-characterized cohort using fine spatial-scale exposure predictions for , we found that chronic exposure was associated with evidence of progressive arterial injury as assessed by both and CP, independent from exposure to and , suggesting a different mechanism from the effect of traffic-related air pollution. The findings were robust to control for all major known CVD risk factors. Strengths of the study include its relatively large sample size, long period of follow-up (), use of advanced methods for estimating individual-level long-term outdoor concentrations, and high-quality individual information on the outcome measures and potential confounding factors.
Findings on the associations of long-term exposure to with mortality in large-scale studies in the United States and Europe have not been consistent. Although respiratory effects of long-term exposure to have been reported (Jerrett et al. 2009), evidence for long-term effects on the cardiovascular system is limited (U.S. EPA 2013). For example, in Jerrett et al. (2009), associations between ozone and cardiovascular mortality were not robust to adjustment for fine particles. concentrations were associated with total mortality (Di et al. 2017) and cardiovascular mortality (Turner et al. 2016) and its subtypes, including ischemic heart disease (Jerrett et al. 2013), cerebrovascular disease (Turner et al. 2016), and mortality risk among participants with congestive heart failure (Zanobetti and Schwartz 2011) in the United States but not with cardiovascular mortality in the United Kingdom (Carey et al. 2013). The causal nature of the association between exposure and cardiovascular risk therefore remains uncertain.
Our paper does not address clinical outcomes or mortality, and focuses on subclinical arterial injury. The pathophysiological mechanisms underlying the associations between exposure and progression of arterial injury are not well elucidated. One proposed pathway is generation of oxidative reaction products from the reaction of with lipids or cellular membranes in the lung, which are subsequently released into the circulatory system and initiate or propagate a systemic inflammatory response. Persistent or repeated activation of this pathway is associated with development of arterial injury (Cosselman et al. 2015). Both human and animal studies support involvement of this pathway. In an animal study using a mouse model, inhaled promoted increased arterial dysfunction, oxidative stress, mitochondrial DNA damage, and atherogenesis (Chuang et al. 2009). In a human study of young, healthy volunteers, exposure to outdoor caused platelet activation and an increase in blood pressure and vascular markers of inflammation relative to clean air after controlling the effects of and (Day et al. 2017).
Progression of and CAC have not been examined previously with regard to exposure in epidemiological studies, but have been investigated with and traffic-related air pollution, where findings have been inconsistent (Adar et al. 2013; Kaufman et al. 2016). Varying associations may be influenced by study design, geography, quality of exposure assessment, characteristics of the study populations, and approaches to analysis and reporting. Compared to the elevated association between and observed in our study, a parallel analysis in the MESA study that used the same set of readings and the same disease models did not find progression of associated with or , but did for CAC (Kaufman et al. 2016), which was not associated with in our study. In that paper, the difference could be attributed to the fact that and CAC are measured in different arterial beds and/or differences in effects due to the different underlying pathophysiologic processes reflected by and CAC measures. reflects early arterial injury from many processes, including inflammation, intimal thickening, and medial hypertrophy (Kiechl and Willeit 1999; Stein et al. 2008), whereas CAC indicates a more advanced stage of atherosclerosis with vessel wall calcification (O'Rourke et al. 2000). , CP, and CAC all predict adverse CVD events (Gepner et al. 2015; Lorenz et al. 2012; Stein et al. 2008). CAC is a more robust predictor of coronary artery disease events, whereas and CP are similar or better predictors of stroke in the general population (Gepner et al. 2015). Also, CAC and may be associated with different risk factors and do not appear to share common genes (Rampersaud et al. 2008). For example, hypertension causes a significant increase in IMT and is considered a major CVD risk factor, whereas hypertension does not appear to contribute strongly to the occurrence of plaque (Baroncini et al. 2015).
Although and may share similar etiologic pathways, their mechanisms of action on the vasculature may differ. Toxicological studies have suggested that cardiac functional changes in response to are different from exposure to PM (Tankersley et al. 2013). In human studies, the effect estimate of personal exposure to on cardio-ankle vascular index, a measure of arterial stiffness, was twice as large as that of exposure in a small-panel study (Wu et al. 2010). In a cross-sectional study, greater exposure to rather than PM was associated with increased risk of IMT in young adulthood (Breton et al. 2012). Indeed, in the MESA cohort, we found the association between exposure and progression of was insensitive to copollutant adjustment, and Kaufman et al. 2016 showed there was no independent effect of over the same time period. Further study is needed to better understand the relative potency of ozone and PM, and pathways of effects on arterial injury.
We observed an association between long-term exposure and CP formation over a decade in the same population as the cohort. CP formation represents a later stage of arterial injury than increased and is more predictive of CVD events than (Lorenz et al. 2012). A clinical consensus statement recommended that ultrasound assessment of the carotid arteries for CVD risk prediction should include CP assessment in addition to (Stein et al. 2008). Our study suggests that chronic exposure may affect both diffuse carotid arterial injury () and focal lesions of carotid atherosclerosis (CP). Exposure to traffic-related air pollution, on the other hand, has not been shown to be associated with plaque progression (Gan et al. 2014).
We found stronger associations between chronic exposure and progression of among the subset of healthier subjects without CP. One potential explanation is that there is less exposure misclassification among healthy subjects who would be expected to spend more time outdoors (confirmed by data on time–activity from the baseline questionnaire) and thus have more personal exposure for a given amount of estimated outdoor residential exposure. Moreover, may become an imprecise indicator of arterial injury in later stages (as better reflected by CP) due to the effect of risk-reducing medications on the end points (Gepner et al. 2015; Lorenz et al. 2012). Therefore, the estimated association between exposure and progression of may be stronger in the early stages of arterial injury.
Our estimates of associations with progression of and CP formation were stronger for long-term concentration averages than with a single-year average exposure. This suggests that specification of exposure over a long time period more accurately captures the critical aspects of exposure on subclinical cardiovascular effects than specifying exposure over shorter time windows.
Our study has some limitations that could affect the findings. First, although we employed advanced statistical modeling methods to produce accurate predictions at the time and location of each residence during follow-up, exposure misclassification remains a concern. is scavenged rapidly by nitric oxide near roadways and therefore varies substantially at local scale. Our measurement data included a large number of sites at the residential locations, some of which were near roadways, but did not use a monitoring design intended to capture fine-scale near-road gradients. Furthermore, indoor concentrations differ from outdoor concentrations because of the rapid deposition and reaction with indoor surfaces and gases. Therefore, outdoor concentrations of do not fully reflect personal exposures (Spalt et al. 2015). Second, because of the time-integrated sampling frame in our monitoring campaigns, our concentrations included the entire 24-h period, which is not directly comparable with the 8-h maximum form of the standard in the United States. However, correlations between daily average and daily 8-h maximum observations have been reported to be high across the U.S. regulatory sites (median ) (U.S. EPA 2013). Third, there may be potential selection bias operating in our analysis because some participants have no follow-up data. However, the percentage with only baseline data is small (9%). Finally, we cannot rule out biases due to unmeasured confounders or changes in neighborhood characteristics that occurred during the study period. Our analysis minimized this concern by incorporating extensive data on potential confounding variables, and we found the results were robust across multiple confounder models.
Conclusion
Chronic exposure to was associated with accelerated progression of and higher risk of CP formation over a decade of follow-up in a cohort of elderly adults. This may indicate that the association between long-term exposure to and cardiovascular mortality that has been observed in some studies is due to arterial injury and acceleration of atherosclerosis. This is the first epidemiological study to provide evidence that might accelerate subclinical arterial disease, and provides insight into a relationship between and CVD risk.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This publication was developed under STAR research assistance agreement, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S EPA, as well as by the University of Washington Center for Clean Air Research (UW CCAR, U.S. EPA RD83479601-01). It has not been formally reviewed by the U.S. EPA. The views expressed in this document are solely those of the authors, and the U.S. EPA does not endorse any products or commercial services mentioned in this publication. MESA was funded by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169; by grants K24ES013195 and P30ES07033 from the National Institute of Environmental Health Sciences; and by grants UL1-TR-000040 and UL1-TR-001079 from National Center for Research Resources (NCRR).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP3325).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Adar SD, Sheppard L, Vedal S, Polak JF, Sampson PD, Diez Roux AV, et al. . 2013. Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the multi-ethnic study of atherosclerosis and air pollution. PLoS Med 10(4):e1001430, PMID: 23637576, 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EEA (European Environment Agency). 2011. Air Quality in Europe 2011. Technical Report No 12/2011. Luxembourg: Publications Office of the European Union. [Google Scholar]
- EEA. 2015. Air Pollution Due to Ozone: Health Impacts and Effects of Climate Change. https://www.eea.europa.eu/data-and-maps/indicators/air-pollution-by-ozone-2/assessment.
- Baroncini LAV, de Castro Sylvestre L, Filho RP. 2015. Carotid intima-media thickness and carotid plaque represent different adaptive responses to traditional cardiovascular risk factors. Int J Cardiol Heart Vasc 9:48–51, PMID: 28785705, 10.1016/j.ijcha.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton CV, Wang X, Mack WJ, Berhane K, Lopez M, Islam TS, et al. . 2012. Childhood air pollutant exposure and carotid artery intima-media thickness in young adults. Circulation 126(13):1614–1620, PMID: 22896588, 10.1161/CIRCULATIONAHA.112.096164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey IM, Atkinson RW, Kent AJ, van Staa T, Cook DG, Anderson HR. 2013. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med 187(11):1226–1233, PMID: 23590261, 10.1164/rccm.201210-1758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang GC, Yang Z, Westbrook DG, Pompilius M, Ballinger CA, White CR, et al. . 2009. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am J Physiol Lung Cell Mol Physiol 297(2):L209–L216, PMID: 19395667, 10.1152/ajplung.00102.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosselman KE, Navas-Acien A, Kaufman JD. 2015. Environmental factors in cardiovascular disease. Nat Rev Cardiol 12(11):627–642, PMID: 26461967, 10.1038/nrcardio.2015.152. [DOI] [PubMed] [Google Scholar]
- Day DB, Xiang J, Mo J, Li F, Chung M, Gong J, et al. . 2017. Association of ozone exposure with cardiorespiratory pathophysiologic mechanisms in healthy adults. JAMA Intern Med 177(9):1344–1353, PMID: 28715576, 10.1001/jamainternmed.2017.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. . 2017. Air pollution and mortality in the Medicare population. N Engl J Med 376(26):2513–2522, PMID: 28657878, 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. . 2001. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 345(2):99–106, PMID: 11450679, 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Allen RW, Brauer M, Davies HW, Mancini GB, Lear SA. 2014. Long-term exposure to traffic-related air pollution and progression of carotid artery atherosclerosis: a prospective cohort study. BMJ Open 4(4):e004743, PMID: 24710134, 10.1136/bmjopen-2013-004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassett AJ, Sheppard L, McClelland RL, Olives C, Kronmal R, Blaha MJ, et al. . 2015. Risk factors for long-term coronary artery calcium progression in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 4(8):e001726, PMID: 26251281, 10.1161/JAHA.114.001726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, et al. . 2015. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging 8(1):e002262, PMID: 25596139, 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Beckerman BS, Turner MC, Krewski D, Thurston G, et al. . 2013. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med 188(5):593–599, PMID: 23805824, 10.1164/rccm.201303-0609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Pope CA 3rd, Ito K, Thurston G, Krewski D, et al. . 2009. Long-term ozone exposure and mortality. N Engl J Med 360(11):1085–1095, PMID: 19279340, 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JD, Adar SD, Allen RW, Barr RG, Budoff MJ, Burke GL, et al. . 2012. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Am J Epidemiol 176(9):825–837, PMID: 23043127, 10.1093/aje/kws169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, et al. . 2016. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet 388(10045):696–704, PMID: 27233746, 10.1016/S0140-6736(16)00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiechl S, Willeit J. 1999. The natural course of atherosclerosis. Part I: incidence and progression. Arterioscler Thromb Vasc Biol 19(6):1484–1490, PMID: 10364079, 10.1161/01.ATV.19.6.1484. [DOI] [PubMed] [Google Scholar]
- Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, Tuomainen TP, et al. . 2012. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet 379(9831):2053–2062, PMID: 22541275, 10.1016/S0140-6736(12)60441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke RA, Brundage BH, Froelicher VF, Greenland P, Grundy SM, Hachamovitch R, et al. . 2000. American College of Cardiology/American Heart Association expert consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. J Am Coll Cardiol 36(1):326–340, PMID: 10898458, 10.1016/S0735-1097(00)00831-7. [DOI] [PubMed] [Google Scholar]
- Rampersaud E, Bielak LF, Parsa A, Shen H, Post W, Ryan KA, et al. . 2008. The association of coronary artery calcification and carotid artery intima-media thickness with distinct, traditional coronary artery disease risk factors in asymptomatic adults. Am J Epidemiol 168(9):1016–1023, PMID: 18805900, 10.1093/aje/kwn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalt EW, Curl CL, Allen RW, Cohen M, Adar SD, Stukovsky KH, et al. . 2015. Time-location patterns of a diverse population of older adults: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). J Expo Sci Environ Epidemiol 26(4):349–355, PMID: 25921083, 10.1038/jes.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. . 2008. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 21(2):93–111, PMID: 18261694, 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Georgakopoulos D, Tang WY, Abston E, Bierman A, Sborz N. 2013. Effects of ozone and particulate matter on cardiac mechanics: role of the atrial natriuretic peptide gene. Toxicol Sci 131(1):95–107, PMID: 22977167, 10.1093/toxsci/kfs273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall MC, Gassett A, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, et al. . 2014. Predictors of carotid thickness and plaque progression during a decade: the Multi-Ethnic Study of Atherosclerosis. Stroke 45(11):3257–3262, PMID: 25213342, 10.1161/STROKEAHA.114.005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MC, Jerrett M, Pope CA 3rd, Krewski D, Gapstur SM, Diver WR, et al. . 2016. Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med 193(10):1134–1142, 10.1164/rccm.201508-1633OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (Environmental Protection Agency). 2013. Integrated Science Assessment (ISA) for Ozone and Related Photochemical Oxidants. EPA/600/R-10/076F. Washington, DC:U.S. EPA. [Google Scholar]
- Wang M, Keller JP, Adar SD, Kim SY, Larson TV, Olives C, et al. . 2015. Development of long-term spatiotemporal models for ambient ozone in six metropolitan regions of the United States: the MESA Air study. Atmos Environ (1994) 123(A):79–87, PMID: 27642250, 10.1016/j.atmosenv.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Kuo IC, Su TC, Li YR, Lin LY, Chan CC, et al. . 2010. Effects of personal exposure to particulate matter and ozone on arterial stiffness and heart rate variability in healthy adults. Am J Epidemiol 171(12):1299–1309, PMID: 20507901, 10.1093/aje/kwq060. [DOI] [PubMed] [Google Scholar]
- Yanez ND 3rd, Kronmal RA, Shemanski LR, Psaty BM; Cardiovascular Health Study. 2002. A regression model for longitudinal change in the presence of measurement error. Ann Epidemiol 12(1):34–38, PMID: 11750238, 10.1016/S1047-2797(01)00280-0. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. 2011. Ozone and survival in four cohorts with potentially predisposing diseases. Am J Respir Crit Care Med 184(7):836–841, PMID: 21700916, 10.1164/rccm.201102-0227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.