Supplemental Digital Content is available in the text.
Key Words: minimally invasive surgical procedures, spinal stenosis, surgical decompression, endoscopic surgical procedure, arthroscopes
Background:
Unilateral biportal endoscopic surgery (UBESS) for severe lumbar central canal stenosis (LCCS) remains challenging.
Objective:
To describe the use of UBESS with a 30-degree arthroscope in patients with severe LCCS.
Materials and Methods:
Working and viewing portals were created in each unilateral paravertebral area at the target interlaminar level. After ensuring the visual field with a 30-degree arthroscope, effective tissue removal was possible through safe access to the bilateral hypertrophic yellow ligament with minimal osteotomy. The authors evaluated 58 patients and analyzed the clinical outcomes using the visual analog scale, Macnab criteria, and self-predicted walking distance.
Results:
The visual analog scale scores for low back and leg pains decreased from 7.1 to 1.9 and from 7.9 to 1.6, respectively, at 18 months after the procedure. According to the Macnab criteria, “excellent,” “good,” and “fair” results were obtained in 51.7%, 41.4%, and 6.9% subjects, respectively. Before surgery, the subjects could walk a mean of 305.8±468.1 m. After surgery, 43.1% of the patients could walk for >1 hour, whereas the remaining patients could walk 1521.8±1831.1 m.
Conclusion:
UBESS using a 30-degree arthroscope can be an efficient and safe intervention in patients with severe LCCS.
Lumbar central canal stenosis (LCCS) is a functional disorder characterized by a gradual narrowing of the spinal canal. The main symptom is difficulty in walking because of pain and weakness. Entrapment and compression of neurovascular structures are thought to be the cause of the symptoms. Diagnosis and treatment are on the basis of pathoanatomic evidence. The typical presenting clinical feature is neurogenic claudication, which is described as a situational pain in the buttocks or lower extremities on standing for a long time or walking, which subsides on sitting down or lumbar flexion.1 The prognostic factors for the final outcomes are unclear, and therapeutic decisions are primarily on the basis of clinical symptoms. Conservative treatment may provide satisfactory results for a short time; however, the results of a randomized and observational cohort study showed that improvement after surgical treatment was more significant.2 LCCS is reportedly the most common cause of lumbar spinal surgery in adults aged more than 65 years.3
The main goal of surgery is to decompress the central canal and foramina, thus relieving neurovascular structures. The traditional method is a laminectomy and facetectomy, through which the posterior spinal elements are largely eliminated or inevitably damaged. Excessive decompression can cause spinal instability and result in secondary spinal fusion.4 To overcome these limitations, in recent years, attempts have been made to develop a minimally invasive approach using endoscopes. Unfortunately, to the best of our knowledge, few reports exist on the efficacy and safety of biportal endoscopic technique.
Therefore, this study aimed to describe the unilateral biportal endoscopic spine surgery (UBESS) technique that is performed using a 30-degree arthroscope and report the results of the clinical application of this technique.
MATERIALS AND METHODS
All the patients were provided with a detailed explanation of the procedure and its attendant complications. Only those who provided a signed informed consent form were included in the study. The study was approved by the institutional ethics committee. All the operations were performed by 1 surgeon (S.B.J.). The clinical assessment and data analysis were performed by another physician (N.K.).
Patient Population
A total of 88 patients with lower back pain and neurogenic claudication because of severe LCCS were enrolled from April 2016 to March 2017. All the subjects were diagnosed as having a clinically significant LCCS on the basis of findings from precise history taking, physical examination, and magnetic resonance imaging (MRI) of the spine. Those with severe and focal stenosis on MRI scans were recruited. Severity was defined as grade 3 on the basis of the LCCS grading method provided by Lee et al.5 Patients were excluded if they showed any of the following signs/symptoms: clinically relevant stenosis at ≥2 levels on MRI, severe degeneration of the intervertebral disks or facet joints on MRI (grade V using the Pfirrmann grading system),6 significant signal changes in the cord or cauda equina on MRI, spondylolisthesis of Meyerding grade >I,7 any signs of upper motor neuron lesion on physical examination, symptom-related lumbosacral bony malformation, history of lumbar spinal surgery, coexisting severe hip or knee joint problem, symptom-related psychological disorder, hematologic disorder, and painful musculoskeletal conditions such as fibromyalgia, myopathy, or polyneuropathy.
Among the 88 patients who were initially selected, 30 were excluded on the basis of the above-mentioned reasons. The remaining 58 patients were scheduled to undergo percutaneous UBESS after they provided informed consent.
Surgical Technique
All the patients underwent the procedure under general anesthesia. During the procedure, we monitored each patient’s blood pressure, heart rate, electrocardiography result, oxygen saturation level, and respiration rate. The patients were placed in the prone position; the back was flexed gently by placing a Wilson frame beneath the abdomen. A soft strap was placed over the trunk and thighs for stabilization. The lumbosacral area was prepped and draped in a sterile manner. Strict asepsis was maintained throughout the procedure. The target stenotic level was confirmed under fluoroscopic guidance. The insertion side was determined on the basis of the more stenotic or symptomatic side.
The level of the target to approach was localized using a fluoroscope. Two portals were marked as follows: 1 cm above and 2 cm below the target interlaminar level (lower margin of the upper lamina) unilaterally, and 0.5 cm lateral to the spinous process midline. The marked skins were opened ~1 cm as a method of transverse incision, which can provide low resistance to the main movement of the inserted devices and less damage to the longitudinal paravertebral muscle fibers. The cranial portal was used for continuous irrigation and endoscopy, whereas the caudal portal was used for working the decompression instruments.
A 30-degree endoscope, which is a general 30-degree arthroscope with a 4-mm diameter, was inserted in the bony lamina after muscle exfoliation was performed using a double-ended separator. A continuous irrigation system was connected and controlled to set a pressure of ~50 mm Hg. After confirming the clear endoscopic view field and the bony contact at the end of the scope, the indicator was inserted in the caudal portal to identify the tip through the endoscopic view. The target point and instrument placement were confirmed again through the fluoroscope (Fig. 1).
FIGURE 1.
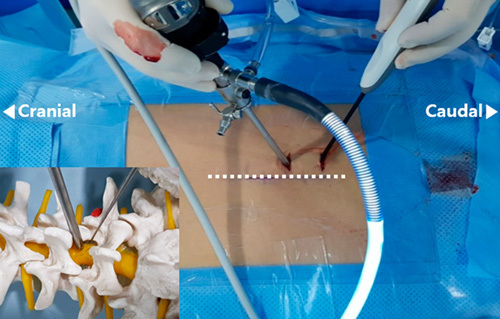
Unilateral biportal endoscopic spine surgery. The dotted line indicates the spinous process line. A viewing portal is placed on the cranial side, and a working portal is placed 4–5 cm caudally from the viewing portal. A schematic diagram using a bony spine model is provided in the left lower corner of the figure.
Next, after muscle-bone separation using the double-ended separator and electrical coagulator, the lower margin of the upper lamina and interlaminar ligament were identified. The interlaminar ligament was ablated 1–1.5 cm transversely with an electrical coblator, and the ligamentum flavum (LF) was separated gently from the lower lamina margin after minimal laminectomy with the oval-shaped arthroscopic burr. After confirmation of the dura mater, the thickened LF was removed, along with a part of the lamina, using the Kerrison punch. The endoscopic view may include the lateral margin of the dura or dural sleeve if sufficient decompression is achieved; a deeply inserted endoscope can also detect the subarticular annulus fibrosus (see Video, Supplemental Digital Content 1, http://links.lww.com/CLINSPINE/A113, which demonstrates surgical decompression). If symptomatic and prominent disk herniation is identified, the surgeon can perform an endoscopic resection. In our cases, the light source of a 30-degree endoscope was rotated 180 degrees to examine the contralateral LF, and removal was possible using the forceps and punch (Fig. 2).
FIGURE 2.
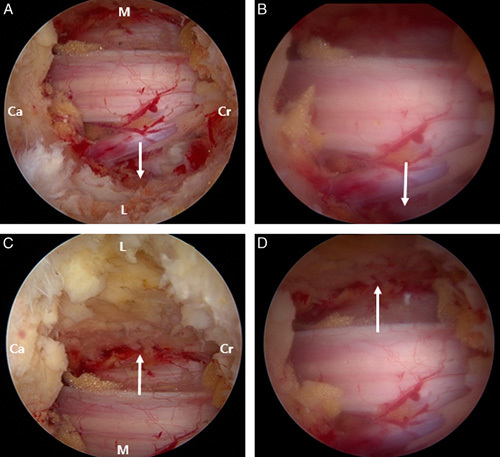
Comparison between the 0-degree and 30-degree arthroscopes. White arrows indicate the lateral recess space. (A) Ipsilateral view with the 30-degree arthroscope. (B) The same view with a 0-degree arthroscope. The contralateral views with a 30-degree (C) and 0-degree arthroscope (D) are shown. Ca indicates caudal; Cr, cranial sides; L, lateral; M, medial.
Bilateral decompression and intact elements were observed closely for dural injury and latent bleeding through the endoscopic views. The level of canal decompression was assessed by examining the normal respiration-induced dural pulsatility and was confirmed through endoscopic observation.
Postoperative Evaluation
All the patients underwent immediate lumbosacral radiography immediately after surgery to evaluate for an unexpected bony fracture and assess the overall skeletal alignment. Postoperative MRI or computer tomography was performed within 72 hours to rule out soft tissue complications such as hematoma or edema, and evaluate the adequacy of the spinal canal decompression.
Assessments including the visual analog scale (VAS), Macnab criteria, and severity of neurogenic claudication were performed. The VAS was used to assess the severity of pain in the back and both legs before the procedure and at follow-up visits scheduled at 6, 12, and 18 months after the procedure. The Macnab criteria were used to assess patient satisfaction regarding the outcome 18 months after the procedure. The severity of neurogenic claudication was assessed on the basis of the self-reported walking distance before and 18 months after the procedure.8,9 We conducted a repeated-measures analysis of variance to analyze the clinical findings before the procedure and the outcomes at each follow-up, and we compared the periodic outcomes using paired t tests at 0.05 significance. We used SPSS 22.0 for Windows (SPSS Korea Data Solution Inc., Seoul, Korea).
RESULTS
Patients
The 58 consecutive patients who satisfied the enrollment criteria underwent UBESS. The patients included 25 men and 33 women, with a mean age of 43.1 years (range, 31–82 y). The mean symptom duration was 45 months (range, 6–240 mo). The levels of the targeted disks were L3/L4 in 10 patients, L4/L5 in 46, and L5/S1 in 2 (Table 1). According to the MRI finding confirmed by a musculoskeletal radiologist, the stenosis severity was grade 3 in the LCCS grading system in all the subjects (Fig. 3).
TABLE 1.
Subjects’ Baseline Characteristics
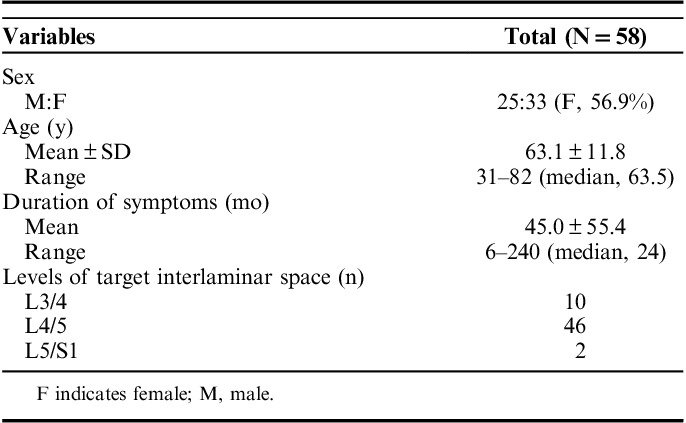
FIGURE 3.
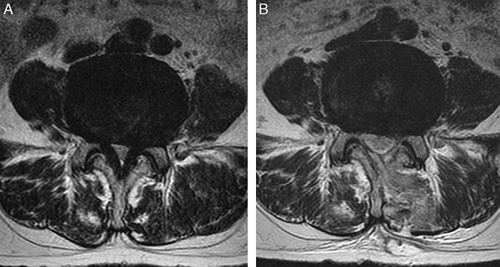
The target spinal level on an axial view of an MRI scan. The patient was a 45-year-old man with grade 3 LCCS who presented with upper gluteal pain radiating to both lower extremities and severe neurogenic claudication with walking ability limited to <100 m. Axial views of L4/L5 in the preoperative state (A) and 1 week after operation (B) are shown. After UBESS, the follow-up MRI scan showed grade 1 LCCS. His clinical symptoms improved and walking capacity increased to 1500 m. LCCS indicates lumbar central canal stenosis; MRI, magnetic resonance imaging; UBESS, unilateral biportal endoscopic spine surgery.
Outcome
After the procedure, we obtained serial follow-up clinical data for all the patients for 18 months. The mean reported back pain level measured using the VAS was 7.1±1.2 and 1.9±1.3 before and 18 months after the procedure, respectively. The VAS score for leg pain was reduced from 7.9±0.8 to 1.6±1.5. Significant improvement was observed in the VAS score for the first 6 months (P<0.001) after the procedure. From 6 to 18 months after the procedure, the improvement was sustained. The patient satisfaction survey according to the Macnab criteria showed “excellent” or “good” results in 93.1% (n=54) of the patients (Fig. 4). Before the surgery, the patients reported a mean walking capacity of 305.8±468.1 m. At 18 months after the operation, 43.1% of the patients could walk for >1 hour, whereas the rest could walk 1521.8±1831.1 m (P<0.001).
FIGURE 4.
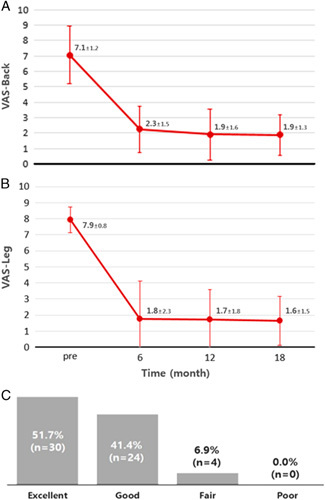
Clinical outcomes. The changes in the VAS scores regarding back (A) and leg symptoms (B) are shown. The preoperative and 6-month follow-up VAS scores show a statistically significant difference (P=0.012). (C) Results of the evaluation on the basis of the Macnab criteria. VAS indicates visual analog scale.
Complications
Two patients had dural tears with symptoms, and 4 patients were suspected to have insufficient decompression on MRI. Two patients required reoperation: one underwent the same procedure again and the other underwent a fixation. Two patients with torn dura attained full recovery within a week after conservative management through the natural draining of epidural space without negative pressure.
DISCUSSION
The primary surgical goal of LCCS is to decompress the impinged neural structures to relieve symptoms and improve function.10 Decompressive laminectomy was reported to have good outcomes regarding clinical efficacy on the basis of the results of some randomized trials and cohort studies.1,11–13 As decompression guarantees a positive outcome of the surgery, removal of the bony tissues is justified. However, several disadvantages, such as massive hemorrhage, postoperative pain, spinal instability, and damage to the paravertebral muscles, have been reported in various reports, especially in surgeries for elderly patients.14,15 In a survey about complications associated with surgery for LCCS in older adults aged more than 65 years, the incidence of life-threatening complications, including mortality, increased with increasing surgical invasiveness.16 In addition, although evidence for better result of more complex surgery for LCCS is lacking, too much information about the spinal pathology in the elderly has increased the complexity of operations.17
Minimally invasive spinal surgery for LCCS is an effective method for treating pathologic structures without disturbing the normal structures. Endoscopy is now used in several surgical situations where a field of view has to be achieved through a very small skin opening.18 An endoscopic procedure for attempting bilateral interlaminar decompression through single-portal access has been already described and has shown good results.19 However, the limitation of manipulating the instrument through 1 small portal and the insufficient decompression caused by this are the reported disadvantages of this procedure.20–23 These are expected to be more pronounced in cases with severe lumbar stenosis, particularly inadequate decompression of the lateral recess. In particular, the single cannula has a relatively large inner diameter to access the contralateral epidural space; otherwise, curved working devices must be used. Failure to obtain a visual field to view the distal end of the device would be expected, and the canal side of the bony lamina could be ablated. In addition, a single-portal method was developed as a system for intervertebral disk prolapse; however, it requires a skilled surgeon to apply it for LF removal of severe stenosis.24
The unilateral biportal approach is well suited to overcome these demerits. In particular, the degree of freedom of manipulating devices is increased through the working portal, thereby increasing the efficiency of tissue removal. In addition, the multidirectional switch of the endoscope has the effect of view-field expansion, and the 30-degree scope used in this study enables visualization of the parts that are usually not visible when using the 0-degree scope. This provides a significant advantage regarding the decompression of severe spinal stenosis.
The 2 portals are used for the cranial viewing scope and caudal working device. If the accessibility of the working device is secured and the visibility is more advantageous, it can be used in reverse. In our experience, it is effective to approach the resection tool caudally to the lower margin of the lamina to access the epidural side of the target lamina. Therefore, the partial lamina may be ablated for dorsocranial entry of the endoscope into the epidural space. We believe that the ablated extent of the lamina is expected to be less with a 30-degree scope than with a 0-degree scope because the inclination or insertion of the 30-degree scope for ensuring the view is minimal. In addition, the side to approach is determined as the symptomatic laterality or the side to require more decompression on MRI because the decompression effect of partial laminectomy is relatively larger. Another consideration in the portals is the handedness of the surgeon. When the side to approach is determined, the position of the operator is also determined accordingly. Considering the patient’s position and the placement of the monitor to display the endoscopic views, the handedness for the caudal working device can be changed. Therefore, it may be beneficial for surgeons to train this procedure using both hands.
With advances in optical and engineering technology, the diameter of the endoscope is becoming smaller, and the field and quality of view are improving. Moreover, the emergence of materials, such as alloys and polymers, makes it possible to develop stronger and more efficient manipulators. Using 1 portal with 2 construction channels, light source, and irrigation system is not likely to increase the degree of freedom of the working device despite the decreasing portal diameter with the development of technology. A 2-portal system allows incorporation of the technological advantage while providing enough space for the operator’s hands.
The biggest disadvantage of this procedure is the relatively high difficulty level. If the surgeon is not familiar with this procedure, then the incidence rate of complications such as dural injuries is increased and the procedure time is prolonged.25,26 One study reported a relatively short learning curve for UBESS; however, in the initial learning period, the overall complication rate was ~10%, which is considered quite high.27
This procedure is contraindicated in patients with severe epidural adhesion. Severe damage is expected in the presence of severe adhesion with dorsal dura and thickened LF.
Sufficient decompression reflected improved symptoms. Especially the radiating and radicular pain recovered obviously. The results were the same in the evaluation of patient satisfaction. Evaluation of neurogenic claudication in the clinical course was assessed on the basis of the working capacity on the basis of some references, which had been well reflected in the patient’s functional status in previous studies.8 Dural tear and inadequate decompression among complications may be because of premature device manipulation or unexpected epidural adhesion. Epidural adhesion requires prior evaluation, and in case of doubt, gentle resection can minimize complications.
This study failed to perform a direct and quantitative comparison with the conventional open surgery and single-portal endoscopic surgery. However, minimal invasiveness is a clear difference from open surgery. Unlike the single-portal method, our method caused no disturbance by the working device because of the distal end of the cannula. The separated portal system has the advantage of setting various directions for tissue removal within a clear view field. The results from performing the procedure by only 1 surgeon at a single center also have a limitation. The absence of an assessment of the degree of postoperative laminectomy may be an important limitation in supporting the less invasiveness of our method as compared with that of the conventional methods. Although the qualitative analysis clearly showed a much smaller range of excision, additional experimental studies are warranted that this is less likely to affect structural stability.
UBESS using a 30-degree scope can be effectively used as a minimally invasive technique for severe LCCS. Sufficient learning and a certain amount of experience are needed to enable safe and fast procedures. A study that compares UBESS with other decompression surgeries, with a quantitative assessment and a long-term follow-up evaluation, is required in the future.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jspinaldisorders.com.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Eng J Med. 2008;358:794–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amundsen T, Weber H, Nordal HJ, et al. Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine (Phila Pa 1976). 2000;25:1424–1435; discussion 1435–1436. [DOI] [PubMed] [Google Scholar]
- 3.Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976). 2005;30:1441–1445; discussion 1446–1447. [DOI] [PubMed] [Google Scholar]
- 4.Caputy AJ, Spence CA, Bejjani GK, et al. The role of spinal fusion in surgery for lumbar spinal stenosis: a review. Neurosurg Focus. 1997;3:e3. [DOI] [PubMed] [Google Scholar]
- 5.Lee GY, Lee JW, Choi HS, et al. A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol. 2011;40:1033–1039. [DOI] [PubMed] [Google Scholar]
- 6.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26:1873–1878. [DOI] [PubMed] [Google Scholar]
- 7.Meyerding H. Spondylolisthesis. Surg Gynecol Obstet. 1932;54:371–377. [Google Scholar]
- 8.Tomkins-Lane CC, Battie MC. Validity and reproducibility of self-report measures of walking capacity in lumbar spinal stenosis. Spine (Phila Pa 1976). 2010;35:2097–2102. [DOI] [PubMed] [Google Scholar]
- 9.Conway J, Tomkins CC, Haig AJ. Walking assessment in people with lumbar spinal stenosis: capacity, performance, and self-report measures. Spine J. 2011;11:816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352:h6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malmivaara A, Slatis P, Heliovaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine (Phila Pa 1976). 2007;32:1–8. [DOI] [PubMed] [Google Scholar]
- 12.Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the Maine lumbar spine study. Spine (Phila Pa 1976). 2005;30:936–943. [DOI] [PubMed] [Google Scholar]
- 13.Katz JN, Lipson SJ, Chang LC, et al. Seven- to 10-year outcome of decompressive surgery for degenerative lumbar spinal stenosis. Spine (Phila Pa 1976). 1996;21:92–98. [DOI] [PubMed] [Google Scholar]
- 14.Bresnahan L, Ogden AT, Natarajan RN, et al. A biomechanical evaluation of graded posterior element removal for treatment of lumbar stenosis: comparison of a minimally invasive approach with two standard laminectomy techniques. Spine (Phila Pa 1976). 2009;34:17–23. [DOI] [PubMed] [Google Scholar]
- 15.Popov V, Anderson DG. Minimal invasive decompression for lumbar spinal stenosis. Adv Orthop. 2012;2012:645321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802–808. [PubMed] [Google Scholar]
- 18.Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine (Phila Pa 1976). 2002;27:432–438. [DOI] [PubMed] [Google Scholar]
- 19.Mobbs RJ, Li J, Sivabalan P, et al. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014;21:179–186. [DOI] [PubMed] [Google Scholar]
- 20.Knotkova H, Fine PG, Portenoy RK. Opioid rotation: the science and the limitations of the equianalgesic dose table. J Pain Symptom Manage. 2009;38:426–439. [DOI] [PubMed] [Google Scholar]
- 21.Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27:E9. [DOI] [PubMed] [Google Scholar]
- 22.Thome C, Zevgaridis D, Leheta O, et al. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine. 2005;3:129–141. [DOI] [PubMed] [Google Scholar]
- 23.Thongtrangan I, Le H, Park J, et al. Minimally invasive spinal surgery: a historical perspective. Neurosurg Focus. 2004;16:E13. [DOI] [PubMed] [Google Scholar]
- 24.Yue JJ, Long W. Full endoscopic spinal surgery techniques: advancements, indications, and outcomes. Int J Spine Surg. 2015;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J. 2009;18:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh K, Tomasino A, Knopman J, et al. Operative results and learning curve: microscope-assisted tubular microsurgery for 1- and 2-level discectomies and laminectomies. Neurosurg Focus. 2008;25:E14. [DOI] [PubMed] [Google Scholar]
- 27.Choi DJ, Choi CM, Jung JT, et al. Learning curve associated with complications in biportal endoscopic spinal surgery: challenges and strategies. Asian Spine J. 2016;10:624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jspinaldisorders.com.


