Abstract
Cataract, the clinical correlate of opacity or light scattering in the eye lens, is usually caused by the presence of high molecular weight protein aggregates or disruption of the lens microarchitecture. In general, genes involved in inherited cataract reflect important processes and pathways in the lens including lens crystallins, connexins, growth factors, membrane proteins, intermediate filament proteins, and chaperones. Usually, mutations causing severe damage to proteins cause congenital cataracts, while milder variants increasing susceptibility to environmental insults are associated with age-related cataracts. These might have different pathogenic mechanisms, with congenital cataracts inducing the UPR and apoptosis, while in age-related cataract denatured crystallins are bound by α-crystallin and form light scattering high molecular weight aggregates. New therapeutic approaches to age related cataracts involve using chemical chaperones to solubilize high molecular weight aggregates, while attempts are being made to regenerate lenses using endogenous stem cells to treat congenital cataracts.
Keywords: Lens, Cataract, Genetics, Crystallin
Introduction
Overview of the eye lens
The eye lens transmits and focuses light on the retina, where photoreceptors detect it and along with the other retinal cell types convert it into visual signals which then undergo initial processing before being transmitted through the optic nerve to the optic cortex. This function is facilitated by the lens structure, which consists of a single layer of anterior epithelial cells that migrate laterally during development towards the lens equator where they invert, elongate, synthesize large amounts of proteins specific to the lens including the lens crystallins, and finally degrade their organelles so as to increase their transparency. They are then overlayed by succeeding lens epithelial cells so that they eventually form an onion like structure of mature fiber cells called the lens nucleus. While this process begins during embryogenesis and is most active during development, it continues more gradually throughout life, so that older fiber cells reside in the lens nucleus, around which younger cortical fiber cells continue to be layered at the equator.
The mechanisms through which lens epithelial cells are transformed to elongated fiber cells lacking organelles is an area of active investigation. There is strong evidence that as the epithelial cells reach the equator they are exposed to increased levels of FGF (Dawes et al. 2018) and possibly oxidative stress (Brennan et al. 2018) to induce lens fiber differentiation. Mechanistically, the loss of organelles has been shown to involve the ubiquitin-proteasome pathway (Wride 2011), although there is increasing evidence that autophagy has an essential role in this process (Costello et al. 2013), However, both the organelle-free nature of lens fiber cells and their precise organization within the lens are critical to lens transparency and vision, as deviations from either can scatter light.
Since lens fiber cells lack ribosomes and other organelles, they cannot repair or replace damaged or modified proteins. This requires that lens crystallins be extremely stable so that they can last the lifetime of the organism, and also requires that the lens maintain strong homeostatic metabolic systems, especially those that contribute to maintenance of a reducing environment, to minimize damage to crystallins and other lens components (Ganea & Harding 2006) or reduce oxidative damage once it occurs (Kantorow et al. 2004), and avoid glycation and osmotic damage to the lens (Linetsky et al. 2008). The central nuclear fiber cells, are mostly dependent on glycolysis as an intrinsic energy source, which limits the energy available to them for these homeostatic activities. However, they receive considerable metabolic support from the anterior epithelia through an intrinsic circulation of fluid that appears to be critical for maintenance of lens homeostasis and transparency (Gao et al. 2018). Thus, the lens represents a delicately balanced anatomical and biochemical system, disruption of any part of which can result in loss of lens transparency or cataracts.
Overview of lens opacity
Opacity or cloudiness of the eye lens, the optical basis of cataract result when the refractive index within the lens varies significantly over distances approximating the wavelength of the transmitted light (Benedek 1971, Delaye & Tardieu 1983). Lens transparency requires both the orderly arrangement of lens cells and the high density and close packing of their protein constituents, primarily the lens crystallins. Variation in the refractive index and hence light scattering can result from changes in lens microarchitecture, the protein constituents, or both (Shiels & Hejtmancik 2017). Breakdown of the lens microarchitecture, including vacuole formation and disarray and degeneration of the lens fiber cells, results in large fluctuations in optical density, causing light scattering and hence cataract. Similarly, light scattering and opacity also can result from increased concentrations of high molecular weight protein aggregates (HMW) larger than 1000 Å in size. As lens crystallins make up over 90% of soluble lens proteins, their short-range ordered packing in a homogeneous phase is critical for lens transparency.
For patients, cataracts become important when they begin to interfere with vision, and they can be categorized by the age at which this occurs, even though some lens opacity probably precedes the clinical recognition. Congenital cataracts are diagnosed in the first year of life, while juvenile cataracts declare themselves between one and ten years of life. Presenile cataracts come to clinical attention before the age of 45–55 years, and cataracts diagnosed after that time are classified as senile or age-related cataract. These definitions are approximate and somewhat arbitrary, so that different studies might shift the limits by 5–10 years. Also, as mentioned, asymptomatic mild cataracts might not be diagnosed for years after they occur. The age of onset of a cataract does not necessarily imply a specific cause. Congenital cataracts might be inherited or secondary to an intrauterine insult (e.g. viral or parasitic disease). Cataracts associated with a systemic metabolic disease such as diabetes or an ophthalmic genetic disease such as retinitis pigmentosa may not occur until the later in life. Age-related cataracts are almost always associated with both a variety of environmental insults accumulated over many years, and the susceptibility to these insults may be modulated directly or indirectly by genetic risk factors (1994, Hammond et al. 2001, Hammond et al. 2000, Heiba et al. 1993, Heiba et al. 1995, Leske et al. 1998, Yonova-Doing et al. 2016).
The genetic origin of an inherited cataract can be suggested by the time it first appears and the specific region of the lens in which it occurs. Opacity should present in lens cells synthesizing large amounts of a mutant protein at the time at which it is being synthesized. One example of this principle is the γ-crystallins, which are synthesized at high levels early in development in fiber cells which are destined to populate the embryonic or central nucleus. For this reason, mutations in γ-crystallins often tend to cause nuclear cataracts, with ~49% of mutations in γ-crystallins listed in CAT-MAP (Shiels et al. 2010) being nuclear and ~15% nuclear lamellar. In spite of these general tendencies, mutations in a variety of genes can cause clinically indistinguishable cataracts and identical mutations in the same gene can cause varying cataract phenotypes. However, one general principle seems to be that mutations in crystallins or other lens proteins are sufficiently severe to cause protein aggregation or directly damage lens cells by themselves, thereby resulting in congenital cataract. In contrast, if mutations merely increase susceptibility to damage from light, hyperglycemia, oxidative stress, or other environmental insults they might contribute to age related cataract.(Hejtmancik & Smaoui 2003) Thus, hereditary congenital cataracts tend to be inherited in a Mendelian fashion with high penetrance, while age-related cataracts tend to be multifactorial, with both multiple genes and environmental factors influencing the phenotype, making them significantly less amenable to classical genetic and biochemical analysis.
Congenital cataract
Estimates of the incidence of congenital cataracts vary from 12 to 136 per 100,000 births, with most countries showing about 72 per 100,000 children. The incidence tends to be higher in less developed countries, probably because of a higher risk of infectious disease and other environmental causes (Gilbert & Foster 2001, Haargaard et al. 2004, Stoll et al. 1992). Overall, between 8.3 and 25 percent of congenital or infantile cataracts are inherited (Francois 1982, Haargaard et al. 2005, Merin 1991). They may be isolated cataracts or may be accompanied by anterior chamber developmental anomalies including microphthalmia, microcornea, or aniridia. Cataracts may also occur as part of multisystem genetic problems including chromosome disorders, developmental defects, or metabolic disorders. There can be overlap between isolated and syndromic cataracts, as in the case of cataracts occurring because of defects in many growth factors. In some patients these may cause cataracts associated with defects in the anterior segment or optic disc in some family members while resulting in isolated cataracts in other members of the same family.
Hereditary Mendelian cataracts are most frequently inherited as an autosomal dominant trait, ~85% in CAT-MAP (Shiels et al. 2010) but can also show autosomal recessive, or X-linked inheritance. As mentioned above clinically similar cataracts can result from mutations in different genes and also may show different inheritance patterns, while phenotypically variable cataracts can result from the same mutation in a single gene, and even be found in a single family (Scott et al. 1994). Several classification systems have been developed based on the clinical presentation and anatomic location of the opacity, with the one proposed by Merin being the most commonly used. In this system cataracts are classified as polar (anterior or posterior), zonular (nuclear, lamellar, sutural, etc.), total (mature or complete), and capsular or membranous (Merin & Crawford 1971). A revision of this system taking anterior segment characteristics into account has recently been proposed (Lin et al. 2016a).
Age-related cataract
Age-related or senile cataracts present after the age of 45–55 years, with the lens clear before that time. They tend to be progressive in nature and are extremely common. Age related cataracts usually represent the effects of various combinations and cumulative damage of environmental effects acting in concert with the genetic predisposition encoded in genes for lens proteins (1994, Hammond et al. 2001, Hammond et al. 2000, Heiba et al. 1993, Heiba et al. 1995, Leske et al. 1998, Yonova-Doing et al. 2016).
Lens crystallins show multiple types of modifications with aging of the lens. Most of the changes are caused or accelerated by oxidative, UV, osmotic, or other types of damage, and these environmental risks are independently associated with cataractogenesis in epidemiological studies (Sharma & Santhoshkumar 2009). Alterations of lens crystallins include proteolysis, an increase in disulfide bridges, phosphorylation, nonenzymatic glycosylation, carbamylation, deamidation of asparagine and glutamine residues, and racemization of aspartic acid residues among others (Sharma & Santhoshkumar 2009). Just as the environmental stresses that cause these changes have been linked epidemiologically to age related cataracts in human populations, the chemical modifications themselves are increased in lenses with cataracts as well as in in vitro or in model systems in which the model animals are subjected to similar environmental insults as seen in human age related cataract (Ottonello et al. 2000).
Crystallins comprise over 90% of the soluble proteins in the eye lens and are the most studied proteins in the aging lens. In humans there are three major classis of lens, α-, β- and γ-crystallins, each of which consist of gene families, the latter two of which share a very stable structure composed of two domains, each comprising two very stable Greek key motifs (Jaenicke & Slingsby 2001). As individuals age, the β- and γ-crystallins are modified, especially under the influence of environmental stress, and start to lose their normally stable protein fold and form irreversible aggregates. The slowly denaturing β- and γ-crystallins are bound by α-crystallins, which have a chaperone-like activity (Rao et al. 1995). However, complexing of the denatured βγ-crystallins by α-crystallins maintains their solubility and thus reduces light scattering, it does not result in their being recycled into the cytoplasm as would happen with true chaperones. Instead, the denatured crystallins are held in complexes with α-crystallins that increase in size over time as increasing amounts of damaged protein are bound until they become high molecular weight aggregates that are large enough to scatter light (Datiles et al. 2008, Rao et al. 1995). With sufficient time and insults, even the high level of α-crystallin in the lens is exhausted and the high molecular weight aggregates precipitate, joining the insoluble protein that increases with age and in cataractous lenses. Whether this process involves complete or partial denaturation or the modified crystallins simply have decreased solubility but intact protein folds is not known currently, and might vary for each crystallin and mutation. However, both mouse models and cell culture studies of mutant proteins associated with human cataracts strongly suggest that the presence of large amounts of denatured protein damages the lens cell, and contributes to cataracts not only through light scattering by protein aggregates, but eventually also through toxicity to lens fiber cells and resultant disruption of cellular architecture (Ma et al. 2011, Wang et al. 2007). Similarly mutations in proteins that maintain intracellular homeostasis can disrupt the intracellular environment of lens cells and eventually cause damage to their constituents, contributing to age related cataract.
Age-related cataracts, while not having as severe an impact on each affected individual as congenital cataracts, have a much greater overall burden on the population because they are extremely common. They are responsible for blinding 17 million persons worldwide, causing just under half of all blindness (Congdon et al. 2003), and are the leading cause of low vision in the United States (Congdon et al. 2003). Cataract surgery is required by about 5% of the American population over 40 years old, making it the most frequently performed surgical procedure. It has been pointed out that due to the advanced age at which age related cataracts are found, delaying their age of onset by only ten years would decrease cataract surgery in the United States by about 45% (Kupfer 1984).
The risk of age-related nuclear cataract is increased by exposure to certain environmental factors such as elevated blood glucose levels, cigarette smoking or chronic exposure to wood smoke, or obesity (Chang et al. 2011, Foster et al. 2003, Leske et al. 1991, Lu et al. 2012, Ye et al. 2012). Similarly, the risk of age related cortical cataracts is increased by ultraviolet light and elevated glucose levels (Brown & Hill 1987, Foster et al. 2003, Group 1991, Hennis et al. 2004, Machan et al. 2012), and the risk of age related PSC is increased by smoking, diabetes, radiation, corticosteroids, and some other drugs (Abe et al. 2012). Alcohol consumption has been suggested to be associated with age-related cataract, but the relationship appears to be complicated and results are somewhat inconsistent (1991, Kanthan et al. 2010). Conversely, exercise, vitamin D, and antioxidant vitamins might have a protective effect, although this has not been borne out by all studies (Chang et al. 2011, West & Valmadrid 1995, Williams 2012) (Park & Choi 2017). One interesting aspect of these studies is that not only have they identified a number of potentially correctable environmental risk factors for age related cataract, but the different risk factors for nuclear, cortical and posterior subcapsular cataracts suggest that they might have distinct pathogenic mechanisms that begin to converge in the final steps of cataractogenesis.
Many epidemiological studies also support a role for genetic factors in age-related cataract (Group 1991, McCarty & Taylor 2001). The Italian American cataract study group (1991) and the Lens Opacity Case Control Study (Leske et al. 1991) both suggest that family history is a risk factor for cortical, combined nuclear and cortical, and posterior subcapsular cataracts. The Framingham Offspring Eye Study (1994) found a threefold increased risk for cataract in individuals with a sibling having a cataract. Results from the Beaver Dam Eye Study (Klein et al. 2001) and Twin Eye Study (Hammond et al. 2001) both suggest that genetic factors could account for as much as 35–48% of the risk for nuclear and 53–75% of the risk for nuclear and cortical cataract, respectively. Finally, a recent twin study suggests that genetic factors might account for 20–39% of intraocular light scattering that didn’t reach the stage of cataract (Benito et al. 2016).
Genes Associated with Congenital or Mendelian Cataracts
While most Mendelian cataracts (whether autosomal dominant, recessive, or X-linked) occur as isolated traits diagnosed at birth or early childhood, some of them can present as late as adulthood, and those will also be covered in this section (Hejtmancik et al. 2001). The most common inheritance pattern is autosomal dominant, although this might be influenced by the tendency for dominant inheritance to result in larger pedigrees with multiple affected individuals. While new cataract loci are being mapped and their genes are being identified at a rapid rate, at this time there are about 60 mapped of which the causative gene has been identified in about 40 (Table 1). Those listed present as isolated cataracts in at least some individuals, although in some cases they can be associated with additional abnormalities in other family members, mostly as part of developmental syndromes affecting the optic disc area. A larger number of inherited cataracts occur as part of syndromes affecting multiple organ systems (Hejtmancik et al. 2001), often caused by mutations in transcription factors or metabolic enzymes. In addition some examples result from genes expressed at high levels in the lens as well as other tissues, such as αB-crystallin mutations which can cause myopathy as well as isolated cataracts, and ferritin, which causes the hyperferritinemia-cataract syndrome (Table 1). Finally, mutations in some genes can lead to extralenticular effects as the result of a developmental sequence, such as crystallin mutations that cause early and severe damage to the lens preventing its normal role in development of the anterior chamber and leading to microcornea and even microphthalmia. Also, if the cataract is early and severe, the resulting visual deprivation during this early critical period inhibits formation of the normal pathways from the retina to the optic cortex with resultant nystagmus or even blindness.
Table 1.
Loci, genes, and phenotypes for nonsyndromic cataract (CTRCT)
| Gene | Cataract phenotype | Inheritance | Associated phenotypes | Phenotype MIM number |
|---|---|---|---|---|
| ? | CTRCT8; multiple types | AD | 115665 | |
| EPHA2 | CTRCT6; multiple types | AD/AR | Age-related cortical cataract, susceptibility to | 116600 |
| ? | CTRCT34; multiple types | AR | With or without microcornea | 612968 |
| GJA8 | CTRCT1; multiple types | AD/AR | With or without microcornea | 116200 |
| ? | CTRCT29; coralliform | AD | 115800 | |
| ? | CTRCT27; nuclear progressive | AD | 607304 | |
| CRYGB | CTRCT39; multiple types | AD | 615188 | |
| CRYBA2 | CTRCT42 | AD | 115900 | |
| CRYGC | CTRCT2; multiple types | AD | With or without microcornea | 604307 |
| CRYGD | CTRCT4; multiple types | AD | With or without microcornea | 115700 |
| FYCO1 | CTRCT18 | AR | 610019 | |
| BFSP2 | CTRCT12; multiple types | AD | Myopia? | 611597 |
| CRYGS | CTRCT20; multiple types | AD | 116100 | |
| WFS1 | CTRCT41 | AD | Wolfram syndrome (DIDMOAD) | 116400 |
| ? | CTRCT28 | ? | Age-related cortical cataract, susceptibility to | 609026 |
| GCNT2 | CTRCT13 | AR | Adult i (blood group) phenotype | 110800 |
| LEMD2 | CTRCT46, juvenile onset | AR | 212500 | |
| AGK | CTRCT38 | AR | Senger’s syndrome | 614691 |
| ? | CTRCT26; multiple types | AR | 605749 | |
| TDRD7 | CTRCT36 | AR | 613887 | |
| VIM | CTRCT30; pulverulent | AD | 116300 | |
| PITX3 | CTRCT11; multiple types | AD | Anterior segment mesenchymal dysgenesis, microphthalmia, neurodevelopmental abnormalities | 610623 |
| CRYAB | CTRCT16; multiple types | AD/AR | Myopathy, multiple types | 613763 |
| MIP | CTRCT15; multiple types | AD | 615274 | |
| ? | CTRCT37; cerulean | AD | 614422 | |
| GJA3 | CTRCT14; multiple types | AD | 601885 | |
| ? | CTRCT32; multiple types | AD | 115650% | |
| ? | CTRCT25 | AD | 605728 | |
| HSF4 | CTRCT5; multiple types | AD/AR | 116800 | |
| MAF | CTRCT21; multiple types | AD | With or without microcornea | 610202 |
| ? | CTRCT24; anterior polar | AD | 601202 | |
| CRYBA1 | CTRCT10; multiple types | AD | 600881 | |
| UNC45B | CTRCT43 | AD | 616279 | |
| ? | CTRCT7 | AD | 115660 | |
| ? | CTRCT35; congenital nuclear | AR | 609376 | |
| SIPAIL3 | CTRCT45 | AR | 616851 | |
| LIM2 | CTRCT19 | AR | 615277 | |
| BFSP1 | CTRCT33; cortical | AR | 611391 | |
| CHMP4B | CTRCT31; multiple types | AD | 605387 | |
| CRYAA | CTRCT9; multiple types | AD/AR | With or without microcornea, age-related nuclear cataract, susceptibility to | 604219 |
| LSS | CTRCT44 | AR | 616509 | |
| CRYBB2 | CTRCT3; multiple types | AD | With or without microcornea | 601547 |
| CRYBB3 | CTRCT22; multiple types | AD/AR | 609741 | |
| CRYBB1 | CTRCT17; multiple types | AD/AR | 611544 | |
| CRYBA4 | CTRCT23 | AD | 610425 | |
| NHS | CTRCT40 | X-linked | Nance-Horan (cataract dental) syndrome | 302200 |
For further information, readers are referred to CAT-MAP (https://cat-map.wustl.edu/). Abbreviations: AD, autosomal dominant; AR, autosomal recessive.
From Table 1 it can be seen that causative genes have not been identified for about a third of mapped cataract loci. The genes that have been identified can be grouped functionally by the processes and pathways to which they belong suggesting that these are critical for the development and homeostasis of the lens, although this analysis is imperfect because it also reflects the predispositions of investigators in their mapping and sequencing efforts. At this time, about 37% of the independent families for whom the causative gene is known show mutations in lens crystallins, about 22% show mutations in connexins, about 14% show mutations in growth or transcription factors, and 3–7% each in intermediate filaments or aquaporin 0, membrane proteins, chaperones or components of the protein degradation apparatus and a mix of other genes, while about 5% are unknown. As mentioned above, Inheritance of the same mutation in different families or even the same mutation within the same family can result in radically different cataract morphologies and severities. This suggests that additional genetic or environmental factors might modify the expression of the primary mutation associated with the cataracts. Conversely, cataracts with similar or identical morphologies can result from mutations in quite different genes, perhaps relating to clinical cataract being a final common pathway for a variety of different initial insults.
Genes encoding crystallins
The importance of lens crystallins in lens biology and especially in transparency is indicated by their accounting for the largest fraction of cataract mutations. α-Crystallins have a dual role in the lens, both as highly expressed lens proteins and as chaperones important to protect the lens from toxicity of denatured βγ-crystallins (Horwitz 2003). Severe mutations in the αA- and αB-crystallin genes that result in a complete loss of the proteins or their chaperone-like activity can cause autosomal recessive cataracts, which suggests that decreased levels of functional α-crystallin are sufficient to maintain lens transparency. A prime example of this is a p.W9X chain termination mutation near the beginning of the protein (Pras et al. 2000), which would be expected to result in nonsense mediated decay and complete absence of the mutant allele without affecting protein synthesized from the normal gene. A p.R54C mutation causes total congenital cataracts in homozygotes, but the effects in carriers are limited to punctate opacities in carriers (Khan et al. 2007), and three families have been identified with autosomal recessive cataracts caused by CRYAB mutations.(Jiao et al. 2015, Safieh et al. 2009) These suggest that even decreased levels of α-crystallin can provide sufficient chaperone-like activity and structural crystallin packing to establish and maintain lens transparency during childhood, consistent with results from αA-crystallin knock-out mice (Brady et al. 1997). In contrast autosomal dominant cataracts, which tend to be associated with nonconservative missense mutations resulting in a change in the amino acid sequence, probably produce a deleterious mutant α-crystallin protein that actively damages the lens cell or inhibits function of the remaining normal α-crystallin in a dominant negative mechanism rather than simply acting through loss of chaperone function as the recessive cataract appears to do (2005). Many CRYAA mutations are also associated with microcornea, possibly due to this gene’s early and abundant expression in the lens causing severe effects during lens formation that might interfere with possible developmental interactions between the lens and the rest of the anterior chamber.
The similar biochemical properties and function of αA- and αB-crystallin and their association into large multimeric complexes might suggest that mutations in CRYAB would have a similar effect to those in CRYAA, at least in the lens. However, the family harboring the first human mutation reported in CRYAB, a missense mutation that reduced αB-crystallin chaperone activity resulting in aggregation and precipitation of the protein under stress, had myofibrillar myopathy but only “discrete” cataracts (Vicart et al. 1998), consistent with the αB-crystallin knockout mouse, which shows myopathy without cataracts (Brady et al. 2001). The myopathy is probably related to high level expression of CRYAB but not CRYAA in muscle cells, where it binds and stabilizes desmin.(Brady et al. 2001) However, multiple missense and frame-shift mutations in CRYAB have been shown to cause autosomal dominant cataracts without myopathy, probably through a similar toxic effect as the dominant CRYAA associated cataracts (Berry et al. 2001).
While mutations in the α-crystallins could cause cataract either by loss of chaperone-like activity or synthesis of a deleterious mutant protein, most mutations in the βγ-crystallins appear to cause cataract through producing an unstable protein that aggregates and then precipitates from the cytosol (Ma et al. 2016c), being bound by α-crystallin or possibly serving as a nidus for additional protein denaturation and precipitation, with resultant toxicity to lens cells and cataract. The exceptions to this are a p.N58TfsX106 CRYBB1 mutation in 3 families (Aldahmesh et al. 2012, Cohen et al. 2007, Khan et al. 2012) and a p.(G65R) CRYBB3 mutation in a two families (Riazuddin et al. 2005), suggesting that these two basic β-crystallins might have a lenticular function beyond being structural crystallins. Consistent with their early expression and high levels in the lens nucleus, γ-crystallin mutations tend to result in nuclear (49%) or zonular (15%) cataracts, although their phenotypes may vary (Shiels et al. 2010). Mutations in the β-crystallins produce phenotypes ranging from dense nuclear (49%) to zonular (16%) pulverulent cataract with or without involvement of the sutures, to cerulean cataracts (Shiels et al. 2010). Zonular or lamellar cataracts might reflect synthesis of the crystallin later in development than the γ-crystallins and for a limited period of time, resulting in a shell of opaque cells surrounded internally by a clear central lens nucleus and externally by relatively clear cortical lens fiber cells (Amaya et al. 2003). However, the occurrence of a variety of cataract phenotypes in a large family with autosomal dominant cataracts resulting from a c.119_123dup mutation emphasizes the importance of modifying genes in the phenotypic expression of these mutations, a point that is supported by variable severity of cataracts resulting from expression of this mutant CRYGC in transgenic mice (Ma et al. 2011).
While most βγ-crystallin mutations appear to cause cataract by destabilizing the structure of the protein, eventually resulting in denaturation, there are exceptions to this general rule. At least 3 separate mutations leave the protein fold intact, but modify the γD-crystallin’s surface characteristics, thus lowering their solubility or enhancing their protein to protein interaction strength so that they come out of solution (Pande et al. 2001). A p.(P23T) mutation of CRYGD increases inter-protein hydrophobic interactions causing protein precipitation, a p.R36S mutation actually results in crystallization of the protein within the lens (Kmoch et al. 2000), and a third p.(R14C), makes the protein susceptible to thiol-mediated aggregation (Pande et al. 2000). These results emphasize that crystallins need not undergo denaturation or other major changes in their protein folds in all cases to cause cataracts.
Genes encoding membrane proteins
As lens epithelia cells elongate dramatically during their transition to fiber cells in a process requiring rapid synthesis of large quantities of membrane lipids and proteins, it is not surprising that membrane-related proteins comprise a large share of cataract genes. One set of genes in this group encode enzymes required for the synthesis of specific membrane lipids including lanosterol synthase, although this might also have a role through its chaperone-like action on denatured crystallins (Zhao et al. 2015). Mutations in acylglycerol kinase (AGK), a lipid kinase that catalyzes synthesis of phosphatidic and lysophosphatidic acids and CYP51A1, an enzyme in the cholesterol synthesis pathway, have been implicated in autosomal recessive cataracts in Saudi Arabia (Aldhamesh et al. 2012).
Mutations in GCNT2, the I-branching enzyme for poly-N-acetyllactosaminoglycans responsible for the adult I blood type, also cause autosomal recessive cataract (Yu et al. 2003). Interestingly, these cataracts are only associated with adult persistence of the childhood i (rather than change to the adult I) phenotype in Japanese rather than Caucasians because of differential splicing of the GCNT2 mRNA in erythrocytes and lens cells and the population specific location of the causative mutations in the two populations. Mutations in CHMP4B, part of the endosomal sorting complex required for transport-III (ESCRT-III) assembly cause posterior polar or subcapsular cataract (Shiels et al. 2007), and mutations in LIM2, a lens-specific membrane protein important for cell junctions cause nuclear cataract (Pras et al. 2002).
Membrane proteins with specialized function in transport of ions or solutes also contribute to this group. Mutations in SLC16A12, a transmembrane transporter active in monocarboxylic acid transport can cause dominant cataracts as well as microcornea and renal glycosuria in some cases (Kloeckener-Gruissem et al. 2008), perhaps by interfering with the circulation of water and small molecules necessary for lens homeostasis and function. Similarly, mutations in aquaporin 0 (also known as AQP0 or MIP), a member of the aquaporin water-channel family, are a common cause of cataract. Two mutations, p.(E134G) and p.(T138R) appear to act by interfering with normal trafficking of AQP0 to the plasma membrane and also with water channel activity by normal AQP0, consistent with a dominant negative mechanism and with dominant inheritance of the cataracts (Francis et al. 2000). Mutations in the ATP-binding cassette transporter ABCA3, a lipid transporter also implicated in respiratory disease (Chen et al. 2014), and the transmembrane protein TMEM114 can also cause cataract (Jamieson et al. 2007), as can DNMBP, a guanine nucleotide exchange factor that regulates the configurations of cell junctions (Ansar et al. 2018).
Gap junctions are critical for nutrition and intercellular communication in the lens, and mutations in the two major gap junction proteins GJA3 and GJA8 (connexins 46 and 50) are responsible for about 22% of cataract families (Shiels et al. 2010). The p.(P88S) mutation in GJA8 modifies the second transmembrane domain so that it fails to form functional gap junction channels (Berthoud et al. 2003). In Xenopus oocytes, incorporation of just one mutant protein molecule into a gap junction inhibits channel function, consistent with a dominant negative effect similar to AQP0 (Minogue et al. 2005). Other mutant connexin proteins, such as an N63S missense mutation also fail to form intercellular channels in paired Xenopus oocytes (Pal et al. 2000), but are unable to participate in gap junction formation at all, and thus do not inhibit channel function by products of the normal gene. The cataracts caused by both GJA3 and GJA8 mutations are phenotypically similar, and predominantly nuclear or zonular pulverulent cataracts (Gong et al. 2007).
Genes encoding cytoskeletal proteins
BFSP1 (filensin, CP115) and BFSP2 (phakinin, CP49) are intermediate filament proteins that are similar to each other but show only low levels of sequence similarity to other members of the intermediate filament family. They too form beaded filaments, only with the chaperone-like assistance of the α-crystallins. Like BFSP1 and BFSP2 themselves, beaded filaments are unique to lens fiber cells. Missense or in-frame deletion mutations in BFSP2 tend to cause nuclear or nuclear lamellar dominant cataracts with some involvement of the sutures, consistent with fiber cell specific expression of the beaded filament proteins (Conley et al. 2000, Jakobs et al. 2000). In contrast, frameshift and nonsense mutations cause recessive cortical cataracts (Aldahmesh et al. 2011, Aldahmesh et al. 2012). Similarly, a missense mutation in BFSP1 causes nuclear cataracts while a frameshift mutation that is probably null has been associated with cystic cortical cataracts (Kumar et al. 2013, Li et al. 2016, Ma et al. 2016a, Ramachandran et al. 2007, Zhai et al. 2017).
Genes encoding transcription or developmental factors
PAX6, a paired box and homeobox domain protein expressed in the developing nervous system and eye, is one of the earliest transcription factors active in eye development (Kamachi et al. 2001). Most PAX6 mutations cause generalized eye developmental defects such as anophthalmia, aniridia or Peter’s anomaly. However, one mild mutation affecting only the C-terminal PST domain has been associated with lamellar cataract and later onset corneal dystrophy (Glaser et al. 1994). Mutations in other transcription factors occurring further down the control pathways for lens development also cause cataract, including VSX2, MAF, FOXE3, EYA1, and PITX3 (Shiels et al. 2010). As these transcription factors influence gene expression in the entire eye field, they can all contribute to a variety of developmental defects in the anterior segment, ranging from microcornea to anterior segment mesenchymal dystrophy (ASMD). NHS has a broader field of activity, and beyond isolated cataracts, mutations in NHS cause the Nance Horan syndrome, characterized by cataracts, dental abnormalities, dysmorphic facies and in some cases mental retardation (Burdon et al. 2003).
In contrast, mutations in HSF4, a member of the heat-shock transcription factor family, appear to cause isolated cataracts not associated with extralenticular effects even though HSF4 is broadly expressed in most eye tissues (Shiels et al. 2010). HSF4 regulates transcription of αB-crystallin in the lens, among other heat shock proteins (Somasundaram & Bhat 2004). HSF4 mutations can cause both autosomal dominant and recessive cataracts. The dominant cataracts are most often lamellar or zonular and most often result from missense mutations in the α-helical DNA-binding domain (Eiberg et al. 1988). In contrast, the recessive cataracts tend to result from homozygous null mutations outside the highly conserved DNA-binding domain and have a congenital onset, ranging in severity from nuclear with some cortical involvement (Forshew et al. 2005) to total lens opacities with secondary nystagmus (Smaoui et al. 2004).
EPHA2, a member of the ephrin receptor subfamily (EPH) of protein-tyrosine kinases, is not itself a transcription factor but is heavily involved in developmental processes in both the eye and the nervous system. Not only can mutations in EPHA2 cause both dominant and recessive congenital cataracts, but have also been shown to contribute to age-related cataract.(Jun et al. 2009, Kaul et al. 2010, Shiels et al. 2008, Sundaresan et al. 2012, Tan et al. 2011, Zhang et al. 2009)
Genes encoding chaperones or protein degradation apparatus
Lens development requires extensive restructuring of the lens itself, and differentiation of lens epithelia cells into cortical and nuclear fiber cells requires elimination of cellular organelles, both of which require high levels of protein degradation. Mutations in FYCO1, a scaffolding protein active in microtubule transport of autophagic vesicles cause autosomal recessive cataracts (Chen et al. 2011), suggesting that autophagic vesicles are important in organelle degradation in developing lens fiber cells among other roles (Brennan et al. 2012). Consistent with this concept, mutations in RRAGA, a GTPase active in the TORC1 pathway are associated with congenital nuclear cataracts (Chen et al. 2016), and mutations in CHMP4B, a member of the endosomal sorting complex that interacts with programmed cell death 6 interacting protein PDCD6IP can cause progressive posterior polar and subcapsular cataracts (Shiels et al. 2007). As the lens fiber cells lack organelles and have no way of turning over damaged proteins, it is not surprising that UNC45B, a co-chaperone with HSP90, can cause juvenile cataracts (Hansen et al. 2014).
Additional genes encoding proteins in biological processes highly active in the lens
Differentiation of lens epithelia and fiber cells requires an extremely high level of protein synthesis, perhaps explaining the role of mutations in TDRD7, a widely expressed component of RNA granules active in RNA processing, in hereditary cataract (Lachke et al. 2011). Finally, the hyperferritinemia-cataract syndrome, characterized by hyperferritinemia in the absence of iron overload and cataracts is the result of inadvertent expression of a non-crystallin protein at crystallin-like levels (Beaumont et al. 1995). Ferritin is widely expressed in the body as well as eye tissues and serves to sequester iron in a soluble and nontoxic state The mutations in the hyperferritinemia-cataract syndrome are in the iron responsive element, a stem loop structure in the 5’ untranslated region of the FTL mRNA, which in the absence of excess iron binds the cytoplasmic iron regulatory protein which inhibit its translation. Overexpression of FTL to levels approaching that of a crystallin occur through loss of this translational control, resulting in crystallization of ferritin in the lens with resultant breadcrumb like opacities in the lens cortex and nucleus (Girelli et al. 1995). This cataract shows that crystallins must be exceptionally soluble to be expressed at high levels in the lens without causing dysfunction.
Genes Associated with Age-Related Cataract
Linkage and family studies
A subset of Mendelian cataract loci and genes identified using classical linkage analysis have a childhood to presenile age of onset or show progression throughout life, including some resulting from mutations in genes that are also implicated in congenital cataract (Shiels et al. 2010). These include BFSP2, in particular 3 families with a p.(E233del) mutation, the a p.(R119C) mutation in HSF4 (Marner, a childhood cataract), p.(890C) and p.(G948W) mutations in EPHA2 (childhood progressive cataracts), p.(T138R) and p.(T138T) mutations in AQP0 (progressive cataracts), the p.(D129V) mutation in CHMP4B (childhood progressive cataracts), the p.(I247M) mutation of GJA8, the p.(Q215X) mutation in SLC16A12, the p.(R37S) mutation in CRYGD, a p.(N115D) mutation in CRYBA2, the p.(I33_A119del) mutation in CRYBA3 (childhood progressive cataracts), the p.(G217AfsX91) and p.(G220PfsX95) mutations in PITX3, the progressive Volkmann cataract, and the CAAR locus are linked to familial adult onset pulverulent cataracts. Only 2 of 7 families with a p.(Q155X) mutation in CRYBB2 have a progressive cataract, the remaining families showing congenital cataract, which suggests the effect of modifying genes or a stochastic biological process. A p.(F104V) mutation in LIM2 causes a presenile cataract as does a p.(G98R) mutation in CRYAA, the remainder of mutations in these genes causing congenital cataracts. Other than the nonsense mutations in CRYBB2 and SLC16A12 and the frameshift mutations in PITX3, mutations associated with late onset or progressive cataracts are missense mutations that might retain some level of function or stability of the original protein.
This concept is exemplified by the p.(G18V) and p.(G18D) mutations in CRYGS, which cause progressive childhood cataracts (Sun et al. 2005, Zhai et al. 2017). The p.(G18V) mutation has been shown to cause minimal perturbation of the CRYGS protein fold under physiological conditions, but to destabilize the crystallin when it is subjected to thermal or chemical stress (Ma et al. 2009), and this is associated with preferential binding of the p.G18V mutant by CRYAB even under relatively benign conditions (Kingsley et al. 2013). Thus, these examples seem consistent with the hypothesis that a mutation that causes major structural damage to the protein or completely disrupts its function might cause highly penetrant Mendelian congenital cataracts, while a mutation resulting in somewhat less severe damage to the same protein or its function might contribute to Mendelian age-related or progressive cataracts. Furthermore, changes causing even milder damage might show reduced penetrance or even require multiple mutant genes and environmental insults for a phenotype, thus showing a complex or multifactorial inheritance pattern. Similarly, mutations causing severe damage to cell architecture or environment seem likely to result in congenital cataracts, while mutations resulting in only mild disruption of lens cell systems might contribute to age-related cataract, especially when combined with environmental stresses.
The reduced penetrance of age-related cataract mutations, especially when combined with their frequent requirement for contributions from multiple genes and environmental factors, as well as their late onset, makes carrying out linkage analysis for this form of inherited cataract much more difficult than for Mendelian congenital cataracts. A genome-wide linkage scan of age-related cortical cataract (Iyengar et al. 2004) identified a locus on chromosome 6p12 that may coincide with a susceptibility locus for type 2 diabetes with retinopathy (OMIM #125853), and a second locus on chromosome 1p near the EPHA2 gene (see below). Other than this, most genes implicated in age related cataract have been identified by association studies, often guided by epidemiological or animal data.
Association studies
Several genes implicated in age-related cataracts support the concept that severe mutations might cause congenital cataracts with Mendelian inheritance while less damaging changes might contribute to age related cataracts along with other genes and environmental stresses (Skalka & Prchal 1980). Deficiency of galactokinase (GALK1), galactose-1-phosphate uridyl transferase (GALT), or deficiencies of galactose epimerase (GALE) cause galactitol accumulation and subsequent osmotic swelling of lens cells resulting in autosomal recessive congenital cataracts (Kinoshita 1965). In 2001 a p. A198V mutation in GALK1, called the “Osaka” variant, was shown to cause instability of the mutant GALK1 protein with resulting low levels of functional galactokinase and an increase in bilateral cataracts in Japanese adults.(Okano et al. 2001) The mutant allele is present in 4.1% of the Japanese population but increases to 7.1% of Japanese with age related cataracts. It appears to be largely confined to the Japanese population with a lower presence (2.8%) in Koreans and much lower in Chinese, but was absent from other ethnic groups tested. In addition, parents of children with galactosemia from GALK1 and GALT deficiency have an increased risk of age related cataract (Stevens et al. 1989). These findings are consistent with the known increased risk of age-related cataract from hyperglycemia, and also with animal data and the finding that susceptibility to cataracts as a diabetic complication in humans is associated with specific allele Z of the microsatellite polymorphism at the 5’-end of the aldose reductase gene (Lee et al. 1995, Lee et al. 2001).
Another gene with a variety of severity and types of mutations resulting in a spectrum of cataract severity is SLC16A12, a creatine transporter also implicated in renal glycosuria. A p.(Q215X) mutation causes juvenile cataracts, microcornea, and glycosuria and appears to act by impairing protein trafficking to the plasma membrane (Castorino et al. 2011, Kloeckener-Gruissem et al. 2008). In contrast, 4 missense mutations in SLC16A12 that decrease creatine have been identified in patients with age related cataract (Staubli et al. 2017). EPHA2, the Eph-receptor type-A2 (EPHA2), functions in the ephrin cell signaling and guidance pathway and lies in the candidate region on chromosome 1p identified by linkage analysis (Iyengar et al. 2004). It is a third gene with strong support for both congenital and age related cataracts, mutations in the coding region being able to cause both dominant and recessive congenital cataracts (Kaul et al. 2010, Shiels et al. 2008, Zhang et al. 2009). In addition, single nucleotide polymorphisms (SNPs) in the EPHA2 region of chromosome 1 are associated with age-related cortical cataract, further suggesting that EPHA2 variants may underlie both inherited and age-related forms of cataract.(Jun et al. 2009, Shiels et al. 2008, Sundaresan et al. 2012, Tan et al. 2011) and the association is consistent with demonstrations that the risk allele of SNP, rs6603883, which is located in in a PAX2 binding-site of the EPHA2 promoter region, decreases EPHA2 expression (Jun et al. 2009, Ma et al. 2017).
Sequence variations in the αA-crystallin gene (CRYAA) have also been associated with age related cataract in a number of studies, and a p.(F71L) amino acid change identified in patients with age-related cataract has been shown to reduce CRYAA chaperone activity (Bhagyalaxmi et al. 2010, Bhagyalaxmi et al. 2009, Liao et al. 2014). In addition, CRYAA was one of two genes identified as associated in a meta-analysis of Asian patients with age related cataracts, the other being KCNAB1 (Liao et al. 2014). Finally, in addition to the mutation analysis described above, there is some functional support for sequence variations decreasing CRYAA expression increasing the risk of age related cataracts. Age related cataract associated SNP rs7278468 lies in a binding site for both KLF10 and Sp1 and the risk T allele increases binding of the former and decreases binding of the latter, which decreases CRYAA expression and provides a possible reason for the increased risk of cataract (Ma et al. 2016b, Zhao et al. 2017).
A number of additional loci and genes have been reported to be associated with age related cataract, although these have not been reproduced in multiple studies and populations. Evidence for one of the first loci associated with age related cataract, the null allele of the GSTM1 locus, has proved to be inconsistent (Sun et al. 2010), as has that for GSTT1 (Zuercher et al. 2010). Polymorphisms in a number of additional genes including MTHFR, PARP1,RNF149, OGG1, EFNA5, NAT2, WRN, P2RY2, ATM, MIP, B3GNT4, GJA3, OSGEP, BMP4, CYP46A1, KLC1, FTO, HSF4, TP53, ACE, MUC16, XRCC1, APOE, and ERCC2, have all been suggested to be associated with age-related cataract (Shiels et al. 2010). While it is highly likely that some of these genes do contribute to age-related cataract, additional work is required to confirm their role.
Thus, the genetic architecture of both inherited congenital and age-related cataracts reflects the developmental and cell biology of the lens. Not only does each novel mutation yield additional insight into the structural or functional biology of the affected protein, each newly identified gene provides similar insight into the lens development, function, and homeostasis. The genes currently associated with cataract are incomplete, with most estimates in the range of 40–50% even for the best characterized populations (Chen et al. 2017), and less for age related cataract, so that much additional information remains to be discovered through further genetic studies.
Hypothesis: Congenital vs. Age Related Cataract Mechanisms
Mechanisms of congenital cataract
It has been shown that alpha-crystallin interacts only with the aggregation prone molten globule state of partially denatured proteins (Rajaraman et al. 1998, Sathish et al. 2004). Thus, while denatured or partially denatured crystallins might normally be bound by α-crystallin, since mutations causing congenital cataract are expected to be particularly severe, it is possible that many or most of are not bound and solubilized by α-crystallin (Moreau & King 2012a). Alternatively, the rapidity and completeness with which the mutant crystallins denature might overwhelm the capability of α-crystallin to buffer the lens from these damaged proteins. Not only would this lead to the presence of high molecular weight aggregates that would scatter light but would also result in free potentially toxic denatured proteins that might damage the systems responsible for lens cell homeostasis and even survival.
The disarray and destruction of lens microarchitecture seen in many experimental cataracts studied by expressing mutant crystallins in model systems supports the concept that lens cells themselves might be damaged in congenital cataracts. This is demonstrated by cataracts caused by a 5-base insertion (c.119_123dup, c.238insGCGGC, p.C42Afs*63) in the CRYGC gene.(Ma et al. 2011, Ren et al. 2000, Scott et al. 1994) Unlike the p.Gly18Val CRYGS mutation, this mutation caused a variable cataract morphology extending from total to lamellar to nuclear pulverulent. The basis of this cataract is expression of an unstable hybrid protein of which the first 41 amino acids are those of CRYGC and the last 62 are random amino acids resulting from the frameshift. Expression of the mutant CRYGC in transgenic mice causes degeneration of lens fiber cells with resultant disruption of lens microarchitecture. Although the lenses initially appear normal, by about 3 weeks of age vacuolization of equatorial epithelial and superficial cortical fiber cells becomes apparent (Ma et al. 2011). This progresses to degeneration of the fiber cells resulting in large intracellular vacuoles and eventually lacunae filled with proteinaceous debris. The mutant protein is found in both the soluble and insoluble fractions of lens cells, consistent with the observation that α-crystallin binds to a molten globule like intermediate in protein unfolding (Rajaraman et al. 1998) and some severe mutations in lens crystallins escape binding by the α-crystallins (Moreau & King 2012a). Taken together histology showing death of lens fiber cells and disruption of lens architecture and biochemical characterization of mutant proteins are most consistent with a direct toxic effect of the mutant protein on the lens cells, either by escaping α-crystallin chaperone action or after saturating it.
Induction of the unfolded protein response and apoptosis
One pathway through which the mutant crystallins might directly damage lens cells is through induction of the unfolded protein response (UPR) followed by apoptosis (Ikesugi et al. 2006). The UPR is a set of adaptive intracellular signaling pathways that reduce stress on the endoplasmic reticulum (ER stress) secondary to large amounts of denatured, protein accumulation in the ER lumen. Under benign conditions, the 70kDa heat shock protein 5, HSPA5 (BiP, GRP78), binds to at least three major sensors in the endoplasmic reticulum, IRE1, ATF6, and EIF2AK3, maintaining them in an inactive state. In the presence of large amounts of unfolded protein HSPA5 dissociates from these three sensors, activating them and initiating the UPR (Schroder & Kaufman 2005). Initially, the UPR attempts to reduce ER stress by decreasing protein synthesis, upregulating levels of endoplasmic reticulum associated degradation proteins (ERAD), and increasing chaperone levels (Sovolyova et al. 2014). In the face of severe ER stress the UPR can fail to achieve homeostasis at which point it can induce apoptosis (Lai et al. 2007, Rasheva & Domingos 2009) through both the intrinsic and mitochondrial-mediated pathways (Gupta et al. 2010, Szegezdi et al. 2008) as well as EIF2AK3 regulation of gene expression. This includes activation of specific gene transcription by ATF6 (Gupta et al. 2012), including induction of XBP1 and IRE1, which cleaves XBP1 activating it and inducing transcription of a broad array of downstream mediators including P58 (Gorman et al. 2012) and DNA-damage-inducible transcript 3 (DDIT3). All of these downstream mediators tend to induce apoptosis, which could easily result in the toxic picture of lens histology seen in many types of congenital cataract.
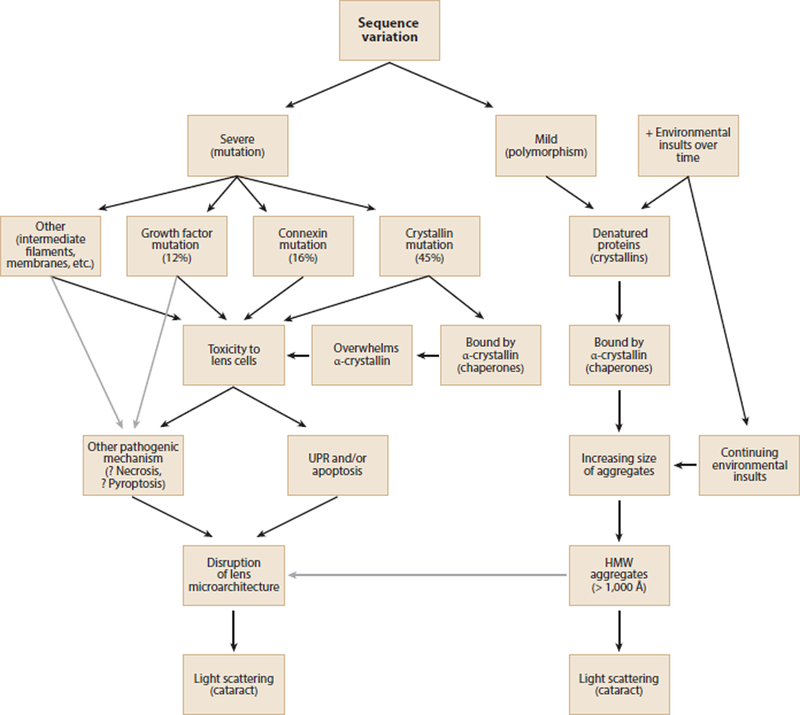
In fact, there is increasing evidence for a potential role for both the UPR and apoptosis in congenital cataract, especially in lens epithelial cells, which have a higher level of metabolic activity than fiber cells. An early example of the UPR in an animal model of cataract was its in lens epithelial cells from galactosemic rats, and cultured transformed lens epithelial cells deprived of glucose (Mulhern et al. 2006). The histologic picture in galactosemic cataracts is similar to that seen in the early stages of the p.C42Afs*63 mutant CRYGC transgenic mouse cataracts mentioned above (Ma et al. 2011) with debris filled vacuoles and cell death. In addition, the UPR also contributes to the selenite induced cataract in rats (Palsamy et al. 2014) as well as cataracts resulting from expression of abnormal collagens in transgenic mice (Firtina et al. 2009). Relating to inherited congenital cataract more directly, apoptosis and elements of the UPR are implicated in cataracts in the knock-in p.Arg49Cys CRYAA mouse (Andley & Goldman 2015) and in cataractous mice expressing p.Ser50Pro and p.Gly22Arg GJA8 mutations (Alapure et al. 2012), although the UPR was only modestly induced in the latter. The UPR and apoptosis were also shown to be induced in cultured lens cells expressing p.V114L CRYBB2 and p.116_118del CRYAA, implicated in autosomal dominant congenital cataracts (Li et al. 2017). Finally, the UPR and all pathways of apoptosis with destruction of the lens microarchitecture were shown to result from expression of a human cataract associated p.Ile33_Ala119del mutant βA3/A1-crystallin protein in transgenic mice (Ma et al. 2016c). Thus, induction of the UPR followed by apoptosis of epithelial and cortical fiber cells appears to be an excellent candidate for the mechanism of severe mutations in lens crystallins or proteins supporting homeostasis of lens cells in causing congenital cataracts. The best evidence supports a different pathogenic mechanism for age-related cataract, although in some cases they might share a final common pathway (Fig. 1).
Figure 1.
Possible pathways for inherited cataracts. Severe mutations would be more likely to cause highly penetrant Mendelian congenital cataracts, while mild changes would be likely to increase susceptibility to environmental insults and lead to multifactorial age-related cataracts. Black arrows show demonstrated pathways and gray arrows show likely pathways.
Most age-related cataracts are the end point of an extended process in which crystallins undergo slow denaturation by a combination of environmental stress, sequence variants causing decreased stability, or disruption of cellular homeostasis (Hejtmancik & Kantorow 2004). Because they are highly expressed in the lens and act like chaperones, α-crystallins, are important in delaying light scattering resulting from this process (Haslbeck et al. 2015). By binding partially denatured βγ-crystallins and holding them in a soluble state in the lens fiber cells, α-crystallins provide protection from the toxic effects of denatured and precipitated proteins (Horwitz 2003). This has been confirmed in human lenses by using dynamic light scattering, which allows measurement of the fraction of free α-crystallin and that complexed with denatured βγ-crystallins in high molecular weight complexes (Datiles et al. 2008). As individuals age, the fraction of unbound α-crystallin decreases about 6-fold in clear lenses. Even when controlled for age, free α-crystallin decreases approximately 10-fold in cataractous lenses as the AREDS nuclear opacity grade increases from 0 to 2 or more. Eventually the α-crystallin is completely consumed so that lenses with nuclear cataracts having AREDS scores greater than 2 have both HMW complexes large enough to scatter light and no remaining free α-crystallin to buffer additional damaged proteins. Thus, it seems likely that most age-related cataracts result from accumulation of HMW protein aggregates large enough to scatter light, with the lens cell architecture largely preserved. In some cases of age-related cataract, after depletion of free α-crystallin and saturation of the HMW complexes, the UPR might be activated by unbound denatured protein. This is also suggested by reports that expression of various parts of the UPR are elevated in age related cataract (Yang et al. 2015). This also would be compatible with a slow increase in opacity over years followed by an accelerated phase over weeks described clinically in some individuals.
Thus, although cataract represents a final common pathway for mutations in a variety of genes in different cellular processes, the molecular mechanisms of many inherited cataracts seem to fall into two general groups. Congenital and age-related cataracts appear to occur by distinct mechanisms, between which there is possibly some overlap. At least some congenital cataracts result from mutations causing severe insults to crystallin stability or dramatic loss of lens cell homeostasis, often with resulting activation of the UPR followed by apoptosis. In contrast, progressive or age-related cataracts proceed through a gradual but progressive denaturation of βγ-crystallins that complex with α-crystallin until this ‘buffer’ is exhausted. The aggregates increase in size until they become HMW aggregates and scatter light or actually precipitate, both resulting in cataract. Although there are exceptions such as amyloid-like fibrils that might contribute to some cataracts (Meehan et al. 2004, Xi et al. 2015), given our current state of knowledge the dual pathway concept is a reasonable hypothesis at this time.
Therapy
Despite the wide availability of highly efficacious surgical treatment, age-related cataract is still a leading cause of low vision and blindness worldwide (Pascolini & Mariotti 2012). With continued aging of populations across the world the resultant increase in age-related cataract is projected to pose a major stress on surgical intraocular lens delivery (Rao et al. 2011, Taylor 2000), and this has prompted the search for non-surgical means to delay, prevent, or reverse cataract formation (Moreau & King 2012b, Toh et al. 2007). Classically, dietary adjustment coupled with avoidance of environmental stress such as UV light, smoking, and hyperglycemia have been suggested to be helpful in preventing or delaying age related cataract, and an inverse association between plasma vitamin C and age-related cataract was shown in India, a population with low vitamin C levels (Ravindran et al. 2011). However, the results of dietary vitamin supplementation in humans have been mixed (Toh et al. 2007), as have oral aspirin, aldose reductase inhibitors and topical N-acetylcarnosine therapy (Abdelkader et al. 2015).
Implication of mutations in LSS, encoding lanosterol synthase, an enzyme in the cholesterol biosynthesis pathway in recessive congenital cataract prompted examination of its role in stabilizing mutant crystallins and suggested a possible topical eye-drop therapy for cataract (Zhao et al. 2015). Because it is soluble in both aqueous and lipids (amphiphatic) and present at high levels in the lens, lanosterol was tested for its ability to solubilize aggregates of mutant and wild-type crystallins associated with inherited and age-related forms of cataract. Not only did lanosterol treatment reverse crystallin aggregation and solubilize amyloid-like fibril formed by denatured crystallins in vitro and in transfected cells but it also improved transparency of rabbit and dog lenses with naturally occurring cataract. Physico-chemical analysis has shown that lanosterol disrupts aggregation of γD-crystallin by binding to the hydrophobic regions? (Kang et al. 2018). However, lanosterol failed to reverse opacities in human lenses removed for age-related cataracts (Shanmugam et al. 2015), although this might relate to the length of time the human cataracts had been present before surgery. Another promising development is the demonstration that a group of pharmacological chaperones can reverse aggregation of mutant αA- and αB-crystallins in vitro as well as in isolated mouse and human lenses (Makley et al. 2015). While these preliminary results with lanosterol and other chaperone-like therapies are promising, much work remains to be done before they are proven clinically, although lanosterol eye drops are currently available for treatment of canine cataracts.
While small molecules that act as chaperones to inhibit protein misfolding and aggregation might show promise for cataract resulting from the accumulation of HMW aggregates that scatter light in intact lens cells, they would not be expected to treat congenital cataracts in which the lens microarchitecture itself is damaged or destroyed. These would require surgical intervention with placement of an intraocular lens within the first few months of life, which fortunately is highly efficacious and widely available. In addition, promising results have been reported using lens regeneration approaches in rabbits, macaques, and human infants (Lin et al. 2016b). While this approach is highly experimental, if proved reliable it promises to preserve refractive and accommodative abilities and greater visual axis transparency than current approaches. Taken together with the small molecule chaperone approach, this progress provides new paradigms in the prevention and therapy of both congenital and age-related cataract.
References
- Needs author Framingham Offspring Eye Study 1994. Familial aggregation of lens opacities: the Framingham Eye Study and the Framingham Offspring Eye Study. American Journal of Epidemiology 140: 555–64 [PubMed] [Google Scholar]
- Needs author 2005. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill [Google Scholar]
- Abdelkader H, Alany RG, Pierscionek B. 2015. Age-related cataract and drug therapy: opportunities and challenges for topical antioxidant delivery to the lens. J Pharm Pharmacol 67: 537–50 [DOI] [PubMed] [Google Scholar]
- Abe T, Furui S, Sasaki H, Sakamoto Y, Suzuki S, et al. 2012. Quantitative evaluation of light scattering intensities of the crystalline lens for radiation related minimal change in interventional radiologists: a cross-sectional pilot study. J Radiat Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alapure BV, Stull JK, Firtina Z, Duncan MK. 2012. The unfolded protein response is activated in connexin 50 mutant mouse lenses. Exp Eye Res 102: 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldahmesh MA, Khan AO, Mohamed J, Alkuraya FS. 2011. Novel recessive BFSP2 and PITX3 mutations: insights into mutational mechanisms from consanguineous populations. Genet Med 13: 978–81 [DOI] [PubMed] [Google Scholar]
- Aldahmesh MA, Khan AO, Mohamed JY, Hijazi H, Al-Owain M, et al. 2012. Genomic analysis of pediatric cataract in Saudi Arabia reveals novel candidate disease genes. Genet Med 14: 955–62 [DOI] [PubMed] [Google Scholar]
- Aldhamesh MA, Khan AO, Mohamed JY, Alghamdi MH, Alkuraya FS. 2012. Identification of a truncation mutation of the acylglycerol kinase (AGK) gene in a novel autosomal recessive cataract locus. Hum Mutat [DOI] [PubMed] [Google Scholar]
- Amaya L, Taylor D, Russell-Eggitt I, Nischal KK, Lengyel D. 2003. The morphology and natural history of childhood cataracts. Surv.Ophthalmol 48: 125–44 [DOI] [PubMed] [Google Scholar]
- Andley UP, Goldman JW. 2015. Autophagy and UPR in alpha-crystallin mutant knock-in mouse models of hereditary cataracts. Biochim Biophys Acta 1860: 234–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar M, Chung HL, Taylor RL, Nazir A, Imtiaz S, et al. 2018. Bi-allelic Loss-of-Function Variants in DNMBP Cause Infantile Cataracts. Am J Hum Genet 103: 568–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont C, Leneuve P, Devaux I, Scoazec JY, Berthier M, et al. 1995. Mutation in the iron responsive element of the L ferritin mRNA in a family with dominant hyperferritinaemia and cataract. Nat.Genet 11: 444–46 [DOI] [PubMed] [Google Scholar]
- Benedek GB. 1971. Theory of transparency of the eye. Applied Optics 10: 459–73 [DOI] [PubMed] [Google Scholar]
- Benito A, Hervella L, Tabernero J, Pennos A, Ginis H, et al. 2016. Environmental and Genetic Factors Explain Differences in Intraocular Scattering. Invest Ophthalmol Vis Sci 57: 163–8 [DOI] [PubMed] [Google Scholar]
- Berry V, Francis P, Reddy MA, Collyer D, Vithana E, et al. 2001. Alpha-B Crystallin Gene (CRYAB) Mutation Causes Dominant Congenital Posterior Polar Cataract in Humans. American Journal of Human Genetics 69: 1141–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud VM, Minogue PJ, Guo J, Williamson EK, Xu X, et al. 2003. Loss of function and impaired degradation of a cataract-associated mutant connexin50. Eur.J.Cell Biol 82: 209–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagyalaxmi SG, Padma T, Reddy GB, Reddy KR. 2010. Association of G>A transition in exon-1 of alpha crystallin gene in age-related cataracts. Oman J Ophthalmol 3: 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagyalaxmi SG, Srinivas P, Barton KA, Kumar KR, Vidyavathi M, et al. 2009. A novel mutation (F71L) in alphaA-Crystallin with defective chaperone-like function associated with age-related cataract. Biochimica et Biophysica Acta 1792: 974–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland D, Duglas-Tabor Y, Robison WG Jr., Groome A, Wawrousek EF. 1997. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc.Natl.Acad.Sci.U.S.A 94: 884–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. 2001. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol.Vis.Sci 42: 2924–34 [PubMed] [Google Scholar]
- Brennan LA, Kantorow WL, Chauss D, McGreal R, He S, et al. 2012. Spatial expression patterns of autophagy genes in the eye lens and induction of autophagy in lens cells. Mol Vis 18: 1773–86 [PMC free article] [PubMed] [Google Scholar]
- Brennan LA, McGreal-Estrada R, Logan CM, Cvekl A, Menko AS, Kantorow M. 2018. BNIP3L/NIX is required for elimination of mitochondria, endoplasmic reticulum and Golgi apparatus during eye lens organelle-free zone formation. Exp Eye Res 174: 173–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NA, Hill AR. 1987. Cataract: the relation between myopia and cataract morphology. Br J Ophthalmol 71: 405–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon KP, McKay JD, Sale MM, Russell-Eggitt IM, Mackey DA, et al. 2003. Mutations in a novel gene, NHS, cause the pleiotropic effects of nance-horan syndrome, including severe congenital cataract, dental anomalies, and mental retardation. American Journal of Human Genetics 73: 1120–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorino JJ, Gallagher-Colombo SM, Levin AV, Fitzgerald PG, Polishook J, et al. 2011. Juvenile cataract-associated mutation of solute carrier SLC16A12 impairs trafficking of the protein to the plasma membrane. Invest Ophthalmol Vis Sci 52: 6774–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JR, Koo E, Agron E, Hallak J, Clemons T, et al. 2011. Risk factors associated with incident cataracts and cataract surgery in the Age-related Eye Disease Study (AREDS): AREDS report number 32. Ophthalmology 118: 2113–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, et al. 2011. Mutations in FYCO1 Cause Autosomal-Recessive Congenital Cataracts. Am J Hum Genet 88: 827–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang Q, Cabrera PE, Zhong Z, Sun W, et al. 2017. Molecular Genetic Analysis of Pakistani Families With Autosomal Recessive Congenital Cataracts by Homozygosity Screening. Invest Ophthalmol Vis Sci 58: 2207–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Huang C, Zhang B, Yin S, Liang J, et al. 2016. Mutations of RagA GTPase in mTORC1 Pathway Are Associated with Autosomal Dominant Cataracts. PLoS Genet 12: e1006090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Dai Y, Wu X, Wang Y, Sun S, et al. 2014. Mutations in the ABCA3 gene are associated with cataract-microcornea syndrome. Invest Ophthalmol Vis Sci 55: 8031–43 [DOI] [PubMed] [Google Scholar]
- Cohen D, Bar-Yosef U, Levy J, Gradstein L, Belfair N, et al. 2007. Homozygous CRYBB1 deletion mutation underlies autosomal recessive congenital cataract. Invest Ophthalmol.Vis.Sci 48: 2208–13 [DOI] [PubMed] [Google Scholar]
- Congdon NG, Friedman DS, Lietman T. 2003. Important causes of visual impairment in the world today. Journal of the American Medical Association 290: 2057–60 [DOI] [PubMed] [Google Scholar]
- Conley YP, Erturk D, Keverline A, Mah TS, Keravala A, et al. 2000. A juvenile-onset, progressive cataract locus on chromosome 3q21-q22 is associated with a missense mutation in the beaded filament structural protein-2. Am.J.Hum Genet 66: 1426–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello MJ, Brennan LA, Basu S, Chauss D, Mohamed A, et al. 2013. Autophagy and mitophagy participate in ocular lens organelle degradation. Exp Eye Res 116: 141–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datiles MB III, Ansari RR, Suh KI, Vitale S, Reed GF, et al. 2008. Clinical detection of precataractous lens protein changes using dynamic light scattering. Archives of Ophthalmology 126: 1687–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes LJ, Shelley EJ, McAvoy JW, Lovicu FJ. 2018. A role for Hippo/YAP-signaling in FGF-induced lens epithelial cell proliferation and fibre differentiation. Exp Eye Res 169: 122–33 [DOI] [PubMed] [Google Scholar]
- Delaye M, Tardieu A. 1983. Short-range order of crystallin proteins accounts for eye lens transparency. Nature 302: 415–17 [DOI] [PubMed] [Google Scholar]
- Eiberg H, Marner E, Rosenberg T, Mohr J. 1988. Marner’s cataract (CAM) assigned to chromosome 16: linkage to haptoglobin. Clinical Genetics 34: 272–75 [DOI] [PubMed] [Google Scholar]
- Firtina Z, Danysh BP, Bai X, Gould DB, Kobayashi T, Duncan MK. 2009. Abnormal expression of collagen IV in lens activates unfolded protein response resulting in cataract. J Biol Chem 284: 35872–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forshew T, Johnson CA, Khaliq S, Pasha S, Willis C, et al. 2005. Locus heterogeneity in autosomal recessive congenital cataracts: linkage to 9q and germline HSF4 mutations. Human Genetics 117: 452–59 [DOI] [PubMed] [Google Scholar]
- Foster PJ, Wong TY, Machin D, Johnson GJ, Seah SK. 2003. Risk factors for nuclear, cortical and posterior subcapsular cataracts in the Chinese population of Singapore: the Tanjong Pagar Survey. Br.J.Ophthalmol 87: 1112–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis P, Chung JJ, Yasui M, Berry V, Moore A, et al. 2000. Functional impairment of lens aquaporin in two families with dominantly inherited cataracts. Hum.Mol.Genet 9: 2329–34 [DOI] [PubMed] [Google Scholar]
- Francois J 1982. Genetics of cataract. Ophthalmologica 184: 61–71 [DOI] [PubMed] [Google Scholar]
- Ganea E, Harding JJ. 2006. Glutathione-related enzymes and the eye. Current Eye Research 31: 1–11 [DOI] [PubMed] [Google Scholar]
- Gao J, Minogue PJ, Beyer EC, Mathias RT, Berthoud VM. 2018. Disruption of the lens circulation causes calcium accumulation and precipitates in connexin mutant mice. Am J Physiol Cell Physiol 314: C492–C503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C, Foster A. 2001. Childhood blindness in the context of VISION 2020--the right to sight. Bull World Health Organ 79: 227–32 [PMC free article] [PubMed] [Google Scholar]
- Girelli D, Corrocher R, Bisceglia L, Olivieri O, De Franceschi L, et al. 1995. Molecular basis for the recently described hereditary hyperferritinemia-cataract syndrome: a mutation in the iron-responsive element of ferritin L-subunit gene (the “Verona mutation”). Blood 86: 4050–3 [PubMed] [Google Scholar]
- Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. 1994. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nature Genetics 7: 463–71 [DOI] [PubMed] [Google Scholar]
- Gong X, Cheng C, Xia CH. 2007. Connexins in lens development and cataractogenesis. J.Membr.Biol 218: 9–12 [DOI] [PubMed] [Google Scholar]
- Gorman AM, Healy SJ, Jager R, Samali A. 2012. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol Ther 134: 306–16 [DOI] [PubMed] [Google Scholar]
- Group TI-ACS. 1991. Risk factors for age-related cortical, nuclear, and posterior subcapsular cataracts. The Italian-American Cataract Study Group. Am J Epidemiol 133: 541–53 [PubMed] [Google Scholar]
- Gupta S, Cuffe L, Szegezdi E, Logue SE, Neary C, et al. 2010. Mechanisms of ER Stress-Mediated Mitochondrial Membrane Permeabilization. Int J Cell Biol 2010: 170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Read DE, Deepti A, Cawley K, Gupta A, et al. 2012. Perk-dependent repression of miR-106b-25 cluster is required for ER stress-induced apoptosis. Cell Death Dis 3: e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haargaard B, Wohlfahrt J, Fledelius HC, Rosenberg T, Melbye M. 2004. Incidence and cumulative risk of childhood cataract in a cohort of 2.6 million Danish children. Invest Ophthalmol Vis Sci 45: 1316–20 [DOI] [PubMed] [Google Scholar]
- Haargaard B, Wohlfahrt J, Rosenberg T, Fledelius HC, Melbye M. 2005. Risk factors for idiopathic congenital/infantile cataract. Invest Ophthalmol Vis Sci 46: 3067–73 [DOI] [PubMed] [Google Scholar]
- Hammond CJ, Duncan DD, Snieder H, de Lange M, West SK, et al. 2001. The heritability of age-related cortical cataract: the twin eye study. Invest Ophthalmol.Vis.Sci 42: 601–05 [PubMed] [Google Scholar]
- Hammond CJ, Snieder H, Spector TD, Gilbert CE. 2000. Genetic and environmental factors in age-related nuclear cataracts in monozygotic and dizygotic twins. New England Journal of Medicine 342: 1786–90 [DOI] [PubMed] [Google Scholar]
- Hansen L, Comyn S, Mang Y, Lind-Thomsen A, Myhre L, et al. 2014. The myosin chaperone UNC45B is involved in lens development and autosomal dominant juvenile cataract. Eur J Hum Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck M, Peschek J, Buchner J, Weinkauf S. 2015. Structure and function of alpha-crystallins: Traversing from in vitro to in vivo. Biochim Biophys Acta 1860: 149–66 [DOI] [PubMed] [Google Scholar]
- Heiba IM, Elston RC, Klein BE, Klein R. 1993. Genetic etiology of nuclear cataract: evidence for a major gene. Am.J.Med.Genet 47: 1208–14 [DOI] [PubMed] [Google Scholar]
- Heiba IM, Elston RC, Klein BE, Klein R. 1995. Evidence for a major gene for cortical cataract. Invest Ophthalmol.Vis.Sci 36: 227–35 [PubMed] [Google Scholar]
- Hejtmancik JF, Kaiser-Kupfer MI, Piatigorsky J. 2001. Molecular biology and inherited disorders of the eye lens In The Metabolic and Molecular Basis of Inherited Disease, ed. Scriver CR, Beaudet AL, Valle D, Sly WS, Childs B, et al. , pp. 6033–62. New York: McGraw Hill [Google Scholar]
- Hejtmancik JF, Kantorow M. 2004. Molecular genetics of age-related cataract. Experimental Eye Research 79: 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejtmancik JF, Smaoui N. 2003. Molecular Genetics of Cataract In Genetics in Ophthalmology, ed. B Wissinger, Kohl S, Langenbeck U, pp. 67–82. Basel: S.Karger; [DOI] [PubMed] [Google Scholar]
- Hennis A, Wu SY, Nemesure B, Leske MC. 2004. Risk factors for incident cortical and posterior subcapsular lens opacities in the Barbados Eye Studies. Archives of Ophthalmology 122: 525–30 [DOI] [PubMed] [Google Scholar]
- Horwitz J 2003. Alpha-crystallin. Experimental Eye Research 76: 145–53 [DOI] [PubMed] [Google Scholar]
- Ikesugi K, Yamamoto R, Mulhern ML, Shinohara T. 2006. Role of the unfolded protein response (UPR) in cataract formation. Exp Eye Res 83: 508–16 [DOI] [PubMed] [Google Scholar]
- Iyengar SK, Klein BE, Klein R, Jun G, Schick JH, et al. 2004. Identification of a major locus for age-related cortical cataract on chromosome 6p12-q12 in the Beaver Dam Eye Study. Proc.Natl.Acad.Sci.U.S.A 101: 14485–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R, Slingsby C. 2001. Lens crystallins and their microbial homologs: structure, stability, and function. Crit Rev.Biochem.Mol.Biol 36: 435–99 [DOI] [PubMed] [Google Scholar]
- Jakobs PM, Hess JF, FitzGerald PG, Kramer P, Weleber RG, Litt M. 2000. Autosomal-dominant congenital cataract associated with a deletion mutation in the human beaded filament protein gene BFSP2. Am.J.Hum Genet 66: 1432–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson RV, Farrar N, Stewart K, Perveen R, Mihelec M, et al. 2007. Characterization of a familial t(16;22) balanced translocation associated with congenital cataract leads to identification of a novel gene, TMEM114, expressed in the lens and disrupted by the translocation. Hum.Mutat 28: 968–77 [DOI] [PubMed] [Google Scholar]
- Jiao X, Khan SY, Irum B, Khan AO, Wang Q, et al. 2015. Missense Mutations in CRYAB Are Liable for Recessive Congenital Cataracts. PLoS One 10: e0137973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun G, Guo H, Klein BE, Klein R, Wang JJ, et al. 2009. EPHA2 is associated with age-related cortical cataract in mice and humans. PLoS.Genet 5: e1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. 2001. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes in Development 15: 1272–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Yang Z, Zhou R. 2018. Lanosterol Disrupts Aggregation of Human gammaD-Crystallin by Binding to the Hydrophobic Dimerization Interface. J Am Chem Soc 140: 8479–86 [DOI] [PubMed] [Google Scholar]
- Kanthan GL, Mitchell P, Burlutsky G, Wang JJ. 2010. Alcohol consumption and the long-term incidence of cataract and cataract surgery: the Blue Mountains Eye Study. Am J Ophthalmol 150: 434–40 e1 [DOI] [PubMed] [Google Scholar]
- Kantorow M, Hawse JR, Cowell TL, Benhamed S, Pizarro GO, et al. 2004. Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress. Proc.Natl.Acad.Sci.U.S.A 101: 9654–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul H, Riazuddin SA, Shahid M, Kousar S, Butt NH, et al. 2010. Autosomal recessive congenital cataract linked to EPHA2 in a consanguineous Pakistani family. Molecular Vison 16: 511–17 [PMC free article] [PubMed] [Google Scholar]
- Khan AO, Aldahmesh MA, Meyer B. 2007. Recessive Congenital Total Cataract with Microcornea and Heterozygote Carrier Signs Caused by a Novel Missense CRYAA Mutation (R54C). Am.J.Ophthalmol [DOI] [PubMed] [Google Scholar]
- Khan AO, Aldahmesh MA, Mohamed JY, Alkuraya FS. 2012. Clinical and molecular analysis of children with central pulverulent cataract from the Arabian Peninsula. Br J Ophthalmol 96: 650–5 [DOI] [PubMed] [Google Scholar]
- Kingsley CN, Brubaker WD, Markovic S, Diehl A, Brindley AJ, et al. 2013. Preferential and specific binding of human alphaB-crystallin to a cataract-related variant of gammaS-crystallin. Structure 21: 2221–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita JH. 1965. Cataracts in galactosemia: the Jonas Friedenwald Memorial Lecture. Invest.Ophthalmol 4: 786–99 [PubMed] [Google Scholar]
- Klein BE, Klein R, Lee KE, Moore EL, Danforth L. 2001. Risk of incident age-related eye diseases in people with an affected sibling: The Beaver Dam Eye Study. Am.J Epidemiol 154: 207–11 [DOI] [PubMed] [Google Scholar]
- Kloeckener-Gruissem B, Vandekerckhove K, Nurnberg G, Neidhardt J, Zeitz C, et al. 2008. Mutation of Solute Carrier SLC16A12 Associates with a Syndrome Combining Juvenile Cataract with Microcornea and Renal Glucosuria. American Journal of Human Genetics [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmoch S, Brynda J, Asfaw B, Bezouska K, Novak P, et al. 2000. Link between a novel human gammaD-crystallin allele and a unique cataract phenotype explained by protein crystallography. Hum Mol.Genet 9: 1779–86 [DOI] [PubMed] [Google Scholar]
- Kumar M, Kaur P, Khokhar S, Dada R. 2013. Molecular and structural analysis of genetic variations in congenital cataract. Mol Vis 19: 2436–50 [PMC free article] [PubMed] [Google Scholar]
- Kupfer C 1984. The Bowman Lecture: The Conquest of cataract: A Global challenge. Survey of Ophthalmology 30: 271. [PubMed] [Google Scholar]
- Lachke SA, Alkuraya FS, Kneeland SC, Ohn T, Aboukhalil A, et al. 2011. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science 331: 1571–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E, Teodoro T, Volchuk A. 2007. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 22: 193–201 [DOI] [PubMed] [Google Scholar]
- Lee AY, Chung SK, Chung SS. 1995. Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc.Natl.Acad.Sci.U.S.A 92: 2780–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Wang Y, Ko GT, Ma RC, Critchley JA, et al. 2001. Risk factors for cataract in Chinese patients with type 2 diabetes: evidence for the influence of the aldose reductase gene. Clinical Genetics 59: 356–59 [DOI] [PubMed] [Google Scholar]
- Leske MC, Chylack LT Jr., He Q, Wu SY, Schoenfeld E, et al. 1998. Risk factors for nuclear opalescence in a longitudinal study. LSC Group. Longitudinal Study of Cataract. American Journal of Epidemiology 147: 36–41 [DOI] [PubMed] [Google Scholar]
- Leske MC, Chylack LT Jr., Wu SY. 1991. The Lens Opacities Case-Control Study. Risk factors for cataract. Archives of Ophthalmology 109: 244–51 [DOI] [PubMed] [Google Scholar]
- Li D, Wang S, Ye H, Tang Y, Qiu X, et al. 2016. Distribution of gene mutations in sporadic congenital cataract in a Han Chinese population. Mol Vis 22: 589–98 [PMC free article] [PubMed] [Google Scholar]
- Li L, Fan DB, Zhao YT, Li Y, Kong DQ, et al. 2017. Two novel mutations identified in ADCC families impair crystallin protein distribution and induce apoptosis in human lens epithelial cells. Sci Rep 7: 17848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Su X, Chen P, Wang X, Xu L, et al. 2014. Meta-analysis of genome-wide association studies in multiethnic Asians identifies two loci for age-related nuclear cataract. Hum Mol Genet 23: 6119–28 [DOI] [PubMed] [Google Scholar]
- Lin H, Lin D, Liu Z, Long E, Wu X, et al. 2016a. A Novel Congenital Cataract Category System Based on Lens Opacity Locations and Relevant Anterior Segment Characteristics. Invest Ophthalmol Vis Sci 57: 6389–95 [DOI] [PubMed] [Google Scholar]
- Lin H, Ouyang H, Zhu J, Huang S, Liu Z, et al. 2016b. Lens regeneration using endogenous stem cells with gain of visual function. Nature 531: 323–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetsky M, Shipova E, Cheng R, Ortwerth BJ. 2008. Glycation by ascorbic acid oxidation products leads to the aggregation of lens proteins. Biochimica et Biophysica Acta 1782: 22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZQ, Sun WH, Yan J, Jiang TX, Zhai SN, Li Y. 2012. Cigarette smoking, body mass index associated with the risks of age-related cataract in male patients in northeast China. Int J Ophthalmol 5: 317–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma AS, Grigg JR, Ho G, Prokudin I, Farnsworth E, et al. 2016a. Sporadic and Familial Congenital Cataracts: Mutational Spectrum and New Diagnoses Using Next-Generation Sequencing. Hum Mutat 37: 371–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Jiao X, Ma Z, Hejtmancik JF. 2016b. Polymorphism rs7278468 is associated with Age-related cataract through decreasing transcriptional activity of the CRYAA promoter. Sci Rep 6: 23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Ma Z, Jiao X, Hejtmancik JF. 2017. Functional non-coding polymorphism in an EPHA2 promoter PAX2 binding site modifies expression and alters the MAPK and AKT pathways. Sci Rep 7: 9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Piszczek G, Wingfield PT, Sergeev YV, Hejtmancik JF. 2009. The G18V CRYGS Mutation Associated with Human Cataracts Increases γS-Crystallin Sensitivity to Thermal and Chemical Stress. Biochemistry 48: 7334–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Yao W, Chan CC, Kannabiran C, Wawrousek E, Hejtmancik JF. 2016c. Human betaA3/A1-crystallin splicing mutation causes cataracts by activating the unfolded protein response and inducing apoptosis in differentiating lens fiber cells. Biochim Biophys Acta 1862: 1214–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Yao W, Theendakara V, Chan CC, Wawrousek E, Hejtmancik JF. 2011. Overexpression of human γC-crystallin 5bp duplication Disrupts Lens Morphology in Transgenic Mice. Invest Ophthalmol Vis.Sci 52: 5269–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machan CM, Hrynchak PK, Irving EL. 2012. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci 89: 1165–71 [DOI] [PubMed] [Google Scholar]
- Makley LN, McMenimen KA, DeVree BT, Goldman JW, McGlasson BN, et al. 2015. Pharmacological chaperone for alpha-crystallin partially restores transparency in cataract models. Science 350: 674–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty CA, Taylor HR. 2001. The genetics of cataract. Invest Ophthalmol.Vis.Sci 42: 1677–78 [PubMed] [Google Scholar]
- Meehan S, Berry Y, Luisi B, Dobson CM, Carver JA, MacPhee CE. 2004. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. Journal of Biological Chemistry 279: 3413–19 [DOI] [PubMed] [Google Scholar]
- Merin S 1991. Inherited Cataracts In Inherited Eye Diseases, ed. Merin S, pp. 86–120. New York: Marcel Dekker,Inc. [Google Scholar]
- Merin S, Crawford JS. 1971. The etiology of congenital cataracts. A survey of 386 cases. Canadian Journal of Ophthalmology 6: 178–82 [PubMed] [Google Scholar]
- Minogue PJ, Liu X, Ebihara L, Beyer EC, Berthoud VM. 2005. An aberrant sequence in a connexin46 mutant underlies congenital cataracts. Journal of Biological Chemistry 280: 40788–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, King JA. 2012a. Cataract-causing defect of a mutant gamma-crystallin proceeds through an aggregation pathway which bypasses recognition by the alpha-crystallin chaperone. PLoS One 7: e37256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, King JA. 2012b. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med 18: 273–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern ML, Madson CJ, Danford A, Ikesugi K, Kador PF, Shinohara T. 2006. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Invest Ophthalmol Vis Sci 47: 3951–9 [DOI] [PubMed] [Google Scholar]
- Okano Y, Asada M, Fujimoto A, Ohtake A, Murayama K, et al. 2001. A genetic factor for age-related cataract: identification and characterization of a novel galactokinase variant, “Osaka,” in Asians. American Journal of Human Genetics 68: 1036–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottonello S, Foroni C, Carta A, Petrucco S, Maraini G. 2000. Oxidative stress and age-related cataract. Ophthalmologica 214: 78–85 [DOI] [PubMed] [Google Scholar]
- Pal JD, Liu X, Mackay D, Shiels A, Berthoud VM, et al. 2000. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am.J.Physiol Cell Physiol 279: C596–C602 [DOI] [PubMed] [Google Scholar]
- Palsamy P, Bidasee KR, Shinohara T. 2014. Selenite cataracts: activation of endoplasmic reticulum stress and loss of Nrf2/Keap1-dependent stress protection. Biochim Biophys Acta 1842: 1794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande A, Pande J, Asherie N, Lomakin A, Ogun O, et al. 2001. Crystal cataracts: human genetic cataract caused by protein crystallization. Proc.Natl.Acad.Sci.U.S.A 98: 6116–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande A, Pande J, Asherie N, Lomakin A, Ogun O, et al. 2000. Molecular basis of a progressive juvenile-onset hereditary cataract. Proc.Natl.Acad.Sci.U.S.A 97: 1993–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Choi NK. 2017. Serum 25-hydroxyvitamin D and Age-Related Cataract. Ophthalmic Epidemiol 24: 281–86 [DOI] [PubMed] [Google Scholar]
- Pascolini D, Mariotti SP. 2012. Global estimates of visual impairment: 2010. Br J Ophthalmol 96: 614–8 [DOI] [PubMed] [Google Scholar]
- Pras E, Frydman M, Levy-Nissenbaum E, Bakhan T, Raz J, et al. 2000. A nonsense mutation (W9X) in CRYAA causes autosomal recessive cataract in an inbred Jewish Persian family. Invest Ophthalmol.Vis.Sci 41: 3511–15 [PubMed] [Google Scholar]
- Pras E, Levy-Nissenbaum E, Bakhan T, Lahat H, Assia E, et al. 2002. A Missense Mutation in the LIM2 Gene Is Associated with Autosomal Recessive Presenile Cataract in an Inbred Iraqi Jewish Family. American Journal of Human Genetics 70: 1363–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaraman K, Raman B, Ramakrishna T, Rao CM. 1998. The chaperone-like alpha-crystallin forms a complex only with the aggregation-prone molten globule state of alpha-lactalbumin. Biochem Biophys Res Commun 249: 917–21 [DOI] [PubMed] [Google Scholar]
- Ramachandran RD, Perumalsamy V, Hejtmancik JF. 2007. Autosomal recessive juvenile onset cataract associated with mutation in BFSP1. Human Genetics: 475–82 [DOI] [PubMed] [Google Scholar]
- Rao GN, Khanna R, Payal A. 2011. The global burden of cataract. Curr Opin Ophthalmol 22: 4–9 [DOI] [PubMed] [Google Scholar]
- Rao PV, Huang QL, Horwitz J, Zigler JS Jr., 1995. Evidence that alpha-crystallin prevents non-specific protein aggregation in the intact eye lens. Biochimica et Biophysica Acta 1245: 439–47 [DOI] [PubMed] [Google Scholar]
- Rasheva VI, Domingos PM. 2009. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 14: 996–1007 [DOI] [PubMed] [Google Scholar]
- Ravindran RD, Vashist P, Gupta SK, Young IS, Maraini G, et al. 2011. Inverse association of vitamin C with cataract in older people in India. Ophthalmology 118: 1958–65 e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Li A, Shastry BS, Padma T, Ayyagari R, et al. 2000. A 5-base insertion in the γC-crystallin gene is associated with autosomal dominant variable zonular pulverulent cataract. Hum Genet 106: 531–37 [DOI] [PubMed] [Google Scholar]
- Riazuddin SA, Yasmeen A, Yao W, Sergeev YV, Zhang Q, et al. 2005. Mutations in βB3-Crystallin Associated with Autosomal Recessive Cataract in Two Pakistani Families. Invest Ophthalmol.Vis.Sci 46: 2100–06 [DOI] [PubMed] [Google Scholar]
- Safieh LA, Khan AO, Alkuraya FS. 2009. Identification of a novel CRYAB mutation associated with autosomal recessive juvenile cataract in a Saudi family. Molecular Vison 15: 980–84 [PMC free article] [PubMed] [Google Scholar]
- Sathish HA, Koteiche HA, McHaourab HS. 2004. Binding of destabilized betaB2-crystallin mutants to alpha-crystallin: the role of a folding intermediate. J Biol Chem 279: 16425–32 [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. 2005. The mammalian unfolded protein response. Annu.Rev.Biochem 74: 739–89 [DOI] [PubMed] [Google Scholar]
- Scott MH, Hejtmancik JF, Wozencraft LA, Reuter LM, Parks MM, Kaiser-Kupfer MI. 1994. Autosomal dominant congenital cataract: Interocular phenotypic heterogeneity. Ophthalmology 101: 866–71 [DOI] [PubMed] [Google Scholar]
- Shanmugam PM, Barigali A, Kadaskar J, Borgohain S, Mishra DK, et al. 2015. Effect of lanosterol on human cataract nucleus. Indian J Ophthalmol 63: 888–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KK, Santhoshkumar P. 2009. Lens aging: effects of crystallins. Biochim Biophys Acta 1790: 1095–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Hejtmancik JF. 2010. Cat-Map: putting cataract on the map. Molecular Vison 16: 2007–15 [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Knopf HL, Maraini G, Li A, et al. 2008. The EPHA2 gene is associated with cataracts linked to chromosome 1p. Molecular Vison 14: 2042–55 [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Knopf HL, Yamada K, Yoshiura K, et al. 2007. CHMP4B, a Novel Gene for Autosomal Dominant Cataracts Linked to Chromosome 20q. American Journal of Human Genetics 81: 596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Hejtmancik JF. 2017. Mutations and mechanisms in congenital and age-related cataracts. Exp Eye Res 156: 95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka HW, Prchal JT. 1980. Presenile cataract formation and decreased activity of galactosemic enzymes. Archives of Ophthalmology 98: 269–73 [DOI] [PubMed] [Google Scholar]
- Smaoui N, Beltaief O, Benhamed S, M’Rad R, Maazoul F, et al. 2004. A Homozygous Splice Mutation in the HSF4 Gene Is Associated with an Autosomal Recessive Congenital Cataract. Invest Ophthalmol.Vis.Sci 45: 2716–21 [DOI] [PubMed] [Google Scholar]
- Somasundaram T, Bhat SP. 2004. Developmentally dictated expression of heat shock factors: exclusive expression of HSF4 in the postnatal lens and its specific interaction with alphaB-crystallin heat shock promoter. Journal of Biological Chemistry 279: 44497–503 [DOI] [PubMed] [Google Scholar]
- Sovolyova N, Healy S, Samali A, Logue SE. 2014. Stressed to death - mechanisms of ER stress-induced cell death. Biol Chem 395: 1–13 [DOI] [PubMed] [Google Scholar]
- Staubli A, Capatina N, Fuhrer Y, Munier FL, Labs S, et al. 2017. Abnormal creatine transport of mutations in monocarboxylate transporter 12 (MCT12) found in patients with age-related cataract can be partially rescued by exogenous chaperone CD147. Hum Mol Genet 26: 4203–14 [DOI] [PubMed] [Google Scholar]
- Stevens RE, Datiles MB, Srivastava SK, Ansari NH, Maumenee AE, Stark WJ. 1989. Idiopathic presenile cataract formation and galactosaemia. Br.J.Ophthalmol 73: 48–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll C, Alembik Y, Dott B, Roth MP. 1992. Epidemiology of congenital eye malformations in 131,760 consecutive births. Ophthalmic Paediatr Genet 13: 179–86 [DOI] [PubMed] [Google Scholar]
- Sun H, Ma Z, Li Y, Liu B, Li Z, et al. 2005. Gamma-S crystallin gene (CRYGS) mutation causes dominant progressive cortical cataract in humans. J.Med.Genet 42: 706–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Xi B, Yu L, Gao XC, Shi DJ, et al. 2010. Association of glutathione S-transferases polymorphisms (GSTM1 and GSTT1) with senile cataract: a meta-analysis. Invest Ophthalmol Vis Sci 51: 6381–6 [DOI] [PubMed] [Google Scholar]
- Sundaresan P, Ravindran RD, Vashist P, Shanker A, Nitsch D, et al. 2012. EPHA2 Polymorphisms and Age-Related Cataract in India. PLoS One 7: e33001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, Herbert KR, Kavanagh ET, Samali A, Gorman AM. 2008. Nerve growth factor blocks thapsigargin-induced apoptosis at the level of the mitochondrion via regulation of Bim. J Cell Mol Med 12: 2482–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Hou S, Jiang Z, Hu Z, Yang P, Ye J. 2011. Association of EPHA2 polymorphisms and age-related cortical cataract in a Han Chinese population. Mol Vis 17: 1553–8 [PMC free article] [PubMed] [Google Scholar]
- Taylor HR. 2000. Cataract: how much surgery do we have to do? Br J Ophthalmol 84: 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh T, Morton J, Coxon J, Elder MJ. 2007. Medical treatment of cataract. Clin Experiment Ophthalmol 35: 664–71 [DOI] [PubMed] [Google Scholar]
- Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, et al. 1998. A missense mutation in the àB-crystallin chaperone gene causes a desmin-related myopathy. Nat.Genet 20: 92–95 [DOI] [PubMed] [Google Scholar]
- Wang K, Cheng C, Li L, Liu H, Huang Q, et al. 2007. GammaD-crystallin associated protein aggregation and lens fiber cell denucleation. Invest Ophthalmol.Vis.Sci 48: 3719–28 [DOI] [PubMed] [Google Scholar]
- West SK, Valmadrid CT. 1995. Epidemiology of risk factors for age-related cataract. Surv.Ophthalmol 39: 323–34 [DOI] [PubMed] [Google Scholar]
- Williams PT. 2012. Walking and Running Are Associated with Similar Reductions in Cataract Risk. Med Sci Sports Exerc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wride MA. 2011. Lens fibre cell differentiation and organelle loss: many paths lead to clarity. Philos Trans R Soc Lond B Biol Sci 366: 1219–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi YB, Chen XJ, Zhao WJ, Yan YB. 2015. Congenital Cataract-Causing Mutation G129C in gammaC-Crystallin Promotes the Accumulation of Two Distinct Unfolding Intermediates That Form Highly Toxic Aggregates. J Mol Biol 427: 2765–81 [DOI] [PubMed] [Google Scholar]
- Yang J, Zhou S, Gu J, Wang Y, Guo M, Liu Y. 2015. Differences in Unfolded Protein Response Pathway Activation in the Lenses of Three Types of Cataracts. PLoS One 10: e0130705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, He J, Wang C, Wu H, Shi X, et al. 2012. Smoking and risk of age-related cataract: a meta-analysis. Invest Ophthalmol Vis Sci 53: 3885–95 [DOI] [PubMed] [Google Scholar]
- Yonova-Doing E, Forkin ZA, Hysi PG, Williams KM, Spector TD, et al. 2016. Genetic and Dietary Factors Influencing the Progression of Nuclear Cataract. Ophthalmology 123: 1237–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LC, Twu YC, Chou ML, Reid ME, Gray AR, et al. 2003. The molecular genetics of the human I locus and molecular background explain the partial association of the adult i phenotype with congenital cataracts. Blood 101: 2081–88 [DOI] [PubMed] [Google Scholar]
- Zhai Y, Li J, Yu W, Zhu S, Yu Y, et al. 2017. Targeted Exome Sequencing of Congenital Cataracts Related Genes: Broadening the Mutation Spectrum and Genotype-Phenotype Correlations in 27 Chinese Han Families. Sci Rep 7: 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Hua R, Xiao W, Burdon KP, Bhattacharya SS, et al. 2009. Mutations of the EPHA2 receptor tyrosine kinase gene cause autosomal dominant congenital cataract. Hum.Mutat 30: E603–E11 [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen XJ, Zhu J, Xi YB, Yang X, et al. 2015. Lanosterol reverses protein aggregation in cataracts. Nature 523: 607–11 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan Q, Zhou P, Ye H, Cai L, Lu Y. 2017. Association of alpha A-crystallin polymorphisms with susceptibility to nuclear age-related cataract in a Han Chinese population. BMC Ophthalmol 17: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuercher J, Neidhardt J, Magyar I, Labs S, Moore AT, et al. 2010. Alterations of the 5’untranslated leader region of SLC16A12 lead to age-related cataract. Invest Ophthalmol.Vis.Sci [DOI] [PMC free article] [PubMed] [Google Scholar]