Abstract
Background:
Oral gingival tissue, especially the junctional epithelium (JE), is constantly exposed to sub-gingival plaque. A key component of gingival health is the regulation of the number of neutrophils that migrate into the gingival crevice to counteract its harmful effects. This report investigates the contribution of innate defense receptors, Toll-like receptor 2 (TLR), TLR4 and both (TLR2/4) to the maintenance of neutrophil homeostasis in the JE.
Methods:
Bacterial composition was analyzed from whole oral swabs collected from 12–14-week-old TLR2, TLR4, TLR2/4 double knock-out (KO) mice using a MiSeq platform targeting the V3-V4 region of the 16S ribosomal RNA gene. Mandibles were histologically examined for quantification of neutrophils in the JE and bone loss. Lastly, total bacterial load was quantitated using quantitative real-time PCR.
Results:
Compared to wild-type (WT), all TLR KO mice displayed significantly increased recruitment of neutrophils (p=0.0079) into the JE. In addition, TLR4 and TLR2/4 KO mice demonstrated a significant increase in the number of bacteria (p=0.022 and p=0.0152, respectively). Lastly, comparative compositional analyses of the oral microbiome revealed that each KO strain harbored unique microbial communities that are distinct from each other but maintained similar levels of alveolar bone.
Conclusions:
Neutrophil migration into healthy mouse JE does not require TLR2 or TLR4. However, a significant increase in the number of neutrophils as well as a significant change in the oral microbial composition in both TLR2 and TLR4 KO mice demonstrate that these TLRs contribute to the homeostatic relationship between bacteria and the host in healthy mice periodontal tissue.
Keywords: Neutrophils, Toll-Like Receptors, Periodontitis, Microbiota, Oral Health, Alveolar Bone Loss
One-sentence summary:
Toll-Like Receptor 2 (TLR) and TLR 4 deficient mice show a significant increase in neutrophil numbers in the junctional epithelium as well as a significant change in the oral microbial composition, demonstrating that both TLR2 and TLR4 contribute to maintaining the homeostatic relationship between bacteria and host in healthy periodontal tissue.
Introduction
The oral cavity is unique in that controlled subclinical levels of inflammation, induced by oral commensal bacteria, leads to marginal alveolar bone loss and is part of the active process of maintaining normal healthy oral homeostasis1–4. Neutrophils are a key component of health, where individuals with deficiencies in neutrophil numbers or defects in leukocyte adhesion (LAD1 and LAD2) develop periodontitis5–7. Similarly, failure to downregulate neutrophil transit resulting in increased neutrophil numbers or prolonged neutrophil exposure has been shown to increase alveolar bone loss8,9. Furthermore, abnormalities in neutrophil activation and function, such as with decreased phagocytosis10,11 or increased superoxide production12, was also found to be associated with this disease. Therefore, in health, neutrophil homeostasis is finely tuned in the oral cavity to prevent commensal dental plaque bacteria overgrowth without over responding to elicit tissue damage.
The relationship between oral bacterial colonization and the establishment of a healthy homeostatic innate defense inflammatory state is not well characterized. However, it is becoming clear that periodontal health is an active and collaborative process between commensal bacteria and host defense, requiring regulated gene expression, controlled inflammatory responses, active neutrophil monitoring, neutralization and repair, amongst others yet still to be identified, all working in homeostasis4,13,14. One mechanism by which host-commensal bacterial interactions contribute to oral health is exemplified by the selective usage of chemotactic ligands targeting chemokine receptor CXCR2 in the process of neutrophil recruitment in periodontal tissue15.
Human gingival tissues express a wide range of toll-like receptors (TLR1–9)16,17, which are part of the innate immune system and are key mediators for inducing innate immunity, inflammation, proliferation and cell survival. TLRs specifically, TLR2 and TLR4, are capable of recognizing an extensive variety of bacteria through highly conserved pathogen-associated-molecular-patterns, such as lipoteichoic acid on gram-positive bacteria and lipopolysaccharide on gram-negative bacteria respectively18. It was previously reported that neutrophil migration into the JE was dependent on commensal colonization and the Myeloid-Differentiation-primary-Response-88 (MyD88) activation pathway4,15, a downstream signaling co-adaptor molecule utilized by interleukin-1 receptor (IL-1R), IL-18R and all TLRs except TLR318. However, it is not known if oral commensal bacteria utilize these TLRs to either elicit or maintain neutrophil homeostasis. To further elucidate the MyD88-dependent mechanism(s) by which oral commensal bacteria contribute to neutrophil trafficking into the JE, we investigated the role of TLR2 and TLR4 or both (TLR2/4) in their potential involvement in neutrophil recruitment in a mouse model.
In this study, specific-pathogen-free (SPF) mice deficient in TLR2, TLR4 or both (TLR2/4) were used to investigate the contribution of these TLRs to healthy homeostasis by measuring neutrophil recruitment in the junctional epithelium (JE), oral microbial composition, and alveolar bone height. Since TLR2 and TLR4 are upstream components of the MyD88 signaling cascade, it was hypothesized that their deficiency would impair neutrophil recruitment into the JE.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Washington, in compliance with established federal and state policies.
Animal resource
Mice with targeted deletions of TLR2 and TLR4 were obtained from Dr. S. Akira19–20 (Osaka, Japan) and backcrossed to C57BL/6 mice§ for at least six generations. TLR2 and 4 double deleted mice were created by crossing TLR2 homozygous deleted and TLR4 homozygous deleted mice21. Genotypes were confirmed with tail-snip DNA.
Thirty specific-pathogen-free (SPF) female and male TLR2, TLR4, TLR2/4 KO and wild-type (WT) C57BL/6 mice (12 to 14 weeks-old and 5 to 6 mice/group) were raised and maintained under SPF conditions at the University of Washington under the direction of the Department of Comparative Medicine.
Histology
Similar to previously published methods22, mice were euthanized by cervical dislocation and tissues were harvested for histological processing. Maxilla and mandibles were immediately fixed overnight on a shaker at 4˚C and rinsed with 70% ethanol daily for 1 week, gradually decreasing the rinses to once every other day until dissection. After dissection, tissues were demineralized in EDTA-Cacodylate decalcification solution and processed following standard histological procedures and embedded in paraffin. Using a microtome, five mandibular halves per group were examined and sectioned (5µm) from mesial to distal in the coronal plane. Two serial sections were collected per charged glass slides resulting in approximately 100 sections per tooth (50 slides per tooth). Slides were numbered and imaged in corresponding order.
Immunohistochemistry
Detection of neutrophils on paraffin embedded samples by immunohistochemistry (IHC). Every 5th slide was picked serially from the root-associated first molar to the root-associated second molar as previously described22, resulting in ~30 sections (60 JE; 15 slides) per half mandible. Tissue sections were deparaffinized and rehydrated using a gradation of diluted ethanol. Sections were blocked in 1.5% hydrogen peroxide in methanol solution and targeted with a primary neutrophil elastase monoclonal antibody** (1µg/mL). Primary antibodies were conjugated to an avidin-biotinylated secondary antibody, developed with a peroxidase substrate and counterstained with hematoxylin. WT samples processed alongside experimental samples with and without the addition of primary antibody served as positive and negative controls, respectively. All quantitative analyses were manually counted under 200x magnification by a single blinded examiner and repeated.
Histomorphometry
Linear distances from the cemento-enamel junction (CEJ) to the alveolar bone crest (ABC) were measured using publically available software†† on all IHC stained sections (150 sections per group) on both buccal and lingual surfaces of the tooth. Images (200x) were acquired blinded‡‡.
Area measurements were determined similarly on blinded images (200x). All measurements were calibrated using a 0.01mm stage micrometer and repeated.
Oral Swabbing and Microbial Genomic DNA Extraction
Bacterial oral swabs of 5 mice per group were obtained from teeth, gingival surfaces and tongue immediately after sacrifice and stored at −80˚C until extraction. Bacterial genomic DNA was extracted using a DNA extraction kit§§ and collected in low bind tubes***. Samples were further purified, concentrated††† and stored at −20˚C until sequencing. Similarly, bacterial oral swabs of 6 mice per group were obtained and genomic DNA was extracted separately from oral swabs collected for sequencing for quantification of total bacterial load.
V3-V4 Amplification and Sequencing Strategy
The V3-V4 hypervariable region of the 16S rRNA was targeted using region specific primers containing an adapter sequence‡‡‡ and purified using magnetic beads§§§. PCR products were verified by gel for visualization of the correct size after each PCR step. Amplicons were then indexed****, purified with magnetic beads§§§, and analyzed by tape station to assess library quality. Concentrations were determined individually with a fluorometer and normalized to a concentration of 3–6nM††††. Ultimately, the library was pooled, denatured with sodium hydroxide and sequenced‡‡‡‡. PhiX control§§§§ was spiked at 20% to serve as an internal control and to balance for possible low diversity and base bias present in the 16S amplicon samples.
Analysis and further downstream applications of sequencing data
300 base pair (bp) paired-end 16S rRNA gene sequences were de-multiplexed, adaptor sequences removed and filtered for quality. The ~130 bp overlapping sequences were merged***** within the V3-V4 region and processed††††† with publically available tools. Sequences were clustered into operational taxonomic units (OTUs) at 97% similarity, aligned and assigned a taxonomy in reference with Greengenes 16S rRNA database version 13_825. OTU tables were rarefied to a sequence depth of 15,200 reads/sample to calculate alpha diversity. For beta diversity, weighted and unweighted Unifrac26 distances were calculated and further analyzed by Principal Coordinates Analysis (PCoA). Significant differences in overall Beta diversity was conducted‡‡‡‡‡ and determined with PERMANOVA(adonis) statistical testing27. Linear discriminant analysis effect size (LEfSe) was used to determine differentially abundant features at the species level28. Publically available packages§§§§§ were used to generate Shannon’s and Fisher’s alpha diversity index charts and pair-wise Wilcoxon Rank Sum test was applied.
Total bacterial load quantification by quantitative real-time PCR
Absolute quantitation of total bacterial load of 6 mice per group was measured by quantitative real-time PCR (qPCR) targeting the 16S ribosomal RNA (16S rRNA) gene. Genomic DNA from mouse oral swabs or nuclease-free water for negative controls or standard (2 µl) were added to 10 µl of mastermix******, 600nM concentrations each of forward primer (5’-CGCTAGTAATCGTGGATCAGAATG-3’) and reverse primer (5’-TGTGACGGGCGGTGTGTA-3’), and 250nM of probe (5’-FAM-CACGGTGAATACGTTCCCGGGC-TAMRA-3’)31. Nuclease-free water was added to bring the total volume of the reaction to 20 µl. qPCR conditions were run at 95ᵒC for 2 min, followed by 40 cycles of 95ᵒC for 15 s and 58ᵒC for 1 min. The number of bacteria was determined using a serially diluted internal standard curve of Fusobacterium nucleatum ATCC 10953 genomic DNA and analyzed using the Second Derivative Maximum method. Internal standards were of high quality, with efficiencies ~2.0 and errors ˂0.20.
Additional statistical analysis
Mann-Whitney test was performed†††††† to determine significance for average neutrophils, total bacterial load and bone loss. A P value below 0.05 was considered significant.
Results
TLR2 and TLR4 contribute to the regulation of neutrophil recruitment into the JE
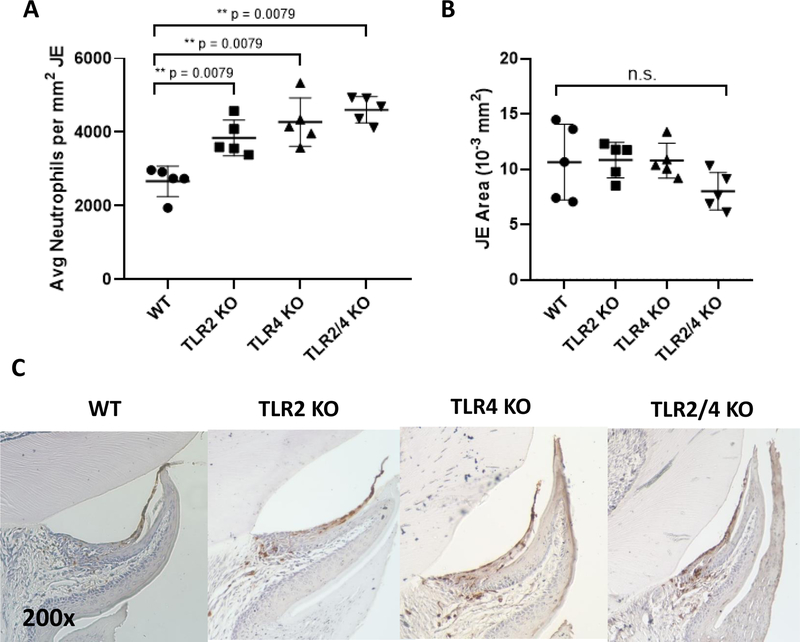
The ability of TLR2, TLR4 and TLR2/4 KO mice to recruit neutrophils into the JE was examined by immunohistochemistry. All TLR KO mice showed a significant increase in average neutrophils/mm2 of JE (p=0.0079) when compared to that of WT mice (Fig. 1A, C). This demonstrates that TLR2 and TLR4, two upstream components in the MyD88-dependent inflammatory cascade, are not necessary for neutrophil migration into the JE. Instead, since the total JE area did not vary between mice strains (Fig. 1B), it is possible that TLR2 and TLR4 may play roles in limiting neutrophil infiltration into the JE. Alternatively, the lack of TLR2 and TLR4 may indirectly affect neutrophil migration and regulation through the alteration of the oral microbiome into a dysbiotic oral community.
Figure 1.
TLR2 and TLR4 are not required for neutrophil recruitment into the junctional epithelium (JE). (A) Scatter dot plot shows average neutrophils standardized to its respective JE area. (B) Scatter dot plot shows average JE area of each mouse. (C) Representative images of histology sections at 200x magnification. Each dot is represented as an average calculated from 30 JE sections / mouse, with 5 mice per group. Significant statistical differences were calculated using the Mann-Whitney test. (*) p < 0.05, (**) p < 0.01, (***) p < 0.001
TLR4 responses prevent overgrowth of the mouse oral microbiota
To address the potential role of an alteration in the oral microbiome for the observed dysregulation of neutrophil recruitment into the JE of the TLR KO mice, genomic DNA from oral swabs were extracted and their total bacterial load was determined.
Bacterial load was significantly greatest (p=0.022) in TLR4 KO mice with ~1.2×105 mean copy numbers (Fig. 2). Similarly, TLR2/4 KO mice also showed an increase in bacterial load with ~5.6×104 mean copy numbers (p=0.0152) compared to WT samples which had ~4.6×104 mean copy numbers (Fig. 2). In contrast, TLR2 KO mouse samples showed no difference in total bacteria present compared to WT samples. This data reveals that the host’s ability to control growth of the microbial community, as measured by total bacterial load, is specifically disrupted in TLR4 KO mice, demonstrating a novel role for TLR4 in regulating oral microbial homeostasis.
Figure 2.
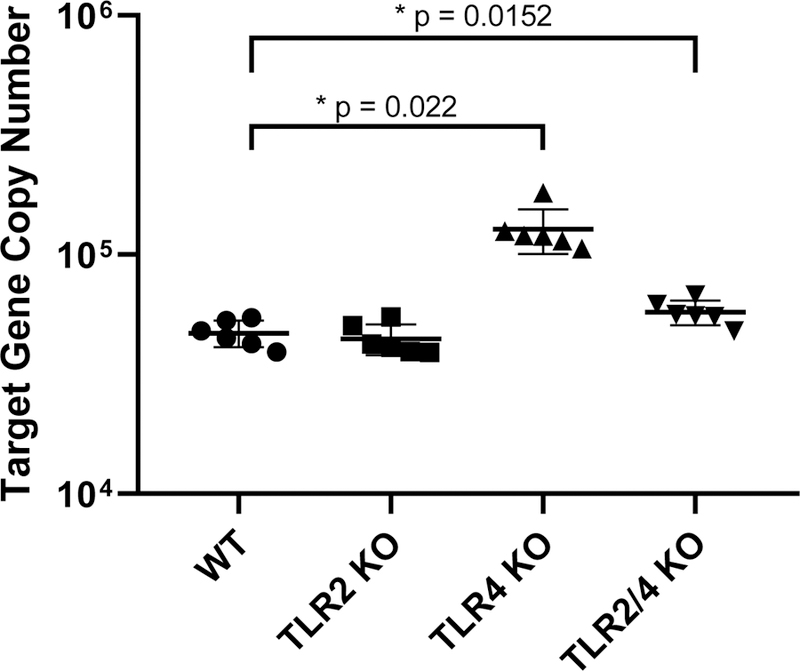
Bacterial load reveals disruption of bacterial homeostasis in some but not all TLR KO mice. Total bacterial load is represented by the copy numbers of 16S rRNA gene for each oral swab sample (n=6 / group) by real-time PCR assays in a scatter dot plot. Significance was determined using the Mann-Whitney test. (*) p < 0.05, (**) p < 0.01, (***) p < 0.001
TLR2, TLR4, TLR2/4 KO and WT mice all display distinct and unique oral microbial compositions
In order to more closely analyze the disruption of bacterial homeostasis observed from the TLR KO mice, the 16S rRNA gene was sequenced from oral swab samples to assess microbial composition.
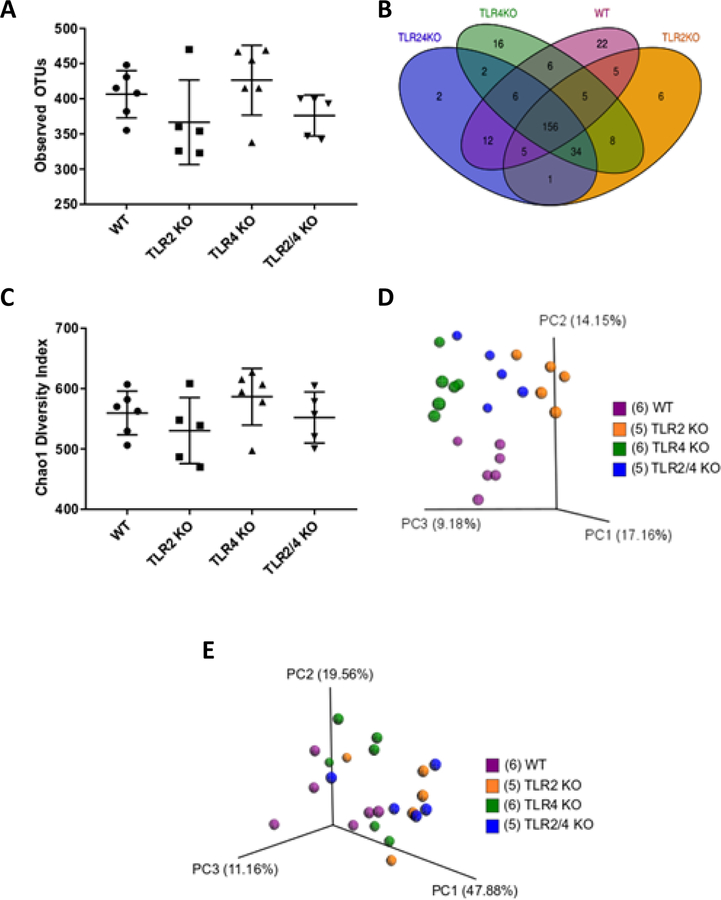
Analyses of alpha diversity measuring total observed operational taxonomic units (OTUs) shows close similarity in the mean number of OTUs in all KO mice groups, with a trend, but no statistical significance towards a higher OTU and Chao1 diversity in TLR4 KO mice (Fig. 3A). Although no significant difference was found in the total number of observed OTUs between TLR KO mice, deeper investigation at the OTU sequence level shows individual KO specific OTU representation (Fig. 3B). A total of 156 OTUs were shared amongst all of the mouse groups, with TLR2/4 KO mice having 2, TLR4 KO mice having 16, TLR2 KO mice having 6 and WT mice having 22 unique OTUs. Moreover, Chao1 diversity analysis shows that TLR2/4 double KO mice ranked the lowest on the diversity index when compared to WT in contrast to the rest of the KO mice groups, which were shown not to be statistically significant (Fig 3C). Similar results were obtained when alpha diversity was measured by the Shannon and Fisher Indexes (see supplementary Figure 1 in online Journal of Periodontology).
Figure 3.
Beta diversity, but not alpha diversity measurements show clear differences in microbial compositions between TLR and WT mice (n=5–6 mice / group).
A. Scatter dot plot comparing total observed operational taxonomic units (OTUs). Alpha diversity measure by total OTUs showed no statistically significant difference between mice groups.
B. Venn diagram comparing OTU specific sequences amongst mice groups reveal the number of both unique and shared OTU representations in and amongst each mouse group.
C. Scatter dot plot comparing the Chao1 diversity index did not show differences in species richness amongst mouse groups.
D. Beta diversity patterns using Principal Coordinate Analysis (PCoA) of unweighted UniFrac distances show clustering of mice within the same group, but spatial segregation between different groups.
E. PCoA of weighted UniFrac distances reveal moderate clustering within each group, but moderate spatial segregation between different groups.
Each dot represents a single mouse and each color represents a mouse group.
Additional analyses of beta diversity by UniFrac Principal Coordinate analyses (PCoA) shows clear spatial segregation and clustering between each TLR KO and WT mice, which is more pronounced in the unweighted (phylogenetic distance alone) than the weighted (combined abundance and phylogenetic distance) PCoA analysis (Fig. 3D). Weighted PCoA analysis shows moderate clustering within each group and minor separation between KO groups (Fig. 3E). The tight clustering of individual mouse samples within each KO strain group and the distinct separation of these groupings from each other in the unweighted PCoA demonstrates that each KO strain is uniquely different from one another. Moreover, the uniqueness of each strain is further reflected by nonparametric multivariate statistical analyses utilizing distances from the unweighted PCoA analysis (employed in Adonis package), which shows a minimum p≤0.05 for all groups, with the most similarity (p=0.022) between TLR2 and TLR2/4 double KO mice and the most dissimilarity (p=0.003) between TLR2 and TLR4 KO mice (see supplementary Figure 2 in online Journal of Periodontology). Altogether, alpha and beta diversity analyses reveal there are clear differences in microbial composition, but not in total number of OTUs present.
Major differences in predominating bacterial genera among mouse groups
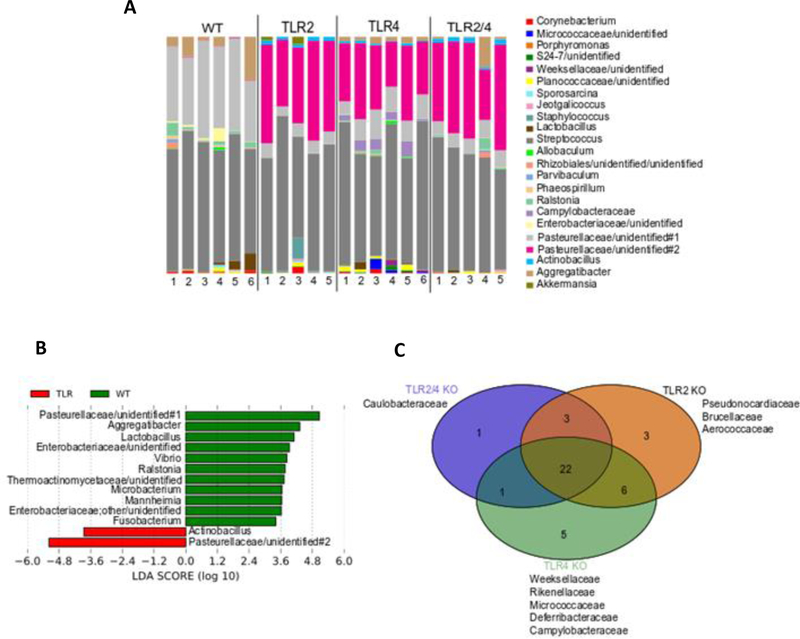
Supporting clear differences represented by the PCoA analyses and the Adonis statistical testing, alterations in bacterial composition are observed at the genus level (Fig. 4A).
Figure 4.
TLR KO mice show unique taxonomic complexity and representation from each other and WT (n=5–6 mice / group).
A. Bacterial composition between TLR2, TLR4, TLR2/4 double KO and WT mice at the genus level. Each color is represented by a genus in the figure legend.
B. Linear Discriminant Analysis Effect Size (LEfSe) analysis showing those OTUs that were significantly differentially abundant between WT (green) and TLR KO (red) mice, ranked by effect size (All LDA scores > 3.4)
C. Venn diagram showing specific family representation amongst the TLR KO mice.
Although the Greengenes 16S rRNA database version 13_8 identified unique genera, limited characterization of some sequences did not allow it to be labeled up its respective genus identity and is referred up its family level identity followed by “unidentified” for its genus level identification.
TLR KO mice exhibit a bacterial composition strikingly different from WT, with all TLR KO groups showing a predominance of Pasteurellaceae/unidentified#2. This TLR KO associated Pasteurellaceae/unidentified#2 is a unique and distinct member from the Pasteurellaceae identified from WT mice, labeled as Pasteurellaceae/unidentified#1. Both identified Pasteurellaceae groups in TLR KO and WT mice are uncharacterized and unnamed distinct genera in the Family Pasteurellaceae.
Focusing on statistically significant differences in bacterial composition, LEfSe analysis confirmed the dominance of the Pasteurellaceaea/unidentified#1 in WT mice compared to that of Pasteurellaceae/unidentified#2 in the TLR KO mice (Fig. 4B). Moreover, WT mice show a high prevalence of both gram-negative and gram-positive members such as Aggregatibacter, Microbacterium, Enterobacteriaceae/unidentified, and Fusobacterium in contrast to TLR KO mice which show a dominance of gram-negative species such as Pasteurellaceae/unidentified#2 and Actinobacillus. Specific differences amongst KO groups can further be observed when analyzed at the broader family level (Fig 4C); where, the presence of 5 unique families (Weeksellaceae, Rikenellaceae, Micrococcaceae, Deferribacteraceae, and Campylobacteraceae) are only found in TLR4 KO mice and absent in the rest of the mouse groups. Furthermore, TLR2 KO mice exclusively harbor Pseudonocardiaceae, Brucellaceae and Aerococcaceae, while TLR2/4 KO mice show an abundance of Caulobacteraceae.
Collectively, analysis of oral bacterial composition between normal, non-infected TLR KO mice show that each TLR KO mouse group establishes a unique oral niche that in each case supports a distinct microbial community.
The predominant members from the Pasteurellaceae family and their abundance are different between WT and TLR KO mice
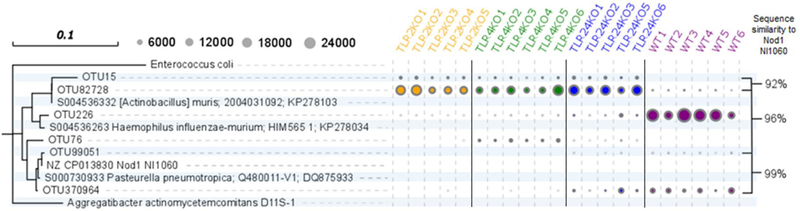
In order to characterize and distinguish between the Pasteurellaceae/unidentified#1 from WT and the Pasteurellaceae/unidentified#2 from TLR KO mice, their respective OTU sequences were aligned and compared in a phylogenetic tree alongside reference sequences and concomitantly with the total observed OTU counts (Fig. 5). Amongst the reference sequences, the 16S rRNA gene sequence of a potentially novel Pasteurellaceae strain, NI1060, was also included into the analysis in an effort to identify and characterize these Pasteurellaceae. NI1060 is a recently identified murine oral bacterium shown to induce periodontitis independently without the cooperation of the oral microbiota in a murine ligature-induced disease model and found to be closely related to Aggregatibacter actinomycetemcomitans32,33, a species with strong correlation to the development of aggressive periodontitis in humans34,35. Lastly, additional Pasteurellaceae OTUs identified in the samples were also included into this analysis to observe for distinctions between the Pasteuerellaceae in TLR KO and WT groups.
Figure 5.
TLR KO mice harbor different species of Pasteurellaceae from WT and WT contains a Pasteurellaceae strain, NI1060.
A phylogenetic tree comparing specific WT and TLR KO identified Pasteurellaceae OTU sequences at the species level and their total sequence counts in relation to reference strains. Sequences from WT from this study were 99% similar to a novel strain of Pasteurellaceae that is closely related to Aggregatibacter actinomycetemcomitans, a species with strong correlation to the development of aggressive periodontitis in humans. Circles represent OTU sequence counts and a sequence similarity of ~97% is accepted as species equivalence.
Sequence analysis shows OTU82728, identified as Pasteurellaceae/unidentified#2 in TLR KO mice samples, and OTU226, identified as Pasteurellaceae/unidentified#1 in WT mice, share a common ancestor, but come from distinctly different lineages that are both closely related to the reference sequence of Actinobacillus muris S004536332. Moreover, sequence count data correlates with previous analyses, where TLR KO OTU82728 is predominant in all TLR KO mice samples, ~12,000 to 24,000 sequence counts, but in low abundance, ~2,000 sequence counts in WT samples. In contrast, WT OTU226 is found in large sequence abundance in all WT samples, but in low abundance in TLR KO samples. Sequence comparison of the Pasteurellaceae OTUs identified in WT and TLR KO mice to the novel NI1060 strain reveal that both TLR KO OTU82728 and WT OTU226 have 96% sequence similarity, where ~97% sequence similarity represents species equivalence. More interestingly, OTU99051 shows 99% sequence similarity and is present in low numbers in all WT and select TLR2/4 double KO samples, but not in TLR2 or TLR4 KO samples. These analyses reveal that the presence of NI1060, a bacterium previously shown to induce periodontal disease36, is also present in the oral cavity of these normal healthy mice. Overall, TLR KO mice have a distinctly different member of Pasteurellaceae from that of WT.
Dysregulation of neutrophil recruitment and an alteration of the microbial composition does not affect alveolar bone levels
To understand the clinical implications of dysregulated neutrophil recruitment and an alteration of the microbial burden and composition, alveolar bone levels were measured histomorphometrically with linear distances from the alveolar bone crest (ABC) to the cemento-enamel junction (CEJ). Histomorphometric analysis of alveolar bone loss at the buccal and lingual sides revealed comparable levels with no statistically significant difference in bone loss between TLR KO and WT mice (Fig. 6). Altogether, our data points out that the dysregulation of neutrophil recruitment and alteration of the oral microbial burden and composition by TLR2 or TLR4 or both TLR2 and 4 does not disrupt alveolar bone levels.
Figure 6.
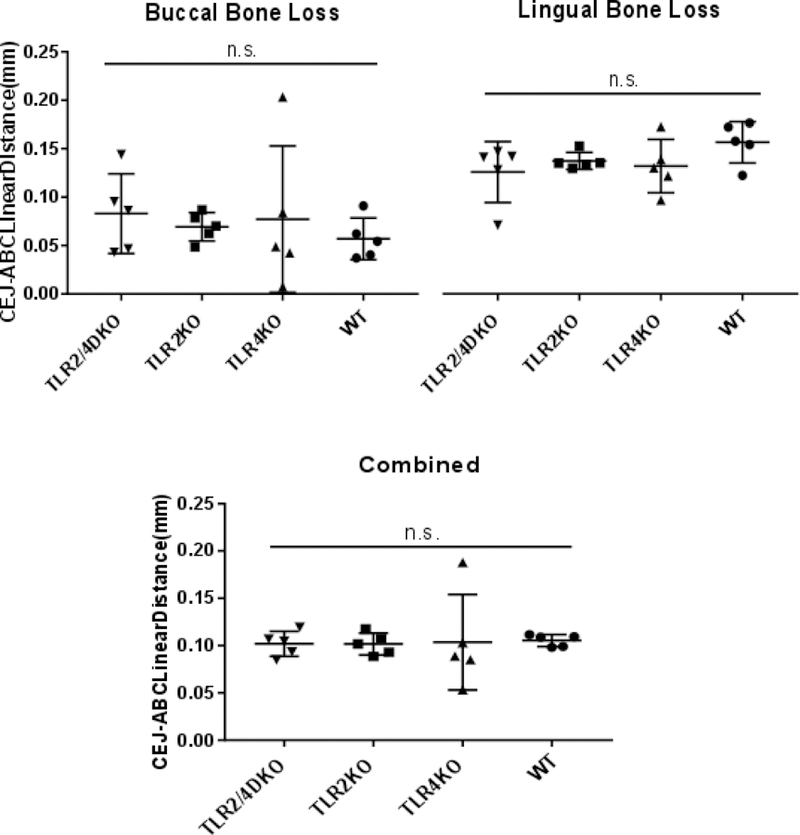
No difference in bone loss was observed between TLR KO and WT mice. Scatter dot plot shows no significant difference in bone loss when determined by histomorphometric analysis of linear distances from the cemento-enamel junction (CEJ) to the alveolar bone crest (ABC) on buccal and lingual surfaces of the tooth (300 JE per group). Measurements of buccal and lingual sides were then combined for analysis of combined measurements. Images were acquired at 200x and measured blinded two independent times by the same observer. (*) p < 0.05, (**) p < 0.01, (***) p < 0.001, (n.s.) not statistically significant.
Discussion
Previous studies demonstrated a significant reduction in neutrophil transit in the JE of MyD88 KO mice4,15. Therefore, it was anticipated that TLR2 and/or TLR4 deficiency would show a reduction in the number of neutrophils that transit into the JE as both signaling pathways engage the TLR adaptor protein MyD8818. However, in contrast to our hypothesis, all KO mice showed a significant increase in average neutrophils/mm2 of JE when compared to WT mice demonstrating that neither TLR2 nor TLR4 are required for neutrophil transit. This increase was not a result of an increase in total circulating blood neutrophils, as TLR deficient mice were reported to show similar numbers of total neutrophils to WT mice39,40. Consistent with the notion that TLRs are not required for neutrophil migration into the JE, but may rather play a role in the modulation of neutrophil trafficking, similar increases in bacterial load and neutrophil numbers in the peritoneal cavity of TLR4 deficient mice were observed in a sepsis model41. Possible explanations for the increase in neutrophil numbers in the JE include the increase in bacterial load observed in the TLR4 and TLR2/4 KO mice and/or the significant change in the oral microbial composition observed in all TLR KO mice.
Oral microbiome analysis revealed that TLR KO mice have characteristically different compositions, with increases in mostly gram-negative species for TLR2 and TLR2/4 KO mice, while TLR4 KO mice show an increase in gram-positive species. In addition, TLR KO mice showed a strong selection for a Pasteurellaceae species more related to Actinobacillus muris, while the WT group has a Pasteurellaceae species more closely related to Haemophilus influenza-murium. At the same time, each group harbored distinctly unique species within each TLR KO group. Cage-to-cage variation may potentially explain the unique compositional differences42 amongst TLR KO and WT mice and remains a limitation of this study. However, if cage to cage variation were to contribute to the observed results, it may explain only a fraction of the differences in composition since samples from each experimental group were collected across different litters and cages over multiple generations and yet still maintained close compositional similarities within the group that were distinct from the others. Therefore, although cage to cage variation remains a limitation of this study, a preponderance of evidence supports the contention that TLR2 and TLR4 significantly shape the oral microbial composition.
Another possible explanation for the increased as opposed to decreased neutrophil infiltration in the JE is that additional MyD88 dependent pathways such as TLRs 1, 5 and 6, and the IL-1 receptor regulate neutrophil transit independent of TLR2 or 4. Alternatively, the absence of TLR2 or TLR4 may indicate a critical role for these TLRs in limiting excessive neutrophil infiltration to sites of infection via dampening chemokine receptor expression or functions. Lending support to this possibility, ex vivo experiments in human neutrophils43,44 and in vivo experiments in mouse models37 demonstrated that TLR2 and TLR4 activation downregulates the cell surface expression of CXCR2 on neutrophils, impairing chemotaxis. Furthermore, it was observed that deficiency of TLR2 prevented CXCR2 downregulation and relieved impairment of neutrophil numbers in lung tissue in a mouse model of sepsis37. Moreover, these mutations may have additional effects on neutrophil lifespan by the inhibition of apoptotic factors and mechanisms and/or inducing mediators which prolong neutrophil survival42. Collectively, our data indicate that TLR2 and TLR4 are not required for neutrophil recruitment, but instead play a key role in regulating neutrophil homeostasis in the oral cavity—possibly directly, by limiting chemotactic responses, or indirectly, by promoting the development of a dysbiotic oral commensal community.
Interestingly, the dysregulated recruitment of neutrophils into the JE and alteration in microbial composition of these TLR KO mice did not affect alveolar bone levels. This result is consistent with previous observations in a mouse model, which found no significant differences in alveolar bone loss between uninfected control TLR2 and TLR4 KO mice43 and also in a rabbit model of disease investigating the contribution of periopathogens to initiate dysbiosis in oral communities, which concluded that not all dysbiotic communities induced bone loss44. This suggests that periodontitis is a complex disease process that requires more than a dysbiotic microbial community and may require engagement of additional components yet to be elucidated.
This study also revealed the potential hazard in the interpretation of data obtained from the current mouse models of periodontitis. It has been shown that one major effect of the addition of Porphyromonas gingivalis is a change in the microbial composition. Clearly, TLR2 and TLR4 KO mice display significantly different oral microbial compositions, therefore differences in the initial microbial composition in the different TLR KO mice preclude definitive conclusions concerning the specific contribution of either TLR2 or TLR4 to P. gingivalis induced disease as has been previously reported45–48. Furthermore, although the presence of N1060, a bacterium shown to initiate periodontitis in mice without the cooperation of the oral microbiota represents a valid study with appropriate conclusions33, the observation that it was not found in all the groups of mice underscores the need for a complete oral microbial analysis of the strains being employed in all periodontitis mouse model of disease.
Conclusions
In conclusion, our findings demonstrate that in health, TLR2 and TLR4 are not required for promoting neutrophil trafficking into the JE, but rather may significantly contribute to shaping the composition of the microbiota, which may in turn modulate neutrophil homeostasis. This study demonstrates that at least in mice, direct recognition of bacterial components by either TLR2 or TLR4 is not required for neutrophil migration into the gingival sulcus. Rather, the key activation pathway that elicits neutrophil migration in healthy gingiva remains unknown, but appears to be dependent upon both MyD8815 and bacteria4,15,22.
Supplementary Material
Acknowledgments
This work was supported by the NIH NIDCR grant DE023453 awarded to R. Darveau†.
The authors thank Jirawan Wade† for her histological assistance and technical support. In addition, the authors thank Dr. Shatha Bamashmous*†, Dr. Sumita Jain† and Dr. Steve Coats† for technical assistance, editing, and proofreading.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
Jackson Laboratories, Bar Harbor, ME
Neutrophil Marker sc-71674, Santa Cruz Biotechnology (Dallas, TX, USA)
ImageJ Version 1.50b, National Institutes of Health (Bethesda, MD, USA)
SPOT CCD camera and software, Diagnostic Instruments (Sterling Heights, MI, USA)
QIAamp DNA Microbiome Kit, Qiagen (Hilden, Germany)
DNA LoBind eppendorf tubes, Eppendorf (Hamburg, Germany)
DNA Clean & Concentrator kit, Zymo Research (Irvine, CA, USA)
Illumina flow cell adapter sequence, Illumina (San Diego, CA, USA)
Agencourt AMPure XP beads, Beckman Coutler Inc. (Pasadena, CA, USA)
Nextera XT Index Kit v2 SetA, Illumina (San Diego, CA, USA)
SequalPrep Normalization Plate Kit, Invitrogen (Carlsbad, CA, USA)
300 paired end MiSeq, Illumina (San Diego, CA, USA)
PhiX Control v3 library, Illumina (San Diego, CA, USA)
Pandaseq23
Qiime24, version 1.9.1
R studio 3.0.2
SsoAdvanced Universal Probes Supermix, Bio-Rad (Hercules, CA)
GraphPad Prism version 7.00 for Windows, GraphPad Software (La Jolla California USA)
References
- 1.Dixon DR, Reife RA, Cebra JJ, Darveau RP. Commensal bacterial influence innate status within gingival tissues: A Pilot Study. J Periodontol 2004; 75:1486–1492. [DOI] [PubMed] [Google Scholar]
- 2.Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 2011; 10:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishii K, Usui M, Yamamoto G, et al. The distribution and expression of S100A8 and S100A9 in gingival epithelium of mice. J Periodontal Res 2013; 48:235–242. [DOI] [PubMed] [Google Scholar]
- 4.Tsukamoto Y, Usui M, Yamamoto G, et al. Role of the junctional epithelium in periodontal innate defense and homeostasis. J Periodontal Res 2012; 47:750–757. [DOI] [PubMed] [Google Scholar]
- 5.Waldrop TC, Anderson DC, Hallmon WW, Schmalstieg F, Jacobs RL. Periodontal manifestations of the heritable Mac-1, LFA-1, deficiency syndrome. Clinical, histopathologic and molecular characteristics. J Periodontol 1987; 58:400–416. [DOI] [PubMed] [Google Scholar]
- 6.Hajishengallis E, Hajishengallis G. Neutrophil homeostasis and periodontal health in children and adults. J Dent Res 2014; 93:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart TC, Shapira L, Van Dyke TE. Neutrophil defects as risk factors for periodontal diseases. J Periodontol 1994; 65:521–9. [DOI] [PubMed] [Google Scholar]
- 8.Eskan MA, Jotwani R, Abe T, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol 2012; 13:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nussbaum G, Shapira L. How has neutrophil research improved our understanding of periodontal pathogenesis? J Clin Periodontol 2011; 38:49–59. [DOI] [PubMed] [Google Scholar]
- 10.Carneiro VM, Bezerra AC, Guimaraes MC, Muniz-Junqueira MI. Decreased phagocytic function I neutrophils and monocytes from peripheral blood in periodontal disease. J Appl Oral Sci 2012; 20:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Dyke TE, Zinney W, Winkel K, Taufiq A, Offenbacher S, Arnold RR. Neutrophil function in localized juvenile periodontitis. Phagocytosis, superoxide production and specific granule release. J Periodontol 1986; 57:703–708. [DOI] [PubMed] [Google Scholar]
- 12.Tapashetti RP, Sharma S, Patil SR, Guvva S. Potential effect of neutrophil functional disorders on pathogenesis of aggressive periodontitis. J Contemp Dent Pract 2013; 14:387–393. [DOI] [PubMed] [Google Scholar]
- 13.Darveau RP. The oral microbial consortium’s interaction with the periodontal innate defense system. DNA Cell Biol 2009; 28:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 2010; 8:481–490. [DOI] [PubMed] [Google Scholar]
- 15.Zenobia C, Luo XL, Hashim A, et al. Commensal bacteria-dependent select expression of CXCL2 contributes to periodontal tissue homeostasis. Cell Microbiol 2013; 15:1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manhanonda R, Pichyangkul S. Toll-like receptors and their role in periodontal health and disease. Periodontol 2000 2007; 43:41–55. [DOI] [PubMed] [Google Scholar]
- 17.Beklen A, Hukkanen M, Richardson R, Konttinen YT. Immunohistochemical localization of toll-like receptors 1–10 in periodontitis. Oral Microbiol Immunol 2008; 23:425–431. [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Res Ther 2005; 7:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 1999; 162:3749–3752. [PubMed] [Google Scholar]
- 20.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999; 11:443–451. [DOI] [PubMed] [Google Scholar]
- 21.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2007; 292:312–22. [DOI] [PubMed] [Google Scholar]
- 22.Greer A, Irie K, Hashim A, et al. Site-specific neutrophil migration and CXCL2 expression in periodontal tissue. J Dent Res 2016; 95:946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD. PANDAseq: paired-end assembler for illumine sequences. BMC Bioinformatics 2012; 13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 2006; 72:5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 2005; 71:8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: 2013. http://www.R-project.org/ [Google Scholar]
- 28.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011; 12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMurdie PJ, Holmes S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013; 8:e61217 DOI: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wickham H ggplot2: Elegant graphics for data analysis Springer-Verlag; New York, 2009. 213 p. [Google Scholar]
- 31.Yoshida A, Suzuki N, Nakano Y, Oho T, Kawada M, Koga T. Development of a 5’ fluorogenic nuclease-based real-time PCR assay for quantitative detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. J Clin Microbiol 2003; 41:863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darzi Y, Jiao Y, Hasegawa M, et al. The genomic sequence of the oral pathobiont strain NI1060 reveals unique strategies for bacterial competition and pathogenicity. PLoS One 2016; 11:e0158866 DOI: 10.1371/journal.pone.0158866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiao Y, Darzi Y, Tawaratsumida K, et al. Induction of bone loss by pathobiont-mediated Nod1 signaling in the oral cavity. Cell Host Microbe 2013; 13:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman MG, Socransky SS. Predominant cultivable microbiota in periodontosis. J Periodontal Res 1977; 12:120–128. [DOI] [PubMed] [Google Scholar]
- 35.Slots J, Reynolds HS, Genco RJ. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect Immun 1980; 29:1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alves-Filho JC, Freitas A, Souto FO, et al. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc Natl Acad Sci U S A 2009; 106:4018–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andonegui G, Bonder CS, Green F, et al. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Invest 2003; 11:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng M, Ma T, Yan Z, et al. Toll-like receptor 4 signaling on dendritic cells suppresses polymorphonuclear leukocyte CXCR2 expression and trafficking via interleukin 10 during intra-abdominal sepsis. J Infect Dis 2015; 213:1280–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoy YE, Bik EM, Lawley TD, et al. Variation in taxanomic composition of the fecal microbiota in an inbred mouse strain across individuals and time. PLoS One 2015; 10:e0142825 DOI: 10.1371/journal.pone.0142825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabroe I, Jones EC, Whyte MKB, Dower S. Regulation of human neutrophil chemokine receptor expression and function by activation of Toll-like receptors 2 and 4. Immunology 2005; 115:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabroe I, Prince LR, Jones EC, et al. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol 2003; 170:5268–5275. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res 2009; 43:25–64. [DOI] [PubMed] [Google Scholar]
- 43.Chukkapalli SS, Velsko IM, Rivera-Kweh MF, Larjava H, Lucas AR, Kesavalu L. Global TLR2 and 4 deficiency in mice impacts bone resorption, inflammatory markers and atherosclerosis to polymicrobial infection. Mol Oral Microbiol 2017; 32:211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zenobia C, Hasturk H, Nguyen D, Van Dyke TE, Kantarci A, Darveau RP. Porphyromonas gingivalis lipid A phosphatase activity is critical for colonization and increasing the commensal load in the rabbit ligature model. Infect Immun 2017; 82:650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lima HR, Gelani V, Fernandes AP, et al. The essential role of toll like receptor-4 in the control of Aggregatibacter actinomycetemcomitans infection in mice. J Clin Periodontol 2010; 37:248–254. [DOI] [PubMed] [Google Scholar]
- 46.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol 2006; 177:8296–8300. [DOI] [PubMed] [Google Scholar]
- 47.Gibson FC III, Ukai T, Genco CA. Engagement of specific innate immune signaling pathways during Porphyromonas gingivalis induce chronic inflammation and atherosclerosis. Front Biosci 2008; 13:2041–2059. [DOI] [PubMed] [Google Scholar]
- 48.Lin M, Hu Y, Wang Y, Kawai T, Wang Z, Han X. Different engagement of TLR2 and TLR4 in Porphyromonas gingivalis vs. ligature-induced periodontal bone loss. Braz Oral Res 2017; 31:e63 DOI: 10.1590/1807-3107BOR-2017.vol31.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.