Abstract
Cold exposure during cycling and recovery enhances PGC-1α transcription, but aspects of mitophagy and a more intense cold exposure without recovery occurring in the cold have not been explored.
Purpose:
Determine the expression of genes related to mitochondrial biogenesis and mitophagy following an acute cycling bout at a temperature below freezing compared to that of room temperature.
Methods:
Eleven male participants cycled at 65% Wmax for 1 h at −2 °C and 20 °C and then recovered at room temperature for 6 h. A muscle biopsy was taken from the vastus lateralis before exercise, 3 h, and 6 h post-exercise for gene expression analysis.
Results:
Exercising heart rate and skin temperature were lower in the cold (p < 0.001; p = 0.004), while core temperature was higher (p = 0.016). Temperature had no effect on gene expression (p > 0.05). BNIP3 and BNIP3L mRNA were not influenced by exercise (p = 0.329; p 0.233). PGC-1α and VEGF were higher after cycling (p < 0.001), but the extent of PGC-1α upregulation was reduced 6 h post-exercise (p 0.006). TFAM increased 6 h post-exercise (p = 0.001). NRF2, ERRα, PINK1, and PARK2 decreased 3 h post-exercise (p 0.035; p = 0.005; p = 0.002; p = 0.001), but this downregulation was diminished after 6 hours of recovery (p = 0.017; p 0.006; p = 0.043; p = 0.047). NRF1 was marginally attenuated with exercise (p = 0.001).
Conclusions:
Exercise induced alterations in gene expression for mitochondrial biogenesis and mitophagy, but these effects were independent of temperature.
Keywords: Cycling, mRNA, PGC-1α, skeletal muscle, human, temperature
Introduction
The metabolic demands of exercise stimulate mitochondrial adaptation, which enhances energy production [23,37,48]. This development is facilitated by simultaneous growth of new mitochondria (termed mitochondrial biogenesis) and at the same time the removal of damaged mitochondria via autophagy (now commonly referred to as mitophagy) [51]. In humans, aging leads to a decline in mitophagy of muscle satellite cells [18], and impaired mitochondrial dynamics are associated with the pathology of neurodegenerative disorders including Parkinson’s [12], Alzheimer’s [29], and Huntington’s disease [8]. Therefore, maintaining a balance between mitochondrial replication and elimination is essential to health.
Mitochondrial biogenesis is primarily driven by peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), which targets a wide-array of downstream transcription factors and nuclear receptors including nuclear respiratory factor 1 (NRF1), nuclear respiratory factor 2 (NRF2), and estrogen-related receptor alpha (ERRα) [13,56]. These regulators stimulate electron transport chain complex expression, duplication of mitochondrial DNA by transcription factor A mitochondrial (TFAM), and activation of vascular endothelial growth factor (VEGF) [13,22,25]. The accumulation of oxidative damage can cause mitochondrial DNA mutations and changes in mitochondrial membrane potential, necessitating the need for mitophagy [30,33]. Mitophagy is coordinated by the transcriptional regulation of several genes, including PTEN-induced putative protein kinase 1 (PINK1), Parkin RBR E3 Ubiquitin Protein (PARK2), Bcl-2/adenovirus E1B 19-kDa interacting protein 3 (BNIP3), and BNIP3-like (BNIP3L) [9,11,28,32,45]. The proteins encoded by these genes accumulate on the outer membrane of disrupted mitochondria to signal for degradation, allow phagophore engulfment, and tether mitochondria to autophagosomes [2,4,26,31,53,57].
Though exercise alone is beneficial for mitochondrial development, the magnitude of this response may be impacted by environmental temperature. Markers of mitochondrial biogenesis are augmented after exercise in the cold [48,49], but mediators of mitophagy have not been fully explored with cold interventions. Though there is a lack of research examining cold exposure and exercise together, acute exercise increases mRNA for select genes linked to mitophagy in humans [24,44]. Animal models exhibit elevated PINK1 protein content after short-term heat stress [17] and decreased protein levels of Parkin with hypothermic recovery from cardiac arrest [36], indicating these markers may be sensitive to temperature. The mitochondrial biogenesis and mitophagy processes must be coordinated carefully to maintain a fully functional intracellular environment, and the creation of temperature-optimized training protocols to stimulate mitochondrial turnover could be beneficial for diseased populations with diminished exercise capacity.
Research examining the transcriptional response to exercise and environmental cold indicates that cycling and a subsequent 3 h recovery at 7 °C leads to a greater enhancement in PGC-1α gene expression compared to 20 °C [48]. However, it is not always feasible or comfortable for individuals to spend a prolonged period after exercise in cold conditions. This differential PGC-1α response to temperature was not found when exercise was performed directly in the cold (7 °C) without further exposure during recovery [46]. By implementing a colder temperature during exercise, a greater thermogenic activation may augment mitochondrial biogenesis and mitophagy without the discomfort of extended cold exposure during recovery from exercise.
The purpose of this investigation was to determine the expression of key genes related to mitochondrial biogenesis and mitophagy following an acute bout of cycling at a temperature below freezing (−2 °C) compared to that of room temperature (20 °C). Temperature therapies that enhance the effectiveness of an acute exercise bout could promote greater muscle function, metabolic health, and training outcomes.
Methods
Participants
Eleven males (age 19–39) partook in this study. Prior to participation, subjects were informed of the experimental procedures, risks and benefits of participation, measures taken to eliminate risks, and their rights as a volunteer in this study. All procedures were approved by the University Institutional Review Board (IRB) and conformed to the standards set by the Declaration of Helsinki. Participants were considered “low risk” for exercise-related cardiac events under ACSM stratification and were recreationally trained, defined by engagement in structured aerobic or resistance exercise at least 3 days a week for the past 3 months.
Preliminary testing
Descriptive data was obtained before completion of exercise trials to assess height (Seca 213 Statiometer, United Kingdom), weight (Befour PS-660 ST Digital Scale, Saukville, WI), body composition, and aerobic capacity. Body composition was determined through hydrodensitometry using correction factors for estimated residual lung volume and gastrointestinal air volume [50]. Body density was used to estimate percent body fat using the Siri equation [47]. Participants then performed a graded exercise test on an electronically braked cycle ergometer (Excalibur Sport, Lode, Groningen, Netherlands). Expired gases were analyzed every 15 sec using a flow and gas calibrated metabolic cart (ParvoMedics, TrueOne Metabolic System, Sandy, Utah) to evaluate peak oxygen uptake (VO2 peak). The cycling protocol started at 95 W, increased by 35 W every 3 min and was performed until volitional exhaustion. Maximum workload (Wmax) was calculated by taking the time completed in the last stage divided by the total stage duration (3 min) multiplied by 35 Watts and added to the watts of the last completed stage. The highest VO2 achieved was recorded using the metabolic cart. Maximum power output (W max) achieved during VO2 peak testing was used to determine workload for experimental trials. See Table 2 for subject characteristics.
Table 2.
Subject characteristics
| Characteristic | Value |
|---|---|
| Age (yr) | 25 ± 2 |
| Height (cm) | 179 ± 2 |
| Weight (kg) | 82.7 ± 3.8 |
| Body fat (%) | 17.9 ± 1.8 |
| VO2peak (1/min) | 3.84 ± 0.16 |
| VO2peak (ml·kg−1·min−1) | 47.0 ± 2.2 |
| Power at VO2 peak (W) | 238 ± 12 |
Data are means ± SE (n = 11). VO2 peak, peak volume of oxygen consumption.
Experimental trials
Experimental and control trials were performed one week apart using a counterbalanced repeated measures study design. Testing was done in July to reduce pre-conditioned cold acclimation (average daily high temperature: 30.8 ± 0.5 °C). Participants were asked to abstain from alcohol, caffeine, and exercise in the 24 h period before completion of the trials. Participants were also asked to record dietary intake and sleep, so it could be replicated for the sec trial. On the trial day, participants arrived at the laboratory in the morning after a 12 h fast. Trials consisted of cycling for 60 min at 65% Wmax in a temperature and humidity-controlled chamber (cold trial: −2.0 ± 0.5 °C, 74 ± 2% relative humidity; room temperature trial: 20.0 ± 0.1 °C, 66 ± 1% relative humidity) (Darwin Chambers Company, St. Louis, MO). The recovery for both trials took place in a room-temperature environment (~20°C). One subject was unable to complete the prescribed intensity, so the workload was reduced but kept consistent between trials. A 500 ml bottle of water was given to drink ad libitum during exercise. More water was given only upon request. Pre-exercise body weight, post-exercise body weight, and water consumption during cycling were measured to calculate sweat rate. The 6 h recovery period began immediately after cessation of cycling. During the first 3 h, participants were directed to remain fasted, avoid extreme temperature, and minimize activity, but water consumption was not limited. Participants were allowed to consume food during the final 3 h of recovery while still limiting activity and avoiding exposure to extreme hot or cold environmental temperatures. Diet was kept consistent between trials. Muscle biopsy samples were extracted before exercise, 3 h post-exercise, and 6 h post-exercise.
Core temperature, skin temperature, and heart rate
Skin temperature (4-channel Thermometer/Datalogger, Extech Instruments, Nashua, NH), core temperature (4-channel Thermometer/Datalogger, Extech Instruments, Nashua, NH), and heart rate (Polar Electronic) were continuously monitored during the 1 h cycling protocol. Skin sensors (SST-2, Physitemp Instruments, Clifton, NJ) were placed on the chest (3 cm superior and lateral to the left nipple) and back (medial portion of the left scapular ridge). Skin temperature was determined by averaging chest and back temperature. Core temperature was measured via rectal probe (RET-1, Physitemp Instruments, Clifton, NJ) inserted 12 cm past the anal sphincter. Temperature data was logged every min to obtain average over the entire 1 h exercise bout. Heart rate was electronically recorded every sec and used to calculate average during exercise.
Rating of perceived exertion
Rating of perceived exertion (RPE) was evaluated using the 6–20 Borg Scale at 15, 30, 45, and 60 min of cycling [6]. All four values were averaged to represent RPE over the entire session.
Muscle biopsies
Muscle biopsies were taken from the vastus lateralis prior to exercise, 3 h post-exercise, and 6 h post-exercise during both the experimental and control trials. We chose the 3 h time-point so that the current results could be closely compared to our previous work using this time-point [46,48]. We chose the 6 h time-point to evaluate the effects of our intervention on the time-course of activation and still be within an expected time frame of peak expression. Additionally, this allowed us to obtain a sample at the 3 h time-point while in a fasted state but allow participants to eat within a reasonable fasting period before the 6 h time-point. All biopsies within a trial were performed on the same leg ~2 cm proximal to the previous biopsy. The leg was chosen in a random, counter-balanced order. First, the biopsy site was sterilized with betadine then numbed with 3–4 ml of 1% lidocaine injected under the skin surface and surrounding the muscle fascia. A small incision was made through the skin. The muscle sample was obtained using a 5 mm Bergstrom percutaneous biopsy needle with the aid of suction [5]. Excess blood, connective tissue, and fat were immediately cleaned from the sample before the muscle was placed in a chemical stabilization agent (All-protect, Qiagen, Hilden, North Rhine-Westphalia, Germany) and stored overnight at 4 °C then transferred to −30 °C for later analysis of gene expression.
mRNA extraction and cDNA synthesis
Muscle homogenization and mRNA extraction were performed as previously detailed [39]. Ten to twenty milligrams (16.6 ± 0.3 mg) of each sample were homogenized in Trizol with an electric blender homogenizer (Bullet Blender, Next Advance Inc., Averill Park, NY) utilizing 1.5 ml Red RINO tubes (Next Advance, Inc). To isolate RNA, chloroform was added, and samples were centrifuged. The aqueous phase containing RNA was transferred to a new tube with glycogen to aid in RNA precipitation. RNA was precipitated by adding isopropyl alcohol, incubating overnight at −20 °C, and centrifuging the next day. After removing supernatant, 75% ethanol was added to wash the RNA pellet. To solubilize the RNA, the supernatant was discarded, the pellet dried, and RNA dissolved in RNA storage solution (Thermo Fisher Scientific, Waltham, MA) to be quantified with a nano-spectrophotometer (nano-drop ND-2000, Thermo Scientific, Wilmington, DE) (average concentration 208.7 ± 9.7 ng/μl). RNA quality was inspected on an Agilent 2100 bioanalyzer according to manufacture instructions for RNA integrity number (RIN: 9.20 ± 0.07) (Agilent Technologies Inc., Santa Clara, CA). Superscript IV-first strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA) was used according to manufacturer’s instructions to synthesize RNA to cDNA.
qRT-PCR
Each RT-PCR 10 μl reaction volume contained 500 nM of primers, a 250 nM probe (PrimeTime qPCR assay, Integrated DNA technologies), 5.0 μl Brilliant III Ultra-Fast QPCR master mix (Agilent Technologies Inc.), 0.15 μl reference dye mixture, and 4.35 μl of sample cDNA (final cDNA dilution: 0.5 μg/μl in each PCR reaction). Samples were analyzed using an Agilent AriaMx Real-Time PCR System (Agilent Technologies Inc.) running 1 cycle at 95 °C for 3 min, then 40 cycles of 95 °C for 5 sec and 60 °C for 20 sec. Mitophagy related genes included PINK1, PARK2, BNIP3, and BNIP3L. Genes involved in mitochondrial biogenesis were PGC-1α, NRF1, GA-binding protein alpha (GABPA) also referred to as NRF2, TFAM, ERRα, and VEGF. Probes and Primers that targeted the specific gene sequences were attained from Integrated DNA technologies (Coralville, Iowa). See Table 1 for all probe and primer sequences used for qRT-PCR.
Table 1:
Probes and primers used for real-time reverse transcription quantitative PCR
| References Gene | Primer 1 | Primer 2 | Probe |
|---|---|---|---|
| ACTB | AAGTCAGTGTACAGGTAAGCC | GTCCCCCAACTTGAGATGTATG | CTGCCTCCACCCACTCCA |
| B2M | ACCTCCATGATGCTGCTTAC | GGACTGGTCTTTCTATCTCTTGT | CCTGCCGTGTGAACCATGTGACT |
| CYC | TCTTTCACTTTGCCAAACACC | CATCCTAAAGCATACGGGTCC | TGCTTGCCATCCAACCACTCAGTC |
| RPS18 | GTCAATGTCTGCTTTCCT CAAC | GTT CCA GCA TAT TTT GCG AGT | TCT TCG GCCCAC ACC CTT AAT GG |
| GAPDH | TGTAGTTGAGGTCAATGAAGGG | ACATCGCTCAGACACCATG | AAGGTCGGAGTCAACGGATTTGGTC |
| Mitophagy Genes | Primer 1 | Primer 2 | Probe |
| PINK1 | GTTGCTTGGGACCTCTCTTG | TGAACACAATGAGCCAGGAG | TGTAAGTGACTGCTCCATACTCCCCA |
| PARK2 | GCTTGGTGGTTTTCTTGATGG | TTGAAGCCTCAGGAACAACT | CCTGCTCGGCGGCTCTTTCA |
| BNIP3 | CCACTAACGAACCAAGTCAGAC | CATCTCTGCTGCTCTCTCAT | AAAGGTGCTGGTGGAGGTTGTCA |
| BNIP3L | CAAACATGATCTGCCCATCTTC | TCCTCATCCTCCATCCACAA | TCTCACTGTGACAGCCCTTCGC |
| Biogenesis Genes | Primer 1 | Primer 2 | Probe |
| PPARGC1A | GCAATCCGTCTTCATCCACA | CCAATCAGTACAACAATGAGCCT | AGCAGTCCTCACAGAGACACTAGACAG |
| NRF1 | GTCATCTCACCTCCCTGTAAC | GATGCTTCAGAATTGCCAACC | ATGGAGAGGTGGAACAAAATT GGGC |
| NRF2 (GABPA) | TGTAGTCTTGGTTCTAGCAGTTTC | TGGAACAGAGAAAGCAGAGTG | TGGTTCATTGAT GTCTATGGCCTGGC |
| TFAM | GCCAAGACAGATGAAAACCAC | TGGGAAGGTCTGGAGCA | CGCTCCCCCTTCAGTTTTGTGTATTT |
| ESRRA | TCTCCGCTTGGTGATCTCA | CTATGGTGTGGCATCCTGTG | TGGTCCTCTTGAAGAAGGCTTTGCA |
| VEGFA | GCGCTGATAGACATCCATGA | CCATGAACTTTCTGCTGTCTTG | TGCTCTACCTCCACCATGCCAAG |
Quantification of mRNA for genes of interest were computed on 3 h and 6 h post-exercise muscle samples relative to pre-exercise using the 2−ΔΔCT method [35]. Normfinder software (Andersen, Jensen, & Orntoft, 2004) was used to determine stable housekeeping genes. Fold-change data was log transformed to create a normal distribution used for statistical analysis. The geometric mean of the following five housekeeping genes were used as the stable reference point: beta-actin (ACTB), beta-2-microglobulin (B2M), cyclophilin (CYC), ribosomal protein S18 (RPS18), and glyceraldehyde-3 phosphate dehydrogenase (GAPDH) for each participant. Average coefficient of variation for housekeeping gene triplicates was 1.81 ± 0.10%.
Statistical analysis
Differences in average core temperature, skin temperature, heart rate, sweat rate, and RPE were analyzed via paired samples t tests. Gene expression was analyzed using repeated measures two-way ANOVA (exercise × temperature) using SPSS for windows Version 25 (Chicago, IL). If a significant F-ratio was detected, Fishers protected least significant difference method was used to determine where differences occurred. A probability of type I error less than 5% (p < 0.05) was deemed significant. Effect size was reported by ηp2 for main effects and by coehn’s d for t tests and pairwise comparisons.
Results
Descriptive data of study participants is depicted in Table 2. During exercise, average skin temperature, heart rate, and sweat rate were lower in the cold compared to room temperature (p = 0.004, d = 0.49; p < 0.001, d = 4.98; p = 0.005, d = 1.27), while average core temperature was slightly higher in the cold (p = 0.016, d = −0.81). Average rating of perceived exertion (RPE) was not different between temperatures (p = 0.179, d = 0.49; Table 3).
Table 3.
Study parameters measured during exercise
| RT | Cold | |
|---|---|---|
| Core temperature (°C) | 38.4 ± 0.2 | 38.9 ± 0.3* |
| Skin temperature (°C) | 33.7 ± 0.3 | 28.0 ± 0.4* |
| HR (bpm) | 160 ± 4 | 154 ± 4* |
| Sweat Rate (l/min) | 0.80 ± 0.08 | 0.42 ± 0.11* |
| RPE | 14 ± 0.5 | 14 ± 0.5 |
Data are means ± SE (n = 11). Values represent 1 h average of entire exercise bout. RT, room temperature; HR, heart rate; RPE, rating of perceived exertion.
p < 0.05 from Room Temperature.
Gene expression for mitochondrial biogenesis
There were no temperature differences (p > 0.05, average ηp2 = 0.086) or exercise by temperature interaction effects (p > 0.05, average ηp2 = 0.097) in gene expression for PGC-1α, VEGF, NRF1, NRF2, ERRα, or TFAM. PGC-1α and VEGF were higher after cycling at the 3 h (p < 0.001, d = 5.52; p < 0.001, d = 2.34) and 6 h post-exercise (p < 0.001, d = 4.95; p < 0.001, d = 3.72) time points. PGC-1α expression was reduced 6 h post-exercise compared to 3 h post-exercise (p = 0.006, d = 0.60) though still above baseline levels. NRF1 was lower after 3 h (p = 0.005, d = 1.27) and 6 h (p < 0.001, d = 1.56) of recovery from exercise. NRF2 and ERRα decreased 3 h post-exercise (p = 0.035, d = 0.71; p = 0.005, d = 1.17) but were higher 6 h post-exercise compared to the 3 h time point (p = 0.017, d = 0.81; p = 0.006, d = 1.01). TFAM increased 6 h post-exercise compared to both pre-exercise (p = 0.028, d = 0.59) and 3 h post-exercise levels (p < 0.001, d = 0.97; Figure 1).
Figure 1.
mRNA response of genes related to mitochondrial biogenesis at 3 h and 6 h post-exercise normalized to pre-exercise conditions. (A) PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (B) VEGF, vascular endothelial growth factor (C) NRF1, nuclear respiratory factor 1 (D) NRF2, nuclear respiratory factor 2 (E) ERRα, estrogen-related receptor alpha (F) TFAM, transcription factor A mitochondrial. Data are expressed as means ± SE (n = 11). * p < 0.05 from pre-exercise; † p < 0.05 from 3 h post-exercise.
Gene expression for mitophagy
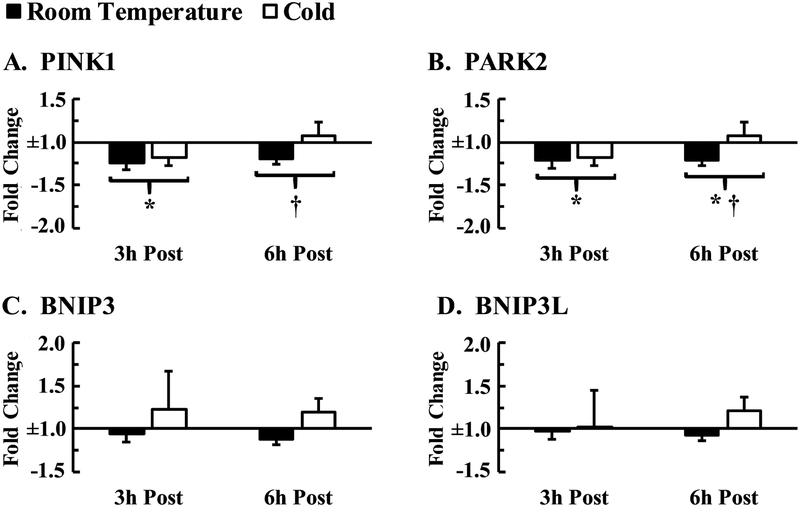
Gene expression of PINK1, PARK2, BNIP3, and BNIP3L was unaffected by temperature (p > 0.05; average ηp2 = 0.032) nor were there any interaction effects between exercise and temperature (p > 0.05; average ηp2 = 0.058). PINK1 and PARK2 decreased 3 h post-exercise compared to pre-exercise (p = 0.002, d = 0.92; p = 0.001, d = 0.90). This downregulation for both PINK1 and PARK2 was diminished after 6 h of recovery (p = 0.043, d = 0.39; p = 0.047, d = 0.36), but PARK2 expression remained lower than pre-exercise levels (p = 0.044, d = 0.49). BNIP3 and BNIP3L mRNA were not influenced by exercise (p = 0.329, ηp2 = 0.105; p = 0.233, ηp2 = 0.135; Figure 2).
Figure 2.
mRNA response of genes related to mitophagy at 3 h and 6 h post-exercise normalized to pre-exercise conditions. (A) PINK1, PTEN-induced kinase 1 (B) PARK2, parkin RBR E3 ubiquitin protein (C) BNIP3, bcl-2/adenovirus E1B 19-kDa interacting protein 3 (D) BNIP3L, bcl-2/adenovirus E1B 19-kDa interacting protein 3-like. Data are expressed as means ± SE (n = 11). * p < 0.05 from pre-exercise; † p < 0.05 from 3 h post-exercise.
Discussion
Mitochondrial dysfunction is implicated in aging and a wide array of chronic diseases [12,18,29]. However, it is well-documented that exercise stimulates beneficial remodeling of the mitochondrial network [3,24,49]. Thus, placing efforts towards the development of specific exercise regimens to heighten mitochondrial adaptation is of great importance, with cold treatments emerging as a potential therapy. Previous human studies report signs of increased mitochondrial biogenesis after training and recovery in a cold (7 °C) environment over that of moderate temperate environments [48,49]. When explored separately, it was discovered that the presence of an exercise stimulus was a critical component to any cold related differences between temperatures [46,58]. While the aforementioned research focuses on mitochondrial biogenesis, markers of mitophagy after these interventions have not been inspected. Both processes are necessary for proper cell functioning as damaged organelles must be eliminated and replaced. Therefore, by exposing participants to below freezing temperatures (−2 °C) during cycling, the present study attempted to induce a greater thermoregulatory response than previous investigations and elicit enhanced activation of genes linked to mitochondrial adaptation compared to exercise at room temperature. Contrary to the previous work, the current study did not observe any statistically significant acute effects of temperature on gene expression.
The whole-body physiological response to exercise and environmental cold may be used to supplement mRNA data and provide potential explanation for why changes in gene signaling may have occurred. Cycling in the cold yielded higher core temperatures compared to that at room temperature. Though this may seem counterintuitive, previous literature reveals either no difference or slightly higher core temperatures in the cold during both exercise and at rest [46,49,58]. We hypothesized that implementing a colder temperature than the aforementioned investigations would limit the rise in core temperature during exercise. However, this did not occur and thereby demonstrates the thermoregulatory capacity of the human body. The subject population consisted of healthy, young individuals who were able to overcome the cold stimulus as the body sought to sustain temperature homeostasis. As expected, skin temperature was lower in the cold. Decrements in skin temperature are instrumental for promoting β3-adrenergic receptor mediated increases in PGC-1α transcription [16], but the exercise stimulus in our study may have been intense enough to override any temperature affiliated modifications. RPE was the same in all conditions despite a lower heart rate in the cold, indicating that subjects experienced the same perceived intensity in both trials. A differential heart rate response to temperature has been affirmed by other studies as well [14,15,20]. It is postulated that changes in heart rate may be due to peripheral vasoconstriction in the cold. This redistributes more blood centrally to the heart leading to enhanced stroke volume and thus a lower heart rate for a given cardiac output [40]. Additionally, sweat production was diminished in the cold, which preserves blood volume and allows the maintenance of a lower heart rate. While the expected physiological variables were altered with the prescribed exercise protocol and temperature stimulus, these deviations did not result in statistically significant changes in gene expression related to mitophagy or mitochondrial biogenesis.
Gene expression for mitochondrial biogenesis
Mitochondrial biogenesis is necessary for the maintenance of a healthy mitochondrial network and is regulated by PGC-1α, which is responsive to metabolic and thermoregulatory stressors. PGC-1α targets downstream factors essential to mitochondrial energy production, including NRF1, NRF2, and ERRα [13,25,56]. Exercise promotes PGC-1α transcription via activation of AMP-activated protein kinase (AMPK), Ca2+/calmodulin-dependent protein kinase IV (CaMKIV), calcineurin (CnA), and p38 mitogen-activated protein kinase (p38 MAPK) whereas cold exposure enhances expression by stimulating B3-adrenergic receptors (B3-AR) [10,16,27]. These receptors arbitrate activation of cAMP response element-binding protein (CREB), thus promoting transcription of PGC-1α [16]. In the present study, PGC-1α mRNA increased with exercise, but there were no significant variations with temperature. Though not statistically significant, it is worth noting that PGC-1α gene expression was slightly higher 3 h post-exercise in the cold trail compared to room temperature (8.7 ± 1.4 vs. 7.1 ± 1.2 fold change). Expression remained non-significantly higher in the cold trail after 6 h of recovery (6.9 ± 1.6 vs. 4.3 ± 0.63 fold change). This cold-induced exacerbation in PGC-1α mRNA at the 3 h post-exercise time point was slightly less robust than observed in previous studies [48,49]; however, a similar pattern is observed. The present investigation offers unique insight by also measuring expression after 6 h of post-exercise recovery, which shows a greater difference (although not statistically significant) between cold and thermoneutral temperatures than at 3 h. Collectively, the data from the current and previous studies suggests that PGC-1α may be augmented to a greater extent, for a longer period of time, or have an altered time course of peak expression following cycling in a cold environment. It is unknown if this difference may lead to a greater increase in mitochondrial quantity and metabolic capacity after repeated bouts of exercise in the cold.
The PGC-1α signaling cascade facilitates NRF1 and NRF2 activation. These transcription factors are involved in electron transport chain complex expression as well as binding to the promotor region of TFAM, which controls the duplication of mitochondrial DNA [25,34,42,52]. Exercise marginally attenuated NRF1 and NRF2 mRNA 3 h post-exercise, but this downregulation was diminished for NRF2 after the 6 h recovery period. Increased transcription for NRF1 and NRF2 after acute exercise is commonly reported [41,54]. However, our subjects appear to be more trained and cycled for a shorter duration, which could have limited the gene response. Additionally, the quantification of mRNA provides insight regarding the transient signaling that occurs prior to protein synthesis, but depending on the availability of cellular resources, mRNA and protein expression are not always perfectly correlated [21]. Given the very small changes in NRF1 mRNA, it is unlikely that it had major implications on overall mitochondrial capacity. Cold exposure during exercise was previously found to have a negative influence on NRF2 gene expression, apparent from muscle biopsies taken 3 h post-exercise [46]. The data from the current study reveals that a longer recovery period, such as 6 h, may lead to different conclusions in that NRF2 was higher at 6 h than 3 h. Congruent with this theme, TFAM was unaltered at 3 h but then was elevated 6 h post-exercise compared to all other time points. This gene has been shown to take 6 h for peak expression [38], which may explain the absence of mRNA changes overserved in response to an exercise bout of similar intensity, duration, and subject fitness level when muscle samples were taken 3 h after exercise [46].
ERRα takes part in fatty acid oxidation and activates VEGF, which promotes increased capillarization as well as import of proteins to the mitochondria to aid in biogenesis [22,43,55]. Expression for ERRα was slightly below baseline 3 h post-exercise, but mRNA levels rebounded from this drop at the 6 h time point in a similar fashion as NRF2 and TFAM. In past studies involving exercise and environmental cold, ERRα transcription was only inhibited in cold trials [46,49] whereas the current data indicates no difference between temperatures. VEGF expression was enhanced with exercise in all conditions, which is in agreement with other research and likely mediated by nitric oxide release to augment blood flow to working muscles [19,46].
Gene expression for mitophagy
The proposed mechanism for thermogenic related alterations in mitophagy is similar to the pathway for cold-mediated mitochondrial biogenesis and occurs via noradrenergic signaling to activate cAMP and PKA [7]. PKA directly inhibits gene expression of lysosomal autophagy pathway elements and also works synergistically with mTORC1 to mitigate nuclear relocation of transcription factors essential to autophagy [1]. Nonetheless, the lack of temperature sensitivity found in the present exploration could be attributed to the length of cold exposure, the tissue type being examined, or the exercise stimulus could have masked any temperature-associated changes. To our knowledge, no research has assessed the impact of resting temperature interventions on skeletal muscle mitophagy in humans, but it has been reported that 3 h of seated rest at 7 °C is not sufficient to activate most genes connected to mitochondrial biogenesis [58].
Mitophagy preserves cell vitality by targeting dysfunctional mitochondrial for degradation and is modulated by several proteins, including PINK, PARK2, BNIP3, and BNIP3Ll. When mitochondrial permeability is disrupted, Pink assembles and triggers Parkin localization to promote envelopment by the phagophore membrane [2,53,57]. BNIP3 and BNIP3L serve as receptors to bind the mitochondria to the autophagosome and allow for engulfment [31] Only 2 of the 4 mitophagy genes inquired were affected by exercise, PINK1 and PARK2. The observed reductions in PINK1 and PARK2 mRNA after 3 h of recovery may serve as an immediate protective mechanism to preserve mitochondria in response to temporary disruptions of the cellular milieu caused by exercise. Overall mRNA levels were restored after 6 h, though still slightly below baseline. Furthermore, it should be noted that these alterations with exercise were very small and it is unknown how it may translate to an applied physiological outcome.
Nutritional status may play an important role in the expression of mitophagy related genes. Participants in the current investigation were approaching a 17 h fast by the 3 h post-exercise biopsy. Interestingly, it has been reported that 2 h of cycling leads to increased gene expression of PARK2, BNIP3, and BNIP3L immediately post-exercise in a fed but not fasted state [44]. Aside from nutritional considerations, part of the paradoxical findings between our study and that by Schwalm et al. [44] may relate to the longer exercise duration or the immediately post-exercise muscle biopsy timing implemented in their study. No direct research has investigated the time-course of exercise induced transcription of mitophagy related genes and indeed it may be possible that the 3 h and 6 h post-exercise biopsies may have been either too early or too late to detect potential alterations. Past research reveals increased BNIP3 and BNIP3L mRNA levels after a 200-km running event [24], further supporting the notion that a greater exercise stimulus may be needed to enhance gene induction concomitant with mitophagy.
A unique observation is that at the 6 h post-exercise time point, expression was non-significantly higher in the cold trial than the room temperature trial for every gene explored. The potentially higher mRNA related to both mitochondrial biogenesis and mitophagy 6 h after exercise in the cold may suggest that the time-course of mitochondrial network remodeling is altered by the cold. Theoretically, enhancing the processes of mitochondrial replication and elimination should improve mitochondrial function and overall health. Future research should also seek to confirm these findings and examine the impact of multiple cold training sessions on parameters such as mitochondrial number, mitochondrial respiratory capacity, protein levels, and whole-body function.
This was the first study to scrutinize aspects of both mitochondrial biogenesis and mitophagy succeeding an environmental cold exercise session in a human population. It was postulated that imposing a greater thermogenic stress than former inquiries would incite cold-activated enhancements in mitochondrial development without the need for a post-exercise cold recovery period. No significant temperature alterations were discovered, but this study provided a promising framework for the development of future temperature therapies to maximize mitochondrial adaptation. When the current data is considered along with our previous work [46,48], it appears that the time of cold exposure may be a more important factor than how cold the environment is in order for cold induced altererations in the transcriptional response to exercise to be observed.
Funding:
This work was supported by the National Institute for General Medical Science [NIGMS P20GM103427], Nebraska IDeA Networks of Biomedical Research Excellence [INBRE], and a Graduate Research and Creative Activity Grant.
Funding sources had no involvement in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- [1].Altshuler-Keylin S, Kajimura S, Mitochondrial homeostasis in adipose tissue remodeling, Sci. Signal 10 (2017) eaai9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ashrafi G, Schwarz TL, The pathways of mitophagy for quality control and clearance of mitochondria, Cell Death Differ 20 (2012) 31–42. https://doi:10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO, Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1, The FASEB Journal 16 (2002) 1879–1886. [DOI] [PubMed] [Google Scholar]
- [4].Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, Mazure NM, Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains, Mol. Cell. Biol 29 (2009) 2570–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bergstrom J, Muscle electrolytes in man determined by neutron activation analysis on needle biopsy specimens, Scandinavian Journal of Clinical and Laboratory Investigation (England) 14 (1962). [Google Scholar]
- [6].Borg GA, Perceived exertion: a note on “history” and methods, Med. Sci. Sports 5 (1973) 90–93. [PubMed] [Google Scholar]
- [7].Cairó M, Villarroya J, Cereijo R, Campderrós L, Giralt M, Villarroya F, Thermogenic activation represses autophagy in brown adipose tissue, Int. J. Obes 40 (2016) 1591. [DOI] [PubMed] [Google Scholar]
- [8].Chen H, Chan DC, Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases, Hum. Mol. Genet 18 (2009) R176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M, Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin, Nature 441 (2006) 1162–1166. [DOI] [PubMed] [Google Scholar]
- [10].Combes A, Dekerle J, Webborn N, Watt P, Bougault V, Daussin FN, Exercise-induced metabolic fluctuations influence AMPK, p38-MAPK and CaMKII phosphorylation in human skeletal muscle, Physiological Reports 3 (2015) 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cookson MR, Parkinsonism due to mutations in PINK1, parkin, and DJ-1 and oxidative stress and mitochondrial pathways, Cold Spring Harbor Perspectives in Medicine 2 (2012) a009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ding W, Yin X, Mitophagy: mechanisms, pathophysiological roles, and analysis, Biol. Chem 393 (2012) 547–564. 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dominy JE, Puigserver P, Mitochondrial biogenesis through activation of nuclear signaling proteins, Cold Spring Harbor Perspectives in Biology 5 (2013) a015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Doubt TJ, Physiology of exercise in the cold, Sports Medicine 11 (1991) 367–381. [DOI] [PubMed] [Google Scholar]
- [15].Febbraio MA, Snow RJ, Stathis CG, Hargreaves M, Carey MF, Blunting the rise in body temperature reduces muscle glycogenolysis during exercise in humans, Experimental Physiology: Translation and Integration 81 (1996) 685–693. [DOI] [PubMed] [Google Scholar]
- [16].Fernandez-Marcos PJ, Auwerx J, Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis–, Am. J. Clin. Nutr 93 (2011) 890S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ganesan S, Pearce SC, Gabler NK, Baumgard LH, Rhoads RP, Selsby JT, Short-term heat stress results in increased apoptotic signaling and autophagy in oxidative skeletal muscle in Sus scrofa, J. Therm. Biol 72 (2018) 73–80. [DOI] [PubMed] [Google Scholar]
- [18].Garcia-Prat L, Martinez-Vicente M, Perdiguero E, Ortet L, Rodriguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Autophagy maintains stemness by preventing senescence, Nature 529 (2016) 37. [DOI] [PubMed] [Google Scholar]
- [19].Gavin TP, Spector DA, Wagner H, Breen EC, Wagner PD, Nitric oxide synthase inhibition attenuates the skeletal muscle VEGF mRNA response to exercise, J. Appl. Physiol 88 (2000) 1192–1198. [DOI] [PubMed] [Google Scholar]
- [20].González-Alonso J, Human thermoregulation and the cardiovascular system, Exp. Physiol 97 (2012) 340–346. [DOI] [PubMed] [Google Scholar]
- [21].Gry M, Rimini R, Strömberg S, Asplund A, Pontén F, Uhlén M, Nilsson P, Correlations between RNA and protein expression profiles in 23 human cell lines, BMC Genomics 10 (2009) 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Heil M, Eitenmüller I, Schmitz-Rixen T, Schaper W, Arteriogenesis versus angiogenesis: similarities and differences, J. Cell. Mol. Med 10 (2006) 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Holloszy JO, Booth FW, Biochemical adaptations to endurance exercise in muscle, Annu. Rev. Physiol 38 (1976) 273–291. 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- [24].Jamart C, Benoit N, Raymackers J, Kim HJ, Kim CK, Francaux M, Autophagy-related and autophagy-regulatory genes are induced in human muscle after ultraendurance exercise, Eur. J. Appl. Physiol 112 (2012) 3173–3177. [DOI] [PubMed] [Google Scholar]
- [25].Jornayvaz FR, Shulman GI, Regulation of mitochondrial biogenesis, Essays Biochem 47 (2010) 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ju J, Jeon S, Park J, Lee J, Lee S, Cho K, Jeong J, Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition, The Journal of Physiological Sciences 66 (2016) 417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jung S, Kim K, Exercise-induced PGC-1α transcriptional factors in skeletal muscle, Integrative Medicine Research 3 (2014) 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kanki T, Nix: A receptor protein for mitophagy in mammals, Autophagy 6 (2010) 433–435. [DOI] [PubMed] [Google Scholar]
- [29].Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF, Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms, Trends Neurosci 40 (2017) 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim I, Rodriguez-Enriquez S, Lemasters JJ, Selective degradation of mitochondria by mitophagy Arch. Biochem. Biophys 462 (2007) 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kubli DA, Gustafsson AB, Mitochondria and mitophagy, Circ. Res 111 (2012) 1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee H, Paik S, Regulation of BNIP3 in normal and cancer cells. Molecules & Cells (Springer Science & Business Media BV) 21 (2006) 1–2. [PubMed] [Google Scholar]
- [33].Lemasters JJ, Nieminen A, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy, Biochimica et Biophysica Acta (BBA)-Bioenergetics 1366 (1998) 177–196. [DOI] [PubMed] [Google Scholar]
- [34].Lin J, Handschin C, Spiegelman BM, Metabolic control through the PGC-1 family of transcription coactivators, Cell Metabolism 1 (2005) 361–370. [DOI] [PubMed] [Google Scholar]
- [35].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method, Methods 25 (2001) 402–408. [DOI] [PubMed] [Google Scholar]
- [36].Lu J, Qian H, Liu L, Zhou B, Xiao Y, Mao J, An G, Rui M, Wang T, Zhu C, Mild hypothermia alleviates excessive autophagy and mitophagy in a rat model of asphyxial cardiac arrest, Neurological Sciences 35 (2014) 1691–1699. [DOI] [PubMed] [Google Scholar]
- [37].Mitchell CR, Harris MB, Cordaro AR, Starnes JW, Effect of body temperature during exercise on skeletal muscle cytochrome c oxidase content, J. Appl. Physiol 93 (2002) 526–530. 10.1152/japplphysiol.00536.2001. [DOI] [PubMed] [Google Scholar]
- [38].Pilegaard H, Saltin B, Neufer PD, Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle, J. Physiol. (Lond.) 546 (2003) 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ross CI, Shute RJ, Ruby BC, Slivka DR, Skeletal muscle mRNA response to hypobaric and normobaric hypoxia after normoxic endurance exercise, High Alt. Med. Biol (2019). [DOI] [PubMed] [Google Scholar]
- [40].Rowell LB, Cardiovascular aspects of human thermoregulation. Circ. Res 52 (1983) 367–379. [DOI] [PubMed] [Google Scholar]
- [41].Saleem A, Hood DA, Acute exercise induces tumour suppressor protein p53 translocation to the mitochondria and promotes a p53–Tfam–mitochondrial DNA complex in skeletal muscle, J. Physiol. (Lond.) 591 (2013) 3625–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Scarpulla RC, Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network, Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1813 (2011) 1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A, The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor α (ERRα), J. Biol. Chem 278 (2003) 9013–9018. [DOI] [PubMed] [Google Scholar]
- [44].Schwalm C, Deldicque L, Francaux M, Lack of Activation of Mitophagy during Endurance Exercise in Human. Med. Sci. Sports Exerc 49 (2017) 1552–1561. [DOI] [PubMed] [Google Scholar]
- [45].Shi R, Zhu S, Li V, Gibson SB, Xu X, Kong J, BNIP3 interacting with LC3 triggers excessive mitophagy in delayed neuronal death in stroke, CNS Neuroscience & Therapeutics 20 (2014) 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shute RJ, Heesch MW, Zak RB, Kreiling JL, Slivka DR, Effects of exercise in a cold environment on transcriptional control of PGC-1α, American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 314 (2018) R857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Siri WE, Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition 9 (1993) 480. [PubMed] [Google Scholar]
- [48].Slivka DR, Dumke CL, Tucker TJ, Cuddy JS, Ruby B, Human mRNA response to exercise and temperature, Int. J. Sports Med 33 (2012) 94–100. [DOI] [PubMed] [Google Scholar]
- [49].Slivka D, Heesch M, Dumke C, Cuddy J, Hailes W, Ruby B, Effects of post-exercise recovery in a cold environment on muscle glycogen, PGC-1α, and downstream transcription factors, Cryobiology 66 (2013) 250–255. [DOI] [PubMed] [Google Scholar]
- [50].Thomas TR, Etheridge GL, Hydrostatic weighing at residual volume and functional residual capacity, J. Appl. Physiol 49 (1980) 157–159. [DOI] [PubMed] [Google Scholar]
- [51].Vainshtein A, Tryon L, Pauly M, Hood D, Acute exercise-induced mitophagy is mediated in part by PGC-1α, The FASEB Journal 29 (2015) 821. [Google Scholar]
- [52].Virbasius JV, Scarpulla RC, Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proceedings of the National Academy of Sciences 91 (1994) 1309–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, PINK1-dependent recruitment of Parkin to mitochondria in mitophagy, Proceedings of the National Academy of Sciences 107 (2010) 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wang L, Psilander N, Tonkonogi M, Ding S, Sahlin K, Similar expression of oxidative genes after interval and continuous exercise, Medicine & Science in Sports & Exercise 41 (2009) 2136–2144. [DOI] [PubMed] [Google Scholar]
- [55].Wright GL, Maroulakou IG, Eldridge J, Liby TL, Sridharan V, Tsichlis PN, Muise-Helmericks RC, VEGF stimulation of mitochondrial biogenesis: requirement of AKT3 kinase, The FASEB Journal 22 (2008) 3264–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yan Z, Lira VA, Greene NP, Exercise training-induced regulation of mitochondrial quality, Exerc. Sport Sci. Rev 40 (2012) 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Youle RJ, Narendra DP, Mechanisms of mitophagy, Nature Reviews Molecular Cell Biology 12 (2011) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zak RB, Shute RJ, Heesch MW, La Salle DT, Bubak MP, Dinan NE, Laursen TL, Slivka DR, Impact of hot and cold exposure on human skeletal muscle gene expression, Applied Physiology, Nutrition, and Metabolism 42 (2016) 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]