Abstract
Signal transmission from the mechanical forces to the various intracellular activities is a fundamental process during tissue development. Despite their critical role, the mechanism of mechanical forces in the biological process is poorly understood. In this study, we demonstrated that in the response to hydrostatic pressure (HP), the piezo type mechanosensitive ion channel component 1 (PIEZO1) is a primary mechanosensing receptor for odontoblast differentiation through coordination of the WNT expression and ciliogenesis. In stem cells from human exfoliated deciduous teeth (SHED), HP significantly promoted calcium deposition as well as the expression of odontogenic marker genes, PANX3 and DSPP, and WNT related-genes including WNT5b and WNT16, whereas HP inhibited cell proliferation and enhanced primary cilia expression. WNT signaling inhibitor XAV939 and primary cilia inhibitor chloral hydrate blocked the HP-induced calcium deposition. The PIEZO1 activator Yoda1 inhibited cell proliferation but induced ciliogenesis and WNT16 expression. Interestingly, HP and Yoda1 promoted nuclear translocation of RUNX2, whereas siRNA-mediated silencing of PIEZO1 decreased HP-induced nuclear translocation of RUNX2. Taken together, these results suggest that PIEZO1 functions as a mechanotransducer that connects HP signal to the intracellular signalings during odontoblast differentiation.
Subject terms: Mesenchymal stem cells, Stress signalling
Introduction
Mechanotransduction is one of the mechanisms by which cells sense the extracellular mechanical stimuli and convert them into intracellular biological signals. This form of sensory communication plays important roles not only in various physiological responses including proprioception, touch, balance, and hearing but also in the fundamental biological functions during organ development, tissue homeostasis, and disease conditions1. In multicellualar organisms, cells are surrounded by the extracellular fluid that is called tissue interstitial fluid. Therefore, the hydrostatic pressure (HP) via the tissue interstitial fluid is considered to be an important mechanical force in cells. However, the underlying mechanism of HP on odontogenesis has not yet established.
The tooth is a specialized tissue that is composed of three hard tissues, enamel, dentin and cementum, and soft tissue of the dental pulp. Tooth development is initiated by epithelial-mesenchymal interaction that mediates invagination of the oral epithelium-derived odontogenic epithelium into the condensed neural crest-derived ectomesenchyme, which results in the formation of tooth bud2. Odontogenic epithelial cells form into the enamel at the crown and cementum at the root. On the other hand, ectodermal mesenchymal cells are responsible for the dentin, the most abundant mineralized tissue in tooth. During dentin formation, the ectodermal mesenchymal cells form into a dental papilla. The peripheral dental papilla cells differentiate into preodontoblasts, creating a contiguous monolayer and expressing a gap junction protein pannexin 3 (PANX3)3. Then, the preodontoblasts differentiate into odontoblasts that secrete collagenous and non-collagenous proteins, including dentin sialophosphoprotein (DSPP) to the enamel side while receding to the dental pulp side4. The deposition of the initial dentin matrix leads to the formation of predentin, which subsequently mineralizes to form a dentin structure5. In this process, runt-related transcription factor 2 (RUNX2) is considered to be an essential transcription factor that regulates the differentiation of dental papilla cells to odontoblasts6,7.
Wnt signaling pathway is an important signal cascade that regulates cell fate determination during development, regeneration, and disease8. In tooth, several of the Wnt family members, including Wnt3, Wnt3a, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, Wnt7b, Wnt10a, and Wnt10b, are identified in dental epithelial and mesenchyme during tooth development9,10. The canonical Wnt signaling pathway depends on β-catenin and is involved in critical morphogenetic signaling at multiple stages of the tooth patterning and development11. The mutation of β-catenin results in the formation of large, misshapen tooth buds and ectopic teeth, whereas the deletion of β-catenin leads to inhibit or arrest of tooth development at the early bud stage12. Furthermore, the activation of the canonical Wnt signaling promotes reparative dentin formation13,14. Thus, the Wnt signaling pathway plays a crucial role in tooth development and dentin repair15.
Almost all eukaryotic cells generate one primary nonmotile cilium that is a specialized protruding structure on the cell surface16 and acts as a mechano/chemosensor17. The primary cilia are formed in quiescent cells and are generated from centrosomes that act a microtubule-organizing center from the G1 to G2 phase of the cell cycle so that primary cilia play an important role in the inhibition of cell proliferation16. Furthermore, abnormal or dysfunctional cilia caused by genetic mutations are associated with ciliopathies that comprise a wide range of symptoms such as primarily retinal degeneration, cerebral anomalies, obesity, and skeletal malformation18. Orofaciodigital syndrome (OFD) and Ellis-van Creveld syndrome, with abnormalities in the oral and maxillofacial region and limbs, are craniofacial ciliopathies, and show abnormal tooth development including dentinogenesis imperfecta19, indicating that primary cilia may be involved in odontoblast differentiation. Recently, several receptors, including Wnt20, sonic hedgehog21, Notch22, and transforming growth factors (TGFβ)23 were identified at the membrane of primary cilia with high density. Thus, primary cilia are also considered to be an essential signaling organelle as an antenna to regulate cell differentiation. However, the molecular mechanisms of ciliogenesis and its role in odontogenesis have not been elucidated.
Piezo type mechanosensitive ion channel component 1 (PIEZO1), also known as FAM38A, is a large transmembrane protein and is conserved among various species24. PIEZO1 plays critical roles in the mammalian physiology, including touch, pain sensation, hearing, and blood pressure regulation25. Mutations of PIEZO1 genes have been shown to cause hereditary stomatocytosis26. PIEZO1 is also involved in the regulation of neural stem cell differentiation and participates in the mechanosensitive lineage choice of neural stem cells27. We have previously reported that PIEZO1 functions as a signal receptor of hydrostatic pressure (HP), and regulates cell fate determination of human mesenchymal stem cell28. Furthermore, Piezo1 is expressed in mouse embryonic stem cells and controls cell proliferation29. These results suggest that PIEZO1, as a mechanosensing receptor, may play a crucial role in odontogenesis from multipotent stem cells in tooth.
In this study, we demonstrated that HP promotes odontoblasts differentiation and mineralization of multipotent stem cells from human exfoliated deciduous teeth (SHED) through Wnt16 expression and ciliogenesis, which is mediated by PIEZO1. We also found that both HP and PIEZO1 activator Yoda1 regulate nuclear translocation of runt-related transcription factor 2 (RUNX2) that is a critical transcription factor for odontoblast differentiation. Our results revealed that PIEZO1, a mechanosensing receptor, acts as a conductor to lead a signaling network connecting between mechanical stimuli to chemical signaling in odontoblast differentiation.
Results
Sustained HP promotes odontogenic differentiation of SHED
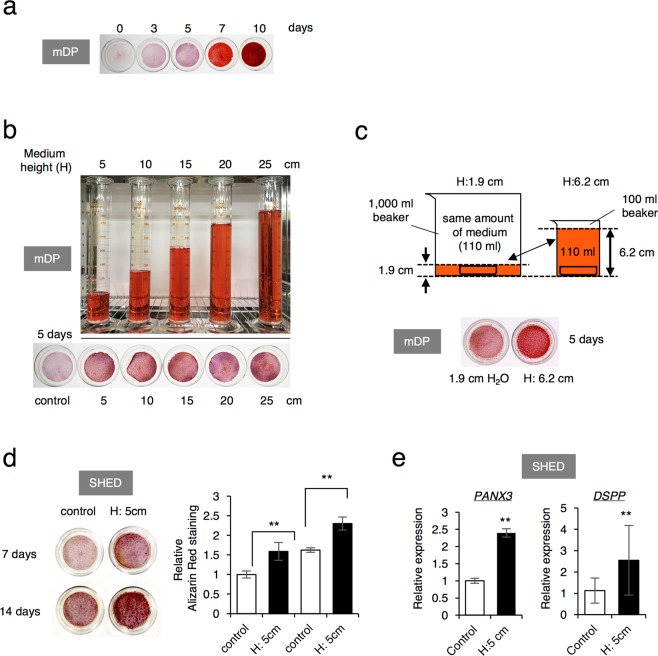
Mouse odontogenic cell line, mDP, is derived from dental pulp and has the potential to differentiate and mineralize in vitro. We cultured these cells in calcification induction media for 10 days and demonstrated their mineralization capacity by staining with Alizarin red S (Fig. 1a). Using this cell line, we first tested whether sustained hydrostatic pressure (HP) affects the mineralization of mDP. To analyze the effect of HP loading on cell culture, we employed the cell culture system where the 35 mm cell culture dish was placed at the bottom of a sterilized syringe and then the media was applied for different heights ranging from 5 to 25 cm (Fig. 1b). Interestingly, we found that at 5 days of culture, the cells exposed to HP loading by the medium height of 5 cm or more showed stronger Alizarin Red S staining than control cells exposed to HP loading by the medium height of 0.3 cm with 2 ml of media (usually an average height for media) (Fig. 1b). Since each dish was cultured with the different volumes of the medium, those differences might affect the promotion of mineralization in the results. Therefore, to clarify whether this result was induced by differences in HP or by differences in the medium volume, the 35 mm cell culture dish was placed in a sterilized 100 ml or 1,000 ml beaker containing the same amount of medium and cultured for 5 days (Fig. 1c). The result showed that cells cultured with the higher level of the medium increased the Alizarin Red S staining when compared to cells with a lower level of medium (Fig. 1c). Thus, we attribute this to the higher HP due to liquid levels of the medium. Therefore, in the following experiments of sustained HP loading, a 35 mm cell culture dish was placed at the bottom of a 100 ml beaker with a medium height of 5 cm.
Figure 1.
Odontoblast differentiation of SHED in the response to the sustained HP. (a) Alizarin Red S staining of the mouse dental mesenchymal cell line mDP cultured with induction (differentiation) medium for 10 days. (b) Effects of the sustained hydrostatic pressure (HP) on the differentiation of mDP cells cultured with a different medium height of 5, 10, 15, 20, and 25 cm. Alizarin red S staining was pefomed after 5 days induction. (c) A schematic diagram shows the sustained HP methods using the same amount of medium at different medium height. (d) Alizarin Red S staining of multipotent stem cells from human exfoliated deciduous teeth (SHED) cultured for 7 and 14 days with or without the HP by the medium height of 5 cm (H: 5cm). The data was representative of 3 independent experiments showed similar results. (e) Expression levels of odontogenic marker genes in SHED. Total RNA was extracted after 72 hrs of odontogenic induction with or without the HP by the medium height of 5 cm (H: 5 cm). Gene expression was analyzed by real-time RT-PCR. The data were pooled from three independent experiments. The error bars indicate standard deviations. Statistical analysis was performed using analysis of variance (***p < 0.01).
To confirm the results of accelerated mineralization by HP loading in mDP cells, multipotent stem cells from human exfoliated deciduous teeth (SHED) were cultured with HP. Alizarin Red S staining revealed that HP promoted mineralization of SHED (Fig. 1d). Furthermore, in SHED, the gene expressions of Pannexin 3 (PANX3), a pre-odontoblast marker3, and dentin sialophosphoprotein (DSPP), a mature odontoblast marker4, was significantly increased by HP (Fig. 1e), indicating that sustained HP promoted cell differentiation of SHED to odontoblasts.
Sustained HP inhibits cell proliferation and induces ciliogenesis in SHED
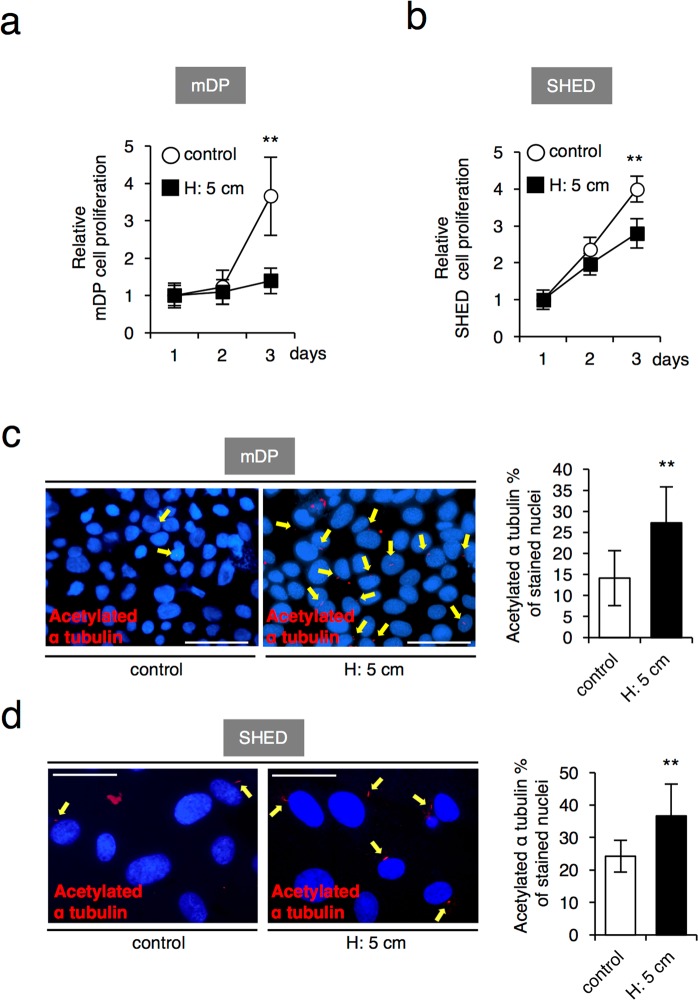
To examine the effect of HP on cell proliferation, mDP cells and SHED were cultured with or without HP for 3 days. The cell proliferation was assessed by cell counting method. The results indicate that HP significantly inhibited the cell proliferation of mDP cells (Fig. 2a) and SHED (Fig. 2b). Next, because primary cilia are derived from centrosomes and play a role in blocking cell division16,17, we examined whether HP induces primary cilia expression. Acetylation at the ε-amino group of K40 of α-tubulin is enriched in primary cilia and is used a cilia marker protein30. We found that HP markedly increased the number of acetylated α-tubulin positive cells in mDP (Fig. 2c) and SHED (Fig. 2d). These results suggest that the inhibition of cell proliferation by HP correlates with HP-induced ciliogenesis.
Figure 2.
Sustained HP inhibits cell proliferation and induces ciliogenesis in SHED. (a) Cell proliferation analysis by a cell counting method in mDP cells (a) and SHED (b). The cells were cultured with or without the HP by the medium height of 5 cm (H: 5cm) for 24, 48 and 72 hrs. (c,d) Immunostaining of acetylated α-tubulin in mDP cells (c), Scale bar: 50 μm; and SHED (d), Scale bar: 25 μm. The cells were cultured with or without the HP by the medium height of 5 cm (H: 5 cm) for 6 hrs, and then immunostaining was perfomed for acetylated α-tubulin. The acetylated α-tubulin-positive cells were counted in twenty randomly selected fields of view under an inverted microscope (20X magnification). The bar graph shows the percentage of α-tubulin-positive cells of total nuclear-stained cells. Red, acetylated α-tubulin; blue, DAPI. The data presented in (a,b) is a representative of three independent experiments showed similar results. (c,d) Represent the mean (±standard deviation, SD) of three independent experiments, and each performed in triplicate. Statistical analysis was performed using analysis of variance (**p < 0.01).
The primary cilium is essential for sustained HP-induced mineralization in SHED
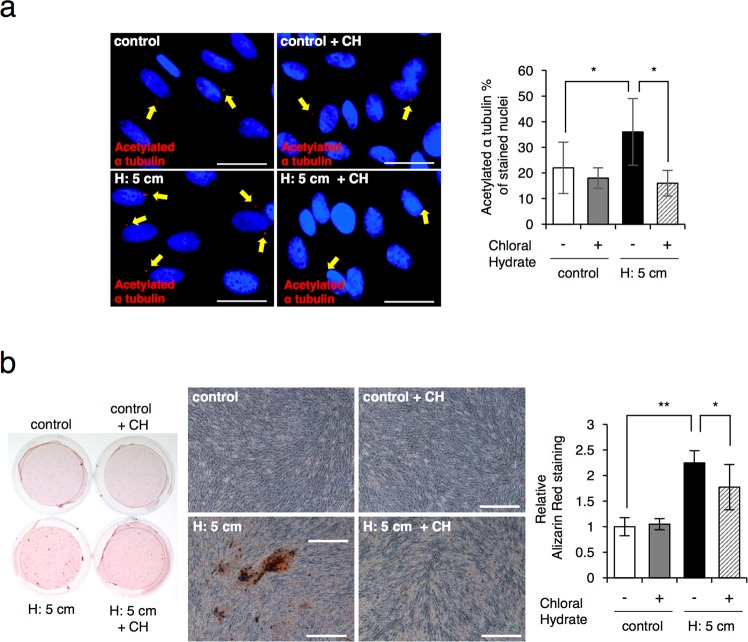
To test whether the HP-induced primary cilia expression is also required for accelerated mineralization of SHED, we treated SHED cells with chloral hydrate (CH) to inhibit primary cilia expression31. We showed that HP-induced primary cilia expression was inhibited by the treatment of 2 μM CH (Fig. 3a). Also, HP-induced calcium deposition decreased in CH-treated cells after 7 days of culture in differentiation media (Fig. 3b). These results suggest that primary cilia expression is involved in the HP-induced mineralization of SHED.
Figure 3.
Ciliogenesis is essential for HP induced mineralizeation in SHED. (a) Immunostaining and quantification of cilia formation. The SHED were cultured in the presence or absence of 2 μM chloral hydrate (CH) and with or without the HP by the medium height of 5 cm (H: 5cm) for 6 hrs. The acetylated α-tubulin-positive cells were counted in twenty randomly selected fields of view under an inverted microscope (20X magnification). The bar graph shows the percentage of acetylated α-tubulin-positive cells from total cells with nuclear staining. Scale bar: 25 μm. (b) Alizarin Red S staining for odontoblast differentiation in SHED cultured in induction media and 2 μM chloral hydrate (CH) for 7 days. Cells were also subjected with or without the HP by the medium height of 5 cm (H: 5 cm). The Alizarin Red-positive areas were quantified using ImageJ. Scale bar, 150 μm. The data, as shown, are the representative of three independent experiments with similar results, and error bars indicate standard deviations. Statistical analysis was performed using analysis of variance.
PIEZO1 is a primary mechanosensing receptor and regulates ciliogenesis in SHED
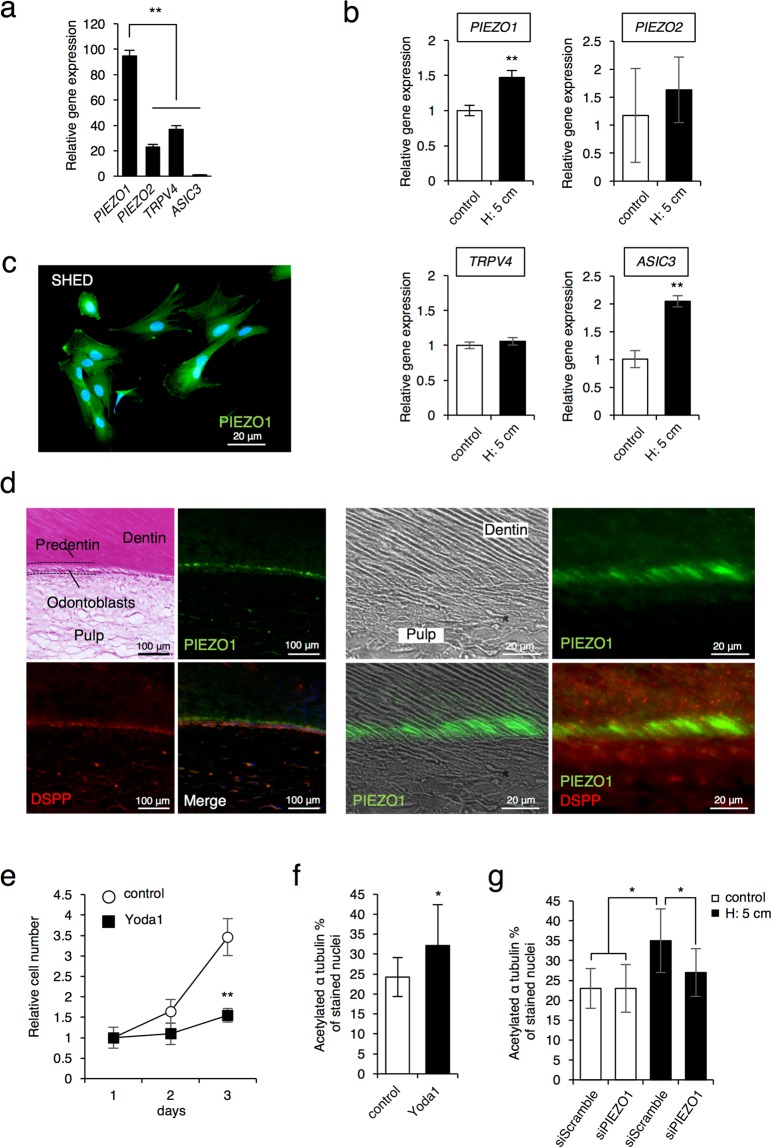
Our findings thus suggest an important role of primary cilia in cell proliferation and differentiation of SHED during HP loading. However, the mechanism of the ciliogenesis by HP loading is not known. Therefore, we analyzed the expression of mechanosensing receptors in SHED to identify the initial response of the receptors to HP. Quantitative gene expression analysis showed that among mechanosensitive receptors tested, piezo type mechanosensitive ion channel component 1 (PIEZO1) strongly expresses in SHED (Fig. 4a). Furthermore, we also found that after 24 hrs of HP loading, the PIEZO1 expression significantly increased (Fig. 4b). Although the expression of ASIC3 was also increased by HP loading, its expression level was low compared to the PIEZO1 expression. Thus, PIEZO1 may function as a primary mechanosensing receptor in SHED. Immunostaining analysis using a PIEZO1 antibody in SHED cells showed that PIEZO1 was localized in the plasma membranes and highly enriched at the cellular process (Fig. 4c). Furthermore, to analyze the expression of PIEZO1 in the human tooth, we performed immunohistochemistry analysis on tissue sections prepared from the extracted tooth, we observed a strong signal of PIEZO1 staining in the process of odontoblasts within predentin (Fig. 4d). These results suggest that PIEZO1 is a mechanosensing receptor in SHED and odontoblasts, and may function in cellular processes in response to extracellular stimuli during tooth development and dentin repair.
Figure 4.
PIEZO1 is primary mechanosensing recepor in SHED and odontoblasts. (a) Expression of mechanosensing receptors in SHED was examined by real-time RT-PCR. (b) Effect of the the HP by the medium height of 5 cm (H: 5 cm) on the expression of PIEZO1, PIEZO2, TRPV4, and ASIC3. SHED cells were cultured with osteogenic differentiation medium at 5 cm medium height (H: 5cm) for 24 hrs. The gene expression was examined by real-time RT-PCR. (c) Cellular localizations of PIEZO1 in SHED was determined by immunostaining. Green, PIEZO1; blue, DAPI; Scale bars, 20 μm. (d) Immunohistochemistry studies in the tooth section using PIEZO1 and DSPP antibodies. Green, PIEZO1; Red, DSPP, a marker of odontoblasts; blue, DAPI; Scale bars, 100 μm and 20 μm. (e) Effect of Yoda1 treatment on cell proliferation. SHED were culutured with or without 5 μM Yoda1 for 24, 48, and 72 hrs, and then cell proliferaiton was assessed by a cell counting method. (f) Effect of Yoda1 treatment on cilia formation. SHED cells were cultured with or without 5 μM Yoda1 for 6 hrs, and acetylated α-tubulin-positive cells were counted in twenty randomly selected fields of view under an inverted microscope (20X magnification). The bar graph shows the percentage of acetylated α-tubulin-expressing cells. (g) Effect of PIEZO1-specific siRNA transfection on cilia formation. SHED cells were transfected with scramble siRNA or PIEZO1-specific siRNA for 6 hrs and then cultured with or without the HP by the medium height of 5 cm (H: 5cm) for 6 hrs. The acetylated α-tubulin-positive cells were counted in twenty randomly selected fields of view under an inverted microscope (20X magnification). The bar graph shows the percentage of acetylated α-tubulin-expressing cells. The data presented in (a,b,e) are the representative of three independent experiments showed similar results. For the analyses, the represented in (f,g) show the data pooled from three independent experiments. The error bars indicate standard deviations. Statistical analysis was performed using analysis of variance (*p < 0.05, **p < 0.01).
To elucidate the function of PIEZO1 in SHED, we used a recently identified novel specific PIEZO1 agonist, Yoda128,32,33. SHED were treated with 5 μM Yoda1 for 3 days, and cell proliferation was determined by a cell counting method. We showed that the number of cells significantly decreased in Yoda1-treated cells when compared the dimethyl sulfoxide (DMSO)-treated control cells (Fig. 4e). Also, Yoda1-induced primary cilia expression (Fig. 4f), but the suppression of endogenous PIEZO1 expression by PIEZO1 siRNA inhibited HP-induced primary cilia expression (Fig. 4g). These results suggest that PIEZO1 regulates ciliogenesis under HP loading.
Sustained HP induced mineralization of SHED through modulation of WNT/β-catenin signaling
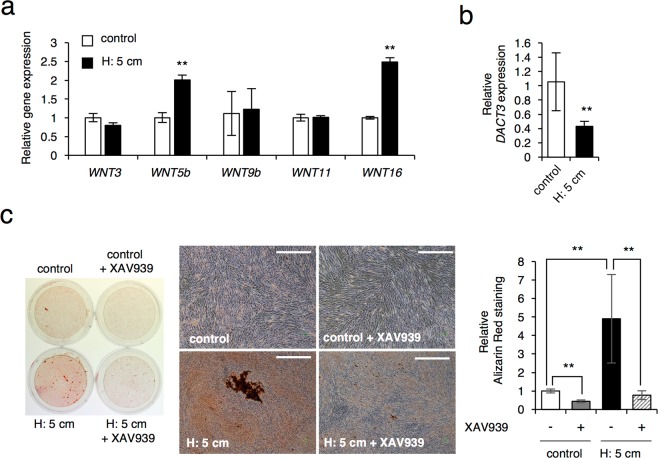
WNT is a secreted protein that is critically implicated in odontogenesis8. Wnt signaling is also mechanosensitive and involves acting downstream of mechanical stimuli in skeletogenesis34. Since HP promoted the odontogenic differentiation and mineralization in SHED (Fig. 1d,e), the expression of human WNT genes such as WNT1, 2, 3, 3a, 5a, 5b, 6, 7a, 7b, 8a, 8b, 9b, 10b, 11 and 16, were examined in SHED after culturing with HP for 24 hrs. We found that HP induced the expression of both WNT5b and WNT16 genes (Fig. 5a). There were no significant differences in the expressions of WNT3, 9b, and 11, and no satisfactory amplification products were obtained in others. Furthermore, Dishevelled, a central component of WNT signaling, interacts with dishevelled-binding antagonist of beta-catenin 3 (DACT3), which results in negative regulation of WNT signaling35. We found that HP significantly inhibited the DACT3 expression (Fig. 5b). These results suggest that the induction of mineralization by HP involves the activation of the WNT signaling pathway. Therefore, to confirm whether the WNT signal is necessary for the HP-induced mineralization of SHED, cells were differentiated in the presence of a WNT/β-catenin signaling selectively inhibitor XAV93936. XAV939, a tankyrase inhibitor, stabilizes axin by suppressing the poly-ADP-ribosylating enzymes tankyrase 1 and tankyrase 2 and is identified as a selective inhibitor of WNT signaling via β-catenin-mediated transcription36. We found that XAV939 markedly inhibited HP-induced mineralization (Fig. 5c), thus suggesting an important role of canonical WNT/β-catenin signal pathway in mineralization of SHED.
Figure 5.
WNT expression is essential for HP induced mineralization in SHED. (a,b) WNT and DACT3 expression in SHED cultured with or without HP by the medium height of 5 cm (H: 5cm) for 24 hrs. Total RNAs prepared from these cells were used for gene expression analysis by real-time RT-PCR for WNT (a) and DACT3 (b). (c) Effect of WNT inhibitor XAV939 on mineralization of SHED by Alizarin Red S staining. SHED were cultured for 7 days in odontogenic induction media with or without 10 μM XAV939 and with or without HP by the medium height of 5 cm (H: 5cm). The Alizarin Red-positive areas were quantified using ImageJ. Scale bar, 150 μm. The data are representative of three independent experiments with similar results, and error bars indicate standard deviations. Statistical analysis was performed using analysis of variance (**p < 0.01).
PIEZO1 regulates WNT16 expression that is involved in mineralization of SHED
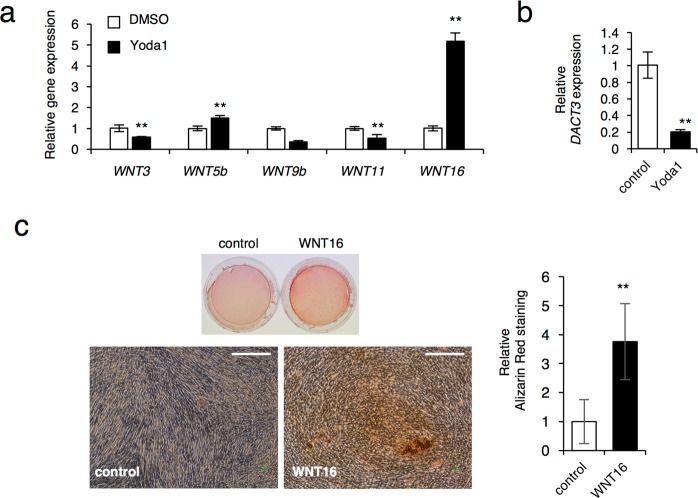
Our results, as shown above, thus suggests that PIEZO1 is a primary mechanosensing receptor in SHED (Fig. 4), which led us to hypothesize that PIEZO1 regulates the expression of WNT-related genes. Quantitative gene expression analysis showed that while the PIEZO1 activator Yoda1 markedly induced WNT16 expression (Fig. 6a), whereas it significantly inhibited the DACT3 expression (Fig. 6b) when compared with DMSO-treated control. These results are similar to those obtained with HP loading (Fig. 5a,b), suggesting that PIEZO1 plays a crucial role in the regulation of WNT gene expression when exposed to HP. However, no report is yet available to indicate that WNT16 is involved in odontogenesis. Therefore, we examined the role of WNT16 during the mineralization of SHED. We found that WNT16 increased Alizarin red S staining in SHED (Fig. 6c). Taken together, these results suggest that PIEZO1 regulates WNT16 expression, which further promotes the differentiation, maturation, and mineralization of SHED.
Figure 6.
Treatment of Yoda1 upregulates expression of WNT16 gene which promotes mineralization of SHED. (a,b) Expression of WNT and DACT3 genes. SHED were cultured with or without 5 μM Yoda1 for 24 hrs. Total RNA was prepared from the cells and analyzed by real-time RT-PCR for WNT expression (a) and DACT3 expression (b). (c) Alizarin Red S staining after WNT16 treatment to SHED. After 7 days of culture of SHED in odontogenic induction media in the presence or absence of 300 ng/ml exogenous WNT16, Alizarin Red staining was performed in SHED. The Alizarin Red-positive areas were quantified using ImageJ. Scale bar, 150 μm. The data, as shown, are representative of three independent experiments with similar results, and error bars indicate standard deviations. Statistical analysis was performed using analysis of variance (**p < 0.01).
Activation of PIEZO1 induces the nuclear translocation of RUNX2
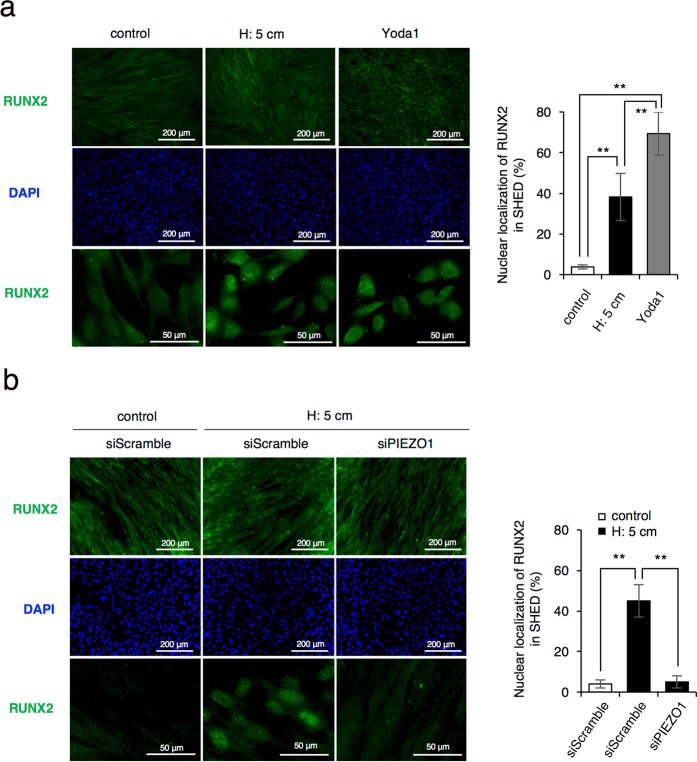
Runt-related transcription factor 2 (RUNX2) is a critical transcription factor for osteoblast and odontoblast differentiation7,37,38. The activity of the canonical Wnt/β-catenin pathway is modulated by Runx239,40. Therefore, we assessed whether HP loading or Yoda1 affects the intracellular localization of RUNX2. Interestingly, we observed that nuclear translocation of RUNX2 siginificantly increased in cells cultured with HP or Yoda1 (Fig. 7a). On the other hand, down-regulation of PIEZO1 expression by the PIEZO1-specific siRNA inhibited the nuclear translocation of RUNX2 in cells cultured with HP (Fig. 7b). These results suggests that under the HP loading, PIEZO1 plays an important role in the regulation of nuclear translocation of RUNX2.
Figure 7.
Nuclear translocation of RUNX2 is regulated by the activation of PIEZO1 in SHED. (a) Imunostaining analysis for nuclear translocation of RUNX2. SHED were cultured with or without HP by the medium height of 5 cm (H: 5cm) or 5 μM Yoda1 for 24 hrs. Green, RUNX2; blue, DAPI; Scale bars, 200 μm and 50 μm. (b) Nuclear translocation of RUNX2 in PIEZO1-specific siRNA transfected cells. The PIEZO1-specific siRNA transfected cells SHED were replaced and cultured with or without HP by the medium height of 5 cm (H: 5cm) for 24 hrs. RUNX2 nuclear translocation was assessed and quantified as the percentage of the DAPI nuclei-positively stained cells to the total cells. Three different siRNAs specific to PIEZO1 were tested, and a similar result was obtained. The data are representative of three independent experiments with similar results and error bars indicate standard deviations. Statistical analysis was performed using analysis of variance (**p < 0.01).
Discussion
In this study, we demonstrated for the first time that hydrostatic pressure (HP) promotes odontoblast differentiation in multipotent stem cells from human exfoliated deciduous teeth (SHED) through both WNT expression and ciliogenesis that mediated by the piezo type mechanosensitive ion channel component 1 (PIEZO1).
Hydrostatic pressure (HP) via the tissue interstitial fluid involes in an important mechanotransduction in cells. Thus far, the importance of HP on cellular activities has been shown in many experimental studies, but many of them used HP by controlling the gaseous phase with a pressurized special chamber41. Previously, we developed a cell culture chamber to control of HP with gaseous phase and found that 0.01MPa (about 75 mmHg) of HP regulates cell fate determination of mesenchymal stem cells28. However, it was difficult to observe the effect of HP below 0.005 MPa (about 37.5 mmHg) on the cell culture with this chamber, because a slight change in the amount of air in the sealed chamber during cell culture affects the stability of HP. The mechanism of movement of tissue interstitial fluid is determined by interstitial fluid pressure that is mediated by the difference of the vascular HP and tissue colloid osmotic pressure42. The mean value of intracapillary HP is 16–18 mmHg43, which acts as a force to push fluid out of the capillaries into the stroma. The average osmotic pressure is approximately 25 mmHg44, which acts as a force to pull fluid back from the stroma into the capillaries. The interstitial fluid pressure is considered to be about 1–3 mmHg45. Furthermore, the sufficient pressure gradient for the fluid movement was reported to be within about 0.5 mmHg46. This exquisite pressure balance suggests that the movement of tissue interstitial fluid in the regulation of cellular activities is physiologically and tightly regulated by a slight change in pressure. Therefore, to observe the effect of the low level of HP on cell differentiation in SHED, the cells were cultured with the modification of the height of the cell culture medium. The HP is determined by an equation: P = r*g*h, where P is the pressure, r (rho) is the density of the liquid, g is the acceleration of gravity and h is the height of the liquid. In cell culture condition, the most remarkable point is that r and g are the same, so the theoretical HP depends on the height of the cell culture medium. Therefore, based on this theoretical concept, we performed cell culture by changing the height of the medium during culture to observe the effect of the low level of HP. As a result, we found that HP with the height of the cell culture medium at only 5 cm (approximately 3.7 mmHg) promoted the mineralization and the expression of odontogenic marker genes such as PANX3 and DSPP in SHED. These results suggest that applying with the medium at only 5 cm height would be a sufficient mechanical force to promote odontoblast differentiation of multipotent stem cells in tooth.
Primary cilia are considered to be a sensory organelle present in most mammalian cells and act as an antenna in the response to mechano/chemo-stimuli16,17. So far, it was shown that the primary cilia formation is regulated by centrosomal and ciliary proteins such as polo-like kinase 4 (Plk4), Cep97, CP110, AurA, and HDAC647–49. Furthermore, in the factors related with the cytoskeleton, actin nucleation-promoting proteins such as cortactin inhibit ciliogenesis50. By contrast, actin-severing factors such as cofilin and gelsolin-family proteins promote ciliogenesis51. Jasplakinolide (Jasp), a potent inducer of actin polymerization, induced ciliogenesis52. These results suggest that the primary cilia formation correlates with mechanotransduction via the cytoskeleton. In this study, we found that among mechanosensing receptors, PIEZO1 is a preferentially expressed in SHED and induced by HP loading. Furthermore, the strong signal of PIEZO1 was observed in the cellular processes of odontoblasts in tooth. These results suggest that PIEZO1 is a primary mechanosensing receptor in SHED and odontoblast. Interestingly, HP and PIEZO1 activator Yoda1 induced primary cilia expression. Conversely, knockdown of endogenous PIEZO1 expression by siRNA inhibited HP-induced primary cilia expression. These results suggest that PIEZO1 plays a role in the expression of primary cilia. It was reported that PIEZO1 activity was higher in membrane bleb-attached patches that lack cytoskeleton than in cell-attached patches that retain connections between the membrane and cytoskeleton53. Furthermore, knocking out filamin, a scaffold protein that binds the actin network to membrane proteins, more activated PIEZO1 in the cell adhesion patch-clamp assay54. Thus, PIEZO1 activation would be correlated with the cytoskeleton and scaffold proteins55,56, suggesting that the transmission of signals via a mechanosensing receptor PIEZO1 may be involved in the actin polymerization, which results in the promotion of the primary cilia formation. Besides, inhibition of primary cilia formation by chloral hydrate treatment significantly inhibited the HP-induced mineralization in SHED. This result suggests that primary cilia and cilia-related signaling pathways are essential for odontogenesis through the mechanochemical transduction mechanism.
WNT signaling is involved in the signaling pathway in primary cilia and also has an essential role in odontogenesis and dentin repair15. Especially, among several types of ciliary-associated signaling pathways, WNT signals are known to be associated with cytoskeleton and cytoskeleton rearrangement57. In this study, we found that HP increased the levels of both WNT5b and WNT16 mRNAs. Notably, PIEZO1 activator Yoda1 strongly induced WNT16 expression. Although the expression and function of WNT16 in tooth were not known, it was reported that WNT16 is associated with cortical bone thickness, cortical porosity, and fracture risk58–60. A mouse model with Wnt16 overexpression in osteoblasts showed an increase of the trabecular bone mass61. Besides, Wnt16 and Wnt5a can also directly regulate osteoclast differentiation62. Thus, WNT16 plays crucial roles in bone homeostasis. Our findings indicate that exogenously added WNT16 promoted mineralization of SHED. These observations suggest that WNT16 plays a key role in HP- and PIEZO1-mediated mechanical signals for odontoblast differentiation in SHED.
WNT signaling can be divided into two pathways: canonical and non-canonical. The canonical WNT pathway leads to regulation of gene transcription by β-catenin, whereas the non-canonical WNT pathway regulates the cytoskeleton in cell morphology63. Several studies have revealed that disruption of ciliated genes leads to aberrant activity in canonical Wnt signaling whilst suppressing non-canonical Wnt signaling64. Therefore, ciliogenesis is thought to induce a switch from canonical signaling towards non-canonical wnt signaling65. In this study, we found that DACT3, a negative regulator of WNT/β-catenin signaling, was dramatically inhibited by both HP and Yoda1. Furthermore, we found that HP-induced mineralization was inhibited by a WNT/β-catenin signaling selectively inhibitor XAV939. These results indicating that canonical WNT/β-catenin signaling pathway may be involved in HP- and PIEZO1-mediated odontoblast differentiation. However, WNT16 can activate both canonical and non-canonical pathways but can regulate to prevent excessive activation of the canonical pathway66. WNT signals would play a pivotal role in the complex processes, involving the stop cell division, primary cilia expression, and the beginning of cell differentiation, by the regulation of the switching of canonical to non-canonical pathways. Thus, PIEZO1 may also regulate HP- induced odontoblast differentiation by modulating WNT signaling.
Finally, to delineate the mechanism between chemical and physical factors in the differentiation process, we focused on RUNX2 that is a critical transcription factor for odontogenesis7,37,38. We found that HP and Yoda1 promoted the nuclear translocation of RUNX2, but the suppression of endogenous PIEZO1 inhibited it. These results indicate that in the response to HP, PIEZO1 could be a regulator of the nuclear translocation of RUNX2 during odontoblast differentiation. However, studies on developing bone have indicated that Wnt/β-catenin signaling positively regulated RUNX267. Although further studies are needed to elucidate whether the nuclear translocation of RUNX2 is directly regulated by PIEZO1 signaling or indirectly induced by WNT expression, PIEZO1 may participate in RUNX2-WNT signaling in odontoblast differentiation.
In conclusion, we showed that PIEZO1, a mechanosensing receptor, receives and transduces HP signal into the cellular biomolecular signal by modulating WNT signaling and ciliogenesis during odontoblast differentiation. Furthermore, we found that HP with the medium height at only 5 cm promoted mineralization of SHED. SHED are important cell sources for regenerative medicine. Thus, our results provide new insights into the molecular mechanisms of mechanical sensitivity in odontoblast differentiation and also would lead to the development of a new therapeutic approach and a novel cell culture device for regenerative medicine.
Material and Methods
Reagents
The Alizarin Red S staining kit was purchased from PG Research (Tokyo, Japan). Yoda1 and XAV939 were purchased from Tocris Bioscience (Bristol, UK) and recombinant human WNT16 protein from R&D systems (Minneapolis, USA). Chloral hydrate was obtained from Tokyo Chemical Industry (Tokyo, Japan).
Teeth collection
Exfoliated deciduous teeth without caries were obtained from 8 healthy children aged between 8–13 years old (5 males and 3 females), and the written informed consent was obtained from their parent based on the guidelines set by the ethics committee of our hospital (Tokushima, Japan). To obtained stem cells from human exfoliated deciduous teeth (SHED), extracted teeth were immediately placed in α-MEM (Wako, Japan), and were transferred to laboratory within 30 min. Ethical approval was obtained from the Ethics Committee of Tokushima University Hospital (approval no. 1799).
Isolation of SHED
The teeth were washed with phosphate balanced saline solution (PBS) and then soaked with 2X Antibiotic-Antimycotic (Anti-Anti, Nacalai tesque, Japan) in PBS. The teeth were mechanically broken into pieces with sterile pliers to collect the pulp tissues, which were then disrupted with a acaple blade and digested in a solution with 3 mg/ml collagenase (Wako, Japan) and 4 mg/ml dispase (Wako, Japan) for 1 hr at 37 °C in a CO2 incubator. After diluting with α-MEM, the cell suspension was passed through a 40 μm cell strainer (Falcon, USA) and centrifuged at 1,000 rpm for 5 min. The cell pellet was resuspended with α-MEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% Anti-anti. Single-cell suspensions were cultured in a regular medium as reported68,69. Cells at 50–60% confluency were passaged with 3–5 times and then used for the experiments. We successfully isolated SHEDs from six indipendent donors and teeth.
Cell culture
SHED were maintained in a growth medium containing α-MEM supplemented with 10% FBS and 1% Anti-anti. A dental mesenchymal cell line (mDP) was culutured in DMEM with 10% FBS and 1% Anti-anti at 37 °C in a humidified chamber with 5% CO2. For the following experiments, cells were seeded at 4.0 × 104 cells/well on the 35 mm glass bottom dish (Matsunami, Japan) with growth medium one day before the treatment. For hydrostatic pressure (HP) loading, the cell culture dish was placed at the bottom of a syringe or beaker and filled the media to the height of 5, 10, 15, 20, and 25 cm in order to expose the sustained HP. The cells cultured with a 0.3 cm height of media under atmospheric pressure were used as the control. Note that in this study, since atmospheric pressure was regarded as the zero reference, HP means gauge pressure. For odontogenic differentiation, induction (differentiation) media contained growth medium and 10 mM β-glycerophosphate, 150 μg/mL ascorbic acid, and 10−8 M dexamethasone, with changing the induction media every 2 days. Calcium deposition, an indicator of mineralization, was determined by Alizarin Red S staining for according to the manufacturer’s protocol. The Alizarin Red S-positive areas were quantified by NIH-ImageJ 1.48v (National Institutes of Health, USA).
Quantitative RT-PCR
Gene expression was analyzed by real-time RT-PCR. Total RNA was extracted from cultured cells using the TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Two micrograms of total RNA were used to generate the first-strand cDNA with the PrimeScript RT Master Mix (Perfect Real Time; Takara, Japan). Real-time PCR was carried out using PCR SYBR Premix Ex Taq II (Takara, Japan) and a Thermal Cycler Dice real-time system (Takara, Japan) with the following conditions: 10 s at 95°C, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s, with a final 5 s at 95°C and 30 s at 60°C. For the DSPP gene expression, PCR condition was at 10 s at 95°C, followed by 45 cycles of 95°C for 5 s and 63°C for 30 s, with a final 5 s at 95°C and 30 s at 60°C. The reactions were run in triplicate and repeated at least three times. The primers sequence are shown in Supplementary Table S1.
Cell proliferation
Cell proliferation was determined by a cell counting methods. Cells were plated at 2.0 × 103 cells/well on the 35 mm glass bottom dish and maintained with or without 5 cm H2O for 24, 48, and 72 hrs. The total cell numbers were counted in twenty randomly selected fields of view under an inverted microscope with 20X magnification.
Immunohistochemistry analysis
For the detection of acetylated α-tubulin and PIEZO1, cultured cells were fixed with 4% paraformaldehyde at room temperature (RT) for 5 min. For the detection of RUNX2, cells were fixed with acetone at −20 °C for 3 min. For acetylated α-tubulin and RUNX2 detecions, the fixed cells were permeabilized with 0.2% TritonX-100 for 30 min. Then, the blocking was performed with 2% Bovine Serum Albumin (Sigma-Aldrich) in PBS for 1 h at RT for acetylated α-tubulin detection, or with Universal Blocking Reagent (Biogenex) for 6 min at RT for PIEZO1 and RUNX2 detection. The tooth sections were deparaffinized in xylene and rehydrated with water prior to antigen retrieval by Liberate Antibody Binding Solution (L.A.B. Solution, Polyscience) and then washed with PBS. The sections were incubated with Universal Blocking Reagent for 6 min and then with the primary antibodies. The following antibodies were used for immunohistochemistry: mouse anti-alpha Tubulin (acetyl K40) antibody [6-11B-1] (Abcam), rabbit anti-PIEZO1 antibody (Novus Biologicals), mouse anti-DSPP antibody (Santa Cruz Biotechnology, Germany), mouse anti-Runx2 (Cbfa1) mAb (Medical & Biological Laboratories). Alexa Fluor 488- or 594- conjugated secondary antibodies (Invitrogen) were used for detecting the primary antibody. Immunofluorescence was analyzed with an Olympus BX50 microscope (Tokyo, Japan).
siRNA experiments
Cells density with 60–80% confluency were transfected with siRNA using Lipofectamine™ RNAiMAX Transfection Reagent (Invtitrogen) according to the manufacturer’s protocol. The following siRNAs were used: ON-TARGET plus Human PIEZO1 siRNA (J-020870-11, 12, and 13; Dharmacon). ON-TARGET plus siCONTROL non-targeting pool siRNA (D-001810-1005; Dharmacon) was used as a control.
Statistical analysis
For the analyses represented in Figs 1e, 2c,d, 4f,g, the data were pooled from three independent experiments. In Figs 1d, 2a, 2b, 3, 4a,b,e, 5, 6 and 7, the data presented is representative of 3 independent experiments showed similar results. The error bars indicate standard deviations. Statistical analysis was performed using analysis of variance (*p < 0.05, **p < 0.01).
Supplementary information
Acknowledgements
We thank Dr. Akihito Yamamoto, Professor in Tokushima University, for providing the technical advice in prepareation of SHED. This work was supported by Grants-in-Aid (17H04414 to T.I.) from the Ministry of Education, Science, and Culture of Japan.
Author Contributions
T.I. conceived and designed this study. A.M., T.K. and T.H. participated in the patient recruitment and SHED preparation. A.M., A.S., K.I., R.K., K.Y., Y.A. and S.F. carried out the molecular analyses and interpreted the data. K.O. and N.I. prepared the tooth secion. T.I. carried out the data analyses and wrote the manuscript. All authors read and approved the manuscript.
Data Availability
The datasets are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-51381-9.
References
- 1.Ernstrom GG, Chalfie M. Genetics of sensory mechanotransduction. Annu Rev Genet. 2002;36:411–453. doi: 10.1146/annurev.genet.36.061802.101708. [DOI] [PubMed] [Google Scholar]
- 2.Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–123. doi: 10.1016/S0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 3.Iwamoto T, et al. Pannexin 3 regulates proliferation and differentiation of odontoblasts via its hemichannel activities. PLoS One. 2017;12:e0177557. doi: 10.1371/journal.pone.0177557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sreenath T, et al. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki, T. & Garant, P. R. Structure and organization of odontoblasts. Anat Rec245, 235–249, doi:10.1002/(SICI)1097-0185(199606)245:2<235::AID-AR10>3.0.CO;2-Q (1996). [DOI] [PubMed]
- 6.D’Souza RN, et al. Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development. 1999;126:2911–2920. doi: 10.1242/dev.126.13.2911. [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki T, et al. Inhibition of the terminal differentiation of odontoblasts and their transdifferentiation into osteoblasts in Runx2 transgenic mice. Arch Histol Cytol. 2008;71:131–146. doi: 10.1679/aohc.71.131. [DOI] [PubMed] [Google Scholar]
- 8.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar L, Sharpe PT. Expression of Wnt signalling pathway genes during tooth development. Mech Dev. 1999;85:197–200. doi: 10.1016/S0925-4773(99)00095-7. [DOI] [PubMed] [Google Scholar]
- 10.Tamura M, Nemoto E. Role of the Wnt signaling molecules in the tooth. Jpn Dent Sci Rev. 2016;52:75–83. doi: 10.1016/j.jdsr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Millar SE. Wnt/beta-catenin signaling in oral tissue development and disease. J Dent Res. 2010;89:318–330. doi: 10.1177/0022034510363373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu F, et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313:210–224. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neves VC, Babb R, Chandrasekaran D, Sharpe PT. Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci Rep. 2017;7:39654. doi: 10.1038/srep39654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babb R, Chandrasekaran D, Carvalho Moreno Neves V, Sharpe PT. Axin2-expressing cells differentiate into reparative odontoblasts via autocrine Wnt/beta-catenin signaling in response to tooth damage. Sci Rep. 2017;7:3102. doi: 10.1038/s41598-017-03145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neves VCM, Sharpe PT. Regulation of Reactionary Dentine Formation. J Dent Res. 2018;97:416–422. doi: 10.1177/0022034517743431. [DOI] [PubMed] [Google Scholar]
- 16.Nachury, M. V. & Mick, D. U. Establishing and regulating the composition of cilia for signal transduction. Nat Rev Mol Cell Biol, 10.1038/s41580-019-0116-4 (2019). [DOI] [PMC free article] [PubMed]
- 17.Goto H, Inaba H, Inagaki M. Mechanisms of ciliogenesis suppression in dividing cells. Cell Mol Life Sci. 2017;74:881–890. doi: 10.1007/s00018-016-2369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugmann SA, Cordero DR, Helms JA. Craniofacial ciliopathies: A new classification for craniofacial disorders. Am J Med Genet A. 2010;152A:2995–3006. doi: 10.1002/ajmg.a.33727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerdes JM, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 21.Huangfu D, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 22.Ezratty EJ, et al. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–1141. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clement CA, et al. TGF-beta signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep. 2013;3:1806–1814. doi: 10.1016/j.celrep.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Costello AP. D employer retiree drug subsidy: inception, implementation and issues. Benefits Q. 2010;26:50–61. [PubMed] [Google Scholar]
- 25.Li J, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grootenboer S, et al. A genetic syndrome associating dehydrated hereditary stomatocytosis, pseudohyperkalaemia and perinatal oedema. Br J Haematol. 1998;103:383–386. doi: 10.1046/j.1365-2141.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- 27.Pathak MM, et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA. 2014;111:16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugimoto A, et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep. 2017;7:17696. doi: 10.1038/s41598-017-18089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Marmol, J. I., Touhara, K. K., Croft, G. & MacKinnon, R. Piezo1 forms a slowly-inactivating mechanosensory channel in mouse embryonic stem cells. Elife7, 10.7554/eLife.33149 (2018). [DOI] [PMC free article] [PubMed]
- 30.Kalebic N, et al. alphaTAT1 is the major alpha-tubulin acetyltransferase in mice. Nat Commun. 2013;4:1962. doi: 10.1038/ncomms2962. [DOI] [PubMed] [Google Scholar]
- 31.Shao YY, Wang L, Welter JF, Ballock RT. Primary cilia modulate Ihh signal transduction in response to hydrostatic loading of growth plate chondrocytes. Bone. 2012;50:79–84. doi: 10.1016/j.bone.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syeda, R. et al. Chemical activation of the mechanotransduction channel Piezo1. Elife4, 10.7554/eLife.07369 (2015). [DOI] [PMC free article] [PubMed]
- 33.Lacroix JJ, Botello-Smith WM, Luo Y. Probing the gating mechanism of the mechanosensitive channel Piezo1 with the small molecule Yoda1. Nat Commun. 2018;9:2029. doi: 10.1038/s41467-018-04405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunt LH, Begg K, Kague E, Cross S, Hammond CL. Wnt signalling controls the response to mechanical loading during zebrafish joint development. Development. 2017;144:2798–2809. doi: 10.1242/dev.153528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue H, et al. Disruption of the Dapper3 gene aggravates ureteral obstruction-mediated renal fibrosis by amplifying Wnt/beta-catenin signaling. J Biol Chem. 2013;288:15006–15014. doi: 10.1074/jbc.M113.458448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlberg T, et al. Structural basis for the interaction between tankyrase-2 and a potent Wnt-signaling inhibitor. J Med Chem. 2010;53:5352–5355. doi: 10.1021/jm100249w. [DOI] [PubMed] [Google Scholar]
- 37.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 38.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/S0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 39.Gaur T, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 40.Holmen SL, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 41.Tworkoski E, Glucksberg MR, Johnson M. The effect of the rate of hydrostatic pressure depressurization on cells in culture. PLoS One. 2018;13:e0189890. doi: 10.1371/journal.pone.0189890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005–1060. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 43.Hargens AR, et al. Normal transcapillary pressures in human skeletal muscle and subcutaneous tissues. Microvasc Res. 1981;22:177–189. doi: 10.1016/0026-2862(81)90087-X. [DOI] [PubMed] [Google Scholar]
- 44.Grande PO, Asgeirsson B, Nordstrom CH. Volume-targeted therapy of increased intracranial pressure: the Lund concept unifies surgical and non-surgical treatments. Acta Anaesthesiol Scand. 2002;46:929–941. doi: 10.1034/j.1399-6576.2002.460802.x. [DOI] [PubMed] [Google Scholar]
- 45.Guyton AC. Pressure-volume relationships in the interstitial spaces. Invest Ophthalmol. 1965;4:1075–1084. [PubMed] [Google Scholar]
- 46.Miki K. Dynamics of the plasma-interstitial fluid distribution and transcapillary pressure difference. Jpn J Physiol. 1981;31:917–929. doi: 10.2170/jjphysiol.31.917. [DOI] [PubMed] [Google Scholar]
- 47.Kleylein-Sohn J, et al. Plk4-induced centriole biogenesis in human cells. Dev Cell. 2007;13:190–202. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Spektor A, Tsang WY, Khoo D, Dynlacht BD. Cep97 and CP110 suppress a cilia assembly program. Cell. 2007;130:678–690. doi: 10.1016/j.cell.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE. MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell. 2010;19:270–283. doi: 10.1016/j.devcel.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, et al. Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat Commun. 2015;6:6781. doi: 10.1038/ncomms7781. [DOI] [PubMed] [Google Scholar]
- 52.Nagai T, Mizuno K. Jasplakinolide induces primary cilium formation through cell rounding and YAP inactivation. PLoS One. 2017;12:e0183030. doi: 10.1371/journal.pone.0183030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cox CD, et al. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun. 2016;7:10366. doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Retailleau K, et al. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep. 2015;13:1161–1171. doi: 10.1016/j.celrep.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 55.Gaub BM, Muller DJ. Mechanical Stimulation of Piezo1 Receptors Depends on Extracellular Matrix Proteins and Directionality of Force. Nano Lett. 2017;17:2064–2072. doi: 10.1021/acs.nanolett.7b00177. [DOI] [PubMed] [Google Scholar]
- 56.Nourse JL, Pathak MM. How cells channel their stress: Interplay between Piezo1 and the cytoskeleton. Semin Cell Dev Biol. 2017;71:3–12. doi: 10.1016/j.semcdb.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.May-Simera HL, Kelley MW. Cilia, Wnt signaling, and the cytoskeleton. Cilia. 2012;1:7. doi: 10.1186/2046-2530-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng HF, et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012;8:e1002745. doi: 10.1371/journal.pgen.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medina-Gomez C, et al. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 locus. PLoS Genet. 2012;8:e1002718. doi: 10.1371/journal.pgen.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moverare-Skrtic S, et al. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat Med. 2014;20:1279–1288. doi: 10.1038/nm.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moverare-Skrtic S, et al. The bone-sparing effects of estrogen and WNT16 are independent of each other. Proc Natl Acad Sci USA. 2015;112:14972–14977. doi: 10.1073/pnas.1520408112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kobayashi Y, et al. Wnt16 regulates osteoclast differentiation in conjunction with Wnt5a. Biochem Biophys Res Commun. 2015;463:1278–1283. doi: 10.1016/j.bbrc.2015.06.102. [DOI] [PubMed] [Google Scholar]
- 63.Nusse R, Clevers H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 64.Abdelhamed ZA, et al. The ciliary Frizzled-like receptor Tmem67 regulates canonical Wnt/beta-catenin signalling in the developing cerebellum via Hoxb5. Sci Rep. 2019;9:5446. doi: 10.1038/s41598-019-41940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMurray RJ, Wann AK, Thompson CL, Connelly JT, Knight MM. Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells. Sci Rep. 2013;3:3545. doi: 10.1038/srep03545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nalesso G, et al. WNT16 antagonises excessive canonical WNT activation and protects cartilage in osteoarthritis. Ann Rheum Dis. 2017;76:218–226. doi: 10.1136/annrheumdis-2015-208577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011;112:750–755. doi: 10.1002/jcb.22994. [DOI] [PubMed] [Google Scholar]
- 68.Sakai K, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80–90. doi: 10.1172/JCI59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsushita Y, et al. Multifaceted therapeutic benefits of factors derived from stem cells from human exfoliated deciduous teeth for acute liver failure in rats. J Tissue Eng Regen Med. 2017;11:1888–1896. doi: 10.1002/term.2086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.