Abstract
In nature, light availability for photosynthesis can undergo massive changes on a very short timescale. Photosynthesis in such dynamic light environments requires that plants can respond swiftly. Expanding our knowledge of the rapid responses that underlie dynamic photosynthesis is an important endeavor: it provides insights into nature's design of a highly dynamic energy conversion system and hereby can open up new strategies for improving photosynthesis in the field. The present review focuses on three processes that have previously been identified as promising engineering targets for enhancing crop yield by accelerating dynamic photosynthesis, all three of them involving or being linked to processes in the chloroplast, i.e. relaxation of non-photochemical quenching, Calvin–Benson–Bassham cycle enzyme activation/deactivation and dynamics of stomatal conductance. We dissect these three processes on the functional and molecular level to reveal gaps in our understanding and critically discuss current strategies to improve photosynthesis in the field.
Keywords: fluctuating light, photosynthesis, protein regulation
Introduction
The biological process photosynthesis provides for most of the atmospheric oxygen and metabolic energy on our planet. It is fueled by the plenty of energy reaching Earth every day in the form of sunlight. In most environments, including farmland, light energy supply for photosynthesis can vary tremendously. Changes in solar position and dynamic movement of plants with the wind can lead to large fluctuations in incident light on very short time scales [1]. A comprehensive understanding of how plant photosynthesis reacts to such strong light fluctuations is warranted on the molecular level. Firstly, this is critical to assess the potential of engineering photosynthetic responses for improving photosynthesis in the field. Enhancing field photosynthesis holds great promise for increasing crop yield and thereby helping address global challenges related to overpopulation and climate change [2,3]. Secondly, it allows basic insights into nature's design for highly dynamic and efficient energy conversion and thereby carries potential for inspiring artificial photosynthesis and, more broadly, energy systems engineering.
While our understanding of the rapid responses of photosynthesis is still limited, the basic processes of plant photosynthesis are well understood (see recent reviews in [1,4–6]). In summary, light energy drives charge separations at the two photosystems in the thylakoid membrane and electrons are transported from water to NADP+ along the electron transport chain, including plastoquinone (PQ), the cytochrome b6f complex and plastocyanin [5]. Concurrently, protons are pumped into the thylakoid lumen, generating the proton motive force (pmf) used by the ATP synthase for ATP production [7]. The chloroplast pmf consists of two components: the proton concentration gradient (ΔpH) and the membrane potential (ΔΨ) [4]. The net proton concentration in the lumen has an additional regulatory effect: a high proton concentration (i.e. low pH) down-regulates light harvesting by activating a non-photochemical quenching (NPQ) mechanism that dissipates absorbed light energy as heat [8–10]. Additionally, high lumen [H+] inhibits plastoquinol oxidation at the Cytb6f complex [11–13]. ATP and NADPH, the products of the thylakoid-localized electron/proton transport reactions, drive cellular metabolism including the carbon fixation reactions of the Calvin–Benson–Bassham (CBB) cycle. NADPH serves an additional regulatory function by donating electrons to the reductive activation of photosynthetic components including the ATP synthase and many CBB enzymes [14,15].
For improving dynamic photosynthesis, three main strategies have been identified [1,16]: (i) the acceleration of NPQ relaxation upon transition to low light. A model of canopy photosynthesis predicted that slow NPQ relaxation kinetics constrain crop photosynthesis by decreasing the quantum efficiency of photosynthesis in low-light periods [17]. (ii) The acceleration of CBB enzyme activation/deactivation. (iii) The acceleration of stomatal dynamics. While the acceleration of NPQ relaxation is expected to improve dynamic photosynthesis of both C3 and C4 plants, alterations to CBB enzyme activation and stomatal conductance mainly apply for improving C3 photosynthesis. This review lays out our current understanding of the molecular processes that underlie NPQ and particularly its relaxation, the activation of CBB enzymes and recent advances in the two light-dependent responses of stomatal opening (red and blue light responses). We point out gaps in our understanding and draw connections to other physiological processes that may be affected by current enhancement approaches.
The acceleration of NPQ relaxation to enhance light use efficiency
Photosynthesis in full sunlight is safeguarded by thermal dissipation of absorbed light in the PSII antenna, the NPQ process which prevents transfer of excess light energy to the photosystems and thus avoids detrimental side reactions (reviewed in [18,19]). In dynamic light environments, however, the efficiency of photosynthesis will depend (at least theoretically) on the speed by which NPQ can be reduced upon shading events. Once cells find themselves in light-limited conditions, any residual NPQ will reduce their photosynthetic efficiency by dissipating some of the available light energy as heat. The idea that slow NPQ relaxation kinetics could represent a limiting factor for plant photosynthesis and crop yield was first inferred from a computational model of light distribution in a crop canopy by Zhu et al. [17]. As a result, the acceleration of NPQ relaxation kinetics has been pushed forward as a key strategy for enhancing photosynthesis in the field [16,20,21].
Mechanisms that contribute to plant NPQ
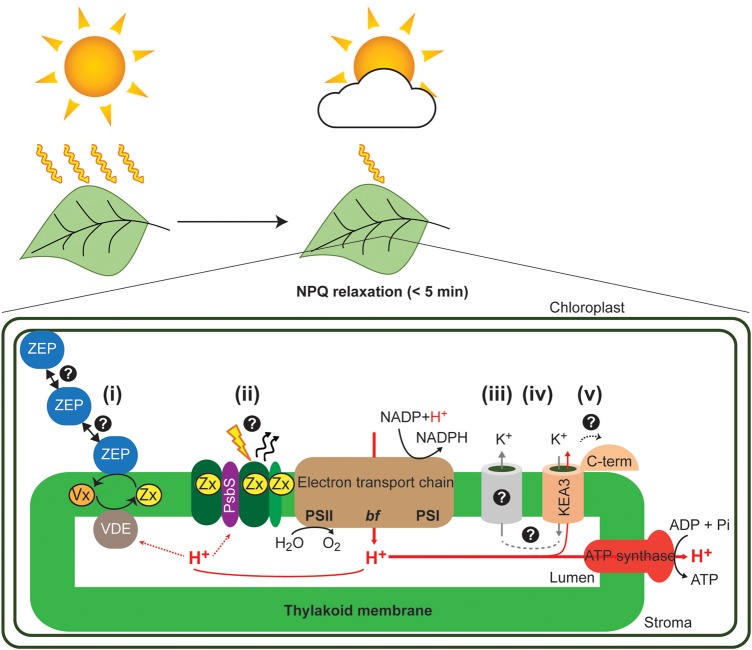
The main contributor to plant NPQ is the energy-dependent quenching (qE, 70–80% of total NPQ [8–10,22–36]. qE is the fastest responding NPQ component and relaxes within 5 min in the dark. More slowly relaxing NPQ components include the zeaxanthin-dependent quenching qZ [23] and the photoinhibitory component qI, the latter relating to the damage of the photosystem II (PSII)-D1 protein by reactive oxygen species and the resulting inactivation of PSII [24–26]. Such photodamaged PSII is a potent quencher of absorbed light energy [27], but in terms of maintaining efficient photosynthesis in dynamic light environments, the targeted destruction of the PSII-D1 protein must be a ‘last resort’ measure, only to be employed when other dissipative mechanisms have failed (reviewed by Ruban [28]). The relaxation of qI, i.e. the repair of photodamaged D1, is energy costly and relatively slow. While the acceleration of PSII repair may theoretically be one option to speed up the recovery of photosynthetic efficiency after prolonged stress, practically this is extremely difficult to approach as many different processes and organizational layers (i.e. protein degradation, expression and complex reassembly) are involved (reviewed by Murchie and Niyogi [29]). While there are additional NPQ components with slower relaxation kinetics than qE [30–32], and their manipulation may well have potential for accelerating NPQ relaxation kinetics, these are outside the scope of this review. We will focus this part of the review on the major NPQ contributor in plant leaves, qE, and its core components PSII subunit S (PsbS), xanthophylls and lumenal pH. We will discuss existing strategies to accelerate NPQ relaxation and point out gaps in our molecular understanding, including (i) PsbS and the molecular mechanism by which light energy gets converted into heat, (ii) the regulation and distribution of the zeaxanthin epoxidase (ZEP) in the chloroplast and (iii) the contribution of thylakoid ion transport to lumenal pH dynamics (Figure 1).
Figure 1. Summary of chloroplast processes involved in NPQ relaxation.
The figure summarizes known components involved in rapid NPQ relaxation and points to five main questions: (i) How is the zeaxanthin epoxidase (ZEP) distributed among the three chloroplast compartments?, (ii) How is light energy converted into thermal energy?, (iii) Which is the molecular identity of the K+ channel in the thylakoid membrane?, (iv) Does KEA3 act in concert with a K+ channel? and (v) How is KEA3 activity regulated? VDE, violaxanthin de-epoxidase; Vx, violaxanthin; Zx, zeaxanthin; PSII, photosystem II; bf, cytochrome b6f complex; PSI, photosystem I; KEA3, K+ efflux antiporter 3.
The role of PsbS and LHCII–PSII supercomplexes in qE
The absorption of light energy by chlorophylls (Chl) results in Chl excited states. When light energy gets absorbed in excess of the photosynthetic electron and proton transfer reactions and ultimately CO2 fixation (and other energy-requiring metabolic processes), Chl excited states are harmlessly dissipated as heat by the qE mechanism. This mechanism requires a conformational change within the PSII–light harvesting supercomplexes (PSII–LHCII) [33]. These supercomplexes are composed of a dimeric oxygen-evolving PSII core complex, four trimers of the major light harvesting antenna proteins (LHCII) Lhcb1, Lhcb2 and Lhcb3, and two of each minor antenna proteins CP24, CP26 and CP29 [34]. The PsbS, a four-helix transmembrane protein from the LHC family, is essential for qE induction [35–37]. The precise localization of PsbS within the supercomplex and its mechanism during qE activation have not been completely elucidated; however, several studies provide insight, which can be summarized as follows. In the dark, inactive PsbS dimers weakly bind to the PSII–LHCII supercomplexes [38,39]. Upon illumination, lumen acidification leads to the protonation of at least two glutamate residues of PsbS at the luminal side [40,41], which may cause PsbS monomerization [39,42,43]. PsbS is activated upon proton binding and induces the reorganization of the PSII–LHCII supercomplex by interacting with Lhcb1 and Lhcb3 [39,44,45]. These interactions are predicted to be responsible for the initial quenching phase that is observed already after short illuminations of one minute or less [46,47]. Upon prolonged light exposure, violaxanthin (Vx) is converted to zeaxanthin (Zx) by the violaxanthin de-epoxidase (VDE), a process also catalyzed by low lumenal pH [10,48]. Zx accumulation correlates with an increased interaction of PsbS with minor antenna proteins, but mainly CP29 [39,45,49]. This interaction likely allows for further rearrangements within the PSII–LHCII supercomplex thereby completing the switch to a thermal dissipating state [46,47]. Altogether, PsbS can be defined as a sensor for lumenal pH, as well as a catalyzer that promotes the conformational reorganization of PSII–LHCII complexes required for qE activation. Conformational changes within the PSII–LHCII complexes occur in response to pH-induced structural changes within the antenna proteins, which may be facilitated by protonated PsbS [50,51]. However, how this mechanism works in detail remains to be elucidated.
PsbS overexpression results in increased qE capacity and thereby reduces photoinhibition [52]. In line with this finding, qE was shown to be important for plant fitness in dynamic light environments [53], i.e. Arabidopsis plants lacking PsbS or VDE set fewer seeds when grown in the field or under controlled light fluctuations [53]. Therefore, in order to generate plants with improved fitness characteristics in the field, PsbS was overexpressed in multiple plant species with interesting and revealing results. In tobacco (Nicotiana tabacum), PsbS overexpression reduces stomatal conductance, resulting in increased water use efficiency [54]. This is likely due to an increase in PSII acceptor availability (i.e. more oxidized PQ pool), which serves as a signal triggering stomata closure [54], a process also referred to as stomata red light response (see the paragraph on Red light response). How exactly the redox state of the PQ pool determines stomatal aperture remains to be determined. However, field-grown tobacco plants that overexpressed PsbS, despite having higher water use efficiency, did not show any increase in biomass [54].
In another study, PsbS was overexpressed in Oryza sativa (rice). These transgenic rice plants displayed increased biomass and grain yield in the field during one growth season [55]. There may be multiple explanations for the discrepancies between the two reports including (i) the effect of PsbS overexpression on plant yield may depend on environmental conditions such as light and water availability and these may have greatly differed between the two reports and (ii) the two species tobacco and rice may respond differently to PsbS overexpression. While increases to NPQ capacity, i.e. by the overexpression of PsbS, may not constitute a robust approach to enhancing plant yield, the acceleration of NPQ induction and particularly NPQ relaxation may pose a promising alternative. Because PsbS (and its rapid function in catalyzing qE) is dependent on lumenal pH, this may be achieved by changing the kinetics of lumenal pH or pH sensitivity and reactivity of the qE components. For such approaches, it will be crucial to understand how PsbS is activated upon protonation and how the resulting conformational change elicits the activation of the qE process at the site of the PSII–LHCII supercomplex.
The role of the xanthophyll cycle in qE and qZ
The xanthophylls Vx, antheraxanthin and Zx are oxygenated β-carotenes and mainly bound at specific sites of PSII major and minor light harvesting antenna proteins [56–58]. Like other carotenoids, xanthophylls are C40 hydrocarbon compounds. Xanthophylls are synthesized through the condensation of isoprenoids and thus have common precursors with the chlorophyll and gibberellic acid biosynthesis pathways [59]. Aside from their important role in plant photoprotection, they also serve as precursors for the plant hormone abscisic acid (ABA) [60]. Thus, xanthophyll cycle enzymes are tightly integrated into metabolic networks contributing to plant hormone biosynthesis. This is important, because any results that derive from the modulation of plant NPQ kinetics by altering the activity of xanthophyll cycle enzymes require careful interpretation as this may additionally affect plant hormone levels. Synthesis of Zx occurs from β-carotene by the carotenoid β-hydroxylase, while Ax and Vx are generated from Zx via a two-step epoxidation reaction catalyzed by the ZEP [61]. In the thylakoid-localized xanthophyll cycle, the formation of Zx from Vx occurs in response to high light or other stresses and as detailed in the previous section is required for a maximum qE response [10,36]. If Zx is produced for a prolonged time, a Zx-dependent sustained quenching component (qZ) is activated, which may be explained by the replacement of minor antenna-bound Vx with Zx [23], a process that is much slower than Vx to Zx exchange at the site of LHCII [62]. The specific role of Zx in qE is still under debate [63,64]. While Zx was first described to be part of the quenching mechanism [65,66], recent evidence suggests that Zx instead plays an indirect role as an allosteric modulator of qE by promoting antenna aggregation [45,64]. Thus, the direct molecular mechanism by which excitation energy is converted to thermal energy remains to be entirely resolved [63]. A recent report suggests that the quenching of excess light energy involves changes in carotenoid structure as the result of lumen pH-induced conformational reorganization of antenna proteins [67].
Upon transition from high to low light, reconversion of Zx to Vx is important for full NPQ relaxation [23]. This reaction is catalyzed by ZEP, which is localized at the stromal side of the thylakoid membrane [68]. In contrast with VDE, relatively little is known about the regulation of ZEP activity. However, it has been shown that high-light exposure down-regulates the xanthophyll epoxidation reaction, which suggests that ZEP is inactivated under such conditions [69]. Interestingly, a recent report identified ZEP as a ‘fast turn-over’ protein, which, similar to the D1 protein of PSII, has a much higher degradation rate than most other plant proteins [29,70]. In line with this report, ZEP activity may be regulated via protein stability. Because of the key role that ZEP plays in both qE and qZ, its manipulation appears as an attractive entry point for accelerating NPQ relaxation. In a previous study, ZEP was overexpressed together with VDE and PsbS and data showed that field-grown plants generated more biomass [71]. However, as outlined above, changes to xanthophyll enzyme levels and activities may also affect plant hormone levels. Fortunately, there may be the possibility to isolate the activity of ZEP involved in NPQ relaxation from its general carotenogenic and ABA-related function. This is because for ZEP to be active in the xanthophyll cycle, it has to be localized at the thylakoid membrane [60,72], whereas its function in carotenoid and ABA biosynthesis is mainly restricted to the chloroplast envelope. Proteomics analyses of chloroplast subfractions found nearly all enzymes upstream and downstream of ZEP at the inner chloroplast envelope [73], spurring the idea that this is the prominent site for ABA and carotenoid syntheses [72]. In fractionated chloroplasts, the ZEP is partitioned about half-and-half between stroma and thylakoid membrane, with envelope levels only detectable when plants are exposed to high light [60]. The thylakoid ZEP has been shown to weakly bind to the stromal side of the membrane via hydrophobic interactions [60,74]. Thus, one possibility for locally increasing ZEP activity in the xanthophyll cycle would be through selectively manipulating its thylakoid-binding properties to achieve binding of a larger pool of ZEP to the thylakoid membrane. This would preferably occur in conditions where ZEP activity is required to rapidly down-regulate qE and qZ, such as in low-light periods. Such manipulation of ZEP activity may appear promising for accelerating NPQ relaxation, however, in order to engineer this property, we currently lack sufficient understanding of (i) how the ZEP binds to the thylakoid membrane (i.e. via interacting proteins or lipids) and (ii) which mechanisms contribute to the formation of different ZEP pools within the chloroplast. Future research is needed to characterize any posttranslational modifications and/or protein interactions, which may determine the localization of the ZEP. ZEP has been found phosphorylated [75] and can be reduced in vitro by the NADPH thioredoxin reductase C (NTRC, [76], see Redox regulation of photosynthesis). However, currently, there is no information about whether these posttranslational modifications affect ZEP binding and/or enzymatic properties.
Overexpression of three key components of qE increases plant biomass in the field
The combined overexpression of three key genetic elements involved in qE, i.e. PsbS and the two xanthophyll cycle enzymes ZEP and VDE, increased the biomass production of field-grown tobacco plants by 15–20% [71]. These so-called VPZ transgenic plants, when analyzed under laboratory conditions, displayed accelerated NPQ relaxation kinetics and increased leaf carbon dioxide uptake after transitions from high to low light. This finding appeared as a breakthrough for work on manipulating NPQ relaxation, because it was the first to show that the acceleration of NPQ relaxation correlated with increased biomass when plants were grown under field conditions. However, several further information are needed to support the notion of Kromdijk et al. [71] that NPQ relaxation is actually causal for their observed increase in biomass: (i) Phenotypes of single or double transgenic lines (not shown in the publication). These will help evaluating (a) what is the contribution of single or double overexpression on NPQ relaxation and growth and (b) whether the speed of NPQ decay directly correlates with the biomass increase. (ii) Hormone levels of VPZ plants. It is known that the overexpression of ZEP increases whole plant levels of ABA [77]. While at high doses, ABA has inhibitory effects on plant growth, it can stimulate shoot growth if supplied at lower doses (reviewed by Humplik et al. [78]). Adding complexity, the overexpression of another carotenogenic enzyme, the lycopene β-cyclase, which acts further upstream of the xanthophyll cycle and draws from the same precursors as the gibberellin biosynthesis pathway, increases levels of the growth-promoting hormone gibberellin, proposedly via a regulatory feedback loop [79]. Thus, it remains to be investigated, whether VPZ plants show altered hormone levels and if so, whether changes in the hormonal status of these plants contribute to the observed increase in biomass. Changes on the levels of plant hormones could either be direct, through ‘push or pull’ effects of the overactive xanthophyll cycle enzymes (i.e. for ABA and gibberellin), or alternatively, as has been proposed recently, indirect by alterations at the levels of ROS signal production in the VPZ plants [24]. Taken together, further experiments are needed to dissect the cross-talk between photoprotection and plant hormone homeostasis via the xanthophyll cycle.
Accelerating NPQ relaxation by speeding up luminal pH kinetics
As discussed above, qE is proportional to the proton concentration of the lumen, because both, the PsbS protein and Zx synthesis via VDE, are activated upon protonation of key residues [40,80]. Thus, a further maybe even superior strategy for the acceleration of NPQ relaxation (when compared with the modification of qE functional components) may be realized by speeding up lumenal pH dynamics after sudden decreases in light intensity. Support for this notion comes from our studies of mutants lacking the putative H+/K+ antiporter in the thylakoid membrane, KEA3 [81,82]. Our data show that, after a transition from high to low light, KEA3 accelerates NPQ relaxation by decreasing the fraction of pmf stored as ΔpH, thereby increasing CO2 fixation [81]. KEA3 activity appears to be tightly regulated via a mechanism involving its C-terminus: KEA3 is inactive in high light, but becomes activated quickly (within seconds) upon transition to lower light intensities [82]. Taken together, these data clearly demonstrate that by means of KEA3 and its regulation, plants possess an intrinsic mechanism to accelerate NPQ relaxation by speeding up lumenal pH dynamics. In fluctuating light, this optimizing mechanism benefits plant growth, as seen by decreased growth of mutants lacking KEA3 when compared with wild-type (WT) specifically under this condition [82]. This prompted us to investigate whether we could speed up NPQ relaxation even further by overexpressing KEA3. This approach was carried out transiently in tobacco and stably in Arabidopsis and, during high to low-light transitions, yielded even faster NPQ relaxation kinetics when compared with control or WT, respectively [82]. The transient decrease in NPQ in leaf cells overexpressing KEA3 was accompanied by increased PSII quantum yield [82]. However, despite lowering NPQ and increasing PSII quantum efficiency during high to low-light transitions, the overexpression of KEA3 did not yield increased growth of Arabidopsis plants in fluctuating light [82]. The reasons for this may be numerous and in the following, we will outline some of the possible explanations.
Photosynthetic or other metabolic constraints downstream of PSII independent of KEA3
An increased PSII quantum efficiency will only translate into growth, if no other reactions downstream of PSII are limiting. Hypothetically, such limitations could be species-dependent and in Arabidopsis could be constraining photosynthetic efficiency downstream of PSII or growth.
Negative effects of high KEA3 activity on ATP production
While Arabidopsis plants with increased KEA3 levels have higher PSII quantum efficiency during high to low-light transitions, they may fail to produce the ATP levels required for increased CO2 fixation. This could have multiple reasons. For example, upon a high to low-light transition, rapid proton efflux from the lumen may be causing an inversion of the membrane potential counteracting further proton efflux via the ATP synthase and thus ATP production. This scenario is supported by data from Stitt et al. [83], which show that right after a high to low-light shift, ATP levels transiently drop. Then, it is also conceivable that the overexpression of KEA3 itself negatively affects the production of ATP. This scenario is unlikely if KEA3 works reversibly by exporting protons from the lumen during high to low-light transitions and subsequently uses the generated K+ gradient to import protons again. If working this way, enhanced KEA3 activity should not disturb the relative production of ATP and NADPH. The situation is different if KEA3 works in concert with a K+ channel, which dissipates the KEA3-generated K+ gradient. Both activities together would result in net pmf loss. Even if temporally separated (KEA3 on during transitions from high to low light, channel on during low to high light), an increase in KEA3 activity should negatively affect pmf and thus ATP production, particularly in highly fluctuating light conditions. At least four experimental reports support the presence of a K+ transporting channel in the thylakoid membrane: (i) light-driven proton influx into the thylakoid lumen was shown to be accompanied by an increase in K+ in the surrounding buffer and (ii) K+ channel activity was independently measured by three groups in isolated thylakoids [84–86]. Additionally, a recent computational simulation of thylakoid K+ channel activity underlines the role of K+ flux for dissipating thylakoid membrane potential (ΔΨ) in the light [87]. Such activity is required for part of the pmf to be stored as ΔpH, and this in turn is necessary for the induction of qE [87]. Another function of thylakoid ion channel activity relates to minimizing charge recombinations within PSII, which are favored by high ΔΨ [25]. While an earlier report found the Arabidopsis Two-Pore K+ channel 3 (TPK3) in the thylakoid membrane [88], we could recently show that TPK3 is instead targeted to the tonoplast (as was shown before [89,90]) and does not affect photosynthesis [91]. Thus, the molecular identity of the thylakoid K+ channel remains to be determined.
Activation of energy-consuming processes by KEA3 overexpression
Additionally, KEA3 may have a function outside of photosynthesis that could counteract the enhancing effects that higher KEA3 levels have on photosynthetic efficiency. In line with such an explanation, KEA3 was shown to positively affect Ca2+ transient generation in response to hyperosmotic stress [92]. Consequently, enhanced KEA3 levels may lower the threshold for Ca2+ transient generation, thereby prematurely activating energy-consuming stress responses. According to this scenario, the surplus in energy generated by the enhancement of photosynthesis by overexpressing KEA3 would be consumed by costly stress responses.
Do these hypotheses for explaining the lack of growth increments in Arabidopsis KEA3 overexpressors in fluctuating light exclude KEA3 as a target for the improvement of photosynthesis? According to hypothesis I (i.e. photosynthetic or metabolic constraints downstream of PSII independent of KEA3), enhancing KEA3 activity may still be a viable option for improving plant growth in species that are not subjected to such constraints. If we consider faster NPQ relaxation in the VPZ tobacco plants [71] as responsible for their increased biomass, and hypothesis I applies in the context of species-dependency, KEA3 overexpression should result in increased biomass accumulation in tobacco (and potentially other species). Hypotheses II and III (i.e. negative effects of high KEA3 activity on ATP production or activation of energy-consuming processes by KEA3 overexpression, respectively) argue against KEA3 overexpression alone as a sustainable strategy to increase biomass production. However, these hypotheses would still allow for strategies targeted at accelerating KEA3 activation, while avoiding higher overall activity. We have collected evidence that KEA3 is specifically activated upon transitions to shade by a mechanism involving its C-terminus [82]. Plants that overexpress a KEA3 version lacking the C-terminus show reduced NPQ in high light, while plants that overexpress the KEA3 full-length version show NPQ levels similar to WT. Upon transition to low light, NPQ relaxation kinetics correlate with levels of KEA3. The KEA3 C-terminus contains the conserved K+ transport nucleotide binding (KTN) domain, which is similar to the regulation of K+ conductance (RCK) domain, with both constituting ubiquitous regulatory components of K+ transport systems [93]. These domains have been shown to gate K+ transport in response to nucleotide binding. In nearly all reported cases, the regulating nucleotide ligand contained an adenosine phosphate moiety (i.e. c-di-AMP [94], ATP [95] or NADH [96]). High-energy adenosine phosphate derivatives in the form of ATP and NADPH are the products of light-dependent photosynthetic reactions. Together with their low-energy counterparts ADP and NADP+, these molecules may serve as reporters of the chloroplast energy status and trigger responses to counteract low energy supply. One of these responses may be the activation of KEA3 activity in order to increase light harvesting and thus energy input into the system. This idea is supported by the analysis of these nucleotides during high to low-light transitions, which demonstrated that (i) the ATP/ADP ratio drops transiently directly after the shift and (ii) NADP+ levels rise permanently in low light [83]. Thus, KEA3 activity after a high to low light transition coincides with decreased ATP/ADP and NADPH/NADP+ ratios. Understanding the mechanism by which KEA3 is regulated may allow for strategies targeted at accelerating activation and deactivation of KEA3. Such versions of KEA3 would optimally accelerate lumenal pH dynamics without increasing net ion flux.
Redox regulation of photosynthesis
The intermediates produced by photosynthetic proton and electron transport, ATP and NADPH, are used in the CBB to regenerate the five-carbon-compound CO2 acceptor molecule, ribulose-1,5 bisphosphate (RuBP). RuBP is carboxylated by the ribulose-1,5 bisphosphate carboxylase oxygenase (Rubisco), with oxygenation of RuBP as a side product. Given the strong dependence of photosynthetic electron and proton transport on light, it is not surprising that the activity of several enzymes in the CBB is light intensity regulated. Posttranslational redox regulation by the thioredoxin (Trx) system is highly conserved throughout life, but it is nowhere as complex and diversified as in chloroplasts [97].
Chloroplast redox regulation has long been considered important for plant photosynthesis in dynamic light conditions, because of its role in the light-dependent activation of CBB enzymes [98,99]. Low CBB enzyme activity in low light and slow reactivation upon shifts from low to high light can lead to a transiently reduced turnover of metabolites in the CBB, thereby reducing carbon gain in fluctuating light [100]. CBB enzymes that are subject to redox control and whose activity may be limiting photosynthesis under fluctuating light are sedoheptulose-1,7 bisphosphatase (SBPase), fructose-1,6 bisphosphatase (FBPase), phosphoribulokinase (PRK) and glyceraldehyde-3-phosphate dehydrogenase (GADPH), as well as Rubisco activase (Rca, see below). Recently, new exciting roles for Trx systems have emerged that are important for photosynthesis in fluctuating light and unrelated to CBB enzyme regulation. As there are several excellent recent reviews on the topic [97,101,102], here we only review some developments that in our view are important for photosynthesis in fluctuating light.
Two thioredoxin systems with differing redox potentials confer flexibility for chloroplast redox regulation
In the ferredoxin (Fd)–Trx system, electrons are transferred from the primary PSI acceptor, Fd, to Trx by engaging the ferredoxin–thioredoxin reductase (FTR). Trxs then pass the electrons onto their target enzymes. As it requires the supply of electrons by photosynthesis, this system is only active in the light. Arabidopsis chloroplasts contain 10 isoforms of Trx: two f, four m, two y and each one x and z-type Trx. These isoforms have a large range of target proteins, some specific and some redundant, and are all reduced by the same FTR [103], albeit with very different efficiencies that correlate with the midpoint redox potentials of the different Trx [15].
A second system engaging the NADPH-dependent thioredoxin reductase (NTRC) combines all functions of the Trx system in one protein: it contains an N-terminal NADPH thioredoxin reductase domain which can oxidize NADPH and a C-terminal Trx domain with which it can reduce its targets. NTRC is active in low light (below ∼150 µmol m−2 s−1 [76,104]), when the Fd–FTR–Trx system is still inactive. Thus, it can already activate enzymes, such as the ATP synthase, the NDH complex and several CBB enzymes in shade conditions [102]. Indeed, plants overexpressing NTRC showed much faster induction of electron transport, and higher net CO2 fixation in low light intensity when compared with WT [105]. These results suggest that crops with increased amounts of NTRC could grow faster under naturally fluctuating light intensities, but this remains to be shown experimentally. Whether NTRC overexpression can increase photosynthesis in fluctuating light may depend on the specific light regime [102].
The Fd–FTR–Trx system gets activated at higher light intensities. It is important for full activation of photosynthesis during the high-light phases of fluctuating light [106]. Double mutants lacking the Trx m1 and m2 isoforms, when grown in fluctuating light, have lower PSII quantum efficiency in high-light periods when compared with WT. However, in low-light periods, they show increased PSII quantum efficiency [106]. Together, this suggests that while lack of Trx m decreases photosynthetic efficiency in high light periods, it also elicits a compensatory response that results in increased light usage in low-light periods.
Together, the two Trx systems are involved in the regulation of CBB enzyme activity, ATP synthase activity, starch synthesis, the malate valve and cyclic electron flow, all of which can exert some control over photosynthesis in fluctuating light [97,101,102]. The manipulation of Trxs may thus play an important role for strategies to improve photosynthesis in dynamic light environments. However, many questions remain unanswered, particularly regarding the target selectivity of the Fd–FTR–Trx system, including (i) which are the targets of the different Trx isoforms? Although many specific Trx-target protein couples have already been identified, clearly, there are more to be found. For example, just recently, the thylakoid STN7 was revealed as a specific target of Trxm [107]. (ii) How specific is the Trx-target interaction and to what extent does redundancy exist and (iii) along these lines, can target selectivity be modified to allow for a selective over- or underactivation of specific enzymes?
Deactivating redox-regulated enzymes upon shading: the role of thiol oxidation
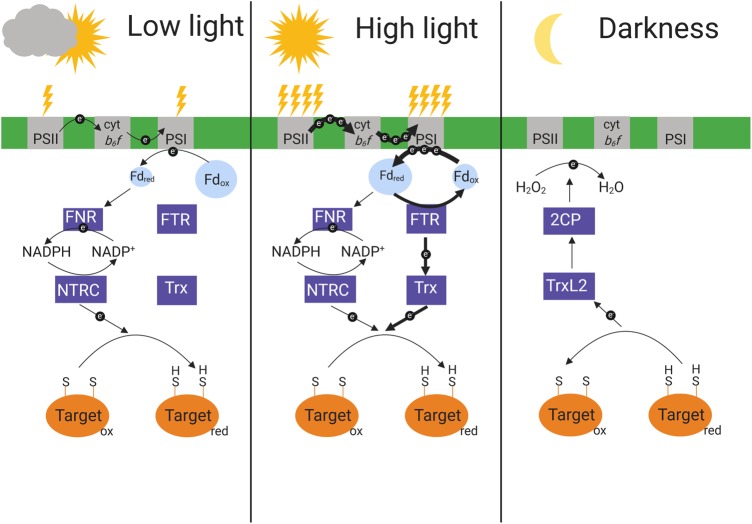
Research on redox regulation has predominantly focused on the activation of chloroplast enzymes by reduction in the light, while the reverse process, their deactivation in the dark or low light by oxidation of redox-sensitive thiols has been unclear. Recently, three landmark publications [108–110] have helped in elucidating this process. Firstly, Ojeda et al. [108] demonstrated that double mutants lacking both A and B isoforms of 2-cys peroxiredoxin (2CP) showed reduced oxidation of several redox-regulated enzymes upon darkening. Concomitant with slower enzyme deactivation in the dark, these plants displayed faster induction of photosynthesis under fluctuating light, compared with WT [108,109]. In vitro, incubating reduced target enzymes with different Trx isoforms, 2CP and H2O2 revealed that reducing equivalents could be successfully transferred from the enzymes to Trx, then to 2CP and finally to H2O2[108,109], suggesting this to be the in vivo oxidation cascade. Additionally, work by Yoshida et al. [110] identified a previously unknown Trx-like protein termed TrxL2 (for Thioredoxin-like2), which has a higher midpoint redox potential (approx. −250 mV) than NTRC (−275 mV) and different Trx isoforms (between −280 and −340 mV), suggesting TrxL2 to be a very efficient oxidant. Also, TrxL2 interacted with many redox-regulated enzymes, but the only enzyme it could reduce, and do so with high efficiency, was 2CP [110]. The overall model emerging from these results was that upon darkening, enzymes are predominantly oxidized by TrxL2, which then reduces 2CP, which finally passes the reducing power on to H2O2 to form H2O ([110], Figure 2). This redox cascade was additionally shown to oxidatively activate the glucose-6-phosphate dehydrogenase, an important enzyme of chloroplast energy metabolism in the dark [111]. These results are clearly a breakthrough for understanding oxidative enzyme deactivation and activation upon decreases in light intensity. Also, the 2cp mutant represents an interesting system for studying the ‘fast phase’ of photosynthetic induction [99]. In WT leaves exposed to low-light intensities, some of the enzymes controlling the rate of RuBP regeneration (FBPase, SBPase, PRK, GADPH) deactivate within 1–2 min and then initially limit the rate of photosynthetic induction after re-illumination [98]. In 2cp, the deactivation of these enzymes in the shade is much slower, conferring faster induction of electron transport after re-illumination [108]. Additionally, it was shown that 2cp plants, which grow smaller than WT under various light conditions, show similar growth as WT under highly fluctuating light, suggesting that the lack of 2CP benefits photosynthesis in dynamic light environments [109]. However, how exactly the lack of 2CP positively affects photosynthesis in fluctuating light and whether this is dependent on changes in the RuBP regeneration activation state remains to be determined.
Figure 2. Summary of chloroplast redox regulation in response to light availability.
Under low irradiance, electrons flow to NADPH, the NTRC is activated and reduces target enzymes. Under high irradiance, reduced ferredoxin (Fdred) is oxidized by the FTR and Trx reduces target enzymes. In the dark, reduced target enzymes are reoxidized by a cascade involving thioredoxin L2 (TrxL2) and 2 Cys-peroxiredoxins (2CP) and a final transfer of electrons to H2O2. PSII, photosystem II; cytb6f, cytochrome b6f complex; PSI, photosystem I; Fdox, oxidized ferredoxin.
Rubisco activation
The enzyme central to photosynthetic CO2 fixation, Rubisco, is a large protein complex with eight active sites. Each active site requires carbamylation (binding of Mg2+ and CO2) at a lysine residue to be catalytically competent. In the absence of carbamylation, RuBP or other sugar phosphates can bind tightly to the uncarbamylated active sites and inhibit their activity [112]. In low light, Rubisco activity decreases slowly due to inhibitor binding at active sites, and reaches a species-dependent residual activation state of 30–50% [113]. The binding of inhibitors in low light may be a protection mechanism to prevent Rubisco degradation [112]. Reactivation of inhibited Rubisco active sites is facilitated by the ATPase Rubisco activase (Rca), which removes inhibitory sugar phosphates [114]. The action of Rca is important to uphold Rubisco activity in the light, but also for reactivation after phases of low light or darkness. Upon shifts from high to low-light intensities, Rca activity decreases, probably due to the formation of higher-order Rca oligomers, which inactivate the enzyme and may act as its storage form [115]. When light intensity increases, Rca is reduced by the Fd–FTR–Trx system and gradually reactivates Rubisco, a process that takes several minutes and therefore limits photosynthetic CO2 fixation under fluctuating light [113].
In many plants, two Rca isoforms exist: one longer α-isoform (46–47 kDa) and one shorter β-isoform (41–43 kDa). Only the α-isoform is redox-regulated and in species containing both isoforms (most species tested so far), the α-isoform regulates the activation state of the β-isoform. Intriguingly, there is large interspecific variation for Rca isoforms: in Arabidopsis, spinach and rice, both isoforms are produced from an identical gene by alternative splicing whereas in maize, cotton and barley, two separate genes exist for the two isoforms [116,117]. Furthermore, in tobacco, only the β-isoform exists, but it is encoded by three separate genes; for a more thorough discussion on Rca isoform distribution, see [118]. Why there is such a large diversity for Rca isoform composition, as well as for the genes that encode these isoforms, is unclear. Also, it remains to be tested whether the diversity in Rca isoform distribution can be used to increase crop photosynthesis in fluctuating light.
Two strategies for manipulating Rca to improve dynamic photosynthesis have been proposed: eliminating Rca redox regulation and changing the Rca/Rubisco ratio. As for the first strategy, in rwt43, transgenic Arabidopsis plants lacking the α-isoform, the β-isoform was not redox-regulated and Rca activation state therefore remained high in low light [119]. Photosynthetic induction after low to high-light transitions was faster in rwt43 compared with WT [113,120]. Interestingly, light use efficiency was higher in rwt34 plants growing under fluctuating light than in rwt43 growing under constant light, while the opposite was demonstrated for WT. However, a functional α-isoform appears to be critical for optimal growth (at least in Arabidopsis), as rwt34 plants were smaller than WT plants under either constant or fluctuating light [118].
The second strategy is based on the idea that there is a tradeoff for protein allocation between Rubisco and Rca: Rubisco makes up 25–40% and ∼14% of total soluble protein in C3 and C4 plants, respectively [118,121,122], while Rca accounts for only 1–2% of total soluble protein [123,124]. Sacrificing small amounts of Rubisco could thus dramatically increase Rca concentrations and may speed up the rate of photosynthetic induction, but this might reduce photosynthesis under constantly high light [125]. A combination of novel in silico studies, using realistic wind-induced leaf movement [126], detailed modeling of photosynthesis dynamics as well as evolutionary algorithms that can predict optimal nitrogen distribution across generations may improve our understanding of optimal Rca/Rubisco ratios. Additionally, to what extent acclimation to different environments affects the Rca/Rubisco ratio is not known.
Genetic variation may be another modulator of the Rca/Rubisco ratio: a recent study in wheat found that Rubisco activation kinetics (measured as the time needed to reach 95% of steady-state carboxylation capacity, Vcmax) varied roughly twofold in the 10 cultivars tested (5.2–9.5 min; [127]). Whether this variation in Vcmax induction times is caused by variation in Rca or Rubisco concentrations remains to be shown, as this was not determined. Also, model calculations suggested that accelerating Vcmax induction could increase diurnal CO2 fixation by at most 3.4% [127]. While this does not seem like a large increment per day, when integrated over a whole growing season, this may cause a large increase in biomass. Further investigations into the natural variation in Rubisco activation dynamics therefore seem promising.
Stomatal conductance and light
Stomata are microscopically small pores on the leaf surface that dynamically balance the influx of CO2 into and the efflux of water vapor out of the leaf. A stoma is formed by two guard cells, which can increase or decrease their turgidity and thereby change the size of the stomatal pore. Stomata continuously balance CO2 supply for photosynthesis against water loss, which for most species and conditions necessitates that they adjust their aperture depending on a large number of environmental factors (e.g. light intensity, light spectrum, humidity, temperature, soil salinity) and intrinsic cues (e.g. photosynthetic electron transport, leaf and plant water status). Stomata typically close in the shade and open upon high-light exposure. Stomatal opening in the light is achieved through import of K+ (using malate2- as counterion) into the guard cells, with subsequent water import leading to guard cell swelling and an increase in stomatal pore size. Given that (i) guard cells contain chloroplasts whose interaction with light plays a major role in regulating stomatal pore width and (ii) stomatal opening has a strong effect on CO2 fixation under fluctuating light [128], here we review the responses of stomata to light. Two separate responses to light are known, one based on light intensity (‘quantitative’ or ‘red light’ response) and one solely responding to the presence of blue light (blue light response; reviewed in [129,130]).
Red light response
When exposed to light not containing blue light, stomatal conductance to water vapor (gs) increases near-linearly up to intensities exceeding full sunlight, a response that has been termed ‘red light’ or ‘quantitative’. The driver for the red light response has been debated heavily, as it was often argued that it must be related to the rate of net CO2 fixation, but several papers showed this assumption to be inconsistent with experimental results (reviewed in [130]). Currently, the tissue-specific origin of the ‘red light signal’ is still unclear. However, recently Busch [131], after a critical review of published data, suggested that the stomatal red light response correlated strongly with the reduction state of the plastoquinone pool and thus the QA redox state. QA is the primary acceptor of electrons from PSII and its availability is expressed by the chlorophyll fluorescence parameter qL [132].
Support for the claim by Busch [131] recently came in the form of experimental and modeling data. When using transgenic tobacco with a range of PsbS concentrations (which strongly affected the QA redox state), PsbS antisense plants showed an increase in 1-qL and gs, whereas PsbS overexpressors showed the opposite ([54], see also the section The role of PsbS and LHCII–PSII supercomplexes in qE). The same study demonstrated a strong linear correlation between qL and gs across genotypes and light intensities, while no such correlation between net CO2 fixation and gs exists [54]. The same authors recently demonstrated that including qL as a factor significantly improved model-based estimations of gs [133]. While these results are potentially exciting for phenotyping and modeling purposes, from our viewpoint, two major questions remain to be addressed: (i) given that qL changes quickly after a change in light intensity and this does not instantaneously translate to gs [134], how long do changes in qL have to persist in order to elicit changes in gs? (ii) —and this may help understand the timeframe of signal transduction from qL to gs—which molecular processes are involved in the signaling cascade?
Blue light response
Provided there is sufficient red background light, stomata strongly increase their pore size in response to blue light. Unlike the red light response, the blue light response saturates at low blue light intensities and is independent of photosynthesis [135]. Blue light is sensed by the phototropins 1 and 2, which start a signaling cascade that ultimately leads to guard cell swelling and stomatal opening [129]. At the beginning of the photoperiod, it is blue light that specifically triggers the breakdown of starch granules in guard cell chloroplasts of Arabidopsis (within 30 min) [136]. This appears to be important for increasing stomatal opening by providing (i) malate to serve as a counterion for K+ uptake, and (ii) sugars to increase guard cell osmotic potential [137].
Recently, the expression of an additional, light-induced K+ channel in Arabidopsis guard cells accelerated stomatal opening under blue light, as well as stomatal closure upon shading, conferring a growth advantage under low light that fluctuates slowly [138]. This result is potentially very exciting, as increasing the kinetics of stomatal opening and closure—instead of gs as such—has been predicted to lead to strongly improved light- and water use efficiencies in the field [139]. However, given that the plants in Papanatsiou et al. [138] were grown under relatively low-light intensity, and that increases in plant growth were observed under conditions where illumination was switched slowly between two relatively low intensities (10 and 150 µmol m−2 s−1, every 60 min), it remains to be investigated whether results can be translated to crops grown in the field. Indeed, stomata of climate-chamber grown WT Arabidopsis plants in this study [138] showed relatively slow opening and closure (half-times of 25–40 min), which is reportedly much slower than that of many crop species grown under natural light conditions (∼2–10 min [140]). Further experiments including the introduction of the light-induced K+ channel into crop species and growing such transgenic plants under controlled conditions of strongly fluctuating light are needed to predict the effect that the synthetic K+ channels may have on stomatal kinetics and photosynthesis under field conditions. Alternatively, such transgenic plants could be analyzed directly under field conditions.
Since the blue light response of stomatal opening saturates at light intensities <1% of full sunlight, it has been suggested to be important for predawn stomatal opening, and increasing stomatal aperture in shaded conditions, which in both cases would contribute to the rapid uptake of CO2 once leaves are exposed to full sunlight [141,142]. However, at least to our knowledge, these hypotheses have not been verified and the ecophysiological role of the stomatal blue light response has not been demonstrated conclusively.
While the modulation of red and blue light responses to increase stomatal opening in low light may be a strategy to accelerate full photosynthesis induction upon transition to high light, such a strategy may be disadvantageous under water-limited conditions. Also, there are several possible explanations for why nature has not already devised faster stomatal responses [139]: (i) in line with our arguments: in a quickly fluctuating light environment, the rapid closure of stomata upon shading would greatly limit CO2 fixation during subsequent high-light periods, i.e. leaves closing their stomata slowly upon shading should be able to use subsequent periods of high light more efficiently [98]. (ii) Leaf temperatures can change strongly in fluctuating light, and evaporative cooling due to larger gs may be in place to buffer temperature fluctuations that may otherwise negatively affect photosynthesis [143]. (iii) Active stomatal movement requires a change in guard cell osmotic potential, which is energy costly [144] and in a fluctuating environment, instantaneously responding stomata may be consuming high amounts of energy, which may be too costly for plant metabolism [145].
Summary and future directions
The aim of this review was to (i) summarize key molecular mechanisms contributing to photosynthesis in dynamic light environments, (ii) reveal gaps in our understanding of dynamic photosynthesis and (iii) discuss existing strategies targeted at improving photosynthesis in the field. For the latter point, two major conclusions can be drawn: firstly, previous attempts to increase dynamic photosynthesis by overexpressing or removing a singular gene have not succeeded in enhancing plant biomass production (as shown for overexpressing PsbS [54], KEA3 [82], NTRC (as discussed in [102]) and removing the Rca α-isoform [113,120]). Secondly and very importantly, processes involved in dynamic photosynthesis tightly interact with other physiological processes. This requires for the future that results, obtained in the context of improving photosynthesis and plant yield, are carefully discussed and integrated into whole plant physiology. Accordingly, rather than changing levels of proteins involved in dynamic photosynthesis, we propose adjusting proteins to activate/deactivate more rapidly in response to light intensity changes, as a promising strategy to improve field photosynthesis. In line with topics discussed in this review, examples for potential target processes include the ZEP activity in the xanthophyll cycle, LHCII–PSII supercomplex reorganization, KEA3-dependent lumenal pH dynamics (Figure 1), the CBB cycle and stomatal aperture. However, currently, we are still lacking important information necessary for the modulation of the kinetics of these processes.
So, how can we fill these gaps in knowledge? One great opportunity lies in exploiting genetic variation. Nature very likely has already devised mechanisms, by which the dynamic responses can be modulated. Already within the Arabidopsis species, photosynthesis acclimation to high light differs greatly between accessions [146]. Much more differences are expected to emerge by cross-species comparisons. Thus, a detailed characterization of photosynthetic responses across diverse species under multiple dynamic light environments is warranted. Such approaches, combined with the mapping of genes that are responsible for phenotypic variation, appear highly promising for increasing our understanding of dynamic photosynthesis.
Furthermore, there may be additional regulatory signals generated in response to light intensity changes (apart from lumenal pH and redox) that have previously been overlooked. For example, KEA3-dependent lumenal pH dynamics may be regulated via ATP/ADP or NADPH/NADP+ binding. It appears plausible that levels of these nucleotides serve as a signal for the energy status of the chloroplast and elicit compensatory responses if needed by directly binding to target proteins. However, whether KEA3 or any other components of photosynthesis are regulated directly via interaction with ATP, ADP, NADPH and/ or NADP+ remains to be determined.
Acknowledgements
We thank Michal Uflewski for help with the figures.
Abbreviations
- 2CP
2-cys peroxiredoxin
- ABA
abscisic acid
- CBB
Calvin–Benson–Bassham cycle
- Chl
chlorophyll
- FBPase
fructose-1,6 bisphosphatase
- Fd
ferredoxin
- FTR
ferredoxin–thioredoxin reductase
- GADPH
glyceraldehyde-3-phosphate dehydrogenase
- gs
stomatal conductance to water vapor
- KEA3
K+ efflux antiporter
- LHCII
light harvesting complex II
- NPQ
non-photochemical quenching
- NTRC
NADPH-dependent thioredoxin reductase
- pmf
proton motive force
- PQ
plastoquinone
- PRK
phosphoribulokinase
- PsbS
PSII subunit S
- PSII
photosystem II
- qE
energy-dependent quenching
- qI
photoinhibition
- qZ
zeaxanthin-dependent quenching
- Rca
Rubisco activase
- Rubisco
ribulose-1,5 bisphosphate carboxylase oxygenase
- RuBP
ribulose-1,5 bisphosphate
- SBPase
sedoheptulose-1,7 bisphosphatase
- TPK3
two-pore K+ channel 3
- Trx
thioredoxin
- Vx
violaxanthin
- WT
wild type
- ZEP
zeaxanthin epoxidase
- Zx
zeaxanthin
Funding
The work has been supported by a DFG research grant to U.A. (AR 808/5-1).
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Kaiser E., Morales A. and Harbinson J. (2018) Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiol. 176, 977–989 10.1104/pp.17.01250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ort D.R., Merchant S.S., Alric J., Blankenship R.E., Bock R., Croce R. et al. (2015) Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl Acad. Sci. U.S.A. 112, 8529–8536 10.1073/pnas.1424031112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simkin A.J., López-calcagno P.E. and Raines C.A. (2019) Feeding the world: improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 70, 1119–1140 10.1093/jxb/ery445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armbruster U., Correa Galvis V., Kunz H.H. and Strand D.D. (2017) The regulation of the chloroplast proton motive force plays a key role for photosynthesis in fluctuating light. Curr. Opin. Plant Biol. 37, 56–62 10.1016/j.pbi.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 5.Johnson M.P. (2016) Photosynthesis. Essays Biochem. 60, 255–273 10.1042/EBC20160016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foyer C.H., Neukermans J., Queval G., Noctor G. and Harbinson J. (2012) Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 63, 1637–1661 10.1093/jxb/ers013 [DOI] [PubMed] [Google Scholar]
- 7.Allen J.F. (2002) Photosynthesis of ATP—Electrons, proton pumps, rotors, and poise. Cell 110, 273–276 10.1016/S0092-8674(02)00870-X [DOI] [PubMed] [Google Scholar]
- 8.Briantais J.M., Vernotte C., Picaud M. and Krause G.H. (1979) A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim. Biophys. Acta 548, 128–138 10.1016/0005-2728(79)90193-2 [DOI] [PubMed] [Google Scholar]
- 9.Demmig-Adams B., Adams Iii W.W., Barker D.H., Logan B.A., Bowling D.R. and Verhoeven A.S. (1996) Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 98, 253–264 10.1034/j.1399-3054.1996.980206.x [DOI] [Google Scholar]
- 10.Niyogi K.K., Grossman A.R. and Björkman O. (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell. 10, 1121–1134 10.1105/tpc.10.7.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishio J.N. and Whitmarsh J. (1993) Dissipation of the proton electrochemical potential in intact chloroplasts (II. The pH gradient monitored by cytochrome f reduction kinetics). Plant Physiol. 101, 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tikhonov A.N., Khomutov G.B., Ruuge E.K. and Blumenfeld L.A. (1981) Electron transport control in chloroplasts. Effects of photosynthetic control monitored by the intrathylakoid pH. Biochim. Biophys. Acta, Bioenergetics. 637, 321–333 10.1016/0005-2728(81)90171-7 [DOI] [Google Scholar]
- 13.Stiehl H.H. and Witt H.T. (1969) Quantitative treatment of the function of plastoquinone in photosynthesis. Z. Naturforsch. 24b, 1588–1598 10.1515/znb-1969-1219 [DOI] [PubMed] [Google Scholar]
- 14.Michelet L., Zaffagnini M., Morisse S., Sparla F., Perez-Perez M.E., Francia F. et al. (2013) Redox regulation of the Calvin–Benson cycle: something old, something new. Front. Plant Sci. 4, 470 10.3389/fpls.2013.00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrillo L.R., Froehlich J.E., Cruz J.A., Savage L.J. and Kramer D.M. (2016) Multi-level regulation of the chloroplast ATP synthase: the chloroplast NADPH thioredoxin reductase C (NTRC) is required for redox modulation specifically under low irradiance. Plant J. 87, 654–663 10.1111/tpj.13226 [DOI] [PubMed] [Google Scholar]
- 16.Zhu X.-G., Long S.P. and Ort D.R. (2010) Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61, 235–261 10.1146/annurev-arplant-042809-112206 [DOI] [PubMed] [Google Scholar]
- 17.Zhu X.G., Ort D.R., Whitmarsh J. and Long S.P. (2004) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J. Exp. Bot. 55, 1167–1175 10.1093/jxb/erh141 [DOI] [PubMed] [Google Scholar]
- 18.Jahns P. and Holzwarth A.R. (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 1817, 182–193 10.1016/j.bbabio.2011.04.012 [DOI] [PubMed] [Google Scholar]
- 19.Ruban A.V. (2016) Nonphotochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 170, 1903–1916 10.1104/pp.15.01935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murchie E.H. and Niyogi K.K. (2011) Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 155, 86–92 10.1104/pp.110.168831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long S.P., Marshall-Colon A. and Zhu X.G. (2015) Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66 10.1016/j.cell.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 22.Wraight C.A. and Crofts A.R. (1970) Energy-dependent quenching of chlorophyll a fluorescence in isolated chloroplasts. Eur. J. Biochem. 17, 319–327 10.1111/j.1432-1033.1970.tb01169.x [DOI] [PubMed] [Google Scholar]
- 23.Nilkens M., Kress E., Lambrev P., Miloslavina Y., Müller M., Holzwarth A.R. et al. (2010) Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim. Biophys. Acta 1797, 466–475 10.1016/j.bbabio.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 24.Foyer C.H. (2018) Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 154, 134–142 10.1016/j.envexpbot.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis G.A., Kanazawa A., Schöttler M.A., Kohzuma K., Froehlich J.E., Rutherford A.W. et al. (2016) Limitations to photosynthesis by proton motive force-induced photosystem II photodamage. eLife 5, e16921 10.7554/eLife.16921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Järvi S., Suorsa M. and Aro E.-M. (2015) Photosystem II repair in plant chloroplasts—regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim. Biophys. Acta, Bioenergetics 1847, 900–909 10.1016/j.bbabio.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 27.Lee H.-Y., Chow W.S. and Hong Y.-N. (1999) Photoinactivation of photosystem II in leaves of Capsicum annuum. Physiol. Plant. 105, 376–383 10.1034/j.1399-3054.1999.105224.x [DOI] [Google Scholar]
- 28.Ruban A.V. (2015) Evolution under the sun: optimizing light harvesting in photosynthesis. J. Exp. Bot. 66, 7–23 10.1093/jxb/eru400 [DOI] [PubMed] [Google Scholar]
- 29.Li L., Aro E.M. and Millar A.H. (2018) Mechanisms of photodamage and protein turnover in photoinhibition. Trends Plant Sci. 23, 667–676 10.1016/j.tplants.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 30.Dall'Osto L., Cazzaniga S., Wada M. and Bassi R. (2014) On the origin of a slowly reversible fluorescence decay component in the Arabidopsis npq4 mutant. Phil. Trans. R. Soc. Lond. Ser. B, Biol. Sci. 369, 20130221 10.1098/rstb.2013.0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malnoë A. (2018) Photoinhibition or photoprotection of photosynthesis? Update on the (newly termed) sustained quenching component qH. Environ. Exp. Bot. 154, 123–133 10.1016/j.envexpbot.2018.05.005 [DOI] [Google Scholar]
- 32.Rochaix J.-D. (2014) Regulation and dynamics of the light-harvesting system. Annu. Rev. Plant Biol. 65, 287–309 10.1146/annurev-arplant-050213-040226 [DOI] [PubMed] [Google Scholar]
- 33.Johnson M.P., Goral T.K., Duffy C.D., Brain A.P., Mullineaux C.W. and Ruban A.V. (2011) Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell 23, 1468–1479 10.1105/tpc.110.081646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagliano C., Nield J., Marsano F., Pape T., Barera S., Saracco G. et al. (2014) Proteomic characterization and three-dimensional electron microscopy study of PSII-LHCII supercomplexes from higher plants. Biochim. Biophys. Acta. 1837, 1454–1462 10.1016/j.bbabio.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 35.Li X.P., Björkman O., Shih C., Grossman A.R., Rosenquist M., Jansson S. et al. (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395 10.1038/35000131 [DOI] [PubMed] [Google Scholar]
- 36.Noctor G., Rees D., Young A. and Horton P. (1991) The relationship between zeaxanthin, energy-dependent quenching of chlorophyll fluorescence, and trans-thylakoid pH gradient in isolated chloroplasts. Biochim. Biophys. Acta, Bioenergetics 1057, 320–330 10.1016/S0005-2728(05)80143-4 [DOI] [Google Scholar]
- 37.Crouchman S., Ruban A. and Horton P. (2006) Psbs enhances nonphotochemical fluorescence quenching in the absence of zeaxanthin. FEBS Lett. 580, 2053–2058 10.1016/j.febslet.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 38.Caffarri S., Kouřil R., Kereϊche S., Boekema E.J. and Croce R. (2009) Functional architecture of higher plant photosystem II supercomplexes. EMBO J. 28, 3052–3063 10.1038/emboj.2009.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Correa Galvis V., Poschmann G., Melzer M., Stühler K. and Jahns P. (2016) Psbs interactions involved in the activation of energy dissipation in Arabidopsis. Nat. Plants 2, 15225 10.1038/nplants.2015.225 [DOI] [PubMed] [Google Scholar]
- 40.Li X.P., Gilmore A.M., Caffarri S., Bassi R., Golan T., Kramer D. et al. (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 279, 22866–22874 10.1074/jbc.M402461200 [DOI] [PubMed] [Google Scholar]
- 41.Liguori N., Campos S.R.R., Baptista A.M. and Croce R. (2019) Molecular anatomy of plant photoprotective switches: the sensitivity of PsbS to the environment, residue by residue. J. Phys. Chem. Lett. 10, 1737–1742 10.1021/acs.jpclett.9b00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergantino E., Segalla A., Brunetta A., Teardo E., Rigoni F., Giacometti G.M. et al. (2003) Light- and pH-dependent structural changes in the PsbS subunit of photosystem II. Proc. Natl Acad. Sci. U.S.A. 100, 15265–15270 10.1073/pnas.2533072100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan M., Li M., Liu Z., Cao P., Pan X., Zhang H. et al. (2015) Crystal structures of the PsbS protein essential for photoprotection in plants. Nat. Struct. Mol. Biol. 22, 729–735 10.1038/nsmb.3068 [DOI] [PubMed] [Google Scholar]
- 44.Kereϊche S., Kiss A.Z., Kouřil R., Boekema E.J. and Horton P. (2010) The PsbS protein controls the macro-organisation of photosystem II complexes in the grana membranes of higher plant chloroplasts. FEBS Let. 584, 759–764 10.1016/j.febslet.2009.12.031 [DOI] [PubMed] [Google Scholar]
- 45.Sacharz J., Giovagnetti V., Ungerer P., Mastroianni G. and Ruban A.V. (2017) The xanthophyll cycle affects reversible interactions between PsbS and light-harvesting complex II to control non-photochemical quenching. Nat. Plants. 3, 16225 10.1038/nplants.2016.225 [DOI] [PubMed] [Google Scholar]
- 46.Holzwarth A., Miloslavina Y., Nilkens M. and Jahns P. (2009) Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem. Phys. Lett. 483, 262–267 10.1016/j.cplett.2009.10.085 [DOI] [Google Scholar]
- 47.Sylak-Glassman E.J., Malnoë A., De Re E., Brooks M.D., Fischer A.L., Niyogi K.K. et al. (2014) Distinct roles of the photosystem II protein psbS and zeaxanthin in the regulation of light harvesting in plants revealed by fluorescence lifetime snapshots. Proc. Natl Acad. Sci. 111, 17498–17503 10.1073/pnas.1418317111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hager A. (1969) Lichtbedingte pH-Erniedrigung in einem Chloroplasten-Kompartiment als Ursache der enzymatischen Violaxanthin-→ Zeaxanthin-Umwandlung; Beziehungen zur Photophosphorylierung. Planta 89, 224–243 10.1007/BF00385028 [DOI] [PubMed] [Google Scholar]
- 49.Betterle N., Ballottari M., Zorzan S., De Bianch S., Cazzaniga S., Dall'osto L. et al. (2009) Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J. Biol. Chem. 284, 15255–15266 10.1074/jbc.M808625200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim E., Watanabe A., Sato R., Okajima K. and Minagawa J. (2019) pH-responsive binding properties of light-Harvesting complexes in a photosystem II supercomplex investigated by thermodynamic dissociation kinetics analysis. J. Phys. Chem. Lett. 10, 3615–3620 10.1021/acs.jpclett.9b01208 [DOI] [PubMed] [Google Scholar]
- 51.Petrou K., Belgio E. and Ruban A.V. (2013) Ph sensitivity of chlorophyll fluorescence quenching is determined by the detergent/protein ratio and the state of LHCII aggregation. Biochim. Biophys. Acta 1837, 1533–1539 10.1016/j.bbabio.2013.11.018 [DOI] [PubMed] [Google Scholar]
- 52.Li X.P., Müller-Moulé P., Gilmore A.M. and Niyogi K.K. (2002) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl Acad. Sci. U.S.A. 99, 15222–15227 10.1073/pnas.232447699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Külheim C., Agren J. and Jansson S. (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297, 91–93 10.1126/science.1072359 [DOI] [PubMed] [Google Scholar]
- 54.Glowacka K., Kromdijk J., Kucera K., Xie J., Cavanagh A.P., Leonelli L. et al. (2018) Photosystem II subunit S overexpression increases the efficiency of water use in a field-grown crop. Nat. Commun. 9, 868 10.1038/s41467-018-03231-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hubbart S., Smillie I.R.A., Heatley M., Swarup R., Foo C.C., Zhao L. et al. (2018) Enhanced thylakoid photoprotection can increase yield and canopy radiation use efficiency in rice. Commun. Biol. 1, 22 10.1038/s42003-018-0026-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bassi R., Croce R., Cugini D. and Sandona D. (1999) Mutational analysis of a higher plant antenna protein provides identification of chromophores bound into multiple sites. Proc. Natl Acad. Sci. U.S.A. 96, 10056–10061 10.1073/pnas.96.18.10056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caffarri S., Croce R., Breton J. and Bassi R. (2001) The major antenna complex of photosystem II has a xanthophyll binding site not involved in light harvesting. J. Biol. Chem. 276, 35924–35933 10.1074/jbc.M105199200 [DOI] [PubMed] [Google Scholar]
- 58.Croce R., Weiss S. and Bassi R. (1999) Carotenoid-binding sites of the major light-harvesting complex II of higher plants. J. Biol. Chem. 274, 29613–29623 10.1074/jbc.274.42.29613 [DOI] [PubMed] [Google Scholar]
- 59.Qin G., Gu H., Ma L., Peng Y., Deng X.W., Chen Z. et al. (2007) Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Res. 17, 471 10.1038/cr.2007.40 [DOI] [PubMed] [Google Scholar]
- 60.Schwarz N., Armbruster U., Iven T., Brückle L., Melzer M., Feussner I. et al. (2015) Tissue-specific accumulation and regulation of zeaxanthin epoxidase in Arabidopsis reflect the multiple functions of the enzyme in plastids. Plant Cell Physiol. 56, 346–357 10.1093/pcp/pcu167 [DOI] [PubMed] [Google Scholar]
- 61.Ruiz-Sola M.Á and Rodríguez-Concepción M. (2012) Carotenoid biosynthesis in Arabidopsis: a colorful pathway. Arabidopsis Book 10, e0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wehner A., Grasses T. and Jahns P. (2006) De-epoxidation of violaxanthin in the minor antenna proteins of photosystem II, LHCB4, LHCB5, and LHCB6. J. Biol. Chem. 281, 21924–21933 10.1074/jbc.M602915200 [DOI] [PubMed] [Google Scholar]
- 63.van Oort B., Roy L.M., Xu P., Lu Y., Karcher D., Bock R. et al. (2018) Revisiting the role of xanthophylls in nonphotochemical quenching. J. Phys. Chem. Lett. 9, 346–352 10.1021/acs.jpclett.7b03049 [DOI] [PubMed] [Google Scholar]
- 64.Tutkus M., Saccon F., Chmeliov J., Venckus O., Ciplys I., Ruban A.V. et al. (2019) Single-molecule microscopy studies of LHCII enriched in Vio or Zea. Biochim. Biophys. Acta, Bioenergetics 1860, 499–507 10.1016/j.bbabio.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 65.Holt N.E., Zigmantas D., Valkunas L., Li X.P., Niyogi K.K. and Fleming G.R. (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307, 433–436 10.1126/science.1105833 [DOI] [PubMed] [Google Scholar]
- 66.Ahn T.K., Avenson T.J., Ballottari M., Cheng Y.C., Niyogi K.K., Bassi R. et al. (2008) Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science 320, 794–797 10.1126/science.1154800 [DOI] [PubMed] [Google Scholar]
- 67.Liguori N., Xu P., van Stokkum I.H.M., van Oort B., Lu Y., Karcher D. et al. (2017) Different carotenoid conformations have distinct functions in light-harvesting regulation in plants. Nat. Commun. 8, 1994 10.1038/s41467-017-02239-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jahns P., Latowski D. and Strzalka K. (2009) Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. Biochim. Biophys. Acta, Bioenergetics 1787, 3–14 10.1016/j.bbabio.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 69.Reinhold C., Niczyporuk S., Beran K.C. and Jahns P. (2008) Short-term down-regulation of zeaxanthin epoxidation in Arabidopsis thaliana in response to photo-oxidative stress conditions. Biochim. Biophys. Acta, Bioenergetics 1777, 462–469 10.1016/j.bbabio.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 70.Li L., Nelson C.J., Trosch J., Castleden I., Huang S. and Millar A.H. (2017) Protein degradation rate in Arabidopsis thaliana leaf growth and development. Plant Cell. 29, 207–228 10.1105/tpc.16.00768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kromdijk J., Glowacka K., Leonelli L., Gabilly S.T., Iwai M., Niyogi K.K. et al. (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354, 857–861 10.1126/science.aai8878 [DOI] [PubMed] [Google Scholar]
- 72.Joyard J., Ferro M., Masselon C., Seigneurin-Berny D., Salvi D., Garin J. et al. (2009) Chloroplast proteomics and the compartmentation of plastidial isoprenoid biosynthetic pathways. Mol. Plant 2, 1154–1180 10.1093/mp/ssp088 [DOI] [PubMed] [Google Scholar]
- 73.Shumskaya M., Bradbury L.M.T., Monaco R.R. and Wurtzel E.T. (2012) Plastid localization of the Key carotenoid enzyme phytoene synthase is altered by isozyme, allelic variation, and activity. Plant Cell. 24, 3725 10.1105/tpc.112.104174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schaller S., Wilhelm C., Strzalka K. and Goss R. (2012) Investigating the interaction between the violaxanthin cycle enzyme zeaxanthin epoxidase and the thylakoid membrane. J. Photochem. Photobiol. 114, 119–125 10.1016/j.jphotobiol.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 75.Bhaskara G.B., Wen T.N., Nguyen T.T. and Verslues P.E. (2017) Protein phosphatase 2Cs and microtubule-associated stress protein 1 control microtubule stability, plant growth, and drought response. Plant Cell. 29, 169–191 10.1105/tpc.16.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naranjo B., Mignée C., Krieger-Liszkay A., Hornero-Méndez D., Gallardo-Guerrero L., Cejudo F.J. et al. (2016) The chloroplast NADPH thioredoxin reductase C, NTRC, controls non-photochemical quenching of light energy and photosynthetic electron transport in Arabidopsis. Plant, Cell Environ. 39, 804–822 10.1111/pce.12652 [DOI] [PubMed] [Google Scholar]
- 77.Park H.Y., Seok H.Y., Park B.K., Kim S.H., Goh C.H., Lee B.H. et al. (2008) Overexpression of Arabidopsis ZEP enhances tolerance to osmotic stress. Biochem. Biophys. Res. Commun. 375, 80–85 10.1016/j.bbrc.2008.07.128 [DOI] [PubMed] [Google Scholar]
- 78.Humplik J.F., Bergougnoux V. and Van Volkenburgh E. (2017) To stimulate or inhibit? that is the question for the function of abscisic acid. Trends Plant Sci. 22, 830–841 10.1016/j.tplants.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 79.Moreno J.C., Cerda A., Simpson K., Lopez-Diaz I., Carrera E., Handford M. et al. (2016) Increased Nicotiana tabacum fitness through positive regulation of carotenoid, gibberellin and chlorophyll pathways promoted by Daucus carota lycopene beta-cyclase (Dclcyb1) expression. J. Exp. Bot. 67, 2325–2338 10.1093/jxb/erw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arnoux P., Morosinotto T., Saga G., Bassi R. and Pignol D. (2009) A structural basis for the pH-dependent xanthophyll cycle in Arabidopsis thaliana. Plant Cell 21, 2036–2044 10.1105/tpc.109.068007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Armbruster U., Carrillo L.R., Venema K., Pavlovic L., Schmidtmann E., Kornfeld A. et al. (2014) Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat. Commun. 5, 5439 10.1038/ncomms6439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Armbruster U., Leonelli L., Correa Galvis V., Strand D., Quinn E.H., Jonikas M.C. et al. (2016) Regulation and levels of the thylakoid K+/H+ antiporter KEA3 shape the dynamic response of photosynthesis in fluctuating light. Plant Cell Physiol. 57, 1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stitt M., Scheibe R. and Feil R. (1989) Response of photosynthetic electron transport and carbon metabolism to a sudden decrease of irradiance in the saturating or the limiting range. Biochim. Biophys. Acta, Bioenergetics 973, 241–249 10.1016/S0005-2728(89)80428-1 [DOI] [Google Scholar]
- 84.Enz C., Steinkamp T. and Wagner R. (1993) Ion channels in the thylakoid membrane (a patch-clamp study). Biochim. Biophys. Acta, Bioenergetics 1143, 67–76 10.1016/0005-2728(93)90217-4 [DOI] [Google Scholar]
- 85.Pottosin I.I. and Schönknecht G. (1996) Ion channel permeable for divalent and monovalent cations in native spinach thylakoid membranes. J. Membr. Biol. 152, 223–233 10.1007/s002329900100 [DOI] [PubMed] [Google Scholar]
- 86.Tester M. and Blatt M.R. (1989) Direct measurement of K(+) channels in thylakoid membranes by incorporation of vesicles into planar lipid bilayers. Plant Physiol. 91, 249–252 10.1104/pp.91.1.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davis G., Rutherford A., W A. and Kramer David M. (2017) Hacking the thylakoid proton motive force for improved photosynthesis: modulating ion flux rates that control proton motive force partitioning into Δψ and ΔpH. Phil. Trans. R. Soc. B: Biol. Sci. 372, 20160381 10.1098/rstb.2016.0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carraretto L., Formentin E., Teardo E., Checchetto V., Tomizioli M., Morosinotto T. et al. (2013) A thylakoid-located two-pore K+ channel controls photosynthetic light utilization in plants. Science 342, 114–118 10.1126/science.1242113 [DOI] [PubMed] [Google Scholar]
- 89.Dunkel M., Latz A., Schumacher K., Müller T., Becker D. and Hedrich R. (2008) Targeting of vacuolar membrane localized members of the TPK channel family. Mol. Plant 1, 938–949 10.1093/mp/ssn064 [DOI] [PubMed] [Google Scholar]
- 90.Voelker C., Schmidt D., Mueller-Roeber B. and Czempinski K. (2006) Members of the Arabidopsis AtTPK/KCO family form homomeric vacuolar channels in planta. Plant J. 48, 296–306 10.1111/j.1365-313X.2006.02868.x [DOI] [PubMed] [Google Scholar]
- 91.Höhner R., Correa Galvis V., Strand D.D., Voelkner C., Kraemer M., Messer M. et al. (2019) Photosynthesis in Arabidopsis thaliana is unaffected by the function of the vacuolar K+ channel TPK3. Plant Physiol. 180, 1322–1335 10.1104/pp.19.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stephan A.B., Kunz H.H., Yang E. and Schroeder J.I. (2016) Rapid hyperosmotic-induced Ca2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters. Proc. Natl Acad. Sci. U.S.A. 113, E5242–E5249 10.1073/pnas.1519555113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choe S. (2002) Potassium channel structures. Nat. Rev. Neurosci. 3, 115–121 10.1038/nrn727 [DOI] [PubMed] [Google Scholar]
- 94.Kim H., Youn S.J., Kim S.O., Ko J., Lee J.O. and Choi B.S. (2015) Structural studies of potassium transport protein ktrA regulator of conductance of K+ (RCK) C domain in complex with cyclic diadenosine monophosphate (c-di-AMP). J. Biol. Chem. 290, 16393–16402 10.1074/jbc.M115.641340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cao Y., Pan Y., Huang H., Jin X., Levin E.J., Kloss B. et al. (2013) Gating of the TrkH ion channel by its associated RCK protein TrkA. Nature 496, 317–322 10.1038/nature12056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roosild T.P., Miller S., Booth I.R. and Choe S. (2002) A mechanism of regulating transmembrane potassium flux through a ligand-mediated conformational switch. Cell 109, 781–791 10.1016/S0092-8674(02)00768-7 [DOI] [PubMed] [Google Scholar]
- 97.Geigenberger P., Thormählen I., Daloso D.M. and Fernie A.R. (2017) The unprecedented versatility of the plant thioredoxin system. Trends Plant Sci. 22, 249–262 10.1016/j.tplants.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 98.Kaiser E., Morales A., Harbinson J., Kromdijk J., Heuvelink E. and Marcelis L.F.M. (2015) Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 66, 2415–2426 10.1093/jxb/eru406 [DOI] [PubMed] [Google Scholar]
- 99.Pearcy R. W., Krall J. P. and Sassenrath-Cole G. F (1996) Photosynthesis in fluctuating light environments In Photosynthesis and the environment. pp. 321–346, Kluwer Academic Publishers, Dordrecht [Google Scholar]
- 100.Sassenrath-Cole G.F. and Pearcy R.W. (1992) The role of ribulose-1,5-bisphosphate regeneration in the induction requirement of photosynthetic CO2 exchange under transient light conditions. Plant Physiol. 99, 227–234 10.1104/pp.99.1.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cejudo F.J., Ojeda V., Delgado-Requerey V., González M. and Pérez-Ruiz J.M. (2019) Chloroplast redox regulatory mechanisms in plant adaptation to light and darkness. Front. Plant Sci. 10, 1–11 10.3389/fpls.2019.00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nikkanen L. and Rintamäki E. (2019) Chloroplast thioredoxin systems dynamically regulate photosynthesis in plants. Biochem. J. 476, 1159–1172 10.1042/BCJ20180707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dietz K.-J. (2008) Redox signal integration: from stimulus to networks and genes. Physiol. Plant. 133, 459–468 10.1111/j.1399-3054.2008.01120.x [DOI] [PubMed] [Google Scholar]
- 104.Yoshida K. and Hisabori T. (2017) Distinct electron transfer from ferredoxin–thioredoxin reductase to multiple thioredoxin isoforms in chloroplasts. Biochem. J. 474, 1347–1360 10.1042/BCJ20161089 [DOI] [PubMed] [Google Scholar]
- 105.Nikkanen L., Toivola J. and Rintamäki E. (2016) Crosstalk between chloroplast thioredoxin systems in regulation of photosynthesis. Plant Cell Environ. 39, 1691–1705 10.1111/pce.12718 [DOI] [PubMed] [Google Scholar]
- 106.Thormählen I., Zupok A., Rescher J., Leger J., Weissenberger S., Groysman J. et al. (2017) Thioredoxins play a crucial role in dynamic acclimation of photosynthesis in fluctuating light. Mol. Plant. 10, 168–182 10.1016/j.molp.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 107.Ancin M., Fernandez-San Millan A., Larraya L., Morales F., Veramendi J., Aranjuelo I. et al. (2019) Overexpression of thioredoxin m in tobacco chloroplasts inhibits the protein kinase STN7 and alters photosynthetic performance. J. Exp. Bot. 70, 1005–1016 10.1093/jxb/ery415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ojeda V., Perez-Ruiz J.M. and Cejudo F.J. (2018) 2-cys peroxiredoxins participate in the oxidation of chloroplast enzymes in the dark. Mol. Plant. 11, 1377–1388 10.1016/j.molp.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 109.Vaseghi M.-J., Chibani K., Telman W., Liebthal M.F., Gerken M., Schnitzer H. et al. (2018) The chloroplast 2-cysteine peroxiredoxin functions as thioredoxin oxidase in redox regulation of chloroplast metabolism. eLife 7, e38194 1–28 10.7554/eLife.38194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoshida K., Hara A., Sugiura K., Fukaya Y. and Hisabori T. (2018) Thioredoxin-like2/2-Cys peroxiredoxin redox cascade supports oxidative thiol modulation in chloroplasts. Proc. Natl Acad. Sci. U.S.A. 115, 8296–8304 10.1073/pnas.1808284115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoshida K., Uchikoshi E., Hara S. and Hisabori T. (2019) Thioredoxin-like2/2-Cys peroxiredoxin redox cascade acts as oxidative activator of glucose-6-phosphate dehydrogenase in chloroplasts. Biochem. J. 476, 1781–1790 10.1042/BCJ20190336 [DOI] [PubMed] [Google Scholar]
- 112.Parry M.A.J., Keys A.J., Madgwick P.J., Carmo-Silva A.E. and Andralojc P.J. (2008) Rubisco regulation: a role for inhibitors. J. Exp. Bot. 59, 1569–1580 10.1093/jxb/ern084 [DOI] [PubMed] [Google Scholar]
- 113.Carmo-Silva A.E. and Salvucci M.E. (2013) The regulatory properties of Rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiol. 161, 1645–1655 10.1104/pp.112.213348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salvucci M.E., Portis A.R. and Ogren W.L. (1985) A soluble chloroplast carboxylase/oxygenase protein catalyzes ribulosebisphosphate activation in vivo. Photosynth. Res. 7, 193–201 10.1007/BF00037012 [DOI] [PubMed] [Google Scholar]
- 115.Serban A.J., Breen I.L., Bui H.Q., Levitus M. and Wachter R.M. (2018) Assembly–disassembly is coupled to the ATPase cycle of tobacco Rubisco activase. J. Biol. Chem. 293, 19451–19465 10.1074/jbc.RA118.005047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Salvucci M.E., van de Loo F.J. and Stecher D. (2003) Two isoforms of Rubisco activase in cotton, the products of separate genes not alternative splicing. Planta 216, 736–744 [DOI] [PubMed] [Google Scholar]
- 117.Yin Z., Zhang Z., Deng D., Chao M., Gao Q., Wang Y. et al. (2014) Characterization of Rubisco activase genes in maize: an alpha-isoform gene functions alongside a beta-isoform gene. Plant Physiol. 164, 2096–2106 10.1104/pp.113.230854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carmo-Silva E., Scales J.C., Madgwick P.J. and Parry M.A.J. (2015) Optimizing Rubisco and its regulation for greater resource use efficiency. Plant, Cell Environ. 38, 1817–1832 10.1111/pce.12425 [DOI] [PubMed] [Google Scholar]
- 119.Zhang N., Kallis R.P., Ewy R.G. and Portis A.R. (2002) Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proc. Natl Acad. Sci. U.S.A. 99, 3330–3334 10.1073/pnas.042529999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaiser E., Morales A., Harbinson J., Heuvelink E., Prinzenberg A.E. and Marcelis L.F.M. (2016) Metabolic and diffusional limitations of photosynthesis in fluctuating irradiance in Arabidopsis thaliana. Sci. Rep. 6, 1–13 10.1038/s41598-016-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Evans J.R. and Poorter H. (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 24, 755–767 10.1046/j.1365-3040.2001.00724.x [DOI] [Google Scholar]
- 122.Ghannoum O., Evans J.R., Chow W.S., Andrews T.J., Conroy J.P. and Caemmerer S.V. (2005) Faster Rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiol. 137, 638–650 10.1104/pp.104.054759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mate C.J., Caemmerer S.V., Evans J.R., Hudson G.S. and Andrews T.J. (1996) The relationship between CO2-assimilation rate, Rubisco carbamylation and Rubisco activase content in activase-deficient transgenic tobacco suggests a simple model of activase action. Planta 198, 604–613 10.1007/BF00262648 [DOI] [PubMed] [Google Scholar]
- 124.Robinson S.P., Streusand V.J., Chatfield J.M. and Portis A.R. (1988) Purification and assay of rubisco activase from leaves. Plant Physiol. 88, 1008–1014 10.1104/pp.88.4.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mott K.A. and Woodrow I.E. (2000) Modelling the role of Rubisco activase in limiting non-steady-state photosynthesis. J. Exp. Bot. 51, 399–406 10.1093/jexbot/51.suppl_1.399 [DOI] [PubMed] [Google Scholar]
- 126.Townsend A.J., Retkute R., Chinnathambi K., Randall J.W.P., Foulkes J., Carmo-Silva E. et al. (2018) Suboptimal acclimation of photosynthesis to light in wheat canopies. Plant Physiol. 176, 1233–1246 10.1104/pp.17.01213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Salter W.T., Merchant A.M., Richards R.A., Trethowan R. and Buckley T.N. (2019) Rate of photosynthetic induction in fluctuating light varies widely among genotypes of wheat. J. Exp. Bot. 70, 2787–2796 10.1093/jxb/erz100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morales A., Kaiser E., Yin X., Harbinson J., Molenaar J., Driever S.M. et al. (2018) Dynamic modelling of limitations on improving leaf CO2 assimilation under fluctuating irradiance. Plant Cell Environ. 41, 589–604 10.1111/pce.13119 [DOI] [PubMed] [Google Scholar]
- 129.Shimazaki K., Doi M., Assmann S.M. and Kinoshita T. (2007) Light regulation of stomatal movement. Annu. Rev. Plant Biol. 58, 219–247 10.1146/annurev.arplant.57.032905.105434 [DOI] [PubMed] [Google Scholar]
- 130.Lawson T., Simkin A.J., Kelly G. and Granot D. (2014) Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 203, 1064–1081 10.1111/nph.12945 [DOI] [PubMed] [Google Scholar]
- 131.Busch F.A. (2014) Opinion: The red-light response of stomatal movement is sensed by the redox state of the photosynthetic electron transport chain. Photosynth. Res. 119, 131–140 10.1007/s11120-013-9805-6 [DOI] [PubMed] [Google Scholar]
- 132.Kramer D.M., Johnson G., Kiirats O. and Edwards G.E. (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 79, 209–218 10.1023/B:PRES.0000015391.99477.0d [DOI] [PubMed] [Google Scholar]
- 133.Kromdijk J., Głowacka K. and Long S.P. (2019) Predicting light-induced stomatal movements based on the redox state of plastoquinone: theory and validation. Photosynth. Res. 141, 83–97 10.1007/s11120-019-00632-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yamori W., Makino A. and Shikanai T. (2016) A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci. Rep. 6, 20147 10.1038/srep20147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hsiao T.C. and Allaway W.G. (1973) Action spectra for guard cell Rb uptake and stomatal opening in Vivia faba. Plant Physiol. 51, 82–88 10.1104/pp.51.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Horrer D., Flütsch S., Pazmino D., Matthews J.S.A., Thalmann M., Nigro A. et al. (2016) Blue light induces a distinct starch degradation pathway in guard cells for stomatal opening. Curr. Biol. 26, 1–9 10.1016/j.cub.2015.12.036 [DOI] [PubMed] [Google Scholar]
- 137.Santelia D. and Lunn J.E. (2017) Transitory starch metabolism in guard cells: unique features for a unique function. Plant Physiol. 174, 539–549 10.1104/pp.17.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Papanatsiou M., Petersen J., Henderson L., Wang Y., Christie J.M. and Blatt M.R. (2019) Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 363, 1456–1459 10.1126/science.aaw0046 [DOI] [PubMed] [Google Scholar]
- 139.Lawson T. and Blatt M.R. (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 164, 1556–1570 10.1104/pp.114.237107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McAusland L., Vialet-Chabrand S., Davey P., Baker N.R., Brendel O. and Lawson T. (2016) Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol. 211, 1209–1220 10.1111/nph.14000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boccalandro H.E., Giordano C.V., Ploschuk E.L., Piccoli P.N., Bottini R. and Casal J.J. (2012) Phototropins but not cryptochromes mediate the blue light-specific promotion of stomatal conductance, while both enhance photosynthesis and transpiration under full sunlight. Plant Physiol. 158, 1475–1484 10.1104/pp.111.187237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zeiger E. and Field C. (1982) Photocontrol of the functional coupling between photosynthesis and stomatal conductance in the intact leaf. Plant Physiol. 70, 370–375 10.1104/pp.70.2.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schymanski S.J., Or D. and Zwieniecki M. (2013) Stomatal control and leaf thermal and hydraulic capacitances under rapid environmental fluctuations. PLoS ONE 8, e54231 10.1371/journal.pone.0054231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vico G., Manzoni S., Palmroth S. and Katul G. (2011) Effects of stomatal delays on the economics of leaf gas exchange under intermittent light regimes. New Phytol. 192, 640–652 10.1111/j.1469-8137.2011.03847.x [DOI] [PubMed] [Google Scholar]
- 145.Lawson T. and Vialet-Chabrand S. (2019) Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 221, 93–98 10.1111/nph.15330 [DOI] [PubMed] [Google Scholar]
- 146.van Rooijen R., Kruijer W., Boesten R., van Eeuwijk F.A., Harbinson J. and Aarts M.G.M. (2017) Natural variation of YELLOW SEEDLING1 affects photosynthetic acclimation of Arabidopsis thaliana. Nat. Commun. 8, 1421 10.1038/s41467-017-01576-3 [DOI] [PMC free article] [PubMed] [Google Scholar]