Abstract
Background:
The southeastern United States consistently has high salmonellosis incidence, but disease drivers remain unknown. Salmonella is regularly detected in this region’s natural environment, leading to numerous exposure opportunities. Rainfall patterns may impact the survival/transport of environmental Salmonella in ways that can affect disease transmission.
Objectives:
This study investigated associations between short-term precipitation (extreme rainfall events) and longer-term precipitation (rainfall conditions antecedent to these extreme events) on salmonellosis counts in the state of Georgia in the United States.
Methods:
For the period 1997–2016, negative binomial models estimated associations between weekly county-level extreme rainfall events ( percentile of daily rainfall) and antecedent conditions (8-week precipitation sums, categorized into tertiles) and weekly county-level salmonellosis counts.
Results:
In Georgia’s Coastal Plain counties, extreme and antecedent rainfall were associated with significant differences in salmonellosis counts. In these counties, extreme rainfall was associated with a 5% increase in salmonellosis risk (95% CI: 1%, 10%) compared with weeks with no extreme rainfall. Antecedent dry periods were associated with a 9% risk decrease (95% CI: 5%, 12%), whereas wet periods were associated with a 5% increase (95% CI: 1%, 9%), compared with periods of moderate rainfall. In models considering the interaction between extreme and antecedent rainfall conditions, wet periods were associated with a 13% risk increase (95% CI: 6%, 19%), whereas wet periods followed by extreme events were associated with an 11% increase (95% CI: 5%, 18%). Associations were substantially magnified when analyses were restricted to cases attributed to serovars commonly isolated from wildlife/environment (e.g., Javiana). For example, wet periods followed by extreme rainfall were associated with a 34% risk increase (95% CI: 20%, 49%) in environmental serovar infection.
Conclusions:
Given the associations of short-term extreme rainfall events and longer-term rainfall conditions on salmonellosis incidence, our findings suggest that avoiding contact with environmental reservoirs of Salmonella following heavy rainfall events, especially during the rainy season, may reduce the risk of salmonellosis. https://doi.org/10.1289/EHP4621
Introduction
Every year, over 9 million cases of foodborne illness occur in the United States. Nontyphoidal Salmonella is estimated to cause 1 million of these cases (with 23,000 hospitalizations and 450 deaths) and is second only to norovirus as the most common foodborne pathogen (Scallan et al. 2011). Infection with nontyphoidal Salmonella is associated with diarrhea, abdominal cramps, and fever and these symptoms are often self limiting (Giannella 1996). Nationwide, Salmonella is also estimated to have a yearly economic burden of (Hoffmann et al. 2015). The southeastern region of the United States consistently has higher incidence rates of salmonellosis compared with other parts of the country (CDC 2016)—its rate was 11% higher than the national rate in 2015 (CDC 2017).
Numerous public health initiatives have been undertaken to better understand the epidemiology of foodborne diseases such as infections from Salmonella. The U.S. Centers for Disease Control and Prevention (CDC) has maintained its Foodborne Disease Active Surveillance Network (FoodNet) since 1995. FoodNet represents a long-standing collaboration between the CDC, the U.S. Food and Drug Administration (FDA), the U.S. Department of Agriculture (USDA), and 10 state health departments. FoodNet conducts rigorous surveillance and promotes behavioral changes to limit the public’s contact with foodborne diseases. However, although the United States has seen a marked decrease in the incidence of certain foodborne illnesses over the past two decades, this reduction has not been observed for salmonellosis. In fact, salmonellosis incidence has experienced an overall 35% increase since 2001—national incidence was approximately 11 cases per 100,000 population in 2001 but in 2015, it was 14.9 (CDC 2016; CDC 2017). In Georgia, there has been an 11% increase since 2001 (data obtained through GA DPH’s Public Health Information Portal: https://dph.georgia.gov/phip-data-request).
There are over 2,500 serovars of Salmonella, but human illnesses have been attributed to fewer than 100 serovars (CDC 2015). Salmonellae live and reproduce in the gastrointestinal tracts of humans and other animals and are shed through feces. Direct or indirect contact with contaminated feces can result in infection. Humans can come in contact with the pathogen through fecal matter in or on food and water and through contact with wild and domesticated animals (Ricke et al. 2013). When Salmonella is isolated from clinical and food samples during outbreaks, certain serovars (e.g., Enteritidis, Heidelberg, Kentucky) are often associated with animal-derived food commodities (Shah et al. 2017) and others (e.g., Muenchen, Javiana), with plant-derived food commodities (Gomba et al. 2016; Reddy et al. 2016). The serovars found on contaminated plant-derived food commodities have also been isolated from non-livestock reservoirs, such as birds, amphibians, water, and soil (Jackson et al. 2013; Micallef et al. 2012; Srikantiah et al. 2004). This may indicate that these serovars are more often associated with environmental reservoirs as opposed to animal-derived food production, and we therefore refer to them here as environmental serovars. These environmental serovars include: Javiana, Litchfield, Mbandaka, Muenchen, Poona, and Senftenberg. In Georgia, many of these serovars are also frequently detected in clinical cases (Maurer et al. 2015). Approximately 19% of the cases from 1997 to 2016 were attributed to these serovars (data obtained through GA DPH’s Public Health Information Portal: https://dph.georgia.gov/phip-data-request). The prevalence of infection with environmental serovars may indicate the potential for human exposure to Salmonella in the environment.
Environmental transmission is further supported by the regular detection of Salmonella in surface water and samples from other environmental sources in Georgia and neighboring states (Antaki et al. 2016; Haley et al. 2009; Lee et al. 2018; Li et al. 2014, 2015; Luo et al. 2015; Strawn et al. 2014). Even in nonhost environments, such as soil and water, Salmonella has been observed to survive and persist for up to 332 d (Islam et al. 2004; Kisluk and Yaron 2012; Maurer et al. 2015; Winfield and Groisman 2003; You et al. 2006) and ultimately to be transported through the soil, into water resources, and even onto produce crops. The fate and transport of Salmonella in the environment can be impacted by various factors, including temperature, soil moisture, nutrients, and microbial competition (Erickson et al. 2014).
Many studies have found that temperature and precipitation can also influence patterns of enteric disease incidence (Carlton et al. 2014, 2016; Levy et al. 2016; Stephen and Barnett 2016). In the United States and Canada, waterborne disease outbreaks have been associated with heavy rainfall events (Cann et al. 2013; Curriero et al. 2001; Jiang et al. 2015; Rose et al. 2001). One study found the risks of salmonellosis were elevated with the increased frequency of extreme rainfall events in Maryland (Jiang et al. 2015), but this association has not been explored for Georgia.
Furthermore, although there appears to be a direct linear relationship between temperature and enteric diseases that is influenced primarily by pathogen taxa (Carlton et al. 2016), based on our general understanding of the fate and transport of environmental pollutants, the influence of rainfall on enteric disease patterns is likely to be more nonlinear in nature. For example, the first flush phenomenon occurs when pollutants accumulate in the environment during dry periods and get dislodged en masse during heavy precipitation events (Bach et al. 2010; Lee et al. 2004). During wet periods, environmental pollutants are constantly diluted and transported; thus, an extreme precipitation event is less likely to result in the movement of a large bolus of accumulated contaminants. This phenomenon is often studied in the urban storm water context for chemical pollutants, but it may also apply to microbial contaminants in both urban and rural settings.
Many previous time-series studies of the climatic drivers of enteric disease have used Poisson and negative binomial regression to investigate associations between cumulative precipitation levels or the presence of extremely high precipitation levels on disease counts (Grjibovski et al. 2013, 2014; Hashizume et al. 2007; Singh et al. 2001). Other studies have assessed the association between extreme rainfall events and diarrheal disease (Bush et al. 2014; Carlton et al. 2014; Jagai et al. 2015). Few studies have explored both extreme rainfall events and cumulative rainfall. One study used logistic regression to individually assess associations between rainfall (cumulative rainfall and an extreme precipitation event) and waterborne outbreaks (Nichols et al. 2009). Of note, a common theme that emerged from a recent literature review on the effects of rainfall on diarrheal diseases is that the effects of heavy rainfall on diarrhea were magnified after dry periods, suggesting that models should incorporate antecedent rainfall conditions (Levy et al. 2016). For example, in a study in Ecuador, heavy rainfall events following dry weather periods were associated with elevated rates of diarrhea, but they were associated with reduced rates of diarrhea when following wet weather periods (Carlton et al. 2014). In a study from India, there was evidence of effect modification of the heavy rainfall–diarrhea association by longer-term rainfall trends, with increased diarrhea prevalence observed when a heavy rainfall event occurred after a 60-d dry period (Mertens et al. 2019). However, both of these studies looked at the incidence of diarrhea and not the incidence of disease from specific pathogens such as Salmonella.
To examine these phenomena, and to better understand relationships between climatic drivers and salmonellosis incidence patterns in Georgia, we analyzed the effect of precipitation on disease incidence using a long-term FoodNet data set for the state. We explored various aspects of rainfall, including overall levels as well as the timing of extreme rainfall events. In particular, we examined the interaction between extreme rainfall events and antecedent rainfall patterns on salmonellosis incidence in Georgia.
Methods
We evaluated a 20-y data set of salmonellosis cases in census tracts by residence in each of the 159 counties of Georgia from January 1997 (when reliable serotyping information became available) to December 2016, which was obtained from the Georgia Department of Public Health (GA DPH), a FoodNet site. In this data set, serotyping was regularly performed on clinical isolates; information on date of symptom onset, county of residence, serotype, whether the case was part of a recognized national or regional foodborne outbreak (based on GA DPH determination), age, gender, race, and ethnicity was available. The races of cases in the GA DPH data set included American Indian/Alaska Native, Asian, black or African American, Hawaiian/Pacific Islander, multiracial, or white. The ethnicities of cases included Hispanic or non-Hispanic. Cases identified as associated with foodborne outbreaks ( of cases), which may be part of a multi-state outbreak or caused by conditions in food production facilities, are less likely to be affected by rainfall conditions in Georgia. These cases were excluded from the analysis in order to focus specifically on cases that could be attributed to environmental sources. Cases that were missing age, gender, race, and ethnicity were still included in the analyses of associations between rainfall and salmonellosis incidence. Cases missing serotype data were included in the analyses of all serovars but not included in the analyses of the environmental serovars.
During the 1997–2016 period, there were 39,535 salmonellosis cases not associated with known outbreaks. We aggregated case data by county to overcome the low counts of salmonellosis for many census tracts. Similarly, although date of symptom onset was available, we aggregated data to weekly disease counts because of the low salmonellosis counts for some counties. Cases without county information were removed from the analyses ( of cases).
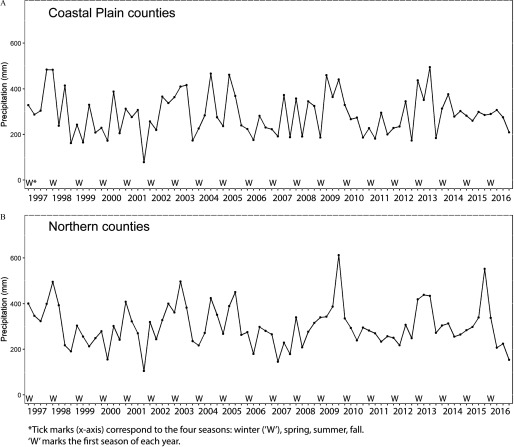
Weather station data were obtained from the 1,098 weather stations in Georgia through NOAA’s National Climatic Data Center (NCDC; https://www.ncdc.noaa.gov/cdo-web/). Daily maximum temperature and precipitation levels for the 1997–2016 period were obtained from each station. We then excluded weather stations with less than 75% completeness in daily precipitation data during the study period. Each county was assigned the closest weather station (with at least 75% completeness) located within of the center of the most populous city of the county. Because of these requirements, some counties were assigned the same weather station. Our final data set used data from 116 weather stations to represent the meteorological conditions in the 159 counties. Median precipitation by year and season for Northern and Coastal Plain counties is depicted in Figure 1.
Figure 1.
Median precipitation (mm) by year and season for (A) Coastal Plain and (B) Northern counties during 1997–2016. Tick marks (x-axis) correspond to the four seasons: winter (W), spring, summer, fall. W marks the first season of each year.
Mean yearly salmonellosis incidence rates [and 95% confidence intervals (CIs)] were estimated by gender, age group (under 5, 5–19, 20–64, and years of age), and race/ethnicity (Hispanic, all races; white, non-Hispanic; Asian, non-Hispanic; black, non-Hispanic). These four race/ethnicity groups were chosen because they accounted for the majority of the state’s population. Negative binomial models with county-specific random intercepts were used to estimate the association between county-level weekly salmonellosis counts and rainfall conditions. Rainfall conditions included: a) the presence of county-specific extreme rainfall events, defined as a daily precipitation level greater than the county-specific 90th percentile (over the 20-y study period); b) cumulative rainfall antecedent to extreme rainfall events (hereafter referred to as antecedent conditions); and c) the interaction between extreme rainfall events and antecedent conditions. Antecedent conditions were determined using 8-week sums of daily precipitation levels occurring in the 8 weeks prior to analysis, following Carlton et al. (2014). Eight-week periods with sums greater than or equal to the county-specific 67th percentile over the 20-y study period were considered antecedent wet periods, those with sums lower than the county-specific 33rd percentile were considered antecedent dry periods, and the rest were considered antecedent moderate rainfall periods. These specific metrics were designed to assess both the overall effects of short- and long-term rainfall, as well as the potential impact of a first-flush phenomenon on salmonellosis counts in Georgia, analyzed using three epidemiologic model formulations as described below.
First, to estimate associations with extreme rainfall events alone, we modeled
| (1) |
where refers to the salmonellosis count in county i during week t. The dichotomous variable Extreme was a county-specific variable that referred to whether at least one extreme rainfall event occurred in county i during the week t-1. Sensitivity analyses investigated associations with extreme precipitation at the 95th and 99th percentiles and of lags of 1–3 weeks. We controlled for weekly county-specific mean Temperature as a linear term; Season using a four-level categorical variable (winter, spring, summer, fall) as categorized by the March equinox, June solstice, September equinox, and December solstice; and long-term trend using natural cubic splines for Week Number in the study, with 20 degrees of freedom for the 20 y of the study. Finally, an offset for Population was included in the models to account for differences in county population. Population data were obtained through GA DPH’s Online Analytical Statistical Information System (https://oasis.state.ga.us/). To account for county-specific baseline risks, we included a random intercept, .
Second, to estimate associations with antecedent rainfall conditions alone, we modeled
| (2) |
where all variables were as defined for Model 1. In place of Extreme, the categorical variable Antecedent was included, which categorized rainfall in county i during the week t-1 as according to whether the antecedent 8-wk periods experienced wet, dry, or moderate rainfall.
Finally, to estimate the interaction of extreme rainfall events and their antecedent conditions, we modeled
| (3) |
where all variables were as defined for Models 1 and 2, with the inclusion of a product term to estimate the interaction of Extreme and Antecedent rainfall conditions. In this model, six permutations of rainfall conditions were considered as exposures because following each of the three 8-week periods of cumulative rainfall (dry/moderate/wet), an extreme rainfall event may or may not have occurred. For each exposure permutation, the associated risk of salmonellosis was estimated. The relative excess risk due to interaction (RERI) and the associated 95% CIs were estimated using methods described by Hosmer and Lemeshow (1992). All analyses were conducted using R (version 3.1.2; R Development Core Team).
The Fall Line of Georgia is a boundary line between two large geologic regions of Georgia, separating the Piedmont region from the Coastal Plain region (Figure 2). It runs from Columbus in the west to Augusta in the east. The Piedmont, Blue Ridge Mountains, and Ridge and Valley regions lie to the north of the Fall Line, whereas the Upper and Lower Coastal Plain lie to the south. Agricultural, household income, climate, and land use data for the counties north and south of the Fall Line are provided in Table 1. Despite the predominance of the poultry production industry in the north of the state, salmonellosis incidence is higher in the Coastal Plain region and most reports of environmental occurrence of Salmonella in Georgia have been reported in this region (Antaki et al. 2016; Haley et al. 2009; Lee et al. 2018; Li et al. 2014; Luo et al. 2015; Maurer et al. 2015). Because of the prevalence of Salmonella in environmental samples from the Coastal Plain, precipitation patterns can affect pathogen survival and transport in the environment and, in turn, have greater effects on exposure to Salmonella among residents of the Coastal Plain. Therefore, we stratified our analyses by county location relative to the Fall Line, in which counties south of the Fall Line were considered to be Coastal Plain counties and those north of the Fall Line were considered to be Northern counties.
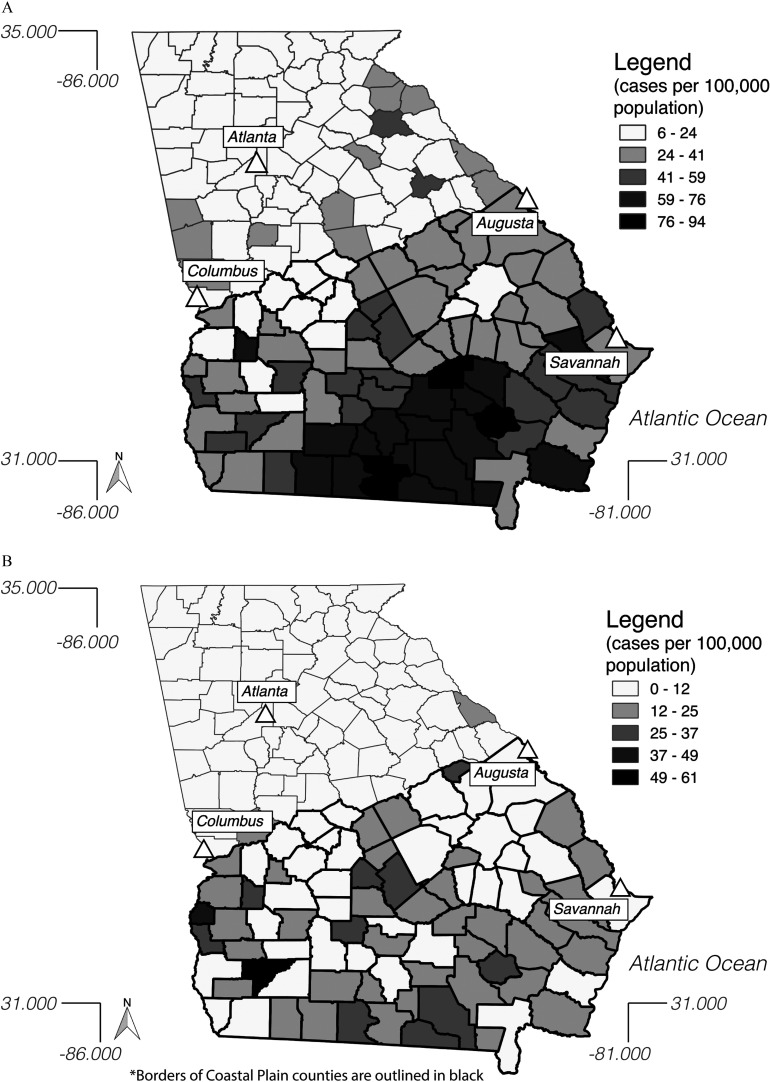
Figure 2.
Mean yearly salmonellosis incidence (cases per 100,000 population) in Georgia by county for (A) all serovars and (B) environmental serovars during 1997–2016. Borders of Coastal Plain counties are outlined in black.
Table 1.
Comparison of demographic, economic, and climatic differences between the Coastal Plain and Northern counties.
| Characteristic | Coastal Plain | Northern |
|---|---|---|
| Total farm gate value (million U.S. dollars)a | ||
| Vegetables | 1,087.9 | 56.3 |
| Livestock | 795.6 | 694.4 |
| Poultry/eggs | 1,331.1 | 4,013.4 |
| High school diploma as highest educational attainment (%)b | 58.2 | 51.4 |
| Median income (U.S. dollars)b | 36,772 | 47,420 |
| Below poverty level (%)b | 24.9 | 18.1 |
| Rural (%)c | 64.2 | 56.3 |
| Climated | ||
| Yearly precipitation (cm) | 129.9 | 168.7 |
| Mean minimum temperature (°C) | 14.4 | 11.3 |
| Mean maximum temperature (°C) | 26.0 | 22.6 |
| Soil temperature (°C) | 22.1 | 18.9 |
Obtained from 2016 Farm Gate Value Report (Wolfe and Stubbs 2017).
Obtained from 2012–2016 American Community 5-Year Estimates (https://data.census.gov).
Obtained from the 2010 U.S. Census: Summary File 1, Table P2 (https://factfinder.census.gov).
Obtained from NOAA’s National Climatic Data Center (NCDC; https://www.ncdc.noaa.gov/cdo-web/).
To investigate the relationship between climatic factors and salmonellosis attributed to serovars more specifically associated with environmental exposure, we ran Models 1 to 3 with county-level weekly salmonellosis counts restricted to several nonlivestock reservoir serovars discussed by Jackson et al. (2013): Javiana, Litchfield, Mbandaka, Muenchen, Poona, and Senftenberg. To differentiate these results from those describing associations of all salmonellosis cases, we hereafter refer to these as the environmental serovar models.
Results
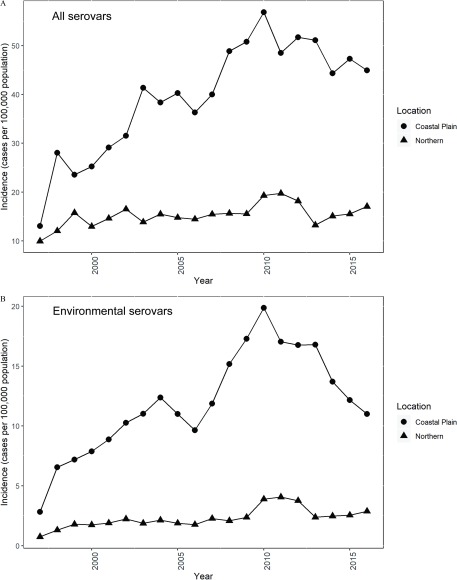
The mean annual incidence of salmonellosis throughout the study period in all counties of Georgia was 21.6 per 100,000 population (95% CI: 20.3, 23.0), with elevated annual incidence in the Coastal Plain counties (39.1 per 100,000; 95% CI: 36.6, 41.6) compared with the Northern counties (15.3 per 100,000; 95% CI: 14.3, 16.2) (Table 2; Figure 2). Salmonellosis incidence rates in Georgia due to all serovars and environmental serovars increased substantially over the study period in the Coastal Plain, but not the Northern, counties (Figure 3).
Table 2.
Comparison of mean yearly salmonellosis case counts (n) and incidence (cases per 100,000 population) and 95% confidence intervals (CIs) in Coastal Plain and Northern counties during 1997–2016.
| Characteristic | Coastal Plain counties (population: 2,938,517) | Northern counties (population: 6,197,440) | All counties (population:9,135,957) | |||
|---|---|---|---|---|---|---|
| Incidence (95% CI) | Incidence (95% CI) | Incidence (95% CI) | ||||
| Serovar | ||||||
| All serovars | 946.9 | 39.1 (36.6, 41.6) | 1,024.7 | 15.3 (14.3, 16.2) | 985.8 | 21.6 (20.3, 23.0) |
| Environmental serovarsa | 251.5 | 10.4 (9.2, 11.7) | 132.0 | 2.0 (1.6, 2.3) | 191.7 | 4.2 (3.6, 4.8) |
| Gender | ||||||
| Male | 472.0 | 39.8 (36.3, 43.4) | 510.0 | 15.5 (14.2, 16.9) | 982.0 | 19.8 (18.6, 21.1) |
| Female | 470.5 | 38.1 (34.8, 41.7) | 508.9 | 14.8 (13.6, 16.2) | 979.4 | 19.0 (17.8, 20.2) |
| Age (y) | ||||||
| 451.5 | 261.8 (238.5, 286.8) | 368.0 | 77.2 (69.5, 85.3) | 819.4 | 113.9 (106.3, 121.9) | |
| 5–9 | 127.3 | 24.2 (20.3, 28.7) | 193 | 13.4 (11.5, 15.4) | 320.3 | 14.6 (13.1, 16.3) |
| 20–64 | 257.7 | 18.1 (15.9, 20.4) | 359.8 | 8.7 (7.9, 9.7) | 617.5 | 10.1 (9.3, 10.9) |
| 106.1 | 35.7 (29.5, 43.2) | 98.2 | 14.6 (11.9, 17.7) | 204.3 | 19.2 (16.7, 22.0) | |
| Race/ethnicity | ||||||
| Asian (non-Hispanic) | 3.8 | 13.9 (4.0, 32.1) | 22.5 | 9.1 (5.9, 13.4) | 13.2 | 9.6 (5.6, 16.2) |
| Black (non-Hispanic) | 205.1 | 23.3 (20.3, 26.7) | 212.8 | 11.6 (10.1, 13.2) | 208.9 | 15.4 (13.4, 17.6) |
| Hispanic | 32.6 | 29.6 (20.6, 41.1) | 64.9 | 11.2 (8.7, 14.1) | 48.7 | 14.1 (10.5, 18.5) |
| White (non-Hispanic) | 558.7 | 41.0 (37.7, 44.4) | 569.0 | 14.4 (13.2, 15.6) | 563.9 | 21.2 (19.5, 22.9) |
| Season | ||||||
| Winter | 80.1 | 3.3 (2.7, 4.1) | 127.6 | 1.9 (1.6, 2.3) | 207.6 | 2.3 (2.0, 2.6) |
| Spring | 156.2 | 6.4 (5.5, 7.5) | 235.4 | 3.5 (3.1, 4.0) | 391.6 | 4.3 (3.9, 4.7) |
| Summer | 427.8 | 17.7 (16.0, 19.4) | 419.2 | 6.2 (5.7, 6.9) | 847.0 | 9.3 (8.7, 9.9) |
| Fall | 282.8 | 11.7 (10.4, 13.1) | 242.6 | 3.6 (3.2, 4.1) | 525.4 | 5.8 (5.3, 6.3) |
Environmental serovars include: Javiana, Litchfield, Mbandaka, Muenchen, Poona, and Senftenberg.
Figure 3.
Median yearly salmonellosis incidence (cases per 100,000 population) in Georgia during 1997–2016 attributed to (A) all serovars and (B) environmental serovars.
Salmonellosis incidence in males (19.8 per 100,000 population; 95% CI: 18.6, 21.1) was similar to incidence in females (19.0 per 100,000 population; 95% CI: 17.8, 20.2) (Table 2). Of the age groups, mean incidence was significantly higher in children under 5 years of age (113.9 per 100,000 population; 95% CI 106.3, 121.9) compared with those between 5 and 19 years of age (14.6 per 100,000 population; 95% CI 13.1, 16.3), those between 20 to 64 years of age (10.1 per 100,000 population; 95% CI: 9.3, 10.9), and those years of age (19.2 per 100,000 population; 95% CI: 16.7, 22.0). For individuals younger than 20 years of age, salmonellosis incidence in males (49.0 per 100,000 population; 95% CI: 45.4, 52.9) was higher than in females (43.7 per 100,000 population; 95% CI: 40.1, 47.4). The opposite was true for individuals years of age: incidence among males (12.5 per 100,000 population; 95% CI: 11.3, 13.8) was lower than incidence among females (14.3 per 100,000 population; 95% CI: 13.1, 15.6). Incidence was significantly higher in the white, non-Hispanic population (21.2 per 100,000 population; 95% CI: 19.5, 22.9) compared with the Hispanic (all races), Asian (non-Hispanic), and black (non-Hispanic) groups (Table 2).
To estimate the overall associations of extreme events and antecedent rainfall conditions with salmonellosis incidence (all serovars), Models 1 and 2 were conducted for all counties, and stratified by region (Coastal Plain counties and Northern counties) (Table 3). When examining incidence in all counties, extreme rainfall events (Model 1) were associated with a 3% increase in risk (i.e., incidence rate ratio) [ (95% CI: 1.00, 1.06)] and antecedent wet periods (Model 2) were associated with a 3% increase in risk [ (95% CI: 1.00, 1.06)], whereas antecedent dry periods (Model 2) were associated with a 7% decrease in risk [ (95% CI: 0.90, 0.96)]. Similar associations were estimated in analyses limited to the Coastal Plain counties. In these Coastal Plain counties, extreme rainfall events (Model 1) were associated with a 5% increase in risk [ (95% CI: 1.01, 1.10)] compared with weeks with no extreme rainfall. Compared with periods with moderate rainfall in the Coastal Plain region, antecedent dry periods (Model 2) were associated with a 9% decrease in risk [ (95% CI: 0.88, 0.95)], whereas antecedent wet periods were associated with a 5% increase [ (95% CI: 1.01, 1.09)] in risk. In contrast to the Coastal Plain counties, in the Northern counties, antecedent dry periods (Model 2) were associated with no increase in risk [ (95% CI: 0.96, 1.04)]. In the Northern counties, there was no significant association between extreme rainfall events (Model 1) and salmonellosis incidence and between antecedent wet conditions and salmonellosis incidence (Model 2), but these associations were similar to those estimated in the Coastal Plain counties.
Table 3.
Salmonellosis (all serovar) incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for six possible combinations of precipitation conditions (extreme precipitation and antecedent conditions) in Model 1 (extreme rainfall) and Model 2 (antecedent rainfall).
| Variable | All counties IRR (95% CI) | Coastal Plain counties IRR (95% CI) | Northern counties IRR (95% CI) |
|---|---|---|---|
| Model 1a | |||
| Extremeb | 1.03 (1.00, 1.06) | 1.05 (1.01, 1.10) | 1.02 (0.98, 1.06) |
| Model 2c | |||
| Antecedentd dry | 0.93 (0.90, 0.96) | 0.91 (0.88, 0.95) | 1.00 (0.96, 1.04) |
| Antecedentd wet | 1.03 (1.00, 1.06) | 1.05 (1.01, 1.09) | 0.99 (0.95, 1.03) |
Model 1 includes terms for extreme precipitation, temperature, season, natural cubic spline for week number, and an offset for population.
Extreme refers to the presence of an extreme rainfall event at the 90th percentile of daily precipitation levels in the week preceding the week of disease incidence (1-week lag).
Model 3 includes terms for antecedent precipitation conditions, temperature, season, natural cubic spline for week number, and an offset for population.
Antecedent refers to the antecedent conditions preceding the week in question (dry or wet periods), corresponding to tertiles of total daily precipitation over the prior 8 weeks and using moderate rainfall periods as the reference group.
To assess the interaction of extreme rainfall events and antecedent rainfall conditions, Model 3 was conducted for all serovars and all counties and stratified by region (Table 4, all serovars results; see also Table S1 for all Model 3 parameter values). In all counties, compared with weeks with no extreme event following moderate rainfall, extreme rainfall events following moderate rainfall were associated with a 9% increase in risk [ (95% CI: 1.04, 1.13)]. Wet periods alone were associated with 8% increase in risk [ (95% CI: 1.03, 1.13)] and wet periods followed by an extreme rainfall event were associated with 9% increase in risk [ (95% CI: 1.05, 1.14)]. In the Coastal Plain, extreme rainfall events were associated with an 11% increase in risk [ (95% CI: 1.05, 1.18)] when they occurred following moderate rainfall and wet periods. Extreme rainfall events following dry periods were not as strongly associated with salmonellosis risk [ (95% CI: 0.98, 1.11)]. In contrast, in the Northern counties, wet periods alone were associated with a 6% decrease in risk [ (95% CI: 0.89, 1.00)]. The risks associated with extreme rainfall events following moderate [ (95% CI: 0.97, 1.09)] and wet periods [ (95% CI: 0.95, 1.07)] were lower than, but not significantly different from, the risks of these precipitation patterns in the Coastal Plain. In the Coastal Plain, we estimated a negative additive interaction between extreme rainfall and wet antecedent conditions [ (95% CI: , )], whereas in the Northern counties, we estimated a more positive additive interaction between extreme rainfall and wet conditions [ (95% CI: , 0.11)].
Table 4.
Incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for six possible combinations of precipitation conditions (extreme precipitation and antecedent conditions) in Model 3 when considering salmonellosis from all serovars and environmental serovars only.
| Extremea | Antecedentb | All counties IRR (95% CI) | Coastal Plain counties IRR (95% CI) | Northern counties IRR (95% CI) |
|---|---|---|---|---|
| All serovars | ||||
| No | Dry | 0.94 (0.90, 0.98) | 0.92 (0.87, 0.98) | 1.01 (0.96, 1.07) |
| Mod | Ref | Ref | Ref | |
| Wet | 1.08 (1.03, 1.13) | 1.13 (1.06, 1.19) | 0.94 (0.89, 1.00) | |
| Yes | Dry | 1.02 (0.97, 1.06) | 1.04 (0.98, 1.11) | 1.04 (0.98, 1.10) |
| Mod | 1.09 (1.04, 1.13) | 1.11 (1.05, 1.18) | 1.03 (0.97, 1.09) | |
| Wet | 1.09 (1.05, 1.14) | 1.11 (1.05, 1.18) | 1.01 (0.95, 1.07) | |
| Environmental serovars | ||||
| No | Dry | 0.97 (0.88, 1.06) | 0.96 (0.86, 1.09) | 0.95 (0.81, 1.11) |
| Mod | Ref | Ref | Ref | |
| Wet | 1.19 (1.09, 1.31) | 1.29 (1.16, 1.44) | 0.90 (0.76, 1.06) | |
| Yes | Dry | 1.12 (1.01, 1.23) | 1.20 (1.06, 1.35) | 1.05 (0.90, 1.22) |
| Mod | 1.18 (1.07, 1.29) | 1.22 (1.09, 1.37) | 1.06 (0.91, 1.24) | |
| Wet | 1.28 (1.17, 1.40) | 1.34 (1.20, 1.49) | 1.14 (0.97, 1.34) | |
Note: Model 3 includes terms for extreme precipitation, antecedent precipitation conditions, temperature, season, natural cubic spline for week number, and an offset for population. Mod, moderate; Ref, reference.
Extreme refers to the presence of an extreme rainfall event at the 90th percentile of daily precipitation levels in the week preceding the week of disease incidence (1-week lag). The presence of an extreme event is indicated as no or yes.
Antecedent refers to the antecedent rainfall conditions preceding the week in question (dry, moderate rainfall, or wet periods), corresponding to tertiles of total daily precipitation over the prior 8 weeks.
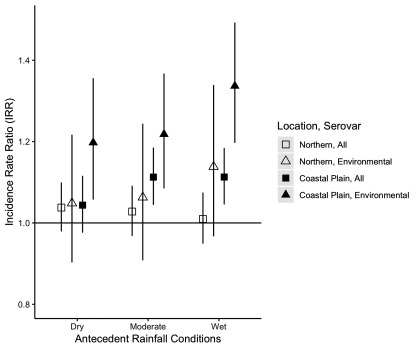
The effects of rainfall conditions on salmonellosis incidence in the Coastal Plain counties were even more pronounced when limiting the analysis to cases associated with environmental serovars (Table 4, environmental serovars results; Figure 4). For all antecedent categories, extreme rainfall events were associated with a significant increase in risk compared with the reference condition of no extreme event during a moderate rainfall period. The increase in risk associated with an extreme rainfall event ranged from 20% during dry periods ( (95% CI: 1.06, 1.35)] to 22% in periods with moderate rainfall [ (95% CI: 1.09, 1.37)] to 34% in wet periods [ (95% CI: 1.20, 1.49)]. In the absence of an extreme rainfall event, wet periods had a 29% higher risk compared with moderate rainfall periods [ (95% CI: 1.16, 1.44)]. When examining the combined effect of extreme rainfall and wet conditions in the Coastal Plain, we estimated a negative additive interaction [ (95% CI: , )]. However, we estimated a positive additive interaction between extreme rainfall and wet conditions in the Northern counties [ (95% CI: , 0.39)].
Figure 4.
Comparison of incidence rate ratios (IRRs) and 95% confidence intervals for the associations between an extreme rainfall event at the 90th percentile (1-week lag) and differing antecedent rainfall conditions (dry, moderate, wet) by county location and serovar type.
In sensitivity analyses of extreme precipitation at the 95th and 99th percentile, the estimated associations with low and high antecedent conditions when followed by no extreme event were similar to the 90th percentile analysis. However, the estimated associations with high antecedent conditions prior to extreme precipitation diminished and IRRs were driven largely by the extreme precipitation events themselves. These trends were observed when examining salmonellosis incidence associated with all serovars and environmental serovars. These results can be found in Tables S2–S3.
In sensitivity analyses of time lags for extreme rainfall, the pattern of observed results was similar, but the magnitude of estimated associations was lower at longer lags (see Table S4).
Discussion
In this analysis, we estimated the potential impacts of both short-term and long-term precipitation conditions and their interaction on salmonellosis incidence using a 20-y data set of weather exposures and disease outcomes across all 159 counties of Georgia. The results of this study indicate a positive association between precipitation and salmonellosis incidence in Georgia. The patterns of associations among Coastal Plain counties were similar to, but stronger than, those for all counties, and associations among Northern counties were consistent with the null. We were particularly interested in the timing of heavy rainfall events, and whether antecedent conditions (defined as rainfall in the prior 8 weeks) modified the association between extreme rainfall events and disease incidence. In the Coastal Plain region, both extreme rainfall events and wet periods (alone and together) trended toward increased risk, although the association of extreme rainfall events and wet conditions combined was not significantly greater than the association of each of these precipitation parameters alone. These results highlight the potential associations of extreme rainfall events and wet periods with salmonellosis risk.
The stronger associations between rainfall patterns and salmonellosis when examining the environmental serovars compared with all serovars further suggest the importance of rainfall-mediated transmission of Salmonella in the environment, particularly in southern Georgia. In the Coastal Plain, extreme rainfall events following wet periods were associated with an 11% increase in risk [ (95% CI: 1.05, 1.18)] of salmonellosis from all serovars; however, when analyzing salmonellosis cases attributed to environmental serovars, extreme rainfall events following wet periods were associated with a 34% increase in risk [ (95% CI: 1.20, 1.49)]. Taken together, these results suggest that environmental sources may contribute to many sporadic cases of salmonellosis, and they support the need to better understand environmental exposure pathways for Salmonella infection and the potential impact of precipitation and other climatic conditions on exposure to pathogens.
The results are consistent with reports of increase in infections from Salmonella and other enteric pathogens such as E. coli O157:H7, Cryptosporidium, and Campylobacter following extreme rainfall events (Jiang et al. 2015; Thomas et al. 2006) and rainy periods (Grjibovski et al. 2014; Nichols et al. 2009). Interestingly, our findings do not support a first flush phenomenon, in contrast with Carlton et al. (2014) and Mertens et al. (2019), who reported stronger associations between all-cause diarrhea and heavy rainfall events during dry compared with wet periods in Ecuador and India. Differences among studies may reflect differences in precipitation-related exposure mechanisms related to agricultural practices, living conditions, water sources, or other factors, or they may reflect differences between Salmonella-specific disease and all-cause diarrhea.
The absence of this first flush phenomenon may also be due to the increased survival of soil bacteria during wet conditions (Cools et al. 2001; Iovieno and Bååth 2008; Yeager and Ward 1981). In addition to promoting microbial survival, high soil moisture content is associated with greater microbial transport (Callahan et al. 2017; Tallon et al. 2007). Soil saturation during wet periods can result in surface water runoff during high rainfall events, which can facilitate the transport of contaminants in the environment (Detty and McGuire 2010; Kibet et al. 2014; Penna et al. 2011). Wet antecedent conditions prior to extreme rainfall may promote the transport of enteric pathogens through the environment and potentially increase the risk of human exposure to these pathogens. Even when soil is not saturated, the intensity of extreme rainfall events can dislodge contaminants and transport them through soil, into water sources, and potentially onto crops (Barak and Liang 2008; Harris et al. 2018; Islam et al. 2004, 2005; Jacobsen and Bech 2012; Keraita et al. 2007; Park et al. 2012).
Human exposure to contaminated surface water, either through recreational use or consumption of crops irrigated with surface water, represents one pathway in which rainfall conditions may increase disease risk. This pathway is of particular concern in regions in Georgia and elsewhere where produce production and livestock production overlap (Wolfe and Stubbs 2017). Further work is necessary to better understand the impact of precipitation patterns—and not just cumulative precipitation levels or the presence of extreme events—on this pathway.
Temperature conditions may also impact Salmonella survival in the environment. Multiple freeze-thaws can limit Salmonella survival in the environment (Holley et al. 2006; Natvig et al. 2002). Thus, even though Salmonella has been detected in Northern counties (Maurer et al. 2015), Salmonella may be more prevalent throughout the year in the Coastal Plain, where average temperatures range from 14.4°C to 26.0°C, compared with 11.3°C to 22.6°C in the Northern counties.
In Georgia, the yearly incidence of salmonellosis associated with all serovars and environmental serovars has increased since 1997. This increase is most notable in the Coastal Plain region (Figure 3). The increase in incidence could be a function of environmental factors such as climatic changes or changes in land management or could also be attributable to improvements in diagnostic procedures and/or improved surveillance; geographic differences in surveillance have been noted in other regions of the United States (Mor et al. 2014).
Another potential explanation for the elevated and rising incidence in the Coastal Plain counties may be a combination of aging private wells and septic systems. Deteriorating wells are vulnerable to intrusion by contaminants and setback distances between septic tanks and wells may be inadequate (Blaschke et al. 2016). Although rural populations throughout Georgia rely on septic systems and private wells, a greater proportion of the Coastal Plain population relies on private wells for drinking water (Johnson and Belitz 2017).
Similar to prior studies of salmonellosis trends by age and sex (CDC 2017; Reller et al. 2008), this study found that salmonellosis incidence in those under 20 years of age is found to be slightly higher in males than in females; among older ages, the incidence in females is higher than males. These differences in incidence may be due to increased contact with wild and domesticated animals and natural environments in male children compared with female children. The higher incidence in females over 20 may be driven by exposure through fresh produce consumption and food preparation (Boore et al. 2015). Future studies could further investigate behavioral factors associated with salmonellosis incidence.
One important caveat to investigating climatic drivers of salmonellosis incidence is the abundance of ways in which people can become exposed to Salmonella. This study explored the association between climate and salmonellosis cases but could not determine specific sources of infection. Many behaviors that are influenced by climatic conditions, such as gardening, swimming, and recent travel, may also increase human exposure to Salmonella. Although we controlled for season to address these types of behaviors, residual confounding is still possible. Better understanding the exposure pathways involved in the transmission of these serovars will help identify leverage points to reduce salmonellosis risk.
One of the strengths of this study was the analysis of salmonellosis incidence by location and serovar, which allowed us to focus more closely on the associations between rainfall and infection with environmental serovars in the Coastal Plain. Infections from Salmonella Javiana, one of the environmental serovars we considered, are most commonly found in the southeastern region of the United States (CDC 2016, 2017). Javiana infections have been associated with wetland presence in Maryland and Georgia and this may be due to the role of wetlands as a habitat for environmental reservoirs, such as reptiles and amphibians (Huang et al. 2017; Srikantiah et al. 2004). Changes in the frequency and duration of droughts and heavy rainfall have the potential to impact wetland quantity and quality. In turn, they can influence the reproductive success and dispersal of reptiles and amphibians and ultimately, the prevalence of Salmonella in the environment (Lind 2008; Walls et al. 2013). Serovar-specific analyses have the potential to elucidate environmental reservoirs and exposure pathways that may be particularly vulnerable to precipitation.
Our findings suggest that extreme rainfall and antecedent conditions are not associated with salmonellosis incidence in the Northern counties of Georgia. Even though poultry production is extensive in the Northern counties of Georgia (Wolfe and Stubbs 2017), salmonellosis incidence is higher in the Coastal Plain counties. These results may indicate that, even if these poultry operations are sources of Salmonella (Berghaus et al. 2013; Trimble et al. 2013), the contamination is either controlled from entering the environment or not affected by the rainfall-related exposure pathways in the Northern counties. For example, these poultry operations may be located far enough away from wells to not pose a threat to drinking water quality.
One major limitation of this study was that we were limited to county-level data because of the low frequency of weekly disease counts by census tract. To estimate county-level weather data, we used data from the weather station closest to the most populous city in each county, which was up to away from that city (this represents the maximum). In addition, even when weather stations were close to the most populous cities, individual salmonellosis cases might have occurred far from these cities. Daily precipitation values can vary widely within a county and, thus, some exposure misclassification for the precipitation data may have resulted. We expect that although the presence of an extreme rainfall event in 1 week may have differed within each county, the categorization of the 8-week antecedent conditions would have been similar throughout the county.
In addition, only cases that were not part of a recognized national or local foodborne outbreak were considered in this analysis. However, we may have included some outbreak cases in the analysis if they were not recognized as such by the GA DPH. We expect though that the inclusion of these cases would have been systematic throughout the state and the study period regardless of precipitation patterns and thus would not have biased our estimates.
Given the contribution of climatic factors to salmonellosis risk, a better understanding of the effects of climate on environmental pathogen transmission is critical for planning adaptation measures for potential changes in climate in the future. Our results suggest that extreme rainfall events and periods of prolonged wetness or dryness may impact salmonellosis risk. Potential increases in periods of drought as anticipated with climate change (Dai 2013; Trenberth 2011) may be protective against exposure and infection, but the anticipated increased frequency and intensity of extreme rainfall events with climate change (Fischer and Knutti 2016; Kunkel et al. 2013; Prein et al. 2017) may increase salmonellosis risk. Under the A2 emissions scenario of the Intergovernmental Panel on Climate Change’s (IPCC) Special Report on Emissions Scenarios (SRES), which assumes continued increases in carbon dioxide () emissions, southern Georgia is expected to experience a 15–20% increase in the number of days when precipitation is greater than 1 in. () by 2041–2070 (compared with 1980–2000) (Kunkel et al. 2013), which is similar to the threshold for extreme rainfall event in our study. This suggests that in this high emissions scenario, there may be a continued elevated risk of salmonellosis in southern Georgia in the future. These results may also be applicable to other regions of the United States and elsewhere where Salmonella is prevalent in the environment that also anticipate increased temperatures and rainfall variability. A comprehensive understanding of these climatic changes and the exposure pathways they influence will help improve public health measures to mitigate salmonellosis risk.
Supplementary Material
Acknowledgments
K.L. was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (grant K01AI103544). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4621).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Antaki EM, Vellidis G, Harris C, Aminabadi P, Levy K, Jay-Russell MT. 2016. Low concentration of Salmonella enterica and generic Escherichia coli in farm ponds and irrigation distribution systems used for mixed produce production in southern Georgia. Foodborne Pathog Dis 13(10):551–558, PMID: 27400147, 10.1089/fpd.2016.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach PM, McCarthy DT, Deletic A. 2010. Redefining the stormwater first flush phenomenon. Water Res 44(8):2487–2498, PMID: 20185157, 10.1016/j.watres.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Barak JD, Liang AS. 2008. Role of soil, crop debris, and a plant pathogen in Salmonella enterica contamination of tomato plants. PLoS One 3(2):e1657, PMID: 18301739, 10.1371/journal.pone.0001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghaus RD, Thayer SG, Law BF, Mild RM, Hofacre CL, Singer RS. 2013. Enumeration of Salmonella and Campylobacter spp. in environmental farm samples and processing plant carcass rinses from commercial broiler chicken flocks. Appl Environ Microbiol 79(13):4106–4114, PMID: 23624481, 10.1128/AEM.00836-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke AP, Derx J, Zessner M, Kirnbauer R, Kavka G, Strelec H, et al. 2016. Setback distances between small biological wastewater treatment systems and drinking water wells against virus contamination in alluvial aquifers. Sci Total Environ 573:278–289, PMID: 27570196, 10.1016/j.scitotenv.2016.08.075. [DOI] [PubMed] [Google Scholar]
- Boore AL, Hoekstra RM, Iwamoto M, Fields PI, Bishop RD, Swerdlow DL. 2015. Salmonella enterica infections in the United States and assessment of coefficients of variation: a novel approach to identify epidemiologic characteristics of individual serotypes, 1996–2011. PLoS One 10(12):e0145416, PMID: 26701276, 10.1371/journal.pone.0145416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush KF, O’Neill MS, Li S, Mukherjee B, Hu H, Ghosh S, et al. 2014. Associations between extreme precipitation and gastrointestinal-related hospital admissions in Chennai, India. Environ Health Perspect 122(3):249–254, PMID: 24345350, 10.1289/ehp.1306807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MT, Micallef SA, Buchanan RL. 2017. Soil type, soil moisture, and field slope influence the horizontal movement of Salmonella enterica and Citrobacter freundii from floodwater through soil. J Food Prot 80(1):189–197, PMID: 28221887, 10.4315/0362-028X.JFP-16-263. [DOI] [PubMed] [Google Scholar]
- Cann KF, Thomas DR, Salmon RL, Wyn-Jones AP, Kay D. 2013. Extreme water-related weather events and waterborne disease. Epidemiol Infect 141(4):671–686, PMID: 22877498, 10.1017/S0950268812001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton EJ, Eisenberg JNS, Goldstick J, Cevallos W, Trostle J, Levy K. 2014. Heavy rainfall events and diarrhea incidence: the role of social and environmental factors. Am J Epidemiol 179(3):344–352, PMID: 24256618, 10.1093/aje/kwt279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton EJ, Woster AP, DeWitt P, Goldstein RS, Levy K. 2016. A systematic review and meta-analysis of ambient temperature and diarrhoeal diseases. Int J Epidemiol 45(1):117–130, PMID: 26567313, 10.1093/ije/dyv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2015. Salmonella. Importance of serotyping. https://www.cdc.gov/salmonella/reportspubs/salmonella-atlas/serotyping-importance.html [accessed 21 February 2018].
- CDC. 2016. National Salmonella Surveillance Annual Report, 2013. Atlanta, GA: U.S. Department of Health and Human Services, CDC. [Google Scholar]
- CDC. 2017. National Salmonella Surveillance Report Annual Report, 2015. Atlanta, GA: U.S. Department of Health and Human Services, CDC. [Google Scholar]
- Cools D, Merckx R, Vlassak K, Verhaegen J. 2001. Survival of E. coli and Enterococcus spp. derived from pig slurry in soils of different texture. Appl Soil Ecol 17(1):53–62, 10.1016/S0929-1393(00)00133-5. [DOI] [Google Scholar]
- Curriero FC, Patz JA, Rose JB, Lele S. 2001. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am J Public Health 91(8):1194–1199, PMID: 11499103, 10.2105/AJPH.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai A. 2013. Increasing drought under global warming in observations and models. Nat Clim Chang 3(1):52–58, 10.1038/nclimate1633. [DOI] [Google Scholar]
- Detty JM, McGuire KJ. 2010. Threshold changes in storm runoff generation at a till-mantled headwater catchment. Water Resour Res 46(7):W07525, 10.1029/2009WR008102. [DOI] [Google Scholar]
- Erickson MC, Habteselassie MY, Liao J, Webb CC, Mantripragada V, Davey LE, et al. 2014. Examination of factors for use as potential predictors of human enteric pathogen survival in soil. J Appl Microbiol 116(2):335–349, PMID: 24224858, 10.1111/jam.12373. [DOI] [PubMed] [Google Scholar]
- Fischer EM, Knutti R. 2016. Observed heavy precipitation increase confirms theory and early models. Nat Clim Chang 6:986–991, 10.1038/nclimate3110. [DOI] [Google Scholar]
- Giannella RA. 1996. Chapter 21 Salmonella. In: Medical Microbiology. Baron S., ed. 4th ed Galveston, TX: University of Texas Medical Branch at Galveston. [Google Scholar]
- Gomba A, Chidamba L, Korsten L. 2016. Prevalence and serovar diversity of Salmonella spp. in primary horticultural fruit production environments. Food Control 69:13–19, 10.1016/j.foodcont.2016.04.026. [DOI] [Google Scholar]
- Grjibovski AM, Bushueva V, Boltenkov VP, Buzinov RV, Degteva GN, Yurasova ED, et al. 2013. Climate variations and salmonellosis in northwest Russia: a time-series analysis. Epidemiol Infect 141(2):269–276, PMID: 22475326, 10.1017/S0950268812000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grjibovski AM, Kosbayeva A, Menne B. 2014. The effect of ambient air temperature and precipitation on monthly counts of salmonellosis in four regions of Kazakhstan, Central Asia, in 2000–2010. Epidemiol Infect 142(3):608–615, PMID: 23816177, 10.1017/S095026881300157X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley BJ, Cole DJ, Lipp EK. 2009. Distribution, diversity, and seasonality of waterborne salmonellae in a rural watershed. Appl Environ Microbiol 75(5):1248–1255, PMID: 19124594, 10.1128/AEM.01648-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CS, Tertuliano M, Rajeev S, Vellidis G, Levy K. 2018. Impact of storm runoff on Salmonella and Escherichia coli prevalence in irrigation ponds of fresh produce farms in southern Georgia. J Appl Microbiol 124(3):910–921, PMID: 29316043, 10.1111/jam.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume M, Armstrong B, Hajat S, Wagatsuma Y, Faruque ASG, Hayashi T, et al. 2007. Association between climate variability and hospital visits for non-cholera diarrhoea in Bangladesh: effects and vulnerable groups. Int J Epidemiol 36(5):1030–1037, PMID: 17664224, 10.1093/ije/dym148. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Maculloch B, Batz M. 2015. Economic Burden of Major Foodborne Illnesses Acquired in the United States. EIB-140. Washington, DC: U.S. Department of Agriculture, Economic Research Service; https://www.ers.usda.gov/webdocs/publications/43984/52807_eib140.pdf?v=0 [accessed 3 September 2019]. [Google Scholar]
- Holley RA, Arrus KM, Ominski KH, Tenuta M, Blank G. 2006. Salmonella survival in manure-treated soils during simulated seasonal temperature exposure. J Environ Qual 35(4):1170–1180, PMID: 16738403, 10.2134/jeq2005.0449. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. 1992. Confidence interval estimation of interaction. Epidemiology 3(5):452–456, PMID: 1391139, 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- Huang JY, Patrick ME, Manners J, Sapkota AR, Scherzinger KJ, Tobin-D’Angelo M, et al. 2017. Association between wetland presence and incidence of Salmonella enterica serotype Javiana infections in selected US sites, 2005–2011. Epidemiol Infect 145(14):2991–2997, PMID: 28803563, 10.1017/S0950268817001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovieno P, Bååth E. 2008. Effect of drying and rewetting on bacterial growth rates in soil. FEMS Microbiol Ecol 65(3):400–407, PMID: 18547324, 10.1111/j.1574-6941.2008.00524.x. [DOI] [PubMed] [Google Scholar]
- Islam M, Doyle MP, Phatak SC, Millner P, Jiang X. 2005. Survival of Escherichia coli O157:H7 in soil and on carrots and onions grown in fields treated with contaminated manure composts or irrigation water. Food Microbiol 22(1):63–70, 10.1016/j.fm.2004.04.007. [DOI] [Google Scholar]
- Islam M, Morgan J, Doyle MP, Phatak SC, Millner P, Jiang X. 2004. Persistence of Salmonella enterica serovar typhimurium on lettuce and parsley and in soils on which they were grown in fields treated with contaminated manure composts or irrigation water. Foodborne Pathog Dis 1(1):27–35, PMID: 15992259, 10.1089/153531404772914437. [DOI] [PubMed] [Google Scholar]
- Jackson BR, Griffin PM, Cole D, Walsh KA, Chai SJ. 2013. Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998–2008. Emerg Infect Dis 19(8):1239–1244, PMID: 23876503, 10.3201/eid1908.121511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen CS, Bech TB. 2012. Soil survival of Salmonella and transfer to freshwater and fresh produce. Food Res Int 45(2):557–566, 10.1016/j.foodres.2011.07.026. [DOI] [Google Scholar]
- Jagai JS, Li Q, Wang S, Messier KP, Wade TJ, Hilborn ED. 2015. Extreme precipitation and emergency room visits for gastrointestinal illness in areas with and without combined sewer systems: an analysis of Massachusetts data, 2003–2007. Environ Health Perspect 123(9):873–879, PMID: 25855939, 10.1289/ehp.1408971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Shaw KS, Upperman CR, Blythe D, Mitchell C, Murtugudde R, et al. 2015. Climate change, extreme events and increased risk of salmonellosis in Maryland, USA: evidence for coastal vulnerability. Environ Int 83:58–62, PMID: 26093493, 10.1016/j.envint.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T, Belitz K. 2017. Domestic well locations and populations served in the contiguous U.S.: 1990. Sci Total Environ 607–608:658–668, PMID: 28709100, 10.1016/j.scitotenv.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Keraita B, Konradsen F, Drechsel P, Abaidoo RC. 2007. Reducing microbial contamination on wastewater-irrigated lettuce by cessation of irrigation before harvesting. Trop Med Int Health 12(Suppl 2):8–14, PMID: 18005310, 10.1111/j.1365-3156.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- Kibet LC, Saporito LS, Allen AL, May EB, Kleinman PJA, Hashem FM, et al. 2014. A protocol for conducting rainfall simulation to study soil runoff. J Vis Exp 86:e51664, PMID: 24748061, 10.3791/51664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisluk G, Yaron S. 2012. Presence and persistence of Salmonella enterica serotype typhimurium in the phyllosphere and rhizosphere of spray-irrigated parsley. Appl Environ Microbiol 78(11):4030–4036, PMID: 22447598, 10.1128/AEM.00087-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel KE, Stevens LE, Stevens SE, Sun L, Janssen E, Wuebbles D, et al. 2013. Regional climate trends and scenarios for the U.S. National Climate Assessment, Part 2. Climate change of the Southeast U.S. NOAA Technical Report NESDIS 142-2. https://scenarios.globalchange.gov/sites/default/files/NOAA_NESDIS_Tech_Report_142-2-Climate_of_the_Southeast_U.S_0.pdf [accessed 3 September 2019].
- Lee D, Tertuliano M, Vellidis G, Harris C, Grossman MK, Rajeev S, et al. 2018. Evaluation of grower-friendly, science-based sampling approaches for the detection of Salmonella in ponds used for irrigation of fresh produce. Foodborne Pathog Dis 15(10):627–636, PMID: 30334659, 10.1089/fpd.2018.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Lau SL, Kayhanian M, Stenstrom MK. 2004. Seasonal first flush phenomenon of urban stormwater discharges. Water Res 38(19):4153–4163, PMID: 15491663, 10.1016/j.watres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Levy K, Woster AP, Goldstein RS, Carlton EJ. 2016. Untangling the impacts of climate change on waterborne diseases: a systematic review of relationships between diarrheal diseases and temperature, rainfall, flooding, and drought. Environ Sci Technol 50(10):4905–4922, PMID: 27058059, 10.1021/acs.est.5b06186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Jackson SA, Gangiredla J, Wang W, Liu H, Tall BD, et al. 2015. Genomic evidence reveals numerous Salmonella enterica serovar Newport reintroduction events in Suwannee watershed irrigation ponds. Appl Environ Microbiol 81(24):8243–8253, PMID: 26386063, 10.1128/AEM.02179-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Vellidis G, Liu H, Jay-Russell M, Zhao S, Hu Z, et al. 2014. Diversity and antimicrobial resistance of Salmonella enterica isolates from surface water in southeastern United States. Appl Environ Microbiol 80(20):6355–6365, PMID: 25107969, 10.1128/AEM.02063-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind AJ. 2008. Amphibians and Reptiles and Climate Change. Washington, DC: U.S. Department of Agariculture, Forest Service, Climate Change Resource Center; https://www.fs.usda.gov/ccrc/topics/amphibians-reptiles-and-climate-change-2008 [accessed 3 September 2019]. [Google Scholar]
- Luo Z, Gu G, Ginn A, Giurcanu MC, Adams P, Vellidis G, et al. 2015. Distribution and characterization of Salmonella enterica isolates from irrigation ponds in the southeastern United States. Appl Environ Microbiol 81(13):4376–4387, PMID: 25911476, 10.1128/AEM.04086-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer JJ, Martin G, Hernandez S, Cheng Y, Gerner-Smidt P, Hise KB, et al. 2015. Diversity and persistence of Salmonella enterica strains in rural landscapes in the southeastern United States. PLoS One 10(7):e0128937, PMID: 26131552, 10.1371/journal.pone.0128937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens A, Balakrishnan K, Ramaswamy P, Rajkumar P, Ramaprabha P, Durairaj N, et al. 2019. Associations between high temperature, heavy rainfall, and diarrhea among young children in rural Tamil Nadu, India: a prospective cohort study. Environ Health Perspect 127(4):47004, PMID: 30986088, 10.1289/EHP3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef SA, Rosenberg Goldstein RE, George A, Kleinfelter L, Boyer MS, McLaughlin CR, et al. 2012. Occurrence and antibiotic resistance of multiple Salmonella serotypes recovered from water, sediment and soil on mid-Atlantic tomato farms. Environ Res 114:31–39, PMID: 22406288, 10.1016/j.envres.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Mor SM, DeMaria A Jr, Naumova EN. 2014. Hospitalization records as a tool for evaluating performance of food- and water-borne disease surveillance systems: a Massachusetts case study. PLoS One 9(4):e93744, PMID: 24740304, 10.1371/journal.pone.0093744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natvig EE, Ingham SC, Ingham BH, Cooperband LR, Roper TR. 2002. Salmonella enterica serovar typhimurium and Escherichia coli contamination of root and leaf vegetables grown in soils with incorporated bovine manure. Appl Environ Microbiol 68(6):2737–2744, PMID: 12039728, 10.1128/AEM.68.6.2737-2744.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols G, Lane C, Asgari N, Verlander NQ, Charlett A. 2009. Rainfall and outbreaks of drinking water related disease and in England and Wales. J Water Health 7(1):1–8, PMID: 18957770, 10.2166/wh.2009.143. [DOI] [PubMed] [Google Scholar]
- Park S, Szonyi B, Gautam R, Nightingale K, Anciso J, Ivanek R. 2012. Risk factors for microbial contamination in fruits and vegetables at the preharvest level: a systematic review. J Food Prot 75(11):2055–2081, PMID: 23127717, 10.4315/0362-028X.JFP-12-160. [DOI] [PubMed] [Google Scholar]
- Penna D, Tromp-van Meerveld HJ, Gobbi A, Borga M, Dalla Fontana G. 2011. The influence of soil moisture on threshold runoff generation processes in an alpine headwater catchment. Hydrol Earth Syst Sci 15(3):689–702, 10.5194/hess-15-689-2011. [DOI] [Google Scholar]
- Prein AF, Rasmussen RM, Ikeda K, Liu C, Clark MP, Holland GJ. 2017. The future intensification of hourly precipitation extremes. Nat Clim Chang 7(1):48–52, 10.1038/nclimate3168. [DOI] [Google Scholar]
- Reddy SP, Wang H, Adams JK, Feng PC. 2016. Prevalence and characteristics of Salmonella serotypes isolated from fresh produce marketed in the United States. J Food Prot 79(1):6–16, PMID: 26735024, 10.4315/0362-028X.JFP-15-274. [DOI] [PubMed] [Google Scholar]
- Reller ME, Tauxe RV, Kalish LA, Mølbak K. 2008. Excess salmonellosis in women in the United States: 1968–2000. Epidemiol Infect 136(8):1109–1117, PMID: 17961280, 10.1017/S0950268807009594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke SC, Koo O-K, Foley S, Nayak R. 2013. Salmonella. In: Guide to Foodborne Pathogens. Labbe R.G. and Garcia S., eds. Chichester, West Sussex, UK: Wiley Blackwell. [Google Scholar]
- Rose JB, Epstein PR, Lipp EK, Sherman BH, Bernard SM, Patz JA. 2001. Climate variability and change in the United States: potential impacts on water- and foodborne diseases caused by microbiologic agents. Environ Health Perspect 109(Suppl 2):211–221, PMID: 11359688, 10.2307/3435011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17(1):7–15, PMID: 21192848, 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah DH, Paul NC, Sischo WC, Crespo R, Guard J. 2017. Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes. Poult Sci 96(3):687–702, PMID: 27665007, 10.3382/ps/pew342. [DOI] [PubMed] [Google Scholar]
- Singh RB, Hales S, de Wet N, Raj R, Hearnden M, Weinstein P. 2001. The influence of climate variation and change on diarrheal disease in the Pacific Islands. Environ Health Perspect 109(2):155–159, PMID: 11266326, 10.1289/ehp.01109155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikantiah P, Lay JC, Hand S, Crump JA, Campbell J, Van Duyne MS, et al. 2004. Salmonella enterica serotype Javiana infections associated with amphibian contact, Mississippi, 2001. Epidemiol Infect 132(2):273–281, PMID: 15061502, 10.1017/S0950268803001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen DM, Barnett AG. 2016. Effect of temperature and precipitation on salmonellosis cases in South-East Queensland, Australia: an observational study. BMJ Open 6(2):e010204, PMID: 26916693, 10.1136/bmjopen-2015-010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn LK, Danyluk MD, Worobo RW, Wiedmann M. 2014. Distributions of Salmonella subtypes differ between two U.S. produce-growing regions. Appl Environ Microbiol 80(13):3982–3991, PMID: 24747908, 10.1128/AEM.00348-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon LK, Si BC, Korber D, Guo X. 2007. Soil wetting state and preferential transport of Escherichia coli in clay soils. Can J Soil Sci 87(1):61–72, 10.4141/S06-004. [DOI] [Google Scholar]
- Thomas KM, Charron DF, Waltner-Toews D, Schuster C, Maarouf AR, Holt JD. 2006. A role of high impact weather events in waterborne disease outbreaks in Canada, 1975–2001. Int J Environ Health Res 16(3):167–180, PMID: 16611562, 10.1080/09603120600641326. [DOI] [PubMed] [Google Scholar]
- Trenberth KE. 2011. Changes in precipitation with climate change. Clim Res 47(1):123–138, 10.3354/cr00953. [DOI] [Google Scholar]
- Trimble LM, Alali WQ, Gibson KE, Ricke SC, Crandall P, Jaroni D, et al. 2013. Prevalence and concentration of Salmonella and Campylobacter in the processing environment of small-scale pastured broiler farms. Poult Sci 92(11):3060–3066, PMID: 24135612, 10.3382/ps.2013-03114. [DOI] [PubMed] [Google Scholar]
- Walls SC, Barichivich WJ, Brown ME, Scott DE, Hossack BR. 2013. Influence of drought on salamander occupancy of isolated wetlands on the southeastern Coastal Plain of the United States. Wetlands (Wilmington) 33(2):345–354, 10.1007/s13157-013-0391-3. [DOI] [Google Scholar]
- Winfield MD, Groisman EA. 2003. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl Environ Microbiol 69(7):3687–3694, PMID: 12839733, 10.1128/aem.69.7.3687-3694.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe K, Stubbs K. 2017. 2016 Georgia Farm Gate Value Report. Athens, Georgia: University of Georgia, College of Agricultural & Environmental Sciences; https://www.caes.uga.edu/content/dam/caes-subsite/caed/publications/annual-reports-farm-gate-value-reports/2016%20Farm%20Gate%20Report.pdf [accessed 22 July 2019]. [Google Scholar]
- Yeager JG, Ward RL. 1981. Effects of moisture content on long-term survival and regrowth of bacteria in wastewater sludge. Appl Environ Microbiol 41(5):1117–1122, PMID: 6789764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Rankin SC, Aceto HW, Benson CE, Toth JD, Dou Z. 2006. Survival of Salmonella enterica serovar Newport in manure and manure-amended soils. Appl Environ Microbiol 72(9):5777–5783, PMID: 16957193, 10.1128/AEM.00791-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.