Abstract
Background:
Emerging studies suggest that ambient temperature during pregnancy may be associated with fetal growth, but the existing evidence is limited and inconsistent.
Objectives:
We aimed to evaluate the association of trimester-specific temperature with risk of being born small for gestational age (SGA) and birth weight—markers of fetal growth—among term births in the contiguous United States.
Methods:
We included data on 29,597,735 live singleton births between 1989 and 2002 across 403 U.S. counties. We estimated daily county-level population-weighted mean temperature using a spatially refined gridded climate data set. We used logistic regression to estimate the association between trimester-specific temperature and risk of SGA and linear regression to evaluate the association between trimester-specific temperature and term birth weight z-score, adjusting for parity, maternal demographics, smoking or drinking during pregnancy, chronic hypertension, and year and month of conception. We then pooled results overall and by geographic regions and climate zones.
Results:
High ambient temperatures ( percentile) during the entire pregnancy were associated with higher risk of term SGA {odds ratio [OR] 1.041 [95% confidence interval (CI): 1.029, 1.054]} and lower term birth weight [standardized to (95% CI: , ) reduction in birth weight for infants born at 40 weeks of gestation]. Low temperatures ( percentile) during the entire pregnancy were not associated with SGA [OR 1.003 (95% CI: 0.991, 1.015)] but were associated with a small decrement in term birth weight [standardized to (95% CI: , )]. Risks of term SGA and birth weight were more strongly associated with temperature averaged across the second and third trimesters, in areas the Northeast, and in areas with cold or very cold climates.
Conclusions:
Above-average temperatures during pregnancy were associated with lower fetal growth. Our findings provide evidence that temperature may be a novel risk factor for reduced fetal growth. https://doi.org/10.1289/EHP4648
Introduction
Birth weight is a marker of fetal growth, an important predictor of neonatal morbidity and mortality, and associated with risk of chronic health problems in later life (Barker 2006; Saigal and Doyle 2008; Whincup et al. 2008; Zhang et al. 2014). Although genetics, lifestyle habits, and socioeconomic factors are known to contribute to reduced fetal growth (Mook-Kanamori et al. 2010; Wollmann 1998), it is difficult to explain the heterogeneity in birth weight worldwide purely based on these identified factors (Wells and Cole 2002).
Several environmental factors have been found to adversely affect fetal growth, including ambient air pollution (Dadvand et al. 2013; Kingsley et al. 2017), household air pollution (Pope et al. 2010; Thompson et al. 2011), features of the built environment (Ebisu et al. 2016; Glazer et al. 2018; Kingsley et al. 2016), and exposure to specific chemicals (Rauch et al. 2012; Zhu et al. 2010). Emerging studies have assessed whether ambient temperature can also affect birth weight, but findings have been mixed, with studies reporting that lower birth weight is associated with both warmer- and colder-than-average temperatures (Ha et al. 2017; Ngo and Horton 2016), associated only with warmer- (Basu et al. 2018; Kloog et al. 2015) or colder-than-average temperatures (Elter et al. 2004; Murray et al. 2000), and not associated with either (Bruckner et al. 2014; Tustin et al. 2004; Wolf and Armstrong 2012). Biologically, temperature extremes may be associated with lower birth weight by increasing oxidative stress and systemic inflammation in response to temperature changes during gestation (Ferguson et al. 2018; Ganesan et al. 2017; Halonen et al. 2010; Kahle et al. 2015).
Given the short- and long-term consequences of reduced fetal growth and the projected increase in ambient temperature associated with continued climate change (IPCC 2014; Melillo et al. 2014), it is important to improve our understanding of the impact of temperature on birth weight. Accordingly, we sought to investigate the association of ambient temperature during pregnancy with risk of term small for gestational age (SGA) and birth weight—both of which are markers of fetal growth—among over 29 million live singleton births from 1989 to 2002 across 403 counties in the contiguous United States. We also examined whether the magnitude of this association varied by trimester, geographic region, and climate zone.
Methods
Study Population
We obtained data on live births occurring in the United States between 1989 and 2002 from the CDC’s National Center for Health Statistics. Data were available only for U.S. resident mothers living in counties with a population of . Exact date of birth is not directly available in these data, so we imputed this variable based on the last menstrual period (LMP), completed weeks of gestation, and the recorded weekday of birth (Sun et al. 2019). We restricted our analyses to the 403 counties with birth data continuously available throughout the study period (see Figure S1).
In order to focus on the direct effects of temperature on fetal growth not mediated through early delivery, we further restricted our analyses to 30,108,870 live singleton births born between 37 and 44 completed weeks of gestation and in which the LMP was recorded. We additionally excluded births with a) a conception date more than 37 weeks before 1 January 1989 or less than 44 weeks before 31 December 2002 to avoid inducing artificial seasonal patterns in gestation length (“fixed cohort bias”) (Strand et al. 2011); b) implausible combinations of birth weight and gestational age according to the Alexander criteria (Alexander et al. 1996); and c) an imputed month of birth that, based on LMP and completed weeks of gestation, differed from the recorded month of birth. The final analytic sample consisted of 29,597,735 births (see Figure S2). Because the data used were de-identified and publicly available, approval by Brown’s institutional review board was not required.
Outcome Definition
Our study outcomes were term SGA and term birth weight z-score. We defined gestational age as the number of completed weeks between the date of LMP and the date of birth. Based on the 1999–2000 U.S. national reference of sex-specific reference percentiles for birth weight at each gestational age (Oken et al. 2003), we classified each infant into corresponding birth weight percentiles and z-scores. Infants with birth weight in the percentile were defined as SGA. Based on this, the U.S. national reference of birth weight, term SGA, and term birth weight z-score remove the contribution of gestational age to fetal growth (Oken et al. 2003).
Geographic Region and Climate Zone
We classified each county into one of six geographic regions (Melillo et al. 2014): Northeast (number of counties, counties), Southeast (), Midwest (), Great Plains (), Northwest (), and Southwest (). In addition, we classified each county into one of five climate zones (U.S. Department of Energy 2015): hot-humid (), mixed-humid (), hot-dry/mixed-dry (), cold/very cold (), and marine () (see Figure S1).
Ambient Temperature Assessment
We obtained estimates of daily mean ambient temperature using the Parameter-elevation Relationships on Independent Slopes Model, a –gridded climate data set consisting of spatially interpolated weather data and accounting for major physiographic features that influence climate patterns, including location, elevation, coastal proximity, topographic facet orientation, vertical atmospheric layer, topographic position, and orographic effectiveness of the terrain (Daly et al. 2008). The gridded PRISM data set offers a more spatially explicit representation of meteorological exposures than observations at individual weather stations (Spangler et al. 2018).
We used gridded estimates of daily mean temperature from PRISM to calculate population-weighted averages of temperature for each day in each county, as previously described (Spangler et al. 2018). Briefly, we first obtained the population centroids for each census tract in each of the 403 counties of interest from the 2000 Census (U.S. Census Bureau 2000). Next, we extracted daily PRISM-predicted temperatures at the grid cell overlaying each of these census tract population centroids. Finally, we used these extracted grid cells to calculate a daily time series of population-weighted mean temperature for each day in each county. Specifically, for each extracted grid cell, we multiplied its daily temperature value by the proportion of the county population falling within that census tract. We then summed the resulting values across all grid cells located within each county to obtain the county population-weighted mean value. An illustration of how the gridded PRISM data intersect with census tracts in Providence County is shown in Figure S3.
For each birth, we averaged daily temperature values from the date of LMP to 13 completed weeks of gestation (first trimester temperature), from 14 to 26 completed weeks of gestation (second trimester temperature), from 27 completed weeks of gestation to birth (third trimester temperature), and from the date of LMP to birth (entire pregnancy). Because individuals adapt to their local climate, in all analyses we considered percentiles of county- and trimester-specific mean temperature rather than absolute values of temperature. Specifically, we categorized and modeled exposure as deciles of county- and trimester-specific mean temperature relative to a reference value defined as the decile spanning the 40th to 50th percentiles (Deschenes et al. 2009; Isen et al. 2017). We defined colder-than-average temperatures as those below the 20th percentile and warmer-than-average temperatures as those above the 80th percentile of the county- and trimester-specific temperature distribution. Tables S1 and S2 show the temperature distribution of the entire pregnancy temperature by county, geographic region, and climate zone.
Air Pollution Assessment
Within each county we estimated monthly levels of particulate matter with aerodynamic diameter less than () from 1989 to 2002 using a spatiotemporal model, as previously described (Yanosky et al. 2009, 2014). This model is informed by meteorological data, location-specific characteristics, and ( measurements obtained from the U.S. EPA’s Air Quality System (https://www.epa.gov/outdoor-air-quality-data), from the Interagency Monitoring of Protected Visual Environments, Stacked Filter Unit Network, and Clean Air Status and Trends Network by accessing the Visibility Information Exchange Web System (http://views.cira.colostate.edu/iwdw/), and prior research studies. The model has a 10-fold cross-validation . We used this model to estimate average levels in each trimester and across the entire pregnancy.
Statistical Analysis
We used a two-stage approach to estimate the association between mean temperature decile and either relative odds of SGA or change in birth weight z-score. In the first stage, we used logistic regression to estimate the odds ratio (OR) of SGA associated with deciles of average temperature, and linear regression to estimate the change in birth weight z-score (continuous outcome) associated with deciles of average temperature. In unadjusted models, we included only the indicator variable of temperature deciles. We then adjusted all models for maternal age (, 25–29, 30–34, or ), marital status (married or unmarried), race (white or nonwhite), years of education (, 9–12, 13–17, or unknown), smoking (yes, no, or unknown) or drinking (yes, no, or unknown) during pregnancy, parity (0, 1, , or unknown), chronic hypertension (yes, no, or unknown), and year and month of conception as categorical variables. Because birth weight z-score and our definition of SGA already account for infant sex and gestational age, we did not further adjust for these variables in our regression models. We conducted sensitivity analyses by including all infants with gestational age ranging from 22 to 44 weeks (i.e., not restricted to term births, ) and, separately, further adjusted for to assess potential confounding by ambient particulate matter.
In the second stage of the analysis, we used random-effects meta-analytic models to combine the county-specific estimates obtained from the first stage (Berkey et al. 1995; Viechtbauer 2010). All results are expressed relative to the reference decile (i.e., the 40th to 50th percentiles of county-specific temperatures). For ease of presentation, we re-express the estimated difference in birth weight z-score to absolute differences in birth weight (in grams) for a hypothetical infant born at 40 completed weeks of gestation, as previously described (Oken et al. 2003).
In secondary analyses, we evaluated whether the associations between temperature, SGA, and birth weight varied by trimester, geographic region, or climate zone. When appropriate, we used a Wald statistic for testing whether observed associations across strata were statistically significantly different (Rothman et al. 2008).
We conducted all analyses in R (version 3.5.1; R Development Core Team). We used the “survival” package (version 2.42-6) for the logistic regression, the “stats” package (version 3.5.1) for the linear regression, and the “metafor” package (version 2.0-0) for the second-stage meta-analysis.
Results
Our analysis was based on records from 29,597,735 live, singleton, term births between 1989 and 2002 in 403 U.S. counties, 10.2% of which were born SGA (Table 1). The average birth weight was [standard deviation (SD): ], and male infants tended to be heavier than female infants [mean (SD) for male vs. female: () vs. ()]. Average birth weight tended to be lower among infants born to mothers who smoked during pregnancy and those born to nonwhite mothers. Infants born in summer, in the Northwest, or in the marine climate zone tended to have higher birth weight. Correlations among overall and trimester-specific temperatures were generally moderate (), except for the correlation between entire pregnancy and the second trimester temperature () (see Table S3).
Table 1.
Demographics of singleton births and distribution of term birth weight and term small for gestational age among 403 U.S. counties from 1989 to 2002.
| Variable | Count (%) | Term mean birth weight (g) () | Term SGA (%) |
|---|---|---|---|
| Total birth | 29,597,735 (100.0) | 10.2 | |
| Child sex | |||
| Male | 15,071,495 (50.9) | 10.1 | |
| Female | 14,526,240 (49.1) | 10.2 | |
| Parity | |||
| 0 | 12,339,520 (41.7) | 12.2 | |
| 1 | 9,564,292 (32.3) | 8.4 | |
| 7,588,896 (25.6) | 9.2 | ||
| Unknown | 105,027 (0.4) | 11.6 | |
| Maternal age (y) | |||
| 10,027,881 (33.9) | 13.2 | ||
| 25–29 | 8,491,989 (28.7) | 9.2 | |
| 30–34 | 7,324,962 (24.7) | 8.1 | |
| 3,752,903 (12.7) | 8.4 | ||
| Marital status | |||
| Married | 20,779,953 (70.2) | 8.2 | |
| Unmarried | 8,817,782 (29.8) | 14.7 | |
| Maternal race | |||
| White | 23,329,456 (78.8) | 8.5 | |
| Nonwhite | 6,268,279 (21.2) | 16.1 | |
| Maternal education (y) | |||
| 1,885,775 (6.4) | 11.3 | ||
| 9–12 | 13,460,319 (45.5) | 12.3 | |
| 13–17 | 13,529,645 (45.7) | 7.8 | |
| Unknown | 721,996 (2.4) | 10.5 | |
| Smoking during pregnancya | |||
| Yes | 2,577,131 (8.7) | 19.1 | |
| No | 19,155,778 (64.7) | 9.1 | |
| Unknown | 7,864,826 (26.6) | 9.7 | |
| Alcohol drinking during pregnancya | |||
| Yes | 384,220 (1.3) | 16.2 | |
| No | 22,176,203 (74.9) | 10.2 | |
| Unknown | 7,037,312 (23.8) | 9.7 | |
| Chronic hypertension | |||
| Yes | 167,815 (0.6) | 15.5 | |
| No | 28,833,979 (97.4) | 10.1 | |
| Unknown | 595,941 (2.0) | 10.7 | |
| Season of conceptionb | |||
| Spring | 6,916,145 (23.4) | 10.5 | |
| Summer | 7,456,193 (25.2) | 9.8 | |
| Fall | 7,679,851 (25.9) | 10.1 | |
| Winter | 7,545,546 (25.5) | 10.2 | |
| Geographic region | |||
| Northeast | 7,605,824 (25.7) | 10.1 | |
| Southeast | 4,807,500 (16.2) | 11.3 | |
| Midwest | 5,788,152 (19.6) | 10.1 | |
| Great Plains | 3,158,660 (10.7) | 10.3 | |
| Northwest | 952,436 (3.2) | 7.7 | |
| Southwest | 7,285,163 (24.6) | 9.8 | |
| Climate zone | |||
| Hot-humid | 4,592,942 (15.5) | 10.8 | |
| Mixed-humid | 7,699,893 (26.0) | 10.9 | |
| Hot-dry/mixed-dry | 5,432,313 (18.4) | 9.7 | |
| Cold/very cold | 9,890,639 (33.4) | 9.8 | |
| Marine | 1,981,948 (6.7) | 8.4 | |
Note: SD, standard deviation; SGA, small for gestational age.
Smoking and drinking status were not recorded on California birth certificate.
Season of conception: spring (March–May), summer (June–August), fall (September–November), and winter (December–February).
Temperature and Term SGA
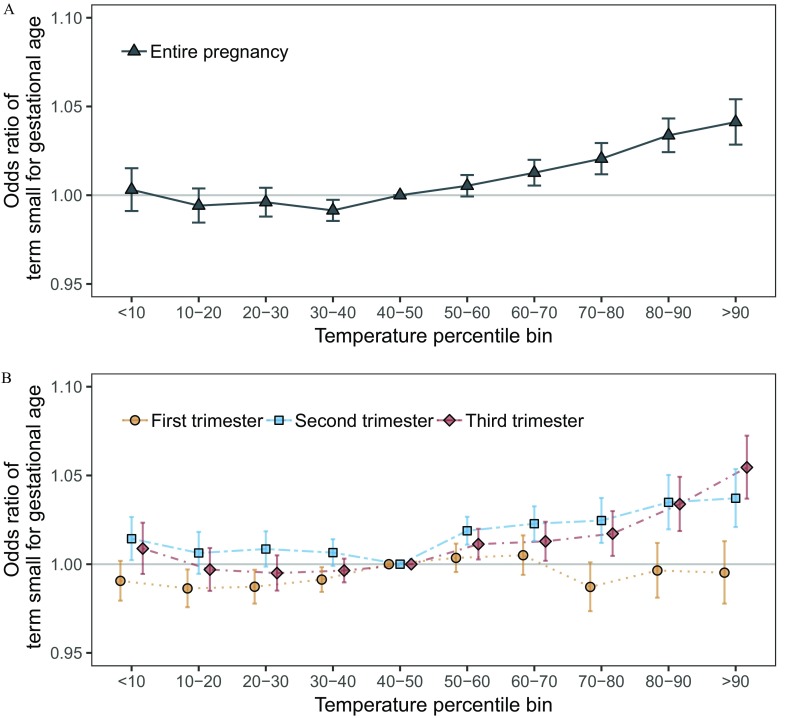
Warmer-than-average temperatures were associated with higher odds of SGA (Table 2; Figure 1A). For example, in adjusted models pregnancy-average temperatures between the county-specific 80th and 90th percentile and above the 90th percentile were associated with a 1.034 (95% CI: 1.024, 1.043) and 1.041 (95% CI: 1.029, 1.054) higher odds of SGA versus temperatures in the reference range defined as the 40th to 50th percentile. Colder-than-average temperatures were not associated with the relative odds of SGA. Results were qualitatively similar in unadjusted models and in models additionally adjusted for (Table 2). Results were also similar in sensitivity analyses not restricted to term births (see Figure S4).
Table 2.
Odds ratio for small for gestational age associated with warmer-than-average and colder-than-average temperatures during the entire pregnancy among 29,597,735 singleton, term births in 403 U.S. counties between 1989 and 2002.
| Temperature (percentile) | Unadjusted model [OR (95% CI)]a | Main model [OR (95% CI)]b | [OR (95% CI)] |
|---|---|---|---|
| 1.053 (1.046, 1.060) | 1.041 (1.029, 1.054) | 1.039 (1.027, 1.053) | |
| 80th–90th | 1.050 (1.044, 1.055) | 1.034 (1.024, 1.043) | 1.032 (1.023, 1.042) |
| 10th–20th | 0.993 (0.987, 0.999) | 0.994 (0.985, 1.004) | 0.995 (0.985, 1.004) |
| 0.996 (0.990, 1.003) | 1.003 (0.991, 1.015) | 1.004 (0.992, 1.016) |
Note: ORs of temperature percentiles were relative to temperatures ranging from the 40th to 50th percentile of each county temperature distribution. CI, confidence interval; OR, odds ratio; , particulate matter with aerodynamic diameter less than .
Unadjusted model included only the indicator variable of temperature deciles.
Main model included parity, maternal age, race, marital status, years of education, smoking or drinking during pregnancy, chronic hypertension, and year and month of conception.
Figure 1.
Exposure–response curves for the association between small for gestational age and (A) average temperatures during the entire pregnancy and (B) trimester-specific temperatures, among 29,597,735 singleton term births in 403 U.S. counties between 1989 and 2002. Models were adjusted for maternal age, race, marital status, years of education, smoking or drinking during pregnancy, parity, chronic hypertension, and year and month of conception.
Results in the second and third trimester were similar to those observed across the entire pregnancy (Figure 1B). For example, temperatures above the county-specific 90th percentile were associated with an OR of SGA 1.037 (95% CI: 1.021, 1.054) in the second trimester and an OR 1.055 (95% CI: 1.037, 1.072) in the third trimester. Average temperatures in the first trimester were not associated with higher odds of SGA.
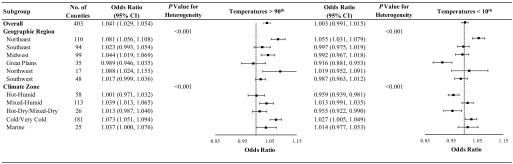
In secondary analyses, we evaluated whether the associations between temperature and SGA varied across subgroups defined by geographic region or climate zone (Figure 2). For temperatures above the county-specific 90th percentile, we observed statistically significant heterogeneity across geographic regions and climate zones, with the strongest impact observed in the Northwest and Northeast regions of the United States and in the marine and cold/very cold climate zones. We also observed statistically significant heterogeneity across locations for county-specific temperatures below the 10th percentile, with the direction of the association varying by both geographic region and climate zone.
Figure 2.
Odds ratio (95% CI) of small for gestational age associated with temperatures above the county-specific 90th percentile and below the county-specific 10th percentile during the entire pregnancy among 29,597,735 singleton term births in 403 U.S. counties between 1989 and 2002, overall and by geographic region and climate zone. Models were adjusted for maternal age, race, marital status, years of education, smoking status, alcohol drinking, parity, chronic hypertension, and year and month of conception. Odds ratios were relative to temperatures ranging from 40th to 50th percentile of each county. CI, confidence interval.
Temperature and Term Birth Weight
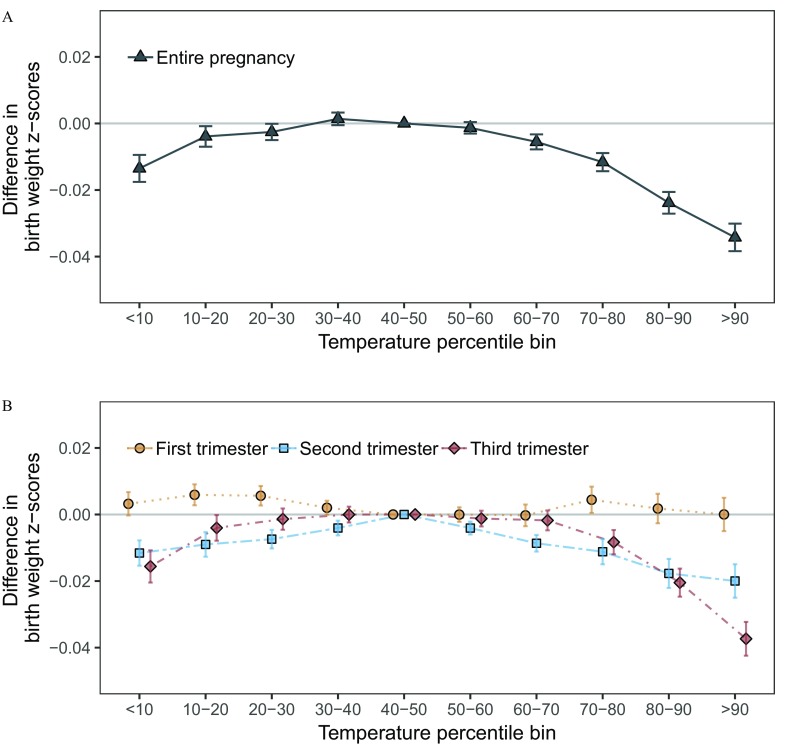
Both warmer-than-average and colder-than-average temperatures during pregnancy were associated with lower birth weight z-score, suggesting an inverse U-shaped relationship (Figure 3A). For example, in adjusted models county-specific temperatures above the 90th percentile and below the 10th percentile were associated with a (95% CI: , ) and (95% CI: , ) lower birth weight (standardized to an infant delivered at 40 weeks of gestation), respectively, versus reference temperatures (Table 3). Results were qualitatively similar in unadjusted models, in models additionally adjusted for , and in sensitivity analyses not restricted to term births (see Figure S4). Results for temperatures in the second and third trimester were similar to those observed for the entire pregnancy, whereas results for the first trimester temperatures were close to the null (Figure 3B).
Figure 3.
Exposure–response curves for the associations between the difference in birth weight z-score and (A) average temperatures during the entire pregnancy and (B) trimester-specific temperatures among 29,597,735 singleton term births in 403 U.S. counties between 1989 and 2002. Models were adjusted for maternal age, race, marital status, years of education, smoking or drinking during pregnancy, parity, chronic hypertension, and year and month of conception.
Table 3.
Difference in birth weight z-score (95% CI) and standardized birth weight (g) (95% CI) associated with warmer-than-average and colder-than-average temperatures during the entire pregnancy among 29,597,735 singleton term births in 403 U.S. counties between 1989 and 2002.
| Temperature (percentile) | Unadjusted modela | Main modelb | |
|---|---|---|---|
| Birth weight z-score | |||
| (, ) | (, ) | (, ) | |
| 80th–90th | (, ) | (, ) | (, ) |
| 10th–20th | 0.004 (0.002, 0.006) | (, ) | (, ) |
| (, 0.000) | (, ) | (, ) | |
| Birth weight (g) | |||
| (, ) | (, ) | (, ) | |
| 80th–90th | (, ) | (, ) | (, ) |
| 10th–20th | 2 (1,3) | (, 0) | (, 0) |
| (, 0) | (, ) | (, ) | |
Note: Difference in birth weight was standardized to the difference in absolute birth weight (in grams) in infants at 40 completed weeks of gestation. The difference in birth weight for temperature percentiles were relative to temperatures ranging from the 40th to 50th percentile of each county temperature distribution. CI, confidence interval; , particulate matter with aerodynamic diameter less than .
Unadjusted model included only the indicator variable of temperature deciles.
Main model included parity, maternal age, race, marital status, years of education, smoking or drinking during pregnancy, chronic hypertension, and year and month of conception.
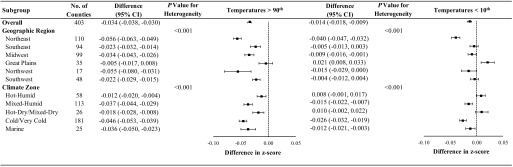
Results stratified by geographic region or climate zone paralleled those observed for SGA (Figure 4). Specifically, temperatures above the 90th percentile were more strongly associated with lower birth weight in the Northeast and Northwest regions and in the marine and cold/very cold climate zones. We also observed statistically significant heterogeneity across locations for county-specific temperatures below the 10th percentile, with the direction of the association varying by both geographic region and climate zone.
Figure 4.
Difference in birth weight z-score associated with county-specific temperatures above the 90th percentile and county-specific temperatures below the 10th percentile during the entire pregnancy among 29,597,735 term births in 403 U.S. counties between 1989 and 2002, overall and by geographic region and climate zone. Models were adjusted for maternal age, race, marital status, years of education, smoking status, alcohol drinking, parity, chronic hypertension, and year and month of conception. Odds ratios were relative to temperatures ranging from 40th to 50th percentile of each county. CI, confidence interval.
Discussion
Leveraging data from nearly 30 million singleton term births across 403 counties in the contiguous United States, we evaluated the relationship between average temperature during pregnancy and two markers of fetal growth: risk of SGA and change in birth weight z-score. We found that warmer-than-average temperatures during pregnancy were associated with lower rates of fetal growth as evidenced by higher risk of term SGA and lower birth weight. On the other hand, colder-than-average temperatures were not associated with altered risk of SGA but were associated with a small decrement in term birth weight. These associations were observed in the second and third trimesters, but they were not evident in the first trimester. Results were not materially different across sensitivity analyses.
Our finding of a positive association between warmer-than-average temperatures and lower birth weight is consistent with results from most prior studies (Ha et al. 2017; Ngo and Horton 2016), as summarized in a recent review (Zhang et al. 2017) as well as with results from a more recent study in California (Basu et al. 2018). For example, in an analysis of 220,572 singleton births from 12 U.S. sites, Ha et al. (2017) reported a 2.5-fold (95% CI: 2.2, 2.8) higher risk of term low birth weight () for pregnancies during periods of high temperatures ( percentile) compared with milder temperature (defined as temperatures ranging from the 5th to the 95th percentiles). Another study conducted in 19 African countries reported that exposure to an extra day with average temperatures during the second trimester was associated with a reduction in birth weight (Grace et al. 2015). On the other hand, studies in Sweden (Bruckner et al. 2014), Germany (Wolf and Armstrong 2012), and New Zealand (Tustin et al. 2004) did not find any impact of warmer-than-average temperatures on markers of fetal growth.
We found that colder-than-average temperatures during pregnancy were not consistently associated with markers of fetal growth. Specifically, colder-than-average temperatures were not associated with risk of SGA, but they were associated with a small decrement in birth weight. Few prior studies were available for direct comparison (Elter et al. 2004; Ha et al. 2017; Murray et al. 2000; Wolf and Armstrong 2012), and results from these studies have been inconsistent. For example, the study conducted in the 12 U.S. sites (Ha et al. 2017) found that compared with milder temperatures, cold ( percentile) in the third trimester was associated with a lower risk of SGA, but cold in mid-to-late pregnancy was associated with higher risk of term low birth weight. The studies in Istanbul (Elter et al. 2004) and Northern Ireland (Murray et al. 2000) found that colder-than-average temperatures during the second trimester were positively associated with lower birth weight, whereas the study in Germany (Wolf and Armstrong 2012) failed to find the association.
Heterogeneity in results across studies may be due to differences in study design, population characteristics, methodologic choices, and/or sample size. For example, our study excluded preterm births, whereas other studies did not (Bruckner et al. 2014; Kloog et al. 2015; Wolf and Armstrong 2012), although our findings in sensitivity analyses not restricted to term births were not materially different. We also allowed the exposure–response function with temperature to be nonlinear, in contrast to some prior studies that constrained this function to be linear (Kloog et al. 2015). We also used SGA and birth weight z-score as indicators of fetal growth (Oken et al. 2003), whereas some prior studies (Bell et al. 2007; Chen and Ho 2016) considered birth weight as the outcome and adjusted for gestational age; these two approaches may yield different results (Lakshmanan et al. 2015; Oken et al. 2003).
Results across studies may also differ based on local factors, including climate. We found that warmer-than-average temperatures were more strongly associated with reduced fetal growth in regions with colder climates. This observation may indicate a lesser degree of adaptation to heat in areas with colder climates and suggests that climate and adaptation may also contribute to heterogeneity across studies.
It is biologically plausible that ambient temperatures during pregnancy can adversely influence fetal growth. Warmer-than-average and colder-than-average temperatures are associated with markers of oxidative stress and systemic inflammation (Ferguson et al. 2018; Ganesan et al. 2017; Halonen et al. 2010; Kahle et al. 2015). Placental function plays a vital role in fetal development, and alterations in placental oxidative capacity enhance the expression of various transcriptional and/or hormonal factors (e.g., reactive oxygen species), which can ultimately lead to reduced fetal growth (Dennery 2010; Rodríguez-Rodríguez et al. 2018). Warmer-than-average and colder-than-average temperatures may also be directly associated with changes in blood viscosity and uterine blood flow (Bouchama and Knochel 2002; Dadvand et al. 2011; Keatinge et al. 1986), which can also influence fetal growth. Biologic plausibility is further supported by studies in experimental animals suggesting that chronic exposure to heat was associated with growth restriction in mice and rats (Galan et al. 1999; Wells 2002).
Identification of critical periods of susceptibility to environmental exposure during pregnancy is important for targeting public health interventions (Selevan et al. 2000). Our findings are consistent with previous studies reporting that temperatures during the second and third trimesters are more strongly associated with fetal growth versus temperatures during the first trimester (Basu et al. 2018; Ha et al. 2017). For example, Basu et al. (2018) reported that term low birth weight was most strongly associated with the third trimester average temperature with increased risk of 15.8% (95% CI: 5.0%, 27.6%) per 5.6°C above 15.6°C, and negatively associated with the average temperature of the first trimester.
Our study has several potential limitations. First, we relied on population-weighted county average temperature for exposure assessment, an approach leading to measurement error in comparison to personal temperature measurements. Nonetheless, we expect that any potential exposure misclassification would be nondifferential and, on average, tend to bias our results towards the null hypothesis of no association. On the other hand, exposure measurement error may have been lower in this study compared with prior studies given our use of population-weighted daily mean temperature estimated from a spatially refined, gridded climate data set rather than data from airport weather stations, which may not fully capture exposures where people tend to live. Second, we were unable to control for residential mobility during pregnancy, which might also have introduced exposure misclassification. However, previous studies suggest that rates of residential mobility among pregnant women are relatively low, with most moves being within the same county (Bell and Belanger 2012; Fell et al. 2004), suggesting limited opportunity for bias in this particular study (Pennington et al. 2017; Pereira et al. 2016). Third, because these data are de-identified, we are not able to account for the correlation among infants born to the same mother. Fourth, although we adjusted for a wide range of covariates, we cannot exclude the possibility of residual confounding. Fifth, although our analysis was limited to the 403 more populous counties of the contiguous United States, accounting for over half of all births in the United States during the study time period, our results may not be generalizable to other counties with smaller populations or to more recent time periods. Similarly, extrapolation of results to future health impacts under continued climate change should be done with caution because the time course over which pregnant women might adapt (physiologically or behaviorally) to the warmer temperatures projected for the future remains unknown (Gosling et al. 2017; Vicedo-Cabrera et al. 2019). On the other hand, to our knowledge this is the largest analysis published to date of the association between ambient temperature during pregnancy and fetal growth, including more than 29 million U.S. singleton births in diverse geographic regions and climate zones.
In summary, among nearly 30 million singleton births across more than 400 populous U.S. counties, we found that warmer-than-average temperatures during pregnancy are associated with lower birth weight and higher risk of being born small for gestational age, especially when considering average temperatures in the second and third trimesters. Notwithstanding results from this and prior studies, it remains unclear how these findings might impact clinical or public health practice. Additional studies are needed to confirm or refute these findings and determine whether any clinical or public health interventions would be warranted during episodes of hotter than usual weather in mid-to-late pregnancy.
Supplementary Material
Acknowledgments
The study was funded by the Institute at Brown for Environment and Society and by grants R21-ES023073 and F32-ES027742 from the National Institutes of Health/National Institute of Environmental Health Sciences.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4648).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. 1996. A United States national reference for fetal growth. Obstet Gynecol 87(2):163–168, PMID: 8559516, 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Barker DJ. 2006. Adult consequences of fetal growth restriction. Clin Obstet Gynecol 49(2):270–283, PMID: 16721106, 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- Basu R, Rau R, Pearson D, Malig B. 2018. Temperature and term low birth weight in California. Am J Epidemiol 187(11):2306–2314, PMID: 29901701, 10.1093/aje/kwy116. [DOI] [PubMed] [Google Scholar]
- Bell ML, Belanger K. 2012. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol 22(5):429–438, PMID: 22617723, 10.1038/jes.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belanger K. 2007. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect 115(7):1118–1124, PMID: 17637932, 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. 1995. A random‐effects regression model for meta‐analysis. Statist Med 14(4):395–411, PMID: 7746979, 10.1002/sim.4780140406. [DOI] [PubMed] [Google Scholar]
- Bouchama A, Knochel JP. 2002. Heat stroke. N Engl J Med 346(25):1978–1988, PMID: 12075060, 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Bruckner TA, Modin B, Vågerö D. 2014. Cold ambient temperature in utero and birth outcomes in Uppsala, Sweden, 1915–1929. Ann Epidemiol 24(2):116–121, PMID: 24332864, 10.1016/j.annepidem.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Chen L-Y, Ho C. 2016. Incense burning during pregnancy and birth weight and head circumference among term births: the Taiwan Birth Cohort Study. Environ Health Perspect 124(9):1487–1492, PMID: 26967367, 10.1289/ehp.1509922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P, Basagaña X, Sartini C, Figueras F, Vrijheid M, De Nazelle A. 2011. Climate extremes and the length of gestation. Environ Health Perspect 119(10):1449–1453, PMID: 21659038, 10.1289/ehp.1003241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA. 2013. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect 121(3):267–373, PMID: 23384584, 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Halbleib M, Smith JI, Gibson WP, Doggett MK, Taylor GH, et al. . 2008. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int J Climatol 28(15):2031–2064, 10.1002/joc.1688. [DOI] [Google Scholar]
- Dennery PA. 2010. Oxidative stress in development: nature or nurture? Free Radic Biol Med 49(7):1147–1151, PMID: 20656021, 10.1016/j.freeradbiomed.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Deschenes O, Greenstone M, Guryan J. 2009. Climate change and birth weight. Am Econ Rev 99(2):211–217, PMID: 29505213, 10.1257/aer.99.2.211. [DOI] [PubMed] [Google Scholar]
- Ebisu K, Holford TR, Bell ML. 2016. Association between greenness, urbanicity, and birth weight. Sci Total Environ 542(Pt A):750–756, PMID: 26546769, 10.1016/j.scitotenv.2015.10.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elter K, Ay E, Uyar E, Kavak ZN. 2004. Exposure to low outdoor temperature in the midtrimester is associated with low birth weight. Aust N Z J Obstet Gynaecol 44(6):553–557, PMID: 15598296, 10.1111/j.1479-828X.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- Fell DB, Dodds L, King WD. 2004. Residential mobility during pregnancy. Paediatr Perinat Epidemiol 18(6):408–414, PMID: 15535816, 10.1111/j.1365-3016.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Kamai EM, Cantonwine DE, Mukherjee B, Meeker JD, McElrath TF. 2018. Associations between repeated ultrasound measures of fetal growth and biomarkers of maternal oxidative stress and inflammation in pregnancy. Am J Reprod Immunol 80(4):e13017, PMID: 29984454, 10.1111/aji.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan HL, Hussey MJ, Barbera A, Ferrazzi E, Chung M, Hobbins JC, et al. . 1999. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am J Obstet Gynecol 180(5):1278–1282, PMID: 10329890, 10.1016/S0002-9378(99)70629-0. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Volodina O, Pearce SC, Gabler NK, Baumgard LH, Rhoads RP, et al. . 2017. Acute heat stress activated inflammatory signaling in porcine oxidative skeletal muscle. Physiol Rep 5(16):e13397, PMID: 28830980, 10.14814/phy2.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer KB, Eliot MN, Danilack VA, Carlson L, Phipps MG, Dadvand P, et al. . 2018. Residential green space and birth outcomes in a coastal setting. Environ Res 163:97–107, PMID: 29433021, 10.1016/j.envres.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling SN, Hondula DM, Bunker A, Ibarreta D, Liu J, Zhang X, et al. . 2017. Adaptation to climate change: a comparative analysis of modeling methods for heat-related mortality. Environ Health Perspect 125(8):087008, PMID: 28885979, 10.1289/EHP634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace K, Davenport F, Hanson H, Funk C, Shukla S. 2015. Linking climate change and health outcomes: examining the relationship between temperature, precipitation and birth weight in Africa. Glob Environ Change 35:125–137, 10.1016/j.gloenvcha.2015.06.010. [DOI] [Google Scholar]
- Ha S, Zhu Y, Liu D, Sherman S, Mendola P. 2017. Ambient temperature and air quality in relation to small for gestational age and term low birthweight. Environ Res 155:394–400, PMID: 28258738, 10.1016/j.envres.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. 2010. Associations between outdoor temperature and markers of inflammation: a cohort study. Environ Health 9:42, PMID: 20653951, 10.1186/1476-069X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Core Writing Team, Pachuri RK, Meyer LA, eds. Geneva, Switzerland:IPCC; https://www.ipcc.ch/site/assets/uploads/2018/05/SYR_AR5_FINAL_full_wcover.pdf [accessed 29 March 2019]. [Google Scholar]
- Isen A, Rossin-Slater M, Walker R. 2017. Relationship between season of birth, temperature exposure, and later life wellbeing. Proc Natl Acad Sci USA 114(51):13447–13452, PMID: 29203654, 10.1073/pnas.1702436114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle JJ, Neas LM, Devlin RB, Case MW, Schmitt MT, Madden MC, et al. . 2015. Interaction effects of temperature and ozone on lung function and markers of systemic inflammation, coagulation, and fibrinolysis: a crossover study of healthy young volunteers. Environ Health Perspect 123(4):310–316, PMID: 25514459, 10.1289/ehp.1307986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge WR, Coleshaw SR, Easton JC, Cotter F, Mattock MB, Chelliah R. 1986. Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. Am J Med 81(5):795–800, PMID: 3776986, 10.1016/0002-9343(86)90348-7. [DOI] [PubMed] [Google Scholar]
- Kingsley SL, Eliot MN, Glazer K, Awad YA, Schwartz JD, Savitz DA, et al. . 2017. Maternal ambient air pollution, preterm birth and markers of fetal growth in Rhode Island: results of a hospital-based linkage study. J Epidemiol Community Health 71(12):1131–1136, PMID: 28947670, 10.1136/jech-2017-208963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley SL, Eliot MN, Whitsel EA, Huang Y-T, Kelsey KT, Marsit CJ, et al. . 2016. Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environ Int 92–93:43–49, PMID: 27058926, 10.1016/j.envint.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Melly SJ, Coull BA, Nordio F, Schwartz JD. 2015. Using satellite-based spatiotemporal resolved air temperature exposure to study the association between ambient air temperature and birth outcomes in Massachusetts. Environ Health Perspect 123(10):1053–1058, PMID: 25850104, 10.1289/ehp.1308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A, Chiu Y-H, Coull BA, Just AC, Maxwell SL, Schwartz J, et al. . 2015. Associations between prenatal traffic-related air pollution exposure and birth weight: modification by sex and maternal pre-pregnancy body mass index. Environ Res 137:268–277, PMID: 25601728, 10.1016/j.envres.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo JM, Richmond T, Yohe GW, eds. 2014. Climate Change Impacts in the United States: The Third National Climate Assessment. U.S. Global Change Research Program. http://s3.amazonaws.com/nca2014/high/NCA3_Climate_Change_Impacts_in_the_United%20States_HighRes.pdf [accessed 29 March 2019].
- Mook-Kanamori DO, Steegers EA, Eilers PH, Raat H, Hofman A, Jaddoe VW. 2010. Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA 303(6):527–534, PMID: 20145229, 10.1001/jama.2010.78. [DOI] [PubMed] [Google Scholar]
- Murray LJ, O’Reilly DP, Betts N, Patterson CC, Davey Smith G, Evans AE. 2000. Season and outdoor ambient temperature: effects on birth weight. Obstet Gynecol 96(5 Pt 1):689–695, PMID: 11042302, 10.1016/S0029-7844(00)01022-X. [DOI] [PubMed] [Google Scholar]
- Ngo NS, Horton RM. 2016. Climate change and fetal health: the impacts of exposure to extreme temperatures in New York City. Environ Res 144(Pt A):158–164, PMID: 26610294, 10.1016/j.envres.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. 2003. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 3(1):6, PMID: 12848901, 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington AF, Strickland MJ, Klein M, Zhai X, Russell AG, Hansen C, et al. . 2017. Measurement error in mobile source air pollution exposure estimates due to residential mobility during pregnancy. J Expo Sci Environ Epidemiol 27(5):513–520, PMID: 27966666, 10.1038/jes.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G, Bracken MB, Bell ML. 2016. Particulate air pollution, fetal growth and gestational length: the influence of residential mobility in pregnancy. Environ Res 147:269–274, PMID: 26918840, 10.1016/j.envres.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope DP, Mishra V, Thompson L, Siddiqui AR, Rehfuess EA, Weber M, et al. . 2010. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiol Rev 32(1):70–81, PMID: 20378629, 10.1093/epirev/mxq005. [DOI] [PubMed] [Google Scholar]
- Rauch SA, Braun JM, Barr DB, Calafat AM, Khoury J, Montesano MA, et al. . 2012. Associations of prenatal exposure to organophosphate pesticide metabolites with gestational age and birth weight. Environ Health Perspect 120(7):1055–1060, PMID: 22476135, 10.1289/ehp.1104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Rodríguez P, Ramiro-Cortijo D, Reyes-Hernández CG, de Pablo ALL, González MC, Arribas SM. 2018. Implication of oxidative stress in fetal programming of cardiovascular disease. Front Physiol 9:602, PMID: 29875698, 10.3389/fphys.2018.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. 2008. Modern Epidemiology. 3rd ed Philadelphia, PA:Lippincott Williams & Wilkins. [Google Scholar]
- Saigal S, Doyle LW. 2008. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371(9608):261–269, PMID: 18207020, 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. 2000. Identifying critical windows of exposure for children’s health. Environ Health Perspect 108(Suppl 3):451–455, PMID: 10852844, 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler KR, Weinberger KR, Wellenius GA. 2018. Suitability of gridded climate datasets for use in environmental epidemiology. J Expo Sci Environ Epidemiol, PMID: 30538298, 10.1038/s41370-018-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand LB, Barnett AG, Tong S. 2011. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol 11:49, PMID: 21501523, 10.1186/1471-2288-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Weinberger KR, Spangler KR, Eliot MN, Braun JM, Wellenius GA. 2019. Ambient temperature and preterm birth: a retrospective study of 32 million US singleton births. Environ Int 126:7–13, PMID: 30776752, 10.1016/j.envint.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LM, Bruce N, Eskenazi B, Diaz A, Pope D, Smith KR. 2011. Impact of reduced maternal exposures to wood smoke from an introduced chimney stove on newborn birth weight in rural Guatemala. Environ Health Perspect 119(10):1489–1494, PMID: 21652290, 10.1289/ehp.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustin K, Gross J, Hayne H. 2004. Maternal exposure to first‐trimester sunshine is associated with increased birth weight in human infants. Dev Psychobiol 45(4):221–230, PMID: 15549686, 10.1002/dev.20030. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2000. Centers of population for Census 2000. https://www.census.gov/geographies/reference-files/2000/geo/2000-centers-population.html [accessed 29 March 2019].
- U.S. Department of Energy. 2015. Volume 7.3. Guide to Determining Climate Regions by County. https://www.energy.gov/sites/prod/files/2015/10/f27/ba_climate_region_guide_7.3.pdf [accessed 9 August 2018].
- Vicedo-Cabrera AM, Sera F, Gasparrini A. 2019. Hands-on tutorial on a modeling framework for projections of climate change impacts on health. Epidemiology 30(3):321–329, PMID: 30829832, 10.1097/EDE.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J Stat Softw 36(3):1–48, 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- Wells JC. 2002. Thermal environment and human birth weight. J Theor Biol 214(3):413–425, PMID: 11846599, 10.1006/jtbi.2001.2465. [DOI] [PubMed] [Google Scholar]
- Wells JC, Cole TJ. 2002. Birth weight and environmental heat load: a between‐population analysis. Am J Phys Anthropol 119(3):276–282, PMID: 12365039, 10.1002/ajpa.10137. [DOI] [PubMed] [Google Scholar]
- Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. . 2008. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 300(24):2886–2897, PMID: 19109117, 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- Wolf J, Armstrong B. 2012. The association of season and temperature with adverse pregnancy outcome in two German states, a time-series analysis. PloS One 7(7):e40228, PMID: 22792247, 10.1371/journal.pone.0040228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann HA. 1998. Intrauterine growth restriction: definition and etiology. Horm Res 49(Suppl 2):1–6, PMID: 9730664, 10.1159/000053079. [DOI] [PubMed] [Google Scholar]
- Yanosky JD, Paciorek CJ, Laden F, Hart JE, Puett RC, Liao D, et al. . 2014. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health 13:63, PMID: 25097007, 10.1186/1476-069X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanosky JD, Paciorek CJ, Suh HH. 2009. Predicting chronic fine and coarse particulate exposures using spatiotemporal models for the Northeastern and Midwestern United States. Environ Health Perspect 117(4):522–529, PMID: 19440489, 10.1289/ehp.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yu C, Wang L. 2017. Temperature exposure during pregnancy and birth outcomes: an updated systematic review of epidemiological evidence. Environ Pollut 225:700–712, PMID: 28284544, 10.1016/j.envpol.2017.02.066. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Kris-Etherton PM, Hartman TJ. 2014. Birth weight and risk factors for cardiovascular disease and type 2 diabetes in US children and adolescents: 10 year results from NHANES. Matern Child Health J 18(6):1423–1432, PMID: 24241968, 10.1007/s10995-013-1382-y. [DOI] [PubMed] [Google Scholar]
- Zhu M, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM. 2010. Maternal low-level lead exposure and fetal growth. Environ Health Perspect 118(10):1471–1475, PMID: 20562053, 10.1289/ehp.0901561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.