Abstract
The neuropeptide Y Y5 receptor subtype has generated great interest, especially regarding its possible involvement in feeding behaviors. However, its distribution and sites of expression in the mammalian brain are, in large part, unknown because of the lack of selective tools. We demonstrate in this study that specific [125I][Leu31,Pro34]PYY binding is competed in a biphasic manner by BIBP3226, a Y1receptor antagonist, demonstrating the existence of sensitive and insensitive sites to BIBP3226. Assays performed by using [125I][Leu31,Pro34]PYY in the presence of 1 μm BIBP3226 to block the Y1 receptor subtype revealed a pharmacological profile highly similar to the cloned Y5 receptor. Moreover, results obtained with GW1229 suggest that the Y4 subtype represents only a very small proportion of the total population of NPY receptors in the rat brain. Quantitative receptor autoradiographic data revealed the discrete distribution of [125I][Leu31,Pro34]PYY/BIBP3226-insensitive Y5 sites in the rat brain, with the external plexiform layer of the olfactory bulb, the lateral septum, the anteroventral thalamic nucleus, the CA3 subfield of the ventral hippocampus, the nucleus tractus solitarius, and the area postrema being most enriched. Rather surprisingly, in the hypothalamus, a key structure modulating food intake, only low densities of Y5 binding sites were detected as well as in most other regions of the rat brain. These data suggest that the Y5 receptor protein is expressed and translated by a small percentage of hypothalamic neurons and that the effect of NPY on feeding behaviors likely is mediated by more than one class of NPY receptors. It also indicates that the Y5 receptor may be involved in other biological actions induced by NPY. Taken together, these data represent the first pharmacological demonstration of the expression and discrete localization of the Y5 receptor protein in the rat brain.
Keywords: NPY/PYY receptor subtypes, Y5 receptor subtype, rat brain, autoradiographic studies, receptor binding assays
Neuropeptide Y (NPY) is a 36-amino-acid peptide that shares high sequence homology with peptide YY (PYY) and the pancreatic polypeptides (PPs) (Tatemoto et al., 1982). NPY is one of the most abundant peptides found in the mammalian brain (Chronwall et al., 1985; DeQuidt and Emson, 1986a,b). Intracerebroventricular injections, as well as direct administration into specific nuclei, of NPY/PYY fragments and analogs induce several biological responses, including increased food intake, modulation of luteinizing hormone-releasing hormone (LHRH) and corticotropin-releasing factor (CRF) releases, regulation of cardiorespiratory parameters, enhanced cognitive function associated with learning and memory, shifts of circadian rhythms, and reduction of anxiety-related behaviors (Dumont et al., 1992; Kalra and Crowley, 1992; Grundemar et al., 1993; Wahlestedt and Reis, 1993; Heilig and Widerlov, 1995; Munglani et al., 1996). Additionally, studies in rodents suggest that NPY and its receptors could have a direct implication in some pathological disorders, including obesity, depression, and epilepsy (Wahlestedt and Reis, 1993; Colmers and Bleakman, 1994; Munglani et al., 1996; Klapstein and Colmers, 1997).
The various biological effects of NPY and homologs are mediated by the activation of at least five classes of receptors termed Y1, Y2, Y4, Y5, and Y6 (Dumont et al., 1992;Wahlestedt and Reis, 1993; Colmers and Bleakman, 1994; Gehlert, 1994;Blomqvist and Herzog, 1997; Michel et al., 1998), all of which have been cloned (Eva et al., 1990; Herzog et al., 1992; Larhammar et al., 1992; Bard et al., 1995; Gerald et al., 1995, 1996; Lundell et al., 1995; Rose et al., 1995; Weinberg et al., 1996). The pharmacology of each of these receptor subtypes has been defined by using several analogs and fragments of NPY, PYY, and PPs (for more details, seeMichel et al., 1998).
Moreover, the recent development of the Y1 nonpeptide antagonists BIBP3226 (Rudolf et al., 1994) and SR 120819A (Serradeil-Le Gal et al., 1995) as well as a Y1 peptidergic antagonist, 1229U91 [Daniels et al. (1995); now known as GW1229; Bitran et al. (1997)], helps to improve our understanding of the role of this receptor subtype in mediating some of the effects of NPY. BIBP3226 has been studied most extensively and has been shown to behave as a competitive, selective, and specific Y1 receptor antagonist in various binding assays and in in vitro and in vivo bioassays (Rudolf et al., 1994; Abounader et al., 1995; Doods et al., 1995; Jacques et al., 1995; Wieland et al., 1995; Lundberg et al., 1996) without any significant activity at the Y2(Rudolf et al., 1994; Jacques et al., 1995), Y4 (Gehlert et al., 1996a,b; Gerald et al., 1996), and Y5 (Gerald et al., 1996) receptor subtypes.
In the mammalian brain the existence of heterogeneous populations of NPY receptor sites has been demonstrated by using, for example, [125I][Leu31,Pro34]PYY and [125I]PYY3–36 as preferential Y1-like and Y2-like radioligands (Dumont et al., 1995, 1996a; Jacques et al., 1997). Interestingly, however, the comparative autoradiographic distribution of specific [3H]BIBP3226 (Y1 antagonist) and [125I][Leu31,Pro34]PYY (Y1-like agonist) binding sites revealed that various areas of the rat brain possessed low to very low amounts of [3H]BIBP3226 binding sites while being comparatively enriched with [125I][Leu31,Pro34]PYY labeling, suggesting that [125I][Leu31,Pro34]PYY could recognize an additional population of sites (Dumont et al., 1996b). These sites could represent the Y4 receptor because [Leu31,Pro34]PYY has rather high affinity for this subtype (Bard et al., 1995; Gehlert et al., 1996a,b). Alternatively, the newly cloned Y5 receptor also possesses rather high affinity for the [Leu31,Pro34]PYY analog and may represent the [125I][Leu31,Pro34]PYY/BIBP3226-resistant sites.
We thus have investigated the precise nature of the [125I][Leu31,Pro34]PYY/BIBP3226-insensitive sites, using a variety of competitors. Our data reveal that these sites demonstrate high affinities for human PP (hPP), but not rat PP (rPP), with a competition binding profile similar to that of the cloned Y5 receptor subtype (Gerald et al., 1996). Moreover, this Y5-like receptor subtype is distributed very discretely in the rat brain, with highest levels seen in the external plexiform layer of the olfactory bulb, the lateral septum, the anteroventral thalamic nucleus, the CA3 subfield of the ventral hippocampus, the nucleus tractus solitarius, and the area postrema. Rather unexpectedly, however, various hypothalamic nuclei are not enriched with specific binding, raising issues as to its critical role in feeding behaviors.
MATERIALS AND METHODS
Materials. Male Sprague Dawley CD rats (200–250 gm) obtained from Charles River Canada (St. Constant, Québec, Canada) were kept on a 12 hr light/dark cycle (light on at 7:00 A.M.) in temperature- and humidity-controlled rooms. Animals were fed with standard laboratory chow and had access to tap water ad libitum. Animal care was given according to protocols and guidelines approved by McGill University and the Canadian Council of Animal Care.
Analogs and fragments of PYY and pNPY were synthesized in our laboratories as previously described (Forest et al., 1990); avian PP (aPP), bovine PP (bPP), rPP, hPP, and [d-Trp32]NPY were purchased from Bachem California (Torrance, CA) and Peninsula Laboratories (Belmont, CA). BIBP3226 and BIBP3435 were generously provided by Karl Thomae GmbH (Germany); GW1229 was a gift from Glaxo Wellcome (Research Triangle Park, NC). Bovine serum albumin (BSA) and iodine-125 were obtained from ICN Biochemicals Canada (Montréal, Québec, Canada), and bacitracin was purchased from Sigma (St. Louis, MO). Schleicher & Schuell #32 glass filters were obtained from Xymotech (Montréal, Québec, Canada). [3H]Hyperfilms and125I-microscale standards were purchased from Amersham (Mississauga, Ontario, Canada). All other chemicals were of analytical grade and obtained from Fisher Scientific (Montréal, Québec, Canada) or Sigma.
Iodine-125 was incorporated into the tyrosine residue of [Leu31,Pro34]PYY and PYY3–36, using the chloramine T method as previously described (Dumont et al., 1995), and the specific activity was assumed to be of the theoretical value (2000 Ci/mmol).
Membrane binding assays. Membranes were prepared as previously described (Dumont et al., 1995). Briefly, rats were decapitated and their brains rapidly removed and homogenized in a Krebs’–Ringer’s phosphate (KRP) buffer at pH 7.4 of the following composition (in mm): NaCl (120), KCl (4.7), CaCl2 (2.2), KH2PO4 (1.2), MgSO4 (1.2), dextrose (5.5), and NaHCO3 (25), using a Brinkman polytron (at setting 6 for 15–20 sec). Homogenates were centrifuged at 49,000 × g for 20 min; supernatants were discarded, and pellets were washed, resuspended, and recentrifuged twice.
All binding assays were initiated by adding 100 μl of membrane preparations in a final volume of 500 μl of KRP containing 0.1% (w/v) BSA, 0.05% (w/v) bacitracin, [125I][Leu31,Pro34]PYY (25–35 pm), and various competitors (pNPY, hPYY, [Leu31,Pro34]NPY, [Leu31,Pro34]PYY, pNPY2–36, NPY13–36, NPY18–36, hPYY3–36, hPYY13–36, [d-Trp32]pNPY, aPP, rPP, hPP, bPP, GW1229, BIBP3435, and BIBP3226) at concentrations ranging from 10−12 to 10−6m. All binding assays were done in the absence or presence of 1 μm BIBP3226 to block the Y1 receptor subtype (see Results). Nonspecific binding was determined in the presence of 1 μm pNPY. After 2 hr the binding reaction was terminated by rapid filtration through Schleicher & Schuell #32 glass filters (previously soaked in 1.0% polyethyleneimine), using a cell harvester filtering apparatus (Brandel Instruments, Gaithersburg, MD). Filters were rinsed three times with 3 ml of cold KRP, and the radioactivity remaining on filters was quantified by using a gamma counter with 85% efficiency (Packard Instruments, Meridian, CT).
All binding experiments were repeated three to six times, each in triplicate, and the results were expressed as a percentage of specific binding representing the mean ± SEM. IC50 values (i.e., the concentration of unlabeled peptide required to compete for 50% of specific binding of the radioligand) of the various peptides and BIBP3226 were calculated from the competition binding assays data, using the GraphPad Prism (GraphPad Software, San Diego, CA).
Quantitative receptor autoradiography. Receptor autoradiography was performed as described in detail elsewhere (Dumont et al., 1993, 1996a). Briefly, rats were decapitated and their brains rapidly removed from the skull, frozen in 2-methylbutane at −40°C for 15 sec, and then kept at −80°C until needed. Sections (20 μm) were obtained with a cryomicrotome at −17°C, mounted on gelatin–chrome–alum-coated slides, dried overnight in a desiccator at 4°C, and then kept at −80°C until use.
On the days of the experiments, adjacent coronal sections were preincubated for 60 min at room temperature in a KRP buffer at pH 7.4 and then incubated for 120 min in a fresh preparation of KRP buffer containing 0.1% BSA, 0.05% bacitracin, 35 pm[125I][Leu31,Pro34]PYY, and various concentrations of BIBP3226 (from 10−10to 10−5m). After a 2 hr incubation, sections were washed four times for 2 min each in ice-cold KRP buffer, then dipped in deionized water to remove salts, and rapidly dried. Nonspecific binding was determined by using 1 μm NPY for both radioligands. Incubated sections were apposed against3H-Hyperfilms for 6 d alongside radioactive standards. Films were developed and quantified as described in detail elsewhere (Dumont et al., 1996a).
RESULTS
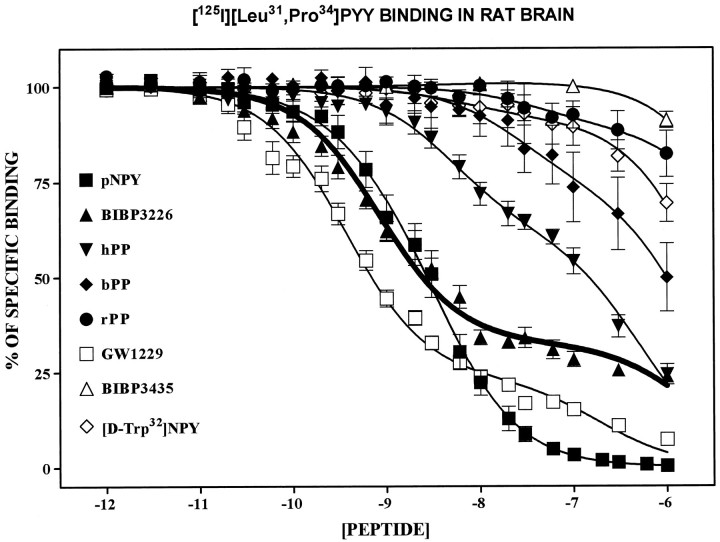
Membrane binding assays in rat brain homogenates revealed that the nonpeptide Y1 antagonist BIBP3226 (Rudolf et al., 1994) competed against [125I][Leu31,Pro34]PYY binding in a clearly biphasic manner (Fig.1). In fact, competition curves were best fit to a two-site model (p < 0.05) with high-affinity (KH 1.2 ± 0.3 nm) and low-affinity (KL > 1000 nm) components (Table 1), with the high-affinity sites representing 65–70% of the binding of [125I][Leu31,Pro34]PYY (Fig. 1). The specificity of BIBP3226 for the Y1 receptor subtype was extended further by its poor ability to compete for [125I]PYY3–36/Y2 binding sites (Table 1). In fact at 1 μm, BIBP3226 competed for <5% of specific [125I]PYY3–36binding (data not shown). As expected, the S-enantiomer of the Y1 antagonist BIBP3435 was inactive at both the Y1-like and Y2-like receptor subtypes labeled with [125I][Leu31,Pro34]PYY and [125I]PYY3–36, respectively (Fig.1, Table 1). The Y1 peptidergic antagonist GW1229 (Daniels et al., 1995) showed a highly complex competition binding profile for [125I][Leu31,Pro34]PYY binding sites (KH and KLof 0.3 and 190 nm, respectively; Table 1) while having only very low affinity for sites labeled with the purported Y2 radioligand, [125I]PYY3–36 (Table 1). In contrast to GW1229 and hPP (see below), the competition of [125I][Leu31,Pro34]PYY binding by BIBP3226 revealed a clear plateau (Fig. 1). Taken together, these results strongly suggest that [125I][Leu31,Pro34]PYY can recognize at least two populations of sites. One is highly sensitive (in nm) to BIBP3226, whereas the other is mostly resistant.
Fig. 1.
Comparative competition binding profiles of BIBP3226 (nonpeptidergic Y1 antagonist), BIBP3435 (S-enantiomer of BIBP3226), GW1229 (peptidergic Y1antagonist), and several pancreatic polypeptides (human, bovine, rat, and avian PP) against [125I][Leu31,Pro34]PYY in rat brain membrane homogenates. Each point represents the mean ± SEM of four to six determinations, each performed in triplicate and expressed as the percentage of specific binding.
Table 1.
Comparative binding parameters of pNPY, PP (avian, rat, bovin, and human), BIBP3226 (a Y1 nonpeptidergic antagonist), BIBP3435 (S-enantiomer of BIBP3226), and GW1229 (a Y1 peptidergic antagonist) against [125I][Leu31, Pro34]PYY and [125I]PYY3-36 binding sites in rat brain membrane homogenates
| Competitors | [125I][Leu31, Pro34]PYY | [125I]PYY3-36 | ||
|---|---|---|---|---|
| IC50(nm) | n | IC50(nm) | n | |
| pNPY | 1.1 ± 0.3 | 0.83 ± 0.03 | 0.1 ± 0.02 | 0.70 ± 0.03 |
| pNPY13-36 | 100 ± 15 | 0.74 ± 0.05 | 0.24 ± 0.1 | 0.77 ± 0.02 |
| [D-Trp32]NPY | 1000 | — | 420 ± 80 | — |
| PYY | 1.7 ± 0.2 | 0.75 ± 0.05 | 0.08 ± 0.02 | 0.75 ± 0.04 |
| PYY3-36 | 780 ± 80 | 0.79 ± 0.04 | 0.3 ± 0.1 | 0.74 ± 0.03 |
| PYY13-36 | 450 ± 120 | 0.71 ± 0.06 | 0.4 ± 0.1 | 0.77 ± 0.05 |
| aPP | 1000 | — | 1000 | — |
| rPP | 1000 | — | 1000 | — |
| bPP | 960 ± 150 | 0.74 ± 0.05 | 1000 | — |
| hPP | KH 5.0 ± 2.3 | 0.49 ± 0.04 | 1000 | — |
| KL 530 ± 160 | ||||
| BIBP3226 | KH 2.1 ± 0.6 | 0.50 ± 0.04 | 1000 | — |
| KL 1000 | ||||
| BIBP3435 | 1000 | — | 1000 | — |
| GW1229 | KH 0.3 ± 0.08 | 0.44 ± 0.02 | 850 ± 230 | — |
| KL 190 ± 50 | ||||
Data represent the mean ± SEM of three to eight determinations. All binding curves were fit to a one- or a two-binding sites model by a nonlinear method of analysis, using GraphPad Prism Software competition data analysis. The goodness of fit between the two models was tested by F test (p = 0.05).KH and KLrepresent the affinity of competitors for the high- and low-affinity sites, respectively. IC50 represents the concentration of competitors needed to inhibit 50% of the specific binding.n represents the Hill coefficient.
Among the various PPs the rPP, bPP, and aPP were poor competitors for binding sites recognized by [125I][Leu31,Pro34]PYY or [125I]PYY3–36, as shown in Figure1 and Table 1. In contrast, hPP competed for [125I][Leu31,Pro34]PYY sites with binding data best fit to a two-site model with high-affinity (5 ± 2.3 nm) and low-affinity (530 ± 160 nm) components (Table 1), the high-affinity portion representing 30% of specific [125I][Leu31,Pro34]PYY binding (Fig. 1). [d-Trp32]NPY, a purported Y5 agonist (Gerald et al., 1996), was almost inactive (IC50 > 1000 nm) in competing for the total population of [125I][Leu31,Pro34]PYY sites (Fig. 1, Table 1) while having an affinity of 320 ± 80 nm for the Y2 receptor subtype (Table 1).
In the next series of experiments, binding assays were performed by using [125I][Leu31,Pro34]PYY in the presence of 1 μm BIBP3226, a concentration sufficient to block all sites (Y1-like) that have a high affinity for this antagonist (Fig. 1). Under such assay conditions the rank order of potency of various fragments and analogs of NPY, PYY, and PP is as follows: NPY = PYY = hPP = [Leu31,Pro34]PYY (low nm range) > NPY2–36, PYY3–36, [Leu31,Pro34]NPY (10–30 nm) > [d-Trp32]NPY (100 nm) > NPY13–36, PYY13–36, rPP, and GW1229 (>300 nm) (Fig.2, Table2). This pharmacological profile is similar to the one reported by Gerald and collaborators (1996) for the cloned Y5 receptor. Additionally, most of the peptides that were tested competed against specific [125I][Leu31,Pro34]PYY/BIBP3226-insensitive binding sites, with Hill coefficients not significantly different from unity (Table 2). Only four competitors ([d-Trp32]NPY, bPP, hPP, and GW1229) demonstrated inhibitory profiles with Hill coefficient values lower than unity (Table 2).
Fig. 2.
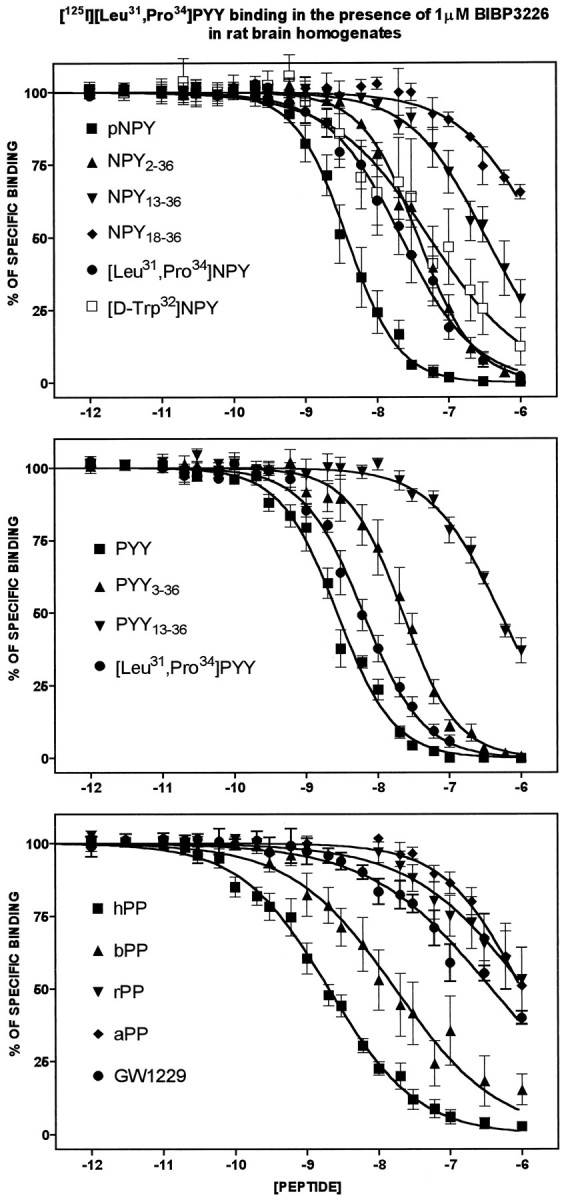
Comparative competition binding profiles of NPY, PYY, PP, and their derivatives as well as GW1229 against [125I][Leu31,Pro34]PYY binding sites in the presence of 1 μm BIBP3226 in rat brain membrane homogenates. Data represent the mean ± SEM of four to nine determinations, each performed in triplicate and expressed as the percentage of specific binding.
Table 2.
Comparative binding parameters of NPY, PYY, PP, and their derivatives as well as BIBP3226, BIBP3435, and GW1229 against [125I][Leu31, Pro34]PYY binding sites in the presence of 1 μm BIBP3226 in rat brain membrane homogenates
| Competitors | [125I][Leu31, Pro34]PYY alone | [125I][Leu31, Pro34]PYY + 1 μm BIBP3226 | ||
|---|---|---|---|---|
| IC50(nm) | n | IC50(nm) | n | |
| pNPY | 1.1 ± 0.3 | 0.83 ± 0.03 | 5.6 ± 1.6 | 1.2 ± 0.1 |
| pNPY2-36 | 42 ± 9 | 0.75 ± 0.04 | 13.2 ± 2.9 | 1.1 ± 0.05 |
| pNPY13-36 | 100 ± 15 | 0.74 ± 0.05 | 450 ± 130 | 0.92 ± 0.07 |
| NPY18-36 | 65 ± 10 | 0.79 ± 0.03 | 1000 | — |
| [Leu31, Pro34]NPY | 2.3 ± 0.6 | 0.71 ± 0.03 | 28 ± 8 | 1.06 ± 0.11 |
| [D-Trp32]NPY | 1000 | — | 100 ± 40 | 0.85 ± 0.14 |
| PYY | 1.7 ± 0.2 | 0.75 ± 0.05 | 2.8 ± 0.4 | 1.08 ± 0.03 |
| PYY3-36 | 780 ± 80 | 0.79 ± 0.04 | 24 ± 4.5 | 0.98 ± 0.05 |
| PYY13-36 | 450 ± 120 | 0.71 ± 0.06 | 510 ± 60 | 1.01 ± 0.08 |
| [Leu31, Pro34]PYY | 2.0 ± 0.9 | 0.82 ± 0.04 | 6.7 ± 1.0 | 1.09 ± 0.06 |
| aPP | 1000 | — | 1000 | — |
| rPP | 1000 | — | 1000 | — |
| bPP | 960 ± 150 | 0.74 ± 0.05 | 48 ± 16 | 0.69 ± 0.08 |
| hPP | KH 5.0 ± 2.3 | 0.49 ± 0.04 | 2.1 ± 0.5 | 0.74 ± 0.07 |
| KL 530 ± 160 | ||||
| BIBP3226 | KH 2.1 ± 0.6 | 0.50 ± 0.04 | 1000 | — |
| KL 1000 | ||||
| BIBP3435 | 1000 | — | 1000 | — |
| GW1229 | KH 0.3 ± 0.08 | 0.44 ± 0.02 | 350 ± 90 | 0.62 ± 0.07 |
| KL 190 ± 50 | ||||
Data represent the mean ± SEM of four to nine determinations. n represents the Hill coefficient. IC50 represents the concentration of competitors needed to inhibit 50% of the specific binding. KHand KL represent, respectively, the affinity of competitors for the high- and low-affinity sites as determined by using a two-binding sites model.
To determine the distribution of BIBP3226-resistant [125I][Leu31,Pro34]PYY binding sites, we performed an autoradiographic study next, using adjacent brain sections incubated in the presence of increasing concentrations (from 10−10 to 10−5m) of BIBP3226. As shown in Figure3, the distribution profile of the total population of [125I][Leu31,Pro34]PYY sites is identical to published data (Dumont et al., 1996a). BIBP3226 competed for [125I][Leu31,Pro34]PYY labeling with high affinity in all cortical areas (Fig.3A–C), the olfactory nuclei, tenia tecta, olfactory tubercle (Fig. 3A), claustrum (Fig. 3A,B), and most thalamic nuclei (Fig. 3B). Similarly, specific [125I][Leu31,Pro34]PYY labeling seen in the dentate gyrus (Fig. 3B), geniculate and medial mamillary nuclei, inferior colliculus, tegmental areas (Fig.3C), cerebellum, basilar artery, vestibular nuclei, and inferior olive (Fig. 3D) is inhibited by low nanomolar concentrations of the Y1 antagonist. However, [125I][Leu31,Pro34]PYY binding in areas such as the external plexiform layer of the olfactory bulb (Fig. 3A), the lateral septum, the anteroventral nucleus of the thalamus (Fig. 3B), the CA3 subfield of the ventral hippocampus (Fig. 3C), the nucleus tractus solitarius, and the area postrema (Fig. 3D) is apparently rather insensitive to BIBP3226 even at concentrations up to 10 μm.
Fig. 3.
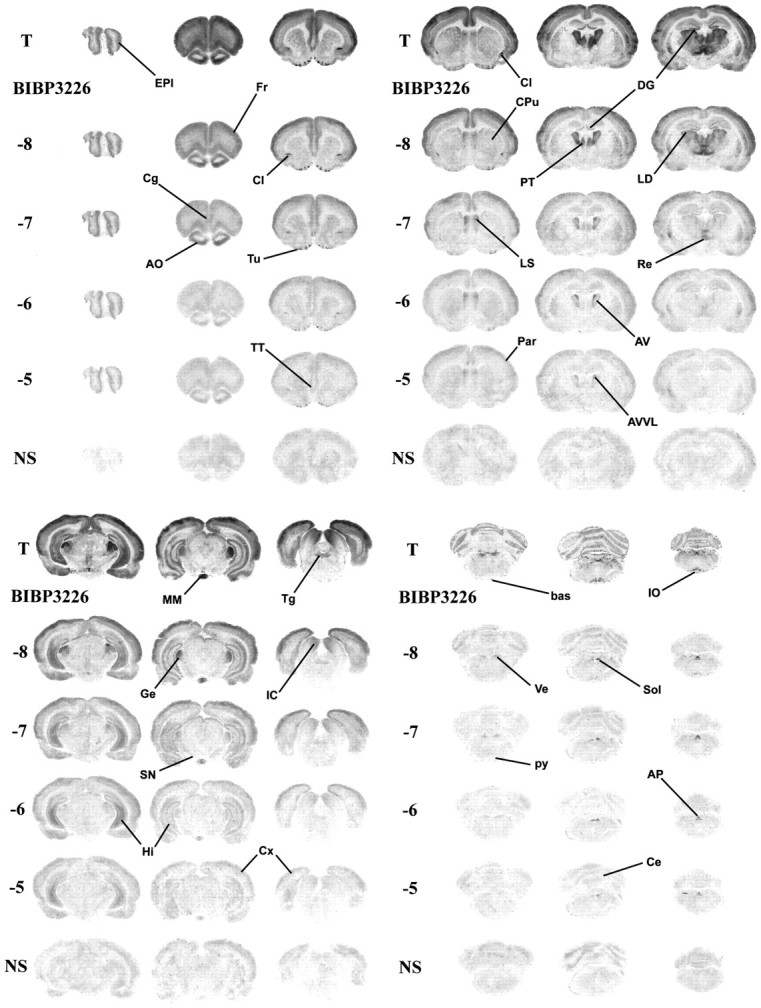
Autoradiographic distribution of [125I][Leu31,Pro34]PYY in the presence of increasing concentrations of BIBP3226. Adjacent coronal rat brain sections were incubated in the presence of 35 pm[125I][Leu31,Pro34]PYY alone (total binding, T) and in the presence of BIBP3226 from 10 to 10,000 nm. Nonspecific binding (NS) was determined by the presence of 1 μm pNPY. AO, Anterior olfactory nuclei;AP, area postrema; AV, anteroventral thalamic nucleus; AVVL, anteroventral thalamic nucleus, ventrolateral part; bas, basilar artery;CA1–3, CA1 and CA3 subfields of the hippocampus;Ce, cerebellum; Cg, cingulate cortex;Cl, claustrum; CPu, caudate putamen (striatum); Cx, cortex; DG, dentate gyrus; EPl, external plexiform layer of the olfactory bulb; Fr1–3, frontal cortex superficial, mid, and deep layers; Ge, geniculate nuclei; GrA, granular cell layer of the olfactory bulb; Hi, hippocampus; HiVe, ventral part of the hippocampus;Hy, hypothalamus; IC, inferior colliculus; IO, inferior olive; LD, laterodorsal thalamic nucleus; LS, lateral septum;MD, mediodorsal thalamic nucleus; MM, mamillary nu-cleus; Par1–3, parietal cortex superficial, mid, and deep layers; PT, paratenial thalamic nucleus; py, pyramidal tract;Re, reuniens thalamic nucleus; SN, substantia nigra; Sol, nucleus of the solitary tract;Tg, tegmental area; TT, tenia tecta;Tu, olfactory tubercle; Ve, vestibular nuclei.
A quantitative autoradiographic analysis of these data confirmed that certain regions of the rat brain are resistant to BIBP3226. For example, in the external plexiform layer of the olfactory bulb >50% of specific [125I][Leu31,Pro34]PYY binding is still seen even in the presence of 10 μmBIBP3226 (Fig. 4). Additionally, the lateral septum, the ventral hippocampus, the nucleus tractus solitarius, and the area postrema also contain significant amounts of [125I][Leu31,Pro34]PYY binding sites that are not competed by BIBP3226 (Fig. 4). In contrast, the Y1 receptor subtype, as defined by specific [125I][Leu31,Pro34]PYY binding sensitive to low nanomolar concentrations of BIBP3226, is highly enriched in regions such as the cortex, claustrum, most thalamic nuclei, the geniculate and medial mamillary nuclei, the inferior colliculus, the cerebellum, and the inferior olive. The hypothalamus apparently is not enriched with significant levels of [125I][Leu31,Pro34]PYY/BIBP3226-resistant sites (see Fig. 3B).
Fig. 4.
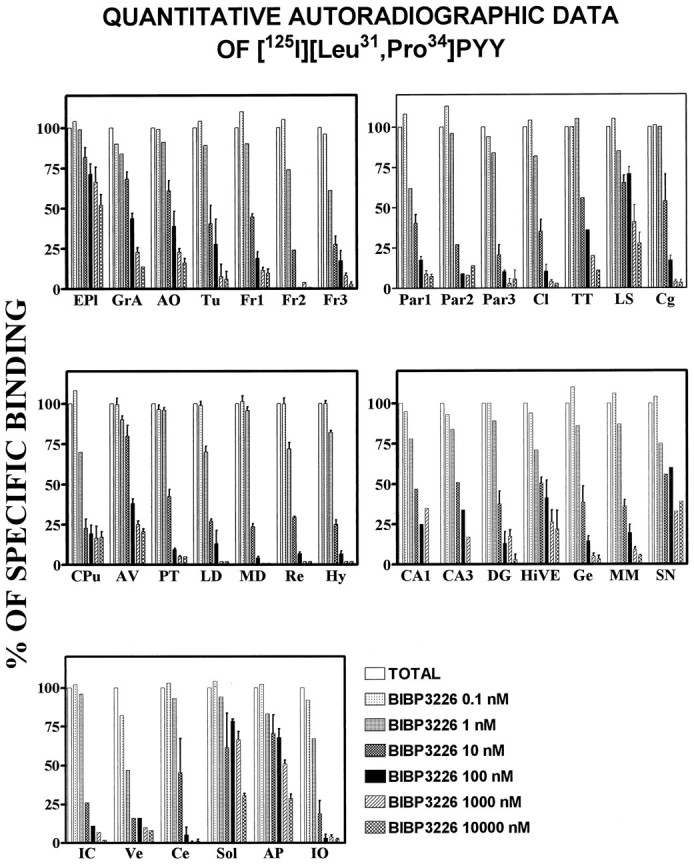
Quantitative autoradiographic data of [125I][Leu31,Pro34]PYY in the presence of increasing concentrations of BIBP3226 (0.1–10,000 nm) in various rat brain regions. See the list of abbreviations in Figure 3 for anatomical identification.
DISCUSSION
This study provides the first direct pharmacological evidence for the presence and unique distribution of the Y5 receptor protein in the rat brain. A few recent studies have attempted to demonstrate the existence of the Y5 receptor subtype in the rat brain but failed to provide conclusive data, mostly because of the limited pharmacological characterization of the binding sites under study or the use of radioligands such as [125I]hPP or [125I]PYY (Trinh et al., 1996; Widdowson, 1997;Widdowson et al., 1997). Our results clearly reveal that [125I][Leu31,Pro34]PYY/BIBP3226-insensitive sites in the rat brain have a pharmacological profile that is very similar to that of the recently cloned Y5 receptor subtype proposed to mediate feeding behaviors (Gerald et al., 1996; Hu et al., 1996) (but see above). Moreover, these sites are distributed in a manner strikingly distinct from those reported earlier for the better characterized Y1 and Y2 receptors (Dumont et al., 1990, 1993, 1996a,b; Aicher et al., 1991; Gehlert et al., 1992;Larsen et al., 1993). Rather surprisingly, however, very low densities of these Y5-like binding sites are expressed in the hypothalamus, a key structure involved in NPY-mediated effects on feeding behaviors.
Studies using membrane receptor binding assays and autoradiography have shown that [125I]PYY can recognize at least two populations of sites: one highly sensitive to [Leu31,Pro34]NPY or [Pro34]NPY and the other having high affinity for NPY13–36 and other C-terminal fragments (Dumont et al., 1990, 1993; Aicher, 1991; Gehlert et al., 1992; Larsen et al., 1993). We also have used [125I][Leu31,Pro34]PYY and [125I]PYY3–36 as radioligands to characterize the Y1 and the Y2 receptor subtype, respectively (Dumont et al., 1995, 1996a; Jacques et al., 1997). However, Hill coefficient values lower than unity of various NPY and PYY analogs in competing against either [125I][Leu31,Pro34]PYY and [125I]PYY3–36 binding sites have suggested further heterogeneity of the sites recognized by these probes (Dumont et al., 1995, 1996b; Gehlert et al., 1996b).
In contrast to most peptidergic molecules, BIBP3226 was able to compete, in a clearly biphasic manner, for 65–70% of specific [125I][Leu31,Pro34]PYY binding sites in the rat brain while failing to act on specific [125I]PYY3–36/Y2-like sites. This selectivity profile of BIBP3226 for the Y1versus the Y2 receptor subtype is in agreement with previous reports that used Y1 and Y2 enriched membrane preparations (Rudolf et al., 1994; Doods et al., 1995; Jacques et al., 1995; Wieland et al., 1995) and in neuroblastoma cell lines expressing the Y1 or the Y2 receptor subtype (Rudolf et al., 1994; Wieland et al., 1995). It also is supported by data obtained in a variety of functional in vitro andin vivo bioassays (see introductory remarks). Moreover, in cells transfected with the Y2, Y4, or Y5 receptor subtype, BIBP3226 failed to antagonize the effects of NPY on cAMP accumulation or to compete for [125I]PYY binding sites (Gehlert et al., 1996a; Gerald et al., 1996). It is thus evident that BIBP3226 is a highly selective Y1 receptor antagonist. In the present study the high affinity of BIBP3226 (5 nm) for specific [125I][Leu31,Pro34]PYY binding sites in the rat brain is similar to data obtained earlier in various binding assays that used [125I]PYY or [125I]BH-NPY as radioligands (Rudolf et al., 1994;Wieland et al., 1995) or directly evaluated by using [3H]BIBP3226 (Entzeroth et al., 1995). As expected, the S-enantiomer BIBP3435 was inactive in the Y1, Y2, and Y5binding assays (Wieland et al., 1995; present study).
The purported Y1 peptide antagonist GW1229 (Daniels et al., 1995; Bitran et al., 1997) revealed a competition binding profile that was also best fit to a two-site model with high-affinity (0.3 nm) and low-affinity (190 nm) components. The proportion of specific [125I][Leu31,Pro34]PYY binding sites that has a high affinity for GW1229 is in the range of that noted for BIBP3226. It recently was shown that GW1229 also has high affinity for the Y4 receptor subtype (Gehlert et al., 1996a,b). The fact that BIBP3226 (devoid of affinity for the Y4 receptor) and GW1229 competed with high affinities for the same proportion of specific [125I][Leu31,Pro34]PYY binding sites suggests that the rat brain does not express high levels of Y4 receptors. This hypothesis is supported by the very restricted distribution and expression of [125I]hPP (Trinh et al., 1996) or [125I]bPP (Gehlert et al., 1997; Whitcomb et al., 1997) sites in the rat brain. Additionally, the rather low affinity of rPP and bPP as compared with hPP in competing for either [125I][Leu31,Pro34]PYY/BIBP3226-sensitive or -insensitive sites further supports the low level of expression of the Y4 receptor in the rat brain, because all mammalian PPs, including rPP, possess very high affinities for the Y4receptor (Bard et al., 1995; Gregor et al., 1996; Lundell et al., 1995;Gehlert et al., 1996a; Gerald et al., 1996). Interestingly, the proportion of specific [125I][Leu31,Pro34]PYY binding sites that is sensitive to nm concentrations of hPP is similar to that which is insensitive to BIBP3226.
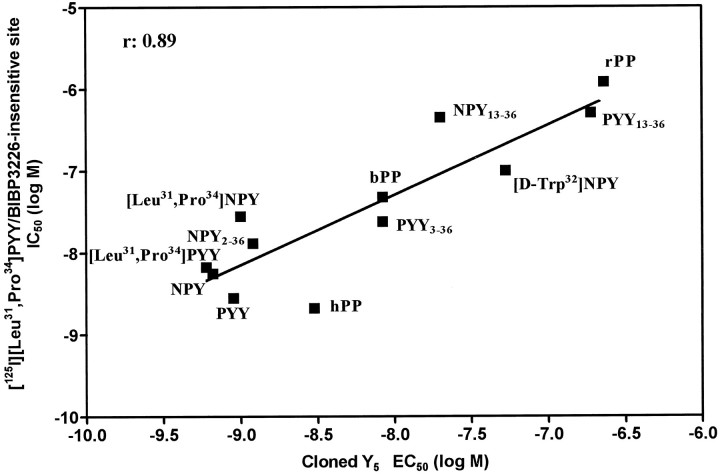
To characterize precisely the pharmacological profile of BIBP3226-insensitive [125I][Leu31,Pro34]PYY binding sites, we performed a series of experiments in the presence of a saturating concentration (1 μm) of BIBP3226. Under such experimental conditions, hPP demonstrated the highest affinity, whereas rPP and aPP were much weaker. This is a key characteristic of the recently cloned Y5 receptor (Gerald et al., 1996; Hu et al., 1996). Moreover, the long C-terminal fragments NPY2–36 and PYY3–36 potently competed for BIBP3226-resistant [125I][Leu31,Pro34]PYY sites, whereas shorter fragments such NPY13–36, NPY18–36, and PYY13–36 did not, again this being similar to the ligand selectivity profile of the cloned Y5 receptor expressed in HEK 293 (Gerald et al., 1995) or COS7 (Hu et al., 1996) cell lines. Additionally, the analog [d-Trp32]NPY demonstrated similar affinities (45 and 100 nm) in the Y5-transfected cells (Gerald et al., 1996) and in our preparation. Moreover, the affinity of GW1229 to compete against [125I][Leu31,Pro34]PYY/BIBP3226-insensitive sites is similar to that reported for the cloned Y5receptor subtype (Schober et al., 1998). In fact, a highly positive correlation (r = 0.89; p < 0.001) is found between the ligand selectivity profile of the cloned and transfected Y5 receptor (Gerald et al., 1996) and [125I][Leu31,Pro34]PYY/BIBP3226-insensitive sites in the rat brain (Fig. 5). Taken together, these data strongly suggest that the binding sites under study represent the genuine protein product of the expression and transduction of the Y5 receptor gene in the rat brain.
Fig. 5.
Comparative affinities and potencies of various analogs of the NPY family for BIBP3226-resistant [125I][Leu31,Pro34]PYY sites (see Table 2) and results reported for the cloned Y5receptor subtype expressed in HEK 293 cells and assessed for cAMP production (Gerald et al., 1996).
Receptor autoradiography confirmed the existence and unique distribution of the putative Y5/BIBP3226-insensitive [125I][Leu31,Pro34]PYY sites in the rat brain. Regions such as the external plexiform layer of the olfactory bulb, the lateral septum, the anteroventral thalamic nucleus, the CA3 subfield of the ventral hippocampus, the nucleus tractus solitarius, and the area postrema were particularly enriched with specific BIBP3226-insensitive [125I][Leu31,Pro34]PYY sites. Most hypothalamic nuclei, including the paraventricular nucleus and the perifornical area, were not enriched with [125I][Leu31,Pro34]PYY/BIBP3226-insensitive sites. These hypothalamic nuclei have been proposed to mediate the potent effect of NPY on food intake-related behaviors (Stanley et al., 1984, 1993), and the Y5 receptor has been proposed to be the “food intake” receptor subtype (Gerald et al., 1996; Hu et al., 1996); an in situ hybridization study that used an oligoprobe revealed abundant NPY Y5 mRNA signals in this area (Gerald et al., 1996), whereas Y5 receptor antisense oligonucleotide-treated rats had reduced appetite (Schaffhauser et al., 1997). Additionally, CGP 71683A, a putative Y5 antagonist, was able to block significantly the effect of NPY on food intake (Hofbauer et al., 1997). Taken together, these results support the hypothesis that the Y5 receptor subtype mediates NPY-induced food intake. In that context it is rather surprising that significant levels of [125I][Leu31,Pro34]PYY/BIBP3226-insensitive sites were not detected in various nuclei of the hypothalamus, including the paraventricular and perifornical nuclei. This apparent discrepancy may be related to (1) a low efficiency in the translation of the Y5 mRNA into its protein; (2) the fact that only a small proportion of hypothalamic neurons indeed expresses and translates the Y5 message into its related protein, a higher resolution technique (electron microscopy) being required to visualize properly the binding signals; and (3) the fact that NPY levels in the hypothalamus are very high. Hence, on neuronal stimulation, high amounts of NPY are released and are sufficient to saturate the low levels of receptors available to elicit a full functional response. This hypothesis is supported by the fact that only low levels of Y1, Y2 (Inui et al., 1989;Lynch et al., 1989; Dumont et al., 1990, 1993; Martel et al., 1990;Aicher, 1991; Gehlert et al., 1992; Larsen et al., 1993), and now Y5 (this study) receptors apparently are expressed in the hypothalamus, even if this brain structure is involved in many NPY-related effects (for review, see Dumont et al., 1992; Kalra and Crowley, 1992; Wahlestedt and Reis, 1993; Heilig and Widerlov, 1995;Munglani et al., 1996). Finally, (4) the translated Y5receptor protein may be located on terminals of projection neurons originating from hypothalamic nuclei. Further investigations that use high-resolution anatomical methods and Y5 receptor antibodies (not available yet) will be required to verify these various possibilities.
Alternatively, it may be that the Y5 receptor subtype is not involved uniquely in food intake behaviors or does act via nonhypothalamic structures to modulate appetite. Already, several recent studies have questioned the role of the Y5 receptor in food intake. For example, Small et al. (1997) reported that the purported Y5 agonist [d-Trp32]NPY was unable to stimulate food intake while being effective to facilitate adrenocorticotropic hormone release. Moreover, L-152804, a molecule reported to act as an orally active Y5 antagonist, failed to block normal or NPY-induced feeding behaviors in rodents (Kanatani et al., 1997). In contrast, numerous laboratories recently have suggested the involvement of the Y1 receptor subtype in NPY-induced feeding behaviors, mostly on the basis of data obtained with antagonists such as BIBP3226, BIBO3304, GW1229, GI264879, and LY353485 (Kanatani et al., 1996; Daniels et al., 1997; Doods et al., 1997; Iyengar et al., 1997;Kalra, 1997; Li et al., 1997) (but see Gerald et al., 1996; Haynes et al., 1997). In fact, it well may be that the potent action of NPY and congeners on appetite involved at least two classes of NPY receptors, namely the Y1 and Y5 subtypes. Additional yet-to-be-characterized fully NPY-related receptors also may be implicated, as suggested by a few recent anatomical (Trinh et al., 1996) and behavioral (O’Shea et al., 1997) studies. Further investigations that use series of potent and fully selective agonists and antagonists (not yet available in most cases) will be necessary to establish fully the role of each receptor subtype in mediating the effects of NPY on food intake.
In summary, our results demonstrate the presence and discrete distribution in the rat brain of a population of sites labeled with [125I][Leu31,Pro34]PYY that are resistant to the Y1 antagonist BIBP3226. One of the major characteristics of this receptor population is its high affinity for hPP, but not for rPP. In fact, the ligand selectivity profile of various NPY, PYY, and PP analogs and fragments against [125I][Leu31,Pro34]PYY/BIBP3226-insensitive sites is most similar to that reported for the cloned Y5receptor subtype. To our knowledge, these results, together with the autoradiographic data, represent the first demonstration of the expression of the Y5 receptor protein in the brain.
Footnotes
This study was supported by the Medical Research Council of Canada. R.Q. and A.F. are Chercheur-Boursiers of the Fonds de la Recherche en Santé du Québec.
Correspondence should be addressed to Dr. Rémi Quirion, Douglas Hospital Research Center, 6875 LaSalle Boulevard, Verdun, Québec, Canada H4H 1R3.
REFERENCES
- 1.Abounader R, Villemure JG, Hamel E. Characterization of neuropeptide Y (NPY) receptors in human cerebral arteries with selective agonists and the new Y1 antagonist BIBP3226. Br J Pharmacol. 1995;116:2245–2250. doi: 10.1111/j.1476-5381.1995.tb15060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aicher SA, Springston M, Berger SB, Reis DJ, Wahlestedt C. Receptor-selective analogs demonstrate NPY/PYY receptor heterogeneity in rat brain. Neurosci Lett. 1991;130:32–36. doi: 10.1016/0304-3940(91)90220-n. [DOI] [PubMed] [Google Scholar]
- 3.Bard JA, Walker MW, Branchek TA, Weinshank RL. Cloning and functional expression of a human Y4 subtype receptor for pancreatic polypeptide, neuropeptide Y, and peptide YY. J Biol Chem. 1995;270:26762–26765. doi: 10.1074/jbc.270.45.26762. [DOI] [PubMed] [Google Scholar]
- 4.Bitran M, Daniels AJ, Boric MP. GW1229, a novel neuropeptide Y Y1 receptor antagonist, inhibits the vasoconstrictor effect of neuropeptide Y in the hamster microcirculation. Eur J Pharmacol. 1997;319:43–47. doi: 10.1016/s0014-2999(96)00832-1. [DOI] [PubMed] [Google Scholar]
- 5.Blomqvist AG, Herzog H. Y-receptor subtypes—how many more? Trends Neurosci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- 6.Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O’Donohue TL. The anatomy of neuropeptide Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- 7.Colmers WF, Bleakman D. Effects of neuropeptide Y on the electrical properties of neurons. Trends Neurosci. 1994;17:373–379. doi: 10.1016/0166-2236(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 8.Daniels AJ, Matthews JE, Slepetis RJ, Jansen M, Viveros OH, Tadepalli A, Harrington W, Heyer D, Landavazo A, Leban JJ, Spaltenstein A. High-affinity neuropeptide Y receptor antagonists. Proc Natl Acad Sci USA. 1995;92:9067–9071. doi: 10.1073/pnas.92.20.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniels AJ, Matthews J, Chance WT, Grizzle M, Heyer D. Food intake inhibition and body weight loss in rats treated with GI264879A and NPY-Y1 receptor antagonist. Regul Pept. 1997;71:212. doi: 10.1016/s0196-9781(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 10.DeQuidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system. I. Radioimmunoassay and chromatographic characterisation. Neuroscience. 1986a;18:527–543. doi: 10.1016/0306-4522(86)90056-4. [DOI] [PubMed] [Google Scholar]
- 11.DeQuidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system. II. Immunohistochemical analysis. Neuroscience. 1986b;18:545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- 12.Doods HN, Wienen W, Entzeroth M, Rudolf K, Eberlein W, Engel W, Wieland HA. Pharmacological characterization of the selective nonpeptide neuropeptide Y Y1 receptor antagonist BIBP3226. J Pharmacol Exp Ther. 1995;275:136–142. [PubMed] [Google Scholar]
- 13.Doods HN, Engel W, Wieland HA, Eberlein W, Rudolf K, Judge M, Hamilton BS. Effects of the novel Y1 antagonist BIBO3304 on feeding in rodents. Regul Pept. 1997;71:212. [Google Scholar]
- 14.Dumont Y, Fournier A, St-Pierre S, Schwartz TW, Quirion R. Differential distribution of neuropeptide Y1 and Y2 receptors in the rat brain. Eur J Pharmacol. 1990;191:501–503. doi: 10.1016/0014-2999(90)94189-5. [DOI] [PubMed] [Google Scholar]
- 15.Dumont Y, Martel JC, Fournier A, St-Pierre S, Quirion R. Neuropeptide Y and neuropeptide Y receptor subtypes in brain and peripheral tissues. Prog Neurobiol. 1992;38:125–167. doi: 10.1016/0301-0082(92)90038-g. [DOI] [PubMed] [Google Scholar]
- 16.Dumont Y, Fournier A, St-Pierre S, Quirion R. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in rat brain. J Neurosci. 1993;13:73–86. doi: 10.1523/JNEUROSCI.13-01-00073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumont Y, Fournier A, St-Pierre S, Quirion R. Characterization of neuropeptide Y binding sites in rat brain membrane preparations using [125I][Leu31,Pro34]PYY and [125I]PYY3–36 as selective Y1 and Y2 radioligands. J Pharmacol Exp Ther. 1995;272:673–680. [PubMed] [Google Scholar]
- 18.Dumont Y, Fournier A, St-Pierre S, Quirion R. Autoradiographic distribution of [125I][Leu31,Pro34]PYY and [125I]PYY3–36 binding sites in the rat brain evaluated with two newly developed Y1 and Y2 receptor radioligands. Synapse. 1996a;22:139–158. doi: 10.1002/(SICI)1098-2396(199602)22:2<139::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Dumont Y, St-Pierre JA, Quirion R. Comparative autoradiographic distribution of neuropeptide Y Y1 receptors visualized with the Y1 receptor agonist [125I][Leu31,Pro34]PYY and the non-peptide antagonist [3H]BIBP3226. NeuroReport. 1996b;7:901–904. doi: 10.1097/00001756-199603220-00013. [DOI] [PubMed] [Google Scholar]
- 20.Entzeroth M, Braunger H, Eberlein W, Engel W, Rudolf K, Wienen W, Wieland HA, Willim KD, Doods HN. Labeling of neuropeptide Y receptors in SK-N-MC cells using the novel nonpeptide Y1 receptor-selective antagonist [3H]BIBP3226. Eur J Pharmacol. 1995;278:239–242. doi: 10.1016/0014-2999(95)00161-d. [DOI] [PubMed] [Google Scholar]
- 21.Eva C, Keinanen K, Monyer H, Seeburg P, Sprengel R. Molecular cloning of a novel G-protein-coupled receptor that may belong to the neuropeptide receptor family. FEBS Lett. 1990;271:81–84. doi: 10.1016/0014-5793(90)80377-u. [DOI] [PubMed] [Google Scholar]
- 22.Forest M, Martel JC, St-Pierre S, Quirion R, Fournier A. Structural study of the N-terminal segment of neuropeptide tyrosine. J Med Chem. 1990;33:1615–1619. doi: 10.1021/jm00168a014. [DOI] [PubMed] [Google Scholar]
- 23.Gehlert DR. Subtypes of receptors for NPY: implications for the targeting of therapeutics. Life Sci. 1994;55:551–562. doi: 10.1016/0024-3205(94)00481-1. [DOI] [PubMed] [Google Scholar]
- 24.Gehlert DR, Gackenheimer SL, Schober DA. [Leu31,Pro34] neuropeptide Y identifies a subtype of 125I-labelled peptide YY binding sites in the rat brain. Neurochem Int. 1992;21:45–67. doi: 10.1016/0197-0186(92)90067-2. [DOI] [PubMed] [Google Scholar]
- 25.Gehlert DR, Schober DA, Beavers L, Gadski R, Hoffman JA, Smiley DL, Chance RE, Lundell I, Larhammar D. Characterization of the peptide binding requirement for the cloned human pancreatic polypeptide-preferring receptor. Mol Pharmacol. 1996a;50:112–118. [PubMed] [Google Scholar]
- 26.Gehlert DR, Gackenheimer SL, Schober DA, Beavers L, Gadski R, Burnett JP, Mayne N, Lundell I, Larhammar D. The neuropeptide Y Y1 receptor selective radioligand, [125I][Leu31,Pro34] peptide YY, is also a high-affinity radioligand for human pancreatic polypeptide receptors. Eur J Pharmacol. 1996b;318:485–490. doi: 10.1016/s0014-2999(96)00797-2. [DOI] [PubMed] [Google Scholar]
- 27.Gehlert DR, Schober DA, Gackenheimer SL, Beavers L, Gadski R, Lundell I, Larhammar D. [125I]Leu31,Pro34-PYY is a high-affinity radioligand for rat PP1/Y4 and Y1 receptors: evidence for heterogeneity of pancreatic polypeptide receptors. Peptides. 1997;18:397–401. doi: 10.1016/s0196-9781(96)00346-4. [DOI] [PubMed] [Google Scholar]
- 28.Gerald C, Walker MW, Vaysse PJJ, Branchek TA, Weinshank RL. Expression, cloning, and pharmacological characterization of a human hippocampal neuropeptide Y/peptide YY Y2 receptor subtype. J Biol Chem. 1995;270:26758–26761. doi: 10.1074/jbc.270.45.26758. [DOI] [PubMed] [Google Scholar]
- 29.Gerald C, Walker MW, Criscione L, Gustafson EL, Batzl-Hartmann C, Smith KE, Vaysse P, Durkin MM, Laz TM, Linemeyer DL, Schaffhauser AO, Whitebread S, Hofbauer KG, Taber RI, Branchek TA, Weinshank RL. A receptor subtype involved in neuropeptide Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- 30.Gregor P, Millham ML, Feng Y, Decarr LB, McCaleb ML, Cornfield LJ. Cloning and characterization of a novel receptor to pancreatic polypeptide, a member of the neuropeptide Y receptor family. FEBS Lett. 1996;381:58–62. doi: 10.1016/0014-5793(96)00067-1. [DOI] [PubMed] [Google Scholar]
- 31.Grundemar L, Sheikh SP, Wahlestedt C. Characterization of receptor types for neuropeptide Y and related peptides. In: Colmers WF, Wahlestedt C, editors. The biology of neuropeptide Y and related peptides. Humana; Totowa, NJ: 1993. pp. 197–240. [Google Scholar]
- 32.Haynes AC, Arch JRS, Wilson S, McClue S, Buckingham RE. The NPY receptor mediating food intake in rats: is it Y5? Regul Pept. 1997;71:221. [Google Scholar]
- 33.Heilig M, Widerlov E. Neurobiology and clinical aspects of neuropeptide Y. Crit Rev Neurobiol. 1995;9:115–136. [PubMed] [Google Scholar]
- 34.Herzog H, Hort YJ, Ball HJ, Hayes G, Shine J, Selbie LA. Cloned human neuropeptide Y receptor couples to two different second messenger systems. Proc Natl Acad Sci USA. 1992;89:5794–5798. doi: 10.1073/pnas.89.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofbauer KG, Schaffhauser AO, Batzl-Hartmann C, Stricker-Krongrad A, Whitebread S, Cumin F, Rigollier P, Yamaguchi Y, Chiesi M, Levens N, Schilling W, Walker MW, Gerald C, Rueeger H, Criscione L. Antisense oligonucleotides targeted against the NPY Y5 receptor and a selective Y5 receptor antagonist inhibit food intake in rodents. Regul Pept. 1997;71:211. [Google Scholar]
- 36.Hu Y, Bloomquist BT, Cronfield LJ, DeCarr LB, Flores-Riveros JR, Friedman L, Jiang P, Lewis-Higgins L, Sadlowski Y, Schaefer J, Velazquez N, McCaleb ML. Identification of a novel hypothalamic neuropeptide Y receptor associated with feeding behavior. J Biol Chem. 1996;271:26315–26319. [PubMed] [Google Scholar]
- 37.Inui A, Okita M, Inoue T, Sakatani N, Oya M, Morioka H, Shii K, Yokono K, Mizuno N, Baba S. Characterization of peptide YY receptors in the brain. Endocrinology. 1989;124:402–409. doi: 10.1210/endo-124-1-402. [DOI] [PubMed] [Google Scholar]
- 38.Iyengar S, Simmons RMA, Li DL, Cantrell B, Lobb KL, Schober D, Helton D, Kallman MJ, Bruns RF, Calligano DO, Hipskind PA, Zimmerman DM, Gehlert DR. Effect of new, structurally novel, neuropeptide Y antagonists on NPY-induced feeding. Soc Neurosci Abstr. 1997;23:1766. [Google Scholar]
- 39.Jacques D, Cadieux A, Dumont Y, Quirion R. Apparent affinity and potency of BIBP3226, a non-peptide neuropeptide Y receptor antagonist, on purported Y1, Y2, and Y3 receptors. Eur J Pharmacol. 1995;278:R3–R5. doi: 10.1016/0014-2999(95)00179-o. [DOI] [PubMed] [Google Scholar]
- 40.Jacques D, Dumont Y, Fournier A, Quirion R. Characterization of neuropeptide Y receptor subtypes in the normal human brain, including the hypothalamus. Neuroscience. 1997;79:129–148. doi: 10.1016/s0306-4522(96)00639-2. [DOI] [PubMed] [Google Scholar]
- 41.Kalra SP. NPY—a novel pleiotropic peptide signal in the CNS. Regul Pept. 1997;71:209. [Google Scholar]
- 42.Kalra SP, Crowley WR. Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormones. Front Neuroendocrinol. 1992;13:1–46. [PubMed] [Google Scholar]
- 43.Kanatani A, Iwaasa H, Isahi S, Tanaka T, Ozaki S, Ihara M. Potent neuropeptide Y Y1 receptor antagonist, 1229U91: blockade of neuropeptide Y-induced and physiological food intake. Endocrinology. 1996;137:3177–3182. doi: 10.1210/endo.137.8.8754736. [DOI] [PubMed] [Google Scholar]
- 44.Kanatani A, Fukami T, Fukuroda T, Iwaasa H, MacNeil D, Van der Poeg L, Ihara M. Y5 receptors are not involved in physiologically relevant feeding in rodents. Regul Pept. 1997;71:212. doi: 10.1016/s0167-0115(98)00096-2. [DOI] [PubMed] [Google Scholar]
- 45.Klapstein GJ, Colmers WF. Neuropeptide Y suppresses epileptiform activity in rat hippocampus in vitro. J Neurophysiol. 1997;78:1651–1661. doi: 10.1152/jn.1997.78.3.1651. [DOI] [PubMed] [Google Scholar]
- 46.Larhammar D, Blomqvist AG, Yee F, Jazin E, Yoo H, Wahlestedt C. Cloning and functional expression of a human neuropeptide Y/peptide YY receptor of the Y1 type. J Biol Chem. 1992;267:10935–10938. [PubMed] [Google Scholar]
- 47.Larsen PJ, Sheikh SP, Jakobsen CR, Schwartz TW, Mikkelsen JD. Regional distribution of putative NPY Y1 receptors and neurons expressing Y1 mRNA in forebrain areas of the rat central nervous system. Eur J Neurosci. 1993;5:1622–1637. doi: 10.1111/j.1460-9568.1993.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 48.Li DL, Simmons RMA, Bruns RF, Gehlert DR, Hipskind PA, Kallman MJ, Helton D, Aaron EB, Lobb KL, Schober D, Iyengar S. Effect of the neuropeptide Y Y1 antagonists BIBP3226 and 1229U91 on NPY-induced feeding in CD-1 mice. Soc Neurosci Abstr. 1997;23:1776. [Google Scholar]
- 49.Lundberg JM, Modin A, Malmstrom RE. Recent developments with neuropeptide Y receptor antagonists. Trends Pharmacol. 1996;17:301–304. [PubMed] [Google Scholar]
- 50.Lundell I, Blomqvist AG, Berglund MM, Schober DA, Johnson D, Statnick M, Gadski RA, Gehlert DR, Larhammar D. Cloning of a human receptor of the NPY receptor family with high affinity for pancreatic polypeptide and peptide YY. J Biol Chem. 1995;270:29123–29128. doi: 10.1074/jbc.270.49.29123. [DOI] [PubMed] [Google Scholar]
- 51.Lynch DR, Walker MW, Miller RJ, Snyder SH. Neuropeptide Y receptor binding sites in rat brain: differential localization with [125I] peptide YY and [125] neuropeptide Y imply receptor heterogeneity. J Neurosci. 1989;9:2607–2619. doi: 10.1523/JNEUROSCI.09-08-02607.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martel JC, Fournier A, St-Pierre S, Quirion R. Quantitative autoradiographic distribution of [125I] Bolton–Hunter neuropeptide Y binding sites in rat brain. Comparison with [125I] peptide YY receptor sites. Neuroscience. 1990;36:225–283. doi: 10.1016/0306-4522(90)90367-d. [DOI] [PubMed] [Google Scholar]
- 53.Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- 54.Munglani R, Hudspith MJ, Hunt SP. The therapeutic potential of neuropeptide Y: analgesic, anxiolytic, and antihypertensive. Drug. 1996;52:371–389. doi: 10.2165/00003495-199652030-00004. [DOI] [PubMed] [Google Scholar]
- 55.O’Shea D, Morgan DGA, Meeran K, Edwards CMB, Turton MD, Choi SJ, Heath MM, Gunn I, Taylor GM, Howard JK, Bloom CI, Small CJ, Haddo O, Ma JJ, Callinan W, Smith DM, Ghatei MA, Bloom SR. Neuropeptide Y induced feeding in the rat is mediated by a novel receptor. Endocrinology. 1997;138:196–202. doi: 10.1210/endo.138.1.4899. [DOI] [PubMed] [Google Scholar]
- 56.Rose PM, Fernandez P, Lynch JS, Frazier ST, Fisher SM, Kodukula K, Kienzle B, Seetha R. Cloning and expression of a cDNA encoding a human type 2 neuropeptide Y receptor. J Biol Chem. 1995;270:22661–22664. doi: 10.1074/jbc.270.39.22661. [DOI] [PubMed] [Google Scholar]
- 57.Rudolf K, Eberlein W, Engel W, Wieland HA, Willim KD, Entzeroth M, Wienen W, Beck-Sickinger AG, Doods HN. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur J Pharmacol. 1994;271:R11–R13. doi: 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- 58.Schaffhauser AO, Stricker-Krongrad A, Brunner L, Cumin F, Gerald C, Whitebread S, Criscione L, Hofbauer MW. Inhibition of food intake by neuropeptide Y Y5 receptor antisense oligonucleotides. Diabetes. 1997;46:1792–1798. doi: 10.2337/diab.46.11.1792. [DOI] [PubMed] [Google Scholar]
- 59.Schober DA, Van Abbema AM, Smiley DL, Bruns RF, Gehlert DR. The neuropeptide Y Y1 antagonist, 1229U91, a potent agonist for the human pancreatic polypeptide-preferring (NPY Y4) receptor. Peptides. 1998;19:537–542. doi: 10.1016/s0196-9781(97)00455-5. [DOI] [PubMed] [Google Scholar]
- 60.Serradeil-Le Gal C, Valette G, Rouby PE, Pellet A, Oury-Donat F, Brossard G, Lespy L, Marty E, Neliat G, deCoitet P, Maffarand JP, Le Fur G. SR 120819A, an orally active and selective neuropeptide Y Y1 receptor antagonist. FEBS Lett. 1995;362:192–196. doi: 10.1016/0014-5793(95)00230-7. [DOI] [PubMed] [Google Scholar]
- 61.Small CJ, Morgan DGA, Meeran K, Heath MM, Gunn I, Edwards CMB, Gardiner J, Taylor GM, Hurley JD, Rossi M, Goldstone AP, O’Shea D, Smith DM, Ghatei MA, Bloom SR. Peptide analogue studies on the hypothalamic neuropeptide Y receptor mediating pituitary adrenocorticotropic hormone release. Proc Natl Acad Sci USA. 1997;94:11686–11691. doi: 10.1073/pnas.94.21.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 63.Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF. The perifornical area: the major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s). Brain Res. 1993;604:304–317. doi: 10.1016/0006-8993(93)90382-w. [DOI] [PubMed] [Google Scholar]
- 64.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y—a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–662. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 65.Trinh T, Dumont Y, Quirion R. High levels of specific neuropeptide Y/pancreatic polypeptide receptors in the rat hypothalamus and brainstem. Eur J Pharmacol. 1996;318:R1–R3. doi: 10.1016/s0014-2999(96)00863-1. [DOI] [PubMed] [Google Scholar]
- 66.Wahlestedt C, Reis DJ. Neuropeptide Y-related peptides and their receptors: are the receptors potential therapeutic drug targets? Annu Rev Pharmacol Toxicol. 1993;32:309–352. doi: 10.1146/annurev.pa.33.040193.001521. [DOI] [PubMed] [Google Scholar]
- 67.Weinberg DH, Sirinathsinghji DJS, Tan CP, Shiao LL, Morin N, Rigby MR, Heavens RH, Rapoport DR, Bayne ML, Cascieri MA, Strader CD, Linemeyer DL, MacNeil DJ. Cloning and expression of a novel neuropeptide Y receptor. J Biol Chem. 1996;271:16435–16438. doi: 10.1074/jbc.271.28.16435. [DOI] [PubMed] [Google Scholar]
- 68.Widdowson PS. Regionally selective down-regulation of NPY receptor subtypes in the obese Zucker rat. Relationship to the Y5 “feeding” receptor. Brain Res. 1997;758:17–25. doi: 10.1016/s0006-8993(97)00160-1. [DOI] [PubMed] [Google Scholar]
- 69.Widdowson PS, Upton R, Henderson L, Buckingham R, Wilson S, Williams G. Reciprocal regional changes in brain NPY receptor density during dietary restriction and dietary-induced obesity in the rat. Brain Res. 1997;774:1–10. doi: 10.1016/s0006-8993(97)81680-0. [DOI] [PubMed] [Google Scholar]
- 70.Wieland HA, Willim KD, Entzeroth M, Wienen W, Rudolf K, Eberlein W, Engel W, Doods HN. Subtype selectivity and antagonistic profile of the nonpeptide Y1 receptor antagonist BIBP3226. J Pharmacol Exp Ther. 1995;275:143–149. [PubMed] [Google Scholar]
- 71.Whitcomb DC, Puccio AM, Vigna SR, Taylor IL, Hoffman GE. Distribution of pancreatic polypeptide receptors in the rat brain. Brain Res. 1997;760:137–149. doi: 10.1016/s0006-8993(97)00295-3. [DOI] [PubMed] [Google Scholar]