Abstract
We have studied the role of actin fiber assembly on calcium signaling in astrocytes. We found that (1) after astrocytes have been placed in culture, it takes several hours for organization of the definitive actin cytoskeleton. Actin organization and the number of cells engaged in calcium signaling increased in parallel. (2) Disruption of the actin cytoskeleton attenuated the calcium wave propagation; cytochalasin D treatment reduced the number of astrocytes engaged in calcium signaling. (3) Propagation of calcium waves depends on cytoskeletal function; inhibition of myosin light chain kinase suppressed wave activity. (4) Astrocytic calcium signaling is mediated by release of ATP and purinergic receptor stimulation, because agents that interfere with this cascade attenuated or reduced calcium signaling. Because purinergic receptors are fully functional shortly after plating and not affected by cytochalasin D, these observations indicate that cytoskeleton organization is a prerequisite for interastrocytic calcium signaling mediated by release of ATP.
Keywords: cytochalasin, actin, myosin, calcium waves, glioma cells
Calcium waves are slowly propagating increments in cytosolic calcium levels among gap junction-coupled cells. These long-distance waves may represent a mechanism by which multicellular tissues coordinate responses to stimuli. In brain, intercellular calcium waves occur among astrocytes and can evoke prolonged elevation of calcium levels in nearby neurons. Hence, astrocytes can modulate the calcium levels and thereby the excitability of nerve cells and are thus an active participant in information processing (Nedergaard, 1994; Parpura et al., 1994, Pasti et al., 1997;Araque et al., 1998; Newman and Zahs, 1998).
Calcium waves are traditionally believed to be transferred from cell to cell by gap junction-mediated diffusion of Ca2+ or IP3 (Sanderson et al., 1994), but an extracellular component may also participate in astrocytic signaling (Hassinger et al., 1996). Cell lines deficient in gap junctions become capable of propagating intercellular calcium signals only after transfection and overexpression of gap junction proteins (Charles et al., 1992; Elfgang et al., 1995; Toyofuku et al., 1998). The present study was prompted by the observation, originally reported by Naus et al. (1992), that forced expression of gap junctions results not only in functional coupling but also in distinct morphological alterations; poorly coupled cell lines are typically composed of compact cells with few cellular contacts, whereas the same cells reorganize into flat epithelioid layers after expression of gap junctions. Thus, acquisition of signaling capability and transformation of phenotype occur in parallel. Likewise, primary cells that transmit robust calcium waves, such as astrocytes and hepatocytes, are organized as flat confluent monolayers in cultures (Cornell-Bell et al., 1990). Because strict cellular organization is critical for an array of cellular signaling mechanisms, such as synaptic function (Prekeris et al., 1996) and receptor-mediated mitogenic signaling (Bohmer et al., 1996), we asked whether gap junction-coupled cells maintained the ability to propagate calcium waves when cellular organization was disrupted. We found that an intact actin cytoskeleton is required for the propagation of astrocytic calcium waves. Furthermore, inhibition of myosin light chain kinase reduced the number of cells engaged in calcium signaling, suggesting that wave propagation depends on cytoskeletal function.
MATERIALS AND METHODS
Astrocytic culture. The procedure for cultured astrocytes has been described in detail elsewhere (Nedergaard et al., 1991; Cotrina et al., 1998a). Briefly, newborn (1 d postnatal) or prenatal (embryonic day 17) rat brains were trypsinized, mechanically triturated, and plated on uncoated glass coverslips or on plastic dishes (1 and 5 × 105 cells/ml, respectively). Cultures were maintained in DMEM-F12 (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (Atlanta Biologica), penicillin and streptomycin (Life Technologies), and 8% glucose (Sigma, St. Louis, MO) in a 5% CO2 humidified incubator at 37°C. Medium was changed every 2–3 d, and the cultures were replated when confluent. Experiments were performed after 10–14 din vitro.
Mice with a null mutation of connexin 43. Heterozygotes of the connexin 43 (Cx43) knock-out line from Jackson Laboratory were used. Pregnant females were killed at 18–20 d after gestation, and the embryonic brains were cultured as described above. To identify homozygotes, heterozygotes, and wild type, we used PCR for amplifying tail-blood genomic DNA flanking the null deletion, according to the protocol of Jackson Laboratory. Also, immunohistochemical mapping of the extent of Cx43 was mapped in conjunction with dye transfer assays. The astrocytes from homozygotes and wild-type mice used in this study were from three different litters.
Actin staining and immunocytochemistry. Cells were plated on 12 mm uncoated coverglasses (0.5–1 × 105cells/ml) and fixed 1–3 d later with 4% paraformaldehyde for 10 min at room temperature. For actin staining, cells were permeabilized with 0.1% Triton X-100 and incubated with Texas Red–phalloidin (Molecular Probes, Eugene, OR) for 30 min. After several washes in PBS, coverslips were mounted in glycerol and examined by confocal microscopy (MRC1000; Bio-Rad, Hercules, CA). For immunodetection of Cx43 and glial fibrillary acidic protein (GFAP), tubulin or myosin, cultures were permeabilized with 0.1% Triton X-100 and blocked with 10% normal goat serum. A polyclonal antibody against Cx43 (1:500) (kindly supplied by Dr. Lau, University of Hawaii), a monoclonal antibody against GFAP (1:100) (GAS; Sigma), a monoclonal antibody against α-tubulin (1:50) (Boehringer-Mannheim, Indianapolis, IN), or a monoclonal antibody against myosin light chain (1:50) (MY-21; Sigma) was applied for 2 hr at room temperature or overnight at 4°C. After three washes in PBS, FITC goat anti-rabbit or goat anti-mouse was applied for 1 hr at room temperature. Reaction was completed by washing with PBS several times and mounting the coverslips in Slow Fade (Molecular Probes).
Actin was quantified by analyzing astrocytic cultures stained with Texas Red–phalloidin with a 20× objective of an IX-50 inverted fluorescence microscope (Olympus). At least 250 cells per field in three different fields were counted in each culture and classified according to their pattern of actin organization. If actin was concentrated in a narrow band under the plasma membrane, the cell was classified as lacking cytoskeletal organization. If the cell contained parallel phalloidin-positive fibers, it was scored as organized. Approximately 5% of the cells could not be classified according to these criteria and were not included in the counting. Data summarize results from three independent experiments scored blindly.
Intercellular calcium waves. Confluent monolayers of astrocytes were loaded for 1 hr with 10 μm fluo-3 AM (Molecular Probes). Waves were elicited by mechanical stimulation (briefly deforming the plasma membrane with an electrode tip) using a hydraulic manipulator (MMO-220; Narishige, Tokyo, Japan). All the experiments were performed in culture medium at room temperature. Excitation was provided by the 488 nm line of the krypton–argon laser of a Bio-Rad MRC1000 confocal-scanning microscope attached to an inverted microscope (Diaphot; Nikon). Images were acquired every 6–8 sec and recorded on an optical disk (LM-D702W; Panasonic). Quantification of calcium waves was performed by measuring the maximal distance traveled by the calcium wave from the point of initiation (radius of the wave). Velocity was calculated by dividing the distance (micrometers) by the time (seconds) from the initial point of stimulation to the second round of cells that showed calcium increases. In some cases, waves were quantified by counting the number of cells in the field that showed calcium increases after stimulation and were expressed as “number of cells per wave.” Occurrence of calcium waves was defined as a 50% increase in ΔF/F (see Measurements of calcium responses to agonists) that propagated for a minimum of 50 μm in at least one direction or as more than six cells engaged in the response to mechanical stimulation (nearest neighbors). Background counts were subtracted from all measurements.
Pharmacological reagents. Stock solutions of cytochalasin D (1 mg/ml; Sigma), nocodazole (1 mg/ml; Sigma), and ML7 (15 mm; Alexis) were prepared in DMSO. 18α-Glycyrrhetinic acid (α-AGA; mm; Sigma) was prepared in ethanol. Treated cultures were incubated for 5 or 10 min with cytochalasin D at 1 μg/ml or for 2 hr with nocodazole at 10 μg/ml at room temperature. Inhibition of myosin light chain kinase activity (MLCK) was accomplished by incubating astrocytes or C6 glioma cells for 1.5 hr with the MLCK inhibitor ML7 (50 μm) (Shrode et al., 1995). After each treatment, cultures were tested for the ability to propagate calcium waves, fixed at the microscope stage, and processed for actin or tubulin staining. Control cultures were incubated with DMSO or ethanol alone (3–5 μl).
Measurement of gap junctional permeability. Gap junction function was assessed by three different assays. (1) In the scrape-loading assay (El-Fouly et al., 1987; Giaume et al., 1991), briefly, several cuts were made with a scalpel while confluent astrocytes were incubated in a calcium-free HBSS containing 10 μm carboxy-dichlorofluorescein (CDCF; Molecular Probes). The solution was removed 90 sec later by several washes. Gap junctional permeability was assessed by confocal microscopy after a 15 min incubation in HBSS. Cytochalasin at 1 μg/ml (5–10 min) or nocodazole at 10 μg/ml (2 hr) was added in selected cultures before the scrape-loading assay. The extent of coupling was evaluated as rows of CDCF-labeled cells visualized by confocal microscopy with the laser adjusted to maximal power and gain and aperture at maximal settings. (2) In the dye transfer technique (adapted from Goldberg et al., 1995), cells were loaded with CDCF diacetate for 5 min, washed, and trypsinized. After resuspension, cells were labeled with 10 μm DiIC18 (excitation, 648 nm; Molecular Probes) for 10 min and mixed with unlabeled cells at a 1:250 ratio. One hour after plating on polylysine-coated dishes, dye transfer from the CDCF- and DiIC18-labeled (donor) cells to unlabeled (recipient) cells was evaluated using confocal-scanning microscopy. The coupling index was calculated as: the fraction of donor cells transferring dye to surroundings × the mean number of receiving cells. (3) In the fluorescence recovery after photobleach procedure (FRAP) (Wade et al., 1986), the cultures were loaded with CDCF diacetate for 5 min, washed, and incubated at room temperature for an additional 20 min. A baseline fluorescence image of the culture was first obtained, and the area of laser scanning was then reduced by 10× zooming. Complete photobleach was achieved after 3–5 scans at full laser power (1 sec each). After returning the settings to initial mode, fluorescence refill was evaluated after 2 min and calculated by: %refill = (I2min −Io)/Io, whereIo is pixel intensity of CDCF fluorescence emission in the target cell before bleach andI2min is pixel intensity in the same cell 2 min after bleach.
Measurements of calcium responses to agonists. Astrocyte cultures were loaded with 10 μm fluo-3 AM for 1 hr. After fluo-3 resting signal was recorded, 25 μm ATP or 25 μm bradykinin (Sigma) was applied. Calcium responses were monitored by confocal microscopy with fixed gain and maximal aperture. For quantification, peak increments were measured, and relative changes in fluorescence (ΔF) were normalized against the baseline fluorescence (F) by ΔF/F.
RESULTS
Dynamics of actin organization in cultured brain astrocytes
Primary astrocytes undergo several morphological transitions, which is paralleled by a complex redistribution of actin after plating. Right after attachment to the substrate, astrocytes are round in shape with few cellular contacts. At this stage, actin is typically found as focal accumulations in the periphery of the cells (Fig.1). Functional coupling indicated by extensive dye diffusion is already evident 1 hr after plating (Fig. 1). During the following hours, the cells lose their initial stage of compaction, flatten, and establish contact with neighbors. Actin fibers form, and parallel arrays of phalloidin-positive fibers radiate in many different directions, both within and between individual cells. Less than 5% of astrocytes have actin fibers at 1 and 2 hr, increasing to 40–50% at 5 hr, whereas essentially all viable astrocytes have organized their cytoskeleton by 24 hr. Some of the actin fibers are very long, spanning >1 mm. These fibers are often arranged in parallel arrays (Fig. 1). Not all parts of a culture are equally well organized, but it is in these well-organized areas that intercellular calcium signaling is most strongly expressed (see below).
Fig. 1.

Left. Temporal pattern of cytoskeletal organization after replating of cortical astrocytes. Differential interference contrast and Texas Red–phalloidin staining detected by confocal microscopy were combined to visualize the cellular arrangement of actin at 1, 8, and 24 hr after plating. At 1 hr, most cells have attached to the substrate but have not yet flattened. Actin aggregates in these rounded compact cells are accumulated in a cortical mantle. At 8 hr, the majority of the cells have flattened, and actin has organized into stress fiber bundles crossing the cells in many different directions. One day after plating, actin is arranged in parallel arrays of long and continuous stress fibers. Inset, Extensive dye coupling is evident 1 hr after plating. The donor astrocytes were prelabeled with the membrane dye DiIC18(red) and CDCF (green; gap junction permeable) and mixed with unlabeled astrocytes. The extent of CDCF diffusion to neighboring recipient cells was visualized 1 hr later. The donor cells appear yellow because of the merge of red and green. In the example shown, three out of six donor cells transferred dye to surrounding cells. Scale bars, 25 μm.
To establish whether multiple astrocytes contribute to the formation of long actin fibers or whether these fibers were contained within individual elongated astrocytes, we combined actin staining with profiling of individual cells by differential interference microscopy. As illustrated in Figure2A, two neighboring astrocytes contributed to the formation of actin fibers. Another approach gave similar results; the astrocytic gap junction protein Cx43 is localized in the plasma membrane, especially at regions of cell-to-cell appositions, and may therefore outline individual astrocytes (Dermietzel et al., 1991). We found that phalloidin-stained fibers were not interrupted at the Cx43-positive cell borders (Fig.2B), supporting the notion that multiple astrocytes contribute to the construction of parallel-arrayed bundles of actin fibers (Abd-El-Basset and Fedoroff, 1994).
Fig. 2.
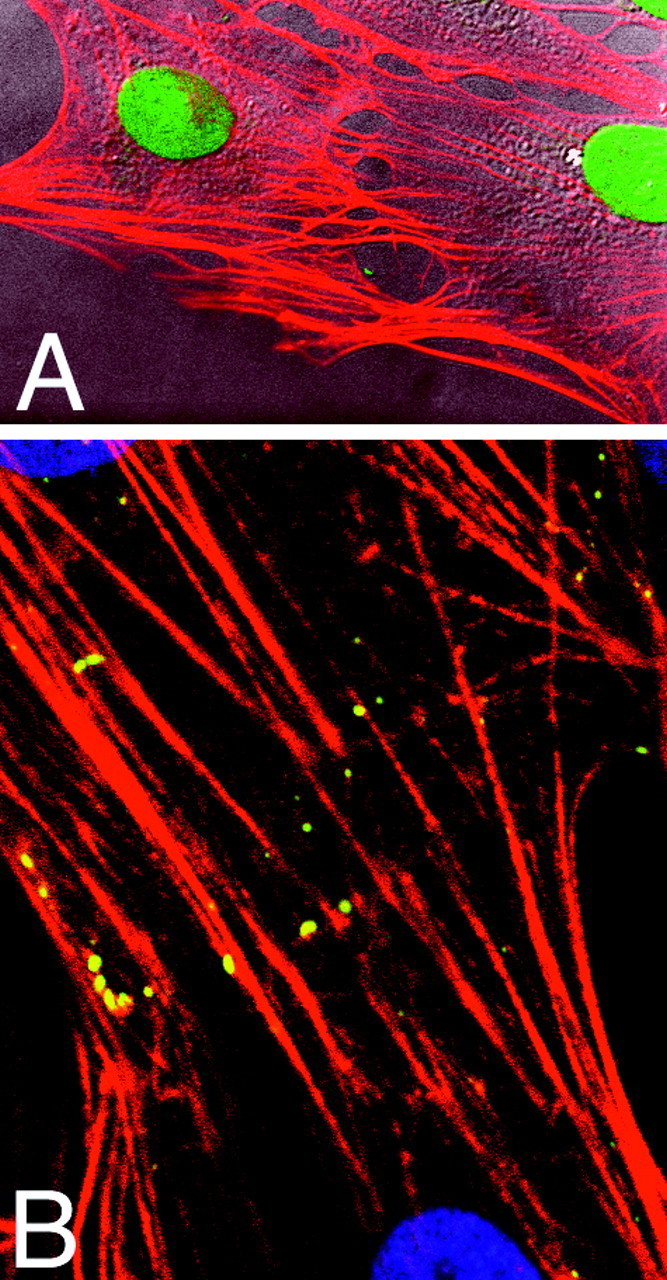
Right. Multiple astrocytes contribute to the formation of long actin fibers. Texas Red–phalloidin staining of stress fibers was combined with outlining of cell-to-cell boundaries to visualize the cellular organization of actin fibers. A, Individual cells were profiled by differential interference contrast microscopy. Two neighboring astrocytes contributed the formation of phalloidin-positive fibers. B, Staining against the astrocytic gap junctional protein Cs43 (fluorescein-labeled) is shown. Cx43-immunoreactive plaques localized in the plasma membrane specially at regions of cell-to-cell appositions. The double staining revealed that actin fibers did not respect Cx43-positive cell borders but spanned uninterrupted from cell to cell. Nuclei were counterstained with Hoechst 33342 (purple). Scale bar, 10 μm.
Astrocytes cannot propagate continuous calcium waves immediately after plating despite extensive gap junction coupling
We next determined when astrocytes became capable of propagating intercellular calcium waves. To this end, calcium waves were evoked by mechanical stimulation in fluo-3–loaded astrocytic cultures at 1, 2, 5, 8, and 24 hr after plating. Calcium wave activity was first observed 5 hr after plating, and the wave radius increased at 8 and 24 hr (Fig.3). No further increase in wave radius was observed at 48–72 hr (data not shown). At the earlier time points studied, calcium signaling was poor or absent (Fig. 3), although extensive cell coupling was established as early as 1 hr after plating (Fig. 1, inset). Presence of functional gap junctions therefore was not sufficient for expression of calcium waves. We can, however, not exclude that coupling increased as a function of time in culture.
Fig. 3.
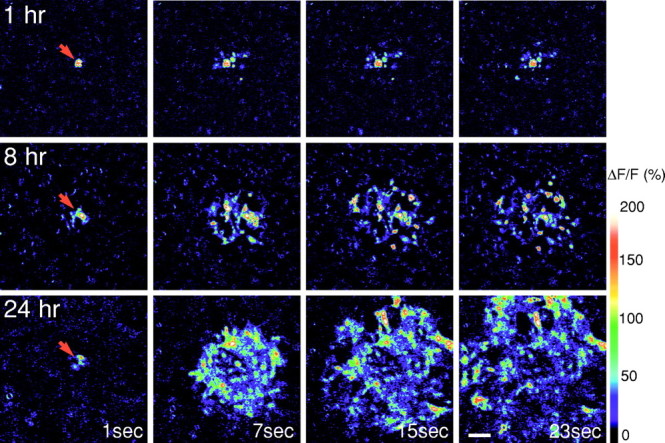
Astrocytic calcium signaling increases as a function of time in culture. Upper row, One hour after plating, mechanical stimulation triggers a robust calcium increase only in the stimulated cell (red arrows) but fails to initiate a calcium wave. Middle row, Eight hours after plating, calcium waves propagated to include several neighboring cells.Lower row, Twenty-four hours after plating, the wave included astrocytes located as far as 300 μm from the stimulation site. Confluent astrocytic cultures were loaded with fluo-3, and calcium waves were evoked by mechanical stimulation. Fluorescence intensities of fluo-3 were monitored 1, 7, 15, and 23 sec after stimulation by confocal microscopy. In all frames, background counts were subtracted. Scale bar, 50 μm.
Formation of actin stress fibers correlates with the occurrence of propagating calcium waves
The extent of calcium signaling was next correlated with cytoskeletal organization by fixing the cultures after calcium imaging and subsequent staining with Texas Red–phalloidin. Analysis of the actin distribution in these cultures revealed that the extent of wave propagation increased as a linear function of the extent of actin stress fiber formation (Fig.4A; r = 0.88). Thus, newly plated astrocytes acquired the capability for propagating calcium signal in parallel with the organization of their cytoskeleton.
Fig. 4.
A, Calcium wave activity correlates with the extent of actin fiber organization after plating of cortical astrocytes. Comparison of the maximal distance traveled by the calcium waves and the fraction of cells with stress fibers 1, 2, 5, 8, or 24 hr after plating is shown. The extent of calcium wave activity and the organization of actin stress fibers increased in parallel. A first-order regression of wave radius as a function of percent actin organization demonstrated that wave radius (micrometers) = 28.6 μm + 2.6 μm × percent actin organization; r = 0.88. The cultures were fixed immediately after calcium imaging and stained with Texas Red–phalloidin to correlate directly the activity of calcium signaling with the extent of actin organization.B, Number of cells engaged in non-contacting calcium signaling as a function of time after plating is shown. Jumping calcium signaling is most pronounced shortly after plating. C, Non-contacting calcium signaling at 2 hr is almost abolished by the ATPase apyrase (40 U/ml); p < 0.01 (Student’s t test). D, The radius of the continuous wave evoked 24 hr after plating is also reduced by apyrase (40 U/ml).
“None-contacting” calcium signaling is prominent shortly after plating but not at later time points
Immediately after plating, calcium increments were often observed in astrocytes that were not in contact with the stimulated cell. Mechanical stimulation evoked “jumping” calcium signaling in approximately five to seven cells the first few hours after plating, one to three cells at 5–8 hr, and none at 24 hr (Fig.4B). However, the number of astrocytes engaged in none-contacting calcium signaling was considerably lower than the number of cells that participated in calcium waves. In comparison, continuous calcium waves at 24 hr engaged 20–60 cells. Of interest, jumping calcium signaling was often observed far away (>80–100 μm) from the contiguous calcium wave. These observations are in accordance with those of Hassinger et al. (1996) who found that calcium waves crossed cell-free lanes (produced by scraping with a glass pipette 4–8 hr earlier).
Astrocytic calcium signaling is reduced by apyrase and suramin
In other cell types, including hepatocytes (Schlosser et al., 1996), neuroepithelial cells (Palmer et al., 1996), osteoblastic cells (Jørgensen et al., 1997), and insulin-secreting cells (Cao et al., 1997), ATP or a related compound has been identified as the diffusable messenger in intercellular signaling. Furthermore, ATP is known to elicit elevation of astrocytic Ca2+i (Kastritsis et al., 1992; Walz et al., 1994; Salter and Hicks, 1995; King et al., 1996; Centemeri et al., 1997). Accordingly, addition of the ATPase apyrase (40 U/ml) attenuated jumping calcium signaling at 2 hr (Fig. 40). Apyrase also reduced the radius of continuous calcium waves at 24 hr, suggesting that ATP participates in both types of calcium signaling (Fig.4D). Apyrase does not interfere with intracellular ATP content or resting membrane potential (data not shown). In support of the notion that ATP participates in astrocytic calcium signaling, the purinergic receptor blocker suramin (100 μm) reduced the extent of astrocytic signaling by 78% (from 27 ± 2 cells per control wave to 6 ± 1 cells per wave in the presence of suramin; n = 5).
Calcium mobilization in response to ATP is not compromised in newly plated astrocytes
Because mechanical stimulation engaged fewer astrocytes shortly after plating than at later time points and apyrase reduced the extent of signaling at both time points, a possible explanation is that the number of functional purinergic receptors is reduced or that the cascade by which receptor activation mobilizes calcium is compromised shortly after plating. However, ATP exposure (25 μm) evoked a calcium increase, expressed as ΔF/Fthat averaged 145 ± 11% 2 hr after plating (n = 18) compared with 138 ± 6% at 24 hr (n = 8). Thus, the astrocytic Ca2+ response to ATP exposure did not change as a function of time after plating.
Disruption of the cytoskeleton attenuates interastrocytic calcium signaling
We then tested whether cytoskeletal disruption in previously established cultures affected their calcium signaling. Calcium waves were evoked in the presence of cytochalasin D (CD), which binds to actin and is associated with a rapid depolymerization of stress fibers (Cooper, 1987). Whereas under control conditions calcium waves propagated at a mean velocity of 12 ± 2 μm/sec and included 25 ± 4 astrocytes (n = 9) (Fig.5, upper row), after a 5 min treatment with CD (1 μg/ml), calcium waves spread to only a few astrocytes in the field, 8 ± 1 cells per wave (p < 0.01; n = 7) (Fig. 5,middle row). A 10 min treatment with CD almost completely blocked the propagation of calcium waves (Fig. 5, bottom row). Only 6 ± 1 astrocytes (p < 0.003; n = 8) exhibited calcium increments after stimulation. The velocity of calcium waves was not significantly affected by CD treatment (12 ± 0.3 or 11 ± 1 μm/sec after 5 or 10 min treatment, respectively). To correlate the extent of calcium signaling directly with the effect of CD on the actin cytoskeleton, we used Texas-Red–phalloidin staining to visualize actin organization. In accordance with previous studies (Yahara et al., 1982), the doses of CD used here caused profound disintegration of actin fibers. Only a few intact stress fibers were found after a 5 min treatment, and essentially none were found after a 10 min CD treatment. Concurrently, actin accumulated in the periphery of the cells (Fig. 5,right).
Fig. 5.

Calcium wave activity is suppressed by cytochalasin D in established astrocytic cultures. Confluent astrocytic cultures were loaded with fluo-3, and calcium waves were evoked by mechanical stimulation. Upper row, A control untreated culture propagates a calcium wave that includes >25 cells.Middle row, Astrocytes pretreated with cytochalasin D for 5 min only propagate calcium increases to a few astrocytes.Lower row, Culture treated with cytochalasin D for 10 min was not capable of propagating a calcium wave, despite a robust calcium increment in the stimulated cell. Actin fibers were visualized in the same field (right, in red) by fixing and staining with Texas Red–phalloidin immediately after analysis of calcium signaling. Note the gradual disappearance of stress fibers in cytochalasin D-treated cultures. Fluorescence intensities of the fluo-3 probe corresponding to relative calcium levels were monitored 1, 7, 15, and 23 sec after stimulation by confocal microscopy. In all frames, background counts were subtracted. Scale bar, 10 μm.
Calcium responses remain unchanged in cytochalasin D-treated astrocytes
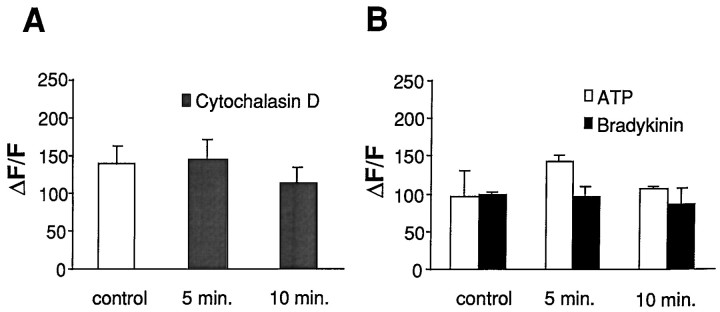
To examine the possibility that CD affected cellular calcium regulation and inhibited calcium signaling by impairing mobilization of calcium, we compared calcium responses in the directly stimulated cell in CD-treated astrocytes versus that in control. The initial increase in intracellular calcium after mechanical stimulation reflects primarily calcium influx (Sanderson et al., 1994; Venance et al., 1997). In control astrocytes, the calcium increase in the stimulated cell, expressed as ΔF/F, was 139 ± 25%, a value not significantly different from those obtained in astrocytes treated with CD for 5 min, 146 ± 25%, or 10 min, 114 ± 19% (Fig.6A). The spread or propagation of calcium waves requires release of calcium from intracellular stores (Venance et al., 1997). Also, changes in calcium levels from activation of receptors that promote IP3-dependent intracellular calcium release showed no significant difference between control calcium responses to ATP-, 97 ± 32%, and CD-treated astrocytic responses, 143 ± 9% (5 min) and 107 ± 2% (10 min). Similar results were obtained when calcium responses were evoked by bradykinin: 99 ± 2% in control versus 96 ± 14% and 86 ± 20% in astrocytes after a 5 or 10 min CD treatment, respectively (Fig. 6B). Thus, cytochalasin D does not alter astrocytic calcium responses after either mechanical stimulation or ATP and bradykinin exposures, supporting the notion that it is one of the most specific cytochalasins for actin (Cooper, 1987).
Fig. 6.
Astrocytic calcium responses to mechanical stimulation or to addition of calcium-mobilizing agonists are not suppressed by cytochalasin D treatment. A, The initial calcium response of the mechanically stimulated cell was compared in control versus cytochalasin D- (5 or 10 min) treated astrocytes.B, Relative calcium increments after application of 25 μm ATP or 25 μm bradykinin are shown. No difference between control and cytochalasin D-treated cultures were observed. Relative calcium levels are indicated as ΔF/F; n = 10.
Cytochalasin D does not alter gap junction permeability
Immunocytochemical studies localized numerous Cx43 gap junctional plaques in areas of cell-to-cell contact together with abundant actin stress fibers in control cultures (Fig.7A). Strong immunoreactivity was also present in astrocytes treated with CD for 5 min (Fig.7C), even after loss of stress fibers. Numerous, although somewhat smaller, immunoreactive plaques at cell membranes were found in cultures treated with CD for 10 min when actin stress fibers were primarily absent (Fig. 7E). The functionality of these gap junctional plaques, assessed by the scrape-loading method, was not altered by a 5 or 10 min exposure to CD (Fig.7B,D,F). Also, fluorescence recovery after the photobleach (FRAP) technique did not reveal any significant changes in gap junction coupling after cytochalasin D treatment; fluorescence recovered to 61 ± 6% (n = 14) in control cultures, whereas recoveries of 59 ± 8% (n = 14) and 56 ± 9% (n = 12) were observed in cultures treated with CD for 5 and 10 min, respectively.
Fig. 7.

Cytochalasin D does not alter the pattern of connexin 43 immunoreactivity or the extent of functional coupling. Control cultures (A, B) or cultures treated for 5 min (C, D) or 10 min (E, F) with cytochalasin D were stained with Texas-Red phalloidin and counterstained with an anti-Cx43 antibody to visualize actin stress fibers (red) and Cx43 plaques (green). Disintegration of actin stress fibers is evident in cytochalasin D-treated cultures, whereas Cx43-immunoreactive plaques remain in the plasma membrane. In sister cultures, the scrape-loading assay revealed that the gap junction plaques were functional because dye diffusion was not reduced by cytochalasin-D when compared with control cells. Scale bars:A, C, E, 10 μm;B, D, F, 50 μm.
Astrocytic calcium waves do not require microtubule organization
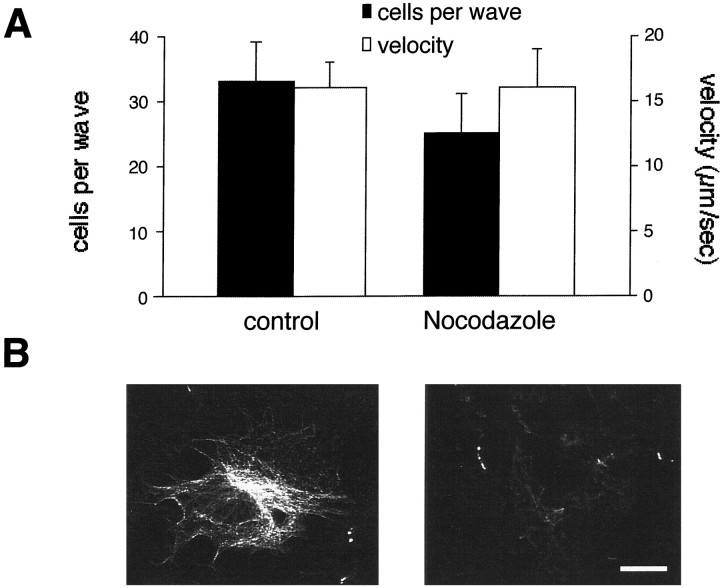
A requirement for both microfilaments and microtubules has been reported for shape change in astrocytes or growth cone movement and exocytosis in neurons (Goldman and Abramson, 1990; Shain et al., 1992;Ashton and Dolly, 1997; Gavin, 1997). In our cultures, α-tubulin immunostaining only stained a fraction of the cells. In these cells, microtubules organized as a fine meshwork of fibers throughout the entire astrocytic cell body (Fig.8B, left). When cultures were treated with nocodazole for 2 hr, tubulin staining was completely absent (Fig. 8B, right). Despite the absence of intact microtubules, nocodazole-treated cultures did not show a significant difference either in the extent of propagation or in the velocity of calcium waves (25 ± 6 cells/wave and 16 ± 3 μm/sec) when compared with control untreated astrocytes (Fig 8A, 33 ± 5 cells/wave and 16 ± 2 μm/sec; p = 0.324, Student’st test).
Fig. 8.
Nocodazole treatment does not alter either the extent or the velocity of astrocytic calcium waves. A, Calcium waves in control astrocytes were compared with waves in astrocytes pretreated for 2 hr with nocodazole. B, Microtubule organization was assessed by immunostaining against α-tubulin. Disappearance of polymerized tubulin is observed in nocodazole-treated astrocytes (right) but not in control astrocytes treated with DMSO alone (left). Scale bar, 10 μm.
Inhibition of myosin light chain kinase activity reduces wave propagation
Actin–myosin complexes mediate contractility events in muscle and nonmuscle cells (Kamm and Stull, 1985). Contractility requires phosphorylation of myosin light chain by its kinase MLCK (for review, see Adelstein and Eisenberg, 1980). To test the requirement for myosin–actin interactions in intercellular signaling, we evoked calcium waves in primary astrocytes that had been preincubated with the MLCK inhibitor ML7 (50 μm). ML7 is specific because it is a 100-fold more potent inhibitor of MLCK than are other kinases (Saitoh et al., 1987; Shrode et al., 1995). ML7 treatment reduced the radius of mechanically induced calcium waves by 36 ± 11% (n = 10) compared with that of DMSO-treated astrocytic cultures (n = 10; p < 0.01). Gap junctional permeability was not altered in ML7-treated cultures when evaluated by the scrape-loading assay (data not shown), and the initial calcium increase in stimulated astrocytes was not reduced compared with control values (185 ± 27 vs 187 ± 21%; n = 10). Thus, MLCK inhibition suppresses calcium signaling without affecting coupling or calcium mobilization.
Astrocytes from Cx43 “knock-out” mice are morphologically indistinguishable from wild type and propagated robust calcium waves
We found that astrocytes derived from transgenic Cx43 null-mutant mice are capable of propagating intercellular calcium waves that are reduced only minimally, if at all, from those propagated by wild-type astrocytes (Fig. 9). Naus et al. (1997)and Spray (1998) have reported previously that astrocytes deficient in Cx43 can propagate Ca2+ waves, although the radius of propagation was less than that from wild-type mice. Lack of Cx43 is not associated with uncoupling, because endogenous expression of at least three other gap junction proteins, Cx40, Cx45, and Cx46, is high enough to maintain 5% residual coupling (Spray, 1998) (data not shown). In this regard, the morphological phenotype of astrocytes from Cx43 knock-out mice is indistinguishable from that of wild-type astrocytes; both are characterized by highly organized arrays of actin filaments (Fig. 9). Possibly, the endogenous expression of connexins other than Cx43 is sufficient to act as a central organizer of the cytoskeleton preserving the normal astrocytic phenotype.
Fig. 9.
Astrocytes from mice with a Cx43 null mutation propagate robust Ca2+ waves. Upper row, Mechanical stimulation induced a calcium wave that propagated >300 μm from the stimulated cell in astrocytes from a Cx43 knock-out mouse. The culture was loaded with fluo-3. Lower row, Texas Red–phalloidin staining of a sister culture revealed well-organized actin fiber bundles. Actin organization in Cx43 knock-out mice did not differ from wild-type astrocytes. Scale bar: Upper row, 75 μm; lower row, 7 μm.
DISCUSSION
This study evaluated the role of actin assembly in astrocytic calcium signaling and found that cytoskeletal organization seems to be a key requirement for the generation of long-distance calcium waves. First, cytoskeletal organization coincided temporally with the number of astrocytes engaged in calcium signaling after plating. Second, disruption of the actin cytoskeleton attenuated calcium signaling in established cultures. The spatial expansion of calcium waves decreased gradually and paralleled the loss of actin fibers after exposure to cytochalasin D. Gap junction coupling or astrocytic capability to mobilize calcium in response to ATP was not compromised by identical treatment, suggesting that the effect was specific to cytoskeletal organization. Several lines of evidence suggest that release of a polyphosphate, possible ATP, is a necessary intermediate in astrocytic signaling. Focal stimulation consistently engaged astrocytes not in contact with the stimulated cell, and the ATPase apyrase attenuated both jumping calcium signaling as well as continuous calcium waves. An inhibitor of purinergic receptors, suramin, also attenuated astrocytic signaling. Combined, these observations support the notion that gap junctions are required for calcium signaling because of their effects on cellular organization, rather than as a route for the exchange of intercellular messengers. As such, the persistence of calcium waves in mice with Cx43 null mutations is a function of their preserved actin superstructure, rather than intercellular gap junction coupling.
At which step do actin stress fibers contribute to the generation of calcium waves? One possibility is that wave propagation is critically dependent on the function of the cytoskeleton as a scaffold for signaling proteins. Actin stress fibers physically bind a number of signaling molecules either directly or via other cytoskeletal-associated proteins (Sastry and Horwitz, 1993). In this regard, phosphatidyl 4,5-bisphosphate (PIP2) and IP3 receptors associate with actin in several systems (Feng and Kraus-Friedmann, 1993; Miki et al., 1996). Possibly, cytochalasin D treatment interferes with the signaling cascade that leads to the generation of IP3 and Ca2+ release from intracellular stores. The observation that Ca2+responses to ATP and bradykinin, agonists that both promote Ca2+ release via the IP3 signaling pathway, were not significantly altered in cytochalasin D-treated astrocytes does not support such a mechanism, but neither does this observation exclude a local effect on sites of Ca2+amplification that may be critical for wave propagation.
Traditionally, actin stress fibers have been implicated mainly in cell shape changes and cell motility by modulation of cell–cell and cell–matrix contacts (Pavalko and Otey, 1994). Assembly of stress fibers is initiated by formation of focal adhesion complexes. These complexes are composed of aggregated adhesion molecules that span the plasma membrane and interact on the outside with other cells or the extracellular matrix and on the inside with bundles of actin fibers and actin-associated components. Numerous observations demonstrate that these complexes, in addition to their functional role in adhesion, also act as “outside-in” signal transduction receptors triggering a number of intracellular cascades, including mobilization of intracellular Ca2+. Cytosolic Ca2+ elevations can in turn feed back as “inside-out” signaling to regulate receptor function. Indeed, phospholipase C- and IP3-dependent pathways have been described in several of these systems including astrocytes (McNamme et al., 1993), and both astrocytes and C6 glioma cells express multiple N-CAMs and integrins (Noble et al., 1985; Bhat and Silberberg, 1987; Malek-Hedayat and Rome, 1992; Tawill et al., 1993). However, although it is established that Ca2+ signaling can be activated after receptor binding of ligands and modulated by an array of growth factors and cytokines, it is not clear how adhesion complexes can contribute to intercellular calcium signaling. One possibility is that adhesion complexes can directly transmit “inside-out-inside” calcium signals and thereby function as a pathway that mediates or supports the propagation of Ca2+ waves. Experimental support for the existence of such a mechanism is lacking, but cell-to-cell signaling has been linked to adhesion complexes in other systems. It is believed that integrin bonds mediate the stretch reflex by which muscle modulates transmitter release from its own motor nerve terminals. A blocking peptide, RGD, that mimics the main integrin binding sites effectively prevents stress-induced changes in transmitter release (Chen and Grinnell, 1995). Integrin bonds might, as a physical link, provide a pathway by which the presynaptic neurons can almost immediately sense changes in muscle length and explain that changes in transmitter release are induced within 10–20 msec of muscle stress. The stress-induced modulation of transmitter release is dependent on intracellular Ca2+ stores, suggesting that integrin bonds mediate an inside-out-inside Ca2+ signal in this system.
Why did inhibition of MLCK suppress wave activity? Forced expression of gap junctions in glioma cells resulted in a profound reorganization, not only of actin, but also of myosin (Cotrina et al., 1998b). Actin and myosin were intimately colocalized in a manner identical to what has been described previously in astrocytes (Abd-El-Basset and Fedoroff, 1994). Isometric contraction is activated by high Ca2+ in both smooth muscle and nonmuscle cells. Formation of a Ca2+–calmodulin complex activates MLCK, which subsequently phosphorylates several sites on myosin light chain, leading to an increase in actin-activated ATPase the activity of which triggers contraction (for review, see Korn and Hammer, 1988; Citi and Kendrick-Jones, 1996). Because astrocytes contain all the elements required for isometric contraction, it is intriguing to speculate that calcium-induced contractility contributes to wave propagation. However, visible manifestation of stress fiber movements has been difficult to prove in other systems. Rather, a large body of evidence supports the idea that isometric tension induced by contractility drives the formation of stress fibers and focal adhesion complexes. Agents that inhibit MLCK, in turn, lead to stress fiber and focal adhesion complex disassembly. Inhibition of MLCK in this study did not result in visible degradation of stress fibers (data not shown), suggesting that ML-7 suppression of wave activity was not secondary to actin disassembly, but further studies are required to establish the role of contractility in the formation of calcium waves.
To date, no clear relationship between actin microfilaments and gap junctional proteins has been established. As for many other ion channel precursors, several observations suggest that connexon precursors are transported to the plasma membrane by actin fibers; an association between connexon precursors and actin fibers has been identified in epithelial cells of the prostate (Tadvalkar and Pinto da Silva, 1983), in outer horizontal cells of the goldfish retina (Kurz-Isler and Worburg, 1988), and in the lens of primates (Lo et al., 1994). However, studies by Wang and Rose (1995) indicate that although cytochalasin suppressed clustering of gap junction hemichannels into functional plaques, the treatment did not affect established plaques. Assuming that the punctate staining observed by immunocytochemistry corresponds to gap junction channels previously assembled in gap junctional plaques (Paul, 1986; Dermietzel et al., 1987; Kumar and Gilula, 1996), our experiments also suggest that existing channels were not affected by cytochalasin treatment. Cytochalasin-treated astrocytes also maintained fully functional gap junctions, as assessed by the scrape-loading method. Consistent with this observation, Vaughan and Lasater (1990) found no effect of cytochalasin on the electrical coupling of fish horizontal cells. Of note, stress fibers and gap junctional plaques did, as a rule, not colocalize in either astrocytes or transfected glioma cells (data not shown).
Extracellular ATP mediates calcium signaling among rat basophilic leukemia cells, hepatocytes, and neuroepithelioma cells (Osipchuk and Cahalan, 1992; Palmer et al., 1996; Schlosser et al., 1996) and possibly also among astrocytes (Hassinger et al., 1996). Transmitter released from astrocytes has primarily been associated with transport systems (Szatkowski et al., 1990; Parpura et al., 1994), but glutamate release via a vesicular-like process in cortical astrocytes has been proposed (Parpura et al., 1995a). The fact that an intact actin cytoskeleton is necessary for calcium-dependent secretion in both neurons and secretory cells (for review, seeTrifaró and Vitale, 1993) and proteins generally involved in regulated exocytosis are expressed in cultured astrocytes (Parpura et al., 1995b; Madison et al., 1996) provides a link between an extracellular component of calcium wave activity and the cytoskeleton. However, the cytoskeleton may also regulate the activity and number of transport proteins in the membrane (Mills and Mandel, 1994).
This study established that cellular organization is a critical constituent for the propagation of intercellular calcium signaling. Astrocytes in culture lost their capability for propagating calcium waves when their cytoskeleton was disrupted by cytochalasin D. Although we do not know at which level stress fibers act, the fact that inhibition of myosin light chain kinase suppressed calcium signaling suggests that a functional cytoskeleton is a prerequisite for interastrocytic calcium signaling.
Footnotes
This study was supported by National Institute of Neurological Disorders and Stroke and National Institutes of Health Grants NS130007 and NS135011. M.N. is an Established Investigator sponsored by The American Heart Association. We thank B. Grafstein for comments on this manuscript.
Correspondence should be addressed to Dr. Maiken Nedergaard, Department of Cell Biology and Anatomy, New York Medical College, Valhalla, NY 10595.
REFERENCES
- 1.Abd-El-Basset E, Fedoroff S. Contractile units in stress fibers of fetal human astroglia in tissue culture. J Chem Neuroanat. 1994;7:113–122. doi: 10.1016/0891-0618(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 2.Adelstein RS, Eisenberg E. Regulation and kinetics of actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- 3.Araque A, Parpura V, Sanzgiri R, Haydon P. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 4.Ashton AC, Dolly O. Microtubules and microfilaments participate in the inhibition of synaptosomal noradrenaline release by tetanus toxin. J Neurochem. 1997;68:649–658. doi: 10.1046/j.1471-4159.1997.68020649.x. [DOI] [PubMed] [Google Scholar]
- 5.Bhat S, Silberberg DH. C6 glioma cells express modified neural-cell adhesion molecule-like glycoproteins. Brain Res. 1987;412:144–147. doi: 10.1016/0006-8993(87)91449-1. [DOI] [PubMed] [Google Scholar]
- 6.Bohmer RM, Scharf E, Assoian RK. Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin D1. Mol Biol Cell. 1996;7:101–111. doi: 10.1091/mbc.7.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao D, Lin G, Westphale E, Beyer E, Steinberg T. Mechanisms for the coordination of intercellular calcium signaling in insulin-secreting cells. J Cell Sci. 1997;110:497–504. doi: 10.1242/jcs.110.4.497. [DOI] [PubMed] [Google Scholar]
- 8.Centemeri C, Bolego C, Abbracchio M, Cattabeni F, Puglisi L, Burnstock G, Nicosia S. Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br J Pharmacol. 1997;121:1700–1706. doi: 10.1038/sj.bjp.0701293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charles A. Glia-neuron intercellular calcium signaling. Dev Neurosci. 1994;16:196–206. doi: 10.1159/000112107. [DOI] [PubMed] [Google Scholar]
- 10.Charles AC, Naus CCG, Zhu D, Kidder GM, Dirksen H, Sanderson MJ. Intercellular calcium signaling via gap junctions in glioma cells. J Cell Biol. 1992;118:195–201. doi: 10.1083/jcb.118.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen B, Grinnell AD. Integrins and modulation of transmitter release from motor nerve terminals by stretch. Science. 1995;269:1578–1580. doi: 10.1126/science.7667637. [DOI] [PubMed] [Google Scholar]
- 12.Citi S, Kendrick-Jones J. Regulation of non-muscle myosin structure and function. Bioessays. 1996;7:155–159. doi: 10.1002/bies.950070404. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 15.Cotrina ML, Kang J, Lin JH, Bueno E, Hansen T, He L, Liu Y, Nedergaard M. Astrocytic gap junctions remain open during ischemic conditions. J Neurosci. 1998a;18:2520–2537. doi: 10.1523/JNEUROSCI.18-07-02520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotrina ML, Lin J, Liu S, Bueno E, Nedergaard M (1998b) Phenotypic transformation of C6 glioma cells following overexpression of gap junction proteins. Soc Neusci Abstr 126.7.
- 17.Dermietzel R, Yancey B, Janssen-Timmen U, Traub O, Willecke K, Revel J-P. Simultaneous light and electron microscopic observation of immunolabeled liver 27 kDa gap junction protein on ultra-thin cryosections. J Histochem Cytochem. 1987;35:387–392. doi: 10.1177/35.3.3029214. [DOI] [PubMed] [Google Scholar]
- 18.Dermietzel R, Hertzberg E, Kessler J, Spray D. Gap junctions between cultured astrocytes: immunocytochemical, molecular, and electrophysiological analysis. J Neurosci. 1991;11:1421–1432. doi: 10.1523/JNEUROSCI.11-05-01421.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elfgang C, Eckert R, Lichten-Frate H, Butterweck A, Traub O, Klein R, Hulser D, Willecke K. Specific permeability and selective formation of gap junction channels in connexin transfected HeLa cells. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Fouly MH, Trosko JE, Chang C. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 21.Feng L, Kraus-Friedmann J. Association of the hepatic IP3 receptor with the plasma membrane: relevance to mode of action. Am J Physiol. 1993;265:C1588–C1596. doi: 10.1152/ajpcell.1993.265.6.C1588. [DOI] [PubMed] [Google Scholar]
- 22.Gavin RH. Microtubule-microfilament synergy in the cytoskeleton. Int Rev Cytol. 1997;173:207–242. doi: 10.1016/s0074-7696(08)62478-x. [DOI] [PubMed] [Google Scholar]
- 23.Giaume C, Marin P, Cordier J, Glowinski J, Premont J. Adrenergic regulation of intercellular communications between cultured striatal astrocytes from the mouse. Proc Natl Acad Sci USA. 1991;88:5577–5581. doi: 10.1073/pnas.88.13.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg S, Bechberger J, Naus CCG. A pre-loading method of evaluating gap junctional communication by fluorescent dye transfer. Biotechniques. 1995;18:490–497. [PubMed] [Google Scholar]
- 25.Goldman JE, Abramson B. Cyclic AMP-induced shape changes of astrocytes are accompanied by rapid depolymerization of actin. Brain Res. 1990;528:189–196. doi: 10.1016/0006-8993(90)91657-3. [DOI] [PubMed] [Google Scholar]
- 26.Hassinger TD, Guthrie PB, Atkinson PB, Bennett MVL, Kater SB. An extracellular signaling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci USA. 1996;93:13268–13273. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jørgensen N, Geist S, Civitelli R, Steinberg T. ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol. 1997;139:497–506. doi: 10.1083/jcb.139.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 29.Kastritsis C, Salm A, McCarthy K. Stimulation of the P2Y purinergic receptor on Type 1 astroglia result in inositol phosphate formation and calcium mobilization. J Neurochem. 1992;58:1277–1284. doi: 10.1111/j.1471-4159.1992.tb11339.x. [DOI] [PubMed] [Google Scholar]
- 30.King B, Neary J, Zhu Q, Wang S, Norenberg M, Burnstock G. P2 purinoceptors in rat cortical astrocytes: expression, calcium-imaging and signaling studies. Neuroscience. 1996;74:1187–1196. doi: 10.1016/0306-4522(96)00209-6. [DOI] [PubMed] [Google Scholar]
- 31.Korn ED, Hammer JA. Myosins of nonmuscle cells. Annu Rev Biophys Chem. 1988;17:23–45. doi: 10.1146/annurev.bb.17.060188.000323. [DOI] [PubMed] [Google Scholar]
- 32.Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 33.Kurz-Isler G, Wolburg H. Light-dependent dynamics of gap junctions between horizontal cells in the retina of the crucian carp. Cell Tissue Res. 1988;251:641–649. doi: 10.1007/BF00214013. [DOI] [PubMed] [Google Scholar]
- 34.Lo W-K, Mills A, Kuck JFR. Actin filament bundles are associated with fiber gap junctions in the primate lens. Exp Eye Res. 1994;58:189–196. doi: 10.1006/exer.1994.1007. [DOI] [PubMed] [Google Scholar]
- 35.Madison DL, Kruger WH, Kin T, Pfeiffer SE. Differential expression of rab3 isoforms in oligodendrocytes and astrocytes. J Neurosci Res. 1996;45:258–268. doi: 10.1002/(SICI)1097-4547(19960801)45:3<258::AID-JNR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 36.Malek-Hedayat S, Rome LH. Expression of multiple integrins and extracellular matrix components by C6 glioma cells. J Neurosci Res. 1992;31:470–478. doi: 10.1002/jnr.490310309. [DOI] [PubMed] [Google Scholar]
- 37.McNamme HP, Ingber DE, Schwartz MA. Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-inositol lipid breakdown. J Cell Biol. 1993;121:673–678. doi: 10.1083/jcb.121.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- 39.Mills JW, Mandel LJ (1994) Cytoskeletal regulation of membrane transport events. FASEB J 1161–1165. [DOI] [PubMed]
- 40.Naus CC, Bechberger JF, Zhang Y, Venance L, Yamasaki H, Juneja SC, Kidder GM, Giaume C. Altered gap junctional communication, intercellular signaling, and growth in cultured astrocytes deficient in connexin43. J Neurosci Res. 1997;49:528–540. doi: 10.1002/(SICI)1097-4547(19970901)49:5<528::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 41.Naus CCG, Zhu D, Todd SDL, Kidder GM. Characteristics of C6 glioma cells overexpressing a gap junction protein. Cell Mol Neurobiol. 1992;12:163–175. doi: 10.1007/BF00713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 43.Nedergaard M, Goldman S, Desai S, Pulsinelli W. Acid-induced death in neurons and glia. J Neurosci. 1991;11:2489–2497. doi: 10.1523/JNEUROSCI.11-08-02489.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noble M, Albrechtsen M, Moller C, Lylis J, Bock E, Goridis C, Watanabe M, Rutishauser U. Glial cells express N-CAM/D2-CAM-like polypeptides in vitro. Nature. 1985;316:725–728. doi: 10.1038/316725a0. [DOI] [PubMed] [Google Scholar]
- 46.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 47.Palmer R, Yule D, Shewach D, Williams J, Fisher S. Paracrine mediation of calcium signaling in human SK-N-MCIXC neuroepithelioma cells. Am J Physiol. 1996;271:C43–C53. doi: 10.1152/ajpcell.1996.271.1.C43. [DOI] [PubMed] [Google Scholar]
- 48.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signaling. Science. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 49.Parpura V, Liu F, Brethorst S, Jeftinija K, Jeftinija S, Haydon PG. Alpha-latrotoxin stimulates glutamate release from cortical astrocytes in cell culture. FEBS Lett. 1995a;360:266–270. doi: 10.1016/0014-5793(95)00121-o. [DOI] [PubMed] [Google Scholar]
- 50.Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett. 1995b;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- 51.Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;15:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul DL. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986;103:123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pavalko FM, Otey CA. Role of adhesion molecule cytoplasmic domains in mediating interactions with the cytoskeleton. Proc Soc Exp Biol Med. 1994;205:282–293. doi: 10.3181/00379727-205-43709. [DOI] [PubMed] [Google Scholar]
- 54.Prekeris R, Mayhew MW, Cooper B, Terrian DM. Identification and localization of an actin-binding motif that is unique to the epsilon isoform of protein kinase C and participates in the regulation of synaptic function. J Cell Biol. 1996;132:77–90. doi: 10.1083/jcb.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 1987;262:7796–7801. [PubMed] [Google Scholar]
- 56.Salter M, Hicks J. ATP causes release of intracellular Ca2+ via phospholipase Cbeta/IP3 pathway in astrocytes from the dorsal spinal cord. J Neurosci. 1995;15:2961–2971. doi: 10.1523/JNEUROSCI.15-04-02961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanderson MJ, Charles AC, Boitano S, Dirksen ER. Mechanisms and function of intercellular calcium signaling. Mol Cell Endocrinol. 1994;98:173–187. doi: 10.1016/0303-7207(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 58.Sastry SK, Horwitz AF. Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr Opin Cell Biol. 1993;5:819–831. doi: 10.1016/0955-0674(93)90031-k. [DOI] [PubMed] [Google Scholar]
- 59.Scemes E, Dermietzel R, Spray DC. Calcium waves between astrocytes from Cx43 knockout mice. Glia. 1998;24:65–73. doi: 10.1002/(sici)1098-1136(199809)24:1<65::aid-glia7>3.0.co;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci USA. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shain W, Bausback D, Fiero A, Madelian V, Turner JN. Regulation of receptor-mediated shape change in astroglial cells. Glia. 1992;5:223–238. doi: 10.1002/glia.440050308. [DOI] [PubMed] [Google Scholar]
- 62.Shrode LD, Klein JD, O’Neill CO, Putnam RM. Shrinkage-induced activation of Na+/H+ exchange in primary rat astrocytes: role of myosin light-chain kinase. Am J Physiol. 1995;269:C257–C266. doi: 10.1152/ajpcell.1995.269.1.C257. [DOI] [PubMed] [Google Scholar]
- 63.Sullivan R, Lo CW. Expression of a connexin 43/β-galactosidase fusion protein inhibits gap junctional communication in NIH3T3 cells. J Cell Biol. 1995;130:419–429. doi: 10.1083/jcb.130.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- 65.Tadvalkar G, Pinto da Silva P. In vitro rapid assembly of gap junctions is induced by cytoskeleton disrupters. J Cell Biol. 1983;96:1279–1287. doi: 10.1083/jcb.96.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tawil N, Wilson P, Carbonetto S. Integrins in point contacts mediate cell spreading: factors that regulate integrin accumulation in point contacts vs. focal contacts. J Cell Biol. 1993;120:261–271. doi: 10.1083/jcb.120.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Intercellular calcium signaling via gap junction in connexin-43-transfected cells. J Biol Chem. 1998;273:1519–1528. doi: 10.1074/jbc.273.3.1519. [DOI] [PubMed] [Google Scholar]
- 68.Trifaró J-M, Vitale ML. Cytoskeleton dynamics during neurotransmitter release. Trends Neurosci. 1993;16:466–472. doi: 10.1016/0166-2236(93)90079-2. [DOI] [PubMed] [Google Scholar]
- 69.Vaughan DK, Lasater EM. Distribution of F-actin in bipolar and horizontal cells of bass retinas. Am J Physiol. 1990;259:C205–C214. doi: 10.1152/ajpcell.1990.259.2.C205. [DOI] [PubMed] [Google Scholar]
- 70.Venance L, Stella N, Glowinski J, Giaume C. Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. J Neurosci. 1997;17:1981–1992. doi: 10.1523/JNEUROSCI.17-06-01981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wade MH, Trosko J, Schindler M. Fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 1986;232:525–528. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- 72.Walz W, Gimpl G, Ohlemeyer C, Kettenman H. Extracellular ATP-induced currents in astrocytes: involvement of a cation channel. J Neurosci Res. 1994;38:12–18. doi: 10.1002/jnr.490380104. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Rose B. Clustering of Cx43 cell-to-cell channels into gap junction plaques: regulation by cAMP and microfilaments. J Cell Sci. 1995;108:3501–3508. doi: 10.1242/jcs.108.11.3501. [DOI] [PubMed] [Google Scholar]
- 74.Yahara I, Harada F, Sekita S, Yoshihira K, Natori S. Correlation between effects of 24 different cytochalasins on cellular structures and cellular events and those on actin in vitro. J Cell Biol. 1982;92:69–78. doi: 10.1083/jcb.92.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]