Abstract
An estimated 499 million curable sexually transmitted infections (STIs; gonorrhea, chlamydia, syphilis, and trichomoniasis) occurred globally in 2008. In addition, well over 500 million people are estimated to have a viral STI such as herpes simplex virus type 2 (HSV-2) or human papillomavirus (HPV) at any point in time. STIs result in a large global burden of sexual, reproductive, and maternal-child health consequences, including genital symptoms, pregnancy complications, cancer, infertility, and enhanced HIV transmission, as well as important psychosocial consequences and financial costs. STI control strategies based primarily on behavioral primary prevention and STI case management have had clear successes, but gains have not been universal. Current STI control is hampered or threatened by several behavioral, biological, and implementation challenges, including a large proportion of asymptomatic infections, lack of feasible diagnostic tests globally, antimicrobial resistance, repeat infections, and barriers to intervention access, availability, and scale-up. Vaccines against HPV and hepatitis B virus offer a new paradigm for STI control. Challenges to existing STI prevention efforts provide important reasons for working toward additional STI vaccines. We summarize the global epidemiology of STIs and STI-associated complications, examine challenges to existing STI prevention efforts, and discuss the need for new STI vaccines for future prevention efforts.
Keywords: Sexually transmitted diseases, Vaccines, Prevention and control
1. Introduction
Sexually transmitted infections (STIs) have a major impact on sexual and reproductive health worldwide. Although more than 30 identified pathogens are known to be transmitted sexually, eight of these have been clearly linked to the greatest amount of morbidity. Three bacterial STIs, Chlamydia trachomatis (chlamydia), Neisseria gonorrhoeae (gonorrhea), and Treponema pallidum (syphilis), and one parasitic STI, Trichomonas vaginalis (trichomoniasis), are currently curable. Four viral STIs, HIV, human papillomavirus (HPV), herpes simplex virus (HSV), and hepatitis B virus (HBV), can be chronic or lifelong, although medications can modify disease course or symptoms. This article focuses on STIs other than HIV.
STIs can cause genital symptoms affecting quality of life, important psychosocial consequences, and serious morbidity and mortality, through pregnancy complications, cancer, infertility, and enhanced HIV transmission. Controlling STIs is a core aspect of the World Health Organization’s (WHO’s) Global Strategy on Reproductive Health [1], and essential for achieving Millennium Development Goals 4 (child health), 5 (maternal health), and 6 (HIV prevention) [2]. However, STI control remains challenging in most settings, particularly in low- and middle-income countries where the health system infrastructure is least developed and the burden of STI-related complications is highest.
Safe and effective vaccines against two STIs have been major advances in global health. The first STI vaccine was developed over 30 years ago against HBV infection, which can be transmitted perinatally and parenterally as well as sexually [3]. HBV vaccine has now been adopted into infant immunization programs in 93% of countries and has already prevented an estimated 1.3 million deaths [4,5]. The second STI vaccine, against HPV, was developed recently and found to be highly efficacious in preventing infection with HPV types causing 70% of cervical cancers [6]. Countries achieving good HPV vaccination coverage have already observed marked benefits against proximal HPV-related outcomes such as genital warts [7,8].
Limitations of available prevention interventions for other STIs provide important reasons for working toward additional STI vaccines as well. The goal of this article is to summarize the global epidemiology of STIs and STI-associated complications, to examine challenges to existing interventions for STI control, and to discuss the need for new STI vaccines for future prevention efforts.
2. STIs: a global snapshot
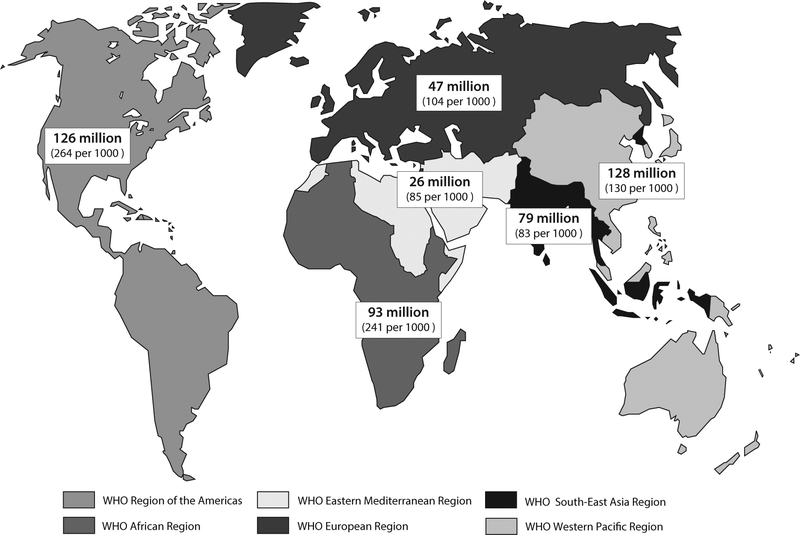
WHO estimates that 499 million new cases of curable STIs occurred in 2008 among 15–49 year-olds globally: 106 million cases of chlamydia, 106 million cases of gonorrhea, 11 million cases of syphilis, and 276 million cases of trichomoniasis [9]. The prevalence of these infections at any point during 2008 was 360 million cases. STI numbers were high across all world regions, but incidence rates were highest in the WHO Region of the Americas and the WHO African Region (Fig. 1) [9]. Men and women were similarly likely to acquire new STIs, with a male to female ratio of 1.14 [9]. The number of new curable STIs does not appear to be decreasing; the 2005 WHO estimate was 448 million cases [9,10].
Fig. 1.
Estimated numbers of new cases and incidence rates per 1000 population of curable sexually transmitted infections (gonorrhea, chlamydia, syphilis, and trichomoniasis) among 15–49 year-olds by WHO region, 2008 [9].
Because viral STIs can be chronic, they comprise a large proportion of prevalent STIs. Approximately 291 million women have an HPV infection at any point in time [11], and it is likely that the numbers of HPV-infected men are similar [12,13]. HSV-2 infection, which is lifelong, affects an estimated 536 million people aged 15–49 years globally [14]. Approximately 360 million people suffer from chronic HBV infections, although most of these were acquired perinatally or in early childhood [3].
It should be noted that global estimates, especially for the curable STIs, have relied on the few regions with systematic STI surveillance along with a relatively small number of prevalence studies among discrete populations (n = 180, WHO 2008 estimates) [9]. Fewer data exist from areas with limited laboratory infrastructure. However, despite data limitations, it is clear that the number of global STIs is large: available estimates suggest that well over a million people acquire an STI every day [9,11,14].
Adolescents and young adults often have the highest rates of incident STIs and account for a disproportionate number of new infections [15]. However, transmission of STIs within populations is affected by a complex interplay of factors, including STI prevalence, which can vary markedly among populations or geographic areas. For example, HSV-2 seroprevalence ranges from 21% among 14–49 year-old women in the United States [16] to more than 80% among young women in parts of sub-Saharan Africa [17]. Chlamydia prevalence among pregnant women attending antenatal care is approximately 7% in sub-Saharan Africa [18], but as high as 25–30% in several Pacific Island countries [19]. In China, syphilis seroprevalence is less than 1% in the general population, but more than 12% among incarcerated female sex workers and almost 15% among men who have sex with men (MSM) [20].
3. STI consequences
STIs can have both short-term and long-term consequences across a broad spectrum of sexual, reproductive, and maternal-child health. The vast majority of STIs are asymptomatic or unrecognized; however, adverse outcomes can occur regardless of the presence of symptoms.
3.1. Genital symptoms
Although most STIs are asymptomatic, some cause genital symptoms that have an important impact on quality of life. Chlamydia, gonorrhea, and trichomoniasis can cause vaginal discharge syndromes in women and urethritis in men. Trichomoniasis, the most common curable STI globally [9], can cause profuse vaginal discharge and irritation. Genital HSV and syphilis infections can cause ulceration. Even if only 10–20% of infections of genital HSV infections are symptomatic [16], more than 50–100 million people around the world may suffer from painful recurrent genital ulceration [14]. HPV infection can cause genital warts, which are not painful but can be distressing and disfiguring [21]. Approximately 7% of women in the United States general population and over 10% of women in Nordic countries report a history of a genital wart diagnosis [22,23]. Genital herpes ulceration and genital warts are more frequent and more severe among HIV-positive persons [24,25].
3.2. Pregnancy complications
All of the curable STIs have been linked with preterm labor, with associated risks to the neonate of pre-term birth, low birth weight, and death [26,27]. Active syphilis during pregnancy results in an estimated 215,000 stillbirths and fetal deaths, 90,000 neonatal deaths, 65,000 infants at increased risk of dying from prematurity or low birth weight, and 150,000 infants with congenital syphilis disease each year, almost all in low-income countries [28]. Chlamydia and gonorrhea infections during pregnancy can lead to neonatal eye infection (ophthalmia neonatorum), which was an important cause of blindness before the use of ocular prophylaxis [29]. Pneumonia can also occur in up to 10–20% of infants born to a mother with untreated chlamydial infection [30].
Perinatal transmission of HSV-2 infection is associated with high morbidity, including long-term neurologic sequelae, and mortality. In the United States, estimates of neonatal herpes incidence range from 1 in 3000 to 1 in 25,000 births; global data are lacking [31,32]. In areas of high HBV endemicity (e.g., East Asia), HBV is most commonly transmitted from mother to child at birth [3]. These infections lead to chronic HBV infection in 80–90% of cases [33].
3.3. Cancer
HPV and HBV are oncogenic. Infection with high-risk types of HPV is a necessary causal factor for cervical cancer [34], and can also cause anal, vulvar, vaginal, penile, and some oropharyngeal cancers. Worldwide, HPV infection results in 530,000 cases of cervical cancer and 275,000 cervical cancer deaths each year, with the vast majority of deaths (88%) occurring in resource-poor settings [35]. In some areas of the world, cervical cancer is the most common cancer and the main cause of cancer death among women. Among women in Eastern Africa, cervical cancer leads to more than twice as many deaths as the next most common cause, breast cancer [35].
Chronic infection with HBV can lead to liver cirrhosis and hepatocellular carcinoma, especially if acquired at birth. Mathematical models have estimated that approximately 600,000 people die from these adverse outcomes of HBV infection annually [36].
3.4. Upper genital tract disease
Chlamydia and gonorrhea can ascend to the upper genital tract in women and cause acute pelvic inflammatory disease (PID), tubal factor infertility, potentially fatal ectopic pregnancy, and chronic pelvic pain. Data on the global STI-related burden of these outcomes are limited. Based on prospective studies in high-income countries, about 10–15% of untreated chlamydia infections lead to clinical PID [37,38], and about 10–15% of clinical PID cases lead to tubal factor infertility [37,39]. Chlamydia can also lead to asymptomatic tubal infection and infertility, but the extent of this is unknown. The proportion of gonorrhea infections leading to PID and infertility may be even higher, especially in areas without access to early treatment [40]. As an estimated 95.5 million cases of chlamydia and gonorrhea occurred among women in 2008 [9], the numbers of women with adverse reproductive outcomes could be sizable. Estimates of global infertility have ranged from 45 million to 186 million couples unable to have a child over 5 years [41,42]. The proportion of infertility that is primarily caused by scarring from genital infection varies by population. In the United States, the proportion of infertility that is tubal factor ranges from 10–40% [43,44]. However, in sub-Saharan Africa, tubal infertility may be the cause of up to 85% of infertility [45].
3.5. Increased HIV risk
Several STIs increase the risk of both acquiring and transmitting HIV. A large body of literature demonstrates that people with HSV-2 infection have a three-fold increased risk of acquiring HIV infection [46]. Further, HIV- and HSV-2-co-infected persons have higher HIV viral loads and are more likely to transmit HIV infection [47,48]. Both ulcerative (syphilis) and inflammatory (chlamydia, gonorrhea, trichomoniasis) curable STIs may also be associated with an increased risk of HIV acquisition, by up to two- to threefold [49,50]. These infections are linked to increased infectiousness among HIV-infected persons; urethritis and cervicitis substantially increase genital HIV shedding [51,52]. HPV might also increase the risk of HIV acquisition [53].
3.6. Psychosocial consequences
In addition to their physical consequences, STIs can have a profound psychosocial impact that is often difficult to quantify. Studies have shown that an STI diagnosis can lead to feelings of stigma, shame, and diminished self-worth, as well as anxiety about sexual relationships and future reproductive health [54–56]. STIs also have an effect on sexual relationships, and can lead to disruption of partnerships and intimate partner violence [55,57].
3.7. Human and financial costs
In the recent Global Burden of Disease Study, curable STIs accounted for almost 11 million disability-adjusted life years (DALYs) lost in 2010: syphilis, 9.6 million DALYs; chlamydia, 714,000 DALYs; gonorrhea, 282,000 DALYS; and trichomoniasis, 167,000 DALYs [58]. HPV-related cervical cancer accounted for another 6.4 million DALYs lost. The 2010 disease burden study did not calculate DALY estimates for HSV-2, which could be substantial given the role of HSV-2 in HIV transmission. Further, study authors have not yet published the specific methods used to calculate DALYs for STIs; global burden estimates have been limited by a paucity of precise data on STI-related complications, especially from low-income settings [59].
STIs also pose a substantial economic burden. In the United States, approximately $3 billion in direct medical costs were spent in 2008 to diagnose and treat 19.7 million cases of STIs and their complications, excluding HIV and pregnancy-related outcomes like stillbirth [60]. The costs associated with adverse STI outcomes are less well documented in resource-poor settings.
4. Challenges to available interventions for STI prevention and control
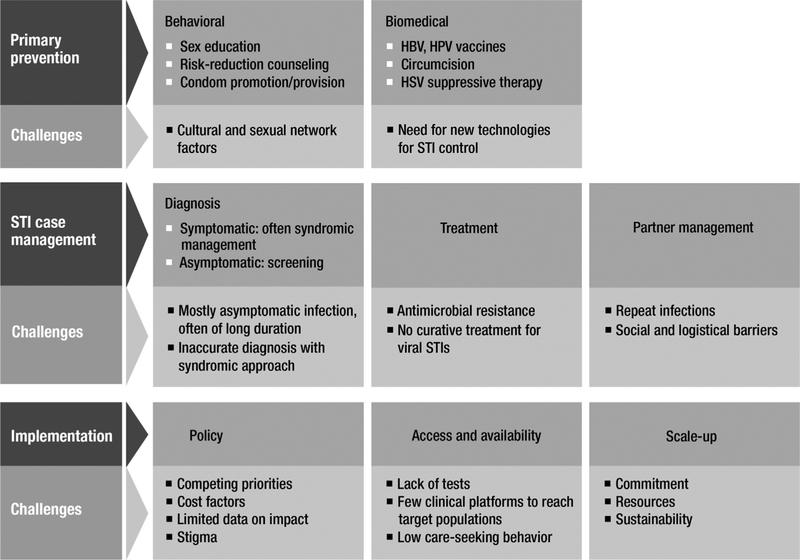
The public health approach to STI control revolves around two main strategies: behavioral and biomedical primary prevention, to prevent STI acquisition among uninfected people, and STI case management, to diagnose and manage infected people to prevent STI complications (secondary prevention) and ongoing transmission (Fig. 2) [61]. Behavioral primary prevention includes comprehensive sex education, risk-reduction counseling, and condom promotion and provision. The main biomedical STI primary prevention interventions are HPV and HBV vaccines. STI case management involves STI diagnosis, provision of effective treatment, notification and treatment of sex partners, and safer sex counseling and condom provision [61]. STI case management can apply to both symptomatic and asymptomatic people. However, in most settings, STI case management is limited to symptomatic people seeking STI care. In addition, because of a lack of laboratory infrastructure, most low- and middle-income countries rely on syndromic management: the use of genital symptom algorithms to guide treatment, without diagnostic tests [61,62]. Where tests are available, affordable, and feasible, they may be used to diagnose symptomatic infections or screen for asymptomatic infections. Several high-income countries recommend screening young women annually for chlamydia, based on evidence that screening reduces the risk of PID [38,63]. Screening pregnant women for syphilis is recommended in virtually all countries [64].
Fig. 2.
Key interventions and challenges for sexually transmitted infection prevention and control.
Several reviews have summarized the efficacy of individual STI prevention interventions [65–68]. Implementation of STI control programs requires not only providing availability and access to these interventions, but also ensuring effective scale-up and sustainability for maximal population impact. The public health approach to STI control has had clear successes, for example, syphilis and gonorrhea infections have decreased dramatically among general populations of several countries with ample resources for STI control [69,70]. However, the gains have not been universal across all infections and all settings. Several important behavioral, biological, and implementation factors influence the potential prevention impact of available interventions (Fig. 2), and are discussed below.
4.1. Behavioral and sexual network factors
Several factors can influence the effectiveness of behavioral primary prevention efforts. Consistent and correct condom use reduces the transmission risk of virtually every STI [65], and some countries have documented declines in STI incidence in concert with implementation of counseling promoting condom use [71]. However, there have been limits to how much progress has been made with condom promotion as the main primary prevention measure for most STIs, especially among young people. Cultural factors impact not only the acceptability of condom use, but also the comfort level with discussing sexual practices and the gender and number of partners and providing STI-related education. In addition, although several randomized trials have demonstrated that behavioral interventions can reduce STI acquisition, none of these assessed sustainability of behavior change past one year [68], which is a key factor in determining long-term impact [72]. Finally, sexual networks reflect how individuals in a population are linked through sexual relationships and thus the pathways through which STIs can be transmitted. In many populations, individual behavior may be less important than network risk, that is, the risk of the individual’s sex partner or STI prevalence in the community [16,72].
4.2. Asymptomatic and long-lasting infection
The vast majority of STIs cause few or no symptoms but can still lead to harmful reproductive sequelae, especially among women. Thus, the standard STI control approach based on symptomatic case management misses the greatest burden of STIs from the outset. Even when STI symptoms occur, they may be nonspecific, which affects syndromic diagnosis. While syndromic management can be more accurate for syndromes such as urethral discharge in men, it performs poorly for nonspecific syndromes like vaginal discharge [73].
STIs that are likely to be symptomatic soon after acquisition, e.g., gonorrhea in men, tend to be treated quickly in areas with quality health services. These infections are removed from the population and transmission is sustained only among groups in which high-risk sexual behaviors are common [69,70]. Infections that are more likely to be asymptomatic and of longer duration may spread more generally through the population, e.g., chlamydia and HPV infections, which can persist without symptoms for a year or more [74,75], and HSV-2 infections, which are lifelong and mostly unrecognized [76]. For these infections, prevention strategies that only partially reduce transmission may have more limited impact at the population level.
4.3. Antimicrobial resistance and treatment barriers
Several efficacious medications exist to treat STIs [65]. However, drug resistance, especially for gonorrhea, is a major threat to STI control globally. Third-generation cephalosporins are the last class of antimicrobials to which <5% of gonorrheal isolates are resistant worldwide, but resistant strains are being increasingly reported [77–79]. Nitroimidazoles are the only class of antimicrobials active against trichomoniasis, and low-level resistance is also on the rise [80,81]. Tetracyclines and macrolides can be used to treat chlamydia, but treatment failures with both have been observed in approximately 10% of cases [82]. In low-income countries, insecure supplies of essential drugs, use of ineffective alternative medications, and treatment in informal settings, such as by drug vendors or traditional healers, all contribute to antimicrobial resistance and hamper STI control efforts.
4.4. Repeat infections
Curable STIs do not result in strong, lasting protective immunity after natural infection. While protective immunity may exist for some infections [83,84], it is easily overcome, and repeat infections are common [85,86]. Repeat infection rates for chlamydia, gonorrhea, and trichomoniasis range from 10–20% after treatment of an initial infection [85,86]. Repeat infection is even more common when little attention is paid to notification and treatment of sex partners of infected patients. Partner management strategies have proven challenging in most settings, especially if resources are limited or partner information is unknown. Data are particularly limited on ways to improve the numbers of partners treated in resource-poor settings [66].
4.5. Health system and implementation factors
Some key challenges exist related to effective implementation of STI control strategies. STIs are often stigmatizing and, in the setting of competing priorities, have often received little public policy attention [66]. These issues are compounded by the fact that many existing STI interventions are either not fully effective by themselves or it has been difficult to directly quantify their impact, making it harder to garner support.
Lack of availability and access to effective interventions hinders STI control in much of the world. Without an effective primary prevention tool such as a vaccine, or a feasible point-of-care diagnostic test with on-site curative treatment and a platform to access large numbers of infected persons, implementation of STI prevention remains challenging. This is especially true in resource-poor settings, where both health infrastructure and care-seeking may be sub-optimal. For example, prior to HPV vaccine, the use of Pap test screening with treatment of cervical cancer precursors dramatically reduced cervical cancer cases and deaths in high-income countries. However, in lower-income countries, without the infrastructure needed for Pap screening, HPV-related cervical cancer remains a major public health problem [35].
For STI case management, availability and access to feasible, affordable diagnostic tests is crucial. New accurate point-of-care diagnostic tests for syphilis are now available and are cheap, easy to use, and make syphilis screening of antenatal and high-risk populations possible even in remote settings [87]. Rapid diagnostic tests for chlamydia, gonorrhea, and trichomoniasis may also be on the horizon [87]. However, availability of accurate tests and other interventions alone does not ensure effective implementation and control [61,88,89]. In addition to needing a platform to access infected persons, it takes commitment, resources, and mechanisms for scale-up, to ensure broad intervention coverage and uptake, steady procurement of supplies, and ongoing sustainability of implementation efforts [61].
5. Needs for the future: working toward new STI vaccines
Vaccines have the potential to overcome many behavioral, biological, and implementation barriers to reducing global STI burden. Here we outline the case for the major new targets for STI vaccine development.
5.1. HSV-2
The large numbers of HSV-2 infections globally [14] are extremely important because of the marked synergy between HSV-2 and HIV infections. In some areas, HSV-2 infection may account for up to 30–50% of new HIV infections [46,90]. Antiviral medications treat HSV-2 symptoms and decrease HSV and HIV genital shedding; however, current regimens do not prevent HIV acquisition or transmission [47,91]. Thus, primary prevention of HSV-2 infection is currently the only way to reduce the excess risk of HIV infection related to HSV-2.
Available primary prevention strategies for HSV-2, such as condom use, use of daily suppressive therapy by symptomatic partners, and medical male circumcision may be useful for individuals. However, efficacy of these interventions ranges from only 30–50% [16,92,93], and interventions like widespread serologic testing and suppressive antiviral therapy are costly and unlikely to be feasible on a large scale. Given mostly asymptomatic, lifelong HSV infection, with widespread transmission in the general population and lack of curative therapy, existing interventions are unlikely to provide broad population impact, and vaccines may be the only option for effective HSV control [76].
Recently, a tenofovir-containing microbicide gel halved the risk of HSV-2 acquisition in one clinical trial; additional trials are ongoing [94]. However, issues related to compliance and acceptability [95], and concerns about HIV resistance with antiretroviral-containing microbicides, remain barriers. A vaccine against HSV-2 infection could have a dramatic impact on HIV spread [96], in addition to preventing neonatal herpes and alleviating suffering associated with genital herpes symptoms, and is a critical need for global public health [97].
5.2. Chlamydia
The global burden of chlamydia-related PID, infertility, ectopic pregnancy, and pregnancy complications has yet to be quantified accurately but is likely very high. In low-income countries without laboratory infrastructure, most chlamydia infections are missed with current control strategies. New rapid diagnostic tests that can be used in remote settings may soon be available, but decisions about whether to screen for asymptomatic infection, among whom, and at what costs will not be completely straightforward [98].
Chlamydia screening programs have been difficult to bring to scale in high-income countries. Even in countries with longstanding chlamydia screening recommendations, the proportion of women screened regularly remains low [89,99]. Although these programs have likely contributed to reductions in PID incidence, their impact on chlamydia incidence is unclear, and they do not appear to have dramatically reduced chlamydia prevalence [88,99]. In addition, while it is clear that screening can reduce clinical PID, the effect of screening on infertility prevention has not been directly assessed, and it is unknown the degree to which some tubal damage has already occurred at the time of screening.
One of the main reasons for ongoing chlamydia transmission is the frequency of repeat infections [85,86]. It has been hypothesized that screening programs might make repeat infections more likely, through reductions in population-wide protective immunity [100]. This is a major concern because animal models show greater tissue destruction during repeat chlamydial infection compared with initial infection, although it is not clear whether repeat infections after screening are inherently more harmful in humans [101].
Improving partner treatment strategies to reduce repeat infections, continued broadening of chlamydia screening coverage where available, and validation of new chlamydia rapid tests are absolutely essential. However, the difficulties in program implementation and reduction of chlamydia prevalence in existing screening programs highlight the complexities of current chlamydia control efforts and the need for continued work toward an effective chlamydia vaccine [102].
5.3. Gonorrhea
Gonorrhea is an STI for which effectively implemented public health control strategies can reduce the burden of infection [69,70]. However, gonorrhea prevention is being threatened by the increasing prevalence of organisms with resistance to cephalosporins, the only class of first-line drugs recommended to treat gonorrhea [77,79]. Given that 106 million cases of gonorrhea occur each year [9], millions could be left at risk of developing gonorrhea-associated PID, infertility, ectopic pregnancy, pregnancy-related complications, and enhancement of HIV transmission.
Rapid development and evaluation of new antibiotics for the treatment of gonorrhea are critical, and two clinical trials of new regimens are ongoing [78]. However, N. gonorrhoeae has successively acquired resistance to four different classes of antibiotics since it was first treatable in the 1940s [78], and the rate of development of resistance appears to be increasing. While efforts are made to find new effective drug regimens for gonorrhea, to improve diagnostic capacity for gonorrhea in low-income settings, and to scale-up existing case management strategies, progress toward a gonorrhea vaccine is also urgently needed [103].
5.4. Trichomoniasis
More cases of trichomoniasis are estimated to occur each year than gonorrhea, chlamydia, and syphilis cases combined [9]. Genital symptoms, especially vaginal discharge and irritation, may have important adverse effects on quality of life. Trichomoniasis is also associated with more serious consequences, including preterm delivery among pregnant women and enhancement of HIV transmission. A lack of available diagnostic tests hampers control efforts globally, but especially in low-income countries. Although not yet at the same level of urgency as for gonorrhea, reports of low-level trichomonal antimicrobial resistance are worrisome, as just one drug class treats trichomoniasis [65]. Additional drug regimens and diagnostic tests for trichomoniasis should be pursued, while continued work is done toward developing trichomoniasis vaccines [104].
5.5. Syphilis
Among the curable STIs, syphilis has the lowest global incidence but accounts for the greatest number of DALYs lost [58], primarily related to the devastating consequences of mother-to-child transmission [28]. More than half a million adverse outcomes of syphilis in pregnancy are estimated to occur each year [28]. Congenital syphilis has been virtually eliminated as a public health problem in most high-income countries [69,70]. However, only about 30% of infected pregnant women in sub-Saharan Africa receive syphilis testing and treatment [28,87]. New point-of-care diagnostic tests, cheap curative treatment with one dose of penicillin, and an ante-natal platform to access infected pregnant women may now make it feasible to prevent a substantial proportion of congenital syphilis outcomes [64,105], and WHO has launched an initiative to eliminate congenital syphilis as a global public health problem [64]. However, if implementation remains challenging and prevention efforts do little to reduce community-wide prevalence, a syphilis vaccine will be an important pursuit [106].
5.6. Implementation of STI vaccines
HPV vaccination has not yet been implemented in low- and middle-income countries with the highest cervical cancer rates. Mathematical models estimate that if 70% vaccination coverage is achieved in low- and middle-income countries, HPV vaccines could prevent the deaths of more than 4 million women vaccinated over the next decade [107]. The GAVI Alliance has approved initial funding for HPV vaccination in eligible low-income countries, which is a major step toward ensuring universal access to HPV vaccine. However, the barriers related to providing a vaccine in early adolescence are even greater than those of including HBV vaccine in the infant immunization schedule. Barriers include difficulties accessing 11–14-year-olds in areas where health-care seeking and school attendance may be low, and parental or societal hesitation related to a vaccine against STIs for adolescents.
A great deal will be learned from current implementation of HPV vaccine to inform delivery of future STI vaccines. Most STI vaccines are being developed for early adolescents, to provide maximal protection before and during the time of highest risk. For some vaccines, there may be compelling reasons for infant vaccination in addition to implementation issues, for example, an HSV vaccine that would also protect against HSV-1 infection. Nonetheless, new adolescent platforms for health intervention delivery are needed to respond to a global agenda to improve adolescent health, especially sexual and reproductive health [108]. HPV vaccine implementation is an opportunity to develop these adolescent platforms, which can be used not only for currently recommended prevention services, but also for future STI vaccines. Given common risk factors, high rates of co-infection, and epidemiologic overlap in STI-related complications, combination STI vaccines for adolescents would be an important future goal. HPV vaccine implementation will also provide insight on monitoring vaccine impact, which will need to be considered for other STI vaccines well in advance of vaccine availability.
6. Conclusion
In the face of almost half a billion curable STIs occurring annually[9], more than half a billion people with a viral STI at any point in time [11,14], and the resulting burden of STI-related complications affecting sexual, reproductive, and maternal-child health, new prevention paradigms are needed. Existing STI prevention interventions can be optimally scaled up within a broad framework of health promotion and wellness, with normalization and integration of STI services into primary and reproductive healthcare settings. This includes strengthening sexual health education and condom promotion, optimizing STI diagnosis and screening where available, implementing effective partner treatment strategies, ensuring syphilis testing for all pregnant women, and broadening delivery of HPV vaccine. At the same time, given the unique obstacles to achieving global STI control for most existing interventions, innovative biomedical solutions are also critical. Validating new rapid diagnostic tests for curable STIs, evaluating new drug regimens for gonorrhea, and testing new microbicides against STIs will be extremely valuable, but these interventions may not fully solve long-term barriers to STI control. Thus, continued advancement of STI vaccines is crucial for sustainable global STI prevention and control.
Acknowledgments
The authors wish to thank Janet Petitpierre for her assistance with the figures.
Footnotes
Conflicts of Interest
We report no conflicts of interest.
Publisher's Disclaimer: Disclaimers
Drs. Newman and Broutet are staff members of the World Health Organization. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions or policies of the World Health Organization.
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].World Health Organization. Reproductive Health Strategy to Accelerate Progress Towards the Attainment of International Development Goals and Targets. Geneva: World Health Organization; 2004, http://www.who.int/reproductivehealth/publications/general/RHR_04_8/en/. [DOI] [PubMed] [Google Scholar]
- [2].UN Millennium Project. Investing in Development: A Practical Plan to Achieve the Millennium Development Goals. New York: United Nations Development Programme; 2005, http://www.who.int/hdp/publications/4b.pdf. [Google Scholar]
- [3].World Health Organization. Hepatitis B vaccines: WHO position paper. Wkly Epidemiol Rec 2009;84(40):405–20.19817017 [Google Scholar]
- [4].World Health Organization. Prevention and Control of Viral Hepatitis Infection: Framework for Global Action. Geneva: World Health Organization; 2012, http://www.who.int/csr/disease/hepatitis/GHP_framework.pdf. [Google Scholar]
- [5].World Health Organization. Hepatitis B: Immunization surveillance, assessment, and monitoring; 2013, http://www.who.int/immunization_monitoring/diseases/hepatitis/en/.
- [6].Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012;30(Suppl. 5):F123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baandrup L, Blomberg M, Dehlendorff C, Sand C, Andersen KK, Kjaer SK. Significant decrease in the incidence of genital warts in young Danish women after implementation of a national human papillomavirus vaccination program. Sex Transm Dis 2013;40(2):130–5. [DOI] [PubMed] [Google Scholar]
- [8].Read TR, Hocking JS, Chen MY, Donovan B, Bradshaw CS, Fairley CK. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect 2011;87(7):544–7. [DOI] [PubMed] [Google Scholar]
- [9].World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections – 2008. Geneva: World Health Organization; 2012, http://www.who.int/reproductivehealth/publications/rtis/stisestimates/en/index.html. [Google Scholar]
- [10].World Health Organization. Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis. Methods and results used by WHO to generate 2005 estimates. Geneva: World Health Organization; 2011, http://www.who.int/reproductivehealth/publications/rtis/9789241502450/en/index.html. [Google Scholar]
- [11].de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007;7(7):453–9. [DOI] [PubMed] [Google Scholar]
- [12].Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis 2006;194(8):1044–57. [DOI] [PubMed] [Google Scholar]
- [13].Smith JS, Gilbert PA, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of human papillomavirus infection in males: a global review. J Adolesc Health 2011;48(6):540–52. [DOI] [PubMed] [Google Scholar]
- [14].Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ 2008;86(10):805–12. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013;40(3):187–93. [DOI] [PubMed] [Google Scholar]
- [16].Centers for Disease Control and Prevention. Seroprevalence of herpes simplex virus type 2 among persons aged 14–49 years–United States, 2005–2008. MMWR Morb Mortal Wkly Rep 2010;59(15):456–9. [PubMed] [Google Scholar]
- [17].Watson-Jones D, Weiss HA, Rusizoka M, et al. Risk factors for herpes simplex virus type 2 and HIV among women at high risk in northwestern Tanzania: preparing for an HSV-2 intervention trial. J Acquir Immune Defic Syndr 2007;46(5):631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chico RM, Mayaud P, Ariti C, Mabey D, Ronsmans C, Chandramohan D. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: a systematic review. JAMA 2012;307(19):2079–86. [DOI] [PubMed] [Google Scholar]
- [19].World Health Organization Regional Office of the Western Pacific. HIV and sexually transmitted infections in the Western Pacific region, 2000–2010. Manila: World Health Organization Regional Office of the Western Pacific; 2012, http://www.wpro.who.int/publications/2012/document_hiv_and_sti_2000-2010.pdf. [Google Scholar]
- [20].Lin CC, Gao X, Chen XS, Chen Q, Cohen MS. China’s syphilis epidemic: a systematic review of seroprevalence studies. Sex Transm Dis 2006;33(12):726–36. [DOI] [PubMed] [Google Scholar]
- [21].Woodhall S, Ramsey T, Cai C, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect 2008;84(3):161–6. [DOI] [PubMed] [Google Scholar]
- [22].Dinh TH, Sternberg M, Dunne EF, Markowitz LE. Genital warts among 18- to 59-year-olds in the United States, national health and nutrition examination survey, 1999–2004. Sex Transm Dis 2008;35(4):357–60. [DOI] [PubMed] [Google Scholar]
- [23].Kjaer SK, Tran TN, Sparen P, et al. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J Infect Dis 2007;196(10):1447–54. [DOI] [PubMed] [Google Scholar]
- [24].Aumakhan B, Gaydos CA, Quinn TC, et al. Clinical reactivations of herpes simplex virus type 2 infection and human immunodeficiency virus disease progression markers. PLoS One 2010;5(4):e9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Denny LA, Franceschi S, de S S, Heard I, Moscicki AB, Palefsky J. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine 2012;30(Suppl. 5):F168–74. [DOI] [PubMed] [Google Scholar]
- [26].Johnson HL, Ghanem KG, Zenilman JM, Erbelding EJ. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner city public sexually transmitted diseases clinics. Sex Transm Dis 2011;38(3):167–71. [DOI] [PubMed] [Google Scholar]
- [27].Marchant T, Willey B, Katz J, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med 2012;9(8):e1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Newman L, Kamb M, Hawkes S, et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS Med 2013;10(2):e1001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schaller UC, Klauss V. Is Crede’s prophylaxis for ophthalmia neonatorum still valid? Bull World Health Organ 2001;79(3):262–3. [PMC free article] [PubMed] [Google Scholar]
- [30].Schachter J, Grossman M, Sweet RL, Holt J, Jordan C, Bishop E. Prospective study of perinatal transmission of Chlamydia trachomatis. JAMA 1986;255(24):3374–7. [PubMed] [Google Scholar]
- [31].Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 2003;289(2):203–9. [DOI] [PubMed] [Google Scholar]
- [32].Dinh TH, Dunne EF, Markowitz LE, Weinstock H, Berman S. Assessing neonatal herpes reporting in the United States, 2000–2005. Sex Transm Dis 2008;35(1):19–21. [DOI] [PubMed] [Google Scholar]
- [33].Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis 1995;20(4):992–1000. [DOI] [PubMed] [Google Scholar]
- [34].Bosch FX, de S S. The epidemiology of human papillomavirus infection and cervical cancer. Dis Markers 2007;23(4):213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].GLOBOCAN. International Agency for Research on Cancer. Cervical cancer incidence and mortality worldwide; 2008, http://globocan.iarc.fr/factsheets/cancers/cervix.asp.
- [36].Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005;34(6):1329–39. [DOI] [PubMed] [Google Scholar]
- [37].Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 2010;201(Suppl. 2):S134–55. [DOI] [PubMed] [Google Scholar]
- [38].Oakeshott P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ 2010;340:c1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992;19(4):185–92. [PubMed] [Google Scholar]
- [40].Hillis SD, Joesoef R, Marchbanks PA, Wasserheit JN, Cates W Jr, Westrom L. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol 1993;168(5):1503–9. [DOI] [PubMed] [Google Scholar]
- [41].Mascarhenas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9(12):e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rutstein SO, Shah IH. Infecundity, infertility, and childlessness in developing countries. DHS Comparative Reports No. 9. Calverton, Maryland, USA: ORC Macro and the World Health Organization; 2004. [Google Scholar]
- [43].Feinberg EC, Larsen FW, Catherino WH, Zhang J, Armstrong AY. Comparison of assisted reproductive technology utilization and outcomes between Caucasian and African American patients in an equal-access-to-care setting. Fertil Steril 2006;85(4):888–94. [DOI] [PubMed] [Google Scholar]
- [44].Macaluso M, Wright-Schnapp TJ, Chandra A, et al. A public health focus on infertility prevention, detection, and management. Fertil Steril 2010;93(1):16–20. [DOI] [PubMed] [Google Scholar]
- [45].Cates W, Farley TM, Rowe PJ. Worldwide patterns of infertility: is Africa different. Lancet 1985;2(8455):596–8. [DOI] [PubMed] [Google Scholar]
- [46].Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006;20(1):73–83. [DOI] [PubMed] [Google Scholar]
- [47].Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010;362(5):427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 2001;357(9263):1149–53. [DOI] [PubMed] [Google Scholar]
- [49].Hayes R, Watson-Jones D, Celum C, van de Wijgert J, Wasserheit J. Treatment of sexually transmitted infections for HIV prevention: end of the road or new beginning. AIDS 2010;24(Suppl 4):S15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sexton J, Garnett G, Rottingen JA. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sex Transm Dis 2005;32(6):351–7. [DOI] [PubMed] [Google Scholar]
- [51].Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 1997;349(9069):1868–73. [DOI] [PubMed] [Google Scholar]
- [52].Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis 2008;35(11):946–59. [DOI] [PubMed] [Google Scholar]
- [53].Houlihan CF, Larke NL, Watson-Jones D, et al. Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. AIDS 2012;26(17):2211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fortenberry JD, McFarlane M, Bleakley A, et al. Relationships of stigma and shame to gonorrhea and HIV screening. Am J Public Health 2002;92(3):378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gottlieb SL, Stoner BP, Zaidi AA, et al. A prospective study of the psychosocial impact of a positive Chlamydia trachomatis laboratory test. Sex Transm Dis 2011;38(11):1004–11. [DOI] [PubMed] [Google Scholar]
- [56].Mortensen GL, Larsen HK. The quality of life of patients with genital warts: a qualitative study. BMC Public Health 2010;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Decker MR, Miller E, McCauley HL, et al. Intimate partner violence and partner notification of sexually transmitted infections among adolescent and young adult family planning clinic patients. Int J STD AIDS 2011;22(6):345–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2197–223. [DOI] [PubMed] [Google Scholar]
- [59].Garnett GP. The theoretical impact of vaccines that protect against sexually transmitted infections and disease. Vaccine 2013, current issue. [DOI] [PubMed] [Google Scholar]
- [60].Owusu-Edusei K Jr, Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013;40(3):197–201. [DOI] [PubMed] [Google Scholar]
- [61].World Health Organization. Global strategy for the prevention and control of sexually transmitted infections: 2006–2015: breaking the chain of transmission. Geneva: World Health Organization; 2007, http://www.who.int/reproductivehealth/publications/rtis/9789241563475/en/index.html. [Google Scholar]
- [62].Lewis DA. HIV/sexually transmitted infection epidemiology, management and control in the IUSTI Africa region: focus on sub-Saharan Africa. Sex Transm Infect 2011;87(Suppl. 2):ii10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Scholes D, Stergachis A, Heidrich FE, Andrilla H, Holmes KK, Stamm WE. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med 1996;334(21):1362–6. [DOI] [PubMed] [Google Scholar]
- [64].World Health Organization. The global elimination of congenital syphilis: rationale and strategy for action. Geneva: World Health Organization; 2007, http://www.who.int/reproductivehealth/publications/rtis/9789241595858/en. [Google Scholar]
- [65].Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines; 2010. MMWR 2010; 59(RR-12). [PubMed] [Google Scholar]
- [66].Low N, Broutet N, Adu-Sarkodie Y, Barton P, Hossain M, Hawkes S. Global control of sexually transmitted infections. Lancet 2006;368(9551):2001–16. [DOI] [PubMed] [Google Scholar]
- [67].Manhart LE, Holmes KK. Randomized controlled trials of individual-level, population-level, and multilevel interventions for preventing sexually transmitted infections: what has worked. J Infect Dis 2005;191(Suppl. 1):S7–24. [DOI] [PubMed] [Google Scholar]
- [68].Wetmore CM, Manhart LE, Wasserheit JN. Randomized controlled trials of interventions to prevent sexually transmitted infections: learning from the past to plan for the future. Epidemiol Rev 2010;32(1):121–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2011. Atlanta, U.S.: Department of Health and Human Services; 2012, http://www.cdc.gov/std/stats11/Surv2011.pdf. [Google Scholar]
- [70].European Centre for Disease Prevention and Control. Sexually transmitted infections in Europe 1990–2010. Stockholm: ECDC; 2012, http://www.ecdc.europa.eu/en/publications/publications/201206-sexually-transmitted-infections-europe-2010.pdf. [Google Scholar]
- [71].Park LS, Siraprapasiri T, Peerapatanapokin W, Manne J, Niccolai L, Kunanusont C. HIV transmission rates in Thailand: evidence of HIV prevention and transmission decline. J Acquir Immune Defic Syndr 2010;54(4): 430–6. [DOI] [PubMed] [Google Scholar]
- [72].Aral SO. Utility and delivery of behavioural interventions to prevent sexually transmitted infections. Sex Transm Infect 2011. December;87(Suppl. 2):ii31–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pettifor A, Walsh J, Wilkins V, Raghunathan P. How effective is syndromic management of STDs? a review of current studies. Sex Transm Dis 2000;27(7):371–85. [DOI] [PubMed] [Google Scholar]
- [74].Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008;26(Suppl. 10):K1–16. [DOI] [PubMed] [Google Scholar]
- [75].Geisler WM. Duration of untreated, uncomplicated Chlamydia trachomatis genital infection and factors associated with chlamydia resolution: a review of human studies. J Infect Dis 2010;201(Suppl. 2):S104–13. [DOI] [PubMed] [Google Scholar]
- [76].Tronstein E, Johnston C, Huang ML, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 2011;305(14):1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Allen VG, Mitterni L, Seah C, et al. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA 2013;309(2):163–70. [DOI] [PubMed] [Google Scholar]
- [78].Kirkcaldy RD, Bolan GA, Wasserheit JN. Cephalosporin-resistant gonorrhea in North America. JAMA 2013;309(2):185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lewis DA, Lukehart SA. Antimicrobial resistance in Neisseria gonorrhoeae and Treponema pallidum: evolution, therapeutic challenges and the need to strengthen global surveillance. Sex Transm Infect 2011;87(Suppl. 2):ii39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bachmann LH, Hobbs MM, Sena AC, et al. Trichomonas vaginalis genital infections: progress and challenges. Clin Infect Dis 2011;53(Suppl. 3):S160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kirkcaldy RD, Augostini P, Asbel LE, et al. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD Surveillance Network, 2009–2010. Emerg Infect Dis 2012;18(6):939–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Manhart LE, Gillespie CW, Lowens MS, et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: a randomized controlled trial. Clin Infect Dis 2013;56(7):934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Batteiger BE, Xu F, Johnson RE, Rekart ML. Protective immunity to Chlamydia trachomatis genital infection: evidence from human studies. J Infect Dis 2010;201(Suppl. 2):S178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Geisler WM, Lensing SY, Press CG, Hook III EW. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. J Infect Dis 2013;207(12):1850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Das A, Pathni AK, Narayanan P, et al. High rates of reinfection and incidence of bacterial sexually transmitted infections in a cohort of female sex workers from two Indian cities: need for different STI control strategies? Sex Transm Infect 2013;89(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis 2009;36(8):478–89. [DOI] [PubMed] [Google Scholar]
- [87].Peeling RW. Applying new technologies for diagnosing sexually transmitted infections in resource-poor settings. Sex Transm Infect 2011;87(Suppl. 2):ii28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Datta SD, Torrone E, Kruszon-Moran D, et al. Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999–2008. Sex Transm Dis 2012;39(2):92–6. [DOI] [PubMed] [Google Scholar]
- [89].Heijne JC, Tao G, Kent CK, Low N. Uptake of regular chlamydia testing by U.S. women: a longitudinal study. Am J Prev Med 2010;39(3):243–50. [DOI] [PubMed] [Google Scholar]
- [90].Freeman EE, Orroth KK, White RG, et al. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect 2007;83(Suppl. 1):i17–24. [DOI] [PubMed] [Google Scholar]
- [91].Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371(9630):2109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Corey L, Ashley R. Prevention of herpes simplex virus type 2 transmission with antiviral therapy. Herpes 2004;11(Suppl. 3):170A–4A. [PubMed] [Google Scholar]
- [93].Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 2009;360(13):1298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Tan D. Potential role of tenofovir vaginal gel for reduction of risk of herpes simplex virus in females. Int J Womens Health 2012;4:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Marrazzo J, Ramjee G, Nair G. VOICE Study Team. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003); 2013, http://www.retroconference.org/2013b/Abstracts/47951.htm.
- [96].Freeman EE, White RG, Bakker R, et al. Population-level effect of potential HSV2 prophylactic vaccines on HIV incidence in sub-Saharan Africa. Vaccine 2009;27(6):940–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Johnston C, Koelle DM, Wald A. Current status and prospects for development of an HSV-2 vaccine. Vaccine 2013, current issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Low N, Cassell JA, Spencer B, et al. Chlamydia control activities in Europe: cross-sectional survey. Eur J Public Health 2012;22(4):556–61. [DOI] [PubMed] [Google Scholar]
- [99].Gottlieb SL, Xu F, Brunham RC. Screening and treating Chlamydia trachomatis genital infection to prevent pelvic inflammatory disease: interpretation of findings from randomized controlled trials. Sex Transm Dis 2013;40(2):97–102. [DOI] [PubMed] [Google Scholar]
- [100].Brunham RC, Rekart ML. The arrested immunity hypothesis and the epidemiology of chlamydia control. Sex Transm Dis 2008;35(1):53–4. [DOI] [PubMed] [Google Scholar]
- [101].Gottlieb SL, Martin DH, Xu F, Byrne GI, Brunham RC. Summary: The natural history and immunobiology of Chlamydia trachomatis genital infection and implications for chlamydia control. J Infect Dis 2010;201(Suppl. 2): S190–204. [DOI] [PubMed] [Google Scholar]
- [102].Hafner LM, Wilson DP, Timms P. Development status and future vaccines for Chlamydia trachomatis infections. Vaccine 2013, current issue. [DOI] [PubMed] [Google Scholar]
- [103].Jerse AE, Bash MC, Russell MW. Vaccines against gonorrhea: current status and future challenges. Vaccine 2013, current issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Smith J, Garber GE. Current status and prospects for development of a vaccine against Trichomonas infections. Vaccine 2013, current issue. [DOI] [PubMed] [Google Scholar]
- [105].Hawkes S, Matin N, Broutet N, Low N. Effectiveness of interventions to improve screening for syphilis in pregnancy: a systematic review and meta-analysis. Lancet Infect Dis 2011;11(9):684–91. [DOI] [PubMed] [Google Scholar]
- [106].Cameron CE, Lukehart SA. Current status of syphilis vaccine development: need, challenges, prospects. Vaccine 2013, current issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Goldie SJ, O’Shea M, Diaz M, Kim SY. Benefits, cost requirements and cost-effectiveness of the HPV16,18 vaccine for cervical cancer prevention in developing countries: policy implications. Reprod Health Matters 2008;16(32):86–96. [DOI] [PubMed] [Google Scholar]
- [108].Broutet N, Lehnert N, Mehl Geal. Effective health interventions for adolescents that could be integrated with human papillomavirus vaccination programs. J Adolesc Health 2013, doi:pii: S1054–139X(13)00144–4. [DOI] [PubMed] [Google Scholar]