Abstract
Background and Objectives
The number of persons living with dementia (PLWD) in the United States will reach 16 million by 2050. Behavioral and psychological symptoms of dementia challenge family caregivers and contribute to negative caregiver outcomes such as burden and depression. Available technology can support the delivery of effective interventions to families providing dementia care at home. The Supporting Family Caregivers with Technology for Dementia Home Care (FamTechCare) randomized controlled trial evaluated the effects of a telehealth intervention on caregiver outcomes.
Research Design and Methods
The FamTechCare intervention provides tailored dementia-care strategies to in-home caregivers based on video recordings caregivers submit of challenging care situations. An expert team reviews the videos and provides individualized interventions weekly for the experimental group. In the telephone-support attention control group, caregivers receive feedback from an interventionist via the telephone based on caregiver retrospective recall of care challenges. Effects of the intervention on caregiver outcomes, including burden, depression, sleep disturbance, competence, desire to institutionalize the PLWD, and caregiver reaction to behavioral symptoms were evaluated by fitting linear mixed regression models to changes in the outcomes measured at 1 and 3 months.
Results
FamTechCare caregivers (n = 42) had greater reductions in depression (p = .012) and gains in competence (p = .033) after 3 months compared to the attention control group (n = 41). Living in rural areas was associated with a reduction in depression for FamTechCare caregivers (p = .002). Higher level of education was associated with greater improvements or lesser declines in burden, competence, and reaction to behavioral symptoms for both the FamTechCare and attention control caregivers.
Discussion and Implications
This research demonstrated benefits of using available technology to link families to dementia care experts using video-recording technology. It provides a foundation for future research testing telehealth interventions, tailored based on rich contextual data to support families, including those in rural or remote locations.
Keywords: Alzheimer disease, Behavioral symptoms, Caregivers, Dementia, Telemedicine
Translational Significance:
The study demonstrated that videos, recorded and submitted by caregivers, provide rich contextual data for determining tailored interventions to meet the needs of specific dyads, effectively linking caregivers to experts for in-home care guidance and reducing negative caregiver outcomes.
Background and Objectives
The population of persons and families living with dementia is projected to expand from 47 million in 2015 to 132 million by 2050.1 In 2017, there were approximately 15.9 million unpaid in-home dementia caregivers who provided an estimated 18.4 billion hours of care, saving the United States $232 billion in health care costs.2 The stress, strain, and burden of dementia caregiving can contribute to negative physical and mental health outcomes for family caregivers further contributing to health care costs.2 Family caregivers of persons living with dementia (PLWD) must cope with the care recipient’s progressive memory loss, self-care impairment, and communication breakdown, which may lead to caregiver depression, insomnia, psychotropic medication use, and increased morbidity and mortality.2–4 There is strong evidence that severity of neuropsychiatric symptoms of PLWD worsens caregiver burden and is often predicted by poor coping, isolation, and insufficient dementia knowledge.5,6 Worldwide, cost-effective dementia care that supports quality of life is considered a public health priority.7
Over the past several decades, research focused on identifying predictors of negative outcomes of caregiving has led to the development and testing of interventions to support family caregivers of PLWD. Many interventions have focused on nonpharmacological management to prevent and reduce challenging behavioral and psychological symptoms of dementia.8–12 However, few of these interventions targeted the dyad (i.e., caregiver + PLWD) itself, but rather the caregiver or PLWD alone.9 Nonpharmacologic interventions have concentrated on a variety of factors such as environment or training and education both indirectly (i.e., caregiver focused) and directly (i.e., PLWD focused).8 Within these factors, the interventions’ emphasis often follows a continuum such as safety versus individualization, social environment versus physical environment, and one-time training versus ongoing training and support.8 Long-term individualized interventions concentrating directly and indirectly on the challenging behaviors that address both the physical and social environment appear to have the best effect on PLWD; however, few of these interventions exist.8 A recent meta-review on nonpharmacological interventions concluded that in order for dementia care interventions to be successful, they need to focus on specific strategies for specific caregiver stressors rather than the current model, which focuses on general coping and care strategies.9
Integrating nonpharmacologic approaches for behavioral management for dementia care involves first describing the behavior, identifying the underlying cause, devising a treatment plan, and evaluating if the intervention was successful.13,14 Traditionally, experts are only able to provide care interventions based on retrospective recall from the family caregiver, rather than through direct observation. However, identifying and managing behavioral and psychological symptoms of dementia requires expertise and relying on retrospective recall may limit the information clinicians can use to form recommendations.15 Due to the stress and strain of caregiving, caregivers may be limited in their historical recall particularly when it comes to identifying and communicating precipitating factors, underlying PLWD needs, environmental stressors, and caregiver triggers. Being unable to accurately identify these factors may limit the advice care providers can provide because addressing the interaction of PLWD needs, environment, neurobiology, and the caregiver itself is the foundation to combating caregiving challenges.13,16
Technology provides an opportunity not only to disseminate evidence-based interventions to caregivers to improve dementia care, but to also allow for new opportunities to tailor caregiver support.17 Currently, a number of technologies are available to support family caregivers, ranging from simple provision of information, to support programs with peers and/or professionals, to actual training, as well as psychotherapy for caregivers.17–22 However, many of these technological interventions may not meet the robust recommendations for effective nonpharmacological interventions including a focus on the dyad with individualized approaches targeting individualized challenges. Caregivers recognized the potential benefits of technology as a resource to assist in caregiving, specifically desiring technology as a resource when it allows for personalized professional consultation and guidance for providing care.23 Caregivers are receptive to using technology, and as younger caregiver cohorts become more tech-savvy, issues ranging from familiarity with technology to privacy and internet access become less prohibitive. Further, the internet now reaches most rural areas, providing a medium for distributing support to more isolated family caregivers.
The Supporting Family Caregivers for Dementia Care (FamTechCare) clinical trial evaluated the effects of a robust technology-based telehealth intervention on caregiver outcomes. The FamTechCare intervention was developed to address the care dyad by providing tailored feedback based on specific care encounters viewed by dementia care experts. The FamTechCare intervention attempts to overcome the challenges in retrospective recall, by allowing experts to see directly into the home for tailored support.
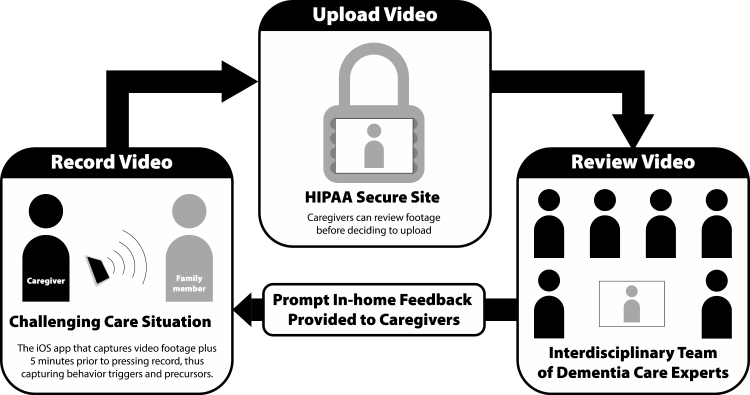
The FamTechCare telehealth intervention uses an innovative video-recording application that allows for enhanced capture of PLWD behaviors and links caregivers to dementia experts for tailored support. In the FamTechCare intervention, caregivers video recorded challenging care situations using a novel application where antecedent behavior is captured via a buffering technology integrated within the application. To ensure privacy, caregivers review recorded videos and elect to upload the videos to a HIPAA-secure website for expert review. If uploaded, an interdisciplinary team of dementia care experts review the recordings and develop tailored interventions that an interventionist communicates back to the caregiver via telephone (Figure 1).
Figure 1.
FamTechCare study procedure. Graphic Design by Chris Lorenzen for Kristine Williams © 2016.
This article reports the main analysis of the Supporting Family Caregivers for Dementia Care clinical trial. The effects on caregiver-focused outcomes for the FamTechCare intervention group were compared to changes in the attention control group (i.e., telephone-support based on caregiver retrospective recall). It was hypothesized that there would be a greater decline in caregiver burden, depression, sleep disturbance, desire to institutionalize, and reaction to behavioral symptoms of the PLWD and greater gains in competence among caregivers who received the FamTechCare intervention compared to caregivers who received the attention control.
Research Design and Methods
Design
A randomized controlled trial was conducted to test the effects of the FamTechCare intervention versus telephone support on caregiver-focused outcomes over a 3-month study period. The study was conducted at two research sites in the Midwest and study procedures were approved by the Institutional Review Board for the protection of Human Subjects at both sites. The trial was registered with ClinicalTrials.gov (NCT02483520).
Study Sample
Participants were recruited between October 2014 and June 2018. Inclusion criteria for PLWD were a dementia diagnosis and living at home. PLWD were excluded if diagnosed with Huntington’s disease, schizophrenia, manic-depressive disorder, deafness, or intellectual disability. Caregivers were required to be in-home family caregivers. To meet recruitment goals, caregiver inclusion criteria were expanded to other family members and formal caregivers that families hired who provide care at least weekly. Informed consent was obtained from all participants or their surrogate decision makers, and assent was obtained from PLWD who were unable to consent independently.
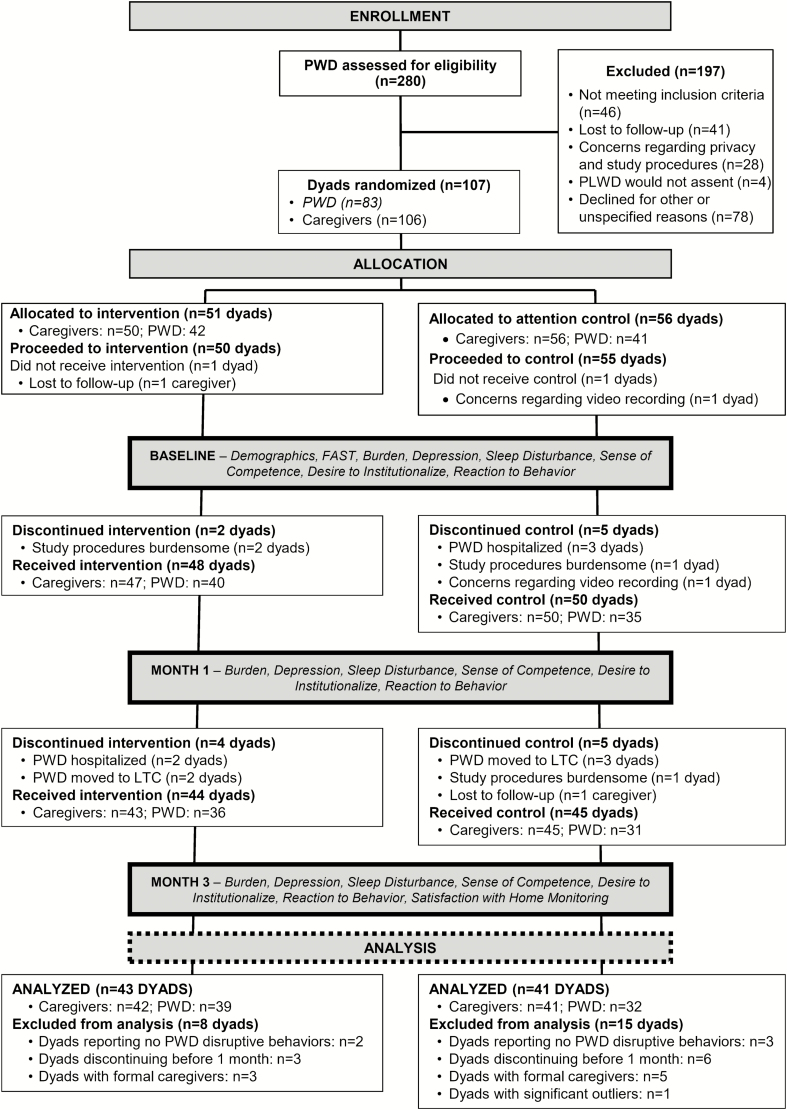
Caregiver-PLWD dyads were randomly assigned to the experimental (FamTechCare) group or the attention control group using a quarter-based blocking strategy with 1:1 allocation developed by the study statistician. Caregivers in multiple dyad homes were cluster randomized to the same group. Power to detect between-group differences in caregiver outcomes was estimated based on published reports of large effect sizes in studies testing psychoeducational interventions similar to FamTechCare.24 Power was estimated at 92% for detecting differences in 70 dyads using an assigned Type I error of 0.05 and assuming average overall effect sizes of 0.81. Figure 2 describes enrollment and attrition. See the published FamTechCare protocol for specific details on participant recruitment and eligibility, the protection of human subjects, intervention development, and study procedures and fidelity.25
Figure 2.
Enrollment and attrition.
FamTechCare Intervention
FamTechCare is a multicomponent video-recording intervention. Following enrollment, caregivers were provided with the telehealth video-monitoring unit (VMU) and trained in the recording and submission process. The VMU included an iPad Mini with the Behavior Capture (the video-recording application), a Bluetooth remote, and an iPad stand. The Behavior Capture application (https://behaviorimaging.com, Boise, ID) utilizes a buffering technology to capture antecedents leading to a challenging care situation. When a caregiver triggers “record” manually on the iPad or via the Bluetooth remote, the Behavior Capture application provides both prospective and retrospective recording, and thus includes the time period immediately leading up the caregiver video activation. Using the iPad on the stand with the iPad connected to a power source allows the application to be upright and constantly running. Caregivers review each recording and decide whether to delete or upload the video to the HIPAA-secure Behavior Connect website for review by the expert team. Upload requires internet access; so, a wireless hotspot was provided free of charge along with the standard study equipment if needed. All materials were provided at no cost to the participants and were returned following completion of the study.
Video recordings uploaded to Behavior Connect were reviewed weekly by a team of dementia care experts. A designated team member reviewed each video within 24 hr of submission to ensure there were no immediate safety concerns and then selected and cued the videos for expert review. Each study site held weekly review meetings with separate expert teams. The video review team included research and health care professionals with substantial dementia care knowledge from the fields of nursing, geriatric psychiatry, social work, and psychology. Other specialties such as speech pathology, dentistry, or occupational therapy were consulted as needed. All video review and subsequent discussions took place as a group and occurred either in-person or remotely using Zoom web conferencing. Zoom provides a share-screen feature, so all group members were able to watch the videos simultaneously and develop tailored interventions through group discussion. Members of the expert team provided feedback and interventions based on their clinical expertise and evidence-based dementia care protocols. To assure consistency of the approach, both sites evaluated videos within the framework of the Need-based Dementia Compromised Behavior Model and used a protocol manual developed by a member of the research team.26,27 The interventionist then relayed the tailored interventions to each caregiver during a scheduled phone call each week during the 3-month trial.
Telephone-Support Attention Control
The telephone-support attention control group (attention control group) received the same interventionist support but without the tailored feedback based on expert review of video recordings. Attention control caregivers had a weekly scheduled phone call with the interventionist in which they relayed challenges retrospectively and received care guidance using the same protocol manual used for the FamTechCare group. Thus, the attention control group was not a true control group as they received tailored interventions relying on caregiver retrospective recall rather than video review. The attention control interventionist was also a member of the expert review team, followed the same protocol manual, and was able to discuss control caregiver challenges with the expert review team. The difference between the two groups was that the attention control feedback was based on retrospective recall rather than video-support. The attention control caregivers were also provided with the VMU and were trained and encouraged to record and submit weekly videos. However, their videos were reviewed by the expert team only at the completion of the 3-month trial and feedback was provided after they completed final outcome assessments.
Data Collection and Outcome Measures
PLWD measures
Demographic information was collected about the PLWD at baseline including age, gender, marital status, education, former occupation, medication utilization, rural residence, type of dementia, dementia severity, and year of diagnosis. Dementia severity was measured via the Functional Assessment Scale (FAST), a 16-item scale assessing function and dementia symptoms. The FAST has adequate intra-rater reliability (Intraclass Correlation Coefficient [ICC] = .86) and inter-rater reliability (ICC = .87),28 adequate concurrent and convergent validity with numerous dementia indicators,29–32 and is not biased by the ceiling and floor effects. Rural residence was determined by percent of rural population in the county in which the PLWD resided. Counties were categorized as rural (≥20% rural population) or urban (<20% rural).
Caregiver measures
Demographic information for the caregiver was collected at baseline and included age, gender, marital status, education, relationship to the PLWD, medication utilization, length of caregiving, and types of care provided. Caregiver-focused outcomes were measured at baseline and repeated at 1 month and 3 months. These included caregiver burden, depression, sleep disturbance, competence, desire to institutionalize, and reaction to behavioral symptoms of the PLWD. Upon completion of the study, caregivers completed a survey about satisfaction with video monitoring.
Caregiver burden was measured using the Modified Zarit Burden Scale,33 that contains 12 items with a 5-point Likert scale (0 = never; 4 = nearly always) adapted from the 22-item Zarit Burden Interview. A higher score indicates greater caregiver burden (range = 0–48). The Modified Zarit Burden Scale has shown adequate internal consistency (α = .88) and excellent concurrent validity (r = .92–.97) with the full Zarit Burden Interview.33
Caregiver depression was measured using the Center for Epidemiologic Studies Depression scale (CES-D).34 The CES-D contains 20 items with a 4-point Likert scale ranging from 0 = rarely or none of the time (i.e., less than 1 day in past week) to 3 = most or all of the time (i.e., 5–7 days in past week). A higher score indicates greater depression (range = 0–60). The CES-D shows adequate internal consistency (α = .84–.90), moderate convergent validity with other depression scales (r = .44–.75),34 and has been shown to effectively measure change in psychoeducational interventions for dementia caregivers.35
Caregiver sleep disturbance was measured by the Pittsburgh Sleep Quality Index (PSQI).36 The PSQI contains 19 items with a 4-point Likert scale across seven domains: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction. A higher global score indicates worse sleep quality (range = 0–21). The PSQI shows adequate internal consistency (α = .83) and adequate validity in differentiating patients with and without sleep disorders.36
Caregiver sense of competence was measured using the Short Sense of Competence Questionnaire (SSCQ).37 The SSCQ contains seven negatively worded items (e.g., “I feel strained in my interaction with…”). Each item is rated on a five-point Likert scale and dichotomized to agree (i.e., agree very strongly, agree, neutral) or disagree (i.e., disagree or strongly disagree). The items where the caregiver disagreed are summed for the total score. A higher score indicates a higher sense of competence (range = 0–7). The SSCQ shows adequate internal consistency (α = .76) and concurrent validity with the original Sense of Competence Questionnaire (r = .88).37
Desire to institutionalize was measured using a modified Desire to Institutionalize Scale.38 The modified Desire to Institutionalize Scale contains six items rated as dichotomous yes or no (1 = yes; 0 = no). A higher score indicates a greater desire to institutionalize (range = 0–6). Internal consistency for the Desire to Institutionalize scale is adequate (α = .69–.77) and has shown adequate construct validity through factor analysis.38
Caregiver reaction to behavioral symptoms of the PLWD was measured by the Revised Memory and Problem Behavior Checklist (RMPBC).39,40 The RMPBC contains 24 items, each with two parts. Each item represents a behavior; if the behavior has been exhibited by the PLWD in the past week, the caregiver reports the behavior as present. If the behavior is present, the caregiver then reports “how much it bothered you?” on a five-point Likert scale (0 = not at all; 4 = extremely). If the behavior was not present, no “bother” score is provided, and the item is scored 0. The items are summed for a total score (range = 0–96) and for three subscales representing bother due to memory-related problems (range = 0–28), depressive symptoms (range = 0–36), and disruptive symptoms (range = 0–32). A higher score indicates a greater negative reaction to behaviors. The RMPBC shows adequate internal consistency (α = .84–.90) and convergent validity with caregiver depression (ρ = .43), leisure time satisfaction (ρ = −.21), and positive aspects of caregiving (ρ = −.23).39,40
Satisfaction with video monitoring was measured with 12 items using a 5-point Likert scale (1 = strongly disagree; 5 = strongly agree) to assess ease of use and perceived satisfaction with the VMU and telehealth feedback. Seven items referred to the VMU and were only rated by the experimental FamTechCare group. Five additional questions assessed interventionist feedback and were rated by both the experimental and attention control group. A higher score indicates greater satisfaction.
Data Analysis
SAS software (version 9.4) was used for all statistical analyses. Descriptive statistics were calculated for sample characteristics and all outcome variables (including caregiver burden, depression, sleep disturbance, competence, desire to institutionalize, and reaction to behavioral symptoms). Outliers and missing data patterns were examined, and adjustments to the data were applied based on the intention-to-treat principle. Intervention and control participants were compared at baseline using the Wilcoxon rank-sum test for continuous variables (i.e., age, length of caregiving, years since dementia diagnosis) and Fisher’s exact test for categorical variables (e.g., gender, race).
Multivariable modeling
Linear mixed models were fitted to changes in outcomes (1 month–baseline and 3 months–baseline) with SAS Procedure Mixed. This approach was chosen because it uses data from repeated measurements to provide more precise estimates of effects.41 A multilevel growth curve model approach was also considered. Unlike repeated measures approach, the growth curve approach treats time as a continuous variable and can be used when individuals are measured at different time points. However, this approach assumes that individual trajectories follow a similar shape across time. The repeated measures approach was deemed more appropriate to the study data because individuals were measured at the same discrete time points and spaghetti plots of the outcomes revealed varying shapes of individual trajectories across the time points.42 All models included fixed effects for group membership (i.e., FamTechCare intervention and attention control), time of change (1 month and 3 months), and interaction between group and time (to estimate separate group means for changes at 1 month and 3 months), whether they were statistically significant or not. All models included a variable representing baseline values for the corresponding outcomes. To account for multiple caregivers for some PLWD (9 out of 71), a random effect of PLWD was included in the models.
The analyses were adjusted for important covariates using a sequential approach appropriate for relatively small sample sizes.43 Potential covariates related to caregivers included age, gender, education (less than bachelor’s degree, bachelor’s degree, and master’s degree or higher), marital status (married vs single/divorced/widowed), years of caregiving (transformed with the natural log function to improve distribution), relationship to PLWD (spouse and child/spouse of child). Potential covariates related to PLWD included age, gender, education (less than bachelor’s degree, bachelor’s degree, and master’s degree or higher), dementia stage (FAST severity category), years with dementia (transformed with the natural log function), primary diagnosis (Alzheimer’s disease, other dementia, and unknown dementia type), and rural residence. The number of videos submitted for each PLWD (transformed with the natural log function) was also considered a potential covariate.
We examined interactions between group membership and selected covariates (i.e., the number of videos submitted and rural residence) to determine if these covariates could be moderating associations between changes in outcomes and group membership. The number of videos submitted was examined as a moderating variable because it may serve as a proxy of intervention utilization. Rural residence was examined as a moderating variable because the effects of the FamTechCare intervention compared to the attention control may be different based on location because dementia caregivers in rural areas typically have access to fewer resources and support.44 Covariates and interactions of group membership with covariates were included in the models if they were significant at α = .05.
Fit of the models was assessed and compared using fit statistics such as Akaike information criterion and Bayesian information criterion. Residuals were examined for outliers and influential observations, and for violations to normality and homogeneity of variance assumptions.45 Finally, efficacy of the FamTechCare intervention versus attention control was expressed as the model-estimated difference between the groups on mean change from baseline to 3 months.
Analysis Sample
Dyads that did not complete baseline data collection (n = 1 FamTechCare intervention and n = 2 attention control), discontinued before 1 month (n = 2 FamTechCare and n = 4 attention control), dyads with a formal caregiver (n = 3 FamTechCare and n = 5 attention control), one dyad with a developmentally delayed caregiver (attention control), and dyads reporting no disruptive dementia behaviors at baseline, 1 month, and 3 months determined with the RMPBC subscale (n = 2 FamTechCare and n = 3 attention control) were excluded from the final analysis. Dyads that withdrew before 1 month (n = 6) were compared to dyads that remained in the study on continuous caregiver and PLWD characteristics. Only one statistically significant difference was identified: PLWD who withdrew had lower FAST scores indicating less severe impairment (4.5 ± 0.4 vs 5.5 ± 1.1; p < .001, corrected for unequal variances). Dyads that did not complete the 3-month measures but completed both the baseline and 1-month measures, were included in the final analysis (n = 4 FamTechCare and n = 5 attention control). For these dyads, their 1-month scores were carried forward as the 3-month values.
Formal caregivers were excluded from final analysis because they differed from family caregivers on most outcomes at baseline. Compared to family caregivers, formal caregivers had greater competence (mean ± SD = 5.9 ± 1.1 vs 3.9 ± 2.0, p = .01) and lower burden (13.6 ± 1.3 vs 27.3 ± 9.3, p < .001), depression (4.6 ± 3.6 vs 13.4 ± 9.7, p < .001), desire to institutionalize (0.5 ± 1.1 vs 1.6 ± 1.6, p < .07), reaction to memory symptoms (1.4 ± 1.7 vs 7.5 ± 5.1, p < .001), reaction to depressive symptoms (3.0 ± 6.5 vs 7.6 ± 7.3, p = .09), and reaction to disruptive symptoms (1.9 ± 1.7 vs 4.9 ± 5.2, p = .001). Dyads with caregivers reporting no disruptive behaviors at baseline, 1 month, and 3 months were excluded from the final analysis because presence of challenging behaviors was an inclusion criteria of the study. During data cleaning, it was noted that five dyads did not report any disruptive behaviors with the RMPBC and were subsequently excluded from the analysis.
Results
Sample Characteristics
The final analysis included 84 dyads made up of 83 caregivers and 71 PLWD (1 caregiver [adult child] provided care for 2 PLWD [parents]). Forty-three dyads received the FamTechCare intervention and 41 dyads received the attention control intervention. The majority of caregivers cared for their spouse (66.3%), were female (71.2%), non-Hispanic (94.0%) white (92.8%), with a bachelor’s degree or higher (59.0%), and a mean age of 64.2 ± 12.8 years (range = 32.0–90.0). Caregivers reported length of caring for the PLWD ranged from 0.3 to 20 years (mean = 4.0 ± 3.2). The majority of PLWD were male (59.2%), non-Hispanic (94.4%) white (95.8%), with a less than a bachelor’s degree (54.9%), and a mean age of 75.7 ± 9.5 years (range = 54.0–93.0). The time since diagnosis ranged from recently diagnosed to 15 years (mean = 4.2 ± 2.9). Over half of PLWD had a primary dementia diagnosis of Alzheimer’s disease (52.1%) and rated as moderately severe (50.7%) dementia on the FAST scale. Demographic characteristics are reported in Table 1 (caregivers) and Table 2 (PLWD). There were no significant differences between groups.
Table 1.
Demographic Characteristics of Caregivers
| Intervention | Control | |||
|---|---|---|---|---|
| Variable | n | Mean (SD) Range |
n | Mean (SD) Range |
| Age (years) | 42 | 64.6 (12.2) 32.0–86.0 |
41 | 63.9 (13.7) 33.0–90.0 |
| Number of years cared for PLWDa | 41 | 4.4 (3.0) 0.3–14.5 |
41 | 3.8 (3.5) 0.3–20.0 |
| n | %d | n | %d | |
| Site | ||||
| Site A | 22 | 52.4 | 20 | 48.8 |
| Site B | 20 | 47.6 | 21 | 51.2 |
| Gender | ||||
| Female | 30 | 71.4 | 29 | 70.7 |
| Male | 12 | 28.6 | 12 | 29.3 |
| Race | ||||
| White | 37 | 88.1 | 40 | 97.6 |
| African American | 4 | 9.5 | 1 | 2.4 |
| More than one race | 1 | 2.4 | 0 | 0.0 |
| Ethnicity | ||||
| Not Hispanic/Latino | 41 | 97.6 | 37 | 90.2 |
| Unknown/Not reported | 1 | 2.4 | 4 | 9.8 |
| Marital status | ||||
| Married | 35 | 83.3 | 38 | 92.7 |
| Single/Widowed/Divorced | 7 | 16.7 | 3 | 7.3 |
| Education level | ||||
| Less than Bachelor’s degree | 19 | 45.2 | 15 | 36.6 |
| Bachelor’s degree | 13 | 31.0 | 20 | 48.8 |
| Master’s degree or higher | 10 | 23.8 | 6 | 14.6 |
| Relationship to PLWDa | ||||
| Spouse | 29 | 69.1 | 26 | 63.4 |
| Child/Spouse of child | 12 | 28.6 | 15 | 36.6 |
| Otherb | 1 | 2.4 | 0 | 0.0 |
| Caregiver helps PLWD with:c | ||||
| Meal preparation | 37 | 88.1 | 38 | 92.7 |
| Preparing medications | 34 | 81.0 | 33 | 80.5 |
| Choosing clothes | 28 | 66.7 | 26 | 63.4 |
| Grooming | 25 | 59.5 | 18 | 43.9 |
| Dressing | 25 | 59.5 | 17 | 41.5 |
| Bathing | 24 | 57.1 | 16 | 39.0 |
| Toileting | 15 | 35.7 | 10 | 24.4 |
| Eating | 13 | 31.0 | 13 | 31.7 |
| Walking | 9 | 21.4 | 9 | 22.0 |
Note: aPLWD = person living with dementia.; bOther relationship with the person with dementia was girlfriend; cMore than one answer may be selected in this category; dPercentages may total more than 100% due to rounding.
Table 2.
Demographics Characteristics of Persons Living with Dementia
| Intervention | Control | |||
|---|---|---|---|---|
| Variable | n | Mean (SD) Range |
n | Mean (SD) Range |
| Age (years) | 39 | 75.5 (9.7) 58.0–92.0 |
32 | 75.9 (9.3) 54.0–93.0 |
| Years since dementia diagnosis | 38 | 4.6 (3.0) 0.0–15.0 |
30 | 3.6 (2.7) 0.0–13.0 |
| n | %b | n | %b | |
| Site | ||||
| Site A | 20 | 51.3 | 18 | 56.3 |
| Site B | 19 | 48.7 | 14 | 43.8 |
| Percent rural | ||||
| ≥ 20% rural | 7 | 18.0 | 11 | 34.4 |
| < 20% rural | 32 | 82.0 | 21 | 65.6 |
| Gender | ||||
| Male | 24 | 61.5 | 18 | 56.3 |
| Female | 15 | 38.5 | 14 | 43.8 |
| Race | ||||
| White | 37 | 94.9 | 31 | 96.9 |
| African American | 2 | 5.1 | 1 | 3.1 |
| Ethnicity | ||||
| Not Hispanic/Latino | 38 | 97.4 | 29 | 90.6 |
| Unknown/not reported | 1 | 2.6 | 3 | 9.4 |
| Education level | ||||
| Less than Bachelor’s degree | 22 | 56.4 | 17 | 53.1 |
| Bachelor’s degree | 5 | 12.8 | 9 | 28.1 |
| Master’s degree or higher | 12 | 30.8 | 6 | 18.8 |
| Number of caregivers in analysis | ||||
| 1 | 35 | 89.7 | 27 | 84.4 |
| 2 | 4 | 10.3 | 2 | 6.3 |
| 3 | 0 | 0.0 | 2 | 6.3 |
| 4 | 0 | 0.0 | 1 | 3.1 |
| Primary dementia diagnosis | ||||
| Alzheimer’s disease | 21 | 53.9 | 16 | 50.0 |
| Other diagnosed dementia | 15 | 38.5 | 9 | 28.1 |
| Unknown | 3 | 7.7 | 7 | 21.9 |
| Type of dementia:a | ||||
| Alzheimer’s disease | 21 | 53.9 | 16 | 50.0 |
| Lewy bodies | 6 | 15.4 | 5 | 15.6 |
| Fronto-temporal | 4 | 10.3 | 2 | 6.3 |
| Parkinson’s related | 3 | 7.7 | 3 | 9.4 |
| Vascular | 2 | 5.1 | 2 | 6.3 |
| Aphasic | 2 | 5.1 | 1 | 3.1 |
| Other dementia | 2 | 5.1 | 2 | 6.3 |
| Unknown dementia type | 3 | 7.7 | 7 | 21.9 |
| FAST disability category | ||||
| Incipient dementia | 0 | 0.0 | 1 | 3.1 |
| Mild dementia | 10 | 25.6 | 10 | 31.3 |
| Moderate dementia | 7 | 18.0 | 6 | 18.8 |
| Moderately severe dementia | 21 | 53.9 | 15 | 46.9 |
| Severe dementia | 1 | 2.6 | 0 | 0.0 |
Note: aMore than one answer may be selected; bPercentages may total more than 100% due to rounding.
Outcome Variables
Descriptive statistics (means, SD, and ranges) are reported in Table 3 for outcome variables at baseline, 1 month, and 3 months for FamTechCare and attention control caregivers. Linear mixed models for changes in outcomes (caregiver burden, depression, sleep disturbance, competence, desire to institutionalize, and reaction to memory, depressive, and disruptive behavioral symptoms) were used to calculate estimated differences in mean changes from baseline to 3 months between the FamTechCare intervention and attention control groups, given in Table 4. Full models are reported in Table 5. Baseline values of the corresponding outcomes were significant predictors of change in all outcomes (p < .05).
Table 3.
Descriptive Statistics for Study Outcomes for FamTechCare Intervention and Attention Control Participants at Each Time Point
| Intervention | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | M ± SD | Range | p j | N | M ± SD | Range | p j | p k | |
| Burdena (12–60) | |||||||||
| Baseline | 42 | 28.0 ± 10.6 | 12.0–49.0 | 41 | 27.2 ± 8.1 | 15.0–43.0 | .989 | ||
| 1 month | 42 | 27.6 ± 9.9 | 13.0–50.0 | .686 | 41 | 26.6 ± 8.5 | 16.0–46.0 | .375 | |
| 3 months | 42 | 26.6 ± 9.8 | 14.0–47.0 | .592 | 41 | 27.4 ± 9.1 | 14.0–46.0 | .949 | |
| Depressionb (0–60) | |||||||||
| Baseline | 42 | 16.0 ± 10.7 | 0.0–44.0 | 41 | 12.0 ± 8.1 | 0.0–32.0 | .085 | ||
| 1 month | 42 | 14.5 ± 11.9 | 0.0–45.0 | .072 | 41 | 12.8 ± 10.2 | 0.0–42.0 | .390 | |
| 3 months | 42 | 13.8 ± 10.6 | 0.0–42.0 | .063 | 41 | 12.3 ± 10.8 | 0.0–39.0 | .848 | |
| Sleep disturbancec (0–21) | |||||||||
| Baseline | 42 | 7.5 ± 3.5 | 1.0–15.0 | 41 | 7.0 ± 3.5 | 2.0–16.0 | .439 | ||
| 1 month | 42 | 7.3 ± 3.6 | 2.0–16.0 | .472 | 41 | 6.9 ± 3.8 | 1.0–16.0 | .709 | |
| 3 months | 42 | 7.3 ± 3.4 | 2.0–17.0 | .530 | 41 | 6.8 ± 4.2 | 1.0–15.0 | .426 | |
| Competenced (0–7) | |||||||||
| Baseline | 42 | 3.7 ± 1.8 | 0.0–7.0 | 41 | 3.9 ± 2.2 | 0.0–7.0 | .690 | ||
| 1 month | 42 | 4.0 ± 2.0 | 0.0–7.0 | .303 | 41 | 3.8 ± 2.1 | 0.0–7.0 | .712 | |
| 3 months | 42 | 4.1 ± 1.9 | 0.0–7.0 | .117 | 41 | 3.5 ± 2.2 | 0.0–7.0 | .676 | |
| Desire to institutionalizee (0–6) | |||||||||
| Baseline | 42 | 1.5 ± 1.7 | 0.0–5.0 | 41 | 1.7 ± 1.5 | 0.0–5.0 | .425 | ||
| 1 month | 42 | 1.6 ± 1.7 | 0.0–6.0 | .599 | 41 | 1.9 ± 2.1 | 0.0–6.0 | .348 | |
| 3 months | 42 | 2.0 ± 2.0 | 0.0–6.0 | .036 | 41 | 2.5 ± 2.3 | 0.0–6.0 | .002 | |
| Reaction to memory symptomsf (0–28) | |||||||||
| Baseline | 41 | 8.4 ± 5.3 | 0.0–25.0 | 40 | 6.6 ± 4.8 | 0.0–19.0 | .097 | ||
| 1 month | 41 | 8.4 ± 6.0 | 0.0–21.0 | .988 | 40 | 6.2 ± 4.3 | 0.0–19.0 | .658 | |
| 3 months | 41 | 7.0 ± 5.8 | 0.0–22.0 | .050 | 40 | 6.5 ± 4.6 | 0.0–19.0 | .572 | |
| Reaction to depression symptomsg (0–36) | |||||||||
| Baseline | 42 | 8.3 ± 7.3 | 0.0–33.0 | 40 | 6.9 ± 6.8 | 0.0–28.0 | .362 | ||
| 1 month | 42 | 6.8 ± 7.6 | 0.0–32.0 | .006 | 40 | 7.6 ± 6.9 | 0.0–34.0 | .291 | |
| 3 months | 42 | 7.3 ± 7.0 | 0.0–28.0 | .176 | 40 | 6.1 ± 6.6 | 0.0–34.0 | .302 | |
| Reaction to disruptive symptomsh (0–32) | |||||||||
| Baseline | 42 | 6.1 ± 5.7 | 0.0–21.0 | 40 | 4.0 ± 4.2 | 0.0–16.0 | .065 | ||
| 1 month | 42 | 5.3 ± 4.7 | 0.0–19.0 | .231 | 40 | 5.1 ± 4.9 | 0.0–17.0 | .038 | |
| 3 months | 42 | 5.4 ± 5.4 | 0.0–19.0 | .195 | 40 | 4.9 ± 5.0 | 0.0–17.0 | .089 | |
| Reaction to symptoms (memory, depression, or disruptive)i (0–96) | |||||||||
| Baseline | 42 | 22.8 ± 15.6 | 3.0–63.0 | 40 | 17.5 ± 11.8 | 0.0–51.0 | .161 | ||
| 1 month | 42 | 20.5 ± 14.3 | 2.0–65.0 | .134 | 40 | 18.9 ± 13.0 | 0.0–63.0 | .458 | |
| 3 months | 42 | 19.8 ± 15.3 | 1.0–59.0 | .043 | 40 | 17.4 ± 12.6 | 1.0–63.0 | .625 | |
Note: aZarit Burden Scale; bCESD; cGlobal Pittsburgh Sleep Quality Index; dCaregiver Competence Rating; eDesire to Institutionalize Scale; fBehavior Symptom Checklist: Memory Subscale; gBehavior Symptom Checklist: Depression Subscale; hBehavior Symptom Checklist: Disruption Subscale; IBehavior Symptom Checklist: Total Score; jbaseline-to-1-month and baseline-to-3.-month changes in outcomes were tested using the Wilcoxon signed-rank test; kintervention and control groups were compared at baseline using the Wilcoxon rank-sum test.
Table 4.
Group Differences of Least Mean Squares for Changes in Outcomes at Month 3
| Outcome | LSM Estimate | 95% CI | t | p > |t|i |
|---|---|---|---|---|
| Burdena | −1.50 | −4.64, 1.64 | −0.96 | .343 |
| Depressionb | −4.79 | −8.51, -1.08 | −2.56 | .012 |
| Sleep disturbancec | 0.12 | −1.08, 1.33 | 0.20 | .839 |
| Competenced | 0.77 | 0.07, 1.47 | 2.17 | .033 |
| Desire to institutionalizee | −0.26 | −1.03, 0.51 | −0.68 | .500 |
| Reaction to memory symptomsf | 0.03 | −2.02, 2.08 | 0.03 | .977 |
| Reaction to depression symptomsg | −0.22 | −2.09, 1.64 | −0.24 | .812 |
| Reaction to disruptive symptomsh | −0.62 | −2.18, 0.94 | −0.79 | .432 |
Note: aZarit Burden Scale; bCESD; cGlobal Pittsburgh Sleep Quality Index; dCaregiver Competence Rating; eDesire to Institutionalize Scale; fBehavior Symptom Checklist: Memory Subscale; gBehavior Symptom Checklist: Depression Subscale; hBehavior Symptom Checklist: Disruption Subscale; ip > |t| = p values for a t-test of significance of an effect or a level of an effect.
Table 5.
Model Results for Changes in Caregiver Outcomes
| Outcome | b | 95% CI | t | p > |t|i |
|---|---|---|---|---|
| Burden a | ||||
| Intercept | 8.43 | 4.26, 12.60 | 4.02 | <.001 |
| Baseline burden | −0.25 | −0.38, −0.12 | −3.80 | <.001 |
| Group: intervention vs control | 0.30 | −2.27, 2.88 | 0.24 | .814 |
| Time: 3 months vs 1 month | 0.80 | −0.94, 2.55 | 0.92 | .361 |
| Group × Time | −1.80 | −4.25, 0.64 | −1.47 | .146 |
| Caregiver education | ||||
| Bachelor’s vs <Bachelor’s | −2.93 | −5.67, −0.18 | −2.12 | .037 |
| Master or higher vs <Bachelor’s | −4.41 | −7.76, −1.06 | −2.62 | .011 |
| Depression b | ||||
| Intercept | 1.77 | −1.06, 4.59 | 1.25 | .216 |
| Baseline depression | −0.16 | −0.30, −0.02 | −2.28 | .025 |
| Group: intervention vs control | 0.39 | −2.93, 3.70 | 0.23 | .816 |
| Time: 3 months vs 1 month | −0.46 | −2.75, 1.82 | −0.40 | .687 |
| Group × Time | −0.25 | −3.46, 2.96 | −0.16 | .877 |
| % rural: ≥20% vs <20% | 4.23 | 0.06, 8.41 | 2.03 | .047 |
| Group × % rural | −9.86 | −16.06, −3.66 | −3.17 | .002 |
| Sleep disturbance c | ||||
| Intercept | 3.23 | 1.69, 4.76 | 4.19 | <.001 |
| Baseline sleep | −0.21 | −0.34, −0.08 | −3.25 | .002 |
| Group: intervention vs control | 0.00 | −0.98, 0.98 | 0.00 | .996 |
| Time: 3 months vs 1 month | −0.10 | −0.80, 0.60 | −0.28 | .783 |
| Group × Time | 0.12 | −0.86, 1.11 | 0.25 | .807 |
| Primary diagnosis | ||||
| Alzheimer’s vs unknown | −2.08 | −3.51, −0.65 | −2.91 | .005 |
| Other dementia vs unknown | −1.90 | −3.42, −0.37 | −2.48 | .016 |
| Competence d | ||||
| Intercept | 3.72 | 2.56, 4.88 | 6.36 | <.001 |
| Baseline competence | −0.59 | −0.74, −0.44 | −7.72 | <.001 |
| Group: intervention vs control | 0.28 | −0.39, 0.95 | 0.84 | .404 |
| Time: 3 months vs 1 month | −0.29 | −0.79, 0.21 | −1.17 | .246 |
| Group × Time | 0.49 | −0.22, 1.19 | 1.38 | .172 |
| Length of caregiving [ln(years)] | −1.27 | −1.78, −0.76 | −4.96 | <.001 |
| % rural: ≥20% vs <20% | −1.27 | −1.96, −0.57 | −3.62 | <.001 |
| Caregiver education | ||||
| Bachelor’s vs <Bachelor’s | 0.78 | 0.13, 1.43 | 2.38 | .020 |
| Master or higher vs <Bachelor’s | 1.35 | 0.57, 2.14 | 3.44 | <.001 |
| Desire to institutionalize e | ||||
| Intercept | 0.06 | −0.88, 0.99 | 0.12 | .901 |
| Baseline desire to institutionalize | −0.24 | −0.43, −0.05 | −2.52 | .014 |
| Group: intervention vs control | 1.39 | −0.20, 2.98 | 1.74 | .086 |
| Time: 3 months vs 1 month | 0.54 | 0.08, 0.99 | 2.33 | .022 |
| Group × Time | −0.11 | −0.75, 0.54 | −0.33 | .740 |
| ln(# of videos uploaded) | 0.29 | −0.16, 0.74 | 1.29 | .203 |
| Group × ln(# of videos uploaded) | −0.68 | −1.32, −0.04 | −2.14 | .037 |
| Reaction to memory symptoms f | ||||
| Intercept | 3.56 | 1.48, 5.64 | 3.40 | .001 |
| Baseline reaction to memory symptoms | −0.41 | −0.59, −0.24 | −4.69 | <.001 |
| Group: intervention vs control | 1.62 | −0.37, 3.61 | 1.62 | .110 |
| Time: 3 months vs 1 month | 0.23 | −0.90, 1.35 | 0.40 | .693 |
| Group × Time | −1.59 | −3.18, 0.00 | −1.99 | .050 |
| Caregiver education | ||||
| Bachelor’s vs <Bachelor’s | −2.57 | −4.53, −0.61 | −2.61 | .011 |
| Master or higher vs <Bachelor’s | −2.75 | −5.07, −0.44 | −2.37 | .021 |
| Reaction to depression symptoms g | ||||
| Intercept | 6.43 | 3.59, 9.28 | 4.49 | <.001 |
| Baseline reaction to depression symptoms | −0.23 | −0.34, −0.12 | −4.19 | <.001 |
| Group: intervention vs control | −2.25 | −4.13, 0.36 | −2.37 | .020 |
| Time: 3 months vs 1 month | −1.50 | −2.97, −0.03 | −2.03 | .045 |
| Group × Time | 2.02 | −0.03, 4.07 | 1.96 | .053 |
| Caregiver education | ||||
| Bachelor’s vs <Bachelor’s | −2.07 | −3.82, −0.33 | −2.36 | .020 |
| Master or higher vs <Bachelor’s | −2.06 | −4.15, 0.03 | −1.96 | .054 |
| Caregiver marital status: married vs single/widowed/divorced | −3.07 | −5.45, −0.68 | −2.56 | .012 |
| Reaction to disruptive symptoms h | ||||
| Intercept | 1.06 | −0.86, 2.98 | 1.10 | .274 |
| Baseline reaction to disruptive symptoms | −0.28 | −0.40, −0.16 | −4.73 | <.001 |
| Group: intervention vs control | 2.49 | −0.59, 5.57 | 1.61 | .111 |
| Time: 3 months vs 1 month | −0.20 | −1.17, 0.77 | −0.41 | .681 |
| Group × Time | 0.25 | −1.10, 1.60 | 0.37 | .716 |
| % rural: ≥20% vs <20% | 1.99 | 0.56, 3.41 | 2.78 | .007 |
| Caregiver education | ||||
| Bachelor’s vs <Bachelor’s | −0.81 | −2.15, 0.53 | −1.20 | .234 |
| Master or higher vs <Bachelor’s | −1.84 | −3.48, −0.21 | −2.24 | .028 |
| ln(# of videos uploaded) | 0.64 | −0.25, 1.53 | 1.43 | .158 |
| Group × ln(# of videos uploaded) | −1.49 | −2.72, −0.26 | −2.41 | .018 |
Note: aZarit Burden Scale; bCESD; cGlobal Pittsburgh Sleep Quality Index; dCaregiver Competence Rating; eDesire to Institutionalize Scale; fBehavior Symptom Checklist: Memory Subscale; gBehavior Symptom Checklist: Depression Subscale; hBehavior Symptom Checklist: Disruption Subscale; ip > |t| = p-values for a t-test of significance of an effect or a level of an effect.
Burden decreased from 28.0 ± 10.6 (mean ± SD) at baseline to 26.6 ± 9.8 at 3 months for FamTechCare caregivers (p = .592) and slightly increased from 27.2 ± 8.1 at baseline to 27.4 ± 9.5 at 3 months for attention control caregivers (p = .949; Table 3). The model-estimated difference in mean changes of −1.50 points was not statistically significant (95% confidence interval [CI] = −4.6, 1.6; p = .343; Table 4). Significant differences were estimated among caregiver education levels: caregivers with less than bachelor’s degrees differed from bachelor’s degree caregivers (p = .037) and master’s or higher degree caregivers (p = .011) in terms of mean changes in burden; caregivers with higher education levels tended to have greater decreases in burden, controlling for other variables (Table 5).
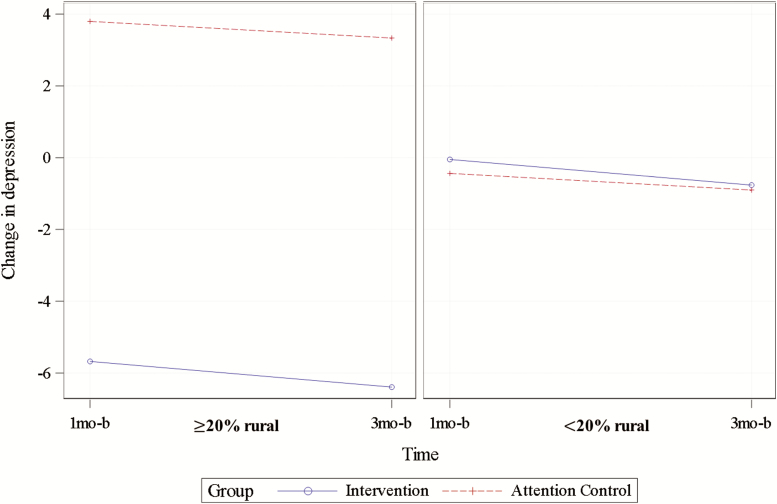
Depression decreased from 16.0 ± 10.7 at baseline to 13.8 ± 10.6 at 3 months for FamTechCare caregivers (p = .063) and increased from 12.0 ± 8.1 at baseline to 12.3 ± 10.8 at 3 months for attention controls (p = .848). The model-estimated difference in mean changes of −4.8 points was statistically significant (95% CI = −8.5, −1.1; p = .012). The model for depression also included a significant interaction effect of group with rural residence, p = .002, illustrated by Figure 3. In rural locations (percent rural ≥ 20%), a decrease in depression for FamTechCare caregivers (n = 8, solid line) and an increase for attention control caregivers (n = 14, dashed line) resulted in a statistically significant model-estimated mean difference of −9.6 points (95% CI = −14.9, −4.3, p < .001) between these groups. However, this relationship did not remain for caregivers in nonrural locations (percent rural < 20%; p = .870).
Figure 3.
Interaction between group and rural residence in the model for change in caregiver depression (plotted for mean baseline depression = 14.01).
Sleep disturbance changed little for each group and the difference between the changes was not statistically significant (p = .839). Significant differences were estimated among PLWD diagnoses; caregivers of PLWD with an unknown dementia etiology differed from caregivers of PLWD with Alzheimer’s disease (p = .005) and caregivers of PLWD with other dementias (p = .016) in terms of mean changes in sleep disturbance. This finding corresponded to an increase in sleep disturbance for caregivers of PLWD with an unknown dementia type (1.7 ± 0.6, n = 11, p = .011) and a minimal decrease for caregivers of PLWD with Alzheimer’s type (−0.4 ± 0.3, n = 46, p = .210) and caregivers of PLWD with other dementia types (−0.2 ± 0.4, n = 27, p = .574), controlling for other variables in the model.
Competence increased from 3.7 ± 1.8 at baseline to 4.1 ± 1.9 at 3 months for FamTechCare caregivers (p = .117) and decreased from 3.9 ± 2.2 at baseline to 3.5 ± 2.2 at 3 months for attention controls (p = .676). The model-estimated difference between groups in terms of mean changes in competence was 0.77 points (95% CI = 0.1, 1.5; p = .033) at 3 months. Longer caregiving was associated with negative changes in competence (p < .001). Compared to caregivers in nonrural locations (percent rural < 20%), caregivers in more rural locations (percent rural ≥ 20%) had a decrease in mean competence (p < .001). Caregivers with less than bachelor’s degrees differed from bachelor’s degree caregivers (p = .02) and master’s or higher degree caregivers (p < .001) in terms of mean changes in competence, so that caregivers with more education tended to have greater increases in self-rated competence, controlling for other variables in the model.
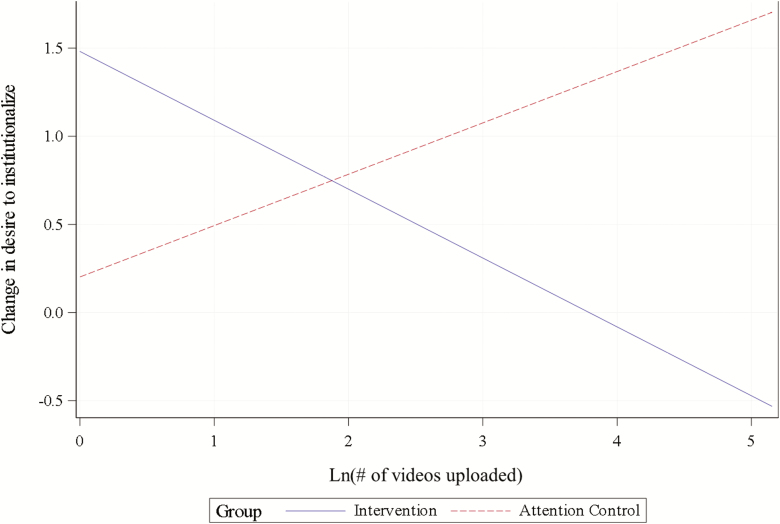
Desire to institutionalize increased from 1.5 ± 1.7 at baseline to 2.0 ± 2.0 at 3 months for FamTechCare caregivers (p = .036) and from 1.7 ± 1.5 at baseline to 2.5 ± 2.3 at 3 months for attention controls (p = .002). The model-estimated difference between increases of −0.3 points was not statistically significant (95% CI = −1.0, 0.5; p = .500). The model for desire to institutionalize also included a significant interaction effect of group with ln(number of videos uploaded), p = .037, illustrated by Figure 4. Specifically, ln(number of videos uploaded) was negatively associated with changes in desire to institutionalize for FamTechCare caregivers and positively associated with changes in desire to institutionalize for attention controls.
Figure 4.
Interaction between group and ln(number of videos uploaded) in the model for change in caregiver desire to institutionalize (plotted for mean baseline desire to institutionalize = 1.63 and change at 3 months).
Reaction to memory symptoms decreased from 8.4 ± 5.3 at baseline to 7.0 ± 5.8 at 3 months for FamTechCare caregivers (p = .050) and from 6.6 ± 4.8 at baseline to 6.5 ± 4.6 at 3 months for attention controls (p = .572). The model-estimated difference in mean changes was not statistically significant (p = .977). Less than bachelor’s degree caregivers differed from bachelor’s degree caregivers (p = .011) and master’s or higher degree caregivers (p = .021) in terms of mean changes in reaction to memory symptoms, so that less than bachelor’s degree caregivers tended to have increases, while those with higher education tended to have decreases, controlling for other variables in the model.
Reaction to depressive symptoms decreased from 8.3 ± 7.3 at baseline to 7.3 ± 7.0 at 3 months for FamTechCare caregivers (p = .176) and from 6.9 ± 6.8 at baseline to 6.1 ± 6.6 at 3 months for attention controls (p = .302). The model-estimated difference in mean changes was not statistically significant (p = .812). Caregivers with less than a bachelor’s degree differed from caregivers with a bachelor’s degree (p = .020) in terms of mean changes in reaction to depressive symptoms, so that less the bachelor’s degree caregivers tended to have increases, while those with higher education tended to have decreases, controlling for other variables. Finally, married caregivers tended to have decreases in reaction to depressive symptoms, while single/widowed/divorced caregivers tended to have increases in reaction to depressive symptoms, which resulted in a statistically significant difference between mean changes (p = .012).
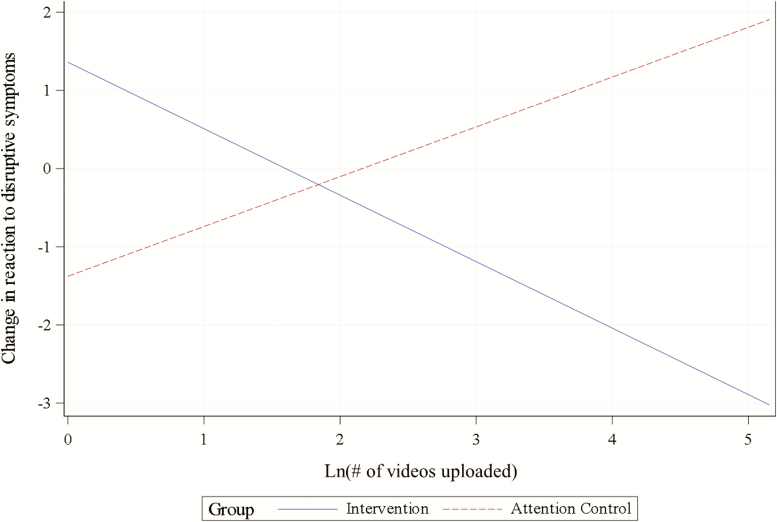
Reaction to disruptive symptoms decreased from 6.1 ± 5.7 at baseline to 5.4 ± 5.4 at 3 months for FamTechCare caregivers (p = .195) and increased from 4.0 ± 4.2 at baseline to 4.9 ± 5.0 at 3 months for attention controls (p = .089). The model-estimated difference in mean changes was not statistically significant (p = .432). Compared to caregivers in nonrural locations (percent rural < 20%), caregivers in more rural locations (percent rural ≥ 20%) tended to have increases in reaction to disruptive symptoms (p = .007). Compared to caregivers with less than bachelor’s degrees, caregivers with master’s or higher degrees tended to have decreases in reaction to disruptive symptoms (p = .028), controlling for other variables in the model. Finally, the model for reaction to disruptive symptoms included a significant interaction effect of group with ln(number of videos uploaded), p = .018, illustrated by Figure 5. Specifically, ln(number of videos uploaded) was negatively associated with changes in reaction to disruptive symptoms for FamTechCare caregivers and positively associated with changes in reaction to disruptive symptoms for attention controls.
Figure 5.
Interaction between group and ln(number of videos uploaded) in the model for change in caregiver reaction to disruptive symptoms (plotted for mean baseline reaction to disruptive symptoms = 5.07, caregivers with bachelor’s degree, percent rural < 20%, and change at 3 months).
Total reaction to memory, depressive, or disruptive symptoms decreased from 22.8 ± 15.6 at baseline to 19.2 ± 15.3 at 3 months for FamTechCare caregivers (p = .043) and from 17.5 ± 11.8 at baseline to 17.4 ± 12.6 at 3 months for attention controls (p = .625). No model was fit to the data, because they represent three different scales combined that were each fit with a corresponding model.
Intervention Fidelity and Intervention Utilization
The caregivers receiving the FamTechCare intervention sent an average of 21.0 ± 27.9 videos. The number of videos submitted from each dyad varied over the 3-month study period, ranging from 1 video to a total of 172 videos. On average, the expert team reviewed and discussed videos related to each PLWD in the FamTechCare intervention group for 84.6 ± 78.5 min (range = 0.0–295.0). The videos from the two dyads that submitted only one video were poor quality and therefore not reviewed, and subsequently, these caregivers did not receive any tailored feedback based on the video submission.
Each dyad in both groups was scheduled to receive one phone call from the interventionist each week. The FamTechCare intervention caregivers received an average of 9.1 ± 2.5 calls (range = 2.0–12.0) over the 3-month study period, totaling an average of 132.8 ± 94.4 min (range = 11.0–357.0). The attention control group similarly had an average of 8.5 ± 3.1 calls (range = 1.0–12.0) and an average of 136.0 ± 110.4 min (range = 15.0–460.0), indicating that both the FamTechCare intervention and attention control group had similar amounts of interventionist feedback.
Similarly, the topics and amount of interventions provided by the interventionist did not vary between groups. When the Typology of Technology-Supported Dementia Care Interventions46 was applied to the weekly interventionist narrative notes, counts of topics of interventions did not differ between groups (p = .280).47 Caregivers in both groups received the similar amounts (p > .05) of interventions related to managing behavioral and psychological symptoms of dementia, knowledge of disease expectations, performing of activities of daily living, enhancing safety, ability of utilize medical care, effective medication use, and caregiver-related interventions focused on social and financial support, self-care, and respite. The only difference in interventions provided to each group was that the attention control caregivers received more positive reinforcement (p = .031) from the interventionist than the FamTechCare caregivers.
Satisfaction
The majority of caregivers receiving the FamTechCare intervention somewhat/strongly agreed that the VMU was easy to setup and use (76.2%) and that having the VMU in their home was acceptable (85.7%). The FamTechCare intervention group was generally more satisfied with the interventionist feedback than the attention control group (76.2% vs 48.4% somewhat/strongly agreed that feedback from the interventionist was helpful, p < .001). Both caregiver groups somewhat/strongly agreed that they would recommend the intervention to others (81.0% for FamTechCare and 81.8% for attention control, p = 1.0). Additional information about the satisfaction and utilization of the FamTechCare intervention will be reported elsewhere.
Withdrawal from the study was greater in the attention control group (21.4%) compared to the FamTechCare intervention group (11.8%). The most frequently cited reason for dyad withdrawal was hospitalization or long-term care placement of the PLWD (55.6%). Only five dyads (two from FamTechCare intervention and three from attention control) withdrew due to finding study procedures burdensome and only two dyads withdrew (both from attention control) withdrew due to concerns regarding video recording.
Discussion and Implications
The FamTechCare intervention addressed the diversity in caregiving by providing tailored support for individual dyad challenges. The models demonstrated that the video-based FamTechCare intervention was effective in reducing some of the negative impacts of caregiving in contrast to the attention control group that received only telephone support. Compared to attention control caregivers, FamTechCare group caregivers had greater reductions in depression and gains in competence. For most outcomes, scores improved for FamTechCare caregivers; depression decreased by 13.8%, and reactions to memory, depressive, and disruptive behavioral symptoms decreased by 16.7%, 12.0%, and 11.5%, respectively. These changes may not be statistically significant but are of clinical significance to the families caring for a loved one with dementia at home. Corresponding changes were less pronounced and sometimes reversed in the attention control group.
The models identified the impact of certain characteristics of caregivers and PLWD on changes in the outcomes. Caregiver education, rural residence, dementia diagnosis, length of caregiving, and caregiver marital status were significant covariates in at least one outcome model. Caregiver education was a consistent predictor of changes. Compared to caregivers with less than a bachelor’s degree, caregivers with higher levels of education tended to have greater improvements or less declines in burden, competence, and reactions to behavioral symptoms, regardless of the intervention group. Future research should assess whether these differences are related to health literacy and then tailor interventions to meet educational needs, such as incorporating demonstration videos into the intervention. In addition, education may have acted as a proxy for socioeconomic status in this study, which was not measured and should be explored in future research.
Rural residence was also a significant covariate in multiple models. Rural residence was a significant moderator of change in depression. There was a substantial difference between rural FamTechCare and attention control caregivers with respect to changes in depression during the study, where FamTechCare caregivers experienced a decline in depression and attention control caregivers experienced an increase in depression. Caregivers living in rural locations typically have fewer resources and FamTechCare may have helped overcome a sense of isolation, thus reducing depression levels, by connecting them to support. Telehealth interventions have the potential to significantly reduce rural health disparities, yet few telehealth studies have focused solely on designing and testing interventions that will specifically aid rural dementia caregivers.44 Living in rural locations was also associated with reductions in competence and increases in reactions to disruptive behavioral symptoms in both groups. Gains in competence for caregivers in nonrural locations may be due to the greater availability of services (e.g., respite) in metropolitan areas. Further research is needed to explore the needs of rural dementia caregivers and test interventions specifically targeted at this population.
The models for changes in desire to institutionalize the PLWD and caregiver reaction to disruptive symptoms included significant interactions of intervention group membership with the number of submitted videos: FamTechCare caregivers who submitted more videos tended to have greater improvement in these two outcomes while for attention controls the relationship was in the opposite direction. This finding indicates that engagement in the FamTechCare intervention utilization may moderate changes in these outcomes. For all outcomes, baseline scores were negatively associated with change during the study, so that caregivers with worse scores at baseline realized greater improvements.
As in other research testing caregiver support interventions, FamTechCare failed to significantly reduce caregiver burden,48–50 which is strongly associated with desire to institutionalize the PLWD48,51,52 that intensified for both groups in our study. It is increasingly clear that caregiver support alone cannot reduce many symptoms that are inherent in caring for a PLWD. Observing the progressive cognitive and functional decline of dementia in a loved one inevitably causes loss of a significant relationship and corresponding grief that even available support cannot eliminate.53 Future interventions should target ways to address burden. Although the FamTechCare intervention did not significantly improve some outcomes, it led to nonsignificant improvements in all outcomes except desire to institutionalize. This is important since caregiver outcomes such as burden have been shown to increase with time.54,55
Two factors may have limited our ability to detect the effects of FamTechCare. First, the dose of the intervention had high variability within dyads, ranging from 11 to 357 min of total interventionist time. Second, the control group was not a true control in that they received an intensive intervention and individualized feedback. Although the control group itself received a weekly intervention, it is important to note that this intervention—based on caregiver retrospective recall—did not result in similar declines in outcome scores and was associated with more negative outcomes. Further, FamTechCare caregivers reported greater satisfaction with their interventionist feedback compared to attention control caregivers.
Overall, the use of video recordings for expert review and feedback to family caregivers was effective in reducing some negative caregiver outcomes. The video recordings provided rich contextual details about in-home care situations for intervention group caregivers (e.g., environmental factors such as noise or clutter), overcoming the need to rely on caregiver description and retrospective recall, and instead allowing dementia care experts to directly view challenging care situations. In addition, the recording application included a buffering feature that captured antecedent events. Although technology has been used to support family caregivers of PLWD,21,56 the FamTechCare trial is the first to capture video recordings of challenging care situations as a basis for tailored feedback for in-home dementia care. Previous telehealth interventions have included video or phone conferencing for caregiver support or in-home telemonitoring for safety.21,56Although such interventions improve access to caregiver support, they do not eliminate reliance on retrospective recall or provide contextually tailored interventions that guide care and reduce negative caregiver outcomes.
Limitations of this study should be addressed in ongoing research. Our sample included only volunteers who agreed to submit videos of in-home care challenges. Although only two dyads withdrew due to privacy concerns once enrolled, and only 14.2% (n = 28 of 197) declined to enroll due to privacy concerns, it is unknown what number of caregivers were not interested in the study due to privacy concerns. The number and quality of videos submitted by families varied, and there were weeks when contact did not occur. Future research is needed to determine the optimal dose for this intervention, which could be evaluated through growth curve analysis by extending the intervention length and adding measurement points. Caregivers may have failed to share videos of extremely uncomfortable situations, limiting the effectiveness of suggested interventions. The FamTechCare intervention group also received more expert discussion on how to address challenges compared to the control group. Although the control interventionist was a member of the expert team and could discuss control dyads with the expert team, the majority of expert team discussion was focused on the FamTechCare intervention dyads and related videos. The sample also had limited diversity so that cultural and ethnic factors that influence the caregiving experience were not evaluated.57 Future research should also evaluate whether caregivers implemented prescribed interventions.
As the population with dementia expands and caregiving becomes recognized as a growing public health crisis, it is important to use all available tools to assist caregivers and minimize negative effects of caregiving.4 The FamTechCare intervention provides tailored interventions to unique caregiving challenges, using innovative technologies that caregivers desire.23 Ongoing research testing the use of technology to support PLWD and their caregivers is needed, and concerns about technological naivety, privacy, and trust issues must be addressed to reach the full potential for support. Additionally, future research is needed to identify caregivers that will benefit from technology-based interventions such as FamTechCare. FamTechCare effectively links caregivers to dementia experts in the home by providing direct observation of care situations, communication, and environmental concerns, providing caregivers with individualized contextually based interventions that reduced caregiver depression and improved caregiver sense of competence.
Funding
This work was supported by the National Institute of Nursing Research of the National Institutes of Health (grant number R01NR014737). The University of Kansas Alzheimer’s Disease Center (P30AG035982) provided essential infrastructure and recruitment support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Clinical Trials registration (NCT02483520).
Conflict of Interest
None reported.
Acknowledgments
The authors thank other research team members who were involved in the conduct of the FamTechCare study including Diane Blyler, Denise Seabold, Michelle Cochran, JoEllen Wurth, Ann Arthur, Michelle Niedens, Phyllis Switzer, Ann Bossen, and Sohyun Kim.
References
- 1. Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M.. World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia. Alzheimer’s Disease International; 2016. Retrieved from https://www.alz.co.uk/research/WorldAlzheimerReport2016.pdf. Accessed January 25, 2019. [Google Scholar]
- 2. Alzheimer’s Association. 2018. Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 2018;14:367–429. doi: 10.1016/j.jalz.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 3. Monin JK, Schulz R. Interpersonal effects of suffering in older adult caregiving relationships. Psychol Aging. 2009;24:681–695. doi: 10.1037/a0016355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Talley RC, Crews JE. Framing the public health of caregiving. Am J Public Health. 2007;97:224–228. doi: 10.2105/AJPH.2004.059337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Isik AT, Soysal P, Solmi M, Veronese N. Bidirectional relationship between caregiver burden and neuropsychiatric symptoms in patients with Alzheimer’s disease: a narrative review. Int J Geriatr Psychiatry. 2019;34:1326–1334. doi: 10.1002/gps.4965 [DOI] [PubMed] [Google Scholar]
- 6. Feast A, Moniz-Cook E, Stoner C, Charlesworth G, Orrell M. A systematic review of the relationship between behavioral and psychological symptoms (BPSD) and caregiver well-being. Int Psychogeriatr. 2016;28:1761–1774. doi: 10.1017/S1041610216000922 [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. (2012). Dementia: A Public Health Priority. World Health Organization; Retrieved from https://www.who.int/mental_health/publications/dementia_report_2012/en/. Accessed January 25, 2019. [Google Scholar]
- 8. Caspar S, Davis ED, Douziech A, Scott DR (2018). Nonpharmacological management of behavioral and psychological symptoms of dementia: what works, in what circumstances, and why? Innovation in Aging. 2018;1:1–10. doi: 10.1093/geroni/igy001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilhooly KJ, Gilhooly ML, Sullivan MP, et al. A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr. 2016;16:106. doi: 10.1186/s12877-016-0280-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kishita N, Hammond L, Dietrich CM, Mioshi E. Which interventions work for dementia family carers?: An updated systematic review of randomized controlled trials of carer interventions. Int Psychogeriatr. 2018;30:1679–1696. doi: 10.1017/S1041610218000947 [DOI] [PubMed] [Google Scholar]
- 11. Ying J, Wang Y, Zhang M, et al. Effect of multicomponent interventions on competence of family caregivers of people with dementia: a systematic review. J Clin Nurs. 2018;27:1744–1758. doi: 10.1111/jocn.14326 [DOI] [PubMed] [Google Scholar]
- 12. Vandepitte S, Van Den Noortgate N, Putman K, Verhaeghe S, Faes K, Annemans L. Effectiveness of supporting informal caregivers of people with dementia: a systematic review of randomized and non-randomized controlled trials. J Alzheimers Dis. 2016;52:929–965. doi: 10.3233/JAD-151011 [DOI] [PubMed] [Google Scholar]
- 13. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ 2015;350:h369. doi: 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012;308:2020–2029. doi: 10.1001/jama.2012.36918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cerejeira J, Lagarto L, Mukaetova-Ladinska EB. Behavioral and psychological symptoms of dementia. Front Neurol. 2012;3:73. doi: 10.3389/fneur.2012.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kolanowski A, Boltz M, Galik E, et al. Determinants of behavioral and psychological symptoms of dementia: a scoping review of the evidence. Nurs Outlook. 2017;65:515–529. doi: 10.1016/j.outlook.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hopwood J, Walker N, McDonagh L, et al. Internet-based interventions aimed at supporting family caregivers of people with dementia: systematic review. J Med Internet Res. 2018;20:e216. doi: 10.2196/jmir.9548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bossen AL, Kim H, Williams KN, Steinhoff AE, Strieker M. Emerging roles for telemedicine and smart technologies in dementia care. Smart Homecare Technol Telehealth. 2015;3:49–57. doi: 10.2147/SHTT.S59500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egan KJ, Pinto-Bruno ÁC, Bighelli I, et al. Online training and support programs designed to improve mental health and reduce burden among caregivers of people with dementia: a systematic review. J Am Med Dir Assoc. 2018;19:200–206.e1. doi: 10.1016/j.jamda.2017.10.023 [DOI] [PubMed] [Google Scholar]
- 20. Scott JL, Dawkins S, Quinn MG, et al. Caring for the carer: a systematic review of pure technology-based cognitive behavioral therapy (TB-CBT) interventions for dementia carers. Aging Ment Health. 2016;20:793–803. doi: 10.1080/13607863.2015.1040724 [DOI] [PubMed] [Google Scholar]
- 21. Waller A, Dilworth S, Mansfield E, Sanson-Fisher R. Computer and telephone delivered interventions to support caregivers of people with dementia: a systematic review of research output and quality. BMC Geriatr. 2017;17:265. doi: 10.1186/s12877-017-0654-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Topo P. Technology studies to meet the needs of people with dementia and their caregivers: a literature review. J Appl Gerontol. 2008;28:5–37. doi: 10.1177/0733464808324019 [DOI] [Google Scholar]
- 23. American Association of Retired Persons. Caregivers & Technology: What they want and need 2016. Retrieved from http://www.aarp.org/content/dam/aarp/home-and-family/personal-technology/2016/04/Caregivers-and-Technology-AARP.pdf. Accessed January 25, 2019.
- 24. Gallagher-Thompson D, Coon DW. Evidence-based psychological treatments for distress in family caregivers of older adults. Psychol Aging. 2007;22:37–51. doi: 10.1037/0882-7974.22.1.37 [DOI] [PubMed] [Google Scholar]
- 25. Williams K, Blyler D, Vidoni ED, et al. A randomized trial using telehealth technology to link caregivers with dementia care experts for in-home caregiving support: FamTechCare protocol. Res Nurs Health. 2018;41:219–227. doi: 10.1002/nur.21869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Niedens M. The Neuropsychiatric Symptoms of Dementia: A Visual Guide to Response Considerations. Leawood, KS: Alzheimer’s Association Heart of America Chapter; 2010. Retrieved from https://ombudsman.ks.gov/docs/default-source/default-document-library/the-neuropsychiatric-symptoms-of-dementia.pdf?sfvrsn=941b3a07_2. Accessed January 25, 2019. [Google Scholar]
- 27. Algase DL, Beck C, Kolanowski A, et al. Need-driven dementia-compromised behavior: an alternative view of disruptive behavior. American Journal of Alzheimer's Disease. 1996;11:10–19. doi: 10.1177/153331759601100603 [DOI] [Google Scholar]
- 28. Sclan SG, Reisberg B. Functional assessment staging (FAST) in Alzheimer’s disease: reliability, validity, and ordinality. Int Psychogeriatr. 1992;4(Suppl 1):55–69. doi: 10.1017/S1041610292001157 [DOI] [PubMed] [Google Scholar]
- 29. Bobinski M, Wegiel J, Tarnawski M, et al. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:414–420. doi: 10.1097/00005072-199704000-00010 [DOI] [PubMed] [Google Scholar]
- 30. Franssen EH, Reisberg B. Neurologic markers of the progression of Alzheimer’s disease. Int Psychogeriatr. 1997;9(Suppl 1):297–306; discussion 317. doi: 10.1017/S1041610297005036 [DOI] [PubMed] [Google Scholar]
- 31. Reisberg B, Ferris SH, Franssen EH, et al. Mortality and temporal course of probable Alzheimer's disease: a 5-year prospective study. Int Psychogeriatr. 1996;8:291–311. doi: 10.1017/S104161029002657 [DOI] [PubMed] [Google Scholar]
- 32. Reisberg B. Global measures: utility in defining and measuring treatment response in dementia. Int Psychogeriatr. 2007;19:421–456. doi: 10.1017/S1041610207005261 [DOI] [PubMed] [Google Scholar]
- 33. Bedard M, Molloy DW, Squire L, Dubois S, Lever JA, O'Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41:652–657. doi: 10.1093/geront/41.5.652 [DOI] [PubMed] [Google Scholar]
- 34. Radloff LS. The CES-D Scale. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 35. Pinquart M, Sörensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr. 2006;18:577–595. doi: 10.1017/S1041610206003462 [DOI] [PubMed] [Google Scholar]
- 36.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 37. Vernooij-Dassen MJ, Felling AJ, Brummelkamp E, Dauzenberg MG, van den Bos GA, Grol R. Assessment of caregiver’s competence in dealing with the burden of caregiving for a dementia patient: a Short Sense of Competence Questionnaire (SSCQ) suitable for clinical practice. J Am Geriatr Soc. 1999;47:256–257. doi: 10.1111/j.1532-5415.1999.tb04588.x [DOI] [PubMed] [Google Scholar]
- 38. McCaskill GM, Burgio LD, Decoster J, Roff LL. The use of Morycz’s desire-to-institutionalize scale across three racial/ethnic groups. J Aging Health. 2011;23:195–202. doi: 10.1177/0898264310381275 [DOI] [PubMed] [Google Scholar]
- 39. Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychol Aging. 1992;7:622–631. doi: 10.1037/0882-7974.7.4.622 [DOI] [PubMed] [Google Scholar]
- 40. Roth DL, Burgio LD, Gitlin LN, et al. Psychometric analysis of the Revised Memory and Behavior Problems Checklist: factor structure of occurrence and reaction ratings. Psychol Aging. 2003;18:906–915. doi: 10.1037/0882-7974.18.4.906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brown H, Prescott R.. Applied Mixed Models in Medicine. 3rd ed. West Sussex, UK: John Wiley & Sons; 2015. doi: 10.1002/9781118778210 [DOI] [Google Scholar]
- 42. Liu S, Rovine MJ, Molenaar PC. Selecting a linear mixed model for longitudinal data: repeated measures analysis of variance, covariance pattern model, and growth curve approaches. Psychol Methods. 2012;17:15–30. doi: 10.1037/a0026971 [DOI] [PubMed] [Google Scholar]
- 43. Cohen J, Cohen P, West SG, Aiken LS.. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3rd ed. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. doi: 10.4324/9780203774441 [DOI] [Google Scholar]
- 44. Ruggiano N, Brown EL, Li J, Scaccianoce M. Rural dementia caregivers and technology: what is the evidence? Res Gerontol Nurs. 2018;11:216–224. doi: 10.3928/19404921-20180628-04 [DOI] [PubMed] [Google Scholar]
- 45. Weiss RE. Modeling Longitudinal Data. New York: Springer Science & Business Media; 2005. doi: 10.1007/0-387-28314-5 [DOI] [Google Scholar]
- 46. Kim S, Shaw C, Williams K, Hein M. Typology of technology-supported dementia care guidance from an in-home Telehealth trial. West J Nurs Res. 2019. (Epub ahead of print). doi: 10.1177/0193945919825861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shaw C, Williams K, Kim S, Hein M, Perkhounkova Y. Identifying priority needs for family caregiver support: do caregivers and dementia care experts agree? Midwest Nursing Research Society Annual Meeting; 2019; Kansas City, MO. [Google Scholar]
- 48. Liu Z, Chen QL, Sun YY. Mindfulness training for psychological stress in family caregivers of persons with dementia: a systematic review and meta-analysis of randomized controlled trials. Clin Interv Aging. 2017;12:1521–1529. doi: 10.2147/cia.S146213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mavandadi S, Wright EM, Graydon MM, Oslin DW, Wray LO. A randomized pilot trial of a telephone-based collaborative care management program for caregivers of individuals with dementia. Psychol Serv. 2017;14:102–111. doi: 10.1037/ser0000118 [DOI] [PubMed] [Google Scholar]
- 50. Williams F, Moghaddam N, Ramsden S, De Boos D. Interventions for reducing levels of burden amongst informal carers of persons with dementia in the community. A systematic review and meta-analysis of randomised controlled trials. Aging Ment Health. 2018:1–14. (Epub ahead of print). doi: 10.1080/13607863.2018.1515886 [DOI] [PubMed] [Google Scholar]
- 51. Cepoiu-Martin M, Tam-Tham H, Patten S, Maxwell CJ, Hogan DB. Predictors of long-term care placement in persons with dementia: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2016;31:1151–1171. doi: 10.1002/gps.4449 [DOI] [PubMed] [Google Scholar]
- 52. Toot S, Swinson T, Devine M, Challis D, Orrell M. Causes of nursing home placement for older people with dementia: a systematic review and meta-analysis. Int Psychogeriatr. 2017;29:195–208. doi: 10.1017/S1041610216001654 [DOI] [PubMed] [Google Scholar]
- 53. Arruda EH, Paun O. Dementia caregiver grief and bereavement: an integrative review. West J Nurs Res. 2017;39:825–851. doi: 10.1177/0193945916658881 [DOI] [PubMed] [Google Scholar]
- 54. Dauphinot V, Ravier A, Novais T, Delphin-Combe F, Mouchoux C, Krolak-Salmon P. Risk factors of caregiver burden evolution, for patients with subjective cognitive decline or neurocognitive disorders: a longitudinal analysis. J Am Med Dir Assoc. 2016;17:1037–1043. doi: 10.1016/j.jamda.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 55. Swinkels JC, Broese van Groenou MI, de Boer A, Tilburg TGV. Male and female partner-caregivers' burden: does it get worse over time? Gerontologist. 2018. (Epub ahead of print). doi: 10.1093/geront/gny132 [DOI] [PubMed] [Google Scholar]
- 56. Chi NC, Demiris G. A systematic review of telehealth tools and interventions to support family caregivers. J Telemed Telecare. 2015;21:37–44. doi: 10.1177/1357633X14562734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Knight BG, Sayegh P. Cultural values and caregiving: the updated sociocultural stress and coping model. J Gerontol B Psychol Sci Soc Sci. 2010;65B:5–13. doi: 10.1093/geronb/gbp096 [DOI] [PubMed] [Google Scholar]