Abstract
Background
Modulating gut microbiota is a potential treatment for irritable bowel syndrome (IBS). This meta-analysis explored whether fecal microbiota transplantation (FMT) is successful in treating IBS.
Methods
A systematic review was performed to find trials on FMT in IBS. Ratios and relative ratios (RR) of improvement for single-arm trials (SATs) and randomized controlled trials (RCTs) were calculated, respectively. Changes in IBS Severity Scoring System (IBS-SSS) and IBS Quality of Life (IBS-QOL) instrument compared to baseline in FMT versus placebo groups were pooled.
Results
In SATs, 59.5% (95% confidence interval (CI) 49.1–69.3) of IBS patients showed significant improvement. In RCTs, there were no differences between FMT and control in improvement (RR=0.93 (95% CI 0.50–1.75)) or changes in the IBS-SSS and IBS-QOL.
Conclusions
FMT was not effective in IBS. Variations in FMT methods and patient factors may contribute to the heterogeneous results of the trials.
Keywords: Fecal microbiota transplantation, irritable bowel syndrome, IBS Symptom Severity Scale, IBS Quality of Life, meta-analysis
Introduction
Irritable bowel syndrome (IBS) is a disorder of gut–brain interaction (DGBI) with multifactorial etiology.1 Motility disturbances, visceral hypersensitivity, altered mucosal permeability, immune activation, or systemic parameters affecting the gut–brain interaction have been considered as underlying mechanisms.2
Dysbiosis plays an important role in the pathogenesis of IBS.3 Accordingly, modulation of gut microbiota with agents such as probiotics, prebiotics, symbiotics, luminal antibiotics, and fecal microbiota transplantation (FMT) have been suggested as treatment options for IBS.4,5 FMT is defined as the transfer of gastrointestinal (GI) microbiota from a healthy donor into the GI tract of a patient with dysbiosis.4 This is a strongly endorsed treatment strategy in refractory or recurrent Clostridioides difficile infection.4 Although not part of a consensus, FMT has been recommended for other GI disorders, including inflammatory bowel disease and IBS.
Studies on the role of FMT in IBS are limited.6–20 Based on a narrative review, several case series showed favorable results for FMT.4 Of five randomized controlled trials (RCTs) on IBS, the majority measured the IBS Symptom Severity Scoring System (IBS-SSS) as an outcome. In contrast, the selection criteria and the route and form of FMT were different between the trials. The results of those studies were inconsistent, and there was a lack of statistical power of the performed trials. Notwithstanding, some of the trials suggested that post-infectious IBS (PI-IBS) and the baseline microbiota status in the donors could predict success for FMT in IBS.4
To overcome the inconsistency of the trials on FMT in IBS, we performed a systematic review and meta-analysis, and the findings are presented in this article.
Methods
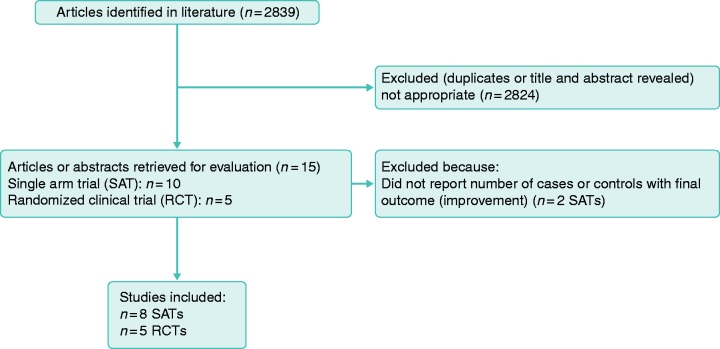
In February 2019, two independent reviewers (M.K. and A.D.) searched PubMed, Embase, Google Scholar, and abstract books of Digestive Disease Week 2010–2018 and United European Gastroenterology Week 2010–2018 using (a) “irritable bowel syndrome,” (b) fecal, (c) stool, (d) microbiota, (e) transplant, (f) transfer, (g) “microb*,” (h) “(((((fecal) OR stool) OR microbiota) OR transplant) OR transfer) OR microb*,” and (i) “irritable bowel syndrome AND (((((fecal) OR stool) OR microbiota) OR transplant) OR transfer) OR microb*.” Single-arm trials (SATs) and RCTs on FMT in IBS were retrieved if the diagnosis of IBS was proven by physicians or was based on ROME I, II, III, or IV criteria when other GI disorders were excluded. Whenever an abstract and a full text of a project were published, data from the full text article were included. Figure 1 shows the flow diagram of study selection.
Figure 1.
Flow diagram of study selection for the meta-analysis of fecal microbiota transplant (FMT) in irritable bowel syndrome (IBS).
Quality assessment
Quality assessment for RCTs was based on the Jadad Scale,21 Cochrane Collaboration’s tool for assessing risk of bias,22 and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) for level of evidence for the variables.23
Data extraction
The total number of studied patients, number of patients with symptom improvement, and the M±SD of the IBS-SSS and IBS-QOL at baseline and after FMT were extracted. Whenever data were presented in figures, data points were reconstructed using the image digitizer Graph Grabber v2.0 (Quintessa, Henley-on-Thames, UK). When M ± SD were not reported, they were estimated, as described previously.24
Statistical analysis
Data analysis was performed using RevMan v5.3 (Cochrane, London, UK) and MedCalc v19 (MedCalc Software, Ostend, Belgium). Response to treatment was defined as number of patients with overall symptom improvement divided by the total number of studied patients and is reported as ratio (R) and 95% confidence interval (95% CI) and relative ratio (RR) and 95% CI for SATs and RCTs, respectively. Changes in IBS-SSS and IBS-QOL in FMT versus control groups were defined as Δ IBS-SSS and Δ IBS-QOL, which were equal to “baseline score” minus “score after treatment.” For Δ IBS-QOL, absolute values were included. IBS-SSS and IBS-QOL were compared using the mean difference (MD) and standard mean difference (SMD) methods, respectively. A positive MD for IBS-SSS indicated a tendency toward better response to FMT compared with placebo (control). Fixed- and random-effects models were used when I2 ≤ 50% and I2 > 50%, respectively. The results are presented as forest plots.
Results
Ten SATs7,11,12,15–18,25–27 and five RCTs10,13,20,28,29 on FMT in IBS were retrieved. Overall, eight SATs7,11,12,16–18,25,26 (n = 90 patients in total) and all five RCTs10,13,20,28,29 (n = 151 patients allocated to FMT and n = 105 controls) were included. One of the RCTs was published in full after the literature review and was later included.6,29 One trial reported separate IBS-SSS for patients treated with frozen or fresh FMT; the mean value for response to both types of FMT was calculated.13
The characteristics of the included SATs and RCTs are summarized in Tables 1 and 2, respectively.
Table 1.
Summary of results of open-label single-arm trials of FMT in IBS.
| References | Country | Sample size (n) | Route of FMT administration | Follow-up | Results |
|---|---|---|---|---|---|
| Morken et al. 200925 | Norway | 10 | Gastroduodenoscopy | 12 months | Antibiotics and bacteriotherapy were ineffective in post-giardiasis IBS-like symptoms |
| Pinn et al. 201418 | USA | 13 | Gastroduodenoscopy | 11 months | 70% of patients experienced resolution of IBS symptoms after FMT |
| Cruz et al. 20157 | Germany | 9 | Colonoscopy | 3 months | FMT was beneficial, even though transient; there were profound microbiome changes in IBS-D patients |
| Hong et al. 201612 | Korea | 10 | Colonoscopy | 12 and 26 weeks | Symptom improvement after FMT in 80% of patients; however, those who showed significant improvement in IBS severity scores during the first month returned to their pre-FMT state after 3 months |
| Syzenko et al. 201626 | Ukraine | 12 | Colonoscopy | NR | Significant rate of clinical improvement in refractory IBS symptoms after FMT (p ≤ 0.01) |
| Mizuno et al. 201717 | Japan | 10 | Colonoscopy | 4 weeks | FMT improved stool form and depressed mood; Bifidobacterium-rich donor feces was related to successful FMT |
| Holvoet et al. 201711 | Belgium | 12 | NR | 12 months | 75% of patients had adequate relief of global IBS symptoms and abdominal bloating; successful donors tended to have higher baseline counts of Streptococcus |
| Mazzawi et al. 201616 | Norway | 16 | Gastroduodenoscopy | 1, 3, 12, and 28 weeks | FMT induced significant changes in gut microbiota and symptom relief in IBS patients |
FMT: fecal microbiota transplant; IBS: irritable bowel syndrome; IBS-D: IBS with diarrhea; NR: Not reported.
Table 2.
RCTs of FMT in IBS.
| References | Holster et al. 2019 | Johnson et al. 2018 | Aroniadis et al. 2018 | Halkjaer et al. 2018 | Holvoet et al. 2018 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Sweden | Norway | USA | Denmark | Belgium | ||||||||
| Enrollment period | May 2014–April 2016 | January 2015– October 2015 | 2015–2017 | October 2016 –December 2016 | December 2015 –September 2017 | ||||||||
| Design | Double-blinded RCT | Prospective double-blinded RCT | Randomized double-blinded multi-center crossover trial | Prospective double-blinded RCT | Prospective double-blinded RCT | ||||||||
| Eligible age group (years) | 18–65 | 18–75 | 18–65 | 18–60 | 18–75 | ||||||||
| Total (n) | 16 | 83 | 48 | 51 | 64 | ||||||||
| Jadad Score | 5 | 5 | 4 | 5 | 5 | ||||||||
| Study group | FMT | Control | FMT | Control | FMT followed by control | Placebo followed by FMT | FMT | Placebo | FMT | Control | |||
| Sample size | 8 | 8 | 55 | 28 | 24 | 24 | 25 | 26 | 42 | 22 | |||
| Mean age (years) | 34 | 39 | 44 | 45 | 38.3 ± 12.3 | 38.5 ± 11.8 | 37.28 | 35.54 | NR | NR | |||
| Sex (M:F) | 5:3 | 3:5 | 9:16 | 19:36 | 15:9 | 15:9 | 8:16 | 8:16 | NR | NR | |||
| Years with IBS | Unknown: 0/1–5 years: 4/>5 years: 4 patients | Unknown: 1/1–5 years: 3/>5 years: 4 patients | 10 (6–16) | 10 (5–19) | 8.0 ± 6.0 | 9.0 ± 7.9 | NR | NR | NR | NR | |||
| ROA | Colonoscopy | Colonoscopy | Colonoscopy | Colonoscopy | Oral capsules | Oral capsules | Oral capsules | Oral capsules | Nasojejunal | Nasojejunal | |||
| Follow-up time | 6 months | 6 months | 12 months | 12 months | 12 weeks | 12 weeks | 6 months | 6 months | 12 weeks | 12 weeks | |||
| IBS subtypes | |||||||||||||
| IBS-C, n (%) | 5 (62.5) | 4 (50) | 0 | 0 | 0 | 0 | 7 (28.0) | 10 (38.5) | NR | NR | |||
| IBS-D, n (%) | 1 (12.5) | 3 (37.5) | 24 (44) | 15 (54) | 24 | 24 | 7 (28.0) | 8 (30.8) | NR | NR | |||
| IBS-M, n (%) | 2 (25) | 1 (12.5) | 31 (56) | 13 (46) | 0 | 0 | 11 (44.0) | 8 (30.8) | NR | NR | |||
| Adverse effects, n (%) | 4 (50) | 7 (78.5) | 4 (72.7)a | 3 (10.7) | NR | NR | 22 (84.6) | 15 (57.7) | NR | NR | |||
One serious adverse effect (nausea/vertigo needing hospitalization).
RCT: randomized controlled trial; NR: not reported; ROA: route of administration; IBS-C: IBS with constipation; IBD: IBS with diarrhea; IBS-M: mixed IBS.
The quality of the trials according to the Jadad Scale was good (score: five in four trials10,13,20,29 and four in one trial28). The risk of bias of the RCTs is shown in Supplemental Figure S1. The level of evidence based on GRADE was very low (see Supplemental Table S1).
Outcome definitions, number of patients, duration of follow-up, and safety profile of the included studies have been discussed in a previous narrative review by our group.4
Two RCTs that used oral capsules had real placebo as a comparator.20,28 The three other RCTs used autologous FMT as controls.10,13,29 The duration of the follow-up in the SATs ranged from 3 weeks to 18 months, while in the RCTs, it was up to 24 months.
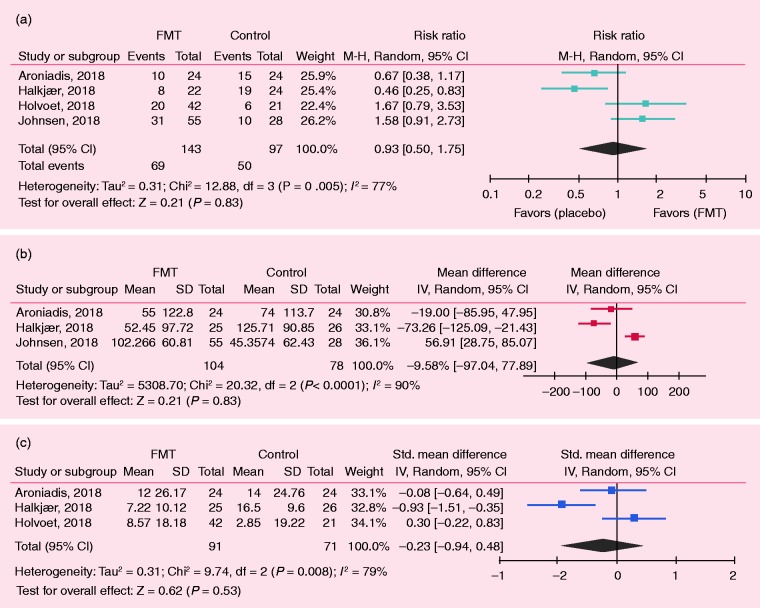
In the SATs, R = 59.5% (95% CI 49.1–69.3) of IBS patients showed significant improvement of IBS symptoms (Figure 2). For the RCTs, 4-, 8-, 12-, and 24-week outcomes were selected for the analysis of IBS-SSS, and 12-week outcomes were selected for IBS-QOL. Holvoet et al. analyzed self-reported adequate relief of general IBS symptoms and bloating, finding that 49% of donor FMT recipients versus 29% of controls (p = 0.004) reached this outcome.10 Discomfort, number of stools, urgency for a bowel movement, abdominal pain, and flatulence were significantly reduced by 19%, 13%, 38%, 26%, and 10%, respectively, in the FMT but not in the control group.10 Aroniadis et al. analyzed the clinical response—defined as a decrease in IBS-SSS by ≥50 points at 12 weeks—and found no differences between the FMT and control groups (48% vs. 63%).28 In the study by Johnsen et al., 36/55 participants receiving FMT and 12/28 receiving placebo showed a response by a decrease of >75 points in IBS-SSS at three months, favoring the active group (p = 0.049).13 Meta-analysis of the RCTs showed no differences between the FMT and control groups in IBS symptom improvement, which was defined as self-reported satisfactory relief of IBS symptoms or decrease in IBS-SSS (RR = 0.93; 95% CI 0.50–1.75; Figure 3(a)) As response to treatment was not standardized in the included studies, changes in IBS-SSS from baseline would provide a better information in terms of response to FMT or control.
Figure 2.
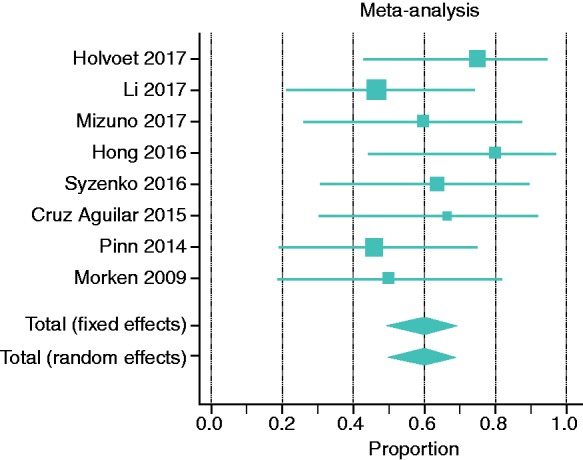
Meta-analysis of single-arm clinical trials (SATs) on the role of FMT in IBS. Data represent proportion (ratio), which is defined as the number of cases with improvement divided by the total studied cases.
Figure 3.
Meta-analysis of randomized clinical trials (RCTs) on the role of FMT in IBS. (a) Risk ratio or relative ratio: events indicate number of cases with improvement of symptoms. (b) Changes in IBS Severity Scoring System (IBS-SSS) after treatment at 12 weeks; negative values indicate decrease in IBS-SSS after treatment or improvement. (c) Crude changes in IBS Quality of Life (IBS-QOL) after treatment.
Four RCTs analyzed IBS-SSS.13,20,28,29 Johnsen et al. and Aroniadis et al.’s findings are described above. Halkjaer et al. used the IBS-SSS as the primary endpoint defining a positive effect in the presence of a 50-point reduction in this scale, showing a significant improvement at the three-month visit, favoring control treatment over FMT: M = 125.71 (SD = 90.85) versus M = 52.45 (SD = 97.72), p = 0.012.20 Holster et al. did not observe a significant difference in IBS-SSS after allogenic and autologous treatments.29 In the meta-analysis of the changes from baseline in the IBS-SSS, there were no differences in the FMT versus control groups: MD = –9.58 (95% CI –77.89 to 97.04) at 12 weeks (Figure 3(b)), as well as at 4, 8, and 24 weeks (Supplemental Figure S2).
Regarding the IBS-QOL, three studies compared the effect on IBS-QOL at 12 weeks.10,20,28 The first one by Aroniadis et al. found an improvement in IBS-QOL at 12 weeks versus baseline in both groups (FMT: 52 ± 19 vs. 64 ± 18; control: 53 ± 18 vs. 67 ± 17), without any difference between the groups.28 Halkjaer et al. showed a significant improvement in FMT and control at 12 weeks, favoring control (–16.50 (9.60)) over FMT (–7.22 (10.12)) in the IBS-QOL.20 Holvoet et al. showed an improvement of the IBS-QOL in the FMT group by 16%, with minimal changes in the control group at 12 weeks. In the meta-analysis of changes of IBS-QOL after treatment, there were no differences between the FMT and control groups (SMD: –0.23; 95% CI –0.94 to 0.48; Figure 3(c)).10
No serious adverse event was reported in any of the RCTs, except for one case in the FMT group requiring a few hours of hospitalization (Table 2).
Discussion
Dysbiosis has been involved in the pathophysiology of functional GI disorders, now called DGBI, such as IBS.30 Dysbiosis can be triggered by antibiotics or after an enteric infection, which can trigger PI-IBS. A study found lower Lactobacillus spp. in stool samples of diarrhea predominant IBS (IBS-D), while constipation predominant IBS (IBS-C) had increased Veillonella spp.31 Kassinen et al. found differences in the genera Coprococcus, Collinsella, and Coprobacillus in IBS versus controls.32 Carroll et al. revealed differences in the luminal and mucosal microbiota between IBS patients and controls and decreased microbial biodiversity in fecal samples of IBS-D patients.33 Rajilic-Stojanovic et al. reported an increased ratio of the Firmicutes to Bacteroidetes phylotypes.34 Tana et al. showed patients with IBS had higher Veillonella and Lactobacillus versus controls.35 Jalanka-Tuovinen et al. identified an index of fecal microbial dysbiosis, which significantly distinguished PI-IBS from controls.36 Therefore, currently, the abnormalities in the microbiota–gut–brain axis are considered an important underlying mechanism in the generation of IBS.37
Bacteriotherapy or FMT, now approved for the treatment of recurrent or refractory C. difficile infection,38,39 has been proposed for the treatment of other disorders such as IBS.4 To our knowledge, during the past few years, 10 SATs7,11,12,15–18,25–27 and five RCTs,6,10,13,20,28,29 on FMT in IBS have been published. In a previous narrative review, we found that FMT for IBS in the SATs was promising, while the RCTs provided conflicting results.4 Two RCTs showed an improvement in IBS symptoms with FMT,10,13 and two other studies provided negative results.20,28 Therefore, we conducted this meta-analysis to explore the real efficacy of FMT in IBS. In summary, eight SATs fulfilled the inclusion criteria. In the SATs, 59.5% of IBS patients had significant improvement of symptoms. In contrast, in the RCTs, there were no differences between FMT and control treatment in IBS symptom, severity, or improvement in quality of life.
The inconsistent results on the RCTs could be related to several factors such as placebo effect. The relative placebo responses for IBS symptom severity is roughly 41.4% (range 25–59%), and for quality of life, it is between 20% and 125%.40 In the RCTs reviewed in this meta-analysis, crude placebo response rates were almost similar to the response to FMT in SATs, suggesting that observations in the SATs would be mainly because of a placebo effect.
The routes and source of FMT could be considered a confounding factor. The two RCTs that used oral capsules did not show an improvement in IBS symptoms compared to placebo.20,28 In contrast, three other trials that used the nasojejunal and colonoscopy approach showed results favoring FMT.10,13,29 Whether FMT into the distal GI tract or the colon is more effective than the oral capsules in IBS needs further investigation. The oral route may result in small intestinal bacterial overgrowth. In addition, the number of bacteria delivered by oral capsules may not be sufficient. Notwithstanding, data from FMT in C. difficile infection have shown that oral capsules are not inferior to FMT delivered by colonoscopy for preventing recurrent infection over 12 weeks.41 In addition, no difference has been found between frozen and fresh FMT.42 This has also been observed when FMT was given orally administering a lyophilized microbiota product compared to a frozen product delivered by enemas.43
Another issue that deserves attention is the effectiveness of FMT according to the IBS subtypes. However, data were not presented based on subtype in the majority of the studies. Although in terms of immunological profile of IBS we did not observe a huge difference between IBS-C and IBS-D,4,44 there is a possibility that a specific IBS subtype would have a better response to FMT.
The presence of dysbiosis might also be important in predicting response to FMT. Recently, Ghoshal et al. showed that treatment directed to manipulate the methanogenic microbiota improved chronic constipation only in the subset of patients colonized by this group.45 As it is not expected that all patients would have dysbiosis, the inclusion of all unselected patients is not likely to yield a positive result with FMT. Another interfering factor is the donors’ and recipients’ fecal microbiota profile. Using patients’ own fecal material may not be considered as a true placebo. The other parameter is PI-IBS. According to Aroniadis et al.,28 a trend toward greater improvement was observed in PI-IBS patients who received FMT, but again we have to consider that the route of FMT administration in the above-mentioned study did not provide a dramatic improvement compared to those studies that used enemas as the route of FMT.
In conclusion, the current systematic review and meta-analysis does not support FMT as a successful treatment strategy in IBS. To address whether FMT would be helpful in IBS, larger studies with appropriate placebo groups and which take into account recipient and donor factors, including baseline microbiota profile, would be necessary. Stratifying data based on IBS subtypes and the presence of PI-IBS would decrease heterogeneity of the observations.
Supplemental Material
Supplemental Material for Fecal microbiota transplantation in irritable bowel syndrome: A systematic review and meta-analysis by Kanchana Myneedu, Abhizith Deoker, Max J Schmulson and Mohammad Bashashati in United European Gastroenterology Journal
Declaration of conflicting interests
M.J.S. received research support from Alfa Wassermann and Takeda Mexico, is a member of the Advisory Board of Alfa Wassermann Mexico, served as a consultant for Commonwealth Diagnostics International Inc., and has been a speaker for Takeda Mexico. K.M., A.D., and M.B. do not have anything to disclose.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Mayer EA, Labus JS, Tillisch K, et al. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol 2015; 12: 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology 2016; 150: 1262–1279. [DOI] [PubMed] [Google Scholar]
- 3.Hong SN, Rhee PL. Unraveling the ties between irritable bowel syndrome and intestinal microbiota. World J Gastroenterol 2014; 20: 2470–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmulson M, Bashashati M. Fecal microbiota transfer for bowel disorders: efficacy or hype? Curr Opin Pharmacol 2018; 43: 72–80. [DOI] [PubMed] [Google Scholar]
- 5.Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol 2014; 109: 1547–1561; quiz 1546, 1562. [DOI] [PubMed] [Google Scholar]
- 6.Holster S, Brummer RJ, Repsilber D, et al. Fecal microbiota transplantation in irritable bowel syndrome and a randomized placebo-controlled trial. Gastroenterology 2017; 152: S101–S102. [Google Scholar]
- 7.Cruz-Aguilar R, Buch T, Bajbouj M, et al. Fecal microbiota transplantation as a novel therapy for irritable bowel syndrome with predominant diarrhea. Neurogastroenterol Motil 2015; 27: 110–110. [Google Scholar]
- 8.Halkjær S, Christensen A, Lo B, et al. Fecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomized, double-blind placebo controlled study. Gastroenterology 2018; 154: S181–S181. [DOI] [PubMed] [Google Scholar]
- 9.Halkjaer SI, Boolsen AW, Gunther S, et al. Can fecal microbiota transplantation cure irritable bowel syndrome? World J Gastroenterol 2017; 23: 4112–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holvoet T, Joossens M, Jerina B, et al. Fecal microbiota transplantation in irritable bowel syndrome with predominant abdominal bloating: results from a double blind, placebo-controlled clinical trial. Gastroenterology 2018; 154: S130–S130. [DOI] [PubMed] [Google Scholar]
- 11.Holvoet T, Joossens M, Wang J, et al. Assessment of faecal microbial transfer in irritable bowel syndrome with severe bloating. Gut 2017; 66: 980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong J, Bang B, Shin Y, et al. Treatment of irritable bowel syndrome with fecal microbiota transplantation: a case series of 10 patients. United European Gastroenterol J 2016;4.
- 13.Johnsen PH, Hilpusch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol 2018; 3: 17–24. [DOI] [PubMed] [Google Scholar]
- 14.Juncadella AC, Moss A. Fecal microbiota transplantation as a possible treatment of irritable bowel syndrome. Ann Transl Med 2017; 5: 506–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Tian H, Ma C, et al. [Efficacy analysis of fecal microbiota transplantation in the treatment of 406 cases with gastrointestinal disorders]. Zhonghua Wei Chang Wai Ke Za Zhi 2017; 20: 40–46. [PubMed] [Google Scholar]
- 16.Mazzawi T, Lied GA, El-Salhy M, et al. Effect of faecal microbiota transplantation on the symptoms and duodenal enteroendocrine cells in patients with irritable bowel syndrome. United European Gastroenterol J 2016; 4: A677–A677. [Google Scholar]
- 17.Mizuno S, Masaoka T, Naganuma M, et al. Bifidobacterium-rich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Digestion 2017; 96: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation the answer for irritable bowel syndrome? A single-center experience. Am J Gastroenterol 2014; 109: 1831–1832. [DOI] [PubMed] [Google Scholar]
- 19.Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol Motil 2015; 27: 19–29. [DOI] [PubMed] [Google Scholar]
- 20.Halkjaer SI, Christensen AH, Lo BZS, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut 2018; 67: 2107–2115. [DOI] [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morken MH, Valeur J, Norin E, et al. Antibiotic or bacterial therapy in post-giardiasis irritable bowel syndrome. Scand J Gastroenterol 2009; 44: 1296–1303. [DOI] [PubMed] [Google Scholar]
- 26.Syzenko G, Budovska L, Puchkov K. Efficiency of FMT in cases of “treatment-resistant” IBS. United European Gastroenterol J 2016; 4: A294–A294. [Google Scholar]
- 27.Borody TJ, George L, Andrews P, et al. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust 1989; 150: 604–604. [DOI] [PubMed] [Google Scholar]
- 28.Aroniadis OC, Brandt LJ, Oneto C, et al. A double-blind, randomized, placebo-controlled trial of fecal microbiota transplantation capsules (FMTC) for the treatment of diarrhea-predominant irritable bowel syndrome (IBS-D). Gastroenterology 2018; 154: S154–S154. [Google Scholar]
- 29.Holster S, Lindqvist CM, Repsilber D, et al. The effect of allogenic versus autologous fecal microbiota transfer on symptoms, visceral perception and fecal and mucosal microbiota in irritable bowel syndrome: a randomized controlled study. Clin Transl Gastroenterol 2019; 10: e00034–e00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enck P, Mazurak N. Dysbiosis in functional bowel disorders. Ann Nutr Metab 2018; 72: 296–306. [DOI] [PubMed] [Google Scholar]
- 31.Malinen E, Rinttila T, Kajander K, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol 2005; 100: 373–382. [DOI] [PubMed] [Google Scholar]
- 32.Kassinen A, Krogius-Kurikka L, Makivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 2007; 133: 24–33. [DOI] [PubMed] [Google Scholar]
- 33.Carroll IM, Ringel-Kulka T, Keku TO, et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2011; 301: G799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajilic-Stojanovic M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011; 141: 1792–1801. [DOI] [PubMed] [Google Scholar]
- 35.Tana C, Umesaki Y, Imaoka A, et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil 2010; 22: 512–519, e114–115. [DOI] [PubMed] [Google Scholar]
- 36.Jalanka-Tuovinen J, Salojarvi J, Salonen A, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014; 63: 1737–1745. [DOI] [PubMed] [Google Scholar]
- 37.De Palma G, Collins SM, Bercik P. The microbiota–gut–brain axis in functional gastrointestinal disorders. Gut Microbes 2014; 5: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konig J, Siebenhaar A, Hogenauer C, et al. Consensus report: faecal microbiota transfer—clinical applications and procedures. Aliment Pharmacol Ther 2017; 45: 222–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughn BP, Rank KM, Khoruts A. Fecal microbiota transplantation: current status in treatment of GI and liver disease. Clin Gastroenterol Hepatol 2019; 17: 353–361. [DOI] [PubMed] [Google Scholar]
- 40.Flik CE, Bakker L, Laan W, et al. Systematic review: the placebo effect of psychological interventions in the treatment of irritable bowel syndrome. World J Gastroenterol 2017; 23: 2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2017; 318: 1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hui W, Li T, Liu W. Fecal microbiota transplantation for treatment of recurrent C. difficile infection: an updated randomized controlled trial meta-analysis. PLoS One 2019; 14: e0210016–e0210016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang ZD, Jenq RR, Ajami NJ, et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: a randomized clinical trial. PLoS One 2018; 13: e0205064–e0205064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bashashati M, Rezaei N, Shafieyoun A, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterol Motil 2014; 26: 1036–1048. [DOI] [PubMed] [Google Scholar]
- 45.Ghoshal UC, Srivastava D, Misra A. A randomized double-blind placebo-controlled trial showing rifaximin to improve constipation by reducing methane production and accelerating colon transit: a pilot study. Indian J Gastroenterol 2018; 37: 416–423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Fecal microbiota transplantation in irritable bowel syndrome: A systematic review and meta-analysis by Kanchana Myneedu, Abhizith Deoker, Max J Schmulson and Mohammad Bashashati in United European Gastroenterology Journal