Abstract
Background
Approximately 10% of the general population and around one third of adult patients in clinical populations suffer from functional somatic symptoms. These take many forms, are often chronic, impair everyday functioning as well as quality of life, and are cost intensive.
Methods
The guideline group (32 medical and psychological professional societies, two patients’ associations) carried out a systematic survey of the literature and analyzed 3795 original articles and 3345 reviews. The aim was to formulate empirically based recommendations that were practical and user friendly.
Results
Because of the variation in course and symptom severity, three stages of treatment are distinguished. In early contacts, the focus is on basic investigations, reassurance, and advice. For persistent burdensome symptoms, an extended, simultaneous and equitable diagnostic work-up of physical and psychosocial factors is recommended, together with a focus on information and self-help. In the presence of severe and disabling symptoms, multimodal treatment includes further elements such as (body) psychotherapeutic and social medicine measures. Whatever the medical specialty, level of care, or clinical picture, an empathetic professional attitude, reflective communication, information, a cautious, restrained approach to diagnosis, good interdisciplinary cooperation, and above all active interventions for self-efficacy are usually more effective than passive, organ-focused treatments.
Conclusion
The cornerstones of diagnosis and treatment are biopsychosocial explanatory models, communication, self-efficacy, and interdisciplinary mangagement. This enables safe and efficient patient care from the initial presentation onwards, even in cases where the symptoms cannot yet be traced back to specific causes.
The German clinical practice guideline on the management of patients with unspecific, functional, and somatoform physical symptoms (1, 2) expired in March 2017. Between November 2016 and July 2018, the guideline was updated and thoroughly revised by a group under the coordination of the German College of Psychosomatic Medicine (Deutsches Kollegium für Psychosomatische Medizin, DKPM) and the German Society of Psychosomatic Medicine and Medical Psychotherapy (Deutsche Gesellschaft für Psychosomatische Medizin und Ärztliche Psychotherapie, DGPM) and in accordance with the requirements of the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft wissenschaftlicher medizinischer Fachgesellschaften, AWMF). Particular attention was paid to user-friendly language and relevance to daily practice. The long version of the guideline and the guideline methods report are available (in German) on the AWMF website (3). The patient guideline is currently undergoing revision.
Characterization of the clinical picture
The term “functional somatic symptoms” refers to a broad spectrum of symptom patterns of greatly varying severity (4– 6):
Persistent unspecific symptoms that are burdensome enough for the patient to consult a doctor but are not classified as disease (“medically unexplained symptoms” or “persistent physical symptoms”). These can nevertheless discernibly impair the patient’s everyday functioning.
Defined symptom clusters present over an extended period in the form of functional somatic syndromes (such as fibromyalgia syndrome or irritable bowel syndrome). These are mostly associated with a significant limitation of everyday functioning.
Conditions that fulfill the criteria of pronounced (multi)somatoform disorders and the newly defined somatic stress disorders. These presuppose considerable impairment of everyday functioning and are also associated with psychobehavioral symptoms.
Functional somatic symptoms as outlined above are to be distinguished from the commonly occurring transitory indispositions that rarely prompt a visit to the doctor and affect everyday functioning only slightly for a limited time, if at all. These are of no medical significance.
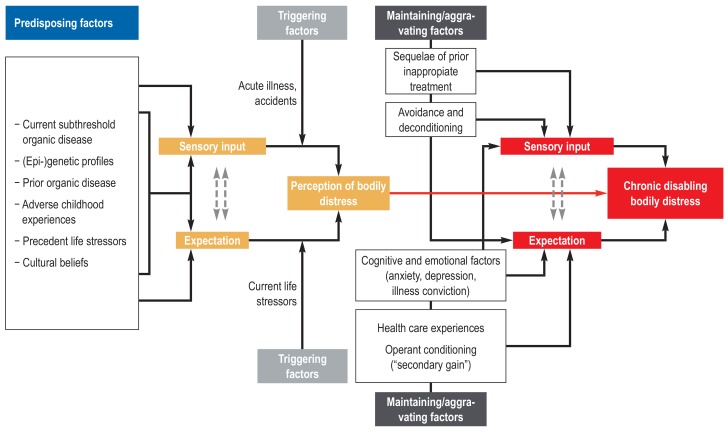
Functional somatic symptoms affect a considerable portion (around 10%) of the general population (e1). In the medical context, rates of 20% to 50% are reported for patients visiting primary care physicians and 25% to 66% in particular specialties (e.g., rheumatology, pain medicine, and gynecology) (e2– e5). Functional somatic symptoms are frequently self-limiting (e6, e7). In at least 20%, more likely 50%, of patients who have multiple somatic symptoms and fulfill the criteria of “(multi)somatoform disorder” or “bodily distress syndrome”, the symptoms are enduring (e8– e11). Over the course of time 50% to 75% of patients report improvement, while in 10% to 30% the symptoms worsen (e10– e12). Life expectancy appears not to be affected, apart from an increased prevalence of suicidal behavior (e13– e17): passive death wishes occur in over half of patients with functional disorders (56%), concrete suicidal thoughts in around one third (24% to 34%); 13% to 18% have attempted to commit suicide earlier in life (e13, e14). Comorbidity with mental disorders (principally anxiety and depression), with a rate of around 50%, occurs just as frequently as the overlapping of different functional syndromes (e18– e28). Moreover, persons with functional somatic symptoms may very well show organic findings, e.g., as normal variants, trivial findings, expression of underlying functional organ dysfunctions, or in the presence of (somatic) illness (comorbidity or differential diagnosis) (4– 6, e29, e30). Swift, unambiguous classification of symptoms as functional is therefore rarely possible. The prevailing etiological models of functional disorders and bodily distress postulate a multifactorial genesis with interaction of biological, psychological, and sociocultural factors in predisposition, triggering, and maintenance (figure 1) (4– 6). Functional somatic symptoms generate high healthcare costs (e31– e33).
Figure 1.
Schematic model of the etiology of bodily distress (from [4] by kind permission of Peter Henningsen)
Methods
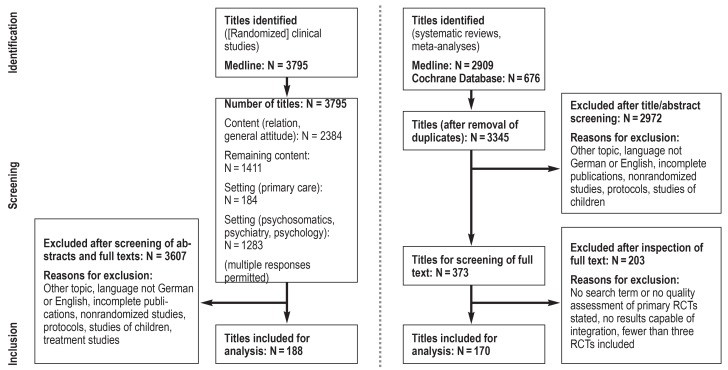
The guideline was revised by the members of a large, representative group of experts from 32 professional medical and psychological societies and two organizations representing the interests of patients (ebox 1). Evidence was derived from an updated systematic literature survey that identified 3795 clinical studies and 3345 systematic reviews, as well as from all relevant source guidelines (eFigure 1, eTable 1). The Table shows the main results of selected reviews on interventions for functional somatic symptoms. A steering group then formulated 109 recommendations, based on the requirements specified by the AWMF and the Center for Quality in Medicine (Ärztliches Zentrum für Qualität in der Medizin) (e37, e38). These recommendations were discussed by the members of the guideline group as a whole in an online Delphi process and at a consensus conference moderated by the AWMF, modified if deemed necessary, and finally adopted. In almost all cases there was a strong consensus for approval. Balancing the great importance of the particularly high degree of interdisciplinary expert consensus for these recommendations against the heterogeneity of the evidence, all recommendations were implemented as “clinical consensus points” (CCP) with the recommendation level “recommended” (e34– e36) (efigure 2). A more detailed description of the methods can be found in eBox 2.
eBOX 1. The participating professional societies and patient organizations (with name of representative[s], if applicable) (from [3]).
German College of Psychosomatic Medicine (Deutsches Kollegium für Psychosomatische Medizin) (coordinating role): Prof. Peter Henningsen
German Society for Psychosomatic Medicine and Medical Psychotherapy (Deutsche Gesellschaft für Psychosomatische Medizin und Ärztliche Psychotherapie) (coordinating role): Prof. Peter Henningsen
German College of General Practitioners and Family Physicians (Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin): Prof. Markus Herrmann
German Society of Internal Medicine (Deutsche Gesellschaft für Innere Medizin): Dr. Timo Specht
German Society of Surgery (Deutsche Gesellschaft für Chirurgie): Prof. Marcus Schiltenwolf
German Society of Orthopedics and Orthopedic Surgery (Deutsche Gesellschaft für Orthopädie und Orthopädische Chirurgie): Prof. Marcus Schiltenwolf
German Association for Psychiatry, Psychotherapy and Psychosomatics (Deutsche Gesellschaft für Psychiatrie, Psychotherapie und Nervenheilkunde): Prof. Kapfhammer
German Society of Psychosomatic Gynecology and Obstetrics (Deutsche Gesellschaft für Psychosomatische Frauenheilkunde und Geburtshilfe): Dr. Friederike Siedentopf
German Society for the Study of Pain (Deutsche Schmerzgesellschaft): Prof. Winfried Häuser
German Neurological Society (Deutsche Gesellschaft für Neurologie): Prof. Marianne Dieterich
German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (Deutsche Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf- und Hals-Chirurgie): Dr. Astrid Marek, Prof. Birgit Mazurek
German Society of Urology / Working Group Psychosomatic Urology and Sexual Medicine (Deutsche Gesellschaft für Urologie/Arbeitskreis Psychosomatische Urologie und Sexualmedizin): Dr. Ulrike Hohenfellner
Society for Phytotherapy (Gesellschaft für Phytotherapie): Prof. Jost Langhorst
German Society of Cardiology (Deutsche Gesellschaft für Kardiologie): Prof. Karl-Heinz Ladwig
German Society for Rheumatology (Deutsche Gesellschaft für Rheumatologie): Prof. Wolfgang Eich
German Society for Gastroenterology, Digestive and Metabolic Diseases (Deutsche Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten): Prof. Hubert Mönnikes
German Society of Dentistry and Oral Medicine / Working Group Psychology and Psychosomatics (Arbeitskreis Psychologie und Psychosomatik der Deutschen Gesellschaft für Zahn-, Mund- und Kieferheilkunde): Prof. Anne Wolowski
German Dermatological Society (Deutsche Dermatologische Gesellschaft): Prof. Uwe Gieler
German Society for Allergology and Clinical Immunology (Deutsche Gesellschaft für Allergologie und Klinische Immunologie): Prof. Uwe Gieler
German Society for Occupational and Environmental Medicine (Deutsche Gesellschaft für Arbeitsmedizin und Umweltmedizin): Prof. Dennis Nowak
German Society of Behavioral Medicine and Behavior Modification (Deutsche Gesellschaft für Verhaltensmedizin und Verhaltensmodifikation): Prof. Winfried Rief
German Society of Medical Psychology (Deutsche Gesellschaft für Medizinische Psychologie): PD Dr. Heide Glaesmer
Commission Psychology and Psychotherapy of the German Society of Psychology (Fachgruppe Klinische Psychologie und Psychotherapie der Deutschen Gesellschaft für Psychologie): Prof. Alexandra Martin
German Psychoanalytical Society (Deutsche Psychoanalytische Vereinigung): Prof. Ulrich Schultz-Venrath
German Society for Clinical Psychotherapy and Psychosomatic Rehabilitation (Deutsche Gesellschaft für Klinische Psychotherapie und Psychosomatische Rehabilitation): Dipl.-Psych. Stefan Schmädeke
German Society of Pediatrics and Adolescent Medicine, Divison for Pediatric Psychosomatic Medicine (Deutsche Gesellschaft für Kinder- und Jugendmedizin/Arbeitskreis Pädiatrische Psychosomatik): Dr. Torsten Lucas
German Association of Self Help Groups (Deutsche Arbeitsgemeinschaft Selbsthilfegruppen e. V.): Jürgen Matzat
Independent Association of Active Pain Patients (Unabhängige Vereinigung aktiver Schmerzpatienten [UVSD]) SchmerzLOS e. V.: Heike Norda
Austrian Association of Psychiatry, Psychotherapy and Psychosomatics (Österreichische Gesellschaft für Psychiatrie, Psychotherapie und Psychosomatik): Prof. Hans-Peter Kapfhammer
German Society of Neurosurgery (Deutsche Gesellschaft für Neurochirurgie): no representative
Society of Hygiene, Environmental and Public Health Sciences (Gesellschaft für Hygiene, Umweltmedizin und Präventivmedizin): no representative
German Society for Social Medicine and Prevention (Deutsche Gesellschaft für Sozialmedizin und Prävention): no representative
German Society of Gynecology and Obstetrics (Deutsche Gesellschaft für Gynäkologie und Geburtshilfe): no representative
German Society for Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy (Deutsche Gesellschaft für Kinder und Jugendpsychiatrie, Psychosomatik und Psychotherapie): no representative
Steering group: Prof. Constanze Hausteiner-Wiehle, Dr. Casper Roenneberg, MHBA; Dipl.-Psych. Heribert Sattel, Prof. Peter Henningsen, Prof. Rainer Schäfert
AWMF representative: Dr. Monika Nothacker
External experts: Prof. Antonius Schneider, Prof. Bernd Löwe
eFigure 1.
Chart describing the updated systematic literature survey: RCT, randomized controlled trial
eTable 2. Passive treatment measures (selection): results at conclusion of treatment compared with control groups. based on recent systematic reviews.
| Intervention | Systematic review | Main study result [95% confidence interval] |
Number of studies (patients) |
Evidence level (EL)/study quality (SQ) |
Signs of lacking acceptance or tolerance |
|
| Analgesics | Pain reduction (if not otherwise specified) |
|||||
| Tension headache | Paracetamol | (e91)*9 | RR 1.3 [1.1; 1.4] (freedom from pain after 2 h) |
23 (8 079) | EL: strong | No |
| Fibromyalgia syndrome |
Nonsteroidal anti-inflammatory drugs |
(e92)*9 | RD −0.04 [−0. 16; 0.08] | 6 (292) | EL: very weak | Yes |
| Myofascial pain syndrome |
Botulinum toxin A | (e93)*9 | SMD −0.36 [−0.86; 0.15]*1 | 4 (233) | SQ: high | Yes |
| Psychopharmaceuticals | Pain reduction (if not otherwise specified) |
|||||
| Functional somatic symptoms in general |
Tricyclic antidepressants | (e94)*9 | SMD −0.13 [−0.39; 0.13] (reduced symptom severity) |
2 (239) | EL: weak | No data |
| Fibromyalgia syndrome | Selective serotonin reuptake inhibitors |
(13)*9 | RD 0.10 [0.01; 0.20] | 6 (343) | EL: very weak | Yes; NB*2 |
| Serotonin–noradrenalin reuptake inhibitor (milnacipran) |
(14)*9 | RR 1.38 [1.22; 1.57]*3, *4 | 3 (1 925) | EL: strong | Yes | |
| Pregabalin | (15)*9 | RR 1.8 [1.4; 2.1]*4 | 5 (1 874) | EL: strong | Yes | |
| Antipsychotic medication (quetiapine) |
(e95)*9 | RD 0.04 [−0.02; 0.10] | 2 (155) | EL: very weak | No | |
| Irritable bowel syndrome |
Tricyclic antidepressants | (26) | RR 0.67 [0.54; 0.82] (“adequate global improvement”)*5 |
23 (1 438) | SQ: unclear | Yes |
| Selective serotonin reuptake inhibitors |
(26) | RR 0.74 [0.52; 1.06] (“adequate global improvement”) |
5 (281) | EL: weak | No data | |
| Functional dyspepsia | Tricyclic antidepressants | (e96) | RR 0.74 [0.61; 0.91] (“symptom improvement”)*6 |
3 (339) | SQ: high | Yes |
| Peripherally acting agents | Various results | |||||
| Irritable bowel syndrome |
Spasmolytics | (26) | RR 0.67 [0.55; 0.80] (“adequate global improvement”) |
22 (2 983) | EL: weak | No data |
| Rifaximine | (27) | OR 1.19 [1.08; 1.32] (reduced symptom severity) |
4 (1 803) | SQ: unclear | No | |
| Soluble dietary fiber | (e97) | RR 0.83 [0.73; 0.94] (“global improvement”) |
14 (907) | No data | No | |
| Lactobacillus | (e98) | RR 17.6 [5.1; 60.7] (clinical improvement)*7 |
3 (273) | SQ: moderate | No data | |
| Linaclotide (irritable bowel syndrome with obstipation) | (26) | RR 0.73 [0.65; 0.82] (“adequate global improvement”) |
3 (1 773) | EL: strong | Yes | |
| Passive physical and physiotherapeutic measures | Pain reduction (if not otherwise specified) |
|||||
| Chronic unspecific back pain | Massage | (e99)*9 | SMD −0.75 [−0.90; −0.60] | 7 (761) | EL: weak | No |
| Fibromyalgia syndrome |
Balneotherapy | (e100) | SMD −0.84 [−1.36; –0.31] | 5 (177) | SQ: low | No |
| Massage | (16) | SMD 0.37 [−0.19; 0.93] | 9 (404) | SQ: unclear | No | |
| Transcutaneous electrical nerve stimulation (TENS) |
(e101) | SMD −1.34 [−3.27; 0.59] | 9 (301) | EL: weak to moderate | No data | |
| Irritable bowel syndrome |
Osteopathic manipulative therapy | (e102) | SMD 0.81 [0.43; 1.19]*1 | 3 (128) | SQ: high | No data |
| Craniomandibular dysfunction | Musculoskeletal manual approach |
(e103) | MD 1.69 [1.09; 2.30] | 5 (184) | SQ: unclear (PEDro) |
No data |
| Tension headache | Multimodal manual therapy | (e104) | MD(w) −0.80 [−1.66; −0.44] (frequency of episodes) |
5 (206) | SQ: moderate (PEDro) |
No data |
| Passive alternative and complementary treatments | Pain reduction (if not otherwise specified) |
|||||
| Chronic unspecific back pain | Acupuncture | (e105) | SMD −0.72 [−0.94; −0.49] | 4 (2911) | SQ: unclear | No data |
| Fibromyalgia syndrome | Acupuncture | (17) | SMD 0.01 [−0.37; 0.35]*8 | 5 (273) | SQ: high | No data |
*1 Own analysis
*2 Safety warning: “black box” warning about use in young adults with major depression und potential suicidality
*3 Daily dose 100 mg. comparable effects for daily dose 200 mg
*4 No recommendation because of the significantly increased risk of severe adverse effects; see guideline on fibromyalgia syndrome (28)
*5 No recommendation because of the significantly increased risk of adverse effects; study discontinued
*6 No recommendation because of the significantly increased risk of adverse effects
*7 No recommendation because of the significant heterogeneity of the studies included
*8 Comparison with sham acupuncture
*9 Cochrane Review
OR. Odds ratio; PEDro. Physiotherapy Evidence Database; RR. relative risk; SMD. standardized mean difference
Target variable/main study result: bold. statistically significant effect size; italic. no recommendation despite statistically significant effect size. Reasons for downgrading given in footnotes.
Table. Findings of selected systematic reviews on interventions to treat functional somatic symptoms.
| Syndrome | Number of systematic reviews (total) | Intervention | Systematic review (exemplary sources) | Target variable/ symptom severity (main study result in exemplary source [95% confidence interval]) |
Evidence level | Signs of lacking acceptance or tolerance |
| Functional somatic symptoms in general | 1 | Self-help interventions | (7) | SMD 0.58 [0.32; 0.84] | Weak | No data |
| 3 | Short-term psychotherapy | (8) | SMD −0.34 [−0.53; −0.16] | Weak | No | |
| Chronic fatigue syndrome | 2 | Exercise*1 | (9) | SMD −0.73 [−1.10; −0.37]*8 | Moderate | No |
| Fibromyalgia syndrome | 6 | Aerobic training | (10) | SMD −0.40 [−0.55; −0.26] | Moderate | No |
| 1 | Multimodal treatment*2 | (11) | SMD −0.42 [−0.58; 0.25] | No data | No | |
| 1 | Tricyclic antidepressants*3 | (12) | SMD −0.53 [−0.78; −0.29] | No data | No | |
| 3 | Selective serotonin reuptake inhibitors*4 | (13) | RD 0.10 [0.01; 0.20] | Very weak | No | |
| 2 | Milnacipran | (14) | RR 1.38 [1.22; 1.57)*9 | Strong | Yes*9 | |
| 2 | Pregabalin | (15) | RR 1.8 [1.4; 2.1] | Strong | Yes*3 | |
| 5 | Passive treatments (massage. transcutaneous electrical nerve stimulation. transcranial magnetic stimulation) | (16) | SMD 0.37 [−0.19; 0.93] | No data | No | |
| 2 | Acupuncture | (17) | SMD 0.01 [−0.37; 0.35]*10 | Strong | No data | |
| 2 | Balneo-/hydro-/spa therapy | (10) | SMD −1.36 [−2.27; −0.44] | Moderate | No | |
| 2 | Cognitive behavioral therapy. hypnotherapy | (18) | SMD −0.29 [−0.47; −0.11] | Weak | No | |
| 1 | Mindfulness-based treatments | (19) | SMD −0.23 [−0.46; −0.01] | No data | No data | |
| Irritable bowel syndrome | 2 | Acupuncture | (20) (21) |
RR 1.29 [1.10; 1.51] SMD −0.11 [−0.35; 0.13] |
Strong Moderate |
No |
| 4 | Low-threshold psychological interventions*5 | (22) | SMD 0.60 [0.33; 0.86] | Weak | No data | |
| 4 | Psychotherapy (in the broad sense) | (23) | SMD 0.69 [0.52; 0.86] | No data | No data | |
| 2 | Cognitive behavioral therapy | (24) | RR 0.60 [0.44; 0.83] | Weak | No data | |
| 3 | Hypnotherapy/hypnosis | (25) | RR 1.69 [1.14; 2.51] | Strong | No | |
| 2 | Tricyclic antidepressants*6 | (24) | RR 0.66 [0.56; 0.79] | Weak | Yes*9 | |
| 2 | Selective serotonin reuptake inhibitors*7 | (26) | RR 0.74 [0.52; 1.06] | Weak | No data | |
| 2 | Spasmolytics | (26) | RR 0.67 [0.55; 0.80] | Weak | No data | |
| 3 | Rifaximine | (27) | OR 1.19 [1.08; 1.32] | No data | No | |
| 3 | Linaclotide in irritable bowel syndrome with predominant obstipation | (26) | RR 0.73 [0.65; 0.82] | Strong | No |
Details of interventions:
*1 Planned. structured. and repeated physical activity
*2 Combination of at least one activating procedure (endurance. strength. flexibility training) with at least one psychological procedure (patient education. behavioral therapy)
*3 Imipramine. amitriptyline. clomipramine. desipramine. dothiepin. nortriptyline. amoxapine. doxepin. protriptyline. trimipramine. maprotiline
*4 Citalopram. fluoxetine. escitalopram. fluvoxamine. paroxetine. sertraline
*5 Mindfulness-based procedures. self-efficacy training or relaxation training
*6 Amitriptyline. desipramine. doxepin. trimipramine
*7 Fluoxetine. paroxetine. citalopram
*8 Daily dose 100 mg. comparable effects for daily dose 200 mg
*9 Leads to downgrading
*10 Comparison with sham acupuncture
OR. Odds ratio; RD. risk difference; RR relative risk; SMD. standardized mean difference; Target variable/main study result: bold. statistically significant effect size; _italic. no recommendation despite statistically significant effect size
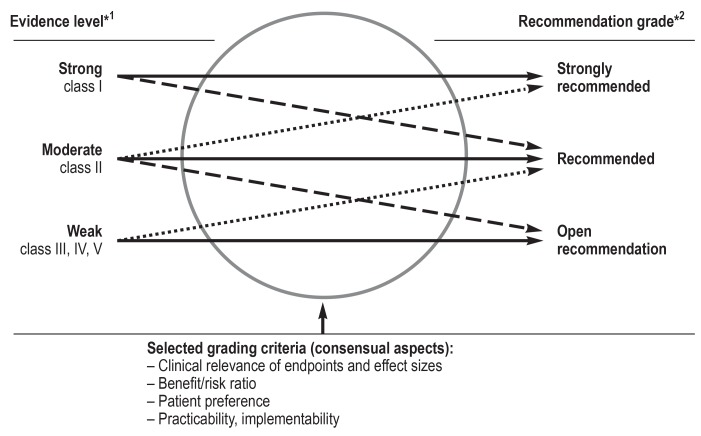
eFigure 2.
*1 Evidence level classes as defined by the Oxford Centre of Evidence-based Medicine
*2 As laid out in the German Program for National Care Guidelines (Programm für Nationale Versorgungsleitlinien)
Upgrading/downgrading of recommendation grade (from [3])
eBOX 2. How the revised guidelines were compiled.
To make the guideline group even more representative for the German-speaking countries, eight new organizations were invited to join the existing group, so that members of 32 medical and psychological societies (including five who did not nominate a representative) and two organizations representing patients’ interests were involved in compiling the guidelines and agreeing on the final version. Two external experts commented on the guidelines; final editing was carried out by the AWMF (ebox 1).
The survey of the literature in PubMed and the Cochrane Library for the period 1 January 2012 to 3 November 2017 was based on the search strategy used for the previous version of the guidelines (ebox 5). The steering group carried out a concluding hand search after the cut-off date to ensure inclusion of new data (date of hand search: 1 July 2018). A total of 3 795 clinical studies and 3 345 systematic reviews were identified (efigure 1). Owing to the high number of treatment studies for individual syndromes, only high-quality systematic reviews were analyzed for this segment (“umbrella review;” inclusion/quality criteria: eBox 6).
Because only a small number of randomized controlled trials (RCTs) were identified for recommendations on attitude, communication, diagnosis, and social medicine aspects, the evidence level of these recommendations is considered weak (evidence class III, IV, V) and was not broken down any further. In contrast, the statements on specific treatments rest on systematic reviews (if any) published within the update period (see above) that covered the findings of at least three RCTs. The detailed results were drawn up into evidence tables. The items reported were, among others, effect sizes for symptom-specific target variables, the number of source studies, the number of patients included, and statements on tolerance and acceptance. Table 1 shows the results of selected reviews on interventions for functional somatic symptoms in general and for chronic fatigue syndrome, fibromyalgia syndrome, and irritable bowel syndrome ((7– (27); a broader selection of treatment evidence can be found in eTables 2 and 3. The evidence level is strong (class I) for all treatment recommendations (efigure 2).
Most treatment studies investigate interventions in particular functional syndromes, which greatly restricts their comparability with each other. Because of this “indirectness of comparison,” in many such cases the strength of evidence was downgraded by one level (e34, e35). If multiple care-relevant criteria (low risks, high patient acceptance, easy implementation, ethical obligation) existed, the strength of evidence was raised by one level (e36) (efigure 2). Because of the importance of the particularly broad and robust expert consensus on the one hand and the heterogeneous nature of the evidence in these guidelines on the other hand, all recommendations were implemented as “clinical consensus points” (CCP).
In accordance with the requirements specified by the AWMF and the Center for Quality in Medicine (Ärztliches Zentrum für Qualität in der Medizin), the steering group formulated, on the basis of the identified evidence and the relevant source guidelines (etable 1), 109 recommendations, divided into 24 key topics, each with source texts, background information (“excursuses”), practical tips (“clinical decision aids”), and references. These recommendations were discussed by the members of the guideline group as a whole in an online Delphi process and a consensus conference moderated by the AWMF, modified if deemed necessary, and finally adopted. In almost all cases there was strong consensus for approval. More than 95% of the participants voted to approve 104 of the recommendations (strong consensus), and for the remaining five recommendations 75% to 95% were in favor (consensus) (etable 4).
Diagnosis and treatment of functional somatic symptoms
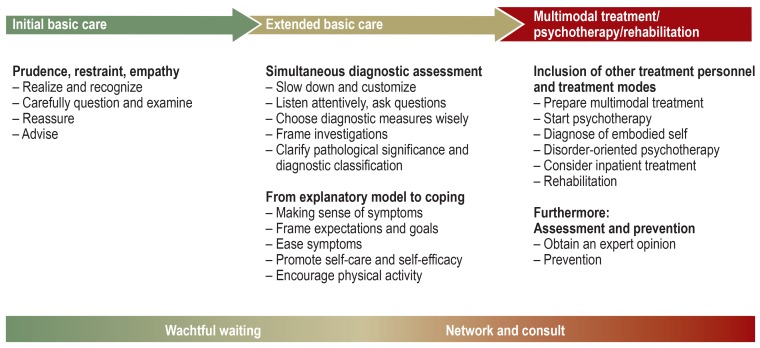
Because of the great variability in the course and severity of functional somatic symptoms, the recommendations are grouped into three stages of treatment (figure 2). Recommendations for the initial stages are still valid for later stages in more severe courses, but are then supplemented by further measures (e39). The assessment of severity is based on the present protective factors and risk factors (green/yellow/red flags) (eBox 3, Figure 2) (1, 2, 4– 6, e40, e41). Basic care is carried out by the primary care physician or the appropriate somatic specialist, who then coordinates any multimodal treatment that may be required later.
Figure 2.
Stepped, collaborative care according to severity:
initial basic care, extended basic care, multimodal treatment/psychotherapy/rehabilitation
eBOX 3. Protective factors, risk factors, and warning signs (shortened from [3]).
| Protective factors for a favorable course (“green flags”) |  |
|
| - | Functional thoughts and attitudes, e.g., humor, self-confidence | |
| - | Active coping strategies, e.g., participation in sports, ability to enjoy oneself and relax | |
| - | Individual resources, e.g., hobbies, general motivation, job-related plans | |
| - | No or only slight psychosocial pressures, e.g., good social support and good workplace conditions | |
| - | No mental comorbidity | |
| - | Predominant preservation of everyday functioning, e.g., ability to work | |
| - | Robust doctor–patient relationship | |
| - | Biopsychosocial treatment strategy avoiding unnecessary diagnostic tests and treatment measures | |
| Indicators/risk factors for a chronic and complicated course (“yellow flags”) |  |
|
| - | Multiple symptoms (polysymptomatic course) | |
| - | Frequently occurring or persisting symptoms | |
| - | Dysfunctional thoughts and attitudes, e.g., catastrophizing thoughts, helplessness/hopelessness, substantial health-related anxiety | |
| - | Passive, overactive, or suppressive behavior, e.g., protective and avoidance behavior, persistent industriousness/perseverance, safety-seeking behavior | |
| - | Moderate to high psychosocial load, e.g., workplace-related stress, despondency, loneliness | |
| - | Mental comorbidity (particularly depression, anxiety disorder, addiction, post-traumatic stress disorder) | |
| - | Significantly reduced everyday functioning, e.g., inability to work, physical deconditioning | |
| - | Therapist–patient relationship experienced as “difficult” on both sides | |
| - | “Iatrogenic somatization” or “chronification” through nocebo messages, endorsement of patients’ passive behaviors and attitudes, and unnecessary diagnostic tests and treatments | |
| Warning signs (“red flags”) |  |
|
| - | Self-harm or suicidality (e.g., malnutrition/underweight, physical consequences of protective behavior, suicidal thoughts and plans) | |
| - | Threat of harm inflicted by others, e.g., physicians, principally as a result of lacking or unsuitable treatment (such as hazardous, non-indicated invasive surgery) | |
| - | Severe mental comorbidity, warning signals of somatic disease (e.g., stool blood in the presence of gastrointestinal symptoms, B-symptoms together with exhaustion) |
The guidelines recommend from the outset an integrative approach, with the systematic consideration of both, somatic and psychosocial aspects of the symptoms (“as well/as attitude”), and alignment of the boundaries between general and specialist medical care and between organic and psychosocial medicine (4– 6, e42– e44). Inappropriate, superfluous, and obsolete drug treatments and invasive interventions are listed in eBox 4.
eBOX 4. Inappropriate, superfluous, and obsolete drugs and invasive measures in the diagnosis and treatment of functional somatic symptoms (selection from [3]).
Passive, pharmakological and invasive measures should be used only after one has thoroughly weighed the benefits (e.g., temporary symptom relief) against the risks (e.g., iatrogenic chronification). Preparations with an elevated risk of (severe) adverse effects should be administered only in strict adherence to the respective guidelines (for instance, pregabalin in fibromyalgia is indicated only for comorbidity with generalized anxiety disorder; check effectiveness after 2 and 4 weeks; discontinue if not effective at 4 weeks). Important examples:
Opiates, cannabis preparations, and benzodiazepines are not indicated for treatment of purely functional somatic symptoms.
Antipsychotics (fluspirilene injection!), anxiolytics, and tranquilizers are justified only in crises or in the presence of corresponding comorbidity.
Long-term administration of antibiotics or chelate-forming drugs and unbalanced diets can have harmful effects on the intestinal flora and on vitamins, metals, and minerals.
Abstention or shielding (e.g., avoidance of public transport or communication media) can have a negative impact on mobility and social relations.
Especially great caution should be exercised in determining the indications for invasive diagnostic procedures and treatments (e.g., catheters, injections, (intrathekal) pain pumps, implanted neurostimulation, neurolysis, milling out the jaws to eliminate amalgam, surgery with no clear indication).
Initial basic care
The recommendations for “initial basic care” (figure 2) advise early consideration of the possible presence of functional somatic symptoms by careful questioning and examination of the patient (consensus: strong; evidence level: weak) (e45– e53). Even in this early stage, diagnostic alertness paired with diagnostic restraint together with empathetic communication of information and reassurance enable a broad diagnostic perspective without fixation on a somatic cause, an informed and calmer attitude on the patient’s side in dealing with the symptoms, and higher treatment satisfaction—and exert a positive impact on the course and prognosis by, for example, amelioration of symptoms and reduced consumption of healthcare resources (consensus) (e54).
Patients should be questioned about their principal symptoms and about any other symptoms or problems. Furthermore, they should be asked how they feel about their symptoms, how the symptoms affect their daily life, and what strategies they use to ease or avoid these symptoms (strong consensus) (4– 6, 28, 29, e43, e55). A thorough physical examination should be carried out to detect further findings or limitations (consensus) (4– 6, 28, 29, e52, e53). During both the initial conversation and the subsequent physical examination, the physician observes the patient’s behavior (e.g., reluctance to perform certain movements, dramatizing symptoms) (strong consensus) (e56, e57). Based on the (preliminary) findings, any further diagnostic testing should be planned in a systematic and reserved manner and communicated with the patient in a reassuring way (strong consensus) (4– 6, 28, 29, e58– e60). On the overall basis of the findings and the information gleaned, signs of an avoidable dangerous course (red flags) or risk factors for a chronic course (yellow flags) are assessed (ebox 3).
If no warning signals are detected, the patient should be reassured, but without playing down or negating the symptoms (strong consensus) (4– 6, 28, 29, e44, e47, e54, e61– e64). The credibility of the symptoms and the carefulness and reliability of the physician’s assessment are conveyed without necessarily using a “diagnostic label” (strong consensus). Therapeutic interventions in the stage of initial basic care are generally restricted to encouraging patients to modify their behavior in terms of a healthy, physically active lifestyle (strong consensus) (4– 7, 28, 29, e65)—ideally activities that they are familiar with and have benefited from in the past. Additionally, a further appointment in 2 to 4 weeks should be offered if required (strong consensus) (e66– e69), while emphasizing that the symptoms will probably resolve, or that there is no need for concern if they should persist (watchful waiting) (strong consensus) (e70).
Extended basic care
Extended basic care begins if a patient presents again because his/her symptoms have persisted or have started to impair quality of life and everyday functioning. It is carried out predominantly by primary care physicians or appropriate somatic specialists and is divided into two phases (figure 2):
Simultaneous diagnostic assessment: extension of physical and psychosocial diagnostic investigations simultaneously and with equal weight (which in itself may have a therapeutic effect)
From explanatory model to coping: integration of all identified issues/problems into an individual explanatory model, from which coping-oriented treatment measures are derived.
In view of the prognostic relevance of reflective management, the extra time required for extended basic care is well invested (e47).
Simultaneous diagnostic assessment
A somewhat less rushed, customized treatment setting can be achieved by reviewing office organization and billing procedures for ways to dedicate more time to patients; a clear schedule with fixed regular appointments in a calm atmosphere irrespective of symptoms, with the potential for delivering measures of “psychosomatic basic care” and other specific training courses; focused management of these patients by the whole treatment team (strong consensus, evidence level: weak) (4– 6, 28, 29, e71– e80).
Careful, attentive listening and questioning, also during physical examinations, strengthen the doctor–patient relationship and yield valuable information about the patient’s previous symptoms and treatments (strong consensus) (e44, e66, e81, e82). If deemed appropriate, clinical and physical examinations should be repeated at regular intervals, also to detect warning signals for (new-onset) somatic disease or any harmful consequences of previous (physical) inactivity or incorrect treatment (strong consensus) (4– 6, 28, 29, e46). Well-considered diagnostic testing and prescribing, advance discussion of examinations (including the anticipation of normal findings), and normalizing explanation of the findings are central aspects of a systematic, stepped diagnostic work-up free of redundancies. The goal is to rule out the presence of serious conditions and complications and to recognize when medical action is required—but not necessarily to define a clear cause for each symptom (consensus). Repeated testing, particularly invasive techniques, should be avoided if they serve primarily to reassure the patient and/or the physician (strong consensus) (4– 6, 28, 29, e83– e85). If a test is unnecessary, the physician should explain clearly why that is the case; necessary investigations should be announced in reassuring fashion, perhaps mentioning the high likelihood of age-appropriate normal findings (strong consensus) (4– 6, 28, 29, e86, e87). Any known previous test results and any incidental or trivial findings with no diagnostic or therapeutic relevance should be interpreted using lay terms, in a reassuring, normalizing manner, with the aid of information materials; occasional “summarizing discussions” can help (re-)evaluate all medical results together with the patient (strong consensus) (e88– e90).
Simultaneous diagnostic assessment concludes with an evaluation of the medical significance of the symptoms and the (suspected) diagnosis/diagnoses, and a decision about further treatment needs. This serves to determine whether treatment is required (strong consensus). If no sound diagnosis can be established, using ICD-10 symptom or health care utilization codes should be preferred over assigning stopgap diagnostic codes (strong consensus).
From explanatory model to coping
Supporting the patient in making individual sense of the symptoms (box 2) plays a central therapeutic role in the context of extended basic care: Even if the the patient’s own attributions seem one-sided or implausible, step by step a comprehensible biopsychosocial explanatory model should be developed that integrates the patient’s subjective assumptions, taking account of individual risk factors as well as context factors (e.g., mental illness). Based on this individual, multifactorial etiological model, therapeutic goals should be developed, consisting of concrete and realistic small-step goals but also establishing superordinate values and motivators (strong consensus) (4– 6, e43, e65– e69, e87). To alleviate the patient’s bodily symptoms, selected symptom-oriented passive measures can be recommended, stressing their generally transitory effects and concomitant role: analgesics, psychopharmaceuticals, as well as primarily peripherally acting medication, passive physical and physiotherapeutic interventions, and passive complementary medicine treatments such as acupuncture and phytotherapy (strong consensus, recommended, evidence level: strong) (etable 1) (4, 10, 12– 17, 21, 24, 26, 27, e91– e105). More sustained effects can be achieved through active coping strategies to reinforce self-efficacy and self-help skills. These include (re)initiating social and particularly physical activity (at the patient’s own initiative, from pleasurable exercise to systematic activation programs; also short-term physiotherapy and ergotherapy), (re)exposure in the case of avoidance and protective behavior, self-help literature and possibly self-help groups, as well as taking advantage of offers beyond the healthcare system, e.g., evening classes, where one is not in the patient role (strong consensus, recommended, evidence level: strong) (etable 2) (4– 7, 9, 10, 19, 22, 24, e100, e106– e108).
BOX 2. An example of user-oriented recommendations with strong consensus: making sense of the symptoms*.
Tell the patient that symptoms very often occur in the absence of physical illness, so they know they are not alone.
Explain psychophysiological processes, e.g., with the aid of “vicious circle models” (“the more pain, the less physical activity— the less physical activity, the more pain”), anatomical illustrations, and recent research findings.
Explain the symptoms, in lay terminology, as physiological expressions (e.g., trembling, pounding heart) of distress (tension, stress, irritability, “out of balance”).
Formulate, together with the patient, a comprehensible personal explanatory model that is multifactorial („as well/as attitude“) and connects with his/her previous assumptions while offering possible strategies for change, especially with respect to the patient’s own attitudes and behaviors (e.g., reduction of avoidance or overloading, resolution of workplace conflicts).
Describe psychosocial burdens as well as (previous or concomitant) physical illness as “conditions”, “triggers”, “refinforcers” or “additional problems”, but not as “causes”. Avoid monocausal, purely psychosocial, or purely somatic attributions.
If diagnoses (including comorbidities) have been made, explain them appropriately. Take advantage of the opportunity to relieve the patient by naming diagnoses and the resulting treatment options.
Distinguish functional and somatoform diagnoses from other known or feared illnesses; explain their descriptive character, tell the patient their life expectancy remains normal, and outline further established sources of information and options for treatment and self-help.
*Reproduced in slightly shortened form from (3)
eTable 1. Source guidelines from Germany and other countries (from [3]).
| German guidelines (selected): Association of the Scientific Medical Societies in Germany (AWMF) | ||||
| Title | Classification | Registry number | Link/reference* | Validity |
| National Care Guideline on Back Pain | S3 | nvl–007 | www.awmf.org/leitlinien/detail/ll/nvl-007.html | Valid until 30. 12. 2021 |
| Fibromyalgia Syndrome: Definition Pathophysiology. Diagnosis. and Treatment |
S3 | 145–004 | www.awmf.org/leitlinien/detail/ll/145–004.html | Valid until 16. 03. 2022 |
| Opioids: Long-term Use for Treatment of Non-tumor-related Pain |
S3 | 145–003 | www.awmf.org/leitlinien/detail/ll/145–003.html | Valid until 01. 10. 2019 |
| Chronic Pain | S1 | 053–036 | www.awmf.org/leitlinien/detail/ll/053–036.html | Expired |
| Irritable Bowel Syndrome: Definition Pathophysiology. Diagnosis and Treatment |
S3 | 021–016 | www.awmf.org/leitlinien/detail/ll/021–016.html | Expired (under revision) |
| Vertigo. Acute. in Primary Care Practise | S3 | 053–018 | www.awmf.org/leitlinien/detail/ll/053–018.html | Expired |
| Thoracic Pain | S3 | 053–023 | www.awmf.org/leitlinien/detail/ll/053–023.html | Expired |
| Chronic Pelvic Pain in Women | S2k | 016–001 | www.awmf.org/leitlinien/detail/ll/016–001.html | Valid until 29. 11. 2020 |
| Fatigue | S3 | 053–002 | www.awmf.org/leitlinien/detail/ll/053–002.html | Expired |
| Chronic Tinnitus | S3 | 017–064 | www.awmf.org/leitlinien/detail/ll/017–064.html | Valid until 27. 02. 2020 |
| Diagnosis and Treatment of Chronic Pruritus | S2k | 013–048 | www.awmf.org/leitlinien/detail/ll/013–048.html | Valid until 30. 05. 2021 |
| Psychosomatic Dermatology (Psychodermatology) |
S2 | 013–024 | www.awmf.org/leitlinien/detail/ll/013–024.html | Expired |
| Acute and Chronic Cough: Diagnosis and Treatment in Adults |
S3 | 020–003 | www.awmf.org/leitlinien/detail/ll/020–003.html | Expired |
| Chronic Pain: Medical Assessment of Persons with Chronic Pain |
S2k | 094–003 | www.awmf.org/leitlinien/detail/ll/094–003.html | Valid until 6. 11. 2022 |
| Assessment of Mental and Psychosomatic Diseases |
S2k | 051–029 | www.awmf.org/leitlinien/detail/ll/051–029.html | Expired |
| General Principles of Medicolegal Assessment | S2k | 094–001 | www.awmf.org/leitlinien/detail/ll/094–001.html | Expired |
| Unipolar Depression— National Care Guideline |
S3 | nvl–005 | /www.awmf.org/leitlinien/detail/ll/_nvl-005.html | Valid until 15. 11. 2020 |
| Anxiety Disorders | S3 | 051–028 | www.awmf.org/leitlinien/detail/ll/051–028.html | Expired |
| Evidenz-based Guideline on Psychotherapy of Somatoform Disorders |
– | – | Martin et al.. 2013 | – |
| Guidelines from other countries (selected) | ||||
| NHG Guideline on Medically Unexplained Symptoms (MUS) |
– | – | https://guidelines.nhg.org/product/_medically-unexplained-symptoms | Since May 2013 |
| Medically Unexplained Symptoms | – | – | www.nhs.uk/conditions/medically-unexplained-symptoms/Pages/Somatisation.aspx | Valid until 24. 11. 2019 |
* The German guidelines are mostly only available in German
Multimodal treatment,psychotherapy,and rehabilitation
The third stage of treatment is required for severe cases with considerable impairment of everyday functioning and high healthcare utilization (figure 2). It involves further forms of treatment, including psychotherapy and rehabilitation, as required and as available as outpatient, inpatient, or day-care treatment (strong consensus, evidence level: strong) (etable 3) (4– 6, 10, 11, e104, e109, e110). To enable the provision of multimodal treatment, an outpatient treatment network should be established, with the treating primary care physician or somatic specialist remaining the principal coordinator (gatekeeper) (strong consensus, recommended, evidence level: strong) (e111, e112). Any referrals that become necessary, e.g., for psychiatric, psychosomatic, or psychotherapeutic treatment, should be prepared with empathy (4– 6).
eTable 3. Active treatment measures (selection): results at conclusion of treatment compared with control groups. based on recent systematic reviews.
| Intervention | Systematic review |
Main study result [95% confidence interval] |
Number of studies (patients) |
Evidence level (EL)/study quality (SQ) |
Signs of lacking acceptance or tolerance |
|
| (Simple) interventions to enhance self-efficacy | ||||||
| Functional somatic symptoms in general |
Self-help interventions |
(7) | SMD 0.58 [0.32; 0.84) (reduced symptom severity) |
17 (1894) | SQ: low | No data |
| Chronic unspecific back pain |
Education | (e106) | RR 1.02 [0.78; 1.33) (back pain prevalence) |
3 (938) | EL: strong | No data |
| Chronic unspecific back pain |
Mindfulness-based stress reduction (MBSR) |
(e107) | SMD −0.46 [−0.70; −0.22) (pain reduction) |
2 (266) | SQ: unclear | Yes*1 |
| Activating procedures | Pain reduction (if not otherwise specified) |
|||||
| Chronic unspecific back pain |
Exercise interventions | (e108) | SMD −0.32 [−0.44; −0.19) | 39 (4109) | SQ: unclear | No data |
| Fibromyalgia syndrome |
Aerobic exercise | (10) | SMD −0.40 [−0.55; −0.26) | 23 (1085) | SQ: moderate | Yes |
| Fibromyalgia syndrome |
Hydrotherapy | (e100) | SMD −0.42 [−0.61; −0.24) | 8 (462) | SQ: unclear | No |
| Chronic fatigue syndrome |
Exercise therapy | (9)*5 | SMD −0.73 [−1.10; −0.37)*2 (reduced fatigue) |
8 (1518) | EL: moderate | No |
| (Outpatient/inpatient) multimodal treatment |
Pain reduction (if not otherwise specified) |
|||||
| Fibromyalgia syndrome |
Multimodal treatment | (11) | SMD −0.42 [−0.58; −0.25) | 14 (927) | SQ: unclear | No |
| Chronic unspecific back pain | Multidisciplinary ‧biopsychosocial ‧rehabilitation | (e110) | SMD 0.54 [0.43; 1.04]*3 | 18 (3430) | SQ: high | No data |
| (Outpatient) psychotherapy | Pain reduction (if not otherwise specified) |
|||||
| Functional somatic symptoms in general | Short-term psychotherapy | (e113) | SMD 0.49 (no data; p < 0.05)*4 (reduced physical symptoms) |
12 (1019) | SQ: low/moderate | No data |
| Fibromyalgia syndrome |
Cognitive behavioral therapy | (18) *5 | SMD −0.29 [−0.47; −0.11] | 20 (1382) | EL: weak | No |
| Guided imagery/hypnosis | (e114) | RD 0.18 [0.02; 0.35] | 7 (387) | EL: weak | No data | |
| Irritable bowel syndrome | Psychological therapies | (23) | SMD 0.69 [0.52; 0.86] _(symptom severity) | 41 (2290) | SQ: unclear | No data |
*1 Predominantly minor and transient adverse effects without study discontinuation in all conditions examined
*2 Own analysis
*3 Median and interquartile range of effect sizes in the studies included. absolute effect size without comparison with a comparator
*4 Estimated from difference between effect sizes on basis of differences before and after the compared interventions
*5 Review from the Cochrane Database of Systematic Reviews
Bold: statistically significant effect size
No data: the review either does not report this information or states that the source trials did not report it.
The tables represent a selection of frequently employed. well established interventions for commonly occurring functional somatic symptoms. Whenever multiple reviews of the same intervention had been published since the previous edition of the guidelines. the most recent review was selected. In the event of publication of two or more reviews in the same year. the review with the largest number of patients and/or the best study quality was chosen. The complete evidence base can be found in the long version of the guidelines (3).
Notes on tabulation of evidence
- The effect sizes given in the tables depend on the study selection and mode of conduct of the systematic review and on the operationalization of the chosen criterion of success in the review. While the quality of the first two items was checked by the guideline group and only high-quality studies were used. the third item depends on the authors of the respective source review. The effect sizes can therefore be used to show the efficacy of a method. but cannot necessarily be compared among various systematic reviews. particularly if these cover different forms of disorders.
- With significant results the direction of the treatment effects is always in favor of the intervention investigated. unless otherwise stated.
- The diagnostic classifications of functional symptoms follow the deliberations of the authors of the systematic reviews. under consideration of recognized (research) diagnostic criteria.
- Whenever possible. the following data were used for assessment of tolerance and acceptance: percentage drop-out rates in the active treatment group and control group; percentage rates of subjectively relevant adverse effects or severe adverse effects.
Empirically significant results regarding efficacy that were not associated with a recommendation because of downgrading are given in italic.
MD/MD(w). Mean difference/mean difference (weighted); OR. odds ratio; RD. risk difference; RR. relative risk; SMD. standardized mean difference: effect size on the basis of validated psychometric instruments
Particularly in the case of major psychosocial stress factors and/or mental comorbidity, relevant dysfunctional disease models, significant functional impairment, or a persistently conflictual therapist–patient relationship, psychotherapy is recommended (consensus). The efficacy of (cognitive) behavioral therapy, psychodynamic psychotherapy, and hypnotherapy is well substantiated in the literature (strong consensus, recommended, evidence level: strong) (etable 3) (4– 6, 8, 18, 23– 25, 30, e113, e114). Further therapeutic elements that have proved efficacious in multimodal treatment models include body-oriented and/or mindfulness-based approaches. Psychotherapy and psychiatric treatments go beyond the usual schemes, so that treatment motivation is the first important treatment goal (strong consensus, recommendation, evidence level: strong) (4– 6, 30, e115).
An extended psychodiagnostic process embraces the following aspects (4– 6, 30):
The altered “embodied self,” i.e., all bodily perceptions, feelings, attitudes, and beliefs
Dysfunctional experience with one’s own illness, role models, (supposed) serious illness in the patient’s environment, traumatic loss, violence, or neglect
Potential disease-maintaining factors, primary or secondary gain (such as ongoing conflict situations, desire to retire from work, or compensation payments)
Mental comorbidities (anxiety, depression, trauma sequelae, addiction, personality disorders (e116).
Psychotherapy for functional somatic symptoms and bodily distress should focus primarily on the somatic symptoms, the existing explanatory model, and symptom-related attitudes and behavior patterns (4– 6, 30). The treatment focuses on positive self-perception and body awareness, self-regulation techniques, interpersonal relationships, encouragement of creativity, and openness to change (strong consensus, recommendation, evidence level: weak) (4– 6, 30, e43, e117). Psychological aspects of symptom formation and maintenance as well as individual vulnerability factors (context, personality, biography) should be addressed only indirectly, or later in the treatment course.
If outpatient treatment is not possible or proves inadequate, multimodal treatment in a suitable day-care or inpatient facility is indicated. If the focus is on improving participation, including maintenance/restoration of ability to work and thus prevention of (further) chronification, one should consider interdisciplinary rehabilitation with sufficient elements of counseling, psychodiagnostics, and psychotherapy, or alternatively psychodynamic treatment (strong consensus, recommendation, evidence level: weak) (4– 6, e118, e119).
Discussion
Functional somatic symptoms are not defined in the same way as diseases with circumscribed organic pathology. Instead, their course is greatly determined by how the symptoms are experienced, coped with, and responded to by physicians. Therefore, many of the recommendations in the updated guidelines relate to the interaction with affected patients, i.e., the comprehension and modification of the individual symptom context and explanatory model. With few exceptions, active therapeutic interventions designed to promote self-efficacy (especially psychoeducation, relaxation and mindfulness, self-help, and physical activation) carry less risk and have more sustained effects than passive, organ-related measures. In severe cases, multimodal treatment and psychotherapy have been shown to be effective. Drug treatment should be reserved for temporary relief of symptoms or management of comorbidity. Much more research is needed into prevention, psychophysiology, and the differential treatment of patients with different manifestations of functional somatic symptoms.
BOX 1. What is new in the revised guidelines?
On the basis of somewhat better evidence, the recommendation levels for reflective discussion and diagnosis (history taking, information, and reassurance), coping-oriented treatment (activation, self-help/self-efficacy), and interdisciplinary cooperation (consultation and discussion, multimodal treatment) have been raised on grounds of their preventive and prognostic relevance and comparatively low risks, low costs, and a tendency towards more durable effects.
In view of their low risk, persistent effect, cost efficiency, and patient preference, and based on positive recent research findings, new body–mind treatment approaches (such as mindfulness-based stress reduction [MBSR]) have been adopted and are now counted among the adjuvant treatments that can be considered.
There are now more research data on the use of various forms of psychotherapy for severe functional somatic symptoms. Cognitive behavioral therapy for a wide range of functional somatic symptoms is still viewed as demonstrably effective, as is hypnosis for irritable bowel syndrome. In addition to continued primary care contacts, or as an element of multimodal treatment, psychotherapy remains an important—albeit not primarily mandatory—treatment component. This slighty moderated recommendation takes into account the still limited availability of and low patient preference for psychotherapy.
Only a small number of commendable passive treatment options have been identified since publication of the previous guideline. The only procedures that can be recommended as adjuvant treatments are balneotherapy/hydrotherapy for syndromes dominated by pain, especially fibromyalgia syndrome.
Given the unchanged evidence with regard to risks, adverse effects, and low acceptance by patients, administration of drugs—particularly psychopharmaceuticals, above all antidepressants—is now recommended only for temporary symptomatic treatment (e.g., of sleep disorders or nervousness), certain pain syndromes (e.g., fibromyalgia syndrome), or in the presence of mental comorbidity. For pharmacological treatment of specific syndromes (e.g., fibromyalgia syndrome, irritable bowel syndrome, and selected pain syndromes, e.g. tension headache), the pertinent specific guidelines should be followed.
Key Messages.
Functional somatic symptoms are a common occurrence and often have a negative impact on everyday functioning and quality of life. The course they take depends greatly on the behavior of the treating physician.
The possible presence of functional somatic symptoms must be considered at an early stage.
A well-planned diagnostic work-up with simultaneous, even-handed consideration of physical and psychosocial factors enables identification and treatment of somatic and mental comorbidities and differential diagnoses without placing undue stress on patients or confining them to a particular diagnosis / binding them to a particular medical professional.
The most important therapeutic interventions are simple and bear little risk: appreciation, reassuring psychophysiological information, joint development of an individual biopsychosocial explanatory model, and promotion of increased self-efficacy, activity, and a healthier lifestyle.
In severe cases with wide-reaching functional impairment and/or psychic comorbidity, multimodal treatment accompanied by other therapeutic measures is indicated.
eBOX 5. Search terms (from [3]).
-
Level 1: Functional somatic symptoms
(somatoform disorder OR somatiz* OR somatis* OR conversion disorder* OR multisomatoform OR medically unexplained* OR organically unexplained* OR psychogenic OR nonorganic OR psychosomatic syndrom* OR bodily distress OR somatic symptom disorder) OR (functional somatic syndrom* OR functional syndrom* OR functional disorder* OR functional illness* OR functional symptom*) OR (bodily distress) OR (fibromyalgia* OR chronic widespread pain* OR widespread musculoskeletal pain* OR myofascial pain) OR (irritable bowel* OR functional bowel* OR functional gastrointestinal*) OR fatigue/*psychology OR chronic fatigue syndrome* OR Fatigue Syndrome, Chronic*) OR (functional dyspepsia* OR nonulcer dyspepsia*) OR (chronic pelvic pain*) OR (functional micturition disorder* OR functional urinary disorder* OR urethral syndrome* OR micturition dysfunction* OR (urinary retention* AND (psychogen* OR psychology) OR irritable bladder* OR painful bladder syndrome*) OR (interstitial cystitis*) OR (food intolerance* OR food allergy) OR ((chronic low back pain* OR back pain*) AND nonspecific) OR (tension-type headache* OR tension headache*) OR (atypical chest pain* OR nonspecific chest pain* OR non-specific chest pain) OR (atypical face pain* OR facial pain* OR myofacial pain syndrome*) OR (panalges* OR (psychogen* AND pain) OR idiopathic pain* OR idiopathic pain disorder*) OR (myalgic encephalomyelitis* OR myalgic encephalopathy* OR chronic epstein barr virus* OR chronic mononucleosis* OR chronic infectious mononucleosis like syndrome* OR chronic fatigue and immune dysfunction syndrome* OR effort syndrome* OR low natural killer cell syndrome* OR neuromyasthenia OR post viral fatigue syndrome* OR postviral fatigue syndrome* OR post viral syndrome* OR postviral syndrome* OR post infectious fatigue* OR postinfectious fatigue*) OR (chronic lyme disease*) OR (candida hypersensitivity* OR candida syndrome* OR candidiasis hypersensitivity) OR (mitral valve prolapse* AND psychology) OR (hypoglycaemia/*psychology) OR (sleep disorder/*psychology OR nonorganic Insomnia*) OR (Multiple chemical sensitivit* OR idiopathic environmental intolerance*) OR (electromagnetic hypersensitivity OR electro-hypersensitivity OR electrosensitiv* OR IEI-EMF OR environmental illness*) OR (Sick Building Syndrome*) OR (Persian gulf syndrome OR gulf war syndrome) OR (Amalgam hypersensitivity* OR Dental Amalgam/*toxicity OR dental amalgam/*adverse effects) OR (silicone breast implant* OR implant intolerance*) OR (temporomandibular joint disorder* OR temporomandibular disorder* OR temporomandibular joint dysfunction* OR temporomandibular joint dysfunction* OR craniomandibular disorder*) OR (atypical odontalgia* OR prosthesis intolerance* OR (psychogen* AND gagging) OR chronic rhinopharyngitis*) OR (burning mouth* OR glossalg* OR glossodyn* OR glossopyr* OR bruxism) OR (globus syndrome* OR globus hystericus*) OR (hyperventilation syndrome*) OR (dysphonia OR aphonia) OR (tinnitus) OR (Vertigo OR Dizziness) OR (repetitive strain injury) OR (chronic whiplash syndrome*) OR (pseudoseizures OR hysterical seizures*) OR (psychogen* AND dystonia) OR (psychogen* AND dysphagia) OR (skin disease* AND (psychology OR psychogen*)) OR (pruritus AND (psychology OR psychogen* OR somatoform)) OR (culture-bound disorder*) OR (aerotoxic syndrome OR sick aeroplane syndrome)
The search terms “somatic symptom disorder,” “bodily distress,” “myofascial pain,” and “aerotoxic syndrome OR sick aeroplane syndrome” were added at the suggestion of the participating societies because of the recent or impending revisions of the major diagnostic classifications DSM-V and ICD-11.
-
Level 2: Setting and content
Primary care or somatic specialist
(Ambulatory Care* OR Primary Health Care* OR Physicians, Family* OR (Specialties, Medical* NOT Psychiatry*) OR general pract* OR family pract* OR family doctor* OR family physician* OR family medicine* OR primary care*)
Psychosomatic medicine, psychiatry, psychology
(Mental Health Services* OR Psychosomatic Medicine OR Psychiatry OR Psychology)
Content: Relationship/own attitude
(Attitude of Health Personnel* OR Communication OR Empathy OR Professional-Patient Relations* OR Physician‘s Practice Patterns* OR Role OR Medical History Taking* OR Decision Making* OR Countertransference OR Disease Attributes* OR Emotions OR interact* OR encounter* OR disposition* OR setting* OR approach* OR engag* OR deal* OR exposure* OR experience* OR handl* OR function* OR attitud* OR declin* OR prejud* OR reject* OR rigid* OR belie* OR concept* OR critic* OR legitim* OR motivat* OR stigma*)
Other content
None of the terms listed under “Content: Relationship/own attitude”
-
Level 3: Treatment
Treatment studies for functional somatic symptoms were identified by combining the pertinent level 1 search term with (treatment OR intervention OR therapy)
eBOX 6. Systematic identification of evidence and aggregated evidence on functional somatic symptoms.
General inclusion and exclusion criteria
Functional somatic symptoms had to be the central topic of the clinical study or review and not, for example, have occurred as adverse drug effects or in the context of nonfunctional symptoms and illnesses.
Studies involving children and adolescents were excluded, as were publications in languages other than English and German.
Recommendations on diagnosis, conduct of discussions with the patient, and basic counseling or the attitude towards functional somatic symptoms, as well as recommendations regarding social medicine aspects, were formulated on the basis of clinical studies. For inclusion, the questions asked in the identified empirical studies had to fit the thematic areas concerned. After scrutiny of the complete updated research data, individual studies were drawn upon to substantiate the respective recommendations.
Recommendations on forms of treatment are based on systematic reviews and meta-analyses examining the identified treatment procedures (umbrella review). The systematic reviews had to fulfill the following criteria:
Each review had to disclose a comprehensible search strategy, have carried out a systematic quality assessment of the primary studies covered, and report the selection and use of established diagnostic criteria of the functional somatic symptoms investigated in the primary studies. If established criteria were not available, this fact had to be stated in the review.
The results had to be presented in a structured and sufficiently differentiated form which permitted the deduction of recommendations regarding the functional somatic symptoms concerned. Reviews permitting the extraction of effect sizes were preferred.
In the presence of several systematic reviews on the same treatment of a particular category of functional somatic symptoms, the number of primary RCTs that were included more than once was determined. If such studies formed a majority, the review was excluded.
Investigation of a given intervention for the described category of functional somatic symptoms had to rest on analysis of three or more primary RCTs with no overlapping of patients. In case of doubt, the study populations were scrutinized for overlap.
eTable 4. Levels of consensus (from [3]).
| Strength of consensus | Agreement of…% of the participants |
| Strong consensus | >95% |
| Consensus | >75–95% |
| Majority agreement | 50–75% |
| No consensus | <50% |
Acknowledgments
Translated from the original German by David Roseveare
Acknowledgments
The authors are grateful to the AWMF and to all colleagues, professional societies, and patient organizations (ebox 1) who helped to compile this revised guideline.
Footnotes
Conflict of interest statement
Dr. Roenneberg, MHBA and Dipl-Psych. Sattel declare that no conflict of interest exists.
Prof. Schäfert has received payment from Springer-Verlag and from the journal Psychotherapeut for authorship.
Prof. Hausteiner-Wiehle and Prof. Henningsen have received payments from the publishers Schattauer and Elsevier for writing textbook chapters on the subject of functional somatic symptoms.
Deutsches Ärzteblatt, in common with many other journals, does not subject German clinical practice guidelines to peer review because they have already been intensively assessed and discussed by experts who have broadly agreed on the final wording.
References
- 1.Hausteiner-Wiehe C, Henningsen P, Häuser W, et al. Klett-Cotta (vormals Schattauer) Vol. 1. Stuttgart: 2013. Umgang mit Patienten mit nicht-spezifischen, funktionellen und somatoformen Körperbeschwerden S3-Leitlinie mit Quellentexten, Praxismaterialien und Patientenleitlinie. [Google Scholar]
- 2.Schaefert R, Hausteiner-Wiehle C, Hauser W, Ronel J, Herrmann M, Henningsen P. Non-specific, functional, and somatoform bodily complaints. Dtsch Arztebl Int. 2012;109:803–813. doi: 10.3238/arztebl.2012.0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.S3-Leitlinie „Funktionelle Körperbeschwerden“. www.awmf.org/leitlinien/detail/ll/051-001.html (last accessed on 4 March 2019) [Google Scholar]
- 4.Henningsen P, Zipfel S, Sattel H, Creed F. Management of functional somatic syndromes and bodily distress. Psychother Psychosom. 2018;87:12–31. doi: 10.1159/000484413. [DOI] [PubMed] [Google Scholar]
- 5.Hausteiner-Wiehle C, Henningsen P. Kein Befund und trotzdem krank? Stuttgart: Klett-Cotta (vormals Schattauer) 2015 doi: 10.1055/s-0041-103867. [DOI] [PubMed] [Google Scholar]
- 6.Henningsen P, Martin A. Somatoforme Störungen, somatische Belastungsstörung Psychotherapie. Funktions- und störungsorientiertes Vorgehen. In: Herpertz S, Caspar F, Lieb K, editors. Elsevier. München: 2016. [Google Scholar]
- 7.van Gils A, Schoevers RA, Bonvanie IJ, Gelauff JM, Roest AM, Rosmalen JG. Self-help for medically unexplained symptoms: a systematic review and meta-analysis. Psychosom Med. 2016;78:728–739. doi: 10.1097/PSY.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 8.van Dessel N, den Boeft M, van der Wouden JC, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev. 2014;11 doi: 10.1002/14651858.CD011142.pub2. CD011142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2016;2 doi: 10.1002/14651858.CD003200.pub4. CD003200. [DOI] [PubMed] [Google Scholar]
- 10.Winkelmann A, Häuser W, Friedel E, et al. Physiotherapy and physical therapies for fibromyalgia syndrome Systematic review, meta-analysis and guideline. Schmerz. 2012;26:276–286. doi: 10.1007/s00482-012-1171-3. [DOI] [PubMed] [Google Scholar]
- 11.Arnold B, Häuser W, Arnold M, et al. Multimodale Therapie des Fibromyalgiesyndroms. Systematische Übersicht, Metaanalyse und Leitlinie. Schmerz. 2012;26:287–290. doi: 10.1007/s00482-012-1173-1. [DOI] [PubMed] [Google Scholar]
- 12.Häuser W, Wolfe F, Tölle T, Uçeyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs. 2012;26:297–307. doi: 10.2165/11598970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Walitt B, Urrútia G, Nishishinya MB, Cantrell SE, Häuser W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015;6 doi: 10.1002/14651858.CD011735. CD011735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cording M, Derry S, Phillips T, Moore RA, Wiffen PJ. Milnacipran for pain in fibromyalgia in adults. Cochrane Database Syst Rev. 2015;10 doi: 10.1002/14651858.CD008244.pub3. CD008244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derry S, Cording M, Wiffen PJ, Law S, Phillips T, Moore RA. Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst Rev. 2016;9 doi: 10.1002/14651858.CD011790.pub2. CD011790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YH, Wang FY, Feng CQ, Yang XF, Sun YH. Massage therapy for fibromyalgia: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089304. e89304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan QL, Wang P, Liu L, et al. Acupuncture for musculoskeletal pain: a meta-analysis and meta-regression of sham-controlled randomized clinical trials. Sci Rep. 2016;6 doi: 10.1038/srep30675. 30675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernardy K, Klose P, Busch AJ, Choy EH, Häuser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev. 2013;9 doi: 10.1002/14651858.CD009796.pub2. CD009796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauche R, Cramer H, Dobos G, Langhorst J, Schmidt S. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res. 2013,;75:500–510. doi: 10.1016/j.jpsychores.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Chao GQ, Zhang S. Effectiveness of acupuncture to treat irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2014;20:1871–1877. doi: 10.3748/wjg.v20.i7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manheimer E, Cheng K, Wieland LS, et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD005111.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manheimer E, Cheng K, Wieland LS, et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2012;5 doi: 10.1002/14651858.CD005111.pub3. CD005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aucoin M, Lalonde-Parsi MJ, Cooley K. Mindfulness-based therapies in the treatment of functional gastrointestinal disorders: a meta-analysis. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/140724. 140724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laird KT, Tanner-Smith EE, Russell AC, Hollon SD, Walker LS. Short-term and long-term efficacy of psychological therapies for irritable bowel syndrome: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:937–947. doi: 10.1016/j.cgh.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Ford AC, Quigley EM, Lacy BE, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1350–1365. doi: 10.1038/ajg.2014.148. [DOI] [PubMed] [Google Scholar]
- 26.Schaefert R, Klose P, Moser G, Häuser W. Efficacy, tolerability, and safety of hypnosis in adult irritable bowel syndrome: systematic review and meta-analysis. Psychosom Med. 2014;76:389–398. doi: 10.1097/PSY.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Lembo A, Sultan S. American Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147:1149–1172e2. doi: 10.1053/j.gastro.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Zhu W, Liu W, Wu Y, Wu B. Rifaximin for irritable bowel syndrome: a meta-analysis of randomized placebo-controlled trials. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000002534. e2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deutsche Schmerzgesellschaft. Definition, Pathophysiologie, Diagnostik und Therapie des Fibromyalgiesyndroms. www.awmf.org/leitlinien/detail/ll/145-004.html (last accessed on 22 February 2019) [Google Scholar]
- 30.Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) Nationale VersorgungsLeitlinie Nicht-spezifischer Kreuzschmerz - Kurzfassung, 2nd edition. Version 1 2017 (last accessed on 22 February 2019) [Google Scholar]
- 31.Martin A, Härter M, Henningsen P, Hiller W, Kröner-Herwig B, Rief W. Evidenzbasierte Leitlinie zur Psychotherapie somatoformer Störungen und assoziierter Syndrome (Vol 4) Göttingen: Hogrefe Verlag. 2013 [Google Scholar]
- E1.Hilderink PH, Collard R, Rosmalen JG, Oude Voshaar RC. Prevalence of somatoform disorders and medically unexplained symptoms in old age populations in comparison with younger age groups: a systematic review. Ageing Res Rev. 2013;12:151–156. doi: 10.1016/j.arr.2012.04.004. [DOI] [PubMed] [Google Scholar]
- E2.Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms: an epidemiological study in seven specialities. J Psychosom Res. 2001;51:361–367. doi: 10.1016/s0022-3999(01)00223-9. [DOI] [PubMed] [Google Scholar]
- E3.Maiden NL, Hurst NP, Lochhead A, Carson AJ, Sharpe M. Medically unexplained symptoms in patients referred to a specialist rheumatology service: prevalence and associations. Rheumatology (Oxford) 2003;42:108–112. doi: 10.1093/rheumatology/keg043. [DOI] [PubMed] [Google Scholar]
- E4.Snijders TJ, de Leeuw FE, Klumpers UM, Kappelle LJ, van Gijn J. Prevalence and predictors of unexplained neurological symptoms in an academic neurology outpatient clinic—an observational study. J Neurol. 2004;251:66–71. doi: 10.1007/s00415-004-0273-y. [DOI] [PubMed] [Google Scholar]
- E5.de Waal MW, Arnold IA, Eekhof JA, van Hemert AM. Somatoform disorders in general practice: prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Br J Psychiatry. 2004;184:470–476. doi: 10.1192/bjp.184.6.470. [DOI] [PubMed] [Google Scholar]
- E6.Verhaak PF, Meijer SA, Visser AP, Wolters G. Persistent presentation of medically unexplained symptoms in general practice. Fam Pract. 2006;23:414–420. doi: 10.1093/fampra/cml016. [DOI] [PubMed] [Google Scholar]
- E7.Rosendal M, Olde Hartman TC, Aamland A, et al. „Medically unexplained“ symptoms and symptom disorders in primary care: prognosis based recognition and classification. BMC Fam Pract. 2017;18 doi: 10.1186/s12875-017-0592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E8.Lieb R, Zimmermann P, Friis RH, Höfler M, Tholen S, Wittchen HU. The natural course of DSM-IV somatoform disorders and syndromes among adolescents and young adults: a prospective longitudinal community study. Eur Psychiatry. 2002;17:321–331. doi: 10.1016/s0924-9338(02)00686-7. [DOI] [PubMed] [Google Scholar]
- E9.Jackson JL, Kroenke K. Prevalence, impact, and prognosis of multisomatoform disorder in primary care: a 5-year follow up study. Psychosom Med. 2008;70:430–434. doi: 10.1097/PSY.0b013e31816aa0ee. [DOI] [PubMed] [Google Scholar]
- E10.Steinbrecher N, Hiller W. Course and prediction of somatoform disorder and medically unexplained symptoms in primary care. Gen Hosp Psychiatry. 2011;33:318–326. doi: 10.1016/j.genhosppsych.2011.05.002. [DOI] [PubMed] [Google Scholar]
- E11.Olde Hartman TC, Borghuis MS, Lucassen PL, van de Laar FA, Speckens AE, van Weel C. Medically unexplained symptoms, somatisation disorder and hypochondriasis: course and prognosis. A systematic review. J Psychosom Res. 2009;66:363–377. doi: 10.1016/j.jpsychores.2008.09.018. [DOI] [PubMed] [Google Scholar]
- E12.Budtz-Lilly A, Vestergaard M, Fink P, Carlsen AH, Rosendal M. The prognosis of bodily distress syndrome: a cohort study in primary care. Gen Hosp Psychiatry. 2015;37:560–566. doi: 10.1016/j.genhosppsych.2015.08.002. [DOI] [PubMed] [Google Scholar]
- E13.Wiborg JF, Gieseler D, Fabisch AB, Voigt K, Lautenbach A, Löwe B. Suicidality in primary care patients with somatoform disorders. Psychosom Med. 2013;75:800–806. doi: 10.1097/PSY.0000000000000013. [DOI] [PubMed] [Google Scholar]
- E14.Kämpfer N, Staufenbiel S, Wegener I, et al. Suicidality in patients with somatoform disorder—the speechless expression of anger? Psychiatry Res. 2016;246:485–491. doi: 10.1016/j.psychres.2016.10.022. [DOI] [PubMed] [Google Scholar]
- E15.Lan CC, Tseng CH, Chen JH, et al. Increased risk of a suicide event in patients with primary fibromyalgia and in fibromyalgia patients with concomitant comorbidities: a nationwide population-based cohort study. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000005187. e5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Calati R, Laglaoui Bakhiyi C, Artero S, Ilgen M, Courtet P. The impact of physical pain on suicidal thoughts and behaviors: meta-analyses. J Psychiatr Res. 2015;71:16–32. doi: 10.1016/j.jpsychires.2015.09.004. [DOI] [PubMed] [Google Scholar]
- E17.Spiegel B, Schoenfeld P, Naliboff B. Systematic review: the prevalence of suicidal behaviour in patients with chronic abdominal pain and irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:183–193. doi: 10.1111/j.1365-2036.2007.03357.x. [DOI] [PubMed] [Google Scholar]
- E18.Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many? Lancet. 1999;354:936–939. doi: 10.1016/S0140-6736(98)08320-2. [DOI] [PubMed] [Google Scholar]
- E19.Olde Hartman TC, Lucassen PL, van de Lisdonk EH, Bor HH, van Weel C. Chronic functional somatic symptoms: a single syndrome? Br J Gen Pract. 2004;54:922–927. [PMC free article] [PubMed] [Google Scholar]
- E20.Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003, 65,:528–533. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- E21.Fink P, Toft T, Hansen MS, Ørnbøl E, Olesen F. Symptoms and syndromes of bodily distress: an exploratory study of 978 internal medical, neurological, and primary care patients. Psychosom Med. 2007;69:30–39. doi: 10.1097/PSY.0b013e31802e46eb. [DOI] [PubMed] [Google Scholar]
- E22.Kanaan RA, Lepine JP, Wessely SC. The association or otherwise of the functional somatic syndromes. Psychosom Med. 2007;69:855–859. doi: 10.1097/PSY.0b013e31815b001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E23.Riedl A, Schmidtmann M, Stengel A, et al. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res. 2008;64:573–582. doi: 10.1016/j.jpsychores.2008.02.021. [DOI] [PubMed] [Google Scholar]
- E24.Fink P, Schröder A. One single diagnosis, bodily distress syndrome, succeeded to capture 10 diagnostic categories of functional somatic syndromes and somatoform disorders. J Psychosom Res. 2010;68:415–426. doi: 10.1016/j.jpsychores.2010.02.004. [DOI] [PubMed] [Google Scholar]
- E25.Lieb R, Meinlschmidt G, Araya R. Epidemiology of the association between somatoform disorders and anxiety and depressive disorders: an update. Psychosom Med. 2007;69:860–863. doi: 10.1097/PSY.0b013e31815b0103. [DOI] [PubMed] [Google Scholar]
- E26.Lowe B, Spitzer RL, Williams JB, Mussell M, Schellberg D, Kroenke K. Depression, anxiety and somatization in primary care: syndrome overlap and functional impairment. Gen Hosp Psychiatry. 2008;30:191–199. doi: 10.1016/j.genhosppsych.2008.01.001. [DOI] [PubMed] [Google Scholar]
- E27.Hanel G, Henningsen P, Herzog W, et al. Depression, anxiety, and somatoform disorders: vague or distinct categories in primary care? Results from a large cross-sectional study. J Psychosom Res. 2009;67:189–197. doi: 10.1016/j.jpsychores.2009.04.013. [DOI] [PubMed] [Google Scholar]
- E28.Kohlmann S, Gierk B, Hilbert A, Brahler E, Lowe B. The overlap of somatic, anxious and depressive syndromes: a population-based analysis. J Psychosom Res. 2016;90:51–56. doi: 10.1016/j.jpsychores.2016.09.004. [DOI] [PubMed] [Google Scholar]
- E29.Hatcher S, Gilmore K, Pinchen K. A follow-up study of patients with medically unexplained symptoms referred to a liaison psychiatry service. Int J Psychiatry Med. 2011;41:217–227. doi: 10.2190/PM.41.3.a. [DOI] [PubMed] [Google Scholar]
- E30.Stone J, Carson A, Duncan R, et al. Symptoms unexplained by organic disease in 1144 new neurology out-patients: how often does the diagnosis change at follow-up? Brain. 2009;132:2878–2888. doi: 10.1093/brain/awp220. [DOI] [PubMed] [Google Scholar]
- E31.Eikelboom EM, Tak LM, Roest AM, Rosmalen JGM. A systematic review and meta-analysis of the percentage of revised diagnoses in functional somatic symptoms. J Psychosom Res. 2016;88:60–67. doi: 10.1016/j.jpsychores.2016.07.001. [DOI] [PubMed] [Google Scholar]
- E32.Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265–275. doi: 10.1159/000337349. [DOI] [PubMed] [Google Scholar]
- E33.Rask MT, Ørnbøl E, Rosendal M, Fink P. Long-term outcome of bodily distress syndrome in primary care: a follow-up study on health care costs, work disability, and self-rated health. Psychosom Med. 2017;79:345–357. doi: 10.1097/PSY.0000000000000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E34.Oxford Centre for Evidence-Based Medicine (2009) Levels of evidence. www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (last accessed on 10 August 2018) [Google Scholar]
- E35.Puhan MA, Schünemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349 doi: 10.1136/bmj.g5630. g5630. [DOI] [PubMed] [Google Scholar]
- E36.Beauchamp TL, Childress JF. Principles of biomedical ethics. Oxford: Oxford University Press. 2013 [Google Scholar]
- E37.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e. V. (AWMF) AWMF-Regelwerk Leitlinien. www.awmf.org/leitlinien/awmf-regelwerk.html (last accessed on 31 August 2018) [Google Scholar]
- E38.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e. V. (AWMF), Ärztliches Zentrum für Qualität in der Medizin (ÄZQ), eds. Deutsches Instrument zur methodischen Leitlinien-Bewertung (DELBI) - Fassung 2005/2006 + Domäne 8. www.leitlinien.de/mdb/edocs/pdf/literatur/delbi-fassung-2005-2006-domaene-8-2008.pdf (last accessed on 10 August 2018) [Google Scholar]
- E39.Berens S, Hausteiner-Wiehle C, Sattel H, et al. Schweregrad-gestuftes, kooperatives und koordiniertes Versorgungsmodell für Patienten mit nicht-spezifischen, funktionellen und somatoformen Körperbeschwerden. Psychologische Medizin: Österreichische Fachzeitschrift für medizinische Psychologie, Psychosomatik und Psychotherapie. 2016;4:13–22. [Google Scholar]
- E40.Almquist E, Toernblom H, Simreìn M. Practical management of irritable bowel syndrome: a clinical review. Minerva gastroenterologica e dietologica. 2016;62:30–48. [PubMed] [Google Scholar]
- E41.van der Feltz-Cornelis CM, Hoedeman R, Keuter EJ, Swinkels JA. Presentation of the multidisciplinary guideline Medically Unexplained Physical Symptoms (MUPS) and somatoform disorder in the Netherlands: disease management according to risk profiles. J Psychosom Res. 2012;72:168–169. doi: 10.1016/j.jpsychores.2011.11.007. [DOI] [PubMed] [Google Scholar]
- E42.Salmon P, Peters S, Stanley I. Patients‘ perceptions of medical explanations for somatisation disorders: qualitative analysis. BMJ. 1999;318:372–376. doi: 10.1136/bmj.318.7180.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E43.Arbeitskreis PISO (Hrsg.) Psychodynamisch-Interpersonelle Therapie bei somatoformen Störungen (PISO). Eine manualisierte Kurzzeitintervention. Göttingen: Hogrefe Verlag. 2012;47 [Google Scholar]
- E44.Schaefert R, Bölter R, Faber R, Claudia Kaufmann C. Tangential, nicht frontal. Annäherung an eine schwierige Patientengruppe. Psychotherapie im Dialog. 2008;9:252–259. [Google Scholar]
- E45.Rask MT, Carlsen AH, Budtz-Lilly A, Rosendal M. Multiple somatic symptoms in primary care patients: a cross-sectional study of consultation content, clinical management strategy and burden of encounter. BMC Fam Pract. 2016;17 doi: 10.1186/s12875-016-0478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E46.Olde Hartman TC, Woutersen-Koch H, van der Horst HE. Medically unexplained symptoms: evidence, guidelines, and beyond. Br J Gen Pract. 2013;63:625–626. doi: 10.3399/bjgp13X675241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E47.Brownell AK, Atkins C, Whiteley A, Woollard RF, Kornelsen J. Clinical practitioners‘ views on the management of patients with Medically Unexplained Physical Symptoms (MUPS): a qualitative study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012379. e012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E48.Murray AM, Toussaint A, Althaus A, Lowe B. The challenge of diagnosing non-specific, functional, and somatoform disorders: a systematic review of barriers to diagnosis in primary care. J Psychosom Res. 2016;80:1–10. doi: 10.1016/j.jpsychores.2015.11.002. [DOI] [PubMed] [Google Scholar]
- E49.Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268:760–765. [PubMed] [Google Scholar]
- E50.Pryor DB, Shaw L, McCants CB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118:81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. [DOI] [PubMed] [Google Scholar]
- E51.Kroenke K. A practical and evidence-based approach to common symptoms: a narrative review. Ann Intern Med. 2014;161:579–586. doi: 10.7326/M14-0461. [DOI] [PubMed] [Google Scholar]
- E52.Evens A, Vendetta L, Krebs K, Herath P. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059–1064. doi: 10.1016/j.amjmed.2015.03.030. [DOI] [PubMed] [Google Scholar]
- E53.Kelly M, Tink W, Nixon L, Dornan T. Losing touch? Refining the role of physical examination in family medicine. Can Fam Physician. 2015;61:1041–1043. [PMC free article] [PubMed] [Google Scholar]
- E54.Pincus T, Holt N, Vogel S, et al. Cognitive and affective reassurance and patient outcomes in primary care: a systematic review. Pain. 2013;154:2407–2416. doi: 10.1016/j.pain.2013.07.019. [DOI] [PubMed] [Google Scholar]
- E55.Mann B, Wilson H. Diagnosing somatisation in adults in the first consultation: moving beyond diagnosis by exclusion. Br J Gen Pract. 2013;63:607–608. doi: 10.3399/bjgp13X674602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E56.Lorenzer A. Interaktion, Sprache und szenisches Verstehen. Psyche. 1983;37:97–115. [PubMed] [Google Scholar]
- E57.Riess H, Kraft-Todd G. EM.P.A.T.H.Y.: a tool to enhance nonverbal communication between clinicians and their patients. Acad Med. 2014;89:1108–1112. doi: 10.1097/ACM.0000000000000287. [DOI] [PubMed] [Google Scholar]
- E58.Axt-Adam P, van der Wouden JC, van der Does E. Influencing behavior of physicians ordering laboratory tests: a literature study. Med Care. 1993;31:784–794. doi: 10.1097/00005650-199309000-00003. [DOI] [PubMed] [Google Scholar]
- E59.van Wijk MA, van der Lei J, Mosseveld M, Bohnen AM, van Bemmel JH. Assessment of decision support for blood test ordering in primary care. a randomized trial. Ann Intern Med. 2001;134:274–281. doi: 10.7326/0003-4819-134-4-200102200-00010. [DOI] [PubMed] [Google Scholar]
- E60.Koch H, van Bokhoven MA, ter Riet G, et al. Ordering blood tests for patients with unexplained fatigue in general practice: what does it yield? Results of the VAMPIRE trial. Br J Gen Pract. 2009;59:e93–e100. doi: 10.3399/bjgp09X420310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E61.Epstein RM, Hadee T, Carroll J, Meldrum SC, Lardner J, Shields CG. “Could this be something serious?” Reassurance, uncertainty, and empathy in response to patients’ expressions of worry. J Gen Intern Med. 2007;22:1731–1739. doi: 10.1007/s11606-007-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E62.Anderson M, Hartz A, Nordin T, et al. Community physicians’ strategies for patients with medically unexplained symptoms. Fam Med. 2008;40:111–118. [PubMed] [Google Scholar]
- E63.Giroldi E, Veldhuijzen W, Leijten C, et al. ‚No need to worry‘: an exploration of general practitioners‘ reassuring strategies. BMC Fam Pract. 2014;15:133. doi: 10.1186/1471-2296-15-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E64.Hasenbring MI, Pincus T. Effective reassurance in primary care of low back pain: what messages from clinicians are most beneficial at early stages? Clin J Pain. 2015;31:133–136. doi: 10.1097/AJP.0000000000000097. [DOI] [PubMed] [Google Scholar]
- E65.Musekamp G, Gerlich C, Ehlebracht-König I, Faller H, Reusch A. Evaluation of a self-management patient education program for patients with fibromyalgia syndrome: study protocol of a cluster randomized controlled trial. BMC Musculoskelet Disord. 2016;17 doi: 10.1186/s12891-016-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E66.Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms: a randomized controlled trial. J Gen Intern Med. 2006;21:671–677. doi: 10.1111/j.1525-1497.2006.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E67.Smith RC, Gardiner JC, Luo Z, Schooley S, Lamerato L, Rost K. Primary care physicians treat somatization. J Gen Intern Med. 2009;24:829–832. doi: 10.1007/s11606-009-0992-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E68.Aiarzaguena JM, Grandes G, Gaminde I, Salazar A, Sanchez A, Arino J. A randomized controlled clinical trial of a psychosocial and communication intervention carried out by GPs for patients with medically unexplained symptoms. Psychol Med. 2007;37:283–294. doi: 10.1017/S0033291706009536. [DOI] [PubMed] [Google Scholar]
- E69.Pols RG, Battersby MW. Coordinated care in the management of patients with unexplained physical symptoms: depression is a key issue. Med J Aust. 2008;188(12 Suppl):133–137. doi: 10.5694/j.1326-5377.2008.tb01877.x. [DOI] [PubMed] [Google Scholar]
- E70.van der Weijden T, van Velsen M, Dinant GJ, van Hasselt CM, Grol R. Unexplained complaints in general practice: prevalence, patients’ expectations, and professionals‘ test-ordering behavior. Med Decis Making. 2003;23:226–231. doi: 10.1177/0272989X03023003004. [DOI] [PubMed] [Google Scholar]
- E71.Heijmans M, Olde Hartman TC, van Weel-Baumgarten E, Dowrick C, Lucassen PL, van Weel C. Experts‘ opinions on the management of medically unexplained symptoms in primary care. A qualitative analysis of narrative reviews and scientific editorials. Fam Pract. 2011;28:444–455. doi: 10.1093/fampra/cmr004. [DOI] [PubMed] [Google Scholar]
- E72.Fink P, Rosendal M, Toft T. Assessment and treatment of functional disorders in general practice: the extended reattribution and management model—an advanced educational program for nonpsychiatric doctors. Psychosomatics. 2002;43:93–131. doi: 10.1176/appi.psy.43.2.93. [DOI] [PubMed] [Google Scholar]
- E73.Larisch A, Schweickhardt A, Wirsching M, Fritzsche K. Psychosocial interventions for somatizing patients by the general practitioner: a randomized controlled trial. J Psychosom Res. 2004;57:507–514. doi: 10.1016/j.jpsychores.2004.04.372. [DOI] [PubMed] [Google Scholar]
- E74.Rief W, Martin A, Rauh E, Zech T, Bender A. Evaluation of general practitioners‘ training: how to manage patients with unexplained physical symptoms. Psychosomatics. 2006;47:304–311. doi: 10.1176/appi.psy.47.4.304. [DOI] [PubMed] [Google Scholar]
- E75.Rosendal M, Olesen F, Fink P, Toft T, Sokolowski I, Bro F. A randomized controlled trial of brief training in the assessment and treatment of somatization in primary care: effects on patient outcome. Gen Hosp Psychiatry. 2007;29:364–373. doi: 10.1016/j.genhosppsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- E76.Smith RC, Gardiner JC, Luo Z, Schooley S, Lamerato L, Rost K. Primary care physicians treat somatization. J Gen Intern Med. 2009;24:829–832. doi: 10.1007/s11606-009-0992-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E77.Weiland A, Blankenstein AH, van Saase JL, et al. Training medical specialists to communicate better with patients with Medically Unexplained Physical Symptoms (MUPS). A randomized, controlled trial. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138342. e0138342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E78.Warner A, Walters K, Lamahewa K, Buszewicz M. How do hospital doctors manage patients with medically unexplained symptoms: a qualitative study of physicians. J R Soc Med. 2017;110:65–72. doi: 10.1177/0141076816686348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E79.Yon K, Habermann S, Rosenthal J, et al. Improving teaching about medically unexplained symptoms for newly qualified doctors in the UK: findings from a questionnaire survey and expert workshop. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-014720. e014720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E80.Schaefert R, Benedikt G, Sauer N, et al. Früherkennung und Behandlung funktioneller/somatoformer Beschwerden in der Allgemeinarztpraxis - das FUNKTIONAL-Forschungsprojekt. Notfall & Hausarztmedizin. 2005;31:527–536. [Google Scholar]
- E81.Rudolf G, Henningsen P. Die psychotherapeutische Behandlung somatoformer Störungen. Z Psychsom Med Psychother. 2003;49:3–19. doi: 10.13109/zptm.2003.49.1.3. [DOI] [PubMed] [Google Scholar]
- E82.Langewitz W. Patientenzentrierte Kommunikation Psychosomatische Medizin. Theoretische Modelle und klinische Praxis. In: Adler RH, Herzog W, Joraschky P, et al., editors. Elsevier, Urban & Fischer. München: 2011. pp. 338–347. [Google Scholar]
- E83.Rolfe A, Burton C. Reassurance after diagnostic testing with a low pretest probability of serious disease: systematic review and meta-analysis. JAMA Intern Med. 2013;173:407–416. doi: 10.1001/jamainternmed.2013.2762. [DOI] [PubMed] [Google Scholar]
- E84.Kroenke K. Diagnostic testing and the illusory reassurance of normal results: comment on „Reassurance after diagnostic testing with a low pretest probability of serious disease“. JAMA Intern Med. 2013;173:416–417. doi: 10.1001/jamainternmed.2013.11. [DOI] [PubMed] [Google Scholar]
- E85.Petrie KJ, Sherriff R. Normal diagnostic test results do not reassure patients. Evid Based Med. 2014;19 doi: 10.1136/eb-2013-101393. [DOI] [PubMed] [Google Scholar]
- E86.Widder B, Dertwinkel R, Egle UT, Foerster K, Schiltenwolf M. Leitlinie für die Begutachtung von Schmerzen. Psychotherapeut. 2007;52:334–346. [Google Scholar]
- E87.Burton C, Lucassen P, Aamland A, Olde Hartman T. Explaining symptoms after negative tests: towards a rational explanation. J R Soc Med. 2015;108:84–88. doi: 10.1177/0141076814559082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E88.Vedsted P, Christensen MB, Sørensen HT, Fink P, Olesen F. Special status consultation for frequent attenders Who are the candidates? J Public Health Med. 2002;24:53–57. doi: 10.1093/pubmed/24.1.53. [DOI] [PubMed] [Google Scholar]
- E89.Fink P, Rosendal M, Toft T. Assessment and treatment of functional disorders in general practice: the extended reattribution and management model—an advanced educational program for nonpsychiatric doctors. Psychosomatics. 2002;43:93–131. doi: 10.1176/appi.psy.43.2.93. [DOI] [PubMed] [Google Scholar]
- E90.Bahrs O. Der Bilanzierungsdialog. Der Mensch. 2007;38:29–32. [Google Scholar]
- E91.Stephens G, Derry S, Moore RA. Paracetamol (acetaminophen) for acute treatment of episodic tension-type headache in adults. Cochrane Database Syst Rev. 2016;6 doi: 10.1002/14651858.CD011889.pub2. CD011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E92.Derry S, Wiffen PJ, Häuser W, et al. Oral nonsteroidal anti-inflammatory drugs for fibromyalgia in adults. Cochrane Database Syst Rev. 2017;3 doi: 10.1002/14651858.CD012332.pub2. CD012332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E93.Soares A, Andriolo RB, Atallah AN, da Silva EM. Botulinum toxin for myofascial pain syndromes in adults. Cochrane Database Syst Rev. 2014;7 doi: 10.1002/14651858.CD007533.pub3. CD007533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E94.Kleinstäuber M, Witthöft M, Steffanowski A, van Marwijk H, Hiller W, Lambert MJ. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev. 2014;11 doi: 10.1002/14651858.CD010628.pub2. CD010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E95.Walitt B, Klose P, Üçeyler N, Phillips T, Häuser W. Antipsychotics for fibromyalgia in adults. Cochrane Database Syst Rev. 2016;6 doi: 10.1002/14651858.CD011804.pub2. CD011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E96.Ford AC, Luthra P, Tack J, Boeckxstaens GE, Moayyedi P, Talley NJ. Efficacy of psychotropic drugs in functional dyspepsia: systematic review and meta-analysis. Gut. 2017;66:411–420. doi: 10.1136/gutjnl-2015-310721. [DOI] [PubMed] [Google Scholar]
- E97.Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109 doi: 10.1038/ajg.2014.195. [DOI] [PubMed] [Google Scholar]
- E98.Tiequn B, Guanqun C, Shuo Z. Therapeutic effects of lactobacillus in treating irritable bowel syndrome: a meta-analysis. Intern Med. 2015;54:243–249. doi: 10.2169/internalmedicine.54.2710. [DOI] [PubMed] [Google Scholar]
- E99.Furlan AD, Giraldo M, Baskwill A, Irvin E, Imamura M. Massage for low-back pain. Cochrane Database Syst Rev. 2015;9 doi: 10.1002/14651858.CD001929.pub3. CD001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E100.Naumann J, Sadaghiani C. Therapeutic benefit of balneotherapy and hydrotherapy in the management of fibromyalgia syndrome: a qualitative systematic review and meta-analysis of randomized controlled trials. Arthritis Res Ther. 2014;16 doi: 10.1186/ar4603. R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E101.Salazar AP, Stein C, Marchese RR, Plentz RD, Pagnussat AS. Electric stimulation for pain relief in patients with fibromyalgia: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20:15–25. [PubMed] [Google Scholar]
- E102.Müller A, Franke H, Resch KL, Fryer G. Effectiveness of osteopathic manipulative therapy for managing symptoms of irritable bowel syndrome: a systematic review. J Am Osteopath Assoc. 2014;114:470–479. doi: 10.7556/jaoa.2014.098. [DOI] [PubMed] [Google Scholar]
- E103.Martins WR, Blasczyk JC, Aparecida Furlan de Oliveira M, Lagoa Goncalves KF, Bonini-Rocha AC, Dugailly PM. Efficacy of musculoskeletal manual approach in the treatment of temporomandibular joint disorder: a systematic review with meta-analysis. Man Ther. 2016;21:10–17. doi: 10.1016/j.math.2015.06.009. [DOI] [PubMed] [Google Scholar]
- E104.Mesa-Jiménez JA, Lozano-López C, Angulo-Díaz-Parreño S, Rodríguez-Fernández ÁL, De-la-Hoz-Aizpurua JL, Fernández-de-Las-Peñas C. Multimodal manual therapy vs pharmacological care for management of tension type headache: a meta-analysis of randomized trials. Cephalalgia. 2015;35:1323–1332. doi: 10.1177/0333102415576226. [DOI] [PubMed] [Google Scholar]
- E105.Lam M, Galvin R, Curry P. Effectiveness of acupuncture for nonspecificchronic low back pain: a systematic review and meta-analysis. Spine (Phila Pa1976) 2013;38:2124–2138. doi: 10.1097/01.brs.0000435025.65564.b7. [DOI] [PubMed] [Google Scholar]
- E106.Ainpradub K, Sitthipornvorakul E, Janwantanakul P, van der Beek AJ. Effect of education on non-specific neck and low back pain: a meta-analysis of randomized controlled trials. Man Ther. 2016;22:31–41. doi: 10.1016/j.math.2015.10.012. [DOI] [PubMed] [Google Scholar]
- E107.Anheyer D, Haller H, Barth J, Lauche R, Dobos G, Cramer H. Mindfulness-based stress reduction for treating low back pain: a systematic review and meta-analysis. Ann Intern Med. 2017;166:799–807. doi: 10.7326/M16-1997. [DOI] [PubMed] [Google Scholar]
- E108.Searle A, Spink M, Ho A, Chuter V. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. 2015;29:1155–1167. doi: 10.1177/0269215515570379. [DOI] [PubMed] [Google Scholar]
- E109.Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014;9 doi: 10.1002/14651858.CD000963.pub3. CD000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E110.Waterschoot FP, Dijkstra PU, Hollak N, de Vries HJ, Geertzen JH, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain. 2014;155:179–189. doi: 10.1016/j.pain.2013.10.006. [DOI] [PubMed] [Google Scholar]
- E111.van der Feltz-Cornelis CM, van Os TW, van Marwijk HW, Leentjens AF. Effect of psychiatric consultation models in primary care. A systematic review and meta-analysis of randomized clinical trials. J Psychosom Res. 2010;68:521–533. doi: 10.1016/j.jpsychores.2009.10.012. [DOI] [PubMed] [Google Scholar]
- E112.Hoedeman R, Blankenstein AH, van der Feltz-Cornelis CM, Krol B, Stewart R, Groothoff JW. Consultation letters for medically unexplained physical symptoms in primary care. Cochrane Database Syst Rev. 2010;12 doi: 10.1002/14651858.CD006524.pub2. CD006524. [DOI] [PubMed] [Google Scholar]
- E113.Koelen JA, Houtveen JH, Abbass A, et al. Effectiveness of psychotherapy for severe somatoform disorder: meta-analysis. Br J Psychiatry. 2014;204:12–19. doi: 10.1192/bjp.bp.112.121830. [DOI] [PubMed] [Google Scholar]
- E114.Zech N, Hansen E, Bernardy K, Häuser W. Efficacy, acceptability and safety of guided imagery/hypnosis in fibromyalgia—a systematic review and meta-analysis of randomized controlled trials. Eur J Pain. 2017;21:217–227. doi: 10.1002/ejp.933. [DOI] [PubMed] [Google Scholar]
- E115.van den Bergh O, Witthöft M, Petersen S, Brown RJ. Symptoms and the body: taking the inferential leap. Neurosci Biobehav Rev. 2017;74(Pt A):185–203. doi: 10.1016/j.neubiorev.2017.01.015. [DOI] [PubMed] [Google Scholar]
- E116.Varinen A, Kosunen E, Mattila K, Koskela T, Sumanen M. The relationship between childhood adversities and fibromyalgia in the general population. J Psychosom Res. 2017;99:137–142. doi: 10.1016/j.jpsychores.2017.06.011. [DOI] [PubMed] [Google Scholar]
- E117.Guthrie E. Adaptation of the psychodynamic-interpersonal model to work with medically unexplained symptoms Psychodynamic interpersonal therapy: a conversational model. In: Barkham M, Guthrie E, Hardy GE, Margison F, editors. Sage. London: 2017. [Google Scholar]
- E118.Probst T, von Heymann F, Zaudig M, et al. Effektivität stationärer psychosomatischer Krankenhausbehandlung - Ergebnisse einer multizentrischen Katamnesestudie. Z Psychosom Med Psychother. 2009;55:409–420. doi: 10.13109/zptm.2009.55.4.409. [DOI] [PubMed] [Google Scholar]
- E119.Beutel ME, von Heymann F, Bleichner F, Tritt K, Hardt J. Wie wirksam ist psychosomatische Therapie bei somatoformen Störungen? Ergebnisse einer Multicenterstudie. Z Psychosom Med Psychother. 2014;60:17–24. [PubMed] [Google Scholar]