Abstract
The breakthrough discoveries of leptin and adiponectin more than two decades ago led to a widespread recognition of adipose tissue as an endocrine organ. Many more adipose tissue-secreted signaling mediators (adipokines) have been identified since then, and much has been learned about how adipose tissue communicates with other organs of the body to maintain systemic homeostasis. Beyond proteins, additional factors, such as lipids, metabolites, noncoding RNAs, and extracellular vesicles (EVs), released by adipose tissue participate in this process. Here, we review the diverse signaling mediators and mechanisms adipose tissue utilizes to relay information to other organs. We discuss recently identified adipokines (proteins, lipids, and metabolites) and briefly outline the contributions of noncoding RNAs and EVs to the ever-increasing complexities of adipose tissue inter-organ communication. We conclude by reflecting on central aspects of adipokine biology, namely, the contribution of distinct adipose tissue depots and cell types to adipokine secretion, the phenomenon of adipokine resistance, and the capacity of adipose tissue to act both as a source and sink of signaling mediators.
Keywords: angiopoietin, angiopoietin-like protein, bone morphogenic protein, chemerin, endotrophin, fibroblast growth factor 21, lipocalin 2, neuregulin 4, fatty acid esters of hydroxy fatty acids, lysophosphatidic acids, sphingolipids, uric acid, uridine, long noncoding ribonucleic acids, micro-ribonucleic acids, extracellular vesicles
THE ENDOCRINE ERA OF ADIPOSE TISSUE
The roles of white adipose tissue (WAT) in long-term energy storage, thermal insulation, and mechanical protection and of brown adipose tissue (BAT) in nonshivering thermogenesis have long been appreciated (1). The concept that adipose tissue could serve as an endocrine organ, however, was only shaped after the discovery of its two most characteristic secretory products, leptin and adiponectin.
Leptin, identified in 1994, is a protein primarily produced by mature adipocytes (2, 3). It signals through the long isoform of the leptin receptor (LEPRb) and exerts the majority of its effects acting on the brain (2, 4–6). Its circulating levels reflect the filling state of adipose tissue depots and thus relate directly to the body’s long-term energy stores (7, 8). The lowering of circulating leptin levels due to a reduction in adipose tissue mass triggers behavioral, metabolic, and endocrine responses that aim at replenishing and preserving the body’s fuel reserves (9, 10). Among these responses are an increase in energy intake, a decrease in energy expenditure, and a reduction or elimination of highly energy-demanding processes, such as reproduction and immune-related processes (9, 10).
Adiponectin, originally described in 1995 as “Acrp30” with additional reports following in 1996, is a protein exclusively produced by mature adipocytes (11–15). It forms low molecular weight trimers, intermediate molecular weight hexamers, and high molecular weight dodeca- to octadecamers (16). It signals through adiponectin receptor (AdipoR)1 and AdipoR2 and binds to the nonsignaling interacting protein, T-cadherin (15). It is found in circulation and critically involved in many signaling events from the adipocyte to other cell types and tissues (11). Its circulating levels are closely tied to the functional integrity of adipose tissue and decline with obesity (17, 18). Adiponectin functions as a powerful insulin sensitizer and suppressor of cell death and inflammation, directly promoting anti-diabetic and anti-atherosclerotic outcomes (16). It acts on the liver to decrease gluconeogenesis, on skeletal muscle to increase fatty acid oxidation, and on pancreatic β-cells and cardiac muscle cells as a key anti-lipotoxic agent, exerting many of these functions on the basis of its effects on sphingolipids (19–22).
Adiponectin and leptin are clearly the two most widely studied adipocyte-derived factors with nearly 50,000 combined citations in PubMed identified with the name of these two adipokines as key search terms. Many reviews cover them extensively, so we do not want to belabor these two adipokines in detail here. However, suffice it to say that much still remains to be learned about both of these factors. While they are unquestionably important, their detailed mechanisms of action at the level of their target cells and organs, the underlying systemic resistance to the effects of these hormones, and their mutual effects on each other are yet to be better understood.
ADIPOSE TISSUE-SECRETED SIGNALING MEDIATORS
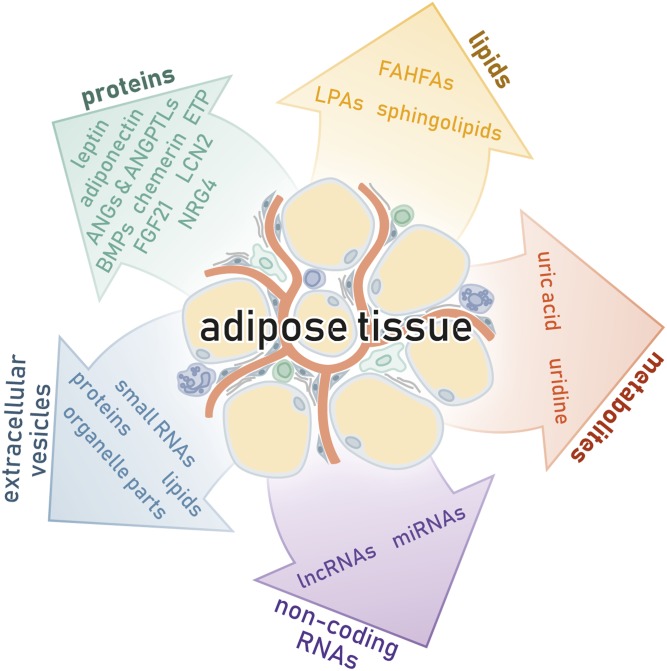
Screening endeavors undertaken in the wake of the discovery of leptin and adiponectin have revealed a vast spectrum of adipose tissue-secreted signaling mediators (see Fig. 1 and Table 1 for a compilation of central factors, some of which are portrayed in detail below) (23). The large diversity of adipose tissue secretory products may partially stem from the complex cellular composition of the tissue, which includes lipid-laden adipocytes, adipose tissue stromal cell populations of different adipogenic potentials, various immune cell populations, endothelial cells, pericytes, and neurons (24). While the term “adipokine” is commonly used to refer to adipose tissue-derived proteins exclusively, it has occasionally been used to refer to the entirety of signaling mediators secreted by adipose tissue, and it is this latter definition that will be applied here.
Fig. 1.
Adipose tissue is a highly dynamic secretory organ that employs a plethora of adipokines (proteins, lipids, metabolites), noncoding RNAs, and EVs to relay information to other organs of the body.
TABLE 1.
Collection of various adipose tissue-derived proteins, lipids, and metabolites with information on essential characteristics and several references for further reading
| Class | Name (Abbreviation) | Characteristics | References |
| Proteins | Angiotensin II (AII) | Extracellular, generated | (453–455, 456–467) |
| Generated from serine protease inhibitor A8/angiotensinogen (SERPINA8/AGT) by combined activity of renin or cathepsins and angiotensin-converting enzyme 1 (ACE1) or chymases | |||
| Signals through G protein-coupled angiotensin receptor (ANGTR)1 and ANGTR2 | |||
| Regulates adipose tissue stromal cell adipogenesis | |||
| Regulates adipose tissue thermogenesis | |||
| Regulates blood pressure | |||
| Regulates cardiac and vascular functions | |||
| Regulates energy expenditure | |||
| Regulates fluid homeostasis | |||
| Regulates glucose tolerance and insulin sensitivity | |||
| Regulates inflammation | |||
| Regulates WAT browning | |||
| May regulate body weight | |||
| Increases adipocyte lipid uptake and lipogenesis | |||
| Increases adipose tissue stromal cell proliferation | |||
| Decreases adipocyte lipolysis | |||
| Proteins | Adiponectin (ACRP30/ADIPOQ) | Extracellular, secreted | (15, 16, 22, 156, 321, 468–476) |
| May be intracellular | |||
| Signals through AdipoR1 and AdipoR2 | |||
| Binds T-cadherin | |||
| Improves glucose tolerance and insulin sensitivity | |||
| Maintains cardiac and vascular functions | |||
| Regulates angiogenesis | |||
| Regulates ceramide metabolism | |||
| May regulate cancer growth and metastasis | |||
| Increases adipocyte and skeletal muscle cell glucose uptake | |||
| Increases adipocyte lipogenesis | |||
| Increases adipose tissue stromal cell adipogenesis | |||
| Increases β-cell survival | |||
| Increases energy expenditure | |||
| Increases hepatocyte and skeletal muscle cell fatty acid oxidation | |||
| May increase β-cell glucose-stimulated insulin secretion | |||
| Decreases adipose tissue stromal cell proliferation | |||
| Decreases atherosclerosis | |||
| Decreases hepatocyte lipogenesis | |||
| Decreases inflammation | |||
| Decreases liver gluconeogenesis | |||
| Decreases liver steatosis | |||
| Proteins | Angiopoietin 1 (ANG1) | Extracellular, secreted | (27, 28, 30, 33, 477–486) |
| Signals through TIE2 and integrin αvβ5 | |||
| Improves glucose tolerance | |||
| Regulates atherosclerosis | |||
| Regulates cancer growth and metastasis | |||
| Regulates inflammation | |||
| Regulates vascular development and functions | |||
| Increases angiogenesis | |||
| Increases lymphangiogenesis | |||
| Increases wound healing | |||
| Decreases body weight gain | |||
| Proteins | Angiopoietin 2 (ANG2) | Extracellular, secreted | (27, 28, 31, 482, 483, 485, 487–494) |
| Signals through TIE2, integrin α3β1, and integrin α5β1 | |||
| Improves glucose tolerance and lipid metabolism | |||
| Regulates atherosclerosis | |||
| Regulates cancer growth and metastasis | |||
| Regulates inflammation | |||
| Regulates vascular development and functions | |||
| Increases angiogenesis | |||
| Increases lymphangiogenesis | |||
| Decreases fibrosis | |||
| Proteins | Angiopoietin-like protein 2 (ANGPTL2) | Intracellular and extracellular, secreted | (34, 35, 37, 38, 495–503) |
| Signals through LILRB2 and integrin α5β1 | |||
| Binds the G protein-coupled angiotensin receptor 1 (AGTR1) (intracellular) | |||
| Furthers glucose intolerance and insulin resistance (chronic exposure) | |||
| Regulates vascular functions | |||
| Regulates hematopoiesis | |||
| Increases atherosclerosis (chronic exposure) | |||
| Increases cancer development, growth, and metastasis | |||
| Increases inflammation | |||
| Increases tissue integrity (acute exposure) | |||
| Decreases tissue integrity (chronic exposure) | |||
| Proteins | Angiopoietin-like protein 4 (ANGPTL4) | Extracellular, secreted | (39, 43, 44, 53, 54) |
| Inhibits LPL and pancreatic lipase | |||
| Cleavage fragments may have signaling functions | |||
| May further glucose intolerance and insulin resistance | |||
| Regulates lipid trafficking | |||
| May increase atherosclerosis | |||
| May increase inflammation | |||
| Decreases lipoprotein breakdown in adipose tissue during fasting | |||
| Proteins | Angiopoietin-like protein 8 (ANGPTL8) | Extracellular, secreted | (39, 50–52) |
| Acts in concert with ANGPTL3 | |||
| Inhibits LPL and endothelial lipase | |||
| May further insulin resistance | |||
| Regulates lipid trafficking | |||
| Decreases lipoprotein breakdown in nonadipose tissues during feeding | |||
| Proteins | Apelin (APLN) | Extracellular, secreted | (504–506, 507–518) |
| Signals through G protein-coupled APLN receptor (APLNR) | |||
| Improves glucose tolerance and insulin sensitivity | |||
| Maintains cardiac functions | |||
| Regulates fluid homeostasis | |||
| May regulate bone mass | |||
| Increases adipocyte and skeletal muscle cell glucose uptake | |||
| Increases adipose tissue thermogenesis | |||
| Increases angiogenesis | |||
| Increases energy expenditure | |||
| Increases lymphangiogenesis | |||
| Increases skeletal muscle cell mitochondrial biogenesis and fatty acid oxidation | |||
| Increases white adipocyte browning | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Decreases blood pressure | |||
| Decreases body weight | |||
| May decrease adipocyte lipolysis | |||
| May decrease inflammation | |||
| May decrease liver steatosis | |||
| Proteins | Autotaxin (ATX) | Extracellular, secreted | (229, 236, 240–245) |
| Exhibits PLD activity | |||
| Generates most extracellular LPAs | |||
| Proteins | Bone morphogenic protein 2 (BMP2) | Extracellular, secreted | (59, 65, 67, 519–526) |
| Signals through ALK3 or ALK6 in complex with BMPR2, ACVR2a, or ACVR2b | |||
| Maintains bone functions | |||
| Regulates embryonic development | |||
| May regulate cancer development, growth, metastasis, and chemoresistance | |||
| May skew adipogenesis toward either white or brown phenotype | |||
| Increases adipose tissue stromal cell adipogenesis | |||
| Proteins | Bone morphogenic protein 3B (BMP3B) | Extracellular, secreted | (65, 79, 80, 527–529) |
| Signals through ALK4 in complex with ACVR2a or ACVR2b | |||
| Improves glucose tolerance and insulin sensitivity | |||
| Maintains neural functions | |||
| Regulates bone development | |||
| Increases activity | |||
| Increases BAT activity | |||
| Increases energy expenditure | |||
| Increases food intake | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Decreases body weight gain | |||
| May decrease bone mass | |||
| Proteins | Bone morphogenic protein 4 (BMP4) | Extracellular, secreted | (59, 65, 67, 68, 76–78, 530–534) |
| Signals through ALK3 or ALK6 in complex with BMPR2, ACVR2a, or ACVR2b | |||
| Improves glucose tolerance and insulin sensitivity | |||
| Regulates embryonic development | |||
| May regulate cancer development, growth, metastasis, and chemoresistance | |||
| May skew adipose tissue stromal cell adipogenesis toward either white or brown phenotype | |||
| Increase adipose tissue stromal cell adipogenesis | |||
| Increases angiogenesis | |||
| Increases BAT whitening | |||
| Increases energy expenditure | |||
| Increases food intake | |||
| Increases WAT browning | |||
| Increases WAT thermogenesis | |||
| Decreases body weight gain | |||
| Decreases brown adipocyte lipolysis | |||
| Decreases BAT thermogenesis | |||
| Proteins | Bone morphogenic protein 8B (BMP8B) | Extracellular, secreted | (65, 81, 82, 535–537) |
| Signals through ALK2, ALK3, or ALK6 in complex with BMPR2, ACVR2a, or ACVR2b | |||
| Maintains reproductive functions | |||
| Increases adipocyte lipolysis | |||
| Increases adipose tissue thermogenesis | |||
| Increases angiogenesis | |||
| Increases brain sympathetic output to adipose tissue | |||
| Increases energy expenditure | |||
| Increases WAT browning | |||
| May increase food intake | |||
| Decreases body weight gain | |||
| Proteins | C1q/TNF-related protein 3 (CTRP3) | Extracellular, secreted | (538, 539–550) |
| May inhibit signaling of bacterial lipopolysaccharide (LPS) through toll-like receptor 4 (TLR4) | |||
| May bind lysosomal-associated matrix protein 1 (LAMP1) and lysosome membrane protein 2 (LIMP2) | |||
| May improve insulin sensitivity | |||
| Maintains cardiac and reproductive functions | |||
| May maintain vascular functions | |||
| May regulate fibrosis | |||
| May regulate liver size | |||
| Increases angiogenesis | |||
| Increases cardiac muscle cell survival | |||
| May increase bone mass | |||
| May increase skeletal muscle stromal cell proliferation | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Decreases inflammation | |||
| Decreases liver gluconeogenesis | |||
| Decreases liver steatosis | |||
| May decrease skeletal muscle stromal cell myogenesis | |||
| Proteins | Chemerin | Extracellular, secreted | (92–94, 106, 109–116) |
| Signals through G protein-coupled CMKLR1 and GPR1 | |||
| Binds chemokine (C-C motif) receptor-like 2 (CCRL2) | |||
| Acts as immune cell chemoattractant | |||
| Impairs vascular functions | |||
| May regulate adipose tissue stromal cell adipogenesis | |||
| May regulate glucose tolerance and insulin sensitivity | |||
| Increases bone mass loss | |||
| Increases skeletal muscle cell insulin resistance | |||
| Proteins | Chemokine (C-C motif) ligand 2/monocyte chemoattractant protein 1 (CCL2/MCP1) | Extracellular, secreted | (551, 552–563) |
| Signals through G protein-coupled chemokine (C-C motif) receptor 2 (CCR2) | |||
| Binds Duffy antigen/chemokine receptor (DARC) | |||
| May further glucose intolerance and insulin resistance | |||
| Acts as immune cell chemoattractant | |||
| Regulates immune cell functions | |||
| May regulate body weight gain | |||
| Increases angiogenesis | |||
| Increases cancer growth and metastasis | |||
| Increases inflammation | |||
| Increases liver steatosis | |||
| Increases wound healing | |||
| Decreases adipocyte and skeletal muscle cell glucose uptake | |||
| Proteins | Complement factor D/adipsin (CFD) | Extracellular, secreted | (564, 565, 566–573) |
| Cleaves complement factor B (CFB) in complex with complement factor 3b (C3b), yielding the C3 convertase (C3bBb) of the alternative pathway of complement activation | |||
| Accelerates C3 cleavage, C3a and C3b generation, as well as C3a signaling through G protein-coupled C3a receptor (C3aR) | |||
| Improves glucose tolerance | |||
| Fulfills crucial functions in immune defense | |||
| Increases adipose tissue stromal cell adipogenesis | |||
| Increases β-cell glucose-stimulated insulin secretion | |||
| Increases cancer stemness and growth | |||
| Proteins | Dipeptidyl peptidase 4 (DPP4) | Extracellular, membrane-bound and secreted | (574, 575, 576–587) |
| Exhibits serine protease activity, processing a variety of other Proteins | |||
| Binds and/or signals through adenosine deaminase (ADA), caveolin 1 (CAV1), caspase recruitment domain-containing protein 11 (CARD11), dipeptidyl peptidase fibroblast activation protein α (FAPα), and others (membrane-bound) | |||
| Binds and/or signals through mannose-6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2R) and G protein-coupled protease-activated receptor 2 (PAR2) (secreted) | |||
| Binds different extracellular matrix components | |||
| Furthers glucose intolerance and insulin resistance | |||
| Alters gastrointestinal microbiome | |||
| Impairs β-cell functions | |||
| Impairs gastrointestinal functions | |||
| May impair cardiac and vascular functions | |||
| Regulates immune cell functions | |||
| May regulate bone mass | |||
| Increases adipose tissue stromal cell proliferation | |||
| Increases atherosclerosis | |||
| Increases body weight gain | |||
| Increases cancer development | |||
| Increases fibrosis | |||
| Increases inflammation | |||
| Increases liver steatosis | |||
| Decreases adipocyte, skeletal muscle cell, and vascular smooth muscle cell insulin sensitivity | |||
| Decreases adipose tissue thermogenesis | |||
| Decreases energy expenditure | |||
| Decreases white adipocyte browning | |||
| Proteins | endotrophin (ETP) | Extracellular, generated | (118, 123–129, 588) |
| C-terminal cleavage fragment of COL6A3 | |||
| Furthers glucose intolerance and insulin resistance | |||
| Increases angiogenesis | |||
| Increases cancer growth, metastasis, and chemoresistance | |||
| Increases fibrosis | |||
| Increases inflammation | |||
| Increases liver steatosis | |||
| May increase adipose tissue stromal cell adipogenesis | |||
| Decreases energy expenditure | |||
| May decrease adipocyte lipolysis | |||
| Proteins | Fatty acid binding protein 4 (FABP4) | Intracellular and extracellular, secreted | (589, 590, 591–602) |
| Binds diverse lipids | |||
| Binds hormonse-sensitive lipase (HSL), PPARγ, and keratin 1 (KRT1) (intracellular) | |||
| Furthers glucose intolerance and insulin resistance | |||
| May maintain brown adipocyte thermogenesis | |||
| Regulates immune cell functions | |||
| Regulates lipid trafficking | |||
| Regulates lipolysis | |||
| Increases angiogenesis | |||
| Increases atherosclerosis | |||
| Increases β-cell glucose-stimulated insulin secretion | |||
| Increases cancer growth and metastasis | |||
| Increases cardiac dysfunction | |||
| Increases inflammation | |||
| Increases liver steatosis | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Proteins | Fibroblast growth factor 21 (FGF21) | Extracellular, secreted | (130, 134–136, 140, 141, 147, 151–155, 158) |
| Signals through FGFR1c and FGFR3c in complex with β-klotho | |||
| Binds FGFR4 in complex with β-klotho | |||
| Improves glucose tolerance and insulin sensitivity (not in humans) | |||
| Regulates circadian rhythm | |||
| Regulates brain sympathetic output to different tissues | |||
| Increases adipose tissue glucose and fatty acid uptake, mitochondrial activity, and thermogenesis | |||
| Increases β-cell glucose-stimulated insulin secretion (acute exposure) | |||
| Increases bone mass loss | |||
| Increases energy expenditure | |||
| Increases hepatocyte fatty acid oxidation | |||
| Increases life span | |||
| Increases liver gluconeogenesis (acute exposure) | |||
| Decreases β-cell glucose-stimulated insulin secretion (chronic exposure) | |||
| Decreases body weight | |||
| Decreases bone mass | |||
| Decreases circulating triglycerides | |||
| Decreases food intake | |||
| Decreases growth | |||
| Decreases hepatocyte lipogenesis | |||
| Decreases liver gluconeogenesis (chronic exposure) | |||
| Decreases liver glycogenolysis | |||
| Decreases sugar and alcohol intake | |||
| Proteins | Intelectin 1/omentin (INTL1/OMT) | Extracellular, secreted | (603, 604–615) |
| Scarcely expressed in mouse adipose tissue | |||
| Binds bacterial glycans | |||
| Binds lactoferrin (LF) | |||
| May partake in bacterial surveillance | |||
| Maintains bone mass | |||
| Maintains cardiac and vascular functions | |||
| Increases adipocyte insulin sensitivity | |||
| Increases adipose tissue stromal cell proliferation and survival | |||
| May increase cancer cell death | |||
| Decreases angiogenesis | |||
| Decreases atherosclerosis | |||
| Decreases inflammation | |||
| May decrease cancer growth | |||
| Proteins | Interleukin 1β (IL1β) | Intracellular and extracellular, secreted or generated | (616–618, 619–630) |
| Generated from pro-IL1β by the NLRP1, NLRP3, NLR family CARD domain-containing 4 (NLRC4), and absent in melanoma 2 (AIM2) inflammasomes | |||
| Alternatively generated from pro-IL1β by various proteases such as proteinase 3 (PRTN3), granzyme A (GZMA), cathepsin G (CG), elastases, chymases, or chymotrypsin | |||
| Signals through IL1 receptor α (IL1Rα) in complex with IL1 receptor accessory protein (IL1RAP) | |||
| Binds IL1 receptor β (IL1Rβ) either alone or in complex with IL1RAP | |||
| Binds soluble IL1Rα | |||
| Binds soluble IL1β either alone or in complex with IL1RAP | |||
| Furthers glucose intolerance and insulin resistance | |||
| Impairs β-cell functions | |||
| Regulates immune cell functions | |||
| May regulate brain sympathetic output to different tissues | |||
| Increases activity | |||
| Increases adipocyte insulin resistance and lipolysis | |||
| Increases β-cell death | |||
| Increases body temperature | |||
| Increases BAT activity | |||
| Increases energy expenditure | |||
| Increases inflammation | |||
| Increases liver steatosis | |||
| May increase adipose tissue stromal cell proliferation | |||
| Decreases adipocyte glucose uptake | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Decreases body weight | |||
| May decrease adipose tissue lipid uptake | |||
| May decrease gastrointestinal lipid uptake | |||
| Proteins | Interleukin 4 (IL4) | Extracellular, secreted | (631, 632–643) |
| Signals through IL4 receptor α (IL4Rα) in complex with IL2 receptor γ (IL2Rγ) or IL13 receptor α1 (IL13Rα1) | |||
| Binds soluble IL4Rα | |||
| Improves glucose tolerance and insulin sensitivity | |||
| Skews adipose tissue stromal cell adipogenesis toward brown phenotype | |||
| Regulates adipocyte lipolysis | |||
| Regulates adipose tissue and skeletal muscle stromal cell adipogenesis | |||
| Regulates body weight gain | |||
| Regulates immune cell functions | |||
| Regulates inflammation | |||
| May regulate atherosclerosis | |||
| Increases WAT browning | |||
| May increase adipose tissue stromal cell proliferation | |||
| May increase energy expenditure | |||
| Proteins | Interleukin 6 (IL6) | Extracellular, secreted | (198, 644–654) |
| Signals through glycoprotein 130 (GP130) in complex with membrane-bound or soluble IL6 receptor (IL6R) | |||
| Binds soluble GP130 and soluble IL6R | |||
| Regulates α- and β-cell functions | |||
| Regulates body weight | |||
| Regulates glucose tolerance and insulin sensitivity | |||
| Regulates immune cell functions | |||
| Regulates inflammation | |||
| Regulates liver steatosis | |||
| Increases adipocyte lipolysis | |||
| Increases body temperature | |||
| Increases cancer development, growth, metastasis, and chemoresistance | |||
| Increases energy expenditure | |||
| Increases skeletal muscle cell fatty acid oxidation | |||
| Increases WAT browning | |||
| Decreases activity | |||
| Decreases food intake | |||
| Proteins | Interleukin 10 (IL10) | Extracellular, secreted | (558, 645, 650, 655, 656–664) |
| Signals through through IL10 receptor α (IL10Rα) in complex with IL10 receptor β (IL10Rβ) | |||
| Maintains cardiac functions | |||
| Regulates glucose tolerance and insulin sensitivity | |||
| Regulates immune cell functions | |||
| Regulates liver steatosis | |||
| May regulate body weight gain | |||
| May increase cancer stemness, growth, and chemoresistance | |||
| Decreases fibrosis | |||
| Decreases inflammation | |||
| May decrease adipose tissue stromal cell adipogenesis | |||
| May decrease adipose tissue thermogenesis | |||
| May decrease energy expenditure | |||
| May decrease WAT browning | |||
| Proteins | Leptin (LEP) | Extracellular, secreted | (5, 9, 665, 666, 667–677) |
| Signals through leptin receptor isoform b (LEPRb) | |||
| Binds short and soluble leptin receptor isoforms (e.g. LEPRa) | |||
| Informs brain on long-term energy stores | |||
| Regulates body weight gain | |||
| Regulates bone mass | |||
| Regulates brain sympathetic output to different tissues | |||
| Regulates food intake and energy expenditure | |||
| Regulates glucose tolerance and insulin sensitivity | |||
| Regulates immune cell functions | |||
| Regulates reproduction | |||
| May regulate body temperature | |||
| May regulate hematopoiesis | |||
| Increases adipocyte lipolysis | |||
| Increases adipocyte, hepatocyte, and skeletal muscle cell fatty acid oxidation | |||
| Increases angiogenesis | |||
| Increases BAT activity | |||
| Increases inflammation | |||
| Increases skeletal muscle cell glucose uptake | |||
| Increases wound healing | |||
| May increase adipose tissue stromal cell proliferation | |||
| May increase blood pressure | |||
| May increase WAT browning | |||
| Decreases adipocyte glucose uptake | |||
| Decreases adipocyte, hepatocyte, and skeletal muscle cell lipogenesis | |||
| Proteins | Lipocalin 2 (LCN2) | Intracellular and extracellular, secreted | (167, 173, 174, 178, 183–187, 190, 192–194) |
| Binds iron-chelating siderophores | |||
| Binds LCN2 receptor and LRP2 | |||
| Regulates intracellular iron stores | |||
| May regulate adipose tissue stromal cell adipogenesis | |||
| May regulate adipocyte glucose uptake | |||
| May regulate body weight gain | |||
| May regulate BAT activity | |||
| May regulate fibrosis | |||
| May regulate glucose tolerance and insulin sensitivity | |||
| May regulate liver steatosis | |||
| May regulate vascular functions | |||
| Proteins | Neuregulin 4 (NRG4) | Extracellular, membrane-bound and secreted | (200, 201, 203–205, 212, 213, 678, 679) |
| Signals through ErbB4 | |||
| Improves glucose tolerance and insulin sensitivity | |||
| Maintains neural functions | |||
| May regulate immune functions | |||
| Increases angiogenesis | |||
| May increase BAT activity | |||
| May increase hepatocyte survival | |||
| Decreases body weight gain | |||
| Decreases hepatocyte lipogenesis | |||
| Decreases inflammation | |||
| Decreases liver steatosis | |||
| May decrease fibrosis | |||
| Proteins | Nicotinamide phosphoribosyltransferase/visfatin (NAMPT) | Intracellular and extracellular, secreted | (680–682, 683–694) |
| Generates nicotinamide mononucleotide (NMN) for NAD synthesis (intracellular) | |||
| Acts as immune cell chemoattractant (extracellular) | |||
| Regulates body weight gain | |||
| Regulates food intake | |||
| Regulates glucose tolerance and insulin sensitivity | |||
| Regulates inflammation | |||
| Increases β-cell glucose-stimulated insulin secretion | |||
| Increases brown adipocyte thermogenesis | |||
| Increases cancer growth and chemoresistance | |||
| Increases immune cell survival | |||
| Increases physical activity | |||
| Decreases fibrosis | |||
| Decreases liver steatosis | |||
| Proteins | Resistin (RETN) | Extracellular, secreted | (695, 696–707) |
| May bind and/or signal through TLR4, cleaved decorin (cDCN), receptor tyrosine kinase-like orphan receptor 1 (ROR1), and adenylyl cyclase-associated protein 1 (CAP1) | |||
| Expressed in mouse adipocytes, but scarcely expressed in human adipocytes | |||
| May be expressed in human immune cells | |||
| Furthers glucose intolerance and insulin resistance (not in humans) | |||
| May regulate brain sympathetic output to different tissues | |||
| Increases adipocyte lipolysis | |||
| Increases angiogenesis | |||
| Increases atherosclerosis | |||
| Increases inflammation | |||
| May increase adipose tissue stromal cell proliferation | |||
| Decreases adipocyte and skeletal muscle cell glucose uptake | |||
| Decreases adipocyte insulin sensitivity | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Proteins | Retinol-binding protein 4 (RBP4) | Extracellular, secreted | (708, 709–720) |
| Binds retinol | |||
| Binds and signals through stimulated by retinoic acid 6 (STRA6) | |||
| Binds RBP4 receptor 2 (RBPR2) | |||
| Signals through TLR4 | |||
| May further glucose intolerance and insulin resistance | |||
| Regulates adipose tissue stromal cell adipogenesis | |||
| Regulates immune cell functions | |||
| Increases cancer stemness and growth | |||
| Increases inflammation | |||
| May increase blood pressure | |||
| May increase liver steatosis | |||
| May increase mitochondrial dysfunction | |||
| Decreases adipocyte insulin sensitivity | |||
| Proteins | Secreted frizzled-related protein 5 (SFRP5) | Extracellular, secreted | (721, 722–731) |
| Inhibits wingless-related integration site (WNT)5a, WNT5b, and WNT11 | |||
| May exhibit additional signaling capacities | |||
| May bind different extracellular matrix components | |||
| Maintains cardiac and vascular functions | |||
| May regulate adipocyte insulin sensitivity | |||
| May regulate adipocyte mitochondrial function | |||
| May regulate adipose tissue stromal cell adipogenesis | |||
| May regulate body weight gain | |||
| May regulate glucose tolerance and insulin sensitivity | |||
| Increases angiogenesis | |||
| Decreases β-cell proliferation | |||
| Decreases inflammation | |||
| Decreases liver steatosis and fibrosis | |||
| Proteins | Serine protease inhibitor A12/vaspin (SERPINA12/VASP) | Extracellular, secreted | (732, 733–744) |
| Inhibits kallikrein 7 (KLK7) | |||
| May inhibit acetylcholine esterase (AChE) | |||
| Signals through GRP78 in complex with DnaJ heat shock protein family member C1 (DNAJC1) and/or voltage-dependent anion channel (VDAC) | |||
| Binds different extracellular matrix components | |||
| Improves glucose tolerance and insulin sensitivity | |||
| Maintains vascular functions | |||
| Maintains β-cell functions | |||
| Increases adipose tissue stromal cell adipogenesis | |||
| Increases skeletal muscle cell glucose uptake and insulin sensitivity | |||
| Increases β-cell glucose-stimulated insulin secretion | |||
| May increase bone mass | |||
| Decreases atherosclerosis | |||
| Decreases food intake | |||
| Decreases ER stress | |||
| Decreases inflammation | |||
| Decreases liver steatosis | |||
| Proteins | Serine protease inhibitor E1/plasminogen activator inhibitor 1 (SERPINE1/PAI1) | Extracellular, secreted | (278, 745, 746, 747–757) |
| Inhibits tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) | |||
| Signals through LRP1 | |||
| Binds and signals through uPA in complex with uPA receptor (uPAR) and LRP1 | |||
| Binds vitronectin and inhibits its binding and signaling through integrin αVβ3, integrin αVβ5, and uPAR | |||
| May further glucose intolerance and insulin resistance | |||
| Maintains cellular senescence | |||
| Regulates angiogenesis | |||
| Regulates cancer growth and metastasis | |||
| Regulates cell migration | |||
| Regulates wound healing | |||
| May regulate adipose tissue stromal cell adipogenesis | |||
| May regulate bone mass | |||
| May regulate ceramide metabolism | |||
| Increases atherosclerosis | |||
| May increase body weight gain | |||
| May increase inflammation | |||
| Decreases fibrinolysis | |||
| Decreases hematopoiesis | |||
| Decreases life span | |||
| May decrease adipocyte glucose uptake | |||
| Proteins | Serine protease inhibitor F1/pigment epithelium-derived factor (SERPINF1/PEDF) | Extracellular, secreted | (758, 759, 760–771) |
| May be intracellular | |||
| No known protease inhibitory functions | |||
| Binds and/or signals through PEDF receptor/adipose tissue triglyceride lipase (PEDFR/ATGL), laminin receptor (LAMR), LRP6, and plexin domain-containing protein (PLXDC)1 and PLXDC2 | |||
| Inhibits cell surface F1-ATPase | |||
| May regulate PPARα (intracellular) | |||
| Binds different extracellular matrix components | |||
| Maintains neuronal functions | |||
| Regulates fibrosis | |||
| Regulates immune cell functions | |||
| Regulates inflammation | |||
| May regulate glucose tolerance and insulin sensitivity | |||
| Increases adipocyte, hepatocyte, and skeletal muscle cell lipolysis | |||
| Increases cancer cell death and differentiation | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Decreases angiogenesis | |||
| Decreases cancer growth and metastasis | |||
| Decreases liver steatosis | |||
| Proteins | Serum amyloid A3 (SAA3) | Extracellular, secreted | (772, 773–783) |
| Not expressed in humans | |||
| Signals through TLR2 and TLR4 | |||
| May bind to HDL | |||
| Acts as immune cell chemoattractant | |||
| May regulate immune cell functions | |||
| May increase body weight gain | |||
| May increase inflammation | |||
| May increase liver steatosis | |||
| Proteins | Transforming growth factor β (TGFβ) | Extracellular, secreted | (784–786, 787–793) |
| Signals through ALK1, ALK2, ALK3, or ALK5 in complex with TGFβ receptor 2 (TGFBR2) | |||
| Binds connective tissue growth factor (CTGF) | |||
| Binds different extracellular matrix components | |||
| Furthers glucose intolerance and insulin resistance | |||
| Increases adipose tissue stromal cell proliferation | |||
| Increases fibrosis | |||
| Increases inflammation | |||
| Increases liver steatosis | |||
| Decreases adipocyte fatty acid oxidation | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Decreases adipose tissue thermogenesis | |||
| Proteins | TNF ligand superfamily member 10/TNF-related apoptosis-inducing ligand (TNFSF10/TRAIL) | Extracellular, membrane-bound and secreted | (794, 795, 796–807) |
| Signals through TRAIL receptor (TRAILR)1 and TRAILR2 | |||
| Binds TRAILR3, TRAILR4, and osteoprotegerin (OPG) | |||
| Improves glucose tolerance and insulin sensitivity | |||
| Regulates adipocyte metabolism | |||
| Regulates immune cell functions | |||
| Increases adipose tissue stromal cell proliferation | |||
| Increases adipose tissue stromal cell and adipocyte inflammation | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Decreases atherosclerosis | |||
| Decreases body weight | |||
| Decreases liver steatosis | |||
| Decreases systemic inflammation | |||
| Proteins | TNF ligand superfamily member 2/TNFα (TNFSF2/TNFA) | Extracellular, membrane-bound and secreted | (802, 808–810, 811–821) |
| Signals through TNF receptor (TNFR)1 and TNFR2 | |||
| Furthers glucose intolerance and insulin resistance | |||
| Regulates immune cell functions | |||
| Increases adipocyte lipolysis | |||
| Increases adipose tissue stromal cell proliferation | |||
| Increases atherosclerosis | |||
| Increases body weight loss | |||
| Increases ER stress | |||
| Increases inflammation | |||
| Increases mitochondrial dysfunction | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Decreases adipose tissue thermogenesis | |||
| Proteins | TNF ligand superfamily member 6/Fas ligand (TNFSF6/FASL) | Extracellular, membrane-bound and secreted | (802, 822, 823–827) |
| Signals through FAS | |||
| Furthers glucose intolerance and insulin resistance | |||
| Regulates immune cell functions | |||
| Increases adipocyte insulin resistance | |||
| Increases adipose tissue stromal cell proliferation | |||
| Increases body weight | |||
| Increases brown adipocyte lipolysis | |||
| Increases inflammation | |||
| Increases liver steatosis | |||
| Increases mitochondrial dysfunction | |||
| Proteins | Vascular endothelial growth factor A (VEGFA) | Extracellular, secreted | (828, 829, 830–841) |
| Maybe intracellular | |||
| Signals through VEGF receptor (VEGFR)1 and VEGFR2 | |||
| May bind to neuropilin 1 (NRP1) | |||
| May bind different extracellular matrix components | |||
| Regulates glucose tolerance and insulin sensitivity | |||
| Regulates vascular permeability | |||
| May regulate adipose tissue stromal cell osteogenesis and adipogenesis | |||
| Increases adipose tissue stromal cell proliferation | |||
| Increases angiogenesis | |||
| Increases brown adipocyte mitochondrial function and survival | |||
| Increases energy expenditure | |||
| Increases vasculogenesis | |||
| Increases white adipocyte browning | |||
| Increases white adipocyte lipolysis | |||
| Increases WAT sympathetic innervation | |||
| Increases WAT vascularization | |||
| May increase inflammation | |||
| Proteins | Vascular endothelial growth factor D (VEGFD) | Extracellular, secreted | (828, 829, 842–845) |
| Signals through VEGFR2 and VEGFR3 | |||
| Acts as immune cell chemoattractant | |||
| Regulates glucose tolerance and insulin sensitivity | |||
| Regulates lymphangiogenesis | |||
| Regulates WAT inflammation | |||
| May regulate liver steatosis | |||
| May regulate vascular permeability | |||
| May increase angiogenesis | |||
| May increase vasculogenesis | |||
| Proteins | Xanthine oxidoreductase (XOR) | Intracellular and extracellular, secreted | (371, 372, 376, 379, 384, 403, 404, 405, 409–414) |
| Exhibits dehydrogenase and oxidase activities | |||
| Interconvertible dehydrogenase and oxidase forms (XDH and XO) | |||
| Generates uric acid | |||
| Can generate reactive oxygen and nitrogen species | |||
| Regulates adipose tissue stromal cell adipogenesis | |||
| Lipids | 12,13-Dihydroxy-9Z-octadecenoic acid (12,13-diHOME) | Intracellular and extracellular | (846–849) |
| Generated from linoleic acid by combined activity of cytochrome P450 oxidases (CYPs) and epoxide hydrolase (EH)1-4 | |||
| May act as peroxisome PPARγ ligand (intracellular) | |||
| Regulates immune cell functions | |||
| Increases brown adipocyte and skeletal muscle cell fatty acid uptake and oxidation | |||
| Increases BAT and skeletal muscle lipid uptake | |||
| Decreases atherosclerosis | |||
| Lipids | 2-Arachidonoylglycerol (2-AG) | Intracellular and extracellular | (850, 851, 852–863) |
| Generated from arachidonic acid (AA)-containing diacylglycerols (DAG) by DAG lipases (DAGL) | |||
| Signals through G protein-coupled cannabinoid receptor (CB)1 and CB2, GPR55, and transient receptor potential cation channel subfamily V member 1 (TRPV1) | |||
| Binds to FABP3, FABP5, and FABP7 | |||
| May act as PPARα and/or PPARγ ligand (intracellular) | |||
| Acts as immune cell chemoattractant | |||
| Regulates brain sympathetic output to different tissues | |||
| Regulates glucose tolerance and insulin sensitivity | |||
| Regulates immune cell functions | |||
| Regulates social and food reward | |||
| Increases adipocyte insulin sensitivity and glucose uptake | |||
| Increases adipose tissue stromal cell adipogenesis | |||
| Increases atherosclerosis | |||
| Increases β-cell glucose-stimulated insulin secretion | |||
| Increases body weight | |||
| Increases food intake | |||
| Increases gastrointestinal energy absorption | |||
| Increases liver steatosis | |||
| Decreases adipose tissue thermogenesis | |||
| Decreases energy expenditure | |||
| Decreases mitochondrial biogenesis | |||
| Decreases white adipocyte browning | |||
| Decreases WAT, liver, and skeletal muscle glycogenesis | |||
| Lipids | 4-Hydroxynonenal (4-HNE) | Intracellular and extracellular | (864–866, 867–878) |
| Generated from unsaturated lipid acyl chains by reactive oxygen species-mediated peroxidation followed by nonenzymatic decomposition | |||
| Strong electrophile that covalently modifies lipids, Proteins, and nucleic acids | |||
| May further glucose intolerance and insulin resistance | |||
| Increases apoptosis | |||
| Increases autophagy | |||
| Increases body weight gain | |||
| Increases ER stress | |||
| Increases mitochondrial dysfunction | |||
| Increases mitophagy | |||
| Increases oxidative stress | |||
| Decrease β-cell glucose-stimulated insulin secretion | |||
| Decreases adipose tissue and skeletal muscle insulin sensitivity | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Decreases adipose tissue stromal cell proliferation | |||
| Lipids | Ceramide-1-phosphates (C1Ps) | Intracellular and extracellular | (251, 296, 345, 879–886) |
| Generated from ceramides by CERK | |||
| Stimulate AA-releasing cytosolic PLA2α | |||
| Inhibit TNF-releasing TACE | |||
| Inhibit acid SMase | |||
| Bind to C1P transfer protein (CPTP) | |||
| Further glucose intolerance | |||
| Regulate immune cell functions | |||
| Regulate inflammation | |||
| Increase body weight gain | |||
| Increase inflammation | |||
| Lipids | Ceramides | Intracellular and extracellular | (251, 256, 261, 264, 268, 273, 290, 296, 297, 299, 301–304, 316) |
| Generated by multiple mechanisms, de novo synhesis and salvage | |||
| Stimulate PP1, PP2A, and PP2C | |||
| Stimulate PKCζ | |||
| Stimulate the NLRP3 inflammasome | |||
| Bind ceramide transfer protein (CERT) | |||
| Further glucose intolerance and insulin resistance | |||
| Increase cancer development | |||
| Increase ER stress | |||
| Increase inflammation | |||
| Increase liver steatosis | |||
| Increase mitochondrial dysfunction | |||
| Increase cell death (various cell types) | |||
| Decrease adipose tissue stromal cell adipogenesis | |||
| Decrease adipose tissue thermogenesis | |||
| Decrease β-cell glucose-stimulated insulin secretion | |||
| Decrease insulin sensitivity (various cell types) | |||
| Decrease WAT browning | |||
| Lipids | cis-Palmitoleic acid | Intracellular and extracellular | (311, 887–889, 890–900) |
| Generated from palmitate by stearoyl-CoA desaturase 1 (SCD1) | |||
| Alternatively generated from stearate or cis-oleate by desaturation and/or chain shortening | |||
| Inhibits SCD1 | |||
| Improves glucose tolerance and insulin sensitivity | |||
| Maintains cardiac and vascular functions | |||
| May regulate liver steatosis | |||
| Increases β-cell proliferation and glucose-stimulated insulin secretion | |||
| Increases hepatocyte and skeletal muscle cell insulin sensitivity | |||
| Increases hepatocyte, skeletal muscle cell, and β-cell survival | |||
| May increase adipocyte and skeletal muscle cell glucose uptake | |||
| May increase adipose tissue stromal cell proliferation and survival | |||
| May increase cancer growth | |||
| Decreases atherosclerosis | |||
| Decreases inflammation | |||
| Lipids | Glucosylceramides | Intracellular and extracellular | (251, 337–344) |
| Generated from ceramides by GCS | |||
| Bind pleckstrin homology domain-containing family A member 8 (PLEKHA8) | |||
| Substrate for complex glycosphingolipid synthesis | |||
| May further glucose intolerance and insulin resistance | |||
| May increase fibrosis | |||
| May increase inflammation | |||
| Lipids | Lysophosphatidic acids (LPAs) | Intracellular and extracellular | (223, 224, 226, 227, 229–231, 233, 236, 238, 239) |
| Generated by multiple mechanisms | |||
| Signal through G protein-coupled LPAR1–6 (extracellular) | |||
| May act as PPARγ ligands (intracellular) | |||
| Intermediates of glyceroplipid synthesis | |||
| Further glucose intolerance and insulin resistance | |||
| Increase adipose tissue stromal cell proliferation | |||
| Decrease adipose tissue stromal cell adipogenesis | |||
| Decrease β-cell glucose-stimulated insulin secretion | |||
| Lipids | Palmitic acid | Intracellular and extracellular | (628, 901–903, 904–914) |
| Taken up from ingested food (exogenous) | |||
| Also generated by multiple mechanisms (endogenous) | |||
| Signals through GPR40 | |||
| Signals through TLR4 (high exposure) | |||
| Also stimulates different PKC isoforms (e.g., PKCε and PKCθ) (likely indirect, high exposure) | |||
| Also stimulates PKR (likely indirect, high exposure) | |||
| Also stimulates the NLRP3 inflammasome (likely indirect, high exposure) | |||
| Binds diverse FABPs, fatty acid transport proteins (FATPs), and fatty acid translocase (FAT) | |||
| Affects lipid membrane properties (e.g., fluidity and permeability) | |||
| Prime substrate for ceramide synthesis | |||
| Substrate for energy generation | |||
| Substrate for structural component and signaling mediator synthesis | |||
| Regulates glucose tolerance and insulin sensitivity | |||
| Regulates immune cell functions | |||
| May regulate adipose tissue stromal cell proliferation and adipogenesis | |||
| May regulate atherosclerosis | |||
| May regulate body weight gain | |||
| May regulate energy expenditure | |||
| May regulate food intake | |||
| May regulate liver steatosis | |||
| Increases β-cell glucose-stimulated insulin secretion (low exposure) | |||
| Increases cell death (various cell types, high exposure) | |||
| Increases ceramide generation (high exposure) | |||
| Increases enteroendocrine cell hormone release (low exposure) | |||
| Increases ER stress (high exposure) | |||
| Increases inflammation (high exposure) | |||
| Increases mitochondrial dysfunction (high exposure) | |||
| Increases oxidative stress (high exposure) | |||
| Lipids | Palmitic acid ester of 5-hydroxystearic acid (5-PAHSA) | Intracellular and extracellular | (215–221, 222) |
| Produced by unknown mechanisms | |||
| May signal through GPR40 and GPR120 | |||
| May improve glucose tolerance and insulin sensitivity | |||
| May increase adipose tissue stromal cell adipogenesis | |||
| May increase adipocyte glucose uptake | |||
| May increase β-cell glucose-stimulated insulin secretion | |||
| May increase L-cell GLP1 secretion | |||
| May decrease inflammation | |||
| Lipids | Palmitic acid ester of 9-hydroxystearic acid (9-PAHSA) | Intracellular and extracellular | (215–221, 222) |
| Produced by unknown mechanisms | |||
| May signal through GPR40 and GPR120 | |||
| May improve glucose tolerance and insulin sensitivity | |||
| May increase adipose tissue stromal cell adipogenesis | |||
| May increase adipocyte glucose uptake | |||
| May increase β-cell glucose-stimulated insulin secretion | |||
| May increase L-cell GLP1 secretion | |||
| May decrease inflammation | |||
| Lipids | Prostaglandin E2 (PGE2) | Intracellular and extracellular | (915, 916–927) |
| Generated from AA by combined activity of cyclooxygenase (COX)1 and COX2 and PGE synthase (PGES)1, PGES2, or PGES3 | |||
| Signals through G protein-coupled PGE receptor (EP)1-4 | |||
| May improve glucose tolerance and insulin sensitivity | |||
| Regulates atherosclerosis | |||
| Regulates fibrosis | |||
| Regulates immune cell functions | |||
| Regulates inflammation | |||
| Regulates liver steatosis | |||
| May regulate lipid trafficking | |||
| Skews adipose tissue stromal cell adipogenesis toward brown phenotype | |||
| May increase activity | |||
| May increase BAT activity | |||
| May increase WAT browning | |||
| Decreases adipose tissue stromal cell adipogenesis | |||
| Decreases white adipocyte lipolysis | |||
| May decrease body weight gain | |||
| May decrease food intake | |||
| Lipids | Sphingomyelins | Intracellular and extracellular | (251, 262, 289, 331–334, 335, 336) |
| Generated from ceramides by SMSs | |||
| May regulate adipose tissue development | |||
| May regulate glucose tolerance and insulin sensitivity | |||
| May regulate liver steatosis | |||
| May regulate mitochondrial functions | |||
| Lipids | Sphingosine-1-phosphate (S1P) | Intracellular and extracellular | (251, 276, 296, 348–350, 353, 355–357, 360, 361, 363, 365) |
| Generated from sphingosine by sphingosine kinases | |||
| Signals through G protein-coupled S1PR1–5 | |||
| Also stimulates CIAP2 | |||
| Also stimulates TRAF2 | |||
| Also inhibits HDAC1 and HDAC2 | |||
| May regulate glucose tolerance and insulin sensitivity | |||
| May regulate vascular functions | |||
| May regulate liver steatosis | |||
| May increase adipose tissue stromal cell proliferation | |||
| May increase β-cell glucose-stimulated insulin secretion | |||
| May increase hepatocyte and skeletal muscle cell glucose uptake | |||
| May increase hepatocyte and β-cell survival | |||
| May increase hepatocyte lipogenesis | |||
| May increase inflammation | |||
| May decrease adipose tissue stromal cell adipogenesis | |||
| Metabolites | Uric acid | Intracellular and extracellular | (371, 372, 376, 379, 385, 393, 395–402) |
| Product of purine base degradation | |||
| Acts as anti-oxidant (extracellular) | |||
| Acts as pro-oxidant (intracellular) | |||
| Stimulates the NLRP3 inflammasome (intracellular) | |||
| Stimulates NOX (intracellular) | |||
| Furthers glucose intolerance and insulin resistance | |||
| Impairs vascular and kidney functions | |||
| Increases blood pressure | |||
| Increases inflammation | |||
| Increases liver steatosis | |||
| Increases mitochondrial dysfunction | |||
| Metabolites | Uridine | Intracellular and extracellular | (418, 419, 421, 424–429) |
| May require metabolism for signaling | |||
| Substrate for RNA and DNA synthesis | |||
| Substrate for glycogen deposition | |||
| Substrate for protein and lipid glycosylation | |||
| Improves glucose tolerance (acute exposure) | |||
| May regulate glucose tolerance and insulin sensitivity (chronic exposure) | |||
| Essential for fasting-induced decrease in body temperature (acute exposure) | |||
| Increases body weight gain (chronic exposure) | |||
| May increase body temperature (low concentration exposure) | |||
| May increase cancer development (chronic exposure) | |||
| May increase liver steatosis (chronic exposure) | |||
| Decreases body temperature (high concentration) | |||
| Decreases energy expenditure (acute exposure) |
References in bold indicate reviews.
Adipose tissue forms circumscribed depots in the body that differ in their cellular composition and character (24, 25). Whereas dermal, subcutaneous, and visceral depots exist in both humans and mice, the occurrence of depots in the bone marrow, skeletal muscle, and pancreas depends on several factors, including species, sex, age, and nutritional state (25). While the cellular differences between these adipose tissue depots immediately suggest quantitatively and possibly even qualitatively distinct patterns of adipokine secretion, thorough assessments of depot-specific production have been carried out for only few adipose tissue-derived factors.
Adipose tissue is highly dynamic and able to respond to changes in nutritional state (e.g., during feeding or fasting or with obesity) with acute and chronic adjustments in both its metabolism and cellularity (26). These metabolic and cellular adjustments are usually accompanied by pronounced shifts in adipokine secretion with immediate effects on systemic homeostasis (26). With obesity, such shifts in adipokine secretion may directly contribute to the development of insulin resistance, hepatic steatosis, type 2 diabetes, and cardiovascular disease (26).
PROTEINS
Angiopoietins and angiopoietin-like proteins
The family of angiopoietins (ANGs) and ANG-like proteins (ANGPTLs) consists of several structurally similar but functionally distinct proteins.
ANG1 and ANG2 regulate angiogenesis and vascular function and exert their effects by signaling through the tyrosine kinase with Ig and epidermal growth factor (EGF) homology domains 2 (TIE2) expressed by endothelial cells and certain populations of monocytes and macrophages, as well as integrins αvβ5, α3β1, and α3β1 expressed by a variety of cells (27, 28). Obesity and fasting decrease ANG1 and ANG2 expression in WAT, while cold exposure increases ANG2 expression in BAT (29–31). Overexpression of ANG1 from injected plasmid DNA slows the body weight gain in obese leptin-deficient ob/ob mice, whereas overexpression of a stabilized ANG1 variant from a viral vector reduces diabetic nephropathy and improves glucose tolerance in obese leptin receptor-deficient db/db mice (30, 32, 33). Inducing adipocyte-specific overexpression of ANG2 in mice elicits increased WAT angiogenesis and an anti-inflammatory secretion profile, offering protection from high-fat diet-induced obesity and improving glucose and lipid metabolism (31). Treating mice with an ANG2-neutralizing antibody conversely decreases WAT angiogenesis, increases WAT inflammation and fibrosis, and results in metabolic deterioration (31). ANG1 and ANG2 thus appear to have beneficial effects on systemic metabolism.
ANGPTL2 also affects vascular function, but does so in a TIE2-independent fashion by engaging integrin α5β1 and the leukocyte Ig-like receptor B2 (LILRB2) (34). ANGPTL2 expression in WAT and BAT is increased with hypoxia, ER stress, and obesity (35, 36). Its circulating levels correlate positively with adiposity and markers of inflammation and insulin resistance (35, 36). In mice, endothelial cell-specific overexpression of ANGPTL2 results in vascular dysfunction and facilitates vascular inflammation and atherosclerosis when combined with ApoE deficiency, whereas adipocyte-specific overexpression causes increased WAT inflammation, glucose intolerance, and insulin resistance (35, 37). ANGPTL2-deficient mice, in turn, exhibit improved insulin sensitivity and are protected from high-fat diet-induced metabolic and vascular deterioration (35, 37, 38). ANGPTL2 thus has detrimental effects on systemic metabolism, at least under the conditions tested.
ANGPTL3, ANGPTL4, and ANGPTL8 regulate triglyceride trafficking and metabolism (39). ANGPTL3 and ANGPTL8 act in concert to inhibit LPL and endothelial lipase, while ANGPTL4 acts alone to inhibit LPL and pancreatic lipase (39–41). ANGPTL3 and ANGPTL4 also undergo proteolytic cleavage, generating C-terminal fragments that may exert alternative signaling functions (42–44). ANGPTL3 is primarily produced by the liver, ANGPTL4 primarily by WAT and BAT, and ANGPTL8 by WAT and BAT as well as the liver (45–47). Fasting increases ANGPTL4 expression in WAT and BAT, suppressing local LPL activity and thus hydrolytic release of fatty acids from triglyceride-rich lipoproteins, redirecting them to other energy-demanding tissues (47). Conversely, upon feeding, ANGPTL3 and ANGPTL8 mediate the suppression of lipases in energy-demanding tissues, allowing white and brown adipocytes to replenish their lipid reserves (39). ANGPTL3- and ANGPTL8-deficient mice display improved triglyceride clearance, but no or only slight improvements in insulin sensitivity, even upon high-fat challenge (48–52). In line with its role in redirecting triglyceride-rich lipoproteins from WAT and BAT to other organs, mice lacking ANGPTL4 exhibit increased fatty acid uptake into WAT during fasting (47). Adipocyte-specific deletion of ANGPTL4 in mice improves triglyceride clearance and glucose tolerance with increased triglyceride uptake into WAT, BAT, and liver (53). In the setting of a high-fat diet, adipocyte-specific deletion of ANGPTL4 improves glucose tolerance and insulin sensitivity, while curbing inflammation and atherosclerosis (53). Specific overexpression of ANGPTL4 in adipocytes, in turn, causes dyslipidemia and exacerbates the detrimental metabolic effects of a high-fat diet (54). Similarly, humans harboring loss-of-function alleles of ANGPTL3, ANGPTL4, or ANGPTL8 display decreased triglyceride levels and increased triglyceride clearance (55–58).
Bone morphogenic proteins
The bone morphogenic protein (BMP) family belongs to the transforming growth factor β (TGFβ) superfamily, and its members have central functions in the development and maintenance of many tissues (59). They signal through complexes of one of seven different type I receptors, the activin receptor-like kinases 1–7 (ALK1–7), with one of three different type II receptors, the BMP receptor 2 (BMPR2) and the activin receptor (ACVR)2a and ACVR2b, that are expressed by a wide range of cells (59). In mice, the specific deletion of ALK3 in brown adipocyte progenitors impairs BAT formation, while its deletion in mature white adipocytes alleviates high-fat diet-induced WAT inflammation and insulin resistance (60, 61).
BMP2 and BMP4 regulate the commitment and differentiation of adipose tissue stromal cells and the maintenance of adipocytes. They signal through ALK3 or ALK6 in conjunction with BMPR2, ACVR2a, or ACVR2b (62–65). BMP2 and BMP4 are expressed in WAT and BAT, and the expression of BMP4 correlates positively with adiposity and adipocyte size (66–68). Both promote the commitment of adipose tissue stromal cells to the adipogenic lineage, which involves the repression of the anti-adipogenic zinc finger protein 521 (ZFP521) and activation of the pro-adipogenic zinc finger protein 423 (ZFP423) (69–73). They also appear to skew adipogenesis toward either a white or brown adipocyte phenotype, although in vitro experiments have been unsuccessful to determine what combination of factors (e.g., dose, time, and duration of treatment, or cell type) determines the exact outcome (66, 68, 74–77). Adipocyte-specific overexpression of BMP4 in mice results in decreased WAT and increased BAT weights, increased WAT angiogenesis and browning, BAT whitening, yet overall increased energy expenditure and improved glucose tolerance and insulin sensitivity (66, 78). Intriguingly, the specific deletion of BMP4 in adipocytes causes increased WAT and BAT weights, decreased WAT angiogenesis, and BAT whitening, as well as disturbed glucose tolerance and insulin sensitivity (66, 78). Similar effects are observed using viral vectors to overexpress BMP4 either systemically or locally in BAT (68, 77).
Another member of the BMP family that is implicated in the regulation of adipose tissue stromal cell adipogenic differentiation is BMP3B. It signals through ALK4 and ACVR2a or ACVR2b, and its production in WAT and BAT increases with obesity (65, 79). Suppressing BMP3B expression in adipose tissue stromal cells increases their adipogenic potential, while overexpressing BMP3B decreases it (79). On a high-fat diet, mice with adipocyte-specific overexpression of BMP3B display decreased WAT weight and adipocyte size, increased BAT thermogenic marker expression, food consumption, activity, and energy expenditure, and improved glucose tolerance and insulin sensitivity (80).
BMP8B is a BMP family member that may particularly regulate BAT function. It signals through a combination of ALK2, ALK3, or ALK6 and BMPR2, ACVR2a, or ACVR2b (65). It is expressed in WAT and BAT, and its expression in BAT is decreased during fasting and increased during feeding and with obesity, as well as upon cold exposure (67, 81). Mice lacking BMP8B display decreased body temperature and impaired cold-induced thermogenesis with reduced oxygen consumption and BAT sympathetic input (81). On a high-fat diet, these mice furthermore exhibit increased body weight gain, but also decreased food intake (81). Apart from directly acting on adipocytes to increase their lipolytic capacity, BMP8B augments the vessel density and neuronal innervation of adipose tissue and prompts the brain to increase the sympathetic output to it (81, 82).
BMP2, BMP3B, BMP4, and BMP8B thus appear to have favorable effects on metabolic homeostasis.
While BMP7 has also been described to have a role in the regulation of BAT formation and function, it has, to our knowledge, never been unambiguously established that it is produced by adipose tissue (67, 74, 76, 83–86).
Chemerin
Chemerin acts as a chemokine and is produced as a pro-protein that undergoes stepwise C-terminal proteolytic processing to generate multiple variants differing greatly in their respective activity (87–90). Chemerin signals through the chemokine-like receptor 1 (CMKLR1) and the G protein-coupled receptor (GPR)1 and also binds to the nonsignaling C-C chemokine receptor-like 2 (CCRL2), all of which are expressed by a variety of cells (91–94). It circulates mostly in its pro-form, and its total circulating levels correlate positively with age, adiposity, triglycerides, and blood pressure (95–102). Apart from its role in immune cell chemotaxis, in vitro experiments implicate chemerin to act on endothelial and vascular smooth muscle cells, promoting vascular dysfunction on skeletal muscle cells fueling insulin resistance and on osteoclasts instigating bone resorption (98, 103–108). A direct action of chemerin on adipose tissue stromal cell adipogenic differentiation or on adipocyte function has remained controversial though (91, 109–111). Chemerin-deficient mice display increased skeletal muscle but decreased WAT insulin sensitivity, as well as mild glucose intolerance; whereas mice overexpressing chemerin specifically in the liver exhibit improved glucose tolerance (112). In contrast, treatment with chemerin exacerbates the obesity-associated glucose intolerance in ob/ob mice, db/db mice, and mice fed a high-fat diet (113). The deletion of CMKLR1 was reported to either not affect or, in another study, decrease glucose tolerance in mice on regular or high-fat diets, while the deletion of GPR1 decreases glucose tolerance in mice on a high-fat diet (110, 111, 114–116). More advanced mouse models may need to be used to clarify the effects of this signaling axis on metabolic homeostasis, such as overexpression or deletion of chemerin or its receptors in a time- and cell type-controlled manner. Such approaches are essential to effectively deconvolute developmental effects from effects on mature cells and tissues (38, 117).
Endotrophin
Endotrophin (ETP) constitutes a C-terminal cleavage fragment of the collagen VI α3 chain (COL6A3) that is released from mature collagen VI (COL6) following secretion (118). While diverse integrins and the chondroitin sulfate proteoglycan 4 (CSPG4) may act as receptors for COL6, a specific receptor for ETP has not yet been identified (118, 119). ETP levels are strongly associated with adipose tissue dysfunction. Similarly, COL6A3 expression in WAT correlates positively with adiposity and with markers of WAT inflammation and is decreased upon anti-diabetic thiazolidinedione treatment (120, 121). Following this pattern, the circulating ETP levels correlate positively with adiposity and markers of insulin resistance, and actually predict the therapeutic response to thiazolidinedione treatment (121). Adipocytes have the unique ability to support the growth of breast cancer cells not only in vitro but also in vivo in the local microenvironment of the mammary gland. COL6A3-derived ETP was singled out as one of the key adipokines involved in this process (122, 123). Studies in the mouse mammary tumor virus/polyomavirus middle T antigen (MMTV-PyMT) model of breast cancer highlighted ETP as a major driver of tumor growth, metastasis formation, and chemoresistance (123–125). In MMTV-PyMT mice, functional elimination of COL6 or treatment with an ETP-neutralizing antibody or with thiazolidinediones decreases tumor growth, metastasis, and chemoresistance (123–125). Mammary epithelial cell-specific overexpression of ETP, in turn, increases tumor inflammation, angiogenesis, and fibrosis, while it also decreases tumor hypoxia and promotes tumor metastasis by initiating epithelial-mesenchymal transition (123–125). Intact TGFβ signaling is required for ETP’s effects on tumor epithelial-mesenchymal transition and is partially required for its effect on tumor fibrosis (124). It is, however, not required for its effects on inflammation and angiogenesis (124). The negative impact of ETP on tumor progression and chemoresistance is in fact highly relevant for human breast cancer as well (126). ETP has more recently also been demonstrated to aggravate the inflammatory and fibrotic consequences of liver damage and advance the development of liver cancer (127). COL6A3 and ETP, moreover, act as drivers of metabolic deterioration in obesity (128). COL6-deficient ob/ob mice and mice fed a high-fat diet exhibit increased WAT adipocyte size and decreased WAT inflammation and liver steatosis, as well as improved triglyceride clearance, glucose tolerance, and insulin sensitivity (128). Consistent with ETP being the key constituent of COL6, adipocyte-specific overexpression of ETP aggravates WAT inflammation and fibrosis, enhances dyslipidemia, liver steatosis, and impaired glucose tolerance and insulin sensitivity in mice fed a high-fat diet, while antibody neutralization of ETP results in the opposite effects (129). ETP thus exerts unfavorable effects on systemic metabolism.
Fibroblast growth factor 21
Fibroblast growth factor (FGF)15/19, FGF21, and FGF23 form the endocrine subgroup of the FGF family (130). They generally have a low heparin- and heparan sulfate-binding capacity, allowing them to leave their place of production and enter circulation (130). FGF21 signals through complexes of FGF receptor (FGFR)1c or FGFR3c with β-klotho as a coreceptor, and binds to, but does not signal through, complexes of FGFR4 with β-klotho (131–133). FGF21 is primarily produced by the liver, but is also expressed in WAT, BAT, and the brain, and possibly skeletal muscle, cardiac muscle, and the pancreas [reviewed in (130)]. Under most conditions, circulating FGF21 primarily derives from the liver where its production increases upon fasting and exercise as well as with high carbohydrate or low protein intake (130). Possible extra-hepatic contributions to the circulating FGF21 levels may occur from BAT upon cold exposure or from skeletal and cardiac muscle upon disturbances of cellular metabolism or mitochondrial function (130). The exact contributions of WAT to circulating pools of FGF21 remain to be clarified. Circulating FGF21 levels are increased with obesity, lipodystrophy, and pancreatitis (130). FGF21 has been extensively studied in mice, monkeys, and humans. Its main effects may relate to decreasing body weight (134–139), sugar and alcohol consumption (140, 141), circulating triglycerides and insulin (134–139), and bone mass (139, 142, 143), while in parallel increasing WAT and BAT glucose uptake, mitochondrial activity, and thermogenesis (136, 144–154) as well as circulating adiponectin (138, 139, 155–157). FGF21 also decreases circulating glucose and improves glucose tolerance and insulin sensitivity in mice (134–136), but may not do so in non-human primates and humans (137–139). Effects on dyslipidemia seem to be preserved in all cases. While FGF21 may exert many of its effects by direct action on the brain, local effects on WAT and BAT nonetheless occur and could be physiologically relevant (140, 141, 147, 150, 158–161). Direct FGF21 signaling was reported to increase white and brown adipocyte glucose uptake (134, 153, 162, 163), thermogenic marker expression (144, 146, 150), and adiponectin secretion (153, 155–157), decrease white adipocyte lipolysis (153, 164), and promote white adipocyte-initiated cold-induced WAT beiging (154), partly through autocrine and paracrine effects of adipocyte-produced FGF21 (146, 154, 157). Other studies failed to demonstrate such effects of direct FGF21 signaling or adipocyte-produced FGF21 (148, 153, 165, 166). Adipocyte-specific deletions of either FGFR1 or β-klotho abolish FGF21’s acute effects on glucose tolerance and insulin sensitivity in mice (137, 145, 153, 155, 161). Adiponectin has been identified as a crucial mediator of FGF21’s glucoregulatory actions (156, 157). We had proposed a direct linear relationship between the activation of PPARγ by thiazolidinediones, local production of FGF21, and local production as well as systemic release of adiponectin, eventually resulting in an effective reduction in blood and tissue ceramide levels with associated improvements in insulin sensitivity (156). It may thus be the absence of the FGF21-triggered adiponectin surge that explains how defects in adipose tissue FGF21 signaling impact its effects on glucose tolerance and insulin sensitivity. Taken together, FGF21 has mostly beneficial effects on systemic metabolism, some of which may, however, not fully translate from rodents to man.
Lipocalin 2
The lipocalin (LCN) family encompasses several structurally similar proteins that bind and transport small hydrophobic molecules, such as retinol, fatty acids, and steroids (167). LCN2 binds iron-chelating siderophores produced by bacterial and mammalian cells, including 2,3-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, and catechol (168–172). LCN2 binds to the LCN2 receptor (LCN2R) and the LDL receptor-related protein (LRP)2, which either increases or decreases intracellular iron stores depending on whether LCN2 is loaded with iron or not (168–172). Human LCN2 can also form covalent homodimers as well as heterodimers with matrix metallopeptidase 9 (MMP9), while murine LCN2 lacks the cysteine residue required for these interactions (173). The circulating LCN levels correlate positively with adiposity, markers of inflammation, and markers of insulin resistance (174–182). Studies with LCN2-deficient mice on either a regular or high-fat diet yielded variable results in that these mice were reported to have increased, decreased, or unchanged body weight gain, altered WAT, BAT, and endothelial cell function, cold intolerance, liver steatosis, and improved, worsened, or unchanged glucose tolerance and insulin sensitivity (178, 183–191). Studies involving either the overexpression of LCN2 or treatment with LCN2 have equally failed to paint a clearer picture of LCN2’s effects on adipose tissue stromal cell adipogenic differentiation, adipocyte function, and metabolic homeostasis (167, 174, 182, 184, 189–194). Surprisingly, despite the central role that iron plays in adipocyte function, the vast majority of these studies on LCN2 did not address iron homeostasis (167, 174, 182, 184, 189–196). As in the case of chemerin, the use of more advanced mouse models enabling an inducible tissue-specific overexpression or deletion of LCN2 or its receptors may be required to refine our assessment of LCN2’s effects on systemic metabolism. We should not be surprised by the wide array of effects reported. This range of phenotypes seen under different conditions is characteristic of what has been observed for many factors involved in inflammatory responses, where beneficial and detrimental effects are in a tug of war, and the net effects differ between acute and chronically challenged states (197–199).
Neuregulin 4
The neuregulin (NRG) family belongs to the EGF superfamily and its members are mostly known for their functions in the development and maintenance of the nervous system (200). Akin to other NRGs, NRG4 is produced as a transmembrane pro-protein that undergoes N-terminal proteolytic processing to release a soluble ligand (201). It signals through the EGF receptor 4 (ErbB4) that is expressed by a wide range of cells (200, 201). NRG4 is expressed in WAT and BAT where its production increases upon cold exposure and decreases with obesity (201–205). Its circulating levels were reported to correlate positively, negatively, or not at all with adiposity and markers of insulin resistance (206–211), yielding a rather unclear picture of its behavior. NRG4-deficient mice fed a high-fat diet display increased body weight gain, decreased WAT and BAT vessel density, increased WAT inflammation, liver steatosis, and impaired glucose tolerance and insulin sensitivity (201, 204, 205). While similar effects occur in high-fat-challenged ErbB4-deficient mice, opposite effects can be achieved by adipocyte- or hepatocyte-specific overexpression of NRG4 (201, 203, 204, 212, 213). This argues for beneficial effects of NRG4 on metabolic homeostasis. Of note though, humans harboring loss-of-function alleles of NRG4 display reduced to nearly absent fasting C-peptide levels, but no apparent alterations of glucose homeostasis, calling for further studies addressing the translatability of above findings (214).
LIPIDS
Fatty acid esters of hydroxy fatty acids
Fatty acid esters of hydroxy fatty acids (FAHFAs) are produced by still poorly understood enzymatic and nonenzymatic processes (215, 216). Differences in acyl chain length, saturation, and hydroxylation of the constituent fatty acids allow for the generation of more than a hundred distinct FAHFA species of which palmitic acid esters of 5- and 9-hydroxystearic acid (5-PAHSA and 9-PAHSA) are the best studied ones (215, 216). 9-PAHSA and possibly also 5-PAHSA signal though the GPR40 and GPR120 expressed by a variety of cells (215, 217, 218). They are produced by BAT and WAT where their production increases with glucose uptake, de novo lipogenesis, and possibly lipid oxidation, and they may be found in food (215, 216, 219, 220). Low circulating 5-PAHSA levels are moreover associated with markers of insulin resistance (215). Administration of 5- and 9-PAHSA to lean or obese mice increases glucagon-like peptide 1 (GLP1) and insulin secretion, decreases circulating glucose levels, and improves glucose tolerance and insulin sensitivity (215, 218). 5-PAHSA and 9-PAHSA may directly act on adipose tissue stromal cells to promote adipogenic differentiation, and in adipocytes to increase insulin-stimulated glucose uptake, in L-cells to increase GLP1 secretion, in β-cells to increase glucose-stimulated insulin secretion, and in macrophages to decrease activation and pro-inflammatory cytokine release (215, 219–221). Of note though, another study featuring both in vitro and in vivo experiments was unable to confirm any of the above-mentioned effects of 5- and 9-PAHSA (217). Whether central aspects of the experimental setups used by individual studies may have contributed to different outcomes remains to be addressed though (222).
Lysophosphatidic acids
Lysophosphatidic acids (LPAs) consist of a glycerol backbone, a phosphate group, and an ester-bound acyl chain of differing length and saturation (223). They are generated both intra- and extracellularly and their circulating levels increase with obesity (223, 224). They can be produced by acylation of glycerol 3-phosphate by glycerol-3-phosphate acyltransferases (GPATs), phosphorylation of monoacylglycerol by acylglycerol kinases (AGKs), head group modification of other lysophospholipids involving phospholipase (PL)D activity, or deacylation of phosphatidic acids involving PLA1 or PLA2 activity (223). LPAs can subsequently be degraded by deacylation involving PLA1 or PLA2 activity, dephosphorylation by lipid phosphate phosphatases (LPPs), or acylation by acylglycerol-3-phosphate acyltransferases (AGPATs) (223). Intracellular LPAs are crucial intermediates of glycerolipid synthesis and may possibly function as endogenous PPARγ ligands, while extracellular LPAs act as lipid mediators signaling through six widely expressed G protein-coupled LPA receptors (LPAR1–6) (223). Administration of LPAs to mice diminishes glucose-stimulated insulin secretion and glucose tolerance (224). LPAs may also directly increase adipose tissue stromal cell proliferation, decrease adipose tissue stromal cell adipogenic differentiation by downregulation of PPARγ2, increase hepatocyte glycogenolysis, and decrease β-cell glucose-stimulated insulin secretion (224–236). Mice deficient in LPAR1 display pronounced developmental defects and delays with a reduced body size and weight, but also increased adipose tissue mass and adipocyte size, enhanced adipocyte glucose transporter 4 (GLUT4) expression, and elevated circulating leptin levels (237–239). Furthermore, when fed a high-fat diet, these mice do not gain body weight or adipose tissue mass and also do not exhibit the expected increase in food intake (238). Chemical inhibition of LPAR1 and LPAR3 in high-fat diet-fed mice increases adipose tissue mass, adipose tissue PPARγ2 expression, and adipocyte size, skeletal muscle glucose utilization, liver glycogen storage, and pancreatic islet mass, and improves glucose tolerance and insulin sensitivity (224, 239). Circulating LPAs are mainly generated from lysophosphatidylcholines by the PLD activity of autotaxin (ATX), an enzyme primarily produced by adipocytes (229, 240–244). ATX secretion by WAT and BAT and circulating ATX levels are increased with obesity and correlate positively with markers of glucose intolerance and insulin resistance (229, 243–249). While a homozygous loss of ATX is embryonically lethal in mice, a heterozygous loss of ATX is tolerated and, upon high-fat feeding, results in decreased circulating LPA levels, body weight gain, and adipose tissue accrual as well as improved glucose tolerance and insulin sensitivity (240–242, 250). Mice with adipocyte-specific deletion of ATX also display decreased circulating LPA levels and improved glucose tolerance, but intriguingly increased adipose tissue accrual, adipose tissue PPARγ2 expression, and adipocyte size (244). Mice overexpressing ATX conversely display increased circulating LPA levels, body weight gain, and adipose tissue accrual, yet no alterations of glucose homeostasis (236).
Taken together, LPAs appear to have mostly detrimental effects on systemic metabolism.
Sphingolipids
The sphingolipid superfamily is characterized by a sphingoid backbone (e.g., sphingosine) and, depending on the respective subfamily, a specific head group, an amide-bound acyl chain, and, in certain cases, also an ester-bound acyl chain (251). Their de novo synthesis begins with the generation of 3-ketodihydrosphingosine from serine and palmitoyl-CoA by serine palmitoyltransferases (SPTs) (251). This is succeeded by a reduction to dihydrosphingosine by 3-ketodihydrosphingosine reductase (KDSR), an acylation to dihydroceramides by (dihydro)ceramide synthases (CERSs), and a conversion to ceramides by (dihydro)ceramide desaturases (DEGSs) (251). Ceramides can be modified further by addition of different head groups, such as phosphatidylcholine by sphingomyelin synthases (SMSs) or glucose by glucosylceramide synthase (GCS) (251). They can alternatively be acylated to acylceramides by diacylglycerol acyltransferases (DGATs), deacylated to sphingosine by ceramidases (CDases), or phosphorylated to ceramide-1-phosphates (C1Ps) by ceramide kinase (CERK) (251). Sphingosine too can be phosphorylated by sphingosine kinases (SPHKs) yielding sphingosine-1-phosphate (S1P) (251). Additional “salvage pathways” exist for ceramide generation from sphingomyelins, glucosylceramides, sphingosine, and C1P that involve SMases, glucosylceramidases (GlcCDases), CERSs, and C1P phosphatases, respectively (251).
While a near-complete reduction of SPT activity due to a homozygous loss of either SPT long chain subunit 1 or 2 (SPTLC1 or SPTLC2) is embryonically lethal in mice, a partial reduction due to heterozygous loss of SPTLC2 or by chemical SPT inhibition alleviates glucose intolerance, insulin resistance, WAT inflammation, liver steatosis, and atherosclerosis, as well as cardiac and vascular dysfunction in different mouse and rat models of obesity, diabetes, and cardiovascular disease (252–269). Highlighting the importance of balanced de novo sphingolipid synthesis for adipose tissue function, mice with adipocyte-specific deletion of SPTLC1 or SPTLC2 display age-dependent lipodystrophy and metabolic deterioration (270, 271). This is, however, a complex pathway, as another study demonstrates that adipocyte-specific deletion of SPTLC2 can also result in protection from high-fat diet-induced metabolic disturbances (268).
Ceramides form a pivotal sphingolipid subfamily that is implicated in causing many of the metabolic sequelae of excessive saturated fatty acid intake (272, 273). Circulating ceramides associate with VLDLs and LDLs, extracellular vesicles (EVs), and possibly also albumin (272). Their levels in circulation as well as in tissues, such as WAT, skeletal muscle, and liver, increase with obesity and correlate positively with markers of inflammation and insulin resistance (268, 274–295). Ceramides can activate protein phosphatase (PP)1, PP2A, and PP2C, protein kinase (PK)Cζ, and the NLR family pyrin domain-containing (NLRP)3 inflammasome, suppress mitochondrial β-oxidation, and promote ER stress (273, 296). They directly decrease the insulin sensitivity of adipose tissue stromal cells, adipocytes, skeletal and cardiac muscle cells, endothelial cells, vascular smooth muscle cells, and kidney cells (256, 261, 264, 297–308). They also decrease adipose tissue stromal cell adipogenic differentiation, white adipocyte browning, and β-cell insulin production, increase adipocyte inflammatory marker expression, and promote β-cell, cardiac muscle cell, and kidney cell death (268, 276, 308–315). Ceramides differ in the length and saturation of their amide-bound acyl chains, mostly resulting from the acyl-CoA preference of the CERS isoform involved in their synthesis (251). CERS1 prefers C18, CERS2 C20–26, CERS5 C16, and CERS6 C14–16 acyl-CoA (251). Mice deficient in either CERS1, CERS5, or CERS6 display improved glucose homeostasis upon high-fat feeding, whereas mice (partially) deficient in CERS2 not only display impaired glucose homeostasis upon high-fat feeding, but also develop liver steatosis and cancer (290, 294, 316–320). Comparable metabolic improvements are seen in high-fat diet-fed mice with either brown adipocyte- or hepatocyte-specific deletions of CERS6 (290). This implicates ceramides with rather short amide-bound C14–C18 acyl chains as prime mediators of saturated fatty acid-induced glucose intolerance and insulin resistance.
Similar to upstream SPT activity, a near-complete reduction of downstream DEGS activity due to a homozygous loss of DEGS1 results in incompletely penetrant embryonic lethality in mice (255). Mice with a heterozygous loss of DEGS1 are viable and display increased insulin sensitivity (255). In line with these observations, chemical DEGS1 inhibition offers partial protection from glucose intolerance and insulin resistance upon high-fat feeding (307).
Ceramide degradation is intimately connected to adiponectin signaling, as the engagement of AdipoR1 and AdipoR2 is associated with increased ceramidase activity, which may stem from the receptors themselves (117, 321–324). Adiponectin-deficient mice display not only impaired glucose tolerance and insulin sensitivity, but also increased ceramide and decreased sphingosine and S1P levels in WAT and liver as well as exacerbated responses upon experimental induction of β-cell and cardiac muscle cell death (117, 321). Treatment with adiponectin or overexpression of it decreases tissue ceramide levels, normalizes glucose homeostasis upon high-fat feeding, and restrains β-cell and cardiac muscle cell death, likely through induction of ceramide degradation and S1P production (321). In mice, WAT-, liver-, or skeletal muscle-restricted overexpression of AdipoR1 or AdipoR2 decreases local ceramide levels and increases local insulin sensitivity (321, 322, 325). When either WAT or liver is targeted, not only local but also distant tissue ceramide levels diminish and glucose tolerance and insulin sensitivity improve, suggesting a dynamic inter-tissue exchange of ceramides (322). In agreement, overexpression of acid ceramidase in either WAT or liver decreases tissue and circulating ceramide levels and augments systemic metabolism (326). Intriguingly, adiponectin itself may play a role in this exchange of ceramides by stimulating the release of ceramide-rich EVs from cells following T-cadherin but not AdipoR1 or AdipoR2 engagement (327). In addition, consistent with adaptor protein containing PH domain, PTB domain, and leucine zipper motif 1 (APPL1) being a key downstream mediator of adiponectin signaling, global APPL1 overexpression decreases cardiac ceramide accumulation, insulin resistance, and damage, and improves systemic metabolism upon high-fat feeding (328).
Sphingomyelins are ceramide derivatives whose circulating levels increase with obesity and, dependent on the length of their amide-bound acyl chain, correlate positively with markers of insulin resistance (251, 276, 287, 288, 291, 329, 330). Mice deficient in SMS1 display incompletely penetrant neonatal lethality, age-dependent lipodystrophy, and disturbed glucose tolerance with pronounced mitochondrial dysfunction and oxidative stress in WAT and pancreas (331, 332). In contrast, mice deficient in SMS2 display augmented glucose tolerance and insulin sensitivity and partial protection from high-fat diet-induced obesity and metabolic deterioration (262, 333, 334). These differences may arise not only from the differential expression of SMS1 and SMS2 in specific tissues, but also from the distinct subcellular localizations of both enzymes, and thus may be due to subcellular differences in sphingomyelin generation (335). Alterations of sphingomyelin synthesis can furthermore influence the levels of ceramides and ceramide derivatives such as glucosylceramides (335). The loss or chemical inhibition of acid SMase, for instance, decreases liver ceramide levels and steatosis, glucose intolerance, and insulin resistance in mice fed a high-fat diet (289, 336).
Glucosylceramides are also ceramide derivatives and themselves form the basis for the synthesis of more complex glycosphingolipids (251). Not much is known about whether and how glucosylceramide levels in circulation and in tissues change with obesity, glucose intolerance, and insulin resistance. A homozygous loss of GCS results in embryonic lethality in mice, and the metabolic consequences of a heterozygous loss of GCS have not yet been studied (337, 338). While hepatocyte-specific deletion of GCS is without apparent impact on systemic metabolism, not even upon high-fat challenge, chemical inhibition of GCS curtails WAT inflammation and liver steatosis, fibrosis, and inflammation and improves glucose tolerance and insulin sensitivity in ob/ob mice and mice fed a high-fat diet (339–344). Thus, while there appears to be an involvement, much remains to be uncovered concerning the role of glucosylceramides and glycosphingolipids in obesity and obesity-associated diseases.
C1Ps and S1P are lipid mediators formed by the phosphorylation of ceramides and sphingosine, respectively (251). C1Ps stimulate the enzymatic activity of the arachidonic acid-releasing cytosolic PLA2α and inhibit those of TNF-releasing TNF-converting enzyme (TACE) and acid SMase (251, 296). Arguing for mostly detrimental effects of C1Ps on systemic metabolism, CERK-deficient mice exhibit decreased body weight gain and decreased WAT adipocyte size, as well as reduced macrophage infiltration and inflammation (345). As a consequence, they show improved glucose tolerance upon high-fat feeding (345).
S1P not only signals through five widely-expressed G protein-coupled S1P receptors (S1PR1–5), but also stimulates the enzymatic activities of TNF receptor-associated factor 2 (TRAF2) and cellular inhibitor of apoptosis 2 (CIAP2) and inhibits those of histone deacetylase (HDAC)1 and HDAC2 (251, 296). In circulation, S1P associates with ApoM on HDL and with albumin (251, 296). Its circulating levels increase with obesity as well as upon fasting and correlate positively with markers of insulin resistance and inflammation (276, 283, 346–351). S1P directly acts on adipose tissue stromal cells to increase proliferation and decrease adipogenic differentiation, prompts adipocytes to increase inflammatory marker expression, triggers hepatocytes to increase inflammatory marker expression, survival, glucose uptake, and lipid accumulation, and leads to an overall decrease in insulin sensitivity (276, 348, 350, 352–361). It also triggers skeletal muscle cells to increase glucose uptake, β-cells to increase survival and glucose-stimulated insulin secretion, vascular smooth muscle cells to increase tone, and endothelial cells to increase immune cell adhesion and permeability (276, 348, 350, 352–361). SPHK1-deficient mice display decreased circulating S1P levels and are variably reported to exhibit either decreased WAT inflammation, liver inflammation and steatosis, and improved glucose tolerance and insulin resistance or increased β-cell death and worsened glucose tolerance and insulin sensitivity (349, 356, 357, 359). While chemical inhibition of SPHK1 has yielded similarly inconsistent results, SPHK1 overexpression from an integrated transgene or from viral vectors was uniformly reported to have beneficial metabolic effects (346, 349, 353, 355, 362). In contrast, not only overexpression but also deletion of SPHK2 results in increased circulating S1P levels and improved glucose tolerance and insulin resistance in mice (361, 363, 364). Targeting S1P signaling rather than S1P production has provided more consistent results. To this end, either combined chemical modulation of S1PR1 and S1PR3–5, chemical inhibition of S1PR2, or deletion of S1PR2 results in augmented glucose homeostasis in different mouse models of obesity and diabetes (348, 350, 360, 365–370).
As in the case of other signaling mediators, more sophisticated models and methods may be required to disentangle acute and chronic effects of altered C1Ps and S1P production and signaling on different cells and tissues.
METABOLITES
Uric acid
Uric acid is a product of purine base degradation, a process that begins with the conversion of adenine and guanine nucleotides to hypoxanthine and xanthine, respectively, and concludes with the conversion of hypoxanthine to xanthine to uric acid (371, 372). Uric acid is produced by adipose tissue, the liver, and skeletal muscle and excreted primarily by the kidneys and secondarily by the liver (372). It is also degraded by uricase, an enzyme that is present in mice and rats, but absent in humans, resulting in overall higher circulating and tissue uric acid levels in the latter (371, 372). The circulating uric acid levels increase with obesity, liver steatosis, type 2 diabetes, and kidney disease and may predict the development of the metabolic syndrome (373–389). Uric acid exerts anti-oxidant effects in the extracellular environment where it can scavenge reactive oxygen and nitrogen species, including superoxide anions (O2−), peroxynitrite anions (ONOO−), and NO, but pro-oxidant effects in the intracellular environment where it can activate the NLRP3 inflammasome and NADPH oxidase (NOX) (379, 385, 390–395). NADPH oxidase activation by uric acid triggers its translocation to the mitochondria, induces mitochondrial oxidative stress, suppresses β-oxidation, and promotes de novo lipogenesis (379, 393, 394). Uric acid directly increases adipocyte and hepatocyte inflammatory marker expression, hepatocyte lipid accumulation, and vascular smooth muscle cell proliferation, and decreases hepatocyte and endothelial cell insulin sensitivity as well as endothelial cell proliferation (376, 379, 385, 393, 395–402). Chemical inhibition of uricase in mice and rats results in elevated circulating uric acid levels, raised blood pressure, diminished WAT, liver skeletal muscle, and vessel insulin sensitivity, evident liver steatosis and inflammation, kidney dysfunction, as well as disturbed glucose tolerance and insulin sensitivity (394, 395, 400, 401).
The final steps of purine base degradation, the conversion of hypoxanthine to xanthine to uric acid, are carried out by the multifunctional enzyme, xanthine oxidoreductase (XOR), that occurs in two distinct forms, a dehydrogenase form (XDH) and an oxidase form (XO) (403, 404). XOR is produced as XDH and can be converted to XO either reversibly by cysteine residue oxidation or irreversibly by limited proteolysis (403, 404). Secreted XDH undergoes rapid turnover to XO, which then binds to the surface of endothelial cells (403, 404). XOR can utilize a wide range of substrates (403, 404). While substrate oxidation by XDH consumes NAD+ to produce NADH, substrate oxidation by XO consumes oxygen (O2) to produce mainly hydrogen peroxide (H2O2) but also O2− (403, 404). Depending on pH, O2 tension, and substrate availability, XDH can also utilize O2 as an electron acceptor and thus act as a source of reactive oxygen species (403, 404). Moreover, both XDH and XO can generate reactive nitrogen species by substrate or NADH oxidation with concomitant reduction of nitrate (NO3−) to nitrite (NO2−) to NO (403, 404). XOR is expressed in WAT, liver, and skeletal muscle, and its production and activity in WAT and liver increase with obesity (382, 385, 405, 406). XOR partakes in the regulation of adipogenesis, and mice with a homozygous loss of XOR display decreased fat mass and early lethality, although comparable XOR deficiency in humans is nonlethal (405, 407, 408). Mice with a heterozygous loss of XOR display age-dependent body and WAT weight gain and WAT dysfunction with increased oxidative stress and inflammation, as well as glucose intolerance and insulin resistance, all of which are exacerbated on a high-fat diet (409). Chemical inhibition of XO, in contrast, not only lowers the circulating uric acid levels, but also preserves WAT and liver function and augments glucose homeostasis in db/db mice as well as mice fed a high-fat diet (376, 379, 384). The outcome of manipulating XOR thus appears to depend on which aspects of XOR biology are targeted.
Uric acid production is tightly linked to fructose intake (371, 372). Following cellular uptake, fructose can undergo unrestrained phosphorylation by ketohexokinase (KHK), which yields fructose-1-phosphate and consumes cellular ATP (371, 372). The accompanying depletion of cellular phosphate triggers an activation of AMP deaminase (AMPD), degradation of adenine nucleotides, XOR-dependent production of uric acid, and uric acid-mediated inhibition of AMP-dependent protein kinase (AMPK) (371, 372). High-fructose feeding of mice and rats causes elevated circulating uric acid levels, cardiac, vascular, and kidney dysfunction with increased oxidative stress, inflammation, and fibrosis, as well as disturbed glucose homeostasis, all of which can be mitigated by chemical XO inhibition (383, 399, 410–414).
Taken together, this argues for mostly detrimental effects of elevated uric acid levels on systemic metabolism.
Uridine
Uridine is the nucleoside of the pyrimidine base, uracil, and provides the basis of substrates that are essential for RNA and DNA synthesis, glycogen deposition, and protein and lipid glycosylation (415). Its de novo synthesis usually begins with the formation of dihydroorotate from glutamine, bicarbonate (HCO3−), ATP, and aspartate by the tri-functional enzyme, carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (CAD2), followed by the conversion of dihydroorotate to orotate by dihydroorotate dehydrogenase (DHODH), of orotate to UMP by the bi-functional enzyme, UMP synthase (UMPS), and of UMP to uridine by 5′-nucleotidase (5NT) (415). Its degradation, in turn, is carried out by uridine phosphorylases (UPPs) (415). Uridine is produced by the liver and WAT and cleared by the liver (416–419). Both endogenous and exogenous uridine (introduced either orally or intraperitoneally) undergo continuous and rapid clearance by the liver, mostly through degradation by Kupffer cells and endothelial cells, but also through biliary excretion by hepatocytes (416–419). The circulating uridine levels are tightly regulated, increase with obesity and upon fasting, exercise, and ingestion of ethanol, glucose, and fructose, and decrease with lipodystrophy and upon ingestion of amino acids (418–421). Strikingly, while the liver produces most of the circulating uridine in the fed state, adipose tissue is doing so in the fasted state (418, 419). Not much is known about how uridine signals and no dedicated uridine receptor has been identified yet. Uridine may indeed exert most of its effects by being metabolized to either UDP, UDP-glucose, or UTP, which can signal through different G protein-coupled purinoreceptors (i.e., P2YR2, P2YR4, P2YR6, and P2YR14) or to UDP-hexosamines and UDP-N-acetyl-hexosamines, which can alter the glycosylation and thus the activity of distinct proteins and lipids (422, 423).
Acute treatment of humans, rats, and mice with a high dose of uridine results in a transient decrease in body temperature, while a low dose may cause a slight increase instead (418, 424, 425). In extension, the fasting-associated decrease in body temperature was found to be critically dependent on uridine production by adipose tissue (418). In mice, uridine treatment also increases the circulating leptin level, decreases the metabolic rate, and improves glucose tolerance in aged and in high-fat diet-fed animals (418). Uridine’s effects on both body temperature and glucose homeostasis apparently involve active leptin signaling, as uridine treatment of ob/ob mice evokes an exacerbated decrease in body temperature, but unexpectedly also worsens glucose homeostasis (418).
Prolonged disturbances of uridine homeostasis in either direction appear to be mostly detrimental. As such, dietary supplementation of uridine in mice for several days to weeks promotes body weight gain, alters liver protein acetylation and glycosylation, stimulates liver glycogen and lipid accumulation, blunts liver insulin sensitivity, and disturbs glucose homeostasis (426–428). Intriguingly, lowering uridine levels by chemical inhibition of DHODH or overexpression of UPP1 also induces liver lipid accumulation and blunts liver and systemic insulin sensitivity, but improves glucose tolerance (426, 427). Elevating uridine levels by UPP1 deletion, in turn, does not affect liver insulin sensitivity, but similarly blunts systemic insulin sensitivity, improves glucose tolerance, and may furthermore promote spontaneous tumorigenesis (427, 429).
The ER stress response, specifically the mRNA splicing-dependent production of the short isoform of X-box binding protein 1 (XBP1s), plays a central role in uridine metabolism (430). In the fed state, XBP1s levels are high in hepatocytes and pro-opiomelanocortin neurons of the arcuate nucleus and low in adipocytes, whereas the opposite can be observed in the fasted state (419, 431, 432). XBP1s upregulation in adipose tissue seems to be tied to active lipolysis with higher XBP1s levels detectable not only upon fasting, but also with obesity and cancer-associated cachexia (419). XBP1s acts as a transcription factor that stimulates uridine de novo synthesis by inducing CAD2 as well as uridine conversion to UDP-hexosamines and UDP-N-acetyl-hexosamines by inducing both glutamine/fructose-6-phosphate aminotransferase 1 (GFPT1) and UDP-glucose 4-epimerase (GALE) (419, 431–433). Highlighting its role in promoting uridine production, adipocyte-specific deletion of XBP1s abolishes the fasting-induced increase in uridine (419). Mice with adipocyte-specific overexpression of XBP1s display elevated circulating and adipose tissue uridine levels, increased activity, energy expenditure, and body heat loss, decreased body weight and body temperature, and protection from obesity upon high-fat feeding or with leptin deficiency (419). Suggesting mostly favorable effects of XBP1s induction, hepatocyte- or pro-opiomelanocortin neuron-specific XBP1s overexpression augments glucose homeostasis, and cardiac muscle cell-specific XBP1s overexpression alleviates ischemia-reperfusion damage in mice (431–433).
Much remains to be learned about how short- and long-term disturbances of uridine homeostasis impact systemic metabolism and about whether manipulations of uridine metabolism may yield therapeutic benefits.
NONCODING RNAs
Long noncoding RNAs
Contrary to lasting assumptions, the majority of the genome is transcribed, at least under some conditions (434). Long noncoding RNAs (lncRNAs) originate from the transcription of intergenic and genic portions of the genome, both in the sense and antisense direction (434). They are, by definition, over 200 nucleotides long, not translated into proteins, and may regulate gene transcription and nuclear domain organization as well as RNA and protein function (434). Most lncRNAs are localized in the nucleus, lowly abundant, and poorly conserved (434). Only a small fraction (hundreds to thousands) of the predicted 20,000–100,000+ lncRNAs in humans may indeed have specific functions (434). lncRNAs are found in EVs, raising the possibility that EV-associated lncRNAs released from adipose tissue function as regulators of distant tissue function (435). Recent reviews provide an excellent overview of the role of lncRNAs in the regulation of adipose tissue function and systemic metabolism (436–438).
MicroRNAs
MicroRNAs (miRNAs) either originate from introns or are transcribed from dedicated genes (439). They are released from pri- and pre-miR precursors by successive processing involving the microprocessor complex in the nucleus as well as DICER in the cytoplasm (439). At the end of their processing, they are 20–24 nucleotides long and incorporated into the RNA-induced silencing complex (RISC). As a RISC component, they regulate mRNA translation and stability, usually resulting in a repression of protein expression (439). They are more conserved than lncRNAs and show a wide range of abundance (439). Strikingly though, only less than 100 of the adipose tissue-expressed miRNAs appear to be regulated by obesity in either humans or mice (439). Distinct populations of miRNAs are released from cells associated mostly with components of the RISC, but also associated with lipoproteins and EVs (440–442). Adipose tissue is a major source of circulating EV-associated miRNAs, and recent reviews offer much insight into how adipose tissue-derived miRNAs shape metabolic homeostasis (439).
EVs
EVs are an eminent means of intercellular communication (435, 443–445). They carry a large variety of cargo, including organelle parts, proteins, and lipids, as well as small coding and noncoding RNAs (e.g., mRNAs, lncRNAs, and miRNAs), delivering them from one cell to another. Cells secrete EVs in an orderly process that is controlled by intra- and intercellular signals, including nutrient-related cues (435, 443–445). Determined by their biogenesis, ectosomes (also called microvesicles) with a diameter of 50–1,000 nm and exosomes with a diameter of 50–150 nm can be distinguished (435, 443–445). While ectosomes bud directly from the plasma membrane, exosomes are generated by inward budding of endosomal membranes to create multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs, i.e., unreleased exosomes) followed by either degeneration through fusion of the ILVs with the MVB membrane, degradation through fusion of the MVBs with lysosomes, or release through fusion of the MVBs with the plasma membrane (435, 443–445). Accordingly, ectosomes are released in an immediate fashion and exosomes in a delayed fashion (435, 443–445). EVs are capable of delivering their cargo to specific cells and tissues by binding to and rolling on target cell surfaces, which subsequently allows for receptor interaction and fusion by fusogenic interactions, endocytosis, macropinocytosis, or phagocytosis (435, 443–445).
Obesity alters the cargo and increases the release of EVs from WAT, while cold exposure does so in BAT and browning WAT (446, 447). Establishing a role for adipose tissue-derived EVs in the regulation of systemic metabolism, EVs collected from WAT of high-fat diet-fed mice elicit glucose intolerance and insulin resistance when injected into wild-type mice and exacerbate atherosclerosis when injected in ApoE-deficient mice (447, 448). Highlighting the contribution of macrophages to these effects, WAT macrophage EVs from high-fat diet-fed mice are sufficient to disrupt glucose homeostasis when injected into wild-type mice, whereas WAT macrophage EVs of regular diet-fed mice are capable of augmenting glucose homeostasis instead (449). WAT EVs of high-fat diet-fed mice may also induce monocyte homing to adipose tissue and the liver, promote local monocyte proliferation and differentiation, and increase macrophage pro-inflammatory cytokine production (447, 448, 450). Taken together, these observations allude to a vicious cycle of obesity-associated shifts in the adipose tissue EV secretion profile, immune cell infiltration, and inflammation.
EVs serve as a means of communication not only between adipose tissue and other organs but also between different cell populations within adipose tissue itself. Regarding this aspect of adipose tissue biology, we recently uncovered an extensive EV-mediated local exchange of cellular components between adipocytes and endothelial cells that is governed by the nutritional state (451). In WAT of mice, the cellular origin and destination as well as the cargo of the transferred EVs changes upon fasting, feeding, and with obesity (451). Fasting, for instance, results in an increased EV-mediated transfer of cellular components from WAT endothelial cells to adipocytes as well as an enrichment of WAT EVs in proteins involved in central cellular signaling pathways, mitochondrial respiration, oxidative stress defense, and hypoxia response and a depletion of WAT EVs in proteins involved in lipid and amino acid metabolism (451). The highly dynamic character of this EV-mediated local exchange alludes to the possibility that it might primarily serve to rapidly and efficiently redistribute cellular components between different cell populations within WAT, thus lending heightened metabolic flexibility to the tissue as a whole.
Altogether, EVs released from adipose tissue into circulation may exert mostly beneficial effects on systemic metabolism in the lean state but detrimental effects in the obese state. Related to this, much remains to be learned about exactly how adipose tissue communicates with other organs by means of EV exchange, the sending cells, and the receiving cells, as well as the nature of the transmitted message.
PERSPECTIVE
There is clearly a wide variety of signaling mediators and mechanisms that adipose tissue utilizes to communicate with other organs of the body. At the systemic level, we deal with adipose tissue as a whole, distributed throughout the body in the form of discrete depots.
The contribution of specific depots and cell populations within them to the overall production frequently remains to be defined for many adipose tissue-derived factors. Likewise, the manner in which physiological and pathophysiological states, such as fasting, aging, and obesity, affect the production of certain factors by distinct depots and cell types awaits elucidation. Particularly with respect to fibrosis and inflammation, it has become clear that the effects observed commonly involve a cross-talk of multiple different cell types with net output from all participating populations. A fundamental impediment to more refined studies of adipose tissue-emergent signals is the present dearth of methods to measure and manipulate the production of distinct signaling mediators by specific adipose tissue depots and cell populations in vivo.
There are several signaling molecules, such as leptin and FGF21, which exert mostly beneficial effects on systemic metabolism, yet also display elevated circulating levels in pathophysiological states tightly associated with metabolic disturbances. The mechanistic basis by which certain pathophysiological states alter the signaling capacity and character of distinct factors is of immense interest. Reduced responsiveness to the metabolically favorable actions of leptin and FGF21 in the obese state, for instance, evoked still controversial ideas of leptin and FGF21 resistance that have been probed in numerous studies, altogether providing no coherent model (130, 452). To reliably define the role that a specific signaling mediator plays in metabolic disease remains challenging. It requires careful modulation of the abundance and/or activity of the respective factor and its receptor(s), while concomitantly monitoring systemic metabolism and cellular signaling. At the same time, focusing unduly on either individual cell types and tissues or isolated signaling pathways has to be avoided. More sophisticated in vivo models that enable these types of modifications are likely to make crucial contributions to such efforts.
Above, we solely discussed signaling mediators that are actively produced by adipose tissue. Adipose tissue is, however, also capable of degrading signaling mediators that derive from or that are destined for other organs (Fig. 2). It thus partakes in inter-organ communication as a source as well as a sink of signals. Future endeavors should thus consider not only the anabolism but also the metabolism and catabolism of signals by adipose tissue when evaluating its contributions to systemic metabolic and cellular homeostasis.
Fig. 2.
Adipose tissue partakes in inter-organ communication by producing new signaling mediators (“signal anabolism”) as well as by converting or degrading signaling mediators reaching it from other organs (“signal metabolism” and “signal catabolism”).
Footnotes
Abbreviations:
- AII
- angiotensin II
- AA
- arachidonic acid
- ACE
- angiotensin-converting enzyme AChE, acetylcholine esterase
- ACRP30/ADIPOQ
- adiponectin
- ACVR
- activin receptor
- ADA
- adenosine deaminase
- AdipoR
- adiponectin receptor
- 2-AG
- 2-arachidonoylglycerol
- AGK
- acylglycerol kinase
- AGPAT
- acylglycerol-3-phosphate acyltransferase
- AGT
- angiotensinogen
- AIM2
- absent in melanoma
- ALK
- activin receptor-like kinase
- AMPD
- AMP deaminase
- AMPK
- AMP-dependent protein kinase ANG, angiopoietin
- ANGPTL
- angiopoietin-like protein
- ANTR
- angiotensin receptor
- APLN
- apelin
- APLNR
- apelin receptor
- APPL1
- adaptor protein containing PH domain, PTB domain, and leucine zipper motif 1
- ATGL
- adipose tissue triglyceride lipase
- ATX
- autotaxin
- BAT
- brown adipose tissue
- BMP
- bone morphogenic protein
- BMPR2
- bone morphogenic protein receptor 2
- C3a
- cleaved complement factor 3 fragment a
- C3aR
- cleaved complement factor 3 fragment a receptor
- C3b
- cleaved complement factor 3 fragment b
- C3bBb
- complement factor 3 convertase
- CAD2
- carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase
- CAP1
- adenylyl cyclase-associated protein 1
- CARD11
- caspase recruitment domain-containing protein 11
- CAV1
- caveolin 1
- CB
- cannabinoid receptor
- CCL2
- chemokine (C-C motif) ligand 2
- CCRL2
- chemokine (C-C motif) receptor-like 2
- CDase
- ceramidase
- cDCN
- cleaved decorin
- CERK
- ceramide kinase
- CERS
- (dihydro)ceramide synthase
- CERT
- ceramide transfer protein
- CF
- complement factor
- CG
- cathepsin G
- CMKLR1
- chemokinelike receptor 1
- COL6
- collagen VI
- COL6A3
- collagen VI a3 chain
- COX
- cyclooxygenase
- C1P
- ceramide-1-phosphate
- CPTP
- ceramide-1-phosphate transfer protein
- CSPG4
- chondroitin sulfate proteoglycan 4
- CTGF
- connective tissue growth factor
- CTRP3
- complement factor 1q/tumor necrosis factor-related protein 3
- CYP
- cytochrome P450 oxidase
- DAG
- diacylglycerol
- DAGL
- diacylglycerol lipase
- DARC
- Duffy antigen/chemokine receptor
- DEGS
- (dihydro)ceramide desaturase
- DHODH
- dihydroorotate dehydrogenase
- 12
- 13-diHOME, 12, 13-dihydroxy-9Z-octadecenoic acid
- DNAJC1
- DnaJ heat shock protein family member C1
- DPP4
- dipeptidyl peptidase 4
- EGF
- epidermal growth factor
- EH
- epoxid hydrolase
- EP
- prostaglandin E receptor
- ErbB4
- epidermal growth factor receptor 4
- ETP
- endotrophin
- EV
- extracellular vesicle
- FABP
- fatty acid binding protein
- FAHFA
- fatty acid esters of hydroxy fatty acids
- FAPα
- dipeptidyl peptidase fibroblast activation protein α
- FASL
- FAS ligand
- FAT
- fatty acid tranlocase
- FATP
- fatty acid transport protein
- FGF
- fibroblast growth factor
- FGFR
- fibroblast growth factor receptor
- GALE
- UDP-glucose-4-epimerase
- GCS
- glucosylceramide synthase
- GFPT1
- glutamine/fructose-6-phosphate aminotransferase 1
- GlcCDase
- glucosylceramidase
- GLP1
- glucagon-like peptide 1
- GP130
- glycoprotein 130
- GPAT
- glycerol-3-phosphate acyltransferase
- GZMA
- granzyme A
- HDAC
- histone deacetylase
- HSL
- hormone-sensitive lipase
- IL
- interleukin
- IL1R
- interleukin 1 receptor
- IL1RAP
- interleukin 1 receptor accessory protein
- IL2Rγ
- interleukin 2 receptor γ
- IL4Rα
- interleukin 4 receptor α
- IL6R
- interleukin 6 receptor
- IL10R
- interleukin 10 receptor
- IL13Rα1
- interleukin 13 receptor α 1
- ILV
- intraluminal vesicle
- INT1
- intelectin 1
- KDSR
- 3-ketodihydrosphingosine reductase
- KHK
- ketohexokinase
- KLK7
- kallikrein 7
- KRT1
- keratin 1
- LAMP1
- lysosomal-associated matrix protein 1
- LAMR
- laminin receptor
- LCN
- lipocalin
- LCN2R
- lipocalin 2 receptor
- LEP
- leptin
- LEPR
- leptin receptor
- LF
- lactoferrin
- LILRB2
- leukocyte Ig-like receptor B2
- LIMP2
- lysosome membrane protein 2
- lncRNA
- long noncoding RNA
- LPA
- lysophosphatidic acid
- LPAR
- lysophosphatidic acid receptor
- LRP
- LDL receptor-related protein
- LPS
- lipopolysaccharide
- LPP
- lipid phosphate phosphatase
- M6P/IGF2R
- mannose-6-phosphate/insulin-like growth factor 2 receptor
- MCP1
- monocyte chemoattractant protein 1
- miRNA
- microRNA
- MMP9
- matrix metallopeptidase 9
- MVB
- multivesicular body
- NAMPT
- nicotinamide phosphoribosyltransferase/visfatin
- NLRC4
- NLR family CARD domain-containing 4
- NLRP
- NLR family pyrin domain-containing
- NMN
- nicotinamide mononucleotide
- NOX
- NADPH oxidase
- NRG
- neuregulin
- NRP1
- neuropilin 1
- OMT
- omentin
- 5NT
- 5’-nucleotidase
- OPT
- osteoprotegerin
- 5-PAHSA
- palmitic acid ester of 5-hydroxystearic acid
- 9-PAHSA
- palmitic acid ester of 5-hydroxystearic acid
- PAI1
- plasminogen activator inhibitor 1
- PAR2
- protease-activated receptor
- PEDF
- pigment epithelium-derived factor
- PEDFR
- pigment epithelium-derived factor
- PGE
- prostaglandin E
- PK
- protein kinase
- PL
- phospholipase
- PLEKHA8
- pleckstrin homology domain-containing family A member 8
- PLXDC
- plexin domain-containing protein
- PP
- protein phosphatase
- PRTN3
- proteinase 3
- RBP4
- retinol-binding protein 4
- RBPR2
- retinol-binding protein 4 receptor 2
- RETN
- resistin
- RISC
- RNA-induced silencing complex
- ROR1
- receptor tyrosine kinase-like orphan receptor 1
- SAA3
- serum amyloid 3
- SCD1
- stearoyl-CoA desaturase 1
- SERPIN
This work was supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Grant 414232833 (J-B.F.); National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK55758, P01-DK088761, and R01-DK099110 (P.E.S.); National Institute on Aging Grant P01-AG051459 (P.E.S.); and an unrestricted grant from the Novo Nordisk Research Foundation (P.E.S.).
REFERENCES
- 1.Rosen E. D., and Spiegelman B. M.. 2014. What we talk about when we talk about fat. Cell. 156: 20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., and Friedman J. M.. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature. 372: 425–432. [DOI] [PubMed] [Google Scholar]
- 3.Zhang F., Basinski M. B., Beals J. M., Briggs S. L., Churgay L. M., Clawson D. K., DiMarchi R. D., Furman T. C., Hale J. E., Hsiung H. M., et al. 1997. Crystal structure of the obese protein leptin-E100. Nature. 387: 206–209. [DOI] [PubMed] [Google Scholar]
- 4.Cohen P., Zhao C., Cai X., Montez J. M., Rohani S. C., Feinstein P., Mombaerts P., and Friedman J. M.. 2001. Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest. 108: 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Luca C., Kowalski T. J., Zhang Y., Elmquist J. K., Lee C., Kilimann M. W., Ludwig T., Liu S. M., and Chua S. C. Jr.. 2005. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J. Clin. Invest. 115: 3484–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo K., McMinn J. E., Ludwig T., Yu Y. H., Yang G., Chen L., Loh D., Li C., Chua S. Jr., and Zhang Y.. 2007. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 148: 3987–3997. [DOI] [PubMed] [Google Scholar]
- 7.Maffei M., Halaas J., Ravussin E., Pratley R. E., Lee G. H., Zhang Y., Fei H., Kim S., Lallone R., Ranganathan S., et al. 1995. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1: 1155–1161. [DOI] [PubMed] [Google Scholar]
- 8.Considine R. V., Sinha M. K., Heiman M. L., Kriauciunas A., Stephens T. W., Nyce M. R., Ohannesian J. P., Marco C. C., McKee L. J., Bauer T. L., et al. 1996. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334: 292–295. [DOI] [PubMed] [Google Scholar]
- 9.Ravussin Y., Leibel R. L., and Ferrante A. W. Jr.. 2014. A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab. 20: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flier J. S., and Maratos-Flier E.. 2017. Leptin’s physiologic role: does the emperor of energy balance have no clothes? Cell Metab. 26: 24–26. [DOI] [PubMed] [Google Scholar]
- 11.Scherer P. E., Williams S., Fogliano M., Baldini G., and Lodish H. F.. 1995. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270: 26746–26749. [DOI] [PubMed] [Google Scholar]
- 12.Maeda K., Okubo K., Shimomura I., Funahashi T., Matsuzawa Y., and Matsubara K.. 1996. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem. Biophys. Res. Commun. 221: 286–289. [DOI] [PubMed] [Google Scholar]
- 13.Hu E., Liang P., and Spiegelman B. M.. 1996. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 271: 10697–10703. [DOI] [PubMed] [Google Scholar]
- 14.Nakano Y., Tobe T., Choi-Miura N. H., Mazda T., and Tomita M.. 1996. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem. 120: 803–812. [DOI] [PubMed] [Google Scholar]
- 15.Straub L. G., and Scherer P. E.. 2019. Metabolic messengers: adiponectin. Nature Metabolism. 1: 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z. V., and Scherer P. E.. 2016. Adiponectin, the past two decades. J. Mol. Cell Biol. 8: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arita Y., Kihara S., Ouchi N., Takahashi M., Maeda K., Miyagawa J., Hotta K., Shimomura I., Nakamura T., Miyaoka K., et al. 1999. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 257: 79–83. [DOI] [PubMed] [Google Scholar]
- 18.Hotta K., Funahashi T., Arita Y., Takahashi M., Matsuda M., Okamoto Y., Iwahashi H., Kuriyama H., Ouchi N., Maeda K., et al. 2000. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 20: 1595–1599. [DOI] [PubMed] [Google Scholar]
- 19.Berg A. H., Combs T. P., Du X., Brownlee M., and Scherer P. E.. 2001. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7: 947–953. [DOI] [PubMed] [Google Scholar]
- 20.Combs T. P., Berg A. H., Obici S., Scherer P. E., and Rossetti L.. 2001. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 108: 1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K., Mori Y., Ide T., Murakami K., Tsuboyama-Kasaoka N., et al. 2001. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7: 941–946. [DOI] [PubMed] [Google Scholar]
- 22.Qi Y., Takahashi N., Hileman S. M., Patel H. R., Berg A. H., Pajvani U. B., Scherer P. E., and Ahima R. S.. 2004. Adiponectin acts in the brain to decrease body weight. Nat. Med. 10: 524–529. [DOI] [PubMed] [Google Scholar]
- 23.Halberg N., Wernstedt-Asterholm I., and Scherer P. E.. 2008. The adipocyte as an endocrine cell. Endocrinol. Metab. Clin. North Am. 37: 753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghaben A. L., and Scherer P. E.. 2019. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 20: 242–258. [DOI] [PubMed] [Google Scholar]
- 25.Schoettl T., Fischer I. P., and Ussar S.. 2018. Heterogeneity of adipose tissue in development and metabolic function. J. Exp. Biol. 221 (Pt. Suppl. 1): doi:10.1242/jeb.162958. [DOI] [PubMed] [Google Scholar]
- 26.Crewe C., An Y. A., and Scherer P. E.. 2017. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest. 127: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Augustin H. G., Koh G. Y., Thurston G., and Alitalo K.. 2009. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10: 165–177. [DOI] [PubMed] [Google Scholar]
- 28.Saharinen P., Eklund L., and Alitalo K.. 2017. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 16: 635–661. [DOI] [PubMed] [Google Scholar]
- 29.Voros G., Maquoi E., Demeulemeester D., Clerx N., Collen D., and Lijnen H. R.. 2005. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology. 146: 4545–4554. [DOI] [PubMed] [Google Scholar]
- 30.Dallabrida S. M., Zurakowski D., Shih S. C., Smith L. E., Folkman J., Moulton K. S., and Rupnick M. A.. 2003. Adipose tissue growth and regression are regulated by angiopoietin-1. Biochem. Biophys. Res. Commun. 311: 563–571. [DOI] [PubMed] [Google Scholar]
- 31.An Y. A., Sun K., Joffin N., Zhang F., Deng Y., Donze O., Kusminski C. M., and Scherer P. E.. 2017. Angiopoietin-2 in white adipose tissue improves metabolic homeostasis through enhanced angiogenesis. eLife. 6: e24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S., Kim W., Moon S. O., Sung M. J., Kim D. H., Kang K. P., Jang K. Y., Lee S. Y., Park B. H., Koh G. Y., et al. 2007. Renoprotective effect of COMP-angiopoietin-1 in db/db mice with type 2 diabetes. Nephrol. Dial. Transplant. 22: 396–408. [DOI] [PubMed] [Google Scholar]
- 33.Jung Y. J., Choi H. J., Lee J. E., Lee A. S., Kang K. P., Lee S., Park S. K., Park T. S., Jin H. Y., Lee S. Y., et al. 2012. The effects of designed angiopoietin-1 variant on lipid droplet diameter, vascular endothelial cell density and metabolic parameters in diabetic db/db mice. Biochem. Biophys. Res. Commun. 420: 498–504. [DOI] [PubMed] [Google Scholar]
- 34.Kadomatsu T., Endo M., Miyata K., and Oike Y.. 2014. Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol. Metab. 25: 245–254. [DOI] [PubMed] [Google Scholar]
- 35.Tabata M., Kadomatsu T., Fukuhara S., Miyata K., Ito Y., Endo M., Urano T., Zhu H. J., Tsukano H., Tazume H., et al. 2009. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 10: 178–188. [DOI] [PubMed] [Google Scholar]
- 36.Kim J., Lee S. K., Jang Y. J., Park H. S., Kim J. H., Hong J. P., Lee Y. J., and Heo Y. S.. 2018. Enhanced ANGPTL2 expression in adipose tissues and its association with insulin resistance in obese women. Sci. Rep. 8: 13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horio E., Kadomatsu T., Miyata K., Arai Y., Hosokawa K., Doi Y., Ninomiya T., Horiguchi H., Endo M., Tabata M., et al. 2014. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler. Thromb. Vasc. Biol. 34: 790–800. [DOI] [PubMed] [Google Scholar]
- 38.Yu C., Luo X., Farhat N., Daneault C., Duquette N., Martel C., Lambert J., Thorin-Trescases N., Rosiers C. D., and Thorin E.. 2014. Lack of angiopoietin-like-2 expression limits the metabolic stress induced by a high-fat diet and maintains endothelial function in mice. J. Am. Heart Assoc. 3: e001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang R. 2016. The ANGPTL3-4-8 model, a molecular mechanism for triglyceride trafficking. Open Biol. 6: 150272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimamura M., Matsuda M., Yasumo H., Okazaki M., Fujimoto K., Kono K., Shimizugawa T., Ando Y., Koishi R., Kohama T., et al. 2007. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 27: 366–372. [DOI] [PubMed] [Google Scholar]
- 41.Mattijssen F., Alex S., Swarts H. J., Groen A. K., van Schothorst E. M., and Kersten S.. 2013. Angptl4 serves as an endogenous inhibitor of intestinal lipid digestion. Mol. Metab. 3: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono M., Shimizugawa T., Shimamura M., Yoshida K., Noji-Sakikawa C., Ando Y., Koishi R., and Furukawa H.. 2003. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J. Biol. Chem. 278: 41804–41809. [DOI] [PubMed] [Google Scholar]
- 43.Lei X., Shi F., Basu D., Huq A., Routhier S., Day R., and Jin W.. 2011. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J. Biol. Chem. 286: 15747–15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McQueen A. E., Kanamaluru D., Yan K., Gray N. E., Wu L., Li M. L., Chang A., Hasan A., Stifler D., Koliwad S. K., et al. 2017. The C-terminal fibrinogen-like domain of angiopoietin-like 4 stimulates adipose tissue lipolysis and promotes energy expenditure. J. Biol. Chem. 292: 16122–16134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nidhina Haridas P. A., Soronen J., Sadevirta S., Mysore R., Quagliarini F., Pasternack A., Metso J., Perttila J., Leivonen M., Smas C. M., et al. 2015. Regulation of angiopoietin-like proteins (ANGPTLs) 3 and 8 by insulin. J. Clin. Endocrinol. Metab. 100: E1299–E1307. [DOI] [PubMed] [Google Scholar]
- 46.Dijk W., Heine M., Vergnes L., Boon M. R., Schaart G., Hesselink M. K., Reue K., van Marken Lichtenbelt W. D., Olivecrona G., Rensen P. C., et al. 2015. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. eLife. 4: e08428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cushing E. M., Chi X., Sylvers K. L., Shetty S. K., Potthoff M. J., and Davies B. S. J.. 2017. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol. Metab. 6: 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y., McNutt M. C., Banfi S., Levin M. G., Holland W. L., Gusarova V., Gromada J., Cohen J. C., and Hobbs H. H.. 2015. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proc. Natl. Acad. Sci. USA. 112: 11630–11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujimoto K., Koishi R., Shimizugawa T., and Ando Y.. 2006. Angptl3-null mice show low plasma lipid concentrations by enhanced lipoprotein lipase activity. Exp. Anim. 55: 27–34. [DOI] [PubMed] [Google Scholar]
- 50.Banfi S., Gusarova V., Gromada J., Cohen J. C., and Hobbs H. H.. 2018. Increased thermogenesis by a noncanonical pathway in ANGPTL3/8-deficient mice. Proc. Natl. Acad. Sci. USA. 115: E1249–E1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y., Quagliarini F., Gusarova V., Gromada J., Valenzuela D. M., Cohen J. C., and Hobbs H. H.. 2013. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA. 110: 16109–16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gusarova V., Alexa C. A., Na E., Stevis P. E., Xin Y., Bonner-Weir S., Cohen J. C., Hobbs H. H., Murphy A. J., Yancopoulos G. D., et al. 2014. ANGPTL8/betatrophin does not control pancreatic beta cell expansion. Cell. 159: 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aryal B., Singh A. K., Zhang X., Varela L., Rotllan N., Goedeke L., Chaube B., Camporez J. P., Vatner D. F., Horvath T. L., et al. 2018. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight. 3: 97918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mandard S., Zandbergen F., van Straten E., Wahli W., Kuipers F., Muller M., and Kersten S.. 2006. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 281: 934–944. [DOI] [PubMed] [Google Scholar]
- 55.Minicocci I., Tikka A., Poggiogalle E., Metso J., Montali A., Ceci F., Labbadia G., Fontana M., Di Costanzo A., Maranghi M., et al. 2016. Effects of angiopoietin-like protein 3 deficiency on postprandial lipid and lipoprotein metabolism. J. Lipid Res. 57: 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robciuc M. R., Maranghi M., Lahikainen A., Rader D., Bensadoun A., Oorni K., Metso J., Minicocci I., Ciociola E., Ceci F., et al. 2013. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler. Thromb. Vasc. Biol. 33: 1706–1713. [Erratum. 2013. Arterioscler. Thromb. Vasc. Biol. 33: e124.] [DOI] [PubMed] [Google Scholar]
- 57.Romeo S., Yin W., Kozlitina J., Pennacchio L. A., Boerwinkle E., Hobbs H. H., and Cohen J. C.. 2009. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 119: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peloso G. M., Auer P. L., Bis J. C., Voorman A., Morrison A. C., Stitziel N. O., Brody J. A., Khetarpal S. A., Crosby J. R., Fornage M., et al. 2014. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am. J. Hum. Genet. 94: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Modica S., and Wolfrum C.. 2013. Bone morphogenic proteins signaling in adipogenesis and energy homeostasis. Biochim. Biophys. Acta. 1831: 915–923. [DOI] [PubMed] [Google Scholar]
- 60.Schulz T. J., Huang P., Huang T. L., Xue R., McDougall L. E., Townsend K. L., Cypess A. M., Mishina Y., Gussoni E., and Tseng Y. H.. 2013. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 495: 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulz T. J., Graja A., Huang T. L., Xue R., An D., Poehle-Kronawitter S., Lynes M. D., Tolkachov A., O’Sullivan L. E., Hirshman M. F., et al. 2016. Loss of BMP receptor type 1A in murine adipose tissue attenuates age-related onset of insulin resistance. Diabetologia. 59: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang Q. Q., Otto T. C., and Lane M. D.. 2004. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA. 101: 9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bowers R. R., Kim J. W., Otto T. C., and Lane M. D.. 2006. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proc. Natl. Acad. Sci. USA. 103: 13022–13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang H., Song T. J., Li X., Hu L., He Q., Liu M., Lane M. D., and Tang Q. Q.. 2009. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA. 106: 12670–12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yadin D., Knaus P., and Mueller T. D.. 2016. Structural insights into BMP receptors: Specificity, activation and inhibition. Cytokine Growth Factor Rev. 27: 13–34. [DOI] [PubMed] [Google Scholar]
- 66.Qian S. W., Tang Y., Li X., Liu Y., Zhang Y. Y., Huang H. Y., Xue R. D., Yu H. Y., Guo L., Gao H. D., et al. 2013. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. USA. 110: E798–E807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gustafson B., Hammarstedt A., Hedjazifar S., Hoffmann J. M., Svensson P. A., Grimsby J., Rondinone C., and Smith U.. 2015. BMP4 and BMP antagonists regulate human white and beige adipogenesis. Diabetes. 64: 1670–1681. [DOI] [PubMed] [Google Scholar]
- 68.Modica S., Straub L. G., Balaz M., Sun W., Varga L., Stefanicka P., Profant M., Simon E., Neubauer H., Ukropcova B., et al. 2016. Bmp4 promotes a brown to white-like adipocyte shift. Cell Reports. 16: 2243–2258. [DOI] [PubMed] [Google Scholar]
- 69.Kang S., Akerblad P., Kiviranta R., Gupta R. K., Kajimura S., Griffin M. J., Min J., Baron R., and Rosen E. D.. 2012. Regulation of early adipose commitment by Zfp521. PLoS Biol. 10: e1001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gupta R. K., Arany Z., Seale P., Mepani R. J., Ye L., Conroe H. M., Roby Y. A., Kulaga H., Reed R. R., and Spiegelman B. M.. 2010. Transcriptional control of preadipocyte determination by Zfp423. Nature. 464: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta R. K., Mepani R. J., Kleiner S., Lo J. C., Khandekar M. J., Cohen P., Frontini A., Bhowmick D. C., Ye L., Cinti S., et al. 2012. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 15: 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shao M., Ishibashi J., Kusminski C. M., Wang Q. A., Hepler C., Vishvanath L., MacPherson K. A., Spurgin S. B., Sun K., Holland W. L., et al. 2016. Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metab. 23: 1167–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hammarstedt A., Gogg S., Hedjazifar S., Nerstedt A., and Smith U.. 2018. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol. Rev. 98: 1911–1941. [DOI] [PubMed] [Google Scholar]
- 74.Tseng Y. H., Kokkotou E., Schulz T. J., Huang T. L., Winnay J. N., Taniguchi C. M., Tran T. T., Suzuki R., Espinoza D. O., Yamamoto Y., et al. 2008. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 454: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suenaga M., Kurosawa N., Asano H., Kanamori Y., Umemoto T., Yoshida H., Murakami M., Kawachi H., Matsui T., and Funaba M.. 2013. Bmp4 expressed in preadipocytes is required for the onset of adipocyte differentiation. Cytokine. 64: 138–145. [DOI] [PubMed] [Google Scholar]
- 76.Xue R., Wan Y., Zhang S., Zhang Q., Ye H., and Li Y.. 2014. Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes. Am. J. Physiol. Endocrinol. Metab. 306: E363–E372. [DOI] [PubMed] [Google Scholar]
- 77.Hoffmann J. M., Grunberg J. R., Church C., Elias I., Palsdottir V., Jansson J. O., Bosch F., Hammarstedt A., Hedjazifar S., and Smith U.. 2017. BMP4 gene therapy in mature mice reduces BAT activation but protects from obesity by browning subcutaneous adipose tissue. Cell Reports. 20: 1038–1049. [DOI] [PubMed] [Google Scholar]
- 78.Tang Y., Qian S. W., Wu M. Y., Wang J., Lu P., Li X., Huang H. Y., Guo L., Sun X., Xu C. J., et al. 2016. BMP4 mediates the interplay between adipogenesis and angiogenesis during expansion of subcutaneous white adipose tissue. J. Mol. Cell Biol. 8: 302–312. [DOI] [PubMed] [Google Scholar]
- 79.Hino J., Miyazawa T., Miyazato M., and Kangawa K.. 2012. Bone morphogenetic protein-3b (BMP-3b) is expressed in adipocytes and inhibits adipogenesis as a unique complex. Int. J. Obes. (Lond.). 36: 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hino J., Nakatani M., Arai Y., Tsuchida K., Shirai M., Miyazato M., and Kangawa K.. 2017. Overexpression of bone morphogenetic protein-3b (BMP-3b) in adipose tissues protects against high-fat diet-induced obesity. Int. J. Obes. (Lond.). 41: 483–488. [DOI] [PubMed] [Google Scholar]
- 81.Whittle A. J., Carobbio S., Martins L., Slawik M., Hondares E., Vazquez M. J., Morgan D., Csikasz R. I., Gallego R., Rodriguez-Cuenca S., et al. 2012. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 149: 871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pellegrinelli V., Peirce V. J., Howard L., Virtue S., Turei D., Senzacqua M., Frontini A., Dalley J. W., Horton A. R., Bidault G., et al. 2018. Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nat. Commun. 9: 4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Townsend K. L., Suzuki R., Huang T. L., Jing E., Schulz T. J., Lee K., Taniguchi C. M., Espinoza D. O., McDougall L. E., Zhang H., et al. 2012. Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. FASEB J. 26: 2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boon M. R., van den Berg S. A., Wang Y., van den Bossche J., Karkampouna S., Bauwens M., De Saint-Hubert M., van der Horst G., Vukicevic S., de Winther M. P., et al. 2013. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS One. 8: e74083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elsen M., Raschke S., Tennagels N., Schwahn U., Jelenik T., Roden M., Romacho T., and Eckel J.. 2014. BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells. Am. J. Physiol. Cell Physiol. 306: C431–C440. [DOI] [PubMed] [Google Scholar]
- 86.McDonald M. E., Li C., Bian H., Smith B. D., Layne M. D., and Farmer S. R.. 2015. Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell. 160: 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wittamer V., Franssen J. D., Vulcano M., Mirjolet J. F., Le Poul E., Migeotte I., Brezillon S., Tyldesley R., Blanpain C., Detheux M., et al. 2003. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 198: 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zabel B. A., Allen S. J., Kulig P., Allen J. A., Cichy J., Handel T. M., and Butcher E. C.. 2005. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 280: 34661–34666. [DOI] [PubMed] [Google Scholar]
- 89.Yamaguchi Y., Du X. Y., Zhao L., Morser J., and Leung L. L.. 2011. Proteolytic cleavage of chemerin protein is necessary for activation to the active form, Chem157S, which functions as a signaling molecule in glioblastoma. J. Biol. Chem. 286: 39510–39519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao L., Yamaguchi Y., Sharif S., Du X. Y., Song J. J., Lee D. M., Recht L. D., Robinson W. H., Morser J., and Leung L. L.. 2011. Chemerin158K protein is the dominant chemerin isoform in synovial and cerebrospinal fluids but not in plasma. J. Biol. Chem. 286: 39520–39527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roh S. G., Song S. H., Choi K. C., Katoh K., Wittamer V., Parmentier M., and Sasaki S.. 2007. Chemerin–a new adipokine that modulates adipogenesis via its own receptor. Biochem. Biophys. Res. Commun. 362: 1013–1018. [DOI] [PubMed] [Google Scholar]
- 92.De Henau O., Degroot G. N., Imbault V., Robert V., De Poorter C., McHeik S., Gales C., Parmentier M., and Springael J. Y.. 2016. Signaling properties of chemerin receptors CMKLR1, GPR1 and CCRL2. PLoS One. 11: e0164179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zabel B. A., Nakae S., Zuniga L., Kim J. Y., Ohyama T., Alt C., Pan J., Suto H., Soler D., Allen S. J., et al. 2008. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J. Exp. Med. 205: 2207–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barnea G., Strapps W., Herrada G., Berman Y., Ong J., Kloss B., Axel R., and Lee K. J.. 2008. The genetic design of signaling cascades to record receptor activation. Proc. Natl. Acad. Sci. USA. 105: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang S. S., Eisenberg D., Zhao L., Adams C., Leib R., Morser J., and Leung L.. 2016. Chemerin activation in human obesity. Obesity (Silver Spring). 24: 1522–1529. [DOI] [PubMed] [Google Scholar]
- 96.Bozaoglu K., Bolton K., McMillan J., Zimmet P., Jowett J., Collier G., Walder K., and Segal D.. 2007. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 148: 4687–4694. [DOI] [PubMed] [Google Scholar]
- 97.Chu S. H., Lee M. K., Ahn K. Y., Im J. A., Park M. S., Lee D. C., Jeon J. Y., and Lee J. W.. 2012. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. PLoS One. 7: e34710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Landgraf K., Friebe D., Ullrich T., Kratzsch J., Dittrich K., Herberth G., Adams V., Kiess W., Erbs S., and Korner A.. 2012. Chemerin as a mediator between obesity and vascular inflammation in children. J. Clin. Endocrinol. Metab. 97: E556–E564. [DOI] [PubMed] [Google Scholar]
- 99.Andersson D. P., Laurencikiene J., Acosta J. R., Ryden M., and Arner P.. 2016. Circulating and adipose levels of adipokines associated with insulin sensitivity in nonobese subjects with type 2 diabetes. J. Clin. Endocrinol. Metab. 101: 3765–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zylla S., Pietzner M., Kuhn J. P., Volzke H., Dorr M., Nauck M., and Friedrich N.. 2017. Serum chemerin is associated with inflammatory and metabolic parameters-results of a population-based study. Obesity (Silver Spring). 25: 468–475. [DOI] [PubMed] [Google Scholar]
- 101.Ebert T., Gebhardt C., Scholz M., Wohland T., Schleinitz D., Fasshauer M., Bluher M., Stumvoll M., Kovacs P., and Tonjes A.. 2018. Relationship between 12 adipocytokines and distinct components of the metabolic syndrome. J. Clin. Endocrinol. Metab. 103: 1015–1023. [DOI] [PubMed] [Google Scholar]
- 102.Zhao L., Yamaguchi Y., Shen W. J., Morser J., and Leung L. L. K.. 2018. Dynamic and tissue-specific proteolytic processing of chemerin in obese mice. PLoS One. 13: e0202780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Watts S. W., Dorrance A. M., Penfold M. E., Rourke J. L., Sinal C. J., Seitz B., Sullivan T. J., Charvat T. T., Thompson J. M., Burnett R., et al. 2013. Chemerin connects fat to arterial contraction. Arterioscler. Thromb. Vasc. Biol. 33: 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Neves K. B., Nguyen Dinh Cat A., Lopes R. A., Rios F. J., Anagnostopoulou A., Lobato N. S., de Oliveira A. M., Tostes R. C., Montezano A. C., and Touyz R. M.. 2015. Chemerin regulates crosstalk between adipocytes and vascular cells through Nox. Hypertension. 66: 657–666. [DOI] [PubMed] [Google Scholar]
- 105.Neves K. B., Nguyen Dinh Cat A., Alves-Lopes R., Harvey K. Y., da Costa R. M., Lobato N. S., Montezano A. C., de Oliveira A. M., Touyz R. M., and Tostes R. C.. 2018. Chemerin receptor blockade improves vascular function in diabetic obese mice via redox-sensitive- and Akt-dependent pathways. Am. J. Physiol. Heart Circ. Physiol. 315: H1851–H1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sell H., Laurencikiene J., Taube A., Eckardt K., Cramer A., Horrighs A., Arner P., and Eckel J.. 2009. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes. 58: 2731–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xie Q., Deng Y., Huang C., Liu P., Yang Y., Shen W., and Gao P.. 2015. Chemerin-induced mitochondrial dysfunction in skeletal muscle. J. Cell. Mol. Med. 19: 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramos-Junior E. S., Leite G. A., Carmo-Silva C. C., Taira T. M., Neves K. B., Colon D. F., da Silva L. A., Salvador S. L., Tostes R. C., Cunha F. Q., et al. 2017. Adipokine chemerin bridges metabolic dyslipidemia and alveolar bone loss in mice. J. Bone Miner. Res. 32: 974–984. [DOI] [PubMed] [Google Scholar]
- 109.Goralski K. B., McCarthy T. C., Hanniman E. A., Zabel B. A., Butcher E. C., Parlee S. D., Muruganandan S., and Sinal C. J.. 2007. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 282: 28175–28188. [DOI] [PubMed] [Google Scholar]
- 110.Rouger L., Denis G. R., Luangsay S., and Parmentier M.. 2013. ChemR23 knockout mice display mild obesity but no deficit in adipocyte differentiation. J. Endocrinol. 219: 279–289. [DOI] [PubMed] [Google Scholar]
- 111.Huang C., Wang M., Ren L., Xiang L., Chen J., Li M., Xiao T., Ren P., Xiong L., and Zhang J. V.. 2016. CMKLR1 deficiency influences glucose tolerance and thermogenesis in mice on high fat diet. Biochem. Biophys. Res. Commun. 473: 435–441. [DOI] [PubMed] [Google Scholar]
- 112.Takahashi M., Okimura Y., Iguchi G., Nishizawa H., Yamamoto M., Suda K., Kitazawa R., Fujimoto W., Takahashi K., Zolotaryov F. N., et al. 2011. Chemerin regulates beta-cell function in mice. Sci. Rep. 1: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ernst M. C., Issa M., Goralski K. B., and Sinal C. J.. 2010. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology. 151: 1998–2007. [DOI] [PubMed] [Google Scholar]
- 114.Ernst M. C., Haidl I. D., Zuniga L. A., Dranse H. J., Rourke J. L., Zabel B. A., Butcher E. C., and Sinal C. J.. 2012. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology. 153: 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gruben N., Aparicio Vergara M., Kloosterhuis N. J., van der Molen H., Stoelwinder S., Youssef S., de Bruin A., Delsing D. J., Kuivenhoven J. A., van de Sluis B., et al. 2014. Chemokine-like receptor 1 deficiency does not affect the development of insulin resistance and nonalcoholic fatty liver disease in mice. PLoS One. 9: e96345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rourke J. L., Muruganandan S., Dranse H. J., McMullen N. M., and Sinal C. J.. 2014. Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice. J. Endocrinol. 222: 201–215. [DOI] [PubMed] [Google Scholar]
- 117.Xia J. Y., Sun K., Hepler C., Ghaben A. L., Gupta R. K., An Y. A., Holland W. L., Morley T. S., Adams A. C., Gordillo R., et al. 2018. Acute loss of adipose tissue-derived adiponectin triggers immediate metabolic deterioration in mice. Diabetologia. 61: 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun K., Park J., Kim M., and Scherer P. E.. 2017. Endotrophin, a multifaceted player in metabolic dysregulation and cancer progression, is a predictive biomarker for the response to PPARgamma agonist treatment. Diabetologia. 60: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cescon M., Gattazzo F., Chen P., and Bonaldo P.. 2015. Collagen VI at a glance. J. Cell Sci. 128: 3525–3531. [DOI] [PubMed] [Google Scholar]
- 120.Pasarica M., Gowronska-Kozak B., Burk D., Remedios I., Hymel D., Gimble J., Ravussin E., Bray G. A., and Smith S. R.. 2009. Adipose tissue collagen VI in obesity. J. Clin. Endocrinol. Metab. 94: 5155–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Karsdal M. A., Henriksen K., Genovese F., Leeming D. J., Nielsen M. J., Riis B. J., Christiansen C., Byrjalsen I., and Schuppan D.. 2017. Serum endotrophin identifies optimal responders to PPARgamma agonists in type 2 diabetes. Diabetologia. 60: 50–59. [DOI] [PubMed] [Google Scholar]
- 122.Iyengar P., Combs T. P., Shah S. J., Gouon-Evans V., Pollard J. W., Albanese C., Flanagan L., Tenniswood M. P., Guha C., Lisanti M. P., et al. 2003. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 22: 6408–6423. [DOI] [PubMed] [Google Scholar]
- 123.Iyengar P., Espina V., Williams T. W., Lin Y., Berry D., Jelicks L. A., Lee H., Temple K., Graves R., Pollard J., et al. 2005. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J. Clin. Invest. 115: 1163–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park J., and Scherer P. E.. 2012. Adipocyte-derived endotrophin promotes malignant tumor progression. J. Clin. Invest. 122: 4243–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Park J., Morley T. S., and Scherer P. E.. 2013. Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Mol. Med. 5: 935–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bu D., Crewe C., Kusminski C. M., Gordillo R., Ghaben A. L., Kim M., Park J., Deng H., Xiong W., Liu X. Z., et al. 2019. Human endotrophin as a driver of malignant tumor growth. JCI Insight. 5: 125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee C., Kim M., Lee J. H., Oh J., Shin H. H., Lee S. M., Scherer P. E., Kwon H. M., Choi J. H., and Park J.. 2018. COL6A3-derived endotrophin links reciprocal interactions among hepatic cells in the pathology of chronic liver disease. J. Pathol. 247: 99–109. [DOI] [PubMed] [Google Scholar]
- 128.Khan T., Muise E. S., Iyengar P., Wang Z. V., Chandalia M., Abate N., Zhang B. B., Bonaldo P., Chua S., and Scherer P. E.. 2009. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29: 1575–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun K., Park J., Gupta O. T., Holland W. L., Auerbach P., Zhang N., Goncalves Marangoni R., Nicoloro S. M., Czech M. P., Varga J., et al. 2014. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat. Commun. 5: 3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Staiger H., Keuper M., Berti L., Hrabe de Angelis M., and Haring H. U.. 2017. Fibroblast growth factor 21-metabolic role in mice and men. Endocr. Rev. 38: 468–488. [DOI] [PubMed] [Google Scholar]
- 131.Suzuki M., Uehara Y., Motomura-Matsuzaka K., Oki J., Koyama Y., Kimura M., Asada M., Komi-Kuramochi A., Oka S., and Imamura T.. 2008. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR)1c and FGFR3c. Mol. Endocrinol. 22: 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kharitonenkov A., Dunbar J. D., Bina H. A., Bright S., Moyers J. S., Zhang C., Ding L., Micanovic R., Mehrbod S. F., Knierman M. D., et al. 2008. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J. Cell. Physiol. 215: 1–7. [DOI] [PubMed] [Google Scholar]
- 133.Kurosu H., Choi M., Ogawa Y., Dickson A. S., Goetz R., Eliseenkova A. V., Mohammadi M., Rosenblatt K. P., Kliewer S. A., and Kuro-o M.. 2007. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 282: 26687–26695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kharitonenkov A., Shiyanova T. L., Koester A., Ford A. M., Micanovic R., Galbreath E. J., Sandusky G. E., Hammond L. J., Moyers J. S., Owens R. A., et al. 2005. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115: 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Coskun T., Bina H. A., Schneider M. A., Dunbar J. D., Hu C. C., Chen Y., Moller D. E., and Kharitonenkov A.. 2008. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 149: 6018–6027. [DOI] [PubMed] [Google Scholar]
- 136.Xu J., Lloyd D. J., Hale C., Stanislaus S., Chen M., Sivits G., Vonderfecht S., Hecht R., Li Y. S., Lindberg R. A., et al. 2009. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 58: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Foltz I. N., Hu S., King C., Wu X., Yang C., Wang W., Weiszmann J., Stevens J., Chen J. S., Nuanmanee N., et al. 2012. Treating diabetes and obesity with an FGF21-mimetic antibody activating the betaKlotho/FGFR1c receptor complex. Sci. Transl. Med. 4: 162ra153. [DOI] [PubMed] [Google Scholar]
- 138.Gaich G., Chien J. Y., Fu H., Glass L. C., Deeg M. A., Holland W. L., Kharitonenkov A., Bumol T., Schilske H. K., and Moller D. E.. 2013. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18: 333–340. [DOI] [PubMed] [Google Scholar]
- 139.Talukdar S., Zhou Y., Li D., Rossulek M., Dong J., Somayaji V., Weng Y., Clark R., Lanba A., Owen B. M., et al. 2016. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 23: 427–440. [DOI] [PubMed] [Google Scholar]
- 140.Talukdar S., Owen B. M., Song P., Hernandez G., Zhang Y., Zhou Y., Scott W. T., Paratala B., Turner T., Smith A., et al. 2016. FGF21 regulates sweet and alcohol preference. Cell Metab. 23: 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.von Holstein-Rathlou S., BonDurant L. D., Peltekian L., Naber M. C., Yin T. C., Claflin K. E., Urizar A. I., Madsen A. N., Ratner C., Holst B., et al. 2016. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 23: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wei W., Dutchak P. A., Wang X., Ding X., Wang X., Bookout A. L., Goetz R., Mohammadi M., Gerard R. D., Dechow P. C., et al. 2012. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc. Natl. Acad. Sci. USA. 109: 3143–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bornstein S., Brown S. A., Le P. T., Wang X., DeMambro V., Horowitz M. C., MacDougald O., Baron R., Lotinun S., Karsenty G., et al. 2014. FGF-21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology. 155: 3516–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hondares E., Rosell M., Gonzalez F. J., Giralt M., Iglesias R., and Villarroya F.. 2010. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 11: 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ding X., Boney-Montoya J., Owen B. M., Bookout A. L., Coate K. C., Mangelsdorf D. J., and Kliewer S. A.. 2012. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 16: 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fisher F. M., Kleiner S., Douris N., Fox E. C., Mepani R. J., Verdeguer F., Wu J., Kharitonenkov A., Flier J. S., Maratos-Flier E., et al. 2012. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Owen B. M., Ding X., Morgan D. A., Coate K. C., Bookout A. L., Rahmouni K., Kliewer S. A., and Mangelsdorf D. J.. 2014. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 20: 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Markan K. R., Naber M. C., Ameka M. K., Anderegg M. D., Mangelsdorf D. J., Kliewer S. A., Mohammadi M., and Potthoff M. J.. 2014. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 63: 4057–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bernardo B., Lu M., Bandyopadhyay G., Li P., Zhou Y., Huang J., Levin N., Tomas E. M., Calle R. A., Erion D. M., et al. 2015. FGF21 does not require interscapular brown adipose tissue and improves liver metabolic profile in animal models of obesity and insulin-resistance. Sci. Rep. 5: 11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Douris N., Stevanovic D. M., Fisher F. M., Cisu T. I., Chee M. J., Nguyen N. L., Zarebidaki E., Adams A. C., Kharitonenkov A., Flier J. S., et al. 2015. Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology. 156: 2470–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kwon M. M., O’Dwyer S. M., Baker R. K., Covey S. D., and Kieffer T. J.. 2015. FGF21-mediated improvements in glucose clearance require uncoupling protein 1. Cell Reports. 13: 1521–1527. [DOI] [PubMed] [Google Scholar]
- 152.Véniant M. M., Sivits G., Helmering J., Komorowski R., Lee J., Fan W., Moyer C., and Lloyd D. J.. 2015. Pharmacologic effects of FGF21 are independent of the “browning” of white adipose tissue. Cell Metab. 21: 731–738. [DOI] [PubMed] [Google Scholar]
- 153.BonDurant L. D., Ameka M., Naber M. C., Markan K. R., Idiga S. O., Acevedo M. R., Walsh S. A., Ornitz D. M., and Potthoff M. J.. 2017. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 25: 935–944.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang Z., Zhong L., Lee J. T. H., Zhang J., Wu D., Geng L., Wang Y., Wong C. M., and Xu A.. 2017. The FGF21-CCL11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunity. Cell Metab. 26: 493–508.e4. [DOI] [PubMed] [Google Scholar]
- 155.Adams A. C., Yang C., Coskun T., Cheng C. C., Gimeno R. E., Luo Y., and Kharitonenkov A.. 2012. The breadth of FGF21’s metabolic actions are governed by FGFR1 in adipose tissue. Mol. Metab. 2: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Holland W. L., Adams A. C., Brozinick J. T., Bui H. H., Miyauchi Y., Kusminski C. M., Bauer S. M., Wade M., Singhal E., Cheng C. C., et al. 2013. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 17: 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lin Z., Tian H., Lam K. S., Lin S., Hoo R. C., Konishi M., Itoh N., Wang Y., Bornstein S. R., Xu A., et al. 2013. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17: 779–789. [DOI] [PubMed] [Google Scholar]
- 158.Bookout A. L., de Groot M. H., Owen B. M., Lee S., Gautron L., Lawrence H. L., Ding X., Elmquist J. K., Takahashi J. S., Mangelsdorf D. J., et al. 2013. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 19: 1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liang Q., Zhong L., Zhang J., Wang Y., Bornstein S. R., Triggle C. R., Ding H., Lam K. S., and Xu A.. 2014. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 63: 4064–4075. [DOI] [PubMed] [Google Scholar]
- 160.Samms R. J., Smith D. P., Cheng C. C., Antonellis P. P., Perfield J. W. 2nd, Kharitonenkov A., Gimeno R. E., and Adams A. C.. 2015. Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell Reports. 11: 991–999. [DOI] [PubMed] [Google Scholar]
- 161.Lan T., Morgan D. A., Rahmouni K., Sonoda J., Fu X., Burgess S. C., Holland W. L., Kliewer S. A., and Mangelsdorf D. J.. 2017. FGF19, FGF21, and an FGFR1/beta-Klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab. 26: 709–718.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu J., Stanislaus S., Chinookoswong N., Lau Y. Y., Hager T., Patel J., Ge H., Weiszmann J., Lu S. C., Graham M., et al. 2009. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models–association with liver and adipose tissue effects. Am. J. Physiol. Endocrinol. Metab. 297: E1105–E1114. [DOI] [PubMed] [Google Scholar]
- 163.Ge X., Chen C., Hui X., Wang Y., Lam K. S., and Xu A.. 2011. Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/Ets-like protein-1 in adipocytes. J. Biol. Chem. 286: 34533–34541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li X., Ge H., Weiszmann J., Hecht R., Li Y. S., Veniant M. M., Xu J., Wu X., Lindberg R., and Li Y.. 2009. Inhibition of lipolysis may contribute to the acute regulation of plasma FFA and glucose by FGF21 in ob/ob mice. FEBS Lett. 583: 3230–3234. [DOI] [PubMed] [Google Scholar]
- 165.Chen M. Z., Chang J. C., Zavala-Solorio J., Kates L., Thai M., Ogasawara A., Bai X., Flanagan S., Nunez V., Phamluong K., et al. 2017. FGF21 mimetic antibody stimulates UCP1-independent brown fat thermogenesis via FGFR1/betaKlotho complex in non-adipocytes. Mol. Metab. 6: 1454–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Keipert S., Kutschke M., Ost M., Schwarzmayr T., van Schothorst E. M., Lamp D., Brachthauser L., Hamp I., Mazibuko S. E., Hartwig S., et al. 2017. Long-term cold adaptation does not require FGF21 or UCP1. Cell Metab. 26: 437–446.e5. [DOI] [PubMed] [Google Scholar]
- 167.Zhang J., Wu Y., Zhang Y., Leroith D., Bernlohr D. A., and Chen X.. 2008. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol. Endocrinol. 22: 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang J., Goetz D., Li J. Y., Wang W., Mori K., Setlik D., Du T., Erdjument-Bromage H., Tempst P., Strong R., et al. 2002. An iron delivery pathway mediated by a lipocalin. Mol. Cell. 10: 1045–1056. [DOI] [PubMed] [Google Scholar]
- 169.Goetz D. H., Holmes M. A., Borregaard N., Bluhm M. E., Raymond K. N., and Strong R. K.. 2002. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell. 10: 1033–1043. [DOI] [PubMed] [Google Scholar]
- 170.Devireddy L. R., Gazin C., Zhu X., and Green M. R.. 2005. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 123: 1293–1305. [DOI] [PubMed] [Google Scholar]
- 171.Devireddy L. R., Hart D. O., Goetz D. H., and Green M. R.. 2010. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 141: 1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bao G., Clifton M., Hoette T. M., Mori K., Deng S. X., Qiu A., Viltard M., Williams D., Paragas N., Leete T., et al. 2010. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat. Chem. Biol. 6: 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xiao X., Yeoh B. S., and Vijay-Kumar M.. 2017. Lipocalin 2: an emerging player in iron homeostasis and inflammation. Annu. Rev. Nutr. 37: 103–130. [DOI] [PubMed] [Google Scholar]
- 174.Yan Q. W., Yang Q., Mody N., Graham T. E., Hsu C. H., Xu Z., Houstis N. E., Kahn B. B., and Rosen E. D.. 2007. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 56: 2533–2540. [DOI] [PubMed] [Google Scholar]
- 175.Milner K. L., van der Poorten D., Xu A., Bugianesi E., Kench J. G., Lam K. S., Chisholm D. J., and George J.. 2009. Adipocyte fatty acid binding protein levels relate to inflammation and fibrosis in nonalcoholic fatty liver disease. Hepatology. 49: 1926–1934. [DOI] [PubMed] [Google Scholar]
- 176.Auguet T., Quintero Y., Terra X., Martinez S., Lucas A., Pellitero S., Aguilar C., Hernandez M., del Castillo D., and Richart C.. 2011. Upregulation of lipocalin 2 in adipose tissues of severely obese women: positive relationship with proinflammatory cytokines. Obesity (Silver Spring). 19: 2295–2300. [DOI] [PubMed] [Google Scholar]
- 177.Liu X., Hamnvik O. P., Petrou M., Gong H., Chamberland J. P., Christophi C. A., Kales S. N., Christiani D. C., and Mantzoros C. S.. 2011. Circulating lipocalin 2 is associated with body fat distribution at baseline but is not an independent predictor of insulin resistance: the prospective Cyprus Metabolism Study. Eur. J. Endocrinol. 165: 805–812. [DOI] [PubMed] [Google Scholar]
- 178.Guo H., Bazuine M., Jin D., Huang M. M., Cushman S. W., and Chen X.. 2013. Evidence for the regulatory role of lipocalin 2 in high-fat diet-induced adipose tissue remodeling in male mice. Endocrinology. 154: 3525–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song E., Fan P., Huang B., Deng H. B., Cheung B. M., Feletou M., Vilaine J. P., Villeneuve N., Xu A., Vanhoutte P. M., et al. 2014. Deamidated lipocalin-2 induces endothelial dysfunction and hypertension in dietary obese mice. J. Am. Heart Assoc. 3: e000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wu G., Li H., Fang Q., Jiang S., Zhang L., Zhang J., Hou X., Lu J., Bao Y., Xu A., et al. 2014. Elevated circulating lipocalin-2 levels independently predict incident cardiovascular events in men in a population-based cohort. Arterioscler. Thromb. Vasc. Biol. 34: 2457–2464. [DOI] [PubMed] [Google Scholar]
- 181.Oberoi R., Bogalle E. P., Matthes L. A., Schuett H., Koch A. K., Grote K., Schieffer B., Schuett J., and Luchtefeld M.. 2015. Lipocalin (LCN) 2 mediates pro-atherosclerotic processes and is elevated in patients with coronary artery disease. PLoS One. 10: e0137924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ye D., Yang K., Zang S., Lin Z., Chau H. T., Wang Y., Zhang J., Shi J., Xu A., Lin S., et al. 2016. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of CXCR2. J. Hepatol. 65: 988–997. [DOI] [PubMed] [Google Scholar]
- 183.Guo H., Jin D., Zhang Y., Wright W., Bazuine M., Brockman D. A., Bernlohr D. A., and Chen X.. 2010. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 59: 1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Law I. K., Xu A., Lam K. S., Berger T., Mak T. W., Vanhoutte P. M., Liu J. T., Sweeney G., Zhou M., Yang B., et al. 2010. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 59: 872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jin D., Guo H., Bu S. Y., Zhang Y., Hannaford J., Mashek D. G., and Chen X.. 2011. Lipocalin 2 is a selective modulator of peroxisome proliferator-activated receptor-gamma activation and function in lipid homeostasis and energy expenditure. FASEB J. 25: 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jun L. S., Siddall C. P., and Rosen E. D.. 2011. A minor role for lipocalin 2 in high-fat diet-induced glucose intolerance. Am. J. Physiol. Endocrinol. Metab. 301: E825–E835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu J. T., Song E., Xu A., Berger T., Mak T. W., Tse H. F., Law I. K., Huang B., Liang Y., Vanhoutte P. M., et al. 2012. Lipocalin-2 deficiency prevents endothelial dysfunction associated with dietary obesity: role of cytochrome P450 2C inhibition. Br. J. Pharmacol. 165: 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang B., Fan P., Xu A., Lam K. S., Berger T., Mak T. W., Tse H. F., Yue J. W., Song E., Vanhoutte P. M., et al. 2012. Improved functional recovery to I/R injury in hearts from lipocalin-2 deficiency mice: restoration of mitochondrial function and phospholipids remodeling. Am. J. Transl. Res. 4: 60–71. [PMC free article] [PubMed] [Google Scholar]
- 189.Asimakopoulou A., Borkham-Kamphorst E., Henning M., Yagmur E., Gassler N., Liedtke C., Berger T., Mak T. W., and Weiskirchen R.. 2014. Lipocalin-2 (LCN2) regulates PLIN5 expression and intracellular lipid droplet formation in the liver. Biochim. Biophys. Acta. 1842: 1513–1524. [DOI] [PubMed] [Google Scholar]
- 190.Zhang Y., Guo H., Deis J. A., Mashek M. G., Zhao M., Ariyakumar D., Armien A. G., Bernlohr D. A., Mashek D. G., and Chen X.. 2014. Lipocalin 2 regulates brown fat activation via a nonadrenergic activation mechanism. J. Biol. Chem. 289: 22063–22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ishii A., Katsuura G., Imamaki H., Kimura H., Mori K. P., Kuwabara T., Kasahara M., Yokoi H., Ohinata K., Kawanishi T., et al. 2017. Obesity-promoting and anti-thermogenic effects of neutrophil gelatinase-associated lipocalin in mice. Sci. Rep. 7: 15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Paton C. M., Rogowski M. P., Kozimor A. L., Stevenson J. L., Chang H., and Cooper J. A.. 2013. Lipocalin-2 increases fat oxidation in vitro and is correlated with energy expenditure in normal weight but not obese women. Obesity (Silver Spring). 21: E640–E648. [DOI] [PubMed] [Google Scholar]
- 193.Principi E., Buschiazzo A., Papait A., Castagnola P., Costa D., Pece R., Maric I., Scussolini M., Marini C., Sambuceti G., et al. 2019. Anthropometric and glucometabolic changes in an aged mouse model of lipocalin-2 overexpression. Int. J. Obes. (Lond.). 43: 189–201. [DOI] [PubMed] [Google Scholar]
- 194.Kamble P. G., Pereira M. J., Sidibeh C. O., Amini S., Sundbom M., Borjesson J. L., and Eriksson J. W.. 2016. Lipocalin 2 produces insulin resistance and can be upregulated by glucocorticoids in human adipose tissue. Mol. Cell. Endocrinol. 427: 124–132. [DOI] [PubMed] [Google Scholar]
- 195.Kusminski C. M., Holland W. L., Sun K., Park J., Spurgin S. B., Lin Y., Askew G. R., Simcox J. A., McClain D. A., Li C., et al. 2012. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 18: 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kusminski C. M., Park J., and Scherer P. E.. 2014. MitoNEET-mediated effects on browning of white adipose tissue. Nat. Commun. 5: 3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wernstedt Asterholm I., Tao C., Morley T. S., Wang Q. A., Delgado-Lopez F., Wang Z. V., and Scherer P. E.. 2014. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 20: 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mauer J., Denson J. L., and Bruning J. C.. 2015. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 36: 92–101. [DOI] [PubMed] [Google Scholar]
- 199.Jia L., Chang X., Qian S., Liu C., Lord C. C., Ahmed N., Lee C. E., Lee S., Gautron L., Mitchell M. C., et al. 2018. Hepatocyte toll-like receptor 4 deficiency protects against alcohol-induced fatty liver disease. Mol. Metab. 14: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mei L., and Nave K. A.. 2014. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 83: 27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang G. X., Zhao X. Y., Meng Z. X., Kern M., Dietrich A., Chen Z., Cozacov Z., Zhou D., Okunade A. L., Su X., et al. 2014. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 20: 1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rosell M., Kaforou M., Frontini A., Okolo A., Chan Y. W., Nikolopoulou E., Millership S., Fenech M. E., MacIntyre D., Turner J. O., et al. 2014. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am. J. Physiol. Endocrinol. Metab. 306: E945–E964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ma Y., Gao M., and Liu D.. 2016. Preventing high fat diet-induced obesity and improving insulin sensitivity through neuregulin 4 gene transfer. Sci. Rep. 6: 26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen Z., Wang G. X., Ma S. L., Jung D. Y., Ha H., Altamimi T., Zhao X. Y., Guo L., Zhang P., Hu C. R., et al. 2017. Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Mol. Metab. 6: 863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nugroho D. B., Ikeda K., Barinda A. J., Wardhana D. A., Yagi K., Miyata K., Oike Y., Hirata K. I., and Emoto N.. 2018. Neuregulin-4 is an angiogenic factor that is critically involved in the maintenance of adipose tissue vasculature. Biochem. Biophys. Res. Commun. 503: 378–384. [DOI] [PubMed] [Google Scholar]
- 206.Dai Y. N., Zhu J. Z., Fang Z. Y., Zhao D. J., Wan X. Y., Zhu H. T., Yu C. H., and Li Y. M.. 2015. A case-control study: Association between serum neuregulin 4 level and non-alcoholic fatty liver disease. Metabolism. 64: 1667–1673. [DOI] [PubMed] [Google Scholar]
- 207.Cai C., Lin M., Xu Y., Li X., Yang S., and Zhang H.. 2016. Association of circulating neuregulin 4 with metabolic syndrome in obese adults: a cross-sectional study. BMC Med. 14: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kang Y. E., Kim J. M., Choung S., Joung K. H., Lee J. H., Kim H. J., and Ku B. J.. 2016. Comparison of serum Neuregulin 4 (Nrg4) levels in adults with newly diagnosed type 2 diabetes mellitus and controls without diabetes. Diabetes Res. Clin. Pract. 117: 1–3. [DOI] [PubMed] [Google Scholar]
- 209.Chen L. L., Peng M. M., Zhang J. Y., Hu X., Min J., Huang Q. L., and Wan L. M.. 2017. Elevated circulating Neuregulin4 level in patients with diabetes. Diabetes Metab. Res. Rev. 33: doi:10.1002/dmrr.2870. [DOI] [PubMed] [Google Scholar]
- 210.Zhang L., Fu Y., Zhou N., Cheng X., and Chen C.. 2017. Circulating neuregulin 4 concentrations in patients with newly diagnosed type 2 diabetes: a cross-sectional study. Endocrine. 57: 535–538. [DOI] [PubMed] [Google Scholar]
- 211.Wang R., Yang F., Qing L., Huang R., Liu Q., and Li X.. 2019. Decreased serum neuregulin 4 levels associated with non-alcoholic fatty liver disease in children with obesity. Clin. Obes. 9: e12289. [DOI] [PubMed] [Google Scholar]
- 212.Zeng F., Wang Y., Kloepfer L. A., Wang S., and Harris R. C.. 2018. ErbB4 deletion predisposes to development of metabolic syndrome in mice. Am. J. Physiol. Endocrinol. Metab. 315: E583–E593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nugroho D. B., Ikeda K., Kajimoto K., Hirata K. I., and Emoto N.. 2018. Activation of neuregulin-4 in adipocytes improves metabolic health by enhancing adipose tissue angiogenesis. Biochem. Biophys. Res. Commun. 504: 427–433. [DOI] [PubMed] [Google Scholar]
- 214.Saleheen D., Natarajan P., Armean I. M., Zhao W., Rasheed A., Khetarpal S. A., Won H. H., Karczewski K. J., O’Donnell-Luria A. H., Samocha K. E., et al. 2017. Human knockouts and phenotypic analysis in a cohort with a high rate of consanguinity. Nature. 544: 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yore M. M., Syed I., Moraes-Vieira P. M., Zhang T., Herman M. A., Homan E. A., Patel R. T., Lee J., Chen S., Peroni O. D., et al. 2014. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 159: 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kuda O., Brezinova M., Silhavy J., Landa V., Zidek V., Dodia C., Kreuchwig F., Vrbacky M., Balas L., Durand T., et al. 2018. Nrf2-mediated antioxidant defense and peroxiredoxin 6 are linked to biosynthesis of palmitic acid ester of 9-hydroxystearic acid. Diabetes. 67: 1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pflimlin E., Bielohuby M., Korn M., Breitschopf K., Lohn M., Wohlfart P., Konkar A., Podeschwa M., Barenz F., Pfenninger A., et al. 2018. Acute and repeated treatment with 5-PAHSA or 9-PAHSA isomers does not improve glucose control in mice. Cell Metab. 28: 217–227.e13. [DOI] [PubMed] [Google Scholar]
- 218.Syed I., Lee J., Moraes-Vieira P. M., Donaldson C. J., Sontheimer A., Aryal P., Wellenstein K., Kolar M. J., Nelson A. T., Siegel D., et al. 2018. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab. 27: 419–427.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hammarstedt A., Syed I., Vijayakumar A., Eliasson B., Gogg S., Kahn B. B., and Smith U.. 2018. Adipose tissue dysfunction is associated with low levels of the novel palmitic acid hydroxystearic acids. Sci. Rep. 8: 15757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vijayakumar A., Aryal P., Wen J., Syed I., Vazirani R. P., Moraes-Vieira P. M., Camporez J. P., Gallop M. R., Perry R. J., Peroni O. D., et al. 2017. Absence of carbohydrate response element binding protein in adipocytes causes systemic insulin resistance and impairs glucose transport. Cell Reports. 21: 1021–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kuda O., Brezinova M., Rombaldova M., Slavikova B., Posta M., Beier P., Janovska P., Veleba J., Kopecky J. Jr., Kudova E., et al. 2016. Docosahexaenoic acid-derived fatty acid esters of hydroxy fatty acids (FAHFAs) with anti-inflammatory properties. Diabetes. 65: 2580–2590. [DOI] [PubMed] [Google Scholar]
- 222.Kuda O. 2018. On the complexity of PAHSA research. Cell Metab. 28: 541–542. [DOI] [PubMed] [Google Scholar]
- 223.Yung Y. C., Stoddard N. C., and Chun J.. 2014. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J. Lipid Res. 55: 1192–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rancoule C., Attane C., Gres S., Fournel A., Dusaulcy R., Bertrand C., Vinel C., Treguer K., Prentki M., Valet P., et al. 2013. Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high-fat diet obese mice. Diabetologia. 56: 1394–1402. [DOI] [PubMed] [Google Scholar]
- 225.Im D. S., Fujioka T., Katada T., Kondo Y., Ui M., and Okajima F.. 1997. Characterization of sphingosine 1-phosphate-induced actions and its signaling pathways in rat hepatocytes. Am. J. Physiol. 272: G1091–G1099. [DOI] [PubMed] [Google Scholar]
- 226.Valet P., Pages C., Jeanneton O., Daviaud D., Barbe P., Record M., Saulnier-Blache J. S., and Lafontan M.. 1998. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. A paracrine signal for preadipocyte growth. J. Clin. Invest. 101: 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pages C., Daviaud D., An S., Krief S., Lafontan M., Valet P., and Saulnier-Blache J. S.. 2001. Endothelial differentiation gene-2 receptor is involved in lysophosphatidic acid-dependent control of 3T3F442A preadipocyte proliferation and spreading. J. Biol. Chem. 276: 11599–11605. [DOI] [PubMed] [Google Scholar]
- 228.Simon M. F., Rey A., Castan-Laurel I., Gres S., Sibrac D., Valet P., and Saulnier-Blache J. S.. 2002. Expression of ectolipid phosphate phosphohydrolases in 3T3F442A preadipocytes and adipocytes. Involvement in the control of lysophosphatidic acid production. J. Biol. Chem. 277: 23131–23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ferry G., Tellier E., Try A., Gres S., Naime I., Simon M. F., Rodriguez M., Boucher J., Tack I., Gesta S., et al. 2003. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J. Biol. Chem. 278: 18162–18169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Simon M. F., Daviaud D., Pradere J. P., Gres S., Guigne C., Wabitsch M., Chun J., Valet P., and Saulnier-Blache J. S.. 2005. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2. J. Biol. Chem. 280: 14656–14662. [DOI] [PubMed] [Google Scholar]
- 231.Noguchi M., Hosoda K., Fujikura J., Fujimoto M., Iwakura H., Tomita T., Ishii T., Arai N., Hirata M., Ebihara K., et al. 2007. Genetic and pharmacological inhibition of Rho-associated kinase II enhances adipogenesis. J. Biol. Chem. 282: 29574–29583. [DOI] [PubMed] [Google Scholar]
- 232.Holmström T. E., Mattsson C. L., Wang Y., Iakovleva I., Petrovic N., and Nedergaard J.. 2010. Non-transactivational, dual pathways for LPA-induced Erk1/2 activation in primary cultures of brown pre-adipocytes. Exp. Cell Res. 316: 2664–2675. [DOI] [PubMed] [Google Scholar]
- 233.Mattsson C. L., Andersson E. R., and Nedergaard J.. 2010. Differential involvement of caveolin-1 in brown adipocyte signaling: impaired beta3-adrenergic, but unaffected LPA, PDGF and EGF receptor signaling. Biochim. Biophys. Acta. 1803: 983–989. [DOI] [PubMed] [Google Scholar]
- 234.Nobusue H., Kondo D., Yamamoto M., and Kano K.. 2010. Effects of lysophosphatidic acid on the in vitro proliferation and differentiation of a novel porcine preadipocyte cell line. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 157: 401–407. [DOI] [PubMed] [Google Scholar]
- 235.Li L., Tam L., Liu L., Jin T., and Ng D. S.. 2011. Wnt-signaling mediates the anti-adipogenic action of lysophosphatidic acid through cross talking with the Rho/Rho associated kinase (ROCK) pathway. Biochem. Cell Biol. 89: 515–521. [DOI] [PubMed] [Google Scholar]
- 236.Federico L., Ren H., Mueller P. A., Wu T., Liu S., Popovic J., Blalock E. M., Sunkara M., Ovaa H., Albers H. M., et al. 2012. Autotaxin and its product lysophosphatidic acid suppress brown adipose differentiation and promote diet-induced obesity in mice. Mol. Endocrinol. 26: 786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Contos J. J., Fukushima N., Weiner J. A., Kaushal D., and Chun J.. 2000. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. USA. 97: 13384–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dusaulcy R., Daviaud D., Pradere J. P., Gres S., Valet P., and Saulnier-Blache J. S.. 2009. Altered food consumption in mice lacking lysophosphatidic acid receptor-1. J. Physiol. Biochem. 65: 345–350. [DOI] [PubMed] [Google Scholar]
- 239.Rancoule C., Dusaulcy R., Treguer K., Gres S., Attane C., and Saulnier-Blache J. S.. 2014. Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie. 96: 140–143. [DOI] [PubMed] [Google Scholar]
- 240.Tanaka M., Okudaira S., Kishi Y., Ohkawa R., Iseki S., Ota M., Noji S., Yatomi Y., Aoki J., and Arai H.. 2006. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 281: 25822–25830. [DOI] [PubMed] [Google Scholar]
- 241.van Meeteren L. A., Ruurs P., Stortelers C., Bouwman P., van Rooijen M. A., Pradere J. P., Pettit T. R., Wakelam M. J., Saulnier-Blache J. S., Mummery C. L., et al. 2006. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 26: 5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fotopoulou S., Oikonomou N., Grigorieva E., Nikitopoulou I., Paparountas T., Thanassopoulou A., Zhao Z., Xu Y., Kontoyiannis D. L., Remboutsika E., et al. 2010. ATX expression and LPA signalling are vital for the development of the nervous system. Dev. Biol. 339: 451–464. [DOI] [PubMed] [Google Scholar]
- 243.Gesta S., Simon M. F., Rey A., Sibrac D., Girard A., Lafontan M., Valet P., and Saulnier-Blache J. S.. 2002. Secretion of a lysophospholipase D activity by adipocytes: involvement in lysophosphatidic acid synthesis. J. Lipid Res. 43: 904–910. [PMC free article] [PubMed] [Google Scholar]
- 244.Dusaulcy R., Rancoule C., Gres S., Wanecq E., Colom A., Guigne C., van Meeteren L. A., Moolenaar W. H., Valet P., and Saulnier-Blache J. S.. 2011. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J. Lipid Res. 52: 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Boucher J., Quilliot D., Praderes J. P., Simon M. F., Gres S., Guigne C., Prevot D., Ferry G., Boutin J. A., Carpene C., et al. 2005. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia. 48: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rancoule C., Dusaulcy R., Treguer K., Gres S., Guigne C., Quilliot D., Valet P., and Saulnier-Blache J. S.. 2012. Depot-specific regulation of autotaxin with obesity in human adipose tissue. J. Physiol. Biochem. 68: 635–644. [DOI] [PubMed] [Google Scholar]
- 247.Rachakonda V. P., Reeves V. L., Aljammal J., Wills R. C., Trybula J. S., DeLany J. P., Kienesberger P. C., and Kershaw E. E.. 2015. Serum autotaxin is independently associated with hepatic steatosis in women with severe obesity. Obesity (Silver Spring). 23: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Reeves V. L., Trybula J. S., Wills R. C., Goodpaster B. H., Dube J. J., Kienesberger P. C., and Kershaw E. E.. 2015. Serum Autotaxin/ENPP2 correlates with insulin resistance in older humans with obesity. Obesity (Silver Spring). 23: 2371–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.D’Souza K., Kane D. A., Touaibia M., Kershaw E. E., Pulinilkunnil T., and Kienesberger P. C.. 2017. Autotaxin is regulated by glucose and insulin in adipocytes. Endocrinology. 158: 791–803. [DOI] [PubMed] [Google Scholar]
- 250.D’Souza K., Nzirorera C., Cowie A. M., Varghese G. P., Trivedi P., Eichmann T. O., Biswas D., Touaibia M., Morris A. J., Aidinis V., et al. 2018. Autotaxin-LPA signaling contributes to obesity-induced insulin resistance in muscle and impairs mitochondrial metabolism. J. Lipid Res. 59: 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hannun Y. A., and Obeid L. M.. 2018. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 19: 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Park T. S., Panek R. L., Mueller S. B., Hanselman J. C., Rosebury W. S., Robertson A. W., Kindt E. K., Homan R., Karathanasis S. K., and Rekhter M. D.. 2004. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 110: 3465–3471. [DOI] [PubMed] [Google Scholar]
- 253.Hojjati M. R., Li Z., and Jiang X. C.. 2005. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim. Biophys. Acta. 1737: 44–51. [DOI] [PubMed] [Google Scholar]
- 254.Hojjati M. R., Li Z., Zhou H., Tang S., Huan C., Ooi E., Lu S., and Jiang X. C.. 2005. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem. 280: 10284–10289. [DOI] [PubMed] [Google Scholar]
- 255.Holland W. L., Brozinick J. T., Wang L. P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., et al. 2007. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5: 167–179. [DOI] [PubMed] [Google Scholar]
- 256.Park T. S., Hu Y., Noh H. L., Drosatos K., Okajima K., Buchanan J., Tuinei J., Homma S., Jiang X. C., Abel E. D., et al. 2008. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49: 2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Park T. S., Rosebury W., Kindt E. K., Kowala M. C., and Panek R. L.. 2008. Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol. Res. 58: 45–51. [DOI] [PubMed] [Google Scholar]
- 258.Yang G., Badeanlou L., Bielawski J., Roberts A. J., Hannun Y. A., and Samad F.. 2009. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 297: E211–E224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ussher J. R., Koves T. R., Cadete V. J., Zhang L., Jaswal J. S., Swyrd S. J., Lopaschuk D. G., Proctor S. D., Keung W., Muoio D. M., et al. 2010. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 59: 2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chun L., Junlin Z., Aimin W., Niansheng L., Benmei C., and Minxiang L.. 2011. Inhibition of ceramide synthesis reverses endothelial dysfunction and atherosclerosis in streptozotocin-induced diabetic rats. Diabetes Res. Clin. Pract. 93: 77–85. [DOI] [PubMed] [Google Scholar]
- 261.Holland W. L., Bikman B. T., Wang L. P., Yuguang G., Sargent K. M., Bulchand S., Knotts T. A., Shui G., Clegg D. J., Wenk M. R., et al. 2011. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 121: 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Li Z., Zhang H., Liu J., Liang C. P., Li Y., Li Y., Teitelman G., Beyer T., Bui H. H., Peake D. A., et al. 2011. Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol. Cell. Biol. 31: 4205–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Russo S. B., Baicu C. F., Van Laer A., Geng T., Kasiganesan H., Zile M. R., and Cowart L. A.. 2012. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Invest. 122: 3919–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhang Q. J., Holland W. L., Wilson L., Tanner J. M., Kearns D., Cahoon J. M., Pettey D., Losee J., Duncan B., Gale D., et al. 2012. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 61: 1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Fillmore N., Keung W., Kelly S. E., Proctor S. D., Lopaschuk G. D., and Ussher J. R.. 2015. Accumulation of ceramide in slow-twitch muscle contributes to the development of insulin resistance in the obese JCR:LA-cp rat. Exp. Physiol. 100: 730–741. [DOI] [PubMed] [Google Scholar]
- 266.Kasumov T., Li L., Li M., Gulshan K., Kirwan J. P., Liu X., Previs S., Willard B., Smith J. D., and McCullough A.. 2015. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS One. 10: e0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Blachnio-Zabielska A. U., Chacinska M., Vendelbo M. H., and Zabielski P.. 2016. The crucial role of C18-Cer in fat-induced skeletal muscle insulin resistance. Cell. Physiol. Biochem. 40: 1207–1220. [DOI] [PubMed] [Google Scholar]
- 268.Chaurasia B., Kaddai V. A., Lancaster G. I., Henstridge D. C., Sriram S., Galam D. L., Gopalan V., Prakash K. N., Velan S. S., Bulchand S., et al. 2016. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 24: 820–834. [DOI] [PubMed] [Google Scholar]
- 269.Zabielski P., Daniluk J., Hady H. R., Markowski A. R., Imierska M., Gorski J., and Blachnio-Zabielska A. U.. 2019. The effect of high-fat diet and inhibition of ceramide production on insulin action in liver. J. Cell. Physiol. 234: 1851–1861. [DOI] [PubMed] [Google Scholar]
- 270.Alexaki A., Clarke B. A., Gavrilova O., Ma Y., Zhu H., Ma X., Xu L., Tuymetova G., Larman B. C., Allende M. L., et al. 2017. De novo sphingolipid biosynthesis is required for adipocyte survival and metabolic homeostasis. J. Biol. Chem. 292: 3929–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lee S. Y., Lee H. Y., Song J. H., Kim G. T., Jeon S., Song Y. J., Lee J. S., Hur J. H., Oh H. H., Park S. Y., et al. 2017. Adipocyte-specific deficiency of de novo sphingolipid biosynthesis leads to lipodystrophy and insulin resistance. Diabetes. 66: 2596–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Xia J. Y., Morley T. S., and Scherer P. E.. 2014. The adipokine/ceramide axis: key aspects of insulin sensitization. Biochimie. 96: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chaurasia B., and Summers S. A.. 2015. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 26: 538–550. [DOI] [PubMed] [Google Scholar]
- 274.Adams J. M. 2nd, Pratipanawatr T., Berria R., Wang E., DeFronzo R. A., Sullards M. C., and Mandarino L. J.. 2004. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 53: 25–31. [DOI] [PubMed] [Google Scholar]
- 275.Straczkowski M., Kowalska I., Nikolajuk A., Dzienis-Straczkowska S., Kinalska I., Baranowski M., Zendzian-Piotrowska M., Brzezinska Z., and Gorski J.. 2004. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes. 53: 1215–1221. [DOI] [PubMed] [Google Scholar]
- 276.Samad F., Hester K. D., Yang G., Hannun Y. A., and Bielawski J.. 2006. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 55: 2579–2587. [DOI] [PubMed] [Google Scholar]
- 277.Straczkowski M., Kowalska I., Baranowski M., Nikolajuk A., Otziomek E., Zabielski P., Adamska A., Blachnio A., Gorski J., and Gorska M.. 2007. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 50: 2366–2373. [DOI] [PubMed] [Google Scholar]
- 278.Shah C., Yang G., Lee I., Bielawski J., Hannun Y. A., and Samad F.. 2008. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J. Biol. Chem. 283: 13538–13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.de Mello V. D., Lankinen M., Schwab U., Kolehmainen M., Lehto S., Seppanen-Laakso T., Oresic M., Pulkkinen L., Uusitupa M., and Erkkila A. T.. 2009. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia. 52: 2612–2615. [DOI] [PubMed] [Google Scholar]
- 280.Haus J. M., Kashyap S. R., Kasumov T., Zhang R., Kelly K. R., Defronzo R. A., and Kirwan J. P.. 2009. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 58: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Chocian G., Chabowski A., Zendzian-Piotrowska M., Harasim E., Lukaszuk B., and Gorski J.. 2010. High fat diet induces ceramide and sphingomyelin formation in rat’s liver nuclei. Mol. Cell. Biochem. 340: 125–131. [DOI] [PubMed] [Google Scholar]
- 282.Amati F., Dube J. J., Alvarez-Carnero E., Edreira M. M., Chomentowski P., Coen P. M., Switzer G. E., Bickel P. E., Stefanovic-Racic M., Toledo F. G., et al. 2011. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 60: 2588–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Majumdar I., and Mastrandrea L. D.. 2012. Serum sphingolipids and inflammatory mediators in adolescents at risk for metabolic syndrome. Endocrine. 41: 442–449. [DOI] [PubMed] [Google Scholar]
- 284.Boon J., Hoy A. J., Stark R., Brown R. D., Meex R. C., Henstridge D. C., Schenk S., Meikle P. J., Horowitz J. F., Kingwell B. A., et al. 2013. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 62: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Brozinick J. T., Hawkins E., Hoang Bui H., Kuo M. S., Tan B., Kievit P., and Grove K.. 2013. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int. J. Obes. (Lond.). 37: 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Turner N., Kowalski G. M., Leslie S. J., Risis S., Yang C., Lee-Young R. S., Babb J. R., Meikle P. J., Lancaster G. I., Henstridge D. C., et al. 2013. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 56: 1638–1648. [DOI] [PubMed] [Google Scholar]
- 287.Weir J. M., Wong G., Barlow C. K., Greeve M. A., Kowalczyk A., Almasy L., Comuzzie A. G., Mahaney M. C., Jowett J. B., Shaw J., et al. 2013. Plasma lipid profiling in a large population-based cohort. J. Lipid Res. 54: 2898–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hanamatsu H., Ohnishi S., Sakai S., Yuyama K., Mitsutake S., Takeda H., Hashino S., and Igarashi Y.. 2014. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr. Diabetes. 4: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Fucho R., Martinez L., Baulies A., Torres S., Tarrats N., Fernandez A., Ribas V., Astudillo A. M., Balsinde J., Garcia-Roves P., et al. 2014. ASMase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage non-alcoholic steatohepatitis. J. Hepatol. 61: 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Turpin S. M., Nicholls H. T., Willmes D. M., Mourier A., Brodesser S., Wunderlich C. M., Mauer J., Xu E., Hammerschmidt P., Bronneke H. S., et al. 2014. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20: 678–686. [DOI] [PubMed] [Google Scholar]
- 291.Bergman B. C., Brozinick J. T., Strauss A., Bacon S., Kerege A., Bui H. H., Sanders P., Siddall P., Kuo M. S., and Perreault L.. 2015. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am. J. Physiol. Endocrinol. Metab. 309: E398–E408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Luukkonen P. K., Zhou Y., Sadevirta S., Leivonen M., Arola J., Oresic M., Hyotylainen T., and Yki-Jarvinen H.. 2016. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol. 64: 1167–1175. [DOI] [PubMed] [Google Scholar]
- 293.Broskey N. T., Obanda D. N., Burton J. H., Cefalu W. T., and Ravussin E.. 2018. Skeletal muscle ceramides and daily fat oxidation in obesity and diabetes. Metabolism. 82: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Turpin-Nolan S. M., Hammerschmidt P., Chen W., Jais A., Timper K., Awazawa M., Brodesser S., and Bruning J. C.. 2019. CerS1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep. 26: 1–10.e7. [DOI] [PubMed] [Google Scholar]
- 295.Kurz J., Parnham M. J., Geisslinger G., and Schiffmann S.. 2019. Ceramides as novel disease biomarkers. Trends Mol. Med. 25: 20–32. [DOI] [PubMed] [Google Scholar]
- 296.Maceyka M., and Spiegel S.. 2014. Sphingolipid metabolites in inflammatory disease. Nature. 510: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Stratford S., Hoehn K. L., Liu F., and Summers S. A.. 2004. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279: 36608–36615. [DOI] [PubMed] [Google Scholar]
- 298.Long S. D., and Pekala P. H.. 1996. Lipid mediators of insulin resistance: ceramide signalling down-regulates GLUT4 gene transcription in 3T3-L1 adipocytes. Biochem. J. 319: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Summers S. A., Garza L. A., Zhou H., and Birnbaum M. J.. 1998. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 18: 5457–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Hajduch E., Turban S., Le Liepvre X., Le Lay S., Lipina C., Dimopoulos N., Dugail I., and Hundal H. S.. 2008. Targeting of PKCzeta and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem. J. 410: 369–379. [DOI] [PubMed] [Google Scholar]
- 301.Blouin C. M., Prado C., Takane K. K., Lasnier F., Garcia-Ocana A., Ferre P., Dugail I., and Hajduch E.. 2010. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes. 59: 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Chavez J. A., Siddique M. M., Wang S. T., Ching J., Shayman J. A., and Summers S. A.. 2014. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J. Biol. Chem. 289: 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Chavez J. A., Holland W. L., Bar J., Sandhoff K., and Summers S. A.. 2005. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J. Biol. Chem. 280: 20148–20153. [DOI] [PubMed] [Google Scholar]
- 304.Chavez J. A., Knotts T. A., Wang L. P., Li G., Dobrowsky R. T., Florant G. L., and Summers S. A.. 2003. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem. 278: 10297–10303. [DOI] [PubMed] [Google Scholar]
- 305.JeBailey L., Wanono O., Niu W., Roessler J., Rudich A., and Klip A.. 2007. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes. 56: 394–403. [DOI] [PubMed] [Google Scholar]
- 306.Powell D. J., Hajduch E., Kular G., and Hundal H. S.. 2003. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol. 23: 7794–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bikman B. T., Guan Y., Shui G., Siddique M. M., Holland W. L., Kim J. Y., Fabrias G., Wenk M. R., and Summers S. A.. 2012. Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis. J. Biol. Chem. 287: 17426–17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Itoh Y., Yano T., Sendo T., Sueyasu M., Hirano K., Kanaide H., and Oishi R.. 2006. Involvement of de novo ceramide synthesis in radiocontrast-induced renal tubular cell injury. Kidney Int. 69: 288–297. [DOI] [PubMed] [Google Scholar]
- 309.Sjoholm A. 1995. Ceramide inhibits pancreatic beta-cell insulin production and mitogenesis and mimics the actions of interleukin-1 beta. FEBS Lett. 367: 283–286. [DOI] [PubMed] [Google Scholar]
- 310.Bielawska A. E., Shapiro J. P., Jiang L., Melkonyan H. S., Piot C., Wolfe C. L., Tomei L. D., Hannun Y. A., and Umansky S. R.. 1997. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am. J. Pathol. 151: 1257–1263. [PMC free article] [PubMed] [Google Scholar]
- 311.Maedler K., Spinas G. A., Dyntar D., Moritz W., Kaiser N., and Donath M. Y.. 2001. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 50: 69–76. [DOI] [PubMed] [Google Scholar]
- 312.Kelpe C. L., Moore P. C., Parazzoli S. D., Wicksteed B., Rhodes C. J., and Poitout V.. 2003. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J. Biol. Chem. 278: 30015–30021. [DOI] [PubMed] [Google Scholar]
- 313.Basnakian A. G., Ueda N., Hong X., Galitovsky V. E., Yin X., and Shah S. V.. 2005. Ceramide synthase is essential for endonuclease-mediated death of renal tubular epithelial cells induced by hypoxia-reoxygenation. Am. J. Physiol. Renal Physiol. 288: F308–F314. [DOI] [PubMed] [Google Scholar]
- 314.Veluthakal R., Palanivel R., Zhao Y., McDonald P., Gruber S., and Kowluru A.. 2005. Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: potential mechanisms underlying ceramide-mediated metabolic dysfunction of the beta cell. Apoptosis. 10: 841–850. [DOI] [PubMed] [Google Scholar]
- 315.Yamashita-Sugahara Y., Tokuzawa Y., Nakachi Y., Kanesaki-Yatsuka Y., Matsumoto M., Mizuno Y., and Okazaki Y.. 2013. Fam57b (family with sequence similarity 57, member B), a novel peroxisome proliferator-activated receptor gamma target gene that regulates adipogenesis through ceramide synthesis. J. Biol. Chem. 288: 4522–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gosejacob D., Jager P. S., Vom Dorp K., Frejno M., Carstensen A. C., Kohnke M., Degen J., Dormann P., and Hoch M.. 2016. Ceramide synthase 5 is essential to maintain C16:0-ceramide pools and contributes to the development of diet-induced obesity. J. Biol. Chem. 291: 6989–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Pewzner-Jung Y., Park H., Laviad E. L., Silva L. C., Lahiri S., Stiban J., Erez-Roman R., Brugger B., Sachsenheimer T., Wieland F., et al. 2010. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J. Biol. Chem. 285: 10902–10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Pewzner-Jung Y., Brenner O., Braun S., Laviad E. L., Ben-Dor S., Feldmesser E., Horn-Saban S., Amann-Zalcenstein D., Raanan C., Berkutzki T., et al. 2010. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. J. Biol. Chem. 285: 10911–10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Park W. J., Park J. W., Merrill A. H., Storch J., Pewzner-Jung Y., and Futerman A. H.. 2014. Hepatic fatty acid uptake is regulated by the sphingolipid acyl chain length. Biochim. Biophys. Acta. 1841: 1754–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Raichur S., Wang S. T., Chan P. W., Li Y., Ching J., Chaurasia B., Dogra S., Ohman M. K., Takeda K., Sugii S., et al. 2014. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20: 687–695. [Erratum. 2014. Cell Metab. 20: 919.] [DOI] [PubMed] [Google Scholar]
- 321.Holland W. L., Miller R. A., Wang Z. V., Sun K., Barth B. M., Bui H. H., Davis K. E., Bikman B. T., Halberg N., Rutkowski J. M., et al. 2011. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Holland W. L., Xia J. Y., Johnson J. A., Sun K., Pearson M. J., Sharma A. X., Quittner-Strom E., Tippetts T. S., Gordillo R., and Scherer P. E.. 2017. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol. Metab. 6: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Tanabe H., Fujii Y., Okada-Iwabu M., Iwabu M., Nakamura Y., Hosaka T., Motoyama K., Ikeda M., Wakiyama M., Terada T., et al. 2015. Crystal structures of the human adiponectin receptors. Nature. 520: 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Vasiliauskaité-Brooks I., Sounier R., Rochaix P., Bellot G., Fortier M., Hoh F., De Colibus L., Bechara C., Saied E. M., Arenz C., et al. 2017. Structural insights into adiponectin receptors suggest ceramidase activity. Nature. 544: 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Patel S. A., Hoehn K. L., Lawrence R. T., Sawbridge L., Talbot N. A., Tomsig J. L., Turner N., Cooney G. J., Whitehead J. P., Kraegen E. W., et al. 2012. Overexpression of the adiponectin receptor AdipoR1 in rat skeletal muscle amplifies local insulin sensitivity. Endocrinology. 153: 5231–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Xia J. Y., Holland W. L., Kusminski C. M., Sun K., Sharma A. X., Pearson M. J., Sifuentes A. J., McDonald J. G., Gordillo R., and Scherer P. E.. 2015. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 22: 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Wang Y., Wang X., Lau W. B., Yuan Y., Booth D., Li J. J., Scalia R., Preston K., Gao E., Koch W., et al. 2014. Adiponectin inhibits tumor necrosis factor-alpha-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation. Circ. Res. 114: 792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Park M., Wu D., Park T., Choi C. S., Li R. K., Cheng K. K., Xu A., and Sweeney G.. 2013. APPL1 transgenic mice are protected from high-fat diet-induced cardiac dysfunction. Am. J. Physiol. Endocrinol. Metab. 305: E795–E804. [DOI] [PubMed] [Google Scholar]
- 329.Eisinger K., Liebisch G., Schmitz G., Aslanidis C., Krautbauer S., and Buechler C.. 2014. Lipidomic analysis of serum from high fat diet induced obese mice. Int. J. Mol. Sci. 15: 2991–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Lemaitre R. N., Yu C., Hoofnagle A., Hari N., Jensen P. N., Fretts A. M., Umans J. G., Howard B. V., Sitlani C. M., Siscovick D. S., et al. 2018. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: the Strong Heart Family Study. Diabetes. 67: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Yano M., Watanabe K., Yamamoto T., Ikeda K., Senokuchi T., Lu M., Kadomatsu T., Tsukano H., Ikawa M., Okabe M., et al. 2011. Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J. Biol. Chem. 286: 3992–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Yano M., Yamamoto T., Nishimura N., Gotoh T., Watanabe K., Ikeda K., Garan Y., Taguchi R., Node K., Okazaki T., et al. 2013. Increased oxidative stress impairs adipose tissue function in sphingomyelin synthase 1 null mice. PLoS One. 8: e61380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mitsutake S., Zama K., Yokota H., Yoshida T., Tanaka M., Mitsui M., Ikawa M., Okabe M., Tanaka Y., Yamashita T., et al. 2011. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J. Biol. Chem. 286: 28544–28555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sugimoto M., Shimizu Y., Zhao S., Ukon N., Nishijima K., Wakabayashi M., Yoshioka T., Higashino K., Numata Y., Okuda T., et al. 2016. Characterization of the role of sphingomyelin synthase 2 in glucose metabolism in whole-body and peripheral tissues in mice. Biochim. Biophys. Acta. 1861: 688–702. [DOI] [PubMed] [Google Scholar]
- 335.Taniguchi M., and Okazaki T.. 2014. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim. Biophys. Acta. 1841: 692–703. [DOI] [PubMed] [Google Scholar]
- 336.Deevska G. M., Rozenova K. A., Giltiay N. V., Chambers M. A., White J., Boyanovsky B. B., Wei J., Daugherty A., Smart E. J., Reid M. B., et al. 2009. Acid sphingomyelinase deficiency prevents diet-induced hepatic triacylglycerol accumulation and hyperglycemia in mice. J. Biol. Chem. 284: 8359–8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Yamashita T., Wada R., Sasaki T., Deng C., Bierfreund U., Sandhoff K., and Proia R. L.. 1999. A vital role for glycosphingolipid synthesis during development and differentiation. Proc. Natl. Acad. Sci. USA. 96: 9142–9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Yamashita T., Wada R., and Proia R. L.. 2002. Early developmental expression of the gene encoding glucosylceramide synthase, the enzyme controlling the first committed step of glycosphingolipid synthesis. Biochim. Biophys. Acta. 1573: 236–240. [DOI] [PubMed] [Google Scholar]
- 339.Aerts J. M., Ottenhoff R., Powlson A. S., Grefhorst A., van Eijk M., Dubbelhuis P. F., Aten J., Kuipers F., Serlie M. J., Wennekes T., et al. 2007. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 56: 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Bijl N., Sokolovic M., Vrins C., Langeveld M., Moerland P. D., Ottenhoff R., van Roomen C. P., Claessen N., Boot R. G., Aten J., et al. 2009. Modulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology. 50: 1431–1441. [DOI] [PubMed] [Google Scholar]
- 341.van Eijk M., Aten J., Bijl N., Ottenhoff R., van Roomen C. P., Dubbelhuis P. F., Seeman I., Ghauharali-van der Vlugt K., Overkleeft H. S., Arbeeny C., et al. 2009. Reducing glycosphingolipid content in adipose tissue of obese mice restores insulin sensitivity, adipogenesis and reduces inflammation. PLoS One. 4: e4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zhao H., Przybylska M., Wu I. H., Zhang J., Maniatis P., Pacheco J., Piepenhagen P., Copeland D., Arbeeny C., Shayman J. A., et al. 2009. Inhibiting glycosphingolipid synthesis ameliorates hepatic steatosis in obese mice. Hepatology. 50: 85–93. [DOI] [PubMed] [Google Scholar]
- 343.Yew N. S., Zhao H., Hong E. G., Wu I. H., Przybylska M., Siegel C., Shayman J. A., Arbeeny C. M., Kim J. K., Jiang C., et al. 2010. Increased hepatic insulin action in diet-induced obese mice following inhibition of glucosylceramide synthase. PLoS One. 5: e11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Jennemann R., Rothermel U., Wang S., Sandhoff R., Kaden S., Out R., van Berkel T. J., Aerts J. M., Ghauharali K., Sticht C., et al. 2010. Hepatic glycosphingolipid deficiency and liver function in mice. Hepatology. 51: 1799–1809. [DOI] [PubMed] [Google Scholar]
- 345.Mitsutake S., Date T., Yokota H., Sugiura M., Kohama T., and Igarashi Y.. 2012. Ceramide kinase deficiency improves diet-induced obesity and insulin resistance. FEBS Lett. 586: 1300–1305. [DOI] [PubMed] [Google Scholar]
- 346.Bruce C. R., Risis S., Babb J. R., Yang C., Kowalski G. M., Selathurai A., Lee-Young R. S., Weir J. M., Yoshioka K., Takuwa Y., et al. 2012. Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet-fed mice. Diabetes. 61: 3148–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Kowalski G. M., Carey A. L., Selathurai A., Kingwell B. A., and Bruce C. R.. 2013. Plasma sphingosine-1-phosphate is elevated in obesity. PLoS One. 8: e72449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Fayyaz S., Henkel J., Japtok L., Kramer S., Damm G., Seehofer D., Puschel G. P., and Kleuser B.. 2014. Involvement of sphingosine 1-phosphate in palmitate-induced insulin resistance of hepatocytes via the S1P2 receptor subtype. Diabetologia. 57: 373–382. [DOI] [PubMed] [Google Scholar]
- 349.Wang J., Badeanlou L., Bielawski J., Ciaraldi T. P., and Samad F.. 2014. Sphingosine kinase 1 regulates adipose proinflammatory responses and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 306: E756–E768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Japtok L., Schmitz E. I., Fayyaz S., Kramer S., Hsu L. J., and Kleuser B.. 2015. Sphingosine 1-phosphate counteracts insulin signaling in pancreatic beta-cells via the sphingosine 1-phosphate receptor subtype 2. FASEB J. 29: 3357–3369. [DOI] [PubMed] [Google Scholar]
- 351.Nagahashi M., Yamada A., Katsuta E., Aoyagi T., Huang W. C., Terracina K. P., Hait N. C., Allegood J. C., Tsuchida J., Yuza K., et al. 2018. Targeting the SphK1/S1P/S1PR1 axis that links obesity, chronic inflammation, and breast cancer metastasis. Cancer Res. 78: 1713–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Bolz S. S., Vogel L., Sollinger D., Derwand R., Boer C., Pitson S. M., Spiegel S., and Pohl U.. 2003. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 108: 342–347. [DOI] [PubMed] [Google Scholar]
- 353.Ma M. M., Chen J. L., Wang G. G., Wang H., Lu Y., Li J. F., Yi J., Yuan Y. J., Zhang Q. W., Mi J., et al. 2007. Sphingosine kinase 1 participates in insulin signalling and regulates glucose metabolism and homeostasis in KK/Ay diabetic mice. Diabetologia. 50: 891–900. [DOI] [PubMed] [Google Scholar]
- 354.Rapizzi E., Taddei M. L., Fiaschi T., Donati C., Bruni P., and Chiarugi P.. 2009. Sphingosine 1-phosphate increases glucose uptake through trans-activation of insulin receptor. Cell. Mol. Life Sci. 66: 3207–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Cantrell Stanford J., Morris A. J., Sunkara M., Popa G. J., Larson K. L., and Ozcan S.. 2012. Sphingosine 1-phosphate (S1P) regulates glucose-stimulated insulin secretion in pancreatic beta cells. J. Biol. Chem. 287: 13457–13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Qi Y., Chen J., Lay A., Don A., Vadas M., and Xia P.. 2013. Loss of sphingosine kinase 1 predisposes to the onset of diabetes via promoting pancreatic beta-cell death in diet-induced obese mice. FASEB J. 27: 4294–4304. [DOI] [PubMed] [Google Scholar]
- 357.Geng T., Sutter A., Harland M. D., Law B. A., Ross J. S., Lewin D., Palanisamy A., Russo S. B., Chavin K. D., and Cowart L. A.. 2015. SphK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes. J. Lipid Res. 56: 2359–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Qi Y., Wang W., Chen J., Dai L., Kaczorowski D., Gao X., and Xia P.. 2015. Sphingosine kinase 1 protects hepatocytes from lipotoxicity via down-regulation of IRE1alpha protein expression. J. Biol. Chem. 290: 23282–23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Chen J., Wang W., Qi Y., Kaczorowski D., McCaughan G. W., Gamble J. R., Don A. S., Gao X., Vadas M. A., and Xia P.. 2016. Deletion of sphingosine kinase 1 ameliorates hepatic steatosis in diet-induced obese mice: Role of PPARgamma. Biochim. Biophys. Acta. 1861: 138–147. [DOI] [PubMed] [Google Scholar]
- 360.Kitada Y., Kajita K., Taguchi K., Mori I., Yamauchi M., Ikeda T., Kawashima M., Asano M., Kajita T., Ishizuka T., et al. 2016. Blockade of sphingosine 1-phosphate receptor 2 signaling attenuates high-fat diet-induced adipocyte hypertrophy and systemic glucose intolerance in mice. Endocrinology. 157: 1839–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Feuerborn R., Besser M., Poti F., Burkhardt R., Weissen-Plenz G., Ceglarek U., Simoni M., Proia R. L., Freise H., and Nofer J. R.. 2018. Elevating endogenous sphingosine-1-phosphate (S1P) levels improves endothelial function and ameliorates atherosclerosis in low density lipoprotein receptor-deficient (LDL-R-/-) Mice. Thromb. Haemost. 118: 1470–1480. [DOI] [PubMed] [Google Scholar]
- 362.Kowalski G. M., Kloehn J., Burch M. L., Selathurai A., Hamley S., Bayol S. A., Lamon S., Watt M. J., Lee-Young R. S., McConville M. J., et al. 2015. Overexpression of sphingosine kinase 1 in liver reduces triglyceride content in mice fed a low but not high-fat diet. Biochim. Biophys. Acta. 1851: 210–219. [DOI] [PubMed] [Google Scholar]
- 363.Ravichandran S., Finlin B. S., Kern P. A., and Ozcan S.. 2019. Sphk2(-/-) mice are protected from obesity and insulin resistance. Biochim. Biophys. Acta Mol. Basis Dis. 1865: 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Lee S. Y., Hong I. K., Kim B. R., Shim S. M., Sung Lee J., Lee H. Y., Soo Choi C., Kim B. K., and Park T. S.. 2015. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology. 62: 135–146. [DOI] [PubMed] [Google Scholar]
- 365.Kendall M. R., and Hupfeld C. J.. 2008. FTY720, a sphingosine-1-phosphate receptor modulator, reverses high-fat diet-induced weight gain, insulin resistance and adipose tissue inflammation in C57BL/6 mice. Diabetes Obes. Metab. 10: 802–805. [DOI] [PubMed] [Google Scholar]
- 366.Imasawa T., Koike K., Ishii I., Chun J., and Yatomi Y.. 2010. Blockade of sphingosine 1-phosphate receptor 2 signaling attenuates streptozotocin-induced apoptosis of pancreatic beta-cells. Biochem. Biophys. Res. Commun. 392: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Moon M. H., Jeong J. K., Lee J. H., Park Y. G., Lee Y. J., Seol J. W., and Park S. Y.. 2012. Antiobesity activity of a sphingosine 1-phosphate analogue FTY720 observed in adipocytes and obese mouse model. Exp. Mol. Med. 44: 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Zhao Z., Choi J., Zhao C., and Ma Z. A.. 2012. FTY720 normalizes hyperglycemia by stimulating beta-cell in vivo regeneration in db/db mice through regulation of cyclin D3 and p57(KIP2). J. Biol. Chem. 287: 5562–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Moon H., Chon J., Joo J., Kim D., In J., Lee H., Park J., and Choi J.. 2013. FTY720 preserved islet beta-cell mass by inhibiting apoptosis and increasing survival of beta-cells in db/db mice. Diabetes Metab. Res. Rev. 29: 19–24. [DOI] [PubMed] [Google Scholar]
- 370.Mauer A. S., Hirsova P., Maiers J. L., Shah V. H., and Malhi H.. 2017. Inhibition of sphingosine 1-phosphate signaling ameliorates murine nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 312: G300–G313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Johnson R. J., Nakagawa T., Sanchez-Lozada L. G., Shafiu M., Sundaram S., Le M., Ishimoto T., Sautin Y. Y., and Lanaspa M. A.. 2013. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 62: 3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Lima W. G., Martins-Santos M. E., and Chaves V. E.. 2015. Uric acid as a modulator of glucose and lipid metabolism. Biochimie. 116: 17–23. [DOI] [PubMed] [Google Scholar]
- 373.Fruehwald-Schultes B., Peters A., Kern W., Beyer J., and Pfutzner A.. 1999. Serum leptin is associated with serum uric acid concentrations in humans. Metabolism. 48: 677–680. [DOI] [PubMed] [Google Scholar]
- 374.Choi H. K., and Ford E. S.. 2007. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am. J. Med. 120: 442–447. [DOI] [PubMed] [Google Scholar]
- 375.Tamba S., Nishizawa H., Funahashi T., Okauchi Y., Ogawa T., Noguchi M., Fujita K., Ryo M., Kihara S., Iwahashi H., et al. 2008. Relationship between the serum uric acid level, visceral fat accumulation and serum adiponectin concentration in Japanese men. Intern. Med. 47: 1175–1180. [DOI] [PubMed] [Google Scholar]
- 376.Baldwin W., McRae S., Marek G., Wymer D., Pannu V., Baylis C., Johnson R. J., and Sautin Y. Y.. 2011. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 60: 1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Ryu S., Chang Y., Kim S. G., Cho J., and Guallar E.. 2011. Serum uric acid levels predict incident nonalcoholic fatty liver disease in healthy Korean men. Metabolism. 60: 860–866. [DOI] [PubMed] [Google Scholar]
- 378.Kim T. H., Lee S. S., Yoo J. H., Kim S. R., Yoo S. J., Song H. C., Kim Y. S., Choi E. J., and Kim Y. K.. 2012. The relationship between the regional abdominal adipose tissue distribution and the serum uric acid levels in people with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Lanaspa M. A., Sanchez-Lozada L. G., Choi Y. J., Cicerchi C., Kanbay M., Roncal-Jimenez C. A., Ishimoto T., Li N., Marek G., Duranay M., et al. 2012. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 287: 40732–40744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Lv Q., Meng X. F., He F. F., Chen S., Su H., Xiong J., Gao P., Tian X. J., Liu J. S., Zhu Z. H., et al. 2013. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 8: e56864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Mangge H., Zelzer S., Puerstner P., Schnedl W. J., Reeves G., Postolache T. T., and Weghuber D.. 2013. Uric acid best predicts metabolically unhealthy obesity with increased cardiovascular risk in youth and adults. Obesity (Silver Spring). 21: E71–E77. [DOI] [PubMed] [Google Scholar]
- 382.Tsushima Y., Nishizawa H., Tochino Y., Nakatsuji H., Sekimoto R., Nagao H., Shirakura T., Kato K., Imaizumi K., Takahashi H., et al. 2013. Uric acid secretion from adipose tissue and its increase in obesity. J. Biol. Chem. 288: 27138–27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Jia G., Habibi J., Bostick B. P., Ma L., DeMarco V. G., Aroor A. R., Hayden M. R., Whaley-Connell A. T., and Sowers J. R.. 2015. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension. 65: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Nakatsu Y., Seno Y., Kushiyama A., Sakoda H., Fujishiro M., Katasako A., Mori K., Matsunaga Y., Fukushima T., Kanaoka R., et al. 2015. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am. J. Physiol. Gastrointest. Liver Physiol. 309: G42–G51. [DOI] [PubMed] [Google Scholar]
- 385.Xu C., Wan X., Xu L., Weng H., Yan M., Miao M., Sun Y., Xu G., Dooley S., Li Y., et al. 2015. Xanthine oxidase in non-alcoholic fatty liver disease and hyperuricemia: one stone hits two birds. J. Hepatol. 62: 1412–1419. [DOI] [PubMed] [Google Scholar]
- 386.Yu T. Y., Jee J. H., Bae J. C., Jin S. M., Baek J. H., Lee M. K., and Kim J. H.. 2016. Serum uric acid: A strong and independent predictor of metabolic syndrome after adjusting for body composition. Metabolism. 65: 432–440. [DOI] [PubMed] [Google Scholar]
- 387.Han T., Meng X., Shan R., Zi T., Li Y., Ma H., Zhao Y., Shi D., Qu R., Guo X., et al. 2018. Temporal relationship between hyperuricemia and obesity, and its association with future risk of type 2 diabetes. Int. J. Obes. (Lond.). 42: 1336–1344. [DOI] [PubMed] [Google Scholar]
- 388.Mazidi M., Katsiki N., Mikhailidis D. P., and Banach M.. 2018. The link between insulin resistance parameters and serum uric acid is mediated by adiposity. Atherosclerosis. 270: 180–186. [DOI] [PubMed] [Google Scholar]
- 389.Mele C., Tagliaferri M. A., Saraceno G., Mai S., Vietti R., Zavattaro M., Aimaretti G., Scacchi M., and Marzullo P.. 2018. Serum uric acid potentially links metabolic health to measures of fuel use in lean and obese individuals. Nutr. Metab. Cardiovasc. Dis. 28: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 390.Gersch C., Palii S. P., Kim K. M., Angerhofer A., Johnson R. J., and Henderson G. N.. 2008. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 27: 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Gersch C., Palii S. P., Imaram W., Kim K. M., Karumanchi S. A., Angerhofer A., Johnson R. J., and Henderson G. N.. 2009. Reactions of peroxynitrite with uric acid: formation of reactive intermediates, alkylated products and triuret, and in vivo production of triuret under conditions of oxidative stress. Nucleosides Nucleotides Nucleic Acids. 28: 118–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Imaram W., Gersch C., Kim K. M., Johnson R. J., Henderson G. N., and Angerhofer A.. 2010. Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry. Free Radic. Biol. Med. 49: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Sautin Y. Y., Nakagawa T., Zharikov S., and Johnson R. J.. 2007. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell Physiol. 293: C584–C596. [DOI] [PubMed] [Google Scholar]
- 394.Sánchez-Lozada L. G., Lanaspa M. A., Cristobal-Garcia M., Garcia-Arroyo F., Soto V., Cruz-Robles D., Nakagawa T., Yu M. A., Kang D. H., and Johnson R. J.. 2012. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp. Nephrol. 121: e71–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Wan X., Xu C., Lin Y., Lu C., Li D., Sang J., He H., Liu X., Li Y., and Yu C.. 2016. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J. Hepatol. 64: 925–932. [DOI] [PubMed] [Google Scholar]
- 396.Corry D. B., Eslami P., Yamamoto K., Nyby M. D., Makino H., and Tuck M. L.. 2008. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 26: 269–275. [DOI] [PubMed] [Google Scholar]
- 397.Yu M. A., Sanchez-Lozada L. G., Johnson R. J., and Kang D. H.. 2010. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J. Hypertens. 28: 1234–1242. [PubMed] [Google Scholar]
- 398.Lanaspa M. A., Cicerchi C., Garcia G., Li N., Roncal-Jimenez C. A., Rivard C. J., Hunter B., Andres-Hernando A., Ishimoto T., Sanchez-Lozada L. G., et al. 2012. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One. 7: e48801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Lanaspa M. A., Sanchez-Lozada L. G., Cicerchi C., Li N., Roncal-Jimenez C. A., Ishimoto T., Le M., Garcia G. E., Thomas J. B., Rivard C. J., et al. 2012. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One. 7: e47948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Choi Y. J., Yoon Y., Lee K. Y., Hien T. T., Kang K. W., Kim K. C., Lee J., Lee M. Y., Lee S. M., Kang D. H., et al. 2014. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 28: 3197–3204. [DOI] [PubMed] [Google Scholar]
- 401.Zhu Y., Hu Y., Huang T., Zhang Y., Li Z., Luo C., Luo Y., Yuan H., Hisatome I., Yamamoto T., et al. 2014. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem. Biophys. Res. Commun. 447: 707–714. [DOI] [PubMed] [Google Scholar]
- 402.Zhang J. X., Zhang Y. P., Wu Q. N., and Chen B.. 2015. Uric acid induces oxidative stress via an activation of the renin-angiotensin system in 3T3-L1 adipocytes. Endocrine. 48: 135–142. [DOI] [PubMed] [Google Scholar]
- 403.Cantu-Medellin N., and Kelley E. E.. 2013. Xanthine oxidoreductase-catalyzed reactive species generation: A process in critical need of reevaluation. Redox Biol. 1: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Battelli M. G., Bortolotti M., Polito L., and Bolognesi A.. 2018. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 1864: 2557–2565. [DOI] [PubMed] [Google Scholar]
- 405.Cheung K. J., Tzameli I., Pissios P., Rovira I., Gavrilova O., Ohtsubo T., Chen Z., Finkel T., Flier J. S., and Friedman J. M.. 2007. Xanthine oxidoreductase is a regulator of adipogenesis and PPARgamma activity. Cell Metab. 5: 115–128. [DOI] [PubMed] [Google Scholar]
- 406.Washio K. W., Kusunoki Y., Murase T., Nakamura T., Osugi K., Ohigashi M., Sukenaga T., Ochi F., Matsuo T., Katsuno T., et al. 2017. Xanthine oxidoreductase activity is correlated with insulin resistance and subclinical inflammation in young humans. Metabolism. 70: 51–56. [DOI] [PubMed] [Google Scholar]
- 407.Vorbach C., Scriven A., and Capecchi M. R.. 2002. The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes Dev. 16: 3223–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Ohtsubo T., Rovira I. I., Starost M. F., Liu C., and Finkel T.. 2004. Xanthine oxidoreductase is an endogenous regulator of cyclooxygenase-2. Circ. Res. 95: 1118–1124. [DOI] [PubMed] [Google Scholar]
- 409.Murakami N., Ohtsubo T., Kansui Y., Goto K., Noguchi H., Haga Y., Nakabeppu Y., Matsumura K., and Kitazono T.. 2014. Mice heterozygous for the xanthine oxidoreductase gene facilitate lipid accumulation in adipocytes. Arterioscler. Thromb. Vasc. Biol. 34: 44–51. [DOI] [PubMed] [Google Scholar]
- 410.Nakagawa T., Hu H., Zharikov S., Tuttle K. R., Short R. A., Glushakova O., Ouyang X., Feig D. I., Block E. R., Herrera-Acosta J., et al. 2006. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Renal Physiol. 290: F625–F631. [DOI] [PubMed] [Google Scholar]
- 411.Sánchez-Lozada L. G., Tapia E., Bautista-Garcia P., Soto V., Avila-Casado C., Vega-Campos I. P., Nakagawa T., Zhao L., Franco M., and Johnson R. J.. 2008. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am. J. Physiol. Renal Physiol. 294: F710–F718. [DOI] [PubMed] [Google Scholar]
- 412.Tapia E., Cristobal M., Garcia-Arroyo F. E., Soto V., Monroy-Sanchez F., Pacheco U., Lanaspa M. A., Roncal-Jimenez C. A., Cruz-Robles D., Ishimoto T., et al. 2013. Synergistic effect of uricase blockade plus physiological amounts of fructose-glucose on glomerular hypertension and oxidative stress in rats. Am. J. Physiol. Renal Physiol. 304: F727–F736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Aroor A. R., Jia G., Habibi J., Sun Z., Ramirez-Perez F. I., Brady B., Chen D., Martinez-Lemus L. A., Manrique C., Nistala R., et al. 2017. Uric acid promotes vascular stiffness, maladaptive inflammatory responses and proteinuria in western diet fed mice. Metabolism. 74: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Lastra G., Manrique C., Jia G., Aroor A. R., Hayden M. R., Barron B. J., Niles B., Padilla J., and Sowers J. R.. 2017. Xanthine oxidase inhibition protects against Western diet-induced aortic stiffness and impaired vasorelaxation in female mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 313: R67–R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Connolly G. P., and Duley J. A.. 1999. Uridine and its nucleotides: biological actions, therapeutic potentials. Trends Pharmacol. Sci. 20: 218–225. [DOI] [PubMed] [Google Scholar]
- 416.Gasser T., Moyer J. D., and Handschumacher R. E.. 1981. Novel single-pass exchange of circulating uridine in rat liver. Science. 213: 777–778. [DOI] [PubMed] [Google Scholar]
- 417.Liu M. P., Beigelman L., Levy E., Handschumacher R. E., and Pizzorno G.. 1998. Discrete roles of hepatocytes and nonparenchymal cells in uridine catabolism as a component of its homeostasis. Am. J. Physiol. 274: G1018–G1023. [DOI] [PubMed] [Google Scholar]
- 418.Deng Y., Wang Z. V., Gordillo R., An Y., Zhang C., Liang Q., Yoshino J., Cautivo K. M., De Brabander J., Elmquist J. K., et al. 2017. An adipo-biliary-uridine axis that regulates energy homeostasis. Science. 355: doi:10.1126/science.aaf5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Deng Y., Wang Z. V., Gordillo R., Zhu Y., Ali A., Zhang C., Wang X., Shao M., Zhang Z., Iyengar P., et al. 2018. Adipocyte Xbp1s overexpression drives uridine production and reduces obesity. Mol. Metab. 11: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Domingo P., Torres-Torronteras J., Pomar V., Giralt M., Domingo J. C., Gutierrez Mdel M., Gallego-Escuredo J. M., Mateo M. G., Cano-Soldado P., Fernandez I., et al. 2010. Uridine metabolism in HIV-1-infected patients: effect of infection, of antiretroviral therapy and of HIV-1/ART-associated lipodystrophy syndrome. PLoS One. 5: e13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Yamamoto T., Koyama H., Kurajoh M., Shoji T., Tsutsumi Z., and Moriwaki Y.. 2011. Biochemistry of uridine in plasma. Clin. Chim. Acta. 412: 1712–1724. [DOI] [PubMed] [Google Scholar]
- 422.Wellen K. E., and Thompson C. B.. 2012. A two-way street: reciprocal regulation of metabolism and signalling. Nat. Rev. Mol. Cell Biol. 13: 270–276. [DOI] [PubMed] [Google Scholar]
- 423.von Kügelgen I., and Hoffmann K.. 2016. Pharmacology and structure of P2Y receptors. Neuropharmacology. 104: 50–61. [DOI] [PubMed] [Google Scholar]
- 424.Peters G. J., van Groeningen C. J., Laurensse E. J., Lankelma J., Leyva A., and Pinedo H. M.. 1987. Uridine-induced hypothermia in mice and rats in relation to plasma and tissue levels of uridine and its metabolites. Cancer Chemother. Pharmacol. 20: 101–108. [DOI] [PubMed] [Google Scholar]
- 425.Peters G. J., van Groeningen C. J., Laurensse E., Kraal I., Leyva A., Lankelma J., and Pinedo H. M.. 1987. Effect of pyrimidine nucleosides on body temperatures of man and rabbit in relation to pharmacokinetic data. Pharm. Res. 4: 113–119. [DOI] [PubMed] [Google Scholar]
- 426.Le T. T., Ziemba A., Urasaki Y., Hayes E., Brotman S., and Pizzorno G.. 2013. Disruption of uridine homeostasis links liver pyrimidine metabolism to lipid accumulation. J. Lipid Res. 54: 1044–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Urasaki Y., Pizzorno G., and Le T. T.. 2014. Uridine affects liver protein glycosylation, insulin signaling, and heme biosynthesis. PLoS One. 9: e99728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Urasaki Y., Pizzorno G., and Le T. T.. 2016. Chronic uridine administration induces fatty liver and pre-diabetic conditions in mice. PLoS One. 11: e0146994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Cao Z., Ma J., Chen X., Zhou B., Cai C., Huang D., Zhang X., and Cao D.. 2016. Uridine homeostatic disorder leads to DNA damage and tumorigenesis. Cancer Lett. 372: 219–225. [DOI] [PubMed] [Google Scholar]
- 430.Scherer P. E. 2016. The multifaceted roles of adipose tissue-therapeutic targets for diabetes and beyond: the 2015 Banting Lecture. Diabetes. 65: 1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Deng Y., Wang Z. V., Tao C., Gao N., Holland W. L., Ferdous A., Repa J. J., Liang G., Ye J., Lehrman M. A., et al. 2013. The Xbp1s/GalE axis links ER stress to postprandial hepatic metabolism. J. Clin. Invest. 123: 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Williams K. W., Liu T., Kong X., Fukuda M., Deng Y., Berglund E. D., Deng Z., Gao Y., Liu T., Sohn J. W., et al. 2014. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 20: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wang Z. V., Deng Y., Gao N., Pedrozo Z., Li D. L., Morales C. R., Criollo A., Luo X., Tan W., Jiang N., et al. 2014. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell. 156: 1179–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Kopp F., and Mendell J. T.. 2018. Functional classification and experimental dissection of long noncoding RNAs. Cell. 172: 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Tkach M., and Thery C.. 2016. Communication by extracellular vesicles: where we are and where we need to go. Cell. 164: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 436.Zhao X. Y., and Lin J. D.. 2015. Long noncoding RNAs: a new regulatory code in metabolic control. Trends Biochem. Sci. 40: 586–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Knoll M., Lodish H. F., and Sun L.. 2015. Long non-coding RNAs as regulators of the endocrine system. Nat. Rev. Endocrinol. 11: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Chen Z. 2015. Progress and prospects of long noncoding RNAs in lipid homeostasis. Mol. Metab. 5: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Arner P., and Kulyte A.. 2015. MicroRNA regulatory networks in human adipose tissue and obesity. Nat. Rev. Endocrinol. 11: 276–288. [DOI] [PubMed] [Google Scholar]
- 440.Arroyo J. D., Chevillet J. R., Kroh E. M., Ruf I. K., Pritchard C. C., Gibson D. F., Mitchell P. S., Bennett C. F., Pogosova-Agadjanyan E. L., Stirewalt D. L., et al. 2011. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA. 108: 5003–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Turchinovich A., Weiz L., Langheinz A., and Burwinkel B.. 2011. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 39: 7223–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Vickers K. C., Palmisano B. T., Shoucri B. M., Shamburek R. D., and Remaley A. T.. 2011. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 13: 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Cocucci E., and Meldolesi J.. 2015. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 25: 364–372. [DOI] [PubMed] [Google Scholar]
- 444.Huang-Doran I., Zhang C. Y., and Vidal-Puig A.. 2017. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol. Metab. 28: 3–18. [DOI] [PubMed] [Google Scholar]
- 445.van Niel G., D’Angelo G., and Raposo G.. 2018. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19: 213–228. [DOI] [PubMed] [Google Scholar]
- 446.Chen Y., Buyel J. J., Hanssen M. J., Siegel F., Pan R., Naumann J., Schell M., van der Lans A., Schlein C., Froehlich H., et al. 2016. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat. Commun. 7: 11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Deng Z. B., Poliakov A., Hardy R. W., Clements R., Liu C., Liu Y., Wang J., Xiang X., Zhang S., Zhuang X., et al. 2009. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 58: 2498–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Xie Z., Wang X., Liu X., Du H., Sun C., Shao X., Tian J., Gu X., Wang H., Tian J., et al. 2018. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J. Am. Heart Assoc. 7: doi:10.1161/JAHA.117.007442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Ying W., Riopel M., Bandyopadhyay G., Dong Y., Birmingham A., Seo J. B., Ofrecio J. M., Wollam J., Hernandez-Carretero A., Fu W., et al. 2017. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 171: 372–384.e12. [DOI] [PubMed] [Google Scholar]
- 450.Zhang Y., Mei H., Chang X., Chen F., Zhu Y., and Han X.. 2016. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J. Mol. Cell Biol. 8: 505–517. [DOI] [PubMed] [Google Scholar]
- 451.Crewe C., Joffin N., Rutkowski J. M., Kim M., Zhang F., Towler D. A., Gordillo R., and Scherer P. E.. 2018. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell. 175: 695–708.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Cui H., Lopez M., and Rahmouni K.. 2017. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 13: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Lu H., Cassis L. A., Kooi C. W., and Daugherty A.. 2016. Structure and functions of angiotensinogen. Hypertens. Res. 39: 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Pahlavani M., Kalupahana N. S., Ramalingam L., and Moustaid-Moussa N.. 2017. Regulation and functions of the renin-angiotensin system in white and brown adipose tissue. Compr. Physiol. 7: 1137–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Schütten M. T., Houben A. J., de Leeuw P. W., and Stehouwer C. D.. 2017. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology (Bethesda). 32: 197–209. [DOI] [PubMed] [Google Scholar]
- 456.Grobe J. L., Grobe C. L., Beltz T. G., Westphal S. G., Morgan D. A., Xu D., de Lange W. J., Li H., Sakai K., Thedens D. R., et al. 2010. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab. 12: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.de Kloet A. D., Krause E. G., Scott K. A., Foster M. T., Herman J. P., Sakai R. R., Seeley R. J., and Woods S. C.. 2011. Central angiotensin II has catabolic action at white and brown adipose tissue. Am. J. Physiol. Endocrinol. Metab. 301: E1081–E1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Yiannikouris F., Karounos M., Charnigo R., English V. L., Rateri D. L., Daugherty A., and Cassis L. A.. 2012. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302: R244–R251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.de Kloet A. D., Pati D., Wang L., Hiller H., Sumners C., Frazier C. J., Seeley R. J., Herman J. P., Woods S. C., and Krause E. G.. 2013. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J. Neurosci. 33: 4825–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Than A., Leow M. K., and Chen P.. 2013. Control of adipogenesis by the autocrine interplays between angiotensin 1–7/Mas receptor and angiotensin II/AT1 receptor signaling pathways. J. Biol. Chem. 288: 15520–15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Palominos M. M., Dunner N. H., Wabitsch M., and Rojas C. V.. 2015. Angiotensin II directly impairs adipogenic differentiation of human preadipose cells. Mol. Cell. Biochem. 408: 115–122. [DOI] [PubMed] [Google Scholar]
- 462.Noll C., Labbe S. M., Pinard S., Shum M., Bilodeau L., Chouinard L., Phoenix S., Lecomte R., Carpentier A. C., and Gallo-Payet N.. 2015. Postprandial fatty acid uptake and adipocyte remodeling in angiotensin type 2 receptor-deficient mice fed a high-fat/high-fructose diet. Adipocyte. 5: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Patel V. B., Mori J., McLean B. A., Basu R., Das S. K., Ramprasath T., Parajuli N., Penninger J. M., Grant M. B., Lopaschuk G. D., et al. 2016. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes. 65: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Chou C. L., Lin H., Chen J. S., and Fang T. C.. 2017. Renin inhibition improves metabolic syndrome, and reduces angiotensin II levels and oxidative stress in visceral fat tissues in fructose-fed rats. PLoS One. 12: e0180712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Graus-Nunes F., Rachid T. L., de Oliveira Santos F., Barbosa-da-Silva S., and Souza-Mello V.. 2017. AT1 receptor antagonist induces thermogenic beige adipocytes in the inguinal white adipose tissue of obese mice. Endocrine. 55: 786–798. [DOI] [PubMed] [Google Scholar]
- 466.Than A., Xu S., Li R., Leow M. K., Sun L., and Chen P.. 2017. Angiotensin type 2 receptor activation promotes browning of white adipose tissue and brown adipogenesis. Signal Transduct. Target. Ther. 2: 17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Quiroga D. T., Munoz M. C., Gil C., Pffeifer M., Toblli J. E., Steckelings U. M., Giani J. F., and Dominici F. P.. 2018. Chronic administration of the angiotensin type 2 receptor agonist C21 improves insulin sensitivity in C57BL/6 mice. Physiol. Rep. 6: e13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Bråkenhielm E., Veitonmaki N., Cao R., Kihara S., Matsuzawa Y., Zhivotovsky B., Funahashi T., and Cao Y.. 2004. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc. Natl. Acad. Sci. USA. 101: 2476–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Shibata R., Ouchi N., Kihara S., Sato K., Funahashi T., and Walsh K.. 2004. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J. Biol. Chem. 279: 28670–28674. [DOI] [PubMed] [Google Scholar]
- 470.Fu Y., Luo N., Klein R. L., and Garvey W. T.. 2005. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 46: 1369–1379. [DOI] [PubMed] [Google Scholar]
- 471.Kim J. Y., van de Wall E., Laplante M., Azzara A., Trujillo M. E., Hofmann S. M., Schraw T., Durand J. L., Li H., Li G., et al. 2007. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117: 2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Yamauchi T., Nio Y., Maki T., Kobayashi M., Takazawa T., Iwabu M., Okada-Iwabu M., Kawamoto S., Kubota N., Kubota T., et al. 2007. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 13: 332–339. [DOI] [PubMed] [Google Scholar]
- 473.Okamoto M., Ohara-Imaizumi M., Kubota N., Hashimoto S., Eto K., Kanno T., Kubota T., Wakui M., Nagai R., Noda M., et al. 2008. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia. 51: 827–835. [DOI] [PubMed] [Google Scholar]
- 474.Landskroner-Eiger S., Qian B., Muise E. S., Nawrocki A. R., Berger J. P., Fine E. J., Koba W., Deng Y., Pollard J. W., and Scherer P. E.. 2009. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin. Cancer Res. 15: 3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Miller R. A., Chu Q., Le Lay J., Scherer P. E., Ahima R. S., Kaestner K. H., Foretz M., Viollet B., and Birnbaum M. J.. 2011. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J. Clin. Invest. 121: 2518–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Ye R., Holland W. L., Gordillo R., Wang M., Wang Q. A., Shao M., Morley T. S., Gupta R. K., Stahl A., and Scherer P. E.. 2014. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes beta-cell regeneration. eLife. 3: doi:10.7554/eLife.03851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Gamble J. R., Drew J., Trezise L., Underwood A., Parsons M., Kasminkas L., Rudge J., Yancopoulos G., and Vadas M. A.. 2000. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ. Res. 87: 603–607. [DOI] [PubMed] [Google Scholar]
- 478.Thurston G., Rudge J. S., Ioffe E., Zhou H., Ross L., Croll S. D., Glazer N., Holash J., McDonald D. M., and Yancopoulos G. D.. 2000. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 6: 460–463. [DOI] [PubMed] [Google Scholar]
- 479.Baffert F., Le T., Thurston G., and McDonald D. M.. 2006. Angiopoietin-1 decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am. J. Physiol. Heart Circ. Physiol. 290: H107–H118. [DOI] [PubMed] [Google Scholar]
- 480.Cho C. H., Sung H. K., Kim K. T., Cheon H. G., Oh G. T., Hong H. J., Yoo O. J., and Koh G. Y.. 2006. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc. Natl. Acad. Sci. USA. 103: 4946–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Bitto A., Minutoli L., Galeano M. R., Altavilla D., Polito F., Fiumara T., Calo M., Lo Cascio P., Zentilin L., Giacca M., et al. 2008. Angiopoietin-1 gene transfer improves impaired wound healing in genetically diabetic mice without increasing VEGF expression. Clin. Sci. (Lond.). 114: 707–718. [DOI] [PubMed] [Google Scholar]
- 482.Falcón B. L., Hashizume H., Koumoutsakos P., Chou J., Bready J. V., Coxon A., Oliner J. D., and McDonald D. M.. 2009. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am. J. Pathol. 175: 2159–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Coxon A., Bready J., Min H., Kaufman S., Leal J., Yu D., Lee T. A., Sun J. R., Estrada J., Bolon B., et al. 2010. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody. Mol. Cancer Ther. 9: 2641–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Jeansson M., Gawlik A., Anderson G., Li C., Kerjaschki D., Henkelman M., and Quaggin S. E.. 2011. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J. Clin. Invest. 121: 2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Woo K. V., Qu X., Babaev V. R., Linton M. F., Guzman R. J., Fazio S., and Baldwin H. S.. 2011. Tie1 attenuation reduces murine atherosclerosis in a dose-dependent and shear stress-specific manner. J. Clin. Invest. 121: 1624–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Lee J., Kim K. E., Choi D. K., Jang J. Y., Jung J. J., Kiyonari H., Shioi G., Chang W., Suda T., Mochizuki N., et al. 2013. Angiopoietin-1 guides directional angiogenesis through integrin alphavbeta5 signaling for recovery of ischemic retinopathy. Sci. Transl. Med. 5: 203ra127. [DOI] [PubMed] [Google Scholar]
- 487.Oliner J., Min H., Leal J., Yu D., Rao S., You E., Tang X., Kim H., Meyer S., Han S. J., et al. 2004. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 6: 507–516. [DOI] [PubMed] [Google Scholar]
- 488.Daly C., Pasnikowski E., Burova E., Wong V., Aldrich T. H., Griffiths J., Ioffe E., Daly T. J., Fandl J. P., Papadopoulos N., et al. 2006. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc. Natl. Acad. Sci. USA. 103: 15491–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Fiedler U., Reiss Y., Scharpfenecker M., Grunow V., Koidl S., Thurston G., Gale N. W., Witzenrath M., Rosseau S., Suttorp N., et al. 2006. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat. Med. 12: 235–239. [DOI] [PubMed] [Google Scholar]
- 490.Felcht M., Luck R., Schering A., Seidel P., Srivastava K., Hu J., Bartol A., Kienast Y., Vettel C., Loos E. K., et al. 2012. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J. Clin. Invest. 122: 1991–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Lee H. S., Oh S. J., Lee K. H., Lee Y. S., Ko E., Kim K. E., Kim H. C., Kim S., Song P. H., Kim Y. I., et al. 2014. Gln-362 of angiopoietin-2 mediates migration of tumor and endothelial cells through association with alpha5beta1 integrin. J. Biol. Chem. 289: 31330–31340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Park S. W., Yun J. H., Kim J. H., Kim K. W., Cho C. H., and Kim J. H.. 2014. Angiopoietin 2 induces pericyte apoptosis via alpha3beta1 integrin signaling in diabetic retinopathy. Diabetes. 63: 3057–3068. [DOI] [PubMed] [Google Scholar]
- 493.Hakanpaa L., Sipila T., Leppanen V. M., Gautam P., Nurmi H., Jacquemet G., Eklund L., Ivaska J., Alitalo K., and Saharinen P.. 2015. Endothelial destabilization by angiopoietin-2 via integrin beta1 activation. Nat. Commun. 6: 5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Theelen T. L., Lappalainen J. P., Sluimer J. C., Gurzeler E., Cleutjens J. P., Gijbels M. J., Biessen E. A., Daemen M. J., Alitalo K., and Yla-Herttuala S.. 2015. Angiopoietin-2 blocking antibodies reduce early atherosclerotic plaque development in mice. Atherosclerosis. 241: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Kim I., Moon S. O., Koh K. N., Kim H., Uhm C. S., Kwak H. J., Kim N. G., and Koh G. Y.. 1999. Molecular cloning, expression, and characterization of angiopoietin-related protein. angiopoietin-related protein induces endothelial cell sprouting. J. Biol. Chem. 274: 26523–26528. [DOI] [PubMed] [Google Scholar]
- 496.Guo D. F., Chenier I., Tardif V., Orlov S. N., and Inagami T.. 2003. Type 1 angiotensin II receptor-associated protein ARAP1 binds and recycles the receptor to the plasma membrane. Biochem. Biophys. Res. Commun. 310: 1254–1265. [DOI] [PubMed] [Google Scholar]
- 497.Zhang C. C., Kaba M., Ge G., Xie K., Tong W., Hug C., and Lodish H. F.. 2006. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat. Med. 12: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Aoi J., Endo M., Kadomatsu T., Miyata K., Nakano M., Horiguchi H., Ogata A., Odagiri H., Yano M., Araki K., et al. 2011. Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 71: 7502–7512. [DOI] [PubMed] [Google Scholar]
- 499.Endo M., Nakano M., Kadomatsu T., Fukuhara S., Kuroda H., Mikami S., Hato T., Aoi J., Horiguchi H., Miyata K., et al. 2012. Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical driver of metastasis. Cancer Res. 72: 1784–1794. [DOI] [PubMed] [Google Scholar]
- 500.Tazume H., Miyata K., Tian Z., Endo M., Horiguchi H., Takahashi O., Horio E., Tsukano H., Kadomatsu T., Nakashima Y., et al. 2012. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 32: 1400–1409. [DOI] [PubMed] [Google Scholar]
- 501.Farhat N., Thorin-Trescases N., Mamarbachi M., Villeneuve L., Yu C., Martel C., Duquette N., Gayda M., Nigam A., Juneau M., et al. 2013. Angiopoietin-like 2 promotes atherogenesis in mice. J. Am. Heart Assoc. 2: e000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Tian Z., Miyata K., Tazume H., Sakaguchi H., Kadomatsu T., Horio E., Takahashi O., Komohara Y., Araki K., Hirata Y., et al. 2013. Perivascular adipose tissue-secreted angiopoietin-like protein 2 (Angptl2) accelerates neointimal hyperplasia after endovascular injury. J. Mol. Cell. Cardiol. 57: 1–12. [DOI] [PubMed] [Google Scholar]
- 503.Odagiri H., Kadomatsu T., Endo M., Masuda T., Morioka M. S., Fukuhara S., Miyamoto T., Kobayashi E., Miyata K., Aoi J., et al. 2014. The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin alpha5beta1, p38 MAPK, and matrix metalloproteinases. Sci. Signal. 7: ra7. [DOI] [PubMed] [Google Scholar]
- 504.Castan-Laurell I., Dray C., Knauf C., Kunduzova O., and Valet P.. 2012. Apelin, a promising target for type 2 diabetes treatment? Trends Endocrinol. Metab. 23: 234–241. [DOI] [PubMed] [Google Scholar]
- 505.O’Carroll A. M., Lolait S. J., Harris L. E., and Pope G. R.. 2013. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J. Endocrinol. 219: R13–R35. [DOI] [PubMed] [Google Scholar]
- 506.Antushevich H., and Wojcik M.. 2018. Review: apelin in disease. Clin. Chim. Acta. 483: 241–248. [DOI] [PubMed] [Google Scholar]
- 507.Dray C., Knauf C., Daviaud D., Waget A., Boucher J., Buleon M., Cani P. D., Attane C., Guigne C., Carpene C., et al. 2008. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 8: 437–445. [DOI] [PubMed] [Google Scholar]
- 508.Kunduzova O., Alet N., Delesque-Touchard N., Millet L., Castan-Laurell I., Muller C., Dray C., Schaeffer P., Herault J. P., Savi P., et al. 2008. Apelin/APJ signaling system: a potential link between adipose tissue and endothelial angiogenic processes. FASEB J. 22: 4146–4153. [DOI] [PubMed] [Google Scholar]
- 509.Yue P., Jin H., Aillaud M., Deng A. C., Azuma J., Asagami T., Kundu R. K., Reaven G. M., Quertermous T., and Tsao P. S.. 2010. Apelin is necessary for the maintenance of insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 298: E59–E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Zhu S., Sun F., Li W., Cao Y., Wang C., Wang Y., Liang D., Zhang R., Zhang S., Wang H., et al. 2011. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol. Cell. Biochem. 353: 305–313. [DOI] [PubMed] [Google Scholar]
- 511.Attané C., Foussal C., Le Gonidec S., Benani A., Daviaud D., Wanecq E., Guzman-Ruiz R., Dray C., Bezaire V., Rancoule C., et al. 2012. Apelin treatment increases complete fatty acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes. 61: 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Than A., Cheng Y., Foh L. C., Leow M. K., Lim S. C., Chuah Y. J., Kang Y., and Chen P.. 2012. Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol. Cell. Endocrinol. 362: 227–241. [DOI] [PubMed] [Google Scholar]
- 513.Sato T., Suzuki T., Watanabe H., Kadowaki A., Fukamizu A., Liu P. P., Kimura A., Ito H., Penninger J. M., Imai Y., et al. 2013. Apelin is a positive regulator of ACE2 in failing hearts. J. Clin. Invest. 123: 5203–5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Wattanachanya L., Lu W. D., Kundu R. K., Wang L., Abbott M. J., O’Carroll D., Quertermous T., and Nissenson R. A.. 2013. Increased bone mass in mice lacking the adipokine apelin. Endocrinology. 154: 2069–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Han S., Englander E. W., Gomez G. A., Rastellini C., Quertermous T., Kundu R. K., and Greeley G. H. Jr.. 2015. Pancreatic islet APJ deletion reduces islet density and glucose tolerance in mice. Endocrinology. 156: 2451–2460. [DOI] [PubMed] [Google Scholar]
- 516.Than A., He H. L., Chua S. H., Xu D., Sun L., Leow M. K., and Chen P.. 2015. Apelin enhances brown adipogenesis and browning of white adipocytes. J. Biol. Chem. 290: 14679–14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Hwangbo C., Wu J., Papangeli I., Adachi T., Sharma B., Park S., Zhao L., Ju H., Go G. W., Cui G., et al. 2017. Endothelial APLNR regulates tissue fatty acid uptake and is essential for apelin’s glucose-lowering effects. Sci. Transl. Med. 9: eaad4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Bertrand C., Pradere J. P., Geoffre N., Deleruyelle S., Masri B., Personnaz J., Le Gonidec S., Batut A., Louche K., Moro C., et al. 2018. Chronic apelin treatment improves hepatic lipid metabolism in obese and insulin-resistant mice by an indirect mechanism. Endocrine. 60: 112–121. [DOI] [PubMed] [Google Scholar]
- 519.Zhang H., and Bradley A.. 1996. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 122: 2977–2986. [DOI] [PubMed] [Google Scholar]
- 520.Schlange T., Andree B., Arnold H. H., and Brand T.. 2000. BMP2 is required for early heart development during a distinct time period. Mech. Dev. 91: 259–270. [DOI] [PubMed] [Google Scholar]
- 521.Ma L., Lu M. F., Schwartz R. J., and Martin J. F.. 2005. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 132: 5601–5611. [DOI] [PubMed] [Google Scholar]
- 522.Tsuji K., Bandyopadhyay A., Harfe B. D., Cox K., Kakar S., Gerstenfeld L., Einhorn T., Tabin C. J., and Rosen V.. 2006. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 38: 1424–1429. [DOI] [PubMed] [Google Scholar]
- 523.Persano L., Pistollato F., Rampazzo E., Della Puppa A., Abbadi S., Frasson C., Volpin F., Indraccolo S., Scienza R., and Basso G.. 2012. BMP2 sensitizes glioblastoma stem-like cells to Temozolomide by affecting HIF-1alpha stability and MGMT expression. Cell Death Dis. 3: e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Du M., Su X. M., Zhang T., and Xing Y. J.. 2014. Aberrant promoter DNA methylation inhibits bone morphogenetic protein 2 expression and contributes to drug resistance in breast cancer. Mol. Med. Rep. 10: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 525.Choi Y. J., Ingram P. N., Yang K., Coffman L., Iyengar M., Bai S., Thomas D. G., Yoon E., and Buckanovich R. J.. 2015. Identifying an ovarian cancer cell hierarchy regulated by bone morphogenetic protein 2. Proc. Natl. Acad. Sci. USA. 112: E6882–E6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Wang M. H., Zhou X. M., Zhang M. Y., Shi L., Xiao R. W., Zeng L. S., Yang X. Z., Zheng X. F. S., Wang H. Y., and Mai S. J.. 2017. BMP2 promotes proliferation and invasion of nasopharyngeal carcinoma cells via mTORC1 pathway. Aging (Albany NY). 9: 1326–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Daluiski A., Engstrand T., Bahamonde M. E., Gamer L. W., Agius E., Stevenson S. L., Cox K., Rosen V., and Lyons K. M.. 2001. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat. Genet. 27: 84–88. [DOI] [PubMed] [Google Scholar]
- 528.Matsumoto Y., Otsuka F., Hino J., Miyoshi T., Takano M., Miyazato M., Makino H., and Kangawa K.. 2012. Bone morphogenetic protein-3b (BMP-3b) inhibits osteoblast differentiation via Smad2/3 pathway by counteracting Smad1/5/8 signaling. Mol. Cell. Endocrinol. 350: 78–86. [DOI] [PubMed] [Google Scholar]
- 529.Li S., Nie E. H., Yin Y., Benowitz L. I., Tung S., Vinters H. V., Bahjat F. R., Stenzel-Poore M. P., Kawaguchi R., Coppola G., et al. 2015. GDF10 is a signal for axonal sprouting and functional recovery after stroke. Nat. Neurosci. 18: 1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Winnier G., Blessing M., Labosky P. A., and Hogan B. L.. 1995. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 9: 2105–2116. [DOI] [PubMed] [Google Scholar]
- 531.Paez-Pereda M., Giacomini D., Refojo D., Nagashima A. C., Hopfner U., Grubler Y., Chervin A., Goldberg V., Goya R., Hentges S. T., et al. 2003. Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a Smad/estrogen receptor crosstalk. Proc. Natl. Acad. Sci. USA. 100: 1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Guo D., Huang J., and Gong J.. 2012. Bone morphogenetic protein 4 (BMP4) is required for migration and invasion of breast cancer. Mol. Cell. Biochem. 363: 179–190. [DOI] [PubMed] [Google Scholar]
- 533.Cao Y., Slaney C. Y., Bidwell B. N., Parker B. S., Johnstone C. N., Rautela J., Eckhardt B. L., and Anderson R. L.. 2014. BMP4 inhibits breast cancer metastasis by blocking myeloid-derived suppressor cell activity. Cancer Res. 74: 5091–5102. [DOI] [PubMed] [Google Scholar]
- 534.Coffman L. G., Choi Y. J., McLean K., Allen B. L., di Magliano M. P., and Buckanovich R. J.. 2016. Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget. 7: 6916–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Zhao G. Q., Deng K., Labosky P. A., Liaw L., and Hogan B. L.. 1996. The gene encoding bone morphogenetic protein 8B is required for the initiation and maintenance of spermatogenesis in the mouse. Genes Dev. 10: 1657–1669. [DOI] [PubMed] [Google Scholar]
- 536.Cheng Z., Cui W., Ding Y., Liu T., Liu W., Qin Y., Xia W., Xu J., Zhang Y., and Zou X.. 2014. BMP8B mediates the survival of pancreatic cancer cells and regulates the progression of pancreatic cancer. Oncol. Rep. 32: 1861–1866. [DOI] [PubMed] [Google Scholar]
- 537.Martins L., Seoane-Collazo P., Contreras C., Gonzalez-Garcia I., Martinez-Sanchez N., Gonzalez F., Zalvide J., Gallego R., Dieguez C., Nogueiras R., et al. 2016. A functional link between AMPK and orexin mediates the effect of BMP8B on energy balance. Cell Reports. 16: 2231–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Li Y., Wright G. L., and Peterson J. M.. 2017. C1q/TNF-related protein 3 (CTRP3) function and regulation. Compr. Physiol. 7: 863–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Weigert J., Neumeier M., Schaffler A., Fleck M., Scholmerich J., Schutz C., and Buechler C.. 2005. The adiponectin paralog CORS-26 has anti-inflammatory properties and is produced by human monocytic cells. FEBS Lett. 579: 5565–5570. [DOI] [PubMed] [Google Scholar]
- 540.Kopp A., Bala M., Buechler C., Falk W., Gross P., Neumeier M., Scholmerich J., and Schaffler A.. 2010. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology. 151: 5267–5278. [DOI] [PubMed] [Google Scholar]
- 541.Peterson J. M., Wei Z., and Wong G. W.. 2010. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J. Biol. Chem. 285: 39691–39701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Yi W., Sun Y., Yuan Y., Lau W. B., Zheng Q., Wang X., Wang Y., Shang X., Gao E., Koch W. J., et al. 2012. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation. 125: 3159–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Schmid A., Kopp A., Hanses F., Karrasch T., and Schaffler A.. 2014. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/-2 phosphorylation in mice in vivo. Biochem. Biophys. Res. Commun. 452: 8–13. [DOI] [PubMed] [Google Scholar]
- 544.Otani M., Furukawa S., Wakisaka S., and Maeda T.. 2015. A novel adipokine C1q/TNF-related protein 3 is expressed in developing skeletal muscle and controls myoblast proliferation and differentiation. Mol. Cell. Biochem. 409: 271–282. [DOI] [PubMed] [Google Scholar]
- 545.Li Y., Ozment T., Wright G. L., and Peterson J. M.. 2016. Identification of putative receptors for the novel adipokine CTRP3 using ligand-receptor capture technology. PLoS One. 11: e0164593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Wolf R. M., Lei X., Yang Z. C., Nyandjo M., Tan S. Y., and Wong G. W.. 2016. CTRP3 deficiency reduces liver size and alters IL-6 and TGFbeta levels in obese mice. Am. J. Physiol. Endocrinol. Metab. 310: E332–E345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Lin J., Liu Q., Zhang H., Huang X., Zhang R., Chen S., Wang X., Yu B., and Hou J.. 2017. C1q/Tumor necrosis factor-related protein-3 protects macrophages against LPS-induced lipid accumulation, inflammation and phenotype transition via PPARgamma and TLR4-mediated pathways. Oncotarget. 8: 82541–82557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Ma Z. G., Yuan Y. P., Xu S. C., Wei W. Y., Xu C. R., Zhang X., Wu Q. Q., Liao H. H., Ni J., and Tang Q. Z.. 2017. CTRP3 attenuates cardiac dysfunction, inflammation, oxidative stress and cell death in diabetic cardiomyopathy in rats. Diabetologia. 60: 1126–1137. [DOI] [PubMed] [Google Scholar]
- 549.Nishimoto H., Yamamoto A., Furukawa S., Wakisaka S., and Maeda T.. 2017. C1q/TNF-related protein 3 expression and effects on adipocyte differentiation of 3T3-L1 cells. Cell Biol. Int. 41: 197–203. [DOI] [PubMed] [Google Scholar]
- 550.Mu Y., Yin T. L., Yin L., Hu X., and Yang J.. 2018. CTRP3 attenuates high-fat diet-induced male reproductive dysfunction in mice. Clin. Sci. (Lond.). 132: 883–899. [DOI] [PubMed] [Google Scholar]
- 551.Rull A., Camps J., Alonso-Villaverde C., and Joven J.. 2010. Insulin resistance, inflammation, and obesity: role of monocyte chemoattractant protein-1 (or CCL2) in the regulation of metabolism. Mediators Inflamm. 2010: doi:10.1155/2010/326580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Boring L., Gosling J., Cleary M., and Charo I. F.. 1998. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 394: 894–897. [DOI] [PubMed] [Google Scholar]
- 553.Gu L., Okada Y., Clinton S. K., Gerard C., Sukhova G. K., Libby P., and Rollins B. J.. 1998. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell. 2: 275–281. [DOI] [PubMed] [Google Scholar]
- 554.Sartipy P., and Loskutoff D. J.. 2003. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA. 100: 7265–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Kamei N., Tobe K., Suzuki R., Ohsugi M., Watanabe T., Kubota N., Ohtsuka-Kowatari N., Kumagai K., Sakamoto K., Kobayashi M., et al. 2006. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 281: 26602–26614. [DOI] [PubMed] [Google Scholar]
- 556.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., et al. 2006. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Weisberg S. P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., Charo I., Leibel R. L., and Ferrante A. W. Jr.. 2006. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Lumeng C. N., Bodzin J. L., and Saltiel A. R.. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Rull A., Escola-Gil J. C., Julve J., Rotllan N., Calpe-Berdiel L., Coll B., Aragones G., Marsillach J., Alonso-Villaverde C., Camps J., et al. 2007. Deficiency in monocyte chemoattractant protein-1 modifies lipid and glucose metabolism. Exp. Mol. Pathol. 83: 361–366. [DOI] [PubMed] [Google Scholar]
- 560.Kirk E. A., Sagawa Z. K., McDonald T. O., O’Brien K. D., and Heinecke J. W.. 2008. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected]. Diabetes. 57: 1254–1261. [DOI] [PubMed] [Google Scholar]
- 561.Pruenster M., Mudde L., Bombosi P., Dimitrova S., Zsak M., Middleton J., Richmond A., Graham G. J., Segerer S., Nibbs R. J., et al. 2009. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat. Immunol. 10: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Tateya S., Tamori Y., Kawaguchi T., Kanda H., and Kasuga M.. 2010. An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice. Endocrinology. 151: 971–979. [DOI] [PubMed] [Google Scholar]
- 563.Sundaram S., and Yan L.. 2019. Adipose-specific monocyte chemotactic protein-1 deficiency reduces pulmonary metastasis of Lewis lung carcinoma in mice. Anticancer Res. 39: 1729–1738. [DOI] [PubMed] [Google Scholar]
- 564.Ricklin D., and Lambris J. D.. 2013. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J. Immunol. 190: 3831–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Ricklin D., and Lambris J. D.. 2013. Complement in immune and inflammatory disorders: therapeutic interventions. J. Immunol. 190: 3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Cook K. S., Min H. Y., Johnson D., Chaplinsky R. J., Flier J. S., Hunt C. R., and Spiegelman B. M.. 1987. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 237: 402–405. [DOI] [PubMed] [Google Scholar]
- 567.Rosen B. S., Cook K. S., Yaglom J., Groves D. L., Volanakis J. E., Damm D., White T., and Spiegelman B. M.. 1989. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 244: 1483–1487. [DOI] [PubMed] [Google Scholar]
- 568.Baldo A., Sniderman A. D., St-Luce S., Avramoglu R. K., Maslowska M., Hoang B., Monge J. C., Bell A., Mulay S., and Cianflone K.. 1993. The adipsin-acylation stimulating protein system and regulation of intracellular triglyceride synthesis. J. Clin. Invest. 92: 1543–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Xu Y., Ma M., Ippolito G. C., Schroeder H. W. Jr., Carroll M. C., and Volanakis J. E.. 2001. Complement activation in factor D-deficient mice. Proc. Natl. Acad. Sci. USA. 98: 14577–14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Lo J. C., Ljubicic S., Leibiger B., Kern M., Leibiger I. B., Moede T., Kelly M. E., Chatterjee Bhowmick D., Murano I., Cohen P., et al. 2014. Adipsin is an adipokine that improves beta cell function in diabetes. Cell. 158: 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Song N. J., Kim S., Jang B. H., Chang S. H., Yun U. J., Park K. M., Waki H., Li D. Y., Tontonoz P., and Park K. W.. 2016. Small molecule-induced complement factor D (adipsin) promotes lipid accumulation and adipocyte differentiation. PLoS One. 11: e0162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Wu X., Hutson I., Akk A. M., Mascharak S., Pham C. T. N., Hourcade D. E., Brown R., Atkinson J. P., and Harris C. A.. 2018. Contribution of adipose-derived factor D/adipsin to complement alternative pathway activation: lessons from lipodystrophy. J. Immunol. 200: 2786–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Goto H., Shimono Y., Funakoshi Y., Imamura Y., Toyoda M., Kiyota N., Kono S., Takao S., Mukohara T., and Minami H.. 2019. Adipose-derived stem cells enhance human breast cancer growth and cancer stem cell-like properties through adipsin. Oncogene. 38: 767–779. [DOI] [PubMed] [Google Scholar]
- 574.Mulvihill E. E., and Drucker D. J.. 2014. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 35: 992–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Röhrborn D., Wronkowitz N., and Eckel J.. 2015. DPP4 in diabetes. Front. Immunol. 6: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Marguet D., Baggio L., Kobayashi T., Bernard A. M., Pierres M., Nielsen P. F., Ribel U., Watanabe T., Drucker D. J., and Wagtmann N.. 2000. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc. Natl. Acad. Sci. USA. 97: 6874–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Conarello S. L., Li Z., Ronan J., Roy R. S., Zhu L., Jiang G., Liu F., Woods J., Zycband E., Moller D. E., et al. 2003. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc. Natl. Acad. Sci. USA. 100: 6825–6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Sauvé M., Ban K., Momen M. A., Zhou Y. Q., Henkelman R. M., Husain M., and Drucker D. J.. 2010. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 59: 1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Kyle K. A., Willett T. L., Baggio L. L., Drucker D. J., and Grynpas M. D.. 2011. Differential effects of PPAR-{gamma} activation versus chemical or genetic reduction of DPP-4 activity on bone quality in mice. Endocrinology. 152: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Shah Z., Kampfrath T., Deiuliis J. A., Zhong J., Pineda C., Ying Z., Xu X., Lu B., Moffatt-Bruce S., Durairaj R., et al. 2011. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 124: 2338–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Femia A. P., Raimondi L., Maglieri G., Lodovici M., Mannucci E., and Caderni G.. 2013. Long-term treatment with Sitagliptin, a dipeptidyl peptidase-4 inhibitor, reduces colon carcinogenesis and reactive oxygen species in 1,2-dimethylhydrazine-induced rats. Int. J. Cancer. 133: 2498–2503. [DOI] [PubMed] [Google Scholar]
- 582.Shimasaki T., Masaki T., Mitsutomi K., Ueno D., Gotoh K., Chiba S., Kakuma T., and Yoshimatsu H.. 2013. The dipeptidyl peptidase-4 inhibitor des-fluoro-sitagliptin regulates brown adipose tissue uncoupling protein levels in mice with diet-induced obesity. PLoS One. 8: e63626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Mulvihill E. E., Varin E. M., Ussher J. R., Campbell J. E., Bang K. W., Abdullah T., Baggio L. L., and Drucker D. J.. 2016. Inhibition of dipeptidyl peptidase-4 impairs ventricular function and promotes cardiac fibrosis in high fat-fed diabetic mice. Diabetes. 65: 742–754. [DOI] [PubMed] [Google Scholar]
- 584.Romacho T., Vallejo S., Villalobos L. A., Wronkowitz N., Indrakusuma I., Sell H., Eckel J., Sanchez-Ferrer C. F., and Peiro C.. 2016. Soluble dipeptidyl peptidase-4 induces microvascular endothelial dysfunction through proteinase-activated receptor-2 and thromboxane A2 release. J. Hypertens. 34: 869–876. [DOI] [PubMed] [Google Scholar]
- 585.Mulvihill E. E., Varin E. M., Gladanac B., Campbell J. E., Ussher J. R., Baggio L. L., Yusta B., Ayala J., Burmeister M. A., Matthews D., et al. 2017. Cellular sites and mechanisms linking reduction of dipeptidyl peptidase-4 activity to control of incretin hormone action and glucose homeostasis. Cell Metab. 25: 152–165. [DOI] [PubMed] [Google Scholar]
- 586.Qin C. J., Zhao L. H., Zhou X., Zhang H. L., Wen W., Tang L., Zeng M., Wang M. D., Fu G. B., Huang S., et al. 2018. Inhibition of dipeptidyl peptidase IV prevents high fat diet-induced liver cancer angiogenesis by downregulating chemokine ligand 2. Cancer Lett. 420: 26–37. [DOI] [PubMed] [Google Scholar]
- 587.Varin E. M., Mulvihill E. E., Beaudry J. L., Pujadas G., Fuchs S., Tanti J. F., Fazio S., Kaur K., Cao X., Baggio L. L., et al. 2019. Circulating levels of soluble dipeptidyl peptidase-4 are dissociated from inflammation and induced by enzymatic DPP4 inhibition. Cell Metab. 29: 320–334.e5. [DOI] [PubMed] [Google Scholar]
- 588.Zhao Y., Gu X., Zhang N., Kolonin M. G., An Z., and Sun K.. 2016. Divergent functions of endotrophin on different cell populations in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 311: E952–E963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Hotamisligil G. S., and Bernlohr D. A.. 2015. Metabolic functions of FABPs–mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 11: 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Prentice K. J., Saksi J., and Hotamisligil G. S.. 2019. Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J. Lipid Res. 60: 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Makowski L., Boord J. B., Maeda K., Babaev V. R., Uysal K. T., Morgan M. A., Parker R. A., Suttles J., Fazio S., Hotamisligil G. S., et al. 2001. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 7: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Furuhashi M., Tuncman G., Gorgun C. Z., Makowski L., Atsumi G., Vaillancourt E., Kono K., Babaev V. R., Fazio S., Linton M. F., et al. 2007. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 447: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Furuhashi M., Fucho R., Gorgun C. Z., Tuncman G., Cao H., and Hotamisligil G. S.. 2008. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J. Clin. Invest. 118: 2640–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Yang R., Castriota G., Chen Y., Cleary M. A., Ellsworth K., Shin M. K., Tran J. L., Vogt T. F., Wu M., Xu S., et al. 2011. RNAi-mediated germline knockdown of FABP4 increases body weight but does not improve the deranged nutrient metabolism of diet-induced obese mice. Int. J. Obes. (Lond.). 35: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Cao H., Sekiya M., Ertunc M. E., Burak M. F., Mayers J. R., White A., Inouye K., Rickey L. M., Ercal B. C., Furuhashi M., et al. 2013. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 17: 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Garin-Shkolnik T., Rudich A., Hotamisligil G. S., and Rubinstein M.. 2014. FABP4 attenuates PPARgamma and adipogenesis and is inversely correlated with PPARgamma in adipose tissues. Diabetes. 63: 900–911. [DOI] [PubMed] [Google Scholar]
- 597.Wu L. E., Samocha-Bonet D., Whitworth P. T., Fazakerley D. J., Turner N., Biden T. J., James D. E., and Cantley J.. 2014. Identification of fatty acid binding protein 4 as an adipokine that regulates insulin secretion during obesity. Mol. Metab. 3: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Burak M. F., Inouye K. E., White A., Lee A., Tuncman G., Calay E. S., Sekiya M., Tirosh A., Eguchi K., Birrane G., et al. 2015. Development of a therapeutic monoclonal antibody that targets secreted fatty acid-binding protein aP2 to treat type 2 diabetes. Sci. Transl. Med. 7: 319ra205. [DOI] [PubMed] [Google Scholar]
- 599.Furuhashi M., Fuseya T., Murata M., Hoshina K., Ishimura S., Mita T., Watanabe Y., Omori A., Matsumoto M., Sugaya T., et al. 2016. Local production of fatty acid-binding protein 4 in epicardial/perivascular fat and macrophages is linked to coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 36: 825–834. [DOI] [PubMed] [Google Scholar]
- 600.Hertzel A. V., Xu H., Downey M., Kvalheim N., and Bernlohr D. A.. 2017. Fatty acid binding protein 4/aP2-dependent BLT1R expression and signaling. J. Lipid Res. 58: 1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Yan F., Shen N., Pang J. X., Zhang Y. W., Rao E. Y., Bode A. M., Al-Kali A., Zhang D. E., Litzow M. R., Li B., et al. 2017. Fatty acid-binding protein FABP4 mechanistically links obesity with aggressive AML by enhancing aberrant DNA methylation in AML cells. Leukemia. 31: 1434–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Hao J., Zhang Y., Yan X., Yan F., Sun Y., Zeng J., Waigel S., Yin Y., Fraig M. M., Egilmez N. K., et al. 2018. Circulating adipose fatty acid binding protein is a new link underlying obesity-associated breast/mammary tumor development. Cell Metab. 28: 689–705.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Watanabe T., Watanabe-Kominato K., Takahashi Y., Kojima M., and Watanabe R.. 2017. Adipose tissue-derived omentin-1 function and regulation. Compr. Physiol. 7: 765–781. [DOI] [PubMed] [Google Scholar]
- 604.Tsuji S., Uehori J., Matsumoto M., Suzuki Y., Matsuhisa A., Toyoshima K., and Seya T.. 2001. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J. Biol. Chem. 276: 23456–23463. [DOI] [PubMed] [Google Scholar]
- 605.Yang R. Z., Lee M. J., Hu H., Pray J., Wu H. B., Hansen B. C., Shuldiner A. R., Fried S. K., McLenithan J. C., and Gong D. W.. 2006. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 290: E1253–E1261. [DOI] [PubMed] [Google Scholar]
- 606.Xie H., Xie P. L., Wu X. P., Chen S. M., Zhou H. D., Yuan L. Q., Sheng Z. F., Tang S. Y., Luo X. H., and Liao E. Y.. 2011. Omentin-1 attenuates arterial calcification and bone loss in osteoprotegerin-deficient mice by inhibition of RANKL expression. Cardiovasc. Res. 92: 296–306. [DOI] [PubMed] [Google Scholar]
- 607.Maruyama S., Shibata R., Kikuchi R., Izumiya Y., Rokutanda T., Araki S., Kataoka Y., Ohashi K., Daida H., Kihara S., et al. 2012. Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism. J. Biol. Chem. 287: 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Akiyama Y., Oshima K., Kuhara T., Shin K., Abe F., Iwatsuki K., Nadano D., and Matsuda T.. 2013. A lactoferrin-receptor, intelectin 1, affects uptake, sub-cellular localization and release of immunochemically detectable lactoferrin by intestinal epithelial Caco-2 cells. J. Biochem. 154: 437–448. [DOI] [PubMed] [Google Scholar]
- 609.Zhang Y. Y., and Zhou L. M.. 2013. Omentin-1, a new adipokine, promotes apoptosis through regulating Sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells. Eur. J. Pharmacol. 698: 137–144. [DOI] [PubMed] [Google Scholar]
- 610.Kataoka Y., Shibata R., Ohashi K., Kambara T., Enomoto T., Uemura Y., Ogura Y., Yuasa D., Matsuo K., Nagata T., et al. 2014. Omentin prevents myocardial ischemic injury through AMP-activated protein kinase- and Akt-dependent mechanisms. J. Am. Coll. Cardiol. 63: 2722–2733. [DOI] [PubMed] [Google Scholar]
- 611.Matsuo K., Shibata R., Ohashi K., Kambara T., Uemura Y., Hiramatsu-Ito M., Enomoto T., Yuasa D., Joki Y., Ito M., et al. 2015. Omentin functions to attenuate cardiac hypertrophic response. J. Mol. Cell. Cardiol. 79: 195–202. [DOI] [PubMed] [Google Scholar]
- 612.Wesener D. A., Wangkanont K., McBride R., Song X., Kraft M. B., Hodges H. L., Zarling L. C., Splain R. A., Smith D. F., Cummings R. D., et al. 2015. Recognition of microbial glycans by human intelectin-1. Nat. Struct. Mol. Biol. 22: 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Hiramatsu-Ito M., Shibata R., Ohashi K., Uemura Y., Kanemura N., Kambara T., Enomoto T., Yuasa D., Matsuo K., Ito M., et al. 2016. Omentin attenuates atherosclerotic lesion formation in apolipoprotein E-deficient mice. Cardiovasc. Res. 110: 107–117. [DOI] [PubMed] [Google Scholar]
- 614.Watanabe K., Watanabe R., Konii H., Shirai R., Sato K., Matsuyama T. A., Ishibashi-Ueda H., Koba S., Kobayashi Y., Hirano T., et al. 2016. Counteractive effects of omentin-1 against atherogenesisdagger. Cardiovasc. Res. 110: 118–128. [DOI] [PubMed] [Google Scholar]
- 615.Rao S. S., Hu Y., Xie P. L., Cao J., Wang Z. X., Liu J. H., Yin H., Huang J., Tan Y. J., Luo J., et al. 2018. Omentin-1 prevents inflammation-induced osteoporosis by downregulating the pro-inflammatory cytokines. Bone Res. 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Garlanda C., Dinarello C. A., and Mantovani A.. 2013. The interleukin-1 family: back to the future. Immunity. 39: 1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Ballak D. B., Stienstra R., Tack C. J., Dinarello C. A., and van Diepen J. A.. 2015. IL-1 family members in the pathogenesis and treatment of metabolic disease: focus on adipose tissue inflammation and insulin resistance. Cytokine. 75: 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Netea M. G., van de Veerdonk F. L., van der Meer J. W., Dinarello C. A., and Joosten L. A.. 2015. Inflammasome-independent regulation of IL-1-family cytokines. Annu. Rev. Immunol. 33: 49–77. [DOI] [PubMed] [Google Scholar]
- 619.Grégoire F., De Broux N., Hauser N., Heremans H., Van Damme J., and Remacle C.. 1992. Interferon-gamma and interleukin-1 beta inhibit adipoconversion in cultured rodent preadipocytes. J. Cell. Physiol. 151: 300–309. [DOI] [PubMed] [Google Scholar]
- 620.Hirsch E., Irikura V. M., Paul S. M., and Hirsh D.. 1996. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc. Natl. Acad. Sci. USA. 93: 11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Kenney M. J., Blecha F., Morgan D. A., and Fels R. J.. 2001. Interleukin-1 beta alters brown adipose tissue but not renal sympathetic nerve responses to hypothermia. Am. J. Physiol. Heart Circ. Physiol. 281: H2441–H2445. [DOI] [PubMed] [Google Scholar]
- 622.Somm E., Henrichot E., Pernin A., Juge-Aubry C. E., Muzzin P., Dayer J. M., Nicklin M. J., and Meier C. A.. 2005. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes. 54: 3503–3509. [DOI] [PubMed] [Google Scholar]
- 623.Lagathu C., Yvan-Charvet L., Bastard J. P., Maachi M., Quignard-Boulange A., Capeau J., and Caron M.. 2006. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia. 49: 2162–2173. [DOI] [PubMed] [Google Scholar]
- 624.García M. C., Wernstedt I., Berndtsson A., Enge M., Bell M., Hultgren O., Horn M., Ahren B., Enerback S., Ohlsson C., et al. 2006. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 55: 1205–1213. [DOI] [PubMed] [Google Scholar]
- 625.Sauter N. S., Schulthess F. T., Galasso R., Castellani L. W., and Maedler K.. 2008. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 149: 2208–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Stienstra R., Joosten L. A., Koenen T., van Tits B., van Diepen J. A., van den Berg S. A., Rensen P. C., Voshol P. J., Fantuzzi G., Hijmans A., et al. 2010. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 12: 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.McGillicuddy F. C., Harford K. A., Reynolds C. M., Oliver E., Claessens M., Mills K. H., and Roche H. M.. 2011. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes. 60: 1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M. T., Brickey W. J., and Ting J. P.. 2011. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12: 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Nov O., Shapiro H., Ovadia H., Tarnovscki T., Dvir I., Shemesh E., Kovsan J., Shelef I., Carmi Y., Voronov E., et al. 2013. Interleukin-1beta regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PLoS One. 8: e53626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Gao D., Madi M., Ding C., Fok M., Steele T., Ford C., Hunter L., and Bing C.. 2014. Interleukin-1beta mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am. J. Physiol. Endocrinol. Metab. 307: E289–E304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Luzina I. G., Keegan A. D., Heller N. M., Rook G. A., Shea-Donohue T., and Atamas S. P.. 2012. Regulation of inflammation by interleukin-4: a review of “alternatives”. J. Leukoc. Biol. 92: 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Huang J. T., Welch J. S., Ricote M., Binder C. J., Willson T. M., Kelly C., Witztum J. L., Funk C. D., Conrad D., and Glass C. K.. 1999. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 400: 378–382. [DOI] [PubMed] [Google Scholar]
- 633.Davenport P., and Tipping P. G.. 2003. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 163: 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Oh-I S., Thaler J. P., Ogimoto K., Wisse B. E., Morton G. J., and Schwartz M. W.. 2010. Central administration of interleukin-4 exacerbates hypothalamic inflammation and weight gain during high-fat feeding. Am. J. Physiol. Endocrinol. Metab. 299: E47–E53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Ricardo-Gonzalez R. R., Red Eagle A., Odegaard J. I., Jouihan H., Morel C. R., Heredia J. E., Mukundan L., Wu D., Locksley R. M., and Chawla A.. 2010. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc. Natl. Acad. Sci. USA. 107: 22617–22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Wu D., Molofsky A. B., Liang H. E., Ricardo-Gonzalez R. R., Jouihan H. A., Bando J. K., Chawla A., and Locksley R. M.. 2011. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 332: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Chang Y. H., Ho K. T., Lu S. H., Huang C. N., and Shiau M. Y.. 2012. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int. J. Obes. (Lond.). 36: 993–998. [DOI] [PubMed] [Google Scholar]
- 638.Dong Y., Silva K. A., Dong Y., and Zhang L.. 2014. Glucocorticoids increase adipocytes in muscle by affecting IL-4 regulated FAP activity. FASEB J. 28: 4123–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Qiu Y., Nguyen K. D., Odegaard J. I., Cui X., Tian X., Locksley R. M., Palmiter R. D., and Chawla A.. 2014. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 157: 1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Tsao C. H., Shiau M. Y., Chuang P. H., Chang Y. H., and Hwang J.. 2014. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J. Lipid Res. 55: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Brestoff J. R., Kim B. S., Saenz S. A., Stine R. R., Monticelli L. A., Sonnenberg G. F., Thome J. J., Farber D. L., Lutfy K., Seale P., et al. 2015. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 519: 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Lee M. W., Odegaard J. I., Mukundan L., Qiu Y., Molofsky A. B., Nussbaum J. C., Yun K., Locksley R. M., and Chawla A.. 2015. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 160: 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Fischer K., Ruiz H. H., Jhun K., Finan B., Oberlin D. J., van der Heide V., Kalinovich A. V., Petrovic N., Wolf Y., Clemmensen C., et al. 2017. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 23: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Wallenius V., Wallenius K., Ahren B., Rudling M., Carlsten H., Dickson S. L., Ohlsson C., and Jansson J. O.. 2002. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 8: 75–79. [DOI] [PubMed] [Google Scholar]
- 645.Kim H. J., Higashimori T., Park S. Y., Choi H., Dong J., Kim Y. J., Noh H. L., Cho Y. R., Cline G., Kim Y. B., et al. 2004. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 53: 1060–1067. [DOI] [PubMed] [Google Scholar]
- 646.Petersen E. W., Carey A. L., Sacchetti M., Steinberg G. R., Macaulay S. L., Febbraio M. A., and Pedersen B. K.. 2005. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am. J. Physiol. Endocrinol. Metab. 288: E155–E162. [DOI] [PubMed] [Google Scholar]
- 647.Ellingsgaard H., Ehses J. A., Hammar E. B., Van Lommel L., Quintens R., Martens G., Kerr-Conte J., Pattou F., Berney T., Pipeleers D., et al. 2008. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc. Natl. Acad. Sci. USA. 105: 13163–13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Park E. J., Lee J. H., Yu G. Y., He G., Ali S. R., Holzer R. G., Osterreicher C. H., Takahashi H., and Karin M.. 2010. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 140: 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Wunderlich F. T., Strohle P., Konner A. C., Gruber S., Tovar S., Bronneke H. S., Juntti-Berggren L., Li L. S., van Rooijen N., Libert C., et al. 2010. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 12: 237–249. [DOI] [PubMed] [Google Scholar]
- 650.Mauer J., Chaurasia B., Goldau J., Vogt M. C., Ruud J., Nguyen K. D., Theurich S., Hausen A. C., Schmitz J., Bronneke H. S., et al. 2014. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 15: 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Wueest S., Item F., Boyle C. N., Jirkof P., Cesarovic N., Ellingsgaard H., Boni-Schnetzler M., Timper K., Arras M., Donath M. Y., et al. 2014. Interleukin-6 contributes to early fasting-induced free fatty acid mobilization in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306: R861–R867. [DOI] [PubMed] [Google Scholar]
- 652.Kraakman M. J., Kammoun H. L., Allen T. L., Deswaerte V., Henstridge D. C., Estevez E., Matthews V. B., Neill B., White D. A., Murphy A. J., et al. 2015. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab. 21: 403–416. [DOI] [PubMed] [Google Scholar]
- 653.Timper K., Denson J. L., Steculorum S. M., Heilinger C., Engstrom-Ruud L., Wunderlich C. M., Rose-John S., Wunderlich F. T., and Bruning J. C.. 2017. IL-6 improves energy and glucose homeostasis in obesity via enhanced central IL-6 trans-signaling. Cell Reports. 19: 267–280. [DOI] [PubMed] [Google Scholar]
- 654.Wueest S., Laesser C. I., Boni-Schnetzler M., Item F., Lucchini F. C., Borsigova M., Muller W., Donath M. Y., and Konrad D.. 2018. IL-6-type cytokine signaling in adipocytes induces intestinal GLP-1 secretion. Diabetes. 67: 36–45. [DOI] [PubMed] [Google Scholar]
- 655.Wang X., Wong K., Ouyang W., and Rutz S.. 2019. Targeting IL-10 family cytokines for the treatment of human diseases. Cold Spring Harb. Perspect. Biol. 11: doi:10.1101/cshperspect.a028548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.den Boer M. A., Voshol P. J., Schroder-van der Elst J. P., Korsheninnikova E., Ouwens D. M., Kuipers F., Havekes L. M., and Romijn J. A.. 2006. Endogenous interleukin-10 protects against hepatic steatosis but does not improve insulin sensitivity during high-fat feeding in mice. Endocrinology. 147: 4553–4558. [DOI] [PubMed] [Google Scholar]
- 657.Hong E. G., Ko H. J., Cho Y. R., Kim H. J., Ma Z., Yu T. Y., Friedline R. H., Kurt-Jones E., Finberg R., Fischer M. A., et al. 2009. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 58: 2525–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Kowalski G. M., Nicholls H. T., Risis S., Watson N. K., Kanellakis P., Bruce C. R., Bobik A., Lancaster G. I., and Febbraio M. A.. 2011. Deficiency of haematopoietic-cell-derived IL-10 does not exacerbate high-fat-diet-induced inflammation or insulin resistance in mice. Diabetologia. 54: 888–899. [DOI] [PubMed] [Google Scholar]
- 659.Gao M., Zhang C., Ma Y., Bu L., Yan L., and Liu D.. 2013. Hydrodynamic delivery of mIL10 gene protects mice from high-fat diet-induced obesity and glucose intolerance. Mol. Ther. 21: 1852–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Dagdeviren S., Jung D. Y., Lee E., Friedline R. H., Noh H. L., Kim J. H., Patel P. R., Tsitsilianos N., Tsitsilianos A. V., Tran D. A., et al. 2016. Altered interleukin-10 signaling in skeletal muscle regulates obesity-mediated inflammation and insulin resistance. Mol. Cell. Biol. 36: 2956–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Nakata M., Yamamoto S., Okada T., Gantulga D., Okano H., Ozawa K., and Yada T.. 2016. IL-10 gene transfer upregulates arcuate POMC and ameliorates hyperphagia, obesity and diabetes by substituting for leptin. Int. J. Obes. (Lond.). 40: 425–433. [DOI] [PubMed] [Google Scholar]
- 662.Xu L., Wang X., Wang J., Liu D., Wang Y., Huang Z., and Tan H.. 2016. Hypoxia-induced secretion of IL-10 from adipose-derived mesenchymal stem cell promotes growth and cancer stem cell properties of Burkitt lymphoma. Tumour Biol. 37: 7835–7842. [DOI] [PubMed] [Google Scholar]
- 663.Dagdeviren S., Jung D. Y., Friedline R. H., Noh H. L., Kim J. H., Patel P. R., Tsitsilianos N., Inashima K., Tran D. A., Hu X., et al. 2017. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. FASEB J. 31: 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Rajbhandari P., Thomas B. J., Feng A. C., Hong C., Wang J., Vergnes L., Sallam T., Wang B., Sandhu J., Seldin M. M., et al. 2018. IL-10 signaling remodels adipose chromatin architecture to limit thermogenesis and energy expenditure. Cell. 172: 218–233.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Naylor C., and Petri W. A. Jr.. 2016. Leptin regulation of immune responses. Trends Mol. Med. 22: 88–98. [DOI] [PubMed] [Google Scholar]
- 666.Stern J. H., Rutkowski J. M., and Scherer P. E.. 2016. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 23: 770–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Bennett B. D., Solar G. P., Yuan J. Q., Mathias J., Thomas G. R., and Matthews W.. 1996. A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 6: 1170–1180. [DOI] [PubMed] [Google Scholar]
- 668.Siegrist-Kaiser C. A., Pauli V., Juge-Aubry C. E., Boss O., Pernin A., Chin W. W., Cusin I., Rohner-Jeanrenaud F., Burger A. G., Zapf J., et al. 1997. Direct effects of leptin on brown and white adipose tissue. J. Clin. Invest. 100: 2858–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Sierra-Honigmann M. R., Nath A. K., Murakami C., Garcia-Cardena G., Papapetropoulos A., Sessa W. C., Madge L. A., Schechner J. S., Schwabb M. B., Polverini P. J., et al. 1998. Biological action of leptin as an angiogenic factor. Science. 281: 1683–1686. [DOI] [PubMed] [Google Scholar]
- 670.Frank S., Stallmeyer B., Kampfer H., Kolb N., and Pfeilschifter J.. 2000. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J. Clin. Invest. 106: 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Pérez C., Fernandez-Galaz C., Fernandez-Agullo T., Arribas C., Andres A., Ros M., and Carrascosa J. M.. 2004. Leptin impairs insulin signaling in rat adipocytes. Diabetes. 53: 347–353. [DOI] [PubMed] [Google Scholar]
- 672.Turner R. T., Kalra S. P., Wong C. P., Philbrick K. A., Lindenmaier L. B., Boghossian S., and Iwaniec U. T.. 2013. Peripheral leptin regulates bone formation. J. Bone Miner. Res. 28: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Simonds S. E., Pryor J. T., Ravussin E., Greenway F. L., Dileone R., Allen A. M., Bassi J., Elmquist J. K., Keogh J. M., Henning E., et al. 2014. Leptin mediates the increase in blood pressure associated with obesity. Cell. 159: 1404–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Brown R. J., Meehan C. A., and Gorden P.. 2015. Leptin does not mediate hypertension associated with human obesity. Cell. 162: 465–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Dodd G. T., Decherf S., Loh K., Simonds S. E., Wiede F., Balland E., Merry T. L., Munzberg H., Zhang Z. Y., Kahn B. B., et al. 2015. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 160: 88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Zeng W., Pirzgalska R. M., Pereira M. M., Kubasova N., Barateiro A., Seixas E., Lu Y. H., Kozlova A., Voss H., Martins G. G., et al. 2015. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 163: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Fischer A. W., Hoefig C. S., Abreu-Vieira G., de Jong J. M. A., Petrovic N., Mittag J., Cannon B., and Nedergaard J.. 2016. Leptin raises defended body temperature without activating thermogenesis. Cell Reports. 14: 1621–1631. [DOI] [PubMed] [Google Scholar]
- 678.Guo L., Zhang P., Chen Z., Xia H., Li S., Zhang Y., Kobberup S., Zou W., and Lin J. D.. 2017. Hepatic neuregulin 4 signaling defines an endocrine checkpoint for steatosis-to-NASH progression. J. Clin. Invest. 127: 4449–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Schumacher M. A., Hedl M., Abraham C., Bernard J. K., Lozano P. R., Hsieh J. J., Almohazey D., Bucar E. B., Punit S., Dempsey P. J., et al. 2017. ErbB4 signaling stimulates pro-inflammatory macrophage apoptosis and limits colonic inflammation. Cell Death Dis. 8: e2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Dahl T. B., Holm S., Aukrust P., and Halvorsen B.. 2012. Visfatin/NAMPT: a multifaceted molecule with diverse roles in physiology and pathophysiology. Annu. Rev. Nutr. 32: 229–243. [DOI] [PubMed] [Google Scholar]
- 681.Garten A., Schuster S., Penke M., Gorski T., de Giorgis T., and Kiess W.. 2015. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 11: 535–546. [DOI] [PubMed] [Google Scholar]
- 682.Yamaguchi S., and Yoshino J.. 2017. Adipose tissue NAD(+) biology in obesity and insulin resistance: from mechanism to therapy. BioEssays. 39: doi:10.1002/bies.201600227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Moschen A. R., Kaser A., Enrich B., Mosheimer B., Theurl M., Niederegger H., and Tilg H.. 2007. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 178: 1748–1758. [DOI] [PubMed] [Google Scholar]
- 684.Revollo J. R., Korner A., Mills K. F., Satoh A., Wang T., Garten A., Dasgupta B., Sasaki Y., Wolberger C., Townsend R. R., et al. 2007. Nampt/PBEF/visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 6: 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Yang H., Yang T., Baur J. A., Perez E., Matsui T., Carmona J. J., Lamming D. W., Souza-Pinto N. C., Bohr V. A., Rosenzweig A., et al. 2007. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 130: 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Li Y., Zhang Y., Dorweiler B., Cui D., Wang T., Woo C. W., Brunkan C. S., Wolberger C., Imai S., and Tabas I.. 2008. Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J. Biol. Chem. 283: 34833–34843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Sun Q., Li L., Li R., Yang M., Liu H., Nowicki M. J., Zong H., Xu J., and Yang G.. 2009. Overexpression of visfatin/PBEF/Nampt alters whole-body insulin sensitivity and lipid profile in rats. Ann. Med. 41: 311–320. [DOI] [PubMed] [Google Scholar]
- 688.Sommer G., Kralisch S., Kloting N., Kamprad M., Schrock K., Kratzsch J., Tonjes A., Lossner U., Bluher M., Stumvoll M., et al. 2010. Visfatin is a positive regulator of MCP-1 in human adipocytes in vitro and in mice in vivo. Obesity (Silver Spring). 18: 1486–1492. [DOI] [PubMed] [Google Scholar]
- 689.Caton P. W., Kieswich J., Yaqoob M. M., Holness M. J., and Sugden M. C.. 2011. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia. 54: 3083–3092. [DOI] [PubMed] [Google Scholar]
- 690.Yoshino J., Mills K. F., Yoon M. J., and Imai S.. 2011. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14: 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Spinnler R., Gorski T., Stolz K., Schuster S., Garten A., Beck-Sickinger A. G., Engelse M. A., de Koning E. J., Korner A., Kiess W., et al. 2013. The adipocytokine Nampt and its product NMN have no effect on beta-cell survival but potentiate glucose stimulated insulin secretion. PLoS One. 8: e54106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Yoon M. J., Yoshida M., Johnson S., Takikawa A., Usui I., Tobe K., Nakagawa T., Yoshino J., and Imai S.. 2015. SIRT1-mediated eNAMPT secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell Metab. 21: 706–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Stromsdorfer K. L., Yamaguchi S., Yoon M. J., Moseley A. C., Franczyk M. P., Kelly S. C., Qi N., Imai S., and Yoshino J.. 2016. NAMPT-mediated NAD(+) biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Reports. 16: 1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Nielsen K. N., Peics J., Ma T., Karavaeva I., Dall M., Chubanava S., Basse A. L., Dmytriyeva O., Treebak J. T., and Gerhart-Hines Z.. 2018. NAMPT-mediated NAD(+) biosynthesis is indispensable for adipose tissue plasticity and development of obesity. Mol. Metab. 11: 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Jamaluddin M. S., Weakley S. M., Yao Q., and Chen C.. 2012. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br. J. Pharmacol. 165: 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Kim K. H., Lee K., Moon Y. S., and Sul H. S.. 2001. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J. Biol. Chem. 276: 11252–11256. [DOI] [PubMed] [Google Scholar]
- 697.Steppan C. M., Bailey S. T., Bhat S., Brown E. J., Banerjee R. R., Wright C. M., Patel H. R., Ahima R. S., and Lazar M. A.. 2001. The hormone resistin links obesity to diabetes. Nature. 409: 307–312. [DOI] [PubMed] [Google Scholar]
- 698.Rajala M. W., Obici S., Scherer P. E., and Rossetti L.. 2003. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J. Clin. Invest. 111: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Kim K. H., Zhao L., Moon Y., Kang C., and Sul H. S.. 2004. Dominant inhibitory adipocyte-specific secretory factor (ADSF)/resistin enhances adipogenesis and improves insulin sensitivity. Proc. Natl. Acad. Sci. USA. 101: 6780–6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Muse E. D., Obici S., Bhanot S., Monia B. P., McKay R. A., Rajala M. W., Scherer P. E., and Rossetti L.. 2004. Role of resistin in diet-induced hepatic insulin resistance. J. Clin. Invest. 114: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Satoh H., Nguyen M. T., Miles P. D., Imamura T., Usui I., and Olefsky J. M.. 2004. Adenovirus-mediated chronic “hyper-resistinemia” leads to in vivo insulin resistance in normal rats. J. Clin. Invest. 114: 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Utzschneider K. M., Carr D. B., Tong J., Wallace T. M., Hull R. L., Zraika S., Xiao Q., Mistry J. S., Retzlaff B. M., Knopp R. H., et al. 2005. Resistin is not associated with insulin sensitivity or the metabolic syndrome in humans. Diabetologia. 48: 2330–2333. [DOI] [PubMed] [Google Scholar]
- 703.Qatanani M., Szwergold N. R., Greaves D. R., Ahima R. S., and Lazar M. A.. 2009. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J. Clin. Invest. 119: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Daquinag A. C., Zhang Y., Amaya-Manzanares F., Simmons P. J., and Kolonin M. G.. 2011. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell. 9: 74–86. [DOI] [PubMed] [Google Scholar]
- 705.Sánchez-Solana B., Laborda J., and Baladron V.. 2012. Mouse resistin modulates adipogenesis and glucose uptake in 3T3–L1 preadipocytes through the ROR1 receptor. Mol. Endocrinol. 26: 110–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Benomar Y., Gertler A., De Lacy P., Crepin D., Ould Hamouda H., Riffault L., and Taouis M.. 2013. Central resistin overexposure induces insulin resistance through Toll-like receptor 4. Diabetes. 62: 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Lee S., Lee H. C., Kwon Y. W., Lee S. E., Cho Y., Kim J., Lee S., Kim J. Y., Lee J., Yang H. M., et al. 2014. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 19: 484–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Kotnik P., Fischer-Posovszky P., and Wabitsch M.. 2011. RBP4: a controversial adipokine. Eur. J. Endocrinol. 165: 703–711. [DOI] [PubMed] [Google Scholar]
- 709.Yang Q., Graham T. E., Mody N., Preitner F., Peroni O. D., Zabolotny J. M., Kotani K., Quadro L., and Kahn B. B.. 2005. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 436: 356–362. [DOI] [PubMed] [Google Scholar]
- 710.Ost A., Danielsson A., Liden M., Eriksson U., Nystrom F. H., and Stralfors P.. 2007. Retinol-binding protein-4 attenuates insulin-induced phosphorylation of IRS1 and ERK1/2 in primary human adipocytes. FASEB J. 21: 3696–3704. [DOI] [PubMed] [Google Scholar]
- 711.Berry D. C., Jin H., Majumdar A., and Noy N.. 2011. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc. Natl. Acad. Sci. USA. 108: 4340–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Norseen J., Hosooka T., Hammarstedt A., Yore M. M., Kant S., Aryal P., Kiernan U. A., Phillips D. A., Maruyama H., Kraus B. J., et al. 2012. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol. Cell. Biol. 32: 2010–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Cheng J., Li Y., Wu G., Zheng J., Lu H., Shi X., and Yang G.. 2014. Ectopic expression of RBP4 impairs the insulin pathway and inguinal fat deposition in mice. J. Physiol. Biochem. 70: 479–486. [DOI] [PubMed] [Google Scholar]
- 714.Moraes-Vieira P. M., Yore M. M., Dwyer P. M., Syed I., Aryal P., and Kahn B. B.. 2014. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 19: 512–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Zemany L., Kraus B. J., Norseen J., Saito T., Peroni O. D., Johnson R. L., and Kahn B. B.. 2014. Downregulation of STRA6 in adipocytes and adipose stromovascular fraction in obesity and effects of adipocyte-specific STRA6 knockdown in vivo. Mol. Cell. Biol. 34: 1170–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Kraus B. J., Sartoretto J. L., Polak P., Hosooka T., Shiroto T., Eskurza I., Lee S. A., Jiang H., Michel T., and Kahn B. B.. 2015. Novel role for retinol-binding protein 4 in the regulation of blood pressure. FASEB J. 29: 3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Lee S. A., Yuen J. J., Jiang H., Kahn B. B., and Blaner W. S.. 2016. Adipocyte-specific overexpression of retinol-binding protein 4 causes hepatic steatosis in mice. Hepatology. 64: 1534–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Liu Y., Mu D., Chen H., Li D., Song J., Zhong Y., and Xia M.. 2016. Retinol-binding protein 4 induces hepatic mitochondrial dysfunction and promotes hepatic steatosis. J. Clin. Endocrinol. Metab. 101: 4338–4348. [DOI] [PubMed] [Google Scholar]
- 719.Gliniak C. M., Brown J. M., and Noy N.. 2017. The retinol-binding protein receptor STRA6 regulates diurnal insulin responses. J. Biol. Chem. 292: 15080–15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Fedders R., Muenzner M., Weber P., Sommerfeld M., Knauer M., Kedziora S., Kast N., Heidenreich S., Raila J., Weger S., et al. 2018. Liver-secreted RBP4 does not impair glucose homeostasis in mice. J. Biol. Chem. 293: 15269–15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Bovolenta P., Esteve P., Ruiz J. M., Cisneros E., and Lopez-Rios J.. 2008. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J. Cell Sci. 121: 737–746. [DOI] [PubMed] [Google Scholar]
- 722.Li Y., Rankin S. A., Sinner D., Kenny A. P., Krieg P. A., and Zorn A. M.. 2008. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 22: 3050–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Ouchi N., Higuchi A., Ohashi K., Oshima Y., Gokce N., Shibata R., Akasaki Y., Shimono A., and Walsh K.. 2010. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 329: 454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Mori H., Prestwich T. C., Reid M. A., Longo K. A., Gerin I., Cawthorn W. P., Susulic V. S., Krishnan V., Greenfield A., and Macdougald O. A.. 2012. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J. Clin. Invest. 122: 2405–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Rebuffat S. A., Oliveira J. M., Altirriba J., Palau N., Garcia A., Esteban Y., Nadal B., and Gomis R.. 2013. Downregulation of Sfrp5 promotes beta cell proliferation during obesity in the rat. Diabetologia. 56: 2446–2455. [DOI] [PubMed] [Google Scholar]
- 726.Carstensen M., Wiza C., Rohrig K., Fahlbusch P., Roden M., Herder C., and Ouwens D. M.. 2014. Effect of Sfrp5 on cytokine release and insulin action in primary human adipocytes and skeletal muscle cells. PLoS One. 9: e85906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Wang R., Hong J., Liu R., Chen M., Xu M., Gu W., Zhang Y., Ma Q., Wang F., Shi J., et al. 2014. SFRP5 acts as a mature adipocyte marker but not as a regulator in adipogenesis. J. Mol. Endocrinol. 53: 405–415. [DOI] [PubMed] [Google Scholar]
- 728.Chatani N., Kamada Y., Kizu T., Ogura S., Furuta K., Egawa M., Hamano M., Ezaki H., Kiso S., Shimono A., et al. 2015. Secreted frizzled-related protein 5 (Sfrp5) decreases hepatic stellate cell activation and liver fibrosis. Liver Int. 35: 2017–2026. [DOI] [PubMed] [Google Scholar]
- 729.Nakamura K., Sano S., Fuster J. J., Kikuchi R., Shimizu I., Ohshima K., Katanasaka Y., Ouchi N., and Walsh K.. 2016. Secreted Frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia/reperfusion injury. J. Biol. Chem. 291: 2566–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Karki S., Ngo D. T. M., Farb M. G., Park S. Y., Saggese S. M., Hamburg N. M., Carmine B., Hess D. T., Walsh K., and Gokce N.. 2017. WNT5A regulates adipose tissue angiogenesis via antiangiogenic VEGF-A165b in obese humans. Am. J. Physiol. Heart Circ. Physiol. 313: H200–H206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Cho Y. K., Kang Y. M., Lee S. E., Lee Y., Seol S. M., Lee W. J., Park J. Y., and Jung C. H.. 2018. Effect of SFRP5 (secreted Frizzled-related protein 5) on the WNT5A (wingless-type family member 5A)-induced endothelial dysfunction and its relevance with arterial stiffness in human subjects. Arterioscler. Thromb. Vasc. Biol. 38: 1358–1367. [DOI] [PubMed] [Google Scholar]
- 732.Weiner J., Zieger K., Pippel J., and Heiker J. T.. Molecular mechanisms of vaspin action - from adipose tissue to skin and bone, from blood vessels to the brain. Adv. Exp. Med. Biol. Epub ahead of print. July 27, 2018; doi:10.1007/5584_2018_241. [DOI] [PubMed] [Google Scholar]
- 733.Hida K., Wada J., Eguchi J., Zhang H., Baba M., Seida A., Hashimoto I., Okada T., Yasuhara A., Nakatsuka A., et al. 2005. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA. 102: 10610–10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Brunetti L., Di Nisio C., Recinella L., Chiavaroli A., Leone S., Ferrante C., Orlando G., and Vacca M.. 2011. Effects of vaspin, chemerin and omentin-1 on feeding behavior and hypothalamic peptide gene expression in the rat. Peptides. 32: 1866–1871. [DOI] [PubMed] [Google Scholar]
- 735.Klöting N., Kovacs P., Kern M., Heiker J. T., Fasshauer M., Schon M. R., Stumvoll M., Beck-Sickinger A. G., and Bluher M.. 2011. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia. 54: 1819–1823. [DOI] [PubMed] [Google Scholar]
- 736.Nakatsuka A., Wada J., Iseda I., Teshigawara S., Higashio K., Murakami K., Kanzaki M., Inoue K., Terami T., Katayama A., et al. 2012. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes. 61: 2823–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Nakatsuka A., Wada J., Iseda I., Teshigawara S., Higashio K., Murakami K., Kanzaki M., Inoue K., Terami T., Katayama A., et al. 2013. Visceral adipose tissue-derived serine proteinase inhibitor inhibits apoptosis of endothelial cells as a ligand for the cell-surface GRP78/voltage-dependent anion channel complex. Circ. Res. 112: 771–780. [DOI] [PubMed] [Google Scholar]
- 738.Liu P., Li G., Wu J., Zhou X., Wang L., Han W., Lv Y., and Sun C.. 2015. Vaspin promotes 3T3-L1 preadipocyte differentiation. Exp. Biol. Med. (Maywood). 240: 1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Lin Y., Zhuang J., Li H., Zhu G., Zhou S., Li W., Peng W., and Xu Y.. 2016. Vaspin attenuates the progression of atherosclerosis by inhibiting ER stress-induced macrophage apoptosis in apoE/ mice. Mol. Med. Rep. 13: 1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Luo X., Li K., Zhang C., Yang G., Yang M., Jia Y., Zhang L., Ma Z. A., Boden G., and Li L.. 2016. Central administration of vaspin inhibits glucose production and augments hepatic insulin signaling in high-fat-diet-fed rat. Int. J. Obes. (Lond.). 40: 947–954. [DOI] [PubMed] [Google Scholar]
- 741.Liu S., Li X., Wu Y., Duan R., Zhang J., Du F., Zhang Q., Li Y., and Li N.. 2017. Effects of vaspin on pancreatic beta cell secretion via PI3K/Akt and NF-kappaB signaling pathways. PLoS One. 12: e0189722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Zieger K., Weiner J., Kunath A., Gericke M., Krause K., Kern M., Stumvoll M., Kloting N., Bluher M., and Heiker J. T.. 2018. Ablation of kallikrein 7 (KLK7) in adipose tissue ameliorates metabolic consequences of high fat diet-induced obesity by counteracting adipose tissue inflammation in vivo. Cell. Mol. Life Sci. 75: 727–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Zieger K., Weiner J., Krause K., Schwarz M., Kohn M., Stumvoll M., Bluher M., and Heiker J. T.. 2018. Vaspin suppresses cytokine-induced inflammation in 3T3-L1 adipocytes via inhibition of NFkappaB pathway. Mol. Cell. Endocrinol. 460: 181–188. [DOI] [PubMed] [Google Scholar]
- 744.Nicholson T., Church C., Tsintzas K., Jones R., Breen L., Davis E. T., Baker D. J., and Jones S. W.. Vaspin promotes insulin sensitivity of elderly muscle and is upregulated in obesity. J. Endocrinol. Epub ahead of print. February 1, 2019; doi:10.1530/JOE-18-0528. [DOI] [PubMed] [Google Scholar]
- 745.Czekay R. P., Wilkins-Port C. E., Higgins S. P., Freytag J., Overstreet J. M., Klein R. M., Higgins C. E., Samarakoon R., and Higgins P. J.. 2011. PAI-1: an integrator of cell signaling and migration. Int. J. Cell Biol. 2011: 562481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Kaji H. 2016. Adipose tissue-derived plasminogen activator inhibitor-1 function and regulation. Compr. Physiol. 6: 1873–1896. [DOI] [PubMed] [Google Scholar]
- 747.Eitzman D. T., Westrick R. J., Xu Z., Tyson J., and Ginsburg D.. 2000. Plasminogen activator inhibitor-1 deficiency protects against atherosclerosis progression in the mouse carotid artery. Blood. 96: 4212–4215. [PubMed] [Google Scholar]
- 748.Morange P. E., Lijnen H. R., Alessi M. C., Kopp F., Collen D., and Juhan-Vague I.. 2000. Influence of PAI-1 on adipose tissue growth and metabolic parameters in a murine model of diet-induced obesity. Arterioscler. Thromb. Vasc. Biol. 20: 1150–1154. [DOI] [PubMed] [Google Scholar]
- 749.Schäfer K., Fujisawa K., Konstantinides S., and Loskutoff D. J.. 2001. Disruption of the plasminogen activator inhibitor 1 gene reduces the adiposity and improves the metabolic profile of genetically obese and diabetic ob/ob mice. FASEB J. 15: 1840–1842. [DOI] [PubMed] [Google Scholar]
- 750.Ma L. J., Mao S. L., Taylor K. L., Kanjanabuch T., Guan Y., Zhang Y., Brown N. J., Swift L. L., McGuinness O. P., Wasserman D. H., et al. 2004. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 53: 336–346. [DOI] [PubMed] [Google Scholar]
- 751.Lijnen H. R. 2005. Effect of plasminogen activator inhibitor-1 deficiency on nutritionally-induced obesity in mice. Thromb. Haemost. 93: 816–819. [DOI] [PubMed] [Google Scholar]
- 752.Lijnen H. R., Alessi M. C., Van Hoef B., Collen D., and Juhan-Vague I.. 2005. On the role of plasminogen activator inhibitor-1 in adipose tissue development and insulin resistance in mice. J. Thromb. Haemost. 3: 1174–1179. [DOI] [PubMed] [Google Scholar]
- 753.Crandall D. L., Quinet E. M., El Ayachi S., Hreha A. L., Leik C. E., Savio D. A., Juhan-Vague I., and Alessi M. C.. 2006. Modulation of adipose tissue development by pharmacological inhibition of PAI-1. Arterioscler. Thromb. Vasc. Biol. 26: 2209–2215. [DOI] [PubMed] [Google Scholar]
- 754.Kortlever R. M., Higgins P. J., and Bernards R.. 2006. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 8: 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Eren M., Boe A. E., Murphy S. B., Place A. T., Nagpal V., Morales-Nebreda L., Urich D., Quaggin S. E., Budinger G. R., Mutlu G. M., et al. 2014. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc. Natl. Acad. Sci. USA. 111: 7090–7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.Khan S. S., Shah S. J., Klyachko E., Baldridge A. S., Eren M., Place A. T., Aviv A., Puterman E., Lloyd-Jones D. M., Heiman M., et al. 2017. A null mutation in SERPINE1 protects against biological aging in humans. Sci. Adv. 3: eaao1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Coudriet G. M., Stoops J., Orr A. V., Bhushan B., Koral K., Lee S., Previte D. M., Dong H. H., Michalopoulos G. K., Mars W. M., et al. 2019. A noncanonical role for plasminogen activator inhibitor type 1 in obesity-induced diabetes. Am. J. Pathol. 189: 1413–1422. [DOI] [PubMed] [Google Scholar]
- 758.Becerra S. P., and Notario V.. 2013. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat. Rev. Cancer. 13: 258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Huang K. T., Lin C. C., Tsai M. C., Chen K. D., and Chiu K. W.. 2018. Pigment epithelium-derived factor in lipid metabolic disorders. Biomed. J. 41: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Dawson D. W., Volpert O. V., Gillis P., Crawford S. E., Xu H., Benedict W., and Bouck N. P.. 1999. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 285: 245–248. [DOI] [PubMed] [Google Scholar]
- 761.Chung C., Doll J. A., Gattu A. K., Shugrue C., Cornwell M., Fitchev P., and Crawford S. E.. 2008. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL). J. Hepatol. 48: 471–478. [DOI] [PubMed] [Google Scholar]
- 762.Crowe S., Wu L. E., Economou C., Turpin S. M., Matzaris M., Hoehn K. L., Hevener A. L., James D. E., Duh E. J., and Watt M. J.. 2009. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metab. 10: 40–47. [DOI] [PubMed] [Google Scholar]
- 763.Wang M., Wang J. J., Li J., Park K., Qian X., Ma J. X., and Zhang S. X.. 2009. Pigment epithelium-derived factor suppresses adipogenesis via inhibition of the MAPK/ERK pathway in 3T3-L1 preadipocytes. Am. J. Physiol. Endocrinol. Metab. 297: E1378–E1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Borg M. L., Andrews Z. B., Duh E. J., Zechner R., Meikle P. J., and Watt M. J.. 2011. Pigment epithelium-derived factor regulates lipid metabolism via adipose triglyceride lipase. Diabetes. 60: 1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Chavan S. S., Hudson L. K., Li J. H., Ochani M., Harris Y., Patel N. B., Katz D., Scheinerman J. A., Pavlov V. A., and Tracey K. J.. 2012. Identification of pigment epithelium-derived factor as an adipocyte-derived inflammatory factor. Mol. Med. 18: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Dai Z., Qi W., Li C., Lu J., Mao Y., Yao Y., Li L., Zhang T., Hong H., Li S., et al. 2013. Dual regulation of adipose triglyceride lipase by pigment epithelium-derived factor: a novel mechanistic insight into progressive obesity. Mol. Cell. Endocrinol. 377: 123–134. [DOI] [PubMed] [Google Scholar]
- 767.Dai Z., Zhou T., Li C., Qi W., Mao Y., Lu J., Yao Y., Li L., Zhang T., Hong H., et al. 2013. Intracellular pigment epithelium-derived factor contributes to triglyceride degradation. Int. J. Biochem. Cell Biol. 45: 2076–2086. [DOI] [PubMed] [Google Scholar]
- 768.Gattu A. K., Swenson E. S., Iwakiri Y., Samuel V. T., Troiano N., Berry R., Church C. D., Rodeheffer M. S., Carpenter T. O., and Chung C.. 2013. Determination of mesenchymal stem cell fate by pigment epithelium-derived factor (PEDF) results in increased adiposity and reduced bone mineral content. FASEB J. 27: 4384–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Gattu A. K., Birkenfeld A. L., Iwakiri Y., Jay S., Saltzman M., Doll J., Protiva P., Samuel V. T., Crawford S. E., and Chung C.. 2014. Pigment epithelium-derived factor (PEDF) suppresses IL-1beta-mediated c-Jun N-terminal kinase (JNK) activation to improve hepatocyte insulin signaling. Endocrinology. 155: 1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770.Lakeland T. V., Borg M. L., Matzaris M., Abdelkader A., Evans R. G., and Watt M. J.. 2014. Augmented expression and secretion of adipose-derived pigment epithelium-derived factor does not alter local angiogenesis or contribute to the development of systemic metabolic derangements. Am. J. Physiol. Endocrinol. Metab. 306: E1367–E1377. [DOI] [PubMed] [Google Scholar]
- 771.Matsui T., Nishino Y., Ojima A., Maeda S., Tahara N., and Yamagishi S. I.. 2014. Pigment epithelium-derived factor improves metabolic derangements and ameliorates dysregulation of adipocytokines in obese type 2 diabetic rats. Am. J. Pathol. 184: 1094–1103. [DOI] [PubMed] [Google Scholar]
- 772.Ye R. D., and Sun L.. 2015. Emerging functions of serum amyloid A in inflammation. J. Leukoc. Biol. 98: 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Kluve-Beckerman B., Drumm M. L., and Benson M. D.. 1991. Nonexpression of the human serum amyloid A three (SAA3) gene. DNA Cell Biol. 10: 651–661. [DOI] [PubMed] [Google Scholar]
- 774.Han C. Y., Subramanian S., Chan C. K., Omer M., Chiba T., Wight T. N., and Chait A.. 2007. Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 56: 2260–2273. [DOI] [PubMed] [Google Scholar]
- 775.Cheng N., He R., Tian J., Ye P. P., and Ye R. D.. 2008. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J. Immunol. 181: 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Hiratsuka S., Watanabe A., Sakurai Y., Akashi-Takamura S., Ishibashi S., Miyake K., Shibuya M., Akira S., Aburatani H., and Maru Y.. 2008. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 10: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 777.Chiba T., Han C. Y., Vaisar T., Shimokado K., Kargi A., Chen M. H., Wang S., McDonald T. O., O’Brien K. D., Heinecke J. W., et al. 2009. Serum amyloid A3 does not contribute to circulating SAA levels. J. Lipid Res. 50: 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Deguchi A., Tomita T., Omori T., Komatsu A., Ohto U., Takahashi S., Tanimura N., Akashi-Takamura S., Miyake K., and Maru Y.. 2013. Serum amyloid A3 binds MD-2 to activate p38 and NF-kappaB pathways in a MyD88-dependent manner. J. Immunol. 191: 1856–1864. [DOI] [PubMed] [Google Scholar]
- 779.den Hartigh L. J., Wang S., Goodspeed L., Ding Y., Averill M., Subramanian S., Wietecha T., O’Brien K. D., and Chait A.. 2014. Deletion of serum amyloid A3 improves high fat high sucrose diet-induced adipose tissue inflammation and hyperlipidemia in female mice. PLoS One. 9: e108564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Lee J. M., Kim E. K., Seo H., Jeon I., Chae M. J., Park Y. J., Song B., Kim Y. S., Kim Y. J., Ko H. J., et al. 2014. Serum amyloid A3 exacerbates cancer by enhancing the suppressive capacity of myeloid-derived suppressor cells via TLR2-dependent STAT3 activation. Eur. J. Immunol. 44: 1672–1684. [DOI] [PubMed] [Google Scholar]
- 781.Sanada Y., Yamamoto T., Satake R., Yamashita A., Kanai S., Kato N., van de Loo F. A., Nishimura F., Scherer P. E., and Yanaka N.. 2016. Serum amyloid A3 gene expression in adipocytes is an indicator of the interaction with macrophages. Sci. Rep. 6: 38697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Tannock L. R., De Beer M. C., Ji A., Shridas P., Noffsinger V. P., den Hartigh L., Chait A., De Beer F. C., and Webb N. R.. 2018. Serum amyloid A3 is a high density lipoprotein-associated acute-phase protein. J. Lipid Res. 59: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Ather J. L., and Poynter M. E.. 2018. Serum amyloid A3 is required for normal weight and immunometabolic function in mice. PLoS One. 13: e0192352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Moustakas A., and Heldin C. H.. 2009. The regulation of TGFbeta signal transduction. Development. 136: 3699–3714. [DOI] [PubMed] [Google Scholar]
- 785.Tan C. K., Chong H. C., Tan E. H., and Tan N. S.. 2012. Getting ‘Smad’ about obesity and diabetes. Nutr. Diabetes. 2: e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Lee M. J. 2018. Transforming growth factor beta superfamily regulation of adipose tissue biology in obesity. Biochim. Biophys. Acta Mol. Basis Dis. 1864: 1160–1171. [DOI] [PubMed] [Google Scholar]
- 787.Petruschke T., Rohrig K., and Hauner H.. 1994. Transforming growth factor beta (TGF-beta) inhibits the differentiation of human adipocyte precursor cells in primary culture. Int. J. Obes. Relat. Metab. Disord. 18: 532–536. [PubMed] [Google Scholar]
- 788.Choy L., Skillington J., and Derynck R.. 2000. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J. Cell Biol. 149: 667–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Choy L., and Derynck R.. 2003. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J. Biol. Chem. 278: 9609–9619. [DOI] [PubMed] [Google Scholar]
- 790.Tsurutani Y., Fujimoto M., Takemoto M., Irisuna H., Koshizaka M., Onishi S., Ishikawa T., Mezawa M., He P., Honjo S., et al. 2011. The roles of transforming growth factor-beta and Smad3 signaling in adipocyte differentiation and obesity. Biochem. Biophys. Res. Commun. 407: 68–73. [DOI] [PubMed] [Google Scholar]
- 791.Yadav H., Quijano C., Kamaraju A. K., Gavrilova O., Malek R., Chen W., Zerfas P., Zhigang D., Wright E. C., Stuelten C., et al. 2011. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 14: 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Babaei R., Schuster M., Meln I., Lerch S., Ghandour R. A., Pisani D. F., Bayindir-Buchhalter I., Marx J., Wu S., Schoiswohl G., et al. 2018. Jak-TGFbeta cross-talk links transient adipose tissue inflammation to beige adipogenesis. Sci. Signal. 11: doi:10.1126/scisignal.aai7838. [DOI] [PubMed] [Google Scholar]
- 793.Petrus P., Mejhert N., Corrales P., Lecoutre S., Li Q., Maldonado E., Kulyte A., Lopez Y., Campbell M., Acosta J. R., et al. 2018;. Transforming growth factor-beta3 regulates adipocyte number in subcutaneous white adipose tissue. Cell Rep. 25: 551–560.e5. [DOI] [PubMed] [Google Scholar]
- 794.Harith H. H., Morris M. J., and Kavurma M. M.. 2013. On the TRAIL of obesity and diabetes. Trends Endocrinol. Metab. 24: 578–587. [DOI] [PubMed] [Google Scholar]
- 795.von Karstedt S., Montinaro A., and Walczak H.. 2017. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer. 17: 352–366. [DOI] [PubMed] [Google Scholar]
- 796.Di Bartolo B. A., Chan J., Bennett M. R., Cartland S., Bao S., Tuch B. E., and Kavurma M. M.. 2011. TNF-related apoptosis-inducing ligand (TRAIL) protects against diabetes and atherosclerosis in Apoe (-)/(-) mice. Diabetologia. 54: 3157–3167. [DOI] [PubMed] [Google Scholar]
- 797.Watt V., Chamberlain J., Steiner T., Francis S., and Crossman D.. 2011. TRAIL attenuates the development of atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 215: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.Bernardi S., Zauli G., Tikellis C., Candido R., Fabris B., Secchiero P., Cooper M. E., and Thomas M. C.. 2012. TNF-related apoptosis-inducing ligand significantly attenuates metabolic abnormalities in high-fat-fed mice reducing adiposity and systemic inflammation. Clin. Sci. (Lond.). 123: 547–555. [DOI] [PubMed] [Google Scholar]
- 799.Di Bartolo B. A., Cartland S. P., Harith H. H., Bobryshev Y. V., Schoppet M., and Kavurma M. M.. 2013. TRAIL-deficiency accelerates vascular calcification in atherosclerosis via modulation of RANKL. PLoS One. 8: e74211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Keuper M., Wernstedt Asterholm I., Scherer P. E., Westhoff M. A., Moller P., Debatin K. M., Strauss G., Wabitsch M., and Fischer-Posovszky P.. 2013. TRAIL (TNF-related apoptosis-inducing ligand) regulates adipocyte metabolism by caspase-mediated cleavage of PPARgamma. Cell Death Dis. 4: e474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Cartland S. P., Erlich J. H., and Kavurma M. M.. 2014. TRAIL deficiency contributes to diabetic nephropathy in fat-fed ApoE−/− mice. PLoS One. 9: e92952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Funcke J. B., Zoller V., El Hay M. A., Debatin K. M., Wabitsch M., and Fischer-Posovszky P.. 2015. TNF-related apoptosis-inducing ligand promotes human preadipocyte proliferation via ERK1/2 activation. FASEB J. 29: 3065–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803.Zoller V., Funcke J. B., Keuper M., Abd El Hay M., Debatin K. M., Wabitsch M., and Fischer-Posovszky P.. 2016. TRAIL (TNF-related apoptosis-inducing ligand) inhibits human adipocyte differentiation via caspase-mediated downregulation of adipogenic transcription factors. Cell Death Dis. 7: e2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804.Cartland S. P., Harith H. H., Genner S. W., Dang L., Cogger V. C., Vellozzi M., Di Bartolo B. A., Thomas S. R., Adams L. A., and Kavurma M. M.. 2017. Non-alcoholic fatty liver disease, vascular inflammation and insulin resistance are exacerbated by TRAIL deletion in mice. Sci. Rep. 7: 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Hirsova P., Weng P., Salim W., Bronk S. F., Griffith T. S., Ibrahim S. H., and Gores G. J.. 2017. TRAIL deletion prevents liver, but not adipose tissue, inflammation during murine diet-induced obesity. Hepatol. Commun. 1: 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806.Zoller V., Funcke J. B., Roos J., Dahlhaus M., Abd El Hay M., Holzmann K., Marienfeld R., Kietzmann T., Debatin K. M., Wabitsch M., et al. 2017. Trail (TNF-related apoptosis-inducing ligand) induces an inflammatory response in human adipocytes. Sci. Rep. 7: 5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Bernardi S., Toffoli B., Tisato V., Bossi F., Biffi S., Lorenzon A., Zauli G., Secchiero P., and Fabris B.. 2018. TRAIL reduces impaired glucose tolerance and NAFLD in the high-fat diet fed mouse. Clin. Sci. (Lond.). 132: 69–83. [DOI] [PubMed] [Google Scholar]
- 808.Cawthorn W. P., and Sethi J. K.. 2008. TNF-alpha and adipocyte biology. FEBS Lett. 582: 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Brenner D., Blaser H., and Mak T. W.. 2015. Regulation of tumour necrosis factor signalling: live or let die. Nat. Rev. Immunol. 15: 362–374. [DOI] [PubMed] [Google Scholar]
- 810.Kalliolias G. D., and Ivashkiv L. B.. 2016. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 12: 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Ron D., Brasier A. R., McGehee R. E. Jr., and Habener J. F.. 1992. Tumor necrosis factor-induced reversal of adipocytic phenotype of 3T3–L1 cells is preceded by a loss of nuclear CCAAT/enhancer binding protein (C/EBP). J. Clin. Invest. 89: 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812.Hotamisligil G. S., Shargill N. S., and Spiegelman B. M.. 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 259: 87–91. [DOI] [PubMed] [Google Scholar]
- 813.Green A., Dobias S. B., Walters D. J., and Brasier A. R.. 1994. Tumor necrosis factor increases the rate of lipolysis in primary cultures of adipocytes without altering levels of hormone-sensitive lipase. Endocrinology. 134: 2581–2588. [DOI] [PubMed] [Google Scholar]
- 814.Xing H., Northrop J. P., Grove J. R., Kilpatrick K. E., Su J. L., and Ringold G. M.. 1997. TNF alpha-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPARgamma without effects on Pref-1 expression. Endocrinology. 138: 2776–2783. [DOI] [PubMed] [Google Scholar]
- 815.Gasic S., Tian B., and Green A.. 1999. Tumor necrosis factor alpha stimulates lipolysis in adipocytes by decreasing Gi protein concentrations. J. Biol. Chem. 274: 6770–6775. [DOI] [PubMed] [Google Scholar]
- 816.Kras K. M., Hausman D. B., and Martin R. J.. 2000. Tumor necrosis factor-alpha stimulates cell proliferation in adipose tissue-derived stromal-vascular cell culture: promotion of adipose tissue expansion by paracrine growth factors. Obes. Res. 8: 186–193. [DOI] [PubMed] [Google Scholar]
- 817.Nisoli E., Briscini L., Giordano A., Tonello C., Wiesbrock S. M., Uysal K. T., Cinti S., Carruba M. O., and Hotamisligil G. S.. 2000. Tumor necrosis factor alpha mediates apoptosis of brown adipocytes and defective brown adipocyte function in obesity. Proc. Natl. Acad. Sci. USA. 97: 8033–8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Borst S. E., Lee Y., Conover C. F., Shek E. W., and Bagby G. J.. 2004. Neutralization of tumor necrosis factor-alpha reverses insulin resistance in skeletal muscle but not adipose tissue. Am. J. Physiol. Endocrinol. Metab. 287: E934–E938. [DOI] [PubMed] [Google Scholar]
- 819.Voros G., Maquoi E., Collen D., and Lijnen H. R.. 2004. Influence of membrane-bound tumor necrosis factor (TNF)-alpha on obesity and glucose metabolism. J. Thromb. Haemost. 2: 507–513. [DOI] [PubMed] [Google Scholar]
- 820.Liang H., Yin B., Zhang H., Zhang S., Zeng Q., Wang J., Jiang X., Yuan L., Wang C. Y., and Li Z.. 2008. Blockade of tumor necrosis factor (TNF) receptor type 1-mediated TNF-alpha signaling protected Wistar rats from diet-induced obesity and insulin resistance. Endocrinology. 149: 2943–2951. [DOI] [PubMed] [Google Scholar]
- 821.Martins L. B., Oliveira M. C., Menezes-Garcia Z., Rodrigues D. F., Lana J. P., Vieira L. Q., Teixeira M. M., and Ferreira A. V. M.. 2018. Paradoxical role of tumor necrosis factor on metabolic dysfunction and adipose tissue expansion in mice. Nutrition. 50: 1–7. [DOI] [PubMed] [Google Scholar]
- 822.Guégan J. P., and Legembre P.. 2018. Nonapoptotic functions of Fas/CD95 in the immune response. FEBS J. 285: 809–827. [DOI] [PubMed] [Google Scholar]
- 823.Wueest S., Rapold R. A., Schumann D. M., Rytka J. M., Schildknecht A., Nov O., Chervonsky A. V., Rudich A., Schoenle E. J., Donath M. Y., et al. 2010. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J. Clin. Invest. 120: 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824.Wueest S., Rapold R. A., Schoenle E. J., and Konrad D.. 2010. Fas activation in adipocytes impairs insulin-stimulated glucose uptake by reducing Akt. FEBS Lett. 584: 4187–4192. [DOI] [PubMed] [Google Scholar]
- 825.Rapold R. A., Wueest S., Knoepfel A., Schoenle E. J., and Konrad D.. 2013. Fas activates lipolysis in a Ca2+-CaMKII-dependent manner in 3T3-L1 adipocytes. J. Lipid Res. 54: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Wueest S., Mueller R., Bluher M., Item F., Chin A. S., Wiedemann M. S., Takizawa H., Kovtonyuk L., Chervonsky A. V., Schoenle E. J., et al. 2014. Fas (CD95) expression in myeloid cells promotes obesity-induced muscle insulin resistance. EMBO Mol. Med. 6: 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.Item F., Wueest S., Lemos V., Stein S., Lucchini F. C., Denzler R., Fisser M. C., Challa T. D., Pirinen E., Kim Y., et al. 2017. Fas cell surface death receptor controls hepatic lipid metabolism by regulating mitochondrial function. Nat. Commun. 8: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Simons M., Gordon E., and Claesson-Welsh L.. 2016. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 17: 611–625. [DOI] [PubMed] [Google Scholar]
- 829.Apte R. S., Chen D. S., and Ferrara N.. 2019. VEGF in signaling and disease: beyond discovery and development. Cell. 176: 1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Elias I., Franckhauser S., Ferre T., Vila L., Tafuro S., Munoz S., Roca C., Ramos D., Pujol A., Riu E., et al. 2012. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 61: 1801–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.Liu Y., Berendsen A. D., Jia S., Lotinun S., Baron R., Ferrara N., and Olsen B. R.. 2012. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J. Clin. Invest. 122: 3101–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Lu X., Ji Y., Zhang L., Zhang Y., Zhang S., An Y., Liu P., and Zheng Y.. 2012. Resistance to obesity by repression of VEGF gene expression through induction of brown-like adipocyte differentiation. Endocrinology. 153: 3123–3132. [DOI] [PubMed] [Google Scholar]
- 833.Sun K., Wernstedt Asterholm I., Kusminski C. M., Bueno A. C., Wang Z. V., Pollard J. W., Brekken R. A., and Scherer P. E.. 2012. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl. Acad. Sci. USA. 109: 5874–5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.Chen G., Shi X., Sun C., Li M., Zhou Q., Zhang C., Huang J., Qiu Y., Wen X., Zhang Y., et al. 2013. VEGF-mediated proliferation of human adipose tissue-derived stem cells. PLoS One. 8: e73673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Sung H. K., Doh K. O., Son J. E., Park J. G., Bae Y., Choi S., Nelson S. M., Cowling R., Nagy K., Michael I. P., et al. 2013. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 17: 61–72. [DOI] [PubMed] [Google Scholar]
- 836.Honek J., Seki T., Iwamoto H., Fischer C., Li J., Lim S., Samani N. J., Zang J., and Cao Y.. 2014. Modulation of age-related insulin sensitivity by VEGF-dependent vascular plasticity in adipose tissues. Proc. Natl. Acad. Sci. USA. 111: 14906–14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.During M. J., Liu X., Huang W., Magee D., Slater A., McMurphy T., Wang C., and Cao L.. 2015. Adipose VEGF links the white-to-brown fat switch with environmental, genetic, and pharmacological stimuli in male mice. Endocrinology. 156: 2059–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.Mahdaviani K., Chess D., Wu Y., Shirihai O., and Aprahamian T. R.. 2016. Autocrine effect of vascular endothelial growth factor-A is essential for mitochondrial function in brown adipocytes. Metabolism. 65: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839.Park J., Kim M., Sun K., An Y. A., Gu X., and Scherer P. E.. 2017. VEGF-A-expressing adipose tissue shows rapid beiging and enhanced survival after transplantation and confers IL-4-independent metabolic improvements. Diabetes. 66: 1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Jin H., Li D., Wang X., Jia J., Chen Y., Yao Y., Zhao C., Lu X., Zhang S., Togo J., et al. 2018. VEGF and VEGFB play balancing roles in adipose differentiation, gene expression, and function. Endocrinology. 159: 2036–2049. [DOI] [PubMed] [Google Scholar]
- 841.Chen Y., Zhao M., Zheng T., Adlat S., Jin H., Wang C., Li D., Zaw Myint M. Z., Yao Y., Xu L., et al. 2019. Repression of adipose vascular endothelial growth factor reduces obesity through adipose browning. Am. J. Physiol. Endocrinol. Metab. 316: E145–E155. [DOI] [PubMed] [Google Scholar]
- 842.Lijnen H. R., Frederix L., Van Hoef B., and Dewerchin M.. 2009. Deficiency of vascular endothelial growth factor-D does not affect murine adipose tissue development. Biochem. Biophys. Res. Commun. 378: 255–258. [DOI] [PubMed] [Google Scholar]
- 843.Karaman S., Hollmen M., Robciuc M. R., Alitalo A., Nurmi H., Morf B., Buschle D., Alkan H. F., Ochsenbein A. M., Alitalo K., et al. 2014. Blockade of VEGF-C and VEGF-D modulates adipose tissue inflammation and improves metabolic parameters under high-fat diet. Mol. Metab. 4: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844.Lammoglia G. M., Van Zandt C. E., Galvan D. X., Orozco J. L., Dellinger M. T., and Rutkowski J. M.. 2016. Hyperplasia, de novo lymphangiogenesis, and lymphatic regression in mice with tissue-specific, inducible overexpression of murine VEGF-D. Am. J. Physiol. Heart Circ. Physiol. 311: H384–H394. [DOI] [PubMed] [Google Scholar]
- 845.Chakraborty A., Barajas S., Lammoglia G. M., Reyna A. J., Morley T. S., Johnson J. A., Scherer P. E., and Rutkowski J. M.. 2019. Vascular endothelial growth factor-D (VEGF-D) overexpression and lymphatic expansion in murine adipose tissue improves metabolism in obesity. Am. J. Pathol. 189: 924–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846.Lynes M. D., Leiria L. O., Lundh M., Bartelt A., Shamsi F., Huang T. L., Takahashi H., Hirshman M. F., Schlein C., Lee A., et al. 2017. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 23: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Stanford K. I., Lynes M. D., Takahashi H., Baer L. A., Arts P. J., May F. J., Lehnig A. C., Middelbeek R. J. W., Richard J. J., So K., et al. 2018. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab. 27: 1111–1120.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 848.Levan S. R., Stamnes K. A., Lin D. L., Fujimura K. E., Ownby D. R., Zoratti E. M., Boushey H. A., Johnson C. C., and Lynch S. V.. 2018. Neonatal gut-microbiome-derived 12,13 DiHOME impedes tolerance and promotes childhood atopy and asthma. bioRxiv. 10.1101/311704. [Google Scholar]
- 849.Park K., Li Q., Lynes M., Yokomizo H., Shinjo T., St-Louis R., Fu J., Rask-Madsen C., Tseng Y. H., and King G. L.. 2018. Endothelial/nitric oxide regulation of brown adipose tissue activating lipokine, 12,13-diHOME and its antiatherogenic actions. Diabetes. 67 (Suppl. 1): doi:10.2337/db18-286-OR. [Google Scholar]
- 850.O’Sullivan S. E. 2016. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 173: 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851.van Eenige R., van der Stelt M., Rensen P. C. N., and Kooijman S.. 2018. Regulation of Adipose Tissue Metabolism by the Endocannabinoid System. Trends Endocrinol. Metab. 29: 326–337. [DOI] [PubMed] [Google Scholar]
- 852.Matias I., Gonthier M. P., Orlando P., Martiadis V., De Petrocellis L., Cervino C., Petrosino S., Hoareau L., Festy F., Pasquali R., et al. 2006. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J. Clin. Endocrinol. Metab. 91: 3171–3180. [DOI] [PubMed] [Google Scholar]
- 853.Migrenne S., Lacombe A., Lefevre A. L., Pruniaux M. P., Guillot E., Galzin A. M., and Magnan C.. 2009. Adiponectin is required to mediate rimonabant-induced improvement of insulin sensitivity but not body weight loss in diet-induced obese mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296: R929–R935. [DOI] [PubMed] [Google Scholar]
- 854.Perwitz N., Wenzel J., Wagner I., Buning J., Drenckhan M., Zarse K., Ristow M., Lilienthal W., Lehnert H., and Klein J.. 2010. Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. Diabetes Obes. Metab. 12: 158–166. [DOI] [PubMed] [Google Scholar]
- 855.Bajzer M., Olivieri M., Haas M. K., Pfluger P. T., Magrisso I. J., Foster M. T., Tschop M. H., Krawczewski-Carhuatanta K. A., Cota D., and Obici S.. 2011. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia. 54: 3121–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856.DiPatrizio N. V., Astarita G., Schwartz G., Li X., and Piomelli D.. 2011. Endocannabinoid signal in the gut controls dietary fat intake. Proc. Natl. Acad. Sci. USA. 108: 12904–12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 857.Jung K. M., Clapper J. R., Fu J., D’Agostino G., Guijarro A., Thongkham D., Avanesian A., Astarita G., DiPatrizio N. V., Frontini A., et al. 2012. 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab. 15: 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.Tam J., Cinar R., Liu J., Godlewski G., Wesley D., Jourdan T., Szanda G., Mukhopadhyay B., Chedester L., Liow J. S., et al. 2012. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 16: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 859.Elmes M. W., Kaczocha M., Berger W. T., Leung K., Ralph B. P., Wang L., Sweeney J. M., Miyauchi J. T., Tsirka S. E., Ojima I., et al. 2015. Fatty acid-binding proteins (FABPs) are intracellular carriers for Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 290: 8711–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 860.Krott L. M., Piscitelli F., Heine M., Borrino S., Scheja L., Silvestri C., Heeren J., and Di Marzo V.. 2016. Endocannabinoid regulation in white and brown adipose tissue following thermogenic activation. J. Lipid Res. 57: 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861.Argueta D. A., and DiPatrizio N. V.. 2017. Peripheral endocannabinoid signaling controls hyperphagia in western diet-induced obesity. Physiol. Behav. 171: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862.Muller T., Demizieux L., Troy-Fioramonti S., Gresti J., Pais de Barros J. P., Berger H., Verges B., and Degrace P.. 2017. Overactivation of the endocannabinoid system alters the antilipolytic action of insulin in mouse adipose tissue. Am. J. Physiol. Endocrinol. Metab. 313: E26–E36. [DOI] [PubMed] [Google Scholar]
- 863.Ruiz de Azua I., Mancini G., Srivastava R. K., Rey A. A., Cardinal P., Tedesco L., Zingaretti C. M., Sassmann A., Quarta C., Schwitter C., et al. 2017. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Invest. 127: 4148–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864.Murdolo G., Bartolini D., Tortoioli C., Piroddi M., Iuliano L., and Galli F.. 2013. Lipokines and oxysterols: novel adipose-derived lipid hormones linking adipose dysfunction and insulin resistance. Free Radic. Biol. Med. 65: 811–820. [DOI] [PubMed] [Google Scholar]
- 865.Hauck A. K., and Bernlohr D. A.. 2016. Oxidative stress and lipotoxicity. J. Lipid Res. 57: 1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866.Hauck A. K., Huang Y., Hertzel A. V., and Bernlohr D. A.. 2019. Adipose oxidative stress and protein carbonylation. J. Biol. Chem. 294: 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 867.Miwa I., Ichimura N., Sugiura M., Hamada Y., and Taniguchi S.. 2000. Inhibition of glucose-induced insulin secretion by 4-hydroxy-2-nonenal and other lipid peroxidation products. Endocrinology. 141: 2767–2772. [DOI] [PubMed] [Google Scholar]
- 868.Zarrouki B., Soares A. F., Guichardant M., Lagarde M., and Geloen A.. 2007. The lipid peroxidation end-product 4-HNE induces COX-2 expression through p38MAPK activation in 3T3-L1 adipose cell. FEBS Lett. 581: 2394–2400. [DOI] [PubMed] [Google Scholar]
- 869.Hill B. G., Haberzettl P., Ahmed Y., Srivastava S., and Bhatnagar A.. 2008. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem. J. 410: 525–534. [DOI] [PubMed] [Google Scholar]
- 870.Singh S. P., Niemczyk M., Saini D., Awasthi Y. C., Zimniak L., and Zimniak P.. 2008. Role of the electrophilic lipid peroxidation product 4-hydroxynonenal in the development and maintenance of obesity in mice. Biochemistry. 47: 3900–3911. [DOI] [PubMed] [Google Scholar]
- 871.Curtis J. M., Grimsrud P. A., Wright W. S., Xu X., Foncea R. E., Graham D. W., Brestoff J. R., Wiczer B. M., Ilkayeva O., Cianflone K., et al. 2010. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 59: 1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872.Curtis J. M., Hahn W. S., Stone M. D., Inda J. J., Droullard D. J., Kuzmicic J. P., Donoghue M. A., Long E. K., Armien A. G., Lavandero S., et al. 2012. Protein carbonylation and adipocyte mitochondrial function. J. Biol. Chem. 287: 32967–32980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873.Pillon N. J., Croze M. L., Vella R. E., Soulere L., Lagarde M., and Soulage C. O.. 2012. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology. 153: 2099–2111. [DOI] [PubMed] [Google Scholar]
- 874.Vladykovskaya E., Sithu S. D., Haberzettl P., Wickramasinghe N. S., Merchant M. L., Hill B. G., McCracken J., Agarwal A., Dougherty S., Gordon S. A., et al. 2012. Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J. Biol. Chem. 287: 11398–11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 875.Dasuri K., Ebenezer P., Fernandez-Kim S. O., Zhang L., Gao Z., Bruce-Keller A. J., Freeman L. R., and Keller J. N.. 2013. Role of physiological levels of 4-hydroxynonenal on adipocyte biology: implications for obesity and metabolic syndrome. Free Radic. Res. 47: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 876.Haberzettl P., and Hill B. G.. 2013. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol. 1: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 877.Zhang X., Wang Z., Li J., Gu D., Li S., Shen C., and Song Z.. 2013. Increased 4-hydroxynonenal formation contributes to obesity-related lipolytic activation in adipocytes. PLoS One. 8: e70663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 878.Elrayess M. A., Almuraikhy S., Kafienah W., Al-Menhali A., Al-Khelaifi F., Bashah M., Zarkovic K., Zarkovic N., Waeg G., Alsayrafi M., et al. 2017. 4-hydroxynonenal causes impairment of human subcutaneous adipogenesis and induction of adipocyte insulin resistance. Free Radic. Biol. Med. 104: 129–137. [DOI] [PubMed] [Google Scholar]
- 879.Gómez-Muñoz A., Kong J. Y., Salh B., and Steinbrecher U. P.. 2004. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J. Lipid Res. 45: 99–105. [DOI] [PubMed] [Google Scholar]
- 880.Pettus B. J., Bielawska A., Subramanian P., Wijesinghe D. S., Maceyka M., Leslie C. C., Evans J. H., Freiberg J., Roddy P., Hannun Y. A., et al. 2004. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J. Biol. Chem. 279: 11320–11326. [DOI] [PubMed] [Google Scholar]
- 881.Tauzin L., Graf C., Sun M., Rovina P., Bouveyron N., Jaritz M., Winiski A., Hartmann N., Staedtler F., Billich A., et al. 2007. Effects of ceramide-1-phosphate on cultured cells: dependence on dodecane in the vehicle. J. Lipid Res. 48: 66–76. [DOI] [PubMed] [Google Scholar]
- 882.Gangoiti P., Granado M. H., Wang S. W., Kong J. Y., Steinbrecher U. P., and Gomez-Munoz A.. 2008. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell. Signal. 20: 726–736. [DOI] [PubMed] [Google Scholar]
- 883.Granado M. H., Gangoiti P., Ouro A., Arana L., and Gomez-Munoz A.. 2009. Ceramide 1-phosphate inhibits serine palmitoyltransferase and blocks apoptosis in alveolar macrophages. Biochim. Biophys. Acta. 1791: 263–272. [DOI] [PubMed] [Google Scholar]
- 884.Hankins J. L., Fox T. E., Barth B. M., Unrath K. A., and Kester M.. 2011. Exogenous ceramide-1-phosphate reduces lipopolysaccharide (LPS)-mediated cytokine expression. J. Biol. Chem. 286: 44357–44366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Lamour N. F., Wijesinghe D. S., Mietla J. A., Ward K. E., Stahelin R. V., and Chalfant C. E.. 2011. Ceramide kinase regulates the production of tumor necrosis factor alpha (TNFalpha) via inhibition of TNFalpha-converting enzyme. J. Biol. Chem. 286: 42808–42817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 886.Simanshu D. K., Kamlekar R. K., Wijesinghe D. S., Zou X., Zhai X., Mishra S. K., Molotkovsky J. G., Malinina L., Hinchcliffe E. H., Chalfant C. E., et al. 2013. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature. 500: 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887.Yilmaz M., Claiborn K. C., and Hotamisligil G. S.. 2016. De novo lipogenesis products and endogenous lipokines. Diabetes. 65: 1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.Frigolet M. E., and Gutierrez-Aguilar R.. 2017. The role of the novel lipokine palmitoleic acid in health and disease. Adv. Nutr. 8: 173S–181S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 889.de Souza C. O., Vannice G. K., Rosa Neto J. C., and Calder P. C.. 2018. Is palmitoleic acid a plausible nonpharmacological strategy to prevent or control chronic metabolic and inflammatory disorders? Mol. Nutr. Food Res. 62: doi:10.1002/mnfr.201700504. [DOI] [PubMed] [Google Scholar]
- 890.Maedler K., Oberholzer J., Bucher P., Spinas G. A., and Donath M. Y.. 2003. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 52: 726–733. [DOI] [PubMed] [Google Scholar]
- 891.Diakogiannaki E., Dhayal S., Childs C. E., Calder P. C., Welters H. J., and Morgan N. G.. 2007. Mechanisms involved in the cytotoxic and cytoprotective actions of saturated versus monounsaturated long-chain fatty acids in pancreatic beta-cells. J. Endocrinol. 194: 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892.Matsuzaka T., Shimano H., Yahagi N., Kato T., Atsumi A., Yamamoto T., Inoue N., Ishikawa M., Okada S., Ishigaki N., et al. 2007. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat. Med. 13: 1193–1202. [DOI] [PubMed] [Google Scholar]
- 893.Cao H., Gerhold K., Mayers J. R., Wiest M. M., Watkins S. M., and Hotamisligil G. S.. 2008. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 134: 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 894.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., et al. 2009. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895.Yang Z. H., Miyahara H., and Hatanaka A.. 2011. Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-Ay mice with genetic type 2 diabetes. Lipids Health Dis. 10: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 896.Guo X., Li H., Xu H., Halim V., Zhang W., Wang H., Ong K. T., Woo S. L., Walzem R. L., Mashek D. G., et al. 2012. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS One. 7: e39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 897.Bolsoni-Lopes A., Festuccia W. T., Farias T. S., Chimin P., Torres-Leal F. L., Derogis P. B., de Andrade P. B., Miyamoto S., Lima F. B., Curi R., et al. 2013. Palmitoleic acid (n-7) increases white adipocyte lipolysis and lipase content in a PPARalpha-dependent manner. Am. J. Physiol. Endocrinol. Metab. 305: E1093–E1102. [DOI] [PubMed] [Google Scholar]
- 898.Talbot N. A., Wheeler-Jones C. P., and Cleasby M. E.. 2014. Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol. Cell. Endocrinol. 393: 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 899.Chan K. L., Pillon N. J., Sivaloganathan D. M., Costford S. R., Liu Z., Theret M., Chazaud B., and Klip A.. 2015. Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK). J. Biol. Chem. 290: 16979–16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 900.Çimen I., Kocaturk B., Koyuncu S., Tufanli O., Onat U. I., Yildirim A. D., Apaydin O., Demirsoy S., Aykut Z. G., Nguyen U. T., et al. 2016. Prevention of atherosclerosis by bioactive palmitoleate through suppression of organelle stress and inflammasome activation. Sci. Transl. Med. 8: 358ra126. [DOI] [PubMed] [Google Scholar]
- 901.Offermanns S. 2014. Free fatty acid (FFA) and hydroxy carboxylic acid (HCA) receptors. Annu. Rev. Pharmacol. Toxicol. 54: 407–434. [DOI] [PubMed] [Google Scholar]
- 902.Ertunc M. E., and Hotamisligil G. S.. 2016. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 57: 2099–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903.Palomer X., Pizarro-Delgado J., Barroso E., and Vazquez-Carrera M.. 2018. Palmitic and oleic acid: the yin and yang of fatty acids in type 2 diabetes mellitus. Trends Endocrinol. Metab. 29: 178–190. [DOI] [PubMed] [Google Scholar]
- 904.Amri E. Z., Ailhaud G., and Grimaldi P. A.. 1994. Fatty acids as signal transducing molecules: involvement in the differentiation of preadipose to adipose cells. J. Lipid Res. 35: 930–937. [PubMed] [Google Scholar]
- 905.Opara E. C., Garfinkel M., Hubbard V. S., Burch W. M., and Akwari O. E.. 1994. Effect of fatty acids on insulin release: role of chain length and degree of unsaturation. Am. J. Physiol. 266: E635–E639. [DOI] [PubMed] [Google Scholar]
- 906.Lee J. Y., Sohn K. H., Rhee S. H., and Hwang D.. 2001. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 276: 16683–16689. [DOI] [PubMed] [Google Scholar]
- 907.Listenberger L. L., Ory D. S., and Schaffer J. E.. 2001. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 276: 14890–14895. [DOI] [PubMed] [Google Scholar]
- 908.Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogi K., Hosoya M., Tanaka Y., Uejima H., et al. 2003. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 422: 173–176. [DOI] [PubMed] [Google Scholar]
- 909.Steneberg P., Rubins N., Bartoov-Shifman R., Walker M. D., and Edlund H.. 2005. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 1: 245–258. [DOI] [PubMed] [Google Scholar]
- 910.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., and Flier J. S.. 2006. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 911.Guo W., Wong S., Xie W., Lei T., and Luo Z.. 2007. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am. J. Physiol. Endocrinol. Metab. 293: E576–E586. [DOI] [PubMed] [Google Scholar]
- 912.Edfalk S., Steneberg P., and Edlund H.. 2008. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 57: 2280–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 913.Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., et al. 2008. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7: 45–56. [DOI] [PubMed] [Google Scholar]
- 914.Eguchi K., Manabe I., Oishi-Tanaka Y., Ohsugi M., Kono N., Ogata F., Yagi N., Ohto U., Kimoto M., Miyake K., et al. 2012. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab. 15: 518–533. [DOI] [PubMed] [Google Scholar]
- 915.Ricciotti E., and FitzGerald G. A.. 2011. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31: 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916.Tsuboi H., Sugimoto Y., Kainoh T., and Ichikawa A.. 2004. Prostanoid EP4 receptor is involved in suppression of 3T3–L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 322: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 917.Babaev V. R., Chew J. D., Ding L., Davis S., Breyer M. D., Breyer R. M., Oates J. A., Fazio S., and Linton M. F.. 2008. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab. 8: 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 918.Vegiopoulos A., Muller-Decker K., Strzoda D., Schmitt I., Chichelnitskiy E., Ostertag A., Berriel Diaz M., Rozman J., Hrabe de Angelis M., Nusing R. M., et al. 2010. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 328: 1158–1161. [DOI] [PubMed] [Google Scholar]
- 919.Inazumi T., Shirata N., Morimoto K., Takano H., Segi-Nishida E., and Sugimoto Y.. 2011. Prostaglandin E(2)-EP4 signaling suppresses adipocyte differentiation in mouse embryonic fibroblasts via an autocrine mechanism. J. Lipid Res. 52: 1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 920.Cai Y., Ying F., Song E., Wang Y., Xu A., Vanhoutte P. M., and Tang E. H.. 2015. Mice lacking prostaglandin E receptor subtype 4 manifest disrupted lipid metabolism attributable to impaired triglyceride clearance. FASEB J. 29: 4924–4936. [DOI] [PubMed] [Google Scholar]
- 921.Tang E. H., Cai Y., Wong C. K., Rocha V. Z., Sukhova G. K., Shimizu K., Xuan G., Vanhoutte P. M., Libby P., and Xu A.. 2015. Activation of prostaglandin E2–EP4 signaling reduces chemokine production in adipose tissue. J. Lipid Res. 56: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 922.Yasui M., Tamura Y., Minami M., Higuchi S., Fujikawa R., Ikedo T., Nagata M., Arai H., Murayama T., and Yokode M.. 2015. The prostaglandin E2 receptor EP4 regulates obesity-related inflammation and insulin sensitivity. PLoS One. 10: e0136304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923.Ceddia R. P., Lee D., Maulis M. F., Carboneau B. A., Threadgill D. W., Poffenberger G., Milne G., Boyd K. L., Powers A. C., McGuinness O. P., et al. 2016. The PGE2 EP3 receptor regulates diet-induced adiposity in male mice. Endocrinology. 157: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 924.Chan P. C., Hsiao F. C., Chang H. M., Wabitsch M., and Hsieh P. S.. 2016. Importance of adipocyte cyclooxygenase-2 and prostaglandin E2-prostaglandin E receptor 3 signaling in the development of obesity-induced adipose tissue inflammation and insulin resistance. FASEB J. 30: 2282–2297. [DOI] [PubMed] [Google Scholar]
- 925.García-Alonso V., Titos E., Alcaraz-Quiles J., Rius B., Lopategi A., Lopez-Vicario C., Jakobsson P. J., Delgado S., Lozano J., and Claria J.. 2016. Prostaglandin E2 exerts multiple regulatory actions on human obese adipose tissue remodeling, inflammation, adaptive thermogenesis and lipolysis. PLoS One. 11: e0153751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 926.Ying F., Cai Y., Cai Y., Wang Y., and Tang E. H. C.. 2017. Prostaglandin E receptor subtype 4 regulates lipid droplet size and mitochondrial activity in murine subcutaneous white adipose tissue. FASEB J. 31: 4023–4036. [DOI] [PubMed] [Google Scholar]
- 927.Zhang X., Luo Y., Wang C., Ding X., Yang X., Wu D., Silva F., Yang Z., Zhou Q., Wang L., et al. 2018. Adipose mTORC1 suppresses prostaglandin signaling and beige adipogenesis via the CRTC2-COX-2 pathway. Cell Reports. 24: 3180–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]