Abstract
Lessons Learned.
The combination of pexidartinib and binimetinib was safe and tolerable and demonstrated encouraging signs of efficacy in two patients with advanced gastrointestinal stromal tumor (GIST) refractory to tyrosine kinase inhibitors (TKIs).
Molecular profiling of GISTs at diagnosis and upon progression may provide insight into the mechanisms of response or resistance to targeted therapies.
Additional trials are needed to further explore combined KIT and MEK inhibition in treatment‐naïve and TKI‐refractory patients with advanced GIST.
Background.
Nearly all patients with advanced gastrointestinal stromal tumor (GIST) develop resistance to imatinib, and subsequent treatments have limited efficacy. Dual inhibition of KIT and MAPK pathways has synergistic antitumor activity in preclinical GIST models.
Methods.
This was an investigator‐initiated, phase I, dose escalation study of the MEK inhibitor binimetinib combined with pexidartinib, a potent inhibitor of CSF1R, KIT, and FLT3, in patients with advanced or metastatic GIST who progressed on imatinib. The primary endpoint was phase II dose determination; secondary endpoints included safety, tolerability, and efficacy. An expansion cohort to further evaluate safety and efficacy was planned.
Results.
Two patients were treated at dose level one (binimetinib 30 mg b.i.d. and pexidartinib 400 mg every morning and 200 mg every evening), after which the study was terminated by the manufacturer. No dose‐limiting toxicities (DLTs) were reported, and treatment was well tolerated. The only grade ≥3 treatment‐emergent adverse event (TEAE) was asymptomatic elevated creatine phosphokinase (CPK). Both patients had a best response of stable disease (SD) by RECIST. Progression‐free survival (PFS) and overall survival (OS) were 6.1 and 14.6 months, respectively, in one patient with five prior lines of therapy. The second patient with NF1‐mutant GIST had a 27% decrease in tumor burden by RECIST and remains on study after 19 months of treatment.
Conclusion.
Pexidartinib combined with binimetinib was tolerable, and meaningful clinical activity was observed in two imatinib‐refractory patients.
Abstract
经验总结
• 在两名对酪氨酸激酶抑制剂 (TKI) 不敏感的晚期胃肠道间质瘤 (GIST) 患者中,pexidartinib联合binimetinib用药具有安全性和可耐受性,并显现出具有一定疗效的迹象,令人振奋。
• 在诊断时及病情进展后对GIST的分子分析可能会就对靶向治疗的反应或抵抗机理提供宝贵意见。
• 还需开展其他试验以进一步研究抑制 KIT 和 MEK 的联合疗法对于从未接受治疗且酪氨酸激酶抑制剂难以控制的晚期GIST患者是否有效。
摘要
背景。几乎所有晚期胃肠道间质瘤 (GIST) 患者均对伊马替尼产生耐药性,而且随后的治疗效果有限。在临床前GIST模型中,对 KIT 和 MAPK 通路的双重抑制表现出协同抗肿瘤活性。
方法。这是一项由研究者发起的 I 期剂量递增研究,旨在评估 MEK 抑制剂binimetinib联合pexidartinib(一种针对 CSF1R、KIT 及 FLT3 蛋白的强效抑制剂)对经伊马替尼治疗后病情进展的晚期或转移性GIST患者是否有效。主要终点是第 II 阶段剂量测定;次要终点包括安全性、耐受性和疗效。计划扩增队列,以进一步评估疗效和安全性。
结果。两名患者接受了一级剂量治疗(binimetinib 30 mg,每日两次,pexidartinib每天早上 400 mg,每天晚上 200 mg),之后,制造商终止了研究。无剂量限制性毒性 (DLT) 报告,治疗耐受性良好。唯一≥ 3 级的需紧急治疗的不良事件 (TEAE) 是无症状性肌酸磷酸激酶 (CPK) 升高。根据实体瘤疗效评定标准 (RECIST),两名患者均出现了疾病稳定 (SD) 的最佳缓解。无进展生存期 (PFS) 和总生存期 (OS) 分别为 6.1 个月和 14.6 个月,其中一名患者既往接受了五线治疗。根据RECIST,另一名具有 NF1 突变的GIST患者肿瘤负荷降低了 27%,在接受治疗 19 个月后仍在参与研究。
结论。在采用伊马替尼难以控制的两名患者中,pexidartinib联合binimetinib呈现良好的耐受性,并观察到具有意义的临床活性。
Discussion
The rationale for combining MEK and KIT inhibitors in advanced GIST is based on preclinical studies demonstrating that MAPK signaling downstream of KIT stabilizes ETV1, a transcriptional regulator essential for GIST cell proliferation [1], [2]. Although a pharmaceutical supporter closed the trial prematurely, two patients were treated with binimetinib and pexidartinib at dose level one. Both tolerated treatment without DLTs. Elevated blood CPK, an expected side effect of binimetinib [3], was the only grade ≥3 TEAE. This TEAE was not clinically significant, and the patient remained asymptomatic without myalgias.
The current standard of care in imatinib‐refractory GIST includes a multitargeted tyrosine kinase inhibitor (TKI), either sunitinib or regorafenib. The median PFS of these agents in phase III trials was less than 7 months [4], [5]. Both patients on this study achieved a clinically meaningful PFS. One has been on treatment for 19 months with a decrease in tumor burden (−27% by RECIST) and remains on treatment. Targeted sequencing of this patient's tumor with MSK‐IMPACT [6] identified a loss‐of‐function mutation in exon 42 (pX2143_splice) of NF1, with no detectable mutation in other GIST‐associated oncogenes.
NF1 loss is associated with the development of GIST in the absence of known genetic drivers [7], [8], and these tumors often have unique clinicopathologic features [9]. Loss‐of‐function of NF1, a negative regulator of RAS [10], leads to constitutive activation of RAS and downstream MEK and ERK. MEK inhibitors contribute to antitumor activity in NF1‐mutant tumors by suppressing downstream ERK [11]. MEK inhibition alone is ineffective in GIST because of MEK inhibitor‐induced feedback reactivation of upstream receptor tyrosine kinases, such as KIT or platelet‐derived growth factor receptor A (PDGFRA), in part through ETV1 [3], [12], [13]. These mechanisms highlight the scientific rationale for using combination targeted treatment in GIST, including in KIT/PDGFRA wild‐type GIST.
The other study patient had received five lines of prior TKI before enrollment. MSK‐IMPACT found a KIT exon 11 founder mutation (D579del) in both the primary and the imatinib‐resistant tumors. Furthermore, the resistant tumor harbored activating mutations in KRAS exon 2 (G12V) and PIK3CA exon 21 (H1047R), which confer resistance to imatinib. This patient achieved a best response of SD (4.3% by RECIST) lasting more than 6 months. A mixed response on the last radiographic assessment led to removal from the study for clinical progression.
Although definitive conclusions cannot be drawn from this trial, clinically meaningful activity was seen in the two patients treated, most strikingly in NF1‐mutant KIT/PDGFRA wild‐type GIST. These clinical responses, each lasting longer than 6 months, support our hypothesis that combined KIT and MAPK pathway inhibition decrease ETV1‐mediated GIST survival. An ongoing study of binimetinib combined with imatinib (NCT01991379) in treatment‐naïve GIST will shed more light on the safety and efficacy of this treatment mechanism. Correlative studies to evaluate pharmacodynamic inhibition of KIT, MAPK signaling, and ETV1 are needed to confirm the hypothesis of this study.
Trial Information
- Disease
GIST
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
1 prior regimen
- Type of Study – 1
Phase I
- Type of Study – 2
3 + 3
- Primary Endpoints
-
Safety
Tolerability
Recommended phase II dose
- Secondary Endpoint
Efficacy
- Additional Details of Endpoints or Study Design
- Phase I Dose Escalation Portion Study Design and Endpoint Assessment: The primary endpoint of the dose escalation portion of the phase I study was to determine the recommended phase II dose of MEK162 and pexidartinib administered in combination in patients with GISTs. The dose escalation study was pursued in standard 3 + 3 format, based on toxicities encountered during the first cycle of therapy. The secondary endpoints of the dose escalation portion were (a) response rate (RR) defined by RECIST 1.1 criteria and by Choi criteria evaluated within 32 weeks and (b) PFS. RR was to be estimated as the proportion of patients who have complete response or partial response for each criterion. PFS was to be calculated using Kaplan‐Meier estimate among all patients enrolled, and median PFS will be estimated. Patients who did not experience the event of interest by the end of the study would be censored at the time of the last follow‐up. The dose escalation portion of the study was to have a minimum sample size of 6 patients and a maximum of 30.
- Investigator's Analysis
Drug tolerable, hints of efficacy
Drug Information
- Drug 1
- Generic/Working Name
Pexidartinib (PLX3397)
- Company Name
Plexxikon
- Drug Type
Small molecule
- Drug Class
FMS, KIT, FLT3
- Dose
Per flat dose
- Route
p.o.
- Drug 2
- Generic/Working Name
Binimetinib (MEK162)
- Trade Name
Mektovi
- Company Name
Array BioPharma
- Drug Class
MEK
- Dose
Per flat dose
- Route
p.o.
Dose Escalation Table (three patients enrolled, two patients evaluable for toxicity)
Patient Characteristics
- Number of Patients, Male
1
- Number of Patients, Female
2
- Stage
IV
- Age
Median (range): 61 (59–78)
- Number of Prior Systemic Therapies
Median (range): 3 (1–5)
- Performance Status: ECOG
-
0 — 1
1 — 2
2 —
3 —
Unknown —
- Cancer Types or Histologic Subtypes
GIST, 3
Primary Assessment Method
- Title
Response rate
- Number of Patients Enrolled
3
- Number of Patients Evaluable for Toxicity
2
- Number of Patients Evaluated for Efficacy
2
- Evaluation Method
RECIST 1.1
- Response Assessment SD
n = 1 (50%)
- Response Assessment PD
n = 1 (50%)
- Outcome Notes
One patient withdrew prior to initiating study treatment. One of two patients continues on study treatment.
Secondary Assessment Method
- Title
Response rate
- Number of Patients Screened
3
- Number of Patients Enrolled
2
- Number of Patients Evaluable for Toxicity
2
- Number of Patients Evaluated for Efficacy
2
- Evaluation Method
Other (specify): Choi
- Response Assessment SD
n = 1 (50%)
- Response Assessment PD
n = 1 (50%)
- Outcome Notes
One patient withdrew consent prior to initiating study treatment. One of two patients remains on study treatment at the time of manuscript submission.
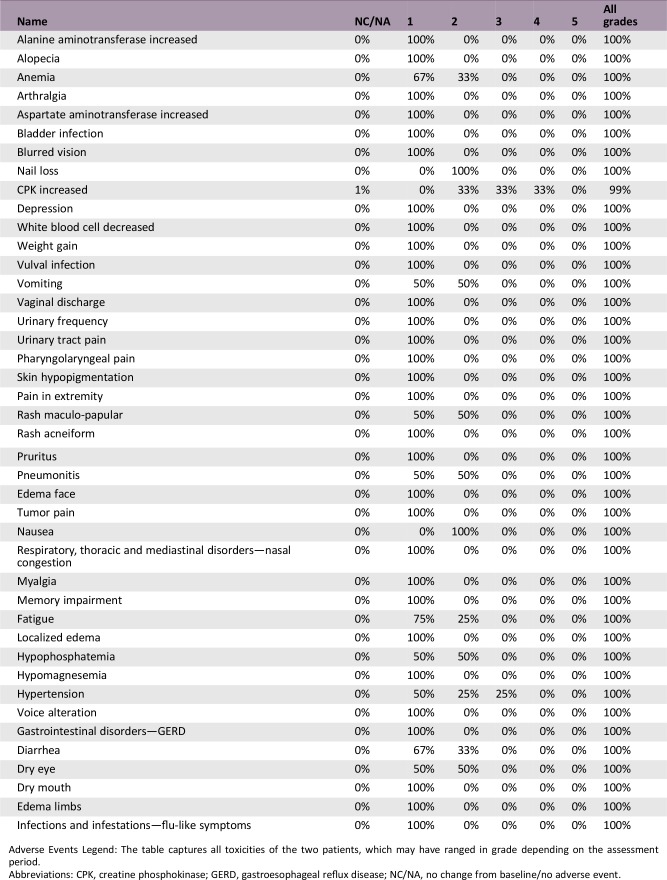
Adverse Events, All Cycles
Adverse Events Legend: The table captures all toxicities of the two patients, which may have ranged in grade depending on the assessment period.
Abbreviations: CPK, creatine phosphokinase; GERD, gastroesophageal reflux disease; NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
- Serious Adverse Events Legend
Both serious adverse events (SAEs) occurred prior to initiation of the dose escalation in the same patient. This patient withdrew consent prior to initiating the dose escalation. There were no treatment‐related SAEs.
Dose‐Limiting Toxicities
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated Reason
Company stopped development
- Investigator's Assessment
Drug tolerable, hints of efficacy
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal (GI) tract arising from the interstitial cells of Cajal (ICC), primordial pacemaker cells located within the muscle layers of the GI tract [14]. Primary GISTs often demonstrate intramural or submucosal growth and remain asymptomatic until they are large enough to cause bowel obstruction, bleeding, or rupture [15]. Approximately 75%–80% of GISTs are characterized by gain‐of‐function mutations in the proto‐oncogene KIT, leading to constitutive activation of the KIT receptor tyrosine kinase [14], [16], [17]. The most common KIT mutation involves the juxtamembrane domain located in exon 11 [14], [18]. Genetic alterations in other oncogenes, including PDGFRA, SDHA/B/C/D, NF1 or BRAF, have been detected in KIT wild‐type cases [17], [19], [20].
Imatinib revolutionized the treatment of advanced GIST by eliciting remarkable clinical responses in a once uniformly fatal and untreatable disease [15], [21], [22]. The overall response rate (ORR) to imatinib approaches 50%, with an additional 25% of patients deriving clinical benefit from treatment [22]. The median progression‐free survival (PFS) and overall survival on first‐line imatinib therapy are approximately 20 and 55 months, respectively [23].
Despite imatinib's remarkable clinical activity, a sizeable portion of patients (10%–15%) harbor primary resistance to therapy, and nearly all patients with advanced GIST demonstrate secondary resistance over time [22]. Whereas patients with KIT exon 11 mutant GIST respond most favorably, fewer responses are noted in KIT exon 9 or PDGFRA exon 18 mutation carriers, and even fewer are seen in patients with wild‐type KIT [18], [24]. Patients with PDGFRA D842V mutation are markedly resistant to imatinib, with a half maximal inhibitory concentration (IC50) 10 ‐ 20 fold higher than other PDGFRA mutant isoforms [18].
Sunitinib and regorafenib are U.S. Food and Drug Administration approved for second‐ and third‐line treatment after imatinib, respectively; but objective responses to these agents are rare, and the duration of response is brief. The ORR to sunitinib is 7% with a median PFS of 6.4 months, whereas the ORR of regorafenib is 4.5% with a median PFS of 4.8 months [4], [5]. The limited response rates of second‐ and third‐line agents represents the emergence of resistance to available tyrosine kinase inhibitor (TKI) therapy, which develops because of secondary mutations, reactivation of signaling pathways downstream of KIT, tumor heterogeneity, or the tumor microenvironment [25], [26]. Novel tyrosine kinase inhibitors, such as avapritinib and ripretinib, are currently under study in patients with primary resistant or TKI‐refractory GISTs (NCT03465722 and NCT03673501) [27], [28].
The ETS family transcription factor ETV1 is required for the development and lineage‐specification of GIST and its precursor ICC; ETV1 is highly expressed in all GISTs at the transcript and protein levels and functions as a master regulator of the transcriptional program in both ICC and GIST [1], [2]. Additionally, activated MAPK signaling, including the RAF‐MEK‐ERK pathway downstream of activated KIT signaling, facilitates GIST oncogenesis by stabilizing ETV1 and augmenting the ETV1‐dependent transcriptome. The stabilized ETV1 protein can enhance KIT expression, and both KIT and ETV1 then cooperate in GIST pathogenesis [2]. In vivo, preclinical GIST models combining imatinib with the MEK inhibitor binimetinib result in the synergistic inhibition of MAPK signaling, a dramatic reduction in GIST tumor size, and durable inhibition of ETV1 protein levels compared with either treatment alone [1]. Thus, targeting the ETV1 protein through dual MEK and KIT inhibition may lead to profound and durable responses in patients with advanced GISTs, regardless of prior exposure to imatinib or KIT/PDGFRA mutational status.
The novel TKI pexidartinib, a potent dual‐specific inhibitor of KIT and FMS, has more anti‐GIST activity compared with imatinib. In transgenic and human GIST xenograft mouse models, pexidartinib reduced tumor weight, resulted in 90% fewer KIT tumor cells, and induced more hypocellularity, necrosis, and fibrosis in GIST tumors than imatinib. The increased potency of this agent led to reduced KIT expression per cell and to decreased downstream mediators of KIT signaling [29]. We hypothesized that the combination of pexidartinib with binimetinib would lead to antitumor activity through durable inhibition of the MAPK pathway and destabilization of the ETV1 protein.
We enrolled three patients onto this phase I dose escalation trial with expansion, prior to its premature closure (Table 1). One patient withdrew consent before starting the treatment combination. The remaining two patients were treated at dose level one with 400 mg of pexidartinib in the morning and 200 mg at night, orally, combined with 30 mg of binimetinib twice daily orally. The most frequent adverse events included fatigue, anemia, leukopenia, diarrhea, dry mouth and dry eye, hypomagnesemia and hypophosphatemia, skin and nail changes, edema, elevated aspartate aminotransferase, and elevated creatine phosphokinase (CPK). Treatment‐emergent adverse events were grade ≤2, except for an asymptomatic grade 3 elevation of CPK. Plexxikon withdrew trial support after the first two patients were treated.
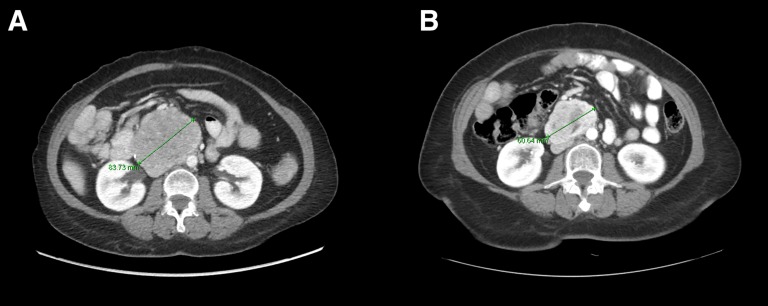
One patient with a loss‐of‐function mutation of NF1 achieved tumor shrinkage (best response—27% by RECIST) and remains on study treatment (Fig. 1). In addition to downregulation of ETV1, MEK inhibition targets the activated MAPK pathway, which results from NF1 loss [12]. The other patient on this study with multiply refractory KIT‐mutant GIST had a PFS of 6.1 months before demonstrating clinical progression.
Figure 1.
Computed tomography scans demonstrate tumor shrinkage of metastatic gastrointestinal stromal tumor, measured on axial image, on pexidartinib and binimetinib. Baseline image (A) and on‐treatment image (B).
Although the investigation of combined pexidartinib and binimetinib was halted, studying alternative treatment combinations incorporating KIT and MEK inhibition in advanced GIST, particularly in NF1‐mutant tumors, is warranted. Pharmacodynamic studies and additional correlative analyses are needed to determine the signaling pathways affected by this treatment combination and to identify other potentially targetable mechanisms of resistance.
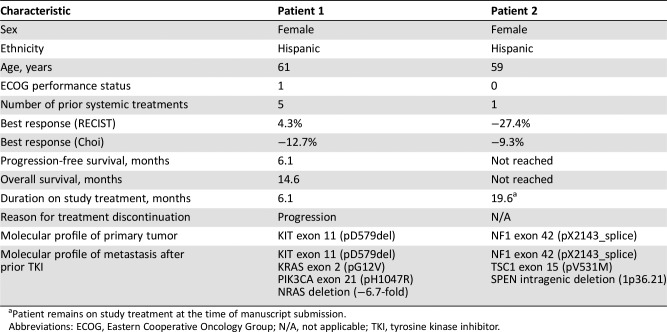
Table
Table 1. Patient characteristics and treatment response.
Patient remains on study treatment at the time of manuscript submission.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; N/A, not applicable; TKI, tyrosine kinase inhibitor.
Acknowledgments
We thank the patients and their families, the investigators, and the clinical and research staff who participated in this study. The study was supported by Plexxikon and Array BioPharma, grants from the National Cancer Institute (P50 CA140146 and P50 CA217694 to Drs. Chi, Qin, Antonescu, Singer, and Tap; P30‐CA008748 to Drs. Chi, Antonescu, Singer, and Tap; GIST Cancer Research Fund to Drs. Chi and Antonescu; the Shuman Fund to Drs. Chi, Antonescu, and Tap; the GIST Cancer Awareness Fund to Dr. Chi; and the Cycle for Survival fund to Drs. Chi and Tap.
Co‐first authors.
Footnotes
ClinicalTrials.gov Identifier: NCT03158103
Sponsor: Memorial Sloan Kettering Cancer Center
Principal Investigator: Ping Chi
IRB Approved: Yes
Contributor Information
William D. Tap, Email: tapw@mskcc.org.
Ping Chi, Email: chip@mskcc.org.
Disclosures
Mark A. Dickson: AADi, Eli Lilly & Co. (RF); Mrinal Gounder: Daiichi Sankyo, Amgen, Karyopharm, Springworks Therapeutics, Bayer, Epizyme (C/A); Sujana Movva: Novartis, Takeda, Eli Lilly & Co.; William D. Tap: Deciphera, Eli Lilly & Co., Eisai, Janssen, Immune Design, Adaptimmune, Daiichi Sankyo, Blueprint, GlaxoSmithKline, Agios, NanoCarrier (C/A), Standard Contract for Clinical Trials Blueprint, Daiichi, Eli Lilly & Co., BioAtla, Deciphera (RF), Certis Oncology Solutions, Atropos Pharmaceuticals (E, SAB), Companion Diagnostic for CDK4 inhibitors ‐ 14/854,329 (IP), Daiichi Sankyo, FDA ODAC Meeting Pexidartinib (ET); Ping Chi: Deciphera (C/A), Novartis, Array, Deciphera (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ran L, Sirota I, Cao Z et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov 2015;5:304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chi P, Chen Y, Zhang L et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature 2010;467:849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mektovi (binimetinib) [package insert]. Boulder, Colorado: Array BioPharma Inc; 2018.
- 4.Demetri GD, Reichardt P, Kang YK et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demetri GD, van Oosterom AT, Garrett CR et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006;368:1329–1338. [DOI] [PubMed] [Google Scholar]
- 6.Cheng DT, Mitchell TN, Zehir A et al. Memorial Sloan Kettering‐integrated mutation profiling of actionable cancer targets (MSK‐IMPACT): A hybridization capture‐based next‐generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015;17:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson J, Sihto H, Meis‐Kindblom JM et al. NF1‐associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am J Surg Pathol 2005;29:1170–1176. [DOI] [PubMed] [Google Scholar]
- 8.Gasparotto D, Rossi S, Polano M et al. Quadruple‐negative GIST is a sentinel for unrecognized neurofibromatosis type 1 syndrome. Clin Cancer Res 2017;23:273–282. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen M, Fetsch JF, Sobin LH et al. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: A clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol 2006;30:90–96. [DOI] [PubMed] [Google Scholar]
- 10.Scheffzek K, Ahmadian MR, Kabsch W et al. The Ras‐RasGAP complex: Structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 1997;277:333–338. [DOI] [PubMed] [Google Scholar]
- 11.Nissan MH, Pratilas CA, Jones AM et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res 2014;74:2340–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ran L, Chen Y, Sher J et al. FOXF1 defines the core‐regulatory circuitry in gastrointestinal stromal tumor. Cancer Discov 2018;8:234–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Y, Cao Z, Wong EW et al. COP1/DET1/ETS axis regulates ERK transcriptome and sensitivity to MAPK inhibitors. J Clin Invest 2018;128:1442–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirota S, Isozaki K, Moriyama Y et al. Gain‐of‐function mutations of c‐kit in human gastrointestinal stromal tumors. Science 1998;279:577–580. [DOI] [PubMed] [Google Scholar]
- 15.Demetri GD. Identification and treatment of chemoresistant inoperable or metastatic GIST: Experience with the selective tyrosine kinase inhibitor imatinib mesylate (STI571). Eur J Cancer 2002;38(suppl 5):S52–S59. [DOI] [PubMed] [Google Scholar]
- 16.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat Rev Cancer 2011;11:865–878. [DOI] [PubMed] [Google Scholar]
- 17.Heinrich MC, Corless CL, Duensing A et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708–710. [DOI] [PubMed] [Google Scholar]
- 18.Heinrich MC, Corless CL, Demetri GD et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342–4349. [DOI] [PubMed] [Google Scholar]
- 19.von Mehren M, Joensuu H. Gastrointestinal stromal tumors. J Clin Oncol 2018;36:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agaram NP, Wong GC, Guo T et al. Novel V600E BRAF mutations in imatinib‐naive and imatinib‐resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer 2008;47:853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Oosterom AT, Judson I, Verweij J et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: A phase I study. Lancet 2001;358:1421–1423. [DOI] [PubMed] [Google Scholar]
- 22.Demetri GD, von Mehren M, Blanke CD et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472–480. [DOI] [PubMed] [Google Scholar]
- 23.Blanke CD, Rankin C, Demetri GD et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626–632. [DOI] [PubMed] [Google Scholar]
- 24.Debiec‐Rychter M, Dumez H, Judson I et al. Use of c‐KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 2004;40:689–695. [DOI] [PubMed] [Google Scholar]
- 25.Antonescu CR, Besmer P, Guo T et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 2005;11:4182–4190. [DOI] [PubMed] [Google Scholar]
- 26.Hemming ML, Heinrich MC, Bauer S et al. Translational insights into gastrointestinal stromal tumor and current clinical advances. Ann Oncol 2018;29:2037–2045. [DOI] [PubMed] [Google Scholar]
- 27.(VOYAGER) Study of avapritinib vs regorafenib in patients with locally advanced unresectable or metastatic GIST. Available at https://clinicaltrials.gov/ct2/show/NCT03465722. Accessed April 15, 2019.
- 28.A study of DCC‐2618 vs sunitinib in advanced GIST patients after treatment with imatinib. Available at https://clinicaltrials.gov/ct2/show/NCT03673501. Accessed April 15, 2019.
- 29.Kim TS, Cavnar MJ, Cohen NA et al. Increased KIT inhibition enhances therapeutic efficacy in gastrointestinal stromal tumor. Clin Cancer Res 2014;20:2350–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]