Effective therapies for advanced head and neck squamous cell carcinoma (HNSCC) are lacking. This article presents what is believed to be the first reported case of a patient harboring a MET mutation who showed a clinical response to crizotinib, highlighting the benefits of comprehensive genomic profiling in HNSCC.
Abstract
Identification of effective targeted therapies for recurrent/metastatic head and neck squamous cell carcinoma (HNSCC) remains an unmet medical need. A patient with platinum‐refractory recurrent oral cavity HNSCC underwent comprehensive genomic profiling (CGP) that identified an activating MET mutation (R1004). The patient was treated with the oral MET tyrosine kinase inhibitor crizotinib with rapid response to treatment.
Based on this index case, we determined the frequency of MET alterations in 1,637 HNSCC samples, which had been analyzed with hybrid capture‐based CGP performed in the routine course of clinical care. The specimens were sequenced to a median depth of >500× for all coding exons from 182 (version 1, n = 24), 236 (version 2, n = 326), or 315 (version 3, n = 1,287) cancer‐related genes, plus select introns from 14 (version 1), 19 (version 2), or 28 (version 3) genes frequently rearranged in cancer. We identified 13 HNSCC cases (0.79%) with MET alterations (4 point mutation events and 9 focal amplification events). MET‐mutant or amplified tumors represent a small but potentially actionable molecular subset of HNSCC.
Key Points.
This case report is believed to be the first reported pan‐cancer case of a patient harboring a MET mutation at R1004 demonstrating a clinical response to crizotinib, in addition to the first documented case of head and neck squamous cell carcinoma (HNSCC) with any MET alteration responding to crizotinib.
The positive response to MET inhibition in this patient highlights the significance of comprehensive genomic profiling in advanced metastatic HNSCC to identify actionable targetable molecular alterations as current treatment options are limited.
Patient Story
A 53‐year‐old never‐smoking male presented with stage I (pT1cN0) oral cavity well‐differentiated squamous cell carcinoma (SCC) of the left anterior tongue. He underwent partial glossectomy with adequate margins, total depth of invasion 2.5 mm, and no signs of lymphovascular invasion. Thirteen months after initial diagnosis, the patient developed loco‐regional nodal recurrence in the left neck, the largest lymph node measuring 2.5 × 2.7 × 2.6 cm; core needle biopsy showed invasive SCC. He underwent an extended radical neck dissection; pathology reported extensive, invasive keratinizing SCC, p16 negative by immunohistochemistry, involving 1/17 left neck level II lymph nodes with extracapsular extension. Because of carotid artery and hypoglossal nerve involvement, the tumor could not be completely resected. Given the residual gross disease, the patient received postoperative chemoradiation (74 Gy with three cycles of bolus cisplatin [100 mg/m2]). Follow‐up positron emission tomography/computed tomography (CT) 3 months after completion of chemoradiation showed no clear evidence of viable disease.
The patient reported symptoms of an increasing and painful mass at his left jaw 5 months after completion of chemoradiation. Because of this, magnetic resonance imaging was ordered, which revealed an unresectable enlarging mass extending into the left parotid space, 5.3 cm in maximum size. Biopsy showed SCC consistent with the initial diagnosis. At this point, a formalin‐fixed paraffin‐embedded tissue sample from the tumor was submitted for hybrid capture‐based comprehensive genomic profiling (CGP). Sample processing and sequencing analysis were performed in a Clinical Laboratory Improvement Amendments‐certified, College of American Pathologists‐accredited, New York State‐accredited laboratory (FoundationOne; Foundation Medicine Inc., Cambridge, MA). At least 50 ng of DNA was extracted and analyzed by next‐generation sequencing on hybridization‐captured, adaptor ligation‐based libraries to high, uniform coverage (>500×) for all coding exons of 315 cancer‐related genes and select introns from 28 genes commonly rearranged in cancer [1].
Molecular Tumor Board
Comprehensive genomic profiling of the tumor identified a missense mutation in the receptor tyrosine kinase (RTK), MET R1004G (3010C>G), as well as TERT promoter (−124C>T) and TP53 R306* (916C>T) mutations. Based on the presence of the MET exon 14 mutation, the patient was started on crizotinib at 250 mg twice a day.
The current standard of care for recurrent/metastatic head and neck squamous cell carcinoma (HNSCC) including oral cavity cancer is palliative chemotherapy, programmed cell death 1 immune checkpoint blockade, and supportive care [2], [3], [4], [5], [6]. Despite recent advances, little progress has been made in targeted treatments for HNSCC, and prognosis remains poor for patients with recurrent or metastatic HNSCC. Specifically, SCC of the oral cavity still has poor prognosis, with a 5‐year overall survival rate of 70% for stage I or II and 54.7% for locally advanced disease [7].
The Cancer Genome Atlas created a comprehensive genomic analysis of somatic alterations by studying 279 patients with HNSCCs. Different risk factors such as human papillomavirus (HPV) status or smoking‐history were associated with specific mutations, where most HPV‐associated HNSCC show PIK3CA mutations, TRAF3 loss, or amplification of E2F1, whereas most smoking‐related HNSCC show loss‐of‐function TP53 mutations and CDKN2A alterations [8], [9]. Genomic alterations in TP53, CDKN2A, and PIK3CA as well as amplification of the RTK epidermal growth factor receptor (EGFR) have been associated with prognostic value in HNSCC [3], [10], [11], [12], [13].
Amplification or mutation of MET, another RTK, is rare in HNSCC and has only been reported in 0%–1.1% of patients [9], [14], [15], [16]. In the Cancer Genome Atlas, only two patients with HNSCC had MET exon 14 skipping mutations. MET mutation and amplification have also been reported at low frequencies in oropharyngeal SCC, esophageal SCC, and head and neck nondifferentiated carcinoma [17], [18], [19]. MET overexpression may also play a role in predicting prognosis in HNSCC, as a recent meta‐analysis found c‐Met overexpression to be an indicator of poor overall survival in patients with HNSCC [20].
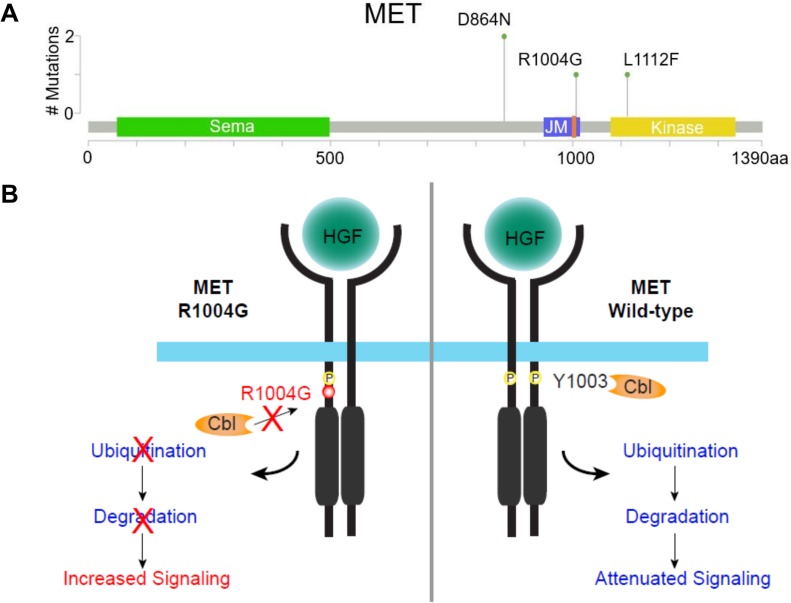
CGP of 1,637 samples from patients with HNSCC, submitted to Foundation Medicine by July 18, 2017, illustrates the rarity of MET alterations in this disease, with only 13 of 1,637 (0.79%) patients with HNSCC identified as having MET alterations. Most of these alterations were focal amplifications (9 of 13, 69.2%), whereas the rest were point mutations (4 of 13, 30.8%; Fig. 1). Our patient was the only clear case identified having an activating MET point mutation, R1004G. The three other point mutations identified are functionally uncharacterized but have been reported as a somatic alteration in the context of cancer.
Figure 1.
Prevalence and model of MET mutations in patients with head and neck squamous cell carcinoma (HNSCC). (A): Lollipop plot depicting frequency and location of somatic mutations detected in samples from patients with HNSCC. MET R1004G, located in JM, is adjacent to the CBL binding site Y1003 (denoted in orange). (B): Model of MET R1004G mutation on downstream signaling pathways. Upon HGF stimulation, MET R1004G (left) inhibits binding of the E3 ligase, CBL. Impaired CBL binding leads to reduced MET receptor ubiquitination and degradation, resulting in sustained signaling of downstream pathways compared with wild‐type MET (right).
Abbreviations: CBL, Casitas B‐lineage lymphoma; HGF, hepatocyte growth factor; JM, juxtamembrane domain.
The MET R1004G alteration is located within exon 14, which encodes a portion of the MET juxtamembrane domain. MET is targeted for degradation by the ubiquitin ligase Casitas B‐lineage lymphocyte (CBL). Changes in the CBL binding site occur with missense mutations R1004, Y1003, or D1002 and are analogous to exon 14 skipping. As a result of these mutations, CBL can no longer bind and degrade MET, leading to MET activation and oncogenesis [21], [22], [23], [24], [25], [26], [27], [28], [29]. MET amplification and/or activating alterations, such as those that lead to MET exon 14 skipping or loss of a CBL binding site, have been reported across various tumor types, including lung, brain, and gastroesophageal, and been shown to respond to MET‐targeted therapies [21], [30], [31], [32], [33], [34], [35].
Crizotinib is U.S. Food and Drug Administration approved for ALK‐positive and ROS1‐positive non‐small cell lung cancer (NSCLC) but was initially developed as a MET inhibitor. MET activation through exon 14 skipping has been studied in NSCLC [22]. In a phase I trial of 16 patients with MET exon 14‐altered (leading to MET activation) NSCLC, crizotinib was shown to have antitumor activity [36]. In addition to MET exon 14 skipping mutations, MET amplification with high copy number gain can also derive clinical benefit and respond to crizotinib in NSCLC [37]. In addition to crizotinib, other MET inhibitors are currently in clinical development (capmatinib, glesatinib) [38]. MET inhibitors such as foretinib and tivantinib have been tested in HNSCC but did not demonstrate activity in such a nonselected HNSCC patient population [39], [40]. Our data in MET‐mutated HNSCC, albeit at a low frequency, support inclusion of patients with HNSCC in pan‐cancer basket trials such as NCI‐MATCH (NCT02465060) that evaluate multiple tumor types with CGP assigning tumors with MET amplification or MET exon 14 skipping mutations to treatment with the MET inhibitor crizotinib.
Patient Update
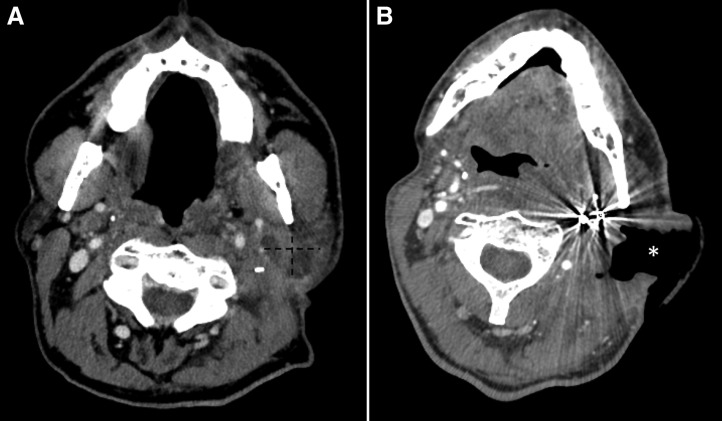
The patient tolerated therapy with rapid response after 1 month of treatment (Fig. 2). CT revealed that the previously identified tumor now had a large defect with significant interval cavitation consistent with response, although residual tumor surrounding the left internal and external carotid artery was also noted (Fig. 2). Because of rapid response and proximity to the carotid artery, crizotinib was held. Nevertheless, shortly thereafter, the patient experienced nonpulsatile bleeding from the neck and eventually passed away from complications of this event.
Figure 2.
Computed tomography angiography scans. (A): Before crizotinib treatment, with parotid tumor demarcated with dotted line. (B): Repeat imaging 1 month of crizotinib treatment, with response to treatment (new defect demonstrating response demarcated with an asterisk (*).
This patient had HNSCC whose tumor harbored a MET point mutation (R1004) and experienced a response to the MET inhibitor, crizotinib. To our knowledge, this is the first case of HNSCC with a MET mutation responding to MET‐targeted therapy.
Glossary of Genomic Terms and Nomenclature
ALK: anaplastic lymphoma kinase
CBL: Casitas B‐lineage lymphoma
EGFR: epidermal growth factor receptor
HGF: hepatocyte growth factor
MET: mesenchymal‐epithelial transition factor
ROS1: ROS proto‐oncogene 1
Contributed equally.
Author Contributions
Data analysis and interpretation: Lisa Pei Chu, Debra Franck, Christine A. Parachoniak, Jeffrey P. Gregg, Michael G. Moore, D. Greg Farwell, Shyam Rao, Andreas M. Heilmann, Rachel L. Erlich, Jeffrey S. Ross, Vincent A. Miller, Siraj Ali, Jonathan W. Riess
Manuscript writing: Lisa Pei Chu, Debra Franck, Christine A. Parachoniak, Jeffrey P. Gregg, Michael G. Moore, D. Greg Farwell, Shyam Rao, Andreas M. Heilmann, Rachel L. Erlich, Jeffrey S. Ross, Vincent A. Miller, Siraj Ali, Jonathan W. Riess
Final approval of manuscript: Lisa Pei Chu, Debra Franck, Christine A. Parachoniak, Jeffrey P. Gregg, Michael G. Moore, D. Greg Farwell, Shyam Rao, Andreas M. Heilmann, Rachel L. Erlich, Jeffrey S. Ross, Vincent A. Miller, Siraj Ali, Jonathan W. Riess
Disclosures
Debra Franck: Foundation Medicine (E, OI); Christine A. Parachoniak: Foundation Medicine: (E,OI); Jeffrey P. Gregg: Foundation Medicine, AstraZeneca (C/A), Foundation Medicine (E), AstraZeneca (RF, SAB); Andreas M. Heilmann: Foundation Medicine (E, OI); Rachel L. Erlich: Foundation Medicine (E, OI); Jeffrey S. Ross: Foundation Medicine (E, OI); Vincent A. Miller: Foundation Medicine (E, OI), Revolution Medicines (SAB), US8501413B2 assigned to Sloan Kettering Institute for Cancer Research (OI [patent royalties]); Siraj M. Ali: Foundation Medicine (OI, E), Incysus (SAB), Revolution Medicines (C/A); Jonathan W. Riess: Merck, AstraZeneca (RF), Celgene, Takeda, Biodesix, Abbvie (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Frampton GM, Fichtenholtz A, Otto GA et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013;31:1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermorken JB, Mesia R, Rivera F et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–1127. [DOI] [PubMed] [Google Scholar]
- 3.Marur S, Forastiere AA. Head and neck cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin Proc 2008;83:489–501. [DOI] [PubMed] [Google Scholar]
- 4.Vermorken JB, Trigo J, Hitt R et al. Open‐label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum‐based therapy. J Clin Oncol 2007;25:2171–2177. [DOI] [PubMed] [Google Scholar]
- 5.Cohen EEW, Soulieres D, Le Tourneau C et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head‐and‐neck squamous cell carcinoma (KEYNOTE‐040): A randomised, open‐label, phase 3 study. Lancet 2019;393:156–167. [DOI] [PubMed] [Google Scholar]
- 6.Ferris RL, Blumenschein G Jr, Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luryi AL, Chen MM, Mehra S et al. Treatment factors associated with survival in early‐stage oral cavity cancer: Analysis of 6830 cases from the National Cancer Data Base. JAMA Otolaryngol Head Neck Surg 2015;141:593–598. [DOI] [PubMed] [Google Scholar]
- 8.Lajer CB, von Buchwald C. The role of human papillomavirus in head and neck cancer. APMIS 2010;118:510–519. [DOI] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Network . Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poeta ML, Manola J, Goldwasser MA et al. TP53 mutations and survival in squamous‐cell carcinoma of the head and neck. N Engl J Med 2007;357:2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed AL, Califano J, Cairns P et al. High frequency of p16 (CDKN2/MTS‐1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res 1996;56:3630–3633. [PubMed] [Google Scholar]
- 12.Sok JC, Coppelli FM, Thomas SM et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res 2006;12:5064–5073. [DOI] [PubMed] [Google Scholar]
- 13.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol 2006;24:2666–2672. [DOI] [PubMed] [Google Scholar]
- 14.Stransky N, Egloff AM, Tward AD et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011;333:1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agrawal N, Frederick MJ, Pickering CR et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011;333:1154–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris LG, Chandramohan R, West L et al. The molecular landscape of recurrent and metastatic head and neck cancers: Insights from a precision oncology sequencing platform. JAMA Oncol 2017;3:244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacroix L, Post SF, Valent A et al. MET geneMichatic abnormalities unreliable for patient selection for therapeutic intervention in oropharyngeal squamous cell carcinoma. PloS One 2014;9:e84319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato H, Arao T, Matsumoto K et al. Gene amplification of EGFR, HER2, FGFR2 and MET in esophageal squamous cell carcinoma. Int J Oncol 2013;42:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choe JY, Yun JY, Nam SJ et al. Expression of c‐Met is different along the location and associated with lymph node metastasis of head and neck carcinoma. Korean J Pathol 2012;46:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vsiansky V, Gumulec J, Raudenska M et al. Prognostic role of c‐Met in head and neck squamous cell cancer tissues: A meta‐analysis. Sci Rep 2018;8:10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrock AB, Frampton GM, Suh J et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol 2016;11:1493–1502. [DOI] [PubMed] [Google Scholar]
- 22.Kong‐Beltran M, Seshagiri S, Zha J et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res 2006;66:283–289. [DOI] [PubMed] [Google Scholar]
- 23.Peschard P, Ishiyama N, Lin T et al. A conserved DpYR motif in the juxtamembrane domain of the Met receptor family forms an atypical c‐Cbl/Cbl‐b tyrosine kinase binding domain binding site required for suppression of oncogenic activation. J Biol Chem 2004;279:29565–29571. [DOI] [PubMed] [Google Scholar]
- 24.Lee JM, Kim B, Lee SB et al. Cbl‐independent degradation of Met: ways to avoid agonism of bivalent Met‐targeting antibody. Oncogene 2014;33:34–43. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Gao CF, Lee CC et al. An alternatively spliced form of Met receptor is tumorigenic. Exp Mol Med 2006;38:565–573. [DOI] [PubMed] [Google Scholar]
- 26.Togashi Y, Mizuuchi H, Tomida S et al. MET gene exon 14 deletion created using the CRISPR/Cas9 system enhances cellular growth and sensitivity to a MET inhibitor. Lung Cancer 2015;90:590–597. [DOI] [PubMed] [Google Scholar]
- 27.Peschard P, Fournier TM, Lamorte L et al. Mutation of the c‐Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol Cell 2001;8:995–1004. [DOI] [PubMed] [Google Scholar]
- 28.Abella JV, Peschard P, Naujokas MA et al. Met/Hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for Hrs phosphorylation. Mol Cell Biol 2005;25:9632–9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray MJ, Kannu P, Sharma S et al. Mutations preventing regulated exon skipping in MET cause osteofibrous dysplasia. Am J Hum Genet 2015;97:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frampton GM, Ali SM, Rosenzweig M et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850–859. [DOI] [PubMed] [Google Scholar]
- 31.Paik PK, Drilon A, Fan PD et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ou SH, Kwak EL, Siwak‐Tapp C et al. Activity of crizotinib (PF02341066), a dual mesenchymal‐epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non‐small cell lung cancer patient with de novo MET amplification. J Thorac Oncol 2011;6:942–946. [DOI] [PubMed] [Google Scholar]
- 33.Le X, Freed JA, VanderLaan PA et al. Detection of crizotinib‐sensitive lung adenocarcinomas with MET, ALK, and ROS1 genomic alterations via comprehensive genomic profiling. Clin Lung Cancer 2015;16:e105–e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lennerz JK, Kwak EL, Ackerman A et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol 2011;29:4803–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung KH, Park BH, Hong SS. Progress in cancer therapy targeting c‐Met signaling pathway. Arch Pharm Res 2012;35:595–604. [DOI] [PubMed] [Google Scholar]
- 36.Drilon AE, Camidge DR, S‐HI Ou et al. Efficacy and safety of crizotinib in patients (pts) with advanced MET exon 14‐altered non‐small cell lung cancer (NSCLC). J Clin Oncol 2016;34(suppl 15):108a. [Google Scholar]
- 37.Caparica R, Yen CT, Coudry R et al. Responses to crizotinib can occur in high‐level MET‐amplified non‐small cell lung cancer independent of MET exon 14 alterations. J Thorac Oncol 2017;12:141–144. [DOI] [PubMed] [Google Scholar]
- 38.Reungwetwattana T, Liang Y, Zhu V et al. The race to target MET exon 14 skipping alterations in non‐small cell lung cancer: The why, the how, the who, the unknown, and the inevitable. Lung Cancer 2017;103:27–37. [DOI] [PubMed] [Google Scholar]
- 39.Saada‐Bouzid E, Le Tourneau C. Beyond EGFR targeting in SCCHN: Angiogenesis, PI3K, and other molecular targets. Front Oncol 2019;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seiwert T, Sarantopoulos J, Kallender H et al. Phase II trial of single‐agent foretinib (GSK1363089) in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Invest New Drugs 2013;31:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]