Abstract
Osteoarthritis (OA) is the leading cause of joint failure, yet the underlying mechanisms remain elusive, and no approved therapies that slow progression exist. Dysregulated integrin function was previously implicated in OA pathogenesis. However, the roles of integrin αVβ3 and the integrin-associated receptor CD47 in OA remain largely unknown. Here, transcriptomic and proteomic analyses of human and murine osteoarthritic tissues revealed dysregulated expression of αVβ3, CD47, and their ligands. Using genetically deficient mice and pharmacologic inhibitors, we showed that αVβ3, CD47, and the downstream signaling molecules Fyn and FAK are crucial to OA pathogenesis. MicroPET/CT imaging of a mouse model showed elevated ligand-binding capacities of integrin αVβ3 and CD47 in osteoarthritic joints. Further, our in vitro studies demonstrated that chondrocyte breakdown products, derived from articular cartilage of individuals with OA, induced αVβ3/CD47-dependent expression of inflammatory and degradative mediators, and revealed the downstream signaling network. Our findings identify a central role for dysregulated αVβ3 and CD47 signaling in OA pathogenesis and suggest that activation of αVβ3 and CD47 signaling in many articular cell types contributes to inflammation and joint destruction in OA. Thus, the data presented here provide a rationale for targeting αVβ3, CD47, and their signaling pathways as a disease-modifying therapy.
Keywords: Immunology, Inflammation
Keywords: Cartilage, Integrins, Osteoarthritis
Integrin αVβ3 and CD47 promote osteoarthritis pathogenesis by promoting expression of inflammatory and degradative mediators in chondrocytes, macrophages, and synoviocytes.
Introduction
Osteoarthritis (OA) is the most prevalent arthritic disease, but its pathogenesis is unclear, and no treatment has been approved to prevent or slow disease progression (1). In articular joints, integrin αVβ3 is expressed by cells in the synovial membrane and by chondrocytes in cartilage. Integrin αVβ3 signaling can promote a variety of processes implicated in OA, such as inflammation, angiogenesis, and bone turnover (2). The integrin-associated glycoprotein CD47 is physically and functionally associated with several integrins including αVβ3 (3, 4). Both integrin αVβ3 and CD47 are considered important therapeutic targets for a number of diseases, but the potential involvement of these receptors in the pathogenesis of OA remains unresolved (5).
Integrins are heterodimeric transmembrane proteins that mediate cell adhesion, ECM organization, mechanosensing, signaling, survival, proliferation and differentiation, and cartilage homeostasis (5). The 18 α and 8 β integrin subunits, each with a large extracellular domain, a single transmembrane domain, and a generally short cytoplasmic tail, can form 24 heterodimers (6, 7). These heterodimers can be classified into several groups based on their interactions with different types of ligands (6, 8). Some integrins recognize ligands that contain an Arg-Gly-Asp (RGD) tripeptide active site — and hence are termed the RGD-binding integrins — while others belong to the Leu-Asp-Val–binding (LDV-binding), laminin/collagen-binding, or other integrin subfamilies (6, 8). Integrins α1β1, α3β1, α5β1, α10β1, αVβ1, αVβ3, and αVβ5 are expressed by articular chondrocytes in healthy adult joints (9). Increased levels of α1β1 and α3β1, as well as α2β1, α4β1, and perhaps α6β1, have been detected in osteoarthritic cartilage (9, 10). These observations, along with evidence from a number of in vitro studies, have pointed to a potential role for integrins in the pathogenesis of OA (5, 10).
The integrin-associated transmembrane protein CD47 is known to be physically and functionally connected to αVβ3 as well as several other integrins, including α2β1 and α4β1 (11–14). CD47 may augment the activities of these integrins in phagocytosis, platelet activation, cell motility and adhesion, leukocyte adhesion, and other cellular functions (15). CD47 also acts as a dominant “don’t eat me” signal for phagocytic cells, playing an important role in cancer cell survival and spread; in blood cell removal in myelodysplastic syndromes (MDSs) (16); in atherosclerosis (17); and in pathological fibrotic conditions such as idiopathic pulmonary fibrosis (IPF), nonalcoholic steatohepatitis (NASH), systemic sclerosis, and peritoneal adhesions (18). Blockade of CD47 in these diseases can lead to phagocytic removal of pathogenic premalignant and malignant cells, of overproliferating fibroblasts, and of arterial smooth muscle cells (17, 19). Although integrin αVβ3 and CD47 are considered important therapeutic targets in cancer and other diseases (20–27), current understanding of the possible contribution of integrin αVβ3 and CD47 to the pathogenesis of OA remains limited (10).
Programmed cell removal is a process by which cells undergoing apoptosis are recognized and cleared by phagocytic macrophages. Recognition of cells undergoing apoptosis allows these cells to be efficiently cleared before rupture and release of potentially pathogenic signals into the extracellular space (28). While programmed cell removal involves the expression and recognition of multiple pro-clearance signals, loss of CD47 expression is also important for promotion of this process. Programmed cell removal can also occur in the absence of apoptosis (28). Previous studies demonstrated that many human cancers evade programmed cell death and removal in part by upregulating CD47 expression and that blockade of CD47-SIRPα interactions can promote clearance of tumor cells (29–33). Therefore, dysregulation of CD47 in OA joints may prevent the clearance of cells that promote production of pathogenic inflammatory and degradative signals.
Here we investigate integrin αVβ3 and CD47 signaling in joint tissues from individuals with OA by transcriptomic and proteomic analyses. Results from these studies, along with those from examination of osteoarthritic joint tissues in the destabilization of the medial meniscus (DMM) mouse model, identified dysregulated CD47 and integrin αVβ3 expression and signaling in OA. We also demonstrated that genetic deficiency in and pharmacological blockade of αVβ3, CD47, and their downstream signaling molecules Fyn and FAK attenuated the progression of OA. Consistent with our findings that αVβ3 and CD47 expression is dysregulated in OA joints, we detected increased ligand-binding capacities in the osteoarthritic joints by microPET/CT imaging of the DMM mouse. Further, in vitro investigations of multiple cell types found in both human and murine joints identified potential mechanisms by which integrin αVβ3 and CD47 signaling contributes to the pathogenesis of OA. Upregulation of CD47 within OA joints may also prevent programmed cell removal of cells expressing pathologic inflammatory and degradative signals. Together, these findings implicate CD47 and integrin αVβ3 signaling as one mechanism through which inflammatory and degradative mediators are stimulated, driving the pathogenesis of OA.
Results
Dysregulated integrin αVβ3 and CD47 signaling in OA.
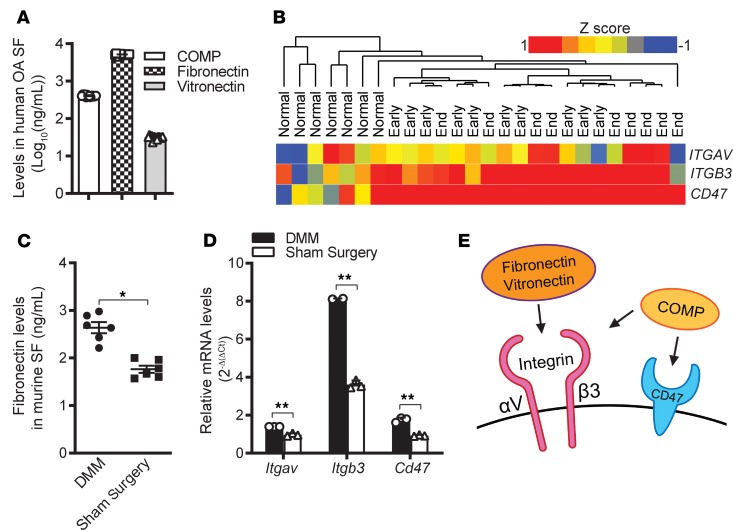
Increased volume of synovial fluids is a hallmark of OA. We sought to identify biomarkers for OA within synovial fluids by performing mass spectrometric analysis of synovial fluids from individuals with OA (n = 5). In our analysis, we detected multiple ligands for integrin αVβ3 and CD47, including cartilage oligomeric matrix protein (COMP), fibronectin, and vitronectin (Table 1). ELISA analysis validated the presence of high concentrations of COMP, fibronectin, and vitronectin in synovial fluids from individuals with OA (Figure 1A). There was little variation in the concentrations of the ligands we measured across the 15 synovial fluid samples analyzed (Figure 1A). Our findings and others indicate that signaling through integrins and binding to the integrin-associated protein CD47 are dysregulated in OA (5). Seeking to gain further insight into the role of integrin αVβ3 and CD47 in OA, we investigated whether expression of αVβ3 and CD47 was dysregulated in OA synovial tissue. We compared mRNA levels in synovial membranes of early- and end-stage OA joints (GEO accession GSE32317) with previously reported expression data from healthy synovial membranes (GEO accession GSE12021; refs. 34, 35). Unsupervised hierarchical clustering analysis revealed upregulated gene expression of integrin subunits αV (ITGAV) and β3 (ITGB3) as well as CD47 in osteoarthritic joint synovial linings (Figure 1B and Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.128616DS1). These findings contrast with previously reported data in which α5β1 was found to promote OA, while αVβ3 downregulated proinflammatory signals contributing to OA (36, 37). We further confirmed the expression of ITGAV, and ITGB3, and CD47 by quantitative PCR (qPCR) analysis of chondrocytes and synoviocytes from osteoarthritic joints (Supplemental Figure 1B). Corroborating these results, higher levels of fibronectin were detected by ELISA in osteoarthritic murine joints that were induced by DMM (38) than in sham-operated joints (Figure 1C). Additionally, these osteoarthritic murine joints also exhibited elevated mRNA levels of Itgav, Itgb3, and Cd47, as revealed by qPCR analyses (Figure 1D). Thus, our findings from these human and mouse studies suggest dysregulated αVβ3 and CD47 signaling in OA as a result of increased levels of both the receptors and their ligands (illustrated in Figure 1E).
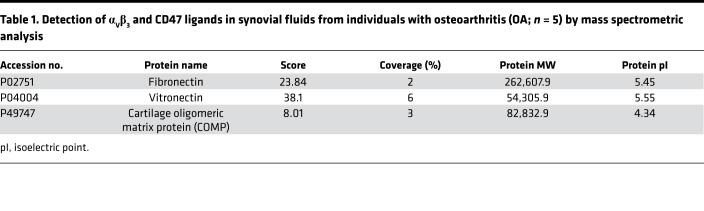
Table 1. Detection of αVβ3 and CD47 ligands in synovial fluids from individuals with osteoarthritis (OA; n = 5) by mass spectrometric analysis.
Figure 1. Dysregulated integrin αVβ3 and CD47 signaling in osteoarthritis.
(A) Ligands for αVβ3 and CD47, including cartilage oligomeric matrix protein (COMP), fibronectin, and vitronectin, were detected by ELISA (n = 15) in synovial fluid (SF) from individuals with osteoarthritis (OA). Data are mean ± SEM of measurements from 15 individual samples. (B) Unsupervised hierarchical clustering of Itgav, Itgb3, and Cd47 expression in microarray data sets on synovial membranes from healthy individuals (n = 7) or those with early- (n =10) or end-stage (n = 9) OA. Scale bar: z score. (C) ELISA analysis of fibronectin levels in synovial fluid from mouse knee joints (n = 6) 20 weeks after destabilization of the medial meniscus (DMM) or sham surgery. Mouse DMM Data are mean ± SEM of duplicates or triplicates from 1 experiment and are representative of 3 independent experiments. *P < 0.05 by Mann-Whitney U test. (D) qPCR analysis of mRNA levels for Itgav, Itgb3, and Cd47 in synovial membrane from mouse knee joints 20 weeks after DMM or sham surgery. Data are the mean ± SEM of duplicates or triplicates and are representative of ≥2 independent experiments. **P < 0.001 by Mann-Whitney U test with multiple-comparison corrections. (E) Schematic of receptor-ligand binding in αVβ3 and CD47 activation in the osteoarthritic joint, as suggested by our data on human and murine OA.
Crucial roles of integrin αVβ3, CD47, and Fyn in murine OA.
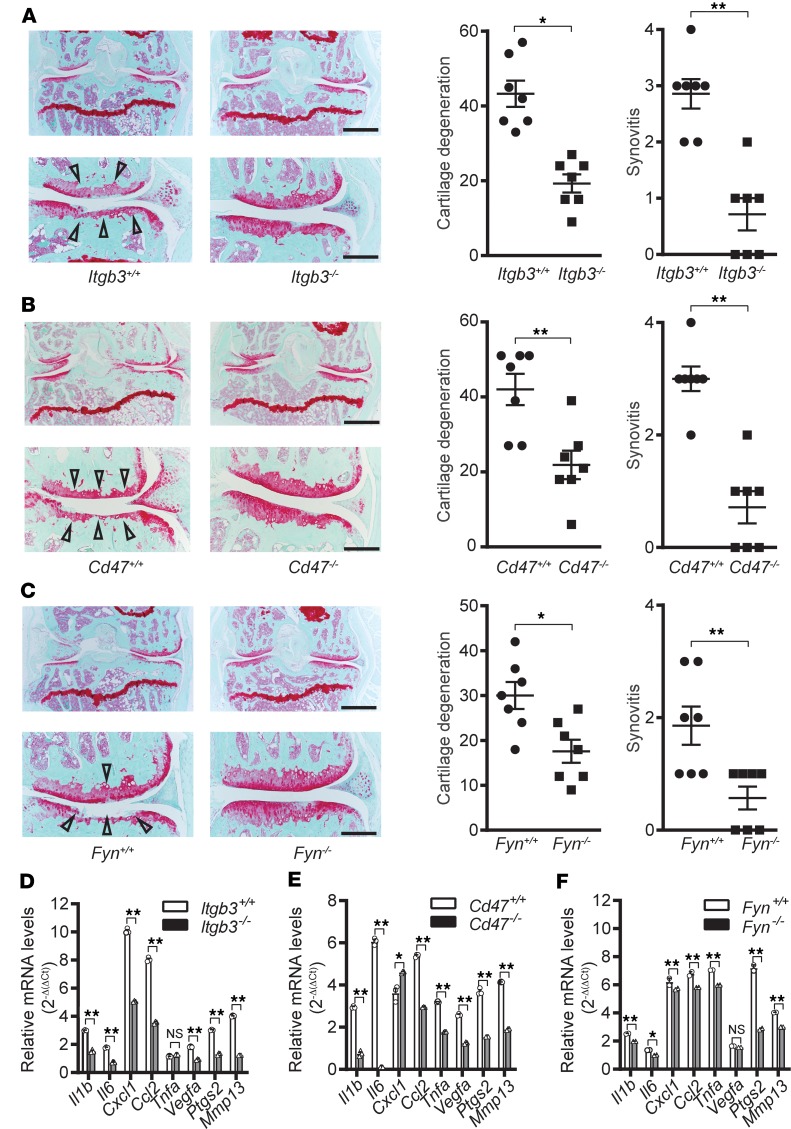
We investigated the roles of αVβ3 and CD47 signaling in the pathogenesis of OA by examining the effects of genetic deficiency in either integrin αVβ3 subunits or CD47 on the DMM mouse model. We first tested the contribution of β3 to OA progression by surgically inducing OA in 20-week-old integrin β3–deficient (Itgb3–/–) and WT (Itgb3+/+) mice. Itgb3–/– mice are fertile but have reduced viability and are susceptible to gastrointestinal bleeding (39). Nevertheless, prior to DMM there were no noticeable differences in joint development between Itgb3–/– and Itgb3+/+ mice. Twenty weeks after DMM, Itgb3–/– mice showed markedly milder cartilage degeneration and synovitis as compared with Itgb3+/+ mice (Figure 2A).
Figure 2. Crucial roles of integrin β3, CD47, and Fyn in the development of mouse osteoarthritis.
(A–C) Representative cartilage degeneration scores in safranin O–stained sections of the medial region of stifle joints from Itgb3+/+ (n = 7) and Itgb3–/– (n = 7) (A), Cd47+/+ (n = 7) and Cd47–/– (n = 7) (B), or Fyn+/+ (n = 7) and Fyn–/– (n = 7) (C) mice 20 weeks after destabilization of the medial meniscus (DMM); and quantification of cartilage degeneration and synovitis. Arrowheads indicate areas of cartilage degeneration. Scale bars in the low-magnification (×4; top) images: 500 μm; scale bars in the high-magnification (×10; bottom) images: 200 μm. Mouse DMM data are mean ± SEM of 1 experiment and are representative of ≥2 independent experiments. NS: P > 0.05; *P < 0.05; **P < 0.01 by Mann-Whitney U test. (D–F) qPCR analysis of relative mRNA expression levels of inflammatory mediators and MMPs in bone marrow–derived macrophages from Itgb3+/+ and Itgb3–/– (D), Cd47+/+ and Cd47–/– (E), and Fyn+/+ and Fyn–/– (F) mice stimulated with lysed WT murine chondrocytes. qPCR data are mean ± SEM of triplicates and are representative of 3 independent experiments. NS: P > 0.01; *P < 0.01; **P < 0.001 by Mann-Whitney U test with multiple-comparison corrections.
Genetic deficiency in the αV subunit leads to embryonic or perinatal lethality (7). Thus, we were unable to test OA progression in Itgav–/– mice. Nevertheless, the β3 subunit dimerizes primarily with either the αV or αIIb subunit. As we found that human OA cartilage and synovium express very little αIIb mRNA (ITGA2B) while expressing high levels of ITGAV mRNA (Supplemental Figure 1A), the protection against OA observed in β3-deficient mice was most likely due to loss of integrin αVβ3.
We then examined the effects of deficiency in either CD47 (Cd47–/–) or FYN (Fyn–/–), a Src family protein tyrosine kinase that is activated downstream of integrin activation (8), in mice. Twenty weeks after DMM, both Cd47–/– and Fyn–/– mice showed significantly reduced cartilage degeneration and synovitis (Figure 2, B and C). While global depletion of these factors in mice was unable to distinguish tissue-specific contributions of αVβ3 and CD47 signaling to the development of OA, these data nevertheless indicate that integrin αVβ3– and CD47-mediated signaling in joints is crucial to the pathogenesis of OA.
Accumulating evidence from recent research indicates the involvement of low-grade, chronic inflammation resulting from the interplay among local tissue damage, metabolic dysfunction (20), immune dysregulation (21), and/or other factors. Therefore, we tested whether genetic deficiencies in the above genes might mitigate DMM-induced OA by dampening the expression of inflammatory and degradative mediators. Products released by chondrocytes that break down during dysregulated cartilage remodeling and repair have been postulated to contribute to joint inflammation and damage in OA (22, 23). Additionally, as the synovial tissues of individuals with OA have been found to contain immune infiltrates of macrophages and other cell types (40), we examined macrophages in these genetically deficient mice following stimulation with breakdown products from chondrocyte lysates. Upon stimulation with chondrocyte breakdown products from WT mice, bone marrow–derived macrophages from Itgb3–/–, Cd47–/–, or Fyn–/– mice expressed lower levels of genes encoding multiple inflammatory (e.g., Il1b, Il6, Cxcl1, Ccl2, Vegfa, and Ptgs2) and degradative (e.g., Mmp13) mediators than macrophages from WT control mice (Figure 2, D–F). These data demonstrate that αVβ3 and CD47 signaling impacts the production of inflammatory and degradative mediators by macrophages.
Attenuation of murine OA by pharmacological inhibition of αVβ3, CD47, or FAK.
As with genetic deficiency in the integrin β3 subunit, pharmacological inhibition of αVβ3 with P11, a hexapeptide antagonist of αVβ3 (41, 42), markedly reduced cartilage degeneration and synovitis in WT mice subjected to DMM (Figure 3A). This recapitulates what we observed in Itgb3–/– mice with DMM-induced OA. An RGD peptide, the minimal integrin recognition sequence in fibronectin, vitronectin, and other ligands (11, 43), displayed no overt effects on this DMM mouse model of OA (Figure 3B). The lack of efficacy of the RGD peptide may be due to low affinity, specificity, or stability of the peptide due to its linear nature (44). As in Cd47–/– mice with DMM-induced OA (Figure 2B), treatment with an anti-CD47 antibody (12) attenuated DMM-induced OA in WT mice (Figure 3C). We also tested the effect of PF-573228 (13), a small molecule that inhibits focal adhesion kinase (FAK), on OA. FAK is a protein tyrosine kinase that is a key mediator of the intracellular signaling pathways activated by integrins (14). Oral administration of PF-573228 mitigated the development of OA in WT mice subjected to DMM (Figure 3D). Together, our findings from genetic and pharmacological studies in mice suggest that integrin αVβ3 and CD47 have a pathogenic role in OA.
Figure 3. Attenuation of mouse osteoarthritis by pharmacological inhibition of integrin αVβ3, CD47, or FAK.
(A–D) Representative cartilage degeneration in safranin O–stained sections of the medial region of stifle joints from mice subjected to destabilization of the medial meniscus (DMM) and immediately treated intraperitoneally or orally for 12 weeks with the αVβ3 antagonist P11 (n = 5) or PBS (control; n = 5) (A), an RGD peptide (n = 7) or PBS (n = 7) (B), anti-CD47 antibody (n = 12) or PBS (n = 8) (C), or an FAK inhibitor, PF-573228 (n = 8), or PBS (n = 8) (D); and quantification of cartilage degeneration and synovitis. Arrowheads indicate areas of cartilage degeneration. Scale bars in high-magnification images (×10): 200 μm. Mouse DMM data are the mean ± SEM of one experiment and are representative of ≥2 independent experiments. NS P > 0.05; *P < 0.05; **P < 0.01 by Mann-Whitney U test.
Elevated ligand-binding capacities of αVβ3 and CD47 in murine osteoarthritic joints.
We used microPET/CT to assess ligand-binding capacities of integrin αVβ3 and CD47 in the articular joints of mice that developed DMM-induced OA. Integrin αVβ3 (as well as other RGD-related integrins) binds to its ligands, such as COMP, fibronectin, and vitronectin, through recognition of their RGD sequence, whereas CD47 binds to COMP via a SFYVVMWK sequence (Pcomp) in its globular C-terminal domain (2). WT mice were subjected to DMM. Twenty weeks later, they were injected intravenously with 68Ga-NODAGA-c(RGDfK), a cyclic radiolabeled specific αVβ3-binding peptide (15), or 68Ga-NODAGA-Pcomp, a radiolabeled specific CD47-bindng peptide (2), and imaged by microPET/CT. Concentrations of 68Ga-NODAGA-c(RGDfK) or 68Ga-NODAGA-Pcomp were significantly higher in the DMM-induced osteoarthritic joints (confirmed by histology; data not shown) than in the sham-operated joints (Figure 4, A and C). Coinjection of competing, unlabeled c(RGDfK) or Pcomp diminished this increase in 68Ga-NODAGA-c(RGDfK) or 68Ga-NODAGA-Pcomp binding, confirming the specificity of the binding that was detected (Figure 4, B and D). The elevated uptake of 68Ga-NODAGA-c(RGDfK) and 68Ga-NODAGA-Pcomp indicates the increased ligand-binding capacities of αVβ3 and CD47 in osteoarthritic joints. Together, these results reflect the upregulation of αVβ3 and CD47 and/or higher activation states of αVβ3 and CD47 in osteoarthritic joints (3, 4).
Figure 4. Increased ligand-binding capacities of integrin αVβ3 or CD47 in the osteoarthritic murine joint.
(A–H) MicroPET/CT imaging of mouse knee joints 20 weeks after they were subjected to destabilization of the medial meniscus (DMM) or sham surgery, and quantification of relative 68Ga levels in these joints. For imaging of αVβ3-binding capacity, mice were injected intravenously with 68Ga-NODAGA-c(RGDfK) alone (n = 6) (A and E), with 68Ga-NODAGA-c(RGDfK) plus unlabeled c(RGDfK) (n = 6) (B), or with 68Ga-NODAGA-c(RGDfK) plus P11 (n = 8) (F); for imaging of CD47-binding capacity, they were injected with 68Ga-NODAGA-Pcomp alone (n = 7) (C and G), with 68Ga-NODAGA-Pcomp plus unlabeled Pcomp (n = 6) (D), or with 68Ga-NODAGA-Pcomp plus an anti-CD47 antibody (n = 5) (H). Mouse DMM data are mean ± SEM of 1 experiment and are representative of ≥2 independent experiments. NS: P > 0.05; **P < 0.01 by Mann-Whitney U test.
It has been shown that c(RGDfK) can bind other RGD integrins including α5β1 (45). Thus, we cannot rule out that the increased uptake of 68Ga-NODAGA-c(RGDfK) following DMM may be, in part, due to increased levels of α5β1 or other integrins. To ensure that the increased uptake of 68Ga-NODAGA-c(RGDfK) is, in fact, specific to αVβ3 binding, we injected mice with P11. Twenty weeks after DMM, injection of P11 reduced the levels of 68Ga-NODAGA-c(RGDfK) detected by microPET/CT imaging in the osteoarthritic joint (Figure 4, E and F), confirming that P11 effectively antagonizes αVβ3-ligand interactions in these mice. Likewise, administration of an anti-CD47 antibody (12) 20 weeks after DMM attenuated CD47-Pcomp binding in the osteoarthritic joints (Figure 4, G and H).
Integrin αVβ3/CD47-dependent expression of inflammatory and degradative mediators induced by chondrocyte breakdown.
We next investigated how αVβ3 and CD47 signaling becomes activated in OA, and the molecular consequences of their activation. Previous studies indicated that synovial tissues are major sources of inflammatory and degradative signals in OA (46). Thus, we investigated whether chondrocyte breakdown could directly induce the production of inflammatory and degradative mediators in synoviocytes through activation of αVβ3/CD47 signaling. Human primary synoviocytes stimulated with chondrocyte breakdown products, both derived from the knee joints of individuals with OA, showed elevated gene expression of multiple inflammatory (e.g., IL1B, IL6, CXCL8 [IL-8], CCL2, VEGFA, and PTGS2) and degradative (e.g., MMP3, MMP13, ADAMTS4, and ADAMTS5) mediators implicated in OA (Figure 5A), as well as elevated protein levels of several of these mediators (Figure 5, B–E). The induction of inflammatory and degradative mediators we observed upon stimulation of synoviocytes with chondrocyte breakdown products was attenuated by treatment with c(RGDfK) (Figure 6A), an anti-CD47 antibody (Figure 6B), PF-573228 (Figure 6C), or an inhibitor of SHP-2 (Figure 6D), indicating a critical role for αVβ3 and CD47 signaling in this process. Further, stimulation of human synoviocytes with COMP increased IL1B gene expression, which was blocked by the addition of c(RGDfK) and/or an anti-CD47 antibody (Figure 6E).
Figure 5. Chondrocyte breakdown products promote production of osteoarthritis-related inflammatory and degradative mediators in human osteoarthritis primary synoviocytes.
(A) qPCR analysis of osteoarthritis-related (OA-related) inflammatory and degradative mediators in human primary synoviocytes stimulated with breakdown products from lysed chondrocytes, both derived from the knee joints of individuals with OA. Data are mean ± SEM of triplicates and are representative of 3 independent experiments. NS: P > 0.01; **P < 0.001 by Mann-Whitney U test with multiple-comparison corrections. (B–E) ELISA analysis of IL-1β (B), IL-6 (C), MMP3 (D), and MMP13 (E) levels in cell culture supernatants. Data are mean ± SEM of triplicates and are representative of 3 independent experiments. *P < 0.05; **P < 0.01 by Mann-Whitney U test.
Figure 6. Integrin αVβ3/CD47-dependent expression of inflammatory and degradative mediators as well as phosphorylation of downstream signaling molecules in human primary synoviocytes induced by chondrocyte breakdown.
(A–D) qPCR analysis of osteoarthritis-related (OA-related) inflammatory and degradative mediators in human primary synoviocytes stimulated with breakdown products from lysed chondrocytes, both derived from knee joints of individuals with OA, in the absence or presence of c(RGDfK) (A), an anti-CD47 antibody (B), PF-573228 (C), or an SHP-2 inhibitor (D). Data are mean ± SEM of duplicates or triplicates and are representative of ≥2 independent experiments. *P < 0.01; **P < 0.001 by Mann-Whitney U test with multiple-comparison corrections. (E) Stimulation with COMP increased IL-1β expression in human OA primary synoviocytes, while the c(RGDfK) peptide and/or an anti-CD47 antibody blocked this effect. Data are mean ± SEM of triplicates and are representative of 3 independent experiments. *P < 0.05 by Mann-Whitney U test.
Stimulating monocyte-derived macrophages from healthy subjects (Figure 7, A–E) or primary chondrocytes from individuals with OA (Figure 8, A–D) with breakdown products from lysed chondrocytes derived from the articular cartilage of osteoarthritic knee joints similarly led to increases in the mRNA and protein levels of several inflammatory and degradative mediators. While both macrophages and chondrocytes exhibited pathogenic responses upon stimulation with chondrocyte breakdown products, differences in these responses may be due to their derivation from healthy and diseased sources, respectively. Stimulation of OA chondrocytes with chondrocyte breakdown products also increased the expression of genes involved in chondrocyte hypertrophy (e.g., COL10A1 and SOX9; Figure 8A), a process integral to the osteoarthritic pathology (24). Treatment with c(RGDfK) or PF-573228, but not a CERVIGTGWVRC peptide that blocks CD47-SIRPα interaction (25), abolished these effects (Figure 7, F–H, and Figure 8, E and F), suggesting that CD47 also contributes to the pathogenesis of OA via mechanisms independent of SIRPα-mediated phagocytosis (26).
Figure 7. Chondrocyte breakdown products stimulate integrin αVβ3– and FAK-dependent expression of osteoarthritis-related inflammatory and degradative mediators in monocyte-derived macrophages.
Healthy human monocyte–derived macrophages were stimulated with lysed osteoarthritis (OA) chondrocyte debris, and then OA-related inflammatory and degradative mediators and αVβ3- and FAK-dependent signaling pathways were characterized. qPCR (A) and ELISA (B–E) analyses of inflammatory and degradative mediators in human monocyte–derived macrophages stimulated with breakdown products from lysed OA chondrocytes. (F–H) qPCR analysis of inflammatory mediators in human monocyte–derived macrophages stimulated with breakdown products from lysed OA chondrocytes, in the absence or presence of an αVβ3 inhibitor c(RGDfK) (F), FAK inhibitor PF-573228 (G), or SIRPα inhibitor CERVIGTGWVRC peptide (H). For all qPCR and ELISA analyses in this figure, data are mean ± SEM of triplicates and are representative of 3 independent experiments. NS: P > 0.01; **P < 0.001 by Mann-Whitney U test with multiple-comparison corrections (A, F–H). *P < 0.05; **P < 0.01, by Mann-Whitney U test (B–E).
Figure 8. Integrin αVβ3– and FAK-dependent expression of osteoarthritis-related inflammatory, degradative, and hypertrophic mediators in human osteoarthritis primary chondrocytes induced by chondrocyte breakdown.
qPCR analysis (A) and ELISA (B–D) of human osteoarthritis (OA) primary chondrocytes stimulated with breakdown products from lysed OA chondrocytes. (E and F) qPCR analysis of relative mRNA expression in human OA primary chondrocytes stimulated with breakdown products from lysed OA chondrocytes in the absence or presence of c(RGDfK) (E) or PF-573228 (F). All data are mean ± SEM of triplicates and are representative of 3 independent experiments. NS: P > 0.01; *P < 0.01; **P < 0.001 by Mann-Whitney U test with multiple-comparison corrections (A, E, and F); *P < 0.05; **P < 0.01, by Mann-Whitney U test (B–D).
Cartilage damage during OA results in both breakdown of the ECM and chondrocyte death, leading to the accumulation of insoluble ECM debris and soluble chondrocyte debris. Both insoluble ECM debris and soluble breakdown products released by chondrocytes have been postulated to contribute to joint inflammation and damage in OA (22, 23). We tested both soluble and insoluble components of chondrocyte lysates for their ability to induce expression of inflammatory and degradative mediators by cell types derived from OA joints. We separated chondrocyte lysates derived from knee OA joints into soluble and insoluble fractions through centrifugation. Stimulation of either primary human synoviocytes or chondrocytes with soluble fractions of the lysates resulted in significantly elevated levels of expression of multiple genes encoding inflammatory and degradative mediators, similar to the elevated expression levels observed upon stimulation with total chondrocyte lysates containing both soluble and insoluble components (Supplemental Figure 2, A and B). Further, stimulation of synoviocytes and chondrocytes with the insoluble fraction led to minor increases in expression of these genes (Supplemental Figure 2, A and B). These results indicate that soluble products resulting from the breakdown of chondrocytes play key roles in promoting the expression of mediators that contribute to the pathogenesis of OA.
Chondrocyte breakdown products induce synoviocyte activation of αVβ3/CD47-dependent signaling molecules.
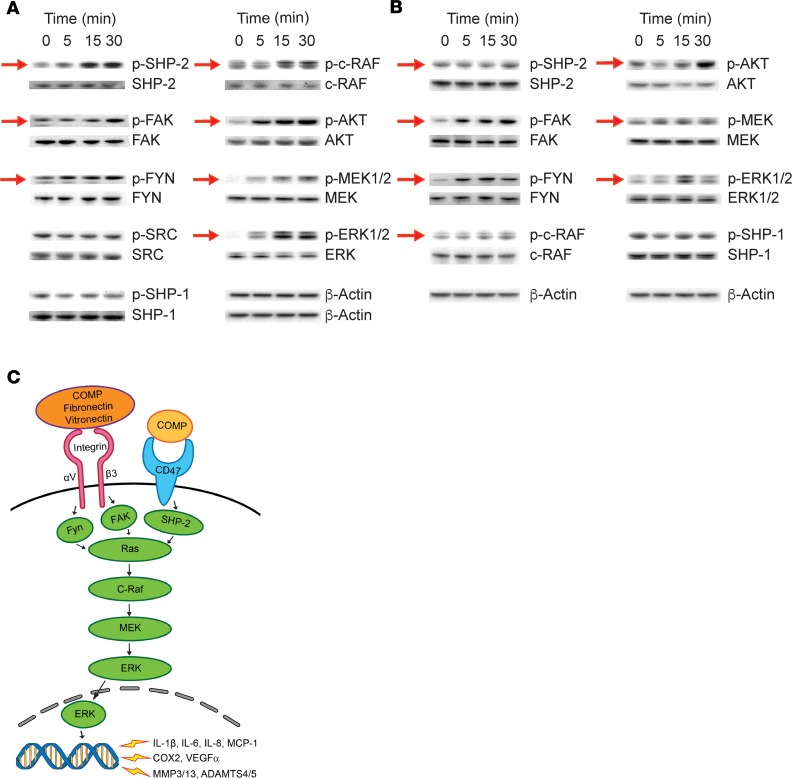
Finally, we examined the signaling network downstream of αVβ3 and CD47 activation in the osteoarthritic joint. To characterize the signaling network, we stimulated human primary synoviocytes or monocyte-derived macrophages in vitro with breakdown products from lysed chondrocytes. Over the course of 30 minutes following stimulation, we detected increased phosphorylation of SHP-2, FAK, FYN, c-RAF, AKT, MEK, and ERK (Figure 9, A and B), all effector molecules in the intracellular signaling pathways that are known to be activated by integrin αVβ3 and CD47 (27, 47, 48). In contrast, no change was observed in the phosphorylation level of SRC or SHP-1 (Figure 9, A and B), confirming specificity of the response (49, 50).
Figure 9. Chondrocyte breakdown products induce activation of αVβ3/CD47-dependent signaling molecules in human primary synoviocytes and monocyte-derived macrophages.
(A and B) Western blot analysis of the phosphorylation of molecules downstream of αVβ3/CD47 signaling in human primary osteoarthritic (OA) synoviocytes (A) and human monocyte–derived macrophages (B) stimulated with breakdown products from lysed chondrocytes derived from knee joints of individuals with OA. Red arrows denote changes in phosphorylation levels over time. (C) Schematic of αVβ3 and CD47 signaling pathways in the pathogenesis of OA.
Discussion
There are currently no effective treatments to prevent the onset of OA or slow its progression, largely due to an incomplete understanding of the underlying mechanisms. Here, by transcriptomic and proteomic analyses of human and murine OA tissues as well as microPET/CT imaging, genetic deficiencies, and pharmacological treatments of the DMM mouse model, we show that integrin αVβ3 and CD47 signaling pathways are pivotal to the pathogenesis of OA. Further, in vitro studies revealed that chondrocyte breakdown products derived from the articular cartilage of individuals with OA induced αVβ3/CD47-dependent expression of inflammatory and degradative mediators through activation of the downstream signaling network. In light of these findings, human trials are necessary to define which of these molecules, individually or in combination, could be targeted to develop safe and efficacious disease-modifying therapies to prevent or treat OA.
Guided by our mass spectrometric analysis and transcriptomic changes in the synovial membrane and fluids in OA, we focused on elucidating the pathologic relevance of integrin αVβ3 and CD47 signaling to OA. We demonstrated that gene expression of both αVβ3 (ITGAV and ITGB3) and CD47 is increased in synovial membranes of OA joints; and that ligands of these receptors, including fibronectin, vitronectin, and COMP, are highly prevalent in OA joints. Further, we demonstrated that induction of OA in mice, through DMM, led to increased uptake of RGD ligand peptides. Importantly, we demonstrate that integrin αVβ3 and CD47- mediated signaling occurs in response to chondrocyte breakdown products, which likely contain the ligands we identified through mass spectrometry. Thus, these findings suggest that the combined effects of increased ligands as a result of cartilage damage, as well as increased αVβ3 and CD47 expression, may enable cells within OA joints to elicit pathogenic responses, and thereby drive the progression of OA. Further, development of OA may result from chronic signaling of inflammatory and degradative mediators as a result of the increased levels of both ligand and receptor components following joint injury (Figure 9C).
Our in vitro studies show that αVβ3- and CD47-mediated signaling, resulting in the expression of inflammatory and degradative mediators, can occur in cells derived from multiple types of joint tissue upon stimulation with chondrocyte breakdown products. Synovial linings, which contain both fibroblasts and macrophages, are known to be significant sources of inflammatory and degradative mediators in OA. Further, mechanical insult to cartilage is known to cause autocrine MMP expression (51). Thus, our findings raise the question, In which tissues is αVβ3/CD47 signaling important for OA pathogenesis? We showed that αVβ3 and CD47 are highly expressed in cartilage and synovium at the transcriptional level, suggesting that signaling in either or both tissues may be important in OA pathogenesis. Cartilage damage can lead to inflammation of synovial membranes and production of cytokines including IL-1, IL-6, and TNF, in addition to signaling in chondrocytes (52, 53). Cytokine production in synovial membranes could further perpetuate OA by promoting the breakdown of cartilage and apoptosis of chondrocytes. Thus, interplay between the two tissues likely contributes significantly to the development of OA, and αVβ3/CD47 signaling in either or both tissues may perpetuate inflammation and degradation leading to joint destruction. Our analysis of mice genetically deficient for β3 or CD47 confirmed functional roles for these proteins in the pathogenesis of OA, but was unable distinguish in which tissues αVβ3 and CD47 are important for OA pathogenesis. As a result, future studies using conditional knockout mice will be needed to parse the contributions of αVβ3 and CD47 signaling in each tissue, as well as to analyze other integrin components (e.g., integrin β1) for which global deletion results in embryonic lethality in mice (54, 55).
In OA, mechanical insult to or overuse of joints is known to cause cytokine expression by articular chondrocytes, with subsequent autocrine MMP expression (51, 56). These cytokines can stimulate unique responses within target cells, but they can also elicit a series of shared intracellular responses that facilitate transcriptional induction of MMPs (51), some of which are known to be mediated by MAPKs (57), which include the ERKs. Phosphorylation of ERK occurs in response to multiple external stimuli, which include IL-1β and TNF-α (41). Further, we and others have shown that in OA, activation of ERK in chondrocytes can be regulated by complement, serpins involved in ECM turnover, and other growth factors (58, 59). Here we demonstrate that αVβ3 and CD47 transduce signals from chondrocyte breakdown, leading to activation of ERK and subsequent expression of MMPs, thereby contributing to the progression of OA in the joint. Further, we show that activation of the ERK/MAPK pathway occurs in synoviocytes and macrophages downstream of FYN, FAK, and SHP-2. As other MAPK pathways, including JNK and p38, have also been implicated in the production of inflammatory mediators in synovial tissue (60, 61), it is possible that they are also activated by αVβ3 and/or CD47 and contribute to the progression of OA.
One noteworthy finding in this report is the distinct function of CD47 as a signaling receptor in promoting the pathology of OA. CD47 is known to interact with SIRPα on phagocytic cells to convey a “don’t eat me” signal (26). CD47 contributes to the prevention of phagocytosis of dangerous, inflamed, and premalignant cells. For example, atherogenic smooth muscle cells, pathological fibroblasts, and cancer cells persist by blocking phagocytosis through the expression of CD47 (18). Macrophages activated via TLRs also make and secrete “eat me” signals on target cells that can resist phagocytosis by CD47 action, which leads to phagocytosis if CD47 signals are blocked (19, 62, 63). Therefore, one action of CD47 could be to allow dangerous cells or cell products to remain in lesions, and blockade of CD47 could enable phagocytic removal of these cells or products. But CD47 can also act as a signaling receptor. For example, when bound to the secreted protein thrombospondin-1 (TSP1), CD47 regulates nitric oxide and cGMP signaling, and controls cell fate, viability, and resistance to stress (64). In the osteoarthritic joint, we found that upon stimulation with chondrocyte breakdown products, signals are likely transduced via SHP-2 but independently of SIRPα to trigger downstream cascades and induce the production of inflammatory and degradative mediators. Future studies are needed to ascertain whether CD47 acting as a phagocytic signal may also play a role in OA, and whether this and/or other mechanisms may also contribute to the attenuation of OA by blockade of CD47 in our DMM mouse model.
CD47 and αVβ3 are known to physically interact and share ligands (65, 66). Here we demonstrate that CD47 and αVβ3 independently contribute to OA pathogenesis by mediating signals from degraded cartilage. However, given that integrin αVβ3 and CD47 likely form a complex on the cell surface, future studies are needed to further define the interactions between αVβ3 and CD47, including the contributions of their independent and combined signaling mechanisms to OA pathogenesis. In addition, future analysis of the relationship among expression levels of CD47, αVβ3, and their ligands and the pathology of human OA will be valuable to gain further insights into the role of CD47 and αVβ3 in OA pathogenesis, and to identify next-generation biomarkers as well as potential targets for disease-modifying therapies to prevent or treat OA.
Multiple limitations to this study exist. First, we were unable to access normal joint synovial fluids for comparison between CD47/αVβ3 ligands present in OA versus normal synovial fluids. However, we demonstrated here that concentrations of fibronectin were elevated in mouse joints following surgical induction of OA through DMM. Furthermore, previous studies have shown that fibronectin and vitronectin are indeed elevated in human OA synovial fluids (67, 68). Second, other integrin pairs, including α5β1, α1β1, α3β1, α2β1, α4β1, and α6β1, have been implicated in OA (5, 9, 69–71). Consistent with these previous studies, we showed that multiple integrin subunits, in addition to αV and β3, were highly expressed in OA cartilage and synovium. Extended transcriptomic analysis of integrin expression demonstrated that other integrin subunits, including α4, αE, αL, αX, β2, β5, β7, and β8, were upregulated in early- and end-stage OA as compared with healthy joints, suggesting that the responses we observe here may be due to activation of other or multiple integrin pairs. We note, however, that αV and β3 were 2 of the most highly upregulated integrin subunits in OA tissues through our analyses. Further, our findings indicate that RGD receptors are disproportionately upregulated in OA joints (Supplemental Figure 1C and ref. 72), suggesting that specific ligands, including fibronectin, vitronectin, and COMP, are crucial to the development of OA. α5β1, an RGD-binding integrin, is known to be upregulated in osteoarthritic cartilage, promote expression of inflammatory signals, and contribute to OA pathogenesis (9, 36, 37, 71, 73). Thus, it is possible that the inflammatory responses demonstrated here are due, in part, to signaling through α5β1. Future studies will need to parse the contributions of αVβ3 and α5β1 to the pathogenesis of OA. Third, our data show that pharmacological blockade of either αVβ3 or CD47 led to partial, but significant suppression of inflammatory and degradative mediators. This partial suppression may be due either to activation of redundant pathways or upregulation of noncanonical pathways to compensate for the loss of signaling through αVβ3 or CD47. These compensatory mechanisms may include pathways that are known contributors to OA, including additional integrin pairs, TLRs, or complement (9, 34, 74, 75). We also found that the breakdown products from lysed chondrocytes utilized in our in vitro experiments contain a diverse range of products that likely stimulate multiple signaling pathways known to contribute to OA. As these lysates better mimic the environment within OA joints than pure proteins, it is unsurprising that multiple signaling pathways were activated in these experiments. These studies, however, indicate that signaling through αVβ3 and CD47 contributes, in part, to the expression of pathogenic mediators. The contributions of additional pathways to the inflammatory response, as well as compensatory upregulation in the absence of αVβ3/CD47 signaling, remain to be determined. Fourth, we demonstrate that expression of proinflammatory cytokines such as IL-1β was upregulated in multiple cell types upon stimulation with chondrocyte breakdown products. One possible explanation for these observations is that the cytokine signals emanated from the chondrocyte lysates themselves, rather than being produced by the stimulated cells. However, analysis of control chondrocytes, which originated from the same OA cartilage as the chondrocyte lysates, revealed that IL-1β was not detectable in these cells prior to treatment with chondrocyte lysates. Therefore, the IL-1β detected in these cultures resulted from stimulation of proinflammatory signaling by chondrocyte breakdown products.
In conclusion, our studies identify mechanisms involving integrin αVβ3 and CD47 signaling that drive the pathogenesis of OA. Cartilage ECM components released by or exposed in osteoarthritic joints may transduce joint trauma or overuse, often in the context of other risk factors, into the activation of integrin αVβ3 and CD47, and subsequently their downstream signaling pathways. This in turn results in production of a myriad of inflammatory, catabolic, and hypertrophic molecules as well as other integrin effectors, all of which may promote osteoarthritic joint pathology (Figure 9C). Specific inhibition of key components of integrin αVβ3 and CD47 cascades attenuates the progression of OA phenotypes. Our findings suggest targeting integrin αVβ3 and CD47 signaling pathways as a disease-modifying therapy for OA and demonstrate the potential for using radiolabeled c(RGDfK) and Pcomp peptides in diagnosing by PET/CT the progression of this disease.
Methods
Transcriptional profiling.
We analyzed RNA from osteoarthritic synovial membranes using Affymetrix Human U133 Plus 2.0 chips (GEO GSE32317). Data from healthy synovial membranes (analyzed on the same platform and array) were downloaded from the NCBI Gene Expression Omnibus (GEO GSE12021) (35). We merged the data sets (76), computed the multichip average expression for the genes, and organized transcript expression profiles by unsupervised hierarchical clustering (Cluster 3.0; http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm).
Proteomic profiling of synovial fluids.
We obtained synovial fluids from individuals with clinically confirmed OA. Synovial fluid proteins were labeled with Cy3 or Cy5, subjected to isoelectric focusing (IEF), and separated on acrylamide gels (2D-DIGE). We selected expressed spots by using DeCyder software (GE Healthcare) based on expression change of ≥1.2-fold over the background levels and a significant t test (P < 0.05). Protein identities were determined by liquid chromatography–tandem mass spectrometry (LC-MS/MS) in conjunction with a Mascot database search (Matrix Science).
ELISA.
We analyzed protein levels of fibronectin in synovial fluids from the osteoarthritic (induced by DMM) and sham-operated mouse joints by the Mouse Fibronectin ELISA Kit (Abcam). Protein levels of fibronectin, vitronectin, and COMP in synovial fluids from individuals with OA were measured by Human Fibronectin, COMP, and Vitronectin Quantikine ELISA Kits (R&D Systems). To analyze protein levels of cytokines and MMPs in human synoviocytes, macrophages, and chondrocytes incubated with or without breakdown products from lysed chondrocytes, we measured levels of human IL-1β and IL6 by ELISA (Peprotech), and levels of human MMP3 or MMP13 by MMP fluorimetric (Anaspec) analysis, according to the manufacturers’ instructions.
qPCR.
We isolated RNA from various cell or tissue types (see below) in human or murine knee joints. We measured mRNA levels by qPCR and normalized them to those of 18s RNA or β-actin (2–ΔΔCt). The primers and probes used were purchased from Applied Biosystems.
Surgical mouse model of OA.
We generated the DMM mouse model of OA according to established methods (38, 77–79) using 20-week-old male mice. Experiments continued for 12–20 weeks after surgery based on a previously described time course analysis demonstrating increased severity of OA pathology at these time points (34). Mice deficient in the integrin β3 subunit (B6.129S2-Itgb3tm1Hyn/JSemJ), in CD47 (B6.129S7-Cd47tm1Fpl/J), or in Fyn (129-Fyntm1Sor/J), as well as WT control (C57BL/6J) mice, were obtained from the Jackson Laboratory. Five to 12 mice were used per experimental arm.
Conjugation and radiolabeling of 68Ga-NODAGA-c(RGDfK) and 68Ga-NODAGA-Pcomp.
Conjugation between the c(RGDfK) (LifeTein) or SFYVVMWK (Pcomp) (Elim Biopharm) peptides and the chelator NODA-GA-NHS (CheMatech) was achieved by the classic click reaction (80). Briefly, c(RGDfK) or Pcomp was dissolved with freshly degassed phosphate buffer (pH 7.4) at a concentration of 1 mg/mL. The bifunctional chelator NODA-GA-NHS ester dissolved in DMSO (10 mM) was added at 20 equivalents per equivalent of the peptide. After mixing the reagents by vortexing them for 12 hours at 4°C, we purified the reaction mixture by HPLC. Characterization of the conjugated products was done by LC–electrospray ionization–MS (LC-ESI-MS). Eight micrograms of NODAGA-c(RGDfK) or NODAGA-Pcomp were radiolabeled with 68Ga by the addition of 203.5–240.5 MBq (5.5–6.5 mCi) 68GaCl3 in 1 M NaOAc (pH 4.5) buffer, followed by a 15-minute incubation at 85°C (81). The radiolabeled complex was then purified by C18 cartridge, dried by nitrogen, and reconstituted in PBS in sterile vials for use in mouse experiments.
MicroPET/CT.
Twenty weeks after DMM, imaging of mice was performed on a Siemens Inveon microPET/CT, using established methods (82) with minor modifications. In one set of experiments, mice were injected with 7.4–14.8 MBq (200–400 μCi) 68Ga-labeled radiotracers (68Ga-NODAGA-c[RGDfK] or 68Ga-NODAGA-Pcomp), in the absence or presence of the corresponding unlabeled blockers (100–300 μg per mouse), via the tail vein. In a second set of experiments, mice were injected with 7.4–14.8 MBq (200–400 μCi) 68Ga-NODAGA-c(RGDfK), in the absence or presence of 100 μg of the integrin αVβ3 antagonist P11, or 7.4–14.8 MBq (200–400 μCi) 68Ga-NODAGA-Pcomp, in the absence or presence of 100 μg of an anti-CD47 antibody (12). Ten minutes after injection, the mice were sacrificed, and both the DMM and sham-operated legs of the mice were then collected and placed near the center of the field of view of the scanner. All the PET images were obtained through 5-minute static acquisition and reconstructed with IRW4.0 software (Siemens) by a 3D ordered-subsets expectation maximization (3DOSEM) algorithm. The degree of radioactivity uptake in the knee joints was evaluated by analyzing the region of interest (ROI) drawn over the whole joint region; this ROI was the same for DMM and sham-operated joints. The degree of radioactivity uptake in the DMM side was calculated as (radioactivity of DMM joint)/(radioactivity of sham-operated) × 100% for each mouse, with uptake in the sham-operated joint set at 100%.
Scoring of cartilage degeneration in murine OA.
We stained sections of mouse joints with safranin O. Cartilage degeneration was blindly scored by a trained examiner using a modified version of a previously described system (83): depth of cartilage degeneration (score of 0–4) × width of cartilage degeneration (with a score of 1 indicating one-third of the surface area; 2, two-thirds of the surface area; 3, the whole surface area) in each third of the femoral-medial and tibial-medial condyles. The scores for the 6 regions were summed. We have previously demonstrated use of this modified scoring system to describe severity of OA following DMM (34, 84, 85).
Scoring of synovitis in murine OA.
To evaluate synovitis, H&E-stained sections were blindly scored by a trained examiner according to a previously described system (86): 0, no changes compared with normal joints; 1, thickening of the synovial lining and some influx of inflammatory cells; 2, thickening of the synovial lining and intermediate influx of inflammatory cells; and 3, profound thickening of the synovial lining (more than 4 cell layers) and maximal observed influx of inflammatory cells. Scores for synovitis were recorded for the femoral-medial and the tibial-medial condyles, and the scores for the 2 regions were summed.
Treatment of mouse OA.
We treated 20-week-old C57BL/6J mice daily intraperitoneally with 100 μg of the αVβ3 integrin antagonist P11 (HSDVHK) (41, 42) or with PBS; intraperitoneally with 100 μg of an anti-CD47 antibody (12) or with PBS; intraperitoneally with 100 μg of an RGD peptide or with PBS; or orally with 50 mg/kg of the FAK inhibitor PF573228 (Wan Qian Pharma) or with PBS, starting 1 week after DMM for 12 weeks.
Isolation, culture, and treatment of murine bone marrow–derived macrophages.
Murine bone marrow–derived macrophages (BMDMs) were prepared as described previously (87). Bone marrow (BM) cells were flushed from tibiae and femurs from male C57BL/6J, Itgb3–/–, Cd47–/–, or Fyn–/– mice with ice-cold PBS, and the suspension was passed through a 70-μm cell strainer. Cells were then treated with red blood cell lysis buffer (Lonza) for 5 minutes at room temperature. After 2 PBS washes, BM cells were cultured in α-MEM with 10% FBS and 25 ng/mL M-CSF for 7–10 days, with medium changed every 2–3 days. After 7–10 days of culture, BMDMs were resuspended and seeded in 12-well plates, with 3 wells of BMDMs per genotype, at a 2 × 105 cells/well concentration and cultured for 24 hours. BMDMs were then cultured in fresh α-MEM with or without 2 × 105 cells/well lysed murine cartilage chondrocytes for another 24 hours followed by cell collection for RNA isolation.
Generation of murine chondrocytes.
Murine cartilage chondrocytes were prepared as described previously (88): anterior rib cages and sternums were isolated from WT newborn C57BL/6J mice and placed into 10-cm petri dishes with PBS on ice. Isolated sternum and rib cages were incubated in DMEM-F12 with 10% FBS and 1 mg/mL collagenase II (Worthington Biochem) for 6 hours at 37°C. After filtration through a 70-μm cell strainer, chondrocytes were washed and resuspended in DMEM with 10% FBS at a 2 × 107 cells/mL concentration.
Isolation and culture of human primary chondrocytes.
Human primary chondrocytes were isolated from osteoarthritic cartilage as described previously (89): articular cartilage was extracted from the osteoarthritic knee joint of individuals undergoing total knee joint replacement (TKR), avoiding areas of erosion. Cartilage was then cut into small pieces (≤1 mm2), seeded in 10-cm petri dishes in DMEM-F12 (Thermo Fisher Scientific) containing 10% FBS and 1 mg/mL collagenase II (Worthington Biochem), and incubated for 24 hours at 37°C. Chondrocytes were then filtered through a 70-μm cell strainer, and washed and resuspended in DMEM with 10% FBS at 2 × 107 cells/mL. Chondrocytes derived from human osteoarthritic cartilage are unable to survive for extended periods of time following extraction from knee joints during surgery. Thus, cells utilized for each experiment involving use of chondrocyte breakdown products or treatment of OA chondrocytes were derived from different donors. Variation in the severity of OA and cartilage degradation across donors may have led to differences in the outcomes of each experiment. These variations across patient samples do not alter our interpretations of the results.
Generation of murine and human chondrocyte breakdown products.
Cartilage comprises a dense ECM interspersed with chondrocytes (90). In OA, cartilage damage results in cartilage ECM breakdown and chondrocyte death, leading to the accumulation of both insoluble ECM debris and soluble chondrocyte debris. Cells within articular joints, including synovial fibroblasts, macrophages, and chondrocytes can be stimulated either to perform phagocytosis and/or to produce inflammatory responses (91). Insoluble ECM debris induces phagocytic responses in these cells, while soluble products released by chondrocytes have been postulated to contribute to joint inflammation and damage in OA (22, 23). In order to assess the roles of αVβ3 and CD47 in promoting inflammatory responses contributing to OA, we stimulated both murine and human cells with soluble chondrocyte breakdown products. In order to enrich for chondrocyte-derived products and to minimize insoluble ECM components, we generated lysates from chondrocytes that were isolated from cartilage explants. Primary chondrocytes (2 × 107 cells/mL) isolated from human osteoarthritic cartilage or WT newborn mice were frozen and thawed 3 times to ensure that all the cells were lysed. Intact synoviocytes, macrophages, and chondrocytes were then treated with 2 × 105 cells/mL lysed chondrocytes as described below.
Isolation, culture, and treatment of human primary synoviocytes.
Human primary synoviocytes were isolated from the synovial tissue of OA patients undergoing TKR, in a manner similar to that described above for chondrocytes (89). Samples were cultured in DMEM (Thermo Fisher Scientific) with 10% FBS and 1 mg/mL collagenase IV (Worthington Biochem). Following filtration, synovial cells were seeded at 1 × 105/well in 12-well plates in DMEM-F12 containing 10% FBS and cultured for 3–5 days, changing the media every other day. Adherent synovial cells were then treated with or without 2 × 105 cells/mL lysed chondrocytes, in the absence or presence of 10 μM c(RGDfK) (Elim Biopharm), 1 μM PF-537228, 20 μg/mL anti-CD47 antibody (12), or 1 μM of the SHP-2 inhibitor NSC 87877 (TOCRIS), either for 24 hours for qPCR analysis and ELISA; or for 0, 5, 15, and 30 minutes for Western blot analysis. In a separate set of experiments, adherent synovial cells were treated with or without 2 μg/mL COMP (R&D Systems), in the absence or presence of 10 μM c(RGDfK) or 20 μM anti-CD47 antibody, for 24 hours for qPCR analysis.
Human macrophage experiments.
Human PBMCs from healthy individuals from the Stanford Blood Bank were isolated using ACCUSPIN (MilliporeSigma). Isolated PBMCs were cultured in RPMI with 10% FBS and 25 ng/mL human M-CSF for 14 days, changing cell medium every 2–3 days. Adherent cells were then resuspended, and 2 × 105 macrophages per well were seeded in 12-well plates in RPMI with 10% FBS and 25 ng/mL M-CSF. After 24 hours of culture, adherent cells were treated with 2 × 105 cells/mL lysed chondrocytes, in the absence or presence of 10 μM c(RGDfK), 1 μM PF-537228, or 10 μM CERVIGTGWVRC peptide (a SIRPα inhibitor; Elim Biopharm), either for 24 hours for qPCR analysis or ELISA, or for 0, 5, 15, and 30 minutes for Western blot analysis.
Western blot analysis.
Western blotting was performed with anti–p-SHP2 (catalog 3751)/anti-SHP2 (catalog 3752), anti–p-FAK (catalog 3284)/anti-FAK (catalog 13009), anti–p-Src (catalog 6943)/anti-Src (catalog 2109), anti–p–c-Raf (catalog 9431)/anti–c-Raf (catalog 12552), anti–p-AKT (catalog 4060)/anti-AKT (catalog 9272), anti–p-MEK (catalog 9154)/anti-MEK (catalog 9126), anti–p-ERK (catalog 9101)/anti-ERK (catalog 4695) antibodies from Cell Signaling Technology; and with anti–p-Fyn (Ab182661)/anti-Fyn (Ab125016) and β-actin (Ab8227) from Abcam.
Statistics.
The significance of our data was assessed by unpaired 2-tailed Mann-Whitney U test for nonparametric data sets. Significance of qPCR data was corrected for multiple comparisons. For single comparisons, P < 0.05 was considered significant. For multiple comparisons, P < 0.01 was considered significant.
Study approval.
We studied human samples under protocols approved by Stanford University and the Hospital for Special Surgery Institutional Review Boards, which required obtaining the subjects’ informed consent (informed consent was obtained after the nature and possible consequences of the studies were explained). Synovial fluids were obtained from individuals with clinically confirmed OA (Kellgren Lawrence [KL] scores of 1–4). Synovial membranes and cartilage were obtained from individuals with end-stage OA (KL scores 3–4) of the knee who underwent TKR. Human monocyte-derived macrophages derived from human PBMCs from healthy individuals were obtained from the Stanford Blood Bank. We performed the mouse studies under protocols approved by the Stanford Committee of Animal Research and in accordance with the NIH Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011) or the equivalent.
Data and materials availability.
Data and materials described in this article are available upon request.
Author contributions
QW, CML, JS, ILW, and WHR initiated the investigation. QW, KO, CL, HW, EEE, RRLC, NH, NL, OS, XZ, DHS, CML, JS, RM, CTC, and HR conducted the studies. CRC and NJG provided human samples. SBW, SSP, and ILW provided the anti-CD47 antibody. KO, JS, ZC, and ILW provided scientific input. QW, MSB, RM, and WHR wrote the manuscript. All authors reviewed the data and approved the final manuscript.
Supplementary Material
Acknowledgments
We thank Tamsin M. Lindstrom for editorial input on this manuscript. Our research was supported by US Department of Veterans Affairs grants I01RX002689, I01BX004713 to WHR and US Department of Defense grant W81XWH-18-1-0590 to CRC.
Version 1. 09/19/2019
Electronic publication
Footnotes
Conflict of interest: ILW is a founder of Forty Seven Inc., in which he and SSP own equity.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(18):e128616.https://doi.org/10.1172/jci.insight.128616.
Contributor Information
Qian Wang, Email: qian957@stanford.edu.
Kazuhiro Onuma, Email: 32726u@ube-ind.co.jp.
Changhao Liu, Email: changhao_liu@hotmail.com.
Heidi Wong, Email: heidihw@stanford.edu.
Michelle S. Bloom, Email: msbloom@stanford.edu.
Richard R.L. Cao, Email: caorichard16@gmail.com.
Nick Hu, Email: nickhu103@gmail.com.
Nithya Lingampalli, Email: nlingampalli@gmail.com.
Orr Sharpe, Email: osharpe@stanford.edu.
Xiaoyan Zhao, Email: xzhao1@gmail.com.
Dong Hyun Sohn, Email: dhsohn@pusan.ac.kr.
Jeremy Sokolove, Email: sokolove@stanford.edu.
Rong Mao, Email: rmao@stanford.edu.
Cecilia T. Cisar, Email: ctcisar@stanford.edu.
Harini Raghu, Email: harini.raghugk@gmail.com.
Nicholas J. Giori, Email: ngiori@stanford.edu.
Susan S. Prohaska, Email: ssp@stanford.edu.
Zhen Cheng, Email: zcheng@stanford.edu.
Irving L. Weissman, Email: irv@stanford.edu.
References
- 1.Felson DT, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Rock MJ, Holden P, Horton WA, Cohn DH. Cartilage oligomeric matrix protein promotes cell attachment via two independent mechanisms involving CD47 and alphaVbeta3 integrin. Mol Cell Biochem. 2010;338(1-2):215–224. doi: 10.1007/s11010-009-0355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. J Cell Biol. 1996;135(2):533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang XQ, Frazier WA. The thrombospondin receptor CD47 (IAP) modulates and associates with alpha2 beta1 integrin in vascular smooth muscle cells. Mol Biol Cell. 1998;9(4):865–874. doi: 10.1091/mbc.9.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian J, Zhang FJ, Lei GH. Role of integrins and their ligands in osteoarthritic cartilage. Rheumatol Int. 2015;35(5):787–798. doi: 10.1007/s00296-014-3137-5. [DOI] [PubMed] [Google Scholar]
- 6.Vicente-Manzanares M, Sánchez-Madrid F. Targeting the integrin interactome in human disease. Curr Opin Cell Biol. 2018;55:17–23. doi: 10.1016/j.ceb.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Bouvard D, Pouwels J, De Franceschi N, Ivaska J. Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat Rev Mol Cell Biol. 2013;14(7):430–442. doi: 10.1038/nrm3599. [DOI] [PubMed] [Google Scholar]
- 8.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94(5):625–634. doi: 10.1016/S0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 9.Loeser RF. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol. 2014;39:11–16. doi: 10.1016/j.matbio.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orazizadeh M, et al. CD47 associates with alpha 5 integrin and regulates responses of human articular chondrocytes to mechanical stimulation in an in vitro model. Arthritis Res Ther. 2008;10(1):R4. doi: 10.1186/ar2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119(Pt 19):3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willingham SB, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109(17):6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slack-Davis JK, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282(20):14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 14.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116(pt 8):1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 15.Pfaff M, et al. Selective recognition of cyclic RGD peptides of NMR defined conformation by alpha IIb beta 3, alpha V beta 3, and alpha 5 beta 1 integrins. J Biol Chem. 1994;269(32):20233–20238. [PubMed] [Google Scholar]
- 16.Pang WW, et al. Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc Natl Acad Sci USA. 2013;110(8):3011–3016. doi: 10.1073/pnas.1222861110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima Y, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536(7614):86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wernig G, et al. Unifying mechanism for different fibrotic diseases. Proc Natl Acad Sci USA. 2017;114(18):4757–4762. doi: 10.1073/pnas.1621375114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao MP, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2(63):63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuo Q, Yang W, Chen J, Wang Y. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol. 2012;8(12):729–737. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 21.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11(1):35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sofat N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int J Exp Pathol. 2009;90(5):463–479. doi: 10.1111/j.1365-2613.2009.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20(5):565–572. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 24.van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthr Cartil. 2012;20(3):223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, et al. Peptide-mediated inhibition of neutrophil transmigration by blocking CD47 interactions with signal regulatory protein alpha. J Immunol. 2004;172(4):2578–2585. doi: 10.4049/jimmunol.172.4.2578. [DOI] [PubMed] [Google Scholar]
- 26.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 27.Oh ES, et al. Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol Cell Biol. 1999;19(4):3205–3215. doi: 10.1128/mcb.19.4.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao MP, Majeti R, Weissman IL. Programmed cell removal: a new obstacle in the road to developing cancer. Nat Rev Cancer. 2011;12(1):58–67. doi: 10.1038/nrc3171. [DOI] [PubMed] [Google Scholar]
- 29.Jaiswal S, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majeti R, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao MP, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chao MP, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71(4):1374–1384. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takimoto CH, et al. The Macrophage ‘Do not eat me’ signal, CD47, is a clinically validated cancer immunotherapy target. Ann Oncol. 2019;30(3):486–489. doi: 10.1093/annonc/mdz006. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17(12):1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber R, et al. Identification of intra-group, inter-individual, and gene-specific variances in mRNA expression profiles in the rheumatoid arthritis synovial membrane. Arthritis Res Ther. 2008;10(4):R98. doi: 10.1186/ar2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Attur MG, Dave MN, Clancy RM, Patel IR, Abramson SB, Amin AR. Functional genomic analysis in arthritis-affected cartilage: yin-yang regulation of inflammatory mediators by alpha 5 beta 1 and alpha V beta 3 integrins. J Immunol. 2000;164(5):2684–2691. doi: 10.4049/jimmunol.164.5.2684. [DOI] [PubMed] [Google Scholar]
- 37.Candela ME, et al. Alpha 5 integrin mediates osteoarthritic changes in mouse knee joints. PLoS ONE. 2016;11(6):e0156783. doi: 10.1371/journal.pone.0156783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glasson SS, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 39.Hodivala-Dilke KM, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103(2):229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y, Kang DK, Chang SI, Han MH, Kang IC. High-throughput screening of novel peptide inhibitors of an integrin receptor from the hexapeptide library by using a protein microarray chip. J Biomol Screen. 2004;9(8):687–694. doi: 10.1177/1087057104268125. [DOI] [PubMed] [Google Scholar]
- 42.Bang JY, et al. Pharmacoproteomic analysis of a novel cell-permeable peptide inhibitor of tumor-induced angiogenesis. Mol Cell Proteomics. 2011;10(8):M110.005264. doi: 10.1074/mcp.M110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickinson CD, et al. Crystal structure of the tenth type III cell adhesion module of human fibronectin. J Mol Biol. 1994;236(4):1079–1092. doi: 10.1016/0022-2836(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 44.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin alphavbeta3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3(5):472–487. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 45.Mondal G, Barui S, Chaudhuri A. The relationship between the cyclic-RGDfK ligand and αvβ3 integrin receptor. Biomaterials. 2013;34(26):6249–6260. doi: 10.1016/j.biomaterials.2013.04.065. [DOI] [PubMed] [Google Scholar]
- 46.Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62(3):647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 47.Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40(12):2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 49.Murata Y, Kotani T, Ohnishi H, Matozaki T. The CD47-SIRPα signalling system: its physiological roles and therapeutic application. J Biochem. 2014;155(6):335–344. doi: 10.1093/jb/mvu017. [DOI] [PubMed] [Google Scholar]
- 50.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23(48):7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 51.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4(3):157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44(6):1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 53.Larsson S, Englund M, Struglics A, Lohmander LS. Interleukin-6 and tumor necrosis factor alpha in synovial fluid are associated with progression of radiographic knee osteoarthritis in subjects with previous meniscectomy. Osteoarthr Cartil. 2015;23(11):1906–1914. doi: 10.1016/j.joca.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 54.Fässler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9(15):1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 55.Stephens LE, et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9(15):1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 56.Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997;40(11):2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- 57.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11(2):211–218. doi: 10.1016/S0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 58.Santoro A, et al. SERPINE2 inhibits IL-1α-induced MMP-13 expression in human chondrocytes: involvement of ERK/NF-κB/AP-1 pathways. PLoS One. 2015;10(8):e0135979. doi: 10.1371/journal.pone.0135979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Otero M, et al. E74-like factor 3 (ELF3) impacts on matrix metalloproteinase 13 (MMP13) transcriptional control in articular chondrocytes under proinflammatory stress. J Biol Chem. 2012;287(5):3559–3572. doi: 10.1074/jbc.M111.265744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neff L, et al. ERK 1/2- and JNKs-dependent synthesis of interleukins 6 and 8 by fibroblast-like synoviocytes stimulated with protein I/II, a modulin from oral streptococci, requires focal adhesion kinase. J Biol Chem. 2003;278(30):27721–27728. doi: 10.1074/jbc.M212065200. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki M, et al. The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from the TNF-alpha- or IL-1beta-stimulated rheumatoid synovial fibroblasts. FEBS Lett. 2000;465(1):23–27. doi: 10.1016/S0014-5793(99)01717-2. [DOI] [PubMed] [Google Scholar]
- 62.Feng M, et al. Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. Proc Natl Acad Sci USA. 2015;112(7):2145–2150. doi: 10.1073/pnas.1424907112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng M, et al. Programmed cell removal by calreticulin in tissue homeostasis and cancer. Nat Commun. 2018;9(1):3194. doi: 10.1038/s41467-018-05211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31(3):162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;111(6 pt 1):2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao AG, Lindberg FP, Dimitry JM, Brown EJ, Frazier WA. Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. J Cell Biol. 1996;135(2):533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chevalier X. Fibronectin, cartilage, and osteoarthritis. Semin Arthritis Rheum. 1993;22(5):307–318. doi: 10.1016/S0049-0172(05)80010-1. [DOI] [PubMed] [Google Scholar]
- 68.Ashton BA, Ashton IK, Marshall MJ, Butler RC. Localisation of vitronectin receptor immunoreactivity and tartrate resistant acid phosphatase activity in synovium from patients with inflammatory or degenerative arthritis. Ann Rheum Dis. 1993;52(2):133–137. doi: 10.1136/ard.52.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lapadula G, et al. Integrin expression on chondrocytes: correlations with the degree of cartilage damage in human osteoarthritis. Clin Exp Rheumatol. 1997;15(3):247–254. [PubMed] [Google Scholar]
- 70.Loeser RF, Carlson CS, McGee MP. Expression of beta 1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. Exp Cell Res. 1995;217(2):248–257. doi: 10.1006/excr.1995.1084. [DOI] [PubMed] [Google Scholar]
- 71.Ostergaard K, Salter DM, Petersen J, Bendtzen K, Hvolris J, Andersen CB. Expression of alpha and beta subunits of the integrin superfamily in articular cartilage from macroscopically normal and osteoarthritic human femoral heads. Ann Rheum Dis. 1998;57(5):303–308. doi: 10.1136/ard.57.5.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morphic Therapeutic Web Site. Integrin Overview. http://morphictx.com/our-technology Accessed August 22, 2019.
- 73.Almonte-Becerril M, Costell M, Kouri JB. Changes in the integrins expression are related with the osteoarthritis severity in an experimental animal model in rats. J Orthop Res. 2014;32(9):1161–1166. doi: 10.1002/jor.22649. [DOI] [PubMed] [Google Scholar]
- 74.Gómez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signalling in osteoarthritis--finding targets for candidate DMOADs. Nat Rev Rheumatol. 2015;11(3):159–170. doi: 10.1038/nrrheum.2014.209. [DOI] [PubMed] [Google Scholar]
- 75.Robinson WH, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(10):580–592. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31(4):e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50(9):2811–2819. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 78.Glasson SS. In vivo osteoarthritis target validation utilizing genetically-modified mice. Curr Drug Targets. 2007;8(2):367–376. doi: 10.2174/138945007779940061. [DOI] [PubMed] [Google Scholar]
- 79.Stanton H, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y, et al. A comparative study of radiolabeled bombesin analogs for the PET imaging of prostate cancer. J Nucl Med. 2013;54(12):2132–2138. doi: 10.2967/jnumed.113.121533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su X, et al. Comparison of two site-specifically (18)F-labeled affibodies for PET imaging of EGFR positive tumors. Mol Pharm. 2014;11(11):3947–3956. doi: 10.1021/mp5003043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strand J, Varasteh Z, Eriksson O, Abrahmsen L, Orlova A, Tolmachev V. Gallium-68-labeled affibody molecule for PET imaging of PDGFRβ expression in vivo. Mol Pharm. 2014;11(11):3957–3964. doi: 10.1021/mp500284t. [DOI] [PubMed] [Google Scholar]
- 83.Kamekura S, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr Cartil. 2005;13(7):632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Raghu H, et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis. 2017;76(5):914–922. doi: 10.1136/annrheumdis-2016-210426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Q, et al. Oral and topical boswellic acid attenuates mouse osteoarthritis. Osteoarthr Cartil. 2014;22(1):128–132. doi: 10.1016/j.joca.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blom AB, et al. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr Cartil. 2004;12(8):627–635. doi: 10.1016/j.joca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Watari K, et al. Impaired differentiation of macrophage lineage cells attenuates bone remodeling and inflammatory angiogenesis in Ndrg1 deficient mice. Sci Rep. 2016;6:19470. doi: 10.1038/srep19470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mirando AJ, Dong Y, Kim J, Hilton MJ. Isolation and culture of murine primary chondrocytes. Methods Mol Biol. 2014;1130:267–277. doi: 10.1007/978-1-62703-989-5_20. [DOI] [PubMed] [Google Scholar]
- 89.Honorati MC, Bovara M, Cattini L, Piacentini A, Facchini A. Contribution of interleukin 17 to human cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthr Cartil. 2002;10(10):799–807. doi: 10.1053/joca.2002.0829. [DOI] [PubMed] [Google Scholar]
- 90.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou C, Zheng H, Buckwalter JA, Martin JA. Enhanced phagocytic capacity endows chondrogenic progenitor cells with a novel scavenger function within injured cartilage. Osteoarthr Cartil. 2016;24(9):1648–1655. doi: 10.1016/j.joca.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.