Abstract
MicroRNAs (miRNAs) have received most of the attention over the last decades in particular for their role in tempering gene expression. An increasing number of studies highlighting the importance of miRNAs in the development and progression of atherosclerosis have been performed. Recently, it was shown that miRNAs exert their role in the pathophysiology of atherosclerosis via the regulation of atherosclerosis-prone genes as well as their impact in regulating post-transcriptional gene expression. Hence, by affecting the level of synthesised protein within cells, they may be significant in driving the dysregulation that affects endothelial cells, smooth muscle cells and leukocytes, which initiates and augments the growth of an atherosclerotic plaque. Furthermore, the circulating levels of vascular cell-enriched miRNAs in patients could serve as a marker of disease severity and phenotypes. The accumulating evidence also indicates that their effects on atherosclerosis may allow us to exploit miRNAs as novel therapeutics or clinical biomarkers that may lead to better management of vascular diseases. Current reports providing insights into the impact of miRNAs and the mechanisms of their influences in atherosclerosis are reviewed here with a particular emphasis on studies that have been recently published in Arteriosclerosis, Thrombosis, and Vascular Biology.
Keywords: microRNA, endothelial cells, smooth muscle, atherosclerosis, vascular diseases
Introduction
MicroRNAs (miRNAs) are defined as short, single-stranded, non-coding RNA molecules, which influence the synthesis of proteins, via their interactions with messenger RNAs (mRNAs)1, 2. They are believed to comprise about 1-5% of the human genome and are theorised to be evolutionarily conserved components, which control the post-transcriptional expression of certain genes2–4. Through this action, the miRNA will be able to inhibit, and hence influence, certain protein expression by attaching to and silencing this gene. The miRNA suppresses the gene expression either via cleaving and degrading its subsequent target mRNA or by inhibiting the translation process. The mode of action employed to silence the gene, is determined by the degree of complementarity that occurs between the miRNA complex and its target mRNA5.
Atherosclerosis refers to the chronic inflammatory disease which involves the accumulation of lipid engorged macrophages in the sub-endothelial layer of arterial vessels6, 7. It is widely recognised that monocyte adhesion is provoked by endothelial dysfunction of the arterial wall, upon which they will differentiate into macrophages that will engulf lipoprotein particles to become foam cells8, 9. This process of lipid accumulation induces the very inflammation which drives and augments the atherosclerotic process, resulting in a fatal positive feedback loop10. This response to endothelial injury is also believed to be cause of restenosis, following treatments for severe atherosclerotic disease11. Arteriosclerosis includes the so-called pathology of accelerated atherosclerosis seen after interventions such as heart transplants, coronary vein grafts and percutaneous coronary interventions12. We can consider three processes attributing to the reduction of a lumen size; elastic recoil, arterial remodelling, and intimal thickening. It is believed that the cause of arteriosclerosis, following these procedures, is attributed to the formation of neointima13. The pathophysiology of this lesion development is believed to be aetiologically similar to the vascular injury response which led to the formation of the primary atherosclerotic lesions, and hence there is an accumulation of smooth muscle cells14. Over time, this continuous growth of the neointima can lead to restenosis and, if it results in full occlusion, will cause ischaemia to the distal tissue and muscles served by this artery. Although a significant progress for the pathogenesis of arteriosclerosis has been made, the molecular mechanisms of cell responses are not fully understood.
As miRNAs play an important role in silencing their target genes, which will subsequently reduce their protein synthesis and hence effect the function of the cells, it has therefore been hypothesised that miRNAs may in fact play a role in endothelial injury and consequent cell attachment, growth and inflammatory responses3, 15–17. Moreover, it has been suggested that miRNAs may act as critical regulators of smooth muscle proliferation and phenotypic change18–21 as well as influencing macrophage activity. Thus, by understanding the influence that miRNAs have on these cells driving the progression of arteriosclerosis, their role in the process of vascular disease and potential use within clinical therapy could be important.
Biogenesis and Target Recognition of miRNAs
MicroRNA biogenesis is a multistep process, beginning from the initial transcription of primary miRNA, which has a hairpin/stem-loop structure. After being transcribed with RNA polymerase II or RNA polymerase III, primary miRNAs were first processed in the nucleus through Drosha/DGCR8 microprocessor complex in a sequence independent but structural-dependent manner to form the precursor miRNA around 60 to 80 nucleotides long22. Afterwards, precursor miRNAs were actively exported to the cytoplasm with Exportin-5 in a Ran-GTP-dependent manner. In the cytoplasm, the transition of GTP to GDP aids to release the precursor miRNAs from Exportin-5. Subsequently, Dicer works cooperatively with TRBP to cleave the precursor miRNA and generate mature miRNAs, which will be incorporated into the RISC complex and then bind to the 3’-untranslated region (3’-UTR) of the target sequence in a complementary manner to induce target mRNA degradation or translation inhibition.
It was determined that the 5’ end of miRNA was more conserved than the 3’ end, with this conservation significantly contributing to better target prediction. Further sequence alignment across different species showed a frequently conserved A in the target mRNA 3’-UTR immediately downstream of the sequence that was complimentary to the seed site23. This conserved A was named A1. Since the nucleotide A could not always bind to the first nucleotide of miRNA in a Watson-Crick base pair matched manner, it was proposed that this conserved nucleotide A could be recognised by proteins in the RISC. Collectively, the target sites described could be separated into 2 groups according to their location on miRNAs. The first group mainly includes the seed site centred ones located at the 5’ end of miRNAs: 8mer site, 7mer-A1 site, 7mer-m8 site and 7mer site, which could be called canonical sites of miRNAs for target prediction. Efforts have also been invested to identify 6mer and offset 6mer sites24. The second group was 3’ supplementary site and 3’ compensatory site, which, as suggested by the nomenclature, locates at the 3’ end of miRNAs.
Apart from the differing sites on miRNAs, including the canonical ones and the non-canonical ones, “seedless” inhibition of miRNA on mRNA was also observed, implying that the whole system of miRNA regulation mechanism might be more complex than we have already described. E2F2 gene could be directly inhibited by miR-24 through seedless 3’-UTRs25. Additionally, as aforementioned, perfect canonical seed sites were not sufficient to predict the functional interaction between miRNA and its target mRNAs. It must be considered that there may also be other factors influencing the interaction process. Argonaute 2 protein is an important component of the RISC complex. It was demonstrated by structural studies that it displayed a flexible structure which could be stabilised by miRNAs binding to it26. Another element that influences the interaction process, is the mRNA accessibility to binding sites27. Binding of the target site on mRNAs with RNA binding protein (RBP) or the inclusion of target site within the stem loop structures of mRNA itself would prevent the target site from being accessible to miRNAs. On the other hand, factors that promote the release of RBP from binding to complimentary sequences, could form the stem loop structure with the target sites that would increase the accessibility of the mRNA target site. The better binding affinity of RISC with target sites, could help release other translational repressors from the target site to increase their accessibility27.
miRNAs and Endothelial Cells
It has been widely accepted that the initial trigger for the formation of atherosclerosis is mediated by the dysfunction of endothelial cells (ECs)28. ECs have many roles within vascular biology, including their production of various factors that influence the aggregation of platelets, smooth muscle growth, inflammation as well as vascular tone29. By introducing the endothelial layer to various noxious stimuli, such as proinflammatory cytokines, hyper-tension/lipidaemia, alteration in fluid shear stress or increased senescence, will have ramifications of increased cell activation and dysfunction28, 30, 31. The activated EC, will begin to express a higher level of adhesion molecules, which will trap leucocytes, such as monocytes and T-cells, and thus the scene has been set, for the continued progression of an atherosclerotic plaque28, 30, 32, 33. MicroRNAs, as they play a crucial role in influencing the gene expression and overall function of ECs, must also be considered as participants in guiding the detrimental effects of damaging the monolayer34.
One way in which miRNAs affect the dysfunction of ECs is through their role in exacerbating endothelial cell senescence, a cellular state which has been attributed as increasing the likelihood of atherogenesis35, 36. There are several miRNAs, including miR-34a, miR-217 and miR-146a, which have been associated with regulating mechanisms involved in EC senescence37. It has been shown that miR-34a influences the proliferation and differentiation amongst many types of cells, including primary ECs, where the level of its expression increases during cellular senescence36–38. This has been attributed to the concept that an overexpression of miR-34a in EC decreases Sirtuin-1 [SIRT1] levels. SIRTI1, is a crucial regulator gene of longevity, which acts by protecting cells against oxidative and genotoxic stress and thus preventing stress-induced senescence36, 38. Various studies have noted an increased level of miR-34a in atherosclerotic arteries, which may be due to this miRNA exacerbating EC dysfunction36. Another miRNA which has been linked to influencing the expression of SIRT1, is miR-217. This is a miRNA expressed in both human aortic and coronary artery ECs and has been found to be progressively expressed in the cells of the endothelium during aging. It targets the 3’UTR of SIRTI1 to inhibit its protein expression and thus reduces its gain of function effects, to improve longevity and vascular disease resistance35, 39, 40. MiR-200c has also been shown to affect, in response to reactive oxygen species (ROS)41, the expression of proteins that influence proliferation arrest, apoptosis and senescence within certain vascular endothelium. They are said to target zinc finger E-box binding homeobox 1, whose reduced expression will influence the pathways responsible for hastening EC senescence42 (Figure 1).
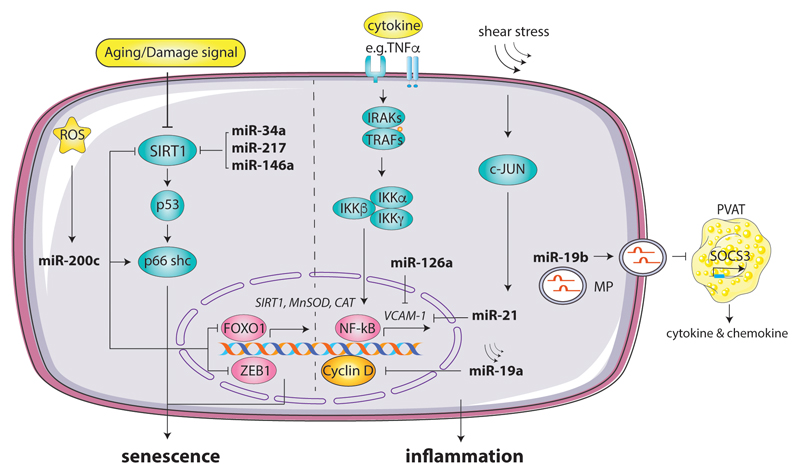
Figure 1. MiRNAs regulate endothelial senscense and inflammation in response to cytokines, metabolic stimuli and disturbed flow.
MiRNAs, such as miR-34a, miR-217, and miR-146, mediate proatherotic factors induced suppression of the key anti-senesence factor Sirtuin 1 (SIRT1) transcript in ECs. Reactive oxygen species (ROS) sensitive miRNA such as miR-200c mediated inhibition of SIRT1, MnSOD and CAT and enhancement in endothelial sensense by downregulating zinc finger E-box binding homeobox 1(ZEB1) or Forkhead box protein O1(FOXO1) transcription. Shear stress enhanced miR-19a impairs the cellular self-repair by inhibiton of cyclin D. Endothelial miR-19a is also transferred in microparticles and mediates proinflammation response in neighboring adipocytes in a paracrine manner. Moreover, disturbed flow and hyperlipidemia decrease miR-21 and mir126 expression in ECs, resulting in derepression of NF-kB medicate VCAM-1 production, which triggers inflammation.
Moreover, as aforementioned, we are aware that several noxious stimuli will result in an increased inflammatory response being induced within the endothelial layer. It has also been suggested that miRNAs are able to control the inflammatory state of vasculature, by influencing the activation and infiltration of leukocytes via the vascular wall34, 43. A key one involved is miR-126, which has been shown to be important in affecting both vascular dysfunction and its inflammatory state42. Studies have shown that miR-126 inhibits vascular cell adhesion molecule 1 (VCAM-1). Thus, an inhibition of miR-126, would result in an increase expression of proinflammatory TNF-α expression, which will increase the activity of NF-κB and stimulate the activity of VCAM-1, resulting in increased leukocyte-EC interactions that drive the formation of lesions29, 44–46. It has also been noted that the expression of different miRNAs will change in response to an alteration in flow patterns within the vasculature46. An introduction of laminar shear stress increases the level of miR-19a, which inhibits the expression of cyclin D1, by binding to its 3’ UTR. This results in the EC being kept in a low proliferative state and is hence unable to repair itself47, 48. MiR-21 was also induced in response to a disruption in flow pattern and is believed to be responsible for increasing the levels of several pro-inflammatory targets, including VCAM-1, which will enhance the dysfunction of the endothelial layer to attract leukocytes, further increasing the likelihood for the initiation of an atherosclerotic plaque34, 42 (Figure 1).
It is also worth exploring the effect of microparticles, which are the main carriers of miRNAs and are predominantly derived from endothelial cells and platelets. A study noted that introduction of an increased level of microparticles carrying MiR-19b, was associated with augmenting the progression of atherosclerosis, by stimulating high levels of macrophage, lipid, and smooth muscle content to enter atherosclerotic plaques49. It is understood that MiR-19b, carried by endothelial derived microparticles, promotes inflammation within perivascular adipose tissue, by down-regulating the expression of its target gene to translate SOCS3. This is a signal transduction inhibitory molecule, which is crucial to negatively regulate the JAK/STAT pathway, to control the inflammatory process occurring within vascular cells. Therefore, by inhibiting the activity of SOCS3 within perivascular adipose tissue, MiR-19b promotes the secretion of inflammatory cytokines and macrophage intrusion into the endothelial layer, promoting the progression of atherosclerotic lesion49.
miRNAs and Smooth Muscle Cells
Smooth muscle cells (SMCs) play a key role in maintaining the functions on vasculature. An alteration leading to apoptosis, senescence or phenotypic changes have all been attributed to promoting inflammation and vascular disease progression50. One particular phenotypic change which SMCs can undergo is the conversion from a ‘contractile’ to a ‘secretory’, which results in SMCs displaying different markers and exhibiting cell functions similar to those of a macrophage51. This phenotypic switching of SMCs may exacerbate atherosclerosis by having an induced ability to take up lipids and necrotic debris, as well as having an impaired autophagy control; all of which will promote the inflammatory environment necessary for the progression of atherosclerosis and hypertension10, 52 53.
Recently, it has been indicated that miR-22 influences both phenotypic modulation and neointima formation involving SMCs with the level of expression of this miRNA, differing between both phenotypic states20, 54, 55. The varying levels of miR-22, was believed to be transcriptionally regulated through the action of TGF-β1, most likely involving a p53-dependant mechanism. Observations of SMCs transfected with miR-22 inhibitor, demonstrated an increased ability of growth, proliferation, and migration. MiR-22 is believed to influence phenotypic change by influencing the expression of three genes: MECP2, HDAC4 and EVI1. It is well understood that miR-22 targets MECP2 during the process of SMC differentiation from stem cells. The inhibition of both MECP2 and HDAC4 in SMCs, mimics the effect of an over-expression of miR-22, during phenotypic modulation. The target gene EVI1, also has its synthesis suppressed via the binding of miR-22 to its 3’UTR. It is believed that EVI1, acts as an inhibitory influence on the expression of 5 SMC genes and 2 transcription factors, one of which has been shown to act as a transcriptional repressor of contractile genes within the muscle cells54. It has thus been concluded that miR-22 does carry an influence over the change of SMCs, from a contractile to synthetic nature, most likely through the effects of the suppression of its various target genes, MECP2, HDAC4 and EVI150, 54.
Other known microRNAs responsible for regulating SMC differentiation include miR-143 and miR-145, both of which are decreased in diseased vessels. This suggests that a reduction in the expression of these microRNAs may contribute to the dedifferentiation of SMCs that influence the formation of the lesion responsible for the progression of atherosclerosis56, 57. Accelerated SMC proliferation can be discerned in both vascular injury and in the early formation of an atherosclerotic plaque50, 58. Mir-21 has been associated with enhancing proliferation and influencing apoptosis in SMCs and has also been associated with being crucial for the expression of SMC specific genes52, 59. It has been observed that diseased vessels, release a myriad of factors involved in the progression of vascular disease, including the aforementioned NFƘβ and angiotensin II, which induces the expression of miR-2156. It is believed that tropomyosin 1 (TPM1) is a target gene of miR-21 within SMCs. TPM1, is part of a family of actin-binding proteins which are essential for the maintenance of the contractile unit, as well for the regulation of the shape and function of the SMCs. Therefore, as miR-21 will down-regulate TPM1 expression, there will be a loss of cytoskeletal stability, which is believed to be responsible for the increased proliferation and migration59. Other miRNA which have been associated with inducing proliferation in SMCs, include miR-26a, miR-34a, miR-130a, miR-22118, however their contribution towards the formation of vascular lesions are yet to be determined (Figure 2).
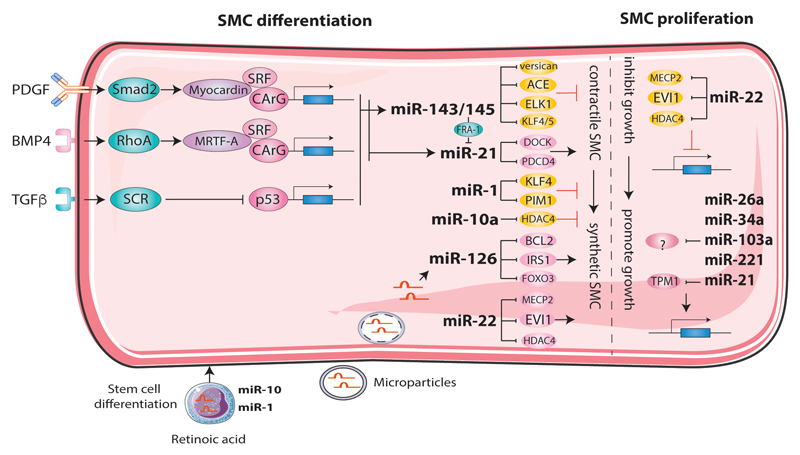
Figure 2. The role of miRNAs in proliferation and differentiation of smooth muscle cell (SMC) in atherosclerosis.
During mechanical, metabolic and inflammation stress, upregulated miR-22 inhibits SMC proliferation and carries SMC transformation from a contractile to synthetic nature. The upregulated miR-21 inhibits tropomyosin 1 (TPM1) expression, which can increase SMC proliferation. During atherosclerosis, microRNAs are induced in SMC and further participate in regulating SMC differentiation either from a contractile to synthetic or vice versa, therefore modulate the progression of disease. miR-143/145, miR-1 and miR-10a are able to halt the diffentiation to synthetic phenotype by suppressing various genes as indicated in the figure. While, miR-126, miR-21 and miR-22 could facilitate the transition and promote atherosclerosis. The increase of miR-126 could also be as a result of microparticle mediated transmission from neighbouring EC. MiR-1 and miR-10 were increased during embryonic stem cells to SMC differentiation.
miRNAs in SMC Differentiation
Recently, accumulating evidence indicates a potential contribution of vascular resident stem cells to SMC accumulation in atherosclerotic lesions60–63. These stem/progenitor cells can migrate into the intima where they differentiate into SMCs via chemokines and cytokines64–70. microRNAs are necessary for the differentiation of SMCs as demonstrated by the study of Dicer deletion experiments which resulted in the failure of microRNA maturation71, 72. Dicer deletion in vascular SMCs caused embryonic lethality at day 16 to 17, with extensive internal haemorrhage generated by reduction of SMC proliferation and impaired contractility which could be partly rescued by overexpression of miR-14571.
MiR-143/145
Both in vitro and in vivo loss-of-function studies have shown that miR-143/145 is important for SMC differentiation73, 74. Through the repression of antagonistic factor (Klf4, Klf5, Elk1, versican and angiotensin converting enzyme) gene expression in the SMC differentiation process, miR-143/145 facilitates expression of SMC specific genes75–79. MiR-143 and miR-145 are expressed in proximity in chromosome 5 and share the same promotor which contains CArG elements, as with most contractile SMC specific genes. Thus miR-143/145 is regulated in an SRF-CArG-dependent pathway. Briefly, for the induction of miR-143/145, TGFβ signals through the Smad2/3 pathway and Myocardin is required to promote binding of SRF to the CArG element, and BMP4 signals through RhoA pathway and MRTF-A translocation to the nucleus is required76. PDGF-BB, which is known as a synthetic SMC phenotype inducer, activates Src through PDGF receptor and as a result, p5380, which is an inducer of miR-143/145 is repressed81. In summary, miR-143/145 is critical for SMC differentiation.
MiR-1
MiR-1 was observed to be steadily increased in the differentiation process from embryonic stem cells to SMCs and loss-of-function study showed that miR-1 was essential to the process. MiR-1 induces SMC differentiation through the repression of Klf482 and Pim-1 (a serine/threonine kinase)83, which also serves as a negative regulator of SMC differentiation. Upstream regulators include Myocardin, whose overexpression resulted in significant induction of miR-183. However, contradictory data was also observed which demonstrated that exogenous miR-1 inhibited contractility of SMCs and repressed the expression of contractile proteins84. Further studies are needed for the elucidation of miR-1 function in SMC differentiation.
MiR-21
MiR-21 was also involved in TGFβ- and BMP4-induced SMC differentiation by downregulating programmed cell death 4 (PDCD4), which is a repressor of SMC contractile genes22. Dedicator of cytokinesis (DOCK) protein superfamily was also identified as the targets of miR-21, whose downregulation inhibited cell migration85. Mature miR-21 could be increased by the recruitment of TGFβ and BMP4 specific SMAD signal transducers to the DROSHA complex which facilitated the processing of pri-miR-21 to precursor miR-2122. However, there was still evidence that miR-21 could be repressed by increased miR-143 through repression of fos-related antigen 1 (FRA-1) in SMC differentiation86 suggesting the complex regulatory role of miR-21. MiR-21 has also been investigated in many tissues outside of the vasculature and its function is suggested to be highly context-dependent87.
MiR-10a
Retinoic acid induces SMC differentiation from embryonic stem cells during which process miR-10a was increased88. This increase is the direct result of retinoic acid-induced nuclear translocation of NF- κB. Upregulated miR-10a binds to the 3’-UTR of HDAC4 which is a negative regulator of SMC differentiation88. The regulatory function of microRNAs in SMC differentiation is summarised in Figure 1-10.
Others
Other microRNAs that contribute to SMC differentiation, proliferation and migration include miR-10089 and miR-125b90, 91. Furthermore, various microRNAs are involved in the promotion of a synthetic phenotype of SMCs, such as miR-2492, miR-26a58, miR-3193, miR-146a94, miR-20495, miR-20896 and miR-22197. In summary, microRNAs play a critical role in SMC differentiation, and represent an important therapeutic choice for cardiovascular diseases98.
miRNAs and Monocytes/Macrophages
The progression of atherosclerosis is driven through the accumulation of lipid-laden macrophage foam cells in the inner arterial wall, which will fuel a state of chronic inflammation that promotes the formation of the plaque99. In order to maintain lipid homeostasis, phagocytosis of lipid residues is critical, and dysregulation can drive various pathological diseases, including atherosclerosis59, 99. One key miRNA involved in lipid phagocytosis is miR-33, which is believed to down-regulate crucial effectors and transcriptional activators including FOXO3 and TFEB, resulting in reduced phagocytosis and lysosomal activity within macrophages. Furthermore, miR-33 also targets ABCA1, which results in the restriction of lipid residue metabolism and cholesterol efflux out of macrophages, potentially influencing the growth of atherosclerotic plaques, due to their effects against macrophage autophagy99, 100. Conversely, miR-302 is also believed to regulate the activity of ABCA1, and promotes the efflux of cholesterol, to act as a potential therapeutic agent to decrease the progression of lipid-laden atherosclerotic plaques99.
It is a well-understood concept that during times of increased stress, a higher abundance of energy substrates is crucial to mount an effective counter-response. For macrophages involved in an atherosclerotic plaque, this stress comes in the form of an accumulation of cholesterol that it must attempt to remove via effective efflux pathways100, 101. This pathway is dependent on efficient and lucrative mitochondrial ATP production. Thus, any dysregulation to this process, within a damaged vessel, can spur on the accumulation of lipid droplets within macrophages, building up the foam cells that fill the growing atherosclerotic plaque. MiR-33 has also been associated with assisting in the down-regulation of HADHB and CROT, both of which are involved in fatty acid oxidation, a crucial component of cellular energy metabolism within a macrophage. Thus, it has been noted, that an inhibition of miR-33, will de-repress certain genes involved in mitochondrial ATP synthesis, which may allow for higher cholesterol efflux out of macrophages, aiding to prevent the progression of an atherosclerotic plaque100, 102.
MiR-21 is found to be highly expressed in the monocyte cellular compartment, particularly in bone-marrow derived macrophages103. It is believed that miR-21 is a crucial signalling mediator between normal and inflammatory states =and that dysregulation of this microRNA may stimulate an intravascular environment which can drive the progression of atherosclerosis104. This has been attributed to miR-21 negatively regulating the expression of various pro-inflammatory mediators including lipopolysaccharides and tumour necrosis factor alpha (TNF-α), to resolve inflammation. Moreover, it is believed that miR-21 also influences the formation of foam cells, macrophage apoptosis and the clearance capacity of phagocytes105. It has been found, that an absence of miR-21 increases the expression of its target gene, MKK3, which will stimulate the induction of both p38 and the JNK signalling pathway. This will encourage macrophage apoptosis as well as down-regulating the expression of ATP-binding cassette transporter G1 (ABCG1). This is a crucial transporter involved in the efflux of cholesterol within macrophages and hence its dysfunction will promote the development of foam cells, thus building up the atherosclerotic plaque99.
Inflammatory macrophages are also known to secrete vesicles, which may carry RNA, protein, or lipids for the purpose of extracellular communication. The transfer of particular extracellular vesicles, carrying miRNAs, has been noted between the cells of atherosclerotic vessels. Some of the confirmed miRNAs that were expressed in atherogenic extracellular vesicles, include miR-146a, miR-128. miR-185, miR-365 and miR-50359, 106, 107. These were all noted to reduce the migration of macrophages. In particular, miR-146a, which had the highest level of expression within atherogenic extracellular vesicles, seemed to greatly affect the ability of the macrophage to migrate. The target genes that were down-regulated by miR-146a, included Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) and human antigen R (HuR)108, which significantly reduces the macrophages ability to migrate109. This can drive the progression of atherosclerosis, by encouraging the entrapment of macrophages within the vessel wall (Figure 3).
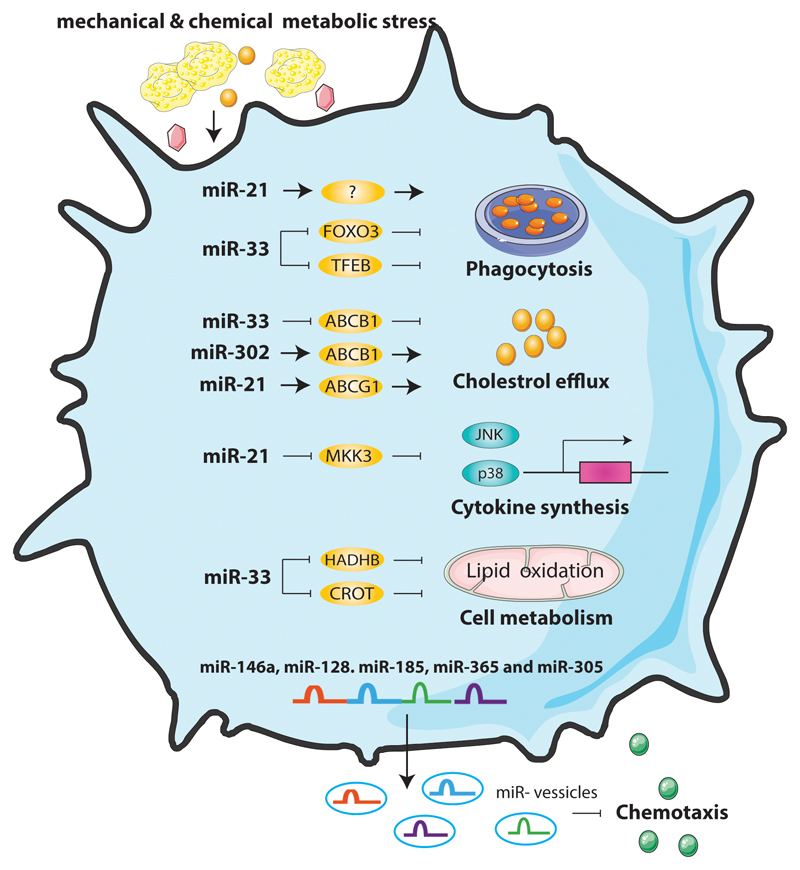
Figure 3. The role of miRNAs in regulation of macrophage functions in atherosclerosis.
During atherosclerosis progression, miR-33 expression levels increase in lesional macrophages, suppressing lipid phagocytosis, cholesterol efflux and mitochondrial fatty acid oxidation, thus promoting foam cell formation and inflammation responses. The increased miR-21 levels protect against atherosclerosis by inhibiting the hyperlipidemia-induced cytokine synthesis and promoting cholesterol efflux. MiR-21 also facilitates higher cholesterol efflux out of macrophages. MiRNAs expressed in extracellular vesicles, such as miR-146a, miR-128. miR-185, miR-365 and miR-503, are able to reduce the migration of macrophages, thus attenuating the progression of atherosclerosis.
Circulating miRNAs
The role of miRNAs as potential biomarkers of vascular disease has been accredited to their high stability and reliable detection within biofluids110. When microRNAs are carried within the circulation via extracellular vesicles, bound to HDL cholesterol particles or to AGO-2 proteins, they are provided with a higher stability against circulating RNAses39, 111. Moreover, it was noted that the levels of miRNAs within the pre-packaged extracellular vesicles, differ from that of the intracellular environment from which the vesicles were expelled. This suggests a process of selective packaging, which may allow us to link the varying miRNAs levels to different diseases. Thus far, circulating miRNAs have been investigated as being effective biomarkers to determine the significance of cardiovascular disease, as well as aiding with the diagnoses of a myocardial infarction112.
The detection of the levels of circulating miRNAs after a complication of vascular disease, has allowed for the consideration of several potential biomarkers. It was observed that several cardiac specific miRNAs, including miR-208, miR-1, miR-133a and miR-133b, were upregulated in patients who had suffered a ST-elevation of myocardial infarction15, 16, 113. Most notably, miR-208 was elevated 4 hours post-myocardial infarction, in all patients that were investigated; increasing the potential of this miRNA to be used as a future biomarker to detect an early myocardial infarction114–116. MiR-155, was also detected at higher levels within the plasma of patients suffering from higher risk coronary syndrome, with an increased level being detected for acute myocardial infarction or unstable angina, rather than those suffering of chest pain syndrome117. Therefore, it is imperative that we continue to gather further data, so that we may be able to solidly differentiate between the sharp rise of circulating miRNAs, seen after an acute coronary syndrome event, and the persistent increased levels that are already present during stable coronary artery disease16 (Table 1).
Table 1. Selected studies on circulating miRNAs and vascular diseases.
| microRNA Increase | microRNA decrease | Disease | Reference |
|---|---|---|---|
| miR-126, miR-223 | miR-122 | ACS | Kaudewitz et al119 |
| miR-21, miR-130a miR-210 |
miR-221, miR-27b miR-222 |
Ischemic limbs |
Li et al121 |
| miRNA-28-3p | miR-15a, miR-126 miR-223 |
Diabetes | Zampetaki et al122 |
| miR-16, miR-20b miR-25, miR-26b |
miR-34 | PAD | Stather et al123 |
| miR-34a, miR-423 | miR-21-3p | HF | De Rosa et al124 |
ACS, acute coronary syndrome; PAD, peripherial artery disease; HF, heart failure.
The assessment of the level of miRNA expression during the presence of these stable plaques, may act as a viable biomarker to assess the severity of the present vascular disease. For example, miR-210 has been detected at lower levels, within the plasma, at the site of carotid artery stenosis. It is believed that the decreased expression of this miRNA, and hence an increased activity of its target gene, results in the substantial reduction of the stability of the fibrous cap overlying an atherosclerotic plaque. Thus, it has been speculated that miR-210 may have a clinical applicability to assess the risk of a carotid artery plaque, particularly in asymptomatic carotid atherosclerosis118.
Moreover, it was identified that platelets contributed to the largest number of circulating miRNAs15. Despite a platelet being enucleated, they possess the intracellular components required to synthesis mature miRNAs from pre-miRNAs. It is known that platelet reactivity is a significant risk factor for thrombotic complications associated with vascular disease. It has been shown that several target genes of various miRNAs regulate platelet function and influence the interaction between circulating cells and vascular endothelium, both factors which can affect the process of cardiovascular diseases such as atherosclerosis. MiRNAs including miR-22, miR-185 and miR-320b, were seen to be upregulated in the distal thrombi, suggesting a transfer of these miRNAs from the original site of platelet aggregation106, 119. The levels of circulating miRNAs were shown to be affected by the use of anti-platelet therapy, including the reduction of miR-223, miR-126, miR-191 and miR-150, after a three week use of both prasugrel and aspirin15, 16, 120. This creates a clinical opportunity for the use of miRNAs to monitor the efficacy of antiplatelet therapy, which is crucial to determine the risk reduction of thrombotic complications within vascular disease. The involvement of circulating miRNAs in vascular diseases has been selectively summarised in Table 1119, 121–124.
miRNA and Atherosclerosis
As aforementioned, atherosclerosis is a chronic inflammatory disease involving the arterial wall, that is characterised by the stenosis of the vascular lumen due to the formation of lipid engorged plaques14. The initiation of an atherosclerotic lesion is as a result of damage to the inner endothelial layer, which results in a phenotypic alteration that increases the expression of adhesion molecules released by ECs. This will result in the increased binding of circulating monocytes to the site of the activated ECs. Here ECs and leukocytes will use the NF-ƘB signalling pathway, to increase the expression of inflammatory genes, including various adhesion molecules, chemoattractant and cytokines, which will stimulate the inflammation of the arterial vessel125. It is due to this orchestra of phenotypic changes and altered gene expression that we have thus far been able to explore the significant impact that miRNAs may have on the progression of atherosclerosis117. It was observed that several miRNAs such as miR-34a, miR-217 and miR-146a, regulate the proliferation and differentiation of ECs and may stimulate cellular senescence, which can act as at trigger for endothelial dysfunction17, 45, 47. The inflammatory state of the vasculature can also be influenced by several miRNAs, including miR-126, which effects both the expression of pro-inflammatory cytokines and the VCAM-1 adhesion molecule that recruits the monocytes which will form the foam cell 126, 127. MiR-19a was also noted to cause a decreased proliferative EC state, increasing the susceptibility of the endothelial layer to vascular injury due to its reduced ability to repair itself45, 46.
Once again, throughout the progression of these steps, miRNAs were also shown to influence the formation of foam cells and the subsequent plaque formation3, 118. It has been observed that miRNAs influenced cholesterol homeostasis, via their effects on the ATP energy availability necessary for cholesterol efflux101, 111. Moreover, both miR-33 and miR-302a, were shown to regulate the expression of the ABC transporters necessary for the efflux of the accumulated cholesterol within macrophages4, 100, 128. The migration and autophagy of intra-arterial macrophages was also shown to be affected through the down-regulation of the target genes affected by miRNAs, factors which both influence the progression of an atherosclerotic plaque. The stability of a plaque has also been speculated to be affected by varying levels of circulating miRNAs. For example, an increase level of miR-155 was detected in more unstable and inflamed plaque lesions. Conversely, miR-210 was found at lower levels within plasma sites at unstable plaques, where direct delivery of miR-210 has been shown to lower cardiac injury117.
Thus, it is important to note that though there are several miRNAs whose regulation can exacerbate an atheroma formation, there are also several miRNAs that affect the expression of their target genes in manners that act to decrease the progression of atherosclerosis. These include, miR-126 which signals for endothelial repair and may prevent the initial trigger for the atheroma formation. This microRNA has also been shown to inhibit the expression of VCAM-1, which will subsequently reduce the interaction between ECs and leukocytes129. MiR-302a has been shown to increase the activity of ABCA1, which encourages the efflux of cholesterol out of macrophages, preventing the formation of the foam cells that would have augmented the growth of the atheromatic plaque105, 118. Furthermore, miR-143 and miR-145, known to be responsible for regulating SMC differentiation, are decreased in diseased vessels, and introduction of them into the vessel wall are believed to prevent the dysregulation of the SMC phenotype, which drives the progression of atherosclerosis18, 19, 53, 118. Hence, we can determine that there are also several miRNAs whose influence over the expression of their target genes, reduces the dysregulation of ECs130, monocytes and SMCs, thus aiding to prevent the progression of atherosclerosis.
Summary and Perspective
MiRNAs ability to regulate protein expression results in their indisputable influence over the initiation and progression of atherosclerosis. We have been able to determine the potential influence of several miRNAs, by linking their target genes to the subsequent effects that occur as a result of lowering the expression of their respective proteins. This has been shown to impact the phenotypic behaviour of several key players within atherosclerosis including that of ECs, SMCs and macrophages, whose dysregulation will initiate and drive the growth of an atherosclerotic plaque.
The understanding of these effects of miRNAs on the specific pathways that lead to atherosclerosis of a vessel, may allow for the development of miRNA based biologic therapy against cardiovascular disease. So far, current miRNA therapy focuses on systemic administration of either anti-miRNA delivery, to decrease the levels of certain microRNAs, or the introduction of synthetic miRNAs, known as miRNA mimics, that are used to increase the levels of those miRNAs which are seen to be down-regulated in some diseases131. For instance, the introduction of miR-126, which is highly concentrated within ECs, has been shown to be enhance the expression of endothelial growth factors and hence is believed to have the potential of being used to stimulate endothelial repair129. Such a therapy, creates the possibility of the earliest form of combat against atherosclerosis: the inhibition of the initial trigger, injury of the endothelial layer, to prevent the subsequent inflammation and attraction of monocytes that will drive the formation of the plaque upon this site of injury. However, it is also important to realise that the current systemic delivery of miRNA comes with the concern of off-target stimulation, which may cause various unpredictable and unwanted side-effects. Therefore, further research must be committed to the method of miRNA administration, perhaps through the insertion via adeno-associated virus vectors, to ensure precise miRNA delivery to the intended site of existing pathology.
The role of miRNAs as potential biomarkers must also be carefully considered. As we have seen, many clinical opportunities exist for the use of circulating microRNAs to determine the presence and progression of vascular disease. This provides us with the ability to differentiate between the level of stenosis, stability of an atherosclerotic plaque and for an early diagnosis of an acute myocardial infarction. It is important to claim the potential of miRNAs, by ensuring that research focuses on the use of them as biomarkers for unmet clinical needs, and not in areas where other plasma markers are sensitive to the same pathology. One of the greatest areas of potential for miRNAs, lies with its ability to determine the severity of vascular disease and hence may be used to determine the level of risk which the patient may be suffering from. This includes the assessment of miR-155, which has been shown to be expressed at increased levels in high-risk diseased vessels versus those with less stenosis or more stable plaques. The same has been shown through the varying expression of miR-210, which is believed to be expressed at higher levels at the site of more stable carotid plaques131. Changes in miRNA expression was also noted after the use of anti-platelet therapy, which may fulfil the clinical need to monitor the effects of this treatment amongst patients and be used as a marker to determine when a satisfactory risk reduction has been achieved. We can see that miRNAs, despite their infancy amongst the research community, have already begun to implement themselves within the aim to provide patients with earlier diagnoses and better therapeutics to combat the progression and potential complications of atherosclerosis.
Sources of Funding
This work is supported by British Heart Foundation (RG/14/6/31144).
Abbreviations
- EC
endothelial cells
- miRNA
microRNA
- ROS
reactive oxygen species
- SMC
smooth muscle cells
- VCAM
vascular cell adhesion molecule
Footnotes
Disclosures
None.
Contributor Information
Yao Lu, Center of Clinical Pharmacology, Third Xiangya Hospital, Central South University, Changsha, China.
Jingjing Cai, Department of Cardiology, Third Xiangya Hospital, Central South University, Changsha, China.
Qingbo Xu, School of Cardiovascular Medicine and Sciences, King’s College London BHF Centre, London, UK.
References
- 1.Thum T, Mayr M. Review focus on the role of microRNA in cardiovascular biology and disease. Cardiovasc Res. 2012;93:543–544. doi: 10.1093/cvr/cvs085. [DOI] [PubMed] [Google Scholar]
- 2.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 3.Maitrias P, Metzinger-Le Meuth V, Nader J, Reix T, Caus T, Metzinger L. The Involvement of miRNA in Carotid-Related Stroke. Arterioscler Thromb Vasc Biol. 2017;37:1608–1617. doi: 10.1161/ATVBAHA.117.309233. [DOI] [PubMed] [Google Scholar]
- 4.Zampetaki A, Mayr M. MicroRNAs in vascular and metabolic disease. Circ Res. 2012;110:508–522. doi: 10.1161/CIRCRESAHA.111.247445. [DOI] [PubMed] [Google Scholar]
- 5.Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153:516–519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015;278:483–493. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116:307–311. doi: 10.1161/CIRCRESAHA.116.301313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vozenilek AE, Navratil AR, Green JM, Coleman DT, Blackburn CMR, Finney AC, Pearson BH, Chrast R, Finck BN, Klein RL, Orr AW, et al. Macrophage-Associated Lipin-1 Enzymatic Activity Contributes to Modified Low-Density Lipoprotein-Induced Proinflammatory Signaling and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:324–334. doi: 10.1161/ATVBAHA.117.310455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Violi F, Carnevale R, Loffredo L, Pignatelli P, Gallin JI. NADPH Oxidase-2 and Atherothrombosis: Insight From Chronic Granulomatous Disease. Arterioscler Thromb Vasc Biol. 2017;37:218–225. doi: 10.1161/ATVBAHA.116.308351. [DOI] [PubMed] [Google Scholar]
- 10.Tabas I. 2016 Russell Ross Memorial Lecture in Vascular Biology: Molecular-Cellular Mechanisms in the Progression of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:183–189. doi: 10.1161/ATVBAHA.116.308036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong RJ, Paulin N, Lemnitzer P, Viola JR, Winter C, Ferraro B, Grommes J, Weber C, Reutelingsperger C, Drechsler M, Soehnlein O. Protective Aptitude of Annexin A1 in Arterial Neointima Formation in Atherosclerosis-Prone Mice-Brief Report. Arterioscler Thromb Vasc Biol. 2017;37:312–315. doi: 10.1161/ATVBAHA.116.308744. [DOI] [PubMed] [Google Scholar]
- 12.Stary HC. The development of calcium deposits in atherosclerotic lesions and their persistence after lipid regression. Am J Cardiol. 2001;88:16E–19E. doi: 10.1016/s0002-9149(01)01713-1. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz RS, Henry TD. Pathophysiology of coronary artery restenosis. Rev Cardiovasc Med. 2002;3(Suppl 5):S4–9. [PubMed] [Google Scholar]
- 14.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 15.Sunderland N, Skroblin P, Barwari T, Huntley RP, Lu R, Joshi A, Lovering RC, Mayr M. MicroRNA Biomarkers and Platelet Reactivity: The Clot Thickens. Circ Res. 2017;120:418–435. doi: 10.1161/CIRCRESAHA.116.309303. [DOI] [PubMed] [Google Scholar]
- 16.Lima J, Jr, Batty JA, Sinclair H, Kunadian V. MicroRNAs in Ischemic Heart Disease: From Pathophysiology to Potential Clinical Applications. Cardiol Rev. 2017;25:117–125. doi: 10.1097/CRD.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 17.Alexandru N, Badila E, Weiss E, Cochior D, Stepien E, Georgescu A. Vascular complications in diabetes: Microparticles and microparticle associated microRNAs as active players. Biochem Biophys Res Commun. 2016;472:1–10. doi: 10.1016/j.bbrc.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Reddy MA, Das S, Zhuo C, Jin W, Wang M, Lanting L, Natarajan R. Regulation of Vascular Smooth Muscle Cell Dysfunction Under Diabetic Conditions by miR-504. Arterioscler Thromb Vasc Biol. 2016;36:864–873. doi: 10.1161/ATVBAHA.115.306770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albinsson S, Sward K. Targeting smooth muscle microRNAs for therapeutic benefit in vascular disease. Pharmacol Res. 2013;75:28–36. doi: 10.1016/j.phrs.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Robinson HC, Baker AH. How do microRNAs affect vascular smooth muscle cell biology? Curr Opin Lipidol. 2012;23:405–411. doi: 10.1097/MOL.0b013e32835719a1. [DOI] [PubMed] [Google Scholar]
- 21.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 22.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 24.Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Research. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lal A, Navarro F, Maher CA, Maliszewski LE, Yan N, O'Day E, Chowdhury D, Dykxhoorn DM, Tsai P, Hofmann O, Becker KG, et al. miR-24 Inhibits Cell Proliferation by Targeting E2F2, MYC, and Other Cell-Cycle Genes via Binding to "Seedless" 3 ' UTR MicroRNA Recognition Elements. Molecular Cell. 2009;35:610–625. doi: 10.1016/j.molcel.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elkayam E, Kuhn CD, Tocilj A, Haase AD, Greene EM, Hannon GJ, Joshua-Tor L. The Structure of Human Argonaute-2 in Complex with miR-20a. Cell. 2012;150:100–110. doi: 10.1016/j.cell.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodersen P, Voinnet O. Revisiting the principles of microRNA target recognition and mode of action. Nature Reviews Molecular Cell Biology. 2009;10:141–148. doi: 10.1038/nrm2619. [DOI] [PubMed] [Google Scholar]
- 28.Feng S, Bowden N, Fragiadaki M, et al. Mechanical Activation of Hypoxia-Inducible Factor 1alpha Drives Endothelial Dysfunction at Atheroprone Sites. Arterioscler Thromb Vasc Biol. 2017;37:2087–2101. doi: 10.1161/ATVBAHA.117.309249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang YZ, Manduchi E, Jimenez JM, Davies PF. Endothelial epigenetics in biomechanical stress: disturbed flow-mediated epigenomic plasticity in vivo and in vitro. Arterioscler Thromb Vasc Biol. 2015;35:1317–1326. doi: 10.1161/ATVBAHA.115.303427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Candela J, Wang R, White C. Microvascular Endothelial Dysfunction in Obesity Is Driven by Macrophage-Dependent Hydrogen Sulfide Depletion. Arterioscler Thromb Vasc Biol. 2017;37:889–899. doi: 10.1161/ATVBAHA.117.309138. [DOI] [PubMed] [Google Scholar]
- 31.Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, Mikhed Y, Munzel T, Daiber A, Forstermann U, Li H. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arterioscler Thromb Vasc Biol. 2016;36:78–85. doi: 10.1161/ATVBAHA.115.306263. [DOI] [PubMed] [Google Scholar]
- 32.Ijaz T, Sun H, Pinchuk IV, Milewicz DM, Tilton RG, Brasier AR. Deletion of NF-kappaB/RelA in Angiotensin II-Sensitive Mesenchymal Cells Blocks Aortic Vascular Inflammation and Abdominal Aortic Aneurysm Formation. Arterioscler Thromb Vasc Biol. 2017;37:1881–1890. doi: 10.1161/ATVBAHA.117.309863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reglero-Real N, Colom B, Bodkin JV, Nourshargh S. Endothelial Cell Junctional Adhesion Molecules: Role and Regulation of Expression in Inflammation. Arterioscler Thromb Vasc Biol. 2016;36:2048–2057. doi: 10.1161/ATVBAHA.116.307610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pankratz F, Hohnloser C, Bemtgen X, et al. MicroRNA-100 Suppresses Chronic Vascular Inflammation by Stimulation of Endothelial Autophagy. Circ Res. 2018;122:417–432. doi: 10.1161/CIRCRESAHA.117.311428. [DOI] [PubMed] [Google Scholar]
- 35.Fiedler J, Thum T. New Insights Into miR-17-92 Cluster Regulation and Angiogenesis. Circ Res. 2016;118:9–11. doi: 10.1161/CIRCRESAHA.115.307935. [DOI] [PubMed] [Google Scholar]
- 36.Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, Lauro R, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 37.Deng S, Wang H, Jia C, Zhu S, Chu X, Ma Q, Wei J, Chen E, Zhu W, Macon CJ, Jayaweera DT, et al. MicroRNA-146a Induces Lineage-Negative Bone Marrow Cell Apoptosis and Senescence by Targeting Polo-Like Kinase 2 Expression. Arterioscler Thromb Vasc Biol. 2017;37:280–290. doi: 10.1161/ATVBAHA.116.308378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Aguda BD, Friedman A. A continuum mathematical model of endothelial layer maintenance and senescence. Theor Biol Med Model. 2007;4:30. doi: 10.1186/1742-4682-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dangwal S, Stratmann B, Bang C, Lorenzen JM, Kumarswamy R, Fiedler J, Falk CS, Scholz CJ, Thum T, Tschoepe D. Impairment of Wound Healing in Patients With Type 2 Diabetes Mellitus Influences Circulating MicroRNA Patterns via Inflammatory Cytokines. Arterioscler Thromb Vasc Biol. 2015;35:1480–1488. doi: 10.1161/ATVBAHA.114.305048. [DOI] [PubMed] [Google Scholar]
- 40.Di Bernardini E, Campagnolo P, Margariti A, Zampetaki A, Karamariti E, Hu Y, Xu Q. Endothelial Lineage Differentiation from Induced Pluripotent Stem Cells Is Regulated by MicroRNA-21 and Transforming Growth Factor beta2 (TGF-beta2) Pathways. J Biol Chem. 2014;289:3383–3393. doi: 10.1074/jbc.M113.495531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nowak WN, Deng J, Ruan XZ, Xu Q. Reactive Oxygen Species Generation and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:e41–e52. doi: 10.1161/ATVBAHA.117.309228. [DOI] [PubMed] [Google Scholar]
- 42.Staszel T, Zapala B, Polus A, Sadakierska-Chudy A, Kiec-Wilk B, Stepien E, Wybranska I, Chojnacka M, Dembinska-Kiec A. Role of microRNAs in endothelial cell pathophysiology. Pol Arch Med Wewn. 2011;121:361–366. [PubMed] [Google Scholar]
- 43.Perez-Sanchez C, Aguirre MA, Ruiz-Limon P, et al. Ubiquinol Effects on Antiphospholipid Syndrome Prothrombotic Profile: A Randomized, Placebo-Controlled Trial. Arterioscler Thromb Vasc Biol. 2017;37:1923–1932. doi: 10.1161/ATVBAHA.117.309225. [DOI] [PubMed] [Google Scholar]
- 44.Lu H, Daugherty A. Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:485–491. doi: 10.1161/ATVBAHA.115.305380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar S, Kim CW, Simmons RD, Jo H. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs. Arterioscler Thromb Vasc Biol. 2014;34:2206–2216. doi: 10.1161/ATVBAHA.114.303425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neth P, Nazari-Jahantigh M, Schober A, Weber C. MicroRNAs in flow-dependent vascular remodelling. Cardiovasc Res. 2013;99:294–303. doi: 10.1093/cvr/cvt096. [DOI] [PubMed] [Google Scholar]
- 47.Sun X, Belkin N, Feinberg MW. Endothelial microRNAs and atherosclerosis. Curr Atheroscler Rep. 2013;15:372. doi: 10.1007/s11883-013-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desjarlais M, Dussault S, Dhahri W, Mathieu R, Rivard A. MicroRNA-150 Modulates Ischemia-Induced Neovascularization in Atherosclerotic Conditions. Arterioscler Thromb Vasc Biol. 2017;37:900–908. doi: 10.1161/ATVBAHA.117.309189. [DOI] [PubMed] [Google Scholar]
- 49.Li C, Li S, Zhang F, Wu M, Liang H, Song J, Lee C, Chen H. Endothelial microparticles-mediated transfer of microRNA-19b promotes atherosclerosis via activating perivascular adipose tissue inflammation in apoE(-/-) mice. Biochem Biophys Res Commun. 2018;495:1922–1929. doi: 10.1016/j.bbrc.2017.11.195. [DOI] [PubMed] [Google Scholar]
- 50.Leeper NJ, Maegdefessel L. Non-coding RNAs: key regulators of smooth muscle cell fate in vascular disease. Cardiovasc Res. 2018;114:611–621. doi: 10.1093/cvr/cvx249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M, Li W, Chang GQ, Ye CS, Ou JS, Li XX, Liu Y, Cheang TY, Huang XL, Wang SM. MicroRNA-21 regulates vascular smooth muscle cell function via targeting tropomyosin 1 in arteriosclerosis obliterans of lower extremities. Arterioscler Thromb Vasc Biol. 2011;31:2044–2053. doi: 10.1161/ATVBAHA.111.229559. [DOI] [PubMed] [Google Scholar]
- 53.Holmberg J, Bhattachariya A, Alajbegovic A, Rippe C, Ekman M, Dahan D, Hien TT, Boettger T, Braun T, Sward K, Hellstrand P, et al. Loss of Vascular Myogenic Tone in miR-143/145 Knockout Mice Is Associated With Hypertension-Induced Vascular Lesions in Small Mesenteric Arteries. Arterioscler Thromb Vasc Biol. 2018;38:414–424. doi: 10.1161/ATVBAHA.117.310499. [DOI] [PubMed] [Google Scholar]
- 54.Yang F, Chen Q, He S, Yang M, Maguire EM, An W, Afzal TA, Luong LA, Zhang L, Xiao Q. miR-22 Is a Novel Mediator of Vascular Smooth Muscle Cell Phenotypic Modulation and Neointima Formation. Circulation. 2018;137:1824–1841. doi: 10.1161/CIRCULATIONAHA.117.027799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albinsson S, Sessa WC. Can microRNAs control vascular smooth muscle phenotypic modulation and the response to injury? Physiol Genomics. 2011;43:529–533. doi: 10.1152/physiolgenomics.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barwari T, Rienks M, Mayr M. MicroRNA-21 and the Vulnerability of Atherosclerotic Plaques. Mol Ther. 2018;26:938–940. doi: 10.1016/j.ymthe.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dangwal S, Thum T. microRNA therapeutics in cardiovascular disease models. Annu Rev Pharmacol Toxicol. 2014;54:185–203. doi: 10.1146/annurev-pharmtox-011613-135957. [DOI] [PubMed] [Google Scholar]
- 58.Leeper NJ, Raiesdana A, Kojima Y, Chun HJ, Azuma J, Maegdefessel L, Kundu RK, Quertermous T, Tsao PS, Spin JM. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol. 2011;226:1035–1043. doi: 10.1002/jcp.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen MA, Karunakaran D, Geoffrion M, Cheng HS, Tandoc K, Perisic Matic L, Hedin U, Maegdefessel L, Fish JE, Rayner KJ. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler Thromb Vasc Biol. 2018;38:49–63. doi: 10.1161/ATVBAHA.117.309795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Worsdorfer P, Mekala SR, Bauer J, Edenhofer F, Kuerten S, Ergun S. The vascular adventitia: An endogenous, omnipresent source of stem cells in the body. Pharmacol Ther. 2017;171:13–29. doi: 10.1016/j.pharmthera.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 62.Yu B, Chen Q, Le Bras A, Zhang L, Xu Q. Vascular Stem/Progenitor Cell Migration and Differentiation in Atherosclerosis. Antioxid Redox Signal. 2018;29:219–235. doi: 10.1089/ars.2017.7171. [DOI] [PubMed] [Google Scholar]
- 63.Zhang L, Issa Bhaloo S, Chen T, Zhou B, Xu Q. Role of Resident Stem Cells in Vessel Formation and Arteriosclerosis. Circ Res. 2018;122:1608–1624. doi: 10.1161/CIRCRESAHA.118.313058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karamariti E, Zhai C, Yu B, et al. DKK3 (Dickkopf 3) Alters Atherosclerotic Plaque Phenotype Involving Vascular Progenitor and Fibroblast Differentiation Into Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2018;38:425–437. doi: 10.1161/ATVBAHA.117.310079. [DOI] [PubMed] [Google Scholar]
- 65.Le Bras A, Yu B, Issa Bhaloo S, Hong X, Zhang Z, Hu Y, Xu Q. Adventitial Sca1+ Cells Transduced With ETV2 Are Committed to the Endothelial Fate and Improve Vascular Remodeling After Injury. Arterioscler Thromb Vasc Biol. 2018;38:232–244. doi: 10.1161/ATVBAHA.117.309853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Templin C, Volkmann J, Emmert MY, et al. Increased Proangiogenic Activity of Mobilized CD34+ Progenitor Cells of Patients With Acute ST-Segment-Elevation Myocardial Infarction: Role of Differential MicroRNA-378 Expression. Arterioscler Thromb Vasc Biol. 2017;37:341–349. doi: 10.1161/ATVBAHA.116.308695. [DOI] [PubMed] [Google Scholar]
- 67.Maguire EM, Xiao Q, Xu Q. Differentiation and Application of Induced Pluripotent Stem Cell-Derived Vascular Smooth Muscle Cells. Arterioscl Thromb Vasc Biol. 2017;37:2026–2037. doi: 10.1161/ATVBAHA.117.309196. [DOI] [PubMed] [Google Scholar]
- 68.Xie Y, Potter CMF, Le Bras A, Nowak WN, Gu W, Bhaloo SI, Zhang Z, Hu Y, Zhang L, Xu Q. Leptin Induces Sca-1(+) Progenitor Cell Migration Enhancing Neointimal Lesions in Vessel-Injury Mouse Models. Arterioscler Thromb Vasc Biol. 2017;37:2114–2127. doi: 10.1161/ATVBAHA.117.309852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diao Y, Mohandas R, Lee P, Liu Z, Sautina L, Mu W, Li S, Wen X, Croker B, Segal MS. Effects of Long-Term Type I Interferon on the Arterial Wall and Smooth Muscle Progenitor Cells Differentiation. Arterioscler Thromb Vasc Biol. 2016;36:266–273. doi: 10.1161/ATVBAHA.115.306767. [DOI] [PubMed] [Google Scholar]
- 70.Xie Y, Fan Y, Xu Q. Vascular Regeneration by Stem/Progenitor Cells. Arterioscler Thromb Vasc Biol. 2016;36:e33–40. doi: 10.1161/ATVBAHA.116.307303. [DOI] [PubMed] [Google Scholar]
- 71.Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol. 2010;30:1118–1126. doi: 10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan Y, Balazs L, Tigyi G, Yue J. Conditional deletion of Dicer in vascular smooth muscle cells leads to the developmental delay and embryonic mortality. Biochem Biophys Res Commun. 2011;408:369–374. doi: 10.1016/j.bbrc.2011.02.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Long X, Miano JM. Transforming growth factor-beta1 (TGF-beta1) utilizes distinct pathways for the transcriptional activation of microRNA 143/145 in human coronary artery smooth muscle cells. J Biol Chem. 2011;286:30119–30129. doi: 10.1074/jbc.M111.258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, Hata A. down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem. 2011;286:28097–28110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet. 2011;4:197–205. doi: 10.1161/CIRCGENETICS.110.958702. [DOI] [PubMed] [Google Scholar]
- 79.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 81.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie C, Huang H, Sun X, Guo Y, Hamblin M, Ritchie RP, Garcia-Barrio MT, Zhang J, Chen YE. MicroRNA-1 regulates smooth muscle cell differentiation by repressing Kruppel-like factor 4. Stem Cells Dev. 2011;20:205–210. doi: 10.1089/scd.2010.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen J, Yin H, Jiang Y, Radhakrishnan SK, Huang ZP, Li J, Shi Z, Kilsdonk EP, Gui Y, Wang DZ, Zheng XL. Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler Thromb Vasc Biol. 2011;31:368–375. doi: 10.1161/ATVBAHA.110.218149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang Y, Yin H, Zheng XL. MicroRNA-1 inhibits myocardin-induced contractility of human vascular smooth muscle cells. J Cell Physiol. 2010;225:506–511. doi: 10.1002/jcp.22230. [DOI] [PubMed] [Google Scholar]
- 85.Kang H, Davis-Dusenbery BN, Nguyen PH, Lal A, Lieberman J, Van Aelst L, Lagna G, Hata A. Bone morphogenetic protein 4 promotes vascular smooth muscle contractility by activating microRNA-21 (miR-21), which down-regulates expression of family of dedicator of cytokinesis (DOCK) proteins. J Biol Chem. 2012;287:3976–3986. doi: 10.1074/jbc.M111.303156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horita HN, Simpson PA, Ostriker A, Furgeson S, Van Putten V, Weiser-Evans MC, Nemenoff RA. Serum response factor regulates expression of phosphatase and tensin homolog through a microRNA network in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:2909–2919. doi: 10.1161/ATVBAHA.111.233585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Y, Liu X, Cheng Y, Yang J, Huo Y, Zhang C. Involvement of MicroRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J Biol Chem. 2009;284:7903–7913. doi: 10.1074/jbc.M806920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang H, Xie C, Sun X, Ritchie RP, Zhang J, Chen YE. miR-10a contributes to retinoid acid-induced smooth muscle cell differentiation. J Biol Chem. 2010;285:9383–9389. doi: 10.1074/jbc.M109.095612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grundmann S, Hans FP, Kinniry S, Heinke J, Helbing T, Bluhm F, Sluijter JP, Hoefer I, Pasterkamp G, Bode C, Moser M. MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation. 2011;123:999–1009. doi: 10.1161/CIRCULATIONAHA.110.000323. [DOI] [PubMed] [Google Scholar]
- 90.Mizuno Y, Yagi K, Tokuzawa Y, Kanesaki-Yatsuka Y, Suda T, Katagiri T, Fukuda T, Maruyama M, Okuda A, Amemiya T, Kondoh Y, et al. miR-125b inhibits osteoblastic differentiation by down-regulation of cell proliferation. Biochem Biophys Res Commun. 2008;368:267–272. doi: 10.1016/j.bbrc.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 91.Goettsch C, Rauner M, Pacyna N, Hempel U, Bornstein SR, Hofbauer LC. miR-125b regulates calcification of vascular smooth muscle cells. Am J Pathol. 2011;179:1594–1600. doi: 10.1016/j.ajpath.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chan MC, Hilyard AC, Wu C, Davis BN, Hill NS, Lal A, Lieberman J, Lagna G, Hata A. Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. EMBO J. 2010;29:559–573. doi: 10.1038/emboj.2009.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X, Cheng Y, Chen X, Yang J, Xu L, Zhang C. MicroRNA-31 regulated by the extracellular regulated kinase is involved in vascular smooth muscle cell growth via large tumor suppressor homolog 2. J Biol Chem. 2011;286:42371–42380. doi: 10.1074/jbc.M111.261065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun SG, Zheng B, Han M, Fang XM, Li HX, Miao SB, Su M, Han Y, Shi HJ, Wen JK. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011;12:56–62. doi: 10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation. 2001;104:790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y, Wang Y, Wang X, Zhang Y, Eisner GM, Asico LD, Jose PA, Zeng C. Insulin promotes vascular smooth muscle cell proliferation via microRNA-208-mediated downregulation of p21. J Hypertens. 2011;29:1560–1568. doi: 10.1097/HJH.0b013e328348ef8e. [DOI] [PubMed] [Google Scholar]
- 97.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scalbert E, Bril A. Implication of microRNAs in the cardiovascular system. Curr Opin Pharmacol. 2008;8:181–188. doi: 10.1016/j.coph.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 99.Ouimet M, Ediriweera H, Afonso MS, Ramkhelawon B, Singaravelu R, Liao X, Bandler RC, Rahman K, Fisher EA, Rayner KJ, Pezacki JP, et al. microRNA-33 Regulates Macrophage Autophagy in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:1058–1067. doi: 10.1161/ATVBAHA.116.308916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karunakaran D, Rayner KJ. Macrophage miRNAs in atherosclerosis. Biochim Biophys Acta. 2016;1861:2087–2093. doi: 10.1016/j.bbalip.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 101.Ouimet M, Hennessy EJ, van Solingen C, et al. miRNA Targeting of Oxysterol-Binding Protein-Like 6 Regulates Cholesterol Trafficking and Efflux. Arterioscler Thromb Vasc Biol. 2016;36:942–951. doi: 10.1161/ATVBAHA.116.307282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karunakaran D, Richards L, Geoffrion M, Barrette D, Gotfrit RJ, Harper ME, Rayner KJ. Therapeutic Inhibition of miR-33 Promotes Fatty Acid Oxidation but Does Not Ameliorate Metabolic Dysfunction in Diet-Induced Obesity. Arterioscler Thromb Vasc Biol. 2015;35:2536–2543. doi: 10.1161/ATVBAHA.115.306404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 104.Huang X, Yue Z, Wu J, Chen J, Wang S, Wu J, Ren L, Zhang A, Deng P, Wang K, Wu C, et al. MicroRNA-21 Knockout Exacerbates Angiotensin II-Induced Thoracic Aortic Aneurysm and Dissection in Mice With Abnormal Transforming Growth Factor-beta-SMAD3 Signaling. Arterioscler Thromb Vasc Biol. 2018;38:1086–1101. doi: 10.1161/ATVBAHA.117.310694. [DOI] [PubMed] [Google Scholar]
- 105.Wang D, Deuse T, Stubbendorff M, et al. Local MicroRNA Modulation Using a Novel Anti-miR-21-Eluting Stent Effectively Prevents Experimental In-Stent Restenosis. Arterioscler Thromb Vasc Biol. 2015;35:1945–1953. doi: 10.1161/ATVBAHA.115.305597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Roxe T, Zeiher AM, Landmesser U, Dimmeler S. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:1392–1400. doi: 10.1161/ATVBAHA.112.300741. [DOI] [PubMed] [Google Scholar]
- 107.Boon RA, Vickers KC. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:186–192. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ray M, Gabunia K, Vrakas CN, Herman AB, Kako F, Kelemen SE, Grisanti LA, Autieri MV. Genetic Deletion of IL-19 (Interleukin-19) Exacerbates Atherogenesis in Il19(-/-)xLdlr(-/-) Double Knockout Mice by Dysregulation of mRNA Stability Protein HuR (Human Antigen R) Arterioscler Thromb Vasc Biol. 2018;38:1297–1308. doi: 10.1161/ATVBAHA.118.310929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kumarswamy R, Volkmann I, Beermann J, Napp LC, Jabs O, Bhayadia R, Melk A, Ucar A, Chowdhury K, Lorenzen JM, Gupta SK, et al. Vascular importance of the miR-212/132 cluster. Eur Heart J. 2014;35:3224–3231. doi: 10.1093/eurheartj/ehu344. [DOI] [PubMed] [Google Scholar]
- 110.Chao CT, Liu YP, Su SF, Yeh HY, Chen HY, Lee PJ, Chen WJ, Lee YM, Huang JW, Chiang CK, Hung KY, et al. Circulating MicroRNA-125b Predicts the Presence and Progression of Uremic Vascular Calcification. Arterioscler Thromb Vasc Biol. 2017;37:1402–1414. doi: 10.1161/ATVBAHA.117.309566. [DOI] [PubMed] [Google Scholar]
- 111.Canfran-Duque A, Lin CS, Goedeke L, Suarez Y, Fernandez-Hernando C. Micro-RNAs and High-Density Lipoprotein Metabolism. Arterioscler Thromb Vasc Biol. 2016;36:1076–1084. doi: 10.1161/ATVBAHA.116.307028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fiedler J, Thum T. MicroRNAs in myocardial infarction. Arterioscler Thromb Vasc Biol. 2013;33:201–205. doi: 10.1161/ATVBAHA.112.300137. [DOI] [PubMed] [Google Scholar]
- 113.Willeit P, Skroblin P, Moschen AR, et al. Circulating MicroRNA-122 Is Associated With the Risk of New-Onset Metabolic Syndrome and Type 2 Diabetes. Diabetes. 2017;66:347–357. doi: 10.2337/db16-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Araldi E, Chamorro-Jorganes A, van Solingen C, Fernandez-Hernando C, Suarez Y. Therapeutic Potential of Modulating microRNAs in Atherosclerotic Vascular Disease. Curr Vasc Pharmacol. 2015;13:291–304. [PubMed] [Google Scholar]
- 115.Lorenzen JM. Vascular and circulating microRNAs in renal ischaemia-reperfusion injury. J Physiol. 2015;593:1777–1784. doi: 10.1113/JP270318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boon RA. Circulating MicroRNAs Link Inflammation to Impaired Wound Healing in Diabetes. Arterioscler Thromb Vasc Biol. 2015;35:1296–1297. doi: 10.1161/ATVBAHA.115.305670. [DOI] [PubMed] [Google Scholar]
- 117.Feinberg MW, Moore KJ. MicroRNA Regulation of Atherosclerosis. Circ Res. 2016;118:703–720. doi: 10.1161/CIRCRESAHA.115.306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eken SM, Jin H, Chernogubova E, et al. MicroRNA-210 Enhances Fibrous Cap Stability in Advanced Atherosclerotic Lesions. Circ Res. 2017;120:633–644. doi: 10.1161/CIRCRESAHA.116.309318. [DOI] [PubMed] [Google Scholar]
- 119.Kaudewitz D, Skroblin P, Bender LH, et al. Association of MicroRNAs and YRNAs With Platelet Function. Circ Res. 2016;118:420–432. doi: 10.1161/CIRCRESAHA.114.305663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gareri C, De Rosa S, Indolfi C. MicroRNAs for Restenosis and Thrombosis After Vascular Injury. Circ Res. 2016;118:1170–1184. doi: 10.1161/CIRCRESAHA.115.308237. [DOI] [PubMed] [Google Scholar]
- 121.Li T, Cao H, Zhuang J, Wan J, Guan M, Yu B, Li X, Zhang W. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin Chim Acta. 2011;412:66–70. doi: 10.1016/j.cca.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 122.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 123.Stather PW, Sylvius N, Wild JB, Choke E, Sayers RD, Bown MJ. Differential microRNA expression profiles in peripheral arterial disease. Circ Cardiovasc Genet. 2013;6:490–497. doi: 10.1161/circgenetics.111.000053. [DOI] [PubMed] [Google Scholar]
- 124.De Rosa S, Eposito F, Carella C, et al. Transcoronary concentration gradients of circulating microRNAs in heart failure. Eur J Heart Fail. 2018;20:1000–1010. doi: 10.1002/ejhf.1119. [DOI] [PubMed] [Google Scholar]
- 125.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 126.Cao WJ, Rosenblat JD, Roth NC, Kuliszewski MA, Matkar PN, Rudenko D, Liao C, Lee PJ, Leong-Poi H. Therapeutic Angiogenesis by Ultrasound-Mediated MicroRNA-126-3p Delivery. Arterioscler Thromb Vasc Biol. 2015;35:2401–2411. doi: 10.1161/ATVBAHA.115.306506. [DOI] [PubMed] [Google Scholar]
- 127.Chen J, Zhu RF, Li FF, Liang YL, Wang C, Qin YW, Huang S, Zhao XX, Jing Q. MicroRNA-126a Directs Lymphangiogenesis Through Interacting With Chemokine and Flt4 Signaling in Zebrafish. Arterioscler Thromb Vasc Biol. 2016;36:2381–2393. doi: 10.1161/ATVBAHA.116.308120. [DOI] [PubMed] [Google Scholar]
- 128.Fernandez-Hernando C, Ramirez CM, Goedeke L, Suarez Y. MicroRNAs in metabolic disease. Arterioscler Thromb Vasc Biol. 2013;33:178–185. doi: 10.1161/ATVBAHA.112.300144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wei Y, Nazari-Jahantigh M, Neth P, Weber C, Schober A. MicroRNA-126, -145, and -155: a therapeutic triad in atherosclerosis? Arterioscler Thromb Vasc Biol. 2013;33:449–454. doi: 10.1161/ATVBAHA.112.300279. [DOI] [PubMed] [Google Scholar]
- 130.Toyama K, Spin JM, Deng AC, et al. MicroRNA-Mediated Therapy Modulating Blood-Brain Barrier Disruption Improves Vascular Cognitive Impairment. Arterioscler Thromb Vasc Biol. 2018;38:1392–1406. doi: 10.1161/ATVBAHA.118.310822. [DOI] [PubMed] [Google Scholar]
- 131.Gadde S, Rayner KJ. Nanomedicine Meets microRNA: Current Advances in RNA-Based Nanotherapies for Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:e73–79. doi: 10.1161/ATVBAHA.116.307481. [DOI] [PMC free article] [PubMed] [Google Scholar]