Abstract
Resistive-based gas sensors have been considered as the most favorable gas sensors for detection of toxic gases and volatile organic compounds (VOCs) because of their simple structure, low cost, high sensitivity, ease of use, and high stability. Unfortunately, wide application of resistive-based gas sensors is limited by their low selectivity. In this article, we present the fabrication of ultrahigh selective NH3 gas sensor based on tin–titanium dioxide/reduced graphene/carbon nanotube (Sn–TiO2@rGO/CNT) nanocomposites. The Sn–TiO2@rGO/CNT nanocomposites with different molar ratios of Sn/Ti (1:10, 3:10, and 5:10) were synthesized via the solvothermal method. Characterizations by scanning electron microscopy, transmission electron microscopy, and X-ray photoelectron spectroscopy confirmed the decoration of Sn–TiO2 nanoparticles on rGO/CNT nanocomposite surfaces. The Sn–TiO2@rGO/CNT nanocomposite gas sensor exhibited high response and ultrahigh selectivity to NH3 against toluene, dimethylformamide, acetone, ethanol, methanol, isopropanol, formaldehyde, hydrogen, carbon dioxide, acetylene, and VOCs in paint thinners at room temperature. The Sn–TiO2@rGO/CNT nanocomposite gas sensor with molar ratio of Sn/Ti = 1:10 showed the highest response to NH3 over other molar ratios of Sn/Ti as well as pure rGO/CNT and Sn–TiO2 gas sensors. The ammonia-sensing mechanisms of the Sn–TiO2@rGO/CNT gas sensor were proposed based on the formation of p–n heterojunctions of p-type rGO/CNT and n-type Sn–TiO2 nanoparticles via a low-temperature oxidizing reaction process.
1. Introduction
Ammonia (NH3) is one of the toxic gases that can cause illness or death when it is inhaled or absorbed by eyes, nose, skin, and the respiratory tract at high concentration.1 NH3 has been widely used in several commercial products and industrial applications such as ice factory, adhesives, rubber cements, automotive fuels, and laboratory solvents.2−4 Moreover, NH3 is also found in the exhaled breath of humans, in which it plays an important biomarker for diagnosis of kidney disorders or ulcers caused by the Helicobacter pylori bacterial stomach infection.5,6 The exhaled breath of patients with kidney disorders and peptic ulcer releases NH3 in the concentration range of 0.82–14.7 ppm.7 Furthermore, NH3 is produced from farming areas or animal agriculture that can affect health of humans, animals, and environment.8 Therefore, detection of NH3 is necessary for life saving, environment protection, and medical applications. Nowadays, sensing nanomaterials and fabrication techniques for NH3 gas sensors have been extensively studied in term of high sensitivity, high selectivity, high stability, fast response, low cost, and ease of use. Metal oxides are one of the most popular NH3-sensing nanomaterials. However, most NH3 gas sensors based on metal oxides require high working temperatures of 150–300 °C and exhibit cross-sensitivity.9,10 The development of room-temperature NH3 gas sensors based on nanocomposites11−14 is a way to overcome these crucial problems but until now no nanocomposites exhibited perfect selectivity to NH3, leading to impractical use in real-world applications based on single gas detection with quantitative purpose.
A nanocomposite is defined as a multicomponent material in which at least one of components are of nanoscale size. It generally comprises multiphase inorganic/organic materials such as metal–metal oxides (Ni60Fe30Mn10, Sn/Cu/ZnO NPs, MoS2–Au, MoS2/ZnO, and Cu–BTC/BNNT),15−19 metal oxide–carbon nanomaterials (TiO2–RGO, CoFe2O4/graphene, and rGO/MoS2),20−22 metal oxide–polymer (Pd–TiO2@PPy, PANI/SnO2, and PPy/Fe2O3),23,24 and metal oxide–carbon nanomaterials–polymer (Pd–PANI–rGO, TiO2@PPy–GN, graphene–PEDOT:PSS, and Pt/PAN–MWCNTs/WGE).25−28 Among them, the hybrid carbon-based nanostructures have become one of the most attractive materials that can provide multidisciplinary applications, including flexible battery, biosensor, solar cell, super-capacitors, chemical sensor, and gas sensing,29−31 due to their unique properties such as low-cost production, strong covalent-bond structures, large specific surface area, high electrical conductivity, low redox potential, and high mechanical strength.32−34 In this work, we focus on new metal oxide–carbon nanocomposites consisting of tin–titanium dioxide/reduced graphene/carbon nanotube (Sn–TiO2@rGO/CNT) for NH3-sensing application at room temperature. The Sn–TiO2@rGO/CNT nanocomposites were synthesized by the solvothermal process and characterized via scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray photoelectron spectroscopy (XPS). The sensing performances of Sn–TiO2@rGO/CNT nanocomposites were systematically investigated. The NH3-sensing mechanism was proposed based on the formation of p–n heterojunctions.
2. Results and Discussion
2.1. Characterization of Sensing Films
The SEM image of Sn–TiO2@rGO/CNT nanocomposite is shown in Figure 1a. It illustrates agglomeration of rGO and CNT leading to self-assembled 3D hierarchical interconnected morphology, whereas the Sn–TiO2 nanoparticles are randomly embedded on the surface of the rGO/CNT nanocomposite. The corresponding TEM image confirms the coexistence of Sn–TiO2 nanoparticles, graphene, and CNTs as demonstrated in Figure 1b. It can be seen that the Sn–TiO2 nanoparticles with diameters of <15 nm are widely coated on graphene and CNT surfaces.
Figure 1.

(a) SEM and (b) TEM images of Sn–TiO2@rGO/CNT nanocomposite.
XPS survey scan measurements were performed to investigate the chemical composition of the Sn–TiO2@rGO/CNT nanocomposite as illustrated in Figure 2. The survey scan XPS spectra of Sn–TiO2@rGO/CNT with different molar ratios of Sn/Ti confirm the presence of C, Ti, Sn, and O in agreement with the expected chemical compositions of the Sn–TiO2@rGO/CNT nanocomposite. The Sn atomic contents of Sn–TiO2@rGO/CNT nanocomposites were calculated from the XPS data to be around 2.9, 3.8, and 5.4 at. % for nanocomposite I, nanocomposite II, and nanocomposite III, respectively. It can be seen that the Sn adding amounts increase approximately linearly with increasing Sn in TiO2@rGO/CNT based on solvothermal method. In addition, all element atomic contents of nanocomposite I were found to be around C 1s (40.7 at. %), Ti (12.6 at. %), Sn (2.9 at. %), and O 1s (43.8 at. %).
Figure 2.
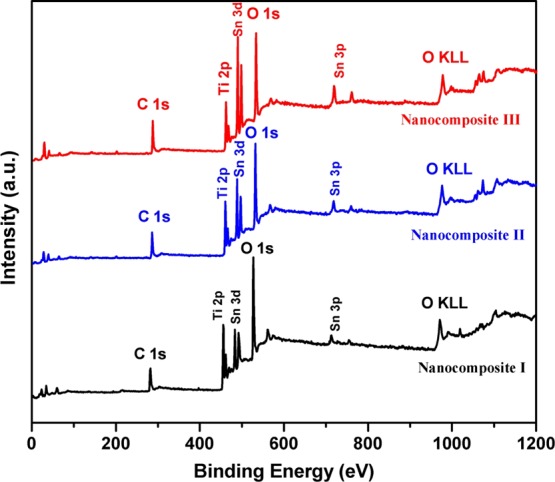
XPS survey scan spectra of Sn–TiO2@rGO/CNT nanocomposite.
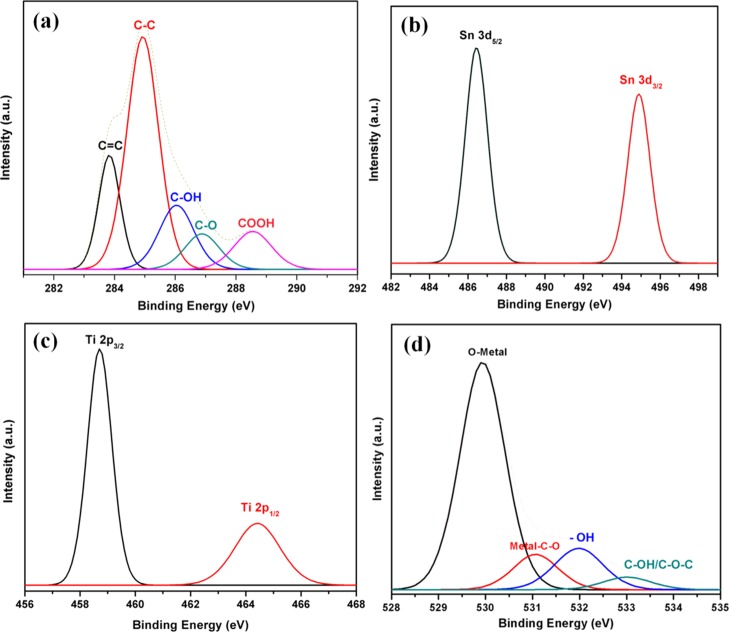
The high-resolution C 1s peak (Figure 3a) can be deconvoluted into five peaks located at 283.3, 284.8, 286.1, 286.9 and 288.6 eV which correspond to C=C, C–C, C–OH, C–O, and COOH groups in rGO/CNT, respectively.35−40 The C=C and C–C components refer to the backbone of the rGO/CNT structure, whereas the C–OH, C–O, and COOH bonds indicate the oxygen-containing functional groups. It can be seen that the C=C and C–C bonds have higher intensity than the oxygen-containing functional groups of C–OH, C–O, and COOH bonds. XPS spectra of Sn 3d and Ti 2p in nanocomposite I are presented in Figure 3b,c, respectively. Two peaks of Sn 3d spectrum at 486.6 and 495.0 eV are attributable to Sn 3d5/2 and Sn 3d3/2 spin orbit peaks of the Sn4+ state in SnO2, confirming the formation of SnO2 nanoparticles in the Sn–TiO2@rGO/CNT nanocomposite.41,42 For Ti 2p spectrum, two spin–orbit peaks of Ti 2p3/2 and Ti 2p1/2 are located at 458.8 and 464.5 eV, respectively. The two prominent peaks can be assigned to the Ti4+ state of TiO2.41,43 The high-resolution O 1s XPS spectrum of the nanocomposite I is shown in Figure 3d, the O 1s peak can be decomposed into four contributions centered at 529.9, 531.1, 531.9 and 532.9 eV, which can be attributed to O–metal (Sn, Ti) bonding,44−46 Sn–O–C or Ti–O–C,47,48 Sn–OH or Ti–OH,49 and C–OH/C–O–C,50 respectively.
Figure 3.
The high-resolution XPS spectra of the nanocomposite I for (a) C 1s, (b) Sn 3d, (c) Ti 2p, and (d) O 1s.
2.2. Gas Sensing Properties
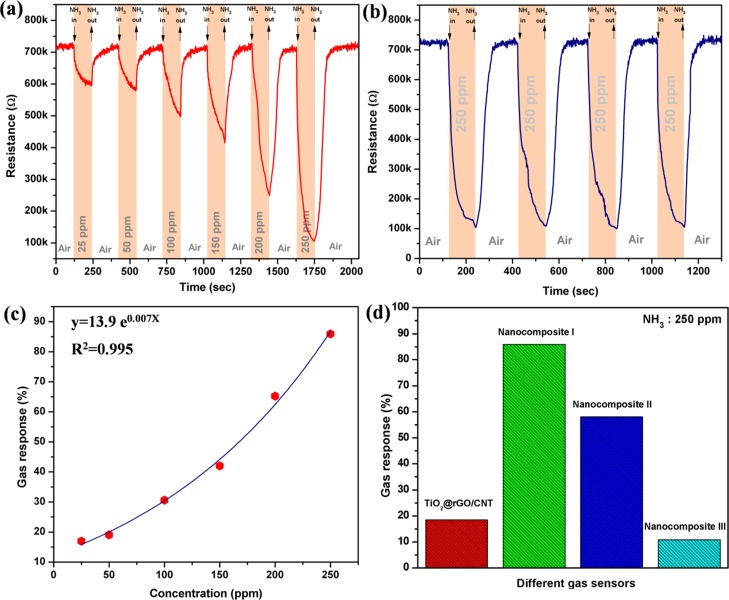
Figure 4a shows the change in resistance of the nanocomposite I gas sensor upon exposure to NH3 vapor with different concentrations ranging from 25 to 250 ppm at room temperature. It is seen that the Sn–TiO2@rGO/CNT gas sensor exhibits a rapid decrease of resistance upon exposure to NH3 vapor before recovering to their baseline values in dry air. The resistance changing behaviors may be attributed to the adsorption and desorption of NH3 molecules of the sensing films. The details of sensing mechanism for the Sn–TiO2@rGO/CNT gas sensor will be discussed in the next section. The nanocomposite I gas sensor has been further tested to evaluate reproducibility under exposure to 250 ppm NH3 vapor at room temperature as demonstrated in Figure 4b. It demonstrates that the Sn–TiO2@rGO/CNT gas sensor can fully recover to their initial baselines for several repeated cycles. Consequently, the Sn–TiO2@rGO/CNT gas sensor indicates its good reproducibility for NH3 detection at room temperature. The stability of the Sn–TiO2@rGO/CNT nanocomposite gas sensor has been investigated with exposure to 250 ppm ammonia for 30 days. Based on storage of gas sensor at room temperature with uncontrolled relative humidity (RH), a slight gas response drop (only about 3%) could be observed after 30 days (see Figure S1 in the Supporting Information). It indicates that the Sn–TiO2@rGO/CNT nanocomposite gas sensor exhibits good stability over 30 days.
Figure 4.
Changes in resistance of (a) nanocomposite I gas sensors to ammonia vapor with various concentrations and (b) four repeated pulses of 250 ppm ammonia at room temperature. (c) Gas responses of nanocomposite I gas sensor to ammonia vapor with various concentrations. (d) Gas responses of nanocomposite I to 250 ppm ammonia vapor at room temperature compared with nanocomposite II, nanocomposite III, and TiO2@rGO/CNT gas sensors.
The nanocomposite I gas
sensor as a function of NH3 concentration
at room temperature is shown in Figure 4c. It can be seen that the gas response increases exponentially
over the NH3 concentration range of 25–250 ppm.
The gas responses of the nanocomposite I gas sensor at 25, 50, 100,
150, 200, and 250 ppm NH3 are 17.0, 19.1, 30.6, 42.0, 65.2
and 85.9%, respectively. The gas response as functions of NH3 concentration (CNH3) can
be fitted by a power law function relation according to the equation: 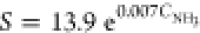 with
a good correlation coefficient (0.995).
The calculated response and recovery times of the nanocomposite I
gas sensor for detection of 250 ppm NH3 are 99 and 66 s,
respectively, which are relatively short for a room-temperature sensor.
with
a good correlation coefficient (0.995).
The calculated response and recovery times of the nanocomposite I
gas sensor for detection of 250 ppm NH3 are 99 and 66 s,
respectively, which are relatively short for a room-temperature sensor.
The effect of different molar ratios of Sn/Ti on the gas response toward 250 ppm NH3 vapor at room temperature was investigated as shown in Figure 4d. It can be observed that the gas response increases from 18.5 to 85.9% after adding Sn/SnO2 to TiO2@rGO/CNT nanocomposites. However, more Sn/SnO2-loading contents into the TiO2@rGO/CNT nanocomposites (nanocomposite II and nanocomposite III) exhibit a decrease in response. Thus, nanocomposite I is the optimal condition of the Sn–TiO2@rGO/CNT gas sensor that yields the highest NH3 response at room temperature. The observed results may be explained based on the formation of p–n heterojunctions. The gas response of the nanocomposite sensor has been improved after adding Sn/SnO2 due to the increasing number of Sn/SnO2 nanoparticles corresponding to the number of p–n heterojunctions on the surfaces. The increasing number of Sn/SnO2 nanoparticles on TiO2@rGO/CNT surfaces may enlarge the specific surface area of the sensing film to absorb more oxygen molecules on the sensing film surface and increase the interface of p–n heterojunction. When the Sn/SnO2 loading amount becomes too high, the Sn/SnO2 molecules may aggregate into larger nanoparticles or cover the active centers of the nanocomposite, leading to a reduced number of p–n heterojunctions, causing a lower response. To distinguish the performance contribution of each ingredient within the nanocomposites, rGO/CNT and Sn–TiO2 gas sensors were fabricated and tested toward 250 ppm NH3 at room temperature (see Figure S2 in the Supporting Information). One can observe that the initial resistances of the rGO/CNT and Sn–TiO2 gas sensors are too low and too high, respectively, whereas the resistance of the Sn–TiO2@rGO/CNT sensor is between the resistance of rGO/CNT and Sn–TiO2 sensors. The resistance of the rGO/CNT sensor increases and Sn–TiO2 sensor resistance decreases in the present of NH3 according to p-type and n-type characteristics, respectively. The gas responses of the rGO/CNT sensor and Sn–TiO2 are calculated to be 30.0 and 28.8%, respectively. These results confirm the benefit of p–n heterojunction formation that gives better gas responses than pure p-type or n-type materials, that is over 55% for sensing NH3 at room temperature.
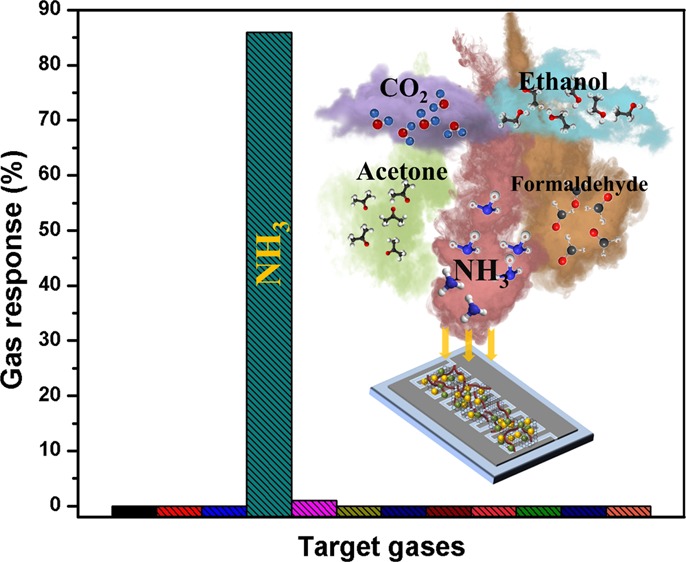
The selectivity of the optimal Sn–TiO2@rGO/CNT gas sensor has been characterized toward various gases including toluene (C7H8), dimethylformamide (DMF), acetone (C3H6O), ammonia (NH3), ethanol (C2H6O), methanol (CH3OH), isopropanol (C3H8O), formaldehyde (CH2O), thinner, hydrogen (H2), carbon dioxide (CO2), and acetylene (C2H2) at concentrations as shown in Figure 5. It can be seen that the optimal Sn–TiO2@rGO/CNT gas sensor exhibits ultrahigh selective NH3 over several tested gases at room temperature. The high selectivity toward NH3 may be related to the interaction between polarity of NH3 molecules and hydroxyl groups on the Sn–TiO2 surface leading to strong chemisorptions, whereas it is too difficult for other tested gases to form hydrogen bonds with these hydroxyl groups on the Sn–TiO2 surface at room temperature.51 Moreover, NH3 molecules were favorable to promoting molecular adsorption on the surface acidity of Sn–TiO2 existing Lewis acid sites (Sn4+ and Ti4+) and Brønsted acid sites (Sn–OH and Ti–OH).52 To investigate the humidity effect, the resistance of the Sn–TiO2@rGO/CNT sensor was measured in real time at various humidity levels from 30 to 90% RH (see Figure S3 in the Supporting Information). It is seen that the sensor resistance does not change significantly in the range of 30–70% RH at room temperature. At high humidity level (RH > 70%), the sensor resistance decreases with increasing RH. However, when humidity response of the Sn–TiO2@rGO/CNT sensor (defined as [R30%RH – RX] × 100/R30%RH, where R30%RH and RX are the sensor resistance values at 30% RH and the target value at another RH, respectively) is calculated, the sensor exhibits only 13.8% at 90% RH that is lower than the NH3 gas response for all tested NH3 concentrations (25–250 ppm). Thus, it can confirm the humidity-independent sensor for normal living condition. Moreover, the NH3-sensing performance of the optimal Sn–TiO2@rGO/CNT gas sensor in this study is superior to other recent reports of room-temperature NH3 as listed in Table 1.
Figure 5.
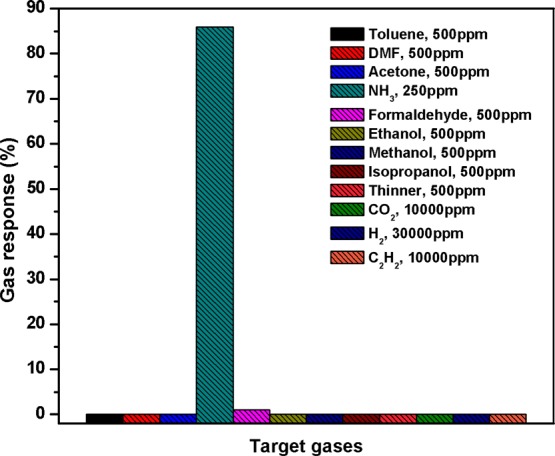
Selectivity histogram of the nanocomposite I gas sensor to various gases/VOCs at room temperature.
Table 1. Comparison between the Present Study and Other Reported Ammonia Gas Sensors Operating at Room Temperature.
| materials | response (%) | ammonia (ppm) | refs |
|---|---|---|---|
| CuSbS2 QDs/rGO composites | 42a | 250 | (53) |
| PANI/Fe3O4 | 47a | 300 | (54) |
| OP-G/G channel | 29.3a | 1000 | (55) |
| CCO–Co(10) | 7.9a | 400 | (56) |
| O2 functional groups on rGO | 3.1a | 300 | (57) |
| 0.1-Pt QDs/WS2 NSs | 14.1b | 500 | (58) |
| TiO2QDs/WS2 nanohybrids | 43.75b | 250 | (59) |
| graphite oxide | 30a | 500 | (60) |
| SnO2/rGO hybrids | 4.73a | 300 | (61) |
| Sn–TiO2@rGO/CNT | 85.9a | 250 | this work |
ΔR/Ra × 100.
ΔI/Ia × 100.
2.3. Sensing Mechanism of Sn–TiO2@rGO/CNT Nanocomposite Gas Sensor
From the results, the resistance changing behaviors may be attributed to the adsorption and desorption of NH3 molecules on the Sn–TiO2@rGO/CNT nanocomposite sensing film. The NH3-sensing mechanisms of the Sn–TiO2@rGO/CNT nanocomposite gas sensor at room temperature may be explained based on the formation of p–n heterojunctions between p-type rGO/CNT and n-type metal–oxide–semiconductor (MOS) of Sn–TiO2.62−64 In air, oxygen species (O2–) chemisorbed on the surface of Sn–TiO2@rGO/CNT nanocomposite film will capture free electrons from the conduction band of Sn–TiO2 and the Fermi level of rGO/CNT according to the surface reaction: (1) O2 (ads) + e– → O2– (ads), leading to the formation of surface depletion layers on the interface of the Sn–TiO2@rGO/CNT sensing film and cause the high resistance of the sensor in air as illustrated in Figure 6a.65−67 Upon exposure to NH3 vapor, NH3 molecules as a reducing gas will react with oxygen species according to the reaction: (2) 4NH3 (gas) + 5O2– (ads) → 4NO (gas) + 6H2O (gas) + 5e–, leading to discharge of electrons into the conduction band of Sn–TiO2 on the sensor surface (as seen in Figure 6a).27,68,69 This process results in a decrease of the thickness of the electron depletion layer leading to decrease in the resistance of the sensor. Moreover, the NH3-sensing process of the Sn–TiO2@rGO/CNT nanocomposite generates a charge between Sn–TiO2 MOS and NH3 molecules to electron transport through the rGO/CNT nanostructure. The rGO/CNT behaves as the electrical conduction pathway between Sn–TiO2 grain boundaries due to the high carrier mobility of rGO/CNT. The Sn–TiO2 nanoparticles are randomly interwoven with the rGO/CNT nanostructure to form a nanocomposite network that can enhance the surface area of the sensing film to increases the ability for adsorption and desorption of gas molecules.
Figure 6.
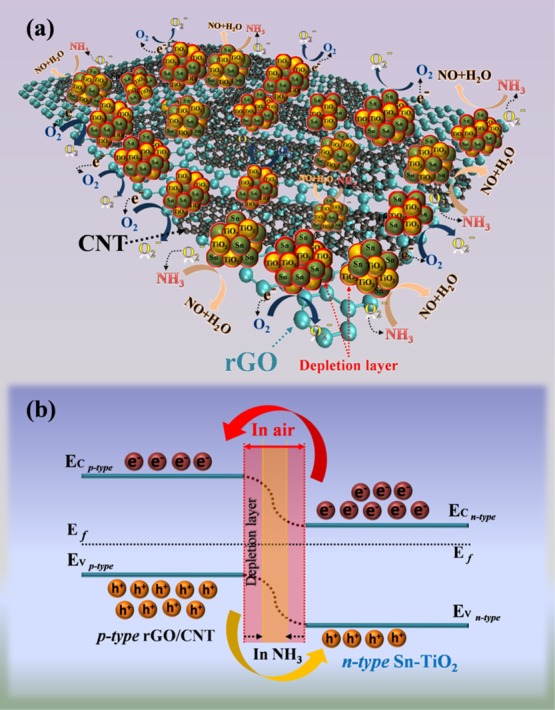
(a) Schematic of the proposed NH3-sensing mechanism and (b) p–n heterojunction of the Sn–TiO2@rGO/CNT nanocomposite gas sensor.
Furthermore, Sn–TiO2 and rGO/CNT are typical n-type MOS and p-type semiconductors, respectively. When the p–n heterojunctions are formed, the electrons in Sn–TiO2 will transfer to rGO/CNT, whereas the holes will transfer from rGO/CNT to Sn–TiO2 because the n-type Sn–TiO2 has a higher Fermi level than the p-type rGO/CNT, as shown in Figure 6b.70−72 Once the Sn–TiO2@rGO/CNT sensor is exposed to NH3 vapor, the reaction between NH3 molecules and oxygen species will occur on the n-type Sn–TiO2 surface, so that the electrons are released from the ionized oxygen species back to the conduction band of n-type Sn–TiO2 and combine with holes of p-type rGO/CNT, leading to reduction of the p–n heterojunction electron depletion layer width and decrease the potential barrier height (ΔΦ). The resistance of the Sn–TiO2@rGO/CNT sensor thus decreases upon exposure to NH3 vapor at room temperature. In addition, after the sensor is exposed to NH3 molecules, the resistance will decrease due to the decrease of the potential barrier height at the p–n heterojunction according to the relationship: R = B exp(ΔΦq/kT), where R is the sensor resistance, B is a constant, q is the electron charge, ΔΦ is an effective the potential barrier height the p–n heterojunction, k is Boltzmann’s constant, and T is the temperature of the sensing layer.39,73
3. Conclusions
In conclusion, Sn–TiO2@rGO/CNT nanocomposites were successfully synthesized by the solvothermal method and systematically characterized for NH3 sensing at room temperature. SEM, TEM, and XPS characterizations confirmed the presence of Sn–TiO2 nanoparticles on the surface of rGO/CNT nanocomposite. As seen from the gas-sensing results, the Sn–TiO2@rGO/CNT gas sensor demonstrated a rapid decrease of resistance upon exposure to NH3 and fully recovered to its baseline values in air with good repeatability. The nanocomposite I gas sensor Sn–TiO2@rGO/CNT with molar ratio of Sn/Ti (1:10) showed the highest response to NH3 at room temperature due to balance of p–n heterojunctions and high active sensing area. Moreover, the Sn–TiO2@rGO/CNT gas sensor exhibited ultrahigh selectivity toward NH3 against various volatile organic compounds (VOCs) and environmental gases at room temperature with no effect of humidity in the range of 30–70% RH. The NH3-sensing mechanism of the Sn–TiO2@rGO/CNT nanocomposite gas sensor has been proposed based on the formation of p–n heterojunctions of p-type rGO/CNT and n-type Sn–TiO2 via the low-temperature oxidizing reaction process. The observed ultrahigh NH3 selectivity, high response, relatively short response, and recovery times proved that the Sn–TiO2@rGO/CNT nanocomposite can be considered as a promising material for practical application of NH3 detection at room temperature.
4. Experimental Details
4.1. Synthesis of Sn–TiO2@rGO/CNT Nanocomposites
The synthesis process of the Sn–TiO2@rGO/CNT nanocomposite is demonstrated in Figure 7. Graphene oxide (GO) was purchased from ACS Material, LLC. CNT (with diameter 20–40 nm, length ≈ 30 μm) were purchased from Timesnano Co., Ltd. (Chengdu, China). Sn–TiO2@rGO/CNT composites were synthesized by the solvothermal method. The amount of 100 mg of GO and CNT was dispersed in 60 mL of ethanol and treated in an ultrasonic processor for 30 min. Then, stannous chloride (SnCl2·2H2O) and 1.5 mL of HCl (1 M) were added into the rGO/CNT solution under mechanical stirring for 30 min. Tetrabutyl titanate (C16H36O4Ti) was mixed into the above suspension and stirred till molar ratios of Sn/Ti is 1:10. This was followed by the addition of 2 mL NaOH (1 M) dropwise and mechanically stirred for 30 min. The solution was then transferred to Teflon-lined stainless-steel autoclave and heated oven at 180 °C for 12 h. At the end of reaction, the precipitates were collected, washed with deionized (DI) water and ethanol 3 times, respectively, and then dried at 80 °C overnight to obtain the as-prepared Sn–TiO2@rGO/CNT nanocomposites. Finally, the well crystalline solid powders were sintered at 500 °C for 3 h. In addition, the Sn–TiO2@rGO/CNT nanocomposite with different molar ratios of Sn/Ti (3:10 and 5:10) were also produced with the same process in order to investigate the effects of Sn content on sensing properties.
Figure 7.
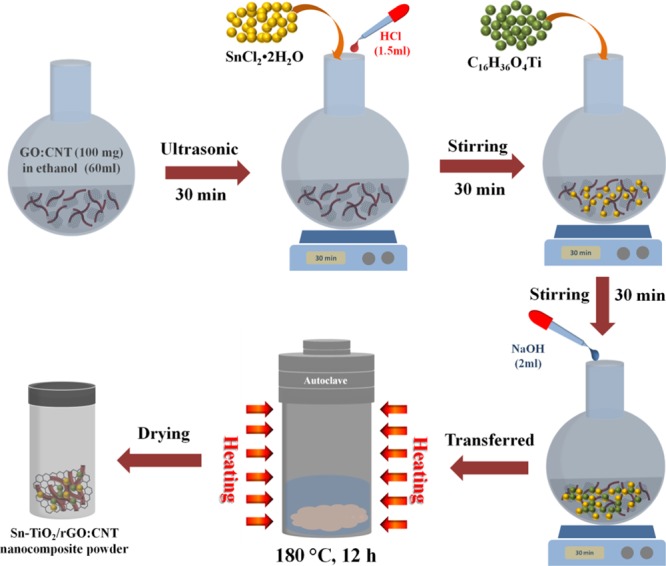
Schematic diagram of Sn–TiO2@rGO/CNT nanocomposite synthesis process.
4.2. Fabrication of Sn–TiO2@rGO/CNT Nanocomposite Gas Sensors
The fabrication process of the Sn–TiO2@rGO/CNT nanocomposite gas sensor is displayed in Figure 8. Sn–TiO2@rGO/CNT nanocomposite powder (100 mg) was dispersed in 1 mL of DI water under ultrasonication for 5 min. The aluminum-interdigitated electrodes with the size of 4 mm × 4 mm and spacing 100 μm were prefabricated on an alumina substrate by conventional photolithography, radio-frequency sputtering, and lift-off processes. Before drop coating of the sensing film, the aluminum interdigitated electrodes on the alumina substrate were treated by O2 plasma in order to improve the adhesion of the sensing film on the substrates and render a hydrophilic surface. Subsequently, the Sn–TiO2@rGO/CNT aqueous dispersion was drop-coated on the aluminum-interdigitated electrodes and dried at 80 °C. The fabricated Sn–TiO2@rGO/CNT gas sensors with different molar ratios of Sn/Ti (1:10, 3:10, and 5:10) were defined as nanocomposite I, nanocomposite II, and nanocomposite III gas sensors, respectively.
Figure 8.
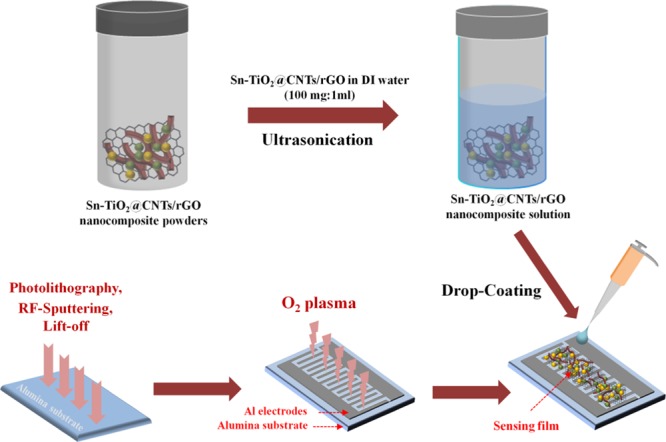
Schematic diagram of Sn–TiO2@rGO/CNT nanocomposite gas sensor fabrication process.
4.3. Gas-Sensing Measurement
The gas-sensing properties of Sn–TiO2@rGO/CNT nanocomposite gas sensors were tested in a Teflon chamber with the dynamic flow measurement. Various gases/VOCs including toluene, DMF, acetone, ammonia, formaldehyde, ethanol, methanol, isopropanol, thinner, carbon dioxide, hydrogen, and acetylene were used to evaluate the response and selectivity of fabricated gas sensors at the room temperature (26 ± 2 °C) with RH (56 ± 2%). The concentrations of test gases were varied using mass flow controllers with a flux of synthetic air. The total gas rate was fixed at 1000 sccm. The baseline of sensors was obtained by clean air for 3 min, and then the tested gas at a particular concentration was introduced into the sensor chamber for 2 min. A simple voltage divider circuit at a fixed voltage of 5 V was employed to measure the sensor resistances. The resistances of gas sensors were recorded every 1 s via a USB NI-DAQ 6008 under our developed LabVIEW software. The performances of the gas sensors were determined by means of gas response and selectivity. The gas response is defined as S (%) = [(Rair – Rgas)/Rair] × 100, where Rair and Rgas are the resistance of the fabricated gas sensor in clean air and the test gas, respectively. Selectivity is the ability of a sensor to identify a target gas that can be evaluated from relative response between different gases. The response time is defined as the time required for the sensor resistance to reach 90% of the final equilibrium signal upon exposure to the target gas, while the recovery time is the time needed to recover 90% of the initial baseline.
Acknowledgments
This work was financially supported by a grant (TRF-KURDI Research Career Development Grant) from Thailand Research Fund and Kasetsart University Research and Development Institute (RSA6180062).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02185.
Stability of gas sensor and changes in resistance of Sn–TiO2 and rGO/CNT gas sensors to 250 ppm ammonia as well as the effect of humidity on sensor resistance (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Timmer B.; Olthuis W.; Berg A. v. d. Ammonia sensors and their applications-a review. Sens. Actuators, B 2005, 107, 666–677. 10.1016/j.snb.2004.11.054. [DOI] [Google Scholar]
- Moos R.; Müller R.; Plog C.; Knezevic A.; Leye H.; Irion E.; Braun T.; Marquardt K.-J.; Binder K. Selective ammonia exhaust gas sensor for automotive applications. Sens. Actuators, B 2002, 83, 181–189. 10.1016/s0925-4005(01)01038-3. [DOI] [Google Scholar]
- Fedoruk M. J.; Bronstein R.; Kerger B. D. Ammonia exposure and hazard assessment for selected household cleaning product uses. J. Exposure Sci. Environ. Epidemiol. 2005, 15, 534–544. 10.1038/sj.jea.7500431. [DOI] [PubMed] [Google Scholar]
- Yang J.; Muroyama H.; Matsui T.; Eguchi K. Development of a direct ammonia-fueled molten hydroxide fuel cell. J. Power Sources 2014, 245, 277–282. 10.1016/j.jpowsour.2013.06.143. [DOI] [Google Scholar]
- Narasimhan L. R.; Goodman W.; Patel C. K. N. Correlation of breath ammonia with blood urea nitrogen and creatinine during hemodialysis. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 4617–4621. 10.1073/pnas.071057598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney D. J.; Hubbard T.; Putnam D. Breath Ammonia Measurement in Helicobacter pylori Infection. Dig. Dis. Sci. 2002, 47, 2523–2530. 10.1023/a:1020568227868. [DOI] [PubMed] [Google Scholar]
- Li H.-Y.; Lee C.-S.; Kim D. H.; Lee J.-H. Flexible Room-Temperature NH3 Sensor for Ultrasensitive, Selective, and Humidity-Independent Gas Detection. ACS Appl. Mater. Interfaces 2018, 10, 27858–27867. 10.1021/acsami.8b09169. [DOI] [PubMed] [Google Scholar]
- Naseem S.; King A. J. Ammonia production in poultry houses can affect health of humans, birds, and the environment-techniques for its reduction during poultry production. Environ. Sci. Pollut. Res. 2018, 25, 15269–15293. 10.1007/s11356-018-2018-y. [DOI] [PubMed] [Google Scholar]
- Sankar Ganesh R.; Navaneethan N.; Patil V. L.; Ponnusamy S.; Muthamizhchelvan C.; Kawasaki S.; Patil P. S.; Hayakawa Y. Sensitivity enhancement of ammonia gas sensor based on Ag/ZnO flower and nanoellipsoids at low temperature. Sens. Actuators, B 2018, 255, 672–683. 10.1016/j.snb.2017.08.015. [DOI] [Google Scholar]
- Liu X.; Chen N.; Han B.; Xiao X.; Chen G.; Djerdj I.; Wang Y. Nanoparticle cluster gas sensor: Pt activated SnO2 nanoparticles for NH3 detection with ultrahigh sensitivity. Nanoscale 2015, 7, 14872–14880. 10.1039/c5nr03585f. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhao Y.; Wang X.; Wang J.; Gaskov A. M.; Akbar S. A. Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens. Actuators, B 2016, 230, 330–336. 10.1016/j.snb.2016.02.069. [DOI] [Google Scholar]
- Mirzaei A.; Janghorban K.; Hashemi B.; Bonyani M.; Leonardi S. G.; Neri G. A novel gas sensor based on Ag/Fe2O3 core-shell nanocomposites. Ceram. Int. 2016, 42, 18974–18982. 10.1016/j.ceramint.2016.09.052. [DOI] [Google Scholar]
- Mani G. K.; Rayappan J. B. B. A highly selective and wide range ammonia sensor—Nanostructured ZnO:Co thin film. Mater. Sci. Eng., B 2015, 191, 41–50. 10.1016/j.mseb.2014.10.007. [DOI] [Google Scholar]
- Patil U. V.; Ramgir N. S.; Karmakar N.; Bhogale A.; Debnath A. K.; Aswal D. K.; Gupta S. K.; Kothari D. C. Room temperature ammonia sensor based on copper nanoparticle intercalated polyaniline nanocomposite thin films. Appl. Surf. Sci. 2015, 339, 69–74. 10.1016/j.apsusc.2015.02.164. [DOI] [Google Scholar]
- Detsi E.; Cook J. B.; Lesel B. K.; Turner C. L.; Liang Y.-L.; Robbennolt S.; Tolbert S. H. Mesoporous Ni60Fe30Mn10-alloy based metal/metal oxide composite thick films as highly active and robust oxygen evolution catalysts. Energy Environ. Sci. 2016, 9, 540–549. 10.1039/c5ee02509e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam V.; Jeyaperumal K. S. Investigations of visible light driven Sn and Cu doped ZnO hybrid nanoparticles for photocatalytic performance and antibacterial activity. Appl. Surf. Sci. 2018, 449, 617–630. 10.1016/j.apsusc.2017.11.167. [DOI] [Google Scholar]
- Zhou Y.; Zou C.; Lin X.; Guo Y. UV light activated NO2 gas sensing based on Au nanoparticles decorated few-layer MoS2 thin film at room temperature. Appl. Phys. Lett. 2018, 113, 082103. 10.1063/1.5042061. [DOI] [Google Scholar]
- Zhou Y.; Gao C.; Guo Y. UV assisted ultrasensitive trace NO2 gas sensing based on few-layer MoS2 nanosheet–ZnO nanowire heterojunctions at room temperature. J. Mater. Chem. A 2018, 6, 10286–10296. 10.1039/c8ta02679c. [DOI] [Google Scholar]
- Xiang C.; Chen T.; Zhang H.; Zou Y.; Chu H.; Zhang H.; Xu F.; Sun L.; Tang C. Growth of copper–benzene-1,3,5-tricarboxylate on boron nitride nanotubes and application of the composite in methane sensing. Appl. Surf. Sci. 2017, 424, 39–44. 10.1016/j.apsusc.2017.02.224. [DOI] [Google Scholar]
- Liu Y. Hydrothermal synthesis of TiO2–rGO composites and their improved photocatalytic activity in visible light. RSC Adv. 2014, 4, 36040–36045. 10.1039/c4ra06342b. [DOI] [Google Scholar]
- Gan L.; Shang S.; Yuen C. W. M.; Jiang S.-x.; Hu E. Hydrothermal synthesis of magnetic CoFe2O4/graphene nanocomposites with improved photocatalytic activity. Appl. Surf. Sci. 2015, 351, 140–147. 10.1016/j.apsusc.2015.05.130. [DOI] [Google Scholar]
- Zou Y.; Wang Q.; Jiang D.; Xiang C.; Chu H.; Qiu S.; Zhang H.; Xu F.; Sun L.; Liu S. Pd-doped TiO2@polypyrrole core-shell composites as hydrogen-sensing materials. Ceram. Int. 2016, 42, 8257–8262. 10.1016/j.ceramint.2016.02.038. [DOI] [Google Scholar]
- Zhou Y.; Liu G.; Zhu X.; Guo Y. Ultrasensitive NO2 gas sensing based on rGO/MoS2 nanocomposite film at low temperature. Sens. Actuators, B 2017, 251, 280–290. 10.1016/j.snb.2017.05.060. [DOI] [Google Scholar]
- Wang S.; Kang Y.; Wang L.; Zhang H.; Wang Y.; Wang Y. Organic/inorganic hybrid sensors: A review. Sens. Actuators, B 2013, 182, 467–481. 10.1016/j.snb.2013.03.042. [DOI] [Google Scholar]
- Zou Y.; Wang Q.; Xiang C.; Tang C.; Chu H.; Qiu S.; Yan E.; Xu F.; Sun L. Doping composite of polyaniline and reduced graphene oxide with palladium nanoparticles for room-temperature hydrogen-gas sensing. Int. J. Hydrogen Energy 2016, 41, 5396–5404. 10.1016/j.ijhydene.2016.02.023. [DOI] [Google Scholar]
- Xiang C.; Jiang D.; Zou Y.; Chu H.; Qiu S.; Zhang H.; Xu F.; Sun L.; Zheng L. Ammonia sensor based on polypyrrole–graphene nanocomposite decorated with titania nanoparticles. Ceram. Int. 2015, 41, 6432–6438. 10.1016/j.ceramint.2015.01.081. [DOI] [Google Scholar]
- Seekaew Y.; Lokavee S.; Phokharatkul D.; Wisitsoraat A.; Kerdcharoen T.; Wongchoosuk C. Low-cost and flexible printed graphene–PEDOT:PSS gas sensor for ammonia detection. Org. Electron. 2014, 15, 2971–2981. 10.1016/j.orgel.2014.08.044. [DOI] [Google Scholar]
- Jin G.-P.; Peng X.; Ding Y.-F.; Liu W.-Q.; Ye J.-M. Electrodeposition of platinum–nickel alloy nanocomposites on polyaniline-multiwalled carbon nanotubes for carbon monoxide redox. J. Solid State Chem. 2009, 13, 967–973. 10.1007/s10008-008-0631-2. [DOI] [Google Scholar]
- Sen T.; Mishra S.; Shimpi N. G. Synthesis and sensing applications of polyaniline nanocomposites: a review. RSC Adv. 2016, 6, 42196–42222. 10.1039/c6ra03049a. [DOI] [Google Scholar]
- Wisitsoraat A.; Pakapongpan S.; Sriprachuabwong C.; Phokharatkul D.; Sritongkham P.; Lomas T.; Tuantranont A. Graphene–PEDOT:PSS on screen printed carbon electrode for enzymatic biosensing. J. Electroanal. Chem. 2013, 704, 208–213. 10.1016/j.jelechem.2013.07.012. [DOI] [Google Scholar]
- Cao F.; Zhao M.; Yu Y.; Chen B.; Huang Y.; Yang J.; Cao X.; Lu Q.; Zhang X.; Zhang Z.; Tan C.; Zhang H. Synthesis of Two-Dimensional CoS1.097/Nitrogen-Doped Carbon Nanocomposites Using Metal–Organic Framework Nanosheets as Precursors for Supercapacitor Application. J. Am. Chem. Soc. 2016, 138, 6924–6927. 10.1021/jacs.6b02540. [DOI] [PubMed] [Google Scholar]
- Stankovich S.; Dikin D. A.; Dommett G. H. B.; Kohlhaas K. M.; Zimney E. J.; Stach E. A.; Piner R. D.; Nguyen S. T.; Ruoff R. S. Graphene-based composite materials. Nature 2006, 442, 282–286. 10.1038/nature04969. [DOI] [PubMed] [Google Scholar]
- Flahaut E.; Peigney A.; Laurent C.; Marlière C.; Chastel F.; Rousset A. Carbon nanotube–metal–oxide nanocomposites: microstructure, electrical conductivity and mechanical properties. Acta Mater. 2000, 48, 3803–3812. 10.1016/s1359-6454(00)00147-6. [DOI] [Google Scholar]
- Haraguchi K. Synthesis and properties of soft nanocomposite materials with novel organic/inorganic network structures. Polym. J. 2011, 43, 223. 10.1038/pj.2010.141. [DOI] [Google Scholar]
- Li W.; Guo J.; Cai L.; Qi W.; Sun Y.; Xu J.-L.; Sun M.; Zhu H.; Xiang L.; Xie D.; Ren T. UV light irradiation enhanced gas sensor selectivity of NO2 and SO2 using rGO functionalized with hollow SnO2 nanofibers. Sens. Actuators, B 2019, 290, 443–452. 10.1016/j.snb.2019.03.133. [DOI] [Google Scholar]
- Lim S. P.; Pandikumar A.; Lim Y. S.; Huang N. M.; Lim H. N. In-situ electrochemically deposited polypyrrole nanoparticles incorporated reduced graphene oxide as an efficient counter electrode for platinum-free dye-sensitized solar cells. Sci. Rep. 2015, 4, 5305. 10.1038/srep05305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami S. K.; Seo J. Y.; Hyeon S.-E.; Shershah M. S. A.; Yoo P.-J.; Chung C.-H. Enhanced capacitive deionization performance by an rGO–SnO2 nanocomposite modified carbon felt electrode. RSC Adv. 2018, 8, 4182–4190. 10.1039/c7ra12764b. [DOI] [Google Scholar]
- Wang X.; Jia Z.; Liu F.; Liang H.; You X.; Wang K.; Lou X.; Shuang W.; Xiao L.; Cai B.; Yang L. The template-free synthesis of hierarchically porous anatase TiO2 via acid-etching for enhancing the cycling stability and reversible capacity of lithium ion batteries. RSC Adv. 2016, 6, 48985–48994. 10.1039/c6ra03821b. [DOI] [Google Scholar]
- Seekaew Y.; Wisitsoraat A.; Phokharatkul D.; Wongchoosuk C. Room temperature toluene gas sensor based on TiO2 nanoparticles decorated 3D graphene-carbon nanotube nanostructures. Sens. Actuators, B 2019, 279, 69–78. 10.1016/j.snb.2018.09.095. [DOI] [Google Scholar]
- Kang Y.; Xue Q.; Jin P.; Jiang J.; Zeng J.; Chen Y. Rhodium Nanosheets–Reduced Graphene Oxide Hybrids: A Highly Active Platinum-Alternative Electrocatalyst for the Methanol Oxidation Reaction in Alkaline Media. ACS Sustainable Chem. Eng. 2017, 5, 10156–10162. 10.1021/acssuschemeng.7b02163. [DOI] [Google Scholar]
- Cheong J. Y.; Kim C.; Jang J. S.; Kim I.-D. Rational design of Sn-based multicomponent anodes for high performance lithium-ion batteries: SnO2@TiO2@reduced graphene oxide nanotubes. RSC Adv. 2016, 6, 2920–2925. 10.1039/c5ra23704a. [DOI] [Google Scholar]
- Zhang B.; Zheng Q. B.; Huang Z. D.; Oh S. W.; Kim J. K. SnO2–graphene–carbon nanotube mixture for anode material with improved rate capacities. Carbon 2011, 49, 4524–4534. 10.1016/j.carbon.2011.06.059. [DOI] [Google Scholar]
- Kumari S.; Shekhar A.; Pathak D. D. Graphene oxide–TiO2 composite: an efficient heterogeneous catalyst for the green synthesis of pyrazoles and pyridines. New J. Chem. 2016, 40, 5053–5060. 10.1039/c5nj03380b. [DOI] [Google Scholar]
- Dang M. T.; Lefebvre J.; Wuest J. D. Recycling Indium Tin Oxide (ITO) Electrodes Used in Thin-Film Devices with Adjacent Hole-Transport Layers of Metal Oxides. ACS Sustainable Chem. Eng. 2015, 3, 3373–3381. 10.1021/acssuschemeng.5b01080. [DOI] [Google Scholar]
- Gupta A.; Dhakate S. R.; Gurunathan P.; Ramesha K. High rate capability and cyclic stability of hierarchically porous Tin oxide (IV)-carbon nanofibers as anode in lithium ion batteries. Appl. Nanosci. 2017, 7, 449–462. 10.1007/s13204-017-0577-8. [DOI] [Google Scholar]
- Ni S.; Guo F.; Wang D.; Jiao S.; Wang J.; Zhang Y.; Wang B.; Feng P.; Zhao L. Modification of TiO2 Nanowire Arrays with Sn Doping as Photoanode for Highly Efficient Dye-Sensitized Solar Cells. Crystals 2019, 9, 113. 10.3390/cryst9020113. [DOI] [Google Scholar]
- Yao Z.; Meng Y.; Zhao Y.; Liu G.; Xia Q.; Wang J.; Jiang Z. A one-step preparation and enhanced electrochemical properties of C-TiO2 composite films. Electrochim. Acta 2017, 254, 320–327. 10.1016/j.electacta.2017.09.107. [DOI] [Google Scholar]
- Ahmad Z.; Najeeb M. A.; Shakoor R. A.; Alashraf A.; Al-Muhtaseb S. A.; Soliman A.; Nazeeruddin M. K. Instability in CH3NH3PbI3 perovskite solar cells due to elemental migration and chemical composition changes. Sci. Rep. 2017, 7, 15406. 10.1038/s41598-017-15841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khore S. K.; Tellabati N. V.; Apte S. K.; Naik S. D.; Ojha P.; Kale B. B.; Sonawane R. S. Green sol–gel route for selective growth of 1D rutile N–TiO2: a highly active photocatalyst for H2 generation and environmental remediation under natural sunlight. RSC Adv. 2017, 7, 33029–33042. 10.1039/c7ra01648d. [DOI] [Google Scholar]
- Zheng J.; Wang Y.; Zhang F.; Yang Y.; Liu X.; Guo K.; Wang H.; Xu B. Microwave-assisted hydrothermal synthesis of solid-state carbon dots with intensive emission for white light-emitting devices. J. Mater. Chem. C 2017, 5, 8105–8111. 10.1039/c7tc01701d. [DOI] [Google Scholar]
- Zhou Y.; Li X.; Wang Y.; Tai H.; Guo Y. UV Illumination-Enhanced Molecular Ammonia Detection Based On a Ternary-Reduced Graphene Oxide–Titanium Dioxide–Au Composite Film at Room Temperature. Anal. Chem. 2019, 91, 3311–3318. 10.1021/acs.analchem.8b04347. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhao Y.; Wang X.; Wang J.; Gaskov A. M.; Akbar S. A. Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens. Actuators, B 2016, 230, 330–336. 10.1016/j.snb.2016.02.069. [DOI] [Google Scholar]
- Liu Y.; Wang H.; Chen K.; Yang T.; Yang S.; Chen W. Acidic Site-Assisted Ammonia Sensing of Novel CuSbS2 Quantum Dots/Reduced Graphene Oxide Composites with an Ultralow Detection Limit at Room Temperature. ACS Appl. Mater. Interfaces 2019, 11, 9573–9582. 10.1021/acsami.8b20830. [DOI] [PubMed] [Google Scholar]
- Chabukswar V. V.; Bora M. A.; Adhav P. B.; Diwate B. B.; Salunke-Gawali S. Ultra-fast, economical and room temperature operating ammonia sensor based on polyaniline/iron oxide hybrid nanocomposites. Polym. Bull. 2019, 10.1007/s00289-019-02703-4. [DOI] [Google Scholar]
- Wu H.; Bu X.; Deng M.; Chen G.; Zhang G.; Li X.; Wang X.; Liu W. A Gas Sensing Channel Composited with Pristine and Oxygen Plasma-Treated Graphene. Sensors 2019, 19, 625. 10.3390/s19030625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S.; Patrike A.; Badadhe S. S.; Bhardwaj M.; Ogale S. Room-Temperature Ammonia Gas Sensing Using Mixed-Valent CuCo2O4 Nanoplatelets: Performance Enhancement through Stoichiometry Control. ACS Omega 2018, 3, 1977–1982. 10.1021/acsomega.7b01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minitha C. R.; Anithaa V. S.; Subramaniam V.; Rajendra Kumar R. T. Impact of Oxygen Functional Groups on Reduced Graphene Oxide-Based Sensors for Ammonia and Toluene Detection at Room Temperature. ACS Omega 2018, 3, 4105–4112. 10.1021/acsomega.7b02085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang C.; Chen Y.; Qin Z.; Zeng D.; Zhang J.; Wang H.; Xie C. Two-dimensional WS2-based nanosheets modified by Pt quantum dots for enhanced room-temperature NH3 sensing properties. Appl. Surf. Sci. 2018, 455, 45–52. 10.1016/j.apsusc.2018.05.148. [DOI] [Google Scholar]
- Qin Z.; Ouyang C.; Zhang J.; Wan L.; Wang S.; Xie C.; Zeng D. 2D WS2 nanosheets with TiO2 quantum dots decoration for high-performance ammonia gas sensing at room temperature. Sens. Actuators, B 2017, 253, 1034–1042. 10.1016/j.snb.2017.07.052. [DOI] [Google Scholar]
- Bannov G. A.; Prášek J.; Jašek O.; Zajíčková L. Investigation of Pristine Graphite Oxide as Room-Temperature Chemiresistive Ammonia Gas Sensing Material. Sensors 2017, 17, 320. 10.3390/s17020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Liu J.; Jiang C.; Liu A.; Xia B. Quantitative detection of formaldehyde and ammonia gas via metal oxide-modified graphene-based sensor array combining with neural network model. Sens. Actuators, B 2017, 240, 55–65. 10.1016/j.snb.2016.08.085. [DOI] [Google Scholar]
- Wei B.-Y.; Hsu M.-C.; Su P.-G.; Lin H.-M.; Wu R.-J.; Lai H.-J. A novel SnO2 gas sensor doped with carbon nanotubes operating at room temperature. Sens. Actuators, B 2004, 101, 81–89. 10.1016/s0925-4005(04)00102-9. [DOI] [Google Scholar]
- Zhang D.; Liu A.; Chang H.; Xia B. Room-temperature high-performance acetone gas sensor based on hydrothermal synthesized SnO2-reduced graphene oxide hybrid composite. RSC Adv. 2015, 5, 3016–3022. 10.1039/c4ra10942b. [DOI] [Google Scholar]
- Li X.; Zhao Y.; Wang X.; Wang J.; Gaskov A. M.; Akbar S. A. Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens. Actuators, B 2016, 230, 330–336. 10.1016/j.snb.2016.02.069. [DOI] [Google Scholar]
- Zhang H.; Feng J.; Fei T.; Liu S.; Zhang T. SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens. Actuators, B 2014, 190, 472–478. 10.1016/j.snb.2013.08.067. [DOI] [Google Scholar]
- Seekaew Y.; Phokharatkul D.; Wisitsoraat A.; Wongchoosuk C. Highly sensitive and selective room-temperature NO2 gas sensor based on bilayer transferred chemical vapor deposited graphene. Appl. Surf. Sci. 2017, 404, 357–363. 10.1016/j.apsusc.2017.01.286. [DOI] [Google Scholar]
- Zhang D.; Liu A.; Chang H.; Xia B. Room-temperature high-performance acetone gas sensor based on hydrothermal synthesized SnO2-reduced graphene oxide hybrid composite. RSC Adv. 2015, 5, 3016–3022. 10.1039/c4ra10942b. [DOI] [Google Scholar]
- Zhang D.; Jiang C.; Sun Y. e. Room-temperature high-performance ammonia gas sensor based on layer-by-layer self-assembled molybdenum disulfide/zinc oxide nanocomposite film. J. Alloys Compd. 2017, 698, 476–483. 10.1016/j.jallcom.2016.12.222. [DOI] [Google Scholar]
- Ye Z.; Tai H.; Xie T.; Su Y.; Yuan Z.; Liu C.; Jiang Y. A facile method to develop novel TiO2/rGO layered film sensor for detecting ammonia at room temperature. Mater. Lett. 2016, 165, 127–130. 10.1016/j.matlet.2015.11.129. [DOI] [Google Scholar]
- Zhang D.; Wu Z.; Zong X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators, B 2019, 289, 32–41. 10.1016/j.snb.2019.03.055. [DOI] [Google Scholar]
- Kim J.-H.; Mirzaei A.; Kim H. W.; Kim S. S. Low power-consumption CO gas sensors based on Au-functionalized SnO2-ZnO core-shell nanowires. Sens. Actuators, B 2018, 267, 597–607. 10.1016/j.snb.2018.04.079. [DOI] [Google Scholar]
- Miller D. R.; Akbar S. A.; Morris P. A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators, B 2014, 204, 250–272. 10.1016/j.snb.2014.07.074. [DOI] [Google Scholar]
- Kim H.-J.; Lee J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators, B 2014, 192, 607–627. 10.1016/j.snb.2013.11.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.