Abstract
This paper represents the third contribution in the Genera of Phytopathogenic Fungi (GOPHY) series. The series provides morphological descriptions, information about the pathology, distribution, hosts and disease symptoms for the treated genera, as well as primary and secondary DNA barcodes for the currently accepted species included in these. This third paper in the GOPHY series treats 21 genera of phytopathogenic fungi and their relatives including: Allophoma, Alternaria, Brunneosphaerella, Elsinoe, Exserohilum, Neosetophoma, Neostagonospora, Nothophoma, Parastagonospora, Phaeosphaeriopsis, Pleiocarpon, Pyrenophora, Ramichloridium, Seifertia, Seiridium, Septoriella, Setophoma, Stagonosporopsis, Stemphylium, Tubakia and Zasmidium. This study includes three new genera, 42 new species, 23 new combinations, four new names, and three typifications of older names.
Key words: DNA barcodes, Fungal systematics, New taxa
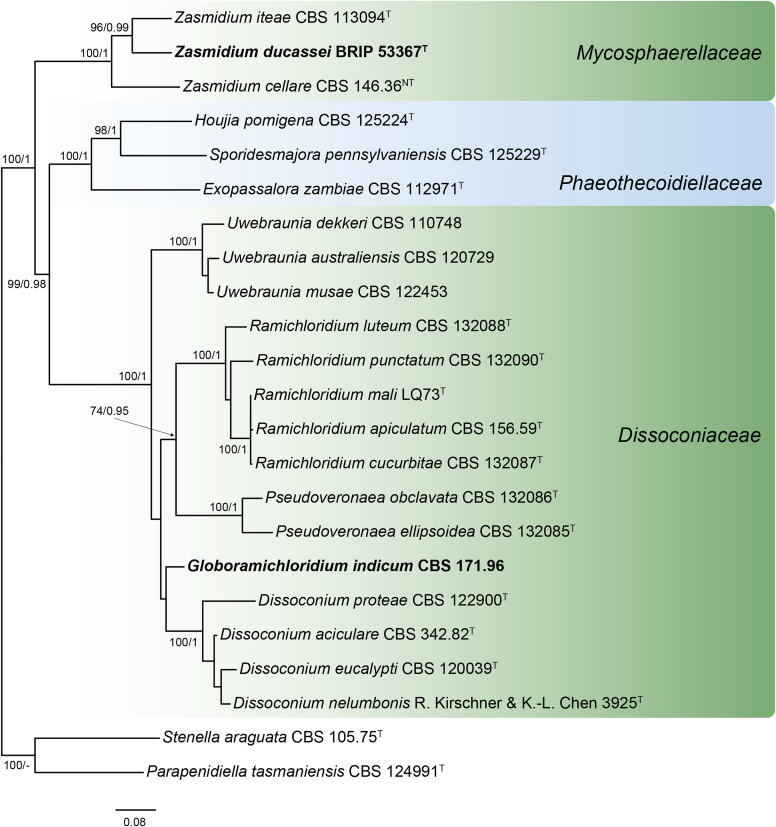
Taxonomic novelties: New genera: Arezzomyces Y. Marín & Crous, Globoramichloridium Y. Marín & Crous, Wingfieldomyces Y. Marín & Crous
New species: Allophoma pterospermicola Q. Chen & L. Cai; Alternaria aconidiophora Iturrieta-González, Dania García & Gené; Alternaria altcampina Iturrieta-González, Dania García & Gené; Alternaria chlamydosporifera Iturrieta-González, Dania García & Gené; Alternaria curvata Iturrieta-González, Dania García & Gené; Alternaria fimeti Iturrieta-González, Dania García & Gené; Alternaria inflata Iturrieta-González, Dania García & Gené; Alternaria lawrencei Iturrieta-González, Dania García & Gené; Alternaria montsantina Iturrieta-González, Dania García & Gené; Alternaria pobletensis Iturrieta-González, Dania García & Gené; Alternaria pseudoventricosa Iturrieta-González, Dania García & Gené; Brunneosphaerella roupeliae Crous; Elsinoe picconiae Crous; Elsinoe veronicae Crous, Thangavel & Y. Marín; Neosetophoma aseptata Crous, R.K. Schumach. & Y. Marín; Neosetophoma phragmitis Crous, R.K. Schumach. & Y. Marín; Neosetophoma sambuci Crous, R.K. Schumach. & Y. Marín; Neostagonospora sorghi Crous & Y. Marín; Parastagonospora novozelandica Crous, Thangavel & Y. Marín; Parastagonospora phragmitis Crous & Y. Marín; Phaeosphaeriopsis aloes Crous & Y. Marín; Phaeosphaeriopsis aloicola Crous & Y. Marín; Phaeosphaeriopsis grevilleae Crous & Y. Marín; Phaeosphaeriopsis pseudoagavacearum Crous & Y. Marín; Pleiocarpon livistonae Crous & Quaedvl.; Pyrenophora avenicola Y. Marín & Crous; Pyrenophora cynosuri Y. Marín & Crous; Pyrenophora novozelandica Y. Marín & Crous; Pyrenophora pseudoerythrospila Y. Marín & Crous; Pyrenophora sieglingiae Y. Marín & Crous; Pyrenophora variabilis Hern.-Restr. & Y. Marín; Septoriella germanica Crous, R.K. Schumach. & Y. Marín; Septoriella hibernica Crous, Quaedvl. & Y. Marín; Septoriella hollandica Crous, Quaedvl. & Y. Marín; Septoriella pseudophragmitis Crous, Quaedvl. & Y. Marín; Setophoma brachypodii Crous, R.K. Schumach. & Y. Marín; Setophoma pseudosacchari Crous & Y. Marín; Stemphylium rombundicum Moslemi, Y.P. Tan & P.W.J. Taylor; Stemphylium truncatulae Moslemi, Y.P. Tan & P.W.J. Taylor; Stemphylium waikerieanum Moslemi, Jacq. Edwards & P.W.J Taylor; Vagicola arundinis Phukhams., Camporesi & K.D. Hyde; Zasmidium thailandicum Crous
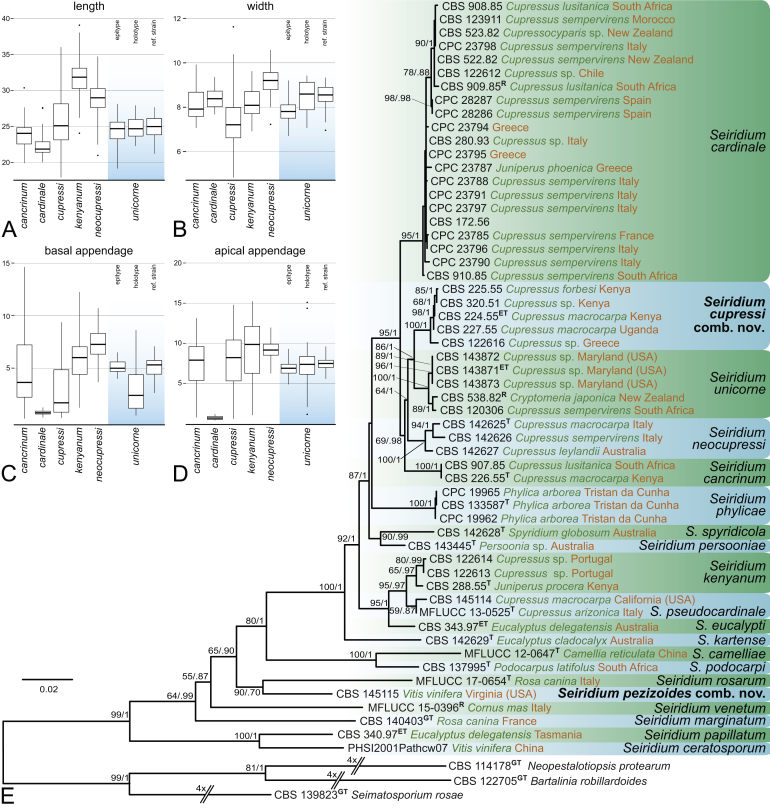
New combinations: Arezzomyces cytisi (Wanas. et al.) Y. Marín & Crous; Globoramichloridium indicum (Subram.) Y. Marín & Crous; Phaeosphaeria phoenicicola (Crous & Thangavel) Y. Marín & Crous; Pyrenophora poae (Baudyš) Y. Marín & Crous; Pyrenophora wirreganensis (Wallwork et al.) Y. Marín & Crous; Seiridium cupressi (Nattrass et al.) Bonthond, Sandoval-Denis & Crous; Seiridium pezizoides (de Not.) Crous; Septoriella agrostina (Mapook et al.) Y. Marín & Crous; Septoriella artemisiae (Wanas. et al.) Y. Marín & Crous; Septoriella arundinicola (Wanas. et al.) Y. Marín & Crous; Septoriella arundinis (W.J. Li et al.) Y. Marín & Crous; Septoriella bromi (Wijayaw. et al.) Y. Marín & Crous; Septoriella dactylidis (Wanas. et al.) Y. Marín & Crous; Septoriella elongata (Wehm.) Y. Marín & Crous; Septoriella forlicesenica (Thambug. et al.) Y. Marín & Crous; Septoriella garethjonesii (Thambug. et al.) Y. Marín & Crous; Septoriella italica (Thambug. et al.) Y. Marín & Crous; Septoriella muriformis (Ariyaw. et al.) Y. Marín & Crous; Septoriella rosae (Mapook et al.) Y. Marín & Crous; Septoriella subcylindrospora (W.J. Li et al.) Y. Marín & Crous; Septoriella vagans (Niessl) Y. Marín & Crous; Wingfieldomyces cyperi (Crous & M.J. Wingf.) Y. Marín & Crous; Zasmidium ducassei (R.G. Shivas et al.) Y. Marín & Crous
New names: Pyrenophora nisikadoi Y. Marín & Crous, Septoriella dactylidicola Y. Marín & Crous, Septoriella neoarundinis Y. Marín & Crous, Septoriella neodactylidis Y. Marín & Crous
Typification: epitypification: Ascochyta chrysanthemi F. Stevens, Pestalotia unicornis Cooke & Ellis, Rhynchosphaeria cupressi Nattrass et al
Introduction
Genera of Phytopathogenic Fungi (GOPHY) is a series of papers with the main focus to provide a stable platform for the taxonomy of phytopathogenic fungi. All genera included here are associated with plant disease, but note that many species treated are not well-known plant pathogens, or Koch’s postulates remain to be completed for them. This series links to a larger initiative known as the “The Genera of Fungi project” (www.GeneraOfFungi.org, Crous et al., 2014a, Crous et al., 2015a, Giraldo et al., 2017), which aims to revise the generic names of all currently accepted fungi (Kirk et al. 2013). Specific aims were detailed by Marin-Felix et al. (2017), when this series was launched. One of the most important aims is to resolve generic and species concepts of the fungi studied, since many taxa have been shown to represent species complexes, or to comprise poly- or paraphyletic genera (Crous et al. 2015b). Other issues to resolve include the fact that type material for many genera and species has not been designated or is missing, and that the vast majority of these taxa were described before the DNA era (Hibbett et al. 2011) and thus lack DNA barcodes (Schoch et al. 2012). Therefore, another important aim is to generate DNA barcodes of type species and type specimens in order to fix the application of these names. Moreover, in cases where no type material has been preserved, taxa need to be recollected, epi- or neotypes designated, and registered in MycoBank to ensure traceability of the nomenclatural act (Robert et al. 2013). Finally, it is necessary to designate a single scientific name for fungi (Crous et al. 2015b) for which sexual-asexual links have been resolved.
Two issues of GOPHY have already been published, in which 41 genera were treated, including a total of two new genera, 46 new species, 15 new combinations and 10 typifications of older names (Marin-Felix et al., 2017, Marin-Felix et al., 2019). In this third contribution, a further 21 genera are treated, resulting in the clarification of their taxonomy and classification, and the introduction of three new genera, 42 new species, 23 new combinations, four new names and the typification of three older names.
For submissions to future issues in the GOPHY series, mycologists are encouraged to contact Pedro Crous (p.crous@wi.knaw.nl) to ensure there is no overlap with activities arising from other research groups. Preference will be given to genera that include novel species, combinations or typifications. Generic contributions published in each issue will also be placed in the database displayed on www.plantpathogen.org.
Material and methods
Isolates and morphological analysis
Descriptions of the new taxa and typifications are based on cultures obtained from the collection at the American Type Culture Collection, Manassas, Virginia, USA (ATCC), the Queensland Plant Pathology Herbarium, Brisbane, Australia (BRIP), the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (CBS), the working collection of P.W. Crous (CPC), housed at the Westerdijk Fungal Biodiversity Institute (WI), the Chinese General Microbiological Culture Collection Center, Beijing, China (CGMCC), the Facultat de Medicina i Ciències de la Salut, University Rovira i Virgili, Reus, Spain (FMR), and the Victorian Plant Pathogen Herbarium, Bundoora, Australia (VPRI). For fresh collections, we followed the procedures previously described in Waksman, 1922, Crous et al., 1991 and Calduch et al. (2004). Colonies were transferred to different media, i.e. cornmeal agar (CMA), 2 % malt extract agar (MEA), potato carrot agar (PCA), 2 % potato dextrose agar (PDA), synthetic nutrient-poor agar (SNA), oatmeal agar (OA), water agar (WA) (Crous et al. 2019b), pine needle agar (PNA; Smith et al. 1996), and incubated under different conditions to induce sporulation. Requirements of media and conditions of incubation are specified for each genus. Reference strains and specimens are maintained at ATCC, BRIP, CBS, CPC, CGMCC, FMR and VPRI.
Vegetative and reproductive structures were mounted in 100 % lactic acid or Shear’s solution either directly from specimens or from colonies sporulating on CMA, MEA, OA, PCA, PDA, PNA, SNA or WA. For cultural characterisation, isolates were grown and incubated on different culture media and temperatures as indicated for each genus. Colour notations were rated according to the colour charts of Kornerup & Wanscher (1978) for Alternaria, and Rayner (1970) for all other genera. Taxonomic novelties were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004).
DNA isolation, amplification and analyses
Fungal DNA was extracted and purified directly from the colonies or host material as specified for each genus. Primers and protocols for the amplification and sequencing of gene loci, and software used for phylogenetic analyses can be found in the bibliographies provided for each genus. Phylogenetic analyses consisted of Maximum-Likelihood (ML) and Bayesian Inference (BI). ML was inferred as described in Hernández-Restrepo et al. (2016b), or by using MEGA v. 6.0 (Tamura et al. 2013). BI was carried out as described by Hernández-Restrepo et al. (2016b), or by using MrBayes on XSEDE v. 3.2.6 on the CIPRES portal (www.phylo.org). Sequence data generated in this study were deposited in GenBank and the alignments and trees in TreeBASE (http://www.treebase.org).
Results
Allophoma Q. Chen & L. Cai, Stud. Mycol. 82: 162. 2015. Fig. 1.
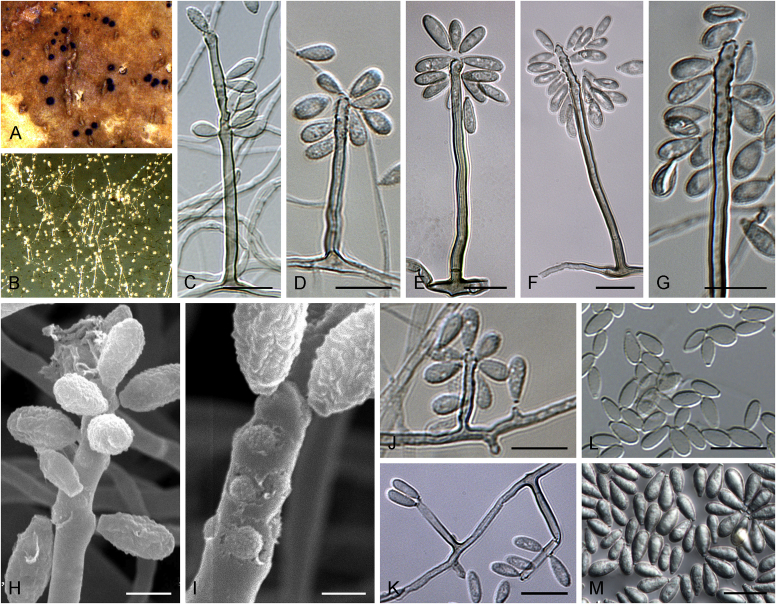
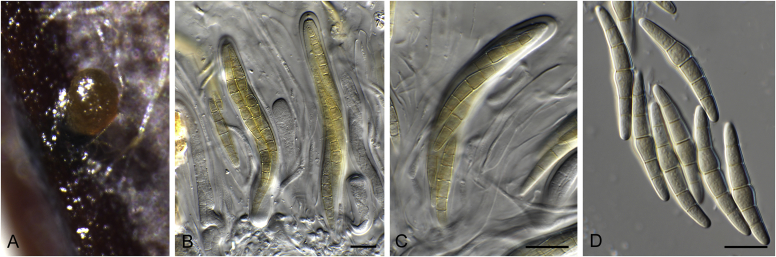
Fig. 1.
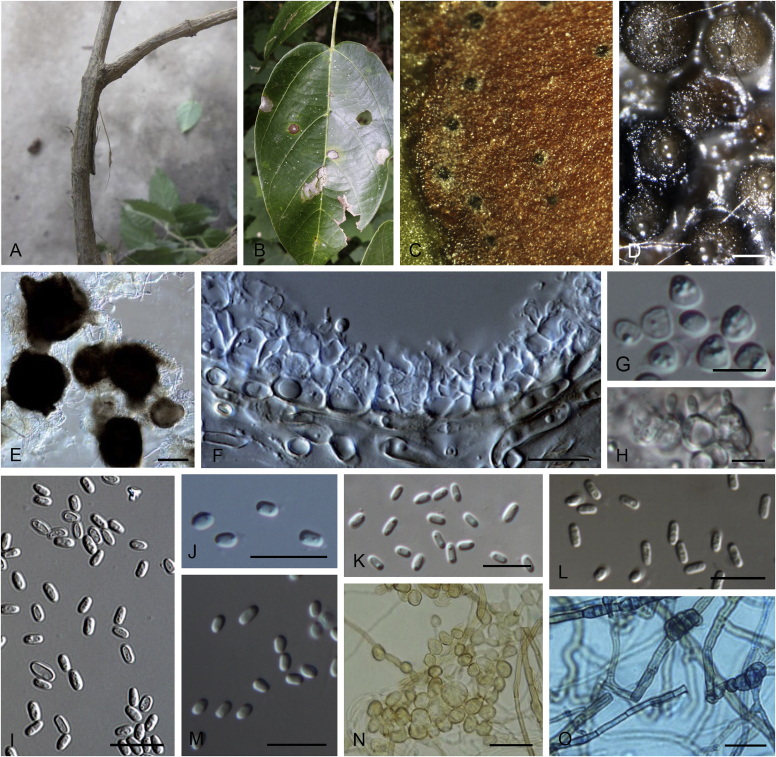
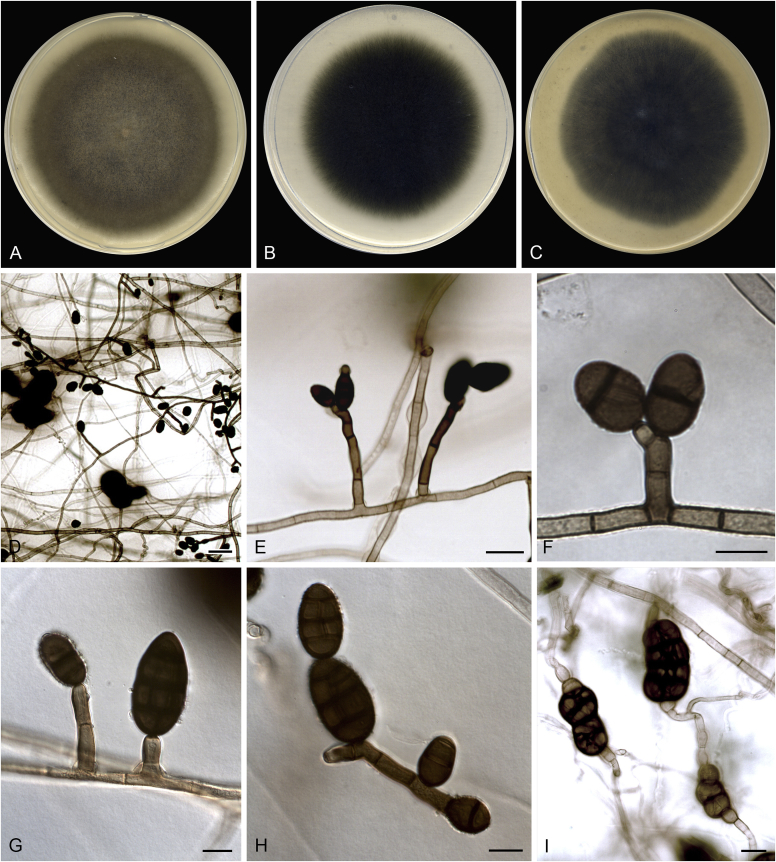
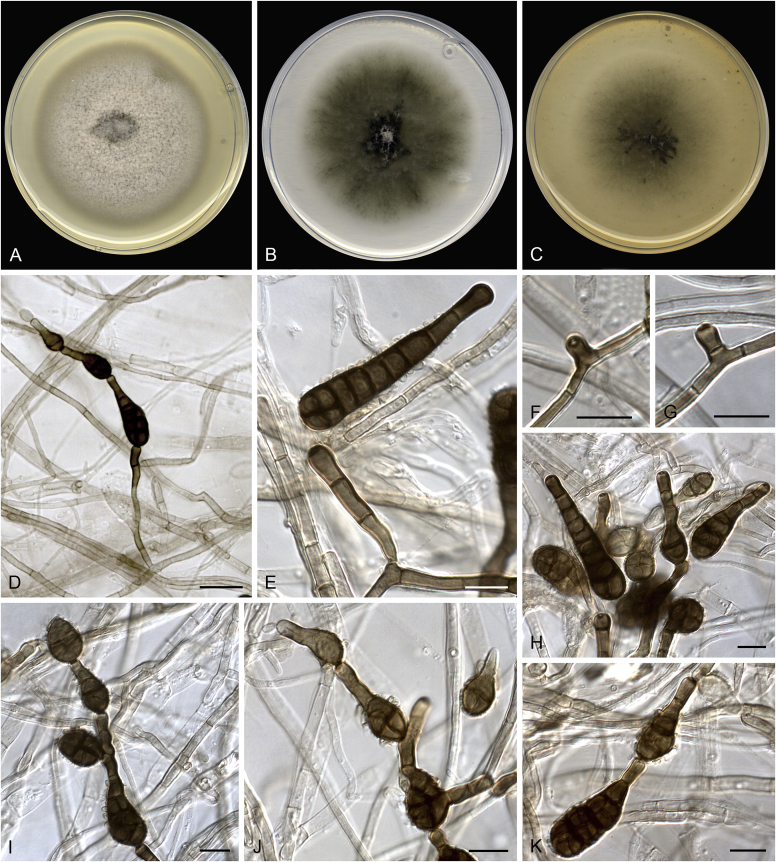
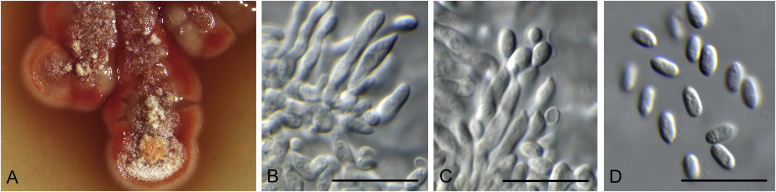
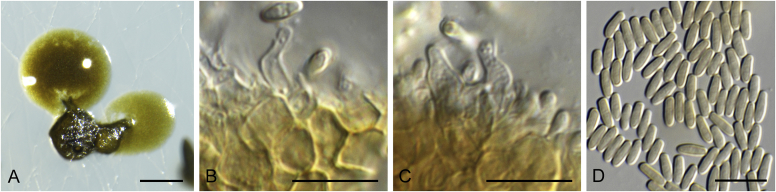
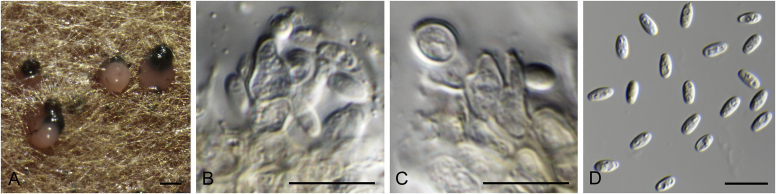
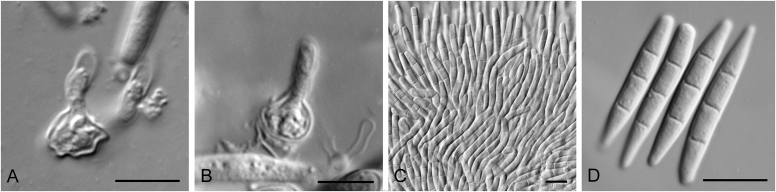
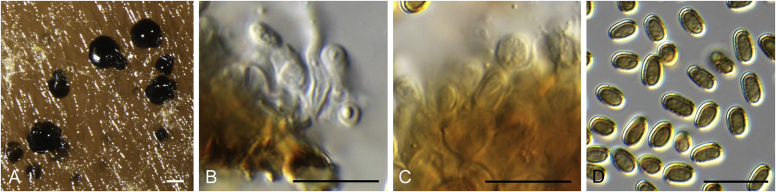
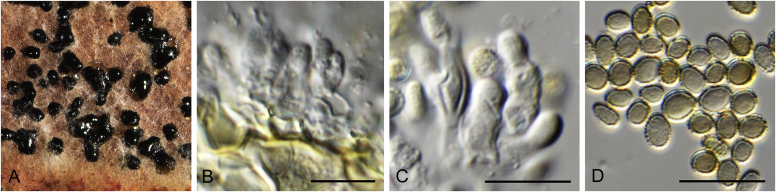
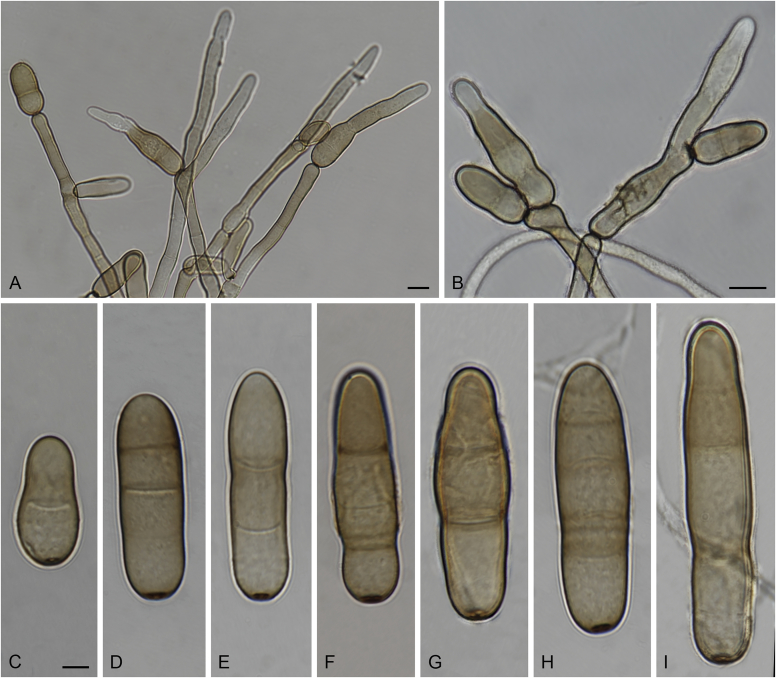
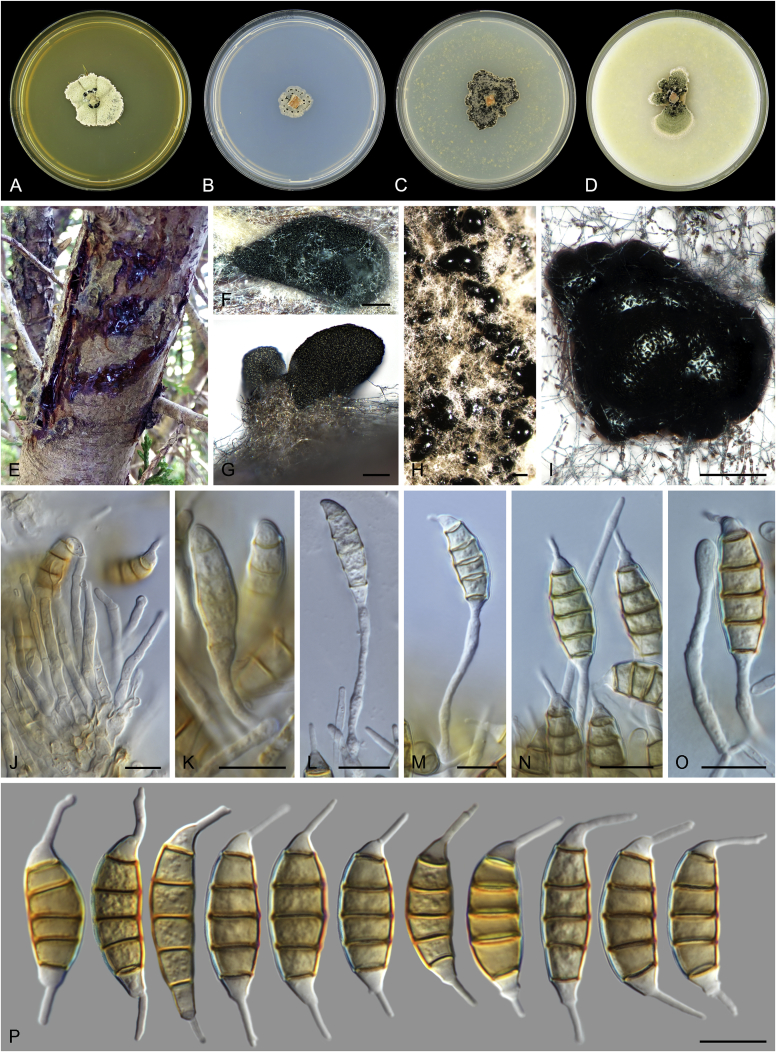
Allophoma spp. A, B. Disease symptoms. A. Symptoms caused by Allophoma hayatii (ex-type CBS 142859) on Lantana camara. B. Symptoms caused by Allophoma pterospermicola (ex-type CGMCC 3.19245) on Pterospermum xylocarpum. C–O. Asexual morph. C. Conidiomata of Allophoma pterospermicola (LC12181) sporulating on Maesa montana. D. Conidiomata of Allophoma oligotrophica (ex-type CGMCC 3.18114) sporulating on OA. E. Conidiomata of Allophoma minor (ex-type CBS 325.82). F. Section of the conidiomatal wall of Allophoma minor (ex-type CBS 325.82). G, H. Conidiogenous cells. G.Allophoma piperis (ex-epitype CBS 268.93). H.Allophoma oligotrophica (ex-type CGMCC 3.18114). I–M. Conidia. I.Allophoma minor (ex-type CBS 325.82). J.Allophoma piperis (ex-epitype CBS 268.93). K.Allophoma oligotrophica (ex-type CGMCC 3.18114). L.Allophoma cylindrispora (ex-type CBS 142453). M.Allophoma nicaraguensis (ex-type CBS 506.91). N. Swollen cells of Allophoma hayatii (ex-type CBS 142859). O. Chlamydospores of Allophoma hayatii (ex-type CBS 142859). Scale bars: D, E = 100 μm; O = 50 μm; N = 20 μm; F, I–M = 10 μm; G, H = 5 μm. Pictures A, N–O taken from Babaahmadi et al. (2018); D, H, K, from Chen et al. (2017); E, F, I from Aveskamp et al. (2010); G, J, M from Chen et al. (2015); L from Valenzuela-Lopez et al. (2018).
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Didymellaceae.
Type species: Allophoma tropica (R. Schneid. & Boerema) Q. Chen & L. Cai, basionym: Phoma tropica R. Schneid. & Boerema, Phytopathol. Z. 83: 361. 1975. Isotype and ex-isotype strain: CBS H-7629, CBS 436.75 = DSM 63365.
DNA barcodes (genus): LSU, ITS.
DNA barcodes (species): rpb2, tub2. Table 1. Fig. 2.
Table 1.
DNA barcodes of accepted Allophoma spp.
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CGMCC: Chinese General Microbiological Culture Collection Center, Beijing, China; LC: Dr Lei Cai's personal culture collection, housed at CAS, China. T,ET and IsoT indicate ex-type,ex-epitype and ex-isotype strains, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial large subunit (28S) nrRNA gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tub2: partial β-tubulin gene.
Fig. 2.
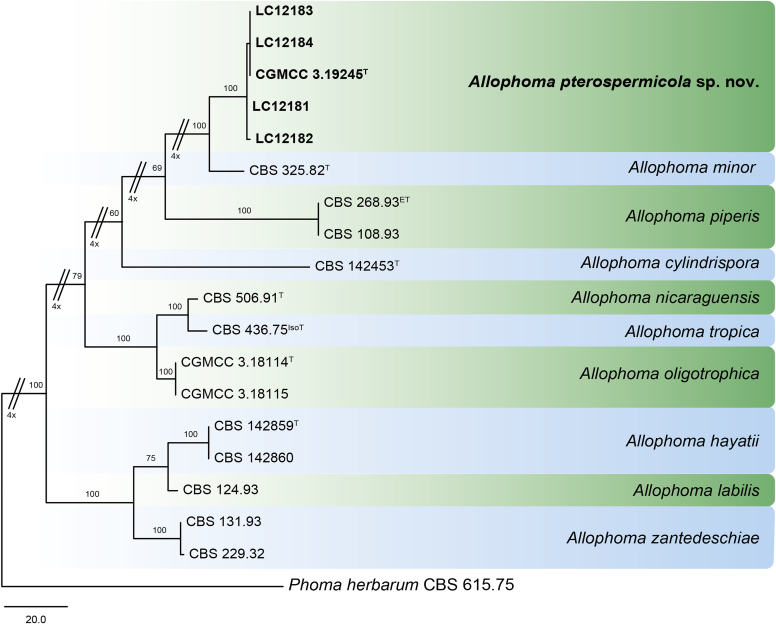
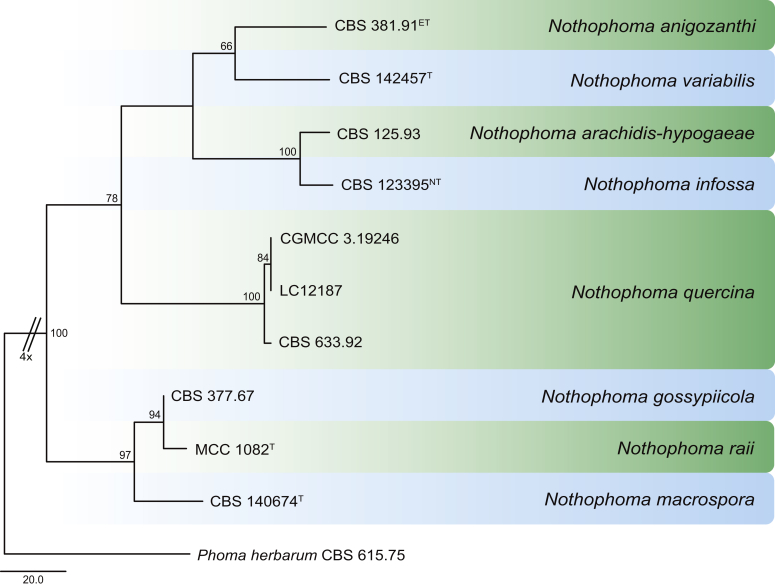
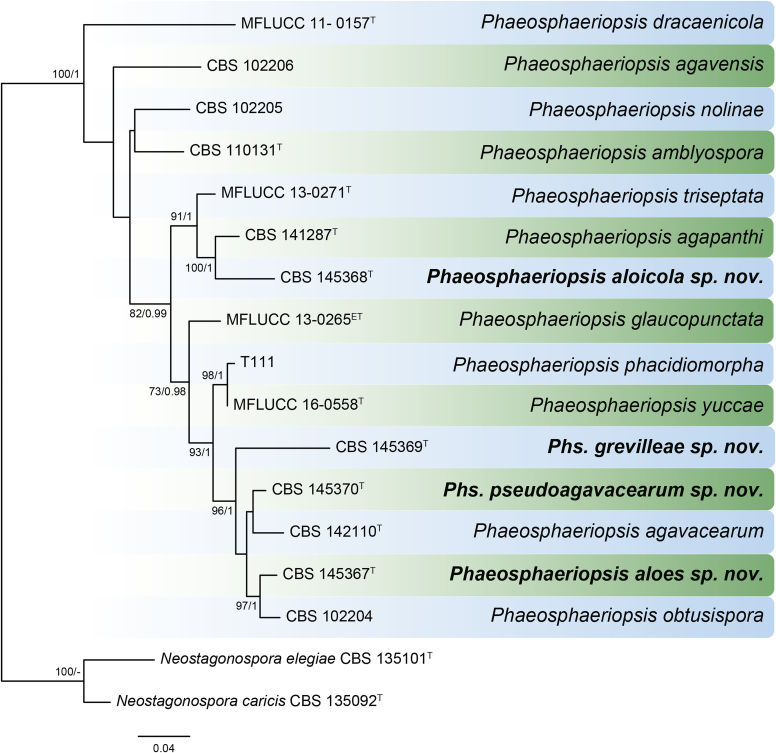
Phylogenetic tree generated from a maximum parsimony analysis based on the combined LSU (860 bp), ITS (480 bp), tub2 (333 bp) and rpb2 (803 bp) sequences of all accepted species of Allophoma. The tree was rooted to Phoma herbarum CBS 615.75. Values above the branches represent parsimony bootstrap support values (> 50 %). Novel sequences and novel taxon are printed in bold. GenBank accession numbers are indicated in Table 1. T, ET and IsoT indicate ex-type, ex-epitype and ex-isotype strains, respectively. TreeBASE: S23493.
Conidiomata pycnidial, globose to flask-shaped, ovoid, superficial or (semi-)immersed, solitary or confluent, ostiolate, sometimes with an elongated neck; conidiomatal wall pseudoparenchymatous, multi-layered. Conidiogenous cells phialidic, hyaline, smooth-walled, ampulliform to doliiform, sometimes flask-shaped or isodiametric. Conidia hyaline, smooth- and thin-walled, aseptate or 1-septate, variable in shape and size, i.e. ovoid, oblong, ellipsoidal to cylindrical, or slightly allantoid, mostly guttulate. Chlamydospores uni- or multicellular (pseudosclerotioid and dictyosporous), solitary or in chains, intercalary or terminal, smooth-walled, brown, where multicellular variable in shape and size. Swollen cells (pseudo-chlamydospores) pale brown, terminal or intercalary, solitary or in clusters, variable in size and shape, commonly in aerial mycelia. Sexual morph unknown (adapted from Chen et al., 2015, Babaahmadi et al., 2018).
Culture characteristics: Colonies on OA white when young, grey to olivaceous or dull green, brown, floccose to woolly, sometimes with rosy-buff tinges near the colony margins or yellow pigment in the sterile sectors, margins regular.
Optimal media and cultivation conditions: OA or sterile pine needles placed on OA under near-ultraviolet light (12 h light, 12 h dark) to promote sporulation at 25 °C.
Distribution: Worldwide.
Hosts: Wide host range, occurring as pathogens or saprobes, on Araceae, Fabaceae, Gesneriaceae, Myrtaceae, Papaveraceae, Piperaceae, Primulaceae, Rosaceae, Rubiaceae, Solanaceae, Sterculiaceae, Verbenaceae and other hosts, including humans.
Disease symptoms: Dieback, tissue necrosis, leaf spots, stem rot, leaf blotch, but also saprobic or isolated from other substrates and environments, e.g. air from karst caves and human infections.
Notes: The genus Allophoma was introduced by Chen et al. (2015) to accommodate five previously described Phoma species, namely Al. labilis (syn. Pho. labilis), Al. minor (syn. Pho. minor), Al. piperis (syn. Pho. piperis), Al. tropica (syn. Pho. tropica) and Al. zantedeschiae (syn. Pho. zantedeschiae), and a new species Al. nicaraguensis. Another four species have been described in the subsequent years, i.e. Al. cylindrispora (Valenzuela-Lopez et al. 2018), Al. hayatii (Babaahmadi et al. 2018), Al. oligotrophica (Chen et al. 2017) and Al. pterospermicola sp. nov. in the present study. Differentiating Allophoma from related phoma-like genera based on morphology alone is sometimes complicated. Furthermore, Allophoma species are morphologically similar and hard to differentiate from one another. Therefore, molecular data are essential for accurate identification of species within this genus, with ITS, LSU, tub2 and rpb2 being the loci selected for this purpose (Chen et al., 2015, Chen et al., 2017, Valenzuela-Lopez et al., 2018). No sexual morph of this genus has been observed to date.
These fungi are generally found in soil, air and regarded as saprobes or as the causal organisms of various diseases of different herbaceous and woody plants, such as some ornamental plants, coffee, etc., and even human eye lesions (Boerema et al., 2004, Aveskamp et al., 2010, Chen et al., 2015, Chen et al., 2017, Babaahmadi et al., 2018, Valenzuela-Lopez et al., 2018).
References: Boerema et al. 2004 (morphology and pathogenicity), Aveskamp et al., 2010, Chen et al., 2015, Chen et al., 2017, Babaahmadi et al., 2018, Valenzuela-Lopez et al., 2018 (morphology, phylogeny and pathogenicity).
Allophoma pterospermicola Q. Chen & L. Cai, sp. nov. MycoBank MB828313. Fig. 3.
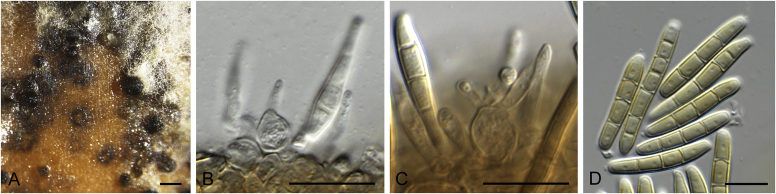
Fig. 3.
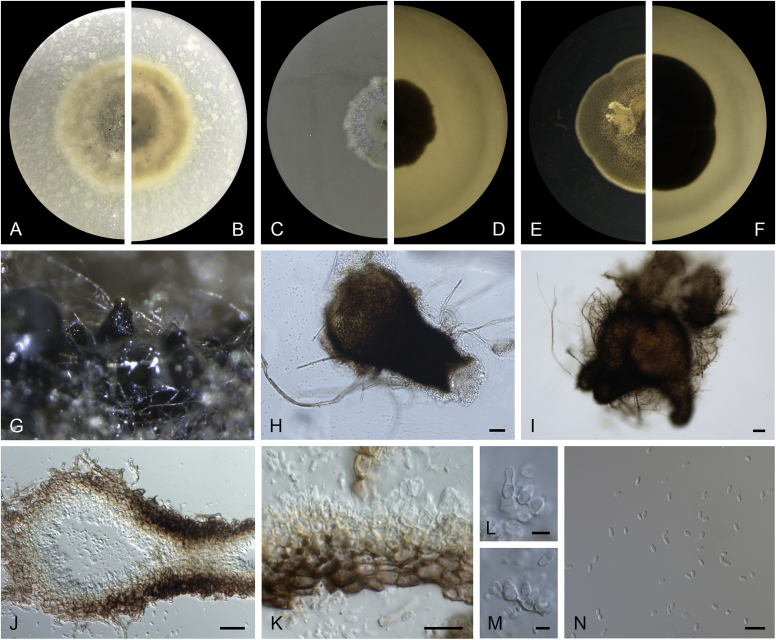
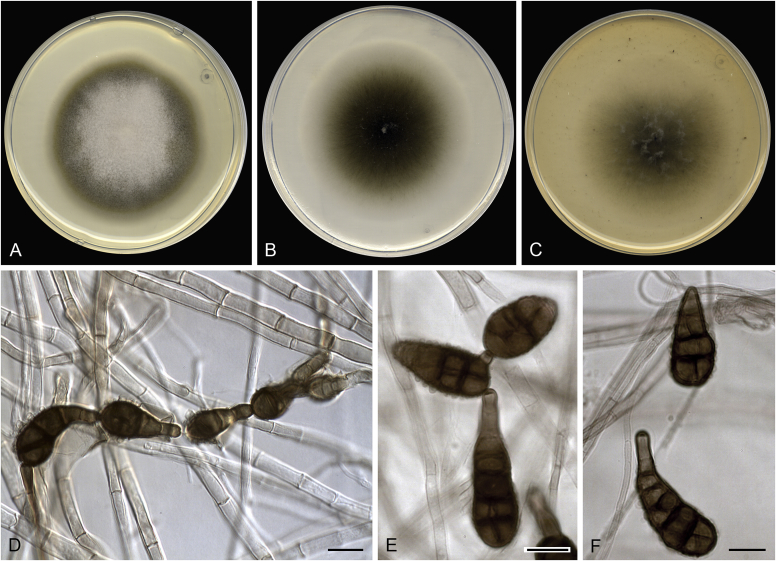
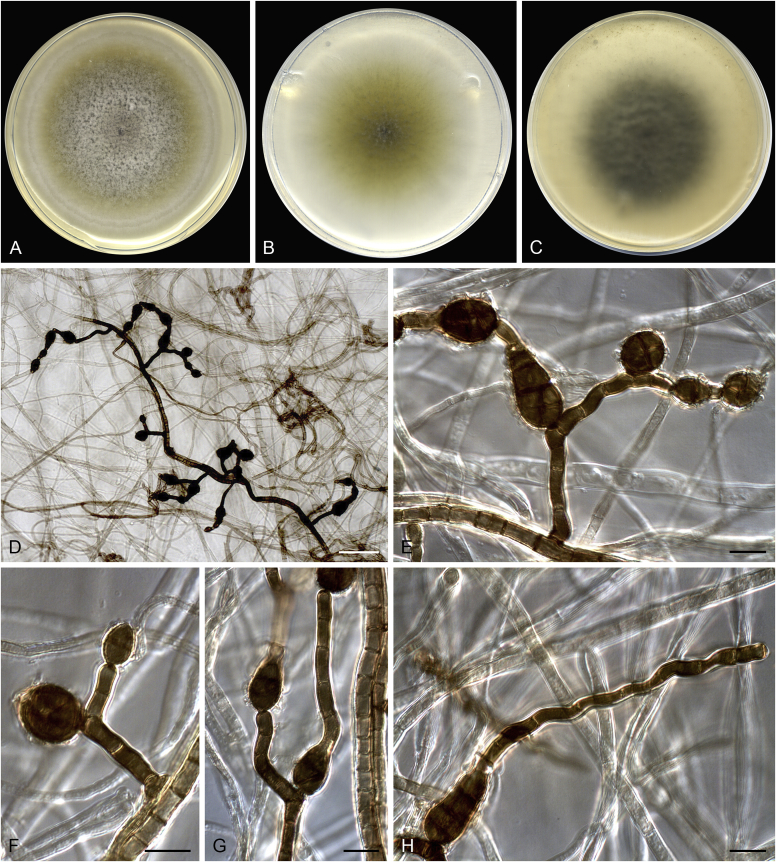
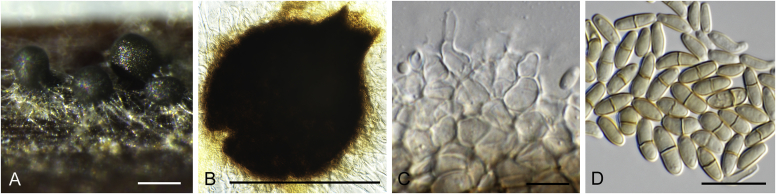
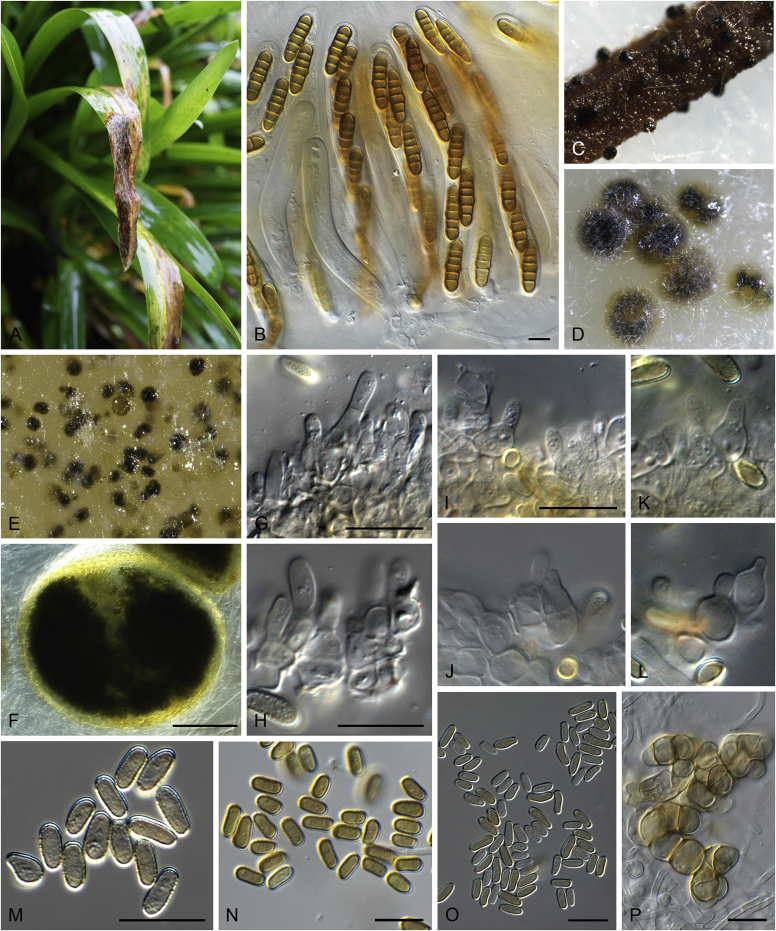
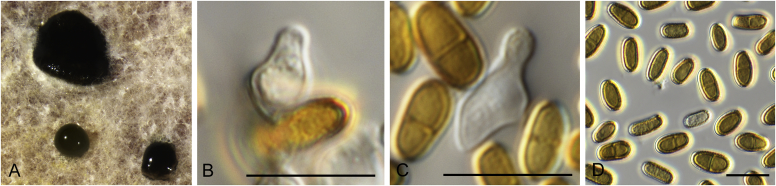
Allophoma pterospermicola (ex-type CGMCC 3.19245). A, B. Colony on OA (front and reverse). C, D. Colony on MEA (front and reverse). E, F. Colony on PDA (front and reverse). G. Conidiomata sporulating on OA. H, I. Conidiomata. J. Section of conidioma. K. Section of conidiomatal wall. L, M. Conidiogenous cells. N. Conidia. Scale bars: H, J = 20 μm; I = 40 μm; K, N = 10 μm; L, M = 5 μm.
Etymology: Name reflects Pterospermum, the host genus from which it was collected.
Conidiomata pycnidial, solitary or aggregated, globose to subglobose, brown, glabrous or with a few hyphal outgrowths, superficial, 60–330 × 67–280 μm, with 1–5 ostioles, sometimes elongated as a long neck, up to 150 μm long, papillate; conidiomatal wall pseudoparenchymatous, 3–5-layered, 12–20 μm thick, composed of isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth-walled, ampulliform to doliiform, 6–10 × 3–6 μm. Conidia oval to oblong, occasionally bacilliform, smooth- and thin-walled, hyaline, aseptate, 3–5.5 × 1.5–2 μm, with 1–2 minute guttules. Conidial matrix cream.
Culture characteristics: Colonies on OA, 33–40 mm diam after 1 wk, margins regular, floccose to woolly, white, pale brownish grey, with a pale salmon concentric ring, pale salmon near the margins, black pycnidia visible; reverse concolourous. Colonies on MEA, 20–25 mm diam after 1 wk, margins regular, aerial mycelium sparse, olivaceous; reverse concolourous. Colonies on PDA, 20–30 mm diam after 1 wk, margins regular, floccose to woolly, olivaceous, white near the margins; reverse dull green, white near the margins. Application of NaOH results in a pale brownish olivaceous discolouration of the agar.
Typus: China, Guangxi, Nonggang National Nature Reserve, on diseased leaves of Pterospermum xylocarpum (Sterculiaceae), Jun. 2017, Z.Y. Ma (holotype HMAS 247983, culture ex-type CGMCC 3.19245 = LC 12185).
Additional materials examined: China, Guangxi, Nonggang National Nature Reserve, on diseased leaves of Pterospermum xylocarpum (Sterculiaceae), Jun. 2017, Z.Y. Ma, LC 12183; ibid., LC 12184; Guangxi, Jingxi, Gulongshan, on diseased leaves of Maesa montana (Primulaceae), Jun. 2017, Z.Y. Ma, LC 12181; ibid., LC 12182.
Notes: Allophoma pterospermicola represents the first report of a species in the family Didymellaceae on the two host genera Pterospermum (Sterculiaceae) and Maesa (Primulaceae). This species is closely related to Al. minor, but differs in producing longer conidiogenous cells [6–10 × 3–6 μm in Al. pterospermicola vs. 4–5.5(–6.2) × 3–4.5(–4.7) in Al. minor] and slightly narrower conidia [3–5.5 × 1.5–2 μm in Al. pterospermicola vs. (3–)3.5–4.5(–5) × 1.8–2.5(–3) μm in Al. minor]. In addition, Al. pterospermicola grows much slower on OA, MEA and PDA than Al. minor, and the latter species has only been recorded on Syzygium aromaticum (Myrtaceae) (Aveskamp et al. 2010).
Authors: Q. Chen & L. Cai
Alternaria Nees, Das System der Pilze und Schwämme: 72. 1816 (1816–1817). Fig. 4.
Fig. 4.
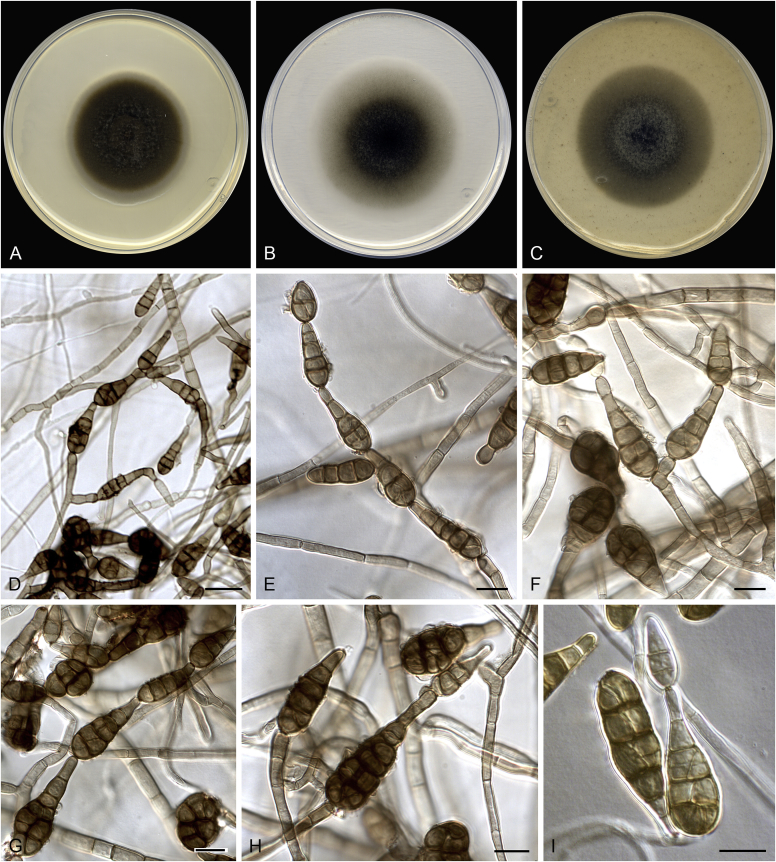
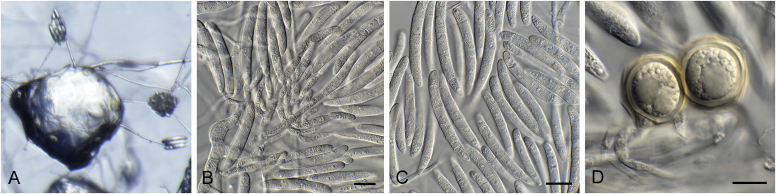
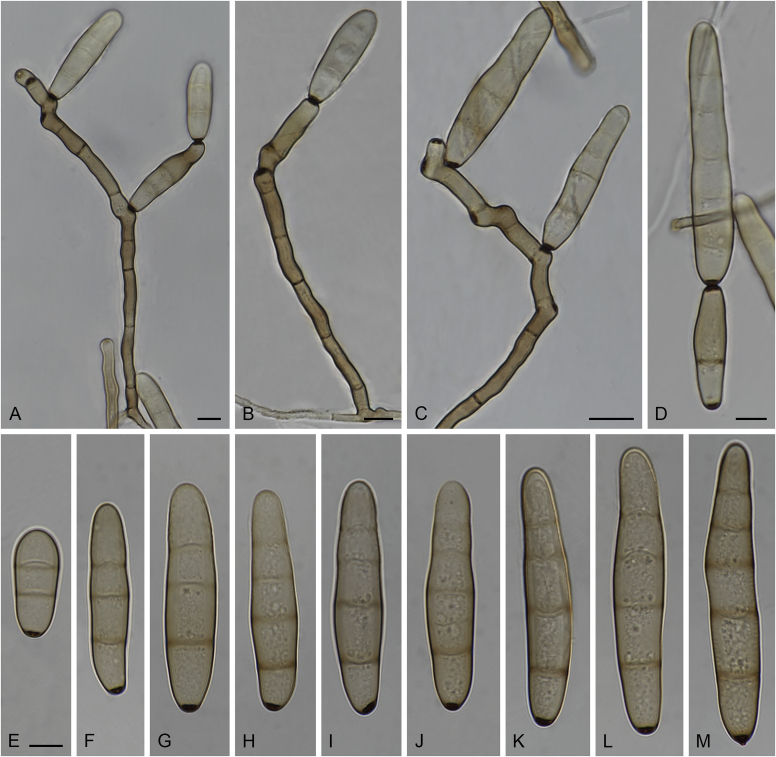
Alternaria spp. A–D. Disease symptoms. A.Alternaria dauci on Daucus carota. B.Alternaria linariae on Solanum lycopersicum. C.Alternaria neoipomoeae on Ipomoeae batatas (Photo A.H. Thompson, ARC, South Africa). D.Alternaria solani on Solanum tuberosum (Photo J.E. van der Waals, University of Pretoria, South Africa). E–V. Asexual morph. E–O. Conidiophores. E.Alternaria caricis. F.Alternaria chartarum. G.Alternaria cinerariae. H.Alternaria conjuncta. I.Alternaria elegans. J.Alternaria embellisia. K.Alternaria indefessa. L.Alternaria japonica. M.Alternaria penicillata. N.Alternaria proteae. O.Alternaria tenuissima. P–T. Conidia. P.Alternaria blumeae. Q.Alternaria calendulae. R.Alternaria perpunctulata. S.Alternaria carotiincultae. T.Alternaria triglochinicola. U, V. Conidia producing secondary conidia. U.Alternaria mimicola. V.Alternaria molesta. Scale bars: 10 μm. Pictures A–D, P, Q taken from Woudenberg et al. (2014); E–O, R–V from Woudenberg et al. (2013).
For synonyms see Woudenberg et al. (2013).
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Pleosporaceae.
Type species: Alternaria alternata (Fries) Keissler, basionym: Torula alternata Fr., Syst. Mycol. (Lundae) 3: 500. 1832 (nom. sanct.); additional synonyms listed in Woudenberg et al. (2015). Neotype designated by Simmons (1967): E.G.S. 11.050. Ex-epitype strain designated by de Hoog & Horré (2002): CBS 916.96 = E.G.S. 34.016.
DNA barcodes (genus): LSU, ITS.
DNA barcodes (species): ITS, ATPase, gapdh, rpb2, tef1. Table 2. Fig. 5, Fig. 6, Fig. 7.
Table 2.
DNA barcodes of accepted Alternaria spp.
CBS: Culture collection of the Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CNU: Culture Collection Center of the Chungnam National University; DAOM: Canadian Collection of Fungal Cultures, Ottawa, Canada; EGS: Personal collection of Dr. E.G. Simmons; FMR: Facultat de Medicina, Universitat Rovira i Virgili, Reus, Spain; IRAN: Fungal Culture Collections of the Iranian Research Institute of Plant Protection; KUMCC, Culture collection of Kunming Institute of Botany, Kunming, China; LEP: Mycological Herbarium of All-Russian Institute of Plant Protection, Saint Petersburg, Russia; MFLU and MFLUCC: Herbarium and culture collection of Mae Fah Luang University, Chiang Rai, Thailand, respectively; PPRI: ARC-Plant Protection Research Institute, Roodeplaat, South Africa. T indicates ex-type strains.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; gapdh: partial glyceraldehyde-3-phosphate dehydrogenase gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; ATPase: partial plasma membrane ATPase gene.
Fig. 5.
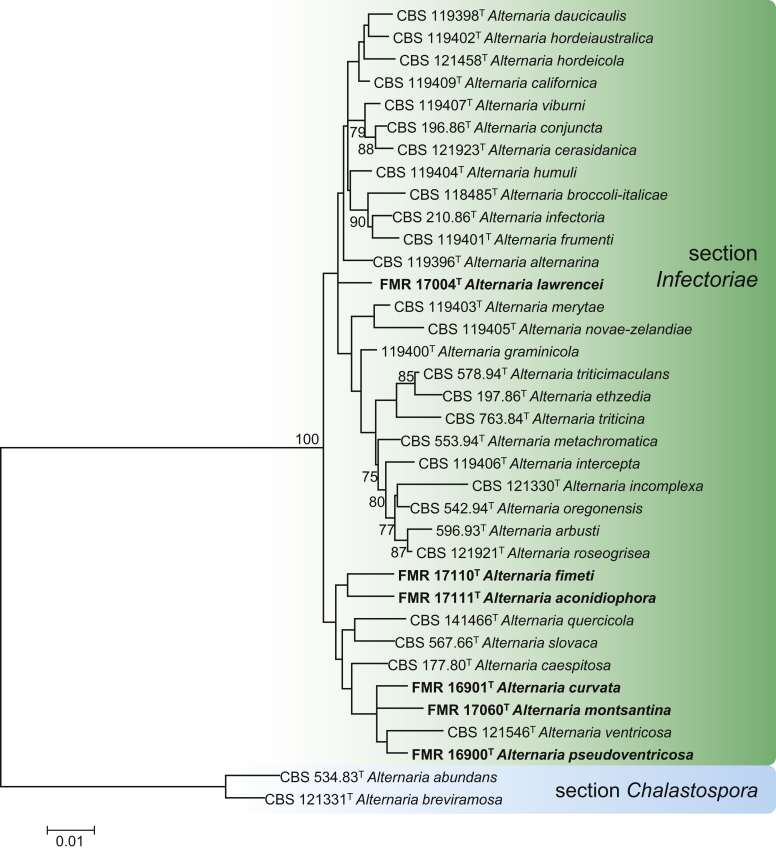
Maximum Likelihood (ML) tree constructed with ITS (529 bp), ATPase (1180 bp), gapdh (489 bp), rpb2 (573 bp) and tef1 (239 bp) sequences of ex-type strains of the species in section Infectoriae. The phylogenetic tree was rooted to Alternaria abundans CBS 534.83 and Alternaria breviramosa CBS 121331 (section Chalastospora). Bootstrap support values above 70 % are shown at the nodes. GenBank accession numbers are indicated in Table 2. The novel species described in this study are indicated in bold. T indicates ex-type strain. TreeBASE: S23786.
Fig. 6.
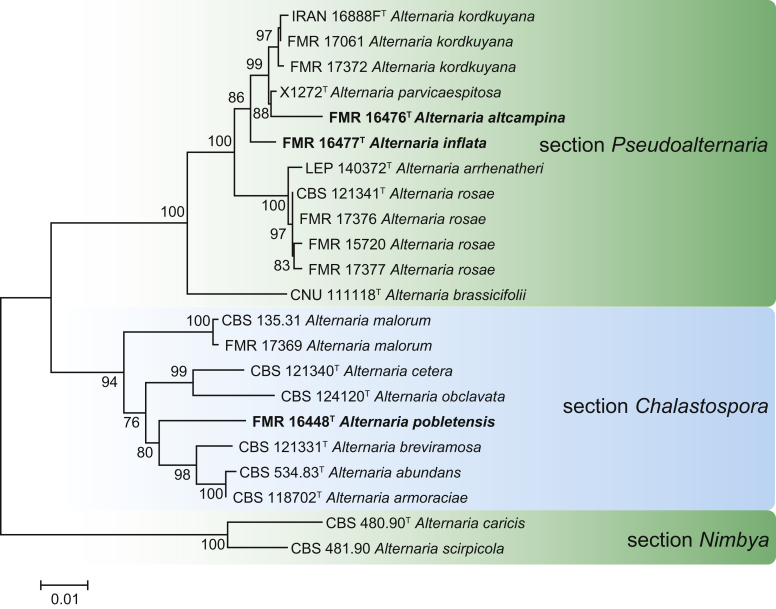
Maximum Likelihood (ML) tree constructed with ITS (576 bp), ATPase (1198 bp) and gapdh (491 bp) sequences of ex-type strains of species in the sections Pseudoalternaria and Chalastospora. The phylogenetic tree was rooted to Alternaria caricis CBS 480.90 and A. scirpicola CBS 481.90 (section Nimbya). Bootstrap support values above 70 % are shown at the nodes. GenBank accession numbers are indicated in Table 2. The novel species described in this study are indicated in bold. T indicates ex-type strain. TreeBASE: S23787.
Fig. 7.
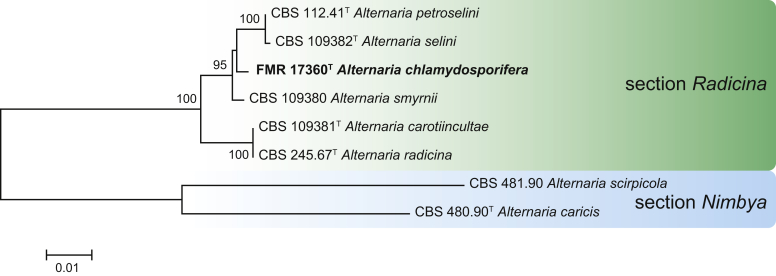
Maximum Likelihood (ML) tree constructed with ITS (523 bp), gapdh (503 bp), rpb2 (860 bp) and tef1 (247 bp) sequences of ex-type strains of species in section Radicina. The phylogenetic tree was rooted to Alternaria caricis CBS 480.90 and A. scirpicola CBS 481.90 (section Nimbya). Bootstrap support values above 70 % are shown at the nodes. GenBank accession numbers are indicated in Table 2. The novel species described in this study are indicated in bold. T indicates ex-type strain. TreeBASE: S23788.
Ascomata small, solitary to clustered, erumpent to almost superficial at maturity, dark brown, globose to ovoid, apically papillate, ostiolate, smooth or setose at maturity, with a thin ascomatal wall; centrum formed by a hamathecium with cellular pseudoparaphyses and asci in basal layer. Asci bitunicate, fissitunicate, uni- or biseriate, (4–6–)8-spored, cylindrical to cylindro-clavate, straight or somewhat curved, with a short furcate pedicel. Ascospores ellipsoid to fusoid, muriform, slightly constricted at septa, 3–7-transverse septa, 1–2 series of longitudinal septa through the two original central segments, end cells without septa, or with one longitudinal or oblique septum, or with a Y-shaped pair of septa, yellow-brown, smooth-walled, without guttules. Conidiophores macronematous or semi-macronematous, mononematous, simple or branched, pale brown or brown. Conidiogenous cells integrated, terminal becoming intercalary, mono- or polytretic and sympodial, cicatrised. Conidia solitary or in simple or branched chains, dry, ovoid, obovoid, cylindrical, narrowly ellipsoid or obclavate, beaked or non-beaked, pale or medium olivaceous brown to brown, smooth-walled or verrucose, with transverse and with or without oblique or longitudinal septa; septa can be thick, dark, an internal cell-like structure can be formed. Species with meristematic growth are known (adapted from Ellis, 1976, Woudenberg et al., 2013, Woudenberg et al., 2014, Grum-Grzhimaylo et al., 2016).
Culture characteristics: Colonies effuse, grey, olivaceous brown, dark blackish brown or black; mycelium immersed or partly superficial, composed of colourless, olivaceous brown or brown hyphae.
Optimal media and cultivation conditions: For morphological examinations the use of PCA and V-8 is recommended, incubated at moderate temperatures (ca. 22–25 ºC) under near-ultraviolet light (8 h light, 16 h dark), without humidity control, for 5–7 d or more if necessary (Simmons 2007). We also recommend microscopic examination of OA cultures due to the alterations observed on the conidial wall when grown on PCA.
Distribution: Worldwide.
Hosts: Mainly pathogens of a wide range of plant families, such as Apiaceae, Asteraceae, Brassicaceae, Cyperaceae, Poaceae, Rosaceae, Rutaceae, Solanaceae, among others (Thomma, 2003, Lawrence et al., 2016). Some are implicated as human pathogens (de Hoog et al. 2011).
Disease symptoms: Most species are foliar pathogens, causing necrotic lesions as brown/black spots or “target spot” with the fungus residing in the central area, but also inducing leaf blight; seed-borne species may attack seedlings, resulting in damping-off, stem lesions or collar rot; sunken and dark lesions are present in roots, tubers, stems and fruits infections; some rots and decay are typical symptoms of post-harvest diseases (Laemmlen, 2001, Thomma, 2003, Lawrence et al., 2008). Phytotoxins are also produced during the invasion process as virulence factors which affect a wide spectrum of plant species. Alternaria toxins diffuse into host tissues resulting in a chlorotic or yellow halo around lesions, exacerbating the severity of the symptoms (Singh et al. 2015).
Notes: Alternaria is characterised mainly by its asexual morph with darkly pigmented multi-celled conidia, which are typically dictyosporous, some phragmosporous, and arranged single or in chains on the conidiophore. Some of these morphological features can also be observed in other closely related genera such as Paradendryphiella (Woudenberg et al. 2013) or Stemphylium (Woudenberg et al. 2017). However, Paradendryphiella mainly differs by its denticulate conidiogenous cells with prominent conidial scars aggregated at the apex of simple or branched conidiophores, and Stemphylium by showing percurrent conidiophores with apically swollen conidiogenous cells.
Extensive morphological investigations of the genus Alternaria were carried out by Emory G. Simmons, which culminated with his monograph on Alternaria species identification (Simmons 2007). Based on the sporulation patterns and conidial morphology, he described several Alternaria species-groups which were typified by representative species (Simmons 1992). In recent years, based on molecular phylogenetic approaches using DNA sequence data, it has been shown that the main morphological groups identified by Simmons represent monophyletic species groups. Lawrence et al. (2013) provided the first strongly supported phylogenetic hypothesis among Alternaria lineages and elevated several of those monophyletic species groups to the taxonomic status of sections, each with a type species. Successive phylogenetic investigations added additional sections within the genus by synonymising genera such as Allewia, Brachycladium, Chalastospora, Chmelia, Crivellia, Embellisia, Nimbya, Pseudoalternaria, Sinomyces, Teretispora, Ulocladium, Undiphilum and Ybotromyces (Woudenberg et al., 2013, Woudenberg et al., 2014, Lawrence et al., 2016). Therefore, the genus Alternaria currently comprises close to 280 species, most of them classified in 27 sections. Taxonomic traits and species composition of all Alternaria sections are summarised in Lawrence et al. (2016).
Considering, however, the overlap of morphological traits among Alternaria sections/species and that the culture conditions can greatly influence the morphology of these fungi, molecular identification is practically mandatory for the classification of Alternaria isolates. Although the ITS barcode is considered a good phylogenetic marker to define sections, it has limited discriminatory power to distinguish species, making multi-locus sequence analysis with several protein-coding loci essential for accurate species identification. While Woudenberg et al. (2013), in addition to the nrDNA regions, used the combination of gapdh, rpb2 and tef1 loci for redefining the genus, the combination of other phylogenetic markers has since been analysed to determine relationships and species delineation in studies on a particular section; i.e. ITS, Alt a-1, endoPG, gapdh, OPA10-2, rpb2 and tef1 for section Alternaria (Woudenberg et al. 2015); ITS, ATPase, tef1 and gapdh for sections Infectoriae and Pseudoalternaria (Andersen et al., 2009, Deng et al., 2018, Poursafar et al., 2018); ITS, Alt a-1, gapdh, rpb2 and tef1 for section Porri (Woudenberg et al. 2014); and ITS, Alt a-1 and gapdh for section Sonchi (Lawrence et al., 2012, Deng et al., 2014). Nevertheless, according to Lawrence et al. (2013) the plasma membrane ATPase, cmdA, and Alt a-1 loci are the most informative markers for Alternaria species delimitation. However, considering that the latter locus unreliably amplifies some species within sect. Infectoriae, they suggested that the most suitable genetic markers for molecular identification at the species level are ATPase and cmdA genes (Lawrence et al., 2013, Lawrence et al., 2016). Unfortunately, the latter marker has not been used for the phylogeny of any of the above-mentioned sections.
Alternaria is a very successful pathogenic genus that causes disease on a great number of economically important plants, causing large economic losses due to the number of plant species affected and worldwide distributions of several Alternaria species (Meena et al. 2017). They are commonly described causing stem canker, leaf blight or leaf spot on a large variety of crops, including cereals, ornamentals, oil crops, vegetables such as broccoli, cauliflower, carrot, onion and potato, and fruits like apple, citrus, pear and strawberry, among others. Species in section Alternaria, such as A. alternata, A. arborescens or A. tenuissima, as well as others from sections Alternantherae, Brassiccicola, Crivellia, Gypsophilae, Nimbya, Radicina or Sonchi, are frequently reported causing such diseases, but the largest group of phytopathogens in the genus is concentrated in section Porri (Lawrence et al., 2016, Meena et al., 2017). The most relevant plant pathogens in this latter section are A. bataticola, A. porri, A. solani and A. tomatophila (Woudenberg et al. 2014). Alternaria species also produce diverse phytotoxins, which affect their host plants at different stages of pathogenesis (Thomma, 2003, Lawrence et al., 2008, Meena et al., 2017). Some of these phytotoxins have been evaluated by the European Food Safety Authority as potentially causing risks to human health (Meena et al. 2017).
In humans, Alternaria species are commonly associated with hypersensitivity pneumonitis, bronchial asthma, allergic sinusitis and rhinitis. To a lesser extent, they have been also described as causing paranasal sinusitis, ocular infections, onychomycosis, cutaneous and subcutaneous infections, granulomatous pulmonary disease, soft palate perforation and disseminated disease (Pastor and Guarro, 2008, de Hoog et al., 2011).
In several surveys of microfungi from Spanish regions with different climates and biodiversity, samples of plant litter (leaves, bark and twigs) and dung of wild and farm herbivore animals (rabbits, rodents, goats, cattle and horses) were collected. From these samples, we found 16 interesting Alternaria isolates, belonging to sections Infectoriae, Pseudoalternaria, Chalastospora and Radicina. The multi-locus phylogenetic analysis based on five above-mentioned gene markers showed that 10 of them were undescribed species for the genus, and the others were identified as A. kourtkuyana, A. rosae and A. malorum (Fig. 5, Fig. 6, Fig. 7). Most of these novel species have been isolated from herbivore dung, which appear to represent a reservoir of interesting Alternaria species which could represent potential plant pathogens.
References: Ellis, 1976, Simmons, 2007 (morphology); Laemmlen, 2001, Thomma, 2003, Lawrence et al., 2008, Meena et al., 2017 (plant infections); Pastor and Guarro, 2008, de Hoog et al., 2011 (human infections); Woudenberg et al., 2013, Woudenberg et al., 2014, Woudenberg et al., 2015, Grum-Grzhimaylo et al., 2016, Lawrence et al., 2016, Poursafar et al., 2018 (morphology and phylogeny).
Alternaria aconidiophora Iturrieta-González, Dania García & Gené, sp. nov. MycoBank MB829626. Fig. 8.
Fig. 8.
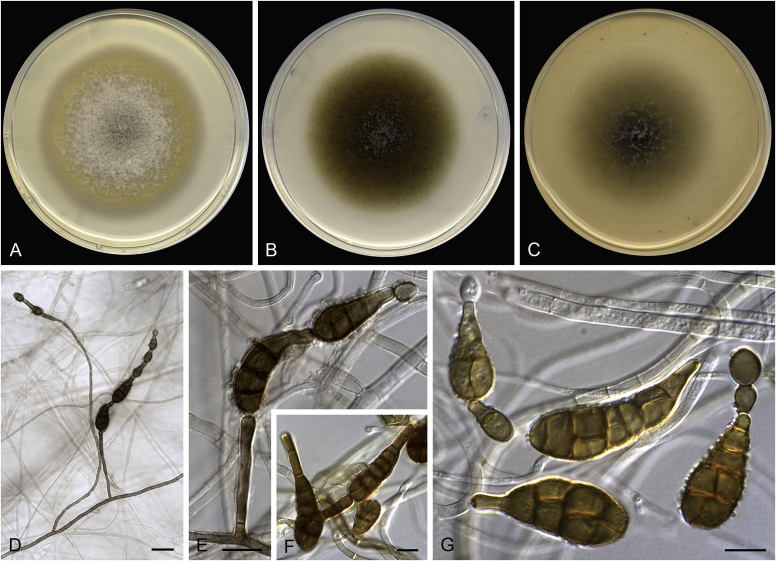
Alternaria aconidiophora (ex-type FMR 17111). A. Colonies on PDA. B. Colonies on PCA. C. Colonies on OA. D–F. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the lack of conidiophores from vegetative hyphae.
Asexual morph on PCA: Mycelium superficial and immersed. Hyphae 1–4 μm wide, septate, branched, hyaline to greyish, smooth-walled. Conidiophores absent. Conidiogenous loci inconspicuous on vegetative hyphae, scarce. Conidia commonly solitary at centre of the colony, globose, ovoid, near ellipsoid or obclavate, 12–31 × 7–12 μm, with some darkened middle transverse septa, 1–5 transverse, 0–1(–2) longitudinal or oblique septa per transverse segment, brown, smooth-walled. Secondary conidiophores present, may be formed apically from the conidial body as a short extension often geniculate, with one or two, terminal or subterminal conidiogenous loci. Sexual morph not observed.
Culture characteristics: Colonies on PDA reaching 64 mm diam after 1 wk at 25 °C, flat, cottony at centre, slightly radially folded towards the periphery, aerial mycelium abundant, margins regular; surface white (1A1); reverse yellowish white (4A2). On PCA attaining 54 mm diam, flat, aerial mycelium scarce, margins regular; surface greyish green to greenish grey (1D3/1B1); reverse greenish grey (1C2/1B1). On OA reaching 61 mm diam, flat, aerial mycelium scarce, margins regular; surface and reverse colourless.
Cardinal temperature for growth: Optimum 25 °C, maximum 37 °C, minimum 5 °C.
Typus: Spain, Catalonia, Alta Ribagorça, Vall de Boí, isolated from forest leaf litter, Dec. 2017, J. Gené (holotype CBS H-23891, culture ex-type CBS 145419 = FMR 17111).
Notes: Alternaria aconidiophora together with A. fimeti, both species introduced here from herbivore dung, are placed in an unsupported clade in Alternaria section Infectoriae (Fig. 5). Morphologically, the latter differs from A. aconidiophora in having conspicuous sporulation with well-differentiated conidiophores and verrucose conidia up to 44 μm long. The conidia of A. aconidiophora are smooth-walled and 12–31 μm long.
Alternaria altcampina Iturrieta-González, Dania García & Gené, sp. nov. MycoBank MB829627. Fig. 9.
Fig. 9.
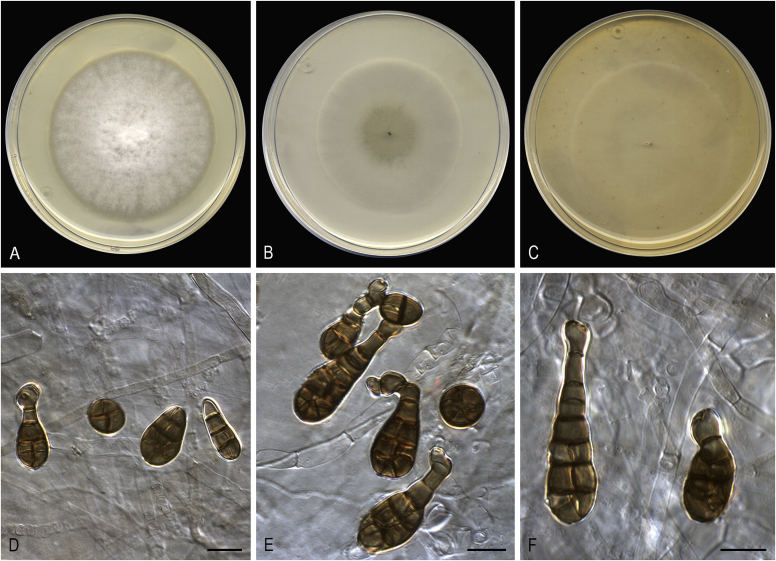
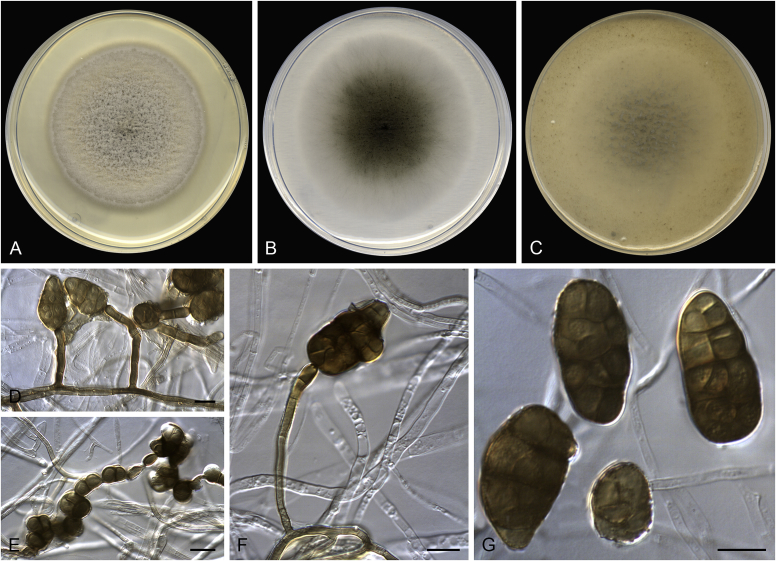
Alternaria altcampina (ex-type FMR 16476). A. Colonies on PDA. B. Colonies on PCA. C. Colonies on OA. D–I. Conidiophores and conidia. Scale bars: D–E = 20 μm; F–I = 10 μm.
Etymology: Name refers to the region of Alt Camp (Catalonia), from where the fungus was collected.
Asexual morph on PCA: Mycelium superficial and immersed. Hyphae 2–4 μm wide, branched, pale yellowish brown to brown, septate, smooth-walled to verruculose. Conidiophores macronematous, arising laterally or terminally from aerial hyphae, erect to slightly flexuous, unbranched, occasionally branched, up to 10-septate, 12–88 × 3–4 μm, brown becoming pale towards apex, smooth-walled, with 1 terminal and up to 3 subterminal conidiogenous loci. Conidia in branched chains, occasionally solitary, ovoid, obclaviform, ellipsoidal or somewhat cylindrical, 9–43 × 6–8 μm, with darkened middle transverse septa, (1–)2–3(–6) transverse, 0–1 longitudinal or oblique septa in up to 4 of the transverse segments, usually inconspicuous, pale yellowish to yellowish brown, verrucose. Secondary conidiophores commonly formed apically as a beak from conidial body, or as a lateral conidiogenous loci from body cells bearing conidia in short chains. Sexual morph not observed.
Culture characteristics: Colonies on PDA reaching 73 mm diam after 1 wk at 25 °C, flat, densely floccose, aerial mycelium abundant, margins fimbriate; surface olive brown to blond (4D3/4C4), white at the periphery; reverse yellowish brown to orange-grey (5E4/5B2). On PCA attaining 66 mm diam, flat, granular, aerial mycelium scarce, margins regular; surface dark green (30F8); reverse dull green (30E4). On OA reaching 70 mm diam, flat, loosely floccose at centre, aerial mycelium scarce, margins regular; surface dark green (28F4); reverse dull green (29E3).
Cardinal temperature for growth: Optimum 25 °C, maximum 37 °C, minimum 5 °C.
Typus: Spain, Catalonia, Alt Camp, isolated from goat dung, Mar. 2017, I. Iturrieta-González, M. Guevara-Suarez & J. Guarro (holotype CBS H-23892, culture ex-type CBS 145420 = FMR 16476).
Notes: Based on the phylogeny of ITS, ATPase and gapdh, A. altcampina is classified in Alternaria section Pseudoalternaria (Fig. 6). It is closely related to the recently described species A. parvicaespitosa, which was isolated from harvested blueberry fruit (California, USA), and A. kordkuyana, isolated from symptomatic wheat heads of Triticum aestivum (Kordkuy, Iran). Alternaria parvicaespitosa differs in having smaller conidia (10–25 × 7–12 μm) with smooth to slightly punctulate outer walls (Gannibal & Lawrence 2016), and A. kordkuyana by its larger conidia [30–50(−60) × 7–11 μm] and shorter conidiophores (10–40 × 3–4 μm) (Poursafar et al. 2018).
Alternaria chlamydosporifera Iturrieta-González, Dania García & Gené, sp. nov. MycoBank MB829628. Fig. 10.
Fig. 10.
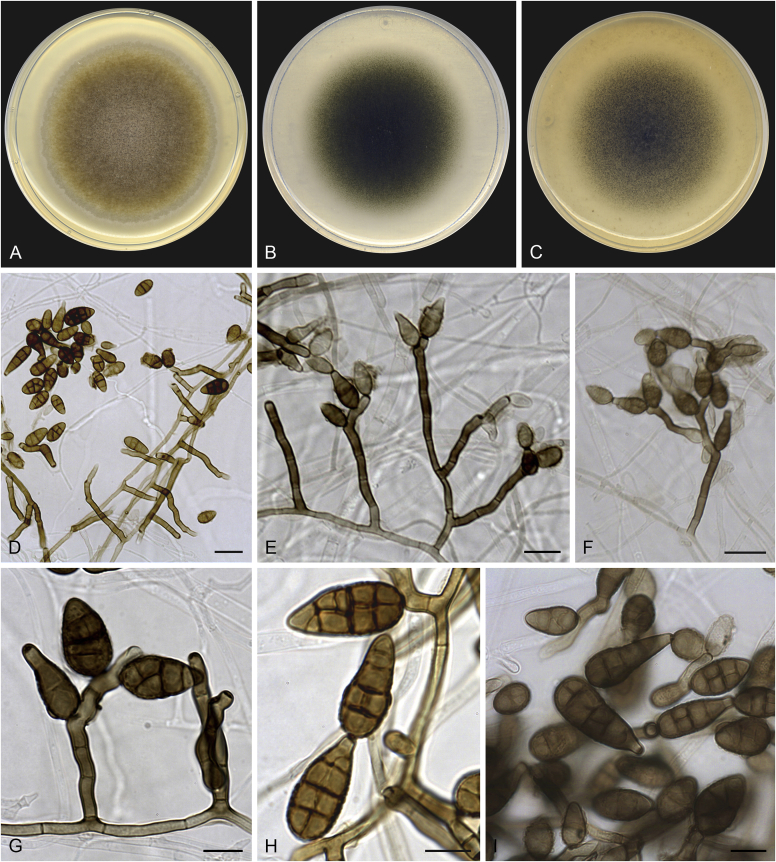
Alternaria chlamydosporifera (ex-type FMR 17360). A. Colonies on PDA. B. Colonies on PCA. C. Colonies on OA. D–H. Conidiophores and conidia. I. Chlamydospores. Scale bars: D = 50 μm; E, I = 20 μm; F–H = 10 μm.
Etymology: Name refers to the production of abundant chlamydospores in culture.
Asexual morph on PCA: Mycelium superficial and immersed. Hyphae 3–6 μm wide, septate, branched, pale brown to brown, smooth-walled. Conidiophores macronematous, arising directly from aerial hyphae, erect to slightly flexuous, occasionally geniculate at apex, 1–4-septate, unbranched or scarcely branched, 14–140 × 3–5 μm, dark brown, verruculose, with 1–2 conidiogenous loci. Conidia mostly solitary, occasionally in short chains with up to two conidia, ellipsoidal or ovoid, occasionally subglobose, 12–41 × 7–20 μm, with darkened middle transverse septa, 1–3(–4) transverse, and 0–1(–2) longitudinal septa per transverse segments, brown to dark brown, verruculose. Secondary conidiophores can be formed apically from conidial body as a beak, geniculate, with 1–3 terminal or lateral conidiogenous loci, bearing solitary or short chains of conidia. Chlamydospores abundant, immersed, intercalary, irregular shape, rarely broadly ellipsoidal or clavate, muriform, sometimes showing central constriction, 60–91 × 32–57 μm, dark brown to black. Sexual morph not observed.
Culture characteristics: Colonies on PDA reaching 82 mm diam after 1 wk at 25 °C, flat, densely floccose, aerial mycelium abundant, margins regular; surface greyish brown (5E3); reverse black to greyish brown (5E3). On PCA attaining 68 mm diam, flat, with granular appearance by the presence of abundant chlamydospores, aerial mycelium scarce, margins regular; surface dark green (29F5); reverse dark green (30F8). On OA reaching 71 mm diam, flat, loosely floccose at centre, slightly granular towards the periphery, aerial mycelium scarce, margins slightly lobate; surface dark green (29F4); reverse dark green (29F4).
Cardinal temperature for growth: Optimum 25 °C, maximum 37 °C, minimum 5 °C.
Typus: Spain, Huesca, Baells, isolated from rabbit dung, Apr. 2018, G. Sisó & D. García (holotype CBS H-23893, culture ex-type CBS 145421 = FMR 17360).
Notes: Alternaria chlamydosporifera belongs to Alternaria section Radicina (Fig. 7). It is included in a well-supported clade (95 % BS) with A. petroselini, A. selini and A. smyrnii, which are pathogens of Apiaceae (Lawrence et al. 2016). Alternaria petroselini and A. selini can be easily differentiated by the lack of chlamydospores in culture and their larger (50–66 μm in A. petroselini vs. 48–65 μm in A. selini) and usually ellipsoidal conidia (Simmons 1995). Although A. smyrnii, the closest relative to A. chlamydosporifera, has been described as producing sclerotial knots in culture that are able to form fertile conidiophores, its conidia are considerably longer (67–96 μm) (Simmons 1995) than those of A. chlamydosporifera (12–41 μm long). In addition, we have never observed conidiophores associated with the chlamydospores of the latter species.
Alternaria curvata Iturrieta-González, Dania García & Gené, sp. nov. MycoBank MB829628. Fig. 11.
Fig. 11.
Alternaria curvata (ex-type FMR 16901). A. Colonies on PDA. B. Colonies on PCA. C. Colonies on OA. D–F. Conidiophore and conidia. Scale bars = 10 μm.
Etymology: Name refers to the presence of curved conidia.
Asexual morph on PCA: Mycelium superficial and immersed. Hyphae 2–6 μm wide, septate, branched, hyaline to yellowish brown to brown, smooth-walled to verruculose. Conidiophores macronematous, arising laterally or terminally from aerial hyphae, erect to slightly flexuous, usually unbranched, up to 14-septate, 23–80 × 3–5 μm, brown to dark brown, smooth-walled, with a terminal or occasionally a sub-terminal conidiogenous loci. Conidia forming branched chains, with up to 5 conidia in unbranched part, ovoid or nearly ellipsoidal, often slightly curved, 13–47(–70) × 4–16 μm, with darkened middle transverse septa, (0–)1–5(–7) transverse, and 0–2(–3) longitudinal or oblique septa per transverse segment, brown to dark brown, verrucose to tuberculate. Secondary conidiophores can be formed apically or laterally from the conidial body as a short extension bearing conidia in short chains. Sexual morph not observed.
Culture characteristics: Colonies on PDA reaching 63 mm diam after 1 wk at 25 °C, flat, densely floccose, aerial mycelium abundant, margins regular; surface white to dull green (1A1/30D4); reverse dark green to olive yellow (30F8/2D6), white at the periphery. On PCA attaining 62 mm diam, flat, loosely floccose, aerial mycelium scarce, margins regular; surface olive (3F4); reverse dark green to grey (29F4/29B1). On OA reaching 61 mm diam, scarce aerial mycelium towards the periphery, margins regular; surface greyish green (30E5), with greyish mycelium tufts at centre; reverse dull green (29E4).
Cardinal temperature for growth: Optimum 25 °C, maximum 37 °C, minimum 5 °C.
Typus: Spain, Catalonia, Tarragona, Els Ports Natural Park, isolated from goat dung, Oct. 2017, G. Sisó & C. González-García (holotype CBS H-23894, culture ex-type CBS 145422 = FMR 16901).
Notes: Alternaria curvata was included in the section Infectoriae, forming an unsupported basal clade together with A. montsantina and A. pseudoventricosa, both introduced here, and A. ventricosa (Fig. 5). Morphologically, the former two species differ from A. curvata in lacking curved conidia. Alternaria ventricosa has asymmetrical conidia, due to the hyperplasia and hypertrophy of cells, especially on one side of the conidia (Roberts 2007). Other morphologically similar species are A. fimeti and A. triticina, which also have curved conidia. However, A. triticina is phylogenetically more distant and its conidia are strongly inequilateral and wider (up to 22 μm) (Simmons 2007) than those of A. curvata (4–16 μm wide). Alternaria fimeti can be differentiated from A. curvata by the production of longer conidiophores (22–182 μm in A. fimeti vs. 23–80 μm in A. curvata) and the absence or scarce development of secondary conidiophores.
Alternaria fimeti Iturrieta-González, Dania García & Gené, sp. nov. MycoBank MB829630. Fig. 12.
Fig. 12.
Alternaria fimeti (ex-type FMR 17110). A. Colonies on PDA. B. Colonies on PCA. C. Colonies on OA. D–G. Conidiophores and conidia. Scale bars: D = 20 μm; E–G = 10 μm.
Etymology: Name refers to the substrate where the species was isolated, herbivore dung.
Asexual morph on PCA: Mycelium superficial and immersed. Hyphae 2–5 μm wide, septate, branched, hyaline to subhyaline to pale yellowish, verruculose. Conidiophores semi- to macronematous, arising laterally or terminally from aerial hyphae, erect to slightly flexuous, unbranched (can be slightly branched on OA), up to 9-septate, 22–182 × 1–5 μm, pale brown, smooth-walled, with 1 terminal conidiogenous locus. Conidia solitary or in short chains of up to six conidia, ovoid, obpyriform or obclavate, some slightly curved, 9–44 × 5–14(–23) μm, with darkened middle transverse septa, 0–5 transverse (up to 7 in OA), and 0–1(–2) longitudinal or oblique septa per segment, brown, verrucose. Secondary conidiophores only scarcely produced on OA as apical or lateral extension from conidial body, up to 25 μm long. Sexual morph not observed.
Culture characteristics: Colonies on PDA reaching 66 mm diam after 1 wk at 25 °C, flat, densely floccose, aerial mycelium abundant, margins fimbriate; surface yellowish grey to yellowish white (3C2/3A2); reverse yellowish brown to light yellow (5E8/4A5). On PCA attaining 65 mm diam, flat, slightly floccose at centre, aerial mycelium scarce, margins regular; surface olive-brown (4F5); reverse olive-brown (4F8/4E4). On OA reaching 64 mm diam, flat, slightly floccose, scarce aerial mycelium, margins regular; surface dull green (30E5) with grey floccose area; reverse dull green (30E4).
Cardinal temperature for growth: Optimum 25 °C, maximum 37 °C, minimum 5 °C.
Typus: Spain, Catalonia, Priorat, Montsant Natural Park, Arbolí, isolated from small rodent dung, Feb. 2018, I. Iturrieta-González, E. Carvalho & J. Gené (holotype CBS H-23895, culture ex-type CBS 145423 = FMR 17110).
Note: Alternaria fimeti is placed in a clade of section Infectoriae together with A. aconidiophora (see notes of this latter species).
Alternaria inflata Iturrieta-González, Dania García & Gené, sp. nov. MycoBank MB829631. Fig. 13.
Fig. 13.
Alternaria inflata (ex-type FMR 16477). A. Colonies on PDA. B. Colonies on PCA. C. Colonies on OA. D, F–G. Conidiophores and conidia. E. Chlamydospores. Scale bars = 10 μm.
Etymology: Name refers to the presence of swollen cells in the conidial body.
Asexual morph on PCA: Mycelium superficial and immersed. Hyphae of 1–3 μm wide, septate, branched, hyaline to pale brown, smooth-walled to verruculose. Conidiophores arising laterally or terminally from aerial hyphae, erect to slightly flexuous, semi- to macronematous, up to 10-septate, commonly unbranched, 9–73(–105) × 2–5 μm, pale brown to brown, smooth-walled, with one terminal conidiogenous loci or up to three subterminal conidiogenous loci. Conidia solitary or in short chains with up to four conidia, broadly ellipsoidal or ovoid, 13–41 × 5–14 μm, often with some swollen cells protruding the conidium outline, some with darkened middle transverse septa, (1–)2–3(–5) transverse septa, and 0–2 longitudinal or oblique septa per transverse segment, brown, verruculose. Secondary conidiophores scarcely produced, as an apical extension up to 15 μm long, bearing conidia in short chains. Chlamydospores present, consisting of intercalary, thick-walled, brown swollen cells, up to 8 × 6 μm, arranged in chains or in clusters. Sexual morph not observed.
Culture characteristics: Colonies on PDA reaching 62 mm diam after 1 wk at 25 °C, flat, aerial mycelium abundant, floccose, margins fimbriate; surface white (1A1); reverse greyish yellow to yellowish white (4C6/4A2). On PCA attaining 67 mm diam, flat, scarce aerial mycelium, margins regular; surface dull green to grey (30E4/30B1); reverse dark green to grey (30E4/30B1). On OA reaching 61 mm diam, flat, loosely floccose, margins regular; surface pale grey (1B1); reverse pale grey (1B1).
Cardinal temperature for growth: Optimum 25 °C, maximum 37 °C, minimum 5 °C.
Typus: Spain, Catalonia, Conca de Barberà, Poblet, isolated from rabbit dung, Mar. 2017, J. Guarro & I. Iturrieta-González (holotype CBS H-23896, culture ex-type CBS 145424 = FMR 16477).
Notes: Our phylogeny shows that A. inflata belongs to section Pseudoalternaria (Fig. 6). It clustered in a well-supported clade (86 % BS) with A. altcampina, A. kordkuyana and A. parvicaespitosa, but formed a single basal lineage representative of a distinct species. Alternaria inflata can be differentiated from all the species in the section by the production of chlamydospores and by the formation of broadly ellipsoidal conidia, usually with swollen cells protruding from the conidial body. In addition, A. altcampina also differs in the production of secondary conidiophores, A. parvicaespitosa in its shorter conidiophores (up to 70 μm) and conidia (10–25 μm) (Gannibal & Lawrence 2016), and A. kordkuyana in the production of longer conidial chains [up 5–8(–10) conidia] and conidia measuring 30–50(–60) × 7–11 μm with up to seven transverse septa (Poursafar et al. 2018).
With the additions of A. altcampina and A. inflata section Pseudoalternaria now comprises seven species. It is of note, however, that most of these taxa are only known from a single collection. In our survey of asexual fungi from herbivore dung, we identified several Spanish isolates belonging to other species in the section, namely A. kordkuyana and A. rosae (Fig. 6). Considering that most of the species in section Pseudoalternaria are mainly associated with herbaceous plants of the families Brassicaceae, Ericaceae, Poacea or Rosaceae, it is not surprising to find these fungi in faeces of herbivorous animals.
Alternaria lawrencei Iturrieta-González, Dania García & Gené, sp. nov. MycoBank MB829632. Fig. 14.
Fig. 14.
Alternaria lawrencei (ex-type FMR 17004). A. Colonies on PDA. B. Colonies on PCA. C. Colonies on OA. D–K. Conidiophores and conidia. Scale bars: D = 20 μm; E–K = 10 μm.
Etymology: Name in honour of Daniel P. Lawrence for his contribution to the taxonomy of the genus Alternaria.
Asexual morph on PCA: Mycelium superficial and immersed. Hyphae 1–3 μm wide, septate, branched, pale brown, smooth-walled. Conidiophores macronematous, solitary, arising directly from aerial hyphae, erect to slightly flexuous, occasionally geniculate at apex, usually unbranched, up to 10-septate, 9–125 × 3–4(–5) μm, brown, smooth-walled, with 1–2 lateral or terminal conidiogenous loci; micronematous conidiophores also present, reduced to intercalary conidiogenous cells with a single conidiogenous locus on hyphae. Conidia solitary or in short chains, up to six conidia in the unbranched part, ovoid, obpyriform or obclavate, 6–71 × 7–15 μm, with darkened middle transverse septa, (1–)2–7(–9) transverse, and 0–2(–3) longitudinal or oblique septa, pale brown to brown, verrucose to tuberculate. Secondary conidiophores commonly formed apically on conidia as a geniculate extension with several conidiogenous loci, or as lateral extensions from cells of conidial body, up to 35 μm long, producing conidia solitary or in short chains. Sexual morph not observed.
Culture characteristics: Colonies on PDA reaching 68 mm diam after 1 wk at 25 °C, low convex, cottony, aerial mycelium abundant, margins regular; surface white (1A1); reverse yellowish brown to greyish yellow (5E7/4B6). On PCA attaining 69 mm diam, low convex, slightly floccose, aerial mycelium relatively abundant at centre, margins regular; surface yellowish grey to olive (2D2/1E3); reverse dark green to olive (30F8/1E3). On OA reaching 63 mm diam, loosely floccose at centre, flat and scarce aerial mycelium towards the periphery, margins regular and diffuse; surface olive (2F4) to olive-grey (2B1); reverse olive to yellowish grey (2F8/2D2).
Cardinal temperature for growth: Optimum 25 °C, maximum 37 °C, minimum 5 °C.
Typus: Spain, Catalonia, Tarragona, Els Ports Natural Park, isolated from goat dung, Oct. 2017, G. Sisó & C. González-García (holotype CBS H-23897, culture ex-type CBS 145425 = FMR 17004).
Notes: Although A. lawrencei is clearly placed in section Infectoriae, the multi-locus analysis did not reveal any phylogenetic relationship with any species included in the analysis (Fig. 4). It is of note, however, that eight species of the section (i.e. A. cerasi, A. cesenica, A. dactylidicola, A. forlicesenensis, A. hampshirensis, A. litorea, A. murispora and A. poaceicola) could not be included in our analysis due to their limited molecular data. Nevertheless, A. cesenica, A. dactylidicola, A. forlicesenensis, A. hampshirensis, A. murispora and A. poaceicola can be distinguished from A. lawrencei by the production of a sexual morph (Ariyawansa et al., 2015b, Liu et al., 2015, Thambugala et al., 2017, Wanasinghe et al., 2018), A. cerasi by its inequilateral conidia (Potebnia, 1907, Simmons, 2007), and A. litorea by the production of shorter primary conidiophores (40–50 μm long) and smooth-walled conidia that are 22–32 μm long (Simmons 2007).
Alternaria montsantina Iturrieta-González, Dania García & Gené, sp. nov. MycoBank MB829633. Fig. 15.
Fig. 15.
Alternaria montsantina (ex-type FMR 17060). A. Colonies on PDA. B. Colonies on PCA. C. Colonies on OA. D–H. Conidiophores and conidia. Scale bars: D = 50 μm; E–H = 10 μm.
Etymology: Name refers to the place, Montsant Natural Park (Catalonia), where the fungus was collected.
Asexual morph on PCA: Mycelium superficial and immersed. Hyphae 1–7 μm wide, septate, branched, usually forming hyphal coils, subhyaline to pale brown, smooth-walled to verruculose. Conidiophores macronematous, arising laterally or terminally from aerial hyphae, erect to slightly flexuous, unbranched, up to 15-septate, 12–137 × 3–6 μm, often with geniculate apical portion containing intercalary and terminal conidiogenous loci, brown, smooth-walled to verruculose. Conidia solitary or in short chains with up to five conidia, subglobose, ovoid or obpyriform, 8–65 × 6–12 μm, with 1–3(–11) transverse septa, and 0–2 longitudinal or oblique septa, brown, verrucose to tuberculate. Secondary conidiophores commonly produced apically as a long, often geniculate extension, up to 105 μm long and 10-septate, bearing terminal conidial chains. Sexual morph not observed.
Culture characteristics: Colonies on PDA reaching 76 mm diam after 1 wk at 25 °C, flat, densely floccose, aerial mycelium abundant, margins regular; surface pastel grey to greyish yellow (1C1/2C4) and with a white edge; reverse blond to white (4C4/1A1). On PCA attaining 70 mm diam, flat, loosely floccose at centre, aerial mycelium moderate, margins regular; surface olive brown to white (4D4/1A1); reverse olive to white (3D4/1A1). On OA reaching 75 mm diam, flat, cottony, margins regular; surface yellowish grey to olive (3D2/2F4) and white edge; reverse olive to white (2F4/1A1).
Cardinal temperature for growth: Optimum 25 °C, maximum 37 °C, minimum 5 °C.
Typus: Spain, Catalonia, Priorat, Montsant Natural Park, Swamp of Siurana, isolated from an unidentified twig, Feb. 2018, I. Iturrieta-González, E. Carvalho & J. Gené (holotype CBS H-23898, culture ex-type CBS 145426 = FMR 17060).
Notes: Alternaria montsantina is placed in a weakly supported basal clade of the section Infectoriae, together with A. curvata, A. pseudoventricosa and A. ventricosa (Fig. 5). Morphologically, this new species can be distinguished from A. curvata and A. ventricosa by the absence of curved or inequilateral inflated conidia. Alternaria montsantina differs from A. pseudoventricosa in the production of longer (12–137 μm) and often geniculate primary and secondary conidiophores, bearing solitary conidia or arranged in short chains (up to five conidia). Conidiophores in A. pseudoventricosa are 30–44 μm long, and the conidial chains include up to 19 conidia.
Alternaria pobletensis Iturrieta-González, Dania García & Gené, sp. nov. MycoBank MB829634. Fig. 16.
Fig. 16.
Alternaria pobletensis (ex-type FMR 16448). A. Colonies on PDA. B. Colonies on PCA. C. Colonies on OA. D–I. Conidiophores and conidia. Scale bars: D = 20 μm; E–I = 10 μm.
Etymology: Name refers to the place, Poblet (Catalonia), from where the species was collected.
Asexual morph on PCA: Mycelium superficial and immersed. Hyphae 2–5 μm wide, branched, pale brown, septate, smooth-walled. Conidiophores semi- to macronematous, solitary, arising directly from aerial hyphae, erect to slightly flexuous, occasionally slightly geniculate at apex, unbranched or branched, up to 8-septate, 14–82 × 4–5(–6) μm, brown, smooth-walled, with 1–2 lateral or terminal conidiogenous loci. Conidia commonly in short, scarcely branched chains, with up to seven conidia, obpyriform or obclavate, some ellipsoidal or subcylindrical, 8–50 × 5–20 μm, (1–)3–7(–9) transverse septa, often middle septa darker, and 0–1(–2) longitudinal or oblique septa per transverse segment, pale brown to brown, smooth-walled or verruculose. Secondary conidiophores commonly produced apically as a short beak up to 11 μm long, or laterally from cells of conidial body, bearing conidia in short chains. Sexual morph not observed.
Culture characteristics: Colonies on PDA reaching 46 mm diam after 1 wk at 25 °C, flat, floccose at the centre, velvety towards the periphery, aerial mycelium moderate, margins regular; surface olive (3F8), whitish at the periphery; reverse black to yellowish brown (5D5). On PCA attaining 58 mm diam, flat, velvety, margins regular; surface dark green to dull green (30F8/28D3); reverse dark green to dull green (30F8/28D3). On OA reaching 55 mm diam, flat, loosely floccose, margins regular; surface greyish green to dull green (29C3/29E4); reverse dark green to dull green (30F8/30E3).
Cardinal temperature for growth: Optimum 25 °C, maximum 35 °C, minimum 5 °C.
Typus: Spain, Catalonia, Conca de Barberà, Poblet, isolated from unidentified herbivore dung, Mar. 2017, J. Guarro & I. Iturrieta-González (holotype CBS H-23899, culture ex-type CBS 145427 = FMR 16448).
Notes: Alternaria pobletensis clustered in section Chalastospora in a single branch clearly separated from the other six species that currently comprise the section (Fig. 7). Other species of section Chalastospora rarely produce conidia with longitudinal septa (Woudenberg et al. 2013); however, the conidia in A. pobletensis usually have two or more longitudinal or oblique septa. Its closest relative is A. breviramosa. This was originally described as Chalastospora ellipsoidea, found on Triticum (Poaceae) in Australia (Crous et al. 2009a), but later its name was changed to avoid confusion with Alternaria ellipsoidea, an already described species from section Gypsophilae (Woudenberg et al. 2013). Section Gypsophilae contains all Alternaria species that occur on Caryophyllaceae (Lawrence et al. 2016). Alternaria breviramosa differs from A. pobletensis by having shorter conidiophores (up to 25 μm), often reduced to conidiogenous cells, and ellipsoidal, subcylindrical to fusoid conidia with only transverse septa (Crous et al. 2009a).
Alternaria pseudoventricosa Iturrieta-González, Dania García & Gené, sp. nov. MycoBank MB829635. Fig. 17.
Fig. 17.
Alternaria pseudoventricosa (ex-type FMR 16900). A. Colonies on PDA. B. Colonies on PCA. C. Colonies on OA. D–F. Conidiophores and conidia. Scale bars: D = 20 μm; E, F = 10 μm.
Etymology: Name refers to the apparent phylogenetic relationship to A. ventricosa.
Asexual morph on PCA: Mycelium superficial and immersed. Hyphae 1–7 μm wide, septate, branched, hyaline to pale brown, smooth-walled. Conidiophores macronematous, arising laterally from aerial hyphae, erect to slightly flexuous, up to 4-septate, unbranched, 30–45 × 4–6 μm, brown, smooth-walled, with one terminal conidiogenous locus. Conidia commonly in unbranched chains, with up to 19 conidia, obpyriform or obclavate, 10–48(–66) × 5–14 μm, with darkened middle transverse septa, 1–7 transverse, 0–1 longitudinal or oblique septa, brown to dark brown, verrucose to tuberculate. Secondary conidiophores scarce, as a beak arising from the conidial body. Sexual morph not observed.
Culture characteristics: Colonies on PDA reaching 64 mm diam after 1 wk at 25 °C, flat, cottony at the centre, floccose towards the periphery, margins regular; surface white (1A1); reverse yellowish white (4A2). On PCA attaining 62 mm diam, flat towards the periphery, margins regular; surface dark green (29F4), with tuft of white aerial mycelium at centre; reverse dark green to grey (29F8/29B1). On OA reaching 67 mm diam, flat, loosely floccose, margins regular; surface dull green (29E4); reverse dull green (29E4).
Cardinal temperature for growth: Optimum 25 °C, maximum 37 °C, minimum 5 °C.
Typus: Spain, Catalonia, Tarragona, Els Ports Natural Park, isolated from horse dung, Oct. 2017, G. Sisó & C. González-García (holotype CBS H-23900, culture ex-type CBS 145428 = FMR 16900).
Notes: Alternaria pseudoventricosa and A. ventricosa clustered in an unsupported monophyletic basal clade in section Infectoriae (Fig. 5). They can be differentiated by their conidial morphology. Conidia in A. ventricosa are usually asymmetric, laterally swollen, and pale cinnamon brown (Roberts 2007). In contrast, those of A. pseudoventricosa are obpyriform or obclavate and brown to dark brown.
Authors: I. Iturrieta-González, D. García, M. Hernández-Restrepo & J. Gené
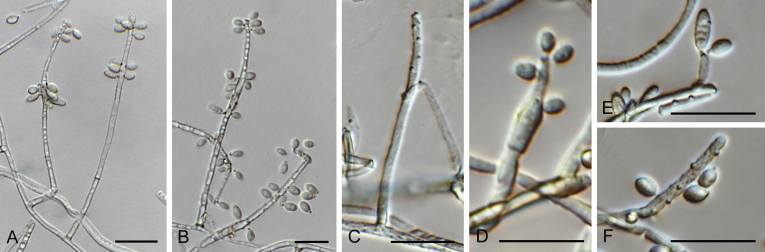
Brunneosphaerella Crous, Stud. Mycol. 64: 31. 2009. Fig. 18.
Fig. 18.
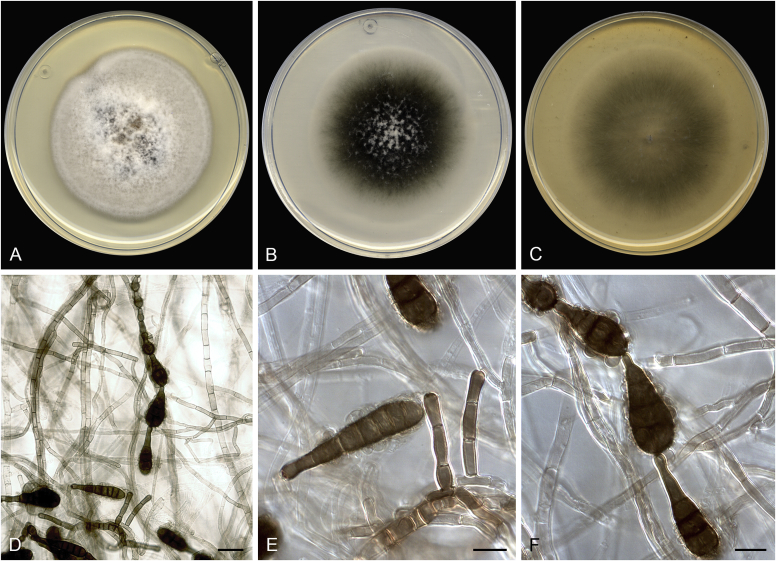
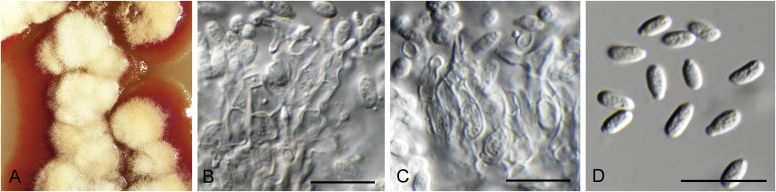
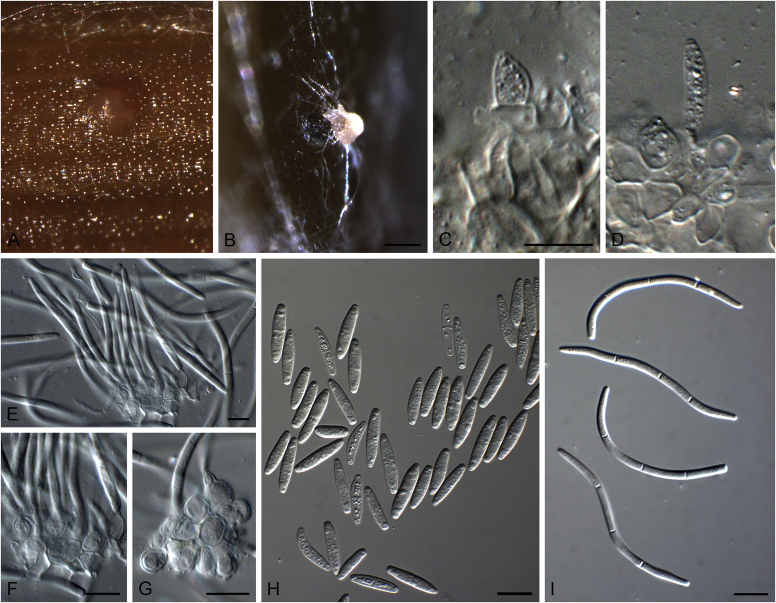
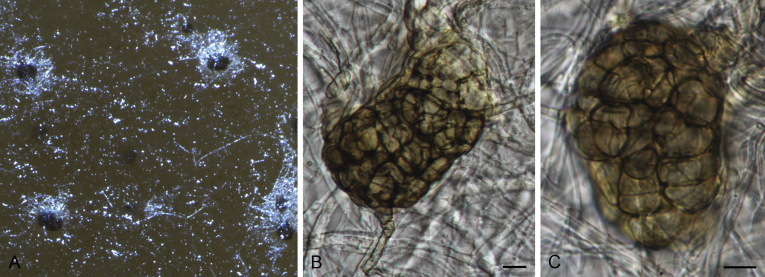
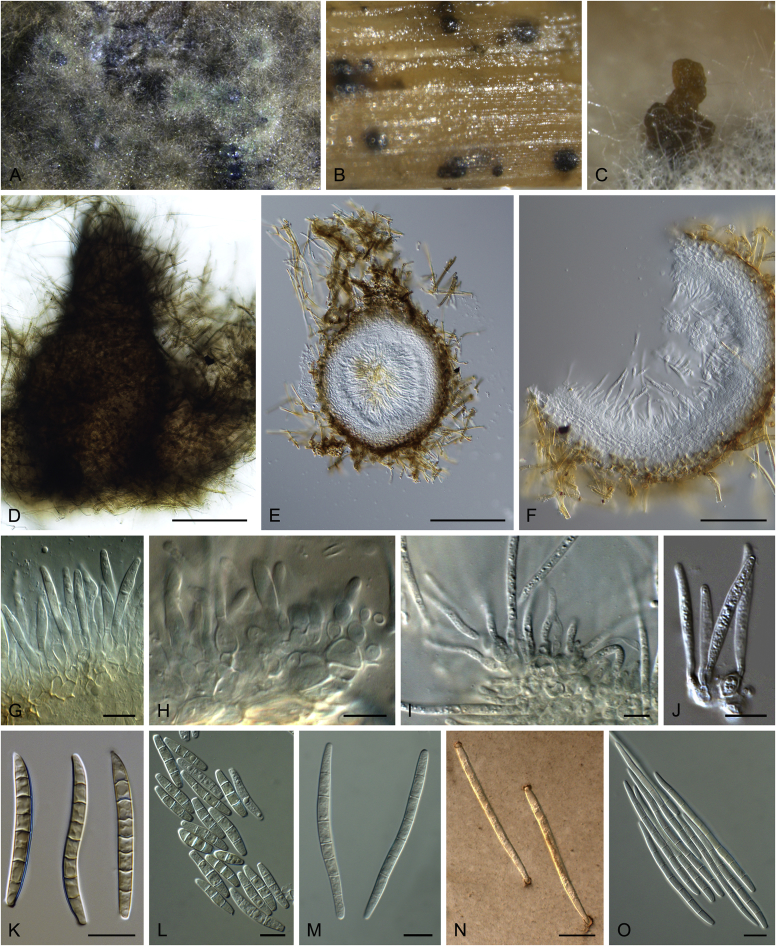
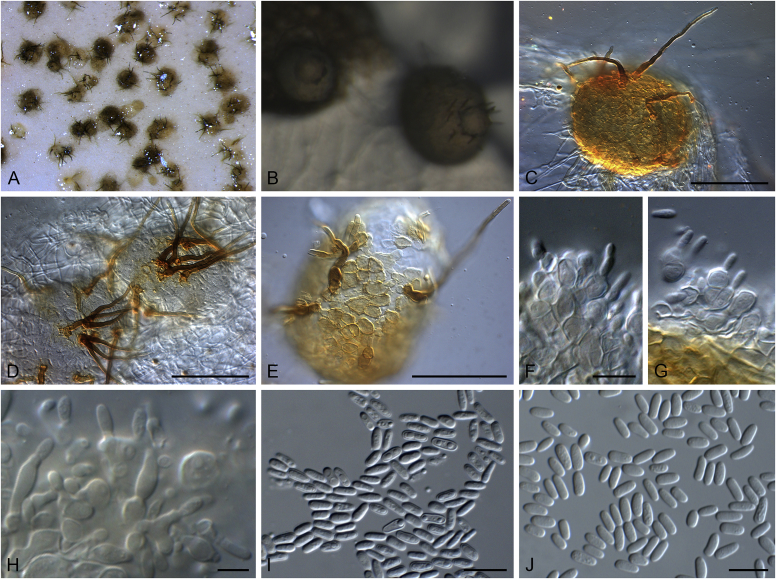
Brunneosphaerella spp. A–E. Disease symptoms. A, B.Brunneosphaerella protearum (epitype CBS H-20335). C.Brunneosphaerella nitidae (holotype CBS H-20334). D, E. Ascomata visible on lesion surface. D.Brunneosphaerella protearum (CBS H-20335). E.Brunneosphaerella nitidae (holotype CBS H-20334). F–H. Vertical sections through ascomata showing wall structure. F.Brunneosphaerella jonkershoekensis (holotype PREM 59447). G, H.Brunneosphaerella protearum (CBS H-20335). I–M. Asci. I.Brunneosphaerella jonkershoekensis (holotype PREM 59447). J, K.Brunneosphaerella nitidae (holotype CBS H-20334). L, M.Brunneosphaerella protearum (CBS H-20335). N–Q. Ascospores. N, O.Brunneosphaerella jonkershoekensis (holotype PREM 59447). P.Brunneosphaerella nitidae (holotype CBS H-20334). Q.Brunneosphaerella protearum (CBS H-20335). R, S. Germinating ascospores. R.Brunneosphaerella nitidae (holotype CBS H-20334). S.Brunneosphaerella protearum (CBS H-20335). Scale bars: G = 75 μm; F, I = 50 μm; H, J–M, P–S = 10 μm; N, O = 5 μm. Pictures A, B, D, F–I, L–O, Q, S taken from Crous et al. (2009b); C, E, J, K, P, R from Crous et al. (2011).
Classification: Dothideomycetes, Dothideomycetidae, Capnodiales, Mycosphaerellaceae.
Type species: Brunneosphaerella protearum (Syd. & P. Syd.) Crous, basionym: Leptosphaeria protearum Syd. & P. Syd. Epitype and ex-epitype strain designated by Crous et al. (2011): CBS H-20335, CBS 130597 = CPC 16338.
DNA barcodes (genus): LSU, ITS.
DNA barcodes (species): chs, rpb2, tef1. Table 3.
Table 3.
DNA barcodes of accepted Brunneosphaerella spp.
| Species | Isolates1 | GenBank accession numbers2 |
References | |||
|---|---|---|---|---|---|---|
| ITS | chs | rpb2 | tef1 | |||
| Brunneosphaerella jonkershoekensis | CPC 13902ET | JN712439 | JN712609 | MF951441 | JN712571 | Crous et al., 2011, Videira et al., 2017 |
| B. nitidae | CBS 130595T | GU214625 | JN712619 | MF951442 | JN712581 | Crous et al., 2009b, Crous et al., 2011, Videira et al., 2017 |
| B. protearum | CBS 130597ET | GU214626 | JN712620 | MF951443 | JN712582 | Crous et al., 2009b, Crous et al., 2011, Videira et al., 2017 |
| B. roupeliae | CBS 144602T | MK539950 | – | MK540080 | – | Present study |
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CPC: Culture collection of Pedro Crous, housed at the Westerdijk Fungal Biodiversity Institute. T and ET indicate ex-type and ex-epitype strains, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; chs: partial chitin synthase-1 gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene.
Ascomata amphigenous, immersed to semi-immersed, black, single, gregarious, substomatal, pyriform or globose with a papillate, periphysate ostiole; ascomatal wall consisting of three strata of slightly compressed textura angularis, an outer stratum of dark brown, thick-walled cells, becoming paler in the central stratum, and hyaline, thin-walled in the inner stratum. Pseudoparaphyses absent. Asci clavate to cylindro-clavate, often curved, tapering to a pedicel, narrowing slightly to a rounded apex with an indistinct ocular chamber, 8-spored, bitunicate with fissitunicate dehiscence. Ascospores biseriate, fusiform, broader at the apical end, initially hyaline and 1-septate, becoming yellow-brown and 3-septate at maturity, slightly constricted at median to supra-median septum (adapted from Crous et al. 2009b).
Culture characteristics: Colonies on OA spreading, flat, with sparse to moderate aerial mycelium, lobate and smooth, rarely feathery, margins. On OA surface pale luteous, smoke grey with submerged iron-grey margins, or olivaceous grey with iron-grey, pale olivaceous grey and smoke grey patches. On PDA surface olivaceous grey, sometimes with pale olivaceous grey to smoke grey patches, or smoke grey with iron-grey margins; reverse iron-grey. On MEA surface pale olivaceous grey, smoke grey, dirty white with patches of smoke grey, or smoke grey with dirty white and olivaceous grey patches and submerged iron-grey margins; reverse iron-grey or olivaceous grey.
Optimal media and cultivation conditions: MEA, OA, PDA and SNA at 25 °C under near-ultraviolet light to promote sporulation.
Distribution: Africa, mainly reported from South Africa. Also reported from Pacific Islands (Hawaii) and Europe (Portugal and Spain).
Hosts: Protea spp. (Proteaceae).
Disease symptoms: Leaf spots and Brunneosphaerella leaf blight.
Notes: Brunneosphaerella was introduced by Crous et al. (2009b) to accommodate Leptosphaeria protearum, which is a major leaf spot and blight pathogen of Protea spp. causing severe losses in plantations of South African Protea spp. wherever they are cultivated. Morphologically, Brunneosphaerella is distinct from Leptosphaeria in that ascospores are always brown at maturity, and asexual morphs have brown, percurrently proliferating conidiogenous cells. A new species isolated from leaves of Protea repens in South Africa, B. jonkershoekensis, was included in the genus when it was introduced (Crous et al. 2009b). This species appears to be a serious pathogen of Pr. repens in the Western Cape Province of South Africa. Subsequently, Crous et al. (2011) described the third species known from the genus, B. nitidae. This was isolated from the same area as B. jonkershoekensis, but B. nitidae was isolated from leaves of Pr. nitida, causing leaf spots on this host. Thus, the genus comprises four species, all of which were isolated from species of Protea in South Africa. The ITS sequences of the four species are highly similar. However, these can be easily delimited based on the chs, rpb2 and tef1 sequences.
References: Crous et al., 2009b, Crous et al., 2011, Videira et al., 2017 (morphology and phylogeny).
Brunneosphaerella roupeliae Crous, sp. nov. MycoBank MB829609. Fig. 19.
Fig. 19.
Brunneosphaerella roupeliae (ex-type CBS 144602). A. Close-up of leaf spot with ascomata. B, C. Asci with ascospores. D. Germinating ascospores. Scale bars = 10 μm.
Etymology: Name refers to Protea roupeliae, the host species from which it was collected.
Leaf spots amphigenous, sub-circular, 5–20 mm diam, medium brown, with raised, dark brown border. Ascomata pseudothecial, amphigenous, black, immersed to erumpent, globose, to 250 μm diam, with apical ostiole; ascomatal wall of 2–3 layers of brown cells of textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, ellipsoid-fusoid, straight to slightly curved, 8-spored, 65–110 × 11–15 μm. Ascospores bi- to triseriate, overlapping, guttulate, thick-walled, straight to slightly curved, obovoid with obtuse ends, widest in middle of apical cell, 3-septate, constricted at median septum, tapering towards both ends, but more prominently towards lower end, (19–)22–23(–25) × 5(–6) μm. Ascospores germinating from both ends, becoming brown and verruculose, constricted at primary septum, with germ tubes parallel to the long axis, ascospore becoming 7–9 μm diam.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium, and even, lobate margins, reaching 10 mm diam after 2 wk at 25 °C. On MEA surface pale olivaceous grey, reverse olivaceous grey; on PDA surface and reverse olivaceous grey, and on OA surface pale luteous.
Typus: South Africa, KwaZulu-Natal Province, Drakensberg, Monks Cowl, on leaves of Protea roupeliae (Proteaceae), 18 Jan. 2010, A. Wood, HPC 1522 (holotype CBS H-23847, culture ex-type CPC 32914 = CBS 144602).
Notes: Brunneosphaerella roupeliae was isolated from the same host genus in South Africa as the other three species of the genus, Protea. The ITS and LSU sequences located this species in the genus Brunneosphaerella since both sequences showed more than 99 % of nucleotide similarity with the ex-type strains of the other three species. The rpb2 sequence showed a nucleotide similarity of 95.86 % with the ex-epitype strain of B. protearum, 95.45 % with the ex-epitype strain of B. jonkershoekensis, and 94.5 % with the ex-type strain of B. nitidae. The tef1 sequence showed a nucleotide similarity of 95.66 % with the ex-epitype strain of B. protearum and the ex-type strain of B. nitidae, and 94.44 % with the epitype strain of B. jonkershoekensis. Brunneosphaerella roupeliae produces the shortest ascospores of the genus [(19–)22–23(–25) μm in B. roupeliae vs. (25–)27–34(–37) μm in B. jonkershoekensis vs. (20–)24–28(–30) μm in B. nitidae vs. (20–)23–26(–30) μm in B. protearum].
Authors: P.W. Crous, J.Z. Groenewald & Y. Marin-Felix
Elsinoe Racib., Parasit. Alg. Pilze Java's (Jakarta) 1: 14. 1900. Fig. 20, Fig. 21.
Fig. 20.
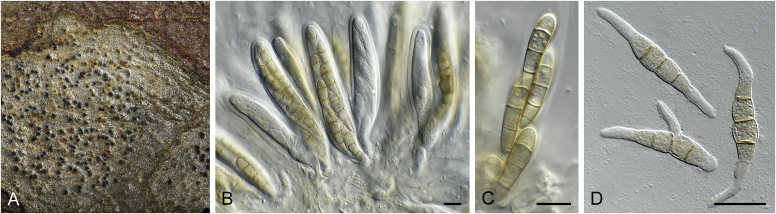
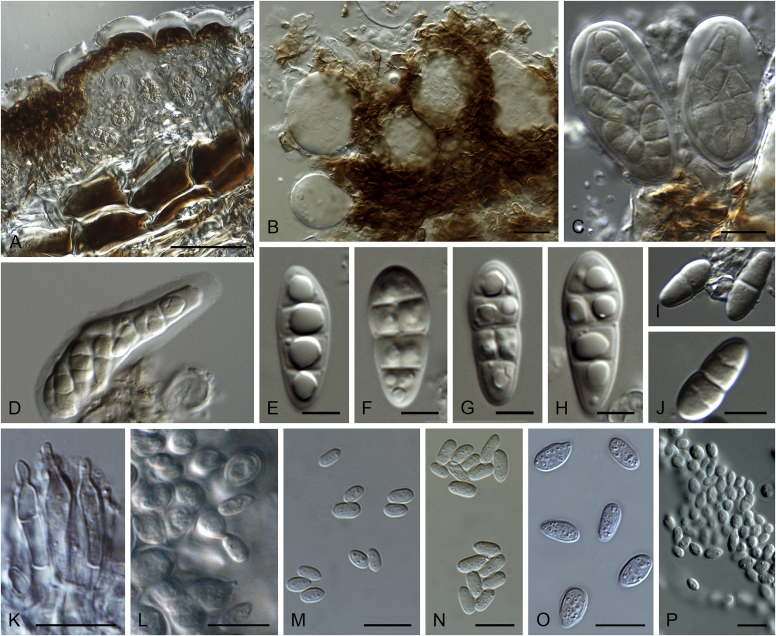
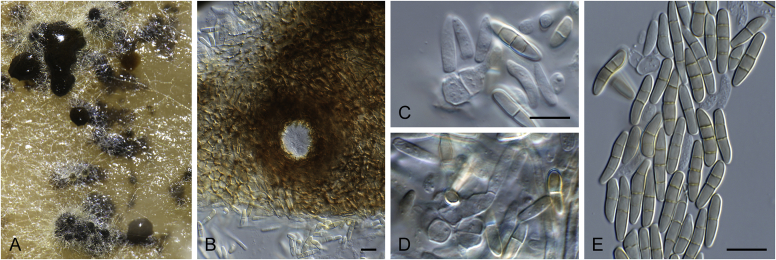
Disease symptoms of Elsinoe spp. A.Elsinoe ampelina on Vitis vinifera. B.Elsinoe asclepiadea on Asclepias mellodora. C.Elsinoe bidentis on Bidens segetum. D.Elsinoe erythrinae on Erythrina sp. E.Elsinoe eucalypticola on Eucalyptus sp. F.Elsinoe fawcettii on Citrus sp. G.Elsinoe freyliniae on Freylinia lanceolata. H.Elsinoe perseae on Persea americana. I.Elsinoe othonnae on Othonna quinquedentata. J.Elsinoe poinsettiae on Euphorbia sp. K.Elsinoe punicae on Punica granatum. L.Elsinoe terminaliae on Terminalia catappa. Pictures taken from Fan et al. (2017).
Fig. 21.
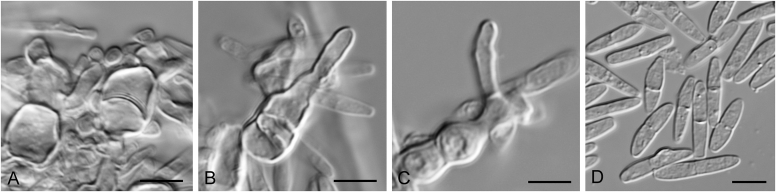
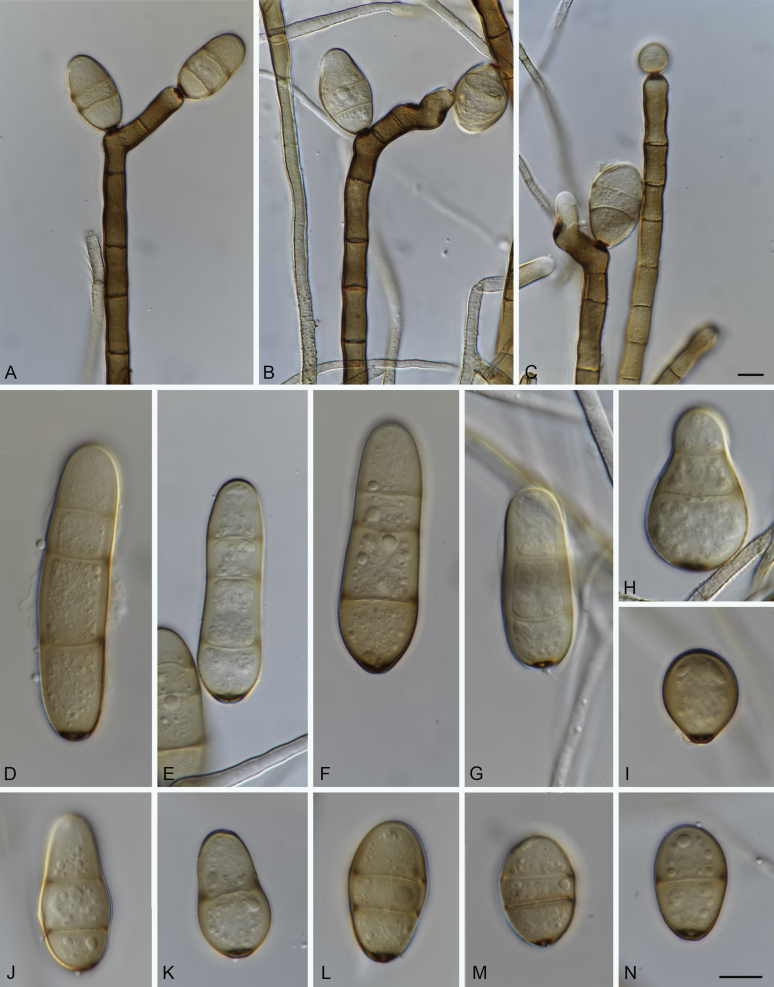
Elsinoe spp. A–J. Sexual morph. A. Subcutilar ascoma of Elsinoe fecunda. B–D. Asci. B.Elsinoe eucalypticola (ex-type CBS 124765). C, D.Elsinoe fecunda (holotype, PREM 56503). E–J. Ascospores. E–H.Elsinoe eucalypticola (ex-type CBS 124765). I, J.Elsinoe fecunda. K–P. Asexual morph. K, L. Conidiophores. K.Elsinoe asclepiadea (ex-type CPC 18544). L.Elsinoe othonnae (ex-type CBS 139910). M–P. Conidia. M.Elsinoe asclepiadea (ex-type CPC 18544). N.Elsinoe erythrinae (ex-epitype CPC 18542). O.Elsinoe tectificae (ex-type CBS 124777). P.Elsinoe othonnae (ex-type CBS 139910). Scale bars: A = 100 μm; B–D, K–U = 10 μm; E–J = 5 μm; C applies to C and B; J applies I and J. Pictures taken from Fan et al. (2017).
Synonyms: Sphaceloma de Bary, Ann. Oenol. 4: 165. 1874.
Manginia Viala & Pacottet, C. r. hebd. Séanc. Acad. Sci., Paris 139: 88. 1904.
Melanobasidium Maubl., Bull. Soc. mycol. Fr. 22: 69. 1906.
Plectodiscella Woron., Mykol. Zentbl. 4: 232. 1914.
Isotexis Syd., in Sydow & Petrak, Annls mycol. 29: 261. 1931.
Melanobasis Clem. & Shear, Gen. fung., Edn 2 (Minneapolis): 224. 1931.
Melanodochium Syd., Annls mycol. 36: 310. 1938.
Bitancourtia Thirum. & Jenkins, Mycologia 45: 781. 1953.
Kurosawaia Hara, List of Japanese Fungi, 4th Edn: 172. 1954.
Uleomycina Petr., Sydowia 8: 74. 1954.
Melanophora Arx, Verh. K. ned. Akad. Wet., tweede sect. 51: 43. 1957.
Classification: Dothideomycetes, Dothideomycetidae, Myriangiales, Elsinoaceae.
Type species: Elsinoe canavaliae Racib. Type or reference material not available.
DNA barcode (genus): LSU.
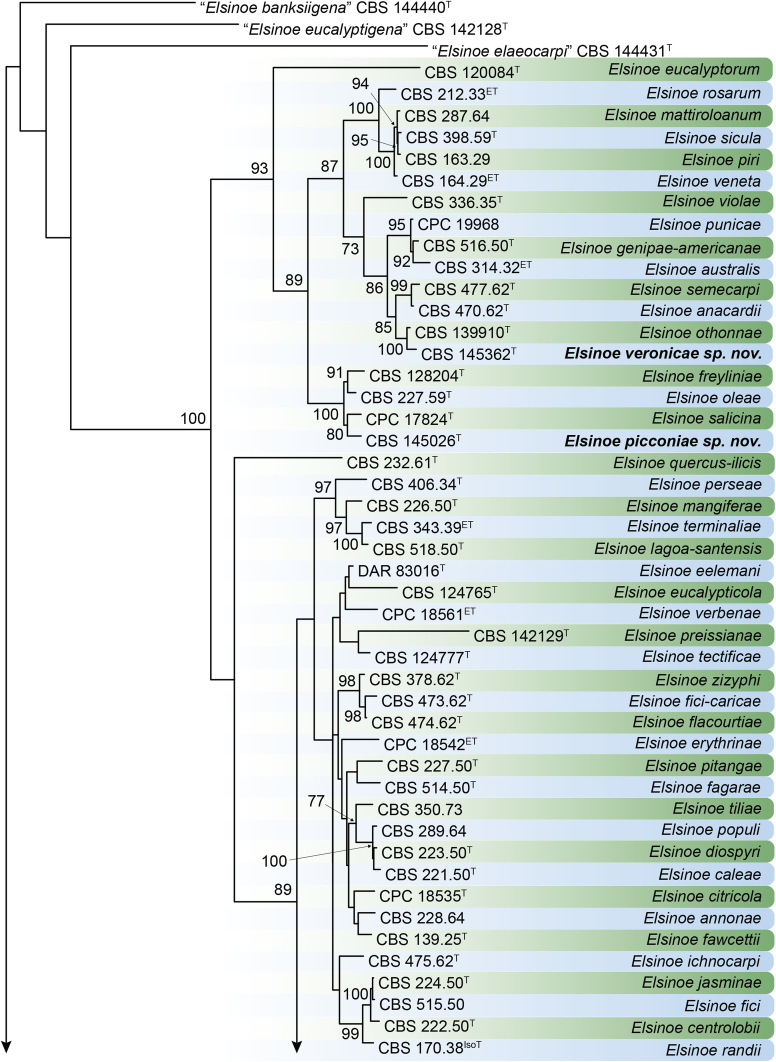
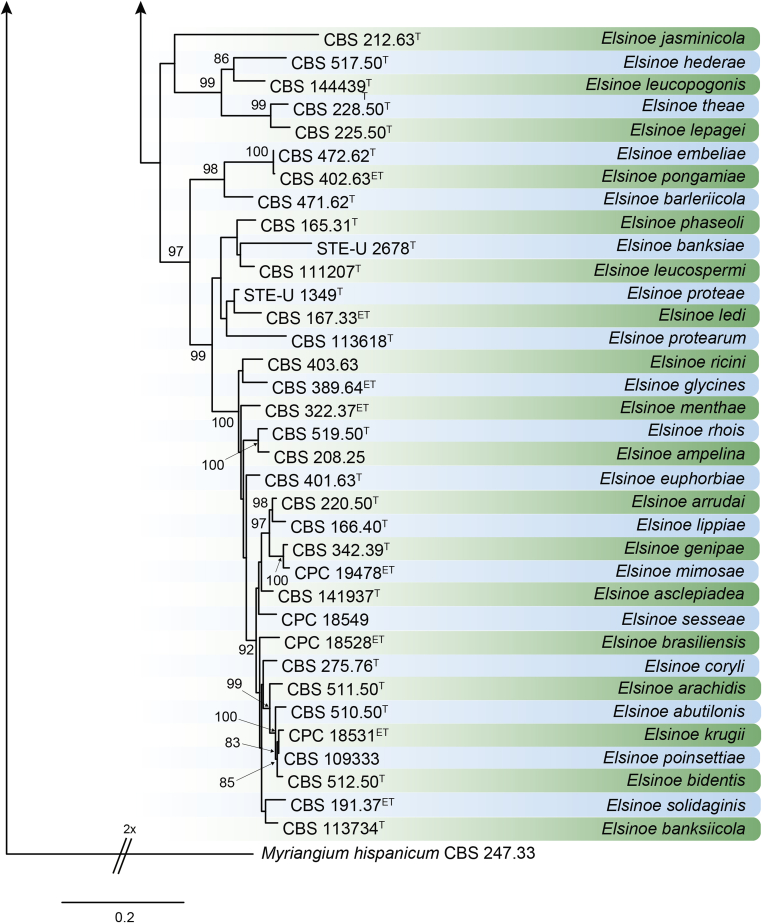
DNA barcodes (species): ITS, rpb2, tef1. Table 4. Fig. 22.
Table 4.
DNA barcodes of accepted Elsinoe spp.
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CPC: Culture collection of Pedro Crous, housed at Westerdijk Fungal Biodiversity Institute; DAR: Plant Pathology Herbarium, New South Wales, Australia; STE-U: Department of Plant Pathology, Stellenbosch University, South Africa. T, ET and IsoT indicate ex-type, ex-epitype and ex-isotype strains, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial large subunit (28S) nrRNA gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene.
Fig. 22.
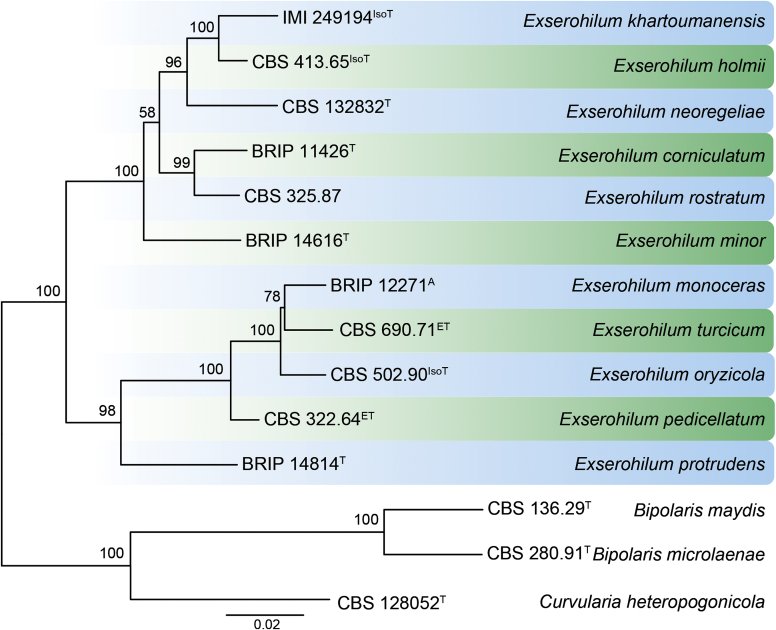
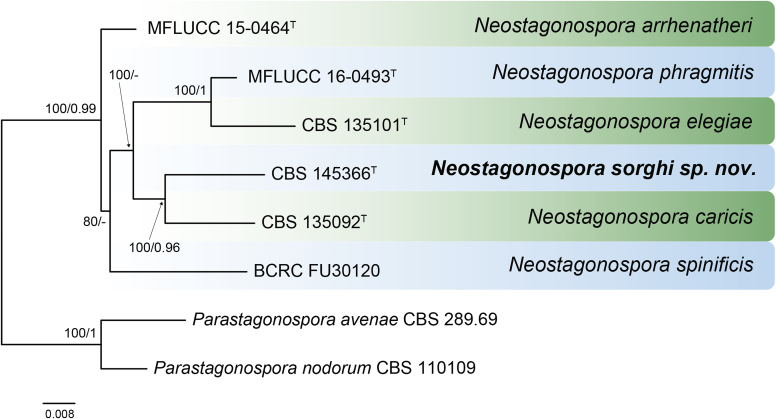
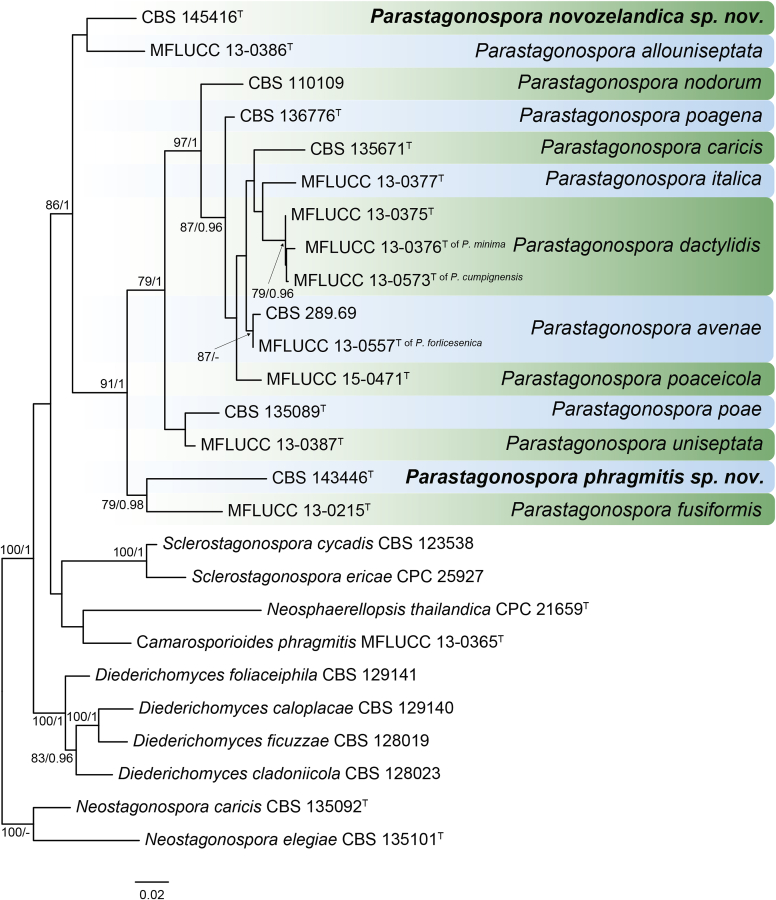
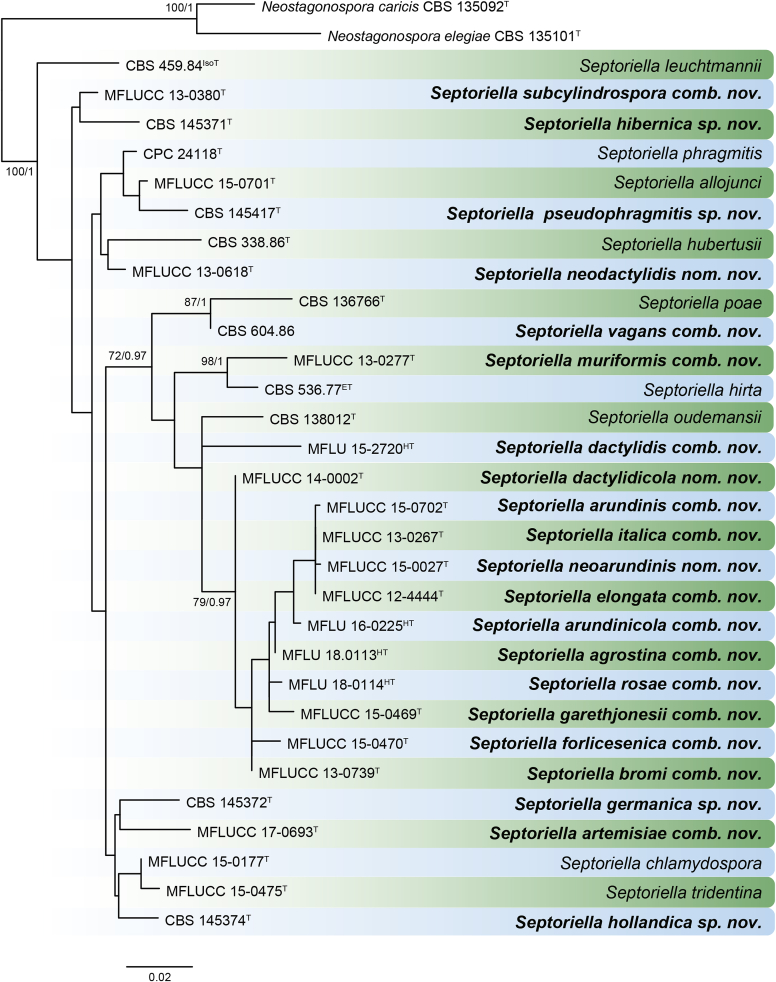
RAxML phylogram obtained from the combined ITS (609 bp), LSU (741 bp), rpb2 (747 bp) and tef1 (422 bp) sequence alignment of all accepted species of Elsinoe. The tree was rooted to Myriangium hispanicum CBS 247.33. The novelties proposed in this study are indicated in bold. RAxML bootstrap support (BS) values above 70 % are shown at the nodes. GenBank accession numbers are listed in Table 4. T, ET and IsoT indicate ex-type, ex-epitype and ex-isotype strains, respectively. TreeBASE: S23834.
Ascostromata solitary, aggregated, or gregarious, wart-like, or as small distinctively coloured elevations, or pulvinate, immersed to semi-immersed, globose to subglobose, white, pale yellow or brown, soft, multi-loculate, locules scattered in upper part of ascostromata; ascostromatal wall composed of pseudoparenchymatous cells of textura globulosa to textura angularis; locules with few to numerous asci inside each locule, ostiolate; ostiole minute. Periphyses absent. Asci 8-spored, bitunicate, fissitunicate, saccate to globose, with a minute pedicel, and ocular chamber. Ascospores irregularly arranged, oblong or fusiform with slightly acutely rounded ends, with 2–3 transverse septa, hyaline, smooth-walled, lacking a sheath. Acervuli or sporodochia subepidermal, pseudoparenchymatous. Conidiophores hyaline to pale brown, polyphialidic. Conidiogenous cells formed directly from the upper cells of the pseudoparenchyma, mono- to polyphialidic, integrated or discrete, determinate, hyaline to pale brown, without visible periclinal thickening. Conidia hyaline, smooth-walled, aseptate, ellipsoidal, guttulate (adapted from Fan et al. 2017).
Culture characteristics: Colonies on MEA, slow growing, raised, irregular, erumpent, folded or cerebriform, smooth and irregular margins, with sparse to moderate white to grey aerial mycelium. On MEA, surface white to pale luteous, cinnamon, sepia, apricot, saffron with or without purplish grey in centre, brown with apricot margins, rosy buff in centre with cinnamon margins, livid red, scarlet red with diffuse red pigment in agar, or iron-grey; reverse umber, ochreous, iron-grey, dark vinaceous, or centre scarlet and orange with cinnamon margins.
Optimal media and cultivation conditions: MEA, OA, PDA, SNA and WA at 22 °C under near-ultraviolet light (12 h light, 12 h dark).
Distribution: Worldwide.
Hosts: Wide range of hosts, including some economically important crops such as avocado, cassava, citrus, grapevines, ornamentals such as poinsettias, field crops and woody hosts.
Disease symptoms: Scab, leaf and fruit spot and anthracnose disease.
Notes: Elsinoe comprises plant pathogenic species that cause scab and spot anthracnose on a wide range of hosts, including some economically important crops and ornamentals. The disease symptoms that these species produce are easily recognisable, being known as “signature-bearing diseases”, for the cork-like appearance of older infected tissues with scab-like appearance. Also, these can produce other disease symptoms often called anthracnose, as in the case of infected grapevines (Barrus and Horsfall, 1928, Jenkins, 1947, Farr et al., 1989, Pan, 1994, Phillips, 1994, Gottwald, 1995). However, the use of this name is confusing since it is used much broader to include diseases caused by Colletotrichum. Although many species of Elsinoe causing scab disease have been described, only few of them cause important diseases (Holliday 1980), having the main impact on the appearance of the harvested product and its market acceptability rather than on crop productivity (Swart et al. 2001). Species of Elsinoe seem to be host-specific since 77 of the 81 species accepted in the present study occur on only one host species or genus.
Elsinoe and its asexual morph, Sphaceloma, were recently reviewed by Fan et al. (2017). In that study, 26 new combinations were proposed for the species originally placed in Sphaceloma. Moreover, eight new species were introduced and 13 epitypes were designated. Based on phylogenetic data, Fan et al. (2017) accepted 75 species in the genus. However, E. banksiae, which was described by Swart et al. (2001), and three species described by Crous et al. (2016a), i.e. E. eelemani, E. eucalyptigena and E. preissianae, were not included in that study. Our phylogenetic analysis corroborated the placement of these species in the main well-supported clade representing the genus Elsinoe, except for E. eucalyptigena, whose placement remains unknown and, therefore it is not considered an accepted species of Elsinoe in the present study. Moreover, another three new species have been subsequently described by Crous et al. (2018), i.e. E. banksiigena, E. elaeocarpi and E. leucopogonis. However, in our phylogenetic analysis, the two first species were not located in the Elsinoe s. str. clade, and are thus excluded from the genus at present. Therefore, hitherto a total of 79 species are accepted, plus the new species described in the present study. Unfortunately, there are no cultures and molecular data of the type species of the genus, E. canavaliae, which needs to be epitypified in order to clarify its phylogenetic position.
References: Fan et al. 2017 (morphology and phylogeny).
Elsinoe picconiae Crous, sp. nov. MycoBank MB829611. Fig. 23.
Fig. 23.
Elsinoe picconiae (ex-type CBS 145026). A. Colony on OA. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Name refers to Picconia, the host from which this fungus was collected.
Conidiomata sporodochial or acervular on leaves, medium brown, up to 250 μm diam; conidiomatal wall composed of textura angularis. Conidiophores subcylindrical to doliiform, hyaline to pale brown, smooth-walled, 0–1-septate, unbranched, 10–15 × 3–4 μm. Conidiogenous cells polyphialidic, hyaline, smooth-walled, subcylindrical to doliiform, 5–8 × 3–4 μm. Conidia hyaline, aseptate, ellipsoid, apex obtuse, base truncate, (4–)5–6(–7) × (2–)2.5 μm.
Culture characteristics: Colonies erumpent, spreading, with sparse aerial mycelium, folded surface, and smooth, lobate margins, reaching 7 mm diam after 2 wk at 25 °C. On MEA surface rust, reverse sienna; on PDA surface coral, reverse bay; on OA surface scarlet with diffuse scarlet pigment.
Typus: Spain, Tenerife, Los Silos, leaf of Picconia excelsa (Oleaceae), 12 Mar. 2017, A. van Iperen, HPC 2063 (holotype CBS H-23848, culture ex-type CBS 145026 = CPC 33648).
Notes: Elsinoe picconiae is related to E. freyliniae, E. oleae and E. salicina. However, E. picconiae can be easily distinguished by its narrower conidia [(2–)2.5 μm vs. (2.5–)3–4 μm in E. freyliniae, 3–6 μm in E. oleae, and (2.5–)3–4.5(–5) μm in E. salicina]. Moreover, E. picconiae is the first species of the genus isolated from Picconia excelsa.
Elsinoe veronicae Crous, Thangavel & Y. Marín, sp. nov. MycoBank MB829610. Fig. 24.
Fig. 24.
Elsinoe veronicae (ex-type CBS 145362). A. Colony on OA. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Name refers to Veronica, the host from which this fungus was collected.
Conidiomata sporodochial, erumpent, 80–200 μm diam, based on a pale brown stroma, giving rise to densely aggregated conidiophores. Conidiophores hyaline to pale brown, smooth-walled, subcylindrical, 1–2-septate, 15–30 × 2.5–3.5 μm, unbranched or branched above. Conidiogenous cells integrated, subcylindrical, hyaline, smooth-walled, 7–10 × 2.5–3.5 μm, polyphialidic. Conidia solitary, aggregating in mucoid mass, aseptate, hyaline, smooth-walled, guttulate, ellipsoid, apex subobtuse, tapering at base to truncate hilum, (4–)5–5.5(–6.5) × 2.5(–3) μm.
Culture characteristics: Colonies erumpent, spreading, surface folded with moderate aerial mycelium, and even, lobate margins, reaching 25 mm diam after 2 wk. On MEA surface brick, reverse cinnamon; on PDA surface brick to scarlet, reverse brick in centre, scarlet in outer region; on OA surface scarlet with diffuse scarlet pigment.
Typus: New Zealand, Auckland, St. John, Morrin Road, on Veronica sp. (Scrophulariaceae), 2013, R. Thangavel (holotype CBS H-23865, culture ex-type CBS 145362 = CPC 34137 = T17_00408D).
Notes: Elsinoe veronicae is closely related to E. othonnae. Although the ITS sequences of both species showed more than 99 % nucleotide similarity, the rpb2 sequences showed only 96.95 % similarity. Morphologically, these are also similar differing mainly in their conidial size [(4–)5–5.5(–6.5) × 2.5(–3) μm in E. veronicae vs. (5–)6–7 × (2.5–)3(–4) μm in E. othonnae]. Moreover, E. veronicae was found on Veronica (Scrophulariaceae) in New Zealand, while E. othonnae has only been reported on Othonna (Asteraceae) in South Africa (Crous et al. 2015c).
Authors: Y. Marin-Felix, R. Thangavel & P.W. Crous
Exserohilum K.J. Leonard & Suggs, Mycologia 66: 290. 1974. Fig. 25.
Fig. 25.
Exserohilum spp. A–E. Sexual morph. A. Ascomata of Exserohilum minor (ex-isotype IMI 294530). B, C. Asci of Exserohilum minor (ex-isotype IMI 294530). D, E. Ascospores. D.Exserohilum minor (ex-isotype IMI 294530). E.Exserohilum khartoumensis (ex-isotype CBS 132708). F–AA. Asexual morph. F–N. Conidiophores and conidia. F, L.Exserohilum oryzicola (ex-isotype CBS 502.90). G.Exserohilum turcicum (ex-epitype CBS 690.71). H, N.Exserohilum holmii (ex-isotype CBS 413.65 and BRIP 12679). I. Exserohilum pedicellatum (CBS 375.76). J, M.Exserohilum rostratum (CBS 120380, CBS 196.29). K.Exserohilum monoceras (CBS 198.29). O. Detail of the conidial hilum of Exserohilum oryzicola (ex-isotype CBS 502.90). P–AA. Conidia. P, Q, Z, AA.Exserohilum rostratum (CBS 128054, CBS 120380, BRIP 11422). R.Exserohilum holmii (BRIP 12679). S.Exserohilum pedicellatum (BRIP 12040). T, U.Exserohilum turcicum (BPI 431157 holotype). V.Exserohilum oryzicola (BRIP 16229). W.Exserohilum protrudens (BRIP 14816). X.Exserohilum corniculatum (ex-type BRIP 11426). Y.Exserohilum neoregelia (CBS 132833). Scale bars A = 50 μm; others = 10 μm; C applies to B and C; E applies to D and E. Pictures taken from Hernández-Restrepo et al. (2018).
Synonyms: Setosphaeria K.J. Leonard & Suggs, Mycologia 66: 294. 1974.
Luttrellia Khokhr. & Gornostaĭ (as ‘Lutrellia’; non Luttrellia Shearer), Vodorosli, Griby i Mkhi Dal’nego Vostoka [Algae, Fungi and Mosses of the Soviet Far-East] (Vladivostok): 80. 1978.
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Pleosporaceae.
Type species: Exserohilum turcicum (Pass.) K.J. Leonard & Suggs, basionym: Helminthosporium turcicum Pass. Ex-epitype and ex-epitype strain designated by Hernández-Restrepo et al. (2018): CBS H-23323, CBS 690.71.
DNA barcodes (genus): LSU, ITS.
DNA barcodes (species): ITS, gapdh, rpb2. Table 5. Fig. 26.
Table 5.
DNA barcodes of accepted Exserohilum spp.
BRIP: Queensland Plant Pathology Herbarium, Brisbane, Australia; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, UK. T, ET, IsoT and A indicate ex-type, ex-epitype, ex-isotype and authentic strains.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial large subunit (28S) nrRNA gene; gapdh: partial glyceraldehyde-3-phosphate dehydrogenase gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene.
Fig. 26.
RAxML phylogram obtained from the combined ITS (793 bp), gapdh (578 bp) and rpb2 (860 bp) sequence alignment of all the accepted species of Exserohilum. The tree was rooted to Curvularia and Bipolaris. RAxML bootstrap support (BS) values above 70 % are shown in the nodes. GenBank accession numbers are indicated in Table 5. T, ET, IsoT and A indicate ex-type, ex-epitype, ex-isotype and authentic strains. TreeBASE: S23834.
Ascomata superficial, immersed or erumpent, globose to ellipsoid, unilocular, dark brown to black, with or without a neck, ostiolate, with simple rigid setae around the ostiolar apex and on the upper half of the ascoma where they are often mixed with hyaline, filiform, septate hyphae; ascomatal wall composed of pseudoparenchymatous cells, dark brown and thick-walled on the outside, but with more or less hyaline cells towards the inside, cells of textura angularis. Pseudoparaphyses filiform, hyaline, septate, branched, anastomosing. Asci arising from a basal cushion of thin-walled pseudoparenchymatous cells, bitunicate, 1–8-spored, cylindrical to cylindrical-clavate, short or moderately long-stalked, thick-walled, with an apical nasse and fissitunicate dehiscence. Ascospores fusoid, hyaline to pale brown, smooth-walled, 2–6 or rarely more transversely septate, constricted at the septa, surrounded by a hyaline mucilaginous sheath which often extends some distance beyond the ends of the spore. Conidiophores macronematous, mononematous, septate, cylindrical, olivaceous brown to brown, smooth-walled to verruculose, often geniculate above. Conidiogenous cells integrated, terminal and intercalary, sympodial, mono- or polytretic, cicatrised; conidiogenous nodes smooth to rough. Conidia fusiform, cylindrical or obclavate, straight to curved, multi-distoseptate, with a protruding hilum (adapted from Hernández-Restrepo et al. 2018).
Culture characteristics: Colonies on PDA brown or grey olivaceous to olivaceous black, sometimes white, pale grey, hairy, cottony to powdery, margins fimbriate.
Optimal media and cultivation conditions: Sterilised Zea mays leaves placed on 1.5 % WA or PDA at 25 °C under near-ultraviolet light (12 h light, 12 h dark) to induce sporulation.
Distribution: Worldwide.
Host: Mainly pathogens of grasses, but some also on non-grass hosts. Other substrates where they can be found include river sediments, soil, grains, plant debris, and humans. Exserohilum rostratum has been reported as a human pathogen.
Disease symptoms: In plants: leaf blight, leaf spots, melting out, root rot, and foot rot, among others. In humans: skin infections, keratitis, non-invasive allergies, invasive sinusitis, and disseminated infections.
Notes: Exserohilum is differentiated from the closely related Bipolaris, Curvularia and Pyrenophora by producing conidia with a protruding hilum (Leonard & Suggs 1974). Recently the taxonomy and phylogeny of Exserohilum has been revisited by Hernández-Restrepo et al. (2018). Based on morphological and molecular data 11 phylogenetic species are accepted (Fig. 26, Table 5). Three species were excluded from the genus, namely Ex. novae-zelandiae relocated to Sporidesmiella, and Ex. paspali and Ex. sorghicola to Curvularia, while another 15 species were retained in Exserohilum, although some were doubtful. Species in Exserohilum are morphologically very variable and a molecular analysis is required for a correct species identification.
The type species of the genus, Ex. turcicum (= Helminthosporium turcicum), was described from Italy causing northern leaf blight of corn (Passerini, 1876, Saccardo, 1886). Other species attacking economically significant crops include Ex. pedicellatum, causing root rot on maize and brown lesions on wheat roots (Henry, 1924, Sivanesan, 1987), and Ex. rostratum, producing leaf spot on banana, maize and wheat, foot rot in wheat, damping off of sugarcane seedlings, blackening and seed germination failure in cereals (Drechsler, 1923, Leonard, 1976, Sivanesan, 1987, Lin et al., 2011).
Previously, three different species, Ex. longirostratum, Ex. macginnisii and Ex. rostratum, were recognised as human pathogens (McGinnis et al., 1986, Padhye et al., 1986, de Hoog et al., 2000, da Cunha et al., 2012). However, a multi-locus phylogenetic analysis (Hernández-Restrepo et al. 2018) demonstrated that they are actually the same phylogenetic species. Exserohilum rostratum has been reported as an agent of phaeohyphomycosis and sometimes causing life-threatening infections in humans (McGinnis et al., 1986, Padhye et al., 1986, Aquino et al., 1995, Adler et al., 2006). This species was recently implicated in an outbreak of fungal meningitis associated with contaminated methylprednisolone in the USA (Kainer et al. 2012).
References: Drechsler, 1923, Drechsler, 1934, Luttrell, 1963, Leonard and Suggs, 1974, Sivanesan, 1987 (taxonomy, morphology and pathogenicity), Leonard 1976 (sexual/asexual connection), de Hoog et al., 2000, da Cunha et al., 2012 (human pathogens), Hernández-Restrepo et al. 2018 (morphology, phylogeny, review).
Author: M. Hernández-Restrepo
Neosetophoma Gruyter et al., Mycologia 102: 1075. 2010. Fig. 27.
Fig. 27.
Neosetophoma lunariae (ex-type CBS 141409). A. Conidiomata on OA. B. Conidiomata showing ostiolar region. C, D. Conidiogenous cells. E. Conidia. Scale bars = 10 μm; C applies to C and D. Pictures taken from Hernández-Restrepo et al. (2016a).
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Phaeosphaeriaceae.
Type species: Neosetophoma samarorum (Desm.) Gruyter et al., basionym: Phoma samarorum Desm. Epitype and ex-epitype strain designated by Gruyter et al. (2010): CBS H-20319, CBS 138.96.
DNA barcode (genus): LSU. Fig. 28.
Fig. 28.
RAxML phylogram obtained from the combined ITS (626 bp) and LSU (852 bp) sequence alignment of members of the family Phaeosphaeriaceae. The tree was rooted to Coniothyrium glycines CBS 124141 and Coniothyrium sidae CBS 135108. The novelties proposed in this study are indicated in bold. RAxML bootstrap support (BS) values above 70 %and Bayesian posterior probability scores above 0.95 are shown at the nodes. Numbers between parentheses correspond to GenBank accession numbers for ITS and LSU sequences, respectively. T, ET, HT, IsoT, LT and NT indicate ex-type, ex-epitype, holotype, ex-isotype, ex-lectotype and ex-neotype strains, respectively. TreeBASE: S23834.
DNA barcodes (species): ITS, rpb2, tef1, tub2. Table 6. Fig. 29.
Table 6.
DNA barcodes of accepted Neosetophoma spp.
| Species | Isolates1 | GenBank accession numbers2 |
References | |||
|---|---|---|---|---|---|---|
| ITS | rpb2 | tef1 | tub2 | |||
| Neosetophoma aseptata | CBS 145363T | MK539953 | MK540084 | – | – | Present study |
| Nph. clematidis | MFLUCC 13-0734T | KP744450 | – | – | – | Liu et al. (2015) |
| Nph. garethjonesii | MFLUCC 14-0528T | KY496758 | – | KY514402a | – | Tibpromma et al. (2017) |
| Nph. guiyangensis | GZCC 18-0111T | MH018134 | – | – | – | Hyde et al. (2018) |
| Nph. iranianum | IBRC-M 30176T | MF684861 | – | – | – | Karunarathna et al. (2017) |
| Nph. italica | MFLUCC 13-0388T | KP711356 | – | – | – | Liu et al. (2015) |
| Nph. lunariae | CBS 141409T | KX306763 | – | – | – | Hernández-Restrepo et al. (2016a) |
| Nph. phragmitis | CBS 145364T | MK539954 | MK540085 | MK540148b | – | Present study |
| Nph. poaceicola | MFLUCC 16-0886T | KY568986 | – | – | – | Thambugala et al. (2017) |
| Nph. rosae | MFLUCC 15-1073T | MG828925 | – | MG829218a | – | Wanasinghe et al. (2018) |
| Nph. rosarum | MFLUCC 17-0308T | MG828927 | – | – | – | Wanasinghe et al. (2018) |
| Nph. rosigena | MFLUCC 17-0768T | MG828928 | – | – | – | Wanasinghe et al. (2018) |
| Nph. samarorum | CBS 138.96ET | KF251160 | KF252168 | KF253119b | KF252655 | Quaedvlieg et al. (2013) |
| Nph. sambuci | CBS 145365T | MK539955 | MK540086 | MK540149b | – | Present study |
| Nph. shoemakeri | MFLUCC 17-0780 | MG844346 | – | MG844352a | – | Hyde et al. (2018) |
| Nph. xingrensis | GZCC 18-0110T | MH018135 | – | – | – | Hyde et al. (2018) |
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; GZCC: Guizhou Academy of Agricultural Sciences Culture Collection, Guiyang, China; IBRC: Herbarium of the Plant bank, Iranian Biological Resource Center, Karaj, Iran; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Ria, Thailand. T and ET indicate ex-type and ex-epitype strains, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial β-tubulin gene. a and b in tef1 column indicate the primers used for sequencing: a: EF1-983F / EF1-2218R; b: EF1-728F / EF-2.
Fig. 29.
RAxML phylogram obtained from the combined ITS (585 bp), LSU (848 bp) and rpb2 (744 bp) sequence alignment of all accepted species of Neosetophoma. The tree was rooted to Phaeosphaeriopsis glaucopunctata MFLUCC 13-0265 and Phaeosphaeriopsis agavacearum CPC 29122. The novelties proposed in this study are indicated in bold. RAxML bootstrap support (BS) values above 70 % and Bayesian posterior probability scores above 0.95 are shown at the nodes. GenBank accession numbers of LSU are listed in Fig. 28, and of the other loci in Table 6, Table 10. T and ET indicate ex-type and ex-epitype strains, respectively. TreeBASE: S23834.
Ascomata ostiolate, globose to subglobose, solitary to gregarious, dark brown to black, immersed to slightly erumpent or superficial, smooth; ascomatal wall composed of 2–4 layers of brown to reddish-brown or dark brown to black cells of textura angularis to textura prismatica. Hamathecium comprising numerous, septate, cellular or filamentous pseudoparaphyses, embedded in a hyaline gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, cylindrical to clavate, short pedicellate, with a furcate pedicel, apically rounded, with a minute or indistinct ocular chamber. Ascospores overlapping, 1–3-seriate, hyaline or subhyaline when young, becoming pale yellow, pale brown or yellowish brown to brown at maturity, 1–5-septate, straight to slightly curved, fusoid or narrowly fusoid, with rounded or acute ends, constricted or not at the septum, enlarged at the second cell below apex, guttulate, smooth-walled, without any mucilaginous sheath and appendages. Conidiomata pycnidial, solitary to confluent, immersed or superficial, globose to subglobose or irregular, with mycelial outgrowths, or confluent, unilocular, occasionally multi-locular, with papillate ostioles, sometimes developing long necks, honey, olivaceous, olivaceous black, pale brown, brown, dark brown, or black, with up to 10 layers of pseudoparenchymatal cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, extending percurrently at apex, hyaline, doliiform to ampulliform, determinate, hyaline, smooth-walled. Conidia hyaline, slightly yellowish or pale brown, 0–3(–4)-septate, ellipsoidal, cylindrical, subcylindrical, fusiform, or tear-drop shape, straight to curved, usually attenuate at one end, or apex and base obtuse, or sometimes with bluntly rounded to truncate base, continuous or constricted at the septa, smooth-walled, often guttulate (asexual morph description adapted from Gruyter et al. 2010).
Culture characteristics: Colonies flat, with a moderate amount of aerial mycelium. On PDA surface fluffy, circular or irregular, margins entire or filiform, white, pale grey, grey, greenish grey, or mouse grey; reverse yellowish, yellowish grey, greyish white, grey olivaceous, or dark brown. On MEA pale grey to almost white, buff, or brown to dark brown; reverse dark brown, or buff with patches of grey olivaceous.
Optimal media and cultivation conditions: CMA, PDA, PNA and OA at 25 °C under continuous near-ultraviolet light to promote sporulation.
Distribution: Asia, Europe and North America.
Hosts: Pathogens or saprophytes found on a wide range of host including Cirsium arvense (Asteraceae), Clematis vitalba (Ranunculaceae), Iris germanica (Iridaceae), Lunaria annuna (Brassicaceae), Malva sp. (Malvaceae), Phlox paniculata (Polemoniaceae), Phragmites australis (Poaceae), Rosa canina (Rosaceae), Sambucus spp., Viburnum opulus (Caprifoliaceae), and Urtica dioica (Urticaceae). Also isolated from soil.
Disease symptoms: Leaf spots.
Notes: Neosetophoma was introduced by de Gruyter et al. (2010) to accommodate Phoma samarorum, which is a pathogen causing leaf spots of grasses. Subsequently, 12 new species have been added to this genus, all of which appear to be saprobes, except for Nph. iranianum, which was isolated from soil (Karunarathna et al. 2017), and Nph. lunariae, which is endophytic (Hernández-Restrepo et al. 2016a).
This genus is characterised by globose to irregular conidiomata with papillate ostioles, and yellowish to brownish conidia usually attenuated at one end, less frequent with apex and base obtuse or with a bluntly rounded to truncate base. The sexual morph was observed for the first time by Tibpromma et al. (2017), when Nph. garethjonesii was introduced. Subsequently, four new species producing a sexual morph have been described, of which only one also produces the asexual morph, namely Nph. shoemakeri (Hyde et al. 2018).
References: de Gruyter et al., 2010, Quaedvlieg et al., 2013, Tibpromma et al., 2017, Hyde et al., 2018, Wanasinghe et al., 2018 (morphology and phylogeny).
Neosetophoma aseptata Crous, R.K. Schumach. & Y. Marín, sp. nov. MycoBank MB829639. Fig. 30.
Fig. 30.
Neosetophoma aseptata (ex-type CBS 145363). A. Conidiomata sporulating on OA. B, C. Conidiogenous cells. D. Conidia. Scale bars: A = 300 μm; others = 10 μm.
Etymology: Name refers to its aseptate conidia, which have never been observed in the other species of the genus.
Conidiomata solitary, brown, erumpent, globose, 250–350 μm diam with 1–3 ostioles; conidiomatal wall of 3–4 layers of brown cells of textura angularis. Conidiophores reduced to conidiogenous cells lining inner cavity, hyaline, smooth-walled, subcylindrical to ellipsoid, phialidic with minute collarette, 4–8 × 4–5 μm. Conidia solitary, aseptate, hyaline, smooth-walled, subcylindrical to ellipsoid, apex obtuse, base truncate, (3.5–)4–5 × (1.5–)2 μm.
Culture characteristics: Colonies flat, spreading, surface folded, with moderate aerial mycelium and even, lobate margins, reaching 50 mm diam after 2 wk. On MEA surface pale olivaceous grey, reverse umber with diffuse umber pigment; on PDA surface olivaceous grey, reverse umber with diffuse umber pigment; on OA surface saffron with patches of grey olivaceous.
Typus: Germany, near Berlin, moist meadow, on Viburnum opulus (Caprifoliaceae), 7 Jun. 2017, R.K. Schumacher, HPC 2131, RKS 123 (holotype CBS H-23866, culture ex-type CBS 145363 = CPC 33919).
Notes: Neosetophoma aseptata was located in a large, well-supported clade (96 % BS / 1 PP) comprising Nph. clematidis, Nph. iranianum, Nph. lunariae, Nph. rosae and Nph. shoemakeri. Neosetophoma aseptata can be easily distinguished by its aseptate conidia, being 1-septate in Nph. shoemakeri, 1–3-septate in Nph. iranianum and Nph. rosae, 3-septate in Nph. clematidis, and (1–)3(–4)-septate in Nph. lunariae. Moreover, Nph. aseptata produces the smallest conidia in the complex [(3.5–)4–5 × (1.5–)2 μm in Nph. aseptata vs. 4–6 × 2–4 μm in Nph. iranianum vs. 7.5–10.5 × 2.5–3 μm in Nph. shoemakeri vs. 8–14 × 1.5–3 μm in Nph. rosae vs. 11–15 × 2–4 μm in Nph. clematidis vs. (10–)14–17(–22) × (2.5–)3 μm in Nph. lunariae]. Neosetophoma clematidis produces the largest conidiomata in this complex, being up to 475 μm diam (up to 300 μm in Nph. aseptata and Nph. lunaria, up to 180 μm in Nph. shoemakeri, up to 130 μm in Nph. rosae, up to 120 μm in Nph. iranianum).
Neosetophoma aseptata is the first species isolated from Viburnum. Neosetophoma samarorum and Nph. sambuci are reported in Sambucus spp., which is a member of the same family, Caprifoliaceae.
Neosetophoma phragmitis Crous, R.K. Schumach. & Y. Marín, sp. nov. MycoBank MB829640. Fig. 31.
Fig. 31.
Neosetophoma phragmitis (ex-type CBS 145364). A. Conidiomata sporulating on SNA. B, C. Conidiogenous cells. D. Conidia. Scale bars: A = 200 μm; others = 10 μm.
Etymology: Name refers to Phragmites, the host from which this fungus was collected.
Conidiomata solitary, pycnidial, brown, globose, 180–200 μm diam, neck papillate with central ostiole; conidiomatal wall of 3–4 layers of brown cells of textura angularis. Conidiophores reduced to conidiogenous cells lining inner cavity, hyaline, smooth-walled, ampulliform, 5–8 × 2.5–3 μm, proliferating percurrently at apex. Conidia solitary, pale brown, smooth-walled, aseptate, straight, apex obtuse, base truncate, (3–)4–5(–6) × 2 μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and feathery margins, covering dish in 2 wk. On MEA surface and reverse ochreous; on PDA surface and reverse hazel; on OA surface hazel.
Typus: Germany, near Berlin, on leaf sheath of Phragmites australis (Poaceae), 16 Apr. 2016, R.K. Schumacher, HPC 1178 (holotype CBS H-23867, culture ex-type CBS 145364 = CPC 30680).
Notes: In the phylogenetic analysis based on ITS, LSU and rpb2 sequences, Nph. phragmitis was located in an independent branch removed from the other species of the genus. This is the first species isolated from Phragmitis australis (Poaceae). Neosetophoma poaceicola is the only species that was reported before on a member of the Poaceae, being isolated from a grass host. However, both species are not related, and Nph. phragmitis only produces the asexual morph, while only the sexual morph was observed in Nph. poaceicola.
Neosetophoma sambuci Crous, R.K. Schumach. & Y. Marín, sp. nov. MycoBank MB829641. Fig. 32.
Fig. 32.
Neosetophoma sambuci (ex-type CBS 145365). A. Conidiomata sporulating on PNA. B. Conidioma on SNA showing papillate neck. C. Conidiogenous cells. D. Conidia. Scale bars: A, B = 200 μm; others = 10 μm.
Etymology: Name refers to the genus Sambucus, the host from which this fungus was collected.
Conidiomata solitary, erumpent, brown, pycnidial, globose, 150–200 μm diam, with central ostiole; conidiomatal wall of 3–4 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth-walled, ampulliform, phialidic, 4–6 × 3–4 μm. Conidia solitary, pale brown, smooth-walled, guttulate, subcylindrical, apex obtuse, base truncate, aseptate, becoming 1-septate and swollen (ellipsoid) with age, (5–)7–8(–10) × (2–)2.5(–3) μm.
Culture characteristics: Colonies flat, spreading, covering dish in 2 wk, with sparse to moderate aerial mycelium. On MEA surface ochreous, reverse umber; on PDA surface and reverse olivaceous grey; on OA surface ochreous.
Typus: Germany, near Berlin, on twig of Sambucus nigra (Caprifoliaceae), 11 Mar. 2016, R.K. Schumacher, HPC 1072 (holotype CBS H-23868, culture ex-type CBS 145365 = CPC 30357).
Notes: In the phylogenetic analysis based on ITS, LSU and rpb2 sequences, Nph. sambuci was located in a well-supported clade (97 % BS / 0.98 PP) together with Nph. garethjonesii, Nph. samarorum and Nph. rosigena. Neosetophoma garethjonesii can be easily distinguished from the other species by only producing a sexual morph. The other three species produce an asexual morph, and can be differentiated by the size of their conidia [4–16 × 1.5–3 μm in Nph. samarorum vs. (5–)7–8(–10) × (2–)2.5(–3) μm in Nph. sambuci vs. 4–6 × 1.5–2.5 μm in Nph. rosigena]. Moreover, conidia in Nph. sambuci are pale brown while in Nph. samarorum they are slightly yellowish and in Nph. rosigena they are olivaceous brown. Neosetophoma sambuci was isolated from twigs of Sambucus nigra (Caprifoliaceae). The only species previously reported on this host was Nph. samarorum.
Authors: Y. Marin-Felix & P.W. Crous
Neostagonospora Quaedvl. et al., Stud. Mycol. 75: 364. 2013. Fig. 33.
Fig. 33.
Neostagonospora spp. A, B. Conidioma forming in culture. A.Neostagonospora caricis (ex-type CBS 135092). B.Neostagonospora elegiae (ex-type CBS 135101). C–G. Conidiogenous cells. C, D.Neostagonospora caricis (ex-type CBS 135092). E–G.Neostagonospora elegiae (ex-type CBS 135101). H, I. Conidia. H.Neostagonospora caricis (ex-type CBS 135092). I.Neostagonospora elegiae (ex-type CBS 135101). Scale bars: B = 150 μm; all others = 10 μm; C applies to C and D. Pictures taken from Quaedvlieg et al. (2013).
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Phaeosphaeriaceae.
Type species: Neostagonospora caricis Quaedvlieg et al. Holotype and ex-type strain: CBS H-21306, CBS 135092.
DNA barcode (genus): LSU. Fig. 28.
DNA barcodes (species): ITS, rpb2, tef1, tub2. Table 7. Fig. 34.
Table 7.
DNA barcodes of accepted Neostagonospora spp.
| Species | Isolates1 | GenBank accession numbers2 |
References | |||
|---|---|---|---|---|---|---|
| ITS | rpb2 | tef1 | tub2 | |||
| Neostagonospora arrhenatheri | MFLUCC 15-0464T | KX926417 | – | MG520901a | – | Phookamsak et al., 2017, Thambugala et al., 2017 |
| Nst. caricis | CBS 135092T | KF251163 | KF252171 | – | KF252658 | Quaedvlieg et al. (2013) |
| Nst. elegiae | CBS 135101T | KF251164 | KF252172 | KF253122b | KF252659 | Quaedvlieg et al. (2013) |
| Nst. phragmitis | MFLUCC 16-0493T | KX926416 | – | MG520902a | – | Phookamsak et al. (2017), Thambugala et al. (2017) |
| Nst. sorghi | CBS 145366T | MK539956 | MK540087 | MK540150b | MK540168 | Present study |
| Nst. spinificis | BCRC FU30120 | KP676045 | LC055104 | – | – | Yang et al. (2016) |
BCRC: Bioresource Collection and Research Centre, Taiwan; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Ria, Thailand. T indicates ex-type strains.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial β-tubulin gene. a and b in tef1 column indicate the primers used for sequencing: a: EF1-983F / EF1-2218R, b: EF1-728F / EF-2.
Fig. 34.
RAxML phylogram obtained from the combined ITS (571 bp), LSU (847 bp), rpb2 (337 bp) and tub2 (304 bp) sequence alignment of all accepted species of Neostagonospora. The tree was rooted to Parastagonospora avenae CBS 289.69 and Parastagonospora nodorum CBS 110109. The novelty proposed in this study is indicated in bold. RAxML bootstrap support (BS) values above 70 % and Bayesian posterior probability scores above 0.95 are shown at the nodes. GenBank accession numbers of LSU are listed in Fig. 28, and of the other loci in Tables 7 and 9. T indicates ex-type strains. TreeBASE: S23834.
Conidiomata immersed, pycnidial, globose, exuding a pale luteous to creamy conidial mass; conidiomatal wall composed of 2–3 layers of pale brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth-walled, aggregated, lining the inner cavity, ampulliform to doliiform, tapering at apex with prominent periclinal thickening. Conidia hyaline, smooth-walled, granular, thin-walled, narrowly fusoid-ellipsoidal to subcylindrical, apex subobtusely rounded, base truncate, widest in middle, aseptate or transversely euseptate, becoming constricted with age (adapted from Quaedvlieg et al. 2013).
Culture characteristics: Colonies flat, spreading, erumpent, circular or undulate, smooth to velvety, even margins, with sparse to moderate aerial mycelium. On PDA, surface dirty white, greyish sepia to isabelline; reverse pale white to pale pink, pale pink with white edge, luteous or olivaceous grey to pale olivaceous grey.
Optimal media and cultivation conditions: PDA and sterilised Carex leaves or Anthriscus stem placed on 1.5 % water agar at 25 °C under continuous near-ultraviolet light to promote sporulation.
Distribution: Africa (South Africa), Asia (Russia and Taiwan), Australia and Europe (Italy and the Netherlands).
Hosts: Arrhenatherum elatius, Phragmites australis, Sorghum halepense and Spinifex littoreus (Poaceae), Carex acutiformis (Cyperaceae) and Elegia cuspidata (Restionaceae).
Disease symptoms: Leaf spots.
Notes: Neostagonospora was introduced by Quaedvlieg et al. (2013) to accommodate two taxa associated with leaf spots on Carex acutiformis (Nst. caricis) and Elegia cuspidata (Nst. elegiae). However, its pathogenicity remains unclear since Koch’s postulates have not been completed. Subsequently, another foliicolous fungus was included in this genus, Nst. spinificis (Yang et al. 2016), associated with green tissues and leaf spots of Spinifex littoreus. The most recently introduced species are both saprobes on members of Poaceae: Nst. arrhenatheri and Nst. phragmitis (Thambugala et al. 2017). Further studies are needed to prove the pathogenicity of members included in this genus.
Neostagonospora is similar to Stagonospora since both produce pycnidial conidiomata with euseptate, hyaline, fusoid-ellipsoidal to subcylindrical conidia, but Neostagonospora is distinguished by having conidiogenous cells that are phialidic, with prominent periclinal thickening (Quaedvlieg et al. 2013).
In our phylogenetic analysis based on ITS and LSU sequences of members of the family Phaeosphaeriaceae (Fig. 28), Nst. artemisiae, which was the most recently described species (Wanasinghe et al. 2018), is not included in the clade that represents the genus Neostagonospora, being located in the Septoriella clade. Therefore, this species is excluded from Neostagonospora and transferred to Septoriella (see Septoriella below).
References: Quaedvlieg et al., 2013, Yang et al., 2016, Thambugala et al., 2017 (morphology and phylogeny).
Neostagonospora sorghi Crous & Y. Marín, sp. nov. MycoBank MB829612. Fig. 35.
Fig. 35.
Neostagonospora sorghi (ex-type CBS 145366). A. Conidiomata sporulating on MEA. B, C. Conidiogenous cells. D. Conidia. Scale bars: A = 200 μm; others = 10 μm.
Etymology: Name refers to the genus Sorghum, the host from which this fungus was collected.
Conidiomata solitary, erumpent, pycnidial, brown, globose, 180–200 μm diam, with central ostiole; conidiomatal wall of 2–3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity. Conidiogenous cells hyaline to pale brown, smooth-walled, ampulliform, phialidic with visible periclinal thickening and collarette, 5–8 × 4–5 μm. Conidia solitary, aseptate, ellipsoid with obtuse ends, straight, guttulate, (4–)5–6 × (2–)2.5(–3) μm.
Culture characteristics: Colonies flat, spreading with moderate aerial mycelium and even, lobate margins, covering dish in 2 wk. On MEA surface hazel to isabelline, reverse isabelline; on PDA surface isabelline with scarlet outer margins, reverse brown vinaceous; on OA surface isabelline with patches of scarlet.
Typus: Australia, Western Australia, Denmark, Mount Lindesay Walk trail, on Sorghum halepense (Poaceae), 19 Sep. 2015, P.W. Crous, HPC 697A (holotype CBS H-23869, culture ex-type CBS 145366 = CPC 29239).
Notes: Neostagonospora sorghi is the first species of the genus reported from Sorghum, and occurring in Australia. It is closely related to Nst. caricis, which is associated with leaf spots on Carex acutiformis. However, Nst. sorghi can be distinguished from this species and from other species of the genus by its aseptate conidia. Moreover, Nst. sorghi produces smaller conidia than Nst. caricis [(4–)5–6 × (2–)2.5(–3) μm in Nst. sorghi vs. (10–)13–16(–19) × (3–)3.5(–4) μm in Nst. caricis].
Authors: Y. Marin-Felix & P.W. Crous
Nothophoma Q. Chen & L. Cai, Stud. Mycol. 82: 212. 2015. Fig. 36.
Fig. 36.
Nothophoma spp. A, B. Disease symptoms. A.Nothophoma quercina (CGMCC 3.19246) on Osmanthus fragrans. B.Nothophoma quercina (LC12187) on Jasminum mesnyi. C–I. Asexual morph. C, D. Conidiomata of Nothophoma anigozanthi (ex-epitype CBS 381.91) sporulating on OA. E–H. Conidia. E.Nothophoma infossa (ex-neotype CBS 123395). F.Nothophoma macrospora (ex-type CBS 140674). G.Nothophoma quercina (CGMCC 3.19246). H.Nothophoma variabilis (ex-type CBS 142457). I. Conidiogenous cells of Nothophoma macrospora (ex-type CBS 140674). Scale bars: C = 200 μm; D = 20 μm; E–I = 10 μm. Pictures C, D taken from Chen et al. (2015); E from Aveskamp et al. (2009); F, I from Crous et al. (2016b); H from Valenzuela-Lopez et al. (2018).
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Didymellaceae.
Type species: Nothophoma infossa (Ellis & Everh.) Q. Chen & L. Cai, basionym: Phoma infossa Ellis & Everh., J. Mycol. 4: 102. 1888. Neotype and ex-neotype strain designated by Aveskamp et al. (2009): CBS H-20145, CBS 123395.
DNA barcodes (genus): LSU, ITS.
DNA barcodes (species): rpb2, tub2. Table 8. Fig. 37.
Table 8.
DNA barcodes of accepted Nothophoma spp.
| Species | Isolates1 | GenBank accession numbers2 |
References | |||
|---|---|---|---|---|---|---|
| LSU | ITS | rpb2 | tub2 | |||
| Nothophoma anigozanthi | CBS 381.91ET | GU238039 | GU237852 | KT389655 | GU237580 | Aveskamp et al., 2010, Chen et al., 2015 |
| Not. arachidis-hypogaeae | CBS 125.93 | GU238043 | GU237771 | KT389656 | GU237583 | Aveskamp et al., 2010, Chen et al., 2015 |
| Not. gossypiicola | CBS 377.67 | GU238079 | GU237845 | KT389658 | GU237611 | Aveskamp et al., 2010, Chen et al., 2015 |
| Not. infossa | CBS 123395NT | GU238089 | FJ427025 | KT389659 | FJ427135 | Aveskamp et al., 2009, Aveskamp et al., 2010, Chen et al., 2015 |
| Not. macrospora | CBS 140674T | LN880537 | LN880536 | LT593073 | LN880539 | Crous et al. (2016b) |
| Not. quercina | CBS 633.92 | EU754127 | GU237900 | KT389657 | GU237609 | Aveskamp et al., 2010, Chen et al., 2015 |
| CGMCC 3.19246 | MK088581 | MK088574 | MK088588 | MK088595 | Present study | |
| LC12187 | MK088582 | MK088575 | MK088589 | MK088596 | Present study | |
| Not. raii | MCC 1082T | – | MF664467 | – | MF664468 | Crous et al. (2017b) |
| Not. variabilis | CBS 142457T | LN907428 | LT592939 | LT593078 | LT593008 | Valenzuela-Lopez et al. (2018) |
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CGMCC: Chinese General Microbiological Culture Collection Center, Beijing, China; LC: Dr Lei Cai's personal culture collection, housed at CAS, China; MCC: National Centre for Microbial Resources (formerly Microbial Culture Collection), Pune, India. T,ET and NT indicate ex-type, ex-epitype and ex-neotype strains, respectively.
LSU: partial large subunit (28S) nrRNA gene; ITS: internal transcribed spacers and intervening 5.8S nrDNA; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tub2: partial β-tubulin gene.
Fig. 37.
Phylogenetic tree generated from a maximum parsimony analysis based on the combined LSU (868 bp), ITS (490 bp), tub2 (336 bp) and rpb2 (845 bp) sequences of all accepted species of Nothophoma. The tree was rooted to Phoma herbarum CBS 615.75. Values above the branches represent parsimony bootstrap support values (> 50 %). GenBank accession numbers are indicated in Table 8. T, ET and NT indicate ex-type, ex-epitype and ex-neotype strains, respectively. TreeBASE: S23494.
Conidiomata pycnidial, globose to elongated, or irregular, superficial or immersed into the agar, solitary or confluent, ostiolate, sometimes with an elongated neck; conidiomatal wall pseudoparenchymatous, multi-layered, outer wall pigmented. Conidiogenous cells phialidic, hyaline, smooth-walled, ampulliform to doliiform, sometimes flask-shaped. Conidia hyaline but incidentally brown, smooth- and thin-walled, aseptate, ovoid or ellipsoidal, eguttulate or guttulate. Chlamydospores elongated barrel-shaped, olivaceous brown, in chains. Sexual morph unknown (adapted from Chen et al., 2015, Crous et al., 2017b).
Culture characteristics: Colonies on OA yellow/green to olivaceous grey/brown, dull green, or translucent, aerial mycelium tenuous, sometimes margins irregular and whitish, flattened or effused, compact, floccose.
Optimal media and cultivation conditions: OA or sterile pine needles placed on OA under near-ultraviolet light (12 h light, 12 h dark) to promote sporulation at 25 °C.
Distribution: Worldwide.
Hosts: Wide host range, mainly occurring as pathogens, and also endophytes or saprobes, on Amaryllidaceae, Anacardiaceae, Fabeceae, Fagaceae, Haemodoraceae, Malvaceae, Oleaceae, Rosaceae, Rhamnaceae and Rutaceae. Also isolated from other substrates and environments, such as soil, fungi and human infections.
Disease symptoms: Leaf spots, stem cankers, brown spot of fruits, shoot canker.
Notes: Nothophoma was one of the genera established recently in order to delineate a more natural classification for the Ascochyta-Didymella-Phoma complex (Chen et al. 2015). Currently this genus comprises nine species, including five Phoma species previously classified in Phoma, and four species that were recently proposed (Crous et al., 2016b, Crous et al., 2017b, Valenzuela-Lopez et al., 2018). Within Nothophoma morphological differences between species are insignificant, and phylogenies based on multi-locus sequence data are primarily used to distinguish species.
Species in this genus are seed- and soil-borne endophytes or pathogens mainly causing leaf spots and stem canker of cultivated crops and plants, such as groundnut and cotton. Some species are mycophylic on other fungi or occur in soil, as well as in the respiratory secretion of a patient with pneumonia or in a human bronchial wash sample (Boerema et al., 2004, Aveskamp et al., 2009, Aveskamp et al., 2010, Chen et al., 2015, Crous et al., 2016b, Crous et al., 2017b, Valenzuela-Lopez et al., 2018).
References: Boerema et al. 2004 (morphology and pathogenicity), Aveskamp et al., 2010, Chen et al., 2015, Crous et al., 2016b, Crous et al., 2017b, Valenzuela-Lopez et al., 2018 (morphology and phylogeny).
Authors: Q. Chen & L. Cai
Parastagonospora Quaedvl. et al., Stud. Mycol. 75: 362. 2013. Fig. 38.
Fig. 38.
Parastagonospora spp. A–D. Sexual morph of Parastagonospora nodorum (CBS H-13909). A, B. Ascomata. C, D. Asci and ascospores. E–M. Asexual morph. E, F. Conidiomata. E.Parastagonospora poagena (ex-type CBS 136776). F.Parastagonospora poae (CBS 135091). G–I. Conidiogenous cells. G.Parastagonospora caricis (ex-type CBS H-21304). H.Parastagonospora poae (CBS 135091). I.Parastagonospora poagena (ex-type CBS 136776). J–M. Conidia. J.Parastagonospora caricis (ex-type CBS H-21304). K.Parastagonospora nodorum (CBS H-13909). L.Parastagonospora poae (CBS 135091). M.Parastagonospora poagena (ex-type CBS 136776). Scale bars: C, D, J–M = 10 μm; G–I = 5 μm. Pictures A–D, F–H, J–L taken from Quaedvlieg et al. (2013); E, I, M from Crous et al. (2014b).
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Phaeosphaeriaceae.
Type species: Parastagonospora nodorum (Berk.) Quaedvlieg et al., basionym: Depazea nodorum Berk. Reference strain: CBS 110109.
DNA barcode (genus): LSU. Fig. 28.
DNA barcodes (species): ITS, rpb2, tef1, tub2. Table 9. Fig. 39.
Table 9.
DNA barcodes of accepted Parastagonospora spp.
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Ria, Thailand. T indicates ex-type strains.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial β-tubulin gene. a and b in tef1 column indicate the primers used in sequencing: a: EF1-983F / EF1-2218R, b: EF1-728F / EF-2.
Fig. 39.
RAxML phylogram obtained from the combined ITS (575 bp), LSU (848 bp), rpb2 (337 bp) and tef1 (866 bp) sequence alignment of all accepted species of Parastagonospora. The tree was rooted to Neostagonospora carici CBS 135092 and Neostagonospora elegiae CBS 135101. The novelties proposed in this study are indicated in bold. RAxML bootstrap support (BS) values above 70 % and Bayesian posterior probability scores above 0.95 are shown at the nodes. GenBank accession numbers of LSU are listed in Fig. 28, and of the others loci are indicated in Table 7, Table 9. T indicates ex-type strains. TreeBASE: S23834.
Ascomata perithecial, immersed, globose, becoming depressed, dark brown to black, with central ostiole with upper region slightly papillate; ascomatal wall thin- or thick-walled, composed of 2–6 layers of brown cells of textura angularis. Pseudoparaphyses filiform, hyaline, septate. Asci bitunicate, clavate, cylindrical, narrowly fusoid or curved, shortly stipitate, thick-walled, 8-spored. Ascospores fusoid or ellipsoidal, hyaline or subhyaline to pale brown, smooth-walled, transversely 3-euseptate, cells above central septum often broader than the lower ones, with acute rounded ends, constricted or not at each septum, sometimes with distinct oil droplets in each cell. Conidiomata pycnidial, brown to black, erumpent or immersed to semi-immersed, subepidermal, globose to subglobose, ampulliform, or obpyriform, with central papillate ostiole, exuding creamy or pinkish conidial mass; conidiomatal wall composed of 2–4 layers of brown cells of textura angularis, or composed of 1–5 outer layers of dark brown cells and 1–3 inner layers of hyaline cells of textura angularis, or composed of an outer layer of brown to dark brown cells of textura globosa and an inner layer of pale brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth-walled, aggregated, lining the inner cavity, ampulliform to subcylindrical, broadly cylindrical or broadly conical, with percurrent proliferation near apex. Conidia hyaline or subhyaline, smooth-walled, thin- or thick-walled, cylindrical, subcylindrical or fusiform, granular to multi-guttulate, with obtuse or subobtuse apex and truncate base, rarely rounded at both ends, straight to gently curved, sigmoid, transversely 1–9-euseptate, sometimes constricted at the septa (adapted from Quaedvlieg et al. 2013).
Culture characteristics: Colonies flat, with aerial mycelium, white to pink, olivaceous, grey or vinaceous buff.
Optimal media and cultivation conditions: Sterilised Carex leaves placed on 1.5 % WA at 25 °C under continuous near-ultraviolet light to promote sporulation.
Distribution: Worldwide.
Hosts: Pathogens or saprophytes of grass (Poaceae). Species of Parastagonospora are directly or indirectly responsible for significant annual crop losses worldwide on wheat, barley and rye.
Disease symptoms: Leaf, glume and node spots.
Notes: Parastagonospora was recently introduced in order to accommodate a clade of several common and serious cereal pathogens that had been previously been placed in the genera Septoria/Stagonospora or Leptosphaeria/Phaeosphaeria (Quaedvlieg et al. 2013). This genus differs from Stagonospora mainly in the sexual morph, being phaeosphaeria-like in Parastagonospora and didymella-like in Stagonospora.
In the phylogenetic analysis based only on the ITS and LSU sequences of representative members of the family Phaeosphaeriaceae (Fig. 28), all the species of Parastagonospora were located in a well-supported clade (0.98 PP), except for P. phoenicicola. The ex-type strain of this latter species clustered in well-supported clade (84 % BS) representing the genus Phaeosphaeria. Therefore, a new combination is proposed for this taxon.
In our phylogenetic analysis (Fig. 39), P. cumpignensis, P. dactylidis and P. minima grouped in the same well-supported clade (79 % BS / 0.96 PP) without significant phylogenetic distance. The three species all have been isolated from Thailand on dead stems of Dactylis (Li et al., 2015, Li et al., 2016a). Only ITS sequences are available for P. cumpignensis and P. dactylidis, and nucleotide similarity for this locus for all three species is 100 %. Therefore, these three species are reduced to synonymy. The same problem is found in P. forlicesenica, which is one of the most recently described species in the genus (Thambugala et al. 2017). Based on ITS, P. forlicesenica shares a nucleotide similarity of 99.8 % with P. avenae. Therefore, further studies should be done to confirm if P. forlicesenica represents a separate species or should be synonymised with P. avenae.
Species of Parastagonospora are pathogens or saprophytes of grasses, being directly or indirectly responsible for significant annual crop losses worldwide. Parastagonospora avenae causes minor leaf blotch of barley and rye, while it is considered an important pathogen of oats (Cunfer 2000). Parastagonospora nodorum is known primarily as a major necrotrophic pathogen of wheat that causes leaf and glume blotch, but also infects barley, on which it is considered as not economically important (Cunfer, 2000, Oliver et al., 2012).
References: Cunfer 2000 (pathology and morphology), Oliver et al. 2012 (pathology, genomics and host resistence), Quaedvlieg et al., 2013, Li et al., 2015, Thambugala et al., 2017 (morphology and phylogeny).
Parastagonospora dactylidis W.J. Li et al., Mycosphere 6: 691. 2015.
Synonyms: Parastagonospora minima W.J. Li, et al., Mycosphere 6: 691. 2015.
Parastagonospora cumpignensis Tibpromma et al., Fungal Diversity 78: 48. 2016.
Typus: Italy, Province of Arezzo, Passo della Consuma, on dead stem of Dactylis sp. (Poaceae), 19 Jun. 2012, Erio Camporesi (holotype MFLU 15-0693, culture ex-type MFLUCC 13-0375 = ICMP 20774 = KUMCC15-0131).
Additional materials: Italy, Province of Arezzo, Passo della Consuma, on dead stem of Dactylis sp. (Poaceae), 19 Jun. 2012, Erio Camporesi, MFLUCC 13-0376 = ICMP 20776 = KUMCC15-0132; ibid., Campigna, Santa Sofia, ForlìCesena Province, on dead stem of Dactylis glomerata (Poaceae), 23 Jun. 2012, Erio Camporesi, MFLUCC 13-0573.
Notes: In our phylogenetic analysis (Fig. 39), the ex-type strains of P. cumpignensis (MFLUCC 13- 0573), P. dactylidis (MFLUCC 13-0375) and P. minima (MFLUCC 13-0376) grouped in the same well-supported clade (79 % BS / 0.96 PP) without significant phylogenetic distance. The ITS sequences of the three species showed a nucleotide similarity of 100 %. Moreover, all of them were isolated from from Thailand on dead stems of Dactylis (Li et al., 2015, Li et al., 2016a). Therefore, the three species are herewith reduced to synonymy.
Parastagonospora novozelandica Crous, Thangavel & Y. Marín, sp. nov. MycoBank MB829668. Fig. 40.
Fig. 40.
Parastagonospora novozelandica (ex-type CPC 29613). A–C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Name refers to New Zealand, the country where this fungus was isolated.
Culture nearly sterile, with only a few conidiomata observed. Conidiomata solitary, pycnidial, dark brown, globose, 180–200 μm diam, with central ostiole; conidiomatal wall with 3–6 layers of pale brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity. Conidiogenous cells hyaline, smooth, ampulliform to subcylindrical, 6–8 × 2.5–5 μm, proliferating percurrently at apex. Conidia solitary, hyaline to pale olivaceous, smooth, guttulate, subcylindrical, straight, apex subobtuse, base truncate, 1-septate, (9–)11–13(–16) × (2–)2.5(–3) μm.
Culture characteristics: Colonies flat, spreading, reaching 60 mm diam after 2 wk, with moderate aerial mycelium, and even, smooth margins. On MEA surface greenish olivaceous to umber, reverse olivaceous to umber; on OA surface brown vinaceous.
Typus: New Zealand, Browns Bay, on unidentified grass (Poaceae), Nov. 2015, R. Thangavel (holotype CBS H-23903, culture ex-type T15–06960B = CPC 29613 = CBS 145416).
Notes: Parastagonospora novozelandica is related to P. allouniseptata. Both species produce 1-septate conidia, but these can be easily distinguished based on their conidial dimensions [(9–)11–13(–16) × (2–)2.5(–3) μm in P. novozelandica vs. 16–22 × 2.5–3.5 μm in P. allouniseptata].
Parastagonospora phragmitis Crous & Y. Marín, sp. nov. MycoBank MB829667. Fig. 41.
Fig. 41.
Parastagonospora phragmitis (ex-type CPC 32075). A, B. Conidiogenous cells. C, D. Conidia. Scale bars = 10 μm.
Etymology: Name reflects the genus Phragmites from which this fungus was isolated.
Conidiomata solitary, pycnidial, brown, globose, 250–300 μm diam, with central ostiole; conidiomatal wall with 3–6 layers of pale brown cells of textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity. Conidiogenous cells hyaline, smooth, ampulliform to doliiform, 7–10 × 8–9 μm, proliferating percurrently at apex. Conidia solitary, hyaline to pale olivaceous, smooth, guttulate, subcylindrical-fusoid, straight to slightly curved, with prominent taper in upper third to subobtuse apex, widest in middle to lower third, base truncate, 3-septate, (18–)23–25(–27) × (3–)4 μm.
Culture characteristics: Colonies flat, spreading, covering dish after 2 wk, with moderate aerial mycelium, and smooth, lobate margins. On MEA surface saffron, reverse sienna; on PDA surface saffron to sienna, reverse sienna; on OA surface pale luteous to saffron.
Typus: Australia, New South Wales, Sussex Inlet, on Phragmites sp. (Poaceae), 27 Nov. 2015, P.W. Crous, HPC 1785 (holotype CBS H-23902, culture ex-type CPC 32075 = CBS 143446).
Notes: Parastagonospora phragmitis is related to P. fusiformis. However, P. phragmitis produces an asexual morph, while in P. fusiformis only the sexual morph has been observed. Moreover, P. phragmitis is the first species of the genus reported on Phragmites.
Phaeosphaeria phoenicicola (Crous & Thangavel) Y. Marín & Crous, comb. nov. MycoBank MB829700.
Basionym: Parastagonospora phoenicicola Crous & Thangavel, Persoonia 37: 349. 2016.
Description and illustration: Crous et al. (2016a).
Typus: New Zealand, Auckland, Botany road, on leaves of Phoenix canariensis (Arecaceae), 2015, R. Thangavel (holotype CBS H-22892, culture ex-type CPC 28711 = CBS 142107).
Notes: In the phylogenetic analysis based on ITS and LSU sequences (Fig. 28), the ex-type strain of this species was located in the well-supported clade (84 % BS) representing the genus Phaeosphaeria. Morphologically, this species produces conidia more similar to Phaeosphaeria than to Parastagonospora, since these are subcylindrical, mostly straight, while in Parastagonospora the conidia tend to be sigmoid and longer than in P. phoenicicola. Based on morphology and molecular data, the new combination, Phaeosphaeria phoenicicola, is herewith proposed.
Authors: Y. Marin-Felix, R. Thangavel & P.W. Crous
Phaeosphaeriopsis M.P.S. Câmara et al., Mycol. Res. 107: 519. 2003. Fig. 42.
Fig. 42.
Phaeosphaeriopsis spp. A. Symptomatic leaves of Agapanthus precox caused by Phaeosphaeriopsis agapanthi. B. Asci and ascospores of Phaeosphaeriopsis agavacearum (ex-type CBS 142110). C–P. Asexual morph. C, D. Conidiomata sporulating on PNA and OA, respectively, of Phaeosphaeriopsis agapanthi (ex-type CBS 141287). E. Conidiomata sporulating on OA of Phaeosphaeriopsis agavacearum (ex-type CBS 142110). F. Conidioma of Phaeosphaeriopsis agavacearum (ex-type CBS 142110). G–L. Conidiogenous cells giving rise to conidia. G, H.Phaeosphaeriopsis agapanthi (ex-type CBS 141287). I–L.Phaeosphaeriopsis glaucopunctata (CBS 653.86). M–O. Conidia. M.Phaeosphaeriopsis agapanthi (ex-type CBS 141287). N.Phaeosphaeriopsis agavacearum (ex-type CBS 142110). O.Phaeosphaeriopsis glaucopunctata (CBS 653.86). P. Chlamydospores of Phaeosphaeriopsis agavacearum (ex-type CBS 142110). Scale bars: F = 100 μm; others = 10 μm; I applies to I–L. Pictures A, C, D, G, H, M taken from Crous et al. (2016b); B, E, F, N, P from Crous et al. (2016a); I–L, O from Quaedvlieg et al. (2013).
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Phaeosphaeriaceae.
Type species: Phaeosphaeriopsis glaucopunctata (Grev.) M.P.S. Câmara et al., basionym: Cryptosphaeria glaucopunctata Grev. [as “glauco-punctata”]. Epitype and ex-epitype strain designated by Thambugala et al. (2014): MFLU 14-0029, MFLUCC 13-0265 = ICMP 20199.
DNA barcode (genus): LSU. Fig. 28.
DNA barcodes (species): ITS, rpb2, tef1, tub2. Table 10. Fig. 43.
Table 10.
DNA barcodes of accepted Phaeosphaeriopsis spp.
| Species | Isolates1 | GenBank accession numbers2 |
References | |||
|---|---|---|---|---|---|---|
| ITS | rpb2 | tef1 | tub2 | |||
| Phaeosphaeriopsis agapanthi | CBS 141287T | KX228260 | MK540094 | MK540157a | MK540173 | Crous et al. (2016b), present study |
| Phs. agavacearum | CBS 142110T | KY173430 | KY173591 | MK540158a | KY173610 | Crous et al. (2016a), present study |
| Phs. agavensis | CBS 102206 | KY090635 | KY090685 | – | – | Ahmed et al. (2017) |
| Phs. aloes | CBS 145367T | MK539959 | MK540090 | MK540153a | – | Present study |
| Phs. aloicola | CBS 145368T | MK539960 | MK540091 | MK540154a | MK540170 | Present study |
| Phs. amblyospora | CBS 110131T | AY188993 | – | – | – | Câmara et al. (2003) |
| Phs. dracaenicola | MFLUCC 11-0157T | KM434273 | KM434309 | KM434301b | – | Phookamsak et al. (2014b) |
| Phs. glaucopunctata | MFLUCC 13-0265ET | KJ522473 | – | MG520918b | – | Thambugala et al., 2014, Phookamsak et al., 2017 |
| Phs. grevilleae | CBS 145369T | MK539961 | MK540092 | MK540155a | MK540171 | Present study |
| Phs. nolinae | CBS 102205 | KY090637 | KY090686 | – | – | Ahmed et al. (2017) |
| Phs. obtusispora | CBS 102204 | KY090636 | KY090687 | – | – | Ahmed et al. (2017) |
| Phs. phacidiomorpha | T111 | FJ462742 | – | – | – | Zhang et al. (unpubl. data) |
| Phs. pseudoagavacearum | CBS 145370T | MK539962 | MK540093 | MK540156a | MK540172 | Present study |
| Phs. triseptata | MFLUCC 13-0271T | KJ522475 | KJ522485 | MG520919b | – | Thambugala et al., 2014, Phookamsak et al., 2017 |
| Phs. yuccae | MFLUCC 16-0558T | KY554482 | – | MG520920b | – | Phookamsak et al. (2017) |
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Ria, Thailand; T: isolate housed in China. T and ET indicate ex-type and ex-epitype strains.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial β-tubulin gene. a and b in tef1 column indicate the primers used in sequencing: a: EF1-728F / EF-2, b: EF1-983F / EF1-2218R.
Fig. 43.
RAxML phylogram obtained from the combined ITS (587 bp), LSU (849 bp), rpb2 (838 bp), tef1 (601 bp) and tub2 (519 bp) sequence alignment of all accepted species of Phaeosphaeriopsis. The tree was rooted to Neostagonospora caricis CBS 135092 and Neostagonospora elegiae CBS 135101. The novelties proposed in this study are indicated in bold. RAxML bootstrap support (BS) values above 70 % and Bayesian posterior probability scores above 0.95 are shown at the nodes. GenBank accession numbers of LSU are listed in Fig. 28, and of the other loci in Tables 7 and 9. T and ET indicate ex-type and ex-epitype strains, respectively. TreeBASE: S23834.
Ascomata solitary or aggregated, immersed, subepidermal to erumpent, pushing up flaps of the epidermis, globose to pyriform, often papillate, solitary or gregarious in a stroma of scleroplectenchyma or dark brown cells of textura angularis, often surrounded by septate, brown hyphae extending into the host tissues. Asci 8-spored, bitunicate, cylindrical to broadly fusoid, short stipitate, with visible apical chamber. Ascospores uni- to triseriate, cylindrical, broadly rounded at apex, tapering to narrowly rounded base, 4–5-septate, first septum submedian, often constricted, medium brown, echinulate, punctate or verrucose. Asexual morph coniothyrium-like or phaeostagonospora-like. Conidiomata pseudoparenchymatous, sometimes of scleroplectenchyma. Conidiogenous cells lining locule, ampulliform, hyaline, proliferating percurrently, resulting in inconspicuous annellations. Conidia cylindrical, with bluntly rounded ends, 0–3-septate, yellowish brown, punctate (Quaedvlieg et al. 2013).
Culture characteristics: Colonies flat or rarely slightly raised, spreading, feathery, velvety or floccose, with sparse to moderate aerial mycelium, circular or lobate, margins smooth or rarely slightly radiating. On PDA, surface white, dirty white, pinkish white, primrose, pale grey or pale luteous; reverse dirty white, light to dark grey, luteous or olivaceous buff. On MEA, surface dirty white, pale luteous, or white to cream at the margins, pale yellowish to yellowish brown in the middle and pale brown to brown or orange-brown at the centre, with small white to grey droplets; reverse luteous, umber with patches of dirty white, isabelline in the middle and cinnamon in outer region, or white to cream at the margins, brown to orange-brown in the middle and pale yellowish at the centre.
Optimal media and cultivation conditions: On MEA, PDA or OA at 25 °C.
Distribution: Worldwide.
Hosts: Pathogens or saprophytes on Agapanthus praecox (Alliaceae), Agave spp. and Yucca spp. (Agavaceae), Aloe sp. (Aloaceae), Dracaena lourieri, Dracaena sp. and Nolina erumpens (Dracaenaceae), Grevillea sp. (Proteaceae), Phormium spp. (Phormiaceae) and Ruscus spp. (Ruscaceae).
Disease symptoms: Leaf spots and leaf blight.
Notes: The genus Phaeosphaeriopsis was introduced by Câmara et al. (2003) to accommodate some species of Paraphaeosphaeria that were not congeneric based on phylogenetic data. Phaeosphaeriopsis is characterised by having uni- or multi-loculate stromata and 4–5-septate ascospores, and coniothyrium-like and phaeostagonospora-like asexual morphs, while Paraphaeosphaeria produces 2-septate ascospores and has a microsphaeropsis-like asexual morph (Câmara et al., 2003, Quaedvlieg et al., 2013). Phaeosphaeriopsis is related to Acericola, which is a genus recently introduced to accommodate a saprobic fungus found on dead twigs of Acer campestre (Hyde et al. 2017). Unfortunately, it appears that the LSU sequence of Acericola is incorrect (Crous et al. 2019a).
In our phylogenetic analysis based on ITS and LSU sequences, 11 species are accepted in the genus Phaeosphaeriopsis, and four strains located in independent branches are introduced as new species.
Species included in Phaeosphaeriopsis are saprobes or presumed pathogens. The type species, Phaeosphaeriopsis glaucopunctata, is associated with leaf spot and necrosis of Ruscus aculeatus (Câmara et al., 2003, Golzar and Wang, 2012). Phaeosphaeriopsis agapanthi and Phs. dracaenicola are also associated with necrotic leaf spots of Agapanthus precox and Dracaena lourieri, respectively (Phookamsak et al., 2014b, Crous et al., 2016b).
References: Câmara et al., 2003, Quaedvlieg et al., 2013, Thambugala et al., 2014 (morphology and phylogeny).
Phaeosphaeriopsis aloes Crous & Y. Marín, sp. nov. MycoBank MB829642. Fig. 44.
Fig. 44.
Phaeosphaeriopsis aloes (ex-type CBS 145367). A. Conidiomata sporulating on MEA. B, C. Conidiogenous cells. D. Conidia. Scale bars: A = 180 μm; all others = 10 μm.
Etymology: Name refers to Aloe, the host from which this fungus was collected.
Conidiomata solitary, brown, pycnidial, globose, 150–180 μm diam, with central ostiole, 30–40 μm diam; conidiomatal wall of 3–4 layers of brown cells of textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth-walled, ellipsoid, phialidic, 4–6 × 3–4 μm. Conidia solitary, aseptate, straight, verruculose, golden-brown, subcylindrical, apex obtuse, base bluntly rounded, (4–)5(–6) × 3(–3.5) μm.
Culture characteristics: Colonies flat, spreading, with sparse to moderate aerial mycelium, covering dish in 2 wk. On MEA surface honey, reverse cinnamon; on PDA surface and reverse sepia; on OA surface saffron.
Typus: USA, California, on leaves of Aloe sp. (Aloaceae), 6 Aug. 2016, P.W. Crous, HPC 1326 (holotype CBS H-23870, culture ex-type CBS 145367 = CPC 31480).
Notes: Phaeosphaeriopsis aloes is related to Phs. obtusispora. This latter species only produces the sexual morph, while only the asexual morph has been observed in our new species.
Phaesphaeriopsis aloes and Phs. aloicola, both described here, are the first species of the genus found on a member of the family Aloaceae, an Aloe sp. However, the two species are not related. Phaeosphaeriopsis aloes produces an asexual morph, while in Phs. aloicola only the sexual morph has been observed.
Phaeosphaeriopsis aloicola Crous & Y. Marín, sp. nov. MycoBank MB829643. Fig. 45.
Fig. 45.
Phaeosphaeriopsis aloicola (ex-type CBS 145368). A. Ascomata sporulating on SNA. B. Asci. C. Pseudoparaphyses. D. Ascospores. Scale bars: A = 200 μm; all others = 10 μm.
Etymology: Name refers to the host genus Aloe from which this fungus was collected.
Ascomata solitary, aggregated, erumpent, brown, globose, 150–200 μm diam, with papillate neck and central ostiole, 40–50 μm diam; ascomatal wall of 4–6 layers of brown cells of textura angularis. Pseudoparaphyses hyaline, smooth-walled, hyphae-like, 2–3 μm diam, anastomosing, branched, intermingled among asci. Asci bitunicate, subcylindrical, apex obtuse with well-defined apical chamber, 2 μm diam, fasciculate, short stipitate, 70–100 × 10–12 μm. Ascospores bi- to triseriate, subcylindrical, straight to slightly curved, (2–)3-septate, at times slightly swollen in second cell from apex, medium brown, verruculose, ends obtuse, (19–)22–25(–26) × (4–)5(–6) μm.
Culture characteristics: Colonies flat, spreading, with sparse to moderate aerial mycelium and even, lobate margins, reaching 30 mm diam on PDA, covering dish on MEA and OA. On MEA surface buff, outer region sienna, reverse sienna; on PDA surface and reverse buff to sienna; on OA surface scarlet.
Typus: USA, California, on leaves of Aloe sp. (Aloaceae), 6 Aug. 2016, P.W. Crous, HPC 1306 (holotype CBS H-23871, culture ex-type CBS 145368 = CPC 31454).
Notes: Phaeosphaeriopsis aloicola is related to Phs. agapanthi and Phs. triseptata. Morphologically, Phs. aloicola is similar to Phs. triseptata since both produce verruculose, 3-septate ascospores. However, both species differ in the size of ascomata (up to 200 μm in Phs. aloicola vs. up to 110 μm in Phs. triseptata), asci (70–100 × 10–12 μm in Phs. aloicola vs. 56−70 × 7.5−9 μm in Phs. triseptata) and ascospores [(19–)22–25(–26) × (4–)5(–6) μm in Phs. aloicola vs. 14.5−18 × 3−4 μm in Phs. triseptata]. Moreover, Phs. triseptata also produces an asexual morph, which has not been observed in Phs. aloicola. Phaeosphaeriopsis agapanthi only produces an asexual morph. The three species were isolated from different hosts in different families. Phaeosphaeriopsis agapanthi was isolated from Agapanthus precox (Amaryllidaceae), Phs. aloicola from Aloe sp. (Aloaceae), and Phs triseptata from Ruscus aculeatus (Asparagaceae). Moreover, Phs. aloicola was found in the USA while the other species have been reported from Europe. Phaeosphaeriopsis aloes was also isolated from Aloe in California. For comparison see notes of Phaeosphaeriopsis aloes.
Phaeosphaeriopsis grevilleae Crous & Y. Marín, sp. nov. MycoBank MB829644. Fig. 46.
Fig. 46.
Phaeosphaeriopsis grevilleae (ex-type CBS 145369). A. Conidiomata sporulating on PDA. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Grevillea from which this fungus was collected.
Conidiomata solitary, pycnidial, scattered, globose, 180–250 μm diam, with central ostiole, exuding black mucoid conidial mass; conidiomatal wall of 2–3 layers of brown cells of textura angularis. Conidiophores reduced to conidiogenous cells lining inner cavity, hyaline, smooth-walled, ampulliform, phialidic, 5–7 × 3–5 μm. Conidia solitary, aseptate, medium brown, verruculose, ellipsoid to ovoid, (4–)5(–6) × (3–)3.5(–4) μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and feathery margins, reaching 35 mm diam on MEA, covering dish on PDA and OA. On MEA surface and reverse scarlet; on PDA surface ochreous, reverse sienna; on OA surface scarlet with patches of ochreous.
Typus: Australia, Queensland, leaves of Grevillea sp. (Proteaceae), 14 Jul. 2009, P.W. Crous, (holotype CBS H-23872, culture ex-type CBS 145369 = CPC 17003).
Notes: In the phylogenetic analysis based on ITS, LSU, rpb2, tef1 and tub2, this species was located in an independent branch. This is the first species reported on Grevillea, which is a member of the Proteaceae.
Phaeosphaeriopsis pseudoagavacearum Crous & Y. Marín, sp. nov. MycoBank MB829645. Fig. 47.
Fig. 47.
Phaeosphaeriopsis pseudoagavacearum (ex-type CBS 145370). A. Conidiomata sporulating on MEA. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Named after its similarity to Phaeosphaeriopsis agavacearum.
Conidiomata solitary, globose, brown, pycnidial, 200–250 μm diam, with central ostiole, 15–20 μm diam; conidiomatal wall of 2–3 layers of brown cells of textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth-walled, ampulliform, phialidic, 5–7 × 3–4 μm. Conidia solitary, golden-brown, verruculose, thick-walled, straight to slightly curved, 1-septate, subcylindrical, apex obtuse, base bluntly rounded, (6–)8–9(–10) × 4(–4.5) μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and even, smooth margins, covering dish in 2 wk. On MEA surface ochreous, reverse bay; on PDA surface sienna with bay outer region, reverse bay; on OA surface sienna with scarlet margins.
Typus: France, Domaine la Fraysse, Valgorge, on leaves of Agave sp. (Asparagaceae), 15 Jul. 2010, P.W. Crous (holotype CBS H-23873, culture ex-type CBS 145370 = CPC 18383).
Notes: Phaeosphaeriopsis pseudoagavacearum is closely related to Phs. agavacearum. Morphologically these species are also similar in producing verruculose, aseptate conidia. However, they differ in the size of their conidiomata (up to 180 μm diam in Phs. agavacearum vs. up to 250 μm diam in Phs. pseudoagavacearum) and conidia [(5–)6–7(–9) × 3(–4) μm in Phs. agavacearum vs. (6–)8–9(–10) × 4(–4.5) μm in Phs. pseudoagavacearum].
Authors: Y. Marin-Felix & P.W. Crous
Pleiocarpon L. Lombard & D. Aiello, IMA Fungus 8: 73. 2017. Fig. 48.
Fig. 48.
Pleiocarpon strelitziae (ex-type CBS 142251). A–F. Disease symptoms. A, B. Wilting and dying Strelitzia reginae plants in the nursery. C–F. Basal rot and wilting of plant induced during the pathogenicity test. G–L. Asexual morph. G, H. Simple conidiophores. I, J. Sporodochia. K. Microconidia. L. Macroconidia. Scale bars: 10 μm; G applies to G–L. Pictures taken from Aiello et al. (2017).
Classification: Sordariomycetes, Hypocreomycetidae, Hypocreales, Nectriaceae.
Type species: Pleiocarpon strelitziae L. Lombard & D. Aiello. Holotype and ex-type strain: CBS H-22967, CBS 142251.
DNA barcode (genus): LSU.
DNA barcodes (species): ITS, his3, rpb2, tef1, tub2. Table 11.
Table 11.
DNA barcodes of accepted Pleiocarpon spp.
| Species | Isolates1 | GenBank accession numbers2 |
References | ||||
|---|---|---|---|---|---|---|---|
| ITS | his3 | rpb2 | tef1 | tub2 | |||
| Pleiocarpon livistonae | CBS 145030T | MK539963 | MK540234 | MK540095 | MK540165 | MK540179 | Present study |
| Pl. strelitziae | CBS 142251T | KY304644 | KY304616 | KY304697 | KY304722 | KY304750 | Aiello et al. (2017) |
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands. T indicates ex-type strains.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; his3: partial histone H3 gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial β-tubulin gene.
Conidiophores simple or aggregated, forming sporodochia; simple conidiophores arising laterally or terminally from aerial mycelium, solitary to loosely aggregated, unbranched or sparsely branched, septate, bearing up to two conidiogenous cells. Conidiogenous cells monophialidic, cylindrical, tapering slightly towards the apex. Macroconidia cylindrical to subcylindrical, hyaline, straight to curved, 1−6-septate, apex or apical cell typically slightly bent to one side and minutely beaked, base with sometimes visible, centrally located or laterally displaced hilum. Microconidia absent or abundant, aseptate, hyaline, ellipsoid to ovoid or subcylindrical, straight to slightly curved, with clearly laterally displaced hilum. Chlamydospores absent or solitary, globose, brown, thich-walled, guttulate. Sexual morph not observed (adapted from Aiello et al. 2017).
Culture characteristics: Colonies on PDA with sparse to moderate aerial mycelium, even, smooth, with lobate margins; surface and reverse umber or cinnamon to honey.
Optimal media and cultivation conditions: On PDA, MEA, OA or SNA with sterile filter paper and carnation leaf pieces at 25 °C.
Distribution: Italy and Sri Lanka.
Host: Livistona rotundifolia (Arecaceae) and Strelitzia reginae (Strelitziaceae).
Disease symptoms: Basal rot and wilt.
Notes: Pleiocarpon was recently introduced by Aiello et al. (2017) to accommodate a new species isolated from potted plants of Strelitzia reginae in an ornamental nursery located in eastern Sicily that had a new basal rot disease. This basal stem rot disease resulted in the detachment of the roots from the stem. Moreover, the diseased plants displayed symptoms of general wilting and rot of the internal foliage. Pathogenicity tests indicated that Pl. strelitziae, was highly aggressive, killing all inoculated test plants within 2 mo (Aiello et al. 2017).
The phylogenetic analysis based on ITS, LSU, tef1 and tub2 demonstrated that Pleiocarpon is closely related to the genus Thelonectria, with both genera being characterised by cylindrocarpon-like asexual morphs (Aiello et al. 2017). Recently, Thelonectria was segregated by introducing three new genera, Cinnamomeonectria, Macronectria and Tumenectria (Salgado-Salazar et al. 2016). These four related genera are mostly found on bark of exposed wood of dead, dying or diseased trees, and are rarely associated with small cankers and root rots (Chaverri et al., 2011, Salgado-Salazar et al., 2016). Moreover, Pleiocarpon can be distinguished from Thelonectria and these three new genera by the absence of a sexual morph.
Hitherto, Pleiocarpon was monospecific. Here we introduce a new species isolated from Livistona rotundifolia (Arecaceae) in Sri Lanka, causing root and corm rot.
References: Aiello et al. 2017 (morphology, pathogenicity and phylogeny).
Pleiocarpon livistonae Crous & Quaedvl., sp. nov. MycoBank MB829613. Fig. 49.
Fig. 49.
Pleiocarpon livistonae (ex-type CBS 145030). A. Sporodochium on SNA. B. Conidiophores with conidiogenous cells. C. Conidia. D. Chlamydospores. Scale bars = 10 μm.
Etymology: Name refers to Livistona, the host from which this fungus was collected.
Conidiophores simple, solitary or aggregated, forming sporodochia, arising from superficial hyphae, branched, 2–4-septate, 50–120 × 6–8 μm. Conidiogenous cells monophialidic, cylindrical, tapering slightly towards apex, 10–30 × 4–5 μm, forming conidia in false chains that eventually aggregate in a mucoid mass. Macroconidia hyaline, smooth-walled, subcylindrical, 1–6-septate, curved, apex subobtuse, with base sometimes visible as lateral hilum; 1–3 septate conidia (21–)28–34(–45) × (5–)6 μm; 4–6-septate conidia (45–)55–65(–70) × (5–)6 μm. Chlamydospores solitary, globose, brown, thick-walled, guttulate, 15–20 μm diam.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margins, reaching 60 mm diam after 2 wk at 25 °C. On MEA surface ochreous, reverse umber; on PDA surface and reverse umber; on OA surface umber.
Typus: Sri Lanka, on Livistona rotundifolia (Arecaceae), W. Quaedvlieg, NAK Tuinbouw, INS-17-20656D (holotype CBS H-23849, culture ex-type CBS 145030 = CPC 34576).
Notes: Pleiocarpon livistonae is distinguished from Pl. strelitziae by the absence of microconidia, the production of chlamydospores, and the septation and size of its macroconidia (1–6-septate, up to 70 μm in Pl. livistonae vs. 1–5-septate, up to 50 μm in Pl. strelitziae). Pleiocarpon livistonae is phylogenetically close but clearly differentiated from Pl. strelitziae based on ITS, his3, rpb2, tef1 and tub2 sequence similarity (96 %, 85 %, 92 %, 94 %, and 91 %, respectively). Moreover, Pl. livistonae was isolated from Livistona rotundifolia (Arecaceae) in Sri Lanka, while Pl. strelitziae was found on Strelitzia reginae (Strelitziaceae) in Italy.
Authors: Y. Marin-Felix, W. Quaedvlieg & P.W. Crous
Pyrenophora Fr., Summa veg. Scand. 2: 397. 1849. Fig. 50.
Fig. 50.
Pyrenophora spp. A–D. Sexual morph. A, B. Sterile ascomata of Pyrenophora campanulata (CBS 127927). C. Protoascomata of Pyrenophora erythrospila on PDA (CBS 312.69). D. Protoascoma of Pyrenophora erythrospila (CBS 108941). E–K. Asexual morph. E–H. Conidiophores. E.Pyrenophora fugax (CBS 509.77). F.Pyrenophora novozelandica (CBS 127934). G.Pyrenophora erythrospila (CBS 312.69). H.Pyrenophora fugax (CBS 509.77). I, J. Conidia. I.Pyrenophora erythrospila (CBS 312.69). J. Pyrenophora fugax (CBS 509.77). K. Chlamydospores of Pyrenophora tetrarrhenae (CBS 127924). Scale bars: A = 50 μm; D = 20 μm; G–K = 10 μm.
Synonyms: Polytrichia Sacc., Syll. fung. (Abellini) 1: 451. 1882.
Pleospora subgen. Scleroplea Sacc., Syll. fung. (Abellini) 2: 277. 1883.
Neilreichina Kuntze, Revis. gen. pl. (Leipzig) 2: 862. 1891.
Scleroplea (Sacc.) Oudem., Verslag. Meded. K. Akad. Wetensch., Afd. Natuurk., ser. 3 9: 152. 1900.
Drechslera S. Ito, Proc. Imp. Acad. Japan 6: 355. 1930.
Marielliottia Shoemaker, Canad. J. Bot. 76: 1559. 1999.
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Pleosporaceae.
Type species: Pyrenophora phaeocomes (Rebent.) Fr., basionym: Sphaeria phaeocomes Rebent. Neotype specimen designated by Shoemaker (1961): UPS 170980. Representative strain: DAOM 222769.
DNA barcodes (genus): LSU, ITS.
DNA barcodes (species): ITS, gapdh, tef1. Table 12. Fig. 51.
Table 12.
DNA barcodes of accepted Pyrenophora spp.
| Species | Isolates1 | GenBank accession numbers2 |
References | |||
|---|---|---|---|---|---|---|
| ITS | LSU | gapdh | rpb2 | |||
| Pyrenophora avenicola | CBS 307.84T | MK539972 | MK540042 | MK540180 | – | Present study |
| Py. biseptata | CBS 307.69 | MK539973 | MK540043 | MK540181 | – | Present study |
| CBS 319.69 | MK539974 | MK540044 | MK540182 | MK540102 | Present study | |
| CBS 108963 | MK539975 | JN712532 | MK540183 | – | Crous et al. (2011), present study | |
| Py. bromi | CBS 311.68 | MK539976 | MH870851 | MK540184 | – | Vu et al. (2019), present study |
| DAOMC 127414 | JN943666 | JN940074 | AY004839 | – | Zhang & Berbee (2001), Hambleton (unpubl. data) | |
| Py. chaetomioides | CBS 279.31A | MK539977 | MK540045 | MK540185 | MK540103 | Present study |
| CBS 195.31 | MK539978 | MH866633 | MK540186 | MK540104 | Vu et al. (2019), present study | |
| CBS 314.68 | MK539979 | MK540046 | MK540187 | MK540105 | Present study | |
| Py. cynosuri | CBS 127918T | MK539980 | MK540047 | MK540188 | MK540106 | Present study |
| Py. dactylidis | DAOMC 92161 | JN943667 | JN940087 | AY004812 | – | Zhang & Berbee (2001), Hambleton (unpubl. data) |
| Py. dictyoides | DAOMC 63666 | JN943653 | JN940080 | AY004836 | – | Zhang & Berbee (2001), Hambleton (unpubl. data) |
| CBS 258.80 | MK539981 | MK540048 | MK540189 | MK540107 | Present study | |
| CBS 967.87 | MK539982 | MK540049 | MK540190 | MK540108 | Present study | |
| CBS 127933 | MH877971 | MK540050 | MK540191 | MK540109 | Vu et al. (2019), present study | |
| Py. erythrospila | CBS 312.69 | MK539983 | MK540051 | MK540192 | – | Present study |
| CBS 108941 | MK539984 | MK540052 | MK540193 | MK540110 | Present study | |
| Py. fugax | CBS 509.77 | MK539985 | MK540053 | MK540194 | MK540111 | Present study |
| Py. grahamii | CBS 315.69 | MK539986 | MK540054 | MK540195 | – | Present study |
| CBS 128043 | MK539987 | MH876230 | MK540196 | MK540112 | Vu et al. (2019), present study | |
| CBS 128044 | MK539988 | MH876231 | MK540197 | MK540113 | Vu et al. (2019), present study | |
| Py. leucospermi | CBS 111083T | JN712467 | JN712533 | MK540198 | MK540114 | Crous et al. (2011), present study |
| CBS 111505 | MK539989 | JN712542 | MK540199 | MK540115 | Crous et al. (2011), present study | |
| CBS 114493 | MK539990 | JN712545 | MK540200 | MK540116 | Crous et al. (2011), present study | |
| Py. lolii | CBS 240.48 | MK539991 | MK540055 | MK540201 | MK540117 | Present study |
| CBS 318.69 | MK539992 | MH871050 | MK540202 | MK540118 | Vu et al. (2019), present study | |
| CBS 128046 | MK539993 | MH876233 | MK540203 | MK540119 | Vu et al. (2019), present study | |
| Py. nisikadoi | CBS 190.29ET | KM257054 | KM243296 | KM257057 | – | Manamgoda et al. (2014) |
| CBS 119213 | EU552124 | MK540056 | MK540204 | MK540120 | Marincowitz et al. (2008), present study | |
| CBS 127912 | MH877963 | MK540057 | MK540205 | MK540121 | Vu et al. (2019), present study | |
| Py. nobleae | CBS 259.80 | MK539994 | MK540058 | MK540206 | MK540122 | Present study |
| CBS 966.87 | MK539995 | MK540059 | MK540207 | MK540123 | Present study | |
| CBS 127936 | MK539996 | MK540060 | MK540208 | MK540124 | Present study | |
| Py. novozelandica | CBS 127934T | MK539997 | MK540061 | MK540209 | MK540125 | Present study |
| Py. phaeocomes | DAOMC 222769 | JN943649 | JN940093 | – | DQ497614 | Hambleton (unpubl. data), James et al. (unpubl. data) |
| Py. poae | CBS 319.68A | MK539998 | MK540062 | MK540210 | MK540126 | Present study |
| CBS 128045 | MK539999 | MH876232 | MK540211 | MK540127 | Vu et al. (2019), present study | |
| DAOMC 145373 | JN943650 | JN940083 | AY004832 | JN993632 | Zhang and Berbee, 2001, Schoch et al., 2012, Hambleton (unpubl. data) | |
| Py. pseudoerythrospila | CBS 127931T | MK540000 | MK540063 | MK540212 | – | Present study |
| Py. semeniperda | DAOMC 213153 | JN943665 | JN940088 | AY004826 | – | Zhang & Berbee (2001), Hambleton (unpubl. data) |
| BRIP 10941 | KJ415564 | KJ415518 | KJ415382 | – | Tan et al. (2014) | |
| CBS 127927 | MK540001 | MK540064 | MK540213 | MK540128 | Present study | |
| Py. sieglingiae | CBS 127930 | MK540002 | MK540065 | MK540214 | MK540129 | Present study |
| Py. teres | CBS 228.76T ofPy. teres f. maculata | MK540003 | MK540066 | MK540215 | MK540130 | Present study |
| CBS 281.31A ofPy. japonica | MK540004 | MK540067 | MK540216 | MK540131 | Present study | |
| CBS 282.31 | MK540005 | MK540068 | MK540217 | MK540132 | Present study | |
| CBS 314.69 | MK540006 | MK540069 | MK540218 | MK540133 | Present study | |
| CBS 336.29 | MK540007 | MH877692 | MK540219 | MK540134 | Vu et al. (2019), present study | |
| CBS 123929 | MK540008 | MK540070 | MK540220 | MK540135 | Present study | |
| CBS 123932 | MK540009 | MK540071 | MK540221 | MK540136 | Present study | |
| Py. tetrarrhenae | DAOMC 171966 | JN943663 | JN940090 | – | JN993620 | Schoch et al. (2012), Hambleton (unpubl. data) |
| CBS 127915 | MK540010 | MH877964 | MK540222 | MK540137 | Vu et al. (2019), present study | |
| CBS 127924 | MK540011 | MH877965 | MK540223 | MK540138 | Vu et al. (2019), present study | |
| Py. trichostoma | CBS 328.53 | MK540012 | MK540072 | MK540224 | MK540139 | Present study |
| CBS 391.54 | MK540013 | MK540073 | MK540225 | – | Present study | |
| CBS 392.54 | MK540014 | MK540074 | MK540226 | MK540140 | Present study | |
| Py. triseptata | CBS 128047 | MK540015 | MH877983 | MK540227 | MK540141 | Vu et al. (2019), present study |
| CBS 128048 | MK540016 | MH876234 | MK540228 | MK540142 | Vu et al. (2019), present study | |
| Py. tritici-repentis | CBS 259.59SynT ofPy. tritici-vulgaris | MK540017 | MK540075 | AM884276 | MK540143 | Lepoint et al. (2010), present study |
| CBS 191.29 | MK540018 | MK540076 | MK540229 | MK540144 | Present study | |
| CBS 127922 | MK540019 | MK540077 | MK540230 | MK540145 | Present study | |
| Py. variabilis | CBS 127920T | MK540020 | MK540078 | MK540231 | MK540146 | Present study |
| Py. wirreganensis | CBS 109896 | MK540021 | MK540079 | MK540232 | MK540147 | Present study |
BRIP: Queensland Plant Pathology Herbarium, Brisbane, Australia; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; DAOMC: Plant Research Institute, Department of Agriculture (Mycology), Ottawa, Canada. A, ET, SynT and T indicate authentic, ex-epitype, ex-syntype and ex-type strains, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial large subunit (28S) nrRNA gene; gapdh: partial glyceraldehyde-3-phosphate dehydrogenase gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene.
Fig. 51.
RAxML phylogram obtained from the combined ITS (788 bp), LSU (862 bp), gapdh (694 bp) and tef1 (860 bp) sequences of all the accepted species of Pyrenophora. Bipolaris panici-miliacei CBS 199.29 and Bipolaris yamadae CBS 202.29 were used as outgroup. The novelties proposed in this study are indicated in bold. RAxML bootstrap support (BS) values above 70 % and Bayesian posterior probability scores ≥ 0.95 are shown in the nodes. GenBank accession numbers were indicated in Table 12 and Manamgoda et al. (2014). A, ET, LT, SynT and T indicate authentic, ex-epitype, ex-lectotype, ex-syntype and ex-type strains, respectively. TreeBASE: S23834.
Ascomata perithecial, immersed, becoming erumpent to near superficial, solitary or scattered, globose to subglobose, broadly or narrowly conical, smooth-walled, with central ostiole; necks papillate, covered with brown to reddish brown setae, which are darkened at the base; ascomatal wall comprising 2–4 layers of brown, thick-walled cells of textura angularis. Pseudoparaphyses not observed. Asci 8-spored, bitunicate, fissitunicate, clavate to subcylindrical, with a short, broad pedicel, with a distinct ocular chamber surrounded by a large apical ring. Ascospores 2–3-seriate, muriform, constricted at the septum, smooth-walled, surrounded by a mucilaginous sheath. Conidiophores semi- to macronematous, mononematous, sometimes caespitose, straight or flexuous, often geniculate, usually unbranched, sometimes branched, pale brown to brown, rarely subhyaline to pale brown. Conidiogenous cells polytretic, integrated, terminal, frequently becoming intercalary, sympodial, cylindrical, smooth-walled, or less frequently verruculose, cicatrised. Conidia solitary, in certain species also sometimes catenate or forming secondary conidiophores which bear conidia, acropleurogenous, simple, straight or curved, cylindrical, ellipsoidal or obclavate, less frequently subglobose, obpyriform or fusiform, tapering towards apex, straw-coloured or pale to dark brown or olivaceous brown, sometimes the end cells are paler than the intermediate ones, smooth-walled or verruculose, pseudoseptate; hila protuberant or flat, darkened, thickened (adapted from Ellis 1971, Ariyawansa et al. 2014).
Culture characteristics: Colonies flat or umbonate, cottony, sometimes granular or powdery, with moderate to abundant aerial mycelium, sometimes with sparse aerial mycelium, margins fringed, sometimes arachnoid. On PDA smoke-grey to olivaceous or olivaceous grey, primrose to greyish yellow-green, greenish grey to olivaceous black, olivaceous black with patches white for the aerial mycelium, honey to isabelline, orange to umber, cinnamon with centre white due to the aerial mycelium, greyish sepia to fuscous black, or fuscous grey with margins buff; reverse olivaceous, olivaceous black with or without margins, primrose or luteous, fuscous black with or without margins transparent or buff, honey to isabelline, sienna to umber with margins luteous, or cinnamon with centre brick to dark brick. On MEA white to pale greenish glaucous or buff, greyish sepia to pale mouse grey or mouse grey, smoke-grey to pale olivaceous grey, glaucous grey to greenish grey, pale vinaceous to vinaceous buff, purplish grey with margins vinaceous buff, luteous with margins white to pale smoke-grey, vinaceous buff to hazel with margins white and saffron, pale greenish grey to greenish grey, or fuscous black with margins luteous; reverse olivaceous black, smoke-grey to olivaceous grey with middle white due to the aerial mycelium, fuscous black with or without margins luteous or olivaceous or buff to cinnamon or honey to isabelline, chestnut with margins luteous to rust, blood colour with margins luteous or scarlet, chestnut with margins luteous, or orange to sienna. On OA smoke-grey, olivaceous, olivaceous black, hazel, buff, cinnamon, olivaceous grey with margins luteous, greyish sepia to fuscous black with margins brick, or orange to umber with margins transparent; reverse olivaceous to olivaceous black, smoke-grey to olivaceous grey, olivaceous black with margins transparent or luteous, leaden grey to leaden black, buff with centre fuscous black and margins olivaceous, isabelline with centre olivaceous, orange to umber or greyish sepia to fuscous black with margins transparent, olivaceous grey to olivaceous black with margins transparent, or fuscous black with margins brick, or transparent with centre brick to dark brick.
Optimal media and cultivation conditions: On PDA, PNA, OA and MEA to induce sporulation of the asexual morph, while for the sexual morph Sach's agar with sterilised rice or wheat straw at 25 °C is used.
Distribution: Worldwide, mainly in Australia, Europe, New Zealand and North America.
Hosts: Wide host range, occurring as pathogens, saprobes or endophytes. Mainly found in members of Poaceae, being pathogens of cereals and grasses, including barley, oats and wheat. The most common genera belonging to Poaceae which this genus is associated to are Agropyron, Agrostis, Avena, Bromus, Dactylis, Festuca, Hordeum, Lolium, Poa and Triticum, among others. Pyrenophora species are also reported from other genera outside this family, such as Protea and Leucospermum in the Proteaceae.
Disease symptoms: Leaf spots, leaf blight, leaf blotch, net blotch, melting out, head rot, foot rot, seed-borne diseases, among others.
Notes: Pyrenophora is characterised by immersed to semi-immersed ascomata with necks covered with brown to reddish brown setae, lack of pseudoparaphyses, clavate to saccate asci, usually with a large apical ring, and muriform terete (cylindrical, frequently circular in section but narrowing to one end) ascospores (Ariyawansa et al. 2014). The asexual morph was known as Drechslera and it is characterised by brown, transversely septate conidia similar to those found in Bipolaris and Curvularia. In order to properly delineate these three genera, phylogenetic analyses using sequence data of different loci (i.e. LSU, SSU, ITS, gapdh and rpb2) were performed (Zhang and Berbee, 2001, Ariyawansa et al., 2014, Manamgoda et al., 2014). The synonymy of Drechslera with Pyrenophora was recently discussed by Ariyawansa et al. (2014). However, there is still a large number of species which await treatment. Three new combinations are introduced here, i.e. Py. nisikadoi, Py. poae and Py. wirreganensis. The main problem encountered is the lack of type material of the already known species, and this resulted in few molecular studies being performed in the past.
Species delimitation in Pyrenophora based on morphology alone is complicated since many species have overlapping characters, similar to what is observed in Bipolaris and Curvularia (Marin-Felix et al. 2017). Therefore, molecular data (ITS, gapdh and rpb2) are essential for an accurate identification of species of Pyrenophora. In our phylogenetic analysis, 21 species are accepted and an additional six are newly described.
Pyrenophora includes saprobic and plant pathogenic species with a worldwide distribution, commonly associated with members of the family Poaceae. Some species are serious plant pathogens, e.g. Py. teres, which is a necrotrophic pathogen causing net blotch in barley (Crous et al., 1995, Louw et al., 1995, Campbell et al., 1999, Campbell et al., 2002), and Py. tritici-repentis, which causes tan spot of wheat (Lamari and Bernier, 1989, Balance et al., 1996, Abdullah et al., 2017) in all the major wheat growing areas of the world resulting in 3–50 % yield losses (Lamari & Bernier 1989).
References: Sivanesan 1987 (morphology and pathogenicity), Zhang and Berbee, 2001, Ariyawansa et al., 2014 (morphology and phylogeny).
Pyrenophora avenicola Y. Marín & Crous, sp. nov. MycoBank MB829614. Fig. 52.
Fig. 52.
Pyrenophora avenicola (ex-type CBS 307.84). A, B. Conidiophores and conidia. C–I. Conidia. Scale bars: A, B = 10 μm; C–I = 5 μm; C applies to C–I.
Etymology: Name refers to the host genus Avena, from which it was isolated.
Hyphae hyaline, branched, septate, verrucose, 2–6(–7.5) μm. Conidiophores arising in groups, septate, straight or flexuous, sometimes geniculate at upper part, usually cells decrease in size towards apex, sometimes branched, cell walls thicker than those of vegetative hyphae, semi- to macronematous, subhyaline to pale brown, usually paler towards apex, not swollen at the base, up to 350 μm long, 4.5–7 μm wide. Conidiogenous cells verruculose, terminal or intercalary, proliferating sympodially, subhyaline to pale brown, subcylindrical to swollen, 14–33.5(–43.5) × 6.5–9.5 μm. Conidia verruculose, straight, middle cells enlarged, cylindrical to obclavate, tapering towards apex, pale brown to brown, end cells rarely slightly paler, 1–4(–5)-distoseptate, 21.5–71.5 × 9.5–15 μm, forming secondary conidiophores or conidia; hila protuberant, darkened, thickened, 3–4.5 μm. Chlamydospores, microconidiation and sexual morph not observed.
Culture characteristics: Colonies on MEA covering dish after 1 wk at 25 °C, with abundant aerial mycelium, umbonate; surface fuscous black, margins luteous; reverse smoke-grey to olivaceous grey, centre white due to the aerial mycelium. Colonies on PDA covering dish, with abundant aerial mycelium; surface smoke-grey to olivaceous grey; reverse fuscous black to black, margins transparent. Colonies on OA covering all dish, with moderate to abundant aerial mycelium; surface smoke-grey to olivaceous grey; reverse olivaceous grey to olivaceous black, margins transparent.
Typus: Sweden, Uppsala, on seed of Avena sp. (Poaceae), unknown date, C. Svensson (holotype CBS H-23840, culture ex-type CBS 307.84).
Notes: Pyrenophora avenicola is closely related to Py. chaetomioides. Moreover, both species have been found on the same host, Avena. However, Py. avenicola can be easily distinguished by its shorter conidiophores (up to 350 μm in Py. avenicola vs. 1 mm in Py. chaetomioides) and smaller conidia (21.5–71.5 × 9.5–15 μm in Py. avenicola vs. 25–140 × 12–22 μm in Py. chaetomioides) with less septa [1–4(–5) in Py. avenicola vs. 2–9 in Py. chaetomioides]. The sexual morph has only been observed in Py. chaetomioides.
Pyrenophora cynosuri Y. Marín & Crous, sp. nov. MycoBank MB829615. Fig. 53.
Fig. 53.
Pyrenophora cynosuri (ex-type CBS 127918). A–C. Conidiophores and conidia. D. Conidium forming secondary conidium. E–M. Conidia. Scale bars: 10 μm; E applies to E–M.
Etymology: Name refers to the host genus Cynosurus, from which it was isolated.
Hyphae hyaline to pale brown, branched, septate, smooth-walled or verrucose, 1.5–5 μm. Conidiophores arising in groups, septate, straight or flexuous, usually geniculate in upper part, size of cells rarely decrease towards apex, rarely branched, cell walls thicker than those of vegetative hyphae, macronematous, rarely micronematous, pale brown to brown, slightly paler towards apex, not swollen at the base, (70–)95–700 × 4.5–8 μm. Conidiogenous cells smooth-walled to slightly verruculose, terminal or intercalary, proliferating sympodially, pale brown to brown, subcylindrical to swollen, 10–30(–37) × 5.5–10 μm. Conidia verruculose, mostly curved, middle cells sometimes enlarged, cylindrical to obclavate, tapering towards apex, subhyaline to pale brown, end cells rarely paler, 2–5-distoseptate, (25–)28–80(–83) × 9–16.5 μm, forming secondary conidiophores or conidia; hila protuberant, darkened, thickened, (3–)3.5–6 μm. Chlamydospores, microconidiation and sexual morph not observed.
Culture characteristics: Colonies on MEA reaching 55–60 mm diam after 1 wk at 25 °C, with moderate aerial mycelium, flat to umbonate, margins arachnoid; surface greyish sepia to mouse grey; reverse blood colour, margins luteous. Colonies on PDA reaching 60–65 mm diam, with sparse aerial mycelium, flat, margins arachnoid; surface greyish sepia to fuscous black; reverse fuscous black. Colonies on OA covering dish, with moderate aerial mycelium; surface greyish sepia to fuscous black, margins brick; reverse fuscous black, margins brick.
Typus: New Zealand, on seeds of Cynosurus cristatus (Poaceae), 1975, E.H.C. McKenzie (holotype CBS H-23841, culture ex-type CBS 127918 = BRIP 12355 a = NZ 14880).
Notes: Pyrenophora cynosuri was isolated from seeds of Cynosurus cristatum, a member of the Poaceae, which includes host genera commonly infected by species of Pyrenophora. However, Cynosurus represents a new host genus for Pyrenophora.
Pyrenophora cynosuri is closely related and morphologically similar to Py. dictyoides. However, both species differ in the size of their conidiophore length (up to 700 μm in Py. cynosuri vs. up to 250 μm in Py. dictyoides) and conidial length (up to 83 μm in Py. cynosuri vs. up to 250 μm in Py. dictyoides), as well as conidial septation (up to 5 in Py. cynosuri vs. up to 15 in Py. dictyoides).
Pyrenophora dictyoides A.R. Paul & Parbery, Trans. Brit. Mycol. Soc. 51: 708. 1968.
Synonyms: Helminthosporium dictyoides Drechsler, J. Agric. Res. 24: 679. 1923.
Helminthosporium dictyoides var. dictyoides Drechsler, J. Agric. Res. 24: 679. 1923.
Helminthosporium dictyoides f. dictyoides Drechsler, J. Agric. Res. 24: 679. 1923.
Drechslera dictyoides (Drechsler) Shoemaker, Canad. J. Bot. 37: 881. 1959.
Helminthosporium dictyoides f. perenne Braverman & J.H. Graham, Phytopathology 50: 695. 1960.
Drechslera andersenii Scharif, Studies on Graminicolous Species of Helminthosporium (Tehran): 29. 1963. (nom. inval., Art. 36.1).
Drechslera andersenii A. Lam, Trans. Brit. Mycol. Soc. 85: 601. 1986.
Notes: Isolates belonging to Py. dictyoides (CBS 127933 and DAOM 63666) and Drechslera andersenii (CBS 258.80 and CBS 967.87) clustered together in a well-supported clade (100 % BS / 1 PP). Moreover, the morphology of the asexual morph is similar in both species, differing only in the production of conidia with much less tapered apices in D. andersenii (Sivanesan 1987). Therefore, we reduce these species to synonymy.
Pyrenophora nisikadoi Y. Marín & Crous, nom. nov. MycoBank MB829616. Fig. 54.
Fig. 54.
Pyrenophora nisikadoi (CBS 119213). A, B. Conidiophores and conidia. C. Chlamydospores. D–K. Conidia. Scale bars: A, B = 10 μm; C = 20 μm; D = 5 μm; D applies to D–K.
Replaced synonym: Helminthosporium brizae Y. Nisik., Ber. Ohara Inst. Landw. Biol.: 121, 133. 1928, non Pyrenophora brizae C. Massal. ex Sacc. 1911.
Additional synonyms: Bipolaris brizae (Y. Nisik.) Shoemaker, Canad. J. Bot. 37: 882. 1959.
Drechslera brizae (Y. Nisik.) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Etymology: Named after the Japanese plant pathologist and mycologist, Y. Nisikado, who first described and named this fungus.
Hyphae hyaline to pale brown, branched, septate, verrucose, 2–5.5 μm. Conidiophores arising in groups, septate, mostly flexuous, rarely straight, geniculate at upper part, sometimes size of cells decrease towards apex, frequently branched, cell walls thicker than those of vegetative hyphae, macronematous, pale brown to brown, sometimes paler towards apex, rarely swollen at the base, 50–330 × 3.5–6.5(–8.5) μm. Conidiogenous cells smooth-walled to verruculose, terminal or intercalary, proliferating sympodially, pale brown to brown, subcylindrical to swollen, 7.5–20.5(–25) × 5–7.5 μm. Conidia verruculose, straight or curved, middle cells enlarged, cylindrical to obclavate, tapering towards apex, pale brown to brown, basal cell sometimes paler, less frequently apical cell also paler, (1–)2–4(–5)-distoseptate, (15–)17.5–42.5 × 8.5–12 μm, not forming secondary conidiophores or conidia; hila flat, darkened, thickened, 2–4 μm. Chlamydospores immersed in all media tested (MEA, OA and PDA), brown to dark brown, lineally or irregularly disposed, verrucose, globose to subglobose, up to 30 μm. Microconidiation and sexual morph not observed.
Culture characteristics: Colonies on MEA reaching 80–90 mm diam after 1 wk at 25 °C, with abundant aerial mycelium, cottony, lobate; surface pale greenish grey to greenish grey; reverse olivaceous black. Colonies on PDA covering dish, with moderate aerial mycelium, cottony, powdery at margins, flat; surface greenish grey to olivaceous black; reverse olivaceous black. Colonies on OA covering the dish, with sparse aerial mycelium, powdery to granular, flat; surface grey olivaceous to olivaceous black; reverse leaden grey to leaden black.
Typus: Japan, from Briza minor (Poaceae), Y. Nisikado [epitype designated by Manamgoda et al. (2014) CBS H-7218, culture ex-epitype CBS 190.29 = MUCL 9613].
Additional materials examined: South Africa, Western Cape Province, J.S. Marais Nature Reserve, from Protea burchellii senescent flowerheads (Proteaceae), 6 Jun. 2000, S. Lee, CBS 119213. New Zealand, Auckland, Waitakere Ranges, from Briza minor (Poaceae), 1 Nov. 1975, E.H.C. McKenzie, CBS 127912 = ICMP 6183.
Notes: This species was originally described in Helminthosporium as Hel. brizae (Nisikado 1928), then transferred to Bipolaris (Shoemaker 1959), and finally placed in Drechslera (Subramanian & Jain 1966). Type material was not available, thus Manamgoda et al. (2014) designated CBS 190.29 as ex-epitype since this strain was isolated by the original author from the same host and location. In our phylogenetic analysis based on ITS, gapdh and rpb2, CBS 190.29 together with other two strains were located in a well-supported clade (100 % BS / 1 PP) within the main clade representing the genus Pyrenophora. Therefore, we propose to transfer this species to Pyrenophora, changing the epithet to Py. nisikadoi since Py. brizae already exists.
Isolate CBS 119213 sporulated, enabling us to conduct a morphological comparison and provide a modern description of this species in PDA. Moreover, Py. nisikadoi was formerly only recorded from Briza minor, a member of the Poaceae, while it is here also recorded from Protea birchellii, which belongs to the Proteaceae.
Pyrenophora novozelandica Y. Marín & Crous, sp. nov. MycoBank MB829620. Fig. 55.
Fig. 55.
Pyrenophora novozelandica (ex-type CBS 127934). A–D. Conidiophores and conidia. E–M. Conidia. Scale bars: A–D = 10 μm; E = 5 μm; E applies to E–M.
Etymology: Name refers to New Zealand, the country from where it was isolated.
Hyphae hyaline to pale brown, branched, septate, verrucose, 1–6(–8.5) μm. Conidiophores arising in groups, septate, straight or flexuous, sometimes geniculate at upper part, size of cells not decreasing towards apex, rarely branched, cell walls thicker than those of vegetative hyphae, macronematous, pale brown to brown, paler towards apex, not swollen at the base, 35–700 × 4.5–7.5 μm. Conidiogenous cells smooth-walled, terminal or intercalary, proliferating sympodially, brown, terminal conidiogenous cells hyaline, cylindrical to subcylindrical, 11–22 × 5–8.5 μm. Conidia smooth-walled, straight, rarely slightly curved, sometimes middle cells slightly enlarged, cylindrical to obclavate, tapering towards apex, pale brown to brown, sometimes basal cell slightly paler, (2–)3–5(–6)-distoseptate, 20.5–58 × 9.5–14 μm, not forming secondary conidiophores or conidia; hila usually inconspicuous, flat, slightly darkened, slightly thickened, 2–4 μm. Chlamydospores, microconidiation and sexual morph not observed.
Culture characteristics: Colonies on MEA reaching 27–30 mm diam after 1 wk at 25 °C, with sparse aerial mycelium, raised, margins fringed; surface grey olivaceous; reverse dark mouse grey, margins buff. Colonies on PDA reaching 38–40 mm diam, with sparse aerial mycelium, flat, margins fringed; surface smoke grey to olivaceous grey; reverse olivaceous black. Colonies on OA reaching 37–40 mm diam, with moderate aerial mycelium, flat, margins fringed; surface grey olivaceous; reverse olivaceous black.
Typus: New Zealand, Wanganui, Palmerston North, Seed Testing Station, on seed of Triticum sp. (Poaceae), 5 Oct. 1976, G.F. Laundon (holotype CBS H-23843, culture ex-type CBS 127934 = LEV 11079b = PDD 50697).
Notes: Pyrenophora novozelandica is similar and closely related to Py. fugax. However, both species can be easily distinguished based on the size of their conidiophores (up to 250 μm in Py. fugax vs. up to 700 μm in Py. novozelandica) and conidia (50–170 × 14–24 μm in Py. fugax vs. 20.5–58 × 9.5–14 μm in Py. novozelandica), as well as conidial septation [4–8(–10) in Py. fugax vs. (2–)3–5(–6) in Py. novozelandica]. Pyrenophora novozelandica is known to occur on Triticum in New Zealand, which is a common host of species belonging to Pyrenophora, including Py. fugax.
Pyrenophora poae (Baudyš) Y. Marín & Crous, comb. nov. MycoBank MB829617.
Basionym: Helminthosporium poae Baudyš, Lotos 63: 104. 1916.
Synonyms: Helminthosporium vagans Drechsler, J. Agric. Res., Washington 24: 688. 1923.
Drechslera vagans (Drechsler) Shoemaker, Canad. J. Bot. 37: 881. 1959.
Drechslera poae (Baudyš) Shoemaker, Canad. J. Bot. 40: 827. 1962.
Description and illustration: Sivanesan (1987).
Materials examined: Canada, Saskatchewan, Saskatoon, from Poa pratensis (Poaceae), Oct. 1973, J.D. Smith, DAOMC 145373. Germany, Husum, from P. pratensis, Aug. 1966, U.G. Schlösser, CBS 319.68. USA, Maryland, Beltsville, from P. pratensis, Apr. 1979, A. Hagan, CBS 128045 = BRIP 12969a.
Notes: Pyrenophora poae was introduced as Helminthosporium poae by Baudys (1916), then transferred to Drechslera (Shoemaker 1959). The original description was based on a specimen isolated from Poa trivialis in the Czech Republic. Type material is not available, but CBS 319.68 is considered here as an authentic strain since was isolated from the same host genus and continent. Unfortunately, it did not sporulate and thus we chose to not designate it as epitype. All the strains identified as D. poae were located in the main clade belonging to Pyrenophora, and a new combination is proposed here.
Pyrenophora pseudoerythrospila Y. Marín & Crous, sp. nov. MycoBank MB829675. Fig. 56.
Fig. 56.
Pyrenophora pseudoerythrospila (ex-type CBS 127931). A. Protoascomata on OA. B, C. Protoascomata. Scale bars = 10 μm.
Etymology: Named after its close phylogenetic relation to Py. erythrospila.
Only forming protoascomata in OA. Protoascomata composed of pale brown to brown cells of up to 100 μm diam, of textura angularis to textura globulosa. Pyrenophora pseudoerythrospila differs from its closest phylogenetic neighbour, Py. erythrospila by unique fixed alleles in three loci based on alignments of the separate loci deposited in TreeBASE (S23834): LSU positions 70 (G), 395 (C), 396 (T), 397 (T), 500 (G), 536 (C), 537 (T); ITS positions 141 (A), 146 (A), 147 (T), 148 (A), 149 (G), 152 (G), 153 (A), 154 (G), 155 (T), 164 (T), 168 (T), 169 (G), 173 (G), 174 (C), 176 (A), 178 (T), 179 (G), 184 (T), 187 (T), 189 (T), 197 (C), 198 (C), 199 (C), 205 (C), 206 (T), 208 (C), 223 (T), 224 (T), 225 (T), 226 (T), 234 (A), 252 (T), 264 (T), 268 (C), 279 (G), 288 (C), 289 (A), 293 (A), 302–304 (indels), 327 (T), 330–332 and 341 (indels),511 (T), 536–540 (indels), 558 (C), 591 (A), 594 (G), 601 (T), 621 (A), 630 (C), 641 (G), 643 (A), 644 (G), 645 and 646 (indels), 659 (T), 660 (G), 664 (T), 666 (A), 667 (T); gapdh positions 30 (C), 49 (A), 54 and 55 (indels), 59 (G), 60 (A), 69 (C), 71 (C), 74 (A), 85 (T), 87 (A), 177 (G), 178 (G), 179 (C), 180 (C), 182 (C), 187 (G), 189 (C), 190 (T), 191 (A), 192 (T), 193 (C), 194 (A), 195 (G), 196 (A), 197 (C), 201 (G), 203 (A), 204 (G), 205 (A), 256 (G), 319 (G), 373 (T), 406 (C), 487 (T), 493 (T), 496 (T), 523 (G), 526 (C), 532 (C), 538 (A).
Culture characteristics: Colonies on MEA reaching 55–60 mm diam after 1 wk at 25 °C, with sparse aerial mycelium, flat, margins fringed; surface pale vinaceous to vinaceous buff; reverse orange to sienna, margins transparent. Colonies on PDA reaching 56–59 mm diam, with sparse aerial mycelium, flat, margins fringed; surface cinnamon, white mycelium in the centre; reverse cinnamon, centre brick to dark brick. Colonies on OA reaching 50–55 mm diam, without aerial mycelium, flat; surface cinnamon; reverse transparent, centre brick to dark brick.
Typus: Germany, West Germany, on Lolium sp. (Poaceae), 9 Sep. 1968, U.G. Schlosser (holotype CBS H-23844, culture ex-type CBS 127931 = DAOMC 126772).
Notes: The ex-type strain of Pyrenophora pseudoerythrospila did not sporulate on any of the media tested, producing only few protoascomata in OA. However, these remained sterile after several months of incubation. Pyrenophora pseudoerythrospila is closely related to Py. erythrospila, which produces both sexual and asexual morphs. The protoascomata were also reported in Py. erythrospila, but these finally developed mature ascospores after 25 wk. Pyrenophora erythrospila is commonly found on Agrostis spp. in Australia and North America, but has also been reported on Lolium in Germany (Farr & Rossman 2019), having the same host and distribution as Py. pseudoerythrospila.
Pyrenophora sieglingiae Y. Marín & Crous, sp. nov. MycoBank MB829618. Fig. 57.
Fig. 57.
Pyrenophora sieglingiae (ex-type CBS 127930). A–C. Sterile ascomata. D. Neck of ascoma. E, F. Conidiophores and conidia. G–O. Conidia. Scale bars: C = 100 μm; D–F = 20 μm; G = 10 μm; G applies to G–O.
Etymology: Name refers to Sieglingia, the host genus from which this fungus was collected.
Hyphae hyaline to pale brown, branched, septate, verrucose, (1–)1.5–5.5 μm. Sterile ascomata solitary or arising in groups, brown to dark brown or black, sometimes apical part of neck yellowish brown, composed of cells of textura intricata, up to 1200 μm long, up to 300 μm wide, conidiophores arising from the body and neck; inside consisting of angular to globose, hyaline cells. Conidiophores arising in groups, septate, straight or flexuous, rarely geniculate in the upper part, cell size rarely decreases towards the apex, unbranched, cell walls thicker than those of vegetative hyphae, macronematous, brown, mostly paler towards apex, not swollen at the base, 100–700 × (5–)7–9(–11) μm. Conidiogenous cells verruculose, terminal or intercalary, proliferating sympodially, pale brown to brown, subcylindrical to slightly swollen, (12–)15–33.5(–36.5) × 9–12(–13.5) μm. Conidia verruculose, straight or curved, sometimes with middle cells enlarged, cylindrical to obclavate, tapering towards apex, pale brown to brown, end cells usually paler, 4–8-distoseptate, 56–108(–120) × 15–23(–25.5) μm, forming secondary conidiophores or conidia; hila not protuberant or flat, darkened, thickened, (4–)4.5–6.5(–7) μm. Chlamydospores, microconidiation and sexual morph not observed.
Culture characteristics: Colonies on MEA reaching 26–29 diam after 1 wk at 25 °C, with abundant aerial mycelium, raised, slightly lobate; surface white to buff; reverse fuscous black. Colonies on PDA reaching 27–30 diam, with abundant aerial mycelium, lobate; surface olivaceous black with patches of white due to aerial mycelium; reverse olivaceous black, margins luteous. Colonies on OA reaching 54–57 diam, with moderate to abundant aerial mycelium, flat; surface smoke-grey to olivaceous grey; reverse smoke-grey to olivaceous grey.
Typus: New Zealand, Auckland, Waikumete, from leaf of Sieglingia decumbens (Poaceae), E.H.C. McKenzie (holotype CBS H-23842, culture ex-type CBS 127930 = ICMP 6170 = PDDCC 6170).
Notes: Pyrenophora sieglingiae is closely related to Py. semeniperda and Py. wirreganensis. Morphologically, these species are similar, producing sterile ascomata with long necks. However, they can be distinguished by the size of their conidiophores (up to 700 μm in Py. sieglingiae vs. up to 180 μm in Py. semeniperda vs. up to 1 000 μm in Py. wirreganensis) and conidia [56–108(–120) × 15–23(–25.5) μm in Py. sieglingiae vs. 70–160 × 13–17 μm in Py. semeniperda vs. (30–)40–80(–100) × (10–)12–19(–22) μm in Py. wirreganensis). Moreover, Py. semeniperda produces conidia with more septa (up to 12 in Py. semeniperda vs. up to 8 in Py. sieglingiae vs. up to 9 in Py. wirreganensis), and it is the only one that produces a sexual morph. Pyrenophora sieglingiae has been isolated from Sieglingia from New Zealand while Py. wirreganensis occurs on Hordeum in Australia. Pyrenophora semeniperda has been isolated from both hosts in both locations, apart from other hosts that are widely distributed, i.e. Agropyron, Avena, Bromus, Cortaderia, Ehrhartia, Pennisetum and Triticum.
Pyrenophora teres Drechsler, J. Agric. Res., Washington 24: 656. 1923.
Synonyms: Helminthosporium secalis Fée, Mém. Soc. Mus. Hist. Nat. Strassbourg 3: 36. 1843.
Alternaria secalis (Fée) Sacc. & Traverso, Syll. fung. (Abellini) 20: 1184. 1911.
Helminthosporium gramineum Rabenh., Klotzschii Herb. Viv. Mycol., Edn Nov, Ser. Sec., Cent. 4: no. 332. 1857.
Brachysporium gracile var. gramineum (Rabenh. ex Schltdl.) Sacc., Syll. fung. (Abellini) 4: 430. 1886.
Drechslera graminea (Rabenh. ex Schltdl.) S. Ito, Proc. Imper. Acad. Tokyo 6: 355. 1930.
Drechslera teres subsp. graminea (Rabenh. ex Schltdl.) Simay, Barley Newsletter 36: 174. 1992.
Helminthosporium teres Sacc., Syll. fung. (Abellini) 4: 412. 1886.
Drechslera teres (Sacc.) Shoemaker, Can. J. Bot. 37: 881. 1959.
Drechslera teres f. teres (Sacc.) Shoemaker, Canad. J. Bot. 37: 881. 1959.
Drechslera teres subsp. teres (Sacc.) Shoemaker, Canad. J. Bot. 37: 881. 1959.
Helminthosporium hordei Eidam, Der Landw. (Schles. Landw. Ztg), Breslau 27: 509. 1891.
Helminthosporium tuberosum G.F. Atk., Bulletin of Cornell University 3: 47. 1897.
Drechslera tuberosa (G.F. Atk.) Shoemaker, Canad. J. Bot. 37: 881. 1959.
Helminthosporium fragosoi Bubák, Hedwigia 57: 13. 1915.
Pyrenophora teres f. teres Drechsler, J. Agric. Res., Washington 24: 656. 1923.
Pyrenophora teres subsp. teres Drechsler, J. Agric. Res., Washington 24: 656. 1923.
Helminthosporium japonicum S. Ito & Kurib., Proc. Imper. Acad. Tokyo 6: 353. 1930.
Pyrenophora japonica S. Ito & Kurib., Proc. Imper. Acad. Tokyo 6: 353. 1930.
Drechslera japonica (S. Ito & Kurib.) Shoemaker, Canad. J. Bot. 37: 881. 1959.
Pyrenophora graminea S. Ito & Kurib., Proc. Imp. Acad. Japan 6: 353. 1930.
Pyrenophora teres subsp. graminea (S. Ito & Kurib.) Simay, Barley Newsletter 36: 174. 1992.
Pyrenophora secalis M.D. Whitehead & J. Dicks., Mycologia 44: 752. 1952.
Drechslera teres f. maculata Smed.-Pet., Arb. Tiflis Bot. Gard.: 139. 1971.
Pyrenophora teres f. maculata Smed.-Pet., The Royal Veterinary and Agricultural University Yearbook: 139. 1971.
Description and illustrations: Sivanesan (1987).
Materials examined: Denmark, from Hordeum vulgare (Poaceae), unknown date, V. Smedegaard-Petersen (ex-type culture of Py. teres f. maculata CBS 228.76). Japan, from H. vulgare (Poaceae), unknown date, S. Ito, CBS 281.31; unkown substrate and date, S. Ito, CBS 282.31; from H. vulgare (Poaceae), unknown date, Y. Nisikado, CBS 336.29 = MUCL 9687. Germany, Niedersachsen, Rotenburg, from H. vulgare (Poaceae), Jul. 1968, U.G. Schlösser, CBS 314.69. Hungary, Eszteragpuszta, from Hordeum vulgare leaf (Poaceae), unknown date, col. M. Csosz, dep. J. Bakonyi, CBS 123929; Taplanszentkereszt, from Hordeum vulgare leaf (Poaceae), unknown date, col. A. Tomcsanyi, dep. J. Bakonyi, CBS 123932.
Notes: In our phylogenetic analysis, isolates identified as Py. teres and Py. graminea were located in the same well-supported clade (100 % BS / 1 PP), suggesting that these represent the same species. In fact, Py. graminea has been recently considered as a subspecies of Py. teres (Simay 1992) since its morphology is similar and both species share the same host, Hordeum. Therefore, we propose the synonymy of both species under the name of Py. teres, which is well established and the most commonly used name for this taxon. Moreover, an authentic strain of Py. japonica was also located in this clade, supporting the synonymy of Py. japonica with Py. teres proposed by Crous et al. (1995), which was based on their morphological, molecular and pathological similarity.
Pyrenophora teres produces net blotch on barley worldwide, causing cell death and feeding off the nutrients released (Sivanesan, 1987, Louw et al., 1995, Campbell et al., 1999, Ellwood et al., 2012). Two different forms of Py. teres were recognised depending on the disease symptoms produced, i.e. Py. teres f. teres producing the net form of net blotch, characterised by elongated lesions where necrosis develops along leaf veins with occasional transverse striations, while Py. teres f. maculata produces the spot form of net blotch, typified by more ovoid lesions, often surrounded by a chlorotic zone (Campbell et al., 1999, Ellwood et al., 2012). However, both forms are considered the same species, Py. teres. This disease becomes systemic in plants infected from seed (Sivanesan 1987).
Pyrenophora variabilis Hern.-Restr. & Y. Marín, sp. nov. MycoBank MB829619. Fig. 58.
Fig. 58.
Pyenophora variabilis (ex-type CBS 127920). A–C. Conidiophores and conidia. D–N. Conidia. Scale bars = 10 μm; C applies to A–C; N applies to D–N.
Etymology: Name refers to the highly variable conidial morphology.
Hyphae hyaline to brown, branched, septate, smooth-walled to verrucose, 2.5–7 μm. Conidiophores arising in groups, septate, straight or flexuous, sometimes geniculate in upper part, simple, cell walls thicker than those of vegetative hyphae, semi- to macronematous, brown, not swollen at the base, up to 321 μm long, 5–10 μm wide. Conidiogenous cells smooth-walled, terminal or intercalary, proliferating sympodially, brown, subcylindrical, 18–27 × 8–10 μm. Conidia smooth-walled, straight to curved, cylindrical, subcylindrical, obclavate, obpyriform to subglobose, pale brown to brown, 1–3-distoseptate, 20–75 × 13–19.5 μm; hila flat, darkened, thickened, 4–7 μm. Chlamydospores and sexual morph not observed.
Typus: Canada, British Columbia, Agassiz Research Station, from leaves of Poa trivialis (Poaceae), Jul. 1972, J.D. Smith (holotype CBS H-23843, culture ex-type CBS 127920 = DAOMC 139513).
Notes: Pyrenophora variabilis was located on an independent branch far removed from the other species in the genus. It can be easily distinguished from all the species of the genus by its highly variable conidial morphology in size and shape, from cylindrical, subcylindrical or obclavate to subglobose or obpyriform. It was isolated from Poa trivialis (Poaceae) leaves in Canada, a common host of Pyrenophora spp.
Pyrenophora wirreganensis (Wallwork et al.) Y. Marín & Crous, comb. nov. MycoBank MB829621.
Basionym: Drechslera wirreganensis Wallwork et al., Mycol. Res. 96. 888. 1992.
Description and illustration: Wallwork et al. (1992).
Material examined: Australia, South Australia, from Hordeum sp. (Poaceae), unknown date, J. Bakonyi, CBS 109896.
Notes: Pyrenophora wirreganensis was introduced as Drechslera wirreganensis by Wallwork et al. (1992) to accommodate a specimen isolated from Hordeum in Australia. In the phylogenetic analysis, the strain CBS 109896 identified as Py. wirreganensis and isolated from the same host and location than the ex-type strain (IMI 348323), was located in an independent branch within the main clade representing the genus Pyrenophora. Therefore, this species is here transferred to the latter genus. For comparison with close species see notes of Py. sieglingiae.
Authors: Y. Marin-Felix, M. Hernández-Restrepo, P.W. Crous
Ramichloridium Stahel ex de Hoog, Stud. Mycol. 15: 59. 1977. Fig. 59.
Fig. 59.
Ramichloridium spp. A.Ramichloridium luteum on apple. B. Sporulating colonies of Ramichloridium luteum (ex-type CBS 132088) on PDA. C–G. Macronematous conidiophores with sympodially proliferating conidiogenous cells, which give rise to a conidium-bearing rachis with crowded and prominent scars. C.Ramichloridium apiculatum (ex-type CBS 156.59). D.Ramichloridium cucurbitae (ex-type CBS 132087). E, F.Ramichloridium luteum (ex-type CBS 132088). G.Ramichloridium punctatum (ex-type CBS 132090). H, I. Scanning electron micrographs of Ramichloridium luteum (ex-type CBS 132088) showing sympodial proliferation with scars on conidiogenous cells. J, K. Conidiophores reduced to conidiogenous cells. J.Ramichloridium cucurbitae (ex-type CBS 132087). K.Ramichloridium luteum (ex-type CBS 132088). L, M. Conidia. L.Ramichloridium apiculatum (ex-type CBS 156.59). M.Ramichloridium punctatum (ex-type CBS 132090). Scale bars: H = 2 μm; I = 1 μm; all others = 10 μm. Pictures C, L taken from Li et al. (2012); all others from Arzanlou et al. (2007).
Classification: Dothideomycetes, Dothideomycetidae, Capnodiales, Dissoconiaceae.
Type species: Ramichloridium apiculatum (J.H. Mill. et al.) de Hoog, basionym: Chloridium apiculatum J.H. Mill. et al. Ex-type strain: CBS 156.59 = ATCC 13211 = IMI 100716 = JCM 6972 = MUCL 15753 = MUCL 7991 = QM 7716.
DNA barcode (genus): LSU.
DNA barcodes (species): ITS, rpb2, tef1. Table 13. Fig. 60.
Table 13.
DNA barcodes of accepted Ramichloridium spp.
| Species | Isolates1 | GenBank accession numbers2 |
References | |||
|---|---|---|---|---|---|---|
| ITS | LSU | rpb2 | tef1 | |||
| Ramichloridium apiculatum | CBS 156.59T | EU041791 | EU041848 | MF951416 | – | Arzanlou et al. (2007), Videira et al. (2017) |
| R. cucurbitae | CBS 132087T | JQ622087 | JQ622095 | – | JQ622112 | Li et al. (2012) |
| R. luteum | CBS 132088T | EU329730 | JQ622099 | MF951417 | JQ622116 | Li et al., 2012, Videira et al., 2017 |
| R. malus | LQ73T | EF627452 | – | – | – | Zhang et al. (2007) |
| R. punctatum | CBS 132090T | JQ622086 | JQ622094 | – | JQ622111 | Li et al. (2012) |
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; LQ: collection not specified in the work in which it was introduced. T indicates ex-type strains.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial large subunit (28S)nrDNA; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial elongation factor 1-alpha gene.
Fig. 60.
RAxML phylogram obtained from the combined ITS (594 bp), LSU (761 bp), rpb2 (819 bp) and tef1 (470 bp) sequence alignment of all accepted species of Ramichloridium and related taxa. The tree was rooted to Parapenidiella tasmaniensis CBS 124991 and Stenella araguata CBS 105.75. The novelties proposed in this study are indicated in bold. RAxML bootstrap support (BS) values above 70 % and Bayesian posterior probability scores above 0.95 are shown at the nodes. GenBank accession numbers are listed in Table 13, Li et al., 2012, Videira et al., 2017 and Chen & Kirschner (2018). T and NT indicate ex-type and ex-neotype strains, respectively. TreeBASE: S23834.
Mycelium consisting of hyaline, subhyaline, pale brown, or brown, smooth-walled, septate, branched, submerged and aerial hyphae. Conidiophores straight to gently curved, subcylindrical, unbranched, smooth- and thick-walled, brown to dark brown, 0–3-septate, or sometimes reduced to intercalary conidiogenous cells. Conidiogenous cells integrated, terminal, pale to medium brown, or golden-brown, apical part subhyaline to pale brown, subcylindrical, rarely somewhat clavate, sometimes with irregular, nodulose swellings along the length of the conidiogenous cells, tapering towards apex, with sympodial proliferation, forming a rachis with slightly thickened and darkened, circular, somewhat protruding scars. Conidia solitary, aseptate, pale brown, smooth-walled to finely verrucose, clavate or oblong to ellipsoid, or obovate to obconical, apex obtuse or subobtuse, base truncate, with a conspicuous, slightly darkened and thickened, not refractive hilum.
Culture characteristics: Colonies after 1 mo at 25 °C in the dark flat, spreading, with sparse to moderate aerial mycelium, margins smooth and even, or lobate and feathery. On SNA grey olivaceous, pale mouse grey, or smoke-grey. On PDA olivaceous grey or smoke-grey; reverse olivaceous grey or iron-grey. On OA olivaceous grey or iron-grey; reverse iron-grey, with pale luteous pigment diffusing into agar. On MEA olivaceous green; reverse olivaceous black, often with a diffusing citron-yellow pigment.
Optimal media and cultivation conditions: MEA, OA, PDA and SNA at 25 °C under near-ultraviolet light.
Distribution: Africa, America and Asia.
Hosts: Aloe sp. (Aloaceae), Cucumis sativus, Cucurbita maxima (Cucurbitaceae), Malus domestica, Malus pumila and Pyrus pyrifolia (Rosaceae). Also isolated from soil.
Disease symptoms: Sooty blotch and flyspeck diseases.
Notes: Ramichloridium was initially introduced by Stahel (1937), designating R. musae as type species. However, the name was invalid due to the lack of Latin description or diagnosis. Subsequently, de Hoog (1977) validated this genus to include species with erect, dark conidiophores and predominantly aseptate conidia, designating as new type species R. apiculatum. In that study, 13 species were recognised in Ramichloridium, and subsequently more species were included. However, several molecular studies demonstrated that some of them belonged to different genera, i.e. Myrmecridium, Pachyramichloridium, Pleurothecium, Radulidium, Rhinocladiella and Zasmidium, and were subsequently transferred (Arzanlou et al., 2007, Cheewangkoon et al., 2009, Videira et al., 2017). Some species still need to be molecularly studied to confirm their phylogenetic position. In the present study, only five species have been demonstrated to belong to Ramichloridium, which is characterised by aseptate, pale brown, smooth-walled to finely verrucose, clavate or oblong to ellipsoid, or obovate to obconical conidia. Based on molecular data, R. indicum is proposed here as a new genus, Globoramichloridium indicum, and R. ducassei as a new combination in Zasmidium (see Zasmidium below). Moreover, R. apiculatum, R. cucurbitae and R. mali were located in a well-supported clade (100 % BS / 1 PP) without phylogenetic distance. Unfortunately, the only loci available and common in the three species are the ITS and LSU. Therefore, other loci should be sequenced to verify their status as separate species.
Species of Ramichloridium cause sooty blotch and flyspeck disease (SBFS) on members of the family Rosaceae, which produces blemishes on the epicuticular wax layer and is regarded as an economically serious disease (Wang et al. 2014). Ramichloridium cucurbitae and R. punctatum have been found as SBFS pathogens only in the USA (Li et al. 2012), while R. apiculatum, R. luteum and R. mali have been reported as causal agents of SBFS in China (Zhang et al., 2007, Li et al., 2012, Wang et al., 2014).
References: Arzanlou et al., 2007, Li et al., 2012 (morphology and phylogeny).
Globoramichloridium Y. Marín & Crous, gen. nov. MycoBank MB829622.
Etymology: Name reflects the characteristic globose conidia produced by this genus.
Illustration: Arzanlou et al. (2007).
Mycelium consisting of submerged and aerial hyphae; submerged hyphae smooth- and thin-walled, hyaline, with thin septa; aerial hyphae coarsely verrucose, olivaceous green, rather thick-walled, with thin septa. Conidiophores arising vertically from creeping hyphae at right angles, straight, unbranched, thick-walled, smooth-walled, dark brown, with up to 10 thin septa, often with inflated basal cells. Conidiogenous cells terminally integrated, smooth-walled, dark brown, sympodially proliferating, rachis straight or flexuose, geniculate or nodose, subhyaline; scars thickened and darkened, clustered at nodes. Microcyclic conidiation observed in culture. Conidia solitary, (0–)1-septate, not constricted at the septum, subhyaline to pale brown, smooth-walled or coarsely verrucose, rather thin-walled, broadly ellipsoidal to globose, with truncate base; hila conspicuous, slightly darkened, not thickened.
Culture characteristics: Colonies on MEA reaching 35 mm diam after 2 wks at 24 °C. Colonies velvety, rather compact, slightly elevated, with entire, smooth, whitish margins, dark olivaceous green in the central part.
Type species: Globoramichloridium indicum (Subram.) Y. Marín & Crous. Holotype: IMI 114625. Representative strain: CBS 171.96.
Notes: This genus is introduced to accommodate R. indicum, which differs from Ramichloridium spp. by its broadly ellipsoidal to globose, mostly 1-septate, smooth-walled or coarsely verrucose conidia, being clavate or oblong to ellipsoid, or obovate to obconical, aseptate, smooth-walled to finely verrucose in Ramichloridium. This genus is related to Dissoconium, but the latter can be easily distinguished by its percurrent and sympodial proliferation, and the ellipsoid to obclavate, smooth-walled conidia.
Globoramichloridium indicum (Subram.) Y. Marín & Crous, comb. nov. MycoBank MB829623.
Basionym: Chloridium indicum Subram., Proc. Indian Acad. Sci., Sect. B 42: 286. 1955.
Synonyms: Veronaea verrucosa Geeson, Trans. Brit. Mycol. Soc. 64: 349. 1975.
Veronaea indica (Subram.) M.B. Ellis, in Ellis, More Dematiaceous Hyphomycetes: 209. 1976.
Ramichloridium indicum (Subram.) de Hoog, Stud. Mycol. 15: 70. 1977.
Description and illustration: Arzanlou et al. (2007).
Material examined: Unknown collection details, Feb. 1996, L. Marvanová, CBS 171.96.
Notes: The strain examined and included in the phylogenetic analysis, CBS 171.96, was not derived from type material. However, the morphology of this strain fits perfectly with the morphology of the holotype IMI 114625 (de Hoog 1977). Therefore, CBS 171.96 is considered here as a representative strain, and we propose the new genus and combination based on the phylogenetic data derived from this isolate, as well as on the morphological differences observed.
Authors: Y. Marin-Felix, J.Z. Groenewald & P.W. Crous
Seifertia Partr. & Morgan-Jones, Mycotaxon 83: 348. 2002. Fig. 61.
Fig. 61.
Seifertia azalae. A, B. Disease symptoms caused on Rhododendron. C–G. Synnemata. H, I. Conidiogenous cells and conidia. J, K. Conidia. Scale bars: F = 100 μm; G = 50 μm; H–K = 10 μm.
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Melanommataceae.
Type species: Seifertia azaleae (Peck) Partr. & Morgan-Jones, basionym: Periconia azaleae Peck. Representative strain: DAOM 239136.
DNA barcode (genus): LSU.
DNA barcodes (species): LSU, tef1. Table 14.
Table 14.
DNA barcodes of accepted Seifertia spp.
| Species | Isolates1 | GenBank accession numbers2 |
References | ||
|---|---|---|---|---|---|
| LSU | ITS | tef1 | |||
| Seifertia azaleae | DAOM 239136 | EU030276 | – | – | Seifert et al. (2007) |
| CPC 35017 | MK540034 | MK539964 | MK540166 | Present study | |
| Sei. shangrilaensis | MFLUCC 16-0238T | KU954100 | – | KU954101 | Li et al. (2016b) |
CPC: Culture collection of Pedro Crous, housed at Westerdijk Fungal Biodiversity Institute; DAOM: Plant Research Institute, Department of Agriculture (Mycology), Ottawa, Canada; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Ria, Thailand. T indicates ex-type strains.
LSU: partial large subunit (28S) nrRNA gene; ITS: internal transcribed spacers and intervening 5.8S nrDNA; tef1: partial translation elongation factor 1-alpha gene.
Mycelium superficial or immersed, composed of branched, septate, smooth- and thin-walled, pale white to white or brown hyphae. Synnemata erect, simple, unbranched or very rarely branched, greenish when young, becoming dark brown to black at maturity, capitate at the apex. Conidiophores macronematous, synnematous, straight or slightly flexuous, unbranched or branched toward the upper region, cylindrical, septate, smooth- and thin-walled, hyaline to pale brown or olivaceous brown to brown. Conidiogenous cells mono- or polyblastic, integrated, terminal, determinate, cylindrical to subclavate or doliiform, smooth-walled. Conidia acropleurogenous, holoblastic, in simple or branched acropetal chains, dry, sometimes aggregated into slimy masses at the apex of the synnema, aseptate or very rarely 1-septate, smooth- and thin-walled, oblong, ellipsoidal, subglobose or fusiform, hyaline to subhyaline, pale brown or olivaceous (adapted from Partridge & Morgan-Jones 2002).
Culture characteristics: Colonies effuse, powdery or cottony to fairy fluffy, grey to dark brown.
Optimal media and cultivation conditions: MEA, OA and PDA at 25 °C.
Distribution: North America, Europe and Asia.
Hosts: Species of Rhododendron.
Disease symptoms: Bud and twig blight; Rhododendron bud blight disease.
Notes: Seifertia was introduced by Partridge & Morgan-Jones (2002) to accommodate Pycnostysanus azaleae based on morphological differences. Seifertia azaleae is morphologically similar to Sorocybe resinae. However, Partridge & Morgan-Jones (2002) decided to erect the new genus Seifertia since Sei. azaleae produces much narrower conidia and has minute denticles on the conidiogenous cells. Seifertia is characterised by erect, simple, and dark synnemata, macronematous conidiophores, holoblastic, integrated, terminal and determinate conidiogenous cells, and unicellular or very rarely 1-septate, pale brown or olivaceous conidia. This cosmopolitan genus occurs on azaleas and rhododendrons causing a disease known as Rhododendron bud blight disease, in which the flower buds die, and twig blight occurs. Infected buds are easily recognisable by the blackening of the bud and the development of numerous synnemata which appear as tiny black spines over the entire surface (Partridge and Morgan-Jones, 2002, Glawe and Hummel, 2006).
This genus, which is relatively poorly studied, was recently placed in Melanommataceae by Li et al. (2016b), when they introduced the second species belonging to Seifertia, Sei. shangrilaensis. However, the relation of Seifertia with Mycopappus and its synasexual morph Xenostigmina, which are foliar pathogens belonging to Melanommataceae, was demonstrated previously by Crous et al. (2009a).
References: Partridge & Morgan-Jones 2002 (morphology), Glawe & Hummel 2006 (pathogenicity), Seifert et al., 2007, Li et al., 2016b (morphology and phylogeny).
Authors: Y. Marin-Felix & P.W. Crous
Seiridium Nees, Das System der Pilze und Schwämme: 22. 1817. Fig. 62.
Fig. 62.
Seiridium spp. A–F. Disease symptoms on Cupressaceae hosts. A–C. Flagging of branches. D. Trunk canker with gummosis. E. Branch canker. F. Conidiomata. G–I.Seiridium pezizoides (CBS 145115). G, H. Acervuli on Cupressaceae sp. I. Conidial masses on artificial media. J, K. Conidiophores and conidiogenous cells. J.Seiridium neocupressi (CBS 142625). K.Seiridium eucalypti (CBS 343.97). L–R. Conidia. L.Seiridium cardinale (CBS 909.85). M.Seiridium spyridicola (CBS 142628). N.Seiridium unicorne (CBS 538.82). O.Seiridium neocupressi (CBS 142625). P.Seiridium eucalypti (CBS 343.97). Q.Seiridium kartense (CBS 142629). R.Seiridium pezizoides (CBS 145115). S–U.Seiridium cupressi (IMI 40096). S, T. Ascomata. U. Ascospores. Scale bars: F = 2 mm; G, H = 50 μm; J–U = 10 μm. Pictures J–U taken from Bonthond et al. (2018).
Synonym: Pestalotia De Not., Mém. Reale Accad. Sci. Torino 3: 80. 1841.
Additional synonyms in Bonthond et al. (2018).
Classification: Sordariomycetes, Xylariomycetidae, Xylariales, Sporocadaceae.
Type species: Seiridium marginatum Nees, Syst. Pilze (Würzburg): 23. 1817. Neotype designated by Shoemaker et al. (1966): K 200376. Epitype and ex-epitype culture designated by Jaklitsch et al. (2016): WU 33575, CBS 140403.
DNA barcode (genus): ITS.
DNA barcodes (species): ITS, rpb2, tef1, tub2. Table 15. Fig. 63.
Table 15.
DNA barcodes of accepted Seiridium spp.
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; MFLU and MFLUCC: Herbarium and culture collection of Mae Fah Luang University, Chiang Rai, Thailand, respectively; PHSI: from Liu et al. (2007). T, ET, GT and R indicate type or ex-type, ex-epitype, ex-generic type and reference strains, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial β-tubulin gene.
Fig. 63.
A–D. Boxplots of conidial measurement data in μm from S. unicorne and other Cupressaceae pathogens. The boxes show the lower and upper quantiles and whiskers extend to 1.5x the interquartile range. Except for the new epitype all measurements are adapted from Bonthond et al. (2018). The ex-epitype strain (CBS 143871), holotype (IMI 5816) and reference strain (CBS 538.82) of S. unicorne are highlighted in blue. E. The best Maximum Likelihood (ML) tree based on four loci (ITS: 616 bp, rpb2: 802 bp, tef1: 633 bp, tub2: 809 bp). Nodes are labelled with ML bootstrap values (BS > 49 %)/Bayesian posterior probabilities (PP > 0.49) using the same model selection, settings and software as in Bonthond et al. (2018). Strains are displayed by number, host and country of collection. GenBank accession numbers are listed in Bonthond et al. (2018) and in Table 15. T, ET, GT and R indicate ex-type, ex-epitype, ex-generic type and reference strains, respectively. TreeBASE: S23390.
Ascomata perithecial, immersed to semi-erumpent, depressed, globose to pyriform, scattered or confluent, with central ostiole; necks slightly papillate, black, periphysate; ascomatal wall dark brown, pseudoparenchymatous. Paraphyses hyaline, smooth-walled, filiform. Asci cylindrical, 8-spored, unitunicate, thin-walled, stipitate, with an apical amyloid ring. Ascospores cylindrical-oblong, euseptate, septa often thicker than the wall, yellow- to dark brown, guttulate. Conidiomata acervuloid to pycnidioid, semi-immersed to erumpent, uni- to plurilocular, brown or black, glabrous, dehiscing by irregular splits in the upper wall. Conidiophores lining the cavity of the conidioma, septate and sparsely branched at the base, or reduced to conidiogenous cells, hyaline, smooth-walled. Conidiogenous cells discrete, integrated, ampulliform to lageniform or subcylindrical, hyaline, smooth-walled, proliferating percurrently at the apex. Conidia fusiform, euseptate (septal pores present or not), end cells hyaline, median cells dark brown to brown, wall thick, smooth or with striations, constricted at septa or not; apical cell with single or multiple, unbranched or branched appendages; basal cell with or without a centric, unbranched or sometimes branched appendage (adapted from Bonthond et al. 2018).
Culture characteristics: Colonies on PDA circular to irregular, reaching 12–68 mm diam after 14 d at 22 °C, mostly flat, in some cultures elevated at margins, often white coloured or with other colours, sporulation rare, with pycnidioid conidiomata. On SNA circular to irregular to rhizoid, reaching 20–54 mm diam after 14 d at 22 °C, mostly flat, white coloured, with moderate to abundant aerial mycelium, sporulation for some species within and others after 2 wk, sporodochia often compact and scattered.
Optimal media and cultivation conditions: Colonies grow well on CMA, MEA, PDA and SNA at 22 °C. Most species sporulate on SNA and some species on CMA, MEA or PDA as well.
Distribution: Worldwide.
Hosts: A diversity of gymnosperms and angiosperms. The genus is most well-known from members of Cupressaceae.
Disease symptoms: Cankers on stems and twigs.
Notes: Pestalotia was introduced in 1841 and is similar to Seiridium (1817), one of the older names in the Sporocadaceae. The genus has been subjected to many rearrangements (reviewed in Sutton 1969) which eventually resulted in the genus accommodating only the type species, Pe. pezizoides. One of the important characters used to separate Pestalotia from the related genera Pestalotiopsis and Truncatella was the production of 5-septate (or 6-celled) conidia. However, this morphology is typical for Seiridium as well, and for this reason it was speculated that Pestalotia and Seiridium could be congeneric (Maharachchikumbura et al. 2014). A fresh collection of Pe. pezizoides from Vitis vinifera collected in the USA was recently obtained (Table 1), which matches in all respects with the type of Pe. pezizoides. DNA sequence data generated here confirm that Pe. pezizoides clusters within Seiridium (Fig. 63). Therefore, Pestalotia is reduced to synonymy with Seiridium. For Seiridium, the here synonymised Pestalotia and related genera, not only the number but also the type of conidial septation has been a commonly reported descriptor. Different authors have interpreted the appearance of the cell walls as either distoseptate (e.g. Nag Raj 1993) or euseptate (e.g. Sutton 1980). Although when examined by light microscopy, conidia can appear as distoseptate, electron microscopic studies on S. cupressi (Roberts & Swart 1980) and S. pezizoides (Griffiths & Swart 1974) have shown that the conidial cell walls are differentiated into multiple zones but arise from a single layered cell wall and are thus euseptate. Since the sexual morph is known for only a few species, the taxonomy in Seiridium has been based mainly on asexual morphology. However, the generic type (S. marginatum) is one of the exceptions where both morphs have been characterised. This species was re-described and epitypified by Jaklitsch et al. (2016), who also provided detailed illustrations of sexual and asexual morphology.
References: Nag Raj 1993 (morphology), Danti & Della Rocca 2017 (pathogenicity), Bonthond et al. 2018 (morphology and phylogeny),
Seiridium cupressi (Nattrass et al.) Bonthond, Sand.-Den. & Crous, comb. nov. MycoBank MB830554.
Basionym: Rhynchosphaeria cupressi Nattrass et al., Trans. Brit. Mycol. Soc. 46: 103. 1963.
Synonyms: Cryptostictis cupressi Guba, Monograph of Monochaetia and Pestalotia: 47. 1961. Nom. inval. Art. 40.3 (Shenzhen).
Lepteutypa cupressi (Nattrass et al.) H.J. Swart, Trans. Brit. Mycol. Soc. 61: 79. 1973.
Seiridium cupressi (Guba) Boesew, Trans. Brit. Mycol. Soc. 80: 545. 1983. Nom. inval. Art. 40.3 (Shenzhen).
Description: Sexual morph Nattrass et al. (1963). Asexual morph Bonthond et al. (2018).
Known distribution: Africa (Kenya, Uganda) and Europe (Greece).
Typus: Africa, Kenya, on Cupressus macrocarpa, July 1954, R.M. Nattrass (holotype of Rhynchosphaeria cupressi IMI 56917); from cankers in branches of Cupressus macrocarpa, 1949, D.R. Jones [epitype of Rhynchosphaeria cupressi designated here IMI 52254, MBT386544 (dried culture), culture ex-epitype CBS 224.55].
Additional materials examined: Europe, Greece, from Cupressus sp., A. Graniti, CBS 122616 = CMW 1646. Africa, Kenya, non-pathogenic isolate from Cupressus sp., collection data unknown, CBS 320.51; on Cupressus macrocarpa, July 1948, R.M. Nattrass, IMI 37158; on Cupressus macrocarpa, Dec. 1949, R.M. Nattrass, IMI 40096; from cankers in branches of Cupressus forbesii, 1949, D.R. Jones, IMI 52255 (dried culture); CBS 225.55.
Notes: This species has been a source of confusion since its introduction (Guba 1961). Bonthond et al. (2018) showed that Guba’s diagnosis included three different species (S. cancrinum, S. cupressi and S. kenyanum) and selected an epi- and lectotype for Cryptosticis cupressi. The latter name, however, was invalidly published (article 40.1). Nattrass et al. (1963) re-examined the material from Guba (1961) and synonymised C. cupressi with Monochaetia unicornis. Despite noting small morphological differences between the asexual stage of R. cupressi and the type of M. unicornis (i.e. smaller and slender conidia), they were not able to confirm the exogenous origin of the basal appendage, which was the main argument for Guba (1961) to place the species in Cryptostictis instead of Monochaetia. In the same study, Nattrass et al. (1963) described the sexual morph of M. unicornis as Rhynchosphaeria cupressi based on three specimens: IMI 37158, IMI 40096 and the holotype IMI 56917. Bonthond et al. (2018) examined each of these specimens but incorrectly cited the holotype as IMI 37158. While only the sexual morph was found in these materials, the original description from Nattrass et al. (1963) includes drawings, photographs and measurements of the conidia. These measurements (22–32 × 6–9.5 μm) fall perfectly within the range documented for the lineage currently assigned to S. cupressi (Guba) Boesew. (18–36 × 5–11.5 μm) (Bonthond et al. 2018). Consequently, being the oldest valid name for this lineage and in accordance with the rule of priority Ry. cupressi is recombined in Seiridium, as S. cupressi (Nattrass et al.) Bonthond, Sand.-Den. & Crous, and an epitype is designated (IMI 52254). The similar species S. cancrinum and S. unicorne show smaller conidia (20–30.5 μm and 19–28 μm, respectively), whereas conidia of S. kenyanum are considerably larger (24–39 μm).
Seiridium pezizoides (De Not.) Crous, comb. nov. MycoBank MB828021. Fig. 64.
Fig. 64.
Seiridium pezizoides (CBS 145115). A–D. Colony morphology in 90-mm-diam Petri dishes after 10 d at 22 °C on MEA, SNA, PDA and CMA, respectively. E–K. Conidiomata on Vitis vinifera. L, M. Conidiophores. N. Conidia. Scale bars: E = 1 mm; F–K = 100 μm; L–N = 10 μm.
Basionym: Pestalotia pezizoides De Not., Mém. Reale Accad. Sci. Torino 2, 3: 80. 1839.
Caulicolous. Isolated from branches of Vitis vinifera. On the host (described in more detail by Nag Raj 1993): Conidiomata irregularly scattered over the surface, gregarious to confluent, discoid to cupulate and occasionally globose, erumpent from tissue, acervular to sporodochial, occasionally with aerial mycelium, black to brown, (300–)350–500(–650) μm. On SNA: Conidiophores tightly aggregated in the conidioma, cylindrical, irregularly branched, hyaline or pale brown at the base, smooth- and thin-walled. Conidiogenous cells discrete, hyaline, cylindrical, smooth- and thin-walled. Conidia lunate to falcate, often curved, 5-septate, not striate, bearing a basal and two or more apical appendages, euseptate with pores sometimes visible, (24–)28–33.5(–38.5) × (6–)7–8(–9) μm, mean ± SD = 30.7 ± 2.8 × 7.5 ± 0.4 μm; basal cell obconic with truncate base, hyaline, smooth-walled, bearing marginal frills, 4–7 μm; four median cells pale brown, smooth-walled, cylindrical to doliiform; second cell from base 3.5–8 μm; third cell 3.5–7 μm; fourth cell 3.5–6.5 μm; fifth cell 3.5–7.5 μm; apical cell conical, hyaline, smooth-walled, 4.5–8.5 μm long; apical appendages single or multiple, centric, branched or unbranched, 8.5–27 μm; basal appendage single, cylindrical, centric, occasionally branched, 5.5–14 μm.
Culture characteristics: Colonies on PDA circular, reaching 34–37 mm diam after 10 d at 22 °C, flat, olivaceous to luteous in the centre, white to brown at the margins, with abundant aerial mycelium at the margins, not sporulating within 10 d. On CMA circular, reaching 39–41 mm diam after 10 d at 22 °C, flat at centre and margins, dark brown to black, without aerial mycelium, not sporulating within 10 d. On MEA circular to slightly irregular, reaching 25–27 mm diam after 10 d at 22 °C, flat, olivaceous to pale green, with a white outer ring, with moderate aerial mycelium, massive spore production in the centre. On SNA circular, reaching 31–33 mm diam after 10 d at 22 °C, raised in the centre, flat at the margins, with moderate aerial mycelium, no sporulation within 10 d.
Distribution: Europe (France, Italy), USA.
Typus: Italy, near Mailand, twig of Vitis vinifera (Vitaceae), leg. Oct. 1838, De Notaris, holotype, RO.
Additional material examined: USA, Virginia, Charlottesville, from a complex hybrid of Vitis æstivalis × Vitis cinerea × Vitis vinifera, 2018, L. Morton, CBS 145115 = CPC 35011.
Notes: After several rearrangements and the introduction of the genera Pestalotiopsis and Truncatella (see Sutton 1969 and references therein), the generic type Pe. pezizoides was the only remaining species in Pestalotia. A specimen of Pe. pezizoides (IMI 83642, from branches of Vitis vinifera, Italy, non-type) was examined and redescribed by Sutton (1980). A more detailed description was provided by Nag Raj (1993), based on several materials, including this specimen. The present study is the first to provide DNA sequence data on this species. We sequenced four loci (ITS, rpb2, tef1 and tub2) and included Pe. pezizoides in an updated phylogeny of Seiridium (Fig. 63) which supports the conclusion that Pestalotia and Seiridium are congeneric. Consequently, the species is transferred to Seiridium as S. pezizoides. The S. pezizoides strain (CBS 145115 = CPC 35011) that was included in this analysis conforms morphologically with the description and was isolated from the same host, Vitis. However, since the specimen was collected in Virginia (USA) and the holotype is from Italy, it is not suitable for epitypification. The phylogeny generated here suggests that S. pezizoides is most closely related to S. rosarum (Rosa canina, Italy).
Seiridium unicorne (Cooke & Ellis) B. Sutton, Mycol. Pap. 138: 74. 1975. Fig. 65.
Fig. 65.
Seiridium unicorne (ex-epitype CBS 143871). A–D. Colony morphology in 90-mm-diam Petri dishes after 10 d at 22 °C on MEA, SNA, PDA and CMA, respectively. E. Symptoms on naturally infected host. F, G. Conidiomata on artificially infected Cupressaceae sp. H. Sporulation on PDA. I. Conidioma on SNA partially immersed in agar. J–O. Conidiophores and conidia. P. Conidia. Scale bars: F–H = 100 μm; I–P = 10 μm.
Basionym: Pestalotia unicornis Cooke & Ellis, Grevillea 7: 6. 1878, as "Pestalozzia".
Synonym: Monochaetia unicornis (Cooke & Ellis) Sacc. & D. Sacc., Syll. Fung. 18: 485. 1906.
Caulicolous. Most commonly isolated from cankers on branches of species from Cupressaceae. Conidiomata on PDA numerous, sporodochial, globose or clavate, mostly solitary, erumpent from agar, partially immersed in mycelium, producing large black spore masses; on SNA, sporodochial, mostly aggregated, erumpent from agar, producing large black spore masses. On SNA: Conidiophores septate, cylindrical, irregularly branched, hyaline or brown, thin-walled, 22–68 μm long, ex-epitype: 22–50 μm long. Conidiogenous cells discrete, hyaline, cylindrical, smooth- and thin-walled, 3.5–29.5 × 1.5–3.5 μm, ex-epitype: 16.2–28.9 × 1.7–3.5 μm, proliferating percurrently, with visible collarettes and minute periclinal thickenings. Conidia lunate to falcate, curved, 5-septate, rarely 4- or 6-septate, not striate, bearing two appendages, euseptate with no visible pores, (19–)22.5–26.5(–28) × (6.5–)7.5–8.5(–9.5) μm, mean ± SD = 24.5 ± 1.8 × 7.9 ± 0.5 μm, ex-epitype: (19–)22.5–26.5(–28) × (6.5–)7.5–8.5(–9) μm, mean ± SD = 24.4 ± 1.9 × 7.8 ± 0.4 μm; basal cell obconic with a truncate base, hyaline, walls smooth, bearing minute marginal frills, 2.5–9.5 μm, ex-epitype: 2.5–6 μm (n = 119); four median cells colour varying from pale to dark brown, smooth-walled, cylindrical to doliiform; second cell from base 3.5–6 μm (n = 152); third cell 3–5.5 μm; fourth cell 3–5.5 μm; fifth cell 3–5.5 μm, ex-epitype: second cell from base 3.5–6 μm; third cell 3–5.5 μm; fourth cell 3–5 μm; fifth cell 3–5.5 μm; apical cell conical, hyaline, smooth-walled, 2–5.5 μm long, ex-epitype: 2–5.5 μm long; apical appendage single, mostly centric, 5–10 μm, ex-epitype: 5–9.5 μm; basal appendage single, cylindrical, mostly excentric, 2.7–7.1 μm, ex-epitype: 4–6.5 μm (adapted from Bonthond et al. 2018).
Culture characteristics: Colonies on PDA irregular, reaching 65–68 mm diam after 14 d at 22 °C, slightly umbonate, colour citrine, olivaceous buff to olivaceous, with compact aerial mycelium on the surface, abundant sporulation surrounding centre and at the margins of the colony. On CMA circular or irregular, reaching 58–59 mm diam after 14 d at 22 °C, flat at the centre and margins, citrine to olivaceous coloured, with moderate aerial mycelium on the surface, sporulating abundantly. On MEA irregular, reaching 35–40 mm diam after 14 d at 22 °C, flat to crateriform, slightly sunk into the agar, buff to olivaceous coloured at the centre becoming white at the margins, with dense mycelium on the surface, sporulating near the centre. On SNA circular to slightly irregular, reaching 20–21 mm diam after 14 d at 22 °C, umbonate, with moderate aerial mycelium, sporulation abundant between centre and margins.
Distribution: New Zealand, South Africa and USA.
Typus: USA, Maryland, Pocomoke City, 38.072952N, 75.555852W, from branch canker of Cupressus sp., 2017, S.A. Krueger-Hadfield (epitype of Pestalotia unicornis designated here CBS H-23739, MBT383596, cultures ex-epitype CBS 143871 = CPC 34650, CBS 143872 = CPC 34649, CBS 143873 = CPC 34651); New Jersey, from Chamaecyparis thyoides, 1878, J.B. Ellis (holotype of Pestalotia unicornis IMI 5816).
Additional materials examined: New Zealand, from Cryptomeria japonica, 1981, H.J. Boesewinkel (CBS H-23151 reference specimen, culture CBS 538.82 = CPC 23783 = NBRC 32684). South Africa, from Cupressus sempervirens, 1999, I. Barnes (culture CBS 120306 = CMW 5596).
Notes: Seiridium unicorne (basionym: Pestalotia unicornis) is the earliest described cypress pathogen (Cooke & Ellis 1878) of Seiridium and was isolated from “cedar wood” collected in New Jersey (USA). The host was later identified by W.W. Wagener as Chamaecyparis thyoides, as indicated on the holotype label (see Bonthond et al. 2018). The genus Seiridium accommodates multiple species infecting Cupressaceae and S. unicorne has traditionally been regarded as a mild pathogen but capable of infecting a broad range of hosts, including plant species beyond the Cupressaceae (Guba 1961). The holotype (IMI 5816) was obtained and examined during a preceding study (Bonthond et al. 2018) and found to be limited to two microscope slides. Therefore, the selection of an epitype for S. unicorne is important to consolidate a stable taxonomic concept for this taxon. Given the occurrence of related species which are pathogenic on the same hosts (i.e. S. cancrinum, S. cardinale, S. cupressi and S. neocupressi) the availability of ex-epitype DNA sequence data provides a valuable reference for the identification of future collections. The specimen we introduce here as epitype (CBS H-23739) was collected from necrotic lesions of a Cupressus sp. in Maryland, USA and matches morphologically in all respects with the holotype (IMI 5816) and the reference strain (CBS 538.82) of S. unicorne. Furthermore, in the four-locus phylogeny (Fig. 63E) the ex-epitype strain clusters under a fully supported node in the clade that was assigned to S. unicorne based on morphology (Bonthond et al. 2018). Conidial measurements strongly overlap between the selected epitype, holotype and reference strain (Fig. 63A–D), although the median width of the epitype being slightly narrower in comparison to the holotype and reference strain. Measurements of basal and apical appendages and distributions of those measurements are highly similar between epitype and reference strain. For both appendages, the variation in measurements is higher for the holotype compared to the reference strain and the epitype, which, however, likely results from the age and condition of the material as we observed that conidial appendages from the holotype were often damaged.
Authors: G. Bonthond, M. Sandoval-Denis, S.A. Krueger-Hadfield, L. Morton, C. Ambers & P.W. Crous
Septoriella Oudem., Ned. kruidk. Archf, ser. 2, 5: 504. 1889. Fig. 66.
Fig. 66.
Septoriella spp. A. Conidiomata on OA of Septoriella hirta (ex-neotype CBS 536.77). B. Conidiomata in vivo of Septoriella phragmitis (ex-epitype CBS 140065). C. Conidial cirrhus of Septoriella phragmitis (ex-epitype CBS 140065). D. Conidioma of Septoriella hirta (ex-neotype CBS 536.77). E, F. Section through conidiomata of Septoriella hirta (ex-neotype CBS 536.77). G–I. Conidiogenous cells. G.Septoriella oudemansii (ex-type CBS 138012). H, I.Septoriella phragmitis (ex-epitype CBS 140065). J. Developing conidia of Septoriella hirta (ex-neotype CBS 536.77). K–O. Conidia. K.Septoriella hirta (ex-neotype CBS 536.77). L.Septoriella oudemansii (ex-type CBS 138012). M.Septoriella poae (ex-type CBS 136766). N, O.Septoriella phragmitis (ex-epitype CBS 140065). Scale bars: D, E = 100 μm; F = 50 μm; all others = 10 μm. Pictures A–F, H–K, N, O taken from Crous et al., 2015a, Crous et al., 2015b, Crous et al., 2015c; G, L from Crous et al. (2014b).
Synonyms: Allophaeosphaeria Ariyaw. et al., Fungal Diversity 72: 137. 2015.
Poaceicola W.J. Li et al., Mycosphere 6: 696. 2015.
Vagicola Chethana & K.D. Hyde, Fungal Diversity 75: 113. 2015.
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Phaeosphaeriaceae.
Type species: Septoriella phragmitis Oudem. Epitype and ex-epitype strain designated by Crous et al. (2015a): CBS H-22281, CBS 140065.
DNA barcode (genus): LSU. Fig. 28.
DNA barcodes (species): ITS, rpb2, tef1, tub2. Table 16. Fig. 67.
Table 16.
DNA barcodes of accepted Septoriella spp.
| Species | Isolates1 | GenBank accession numbers2 |
References | |||
|---|---|---|---|---|---|---|
| ITS | rpb2 | tef1 | tub2 | |||
| Septoriella agrostina | MFLU 18-0113HT | MG828945 | – | MG829227a | – | Wanasinghe et al. (2018) |
| Sep. allojunci | MFLUCC 15-0701T | KU058718 | – | MG520935a | – | Li et al., 2015, Phookamsak et al., 2017 |
| Sep. artemisiae | MFLUCC 17-0693T | MG828929 | – | – | – | Wanasinghe et al. (2018) |
| Sep. arundinicola | MFLU 16-0225HT | MG828946 | MG829261 | MG829228a | – | Wanasinghe et al. (2018) |
| Sep. arundinis | MFLUCC 15-0702T | KU058716 | – | MG520921a | – | Li et al., 2015, Phookamsak et al., 2017 |
| Sep. bromi | MFLUCC 13-0739T | KU058717 | – | – | – | Li et al. (2015) |
| Sep. chlamydospora | MFLUCC 15-0177T | KU163658 | – | – | – | Hyde et al. (2018) |
| Sep. dactylidicola3 | MFLUCC 14-0002T | – | – | – | – | – |
| Sep. dactylidis | MFLU 15-2720HT | KU163657 | – | – | – | Jayasiri et al. (2015) |
| Sep. elongata | MFLUCC 12-4444T | KM491546 | – | – | – | Li et al. (2015) |
| Sep. forlicesenica | MFLUCC 15-0470T | KX926422 | KY131966 | MG520922a | – | Phookamsak et al., 2017, Thambugala et al., 2017 |
| Sep. garethjonesii | MFLUCC 15-0469T | KX926425 | KX898363 | MG520923a | – | Phookamsak et al., 2017, Thambugala et al., 2017 |
| Sep. germanica | CBS 145372T | MK539965 | MK540096 | MK540159b | MK540174 | Present study |
| Sep. hibernica | CBS 145371T | MK539966 | MK540097 | – | – | Present study |
| Sep. hirta | CBS 536.77ET | KR873249 | KR873324 | – | – | Crous et al. (2015a) |
| Sep. hollandica | CBS 145374T | MK539967 | MK540098 | MK540160b | MK540175 | Present study |
| Sep. hubertusii | CBS 338.86T | KF251230 | KF252235 | – | KF252717 | Quaedvlieg et al. (2013) |
| Sep. italica | MFLUCC 13-0267T | KX926421 | KX891169 | MG520924a | – | Phookamsak et al., 2017, Thambugala et al., 2017 |
| Sep. leuchtmannii | CBS 459.84IsoT | KF251188 | KF252195 | KF253144b | KF252682 | Quaedvlieg et al. (2013) |
| Sep. muriformis | MFLUCC 13-0277T | KX926415 | KX863710 | – | – | Thambugala et al. (2017) |
| Sep. neoarundinis | MFLUCC 15-0027T | KY706139 | – | MG520936a | – | Phookamsak et al., 2017, Thambugala et al., 2017 |
| Sep. neodactylidis | MFLUCC 13-0618T | KP744432 | – | – | – | Liu et al. (2015) |
| Sep. oudemansii | CBS 138012T | KR873250 | – | – | – | Crous et al. (2015a) |
| Sep. phragmitis | CBS 140065ET | KR873251 | – | – | – | Crous et al. (2015a) |
| Sep. poae | CBS 136766T | KJ869111 | KJ869233 | – | – | Crous et al. (2014b) |
| Sep. pseudophragmitis | CBS 145417T | MK560161 | MK559450 | MK559452b | MK559451 | Present study |
| Sep. rosae | MFLU 18-0114HT | MG828948 | – | MG829230a | – | Wanasinghe et al. (2018) |
| Sep. subcylindrospora | MFLUCC 13-0380T | KT314184 | – | – | – | Ariyawansa et al. (2015a) |
| Sep. tridentina | MFLUCC 15-0475T | KX926424 | KX891171 | – | – | Thambugala et al. (2017) |
| Sep. vagans | CBS 604.86 | KF251193 | KF252200 | KF253149b | KF252687 | Quaedvlieg et al. (2013) |
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; MFLU and MFLUCC: Herbarium and culture collection of Mae Fah Luang University, Chiang Rai, Thailand, respectively. T, ET, HT and IsoT indicate ex-type, ex-epitype, holotype and ex-isotype strains, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial β-tubulin gene. a and b in tef1 column indicate the primers used in sequencing: a: EF1-983F, EF1-2218R, b: EF1-728F, EF-2.
Only LSU and SSU sequences available: KY657264 and KY657265, respectively (Thambugala et al. 2017).
Fig. 67.
RAxML phylogram obtained from the combined ITS (580 bp), LSU (849 bp) and rpb2 (1083 bp) sequence alignment of all accepted species of Septoriella. The tree was rooted to Neostagonospora caricis CBS 135092 and Neostagonospora elegiae CBS 135101. The novelties proposed in this study are indicated in bold. RAxML bootstrap support (BS) values above 70 % and Bayesian posterior probability scores above 0.95 are shown at the nodes. GenBank accession numbers of LSU are listed in Fig. 28, and of the other loci in Tables 7 and 16. T, ET, HT and IsoT indicate ex-type, ex-epitype, holotype and ex-isotype strains, respectively. TreeBASE: S23834.
Ascomata ostiolate, solitary to gregarious, immersed to semi-immersed or superficial, broadly ellipsoidal to globose, subglobose, or obpyriform, brown to dark brown or black, smooth-walled, coriaceous, uni- to biloculate; necks central, flush to papillate, brown to dark brown or black, with or without periphyses, rarely comprising short, hyaline setae; ascomatal wall thin-walled, outer layers composed of brown to dark brown or blackish cells of textura angularis, inner layers composed of brown cells of textura prismatica, or of hyaline or brown cells of textura angularis, rarely composed of hyaline gelatinous cells. Hamathecium composed of numerous, 1–3 μm wide, filiform to broadly cylindrical, septate, cellular pseudoparaphyses, or lacking pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical, broadly cylindrical, cylindrical-fusiform, cylindrical-clavate, clavate, or broadly clavate, pedicellate, apically rounded with an ocular chamber. Ascospores overlapping, 1–3-seriate, hyaline to yellowish brown or pale brown, brown, golden brown, or reddish brown, narrowly or broadly fusiform or oblong to narrowly oblong, straight or slightly curved, smooth-walled or echinulate, multi-septate, with transverse septa, with or without longitudinal septa, sometimes with enlarged medium cells, constricted or not at septa, conical or obtuse, rounded ends, with or without sheath. Conidiomata pycnidial, solitary or aggregated, immersed to semi-immersed, globose to subglobose, unilocular, pale brown to brown or dark brown, with central, papillate, circular ostiole; conidiomatal wall of brown cells of textura angularis, rarely of textura oblita, inner layers becoming hyaline. Conidiophores lining the inner cavity, reduced to conidiogenous cells, invested in mucus. Conidiogenous cells ampulliform to lageniform, hyaline, smooth-walled, proliferating via inconspicuous percurrent proliferations near apex. Conidia cylindrical to subcylindrical, fusiform, or subfusiform, apex obtuse to subobtuse, base truncate, straight or curved, euseptate, pale brown to brown, thin-walled, smooth-walled or minutely verruculose, bearing mucoid appendages at both ends (type H sensu Nag Raj 1993) (description of asexual morph adapted from Crous et al. 2015a).
Culture characteristics: Colonies with moderate to abundant aerial mycelium, variable in colour, circulate or lobate. On PDA surface white to iron-grey or grey olivaceous, greyish white, pale olivaceous grey, or dull green margins, mouse grey in the middle and pale mouse grey at the center; reverse grey, olivaceous grey, dull green, buff to dark brown, olivaceous to pale brown or black, brown or olivaceous brown to black. On MEA surface white, dirty white, or white to pale yellow or iron-grey; reverse yellow, umber, buff, or dark grey to black.
Optimal media and cultivation conditions: CMA, MEA, OA and PDA at 25 °C.
Distribution: Mostly Europe, but also reported in Asia and North America.
Hosts: Mostly saprophytes of grasses (Poaceae), including Arundo spp., Agrostis stolonifera, Bromus sterilis, Calamagrostis spp., Dactylis glomerata, Elymus glaucus, E. repens, Poa sp., Phragmites spp. and Setaria verticillata. Also found on Rosa canina (Rosaceae) and Juncus sp. (Juncaceae), and others hosts not molecularly corroborated. Septoriella hirta is considered and important secondary pathogen of grasses, including Agropyron spp., Bromus spp., Dactylis glomerata, Festuca spp., Poa spp., Stipa spp., and Triticum spp., among others.
Disease symptoms: Secondary foot rot and rot of mature straw. Discoloured culms and predisposition of the plant to premature collapse.
Notes: Septoriella was considered an asexual genus characterised by pycnidial, unilocular conidiomata, and cylindrical to fusoid, euseptate conidia bearing mucoid appendages at both ends (Crous et al. 2015a). However, in our phylogenetic studies based on ITS and LSU, and on the combined dataset, the ex-type strains of the sexual genera Allophaeosphaeria, Poaceicola and Vagicola were located in the clade representing the genus Septoriella. Therefore, these genera are synonymised with Septoriella in the present study.
Allosphaeosphaeria was recently introduced by Liu et al. (2015) to incorporate two new saprophytic species found on Dactylis glomerata from Italy, i.e. Al. dactylidis and Al. muriformia, the latter designated as type species. These species only produce the sexual morph characterised by ascospores with transverse and longitudinal septa, and a gelatinous sheath. Subsequently, three other new species were introduced in the genus, i.e. Al. clematidis, Al. cytisi and Al. subcylindrospora. Allosphaeosphaeria clematidis and Al. cytisi only produce the sexual morph, while Al. subcylindrospora only produces an asexual morph. The morphology of this asexual morph fits perfectly in the description of Septoriella, corroborating the synonymy proposed in the present study based on the phylogenetic data. Allosphaeosphaeria clematidis was recently excluded from the genus and transferred to the new genus Embarria (Wanasinghe et al. 2018). Moreover, in our phylogenetic studies, Al. cytisi formed an independent lineage in Phaeosphaeriaceae far from the clade representing Septoriella. Therefore, a new genus is proposed to accommodate this species.
The genus Poaceicola was introduced by Li et al. (2015) to accommodate Phaeosphaeria elongata and two new species, i.e. Po. arundinis and Po. bromi. The two latter species are characterised by the production of an asexual morph similar to Septoriella. Poaceicola elongata produces a sexual morph characterised by ascospores with transverse septa. The presence of only transverse septa could be a morphologic difference from Allophaeosphaeria. However, seven more new species have been included in the genus, including one species presenting ascospores with transverse and longitudinal septa, Po. arundinis, demonstrating that the longitudinal septation of the ascospores is not phylogenetically informative in these genera.
Vagicola was recently introduced by Ariyawansa et al. (2015a), in the same year as the other two genera. Ariyawansa et al. (2015a) raised the subgenus Vagicola (Shoemaker & Babcock 1989) to generic rank to accommodate Phaeosphaeria vagans, a species characterised by a sexual morph similar to the species of Poaceicola, having ascospores with transverse septa only. Subsequently, Jayasiri et al. (2015) introduced two new species: V. chlamydospora, which presents both morphs, and V. dactylidis, which produces only the sexual morph. The sexual morph of V. chlamydospora is similar to the two former species of the genus, while V. dactylidis produces ascospores with transverse and longitudinal septa as seen in species of Allophaeosphaeria, indicating again that the longitudinal septation of ascospores is not phylogenetically informative. Vagicola chlamydospora was recently transferred to Septoriella based on phylogenetic data (Jayasiri et al. 2015). Surprisingly, the asexual morph reported in that species does not fit with the morphology of Septoriella, since it produces micro- to macronematous conidiophores and chlamydospore-like conidia. Recently, Thambugala et al. (2017) introduced the last species of the genus, V. arundinis, which produces both morphs and is characterised by ascospores with transverse septa and an asexual morph similar to Septoriella, which demonstrates the link of Vagicola with Septoriella. This last species was invalid because two holotypes were designated. Therefore, this taxon is validated in the present study.
Moreover, in our phylogenetic analyses, the ex-type strain of the most recently described species of Neostagonospora was located in the clade representing Septoriella. This species is characterised by the production of conidia that are subcylindrical or fusiform, euseptate, with a subobtuse apex and truncate base. However, the presence of mucoid appendages at both ends, as the other species of Septoriella, has not been reported. Septoriella artemisiae is saprobic or weakly necrotrophic on dead and dying stems of Artemisia austriaca.
Most of the species now included in Septoriella are saprophytes, except for Sep. hirta, which is an important secondary pathogen of grasses (Sprague 1950). This species is often found in association with other fungi such as Gaeumannomyces graminis (Johnston et al. 2014) and Oculimacula yallundae causing foot rot of wheat (Crous et al., 2003, Crous et al., 2015a). Other disease symptoms observed in plants affected by Sep. hirta are discoloured culms and predisposition to premature collapse, especially in rainy and windy seasons, since this species produces a weakness in the culms of plants with ripe grains. The result of all these symptoms resulted in the increasing of the cost of harvesting and decreasing of the grain quality (Sprague 1950).
References: Sprague 1950 (pathogenicity), Crous et al., 2014b, Ariyawansa et al., 2015a, Li et al., 2015, Liu et al., 2015, Thambugala et al., 2017 (morphology and phylogeny).
Septoriella agrostina (Mapook et al.) Y. Marín & Crous, comb. nov. MycoBank MB829676.
Basionym: Poaceicola agrostina Mapook et al., Fungal Diversity 89: 132. 2018.
Description and illustration: Wanasinghe et al. (2018).
Septoriella artemisiae (Wanas. et al.) Y. Marín & Crous, comb. nov. MycoBank MB829677.
Basionym: Neostagonospora artemisiae Wanas. et al, Fungal Diversity 89: 130. 2018.
Description and illustration: Wanasinghe et al. (2018).
Septoriella arundinicola (Wanas. et al.) Y. Marín & Crous, comb. nov. MycoBank MB829678.
Basionym: Poaceicola arundinicola Wanas. et al., Fungal Diversity 89: 135. 2018.
Description and illustration: Wanasinghe et al. (2018).
Septoriella arundinis (W.J. Li et al.) Y. Marín & Crous, comb. nov. MycoBank MB829679.
Basionym: Poaceicola arundinis W.J. Li et al., Mycosphere 6: 698. 2015.
Description: Li et al. (2015).
Septoriella bromi (Wijayaw. et al.) Y. Marín & Crous, comb. nov. MycoBank MB829680.
Basionym: Poaceicola bromi Wijayaw. et al., Mycosphere 6: 698. 2015.
Description and illustration: Li et al. (2015).
Septoriella dactylidicola Y. Marín & Crous, nom. nov. MycoBank MB829681.
Replaced synonym: Poaceicola dactylidis Tibpromma et al., Mycosphere 8: 755. 2017, non Septoriella dactylidis (Wanas. et al.) Y. Marín & Crous. 2019.
Description and illustration: Thambugala et al. (2017).
Septoriella dactylidis (Wanas. et al.) Y. Marín & Crous, comb. nov. MycoBank MB829682.
Basionym: Vagicola dactylidis Wanas. et al., Phytotaxa 6: 725. 2015.
Description and illustration: Jayasiri et al. (2015).
Septoriella elongata (Wehm.) Y. Marín & Crous, comb. nov. MycoBank MB829683.
Basionym: Leptosphaeria elongata Wehm., Mycologia 44: 633. 1952.
Synonym: Poaceicola elongata (Wehm.) W.J. Li et al., Mycosphere 6: 701. 2015.
Description and illustration: Wehmeyer (1952).
Septoriella forlicesenica (Thambug. et al.) Y. Marín & Crous, comb. nov. MycoBank MB829684.
Basionym: Poaceicola forlicesenica Thambug et al., Mycosphere 8: 756. 2017.
Description and illustration: Thambugala et al. (2017).
Septoriella garethjonesii (Thambug. et al.) Y. Marín & Crous, comb. nov. MycoBank MB829685.
Basionym: Poaceicola garethjonesii Thambug et al., Mycosphere 8: 756. 2017.
Description and illustration: Thambugala et al. (2017).
Septoriella germanica Crous, R.K. Schumach. & Y. Marín, sp. nov. MycoBank MB829701. Fig. 68.
Fig. 68.
Septoriella germanica (ex-type CBS 145372). A. Conidiomata sporulating on PNA. B, C. Conidiogenous cells. D. Conidia with mucoid caps. Scale bars: A = 200 μm; all others = 10 μm.
Etymology: Name refers to Germany, from where this fungus was isolated.
Conidiomata solitary, pycnidial, erumpent, globose, brown, 180–220 μm diam, in vivo gregarious, caespitose or in rows, but also pseudostromatic, up to 300 μm diam, with central ostiole, 30–40 μm diam; conidiomatal wall of 3–4 layers of brown cells of textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth-walled, globose to ampulliform, phialidic, 4–6 × 4–5 μm. Conidia solitary, scolecosporous, fusoid to subcylindrical, apex subobtuse, base truncate, straight to sligthly curved, 3–6-septate, golden-brown, smooth-walled, granular with mucoid caps at each end, (35–)37–42(–46) × 3(–3.5) μm, in vivo 29–46 × 3–4.5 μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium, covering dish in 2 wk. On MEA, PDA and OA surface olivaceous grey, reverse iron-grey.
Typus: Germany, near Berlin, on dead culm of Phragmites australis (Poaceae), 16 Apr. 2016, R.K. Schumacher, HPC 1168 (holotype CBS H-23875, culture ex-type CBS 145372 = CPC 30511).
Notes: Septoriella germanica is related to Sep. artemisiae. However, both species differ in the size of the conidia [15–25 × 2–2.5 μm in Sep. artemisiae vs. (35–)37–42(–46) × 3(–3.5) μm in Sep. germanica], as well as in the conidial septation (2–4 in Sep. artemisiae vs. 3–6 μm in Sep. germanica). Septoriella germanica was isolated from Phragmites australis (Poaceae), while Sep. artemisiae was found on Artemisia austriaca (Asteraceae).
Septoriella hibernica Crous, Quaedvl. & Y. Marín, sp. nov. MycoBank MB829703.
Etymology: Name refers to Ireland, where this fungus was collected.
Culture sterile. Septoriella hibernica differs from its closest phylogenetic neighbour, Septoriella subcylindrispora by unique fixed alleles in the ITS locus based on the alignment deposited in TreeBASE (S23834): positions 5 (T), 33 (T), 34 (A), 46 (T), 61 (A), 89 (T), 477 (A), 479 (T), 480 (A), 512 (T), 528 (G), 534 (G).
Culture characteristics: Colonies erumpent, spreading, covering dish in 2 wk, with fluffy aerial mycelium and even margins. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: Ireland, on unidentified grass species (Poaceae), Mar. 2014, W. Quaedvlieg (holotype CBS H-23874, culture ex-type CBS 145371 = CPC 24290).
Notes: Septoriella hibernica remained sterile on all media tested. It is related to Sep. subcylindrispora, but the ITS sequences of both species showed only 97.75 % of nucleotide similarity (Identities = 522/538, 3 gaps).
Septoriella hollandica Crous, Quaedvl. & Y. Marín, sp. nov. MycoBank MB829702. Fig. 69.
Fig. 69.
Septoriella hollandica (ex-type CBS 145374). A. Ascomata sporulating on PNA. B, C. Asci. D. Ascospores. Scale bars = 10 μm.
Etymology: Name refers to the Netherlands, where this fungus was collected.
Ascomata solitary, erumpent, globose, brown, 150–180 μm diam with central ostiole; conidiomatal wall of 3–4 layers of brown cells of textura angularis. Pseudoparaphyses hyphae-like, hyaline, smooth-walled, branched, septate, 1.5–2 μm diam. Asci subcylindrical, flexuous, bitunicate, with well-defined apical chamber, 1–1.5 μm diam, fasciculate, short stipitate, 70–90 × 8–10 μm. Ascospores bi- to triseriate, fusoid-ellipsoid, 5-septate, constricted at median septum, medium brown, smooth-walled, guttulate, widest above median septum, (27–)28–30(–32) × (4–)4.5(–5) μm.
Culture characteristics: Colonies flat, spreading, covering dish in 2 wk with moderate aerial mycelium. On MEA surface vinaceous buff, reverse sienna; on PDA surface isabelline, reverse hazel; on OA surface saffron.
Typus: The Netherlands, Oosterbeek, on leaves of Phragmites australis (Poaceae), 24 Jan. 2014, W. Quaedvlieg (holotype CBS H-23877, culture ex-type CBS 145374 = CPC 24109).
Notes: Septoriella hollandica is related to Sep. chlamydospora and Sep. tridentina. Septoriella hollandica, as well as Sep. chlamydospora and Sep. tridentina, produce sexual morphs in culture. Septoriella hollandica can be easily distinguished from Sep. chlamydospora by its 5-septate ascospores, being 9-septate in Sep. chlamydospora. Septoriella tridentina is the only species of this complex that produces ascospores surrounded by a mucilaginous sheath. The asexual morph was reported only for Sep. chlamydospora. However, as it was mentioned above, the asexual morph described in Sep. chlamydospora (Jayasiri et al. 2015) corresponds to chains of chlamydospores instead of scolecosporous conidia typical of Septoriella. Septoriella hollandica was isolated from Phragmites australis, while the other two species were found on Dactylidis spp.
Septoriella italica (Thambug. et al.) Y. Marín & Crous, comb. nov. MycoBank MB829704.
Basionym: Poaceicola italica Thambug. et al., Mycosphere 8: 759. 2017.
Description: Thambugala et al. (2017).
Septoriella muriformis (Ariyaw. et al.) Y. Marín & Crous, comb. nov. MycoBank MB829705.
Basionym: Allophaeosphaeria muriformis Ariyaw. et al., Fungal Diversity 72: 137.
Descriptions and illustrations: Liu et al., 2015, Thambugala et al., 2017.
Septoriella neoarundinis Y. Marín & Crous, nom. nov. MycoBank MB829706.
Replaced synonym: Vagicola arundinis Phukhams., Camporesi & K.D. Hyde, sp. nov. MycoBank MB831056, non Septoriella arundinis (W.J. Li et al.) Y. Marín & Crous. 2019.
Synonym: Vagicola arundinis Phukhams. et al. Mycosphere 8: 763. 2017. (nom. inval., Art. 40).
Etymology: Name reflects the host genus Arundo from which it was isolated.
Description and illustration: Thambugala et al. (2017).
Typus: Italy, Province of Marsignano, Predappio, on a dead stem of Arundo plinii (Poaceae), 10 Nov. 2014, E. Camporesi IT 2223A (holotype MFLU 17-0016, ex-type living culture MFLUCC 15-0027).
Notes: This species was initially introduced by Thambugala et al. (2017) as Vagicola arundinis. However, it was invalid since two different holotype numbers were cited. Therefore, this species is validated here and a new name in Septoriella is proposed, using a new epithet since Sep. arundinis is occupied.
Septoriella neodactylidis Y. Marín & Crous, nom. nov. MycoBank MB829707.
Replaced synonym: Allophaeosphaeria dactylidis Wanas. et al., Fungal Diversity 72: 137. 2015, non Septoriella dactylidis (Wanas. et al.) Y. Marín & Crous. 2019.
Description and illustration: Liu et al. (2015).
Septoriella pseudophragmitis Crous, Quaedvl. & Y. Marín, sp. nov. MycoBank MB829708. Fig. 70.
Fig. 70.
Septoriella pseudophragmitis (ex-type CPC 24166). A. Conidiomata sporulating on MEA. B, C. Conidiogenous cells. D. Conidia. Scale bars: A = 250 μm; all others = 10 μm.
Etymology: Name refers to its morphological similarity with Sep. phragmitis, which occurs on the same host.
Conidiomata solitary, pycnidial, erumpent, globose, brown-black, 200–250 μm diam with central ostiole; conidiomatal wall of 6–8 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth-walled, globose to ampulliform, phialidic, 4–10 × 4 μm. Conidia solitary, subcylindrical, golden-brown, guttulate, smooth-walled, apex obtuse, base truncate, 3(–6)-septate, with mucoid caps at each end, (20–)24–28(–32) × (3–)3.5(–4) μm.
Culture characteristics: Colonies erumpent, spreading, covering dish after 2 wk at 25 °C, with moderate aerial mycelium and feathery margins. On MEA surface olivaceous grey, reverse iron-grey.
Typus: The Netherlands, on leaves of Phragmites sp. (Poaceae), 5 Mar. 2014, W. Quaedvlieg (holotype CBS-H 23904, culture ex-type CBS 145417 = CPC 24166).
Notes: Septoriella pseudophragmitis is similar to Sep. phragmitis, which is reported from the same host, Phragmites (Poaceae). These species differ in the size of their conidiomata (up to 250 μm diam in Sep. pseudophragmitis vs. 350 μm diam in Sep. phragmitis) and conidia [(20–)24–28(–32) × (3–)3.5(–4) μm in Sep. pseudophragmitis vs. (29–)32–40(–46) × 3(–3.5) μm diam in Sep. phragmitis], as well as in the conidial septation, being mostly 3-septate in Sep. pseudophragmitis and 5-septate in Sep. phramitis. Based on our phylogenetic analysis, Septoriella pseudophragmitis is related to Sep. allojunci. However, Sep. allojunci produces smaller conidiomata (up to 150 μm) and larger conidia (48–70 × 3–6.6 μm). Moreover, Sep. allojunci was isolated from Juncus (Juncaceae).
Septoriella rosae (Mapook et al.) Y. Marín & Crous, comb. nov. MycoBank MB829713.
Basionym: Poaceicola rosae Mapook et al., Fungal Diversity 89: 136. 2018.
Description and illustration: Wanasinghe et al. (2018).
Septoriella subcylindrospora (W.J. Li et al.) Y. Marín & Crous, comb. nov. MycoBank MB829709.
Basionym: Allophaeosphaeria subcylindrospora W.J. Li et al., Fungal Diversity 75: 100. 2015.
Description and illustration: Ariyawansa et al. (2015a).
Septoriella vagans (Niessl) Y. Marín & Crous, comb. nov. MycoBank MB829710.
Basionym: Pleospora vagans Niessl, Verh. nat. Ver. Brünn 14: 174. 1876.
Synonym: Vagicola vagans (Niessl) O.E. Erikss. et al., Fungal Diversity 75: 115. 2015.
Description and illustration: Jayasiri et al. (2015).
Arezzomyces Y. Marín & Crous, gen. nov. MycoBank MB829711.
Etymology: Name reflects the Italian province Arezzo where it was collected.
Ascomata solitary, scattered, immersed to erumpent, obpyriform, dark brown to black, coriaceous, with ostiole filled with hyaline cells, appearing as a white ring around ostiole; necks papillate, black, smooth; ascomatal wall comprising 6–8 layers, outer layer heavily pigmented, comprising blackish to dark brown, thick-walled cells of textura angularis, inner layer composed of brown, thin-walled cells of textura angularis. Hamathecium comprising numerous, filamentous, branched, septate pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical, pedicel furcate, rounded and thick-walled at the apex, with an ocular chamber. Ascospores mostly uniseriate, initially hyaline, becoming yellowish brown at maturity, ellipsoidal, muriform, with 6–8 transverse septa, 3–7 vertical septa, strongly constricted at the central septa, weakly constricted at the other septa, with conical and narrowly rounded ends, lacking a mucilaginous sheath. Asexual morph not observed.
Culture characteristics: Colonies spreading, surface erumpent, with moderate aerial mycelium, and feathery margins. On MEA, PDA and OA surface dirty white; reverse dirty white to luteous.
Type species: Arezzomyces cytisi (Wanas. et al.) Y. Marín & Crous. Holotype and ex-type cultures: MFLU 15-1502, MFLUCC 15-0649.
Notes: Arezzomyces is introduced to accommodate Allophaeosphaeria cytisi since, based on phylogenetic data, it is located in an independent lineage distant to the clade representing the genus Septoriella. Moreover, based on a megablast search using the ITS sequence, the closest matches in NCBIs GenBank nucleotide database were Ophiosimulans tanaceti [GenBank KU738890; Identities = 534/586 (91 %), 11 gaps (1 %)], Ophiobolus cirsii [GenBank KM014664; Identities = 514/566 (91 %), 22 gaps (1 %)], and Chaetosphaeronema hispidulum [GenBank KX096655; Identities 535/588 (91 %), 22 gaps (3 %)]. Arezzomyces cytisi is a saprobe found on dead herbaceous branches of Cytisus.
Arezzomyces cytisi (Wanas. et al.) Y. Marín & Crous, comb. nov. MycoBank MB829712.
Basionym: Allophaeosphaeria cytisi Wanas. et al., Fungal Diversity 75: 97. 2015.
Description and illustration: Ariyawansa et al. (2015a).
Typus: Italy, Arezzo Province, Casuccia di Micheli in Quota, dead and hanging branches of Cytisus sp. (Fabaceae), 20 Jun. 2012, E. Camporesi (holotype MFLU 15-1502, culture ex-type MFLUCC 15-0649).
Authors: Y. Marin-Felix, W. Quaedvlieg, R.K. Schumacher & P.W. Crous
Setophoma Gruyter et al., Mycologia 10: 1077. 2010. Fig. 71.
Fig. 71.
Setophoma spp. A, B. Conidioma forming in culture. A.Setophoma chromolaenae (ex-type CBS 135105). B.Setophoma vernoniae (ex-type CBS 137988). C–E. Conidiomata with setae of Setophoma chromolaenae (ex-type CBS 135105). F–H. Conidiogenous cells. F, G.Setophoma chromolaenae (ex-type CBS 135105). H.Setophoma vernoniae (ex-type CBS 137988). I, J. Conidia. I.Setophoma chromolaenae (ex-type CBS 135105). J.Setophoma vernoniae (ex-type CBS 137988). Scale bars: C–E = 20 μm; all others = 10 μm; F applies to F and G.
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Phaeosphaeriaceae.
Type species: Setophoma terrestris (H.N. Hansen) Gruyter et al., basionym: Phoma terrestris H.N. Hansen. Lectotype and ex-lectotype strain designated by de Gruyter et al. (2010): CBS H-20311, CBS 335.29.
DNA barcode (genus): LSU. Fig. 28.
DNA barcodes (species): ITS, rpb2, tef1, tub2. Table 17. Fig. 72.
Table 17.
DNA barcodes of accepted Setophoma spp.
| Species | Isolates1 | GenBank accession numbers2 |
References | |||
|---|---|---|---|---|---|---|
| ITS | rpb2 | tef1 | tub2 | |||
| Setophoma brachypodii | CBS 145418T | MK539968 | MK540099 | MK540161 | – | Present study |
| Set. chromolaenae | CBS 135105T | KF251244 | KF252249 | KF253195 | KF252728 | Quaedvlieg et al. (2013) |
| Set. pseudosacchari | CBS 145373T | MK539969 | MK540100 | – | MK540176 | Present study |
| Set. sacchari | CBS 333.39ET | KF251245 | KF252250 | – | – | Quaedvlieg et al. (2013) |
| Set. terrestris | CBS 335.29LT | KF251246 | KF252251 | KF253196 | KF252729 | Quaedvlieg et al. (2013) |
| Set. vernoniae | CBS 137988T | KJ869141 | – | MK540162 | MK540177 | Crous et al. (2014b), present study |
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands. T, ET and LT indicate ex-type strains, ex-epitype and ex-lectotype, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial β-tubulin gene.
Fig. 72.
RAxML phylogram obtained from the combined ITS (589 bp), LSU (835 bp), tef1 (788 bp) and tub2 (532 bp) sequence alignment of all accepted species of Setophoma. The tree was rooted to Neostagonospora caricis CBS 135092 and Neostagonospora elegiae CBS 135101. The novelties proposed in this study are indicated in bold. RAxML bootstrap support (BS) values above 70 % and Bayesian posterior probability scores above 0.95 are shown at the nodes. GenBank accession numbers of LSU are listed in Fig. 28, and of the other loci in Table 7, Table 17 and 18. T, ET and LT indicate ex-type strains, ex-epitype and ex-lectotype, respectively. TreeBASE: S23834.
Ascomata scattered, gregarious, immersed, visible as raised, minute black dots on host surface, uniloculate, globose to subglobose, glabrous, brown to dark brown, ostiole central, with minute papilla; ascomatal wall thin, of equal thickness, composed of pseudoparenchymatous cells, arranged in flattened textura angularis to textura prismatica. Hamathecium composed of numerous, filiform, broad cellular pseudoparaphyses, with distinct septa, anastomosing at apex. Asci 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, short pedicellate, apically rounded, with well-developed narrowly ocular chamber. Ascospores overlapping, 2-seriate, phragmosporous, cylindrical to cylindrical-clavate, hyaline, 3-septate, usually enlarged at the second cell from apex, smooth-walled with large guttules. Conidiomata pycnidial, solitary to confluent, superficial or submerged in agar, globose to subglobose, setose, with papillate necks, honey to olivaceous or olivaceous black, with 2–7(–11) layers of pseudoparenchymatal cells. Conidiogenous cells hyaline, monophialidic. Conidia aseptate, ellipsoidal to subcylindrical or subfusoid, guttulate (adapted from Quaedvlieg et al., 2013, Phookamsak et al., 2014a).
Culture characteristics: Colonies spreading with sparse to moderate aerial mycelium, smooth or folded surface, even or lobate margins. On PDA surface iron-grey or grey olivaceous with outer region iron-grey; reverse olivaceous grey or iron-grey. On MEA surface olivaceous grey or umber with patches of apricot and dirty white; reverse ochreous or cinnamon with patches of olivaceous grey. On OA surface isabelline or iron-grey surrounded by orange to apricot diffuse pigment layer in agar.
Optimal media and cultivation conditions: OA and SNA at 25 °C under continuous near-ultraviolet light to promote sporulation. Sterile bamboo pieces on WA to induce sporulation of the sexual morph.
Distribution: Worldwide.
Hosts: Mainly on members of Poaceae, but also of Amaryllidaceae and Asteraceae, among others.
Disease symptoms: Leaf spots and necrosis, leaf dieback, and pink root.
Notes: Setophoma was introduced by de Gruyter et al. (2010) to accommodate two species previously placed in Pyrenochaeta, i.e. Pyr. sacchari and Pyr. terrestris. Setophoma is characterised by pycnidial conidiomata covered by setae, phialidic conidiogenous cells, and hyaline, ellipsoidal to subcylindrical, aseptate, guttulate conidia (de Gruyter et al., 2010, Quaedvlieg et al., 2013). When Setophoma was introduced, the sexual morph had not been observed. Subsequently, Phookamsak et al. (2014a) reported the sexual morph of this genus. It was found causing leaf spots of sugarcane (Saccharum officinarum), and based on the phylogenetic data it was shown to be the sexual morph of Set. sacchari. This sexual morph is similar to Phaeosphaeria species, producing ascospores with three septa with the second cell from the apex being swollen; these cells differ in colour (hyaline in Setophoma vs. yellowish to brown in Phaeosphaeria).
Setophoma encompasses pathogenic or saprobic species associated with monocotyledonous plants (de Gruyter et al. 2010). The type species, Set. terrestris, causes pink root on Allium spp., and also on Zea mays and Oryza sativa, but it is asymptomatic on other hosts (Farr & Rossman 2019). Setophoma vernoniae produces leaf spots on Vernonia polyanthes (Crous et al. 2014b), while Set. sacchari is considered a weak pathogen of members of the Poaceae that is only noticeable when conditions are favourable for disease spread, and causes leaf spots and necrosis and leaf dieback (Farr & Rossman 2019).
In our phylogenetic analysis based on ITS and LSU (Fig. 28), the clade representing the genus Setophoma is well-supported (95 % BS / 1 PP). However, the most recently described species, Set. cyperi, is not located in that clade, representing a new genus in the family Phaeosphaeriaceae.
At the proof stage of this paper, a new publication appeared on Setophoma (Liu et al. 2019), which contains four new species.
References: de Gruyter et al., 2010, Quaedvlieg et al., 2013, Phookamsak et al., 2014a (morphology and phylogeny).
Setophoma brachypodii Crous, R.K. Schumach. & Y. Marín, sp. nov. MycoBank MB829669.
Etymology: Name reflects the host genus Brachypodium from which it was isolated.
Culture sterile. Setophoma brachypodii differs from its closest phylogenetic neighbour, Setophoma terrestris by unique fixed alleles in two loci based on alignments of the separate loci deposited in TreeBASE (S23834): LSU positions 42 (G), 67 (C), 75 (C), 77 (T), 79 (G), 81 (C), 82 (A), 89 (C), 133 (C), 144 (G), 145 (C), 146 (C), 147 (T), 150 (G), 302 (C), 348 (T), 380 (C), 392 (A), 437 (T), 445 (T), 446 (C), 473 (A), 477 (G), 601 (G), 636 (T), 637 (T), 638 (A); ITS positions 32 (C), 34 and 35 (indels), 36 (T), 37 (T), 38 (T), 42 (G), 43 (T), 44 (A), 54 (C), 56 (G), 57 (T), 58 (T), 59 (C), 60 (G), 61 (C), 62 (T), 63 (G), 64 (T), 66 (G), 67 (T), 72 (T), 77 (G), 78 (T), 80 (T), 99 (T), 100 (G), 101 (A), 103 (C), 114 (C), 117 (G), 118 (T), 119 (A), 121 (C), 122 (T), 124 (C), 130 (A) 138 (C), 140 (A), 143 (T), 146 (A), 148 (C), 172 (A), 176 (T), 178 (A), 180 (T), 182 (A), 186 (indel), 354 (T), 379 (T), 381 (indel), 388 (T), 389 (G), 390 (G), 391 (T), 392 (C), 393 (C), 394 (T), 395 (C), 396 (T), 399 (G), 400 (A), 401 (C), 402 (C), 409 (A), 418 (A), 419 (T), 433 (G), 434 (T), 435 (A), 441 (G), 444 (T), 467 (A), 470 (indel), 473 (T), 475 (C), 477 (A), 478 (C), 479 (T), 482 (A), 485 (C), 486 (C), 490 (A), 495–498 (indels), 499 (C), 500 (C), 502 (T), 504 (A), 506 (T), 507 (A), 511 (C).
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and even, lobate margins, reaching 60 mm diam after 2 wk. On MEA, PDA and OA, surface and reverse olivaceous grey.
Typus: Belgium, Dinant, 173 m a.s.l., on border of calcareous meadow, on a dead and attached leaf of Brachypodium sylvaticum (Poaceae), 2 Nov. 2016, L. Bailly & R.K. Schumacher, HPC 1503, RKS 1 (holotype CBS H-23905, culture ex-type CBS 145418 = CPC 32492).
Notes: Setophoma brachypodii remained sterile on all media tested, and the original specimen was depleted, hence we could not describe it based on morphology. This is the first species of Setophoma reported on Brachypodium.
Setophoma pseudosacchari Crous & Y. Marín, sp. nov. MycoBank MB829670. Fig. 73.
Fig. 73.
Setophoma pseudosacchari (ex-type CBS 145373). A. Ascomata sporulating on OA. B. Asci with ascospores. C. Conidia. Scale bars: A = 300 μm; all others = 10 μm.
Etymology: Named after its closely phylogenetic relation to Setophoma sacchari.
Ascomata developing on OA, solitary, erumpent, brown, 200–300 μm diam, globose, with large central ostiole, 30–40 μm diam; ascomatal wall of 3–4 layers of brown cells of textura angularis, ascomata setose; setae brown, flexuous, thick-walled, septate, base verruculose, with slight taper to obtuse apex, up to 150 μm long. Pseudoparaphyses hyphae-like, anastomosing, branched, septate, hyaline, occurring intermingled among asci. Asci bitunicate, ellipsoid to subcylindrical, hyaline, curved to straight, fasciculate, apex obtuse, with well-defined ocular chamber, 2 μm diam, stipitate, 70–100 × 10–13 μm. Ascospores bi- to triseriate, fusoid-ellipsoid with subobtuse ends, straight, 3-septate, widest in second cell from apex, prominently guttulate, hyaline, smooth-walled, (22–)25–30 × (5.5–)6 μm. Conidiomata developing on SNA, solitary to aggregated, erumpent, brown, globose, 200–300 μm diam, with 1–2 ostioles, lacking setae; conidiomatal wall of 2–3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells lining inner cavity, dissolving at maturity, hyaline, smooth-walled, globose to ampulliform, phialidic, 4–6 × 5–6 μm. Conidia solitary, aseptate, straight to slightly curved, subcylindrical to fusoid-ellipsoid, apex obtuse, base truncate, hyaline, smooth-walled, guttulate, (8–)11–12(–14) × (3–)4 μm.
Culture characteristics: Colonies erumpent, spreading, surface folded, with moderate aerial mycelium and even, lobate margins, reaching 55 mm diam. On MEA surface peach, outer region scarlet, reverse sienna; on PDA surface umber, outer region saffron, reverse sienna with patches of saffron; on OA surface sienna with patches of saffron.
Typus: France, La Réunion Island, leaf spots on Saccharum officinarum (Poaceae), May 2015, P.W. Crous, HPC 296 (holotype CBS H-23876, CBS 145373 = CPC 26421).
Notes: This species is closely related to Nph. sacchari, which is a species also isolated from sugarcane. However, the ITS sequences of the type material of both species showed only a 97.68 % of nucleotide similarity. Unfortunately, tef1 and tub2 sequences of Nph. sacchari are not available in order to compare both species. These species produce both the sexual and asexual morphs, with morphological differences most obvious in the sexual morph. Neosetophoma pseudosacchari can be easily distinguished by its larger ascomata (up to 300 μm diam in Nph. pseudosacchari vs. up to 180 μm diam in Nph. sacchari), asci [70–100 × 10–13 μm in Nph. pseudosacchari vs. 60–75(–85) × 12–15(–17) μm in Nph. sacchari] and ascospores [(22–)25–30 × (5.5–)6 μm in Nph. pseudosacchari vs. 20–23(–25) × 5–6 μm in Nph. sacchari].
Wingfieldomyces Y. Marín & Crous, gen. nov. MycoBank MB829671. Table 18. Fig. 74.
Table 18.
DNA barcodes of the accepted Wingfieldomyces sp.
| Species | Isolates1 | GenBank accession numbers2 |
References | |||
|---|---|---|---|---|---|---|
| ITS | rpb2 | tef1 | tub2 | |||
| Wingfieldomyces cyperi | CBS 141450T | KX228286 | MK540101 | MK540163 | MK540178 | Crous et al. (2016b), present study |
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands. T indicates ex-type strain.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial β-tubulin gene.
Fig. 74.
Wingfieldomyces cyperi (ex-type CBS 141450). A. Symptomatic leaf of Cyperus sphaerocephala. B–D. Asci. E. Pseudoparaphyses. F–I. Ascospores. Scale bars: 10 μm; F applies to F–I. Pictures B, C, E–I taken from Crous et al. (2016b).
Etymology: Named in honour of its collector, Prof. dr M.J. Wingfield, who contributed greatly to the elucidation of African fungal biodiversity.
Ascomata immersed on host, erumpent in culture, black, globose, with central ostiole; ascomatal wall of 3–4 layers of dark brown cells of textura angularis. Pseudoparaphyses intermingled among asci, hyaline, septate, branched prominently, constricted at septa. Asci bitunicate with apical chamber, subcylindrical, hyaline, smooth, fasciculate, stipitate, 8-spored. Ascospores tri- to multiseriate, fusoid with subobtusely rounded ends, finely verruculose, red-brown, guttulate, 2-septate, slightly constricted at septa, with central cell somewhat swollen.
Culture characteristics: Colonies spreading, erumpent surface, with moderate aerial mycelium, margins feathery. On MEA, PDA and OA surface dirty white; reverse dirty white to luteous.
Type species: Wingfieldomyces cyperi (Crous & M.J. Wingf.) Y. Marín & Crous. Holotype and ex-type cultures: CBS H-22622, CBS 141450 = CPC 25702.
Notes: Wingfieldomyces is introduced to accommodate Set. cyperi since, based on phylogenetic data, it is located in an independent lineage distant to the clade representing the genus Setophoma. Moreover, based on a megablast search using the ITS sequence, the closest matches in NCBIs GenBank nucleotide database were Pringsheimia euphorbiae [GenBank NR_145344; Identities = 456/500 (91 %), 8 gaps (1 %)] and Phaeosphaeria caricis [GenBank KY090633; Identities 439/485 (91 %), 12 gaps (2 %)]. It only produces a sexual morph in culture, characterised by tri- to multiseriate, 2-septate, red-brown ascospores, while Setophoma produces both morphs and 2-seriate, 3-septate, hyaline ascospores with the second cell from the apex becoming swollen. Wingfieldomyces is associated with leaf scorch symptoms on Cyperus.
Wingfieldomyces cyperi (Crous & M.J. Wingf.) Y. Marín & Crous, comb. nov. MycoBank MB829672. Fig. 74.
Basionym: Setophoma cyperi Crous & M.J. Wingf., Persoonia 36: 385. 2016.
Description: Crous et al. (2016b).
Typus: South Africa, Eastern Cape Province, Haga Haga, on leaves of Cyperus sphaerocephala (Cyperaceae), Dec. 2014, M.J. Wingfield (holotype CBS H-22622, culture ex-type CPC 25702 = CBS 141450).
Authors: Y. Marin-Felix & P.W. Crous
Stagonosporopsis Died., Ann. Mycol. 10: 142. 1912. Emend. Aveskamp et al., Stud. Mycol. 65: 44. 2010. Fig. 75.
Fig. 75.
Stagonosporopsis spp. A, B. Disease symptoms of Stagonosporopsis tanaceti (ex-type CBS 131484). A. Leaf necrosis. B. Drooping flower heads. C–G. Sexual morph of Stagonosporopsis inoxydabilis (ex-type CBS 425.90). C. Close-up of ascoma with darkened ostiolar area. D, E. Stipitate, bitunicate asci. F, G. Ascospores (arrows denote sheath). H–T. Asexual morph. H, I. Colony sporulating on OA. H.Stagonosporopsis chrysanthemi (CBS 500.63). I.Stagonosporopsis tanaceti (ex-type CBS 131484). J. Close-up of pycnidial conidiomata of Stagonosporopsis tanaceti (ex-type CBS 131484). K. Close-up of darkened ostiolar area of Stagonosporopsis chrysanthemi (CBS 500.63). L–Q. Conidiogenous cells. L–N.Stagonosporopsis chrysanthemi (CBS 500.63). O–Q.Stagonosporopsis tanaceti (ex-type CBS 131484). R, S. Conidia. R.Stagonosporopsis chrysanthemi (CBS 500.63). S.Stagonosporopsis tanaceti (ex-type CBS 131484). T. Chain of chlamydospores of Stagonosporopsis tanaceti (ex-type CBS 131484). Scale bars: C = 35 μm; J = 150 μm; all others = 10 μm; D applies to D and E; L applies to L–N; O applies to O–Q. Pictures taken from Vaghefi et al. (2012).
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Didymellaceae.
Type species: Stagonosporopsis boltshauseri (Sacc.) Died., designated as lectotype by Clements & Shear (1931), basionym: Ascochyta boltshauseri Sacc. = Stagonosporopsis hortensis (Sacc. & Malbr.) Petr., basionym: Hendersonia hortensis Sacc. & Malbr. Representative strain of Sta. hortensis: CBS 572.85 = PD 79/269.
DNA barcode (genus): LSU.
DNA barcodes (species): ITS, rpb2, tub2. Table 19. Fig. 76.
Table 19.
DNA barcodes of accepted Stagonosporopsis spp.
ATCC: American Type Culture Collection, Virginia, USA; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CGMCC: Chinese General Microbiological Culture Collection Center, Beijing, China; DAR: New South Wales Plant Pathology Herbarium, NSW, Australia; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand. T, ET, IsoT and NT indicate ex-type, ex-epitype, ex-isotype and ex-neotype strains, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial large subunit (28S) nrRNA gene; act: partial actin gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit; tub2: partial β-tubulin gene.
Q-bank: Sequences retrieved from Q-bank Fungi database (http://www.q-bank.eu/fungi/).
Fig. 76.
The majority rule consensus phylogram of Stagonosporopsis spp. inferred from the concatenated LSU (876 bp), ITS (459 bp), tub2 (299 bp) and rpb2 (596 bp) sequence alignment using Bayesian Inference. The tree is rooted to Boeremia exigua var. exigua CBS 431.74. Bootstrap support values > 75 % and PP values > 0.90 are shown above or below the branches. T,ET,IsoT and NT indicate ex-type, ex-epitype, ex-isotype and ex-neotype strains, respectively. Genbank accession numbers are indicated in Table 19, Aveskamp et al. (2010) and Vaghefi et al. (2012). TreeBASE: S23800.
Ascomata pseudothecial, globose to subglobose, sometimes with a somewhat conical neck. Asci cylindrical or subclavate, 8-spored, biseriate. Ascospores ellipsoid, fusoid or obovoid, uniseptate, guttulate, sometimes with a gelatinous sheath. Conidiomata pycnidial, globose to subglobose, glabrous or with hyphal outgrowths, superficial on agar surface or immersed, solitary or confluent, ostiolate or poroid, occasionally papillate; conidiomatal wall pseudoparenchymatous, 2–6 layered, with an outer wall composed of 1–3 layers of brown to olivaceous cells. Conidiogenous cells phialidic, hyaline, simple, smooth-walled, ampulliform or doliiform. Conidia often dimorphic: mainly aseptate, hyaline, ellipsoid to subglobose, thin-walled, smooth-walled, eguttulate or with several polar or scattered guttules; second type of conidia larger in size, can be produced both in vivo and in vitro in the same pycnidia as the other type of conidia, 0–3-septate.
Culture characteristics: Colonies on OA regular to somewhat irregular, colourless, buff, luteous to ochraceous or amber, or olivaceous grey to greenish grey, with scarce or abundant floccose white to pale salmon, or olivaceous grey aerial mycelium.
Optimal media and cultivation conditions: On OA at 20–24 °C under near-ultraviolet light (13 h light, 11 h dark) to induce sporulation of the asexual morph, while MEA stimulates pigmentation and crystal formation. Changes in colour of the fungal cultures upon a sudden increase of pH (NaOH spot test), which may be used for taxonomic characterisation, are best observed on OA.
Distribution: Worldwide.
Hosts: Associated with at least 30 plant genera in Asteraceae, Brassicaceae, Campanulaceae, Caricaceae, Cucurbitaceae, Fabaceae, Lamiaceae, Ranunculaceae, Solanaceae and Valerianaceae as saprobes or pathogens. Stagonosporopsis oculo-hominis is the only species that is not associated with a plant host and was isolated from human corneal ulcer in the USA.
Disease symptoms: Plant stunting, seedling damping-off, leaf spots and dieback, crown rot, stem canker, flower blight, and fruit rot.
Notes: Many species of Stagonosporopsis are phytopathogens, causing devastating diseases on plants from various families. Some species have a worldwide distribution, e.g., Sta. cucurbitacearum on Cucurbitaceae, and Sta. hortensis on Fabaceae, while others represent important quarantine plant pathogens limited to certain geographical areas. For example, Sta. andigena and Sta. chrysanthemi are classified as A1 and A2 quarantine pathogens, respectively, by the European and Mediterranean Plant Protection Organisation (EPPO; 2016). Stagonosporopsis tanaceti is a destructive pathogen of pyrethrum (Tanacetum cinerariifolium) in Australia but has not been reported elsewhere in the world (Vaghefi et al. 2012). Some Stagonosporopsis species have been isolated from plants but their pathogenicity has not been established. For example, Sta. dennisii has been reported from dead stems of Solidago spp. but no data are available on its pathogenicity (Boerema et al. 2004).
Species identification based on only morphology is unreliable. Stagonosporopsis was originally separated from Ascochyta based on occasional formation of multi-septate (stagonospora-like) conidia (Diedicke 1912). However, later phylogenetic studies revealed that some Stagonosporopsis spp. lack the stagonospora-like spores or any features except for globose pycnidial conidiomata, and aseptate, hyaline conidia (Aveskamp et al. 2010). Thus, multi-locus sequence typing is essential for identification of Stagonosporopsis species. The emended description of the genus Stagonosporopsis by Aveskamp et al. (2010) states that the sexual morph of Stagonosporopsis, if present, occurs only in vivo. However, some strains of Sta. chrysanthemi, Sta. caricae and Sta. inoxydabilis have been shown to produce pseudothecial ascomata intermingled with pycnidial conidiomata on agar media (Boerema et al., 2004, Vaghefi et al., 2012).
Currently, more than 40 species are linked to the genus Stagonosporopsis. However, only 27 species are recognised based on molecular data (Table 19). Previous phylogenetic studies have used one locus (act in De Gruyter et al. 2012), three loci (LSU, ITS and tub2 in Aveskamp et al. 2010), four loci (LSU, ITS, tub2 and act in Hyde et al. 2014; LSU, ITS, tub2 and rpb2 in Chen et al. 2015; ITS, tub2, chs and cal in Stewart et al. 2015) and five loci (LSU, ITS, tub2, act and tef1 in Vaghefi et al. 2012) for phylogenetic species recognition in Stagonosporopsis. However, in most cases, ITS and tub2 sequences are sufficient for achieving resolution to species level. While ITS sequences alone may be used to distinguish Stagonosporopsis as a monophyletic clade within Didymellaceae, tub2 fails to distinguish Stagonosporopsis and, thus, needs to be always combined with ITS (Chen et al. 2015). A phylogeny produced by rpb2 alone is highly similar to the combined four-locus phylogeny based on LSU, ITS, tub2 and rpb2. However, amplification of rpb2 has not been successful for many Stagonosporopsis spp. (Chen et al. 2015). Likewise, while partial cal sequences provide high resolution for Stagonosporopsis species delineation, it has not been successfully amplified in some strains (Aveskamp et al., 2010, Vaghefi et al., 2012). Thus, the use of ITS and tub2 is recommended as they will provide sufficient resolution for almost all Stagonosporopsis species, are easier to amplify, and are available for the majority of Stagonosporopsis spp. described to date (Table 19). The only two species that cannot be separated based on the LSU-ITS-tub2 phylogeny are S. bomiensis and S. papillata, for which sequencing of rpb2 was necessary (Chen et al. 2017).
References: Boerema et al. 2004 (morphology and distribution); Aveskamp et al., 2010, Chen et al., 2015 (morphology and phylogeny).
Stagonosporopsis chrysanthemi (F. Stevens) Crous et al., Australas. Pl. Pathol. 41: 681. 2012.
Basionym: Ascochyta chrysanthemi F. Stevens, Bot. Gaz. 44: 246. 1907.
Synonyms: Mycosphaerella ligulicola, K.F. Baker et al., Phytopathology 39: 799. 1949.
Didymella ligulicola (K.F. Baker et al.) Arx, Beitr. Kryptfl. Schweiz 11: 364. 1962.
Didymella ligulicola var. ligulicola (K.F. Baker et al.) Arx, Stud. Mycol. 32: 199. 1990.
Phoma ligulicola var. ligulicola Boerema, Stud. Mycol. 32: 9. 1990.
Stagonosporopsis ligulicola var. ligulicola (K.F. Baker et al.) Aveskamp et al., Stud. Mycol. 65: 46. 2010.
Typus: USA, North Carolina, West Raleigh, on Chrysanthemum indicum, Dec. 1906, F.L. Stevens (Bartholomew, Fungi Columbiani no. 2502, Field Museum of Natural History, C0004169F; designated here as lectotype, MBT385563); on Chrysanthemum morifolium, 1949, L.H. Davis [epitype of Ascochyta chrysanthemi designated here ATCC 10748, MBT385567 (preserved in a metabolically inactive state)].
Notes: Stevens (1907) described Ascochyta chrysanthemi on Chrysanthemum indicum from North Carolina; however, he did not refer to a holotype specimen in the original description. A specimen at Field Museum of Natural History (C0004169F) is chosen as lectotype, among numerous other duplicates deposited at BPI, CUP, NYBG, MSC, and various other herbaria that include collections distributed as E. Bartholomew, Fungi Columbiani 2502. Since no living cultures derived from these specimens are available, we designate ATCC 10748, isolated from Chrysanthemum morifolium from North Carolina, as ex-epitype culture of Ascochyta chrysanthemi here.
Authors: N. Vaghefi, Y. Marin-Felix, P.W. Crous & P.W.J. Taylor
Stemphylium Wallr., Flora Cryptogamica Germaniae 2: 300. 1833. Fig. 77.
Fig. 77.
Stemphylium spp. A. Disease symptoms caused by Stemphylium vesicarium (BRIP 65181) on pyrethrum leaves. B, C. Pseudothecial ascomata of Stemphylium vesicarium (BRIP 65181) on pyrethrum flower stems in vivo and in vitro respectively. D–H. Conidia of Stemphylium spp. D, H.Stemphylium vesicarium (BRIP 65181). E.Stemphylium truncatulae (holotype BRIP 14850). F, G.Stemphylium waikerieanum (ex-type VPRI 21969). I–K. Conidiophores of Stemphylium spp. I. 1-branched conidiophore of Stemphylium vesicarium (BRIP 65181). J. Simple conidiophore of Stemphylium truncatuale (holotype BRIP 14850). K. Branched and immature conidiophores of Stemphylium waikerieanum (ex-type VPRI 21969). L–N. Sexual morphs. L, M. Asci and ascospores of Stemphylium vesicarium isolated from the dead flower stems of pyrethrum. N. Immature pseudothecial ascomata of Stemphylium truncatulae on SNA after 1 wk. Scale bars: D–K = 20 μm; L, M = 100 μm. Pictures A–D, H, I, M taken from Moslemi et al. (2017).
Synonyms: Scutisporium Preuss, Linnaea 24: 112. 1821.
Epochniella Sacc., Michelia 2: 127. 1880.
Soreymatosporium Sousa da Câmara, Proposta Stemphylium: 18. 1930.
Thyrodochium Werderm., Annls mycol. 22: 188. 1942.
Classification: Dothideomycetes, Pleosporomycetidae, Pleosporales, Pleosporaceae.
Type species: Stemphylium botryosum Wallr. = Pleospora tarda E.G. Simmons. Holotype of Ste. botryosum: "Ad sparagam" in herb. Wallroth, STR. Ex-type strain of Ple. tarda: CBS 714.68.
DNA barcode (genus): ITS.
DNA barcodes (species): cmdA, gapdh. Table 20. Fig. 78.
Table 20.
DNA barcodes of accepted Stemphylium spp.
BRIP: Queensland Plant Pathology Herbarium, Brisbane, Queensland, Australia; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; VPRI: Victorian Plant Pathology Herbarium, Bundoora, Victoria, Australia. T, ET, HT and NT indicate ex-type, ex-epitype, holotype and ex-neotype strains, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; gapdh: partial glyceraldehyde-3-phosphate dehydrogenase gene; cmdA: partial calmodulin gene.
Fig. 78.
Bayesian phylogenetic tree inferred from ITS (565 bp), gapdh (613 bp) and cmdA (863) using partitioned analysis with N92+G+I substituition model for ITS and gapdh and GTR+G+I for cmdA. Highest log likelihood -34098.05. The analysis involved 122 nucleotide sequences including 98 sequences obtained from GenBank and 24 sequences obtained in the present study. Scale bar indicates expected changes per site. The tree was rooted to Alternaria alternata (GV14 634a1). The novel species described in this study are shown in bold. Bayesian posterior probability scores above 0.95 are shown at the nodes. GenBank accession numbers are indicated in Table 20. T, ET, HT and NT indicate ex-type, ex-epitype, ex-holotype and ex-neotype strains, respectively. TreeBASE:S23794.
Ascomata pseudothecial, globose or ovoid, membranous, dark brown to black, sometimes with a slender neck. Asci oblong to clavate, with distinct outer and inner walls. Ascospores elongate to oval, with 7 horizontal and 3–5 longitudinal septa, yellowish to brown, muriform on maturity. Conidiophores dark due to percurrent proliferation forming phaeodictyospores, mostly solitary, straight or flexuous, short or long, branched or unbranched, aseptate or septate. Conidiophores proliferate further after a conidium is produced, producing new cells and new conidia. Conidiogenous cells swollen at apex, single or in group. Conidia olive, dark or pale brown, verrucose, oblong or muriform, with 3 or more constricted transverse and 1–2 longitudinal or oblique septa.
Culture characteristics: Stemphylium colonies grow rapidly on a variety of media. On most media, the colonies are velvety to cottony in texture with a pale or dark olivaceous grey, brown or brownish black colour and black pigmentation on the colony reverse. Conidial density is low in cultures produced under laboratory conditions even when the isolate is grown under alternate cycle of 12 h light and 12 h darkness on PDA. Aerial mycelia flat/effuse, woolly or compact. Margins smooth and sharp or crenate and lobate.
Optimal media and cultivation conditions: SNA for pigmentation and morphological identification, PCA for morphological identification and alternatively PDA for pigmentation and morphological identification. Incubation for 1–2 wk at moderate temperatures from 23–27 °C (depending on the species) under cool white florescent light with an 8-hr or 12-hr photoperiod.
Distribution: Worldwide.
Hosts: Many Stemphylium species are saprophytes and grow on plant debris and cellulose material. However, plant pathogenic species, such as Ste. beticola, Ste. botryosum, Ste. loti, Ste. solani and Ste. vesicarium, can cause devastating damage and significant loss of agriculturally important crops annually. Stemphylium spp. are pathogenic to a wide range of hosts, such as tomato, garlic, asparagus, alfalfa, lupin, lentil and cotton. The ability of pathogens to infect a wide range of crops reflects its adaptability to wide range of climatic conditions and provides better survival chances.
Disease symptoms: Leaf spot, defoliation, curling and bending of the leaf margins and stems. Lesion size differs in various hosts and can grow to encompass the entire leaf and reduce photosynthesis.
Notes: Simmons (1967) established criteria for morphological identification of various Stemphylium spp. and introduced Pleospora herbarum as the sexual morph of Ste. botryosum. However, Simmons (1985) subsequently reclassified Stemphylium/Pleospora holomorphs and reported P. tarda as the sexual morph of Ste. botryosum and P. herbarum the sexual morph of Ste. herbarum (Moslemi et al. 2017). The asexual morph Stemphylium has been well studied, though the sexual morph Pleospora is poorly defined. The number of Pleospora spp. identified may be as many as 1000 and they are reported to be polyphyletic. Stemphylium is morphologically similar to the closely related genus Alternaria. However, unlike Alternaria in which its conidia remain attached and form a chain, Stemphylium conidia are always solitary, arising from a conidiogenous cell with a swollen apex (Inderbitzin et al. 2009). Morphological features such as spore shape and size, conidiophores, ascospores and the size and time of pseudothecial maturation are important characteristics in species identification. Other features such as variation in conidial wall ornamentation and septum development are not considered as important parameters in Stemphylium identification (Câmara et al. 2002).
For phylogenetic analyses of Stemphylium species, cmdA and gapdh were identified as the most informative genes, and rpb2 and actA as the least informative (Woudenberg et al. 2017). Among ITS, cmdA and gapdh, cmdA provides the highest resolution; however, more significant support is obtained when all three loci are combined.
Environmental factors such as temperature and moisture are key in Stemphylium disease development. Plant debris and seeds are primary sources of inoculum of Stemphylium in most host plant species. When environmental conditions are favourable, the pathogen can cause significant loss to various agricultural crops such as lupin and cotton (Boshuizen et al. 2004).
References: Simmons, 1967, Ellis, 1971, Bayaa and Erskine, 1998, Câmara et al., 2002 (morphology); Bashi and Rotem, 1975, Mwakutuya, 2006 (culture characteristics); Boshuizen et al. (2004) (biology and life cycle); Wang et al. (2010) (host range); Woudenberg et al., 2017, Crous et al., 2019b (optimal media and growing conditions).
Stemphylium rombundicum Moslemi, Y.P. Tan & P.W.J. Taylor, sp. nov. MycoBank MB829291. Fig. 79.
Fig. 79.
Stemphylium rombundicum (ex-type BRIP 27486). A–J. Conidia. K–S. Verrucose conidiogenous cells and straight and simple conidiophores with conidia attached. Scale bars: A–N, P = 20 μm; others 100 μm.
Etymology: Named after the famous beverage, Bundaberg Rum (Bundy Rum), produced in Bundaberg, Queensland, Australia, where the fungus was first isolated.
Conidiophores long, solitary, straight, septate, verrucose, light or dark brown, (62–)111–258(–307) μm, bearing one thickened, darkened, percurrent rejuvenation site. Conidiogenous cells swollen at apex, darkened, (5–)6–9(–10) μm wide. Conidia solitary, conidium body light brown to golden, turning into dark brown around longitudinal and transverse septa, verrucose, oblong or cylindrical, occasionally ovoid with curved apex, (27.5–)35–55.5(–61) × (10–)12.5–24(–26.5) μm, with 3–4 transverse septa, 1–2(–3) longitudinal or oblique septa per transverse sector. Sexual morph not observed.
Culture characteristics: Colonies on SNA after 1 wk reaching 35 mm diam, effuse, hairy or velvety, white, colourless, mycelia mostly immersed in the agar. On PDA reaching 35 mm diam, fast growing, with compact, entire, aerial mycelium, fine, woolly on the surface; reverse dark orange to dark brown in the centre with central pale brown rings growing towards the sides; thick yellow margins, and grey zones can also be seen.
Typus: Australia, Queensland, Bundaberg (Burnett Heads), from fruit lesions of Solanum lycopersicum (Solanaceae), 9 Aug. 2000, J. Maltby (holotype BRIP 27486, culture ex-type BRIP 27486).
Notes: Colonies of Ste. lycopersici produce a yellow dark red pigmentation diffusing out in PDA and other media (Yamamoto 1960). As Ste. rombundicum is closely related to species in the Ste. lycopersici complex, similar physiological characters can be observed on PDA. Colonies only sporulated at 23 °C under 12 h photoperiod on PDA. Against Ste. lycopersici in which the conidia are mostly ovoid with a pointed apex, Ste. rombondicum mostly contains cylindrical or oblong conidia. It is difficult to observe the longitudinal septa in conidia of Ste. rombundicum. Conidiophores are significantly long compared to the type Ste. lycopersici in which the conidiophores length do not exceed 140 μm. Conidia of Ste. lycopersici are significantly longer (50–74 μm × 16–23 μm) than those of Ste. rombundicum.
Stemphylium truncatulae Moslemi, Y.P. Tan & P.W.J. Taylor, sp. nov. MycoBank MB829282. Fig. 80.
Fig. 80.
Stemphylium truncatulae (ex-type BRIP 14850). A–H. Asexual morph. A–D. Conidia on SNA. E–H. Straight and simple or multibranched conidiophores and conidiogenous cells. I–J. Sexual morph. I. Immature pseudothecium. J. Ascomatal wall. Scale bars: I = 100 μm; others 20 μm.
Etymology: Named after the host species, Medicago truncatula, from which it was first collected.
Immature ascomata observed on SNA and PDA embedded in the agar. Ascomata pseudothecial, dark brown to black, globose or flask shaped, solitary or aggregated in groups of 3–5, (83.5–)185.5–186(–304) × (351–)453–483(–603) μm, with outgrowing dark mycelia; ascomatal wall 27–32 μm thick. Conidiophores solitary, straight to flexuous, mostly branched, septate or occasionally aseptate, smooth-walled, light brown, mostly 7–11 μm length, some 17–39 μm in length, bearing 2–6 thickened, pale, percurrent rejuvenation sites. Conidiogenous cells slender or slightly swollen at apex, pale, (5.5–)6.5–7(–9.5) μm. Conidia solitary, conidium body pale brown to golden or olivaceous brown, mostly smooth-walled, sometimes minutely verrucose, usually ovoid, occasionally with pointed apex, (8–)9.5–18(–21) × (14–)15.5–29.5(–32) μm, with 2–4 transverse septa, one longitudinal or oblique septa per transverse sector.
Culture characteristics: Colonies on SNA reaching 21–25 mm diam after 1 wk, with white and fluffy aerial mycelia in the centre; reverse colourless with pale olivaceous grey centre. Colonies on PDA reaching 25 mm diam after 1 wk, with white fluffy mycelia in the centre; reverse dark green to grey with thin white margins.
Typus: Australia, Victoria, from seeds of Medicago truncatula (Fabaceae), 10 Sep. 1982, M. Mebalds (holotype BRIP 14850, culture ex-type BRIP 14850).
Notes: Differs from the type species Ste. botryosum described by Simmons (1969) by producing significantly smaller conidia. According to Simmons (1969), conidia of Ste. botryosum are 24–26 μm wide and 33–35 μm long. Additionally, colonies of Ste. botryosum grow rapidly to 48 mm diam after 6 d of incubation at 25 °C (Hosen et al. 2013), while Ste. truncatulae is slow growing. The morphological identifications along with sequence analyses support Ste. truncatulae as a unique taxon closely related to the type species Ste. botryosum (CBS 714.68).
Stemphylium waikerieanum Moslemi, Jacq. Edwards & P.W.J Taylor, sp. nov. MycoBank MB829283. Fig. 81.
Fig. 81.
Stemphylium waikerieanum (ex-type VPRI 21969). A. Dried leaf of Allium cepa showing leaf lesions caused by the pathogen. B. Dried type culture. C. Revived colony after 1 wk on PDA. D–K. Asexual morph. D–H. Simple or 1-branched conidiophores on PDA and SNA. I–K. Phaeodictyospores. L–N. Sexual morph. L, M. Immature ascomata on SNA after 1 wk. N. Ascomatal wall. Scale bars: N = 100 μm; others 20 μm.
Etymology: Named after the location, Waikerie in South Australia, from where it was collected.
Immature ascomata observed on SNA after 2 wk embedded in the agar. Ascomata pseudothecial, dark brown to black, mostly flask-shaped or occasionally globose, solitary or aggregated in groups of 4–6, (267.5–)292–349.5(–374) × (191.5–)235.5–337(–381) μm, with outgrowing mycelia; ascomatal wall thin, 10–15 μm thick. Conidiophores solitary, straight, simple or occasionally 1-branched, septate, smooth-walled, pale brown, (29–)42–85(–98) μm long, cylindrical, enlarging apically to the site of conidium production. Conidiogenous cells swollen at apex, darkened, (6–)6.5–7(–7.5) μm wide. Conidia solitary, dark reddish brown, verrucose, ovoid, oblong or cylindrical, (18–)25–49(–52) × (10.5–)14–26(–30) μm, with 2–5 transverse septa, 1–2(–3) longitudinal or oblique septa per transverse sector, constricted at multiple darkened transverse septa.
Culture characteristics: Colonies on SNA reaching 28 mm diam after 1 wk with flat, entire and fluffy aerial mycelia in the centre, sub-hyaline. On PDA reaching 20 mm diam after 1 wk, compact, entire, aerial mycelium white, woolly, with rings of light olivaceous grey in the centre and dark olivaceous grey to black on the reverse side, margins regular, thick and white.
Typus: Australia, South Australia, Waikerie, from leaf spots on Allium sativum (Alliaceae), 14 Nov. 1997, H. Suheri (holotype VPRI 21969, culture ex-type VPRI 21969).
Notes: Stemphylium waikerieanum is morphologically similar to species in the Ste. vesicarium species complex. However, the conidium length in Ste. vesicarium complex does not exceed 45 μm (Simmons 1969), whereas conidia of Ste. waikerieanum were observed up to 49 μm long. The multigene phylogenetic analysis of ITS, gapdh and cmdA also support (PP value =1) this species as a novel taxon.
Authors: A. Moslemi, J. Edwards, Y.P. Tan & P.W.J. Taylor
Tubakia B. Sutton, Trans. Brit. Mycol. Soc. 60: 164. 1973. Fig. 82.
Fig. 82.
Tubakia spp. A–E. Disease symptoms. A.Tubakia californica on Tanoak tree (Notholithocarpus densiflorus). B.Tubakia californica on California black oak (Quercus kelloggii). C.Tubakia sierrafriensis (holotype CFNL 2944) on Quercus eduardi. D.Tubakia japonica (epitype NBRC H-11611) on Castanea crenata. E.Tubakia melnikiana (holotype HAL 3179 F) causing necrotic leaf lesion. F–N. Asexual morph. F. Scutellum of Tubakia paradryinoides (holotype TFM:FPH 3923). G. Central columella of Tubakia oblongispora (holotype NBRC H-11881). H–J. Conidiophores. H.Tubakia japonica (epitype NBRC H-11611). I.Tubakia oblongispora (holotype NBRC H-11881). J.Tubakia paradryinoides (holotype TFM:FPH 3923). K–M. Conidia. K.Tubakia dryinoides (holotype NBRC H-11618). L.Tubakia oblongispora (holotype NBRC H-11881). M.Tubakia paradryinoides (holotype TFM:FPH 3923). N. Microconidia of Tubakia dryinoides (holotype NBRC H-11618). Scale bars: 10 μm. Pictures taken from Braun et al. (2018).
Synonyms: Actinopelte Sacc., Annls mycol. 11: 315. 1913.
Classification: Dothideomycetes, Diaporthomycetidae, Diaporthales, Tubakiaceae.
Type species: Tubakia japonica (Sacc.) B. Sutton, basionym: Actinopelte japonica Sacc. Epitype and ex-epitype strains designated by Braun et al. (2018): NBRC H-11611, NBRC 9268 = MUCC2296 = ATCC 22472.
DNA barcodes (genus): ITS, LSU, rpb2.
DNA barcodes (species): ITS, tef1, tub2. Table 21.
Table 21.
DNA barcodes of accepted Tubakia spp.
CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CPC: Culture collection of Pedro Crous, housed at the Westerdijk Fungal Biodiversity Institute; NBRC: Biological Resource Center, NITE, Chiba, Japan. T, ET and IsoT indicate ex-type, ex-epitype and ex-isotype strains, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial β-tubulin gene.
Ascomata perithecial, pigmented, dark, on fallen overwintered leaves, rostrate, beak short, usually lateral-eccentric, slightly protuberant, with periphysate ostiolate; ascomatal wall variable in thickness, paler than stromatic layers, polyascal. Paraphyses lacking. Asci unitunicate, 8-spored, oblong-ellipsoid, stalk short to oblong, ascal apex with two refractive conoid structures, asci deliquescing at maturity. Ascospores more or less uniseriate, becoming irregularly biseriate, one-celled, hyaline, ellipsoid to fusiform, often inequilateral or slightly curved, wall finely ornamented, content granular-guttulate. Conidiomata pycnothyrial, usually circular or subcircular when viewed from above, superficial, easily removable, scutellate, fixed to the leaf by a central columella; scutellum composed of loose to dense hyphal strands, mostly branched, thick-walled, pigmented, margin compact or outer portions of the radiating hyphal strands looser to free, tips rounded, truncate or pointed, margin usually not recurved. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, usually subcylindrical-conical, lageniform, hyaline to pale brown, arising from small, colourless fertile cells around the upper part of the central pycnothyrial columella, percurrently proliferating, sometimes forming indistinct periclinal thickenings or annellations (collarettes). Conidia formed singly, globose to broad ellipsoid-obovoid, sometimes subcylindrical or somewhat irregular, wall thin to somewhat thickened, smooth to faintly rough, hyaline to pigmented, apex rounded, base rounded to attenuated, sometimes with distinct frill or peg-like basal hilum (adapted from Braun et al. 2018).
Culture characteristics: Colonies on MEA flat, with dense or fluffy, sparse to moderate aerial mycelium, margins regular or scalloped; surface ivory white, dingy white to pale yellow, straw, cream to light grey, smoke grey, white with center green olive and brown hyphal stripes, creamy white with or without concentric rings of olivaceous mycelium, or white to grey with wet conidial masses olive green to black; reverse pale grey, greyish white with olivaceous margins, smoke grey with olivaceous grey margins, golden yellow to slightly darker, yellowish grey with concentric rings, yellow with dark grey concentric rings, straw with dark brown concentric rings, or middle dark grey and yellow to medium brown towards the rim.
Optimal media and cultivation conditions: MEA, OA, PDA and PNA at 25 °C under near-ultraviolet light.
Distribution: North America, Asia, Australia and Europe.
Hosts: Castanea spp., Chrysolepis chrysophylla, Lithocarpus densiflorus and Quercus spp (Fagaceae), Liquidambar styraciflua (Hamamelidaceae), Lindera glauca (Lauraceae), and Pinus tabuliformis (Pinaceae). Reported from other hosts not verified such as Acer spp. (Aceraceae), Carya spp. (Juglandaceae) and Fraxinus spp. (Oleaceae).
Disease symptoms: Leaf spots, necrosis and death. Tubakia iowensis also causes petiole necrosis and death of whole leaves on bur oak (bur oak blight).
Notes: Tubakia was recently revised by Braun et al. (2018), resulting in the introduction of five new genera to accommodate species previously placed in Tubakia, i.e. Apiognomonioides, Involutiscutellula, Oblongisporothyrium, Paratubakia and Saprothyrium. All these genera together with Racheliella and Sphaerosporithyrium, both genera also described during the revision of the genus Tubakia, were accommodated in the new family Tubakiaceae (Braun et al. 2018). Presently, 16 species are accepted in the genus based on molecular data (ITS, tef1 and tub2 sequences). These species may form different types of asexual morphs, being the punctiform conidiomata (pycnothyria) composed of convex scutella with radiating threads of cells fixed to the substratum by a central columella the most common and characteristic structure formed. Other asexual morphs include sporodochial conidiomata and crustose or pustulate pycnidioid conidiomata. The conidia are globose to broad ellipsoid-obovoid, sometimes subcylindrical or somewhat irregular, aseptate, hyaline, subhyaline to pigmented. Tubakia suttoniana is the only species that produces sexual morph characterised by ostiolate ascomata, unitunicate asci with two refractive conoid structures in the ascal apex, and one-celled, hyaline ascospores.
Species of Tubakia are endophytes and/or pathogens in leaves and twigs of many tree species, causing distinct leaf lesions in different hosts including oak (Quercus spp.), chestnut (Castanea spp.) and other hardwood species. Moreover, T. iowensis is also capable of causing petiole necrosis and death of whole leaves on bur oak (Q. macrocarpa), sometimes killing nearly every leaf on a susceptible tree. This disease is known as bur oak blight and is most common in Iowa and Minnesota, but it has been noted in western and southern Wisconsin, northern Illinois, northeast Kansas, eastern Nebraska, and eastern South Dakota, with isolated groups of affected trees in counties of Illinois and Missouri that border Iowa (Harrington & McNew 2016).
References: Harrington et al. 2012 (morphology and pathogenicity), Harrington & McNew 2016 (pathogenicity, bur oak blight), Harrington & McNew 2018 (morphology and phylogeny), Braun et al. 2018 (morphology, pathogenicity and phylogeny).
Authors: Y. Marin-Felix & P.W. Crous
Zasmidium Fr., Summa veg. Scand., Sectio Post. (Stockholm): 407. 1849. Fig. 83.
Fig. 83.
RAxML phylogram obtained from the combined ITS (534 bp), LSU (741 bp) and rpb2 (739 bp) sequence alignment of all accepted species of Zasmidium. The tree was rooted to Anellosympodiella juniperi CBS 137992 and Neopenidiella nectandrae CBS 734.78. The novelties proposed in this study are indicated in bold. RAxML bootstrap support (BS) values above 70 % and Bayesian posterior probability scores above 0.95 are shown at the nodes. GenBank accession numbers are listed in Table 22 and Videiraet al. (2017). T, ET, HT and NT indicate ex-type, ex-epitype, holotype and ex-neootype strains, respectively. TreeBASE: S23834.
Synonyms: Periconiella Sacc., Atti Ist. Veneto Sci. Lett. Arti 3: 727. 1885.
Biharia Thirum. & Mishra, Sydowia 7: 79. 1953.
Stenellopsis B. Huguenin, Bull. Trimestriel Soc. Mycol. France 81: 695. 1966.
Verrucispora D.E. Shaw & Alcorn, Proc. Linn. Soc. New South Wales 92: 171. 1967. (nom. illegit., Art. 53.1).
Verrucisporota D.E. Shaw & Alcorn, Austral. Syst. Bot. 6: 273. 1993.
Classification: Dothideomycetes, Dothideomycetidae, Capnodiales, Mycosphaerellaceae.
Type species: Zasmidium cellare (Pers.) Fr., basionym: Racodium cellare Pers. Neotype designated by Videira et al. (2017): CBS 146.36 (duplicate cultures ATCC 36951 = IFO 4862 = IMI 044943 = LCP 52.402 = LSHB BB274 = MUCL 10089).
DNA barcode (genus): LSU.
DNA barcodes (species): ITS, rpb2, act, tef1, tub2. Table 22. Fig. 84.
Table 22.
DNA barcodes of accepted Zasmidium spp.
BRIP: Queensland Plant Pathology Herbarium, Brisbane, Australia; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CPC: Culture collection of Pedro Crous, housed at the Westerdijk Fungal Biodiversity Institute; MFLU: Mae Fah Luang University herbarium, Chiang Rai, Thailand; rapssd and ratstk were not specified in the original publications. T, ET, HT and NT indicate ex-type, ex-epitype, holotype and ex-neotype strains, respectively.
ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial large subunit (28S) nrRNA gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; act: partial actin gene; tef1: partial translation elongation factor 1-alpha gene; tub2: partial β-tubulin gene.
Fig. 84.
Zasmidium spp. A–C. Disease symptoms caused by Zasmidium cyatheae (ex-type CPC 24725). A, B. Frond spots on Cyathea delgadii. C. Erumpent subcuticular ascomata, fruiting epiphyllous. D–F. Sexual morph of Zasmidium cyatheae (ex-type CPC 24725). D. Ascoma. E. Asci. F. Ascospores. G–R. Asexual morph. G–L. Conidiophores. G. Zasmidium biverticillatum (CBS 335.36). H. Zasmidium citri-griseum (ex-epitype CBS 139467). I. Zasmidium fructigenum (ex-type CBS 139626). J. Zasmidium indonesianum (ex-type CBS 139627). K. Zasmidium musigenum (ex-type CBS 365.36). L. Zasmidium strelitziae (ex-type CBS 121711). M–Q. Conidiophores. M. Zasmidium biverticillatum (CBS 335.36). N. Zasmidium biverticillatum (CBS 335.36). O. Zasmidium citri-griseum (ex-epitype CBS 139467). P. Zasmidium fructicola (ex-type CBS 139625). Q. Zasmidium musigenum (ex-type CBS 365.36). R. Primary and secondary conidia of Zasmidium cellare (ex-neotype CBS 146.36). Scale bars: 10 μm. Pictures A–F taken from Guatimosim et al. (2016); G, K–N, Q, R from Arzanlou et al. (2007); H–J, O, P from Huang et al. (2015).
Ascomata pseudothecial, amphigenous or epiphyllous, dark brown or black, globose, single to aggregated; necks rarely perceptible, usually a paler coloured circular area, composed of convergent yellow hyphae; ascomatal wall composed of 2–3 layers of cells of textura angularis. Asci bitunicate, fasciculate, subsessile, obpyriform, obovoid to ellipsoidal, obclavate to fusoid-ellipsoidal, saccate or clavate to cylindrical, aparaphysate, 8-spored. Ascospores 2–3-seriate to multiseriate, hyaline, smooth-walled, guttulate, without sheath, fusoid to ellipsoidal with obtuse ends, straight to slightly curved, uniseptate, constricted or not at the septum, widest in the middle of apical cell. In plant pathogenic species, mycelium mostly immersed as well as superficial, rarely only immersed; hyphae branched, septate, hyaline or almost so to pigmented, pale olivaceous to brown, wall thin to somewhat thickened, immersed hyphae smooth-walled or almost so to faintly rough, external hyphae distinctly verruculose to verrucose (in culture immersed hyphae usually smooth-walled or almost so, aerial hyphae verruculose). Stromata lacking to well-developed, pigmented. Conidiophores solitary, arising from superficial hyphae, lateral, occasionally terminal, in vivo (in plant pathogenic taxa) sometimes also fasciculate, arising from internal hyphae or stromata, semimacronematous to macronematous, in culture occasionally micronematous, cylindrical, filiform, subuliform, straight to strongly geniculate-sinuous, mostly unbranched, aseptate, i.e. reduced to conidiogenous cells, to pluriseptate, subhyaline to pigmented, pale olivaceous to medium dark brown, wall thin to somewhat thickened, smooth to verruculose. Conidiogenous cells integrated, terminal, occasionally intercalary, rarely pleurogenous, or conidiophores reduced to conidiogenous cells, mostly polyblastic, sympodial, with conspicuous, somewhat thickened and darkened-refractive, planate loci. Conidia solitary or catenate, in simple or branched acropetal chains, shape and size variable, ranging from amero- to scolecosporous, aseptate to transversely plurieuseptate, subhyaline to pigmented, pale olivaceous to brown, wall thin to somewhat thickened, smooth or almost so to usually distinctly verruculose (in plant pathogenic species without superficial mycelium always verruculose); hila somewhat thickened and darkened-refractive, planate, conidial secession schizolytic (asexual morph description adapted from Braun et al. 2013).
Culture characteristics: Colonies slow growing, with sparse to moderate aerial mycelium, rarely with aerial mycelium absent, sometimes with mucoid exudate, margins smooth and regular, lobate or feathery. On MEA olivaceous, olivaceous green with margins whitish, brown olivaceous, olivaceous grey, iron mouse grey, grey, cream, yellowish brown, vinaceous buff to olivaceous buff, dark brown, or dark brown with margins grey; reverse pale or dark grey olivaceous, olivaceous black, mouse grey, iron-grey, greenish black, pale orange, isabelline, buff, dark brown, or brown vinaceous. On PDA pale white with margins pale olivaceous grey, pale mouse grey, olivaceous grey, iron-grey, iron-grey with patches orange, or brown olivaceous; reverse olivaceous grey, iron-grey, iron-grey with patches orange, or isabelline. On OA mouse grey, olivaceous grey, smoke grey with margins olivaceous grey, iron-grey, or iron-grey with broad margins of orange; reverse olivaceous, olivaceous grey, dark mouse grey, or iron-grey.
Optimal media and cultivation conditions: MEA, OA, PDA and SNA at 25 °C under near-ultraviolet light.
Distribution: Worldwide.
Hosts: Wide range of hosts belonging to 25 different families, including Alocasia odora and Anthurium sp. (Araceae), Aporosa villosa (Euphorbiaceae), Brabejum stellatifolium, Grevillea spp. and Hakea undulata (Proteaceae), Citrus spp. (Rutaceae), Cyathea delgadii (Cyatheaceae), Dasypogon sp. (Dasypogonaceae), Daviesia latifolia (Fabaceae), Elaeocarpus kirtonii (Elaeocarpaceae), Eucalyptus spp. (Myrtaceae), Gahnia sieberiana (Cyperaceae), Geniostoma rupestre (Loganiaceae), Itea parvifolia (Escalloniaceae), Lonicera japonica (Caprifoliaceae), Maclura cochinchinensis (Moraceae), Malus spp. (Rosaceae), Pittosporum tenuifolium (Pittosporaceae), Podocarpus sp. (Podocarpaceae), Pseudotsuga menziesii and Tsuga heterophylla (Pinaceae), Restio subverticillatus (Restionaceae), Rothmannia engleriana (Rubiaceae), Sasa sp. (Poaceae), Scaevola taccada (Goodeniaceae), Schinus terebinthifolius (Anacardiaceae), Strelitzia sp. (Strelitziaceae), and Syzygium cordatum (Myrtaceae).
Disease symptoms: Causing various lesions, ranging from yellowish discolorations to distinct leaf spots. Also associated with sooty blotch and flyspeck diseases. Also isolated from wall in wine cellar.
Notes: The genus Zasmidium is morphologically similar to Stenella, producing thickened and darkened conidiogenous loci and hila (Braun et al. 2013). However, these genera differ in the conidial hila and scars, being flat in Stenella and planate and somewhat thickened, darkened in Zasmidium. Moreover, Stenella belongs to Teratosphaeriaceae while Zasmidium is located within Mycosphaerellaceae (Arzanlou et al., 2007, Quaedvlieg et al., 2013, Videira et al., 2017).
A recent phylogenetic analysis based on LSU, ITS and rpb2 showed that species belonging to other genera, i.e. Mycosphaerella, Parastenella, Periconiella, Ramichloridium, Rasutoria, Stenella and Verrucisporota, were located in the monophyletic clade representing the genus Zasmidium (Videira et al. 2017). Therefore, 12 new combinations were introduced, and the genera Periconiella and Verrucisporota reduced to synonymy with Zasmidium. Based on our phylogenetic analysis, 48 species are accepted in the genus together with one new species described here, and a new combination based on Ramichloridium ducassei.
The type species of the genus, Z. cellare, has been isolated from wine cellars in Europe and America, while the other species of the genus are associated to plants as saprobic or mostly biotrophic, usually foliicolous, symptomless or causing various lesions, ranging from yellowish discolorations to distinct leaf spots. Zasmidium spp. are pathogens of a wide range of hosts such as Z. biverticillatum and Z. musigenum, which cause tropical speckle disease on members of Musaceae (Stahel, 1937, Jones, 2000), and Z. fructicola and Z. fructigenum, both pathogens of Citrus causing a disease known as citrus greasy spot (Huang et al. 2015).
References: Arzanlou et al., 2007, Videira et al., 2017 (morphology and phylogeny), Braun et al. 2013 (morphology).
Zasmidium ducassei (R.G. Shivas et al.) Y. Marín & Crous, comb. nov. MycoBank MB829646.
Basionym: Ramichloridium ducassei R.G. Shivas et al., Australas. Pl. Path. 40: 63. 2010.
Description and illustration: Shivas et al. (2011).
Typus: Australia, Queensland, Daintree, on leaves of Musa acuminata × balbisiana (Musaceae), 14 Apr. 2010, M. Berridge & K.R.E. Grice (holotype BRIP 53367, culture ex-type BRIP 53367).
Additional material examined: Malaysia, on leaves of Musa sp., 2016, P.W. Crous, CPC 32929.
Notes: This species was initially introduced as R. ducassei to accommodate some isolates associated with a severe leaf speckle disease of Ducasse banana (Musa acuminata × babisiana cv. Pisang awak) in northern Queensland (Shivas et al. 2011). The authors noticed that this species was similar to Zasmidium in having pigmented conidiophores with integrated conidiogenous cells that sympodially proliferate near the apex, with slightly thickened and refractive scars and aseptate, subhyaline conidia also with slightly thickened and refractive hila. However, it was classified in Ramichloridium in preference to Zasmidium because, at the time, Zasmidium was a paraphyletic genus in the Mycosphaerellaceae. In our phylogenetic analysis based on the combined dataset, the ex-type strain of this species was located in the well-supported clade (100 % BS / 1 PP) representing Zasmidium, and therefore a new combination Z. ducassei is proposed. Moreover, additional isolates belonging to this species were obtained from the same host genus, but from different locale, Malaysia.
Zasmidium thailandicum Crous, sp. nov. MycoBank MB829647. Fig. 85.
Fig. 85.
Zasmidium thailandicum (ex-type CBS 145027). A–C. Conidiophores sporulating on SNA. D–F. Conidiogenous cells with apical rachis giving rise to conidia. Scale bars = 10 μm.
Etymology: Named reflects the country from where it was collected, Thailand.
On SNA. Conidiophores solitary, arising from superficial hyphae, subcylindrical, pale brown, 1–3-septate, unbranched or branched below, 20–100 × (1.5–)2 μm. Conidiogenous cells subhyaline, smooth-walled, subcylindrical, apical and intercalary, apical part with well-defined rachis bearing minute (0.5 μm diam) slightly darkened scars, 10–30 × 1.5–2 μm. Ramoconidia fusoid to obclavate, hyaline, smooth-walled, aseptate, guttulate, 8–12(–17) × 2.5–3 μm. Conidia solitary, hyaline, smooth-walled, guttulate, aseptate, ellipsoid, apex obtuse, base protruding, truncate, 0.5–1 μm diam, (3–)4–4.5(–5) × (2–)2.5 μm.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium and smooth, lobate margins, reaching 20 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface pale mouse grey, reverse mouse grey.
Typus: Thailand, Rachaburi province, Bangkok, on leaves of Musa sp. (Musaceae), 2010, P.W. Crous, HPC 2158 (holotype CBS H-23850, culture ex-type CBS 145027 = CPC 33960).
Notes: Zasmidium thailandicum is closely related to Z. ducassei. Moreover, both species have been reported from the same host genus, Musa, causing leaf spots on banana leaves. However, these species can be easily distinguished by the length of their conidia [5–10 μm in Z. ducassei vs. (3–)4–4.5(–5) μm in Z. thailandicum].
Authors: P.W. Crous, J.Z. Groenewald, J.J. Luangsa-ard & Y. Marin-Felix
Acknowledgements
Sincere thanks are due to the curators Tara Rintoul (DAOM) and Bevan Weir (ICMP and PDD). We also thank the MycoBank curator Konstanze Bench, and the technical staff at the Westerdijk Institute, Arien van Iperen (cultures and deposit of herbarium samples), Mieke Starink-Willemse (DNA isolation and sequencing) and Trix Merkx (deposit of isolates) for their invaluable assistance. Jacqueline Edwards thanks Robyn Brett, VPRI curatorial assistant, for maintaining and culturing the VPRI specimens and Tonya Wiechel for extracting DNA and performing PCR on the VPRI specimens. Sampling in Maryland was supported by start-up funds from the University of Alabama at Birmingham to S.A. Krueger-Hadfield. We are thankful to Dr. Paul Kirk for helpful advice regarding the treatment of the invalid name Seiridium cupressi (Guba) Boesew. The study of the genus Alternaria was supported by the Spanish Ministerio de Economía y Competitividad, Grant CGL2017-88094-P.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
Contributor Information
Y. Marin-Felix, Email: y.marin@wi.knaw.nl.
P.W. Crous, Email: p.crous@wi.knaw.nl.
References
- Abdullah S., Sehgal S.K., Ali S. Characterization of Pyrenophora tritici-repentis (tan spot of wheat) races in Baltic States and Romania. The Plant Pathology Journal. 2017;33:133–139. doi: 10.5423/PPJ.OA.10.2016.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler A., Yaniv I., Samra Z. Exserohilum: an emerging human pathogen. European Journal of Clinical Microbiology and Infectious Diseases. 2006;25:247–253. doi: 10.1007/s10096-006-0093-3. [DOI] [PubMed] [Google Scholar]
- Ahmed S.A., Hofmüller W., Seibold M. Tintelnotia, a new genus in Phaeosphaeriaceae harbouring agents of cornea and nail infections in humans. Mycoses. 2017;60:244–253. doi: 10.1111/myc.12588. [DOI] [PubMed] [Google Scholar]
- Aiello D., Polizzi G., Crous P.W. Pleiocarpon gen. nov. and a new species of Ilyonectria causing basal rot of Strelitzia reginae in Italy. IMA Fungus. 2017;8:65–76. doi: 10.5598/imafungus.2017.08.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaradasa B.S., Madrid H., Groenewald J.Z. Porocercospora seminalis gen. et comb. nov. the causal organism of buffalo grass false smut. Mycologia. 2014;106:77–85. doi: 10.3852/13-147. [DOI] [PubMed] [Google Scholar]
- Andersen B., Sørensen J.L., Nielsen K.F. A polyphasic approach to the taxonomy of the Alternaria infectoria species-group. Fungal Genetics and Biology. 2009;46:642–656. doi: 10.1016/j.fgb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Aquino V.M., Norvell J.M., Krisher K. Fatal disseminated infection due to Exserohilum rostratum in a patient with aplastic anemia: case report and review. Clinical Infectious Diseases. 1995;20:176–178. doi: 10.1093/clinids/20.1.176. [DOI] [PubMed] [Google Scholar]
- Ariyawansa H.A., Hyde K.D., Jayasiri S.C. Fungal diversity notes 111–252 – taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity. 2015;75:27–274. [Google Scholar]
- Ariyawansa H.A., Kang J.C., Alias S.A. Pyrenophora. Mycosphere. 2014;5:351–362. [Google Scholar]
- Ariyawansa H.A., Thambugala K.M., Manamgoda D.S. Towards a natural classification and backbone tree for Pleosporaceae. Fungal Diversity. 2015;71:85–139. [Google Scholar]
- Arzanlou M., Groenewald J.Z., Fullerton R.A. Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia. 2008;20:19–37. doi: 10.3767/003158508X302212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzanlou M., Groenewald J.Z., Gams W. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology. 2007;58:57–93. doi: 10.3114/sim.2007.58.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveskamp M.M., de Gruyter J., Woudenberg J.H.C. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology. 2010;65:1–60. doi: 10.3114/sim.2010.65.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveskamp M.M., Verkley G.J.M., de Gruyter J. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia. 2009;101:363–382. doi: 10.3852/08-199. [DOI] [PubMed] [Google Scholar]
- Babaahmadi G., Mehrabi-Koushki M., Hayati J. Allophoma hayatii sp. nov., an undescribed pathogenic fungus causing dieback of Lantana camara in Iran. Mycological Progress. 2018;17:365–379. [Google Scholar]
- Balance G.M., Lamari L., Kowatsch R. Cloning, expression and occurrence of the gene encoding the Ptr necrosis toxin from Pyrenophora tritici-repentis. Molecular Plant Pathology. 1996 Online publication no. 1996/1209ballance. [Google Scholar]
- Barrus M.F., Horsfall J.G. Preliminary note on snowberry anthracnose. Phytopathology. 1928;18:797–803. [Google Scholar]
- Bashi E., Rotem J. Sporulation of Stemphylium botryosum f. sp. lycopersici in tomatoes and of Alternaria porri f. sp. solani in potatoes under alternating wet-dry regimes. Phytopathology. 1975;65:532–535. [Google Scholar]
- Baudys E. Ein Beitrag zur Kenntnis der Mikromyceten in Böhmen. Lotos Prague. 1916;63:103–112. [Google Scholar]
- Bayaa B., Erskine W. Lentil Pathology. In: Allen D.J., Lenné J.M., editors. The pathology of food and pasture legumes. Andhra Pradesh; India: 1998. pp. 423–472. (CAB International in association with ICRISAT). [Google Scholar]
- Boerema G.H., de Gruyer J., Noordeloos M.E. CABI publishing; Wallingford, UK: 2004. Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. [Google Scholar]
- Bonthond G., Sandoval-Denis M., Groenewald J.Z. Seiridium (Sporocadaceae): an important genus of plant pathogenic fungi. Persoonia. 2018;40:96–118. doi: 10.3767/persoonia.2018.40.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshuizen A., de Jong P., Heijne B. Modelling Stemphylium vesicarium on pear: an hourly-based infection model. In: Braun P., editor. International Symposium on Modelling in Fruit Research and Orchard Management. 2004. pp. 205–209. Copenhagen, Denmark. [Google Scholar]
- Braun U., Nakashima C., Crous P.W. Cercosporoid fungi (Mycosphaerellaceae) 1. Species on other fungi, Pteridophyta and Gymnospermae. IMA Fungus. 2013;4:265–345. doi: 10.5598/imafungus.2013.04.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Nakashima C., Crous P.W. Phylogeny and taxonomy of the genus Tubakia s. lat. Fungal Systematics and Evolution. 2018;1:41–99. doi: 10.3114/fuse.2018.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calduch M., Gené J., Stchigel A. Ramophialophora, a new anamorphic genus of Sordariales. Studies in Mycology. 2004;50:83–88. [Google Scholar]
- Câmara M.P.S., O'Neill N.R., van Berkum P. Phylogeny of Stemphylium spp. based on ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 2002;94:660–672. doi: 10.1080/15572536.2003.11833194. [DOI] [PubMed] [Google Scholar]
- Câmara M.P.S., Ramaley A.W., Castlebury L.A. Neophaeosphaeria and Phaeosphaeriopsis segregates of Paraphaeosphaeria. Mycological Research. 2003;107:516–522. doi: 10.1017/s0953756203007731. [DOI] [PubMed] [Google Scholar]
- Campbell G.F., Crous P.W., Lucas J.A. Pyrenophora teres f. maculata, the cause of Pyrenophora leaf spot of barley in South Africa. Mycological Research. 1999;103:257–267. [Google Scholar]
- Campbell G.F., Lucas J.A., Crous P.W. Genetic diversity amongst net- and spot-type populations of Pyrenophora teres in South Africa as determined by RAPD analysis. Mycological Research. 2002;106:602–608. [Google Scholar]
- Chaverri P., Salgado C., Hirooka Y. Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with cylindrocarpon-like anamorphs. Studies in Mycology. 2011;68:57–78. doi: 10.3114/sim.2011.68.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheewangkoon R., Groenewald J.Z., Summerell B.A. Myrtaceae, a cache of fungal biodiversity. Persoonia. 2009;23:55–85. doi: 10.3767/003158509X474752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.-L., Kirschner R. Fungi from leaves of lotus (Nelumbo nucifera) Mycological Progress. 2018;17:275–293. [Google Scholar]
- Chen Q., Hou L.W., Duan W.J. Didymellaceae revisited. Studies in Mycology. 2017;87:105–159. doi: 10.1016/j.simyco.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Jiang J.R., Zhang G.Z. Resolving the Phoma enigma. Studies in Mycology. 2015;82:137–217. doi: 10.1016/j.simyco.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary A., Hagen F., Curfs-Breuker I. In vitro activities of eight antifungal drugs against a global collection of genotyped Exserohilum isolates. Antimicrobial Agents and Chemotherapy. 2015;59:6642–6645. doi: 10.1128/AAC.01218-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements F., Shear C. Wilson; New York, USA: 1931. The Genera of Fungi. [Google Scholar]
- Cooke M.C., Ellis J.B. New Jersey fungi. Grevillea. 1878;7:37–42. [Google Scholar]
- Crous P.W., Braun U., Wingfield M.J. Phylogeny and taxonomy of obscure genera of microfungi. Persoonia. 2009;22:139–161. doi: 10.3767/003158509X461701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Carris L.M., Giraldo A. The Genera of Fungi – fixing the application of the type species of generic names – G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus. 2015;6:163–198. doi: 10.5598/imafungus.2015.06.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Giraldo A., Hawksworth D.L. The Genera of Fungi: fixing the application of type species of generic names. IMA Fungus. 2014;5:141–160. doi: 10.5598/imafungus.2014.05.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Groenewald J.Z., Gams W. Eyespot of cereals revisited: ITS phylogeny reveals new species relationships. European Journal of Plant Pathology. 2003;109:841–850. [Google Scholar]
- Crous P.W., Hawksworth D.L., Wingfield M.J. Identifying and naming plant pathogenic fungi: past, present, and future. Annual Review of Phytopathology. 2015;53:247–267. doi: 10.1146/annurev-phyto-080614-120245. [DOI] [PubMed] [Google Scholar]
- Crous P.W., Janse B.J.H., Tunbridge J. DNA homology of Pyrenophora japonica and P. teres. Mycological Research. 1995;99:1098–1102. [Google Scholar]
- Crous P.W., Phillips A.J.L., Wingfield M.J. The genera Cylindrocladium and Cylindrocladiella in South Africa, with special reference to forest nurseries. South African Journal of Forestry. 1991;157:69–85. [Google Scholar]
- Crous P.W., Schoch C.L., Hyde K.D. Phylogenetic lineages in the Capnodiales. Studies in Mycology. 2009;64 doi: 10.3114/sim.2009.64.02. 17–47-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Schumacher R.K., Akulov A. New and interesting fungi. 2. Fungal Systematics and Evolution. 2019;3:57–134. doi: 10.3114/fuse.2019.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Shivas R.G., Quaedvlieg W. Fungal Planet description sheets: 214–280. Persoonia. 2014;32:184–306. doi: 10.3767/003158514X682395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Shivas R.G. Fungal Planet description sheets: 107–127. Persoonia. 2012;28:138–182. doi: 10.3767/003158512X652633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Swart L. Fungal pathogens of Proteaceae. Persoonia. 2011;27:20–45. doi: 10.3767/003158511X606239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z. Westerdijk Fungal Biodiversity Institute; Utrecht, the Netherlands: 2019. Westerdijk Laboratory Manual Series 1: Fungal Biodiversity. [Google Scholar]
- Crous P.W., Wingfield M.J., Burgess T.I. Fungal Planet description sheets: 469–557. Persoonia. 2016;37:218–403. doi: 10.3767/003158516X694499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Burgess T.I. Fungal Planet description sheets: 558–624. Persoonia. 2017;38:240–384. doi: 10.3767/003158517X698941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Burgess T.I. Fungal Planet description sheets: 625–715. Persoonia. 2017;39:270–467. doi: 10.3767/persoonia.2017.39.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Burgess T.I. Fungal Planet description sheets: 716–784. Persoonia. 2018;40:240–393. doi: 10.3767/persoonia.2018.40.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J. Fungal Planet description sheets: 154–213. Persoonia. 2013;31:188–296. doi: 10.3767/003158513X675925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J. Fungal Planet description sheets: 320–370. Persoonia. 2015;34:167–266. doi: 10.3767/003158515X688433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Mansilla J.P. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology. 2006;55:99–131. doi: 10.3114/sim.55.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Richardson D.M. Fungal Planet description sheets: 400–468. Persoonia. 2016;36:316–458. doi: 10.3767/003158516X692185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Schumacher R.K. Fungal Planet Description Sheets: 281–319. Persoonia. 2014;33:212–289. doi: 10.3767/003158514X685680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunfer B.M. Stagonospora and Septoria diseases of barley, oat, and rye. Canadian Journal of Plant Pathology. 2000;22:332–348. [Google Scholar]
- da Cunha K.C., Sutton D.A., Gené J. Molecular identification and in vitro response to antifungal drugs of clinical isolates of Exserohilum. Antimicrobial Agents and Chemotherapy. 2012;56:4951–4954. doi: 10.1128/AAC.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danti R., Della Rocca G. Epidemiological history of Cypress Canker Disease in source and invasion sites. Forests. 2017;8:121–146. [Google Scholar]
- de Gruyter J., Woudenberg J.H.C., Aveskamp M.M. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia. 2010;102:1066–1081. doi: 10.3852/09-240. [DOI] [PubMed] [Google Scholar]
- de Gruyter J., van Gent-Pelzer M.P., Woudenberg J.H.C. The development of a validated real-time (TaqMan) PCR for detection of Stagonosporopsis andigena and S. crystalliniformis in infected leaves of potato and tomato. European Journal of Plant Pathology. 2012;134:301–313. [Google Scholar]
- de Hoog G.S. Rhinocladiella and allied genera. Studies in Mycology. 1977;15:1–140. [Google Scholar]
- de Hoog G.S., Horré R. Molecular taxonomy of the Alternaria and Ulocladium species and their identification in the routine laboratory. Mycoses. 2002;45:259–276. doi: 10.1046/j.1439-0507.2002.00747.x. [DOI] [PubMed] [Google Scholar]
- de Hoog G.S., Guarro J., Gené J. 2nd ed. CBS-KNAW Fungal Biodiversity Centre; Utrecht, the Netherlands: 2000. Atlas of Clinical Fungi. [Google Scholar]
- de Hoog G.S., Guarro J., Gené J. CBS-KNAW Fungal Biodiversity Centre; Utrecht, the Netherlands: 2011. Atlas of clinical fungi. CD-ROM version 3.1. [Google Scholar]
- Deng J.X., Cho H.S., Paul N.C. A novel Alternaria species isolated from Peucedanum japonicum in Korea. Mycobiology. 2014;42:12–16. doi: 10.5941/MYCO.2014.42.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J.X., Lib M.J., Paul N.C. Alternaria brassicifolii sp. nov. isolated from Brassica rapa subsp. pekinensis in Korea. Mycobiology. 2018;46:172–176. doi: 10.1080/12298093.2018.1468054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedicke H. Die Abteilung Hyalodidymae der Sphaerioideen. Annales Mycologici. 1912;10:135–152. [Google Scholar]
- Drechsler C. Some graminicolous species of Helminthosporium: I. Journal of Agricultural Research. 1923;24:641–739. [Google Scholar]
- Drechsler C. Phytopathological and taxonomic aspects of Ophiobolus, Pyrenophora and Helminthosporium and a new genus, Cochliobolus. Phytopathology. 1934;24:953–983. [Google Scholar]
- Ellis M.B. Commonwealth Mycological Institute; Kew, UK: 1971. Dematiaceous Hyphomycetes. [Google Scholar]
- Ellis M.B. Commonwealth Mycological Institute; Kew, UK: 1976. More Dematiaceous Hyphomycetes. [Google Scholar]
- Ellwood S.R., Syme R.A., Moffat C.S. Evolution of three Pyrenophora cereal pathogens: Recent divergence, speciation and evolution of non-coding DNA. Fungal Genetics and Biology. 2012;49:825–829. doi: 10.1016/j.fgb.2012.07.003. [DOI] [PubMed] [Google Scholar]
- EPPO . 2016. European and Mediterranean Plant Protection Organisation database on quarantine pests.http://www.eppo.int/DATABASES/databases.htm [Google Scholar]
- Fan X.L., Barreto R.W., Groenewald J.Z. Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes) Studies in Mycology. 2017;87:1–41. doi: 10.1016/j.simyco.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr D.F., Bills G.F., Chamuris G.P. 1st edn. American Phytopathological Society; USA: 1989. Fungi on plants and plant products in the United States. [Google Scholar]
- Farr D.F., Rossman A.Y. 2019. Fungal Databases, U.S. National Fungus Collections, ARS, USDA.https://nt.ars-grin.gov/fungaldatabases/ Retrieved January 5, 2019, from. [Google Scholar]
- Gannibal P.B., Lawrence D.P. Distribution of Alternaria species among sections. 3. Sections Infectoriae and Pseudoalternaria. Mycotaxon. 2016;131:781–790. [Google Scholar]
- Giraldo A., Crous P.W., Schumacher R.K. The Genera of Fungi – G3: Aleurocystis, Blastacervulus, Clypeophysalospora, Licrostroma, Neohendersonia and Spumatoria. Mycological Progress. 2017;16:325–348. [Google Scholar]
- Glawe D.A., Hummel R.L. New North American host records for Seifertia azaleae, cause of Rhododendron blud blight disease. Pacific Northwest Fungi. 2006;1:1–6. [Google Scholar]
- Golzar H., Wang C. First report of Phaeosphaeriopsis glaucopunctata as the cause of leaf spot and necrosis on Ruscus aculeatus in Australia. Australasian Plant Disease Notes. 2012;7:13–15. [Google Scholar]
- Gottwald T.R. Spatio-temporal analysis and isopath dynamics of citrus scab in nursery plots. Phytopathology. 1995;85:1082–1092. [Google Scholar]
- Griffiths D.A., Swart H.J. Conidial structure in Pestalotia pezizoides. Transactions of the British Mycological Society. 1974;63:169–173. [Google Scholar]
- Grum-Grzhimaylo A.A., Georgieva M.L., Bondarenko S.A. On the diversity of fungi from soda soils. Fungal Diversity. 2016;76:27–74. [Google Scholar]
- Guatimosim E., Schwartsburd P.B., Barreto R.W. Novel fungi from an ancient niche: cercosporoid and related sexual morphs on ferns. Persoonia. 2016;37:106–141. doi: 10.3767/003158516X690934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guba E.F. Harvard University Press; Cambridge, Massachusetts, USA: 1961. Monograph of Monochaetia and Pestalotia. [Google Scholar]
- Harrington T.C., McNew D. Bur oak blight. In: Bergdahl A.D., Hill A., editors. Diseases of the Great Plains. USDA Forest Service RMRS-GTR-335; Fort Collins, Colorado, USA: 2016. pp. 16–19. [Google Scholar]
- Harrington T.C., McNew D. A re-evaluation of Tubakia, including three new species on Quercus and six new combinations. Antonie van Leeuwenhoek. 2018;111:1003–1022. doi: 10.1007/s10482-017-1001-9. [DOI] [PubMed] [Google Scholar]
- Harrington T.C., McNew D., Yun H.Y. Bur oak blight, a new disease on Quercus macrocarpa caused by Tubakia iowensis sp. nov. Mycologia. 2012;104:79–92. doi: 10.3852/11-112. [DOI] [PubMed] [Google Scholar]
- Henry A.W. Root-rots of wheat. University of Minnesota Agricultural Experiment Station Technical Bulletin. 1924;22:1–71. [Google Scholar]
- Hernández-Restrepo M., Schumacher R.K., Wingfield M.J. Fungal Systematics and Evolution: FUSE 2. Sydowia. 2016;68:193–230. [Google Scholar]
- Hernández-Restrepo M., Groenewald J.Z., Elliott M.L. Take-all or nothing. Studies in Mycology. 2016;83:19–48. doi: 10.1016/j.simyco.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Restrepo M., Madrid H., Tan Y.P. Multi-locus phylogeny and taxonomy of Exserohilum. Persoonia. 2018;41:71–108. doi: 10.3767/persoonia.2018.41.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett D.S., Ohman A., Glotzer D. Progress in molecular and morphological taxon discovery in Fungi and options for formal classification of environmental sequences. Fungal Biology Reviews. 2011;25:38–47. [Google Scholar]
- Holliday P. Cambridge University Press; Cambridge, UK: 1980. Fungal diseases of tropical crops. [Google Scholar]
- Hosen M.I., Ahmed A.U., Zaman J. Cultural and physiological variation between isolates of Stemphylium botryosum the causal of Stemphylium blight disease of lentil (Lens culinaris) World Journal of Agricultural Sciences. 2013;5:94–98. [Google Scholar]
- Huang F., Groenewald J.Z., Zhu L. Cercosporoid diseases of Citrus. Mycologia. 2015;107:1151–1171. doi: 10.3852/15-059. [DOI] [PubMed] [Google Scholar]
- Hyde K.D., Chaiwan N., Norphanphoun C. Mycosphere notes 169–224. Mycosphere. 2018;9:271–430. [Google Scholar]
- Hyde K.D., Hongsanan S., Jeewon R. Fungal diversity notes 367–491: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity. 2016;80:1–270. [Google Scholar]
- Hyde K.D., Nilsson R.H., Alias S.A. One stop shop: backbones trees for important phytopathogenic genera: I. Fungal Diversity. 2014;67:21–125. [Google Scholar]
- Hyde K.D., Norphanphoun C., Abreu V.P. Fungal diversity notes 603–708: taxonomic and phylogenetic notes on genera and species. Fungal Diversity. 2017;87:1–235. [Google Scholar]
- Inderbitzin P., Mehta Y.R., Berbee M.L. Pleospora species with Stemphylium anamorphs: a four locus phylogeny resolves new lineages yet does not distinguish among species in the Pleospora herbarum clade. Mycologia. 2009;101:329–339. doi: 10.3852/08-071. [DOI] [PubMed] [Google Scholar]
- Jaklitsch W., Gardiennet A., Voglmayr H. Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria. Persoonia. 2016;37:82–105. doi: 10.3767/003158516X690475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasiri S.C., Wanasinghe D.N., Ariyawansa H.A. Two novel species of Vagicola (Phaeosphaeriaceae) from Italy. Mycosphere. 2015;6:716–728. [Google Scholar]
- Jenkins A.E. A specific term for diseases caused by Elsinoë and Sphaceloma. Plant Disease Reporter. 1947;31:71. [Google Scholar]
- Johnston P.R., Seifert K.A., Stone J.K. Recommendations on generic names competing for use in Leotiomycetes (Ascomycota) IMA Fungus. 2014;5:91–120. doi: 10.5598/imafungus.2014.05.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.D. Tropical speckle. In: Jones R.D., editor. Disease of Banana, Abaca and Enset. CABI Publishing; Wallingford, UK: 2000. pp. 116–120. [Google Scholar]
- Kainer M.A., Reagan D.R., Nguyen D.B. Fungal infections associated with contaminated methylprednisolone in Tennessee. New England Journal of Medicine. 2012;367:2194–2203. doi: 10.1056/NEJMoa1212972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunarathna A., Papizadeh M., Senanayake I.C. Novel fungal species of Phaeosphaeriaceae with an asexual/sexual morph connection. Mycosphere. 2017;8:1818–1834. [Google Scholar]
- Kirk P.M., Stalpers J.A., Braun U. A without-prejudice list of generic names of fungi for protection under the International Code of Nomenclature for algae, fungi and plants. IMA Fungus. 2013;4:381–443. doi: 10.5598/imafungus.2013.04.02.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup A., Wanscher J.H. 3rd ed. Methuen; London, UK: 1978. Methuen Handbook of Colour. [Google Scholar]
- Laemmlen F. Publication 8040, University of California, Agriculture and Natural Resources; Oakland, USA: 2001. Alternaria diseases.http://anrcatalog.ucdavis.edu [Google Scholar]
- Lamari L., Bernier C.C. Evaluation of wheat lines and cultivars to tan spot (Pyrenophora tritici-repentis) based on lesion type. Canadian Journal of Plant Pathology. 1989;11:49–56. [Google Scholar]
- Lawrence C.B., Mitchell T.K., Craven K.D. At death’s door: Alternaria pathogenicity mechanisms. The Plant Pathology Journal. 2008;24:101–111. [Google Scholar]
- Lawrence D.P., Gannibal P.B., Peever T.L. The sections of Alternaria: formalizing species-group concepts. Mycologia. 2013;105:530–546. doi: 10.3852/12-249. [DOI] [PubMed] [Google Scholar]
- Lawrence D.P., Park M.S., Pryor B.M. Nimbya and Embellisia revisited, with nov. comb. for Alternaria celosiae and A. perpunctulata. Mycological Progress. 2012;11:799–815. [Google Scholar]
- Lawrence D.P., Rotondo F., Gannibal P.B. Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycological Progress. 2016;15:1–22. [Google Scholar]
- Leonard K.J. Synonymy of Exserohilum halodes with E. rostratum, and induction of the ascigerous state, Setosphaeria rostrata. Mycologia. 1976;68:402–411. [Google Scholar]
- Leonard K.J., Suggs E.G. Setosphaeria prolata, the ascigerous state of Exserohilum prolatum. Mycologia. 1974;66:281–297. [Google Scholar]
- Lepoint P., Renard M.E., Legreve A. Genetic diversity of the mating type and toxin production genes in Pyrenophora tritici-repentis. Phytopathology. 2010;100:474–483. doi: 10.1094/PHYTO-100-5-0474. [DOI] [PubMed] [Google Scholar]
- Li G.J., Hyde H.D., Zhao R.L. Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity. 2016;78:1–237. [Google Scholar]
- Li H.Y., Sun G.Y., Zhai X.R. Dissoconiaceae associated with sooty blotch and flyspeck on fruits in China and the United States. Persoonia. 2012;28:113–125. doi: 10.3767/003158512X651157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Phookamsak R., Mapook A. Seifertia shangrilaensis sp. nov. (Melanommataceae), a new species from Southwest China. Phytotaxa. 2016;273:34–42. [Google Scholar]
- Li W.J., Bhat D.J., Camporesi E. New asexual morph taxa in Phaeosphaeriaceae. Mycosphere. 2015;6:681–708. [Google Scholar]
- Lin S.-H., Huang S.-L., Li Q.-Q. Characterization of Exserohilum rostratum, a new causal agent of banana leaf spot disease in China. Australasian Plant Pathology. 2011;40:246–259. [Google Scholar]
- Liu A.R., Xu T., Guo L.D. Molecular and morphological description of Pestalotiopsis hainanensis sp. nov., a new endophyte from a tropical region of China. Fungal Diversity. 2007;24:23–36. [Google Scholar]
- Liu F., Wang J., Li H. Setophoma spp. on Camellia sinensis. Fungal Systematics and Evolution. 2019;4:43–57. doi: 10.3114/fuse.2019.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.K., Hyde K.D., Jones E.B.G. Fungal diversity notes 1–100: taxonomic and phylogenetic contributions to fungal species. Fungal Diversity. 2015;72:1–197. [Google Scholar]
- Louw J.P.J., Victor D., Crous P.W. Characterization of Pyrenophora isolates associated with spot and net type lesions on barley in South Africa. Journal of Phytopathology. 1995;143:129–134. [Google Scholar]
- Luttrell E.S. Taxonomic criteria in Helminthosporium. Mycologia. 1963;55:643–674. [Google Scholar]
- Maharachchikumbura S.S., Camporesi E., Liu Z.Y. Seiridium venetum redescribed, and S. camelliae, a new species from Camellia reticulata in China. Mycological Progress. 2015;14:85. [Google Scholar]
- Maharachchikumbura S.S., Hyde K.D., Groenewald J.Z. Pestalotiopsis revisited. Studies in Mycology. 2014;79:121–186. doi: 10.1016/j.simyco.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manamgoda D.S., Rossman A.Y., Castlebury L.A. The genus Bipolaris. Studies in Mycology. 2014;79:221–288. doi: 10.1016/j.simyco.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Felix Y., Groenewald J.Z., Cai L. Genera of phytopathogenic fungi: GOPHY 1. Studies in Mycology. 2017;86:99–216. doi: 10.1016/j.simyco.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Felix Y., Hernández-Restrepo M., Wingfield M.J. Genera of phytopathogenic fungi: GOPHY 2. Studies in Mycology. 2019;92:47–133. doi: 10.1016/j.simyco.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincowitz S., Crous P.W., Groenewald J.Z. CBS-KNAW Fungal Biodiversity Centre; Utrecht, the Netherlands: 2008. Microfungi occurring on Proteaceae in the fynbos. CBS Biodiversity Series 7. [Google Scholar]
- McGinnis M.R., Rinaldi M.G., Winn R.E. Emerging agents of phaeohyphomycosis: pathogenic species of Bipolaris and Exserohilum. Journal of Clinical Microbiology. 1986;24:250–259. doi: 10.1128/jcm.24.2.250-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena M., Gupta S.K., Swapnil P. Alternaria toxins: potential virulence factors and genes related to pathogenesis. Frontiers in Microbiology. 2017;8:1451. doi: 10.3389/fmicb.2017.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moslemi A., Ades P.K., Groom T. Alternaria infectoria and Stemphylium herbarum, two new pathogens of pyrethrum (Tanacetum cinerariifolium) in Australia. Australasian Plant Pathology. 2017;46:91–101. [Google Scholar]
- Mwakutuya E. Department of Plant Sciences, University of Saskatchewan; Saskatoon, Canada: 2006. Epidemiology of Stemphylium blight on lentil (Lens culinaris) in Saskatchewan. Ph.D. dissertation. [Google Scholar]
- Nag Raj T. Mycologue Publications; Waterloo, Canada: 1993. Coelomycetous anamorphs with appendage-bearing conidia. [Google Scholar]
- Nattrass R.M., Booth C., Sutton B.C. Rhynchosphaeria cupressi sp. nov., the causal organism of Cupressus canker in Kenya. Transactions of the British Mycological Society. 1963;46:102–106. [Google Scholar]
- Nees von Esenbeck C.G.D. Würzburg; Germany: 1817. System der Pilze und Schwämme. [Google Scholar]
- Nisikado Y. Studies on Helminthosporium diseases of Graminae in Japan. Reports of the Ohara Institute of Agricultural Research. 1928;4:1–384. (in Japanese) [Google Scholar]
- Oliver R.P., Friesen T.L., Faris J.D. Stagonospora nodorum: from pathology to genomics and host resistance. Annual Review of Phytopathology. 2012;50:23–43. doi: 10.1146/annurev-phyto-081211-173019. [DOI] [PubMed] [Google Scholar]
- Padhye A.A., Ajello L., Wieden M.A. Phaeohyphomycosis of the nasal sinuses caused by a new species of Exserohilum. Journal of Clinical Microbiology. 1986;24:245–249. doi: 10.1128/jcm.24.2.245-249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L. The symptoms of five grape disease and main control methods. Bulletin of Agricultural Science and Technology. 1994;1:27–28. [Google Scholar]
- Partridge E.C., Morgan-Jones G. Notes on Hyphomycetes. LXXXVIII. New genera in which to classify Alysidium resinae and Pycnostysanus azaleae, with a consideration of Sorocybe. Mycotaxon. 2002;83:335–352. [Google Scholar]
- Passerini G. La nebbia del grano turco. Bolletino del Comizio Agrario Parmense. 1876;10:1–3. [Google Scholar]
- Pastor F.J., Guarro J. Alternaria infections: laboratory diagnosis and relevant clinical features. Clinical Microbiology and Infection. 2008;14:734–746. doi: 10.1111/j.1469-0691.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- Phengsintham P., Chukeatirote E., McKenzie E.H.C. Monograph of Cercosporoid fungi from Laos. Current Research in Environmental & Applied Mycology. 2013;3:34–158. [Google Scholar]
- Phillips A.J.L. A comparison of methods for inoculating bean plants with Elsinoë phaseoli and some factors affecting infection. Annals of Applied Biology. 1994;125:97–104. [Google Scholar]
- Phookamsak R., Liu J.-K., Manamgoda D.S. The sexual state of Setophoma. Phytotaxa. 2014;176:260–269. [Google Scholar]
- Phookamsak R., Liu J.-K., McKenzie E.H.C. Revision of Phaeosphaeriaceae. Fungal Diversity. 2014;68:159–238. [Google Scholar]
- Phookamsak R., Wanasinghe D.N., Hongsanan S. Towards a natural classification of Ophiobolus and ophiobolus-like taxa; introducing three novel genera Ophiobolopsis, Paraophiobolus and Pseudoophiobolus in Phaeosphaeriaceae (Pleosporales) Fungal Diversity. 2017;87:299–339. [Google Scholar]
- Potebnia A. Mycologische Studien. Annales Mycologici. 1907;5:1–28. [Google Scholar]
- Poursafar A., Ghosta Y., Orina A.S. Taxonomic study on Alternaria sections Infectoriae and Pseudoalternaria associated with black (sooty) head mold of wheat and barley in Iran. Mycological Progress. 2018;17:343–356. [Google Scholar]
- Quaedvlieg W., Binder M., Groenewald J.Z. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia. 2014;33:1–40. doi: 10.3767/003158514X681981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W., Kema G.H.J., Groenewald J.Z. Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia. 2011;26:57–69. doi: 10.3767/003158511X571841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W., Verkley G.J.M., Shin H.D. Sizing up Septoria. Studies in Mycology. 2013;75:307–390. doi: 10.3114/sim0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner R.W. Commonwealth Mycological Institute; Kew, UK: 1970. A mycological colour chart. [Google Scholar]
- Robert V., Vu D., Amor A.B.H. MycoBank gearing up for new horizons. IMA Fungus. 2013;4:371–379. doi: 10.5598/imafungus.2013.04.02.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D.C., Swart H.J. Conidium wall structure in Seiridium and Monochaetia. Transactions of the British Mycological Society. 1980;74:289–296. [Google Scholar]
- Roberts R.G. Two new species of Alternaria from pear fruit. Mycotaxon. 2007;100:159–167. [Google Scholar]
- Saccardo P.A. Sylloge Hyphomycetum. Sylloge Fungorum. 1886;4:1–807. [Google Scholar]
- Salgado-Salazar C., Rossman A.Y., Chaverri P. The genus Thelonectria (Nectriaceae, Hypocreales, Ascomycota) and closely related species with cylindrocarpon-like asexual morphs. Fungal Diversity. 2016;80:411–455. [Google Scholar]
- Schoch C.L., Seifert K.A., Huhndorf S. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences, USA. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K.A., Hughes S.J., Boulay H. Taxonomy, nomenclature and phylogeny of three cladosporium-like hyphomycetes, Sorocybe resinae, Seifertia azaleae and the Hormoconis anamorph of Amorphotheca resinae. Studies in Mycology. 2007;58:235–245. doi: 10.3114/sim.2007.58.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivas R.G., Grice K.R.E., Young A.J. Ramichloridium spp. on Musa in northern Queensland: introducing Ramichloridium ducassei sp. nov. on leaf streaks of Ducasse banana. Australasian Plant Pathology. 2011;40:61–65. [Google Scholar]
- Shivas R.G., Young A.J., McCallie K.J. Zasmidium macluricola R.G. Shivas, A.J. Young & U. Braun, sp. nov. Fungal Planet. 39. Persoonia. 2009;23:190–191. [Google Scholar]
- Shoemaker R.A. Nomenclature of Drechslera and Bipolaris, grass parasites segregated from 'Helminosporium'. Canadian Journal of Botany. 1959;37:879–887. [Google Scholar]
- Shoemaker R.A. Pyrenophora phaeocomes (Reb. ex Fr.) Fr. Canadian Journal of Botany. 1961;39:901–908. [Google Scholar]
- Shoemaker R.A., Babcock C.E. Phaeosphaeria. Canadian Journal of Botany. 1989;67:1500–1599. [Google Scholar]
- Shoemaker R.A., Müller E., Morgan-Jones G. Fuckel's Massaria marginata and Seiridium marginatum Nees ex Steudel. Canadian Journal of Botany. 1966;44:247–254. [Google Scholar]
- Simay E.I. Are Pyrenophora teres and Pyrenophora graminea different species? Barley Newsletter. 1992;36:173–174. [Google Scholar]
- Simmons E.G. Typification of Alternaria, Stemphylium and Ulocladium. Mycologia. 1967;59:67–92. [PubMed] [Google Scholar]
- Simmons E.G. Perfect states of Stemphylium. Mycologia. 1969;61:1–26. [PubMed] [Google Scholar]
- Simmons E.G. Perfect states of Stemphylium II. Sydowia. 1985;38:284–293. [Google Scholar]
- Simmons E.G. Alternaria taxonomy: current status, viewpoint, challenge. In: Chelkowski J., Visconti A., editors. Alternaria: biology, plant diseases, and metabolites. Elsevier; New York, USA: 1992. pp. 1–35. [Google Scholar]
- Simmons E.G. Alternaria chronology and catalogue raisonné. Part I: 1796–1871. Mycotaxon. 1995;55:1–53. [Google Scholar]
- Simmons E.G. CBS Fungal Biodiversity Centre; Utrecht, the Netherlands: 2007. Alternaria. An identification manual. CBS Biodiversity Series 6. [Google Scholar]
- Singh V., Shrivastava A., Jadon S. Alternaria diseases of vegetable crops and its management control to reduce the low production. International Journal of Agriculture Sciences. 2015;7:834–840. [Google Scholar]
- Sivanesan A. Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycological Papers. 1987;158:1–261. [Google Scholar]
- Smith H., Wingfield M.J., Crous P.W. Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. South African Journal of Botany. 1996;62:86–88. [Google Scholar]
- Sprague R. The Ronald Press Company; New York, USA: 1950. Diseases of cereals and grasses in North America. [Google Scholar]
- Stahel G. The banana leaf speckle in Surinam caused by Chloridium musae nov. spec. and another related banana disease. Tropical Agriculture. 1937;14:42–44. [Google Scholar]
- Stewart J.E., Turner A.N., Brewer M.T. Evolutionary history and variation in host range of three Stagonosporopsis species causing gummy stem blight of cucurbits. Fungal Biology. 2015;119:370–382. doi: 10.1016/j.funbio.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Stevens L.F. The Chrysanthemum ray blight. Botanical Gazzette. 1907;44:241–258. [Google Scholar]
- Subramanian C.V., Jain B.L. A revision of some graminicolous Helminthosporia. Current Science. 1966;35:352–355. [Google Scholar]
- Sutton B.C. Forest microfungi. III. The heterogeneity of Pestalotia de Not. section sexloculatae Klebahn sensu Guba. Canadian Journal of Botany. 1969;47:2083–2094. [Google Scholar]
- Sutton B.C. Commonwealth Mycological Institute; Kew, UK: 1980. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. [Google Scholar]
- Swart L., Crous P.W., Kang J.C. Differentiation of species of Elsinoë associated with scab disease of Proteaceae based on morphology symptomatology and ITS sequence phylogeny. Mycologia. 2001;93:366–379. [Google Scholar]
- Tamura K., Stecher G., Peterson D. MEGA 6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.P., Madrid H., Crous P.W. Johnalcornia gen. et. comb. nov., and nine new combinations in Curvularia based on molecular phylogenetic analysis. Australasian Plant Pathology. 2014;43:589–603. [Google Scholar]
- Thambugala K.M., Camporesi E., Ariyawansa H.A. Phylogeny and morphology of Phaeosphaeriopsis triseptata sp. nov., and Phaeosphaeriopsis glaucopunctata. Phytotaxa. 2014;176:238–250. [Google Scholar]
- Thambugala K.M., Wanasinghe D.N., Phillips A.J.L. Mycosphere notes 1–50: Grass (Poaceae) inhabiting Dothideomycetes. Mycosphere. 2017;8:697–796. [Google Scholar]
- Thomma B.P.H.J. Alternaria spp.: from general saprophyte to specific parasite. Molecular Plant Pathology. 2003;4:225–236. doi: 10.1046/j.1364-3703.2003.00173.x. [DOI] [PubMed] [Google Scholar]
- Tibpromma S., Hyde K.D., Jeewon R. Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity. 2017;83:1–261. [Google Scholar]
- Vaghefi N., Pethybridge S.J., Ford R. Stagonosporopsis spp. associated with ray blight disease of Asteraceae. Australasian Plant Pathology. 2012;41:675–686. [Google Scholar]
- Valenzuela-Lopez N., Cano-Lira J.F., Guarro J. Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Studies in Mycology. 2018;90:1–69. doi: 10.1016/j.simyco.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira S.I.R., Groenewald J.Z., Nakashima C. Mycosphaerellaceae – Chaos or clarity? Studies in Mycology. 2017;87:257–421. doi: 10.1016/j.simyco.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D., Groenewald M., de Vries M. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology. 2019;92:135–154. doi: 10.1016/j.simyco.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman S.A. A method for counting the number of fungi in the soil. Journal of Bacteriology. 1922;7:339–341. doi: 10.1128/jb.7.3.339-341.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallwork H., Lichon A., Sivanesan A. Drechslera wirreganensis — a new hyphomycete affecting barley in Australia. Mycological Research. 1992;96:886–888. [Google Scholar]
- Wanasinghe D.N., Phukhamsakda C., Hyde K.D. Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Diversity. 2018;89:1–236. [Google Scholar]
- Wang L., Du Y., Ju L. Ramichloridium apiculatum, a new record for China, causing sooty blotch and flyspeck. Mycotaxon. 2014;127:121–127. [Google Scholar]
- Wang Y., Geng Y., Pei Y.F. Molecular and morphological description of two new species of Stemphylium from China and France. Mycologia. 2010;102:708–717. doi: 10.3852/09-208. [DOI] [PubMed] [Google Scholar]
- Wehmeyer L.E. The genera Leptosphaeria, Pleospora and Clathrospora in Mt. Rainier National Park. Mycologia. 1952;44:621–655. [Google Scholar]
- Wijayawardene N.N., Hyde K.D., Wanasinghe D.N. Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Diversity. 2016;77:1–316. [Google Scholar]
- Winton L.M., Stone J.K., Hansen E.M. The systematic position of Phaeocryptopus gaeumannii. Mycologia. 2007;99:240–252. doi: 10.3852/mycologia.99.2.240. [DOI] [PubMed] [Google Scholar]
- Woudenberg J.H.C., Groenewald J.Z., Binder M. Alternaria redefined. Studies in Mycology. 2013;75:171–212. doi: 10.3114/sim0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg J.H.C., Hanse B., van Leeuwen G.C.M. Stemphylium revisited. Studies in Mycology. 2017;87:77–103. doi: 10.1016/j.simyco.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg J.H.C., Seidl M.F., Groenewald J.Z. Alternaria section Alternaria: Species, formae speciales or pathotypes? Studies in Mycology. 2015;82:1–21. doi: 10.1016/j.simyco.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg J.H.C., Truter M., Groenewald J.Z. Large-spored Alternaria pathogens in section Porri disentangled. Studies in Mycology. 2014;79:1–47. doi: 10.1016/j.simyco.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto W. Synonymous species of Alternaria and Stemphylium in Japan. Transactions of the Mycological Society in Japan. 1960;2:88–93. [Google Scholar]
- Yang J.W., Yeh Y.H., Kirschner R. A new endophytic species of Neostagonospora (Pleosporales) from the coastal grass Spinifex littoreus in Taiwan. Botany. 2016;94:593–598. [Google Scholar]
- Zhang G., Berbee M.L. Pyrenophora phylogenetics inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 2001;93:1048–1063. [Google Scholar]
- Zhang R., Zhang Z., Zhai X.R. A new species of Dissoconium from China colonizing apples. Mycotaxon. 2007;101:165–172. [Google Scholar]