Summary
Activated T cells are pathological in various autoimmune and inflammatory diseases including Psoriasis, and also in graft rejection and graft‐versus‐host‐disease. In these pathological conditions, selective silencing of activated T cells through physiological receptors they express remains a clinical challenge. In our previous studies we found that activation of dopamine receptors (DRs) in resting human T cells activates these cells, and induces by itself many beneficial T cell functions. In this study, we found that normal human T cells express all types of DRs, and that expression of D1R, D4R and D5R increases profoundly after T cell receptor (TCR) activation. Interestingly, DR agonists shift the membrane potential (V m) of both resting and activated human T cells, and induces instantaneous T cell depolarization within 15 seconds only. Thus, activation of DRs in T cells depolarize these immune cells, alike activation of DRs in neural cells. The skin of Psoriasis patients contains 20‐fold more D1R+ T cells than healthy human skin. In line with that, 25‐fold more D1R+ T cells are present in Psoriasis humanized mouse model. Highly selective D1‐like receptor agonists, primarily Fenoldopam (Corlopam) – a D1‐like receptor agonist and a drug used in hypertension, induced the following suppressive effects on activated T cells of Psoriasis patients: reduced chemotactic migration towards the chemokine SDF‐1/CXCL12; reduced dramatically the secretion of eight cytokines: tumor necrosis factor‐α, interferon‐γ, interleukin‐1β (IL‐1β), IL‐2, IL‐4, IL‐6, IL‐8 and IL‐10; and reduced three T cell activation proteins/markers: CD69, CD28 and IL‐2. Next, we invented a novel topical/dermal Fenoldopam formulation, allowing it to be spread on, and providing prolonged and regulated release in, diseased skin. Our novel topical/dermal Fenoldopam: reduced secretion of the eight cytokines by activated human T cells; reduced IL‐1β and IL‐6 secretion by human lipopolysaccharide‐inflamed skin; eliminated preferentially >90% of live and large/proliferating human T cells. Together, our findings show for the first time that both resting and activated T cells are depolarized instantaneously via DRs, and that targeting D1‐like receptors in activated T cells and inflamed human skin by Fenoldopam, in Psoriasis, and potentially in other T cell‐mediated diseases, could be therapeutic. Validation in vivo is required.
Keywords: dopamine receptors, dopamine D1‐like receptors, depolarization, Fenoldopam, Psoriasis, T cells
Introduction
Psoriasis and T cells
Psoriasis is a chronic inflammatory skin disorder characterized by abnormal activation of T cells and their migration into the skin, leading to the accumulation of inflammatory cells. The most current disease model of Psoriasis considers that the main driver of Psoriasis is the type 17 (Th17, Tc17) T cells, combined with specific auto‐antigens.1, 2, 3 Patients with moderate to severe forms of Psoriasis undergo systemic treatment with increasing risk of adverse effects. For all these reasons, combined with the fact that psoriatic lesions may have a significant impact on a patient's quality of life, it is obvious that there is an urgent need to discover and develop new suitable therapeutic strategies for individuals with mild to moderate Psoriasis.
In this study, we studied the expression of D1‐like receptors on activated T cells of Psoriasis patients, in comparison to T cells of healthy individuals. We also studied the ability of D1‐like receptor agonists to suppress activated T cells of Psoriasis patients, and inflamed human skin. We were especially interested in studying the potential suppression induced by the D1‐like receptor agonist and drug: Fenoldopam (Corlopam), either in its soluble form (alike used for iv injections to patients with hypertension), or in its new topical formulation, that we designed and produced for application on the skin.
Dopamine and its receptors in the immune system
Dopamine is a key neurotransmitter, signaling via five types of dopamine receptors (DRs): D1R–D5R, expressed in variable levels and combinations in many cells types, within many organs and tissues. The DRs are sub‐grouped into two broad DR families: the D1‐like receptors: D1R and D5R; and the D2‐like receptors: D2R, D3R and D4R. In the immune system, the DRs are expressed in many types of immune cells, among them: T cells of several subtypes, B cells, Natural Killer cells, Dendritic cells, Monocytes, Macrophages, Microglia and Neutrophils (see for examples refs 4, 5, 6, 7, 8, 9 and other papers on this topic).
As for T cells, the focus of the current study: dopamine itself at low physiological concentrations of ~10−8–10−7 M (10–100 nM), as well as selective D2R, D3R and D4R agonists at ~10−8–10−6 M, usually activate on their own resting normal human T cells, and induce multiple potent and beneficial T‐cell effects. The dopamine‐induced beneficial effects on resting T cells include, but not limited to: T cell adhesion to glycoproteins of the extracellular matrix, chemotactic migration, homing, cytokine secretion, activation of downstream signaling, increased expression on key receptors on the cell surface, and various other effects (reviewed, for example, in refs 5, 6, 8, 9, 10, 11).
In contrast to dopamine's activation of resting T cells, dopamine and some of its receptor agonists supress activated T cells. This was shown by several different studies. Thus, when T cells are already activated before the encounter with dopamine or with DR agonists, i.e. when before they encounter dopamine, the T cells are pre‐activated by either mitogens, CD3/CD28 antibodies, T cell‐activating cytokines or other stimuli, or even if the T cells are simultaneously exposed to both dopamine or DR agonist and a different type of T cell‐stimulating molecule, the final outcome is suppression of T cells.8
Hence, whereas either dopamine on its own at low physiological concentrations, or several DR agonists on their own, are potent activators of resting T cells, they are potent suppressors of activated T cells, and of other activated immune cells (for review citing many findings revealed by various groups worldwide, see refs 8, 9, 12 and papers cited therein).
This shows that the effects of dopamine and its receptors on T cells are ‘a matter of context’ and usually the opposite for resting and activated T cells.8
Various diseases of the nervous system or immune system, are accompanied by (or perhaps even caused by) abnormal expression of DRs on immune cells, and/or abnormal responsiveness to dopamine and DR analogues. A variety of evidence supporting this notion has been reviewed.8, 9 Moreover, dopamine analogues/drugs have strong effects on various immunological diseases among them autoimmune diseases (for review see, 12, 13 in addition to their use in diseases of the central nervous system (for reviews read, for example, Refs 8, 9, 10, 13, 14).
The dopamine D1 receptor in activated immune cells is a deliverer of suppressive signals to these cells
We chose to focus in this study on the D1‐like receptors in T cells, as previous studies have shown that the binding of these receptors in some activated immune cells by selective D1‐like receptor analogues (i.e. the analogues being defined as either DR agonists or antagonists, based on their effects on other non‐immune cells, primarily neural cells) induces potent suppressive effects on these cells.11, 12, 13, 14, 15, 16, 17, 18, 19
The suppressive effects mediated by D1‐like receptors expressed on the cell surface of activated immune cells in general, and activated T cells in particular, were revealed in several studies by different research groups, among them the ones cited below. First, activation of D1‐like receptors in activated normal human T cells inhibits their proliferation by elevating intracellular cAMP ¸and down‐regulating expression of important signaling intermediates of the T cell receptor (TCR) activation pathway.15, 16 Second, stimulation of D1‐like receptors in activated regulatory T cells [i.e. regulatory T cells pre‐activated by irradiated T cell‐depleted spleen cells as accessory cells, anti‐CD3 antibodies and IL‐2], suppress the activity and adhesive and migratory abilities of these cells.17 Third, activation of D1‐like receptors in activated regulatory T cells (i.e. pre‐activated by anti‐CD3/CD28 antibodies) suppress their cytokine secretion and function.18 Fourth, stimulation of D1‐like receptors (and not D2‐like receptors) by mitogen [lipopolysaccharide (LPS)] ‐activated bone marrow‐derived macrophages, inhibits inflammation by inhibiting the activation of NLRP3 inflammasome, and also reduces the LPS‐induced secretion of inflammasome‐dependent cytokines: IL‐1β and IL‐18.19 Based on these findings, Yan et al. suggested that D1Rs suppress inflammation in ‘stressed’ conditions, and can be a potential target for treatment of inflammasome‐driven diseases.19
Interestingly, several other studies revealed strong suppressive effects of D1‐like receptors on various immune‐mediated pathologies, when these receptors are bound to, or blocked by, D1‐like receptor antagonists (not agonists).20, 21, 22 The findings of another relevant study suggested that D1‐like receptors mediate immediate and late‐phase skin reactions, by promoting Th2 induction and mast cell degranulation.23
In addition to all these studies, Borcherding et al.24 revealed a different type of evidence for the ability of D1‐like receptors to deliver suppressive signals to pathological cells that express these receptors. In their study, Borcherding et al. discovered strong to moderate immunoreactive D1R expression in 30% of 751 patients with primary breast carcinomas, and that this overexpression of D1R was associated with larger tumors, higher tumor grades, node metastasis and shorter patient survival. Furthermore, selective D1‐like receptor agonists, signaling through the cGMP/protein kinase G pathway, suppressed cell viability, inhibited invasion and induced apoptosis in multiple breast cancer cell lines.24 In addition, Fenoldopam, a peripheral D1‐like receptor agonist and drug (Corlopam) for hypertension, dramatically suppressed tumor growth in two mouse models with D1R‐expressing xenografts by increasing both necrosis and apoptosis.24
On the background of all the above, the focus of our study was dopamine D1‐like receptors in human T cells. With this focus in mind, our main inter‐connected aims have been the following seven aims: (i) Confirm that D1‐like receptors are elevated in activated normal human T cells, compared to resting T cells. (ii) Reveal whether patients with Psoriasis have an enriched population of D1R+ T cells. (iii) Find out if dopamine D1‐like receptor agonists suppress activated human T cells, first of healthy individuals and then of patients with Psoriasis, to explore a justification for silencing activated T cells of patients with Psoriasis via their dopamine D1‐like receptors. (iv) Discover whether dopamine D1‐like receptor agonists (and also agonists of other DRs) induce extremely rapid shifts in the T cell's membrane potential, which could subsequently trigger rapidly other T cell functions and dialogues with other cells. (v) Discover whether Fenoldopam – a selective dopamine D1‐like receptor agonist and drug for hypertension, that does not cross the blood–brain barrier, suppresses human T cells of both healthy individuals and Psoriasis. (vi) Develop new dermal/topical formulation of Fenoldopam, for potential therapeutic use in Psoriasis. (vii) Test whether our novel topical/dermal formulation of Fenoldopam suppresses both activated human T cells and inflamed human skin.
Materials and methods
Purification of normal human CD3+ T cells of healthy blood donors
Normal peripheral human T cells were purified from peripheral blood of healthy blood donors, purchased from the blood bank, with all related ethical permission and documentation.
First, all the peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep™ density gradient (Axis‐Shield; Alere Technologies, Oslo, Norway). Then, CD3+ T cells were isolated from the PBMCs by MACS® CD3 separation technique and reagents (Miltenyi Biotec, https://www.miltenyibiotec.com), according to the manufacturer's instructions, using CD3 MicroBeads, conjugated to monoclonal anti‐human CD3 antibodies (isotype: mouse IgG2a), MACS Columns and MACS Separator.
CD3/CD28 activation of normal human CD3+ T cells
Normal human CD3+ T cells, purified from leukocytes of healthy blood donors, were seeded in 24‐well plates at a concentration of 1 × 106–2 × 106/ml, and activated by mouse anti human anti‐CD3 and anti‐CD28 antibodies (BD Pharmingen, https://www.bdbiosciences.com/en-us), according to the manufacturer's instructions.
Immunofluorescence staining of dopamine receptors on the cell surface of human T cells
In single‐staining experiments, normal human CD3+ T cells (isolated from fresh human blood samples of healthy blood donors) were stained with rabbit‐anti‐human D1R, D2R, D3R, D4R and D5R polyclonal antibodies (Calbiochem, http://www.merckmillipore.com/) (1 : 50), or with rabbit isotype control (1 : 50), and then with fluorescein isothiocyanate (FITC)‐conjugated goat anti‐rabbit antibody (Jackson Laboratories, https://www.jacksonimmuno.com) (1 : 100). In double‐staining experiments, in addition to the above‐mentioned staining steps, the T cells were stained also with phycoerythrin (PE)‐conjugated mouse anti‐human CD4 or CD8 antibodies (BD Pharmingen), or with PE‐conjugated isotype control. Fluorescence profiles were recorded in a fluorescence‐activated cell sorter (FACS).
Immunofluorescence staining of D1R on the cell surface of mouse T cells
T cells isolated from the DBA1 mice were either left resting, or activated by anti‐mouse CD3 and anti‐CD28 antibodies (BD Pharmingen), and then immunostained with anti‐mouse D1R antibody (Epitomics, www.epitomics.com) according to the manufacturer's instructions, and then with a Cy5‐secondary antibody.
Chemotactic migration of normal human CD3+ T cells
The chemotactic migration of normal human CD3+ T cells was performed by Boyden Chamber Transwell migration assay (Transwell 5·0 lm, 6.5 mm insert, 24‐well plate, Costar Corning, NY), as described in our previous studies (see for example25). In this study, 200 000 normal human T cells in 100 μl RPMI‐1640 + 0·1% bovine serum albumin, either untreated or pre‐treated with a D1/5 receptor agonist (1 hr, 37°), were added to the respective upper chambers of the transwells. The lower chamber contained 400 μl RPMI‐1640 + 0·1% bovine serum albumin, into which human SDF‐1 (150 ng/ml; Peprotech, Rocky Hill, NJ) was added. The migration plate was then placed in a tissue‐culture incubator (37°) for 4 hr. Then, the upper chambers were removed gently, and the 400 μl content of the lower chamber – containing the T cells that had migrated to the lower chamber through the microporous membrane – were transferred into a clean test tube. Next, 600 μl of phosphate‐buffered saline (PBS) was added to the 400 μl of the lower chamber medium, to reach a final volume of 1 ml. FACS was used to count the number of T cells present in each tube, by counting the number of cells within a given sample during a desired pre‐determined time (fixed for 1 min). This gave the number of T cells counted in each sample during 1 min. Since the volume aspirated and analyzed by the FACS during 1 min was only 100 μl (i.e. 10% of the total 1ml), the number of T cells counted in 1min was multiplied by 10, to obtain the total number of migrating T cells.
Measuring rapid shifts in the membrane potential of normal human CD3+ T cells, by a voltage‐sensitive fluorescent dye and flow cytometry
In principle, we used a voltage‐sensitive dye and flow cytometry to follow the membrane potential of T cells, as in our previous study,26 but in the present study, the experiment's aims, set‐up and performance were different, and required a new protocol. In practice, purified normal human CD3+ T cells (~ 1 × 106/ml) were suspended in RPMI‐1640 medium (without any serum) for 1 hr (37°), to ensure steady‐state conditions. Then, the cells were washed and re‐suspended in PBS, and voltage‐sensitive fluorescent dye: bis‐(1,3‐dibutylbarbituric acid) trimethine oxonol (DiBAC4[3]), 300 nM (Molecular Probes), was added to the cells (15–30 min at room temperature, in the dark). Then, an equal volume of 450 μl containing ~450 000 DiBAC4[3]‐labeled T cells, was transferred into each of dozens of similar empty FACS tubes. Every three tubes were marked similarly, for later triplicate tests. Additional preparation for the experiment included placing all the following beside the FACSsort: (i) Eppendorf tubes with different D1/5 agonists at a 10× concentration of the final desired concentration. These dilutions of the DR agonists were freshly prepared from stocks of 10–100 mM stored at −80°, and placed before the experiment in an ice bucket, (ii) a bottle of sterile PBS, (iii) a timer set for 15 seconds, (iv) a Pipetor set for 50 μl, (v) clean tips.
In the experiment itself, the fluorescence of the voltage‐sensitive dye, DiBAC4[3], was measured by FACSort (Becton Dickinson, https://www.bd.com/en-us/company) at 488 nm. The fluorescence of each individual numbered FACS tube containing the untreated DiBAC4[3]‐labeled T cells was tested twice: first before adding anything, and then exactly 15 seconds after addition of 50 μl of the chosen D1‐like receptor agonist at a 10× concentration of the final desired concentration to 450 μl of cells. The effect of each D1/5R agonist was tested in this way (i.e. before and after addition of the respective DR agonist) in three replicate tubes. For control untreated T cells, we tested another set of three tubes before and exactly 15 seconds after adding PBS. In the analysis of the results, the DiBAC4[3] fluorescence profile of the T cells in each FACS tube was analyzed by flowjo, and overlay figures of the fluorescence curves before and after the addition of the respective DR agonist, were drawn. The mean, geometric mean and median of the fluorescence curves before and after treatment were compared, allowing quantitative comparison and detection of the rapid shifts in membrane potential. Genuine shifts induced by D1/5R agonists were then compared with the non‐specific control shifts in membrane potential due to the addition of buffer (PBS).
Skin and blood samples of healthy volunteers and patients with Psoriasis
Two healthy female volunteers aged 42 ± 7 years (36 and 47 years) were included in this study. Volunteers underwent surgical procedures at the Department of Plastic Surgery, Rambam Medical Center, Haifa, Israel. Normal healthy donor skin was transplanted onto SCID/Beige mice as previously described.27, 28 Twenty ml of venous blood was obtained from the same donors. The study was approved by the institutional ethics committee of the Rambam Medical Center. For immunofluorescence analysis, biopsies were obtained from lesional areas of six patients with Psoriasis aged 42 ± 4 years (range 32–49 years), and of four healthy volunteers for the control specimens, aged 36 ± 6 years (range 29–43 years).
Studying the expression of D1R in T cells within skin sections derived from skin of patients with Psoriasis
Five‐micrometer paraffin sections of patients with Psoriasis were subjected to antigen retrieval (EDTA, pH 8) for 20 min in a microwave, and were cooled at room temperature for 25 min. Sections were then incubated with goat anti‐human D1R antibody (Santa Cruz Biotechnology, Dallas, TX) and mouse anti‐human CD4 and CD8 antibodies (Dako, https://www.agilent.com/en/dako-products) for 14 hr at 4°. This was followed by incubation with secondary antibodies: Alexa Fluor 488 anti‐mouse, and Alexa 594 donkey anti‐goat (Molecular Probes). DAPI staining was performed for visualization of nuclei. Slides were visualized by confocal microscopy (LSM 700; Zeis).
For cell counting, positive cells were counted in an area of 0·66 mm2. Data are presented as the mean ± SEM. The study parameters of each group were compared using Student's t‐test with statistical significance set at P < 0·05.
Phenotypic characterization of PBMCs of patients with Psoriasis, with and without culturing with Fenoldopam
Peripheral blood mononuclear cells from individuals with Psoriasis (three men, two women, mean age 32 years) were activated in culture with IL‐2, with and without Fenoldopam as described above. After 14 days, the cells were centrifuged, and treated with Fenoldopam at 10–6 M (from powder; 48 hr incubation). Following this incubation, cells were immunostained and analyzed by flow cytometer (FACS). Two‐color flow cytometry was performed by incubation of 5 × 105 PBMCs with the indicated monoclonal antibodies. Cells were stained with mouse anti‐human CD69 (Brilliant Violet 421; BioLegend, San Diego, CA), mouse anti‐human CD28 (Brilliant Violet 421; BioLegend), mouse anti‐human IL‐2 (Brilliant Violet; BioLegend), and mouse anti‐human CD3 (PE; BioLegend).
The lymphocyte population was assessed using flowjo software (Tree Star, Ashland, OR). Isotype‐matched control antibodies conjugated to PE and Brilliant Violet 421' were used to exclude non‐specific binding.
Humanized mouse model of Psoriasis
The humanized psoriatic SCID/Beige mouse model uses healthy human skin grafts, and PBMCs from patients with Psoriasis that were cultured in the presence of high‐dose IL‐2 (IL‐2‐activated PBMCs). This promotes the growth of different PBMCs that are enriched for activated T cells bearing Natural Killer (NK) receptors.27, 28
Ten C.B‐17/IcrHsd‐scid‐bg mice were used (Harlan, Jerusalem, Israel). Animal handling was in accordance with the national guidelines, and approved by the Technion Animal Care and Use Committee. Mice were housed with an artificial 12‐hr/12‐hr light/dark cycle at constant temperature, and were fed with standard chow and water ad libitum. The mice were acclimatized for 1 week before experimentation.
Following skin transplantation, PBMCs were isolated from donors with Psoriasis by centrifugation of Ficoll/Hypaque (Pharmacia; Amershem Pharmacia Biotech, Uppsala, Sweden). The PBMCs were cultured for 14 days with 100 U/ml human IL‐2 (PROSPEC, Protein Specialists, https://www.prospecbio.com), in filtered medium composed of RPMI‐1640, 10% human AB serum (Sigma‐Aldrich), 1% l‐glutamine and 1% penicillin‐streptomycin (media components; Biological Industries, Kibbutz Beit Haemeck, Israel). The medium was changed as needed. The cells were then injected intradermally (7 × 106) into the grafts.
Induction of inflammation by LPS in normal human skin derived from healthy donors undergoing cosmetic surgery
Skin samples were obtained from healthy adult individuals undergoing elective cosmetic surgery (abdominoplasty), as approved by the Hadassah University Hospital Ethics Committee (#0639‐12HMO). The samples were cultured with the dermal side immersed in the medium, and the epidermis exposed to air.27 LPS (Santa Cruz Biotechnology, Inc.) was added to the growth medium at a final concentration of 10 μg/ml, to induce inflammation, and the gel formulation containing increasing concentrations of Fenoldopam (2 μl) was applied topically. The levels of the cytokines released into the medium were determined by solid‐phase enzyme‐linked immunosorbent assay (ELISA), using specific immunoassay kits [ELISA MAX; BioLegend (IL‐1β, IL‐6 and IL‐8); Milliplex Assay, Millipore Corp., Merck, http://www.merckmillipore.com/ [(IL‐1β, IL‐2, IL‐8, IL‐10, tumor necrosis factor‐α (TNF‐α)] and interferon‐γ (IFN‐γ). The experiments were performed in quadruplicates, from several independent donors.
Preparation of Fenoldopam topical formulations
Ointment formulations of Fenoldopam mesylate, i.e. ointment with water‐washable base containing 1% weight/weight Fenoldopam was prepared by the fusion method.29 Propylene glycol (PG) was used as a solubilizer phase, and citric acid was used as an acidic adjuster, in all these formulations. Briefly, the drug and citric acid were dissolved in PG, and the components of base were melted at a temperature not more than 60°, and then cooled to room temperature so that the base solidifies. Then the PG mixture was incorporated into the solidified base and mixed thoroughly to form a homogeneous ointment.
Simultaneous multiplex measuring of multiple cytokines secreted in vitro by human T cells or isolated human skin
Simultaneous evaluation of the levels of multiple cytokines in the medium of either T cells, or isolated human skin (treated versus untreated), was performed by ‘Human bead‐based multiplex assay – Luminex High Performance Assay’ (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions. In principle, Luminex Assays and High‐Performance Assays use color‐coded superparamagnetic beads coated with cytokine‐specific antibodies. Beads recognizing different target cytokines are mixed together and incubated with the sample. Captured cytokines are subsequently detected using a cocktail of biotinylated detection antibodies and a streptavidin–phycoerythrin conjugate.
Determination of pro‐inflammatory cytokine levels in skin samples of patients with Psoriasis
Psoriasis is characterized by altered levels of key inflammatory cytokines like IL‐17, IL‐23 and TNF‐α. The skin biopsies treated with different formulations were collected at the end of the efficacy study and stored at −80° until use. Briefly, collected skin tissues were homogenized in double the amount of extraction buffer containing 10 mM Tris–HCl pH 7·4, 150 mM NaCl, 1% Triton‐X‐100 using tissue homogenizer (RQ‐127A/D; REMI Elektrotechnik Ltd., Thane, India), at 3000 rpm for 5 min.30 The homogenates were centrifuged at 13 000 rpm for 15 min at 4° and the supernatant obtained was analyzed for estimation of cytokine levels using respective quantikine mouse ELISA kits (R&D Systems).
Statistics
Statistical analysis for each set of results was performed by the appropriate method specified in the respective figure legends. Collectively, the methods included the analysis of variance (anova) followed by Bonferroni's test for multiple comparisons, and Kruskal–Wallis non‐parametric test for independent samples.
Results
Normal human T cells of healthy subjects express all types of dopamine receptors – D1R‐D5R, and activation of T cells increases significantly D1‐like receptors: D1R and D5R, and also D4R
A previous study by Kustrimovic et al.7 revealed already that human T cells express all DR types, but that there is a remarkable variability between different human subjects with regards to the exact level of each DR type. In 15 individuals whose T cells were studied, the levels were : D1‐like receptors: D1R 2·5–22·8%, D5R 2–20·9%; and D2‐like receptors: D2R 1·1–7·9%, D3R 1·9–15% and D4R 0·8–17%.7
We wished to confirm the expression of all DR types in resting and TCR‐activated human T cells. For this purpose, normal human CD3+ T cells were purified from blood of healthy individuals (blood donors). Thereafter, some of the T cells were left resting and untreated, whereas some were activated for 72 hr by commercial anti‐CD3 and anti‐CD28 antibodies. Then, the cell surface expression level of all types of DRs: D1R–D5R, was measured by immunostaining, using highly selective antibodies to each DR type, and analyzed by flow cytometry. Figure 1, presenting the results of one representative experiment, shows that both resting T cells (Fig 1a–e) and activated (Fig 1f–j) CD3+ T cells express all DR types. Furthermore, the level of D1‐like receptors, D1R and D5R, and that of D4R (D2‐like receptor) were significantly higher in the CD3/CD28‐activated T cells than in the resting T cells: D1R+ 38·5% in resting versus 60·5% in activated T cells (1·57‐fold increase); D5R+ 38·5% in resting versus 52·2% in activated T cells (1·35‐fold increase); D4R+ 38% in resting versus 65·3% in activated T cells (1·71‐fold increase).
Figure 1.
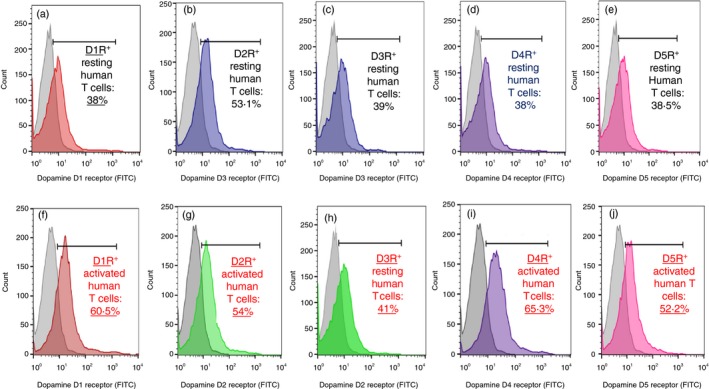
Normal human CD3+ T cells express all types of dopamine receptors (DRS) – D1R‐D5R on their cell surface, and the level of D1R, D5R and D4R are higher in activated T cells than in resting T cells. (a–e; Upper Figs) Expression of D1R–D5R on resting normal human CD3+ T cells. (f–j; Lower Figs) Expression of D1R–D5R on CD3/CD28‐activated normal human CD3+ T cells. The colored graphs show the levels of immunofluorescence staining, first with rabbit‐anti‐human antibodies selective to each DR type, and then with a secondary‐FITC‐conjugated goat anti‐rabbit antibody. The control gray fluorescence profiles in each graph represent the control non‐specific staining, first with rabbit isotype control, and then with the same secondary antibody. The results are of one representative experiment, of two performed on T cells of different healthy human individuals. In both experiments the same pattern of results was observed, but the actual numbers/expression levels were different. This observation is in line with the findings of Kustrimovic et al.,7 that human T cells express all DR types, but that there is a remarkable variability between different people with regard to the exact level of each DR type expressed on the cell surface of their T cells.
These results are in line with previous findings of other research groups, revealing that DRs are expressed on the cell surface of most, if not all, T cell subpopulations,4 and that T cell activation elevates their expression levels.7
Further double staining of each DR type, and of either CD4+ helper T cells, or CD8+ cytotoxic T cells, revealed that both CD4+ and CD8+ resting normal human T cells express all types of DRs on their cell surface. Yet, the CD8+ T cells have slightly higher DR levels: 15·28% CD8+ D1R+ versus 11·5% CD4+ D1R+ T cells; 14% CD8+ D2R+ versus 12·1% CD4+ D2R+ T cells; 13·1% CD8+ D3R+ versus 11·4% CD4+ D3R+; 13·1% CD8+ D4R+ versus 11·4% CD4+ D3R+ T cells; and 13·8% CD8+ D5R+ versus 10·7% CD4+ D5R+ T cells (immunostaining figures not shown). These results were not statistically significant, but they showed a tendency which is in line with previous findings showing that: (i) the percentage of CD8+ single‐positive T cells expressing DRs is significantly higher than that of CD4+ T cells,31 and (ii) dopamine, via D3R, induces selective migration and homing of naive CD8+ T cells.32
Seven highly selective DR agonists shift the membrane potential and induce depolarization of normal human T cells, within 15 seconds only
The membrane potential is the difference in electric potential between the interior and the exterior of a biological cell. Very rapid shifts in the membrane potential – either depolarization (shift towards more positive interior) or hyperpolarization (shift towards more negative interior) – occur characteristically within milliseconds to seconds in excitable cells, like neurons and muscle cells, in response to neurotransmitters, several other specific stimuli, or changes in the extracellular milieu of the cells.
These rapid membrane potential shifts, especially the depolarizations, are then responsible for eliciting important fast changes within the cells, communication between cells, and key functional responses essential for the overall physiology of the organism.
The membrane potential shifts occur characteristically in biological cells by rapid gating – opening or closing – of specific ion channels, mainly Na+, K+ and Ca2+ and Cl− channels.
Many neurotransmitters bind to their specific receptors in excitable target cells, shift their membrane potential, and by doing so initiate transmission of important physiological signals to other cells, and elicit various cellular functions
In this study we tested if activation of each of the DRs in human T cells could lead by itself to very rapid shifts in the membrane potential of human T cells, although these immune cells are of course not considered electrically‐excitable cells.
For testing this, we designed a new protocol. First, freshly purified normal human T cells were labeled with a voltage‐sensitive fluorescent dye, DiBAC4[3], and then distributed into individual tubes. Second, using flow cytometry, we read the voltage‐sensitive fluorescence of the DiBAC4[3]‐labeled T cells in every individual tube twice: first before, and then again exactly 15 seconds after, adding the respective DR agonist.
A later FACS analysis and comparison of the DiBAC4[3] fluorescent curves and intensity (expressed by the geometric mean) before and after such addition of each DR agonist to each individual tube, was used to detect the membrane potential shifts. Each test was in triplicate, and the entire experiment was performed on T cells of different healthy human participants.
Representative results (Figs 2, 3 and Table 1) show that seven highly selective DR agonists shifted the membrane potential of both resting and activated human T cells towards a more depolarized potential, within 15 seconds only.
Figure 2.
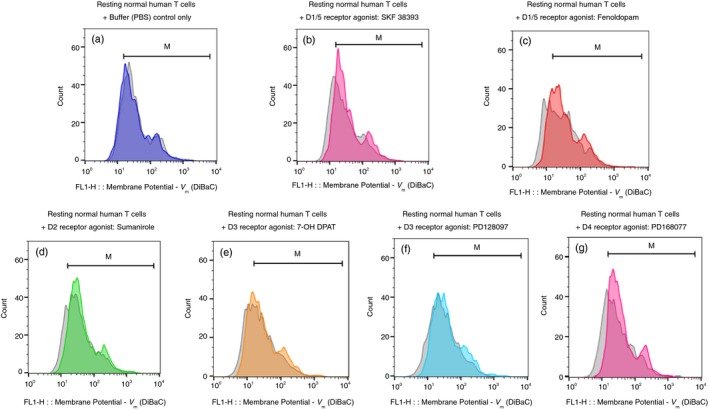
Selective D1/5R agonists induce depolarization of resting CD3+ normal human T cells within 15 seconds. (a–g) The fluorescence profiles of the voltage‐sensitive fluorescence dye DiBac3 inside resting normal human T cells, both before (gray) and then again 15 seconds after (colored) addition of either: Control buffer (PBS) only (a), D1R/D15R (D1‐like) agonists: SKF 38393 (b) and Fenoldopam (c); D2R agonist: Sumanirole (d); D3R agonists: 7‐OH DPAT (e) and PD 128907 (f); D4R agonist: PD 168077 (g). All the DR agonists were used in a final concentration of 10−7 M (100 nM), prepared freshly before each experiment, from either their powders or very concentrated stocks stored in at –80°. The corresponding intensity of the voltage‐sensitive fluorescence dye DiBac3 was determined by the geometric mean (GM) of the fluorescence (FL1), in each tube before and after addition of each agonist, and all the GMs and fold change/shift are shown in Table 1. The results shown in the figure are of one representative experiment, of two performed altogether, on T cells of different healthy human participants. In both experiments the same pattern of results was observed, but the actual extent of the depolarization varied. This was not surprising in view of the differences between individuals with regard to the level of DRs on the cell surface of their T cells (found in 7 and also in the current study (data not shown).
Figure 3.
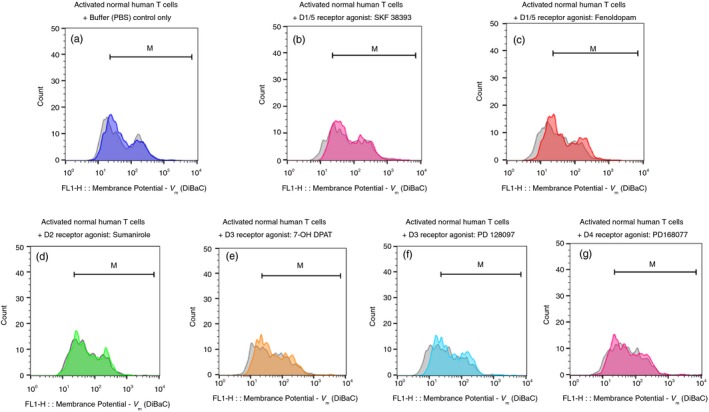
Selective D1/5R agonists induce depolarization of activated CD3+ normal human T cells within 15 seconds. (a–g) The fluorescence profiles of the voltage‐sensitive fluorescence dye DiBac3 inside activated normal human T cells, both before (gray) and then again 15 seconds after (colored) addition of either: Control buffer (PBS) only (a), D1R/D15R (D1‐like) agonists: SKF 38393 (b) and Fenoldopam (c); D2R agonist: Sumanirole (d); D3R agonists: 7‐OH DPAT (e) and PD 128907 (f); or D4R agonist: PD 168077 (g). All the DR agonists were used in a final concentration of 10−7 M (100 nM), prepared freshly before each experiment, from either their powders or very concentrated stocks stored at −80°. The corresponding intensity of the voltage‐sensitive fluorescence dye DiBac3 was determined by the geometric mean (GM) of the fluorescence (FL1), in each tube before and after addition of each agonist, and all the GMs and fold change/shift are shown in Table 1. As for the depolarization of resting T cells shown in Fig 2, the results of the activated T cells shown in the figure are of one representative experiment, of two performed altogether, on T cells of different healthy human paticipants. In both experiments the same pattern of results was observed, but the actual extent of the depolarization varied. This was not surprising in view of the differences between individuals with regard to the level of DRs on the cell surface of their T cells found in7 and also in the present study (data not shown).
Table 1.
Dopamine receptor agonists (100 nm) by themselves shift the membrane potential (V m) of normal human T cells, within 15 seconds only.
| + Control buffer (PBS) only | + D1/5 receptor agonist: SKF 38393 | + D1/5 receptor agonist: Fenoldopam | + D2 receptor agonist: Sumanirole | + D3 receptor agonist: 7‐OH‐ DPAT | + D3 receptor agonist: PD 128097 | + D4 receptor agonist: PD168907 | |
|---|---|---|---|---|---|---|---|
| Resting normal human CD3+ T cells | V m before (G.M.) 35·9 | V m before (G.M.) 29·2 | V m before (G.M.) 28 | V m before (G.M.) 36·2 | V m before (G.M.) 25·1 | V m before (G.M.) 30·2 | V m before (G.M.) 29·7 |
| V m after (G.M.) 32·4 | V m after (G.M.) 34·9 | V m after (G.M.) 34·8 | V m after (G.M.) 45·6 | V m after (G.M.) 30·3 | V m after (G.M.) 39·7 | V m after (G.M.) 40·3 | |
| Shift in V m: G.M. after– before = 3·5 | Shift in V m: G.M. after– before = 5·7 | Shift in V m: G.M. after– before = 6·8 | Shift in V m: G.M. after– before = 9·4 | Shift in V m: G.M. after– before = 5·2 | EShift in V m: G.M. after– before = 9·5 | EShift in V m: G.M. after– before = 10·6 | |
| Activated normal human CD3+ T cells | V m before (G.M.) 48·2 | V m before (G.M.) 60·5 | V m before (G.M.) 32·1 | V m before (G.M.) 54·7 | V m before (G.M.) 42·8 | V m before (G.M.) 33·8 | V m before (G.M.) 52·4 |
| V m after (G.M.) 50·9 | V m after (G.M.) 71·8 | V m after (G.M.) 45·1 | V m after (G.M.) 59·6 | V m after (G.M.) 51·5 | V m after (G.M.) 46·9 | V m after (G.M.) 59·1 | |
| Shift in V m: G.M. after– before = 2·7 | Shift in V m: G.M. after– before = 11·3 | Shift in V m: G.M. after– before = 13 | Shift in V m: G.M. after– before = 4·9 | Shift in V m: G.M. after– before = 8·7 | EShift in V m: G.M. after– before = 13·1 | EShift in V m: G.M. after– before = 6·7 |
The seven DR agonists were: D1R/D15R (D1‐like) agonists: SKF 38393 (Figs 2b and 3b) and Fenoldopam (Figs 2c and 3c); D2R agonist: Sumanirole (Fig 2d and 3d); D3R agonists: 7‐OH DPAT (Fig 2e and 3e) and PD 128907 (Fig 2f and 3f); and D4R agonist: PD 168077 (Figs 2g and 3g). All these DR agonists were tested in a final concentration of 10−7 M (100 nM), prepared just before the experiment from fresh stocks of much higher concentration (usually 100 mM) frozen in −80°.
Of note, to the best of our knowledge there are still no agonists or antagonists that can distinguish between the two D1‐like receptors: D1R or D5R, in contrast to the availability of antibodies that can.
The concentration of the DR agonists was chosen based on previous studies of our own group and of others, showing that 10−8–10−6 M (10 nM to 1 μM ) is the optimal concentration range of multiple neurotransmitters and neuropeptides and of their receptor agonists, among them: dopamine and its receptor agonists, and glutamate and its agonists and many others, in which they induce direct beneficial effects on resting normal human T cells (see for example refs 5, 6, 24). In addition, in the study of Borcherding et al.24, the D1‐like receptor agonist Fenoldopam at a low concentration range of 10−10–10−8 M suppressed cAMP and stimulated cGMP in breast cancer cells, and markedly reduced their viability.
Representative results are presented in Figs 2 and 3 (a–g), showing overlays of the DiBAC4[3] fluorescence profiles of resting (Fig. 2) or activated (Fig. 3) T cells, before and 15 seconds after the addition of either control buffer PBS (Figs 2a and 3a), or each DR agonist.
Table 1 shows the geometric mean of all the DiBAC4[3] fluorescence profiles of the resting and activated T cells whose fluorescence profiles are presented in Figs 2 and 3.
These results showed that activation of all DRs by their respective agonists, induced mild‐moderate depolarization of normal human T cells within 15 seconds only. It could be that dopamine itself (not tested here), and different conc. of dopamine agonists (also not tested), would induce stronger T cell depolarizations.
In view of the fact that in the rest of the study we focused only on the D1‐like receptors in T cells, in an additional experiment, whose representative results are shown in Fig. 4(a–d), we confirmed that three D1‐like receptor agonists depolarize normal human T cells within 15 seconds. These included: Corlopam – the Fenoldopam soluble drug (diluted from the original drug ampoule), SKF 38393 and A77636, all at 10−7 M (100 nM).
Figure 4.
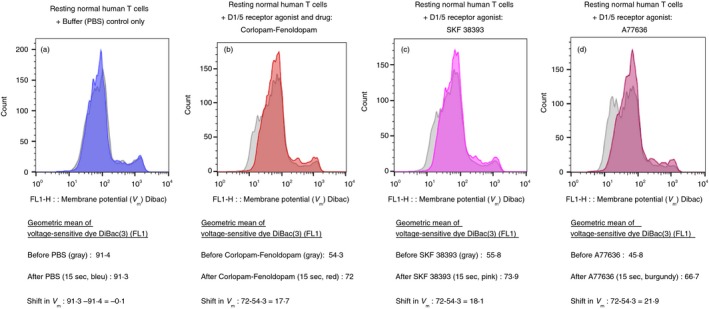
Three selective agonists of D1‐like receptors (D1R and D5R) induce depolarization of CD3+ normal human T cells, within 15 seconds. (a–d) The fluorescence profiles of the voltage‐sensitive fluorescence dye DiBac3 inside normal human T cells, before (gray) and then 15 seconds after (colored) either: Control buffer (PBS) only (a), Corlopam, the Fenoldopam drug (from its original drug ampoule) (b), SKF 38393 (c) and A77636 (d). All the DR agonists were used in a final concentration of 10−7 M (100 nM), prepared freshly before each experiment, from either their powders or from concentrated stocks stored at −80°. The corresponding intensity of the voltage‐sensitive fluorescence dye DiBac3 was determined by the geometric mean (GM) of the fluorescence (FL1) in each tube before and after addition of each agonist. The GMs and fold shift in each case are shown in the figures below the corresponding fluorescence profiles. As for the depolarization of resting T cells shown in Fig 2, and for activated T cells shown in Fig. 3, here too the results shown are for one representative experiment, of two performed altogether, on T cells of different healthy human participants. In both experiments the same pattern of results was observed, but the actual extent of the depolarization varied. This was not surprising in view of the differences between individuals with regard to the level of DRs on the cell surface of their T cells found in7 and also in the present study (data not shown).
To the best of our knowledge, this is the first demonstration of depolarization of T cells by activation of neurotransmitter receptors in general, and DRs in particular.
Skin of patients with Psoriasis contains ~20‐fold more CD3+ D1R+ T cells than healthy human skin. Dramatic elevation of 25‐fold in D1R+ T cells is also evident in the Psoriasis humanized mouse model
From this point onwards, we focused only on the D1‐like receptors in human T cells and skin, based on the evidence cited in the Introduction, for the ability of these receptors to deliver suppressive signals to activated immune cells.17, 18, 19
We first asked if T cells expressing D1R are elevated in the skin in Psoriasis – a disease mediated by activated T cells.33, 34, 35 Psoriasis is discussed further in the last part of the Discussion.
We hypothesized that human psoriatic skin may contain elevated levels of D1R+ T cells, and that if so, targeting D1Rs by their selective D1R analogues may be beneficial and therapeutic in Psoriasis. On these grounds, we stained and then quantified the D1R+ CD4+ T cells and D1R+ CD8+ T cells present in the skin of patients with Psoriasis, and of control healthy human volunteers, by immunohistochemical staining (Fig. 5a: immunohistochemical images, Fig. 5c: quantitative graphs).
Figure 5.
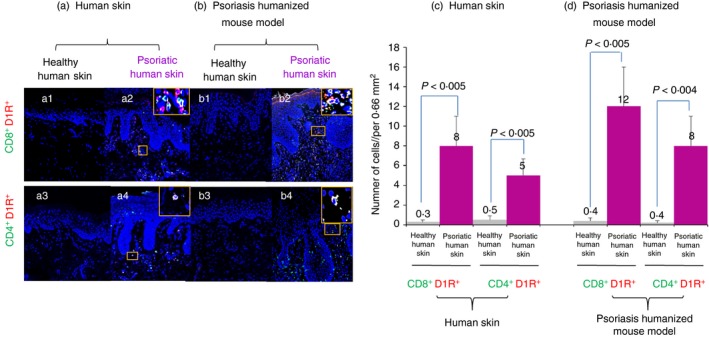
Immunofluorescence analysis of T cells expressing D1R in human psoriatic skin, and in human psoriasiform skin grafts of Psoriasis humanized mouse model in SCID/Beige mice. (a) CD8+/D1R+ and CD4+/D1R+ T cells are more numerous in involved skin from patients with Psoriasis than in healthy skin. (b) These CD8+/D1R+ and CD4+/D1R+ double‐positive T cells are also elevated in psoriatic human skin of Psoriasis humanized mouse model, compared with healthy human skin. (c) Quantitative analysis of double‐positive CD8+/D1R+ and CD4+/D1R+ T cells in human Psoriasis and in (d) psoriatic and healthy skin of Psoriasis humanized mouse model. The data represent mean + SD of six Psoriasis patients. Statistical analysis used the Kruskal–Wallis test. Statistical significance was set at P < 0·05.
The results were striking: the patient's psoriatic skin contained ~20‐fold more D1R+ T cells than healthy human skin. The psoriatic human skin contained significantly more of both cytotoxic CD8+ D1R+ T cells (Fig. 5a, upper panel: a2 versus a1; Fig. 5c, two left histograms), and helper CD4+ D1R+, compared with the healthy human skin, (Fig. 5a, lower panel: a4 versus a3; Fig. 5c, two right histograms), but there were more CD8+ D1R+ T cells.
Subsequently, we performed a similar investigation of D1R+ T cells in a Psoriasis humanized mouse model. In this in vivo human–mouse model, Psoriasis‐like disease is induced by a biological method in human skin grafts transplanted on SCID/Beige mice (see Methods).
The model is composed of normal human skin injected with PBMCs cultured with a high dose of IL‐2 (IL‐2‐enriched PBMCs leading to the appearance of T cells bearing NK receptors36, 37). The model is used widely as a preclinical tool to test a possible therapeutic effect in Psoriasis.36, 37, 38, 39, 40, 41
Strikingly, in this Psoriasis mouse model, we revealed a dramatic 25‐fold increase of D1R+ T cells in the psoriatic human skin graft, compared with the control healthy human skin graft (Fig. 5b, immunohistochemical images; Fig. 5d, quantitative graphs). Once again, the psoriatic skin contained significantly more of both cytotoxic CD8+ D1R+ T cells (Fig. 5b, upper panel: b2 versus b1; Fig. 5d, two left histograms), and helper CD4+ D1R+ T cells (Fig. 5b, lower panel: b4 versus b3; Fig. 5d, two right histograms) than the healthy skin, but there were more CD8+ D1R+ T cells.
Fenoldopam and additional dopamine D1‐like receptor agonists, reduce significantly the chemotactic migration of activated normal human T cells towards the chemokine SDF‐1/CXCL12
An important function of T cells is their ability to migrate towards chemokines secreted in distant places. The chemotactic migration (chemotaxis) of T cells is mediated by their chemokine receptors, and enables T cells to move and home in a directed manner towards target organs and tissues, and subsequently penetrate them.
While T cell chemotactic migration is an essential and beneficial function under physiological conditions, it is detrimental in various pathological conditions, among them: T cell mediated autoimmune diseases, T cell cancers (T cell leukemia and T cell lymphoma), and other diseases caused by detrimental T cells. In T cell‐mediated autoimmune diseases, the chemotactic migration of autoimmune T cells towards chemokines present in the loci bearing their target autoantigen, contributes substantially to the pathological effects of these T cells, and ultimately to the overall autoimmune disease.
On the basis of all the above, we tested whether the binding of D1‐like receptors in T cells by three highly selective D1‐like receptor agonists: Fenoldopam, SKF 38393 and A77636, could reduce the chemotactic migration of CD3/CD28‐activated normal human T cells.
We chose to study T cell chemotactic migration towards SDF‐1/CXCL12, as this pleiotropic chemokine and its receptor CXCR4 are extremely important for many cellular functions in health and disease, and participate in the regulation of tissue homeostasis, immune surveillance, autoimmunity and cancer. SDF‐1/CXCL12 is constitutively expressed in the bone marrow and various tissues, and regulates trafficking and localization of immature and maturing leukocytes, including bone marrow stem cells, neutrophils, monocytes and T cells.42 In addition, CXCL12 is key regulator for early development of the central nervous system (CNS), but also participates in the pathogenesis of CNS disorders.43
Figure 6(a–c), presenting representative results of three independent experiments performed on T cells of three healthy human participants, show that 1 hr pre‐incubation with Fenoldopam solution (10−7 M) (prepared from Fenoldopam powder) reduced by 41·4% (Fig. 6a), and with Fenoldopam/Corlopam (the FDA‐approved Fenoldopam drug; 10−7 M) reduced by 55·2% in one experiment (Fig. 6a), by 52% (Fig. 6b) in a second, and by 27% in a third (Fig. 6c), the subsequent chemotactic migration of CD3/CD28‐activated CD3+ normal human T cells towards SDF‐1/CXCL12.
Figure 6.
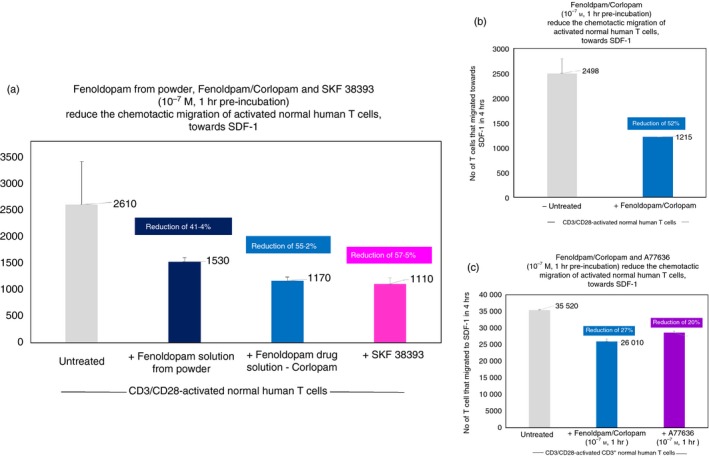
A highly selective D1‐like receptor agonist and drug – Fenoldopam mesylate/Corlopam (solutions prepared from two sources: powder and the Corlopam drug), as well as two other highly selective D1‐like receptor agonists: SKF 38393 and A77636, reduce the in vitro the chemotactic migration of activated human T cells. (a–c) Representative results of three independent experiments performed on T cells of three healthy human participants. Each treatment/test shown in the histograms was performed in triplicates. The CD3/CD28‐activated normal human T cells were incubated for 1 hr only with the D1‐like receptor agonists, before the onset of the 4‐hr migration assay. The extent of T cell migration was evaluated in a Boyden Chamber Assay. The results represent mean ± SD (n = 3) chemotactic migration of activated normal human T cells towards the chemokine SDF‐1. The results shown in the figure are of one representative experiment, of two performed together, on T cells of different healthy human individuals. In both experiments, the same pattern of results was observed, but the actual extent of the migration and of the suppressive effects induced by the D1‐like receptor agonists varied between individuals. This was not surprising in view of the differences between different individuals with regard to the level of DRs on the cell surface of their T cells [found in7 and also in the present study (data not shown)].
The two other D1‐like receptor agonists (both at similar concentrations of 10−7 m) also reduced the chemotactic migration of the activated T cells: SKF 38393 by 57·5% (Fig. 6a) and A776363 by 20% (Fig. 6c).
To begin with, the T cells of the third individual tested (Fig. 6c), migrated much more than those of the first and second individuals. It is noteworthy that we did not test in these experiments whether longer pre‐incubations (i.e. more than 1 hr) of the T cells with the D1‐like receptor agonists would reduce the chemotactic migration even more.
Fenoldopam mesylate reduces dramatically the in vitro secretion of eight cytokines by activated lymphocytes of patients with Psoriasis
Patients with Psoriasis have detrimental T cells that perform pathological autoimmune and inflammatory activities leading to the Psoriasis‐associated tissue damage.44 Based on that, on prior knowledge of the key role played by lymphocyte‐derived cytokines in Psoriasis,45 and on our novel results described in the preceding paragraphs, we moved to T cells of patients with Psoriasis, and tested if Fenoldopam can reduce the secretion of eight cytokines by patient's IL‐2‐activated lymphocytes: TNF‐α, IFN‐γ, IL‐1β, IL‐2, IL‐4, IL‐6, IL‐8 and IL‐10.
First, as shown in Fig. 7 (findings of a representative Psoriasis patient), IL‐2 elevated dramatically the secretion of all eight cytokines by the patient's peripheral blood lymphocytes (PBLs) : TNF‐α: from 27·5 to 713 pg/ml – a 26‐fold increase; IFN‐γ: from 609 to 16 970 pg/ml – a 28‐fold increase; IL‐1β: from 1·5 to 79 pg/ml – a 54‐fold increase; IL‐2: from 545 to 2669 pg/ml – a 9‐fold increase; IL‐4: from 13·6 to 53 pg/ml – a 3·9‐fold increase; IL‐6: from 6·9 to 186 pg/ml – a 27‐fold increase; IL‐8: from 622 to 7331 pg/ml – an 11‐fold increase, and IL‐10: from 1·5 to 6·2 pg/ml – a 4·3‐fold increase.
Figure 7.
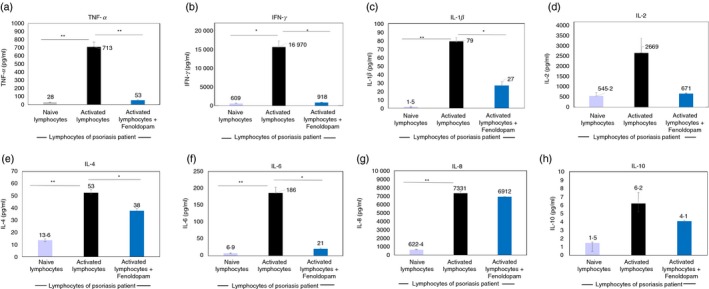
Fenoldopam in solution induces robust reduction of the secretion of eight key cytokines by Psoriasis patient's activated PBMCs . Incubation of activated PBMCs of patients with Psoriasis with Fenoldopam (48 hr) reduced dramatically the levels of tumor necrosis factor‐α (TNF‐α) (a), interferon‐γ (IFN‐γ) (b), interleukin‐1β (IL‐1β) (c), IL‐2 (d), IL‐4 (e), IL‐6 (f), IL‐8 (g) and IL‐10 (h). The graphs show representative results of one Psoriasis patient, of two tested. The levels of all these cytokines in the cell culture media was measured simultaneously by Luminex high performance assays (see Methods), allowing simultaneous detection of multiple desired cytokines. Statistical analysis was performed by Kruskal–Wallis test. Statistical significance was set at P < 0·05.
Second, Fenoldopam (48 hr incubation) decreased dramatically the secretion of five cytokines by the Psoriasis pateint's IL‐2‐activated PBLs (Fig. 7): TNF‐α: from 713 to 53 pg/ml – decrease of 93%; IFN‐γ: from 16 970 to 918 pg/ml – decrease of 95%; IL‐1β: from 79 to 27 pg/ml – decrease of 66%; IL‐2: from 2669 to 671 pg/ml – decrease of 75%; and IL‐6: from 186 to 21 pg/ml – decrease of 89%.
The effect of Fenoldopam was also evident, but smaller, for the two other cytokines: IL‐4: from 53 to 38 pg/ml – decrease of 28%; and IL‐10: from 6·2 to 4·1 pg/ml – decrease of 34%. In contrast, the effect on IL‐8 was negligible: from 7331 to 6912 pg/ml – decrease of only 6% (Fig. 7).
In summary of these findings: Fenoldopam induced potent inhibitory effects on the secretion of multiple key cytokines by activated lymphocytes of patients with Psoriasis, and it is likely (but not yet tested) that it can also suppress the secretion of IL‐17 and IL‐23, which are important in Psoriasis.46, 47, 48, 49
It is also noteworthy that we previously demonstrated that PBMCs co‐cultured with high doses of IL‐2 produced large amounts of IL‐17,37, 50 Furthermore, immunohistochemical staining revealed significantly increased expression of IL‐17 and IL‐23 in the psoriatic‐induced skin grafts.50
Fenoldopam reduces markedly the level of three T cell activation proteins/markers, in CD3+ T cells of patients with Psoriasis
We then studied if Fenoldopam decreases significantly also the levels of key T cell activation‐related proteins/markers: CD69, CD28 and IL‐2, in CD3+ T cells of patients with Psoriasis.
CD69 is one of the earliest cell surface antigens expressed by T cells following activation, and is typically detectable within 1 hr of ligation of the TCR/CD3 complex. Once expressed, CD69 acts as a co‐stimulatory molecule for T cell activation and proliferation.
CD28, also expressed on the surface of T cells, is involved in T cell activation, and in the related induction of cell proliferation, cytokine production and promotion of T cell survival. T cell stimulation through CD28, in addition to through the TCR, can provide a potent signal for the production of various interleukins.
Figure 8 (representative FACS images) shows that Fenoldopam (48 hr) induced a robust down‐regulation of CD69 in human activated T cells of patients with Psoriasis. Hence, when Fenoldopam was added for 48 hr to T cells of Psoriasis patients that were pre‐activated in vitro with IL‐2 (72 hr) before the Fenoldopam treatment, there was a reduction of 92% in the percentage of CD3+ CD69+ T cells: from 76% (Fig. 8a1) to 7% (Fig. 8b1).
Figure 8.
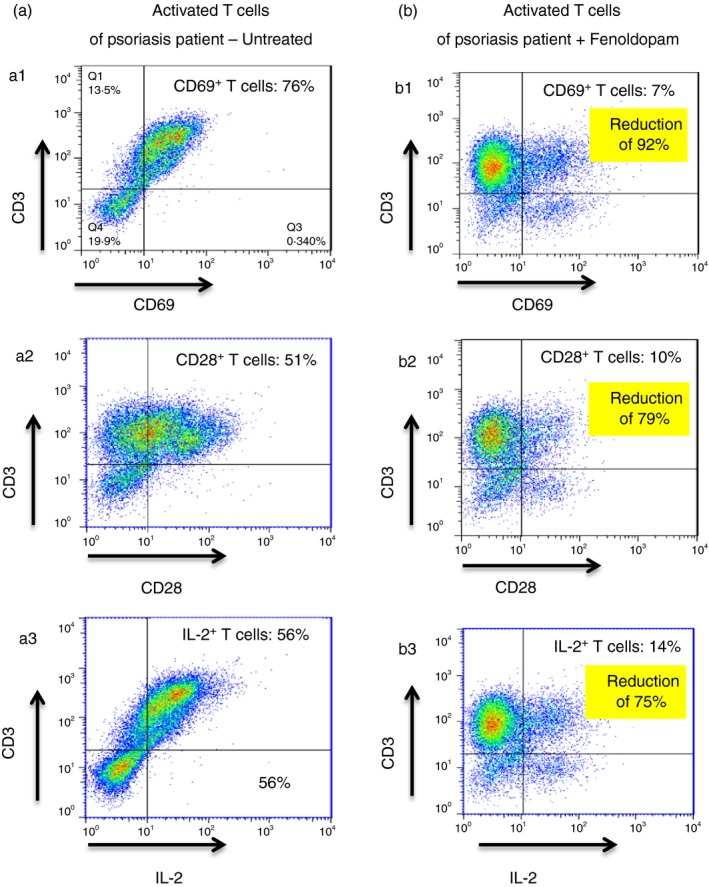
Fenoldopam induces dramatic reduction in 3 activation‐related molecules: CD69, CD28 and IL‐2, in activated T cells of Psoriasis patients. Immunostainig and flow cyometry show the following: (a1) untreated IL‐2 activated Psoriasis patient's PBMCs contain 76% of CD3+ T cells that are positive to CD69 (a2), 51% of CD3+ T cells that are positive to CD28, and (a3) 56% of CD3+ T cells that are positive to IL‐2. Following 48 hr incubation of Psoriasis patient's activated PBMCs with Fenoldopam there was a dramatic reduction in the edxdpression level of all theree T cell activation markers: (b1) Only 7% of CD3+ T cells are positive to CD69, (b2) only 10% of CD3+ T cells are positive to CD28, and (b3) only 14% of CD3+ T cells are positive to IL‐2. For all the flow cytometric analysis plots, isotype‐matched control antibodies were used to determine non‐specific staining. Data represent mean ± SD (n = 5). Statistical analysis was performed using the Kruskal–Wallis test. Statistical significance was set at P < 0·05.
Fenoldopam reduced profoundly also CD28 in these activated T cells of the patient with Psoriasis. Hence, Fenoldopam reduced the percentage of CD3+ CD28+ T cells of the patient by 80%; from 51% (Fig. 8a2) to 10% (Fig. 8b2).
Furthermore, Fenoldopam down‐regulated markedly the expression of intracellular IL‐2 in the activated T cells of the patient with Psoriasis by 75%; from 56% (Fig. 8a3) to 14% (Fig. 8b3).
Importantly, Fenoldopam induced all of these inhibitor effects without killing the T cells.
Together, these results demonstrate that Fenoldopam reduces dramatically the level of activation of T cells of patients with Psoriasis.
Novel topical/dermal formulation of Fenoldopam was invented, produced and found to reduce dramatically the secretion of multiple cytokines by activated normal human T cells
Fenoldopam in solution, prepared from Fenoldopam powder, as well as the Corlopam solution – the Fenoldopam drug used clinically in a continuous intravenous infusion for 24–48 hr, are unstable, with a short half‐life of only 5 min. As such, Fenoldopam does not suit potential/putative dermal treatment of Psoriasis, or of any other skin disease that might be mediated by autoimmune, inflammatory or cancerous D1R+ T cells.
These diseases necessitate new topical/dermal formulations of Fenoldopam that can be spread on the skin, penetrate its relevant disease‐affecting layers, and thereafter be released there in a regulated and continuous manner, for a chosen time period.
Meeting these criteria was a difficult challenge, in view of the stability issues of the drug, and its susceptibility to oxidation, pH sensitivity, poor transdermal flux, and the natural barrier properties of the skin.
To overcome these obstacles, we invented, prepared and performed extensive chemical and pharmacological studies of novel topical/dermal stable formulations of Fenoldopam – a polyethylene glycol‐based water washable ointment, and glycerin‐based carbopol anhydrous gel. These novel topical/dermal formulations of Fenoldopam allowed its spread on, and its subsequent continuous and controlled release in, the diseased skin.
The successful development of the novel topical/dermal Fenoldopam formulations, the multiple pharmacological in vivo and in vitro experiments performed with it, and the promising results we obtained in these chemical studies, are all included in a separate paper (still unpublished).
Here, we only tested the in vitro effects of the novel topical formulations of Fenoldopam on three functions and features, in 3 experimental setups: (i) the cytokine secretion by activated normal human T cells of healthy individuals, (ii) The number, size and granularity of live big/proliferating vis‐a ‐vis small T cells, within a population of either resting or activated normal human cells, and (iii) Pro‐inflammatory cytokine secretion by activated/inflamed normal human skin.
As for our choice of concentration of the topical Fenoldopam: Fenoldopam is stable for years in water at pH 3·5 or below, or as dry powder, or as an anhydrous gels or ointments. Topical drug formulations commonly contain 1% or less of active material. Hence, we tested 1%, but also lower (0·1%) and higher (5%) Fenoldopam in the carrier base, that contain a solid acid such as citric acid, to maintain low pH.
We spread either the new topical formulation of Fenoldopam at three different concentrations (0·1%, 1% and 5%), or the empty formulation control (i.e. the identical formulation but without Fenoldopam) at the bottom of tissue‐culture plates, and then added CD3/CD28‐activated normal human T cells of healthy individuals for 48 hr of incubation. Then the T cell medium was collected from the tissue‐culture wells and tested for eight cytokines: TNF‐α, IFN‐γ, IL‐1β, IL‐2, IL‐4, IL‐6, IL‐8 and IL‐10.
The results, presented in Fig. 9, show that the novel topical/dermal formulation of Fenoldopam at 1% and 5%, dramatically reduced the secretion of all eight cytokines by the CD3/CD28‐activated CD3+ normal human T cells, compared with the empty formulation control.
Figure 9.
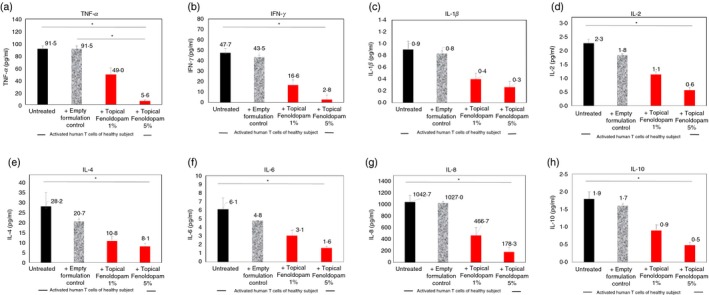
Novel topical/dermal Fenoldopam mesylate formulations reduce significantly the in vitro secretion of eight cytokines: tumor necrosis factor‐α (TNF‐α), interferon‐γ (IFN‐γ), interleukin‐1β (IL‐1β), IL‐2, IL‐4, IL‐6, IL‐8 and IL‐10, by CD3/CD28‐activated CD3+ normal human T cells of healthy subjects. Data represent mean ± SD (n = 3). Statistical analysis was performed by Kruskal–Wallis test. Statistical significance was set at P < 0·05.
The reductions induced by the topical Fenoldopam 1% were as follows: TNF‐α: from 91·5 ± 4·8 to 49 ± 10·9 pg/ml, a decrease of 46%; IFN‐γ: from 47·7 ± 4 to 16·6 ± 4·9 pg/ml, a decrease of 65%; IL‐1β: from 0·9 ± 4 to 0·1 ± 0·4 pg/ml, a decrease of 55%; IL‐2: from 2·3 ± 0·3 to 1·1 ± 0·2 pg/ml, a decrease of 50%; IL‐4: from 28·2 ± 6·9 to 10·8 ± 2·2 pg/ml, a decrease of 61%; IL‐6: from 6·1 ± 1·3 to 3·1 ± 0·6 pg/ml, a decrease of 50%; IL‐8: from 1042 ± 114 to 466 ± 134 pg/ml, a decrease of 55%; and IL‐10: from 1·9 ± 0·2 to 0·9 ± 0·2 pg/ml, a decrease of 50% (Fig. 9). The extent of reductions induced by the topical Fenoldopam 5% were even greater (Fig. 9).
The novel topical/dermal formulation of Fenoldopam eliminates preferentially in vitro >90% of the alive big normal human T cells, while sparing most of the alive small T cells
The effect of the novel topical/dermal Fenoldopam formulation on the number, size [reflected in flow cytometry by the forward scatter (FSC)], and granularity [reflected by the side scatter (SSC)], of both resting and CD3/CD28‐activated normal human CD3+ T cells of healthy human participants, was then studied by flow cytometry (without immunostaining).
The results are presented in Figs 10 and 11. Figure 10 shows the results in representative FACS images, while Fig. 11 shows summary graphs of the mean ± SD of the number of T cells in each group.
Figure 10.
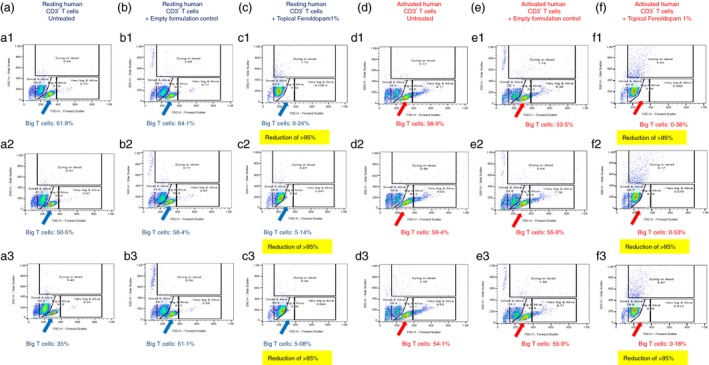
Novel topical/dermal Fenoldopam formulations eliminate preferentially the big alive (i.e. having high FSC and low SSC) normal human T cells from cultures of both resting and activated normal human T cells, while sparing the small alive (i.e. having low FSC and low SSC) T cells. Three individual FACS images are shown for each experimental group: Resting human T cells: Untreated (a1–3), treated with the empty formulation control (b1–3), and treated with the novel topical Fenoldopam 1% formulation (c1–3). CD3/CD28 activated human T cells: untreated (d1–3), treated with the empty formulation control (e1–3), and treated with the novel topical Fenoldopam 1% formulation (f1–3). The original FACS images, and the windows added on them during the analysis of the results, reveal two major subpopulations: (i) Small (low FSC) and not granular (low SSC) T cells, termed ‘Small and alive T cells’, and (ii) Big (high FSC) and not granular (low SSC) T cells, termed ‘Big and alive T cells’, as well as two additional minor subpopulations: (iii) very big (very high FSC) and not granular (low SSC) T cells, termed ‘Very big and alive T cells’; This minor T‐cell population was present mainly in the activated T cells, not in the resting ones, and (iv) small (low FSC) and very granular (very high SSC) T cells, termed ‘Dying or dead T cells’. The number of these cells was extremely low in the untreated resting and activated T cells. The percentage of T cells in each of these subpopulations in each treatment is shown inside the windows.
Figure 11.
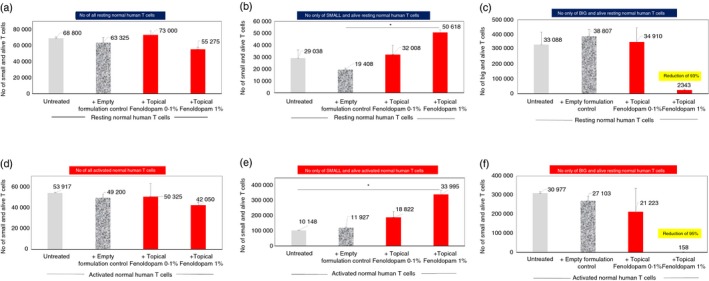
Novel topical formulation of Fenoldopam eliminates preferentially, or reduce profoundly the size of, >90 of % big alive normal human T cells within 48 hr, while sparing most of the small alive T cells. See the corresponding FACS images in Fig 10 and the figure legend describing the experimental groups, and the different subpopulation of T cells. (a–c) Number of resting T cells: either of all resting T cells (a), or only of the small alive resting T cells (b), or only of the big alive resting T cells (c). (d–f) Number of activated T cells: either all activated T cells (d), or only of the small alive activated T cells (e), or only of the big alive activated T cells (f). The numbers represent mean and SD of three independent experiments (n = 3).
In Fig. 10 three individual FACS images are shown for each of the following experimental groups: Resting normal human T cells: (i) untreated (Fig. 10a1–3), (ii) treated with the empty formulation control (Fig. 10b1–3), and (iii) treated with the novel topical Fenoldopam 1% formulation (Fig. 10c1–3). Activated human T cells: (i) untreated (Fig. 10d1–3), (ii) treated with the empty formulation control (Fig. 10e1–3), and (iii) treated with the novel topical Fenoldopam 1% formulation (Fig. 10f1–3). All treatments in vitro were for 48 hr.
As can be seen in all the FACS images in Fig. 10, the entire population of CD3+ T cells was composed of two distinct major subpopulations: (i) small (low FSC) and not granular (low SSC) T cells, which we called ‘Small and alive T cells’ (Fig. 10 low left boxes), and (ii) big (high FSC) and not granular (low SSC) T cells, which we called ‘Big and alive T cells’ (Fig. 10, low middle boxes).
In addition, there were two minor subpopulations: (i) very big (very high FSC) and not granular (low SSC) T cells, which we called ‘Very big and alive T cells’ (Fig. 10, low right boxes). Note that these very big T cells were present mainly in the activated T cells, not in the resting ones, and we therefore assume that these T cells are activated T cells with high proliferative potential; (ii) small (low FSC) and very granular (very high SSC) T cells, which we called ‘Dying or dead T cells’ (Fig. 10 upper boxes), whose number was extremely low in the untreated resting and activated T cells. This showed that the overall condition of the normal human T cells was good.
The FACS images in Fig. 10 show the percentage of CD3+ normal human T cells in each of these four subpopulations, in each experimental group, and reveal a striking finding: the topical formulation of Fenoldopam 1% (48 hr) preferentially eliminated, or reduced the size of, the vast majority of the ‘Big and alive T cells’. This was evident in the resting normal human T cells (compare resting T cells + Topical Fenoldopam 1% (Fig 10c1–3) with the untreated resting T cells (Fig. 10a1–3), and with resting T cells treated with the empty formulation control (Fig. 10b1–3)). This was also evident in the activated T cells, where the topical formulation of Fenoldopam 1% eliminated both the ‘Big and alive T cells’ and the ‘Very big and alive T cells’ (compare Fig 10f1–3 to d13 and e1–3), while minimally affecting, if at all, the ‘Small and alive T cells’ (having low FSC and low SSC) (Fig. 10).
Concomitantly with all these effects, the incubation of the T cells with Fenoldopam increased significantly the number of the highly granular (high SSC) dying/dead T cells (Fig. 10). The topical Fenoldopam at lower concentration of 0·1% had a milder effect, while higher concentration of Fenoldopam (5%) had a much stronger effect than 1%, but they were all followed the same pattern (data not shown).
Figure 11(a–f) shows the mean ± SD of the numbers of T cells, either of all T cells (Fig. 11a, resting, Fig. 11c, activated), only of the ‘Small and alive T cells’ (Fig. 11b, resting, Fig. 11d, activated), or only of the ‘Big and alive T cells’ (Fig. 11c, resting, Fig. 11e, activated).
The main findings of these experiments are that the topical Fenoldopam 1% reduced by 93% the number of big and alive resting T cells (Fig. 11c), and by 95% the number of big and alive activated T cells (Fig. 11f). Concomitantly, there was an increase in the number of ‘Small and alive T cells’.
Thus, Fenoldopam preferentially eliminated, or reduced substantially the size of, big alive T cells, which are typically considered as the activated proliferating T cells, or T cell blasts – as activated lymphocytes must increase in size and duplicate their contents (cell growth) before they can divide.
The novel topical formulation of Fenoldopam significantly reduced, in a dose‐dependent manner, the in vitro cytokine secretion by LPS‐activated ‘inflamed‐like’ human skin
Next, the ex vivo effect of the novel topical Fenoldopam formulation on samples of normal human skin was studied. These healthy skin samples were obtained from healthy adult individuals undergoing elective cosmetic surgery.27
Activation and inflammation were then induced in these skin samples by LPS – a well‐known component of the outer membrane of Gram‐negative bacteria that elicits very strong immune responses. The novel topical formulation of Fenoldopam at increasing concentrations was spread topically on the human skin pieces. After 48 hr of incubation, viability was determined by MTT assay, and the levels of multiple cytokines released into the medium were determined simultaneously by ELISA and multiplex assay (see Methods).
First, the results showed that Fenoldopam applied in a topical gel formulation was not toxic to the skin in vitro (data not shown).
Second, as expected, induction of skin activation/inflammation by LPS, increased significantly the secretion of IL‐1β, IL‐6 and IL‐8 (Fig. 12c,f,g; P < 0·05 for all) by the human skin, in line with previously reported findings.28 In contrast, LPS did not elevate significantly the levels of TNF‐α, IFN‐γ, IL‐2, IL‐4 and IL‐10 secreted by the skin (Fig. 12a,b,d,e,h).
Figure 12.
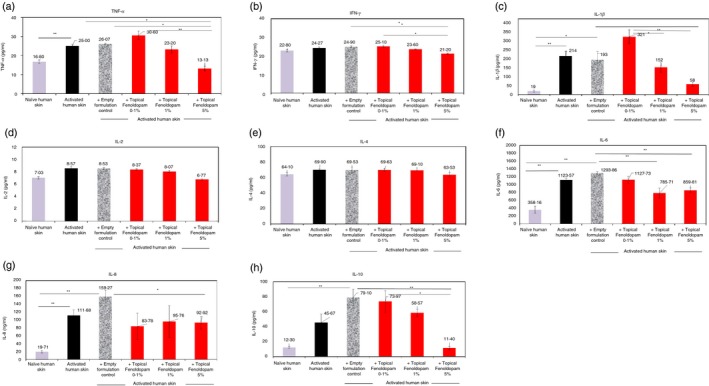
Novel topical/dermal formulation of Fenoldopam induces profound reduction in the levels of several cytokines: tumor necrosis factor‐α (TNF‐α), interleukin‐1β (IL‐1β), IL‐8, IL‐6 and IL‐10, secreted in vitro by lipopolysaccharide (LPS) ‐activated normal human skin samples. All the data represent mean ± SD of three independent experiments. Statistical analysis was performed by Kruskal–Wallis [IL‐2, IL‐4, IL‐10, TNF‐α, interferon‐γ (IFN‐γ), and analysis of variance (IL‐1β, IL‐6, IL‐8). Statistical significance was set at P < 0·05.
Third, compared with the control untreated human skin, and/or the empty formulation control‐treated LPS‐activated skin, the following effects were revealed: (i) The topical Fenoldopam 5% reduced significantly the levels of TNF‐α, IFN‐γ, IL‐1β, IL‐6, IL‐8 and IL‐10 (Fig. 12a,b,c,f,g,h; P < 0·05 for all). (ii) The topical Fenoldopam 1% reduced significantly only the levels of IL‐1β and IL‐6 (Fig. 12c,f). (iii) The topical Fenoldopam 0·1% failed to induce statistically significant effects on any of the cytokines.
Taken together, these results showed that the novel topical formulation of Fenoldopam could in principal inhibit the secretion of several key pro‐inflammatory cytokines by activated/inflamed human skin.
Discussion
Human psoriatic skin harbors elevated levels of D1R+ T cells, and D1‐like receptor agonists, primarily Fenoldopam, suppress activated human T cells and activated human skin
In this study we revealed that skin of patients with Psoriasis contains 20‐fold more D1R+ T cells than healthy human skin (Fig. 5). In line with this, there is a 25‐fold increase in human D1R+ T cells in human skin grafts that were transplanted in immunodeficient mice, and in which Psoriasis‐like disease was induced (Fig. 5).
The dramatic elevation in D1R+ T cells in the human psoriatic skin is evident for both cytotoxic CD8+ D1R+ and helper CD4+ D1R+ T cells, but there are more of the cytotoxic T cells (Fig. 5).
Having revealed these findings, we humbly emphasize that our results by no means imply that the enriched T cells in the human psoriatic skin do not express other DRs beyond D1R. The presence in the psoriatic skin of T cells expressing other DRs was not tested in our experiments, because we focused on D1R in our functional studies. But even without testing this, our findings seem already sufficient to suggest indirectly that, first: Helper and cytotoxic T cells in psoriatic skin may need D1R for their own purposes and therefore express this receptor, and second: D1R in T cells could become a drug target in Psoriasis and potentially additional diseases, that are caused by, or perpetuated by, detrimental T cells that are present in the diseased organ and that express this receptor.
In accordance with the second suggestion, we revealed that Fenoldopam mesylate – a highly selective D1‐like receptor agonist and a drug for hypertension named Corlopam, have potent suppressive effects on activated human T cells and inflamed human skin. Specifically, we revealed that Fenoldopam at a relatively low dose of 10−7 M (100 nM), either in a soluble solution prepared from Fenoldopam powder, or diluted from Corlopam – the original soluble drug ampoule, or in our new topical/dermal formulation, is sufficient to induce all the following effects: (i) depolarize T cells (15 seconds), (ii) reduce significantly the chemotactic migration of normal human T cells (1 hr pre‐incubation with Fenoldopam, followed by 4 hr migration assay), (iii) down‐regulate profoundly, by 75–90%, the expression level of three highly important T cell activation‐associated proteins: CD69 and CD28 on the cell surface, and IL‐2 intracellularly, in activated T cells of patients with Psoriasis (24–72 hr), (iv) reduce profoundly the secretion of multiple principal cytokines by: (i) IL‐2‐activated T cells and PBMCs of patients with Psoriasis, (ii) CD3/CD38‐activated normal human T cells, (iii) LPS‐activated healthy human skin (24 hr), (iv) eliminates preferentially in vitro >90% of the alive large big normal human T cells, while sparing most of the live small T cells (48 hr).
The downstream signaling of the D1‐like receptors in activated T cells has been studied extensively before by several research groups, and found to involve cAMP 16, 18, 51, 52 and phosphorylation of the extracellular signal‐regulated kinase 1/2 (ERK1/2) signaling pathway.17 Although we did not study by which downstream signaling pathway Fenoldopam induces all the suppressive effects on activated T cells and inflammed skin of healthy individuals and patients with Psoriasis, we assume that the downstream signaling mediating these effects would also involve cAMP and ERK1/2. In addition, we raise the possibility that the downstream events occurring in activated T cells and in inflamed skin after binding Fenoldopam, may also involve the D1‐like receptor‐associated downstream pathways revealed in other cells, by the four below cited studies (of many more). First, Borcherding et al. 24 revealed that in several breast cancer cells, dopamine and also D1R agonists, among them Fenoldopam, signal through the cGMP/protein kinase G pathways. Second, Yan et al. 19 revealed that in mouse LPS‐primed bone marrow‐derived macrophages, D1R activation inhibits the activation of the NLRP3 inflammasome via cAMP, which binds to NLRP3 and promotes its ubiquitination and degradation via the E3 ubiquitin ligase MARCH7. Third, Gao et al. found that in OS732 cells, overexpression of DR1 increased the rate of apoptosis, caspase‐9 and caspase‐3 expression, and the release of cytochrome c, reduced Bcl‐2 expression, inhibited ERK1/2 phosphorylation, and induced phosphorylation of p38 mitogen‐activated protein kinase (MAPK) and c‐Jun N‐terminal kinase (JNK).53 These results suggest that activation of D1R induces osteosarcoma cell apoptosis via changes in the MAPK pathway. Fourth, Takeuchi and Fukunaga 54 revealed that stimulation of NG108‐15 cells transfected with D1R and stably expressing this receptor, with the selective D1‐like receptor agonist SKF 38393, decreased nuclear factor‐κB and serum response element activity, but increased CRE activity.
Interestingly, several studies have shown that the D1R undergoes active trafficking and that the cell surface expression of D1R in living cells is dynamic and changes rapidly via internalization in response to various stimuli.55, 56, 57 Some evidences for this are summarized below: The D1R undergoes phosphorylation‐mediated desensitization and internalization.55 Protein kinase D1‐mediated phosphorylation of serine 421 (S421) of D1R promotes surface localization of D1R, and enhances downstream extracellular signal‐regulated kinase signaling in D1R‐transfected HEK 293 cells.55 Agonist stimulation induces internalization of D1R, but not of D3R, and heterodimerization with D3R abolishes agonist‐induced D1R cytoplasmic sequestration induced by selective D1R agonists, and enables internalization of the D1R/D3R complex in response to the paired stimulation of both D1R and D3R.56 Internalization of agonist‐activated D1R is regulated by both sorting nexin 5 (SNX5) and G‐protein‐coupled receptor kinase 4 (GRK4), and SNX5, is critical to the recycling of the receptor to the plasma membrane57. Dendritic and axonal D1Rs are internalized after agonist stimulation, and targeted to the recycling pathway, demonstrating that the machinery involved in G protein‐coupled receptor endocytosis and recycling is functional both in dendrites and in axons58.
Our findings of the suppressive effects of Fenoldopam on activated T cells are in line with the findings of various groups, among them those cited in the introduction herein, showing that once selective D1‐like receptor analogues (considered as either agonists or antagonists of D1‐like receptors, only based on their effects on other cells, not T cells!), bind to D1‐like receptors expressed in immune cells, they can lead to significant suppression of various immune pathways and functions, and to down‐regulation of systemic experimental immune/autoimmune diseases in vivo.19, 20, 21, 22, 59
Resting and activated normal human T cells express all DR types, and some are markedly elevated after T cell activation
In this study we confirmed that normal human T cells express all DR types, and that the expression of some of them, primarily the D1‐like receptors: D1R and D5R, and also D4R, are elevated significantly after the T cells have been activated via their TCR–CD3 complex. These results are in accordance with findings of few other groups, primarily of Kustrimovic et al.,7 showing that: (i) CD4+ T cells of healthy subjects always express all DRs, (ii) T cells of different people express different levels of each DR, (iii) stimulation with anti‐CD3/anti‐CD28 antibodies increase the expression of D1‐like DRs in T cells by 71–84%, and of D2‐like DRs by 55–97%.
Together, these findings by others and ourselves indicate that there is a functional dialogue in T cells between the antigen–receptor, i.e. the TCR, and neurotransmitter receptors, i.e. DRs in this case, in view of the findings that activation of the former leads to elevation of the latter. The dicovery of this dialogue has of course various important implications, beyond those addressed and discussed in this paper.
Activation of DRs in T cells shifts the membrane potential of T cells within 15 seconds
The vast majority of plasma membranes have an electrical potential across them, with the inside usually being negative with respect to the outside. The membrane potential provides power to operate multiple ‘molecular devices’ embedded in the membrane. In addition, in electrically excitable cells such as neurons and muscle cells, shifts in the membrane potential occur within milliseconds to seconds after binding of certain stimuli, primarily neurotransmitters, to their specific receptors expressed on the cell surface of the target cells, or after various changes in the extracellular milieu (e.g. elevation of extracellular K+). Subsequently, the membrane potential shifts lead to: (i) various rapid changes within the target cells, (ii) transmission of important signals to other cells, and (iii) initiation, or rather blocking, of functionally important responses.
Many studies on dopamine in the nervous system have shown that dopamine induces rapid direct and indirect effects on the membrane potential of various types of excitable neurons, and thereby modulates neuronal excitability. The dopamine‐induced shifts in the resting membrane potential of neural cells are either hyperpolarization or depolarization, depending on the type of cell, the specific DR being activated, and the time after DR activation. For example, the readers are referred to the study by Yasumoto et al., 60 and to the papers cited therein.
We found in this study for the very first time that activation of DRs in normal human T cells, which are of course considered classicaly as non‐excitable cells, leads to very rapid shifts in their membrane potential. Thus, in this specific respect, T cells behave as excitable cells, and resemble neural cells are noteworthy. Having found these effects, a few words of caution. First, subsequent electrophysiological studies on T cells are needed to confirm the DR‐mediated depolarizations, which were detected in our study only indirectly, i.e. by a voltage‐sensitive fluorescent dye. Second, although we found that activation of DRs in normal human T cells leads to moderate depolarization within 15 seconds, these findings do not teach us: (i) if DR agonists at higher concentrations would induce stronger depolarizations, (ii) if 15 seconds is the optimal time to detect T cell depolarization induced by activation of the T cell's DRs, and how long does the depolarization last, (iii) if an even earlier event of hyperpolarization took place before the depolarization, or followed it, (iv) which ion currents are responsible for the depolarization of T cells in response to DRs activation; or (v) are the rapid V m shifts in human T cells a direct trigger to any of the subsequent functional effects induced by selective DR agonists.
Interestingly, and with relevance to depolarization of T cells, in a previous study we found that normal human T cells are depolarized by elevated levels of extracellular K+ ions in the extracellular milieu, 26 and that this T cell depolarization event is sufficient by itself (i.e. without any other stimuli) to trigger integrin‐mediated T cell adhesion to extracellular matrix glycoproteins.26 Hence, depolarization of T cells has important functional consequences.
In general terms, depolarization – a shift towards a more positively charged cell interior – is caused usually either from rapid opening of calcium (Ca2+) or sodium (Na+) channels, and subsequent inward currents of Ca2+ and/or Na+ ions along their concentration gradients, or from transient closing of potassium (K+) channels, and subsequent prevention of outward currents of K+ ions, along their own concentration gradient.
A potentially relevant study to our present one is that of Podda et al.,61 revealing that D1‐like receptor activation depolarizes medium spiny neurons of mouse nucleus accumbens, by inhibiting inwardly rectifying K+ currents, through a cAMP‐dependent protein kinase.
The nature of the ion currents underlying the membrane potential shifts occurring in normal human T cells after activation of DRs (revealed herein) was not investigated in our present study, and calls for subsequent studies. These future studied could only be performed with appropriate sophisticated methodologies that combine electrophysiology and ion‐sensitive detection methodologies, and that could monitor simultaneously and continuously both the membrane potential and the levels of Ca2+, Na+, K+ (and preferably Cl−) in T cells, before and immediately after DR activation in the same T cells.
Meanwhile, the newly designed experiments performed in the present study on the membrane potential of T cells have broader implications and novelty beyond showing that T cells respond to DR agonists in an extremely rapid manner, because they show that one can detect rapid depolarization or hyperpolarization of live T cells (or in fact any other immune cell) in suspension (as opposed for example to neural cells or tissue), and that this can be done using immunofluorescence and flow cytometry. This new technology can of course be combined with other simultaneous immunostaining of any other protein in the same cell.
Psoriasis – an autoimmune disease mediated by T cells
Psoriasis is one of the most common chronic diseases, affecting 2–3% of the adult population, with tremendous impact on patients’ quality of life.62 The disease, which is considered to be an autoimmune disorder,33, 34, 35 develops due to a close interaction between several mediators, cells of the innate and adaptive immune systems, keratinocytes and endothelial cells.63, 64 However, there is a general consensus among scientists that T cells play crucial roles in the pathogenesis of Psoriasis.33, 34, 35 For example, an impairment of the regulatory T cell activity in peripheral blood, enhances effector T cell functions in patients with Psoriasis,65 further indicating the critical role of T cells in development of Psoriasis.
During the last decade it has become clear that Th17 cells, and the IL‐23/IL‐17 axis, are crucial factors in the pathogenesis of Psoriasis.46, 47, 48, 49
The advances in understanding Psoriasis etiopathogenesis have resulted in the development of new immunobiological treatments. As Psoriasis is known to be a chronic disease that often requires long‐term treatments, the biological agents are given for long periods of time. Such a mode of long‐term administration is not always recommended, due to the potential for patients with Psoriasis to experience cumulative toxicity, and may also raise the already considerable cost of managing the disease.66
There are cases, in which patients with Psoriasis developed diseases requiring temporary or permanent discontinuation of therapy.66 A recent study demonstrated low adherence and high discontinuation rates in patients with Psoriasis treated with biological agents.66 Therefore, there is still urgent need for developing rational, effective and acceptable strategies to manage Psoriasis treatments.
Based on the findings presented in this study, we humbly propose that targeting the D1‐like receptors in pathological T cells that express these receptors, either by Fenoldopam if the T cells are in the periphery, or by our novel topical/dermal formulation of Fenoldopam if they are in the skin, could be therapeutic.
These proposed therapeutic effects of Fenoldopam would be validated only if Fenoldopam induces in vivo similar suppressive effects to those induced in this study in vitro. In these needed in vivo experiements in animal models: The effects of topical/deramal Fenoldopam should of course be tested, by applying it to the skin of mice suffering from Psoriasis‐like disease that should resemble the human Psoriasis as much as possible.
If the in vivo findings would validate our in vitro findings, then topical/dermal Fenoldopam could be therapeutic in Psoriasis, and potentially in additional skin pathologies mediated by D1R‐expressing activated T cells.
Disclosure
The authors declare that they are not aware of any conflict of interest, and so have none.
Acknowledgements
The authors thank Dr Tomer Zur, Department of Plastic, Reconstructive and Hand Surgery, Hadassah University Medical Center, Jerusalem, Israel, for supplying the human skin of healthy individuals undergoing cosmetic surgery.
References
- 1. Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun 2015; 64:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim J, Krueger JG. Highly effective new treatments for psoriasis target the IL‐23/Type 17 T cell autoimmune axis. Annu Rev Med 2017; 68:255–69. [DOI] [PubMed] [Google Scholar]
- 3. Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour J‐P et al Risankizumab versus ustekinumab for moderate‐to‐severe plaque psoriasis. N Engl J Med 2017; 376:1551–60. [DOI] [PubMed] [Google Scholar]
- 4. McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen‐Jones D et al Dopamine receptor expression on human T‐ and B‐lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol 2002; 132:34–40. [DOI] [PubMed] [Google Scholar]
- 5. Levite M, Chowers Y, Ganor Y, Besser M, Hershkovits R, Cahalon L. Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates β1 integrin function. Eur J Immunol 2001; 31:3504–12. [DOI] [PubMed] [Google Scholar]
- 6. Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T‐cells and triggers the selective secretion of either IL‐10, TNFα or both. J Neuroimmunol 2005; 169:161–71. [DOI] [PubMed] [Google Scholar]
- 7. Kustrimovic N, Rasini E, Legnaro M, Marino F, Cosentino M. Expression of dopaminergic receptors on human CD4+ T lymphocytes: flow cytometric analysis of naive and memory subsets and relevance for the neuroimmunology of neurodegenerative disease. J Neuroimmune Pharmacol 2014; 9:302–12. [DOI] [PubMed] [Google Scholar]
- 8. Levite M. Dopamine in the immune system: dopamine receptors in immune cells, potent effects, endogenous production and involvement in immune and neuropsychiatric diseases Nerve Driven Immunity: Neurotransmitters and Neuropeptides in the Immune System. New York: Springer, 2012: pp 1–45. [Google Scholar]
- 9. Levite M. Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol 2016; 216:42–89. [DOI] [PubMed] [Google Scholar]
- 10. Pacheco R, Prado CE, Barrientos MJ, Bernales S. Role of dopamine in the physiology of T‐cells and dendritic cells. J Neuroimmunol 2009; 216:8–19. [DOI] [PubMed] [Google Scholar]
- 11. Franz D, Contreras F, González H, Prado C, Elgueta D, Figueroa C et al Dopamine receptors D3 and D5 regulate CD4+ T‐cell activation and differentiation by modulating ERK activation and cAMP production. J Neuroimmunol 2015; 284:18–29. [DOI] [PubMed] [Google Scholar]
- 12. Vidal PM, Pacheco R. Targeting the dopaminergic system in autoimmunity. J Neuroimmune Pharmacol. 2019; 10.1007/s11481-019-09834-5 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13. Pacheco R, Contreras F, Zouali M. The dopaminergic system in autoimmune diseases. Front Immunol 2014; 5:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levite M, Marino F, Cosentino M. Dopamine, T cells and multiple sclerosis (MS). J Neural Transm (Vienna) 2017; 124:525–42. [DOI] [PubMed] [Google Scholar]
- 15. Basu B, Sarkar C, Chakroborty D, Ganguly S, Shome S, Dasgupta PS et al D1 and D2 dopamine receptor‐mediated inhibition of activated normal T cell proliferation is lost in Jurkat T leukemic cells. J Biol Chem 2010; 285:27026–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghosh MC, Mondal AC, Basu S, Banerjee S, Majumder J, Bhattacharya D et al Dopamine inhibits cytokine release and expression of tyrosine kinases, Lck and Fyn in activated T cells. Int Immunopharmacol 2003; 3:1019–26. [DOI] [PubMed] [Google Scholar]
- 17. Kipnis J, Cardon M, Avidan H, Lewitus GM, Mordechay S, Rolls A et al Dopamine, through the extracellular signal‐regulated kinase pathway, downregulates CD4+ CD25+ regulatory T‐cell activity: implications for neurodegeneration. J Neurosci 2004; 24:6133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E et al Human CD4+ CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 2007; 109:632–42. [DOI] [PubMed] [Google Scholar]
- 19. Yan Y et al Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015; 160:62–73. [DOI] [PubMed] [Google Scholar]
- 20. Nakashioya H, Nakano K, Watanabe N, Miyasaka N, Matsushita S, Kohsaka H. Therapeutic effect of D1‐like dopamine receptor antagonist on collagen‐induced arthritis of mice. Mod Rheumatol 2010; 23:260–6. [DOI] [PubMed] [Google Scholar]
- 21. Nakano K, Yamaoka K, Hanami K, Saito K, Sasaguri Y, Yanagihara N et al Dopamine induces IL‐6‐dependent IL‐17 production via D1‐like receptor on CD4 naive T cells and D1‐like receptor antagonist SCH‐23390 inhibits cartilage destruction in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol 2011; 186:3745–52. [DOI] [PubMed] [Google Scholar]
- 22. Nakagome K, Imamura M, Okada H, Kawahata K, Inoue T, Hashimoto K et al Dopamine D1‐like receptor antagonist attenuates Th17‐mediated immune response and ovalbumin antigen‐induced neutrophilic airway inflammation. J Immunol 2011; 186:5975–82. [DOI] [PubMed] [Google Scholar]
- 23. Mori T, Kabashima K, Fukamachi S, Kuroda E, Sakabe J, Kobayashi M et al D1‐like dopamine receptors antagonist inhibits cutaneous immune reactions mediated by Th2 and mast cells. J Dermatol Sci 2013; 71:37–44. [DOI] [PubMed] [Google Scholar]
- 24. Borcherding DC, Tong W, Hugo ER, Barnard DF, Fox S, LaSance K et al Expression and therapeutic targeting of dopamine receptor‐1 (D1R) in breast cancer. Oncogene 2015; 35:3103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saussez S, Laumbacher B, Chantrain G, Rodriguez A, Gu S, Wank R et al Towards neuroimmunotherapy for cancer: the neurotransmitters glutamate, dopamine and GnRH‐II augment substantially the ability of T cells of few head and neck cancer patients to perform spontaneous migration, chemotactic migration and migration towards the autologous tumor, and also elevate markedly the expression of CD3zeta and CD3epsilon TCR‐associated chains. J Neural Transm 2014; 121:1007–27. [DOI] [PubMed] [Google Scholar]
- 26. Levite M, Cahalon L, Peretz A, Hershkoviz R, Sobko A, Ariel A et al Extracellular K+ and opening of voltage‐gated potassium channels activate T cell integrin function: physical and functional association between Kv1.3 channels and β1 integrins. J Exp Med 2000; 191:1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Portugal‐Cohen M, Soroka Y, Ma'or Z, Oron M, Zioni T, Brégégère FM et al Protective effects of a cream containing Dead Sea minerals against UVB‐induced stress in human skin. Exp Dermatol 2009; 18:781–8. [DOI] [PubMed] [Google Scholar]
- 28. Frusic‐Zlotkin M, Soroka Y, Tivony R, Larush L, Verkhovsky L, Brégégère FM et al Penetration and biological effects of topically applied cyclosporin A nanoparticles in a human skin organ culture inflammatory model. Exp Dermatol 2012; 21:938–43. [DOI] [PubMed] [Google Scholar]
- 29. De Villiers M. Ointment Bases In: Thompson JE. A Practical Guide to Contemporary Pharmacy Practice, 3rd edn, Chapter: 23. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:277–90. [Google Scholar]
- 30. Scott KA, Moore RJ, Arnott CH, East N, Thompson RG, Scallon BJ et al An anti‐tumor necrosis factor‐α antibody inhibits the development of experimental skin tumors. Mol Cancer Ther 2003; 2:445–51. [PubMed] [Google Scholar]
- 31. Mignini F, Sabbatini M, Capacchietti M, Amantini C, Bianchi E, Artico M et al T‐cell subpopulations express a different pattern of dopaminergic markers in intra‐ and extra‐thymic compartments. J Biol Regul Homeost Agents 2013; 27:463–75. [PubMed] [Google Scholar]
- 32. Watanabe Y, Nakayama T, Nagakubo D, Hieshima K, Jin Z, Katou F et al Dopamine selectively induces migration and homing of naive CD8+ T cells via dopamine receptor D3. J Immunol 2006; 176:848–56. [DOI] [PubMed] [Google Scholar]
- 33. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361:496–509. [DOI] [PubMed] [Google Scholar]
- 34. Lowes MA, Suarez‐Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol 2014; 32:227–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boehncke WH, Schon MP. Psoriasis. Lancet 2015; 386:983–94. [DOI] [PubMed] [Google Scholar]
- 36. Gilhar A, Bergman R, Assay B, Ullmann Y, Etzioni A. The beneficial effect of blocking Kv1.3 in the psoriasiform SCID mouse model. J Invest Dermatol 2011; 131:118–24. [DOI] [PubMed] [Google Scholar]
- 37. Zaretsky M, Etzyoni R, Kaye J, Sklair‐Tavron L, Aharoni A. Directed evolution of a soluble human IL‐17A receptor for the inhibition of psoriasis plaque formation in a mouse model. Chem Biol 2013; 20:202–11. [DOI] [PubMed] [Google Scholar]
- 38. Chiricozzi A, Suárez‐Fariñas M, Fuentes‐Duculan J, Cueto I, Li K, Tian S et al Increased expression of interleukin‐17 pathway genes in nonlesional skin of moderate‐to‐severe psoriasis vulgaris. Br J Dermatol 2016; 174:136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schafer PH, Parton A, Gandhi AK, Capone L, Adams M, Wu L et al Apremilast, a cAMP phosphodiesterase‐4 inhibitor, demonstrates anti‐inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol 2010; 159:842–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nousbeck J, Ishida‐Yamamoto A, Bidder M, Fuchs D, Eckl K, Hennies HC et al IGFBP7 as a potential therapeutic target in psoriasis. J Invest Dermatol 2011; 131:1767–70. [DOI] [PubMed] [Google Scholar]
- 41. Bracke S, Carretero M, Guerrero‐Aspizua S, Desmet E, Illera N, Navarro M et al Targeted silencing of DEFB4 in a bioengineered skin‐humanized mouse model for psoriasis: development of siRNA SECosome‐based novel therapies. Exp Dermatol 2014; 23:199–201. [DOI] [PubMed] [Google Scholar]
- 42. Karin N. The multiple faces of CXCL12 (SDF‐1α) in the regulation of immunity during health and disease. J Leukoc Biol 2010; 88:463–73. [DOI] [PubMed] [Google Scholar]
- 43. Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol 2008; 84:116–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatol Clin 2015; 33:13–23. [DOI] [PubMed] [Google Scholar]
- 45. Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG et al The emerging role of IL‐17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol 2013; 133:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puig L. The role of IL 23 in the treatment of psoriasis. Expert Rev Clin Immunol 2017; 13:525–34. [DOI] [PubMed] [Google Scholar]
- 47. Belge K, Bruck J, Ghoreschi K. Advances in treating psoriasis. F1000PrimeRep 2014; 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE et al Generation of pathogenic TH17 cells in the absence of TGF‐β signalling. Nature 2010; 467:967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eberle FC, Brück J, Holstein J, Hirahara K, Ghoreschi K. Recent advances in understanding psoriasis. F1000Res 2016; 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keren A, Shemer A, Ginzburg A, Ullmann Y, Schrum AG, Paus R et al Innate lymphoid cells 3 induce psoriasis in xenotransplanted healthy human skin. J Allergy Clin Immunol. 2018. [DOI] [PubMed] [Google Scholar]
- 51. Cosentino M, Zaffaroni M, Trojano M, Giorelli M, Pica C, Rasini E et al Dopaminergic modulation of CD4+ CD25high regulatory T lymphocytes in multiple sclerosis patients during interferon‐β therapy. NeuroImmunoModulation 2012; 19:283–92. [DOI] [PubMed] [Google Scholar]
- 52. Saha B, Mondal AC, Majumder J, Basu S, Dasgupta PS. Physiological concentrations of dopamine inhibit the proliferation and cytotoxicity of human CD4+ and CD8+ T cells in vitro: a receptor‐mediated mechanism. NeuroImmunoModulation 2001; 9:23–33. [DOI] [PubMed] [Google Scholar]
- 53. Gao J, Gao F. Dopamine D1 receptors induce apoptosis of osteosarcoma cells via changes of MAPK pathway. Clin Exp Pharmacol Physiol. 2017; 44(11):1166–8. [DOI] [PubMed] [Google Scholar]
- 54. Takeuchi Y, Fukunaga K. Differential regulation of NF‐κB, SRE and CRE by dopamine D1 and D2 receptors in transfected NG108‐15 cells. J Neurochem 2003; 85:729–39. [DOI] [PubMed] [Google Scholar]
- 55. Wang N, Su P, Zhang Y, Lu J, Xing B, Kang K et al Protein kinase D1‐dependent phosphorylation of dopamine D1 receptor regulates cocaine‐induced behavioral responses. Neuropsychopharmacology 2014; 39:1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol 2008; 74:59–69. [DOI] [PubMed] [Google Scholar]
- 57. Villar VA, Armando I, Sanada H, Frazer LC, Russo CM, Notario PM et al Novel role of sorting nexin 5 in renal D(1) dopamine receptor trafficking and function: implications for hypertension. FASEB J. 2013; 27:1808–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martin‐Negrier ML, Charron G, Bloch B. Receptor recycling mediates plasma membrane recovery of dopamine D1 receptors in dendrites and axons after agonist‐induced endocytosis in primary cultures of striatal neurons. Synapse 2006; 60:194–204. [DOI] [PubMed] [Google Scholar]
- 59. Nakano K, Higashi T, Hashimoto K, Takagi R, Tanaka Y, Matsushita S. Antagonizing dopamine D1‐like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun 2008; 373:286–91. [DOI] [PubMed] [Google Scholar]
- 60. Yasumoto S, Tanaka E, Hattori G, Maeda H, Higashi H. Direct and indirect actions of dopamine on the membrane potential in medium spiny neurons of the mouse neostriatum. J Neurophysiol 2002; 87:1234–43. [DOI] [PubMed] [Google Scholar]
- 61. Podda MV, Riccardi E, D'Ascenzo M, Azzena GB, Grassi C. Dopamine D1‐like receptor activation depolarizes medium spiny neurons of the mouse nucleus accumbens by inhibiting inwardly rectifying K+ currents through a cAMP‐dependent protein kinase A‐independent mechanism. Neuroscience 2010; 167:678–90. [DOI] [PubMed] [Google Scholar]
- 62. Abrouk M, Nakamura M, Zhu TH, Farahnik B, Koo J, Bhutani T. The impact of PASI 75 and PASI 90 on quality of life in moderate to severe psoriasis patients. J Dermatolog Treat 2017; 28:488–91. [DOI] [PubMed] [Google Scholar]
- 63. Heidenreich R, Rocken M, Ghoreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol 2009; 90:232–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yazdi AS, Rocken M, Ghoreschi K. Cutaneous immunology: basics and new concepts. Semin Immunopathol 2016; 38:3–10. [DOI] [PubMed] [Google Scholar]
- 65. Chen H, Liu H, Lu C, Wang M, Li X, Zhao H et al PSORI‐CM02 formula increases CD4+ Foxp3+ regulatory T cell frequency and ameliorates imiquimod‐induced psoriasis in mice. Front Immunol 2017; 8:1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Doshi JA, Takeshita J, Pinto L, Li P, Yu X, Rao P et al Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US Medicare population. J Am Acad Dermatol 2016; 74:1057–65. e1054. [DOI] [PMC free article] [PubMed] [Google Scholar]


