Abstract
Background:
Previous studies have neglected to report the specific action of different probiotic genera in preterm infants. To evaluate the efficacy and safety of specific probiotic genera, we performed a network meta-analysis (NMA) to identify the best prevention strategy for necrotizing enterocolitis in preterm infants.
Methods:
MEDLINE, PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials had been searched for randomized control trials reporting the probiotics strategy for premature infants.
Results:
We identified 34 eligible studies of 9161 participants. The intervention in the observation group was to add probiotics for feeding: Lactobacilli in 6 studies; Bifidobacterium in 8 studies; Bacillus in 1 study; Saccharomyces in 4 studies and probiotic mixture in 15 studies. This NMA showed a significant advantage of probiotic mixture and Bifidobacterium to prevent the incidence of necrotizing enterocolitis in preterm infants. A probiotic mixture showed effectiveness in reducing mortality in preterm infants.
Conclusion:
The recent literature has reported a total of 5 probiotic strategies, including Bacillus, Bifidobacterium, Lactobacillus, Saccharomyces, and probiotic mixture. Our thorough review and NMA provided a piece of available evidence to choose optimal probiotics prophylactic strategy for premature infants. The results indicated that probiotic mixture and Bifidobacterium showed a stronger advantage to use in preterm infants; the other probiotic genera failed to show an obvious effect to reduce the incidence of NEC, sepsis and all-cause death. More trials need to be performed to determine the optimal probiotic treatment strategy to prevent preterm related complications.
Keywords: necrotizing enterocolitis, preterm infants, probiotics, sepsis
1. Introduction
Necrotizing enterocolitis (NEC) is one of the most common and serious preterm-related complications with high surgical rate and mortality in premature infants. The morbidity of NEC can be as high as 28% in very low birthweight infants.[1,2] Besides that, although accept medical treatment, patients with NEC tend to suffer from long-term complications which included chronic nutritional intolerance, short bowel syndrome, and growth retardation.[3] Therefore, the prevention of the incidence of NEC seems to be more important and effective than its treatment. Some studies reported that key risk factors for NEC include an overgrowth of pathogenic microflora in premature infants, primarily because of the immature mucosal barrier and immune response in preterm newborns, together with their exposure high-risk hospital milieu with bacterial pathogens.[4–6] Recently, the probiotic product has been reported to be beneficial for decreasing the morbidity of NEC in the literature. Probiotics, as live microbial supplements, might to improve the function of the intestinal mucosal barrier and competitively inhibit the growth of gastrointestinal pathogenic bacteria in preterm infants.[7,8]
Many clinical RCTs have proved probiotics are effective preparations for the prevention of NECs but few studies have examined the effect of different genera.[9] The function of probiotics is species specific, depending on morphological, physiological, and biochemical characteristics of the different probiotic genera.[7] Recent review studies showed that Bifidobacterium significantly decreases the incidence of NEC and mortality rates.[10]Bifidobacterium breve may improve intestinal tolerance, which was reported by Kitajima et al.[11] Additionally, in other literature, the effect of a single strain might also differ from combined strains in NEC.[12] Reports showed that supplements of a combination of strains of Bifidobacterium species[13] or a combination of Bifidobacterium and Lactobacillus acidophilus[14] might achieve an earlier total gastrointestinal nutrition. Moreover, other clinical trials considered different strains of probiotics including Lactobacillus[15,16] and Saccharomyces boulardii.[8] However, the current studies and systematic analysis have failed to recommend an optimal prevention strategy to reduce the incidence of NEC.
To evaluate the efficacy and safety of different genera of probiotics, we sum up available clinical evidence from randomized controlled trials (RCTs) related to this topic and performed a network meta-analysis (NMA) to identify the best prevention strategy for NEC in preterm infants.
2. Materials and methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and reported a net-meta analysis of the RCTs.
2.1. Literature searches
Two independent reviewers systematically searched the following electronic databases: PubMed, MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials to identify literature on probiotics for NEC in premature infants before January 2019. We used PubMed medical subject heading terms and free-text words in combination with Boolean operators as comprehensively as possible: (premature birth OR preterm birth OR preterm infants) AND (RCT OR controlled clinical trial OR randomly) AND (probiotics OR probiotic treatment). Besides, we further searched other databases (EBSCO Information Services, Web of Science, and Google Scholars) to identify potentially available studies. The process was completed when no further trials could be determined. The review was limited to RCTs. The ethical approval is not necessary.
2.2. Criteria for study inclusion
All enrolled RCTs met the following inclusion criteria:
-
1.
premature infants with a low birth weight (<2500 g);
-
2.
intervention: probiotics;
-
3.
comparison intervention: placebo or negative control;
-
4.
outcomes, including more than one of the following outcomes; and
-
5.
only clinical studies.
Exclusion criteria were as follows:
-
1.
non-probiotic interventions;
-
2.
non-English language literature;
-
3.
animal studies; and
-
4.
studies including infants who also had other congenital diseases (e.g., intestinal atresia).
The process was completed by two independent investigators.
The five eligible probiotic strategies were
-
1.
Lactobacilli included Lactobacillus rhamnosus GG (L casei), Lactobacillus reuteri (L reuteri), and Lactobacillus sporogenes;
-
2.
Bifidobacterium included Bifidobacterium longum (B longum), Bifidobacterium breve (B breve), Bifidobacterium bifidum (B bifidum), and Bifidobacterium lactis (B lactis);
-
3.
Bacillus included Bacillus clausii (B clausii);
-
4.
Saccharomyces included Saccharomyces boulardii (S boulardii);
-
5.
probiotic mixture included the combination of the different probiotic strains.
Their control treatment included blank or placebo control.
2.3. Primary and secondary outcomes
In this study, the primary outcome is NEC incidence rate (NEC was diagnosed and classified according to the classification of Bell). The secondary outcomes included the incidence of sepsis (which is diagnosed by positive blood culture results) and all-cause mortality.
2.4. Data extraction and risk of bias assessment
Data from all included RCTs was collected: author's name, year of publication, sample size, patient characteristics, probiotic type, intervention time, dose, and outcomes. Any dispute arising from the data collection shall be negotiated by two researchers and determined by the third evaluator. When the specific number is not reported, the relevant incidence rate is extracted from the article and the required data are calculated.
We assessed the quality of randomized controlled studies using the Cochrane Collaboration's Risk of Bias Tool.[17] The main evaluation contents included:
-
1.
the random sequence generation,
-
2.
the allocation sequence concealment,
-
3.
blinding of participants and personnel,
-
4.
the blinding of outcome assessment,
-
5.
the completeness of outcome data,
-
6.
selective reporting,
-
7.
other sources of bias.
Each evaluation of these 7 items was mainly divided into three options of low, high, and unclear. The evaluation process was mainly evaluated by two authors independently.
2.5. Data synthesis and analysis
STATA V13 software was used for the meta-analysis. The Mantel–Haenszel method was used for continuous variables, and the combined odds ratios (ORs) and 95% confidence intervals (CIs) were used for dichotomous variables. The difference was statistically significant when the P-value was <.05. The pooled mean difference (MD) was measured in the meta-analysis using the inverse variance method. Inferred heterogeneity was determined according to I2. When I2 was <50%, there was no obvious heterogeneity in the analysis, and the fixed effect model was used. When the I2 was ≥50%, there was significant heterogeneity among the analyses, and the random effect model was selected.
We performed a Bayesian hierarchical random-effects NMA to assess all probiotic preventions of primary outcomes simultaneously with the use of Markov chain Monte Carlo simulation with a prior distribution. The analyses used generalized linear models with a logit link function with 4 chains and 50,000 iterated simulations discarding the initial 20,000 iterations as burn-in. Convergence was assessed using the Brooks–Gelman–Rubin method. We calculated the ORs and 95% CI to compare the pairwise relative treatment efficacy of the competing interventions. In addition, we ranked all interesting treatments with each endpoint and assessed the probabilities. The surface under the cumulative ranking curve (SUCRA) was used. SUCRA is a simple summary index indicating the degree to which an intervention is better or worse than others, taking a value between 0 (certainly the worst intervention) and 1 (certainly the best intervention).[18] We planned to use the node-splitting to evaluate the incoherence between direct and indirect comparison. Finally, the funnel plots were used to assess the publication bias. We produced summary results for all outcomes. We performed an NMA using Stata version 13.1 and WinBUGS 1.4.3.
2.6. Quality-of-evidence assessment
We used the GRADE method to assess the quality of the evidence of direct, indirect and network results.[19,20] The contents of the evaluation included the following five factors: risk of bias; indirectness; inconsistency; imprecision; publication bias. As for indirect results, we chose the optimal indirect comparison approach and assessed separately the quality of the evidence of each group in the approach. The lower level of evidence is used as the level of evidence for the indirect comparison. If only indirect evidence existed, the level of evidence for indirect comparison represented the level of evidence. If the direct and indirect evidence both existed, the higher level of the evidence is used as the level of evidence for the NMA results. According to established guidelines, we finally assessed the strength of evidence as high, moderate, low, or insufficient.
3. Results
3.1. The results of the literature search
A total of 1630 related literature were obtained by a preliminary literature search. 1558 trials were identified by scanning the titles and abstracts, and 112 were identified after reading the full text. 78 trials were excluded because the data were not available, the same trial article or the patient had other related conditions. After literature retrieval and total text examination, 34 RCTs were finally included in our analysis (Fig. 1).[3,8,11,13–16,21–48]
Figure 1.
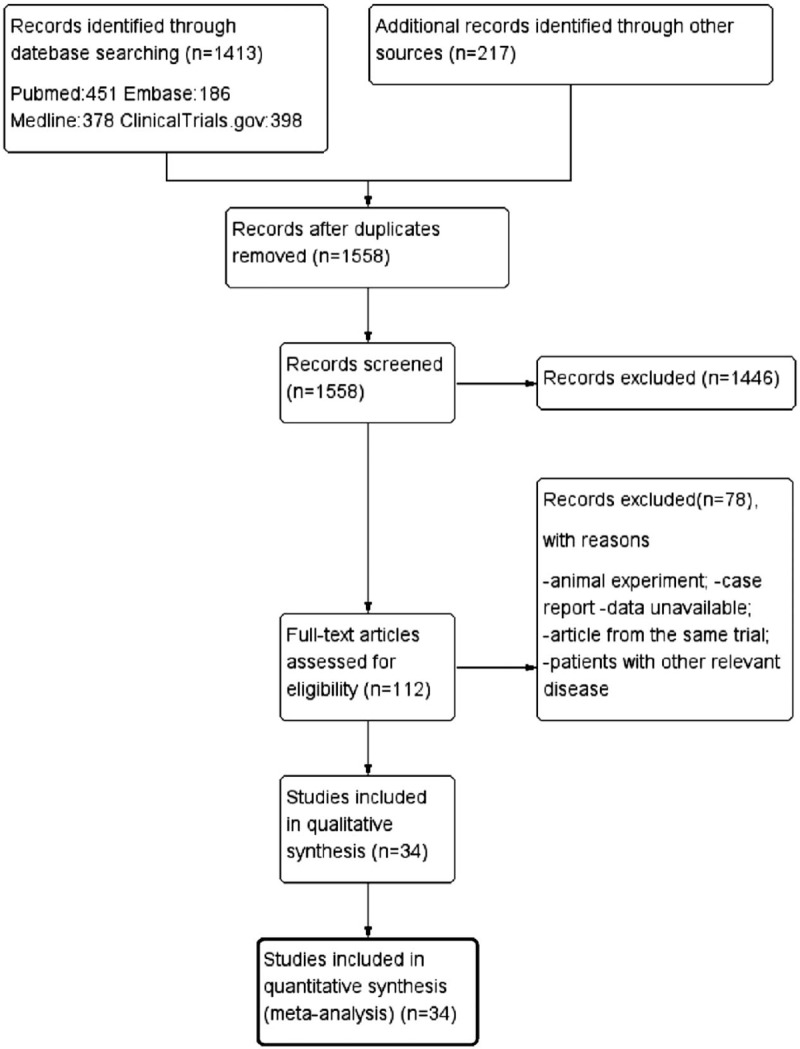
Flow chart showing the search strategy and search results. The relevant number of papers at each point is given.
3.2. Characteristics of included studies
All the 34 studies were RCTs with a total of 9171 objects. The intervention in the observation group was to add probiotics for feeding: Lactobacilli in 6 studies; Bifidobacterium in 8 studies; Bacillus in 1 study; Saccharomyces in 4 studies; and probiotic mixture in 15 studies. In all studies, there are 443 cases of NEC, 1304 cases of sepsis, and 544 deaths. More details of the included RCTs are shown in Tables 1 and 2. The network structure of evidence reporting on the probiotic strategy to prevent NEC in preterm infants is illustrated using network plots in Figure 2.
Table 1.
Characteristics of the included RCTs in our network meta-analysis.
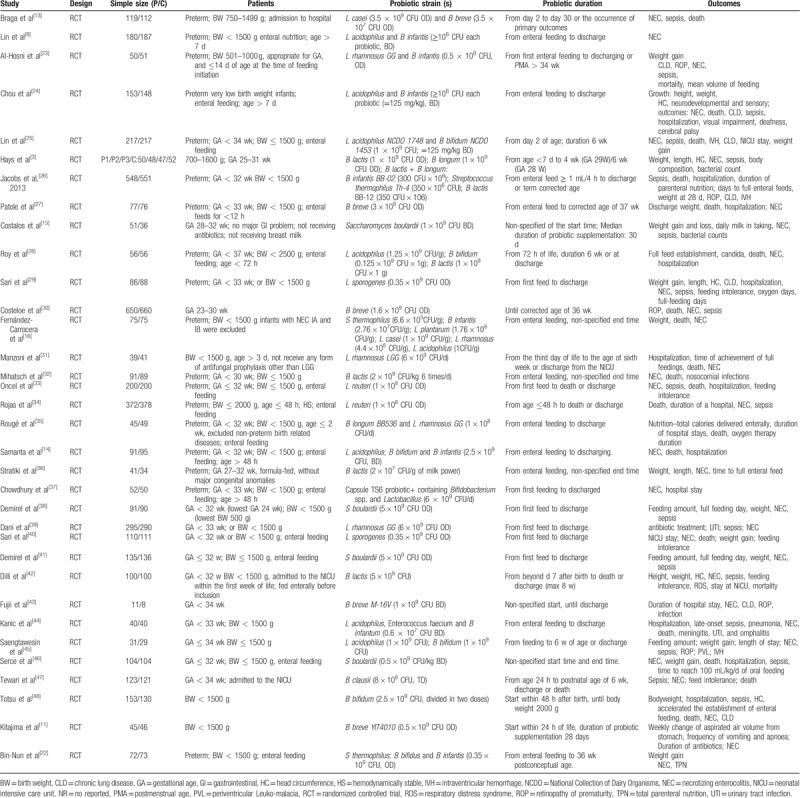
Table 2.
The incidence of NEC (a), sepsis (b) and all-cause mortality (c).
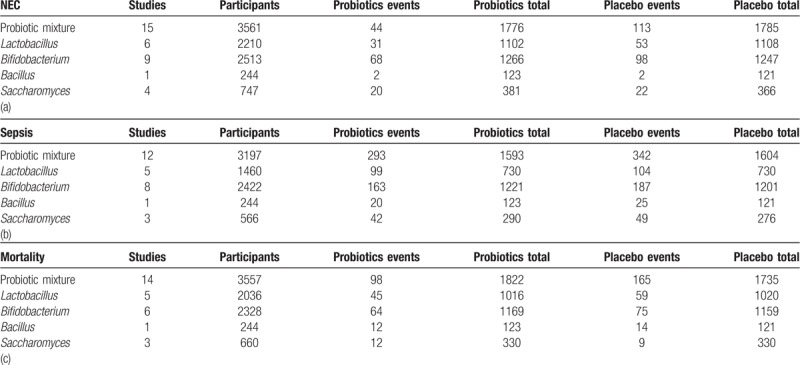
Figure 2.
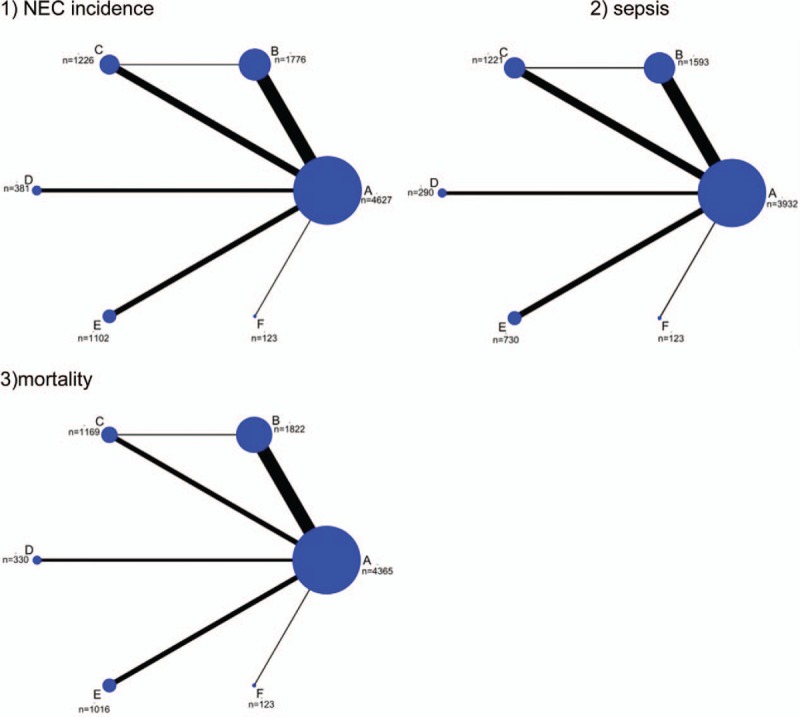
Network plot of RCTs comparing different probiotic treatment strategies for preterm-related complications. The width of the lines is proportional to the number of trials comparing each pair of treatments with numbers on the lines illustrating the exact number. The size of the circles represents the cumulative number of patients for each intervention. A: Placebo; B: Probiotic mixture; C: Bifidobacterium; D: Saccharomyces; E: Lactobacillus; F: Bacillus.
3.3. Risk of bias of included studies
The results showed that most of the included trials followed a strict blind procedure for the researchers, outcome evaluators, and intervention participants, but one of the trials had a high risk of randomization and blindness.[43] The risk assessment of all included RCTs bias is shown in Figure 3.
Figure 3.
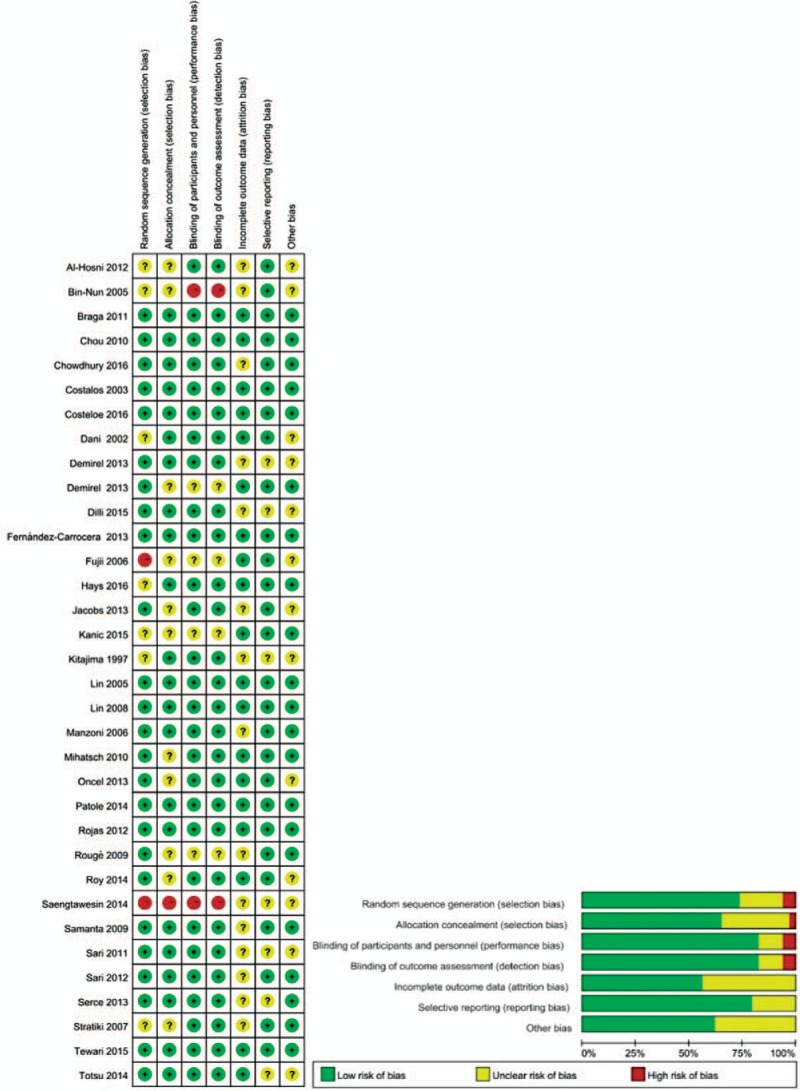
Risk of bias summary and graph showing authors judgement about each risk of bias item for the randomized trial.
3.4. Meta-analysis results for NEC incidence, gut associated sepsis and mortality
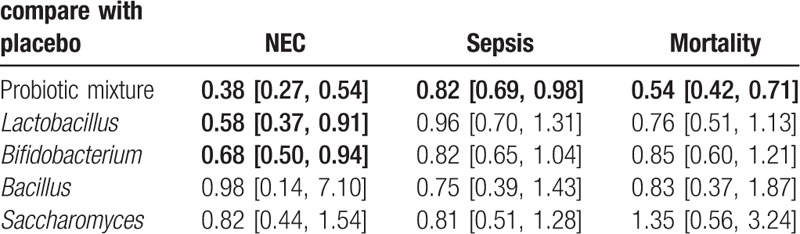
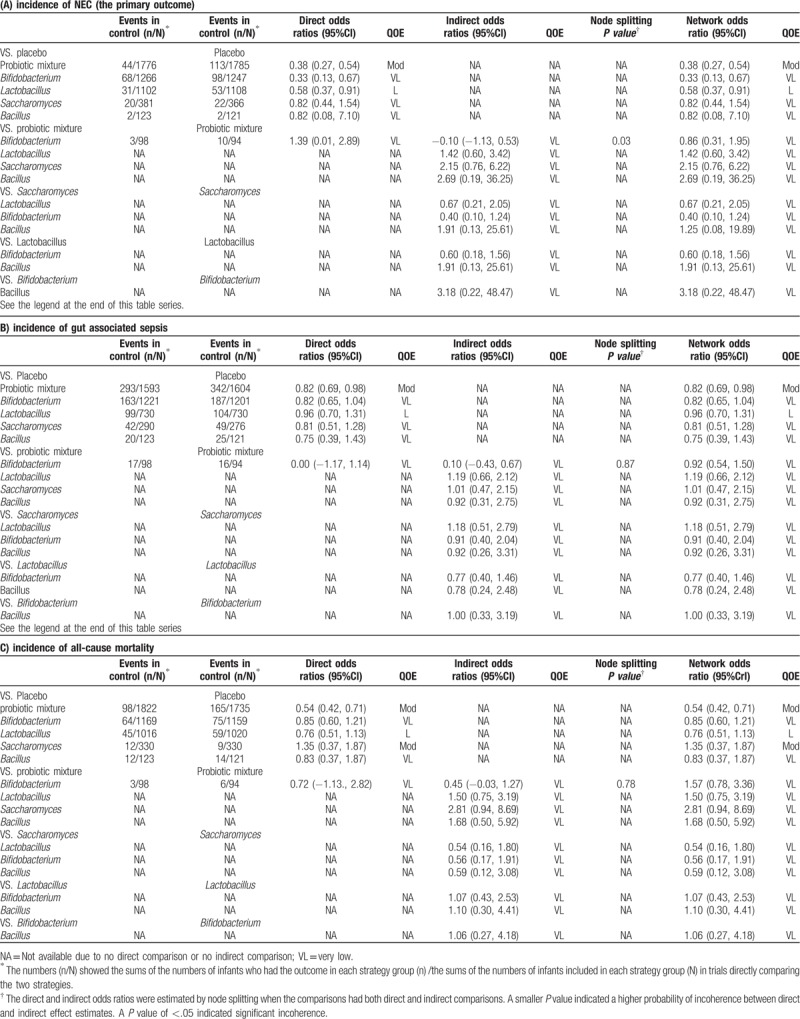
The result showed that the risk of incidence of NEC (OR = 0.38, 95%CI: 0.27–0.54), gut associated sepsis (OR = 0.82, 95%CI: 0.69–0.98) and mortality (OR = 0.54, 95%CI: 0.42–0.71) were significantly reduced after the administration of probiotic mixture. In addition, Lactobacillius (OR = 0.58, 95%CI: 0.37–0.91) and Bifidobacterium (OR = 0.68, 95%CI: 0.50–0.94) both reduced the risk of incidence of NEC compared with the placebo. However, other results of our analysis showed no significant statistical difference (Table 3).
Table 3.
Meta-analysis results for incidence of NEC, sepsis and all-cause mortality.
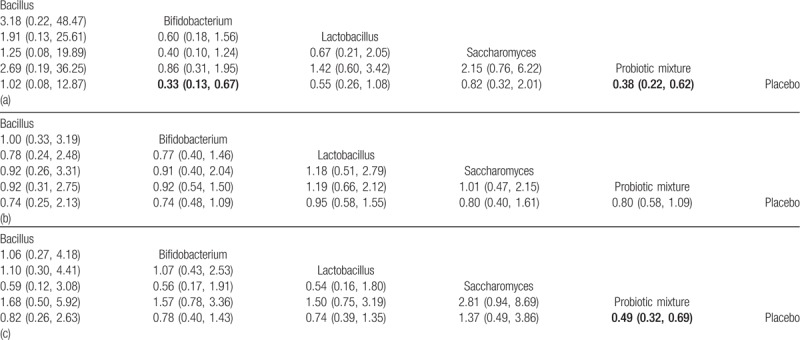
3.5. NMA results for NEC incidence, gut associated sepsis and mortality
Preterm infants fed with Bifidobacterium or probiotic mixture showed a significantly lower risk of the incidence of NEC when compared with those with placebo (Bifidobacterium: OR = 0.33, 95%CI: 0.13–0.67; probiotic mixture: OR = 0.38, 95%CI: 0.22–0.61, Table 4). However, we found no significant difference between probiotic supplement and placebo in the incidence of gut associated sepsis (Table 4). Furthermore, there is a significant decrease in preterm infants’ mortality with the probiotic mixture when compared placebo (probiotic mixture: OR = 0.49, 95%CI: 0.32–0.69, Table 4).
Table 4.
Network meta-analysis results for the incidence of NEC (a), sepsis (b) and all-cause mortality (c).
3.6. Ranking of 5 probiotic strategies and cluster analysis
We used the SUCRA value for each probiotic supplement to show their potential ranks for each outcome (Table 5). Bifidobacterium exhibited the highest SUCRA values with respect to NEC incidence (SUCRA = 0.50). Bacillus showed a potential advantage in reducing the risk of gut associated sepsis incidence with highest SUCRA values (SUCRA = 0.38). Additionally, the performance of the probiotic mixture appeared to have the highest SUCRA value under the outcome mortality (SUCRA = 0.66). The funnel plots showed a clear visual asymmetry (Fig. 4). We identified no strong evidence of publication bias in our study.
Table 5.
SUCRA values for the treatments under 3 endpoints.
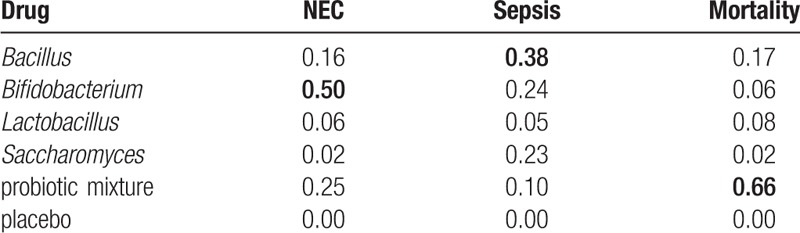
Figure 4.
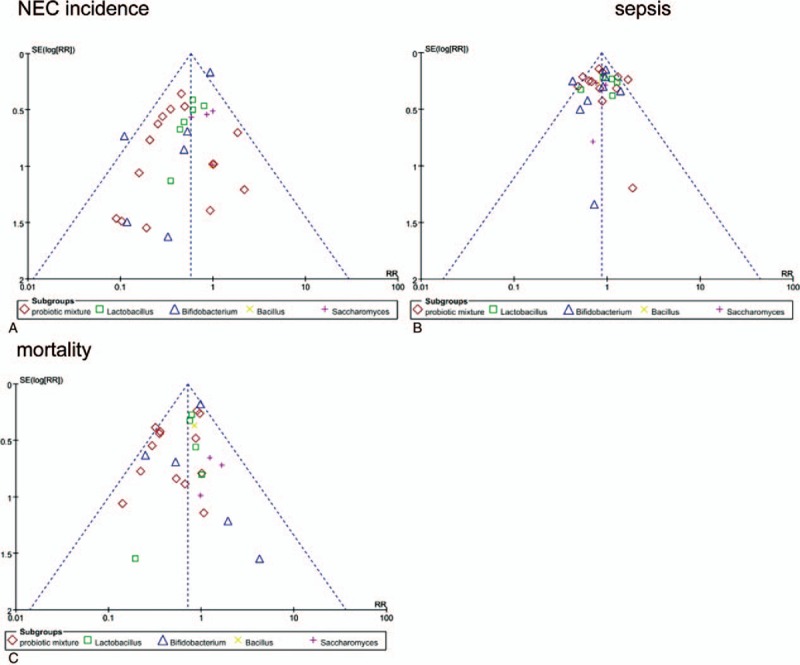
Comparison funnel plots for publication bias analysis.
3.7. Quality of evidence evaluation
Among all 45 direct and indirect comparisons for outcomes, the quality of evidence was down because of serious publication bias in all comparisons, for serious imprecision in 41 comparisons, and serious inconsistency in 35 comparisons. Node splitting found an obvious incoherence in comparisons for the NEC incidence (probiotic mixture vs Bifidobacterium), whereas no significant incoherence for other outcomes (sepsis and mortality). Ultimately, we determined the level of evidence for NMA in the 4 comparisons to be moderate, 3 to be low, and 38 to be very low (Table 6).
Table 6.
Network meta-analysis for all outcomes and quality-of-evidence assessment.
4. Discussion
The intestine of the preterm infants easily trended to colonized by pathogenic bacteria in the neonatal intensive care units (NICUs), which may be because of the delayed breastfeeding, early antibiotic intervention, and/or total parenteral nutrition.[20] This contributed to the higher incidence of NEC and gut associated sepsis. The recent literature reported the incidence of NEC up to 10% and the NEC-related mortality rate of up to 20%, which greatly affects the health and survival of preterm infants especially in NICUs.[20,49] There has been evidence that probiotics have the effect to prevent the severe complications of the preterm infants such as NEC, sepsis and mortality.[36,37,43] Probiotics act through many different mechanisms which are genera-specific. Unfortunately, there is no trial to explore the comparison of the effect of different strains to prevent preterm related complications. Network meta-analysis allowed comparisons of relative effectiveness between interventions that have never been compared head to head. Therefore, we performed an NMA to address the relative efficacy of different probiotic genera strategy of preventing severe complications in preterm infants.
Neither the traditional meta results or the NMA results both indicated that probiotic mixture and Bifidobacterium might show a greater availability to prevent the NEC incidence in preterm infants. Similarly, the performance of the probiotic mixture was still superior to other probiotic supplements under the outcome of all-cause mortality. The possible explanation is because normal flora is diverse in the gut, it might mean that the combination of probiotic strains is more rational, and our conclusions appear to support this hypothesis. The recent systematic review draws a same conclusion that the multistrain products performed more significant decline of NEC incidence when compared with a single organism.[16] Similarly, Bifidobacterium has its unique advantages to prevent the NEC incidence and inflammatory reactions in preterm infants in recent literature. Because Bifidobacterium might have more affinity with immature intestine, and reduce the butyric acid and up-regulate transforming growing factor A1 (that included potent anti-inflammatory effects and promoted epithelial cell proliferation and differentiation) to provide protection from preterm related complications.[11,22,27,36] In our analysis, traditional results revealed that Lactobacillus had the ability to reduce the incidence of NEC, while the NMA showed that it had no effect. We failed to confirm the accurate results from these paradoxical statistic results. These might provide a possibility that Lactobacillus was worth of deep investigation. Therefore, it might imply that probiotic mixture and Bifidobacterium could be the better option for preterm infants.
Furthermore, selecting an appropriate probiotic strategy merely according to the efficacy for preventing NEC and mortality might result in biased results. This is of specific importance, considering that safety remains a concern in the use of probiotics because probiotics are live bacteria supplement. It is reported that probiotics had the potential to cause probiotics related sepsis. We aimed at the end point of gut related sepsis to perform an analysis. The performance of all elected probiotic genus was superior to placebo under the outcome of sepsis. However, it did not mean that probiotics are safe absolutely. As with our concern, a few reported Lactobacillus bacteremia cases occurred in extremely sick infants who accessed to high doses of Lactobacillus.[50]Lactobacillus should be used with caution because excessive ingestion may cause a high risk of sepsis, and this may cause adverse effects on preterm infants.
In spite of the value of this question (namely, which is the best probiotic strategy to prevent preterm related complications), the evaluated data was not comprehensive. The statistical power of our network meta-analyses is relatively low because of a few direct comparisons as well as a few studies and patients in each indirect comparison.
Our study only aimed at the genera of probiotics to analysis, which may merely provide a research direction, not a specific probiotic treatment strategy. None of the studies reported the results according to dose categories albeit excess of probiotics might be connected with safety. Similarly, it is impossible to perform a subanalysis with small patient cohorts according to birthweight categories in every probiotic group. Considering the known increased morbidity of the preterm related complications for the very low birth weight infants, probiotic intervention in this subgroup might be even more hazardous and less efficacious. In addition, we found only one study reported long-term outcomes of oral probiotics and the study found no effect on neurodevelopment and growth.[29] Their long-term outcomes remain to be evaluated.
The outcomes of our network meta-analysis suggested that further investigations are necessary to explore the suitable probiotic treatment strategy, the optimal dose, the long-term safety and the preterm infants who benefit the most.
5. Conclusion
The recent literature has reported a total of 5 probiotic strategies, including Bacillus, Bifidobacterium, Lactobacillus, Saccharomyces, and probiotic mixture. Our thorough review and NMA provided a piece of available evidence to choose optimal probiotics prophylactic strategy for premature infants. The results indicated that probiotic mixture and Bifidobacterium showed a stronger advantage to use in preterm infants; the other probiotic genera failed to show an obvious effect to reduce the incidence of NEC, sepsis and all-cause death. More trials need to be performed to determine the optimal probiotic treatment strategy to prevent preterm related complications.
Acknowledgments
We wish to thank Tianjin Medical University for providing us with databases.
Author contributions
LW Bi contributed the most to the study and should be considered first author. BL Yan contributed the same as the first authors. Le-wee Bi conceptualized and designed the study, drafted the initial manuscript, and interpreted the data; Bei-lei Yan conducted the initial analyses and drafted the initial manuscript; Qian-yu Yang conceptualized and designed the study and supervised the analysis; Miao-miao Li conducted the meta-analyses, interpreted the data, and reviewed the manuscript; and Hua-lei Cui conceptualized and designed the study, supervised the analysis, interpreted the data, and reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conceptualization: Le-wee Bi, Bei-lei Yan, Hua-lei Cui.
Data curation: Bei-lei Yan, Qian-yu Yang, Miao-miao Li.
Formal analysis: Le-wee Bi, Hua-lei Cui.
Methodology: Le-wee Bi, Bei-lei Yan, Qian-yu Yang, Miao-miao Li.
Supervision: Qian-yu Yang.
Validation: Qian-yu Yang, Hua-lei Cui.
Visualization: Miao-miao Li, Hua-lei Cui.
Writing – original draft: Le-wee Bi, Bei-lei Yan, Qian-yu Yang.
Writing – review & editing: Miao-miao Li, Hua-lei Cui.
Footnotes
Abbreviations: CIs = confidence intervals, MD = mean difference, NEC = Necrotizing enterocolitis, NICU = neonatal intensive care unit, NMA = network meta-analysis, OR = odds ratios, PRISMA = the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized controlled trial, SUCRA = Surface under the cumulative ranking curve.
How to cite this article: Bi Lw, Yan Bl, Yang Qy, Li Mm, Cui Hl. Which is the best probiotic treatment strategy to prevent the necrotizing enterocolitis in premature infants. Medicine. 2019;98:41(e17521).
L-wB and B-lY contributed the same as the first author.
This work was supported by funding from standardization of endoscopic treatment of acute abdomen in children (no:14RCGFSY00150).
The authors have no conflicts of interest to disclose.
References
- [1].Kafetzis DA, Skevaki C, Costalos C. Neonatal necrotizing enterocolitis: an overview. Curr Opin Infect Dis 2003;16:349–55. [DOI] [PubMed] [Google Scholar]
- [2].Neu J. Necrotizing enterocolitis. World Rev Nutr Diet 2014;110:253–63. [DOI] [PubMed] [Google Scholar]
- [3].Hays S, Jacquot A, Gauthier H, et al. Probiotics and growth in preterm infants: a randomized controlled trial, PREMAPRO study. Clin Nutr 2016;35:802–11. [DOI] [PubMed] [Google Scholar]
- [4].Goldmann DA, Leclair J, Macone A. Bacterial colonization of neonates admitted to an intensive care environment. J Pediatr 1978;93:288–93. [DOI] [PubMed] [Google Scholar]
- [5].Magne F, Suau A, Pochart P, et al. Fecal microbial community in preterm infants. J Pediatr Gastroenterol Nutr 2005;41:386–92. [DOI] [PubMed] [Google Scholar]
- [6].Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol 2013;40:27–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Verdu EF. Probiotics effects on gastrointestinal function: beyond the gut? Neurogastroenterol Motil 2009;21:477–80. [DOI] [PubMed] [Google Scholar]
- [8].Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol 2008;32:70–82. [DOI] [PubMed] [Google Scholar]
- [9].Alfaleh K, Anabrees J, Bassler D, et al. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database System Rev 2011;Cd005496. [DOI] [PubMed] [Google Scholar]
- [10].AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid Based Child Health 2014;9:584–671. [DOI] [PubMed] [Google Scholar]
- [11].Kitajima H, Sumida Y, Tanaka R, et al. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Archives of disease in childhood. Fetal Neonatal Ed 1997;76:F101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guthmann F, Kluthe C, Buhrer C. Probiotics for prevention of necrotising enterocolitis: an updated meta-analysis. Klinische Padiatrie 2010;222:284–90. [DOI] [PubMed] [Google Scholar]
- [13].Braga TD, da Silva GA, de Lira PI, et al. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 2011;93:81–6. [DOI] [PubMed] [Google Scholar]
- [14].Samanta M, Sarkar M, Ghosh P, et al. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr 2009;55:128–31. [DOI] [PubMed] [Google Scholar]
- [15].Costalos C, Skouteri V, Gounaris A, et al. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum Dev 2003;74:89–96. [DOI] [PubMed] [Google Scholar]
- [16].Fernández-Carrocera LA, Solis-Herrera A, Cabanillas-Ayón M, et al. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2013;98:F5–9. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–7. [DOI] [PubMed] [Google Scholar]
- [19].Guyatt GH, Oxman AD, Vist GE, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Puhan MA, Schunemann HJ, Murad MH, et al. A grade working group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- [21].Lin HC, Su BH, Chen A-C, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005;115:1–4. [DOI] [PubMed] [Google Scholar]
- [22].Bin-Nun A, Bromiker R, Wilschanski M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 2005;147:192–6. [DOI] [PubMed] [Google Scholar]
- [23].Al-Hosni M, Duenas M, Hawk M, et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J Perinatol 2012;32:253–9. [DOI] [PubMed] [Google Scholar]
- [24].Chou IC, Kuo HT, Chang J-S, et al. Lack of effects of oral probiotics on growth and neurodevelopmental outcomes in preterm very low birth weight infants. J Pediatr 2010;156:393–6. [DOI] [PubMed] [Google Scholar]
- [25].Lin HC, Hsu CH, Chen HL, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 2008;122:693–700. [DOI] [PubMed] [Google Scholar]
- [26].Jacobs SE, Tobin JM, Opie GF, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 2013;132:1055–62. [DOI] [PubMed] [Google Scholar]
- [27].Patole S, Keil AD, Chang A, et al. Effect of Bifidobacterium breve m-16 v supplementation on fecal bifidobacteria in preterm neonates – a randomised double blind placebo controlled trial. PLoS One 2014;9:e89511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Roy A, Chaudhuri J, Sarkar D, et al. Role of enteric supplementation of probiotics on late-onset sepsis by Candida species in preterm low birth weight neonates: a randomized, double blind, placebo-controlled trial. N Am J Med Sci 2014;6:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sari FN, Eras Z, Dizdar EA, et al. Do oral probiotics affect growth and neurodevelopmental outcomes in very low-birth-weight preterm infants? Am J Perinatol 2012;29:579–86. [DOI] [PubMed] [Google Scholar]
- [30].Costeloe K, Hardy P, Juszczak E, et al. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. The Lancet 2016;387:649–60. [DOI] [PubMed] [Google Scholar]
- [31].Manzoni P, Mostert M, Leonessa ML, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis 2006;42:1735–42. [DOI] [PubMed] [Google Scholar]
- [32].Mihatsch WA, Vossbeck S, Eikmanns B, et al. Effect of bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology 2010;98:156–63. [DOI] [PubMed] [Google Scholar]
- [33].Oncel MY, Sari FN, Arayici S, et al. Lactobacillus reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2014;99:F110–115. [DOI] [PubMed] [Google Scholar]
- [34].Rojas MA, Lozano JM, Rojas MX, et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics 2012;130:e1113–20. [DOI] [PubMed] [Google Scholar]
- [35].Rouge C, Piloquet H, Butel MJ, et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2009;89:1828–35. [DOI] [PubMed] [Google Scholar]
- [36].Stratiki Z, Costalos C, Sevastiadou S, et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev 2007;83:575–9. [DOI] [PubMed] [Google Scholar]
- [37].Chowdhury T, Ali MM, Hossain MM, et al. Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: a double-blind randomized controlled trial. J Coll Physicians Surg Pak 2016;26:770–4. [PubMed] [Google Scholar]
- [38].Demirel G, Celik IH, Erdeve O, et al. Prophylactic Saccharomyces boulardii versus nystatin for the prevention of fungal colonization and invasive fungal infection in premature infants. Eur J Pediatr 2013;172:1321–6. [DOI] [PubMed] [Google Scholar]
- [39].Dani C, Biadaioli R, Bertini G, et al. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 2002;82:103–8. [DOI] [PubMed] [Google Scholar]
- [40].Sari FN, Dizdar EA, Oguz S, et al. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr 2011;65:434–9. [DOI] [PubMed] [Google Scholar]
- [41].Demirel G, Erdeve O, Celik IH, et al. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatr 2013;102:e560–5. [DOI] [PubMed] [Google Scholar]
- [42].Dilli D, Aydin B, Fettah ND, et al. The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr 2015;166:545–51. e541. [DOI] [PubMed] [Google Scholar]
- [43].Fujii T, Ohtsuka Y, Lee T, et al. Bifidobacterium breve enhances transforming growth factor beta1 signaling by regulating smad7 expression in preterm infants. J Pediatr Gastroenterol Nutr 2006;43:83–8. [DOI] [PubMed] [Google Scholar]
- [44].Kanic Z, Micetic Turk D, Burja S, et al. Influence of a combination of probiotics on bacterial infections in very low birthweight newborns. Wien Klin Wochenschr 2015;127Suppl 5:S210–5. [DOI] [PubMed] [Google Scholar]
- [45].Saengtawesin V, Tangpolkaiwalsak R, Kanjanapattankul W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J Med Assoc Thai 2014;97Suppl 6:S20–5. [PubMed] [Google Scholar]
- [46].Serce O, Benzer D, Gursoy T, et al. Efficacy of Saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: a randomised controlled trial. Early Hum Dev 2013;89:1033–6. [DOI] [PubMed] [Google Scholar]
- [47].Tewari VV, Dubey SK, Gupta G. Bacillus clausii for prevention of late-onset sepsis in preterm infants: a randomized controlled trial. J Trop Pediatr 2015;61:377–85. [DOI] [PubMed] [Google Scholar]
- [48].Totsu S, Yamasaki C, Terahara M, et al. Probiotics Study Group in, J. Bifidobacterium and enteral feeding in preterm infants: cluster-randomized trial. Pediatr Int 2014;56:714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lee JS, Polin RA. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol 2003;8:449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Land MH, Rouster-Stevens K, Woods CR, et al. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005;115:178–81. [DOI] [PubMed] [Google Scholar]


