Abstract
Background
In 2011, South Africa committed to promoting exclusive breastfeeding (EBF) for six months for all mothers, regardless of HIV status, in line with World Health Organization recommendations. This was a marked shift from earlier policies, and with it, average EBF rates increased from less than 10% in 2011 to 32% by 2016.
Objectives
The aim of this mixed-methods systematic review was to describe EBF practices in South Africa and their multi-level influences over four policy periods.
Methods
We applied PRISMA guidelines according to a published protocol (Prospero: CRD42014010512). We searched seven databases [Africa-Wide, PubMed, Popline, PsychINFO, CINAHL, Global Health, and The Cochrane Library] and conducted hand searches for eligible articles (all study designs, conducted in South Africa and published between 1980–2018). The quality of articles was assessed using published tools, as appropriate. Separate policy analysis was conducted to delineate four distinct policy periods. We compared EBF rates by these periods. Then, applying a three-level ecological framework, we analysed EBF influences concurrently by method. Finally, the findings were synthesized to compare breastfeeding influences by policy period, maintaining an ecological framework.
Results
From an initial sample of 20,226 articles, 72 unique articles were reviewed, three of which contributed to both quantitative and qualitative analysis. Despite the large sample, several provinces were poorly represented (if at all) and many studies were assessed as low to moderate quality. Despite these limitations, our historical lens enabled us to explore why South African progress on increasing EBF practices has been slow. The review reflects a context that increasingly supports EBF, but falls short in accounting for family, community, and workplace influences. The findings also highlight the unintended damage caused by rapidly adopting and introducing global guidelines to an unsupported health workforce.
Conclusions
From a South African perspective, we identified geographic and methodological biases, as well as gaps in our understanding and potential explanations of inequities in EBF. Our recommendations relate to policy, programming, and research to inform changes that would be required to further improve EBF practice rates in South Africa. While our review is South Africa-specific, our findings have broader implications for investing in multi-level interventions and limiting how often infant feeding guidelines are changed.
Introduction
Children depend on their families and communities to make dietary decisions on their behalf. The contexts of such decisions change over time, as do guidelines on what is the best for infants [1]. The current public health consensus is that exclusive breastfeeding (EBF) for the first six months is the best start for health and development [2]. The World Health Organization (WHO) defines EBF as infants consuming only breast milk, with the exception of oral rehydration solutions (ORS), drops or syrups [3]. EBF forms part of a broader definition of optimal infant and young child feeding (IYCF) practices, which also include initiation of breastfeeding within the first hour and continued breastfeeding for two years with the introduction of safe, adequate and appropriate complementary foods from six months [3]. A large volume of breastfeeding research has been conducted in South Africa, but this has not been systematically reviewed to identify factors that promote EBF. Identifying factors that promote (or inhibit) behavior is an essential step for the design of evidence-based policies and interventions that are sensitive to context [4].
Regrettably, scientific knowledge of the life-saving benefits of EBF has not translated into practice in South Africa. Globally, exclusively breastfed infant’s risk of death is 12% that of infants who are not breastfed [2]. The same authors estimate that universal EBF would avert an estimated 13.8% of deaths of children below age two. However, South Africa’s average EBF rate for infants below six months only recently rose to 32% [5] from rates closer to 7% in the 1998 national survey [6]. In fact, the national rates are even lower if one considers that only 23.7% of infants between four and five months were exclusively breastfed [5].
One explanation for South Africa’s low rates of EBF is the country’s high HIV prevalence, with 30.8% of mothers attending antenatal care testing HIV positive [7]. As epidemiologists tried to identify the risk of mother to child transmission early in the epidemic, government health facilities provided eligible HIV positive mothers with the option of receiving free commercial formula to support that choice [8, 9]. As antiretroviral therapy (ART) became more available and transmission risk dropped, South Africa’s Department of Health proclaimed the 2011 Tshwane Declaration for the promotion of breastfeeding and discontinued its free formula program [10].
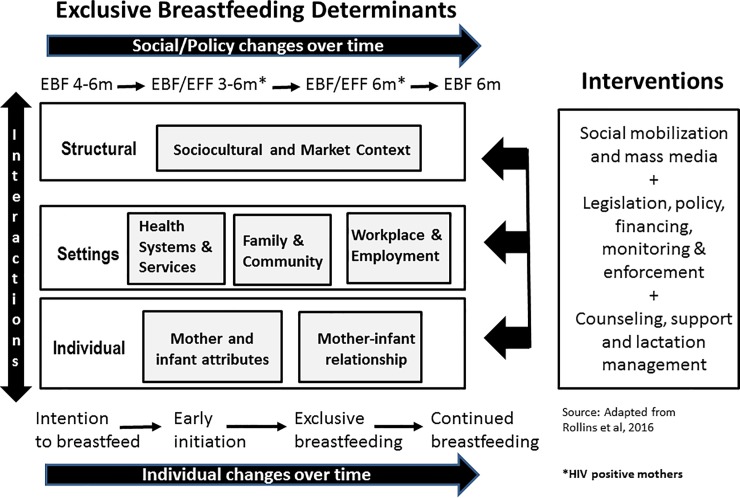
However, the HIV epidemic and free formula can be only part of the explanation for low EBF rates [11], with culturally established mixed feeding practices reported from well before the epidemic [12–14]. Consistent with an understanding that multiple factors shape infant feeding, scholars have argued for comprehensive interventions [4, 15]. Given the complex mix of factors that influence infant feeding, we adapted an ecological conceptual framework proposed by Rollins and colleagues [4] to synthesize and analyze factors that influence EBF. Our adaptation was to account for policy changes over time in addition to the original time element for the mother/infant dyad (see Fig 1).
Fig 1. Adapted ecological model for breastfeeding determinants and interventions.
As we were particularly interested in infant feeding guideline changes, this systematic review analyzed four critical policy periods, summarized in Table 1. These highlight policies before the pediatric HIV epidemic (Period 1), during the early years of the epidemic (Periods 2 and 3), and into the antiretroviral period (Period 4) when South Africa reverted to universal breastfeeding guidelines for all women. While the HIV epidemic became a lens for differentiating policy periods, national IYCF guidelines were also consulted as a way to reflect the broader policy environment.
Table 1. Infant feeding policy periods in South Africa.
| Policy Period | Years | Characteristics |
|---|---|---|
| Period 1 | 1980–1999 | EBF for 4–6 months promoted for all mothers |
| Period 2 | 2000–2007 | EBF 6 months promoted for HIV negative mothers; EBF for 3–6 months promoted or exclusive formula feeding (EFF) (if AFASS* met) for 6 months for HIV positive mothers |
| Period 3 | 2008–2011 | EBF for 6 months promoted for HIV negative mothers EBF or EFF for 6 months promoted HIV positive mothers |
| Period 4 | 2012–2018 | EBF for 6 months promoted for all mothers |
*AFASS = Acceptable, Feasible, Affordable, Sustainable and Safe
As summarized in Table 1, while all mothers received the same advice during Period 1 and Period 4, between 2000 and 2011 different advice was provided to HIV positive and HIV uninfected mothers. Differences in advice went as far as indicating different durations of breastfeeding by HIV status and content (breastmilk or formula). For instance, during Period 2, HIV-positive mothers were officially counselled to either EBF for 3–6 months or EFF for 6 months before rapidly weaning [9], while HIV uninfected mothers were instructed to EBF for 6 months. South Africa’s prevention of mother to child transmission (PMTCT) guidance was amended in 2008 to encourage HIV-positive mothers to EBF for 6 months [8] in alignment with the country’s 2007 IYCF Policy [16]. Still, HIV positive mothers continued to be counselled that EFF for the first six months was an optimal option if they met AFASS criteria. Period 4 began in 2012, after South Africa declared itself a country that actively promoted, protected and supported EBF for all [10]. Free formula at public clinics was discontinued, with the exception of approved medical conditions. Currently, all health workers are expected to promote EBF for six months, regardless of the mother’s HIV status. If mothers decide to formula feed infants after counselling and they meet AFASS requirements, health workers can support them.
The overarching research question that the review sought to answer was ‘What factors support EBF for six months postpartum in South Africa?’ In order to inform policy and interventions, the review sought to explore EBF rates over time, and similarities or differences in the profiles or contexts of women who managed to exclusively breastfeed.
Methods
Protocol registration
The protocol first was registered with PROSPERO (International Prospective Register of Ongoing Systematic Reviews; http://www.crd.york.ac.uk/PROSPERO/ [registration number CRD42014010512). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used to guide the review [17].
PICO (Population, Intervention, Comparator and Outcome) criteria
The key population of interest was mothers in South Africa. To be eligible, the mother had to have given birth to a healthy baby with known infant feeding practices through the first month postpartum. The intervention and/or exposures (referred to hereafter as factors) were left open intentionally, as these were the object of exploration. Significant factors and themes were noted from both observational studies and intervention evaluations, e.g. randomized controlled trials (RCTs) and quasi-experimental designs. The key comparator of interest was EBF. Overall, we looked at any breastfeed (yes vs. no) and, among those breastfeeding, the duration (point at which EBF was measured, e.g. 3 months vs. 6 months). For those studies that had the data, we disaggregated by the mother’s HIV status. The key outcome of interest was EBF for up to six months postpartum.
Inclusion and exclusion criteria
To be included in the review, a number of criteria had to be met, as summarized in Table 2.
Table 2. Review selection criteria.
| Selection Criteria | Standard |
|---|---|
| Study type | Peer-reviewed primary studies employing quantitative and/or qualitative methods as well as primary studies reported in the grey literature. Commentaries and opinion pieces will not be included. Studies where informed consent was not obtained were also excluded. |
| Languages | English, Spanish and French |
| Settings | South Africa. Multi-country studies will be permitted, but only data from South Africa will be extracted. |
| Publishing date | 1980 to 2018 (data collection dates not older than 1975) |
| Outcomes | Breastfeeding practices (not intention to breastfeed) |
Search strategy
Seven databases were consulted through the University of the Witwatersrand (Wits) library: Africa-Wide, PubMed, Popline, PsychINFO, CINAHL, Global Health, and The Cochrane Library. Search strategies for each of the search engines were developed in consultation with two experts; the full search strategies can be found in S1 File. The search terms included variations of the following: infant feeding, breast feeding, bottle feeding, mixed feeding, solid feeding and South Africa, restricted for the period 1980 to 2018. The citations of relevant articles were also searched and content experts, e.g. UNICEF and South African Department of Health, were consulted to ensure all relevant articles were included.
Selection and data extraction
The database searches were replicated independently by two of the authors (SJN and CBN) to reduce bias. The search results were independently saved as EndNote libraries and merged into a single library (n = 20,217) to remove duplicates; an additional 7 documents identified through consultations with experts, of which only one met eligibility criteria [18]. After removing duplicates (n = 2,528), SJN excluded 16,966 articles using titles and a further 293 using abstracts based on the selection criteria. SJN and CBN conducted full text screening on the remaining 101 eligible articles to arrive at the final list of 72 articles. All authors were consulted on the final list before data extraction was completed.
As a mixed-methods systematic review, qualitative and quantitative data were extracted and analyzed separately. All studies were uploaded into NVivo 10.0 software for systematic data extraction and analysis. Summary data for quantitative studies were extracted by both CBN and SJN into Microsoft Excel and SJN extracted qualitative study data. Both authors undertook random quality checks for each other. For both methods, we extracted information summarizing the study year, study design, study setting, sample, and infant age (signifying feeding duration). The policy period during which data were collected were also noted. This was based on separate identification of policy changes over time by SJN, already described in the introduction.
Some data extraction was specific to the methodology. For instance, analysis techniques were only noted for qualitative studies. A framework analysis approach [19] was applied to guide meta-synthesis of qualitative themes, which began by using the three socio-ecological levels and quantitative finding framings to categorize themes, only thereafter reporting on novel themes that were not included in the quantitative studies. The rationale for this approach was to facilitate a broader synthesis of findings from the two methodologies. For quantitative studies, two forms of summary measures were extracted. The primary descriptive measure for all studies was the EBF rate, which was presented as a percentage with notation for the duration that breastfeeding was measured. For the sub-set of intervention studies, the strongest analytic summary measure was extracted, most often in the form of odds ratios or hazard ratios. These were presented with confidence intervals (or p-values).
Methodological quality and level of evidence assessment
The quality of studies was critically assessed using a range of tools: Grading of Recommendations Assessment, Development and Evaluation (GRADE) for RCTs [20–22], the Newcastle-Ottawa Scale (NOS) [23] for cohort studies, and an adaptation of NOS for observational studies. These were summarized as high, medium or low for the sake of comparison in the results summary table. CERQual [24, 25] was applied to critically assess qualitative studies. Although some qualitative researchers have argued against applying quality criteria for meta-synthesis or meta-ethnography, as it counters epistemological values of relativism, we adopted the position that basic standards of qualitative rigor (transferability, credibility, dependability and confirmability) should be present before the application of meta-synthesis [26]. For mixed methods studies, the appropriate tools already listed were applied to the different methodology and design. See S2 File for a full description of critical assessment methods and the assessments of each article.
Results
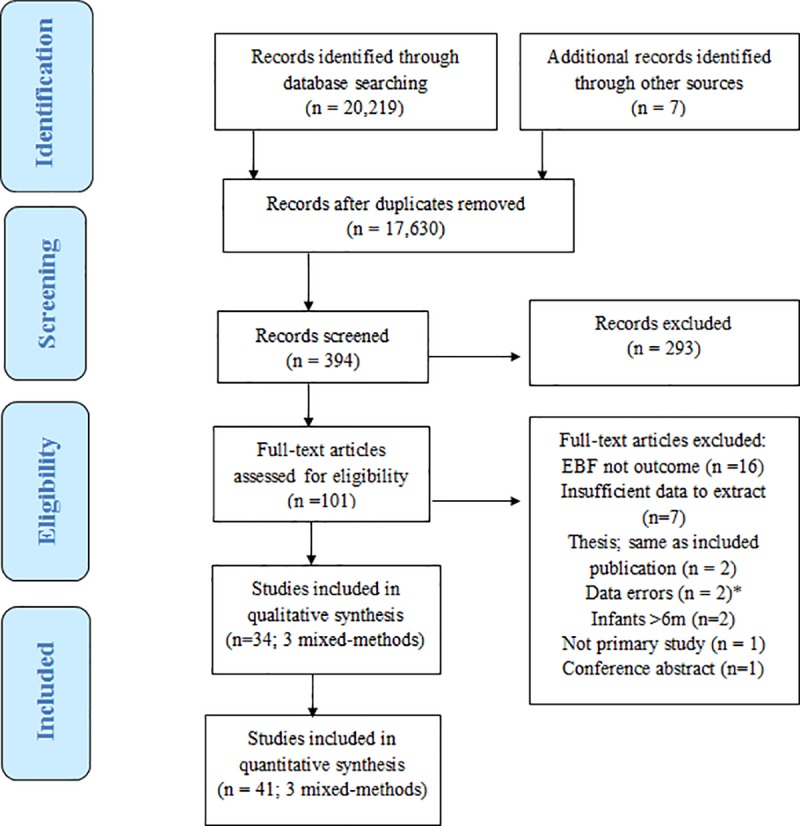
A total of 72 unique articles were reviewed; as three mixed-methods studies contributed to both sets of analyses, 41 articles were included in quantitative analysis and 34 articles were included in qualitative analysis (see Fig 2).
Fig 2. PRISMA flow chart on EBF supports in the first 6 months postpartum.
Observations on excluded articles
As shown in Fig 2, articles were excluded mostly because EBF was not clearly defined as an outcome (n = 16) [27–43]or because the data were not presented in a format that aligned with preset review criteria (n = 7) [44–50]. This was particularly true for articles written in Periods 1 and 2 [20–29], when EBF was less a feature of “optimal feeding” definitions. The data from the two masters theses [51, 52] which were excluded are still represented in the review [53–55]. Whenever possible we retained articles, even if we had to recalculate EBF rates to enable comparison, e.g. when the denominator of EBF rates for HIV positive mothers excluded those who had opted to formula feed [11].
Quantitative results
A total of 41 articles were identified using quantitative methods to explore EBF and one or more related determinants (see S1 Table).
Quantitative studies characteristics
The quantitative studies on EBF in South Africa were highly varied in terms of both study design and quality. There was a relatively even spread of articles by policy period, demonstrating South Africa’s persistent scholarly interest in the topic of infant feeding. As detailed in S1 Table, while observational study designs dominated the mix (18 cross-sectional surveys, 10 cohort designs and one record review), there were also a number of intervention studies (four quasi-experimental designs, one trial and eight RCTs), with most RCTs in the last two policy periods.
Geographic biases were also clear in terms of where EBF was studied, clustering in Kwa-Zulu Natal (KZN; 17) [11, 18, 56–70], the Western Cape (WC; 13) [11, 53–55, 65, 71–78], and Gauteng provinces (GP; 10) [79–88]. Five studies reported data from the Eastern Cape (EC) [11, 65, 87, 89, 90]. The other provinces were poorly represented, with one study each in the Northwest (NW) [87], Mpumalanga (MP) [91] and Free State (FS) [87]. No studies were conducted in Limpopo (LP) or the Northern Cape (NC).
Many study sampling strategies introduced selection bias, likely to overestimate EBF (also see S2 File). For instance, in nine studies only mothers who had an intention to breastfeed or who were breastfeeding were included [54, 55, 65, 68, 73, 76, 84, 88, 89]; in these studies, exclusivity or introduction of complementary feeding were the main objects of interest. This was particularly true in research with HIV positive mothers during Policy Periods 2 and 3, as EFF was considered an acceptable alternative to breastfeeding [11, 18, 56, 57, 59, 61, 72, 89, 91]. Nearly all studies introduced selection bias by recruiting mothers from health facilities, often from prevention of mother to child transmission (PMTCT) programs or baby-friendly institutions, versus the community [62, 64, 66, 74]. The samples themselves also varied, though most involved mothers of young infants. In analysis, mothers were disaggregated by age, HIV status, race and/or in relationship to their exposure to an intervention. In a few cases, infant caregivers (versus mothers only) were sampled [53, 58, 87].
Most studies were scored as low to moderate quality (see S2 File). The introduction of bias in sampling was the most frequent reason for low or moderate scores. Other sources of weakness included small sample sizes, not powered to pick up differences, and limited use of advanced analytic techniques, such as regression analysis, to address confounding. Reliance on simple descriptive statistics or measures of association was the norm in studies conducted in the 1980s and 1990s, as compared with more recent studies. In addition, earlier studies were more likely to exclude details of the study, such as the relationship of the researchers to the sample and blinding for RCTs.
EBF rates over time
There was a high level of hereogeneity in EBF durations reported (see S1 Table). Many early studies restricted measure of EBF to 12–16 weeks or less [58, 60, 61, 83], reflecting sampling strategies and definitions of optimal feeding at the time. The rates of EBF also varied widely by study sample and setting, even when we took duration into account. The articles presented EBF measures for different durations and used different definitions, such as including or excluding the use of non-prescribed medicine [58]. Only the strongest and longest duration measures of EBF are presented in Table 3 in order to assess EBF more critically. We summarized EBF prevalence ranges by policy period and study design (see Table 3, based on S1 Table).
Table 3. EBF ranges by policy period and study design.
| Policy Period | EBF Ranges (Design) | Infant age at measurement |
|---|---|---|
| 1. 1980–1999 | 0.0% to 32.0% (Cross-sectional) | 6 months |
| 0.8%; 3.0% to 23.7% (Cohort) | 6 months; 12–16 weeks | |
| 32.7% to 38.7% (Quasi-experimental) | 12 weeks | |
| 2. 2000–2007 | 0.0% to 5.0% (Cross-sectional) | 6 months |
| 10.8% to 13%; 14.0% to 18.0% (Cohort) | 6 months; 14 weeks | |
| 45.0% (Quasi-experimental) | 6 months | |
| 3. 2008–2011 | 6.0% to 35.6% (Cross-sectional) | <6 months |
| 1.5% to 6.4%; 20.1% to 20.8% (RCTs) | 6 months; 12 weeks | |
| 4. 2012–2018 | 12.0%; 14.0% (Cross-sectional) | 6 months; 14 weeks |
| 13.0% (Cohort) | 6 months | |
| 21.6% to 43.7% (RCTs) | 6 months |
While there was an upward trend in EBF over time, the rates varied widely within and across policy periods. For instance, during the first period, EBF for six months rates ranged from 0.0–32.0%. Interventions to increase EBF during this period did not measure to six months because guidelines at the time recommended 4–6 months. The next policy period, when PMTCT programs began, reported lower rates of EBF for six months (0.0–13.0%) across observational study designs, but a higher rate for the one quasi-experimental study [57]. Rates declined further during the third policy period in studies that measured EBF up to 6 months (1.5–6.4%). In policy period 4, after the Tshwane Declaration, EBF increased between 12.0–43.7%, with particularly high rates measured for RCTs specifically seeking to improve EBF for six months [68, 76].
Quantitative findings on factors associated with EBF
A range of factors across individual, settings and structural levels were explored in relation to their influence on EBF. The factors identified in observational studies (cross-sectional, cohort and record reviews), significantly associated with EBF either as facilitators or barriers, were systematically documented according to the conceptual framework (see S2 Table). Where the findings were mixed, both barrier and facilitator symbols were used. Where regression analyses were not conducted with EBF as the outcome, only descriptive statistics could be used to characterize the study findings for EBF. In a few instances, secondary analyses from RCTs were integrated into this table.
The factors influencing EBF represented all three levels of the socio-ecological framework, with most related to mother attributes, hospital and family settings, and socio-cultural factors at the structural level. As quantitative studies can only report results on what they measure, the number of studies finding significant results for any given variable should be read with caution. For instance, only one study measured depression among mothers [69], but it found significant associations.
The quality of the studies and persistence of factors over time are both worth considering. A general trend in study quality was observed with earlier studies being less sophisticated, particularly with analysis, than those published in the last two policy periods. Some variable outcomes changed over time. For instance, while fear of HIV transmission used to be a barrier to EBF, more recently it has become a facilitator, with increased access to ART. This will be discussed further with the triangulation of quantitative and qualitative findings.
Quantitative intervention results
Findings related to interventions are presented in Table 4, with final regression model results presented where multiple models were tested.
Table 4. EBF promotion intervention results.
| Article and study design | Intervention description | Primary outcome | Results |
|---|---|---|---|
| Policy period 1 | |||
| Hoffman et al, 1984b [55] Pre-post, quasi-experimental |
The intervention comprised six steps: 1. The birth notification form was duplicated so that one copy could be sent to the appropriate health visitor immediately after a birth while the other copy could be processed through the normal channels. 2. Breastfeeding clinics were introduced, allowing prospective mothers to see breastfeeding in action and learn by example. 3. Community talks on the advantages of breastfeeding. 4. Cape Town Breastfeeding Association contact numbers of volunteers available to help mothers with problems were circulated to all clinics in the area. 5. A letter to all the local family practitioners, encouraging breastfeeding promotion 6. Health visitors and nurses at the local clinic encouraged to promote breastfeeding. Their subject knowledge enhanced by lectures, demonstrations and a symposium. |
‘Fully breastfed’ | Significant findings observed p<0.01 (chi-square test) Pre-intervention <6w: 52.7% Post-intervention <6w: 75.8% p<0.01 (chi-square test) Pre-intervention 6–12w: 23.7% Post-intervention 6–12w: 38.7% |
| Nikodem et al, 1993 [83] Randomised control trial |
Breastfeeding mothers were allocated, by means of randomly ordered cards in sealed opaque envelopes, to view one of two health education video programmes within 72 hours after delivery. The first programme gave information and specific motivation concerning the importance of breastfeeding and the correct positioning of the baby. The second gave information about healthy eating habits for adults. Results were tested 6 weeks postpartum through a blinded questionnaire interview, with 47.6% follow up rate. | ‘Breastfed only’ at 6 weeks | Not significant for EBF at 6 w OR 1.38 (.68–2.81); p = 0.431 Study: 30/83 (36.1%) Control: 23/79 (29.1%) |
| Policy period 2 | |||
| Baek et al., 2007 [18] Pre-post, quasi-experimental study design |
Three evaluation sites in the Pietermaritzburg area of KZN recruited urban and peri-urban mothers (18–49) who knew their HIV status and were either 6–9 months pregnant or 12 weeks or less postpartum. Two cross-sectional surveys were conducted, baseline prior to mothers to mothers (m2m) intervention and another one year after m2m was introduced. At baseline data collection in 2005 before m2m was introduced. m2m was a peer support program that provided education and psychosocial support to HIV-positive pregnant women and new mothers through health talks, counselling & support groups and outreach. Two or more contacts were counted as study exposure. | Exclusive feeding practices: EBF |
Not significant for EBF p>0.05 (bivariate) for EBF Study: 11% Control: 15% |
| EFF |
p<0.01 (bivariate) for EFF Study: 78% Control: 61% |
||
| Bland et al, 2008 [56] Non-randomized intervention cohort |
Lay counsellors trained on the WHO/UNICEF Breastfeeding Counselling Course visited HIV-positive and HIV-negative women in KZN to support EBF: four times antenatally and once within 72 hours of birth. Mothers initiating breast- feeding received a further three home visits in the first 2 weeks and fortnightly thereafter for 6 months. |
EBF* for six months *WHO definition |
Significant for EBF at 4 months aOR 2.07 (1.56–2.74), P<0.0001 HIV- mothers receiving visits aOR 2.86 (2.13–3.83), P<0.0001 HIV+ mothers receiving visits |
| Policy period 3 | |||
| Ijumba et al, 2015 [62] Cluster-randomized trial |
The intervention was provision of community-based counselling during the first 12 weeks after birth. The intervention was delivered by 15 trained CHW living in the clusters though a structured home visiting schedule. Each visit was designated to cover specific topics related to the outcomes of the study. Visits in the intervention arm included two home visits during pregnancy, one in the first 48 h after delivery, then at 3–4 d, 10–14 d, 3–4 weeks and a final visit at 8–9 weeks. All neonates with low birth weight (≤2500 g) received two extra visits during the first week. | EBF (24h recall) for the first 12 weeks Adjusted for cluster, household asset score level and maternal education level |
Significant for EBF at 12w Total: aOR 2.31 (1.82–2.93) Study: 441/1629 (27.1%) Control: 260/1865 (13.9%) HIV-: aOR 2.70 (2.01–3.70) HIV+: aOR 1.70 (1.32–2.20) |
| Rotheram-Borus et al., 2014 [74] Cluster-randomized trial |
The intervention was a home visiting intervention by community-based workers (CBWs) trained in cognitive-behavioural strategies to address health risks (by the Philani MCH and Nutrition Programme), in addition to clinic care (the control). CBW home visitors were selected from community role models prior to training. | EBF for six months | Significant for EBF at 6m OR = 3.59 (1.91–6.75); p<0.001 Study: 10.3% Control: 3.1% |
| Some et al, 2017 [89] Clinical trial (RCT) |
The intervention was provision of infant prophylaxis in the breastfeeding period plus one week from day 7 to 50 weeks of age with either lopinavir/ritonavir or lamivudine in four countries. HIV-1 positive mothers enrolled in the RCT were not eligible for HAART due to CD4 counts >350 cells/mm3. Country-specific hazard ratios for shorter duration of EPBF were calculated for a number of variables. | Combined EBF and Predominant BF into one group (EPBF) * *PBF very low |
Significant for shorter EPBF aHR 3.0 (1.6–5.5) for lop/rit aHR 1.4 (1.1–1.9) age 25–30 aHR 1.6 (1.2–2.1) married aHR 1.3 (1.0–1.6) employed aHR 1.6 (1.2–2.1) multiparous |
| Tomlinson et al, 2014 [64] Community-based cluster RCT |
Goodstart was a structured home visiting intervention where study CHWs provided two pregnancy visits and five post-natal home visits in Umlazi, Durban, South Africa. CHWs were living in the mothers’ neighbourhoods and received a10-day training on PMTCT, Integrated Management of Childhood Illness, lactation counselling and newborn care guidelines. They were also trained on motivational interviewing techniques. Control CHWs provided information and support on accessing social welfare grants and conducted three home-based visits: one during pregnancy and two during weeks 4–6 and 10–12 post-delivery. | EBF for 12 weeks | Significant for EBF at 12 weeks RR 1.92 (1.59–2.33); all Study: 28.6% EBF Control: 14.9% EBF RR 1.53 (1.22–1.94); HIV+ RR 2.16 (1.71–2.73) ; HIV1 |
| Tylleskar et al, 2011 [65] Cluster RCT |
In the PROMISE-EBF intervention group, peers living around the study areas were trained for one week. Study peers provided 1 antenatal breastfeeding visit and 4 post-delivery visits. Control peer cousellors followed the same schedule but assisted families in obtaining birth certificates and social welfare grants. The peer counsellors for the intervention and control clusters were kept separate during the study. | EBF at 12 and 24 weeks using 24h and 7 days recall measures Adjusted for clustering and site |
Significant for all EBF measures; 24 weeks prevalence ratios (PR) shown PR 5.70 (1.33–24.26); 24 hr PR 9.83 (1.40–69.14); 7 day Study: 2% (both 24h & 7 day) Control: <1% (both 24h & 7 day) |
| Policy Period 4 | |||
| Horwood et al, 2017 [66] Cluster RCT |
The continuous quality improvement (CQI) intervention, CHWs provided home-based education and support to pregnant women and mothers. All CHWs received a10-day government training on community-based care of women and infants. Intervention CHWs received a 2-week training in WHO Community Case Management followed by 12 months of mentoring. | EBF for 6 weeks | Significant for EBF at 6 weeks OR 1.7 (1.1–2.7); at follow-up Study: 76.7% Control: 65.1% OR 2.3 (1.4–4.0); change before and after intervention |
| Myer et al, 2018 [76] Parallel arm RCT |
The MCH-ART intervention provided integrated postnatal service to HIV+ mothers and their infants within the MCH clinic. At each postnatal visit nurse-midwives asked questions about infant feeding. The local standard of care acted as a control and involved immediate postnatal referral of HIV+ women on ART to general adult ART services and their infants to separate routine infant follow-up. | EBF at 6 months | Significant for EBF 6 months p<0.001 Study: 67/211 (31.8%) Control: 26/219 (11.9%) |
| Reimers et al, 2017 [68] Cluster RCT |
For this intervention, HIV+ mothers identified “Feeding Buddies” (FB) to support them. Two hour-long training ses- sions were scheduled prior to delivery at regular ANC visits. Two follow-up training sessions occurred at the 3 day and 6 week well-baby clinic visits. Where possible, the mother and her buddy were trained together. Mother-buddies were also given a take-home booklet to reinforce messages. |
EBF at 22 weeks | Not significant for EBF at 22 w p = 0.67 Study: 109/255 (42.75%) Control: 105/235 (44.68%) |
| Tuthill et al, 2017 [70] RCT |
The Information–Motivation–Behavioural Skills (IMB) model was applied for HIV+ women on ART during their third trimester of pregnancy. The intervention was a one-time, 45-minute tailored, one-on-one motivational interviewing counselling session with a trained female counsellor. The control was standard of care. | EBF at 6 weeks | Not significant for EBF at 6 w p = 1.00 Study: 81.5% Control: 81.5% |
While most interventions showed statistically significant improvements in EBF (9/13), the variability of approaches made any prospect of meta-analysis impossible. As shown in Table 4, four studies did not observe significant differences between intervention and control groups. Short once-off interventions, such as playing a video [83] or a one-time counselling session [70], failed outright. Postnatal support by “peers” [18] or individuals nominated by mothers, “buddies” [68], had less success than interventions that used already-employed community health workers (CHWs) [56, 62, 64, 66]. The two RCTs that employed peer workers [65, 74] reported lower EBF rates than others. In other words, while their findings were statistically significant, the population-level benefit of increasing EBF to the levels they reported is questionable. Postnatal support provided by CHWs with at least 10 days training and between four to seven visits reported the most impressive EBF increases [64]. RCTs that applied health system reforms, such as integrated care for mothers and infants [76] or educating health staff [55], also resulted in significant EBF improvements.
Qualitative findings
A total of 34 qualitative studies (including nine mixed-methods studies from which qualitative data were extracted), representing 31 distinct studies, were reviewed covering all policy periods (see S5 Table). Four studies were conducted in the first period [92–95], three led by the sociologist Gill Seidel in KZN. Most studies were conducted during Period 2 (n = 14) [96–108] and Period 3 (n = 11) [53, 63, 109–117], highlighting the increased value placed on qualitative inquiry. In Period 4, five studies using qualitative methods were published on the influences on infant feeding in South Africa [118–122].
Qualitative study characteristics
As with the quantitative studies, the range of qualitative study designs and settings was broad. Specific qualitative study designs included ethnography [96, 97, 107] and phenomenology [116], although 17/25 publications did not name a design or simply referred to their methods as qualitative. In terms of settings, studies were conducted in urban, peri-urban/township, and rural settings, with a bias for peri-urban and township environments. Mirroring the quantitative biases, the most popular study provinces were KZN (n = 13), WC (n = 13), GP (n = 8), and the EC (n = 6) followed by LP (n = 3) and the NW (n = 1) (see S3 Table for details). No qualitative studies meeting search criteria were published from Mpumalanga, Free State, or the Northern Cape provinces.
The people sampled for qualitative studies were strongly biased towards exploring the feeding influences of HIV positive mothers (23/34). Only 12 studies included mothers who were HIV negative or had an unspecified HIV status. Among mothers, some were purposively sampled based on age, parity, work status or feeding preference. The ages of the infants were not stated in all cases, and there was considerable variation when they were specified: neonates [110], mothers whose infants had an average age older than 6 months [99]; some whose infants had died [95]. This is relevant in terms of recall bias and how mothers may have described feeding influences. Health workers, particularly counsellors, were included in nine qualitative studies [53, 93, 94, 96–99, 108, 111]. Five studies included family members, usually fathers or grandmothers [53, 96, 97, 111, 112]. Some studies also included PTMCT or intervention program staff in their samples.
A total of 21 (61.8%) studies used interviews for data collection, with group discussions being the second most popular form of data collection (15/34), often with a mixture of the two. Novel [93–95] and participatory [108] methods were used in a few instances. Qualitative data were analyzed in a number of ways, with variations of thematic, content, and framework analyses the most common.
A meta-synthesis of themes from the qualitative studies was conducted by socio-ecological level prior to synthesis of the two methods (see S5 Table). The HIV epidemic and prevention efforts emerged strongly as an important part of the breastfeeding narrative. Following HIV positive mother narratives over time helped us understand their breastfeeding decision-making. Specifically, they helped us gain insight into how, as guidance about transmission risk changed from EFF to EBF, mothers’ behaviors also changed to provide the best health prospects for their infants. Gender and mixed feeding norms also emerged as strong infant feeding influences over time. To avoid repetition, these themes are synthesized below with the quantitative findings in order to highlight how the combined sources provide a more context-rich picture of infant feeding in South Africa.
Synthesis of qualitative and quantitative findings across ecological levels
The synthesis of quantitative and qualitative study findings is presented in Table 5, with only statistically significant quantitative results or strong qualitative themes linked to EBF reported. The two unique features of this table are that, firstly, it accounts for variations in influences across time, and secondly these influences are described in relation to what the evidence found in relation to support for or against EBF. Where the data for influences were unclear, supporting EBF in some instances while acting as barriers in other studies, this is also noted.
Table 5. Synthesis of key influences on EBF from 1980–2018 (based on S2 and S4 Tables).
| EBF influences by ecological level | Policy Periods | Evidence synthesis in relation to support (or not) for EBF |
|---|---|---|
| STRUCTURAL–Sociocultural/ Market | ||
| Breastfeeding norms | All | Consistently supported EBF, with promotion project-based |
| Breastfeeding promotion | 1–2 only | |
| HIV stigma against formula | All | Supports breastfeeding, but not exclusivity (see next influence) |
| HIV stigma around exclusive feeding | All | Undermins EBF; Exclusivity perceived as proxy for HIV |
| Mixed feeding norms before six months | All | Strong influence undermining EBF, with formula culture reinforcing norm |
| Commercial formula “culture” | 3–4 only | |
| Social media/Internet | 3–4 only | Reinforces existing biases/practices |
| Motherhood expectations and exclusive feeding | All | Sometimes, but not always, associated with “good” motherhood |
| SETTINGS–Healthcare | ||
| Postnatal visits/support by HWs | All | Proactive visits strongly support EBF |
| Health worker counselling/advice | All | Strong influence; support of EBF depends on consistency and content |
| Separating mothers and infants | All | Consistently undermine EBF |
| Pre-lacteal feeds | 1–2 only | |
| Free formula program | 1–2 only | |
| SETTINGS–Household | ||
| Support for mother after HIV disclosure | All | Supports EBF; for HIV-positive only |
| Family advice & caregiving support | All | Strong influence; EBF support depends on family preferences |
| Gender & power relations | All | Consistently undermine EBF, with rituals specific to only some cultures |
| Infant cleansing rituals | All | |
| SETTINGS–Community | ||
| Community-based EBF support efforts | All | Strong influence; linked to projects |
| HIV stigma/gossip | All | Consistently undermine EBF; stigma fears strong for HIV-positive mothers |
| Work/school environments | All | |
| INDIVIDUAL—Infant Attributes | ||
| Infant Growth | All | Healthy growth and calm disposition reinforce selected feeding practices |
| Disposition (crying, calm, etc.) | All | |
| Negative health events, e.g. HIV conversion | 1–2 only | Negative infant responses undermine EBF |
| Breastmilk refusal | 3–4 only | |
| INDIVIDUAL—Mother attributes | ||
| Self-efficacy/confidence | 2–4 only | Support EBF consistently |
| Knowledge of breastfeeding benefits | All | |
| Fear of HIV transmission (for HIV positive mothers only) | All | Strong influence for/against EBF; dependent on advice received |
| Past feeding experience | All | Strong influence for/against EBF; dependent on experience |
| Milk contamination beliefs | 1–2 only | Consistently undermine EBF; milk contamination beliefs include HIV and other factors, such as not feeding breastmilk after sexual dreams |
| Antenatal depression | 4 only | |
| Milk insufficiency beliefs | All | |
| Employed or in school | All | |
| Young and dependent on family | All | |
Table 5 shows how most multi-level EBF influences persisted across all policy periods. Common influences that supported EBF were norms encouraging breastfeeding, postnatal support (from healthcare settings, community and households) and knowledge of breastfeeding benefits. Common EBF barriers included mixed feeding norms, the separation of mothers and infants after delivery, unsupportive workplaces (and schools), and milk insufficiency beliefs. The changes in influence that occurred between policy periods were concentrated in the healthcare setting and structural levels. In the healthcare setting we observed a move towards more breastfeeding-friendly environments, e.g. reducing pre-lacteal feeds and removing free formula. In contrast, the past decade has seen formula emerge as a “culture” and social media becoming increasingly important, coinciding with increased reports of infants refusing breastmilk.
True to our conceptual framework, some factors interacted across multiple levels of influence. For instance, HIV was seen with stigma at the sociocultural level, in how health workers advised mothers in the healthcare setting (often in conflicting ways), and through familial support (or not) after disclosure. At the individual level HIV manifested in how knowledge about breastfeeding benefits interacted with knowledge about transmission risks; these were dynamic. For instance, the fear of HIV transmission informed the choices of most HIV-positive mothers consistently, while the advice they received on the topic changed based on shifting PMTCT guidelines. Mothers with a recent HIV diagnosis appeared to be more influenced by infant feeding recommendations by health workers (who in Period 4 are more likely to promote EBF) than those with an older diagnosis.
Living in HIV endemic communities has influenced intentions to breastfeed and public performances of breastfeeding, even among non-infected women. For HIV-positive mothers, intentions to follow health advice were mitigated by fears that their HIV status would be disclosed inadvertently by their feeding practices; this was particularly clear in the qualitative studies [97, 100, 104, 112]. While active disclosure to intimate partners and family members was often described as a positive experience, some HIV-positive mothers avoided disclosure for fear of violence or abandonment [98]. When the government provided formula (Period 2), mothers did not want to be seen with the government brand of formula, Pelargon, and at times sold it to buy other brands or transferred the formula into empty tins of commercial brands [99, 104, 113]. Even when the free formula program ended, the practice of exclusive feeding (formula or breastmilk) was described with trepidation because avoidance of mixed feeding was perceived by community members to be linked to HIV [112], to the extent that HIV negative mothers report pretending to mix feed to avoid being labelled as HIV positive.
Other factors also operated across levels. Specifically, young and unemployed mothers were particularly vulnerable to abandoning EBF for reasons of gendered cultural expectations in their households and low perceived power. Those who wished to return to school opted for formula; expressing milk was not mentioned as a viable option, nor did any studies mention negotiating breastfeeding in the school setting. It was common for such young mothers to pass on child rearing responsibilities to their mothers; in doing so, they surrendered any ability to influence and determine decisions over infant feeding [112]. Within patriarchal kinship networks, young women faced expectations of unquestioning submission to the instructions of their elders, with mother-in-laws wielding particular power (if married). When elders differed in their beliefs about feeding, young mothers described deferring to family wishes and lying to health workers as their most common strategy to negotiate infant feeding. Financial dependence created added pressure to adhere to family wishes. In some instances, young mothers wanted to keep the baby’s father involved by requesting that he provide formula. Concerns about their body, e.g. saggy breasts, were also mentioned as barriers by younger women, although these concerns were mentioned less often than feelings of powerlessness in the family unit.
Older mothers faced their own challenges. Those who were employed or looking for work opted for formula or mixed feeding. Those with more than one child often based their decisions on how the firstborn fared. If a previous child survived mixed feeding, shifting to exclusive feeding was less likely, especially because of community mixed feeding norms.
Practical considerations were often raised. Mothers who felt that breastmilk was cheap and easy were most likely to breastfeed, whereas those who experienced pain and did not mind preparing formula went the opposite way. The discomfort and demands of breastfeeding, ranging from complaints of breast problems to sleepless nights, were rarely disclosed to new mothers; this lack of preparation was described as a reason for abandoning breastfeeding. These types of concerns have persisted over time.
As indicated, both young and older mothers reported family and community pressure to mixed feed across all four policy periods. Some of this advice comes from traditional practices or requirements. The most consistent reasons for complying with family pressure related to seeking to quiet a crying baby or fulfilling expectations about what an infant “needs.” Common beliefs about milk being insufficient also played a strong role at both household and community levels, reflecting and influencing mixed feeding norms. All of these factors contribute to whether a mother felt confident or had self-efficacy to EBF, which was strongly linked to the practice. One recent study also highlighted the role of mental health, particularly antenatal depression, as a barrier to EBF [69].
The healthcare setting has been noted as a critical space for establishing breastfeeding or not and as a source for breastfeeding (and HIV transmission) knowledge. Inconsistent and inadequate counselling by health workers was most often associated with abandoning EBF. Confused mothers often referred back to lay knowledge and direct observations of their children’s growth in a context where they were not given clear and consistent messages. This was most pronounced for HIV positive mothers. The free formula program, most often discussed during policy periods 2 and 3, eroded trust in EBF. While it no longer directly influences choice, confusion about optimal feeding among both mothers and health workers has carried into the current policy period. Other practices related to mother-baby friendly initiative (MBFI) hospitals were also reported across all periods. MBFI is South Africa’s localized version of the global Baby-Friendly Hospital Initiative (BFHI) [123]. While the practice of pre-lacteal feeds (health workers feeding infants formula before breastfeeding could be established) was only reported in the first two periods, experiences of mothers being separated from their infants after delivery persist. On the positive side, health services provided during the postnatal period, including home visits and support groups, were linked to increased and longer EBF, particularly when CHWs were involved. Our synthesis identified that neither school nor work settings promote EBF. Commercial formula marketing and the media space in general were understudied.
Discussion
Our finding of highly variable rates of EBF over time was recently confirmed by spatial scientists as a trend both in South Africa and throughout the region [124]. While different policies and guidelines have influenced infant feeding, most obviously through health workers counselling practices and the free formula program for HIV positive mothers, this systematic review has highlighted persistent multi-level influences of EBF in South Africa over time for a larger population of mothers. The observation that complex factors constitute infant feeding decision-making [15] is clearly illustrated in this review.
At the structural level, norms that favor both breastfeeding and mixed feeding can partly explain high levels of breastfeeding initiation but early introduction of complementary feeding in South Africa [5]. Norms also influence gender identities, with one meta-ethnographic synthesis finding a narrative that breastfeeding was described as synonymous with “good mothering” [125]. The South African studies we reviewed suggest that this narrative is contested, as has also been found in the U.K. [126, 127]; for instance, some mothers who EBF based on health worker advice are chastised in the community setting for depriving their hungry infants of complementary foods [119]. Added to this, the mainstreaming of formula combined with the growth of social media present challenges to enforcing Code infringements [128].
The evidence suggests that the healthcare setting is moving towards more mother- and baby-friendly spaces, demonstrated in provincial plans [129]. The Tshwane Declaration has contributed to this shift. Our finding that both HW-led support and lay support can increase EBF compared to standard care has also been noted in two recent systematic reviews of the global literature, with health worker interventions showing the best results [130, 131]. While some factors influencing EBF require health systems responses, such as limiting the time mothers and infants are separated after delivery, many factors fall well outside of the health system’s purview, such as shifting norms.
Referring back to the adapted conceptual framework (see Fig 1) and synthesized results (see Table 5), a range of interventions are needed to address barriers to EBF and build on existing opportunities and efforts. These recommendations are made by level and draw on evidence from the reviewed articles, as well as, global literature.
Interventions at the individual level to support mothers and infants remain important as barriers related to milk insufficiency beliefs and the incompatibility of EBF with schooling and employment persist. Counselling and information sharing that clarifies the benefits of EBF for all infants [2] are needed for mothers, HWs as well as family members. For HIV positive mothers and the HWs treating them, the low risk of HIV transmission through breastfeeding while on ART needs emphasis [132]. Specific attention needs to be paid to how counsellors speak to all mothers about past feeding experiences, e.g. latching problems, and how the each infant is different. Ongoing efforts, such as a pediatric dietary guideline [133], are welcome in this context. Early diagnosis and treatment of depression among women postpartum is also likely to help [69]. For mothers facing HIV disclosure and the potential of violence or abandonment, specialized attention is needed. For young mothers, engagement with their families and school environments is most likely to increase EBF rates.
Within the community, norms around mixed-feeding, which is expected to be performed in public, must be addressed. The successful mass media and community mobilization campaigns by Alive and Thrive in Vietnam and Bangladesh are good models of how this might be done [134]. We agree with recommendations to address school and work policies and environments to shift perceptions that they are incompatible with breastfeeding [135, 136]. In South Africa, there is also clear scope to continue HIV stigma reduction efforts. This remains a huge barrier to HIV-positive mothers and a disincentive to EBF also for HIV-negative mothers, both in South Africa and in other HIV endemic settings.
The hospital setting presents a number of opportunities for institutions to align with MBFI practices, otherwise known as BFHI, particularly around keeping mothers and infants together after delivery. If this is not done, the practice of hospital staff separating mothers and infants will continue to undermine breastfeeding, as noted globally [4]. The importance of clear and compassionate counselling has been emphasized across all policy periods to promote (or discourage) EBF. This needs to include content clarity on duration and how to potentially overcome the challenges that may present early on.
Our synthesis highlights how families, particularly partners and surrogate caregivers, need to be engaged on how to support optimal feeding choices. They wield tremendous influence over infant feeding practices and by targeting these support structures, individual mothers could experience less pressure to negotiate exclusive feeding in a context where it is culturally not accepted. Strategies on how to acknowledge the source of socio-cultural beliefs and respect them, without endangering the infant’s health, are also needed. This process has already started, taking direction from the Tshwane Declaration and Regulation 991 [10, 137], and through campaigns such as Side by Side (sidebyside.co.za). These could be expanded.
A limitation of this review is that only one author did the initial screening of the full sample to identify potentially eligible articles, while pairs for the entire process are recommended [138]. Despite the large number of articles in the final review, significant population groups have not been studied. Almost all studies are with African women. Cultural considerations of different ethnic groups, including Indian populations (both Muslim and Hindu) and whites, are absent from these studies. Higher socio-economic status women have been largely neglected as well. Few studies have been conducted in four provinces of South Africa’s nine provinces. As mentioned earlier, there has also been a much stronger focus on mothers, leading to gaps in our understanding about work and school environments, and accommodation of infant feeding. The role of the media in promoting or undermining breastfeeding is also missing from conceptual frameworks. All of these gaps provide opportunities for future research. Many studies did not meet PICO requirements but still addressed many of the questions raised about EBF. Finally, the quality of studies was highly variable. We applied an exploratory approach to uncover as many factors as possible, rather than excluding factors based on poor study design, except in a minority of cases discussed earlier. However, the variability of designs meant that we were unable to conduct a meta-analysis.
Conclusion
Exclusive breastfeeding for six months is possible, but it is challenging in the South African context. Multi-level barriers to EBF, highlighted globally [4], were described across all four policy periods. These included milk insufficiency beliefs, separating mothers and infants after delivery, and mother returning to work. In addition, South Africans contend with strong mixed feeding norms and an HIV epidemic that permeates all levels [139]. South Africa is also extremely diverse, with 11 official languages and myriad cultural permutations not reflected in our literature. This review highlights gaps in our understanding of infant feeding in various populations, both geographically and in terms of sociodemographic backgrounds. It also highlights potential blindspots in our focus on the market context where formula and breastfeeding are promoted (or not) and that mixed feeding norms require creative approaches that go beyond simply counselling individuals.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank John Eyers and Nigel Rollins for their early support in conceptualising the systematic review scope and search strategies. This research was supported by the Consortium for Advanced Research Training in Africa (CARTA). CARTA is jointly led by the African Population and Health Research Center and the University of the Witwatersrand and funded by the Carnegie Corporation of New York (Grant No—B 8606.R02), Sida (Grant No:54100113), the DELTAS Africa Initiative (Grant No: 107768/Z/15/Z) and Deutscher Akademischer Austauschdienst (DAAD). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (UK) and the UK government. The statements made and views expressed are solely the responsibility of the first author, who was a CARTA Fellow at the time of the study. SAN is supported by the DST-NRF Centre of Excellence in Human Development at the University of the Witwatersrand, Johannesburg, South Africa.
Data Availability
All relevant data are contained within the manuscript and Supporting Information files.
Funding Statement
SJN received PhD funding support from the Consortium for Advanced Research Training in Africa (CARTA: www.cartafrica.org). CARTA is jointly led by the African Population and Health Research Center and the University of the Witwatersrand and funded by the Carnegie Corporation of New York (Grant No--B 8606.R02), Sida (Grant No:54100113), the DELTAS Africa Initiative (Grant No: 107768/Z/15/Z) and Deutscher Akademischer Austauschdienst (DAAD). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (UK) and the UK government. CARTA had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Du Plessis L, Peer N, Honikman S, English R. Breastfeeding in South Africa: Are we making progress? In: Padarath A, King JF, Mackie E, Casciola J, editors. S Afr Health Rev. Durban: Health Systems Trust; 2016. pp. 109–23. [Google Scholar]
- 2.Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017): 475–90. 10.1016/S0140-6736(15)01024-7 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Indicators for assessing infant and young child feeding practices: Conclusions of a consensus meeting held 6–8 November 2007 in Washington DC Geneva: WHO; 2008. [Google Scholar]
- 4.Rollins NC, Bhandari N, Hajeebhoy N, Horton S, Lutter CK, Martines JC, et al. Why invest, and what it will take to improve breastfeeding practices? Lancet. 2016;387(10017): 491–504. 10.1016/S0140-6736(15)01044-2 [DOI] [PubMed] [Google Scholar]
- 5.National Department of Health, Statistics South Africa, South African Medical Research Council, ICF. South Africa demographic and health survey 2016: Key indicators Pretoria, South Africa and Rockville, Maryland, USA: NDoH, Stats SA, SAMRC, and ICF, 2017. [Google Scholar]
- 6.South African National Department of Health, Medical Research Council of South Africa, Demographic and Health Surveys, Macro International Inc. South African demographic and health survey, 1998 report Pretoria, South Africa and Rockville, Maryland, USA: NDoH, SAMRC, and Macro, 1998. [Google Scholar]
- 7.National Department of Health. The 2015 National antenatal sentinel HIV & syphilis survey, South Africa Pretoria: National Department of Health, 2017. [Google Scholar]
- 8.National Department of Health. Policy and guidlines for the implemention of the PMTCT programme. Pretoria: National Department of Health, 2008. [Google Scholar]
- 9.National Department of Health. Protocol for providing a comprehensive package of care for the prevention of mother to child transmission of HIV (PMTCT) in South Africa Pretoria: South African National Department of Health; 2001. [Google Scholar]
- 10.Department of Health. The Tshwane Declaration of support for breastfeeding in South Africa. South Afr J Clin Nutr. 2011;24(4):214. [Google Scholar]
- 11.Goga AE, Doherty T, Jackson DJ, Sanders D, Colvin M, Chopra M, et al. Infant feeding practices at routine PMTCT sites, South Africa: Results of a prospective observational study amongst HIV exposed and unexposed infants—birth to 9 months. Int Breastfeed J. 2012. April;7(1): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter M. Reaction to conquest: Effects of contact with Europeans on the Pondo of South Africa, second edition London: Oxford University Press; 1961. [Google Scholar]
- 13.Kruger R, Gericke GJ. A qualitative exploration of rural feeding and weaning practices, knowledge and attitudes on nutrition. Public Health Nutr. 2003. April;6(2): 217–23. 10.1079/PHN2002419 [DOI] [PubMed] [Google Scholar]
- 14.Schoub BD, Greeff A, Lecatsas G, Prozesky O, Hay I, Prinsloo J, et al. A microbiological investigation of acute summer gastroenteritis in black South African infants. Epidemiol Infect. 1977;78(3): 377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazarus R, Struthers H, Violari A. Promoting safe infant feeding practices–the importance of structural, social and contextual factors in Southern Africa. J Int AIDS Soc. 2013. January;16(1): 0–7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Department of Health UNICEF. Infant and young child feeding policy Pretoria: Department of Health Nutrition Directorate; 2007. p. 45. [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7): e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baek C, Mathambo V, Mkhize S, Friedman I, Apicella L. Key findings from an evaluation of the mothers2mothers program in Kwazulu-Natal, South Africa Washington, D.C: Population Council, Horizons, 2007. [Google Scholar]
- 19.Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013. September 18;13:117 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. Br Med J (Clin Res Ed). 2008;336(7650): 924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64: 401–6. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 22.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADe guidelines: 1. Introduction-grade evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64: 383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 23.Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Ottawa (ON): Ottawa Hospital Research Institute, 2009. [Google Scholar]
- 24.Lewin S, Glenton C, Munthe-Kaas H, Carlsen B, Colvin CJ, Gülmezoglu M, et al. Using qualitative evidence in decision making for health and social interventions: An approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERLQUAL). PLoS Med. 2015;12(10):e1001895 10.1371/journal.pmed.1001895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewin S, Booth A, Glenton C, Munthe-Kaas H, Rashidian A, Wainwright M, et al. Applying GRADE-CERQUAL to qualitative evidence synthesis findings: Introduction to the series. Implement Sci. 2018. January 25;13(1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downe S. Metasynthesis: A guide to knitting smoke. Evid Based Midwif. 2008;6: 4–8. [Google Scholar]
- 27.Ross SM, Loening WEK, Van Middelkoop A. Breast-feeding-evaluation of a health education programme. S Afr Med J. 1983;64(10): 361–3. [PubMed] [Google Scholar]
- 28.Myburgh J, Price S. To breastfeed or not to breastfeed? That is the question. Curationis. 1985;8(1): 32 [PubMed] [Google Scholar]
- 29.Mkhasibe C, Wagstaff L. Breast feeding practices of nurses at Baragwanath, Coronation and Johannesburg hospitals. Curationis. 1988. December;11(4): 19–21. [PubMed] [Google Scholar]
- 30.Ransome OJ, Chalmers B, Herman AAB, Reinach SG. Infant feeding in an urban community. S Afr Med J. 1988;74(8): 393–5. [PubMed] [Google Scholar]
- 31.Ransome OJ, Chalmers B, Herman AAB, Reinach SG. Factors influencing breast-feeding in an urban community. S Afr Med J. 1989;76(8): 431–3. [PubMed] [Google Scholar]
- 32.Zöllner E, Carlier ND. Breast-feeding and weaning practices in Venda, 1990. S Afr Med J. 1993;83(8): 580–3. [PubMed] [Google Scholar]
- 33.Roberts GJ, Cleaton-Jones PE, Richardson BD, Sinwell RE, Lucas VS. Breast and bottle feeding in rural and urban South African children. J Hum Nutr Diet. 1995. Jan 1;8(4): 255–63. [Google Scholar]
- 34.Mamabolo RL, Alberts M, Mbenyane GX, Steyn NP, Nthangeni NG, Delemarre-Van De Waal HA, et al. Feeding practices and growth of infants from birth to 12 months in the central region of the Limpopo province of South Africa. Nutr. 2004;20(3): 327–33. [DOI] [PubMed] [Google Scholar]
- 35.Whitfield M. Breastfeeding in South Africa: Why the lack of support?: Research hot off the press. Health Systems Trust Update. 1996. January 1;17(19): 5–6. [Google Scholar]
- 36.Kruger R, Gericke G. Feeding practices of children [aged 0–2 years] in a rural area in North West province in South Africa. South Afr J Clin Nutr. 2000;13(3):127. [Google Scholar]
- 37.Ukpe IS, Blitz J, Hugo J, Theledi M. The infant feeding practices of mothers enrolled on the prevention of mother to child transmission of HIV programme at a primary health care clinic in Mpumalanga province, South Africa. S Afr Fam Pract. 2009;51(4): 337–9. [Google Scholar]
- 38.Marais D, Koornhof HE, du Plessis LM, Naude CE, Smit K, Hertzog E, et al. Breastfeeding policies and practices in health care facilities in the Western Cape province, South Africa. South Afr J Clin Nutr. 2010;23(1): 40–5. [Google Scholar]
- 39.Kassier SM, Veldman FJ. Cry, the beloved bottle: Infant-feeding knowledge and the practices of mothers and caregivers in an urban township outside Bloemfontein, Free State province. South Afr J Clin Nutr. 2013;26(1): 17–22 [Google Scholar]
- 40.Morgan JJ, Jeggels JD. Factors influencing the infant feeding choices of HIV-positive mothers at a level two hospital in Cape Town. Afr J Midwifery Womens Health. 2015;9(2): 66–70. [Google Scholar]
- 41.Kyei K, Netshikweta M, Spio K. Breastfeeding in the Vhembe district of Limpopo province, South Africa: Duration and factors. Studies on Ethno-Medicine. 2014;8(3): 317–24. [Google Scholar]
- 42.Matji JN, Wittenberg DF, Makin JD, Jeffery B, MacIntyre UE, Forsyth BW. Psychosocial and economic determinants of infant feeding intent by pregnant HIV-infected women in Tshwane/Pretoria. SAJCH. 2008. January 1;2(3): 114–18. [Google Scholar]
- 43.Zunza M, Theron GB, Harvey C. Compliance with infant formula feeding by HIV-positive women one week after delivery in khayelitsha, South Africa. South Afr J Epidemiol & Infect. 2011;26(4): 274–9. [Google Scholar]
- 44.Ross SM, Middelkoop AV, Khoza NC. Breast-feeding practices in a black community. S Afr Med J. 1983;63(1): 23–5. [PubMed] [Google Scholar]
- 45.Ross SM, Loening WEK, Mbele BE. Breast-feeding support. S Afr Med J. 1987;72(5): 357–8. [PubMed] [Google Scholar]
- 46.Doherty T, Chopra M, Jackson D, Goga A, Colvin M, Persson L-A. Effectiveness of the WHO/UNICEF guidelines on infant feeding for HIV-positive women: Results from a prospective cohort study in South Africa. AIDS. 2007;21(13):1791–7. 10.1097/QAD.0b013e32827b1462 [DOI] [PubMed] [Google Scholar]
- 47.Bork K, Cames C, Cournil A, Musyoka F, Ayassou K, Naidu K, et al. Infant feeding modes and determinants among HIV-1-infected African women in the Kesho Bora study. J Acquir Immune Defic Syndr. 2013. January 1;62(1): 109–18. 10.1097/QAI.0b013e318277005e [DOI] [PubMed] [Google Scholar]
- 48.Kuhn L, Mathews C, Fransman D, Dikweni L, Hussey G. Child feeding practices of HIV-positive mothers in Cape Town, South Africa. AIDS. 1999. January 14;13(1):144–6. [PubMed] [Google Scholar]
- 49.Mostert D, Steyn NP, Temple NJ, Olwagen R. Dietary intake of pregnant women and their infants in a poor black South African community. Curationis. 2005. November;28(4):12–9. 10.4102/curationis.v28i4.1002 [DOI] [PubMed] [Google Scholar]
- 50.Engebretsen IMS, Nankabirwa V, Doherty T, Diallo AH, Nankunda J, Fadnes LT, et al. Early infant feeding practices in three african countries: The PROMISE-EBF trial promoting exclusive breastfeeding by peer counsellors. Int Breastfeed J. 2014;9(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman M. A programme to promote breast feeding in a socio-economically disadvantaged area of Cape Town SALDRU, School of Economics, University of Cape Town, 1984. [PubMed] [Google Scholar]
- 52.Goosen C. Factors influencing feeding practices of primary caregivers of infants [0–5.9 months] in Avian Park and Zwelethemba, Western Cape, South Africa. Doctoral dissertation, Stellenbosch University, 2013.
- 53.Goosen C, McLachlan MH, Schübl C. Infant feeding practices during the first 6 months of life in a low-income area of the Western Cape province. SAJCH. 2014. May 21;8(2): 50–4. [Google Scholar]
- 54.Hoffman MN, Durcan NM, Disler PB. Breast-feeding in a socio-economically disadvantaged area of Cape Town. Part I. Analysis of breast-feeding patterns among clinic attenders. S Afr Med J. 1984. July 14;66(2): 64–5. [PubMed] [Google Scholar]
- 55.Hoffman MN, Durcan NM, Disler PB. Breast-feeding in a socio-economically disadvantaged area of Cape Town. Part II. The introduction of an educational and support programme. S Afr Med J. 1984. July 14;66(2):66–7. [PubMed] [Google Scholar]
- 56.Bland RM, Little KE, Coovadia HM, Coutsoudis A, Rollins NC. Intervention to promote exclusive breast-feeding for the first 6 months of life in a high HIV prevalence area. AIDS. 2008. April 23;22(7): 883–91. 10.1097/QAD.0b013e3282f768de [DOI] [PubMed] [Google Scholar]
- 57.Bland RM, Rollins NC, Coovadia HM, Coutsoudis A, Newell ML. Infant feeding counselling for HIV-infected and uninfected women: Appropriateness of choice and practice. Bull World Health Organ. 2007. April;85(4): 289–96. 10.2471/BLT.06.032441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bland RM, Rollins NC, Coutsoudis A, Coovadia HM. Breastfeeding practices in an area of high HIV prevalence in rural South Africa. Acta Paediatr. 2002;91(6): 704–11. 10.1080/080352502760069151 [DOI] [PubMed] [Google Scholar]
- 59.Bork K, Cames C, Cournil A, Musyoka F, Ayassou K, Naidu K, et al. Infant feeding modes and determinants among HIV-1-infected African women in the Kesho Bora study. J Acquir Immune Defic Syndr. 2013. January 1;62(1): 109–18. 10.1097/QAI.0b013e318277005e [DOI] [PubMed] [Google Scholar]
- 60.Ghuman MR, Saloojee H, Morris G. Infant feeding practices in a high HIV prevalence rural district of Kwazulu-Natal, South Africa. South Afr J Clin Nutr. 2009;22(2): 74–9. [Google Scholar]
- 61.Kassier S, Maunder E, Senekal M. Infant feeding practices in Kwazulu-Natal: An exploratory study of current infant feeding practices of mothers with 0–6 month old infants attending PMTCT and non-PMTCT clinics in central Durban. Durban Institute of Technology, 2003. [Google Scholar]
- 62.Ijumba P, Doherty T, Jackson D, Tomlinson M, Sanders D, Swanevelder S, et al. Effect of an integrated community-based package for maternal and newborn care on feeding patterns during the first 12 weeks of life: A cluster-randomized trial in a South African township. Public Health Nutr. 2015; 18(14): 2660–8 10.1017/S1368980015000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swarts S, Kruger HS, Dolman RC. Factors affecting mothers' choice of breastfeeding vs. Formula feeding in the lower Umfolozi District War Memorial hospital, Kwazulu-Natal. Health SA: interdisciplinary research journal. 2010. January 1;15(1):119–26. [Google Scholar]
- 64.Tomlinson M, Doherty T, Ijumba P, Jackson D, Lawn J, Persson L, et al. Goodstart: A cluster randomised effectiveness trial of an integrated, community-based package for maternal and newborn care, with prevention of mother-to-child transmission of HIV in a South African township. Trop Med Int Health. 2014; 19(3): 256–66 10.1111/tmi.12257 [DOI] [PubMed] [Google Scholar]
- 65.Tylleskär T, Jackson D, Meda N, Engebretsen IM, Chopra M, Diallo AH, et al. Exclusive breastfeeding promotion by peer counsellors in sub-saharan africa (PROMISE-EBF): A cluster-randomised trial. Lancet. 2011; 378(9789): 420–7 10.1016/S0140-6736(11)60738-1 [DOI] [PubMed] [Google Scholar]
- 66.Horwood C, Butler L, Barker P, Phakathi S, Haskins L, Grant M, et al. A continuous quality improvement intervention to improve the effectiveness of community health workers providing care to mothers and children: A cluster randomised controlled trial in South Africa. Hum Resour Health. 2017. June 13;15(1): 39 10.1186/s12960-017-0210-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pillay S, Sibanda W, Ghuman MR, Coutsoudis A. Infant feeding practices of teenage mothers attending a well-baby clinic in a public hospital in Umlazi, Kwazulu-Natal, South Africa. South Afr J Clin Nutr. 2018;31(1): 14–9. [Google Scholar]
- 68.Reimers P, Israel-Ballard K, Craig M, Spies L, Thior I, Tanser F, et al. A cluster randomised trial to determine the efficacy of the 'feeding buddies' programme in improving exclusive breastfeeding rates among HIV-infected women in rural Kwazulu-Natal, South Africa. AIDS Behav. 2018. January 1;22(1): 212–23. 10.1007/s10461-017-1865-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tuthill EL, Pellowski JA, Young SL, Butler LM. Perinatal depression among HIV-infected women in Kwazulu-Natal South Africa: Prenatal depression predicts lower rates of exclusive breastfeeding. AIDS Behav. 2017. June;21(6): 1691–8. 10.1007/s10461-016-1557-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tuthill EL, Butler LM, Pellowski JA, McGrath JM, Cusson RM, Gable RK, et al. Exclusive breast-feeding promotion among HIV-infected women in South Africa: An Information-Motivation-Behavioural skills model-based pilot intervention. Public Health Nutr. 2017. June;20(8): 1481–90. 10.1017/S1368980016003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van der Elst CW, Pick W, Isaacs S, Malan AF. Current trends in infant feeding. S Afr Med J. 1989. October 21;76(8): 434–7. [PubMed] [Google Scholar]
- 72.Petrie KEM, Schmidt SD, Schwarz CE, Koornhof HE, Marais D. Knowledge, attitudes and practices of women regarding the prevention of mother-to-child transmission (PMTCT) programme at the Vanguard Community Health Centre, Western Cape—a pilot study. South Afr J Clin Nutr. 2007:20(2): 71–8. [Google Scholar]
- 73.Sibeko L, Dhansay MA, Charlton KE, Johns T, Gray-Donald K. Beliefs, attitudes, and practices of breastfeeding mothers from a periurban community in South Africa. J Hum Lact. 2005. February;21(1): 31–8. 10.1177/0890334404272388 [DOI] [PubMed] [Google Scholar]
- 74.Rotheram-Borus MJ, Richter LM, Heerden Av, Rooyen Hv, Tomlinson M, Harwood JM, et al. A cluster randomized controlled trial evaluating the efficacy of peer mentors to support South African women living with HIV and their infants. PLoS ONE. 2014;9(1): e84867–e. 10.1371/journal.pone.0084867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Budree S, Goddard E, Brittain K, Cader S, Myer L. Infant feeding practices in a South African birth cohort-a longitudinal study. Matern Child Nutr. 2017. July;13(3): e12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Myer L, Phillips TK, Zerbe A, Brittain K, Lesosky M, Hsiao N, et al. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial. PLoS Med. 2018;15(3):e1002547–e. 10.1371/journal.pmed.1002547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steyn N, Zunza M, Decloedt EH. A cross-sectional descriptive study of breastfeeding behaviour and galactogogue use among private-sector patients in Cape Town, South Africa. SAJOG. 2017;23(1):20–3. [Google Scholar]
- 78.Thomas E, Kuo C, Cohen S, Hoare J, Koen N, Barnett W, et al. Mental health predictors of breastfeeding initiation and continuation among HIV infected and uninfected women in a South African birth cohort study. Prev Med. 2017;102:100–11. 10.1016/j.ypmed.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chalmers B. Urban/rural differences in infant feeding practices among Pedi women. S Afr Med J. 1986. July 5;70(1): 63–4. [PubMed] [Google Scholar]
- 80.Chalmers B, Ransome OJ, Herman A. Psychosocial factors related to infant feeding patterns. J Reprod Infant Psychol. 987 Jul-Sep;5(3): 153–64. [Google Scholar]
- 81.Ellison GT, Wagstaff L, Cameron N, de Wet T. Geographical differences in infant feeding patterns in disadvantaged communities. S Afr Med J. 1997;87(8): 1025–6. [PubMed] [Google Scholar]
- 82.MacIntyre UE, de Villiers FP, Baloyi PG. Early infant feeding practices of mothers attending a postnatal clinic in Ga-rankuwa. SAJCN. 2005. September;18(2): 70–5. [Google Scholar]
- 83.Nikodem VC, Hofmeyr GJ, Kramer TR, Gulmezoglu AM, Anderson A. Audiovisual education and breastfeeding practices: A preliminary report. Curationis. 1993;16(4): 60–3. [Google Scholar]
- 84.du Plessis D. Breastfeeding: Mothers and health practitioners in the context of private medical care in Gauteng. Health SA Gesondheid. 2009;14(1): 39–48. [Google Scholar]
- 85.Mnyani CN, Tait CL, Armstrong J, Blaauw D, Chersich MF, Buchmann EJ, et al. Infant feeding knowledge, perceptions and practices among women with and without HIV in Johannesburg, South Africa: A survey in healthcare facilities. Int Breastfeeding J. 2016;12(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sepeng L, Ballot DE. Audit of feeding practices in the neonatal wards at the Charlotte Maxeke Johannesburg academic hospital. SAJCH. 2016;9(4): 133–6. [Google Scholar]
- 87.Siziba LP, Jerling J, Hanekom SM, Wentzel-Viljoen E. Low rates of exclusive breastfeeding are still evident in four South African provinces. South Afr J Clin Nutr. 2016;28(4): 170–9. [Google Scholar]
- 88.Delport SD, Bergh AM, Hay IT. Breast-feeding practices in a private maternity hospital. S Afr Med J. 1988. October 15;74(8): 396–9. [PubMed] [Google Scholar]
- 89.Some EN, Engebretsen IM, Nagot N, Meda N, Lombard C, Vallo R, et al. Breastfeeding patterns and its determinants among mothers living with human immuno-deficiency virus -1 in four African countries participating in the ANRS 12174 trial. Int Breastfeed J. 2017. May 2;12(22):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yako EM, Nzama NP. Maintenance of the selected infant feeding methods amongst postnatal mothers at risk of HIV in the Eastern Cape province, South Africa. Health SA Gesondheid. 2013. April 29;18(1): 7 [Google Scholar]
- 91.Ladzani R, Peltzer K, Mlambo MG, Phaweni K. Infant‐feeding practices and associated factors of HIV‐positive mothers at Gert Sibande, South Africa. Acta Paediatr. 2011;100(4): 538–42. 10.1111/j.1651-2227.2010.02080.x [DOI] [PubMed] [Google Scholar]
- 92.Kruger R, Gericke G. Breast feeding practices of mothers with children [aged 0–36 months] in a rural area of South Africa a qualitative approach. Journal of Family Ecology and Consumer Sciences. 2001. March15; 29(1): 60–70. [Google Scholar]
- 93.Seidel G. Reconceptualising issues around HIV & breastfeeding advice: Findings from Kwazulu‐Natal, South Africa. Rev Afr Pol Econ. 2000;27(86): 501–18. [Google Scholar]
- 94.Seidel G. Decisions and advice about infant feeding: Findings from sociological work in Kwazulu-Natal, South Africa. Afr J AIDS Res. 2004. June 17; 3(2):167–77. 10.2989/16085900409490331 [DOI] [PubMed] [Google Scholar]
- 95.Seidel G, Sewpaul V, Dano B. Experiences of breastfeeding and vulnerability among a group of HIV-positive women in Durban, South Africa. Health Policy Plan. 2000;15(1): 24–33. 10.1093/heapol/15.1.24 [DOI] [PubMed] [Google Scholar]
- 96.Buskens I, Jaffe A. Demotivating infant feeding counselling encounters in Southern Africa: Do counsellors need more or different training? AIDS Care. 2008;20(3): 337–45. 10.1080/09540120701660346 [DOI] [PubMed] [Google Scholar]
- 97.Buskens I, Jaffe A, Mkhatshwa H. Infant feeding practices: Realities and mind sets of mothers in Southern Africa. AIDS Care. 2007;19(9):1101–9. 10.1080/09540120701336400 [DOI] [PubMed] [Google Scholar]
- 98.Chopra M, Piwoz E, Sengwana J, Schaay N, Dunnett L, Saders D. Effect of a mother-to-child HIV prevention programme on infant feeding and caring practices in South Africa. S Afr Med J. 2002. April;92(4): 298–302. [PubMed] [Google Scholar]
- 99.Doherty T, Chopra M, Nkonki L, Jackson D, Greiner T. Effect of the HIV epidemic on infant feeding in South Africa: "When they see me coming with the tins they laugh at me". Bull World Health Organ. 2006. February;84(2): 90–6. 10.2471/blt.04.019448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doherty T, Chopra M, Nkonki L, Jackson D, Persson L-A. A longitudinal qualitative study of infant-feeding decision making and practices among HIV-positive women in South Africa. J Nutr. 2006;136(9): 2421–6. 10.1093/jn/136.9.2421 [DOI] [PubMed] [Google Scholar]
- 101.Nor B, Ahlberg BM, Doherty T, Zembe Y, Jackson D, Ekströ, et al. Mother's perceptions and experiences of infant feeding within a community-based peer counselling intervention in South Africa. Matern Child Nutr. 2012;8(4): 448–58. 10.1111/j.1740-8709.2011.00332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nor B, Zembe Y, Daniels K, Doherty T, Jackson D, Ahlberg BM, et al. "Peer but not peer": Considering the context of infant feeding peer counseling in a high HIV prevalence area. J Hum Lact. 2009;25(4): 427–34. 10.1177/0890334409341050 [DOI] [PubMed] [Google Scholar]
- 103.Du Plessis D. Breastfeeding: Mothers and health practitioners in the context of private medical care in Gauteng. Health SA. 2009;14(1): 1–10. [Google Scholar]
- 104.Mackowski AM. An assessment of the factors that influence the infant feeding practices of HIV-positive mothers in the mothers' programmes: A qualitative study. Doctoral dissertation, University of Cape Town, 2005.
- 105.Sibeko L, Coutsoudis A, Nzuza S, Gray-Donald K. Mothers' infant feeding experiences: Constraints and supports for optimal feeding in an HIV-impacted urban community in South Africa. Public Health Nutr. 2009. November;12(11): 1983–90. 10.1017/S1368980009005199 [DOI] [PubMed] [Google Scholar]
- 106.Stinson K, Myer L. HIV-infected women's experiences of pregnancy and motherhood in Cape Town, South Africa. Vulnerable Child Youth Stud. 2012;7(1): 36–46. [Google Scholar]
- 107.Thairu LN, Pelto GH, Rollins NC, Bland RM, Ntshangase N. Sociocultural influences on infant feeding decisions among HIV-infected women in rural Kwa-zulu Natal, South Africa. Mat Child Nutr. 2005;1(1): 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Varga C, Brookes H. Preventing mother-to-child HIV transmission among South African adolescents. J Adolesc Res. 2008;23(2): 172–205. [Google Scholar]
- 109.Andreson J, Dana N, Hepfer B, Kingori E, Oketch J, Wojnar D, et al. Infant feeding buddies: A strategy to support safe infant feeding for HIV-positive mothers. J Hum Lact. 2013. February;29(1): 90–3. 10.1177/0890334412469056 [DOI] [PubMed] [Google Scholar]
- 110.Chaponda A, Goon DT, Hoque ME. Infant feeding practices among HIV-positive mothers at Tembisa Hospital, South Africa. Afr J Prim Health Care Fam Med. 2017. July 27;9(1):e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goosen C, McLachlan MH, Schübl C. Factors impeding exclusive breastfeeding in a low-income area of the Western Cape province of South Africa. Afr J Nurs Midwifery. 2014;16(1):13–31. [Google Scholar]
- 112.Ijumba P, Doherty T, Jackson D, Tomlinson M, Sanders D, Persson L-Å. Social circumstances that drive early introduction of formula milk: An exploratory qualitative study in a peri-urban South African community. Mat Child Nutr. 2014;10(1): 102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Laher F, Cescon A, Lazarus E, Kaida A, Makongoza M, Hogg RS, et al. Conversations with mothers: Exploring reasons for prevention of mother-to-child transmission [PMTCT] failures in the era of programmatic scale-up in Soweto, South Africa. AIDS Behav. 2012. January 1;16(1): 91–8. 10.1007/s10461-010-9875-9 [DOI] [PubMed] [Google Scholar]
- 114.Madiba S, Langa J. Cultural practices interfere with adherence to exclusive infant feeding: A qualitative study among HIV positive post natal women in Hammanskraal, South Africa: Child nutrition and feeding practices. Afr J Phys Health Educ Recreat Dance. 2014;20(S1): 264–78. [Google Scholar]
- 115.Madiba S, Letsoalo R. HIV disclosure to partners and family among women enrolled in prevention of mother to child transmission of HIV program: Implications for infant feeding in poor resourced communities in South Africa. Glob J Health Sci. 2013. July;5(4): 1 10.5539/gjhs.v5n4p1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ramara NS, Maputle MS, Lekhuleni ME. Infant feeding and HIV positive mothers in the Capricorn district of Limpopo province. Curationis. 2010. March;33(1): 5–16. 10.4102/curationis.v33i1.1000 [DOI] [PubMed] [Google Scholar]
- 117.Zulliger R, Abrams EJ, Myer L. Diversity of influences on infant feeding strategies in women living with HIV in Cape Town, South Africa: A mixed methods study. Trop Med Int Health. 2013;18(12):1547–54. 10.1111/tmi.12212 [DOI] [PubMed] [Google Scholar]
- 118.Hunter-Adams J, Myer L, Rother HA. Perceptions related to breastfeeding and the early introduction of complementary foods amongst migrants in Cape Town, South Africa. Int Breastfeed J. 2016;11(1): 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jama NA, Wilford A, Masango Z, Haskins L, Coutsoudis A, Spies L, et al. Enablers and barriers to success among mothers planning to exclusively breastfeed for six months: A qualitative prospective cohort study in Kwazulu-Natal, South Africa. Int Breastfeed J. 2017;12: 43 10.1186/s13006-017-0135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Modiba LM. Dilemma for choosing exclusive replacement feeding for HIV positive mothers of infants at a public hospital in Gauteng province, South Africa. Afr J Phys Health Educ Recreat Dance. 2015. 08/31/;21(3): 1–11. [Google Scholar]
- 121.Mushaphi LF, Mahopo TC, Nesamvuni CN, Baloyi B, Mashau E, Richardson J, et al. Recommendations for infant feeding policy and programs in Dzimauli Region, South Africa: Results from the MAL-ED birth cohort. Food Nutr Bull. 2017;38(3): 428–40. 10.1177/0379572117696662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ntuli B, Modibedi K. Decision-making and practice on infant feeding: A case study of women in a prevention of mother to child transmission programme in Gauteng province, South Africa: Public health intervention for maternal and child health. Afr J Phys Health Educ Recreat Dance. 2015;21(Suppl. 2.1): 12–24. [Google Scholar]
- 123.World Health Organization, UNICEF. Baby-friendly hospital initiative: Revised, updated and expanded for integrated care. 2009. [PubMed]
- 124.Bhattacharjee NV, Schaeffer LE, Marczak LB, Ross JM, Swartz SJ, Albright J, et al. Mapping exclusive breastfeeding in Africa between 2000 and 2017. Nat Med. 2019. August;25(8):1205–12. 10.1038/s41591-019-0525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burns E, Schmied V, Sheehan A, Fenwick J. A meta-ethnographic synthesis of women's experience of breastfeeding. Mat Child Nutr. 2010;6(3): 201–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marshall JL, Godfrey M, Renfrew MJ. Being a ‘good mother’: Managing breastfeeding and merging identities. Soc Sci Med. 2007. November;65(10): 2147–59. 10.1016/j.socscimed.2007.06.015 [DOI] [PubMed] [Google Scholar]
- 127.Leeming D, Williamson I, Lyttle S, Johnson S. Socially sensitive lactation: Exploring the social context of breastfeeding. Psych Health. 2013;28(4): 450–68. [DOI] [PubMed] [Google Scholar]
- 128.Abrahams SW. Milk and social media:Online communities and the international code of marketing of breast-milk substitutes. J Hum Lact. 2012;28(3): 400–6. 10.1177/0890334412447080 [DOI] [PubMed] [Google Scholar]
- 129.Kwazulu-Natal provincial guidelines on mother-baby friendly initiative implementation in Department of Health healthcare facilities, Pietersmaritzburg: KZN Department of Health, 2012.
- 130.Balogun OO, O'Sullivan EJ, McFadden A, Ota E, Gavine A, Garner CD, et al. Interventions for promoting the initiation of breastfeeding. Cochrane Database Syst Rev. 2016. November:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McFadden A, Gavine A, Renfrew MJ, Wade A, Buchanan P, Taylor JL, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.World Health Organization, United Nations Children’s Fund. Guideline: Updates on HIV and infant feeding: The duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 133.Du Plessis L, Pereira C. Commitment and capacity for the support of breastfeeding in South Africa. South Afr J Clin Nutr. 2013;26(S): S120–S8. [Google Scholar]
- 134.Nguyen PH, Headey D, Frongillo EA, Tran LM, Rawat R, Ruel MT, et al. Changes in underlying determinants explain rapid increases in child linear growth in Alive & Thrive study areas between 2010 and 2014 in Bangladesh and Vietnam. J Nutr. 2017. March;147(3): 462–9. 10.3945/jn.116.243949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martin-Wiesner P. A policy review: South Africa’s progress in systematising its international and national responsibilities to protect, promote and support breastfeeding Johannesburg: DST-NRF Centre of Excellence in Human Development, 2018. [Google Scholar]
- 136.Pillay N. Pathways to school completion for young mothers: Are we winning the fight? SAJCH. 2018;2018(Special Edition):s15–s8. [Google Scholar]
- 137.Guidelines to industry and health care personnel: The regulations relating to foodstuffs for infants and young children, Resolution 911, Pretoria; 2012
- 138.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358: j4008 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.South African National AIDS Council. Let our actions count: South Africa’s National Strategic Plan for HIV, TB and STIs 2017–2022. South African National AIDS Council Pretoria; 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are contained within the manuscript and Supporting Information files.