Abstract
Cardiovascular disease (CVD) is among the leading causes of mortality worldwide. The shortage of donor hearts to treat end-stage heart failure patients is a critical problem. An average of 3,500 heart transplant surgeries are performed globally, half of these transplants are performed in the US alone. Stem cell therapy is growing rapidly as an alternative strategy to repair or replace the damaged heart tissue after a myocardial infarction (MI). Nevertheless, the relatively poor survival of the stem cells in the ischemic heart is a major challenge to the therapeutic efficacy of stem-cell transplantation. Recent advancements in tissue engineering offer novel biomaterials and innovative technologies to improve upon the survival of stem cells as well as to repair the damaged heart tissue following a myocardial infarction (MI). However, there are several limitations in tissue engineering technologies to develop a fully functional, beating cardiac tissue. Therefore, the main goal of this review article is to address the current advancements and barriers in cardiac tissue engineering to augment the survival and retention of stem cells in the ischemic heart.
Keywords: stem cells, biomaterials, nanofibers, tissue engineering, hydrogels, drug screening, cardiac, 3D printing
Introduction
Cardiovascular disease (CVD) is a major cause of deaths in the world. According to the Global Burden of Diseases, Injuries, and Risk Factors 2015 report, there were 17.92 million deaths due to CVD worldwide [1]. The American Heart Association (AHA) estimated that 46.1% of US population will have some form of CVD by 2035 at which time, the total cost associated with CVD will be 1.1 trillion USD [2]. Among CVD in the US, coronary heart disease (CHD) accounts for 43.5% deaths followed by stroke (16.8%), high blood pressure (9.4%), heart failure (9.0%), diseases of the arteries (3.1%), and other CVDs (17.9%). CHD and stroke were found to be first and fifth leading cause of deaths overall in the US [2].
CHD (aka ischemic heart disease) is caused by a buildup of plaque in the coronary artery (i.e. atherosclerosis), which gradually leads to partial or complete blockage of the artery with a concomitant reduction in blood flow to the heart muscle. The consequence can range from angina, shortness of breath, or myocardial infarction (MI) to heart failure in extreme cases [3]. As a result, medical and scientific communities worldwide are investigating strategies to reduce the burden of this disease. A major limitation with the conventional treatment strategies for CHD is that the heart muscle has little or no capability to recover after an episode of cardiac arrest, which ultimately leads to heart failure. Thus, heart transplantation remains the only viable option for treating end-stage heart disease. However, due to the low supply of donor hearts and a high demand for transplantation [2,4], there is an pressing need to develop alternative strategies to treat CHD patients.
Cellular cardiomyoplasty in combination with tissue engineering offers a potential therapeutic option to repair the failing heart. In cell therapy, stem cells are injected into the infarcted heart to replace the damaged cells [5]. A recent progress report by Singh et al., summarized the a importance of variety of stem cells namely, mesenchymal stem cells (MSCs), cardiospheres derived cells (CDC) and cardiac stem cells for myocardial repair [6]. Additionally, these transplanted cardiac stem/progenitor cells can differentiate into variety of cells like cardiomyocytes, endothelial and smooth muscle cells in the ischemic heart. Although stem cells have been successfully delivered into the infarcted region, the main mechanism of action is linked to the paracrine factors released by the transplanted cells, rather than cardiac regeneration [7]. To enhance the therapeutic effectiveness of stem cells, additional support in the form of scaffold/matrix is employed to improve the cell adhesion, viability and tissue regeneration [8]. Thus, nanofiber-based scaffolds and hydrogel-based extracellular matrices could play an important role in enhancing cell viability, retention and regenerative capability of transplanted stem cells in the damaged ischemic heart tissue.
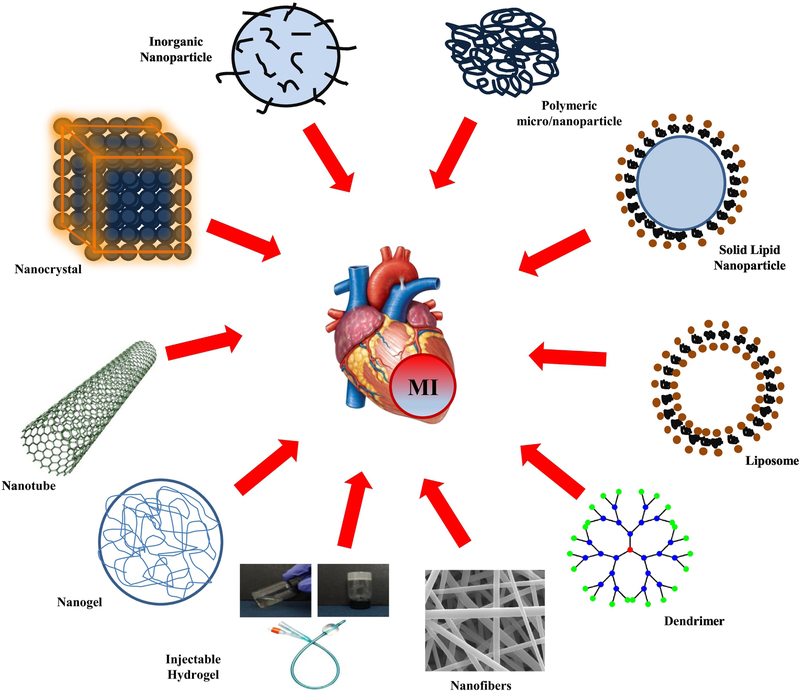
In contrast to cell therapy alone, cardiovascular tissue engineering offers a promising integrative approach to build an artificial heart muscle using a combination of stem cells and biomimetic scaffolds [9]. Recent advances in nanotechnology and biomaterials provide a variety of different approaches to formulate scaffolds for tissue engineering, which include nanofibers [8], metallic nanoparticles [10], hydrogels [11], injectable gels [12], polymeric microparticles [13], nanogels [14], liposomes [15], and dendrimers [16], as shown in Figure 1. Therefore, the main focus of this review article is to address the current limitations for treating CHD and novel tissue engineering approaches for cardiac regeneration as shown in Table 1. Furthermore, we will also discuss the current barriers in cell therapy and cardiac tissue engineering.
Figure 1.
Fabrication of different biomaterials for cell delivery, imaging and scaffold development for cardiac tissue engineering.
Table 1.
Methods for Cardiac Tissue Engineering.
| Tissue Engineering Methods | Properties | Drawbacks | Challenges | References |
|---|---|---|---|---|
| Nanofibers | • Provide natural ECM for cell migration and proliferation. • Aligned structure, provide good mechanical support if they are fabricated with metallic particles. |
• Need special fabrication instrument. • Difficult to get uniform nanofibers sheet. • Need to be implanted-an • Invasive procedure. • Need of toxic solvents |
•To obtain the desired porosity. •Fabricating a thick nanofiber patch. •Oxygen & nutrient diffusion in a thick patch. |
[9,171–174] |
| Hydrogels | • 3D Natural ECM like structure. • Can deliver growth factors. • Easy surface modification. • Versatile design. |
• Need to be implanted • mostly invasive procedure. • Very fast release kinetics |
• Optimizing an optimal non-toxic cross-linkers for hydrogel gelation • Electrical conductance |
[142,144,146–149,152,154,156,158] |
| Injectable gels | • Minimally invasive and • Injected using catheter. • Thermo-sensitive and • pH sensitive properties. • 3D Natural ECM like structure. • Can deliver growth factors. |
• Chance of blocking the vessels due to uncontrolled gelation • Very fast release kinetics |
• Tuning an optimum gelation kinetics. • Electrical conductance |
[73,141,151,183] |
| Decellularization | • Preserved architecture and mechanical properties. • Electrical coupling with the host tissue. |
• Availability of tissues, • Washing step-very critical. • Immune rejection. |
• Possibility of growth factor delivery • Injectability |
[148,162–166] |
| 3D printing | • Can lead to scaffolds with defined geometric dimensions • Surface engineering possible • Improved precision, can be coupled with microfluidics to develop heart-on-a-chip |
• Specialized skills needed. • Maintaining a sterile • setup is not easy |
• Developing a fully • function heart Alignment of cardiomyocytes in a 3D • printing setup |
[168–78,180,182] |
1. The conventional treatments for CHD and their limitations
Conventionally, there are three common ways to treat CHD: pharmacological treatment, surgical interventions with/without medical device implants and heart transplantation. Pharmacological treatments are commonly used to alleviate the early symptoms of angina, atrial fibrillation, peripheral artery diseases, swelling, dizziness and to prevent CHD. Some of the most widely prescribed medicines for the above said complications fall under the category of anticoagulants, platelet inhibitors, cholesteryl ester transfer protein (CETP) inhibitors [17–19]. Additionally, β-blockers are administered to reduce metabolic demands of the heart and to stabilize cell membrane [18], which reduces the risk of ventricular fibrillation (VF).
Medical devices and implants are primarily used to restore the heart functions. For instance, to restore cardiac rhythm, a pacemaker is used. Similarly, to restore blood flow and to reduce the shear stress in an occluded artery, drug eluting stents are used [17]. Surgical interventions like coronary artery bypass graft surgery (CABG) are performed to treat arteriosclerosis [20]. In case of ventricular tachycardia (VT) and VF, an implantable cardioverter defibrillator (ICD) is used to stop the cardiac fibrillation, which prevents scar formation and restores the cardiac rhythm. However, ICDs cannot treat all the cases of VT and VF, which are associated with enhanced mortality and heart failure [24].
Overall, current therapies to treat CHD cannot sufficiently manage the progression and prevention of cardiovascular diseases; especially, pharmacological treatments and medical devices with interventional therapies are not a permanent solution and a number of follow-up surgeries are required. The only remaining option is heart transplantation, to replace the damaged heart with a healthy heart from a donor. However, several complications like immune reaction, organ rejection, immunosuppressive therapy and limited availability of donors hinder the success of heart transplantation. Due to these complications, heart transplantation remains an insufficient methodology and additional strategies are of utmost priority [21,22]. One such strategy is to engineer heart tissues and/or build a whole human heart using a combination of biomaterials and cells of right type. Researchers across the globe are actively working in the area of bioengineering to develop functional human heart tissue. Before we discuss about recent strategies, we will discuss in brief about the inherent challenges in tissue engineering to fabricate a functional cardiac tissue.
2. Challenges in cardiac tissue engineering
Current challenges in cardiac tissue engineering include the following: alignment and adhesion of cells on scaffolds, conduction of electrical impulse between the cells, optimization of scaffold mechanics to support cell mass and facilitate contraction, development of tissue junctions, thickness of the tissue and supply of oxygen [23–25]. It is extremely challenging to replicate the anisotropic structure of human heart tissue, as it requires an intricate, hierarchical control over cell alignment, cell-cell adhesion and electrical conduction. Therefore, scaffolds should be designed to encourage cell adhesion and promote alignment of cardiomyocytes uniformly across the entire scaffold. Furthermore, acquiring a uniform contractility/cardiac rhythm from the seeded cardiomyocytes on a scaffold is very challenging as cardiomyocytes exist in an isolated form and exhibit mismatch with each other due to differences in their adhesion and cell-cell junctions [25]. Consequently, synchronized contractions of cardiomyocytes are critical for their response to electrical impulses and ability to propagate an electric impulse [26,27]. Therefore, for a newly engineered cardiac tissue constructs to respond synchronously with the native tissue requires an impulse generator with the same frequency as that of the existing cardiac tissue [28]. The alignment of cardiomyocytes in the direction of extracellular matrix (ECM) and scaffolds fibers is crucial as the scaffold fibers have to contract and relax along with the cells accompanied with no or minimal phase lag. The highly compact and aligned nature of cardiomyocytes in the myocardium result in cardiac tissue with anisotropic mechanical properties. Their alignment is not unilateral as in skeletal muscle, rather cardiac tissue shows complex patterns of cells that shifts to follow the principal direction of stress [29]. During a cardiac contractility, cardiomyocytes contract in the direction of their alignment and expand radially outward, resulting in their host tissue following a similar pattern. Therefore, it is very challenging to design scaffolds, which could match the complexity, alignment and functionalities of the cardiomyocytes in the native heart tissue [30].
In addition to the above mentioned barriers, the thickness of the engineered cardiac patch is very critical [31]. It is easy to fabricate cardiac patches with the required surface area; however, fabricating a cardiac patch several millimeters thick is very challenging. It is reported that multilayered patches exhibit improved performance (in regards with uniform pulsations) when compared to single sheets of cardiomyocytes[32]. As the oxygen diffusion is limited to a thin sheet of cardiac patch, vascularization may be required for implantation of thick sheets. For instance, a 100 µm thick neonatal cardiomyocyte sheets (4– 6 layers) was subcutaneously implanted into athymic nude rats showed survival for one year along with angiogenesis [33]. In another study, in order to have thickness closer to the normal human diastolic, left ventricular myocardium [34], four 100 µm thick neonatal cardiomyocyte sheets were implanted. However, poor angiogenesis was shown and central necrosis was observed due to insufficient oxygen supply [35]. The supply of oxygen and nutrients to cardiomyocytes deep inside of the engineered patch is a major challenge [8,25] as most cardiac patches depend on diffusion to meet their oxygen demand [25]. Diffusion is not sufficient as there is a 200 µm tissue depth limit for the diffusion of oxygen [34]. Thus, diffusion is only sufficient in in vitro models where a thin layer of cardiomyocytes is in continuous contact with culture media [9,36]. It was reported that even for tissue constructs bathed in culture medium with harmonic or circulatory motion in case of vibratory or rotating bioreactors respectively, diffusion is the only mechanism to control oxygen exchange inside the construct [37–39]. The problem of oxygen supply can be resolved by vascularization of the synthetic cardiac patch via co-culture with endothelial cells and/or release of angiogenesis growth factors [25,40]. While these neovascularization strategies have been widespread in tissue engineering, problems remain with inosculation of the in vitro formed vessels with the host vessels. Moreover, this requires establishing perfusion with multiple blood arteries and veins, and maintenance of co-culture environment, in addition to coupling of electromechanical beating with host tissue [41]. To gain these functions, external electric stimulations and use of electro-conductive scaffold materials are required to improve the involuntary and synchronized beating of synthetic heart tissues [27,42,43]. Attaining precise control of all of these factors and optimizing them with native host tissue is very challenging and efforts continue in all these fronts to develop fully functional artificial heart constructs.
The other key challenges are the choice of right stem cell sources and their maturation aspect and incidence of cardiac arrhythmia after transplantation. We shall discuss briefly about these issues in the following paragraphs.
2.1. Choice of stem cell sources and their maturation aspect for cardiac repair
The typical choice of donor cell sources for cardiac tissue engineering are: 1) Somatic muscle cells including fetal/neonatal cardiomyocytes and skeletal myoblasts. 2) Myocardium generating cells: Embryonic stem cells, pluripotent stem cells, adipose derived stem cells, bone-marrow derived stem cells and cardiac progenitor cells, and 3) Angiogenesis-inducing cells such as fibroblasts and endothelial progenitor cells [44,45].
A growing body of evidence points toward the fact that the maturation stage of the stem cells play a vital role in their survival and host tissue integration [46–53]. An ideal maturation stage required for stem cell transplantation and cardiac tissue engineering studies is highly debatable. In case of cardiomyocytes, early stage cardiomyocytes (fetal/neonatal CM) were considered better candidate than fully matured cardiomyocytes because of their better survival in vivo [54]. However, there are very few studies comparing the efficacy of these cell sources. One recent comparative study by Fernandes et al [55] concluded that the human embryonic stem cells derived cardiomyocytes (hESC-CMs) and mesodermal cardiovascular progenitors (CPCs) performed better than the human bone marrow mononuclear cells (hBMMNCs), which is one of the most widely tested cells in the clinical trials. Interestingly, this study did not observe any difference in cardiac repair efficacy when hESC-CMs (more-matured) and CPC (less-matured) were compared [55]. However, this study had one limitation, it was not well established how matured hESC-CMs (with respect to their structure, sarcomere size, gene expression and electrical conductance), when compared to the normal adult human cardiomyocytes. Similarly, another study demonstrated that induced pluripotent stem cells derived cardiomyocytes (iPSC-CMs) were typically immature in nature, when compared to adult cardiomyocytes [56]. Furthermore, these cells differ in terms of their structure/function, fetal gene expression, uneven morphology, dependence on glycolytic metabolism and poor contractile properties, which act as a critical barrier for in vivo survival of stem cells and host tissue integration [48,57–59]. Nonetheless, numerous studies have demonstrated various in vitro strategies to enhance maturation of cardiomyocytes by biochemical stimulations (using cytokines, hormones and other small molecules), biophysical stimulations (electric current and mechanical) and by providing necessary 3D microenvironment [48,51–53,56,60–62]. Overall, there is a paucity of information about desired maturation stage for successful engraftment of transplanted stem cells into the ischemic heart and further studies are warranted to compare transplantation of mature vs immature cells (human IPS-derived cardiomyocytes/CPCs) to establish the therapeutic efficacy of the stem cells for cardiac repair.
2.2. Transplantation of stem-cell patches and incidence of cardiac arrhythmias
The incidence of cardiac arrhythmias after transplantation of ESC/iPSC-derived cardiomyocytes cannot be ruled out [63–65]. Interestingly, arrhythmias were predominantly observed only in non-human primate models [64,65] and not in small animal models [66–68]. In the pigtail macaques MI model, Chong et al. demonstrated significant remuscularization of the infarcted heart upon intramyocardial injection of one-billion hESC-CMs. However, they also observed non-fatal ventricular arrhythmias in hESC-CM engrafted primates [65]. In another study by Liu et al., similar graft-associated ventricular arrhythmias were observed when ~750 million hESC-CMs were intramyocardially injected in a non-human primate model [64]. In contrary, it has also been reported that the cells grown on the patch showed a better host-tissue integration. For instance, a study by Ye et al. demonstrated that trilineage (2 million cells each; hiPSC-derived cardiomyocytes, endothelial cells and smooth muscle cells) 3D fibrin patch loaded with insulin growth factor (IGF)-encapsulated microspheres resulted in significant improvement in the cardiac function. Furthermore, this study used a low dose of hiPSC-CMs (~100 fold lower than that used by Chong et al [65]) and did not observe any cardiac arrhythmias [69]. Similarly, another study demonstrated that the cells grown on a patch caused a lower frequency of arrhythmias since the cells grown on a patch have structural and functional similarity to the host cardiac tissue [61]. A recent study by Shadrin et al. developed a large functional 3D patch called “Cardiopatch” cultured with hiPSC-CMs on a hydrogel and matured for up to 5 weeks. Using this approach, they fabricated highly functional clinically relevant cardiac patch of large dimension (36 mm × 36 mm). Additionally, these cardiac patches (0.5 mm, 7 × 7 mm) implanted onto rat heart following an MI showed improved cardiac function, with no incidence of cardiac arrhythmias [70]. Moreover, the cells seeded on the cardiac patches can be tuned to match the host- tissue structure and dynamics through designing a highly conductive non-isotropic cardiac patch. The conduction property of the patches can be enhanced by fabricating the patches with conducting materials like gold nanowires [71], carbon nanotubes [72], silica nanomaterials [73] or other conducting polymers [74,75]. These cardiac patches can also be functionalized to have growth factors or other chemotactic agents [69,76], which can attract host cells within the patch and assist in superior tissue integration and electrical conductance. Menasche et al. in a recent clinical safety study demonstrated the successful transplantation of hESC derived cardiac progenitor cells in six patients having severe ischemic left ventricular dysfunction. The hESC derived cardiac progenitor cells co-expressing stage specific embryonic antigen-1 (SSEA-1) and cardiac transcription factor ISL-1 markers were specifically generated, encapsulated in fibrin patch and transplanted into patient’s heart through epicardial delivery (median dose of 8.2 million cells) during a coronary artery bypass procedure. One patient died immediately after the surgery due to treatment-unrelated comorbidities. This study did not observe any tumors or arrhythmias in all of the remaining patient’s follow-up for one year, which was the primary end point of their experiment. All treated patients showed improvement in their systolic motion, although one patient died due to heart failure after 22 months. This is the first study to demonstrate the safety and efficacy of hESC based cell therapy in an encapsulated fibrin patch [77].
In summary, we need to consider several factors to see beneficial effects of cardiac cell therapy and it is very crucial to examine the maturation stage of cardiomyocytes, and the total number of cells before implantation into the ischemic heart. Moreover, combination of a multiple lineage cells in a patch could have a significant clinical impact on the therapeutic outcomes for treating failing heart. Other considerations might be using a 3D conductive cardiac patch, which could improve the electrical conduction with the host-tissue and decrease the incidence of cardiac arrhythmias.
3. Modern trends in cardiac tissue engineering
3.1. Scaffold-free cardiac tissue engineering approaches
After an MI, there is loss of up to one billion cardiomyocytes in the infarct zone. Thus the idea of myocardial regeneration by cell injection has emerged and was tested in several in vivo studies over the past two decades [54]. Scaffold-free approaches involve injection of cells either in 2D or 3D structures onto the infarct zone[78]. Scaffold-free approaches involve fabrication of tissues by mimicking the tissue developmental processes, which follows a pattern of sequences like cell condensation, proliferation, differentiation, ECM production and tissue maturation. Furthermore, self-organization and self-assembly are two distinct categories reported as scaffold-free tissue engineering. Self-organization is a process in which cellular organization is imparted via an external energy or force is given to the system, which includes bioprinting and cell-sheet engineering. Cell aggregates are often formed in culture by application of rotational force to suspended cells or by utilizing a non-adherent cell culture conditions, which can be categorized as self-organization. Due to this rotational motion, these culture experience improved diffusion and nutrient/gas exchange when compared to static cultures. In contrast, self-assembly does not require outside forces to form tissues. The tissue maturation follows a track which is identical to that of native tissue development, where cellular interactions and coming together (e.g. high-density cell culture) are directed by spontaneous minimization of free energy, followed by tissue-specific extracellular matrix (ECM) production via maturation process to form functional tissue [79].
These approaches of self-organization and self-assembly involve two methods which includes engineering cell sheets and spheroids respectively [80,81]. Cell sheet engineering technique involves generation of sheet of confluent cells, which can be stacked on top of one another to develop a thick cardiac patch. Cell sheets are developed by seeding cells on a culture dish coated with temperature responsive polymeric layer [80]. Once a complete sheet is formed, the culture dish is placed in a chamber at reduced temperatures (below the polymer’s LCST temperature) which alters the hydrophobicity of the polymer substrate and reduces the adhesion between cells sheet and the cell culture dish [82]. The sheet of cells is removed and stacked one another to assemble into a myocardium-like tissue. Vascularization in this tissue can be attained by sandwiching endothelial cell sheet between two cardiac cell sheets. Previously, an in vitro pre-vascularized 3D tissue construct was reported by using cell sheets developed with fibroblast and endothelial cells [83]. Similarly, cell sheet with aligned cells in specific direction to develop anisotropic tissue construct was produced by using a patterned substrate [84]. These cell sheets can be configured into different shapes such as tubes or patches to attain the desired mechanical strength and functions in vivo without any synthetic polymer scaffold [85,86].
The second scaffold-free technique of tissue engineering works through the generation of multicellular masses of cells called spheroids [87,88]. Uniform embryoid bodies are obtained by seeding cells in microwells formed in containers comprised of polymers like PDMS and poly(ethylene glycol) (PEG) [89,90]. The physiology and morphology of these spheroids are based on the nature of seeding cells type, their density and the dimensions of the container (size, shape, volume). These resulted spheroids/embryoids have the ability to differentiate into variety of cell types including cardiac cells, endothelial cells and nerve cells [91–93]. In a recent study, embryoids with a diameter of 450 μm demonstrated enhanced cardiogenesis, which was confirmed by increased expression of sarcomeric α-actinin and cardiomyogenic genes. Embryoids can be an important tool to study initial phase of embryonic development and can mimic the cardiogenesis in vitro [94].
Multicellular spheroids are mostly used in scaffold-free tissue engineering due to their ease of handling and fast growth [95,96]. Various cell culture techniques utilized to prepare these multicellular spheroids include non-adherent cell culture plates, suspension cell culture, hanging drops and microfluidics devices [97,98]. These spheroids offer extra tissue regeneration capacity due to their intense cell-cell interactions and native ECM structure similar to 3D scaffold [99]. Furthermore, they vascularize rapidly in vivo due to their high vasculogenic and angiogenic potential [100]. Recently, usage of spheroids for computer-aided tissue engineering technologies like 3D bioprinting have been reported. A complex computer-aided design (CAD) with full control on assembly of spheroids is now being used to develop fully functional 3D construct [101].
Although scaffold free techniques offer advantages such as high levels of cell-cell interactions and production of native ECM, this strategy has limitations and further studies are warranted to optimize these techniques. Although, scaffold-free tissue engineering techniques showed success to some extent, they lack the native myocardium arrangement which is required to perform cardiac rhythmic contraction in vivo [102]. Other limitations include weak mechanical strength of its individual blocks and requirement of high initial cell density for spheroids [103]. In order to overcome limitations of mechanical strength, initial cell density, cells adhesions and ECM depositions in scaffold free tissue engineering, scaffold-based tissue engineering approaches were developed as described below.
3.2. Scaffold based cardiac tissue engineering approaches
Cell therapy studies for MI treatment resulted in poor survival of stem cells in the ischemic heart and majority of the transplanted cells were lost within hours to days after transplantation. Therefore, attempts have been made to overcome this challenge by using scaffolds to enhance the survival and retention and of transplanted stem cells in the infarcted tissue. These materials can be synthetic/natural in origin or a combination of both, they can further be modulated by surface modifications or by conjugating them with other required biological molecules for stimulating survival and proliferation of cells. These scaffolds can be seeded with autologous/allogeneic cells and transplanted onto the damaged site to promote tissue regeneration [104].
A myocardial tissue is dynamic in nature with continuous exposure to forces which alternately contract and relax the tissue. Thus, an ideal scaffold for cardiac tissue engineering should possess excellent mechanical properties and electrical conductivity to perform normal physiological functions of heart [36,105]. Over a period of past two decades, a variety of scaffolds with high mechanical stability, electrical conductivity and biodegradability have been developed. Table 2 & 3 lists most widely used biomaterials (natural and synthetic) for cardiac tissue engineering with their properties. Materials utilized for cardiac tissue engineering include biocompatible natural polymers like gelatin, collagen, alginate, chitosan, fibrin, and hyaluronic acid [51,106–109]. Synthetic biodegradable polymers such as PGA, PCL, PLA, and PLGA have also been widely used for cardiac tissue engineering [8,93,106,110]. The major advantage of synthetic polymers as compared to natural polymers is that they can be synthesized in a controlled manner and their properties can be fine-tuned with respect to the desired applications. For instance, PLGA degradation rate can be tuned by varying the ratio of glycolic acid to lactic acid [8]. This control of synthetic scaffold makes it possible to initially provide required cells support and then slowly degrade with the development of tissue and finally lead to the development of fully functional tissue. Furthermore, there are reports in which cell adhesion domains, biological cross linking proteins/peptides and enzymatic cleavage site are added to enhance cell attachment, migration, and to naturally dissolve the scaffolds [111].
Table 2.
Natural polymers for cardiac tissue engineering.
| Natural Polymers | ||||
|---|---|---|---|---|
| Polymer | Properties | Scaffold Types | Functional Outcomes | References |
| Alginate | Polysaccharide, Biocompatible, Biodegradable. | Nanofibers, Hydrogels, Injectable gels | • Improved cardiac function, • ventricular wall thickness, • angiogenesis, cell survival, and decreased fibrosis and inflammation in the infarct zone. • Sequential delivery of VEGF-A165 and PDGF-BB, increased vessels • density and improved cardiac function. |
[156] |
| Chitosan | Polysaccharide, Biocompatible, Biodegradable. | Nanofibers, Hydrogels, Injectable gels | • Enhanced cardiac differentiation of adipose derived stem cells (BADS Cs), increased angiogenesis. | [154,155] |
| Hyaluronic acid | Glycosaminoglycan, part of ECM, Support cell migration and proliferation | Nanofibers, Hydrogels, Injectable gels | • Increased cell adhesion and proliferation | [112] |
| Collagen | Protein, ECM main component. | Nanofibers, Hydrogels, Injectable gels | • Increased maturation of hESC-CMs. Increased cardiac re-modeling and angiogenesis. • Cardiac differentiation of hESCs without the addition of soluble factors. |
[51,107,112,123,148,151,152,175] |
| Gelatin | Peptide, Produced from collagen hydrolysis. | Nanofibers, Hydrogels | • Increased cell attachment, proliferation, differentiation, alignment and contraction. • Enhanced paracrine mechanisms such as tissue preservation and neovascularization. |
[88,115,142,178] |
| Fibrin | Protein, Water insoluble. | Nanofibers, Hydrogels | • Increased mechanical coupling of E CM. Improved cardiac function, ventricular wall thickness, angiogen esis, cell survival, decreased fibrosis and inflammation. | [116,144] |
| Cellulose | Polysaccharide, Strong mechanical properties. | Nanofibers, Hydrogels | • Increased anisotropy. | [127] |
Table 3.
Synthetic polymers for cardiac tissue engineering
| Synthetic Polymers | ||||
|---|---|---|---|---|
| Polymer | Properties | Scaffold Types | Functional Outcomes | References |
| PLGA | FDA approved co-polymer, Biocompatible and biodegradable. | Nanofibers, Hydrogels, Microfluidics devices | Increased cardiomyocyte proliferation, decreased LV remodeling, and enhanced cardiac function. | [8,61] |
| PCL | Semi-crystalline and biodegradable polymer. Sustained drug delivery. High mechanical stability to support cardiac contractions. | Hydrogels, Nanofibers, Nanoparticles, Microfluidics devices | Increased alignment and maturation of cardiomyocytes. | [13,122] |
| PNIPAAM | Thermo-responsive, LCST can be tuned to 37°C. On-demand drug delivery. | Nanofibers, Nanogels, Hydrogels | Increased maturation and vascularization. | [14,153] |
| Gold Nanoparticles | Improved electrical conduction and enhanced coupling of tissue construct with host tissues. | Nanofibers, Hydrogels | Enhanced cardiomyogenic differentiation, enhanced MSC proliferation and differentiation into cardiomyocytes, increased contractile proteins synthesis and help to achieve typical cardiac phenotype. | [114,125,135] |
| Polypyrrole | Stimulating cardiomyocytes, regeneration of infarct myocardium and cardiac defects | Nanofibers | Promote cell attachment, proliferation, interaction, and expression of cardiac-specific proteins. | [132] |
| PVA | Water soluble, transparent, biodegradable and biocompatible. Co-culture membrane and excellent coating material. | Nanofibers Hydrogels | Stabilized cardiac function and attenuated dilatation. | [126,127] |
| CNT | Stimulating the proliferation of human cardiomyocyte, support improved alignment of cardiomyocyte, higher expression of cardiac-specific genes, in vitro cardio-myogenesis. | Nanofibers, Hydrogels | Enhanced cardiac differentiation and increased synthesis of contractile protein (cardiac myosin heavy chain). | [128,129] |
To develop functional cardiac tissue, scaffold must provide sufficient porosity for cell ingrowth, an architecture that aligns its cellular constituents, mechanical properties sufficient to facilitate surgical implantation, maintain/encourage the desired cell phenotype, and suitable surface characteristics to encourage firm cell adhesion. In order to fabricate such a scaffold, hybrid or composite scaffolds comprising of both natural and synthetic materials are currently developed. For instance, semi-interpenetrating networks of photo-crosslinkable methacrylated hyaluronic acid (MeHA) and collagen scaffold have been fabricated to attain better mechanical strength than collagen or MeHA alone, to maintain biocompatibility and cell adhesion [112]. Biomaterials have great potential to treat both myocardial conduction (AV block/VA) and contraction disorders. Polymers that exhibit high electrical conduction or composite materials with metallic nanoparticles can restore conduction pathways blocked either by ischemic heart diseases or by other physiological reasons. Extracellular matrix (ECM)-like structures made using conductive polymers can promote cell survival while establishing electrical bridges between the cells [71]. Researchers are trying to develop biological pacemakers, which can act as a permanent pacemaker. This proposed biological pacemaker will be able to respond to endogenous stimuli, which is triggered during increased/decreased cardiac activity [113].
3.2.1. Synthetic Scaffolds for Tissue Engineering
3.2.1.1. Nanofibers
Nanofibers can be defined as fibers that mimic the natural architecture of the extracellular matrix (ECM) and promotes a platform for cell adhesion in a 3-D microenvironment. They are fabricated using different methods, which include; electrospinning, self-assembly, drawing, template synthesis, and thermal-induced phase separation [114–116]. Nanofibers produced by these methods have a morphological resemblance to natural ECM [8] and provide special structural support for cell attachment, migration, and differentiation. The morphology, topology, and geometry of nanofibers are important for proper transport of biophysical factors and thus to maintain the functional cardiomyocyte physiology. Aligned nanofiber scaffolds made using Poly(L-lactic acid) (PLA) blended with polyaniline (PANI) have been reported to have enhanced conductivity with better beat frequency and good cell viability. The H9c2 cardiomyoblasts grown on these scaffolds were also found to have improved maturation and fusion index as well as better spontaneous beating due to the ECM mimicking properties of this scaffold [117]. To understand the mechanism by which cells interact with nanofibers, Balashov et al., performed a recent study to understand a single cell-single fiber interaction. They observed that cardiomyocyte with its cytoplasm, covers the entire nanofiber or nanofibers bundles in certain cases, forming a sheath like structure, which enables the cell attachment over the entire fiber [118].
There are various electrospinning techniques to obtain nanofibers with different dimensions, morphologies and porosity to most closely match natural ECM as shown in Figure 2. Furthermore, high level of porosity and improved oxygen supply to cardiomyocytes was observed in bioengineered engineered scaffolds. PCL based nanofiber scaffolds are commonly used in tissue engineering applications, PCL nanofibers with its surface modified with gelatin coating increases its hydrophilicity, thereby enhancing cardiomyocyte attachment and proliferation [110]. In another study, polyethylene glycol (PEG), carboxylated PCL (CPCL), and PCL hybrid scaffold has been reported to develop embryoid bodies (EBs) from murine ESCs (mESCs). Additionally, this scaffold also facilitated the differentiation of mESCs towards functional cardiomyocytes. It was also found that the enhanced CM differentiation was directly linked to PCL-3D nanofiber scaffold and was triggered by the stimulation of canonical Wnt/β-catenin signaling during early differentiation [119]. A composite Polypyrrole (PPy), PCL and gelatin-based nanofibers were fabricated by mixing different concentrations of polypyrrole (PPy) to PCL/gelatin (PG) solution and tested for cardiac tissue engineering. This study showed a significant enhancement in attachment, proliferation, differentiation, and interactions of cardiomyocytes [120].
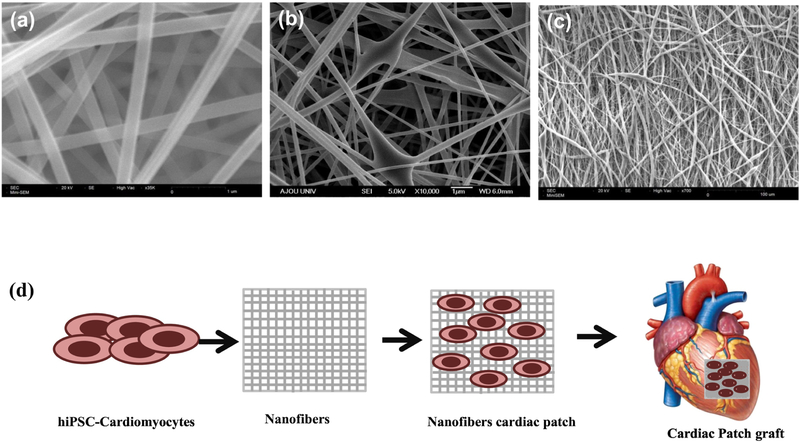
Figure 2.
Electrospinning techniques of nanofibers for cardiac applications. (a) Uniform nanofibers-having uniform size distribution (b) Hybrid-combination of nano and microfibers (c) Aligned nanofibers (d) Schematic of cells incorporated nanofiber based cardiac patch.
As discussed earlier, the alignment of nanofibers in scaffold is also important for cardiac tissue engineering as shown in Figure 3 [121]. Han et al., investigated the effect of aligned nanofibers on maturation of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs). The aligned nanofibers sheet showed highly synchronized sarcomeric organization, when compared to controls. The morphology of cells on aligned nanofibrous scaffold was also uniform and cell size was larger than controls (864 ± 37 μm2 versus 745 ± 37 μm2). The hiPSC-CMs grown on aligned nanofibrous scaffold showed an increase in cardiomyocyte associated genes and protein expression as well as rapid response to treatment with cardiovascular drugs. However, modest changes were observed in regard to structural maturation as well as calcium transient were much slower than the control cells [122]. This study demonstrated that the aligned nanofibrous scaffolds despite of its structural advantages, did not show any positive effects on the maturation of hiPSC-CMs. Similarly, Joanne et al., showed that collagen based nanofibrous scaffold seeded with hiPSC-CMs attenuate dilated cardiomyopathy (DCM) mouse model. At two weeks of patch implantation, the cardiac function decreased in control group (cell-free scaffold) while it was maintained in the experimental group. The main reason attributed for this difference was due to an increased angiogenic activity of the cell-loaded scaffold, when compared to the control. This study confirmed the potential use of hiPSC-CMs loaded nanofibrous scaffold to stabilize the DCM condition [123].
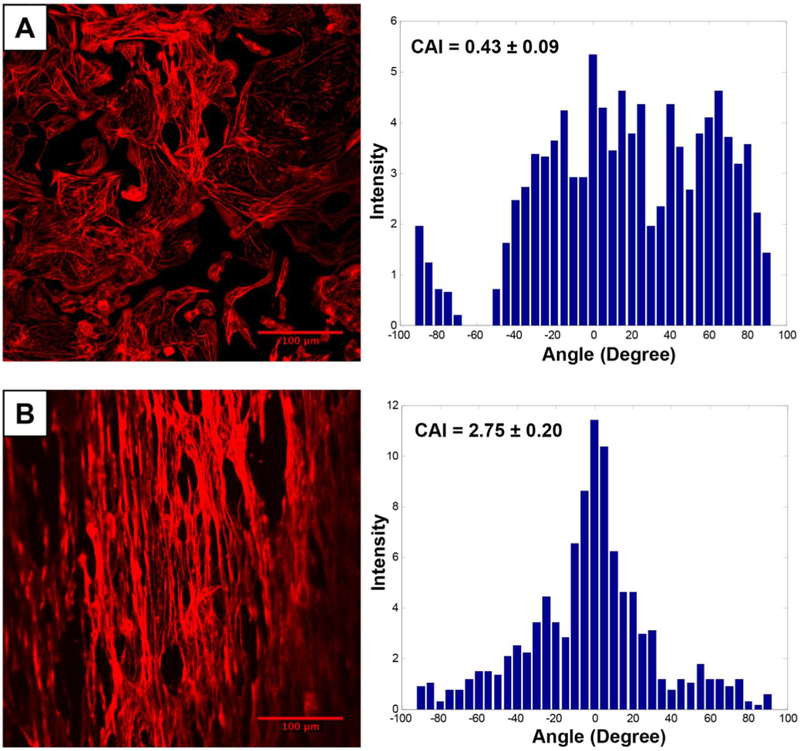
Figure 3.
Fluorescence imaging of hiPSC-CM labeled with phalloidin dye on a PLGA nanofiber scaffold. Cardiomyocytes seeded on the aligned nanofiber scaffold were aligned and elongated in the direction of the nanofiber, when compared to cells seeded on a flat cell culture plate. Alignment of cells on the scaffold was further confirmed by enhanced cell anisotropy index (CAI). Reprinted from Khan et al., PLOS One [121].
In another study, hiPSC-CMs were also used to develop cardiac tissue-like constructs (CTLCs) by seeding them on a thin layer of poly (D, L-lactic-co-glycolic acid) (PLGA) nanofibers. These CTLCs exhibited rapid host electrical integration with coupling times of about 81 ± 49 min. The results from this study showed that the CTLC could integrate with the host CM sheets and help in establishing rapid electrical coupling between the interrupted areas in the heart tissue, suggesting potential role of CTLCs in healing re-entrant cardiac arrhythmias [61]. They also tested and confirmed that CTLCs can be a better in vitro cardiac tissue model than the normal 2D culture [61]. Similarly, a recent study from our lab demonstrated alignment of hiPSC-CM on a PLGA aligned nanofiber scaffold as shown in Figure 3. Cardiomyocytes seeded on this scaffold showed well organized sarcomeres and shorter calcium transients at 2 weeks of culture [121]. The successful integration of these scaffolds relies on their electrical coupling with the host myocardial tissues and they must facilitate the cell bundle extension to establish that connection [124]. Hybrid nanofibrous scaffold with metallic particles and nanowires are reported to increase cell attachment, migration, proliferation, and electrical coupling with the host cells [72,125–129]. Coiled fiber scaffold with gold nanowire has been reported to enhance cardiac contraction, beating rate and lower excitation thresholds. Gold nanowires help the cardiomyocytes to interact with each other and establish conduction bundles. Cardiac tissue grown on this hybrid scaffold showed anisotropic conduction of electrical signals with strong contraction force. These coiled nanofibrous scaffold with nanowires was also reported to have better mechanical strength. These fibers stretch and re-coil with the heart muscle and reinforce them with required mechanical strength for continuous rhythmic movements and conduction of impulses [130].
Fabrication of electrically conductive scaffolds having suitable mechanical strength, which can induce proper assembly of functioning cardiac tissue is a key challenge in the tissue engineering of heart [4]. Both natural and synthetic materials have their own limitations if they are used alone to fabricate scaffolds. The natural polymers suffer from low mechanical strength and immune reaction, while synthetic materials have issues of biocompatibility, biodegradability and low cell adhesions [5–8]. Therefore, hybrid nanofibrous scaffold made of biocompatible, biodegradable polymers and metallic nanoparticles help to overcome the shortcoming of individual material scaffold [72,131,132]. For instance, to develop a scaffold with robust mechanical properties, carbon nanotubes are combined with biocompatible polymer, chitosan, and this scaffold showed efficient electrical conduction with a conductivity of 0.25 ± 0.09 S/m and superior mechanical strength to support cardiac tissue engineering. Furthermore, the carbon nanotubes impregnated chitosan scaffold was highly porous and had an elastic modulus of 28.1 ± 3.3 kPa, which was similar to rat myocardium. This study confirmed that the introduction of conductive materials in nanofibrous scaffold increase their potential to mimic natural ECM especially in terms of their electrical conduction [42]. Further step ahead, small molecules like peptides [133] and functionalized gold nanoparticles were integrated in nanofibrous scaffold of polymethylglutarimide (PMGI), these gold nanoparticles were functionalized with adhesive peptides to enhance the cell attachment. Additionally, these scaffolds were also found to promote the differentiation of hiPSCs towards cardiomyocytes [134]. Gold-nanoparticles (AuNP) have also been introduced in collagen nanofibrous scaffold to develop a hybrid scaffold (AuNP–Col). Interestingly, no differences were observed in the elastic modulus between the AuNP-Col and collagen only scaffold. However, they observed a significant difference in the local nanoscale stiffness between them, which might have contributed to the AuNP-Col’s improved organization of cardiomyocyte and intercalated discs (ID) assembly [135]. In another study, the scaffold moduli were found to influence hiPSCs differentiation into cardiomyocytes. When maturation of hiPSC-CMs over two scaffolds: Matrigel coated PDMS membranes and matrigel coated glass slide were compared, it was found that the action potentials was two-fold higher for cells grown on the low modulus PDMS membrane. However, the obtained action potential values were found to be just 57% of the standard in vivo value [136]. Robust iPSCs differentiation into cardiomyocytes was reported through trigger of Wnt/Catenin signaling via inhibition of GSK3 in iPSCs grown on gelatin-coated PCL nanofiber scaffolds [110].
Although nanofibers-based scaffolds have tremendous potential, implantation of these scaffolds in the infarct heart requires an invasive surgery. Therefore, hydrogels are currently being explored to mimic ECM for survival and retention of transplanted stem cells in the infarct heart. In the below sections, we will discuss in detail various hydrogel-based strategies for cardiac tissue engineering.
3.2.1.2. Hydrogels
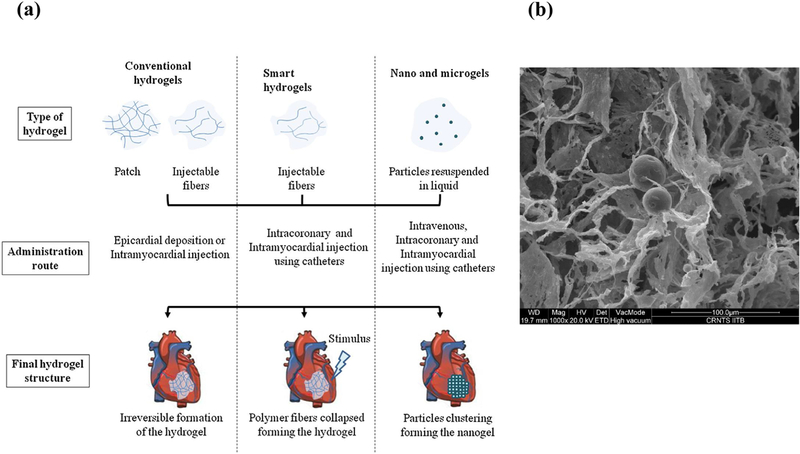
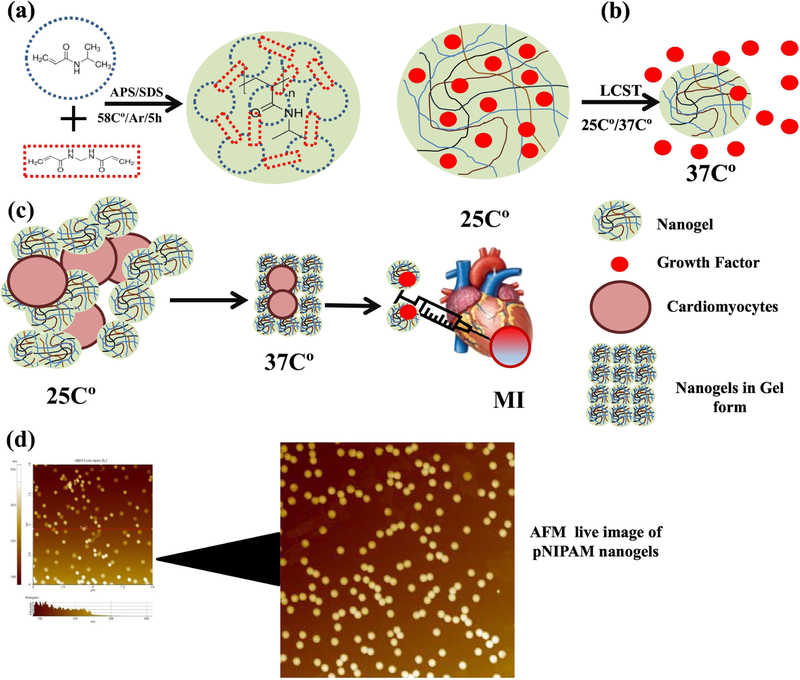
Hydrogels are three-dimensional hydrophilic polymeric networks, which are cross-linked by physical or/and chemical means and that can swell upon interaction with water [11]. They typically have a semi solid/gel nature with very large content of water that mimics the natural ECM. Hydrogels can be fabricated using different polymers and their surface can be modified easily by the addition of co-monomers or cross linkers [14]. There are variety of approaches presented in the literature to use hydrogels in tissue engineering applications using natural and synthetic polymer-based hydrogels for cell delivery, synthetic peptide-based hydrogels to improve cell delivery [137,138], decellularized-heart derived hydrogels to enhance endogenous cardiac repair [139], ECM peptide-derived hydrogel for promoting endogenous cardiac repair and growth factor-releasing hydrogels for improved recovery from injury [140]. Hydrogels are typically used in two different forms: bulk/conventional hydrogels and injectable hydrogels/gels as shown in Figure 4a [141]. Bulk hydrogels or interchangeably known as hydrogels, which are pre-formed ex situ as a semi solid/gel structure and are typically implanted at the site [142]. On the other hand, Injectable hydrogels, are hydrophilic materials, which are in liquid form before injection and transform into a gel in the infarct region in response to change in physiological stimuli such as temperature/pH. The main advantage of these injectable gels is that they can be delivered to the target site through a minimally invasive procedure via catheter as shown in Figure 5 [143].
Figure 4.
(a) Hydrogel types and their delivery modes. Reprinted with permission from reference [143] (b) An example of composite injectable gels which has PCL-micro particles embedded in an injectable Chitosan hydrogel.
Figure 5.
(a) Nanogels fabrications, (b) biological molecules delivery from pNIPAM nanogels based on its LCST (c) Nanogels loaded with cardiac stem cells for MI treatment, (d) AFM images of pNIPAM nanogels.
In tissue engineering applications, hydrogels play a vital role in cell attachment, proliferation and delivery of angiogenic growth factors [143,144]. In recent years, hydrogels have been used for a wide variety of cardiac applications. For instance, they are used to deliver vascular growth factors like VEGF, for enhanced angiogenesis inside the engineered cardiac patches, and to guide the alignment of cardiac cells to improve their functions [11,143,145]. In case of drug delivery applications, these hydrogels are combined with micro/nanoparticles, to form a composite hydrogel system as shown in Figure 4b [146–148]. These composite hydrogels can deliver multiple biomolecules for longer duration in a controlled manner [149]. Some of the most commonly used hydrogels include alginate, matrigel, collagen, gelatin, chitosan, hyaluronic acid, fibrin, poly(2-hydroxyethyl methacrylate) (PHEMA), poly vinyl alcohol (PVA), PLGA, poly(Nisopropylacrylamide) (pNIPAAM), and poly(ethylene glycol) (PEG) [143,150,151]. These materials are used alone or in combination with other polymers and cells. Furthermore, hydrogel platforms can be further augmented by incorporation of biological molecules, which act as a cross-linker for imparting biodegradable properties to the hydrogels [11]. Carbon nanotubes (CNT) can be combined with collagen hydrogels to enhance the electro-mechanical properties of the hydrogel and to improve cells viability of cardiac patch [152]. Additionally, implantation of cell-free hydrogels have shown to improve cardiac function [11]. Hydrogels fabricated using pNIPAAM, a temperature-sensitive material, hydroxyethyl methacrylate-poly (trimethylene carbonate) and acrylic acid (AAc) was injected into infarct heart showed decreased left ventricular dilation and improved cardiac function. Similarly, pHEMA plastic-reinforced gels delivered in a canine model controlled the inflammation for one year and showed its potential as pericardial substitutes [11].
Hydrogels in nano-size are called nanogels, they can be used with nanofiber as hybrid materials for the development of artificial tissue engineered constructs [143]. A recent study demonstrated that cardiac stem cells encapsulated in the nanogels (pNIPAM-AA) showed improved cell retention and decreased scar formation. Furthermore, the combination of cardiac stem cells and nanogels did not elicit immune reaction in mouse and pig MI model [153]. Similarly, chitosan based hydrogels also reportedly improved the healing after MI [154,155]. Though the clinical trials of biomaterials-based product for cardiac tissue engineering are rare, a recent clinical study on the alginate-based hydrogel along with standard medical therapy (SMT) to treat patients with advanced heart failure. Six months follow-up results showed that alginate-hydrogel is more effective in improving subjects exercising ability and as compared to standard medical treatment alone [156]. This clinical study suggests further potential clinical applications of hydrogels for cardiac repair. Decellularized heart matrix (decellularized ECM) and collagen type I hydrogels are reported to differentiate human embryonic stem cells (hESCs) to cardiomyocytes without addition of growth factors. At two weeks after cell-seeding, improved contractile function and increased mRNA (cTnT) protein expression was observed in embryoid bodies. Therefore, the ability of native ECM-hydrogel for inducing hESCs cardiac differentiation in the absence of any soluble factors makes it an ideal candidate for cardiac repair applications [148].
On the other hand, Injectable gels are much more attractive candidates than the hydrogels alone as they are easily translatable and can be delivered with minimally invasive delivery strategies. Smart polymers, which respond to either extrinsic or intrinsic stimuli as mentioned before are explored as an injectable gelling system. The following are the list of various polymers (both natural as well as synthetic) which are used as injectable gels: alginate, chitosan, hyaluronic acid, matrigel, decellularized extracellular matrices, polyethylene glycol based copolymers, pNIPAAM based copolymers, Poly (2-hydroxyethyl methacrylate) (pHEMA), Poly(glycerol sebacate) (PGS), and self-assembling peptides. However, the main challenge in using these injectable hydrogels is the difficulty in tuning their viscosity, fluidity and gelation kinetics, which could lead blockade of blood vessel [143,157].
A recent study by Li et al., addressed the problem of hydrogel in blocking vessels by controlling the gelation time of hydrogel. PAA, HEMAoTMC, MA-PEG, and NIPAAM were combined and polymerized to synthesize Poly (NIPAAm-co-PAA-co-HEMAoTMC-co-MA-PEG) hydrogel with free radical polymerization method. This smart polymer behaves like liquid at physiological pH of blood (pH 7.4) and behave like gel on pH of infarcted heart (6–7). As this hydrogel behaves like liquid at physiological pH of blood and gels at pH of an infarcted heart, it can be delivered safely to the infarct heart using a catheter. Furthermore, this study also demonstrated successful loading, delivery and differentiation of cardiosphere-derived cells (CDCs) in this hydrogel. CDCs were viable in this hydrogel for over 7 days period and increased maturation of these cells into cardiac cells was also observed [158]. In another study, hydrogel-molding method was combined with dynamic culture to develop an in vitro human cardiac tissue maturation platform (cardiopatches) without exogenous stimulation. In this method, human fibrinogen-based hydrogel-cells combination was added on to a PDMS mold (9×9mm) with a Nylon frame and it was crosslinked using thrombin. The cardiopatches thus obtained were removed from the mold and cultured on a 12-well plate with a rocking platform in presence of cardiac medium containing aminocaproic acid to prevent fibrin degradation. Using multiple hiPSCs cell lines, it was shown that cardiopatches exhibit both electrical and mechanical properties similar to those of the adult human myocardium. Additionally, the scalability of the technique is shown by the developing a patch with a size of 4 × 4 cm that is clinically relevant, maintaining maturation and functional properties [70]. Recently, a cylindrical heart tissue was developed by using free-floating dynamic cell culture. The resulted cardiac tissue mimicked the physiology and maturation as that of an adult cardiac tissue [159]. Although these laboratories-generated scaffolds have some success, they are not found to have the natural architecture as well as complex signaling molecules as that of natural cardiac ECM. In order to emulate these properties in the scaffolds, decellularized scaffolds are developed, which we shall discuss in more details in the following section.
3.2.1. Decellularized Scaffold
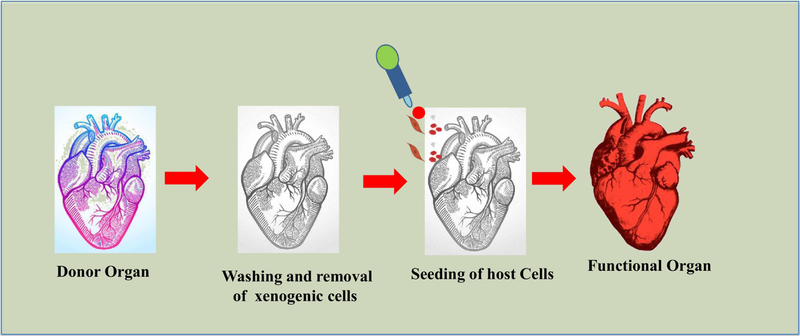
Decellularization is a process of isolating the ECM from the tissue after removing the majority of cellular debris. Unlike material based scaffolds, decellularization based scaffolds are made using natural decellularized matrix and repopulated with desired cell types [160]. Over the past decade, a variety of decellularization protocols have been successfully developed [161]. In decellularization process, the xenogenics are washed out from sacrificial tissue/organ using surfactants like sodium dodecyl sulfate (SDS), Triton X-100, or enzymes like trypsin and collagenase type I and II, as shown in Figure 6 to get cell-free scaffold [162]. It is prerequisite that the washing process for decellularization has to be repeated until complete removal of the xenogenic materials, without damaging the ECM composition and integrity. The key advantage of the decellularized scaffolds over their synthetic counterparts is that they maintain the native ECM with site-specific proteins for cellular adhesion. The decellularized xenogenic or allogenic ECM-based scaffolds have been proved to be less immunogenic and were found to display precise biophysical stimuli and signaling molecules which could stimulate normal physiology of tissues. Moreover, the decellularized scaffolds also mimic the natural ECM and thus have better biocompatibility [148]. Therefore, manipulation of the decellularized heart as a scaffold for heart tissue engineering is an ideal case considering the complexity to regenerate heart tissues. For instance, improvement in cardiac function and its pumping capacity was observed when a decellularized heart ECM was used as a scaffold for seeding of rat CMs and ECs [163]. In another study, decellularized mouse hearts have been used as scaffold to culture human iPSCs-derived multi-potential cardiovascular progenitor cells, which showed uniform migration, proliferation, and differentiation into CMs, ECs and SMCs [164]. Similarly, porcine heart slices (150 µm thick) were decellularized and seeded with either rat or human cardiac cells. This study demonstrated that engineered heart tissues (EHTs) beat synchronously at 5 days after seeding with cardiac cells and displayed a robust length-dependent activation at 21 days. The cells were aligned in the direction of the fiber exhibiting physiological contractile anisotropy [165].
Figure 6.
Schematic representation of decellularization process for a human heart.
To evaluate the potential of decellularized plant tissues to regenerate cardiac tissues, researchers have used decellularized plant leaves as scaffolds. Gershlak et al., used vascularized leaves surface as scaffold with fluid perfusion ability. They used various types of leaves with different vascular patterns and found that these leaves remained intact as decellularized scaffold and their perfusion channels were able to transport fluids. Furthermore, hiPSC-CMs, human endothelial cells (HUVECs) and HMSCs were seeded on the leaf scaffold. After few days of culture, HUVEC occupied the inner surface of leaf vasculature and other two types of cells migrated to outer surface of leaf. After three weeks, hiPSC-CMs showed spontaneous contraction and expressed calcium handling properties. In addition, the hiPSC-CMs adhere to surface of leaf and formed clusters after three days and showed contraction one day after the culture [166].
Despite successful improvement in the functions of the heart using decellularized heart-based scaffolds, there are several drawbacks associated with it as follows: 1) the site-specific repopulation of cells and obtaining the appropriate mechanical strength for pumping the blood [139,160,167]; 2) limited organ donors to get decellularized scaffolds and cross species source may face potential immune rejection. Therefore, researchers around the world are investigating newer synthetic biodegradable scaffolds and technologies, which could mimic natural ECM. One such approach is the 3D bioprinting of cardiac tissue, which will be discussed in greater details in the following section below.
3D bio-printing based cardiac tissue engineering approaches
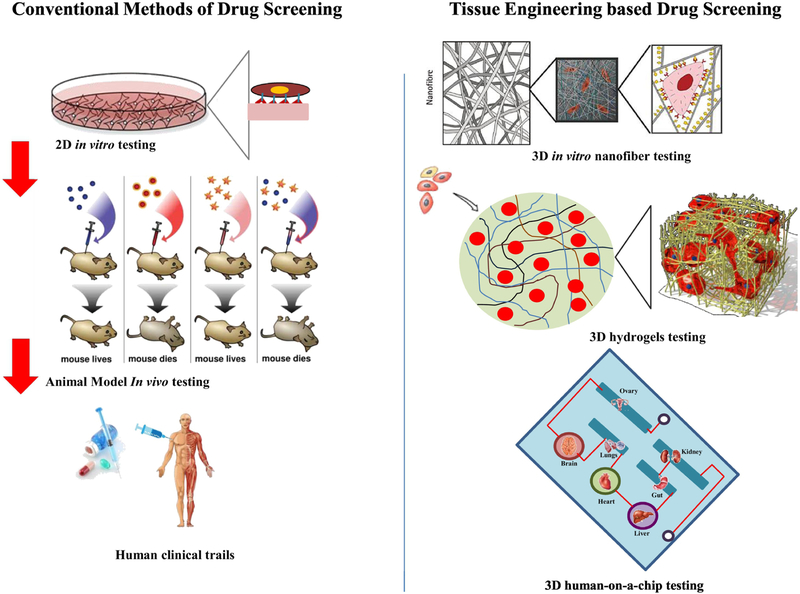
3D bio-printing is a computer-controlled process in which materials are combined in a 3D pattern to form a 3D object [168,169]. A recent study demonstrated 3D printing technique to improve tissue vascularization and rapid vascular integration in vivo [170]. The scaffolds with defined geometric dimensions, which mimic the natural ECM morphology are efficiently developed using 3D printing technologies [171]. Surface engineering, also known as topological or surface guidance in 3D printing are used to print grooves and channels in scaffolds, which are found to influence the cell migration, differentiation, morphology, phenotype, and physiology [169,172,173]. In regards to this, there are reports in the literature which showed a resolution of ≤ 1 micron and thus are able to maintain intact and distinct channels [174,175]. Similarly, CMs seeded on a 3D printed scaffold showed synchronized contractions with improved cell adhesion within the scaffold [176]. In another technique called 3D sacrificial molding, a rigid 3D lattice of sugar filaments are printed and embedded within an ECM-like matrix with cells and subsequently dissolved to leave behind a micro-channel network for endothelialization [177]. Similarly, 3D printed gelatin hydrogel cross-linked with an enzyme mTgase with microchannels was developed. These microchannels in the hydrogel was found to orient hMSCs, promote myocardial lineage commitment and improve the cardiomyocyte functionality and orientation [178]. One-step ahead to develop a fully functional heart, researchers have utilized 3D bio-printing to develop a microfluidic chip based in vitro model of heart as shown in the schematic in Figure 7 [174,179]. Another study by Lind et al., demonstrated a 3D printed microfluidics device, which could continuously monitor electronic readouts of the contractile stress in 3D printed microfluidics channels. The 3D printed microfluidics device imparts a successful platform to record contractile function of cardiac tissues and drug screening platform in a controlled microenvironment [180].
Figure 7.
A) Conventional and B) 3D-tissue engineering based approaches for drug screening applications.
In another strategy, bioink which is a mixture of cells and desired hydrogel polymers, are directly printed as scaffolds to develop partial or full organ [168]. A recent study by Gao et al., showed that a novel way of 3D printing technique using multiphoton-excitation to print ECM based scaffold with unique high resolution. The 3D printed scaffold was used to develop a human cardiomyocytes patch, and implantation of this patch onto the mouse MI heart showed increased engraftment of transplanted cells in the patch [181]. Zhang et al., used the composite bioink composed of endothelial cells and microfibrous hydrogel in 3D printing to fabricate a myocardium. Due to gravity effect and difference in density, endothelial cells aligned with the periphery of microfibers and form a symmetrical endothelium layer. Above this layer, a layer consisting of cardiomyocytes was fabricated with controlled anisotropy which gave rise to an aligned functional myocardium with synchronized rhythmic beating [174]. To develop a functional cardiac tissue, a hybrid hydrogel system based on three components i.e. PCL, sacrificial hydrogel and cell-laden hydrogel was developed. The sacrificial and cell-laden hydrogel was made using fibrin and cross-linked using thrombin. This hydrogel along with 10 × 106 cells/ml acted as a bioink to print a 3D cardiac construct at 18 °C. During the 3D printing process, three different dispensing modules were used for each type of hydrogel and PCL. Overall, the bioengineered cardiac tissue patches showed a synchronized beatings in culture, indicating potential for cardiac tissue maturation in vitro [169].
Use of biomaterials or polymers as constituents of bioink have issues associated with their biodegradability, immune reactions, and toxicities. In order to address these drawbacks, Ong et al., demonstrated a unique strategy to directly deliver stem cells using 3D bio-printing technique without addition of biomaterials. To develop this cardiac patch, hiPSC-CMs, fibroblast and endothelial cells (ECs) were combined in ratios (CM: FB: EC=70:15:15, 70:0:30, 45:40:15) to create spheroids and these spheroids were used for 3D printing of cardiac patches. At day 3, these patches initiated its beating and fusion of voids between these patches was also observed resulting in electrical integration of the patch. Additionally, these cardiac patches demonstrated action potential waveforms similar to ventricular cardiomyocytes, along with constant cell alignment and electrical conduction in the patch [182].
Conclusions
Several potential tissue-engineering approaches have been employed to treat the damaged heart via a combination of stem cells and biomaterials-based 3D scaffolds. However, replicating the anisotropy of a fully functional cardiac tissue is a major challenge in the field of cardiac tissue engineering. Further studies are needed to optimize the scaffold and the scaffold-free approaches of tissue engineering. There is an unmet need to develop biomaterials, which could rapidly integrate with the body’s host immune system and synchronize with native cardiac tissue for electro-mechanical coupling. Furthermore, developing scaffolds with angiogenic properties will promote vascularization to supply oxygen and nutrients to the ischemic cardiac tissue. Scaffolds also need to have properties to support native ECM development and recruitment of appropriate number of cells to promote regeneration. The 3D printing of scaffolds offers a promising approach to develop functional cardiac tissue. Overall, the current research trends in cardiac tissue engineering are pointing towards a new hope for developing novel biomimetic cardiac scaffolds in near future for treating damaged heart tissue.
Acknowledgments
We would like to acknowledge funding from National Institute of Health grant (HL136232, MK) and OSU start-up funds to MK.
Abbreviations:
- CVDs
Cardiovascular diseases
- WHO
World health organization
- CHD
Coronary heart diseases
- CDC
cardio sphere derived cells
- 3D
Three-dimensional
- MI
Myocardial infarction
- VF
Ventricular fibrillation
- VT
Ventricular tachycardia
- VA
Ventricular arrhythmias
- ICD
Implant of cardioverter defibrillator
- SA
Sinoatrial
- AV
Atrioventricular
- ECM
Extracellular matrix
- PDMS
Polydimethylsiloxane
- PEG
poly(ethylene glycol)
- iPSCs
Induced pluripotent stem cells
- ESCs
Embryonic stem cells
- GSK3
Glycogen synthase kinase 3
- PGA
Poly (glycolic acid)
- PGA
Poly (glycolic acid)
- PCL
Polycaprolactone
- PLA
Poly (lactic acid)
- PLGA
Poly (D,L-lactic-co-glycolic acid)
- MeHA
Methacrylated hyaluronic acid
- GelMA
Gelatin methacrylate
- CMs
Cardiomyocytes
- PEG
Polyethylene glycol
- CPCL
carboxylated PCL
- EBs
Embryoid bodies
- mESCs
murine ESCs
- PPy
Polypyrrole
- hiPSC-CMs
Human induced pluripotent stem cell-derived cardiomyocytes
- DCM
Dilated cardiomyopathy
- CTLCs
Cardiac tissue-like constructs
- PMGI
Polymethylglutarimid
- AuNP
Gold-nanoparticle
- IDs
Intercalated discs
- TEM
Transmission electron microscopy
- CLSM
Confocal laser scanning microscopy
- SPNT
Scanning probe nanotomography
- PHEMA
Poly(2-hydroxyethyl methacrylate)
- PVA
Poly vinyl alcohol
- pNIPAAM
Poly(Nisopropylacrylamide)
- AAc
Acrylic acid
- pNIPAM-AA
Poly N-isopropylacrylamineco- acrylic acid
- SMT
Standard medical therapy
- hESCs
Human Embryonic Stem Cells
- hESC-CMs
Human embryonic stem cells derived cardiomyocytes
- cTnT
Cardiac troponin T
- ECs
Endothelial cells
- TEHV
Tissue engineered heart valves
- HAVIC
Human aortic valve interstitial cells
- hCMP
Human cardiomyocytes patches
- HMSCs
Human mesenchymal stem cells
- HEMSCs
Human embryonic stem cells
- HUVECs
Human endothelial cells
- PFC
Perfluorocarbon
- MTFs
Muscular thin films
- ADME
Absorption, distribution, metabolism, elimination
- ECTCs
Embryonic cardiac constructs
- LCST
Lower Critical Solution Temperature
- Az-chitosan/Acr-PEG-RGD
Azidobenzoic acid modified Chitosan with acryloyl-poly(ethylene glycol)-RGDS
- OAC-PEG-OAC
Oligo(acryloyl carbonate)–poly(ethylene glycol)–oligo(acryloyl carbonate) copolymer, PANi-Polyaniline
- PPy
Polypyrrole
- S/m
siemens per metre
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All the authors of this manuscript have declared no conflict of interest.
References:
- [1].Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Ketema E. Belay, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi JY, Christensen H, Cirillo M, Cooper L, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Milicevic M. Santric, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Atnafu N. Tadele, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C, Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015, J. Am. Coll. Cardiol 70 (2017) 1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association, 2018. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed]
- [3].Anderson JL, Morrow DA, Acute Myocardial Infarction, N. Engl. J. Med 376 (2017) 2053–2064. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- [4].White SL, Hirth R, Mahíllo B, Domínguez-Gil B, Delmonico FL, Noel L, Chapman J, Matesanz R, Carmona M, Alvarez M, Núñez JR, Leichtman A, The global diffusion of organ transplantation: trends, drivers and policy implications, Bull. World Health Organ 92 (2014) 826–835. doi: 10.2471/BLT.14.137653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hastings CL, Roche ET, Ruiz-Hernandez E, Schenke-Layland K, Walsh CJ, Duffy GP, Drug and cell delivery for cardiac regeneration, Adv. Drug Deliv. Rev 84 (2015) 85–106. doi: 10.1016/j.addr.2014.08.006. [DOI] [PubMed] [Google Scholar]
- [6].Singh A, Singh A, Sen D, Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010–2015), Stem Cell Res. Ther 7 (2016) 82. doi: 10.1186/s13287-016-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].O’Neill HS, Gallagher LB, O’Sullivan J, Whyte W, Curley C, Dolan E, Hameed A, O’Dwyer J, Payne C, O’Reilly D, Ruiz-Hernandez E, Roche ET, O’Brien FJ, Cryan SA, Kelly H, Murphy B, Duffy GP, Biomaterial-Enhanced Cell and Drug Delivery: Lessons Learned in the Cardiac Field and Future Perspectives, Adv. Mater 28 (2016) 5648–5661. doi: 10.1002/adma.201505349. [DOI] [PubMed] [Google Scholar]
- [8].Prabhakaran MP, Kai D, Ghasemi-Mobarakeh L, Ramakrishna S, Electrospun biocomposite nanofibrous patch for cardiac tissue engineering, Biomed. Mater 6 (2011). doi: 10.1088/1748-6041/6/5/055001. [DOI] [PubMed] [Google Scholar]
- [9].Kitsara M, Agbulut O, Kontziampasis D, Chen Y, Menasché P, Fibers for hearts: A critical review on electrospinning for cardiac tissue engineering, Acta Biomater 48 (2017) 20–40. doi: 10.1016/j.actbio.2016.11.014. [DOI] [PubMed] [Google Scholar]
- [10].Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini A, Chen P, Biocompatibility of engineered nanoparticles for drug delivery, J. Control. Release 166 (2013) 182–194. doi: 10.1016/j.jconrel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- [11].Camci-Unal G, Annabi N, Dokmeci MR, Liao R, Khademhosseini A, Hydrogels for cardiac tissue engineering, NPG Asia Mater 6 (2014) e99–12. doi: 10.1038/am.2014.19. [DOI] [Google Scholar]
- [12].Arunkumar P, Indulekha S, Vijayalakshmi S, Srivastava R, Poly (caprolactone) microparticles and chitosan thermogels based injectable formulation of etoricoxib for the potential treatment of osteoarthritis, Mater. Sci. Eng. C 61 (2016) 534–544. doi: 10.1016/j.msec.2015.12.039. [DOI] [PubMed] [Google Scholar]
- [13].Arunkumar P, Indulekha S, Vijayalakshmi S, Srivastava R, Synthesis, characterizations, in vitro and in vivo evaluation of Etoricoxib-loaded Poly (Caprolactone) microparticles-a potential Intra-articular drug delivery system for the treatment of Osteoarthritis, J. Biomater. Sci. Polym. Ed 27 (2016) 303–316. doi: 10.1080/09205063.2015.1125564. [DOI] [PubMed] [Google Scholar]
- [14].Qasim M, Baipaywad P, Udomluck N, Na D, Park H, Enhanced therapeutic efficacy of lipophilic amphotericin B against Candida albicans with amphiphilic poly(N-isopropylacrylamide) nanogels, Macromol. Res 22 (2014) 1125–1131. doi: 10.1007/s13233-014-2162-2. [DOI] [Google Scholar]
- [15].Katsuki S, Matoba T, Koga J, Nakano K, Egashira K, Anti-inflammatory Nanomedicine for Cardiovascular Disease, Front. Cardiovasc. Med 4 (2017). doi: 10.3389/fcvm.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shaunak S, Thomas S, Gianasi E, Godwin A, Jones E, Teo I, Mireskandari K, Luthert P, Duncan R, Patterson S, Khaw P, Brocchini S, Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation, Nat. Biotechnol 22 (2004) 977–984. doi: 10.1038/nbt995. [DOI] [PubMed] [Google Scholar]
- [17].Van De Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Aguirre FV, Al-Attar N, Alegria E, Andreotti F, Benzer W, Breithardt O, Danchin N, Di Mario C, Dudek D, Gulba D, Halvorsen S, Kaufmann P, Kornowski R, Lip GYH, Rutten F, Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation, Eur. Heart J 29 (2008) 2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- [18].Huffman MD, Bhatnagar D, Novel treatments for cardiovascular disease prevention., Cardiovasc. Ther 30 (2012) 257–63. doi: 10.1111/j.1755-5922.2011.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ, Cardioprotection and pharmacological therapies in acute myocardial infarction: Challenges in the current era., World J. Cardiol 6 (2014) 100–6. doi: 10.4330/wjc.v6.i3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cui Z, Yang B, Li RK, Application of Biomaterials in Cardiac Repair and Regeneration, Engineering 2 (2016) 141–148. doi: 10.1016/J.ENG.2016.01.028. [DOI] [Google Scholar]
- [21].DiNella JV, Bowman J, Heart Transplantation, Crit. Care Nurs. Clin. North Am 23 (2011) 471–479. doi: 10.1016/j.ccell.2011.08.005. [DOI] [PubMed] [Google Scholar]
- [22].Crudele V, Picascia A, Infante T, Grimaldi V, Maiello C, Napoli C, Repeated immune and non immune insults to the graft after heart transplantation, Immunol. Lett 141 (2011) 18–27. doi: 10.1016/j.imlet.2011.07.004. [DOI] [PubMed] [Google Scholar]
- [23].Abramson S, Ackermann DM, Akins R, Anders R, Andersen PJ, Anderson JM, Ankrum JA, Anseth KS, Antonucci J, Atzet S, Badylak SF, Baura GD, Bellamkonda RV, Best SM, Bhumiratana S, Bianco RW, Bokros JC, Borovetz HS, Boskey AL, Brown JL, Brown BN, Brown SA, Brunski JB, Cahn F, Ritchie AC, Caplan AI, Carpenedo RL, Chilkoti A, Chung S, Cimetta E, Cleary G, Clements IP, Colas A, Coleman KP, Conway DE, Cooper SL, Costerton B, Coury AJ, Cunanan C, Curtis J, D’Amore A, DeMeo P, Desai TA, Dickens S, Domingo G, Duncan E, Eskin SG, Feigal DW, Ferreira L, Fuller J, Gallegos RP, Gawalt E, Ghosh K, Ghosn B, Gilbert TW, Glaser DE, Godier-Furnemont A, Gombotz WR, Grainger DW, Grunkemeier GL, Hacking SA, Hallab NJ, Hall-Stoodley L, Hanson SR, Haubold AD, Hauch KD, Hawkins KR, Heath DE, Helm DL, Hench LL, Hensten A, Hill RT, Hobson C, Hoerstrup SP, Hoffman AS, Horbett TA, Hubbell JA, Humayun MS, Ideker R, Ingber DE, Jain R, Jacob J, Jacobs JJ, Jacobsen N, Jin R, Johnson RJ, Karp JM, Kasper FK, Kathju S, Khademhosseini A, Kim S, King MW, Kleiner LW, Kohn J, Koschwanez HE, Kumbar SG, Kuo CK, LaFleur L, Lahti MT, Lambert B, Langer R, Laurencin CT, Lee-Parritz D, Lemons JE, Levin M, Levy RJ, Lewerenz GM, Li W-J, Lin C-C, Liu F, Lowrie WG, Lu Y, Lysaght MJ, Maidhof R, Mansbridge JN, Cristina M, Martins L, Martin J, Mayesh JP, McDevitt TC, McIntire LV, Merrit K, Migliaresi C, Mikos AG, Misch CE, Mitchell RN, More RB, Moss CW, Munson JM, Navarro M, Nerem RM, Ogawa R, Orgill BD, Orgill DP, Padera RF, Pandit A, Park K, Patel AS, Peck RB, Peckham PH, Peppas NA, Pereira MN, Planell J, Popat KC, Prestwich GD, Pun SH, Rabolt J, Rainbow RS, Rajab T, Ratner BD, Reichert WM, Rivard AL, Rowley AP, Ruan G, Sacks M, Sarkar D, Schaefer S, Schmidt CE, Schoen FJ, Schutte SC, Sefton MV, Shalaby SW, Shirtliff M, Simon MA, Singh M, Slack SM, Spelman FA, Starr A, Stayton PS, Steinert R, Stoodley P, Suri S, Swi Chang TM, Tandon N, Tanguay AR, Taylor MS, Teo GSL, Thodeti CK, Tolkoff J, Treiser M, Tuan RS, Tucker EI, Venugopalan R, Vicari AR, Viney C, Voight JM, Vunjak-Novakovic G, Wagner WR, Wang L, Wasiluk KR, Watts DC, Weigl BH, Weiland JD, Whalen JJ, Williams DF, Williams RL, Wilson JT, Wilson CG, Winter J, Wolf MF, Wright JC, Yager P, Zhao W, Contributors, Biomater. Sci (2013) xiii–xix. doi: 10.1016/B978-0-08-087780-8.00150-9. [DOI]
- [24].Kluin J, Talacua H, Smits AIPM, Emmert MY, Brugmans MCP, Fioretta ES, Dijkman PE, S??ntjens SHM, Duijvelshoff R, Dekker S, Janssen-van den Broek MWJT, Lintas V, Vink A, Hoerstrup SP, Janssen HM, Dankers PYW, Baaijens FPT, Bouten CVC, In situ heart valve tissue engineering using a bioresorbable elastomeric implant ??? From material design to 12 months follow-up in sheep, Biomaterials 125 (2017) 101–117. doi: 10.1016/j.biomaterials.2017.02.007. [DOI] [PubMed] [Google Scholar]
- [25].Vunjak-novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, Radisic M, Challenges in Cardiac Tissue Engineering 1, 16 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lancaster JJ, Juneman E, Arnce SA, Johnson NM, Qin Y, Witte R, Thai H, Kellar RS, Ek Vitorin J, Burt J, Gaballa MA, Bahl JJ, Goldman S, An electrically coupled tissue-engineered cardiomyocyte scaffold improves cardiac function in rats with chronic heart failure, J. Hear. Lung Transplant 33 (2014) 438–445. doi: 10.1016/j.healun.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Monteiro LM, Vasques-Nóvoa F, Ferreira L, Pinto-do-Ó P, Nascimento DS, Restoring heart function and electrical integrity: closing the circuit, Npj Regen. Med 2 (2017) 9. doi: 10.1038/s41536-017-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tandon N, Cannizzaro C, Chao PP-HG, Maidhof R, Marsano A, Au HTH, Radisic M, Vunjak-Novakovic G, Electrical stimulation systems for cardiac tissue engineering, Nat. Protoc 4 (2009) 155–173. doi: 10.1038/nprot.2008.183.Electrical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH, Developmental patterning of the myocardium., Anat. Rec 258 (2000) 319–37. [DOI] [PubMed] [Google Scholar]
- [30].Zhang S, Crow JA, Yang X, Chen J, Borazjani A, Mullins KB, Chen W, Cooper RC, McLaughlin RM, Liao J, The Correlation of 3D DT-MRI Fiber Disruption with Structural and Mechanical Degeneration in Porcine Myocardium, Ann. Biomed. Eng 38 (2010) 3084–3095. doi: 10.1007/s10439-010-0073-8. [DOI] [PubMed] [Google Scholar]
- [31].Joshi J, Kothapalli CR, Nanofibers based tissue engineering and drug delivery approaches for myocardial regeneration., Curr. Pharm. Des 21 (2015) 2006–2020. [DOI] [PubMed] [Google Scholar]
- [32].Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T, Fabrication of Pulsatile Cardiac Tissue Grafts Using a Novel 3-Dimensional Cell Sheet Manipulation Technique and Temperature-Responsive Cell Culture Surfaces, Circ. Res 90 (2002) 40–48. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- [33].Shimizu T, Sekine H, Isoi Y, Yamato M, Kikuchi A, Okano T, Long-Term Survival and Growth of Pulsatile Myocardial Tissue Grafts Engineered by the Layering of Cardiomyocyte Sheets, Tissue Eng 12 (2006) 499–507. doi: 10.1089/ten.2006.12.499. [DOI] [PubMed] [Google Scholar]
- [34].Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G, Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue, Biotechnol. Bioeng 93 (2006) 332–343. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- [35].Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T, Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues, FASEB J 20 (2006) 708–710. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- [36].Xu Y, Patnaik S, Guo X, Li Z, Lo W, Butler R, Claude A, Liu Z, Zhang G, Liao J, Anderson PM, Guan J, Cardiac differentiation of cardiosphere-derived cells in scaffolds mimicking morphology of the cardiac extracellular matrix, Acta Biomater 10 (2014) 3449–3462. doi: 10.1016/j.actbio.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maxson S, Orr D, Burg KJL, Bioreactors for tissue engineering, Third Edit, Elsevier, 2011. doi: 10.1007/978-3-642-02824-3_10. [DOI] [Google Scholar]
- [38].Hollweck T, Akra B, Häussler S, Überfuhr P, Schmitz C, Pfeifer S, Eblenkamp M, Wintermantel E, Eissner G, A Novel Pulsatile Bioreactor for Mechanical Stimulation of Tissue Engineered Cardiac Constructs, J. Funct. Biomater 2 (2011) 107–118. doi: 10.3390/jfb2030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vunjak-Novakovic G, Radisic M, Obradovic B, Cardiac tissue engineering: Effects of bioreactor flow environment on tissue constructs, J. Chem. Technol. Biotechnol 81 (2006) 485–490. doi: 10.1002/jctb.1467. [DOI] [Google Scholar]
- [40].Xu HL, Yu WZ, Lu CT, Li XK, Zhao YZ, Delivery of growth factor-based therapeutics in vascular diseases: Challenges and strategies, Biotechnol. J 12 (2017) 1–19. doi: 10.1002/biot.201600243. [DOI] [PubMed] [Google Scholar]
- [41].Dubey P, Boccaccini AR, Roy I, Novel cardiac patch development using natural biopolymers, ACS Symp. Ser 1175 (2014) 159–175. doi: 10.1021/bk-2014-1175.ch010. [DOI] [Google Scholar]
- [42].Martins AM, Eng G, Caridade SG, Mano JF, Reis RL, Vunjak-Novakovic G, Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering, Biomacromolecules 15 (2014) 635–643. doi: 10.1021/bm401679q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tandon N, Taubman A, Cimetta E, Saccenti L, Vunjak-Novakovic G, Portable bioreactor for perfusion and electrical stimulation of engineered cardiac tissue., Conf. Proc. …Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf 2013 (2013) 6219–23. doi: 10.1109/EMBC.2013.6610974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chaudhuri R, Ramachandran M, Moharil P, Harumalani M, Jaiswal AK, Biomaterials and cells for cardiac tissue engineering: Current choices, Mater. Sci. Eng. C 79 (2017) 950–957. doi: 10.1016/j.msec.2017.05.121. [DOI] [PubMed] [Google Scholar]
- [45].Hao M, Wang R, Wang W, Cell Therapies in Cardiomyopathy: Current Status of Clinical Trials, Anal. Cell. Pathol 2017 (2017) 1–20. doi: 10.1155/2017/9404057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Karakikes I, Ameen M, Termglinchan V, Wu JC, Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Insights into Molecular, Cellular, and Functional Phenotypes, Circ. Res 117 (2015) 80–88. doi: 10.1161/CIRCRESAHA.117.305365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jiang Y, Park P, Hong S-M, Ban K, Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells: Current Strategies and Limitations, Mol. Cells 41 (2018) 0–0. doi: 10.14348/molcells.2018.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yoshida S, Miyagawa S, Fukushima S, Kawamura T, Kashiyama N, Ohashi F, Toyofuku T, Toda K, Sawa Y, Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes by Soluble Factors from Human Mesenchymal Stem Cells, Mol. Ther 26 (2018) 2681–2695. doi: 10.1016/j.ymthe.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Otsuji TG, Minami I, Kurose Y, Yamauchi K, Tada M, Nakatsuji N, Progressive maturation in contracting cardiomyocytes derived from human embryonic stem cells: Qualitative effects on electrophysiological responses to drugs, Stem Cell Res 4 (2010) 201–213. doi: 10.1016/j.scr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- [50].Lundy SD, Zhu W-Z, Regnier M, Laflamme MA, Structural and Functional Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells, Stem Cells Dev 22 (2013) 1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang W, Kong CW, Tong MH, Chooi WH, Huang N, Li RA, Chan BP, Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: Effects of niche cell supplementation and mechanical stimulation, Acta Biomater 49 (2017) 204–217. doi: 10.1016/j.actbio.2016.11.058. [DOI] [PubMed] [Google Scholar]
- [52].Besser RR, Ishahak M, Mayo V, Carbonero D, Claure I, Agarwal A, Engineered microenvironments for maturation of stem cell derived cardiac myocytes, Theranostics 8 (2018) 124–140. doi: 10.7150/thno.19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N, Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes, Biomaterials 34 (2013) 5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Reinecke H, Zhang M, Bartosek T, Murry CE, Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts., Circulation 100 (1999) 193–202. [DOI] [PubMed] [Google Scholar]
- [55].Fernandes S, Chong JJH, Paige SL, Iwata M, Torok-Storb B, Keller G, Reinecke H, Murry CE, Comparison of human embryonic stem cell-derived cardiomyocytes, cardiovascular progenitors, and bone marrow mononuclear cells for cardiac repair, Stem Cell Reports 5 (2015) 753–762. doi: 10.1016/j.stemcr.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Machiraju P, Greenway SC, Current methods for the maturation of induced pluripotent stem cell-derived cardiomyocytes, World J. Stem Cells 11 (2019) 33–43. doi: 10.4252/wjsc.v11.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y, Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model, Circulation 126 (2012) 29–37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- [58].Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Ito E, Sougawa N, Kawamura T, Daimon T, Shimizu T, Okano T, Toda K, Sawa Y, Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart, Circulation 128 (2013) 87–94. doi: 10.1161/CIRCULATIONAHA.112.000366. [DOI] [PubMed] [Google Scholar]
- [59].Koivumäki JT, Naumenko N, Tuomainen T, Takalo J, Oksanen M, Puttonen KA, Lehtonen Š, Kuusisto J, Laakso M, Koistinaho J, Tavi P, Structural immaturity of human iPSC-derived cardiomyocytes: In silico investigation of effects on function and disease modeling, Front. Physiol 9 (2018) 1–17. doi: 10.3389/fphys.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yang X, Pabon L, Murry CE, Engineering Adolescence, Circ. Res 114 (2014) 511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Li J, Minami I, Shiozaki M, Yu L, Yajima S, Miyagawa S, Shiba Y, Morone N, Fukushima S, Yoshioka M, Li S, Qiao J, Li X, Wang L, Kotera H, Nakatsuji N, Sawa Y, Chen Y, Liu L, Human Pluripotent Stem Cell-Derived Cardiac Tissue-like Constructs for Repairing the Infarcted Myocardium, Stem Cell Reports 9 (2017) 1546–1559. doi: 10.1016/j.stemcr.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Teles D, Yazawa M, Vunjak-Novakovic G, Song L, Yeager K, Chen T, Sirabella D, Ma SP, Ronaldson-Bouchard K, Morikawa K, Advanced maturation of human cardiac tissue grown from pluripotent stem cells, Nature (2018). doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed]
- [63].Ehman EC, Johnson GB, Villanueva-meyer JE, Cha S, Leynes AP, Eric P, Larson Z, Hope TA, HHS Public Access, 46 (2017) 1247–1262. doi: 10.1002/jmri.25711.PET/MRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Liu Y-W, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, Couture L, Vogel KW, Astley CA, Baldessari A, Ogle J, Don CW, Steinberg ZL, Seslar SP, Tuck SA, Tsuchida H, Naumova AV, Dupras SK, Lyu MS, Lee J, Hailey DW, Reinecke H, Pabon L, Fryer BH, MacLellan WR, Thies RS, Murry CE, Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates., Nat. Biotechnol 36 (2018) 597–605. doi: 10.1038/nbt.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chong JJH, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE, Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts, Nature 510 (2014) 273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE, Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats, J. Mol. Cell. Cardiol 49 (2010) 941–949. doi: 10.1016/j.yjmcc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA, Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts, Nature 489 (2012) 322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE, Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts, Nat. Biotechnol 25 (2007) 1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- [69].Ye L, Chang Y, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Dutton JR, Zhang JJ, Kamp TJ, Kaufman DS, Ge Y, Zhang JJ, Cardiac Repair in a Porcine Model of Acute Myocardial Infarction with Human Induced Clinical Progress Cardiac Repair in a Porcine Model of Acute Myocardial Infarction with Human Induced Pluripotent Stem Cell-Derived Cardiovascular Cells, Stem 15 (2014) 750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, Bursac N, Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues, Nat. Commun 8 (2017). doi: 10.1038/s41467-017-01946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ganji Y, Li Q, Quabius ES, Böttner M, Selhuber-Unkel C, Kasra M, Cardiomyocyte behavior on biodegradable polyurethane/gold nanocomposite scaffolds under electrical stimulation, Mater. Sci. Eng. C 59 (2016) 10–18. doi: 10.1016/j.msec.2015.09.074. [DOI] [PubMed] [Google Scholar]
- [72].Kharaziha M, Shin SR, Nikkhah M, Topkaya SN, Masoumi N, Annabi N, Dokmeci MR, Khademhosseini A, Tough and flexible CNT-polymeric hybrid scaffolds for engineering cardiac constructs, Biomaterials 35 (2014) 7346–7354. doi: 10.1016/j.biomaterials.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Waters R, Alam P, Pacelli S, Chakravarti AR, Ahmed RPH, Paul A, Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue, Acta Biomater 69 (2018) 95–106. doi: 10.1016/j.actbio.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jackman CP, Ganapathi AM, Asfour H, Qian Y, Allen BW, Li Y, Bursac N, Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation, Biomaterials 159 (2018) 48–58. doi: 10.1016/j.biomaterials.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ku SH, Lee SH, Park CB, Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation, Biomaterials 33 (2012) 6098–6104. doi: 10.1016/j.biomaterials.2012.05.018. [DOI] [PubMed] [Google Scholar]
- [76].Rodness J, Mihic A, Miyagi Y, Wu J, Weisel RD, Li RK, VEGF-loaded microsphere patch for local protein delivery to the ischemic heart, Acta Biomater 45 (2016) 169–181. doi: 10.1016/j.actbio.2016.09.009. [DOI] [PubMed] [Google Scholar]
- [77].Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Parouchev A, Cacciapuoti I, Al-Daccak R, Benhamouda N, Blons H, Agbulut O, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Charron D, Tartour E, Tachdjian G, Desnos M, Larghero J, Transplantation of Human Embryonic Stem Cell–Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction, J. Am. Coll. Cardiol 71 (2018) 429–438. doi: 10.1016/j.jacc.2017.11.047. [DOI] [PubMed] [Google Scholar]
- [78].Dikina AD, Strobel HA, Lai BP, Rolle MW, Alsberg E, Engineered cartilaginous tubes for tracheal tissue replacement via self-assembly and fusion of human mesenchymal stem cell constructs, Biomaterials 52 (2015) 452–462. doi: 10.1016/j.biomaterials.2015.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shimomura K, Ando W, Fujie H, Hart DA, Yoshikawa H, Nakamura N, Scaffold-free tissue engineering for injured joint surface restoration., J. Exp. Orthop 5 (2018) 2. doi: 10.1186/s40634-017-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tang Z, Akiyama Y, Okano T, Temperature-responsive polymer modified surface for cell sheet engineering, Polymers (Basel) 4 (2012) 1478–1498. doi: 10.3390/polym4031478. [DOI] [Google Scholar]
- [81].Rezai KA, Farrokh-Siar L, Godowski K, Patel SC, Ernest JT, A model for xenogenic immune response, Graefes Arch. Clin. Exp. Ophthalmol 238 (2000) 352–358. doi: 10.1007/s004170050364. [DOI] [PubMed] [Google Scholar]
- [82].Owaki T, Shimizu T, Yamato M, Okano T, Cell sheet engineering for regenerative medicine: Current challenges and strategies, Biotechnol. J 9 (2014) 904–914. doi: 10.1002/biot.201300432. [DOI] [PubMed] [Google Scholar]
- [83].Asakawa N, Shimizu T, Tsuda Y, Sekiya S, Sasagawa T, Yamato M, Fukai F, Okano T, Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering, Biomaterials 31 (2010) 3903–3909. doi: 10.1016/j.biomaterials.2010.01.105. [DOI] [PubMed] [Google Scholar]
- [84].Takahashi H, Okano T, Cell Sheet-Based Tissue Engineering for Organizing Anisotropic Tissue Constructs Produced Using Microfabricated Thermoresponsive Substrates, Adv. Healthc. Mater 4 (2015) 2388–2407. doi: 10.1002/adhm.201500194. [DOI] [PubMed] [Google Scholar]
- [85].Masuda S, Shimizu T, Three-dimensional cardiac tissue fabrication based on cell sheet technology, Adv. Drug Deliv. Rev 96 (2016) 103–109. doi: 10.1016/j.addr.2015.05.002. [DOI] [PubMed] [Google Scholar]
- [86].Matsuura K, Masuda S, Shimizu T, Cell sheet-based cardiac tissue engineering, Anat. Rec 297 (2014) 65–72. doi: 10.1002/ar.22834. [DOI] [PubMed] [Google Scholar]
- [87].Ashur C, Frishman WH, Cardiosphere-derived cells and ischemic heart failure, Cardiol. Rev 26 (2018) 8–21. doi: 10.1097/CRD.0000000000000173. [DOI] [PubMed] [Google Scholar]
- [88].Cheng K, Blusztajn A, Shen D, Li TS, Sun B, Galang G, Zarembinski TI, Prestwich GD, Marbán E, Smith RR, Marbán L, Functional performance of human cardiosphere-derived cells delivered in an in situ polymerizable hyaluronan-gelatin hydrogel, Biomaterials 33 (2012) 5317–5324. doi: 10.1016/j.biomaterials.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh K-Y, Cheng J, Mahdavi A, Borenstein J, Langer R, Khademhosseini A, Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells, Lab Chip 7 (2007) 786. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- [90].Crouzier T, Jang H, Ahn J, Stocker R, Ribbeck K, Cell patterning with mucin biopolymers., Biomacromolecules 14 (2013) 3010–6. doi: 10.1021/bm400447z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ, Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview., Circ. Res 111 (2012) 344–58. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bratt-Leal AM, Carpenedo RL, McDevitt TC, Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation., Biotechnol. Prog 25 (2009) 43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Liu B-H, Yeh H-Y, Lin Y-C, Wang M-H, Chen DC, Lee B-H, Hsu S-H, Spheroid formation and enhanced cardiomyogenic potential of adipose-derived stem cells grown on chitosan., Biores. Open Access 2 (2013) 28–39. doi: 10.1089/biores.2012.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Cha JM, Bae H, Sadr N, Manoucheri S, Edalat F, Kim K, Kim SB, Kwon IK, Hwang YS, Khademhosseini A, Embryoid body size-mediated differential endodermal and mesodermal differentiation using polyethylene glycol (PEG) microwell array, Macromol. Res 23 (2015) 245–255. doi: 10.1007/s13233-015-3034-0. [DOI] [Google Scholar]
- [95].Jakab K, Neagu A, Mironov V, Markwald RR, Forgacs G, Engineering biological structures of prescribed shape using self-assembling multicellular systems, Proc. Natl. Acad. Sci 101 (2004) 2864–2869. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR, Organ printing: Tissue spheroids as building blocks, Biomaterials 30 (2009) 2164–2174. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Mehesz AN, Brown J, Hajdu Z, Beaver W, da Silva JVL, Visconti RP, Markwald RR, Mironov V, Scalable robotic biofabrication of tissue spheroids, Biofabrication 3 (2011) 025002. doi: 10.1088/1758-5082/3/2/025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, de Boer J, Spheroid culture as a tool for creating 3D complex tissues, Trends Biotechnol 31 (2013) 108–115. doi: 10.1016/j.tibtech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- [99].Laschke MW, Menger MD, Life is 3D: Boosting Spheroid Function for Tissue Engineering, Trends Biotechnol 35 (2017) 133–144. doi: 10.1016/j.tibtech.2016.08.004. [DOI] [PubMed] [Google Scholar]
- [100].Laschke MW, Menger MD, Spheroids as vascularization units: From angiogenesis research to tissue engineering applications, Biotechnol. Adv 35 (2017) 782–791. doi: 10.1016/j.biotechadv.2017.07.002. [DOI] [PubMed] [Google Scholar]
- [101].Danilevicius P, Rezende RA, Pereira FDAS, Selimis A, Kasyanov V, Noritomi PY, da Silva JVL, Chatzinikolaidou M, Farsari M, Mironov V, Burr-like, laser-made 3D microscaffolds for tissue spheroid encagement, Biointerphases 10 (2015) 021011. doi: 10.1116/1.4922646. [DOI] [PubMed] [Google Scholar]
- [102].Zhang YS, Aleman J, Arneri A, Bersini S, Piraino F, Shin SR, Dokmeci MR, Khademhosseini A, From cardiac tissue engineering to heart-on-a-chip: beating challenges, Biomed. Mater 10 (2015) 034006. doi: 10.1088/1748-6041/10/3/034006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Taniguchi D, Matsumoto K, Tsuchiya T, Machino R, Takeoka Y, Elgalad A, Gunge K, Takagi K, Taura Y, Hatachi G, Matsuo N, Yamasaki N, Nakayama K, Nagayasu T, Scaffold-free trachea regeneration by tissue engineering with bio-3D printing†, Interact. Cardiovasc. Thorac. Surg 26 (2018) 745–752. doi: 10.1093/icvts/ivx444. [DOI] [PubMed] [Google Scholar]
- [104].Hinderer S, Layland SL, Schenke-Layland K, ECM and ECM-like materials - Biomaterials for applications in regenerative medicine and cancer therapy, Adv. Drug Deliv. Rev 97 (2016) 260–269. doi: 10.1016/j.addr.2015.11.019. [DOI] [PubMed] [Google Scholar]
- [105].Galperin A, Long TJ, Ratner BD, A Degradable, Thermo-sensitive Poly(N-isopropyl acrylamide)- Based Scaffold with Controlled Porosity for Tissue Engineering Applications, Biomacromolecules 11 (2011) 2583–2592. doi: 10.1021/bm100521x.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Liu Q, Tian S, Zhao C, Chen X, Lei I, Wang Z, Ma PX, Porous nanofibrous poly(l-lactic acid) scaffolds supporting cardiovascular progenitor cells for cardiac tissue engineering, Acta Biomater 26 (2015) 105–114. doi: 10.1016/j.actbio.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Nistor M-T, Chiriac AP, Nita LE, Vasile C, Verestiuc L, Upon the characterization of semi-synthetic hydrogels based on poly (NIPAM) inserted onto collagen sponge, Compos. Part B Eng 43 (2012) 1508–1515. doi: 10.1016/j.compositesb.2011.10.015. [DOI] [Google Scholar]
- [108].Qasim Muhammad, Lim D-J, Park H, Na D, Nanotechnology for Diagnosis and Treatment of Infectious Diseases, J. Nanosci. Nanotechnol 14 (2014) 7374–7387. doi: 10.1166/jnn.2014.9578. [DOI] [PubMed] [Google Scholar]
- [109].Yeo Y, Geng W, Ito T, Kohane DS, Burdick JA, Radisic M, Photocrosslinkable hydrogel for myocyte cell culture and injection, J. Biomed. Mater. Res. Part B Appl. Biomater 81B (2007) 312–322. doi: 10.1002/jbm.b.30667. [DOI] [PubMed] [Google Scholar]
- [110].Chen Y, Zeng D, Ding L, Li X-L, Liu X-T, Li W-J, Wei T, Yan S, Xie J-H, Wei L, Zheng Q-S, Three-dimensional poly-(ε-caprolactone) nanofibrous scaffolds directly promote the cardiomyocyte differentiation of murine-induced pluripotent stem cells through Wnt/β-catenin signaling., BMC Cell Biol 16 (2015) 22. doi: 10.1186/s12860-015-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].McDevitt TC, Woodhouse KA, Hauschka SD, Murry CE, Stayton PS, Spatially organized layers of cardiomyocytes on biodegradable polyurethane films for myocardial repair, J. Biomed. Mater. Res 66A (2003) 586–595. doi: 10.1002/jbm.a.10504. [DOI] [PubMed] [Google Scholar]
- [112].Brigham MD, Bick A, Lo E, Bendali A, Burdick JA, Khademhosseini A, Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks., Tissue Eng. Part A 15 (2009) 1645–53. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Robinson RB, Engineering a biological pacemaker: In vivo, in vitro and in silico models, Drug Discov. Today Dis. Model 6 (2009) 93–98. doi: 10.1016/j.ddmod.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ravichandran R, Sridhar R, Venugopal JR, Sundarrajan S, Mukherjee S, Ramakrishna S, Gold nanoparticle loaded hybrid nanofibers for cardiogenic differentiation of stem cells for infarcted myocardium regeneration, Macromol. Biosci 14 (2014) 515–525. doi: 10.1002/mabi.201300407. [DOI] [PubMed] [Google Scholar]
- [115].Aldana AA, Abraham GA, Current advances in electrospun gelatin-based scaffolds for tissue engineering applications, Int. J. Pharm 523 (2017) 441–453. doi: 10.1016/j.ijpharm.2016.09.044. [DOI] [PubMed] [Google Scholar]
- [116].Yuan Ye K, Sullivan KE, Black LD, Encapsulation of Cardiomyocytes in a Fibrin Hydrogel for Cardiac Tissue Engineering, J. Vis. Exp (2011). doi: 10.3791/3251. [DOI] [PMC free article] [PubMed]
- [117].Wang L, Wu Y, Hu T, Guo B, Ma PX, Electrospun Conductive Nanofibrous Scaffolds for Engineering Cardiac Tissue and 3D Bioactuators, Acta Biomater (2017). doi: 10.1016/j.actbio.2017.06.036. [DOI] [PubMed]
- [118].Balashov V, Efimov A, Agapova O, Pogorelov A, Agapov I, Agladze K, High resolution 3D microscopy study of cardiomyocytes on polymer scaffold nanofibers reveals formation of unusual sheathed structure, Acta Biomater 68 (2018) 214–222. doi: 10.1016/j.actbio.2017.12.031. [DOI] [PubMed] [Google Scholar]
- [119].Gupta MK, Walthall JM, Venkataraman R, Crowder SW, Jung DK, Yu SS, Feaster TK, Wang X, Giorgio TD, Hong CC, Baudenbacher FJ, Hatzopoulos AK, Sung HJ, Combinatorial polymer electrospun matrices promote physiologically-relevant cardiomyogenic stem cell differentiation, PLoS One 6 (2011) 20–22. doi: 10.1371/journal.pone.0028935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Kai D, Prabhakaran MP, Jin G, Ramakrishna S, Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering, J. Biomed. Mater. Res. Part A 99A (2011) 376–385. doi: 10.1002/jbm.a.33200. [DOI] [PubMed] [Google Scholar]
- [121].Khan M, Xu Y, Hua S, Johnson J, Belevych A, Janssen PML, Gyorke S, Guan J, Angelos MG, Evaluation of changes in morphology and function of human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) cultured on an aligned-nanofiber cardiac patch, PLoS One 10 (2015) 1–19. doi: 10.1371/journal.pone.0126338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Han J, Wu Q, Xia Y, Wagner MB, Xu C, Cell alignment induced by anisotropic electrospun fibrous scaffolds alone has limited effect on cardiomyocyte maturation, Stem Cell Res 16 (2016) 740–750. doi: 10.1016/j.scr.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Joanne P, Kitsara M, Boitard SE, Naemetalla H, Vanneaux V, Pernot M, Larghero J, Forest P, Chen Y, Menasché P, Agbulut O, Nanofibrous clinical-grade collagen scaffolds seeded with human cardiomyocytes induces cardiac remodeling in dilated cardiomyopathy, Biomaterials 80 (2016) 157–168. doi: 10.1016/j.biomaterials.2015.11.035. [DOI] [PubMed] [Google Scholar]
- [124].Borriello A, Guarino V, Schiavo L, Alvarez-Perez MA, Ambrosio L, Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle, J. Mater. Sci. Mater. Med 22 (2011) 1053–1062. doi: 10.1007/s10856-011-4259-x. [DOI] [PubMed] [Google Scholar]
- [125].Sridhar S, Venugopal JR, Sridhar R, Ramakrishna S, Cardiogenic differentiation of mesenchymal stem cells with gold nanoparticle loaded functionalized nanofibers, Colloids Surfaces B Biointerfaces 134 (2015) 346–354. doi: 10.1016/j.colsurfb.2015.07.019. [DOI] [PubMed] [Google Scholar]
- [126].Guex AG, Frobert A, Valentin J, Fortunato G, Hegemann D, Cook S, Carrel TP, Tevaearai HT, Giraud MN, Plasma-functionalized electrospun matrix for biograft development and cardiac function stabilization, Acta Biomater 10 (2014) 2996–3006. doi: 10.1016/j.actbio.2014.01.006. [DOI] [PubMed] [Google Scholar]
- [127].Peresin MS, Vesterinen AH, Habibi Y, Johansson LS, Pawlak JJ, Nevzorov AA, Rojas OJ, Crosslinked PVA nanofibers reinforced with cellulose nanocrystals: Water interactions and thermomechanical properties, J. Appl. Polym. Sci 131 (2014) 1–12. doi: 10.1002/app.40334. [DOI] [Google Scholar]
- [128].Jeong JS, Moon JS, Jeon SY, Park JH, Alegaonkar PS, Yoo JB, Mechanical properties of electrospun PVA/MWNTs composite nanofibers, Thin Solid Films 515 (2007) 5136–5141. doi: 10.1016/j.tsf.2006.10.058. [DOI] [Google Scholar]
- [129].Mooney E, Mackle JN, Blond DJP, O’Cearbhaill E, Shaw G, Blau WJ, Barry FP, Barron V, Murphy JM, The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs, Biomaterials 33 (2012) 6132–6139. doi: 10.1016/j.biomaterials.2012.05.032. [DOI] [PubMed] [Google Scholar]
- [130].Fleischer S, Shevach M, Feiner R, Dvir T, Coiled fiber scaffolds embedded with gold nanoparticles improve the performance of engineered cardiac tissues, Nanoscale 6 (2014) 9410–9414. doi: 10.1039/C4NR00300D. [DOI] [PubMed] [Google Scholar]
- [131].Moura RM, de Queiroz AAA, Dendronized Polyaniline Nanotubes for Cardiac Tissue Engineering, Artif. Organs 35 (2011) 471–477. doi: 10.1111/j.1525-1594.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- [132].Kai D, Prabhakaran MP, Jin G, Ramakrishna S, Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering, J. Biomed. Mater. Res. - Part A 99 A (2011) 376–385. doi: 10.1002/jbm.a.33200. [DOI] [PubMed] [Google Scholar]
- [133].Yuan X, He B, Lv Z, Luo S, Fabrication of self-assembling peptide nanofiber hydrogels for myocardial repair, RSC Adv 4 (2014) 53801–53811. doi: 10.1039/c4ra08582e. [DOI] [Google Scholar]
- [134].Jung D, Minami I, Patel S, Lee J, Jiang B, Yuan Q, Li L, Kobayashi S, Chen Y, Lee KB, Nakatsuji N, Incorporation of functionalized gold nanoparticles into nanofibers for enhanced attachment and differentiation of mammalian cells, J. Nanobiotechnology 10 (2012) 1–10. doi: 10.1186/1477-3155-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Li Y, Shi X, Tian L, Sun H, Wu Y, Li X, Li J, Wei Y, Han X, Zhang J, Jia X, Bai R, Jing L, Ding P, Liu H, Han D, AuNP–Collagen Matrix with Localized Stiffness for Cardiac-Tissue Engineering: Enhancing the Assembly of Intercalated Discs by β1-Integrin-Mediated Signaling, Adv. Mater 28 (2016) 10230–10235. doi: 10.1002/adma.201603027. [DOI] [PubMed] [Google Scholar]
- [136].Herron TJ, Da Rocha AM, Campbell KF, Ponce-Balbuena D, Willis BC, Guerrero-Serna G, Liu Q, Klos M, Musa H, Zarzoso M, Bizy A, Furness J, Anumonwo J, Mironov S, Jalife J, Extracellular Matrix-Mediated Maturation of Human Pluripotent Stem Cell-Derived Cardiac Monolayer Structure and Electrophysiological Function., Circ. Arrhythm. Electrophysiol 9 (2016) e003638. doi: 10.1161/CIRCEP.113.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Li Y, Wang F, Cui H, Peptide-based supramolecular hydrogels for delivery of biologics, Bioeng. Transl. Med 1 (2016) 306–322. doi: 10.1002/btm2.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Tatman PD, Muhonen EG, Wickers ST, Gee AO, Kim E-S, Kim D-H, Self-assembling peptides for stem cell and tissue engineering., Biomater. Sci 4 (2016) 543–54. doi: 10.1039/c5bm00550g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wang RM, Christman KL, Decellularized myocardial matrix hydrogels: In basic research and preclinical studies, Adv. Drug Deliv. Rev (2016). doi: 10.1016/j.addr.2015.06.002. [DOI] [PMC free article] [PubMed]
- [140].Xu H-L, Tian F-R, Xiao J, Chen P-P, Xu J, Fan Z-L, Yang J-J, Lu C-T, Zhao Y-Z, Sustained-release of FGF-2 from a hybrid hydrogel of heparin-poloxamer and decellular matrix promotes the neuroprotective effects of proteins after spinal injury., Int. J. Nanomedicine 13 (2018) 681–694. doi: 10.2147/IJN.S152246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lian S, Xiao Y, Bian Q, Xia Y, Guo C, Wang S, Lang M, Injectable hydrogel as stem cell scaffolds from the thermosensitive terpolymer of NIPAAm/AAc/HEMAPCL., Int. J. Nanomedicine 7 (2012) 4893–905. doi: 10.2147/IJN.S32645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A, Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels, Biomaterials (2015). doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed]
- [143].Saludas L, Pascual-Gil S, Prósper F, Garbayo E, Blanco-Prieto M, Hydrogel based approaches for cardiac tissue engineering, Int. J. Pharm 523 (2017) 454–475. doi: 10.1016/j.ijpharm.2016.10.061. [DOI] [PubMed] [Google Scholar]
- [144].Awada HK, Johnson NR, Wang Y, Sequential delivery of angiogenic growth factors improves revascularization and heart function after myocardial infarction, J. Control. Release 207 (2015) 7–17. doi: 10.1016/j.jconrel.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hasan A, Khattab A, Islam MA, Hweij KA, Zeitouny J, Waters R, Sayegh M, Hossain MM, Paul A, Injectable Hydrogels for Cardiac Tissue Repair after Myocardial Infarction, Adv. Sci (2015). doi: 10.1002/advs.201500122. [DOI] [PMC free article] [PubMed]
- [146].Zhao XL, Ding XB, Deng ZH, Zheng ZH, Peng YX, Long XP, Thermo switchable electronic properties of a gold nanoparticle/hydrogel composite, Macromol. Rapid Commun 26 (2005) 1784–1787. doi:DOI 10.1002/marc.200500534. [DOI] [Google Scholar]
- [147].Wu S, Duan B, Qin X, Butcher JT, Living nano-micro fibrous woven fabric/hydrogel composite scaffolds for heart valve engineering, Acta Biomater 51 (2017) 89–100. doi: 10.1016/j.actbio.2017.01.051. [DOI] [PubMed] [Google Scholar]
- [148].Duan Y, Liu Z, O’Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G, Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors., J. Cardiovasc. Transl. Res 4 (2011) 605–15. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Dai W, Zhang Y, Du Z, Ru M, Lang M, The pH-induced thermosensitive poly (NIPAAm-co-AAc-co-HEMA)-g-PCL micelles used as a drug carrier, J. Mater. Sci. Mater. Med 21 (2010) 1881–1890. doi: 10.1007/s10856-010-4049-x. [DOI] [PubMed] [Google Scholar]
- [150].Wang W, Tan B, Chen J, Bao R, Zhang X, Liang S, Shang Y, Liang W, Cui Y, Fan G, Jia H, Liu W, An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction, Biomaterials 160 (2018) 69–81. doi: 10.1016/j.biomaterials.2018.01.021. [DOI] [PubMed] [Google Scholar]
- [151].Xu G, Wang X, Deng C, Teng X, Suuronen EJ, Shen Z, Zhong Z, Injectable biodegradable hybrid hydrogels based on thiolated collagen and oligo(acryloyl carbonate)–poly(ethylene glycol)–oligo(acryloyl carbonate) copolymer for functional cardiac regeneration, Acta Biomater 15 (2015) 55–64. doi: 10.1016/j.actbio.2014.12.016. [DOI] [PubMed] [Google Scholar]
- [152].Sherrell PC, Cieślar-Pobuda A, Ejneby MS, Sammalisto L, Gelmi A, de Muinck E, Brask J, Łos MJ, Rafat M, Rational Design of a Conductive Collagen Heart Patch, Macromol. Biosci 17 (2017) 1–10. doi: 10.1002/mabi.201600446. [DOI] [PubMed] [Google Scholar]
- [153].Tang J, Cui X, Caranasos TG, Hensley MT, Hartanto Y, Shen D, Zhang H, Zhang J, Cheng K, Heart Repair Using Nanogel-Encapsulated, (2017). doi: 10.1021/acsnano.7b01008. [DOI] [PMC free article] [PubMed]
- [154].Xu B, Li Y, Deng B, Liu X, Wang L, Zhu QL, Chitosan hydrogel improves mesenchymal stem cell transplant survival and cardiac function following myocardial infarction in rats, Exp. Ther. Med 13 (2017) 588–594. doi: 10.3892/etm.2017.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Wang H, Shi J, Wang Y, Yin Y, Wang L, Liu J, Liu Z, Duan C, Zhu P, Wang C, Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction, Biomaterials 35 (2014) 3986–3998. doi: 10.1016/j.biomaterials.2014.01.021. [DOI] [PubMed] [Google Scholar]
- [156].Anker SD, Coats AJS, Cristian G, Dragomir D, Pusineri E, Piredda M, Bettari L, Dowling R, Volterrani M, Kirwan B-A, Filippatos G, Mas J-L, Danchin N, Solomon SD, Lee RJ, Ahmann F, Hinson A, Sabbah HN, Mann DL, FH R, S. J, S. P, S. J, T. PT, V. AA, Z. F, Z. A, A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial), Eur. Heart J 36 (2015) 2297–2309. doi: 10.1093/eurheartj/ehv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Radhakrishnan J, Krishnan UM, Sethuraman S, Hydrogel based injectable scaffolds for cardiac tissue regeneration, Biotechnol. Adv 32 (2014) 449–461. doi: 10.1016/j.biotechadv.2013.12.010. [DOI] [PubMed] [Google Scholar]
- [158].Li Z, Fan Z, Xu Y, Lo W, Wang X, Niu H, Li X, Xie X, Khan M, Guan J, pH-Sensitive and Thermosensitive Hydrogels as Stem-Cell Carriers for Cardiac Therapy, (2016) 10752–10760. doi: 10.1021/acsami.6b01374. [DOI] [PMC free article] [PubMed]
- [159].Jackman CP, Carlson AL, Bursac N, Dynamic culture yields engineered myocardium with near-adult functional output, Biomaterials 111 (2016) 66–79. doi: 10.1016/j.biomaterials.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Tapias LF, Ott HC, Decellularized scaffolds as a platform for bioengineered organs., Curr. Opin. Organ Transplant 19 (2014) 145–52. doi: 10.1097/MOT.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Gilpin A, Yang Y, Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications., Biomed Res. Int 2017 (2017) 9831534. doi: 10.1155/2017/9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Wang Q, Yang H, Bai A, Jiang W, Li X, Wang X, Mao Y, Lu C, Qian R, Guo F, Ding T, Chen H, Chen S, Zhang J, Liu C, Sun N, Functional engineered human cardiac patches prepared from nature’s platform improve heart function after acute myocardial infarction, Biomaterials 105 (2016) 52–65. doi: 10.1016/j.biomaterials.2016.07.035. [DOI] [PubMed] [Google Scholar]
- [163].Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, Taylor DA, Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart, Nat. Med 14 (2008) 213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- [164].Lu T-Y, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L, Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells, Nat. Commun 4 (2013) 1–11. doi: 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]
- [165].Schwan J, Kwaczala AT, Ryan TJ, Bartulos O, Ren Y, Sewanan LR, Morris AH, Jacoby DL, Qyang Y, Campbell SG, Anisotropic engineered heart tissue made from laser-cut decellularized myocardium, Sci. Rep 6 (2016) 32068. doi: 10.1038/srep32068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Gershlak JR, Hernandez S, Fontana G, Perreault LR, Hansen KJ, Larson SA, Binder BYK, Dolivo DM, Yang T, Dominko T, Rolle MW, Weathers PJ, Medina-Bolivar F, Cramer CL, Murphy WL, Gaudette GR, Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds, Biomaterials 125 (2017) 13–22. doi: 10.1016/j.biomaterials.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Badylak SF, Brown BN, Gilbert TW, Tissue Engineering with Decellularized Tissues, Third Edit, Elsevier, 2013. doi: 10.1016/B978-0-08-087780-8.00140-6. [DOI] [Google Scholar]
- [168].Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, Pi Q, Byambaa B, Dokmeci MR, Shin SR, Khademhosseini A, Direct 3D bioprinting of perfusable vascular constructs using a blend bioink, Biomaterials 106 (2016) 58–68. doi: 10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Wang Z, Lee SJ, Cheng H-J, Yoo JJ, Atala A, 3D Bioprinted Functional and Contractile Cardiac Tissue Constructs, Acta Biomater (2018). doi: 10.1016/j.actbio.2018.02.007. [DOI] [PMC free article] [PubMed]
- [170].Zhu W, Qu X, Zhu J, Ma X, Patel S, Liu J, Wang P, Lai CSE, Gou M, Xu Y, Zhang K, Chen S, Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture, Biomaterials 124 (2017) 106–115. doi: 10.1016/j.biomaterials.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Usprech J, Romero DA, Amon CH, Simmons CA, Combinatorial screening of 3D biomaterial properties that promote myofibrogenesis for mesenchymal stromal cell-based heart valve tissue engineering, Acta Biomater 58 (2017) 34–43. doi: 10.1016/j.actbio.2017.05.044. [DOI] [PubMed] [Google Scholar]
- [172].Bourget J, Guillemette M, Veres T, Auger F. a, Germain L, Alignment of Cells and Extracellular Matrix Within Tissue-Engineered Substitutes, Adv. Biomater. Sci. Biomed. Appl. Ref (2013) 365–390. doi: 10.5772/54142. [DOI]
- [173].Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, Cerino G, Redaelli A, Rasponi M, Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues, Lab Chip 16 (2016) 599–610. doi: 10.1039/C5LC01356A. [DOI] [PubMed] [Google Scholar]
- [174].Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A, Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip, Biomaterials 110 (2016) 45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Bell A, Kofron M, Nistor V, Multiphoton crosslinking for biocompatible 3D printing of type I collagen, Biofabrication 7 (2015) 035007. doi: 10.1088/1758-5090/7/3/035007. [DOI] [PubMed] [Google Scholar]
- [176].Sidorov VY, Samson PC, Sidorova TN, Davidson JM, Lim CC, Wikswo JP, I-Wire Heart-on-a-Chip I: Three-dimensional cardiac tissue constructs for physiology and pharmacology, Acta Biomater 48 (2017) 68–78. doi: 10.1016/j.actbio.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Mirabella AT, Macarthur JW, Cheng D, Ozaki CK, Yoo YJ, Yang M, Title : 3D printed vascular networks direct therapeutic angiogenesis in ischemia, Nat. Biomed. Eng 0083 (2017) 1–6. doi: 10.1038/s41551-017-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Tijore A, Irvine SA, Sarig U, Mhaisalkar P, Baisane V, Venkatraman SS, Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel., Biofabrication 10 (2017) 025003. doi: 10.1088/1758-5090/aaa15d. [DOI] [PubMed] [Google Scholar]
- [179].Kobuszewska A, Tomecka E, Zukowski K, Jastrzebska E, Chudy M, Dybko A, Renaud P, Brzozka Z, Heart-on-a-Chip: An Investigation of the Influence of Static and Perfusion Conditions on Cardiac (H9C2) Cell Proliferation, Morphology, and Alignment, SLAS Technol 22 (2017) 536–546. doi: 10.1177/2472630317705610. [DOI] [PubMed] [Google Scholar]
- [180].Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park SJ, Kotikian A, Nesmith AP, Campbell PH, Vlassak JJ, Lewis JA, Parker KK, Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing, Nat. Mater 16 (2017) 303–308. doi: 10.1038/nmat4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Gao L, Kupfer ME, Jung JP, Yang L, Zhang P, Da Sie Y, Tran Q, Ajeti V, Freeman BT, Fast VG, Campagnola PJ, Ogle BM, Zhang J, Myocardial Tissue Engineering with Cells Derived from Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold, Circ. Res 120 (2017) 1318–1325. doi: 10.1161/CIRCRESAHA.116.310277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Ong CS, Fukunishi T, Zhang H, Huang CY, Nashed A, Blazeski A, Disilvestre D, Vricella L, Conte J, Tung L, Tomaselli GF, Hibino N, Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes, Sci. Rep 7 (2017) 2–12. doi: 10.1038/s41598-017-05018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Wang W, Tan B, Chen J, Bao R, Zhang X, Liang S, Shang Y, Liang W, Cui Y, Fan G, Jia H, Liu W, An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction, Biomaterials (2018). doi: 10.1016/j.biomaterials.2018.01.021. [DOI] [PubMed]