INTRODUCTION
Measles is a highly communicable disease with a high burden among children in low- and middle- income countries (LMICs).[1] Edmonston-derived vaccines strains are the most commonly used measles-containing vaccines (MCV) in the US and Europe. China uses a vaccine from a locally-isolated strain, the Shanghai- (or Hu-) 191 strain, which has been shown to have comparable safety and immunogenicity to the Edmonston-derived vaccine.[2,3]. With these vaccines, measles has been targeted for elimination efforts across the globe.[4,5] Despite significant improvement in measles vaccination coverage, progress toward elimination has slowed in recent years.[6] Achieving elimination is complicated by the high communicability of measles, which requires high vaccination coverage in tandem with high vaccine efficacy.
Vaccine effectiveness (VE) of a single MCV dose, under ideal conditions, has ranged from 85% when administered at 9 months to 90–95% when given at 12 months.[7,8] The immune response to vaccination can be influenced by various host factors, including antibodies acquired through maternal antibody transfer, HIV infection, malnutrition, or other forms of immunosuppression.[8] Given this variability of immune response, a universal recommendation to implement a two-dose MCV schedule was put forth by WHO in 2009.[9] Whereas VE of MCV1 was reported to be as low as 73% in the WHO African region and 77% in the South-East Asian Region, after widespread adoption of the two-dose schedule, median VE was reported to be 94.1% (IQR, 88.3%–98.3%). [10] Despite very high VE under test conditions and significant utilization of the two-dose schedule globally, VE is lower and much more variable than originally anticipated. Further investigations are necessary as to the cause of this discrepancy to reach immunity thresholds necessary for global elimination.
In China, children receive MCV at 8 months, 18–24 months, and in some large urban areas, including Beijing, Tianjin, and Shanghai, a third dose is provided at 4–6 years of age; vaccination coverage is high among children (>97% for the 1st and 2nd dose in Tianjin), although vaccination records for adults are unavailable.[11] Public health officials in China are concerned about the high number of measles cases which are occurring among individuals with multiple vaccinations (Personal communication, Ding Yaxing, Expanded Programs on immunization, Tianjin Centers for Disease Control and Prevention, November 12, 2018). This study sought to characterize the prevalence of vaccine failure by exploring (1) the vaccination history of childhood measles cases and (2) the differences in time-to-measles-diagnosis based on vaccination history in Tianjin.
METHODS
This study includes data from two datasets. The Tianjin surveillance dataset is described in detail elsewhere.[12] Measles is a nationally notifiable disease in China, and data regarding cases are reported into the China Information System for Disease Control and Prevention (CISDCP), a web-based communicable disease surveillance system. After a diagnosis of laboratory-confirmed measles, health care providers report the case through CISDCP. National, provincial, and district-level Centers for Disease Control and Prevention (CDCs) in China have access to disease data within their jurisdiction. This analysis includes measles data from 2009–2013 within Tianjin.
The International Collaborations in Infectious Diseases Research (ICIDR) dataset is described in detail elsewhere.[11] During the study period of 2011–2015, measles cases were enrolled into a case-control study. Every week, staff at the Tianjin CDC downloaded cases reported into CISDCP from the past week and randomly selected between 1 and 37 cases to enroll into this study based on overall case-load. For weeks with few cases, most cases would be enrolled, for weeks with more than 40 cases, only a fraction would be approached. This dataset did not contain individual birthdate records, only the age of the individual at the survey date, and their vaccination history. Birthdates were imputed based on the age at the survey date to approximate age at measles diagnosis. Age at case-diagnosis was determined by subtracting the imputed birth date from the date of diagnosis. The surveillance system collected information on whether the individual had 0, 1, or 2 or more doses, whereas in the case-series, selection options were 0, 1, 2, 3, or 4 doses given. More details about the surveillance system and the ICIDR study are available at https://doi.org/10.6084/m9.figshare.7597277.
This study focuses on individuals 8 months-19 years of age. Vaccination status was assessed through dates on vaccination cards, and this age group was selected to encompass those who were old enough to have received the measles vaccine based on Tianjin’s vaccination schedule, but were not so old as to lack vaccination data. We reasoned this age group represented the population segment most likely to be vaccinated and not yet experiencing waning immunity from the vaccine; thus their vaccinations would be the best indication of protection against measles. Within this age range, the distribution of cases is reported by more specific age groupings, and the impact of vaccination is assessed by calculating the time interval between age at vaccination and age at disease, stratified by number of MCV doses. Statistical analysis was conducted in SAS version 9.4 and R version 3.3.3 using the survival & survminer packages.
Ethical Consideration
This study was approved by the University of Michigan Institutional Review Board and the Tianjin Centers for Disease Control and Prevention Ethics Committee.
RESULTS
The surveillance dataset contained data for 2,945 measles cases from 2009–2013 and the case series dataset contained 500 measles cases from 2011–2015. Most cases were ≥19 years of age (61.1% and 78.0% in the surveillance and case series datasets, respectively). 19.0% of the cases in the surveillance dataset and 12.4% of the cases in the case series were in the study’s target age range, 8 months-19 years (Table S1). Among the target age group, in the surveillance dataset, 57.4% of cases did not receive the measles vaccine, 29.0% received at least one dose MCV, and the remaining 13.6% did not know their vaccination status. In the case series, 43.5% of cases were unvaccinated and 54.4% of cases had received at least one dose of MCV, with 1 case unaware of vaccination status.
Analysis of the time-to-measles diagnosis stratified by vaccination status revealed increasing time-to-measles diagnosis with each successive dose for the case series and surveillance datasets (Table 1). Within the surveillance dataset, the median time-to-diagnosis was considerably longer for those who received two vaccines versus a single dose of MCV: 4.26 years vs. 1 year, compared to 0.82 years, or ~9.8 months for those who were unvaccinated. The minimum time-to-diagnosis since the first measles vaccination (administered at ~8 months) also increased with the number of doses received. A similar trend was observed in the case-control dataset, with median time-to-diagnosis increasing from 301 days for those who received only one vaccine (approximately 9.8 months), to 7.49 years for those who received two or more vaccinations.
Table 1.
Distribution of Measles time-to-diagnosis since in days (and years) stratified by measles vaccination history, children aged 8 months – 19 years, surveillance and case-control datasets
| Measles Diagnosis Time Since Vaccine | Minimum | Median | Mean | Maximum |
|---|---|---|---|---|
| Case-Control Dataset (2011–2015) | ||||
| Missing/No Dose Information (n = 21) | 247 (0.68) | 518 (1.42) | 1136 (3.11) | 6381 (17.48) |
| 1 Dose Received (n = 13) | 248 (0.68) | 301 (0.82) | 1270 (3.48) | 5657 (15.50) |
| Diagnosis time since vaccination (n = 12)* | 4 (0.01) | 41 (0.08) | 649 (1.78) | 4222 (11.57) |
| At least 2 doses received (n = 12) | 560 (1.53) | 2735 (7.49) | 2434 (6.67) | 4935 (13.52) |
| Diagnosis time since vaccination (n = 12)* | 372 (1.02) | 2349 (6.44) | 2134 (5.86) | 4593 (12.58) |
| Surveillance Dataset (2009–2013) | ||||
| Missing/No Dose Information (n = 76) | 272 (0.75) | 5655 (15.49) | 4011 (10.99) | 6909 (18.93) |
| Non-Vaccinated (n = 321) | 242 (0.66) | 298 (0.82) | 601 (1.65) | 6919 (18.96) |
| 1 Dose Received (n = 114) | 242 (0.66) | 364 (1.00) | 664 (1.82) | 6291 (17.24) |
| Diagnosis time since vaccination (n = 99)* | 0 (0) | 24 (0.07) | 186 (0.51) | 3253 (8.91) |
| At least 2 doses received (n = 48) | 485 (1.33) | 1557 (4.26) | 2439 (6.68) | 6871 (18.82) |
| Diagnosis time since vaccination (n = 33)* | 301 (0.82) | 1192 (3.27) | 1778 (4.87) | 5806 (15.91) |
Additionally, for those who received any doses of the measles vaccine, we report the distribution of time-to infection since the first dose of measles vaccine in days (i.e. at approximately 8 months). This was included to clarify the time to infection since initiating the vaccination schedule.
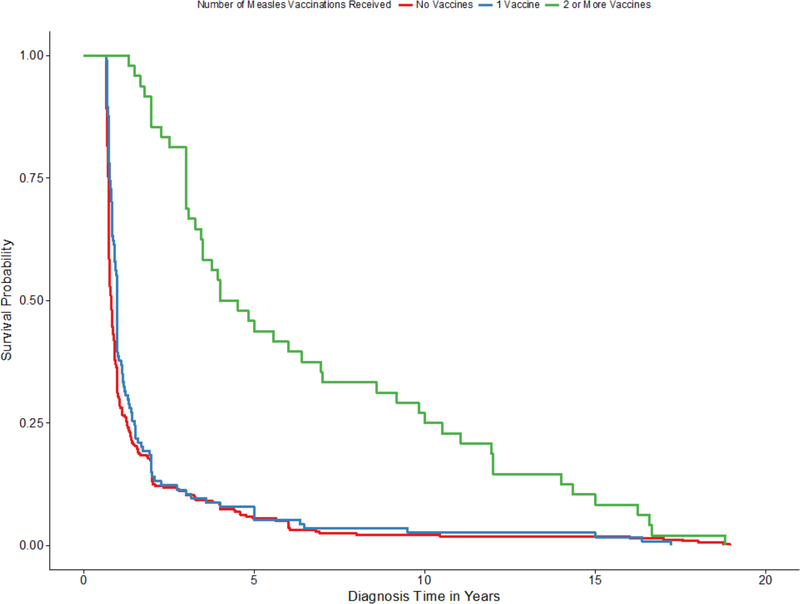
Kaplan-Meier survival curves from the Tianjin surveillance dataset (Fig. 1) corroborate the findings in Table 1, and reveal that those who received zero measles vaccinations were the youngest at diagnosis. The results showed statistically significantly different time-to-disease onset by dose (log-rank p-value < 0.0001). While one dose of measles vaccine somewhat delayed the diagnostic onset for measles, having ≥2 vaccinations significantly delayed the time-to-diagnosis.
Figure 1.
Product-Limit Survival Estimates of diagnosis time, in years, of measles diagnosis, stratified by the number of measles vaccinations received: 0, 1, or 2 or more for the Tianjin surveillance dataset among individuals aged 8 months – 19 years.
DISCUSSION
This analysis revealed that over a quarter of cases of measles in Tianjin, China had received ≥1 dose prior to contracting the disease. Time-to-event analysis also showed that those with two doses or more of MCV had significantly delayed onset of measles, though the onset of measles for those who received only one dose MCV was similar to those who were unvaccinated. This evidence points to the fact that one vaccine is insufficient, and perhaps that the dose administered at 8 months of age, one of the earliest suggested dose times in the world for MCV, may have a reduced immune response, producing the similar time-to-measles diagnosis curves. Accordingly, the second dose at 18–24 months is necessary for full protection.
This study reveals that within a small subset of measles cases in Tianjin who have recorded vaccination history, there is a high burden of measles among those who have been vaccinated. As such, a vaccine effectiveness (VE) study may be warranted to explore potential reasons for these breakthrough cases. Measles VE studies have found variable effectiveness of the measles vaccine, ranging from 23.1% for one dose and 63.4% for two doses in Micronesia [13], to 70% in Uganda [14] to 96.7% for one dose and 99.7% for two doses with waning immunity in Australia. [15] Potential reasons for variable VE and vaccine failure were suspected to be due to improper cold chain management [13,16] and waning immunity [13,15]. Our findings are similar to those of Durrheim et al., in which multiple countries approaching elimination found that a large proportion of cases were vaccinated. [16] Long-term VE could also vary by age at administration. A recent study in China found similar immunogenicity of measles-containing vaccine given at 8 versus 12 months [17], but a long-term study of measles antibodies in children who had been vaccinated early (at 9 months) during an outbreak in The Netherlands found that children vaccinated at 9 months had significantly lower concentrations of measles antibodies compared to a control group at 4 years of age. [18]
This study has limitations: first, these two datasets may not be representative beyond Tianjin to all of China, limiting the generalizability of the findings. Given the small number of cases for whom there was available vaccination data in both datasets, there may be bias due to missing data. Additionally, potential measurement error may have resulted in misclassification of the number of doses received or if vaccination status was unknown, small numbers of cases in certain groupings decrease potential for statistical significance testing, and there may be duplicate cases between the two datasets. There may also be bias in the selection of cases into the ICIDR case-series. Finally, the lack of more recent data from Tianjin limits the potential to explore trends in measles vaccination and subsequent disease. Given these limitations, this study can primarily serve for hypotheses generation indicating areas for further study.
CONCLUSIONS
This analysis of measles cases in Tianjin found that among children with a recorded vaccine history, a substantial number of those who contracted measles had received at least one MCV dose. Although time-to-diagnosis following vaccination increases with receipt of each successive dose of measles vaccine, the fact that 8.5% of cases in the surveillance dataset and 26% in the case series contracted measles despite 2 or more doses of MCV is surprising. This also has implications for the VE of the measles vaccine series in Tianjin. Future research is needed to identify whether this is due to primary or secondary vaccine failure, and whether cold chain management, low vaccine efficacy, scheduled dose timing, or host factors such as co-morbidities and waning immunity might be responsible. This analysis motivates further research to discern the cause of these breakthrough cases in both outbreak and isolated case settings.
Supplementary Material
Funding Statement:
This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Disease, Division of Microbial and Infectious Diseases [U01-AI-088671].
ALW was supported by the PhRMA Foundation (Health Outcomes Post Doctoral Fellowship). The PhRMA Foundation did not have any role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication. We report no other external funding for this manuscript.
Abbreviations
- LMICs
Low and middle income countries
- MCV
Measles-containing vaccine
- VE
Vaccine effectiveness
Footnotes
Conflict of Interest Statement: The authors have no potential, perceived, or real conflicts of interest relevant to this article to disclose. NBM wrote the first draft of the article.
REFERENCES
- [1].Centers for Disease Control and Prevention (CDC). Measles In: Hamborsky J, Kroger A, Wolfe SE, editor. Epidemiol. Prev. Vaccine-Preventable Dis. 13th Ed. 13th ed, Washington D.C.: Public Health Foundation; 2015, p. 209–29. [Google Scholar]
- [2].Xiang J, Chen Z. Measles Vaccine in the People’s Republic of China. Rev Infect Dis 1983;5:506–10. [PubMed] [Google Scholar]
- [3].Fu J, He H, Chen E, Yan C, Yan R, Yin Z, et al. Safety and Immunogenicity of the Imported and Domestic Measles, Mumps and Rubella Combined Attenuated Live Vaccine. Chin J Vaccine Immun 2013;19:222–249 [in Chinese]. [Google Scholar]
- [4].Moss WJ, Griffin DE. Global measles elimination. Nat Rev Microbiol 2006;4:900–8. doi: 10.1038/nrmicro1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].World Health Organization. Global Measles and Rubella Strategic Plan 2012–2020. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- [6].Perry RT, Murray JS, Gacic-Dobo M, Dabbagh A, Mulders MN SP et al. Progress toward regional measles elimination - worldwide, 2000–2014. MMWR Morb Mortal Wkly Rep 2015;64:1245–51. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- [7].Cuffs FT, Grabowsky M, Markowitz LE. The effect of dose and strain of live attenuated measles vaccines on serological responses in young infants. Biologicals 1995;23:95–106. doi: 10.1016/1045-1056(95)90018-7. [DOI] [PubMed] [Google Scholar]
- [8].World Health Organization (WHO). Measles: The immunological basis for immunization, 2009. n.d.
- [9].World Health Organization (WHO). Measles Position Paper. 2009.
- [10].Uzicanin A, Zimmerman L. Field effectiveness of live attenuated measles-containing vaccines: a review of published literature. J Infect Dis 2011;204:S133–48. doi: 10.1093/infdis/jir102. [DOI] [PubMed] [Google Scholar]
- [11].Zhang Y, Wagner AL, Wang X, Carlson BF, Ding Y, Montgomery JP, et al. Risk factors for measles among infants in Tianjin, China. Public Health 2017;151. doi: 10.1016/j.puhe.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang X, Boulton ML, Montgomery JP, Carlson B, Zhang Y, Gillespie B, et al. The epidemiology of measles in Tianjin, China, 2005–2014. Vaccine 2015;33:6186–91. doi: 10.1016/j.vaccine.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hales CM, Johnson E, Helgenberger L, Papania MJ, Larzelere M, Gopalani SV., et al. Measles outbreak associated with low vaccine effectiveness among adults in Pohnpei State, Federated States of Micronesia, 2014. Open Forum Infect Dis 2016;3:1–7. doi: 10.1093/ofid/ofw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nsubuga F, Bulage L, Ampeire I, Matovu JKB, Kasasa S, Tanifum P, et al. Factors contributing to measles transmission during an outbreak in Kamwenge District, Western Uganda, April to August 2015. BMC Infect Dis 2018;18:21. doi: 10.1186/s12879-017-2941-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pillsbury A, Quinn H. An assessment of measles vaccine effectiveness, Australia, 2006–2012. West Pacific Surveill Response J 2015;6:43–50. doi: 10.5365/wpsar.2015.6.2.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zahidie A, Wasim S, Fatmi Z. Vaccine effectiveness and risk factors associated with measles among children presenting to the hospitals of Karachi, Pakistan. J Coll Physicians Surg Pak 2014;24:882–8. doi:12.2014/JCPSP.882888. [PubMed] [Google Scholar]
- [17].He H, Chen E, Chen H, Wang Z, Li Q, Yan R, et al. Similar immunogenicity of measles-mumps-rubella (MMR) vaccine administrated at 8 months versus 12 months age in children. Vaccine 2014;32:4001–5. doi: 10.1016/j.vaccine.2014.04.044. [DOI] [PubMed] [Google Scholar]
- [18].Brinkman ID, de Wit J, Smits GP, Jongerius MC, Taymara C, van Binnendijk RS. Early measles vaccination during an outbreak in The Netherlands: reduced short and long-term antibody responses in children vaccinated before 12 months of age. J Infect Dis 2019:pii: jiz159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.