Abstract
Young adult-born granule cells (abGCs) in the dentate gyrus (DG) have a profound impact on cognition and mood. However, it remains unclear how abGCs distinctively contribute to local DG information processing. We found that the actions of abGCs in the DG depend on the origin of incoming afferents. In response to lateral entorhinal cortex (LEC) inputs, abGCs exert monosynaptic inhibition of mature granule cells (mGCs) through group II metabotropic glutamate receptors. By contrast, in response to medial entorhinal cortex (MEC) inputs, abGCs directly excite mGCs through N-methyl-D-aspartate receptors. Thus, a critical function of abGCs may be to regulate the relative synaptic strengths of LEC-driven contextual information versus MEC-driven spatial information to shape distinct neural representations in the DG.
Young adult-born granule cells (abGCs) (≤6 weeks old) are essential for hippocampal-dependent tasks involving behavioral pattern separation, cognitive flexibility, and forgetting (1, 2). The behavioral impact of neurogenesis may be accounted for by the increased plasticity of abGCs and/or by abGC-mediated modulation of mature granule cells (mGCs) (≥8 weeks old) (1, 2). Feedback inhibition of mGCs by means of local interneurons is the only known abGC-driven modulatory pathway in the DG, but its importance to mGC excitability is debatable (3, 4).
The dentate gyrus (DG) is primarily activated by synaptic inputs from the lateral entorhinal cortex (LEC) and medial entorhinal cortex (MEC) via the lateral and medial performant paths (LPPs and MPPs), respectively (5). LPP inputs tend to signal nonspatial contextual features, whereas MPP inputs are mostly related to spatial information (6, 7). Thus, determining how abGCs affect mGCs in response to LPP versus MPP synaptic activation is necessary for understanding their contribution to DG information processing.
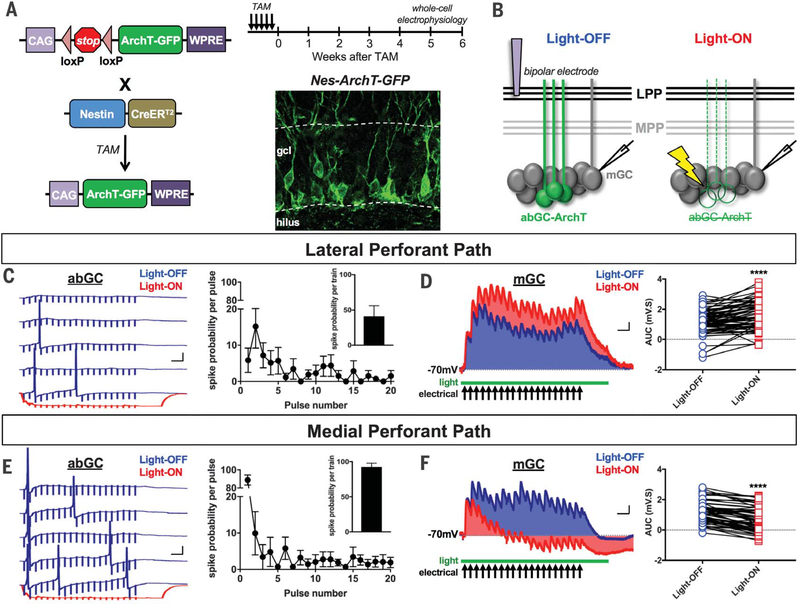
For these experiments, we crossed mice expressing the tamoxifen (TAM)–inducible recombinase CreERT2 under the Nestin (Nes) promoter with a floxed-stop archaerhodopsin T (ArchT) line (referred to as Nes-ArchT) (Fig. 1A). We selected this transgenic line because of its high recombination efficiency and specificity for labeling neural stem cells in the DG (4, 8). This allowed us to optogenetically silence a large number of young abGCs and assess their concerted actions on mGC excitability in DG slices (Fig. 1).
Fig. 1. Bidirectional abGC-driven modulation of LPP- and MPP-evoked mGC responses.
(A) Breeding strategy and TAM-induction schedule. Confocal image of abGCs in the DG of a Nes-ArchT-GFP mouse. gcl, granule cell layer; GFP, green fluorescent protein; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element. (B) Experimental protocol: A bipolar stimulating electrode was positioned in the LPP or MPP Whole-cell current clamp recordings were performed on GCs before (Light-OFF) and during (Light-ON) optogenetic silencing of abGCs with a 532-nm green laser projected through a 40× objective. (C and E) (Left) Five traces from a representative abGC spiking in response to 40-Hz train stimulation. Stimulus artifacts show 20 pulses (0.1 ms) in each trial. Blue and red traces indicate spike activity during Light-OFF and Light-ON conditions, respectively. (Right) Average abGC spike probability for each pulse in the train. (Inset) Average abGC spike probability per train. Error bars indicate SEM. (D and F) (Left) Average evoked synaptic potentials in a representative mGC. Shaded blue (Light-OFF) and red (Light-ON) areas indicate AUC. AUC = area above baseline (depolarization) minus area below baseline (hyperpolarization). (Right) Plots comparing Light-OFF versus Light-ON AUCs for each mGC. Scale bars: 20 mV, 50 ms [(C) and (E)]; 0.5 mV, 50 ms [(D) and (F)].
We performed whole-cell current clamp recordings on abGCs and mGCs and recorded synaptic potentials evoked by LPP or MPP electrical stimulation before (Light-OFF) and after (Light-ON) silencing abGCs (Fig. 1B). To prevent nonspecific excitation of DG cells and limit current spread to the LPP or MPP, we used the lowest stimulation intensity that elicited 100% synaptic responses. We measured paired-pulse ratios (interstimulus interval = 25 ms) on the basis of the first two pulses in our stimulation protocol to verify that we were stimulating the LPP (ratio > 1: mean ± SEM = 1.55 ± 0.08; n = 78 cells) or MPP (ratio < 1: 0.84 ± 0.04; n = 65 cells) (9).
In vivo studies have shown that the entorhinal cortex transmits behaviorally relevant information to the DG as 10- to 40-Hz synaptic bursts (10). We therefore used a 40-Hz train stimulation of the LPP or MPP to recapitulate these conditions. We found that abGCs had a significantly lower spike probability per train in response to the LPP (41 ± 15%; n = 7) than the MPP (92 ± 5; n = 8; P < 0.01) (Fig. 1, C and E). We also found that MPP-evoked spikes occurred mostly at the first pulse (89 ± 6%, n = 8) (Fig. 1E), whereas LPP-evoked spikes were more temporally dispersed with the highest probability at the second pulse (15 ± 8%, n = 7) (Fig. 1C). These results suggest that larger numbers of abGCs would spike in response to the MPP than to the LPP. Moreover, there is a greater probability that multiple abGCs would fire simultaneously in response to the MPP than to the LPP. Optogenetic inhibition robustly hyperpolarized abGCs (−30.79 ± 1.95 mV; n = 6), effectively blocking all of their action potentials (Fig. 1, C and E).
We found that silencing abGCs had opposite effects on LPP-versus MPP-evoked mGC synaptic responses [measured using area under the curve (AUC) in millivolt-seconds]; AUC captures the cumulative effect of the shifts in depolarization and hyperpolarization in a train (Fig. 1, D and F). Silencing abGCs increased the magnitude of mGC responses elicited by 40-Hz LPP trains (Light-OFF = 1.27 ± 0.13 mV·s; Light-ON = 1.74 ± 0.12 mV·s; n = 78; P < 0.0001; Fig. 1D), indicating that abGCs inhibit mGCs; 10-Hz and 20-Hz trains gave similar results (fig. S1). By contrast, silencing abGCs decreased MPP-evoked response magnitudes (Light-OFF = 1.12 ± 0.09 mV·s; Light-ON = 0.63 ± 0.10 mV·s; n = 65; P < 0.0001; Fig. 1F) (also 10 Hz and 20 Hz; fig. S1), indicating that in these conditions abGCs excite mGCs. In fact, Light-ON responses would often dip below baseline (Fig. 1F), likely because of inhibitory interneurons hyperpolarizing mGCs.
abGCs could modulate mGCs through gamma-aminobutyric acid receptor (GABAAR)-mediated feedback inhibition (3, 4). However, GABAAR antagonists failed to alter the effects of silencing abGCs on LPP- or MPP-evoked responses (fig. S2).
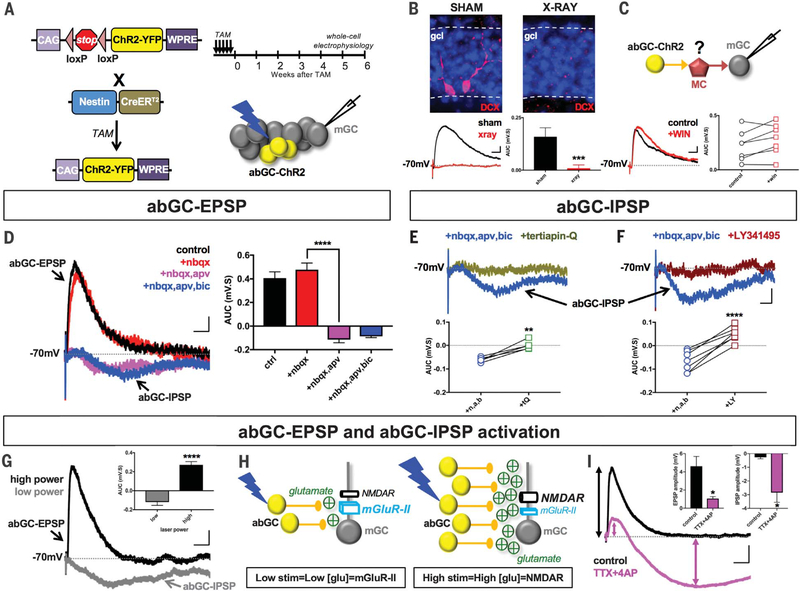
We therefore assessed what other receptors abGCs utilized to modulate mGCs. We used mice expressing Nes-CreERT2 and a floxed-stop channel-rhodopsin2 (Nes-ChR2) to optogenetically excite abGCs (Fig. 2A). We recorded strong excitatory postsynaptic potentials (referred to as abGC-EPSPs) in mGCs (0.30 ± 0.04 mV·s; n = 58) (Fig. 2B). These responses were not present in mice whose DGs were focally X-irradiated (Fig. 2B), indicating that they were neurogenesis dependent (8). GCs do not normally show recurrent excitatory connections (11), suggesting that abGC-EPSPs may arise from feedback excitation involving the other principal excitatory neuron in the DG: hilar mossy cells (MCs) (12). However, abGC-EPSPs were insensitive to WIN 55,212–2, an agonist of the cannabinoid-1 receptors expressed in MC axonal terminals but not in GCs (13) (Fig. 2C). Furthermore, these properties were specific to abGCs because optogenetic excitation of mGCs did not evoke EPSPs in other mGCs (fig. S3). Thus, abGC-EPSPs appear to arise from monosynaptic excitation of mGCs by abGCs.
Fig. 2. Pharmacological analysis of abGC-mediated excitation and inhibition of mGCs.
(A) Breeding strategy and TAM-induction schedule for Nes-ChR2-YFP mice. Experimental protocol: abGCs were optogenetically stimulated (473-nm blue laser; 1-ms pulse) and evoked responses recorded in mGCs. YFP, yellow fluorescent protein. (B) Doublecortin (DCX) labeling in sham versus X-irradiated mice. Sham = 0.16 ± 0.04 mV·s, n = 9; x-ray = 0.01 ± 0.02 mV·s, n = 7. Unpaired t test ***P < 0.001. All stimulus artifacts indicate onset of laser pulse. (C) Inhibiting MC transmission with WIN 55,212–2 (5 μM) did not block abGC-evoked EPSPs. Control = 0.22 ± 0.05 mV·s, n = 7; WIN = 0.28 ± 0.05 mV·s, n = 7. Paired t test P = 0.08. (D) APV (50 μM), not NBQX (20 μM), inhibited abGC-EPSPs and unmasked bicuculline (BIC; 20 μM)–insensitive abGC-IPSPs. One-way analysis of variance (ANOVA), F3,58 = 47.12 (P < 0.0001). Control (ctrl) = 0.41 ± 0.05 mV·s, n = 17; +NBQX = 0.48 ± 0.06 mV·s, n = 17; +NBQX,APV = −0.11 ± 0.03 mV·s, n = 17; +NBQX,APV,BIC = −0.09 ± 0.01 mV·s, n = 8. Fisher’s least significant difference (LSD) post hoc test: Control × NBQX P = 0.26; control × NBQX,APV P < 0.0001; control × NBQX,APV,BIC P < 0.0001; NBQX × NBQX,APV ****P < 0.0001; NBQX × NBQX,APV,BIC P < 0.0001; NBQX,APV × NBQX,APV,BIC P = 0.72. (E and F) abGC-IPSPs were blocked by Tertiapin-Q (0.75 μM) [+NBQX,APV,BIC (n,a,b) = −0.06 ± 0.00 mV·s, n = 5; + Tertiapin-Q = 0.00 ± 0.01 mV·s, n = 5; paired t test **P < 0.01] and LY3414955 (LY; 0.5 μM) (+NBQX, APV, BIC = −0.07 ± 0.02 mV·s, n = 7; +LY341495 = 0.05 ± 0.01 mV·s, n = 7; paired t test ****P < 0.0001). (G) abGC-IPSPs were recorded at low (~2 mW) and abGC-EPSPs at high (~5 mW) laser power. (H) Model of mGlu-II and NMDAR activation in mGCs based on glutamate released by small versus large numbers of abGCs. (I) abGC-evoked EPSP and IPSP amplitudes before and after bath application of TTX (1 μM) and 4-AP (1 mM). EPSP: control = 4.61 ± 1.09 mV, n = 8; TTX+4AP = 1.07 ± 1.09 mV, n = 8; paired t test *P < 0.05. IPSP: control = −0.27 ± 0.11, n = 8; TTX+4AP = −2.85 ± 0.71, n = 8; *P < 0.05. Scale bars: 0.5 mV, 25 ms [(B) and (C)]; 0.25 mV, 50 ms [(D) to (F)]; 1 mV, 50 ms [(G) and (I)]. All values = mean ± SEM.
Typically, EPSPs are mediated by glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) at resting membrane potentials. However, the AMPAR antagonist NBQX [2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline] failed to inhibit abGC-EPSPs (Fig. 2D). Instead, abGC-EPSPs were blocked by the N-methyl-D-aspartate receptor (NMDAR) antagonist D,L-2-amino-5-phosphonovaleric acid (APV) (Fig. 2D). Increasing extracellular magnesium from 1.0 mM to less physiological 1.3 mM (14) blocked abGC-EPSPs, further indicating that they were driven by NMDARs (fig. S3). These NMDARs likely contain the NR3 rather than the more common NR2 subunits because they were active at more hyperpolarized potentials (fig. S4). NR3-containing receptors are mostly found outside the postsynaptic density (15, 16), suggesting that abGCs may excite mGCs extrasynaptically.
Blocking abGC-EPSPs revealed an underlying abGC-driven inhibitory postsynaptic potential in mGCs (−0.11 ± 0.03 mV·s; n = 20) (Fig. 2, D to F). X-irradiated mice lacked abGC inhibitory postsynaptic potentials (abGC-IPSPs) (Fig. 2B), indicating that this response was also neurogenesis dependent (8). A disynaptic pathway involving inhibitory interneurons could not underlie abGC-IPSPs because they were insensitive to antagonists against AMPAR, NMDAR, and GABAAR (Fig. 2, D to F).
Instead, abGC-IPSPs were blocked by the G protein–coupled inwardly rectifying potassium channel (GIRK) antagonist Tertiapin-Q (Fig. 2E) and the group II metabotropic glutamate receptor (mGlu-II) blocker LY341495 (Fig. 2F), indicating that the source of abGC-IPSPs was mGlu-II–coupled GIRK channels. This form of inhibition was exclusively driven by abGCs because optogenetic excitation of mGCs did not evoke mGlu-II–dependent IPSPs in other mGCs (fig. S3). Like NR3, mGlu-IIs in mGCs are usually located extrasynaptically (17).
mGlu-IIs have a higher affinity for glutamate than NMDARs have (18, 19), so they would likely be activated even when abGC-driven glutamate release is low. Indeed, at the lowest laser power that elicited reliable responses (~2 mW), we primarily recorded abGC-IPSPs in mGCs (−0.12 ± 0.03 mV·s, n = 10) (Fig. 2G). At higher intensities (~5 mW), abGC-EPSPs were the major response (0.25 ± 0.03 mV·s, n = 10; P < 0.0001) (Fig. 2G). Experiments using paired whole-cell recordings of single abGCs and neighboring mGCs, as well as optogenetic experiments using a transgenic line (ASCL1-ChR2) in which fewer abGCs are stimulated than in the Nes-ChR2 mice (8, 20), indicate that multiple abGCs are likely required to elicit abGC-IPSPs and abGC-EPSPs in an mGC (fig. S5).
We therefore propose that when a small number of abGC are active, relatively low levels of glutamate are released, and mostly mGlu-IIs are monosynaptically activated in mGCs (Fig. 2H). By contrast, when a large population of abGCs is active, enough extrasynaptic NMDARs are directly activated to override mGlu-II (Fig. 2H). To test this model, we isolated monosynaptic responses in mGCs by blocking action-potential firing in the DG with tetrodotoxin (TTX) and preserving ChR2-driven abGC presynaptic release with 4-aminopyridine (4-AP) (21). This manipulation also results in attenuated glutamate release from these ChR2-expressing abGC terminals (21). Consequently, we found that abGC-EPSP amplitudes decreased while abGC-IPSPs were unmasked (Fig. 2I), suggesting that low glutamate release favors inhibition over excitation, as proposed in our model (Fig. 2, G and H). Importantly, the fact that mGC excitation and inhibition remained in the presence of TTX and 4-AP (Fig. 2I) indicates that they must both arise from abGCs directly releasing glutamate onto mGCs without having to activate an intermediary neuron (Fig. 2H).
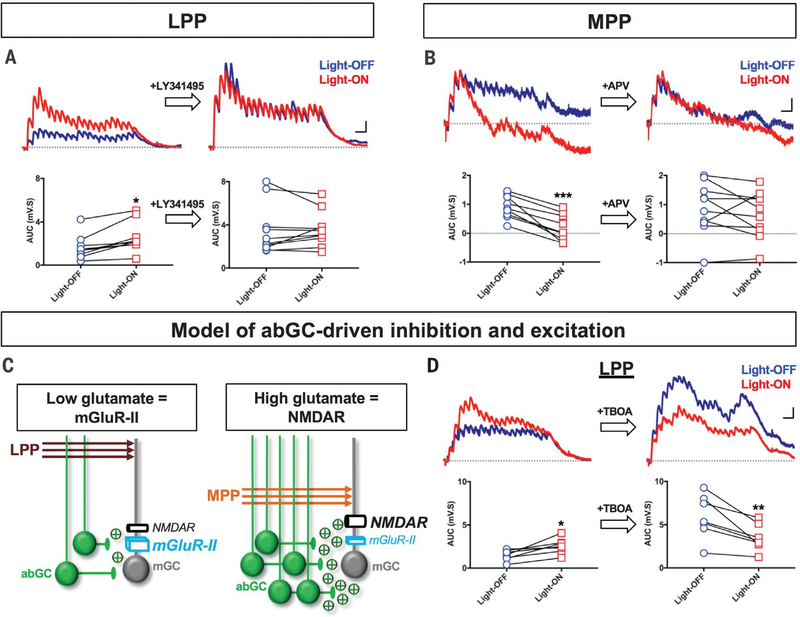
We hypothesized that mGlu-IIs may account for abGC-mediated inhibition of LPP-evoked mGC responses, whereas NMDARs may underlie abGC-mediated excitation of MPP-evoked mGC responses in Nes-ArchT mice (Fig. 1). We tested the effects of the mGlu-II antagonist LY341495 on LPP-evoked mGC responses and found that the increase in these signals as a result of silencing abGCs was blocked by LY341495 (Fig. 3A) but not by the NMDA antagonist APV (fig. S6). Additionally, when we replaced the potassium in our pipette solution with cesium, we blocked the effects of silencing abGCs (fig. S7), thereby demonstrating that postsynaptic, and not presynaptic, mGlu-II–coupled GIRK channels underlie abGC-driven inhibition of LPP-evoked responses. We then tested the effects of APV on MPP-evoked responses and found that the decrease in MPP-evoked responses due to abGC silencing was significantly attenuated with APV (Fig. 3B) but not LY341495 (fig. S6), indicating that NMDAR underlie abGC-driven excitation of mGCs.
Fig. 3. mGlu-IIs mediate abGC-driven inhibition of LPP-evoked responses, and NMDARs mediate abGC-driven excitation of MPP-evoked responses.
(A) Average traces and plots showing abGC-driven inhibition of LPP responses before and after bath application of LY341495. Repeated-measures two-way ANOVA showed a main effect of light (F1,8 = 10.07; P < 0.05; n = 9), a main effect of drug treatment (F1,8 = 29.13; P < 0.001; n = 9), and a significant interaction between both factors (F1,8 = 5.38; P < 0.05; n = 9). Fisher’s LSD post hoc test, control: Light-OFF = 1.32 ± 0.47 mV·s, n = 9; Light-ON = 2.30 ± 0.53 mV·s, n = 9; *P < 0.05. LY341495: Light-OFF = 3.68 ± 0.79 m·s, n = 9; Light-ON = 3.56 ± 0.57 mV·s, n = 9; P = 0.72. (B) Average traces and plots showing abGC-mediated excitation of MPP responses before and after APV. Repeated-measures two-way ANOVA showed a main effect of light (F1,9 = 11.95; P < 0.01; n = 10), no main effect of APV (F1,9 = 1.66; P = 0.23; n = 10), and a significant interaction between both factors (F1,9 = 7.19; P < 0.05; n = 10). Fisher’s LSD post hoc test, control: Light-OFF = 0.84 ± 0.14 mV·s, n = 10; Light-ON = 0.12 ± 0.15 mV·s, n = 10; ***P < 0.001. APV: Light-OFF = 0.88 ± 0.32 mV·s, n = 10; Light-ON = 0.64 ± 0.25 mV·s, n = 10; P = 0.09. (C) Model postulating that the LPP evokes low glutamate release from a small number of abGC, resulting in mGlu-II–dependent inhibition of mGCs. MPP inputs elicit high glutamate release from a larger population of abGCs, leading to NMDAR-dependent excitation of mGCs. (D) Average traces and plots showing the effects of the glutamate uptake blocker TBOA (30 μM) on LPP-evoked responses. Repeated-measures two-way ANOVA showed a main effect of light (F1,8 = 6.81; P < 0.05; n = 9), a main effect of TBOA (F1,8 = 11.38; P < 0.01; n = 9), and a significant interaction between both factors (F1,8 = 64.84; P < 0.0001; n = 9). Fisher’s LSD post hoc test, control: Light-OFF = 1.69 ± 0.23 mV·s, n = 9; Light-ON = 2.63 ± 0.27 mV·s, n = 9; *P < 0.05; TBOA: Light-OFF = 9.19 ± 1.96 mV·s, n = 9; Light-ON = 6.22 ± 1.41 mV·s, n = 9; ****P < 0.0001). Scale bars: 2mV, 50 ms [(A) and (D)]; 0.5 mV, 50 ms (B). All values = mean ± SEM.
These results suggest that the LPP and MPP would evoke low and high abGC-driven glutamate release, respectively (Fig. 3C). Indeed, we had shown that larger numbers of abGCs would spike in response to the MPP than to the LPP and that these MPP-activated abGCs have a higher probability of spiking simultaneously than LPP-activated abGCs (Fig. 1, C and E). MPP-evoked abGC spiking should therefore result in greater amounts of glutamate released than LPP activation would. We tested this hypothesis by using the glutamate transport inhibitor DL-threo-β-benzyloxyaspartic acid (TBOA) to artificially increase extracellular glutamate during LPP stimulation experiments. We found that TBOA reversed the effects of silencing abGCs on LPP-evoked mGC responses (Fig. 3D). This is likely because increased ambient glutamate allows for abGC-driven NMDAR excitation to supersede mGlu-II inhibition. As a result, silencing abGCs decreases LPP-evoked responses just like it decreases MPP-evoked responses. However, given the global changes in extracellular glutamate caused by TBOA, the potential contribution of other pre- and postsynaptic mechanisms cannot be fully excluded.
Our findings would predict that during behaviors in which MEC activity predominates, abGCs provide additional excitation to mGCs; but when the LEC is active, abGCs instead inhibit mGCs. MEC axons (MPP) are more abundant in the inferior blade of the DG than the superior blade, whereas LEC axons (LPP) are more pronounced in the superior blade (fig. S8) (5). Thus, ablating neurogenesis should decrease MPP excitation, resulting in decreased activity, mostly in the inferior blade. Conversely, ablating neurogenesis should decrease LPP inhibition, resulting in increased activity, mostly in the superior blade. Consistent with these hypotheses, our electrophysiological experiments show that abGCs provide stronger excitation to MPP-evoked responses of mGCs in the inferior blade than in the superior blade. By contrast, abGC more strongly inhibited LPP-evoked responses of mGCs in the superior blade than in the inferior blade (fig. S9).
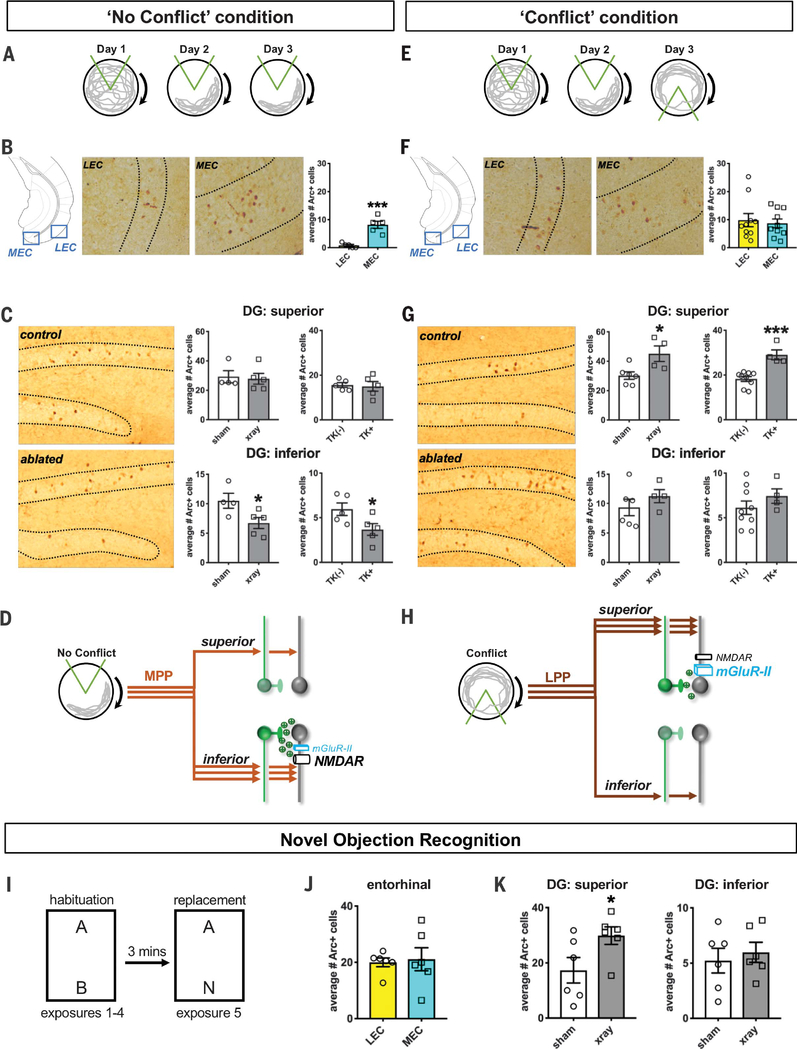
As an in vivo proof of concept, we used X-irradiation to completely ablate neurogenesis in the DG superior and inferior blades (fig. S10) (8, 22, 23). We then tested sham and X-irradiated mice in a neurogenesis-dependent active place-avoidance task wherein mice were placed on a rotating circular platform and initially trained to avoid a stationary 60° shock zone defined by cues in the room (the “No Conflict” condition) (Fig. 4A and fig. S11) (22, 24). On the basis of the expression of the immediate-early gene Arc, we found that the MEC was much more active than the LEC during avoidance of the initial shock zone (Fig. 4B). There were also significantly fewer Arc+ cells in the inferior blade of X-irradiated mice compared with sham, with no differences seen in the superior blade (Fig. 4C). Complementary experiments in which we pharmacogenetically ablated abGCs using the GFAP-TK mouse line (fig. S10) (22, 23) revealed the same behavior and DG activity patterns (Fig. 4C and fig. S11) as with X-irradiation, confirming that these behaviors and activation profiles were not confounded by potential side effects of X-irradiation. All these results are consistent with studies showing a reduction in DG excitability in X-irradiated mice (24) even though their performance was unimpaired in this variant of the task (fig. S11) (22, 24). Thus, during behaviors in which MEC inputs predominate, abGCs appear to excite mGCs, mostly in the inferior blade (Fig. 4D).
Fig. 4. Differential abGC-dependent modulation in an active place-avoidance task.
(A) “No Conflict” protocol: 13 sham and 13 x-ray mice were placed on a rotating arena. Arrows indicate direction of rotation. On days 1 and 2, mice learned to avoid a stationary shock zone (area between orange lines). On day 3, mice were given two additional training trials to the same shock zone. A subset of mice was perfused 70 min after the beginning of the first training trial on day 3 for Arc+ immunohistochemical labeling. Gray lines illustrate the path of a mouse. (B) Average Arc+ cells counted from four coronal hemisections of layer II LEC and MEC from each sham and X-irradiated mouse in the No Conflict condition. MEC = 8.16 ± 1.28 cells, n = 5 mice; LEC = 0.80 ± 0.37 cells, n = 5 mice. Unpaired t test ***P < 0.001. (C) Average number of Arc+ cells counted from eight coronal dorsal DG hemisections in each sham, X-irradiated, GFAP-TK(−), and GFAP-TK+ mouse in the No Conflict condition. Superior blade: Sham = 29.25 ± 4.13 cells, n = 4 mice; x-ray = 27.85 ± 3.66 cells, n = 5 mice; unpaired t test P = 0.81. TK(−) = 15.56 ± 1.08 cells, n = 5 mice; TK+ = 14.97 ± 2.14, n = 5; P = 0.80. Inferior blade: Sham = 10.50 ± 1.26 cells, n = 4 mice; x-ray = 6.72 ± 0.93 cells, n = 5 mice; unpaired t test *P < 0.05. TK(−) = 5.94 ± 0.70 cells, n = 5 mice; TK+ = 3.67 ± 0.66, n = 5; *P < 0.05. (D) Model of the No Conflict condition circuitry. The DG is primarily activated by the MEC (via the MPP) resulting in abGC-mediated (NMDAR) excitation of mGCs mostly in the inferior blade. (E) Conflict protocol: Behavioral protocol and number of mice tested were the same as in the No Conflict version except that on day 3, the shock zone was moved 180° from the initial location. Mice were tested in two trials with this new shock zone. A subset of mice was perfused 70 min after the beginning of the first Conflict trial on day 3. (F) Average layer II LEC versus MEC Arc+ cells counted from four coronal hemisections from each sham and X-irradiated mouse in the Conflict condition. MEC = 8.71 ± 1.54 cells, n = 10 mice; LEC = 9.84 ± 2.33 cells, n = 10 mice. Unpaired t test P = 0.69. (G) Arc+ cells counted from eight coronal dorsal DG hemisections in each mouse in the Conflict condition. Superior blade: Sham = 30.23 ± 2.57 cells, n = 6 mice; x-ray = 45.09 ± 5.29 cells, n = 4 mice; unpaired t test *P < 0.05. TK(−) = 18.26 ± 1.10 cells, n = 9 mice; TK+ = 29.04 ± 2.20, n = 4; ***P < 0.001. Inferior blade: Sham = 9.42 ± 1.47 cells, n = 6 mice; x-ray = 11.25 ± 1.12 cells, n = 4 mice; unpaired t test P = 0.39. TK(−) = 6.12 ± 0.75 cells, n = 9 mice; TK+ = 743 ± 0.82, n = 4; P = 0.32. (H) Model of the Conflict condition circuitry. The DG receives strong excitation from the LEC (via the LPP) resulting in abGC-mediated (mGlu-II) inhibition of mGCs mostly in the superior blade. (I) NOR experimental paradigm. Exposures 1 through 4 were habituation sessions with two objects “A” and “B” placed symmetrically on either end of the arena. In exposure 5, object B was replaced with a novel object “N.” (J) LEC and MEC activity as measured by average number of Arc+ cells counted from four coronal hemisections in each mouse. LEC = 20.00 ± 1.57 Arc+ cells, n = 6 mice; MEC = 21.12 ± 4.09 Arc+ cells, n = 6 mice. Unpaired t test P = 0.80. (K) Average Arc+ cells from eight coronal and eight hemisections in each sham and X-irradiated mouse. Superior blade: sham = 17.31 ± 4.64 Arc+ cells, n = 6 mice; x-ray = 29.88 ± 3.18 Arc+ cells, n = 6 mice; unpaired t test *P < 0.05. Inferior blade: sham = 5.23 ± 1.11 Arc+ cells, n = 6 mice; x-ray = 5.98 ± 0.91 Arc+ cells, n = 6 mice; unpaired t test P = 0.61. All values = mean ± SEM.
When the shock zone was relocated 180° (the “Conflict” condition) (Fig. 4E and fig. S11), LEC Arc+ cell counts increased to levels similar to the MEC (Fig. 4F). We found impaired performance in X-irradiated mice (fig. S11) and significantly more Arc+ cells in the superior blade of X-irradiated mice than in sham animals, with no group differences seen in the inferior blade (Fig. 4G). We again found similar results using the GFAP-TK mouse line (Fig. 4G and fig. S11) (22, 23). These results indicate that during behaviors in which the LEC is active, abGCs likely inhibit mGCs, mostly in the superior blade (Fig. 4H).
The LEC is one of the key areas responsible for transmitting novel information to the hippocampus, a function that also appears to be neurogenesis dependent (25, 26). Indeed, we have found that X-irradiated mice are impaired in a novel object recognition task (NOR) (Fig. 4I and fig. S12). Importantly, we also found that X-irradiated mice tested in the NOR had the same LEC and DG superior blade activation profiles (Fig. 4, J and K) as in the Conflict condition (Fig. 4, F and G). It thus appears that abGC-driven inhibition of LPP-evoked responses contributes to shaping DG activation patterns in these seemingly distinct tasks. Moreover, our findings suggest that novelty—which results in increased LEC activity—is a key feature that could drive abGC-mediated inhibition of mGCs.
The impaired performance of X-irradiated mice in the Conflict condition (fig. S11) and NOR (fig. S12) suggests that reducing abGC-mediated inhibition is behaviorally more impactful than decreasing abGC-mediated excitation. This may be due in part to the greater number of cells affected in the superior than the inferior blade as well as previous findings indicating that abGCs receive preferential innervation from the LEC relative to the MEC (27, 28). However, the potential contribution of other brain regions cannot be discounted from our results. Furthermore, generating inducible knockouts of the right mGlu-II and NMDAR subunits specifically in mGCs will be necessary to establish the causal role of both abGC-evoked IPSPs and EPSPs on neurogenesis-dependent behaviors.
In summary, we have identified two mechanisms by which abGCs directly modulate the activity of mGCs: inhibition via mGlu-IIs and excitation via NMDARs. These monosynaptic mechanisms are differentially recruited depending on whether the DG is activated by the LPP or MPP, which suggests that abGCs can rapidly shift the balance between contextual information provided by the LPP and spatial information carried by the MPP (6, 7). These mechanisms are not utilized by mGCs (fig. S3), which is consistent with the fact that mGCs do not form direct connections with each other under normal conditions (11, 29).
Adult hippocampal neurogenesis is considered an adaptive mechanism that enables animals to adjust their behaviors to the changing cognitive demands in the environment (1, 2). How abGCs accomplish this function is unclear. We propose that increased neurogenesis due to, for instance, enriched environments (8, 30) could lead to increased abGC inhibition of mGC responses to LPP inputs. This inhibitory control may promote sparse neural activity, a property that could enhance pattern separation and cognitive flexibility (1, 2). Such an improvement in discrimination behavior may be beneficial in enriched environments where highly similar rewards abound. By contrast, neurogenesis levels decrease in stressful environments (2). Consequently, abGC inhibition of LPP-evoked responses would decrease, leading to decreased sparseness (i.e., increased neural activity) and an increased probability of similar contexts being represented as overlapping neural ensembles. These processes could manifest as enhanced generalization, which may be necessary for situations in which detection (e.g., predators) is more important than discrimination. On the other hand, the precise role of abGC excitation of MPP inputs is less clear but perhaps involves reinforcing the spatial features of an environment (e.g., boundaries, edges, and guide-posts), so they would persist over prolonged periods of time.
Our results show that abGCs bidirectionally shape DG neuronal activity on the basis of the type of incoming synaptic information. Such a modulatory function is specific to abGCs and may underlie their pivotal role in DG-dependent regulation of cognition and mood (1, 2).
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Hamilton, A. Harris, I. Pavlova, S. Tuncdemir, and G. Turi for their technical expertise. We thank S. Siegelbaum for helpful comments on the manuscript.
Funding: This work was supported by National Institutes of Health grants to V.M.L. (K01 AG054765), C.A. (K99 MH108719), N.S.B. (G12MD007599), C.A.D. (DP5 OD017908), A.A.F. (R01 AG043688), H.E.S. (R01 NS081203), and R.H. (R37 MH068542, R01 MH083862, R01 AG043688, R01 NS081203, and T32 MH01574). A.Ma. was supported by a Rotary Global Grant (GG1864162) and a Sackler award. C.A.D. was supported by a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (P&S Investigator). C.A.D. and R.H. were supported by NYSTEM (C029157). R.H. was also supported by the Hope for Depression Research Foundation (RGA-13-003).
Footnotes
Competing interests: The authors have no competing interests.
Data and materials availability: All data are available in the manuscript or the supplementary materials.
SUPPLEMENTARY MATERIALS
science.sciencemag.org/content/364/6440/578/suppl/DC1
Materials and Methods
Figs. S1 to S12
References (31–40)
REFERENCES AND NOTES
- 1.Deng W, Aimone JB, Gage FH, Nat. Rev. Neurosci 11, 339–350 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anacker C, Hen R, Nat. Rev. Neurosci 18, 335–346 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temprana SG et al. , Neuron 85, 116–130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drew LJ et al. , Hippocampus 26, 763–778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witter MP, Prog. Brain Res 163, 43–61 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Knierim JJ, Neunuebel JP, Deshmukh SS, Philos. Trans. R. Soc. B 369, 20130369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hargreaves EL, Rao G, Lee I, Knierim JJ, Science 308, 1792–1794 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Dranovsky A et al. , Neuron 70, 908–923 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen RP et al. , Neuroscience 252, 154–168 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Pernía-Andrade AJ, Jonas P, Neuron 81, 140–152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amaral DG, Scharfman HE, Lavenex P, Prog. Brain Res 163, 3–22 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scharfman HE, Nat. Rev. Neurosci 17, 562–575 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chancey JH, Poulsen DJ, Wadiche JI, Overstreet-Wadiche L, J. Neurosci 34, 2349–2354 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L et al. , Magnes. Res 22, 266–272 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Henson MA, Roberts AC, Perez-Otano I, Philpot BD, Prog. Neurobiol 91, 23–37 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-Otaño I, Larsen RS, Wesseling JF, Nat. Rev. Neurosci 17, 623–635 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Brunner J et al. , J. Neurosci 33, 7285–7298 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conn PJ, Pin JP, Annu. Rev. Pharmacol. Toxicol 37, 205–237 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Yao Y, Mayer ML, J. Neurosci 26, 4559–4566 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang SM, Alvarez DD, Schinder AF, J. Neurosci 35, 15379–15390 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petreanu L, Mao T, Sternson SM, Svoboda K, Nature 457, 1142–1145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burghardt NS, Park EH, Hen R, Fenton AA, Hippocampus 22, 1795–1808 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxe MD et al. , Proc. Natl. Acad. Sci. U.S.A 104, 4642–4646 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park EH, Burghardt NS, Dvorak D, Hen R, Fenton AA, J. Neurosci 35, 11656–11666 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jessberger S et al. , Learn. Mem 16, 147–154 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suárez-Pereira I, Carrión AM, Sci. Rep 5, 13993 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vivar C et al. , Nat. Commun 3, 1107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods NI et al. , J. Neurosci 38, 5843–5853 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendricks WD, Chen Y, Bensen AL, Westbrook GL, Schnell E, J. Neurosci 37, 5722–5735 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Praag H, Kempermann G, Gage FH, Nat. Rev. Neurosci 1, 191–198 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.