Abstract
BACKGROUND
Atrial fibrillation (AF) is sustained by reentrant mechanisms that depend, in part, on atrial structural remodeling. Increased Ca2+/calmodulin-dependent protein kinase II (CaMKII) activity occurs in persistent AF. A general consensus has been that electrophysiological actions of CaMKII must be the contributing factor, but electrical remodeling in AF differs considerably with electrophysiological effects of CaMKII. CaMKII has been associated with structural remodeling in several tissues, but not the cardiac atria. The role of CaMKII in sustaining AF remains undefined.
OBJECTIVE
The purpose of this study was to assess the effects of CaMKII on AF-related structural remodeling.
METHODS
We evaluated the objective in a porcine AF-heart failure model using atrial gene transfer of the CaMKII inhibitory peptide CaMKIIn. We used conventional methods including in vivo electrophysiological study, telemetry, western blot, echo-cardiography, and histology to quantify rhythm, function, micro-structure, and signaling pathways relevant to CaMKII and structural remodeling.
RESULTS
CaMKII levels and activity increased progressively in the early stages of AF-heart failure. Inhibiting CaMKII preserved atrial contractile function and attenuated atrial hypertrophy, fibrosis, and apoptosis but did not affect inflammation or myolysis. These effects were accompanied by significantly decreased phosphorylation of HDAC4, decreased expression of p38MAP-kinase, and alterations in the phosphorylation pattern and relative ratios of JNK isoforms.
CONCLUSION
Our findings suggest that CaMKII mediates signaling pathways related to atrial contractile function and structural remodeling in AF. CaMKII inhibition is potentially a novel therapy for AF. These findings are of importance because no clinically relevant mediators of either atrial contractile function or structural remodeling have yet been identified.
Keywords: Apoptosis, Atrial fibrillation, Ca/calmodulin-dependent protein kinase, Fibrosis, Gene therapy
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, affecting millions of patients worldwide.1 Currently available therapies have limited efficacy and significant risks of toxicity, suggesting the need for novel approaches to treat AF.1–3 Any new AF therapy will likely need to reverse structural and electrical remodeling that play central roles in AF. Electrical remodeling has been reliably reversed with drugs and in preclinical studies with gene therapy.4–7 Prolongation of atrial action potential duration (APD) prevents AF in the short term. In most patients, however, APD-prolonging drugs are insufficient to prevent the inevitable progression to permanent AF,8 suggesting that structural remodeling must be interrupted for long-term AF elimination.
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a multifunctional serine-threonine kinase. Total, phosphorylated, and oxidized CaMKII are increased in persistent AF.9,10 CaMKII activity has been connected to triggered arrhythmias from diastolic sarcoplasmic reticular calcium leak, but data suggest that the sustaining mechanism for persistent AF is reentry and not triggered activity.11 Other than diastolic calcium leak, the electrical remodeling associated with AF differs drastically from that described for CaMKII,12–14 suggesting that the contribution of CaMKII to persistent AF is likely independent of its electrophysiological effects. Because known elements of electrical remodeling are insufficient to connect CaMKII hyperactivity to reentry and persistent AF, we hypothesized that increased CaMKII activity is a cause of structural remodeling in AF.
Methods
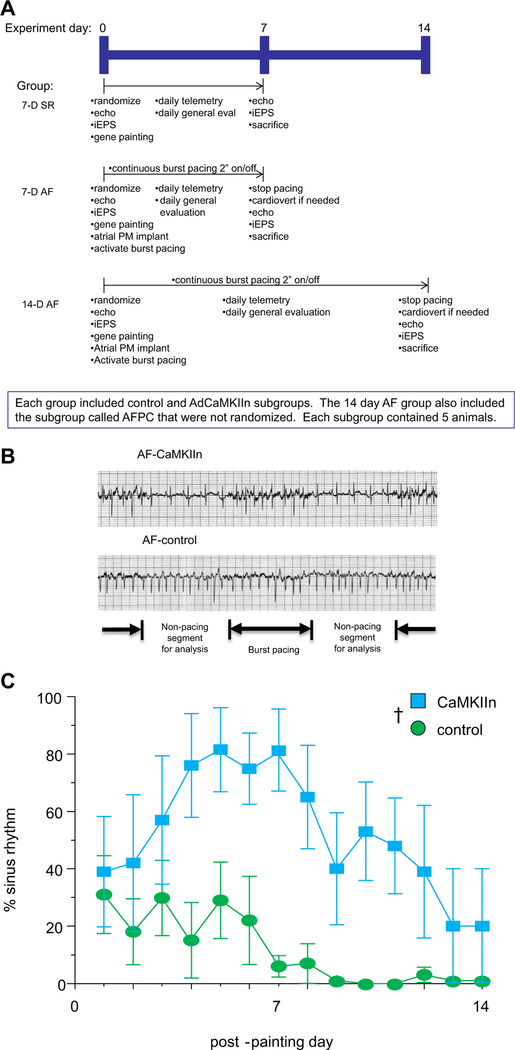
We addressed our hypothesis using our porcine model of persistent AF and heart failure,15 and our previously reported atrial gene painting technique with CaMKIIn, a specific and potent peptide inhibitor of CaMKII.16,17 A full description of methods is included in the Supplemental Methods. The overall study protocol is described graphically in Figure 1A. A total of 35 Yorkshire pigs were divided into 3 groups: (1) 7-day sinus rhythm (SR); (2) 7-day AF; and (3) 14-day AF. The 7-day time points were used to assess effects during peak adenovirus-mediated transgene expression,18,19 and the 14-day animals gave addition perspective on the time course of structural remodeling. Animals in each group were randomized to receive atrial gene painting with either an adenovirus encoding CaMKIIn (AdCaMKIIn) or saline, delivered in a mixture with 20% poloxamer F127 and 0.5% trypsin. Each of these subgroups contained 5 animals. Rhythm was checked with daily telemetry recordings (Figure 1B). When subgroup differences in AF burden became apparent (Figure 1C), we added another 14-day AF subgroup of 5 animals that received AdCaMKIIn and adjusted the burst pacing algorithm daily to reproduce the average daily AF/burst pacing load experienced by the 14-day AF control animals (Supplemental Figure 1). To distinguish from animals receiving AdCaMKIIn with standard 2-second on/off burst pacing, these additional animals were called the AF pacing control (AFPC) subgroup.
Figure 1.
A: Overall study design. B: Representative telemetry recordings at day 7 show the 2-second burst pacing and 2-second nonburst pacing intervals. Rhythm was recorded for each nonburst pacing interval, and the overall percentage of time in sinus rhythm (SR) was calculated by dividing the number of segments showing SR by the total number of assessed segments. C: Percentage of time in SR after gene transfer. Compared to controls, animals exposed to CaMKIIn had a significant increase in the percent with SR. †P <.01 comparing CaMKIIn to control over the time course of the study. AF = atrial fibrillation.
All animals underwent an initial procedure on study day 0 that included echocardiogram, invasive electrophysiology study, and gene painting. In the AF animals, an atrial pacemaker was implanted at this time, and burst pacing was started immediately after the procedure. Animals underwent daily telemetry recording to evaluate rhythm in 30 non-paced segments while the atrial burst pacing continued uninterrupted (Figure 1A). At sacrifice, animals were cardioverted to SR if necessary, and then they underwent repeat electrophysiology study and echocardiogram before sacrifice for tissue analysis. Testing after sacrifice included western blot, histology, and TUNEL staining using conventional methods.
Results
Time course of structural remodeling in the porcine model of AF and heart failure
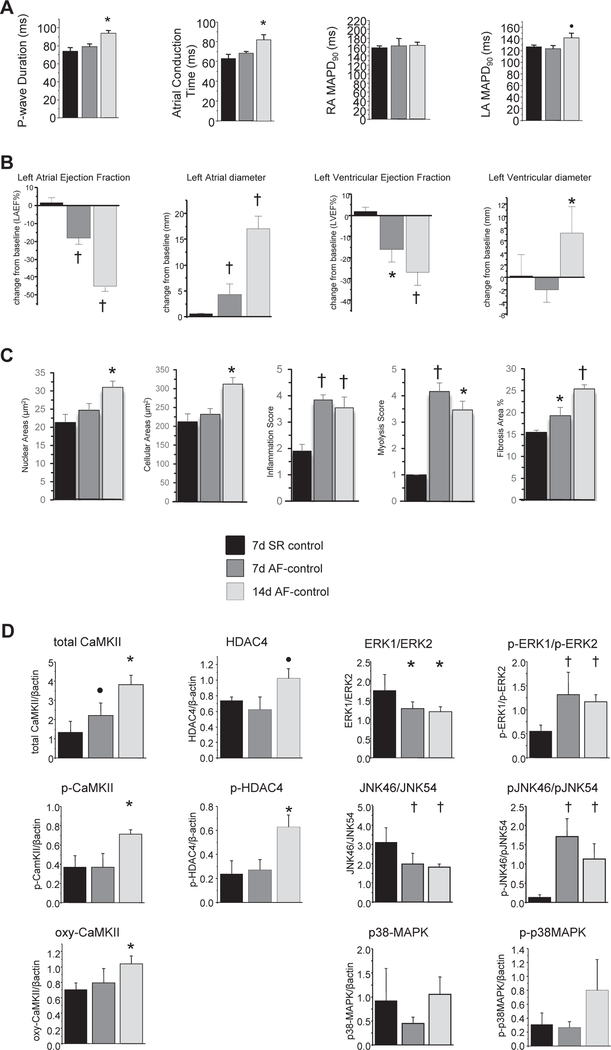
To define the early time course of structural remodeling in our porcine AF-heart failure model, we compared the control subgroups (animals that did not receive an active transgene) at each time point, evaluating SR, 1-week AF, and 2-week AF animals.
Measures of cardiac electrophysiology were variably affected by the combination of AF and heart failure (Figure 2A). Measures of conduction progressively worsened. Monophasic APD was not significantly changed across time points, although there was a trend toward increased left atrial APD at 2 weeks (P = .1).
Figure 2.
Time course of remodeling during the early stages of persistent atrial fibrillation (AF) and heart failure. A: In vivo electrophysiological measurements demonstrated progressive worsening of conduction, no change in right atrial repolarization, and a trend toward prolongation of left atrial action potential duration. B: Echocardiographic assessment of cardiac structure and function revealed progressive worsening of atrial and ventricular ejection fractions, progressive increase in left atrial diameter, and delayed left ventricular dilation. C: Histological assessment showed progressive increase in nuclear and whole cell measured area, persistent elevation in inflammation and myolysis scores (see Methods for scoring criteria), and progressive increase in fibrosis. D: Western blot analysis indicated progressive increases in total and oxidized CaMKII, delayed increases in phospho-CaMKII and phospho-HDAC4, and alterations in isoform ration and phosphorylation patterns of JNK and ERK. ●P <.10; *P <.05; †P <.01. LA = left atrium; MAPD = monophasic action potential duration; RA = right atrium; SR = sinus rhythm.
On echocardiographic examination, we found evidence of significant atrial dilation and decreased atrial and ventricular contractile function at 1 week that worsened at 2 weeks of AF (Figure 2B). Ventricular end-diastolic diameter was unchanged in the 1-week AF animals compared to SR animals, but ventricular dilation was present at 2 weeks.
Analysis of atrial histological samples showed increased nuclear and myocyte size, fibrosis, myolysis, and inflammation at 1 and 2 weeks of AF compared to SR (Figure 2C). Apoptosis was evident at 1 week (0.5% 6 0.1% of myocytes TUNEL positive) and worse at 2 weeks (1.9% 6 0.3% of myocytes TUNEL positive).
Western blot analysis showed that several proteins implicated in CaMKII signaling were altered (Figure 2D). Total CaMKII progressively increased. Phosphorylated and oxidized CaMKII were unchanged at 1 week and increased at 2 weeks of AF. Phosphorylated-HDAC4 increased, and there was a trend toward increased total HDAC4 after 2 weeks of AF. Total JNK was unchanged, but the ratio of JNK46 to JNK54 decreased, and phospho-JNK46:phospho-JNK54 increased. Likewise, total ERK was unchanged, but ERK1:ERK2 decreased, and phospho-ERK1:phospho-ERK2 increased. There were no significant changes in either total or phosphorylated p38MAPK.
CaMKII in AF-heart failure animals
We have previously shown complete transmural atrial gene transfer of the atrial free walls after epicardial gene painting.16 To verify successful transgene expression in this study, we quantified CaMKIIn mRNA with quantitative polymerase chain reaction analysis on the 7-day animals (timed to peak transgene expression with adenovirus).18,19 We found transgene expression in the CaMKIIn group (right atrium 0.09 ± 0.02; left atrium 0.05 ± 0.02 mRNA copies of CaMKIIn per GAPDH mRNA) and no evidence of CaMKIIn expression in control animals. In the 2-week animals, CaMKIIn mRNA was below the detection threshold, indicating diminished transgene expression at that point.
On rhythm analysis, we saw maintenance of SR in animals receiving AdCaMKIIn (Figure 1C). Because structural remodeling is dependent on AF burden, the difference in rhythm between the AF-CaMKIIn and AF-control animals was a potential confounder. To distinguish between AF burden and CaMKII inhibition effects, we created the AFPC subgroup. The AFPC animals received AdCaMKIIn by atrial gene painting. Rather than standard 2-second on/off burst pace cycling, in the AFPC animals burst pacing was adjusted daily so that the combined burden of AF and burst pacing in the AFPC animals equaled the total AF/burst pacing burden of the AF-control group (Supplemental Figure 1).
We assessed gross electrophysiological actions of CaMKIIn with in vivo electrophysiology study, comparing CaMKIIn-treated to control animals (Supplemental Figure 2). We saw no between-group differences in APD. The CaMKIIn-treated animals had decreased SR P-wave duration and sinus node-to-left atrial appendage conduction time compared to control animals. These conduction changes likely represented an effect of AF burden because they were not present in the AFPC group, suggesting that CaMKIIn had no direct effects on atrial conduction properties.
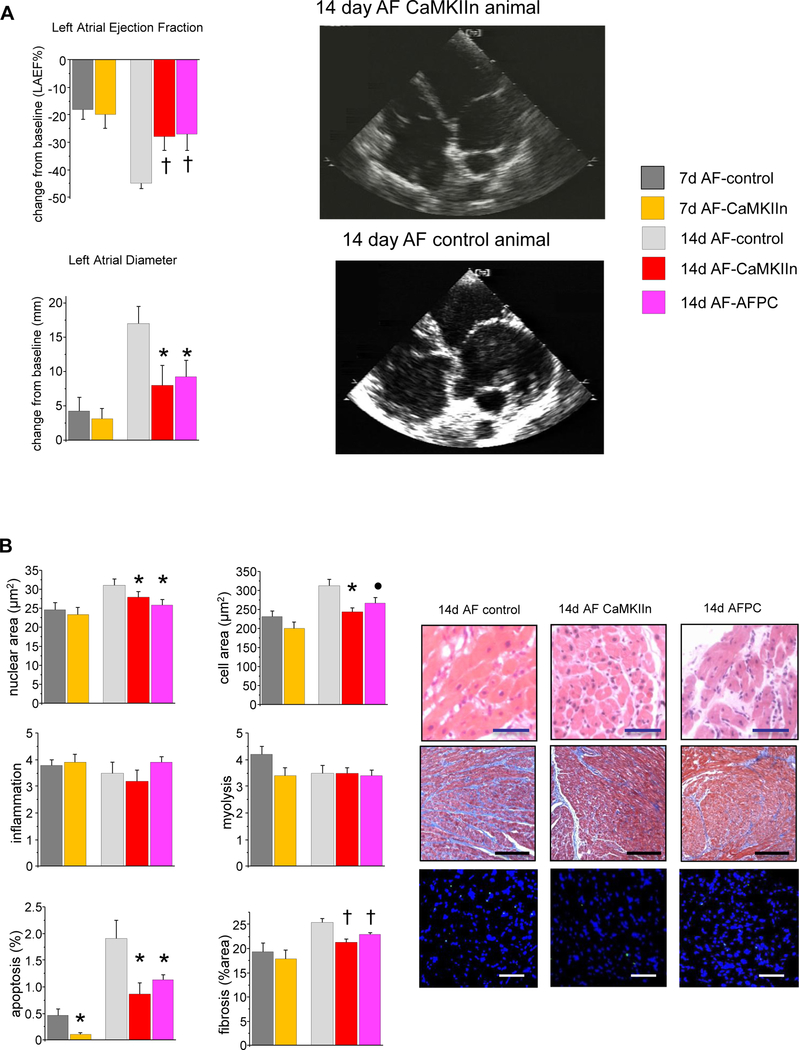
Echocardiographic assessment showed significantly smaller atrial size and better function in CaMKIIn-treated animals relative to controls (Figure 3A). These findings were also present in the AFPC group, confirming that the improvement came from CaMKIIn expression and not from differences in arrhythmia burden. These changes were atrial specific. No differences existed between groups with regard to left ventricular structure or function.
Figure 3.
Effects of CaMKII inhibition on structural remodeling. A: Echocardiography showed delayed progression in atrial dilation and dysfunction in subgroups receiving CaMKIIn. No change in ventricular structure or function was observed (data not shown). Right: Apical 4-chamber echocardiographic views illustrate the smaller atrial size in the CaMKIIn animals. B: Histological analyses show significantly less nuclear and cellular hypertrophy, apoptosis, and fibrosis in CaMKIIn-treated animals at 2 weeks, and decreased apoptosis at 1 week; otherwise, no significant changes were seen at that time point, and no effect of CaMKIIn gene transfer on myolysis or inflammation. Right: Representative images of right atrial microsections. Magnification bar indicates 50 μm in hematoxylin/eosin-and TUNEL-stained images and 200 μm in Masson trichrome images. ●P <.10; *P <.05; †P <.01. AF = atrial fibrillation; AFPC = atrial fibrillation pacing control.
Histological analyses showed reductions in apoptosis for the CaMKIIn-treated animals relative to the controls at both 7-and 14-day time points (Figure 3B). There was no difference between the AF-control and AF-CaMKIIn groups at 7 days with regard to nuclear hypertrophy, cellular hypertrophy, or fibrosis, but each was significantly attenuated at 14 days. No differences at any of the time points were seen for myolysis or inflammation. The AFPC animals also had significantly decreased nuclear hypertrophy, fibrosis, and apoptosis with a trend toward decreased cellular hypertrophy, suggesting that these changes were caused by CaMKIIn expression and not by differences in arrhythmia burden.
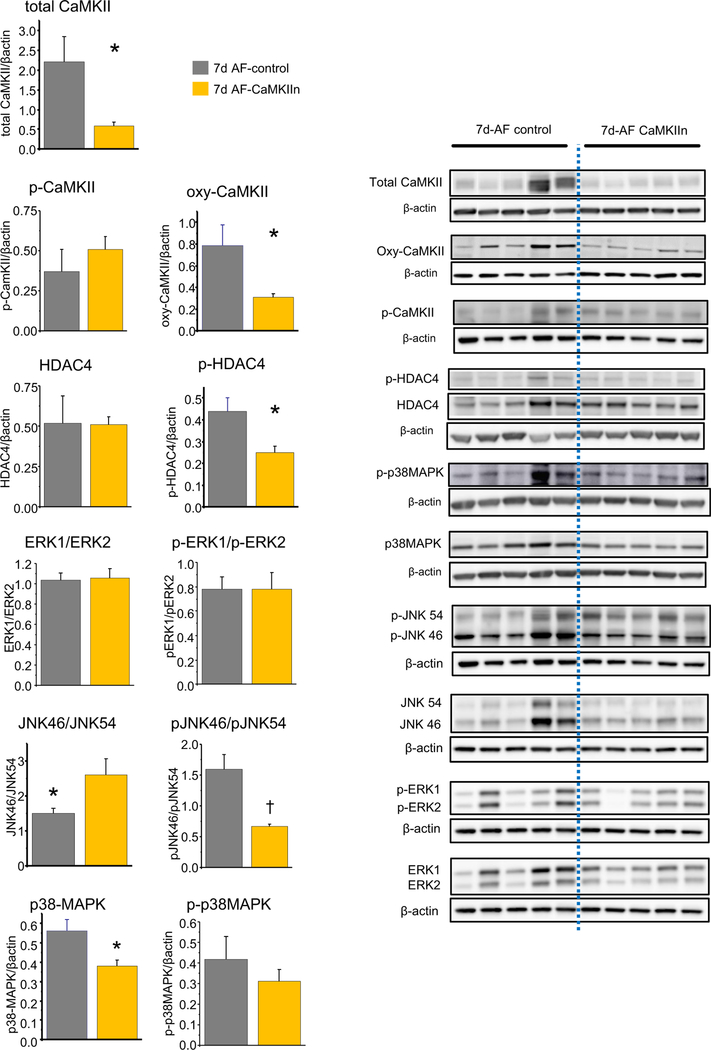
To connect CaMKII-associated signaling pathways to atrial structural remodeling, we compared expression levels of signaling pathway proteins between CaMKIIn and control-AF groups at peak transgene expression (Figure 4). CaMKIIn animals had decreased total and oxidized CaMKII, decreased phospho-HDAC4, and decreased total p38MAPK. CaMKIIn also prevented the changes in JNK and phospho-JNK subtype ratios. There were no between-group differences in phospho-CaMKII, total HDAC4, phospho-p38MAPK, and either total- or phospho-ERK. There were no between-group differences in western blot analyses for the 14-day animals, likely resulting from the minimal transgene expression at that point (Supplemental Figure 3).
Figure 4.
Effects of CaMKII inhibition on signaling pathway protein expression at peak transgene expression, 7 days after gene transfer. Quantified protein expression shows changes in total and oxidized CaMKII, phospho-HDAC4, phospho-p38MAPK, and the JNK isoform ratio and phosphorylation pattern. Right: Representative lanes from western blots for the indicated protein in each group. *P <.05; †P <.01. AF = atrial fibrillation.
CaMKII in SR animals
In the SR animals, we saw no differences between the CaMKIIn and control groups for any measurement. All animals remained in SR without atrial or ventricular ectopy. We saw no significant differences between groups with regard to echo-cardiographic measures of chamber size or function (Supplemental Figure 4A), histological characteristics (Supplemental Figure 4B), or electrophysiological measures (Supplemental Figure 4C). Western blot measures of CaMKII and various downstream targets were also not significantly different between groups (Supplemental Figure 5).
Discussion
In the porcine AF-heart failure model, rapid and aggressive atrial structural remodeling occurs within 2 weeks of AF and heart failure. The results of our current study extend our previous studies in which we found atrial fibrosis and reduced conduction velocity after 1 week and severe 4-chamber dilation and failure with extensive structural remodeling after 3 and 6 weeks of AF and heart failure.6,15 Pulling together these various studies, we see a time course of structural remodeling, including hypertrophy, myolysis, apoptosis, inflammation, fibrosis, contractile dysfunction, and reduced conduction velocity, which progresses over the first 2–3 weeks and then stabilizes out to 6 weeks. Based on our western blot studies, earlier events in the time course of structural remodeling include increased total CaMKII expression and switch in the JNK and ERK isotype ratios, and relatively later events include increased phospho- and oxy-CaMKII, HDAC4, and phospho-HDAC-4. The time course and severity of structural remodeling in our pigs with persistent AF and heart failure are similar to, although considerably more aggressive than, the results of Ausma et al,20 who studied goats with persistent lone AF, suggesting that AF and heart failure are synergistic in their effects on atrial structural remodeling.
We found that CaMKII inhibition had no detectable effect in SR animals, but it preserved left atrial contractile function, reduced several measures of structural remodeling, and had a modest but significant antiarrhythmic effect in our clinically relevant model of persistent AF and heart failure. These results correlated with CaMKIIn-induced decreases in total and oxidized CaMKII, phospho-HDAC4, total p38MAPK protein expression, and preservation of the SR pattern of JNK isoform expression and phosphorylation pattern. The timing of CaMKIIn effects relative to duration of transgene expression and time course of structural remodeling supports the idea that CaMKII is an upstream effector, allowing the effects of the inhibition to potentially last longer than transgene expression. If we consider the antiarrhythmic effect a marker of the timing and intensity of transgene expression, the likely time course is peak expression around days 4–7, with progressive loss of expression on days 8–12 and potentially no expression on days 13–14.
We observed an immediate antifibrillatory effect with CaMKIIn that could not be explained by alterations in either conduction or repolarization properties of the atria because measures of both were unaffected by CaMKIIn. It could also not be explained by the effects of CaMKIIn on structural remodeling because prevention of AF was evident before structural remodeling occurred. Further study is required to explain this antiarrhythmic action.
The absence of significant between-group differences in the SR animals suggests that CaMKII is minimally active with respect to atrial structure or electrical function in the absence of stress. The reduction in atrial structural remodeling with CaMKIIn treatment of our AF animals supports the hypothesis that increased CaMKII activity is a cause of structural remodeling in AF.
CaMKII and electrical remodeling in AF
CaMKII is increased in persistent AF, but most of the described electrophysiological effects of CaMKII are opposite the described electrophysiological effects of persistent AF. CaMKII alters INa inactivation kinetics, increases INa,late, increases ICa,L amplitude, delays ICa,L inactivation, decreases IK1 and Ito,fast, but increases Ito,slow.12 In contrast, AF does not change INa, decreases lCa,L and Ito, and increases IK1. CaMKII increases RYR leak, which is a consistent finding in AF as well. The stark differences between most of the electrical effects of CaMKII and AF suggest that CaMKII is not a key driver of electrical remodeling in AF, consistent with our data showing no change in APD or macroscopic conduction with CaMKII inhibition.
CaMKII and structural remodeling in AF
CaMKII has been connected to cellular hypertrophy, inflammation, apoptosis, and fibrosis in several organ systems,21–24 but a PubMed search failed to identify any previous investigation of CaMKII and structural remodeling in AF. Suggestion of a role for CaMKII in atrial structural remodeling can be found in a report by Li et al,25 who evaluated a transgenic mouse line characterized by increased sarcoplasmic reticulum diastolic calcium leak, atrial dilation, and decreased electrical conduction leading to increased AF susceptibility. They found that preventing RYR2 phosphorylation at the putative CaMKII site attenuated the atrial electrical and structural phenotype of the model.25 The data of Li et al data suggested that CaMKII activity, potentially working through RYR2 phosphorylation and increased diastolic calcium in the cytoplasm, may play a role in atrial dilation and conduction disturbances.
Our study more directly assesses the role of CaMKII in atrial structural remodeling. We found that CaMKII inhibition significantly reduced but incompletely prevents atrial dilation, myocyte hypertrophy, apoptosis, and fibrosis, suggesting that CaMKII plays an important role but that other drivers are likely active in these processes. Because cytoplasmic calcium overload also activates the calcineurin signaling pathway, future investigation of the calcineurin pathway in combination with CaMKII block may yield additional benefit with an ultimate goal of eliminating all AF-related structural remodeling.
Molecular basis of CaMKII-induced atrial structural remodeling
Structural remodeling in other organ systems has been connected to CaMKII through signaling pathways involving HDAC4, JNK, ERK1/2, and p38MAPK.26–30 The components of these signaling pathways have, to a large extent, been worked out. For this work, we wondered which of those pathways might be playing a role in our observed reductions in hypertrophy, apoptosis, and fibrosis. We used western blot analysis to assay the entry points for each of these pathways and examined their expression and phosphorylation levels. Future study can further delineate the time course and sequence of events within these signaling pathways to refine the connection between our observed phosphorylation patterns, the isoform switch of JNK and ERK, and potentially other calcium signaling pathways (eg, calcineurin) with AF-induced structural remodeling, with the possibility of identifying therapeutic targets that could have synergistic effects with CaMKIIn.
Previous studies have connected cellular hypertrophy to HDAC4, and apoptosis, inflammation, and fibrosis to ERK, JNK, and p38MAPK in various models.28–33 We found increased phospho-HDAC4 with AF that was prevented by CaMKII inhibition, supporting the hypothesis that CaMKII effects on atrial hypertrophy are, at least partially, driven by HDAC4 phosphorylation in the atria. We saw shifts in JNK isoform expression and phosphorylation patterns with AF that were prevented by CaMKII inhibition, implicating CaMKII in JNK signaling and, in turn, JNK signaling in AF-induced apoptosis, inflammation, and fibrosis. Although we saw decreases in the ratio of ERK1:ERK2 and increases in p-ERK1:ERK2 with AF, these were not affected by CaMKII inhibition. Our results implicate ERK signaling in atrial hypertrophy but suggest that CaMKII is not a significant driver of this signaling pathway in the atria during AF.
We did not see significant changes in p38MAPK with AF, but CaMKIIn decreased total p38MAPK. Our data are insufficient to explain a role for p38MAPK in AF-related structural remodeling. It may be relevant, or it may be a bystander affected by CaMKII inhibition but not active in atrial structural remodeling. Aschar-Sobbi et al34 studied AF with endurance exercise in mice and found that direct inhibition of p38MAPK prevented atrial fibrosis, inflammation, and AF vulnerability, suggesting that p38MAPK may play a role. Further study is required to determine this 9 discrepancy.
The absence of any significant effect on inflammation in our model may indicate either that cellular drivers of the inflammatory response are not affected by CaMKII signaling or that the relevant cells are exogenous to the atrial myocardium at the time of gene transfer, so they were not transduced with CaMKIIn and therefore unaffected by our intervention. This finding clearly requires more investigation to fully understand, but it could potentially inform the debate about local vs recruited sources of inflammatory and fibrotic cells responsible for structural remodeling in AF. Our results show that this presumed lack of anti-inflammatory effect does not limit the improved function and otherwise reduced structural remodeling that we see with CaMKIIn.
Conclusion
We showed in a clinically relevant large mammalian model of persistent AF and heart failure that atrial gene painting with CaMKIIn reduced atrial structural remodeling and prevented AF. Our results are directly relevant to the patient with uncontrolled ventricular response to AF and tachycardiomyopathy and likely also relevant to patients with AF and heart failure of other etiologies. Our data suggest that atria-specific gene therapy with CaMKII inhibitor peptide could be a novel paradigm for AF treatment.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health Grants R01-HL93486 and R01-HL130376 to Dr Donahue; R01-HL113640 and R01-AA024769 to Dr Ai; R01-HL079031, R01-HL070250, R01-HL096652, and R01-HL113001 to Dr Anderson; and the Fondation LeDucq Alliance for CaMKII Signaling to Drs Ai, Anderson, and Donahue. Pacemaker equipment was donated by Medtronic. Dr Donahue is inventor on gene therapy patents issued to Johns Hopkins University; otherwise he has no conflicts to declare. Dr Anderson is co-founder of Allosteros Therapeutics, aiming to develop CaMKII inhibitor therapies; but did not control experimental design or data analysis.
Footnotes
Appendix
Supplementary data
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrthm.2019.01.013.
The remaining authors report no conflicts relevant to the contents of this paper to disclose.
References
- 1.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 2.Camm AJ. Safety considerations in the pharmacological management of atrial fibrillation. Int J Cardiol 2008;127:299–306. [DOI] [PubMed] [Google Scholar]
- 3.Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–38. [DOI] [PubMed] [Google Scholar]
- 4.Pederson O, Bagger H, Keller N, Marchant B, Kober L, Torp-Peterson C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mor- tality on dofetilide (DIAMOND) substudy. Circulation 2001;104:292–296. [DOI] [PubMed] [Google Scholar]
- 5.Amit G, Kikuchi K, Greener ID, Yang L, Novack V, Donahue JK. Selective molecular potassium channel blockade prevents atrial fibrillation. Circulation 2010; 121:2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Igarashi T, Finet JE, Takeuchi A, et al. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation 2012;125:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Hutt JA, Rajeshkumar B, Azuma Y, Duan KL, Donahue JK. Preclinical efficacy and safety of KCNH2-G628S gene therapy for postoperative atrial fibril- lation. J Thorac Cardiovasc Surg 2017;154:1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AFFIRM First Antiarrhythmic Drug Substudy Investigators. Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. J Am Coll Cardiol 2003;42:20–29 [DOI] [PubMed] [Google Scholar]
- 9.Voigt N, Li N, Wang Q, et al. Enhanced sarcoplasmic reticulum Ca21 leak and increased Na1-Ca21 exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125:2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purohit A, Rokita AG, Guan X, et al. Oxidized Ca(21)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 2013;128:1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atienza F, Almendral J, Moreno J, et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation 2006;114:2434–2442. [DOI] [PubMed] [Google Scholar]
- 12.Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J Cardiovasc Pharmacol 2009;54:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysi- ology of atrial fibrillation initiation, maintenance, and progression. Circ Res 2014;114:1483–1499. [DOI] [PubMed] [Google Scholar]
- 14.Heijman J, Voigt N, Wehrens XH, Dobrev D. Calcium dysregulation in atrial fibrillation: the role of CaMKII. Front Pharmacol 2014;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer A, McDonald AD, Donahue JK. Pathophysiological findings in a model of persistent atrial fibrillation and severe congestive heart failure. Cardiovasc Res 2004;61:764–770. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi K, McDonald AD, Sasano T, Donahue JK. Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation 2005;111:264–270. [DOI] [PubMed] [Google Scholar]
- 17.Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci U S A 1998;95:10890–10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu JC, Inubushi M, Sundaresan G, Schelbert HR, Gambhir SS. Optical imaging of cardiac reporter gene expression in living rats. Circulation 2002; 105:1631–1634. [DOI] [PubMed] [Google Scholar]
- 19.Muhlhauser J, Jones M, Yamada I, et al. Safety and efficacy of in vivo gene transfer into the porcine heart with replication-deficient, recombinant adenovirus vectors. Gene Ther 1996;3:145–153. [PubMed] [Google Scholar]
- 20.Ausma J, Litjens N, Lenders M, Duimel H, Mast F, Wouters L, Ramaekers F, Allessie M, Borgers M. Time course of atrial fibrillation- induced cellular structural remodeling in atria of the goat. J Mol Cell Cardiol 2001;33:2083–2094. [DOI] [PubMed] [Google Scholar]
- 21.Singh MV, Kapoun A, Higgins L, et al. Ca21/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor B gene expression in the mouse heart. J Clin Invest 2009;119:986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T, Maier LS, Dalton ND, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res 2003;92:912–919. [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, Khoo MS, Wu Y, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med 2005;11:409–417. [DOI] [PubMed] [Google Scholar]
- 24.Vila-Petroff M, Salas MA, Said M, et al. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res 2007;73:689–698. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Chiang DY, Wang S, et al. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation 2014;129:1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bers DM. Ca(2)-calmodulin-dependent protein kinase II regulation of cardiac excitation-transcription coupling. Heart Rhythm 2011;8:1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol 2011;51:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olofsson MH, Havelka AM, Brnjic S, Shoshan MC, Linder S. Charting calcium-regulated apoptosis pathways using chemical biology: role of calmodulin kinase II. BMC Chem Biol 2008;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palomeque J, Rueda OV, Sapia L, et al. Angiotensin II-induced oxidative stress resets the Ca21 dependence of Ca21-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ Res 2009; 105:1204–1212. [DOI] [PubMed] [Google Scholar]
- 30.Timmins JM, Ozcan L, Seimon TA, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest 2009;119:2925–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Backs J, Backs T, Neef S, et al. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A 2009;106:2342–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreusser MM, Lehmann LH, Keranov S, et al. Cardiac CaM Kinase II genes delta and gamma contribute to adverse remodeling but redundantly inhibit calcineurin-induced myocardial hypertrophy. Circulation 2014;130:1262–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voelkers M, Salz M, Herzog N, et al. Orai1 and Stim1 regulate normal and hyper-trophic growth in cardiomyocytes. J Mol Cell Cardiol 2010;48:1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aschar-Sobbi R, Izaddoustdar F, Korogyi AS, et al. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFalpha. Nat Commun 2015;6:6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.