Abstract
Traumatic brain injury (TBI) is a major risk factor for acquired epilepsy. Post-traumatic epilepsy (PTE) develops over time in up to 50% of patients with severe TBI. PTE is mostly unresponsive to traditional anti-seizure treatments suggesting distinct, injury-induced pathomechanisms in the development of this condition. Moderate and severe TBIs cause significant tissue damage, bleeding, neuron and glia death, as well as axonal, vascular, and metabolic abnormalities. These changes trigger a complex biological response aimed at curtailing the physical damage and restoring homeostasis and functionality. Although a positive correlation exists between the type and severity of TBI and PTE, there is only an incomplete understanding of the time-dependent sequelae of TBI pathobiologies and their role in epileptogenesis. Determining the temporal profile of protein biomarkers in the blood (serum or plasma) and cerebrospinal fluid (CSF) can help to identify pathobiologies underlying the development of PTE, high-risk individuals, and disease modifying therapies. Here we review the pathobiological sequelae of TBI in the context of blood- and CSF-based protein biomarkers, their potential role in epileptogenesis, and discuss future directions aimed at improving the diagnosis and treatment of PTE.
Keywords: Traumatic brain injury, Epilepsy, Epileptogenesis, Post-traumatic epilepsy, Protein biomarkers, Primary injury, Secondary injury, Cerebrospinal fluid, Serum
1. Introduction
Traumatic brain injury (TBI) triggers an array of dynamic and potentially long-term pathobiological responses (Ahmed et al., 2015; Blennow et al., 2016; 2012), making TBI a major risk factor for neurodegenerative and other chronic conditions such as post-traumatic epilepsy (PTE). Sudden mechanical impact to the brain causes macro- and micro-structural changes that include injury to axons, glia cells, and vasculature, as well as altered metabolism and water dysregulation (McGinn and Povlishock, 2016). While the effects of the primary injury, i.e., the direct physical damage to the head/brain, are instantaneous, the secondary injury process is a highly complex biological response that takes place over days, weeks, and even months after the insult (Grafman and Salazar, 2015; Wilson et al., 2017). Importantly, the onset and magnitude of these pathobiological mechanisms (e.g., neuroinflammation, cellular proliferation, synaptic remodeling) may significantly differ in relation to injury time.
The same secondary injury process, initially aimed at limiting the extent of the damage and restoring homeostasis and functionality (Kochanek et al., 2013), can last beyond the repair period and become pathogenic as in PTE. Epileptogenesis is a process that leads to the development of epilepsy, a chronic disorder characterized by recurrent seizures, following various forms of innate or acquired brain pathology including TBI (Choi and Koh, 2008; Diaz-Arrastia et al., 2009; Scharfman, 2000; Webster et al., 2017). The role of secondary injury processes, such as neuroinflammation, in epileptogenesis and the development of spontaneous seizure activity after TBI is currently poorly understood (Webster et al., 2017). Protein biomarkers offer a reliable and objective means to identify individual molecular pathologies and their temporal profiles during the secondary injury process, and in turn provide opportunities for therapeutic intervention that can limit damage, improve functional recovery, and prevent injury-induced chronic conditions.
2. Traumatic brain injury
The pathobiological processes that ensue the physical damage vary depending on the type (closed head vs. penetrating, focal vs. diffuse) and intensity (mild vs. severe) of the primary injury (Salazar and Grafman, 2015). The primary injury causes structural damage to the skull, dura, brain parenchyma and its various cellular elements including axons, blood vessels, neurons and glia (Hawryluk and Manley, 2015; Manley and Maas, 2013; Manley et al., 2017). At the molecular level, receptors, surface molecules, ion channels, as well as intracellular structural proteins and signaling molecules of damaged cells are dislocated (Potts et al., 2006), thereby affecting signaling processes and cerebral metabolism (Buitrago Blanco et al., 2016). The secondary injury process begins almost immediately after the physical impact and includes neuroinflammation (Kelso and Gendelman, 2014) to demark damaged foci and eliminate cellular debris (Simon et al., 2017), as well as regenerative mechanisms to promote cellular proliferation and synaptic reorganization (Abbott and Videnovic, 2016; Baker, 2014; Takase et al., 2018).
Clinical and experimental studies have shown that each of these injury processes has a temporal profile that is both distinct and dynamic, especially during the acute to subacute post-injury phase where primary (damage) and secondary (repair) processes overlap (Adams et al., 2017; Bogoslovsky and Diaz-Arrastia, 2016; Bramlett et al., 1997; Cernak et al., 2002; Hicks et al., 1996; Li et al., 2013; O’Connor et al., 2006; Schuhmann et al., 2003). In the chronic post-injury phase, cell loss may continue due to neuroinflammation directly and indirectly attacking neurons and glia (Balu, 2014; Cederberg and Siesjo, 2010; Hinson et al., 2015; Kumar and Loane, 2012; Potts et al., 2006). Ongoing neuroinflammation is believed to be one of the main pathomechanisms underlying long-term complications including PTE (Aronica and Crino, 2011; Choi and Koh, 2008; Vezzani and Friedman, 2011; Webster et al., 2017).
3. Post-traumatic epilepsy
Post-traumatic epilepsy (PTE), characterized by repeated posttraumatic seizures (PTS), can occur at various time points after injury (Diaz-Arrastia et al., 2009; Kharatishvili and Pitkanen, 2010; Pitkanen et al., 2014; Prince et al., 2012; Wilson et al., 2018). Immediate PTS are defined as typically occurring within 24 hours of brain injury, early PTS within a week post-injury, and late PTS occurring more than a week after injury. The definition, however, can vary; “immediate” may be used to describe seizures that occur within minutes after injury and “late” after a month or longer (Englander et al., 2003; Lucke-Wold et al., 2015; Ritter et al., 2016; Temkin, 2003). Early (and immediate) PTS are likely provoked by an altered threshold to stimuli and abnormal signaling that are directly caused by physical injury to the brain parenchyma (Christensen, 2015; Diaz-Arrastia et al., 2009; Frey, 2003). The ensuing hemorrhage, cerebral edema, metabolic crisis, and impaired energy production alter the extracellular ion milieu causing excessive glutamate release that overloads the already compromised astroglia. Early seizures are more frequent in kids (< 5 years) and in the presence of risk factors such as hematomas (both subdural and intracerebral), edema, and skull fractures (Prince et al., 2012), while late seizures are more frequent in adults over 65.
Late onset PTS is likely due to the lasting structural and molecular changes caused by the combination of initial physical damage and subsequent secondary injury processes (Christensen, 2015; Diaz-Arrastia et al., 2009; Frey, 2003). The resulting glia scarring, aberrant synapses, and abnormal rewiring of neuronal networks culminates in an imbalance between excitatory and inhibitory signaling that is manifested in unprovoked and recurring seizures or PTE (Pitkanen et al., 2009; Pitkanen et al., 2014). Epileptic seizures are defined as a “transient occurrence of signs and/or symptoms due to abnormal, excessive or asynchronous neuronal activity in the brain” (Falco-Walter et al., 2018). Late onset PTS/PTE can develop up to 2 years post-injury, and early PTS, hematomas, bleeding, penetrating injury, focal contusions, and injury severity are considered major risk factors (Diaz-Arrastia et al., 2009; Frey, 2003; Xu et al., 2017). The prevalence of PTE is not only correlated with injury type and age of subjects, but also with TBI severity (Ritter et al., 2016; Temkin, 2003).
The current classification of TBI severity is based on clinical symptoms such as Glasgow Coma Scale (GCS), loss of consciousness (LOC), post-traumatic amnesia (PTA), and basic neuroimaging (mainly computed tomography, CT). The incidence of PTE after mild TBI (GCS 13–15, LOC < 30 minutes, PTA < 1 hour, and normal neuroimaging) is low, about 1–3% (Ritter et al., 2016). Patients who have suffered moderate TBIs (GCS 9–12, LOC > 30 minutes and PTA between 1 and 7 days, with or without abnormal imaging) have the second highest, or in some studies (Englander et al., 2003) the highest, risk for developing PTE. In addition to TBI severity based on GCS, LOC, and PTA, epidemiology studies have identified dura penetration with bone fragments, subdural hematoma, intraparenchymal bleeding, and injury with contusions as major risk factors for PTE (Ding et al., 2016; Lucke-Wold et al., 2015; Salazar and Grafman, 2015). In fact, the incidence of PTE after severe TBI (GCS 3–8, LOC > 24 hours, PTA > 7 days, and typically abnormal neuroimaging) can be as high as 50% in patients with a penetrated dura (Christensen, 2015; Salazar and Grafman, 2015). Abnormal imaging indicating contusion and/or hematoma puts these patients at the highest risk for both early and late onset PTE. It should be noted that this injury group also has the highest mortality rate, which impacts our ability to accurately account for the incidence of late onset PTE.
4. Protein biomarkers in TBI
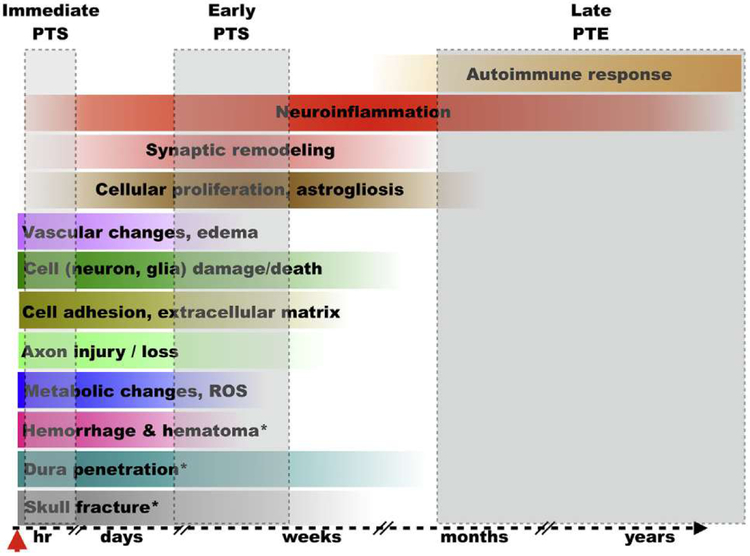
The same clinical symptomatology described above can result from different injury types (i.e., penetrating/focal vs. closed-head/diffuse) with disparate primary injuries and heterogeneous secondary injury processes (Saatman et al., 2008). Penetrating head injury generally presents a greater risk for PTE development than diffuse injury of a similar severity (Ding et al., 2016; Lucke-Wold et al., 2015; Salazar and Grafman, 2015). In penetrating/focal TBIs, kinetic forces affect a small area of the brain causing massive local damage. Conversely, closed-head/diffuse TBIs affect larger areas of the brain, particularly at the interface of tissues with varying densities (e.g., white and gray matter). Protein biomarkers measured in CSF and serum have played a critical role in determining TBI severity, identifying molecular changes related to damage and repair, and their temporal profiles (Agoston and Elsayed, 2012; Agoston et al., 2017; Strathmann et al., 2014; Wang et al., 2005; Wang et al., 2018; Zetterberg and Blennow, 2016; Zetterberg et al., 2013). Biomarkers have the potential to monitor disease progression and clinical outcomes, and to provide molecular-level information about the pathobiologies that can lead to long-term consequences/complications after TBI. The specific serum and/or CSF-based protein biomarker “footprints” have already been identified for a number of these processes. Because the incidence of PTE after mild TBI and concussion is very low, here we focus on the pathobiologies of moderate and severe TBI and related protein biomarkers. We list the main pathobiological events triggered by penetrating, focal TBI and closed head, diffuse TBI and discuss in the context of epileptogenesis and PTE (Fig. 1). While most of the injury mechanisms described below occur in both TBI types, their onset, duration, and contribution to the overall pathology can significantly vary (Table 1).
Fig. 1.
The onset and extent of select pathological changes and biological responses after TBI (a hypothetical model). The colored horizontal bars illustrate the approximate onset and scale of the individual pathological changes following injury; color gradients reflect time-dependent changes in the intensity of individual pathobiologies. The grey shaded boxes indicate the relative onset of post-traumatic seizures (PTS) and post-traumatic epilepsy (PTE). * pathological changes that occur predominantly or exclusively after penetrating TBI; hr = hours; ROS = reactive oxygen species.
Table 1.
List of major pathobiological events and affected structures, injury mechanism(s), and candidate protein biomarkers of epileptogenicity after TBI.
| Event and affected structure(s) | Injury mechanism(s) | Candidate protein biomarkers |
|---|---|---|
| Skull fracturea | Primary (mechanotransductive mechanisms) | Alkaline phosphatase, BMPs, OPN |
| Dura penetration or damagea | Primary | Leptomeningeal proteins (β-trace), glycosaminoglycans, inflammatory molecules |
| Hemorrhage, hematomaa | Primary | Hemoglobin (CSF), fibrinogen (CSF) |
| Metabolic changes, reactive oxygen species | Primary (hypoxia, altered energy demands, ion homeostasis and neurotransmission, toxic substances) | HIF1α, HNE, SOD2, Ceruloplasmin, HSP70 |
| Axonal injury and/or loss | Primary and secondary (e.g., inflammation) | NF-L, NF-H, tau, p-tau |
| Disruption of cell adhesion and extracellular matrix | Primary and secondary (e.g., inflammation) | MMP9, Integrin-α6, 1TMP1, TIMP4, Connexin-43, NCAD, ICAM1, NCAM1 |
| Neuronal and glial damage or death | Primary and secondary (metabolic dysregulation and neuroinflammation) | Neuronal: UCHL1, NSE, CK-BB Glial: GFAP, ALDOC, BLBP, PEA15, GS, S100β, MBP |
| Vascular changes, edema | Primary and secondary (neuroinflammation, BBB disruption, metabolic stress, toxic substances) | VEGF, VEGF-R1 (Flk1), pVEGF-R1, Claudin-5, vWF, AQP4, Occludin, VCAM1, CSF/serum albumin ratio |
| Cellular proliferation, astrogliosis | Secondary | GFAP, BDNF, glial cell-derived neurotrophic factor (GDNF) |
| Synaptic remodeling | Secondary | BDNF, GDNF |
| Neuroinflammation | Secondary | Chemo/cytokines, Interleukins (IL1β in CSF), TLR signaling molecules, OPN, fibrinogen, and DAMPs (HSP70, IIMGB1, S100β) |
| Autoimmune response | Secondary | MBP (in CSF), Immunoglobulin G (CSF), autoantibodies |
Pathological changes that occur predominantly or exclusively after penetrating TBI. Bold faced markers have shown prognostic value in PTE.
4.1. Skull fracture and fragmentation
Epidemiology studies identified skull fracture and dura penetration as the most frequent underlying cause of PTE (Lucke-Wold et al., 2015; Salazar and Grafman, 2015; Xu et al., 2017). TBI with concomitant bone fracture triggers a highly complex molecular response involving osteogenesis and ossification (Brady et al., 2016a; 2017; 2016b). Accordingly, the penetration of the skull and dura induces pathobiological changes unique to this form of TBI (Huang et al., 2018). Penetrating TBIs result in the deposition of bone particles in the brain parenchyma; these foreign bodies, along with metal fragments after ballistic TBI, are among the greatest risk factors for epileptogenesis and PTE (Ommaya et al., 1996; Raymont et al., 2010; Salazar et al., 1995). Bone fracture results in bleeding, followed by inflammation aimed at demarcating the lesion, which is followed by a repair process that involves partial reactivation of the developmental process (Bajwa et al., 2018; Yao et al., 2004). Levels of inflammatory proteins, both chemo- and cytokines, osteogenic proteins (Brady et al., 2017; Huang et al., 2018), mesenchymal proteins and their regulators (Gu et al., 2016), and bone morphogenic proteins (BMPs) (Agarwal et al., 2017) increase in the systemic circulation. These may be used to indicate the extent of the damage and the status of the repair process (Heggeness et al., 2017). Osteopontin (OPN) is an extracellular matrix protein and proinflammatory cytokine that plays a critical role in bone formation and immunomodulation (Butler, 1989; Shin, 2012). Up-regulation of OPN has been found to respond to mechanical forces, such as those of the primary injury, making OPN a potentially important differentiator of injury type and related patomechanisms.
4.2. Dura penetration and/or damage
Piercing the dura, especially with intraparenchymal bone and/or metal fragments, substantially increases the risk for developing PTE. The dura mater is heavily vascularized; perforating it disrupts blood vessels and venous sinuses resulting in hemorrhage. Dura penetration also damages and/or destroys fibroblast cells which then release their structural proteins, collagens, reticular fibers and glycosaminoglycans into the CSF and systemic circulation. The dura hosts various immune cells, lymphocytes, plasma and mast cells that are involved in the response to tissue damage and mediation of the inflammatory process. Upon activation, these cells release a whole array of chemo- and cytokines. While dura penetration and the extent of damage may be clinically obvious without measuring protein biomarker levels in the CSF (and/or serum), CSF levels of dura markers, fibroblast-derived and inflammatory molecules can indicate the magnitude of the neuroinflammatory response. Determining the temporal profiles of such changes can aid in the diagnosis and prognosis of epileptogenesis, and improve the predictability of PTE.
4.3. Hemorrhage/hematoma
Hemorrhage and hematoma are among the most important risk factors for developing PTE (Lucke-Wold et al., 2015; Pitkanen et al., 2016; Salazar and Grafman, 2015; Xu et al., 2017). The presence, extent, and location of hematoma and/or hemorrhage are diagnosable by various neuroimaging techniques. Serial imaging provides critical information about time-related changes that can be used to direct surgical intervention and help assess outcome. Similarly, determining hemoglobin levels and its breakdown products in the CSF- along with vascular, metabolic, and inflammatory markers-can be an important addition to imaging-based decision making and prognosis for PTE. Ceruloplasmin, also called ferroxidase, is an acute phase protein that plays a protective role after infection and injury (Hellman and Gitlin, 2002). In addition to binding 95% of all copper in plasma, ceruloplasmin also plays a role in iron metabolism through its copper-dependent oxidase activity. Ceruloplasmin oxidizes ferrous iron (+2) into ferric iron (+3), which is important because transferrin can only carry iron in the ferric state. Iron accumulation due to bleeding and subsequent hemolysis in the brain is a major risk factor for PTE (Ding et al., 2016; Salazar and Grafman, 2015). Iron is cytotoxic, resulting in oxidative stress through the generation of free radicals and mitochondrial pathology. Importantly, it has been shown that TBI patients with abnormally low ceruloplasmin plasma levels develop increased intracranial pressure (Ayton et al., 2014; Dash et al., 2010).
4.4. Vascular changes
TBI can cause vascular damage of varying severity: from transient increases in blood-brain-barrier (BBB) permeability and microbleeds to subdural or intracerebral hemorrhage. Cerebral contusion is associated with vascular injury and/or microhemorrhage that also significantly increases the probability of developing PTE. Increased serum and/or CSF levels of proteins such as occludin or claudin-5 are good indicators of damage to the endothelial tight junctions (Jiao et al., 2011). Cerebral endothelial cells and their astrocytic footprocesses express high numbers of aquaporin-4 (AQP4), the main water channel in the brain (Arcienega et al., 2010; Thrane et al., 2011). AQP4 functions are critical for maintaining the balance between extra- and intracellular water in the CNS. Elevated serum and CSF levels of AQP4 are detected after TBI, indicating damage to the molecular machinery of water homeostasis (Ahmed et al., 2012; Ahmed et al., 2015). Cerebral edema and vasospasm are hallmarks of severe TBI, indicating major changes in water transport and altered endothelial/vascular reactivity. Stressed or injured endothelial cells increase the expression of von Willebrand factor (vWF), an endothelium-specific glycoprotein that plays a major role in blood coagulation and platelet adhesion to wound sites (De Oliveira et al., 2007; Yokota et al., 2007). Elevated vWF levels in the serum indicate endothelial activation in response to TBI, to counter injury-induced vascular damage, increased permeability, and microbleeding. In response to stress and injury, endothelial cells also express elevated levels of vascular endothelial growth factor (VEGF) which promotes proliferation and vascular regrowth (Croll et al., 2004; Lee and Agoston, 2005; Lee and Agoston, 2009). Elevated serum or CSF levels of VEGF after TBI can be indicative of endothelial damage or repair, depending on the tested VEGF isoform (anti-angiogenic vs. pro-angiogenic) and injury phase (Ahmed et al., 2014; 2015; 2013).
4.5. Cell surface and extracellular matrix changes
Sudden acceleration/deceleration in closed head injury causes massive lateral movement of adjacent membrane surfaces. Cell to cell junctions are disrupted and opposing cell membrane surfaces are dislocated affecting extracellular matrix molecules, junctional proteins, receptors, ion channels, and so forth. Increased CSF and/or serum levels of cell adhesion and cell surface molecules such as matrix metalloproteinase-9 (MMP9), Integrin-α6, tissue inhibitor of metalloproteinases, TIMP1 and TIMP4, connexin-43, neural cadherin (NCAD), intercellular adhesion molecule-1 (ICAM1), and neural cell adhesion molecule-1 (NCAM1) indicate alterations in cell-cell adhesion and cellular connectivity (Karkela et al., 1993; Park and Biederer, 2013). TIMP4 is an inhibitor of matrix metalloproteinases involved in the degradation of the extracellular matrix (Pullen et al., 2012; Rorive et al., 2010). NCAD is a multifunctional molecule involved in mediating cell adhesion; in the CNS, it is involved in stabilizing synapses, thus playing an important role in learning and memory (Jang et al., 2009; Kanemaru et al., 2013). Increased CSF and/or serum levels of NCAD indicate altered cell adhesion including synapse disruptions. NCAD is also involved in the repair process due to its involvement in mediating astrogliosis.
4. 6. Cellular proliferation, astrogliosis
While neurons in the adult CNS are post-mitotic, there is a limited population of reserve neural stem/progenitor cells, located mostly in the subgranular layer of the dentate gyrus of the hippocampus (Palmer et al., 1997; Song et al., 2002). This population responds to injury with proliferation and some of the new cells can differentiate in to functional neurons (Hess and Borlongan, 2008; Ming and Song, 2005; Picard-Riera et al., 2004). This adult neurogenesis is regulated by several soluble molecules including VEGF-A, a pro-angiogenic isoform of the endothelial growth factor (Lee and Agoston, 2009). However, there is experimental evidence that de novo neurogenesis following TBI may increase neuronal excitability and seizure susceptibility (Kuruba and Shetty, 2007; Neuberger et al., 2017; Parent, 2003; Yu et al., 2014a). Cellular proliferation, the response of endothelial cells, oligodendrocytes, astro- and microglia to TBI, is an important part of the secondary injury process (Chirumamilla et al., 2002). Of these proliferative events, reactive astrogliosis is a major risk factor for epileptogenesis (Cotrina et al., 2014; Eng and Ghirnikar, 1994; Little and O’Callagha, 2001; Takano et al., 2014). Proliferation and differentiation of astrocytes into the reactive, morphologically distinct, stellar shape is part of the secondary injury mechanism. Their primary biological function is to demarcate the injury site and mediate neuroinflammatory response by controlling the access of inflammatory cells to the lesion, rebuild the BBB, and reduce oxidative stress (Fitch and Silver, 2008). However, glial scar formation creates a physical and chemical barrier in the injured brain, thereby preventing select regenerative efforts (e.g., axonal regeneration) and contributing to the epileptogenicity of brain structures after TBI. The signaling molecules that trigger astroglia proliferation include several growth factors such as epidermal growth factor (EGF), fibroblast growth factor (FGF), and endothelin-1 (Okada et al., 2018; Pekny and Pekna, 2014; Sofroniew, 2014). In addition to these soluble regulatory molecules, several chemo- and cytokines including interleukin-1β (IL1β), interleukin-6 (IL6), and tumor necrosis factor-α (TNFα), produced by microglia and invading leukocytes, also act as regulators of astrogliosis.
4.7. Metabolic changes
Metabolic dysregulation is implicated in all TBIs, albeit the molecular nature, magnitude, and duration depend on the primary injury mechanism and severity (Buitrago Blanco et al., 2016; Giza and Hovda, 2001; Hillered et al., 2006; Hovda et al., 1995; Ott et al., 1987; Vespa et al., 2005). Metabolic depression, i.e., altered cerebral glucose metabolism, is a key component of cerebral vulnerability that underlies many of the clinical symptomatologies observed after TBI (Buitrago Blanco et al., 2016). Reduced cerebral glucose metabolism leads to overproduction of reactive oxygen species (ROS) and, in turn, oxidative stress (Chong et al., 2005; Martin et al., 2005; Potts et al., 2006). The onset and extent of oxidative stress can be determined by monitoring 4-hydroxynonenal (HNE), hypoxia-inducible factor 1α (HIF1α), and mitochondrial superoxide dismutase (SOD2) levels preferably in CSF or in serum. HNE is a byproduct of lipid peroxidation, and a sensitive and specific marker of oxidative stress (Abdul-Muneer et al., 2013; Hall et al., 2004; Versace et al., 2013). Increased CSF levels of HNE are good indicators of the extent of oxidative stress in the CNS. HIF1α is a transcription factor that plays a critical role in the adaptive response to hypoxia. Its levels in the CSF are elevated in TBI and in stroke; elevated serum levels, however, can be the result of CNS as well as peripheral/systemic response to hypoxic conditions. Astroglia and endothelial cells play important roles in glucose metabolism and transport, thus elevated CSF and/or serum levels of glial fibrillary acidic protein (GFAP), various astroglia-associated and endothelium-specific proteins, as well as vascular factors (see in detail under respective sections) can provide insight into more than one pathobiology.
4.8. Neuronal, axonal and glial damage/loss
Closed or penetrating head trauma can cause structural brain injury, directly killing neurons and glia and damaging axons (Blennow et al., 2012). Proteins that are (mostly) specific to neural cells (e.g., neuron-specific enolase, NSE, or GFAP) are released from damaged and/or dying cells; therefore, serum and CSF levels of these proteins are good indicators of injury severity (Agoston and Elsayed, 2012; Agoston et al., 2017; Wright et al., 2016; Zetterberg and Blennow, 2015). Cell damage or death, and consequent release of damage-associated proteins, can continue during the secondary injury process due to neuroinflammation causing extended cell death. Chronically elevated serum and CSF levels of these biomarkers indicate poor prognosis. Ubiquitin C-terminal hydrolase-Ll (UCHL1) is a (mostly) neuron-specific cytoplasmic enzyme involved in protein ubiquitination and elimination (Bishop et al., 2016). Increased serum levels of UCHL1 in the acute phase of TBI have been associated with injury severity (Brophy et al., 2011; Li et al., 2015; Liu et al., 2010; Mondello et al., 2012). In conjunction with elevated GFAP serum levels, these two markers are positively correlated with injury severity (Diaz-Arrastia et al., 2014; Diaz-Arrastia et al., 2013). However, there are controversies about how well their serum levels reflect injury severity (Diaz-Arrastia et al., 2014; Diaz-Arrastia et al., 2013). Elevated serum and CSF levels as well as the temporal pattern of myelin basic protein (MBP), respectively, can indicate the extent and kinetics of white matter damage, and help to predict outcome (Berger et al., 2005; Derkus et al., 2017; Thomas et al., 1978). Increased serum levels of additional astroglia-enriched markers (aldolase C, ALDOC; its 38kD breakdown product, BOP; brain lipid-binding protein, BLBP; phosphoprotein enriched in astrocytes-15, PEA15; glutamine synthetase, GS; and 18–25kD-GFAP-BDPs) have shown positive correlation with injury severity (Halford et al., 2017). Elevated serum and/or CSF levels of axonal markers including various isoforms of neurofilament (NF), tau, as well as its phosphorylated form (p-tau) have been extensively used as biomarkers of TBI type and severity (Liliang et al., 2010; Wang et al., 2016a; Zemlan et al., 2002; Zetterberg, 2017). Like other markers, the diagnostic and prognostic value of these proteins is greatly dependent on their temporal pattern, e.g., an early peak in NF heavy chain (NF-H) serum levels indicates severe TBI and poor outcome, whereas a gradual increase reflects mild to moderate injury (Gyorgy et al., 2011). S100β is one of the most studied, albeit controversial serum biomarkers in TBI (Thelin et al., 2013) versus (Papa et al., 2014). However, the temporal profile of serum S100β levels has been found to be correlated with injury severity and outcome (Thelin et al., 2014), but increased serum S100β levels can also be due to non-neuronal sources (Unden et al., 2005). It should be noted that S100β is an important member of damage associated molecular patterns (DAMPs) mostly originating from non-neural source (see Neuroinflammation).
4.9. Synaptic remodeling
Damage to neurons, especially in focal injury, results in axonal degeneration and loss of synapses as indicated by the reduced number of dendritic spines observable within a few days after the insult (Biennow et al., 2016; Blennow et al., 2012). These adverse changes are followed by axonal sprouting as part of the regenerative attempt along with de novo neurogenesis (Parent, 2003; Richardson et al., 2007; Sun, 2014) and angiogenesis (Morgan et al., 2007; Thau-Zuchman et al., 2012). Axonal sprouting and synaptic remodeling are regulated by neuronal growth factors, like nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), through various tyrosine receptor kinase (trk) signaling pathways and neuronal adhesion molecules (da Silva Meirelles et al., 2017; Lorente, 2017; Werner and Stevens, 2015). It should be noted that the availability of BDNF after penetrating TBI is a critical determinant of functional recovery; diminished BDNF signaling (e.g., caused by mutation) results in poor functional outcome (Rostami et al., 2011). The process is a partial reenactment of the developmental process of axonal growth and synapse formation regulated by the same molecules: NCAD, polysialylated-neural adhesion molecule (PSA-NCAM), NCAM, neuropilin, and various semaphorins, ephrins, and neuroexins (Park and Biederer, 2013). CSF levels of these molecules may be used to assess the extent of axonal regrowth and synaptic remodeling (Pleines et al., 1998; Whalen et al., 1998).
4.10. Neuroinflammation
Inflammation is a fundamental defense mechanism after injury, and potential disease mechanism in moderate and severe TBI (Balu, 2014; Corrigan et al., 2016; Hinson et al., 2015; Simonet al., 2017). In fact, neuroinflammation is one of the main candidate pathomechanisms in the development of PTE (Webster et al., 2017). Several of the above described injury-induced changes, such as bleeding, contusion/cell loss, and metabolic changes (especially reactive oxygen species) can activate the neuroinflammatory process within minutes after insult. Following injury, resident microglia and astrocytes are activated, and leukocytes from the systemic circulation invade the brain parenchyma (Simon et al., 2017). During the acute phase of injury, soluble cytokines (IL1, IL6, and TNF) and chemokines (monocyte chemoattractant protein-1, MCP1; macrophage inflammatory protein-1, MIP1; and regulated on activation, normal T cell expressed and secreted, RANTES) are released to guide immune cells to injured brain regions. Significant increases in the CSF and serum levels of several inflammatory markers have been demonstrated in experimental TBI as well as in clinical TBI (Woodcock and Morganti-Kossmann, 2013). For instance, injury-induced increases in serum levels of the toll-like receptor (TLR) family of proteins and MCP1 (Semple et al., 2010). MCP1, a member of the CC chemokine family, is involved in attracting monocytes and regulating the permeability of the BBB (Yadav et al., 2010). Neuroinflammation may play an important role in maintaining PTE because seizures can increase BBB permeability, allowing the entry of peripheral immune cells into the brain parenchyma and the release of inflammatory molecules that creates a vicious, self-sustaining cycle.
Increased serum levels of cytokine-induced neutrophil chemoattractant-1α (CINC1α), CD53, and TLR9 have also been found in various TBI models (Katayama et al., 2009; Vollmer, 2006). CINC1α is produced by astrocytes in response to oxidative stress, linking it to the neuroinflammatory response (Anada et al., 2018). CD53 is a cell-surface protein expressed by microglia that mediates cell growth and immune response. Elevated CSF levels of CD53 are indicative of an ongoing neuroinflammatory process that involves microglia (Woodcock and Morganti-Kossmann, 2013). Elevated serum levels of fibrinogen, a plasma glycoprotein, are detected during neuroinflarnmation (Davalos and Akassoglou, 2012; Muradashvili and Lominadze, 2013; Ryu et al., 2009). Increased fibrinogen levels increase blood viscosity and vascular permeability by activating the extracellular signal-regulated kinase 1/2 (ERK1/2) pathways. The pro-inflammatory chemokine MIP1α is produced by a variety of immune cells including macrophages and microglia (McManus et al., 1998). Elevated MIPla serum levels after TBI have been found to indicate a systemic inflammatory response (Hsieh et al., 2008). Importantly, activated glia cells can be found months to years following TBI (Gentleman et al., 2004; Johnson et al., 2013) further implicating neuroinflammation in the pathomechanism of PTE. A genetic and biomarker cohort study by (Diamond et al., 2014) has shown that elevated CSF/serum IL1β ratios were associated with increased risk for PTE.
Damage associated molecular patterns (DAMPs) are released from damaged and dying cells after TBI and initiate the innate immune reaction in response to injury (Liesz et al., 2015). DAMPs are highly heterogenous molecules and include heat shock proteins (HSPs), high mobility group (HMG) proteins, extracellular proteins, as well as non-proteins like deoxyribonucleic acid (DNA), heparin sulfate, and adenosine triphosphate (Pandolfi et al., 2016). DAMPs bind to pattern recognition receptors and activate immune response (Kigerl et al., 2014). High mobility group box 1 protein (HMGB1) is an intracellular DNA binding protein that elicits an inflammatory response upon release from damaged cells by activating glia, endothelial and peripheral immune cells (Asavarut et al., 2013; Parker et al., 2017). HMGB1 can bind to multiple receptors, including TLR2 and TLR4, and regulate the expression of pro-inflammatory cytokines IL1α, IL1β, IL6, TNFα, and interferon-gamma (IFNγ) through the nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) signaling pathway. Heat shock proteins (HSP27, HSP40, HSP60, HSP70, HSP100 and Crystallin alpha-B) are molecular chaperons involved in stabilizing damaged proteins in injured cells (Kim and Yenari, 2013; Stetler et al., 2010).
Extracellular HSP70, like HMGB1, also binds to TLR2 and TLR4 receptors and can induce the release of cytokines from activated immune cells. HSP70 may play a neuroprotective role in TBI by blocking pro-inflammatory signaling in astrocytes. CSF levels of HSP60, a mitochondrial protein, are significantly increased after severe TBI (measured in pediatric patients) indicating mitochondrial/metabolic stress (Lai et al., 2006). Serum levels of HSP60 also increased in temporal lobe epilepsy; levels showed positive correlation with the activation of immune cells and the secretion of cytokines (Marino Gammazza et al., 2015). S100β is a calcium-binding protein that is mainly (but not exclusively) expressed and released by astrocytes (Donato, 1999). S100β is neurotrophic at low concentrations, but in micromolar doses S100β is neurotoxic and promotes neuroinflammation by binding to the receptor for advanced glycation endproducts (RAGE) and activating NFkB signaling (Kleindienst et al., 2007; Kleindienst and Ross Bullock, 2006). Other members of the S100 family, S100A8 and S100A9 are also involved in mediating neuroinflammation but their role is less well understood (Sorci et al., 2000). Osteopontin is an extracellular matrix protein with multiple functions that include immunoactivation (Butler, 1989; Shin, 2012). Elevated serum levels of osteopontin have been found after TBI, and it has been implicated in autoimmune diseases such as multiple sclerosis (MS), bridging between innate and adaptive immunity (Agah et al., 2018).
4.11. Autoimmune response
Autoimmune epilepsy (AE) is considered a separate disease entity that is mostly resistant to traditional anti-epileptic drugs (Britton, 2016; Gaspard, 2016; Greco et al., 2016). AE can be caused by a whole array of pathologies including malignancies, infections, and by TBI. TBI, especially its severe form, causes significant tissue damage releasing extra- as well as intracellular proteins into the interstitium, CSF, and eventually systemic circulation. The accompanying vascular damage and/or major breakdown of BBB enable peripheral immune cells to be exposed to the proteins and/or their degradation products triggering an autoimmune response. Some of the released proteins undergo post-translational modifications, such as deimination (also called citrullination), creating novel, highly antigenic epitopes that can elicit autoimmune responses. One example is GFAP that is citrullinated after release from injured astrocytes following TBI (Attilio et al., 2017; Wang et al., 2016b). CSF- and serum-based biomarkers of autoimmune response that can significantly contribute to PTE include autoantibodies against channel and receptor proteins, especially against N-Methyl-D-aspartate, voltage-gated potassium receptor complex, and voltage-gated calcium channel proteins, as well as against glutamic acid decarboxylase-65 (GAD65). (Fang et al., 2017). Important considerations are: autoantibodies are not the best biomarkers because autoantibody levels can remain elevated even after treatments that alleviate the symptoms; the production of autoantibodies takes time, typically beyond the time frame of preclinical TBI studies and clinical observations. Additional biomarkers, ideally measured in the CSF, include degradation products of the released protein(s), enzymes like MMP2 and MMP9 that are indicators of ongoing proteolytic process and inflammatory markers, cyto- and chemokines, and other indicators of immune system activation like IL6 and TNFα (Britton, 2016; Gaspard, 2016; Greco et al., 2016).
5. Considerations for biomarker studies of PTE
5.1. The time factor
PTE can develop at various post-injury time points (Ding et al., 2016; Lucke-Wold et al., 2015; Pitkanen and Immonen, 2014; Pitkanen et al., 2009; Wilson et al., 2018). The onset of early, provoked seizures vs. unprovoked and recurrent seizures is related to distinct pathobiological events of the primary and secondary injury mechanisms. Therefore, the pathomechanisms (and candidate biomarkers) for immediate and early PTS vs. late onset PTE are likely very different (Fig. 1). As discussed above, the acute phase of TBI is dominated by the consequences of the primary injury, and changes in protein biomarker levels in CSF and/or serum can reflect the underlying pathologies. Neuron- and glia-specific proteins such as UCHL1, GFAP, and NSE are released from damaged and dying cells and their levels peak in biofluids at the earliest measured time point (Agoston and Kamnaksh, 2015; Diaz-Arrastia et al., 2014; Rostami et al., 2015; Wang et al., 2018). Similarly, axonal damage is reflected in the peak levels of p-tau and NF proteins (Rubenstein et al., 2017; Wang et al., 2016a). While metabolic changes in the cerebrum are best measured by other clinical methods (Agoston and Kamnaksh, 2015), protein biomarkers like HNE and SOD2 can also indicate drastic changes in oxidative stress during the acute phase (Ahmed et al., 2015). The combination of disrupted connectivity due to cell damage/death and metabolic dysregulation are likely the main cause of immediate and early onset seizures (Diaz-Arrastia et al., 2009; Ding et al., 2016; Tubi et al., 2018). Late onset PTE, however, is likely caused by the combination of initial damage to the brain parenchyma, loss of cells and physically disrupted connectivity, as well as the biological responses of the secondary injury mechanism (Diaz-Arrastia et al., 2009; Dulla et al., 2016; Salazar and Grafman, 2015; Wilson et al., 2018). The exact onset and time course of these pathobiological responses is currently not well known. But an acute inflammatory response to injury can become chronic. Combined with an autoimmune response, neuroinflammation is probably the most important pathomechanism underlying epileptogenesis and sustaining PTE (Lucke-Wold et al., 2015). Studies have found dynamic changes in protein biomarker levels and biofluid-specific temporal profiles of prognostic value (Ahmed et al., 2012; Gyorgy et al., 2011; Thelin et al., 2014), for that reason biofluids should be obtained for analysis at multiple post-injury time points.
5.2. Biofluids
The importance of CSF for proteomic analysis.
The functional relationship between intracranial and extracranial fluid dynamics is very complex, and intracranial changes may not be truly reflected in the systemic blood (Nakada and Kwee, 2018). Therefore, biofluids selected for routine clinical analysis each has its pros and cons. The advantages of using blood as “the biofluid” for biomarker analysis are obvious: obtaining blood is minimally invasive and it can be repeated frequently. However, blood (serum or plasma) has limitations for the initial phase of PTE protein biomarker discovery for several reasons (Agoston et al., 2017). Protein biomarkers that are indicative of certain pathomechanisms (e.g., inflammation) are not specific to the central nervous system (CNS) (Zetterberg and Blennow, 2016), and their concentrations in serum or plasma are typically very low. Furthermore, there is a temporal mismatch (even under normal physiologic conditions) between blood and CSF levels of the same protein(s) due to blood-CSF concentration gradient and molecular size (Huhmer et al., 2006). The BBB is almost always compromised after moderate to severe TBI. An “open BBB” allows protein biomarkers to enter the systemic circulation thereby “diluting”/reducing their CSF levels (Abbott and Friedman, 2012). The BBB thus plays a dual role in the pathobiology of TBI; BBB breakdown majorly contributes to the propagation of the injury intracranially, and it permits the movement of protein biomarkers from the brain parenchyma into systemic circulation (Simon and Iliff, 2016). Intracranial fluid movements, including protein and metabolite clearance from the injured brain parenchyma, involve the glymphatic system (Benveniste et al., 2017). The exact role of the glymphatic system in clearing proteins from the interstitium is currently poorly understood and little is known about how TBI affects the structural elements of the glymphatic system and consequently glymphatic flow (Asgari et al., 2016). This provides a strong rationale for comparing protein bio-marker levels in serial blood and CSF samples collected from the same patients at identical timepoints.
Severely injured TBI patients are typically admitted to neurointensive care units where CSF is routinely obtained for various clinical diagnostics (Agoston, 2015). Patients are clinically monitored even after discharge; thus, CSF samples can be obtained via lumbar puncture at several, later time points. This patient population is particularly valuable for identifying and profiling time-related changes in CSF protein biomarkers to determine the pathomechanism(s) of epileptogenesis and onset of PTE (Agoston et al., 2017). The value of analyzing CSF to identify risk factors for PTE has been demonstrated by several studies (Aronica and Crino, 2011; Britton, 2016; Diamond et al., 2014; Hegde and Lowenstein, 2014; Nordby and Urdal, 1982; Vezzani and Friedman, 2011; Xi et al., 2015; Yu et al., 2014b). Relative to blood serum or plasma, the biochemical properties and physiologic compartmentalization of CSF makes it a “cleaner” biomaterial for proteomic analysis. A significant proportion of patients admitted to the neurointensive care unit with moderate to severe TBI receive whole blood transfusions, fresh frozen plasma, cryoprecipitate, and factor VII among other substances. What was infused, how much, the rate and duration of trans-fusion (relative to the time blood is drawn for biomarker analysis) can markedly impact the biochemistry of serum and plasma samples. Therefore, CSF offers a relatively undisturbed “window” into the brain’s physiologic (or pathologic) state as well as its surrounding environment. Nevertheless, the benefits of using CSF for proteomic analysis come with important caveats. Despite its closeness to the brain parenchyma, CSF levels of protein biomarkers, especially after moderate to severe TBI, can be substantially affected by brain edema, poor CSF circulation, and altered BBB function. Cerebral edema is one of the leading consequences of moderate to severe TBI; intracranial fluid movements, including the production, circulation, and absorption of CSF, can be majorly affected by the swelling of the brain (Donkin and Vink, 2010; Pasco et al., 2006). Therefore, brain edema and altered cerebral blood flow can also change protein biomarker levels (Simon and Iliff, 2016). Furthermore, it is unrealistic to expect that CSF sampling will be widely and routinely used, so the value of identifying blood-based protein biomarkers for PTE is paramount. An essential first step toward this goal is to establish how injury-induced changes in the CSF levels of protein biomarkers are reflected in systemic circulation. This can be achieved by comparing protein biomarker levels in the CSF vs. serum/plasma obtained from the same patients at identical time-points, at least for the duration of their stay in neurointensive care as in the Epilepsy Bioinformatics Study for Antiepileptic Therapy (EpiBioS4Rx).
5.3. Protein biomarker studies in rodents vs. human
Protein biomarker studies of TBI animal models have provided critical information about the underlying pathobiological changes that can contribute to epileptogenicity after TBI (Agoston and Elsayed, 2012; Kobeissy et al., 2008; Kovesdi et al., 2010; Mondello et al., 2017; Thelin et al., 2017). As discussed above, serial sampling is important to ensure that the onset and evolution of pathobiological changes are not missed. Determining the temporal profile of protein biomarkers in different biofluids would enable a timelier PTE diagnosis and better prognosis. Combined with systems biology, establishing the time-dependent changes of protein biomarkers in the CSF and/or in serum has the potential to identify the precise chain of molecular events that lead to PTE. Age is another important risk factor for PTE (Bruns Jr. and Hauser, 2003); therefore, animal studies should account for age effects and design experiments accordingly. An important yet poorly understood relationship is how rodent and human timescales compare. All available data point to accelerated biological and pathological processes in rodents as compared to humans (Agoston, 2013; Agoston, 2017). Unfortunately, there is no simple “conversion factor” to translate between rodent and human times, even for normal biological processes. Based on limited experimental data from rodent and human TBI studies that have used comparable outcome measures, the difference between rodent and human time appears to be process-dependent making the translation between rodent and human timeframes challenging.
5.4. Limitations of protein biomarker studies
Virtually all biomarker assay platforms are dependent on the availability of specific antibodies. Proteases normally present in the various biofluids can damage epitopes and result in false negative data. Therefore, sample collection, handling, and preparation (e.g., serum or plasma isolation) are critical for proteomic analysis, yet there are no standardized protocols or internal controls to indicate the intactness of proteins. Sample quality and stability can significantly affect biomarker values, especially when ultra-high sensitivity platforms such as Simoa are used (Ambroz, 2011; Signore et al., 2017; Solier and Langen, 2014). The alternative to antibody-based analysis platforms, mass spectrometry, is currently not fast enough. While protein biomarker studies can provide important molecular level information about post-injury pathobiological events and their temporal patterns, they lack spatial precision. Elevated CSF and/or serum levels of protein markers can be the result of massive focal damage or smaller but more diffuse damage. This information can be provided by neuroimaging, and the combination of protein biomarker data and imaging data provides high diagnostic value and specificity for PTE. Similarly, protein biomarker data needs to be analyzed in the context of functional, neuropsychiatric, and electroencephalography (EEG) data for a better understanding of how macrostructural and molecular abnormalities translate into functional changes.
6. Conclusion
Protein biomarkers of the acute and subacute phase of TBI have been identified, but relatively little is known about their potential role in PTE development. The temporal profiles of protein biomarkers in CSF and serum of these early post-injury phases indicate complex and dynamic pathobiological processes. Much needs to be learned about the long-term changes. Longitudinal studies, both experimental and clinical, such as the multinational, multicenter consortium EpiBioS4Rx are needed to identify specific and reliable biomarkers for PTE. In combination with other biomarkers, imaging and EEG, protein biomarkers will help to identify individuals at increased risk for PTE, elucidate the exact molecular pathologies underlying epileptogenesis, and guide the development of evidence-based therapeutic interventions.
Footnotes
Disclosure
The authors declare no competing financial interests.
References
- Abbott NJ, Friedman A, 2012. Overview and introduction: the blood-brain barrier in health and disease. Epilepsia 53 (Suppl 6), 1–6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SM, Videnovic A, 2016. Chronic sleep disturbance and neural injury: links to neurodegenerative disease. Nat. Sci. Sleep 8, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Muneer PM, et al. , 2013. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic. Bioi. Med 60, 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams H, et al. , 2017. Temporal profile of intracranial pressure and cerebrovascular reactivity in severe traumatic brain injury and association with fatal outcome: an observational study. PLoS Med 14, el002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agab E, et al. , 2018. Osteopontin (OPN) as a CSF and blood biomarker for multiple sclerosis: a systematic review and meta-analysis. PLoS ONE 13, e0190252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, et al. , 2017. Strategic targeting of multiple BMP receptors prevents trauma-induced heterotopic ossification. Mol. Ther 25, 1974–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoston DV, 2013. Of Timescales, animal models, and human disease: The 50th arutiversary of C. elegans as a biological model. Front. Neurol 4, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoston D, 2015. Bench-to-bedside and bedside back to the bench: seeking a better understanding of the acute pathophysiological process in severe traumatic brain injury. Front. Neurol 6, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoston DV, 2017. How to translate time? The temporal aspect of human and rodent biology. Front Neurol 8 (92), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoston DV, Elsayed M, 2012. Serum-Based Protein Biomarkers in Blast-Induced Traumatic Brain Injury Spectrum Disorder. Front. Neurol 3, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoston DV, Kamnaksh A, 2015. Modeling the neurobebavioral consequences of blast-induced traumatic brain injury spectrum disorder and identifying related biomarkers In: Kobeissy FH (Ed.), Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. CRC Press/Taylor & Francis (c) 2015 by Taylor & Francis Group; LLC, Boca Raton (FL). [PubMed] [Google Scholar]
- Agoston DV, et al. , 2017. Biofluid biomarkers of traumatic brain injury. Brain Inj 31, 1195–1203. [DOI] [PubMed] [Google Scholar]
- Ahmed F, et al. , 2012. Time-dependent changes of protein biomarker levels in the cerebrospinal fluid after blast traumatic brain injury. Electrophoresis 33, 3705–3711. [DOI] [PubMed] [Google Scholar]
- Ahmed FA, et al. , 2013. Long-term consequences of single and multiple mild blast exposure on select physiological parameters and blood-based biomarkers. Electrophoresis 34, 2229–2233. [DOI] [PubMed] [Google Scholar]
- Ahmed F, et al. , 2014. Time-Dependent Changes of Protein Biomarker levels in the Serum and Cerebrospinal Fluid in a Large, Gyrencephalic Animal Model of Blast-Induced Mild Traumatic Brain Injury Journal of Neurotrauma. Submitted. [Google Scholar]
- Ahmed F, et al. , 2015. The Temporal Pattern of Changes in Serum Biomarker levels Reveals Complex and Dynamically Changing Pathologies After Exposure Toe a Single Low Intensity Blast in Mice Frontiers in Neurology. 6, Article 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroz K, 2011. Impact of blocking and detection chemistries on antibody performance for reverse phase protein arrays. Methods Mol. Bioi 785, 13–21. [DOI] [PubMed] [Google Scholar]
- Anada RP, et al. , 2018. Panel of serum protein biomarkers to grade the severity of traumatic brain injury. Electrophoresis 23, 1–8. [DOI] [PubMed] [Google Scholar]
- Arcienega II, et al. , 2010. Cell locations for AQP1, AQP4 and 9 in the non-human primate brain. Neuroscience 167, 1103–1114. [DOI] [PubMed] [Google Scholar]
- Aronica E, Crino PB, 2011. Inflammation in epilepsy: clinical observations. Epilepsia 52 (Suppl 3), 26–32. [DOI] [PubMed] [Google Scholar]
- Asavarut P, et al. , 2013. The role of HMGB1 in inflammation-mediated organ injury. Acta Anaesthesiol. Taiwanica 51, 28–33. [DOI] [PubMed] [Google Scholar]
- Asgari M, et al. , 2016. Glymphatic solute transport does not require bulk flow. Sci. Rep 6, 38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attilio PJ, et al. , 2017. The effects of blast exposure on protein deimination in the brain. Oxidative Med. Cell. Longev 2017, 8398072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S, et al. , 2014. Ceruloplasmin and beta-amyloid precursor protein confer neuroprotection in traumatic brain injury and lower neuronal iron. Free Radic. Bioi. Med 69, 331–337. [DOI] [PubMed] [Google Scholar]
- Bajwa NM, et al. , 2018. Long-term consequences of traumatic brain injury in bone metabolism. Front. Neurol 9, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MS, 2014. Casualties of the global war on terror and their future impact on health care and society: a looming public health crisis. Mil. Med 179, 348–355. [DOI] [PubMed] [Google Scholar]
- Balu R, 2014. lnflanunation and immune system activation after traumatic brain injury. Curr. Neurol. Neurosci. Rep 14, 484. [DOI] [PubMed] [Google Scholar]
- Benveniste H, et al. , 2017. The Glymphatic pathway. Neuroscientist 23, 454–465 (1073858417691030). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RP, et al. , 2005. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J. Neurosurg 103, 61–68. [DOI] [PubMed] [Google Scholar]
- Bishop P, et al. , 2016. Ubiquitin C-temtinal hydrolase L1 (UCH-L1): structure, distribution and roles in brain function and dysfunction. Biochem. J 473, 2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, et al. , 2012. The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899. [DOI] [PubMed] [Google Scholar]
- Blennow K, et al. , 2016. Traumatic brain injuries. Nat. Rev. Dis. Prim 2, 16084. [DOI] [PubMed] [Google Scholar]
- Bogoslovsky T, Diaz-Arrastia R, 2016. Dissecting temporal profiles of neuronal and axonal damage after mild traumatic brain injury. JAMA Neurol 73, 506–507. [DOI] [PubMed] [Google Scholar]
- Brady RD, et al. , 2016a. Sodium selenate treatment mitigates reduction of bone volume following traumatic brain injury in rats. J. Musculoskelet. Neuronal Interact 16, 369–376. [PMC free article] [PubMed] [Google Scholar]
- Brady RD, et al. , 2016b. Experimental traumatic brain injury induces bone loss in rats. J. Neurotrauma 33, 2154–2160. [DOI] [PubMed] [Google Scholar]
- Brady RD, et al. , 2017. Neurological heterotopic ossification: current understanding and future directions. Bone 109, 35–42. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, et al. , 1997. Temporal and regional patterns of axonal damage following traumatic brain injury: a beta-amyloid precursor protein immunocytochemical study in rats. J. Neuropathol. Exp. Neurol 56, 1132–1141. [DOI] [PubMed] [Google Scholar]
- Britton J, 2016. Autoimmune epilepsy. Handb. Clin. Neurol 133, 219–245. [DOI] [PubMed] [Google Scholar]
- Brophy GM, et al. , 2011. Biokinetic analysis of ubiquitin C-terminal hydrolase-L1 (UCH-L1) in severe traumatic brain injury patient biofluids. J. Neurotrauma 28, 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns J Jr., Hauser WA, 2003. The epidemiology of traumatic brain injury: a review. Epilepsia 44 (Suppl 10), 2–10. [DOI] [PubMed] [Google Scholar]
- Buitrago Blanco MM, et al. , 2016. Cerebral metabolism and the role of glucose control in acute traumatic brain injury. Neurosurg. Clin. N. Am 27, 453–463. [DOI] [PubMed] [Google Scholar]
- Butler WT, 1989. The nature and significance of osteopontin. Connect. Tissue Res. 23, 123–136. [DOI] [PubMed] [Google Scholar]
- Cederberg D, Siesjo P, 2010. What has inflammation to do with traumatic brain injury? Childs Nerv. Syst 26, 221–226. [DOI] [PubMed] [Google Scholar]
- Cernak I, et al. , 2002. Temporal characterisation of pro- and anti-apoptotic mechanisms following diffuse traumatic brain injury in rats. J. Clin. Neurosci 9, 565–572. [DOI] [PubMed] [Google Scholar]
- Chirumamilla S, et al. , 2002. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J. Neurotrauma 19, 693–703. [DOI] [PubMed] [Google Scholar]
- Cboi J, Koh S, 2008. Role of brain inflammation in epileptogenesis. Yonsei Med. J 49, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, et al. , 2005. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol 75, 207–246. [DOI] [PubMed] [Google Scholar]
- Christensen J, 2015. The Epidemiology of Posttrawnatic Epilepsy. Semin. Neurol 35, 218–222. [DOI] [PubMed] [Google Scholar]
- Corrigan F, et al. , 2016. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J. Neuroinflarnmation 13, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, et al. , 2014. Effects of traumatic brain injury on reactive astrogliosis and seizures in mouse models of Alexander disease. Brain Res 1582, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll SD, et al. , 2004. Vascular endothelial growth factor (VEGF) in seizures: a double-edged sword. Adv. Exp. Med. Bioi 548, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Meirelles L, et al. , 2017. Neurotrauma: the crosstalk between neurotrophins and inflammation in the acutely injured brain. Int. J. Mol. Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, et al. , 2010. Serum ceruloplasmin and copper are early biomarkers for traumatic brain injury-associated elevated intracranial pressure. J. Neurosci. Res 88, 1719–1726. [DOI] [PubMed] [Google Scholar]
- Davalos D, Akassoglou K, 2012. Fibrinogen as a key regulator of inflammation in disease. Semin. Immunopathol 34, 43–62. [DOI] [PubMed] [Google Scholar]
- De Oliveira CO, et al. , 2007. Plasma von Willebrand factor levels correlate with clinical outcome of severe traumatic brain injury. J. Neurotrauma 24, 1331–1338. [DOI] [PubMed] [Google Scholar]
- Derkus B, et al. , 2017. Simultaneous quantification of Myelin Basic Protein and Tau proteins in cerebrospinal fluid and serum of Multiple Sclerosis patients using nanoimmunosensor. Biosens. Bioelectron 89, 781–788. [DOI] [PubMed] [Google Scholar]
- Diamond ML, et al. , 2014. IL-1beta associations with posttraumatic epilepsy development a genetics and biomarker cohort study. Epilepsia 55, 1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Arrastia R, et al. , 2009. Posttraumatic epilepsy: the endophenotypes of a human model of epileptogenesis. Epilepsia 50 (Suppl 2), 14–20. [DOI] [PubMed] [Google Scholar]
- Diaz-Arrastia R, et al. , 2013. Acute biomarkers of traumatic brain injury: relationship between plasma levels of Ubiquitin C-Temtinal Hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 31, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Arrastia R, et al. , 2014. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-temtinal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 31, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K, et al. , 2016. Epilepsy after traumatic brain injury In: Laskowitz D, Grant. G (Eds.), Translational Research in Traumatic Brain Injury. CRC Press/Taylor and Francis Group (c) 2016 by Taylor & Francis Group; LLC, Boca Raton (FL). [Google Scholar]
- Donato R, 1999. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim. Biophys. Acta 1450, 191–231. [DOI] [PubMed] [Google Scholar]
- Donkin JJ, Vink R, 2010. Mechanisms of cerebral edema in traumatic brain injury: therapeutic developments. Curr. Opin. Neurol 23, 293–299. [DOI] [PubMed] [Google Scholar]
- Dulla CG, et al. , 2016. From molecular circuit dysfunction to disease: case studies in epilepsy, traumatic brain injury, and alzheimer’s disease. Neuroscientist 22, 295–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF, Ghimikar RS, 1994. GFAP and astrogliosis. Brain Pathol 4, 229–237. [DOI] [PubMed] [Google Scholar]
- Englander J, et al. , 2003. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch. Phys. Med. Rehabil 84, 365–373. [DOI] [PubMed] [Google Scholar]
- Falco-Walter JJ, et al. , 2018. The new definition and classification of seizures and epilepsy. Epilepsy Res 139, 73–79. [DOI] [PubMed] [Google Scholar]
- Fang Z, et al. , 2017. Advances in autoimmune epilepsy associated with antibodies, their potential pathogenic molecular mechanisms, and current recommended immunotherapies. Front. Immunol 8, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J, 2008. CNS injury, glial scars, and inflammation: inhibitory extra-cellular matrices and regeneration failure. Exp. Neurol 209, 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey LC, 2003. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia 44 (Suppl 10), 11–17. [DOI] [PubMed] [Google Scholar]
- Gaspard N, 2016. Autoimmune epilepsy. Continuum. (Minneap Minn) 22, 227–245. [DOI] [PubMed] [Google Scholar]
- Gentleman SM, et al. , 2004. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci. Int 146, 97–104. [DOI] [PubMed] [Google Scholar]
- Giza CC, Hovda DA, 2001. The neurometabolic cascade of concussion. J. Athl. Train 36, 228–235. [PMC free article] [PubMed] [Google Scholar]
- Grafman J, Salazar AM, 2015. The ebb and flow of traumatic brain injury research. Handb. Clin. Neurol 128, 795–802. [DOI] [PubMed] [Google Scholar]
- Greco A, et al. , 2016. Autoimmune epilepsy. Autoimmun. Rev 15, 221–225. [DOI] [PubMed] [Google Scholar]
- Gu XC, et al. , 2016. Neuropeptide Y accelerates post-fracture bone healing by promoting osteogenesis of mesenchymal stem cells. Neuropeptides 60, 61–66. [DOI] [PubMed] [Google Scholar]
- Gyorgy A, et al. , 2011. Time-dependent changes in serum biomarker levels after blast traumatic brain injury. J. Neurotrauma 28, 1121–1126. [DOI] [PubMed] [Google Scholar]
- Halford J, et al. , 2017. New astroglial injury-defined biomarkers for neurotrauma assessment. J. Cereb. Blood Flow Metab 37, 3278–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, et al. , 2004. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J. Neurotrauma 21, 9–20. [DOI] [PubMed] [Google Scholar]
- Hawryluk GW, Manley GT, 2015. Classification of traumatic brain injury: past, present, and future. Handb. Clin. Neurol 127, 15–21. [DOI] [PubMed] [Google Scholar]
- Hegde M, Lowenstein DH, 2014. The search for circulating epilepsy biomarkers. Biomark. Med 8, 413–427. [DOI] [PubMed] [Google Scholar]
- Heggeness MH, et al. , 2017. Quiescent pluripotent stem cells reside within murine peripheral nerves that can be stimulated to proliferate by recombinant human bone morphogenic protein 2 or by nerve trauma. Spine J 17, 252–259. [DOI] [PubMed] [Google Scholar]
- Hellman NE, Gitlin JD, 2002. Ceruloplasmin metabolism and function. Annu. Rev. Nutr 22, 439–458. [DOI] [PubMed] [Google Scholar]
- Hess DC, Borlongan CV, 2008. Stem cells and neurological diseases. Cell Prolif 41 (Suppl 1), 94–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks R, et al. , 1996. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol 91, 236–246. [DOI] [PubMed] [Google Scholar]
- Hillered L, et al. , 2006. Continuous monitoring of cerebral metabolism in traumatic brain injury: a focus on cerebral microdialysis. Curr. Opin. Crit. Care 12, 112–118. [DOI] [PubMed] [Google Scholar]
- Hinson HE, et al. , 2015. Clinical evidence of inflammation driving secondary brain injury: a systematic review. J. Trauma Acute Care Surg 78, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovda DA, et al. , 1995. The neurochemical and metabolic cascade following brain injury: moving from animal models to man. J. Neurotrauma 12, 903–906. [DOI] [PubMed] [Google Scholar]
- Hsieh CH, et al. , 2008. The role of MIP-1 alpha in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J. Immunol 181, 2806–2812. [DOI] [PubMed] [Google Scholar]
- Huang H, et al. , 2018. Relationship between heterotopic ossification and traumatic brain injury: why severe traumatic brain injury increases the risk of heterotopic ossification. J. Orthop. Translat 12, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhmer AF, et al. , 2006. Protein analysis in human cerebrospinal fluid: physiological aspects, current progress and future challenges. Dis. Markers 22, 3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YN, et al. , 2009. Calpain-mediated N-cadherin proteolytic processing in brain injury. J. Neurosci 29, 5974–5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H, et al. , 2011. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J. Mol. Neurosci 44, 130–139. [DOI] [PubMed] [Google Scholar]
- Johnson VE, et al. , 2013. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemaru K, et al. , 2013. Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc. Natl. Acad. Sci. U. S. A 110, 11612–11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkela J, et al. , 1993. CSF and serum brain-specific creatine kinase isoenzyme (CK-BB), neuron-specific enolase (NSE) and neural cell adhesion molecule (NCAM) as prognostic markers for hypoxic brain injury after cardiac arrest in man. J. Neurol. Sci 116, 100–109. [DOI] [PubMed] [Google Scholar]
- Katayama T, et al. , 2009. Neuronal injury induces cytokine-induced neutrophil chemoattractant-1 (CJNC-1) production in astrocytes. J. Pharmacol. Sci 109, 88–93. [DOI] [PubMed] [Google Scholar]
- Kelso ML, Gendelman HE, 2014. Bridge between neuroimmunity and traumatic brain injury. Curr. Pharm. Des 20, 4284–4298. [PMC free article] [PubMed] [Google Scholar]
- Kharatishvili I, Pitkanen A, 2010. Posttraumatic epilepsy. Curr. Opin. Neurol 23, 183–188. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, et al. , 2014. Pattern recognition receptors and central nervous system repair. Exp. Neurol 258, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Yenari MA, 2013. The immune modulating properties of the heat shock proteins after brain injury. Anat. Cell Bioi 46, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst A, Ross Bullock M, 2006. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J. Neurotrauma 23, 1185–1200. [DOI] [PubMed] [Google Scholar]
- Kleindienst A, et al. , 2007. The neurotrophic protein S100B: value as a marker of brain damage and possible therapeutic implications. Prog. Brain Res 161, 317–325. [DOI] [PubMed] [Google Scholar]
- Kobeissy FH, et al. , 2008. Neuroproteomics and systems biology-based discovery of protein biomarkers for traumatic brain injury and clinical validation. Proteomics Clio. Appl 2, 1467–1483. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, et al. , 2013. Screening of biochemical and molecular mechanisms of secondary injury and repair in the brain after experimental blast-induced traumatic brain injury in rats. J. Neurotrauma 30, 920–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi E, et al. , 2010. Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir 152, 1–17. [DOI] [PubMed] [Google Scholar]
- Kumar A, Loane DJ, 2012. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun 26, 1191–1201. [DOI] [PubMed] [Google Scholar]
- Kuruba R, Shetty AK, 2007. Could hippocampal neurogenesis he a future drug target for treating temporal lobe epilepsy? CNS Neurol. Disord. Drug Targets 6, 342–357. [DOI] [PubMed] [Google Scholar]
- Lai Y, et al. , 2006. Mitochondrial heat shock protein 60 is increased in cerebrospinal fluid following pediatric traumatic brain injury. Dev. Neurosci 28, 336–341. [DOI] [PubMed] [Google Scholar]
- Lee C, Agoston DV, 2005. Does vascular endothelial growth factor (VEGF) mediates traumatic brain injury-induced de novo neurogenesis in the adult rat brain? Soc. Neurosci 31. [Google Scholar]
- Lee C, Agoston DV, 2009. Inhibition of VEGF receptor 2 increased cell death of dentate hilar neurons after traumatic brain injury. Exp. Neurol 220, 400–403. [DOI] [PubMed] [Google Scholar]
- Li S, et al. , 2013. Temporal profiles of axonal injury following impact acceleration traumatic brain injury in rats-a comparative study with diffusion tensor imaging and morphological analysis. Int. J. Legal Med 127, 159–167. [DOI] [PubMed] [Google Scholar]
- Li J, et al. , 2015. Serum ubiquitin C-terminal hydrolase L1 as a biomarker for traumatic brain injury: a systematic review and meta-analysis. Am. J. Emerg. Med 33, 1191–1196. [DOI] [PubMed] [Google Scholar]
- Uesz A, et al. , 2015. DAMP signaling is a key pathway inducing immune modulation after brain injury. J. Neurosci 35, 583–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liliang PC, et al. , 2010. Tau proteins in serum predict outcome after severe traumatic brain injury. J. Surg. Res 160, 302–307. [DOI] [PubMed] [Google Scholar]
- Little AR, O’Callagha JP, 2001. Astrogliosis in the adult and developing CNS: is there a role for proinflammatory cytokines? Neurotoxicology 22, 607–618. [DOI] [PubMed] [Google Scholar]
- Liu MC, et al. , 2010. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur. J. Neurosci 31, 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente L, 2017. Biomarkers associated with the outcome of traumatic brain injury patients. Brain Sci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke-Wold BP, et al. , 2015. Traumatic brain injury and epilepsy: underlying mechanisms leading to seizure. Seizure 33, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GT, Maas AI, 2013. Traumatic brain injury: an international knowledge-based approach. JAMA 310, 473–474. [DOI] [PubMed] [Google Scholar]
- Manley GTMDPD, et al. , 2017. The traumatic brain injury endpoints development (ted) initiative: progress on a public-private regulatory collaboration to accelerate diagnosis and treatment of traumatic brain injury. J. Neurotrauma 34, 2721–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino Gammazza A, et al. , 2015. Hsp60 response in experimental and human temporal lobe epilepsy. Sci. Rep 5, 9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, et al. , 2005. Pyruvate dehydrogenase complex: metabolic link to ischemic brain injury and target of oxidative stress. J. Neurosci. Res 79, 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn MJ, Povlishock JT, 2016. Pathophysiology of traumatic brain injury. Neurosurg. Clin. N. Am 27, 397–407. [DOI] [PubMed] [Google Scholar]
- McManus CM, et al. , 1998. Cytokine induction of MIP-1 alpha and MIP-1 beta in human fetal microglia. J. Immunol 160, 1449–1455. [PubMed] [Google Scholar]
- Ming GL, Song H, 2005. Adult neurogenesis in the mammalian centraJ nervous system. Annu. Rev. Neurosci 28, 223–250. [DOI] [PubMed] [Google Scholar]
- Mondello S, et al. , 2012. Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery 70, 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello S, et al. , 2017. Blood-based protein biomarkers for the management of traumatic brain injuries in adults presenting with mild head injury to emergency departments: a living systematic review and meta-analysis. J. Neurotrauma 34, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R, et al. , 2007. Neovascularization following traumatic brain injury: possible evidence for both angiogenesis and vasculogenesis. Neurol Res 29, 375–381. [DOI] [PubMed] [Google Scholar]
- Muradashvili N, Lominadze D, 2013. Role of fibrinogen in cerebrovascular dysfunction after traumatic brain injury. Brain Inj 24, 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada T, Kwee IL, 2018. Fluid dynamics inside the brain barrier: current concept of interstitial flow, glymphatic flow, and cerebrospinal fluid circulation in the brain. Neuroscientist 6, 1–12 (1073858418775027). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger EJ, et al. , 2017. Enhanced dentate neurogenesis after brain injury undermines long-term neurogenic potential and promotes seizure susceptibility. Stem Cell Rep 9, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordby HK, Urdal P, 1982. The diagnostic value of measuring creatine kinase BB activity in cerebrospinal fluid following acute head injury. Acta Neurocbir 65, 93–101. [DOI] [PubMed] [Google Scholar]
- O’Connor CA, et al. , 2006. The temporal profile of edema formation differs between male and female rats following diffuse traumatic brain injury. Acta Neurochir. Suppl 96, 121–124. [DOI] [PubMed] [Google Scholar]
- Okada S, et al. , 2018. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res 126, 39–43. [DOI] [PubMed] [Google Scholar]
- Ommaya AK, et al. , 1996. Causation, incidence, and costs of traumatic brain injury in the U.S. military medical system. J. Trauma 40, 211–217. [DOI] [PubMed] [Google Scholar]
- Ott L, et al. , 1987. The metabolic response to brain injury. JPEN J. Parenter. Enteral Nutr 11, 488–493. [DOI] [PubMed] [Google Scholar]
- Palmer TD, et al. , 1997. The adult rat hippocampus contains primordial neural stem cells. Mol. Cell. Neurosci 8, 389–404. [DOI] [PubMed] [Google Scholar]
- Pandolfi F, et al. , 2016. Key role of DAMP in inflammation, cancer, and tissue repair. Clin. Ther 38, 1017–1028. [DOI] [PubMed] [Google Scholar]
- Papa L, et al. , 2014. GFAP out-performs S100beta in detecting traumatic intracranial lesions on computed tomography in trauma patients with mild traumatic brain injury and those with extracranial lesions. J. Neurotrauma 31, 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, 2003. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist 9, 261–272. [DOI] [PubMed] [Google Scholar]
- Park K, Biederer T, 2013. Neuronal adhesion and synapse organization in recovery after brain injury. Future Neurol 8, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker TM, et al. , 2017. The danger zone: Systematic review of the role of HMGB1 danger signalling in traumatic brain injury. Brain Inj 31, 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco A, et al. , 2006. Dynamics of cerebral edema and the apparent diffusion coefficient of water changes in patients with severe traumatic brain injury. A prospective MRI study. Eur. Radiol 16, 1501–1508. [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M, 2014. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol. Rev 94, 1077–1098. [DOI] [PubMed] [Google Scholar]
- Picard-Riera N, et al. , 2004. Endogenous adult neural stem cells: limits and potential to repair the injured central nervous system. J. Neurosci. Res 76, 223–231. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Immonen R, 2014. Epilepsy related to traumatic brain injury. Neurotherapeutics 11, 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, et al. , 2009. From traumatic brain injury to posttraumatic epilepsy: what animal models tell us about the process and treatment options. Epilepsia 50 (Suppl 2), 21–29. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, et al. , 2014. Posttraumatic epilepsy disease or comorbidity? Epilepsy Behav 38, 19–24. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, et al. , 2016. Advances in the development of biomarkers for epilepsy. Lancet Neurol 15, 843–856. [DOI] [PubMed] [Google Scholar]
- Pleines UE, et al. , 1998. Soluble ICAM-1 in CSF coincides with the extent of cerebral damage in patients with severe traumatic brain injury. J. Neurotrauma 15, 399–409. [DOI] [PubMed] [Google Scholar]
- Potts MB, et al. , 2006. Traumatic injury to the immature brain: inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. NeuroRx 3, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA, et al. , 2012. Traumatic brain injury and posttraumatic epilepsy In: Noebels JL (Ed.), Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information (US). [PubMed] [Google Scholar]
- Pullen NA, et al. , 2012. Matrix metalloproteinase-1 expression enhances tumorigenicity as well as tumor-related angiogenesis and is inversely associated with TIMP-4 expression in a model of glioblastoma J. Neuro-Oncol. 106, 461–471. [DOI] [PubMed] [Google Scholar]
- Raymont V, et al. , 2010. Correlates of posttraumatic epilepsy 35 years following combat brain injury. Neurology 75, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RM, et al. , 2007. Neurogenesis after trauroatic brain injury. Neurosurg. Clin. N. Am 18, 169–181 (xi). [DOI] [PubMed] [Google Scholar]
- Ritter AC, et al. , 2016. Incidence and risk factors of posttraumatic seizures following traumatic brain injury: a traumatic brain injury model systems study. Epilepsia 57, 1968–1977. [DOI] [PubMed] [Google Scholar]
- Rorive S, et al. , 2010. TlMP-4 and CD63: new prognostic biomarkers in human astrocytomas. Mod. Pathol 23, 1418–1428. [DOI] [PubMed] [Google Scholar]
- Rostami E, et al. , 2011. BDNF polymorphism predicts general intelligence after penetrating trauroatic brain injury. PLoS ONE 6, e27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami E, et al. , 2015. Time-dependent changes in serum level of protein biomarkers after focal traumatic brain injury. Int. J. Neurorehabil 2, 2–6. [Google Scholar]
- Rubenstein R, et al. , 2017. Comparing plasma phospho tau, total tau, and phospho tau-total tau ratio as acute and chronic traumatic brain injury biomarkers. JAMA Neurol 74, 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, et al. , 2009. Fibrinogen signal transduction in the nervous system. J. Thromb. Haemost 7 (Suppl 1), 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE, et al. , 2008. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 25, 719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar AM, Grafman J, 2015. Post-traumatic epilepsy: clinical clues to pathogenesis and paths to prevention. Handb. Clin. Neurol 128, 525–538. [DOI] [PubMed] [Google Scholar]
- Salazar AM, et al. , 1995. Penetrating injuries in the Vietnam war. Traumatic unconsciousness, epilepsy, and psychosocial outcome. Neurosurg. Clio. N. Am 6, 715–726. [PubMed] [Google Scholar]
- Scharfman HE, 2000. Epileptogenesis in the parahippocampal region. Parallels with the dentate gyrus. Ann. N. Y. Acad. Sci 911, 305–327. [DOI] [PubMed] [Google Scholar]
- Schuhmann MU, et al. , 2003. Temporal profiles of cerebrospinal fluid leukotrienes, brain edema and inflammatory response following experimental brain injury. Neurol. Res 25, 481–491. [DOI] [PubMed] [Google Scholar]
- Semple BD, et al. , 2010. Role ofCCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/−mice. J. Cereb. Blood Flow Metab 30, 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, 2012. Osteopontin as a two-sided mediator in acute neuroinflammation in rat models. Acta Histochem 114, 749–754. [DOI] [PubMed] [Google Scholar]
- Signore M, et al. , 2017. Antibody validation by western blotting; molecular profiling. Methods Mol. Bioi 1606, 51–70. [DOI] [PubMed] [Google Scholar]
- Simon MJ, Iliff JJ, 2016. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta 1862, 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DW, et al. , 2017. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol 13, 171–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, 2014. Astrogliosis. Cold Spring Harb. Perspect. Biol 7, a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solier C, Langen H, 2014. Antibody-based proteomics and biomarker research - current status and limitations. Proteomics 14, 774–783. [DOI] [PubMed] [Google Scholar]
- Song HJ, et al. , 2002. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat. Neurosci 5, 438–445. [DOI] [PubMed] [Google Scholar]
- Sorci G, et al. , 2000. Effects of S100A1 and S100B on microtubule stability. An in vitro study using triton-cytoskeletons from astrocyte and myoblast cell lines. Neuroscience 99, 773–783. [DOI] [PubMed] [Google Scholar]
- Stetler RA, et al. , 2010. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Prog. Neurobiol 92, 184–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathmann FG, et al. , 2014. Blood-based biomarkers for traumatic brain injury: evaluation of research approaches, available methods and potential utility from the clinician and clinical laboratory perspectives. Clin. Biochem 47, 876–888. [DOI] [PubMed] [Google Scholar]
- Sun D, 2014. The potential of endogenous neurogenesis for brain repair and regeneration following traumatic brain injury. Neural Regen. Res 9, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, et al. , 2014. Rapid manifestation of reactive astrogliosis in acute hippocampal brain slices. Glia 62, 78–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase H, et al. , 2018. Oligodendrogenesis after traumatic brain injury. Behav. Brain Res 340, 205–211. [DOI] [PubMed] [Google Scholar]
- Temkin NR, 2003. Risk factors for posttraumatic seizures in adults. Epilepsia 44 (Suppl 10), 18–20. [DOI] [PubMed] [Google Scholar]
- Thau-Zuchman O, et al. , 2012. Subacute treatment with vascular endothelial growth factor after traumatic brain injury increases angiogenesis and gliogenesis. Neuroscience 202, 334–341. [DOI] [PubMed] [Google Scholar]
- Thelin EP, et al. , 2013. S100B is an important outcome predictor in traumatic brain injury. J. Neurotrauma 30, 519–528. [DOI] [PubMed] [Google Scholar]
- Thelin EP, et al. , 2014. Secondary peaks of S100B in serum relate to subsequent radiological pathology in trauroatic brain injury. Neurocrit. Care 20, 217–229. [DOI] [PubMed] [Google Scholar]
- Thelin EP, et al. , 2017. Serial sampling of serum protein biomarkers for monitoring human traumatic brain injury dynamics: a systematic review. Front. Neurol 8, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DG, et al. , 1978. Serum-myelin-basic-protein assay in diagnosis and prognosis of patients with head injury. Lancet 1, 113–115. [DOI] [PubMed] [Google Scholar]
- Thrane AS, et al. , 2011. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc. Natl. Acad. Sci. U. S. A 108, 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubi MA, et al. , 2018. Early seizures and temporal lobe trauma predict post-traumatic epilepsy: a longitudinal study. Neurobiol. Dis (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden J, et al. , 2005. Raised serum S100B levels after acute bone fractures without cerebral injury. J. Trauma 58, 59–6l. [DOI] [PubMed] [Google Scholar]
- Versace A, et al. , 2013. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Mol. Psychiatry 19, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P, et al. , 2005. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 25, 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Friedman A, 2011. Brain inflammation as a biomarker in epilepsy. Biomark. Med 5, 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer J, 2006. TLR9 in health and disease. Int. Rev. Immunol 25, 155–181. [DOI] [PubMed] [Google Scholar]
- Wang KK, et al. , 2005. Proteomic identification of biomarkers of traumatic brain injury. Expert. Rev. Proteomics 2, 603–614. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. , 2016a. Serum tau protein as a potential biomarker in the assessment of traumatic brain injury. Exp. Ther. Med 11, 1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK, et al. , 2016b. Plasma anti-glial fibrillary acidic protein autoantibody levels during the acute and chronic phases of traumatic brain injury: a transforming research and clinical knowledge in traumatic brain injury pilot study. J. Neurotrauma 33, 1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK, et al. , 2018. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert. Rev. Mol. Diagn 18, 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KM, et al. , 2017. Inflammation in epileptogenesis after traumatic brain injury. J. Neuroinflammation 14, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JK, Stevens RD, 2015. Traumatic brain injury: recent advances in plasticity and regeneration. Curr. Opin. Neurol 28, 565–573. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, et al. , 1998. Soluble adhesion molecules in CSF are increased in children with severe head injury. J. Neurotrauma 15, 777–787. [DOI] [PubMed] [Google Scholar]
- Wilson L, et al. , 2017. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol 16, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CD, et al. , 2018. Early and late posttraumatic epilepsy in the setting of traumatic brain injury: a meta-analysis and review of antiepileptic management. World Neurosurg 110, e901–e906. [DOI] [PubMed] [Google Scholar]
- Woodcock T, Morganti-Kossmann MC, 2013. The role of markers of inflammation in traumatic brain injury. Front. Neurol 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DK, et al. , 2016. Behavioral, blood, and magnetic resonance imaging biomarkers of experimental mild traumatic brain injury. Sci. Rep 6, 28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZQ, et al. , 2015. Fibronectin is a potential cerebrospinal fluid and serum epilepsy biomarker. Epilepsy Behav 48, 66–69. [DOI] [PubMed] [Google Scholar]
- Xu T, et al. , 2017. Risk factors for posttraumatic epilepsy: a systematic review and meta-analysis. Epilepsy Behav 67, 1–6. [DOI] [PubMed] [Google Scholar]
- Yadav A, et al. , 2010. MCP-1: chemoattractant with a role beyond immunity: a review. Clin. Chim. Acta 411, 1570–1579. [DOI] [PubMed] [Google Scholar]
- Yao Z, et al. , 2004. Increase of both angiogenesis and bone mass in response to exercise depends on VEGF. J. Bone Miner. Res 19, 1471–1480. [DOI] [PubMed] [Google Scholar]
- Yokota H, et al. , 2007. Cerebral endothelial injury in elderly patients with severe head injury measured by serum thrombomodulin and von Willebrand factor. Neural. Med. Chir (Tokyo) 47, 383–388 discussion 388. [DOI] [PubMed] [Google Scholar]
- Yu TS, et al. , 2014a. Injury-induced neurogenesis: mechanisms and relevance. Neuroscientist 22, 61–71. [DOI] [PubMed] [Google Scholar]
- Yu W, et al. , 2014b. Altered cerebrospinal fluid concentrations of TGFbeta1 in patients with drug-resistant epilepsy. Neurochem. Res 39, 2211–2217. [DOI] [PubMed] [Google Scholar]
- Zemian FP, et al. , 2002. C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res 947, 131–139. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, 2017. Review: Tau in biofluids - relation to pathology, imaging and clinical features. Neuropathol. Appl. Neurobiol 43, 194–199. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Blennow K, 2015. Fluid markers of traumatic brain injury. Mol. Cell. Neurosci 66, 99–102. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Blennow K, 2016. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat. Rev. Neurol 12, 563–574. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, et al. , 2013. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev. Neurol 9, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]