Abstract
Over the past two decades, a low frequency variant (rs1800012) within the first intron of the type I collagen alpha 1 (COLIA1) gene has been implicated in lower areal BMD (aBMD) and increased risk of osteoporotic fracture. This association is particularly strong in postmenopausal women, in whom net bone loss arises in the context of high bone turnover. High bone turnover also accompanies childhood linear growth; however, the role of rs1800012 in this stage of net bone accretion is less well understood. Thus, we assessed the association between rs1800012 and aBMD and bone mineral content (BMC) Z-scores in the Bone Mineral Density in Childhood Study, a mixed-longitudinal cohort of children and adolescents (total n=804 girls and 771 boys; n=19 girls and 22 boys with the TT genotype). Mixed effects modeling, stratified by sex, was used to test for associations between rs1800012 and bone Z-scores and for pubertal stage interactions. Separately, SITAR growth modeling of aBMD and BMC reduced the complex longitudinal bone accrual curves into three parameters representing a-size, b-timing, and c-velocity. We tested for differences in these three parameters by rs1800012 genotype using t-tests. Homozygous TT girls had significantly lower bone Z-scores prior to puberty completion (e.g. spine aBMD-Z P-interaction=1.0×10−6), but this association was attenuated post-puberty. SITAR models revealed that homozygous TT girls began pubertal bone accrual later (b-timing; e.g. total hip BMC, P=0.03). BMC and aBMD Z-scores also increased across puberty in TT homozygous boys. Our data, along with previous findings in post-menopausal women, suggest that rs1800012 principally affects female bone density during periods of high turnover. Insights into the genetics of bone gain and loss may be masked during the relatively quiescent state in mid-adulthood, and discovery efforts should focus on early and late life.
Keywords: bone mineral density, alpha1 type 1 collagen, longitudinal study, bone development, puberty
Introduction
Twenty years ago, a variant (rs1800012) within an Sp1 binding site embedded in the first intron of the type I collagen alpha 1 (COLIA1) gene was implicated in accelerated bone loss and increased rate of fracture in post-menopausal women(1). In contrast to its marked effect after menopause, this variant has only a modest impact on peak BMD in pre-menopausal women (with TT homozygous minor allele carriers having an average of 25 mg/cm2 lower BMD at the femoral neck)(2,3), suggesting a stronger effect during high bone turnover periods.
Similar to post-menopause, childhood and adolescence are also periods of high bone turnover, resulting in net bone gain. Bone mass acquisition during this critical developmental window has consequences for lifelong skeletal health(4). While the implications of rs1800012 in later life are well-established(1,5–7), the effect of this variant during bone development has not been clearly delineated. Three studies have tested the association between this variant and BMD among children and adolescents, but these studies were hampered by small sample sizes, particularly of carriers of the minor homozygous genotype, and did not account for changes across puberty(8–10). Furthermore, these studies arrived at conflicting conclusions, with two reporting an association of this variant with lower childhood BMD(8,10) and one finding no evidence of association(9).
Therefore, to address the limitations of previous studies and clarify the role of rs1800012 during growth, we assessed the association of this variant with areal-BMD (aBMD) and BMC and tested for puberty interactions in the Bone Mineral Density in Childhood Study (BMDCS), a mixed-longitudinal cohort of 1,575 healthy children and adolescents with annual DXA and puberty measurements.
Materials and Methods
Study subjects
The BMDCS was a prospective, longitudinal study of healthy children and adolescents aimed at establishing norms for BMC and aBMD for children 5 to 20 years of age in the United States. Subjects were recruited in 2002–2003 at 5 US study centers (Los Angeles, CA; Cincinnati, OH; Omaha, NE; Philadelphia, PA; and New York, NY). Children were recruited in two phases, including a longitudinal and cross-sectional cohort. In the longitudinal cohort, girls (aged 6–15 yr) and boys (aged 6–16 yr) were followed annually for up to 6 yr, with a maximum of 7 study visits per individual. In 2006–2007, individuals aged 5 and 19 yr were also enrolled, and were subsequently followed for 2 years annually. In the cross-sectional cohort, intended as a replication dataset, children of European descent aged 5–18 yr were enrolled at two of the study centers (Cincinnati, OH and Omaha, NE). The enrollment criteria were term birth (≥37 weeks gestation), birth weight > 2.3 kg, no evidence of precocious or delayed puberty, and height and weight or BMI in the 3rd to 97th percentiles for age and sex. Subjects were excluded if they had been exposed to medications or medical conditions known to affect bone accrual, had been on extended bed rest, or had a history of multiple fractures. At the final visit, a saliva or blood sample was taken for DNA extraction. In this study of rs1800012, only participants of non-African American ancestry were included (n=804 girls and 771 boys), as the homozygous recessive genotype for rs1800012 was not present among African Americans.
Phenotypes
aBMD was measured by DXA bone densitometers (Hologic, Bedford, MA; QDR4500A, QDR4500W, Delphi A, and Discovery models). Scans were performed at the lumbar spine, proximal femur, forearm, and total body by trained research technicians using standardized protocols that followed the manufacturer’s guidelines. The scans were centrally analyzed by the DXA Core Laboratory (University of California, San Francisco, CA) with Hologic software v.12.3 for baseline scans and Apex 2.1 for follow-up scans, as previously reported(11). Scan results were adjusted for clinical center differences and longitudinal drift. Age, sex and ancestry (African American vs. non-African American) specific Z-scores for the spine, total hip, femoral neck, and distal 1/3 radius, and BMC of the total body less head (TBLH) were calculated and adjusted for height-for-age Z-scores(12).
Height was measured using a stadiometer, and height Z-scores were calculated using CDC 2000 growth charts(12). Pubertal stage was assessed by trained physicians or nurses, and study subjects were designated as pre-pubertal (Tanner stage I), pubertal (Tanner II-IV), or post-pubertal (Tanner V) based on breast development in girls and testicular volume in boys(13,14). Dietary calcium was assessed with a semi-quantitative food frequency questionnaire (Block Dietary Data Systems, Berkeley, CA). Physical activity was estimated using a modified version of the Slemenda questionnaire that queried over 40 different weight and non-weight bearing physical activities. The time spent in each activity was summed and expressed as hours of physical activity per week.
Genotyping
High-throughput genome-wide genotyping was performed using the Illumina Infinium II OMNI Express plus Exome BeadChip (Illumina, San Diego, CA) at the Children’s Hospital of Philadelphia, Center for Applied Genomics (Philadelphia, PA). Quality control included filtering out SNPs with call rate <95%, minor allele frequency <1%, missing genotype rate >2%, and Hardy-Weinberg P <1×10−5, as previously described(15).
Mixed-Effects Modeling
We tested the effect of homozygous (TT) minor allele groups for rs1800012 against the common homozygotes (GG) and the heterozygous (TG) allele carriers on height-for-age adjusted aBMD and BMC at the hip neck, total hip, lumbar spine, distal 1/3 radius, and total body less head (TBLH) using linear mixed effects models, with random intercepts, accounting for multiple measurements per individual. Given the established association between skeletal maturation and linear growth, we also tested the effect of genotype on height Z-score. We assessed males and females separately. The models included age, cohort (“discovery” or “replication”), recruitment site, BMI Z-score, dietary calcium, and physical activity level as covariates. STATA v 14 or 15 (StataCorp, College Station, TX) were used to perform all analyses. We further tested for pubertal stage interactions between the three pubertal stage groups (Group I, pre-pubertal (Tanner stage 1); Group II, pubertal (Tanner stages 2–4); and Group III, post-pubertal (Tanner stage 5)) and genotype in an additive model.
SITAR modeling
To further assess the impact of carrying the rare homozygous genotype on longitudinal bone accrual, growth modeling was performed with SITAR (Shape Invariant Translation And Rotation), as previously described(16,17), using the R package ‘sitar’. Here, we extracted the bone accrual curves for the TT homozygous minor allele carriers vs. TG and GG carriers for aBMD and BMC at the hip neck, total hip, lumbar spine, distal 1/3 radius, and TBLH, as well as height gain. We tested the differences between carrier groups (GG vs. TT and GT vs. TT) for a-size, b-tempo, and c-velocity with unpaired t-tests in R.
Results
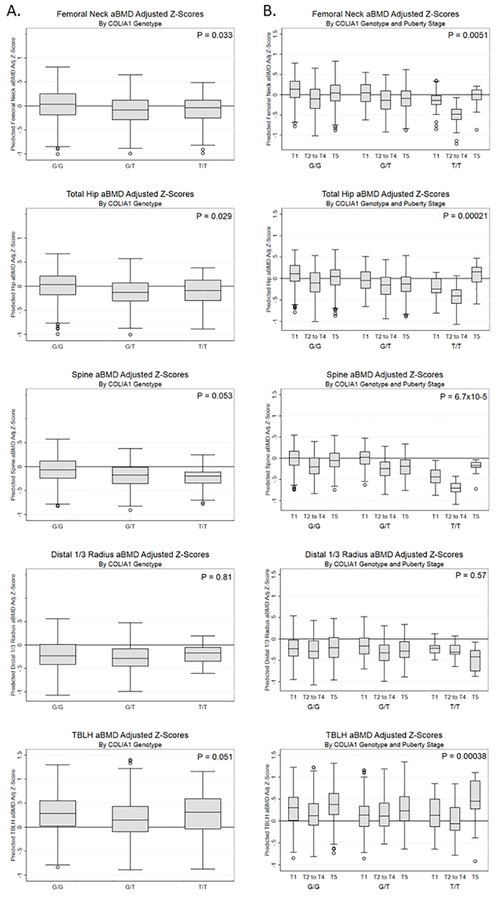
In our cohort, there were 19 girls with up to 63 observations per skeletal site and 22 boys with up to 92 observations per skeletal site who carried the TT genotype (Supplementary Table 1). Overall, aBMD was slightly lower in female T carriers at the femoral neck (per copy of T, beta (SE)=−0.126 (0.059), P=0.033) and total hip (per T, beta (SE)=−0.134 (0.062), P=0.029) (Fig. 1A, Supplementary Fig. 1). However, we found significant (P-interaction < 0.05) variant-pubertal status interactions at all skeletal sites (except for distal 1/3 radius) for girls (Fig. 1B). Specifically, TT homozygotes had significantly lower predicted aBMD and BMC Z-scores prior to puberty completion at many skeletal sites (e.g. spine aBMD-Z, Tanner group 3 vs 2, −0.44 [95% CI: −0.69, −0.20], P<0.001). While male carriers of the TT genotype did not have lower aBMD or BMC in the main interactions (Supplementary Fig. 2), we observed similar variant-Tanner interactions in boys (all P-interaction<0.05; Supplementary Fig. 3).
Figure 1.
Sp1 variant in COLIA1 affects bone density prior to and during puberty in females. aBMD and BMC age, sex, and population ancestry-specific Z-scores were calculated and further adjusted for height-for-age Z-scores. A. Main effects by genotype for lumbar spine and femoral neck aBMD Z-scores. B. Puberty interactions by genotype for lumbar spine and femoral neck aBMD Z-scores. T1, Tanner stage 1 (pre-pubertal); T2-T4, Tanner stages 2 to 4 (pubertal); T5, Tanner stage 5 (post-pubertal). P-values in column A are for the main variant associations; P-values in column B are for the variant-puberty stage interactions. TBLH, total body less head.
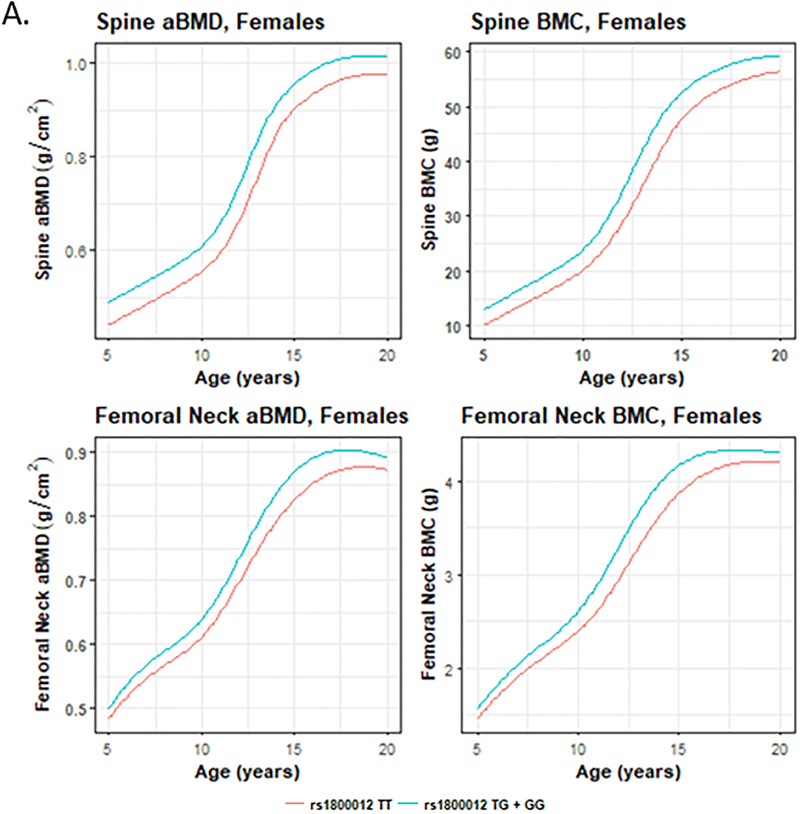
SITAR longitudinal modeling of aBMD and BMC gain revealed that girls who were TT genotype carriers had lower aBMD and BMC prior to puberty (femoral neck and spine, shown in Fig. 2; all skeletal sites shown in Supplementary Fig. 4), began pubertal bone accrual (b-timing; e.g. total hip BMC P=0.03) and their growth spurt (height P=0.007) later (Supplementary Fig. 5). These girls on average demonstrated partial “catch up” in BMC and aBMD at most skeletal sites post-puberty. In boys, the aBMD and BMC SITAR curves of TT carriers were similar to individuals with the TG or GG genotypes (Supplementary Fig. 4). However, boys had reduced height, even post-puberty (Supplementary Fig. 5). At the same time, the stature of TT girls caught up to GG and GT carriers (Supplementary Fig. 5).
Figure 2.
SITAR curves for lumbar spine and femoral neck aBMD and BMC for homozygotes for the minor T effect allele (red) versus TG and GG genotype carriers (blue).
Discussion
Genetic studies to date have implicated dozens of variants impacting BMD at various times throughout the lifespan(18,19). Among the first genetic variants to be described was a polymorphism upstream of the COLIA1 gene that affects a binding site for the transcription factor Sp1(1,5). While the implications of this variant are clear in later life, its impact during bone development has not been clearly delineated. The current study helps fill this gap in knowledge regarding the role of this COLIA1 variant in childhood by utilizing a larger sample size than previous studies, adopting a longitudinal design, and testing for sex differences and puberty effects. We observed that individuals homozygous for the minor genotype (i.e., TT carriers) had delayed bone accrual during pubertal development. In contrast to previous pediatric studies(8–10), we analyzed boys and girls separately, and found that TT girls had lower aBMD before puberty and began pubertal bone accrual later. These girls attained some degree of “catch up” in BMC and aBMD at most skeletal sites post-puberty, consistent with the modest association of this genotype with pre-menopausal BMD(2,3). While previous reports on the effect of this variant focused on the femoral neck and lumbar spine, we observed similar reductions across puberty at the other skeletal sites assessed.
In boys, while the same pattern of increasing aBMD and BMC was evident across puberty only in the minor allele homozygotes, we observed that predicted post-pubertal height-adjusted values were actually quite high. This may be partly explained by the reduced height seen in TT boys(10), even post-puberty (Supplementary Fig. 5). Since our aBMD and BMC phenotypes were adjusted for height-for-age Z-scores, shorter height may result in inflated bone strength surrogates (i.e., Z-scores). Although our study contains the largest number of TT homozygotes in any pediatric study to date by an order of magnitude, these numbers are still small (n=19 girls and 22 boys) and could impact the results.
Given the observed effects of this variant on both female pubertal bone acquisition and postmenopausal bone loss, effects that are partly masked during the relative quiescence in pre-menopausal adulthood, our findings suggest that high bone turnover states may be important windows into bone biology that provide valuable insight beyond peak bone density. Investigating the genetic determinants of bone health during high bone turnover states may reveal biological factors that impact the balance between osteoblasts and osteoclasts and represent candidate targets for osteoporosis prevention and therapy(20).
Conclusions
The genetic regulation of bone gains and losses may be masked during the relative quiescence of adulthood. Our findings strongly support investigating the interaction between genetics, maturational phase, and the hormonal milieu during periods of high bone turnover, particularly in childhood and adolescence, to fully understand the pathogenesis of osteoporosis.
Supplementary Material
Acknowledgements
This work was funded by R01 HD58886. D.L.C. is supported by American Diabetes Association grant #1–17-PDF-077. J.M. is supported by NIH/NHLBI grant K01HL123612. S.F.A.G. is supported by the Daniel B. Burke Endowed Chair for Diabetes Research
References
- 1.Uitterlinden AG, Burger H, Huang Q, Yue F, McGuigan FEA, Grant SFA, Hofman A, JPTM van Leeuwen, Pols HAP, Ralston SH. Relation of Alleles of the Collagen Type Iα1 Gene to Bone Density and the Risk of Osteoporotic Fractures in Postmenopausal Women. New England Journal of Medicine. 1998. April 9;338(15):1016–21. [DOI] [PubMed] [Google Scholar]
- 2.Mann V, Ralston S. Meta-analysis of COL1A1 Sp1 polymorphism in relation to bone mineral density and osteoporotic fracture. Bone. 2003. June;32(6):711–7. [DOI] [PubMed] [Google Scholar]
- 3.Ralston SH, Uitterlinden AG, Brandi ML, Balcells S, Langdahl BL, Lips P, Lorenc R, Obermayer-Pietsch B, Scollen S, Bustamante M, Husted LB, Carey AH, Diez-Perez A, Dunning AM, Falchetti A, Karczmarewicz E, Kruk M, van Leeuwen JPTM, van Meurs JBJ, Mangion J, McGuigan FEA, Mellibovsky L, del Monte F, Pols HAP, Reeve J, Reid DM, Renner W, Rivadeneira F, van Schoor NM, Sherlock RE, Ioannidis JPA, for the GENOMOS investigators. Large-Scale Evidence for the Effect of the COLIA1 Sp1 Polymorphism on Osteoporosis Outcomes: The GENOMOS Study. Lewis C, editor. PLoS Medicine. 2006. February 21;3(4):e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Hangartner TN, Huang X, Frederick MM, Winer KK, Zemel BS. Tracking of Bone Mass and Density during Childhood and Adolescence. The Journal of Clinical Endocrinology & Metabolism. 2010. April;95(4):1690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant SF, Reid DM, Blake G, Herd R, Fogelman I, Ralston SH. Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I alpha 1 gene. Nat. Genet 1996. October;14(2):203–5. [DOI] [PubMed] [Google Scholar]
- 6.Langdahl BL, Ralston SH, Grant SFA, Eriksen EF. An Sp1 Binding Site Polymorphism in the COLIA1 Gene Predicts Osteoporotic Fractures in Both Men and Women. Journal of Bone and Mineral Research. 1998. September 1;13(9):1384–9. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald HM, McGuigan FA, New SA, Campbell MK, Golden MHN, Ralston SH, Reid DM. COL1A1 Sp1 Polymorphism Predicts Perimenopausal and Early Postmenopausal Spinal Bone Loss. Journal of Bone and Mineral Research. 2001. September 1;16(9):1634–41. [DOI] [PubMed] [Google Scholar]
- 8.Sainz J, Van Tornout JM, Sayre J, Kaufman F, Gilsanz V. Association of Collagen Type 1 α1 Gene Polymorphism with Bone Density in Early Childhood 1. The Journal of Clinical Endocrinology & Metabolism. 1999. March;84(3):853–5. [DOI] [PubMed] [Google Scholar]
- 9.Berg JP, Lehmann EH, Stakkestad JA, Haug E, Halse J. The Sp1 binding site polymorphism in the collagen type I alpha 1 (COLIA1) gene is not associated with bone mineral density in healthy children, adolescents, and young adults. Eur. J. Endocrinol 2000. August;143(2):261–5. [DOI] [PubMed] [Google Scholar]
- 10.van der Sluis IM, Muinck Keizer-Schrama SMPF, Pols HAP, Lequin MH, Krenning EP, Uitterlinden AG. Collagen Ia1 Polymorphism is Associated with Bone Characteristics in Caucasian Children and Young Adults. Calcified Tissue International. 2002. November 1;71(5):393–9. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell JA, Chesi A, Elci O, McCormack SE, Kalkwarf HJ, Lappe JM, Gilsanz V, Oberfield SE, Shepherd JA, Kelly A, Zemel BS, Grant SF. Genetics of Bone Mass in Childhood and Adolescence: Effects of Sex and Maturation Interactions. Journal of Bone and Mineral Research. 2015. September;30(9):1676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ. Height Adjustment in Assessing Dual Energy X-Ray Absorptiometry Measurements of Bone Mass and Density in Children. The Journal of Clinical Endocrinology & Metabolism. 2010. March;95(3):1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child 1970. February;45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch. Dis. Child 1969. June;44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakonarson H, Grant SFA, Bradfield JP, Marchand L, Kim CE, Glessner JT, Grabs R, Casalunovo T, Taback SP, Frackelton EC, Lawson ML, Robinson LJ, Skraban R, Lu Y, Chiavacci RM, Stanley CA, Kirsch SE, Rappaport EF, Orange JS, Monos DS, Devoto M, Qu H-Q, Polychronakos C. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007. August;448(7153):591–4. [DOI] [PubMed] [Google Scholar]
- 16.Cole TJ, Donaldson MDC, Ben-Shlomo Y. SITAR—a useful instrument for growth curve analysis. International Journal of Epidemiology. 2010. December;39(6):1558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormack SE, Cousminer DL, Chesi A, Mitchell JA, Roy SM, Kalkwarf HJ, Lappe JM, Gilsanz V, Oberfield SE, Shepherd JA, Winer KK, Kelly A, Grant SFA, Zemel BS. Association Between Linear Growth and Bone Accrual in a Diverse Cohort of Children and Adolescents. JAMA Pediatrics. 2017. September 5;171(9):e171769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemp JP, Medina-Gomez C, Tobias JH, Rivadeneira F, Evans DM. The case for genome-wide association studies of bone acquisition in paediatric and adolescent populations. BoneKEy Reports [Internet]. 2016 May 25 [cited 2018 Jul 9];5. Available from: http://www.portico.org/Portico/article?article=pgk3b8tmgjk [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina-Gomez C, Kemp JP, Trajanoska K, Luan J, Chesi A, Ahluwalia TS, Mook-Kanamori DO, Ham A, Hartwig FP, Evans DS, Joro R, Nedeljkovic I, Zheng H-F, Zhu K, Atalay M, Liu C-T, Nethander M, Broer L, Porleifsson G, Mullin BH, Handelman SK, Nalls MA, Jessen LE, Heppe DHM, Richards JB, Wang C, Chawes B, Schraut KE, Amin N, Wareham N, Karasik D, Van der Velde N, Ikram MA, Zemel BS, Zhou Y, Carlsson CJ, Liu Y, McGuigan FE, Boer CG, Bønnelykke K, Ralston SH, Robbins JA, Walsh JP, Zillikens MC, Langenberg C, Li-Gao R, Williams FMK, Harris TB, Akesson K, Jackson RD, Sigurdsson G, den Heijer M, van der Eerden BCJ, van de Peppel J, Spector TD, Pennell C, Horta BL, Felix JF, Zhao JH, Wilson SG, de Mutsert R, Bisgaard H, Styrkársdóttir U, Jaddoe VW, Orwoll E, Lakka TA, Scott R, Grant SFA, Lorentzon M, van Duijn CM, Wilson JF, Stefansson K, Psaty BM, Kiel DP, Ohlsson C, Ntzani E, van Wijnen AJ, Forgetta V, Ghanbari M, Logan JG, Williams GR, Bassett JHD, Croucher PI, Evangelou E, Uitterlinden AG, Ackert-Bicknell CL, Tobias JH, Evans DM, Rivadeneira F. Life-Course Genome-wide Association Study Meta-analysis of Total Body BMD and Assessment of Age-Specific Effects. The American Journal of Human Genetics. 2018. January;102(1):88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown JP, Albert C, Nassar BA, Adachi JD, Cole D, Davison KS, Dooley KC, Don-Wauchope A, Douville P, Hanley DA, Jamal SA, Josse R, Kaiser S, Krahn J, Krause R, Kremer R, Lepage R, Letendre E, Morin S, Ooi DS, Papaioaonnou A, Ste-Marie L-G. Bone turnover markers in the management of postmenopausal osteoporosis. Clinical Biochemistry. 2009. July;42(10–11):929–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.