Abstract
The ε4 allele of APOE is a well-established genetic risk factor for cognitive aging and dementia, although its influence on early life cognition is unknown. Consequently, we assessed associations of APOE genotypes with cognitive performance during 7, 12 and 16 year-assessments in our ongoing Colorado Adoption/Twin Study of Lifespan behavioral development (CATSLife). In general, APOE ε4 was associated with lower Verbal, Performance and Full Scale IQ scores during childhood and adolescence (e.g., Full Scale IQ was lower by 1.91 points per ε4 allele, d = −.13), with larger effects in females (e.g., average Full Scale IQ scores were 3.41 points lower in females per each ε4 allele, versus .33 points lower in males). Thus, these results suggest that deleterious effects of the APOE ε4 allele are manifested prior to adulthood, especially in females, and support both early origin theories and differential life-course vulnerabilities for later cognitive impairment.
Keywords: early origins, APOE, cognitive development
1. Introduction
Although the origins of late-life cognitive health may begin at conception (Barnett et al., 2013), the extent to which cognitive dysfunction in adulthood is presaged during early life is currently unknown. Individual differences in general cognitive ability occur early in development and are stable longitudinally (Plomin et al., 1988; Walhovd et al., 2016); nevertheless, the effects of early life factors may accumulate over the lifespan and impact subsequent cognitive aging (Liu et al., 2010). In order to assess the saliency of some early life genetic factors, we evaluated the associations of APOE genotypes with cognitive development from childhood to adolescence in the Colorado Adoption/Twin Study of Lifespan Behavioral Development (CATSLife).
The APOE gene encodes the brain’s primary cholesterol transporter, apolipoprotein E, which may play additional roles in synaptic plasticity and cell signaling (Holtzman et al., 2012). There are three common APOE alleles, ε2, ε3 and ε4 that vary in allele frequency with mean values across populations at about 6.4% (SD = 5.1), 78.3% (SD = 12.1), and 14.5% (SD = 8.5) (Eisenberg et al., 2010). The APOE ε4 variant is implicated in Aβ deposition and tau-pathologies (Shi et al., 2017), respective features in brain plaques and tangles. The APOE ε4 allele is an established risk factor for late-onset Alzheimer’s Disease (AD) (Liu et al., 2013), with a dose-dependent association between the number of ε4 alleles and AD risk and age of onset (Liu et al., 2013). APOE ε4 is also implicated in non-pathological cognitive aging, including general cognitive ability, episodic and working memory, verbal, spatial, and perceptual speed abilities (Davies et al., 2014; Reynolds et al., 2006; Schiepers et al., 2012). The least common APOE ε2 allele has been associated with reduced risk of AD and is thought to be possibly neuroprotective (Conejero-Goldberg et al., 2014; Liu et al., 2013). Though of keen interest, the role of APOE on cognition in childhood and adolescence is inconclusive (Chang et al., 2016; Ihle et al., 2012; Khan et al., 2014; Weissberger et al., 2018). However, it has been suggested that structural brain differences in ε4 carriers’ volumes may appear in infancy, including lower hippocampal, frontal and temporal lobe volumes, as well as gray and white matter (Dean et al., 2014; Knickmeyer et al., 2013). A recent large cross-sectional imaging and neuropsychological study of 1187 children and youth, aged 3 to 20 years, suggested a number of differences in brain volume, fractional anisotropy, or thinning by APOE genotypes as well as cognitive ability performance (Chang et al., 2016). For example, smaller hippocampal volumes among ε4/ε4 individuals were associated with poorer performance on attention and working memory tasks (Chang et al., 2016). A recent comparison of mice from lines generated to overexpress 1N4R human tau via a P301S mutation and to either express human ApoE (ε2, ε3, or ε4 knock-ins) or not to express ApoE (knockouts) suggested that tau-related atrophy was observed in those expressing ApoE ε4 at 9 months of age, approximately equivalent to human middle adulthood (Shi et al., 2017). Indeed, the P301S/ε4 mice showed greater tau hyperphosphorylation in the hippocampus at 3 months of age, approximately equivalent to human early adulthood, and a bigger loss of neurons in the hippocampal CA1 region at 9 months of age (Shi et al., 2017). Taken together, results of recent studies are consistent with the proposition that early life factors may be associated with later cognitive health, such that the APOE ε4 variant is implicated in cognitive health starting in early life.
Whether differential vulnerability is characteristic of all individuals who carry one or two copies of the APOE ε4 allele, or whether women are more vulnerable is of interest beyond differential mortality explanations. A recent meta-analysis (Neu et al., 2017) suggests that women with the ε3/ε4 genotype may be at greater risk than men for developing Mild Cognitive Impairment (MCI) between ages 55 and 70 years and at greater risk of Alzheimer’s disease between 65-75 years but are not at differential risk at later ages. Whether sex differences in the association between APOE ε4 and cognitive profiles appear earlier in the lifespan is unclear, particularly in childhood and adolescence, although studies of childhood IQ suggest possibly differential associations for females than males on IQ performance (Calderon-Garciduenas et al., 2016; Taylor et al., 2011).
While the extant literature and early origins theories (Barnett et al., 2013; Liu et al., 2010) of adult-onset disease risk (Barker and Martyn, 1992) suggest that APOE-cognition associations might emerge with development, there is little longitudinal data to resolve this question (Ihle et al., 2012). Most childhood studies have been cross-sectional (e.g., Calderon-Garciduenas et al., 2016; Chang et al., 2016; Ihle et al., 2012; Khan et al., 2014) or limited in assessments (i.e., two waves(Taylor et al., 2011)). Indeed, to our knowledge, no childhood studies considering APOE and general cognitive ability or IQ have evaluated more than a single occasion, despite knowledge that multiple assessment affords greater reliability to evaluate and observe a possible relationship. We examined associations of APOE genotypes with cognitive performance, evaluating whether APOE ε4 conferred a disadvantage for IQ performance evaluated at three assessments between childhood and adolescence. We also explore moderation of APOE ε4 by sex in associations with IQ performance.
2. Materials and Methods
2.1. Participants
The CATSLife project includes participants from the Colorado Adoption Project (CAP; (Rhea et al., 2013a)), which was initiated in 1975 and enrolled 245 adoptive and 245 control families, and the Longitudinal Twin Study (LTS; (Rhea et al., 2013b)), which was initiated in 1985 and enrolled 483 twin pairs (265 MZ, 218 DZ). These studies used nearly parallel assessments between infancy and adolescence conducted on a nearly annual basis, and with periodic assessments into adulthood. Age descriptives by sample are reported in Table 1. The current analysis sample of 1321 includes individuals (nested within 773 families) who ranged between the ages of 6.50 years at the year 7 assessment and up to 17.99 years of age at the year 16 assessment. Genotyping was conducted on archival DNA samples and samples obtained from the ongoing CATSLife study (NCAP = 472, 48.7% male; 44.92% adoptive families; NLTS = 849, 48.8% male, 54.42% MZ twins). Self-reported race and ethnicity of the sample is 92.13% white and 7.87% non-white (American Indian/Alaska Native, Asian, Native Hawaiian/Pacific Islander, Black/African-American, More than one race, Unknown/Not reported), with 5.90% of the analysis sample identifying as Hispanic. These numbers are representative of the Colorado Front Range ethnic composition at the time participants were recruited into the foundational CAP and LTS studies.
Table 1.
Analysis sample age descriptives by study.
| Assessment | CAP N=472 |
MAGE | SDAGE | Min | Max | LTS N=849 |
MAGE | SDAGE | Min | Max |
|---|---|---|---|---|---|---|---|---|---|---|
| Year 7 | 397 | 7.42 | 0.37 | 6.50 | 8.42 | 780 | 7.43 | 0.36 | 6.67 | 8.50 |
| Year 12 | 403 | 12.45 | 0.41 | 11.67 | 14.17 | 738 | 12.43 | 0.37 | 11.33 | 14.00 |
| Year 16 | 451 | 16.34 | 0.48 | 16.00 | 17.99 | 724 | 16.37 | 0.41 | 16.00 | 17.92 |
Note. CAP = Colorado Adoption Project; LTS = Longitudinal Twin Study.
The protocol was approved by the two respective institutional review boards of the authors’ institutions, the University of Colorado at Boulder and the University of California at Riverside.
2.2. Measures
The year 7 and 12 cognitive assessments included WISC IQ measures (WISC-R or WISC-III; Wechsler, 1974, 1991) and the age 16 and CATSLife assessments included WAIS IQ measures (WAIS-R or WAIS-III; Wechsler, 1981, 1997) (see Table 2). The WISC-R and WISC-III tests are similar in item content and subtest composition (12/13 subtests overlap) with equivalent covariance structure among the subtests across the two versions (Dixon and Anderson, 1995). The WAIS-R and WAIS-III tests are likewise very similar, with respective correlations of Verbal IQ (VIQ), Performance IQ (PIQ) and Full Scale IQ scores exceeding .80 across versions (Strauss et al., 2006; Tulsky et al., 1997). Last, IQ scores calculated from child versus adult batteries are comparable and strongly correlated, e.g., WISC-III versus WAIS-III scores among 16-year old’s are correlated .78 to .88 (Strauss et al., 2006). IQ scores are scaled by age-based norms, and the general expectation would be, therefore, that age effects ought to be minimal; given the shift in the test batteries associated with age and cohort, however, we included study and age covariates in analyses as described further below.
Table 2.
IQ Assessments by study.
| Assessment | Year 7 | Year 12 | Year 16 |
|---|---|---|---|
| WISC - R | CAP/LTS | CAP | |
| WISC - III | LTS | ||
| WAIS - R | CAP | ||
| WAIS - III | LTS |
Note. CAP = Colorado Adoption Project; LTS = Longitudinal Twin Study.
2.2.1. APOE Genotyping
Taqman assays of APOE SNPS, rs7412 and rs429358, were performed using buccal cell derived DNA. The success rate was 97%. APOE genotypes were formed from the two SNPs according to common practice (see Table 3). Where MZ twins were un-typed (N=4), they were assigned their cotwin’s APOE genotype. Where one of the two APOE SNP assays failed we took the MZ cotwins genotype for that SNP (N=17). Hardy Weinberg Equilibrium (HWE) was achieved for both SNPs in both samples, selecting one sibling in each twin pair or sibship (all p ≥ .088). In addition, the APOE genotypes formed from both SNPs achieved HWE in each sample and when combined across the two samples (all p > .154). Supplemental Table S1 presents the distribution of independent APOE alleles in a selection of one sibling or twin member from each pair/sibship. Overall, the ε4 allele is more frequent (13.95%) than the ε2 allele (7.89%), as expected (see Supplemental Table S1).
Table 3.
APOE genotype frequencies in analysis sample by study.
| CAP | LTS | |||||
|---|---|---|---|---|---|---|
| APOE | rs429358 | rs7412 | N | Percent | N | Percent |
| ε22 | T/T | T/T | 6 | 1.27 | 9 | 1.06 |
| ε23 | T/T | C/T | 50 | 10.59 | 104 | 12.25 |
| ε24 | C/T | C/T | 13 | 2.75 | 16 | 1.88 |
| ε33 | T/T | C/C | 283 | 59.96 | 524 | 61.72 |
| ε34 | C/T | C/C | 110 | 23.31 | 189 | 22.26 |
| ε44 | C/C | C/C | 10 | 2.12 | 7 | 0.82 |
| Current N | 1321 | 472 | 849 | |||
Note. CAP = Colorado Adoption Project; LTS = Longitudinal Twin Study.
2.3. Analyses
Multi-level regression analyses of WISC and WAIS IQ measures were carried out using SAS Proc Mixed 9.4 (SAS Inc, Cary NC), using full maximum likelihood estimation. In Model I, the main effects model, IQ scores were predicted by the number of APOE ε4 alleles (0, 1 or 2), adjusting for the number of APOE ε2 alleles (0, 1 or 2) given its possible neuroprotective effect. The reference APOE genotype was therefore ε3/ε3. Additional covariates included study, sex, adopted status, and age, with coding as follows. Study was effects-coded as Colorado Adoption Project (CAP = −.5), Longitudinal Twin Study (LTS = .5). Sex was dummy coded and reflected effects for females (Male = 0, Female = 1). Adopted was dummy coded and reflects adoption status (0 = Not, 1 = Adopted). Age was centered at 16 years and reflected any possible age differences within or across longitudinal assessments, but also possible differences due to IQ battery as described above. Thus, in Model I, the fixed effect intercept reflects the expected mean IQ score for males, who were not adopted, at age 16 years, for those who are APOE ε3/ε3, controlling for study. In Model II, interaction terms with sex and APOE ε4 alleles were entered to evaluate possible sex moderation of APOE ε4 on IQ, adjusting for the interaction of sex and number of ε2 alleles. Hence in Model II, the APOE ε4 effect reflects the ε4 effect for males and the APOE ε4*Sex interaction reflects the APOE ε4 effect for females.
Additional sensitivity tests included fitting the described Models I and II to IQ data at assessment year 7 alone when the WISC-R battery was given to both CAP and LTS participants. Additional sensitivity analyses of the longitudinal IQ results included covariate adjustments by race and ethnicity, and a sex by study interaction. Race and ethnicity were coded as white (1 = white, 0 = non-white) and Hispanic (1 = Hispanic, 0 = non-Hispanic). Last, we explored possible non-additive effects given hints of non-additivity in the mean IQ patterns; this was done for Model I given sex by APOE genotype frequencies would be limited for the rarer genotypes. We recoded ε2 and ε4 additive terms where 0 alleles = −1, 1 allele = 0, and 2 alleles = 1 (Model I.a). We coded dominance effects for ε2 alleles (Model I.b) and ε4 alleles (Model I.c) as follows: 0 alleles = 0, 1 allele = 1 and 2 alleles = 0. Models I.b and I.c respectively allow for heterozygotes to deviate non-additively from respective ε2 and ε4 homozygotes and both differentiate ε24 from ε34.
All analyses adjusted for nesting of individuals within family type, to account for dependencies in the data that could affect the standard errors of the regression parameters of the fixed effect predictors and covariates. Specifically, random effect variances were estimated for the intercept, decomposed into within-sibling and between-sibling variance, as well as residual variance, each by family/sibling type: i.e., siblings were from adoptive (A) or non-adoptive control (C) families, or were dizygotic twins (DZ) or monozygotic twins (MZ). Thus, the analysis accounted for differential dependencies between MZ twins, who share identical genotypes, from DZ twins who share on average 50% of segregating genes in common, as well as for differential dependencies between genetically-unrelated siblings and genetically-related siblings. The between sibling intercept variance represents similarity among siblings in IQ performance that is systematic across longitudinal assessments of IQ and vary by sibling type (,,,) (see Tables S2, S4, S6). The corresponding within-sibling intercept variance represents differences in IQ among siblings that are systematic across longitudinal assessments of IQ and vary by sibling type (,,,). Last, the residual variance represents within-person variability in performance that is occasion-specific (,,,). No constraints were placed on the magnitudes of random effects estimated by sibling types. In sensitivity analyses of the IQ data at the year 7 assessment, the decomposition of the random effects of the intercept included between pair random effects (,,,) and residual within pair random effects of IQ (denoted ,,,); in these models age was centered at age 7.
Regression parameter tests of significance were as reported via SAS Proc MIXED 9.4 (SAS Inc, Cary NC), which included asymptotically distributed t-statistics formed by taking the parameter estimate over its standard error, with degrees of freedom estimated using the between-within option. For Model I, 1-tailed tests of significance are reported for APOE ε4 effects given our hypothesized direction of effect while Model II reported 2-tailed tests of significance for APOE ε4 by sex interactions. We report 95% confidence intervals for all parameters for both Models I and II.
The sample size is appropriate for tests of association. Our expected power for an overall main effect association exceeds .94 assuming an effect size contribution of 1% to the outcome, a sibling correlation of .40, and an ε4 frequency of 15% (Purcell et al., 2003). Under the same conditions, an expected power of .80 is achieved with an effect size contribution of 0.65%. Moreover, the multiple longitudinal assessments of IQ provide increased reliability to evaluate and observe a possible relationship.
3. Results
Unadjusted descriptives of IQ performance at each assessment by APOE ε4 status are suggestive of differential performance by APOE ε4 (see Table 4), with reduced performance particularly for those carrying one APOE ε4 allele in the total sample. When considering sex, a pattern of lower mean IQ performance per APOE ε4 allele is observable in females but not for males, though notably the sample sizes for APOE ε4/ε4 are small.
Table 4.
Age and IQ test performance by APOE ε4 alleles across assessments at Years 7, 12, and 16.
| Total Sample | Variable | APOEε4=0 | APOEε4=1 | APOEε4= 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | ||
| Year 7 | Verbal IQ | 865 | 106.31 | 14.32 | 296 | 105.72 | 14.65 | 15 | 107.40 | 10.44 |
| Performance IQ | 866 | 110.78 | 12.54 | 296 | 108.68 | 13.62 | 15 | 110.53 | 10.33 | |
| Full Scale IQ | 865 | 109.24 | 12.99 | 296 | 107.77 | 13.50 | 15 | 109.60 | 7.87 | |
| Age | 871 | 7.42 | 0.37 | 296 | 7.45 | 0.36 | 15 | 7.38 | 0.40 | |
| Year 12 | Verbal IQ | 839 | 105.89 | 13.19 | 288 | 105.58 | 12.76 | 14 | 108.07 | 14.07 |
| Performance IQ | 839 | 105.85 | 13.75 | 287 | 104.36 | 13.59 | 13 | 109.62 | 8.44 | |
| Full Scale IQ | 839 | 106.38 | 12.95 | 287 | 105.40 | 12.74 | 13 | 109.08 | 10.87 | |
| Age | 841 | 12.44 | 0.39 | 288 | 12.45 | 0.36 | 14 | 12.40 | 0.42 | |
| Year 16 | Verbal IQ | 873 | 103.70 | 12.58 | 288 | 102.94 | 11.58 | 14 | 103.43 | 10.91 |
| Performance IQ | 873 | 104.25 | 12.44 | 288 | 103.22 | 11.88 | 14 | 105.86 | 14.20 | |
| Full Scale IQ | 873 | 104.17 | 11.75 | 288 | 103.20 | 10.63 | 14 | 104.71 | 10.31 | |
| Age | 873 | 16.36 | 0.44 | 289 | 16.36 | 0.43 | 14 | 16.55 | 0.41 | |
| Males | Variable | N | Mean | SD | N | Mean | SD | N | Mean | SD |
| Year 7 | Verbal IQ | 430 | 105.81 | 14.49 | 146 | 107.03 | 14.17 | 8 | 106.88 | 11.31 |
| Performance IQ | 430 | 111.14 | 12.72 | 146 | 110.82 | 13.08 | 8 | 115.38 | 10.93 | |
| Full Scale IQ | 430 | 109.13 | 13.29 | 146 | 109.72 | 13.11 | 8 | 111.88 | 7.88 | |
| Age | 432 | 7.47 | 0.39 | 146 | 7.51 | 0.36 | 8 | 7.54 | 0.40 | |
| Year 12 | Verbal IQ | 407 | 106.61 | 13.36 | 142 | 107.55 | 13.41 | 7 | 114.57 | 11.31 |
| Performance IQ | 407 | 104.89 | 13.80 | 142 | 106.12 | 13.49 | 7 | 111.71 | 6.32 | |
| Full Scale IQ | 407 | 106.29 | 12.84 | 142 | 107.56 | 13.33 | 7 | 114.71 | 7.95 | |
| Age | 409 | 12.48 | 0.40 | 142 | 12.51 | 0.38 | 7 | 12.58 | 0.45 | |
| Year 16 | Verbal IQ | 421 | 104.49 | 13.05 | 147 | 104.46 | 11.13 | 6 | 109.50 | 11.36 |
| Performance IQ | 421 | 103.97 | 12.94 | 147 | 104.99 | 12.40 | 6 | 114.83 | 14.96 | |
| Full Scale IQ | 421 | 104.54 | 12.05 | 147 | 104.98 | 10.86 | 6 | 112.33 | 8.96 | |
| Age | 421 | 16.39 | 0.48 | 148 | 16.41 | 0.44 | 6 | 16.64 | 0.47 | |
| Females | Variable | N | Mean | SD | N | Mean | SD | N | Mean | SD |
| Year 7 | Verbal IQ | 435 | 106.81 | 14.14 | 150 | 104.44 | 15.04 | 7 | 108.00 | 10.21 |
| Performance IQ | 436 | 110.42 | 12.37 | 150 | 106.60 | 13.86 | 7 | 105.00 | 6.53 | |
| Full Scale IQ | 435 | 109.34 | 12.70 | 150 | 105.87 | 13.64 | 7 | 107.00 | 7.57 | |
| Age | 439 | 7.37 | 0.33 | 150 | 7.39 | 0.35 | 7 | 7.19 | 0.35 | |
| Year 12 | Verbal IQ | 432 | 105.20 | 13.01 | 146 | 103.66 | 11.83 | 7 | 101.57 | 14.22 |
| Performance IQ | 432 | 106.75 | 13.66 | 145 | 102.64 | 13.52 | 6 | 107.17 | 10.48 | |
| Full Scale IQ | 432 | 106.45 | 13.06 | 145 | 103.28 | 11.81 | 6 | 102.50 | 10.56 | |
| Age | 432 | 12.40 | 0.38 | 146 | 12.39 | 0.33 | 7 | 12.21 | 0.31 | |
| Year 16 | Verbal IQ | 452 | 102.96 | 12.10 | 141 | 101.36 | 11.87 | 8 | 98.88 | 8.58 |
| Performance IQ | 452 | 104.51 | 11.96 | 141 | 101.38 | 11.07 | 8 | 99.13 | 9.67 | |
| Full Scale IQ | 452 | 103.82 | 11.46 | 141 | 101.34 | 10.10 | 8 | 99.00 | 7.27 | |
| Age | 452 | 16.32 | 0.40 | 141 | 16.31 | 0.40 | 8 | 16.47 | 0.39 | |
3.1. Multilevel Regression Models I and II
APOE ε4 associations with longitudinal IQ performance at the year 7, 12 and 16 assessments were evaluated via multilevel regression models fitted to longitudinal IQ scores (N = 1321, 48.86% male; age range 6.50 to 17.99 years), adjusting for sex, age, adopted status, study sample, and the number of ε2 alleles. Parameters from the full multi-level main effects model fitted with covariates (Model I) are presented in Tables 5 (fixed effects) and S2 (random effects). With respect to prediction by APOE genotype, 1-tailed tests were selected for ε4 under Model I given the hypothesized directionality. Results suggested that for each ε4 allele, Full scale IQ scores were lower by 1.91 points compared to ε33 homozygotes (p = 0.0051/2 = 0.0026, 1-tailed); this corresponds to an estimated d effect size of −.13 using the expected SD for IQ scores of 15 (i.e., 1.91/15). Consistent effects were observed for Verbal (B = −1.60, p = 0.0224/2 = 0.0112 1-tailed, d = −.11) and Performance IQ (B = −1.78, p=0.0118/2 = 0.0059 1-tailed, d=−.12). False Discovery Rate (FDR) adjusted 1- tailed p-values remain significant: Full scale IQ, p = 0.0077; Verbal IQ, p = 0.0112; and Performance IQ, p = 0.0089.
Table 5.
Multilevel fixed effects: APOE ε4 effects on IQ across Year 7, Year 12 and Year 16 assessments.
| Verbal IQ | Model I | Model II | ||||||
|---|---|---|---|---|---|---|---|---|
| Fixed Effects | B | se | LL | UL | B | se | LL | UL |
| Intercept | 106.42 | 0.64 c | 105.16 | 107.67 | 105.89 | 0.69 c | 104.54 | 107.23 |
| Study | −6.28 | 0.88 c | −8.01 | −4.55 | −6.21 | 0.88 c | −7.95 | −4.48 |
| Adopted | −3.64 | 0.98 c | −5.55 | −1.72 | −3.56 | 0.98 c | −5.47 | −1.64 |
| Sex | −2.36 | 0.71 c | −3.75 | −0.96 | −1.30 | 0.87 | −3.00 | 0.39 |
| Age | −0.36 | 0.03 c | −0.43 | −0.30 | −0.36 | 0.03 c | −0.43 | −0.30 |
| ε2 | −0.42 | 0.86 | −2.12 | 1.27 | 0.30 | 1.22 | −2.09 | 2.69 |
| ε4 | −1.60 | 0.70 a,f | −2.97 | −0.23 | −0.23 | 0.95 | −2.09 | 1.63 |
| Sex*ε2 | -- | -- | -- | -- | −1.37 | 1.60 | −4.50 | 1.77 |
| Sex*ε4 | -- | -- | -- | -- | −2.95 | 1.38 a,t | −5.65 | −0.25 |
| Performance IQ | Model I | Model II | ||||||
| Fixed Effects | B | se | LL | UL | B | se | LL | UL |
| Intercept | 105.76 | 0.61 c | 104.56 | 106.96 | 105.33 | 0.66 c | 104.02 | 106.63 |
| Study | −8.17 | 0.81 c | −9.76 | −6.58 | −8.11 | 0.81 c | −9.70 | −6.51 |
| Adopted | −1.56 | 1.06 | −3.63 | 0.51 | −1.47 | 1.06 | −3.55 | 0.60 |
| Sex | −1.16 | 0.70 | −2.53 | 0.22 | −0.30 | 0.86 | −1.99 | 1.38 |
| Age | −0.79 | 0.03 c | −0.86 | −0.72 | −0.79 | 0.03 c | −0.86 | −0.72 |
| ε2 | −0.17 | 0.84 | −1.82 | 1.48 | −0.64 | 1.24 | −3.06 | 1.79 |
| ε4 | −1.78 | 0.71 a,f | −3.16 | −0.39 | −0.13 | 0.97 | −2.03 | 1.77 |
| Sex*ε2 | -- | -- | -- | -- | 0.85 | 1.65 | −2.38 | 4.08 |
| Sex*ε4 | -- | -- | -- | -- | −3.48 | 1.40 a,t | −6.23 | −0.73 |
| Full Scale IQ | Model I | Model II | ||||||
| Fixed Effects | B | se | LL | UL | B | se | LL | UL |
| Intercept | 106.60 | 0.61 c | 105.41 | 107.80 | 106.11 | 0.65 c | 104.82 | 107.39 |
| Study | −7.81 | 0.83 c | −9.44 | −6.17 | −7.75 | 0.83 c | −9.38 | −6.11 |
| Adopted | −2.97 | 0.99 b | −4.92 | −1.02 | −2.90 | 0.99 b | −4.84 | −0.95 |
| Sex | −2.05 | 0.69 b | −3.41 | −0.70 | −1.06 | 0.84 | −2.71 | 0.59 |
| Age | −0.63 | 0.03 c | −0.69 | −0.58 | −0.63 | 0.03 c | −0.69 | −0.58 |
| ε2 | −0.45 | 0.83 | −2.09 | 1.18 | −0.35 | 1.19 | −2.67 | 1.98 |
| ε4 | −1.91 | 0.68 b,f | −3.25 | −0.58 | −0.33 | 0.92 | −2.14 | 1.48 |
| Sex*ε2 | -- | -- | -- | -- | −0.22 | 1.56 | −3.28 | 2.84 |
| Sex*ε4 | -- | -- | -- | -- | −3.41 | 1.35 a,t | −6.05 | −0.77 |
p < .05,
p < .01,
p < .001;
p<.05 1-tailed, FDR corrected,
p< .05 2-tailed, FDR corrected.
Note. N = 1321. Study (CAP = −.5, LTS = .5), Adopted (0=Not, 1=Adopted), Sex (Male = 0, Female = 1); Age was centered at 16 years; ε2 = number of alleles (0,1,2); ε4 = number of alleles (0,1,2). Model I refers to main effects models with APOE and Model II includes interaction effects with sex and APOE. LL, UL = lower and upper 95% confidence interval.
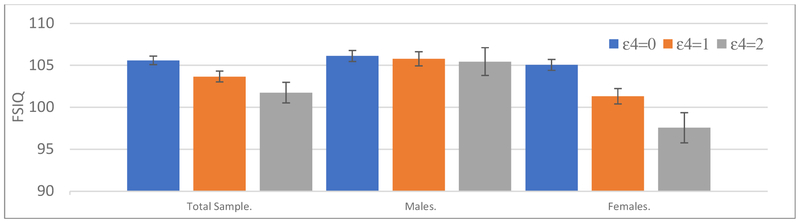
Figure 1 presents expected mean Full Scale IQ scores as a function of the number of APOE ε4 alleles in the total sample.
Figure 1. Mixed Model estimates: APOE ε4 effects on Full Scale IQ based on child and adolescent assessments (years 7, 12, and 16).
Note. Analyses adjusted for nesting of individuals within family type, number of APOE ε2 alleles, sex, age, adoption status, and study (CAP or LTS). APOE ε3/ε3 was the referent group. The ‘Total Sample’ estimates were averaged across sex (i.e., sex = .5), with all other effects at centered values. Standard errors shown.
Next, to evaluate sex differences with respect to APOE effects, an interaction term with sex and APOE ε4 alleles was entered and evaluated under Model II using a 2-tailed test (see Table 5, Model II), adjusting for the interaction of sex and ε2 alleles. For Verbal IQ, the ε4 by sex interaction was significant (p =.0324 2-tailed), suggesting the ε4 effect may be larger in females than males, while the main effect of ε4, now reflecting male performance, was non-significant. Specifically, the ε4 effect was −.23 (se = .95; d = −.02) in males and −2.95 (se = 1.38; d = −.20) in females. The ε4 effects for Performance IQ and Full Scale IQ may be especially pronounced for females (ε4 × Sex p ≤ 0.0132 2-tailed), while the main effect of ε4, reflecting differential male performance, was non-significant. For Full Scale IQ, the ε4 effect was −0.33 (se = .92; d = −.02) in males and −3.41 (se = 1.35; d = −.23) in females (see Figure 1). For Performance IQ, the ε4 effect was −0.13 (se = .97; d = −.01) in males and −3.48 (se = 1.40; d = −.23) in females. FDR adjusted 2-tailed p-values for the ε4 × Sex effects remain significant: Full scale IQ, p = 0.0198; Verbal IQ , p = .0324, and Performance IQ, p = 0.0198.
Figure 1 represents expected performance differences for Full Scale IQ by the number of APOE ε4 alleles in males and females.
3.2. Sensitivity Analyses
We performed a sensitivity test to evaluate whether APOE effects are determinable at the youngest assessment at year 7 alone (N = 1176-1177; see Supplementary Tables S3-S4); a further benefit was that the WISC-R battery was implemented for both study samples. We observed significant main effects of APOE for Performance and Full Scale IQ (FDR adjusted 1-tailed p = .0156 for both) with consistent, but non-significant, effects for Verbal IQ (FDR adjusted 1-tailed p = .0749) (see Table S3, Model I). While the models including sex interactions did not achieve significance (all p ≥ .0973; see Table S3, Model II), the pattern of effect sizes was consistent to that observed in the models fitted to longitudinal IQ above.
We also performed a follow-up sensitivity analysis of the longitudinal IQ performance models, with the inclusion of additional covariates, i.e., a sex by study interaction and self-reported race and ethnicity. Adding these covariates did not alter conclusions for longitudinal IQ across assessment years 7, 12 and 16 as detailed above with all tests remaining significant at adjusted FDR p-values: Model I, 1-tailed p = .0064 to p = .0041; and Model II, 2-tailed p = .0397 to p = .0264 (see Supplementary Tables S5-S6).
3.2.1. Tests of non-additivity
We explored the possibility of non-additive effects in Model I, given hints of non-additivity in the mean IQ patterns by APOE ε4 alleles. We fitted Model I.a, with all covariates, entering recoded additive effects for ε2 and ε4 alleles (see Supplementary Tables S7, Model I.a). Subsequently, we added the dominance effect for ε2 alleles. Results suggested that the ε2 dominance effect was negative and significant for Verbal, Performance and Full Scale IQ (p = .0003, .0247, and .0006, respectively, 2-tailed), suggesting reduced performance for ε2 heterozygotes (ε2/ε3, ε2/ε4) than expected from an additive model (see Supplementary Tables S6, Model I.b). FDR adjusted 2-tailed p-values for the ε2 dominance effects remained significant: p = 0.0247 to 0.0009). Moreover, the additive effect for ε2 became significant and positive for Verbal (p = .0090) and Full Scale IQ (p = .0159), with the same direction for Performance IQ (p = .1015), suggesting a benefit in IQ performance for ε2/ε2 homozygotes. FDR adjusted 2-tailed p-values remained significant for Verbal and Full Scale IQ, both p = 0.0239. Last, we added the ε4 dominance effect, which allows ε4 heterozygotes (ε2/ε4, ε3/ε4) to deviate from that expected under an additive model. Results suggested that adding the ε4 dominance effect was not significant (all p ≥ 0.2917) (see Supplementary Table S7, Model I.c). The more parsimonious Model I.b best represents the contributions of additive (ε2 and ε4) and nonadditive (ε2) influences. Supplementary figure S1 displays Full scale IQ by APOE genotypes, based on Models I.a and Model I.b, as well as covariate-adjusted least-squares means. See supplement for further details.
4. Discussion
APOE genotypes may be associated with general cognitive performance earlier than midlife. We observed small detriments in IQ performance across childhood and adolescence with nearly a 2-point decrement for each ε4 allele compared to those with APOE ε3/ε3 genotypes. Performance and Verbal IQ showed consistent effects, with the smaller effect sizes for Verbal IQ. Hence, APOE ε4 genotypes may be associated with lower IQ as early as childhood. Moreover, APOE may show stronger (or earlier) effects on IQ in females than males, particularly for Performance and Full Scale IQ.
The role of APOE on cognition between childhood and early adulthood is inconclusive, with most childhood studies cross-sectional in design (Calderon-Garciduenas et al., 2016; Chang et al., 2016; Ihle et al., 2012; Khan et al., 2014) and limited in assessments of IQ (Taylor et al., 2011). A recent large cross-sectional imaging and neuropsychological study of individuals aged 3 to 20 years (Chang et al., 2016) suggested potential differences in brain and cognitive development for those with particular APOE genotypes. For example, those with APOE ε2/ε4 evidenced smaller hippocampal volumes, and those with ε4/ε4 evidenced lower hippocampal fractional anisotropy at age 8 and younger (Chang et al., 2016). In addition, differential executive functioning and working memory performance were reported for APOE ε4/ε4 and attentional processing for APOE ε2/ε4 (Chang et al., 2016); the authors did not test associations with broader constructs, however. The Lothian Birth Cohort included a single childhood cognitive ability assessment with follow-ups in late adulthood at age 70 and beyond; they report a nonsignificant negative APOE ε4 effect for their measure of general verbal cognitive ability and reasoning assessed at age 11 (Luciano et al., 2009). In the current study, the weakest effect we observed was for Verbal IQ with larger APOE ε4 effects for Performance and Full Scale IQ. Notably absent from a recent large-scale GWAS of intelligence is implication of the APOE region (Savage et al., 2017); only about 5.7% of the nearly 280,000 samples included in this GWAS were from children or young adults. In this, and in similar GWAS, age and sex tend to be treated as covariates, and not leveraged per se to evaluate whether particular variants or gene sets are associated at certain age periods. Cautions in relying on imputations of APOE genotypes from GWAS have also been noted, although in recent years imputation has become more reliable (Radmanesh et al., 2014; Roses et al., 2016).
Opportunities to evaluate earlier life cognitive functioning are uncommon in studies of cognitive aging, yet cognitive development across childhood and adolescence may represent a salient period when cognitive reserves are being formed. Cognitive reserve theories suggest that individuals may differ in their capacity to withstand aging-related pathologies due to cognitive processing optimizations that boost functioning, and may allow individuals to weather aging and disease-related neural changes (Barulli and Stern, 2013). Likewise, early origin theories of late-life cognitive health (Barnett et al., 2013; Liu et al., 2010), stimulated in part by the “Barker hypothesis” of prenatal and early-life developmental determinants of adult-onset disease risk, have led to interests in modifiable features and life course mediators and moderators of cognitive aging and dementia. Our results suggest that cognitive differences associated with APOE may emerge early and become magnified later in the life course, and if so, childhood represents a key period of intervention to invest in and boost reserves.
APOE ε4 effects may be larger in females than in males, particularly for Full Scale IQ and Performance IQ. Recent cross-sectional work described in children ages 3-20 years (Chang et al., 2016) treated sex as a covariate but did not evaluate sex as a moderator of observed APOE effects. A report of 5995 British 8-year-old children from the ALSPAC study failed to find APOE associations with cross-sectional WISC Verbal, Performance or Full Scale IQ (Taylor et al., 2011). However, trends for sex-stratified effects were observed where females with rare APOE genotypes, ε2/ε2 and ε4/ε4, showed higher average scores than ε3/ε3 females, while those with ε2/ε4 and ε3/ε4 genotypes showed worse scores than ε3/ε3 females for Verbal (p = .03) and Total IQ (p = .02). Moreover, a recent cross-sectional study evaluating 105 12-year old children (SD = 5.4 years) living in Mexico City reported findings of an increased vulnerability to poorer performance in female carriers of APOE ε4 on Total and Performance IQ, consistent with our report (Calderon-Garciduenas et al., 2016). Recent literature suggests that differential risks for MCI and AD in females may appear in proscribed age periods and may not extend over the lifespan (Neu et al., 2017). Our findings suggest that such windows may extend to earlier stages of development, particularly for reasoning traits represented by performance IQ, before any notable cognitive differences raise clinical concerns. It will be important to track whether such early differences may become amplified perhaps due to differential cognitive reserves (Pettigrew et al., 2013; Runge et al., 2014) although early life cognitive differences associated with APOE have yet to be directly linked to later cognitive reserves.
Explorations of non-additive effects suggest a possible advantage for APOE ε2/ε2 individuals, followed by ε3/ε3,and the lowest performance among those who carry one or more ε4 alleles. While the observed patterns by genotype are consistent with patterns of dementia risk (Farrer et al., 1997; Neu et al., 2017), and congruent with possible cognitive advantages and detriments observed in child samples (Chang et al., 2016) (c.f., females, Taylor et al., 2011), we note the ε2/ε2 and ε4/ ε4 genotypes are relatively rare and necessitates some caution in interpretation of their means relative to the other more common genotypes. Further examination of differential benefits and vulnerabilities of APOE genotypes, and sex moderation in larger samples, where it can be fully interrogated, is warranted.
The point estimates and d-effect size estimates we observed indicate that the effects on IQ are small per APOE ε4 allele, up to a few points. In terms of clinical relevance, a difference of a few points, while small, may be potentially relevant in terms of cognitive reserve for which any disadvantages could become magnified later in adulthood (c.f., Barulli and Stern, 2013; Liu et al., 2010). This bears further study. Moreover, childhood IQ is predictive of biological age as well as the pace of aging at midlife (Belsky et al., 2017; Schaefer et al., 2016), with lower childhood IQ associated with increased biological aging, as well as increased cardiovascular disease (CVD) risks before age 65 (Hart et al., 2004) even after adjusting for covariates, suggesting that there may be physical health pathways to consider. With respect to APOE, this would include cholesterol-lipid pathways. Finally, for further perspective, the small differences in IQ that we observed are similar to the effect sizes noted in a recent meta-analysis of over 600,000 individuals from 42 studies across the lifespan suggesting that each additional year of achieved education may be causally associated with a 1 to 5 IQ point gain (Ritchie and Tucker-Drob, 2018). Echoing Ritchie and Tucker-Drob (2018), here in the context of APOE ε4, a few points may not be consequential for any one individual, but could be meaningful in terms of early interventions to allay accelerated cognitive aging or dysfunction in a population sense.
Cognitive decline is a devastating and feared aspect of aging, yet its developmental origins have become a focus only recently. Cognitive development during childhood and adolescence may contribute to the formation of crucial cognitive reserves that may hold a unique saliency to later cognitive functioning. Understanding the emergence and phenomena of differential cognitive growth in early life and differential maintenance in functioning in adulthood is critical to evaluating the promise of interventions. Additional longitudinal studies are warranted to consider early origins of cognitive health, and the possibility of developmental effects that emerge in this age span. To this end, we are in the process of collecting additional Full Scale IQ and specific cognitive abilities data on the entire CATSLife sample between 28-46 years of age with an expected completion in 2020.
Supplementary Material
Highlights.
APOE ε4 associated with lower intelligence (IQ) scores from childhood to adolescence
Females may be at greater risk to show these early life effects
Effects of the ε4 allele in childhood support early origin theories
Distinctive age-related profiles may be important to later cognitive impairment
Acknowledgments
The authors gratefully acknowledge support from the National Institutes of Health, NIH AG046938 [MPIs, Chandra A. Reynolds (Contact), Sally J. Wadsworth]. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the CAP and LTS staff, participants and their families who have graciously participated over many years.
Footnotes
Data Statement
Analysis code and outputs are provided at the Open Science Framework platform (https://osf.io/fn9gk/?view_only=f50599adacfc4abdbfda47aa926387eb). We will make available all data used in this manuscript, except where participant directives do not permit us to do so. Data will be made available publicly after the current CATSLife testing is completed and, in the interim, can be re-analyzed in a collaborative effort with our group by qualified investigators.
Disclosure Statement
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker DJ, Martyn CN, 1992. The maternal and fetal origins of cardiovascular disease. J Epidemiol Community Health 46(1), 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Hachinski V, Blackwell AD, 2013. Cognitive health begins at conception: addressing dementia as a lifelong and preventable condition. BMC Med 11, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barulli D, Stern Y, 2013. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci 17(10), 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Cohen HJ, Kraus WE, Ramrakha S, Poulton R, Moffitt TE, 2017. Impact of early personal-history characteristics on the Pace of Aging: implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell 16(4), 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Jewells V, Galaz-Montoya C, van Zundert B, Perez-Calatayud A, Ascencio-Ferrel E, Valencia-Salazar G, Sandoval-Cano M, Carlos E, Solorio E, Acuna-Ayala H, Torres-Jardon R, D'Angiulli A, 2016. Interactive and additive influences of Gender, BMI and Apolipoprotein 4 on cognition in children chronically exposed to high concentrations of PM2.5 and ozone. APOE 4 females are at highest risk in Mexico City. Environ Res 150, 411–422. [DOI] [PubMed] [Google Scholar]
- Chang L, Douet V, Bloss C, Lee K, Pritchett A, Jernigan TL, Akshoomoff N, Murray SS, Frazier J, Kennedy DN, Amaral DG, Gruen J, Kaufmann WE, Casey BJ, Sowell E, Ernst T, Pediatric Imaging, N., Genetics Study, C., 2016. Gray matter maturation and cognition in children with different APOE epsilon genotypes. Neurology 87(6), 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejero-Goldberg C, Gomar JJ, Bobes-Bascaran T, Hyde TM, Kleinman JE, Herman MM, Chen S, Davies P, Goldberg TE, 2014. APOE2 enhances neuroprotection against Alzheimer's disease through multiple molecular mechanisms. Mol Psychiatry 19(11), 1243–1250. [DOI] [PubMed] [Google Scholar]
- Davies G, Harris SE, Reynolds CA, Payton A, Knight HM, Liewald DC, Lopez LM, Luciano M, Gow AJ, Corley J, Henderson R, Murray C, Pattie A, Fox HC, Redmond P, Lutz MW, Chiba-Falek O, Linnertz C, Saith S, Haggarty P, McNeill G, Ke X, Ollier W, Horan M, Roses AD, Ponting CP, Porteous DJ, Tenesa A, Pickles A, Starr JM, Whalley LJ, Pedersen NL, Pendleton N, Visscher PM, Deary IJ, 2014. A genome-wide association study implicates the APOE locus in nonpathological cognitive ageing. Mol Psychiatry 19(1), 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC Iii, Jerskey BA, Chen K, et al. , 2014. Brain differences in infants at differential genetic risk for late-onset alzheimer disease: A cross-sectional imaging study. JAMA Neurology 71(1), 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WE, Anderson T, 1995. Establishing Covariance Continuity between the Wisc-R and the Wisc-Iii. Psychol Assessment 7(1), 115–117. [Google Scholar]
- Eisenberg DT, Kuzawa CW, Hayes MG, 2010. Worldwide allele frequencies of the human apolipoprotein E gene: climate, local adaptations, and evolutionary history. Am J Phys Anthropol 143(1), 100–111. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, PericakVance MA, Risch N, vanDuijn CM, 1997. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease - A meta-analysis. Jama-J Am Med Assoc 278(16), 1349–1356. [PubMed] [Google Scholar]
- Hart CL, Taylor MD, Smith GD, Whalley LJ, Starr JM, Hole DJ, Wilson V, Deary IJ, 2004. Childhood IQ and cardiovascular disease in adulthood: prospective observational study linking the Scottish Mental Survey 1932 and the Midspan studies. Soc Sci Med 59(10), 2131–2138. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Herz J, Bu G, 2012. Apolipoprotein e and apolipoprotein e receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med 2(3), a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle A, Bunce D, Kliegel M, 2012. APOE epsilon4 and cognitive function in early life: a meta-analysis. Neuropsychology 26(3), 267–277. [DOI] [PubMed] [Google Scholar]
- Khan W, Giampietro V, Ginestet C, Dell'acqua F, Bouls D, Newhouse S, Dobson R, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Conrod P, Flor H, Frouin V, Garavan H, Gowland P, Heinz A, Ittermann B, Lemaitre H, Nees F, Paus T, Pausova Z, Rietschel M, Smolka MN, Strohle A, Gallinat J, Westman E, Schumann G, Lovestone S, Simmons A, 2014. No Differences in Hippocampal Volume between Carriers and Non-Carriers of the ApoE epsilon4 and epsilon2 Alleles in Young Healthy Adolescents. Journal of Alzheimer's disease : JAD 40(1), 37–43. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Wang J, Zhu H, Geng X, Woolson S, Hamer RM, Konneker T, Lin W, Styner M, Gilmore JH, 2013. Common Variants in Psychiatric Risk Genes Predict Brain Structure at Birth. Cerebral cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G, 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9(2), 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Jones R, Glymour M, 2010. Implications of Lifecourse Epidemiology for Research on Determinants of Adult Disease. Public Health Reviews 32, 489–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Gow AJ, Harris SE, Hayward C, Allerhand M, Starr JM, Visscher PM, Deary IJ, 2009. Cognitive Ability at Age 11 and 70 Years, Information Processing Speed, and APOE Variation: The Lothian Birth Cohort 1936 Study. Psychol Aging 24(1), 129–138. [DOI] [PubMed] [Google Scholar]
- Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, Wang LS, Romero K, Arneric SP, Redolfi A, Orlandi D, Frisoni GB, Au R, Devine S, Auerbach S, Espinosa A, Boada M, Ruiz A, Johnson SC, Koscik R, Wang JJ, Hsu WC, Chen YL, Toga AW, 2017. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol 74(10), 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew C, Soldan A, Li S, Lu Y, Wang MC, Selnes OA, Moghekar A, O'Brien R, Albert M, The Biocard Research T, 2013. Relationship of cognitive reserve and APOE status to the emergence of clinical symptoms in preclinical Alzheimer's disease. Cogn Neurosci 4(3–4), 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Fulker DW, 1988. Nature and nurture during infancy and early childhood. Cambridge University Press; (1988) xiii, 345 pp., New York, NY. [Google Scholar]
- Purcell S, Cherny SS, Sham PC, 2003. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 19(1), 149–150. [DOI] [PubMed] [Google Scholar]
- Radmanesh F, Devan WJ, Anderson CD, Rosand J, Falcone GJ, Alzheimer's Disease Neuroimaging I, 2014. Accuracy of imputation to infer unobserved APOE epsilon alleles in genome-wide genotyping data. Eur J Hum Genet 22(10), 1239–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CA, Prince JA, Feuk L, Brookes AJ, Gatz M, Pedersen NL, 2006. Longitudinal memory performance during normal aging: twin association models of APOE and other Alzheimer candidate genes. Behav Genet 36(2), 185–194. [DOI] [PubMed] [Google Scholar]
- Rhea SA, Bricker JB, Wadsworth SJ, Corley RP, 2013a. The Colorado Adoption Project. Twin Res Hum Genet 16(1), 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea SA, Gross AA, Haberstick BC, Corley RP, 2013b. Colorado twin registry: an update. Twin Res Hum Genet 16(1), 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Tucker-Drob EM, 2018. How Much Does Education Improve Intelligence? A Meta-Analysis. Psychol Sci 29(8), 1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses A, Sundseth S, Saunders A, Gottschalk W, Burns D, Lutz M, 2016. Understanding the genetics of APOE and TOMM40 and role of mitochondrial structure and function in clinical pharmacology of Alzheimer's disease. Alzheimers Dement 12(6), 687–694. [DOI] [PubMed] [Google Scholar]
- Runge SK, Small BJ, McFall GP, Dixon RA, 2014. APOE Moderates the Association between Lifestyle Activities and Cognitive Performance: Evidence of Genetic Plasticity in Aging. J Int Neuropsych Soc 20(5), 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, Nagel M, Awasthi S, Barr PB, Coleman JRI, Grasby KL, Hammerschlag AR, Kaminski J, Karlsson R, Krapohl E, Lam M, Nygaard M, Reynolds CA, Trampush JW, Young H, Zabaneh D, Hägg S, Hansell NK, Karlsson IK, Linnarsson S, Montgomery GW, Munoz-Manchado AB, Quinlan EB, Schumann G, Skene N, Webb BT, White T, Arking DE, Attix DK, Avramopoulos D, Bilder RM, Bitsios P, Burdick KE, Cannon TD, Chiba-Falek O, Christoforou A, Cirulli ET, Congdon E, Corvin A, Davies G, Deary IJ, DeRosse P, Dickinson D, Djurovic S, Donohoe G, Drabant Conley E, Eriksson JG, Espeseth T, Freimer NA, Giakoumaki S, Giegling I, Gill M, Glahn DC, Hariri AR, Hatzimanolis A, Keller MC, Knowles E, Konte B, Lahti J, Le Hellard S, Lencz T, Liewald DC, London E, Lundervold AJ, Malhotra AK, Melle I, Morris D, Need AC, Ollier W, Palotie A, Payton A, Pendleton N, Poldrack RA, Räikkönen K, Reinvang I, Roussos P, Rujescu D, Sabb FW, Scult MA, Smeland OB, Smyrnis N, Starr JM, Steen VM, Stefanis NC, Straub RE, Sundet K, Voineskos AN, Weinberger DR, Widen E, Yu J, Abecasis G, Andreassen OA, Breen G, Christiansen L, Debrabant B, Dick DM, Heinz A, Hjerling-Leffler J, Ikram MA, Kendler KS, Martin NG, Medland SE, Pedersen NL, Plomin R, Polderman TJC, Ripke S, van der Sluis S, Sullivan PF, Tiemeier H, Vrieze SI, Wright MJ, Posthuma D, 2017. GWAS meta-analysis (N=279,930) identifies new genes and functional links to intelligence. bioRxiv. [Google Scholar]
- Schaefer JD, Caspi A, Belsky DW, Harrington H, Houts R, Israel S, Levine ME, Sugden K, Williams B, Poulton R, Moffitt TE, 2016. Early-Life Intelligence Predicts Midlife Biological Age. J Gerontol B-Psychol 71(6), 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers OJ, Harris SE, Gow AJ, Pattie A, Brett CE, Starr JM, Deary IJ, 2012. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry 17(3), 315–324. [DOI] [PubMed] [Google Scholar]
- Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg T, Rojas JC, Gallardo G, Wang K, Roh J, Robinson G, Finn MB, Jiang H, Sullivan PM, Baufeld C, Wood MW, Sutphen C, McCue L, Xiong C, Del-Aguila JL, Morris JC, Cruchaga C, Alzheimer's Disease Neuroimaging I, Fagan AM, Miller BL, Boxer AL, Seeley WW, Butovsky O, Barres BA, Paul SM, Holtzman DM, 2017. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549(7673), 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EM, Spreen O, 2006. A compendium of neuropsychological tests: Administration, norms, and commentary. , Third edition ed. Oxford University Press, New York. [Google Scholar]
- Taylor AE, Guthrie PA, Smith GD, Golding J, Sattar N, Hingorani AD, Deanfield JE, Day IN, 2011. IQ, educational attainment, memory and plasma lipids: associations with apolipoprotein E genotype in 5995 children. Biol Psychiatry 70(2), 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsky D, Zhu J, Ledbetter MF, 1997. WAIS-III/WMS-III Technical Manual. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Walhovd KB, Krogsrud SK, Amlien IK, Bartsch H, Bjornerud A, Due-Tonnessen P, Grydeland H, Hagler DJ Jr., Haberg AK, Kremen WS, Ferschmann L, Nyberg L, Panizzon MS, Rohani DA, Skranes J, Storsve AB, Solsnes AE, Tamnes CK, Thompson WK, Reuter C, Dale AM, Fjell AM, 2016. Neurodevelopmental origins of lifespan changes in brain and cognition. Proc Natl Acad Sci U S A 113(33), 9357–9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1974. Wechsler intelligence scale for children—revised. Psychological Corporation, New York. [Google Scholar]
- Wechsler D, 1981. Manual for the Wechsler Adult Intelligence Scale, R. Psychological Corporation, New York. [Google Scholar]
- Wechsler D, 1991. The Wechsler intelligence scale for children—third edition manual. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wechsler D, 1997. Wechsler Adult Intelligence Scale - Third Edition (WAIS-III). Psychological Corporation, New York. [Google Scholar]
- Weissberger GH, Nation DA, Nguyen CP, Bondi MW, Han SD, 2018. Meta-analysis of cognitive ability differences by apolipoprotein e genotype in young humans. Neurosci Biobehav Rev 94, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.