Abstract
Genome-wide association studies linked diacylglycerol kinase eta (DGKH) and iota (DGKI) to mood disorders, including bipolar disorder and schizophrenia, and both genes are expressed throughout the brain. Here, we generated and behaviorally characterized female mice lacking Dgkh alone, Dgki alone, and double Dgkh/Dgki knockout (dKO) mice. We found that fewer than 30% of newborn pups raised by dKO females survived to weaning, while over 85% of pups survive to weaning when raised by wild type (WT) females. Poor survival under the care of dKO mothers was unrelated to pup genotype. Moreover, pups from dKO dams survived when fostered by WT dams, suggesting the poor survival rate of dKO-raised litters was related to impaired maternal care by dKO dams. Nest building was similar between WT and dKO dams; however, some dKO females failed to retrieve any pups in a retrieval assay. Pups raised by dKO dams had smaller or absent milk spots and reduced weight, indicative of impaired nursing. Unlike WT females, postpartum dKO females showed erratic, panicked responses to cage disturbances. Virgin dKO females showed behavioral signs of anxiety and mania, which were not seen in mice lacking either Dgkh or Dgki alone. Our research indicates that combined deletion of Dgkh and Dgki impairs maternal behavior in the early postpartum period, and suggests female dKO mice model symptoms of mania and anxiety.
1 ∣. INTRODUCTION
Diacylglycerol kinases play an important role in mammalian physiology by altering the activity of the substrate diacylglycerol (DAG) and other effectors that control cellular functions.1,2 All ten mammalian Dgk genes are expressed in the brain, each with a distinct regional expression pattern.3,4 Many Dgk genes have been implicated in the regulation of neuron physiology and mouse behavior.3-6 Dgkb deletion and Dgkz knockdown were found to reduce spine formation and maintenance, respectively, and Dgkk knockdown reduced spine maturation and stability.7-9 Dgkb, Dgkz, Dgki, and Dgkk have each been shown to regulate synaptic plasticity.9-12 Alterations in Dgkb, Dgkz, Dgke, or Dgkk coincided with differences in lipid levels in various neuronal tissues.9,10,13,14 On the behavioral level, seizure susceptibility was enhanced by Dgkd knockdown and Dgkb knockout15,16 and reduced by Dgke loss.14 Dgkb−/− and Dgkh−/− male mice showed neurological phenotypes including hyperactivity and reduced depression,7,17 while Dgkk-deficient mice showed Fragile X syndrome-like social deficits and stereotypic behaviors.9 Therefore, changing the balance of lipids in this network by targeting various Dgk genes appears to influence neurophysiology.
DGKH and DGKI are linked to mental and cognitive disorders, including schizophrenia, bipolar disorder, depression, attention deficit hyperactivity disorder, and autism spectrum disorders.18-23 Recently, researchers examined the function of DGKH in male mice.17 Overall, they found that Dgkh-knockout males displayed mania-like behaviors that could be reversed with lithium treatment. Using Dgki-knockout mice24 others found that DGKI loss caused no changes in learning, anxiety, or motor phenotypes other than a slightly diminished habituation to an open field.12 Note that the sex of the mice in this latter study was not indicated.
Here, using a new Dgkh mutant line, we show that double knockout (dKO) females lacking expression of both Dgkh and Dgki have deficits in maternal care, resulting in poor offspring survival. The dKO females also demonstrate mania- and anxiety-like behaviors. These phenotypes were not seen in mice missing only Dgkh or Dgki. Overall, this work highlights the impact of disrupting both Dgkh and Dgki on mood-disorder-related phenotypes and on maternal behavior.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Mice
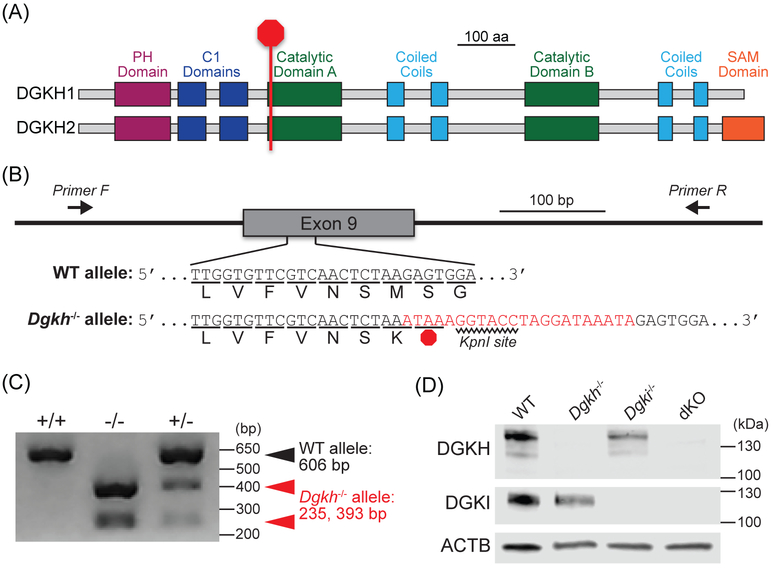
All procedures used in this study were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. We generated a Dgkh-knockout mouse using CRISPR/Cas9 technology.25 To arrest translation at the start of the first catalytic domain of DGKH (Figure 1A), we designed a guide RNA (GTGTTCGTCAACTCTAAGAGTGG) and a homology-directed repair donor template (TTTCGTTCTGTGTCAGCCCCCTCTTGGTGTTCGTCAACTCTAAATAAAGGTACCTAGGATAAATAGAGTGGAGATAATCAGGGAGTGAAGTTCCTTCGTCGCTTTAAA) to insert a three-frame stop cassette (bold) and KpnI site (bold underlined) in exon 9 of Dgkh (Figure 1B). Pronuclear injections of the guide RNA and donor template produced founder mice from which we cloned and sequenced Dgkh alleles to demonstrate successful integration of the stop cassette. Dgkh−/− mice present no gross anatomical or overt motor phenotypes.
Figure 1. Generation of Dgkh-knockout mice using CRISPR/Cas9.
A, Integration of a STOP cassette arrests translation at the start of the first catalytic domain of DGKH. PH = pleckstrin homology. C1 = cysteine-rich (diacylglycerol-binding). SAM = sterile alpha motif. B, The 22-base-pair cassette (red), containing a STOP codon and KpnI restriction site, was inserted into Exon 9 of the Dgkh gene. C, PCR amplification of tail DNA and digestion with KpnI. Without KpnI digestion, the amplified fragment from the Dgkh−/− allele is 628 bp. D, Western blot using 30 μg of protein isolated from cerebral cortices of WT, Dgkh−/−, Dgki−/−, and dKO female mice.
We acquired a Dgki−/− mouse line described previously,24 which was on a mixed 129 and C57BL/6 background. All data presented here from both the Dgki−/− and dKO lines were from mice that were backcrossed with C57BL/6J mice for at least five generations.
Mice were maintained on a 12 h:12 h light:dark cycle (lights on 7 AM to 7 PM) and given food (Teklad 2020X, Envigo, Huntingdon, United Kingdom) and water ad libitum. For maternal behavior experiments, timed matings were set up in the evening within 2 hours of the start of the dark cycle, using one male mouse and one or two female mice per breeding cage. The male mouse was separated from the female mice after 48 or 72 hours. Female mice were single-housed at least one week prior to giving birth. Pup retrieval experiments and observations of pup survival, weight, and milk spots were conducted in the evening within 3 hours of the start of the dark cycle. When handling pre-weanling mice, care was taken to rub gloves with used bedding from the home cage before touching the animals, particularly when removing pups for weighing or testing in the retrieval assay.
Because of the large number of animals required for the tests of psychopathological behaviors, the three genetic models (Dgkh−/−, Dgki−/−, and dKO) were tested in separate cohorts, each with an age-matched wild type (WT) cohort. For the elevated plus maze, open field, forced swim test, and acoustic startle and prepulse inhibition experiments (tested in that order), we tested 2- to 4-month-old virgin female mice during the second half of the light phase of their light:dark cycle. Mice were group-housed with 3 to 5 mice per cage. Mice were given at least 48 hours to recover after the elevated plus maze and open field assays, and at least one week to recover after the forced swim test. For these assays, 14 WT mice were tested with 14 Dgkh−/− mice; 15 WT mice were tested with 16 Dgki−/− mice; and 19 WT mice were tested with 19 dKO mice (only 14 mice of each genotype from the dKO cohort were tested in the acoustic startle and prepulse inhibition experiments). For the sucrose preference and sleep phenotyping experiments (tested in that order, separated by 1 week), we tested 2-month-old virgin female mice. The 9 WT and 8 dKO females were individually housed for both experiments; mice were group housed (3 to 5 mice per cage) before testing for sucrose preference and were again group housed with their original cage mates for the 1 week before assessing sleep patterns. For each of these experiments, outliers were identified using the interquartile range (IQR = Q3 – Q1). Any data points that were 1.5×IQR below Q1 or 1.5×IQR above Q3 were excluded.
2.2 ∣. Genotyping
We performed PCR with genomic DNA isolated from tail clips to confirm genotypes of our mice. Dgki was analyzed as described previously.24 A region of Dgkh containing exon 9 was amplified using primers 5’-GCAGATACTGAACCGTTTAGCCAG-3’ and 5’-CGCATGAGAGCAACAAAGATGTC-3’, on a PCR cycle that consisted of 35 rounds of 15 s of denaturing at 95°C, 15 s of annealing at 55°C, and 60 s of extending at 72°C. The products of this reaction were PCR purified and digested with KpnI (R0142, New England BioLabs, Ipswich, Massachusetts) and run on a 2% agarose gel containing SybrSafe (S33102, Invitrogen, Carlsbad, California; Figure 1C).
2.3 ∣. Western blotting
Frontal cerebral cortex tissue was dissected from WT, Dgkh−/−, Dgki−/−, and dKO female mice and lysed on ice for 10 min in a buffer containing 50 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA pH 8.0, 1% v/v Triton-X100, 1 mM phenylmethanesulfonyl fluoride, 1 mM sodium deoxycholate, 1× cOmplete Mini EDTA-free Protease Inhibitor Cocktail (4693159001, Roche, Basel, Switzerland). Following sonication on ice for 10 s, lysates were centrifuged at 10,000g at 4°C for 15 min to separate the debris. Protein (30 μg) from each lysate was separated on a 4-20% SDS/PAGE gel (456-1094, Bio-Rad, Hercules, California) and transferred to a 0.2 μm nitrocellulose membrane (1620095, Bio-Rad). Following 1 h of blocking in a solution of 5% w/v milk (170-6404, Bio-Rad) in tris-buffered saline with Tween 20 (100 mM Tris pH 7.5, 165 mM NaCl, 0.1% v/v Tween 20; TBST) at approximately 23°C, membranes were washed for 15-20 h at 4°C with primary antibodies for β-actin (1:3,000 mouse anti-ACTB, ab6276, Abcam, Cambridge, United Kingdom) and either DGKH (1:1,000 rabbit anti-DGKH, HPA040355, Sigma, St. Louis, Missouri) or DGKI (1:1,000 rabbit anti-DGKI, LS-C118721, Lifespan Biosciences, Seattle, Washington) in a solution of 5% w/v bovine serum albumin (A3912, Sigma) in TBST. Blots were probed with secondary antibodies of 1:10,000 IRDye 680RD-conjugated goat anti-mouse (925-68070, LI-COR Biosciences, Lincoln, Nebraska) and 1:10,000 IRDye 800RD-conjugated donkey anti-rabbit (926-32213, LI-COR) in 5% w/v milk in TBST for 2 h at approximately 23°C. Blots were imaged on a LI-COR Odyssey system and confirmed loss of DGKH and DGKI protein (Figure 1D).
2.4 ∣. Fostering
Within an hour after the birth of the last pup from dKO female litters, the dKO dam was removed and replaced with a foster WT dam. Foster WT dams had given birth to a litter no more than seven days prior to being used for fostering. The 8 litters came from 7 different dKO mothers and were fostered with 8 different foster dams.
2.5 ∣. Pup retrieval
To examine pup retrieval,26-28 in a cage with a dam and her litter, the pups were removed from the home cage and kept warm on a SpaceGel heating pad (Braintree Scientific, Braintree, Massachusetts). With the nest in one corner of the home cage, one pup was placed in each of the three remaining corners. The mother was then placed in the cage at the site of the nest, and her latency to retrieve each pup and place them in the nest was timed. This assay was only performed when litters had at least three live pups. Mothers were tested on the day of or day after birth of the litter. After 15 min, all pups were returned to the nest by the experimenter. At P0, 22 WT mothers were assay with 25 litters, and 12 dKO mothers were assayed with 14 litters. At P1, 18 WT mothers were assay with 21 litters, and 8 dKO mothers were assayed with 9 litters. No mother was tested with more than 2 of her litters.
2.6 ∣. Acoustic startle response and prepulse inhibition
To assess sensorimotor gating and startle reflex in these mice,29,30 mice were placed into an acrylic tube (7 cm long × 3.75 cm inner diameter) that was paired with a piezoelectric transducer that measured flinch responses. The tube and transducer were housed in a 29 cm3 sound-attenuating chamber with a light, fan, and speaker. Responses to a 40-ms, 120-dB acoustic stimulus were measured, alone or with a 20-ms prepulse tone played 100 ms preceding the 120-dB stimulus. Following a 5-min acclimation to the startle chamber, testing sessions were 10 min and consisted of 42 randomized trials, 6 trials each of the following 7 conditions: 1) no acoustic stimulus, 2) 120-dB startle tone alone, or 3) prepulse tone of 74, 78, 82, 86, or 90 dB followed by the 120-dB startle tone. The startle response was measured and analyzed with SR-LAB startle response system apparatus and software (San Diego Instruments, San Diego, California). The degree to which the prepulse tone inhibited the startle response to the 120-dB tone was calculated as: 100 – [(response to startle stimulus post-prepulse)/(response to startle stimulus alone) × 100]. Mouse weights were measured at the end of the session; however, neither Dgkh−/−, Dgki−/−, nor dKO mice differed in weight from their simultaneously-tested WT cohort, so we did not adjust the startle responses for weight in our analyses.
2.7 ∣. Forced swim test
To model depression (despair behavior) or mania (goal-directed behavior),31,32 activity was monitored in the forced swim test. Mice were placed into a 28 cm tall cylinder of 20 cm diameter filled to approximately 15 cm with 24-26°C water for 6 min. Activity was video recorded. The time spent immobile during the final 4 min in the chamber was tracked and scored using EthoVision XT 7.0 software (Noldus Information Technology, Leesburg, Virginia). Floating without actively swimming was counted as immobility. Animals were monitored during the experiment period to ensure their head stayed above the water.
2.8 ∣. Elevated plus maze
To model anxiety-like behavior,33 mice were placed into the 7.5 cm2 center of a 52 cm high elevated plus maze, the two closed and two open arms of which are each 30 cm long and 7.5 cm wide. The height of the walls of the closed arms was 20 cm, and the lip surrounding the open arms was 1.5 cm high. Activity of the freely-exploring mouse was monitored by a human observer for 5 min. Time spent in the closed or open arms or the center of the maze was measured. Entry into the center or any arm was scored when three of the animal’s paws crossed the threshold between each area.
2.9 ∣. Open field
To test for exploratory behavior and activity levels,34 mice were monitored in a 40 cm2 open field of 28 cm depth, enclosed in a box with a light above the open field, for 60 min. Activity was analyzed using the VersaMax animal activity monitoring system (AccuScan Instruments, Columbus, Ohio) to determine total distance moved horizontally in the entire arena, the number of vertical movements, and the total distance covered and total time spent in the 25 cm × 25 cm center region, binned in 5-min segments.
2.10 ∣. Sucrose preference
To test for depressed (anhedonia) or manic (reward-seeking) behavior,35,36 mice were assessed for their consumption of water and sucrose. Mice were individually housed in a cage with 2 identical water bottles for a 24-h acclimation period. After 24 h, each mouse cage and one control cage with no mouse was given 1 bottle of water and 1 bottle of 1% (w/v) sucrose in water. Each bottle was weighed before placing on the cages. The sucrose bottle was placed on the left or right at random. All cages were placed together on the same rack. Each bottle was weighed after 24 h to determine the amount of liquid consumed by the mouse or lost in the control cage. The amount of water or sucrose lost in the control cage was subtracted from the water or sucrose consumption by each mouse to determine water or sucrose intake, respectively. Sucrose preference was calculated for each mouse individually as: (sucrose intake)/(sucrose intake + water intake) × 100.
2.11 ∣. Sleep phenotyping
To survey sleep and wake patterns, mice were moved to a room with a 12 h:12 h light:dark cycle (lights on 8 AM to 8 PM) with a 1-h shift in Zeitgeber time (ZT) relative to their previous housing. Mice were given two full dark phases to acclimate before recording sleep data. No other mice were housed in the room during the acclimation and test periods. Sleep and wake behavior were monitored continuously for eight days with a non-invasive piezoelectric movement monitoring system described in detail previously.37-39 Animals were individually housed with bedding, food, and water in 15.5 cm2 cages with a pressure-sensitive detector pad below each cage that transmitted activity signals to monitoring software (PiezoSleep 2.0, Signal Solutions, Lexington, Kentucky). Activity signals were analyzed in sliding 2-s intervals, with activity patterns of approximately 3 Hz—representing regular respiration consistent with sleep—coded as sleep and irregular patterns coded as wake. Sleep-wake thresholds were calculated automatically for each individual based on decision statistics. Sleep and wake behavior were analyzed with customized statistics software (SleepStats, Signal Solutions).
2.12 ∣. Statistics
After processing by the software programs mentioned above, data were analyzed with Prism version 7.04 (GraphPad Software Inc., La Jolla, California). Survival curves of WT and dKO pups were compared with a log-rank (Mantel-Cox) test. The proportion of mothers retrieving all three pups was compared between genotypes with a binomial test. Open field metrics were compared between WT and other genotypes using two-way repeated measures analysis of variance (ANOVA), with Sidak's multiple comparisons tests used for pairwise comparisons within 5-minute time bins. All other assays were tested for significance using two-tailed t-tests with Welch’s correction to compare WT and Dgkh−/−, Dgki−/−, or dKO, or to compare dKO and dKO Fostered, in the case of litter survival.
3 ∣. RESULTS
3.1 ∣. dKO mice lack DGKH and DGKI protein
We confirmed DGKH and/or DGKI protein loss in brain tissue from Dgkh−/−, Dgkh−/−, and dKO female mice via immunoblotting (Figure 1D). To address the potential for compensation, we examined protein levels of DGKH or DGKI in Dgki−/− or Dgkh−/− mice, respectively. We found no upregulation of DGKH in Dgki−/− mice and no upregulation of DGKI in Dgkh−/− mice relative to WT mice (data not shown).
3.2 ∣. Poor survival rates of offspring raised by dKO mothers
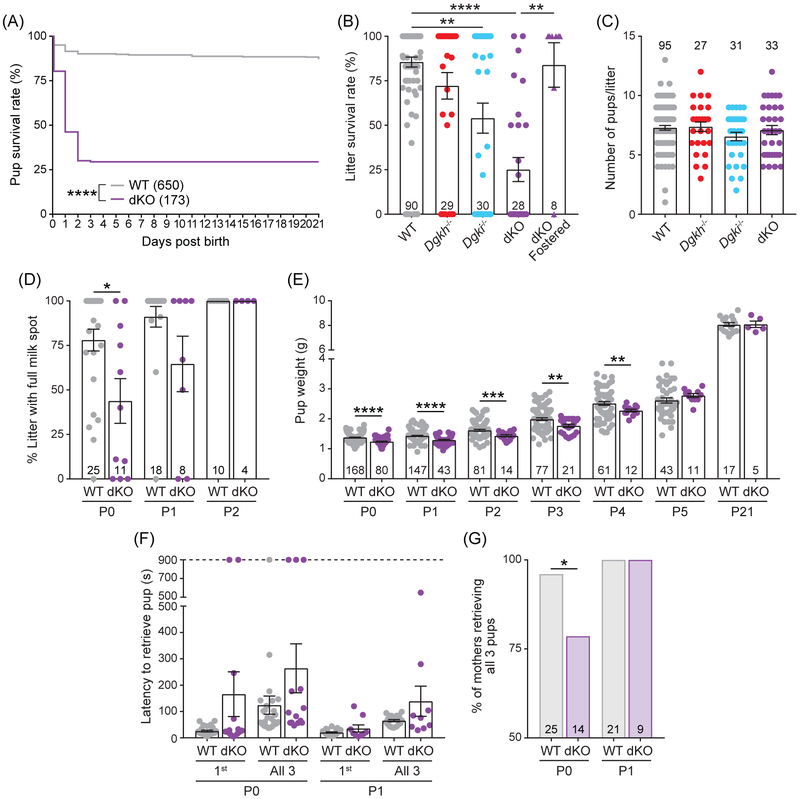
While maintaining WT, Dgkh−/−, Dgki−/−, and dKO mice, we found that offspring of dKO mothers showed a significant decrease in survival within the first two days after birth (Figure 2A). Pups raised by WT mothers had a survival rate of 87.1%, whereas dKO-raised pups had a 29.5% survival rate (Χ21 = 287.9, P < 0.0001). To evaluate the relative contribution of each Dgk gene to this phenotype, we examined survival rates of litters born from mothers of each genotype (Figure 2B). When raised by WT females, an average of 85.5% of the litter survived to weaning age, whereas dKO-raised litters had an average survival rate of 25.1% (t37 = 8.253, P < 0.0001). At 72.1% average survival, litters raised by Dgkh−/− did not significantly differ from WT litters. At 54.0%, litters raised by Dgki−/− mothers fared slightly worse than those raised by WT dams (t36 = 3.546, P = 0.0011), but the difference was most pronounced when both Dgkh and Dgki were deleted (dKO). The severity of this phenotype was not dependent on whether the dam was a new or experienced mother, the age of the mother, the genotype of the pup, or the size of the litter (data not shown).
Figure 2. Poor survival of offspring raised by dKO females.
A, Survival rate of pups born from dKO dams was significantly reduced after birth relative to those born from WT dams. B, The average proportion of each litter that survived to weaning was dependent on genotype of the mother. dKO fostered = litters born from dKO dams fostered with recently postpartum WT dams. C, Litter size on the day of birth based on the genotype of the mother. D, Percentage of slitter with a milk spot present, shown by genotype of the mother. E, Weight of pups raised by WT or dKO mothers. F, Time taken to retrieve the first pup and all three pups in the pup retrieval assay, based on the genotype of the mother. G, Percentage of mothers, tested in (F), that retrieved all three pups to the nest. Number of pups indicated on graphs in (A) and (E). Number of litters indicated on graphs in (B-D). Bars in (B-F) represent mean ± SEM. P < *0.05, **0.01, ***0.001, ****0.0001.
3.3 ∣. dKO dams show signs of deficient postnatal care
To determine if the poor survival rate was due to deficiencies in prenatal development or postnatal care, we fostered pups from dKO dams to recently postpartum WT dams. When fostered to a WT mother, an average of 83.9% of dKO-born pups survived (dKO Fostered; Figure 2B), which was a significant improvement over dKO-raised litters (t11 = 4.134, P = 0.0015). These data suggest dKO-born pups can nurse and grow normally when under the care of a WT mother. Moreover, the normal litter size at birth (Figure 2C) ruled out the possibility that pup survival was impaired prenatally.
3.4 ∣. Newborn offspring consume less milk when reared by dKO female mice
Since our data suggested poor pup survival was due to deficient maternal care, we next assayed an array of maternal behaviors. From observing their cages, we found that WT and dKO females made protective nests, with tall sides and top coverings that fully enveloped both the dam and the litter. The protective nests prevented the observation of nursing behavior directly, so we monitored the presence of milk spots in the pups to determine if the pups were consuming milk. Due to their transparent skin, milk in the stomach of newborn mice can be seen as a white spot on the abdomen. At P0, on average 43.8% of pups from dKO-born litters had visible milks spots, compared to litters from WT dams with an average of 78.0% (t15 = 2.451, P = 0.0270; Figure 2D). All dKO-raised pups had milk spots at P2, likely explaining why dKO pups that survived to P2 then survived to weaning. Moreover, the presence of milk spots ruled out the possibility that dKO females were unable to produce milk.
3.5 ∣. Offspring of dKO mothers gain weight slowly in early postnatal period
This transient phenotype at birth had a measurable impact on the body weight of mice raised by dKO mothers (Figure 2E). Pups raised by dKO dams weighed significantly less than those raised by WT dams at P0 (1.24 g and 1.38 g, respectively; t246 = 6.887, P < 0.0001), P1 (1.29 g and 1.43 g; t95 = 5.568, P < 0.0001), and P2 (1.43 g and 1.62 g; t35 = 3.83, P = 0.0005). The weight difference between dKO and WT pups began to normalize by P3 (1.77 g and 1.99 g, respectively; t52 = 3.101, P = 0.0031) and P4 (2.28 g and 2.51 g; t53 = 3.002, P = 0.0041). From P5 to P21 (typical weaning age) there were no significant differences in offspring weight based on maternal genotype. Weight gain was preceded by the presence of milk spots at P2 (Figure 2D) and coincided with a decrease in lethality (Figure 2A).
3.6 ∣. dKO mothers show variable pup retrieval behavior
Dams retrieve pups that have strayed from the nest. In this assay of this maternal behavior, the latency to retrieve three stray pups was tested in WT and dKO moms on the day of (P0) or the day after (P1) birth of a new litter (Figure 2F). The average latency to return stray pups to the nest was higher in dKO dams at P0 (not significant) relative to WT (263.9 s and 123.9 s, respectively) but was skewed by a subset of dKO mothers that failed to retrieve any pups during the entire 900-s assay period. Of the 14 P0 litters assayed with dKO mothers, 11 successfully retrieved all 3 pups (78.6%), compared to a success rate of 96.0% (24 out of 25) in trials with WT mothers (P = 0.0167; Figure 2G). All dams retrieved all three pups at P1 (Figure 2G), with comparable latencies between WT and dKO dams (Figure 2F).
During the pup retrieval assay, as well as while working with dKO females in the animal housing facility, we noticed that dKO females would dart around the cage, rear frequently, and not settle down like WT females, especially after opening or disturbing the cage. And, during the pup retrieval assay, the dKO females that failed to return all three pups to the nest (Figure 2F) displayed this manic-like exploratory behavior for the entire testing period. We speculate that this manic-like behavior might make it difficult for newborn pups to nurse, and hence could contribute to poor pup survival.
3.7 ∣. Loss of Dgkh and/or Dgki in females does not alter responses to startling acoustic stimuli
Given our findings above and the genetic linkage of DGKH and DGKI to mood disorders in humans, we next tested WT, Dgkh−/−, Dgki−/−, and dKO female mice with neuropsychiatric disorder-related behavioral assays. We used virgin females for these studies because it is not practical to generate large cohorts of age-matched and postpartum-time-matched females for behavioral studies. Additionally, since deficient maternal care of pups is known to impair behaviors of offspring in adulthood,40-44 the dKO females used in these behavioral studies were raised by WT foster moms.
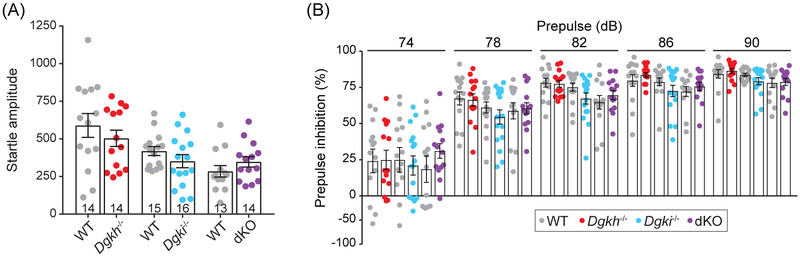
Deficits in prepulse inhibition are used to model sensorimotor gating, a symptom of schizophrenia.45 We found that responses to a startling acoustic tone of 120 dB did not differ based on genotype (Figure 3A), nor did the inhibition of the startle response when paired with a softer prepulse tone of varying loudness (Figure 3B). This suggests that startle reflexes and sensorimotor gating are not dependent on Dgkh and/or Dgki expression in female mice.29
Figure 3. Deletion of Dgkh and/or Dgki does not alter responses or habituation to acoustic startle tone in female mice.
A, Startle responses to a 120-decibel (dB) tone. B, The % decrease in startle response from (A) when the 120-dB tone was preceded by a non-startling tone of 74, 78, 82, 86, or 90 dB. Same mice were used in (A) and (B); number of mice indicated on graph in (A). Bars represent mean ± SEM.
3.8 ∣. dKO females exhibit decreased immobility in the forced swim test
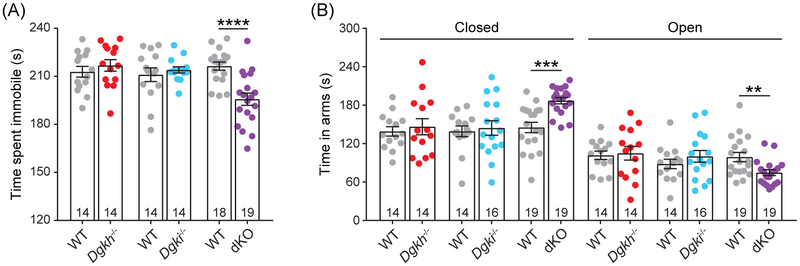
In the forced swim test, neither Dgkh−/− nor Dgki−/− females differed from WT females, but the dKO females showed decreased immobility relative to WT female mice (195.7 s and 216.4 s, respectively; t31 = 4.521, P < 0.0001; Figure 4A). Reduced immobility in this assay, i.e. enhanced escape drive, models the goal-directed vigor domain of mania.32
Figure 4. dKO females show mood-disorder-like phenotypes.
A, In the forced swim test, mice were placed in a large cylinder of water for six minutes. Time spent immobile (i.e. not swimming) was measured for the final four minutes. B, Time in closed arm versus aversive open arms of an elevated plus maze was measured in a five-minute period. Number of mice indicated on graphs. Bars represent mean ± SEM. P < **0.01, ***0.001, ****0.0001.
3.9 ∣. dKO females prefer the closed arms in the elevated plus maze
The elevated plus maze was used to test for anxiety-like behavior. In this test, we measured how much time mice spent in the protective closed arms versus the aversive open arms (Figure 4B). Deletion of Dgkh or Dgki alone had no effect on behavior in this assay; however, females lacking both Dgkh and Dgki spent more time in the closed arms of the maze (187.0 s for dKO and 145.2 s for WT; t29 = 4.409, P = 0.0001) and less time in the open arms (74.7 s for dKO and 99.0 s for WT; t30 = 2.86, P = 0.0076), considered a representation of anxiety-like behavior.33
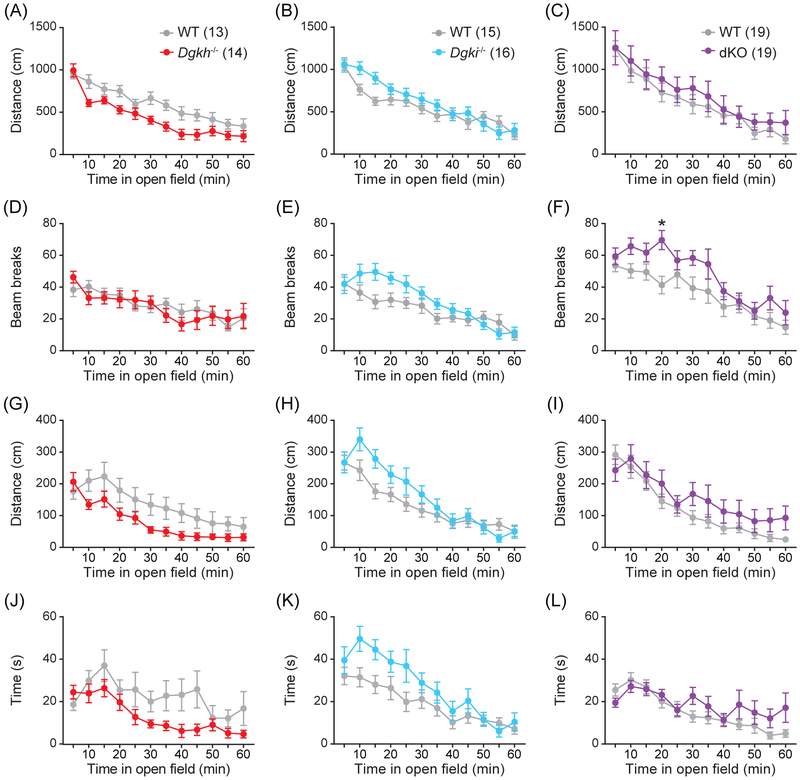
3.10 ∣. Loss of Dgkh and/or Dgki in females does not affect behavior in an open field
In the open field test, none of the three genetic mouse models showed differences in horizontal (Figure 5A-C) or vertical (Figure 5D-F) locomotion relative to WT females, apart from a slight increase in dKO rearing activity in a single 5-minute bin (t420 = 3.306, P = 0.0123; Figure 5F). Additionally, the distance traveled (Figure 5G-I) and time spent (Figure 5J-L) in the center of the open field did not significantly differ with genotype. The behavior of dKO females in the open field suggested that their increased activity in the forced swim test (Figure 4A) was indicative of mania-like behavior, not general hyperactivity,46 and the phenotype of the dKO females in the elevated plus maze (Figure 4B) represented anxiety and not simply hypo-exploratory behavior.47
Figure 5. Locomotion and center behavior in an open field were unaffected by Dgkh and/or Dgki loss in female mice.
In the 60-minute test, the total distance covered (A-C), vertical rearing activity (D-F), and the distance covered (G-I) and time spent (J-L) in the center of an open field were tracked. Number of mice indicated in graphs. Data represent mean ± SEM. P < *0.05.
3.11 ∣. Loss of both Dgkh and Dgki in females enhances sucrose preference
We performed additional behavioral tests to evaluate mania- and anxiety-like phenotypes in dKO females. Because neither Dgkh−/− nor Dgki−/− females differed from WT in previous assays, subsequent analyses were performed with only WT and dKO female mice.
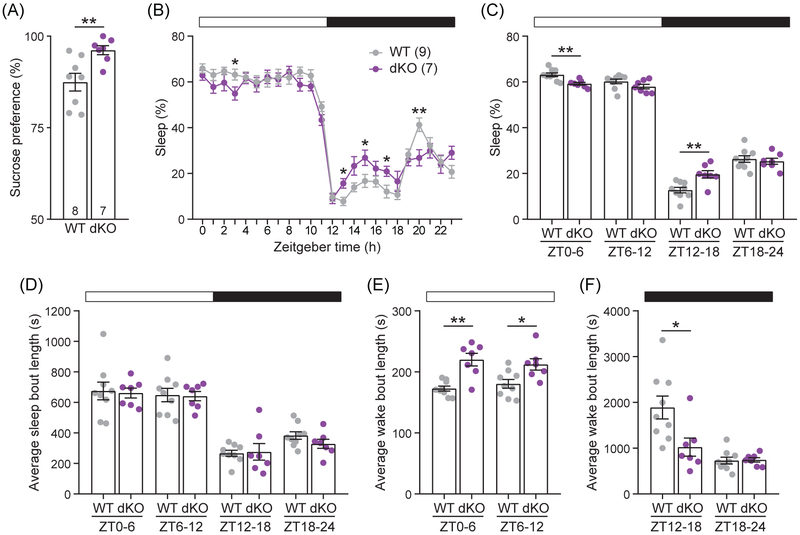
The degree to which mice prefer to drink a sucrose solution over water can indicate mood-related phenotypes. After monitoring water and sucrose consumption over 24 h, we found dKO female mice have a significantly greater preference for sucrose over water than WT females (96.2% vs. 87.5%, respectively; t10 = 3.216, P = 0.0089; Figure 6A), a phenotype analogous to the reward-seeking symptom of mania.36
Figure 6. Sucrose preference and sleep patterns were disrupted in dKO female mice.
A, The preference of a 1% sucrose solution over water was measured over 24 h. B-F, Wake and sleep behaviors were measured continuously for eight days, and the following measurements for each animal were calculated by averaging across days. The average percentage of time each mouse spent asleep was calculated in 1-h (B) and 6-h (C) bins over the 12-h light and 12-h dark phases. The average length of sleep bouts in the light and dark phases (D) and the average length of wake bouts in light (E) and dark (F) phases were calculated in 6-h bins. Number of mice indicated on graphs in (A) and (B). Same mice from (B) are represented in (C-F). Bars represent mean ± SEM. P < *0.05, **0.01.
3.12 ∣. dKO females have disrupted sleep patterns
Sleep is impaired in anxious and manic human patients as well as in rodent models of mania and anxiety.48-55 To determine the effect of Dgkh and Dgki loss on sleep, the patterns of wakefulness and sleep throughout the light and dark phases were measured continuously for eight days in WT and dKO female mice. The proportion of time dKO female mice spend asleep differs from WT mice during multiple 1-h blocks (Figure 6B) of both the light phase (ZT3, t13 = 2.223, P = 0.0451) and the dark phase (ZT13, t13 = 2.849, P = 0.0138; ZT15, t13 = 2.306, P = 0.0385; ZT17, t14 = 2.491, P = 0.0260; ZT20, t13 = 3.131, P = 0.0082). Larger trends in sleep patterns are visible in 6-h blocks (Figure 6C). Relative to WT, dKO females spend less of their time asleep during the first half of the light phase (59.3% vs. 63.2%, respectively, at ZT0-6; t14 = 3.825, P = 0.0019) but sleep more during the first half of the dark phase (19.7% vs. 12.8% at ZT12-18; t12 = 3.391, P = 0.0055).
Although there are variations in the total amount of time spent asleep (Figure 6C), the average length of individual sleep bouts did not differ (Figure 6D). However, the average wake bout was longer for dKO than WT mice throughout the light phase (Figure 6E) at ZT0-6 (220.0 s vs. 172.7 s, respectively; t8 = 4.263, P = 0.0028) and ZT6-12 (212.3 s vs. 180.4 s; t12 = 2.717, P = 0.0187) and was shorter in the first half of the dark phase at ZT12-18 (1024 s vs. 1889 s; t14 = 2.719, P = 0.0167; Figure 6F). Together, these data suggest that WT and dKO mice differ in the total proportion of time spent asleep because of differences in how long the mice stay awake between sleep bouts.
Mania is strongly linked to reduced sleep in humans and rodents.50,53,55 Disrupted sleep-related symptoms are connected to anxiety, although the direction of the disruption is ambiguous in anxious patients.48,49,51,52,55 Both fatigue and restlessness are diagnostic criteria of anxiety,55 and anxious patients report insomnia and hypersomnia.48,51,52 In dKO females, reduced sleep in the light phase—the phase in which the most sleep occurs—could either represent a decreased need for sleep (a symptom of mania) or a decreased ability to sleep (a symptom of anxiety). The subsequent increase in sleep in the dark phase may reflect fatigue or a lack of sufficient rest during the light phase, suggesting a more anxiety-like phenotype. Indeed, a mouse model of high anxiety lacked a light-phase sleep phenotype, but slept more during the dark phase.54 Overall, the light-phase sleep behavior more closely mimics rodent mania models,50,53 while the dark-phase sleep behavior more closely mimics anxious rodents.54
4 ∣. DISCUSSION
Here, we show that global loss of both Dgkh and Dgki in female mice causes anxiety-and mania-like behaviors—phenotypes not seen from loss of Dgkh or Dgki alone. These behavioral phenotypes were paired with a significant deficit in early maternal care. The results of the behaviors analyzed in the three genetic mouse models are summarized in Table 1. Poor offspring survival was paired with decreases in milk consumption and pup retrieval in dKO-raised litters. Anxious behaviors were seen in dKO female mice in the closed-arm preference in elevated plus maze and through disrupted sleep patterns. The dKO females demonstrated mania-like behavior through reduced immobility in the forced swim test, enhanced sucrose preference, and reduced light-phase sleep.
Table 1.
Summary of behavioral results for Dgkh−/−, Dgki−/−, and dKO female mice.
| Phenotype | Dgkh−/− | Dgki−/− | dKO |
|---|---|---|---|
| Offspring survival | = | ↓** | ↓**** |
| Offspring milk consumption @ P0 | NT | NT | ↓* |
| Pup retrieval @ P0 | NT | NT | ↓* |
| Acoustic startle response | = | = | = |
| Prepulse inhibition | = | = | = |
| Forced swim test: Immobility time | = | = | ↓**** |
| Elevated plus maze: Closed arm time | = | = | ↑*** |
| Open field: Total distance | = | = | = |
| Open field: Center distance | = | = | = |
| Sucrose preference | NT | NT | ↑** |
| Light phase sleep | NT | NT | ↓** |
| Dark phase sleep | NT | NT | ↑** |
Relative to WT females. “=” symbolizes no difference from WT. “NT” means mice of the indicated genotype were not tested for an individual assay. P < **0.01, ***0.001, ****0.0001.
In human patients, the co-occurrence of mania and anxiety is debated. Some symptoms of manic episodes (e.g. euphoric mood, high energy, and goal-directed behavior) oppose symptoms of anxiety (e.g. worrying, fatigue, impaired concentration).55 Additionally, many rodent models of mania have reduced anxiety.56,57 On the other hand, some of the symptoms of mania and anxiety overlap, such as irritability and aberrant sleep patterns.55,58 Anxiety disorders and bipolar disorder (BD) are highly comorbid59-63 and have significantly greater co-occurrence than anxiety disorders and unipolar depression,61,62 suggesting that mania may drive the BD-anxiety comorbidity. The prevalence of comorbid anxiety in BD patients in a manic state is ambiguous, as it has been found to be both higher64 and lower65 than in other BD patients. However, manic episodes strongly predicted the diagnosis of an anxiety disorder at a three-year follow-up.66 Because of the inconclusive evidence for the co-occurrence of mania and anxiety, and without testing all features of both disorders, we hesitate to suggest that the dKO female mice are models of anxiety and/or mania. These are complex human conditions that would be difficult to wholly represent in a mouse. Instead, we argue that dKO female mice model some features of anxiety and mania. As the comorbidity of anxiety and mania are under debate, these mice may be useful tools for analyzing how phenotypes of both conditions could present in the same individual.
While a detailed exploration of the molecular, cellular, and circuit-based mechanisms for these phenotypes is beyond the scope of this study, DGKH and DGKI are known to regulate multiple signaling pathways that have been linked to the phenotypes we uncovered. Activation of Gαq-protein-coupled receptors (Gαq-GPCRs) induces calcium release and production of DAG, together leading to neuronal activity.3,67 We previously found that overexpression of Dgkh in HEK293 cells prolongs Gαq-GPCR-stimulated calcium mobilization by attenuating the activation of protein kinase C (PKC).68 Using hippocampal slices from neonatal (2-week-old) Dgki-knockout mice, others found that metabotropic glutamate receptor-dependent long-term depression (mGluR-LTD) was dampened; although, they did not find this effect in adult tissue.12 Impaired mGluR-LTD (a Gαq-GPCR-dependent process) in the Dgki−/− mouse tissue required increased PKC activation, suggesting a mechanism analogous to that which we previously showed for DGKH. In addition to negatively regulating PKC, both DGKH and DGKI were found to positively regulate ERK signaling.24,69,70 Increased PKC activity has been shown in a mouse model of mania and anxiety71,72 and in bipolar manic patients,73 and PKC-null (Prkcg−/− and Prkce−/−) mice demonstrate reduced anxiety.74,75 ERK dysfunction leads to poor maternal care and mania- and anxiety-like behavior in rodents.27,76,77 Mood stabilizers that manage mania in human patients can decrease PKC activity73 or increase ERK signaling78 in neurons. Thus, loss of DGKH and DGKI has the potential to affect behavior by enhancing PKC activity and/or attenuating ERK activity.
PKC and ERK have many signaling targets that could contribute to impaired behavior in dKO female mice. PKC modulates the function of GABAA and 5-HT2A receptors,79,80 both of which regulate maternal behavior81,82 and anxiety.83,84 Altered PKC or ERK function resulting from DGKH and DGKI loss may induce behavioral changes by modulating phosphorylation of GSK3β,85,86 an enzyme implicated in the pathology of multiple mood disorders.87,88 Indeed, other researchers found that phosphorylation (i.e. inactivation) of GSK3β was decreased in brains of their Dgkh−/− male mice (a different mutant than the Dgkh−/− females used in this study), which showed manic behaviors.17 Further investigations are needed to determine which signaling pathways are disrupted in the brains of dKO female mice.
Based on gene expression data from the Allen Brain Atlas,89 Dgkh and Dgki have highly overlapping regional expression patterns in the brain.3 Dgkh and Dgki expression are enriched in the cerebral cortex, hippocampus, and striatum.3,90 Human and animal studies connect the pathology of both mania and anxiety to cortical and hippocampal function,91-96 whereas striatal function has been implicated in the regulation of nurturing behaviors in rodents.97-99 Given the known links between different brain regions and the phenotypes we have shown here, it is possible that Dgkh and Dgki loss could affect mood by altering hippocampal and cortical activity, while their effects on maternal behavior may arise in the striatum. On the other hand, other mouse models of deficient maternal behavior have likewise shown comorbid psychopathological phenotypes, suggesting potential overlap in the pathology behind these behaviors. Mice lacking the δ subunit of GABAA receptors showed poor maternal care, anxiety, and depression.81 Mice with loss of ephrin-A5 or central nervous system-specific ERK2 deletion had decreased nurturing behaviors and anxiety,27,100 and mice bred for low anxiety were less maternal than their high-anxiety counterparts.101 While these studies show variability in the phenotypes in each mouse model, they demonstrate that aberrant mood-related behaviors commonly coincide with disrupted maternal behavior in mice.
Parturition can induce depression and anxiety in mice and humans81,102,103 and, importantly, frequently exacerbates symptoms in patients with prepartum mood disorders.104 Having found that virgin dKO females show signs of anxiety and mania, an interesting future direction would be to investigate how parturition influences the mood-related phenotypes seen in dKO mice. Extensive research has demonstrated that receiving poor maternal care as a young rodent increases the likelihood of developing anxiety-like behaviors as an adult.40-44 However, the influence of a rodent’s mood on its ability to provide maternal care is underappreciated. One possible explanation of the poor survival of dKO-raised litters is that dKO females have exaggerated responses to stressful situations, including delivery or the presence of new pups in the cage, which cause them to neglect their offspring. Because the milk consumption and survival rate of dKO-raised pups improved after P2, the deficits in nurturing behavior by dKO dams may be specific to the early postpartum period. If symptoms of anxiety or mania impair maternal care, and mood-related symptoms worsen for a short period immediately after parturition, this may explain the apparent transience in the survival phenotype. Our data rule out the possibility that maternal neglect persists throughout the preweaning period, as survival of pups to dKO mice is not affected after P2.
Whereas the Dgkh−/− males tested by other researchers17 were generated via a different mutation than the Dgkh−/− female mice presented here, differences between these mouse models are interesting to note. Dgkh−/− male mice presented with manic behavior in the tail suspension test, reduced anxiety in the elevated plus maze, and slightly increased activity in the open field.17 None of these phenotypes were present in our Dgkh−/− female mice (using the forced swim test in place of tail suspension). Evidence of mutation- and sex-dependent variations in how Dgkh controls behavior suggests that both Dgkh−/− mouse lines may be useful tools for understanding the psychopathological conditions to which DGKH has been linked in human patients.
In summary, we found that dKO mothers showed poor nurturing behavior, evidenced by fewer milk spots, reduced weight, and impaired survival of dKO-raised litters. Additionally, we observed that dKO females—but not Dgkh−/− or Dgki−/− females—showed mania- and anxiety-like behaviors, without disrupting activity or exploration of a novel environment, startle reflexes, or sensorimotor gating. Our research using Dgkh−/−, Dgki−/−, and dKO female mice suggests that Dgkh and Dgki have functional compensation in each other’s absence, perhaps due to the high degree of overlapping expression. Further experiments are needed to understand the mechanism by which loss of these genes induces deficits in maternal care and manic and anxious behavior. Moreover, defining how phosphorylation of DAG by DGKH and DGKI modulates behavior may help to identify new pharmacological treatments for symptoms of mania and anxiety, particularly during the postpartum period.
ACKNOWLEDGEMENTS
We thank the Animal Models Core at the University of North Carolina at Chapel Hill for generating Dgkh−/− founder mice, Matthew Topham at the University of Utah for providing sperm from Dgki−/− mice, the Mutant Mouse Resource and Research Centers at the University of North Carolina at Chapel Hill for performing in vitro fertilization to recover the Dgki−/− mouse line, Sheryl Moy and Natallia Riddick of the Behavioral Phenotyping core (supported by NICHD, U54HD079124) for teaching us how to perform neuropsychiatric behavioral tasks, and Thomas Kash for sharing his equipment for the analysis of sucrose preference. This research was supported by the National Institute of Neurological Disorders and Stroke (R01NS081127, M.J.Z.) and (F31NS098600, V.B.B.) and by the Simons Foundation Autism Research Initiative (Bridge to Independence Award #385188, G.H.D.).
REFERENCES
- 1.Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409(1):1–18. [DOI] [PubMed] [Google Scholar]
- 2.Topham MK, Epand RM. Mammalian diacylglycerol kinases: molecular interactions and biological functions of selected isoforms. Biochim Biophys Acta. 2009;1790(6):416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tu-Sekine B, Raben DM. Regulation and roles of neuronal diacylglycerol kinases: a lipid perspective. Crit Rev Biochem Mol Biol. 2011;46(5):353–364. [DOI] [PubMed] [Google Scholar]
- 4.Shirai Y, Saito N. Diacylglycerol kinase as a possible therapeutic target for neuronal diseases. J Biomed Sci. 2014;21:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K, Yang J, Kim E. Diacylglycerol kinases in the regulation of dendritic spines. J Neurochem. 2010;112(3):577–587. [DOI] [PubMed] [Google Scholar]
- 6.Lee D, Kim E, Tanaka-Yamamoto K. Diacylglycerol kinases in the coordination of synaptic plasticity. Front Cell Dev Biol. 2016;4(92). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakefuda K, Oyagi A, Ishisaka M, et al. Diacylglycerol kinase beta knockout mice exhibit lithium-sensitive behavioral abnormalities. PLoS One. 2010;5(10):e13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frere SG, Di Paolo G. A lipid kinase controls the maintenance of dendritic spines. EMBO J. 2009;28(8):999–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabet R, Moutin E, Becker JAJ, et al. Fragile X Mental Retardation Protein (FMRP) controls diacylglycerol kinase activity in neurons. Proc Natl Acad Sci U S A. 2016;113(26):E3619–E3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirai Y, Kouzuki T, Kakefuda K, et al. Essential role of neuron-enriched diacylglycerol kinase (DGK), DGKβ in neurite spine formation, contributing to cognitive function. PLoS One. 2010;5(7):e11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo J, Kim K, Jang S, Han S, Choi SY, Kim E. Regulation of hippocampal long-term potentiation and long-term depression by diacylglycerol kinase zeta. Hippocampus. 2012;22(5):1018–1026. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Seo J, Nair R, et al. DGKi regulates presynaptic release during mGluR-dependent LTD. EMBO J. 2011;30(1):165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K, Yang J, Zhong X-P, et al. Synaptic removal of diacylglycerol by DGKζ and PSD-95 regulates dendritic spine maintenance. EMBO J. 2009;28(8):1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez de Turco EB, Tang W, Topham MK, et al. Diacylglycerol kinase ɛ regulates seizure susceptibility and long-term potentiation through arachidonoyl–inositol lipid signaling. Proc Natl Acad Sci U S A. 2001;98(8):4740–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leach NT, Sun Y, Michaud S, et al. Disruption of diacylglycerol kinase delta (DGKD) associated with seizures in humans and mice. Am J Hum Genet. 2007;80(4):792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishisaka M, Tsuruma K, Shimazawa M, Shirai Y, Saito N, Hara H. Increased seizure susceptibility in a mouse with diacylglycerol kinase β deficiency. Neurosci Med. 2013;04(02):117–122. [Google Scholar]
- 17.Isozaki T, Komenoi S, Lu Q, et al. Deficiency of diacylglycerol kinase eta induces lithium-sensitive mania-like behavior. J Neurochem. 2016;138(3):448–456. [DOI] [PubMed] [Google Scholar]
- 18.Baum AE, Akula N, Cabanero M, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13(2):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moskvina V, Craddock N, Holmans P, et al. Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Mol Psychiatry. 2009;14(3):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber H, Kittel-Schneider S, Gessner A, et al. Cross-disorder analysis of bipolar risk genes: further evidence of DGKH as a risk gene for bipolar disorder, but also unipolar depression and adult ADHD. Neuropsychopharmacology. 2011;36(10):2076–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griswold AJ, Dueker ND, Van Booven D, et al. Targeted massively parallel sequencing of autism spectrum disorder-associated genes in a case control cohort reveals rare loss-of-function risk variants. Mol Autism. 2015;6(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruderfer DM, Ripke S, McQuillin A, et al. Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell. 2018;173(7):1705–1715.e1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regier DS, Higbee J, Lund KM, Sakane F, Prescott SM, Topham MK. Diacylglycerol kinase ι regulates Ras guanyl-releasing protein 3 and inhibits Rap1 signaling. Proc Natl Acad Sci U S A. 2005;102(21):7595–7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Yang H, Shivalila Chikdu S, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153(4):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86(2):297–309. [DOI] [PubMed] [Google Scholar]
- 27.Satoh Y, Endo S, Nakata T, et al. ERK2 contributes to the control of social behaviors in mice. J Neurosci. 2011;31(33):11953–11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Storm DR. Maternal behavior is impaired in female mice lacking type 3 adenylyl cyclase. Neuropsychopharmacology. 2011;36(4):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swerdlow NR, Braff DL, Geyer MA. Cross-species studies of sensorimotor gating of the startle reflex. Ann N Y Acad Sci. 1999;877:202–216. [DOI] [PubMed] [Google Scholar]
- 30.Valsamis B, Schmid S. Habituation and prepulse inhibition of acoustic startle in rodents. J Visualized Exp. 2011(55):3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- 32.Flaisher-Grinberg S, Einat H. A possible utilization of the mice forced swim test for modeling manic-like increase in vigor and goal-directed behavior. J Pharmacol Toxicol Methods. 2009;59(3):141–145. [DOI] [PubMed] [Google Scholar]
- 33.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh RN, Cummins RA. The open-field test: a critical review. Psychol Bull. 1976;83(3):482–504. [PubMed] [Google Scholar]
- 35.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-Induced Anhedonia in Mice is Associated with Deficits in Forced Swimming and Exploration. Neuropsychopharmacology. 2004;29:2007. [DOI] [PubMed] [Google Scholar]
- 36.Flaisher-Grinberg S, Overgaard S, Einat H. Attenuation of high sweet solution preference by mood stabilizers: A possible mouse model for the increased reward-seeking domain of mania. J Neurosci Methods. 2009;177(1):44–50. [DOI] [PubMed] [Google Scholar]
- 37.Donohue KD, Medonza DC, Crane ER, O'Hara BF. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomedical engineering online. 2008;7:14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mang GM, Nicod J, Emmenegger Y, Donohue KD, O'Hara BF, Franken P. Evaluation of a piezoelectric system as an alternative to electroencephalogram/ electromyogram recordings in mouse sleep studies. Sleep. 2014;37(8):1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison JL, Rowe RK, Ellis TW, et al. Resolvins AT-D1 and E1 differentially impact functional outcome, post-traumatic sleep, and microglial activation following diffuse brain injury in the mouse. Brain Behav Immun. 2015;47:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. 2003;43(5):561–567. [DOI] [PubMed] [Google Scholar]
- 41.Weaver ICG, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103(9):3480–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen C, Vadlamudi S, Boccia M, Moy S. Variations in maternal behavior in C57BL/6J mice: behavioral comparisons between adult offspring of high and low pup-licking mothers. Front Psychiatry. 2011;2(42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishikawa J, Nishimura R, Ishikawa A. Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats. Eur J Neurosci. 2015;41(4):442–453. [DOI] [PubMed] [Google Scholar]
- 44.van der Kooij MA, Grosse J, Zanoletti O, Papilloud A, Sandi C. The effects of stress during early postnatal periods on behavior and hippocampal neuroplasticity markers in adult male mice. Neuroscience. 2015;311:508–518. [DOI] [PubMed] [Google Scholar]
- 45.Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull. 1987;13(4):643–668. [DOI] [PubMed] [Google Scholar]
- 46.Goto SH, Conceicao IM, Ribeiro RA, Frussa-Filho R. Comparison of anxiety measured in the elevated plus-maze, open-field and social interaction tests between spontaneously hypertensive rats and Wistar EPM-1 rats. Rev Bras Pesqui Med Biol. 1993;26(9):965–969. [PubMed] [Google Scholar]
- 47.Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134(1-2):49–57. [DOI] [PubMed] [Google Scholar]
- 48.Ford DE, Kamerow DB. Epidemiologic Study of Sleep Disturbances and Psychiatric Disorders: An Opportunity for Prevention? JAMA. 1989;262(11):1479–1484. [DOI] [PubMed] [Google Scholar]
- 49.Fuller KH, Waters WF, Binks PG, Anderson T. Generalized anxiety and sleep architecture: a polysomnographic investigation. Sleep. 1997;20(5):370–376. [DOI] [PubMed] [Google Scholar]
- 50.Naylor E, Bergmann BM, Krauski K, et al. The Circadian Clock Mutation Alters Sleep Homeostasis in the Mouse. The Journal of Neuroscience. 2000;20(21):8138–8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staner L. Sleep and anxiety disorders. Dialogues Clin Neurosci. 2003;5(3):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mellman TA. Sleep and Anxiety Disorders. Psychiatr Clin North Am. 2006;29(4):1047–1058. [DOI] [PubMed] [Google Scholar]
- 53.Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol. 2011;164(4):1263–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jakubcakova V, Flachskamm C, Landgraf R, Kimura M. Sleep Phenotyping in a Mouse Model of Extreme Trait Anxiety. PLoS One. 2012;7(7):e40625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 56.Roybal K, Theobold D, Graham A, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104(15):6406–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaltiel G, Maeng S, Malkesman O, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13(9):858–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suppes T, Eberhard J, Lemming O, Young AH, McIntyre RS. Anxiety, irritability, and agitation as indicators of bipolar mania with depressive symptoms: a post hoc analysis of two clinical trials. International Journal of Bipolar Disorders. 2017;5(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freeman MP, Freeman SA, McElroy SL. The comorbidity of bipolar and anxiety disorders: prevalence, psychobiology, and treatment issues. J Affect Disord. 2002;68(1):1–23. [DOI] [PubMed] [Google Scholar]
- 60.Henry C, Van den Bulke D, Bellivier F, Etain B, Rouillon F, Leboyer M. Anxiety disorders in 318 bipolar patients: prevalence and impact on illness severity and response to mood stabilizer. J Clin Psychiatry. 2003;64(3):331–335. [PubMed] [Google Scholar]
- 61.Dilsaver SC, Benazzi F, Akiskal KK, Akiskal HS. Differential patterns of lifetime multiple anxiety disorder comorbidity between Latino adults with bipolar I and major depressive disorders. Bull Menninger Clin. 2008;72(2):130–148. [DOI] [PubMed] [Google Scholar]
- 62.Schaffer A, Cairney J, Veldhuizen S, Kurdyak P, Cheung A, Levitt A. A population-based analysis of distinguishers of bipolar disorder from major depressive disorder. J Affect Disord. 2010;125(1–3):103–110. [DOI] [PubMed] [Google Scholar]
- 63.Hawke LD, Provencher MD, Parikh SV, Zagorski B. Comorbid Anxiety Disorders in Canadians with Bipolar Disorder: Clinical Characteristics and Service Use. The Canadian Journal of Psychiatry. 2013;58(7):393–401. [DOI] [PubMed] [Google Scholar]
- 64.Das A Anxiety disorders in bipolar I mania: prevalence, effect on illness severity, and treatment implications. Indian J Psychol Med. 2013;35(1):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yapici Eser H, Kacar AS, Kilciksiz CM, Yalçinay-Inan M, Ongur D. Prevalence and Associated Features of Anxiety Disorder Comorbidity in Bipolar Disorder: A Meta-Analysis and Meta-Regression Study. Front Psychiatry. 2018;9:229–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olfson M, Mojtabai R, Merikangas KR, et al. Reexamining associations between mania, depression, anxiety and substance use disorders: results from a prospective national cohort. Mol Psychiatry. 2016;22:235. [DOI] [PubMed] [Google Scholar]
- 67.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21(1):13–26. [DOI] [PubMed] [Google Scholar]
- 68.Rittiner JE, Brings VE, Zylka MJ. Overexpression of diacylglycerol kinase eta enhances Gaq-coupled G protein-coupled receptor signaling. Mol Pharmacol. 2014;85(5):800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yasuda S, Kai M, Imai S-i, et al. Diacylglycerol kinase η augments C-Raf activity and B-Raf/C-Raf heterodimerization. J Biol Chem. 2009;284(43):29559–29570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakano T, Iravani A, Kim M, et al. Diacylglycerol kinase eta modulates oncogenic properties of lung cancer cells. Clin Transl Oncol. 2014;16(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garzón-Niño J, Rodríguez-Muñoz M, Cortés-Montero E, Sánchez-Blázquez P. Increased PKC activity and altered GSK3β/NMDAR function drive behavior cycling in HINT1-deficient mice: bipolarity or opposing forces. Sci Rep. 2017;7:43468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varadarajulu J, Lebar M, Krishnamoorthy G, et al. Increased anxiety-related behaviour in Hint1 knockout mice. Behav Brain Res. 2011;220(2):305–311. [DOI] [PubMed] [Google Scholar]
- 73.Hahn CG, Friedman E. Abnormalities in protein kinase C signaling and the pathophysiology of bipolar disorder. Bipolar Disord. 1999;1(2):81–86. [DOI] [PubMed] [Google Scholar]
- 74.Bowers BJ, Collins AC, Tritto T, Wehner JM. Mice lacking PKCγ exhibit decreased anxiety. Behav Genet. 2000;30(2):111–121. [DOI] [PubMed] [Google Scholar]
- 75.Hodge CW, Raber J, McMahon T, et al. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cε. J Clin Invest. 2002;110(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ailing F, Fan L, Li S, Manji S. Role of extracellular signal-regulated kinase signal transduction pathway in anxiety. J Psychiatr Res. 2008;43(1):55–63. [DOI] [PubMed] [Google Scholar]
- 77.Engel SR, Creson TK, Hao Y, et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry. 2009;14(4):448–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen G, Manji HK. The extracellular signal-regulated kinase pathway: an emerging promising target for mood stabilizers. Curr Opin Psychiatry. 2006;19(3):313–323. [DOI] [PubMed] [Google Scholar]
- 79.Brandon NJ, Delmas P, Kittler JT, et al. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275(49):38856–38862. [DOI] [PubMed] [Google Scholar]
- 80.Bhattacharyya S, Puri S, Miledi R, Panicker MM. Internalization and recycling of 5-HT2A receptors activated by serotonin and protein kinase C-mediated mechanisms. Proc Natl Acad Sci U S A. 2002;99(22):14470–14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59(2):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao J, Wu R, Davis C, Li M. Activation of 5-HT2A receptor disrupts rat maternal behavior. Neuropharmacology. 2018;128:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nuss P Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat. 2015;11:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aznar S, Hervig ME-S. The 5-HT2A serotonin receptor in executive function: Implications for neuropsychiatric and neurodegenerative diseases. Neurosci Biobehav Rev. 2016;64:63–82. [DOI] [PubMed] [Google Scholar]
- 85.Goode N, Hughes K, Woodgett JR, Parker PJ. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992;267(24):16878–16882. [PubMed] [Google Scholar]
- 86.Ding Q, Xia W, Liu JC, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19(2):159–170. [DOI] [PubMed] [Google Scholar]
- 87.Jope RS, Roh M-S. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets. 2006;7(11):1421–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Latapy C, Rioux V, Guitton MJ, Beaulieu J-M. Selective deletion of forebrain glycogen synthase kinase 3β reveals a central role in serotonin-sensitive anxiety and social behaviour. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2012;367(1601):2460–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2006;445:168. [DOI] [PubMed] [Google Scholar]
- 90.Sommer W, Arlinde C, Caberlotto L, Thorsell A, Hyytia P, Heilig M. Differential expression of diacylglycerol kinase iota and L18A mRNAs in the brains of alcohol-preferring AA and alcohol-avoiding ANA rats. Mol Psychiatry. 2001;6(1):103–108. [DOI] [PubMed] [Google Scholar]
- 91.Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin North. 2009;32(3):549–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blanco E, Castilla-Ortega E, Miranda R, et al. Effects of medial prefrontal cortex lesions on anxiety-like behaviour in restrained and non-restrained rats. Behav Brain Res. 2009;201(2):338–342. [DOI] [PubMed] [Google Scholar]
- 93.Kheirbek MA, Drew LJ, Burghardt NS, et al. Differential control of learning and anxiety along the dorso-ventral axis of the dentate gyrus. Neuron. 2013;77(5):955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abé C, Ekman C-J, Sellgren C, Petrovic P, Ingvar M, Landén M. Manic episodes are related to changes in frontal cortex: a longitudinal neuroimaging study of bipolar disorder 1. Brain. 2015;138(11):3440–3448. [DOI] [PubMed] [Google Scholar]
- 95.Park J, Wood J, Bondi C, Del Arco A, Moghaddam B. Anxiety evokes hypofrontality and disrupts rule-relevant encoding by dorsomedial prefrontal cortex neurons. J Neurosci. 2016;36(11):3322–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee Y, Zhang Y, Kim S, Han K. Excitatory and inhibitory synaptic dysfunction in mania: an emerging hypothesis from animal model studies. Exp Mol Med. 2018;50(4):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav. 1991;39(1):71–77. [DOI] [PubMed] [Google Scholar]
- 98.Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30(1):46–64. [DOI] [PubMed] [Google Scholar]
- 99.Henschen CW, Palmiter RD, Darvas M. Restoration of dopamine signaling to the dorsal striatum is sufficient for aspects of active maternal behavior in female mice. Endocrinology. 2013;154(11):4316–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sheleg M, Yu Q, Go C, Wagner GC, Kusnecov AW, Zhou R. Decreased maternal behavior and anxiety in ephrin-A5−/− mice. Genes Brain Behav. 2017;16(2):271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kessler MS, Bosch OJ, Bunck M, Landgraf R, Neumann ID. Maternal care differs in mice bred for high vs. low trait anxiety: impact of brain vasopressin and cross-fostering. Soc Neurosci. 2011;6(2):156–168. [DOI] [PubMed] [Google Scholar]
- 102.O'Hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry. 1996;8(1):37–54. [Google Scholar]
- 103.C. R, K. S, M. B, et al. Prevalence, onset and comorbidity of postpartum anxiety and depressive disorders. Acta Psychiatr Scand. 2008;118(6):459–468. [DOI] [PubMed] [Google Scholar]
- 104.Katon W, Russo J, Gavin A. Predictors of postpartum depression. J Womens Health. 2014;23(9):753–759. [DOI] [PubMed] [Google Scholar]