Abstract
Background
Strain is a relative deformation and has three dimensions, in the left ventricle (LV) usually longitudinal (εL), transmural (εT) and circumferential (εC) strain. All three components can be measured generically by the basic systolic and diastolic dimension measures of LV wall length, wall thickness and diameter. In this observational study we aimed to study the relations of normal generic strains to age, body size and gender, as well as the interrelations between the three strain components.
Methods
Generic strains derived from dimension measures by longitudinal and cross-sectional M-mode in all three dimensions were measured in 1266 individuals without heart disease from the Nord-Trøndelag Health Study.
Results
The mean εL was −16.3%, εC was −22.7% and εT was 56.5%. Normal values by age and gender are provided. There was a gradient of εC from the endocardial, via the midwall to the external level, lowest at the external. All strains decreased in absolute values by increasing body surface area (BSA) and age, relations were strongest for εL. Gender differences were mainly a function of BSA differences. The three strain components were strongly interrelated through myocardial incompressibility.
Conclusions
Global systolic strain is the total deformation of the myocardium; the three strain components are the spatial coordinates of this deformation, irrespective of the technology used for measurement. Normal values are method-dependent and not normative across methods. Interrelation of strains indicates a high degree of myocardial incompressibility and that longitudinal strain carries most of the total information.
Keywords: cardiac function, echocardiography, epidemiology
Key questions.
What is already known about this subject?
Myocardial systolic strain is a relative deformation, but has been linked to complex ultrasound technologies as tissue Doppler and speckle tracking.
Strain is three-dimensional, but the three strain components have been linked to specific fibre directions in the heart.
Longitudinal strain has been shown to be age-dependent and body size-dependent.
What does this study add?
This study emphasises the simplicity of the strain concept and shows that strain is a basic deformation that can be measured by standard ultrasound methods, and is demonstrated in a large normal study, providing age-related and gender-related reference values.
It also emphasises that even with simple measures, actual strain values are dependent on basic definitions.
The paper clarifies that the three strain components in reality are spatial coordinates of the myocardial deformation as a single object.
The present study shows that transmural and circumferential strains are also age-dependent and body size-dependent, and that they are interdependent through a high degree of incompressibility of the myocardium.
How might this impact on clinical practice?
It shows that strain can be assessed by basic, vendor independent ultrasound tools, and contributes to the basic understanding of the physiology of myocardial deformation.
Due to the interdependence of the three strain components, the longitudinal strain may carry most of the information about global myocardial function.
The relations to age-related myocardial hypertrophy may be relevant also for other hypertrophic conditions with preserved ejection fraction.
Introduction
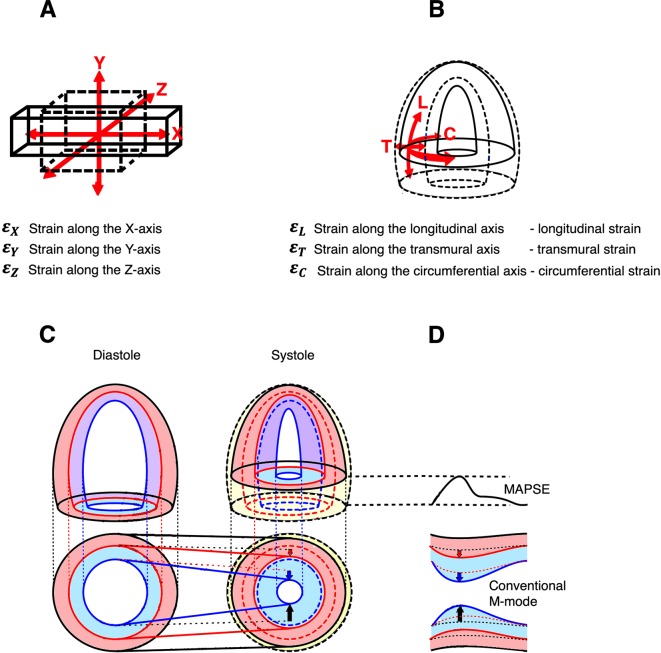
Left ventricular (LV) systolic myocardial strain (ε) is a relative systolic deformation: ε = (L−L0)/L0 = ΔL/L0, where L0 and L are the length before and after deformation, respectively. Thus, shortening is a negative and lengthening is a positive strain, usually given in per cent.1 LV systolic deformation is usually described as three normal components: longitudinal (εL), transmural (εT) and circumferential (εC) strain,2 as illustrated in figure 1. (‘Transmural strain’ is often termed ‘radial’, but this term is ambiguous, also meaning ‘in the direction of the ultrasound beam’.) εL is negative (longitudinal shortening), εT is positive (wall thickening) and εC is negative (circumferential shortening). Thus, all strains are basically given by simple end-systolic and end-diastolic dimension measurements, as shown in figure 2. Depending on the degree of myocardial compressibility, the three components interrelate; LV systolic shortening will result in LV thickening, which again will result in inward motion of both midwall and endocardial circumferences, as shown in figure 1. If the myocardium is totally incompressible, [εL + 1] × [εC + 1] × [εT + 1]=1, as shown in the online supplementary appendix 1. However, this has not, to our knowledge, been evaluated with echocardiography.
Figure 1.
Deformation in three dimensions. (A) Deformation in three dimensions in Cartesian coordinate system. A cube is deformed simultaneously in all three directions, in this case expanding (positive strain) along the x axis, and shrinking (negative strain) along the y and z axes. If the cube is incompressible, the three strain components are interrelated, so (1+ ) × (1 + ) × (1 + ) = 1. (B) Deformation in three dimensions of a hollow ellipsoid. In this case a coordinate system of longitudinal, transmural and circumferential strains is more convenient. The ellipsoid shortens in the longitudinal and circumferential directions (negative strain), and expands in the transmural direction. If the object is incompressible, the three strain coordinates are interrelated in the same way: (εL + 1) × (εC + 1) × (εT + 1) = 1. Thus it is evident that the three strain components are coordinates of the complete three-dimensional deformation of a single object. (C) Myocardial strains explained by the ellipsoid model. There is systolic shortening of the ventricular length (longitudinal strain, εL) and external circumference (external circumferential shortening). The total volume reduction is shown in yellow, and the changes in external contours in black. As the wall shortens, it must thicken in order to conserve the volume, depending on the degree of myocardial compressibility. The thickening is thus mainly a function of the longitudinal shortening. As the external contour decreases, the thickening has to occur inwards. External circumferential shortening will also, to a certain degree, push the wall inwards into more limited space, thus causing some thickening. The thickening of the external layer (red) will also displace the inner layer (blue) into a region with less space, so there is more thickening of the inner layer, due to both shortening and inward displacement. Thus, there is a gradient of wall thickening (transmural strain, εT) from the external to the inner layers. The thickening of the two layers and total thickening (black) is shown by the length of the arrows. The external circumferential shortening is a real contraction, but is only a partial contributor to the shortening of the inner circumference. As the outer layer thickens, the midwall circumference is pushed inwards, and thus shortens more due to the wall thickening, and as there is more thickening of the inner layer the endocardial circumference shortens even more, and thus there is also a gradient of circumferential strain (εC). The inward movement of outer (black), midwall (red) and endocardial (blue) circumferences are indicated by the unbroken straight lines. (D) Relation to M-mode measurements. Longitudinal shortening can be measured by mitral annular plane systolic excursion (MAPSE) and longitudinal strain derived by dividing by the wall length. Transmural strain is simply relative wall thickening, which is available from transverse M-mode, while circumferential shortening equals diameter shortening, that is, shortening—endocardial, midwall and external as explained in the text.
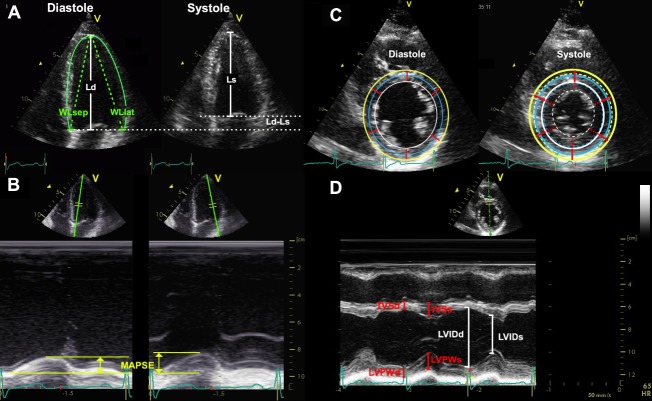
Figure 2.
Relations of the strains to the systolic deformation of the left ventricle. (A) Longitudinal shortening. Longitudinal strain can be measured by systolic and diastolic left ventricle (LV) lengths (Ls and Ld, respectively, white lines), εL = . (B) Absolute longitudinal shortening can be measured as the mitral annular plane systolic excursion (MAPSE) in each point (yellow lines), and divided by either Left ventricular diastolic length (LVd) or mean diastolic wall length (WLd)for calculation of longitudinal strain. As shown in the text Wall length (WL) should be mean of at least four walls, here is only shown the septal (WLsep) and lateral (WL lat). WL again can either be estimated by the straight lines from the apex to the annular point (dashed green lines). As these alternatives will give different lengths, and thus different denominators, the strain values will differ as well. (C) Transmural and circumferential strain. The wall thicknesses (red, straight lines) and the increase in thickness in systole (dotted red segments) are shown. This shows transmural strain to be a truly segmental measure, the quantitative equivalent of wall motion score. Outer (yellow), midwall (blue) and endocardial (white) circumferences are shown in the diastolic frame, and transferred to the systolic frame for reference. Systolic circumferences are shown in the same colours, as dotted circles. The circumferential strains can be seen to be related to outer circumferential shortening as well as wall thickening, and endocardial circumference can be seen to move most, external most. (D) As circumferences can be calculated from diameters, circumferential strains can be calculated from fractional shortening, calculated from endocardial diameters in systole (LVIDs) and diastole (LVIDd). Midwall and external circumferential strains were calculated from endocardial diameters and wall thicknesses, by addiand half and full wall thickeness of the septum in systole (IVSs) and diastole (IVSd) and posterior wall (LVPWs and LVPWd, respectively). They were not measured directly, as explained in the text.
openhrt-2019-001050supp001.pdf (270.9KB, pdf)
εL can be measured by simple length measurements,3 4 tissue Doppler,5 speckle tracking6–8 or a combination of both the latter.9 It has been shown to depend on age, gender and body size3 5–11 regardless of the method used, except in one larger meta analysis.12 However, gender difference seems to be due to body size.3 There is less information about the two other strain components in normal subjects. There is also a gradient of εC and εT across the myocardial wall, from the subendocardial layer to the subepicardial layer.13
The aims of the present normal study were first to evaluate the three normal strain components and their interrelations by a generic method, and second to study the relations of strains with age, gender and body size in a healthy population.
Methods
Study subjects
The study population was recruited from the Nord-Trøndelag Health Study (HUNT3) in Norway in the years 2006–2008 as previously described.9 The study group consisted of 1266 subjects, without evidence of heart disease, diabetes or hypertension, after exclusion of 30 subjects with abnormal echo findings. They were aged 19–89 years. The population has been extensively described previously.9 14 15 The basic characteristics are provided in table 1.
Table 1.
Basic measurements of the study population
| Women | Men | Total | |
| n | 663 | 603 | 1266 |
| Age (years) | 47.8 (13.5) | 50.5 (13.7) | 49.1 (13.7) |
| Height (m) | 1.65 (0.11) | 1.79 (0.07) | 1.72 (0.12) |
| Weight (kg) | 71.6 (14.0) | 87.1 (30.7) | 79.0 (24.7) |
| BMI (kg/m2) | 25.9 (4.2) | 26.8 (3.5) | 26.3 (3.9) |
| BSA (kg/m2) | 1.79 (0.16) | 2.05 (0.16) | 1.91 (0.20) |
| BP systolic/diastolic (mm Hg) | 127/71 (17/10) | 133/77 (14/10) | 130/74 (16/10) |
Measurements are mean (SD).
BMI, body mass index; BP, blood pressure; BSA, body surface area; n, number of subjects.
Echocardiography
Subjects were examined in the left lateral supine position with a Vivid 7 scanner (V.BT06, GE Ultrasound, Horten, Norway), with phased-array matrix transducers (M3S and M4S). One experienced echocardiographer (HD) did all the examinations, which included parasternal and all apical views. For each view, at least three consecutive cardiac cycles were recorded during quiet respiration. The mean B-mode frame rate was 44 frames per second. Diastolic wall length (WL) was measured as the straight lines from the epicardial apex to the mitral point in the six LV walls in diastole (figure 2A). Similarly, mitral annular plane systolic excursion (MAPSE) was measured from reconstructed longitudinal M-mode of the mitral annulus (figure 2B) in all six walls. Septal and posterior wall thicknesses in diastole (IVSd, LVPWd) and systole (IVSs, LVPWs) and diastolic and systolic LV internal chamber diameters (LVIDd and LVIDs) were measured in parasternal M-mode at the tip of the mitral leaflets (close to the papillary muscles) in end-diastole (figure 2D). Mean wall thicknesses in diastole and systole (WTd and WTs) were calculated as the mean of LVPW and IVS in diastole and systole, respectively.
Calculations and statistics
Calculations and statistics were performed in SPSS (v. 23, IBM corp). εL was calculated as MAPSE / WL (figure 2A,B) for each of the six walls and averaged into means of four (from two-chamber and four-chamber views) and six (from all three apical views) walls. MAPSE and εL by the generic strain method have been published previously,3 but are included here for the totality of the three-dimensional strain analysis.
Global εT was calculated as relative wall thickening, from WTd and WTs under assumption of a symmetric and circular left ventricle. Endocardial diameter was measured directly, and outer diameter (LVED) was calculated as LVED=LVID+IVS+LVPW both in diastole (LVEDd) and systole (LVEDs). Endocardial and external fractional shortening (FS) was calculated from internal and external diameters in diastole and systole in the ordinary way. Midwall FS was calculated from LVID + (2×1/2 WT) in systole and diastole, respectively. εC equals the negative value of fractional diameter shortening as shown in online supplementary appendix 1. Thus, εC was calculated as FS for external, midwall and endocardial εC.
Mean and SD are provided. Significance of differences between measures was tested by one-sample Student’s t-test, between genders by independent-samples t-test, and differences between age groups by one-way analysis of variance, with Bonferroni post-hoc comparisons. Correlations were tested by Pearson’s correlation and multiple linear regression. Repeatability of single measures in this population has been extensively studied previously.3 9 14–16 The myocardium is generally considered fairly incompressible. The product [εL + 1] × [εC + 1] × [εT + 1] was calculated for each subject, with mean, SD and SEM. Strain values are given as arithmetic values, but the relative magnitudes between strains as well as relations to age and body size are discussed in absolute (numeric) values.
Results
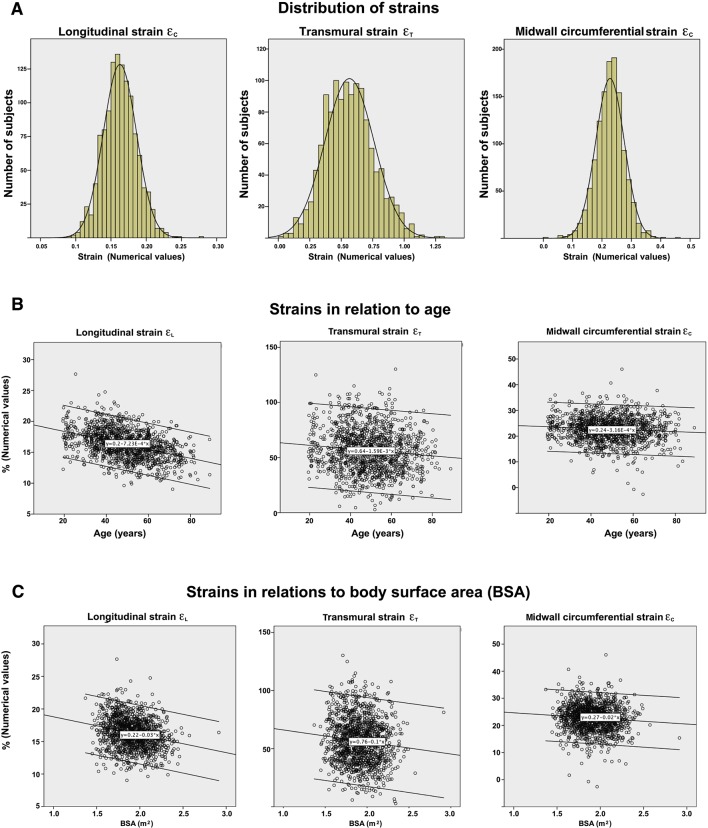
All three strain measures were near normally distributed, with skewness of 0.17, 0.28 and −0.27 for εL, εT and εC, respectively (figure 3A). Strain measurements according to age and gender are shown in table 2. εL by this method has been published previously3 but must be included for the analysis of the interrelations of strains. Variability, as seen by the relative SD, was highest in εT and lowest in εL.
Figure 3.
The distribution and relation with age and body surface area (BSA) of the three normal strains. Diagrams show numerical values. (A) Normal distribution of all the three strains (normal distribution curve is added for comparison) in the population. (B) Relations to age. Numerical values of all strains decrease with age. The effect was most profound for longitudinal strain. (C) Relations to BSA. Numerical values of all strains are inversely related to BSA, despite strain being relative to heart size. Again, the relation was strongest for longitudinal strain.
Table 2.
Mean (SD) longitudinal, transmural and circumferential strains from B-mode and M-mode recordings, all in %
| Age (years) | n | εL 4 walls |
εL 6 walls |
Septal εT | Inferolateral εT | Mean εT | Endocardial εC | Midwall εC | External εC |
| Women | |||||||||
| <40 | 208 | −18.1 (2.0) | −17.8 (2.4) | 45.8 (25.7) | 77.5 (29.4) | 61.7 (20.2) | −36.6 (6.1) | −23.9 (4.1) | −14.1 (3.3) |
| 40–60 | 336 | −17.0 (2.2) | −16.6 (2.2) | 44.6 (23.7) | 71.2 (27.6) | 57.9 (19.6) | −36.5 (6.9) | −23.2 (4.8) | −13.2 (4.2) |
| >60 | 119 | −14.8 (2.1) | −14.3 (2.4) | 43.7 (22.6) | 65.2 (30.4) | 54.5 (19.8) | −36.0 (9.1) | −22.3 (5.6) | −12.1 (4.2) |
| Total | 663 | −17.0 (2.4) | −16.6 (2.5) | 44.8 (24.1) | 72.2 (28.9) | 58.5 (19.9) | −36.4 (7.1) | −23.2 (4.8) | −13.3 (4.0) |
| Men | |||||||||
| <40 | 126 | −16.5 (2.0) | −16.6 (2.0) | 44.5 (19.9) | 68.3 (29.8) | 56.4 (19.1) | −35.5 (6.9) | −22.4 (4.6) | −12.6 (3.7) |
| 40–60 | 327 | −15.4 (1.9) | −15.0 (2.0) | 44.1 (22.6) | 65.2 (27.0) | 54.6 (19.7) | −35.8 (7.4) | −22.2 (4.9) | −12.2 (3.8) |
| >60 | 150 | −14.9 (1.9) | −14.3 (2.4) | 41.3 (18.8) | 62.2 (23.4) | 51.8 (16.4) | −36.0 (8.0) | −21.9 (5.2) | −11.8 (4.4) |
| Total | 603 | −15.5 (2.0) | −15.1 (2.2) | 43.5 (21.1) | 65.2 (26.8) | 54.2 (18.8) | −35.8 (7.5) | −22.2 (4.9) | −12.2 (3.9) |
| All | 1266 | −16.3 (2.4) | −15.9 (2.5) | 44.2 (22.7) | 68.9 (28.1) | 56.5 (19.6) | −36.1 (7.3) | −22.7 (4.9) | −12.8 (4.0) |
| Relative SD | 0.15 | 0.16 | 0.51 | 0.41 | 0.35 | 0.20 | 0.22 | 0.31 | |
Measurements are mean (SD).
εC, circumferential strain; εL, longitudinal strain; εT, transmural strain.n, number of subjects;
In summary, the mean εL was −16.3%, εC was −22.7% and εT was 56.5%. There was a gradient of εC from the endocardial, via the midwall to the external level (p<0.001). εT was higher in the posterior wall compared with the septum (p<0.001). The product [εL + 1] × [εC + 1] × [εT + 1]was calculated and was equal to 1.009 (SD=0.119, SEM=0.003) for εL averaged from four walls and 1.015 (SD=0.120, SEM=0.003) for εL from six walls. There were no relations of the strain product to age, gender or body surface area (BSA).
Relations of the strains to age and BSA are shown in figure 3B,C and table 3. In brief, there were independent, negative correlations of the absolute magnitude of all strains with both age and BSA. The correlations with age was r=−0.41, r=–0.11 and r=−0.09 for εL, εT and εC, respectively (all p<0.001).
Table 3.
Relations between strains, age and body size in multiple linear regressions
| Measure | r (univariate) | Β coefficient | P value | |
| εL | Age | −0.41 | −0.43 | <0.001 |
| BSA | −0.23 | −0.25 | <0.001 | |
| εT | Age | −0.11 | −0.12 | <0.001 |
| BSA | −0.11 | −0.11 | <0.001 | |
| Midwall εC | Age | −0.09 | −0.09 | 0.001 |
| BSA | −0.09 | −0.09 | 0.001 |
BSA, body surface area; εC, circumferential strain; εL, longitudinal strain; εT, transmural strain.
The difference between age groups was significant for all strains (p<0.01), but in post-hoc analysis the difference in εT between the middle and oldest age groups was only borderline significant (p=0.06).
All strains were lower (absolute values) with higher BSA, and all strains were higher in women compared with men (p<0.01), but in multiple linear regression with gender and BSA, only BSA was significant for any of the strains.
Endocardial εC showed no correlation with age, and there was no difference between age groups. For midwall εC, only the difference between the youngest and the oldest age groups was significant (p<0.05), while external εC was significantly different for all age groups (p<0.001 overall and p<0.05 for all pairwise comparisons). Correlations with age were r=−0.09 and r=−0.15 for midwall and external εC, respectively.
Systolic (SBP) and diastolic (DBP) blood pressure both showed a negative correlation with εL (r=−0.33 and r=−0.34, respectively, both p<0.001). SBP showed no correlations with εC or εT, and DBP was very modest, although with significantly (p<0.05) negative correlations of r=–0.08 and r–0.13, respectively.
Discussion
The following are the main findings of this study:
Under assumptions of LV symmetry, all three strain components can be measured generically by simple dimension measures.
There is a gradient of circumferential strain across the wall, being highest in the endocardial layer and lowest in the external layer.
All the three strains are interrelated and the interrelations indicate that there is little systolic compression of the myocardium.
Normal values by age and gender are provided. All three strain components are lower with higher age and BSA, but with the strongest association for longitudinal strain. Gender differences are explained by different BSA.
Myocardial strains
Importantly, strains are simple deformation measures, that is, relative systolic change in dimensions, not dependent on specific techniques as tissue Doppler or speckle tracking. Strain is a single deformation in three dimensions, and the separation of the three normal strain components is somewhat artificial. Strain is one single three-dimensional tensor, and εL, εT and εC are components of this single tensor, as discussed in online supplementary appendix 1. Thus, the strain components are simply the spatial coordinates of the three-dimensional deformation of the LV myocardium, as illustrated in figure 1. Most myocardial fibres are running in a spiral course, with varying angles with the major axes of the ventricle.17 Only few fibres are completely longitudinal or circumferential, and none are transmural. Thus, the individual strain components do not relate directly to specific myocardial fibre functions, and the total three-dimensional deformation is the resultant shortening of all fibres. While the individual myocardial fibre shortening is relatively uniform, the varying course of the fibres will result in different strains in different directions.
All strain measurements are method-specific. A simple generic method for εL was used in this study, as shown in figure 2A—other methods may differ even more. Thus, there is no universal ‘ground truth’ for strains, and the current guidelines do not (and should not) recommend normative values for strain.18 However, the relation between strain components and the relations to age and body size may be expected to be universal.
εL is the most extensively used and studied index of global LV function, assessed by averaging the longitudinal strain of all walls. MAPSE is usually measured only in two or four points of the mitral plane, from four-chamber and two-chamber views,19 20 but the difference was only 0.4% points if εL was assessed from four versus six walls (table 2).3 εL in the present study is similar both in method and results from the normal subjects in the study of Aurich et al.4 It is also in accordance with the values found in the present material by the segmental combined speckle tracking–tissue Doppler method.9 In the study by Aurich et al,4 the normal subjects showed higher strain values with speckle tracking, which seems to be the case for most speckle tracking studies.7 8 10–12 However, speckle tracking-derived εL varies between vendors21–23 as well. Recently, a collaborative work has unified some definitions,24 but the basic technical differences in the underlying algorithms for tracking nor the validation issues of these are not addressed. The relation using other technology, for instance cardiac magnetic resonance (CMR), has not been addressed. This is extensively discussed in a recent paper,25 showing that the problem is not solved yet.
εT is a regional measure of local, wall thickening. It is mainly a result of ventricular shortening, but external circumferential shortening will also contribute (figure 1). There is no real ‘radial function’ in terms of fibre shortening. In regional dysfunction, εT is a segmental, quantitative alternative to wall motion score (figure 2C). In the present study, findings are in accordance with older studies of wall thickening, both by M-mode26–29 and B-mode,30 as well as magnetic resonance and ultrasonomicrometry.31 The finding of a notable difference in transmural strain between the septum and the posterior wall is in accordance with older findings.30 The speckle tracking studies of Sun et al,7 Sugimoto et al11 and Yingchoncharoen et al12 show lower values of 42.6%, 37.4% and 47.3%, respectively. Kaku et al,8 on the other hand, found an εT of 88%, far in excess of normal wall thickening. The discrepancy of speckle tracking-based εT and normal wall thickening has not been discussed previously to our knowledge.
εC is the percentage circumferential shortening. Circumferential fibre shortening causes only the external εC of 12.8% in the present study. Wall thickening will push the midwall and endocardial circumferences inwards, and thus midwall and endocardial εC are increasingly a function of εT and hence εL (figure 1C). Inner layers are situated in a more limited space and will thicken more, causing the external to endocardial gradient of εC and εT, which has been observed previously13 and is evident in table 2. This is simply a function of geometry. Thus, εC is ambiguous, unless the level of measurement is defined. Midwall εC is most closely related to mean εC, the global measure. Assessing global εC from one FS measure, however, depends on the assumption of symmetry. The true global measure is the mean of three planes. Midwall εC in the present study is similar to εC in the speckle tracking studies of Sun et al7 and Yingchoncharoen et al.12 The NORRE study11 shows slightly higher numerical εC value of −31.9%, and the study of Kaku et al8 is in between.
[εL + 1] × [εC + 1] × [εT + 1] is ≈ 1. This has two implications. First, the three components are tightly interrelated in each individual. Most of the global deformation can be seen from εL alone, and it is dubious whether the other strain components add much information. Second, the strain product being so close to 1 would indicate that there is little systolic compression of the myocardium. However, incompressibility of the myocardium is an approximation only, as some degree of compression of capillaries and crypts is to be expected. In the studies of Sun et al,7 Sugimoto et al11 and Yingchoncharoen et al,12 this strain product (calculated from the reported means) was 0.87, 0.73 and 0.91. The cause for the differences was the higher numerical values (more negative) of εL, as well as the lower values of εT as discussed above. The study by Kaku et al8 gave 1.07, indicating systolic myocardial expansion, which is improbable and is due to the unrealistically high εT.
Relations to age, BSA and blood pressure
εL was inversely related to higher age as previously shown.3 5–11 An exception was one larger meta-analysis probably due to the heterogeneity of the studies included.12 We found all strain components to be dependent on age and BSA (figure 3B,C, tables 2 and 3). As εT and εC are related to εL, this was expected. However, in the NORRE study, age dependency was only shown for women with a decrease in εL and an increase in εC, but differences were small and not present in men.11
In our study, endocardial εC is preserved in increasing age, in line with what has previously been shown for FS.27–29 32 33 Age correction for FS is not recommended in current guidelines.18 In addition, the myocardial wall thickens with age.15 27–29 This also will result in a decrease in εT and εC, relative to wall thickness. Although the age-related reduction of εC is less than εL, the main point is that there was no compensatory increase in transverse or circumferential function with age in the present study. This has previously been offered as an explanation for preserved ejection fraction (EF) in the presence of declining longitudinal function with age.34 In that study, reduced longitudinal velocity and unchanged EF with age were found, and an increase in either radial or circumferential function was postulated. Interestingly, FS was not reported, despite being mentioned in the methods. Increased εC or εT is not a prerequisite for preserved EF. Preservation of EF with simultaneous reduction of all strains has also been demonstrated in other hypertrophic states even in heart failure with preserved EF as well.35–37 Thus, the findings may have relevance for other hypertrophic states with preserved EF.
We have previously shown in this material that even though chamber diameter remains unchanged, LV length decreases with increasing age,15 as has newer MRI studies.38 This is consistent with decreasing end-diastolic volume (EDV). The present guidelines report a decrease in both EDV and end-systolic volume (ESV) with age.18 This may in itself be a sufficient explanation for preserved EF, depending on the stroke volume (SV). The NORRE study39 reports a significant decrease in both EDV and ESV, which results in, on the average, increased SV and actually an increased EF. Reduced length and unchanged diameter will increase the sphericity of the cavity, but as this is caused by reduced length, not increased diameter, it does not result in increased FS (endocardial εC), and sphericity is not part of the strain calculations.
All strains were BSA-dependent (figure 3C and table 3), while gender dependency was simply a function of BSA. This negative correlation is a systematic error, as each strain component corrects for one dimension, while the strain is three-dimensional. εL correlated to both SBP and DBP, as shown also previously in this material by another method.14 Even though εL is afterload-dependent, the similar relation to both pressures is more consistent to a sensitivity to subclinical changes with increasing blood pressure, even in a presumably healthy population. This is seen also with other risk factors and discussed in detail earlier.14 The low/absent correlations with the two other strains probably reflect lower sensitivity of the two measures, reinforcing the view that εL is the most important measure.
Strengths and limitations
The main strength of the study is the size, being to our knowledge the largest normal strain study and one of the largest normal echocardiographic studies as well. Due to the large size, measurements are done with a limited set of simplified measurements, and thus with limited access to regional measurements. M-mode measures are prone to systematic errors. εL is measured by reconstructed M-mode. This means the temporal resolution is low, compared with real-time M-mode, but so is the B-mode frame rate, as are speckle tracking methods. Tissue Doppler-based εL will have about twice that. εT and εC from M-mode are real-time, that is, 500–1000 frames per second (FPS). Both, however, are based on M-mode through the septum and inferolateral wall only, relying heavily on assumptions of symmetry, excluding regional dysfunction. Findings in this study have less validity for any regional dysfunction that reduces annular motion. Angle dependency of measurements may also give a systematic overestimation of lengths, wall thicknesses and chamber diameters. Strains, however, will not be similarly affected, as the overestimation will be the same for systolic and diastolic measures and the relative difference (ie, strain) will be correct. Also, the motion of the base may tend towards an overestimation of especially external εC. The analyses are done by one single experienced analyser, ensuring homogeneity of data. The high reproducibility has been published previously.3 9 14–16 Finally, the study shows very little skewness in the strain data. As the conventional LV dimensions and FS in this material15 (data not shown) are in line with others, the population seems to be fairly representative.19 27–29 In line with others the M-mode-derived values in this study are slightly higher than normal values by two-dimensional echocardiography,18 39 which is customary for M-mode-derived values.
The population in the HUNT Study is ethnically homogeneous, but as the values are not intended to be normative, this is of less importance. As seen from table 1, the average BMI is at the upper normal limit, indicating that half the population is overweight. However, this is a common finding in many countries today, and compared with the general HUNT3 population the values for BMI were slightly lower in the echocardiographic substudy.40 Finally, as this is a cross-sectional study, the age differences are between cohorts and not related to true ageing.
The study does not address torsion. This is related to the limitation of the acquisitions, as this would need speckle tracking in multiple cross-sections. However, torsion is a shear strain (circumferential-transmural), not one of the three normal strains.
Finally, it must be emphasised that the present study addresses normal and age-related changes in global LV function only. Regional strain and strain rate, on the other hand, will add diagnostic information where they show patterns of uneven distribution.
Conclusions
Global systolic strain is the total deformation of a three-dimensional object; the three strain components are the coordinates of this deformation. Global strains in symmetric ventricles can be measured by simple generic measures, where circumferential strain is simply the negative value of FS. However normal values are not normative across methods. All strains decrease with age and there was no compensatory increase seen in circumferential or radial function to account for preservation of ejection fraction with increasing age. The three normal global strains are tightly interrelated, indicating a high degree of myocardial incompressibility. Thus, there seems to be little supplementary information from transmural and circumferential global strain compared with longitudinal strain.
Acknowledgments
The HUNT Study is a collaboration between the HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology (NTNU)), Nord-Trøndelag County Council and the Norwegian Institute of Public Health. We thank the people of Nord-Trøndelag for endurance and participation.
Footnotes
Twitter: @strain_rate
Contributors: AS and HD participated in the study design. HD has done the actual acquisitions. HD and HEM have done the measurements. AS has done the data analysis, interpretation and draft of the paper. All three authors participated in critical revision and approved the final manuscript. All authors accept responsibility for the final content.
Funding: The study was fully sponsored by the Norwegian University of Science and Technology as a PhD grant, as well as the HUNT Study providing the infrastructure for the echocardiography in the HUNT3 Study.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: All subjects gave written consent to participate in the study and the study was approved by the ethical committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1.Mirsky I, Parmley WW. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ Res 1973;33:233–43. 10.1161/01.RES.33.2.233 [DOI] [PubMed] [Google Scholar]
- 2.D'hooge J, et al. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr 2000;1:154–70. 10.1053/euje.2000.0031 [DOI] [PubMed] [Google Scholar]
- 3.Støylen A, Mølmen HE, Dalen H. Relation between mitral annular plane systolic excursion and global longitudinal strain in normal subjects: the HUNT study. Echocardiography 2018;35:603–10. 10.1111/echo.13825 [DOI] [PubMed] [Google Scholar]
- 4.Aurich M, Fuchs P, Müller-Hennessen M, et al. Unidimensional longitudinal strain: a simple approach for the assessment of longitudinal myocardial deformation by echocardiography. J Am Soc Echocardiogr 2018;31:733–42. 10.1016/j.echo.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 5.Kuznetsova T, Herbots L, Richart T, et al. Left ventricular strain and strain rate in a general population. Eur Heart J 2008;29:2014–23. 10.1093/eurheartj/ehn280 [DOI] [PubMed] [Google Scholar]
- 6.Marwick TH, Leano RL, Brown J, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging 2009;2:80–4. 10.1016/j.jcmg.2007.12.007 [DOI] [PubMed] [Google Scholar]
- 7.Sun JP, Lee AP-W, Wu C, et al. Quantification of left ventricular regional myocardial function using two-dimensional speckle tracking echocardiography in healthy volunteers-a multi-center study. Int J Cardiol 2013;167:495–501. 10.1016/j.ijcard.2012.01.071 [DOI] [PubMed] [Google Scholar]
- 8.Kaku K, Takeuchi M, Tsang W, et al. Age-Related normal range of left ventricular strain and torsion using three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr 2014;27:55–64. 10.1016/j.echo.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 9.Dalen H, Thorstensen A, Aase SA, et al. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr 2010;11:176–83. 10.1093/ejechocard/jep194 [DOI] [PubMed] [Google Scholar]
- 10.Menting ME, McGhie JS, Koopman LP, et al. Normal myocardial strain values using 2D speckle tracking echocardiography in healthy adults aged 20 to 72 years. Echocardiography 2016;33:1665–75. 10.1111/echo.13323 [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto T, Dulgheru R, Bernard A, et al. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2017;18:833–40. 10.1093/ehjci/jex140 [DOI] [PubMed] [Google Scholar]
- 12.Yingchoncharoen T, Agarwal S, Popović ZB, et al. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 2013;26:185–91. 10.1016/j.echo.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 13.Adamu U, Schmitz F, Becker M, et al. Advanced speckle tracking echocardiography allowing a three-myocardial layer-specific analysis of deformation parameters. Eur J Echocardiogr 2009;10:303–8. 10.1093/ejechocard/jen238 [DOI] [PubMed] [Google Scholar]
- 14.Dalen H, Thorstensen A, Romundstad PR, et al. Cardiovascular risk factors and systolic and diastolic cardiac function: a tissue Doppler and speckle tracking echocardiographic study. J Am Soc Echocardiogr 2011;24:322–32. 10.1016/j.echo.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 15.Støylen A, Mølmen HE, Dalen H. Importance of length and external diameter in left ventricular geometry. normal values from the HUNT study. Open Heart 2016;3:e000465 10.1136/openhrt-2016-000465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorstensen A, Dalen H, Amundsen BH, et al. Reproducibility in echocardiographic assessment of the left ventricular global and regional function, the HUNT study. Eur J Echocardiogr 2010;11:149–56. 10.1093/ejechocard/jep188 [DOI] [PubMed] [Google Scholar]
- 17.Streeter DD, Spotnitz HM, Patel DP, et al. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res 1969;24:339–47. 10.1161/01.RES.24.3.339 [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–71. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 19.Simonson JS, Schiller NB. Descent of the base of the left ventricle: an echocardiographic index of left ventricular function. J Am Soc Echocardiogr 1989;2:25–35. 10.1016/S0894-7317(89)80026-4 [DOI] [PubMed] [Google Scholar]
- 20.Höglund C, Alam M, Thorstrand C. Effects of acute myocardial infarction on the displacement of the atrioventricular plane: an echocardiographic study. J Intern Med 1989;226:251–6. 10.1111/j.1365-2796.1989.tb01389.x [DOI] [PubMed] [Google Scholar]
- 21.Farsalinos KE, Daraban AM, Ünlü S, et al. Head-To-Head comparison of global longitudinal strain measurements among nine different vendors. J Am Soc Echocardiogr 2015;28:1171–81. 10.1016/j.echo.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 22.Nagata Y, Takeuchi M, Mizukoshi K, et al. Intervendor variability of two-dimensional strain using Vendor-Specific and Vendor-Independent software. Journal of the Echocardiogr 2015;28:630–41. 10.1016/j.echo.2015.01.021 [DOI] [PubMed] [Google Scholar]
- 23.Mirea O, Pagourelias ED, Duchenne J, et al. EACVI-ASE-Industry standardization Task force. Intervendor differences in the accuracy of detecting regional functional abnormalities: a report from the EACVI-ASE strain standardization Task force. JACC Cardiovasc Imaging 2017. [DOI] [PubMed] [Google Scholar]
- 24.Voigt J-U, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015;16:1–11. 10.1093/ehjci/jeu184 [DOI] [PubMed] [Google Scholar]
- 25.Amzulescu MS, De Craene M, Langet H, et al. Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging 2019;20:605–19. 10.1093/ehjci/jez041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St John Sutton MG, Reichek N, Kastor JA, et al. Computerized M-mode echocardiographic analysis of left ventricular dysfunction in cardiac amyloid. Circulation 1982;66:790–9. 10.1161/01.CIR.66.4.790 [DOI] [PubMed] [Google Scholar]
- 27.Ganau A, Saba PS, Roman MJ, et al. Ageing induces left ventricular concentric remodelling in normotensive subjects. J Hypertens 1995;13:1818–22. 10.1097/00004872-199512010-00058 [DOI] [PubMed] [Google Scholar]
- 28.Gerstenblith G, Frederiksen J, Yin FC, et al. Echocardiographic assessment of a normal adult aging population. Circulation 1977;56:273–8. 10.1161/01.CIR.56.2.273 [DOI] [PubMed] [Google Scholar]
- 29.Knutsen KM, Stugaard M, Michelsen S, et al. M-mode echocardiographic findings in apparently healthy, non-athletic Norwegians aged 20-70 years. Influence of age, sex and body surface area. J Intern Med 1989;225:111–5. 10.1111/j.1365-2796.1989.tb00049.x [DOI] [PubMed] [Google Scholar]
- 30.Pandian NG, Skorton DJ, Collins SM, et al. Heterogeneity of left ventricular segmental wall thickening and excursion in 2-Dimensional echocardiograms of normal human subjects. Am J Cardiol 1983;51:1667–73. 10.1016/0002-9149(83)90207-2 [DOI] [PubMed] [Google Scholar]
- 31.Lima JAC, Jeremy R, Guier W, et al. Accurate systolic wall thickening by nuclear magnetic resonance imaging with tissue tagging: correlation with sonomicrometers in normal and ischemic myocardium. J Am Coll Cardiol 1993;21:1741–51. 10.1016/0735-1097(93)90397-J [DOI] [PubMed] [Google Scholar]
- 32.Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation 1980;62:1054–61. 10.1161/01.CIR.62.5.1054 [DOI] [PubMed] [Google Scholar]
- 33.Devereux RB, Lutas EM, Casale PN, et al. Standardization of M-mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol 1984;4:1222–30. 10.1016/S0735-1097(84)80141-2 [DOI] [PubMed] [Google Scholar]
- 34.Nikitin NP, Witte KKA, Ingle L, et al. Longitudinal myocardial dysfunction in healthy older subjects as a manifestation of cardiac ageing. Age Ageing 2005;34:343–9. 10.1093/ageing/afi043 [DOI] [PubMed] [Google Scholar]
- 35.Kraigher-Krainer E, Shah AM, Gupta DK, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63:447–56. 10.1016/j.jacc.2013.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizuguchi Y, Oishi Y, Miyoshi H, et al. Concentric left ventricular hypertrophy brings deterioration of systolic longitudinal, circumferential, and radial myocardial deformation in hypertensive patients with preserved left ventricular pump function. J Cardiol 2010;55:23–33. 10.1016/j.jjcc.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 37.Serri K, Reant P, Lafitte M, et al. Global and regional myocardial function quantification by two-dimensional strain: application in hypertrophic cardiomyopathy. J Am Coll Cardiol 2006;47:1175–81. 10.1016/j.jacc.2005.10.061 [DOI] [PubMed] [Google Scholar]
- 38.Hees PS, Fleg JL, Lakatta EG, et al. Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol 2002;90:1231–6. 10.1016/s0002-9149(02)02840-0 [DOI] [PubMed] [Google Scholar]
- 39.Kou S, Caballero L, Dulgheru R, et al. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging 2014;15:680–90. 10.1093/ehjci/jet284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Midthjell K, Lee CMY, Langhammer A, et al. Trends in overweight and obesity over 22 years in a large adult population: the HUNT Study, Norway. Clin Obes 2013;3:12–20. 10.1111/cob.12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2019-001050supp001.pdf (270.9KB, pdf)