CRISPR/Cas9-assisted recombineering is restricted in lactic acid bacteria because of the lack of available antibiotics and vectors. In this study, a seamless genome editing method was carried out in Lactobacillus plantarum using CRISPR/Cas9-assisted double-stranded DNA (dsDNA) and single-stranded DNA (ssDNA) recombineering, and recombination efficiency was effectively improved by endogenous adenine-specific methyltransferase overexpression. L. plantarum WCFS1 produced 797.3 mg/liter N-acetylglucosamine (GlcNAc) through reinforcement of the GlcNAc pathway, without introducing exogenous genes or plasmids. This seamless editing strategy, combined with the potential exogenous GlcNAc-producing pathway, makes this strain an attractive candidate for industrial use in the future.
KEYWORDS: Lactobacillus plantarum WCFS1, CRISPR/Cas9, recombineering, N-acetylglucosamine, metabolic engineering
ABSTRACT
Lactobacillus plantarum is a potential starter and health-promoting probiotic bacterium. Effective, precise, and diverse genome editing of Lactobacillus plantarum without introducing exogenous genes or plasmids is of great importance. In this study, CRISPR/Cas9-assisted double-stranded DNA (dsDNA) and single-stranded DNA (ssDNA) recombineering was established in L. plantarum WCFS1 to seamlessly edit the genome, including gene knockouts, insertions, and point mutations. To optimize our editing method, phosphorothioate modification was used to improve the dsDNA insertion, and adenine-specific methyltransferase was used to improve the ssDNA recombination efficiency. These strategies were applied to engineer L. plantarum WCFS1 toward producing N-acetylglucosamine (GlcNAc). nagB was truncated to eliminate the reverse reaction of fructose-6-phosphate (F6P) to glucosamine 6-phosphate (GlcN-6P). Riboswitch replacement and point mutation in glmS1 were introduced to relieve feedback repression. The resulting strain produced 797.3 mg/liter GlcNAc without introducing exogenous genes or plasmids. This strategy may contribute to the available methods for precise and diverse genetic engineering in lactic acid bacteria and boost strain engineering for more applications.
IMPORTANCE CRISPR/Cas9-assisted recombineering is restricted in lactic acid bacteria because of the lack of available antibiotics and vectors. In this study, a seamless genome editing method was carried out in Lactobacillus plantarum using CRISPR/Cas9-assisted double-stranded DNA (dsDNA) and single-stranded DNA (ssDNA) recombineering, and recombination efficiency was effectively improved by endogenous adenine-specific methyltransferase overexpression. L. plantarum WCFS1 produced 797.3 mg/liter N-acetylglucosamine (GlcNAc) through reinforcement of the GlcNAc pathway, without introducing exogenous genes or plasmids. This seamless editing strategy, combined with the potential exogenous GlcNAc-producing pathway, makes this strain an attractive candidate for industrial use in the future.
INTRODUCTION
Lactobacillus plantarum is a widespread member of the Gram-positive lactic acid bacteria (LAB) and is commonly found in fermented food and in the gastrointestinal tract. It is commonly used in the food industry as a potential starter probiotic, in the pharmaceutical industry for its health-promoting and anti-inflammatory properties, and as a delivery vehicle for therapeutic compounds (1). Comprehensive metabolic and genetic data have been intensively studied for this bacterium (2). Through metabolic engineering and synthetic biology, many species of lactic acid bacteria are now used as cell factories for value-added chemical production (3–5). However, genetic manipulation of L. plantarum is in its early stages, especially regarding the tools used for seamless engineering in the food and pharmaceutical industries.
In Gram-positive LAB, recombineering was initially developed using the traditional vector-based double-crossover method with endogenous RecA (6). Low vector excision efficiency and an inherent allelic replacement frequency made it difficult to screen prospective mutants using this method. Red/RecET-mediated double-stranded DNA (dsDNA) recombineering has greatly facilitated rapid and precise functional genomic manipulation in Escherichia coli and other microorganisms (7–10). However, applying the Red/RecET system in many bacteria other than E. coli has seen limited success (11, 12). Only in the last 2 years has a double-crossover method using counterselectable markers such as ddl or pheS* with the Red/RecET system or the temperature-sensitive plasmid pG+ host9 to efficiently mediate recombineering been developed in LAB (13, 14). Moreover, Yang et al. identified potential analogs of Gam, Beta, and Exo in L. plantarum WCFS1 and demonstrated their effectiveness in dsDNA recombineering (15). This dsDNA recombineering system has the advantage of easily screening mutants, including gene deletions and insertions. However, this method employs loxP/Cre to remove antibiotic genes. The phage protein Cre catalyzes site-specific recombination between its two loxP recognition sites. DNA sequences flanked by loxP sites are excised when the loxP sites are convergently oriented and inverted when the loxP sites are divergently oriented (16, 17). This leaves a lox scar on the genome. Recent studies in Lactobacillus casei developed a CRISPR/Cas9-assisted double-crossover system. Assisted by CRISPR/Cas9, the double-crossover method in L. casei can only be used for gene deletion/insertion (18).
In recent years, single-stranded DNA (ssDNA) recombineering that enables subtle modification of the genome (e.g., point mutations) has been developed in Lactobacillus reuteri and Lactococcus lactis (19, 20). When assisted by RecT (a prophage-derived ssDNA-binding protein)-mediated recombineering, the efficiencies of introduced mutagenesis ranged from 0.4 to 19%. Since ssDNA recombineering has been combined with the type II CRISPR system of Streptococcus pyogenes and is widely used for eliminating cells with unedited genomes (21, 22), recent studies in L. reuteri developed CRISPR/Cas9-assisted ssDNA recombineering. ssDNA recombineering in L. reuteri is highly efficient, but only for point mutations and short fragment deletions (<1 kb) (23).
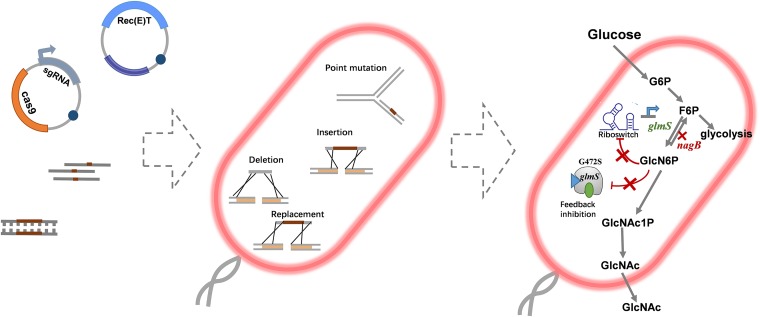
CRISPR/Cas9-assisted recombineering is restricted in LAB species, including L. plantarum, because of the lack of available antibiotics and vectors. A recent study developed Cas9-based editing methods for L. plantarum using two approaches, ssDNA recombineering and vector-based double-crossover for genome editing in different L. plantarum strains. The double-crossover method was only accomplished in strain WJL. In strain WCFS1, only a point mutation was achieved (24). In metabolic engineering, multiple genome manipulations must be achieved within one strain to acquire optimized target pathway flux. In this study, we developed a set of seamless genome manipulation methods via CRISPR/Cas9-assisted recombineering, comprising gene knockouts, insertions, and point mutations in L. plantarum WCFS1. Using prophage recombinase-based gene insertions and knockouts, introduction of DNA adenine methylase (Dam) effectively increased the point mutation recombination efficiency, and the whole method was applied to metabolic engineering. To validate the effectiveness of our methods, we modified the N-acetylglucosamine (GlcNAc) biosynthesis pathway. GlcNAc is a precursor of glycosaminoglycan, which plays an important role in maintaining healthy cartilage and joint tissue function (25). Thus, it is widely used as a food supplement for managing osteoarthritis (26, 27) and in the cosmetic and pharmaceutical fields (26). GlcNAc production via fermentation has been developed in E. coli (28) and Bacillus subtilis (29) but not in LAB. Until now, no probiotics have been reported to produce GlcNAc. GlcNAc can be synthesized from fructose-6-phosphate (F6P) by the following three enzymes: (i) GlmS, an l-glutamine–F6P aminotransferase, which catalyzes the conversion of F6P into glutamate and glutamine into glucosamine 6-phosphate (GlcN-6P); (ii) phosphoglucosamine mutase (GlmM), which catalyzes the interconversion of glucosamine 1-phosphate (GlcN-1P) and GlcN-6P; and (iii) GlcN-1P acetyltransferase (GlmU), which catalyzes the conversion of GlcN-1P and acetyl-coenzyme A (CoA) into N-acetylglucosamine-1-phosphate (GlcNAc-1P), which then can be hydrolyzed into GlcNAc (Fig. 1). Here, by using the editing method we developed, we designed and constructed a GlcNAc-producing L. plantarum engineered strain. By inactivating nagB and replacing the riboswitch and promoter in front of glmS1 and the point mutation of glmS1, GlcNAc was produced in the resulting strain without introducing exogenous genes or plasmids.
FIG 1.
The schematic diagram of CRISPR/Cas9-assisted seamless genome editing for N-acetylglucosamine metabolic engineering in Lactobacillus plantarum.
RESULTS AND DISCUSSION
Gene knockout via CRISPR/Cas9-assisted dsDNA recombineering.
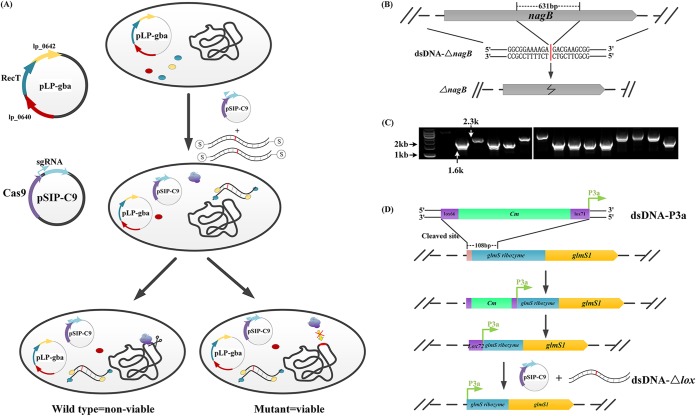
CRISPR/Cas9-assisted dsDNA recombineering was developed in L. plantarum WCFS1 as per our previous work. Genes lp_0642, lp_0641, and lp_0640, encoding an exonuclease analogue, a single-stranded annealing protein, and a potential host-nuclease inhibitor, respectively, were identified from a prophage P1 locus in L. plantarum WCFS1. Plasmid pLP-gba (15), harboring these three genes, was first transformed into cells to mediate effective homologous recombination between a heterologous dsDNA substrate and the host genomic DNA (15). Here, we used the CRISPR/Cas9 system to induce double-stranded breaks at the editing site and eliminate the unedited cells other than antibiotic markers and loxP/Cre (Fig. 2A). An artificially designed chimeric single guide RNA (sgRNA) (30) was adopted in this study to replace the complex and inconvenient trans-activating CRISPR RNA (tracrRNA) and CRISPR RNA (crRNA) duplex (23). A 631-bp DNA fragment of the nagB gene was knocked out. The nagB gene, which encodes glucosamine-6-phosphate (GlcN-6P) isomerase/deaminase, catalyzes the reverse reaction of fructose-6-phosphate (F6P) to GlcN-6P. A dsDNA was designed and named dsDNA-ΔnagB (1.5-kb fragment flanking the 631-bp deletion region; Fig. 2B). A protospacer-adjacent motif (PAM) site was selected in this nagB knockout fragment. An sgRNA was designed from this PAM. cas9 and the designed sgRNA sequence were cloned into plasmid pSIP403 (31) under the control of promoter-induced sppA (PsppA) and endogenous constitutive promoter 3a (P3a), respectively, forming pSIP-C9(nagB).
FIG 2.
Schematic of CRISPR/Cas9-assisted dsDNA recombineering. (A) Schematic diagram showing the CRISPR/Cas9-assisted dsDNA recombineering process. A strain harboring pLP-gba that expressed the recombination system was induced by sakacin P-inducing peptide (IP), followed by the cotransformation of pSIP-C9 and dsDNA fragments containing mutations or gene deletions. The recombination system then mediated allelic replacement, resulting in mutations or gene deletion. The Cas9 nucleases induced by IP and sgRNA were constitutively expressed. sgRNA guided Cas9 to the designated site and caused a double-strand break. Cells survived Cas9 cleavage when the protospacer-adjacent motif (PAM) site was modified by dsDNA recombineering, whereas unmodified cells did not survive. Differently colored dots represent different enzymes expressed from the correspondingly colored genes in plasmids. (B) Schematic diagram illustrating the seamless deletion of the nagB gene. (C) PCR verification result of nagB knockout using primers nagB-cf and nagB-cr. Wild type, 2.3 kb; knockout strain, 1.6 kb. (D) Insertion of promoter 3a in front of the glmS ribozyme by a two-step method. The result was verified by PCR using primers 3a-cf/3a-cr.
Initially, about 2 μg of the dsDNA substrate and 0.2 μg of the CRISPR/Cas9 vector were used for electroporation after inducing the pLP-gba-harboring cells via sakacin P-inducing peptide (IP). Approximately 12 colonies were obtained per plate, with a positive rate of approximately 25%. As a modification, phosphorothioate bonds were added to the 5′ ends of the dsDNA to protect against cleavage by intracellular exonucleases. This modification greatly improved the efficiency. In total, 45 colonies were obtained on the plate. We tested 15 colonies using colony PCR; among them, 8 were correct mutants (Fig. 2C). The positive rate was 53.3%.
Gene insertion via CRISPR/Cas9-assisted dsDNA recombineering.
For DNA insertion, we designed a one-step method to directly substitute/insert the DNA fragment on the genome without using resistance genes or loxP sites. The dsDNA recombineering was performed by cotransforming the CRISPR/Cas9 system and the dsDNA into L. plantarum expressing the three recombinases. However, we failed to directly substitute the DNA fragment on the L. plantarum WCFS1 genome using dsDNA recombineering. Very few strains grew, and no recombinant mutant was obtained. Thus, a two-step recombineering method was adopted. First, antibiotic-dependent dsDNA recombineering was performed to insert the target DNA fragment with the antibiotic gene and loxP site (Fig. 2D). After excising the antibiotic via the loxP/Cre system, we used the CRISPR/Cas9-assisted gene knockout method to remove the loxP site. The sgRNA-targeting loxP sites were cloned into plasmid pSIP-C9, forming pSIP-C9(lox). This method was used to insert promoter 3a in front of glmS1 for high expression, while the main functional area (108 bp, including the self-cleaved site) of the predicted glmS1 ribozyme was simultaneously deleted (32). The expression of glmS1 was enhanced and relieved from ribozyme cleavage. This method may be somewhat complex, but it is a practicable and efficient method for seamlessly inserting genes into L. plantarum. Because sgRNA targets the loxP site, CRISPR-expressing plasmids do not require rebuilding each time, making this method more convenient. The efficiency of the two-step gene insertion was also divided into two parts. The chloramphenicol resistance gene, loxP sites, and the 3a promoter were inserted with a positive rate of 82%. The second step was CRISPR-assisted seamless genome knockout of the loxP sites, in which 57 colonies were obtained on the plate; 24 of these were tested, and 14 were correct mutants as identified by colony PCR and sequencing, with a positive rate of 58.3% (Fig. S1).
Point mutations via CRISPR/Cas9-assisted ssDNA recombineering.
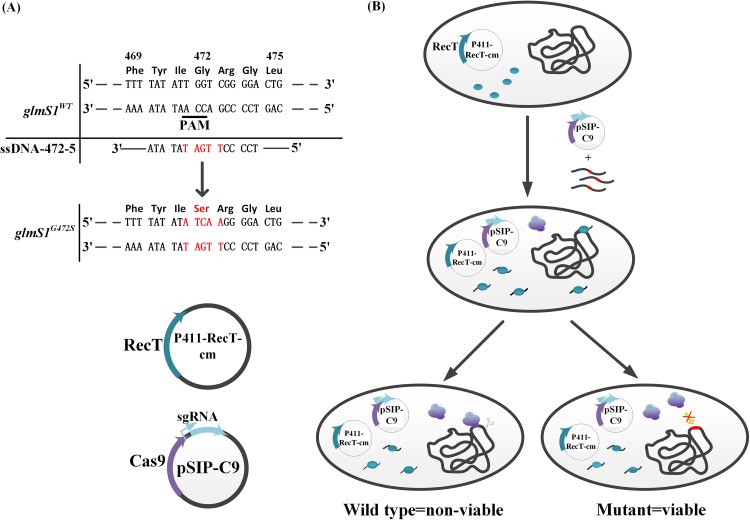
CRISPR/Cas9-assisted dsDNA recombineering without antibiotic markers performs poorly in point mutations. After cotransforming the point mutation dsDNA and CRISPR system, the positive rate was only approximately 1/20. Therefore, we attempted to develop an efficient point mutation method. The CRISPR/Cas9-assisted ssDNA recombineering system in L. reuteri has been successfully constructed (23). Thus, we used Lp0641, which has 46% identity with the RecT of L. reuteri, to mediate ssDNA recombineering. Gene Lp0641 was cloned into plasmid p411 to yield p411-RecT.
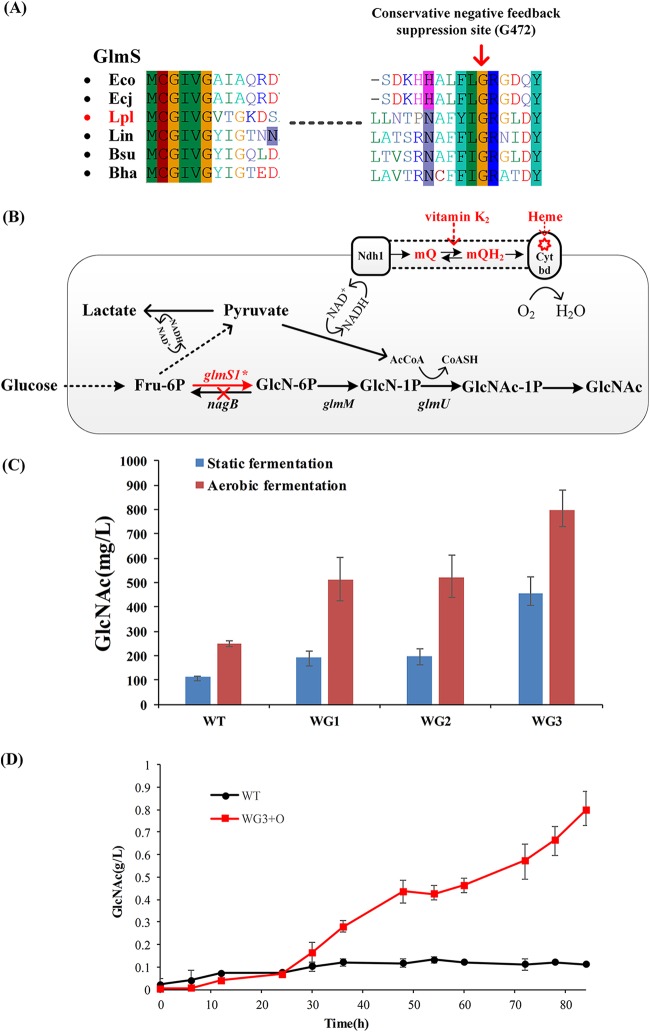
To validate this ssDNA recombineering method, we chose the glmS1 gene point mutation as an example. Several point mutations in GlmS of E. coli were reported to depress the feedback inhibition caused by GlcN-6P and improve the catalytic activity and solubility (33). Comparing the glmS amino acid sequences revealed that the amino acid was highly conserved in this alignment (see Fig. 5A), and G471S generated product resistance. Therefore, we chose this conserved site mutation (G472 in Lactobacillus plantarum WCFS1) to conduct recombineering using a designed ssDNA. The sgRNA was designed to target the PAM sequence NGG (underlined in Fig. 3A), which landed in site 472 of glycine. The G472S mutant avoided the PAM site, and thus the cell escaped Cas9 cleavage. ssDNA [35 μg; ssDNA-472-5 and pSIP-C9(472)] was electrotransformed into cells expressing p411-RecT. By sequencing the PCR amplicon, we obtained 32 monoclonal strains per plate, including 20 mutants, with a positive rate of near 62.5%.
FIG 5.
Increase of GlcNAc production by strain and process optimization. (A) Amino acid sequence comparison of GlmS to six other bacterial strains. In E. coli, G471S was proved to effectively relieve product resistance. By comparison of the amino acid sequences of glmS, G471 is highly conserved in these alignments. Therefore, we chose this conserved site (G472 in Lactobacillus plantarum WCFS1) mutation to conduct recombineering using a designed ssDNA. The G472 site is indicated by a red arrow. Organism abbreviations: Eco, E. coli K-12 MG1655; Ecj, E. coli K-12 W3110; Lpl, L. plantarum WCFS1; Lin, Listeria innocua (serotype 6a); Bsu, Bacillus subtilis subsp. subtilis 168; Bha, Bacillus halodurans. (B) Optimized metabolic pathways for GlcNAc production. Optimization was accomplished via mutation of glmS1 and knockout of nagB. Heme and menaquinone were external sources and are shown in red. (C) Fermentation production of GlcNAc in each individual engineered strain. Aerobic fermentation was conducted in MRS medium at 37°C and 220 rpm, and pH was maintained at approximately 6.5. Results are averages from three independent experiments. (D) Fermentation profiles of wild-type (WT) L. plantarum WCFS1 under anaerobic conditions and WG3 under aerobic conditions.
FIG 3.
CRISPR/Cas9-assisted ssDNA recombineering in Lactobacillus plantarum WCFS1. (A) The gene sequences of wild-type glmS1 and mutant glmS1G472S are shown as two alphabetic strings. The ssDNA as lagging strand is shown under the genome of the wild type, using an alphabetic string and solid line, and the mutated bases are marked in red. (B) The operation process of ssDNA recombineering is similar to that of dsDNA recombineering; only the recombinase and exogenous DNA are different. The strain harboring plasmid p411-recT-cm expresses RecT protein induced by IP, followed by the cotransformation of the plasmid expressing Cas9 and sgRNA by the inducing of IP along with the ssDNA fragment containing desired mutations. Then, the single-strand annealing protein RecT bonds and protects the ssDNA from degradation by host nucleases and promotes annealing of cDNA strands. The sgRNA guides the Cas9 to the designated site and causes a double-strand break. Strains whose genomes are modified by ssDNA recombineering survive the Cas9 incision, while those not modified by ssDNA recombineering are nonviable. The result was verified by PCR using primers 472-cf and 472-cr. Sequencing results are shown in Fig. S2.
Redesign of ssDNA recombineering using adenine-specific methyltransferase.
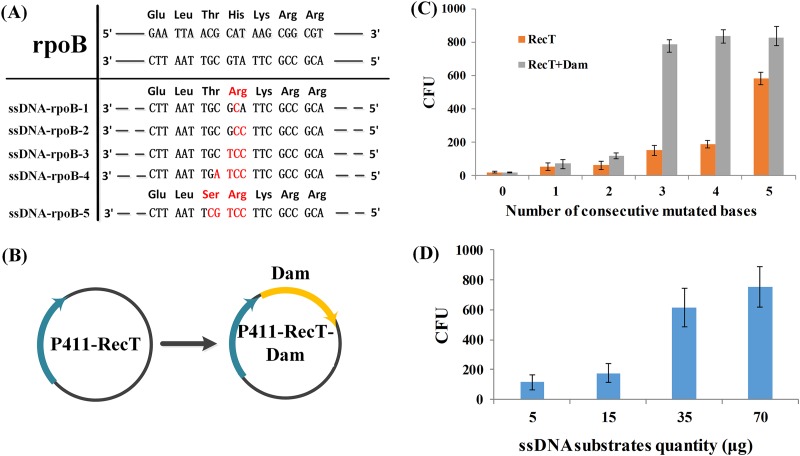
The CRISPR/Cas9-assisted ssDNA recombineering efficiency has been designed to be improved. Mutation of DNA-directed RNA polymerase (rpoB) (H487R or T486S/H487R) yielded a rifampin-resistant strain (34). Thus, the rpoB gene was targeted for point mutation through ssDNA recombineering, and the rifampin-resistant recombinants represent the recombination efficiency. Recombineering efficiency is often affected by the methyl-directed mismatch repair system (MMR) in E. coli; however, multiple adjacent mismatches can avoid the MMR. Thus, a series of ssDNAs were designed (Fig. 4A) with one to five consecutive mismatches. Five consecutive mismatches had the best recombination efficiency among the tested mismatches and obtained 595 rifampin-resistant colonies. The number of colonies with five consecutive mismatches was 5-fold higher than the number of colonies with the other consecutive mismatches (Fig. 4C). We also tried the designed ssDNA with interval mismatches, but with less recombination efficiency (Fig. S3).
FIG 4.
The optimization design of ssDNA recombineering. (A) The ssDNA with various mutant bases (marked in red) was used to mutate the rpoB gene on the genome, resulting in a rifampin-resistant phenotype. The number of consecutive mismatches from 1 to 5 was designed. (B) The DNA adenine methylase gene dam was cloned into vector p411-RecT-cm to yield p411-RecT-Dam-cm. The promoter of Dam is the same as that of RecT and is induced by IP. (C) The efficiency of ssDNA recombineering resulting in various mismatches is assumed by the CFU of mutants with rifampin resistance. The 5 consecutive mismatches are the most efficient when only expressing RecT. With the help of the methylase Dam, efficiency was obviously enhanced, especially that with 3 consecutive mismatches. (D) The recombination efficiency improved as the ssDNA concentration increased.
Consecutive nucleotide mismatches of greater than 5 bases can improve efficiency, while in some circumstances, fewer nucleotide changes are needed (e.g., 1, 2, or 3). Previous reports showed that the destroyed MMR increased the ssDNA recombineering efficiency, but the strain with defective MMR had approximately 1,000-fold-higher spontaneous mutation rates than the wild type (35, 36). Hong et al. used conditionally activated mutations of the MMR proteins MutS and MutL to generate temperature-sensitive mismatch repair activity (37). Thus, recombineering can be achieved by adjusting the temperature. A recent report showed that DNA adenine methylase (Dam) may improve ssDNA recombineering efficiency (38). Therefore, the L. plantarum WCFS1 endogenous adenine-specific methyltransferase subunit gene (lp_2243) was cloned into plasmid p411-RecT under the control of the sakacin P-inducing peptide, resulting in plasmid p411-RecT-Dam. The results showed that Dam remarkably improved the mutation efficiencies of ssDNA recombineering of all five consecutive mismatches, especially that of the ssDNA with three consecutive mismatches (Fig. 4C). Dam overexpression can transiently disable MMR during induction because Dam binds the mismatched bases or its adjacent bases faster than does MMR. Thus, the MMR could not repair the mismatched bases in the methylated DNA chain (28).
We suspected that different bases have different mismatch repair efficiencies. The fourth base of the four consecutive mismatches in ssDNAs is a degenerate base; therefore, we designed ssDNAs in which the fourth base is A, G, C, or T (Fig. S4A). After transforming the ssDNA, colonies growing on rifampin plates were counted to obtain the mutation frequency toward different bases. The highest mismatch efficiency of the fourth base was A, for which approximately 800 colonies were obtained on the rifampin-resistant plates, followed by C, T, and G, with 710, 430, and 330 rifampin-resistant colonies, respectively. We then determined the mismatch efficiency of different bases at other sites. We designed an ssDNA named ssDNA-rpoB-3+N (N = A, G, C, or T) to calculate the mismatch efficiency of this site. The mismatch efficiency of base C was the highest, while that of base G was the lowest (Fig. S4B).
There are eight possible mismatch pairs. Costantino and Court reported that the efficiency of the eight mismatches was retained in the following order: G·T, A·C, A·A, G·G < T·T, T·C, A·G < C·C (39). Our experiment was consistent with this result, where the C·T mismatch was the most easily retained, with the highest recombination efficiency among these mutations. Therefore, if conditions permit, the mutation efficiency can be effectively increased by changing the mismatched base to one more likely to be retained.
To further verify the function of Dam, GlmS1 G472S was again chosen as the recombineering target. ssDNA with three consecutive mismatches was used for CRISPR/Cas9-assisted recombineering under Dam overexpression. We obtained 35 monoclonal strains per plate, with a positive rate of approximately 71.4%. This method was much more efficient than that without Dam.
Seamless engineering of L. plantarum toward GlcNAc production.
First, we knocked out the nagB gene, whose product catalyzes the reversal reaction of GlmS, via CRISPR/Cas9-assisted dsDNA recombineering. The strain was named WG1, and GlcNAc production increased to 193 mg/liter from 105 mg/liter. The glmS ribozyme in front of the glmS1 gene inhibits glmS1 expression via mRNA self-cleavage when sufficient GlcN-6P bonds to the ribozyme (32). We deleted the core region of the glmS ribozyme, including the cleavage site, and replaced it with the endogenous strong promoter 3a in a two-step method (Fig. 2D), resulting in strain WG2. The production was not further improved. We suspect that GlmS is the rate-limiting enzyme in GlcNAc production (33) and is tightly regulated at both the transcriptional and posttranscriptional levels. Mutations in glmS in E. coli can depress feedback inhibition (33). Compared with other homologous proteins, glycine 472 of GlmS1 in L. plantarum WCFS1 was highly conserved in the compared strains (Fig. 5A). Product feedback inhibition can be eliminated by mutating this site in E. coli; therefore, G472S was mutated to improve GlmS1 catalytic capacity. The resulting WG3 accumulated 456 mg/liter of GlcNAc. GlcNAc synthesis requires one precursor, acetyl-CoA, which is transferred onto GlcN-1P and forms GlcNAc-1P. Excess NADH generated through pyruvate decarboxylation to acetyl-CoA cannot be oxidized because L. plantarum normally relies on a fermentative metabolism. Reports have shown that L. plantarum WCFS1 induces respiration-like behavior when heme and menaquinone are in the fermentation medium (36, 37). The redundant NADH can be consumed by the electron transport chain as shown in Fig. 5B. By optimizing the cultivation conditions, this engineered strain produced 797.3 mg/liter of GlcNAc using glucose as the sole carbon source (Fig. 5D).
Conclusions.
In this study, a series of CRISPR/Cas9-assisted genome engineering methods were developed. Using CRISPR/Cas9 as the screening tool simplified the entire genetic manipulation process, and combining dsDNA/ssDNA recombineering yielded seamless genome modifications. Introducing DNA adenine methylase (Dam) significantly improved ssDNA recombineering efficiency in L. plantarum WCFS1. Using this genome editing tool, we constructed a GlcNAc-producing L. plantarum strain. The final strain yielded 797.3 mg/liter of GlcNAc without introducing exogenous marker genes or plasmids. Our strategy is a powerful method of extending the food and medical applications of L. plantarum.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Table S1 lists all plasmids and bacterial strains used in this study. L. plantarum WCFS1 was cultivated in de Man, Rogosa, and Sharpe (MRS) broth (Oxoid, England) at 37°C under static conditions. L. lactis MG1363 was used for pSIP411-based (31) plasmid amplification and was cultivated in M17 broth (Oxoid) supplemented with 1% glucose at 30°C under static conditions. E. coli XL1-Blue was used for shuttle vector construction and was cultivated in Luria-Bertani (LB) medium at 37°C and 220 rpm. For Lactobacillus and E. coli, the erythromycin concentrations were 5 μg/ml and 250 μg/ml and the chloramphenicol concentrations were 5 μg/ml and 10 μg/ml, respectively. Sakacin P-inducing peptide (GenScript) was added to the medium at 50 ng/ml.
Molecular techniques.
Plasmid DNA was extracted using plasmid minikits from Tiangen (Beijing, China). The genome was extracted using the TIANamp bacteria DNA kit from Tiangen (Beijing, China). E. coli competent cells were purchased from TransGen (Beijing, China). Phanta Max super-fidelity DNA Polymerase from Vazyme (Nanjing, China) was used for the PCR amplifications and assays. DNA was purified by the gel extraction kit and Cycle-Pure kit from Omega (USA). The DNA restriction enzymes for cloning were from Thermo Fisher Scientific (USA). The Gibson assembly method was used to assemble DNA fragments.
Lactobacillus plasmids and DNA fragments were electrotransformed as follows. Briefly, 100 μl of overnight cultures and induction peptide were inoculated into 5 ml SGMRS (MRS with 0.75 M sorbitol and 1% glycine) and cultivated at 37°C until the cell density at an optical density at 600 nm (OD600) reached 0.6. Then the cells were centrifuged and washed twice using SM buffer (952 mM sucrose and 3.5 mM MgCl2). Finally, the cells were centrifuged and resuspended in 80 μl SM buffer for electrotransformation. After standing on ice for 10 min, plasmids and/or DNA fragments mixed with the competent cells were added into 0.2-cm cuvettes (Bio-Rad) and electroporated with a Gene Pulser (2,000 V, 25 μF, 400 Ω; Bio-Rad). The mixture was then transferred to 1 ml SMRS broth (MRS with 0.5 M sucrose and 0.1 M MgCl2) and cultured at 37°C for 2h to recover unless otherwise indicated. Finally, the recovery medium that contained cells was plated onto MRS agar plates with the required antibiotics after being centrifuged and resuspended.
Plasmid construction and DNA fragment amplification.
Table S2 shows the sequences of all oligonucleotides used in this study. The CRISPR/Cas9-assisting plasmid was constructed as follows. The cas9 gene and sgRNA sequence from plasmid p99S-Cas9 were amplified by the primer pairs 403-Cas9-f/Cas9-3a-r and 3a-sgRNA(472)-f/sgRNA-403-r, respectively. The two fragments were cloned into pSIP403, digested with NcoI and XhoI by Gibson assembly to generate pSIP-C9(472), and transformed in E. coli XL1-Blue cells. The Cas9-3a-r and 3a-sgRNA(472) primers contained the endogenous constitutively expressed promoter 3a, and 3a-sgRNA(472) contained a 30-bp spacer. Plasmid pSIP-C9(nagB) and pSIP-C9(lox) construction was similar to that for pSIP-C9(472); only the spacer sequences differed. Plasmids used for recombineering were constructed as follows. The RecT gene and plasmid pSIP411 were amplified via RecT-f/RecT-r and p411-f/p411-r, respectively. The two amplicons were fused by Gibson assembly to generate plasmid p411-RecT-em. The erythromycin resistance gene was replaced by the chloramphenicol resistance gene with primers cm-f/cm-r and p411-cm-f/p411-cm-r, generating p411-RecT-cm. The adenine-specific methyltransferase subunit gene (lp_2243) was amplified by the primer pair Dam-f/Dam-r and was inserted into the p411-RecT-cm cut with EcoRI, generating p411-RecT-Dam-cm.
The dsDNA fragment was amplified as follows. dsDNA-ΔnagB was amplified and overlapped by primers nagB-f/nagB-r and nagB-f2/nagB-r2. dsDNA-ΔP3a was amplified and overlapped by primers 3a-f1/3a-r1, 3a-f2/3a-r2, and 3a-f3/3a-r3. dsDNA-Δlox was amplified and overlapped by primers 3a-f1/lox-r1 and lox-f2/3a-r3.
Strain cultivation.
To perform GlcNAc batch fermentation, a single colony was inoculated into 5 ml of MRS medium containing the appropriate antibiotics and incubated for 12 h at 37°C without shaking. The preculture was then inoculated into 50 ml of fresh MRS medium containing 30 g/liter glucose. Fermentation was performed at 37°C without shaking, and the pH was maintained at approximately 6.5 using 30% NH4OH and 4 mM H2SO4. The inducing peptides were added after 12 h.
GlcNAc synthesis requires acetyl-CoA as a precursor. Acetyl-CoA, which is derived from pyruvate decarboxylation, is transferred to GlcN-1P to form GlcNAc-1P. L. plantarum normally relies on fermentative metabolism, of which respiration (the electron transport chain) is not activated; thus, excess NADH generated through pyruvate decarboxylation cannot be oxidized. LAB can respire when hemin (an essential cofactor of cytochrome oxidase) and methylnaphthoquinone are present (40, 41). Thus, redundant NADH can be consumed by the electron transport chain, as shown in Fig. 5B (42, 43). L. plantarum aerobic culture conditions were cultivated in medium containing heme (20 μM) and menaquinone (10 μM) at 37°C and 220 rpm.
Analytical methods.
Samples were centrifuged at 12,000 rpm for 5 min; the cells were then resuspended with distilled water. The cell density was measured at 600 nm using a spectrophotometer (Shimazu, Japan). The supernatant was used to detect extracellular metabolites. Glucose, pyruvate, and GlcNAc were quantitatively determined via a high-performance liquid chromatograph (Shimadzu, Japan) equipped with a refractive index detector (RID-10A; Shimadzu, Japan) and an Aminex HPX-87H ion exclusion column (Bio-Rad, USA), as described previously (44, 45).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (grants 31670077 and 31670047) and the Natural Science Foundation of Shandong Province (grant ZR2017ZB0210).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01367-19.
REFERENCES
- 1.Van Huynegem K, Loos M, Steidler L. 2009. Immunomodulation by genetically engineered lactic acid bacteria. Front Biosci 14:4825–4835. doi: 10.2741/3571. [DOI] [PubMed] [Google Scholar]
- 2.Teusink B, Smid EJ. 2006. Modelling strategies for the industrial exploitation of lactic acid bacteria. Nat Rev Microbiol 4:46–56. doi: 10.1038/nrmicro1319. [DOI] [PubMed] [Google Scholar]
- 3.Wegkamp A, de Vos WM, Smid EJ. 2009. Folate overproduction in Lactobacillus plantarum WCFS1 causes methotrexate resistance. FEMS Microbiol Lett 297:261–265. doi: 10.1111/j.1574-6968.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- 4.Hugenholtz J. 2008. The lactic acid bacterium as a cell factory for food ingredient production. Int Dairy J 18:466–475. doi: 10.1016/j.idairyj.2007.11.015. [DOI] [Google Scholar]
- 5.Gaspar P, Carvalho AL, Vinga S, Santos H, Neves AR. 2013. From physiology to systems metabolic engineering for the production of biochemicals by lactic acid bacteria. Biotechnol Adv 31:764–788. doi: 10.1016/j.biotechadv.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Biswas I, Gruss A, Ehrlich SD, Maguin E. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J Bacteriol 175:3628–3635. doi: 10.1128/jb.175.11.3628-3635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta S, Costantino N, Zhou X, Court DL. 2008. Identification and analysis of recombineering functions from Gram-negative and Gram-positive bacteria and their phages. Proc Natl Acad Sci U S A 105:1626–1631. doi: 10.1073/pnas.0709089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomason LC, Sawitzke JA, Li X, Costantino N, Court DL. 2014. Recombineering: genetic engineering in bacteria using homologous recombination. Curr Prot Mol Biol 106:1.16.1–1.16.39. doi: 10.1002/0471142727.mb0116s106. [DOI] [PubMed] [Google Scholar]
- 9.Swingle B, Bao Z, Markel E, Chambers A, Cartinhour S. 2010. Recombineering using RecTE from Pseudomonas syringae. Appl Environ Microbiol 76:4960–4968. doi: 10.1128/AEM.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin J, Zhu H, Xia L, Ding X, Hoffmann T, Hoffmann M, Bian X, Muller R, Fu J, Stewart AF, Zhang Y. 2015. A new recombineering system for Photorhabdus and Xenorhabdus. Nucleic Acids Res 43:e36. doi: 10.1093/nar/gku1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunny K, Liu J, Roth J. 2002. Phenotypes of lexA mutations in Salmonella enterica: evidence for a lethal lexA null phenotype due to the Fels-2 prophage. J Bacteriol 184:6235–6249. doi: 10.1128/jb.184.22.6235-6249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu S, Fu J, Huang F, Ding X, Stewart AF, Xia L, Zhang Y. 2014. Genome engineering of Agrobacterium tumefaciens using the lambda Red recombination system. Appl Microbiol Biotechnol 98:2165–2172. doi: 10.1007/s00253-013-5412-x. [DOI] [PubMed] [Google Scholar]
- 13.Xin Y, Guo T, Mu Y, Kong J. 2017. Development of a counterselectable seamless mutagenesis system in lactic acid bacteria. Microb Cell Fact 16:116. doi: 10.1186/s12934-017-0731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Oh JH, Alexander LM, Ozcam M, van Pijkeren JP. 2018. d-Alanyl-d-alanine ligase as a broad-host-range counterselection marker in vancomycin-resistant lactic acid bacteria. J Bacteriol 200:e00607-17. doi: 10.1128/JB.00607-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang P, Wang J, Qi Q. 2015. Prophage recombinases-mediated genome engineering in Lactobacillus plantarum. Microb Cell Fact 14:154. doi: 10.1186/s12934-015-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abremski K, Hoess R. 1984. Bacteriophage P1 site-specific recombination: purification and properties of the Cre recombinase protein. J Biol Chem 259:1509–1514. [PubMed] [Google Scholar]
- 17.Lambert JM, Bongers RS, Kleerebezem M. 2007. Cre-lox-based system for multiple gene deletions and selectable-marker removal in Lactobacillus plantarum. Appl Environ Microbiol 73:1126–1135. doi: 10.1128/AEM.01473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song X, Huang H, Xiong Z, Ai L, Yang S. 2017. CRISPR-Cas9D10A nickase-assisted genome editing in Lactobacillus casei. Appl Environ Microbiol 31:e01259-17. doi: 10.1128/AEM.01259-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Pijkeren JP, Britton RA. 2014. Precision genome engineering in lactic acid bacteria. Microb Cell Fact 13(Suppl 1):S10. doi: 10.1186/1475-2859-13-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Pijkeren JP, Neoh KM, Sirias D, Findley AS, Britton RA. 2012. Exploring optimization parameters to increase ssDNA recombineering in Lactococcus lactis and Lactobacillus reuteri. Bioengineered 3:209–217. doi: 10.4161/bioe.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. 2013. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat Biotechnol 31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh JH, van Pijkeren JP. 2014. CRISPR-Cas9-assisted recombineering in Lactobacillus reuteri. Nucleic Acids Res 42:e131. doi: 10.1093/nar/gku623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leenay RT, Vento JM, Shah M, Martino ME, Leulier F, Beisel CL. 2019. Genome editing with CRISPR-Cas9 in Lactobacillus plantarum revealed that editing outcomes can vary across strains and between methods. Biotechnol J 14:e1700583. doi: 10.1002/biot.201700583. [DOI] [PubMed] [Google Scholar]
- 25.Tamai Y, Miyatake K, Okamoto Y, Takamori Y, Sakamoto K, Minami S. 2003. Enhanced healing of cartilaginous injuries by N-acetyl-d-glucosamine and glucuronic acid. Carbonhyd Polym 54:251–262. doi: 10.1016/S0144-8617(03)00170-X. [DOI] [Google Scholar]
- 26.Chen J-K, Shen C-R, Liu C-L. 2010. N-acetylglucosamine: production and applications. Mar Drugs 8:2493–2516. doi: 10.3390/md8092493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubomura D, Ueno T, Yamada M, Nagaoka I. 2017. Evaluation of the chondroprotective action of N-acetylglucosamine in a rat experimental osteoarthritis model. Exp Ther Med 14:3137–3144. doi: 10.3892/etm.2017.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng M-D, Severson DK, Grund AD, Wassink SL, Burlingame RP, Berry A, Running JA, Kunesh CA, Song L, Jerrell TA, Rosson RA. 2005. Metabolic engineering of Escherichia coli for industrial production of glucosamine and N-acetylglucosamine. Metab Eng 7:201–214. doi: 10.1016/j.ymben.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Liu L, Shin H-D, Chen RR, Li J, Du G, Chen J. 2013. Pathway engineering of Bacillus subtilis for microbial production of N-acetylglucosamine. Metab Eng 19:107–115. doi: 10.1016/j.ymben.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Su T, Jin H, Zheng Y, Zhao Q, Chang Y, Wang Q, Qi Q. 2018. Improved ssDNA recombineering for rapid and efficient pathway engineering in Corynebacterium glutamicum. J Chem Technol Biotechnol 93:3535–3542. doi: 10.1002/jctb.5726. [DOI] [Google Scholar]
- 31.Sorvig E, Mathiesen G, Naterstad K, Eijsink VG, Axelsson L. 2005. High-level, inducible gene expression in Lactobacillus sakei and Lactobacillus plantarum using versatile expression vectors. Microbiology 151:2439–2449. doi: 10.1099/mic.0.28084-0. [DOI] [PubMed] [Google Scholar]
- 32.Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, Lee M, Roth A, Sudarsan N, Jona I, Wickiser JK, Breaker RR. 2004. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc Natl Acad Sci U S A 101:6421–6426. doi: 10.1073/pnas.0308014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng M-D, Grund AD, Wassink SL, Peng SS, Nielsen KL, Huckins BD, Walsh BL, Burlingame RP. 2006. Directed evolution and characterization of Escherichia coli glucosamine synthase. Biochimie 88:419–429. doi: 10.1016/j.biochi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 34.van Pijkeren JP, Britton RA. 2012. High efficiency recombineering in lactic acid bacteria. Nucleic Acids Res 40:e76. doi: 10.1093/nar/gks147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glickman BW, Radman M. 1980. Escherichia coli mutator mutants deficient in methylation-instructed DNA mismatch correction. Proc Natl Acad Sci U S A 77:1063–1067. doi: 10.1073/pnas.77.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horst JP, Wu TH, Marinus MG. 1999. Escherichia coli mutator genes. Trends Microbiol 7:29–36. doi: 10.1016/S0966-842X(98)01424-3. [DOI] [PubMed] [Google Scholar]
- 37.Hong ES, Yeung A, Funchain P, Slupska MM, Miller JH. 2005. Mutants with temperature-sensitive defects in the Escherichia coli mismatch repair system: sensitivity to mispairs generated in vivo. J Bacteriol 187:840–846. doi: 10.1128/JB.187.3.840-846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lennen RM, Nilsson Wallin AI, Pedersen M, Bonde M, Luo H, Herrgard MJ, Sommer MO. 2016. Transient overexpression of DNA adenine methylase enables efficient and mobile genome engineering with reduced off-target effects. Nucleic Acids Res 44:e36. doi: 10.1093/nar/gkv1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costantino N, Court DL. 2003. Enhanced levels of lambda Red-mediated recombinants in mismatch repair mutants. Proc Natl Acad Sci U S A 100:15748–15753. doi: 10.1073/pnas.2434959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koebmann B, Blank LM, Solem C, Petranovic D, Nielsen LK, Jensen PR. 2008. Increased biomass yield of Lactococcus lactis during energetically limited growth and respiratory conditions. Biotechnol Appl Biochem 50:25–33. doi: 10.1042/BA20070132. [DOI] [PubMed] [Google Scholar]
- 41.Tachon S, Brandsma JB, Yvon M. 2010. NoxE NADH oxidase and the electron transport chain are responsible for the ability of Lactococcus lactis to decrease the redox potential of milk. Appl Environ Microbiol 76:1311–1319. doi: 10.1128/AEM.02120-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Wang Z, Kandasamy V, Lee SY, Solem C, Jensen PR. 2017. Harnessing the respiration machinery for high-yield production of chemicals in metabolically engineered Lactococcus lactis. Metab Eng 44:22–29. doi: 10.1016/j.ymben.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Brooijmans RJ, de Vos WM, Hugenholtz J. 2009. Lactobacillus plantarum WCFS1 electron transport chains. Appl Environ Microbiol 75:3580–3585. doi: 10.1128/AEM.00147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang P, Wang J, Pang Q, Zhang F, Wang J, Wang Q, Qi Q. 2017. Pathway optimization and key enzyme evolution of N-acetylneuraminate biosynthesis using an in vivo aptazyme-based biosensor. Metab Eng 43:21–28. doi: 10.1016/j.ymben.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Kang J, Gu P, Wang Y, Li Y, Yang F, Wang Q, Qi Q. 2012. Engineering of an N-acetylneuraminic acid synthetic pathway in Escherichia coli. Metab Eng 14:623–629. doi: 10.1016/j.ymben.2012.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.